User login
Combination treatment shows promise for men with advanced prostate cancer
The findings were specific to patients not yet been treated with chemotherapy and whose tumors were positive for homologous recombination deficiency (HRD). However, for patients whose tumors were negative for HRD, the clinical activity was limited, said Daniel P. Petrylak, MD, Yale University, New Haven, Conn., and lead investigator for the study called CheckMate 9KD (NCT03338790) .
The patients who were included in all CheckMate 9KD cohorts had no prior treatment with targeted T-cell co-stimulation or immune checkpoint pathways. They had metastatic castrate resistant prostate cancer with documented disease progression, ECOG performance status of 0-1, and tissue available for HRD testing.
Dr. Petrylak offered an updated analysis of cohort A2 with 71 patients (median age 73 years), all of whom had received 1-2 prior new hormonal therapies in the pre-chemotherapy setting. Patients who had received prior PARP inhibitors were ineligible, as were those who refused chemotherapy treatment.
ORR/PSA RR primary endpoints
Patients received nivolumab and rucaparib, nivolumab at 480 mg (q4 weeks up to 2 years) and rucaparib at 600 mg b.i.d., until disease progression or unacceptable toxicity. Objective response rate and PSA response rate (PSA-RR) were the primary endpoint, with overall survival as a secondary endpoint, along with time to objective response, duration of objective response, time to PSA progression, safety, and radiographic progression-free survival.
Median follow-up was 17.5 months with median treatment duration of 4.6 months in the nivolumab group and 5.5 months for rucaparib. At the time of the final database lock in March 2021, 65 patients (91.5%) had discontinued treatment, most often for disease progression (n = 43; 60.6%) or study drug toxicity (n = 8; 11.3%). Four patients (5.6%) remained on treatment.
Better responses for HRD and BRCA 1/2 positive
Stratifying response outcomes showed higher rates for patients who were HRD positive and BRCA1/2 positive for confirmed objective response rate (HRD+ 25.0%, BRCA 1/2+ 33.3%, HRD-/not evaluable 5.3%, all patients 15.4%) and for PSA response (HRD+ 41.9%, BRCA 1/2+ 84.6%, HRD-/not evaluable 14.3%, all patients 27.3%). Partial response rates were 33.3% for BRCA 1/2, 25.0% for HRD positive, 5.3% for HRD- and 15.4% for all patients. Radiographic progression-free survival was longer in the HRD positive group at a median of 10.9 months (95% CI 6.7-12.0), compared with 5.6 months (3.7-9.1) in the HRD-/not evaluable group. Overall survival was similar in the HRD negative group/not evaluable group at 19.0 months (8.2-22.1) and the HRD positive group at 22.7 months (14.1-NE).
Safety profile as expected
Treatment-related adverse events were reported for most patients (64/71, 90.1%), with grade 3-4 events in about half (50.7%). The most common event was grade 1-2 nausea (40.8%), with anemia at 32.4% and alanine aminotransferase (ALT) increases and fatigue both at 28.2%. Adverse events led to discontinuation in 23.9% of patients, with anemia and increased ALT leading both at 4.2%. Grade 3-4 adverse events led to discontinuation in 15.5% of patients. Investigators reported no treatment-related deaths. “The safety profile of nivolumab plus rucaparib was as expected based on the individual components with no new safety signals,” Dr. Petrylak said.
Longer follow-up is needed, Dr. Petrylak added, to better characterize the clinical benefits of adding nivolumab to rucaparib for this population.
Discussion moderator Guilia Baciarello, MD, Milan, asked how much nivolumab added to the rucaparib benefit. Dr. Petrylak responded, “We really can’t determine how much it’s adding because the single-agent data, particularly with the checkpoints, is generally very low. I can’t recall any published data with nivolumab as a single agent, but for example with pembrolizumab or atezolizumab in unselected patients it’s 5%-10%. So, we really can’t tell how much nivolumab added in the BRCA positive patients.”
Dr. Baciarello asked, “Will there be a nivolumab versus rucaparib trial in HRD positive patients?”
“I think that’s something that needs to be considered. I think we may also want to consider doing a broader phase II in that group of patients to really nail down the signal. That’s under discussion,” Dr. Petrylak said.
The study was funded by Bristol Myers Squibb. Dr. Petrylak disclosed numerous financial interests including personal and consulting fees.
This article was updated Sept. 24, 2021.
The findings were specific to patients not yet been treated with chemotherapy and whose tumors were positive for homologous recombination deficiency (HRD). However, for patients whose tumors were negative for HRD, the clinical activity was limited, said Daniel P. Petrylak, MD, Yale University, New Haven, Conn., and lead investigator for the study called CheckMate 9KD (NCT03338790) .
The patients who were included in all CheckMate 9KD cohorts had no prior treatment with targeted T-cell co-stimulation or immune checkpoint pathways. They had metastatic castrate resistant prostate cancer with documented disease progression, ECOG performance status of 0-1, and tissue available for HRD testing.
Dr. Petrylak offered an updated analysis of cohort A2 with 71 patients (median age 73 years), all of whom had received 1-2 prior new hormonal therapies in the pre-chemotherapy setting. Patients who had received prior PARP inhibitors were ineligible, as were those who refused chemotherapy treatment.
ORR/PSA RR primary endpoints
Patients received nivolumab and rucaparib, nivolumab at 480 mg (q4 weeks up to 2 years) and rucaparib at 600 mg b.i.d., until disease progression or unacceptable toxicity. Objective response rate and PSA response rate (PSA-RR) were the primary endpoint, with overall survival as a secondary endpoint, along with time to objective response, duration of objective response, time to PSA progression, safety, and radiographic progression-free survival.
Median follow-up was 17.5 months with median treatment duration of 4.6 months in the nivolumab group and 5.5 months for rucaparib. At the time of the final database lock in March 2021, 65 patients (91.5%) had discontinued treatment, most often for disease progression (n = 43; 60.6%) or study drug toxicity (n = 8; 11.3%). Four patients (5.6%) remained on treatment.
Better responses for HRD and BRCA 1/2 positive
Stratifying response outcomes showed higher rates for patients who were HRD positive and BRCA1/2 positive for confirmed objective response rate (HRD+ 25.0%, BRCA 1/2+ 33.3%, HRD-/not evaluable 5.3%, all patients 15.4%) and for PSA response (HRD+ 41.9%, BRCA 1/2+ 84.6%, HRD-/not evaluable 14.3%, all patients 27.3%). Partial response rates were 33.3% for BRCA 1/2, 25.0% for HRD positive, 5.3% for HRD- and 15.4% for all patients. Radiographic progression-free survival was longer in the HRD positive group at a median of 10.9 months (95% CI 6.7-12.0), compared with 5.6 months (3.7-9.1) in the HRD-/not evaluable group. Overall survival was similar in the HRD negative group/not evaluable group at 19.0 months (8.2-22.1) and the HRD positive group at 22.7 months (14.1-NE).
Safety profile as expected
Treatment-related adverse events were reported for most patients (64/71, 90.1%), with grade 3-4 events in about half (50.7%). The most common event was grade 1-2 nausea (40.8%), with anemia at 32.4% and alanine aminotransferase (ALT) increases and fatigue both at 28.2%. Adverse events led to discontinuation in 23.9% of patients, with anemia and increased ALT leading both at 4.2%. Grade 3-4 adverse events led to discontinuation in 15.5% of patients. Investigators reported no treatment-related deaths. “The safety profile of nivolumab plus rucaparib was as expected based on the individual components with no new safety signals,” Dr. Petrylak said.
Longer follow-up is needed, Dr. Petrylak added, to better characterize the clinical benefits of adding nivolumab to rucaparib for this population.
Discussion moderator Guilia Baciarello, MD, Milan, asked how much nivolumab added to the rucaparib benefit. Dr. Petrylak responded, “We really can’t determine how much it’s adding because the single-agent data, particularly with the checkpoints, is generally very low. I can’t recall any published data with nivolumab as a single agent, but for example with pembrolizumab or atezolizumab in unselected patients it’s 5%-10%. So, we really can’t tell how much nivolumab added in the BRCA positive patients.”
Dr. Baciarello asked, “Will there be a nivolumab versus rucaparib trial in HRD positive patients?”
“I think that’s something that needs to be considered. I think we may also want to consider doing a broader phase II in that group of patients to really nail down the signal. That’s under discussion,” Dr. Petrylak said.
The study was funded by Bristol Myers Squibb. Dr. Petrylak disclosed numerous financial interests including personal and consulting fees.
This article was updated Sept. 24, 2021.
The findings were specific to patients not yet been treated with chemotherapy and whose tumors were positive for homologous recombination deficiency (HRD). However, for patients whose tumors were negative for HRD, the clinical activity was limited, said Daniel P. Petrylak, MD, Yale University, New Haven, Conn., and lead investigator for the study called CheckMate 9KD (NCT03338790) .
The patients who were included in all CheckMate 9KD cohorts had no prior treatment with targeted T-cell co-stimulation or immune checkpoint pathways. They had metastatic castrate resistant prostate cancer with documented disease progression, ECOG performance status of 0-1, and tissue available for HRD testing.
Dr. Petrylak offered an updated analysis of cohort A2 with 71 patients (median age 73 years), all of whom had received 1-2 prior new hormonal therapies in the pre-chemotherapy setting. Patients who had received prior PARP inhibitors were ineligible, as were those who refused chemotherapy treatment.
ORR/PSA RR primary endpoints
Patients received nivolumab and rucaparib, nivolumab at 480 mg (q4 weeks up to 2 years) and rucaparib at 600 mg b.i.d., until disease progression or unacceptable toxicity. Objective response rate and PSA response rate (PSA-RR) were the primary endpoint, with overall survival as a secondary endpoint, along with time to objective response, duration of objective response, time to PSA progression, safety, and radiographic progression-free survival.
Median follow-up was 17.5 months with median treatment duration of 4.6 months in the nivolumab group and 5.5 months for rucaparib. At the time of the final database lock in March 2021, 65 patients (91.5%) had discontinued treatment, most often for disease progression (n = 43; 60.6%) or study drug toxicity (n = 8; 11.3%). Four patients (5.6%) remained on treatment.
Better responses for HRD and BRCA 1/2 positive
Stratifying response outcomes showed higher rates for patients who were HRD positive and BRCA1/2 positive for confirmed objective response rate (HRD+ 25.0%, BRCA 1/2+ 33.3%, HRD-/not evaluable 5.3%, all patients 15.4%) and for PSA response (HRD+ 41.9%, BRCA 1/2+ 84.6%, HRD-/not evaluable 14.3%, all patients 27.3%). Partial response rates were 33.3% for BRCA 1/2, 25.0% for HRD positive, 5.3% for HRD- and 15.4% for all patients. Radiographic progression-free survival was longer in the HRD positive group at a median of 10.9 months (95% CI 6.7-12.0), compared with 5.6 months (3.7-9.1) in the HRD-/not evaluable group. Overall survival was similar in the HRD negative group/not evaluable group at 19.0 months (8.2-22.1) and the HRD positive group at 22.7 months (14.1-NE).
Safety profile as expected
Treatment-related adverse events were reported for most patients (64/71, 90.1%), with grade 3-4 events in about half (50.7%). The most common event was grade 1-2 nausea (40.8%), with anemia at 32.4% and alanine aminotransferase (ALT) increases and fatigue both at 28.2%. Adverse events led to discontinuation in 23.9% of patients, with anemia and increased ALT leading both at 4.2%. Grade 3-4 adverse events led to discontinuation in 15.5% of patients. Investigators reported no treatment-related deaths. “The safety profile of nivolumab plus rucaparib was as expected based on the individual components with no new safety signals,” Dr. Petrylak said.
Longer follow-up is needed, Dr. Petrylak added, to better characterize the clinical benefits of adding nivolumab to rucaparib for this population.
Discussion moderator Guilia Baciarello, MD, Milan, asked how much nivolumab added to the rucaparib benefit. Dr. Petrylak responded, “We really can’t determine how much it’s adding because the single-agent data, particularly with the checkpoints, is generally very low. I can’t recall any published data with nivolumab as a single agent, but for example with pembrolizumab or atezolizumab in unselected patients it’s 5%-10%. So, we really can’t tell how much nivolumab added in the BRCA positive patients.”
Dr. Baciarello asked, “Will there be a nivolumab versus rucaparib trial in HRD positive patients?”
“I think that’s something that needs to be considered. I think we may also want to consider doing a broader phase II in that group of patients to really nail down the signal. That’s under discussion,” Dr. Petrylak said.
The study was funded by Bristol Myers Squibb. Dr. Petrylak disclosed numerous financial interests including personal and consulting fees.
This article was updated Sept. 24, 2021.
FROM ESMO 2021
Datopotamab deruxtecan for advanced NSCLC encouraging so far
according to Edward B. Garon, MD, of the University of California, Los Angeles. Prior results from TROPION-PanTumor01, have demonstrated similarly encouraging activity and a manageable safety profile for Dato-DXd, Dr. Garon said in a 2021 European Society for Medical Oncology Congress virtual oral presentation on Sept. 19 (abstract LBA49).
Limited benefit from existing treatments
Once tyrosine kinase inhibitors and platinum chemotherapy have failed, patients with advanced/metastatic NSCLC with AGAs (e.g., EGFR or ALK mutations) derive limited benefit from existing treatments, Dr. Garon observed. Datopotamab deruxtecan is an antibody-drug conjugate composed of a humanized anti-TROP2 monoclonal antibody conjugated to a potent topoisomerase I inhibitor payload via a stable tetrapeptide-based cleavable linker. TROP2 is highly expressed in NSCLC, regardless of genomic mutation status and has been associated with poor prognosis. Patients in TROPION-PanTumor01 were not selected based on TROP2 expression or AGA status, Dr. Garon noted.
TROPION-PanTumor01 (NCT03401385), an ongoing multicenter, open-label, dose-expansion study evaluating datopotamab deruxtecan in solid tumors, including NSCLC in 210 patients, is assessing safety, pharmacokinetics, antitumor activity, and biomarkers. All included patients (n = 180; median age, 62 years; 56% female) had progressed after standard treatment or had measurable disease and had no standard treatment available. Stable/treated brain metastases were permitted.
Subgroup with AGAs
The current report includes outcomes from the subgroup of 34 patients with AGAs, who were treated with 4 (n = 8), 6 (n = 10), and 8 mg/kg (n = 16) of datopotamab deruxtecan. AGAs were EGFR in 29 patients, ALK in 3, and ROS1 and RET in 1 each. Most patients (82%) had received three or more prior regimens; 85% had prior TKI, and among EGFR mutation patients, 69% had received osimertinib. Prior systemic treatment consisted of immunotherapy in 41%, platinum-based chemotherapy in 91%, and tyrosine kinase inhibitor in 85%. The primary objectives were to establish the maximum tolerated dose, safety, and tolerability. Efficacy was a secondary outcome.
Treatment-emergent adverse events were reported in all patients, with grade 3 or higher events in 53%. Most common were grade 1-2 nausea, stomatitis, fatigue, and alopecia. Drug-attributed events in 88% were grade 3 or higher in 38%. Treatment-emergent adverse events led to discontinuation in 15%, dose interruption in 27% and dose reductions in 15%. One case of grade 5 interstitial lung disease, in the 8-mg group, was adjudicated as drug related. “The safety profile of Dato-DXd was manageable and consistent with that observed in the overall NSCLC population in TROPION-PanTumor01,” Dr. Garon said, “and were primarily nonhematologic.”
The objective response rate was 35%, all partial responses. The stable disease rate was 41%; the progressive disease rate was 6%. Median duration of response was 9.5 months (95% confidence interval, 3.3-NE). Dr. Garon noted that clinical activity was observed in EGFR (Ex 19del, L858R) including after osimertinib and across other AGAs.
Further evaluation ongoing
Further evaluation of datopotamab deruxtecan is ongoing in the TROPION-Lung05 study among NSCLC patients with AGAs after targeted therapy and platinum-based chemotherapy options have been exhausted. Eligible AGAs include EGFR (including exon 20 insertions), ALK, ROS1, RET, BRAF, NTRK and MET exon 14 skipping.
Session moderator David Gandara, MD, University of California Davis Health, questioned the rationale for targeting oncogene driven cancers with this particular drug: “Is this just because this is felt to be an unmet need, or is there higher expression or some other biologic rationale?”
Dr. Garon responded, “Why are we looking at these driver mutation–positive patients? I think it has less to do with mechanism and more to do with the differences in treatment between these driver mutation positive patients and the rest of the population. This is a group of patients which has TROP2, but TROP2 expression is seen really across non–small cell lung cancer. But, in fact, one of the reasons it has been postulated that TROP2 is not a good biomarker for this class of drugs to date, is that its expression is so ubiquitous in the disease.”
The study was funded by Daiichi Sankyo. Dr. Garon disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
according to Edward B. Garon, MD, of the University of California, Los Angeles. Prior results from TROPION-PanTumor01, have demonstrated similarly encouraging activity and a manageable safety profile for Dato-DXd, Dr. Garon said in a 2021 European Society for Medical Oncology Congress virtual oral presentation on Sept. 19 (abstract LBA49).
Limited benefit from existing treatments
Once tyrosine kinase inhibitors and platinum chemotherapy have failed, patients with advanced/metastatic NSCLC with AGAs (e.g., EGFR or ALK mutations) derive limited benefit from existing treatments, Dr. Garon observed. Datopotamab deruxtecan is an antibody-drug conjugate composed of a humanized anti-TROP2 monoclonal antibody conjugated to a potent topoisomerase I inhibitor payload via a stable tetrapeptide-based cleavable linker. TROP2 is highly expressed in NSCLC, regardless of genomic mutation status and has been associated with poor prognosis. Patients in TROPION-PanTumor01 were not selected based on TROP2 expression or AGA status, Dr. Garon noted.
TROPION-PanTumor01 (NCT03401385), an ongoing multicenter, open-label, dose-expansion study evaluating datopotamab deruxtecan in solid tumors, including NSCLC in 210 patients, is assessing safety, pharmacokinetics, antitumor activity, and biomarkers. All included patients (n = 180; median age, 62 years; 56% female) had progressed after standard treatment or had measurable disease and had no standard treatment available. Stable/treated brain metastases were permitted.
Subgroup with AGAs
The current report includes outcomes from the subgroup of 34 patients with AGAs, who were treated with 4 (n = 8), 6 (n = 10), and 8 mg/kg (n = 16) of datopotamab deruxtecan. AGAs were EGFR in 29 patients, ALK in 3, and ROS1 and RET in 1 each. Most patients (82%) had received three or more prior regimens; 85% had prior TKI, and among EGFR mutation patients, 69% had received osimertinib. Prior systemic treatment consisted of immunotherapy in 41%, platinum-based chemotherapy in 91%, and tyrosine kinase inhibitor in 85%. The primary objectives were to establish the maximum tolerated dose, safety, and tolerability. Efficacy was a secondary outcome.
Treatment-emergent adverse events were reported in all patients, with grade 3 or higher events in 53%. Most common were grade 1-2 nausea, stomatitis, fatigue, and alopecia. Drug-attributed events in 88% were grade 3 or higher in 38%. Treatment-emergent adverse events led to discontinuation in 15%, dose interruption in 27% and dose reductions in 15%. One case of grade 5 interstitial lung disease, in the 8-mg group, was adjudicated as drug related. “The safety profile of Dato-DXd was manageable and consistent with that observed in the overall NSCLC population in TROPION-PanTumor01,” Dr. Garon said, “and were primarily nonhematologic.”
The objective response rate was 35%, all partial responses. The stable disease rate was 41%; the progressive disease rate was 6%. Median duration of response was 9.5 months (95% confidence interval, 3.3-NE). Dr. Garon noted that clinical activity was observed in EGFR (Ex 19del, L858R) including after osimertinib and across other AGAs.
Further evaluation ongoing
Further evaluation of datopotamab deruxtecan is ongoing in the TROPION-Lung05 study among NSCLC patients with AGAs after targeted therapy and platinum-based chemotherapy options have been exhausted. Eligible AGAs include EGFR (including exon 20 insertions), ALK, ROS1, RET, BRAF, NTRK and MET exon 14 skipping.
Session moderator David Gandara, MD, University of California Davis Health, questioned the rationale for targeting oncogene driven cancers with this particular drug: “Is this just because this is felt to be an unmet need, or is there higher expression or some other biologic rationale?”
Dr. Garon responded, “Why are we looking at these driver mutation–positive patients? I think it has less to do with mechanism and more to do with the differences in treatment between these driver mutation positive patients and the rest of the population. This is a group of patients which has TROP2, but TROP2 expression is seen really across non–small cell lung cancer. But, in fact, one of the reasons it has been postulated that TROP2 is not a good biomarker for this class of drugs to date, is that its expression is so ubiquitous in the disease.”
The study was funded by Daiichi Sankyo. Dr. Garon disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
according to Edward B. Garon, MD, of the University of California, Los Angeles. Prior results from TROPION-PanTumor01, have demonstrated similarly encouraging activity and a manageable safety profile for Dato-DXd, Dr. Garon said in a 2021 European Society for Medical Oncology Congress virtual oral presentation on Sept. 19 (abstract LBA49).
Limited benefit from existing treatments
Once tyrosine kinase inhibitors and platinum chemotherapy have failed, patients with advanced/metastatic NSCLC with AGAs (e.g., EGFR or ALK mutations) derive limited benefit from existing treatments, Dr. Garon observed. Datopotamab deruxtecan is an antibody-drug conjugate composed of a humanized anti-TROP2 monoclonal antibody conjugated to a potent topoisomerase I inhibitor payload via a stable tetrapeptide-based cleavable linker. TROP2 is highly expressed in NSCLC, regardless of genomic mutation status and has been associated with poor prognosis. Patients in TROPION-PanTumor01 were not selected based on TROP2 expression or AGA status, Dr. Garon noted.
TROPION-PanTumor01 (NCT03401385), an ongoing multicenter, open-label, dose-expansion study evaluating datopotamab deruxtecan in solid tumors, including NSCLC in 210 patients, is assessing safety, pharmacokinetics, antitumor activity, and biomarkers. All included patients (n = 180; median age, 62 years; 56% female) had progressed after standard treatment or had measurable disease and had no standard treatment available. Stable/treated brain metastases were permitted.
Subgroup with AGAs
The current report includes outcomes from the subgroup of 34 patients with AGAs, who were treated with 4 (n = 8), 6 (n = 10), and 8 mg/kg (n = 16) of datopotamab deruxtecan. AGAs were EGFR in 29 patients, ALK in 3, and ROS1 and RET in 1 each. Most patients (82%) had received three or more prior regimens; 85% had prior TKI, and among EGFR mutation patients, 69% had received osimertinib. Prior systemic treatment consisted of immunotherapy in 41%, platinum-based chemotherapy in 91%, and tyrosine kinase inhibitor in 85%. The primary objectives were to establish the maximum tolerated dose, safety, and tolerability. Efficacy was a secondary outcome.
Treatment-emergent adverse events were reported in all patients, with grade 3 or higher events in 53%. Most common were grade 1-2 nausea, stomatitis, fatigue, and alopecia. Drug-attributed events in 88% were grade 3 or higher in 38%. Treatment-emergent adverse events led to discontinuation in 15%, dose interruption in 27% and dose reductions in 15%. One case of grade 5 interstitial lung disease, in the 8-mg group, was adjudicated as drug related. “The safety profile of Dato-DXd was manageable and consistent with that observed in the overall NSCLC population in TROPION-PanTumor01,” Dr. Garon said, “and were primarily nonhematologic.”
The objective response rate was 35%, all partial responses. The stable disease rate was 41%; the progressive disease rate was 6%. Median duration of response was 9.5 months (95% confidence interval, 3.3-NE). Dr. Garon noted that clinical activity was observed in EGFR (Ex 19del, L858R) including after osimertinib and across other AGAs.
Further evaluation ongoing
Further evaluation of datopotamab deruxtecan is ongoing in the TROPION-Lung05 study among NSCLC patients with AGAs after targeted therapy and platinum-based chemotherapy options have been exhausted. Eligible AGAs include EGFR (including exon 20 insertions), ALK, ROS1, RET, BRAF, NTRK and MET exon 14 skipping.
Session moderator David Gandara, MD, University of California Davis Health, questioned the rationale for targeting oncogene driven cancers with this particular drug: “Is this just because this is felt to be an unmet need, or is there higher expression or some other biologic rationale?”
Dr. Garon responded, “Why are we looking at these driver mutation–positive patients? I think it has less to do with mechanism and more to do with the differences in treatment between these driver mutation positive patients and the rest of the population. This is a group of patients which has TROP2, but TROP2 expression is seen really across non–small cell lung cancer. But, in fact, one of the reasons it has been postulated that TROP2 is not a good biomarker for this class of drugs to date, is that its expression is so ubiquitous in the disease.”
The study was funded by Daiichi Sankyo. Dr. Garon disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
FROM ESMO 2021
TULIP trial shows extended survival in HER2+ metastatic breast cancer
, according to Cristina Saura Manich, MD, Hospital Universitario Valle de Hebrón, Barcelona. In TULIP, trastuzumab duocarmazine (SYD985, Byondis B.V., NL) was compared with physician’s choice of chemotherapy, Dr. Saura said at the virtual European Society for Medical Oncology Congress 2021 on Sept. 18 (abstract LBA15).
Trastuzumab duocarmazine, Dr. Manich noted, is a novel HER2-targeting antibody–drug conjugate based on trastuzumab and a cleavable linker-duocarmycin (vc-seco-DUBA) payload. Its three-way mechanism of action includes uptake of the antibody–drug conjugate by internalization and intracellular release of the payload, and two bystander effects: proteolytic cleavage and subsequent release of payload in the tumor microenvironment and diffusion of active payload to neighboring tumor cells.
Two or more prior therapies for metastatic breast cancer
TULIP investigators enrolled 437 patients from 83 sites in 11 countries with HER2-positive locally advanced or metastatic breast cancer who had received two or more therapies for metastatic disease (treatment for brain metastases allowed). They were randomized 2:1 to SYD985 (1.2 mg/kg IV every 21 days [n = 291]) or physician’s choice (PC) [n = 146] of one of three trastuzumab-containing combinations or lapatinib plus capecitabine. Treatment was continued until progression or unacceptable toxicity. The primary endpoint was centrally assessed PFS.
Longer progression-free survival with SYD985
Median age was 57 years, and the median number of prior metastatic breast cancer regimens was 4.7. Centrally reviewed progression-free survival was significantly longer in the SYD985 group at 7.0 months (5.4-7.2) versus 4.9 months (4.0-5.5) for PC (hazard ratio [HR], 0.64, 95% confidence interval [CI], 0.49-0.84, P = .002). Subgroup analysis, also centrally reviewed, revealed numerical advantage for SYD985 over physician choice across all categories (except for ECOG status 2). Analysis of progression-free survival by investigators showed a similar benefit for SYD985 (6.9 months versus 4.6 months, HR, 0.60, P < .001).
A first look at median overall survival showed a nonsignificant advantage for SYD985 (20.4 months versus 16.3 months (HR, 0.83, 95% CI, 0.62-1.09, P = .153). The overall response rate (partial or complete response) was similar between groups at 27.8% for SYD985 and 29.5% for PC, with reductions in target lesion measurement at 70.2% and 32.2% for SYD985 and physician choice, respectively. The clinical benefit rates were 38.5% for SYD985 and 32.2% for physician choice.
Ocular toxicity
Most patients had at least one treatment-related adverse event (96.5% SD985, 96.4% PC), and grade 3 or higher event rates were similar between groups (52.8% SYD985, 48.2% PC). The most frequently reported adverse events for SYD985 were ocular toxicity, with conjunctivitis reported in 38.2%, and keratitis in 38.2%, with fatigue at 33.3%; for physician’s choice these were diarrhea (35.8%), nausea (31.4%), and fatigue (29.9%). Interstitial lung disease pneumonitis was reported for 7.6% (5.2% grade 1-2) of patients treated with SYD985, including two grade 5 events. Eye toxicity led to discontinuations in 20.8% of SYD985 patients, dose modifications in 22.9%, with dose modifications for interstitial lung disease/pneumonitis in 5.2% of SYD985 patients. Six fatalities (2.1%) were reported in the SYD985 group, with four attributed to treatment. Assessment of health-related quality of life showed no significant difference between groups.
Dr. Manich outlined risk mitigation strategies. Patients with prior keratitis were excluded and patients were given prophylactic lubricating eye drops and regular eye exams by ophthalmologists. Treatment was discontinued if grade 3 or higher keratitis developed, and was delayed if grade 3 conjunctivitis developed until it reduced to grade 2. Also, patients with prior pneumonitis were excluded and CT lung scans were evaluated for lung changes. New or worsening respiratory symptoms triggered a full diagnostic workup. Treatment was discontinued for grade 2 or higher pneumonitis and delayed until resolution for grade 1 pneumonitis.
Another option
“It is encouraging to observe clinically meaningful and potentially practice changing PFS improvements in patients receiving treatment in the third line and beyond,” said Aditya Bardia, MD, of Massachusetts General Hospital and Harvard Medical School, Boston. “Several agents have been approved as treatments for HER2-positive metastatic breast cancer in recent years – including T-DXd, neratinib, tucatinib, and margetuximab – and [vic-]trastuzumab duocarmazine could eventually be another option.”
“At this time, there is only a minor 2-month difference in progression-free survival and a nonsignificant overall survival difference,” said Fatima Cardoso, MD, of Champalimaud Cancer Center, Lisbon, Portugal. “With the high incidence of ocular toxicity and four toxic deaths, we cannot recommend this drug for clinical practice, in my opinion.”
Dr. Manich concluded, “SYD985 can provide a new treatment option for patients with pretreated locally advanced or metastatic HER2-positive metastatic breast cancer.”
The study was funded by Byondis B.V. The authors disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
, according to Cristina Saura Manich, MD, Hospital Universitario Valle de Hebrón, Barcelona. In TULIP, trastuzumab duocarmazine (SYD985, Byondis B.V., NL) was compared with physician’s choice of chemotherapy, Dr. Saura said at the virtual European Society for Medical Oncology Congress 2021 on Sept. 18 (abstract LBA15).
Trastuzumab duocarmazine, Dr. Manich noted, is a novel HER2-targeting antibody–drug conjugate based on trastuzumab and a cleavable linker-duocarmycin (vc-seco-DUBA) payload. Its three-way mechanism of action includes uptake of the antibody–drug conjugate by internalization and intracellular release of the payload, and two bystander effects: proteolytic cleavage and subsequent release of payload in the tumor microenvironment and diffusion of active payload to neighboring tumor cells.
Two or more prior therapies for metastatic breast cancer
TULIP investigators enrolled 437 patients from 83 sites in 11 countries with HER2-positive locally advanced or metastatic breast cancer who had received two or more therapies for metastatic disease (treatment for brain metastases allowed). They were randomized 2:1 to SYD985 (1.2 mg/kg IV every 21 days [n = 291]) or physician’s choice (PC) [n = 146] of one of three trastuzumab-containing combinations or lapatinib plus capecitabine. Treatment was continued until progression or unacceptable toxicity. The primary endpoint was centrally assessed PFS.
Longer progression-free survival with SYD985
Median age was 57 years, and the median number of prior metastatic breast cancer regimens was 4.7. Centrally reviewed progression-free survival was significantly longer in the SYD985 group at 7.0 months (5.4-7.2) versus 4.9 months (4.0-5.5) for PC (hazard ratio [HR], 0.64, 95% confidence interval [CI], 0.49-0.84, P = .002). Subgroup analysis, also centrally reviewed, revealed numerical advantage for SYD985 over physician choice across all categories (except for ECOG status 2). Analysis of progression-free survival by investigators showed a similar benefit for SYD985 (6.9 months versus 4.6 months, HR, 0.60, P < .001).
A first look at median overall survival showed a nonsignificant advantage for SYD985 (20.4 months versus 16.3 months (HR, 0.83, 95% CI, 0.62-1.09, P = .153). The overall response rate (partial or complete response) was similar between groups at 27.8% for SYD985 and 29.5% for PC, with reductions in target lesion measurement at 70.2% and 32.2% for SYD985 and physician choice, respectively. The clinical benefit rates were 38.5% for SYD985 and 32.2% for physician choice.
Ocular toxicity
Most patients had at least one treatment-related adverse event (96.5% SD985, 96.4% PC), and grade 3 or higher event rates were similar between groups (52.8% SYD985, 48.2% PC). The most frequently reported adverse events for SYD985 were ocular toxicity, with conjunctivitis reported in 38.2%, and keratitis in 38.2%, with fatigue at 33.3%; for physician’s choice these were diarrhea (35.8%), nausea (31.4%), and fatigue (29.9%). Interstitial lung disease pneumonitis was reported for 7.6% (5.2% grade 1-2) of patients treated with SYD985, including two grade 5 events. Eye toxicity led to discontinuations in 20.8% of SYD985 patients, dose modifications in 22.9%, with dose modifications for interstitial lung disease/pneumonitis in 5.2% of SYD985 patients. Six fatalities (2.1%) were reported in the SYD985 group, with four attributed to treatment. Assessment of health-related quality of life showed no significant difference between groups.
Dr. Manich outlined risk mitigation strategies. Patients with prior keratitis were excluded and patients were given prophylactic lubricating eye drops and regular eye exams by ophthalmologists. Treatment was discontinued if grade 3 or higher keratitis developed, and was delayed if grade 3 conjunctivitis developed until it reduced to grade 2. Also, patients with prior pneumonitis were excluded and CT lung scans were evaluated for lung changes. New or worsening respiratory symptoms triggered a full diagnostic workup. Treatment was discontinued for grade 2 or higher pneumonitis and delayed until resolution for grade 1 pneumonitis.
Another option
“It is encouraging to observe clinically meaningful and potentially practice changing PFS improvements in patients receiving treatment in the third line and beyond,” said Aditya Bardia, MD, of Massachusetts General Hospital and Harvard Medical School, Boston. “Several agents have been approved as treatments for HER2-positive metastatic breast cancer in recent years – including T-DXd, neratinib, tucatinib, and margetuximab – and [vic-]trastuzumab duocarmazine could eventually be another option.”
“At this time, there is only a minor 2-month difference in progression-free survival and a nonsignificant overall survival difference,” said Fatima Cardoso, MD, of Champalimaud Cancer Center, Lisbon, Portugal. “With the high incidence of ocular toxicity and four toxic deaths, we cannot recommend this drug for clinical practice, in my opinion.”
Dr. Manich concluded, “SYD985 can provide a new treatment option for patients with pretreated locally advanced or metastatic HER2-positive metastatic breast cancer.”
The study was funded by Byondis B.V. The authors disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
, according to Cristina Saura Manich, MD, Hospital Universitario Valle de Hebrón, Barcelona. In TULIP, trastuzumab duocarmazine (SYD985, Byondis B.V., NL) was compared with physician’s choice of chemotherapy, Dr. Saura said at the virtual European Society for Medical Oncology Congress 2021 on Sept. 18 (abstract LBA15).
Trastuzumab duocarmazine, Dr. Manich noted, is a novel HER2-targeting antibody–drug conjugate based on trastuzumab and a cleavable linker-duocarmycin (vc-seco-DUBA) payload. Its three-way mechanism of action includes uptake of the antibody–drug conjugate by internalization and intracellular release of the payload, and two bystander effects: proteolytic cleavage and subsequent release of payload in the tumor microenvironment and diffusion of active payload to neighboring tumor cells.
Two or more prior therapies for metastatic breast cancer
TULIP investigators enrolled 437 patients from 83 sites in 11 countries with HER2-positive locally advanced or metastatic breast cancer who had received two or more therapies for metastatic disease (treatment for brain metastases allowed). They were randomized 2:1 to SYD985 (1.2 mg/kg IV every 21 days [n = 291]) or physician’s choice (PC) [n = 146] of one of three trastuzumab-containing combinations or lapatinib plus capecitabine. Treatment was continued until progression or unacceptable toxicity. The primary endpoint was centrally assessed PFS.
Longer progression-free survival with SYD985
Median age was 57 years, and the median number of prior metastatic breast cancer regimens was 4.7. Centrally reviewed progression-free survival was significantly longer in the SYD985 group at 7.0 months (5.4-7.2) versus 4.9 months (4.0-5.5) for PC (hazard ratio [HR], 0.64, 95% confidence interval [CI], 0.49-0.84, P = .002). Subgroup analysis, also centrally reviewed, revealed numerical advantage for SYD985 over physician choice across all categories (except for ECOG status 2). Analysis of progression-free survival by investigators showed a similar benefit for SYD985 (6.9 months versus 4.6 months, HR, 0.60, P < .001).
A first look at median overall survival showed a nonsignificant advantage for SYD985 (20.4 months versus 16.3 months (HR, 0.83, 95% CI, 0.62-1.09, P = .153). The overall response rate (partial or complete response) was similar between groups at 27.8% for SYD985 and 29.5% for PC, with reductions in target lesion measurement at 70.2% and 32.2% for SYD985 and physician choice, respectively. The clinical benefit rates were 38.5% for SYD985 and 32.2% for physician choice.
Ocular toxicity
Most patients had at least one treatment-related adverse event (96.5% SD985, 96.4% PC), and grade 3 or higher event rates were similar between groups (52.8% SYD985, 48.2% PC). The most frequently reported adverse events for SYD985 were ocular toxicity, with conjunctivitis reported in 38.2%, and keratitis in 38.2%, with fatigue at 33.3%; for physician’s choice these were diarrhea (35.8%), nausea (31.4%), and fatigue (29.9%). Interstitial lung disease pneumonitis was reported for 7.6% (5.2% grade 1-2) of patients treated with SYD985, including two grade 5 events. Eye toxicity led to discontinuations in 20.8% of SYD985 patients, dose modifications in 22.9%, with dose modifications for interstitial lung disease/pneumonitis in 5.2% of SYD985 patients. Six fatalities (2.1%) were reported in the SYD985 group, with four attributed to treatment. Assessment of health-related quality of life showed no significant difference between groups.
Dr. Manich outlined risk mitigation strategies. Patients with prior keratitis were excluded and patients were given prophylactic lubricating eye drops and regular eye exams by ophthalmologists. Treatment was discontinued if grade 3 or higher keratitis developed, and was delayed if grade 3 conjunctivitis developed until it reduced to grade 2. Also, patients with prior pneumonitis were excluded and CT lung scans were evaluated for lung changes. New or worsening respiratory symptoms triggered a full diagnostic workup. Treatment was discontinued for grade 2 or higher pneumonitis and delayed until resolution for grade 1 pneumonitis.
Another option
“It is encouraging to observe clinically meaningful and potentially practice changing PFS improvements in patients receiving treatment in the third line and beyond,” said Aditya Bardia, MD, of Massachusetts General Hospital and Harvard Medical School, Boston. “Several agents have been approved as treatments for HER2-positive metastatic breast cancer in recent years – including T-DXd, neratinib, tucatinib, and margetuximab – and [vic-]trastuzumab duocarmazine could eventually be another option.”
“At this time, there is only a minor 2-month difference in progression-free survival and a nonsignificant overall survival difference,” said Fatima Cardoso, MD, of Champalimaud Cancer Center, Lisbon, Portugal. “With the high incidence of ocular toxicity and four toxic deaths, we cannot recommend this drug for clinical practice, in my opinion.”
Dr. Manich concluded, “SYD985 can provide a new treatment option for patients with pretreated locally advanced or metastatic HER2-positive metastatic breast cancer.”
The study was funded by Byondis B.V. The authors disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
FROM ESMO 2021
ctDNA may be a better surrogate for survival than RECIST
according to Alexander Noor Shoushtari, MD, Memorial Sloan Kettering Cancer Center, New York.
Tebentafusp is the first therapy to demonstrate an overall survival (OS) benefit in uveal melanoma, Dr. Shoushtari noted in a 2021 European Society of Medical Oncology Congress virtual oral presentation Sept. 17 (abstract 17570). He noted further that, in prior research, OS was improved regardless of RECISTv1.1 best response, suggesting that better surrogate efficacy endpoints are needed.
Uveal melanoma is a rare melanoma type with low mutational burden, but frequent liver metastases. Benefit from immune checkpoint inhibitors is poor, and there is no established standard of care once the disease becomes metastatic. “Immune checkpoint inhibitors are not as good for treating this type of melanoma as they are for treating cutaneous disease, and traditionally preferred treatment is within clinical trials,” Dr. Shoushtari said. In frontline trials, 1-year survival has been in the 50% range. Tebentafusp is an investigational, first-in-class bispecific soluble T-cell receptor (TCR) therapeutic. It is designed to target gp100 (a melanoma-associated antigen) through a high-affinity TCR-binding domain and an anti-CD3 T-cell–engaging domain, which redirects T cells to kill gp100 positive melanocytic expressing tumor cells.
Prior research has demonstrated a disconnect between RECIST response classification and tebentafusp OS benefit. In the IMCgp100-202 study among patients treated first-line for metastatic uveal melanoma with tebentafusp or investigator choice, intent-to-treat analysis showed a survival probability benefit for tebentafusp (hazard ratio, 0.51; 95% CI, 0.37-0.71), with a best response of progressive disease population HR of 0.43 (95% CI, 0.27-0.68). While the RECIST response rate was only 9.1%, the HR for progression-free survival was 0.73 (95% CI, 0.58-0.94). “That suggests that RECIST is not a fantastic way to predict who will benefit from this drug,” Dr. Shoushtari stated.
Similarly in the IMCgp100-102 study of tebentafusp monotherapy in second-line metastatic uveal melanoma (n = 127), the RECIST response rate was 5%. Duration of response was 8.7 months and median OS was 16.8 months. Historical second-line OS has been reported at 7.8 months. The 1- and 2-year survival (62%/37%) compared favorably with historical rates (37%/15%), as well. Dr. Shoushtari noted that 92% of patients had detectable ctDNA with mutations in known uveal melanoma oncogenes. He pointed out that baseline ctDNA levels significantly correlated with tumor burden. Also, 70% of evaluable patients had any ctDNA reduction, with 0.5-3.2 log reduction in 99.9%, a 0.5 log reduction in 68% and 3 log reduction (cleared) in 14% of patients. ctDNA reduction, Dr. Shoushtari said, was associated with greater mean tumor shrinkage and with less tumor growth. Importantly, there was a linear correlation between ctDNA reduction and better OS (R2, 0.88, P < .0001).
Among all evaluable patients, comparing those with less than 0.5 log ctDNA reduction with those with at least a 0.5 log reduction showed a hazard ratio of 0.56 (95% CI, 0.32-0.95; P = .03). Among those whose best response was progressive disease, 35% had at least a 0.5 log reduction in ctDNA with an OS hazard ratio of 0.44 (95% CI, 0.2-0.94; P = .027), compared with less than a 0.5 log reduction. Among those whose best response was stable disease, 28% had at least 1 log reduction with a hazard ratio of 0.48 (95% CI 0.16-1.43; P = .16) for OS, compared with those with less than 1 log reduction. Dr. Shoushtari pointed out that “14% of patients cleared ctDNA, including some (n = 12) with best RECIST responses of stable or progressive disease. All patients with ctDNA clearance were alive beyond 1 year; with a hazard ratio, compared to those who had not cleared ctDNA, of 0.14 (95% CI, 0.03-0.57).
Summing up, Dr. Shoushtari said that ctDNA was detectable in more than 90% of second-line tebentafusp-treated patients with metastatic uveal melanoma and correlated with tumor burden. About 70% had ctDNA reduction versus 39% with tumor shrinkage and 5% RECIST response. The linear correlation between the magnitude of ctDNA reduction and improved OS on tebentafusp, was uncoupled from best RECIST response. “For tebentafusp, ctDNA reduction may be a better surrogate of overall survival than RECIST response.”
The ESMO-appointed discussant for the study, Christian Rolfo, MD, PhD, MBA, Icahan School of Medicine at Mount Sinai, New York, examined the tebentafusp study author’s claim that the radiographic assessment of tumors may underestimate the effect of tebentafusp, compared with ctDNA. The strengths of the study include, he said, that it is a drug- and tumor-specific evaluation of the role of ctDNA as a surrogate of response. “Its strength is that it shows an important correlation between ctDNA levels and overall survival, and that response rate is evaluated better with ctDNA.” A question that remains open, Dr. Rolfo added, is whether RECIST criteria are still a good comparator for biologic response.
The study was funded by Immunocore Dr. Shoushtari disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
according to Alexander Noor Shoushtari, MD, Memorial Sloan Kettering Cancer Center, New York.
Tebentafusp is the first therapy to demonstrate an overall survival (OS) benefit in uveal melanoma, Dr. Shoushtari noted in a 2021 European Society of Medical Oncology Congress virtual oral presentation Sept. 17 (abstract 17570). He noted further that, in prior research, OS was improved regardless of RECISTv1.1 best response, suggesting that better surrogate efficacy endpoints are needed.
Uveal melanoma is a rare melanoma type with low mutational burden, but frequent liver metastases. Benefit from immune checkpoint inhibitors is poor, and there is no established standard of care once the disease becomes metastatic. “Immune checkpoint inhibitors are not as good for treating this type of melanoma as they are for treating cutaneous disease, and traditionally preferred treatment is within clinical trials,” Dr. Shoushtari said. In frontline trials, 1-year survival has been in the 50% range. Tebentafusp is an investigational, first-in-class bispecific soluble T-cell receptor (TCR) therapeutic. It is designed to target gp100 (a melanoma-associated antigen) through a high-affinity TCR-binding domain and an anti-CD3 T-cell–engaging domain, which redirects T cells to kill gp100 positive melanocytic expressing tumor cells.
Prior research has demonstrated a disconnect between RECIST response classification and tebentafusp OS benefit. In the IMCgp100-202 study among patients treated first-line for metastatic uveal melanoma with tebentafusp or investigator choice, intent-to-treat analysis showed a survival probability benefit for tebentafusp (hazard ratio, 0.51; 95% CI, 0.37-0.71), with a best response of progressive disease population HR of 0.43 (95% CI, 0.27-0.68). While the RECIST response rate was only 9.1%, the HR for progression-free survival was 0.73 (95% CI, 0.58-0.94). “That suggests that RECIST is not a fantastic way to predict who will benefit from this drug,” Dr. Shoushtari stated.
Similarly in the IMCgp100-102 study of tebentafusp monotherapy in second-line metastatic uveal melanoma (n = 127), the RECIST response rate was 5%. Duration of response was 8.7 months and median OS was 16.8 months. Historical second-line OS has been reported at 7.8 months. The 1- and 2-year survival (62%/37%) compared favorably with historical rates (37%/15%), as well. Dr. Shoushtari noted that 92% of patients had detectable ctDNA with mutations in known uveal melanoma oncogenes. He pointed out that baseline ctDNA levels significantly correlated with tumor burden. Also, 70% of evaluable patients had any ctDNA reduction, with 0.5-3.2 log reduction in 99.9%, a 0.5 log reduction in 68% and 3 log reduction (cleared) in 14% of patients. ctDNA reduction, Dr. Shoushtari said, was associated with greater mean tumor shrinkage and with less tumor growth. Importantly, there was a linear correlation between ctDNA reduction and better OS (R2, 0.88, P < .0001).
Among all evaluable patients, comparing those with less than 0.5 log ctDNA reduction with those with at least a 0.5 log reduction showed a hazard ratio of 0.56 (95% CI, 0.32-0.95; P = .03). Among those whose best response was progressive disease, 35% had at least a 0.5 log reduction in ctDNA with an OS hazard ratio of 0.44 (95% CI, 0.2-0.94; P = .027), compared with less than a 0.5 log reduction. Among those whose best response was stable disease, 28% had at least 1 log reduction with a hazard ratio of 0.48 (95% CI 0.16-1.43; P = .16) for OS, compared with those with less than 1 log reduction. Dr. Shoushtari pointed out that “14% of patients cleared ctDNA, including some (n = 12) with best RECIST responses of stable or progressive disease. All patients with ctDNA clearance were alive beyond 1 year; with a hazard ratio, compared to those who had not cleared ctDNA, of 0.14 (95% CI, 0.03-0.57).
Summing up, Dr. Shoushtari said that ctDNA was detectable in more than 90% of second-line tebentafusp-treated patients with metastatic uveal melanoma and correlated with tumor burden. About 70% had ctDNA reduction versus 39% with tumor shrinkage and 5% RECIST response. The linear correlation between the magnitude of ctDNA reduction and improved OS on tebentafusp, was uncoupled from best RECIST response. “For tebentafusp, ctDNA reduction may be a better surrogate of overall survival than RECIST response.”
The ESMO-appointed discussant for the study, Christian Rolfo, MD, PhD, MBA, Icahan School of Medicine at Mount Sinai, New York, examined the tebentafusp study author’s claim that the radiographic assessment of tumors may underestimate the effect of tebentafusp, compared with ctDNA. The strengths of the study include, he said, that it is a drug- and tumor-specific evaluation of the role of ctDNA as a surrogate of response. “Its strength is that it shows an important correlation between ctDNA levels and overall survival, and that response rate is evaluated better with ctDNA.” A question that remains open, Dr. Rolfo added, is whether RECIST criteria are still a good comparator for biologic response.
The study was funded by Immunocore Dr. Shoushtari disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
according to Alexander Noor Shoushtari, MD, Memorial Sloan Kettering Cancer Center, New York.
Tebentafusp is the first therapy to demonstrate an overall survival (OS) benefit in uveal melanoma, Dr. Shoushtari noted in a 2021 European Society of Medical Oncology Congress virtual oral presentation Sept. 17 (abstract 17570). He noted further that, in prior research, OS was improved regardless of RECISTv1.1 best response, suggesting that better surrogate efficacy endpoints are needed.
Uveal melanoma is a rare melanoma type with low mutational burden, but frequent liver metastases. Benefit from immune checkpoint inhibitors is poor, and there is no established standard of care once the disease becomes metastatic. “Immune checkpoint inhibitors are not as good for treating this type of melanoma as they are for treating cutaneous disease, and traditionally preferred treatment is within clinical trials,” Dr. Shoushtari said. In frontline trials, 1-year survival has been in the 50% range. Tebentafusp is an investigational, first-in-class bispecific soluble T-cell receptor (TCR) therapeutic. It is designed to target gp100 (a melanoma-associated antigen) through a high-affinity TCR-binding domain and an anti-CD3 T-cell–engaging domain, which redirects T cells to kill gp100 positive melanocytic expressing tumor cells.
Prior research has demonstrated a disconnect between RECIST response classification and tebentafusp OS benefit. In the IMCgp100-202 study among patients treated first-line for metastatic uveal melanoma with tebentafusp or investigator choice, intent-to-treat analysis showed a survival probability benefit for tebentafusp (hazard ratio, 0.51; 95% CI, 0.37-0.71), with a best response of progressive disease population HR of 0.43 (95% CI, 0.27-0.68). While the RECIST response rate was only 9.1%, the HR for progression-free survival was 0.73 (95% CI, 0.58-0.94). “That suggests that RECIST is not a fantastic way to predict who will benefit from this drug,” Dr. Shoushtari stated.
Similarly in the IMCgp100-102 study of tebentafusp monotherapy in second-line metastatic uveal melanoma (n = 127), the RECIST response rate was 5%. Duration of response was 8.7 months and median OS was 16.8 months. Historical second-line OS has been reported at 7.8 months. The 1- and 2-year survival (62%/37%) compared favorably with historical rates (37%/15%), as well. Dr. Shoushtari noted that 92% of patients had detectable ctDNA with mutations in known uveal melanoma oncogenes. He pointed out that baseline ctDNA levels significantly correlated with tumor burden. Also, 70% of evaluable patients had any ctDNA reduction, with 0.5-3.2 log reduction in 99.9%, a 0.5 log reduction in 68% and 3 log reduction (cleared) in 14% of patients. ctDNA reduction, Dr. Shoushtari said, was associated with greater mean tumor shrinkage and with less tumor growth. Importantly, there was a linear correlation between ctDNA reduction and better OS (R2, 0.88, P < .0001).
Among all evaluable patients, comparing those with less than 0.5 log ctDNA reduction with those with at least a 0.5 log reduction showed a hazard ratio of 0.56 (95% CI, 0.32-0.95; P = .03). Among those whose best response was progressive disease, 35% had at least a 0.5 log reduction in ctDNA with an OS hazard ratio of 0.44 (95% CI, 0.2-0.94; P = .027), compared with less than a 0.5 log reduction. Among those whose best response was stable disease, 28% had at least 1 log reduction with a hazard ratio of 0.48 (95% CI 0.16-1.43; P = .16) for OS, compared with those with less than 1 log reduction. Dr. Shoushtari pointed out that “14% of patients cleared ctDNA, including some (n = 12) with best RECIST responses of stable or progressive disease. All patients with ctDNA clearance were alive beyond 1 year; with a hazard ratio, compared to those who had not cleared ctDNA, of 0.14 (95% CI, 0.03-0.57).
Summing up, Dr. Shoushtari said that ctDNA was detectable in more than 90% of second-line tebentafusp-treated patients with metastatic uveal melanoma and correlated with tumor burden. About 70% had ctDNA reduction versus 39% with tumor shrinkage and 5% RECIST response. The linear correlation between the magnitude of ctDNA reduction and improved OS on tebentafusp, was uncoupled from best RECIST response. “For tebentafusp, ctDNA reduction may be a better surrogate of overall survival than RECIST response.”
The ESMO-appointed discussant for the study, Christian Rolfo, MD, PhD, MBA, Icahan School of Medicine at Mount Sinai, New York, examined the tebentafusp study author’s claim that the radiographic assessment of tumors may underestimate the effect of tebentafusp, compared with ctDNA. The strengths of the study include, he said, that it is a drug- and tumor-specific evaluation of the role of ctDNA as a surrogate of response. “Its strength is that it shows an important correlation between ctDNA levels and overall survival, and that response rate is evaluated better with ctDNA.” A question that remains open, Dr. Rolfo added, is whether RECIST criteria are still a good comparator for biologic response.
The study was funded by Immunocore Dr. Shoushtari disclosed numerous pharmaceutical-related financial interests.
This article was updated Sept. 24, 2021.
FROM ESMO 2021
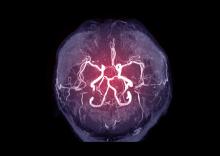
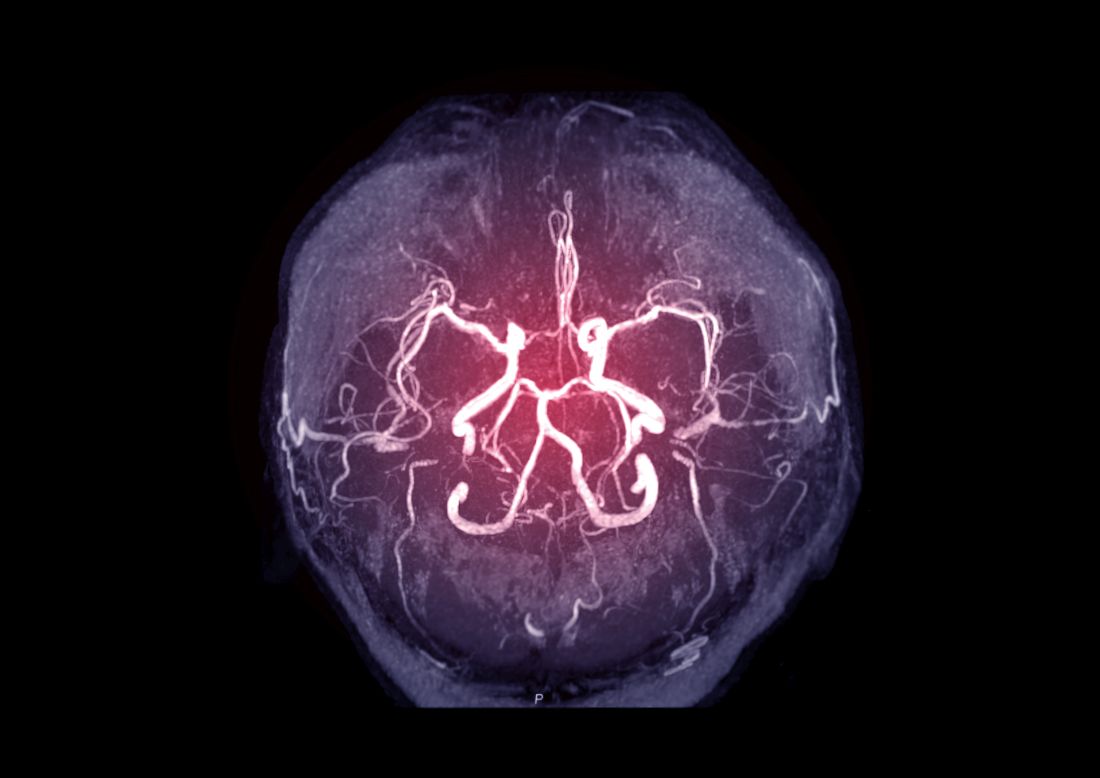
Intracranial atherosclerosis finding on MRA linked to stroke
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
An incidental diagnosis of intracranial atherosclerotic stenosis in stroke-free individuals should trigger a thorough assessment of vascular health, according to the authors of a study identifying risk factors and vascular event risk in asymptomatic ICAS.
That conclusion emerged from data collected on more than 1,000 stroke-free participants in NOMAS (Northern Manhattan Study), a trial that prospectively followed participants who underwent a brain magnetic resonance angiogram (MRA) during 2003-2008.
In ICAS patients with stenosis of at least 70%, even with aggressive medical therapy, the annual stroke recurrence rate is 10%-20% in those with occlusions and at least three or more vascular risk factors. This high rate of recurrent vascular events in patients with stroke caused by ICAS warrants greater focus on primary prevention and targeted interventions for stroke-free individuals at highest risk for ICAS-related events, the investigators concluded.
Identify high-risk ICAS
Using NOMAS data, the investigators, led by Jose Gutierrez, MD, MPH, tested the hypothesis that stroke-free subjects at high risk of stroke and vascular events could be identified through the presence of asymptomatic ICAS. NOMAS is an ongoing, population-based epidemiologic study among randomly selected people with home telephones living in northern Manhattan.
During 2003-2008, investigators invited participants who were at least 50 years old, stroke free, and without contraindications to undergo brain MRA. The 1,211 study members were followed annually via telephone and in-person adjudication of events. A control group of 79 patients with no MRA was also identified with similar rates of hypertension, diabetes, hypercholesterolemia and current smoking.
Mean age was about 71 years (59% female, 65% Hispanic, 45% any stenosis). At the time of MRA, 78% had hypertension, 25% had diabetes, 81% had hypercholesterolemia, and 11% were current smokers.
Researchers rated stenoses in 11 brain arteries as 0, with no stenosis; 1, with less than 50% stenosis or luminal irregularities; 2, 50%-69% stenosis; and 3, at least 70% stenosis or flow gap. Outcomes included vascular death, myocardial infarction, ischemic stroke, cardioembolic stroke, intracranial artery disease stroke (which combined intracranial small and large artery disease strokes), and any vascular events (defined as a composite of vascular death, any stroke, or MI).
Greater stenosis denotes higher risk
Analysis found ICAS to be associated with older age (odds ratio, 1.02 per year; 95% confidence interval, 1.01-1.04), hypertension duration (OR, 1.01 per year; 95% CI, 1.00-1.02), higher number of glucose-lowering drugs (OR, 1.64 per each medication; 95% CI, 1.24-2.15), and HDL cholesterol(OR, 0.96 per mg/dL; 95% CI, 0.92-0.99). Event risk was greater among participants with ICAS of at least 70% (5.5% annual risk of vascular events; HR, 2.1; 95% CI, 1.4-3.2; compared with those with no ICAS), the investigators reported in the Journal of the American College of Cardiology.
Furthermore, 80% of incident strokes initially classified as small artery disease occurred among individuals with evidence of any degree of ICAS at their baseline MRI, the investigators noted. They found also that individuals with ICAS who had a primary care physician at the time of their initial MRI had a lower risk of events. Frequent primary care visits, they observed, might imply greater control of risk factors and other unmeasured confounders, such as health literacy, health care trust, access, and availability.
Incidental ICAS should trigger vascular assessment
An incidental diagnosis of ICAS in stroke-free subjects should trigger a thorough assessment of vascular health, the investigators concluded. They commented also that prophylaxis of first-ever stroke at this asymptomatic stage “may magnify the societal benefits of vascular prevention and decrease stroke-related disability and vascular death in our communities.”
“The big gap in our knowledge,” Tanya N. Turan, MD, professor of neurology at Medical University of South Carolina, Charleston, wrote in an accompanying editorial “is understanding the pathophysiological triggers for an asymptomatic stenosis to become a high-risk symptomatic stenosis. Until that question is answered, screening for asymptomatic ICAS is unlikely to change management among patients with known vascular risk factors.” In an interview, she observed further that “MRI plaque imaging could be a useful research tool to see if certain plaque features in an asymptomatic lesion are high risk for causing stroke. If that were proven, then it would make more sense to screen for ICAS and develop specific therapeutic strategies targeting high-risk asymptomatic plaque.”
Focus on recurrent stroke misplaced
Dr. Gutierrez said in an interview: “In the stroke world, most of what we do focuses on preventing recurrent stroke. Nonetheless, three-fourths of strokes in this country are new strokes, so to me it doesn’t make much sense to spend most of our efforts and attention to prevent the smallest fractions of strokes that occur in our society.”
He stressed that “the first immediate application of our results is that if people having a brain MRA for other reasons are found to have incidental, and therefore asymptomatic, ICAS, then they should be aggressively treated for vascular risk factors.” Secondly, “we hope to identify the patients at the highest risk of prevalent ICAS before they have a stroke. Among them, a brain MRI/MRA evaluating the phenotype would determine how aggressively to treat LDL.”
Dr. Gutierrez, professor of neurology at Columbia University Irving Medical Center, New York, noted that educating patients of their underlying high risk of events may have the effect of engaging them more in their own care. “There is evidence that actually showing people scans increases compliance and health literacy. It’s not yet standard of care, but we hope our future projects will help advance the field in the primary prevention direction,” he said.
This work was supported by the National Institutes of Health. The authors reported that they had no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Esophageal cancer: Preoperative chemoradiotherapy benefit in CROSS persists over 10 years
Among patients with locally advanced resectable esophageal or junctional cancer, the overall survival benefit conferred by preoperative chemoradiotherapy persists for at least 10 years, according to long-term results of the Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study (CROSS). As a result of earlier publication of CROSS data, chemoradiotherapy followed by surgery has become one of the standards of care for patients with locally advanced resectable esophageal cancer, stated lead author Ben M. Eyck, MD, of Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues in the Journal of Clinical Oncology.
In the multicenter, randomized trial, initiated in 2004, 178 patients randomized to chemoradiotherapy with subsequent surgery and 188 patients randomized to surgery alone were followed with overall survival as the primary, and cause-specific survival and risks of locoregional and distant relapse as the secondary endpoints. Chemoradiotherapy consisted of 5 weekly cycles of carboplatin (area under the curve of 2 mg/mL/min) and paclitaxel (50 mg/m2 body surface area on days 1, 8, 15, 22, and 29) with concurrent radiotherapy (41.4 Gy in 23 fractions, 5 days per week. Mean age was 60 years (around 78% male), with squamous cell carcinoma (23%) and adenocarcinoma (75%) as the predominant histologies.
The first analysis showed low short-term toxicity and 2-year survival increased from 50% for patients receiving surgery alone to 67% for neoadjuvant chemoradiotherapy plus surgery. Five-year follow-up data were consistent with initial reporting. Long-term benefits and harms of this regimen remain unclear, according to the researchers. Neoadjuvant chemoradiotherapy’s side effects could lead to long-term death from other causes than esophageal cancer, and may not be preventing but rather merely postponing cancer-related death. The aim of the current analysis was to determine whether the observed benefits persisted beyond 5 years.
As of Dec. 31, 2018, 117/178 patients in the chemoradiotherapy-surgery arm and 144/188 in the surgery arm had died. Median follow-up for surviving patients was 147 months. Patients in the chemoradiotherapy surgery arm had better overall survival than patients in the surgery arm (hazard ratio, 0.70; 95% confidence interval, 0.55-0.89; P = .004), with a 10-year overall survival of 38% (95% CI, 31-45) and 25% (95% CI, 19-32), respectively. No significant subgroup differences were observed for overall survival. Also, there was no evidence of a time-dependent effect of neoadjuvant chemoradiotherapy on overall survival. The major effect of neoadjuvant chemoradiotherapy, landmark analyses showed, was in the first 5 years of follow-up, with the effect on overall survival stabilized thereafter, with a hazard ratio approaching 1.00.
Cause-specific mortality
Eighty-four of 178 patients in the chemoradiotherapy-surgery arm died of esophageal cancer, with 32 dying of other causes. In the surgery arm, 121/188 died of esophageal cancer and 22 of other causes. The hazard ratio for esophageal cancer death in the chemoradiotherapy-surgery arm was 0.60 (95% CI, 0.46 to 0.80), with 10-year absolute risks of 47% (95% CI, 40-54) and 64% (95% CI,57-71), respectively, in the two arms. Death from other causes was comparable, with 10-year absolute risks of 15% (95% CI, 10-21) and 11% (95% CI, 7-16), respectively, for chemoradiotherapy-surgery versus surgery alone.
Locoregional relapse
Locoregional relapse rates were 8% (15/178) and 18% (33/188) in the chemoradiotherapy-surgery and surgery arms, respectively (HR, 0.39; 95% CI, 0.21-0.72). Eighty-seven percent of those developed within 3 years of follow-up in the chemoradiotherapy arm, with the median relapse-free interval at 3.9 months. In the surgery arm, 28 of 33 relapses (85%) developed within 3 years and the median relapse-free interval was 7.1 months. Beyond 6 years, there were no further relapses in either arm.
While synchronous distant plus locoregional relapse developed in 23 of 178 patients (13%) in the chemoradiotherapy-surgery arm and in 42 of 188 patients (22%) in the surgery arm (HR, 0.43; 95% CI, 0.26-0.72), isolated distant relapse developed at similar rates (around 27.5%) in both groups. Risk of distant relapse (with or without locoregional relapse) was lower in the chemoradiotherapy-surgery arm (HR, 0.61; 95%CI, 0.45-0.84). The median relapse-free interval was 15.1 months (interquartile range, 9.3-27.6) in the chemoradiotherapy-surgery arm and 9.0 months (IQR, 5.3-19.7) in the surgery arm.
Safety and health-related quality of life
The combination of paclitaxel and carboplatin with concurrent 41.4 Gy radiotherapy before surgery seems safe in the long term and does not significantly increase the risk of toxicity-related death, the researchers stated. Within the CROSS trial, short-term and long-term health-related quality of life after neoadjuvant chemoradiotherapy plus surgery for surviving patients was comparable to that after surgery alone.
Long-term persistent overall survival benefit
Ten-year CROSS results show that “for locally advanced resectable cancer of the esophagus or esophagogastric junction, preoperative chemoradiotherapy induces a long-term persistent improvement in overall survival.” Also, neoadjuvant chemoradiotherapy does not lead to an increased risk of death from other causes, and the survival benefit of long-term survivors is not compromised, compared with surgery alone. Furthermore, neoadjuvant chemoradiotherapy plus surgery according to CROSS can still be regarded as a standard of care, the researchers added.
Dr. Eyck and colleagues are currently performing the phase II TNT-OES-1 trial. It combines FLOT (fluorouracil, leucovorin, oxaliplatin and docetaxel) chemotherapy followed by CROSS chemoradiotherapy in patients with advanced esophageal and junctional adenocarcinoma. If this regimen appears to be safe in advanced cancer, they plan to perform a phase III trial with this regimen in locally advanced cancer. In addition, they are currently evaluating the implementation of adjuvant nivolumab in clinical practice for patients with pathologically residual disease after CROSS + surgery, based on the recently published CheckMate 577 trial .
“If possible, we prefer adding better systemic therapy to chemoradiotherapy rather than replacing chemoradiotherapy with systemic therapy alone,” Dr. Eyck said in an interview. “The reason for this is that we would like to allow patients with a complete response to neoadjuvant therapy to undergo active surveillance instead of surgery in the near future. … Since the pathologically complete response rate after regimens containing radiotherapy is substantially higher, we still prefer the addition of radiotherapy.”
The study was funded by the Dutch Cancer Foundation (KWF Kankerbestrijding). Dr. Eyck reported no disclosures. Several of the coauthors reported consulting and advisory roles with a variety of pharmaceutical companies.
Among patients with locally advanced resectable esophageal or junctional cancer, the overall survival benefit conferred by preoperative chemoradiotherapy persists for at least 10 years, according to long-term results of the Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study (CROSS). As a result of earlier publication of CROSS data, chemoradiotherapy followed by surgery has become one of the standards of care for patients with locally advanced resectable esophageal cancer, stated lead author Ben M. Eyck, MD, of Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues in the Journal of Clinical Oncology.
In the multicenter, randomized trial, initiated in 2004, 178 patients randomized to chemoradiotherapy with subsequent surgery and 188 patients randomized to surgery alone were followed with overall survival as the primary, and cause-specific survival and risks of locoregional and distant relapse as the secondary endpoints. Chemoradiotherapy consisted of 5 weekly cycles of carboplatin (area under the curve of 2 mg/mL/min) and paclitaxel (50 mg/m2 body surface area on days 1, 8, 15, 22, and 29) with concurrent radiotherapy (41.4 Gy in 23 fractions, 5 days per week. Mean age was 60 years (around 78% male), with squamous cell carcinoma (23%) and adenocarcinoma (75%) as the predominant histologies.
The first analysis showed low short-term toxicity and 2-year survival increased from 50% for patients receiving surgery alone to 67% for neoadjuvant chemoradiotherapy plus surgery. Five-year follow-up data were consistent with initial reporting. Long-term benefits and harms of this regimen remain unclear, according to the researchers. Neoadjuvant chemoradiotherapy’s side effects could lead to long-term death from other causes than esophageal cancer, and may not be preventing but rather merely postponing cancer-related death. The aim of the current analysis was to determine whether the observed benefits persisted beyond 5 years.
As of Dec. 31, 2018, 117/178 patients in the chemoradiotherapy-surgery arm and 144/188 in the surgery arm had died. Median follow-up for surviving patients was 147 months. Patients in the chemoradiotherapy surgery arm had better overall survival than patients in the surgery arm (hazard ratio, 0.70; 95% confidence interval, 0.55-0.89; P = .004), with a 10-year overall survival of 38% (95% CI, 31-45) and 25% (95% CI, 19-32), respectively. No significant subgroup differences were observed for overall survival. Also, there was no evidence of a time-dependent effect of neoadjuvant chemoradiotherapy on overall survival. The major effect of neoadjuvant chemoradiotherapy, landmark analyses showed, was in the first 5 years of follow-up, with the effect on overall survival stabilized thereafter, with a hazard ratio approaching 1.00.
Cause-specific mortality
Eighty-four of 178 patients in the chemoradiotherapy-surgery arm died of esophageal cancer, with 32 dying of other causes. In the surgery arm, 121/188 died of esophageal cancer and 22 of other causes. The hazard ratio for esophageal cancer death in the chemoradiotherapy-surgery arm was 0.60 (95% CI, 0.46 to 0.80), with 10-year absolute risks of 47% (95% CI, 40-54) and 64% (95% CI,57-71), respectively, in the two arms. Death from other causes was comparable, with 10-year absolute risks of 15% (95% CI, 10-21) and 11% (95% CI, 7-16), respectively, for chemoradiotherapy-surgery versus surgery alone.
Locoregional relapse
Locoregional relapse rates were 8% (15/178) and 18% (33/188) in the chemoradiotherapy-surgery and surgery arms, respectively (HR, 0.39; 95% CI, 0.21-0.72). Eighty-seven percent of those developed within 3 years of follow-up in the chemoradiotherapy arm, with the median relapse-free interval at 3.9 months. In the surgery arm, 28 of 33 relapses (85%) developed within 3 years and the median relapse-free interval was 7.1 months. Beyond 6 years, there were no further relapses in either arm.
While synchronous distant plus locoregional relapse developed in 23 of 178 patients (13%) in the chemoradiotherapy-surgery arm and in 42 of 188 patients (22%) in the surgery arm (HR, 0.43; 95% CI, 0.26-0.72), isolated distant relapse developed at similar rates (around 27.5%) in both groups. Risk of distant relapse (with or without locoregional relapse) was lower in the chemoradiotherapy-surgery arm (HR, 0.61; 95%CI, 0.45-0.84). The median relapse-free interval was 15.1 months (interquartile range, 9.3-27.6) in the chemoradiotherapy-surgery arm and 9.0 months (IQR, 5.3-19.7) in the surgery arm.
Safety and health-related quality of life
The combination of paclitaxel and carboplatin with concurrent 41.4 Gy radiotherapy before surgery seems safe in the long term and does not significantly increase the risk of toxicity-related death, the researchers stated. Within the CROSS trial, short-term and long-term health-related quality of life after neoadjuvant chemoradiotherapy plus surgery for surviving patients was comparable to that after surgery alone.
Long-term persistent overall survival benefit
Ten-year CROSS results show that “for locally advanced resectable cancer of the esophagus or esophagogastric junction, preoperative chemoradiotherapy induces a long-term persistent improvement in overall survival.” Also, neoadjuvant chemoradiotherapy does not lead to an increased risk of death from other causes, and the survival benefit of long-term survivors is not compromised, compared with surgery alone. Furthermore, neoadjuvant chemoradiotherapy plus surgery according to CROSS can still be regarded as a standard of care, the researchers added.
Dr. Eyck and colleagues are currently performing the phase II TNT-OES-1 trial. It combines FLOT (fluorouracil, leucovorin, oxaliplatin and docetaxel) chemotherapy followed by CROSS chemoradiotherapy in patients with advanced esophageal and junctional adenocarcinoma. If this regimen appears to be safe in advanced cancer, they plan to perform a phase III trial with this regimen in locally advanced cancer. In addition, they are currently evaluating the implementation of adjuvant nivolumab in clinical practice for patients with pathologically residual disease after CROSS + surgery, based on the recently published CheckMate 577 trial .
“If possible, we prefer adding better systemic therapy to chemoradiotherapy rather than replacing chemoradiotherapy with systemic therapy alone,” Dr. Eyck said in an interview. “The reason for this is that we would like to allow patients with a complete response to neoadjuvant therapy to undergo active surveillance instead of surgery in the near future. … Since the pathologically complete response rate after regimens containing radiotherapy is substantially higher, we still prefer the addition of radiotherapy.”
The study was funded by the Dutch Cancer Foundation (KWF Kankerbestrijding). Dr. Eyck reported no disclosures. Several of the coauthors reported consulting and advisory roles with a variety of pharmaceutical companies.
Among patients with locally advanced resectable esophageal or junctional cancer, the overall survival benefit conferred by preoperative chemoradiotherapy persists for at least 10 years, according to long-term results of the Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study (CROSS). As a result of earlier publication of CROSS data, chemoradiotherapy followed by surgery has become one of the standards of care for patients with locally advanced resectable esophageal cancer, stated lead author Ben M. Eyck, MD, of Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues in the Journal of Clinical Oncology.
In the multicenter, randomized trial, initiated in 2004, 178 patients randomized to chemoradiotherapy with subsequent surgery and 188 patients randomized to surgery alone were followed with overall survival as the primary, and cause-specific survival and risks of locoregional and distant relapse as the secondary endpoints. Chemoradiotherapy consisted of 5 weekly cycles of carboplatin (area under the curve of 2 mg/mL/min) and paclitaxel (50 mg/m2 body surface area on days 1, 8, 15, 22, and 29) with concurrent radiotherapy (41.4 Gy in 23 fractions, 5 days per week. Mean age was 60 years (around 78% male), with squamous cell carcinoma (23%) and adenocarcinoma (75%) as the predominant histologies.
The first analysis showed low short-term toxicity and 2-year survival increased from 50% for patients receiving surgery alone to 67% for neoadjuvant chemoradiotherapy plus surgery. Five-year follow-up data were consistent with initial reporting. Long-term benefits and harms of this regimen remain unclear, according to the researchers. Neoadjuvant chemoradiotherapy’s side effects could lead to long-term death from other causes than esophageal cancer, and may not be preventing but rather merely postponing cancer-related death. The aim of the current analysis was to determine whether the observed benefits persisted beyond 5 years.
As of Dec. 31, 2018, 117/178 patients in the chemoradiotherapy-surgery arm and 144/188 in the surgery arm had died. Median follow-up for surviving patients was 147 months. Patients in the chemoradiotherapy surgery arm had better overall survival than patients in the surgery arm (hazard ratio, 0.70; 95% confidence interval, 0.55-0.89; P = .004), with a 10-year overall survival of 38% (95% CI, 31-45) and 25% (95% CI, 19-32), respectively. No significant subgroup differences were observed for overall survival. Also, there was no evidence of a time-dependent effect of neoadjuvant chemoradiotherapy on overall survival. The major effect of neoadjuvant chemoradiotherapy, landmark analyses showed, was in the first 5 years of follow-up, with the effect on overall survival stabilized thereafter, with a hazard ratio approaching 1.00.
Cause-specific mortality
Eighty-four of 178 patients in the chemoradiotherapy-surgery arm died of esophageal cancer, with 32 dying of other causes. In the surgery arm, 121/188 died of esophageal cancer and 22 of other causes. The hazard ratio for esophageal cancer death in the chemoradiotherapy-surgery arm was 0.60 (95% CI, 0.46 to 0.80), with 10-year absolute risks of 47% (95% CI, 40-54) and 64% (95% CI,57-71), respectively, in the two arms. Death from other causes was comparable, with 10-year absolute risks of 15% (95% CI, 10-21) and 11% (95% CI, 7-16), respectively, for chemoradiotherapy-surgery versus surgery alone.
Locoregional relapse
Locoregional relapse rates were 8% (15/178) and 18% (33/188) in the chemoradiotherapy-surgery and surgery arms, respectively (HR, 0.39; 95% CI, 0.21-0.72). Eighty-seven percent of those developed within 3 years of follow-up in the chemoradiotherapy arm, with the median relapse-free interval at 3.9 months. In the surgery arm, 28 of 33 relapses (85%) developed within 3 years and the median relapse-free interval was 7.1 months. Beyond 6 years, there were no further relapses in either arm.
While synchronous distant plus locoregional relapse developed in 23 of 178 patients (13%) in the chemoradiotherapy-surgery arm and in 42 of 188 patients (22%) in the surgery arm (HR, 0.43; 95% CI, 0.26-0.72), isolated distant relapse developed at similar rates (around 27.5%) in both groups. Risk of distant relapse (with or without locoregional relapse) was lower in the chemoradiotherapy-surgery arm (HR, 0.61; 95%CI, 0.45-0.84). The median relapse-free interval was 15.1 months (interquartile range, 9.3-27.6) in the chemoradiotherapy-surgery arm and 9.0 months (IQR, 5.3-19.7) in the surgery arm.
Safety and health-related quality of life
The combination of paclitaxel and carboplatin with concurrent 41.4 Gy radiotherapy before surgery seems safe in the long term and does not significantly increase the risk of toxicity-related death, the researchers stated. Within the CROSS trial, short-term and long-term health-related quality of life after neoadjuvant chemoradiotherapy plus surgery for surviving patients was comparable to that after surgery alone.
Long-term persistent overall survival benefit
Ten-year CROSS results show that “for locally advanced resectable cancer of the esophagus or esophagogastric junction, preoperative chemoradiotherapy induces a long-term persistent improvement in overall survival.” Also, neoadjuvant chemoradiotherapy does not lead to an increased risk of death from other causes, and the survival benefit of long-term survivors is not compromised, compared with surgery alone. Furthermore, neoadjuvant chemoradiotherapy plus surgery according to CROSS can still be regarded as a standard of care, the researchers added.
Dr. Eyck and colleagues are currently performing the phase II TNT-OES-1 trial. It combines FLOT (fluorouracil, leucovorin, oxaliplatin and docetaxel) chemotherapy followed by CROSS chemoradiotherapy in patients with advanced esophageal and junctional adenocarcinoma. If this regimen appears to be safe in advanced cancer, they plan to perform a phase III trial with this regimen in locally advanced cancer. In addition, they are currently evaluating the implementation of adjuvant nivolumab in clinical practice for patients with pathologically residual disease after CROSS + surgery, based on the recently published CheckMate 577 trial .
“If possible, we prefer adding better systemic therapy to chemoradiotherapy rather than replacing chemoradiotherapy with systemic therapy alone,” Dr. Eyck said in an interview. “The reason for this is that we would like to allow patients with a complete response to neoadjuvant therapy to undergo active surveillance instead of surgery in the near future. … Since the pathologically complete response rate after regimens containing radiotherapy is substantially higher, we still prefer the addition of radiotherapy.”
The study was funded by the Dutch Cancer Foundation (KWF Kankerbestrijding). Dr. Eyck reported no disclosures. Several of the coauthors reported consulting and advisory roles with a variety of pharmaceutical companies.
FROM JOURNAL OF CLINICAL ONCOLOGY
Superior survival with sintilimab in squamous NSCLC
Sintilimab improved both overall survival (OS) and progression-free survival (PFS), according to Yuankai Shi, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College in Beijing.
Dr. Shi presented these findings, from the ORIENT-3 study, at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT041).
ORIENT-3 enrolled and randomized 290 patients with stage IIIB/IIIC or IV sqNSCLC and disease progression during or after first-line platimum-based chemotherapy. They were randomized 1:1 to receive sintilimab at 200 mg or docetaxel at 75 mg/m2intravenously every 3 weeks until disease progression or intolerable toxicity.
The median age was 60 years in the sintilimab arm and 61 years in the docetaxel arm. A majority of patients were men (94% in the sintilimab arm and 90% in the docetaxel arm), most were current or former smokers (90% and 80%, respectively), and more than three-quarters had an ECOG performance status of 1 (76% and 77%, respectively). More than half of patients had a PD-L1 tumor proportion score (TPS) of 1% or greater (57% and 47%, respectively), and 81% of patients in both arms had stage IV disease.
Results: Survival and safety
Patients in the sintilimab arm received a median of 8.0 cycles of therapy (range, 1-45), and those in the docetaxel arm received a median of 2.0 cycles of therapy (range, 1-15).
At a median follow-up of 23.56 months, the median OS was significantly longer in the sintilimab arm than in the docetaxel arm – 11.79 months and 8.25 months, respectively (hazard ratio, 0.74; P = .02489). OS benefits were generally consistent across subgroups.
The secondary endpoints of PFS and objective response rate also favored sintilimab, Dr. Shi reported.
The median PFS was 4.30 months in the sintilimab arm and 2.79 months in the docetaxel arm (HR, 0.52; P < .00001). Confirmed objective response rates were 25.5% and 2.2%, respectively; the median duration of response was 12.45 months and 4.14 months, respectively; and disease control rates were 65.5% and 37.8%, respectively.
“Sintilimab had a favorable safety profile over docetaxel, with a lower frequency of grade 3 or higher treatment-related adverse events, with no new safety signals observed,” Dr. Shi said.
Treatment-related adverse events (TRAEs) occurred in 84.7% of patients receiving sintilimab and 83.1% of those receiving docetaxel. Hypothyroidism was the most common TRAE in the sintilimab arm (18.1%), and alopecia was the most common TRAE in the docetaxel arm (34.6%).
Grade 3 or higher TRAEs were less frequent in the sintilimab arm than in the docetaxel arm (18.1% vs. 36.2%). Rates of discontinuation because of TRAEs were 12.5% and 5.4% in the sintilimab and docetaxel arms, respectively. TRAEs leading to death occurred in five patients in the sintilimab arm and one in the docetaxel arm.
Use in the real world
Noting sintilimab’s significant OS and PFS benefits as well as superior response rate and duration of response, Dr. Shi concluded, “Sintilimab might provide an alternative second-line treatment option for advanced and metastatic sqNSCLC.”
AACR moderator Marina Garassino, MD, of the University of Chicago, commented on the potential utility of sintilimab and tislelizumab, another checkpoint inhibitor that was evaluated in NSCLC in the RATIONALE 303 trial (AACR 2021, Abstract CT039). Dr. Garassino observed that both drugs have demonstrated superiority to docetaxel as second-line therapy in NSCLC.
Although there have been no head-to-head trials, sintilimab and tislelizumab appear to be very similar to the already approved immune checkpoint inhibitors, which are currently being used as first-line treatment.
“That similarity would make them inappropriate for second-line treatment, except in countries where immune checkpoint inhibitors are not yet approved for first-line therapy,” Dr. Garassino noted.
When asked to comment on the higher treatment-related death rate observed with sintilimab, Dr. Garassino said, “We need to remember that these drugs were developed in China with a population that may have a side effect profile differing from that of a Western population. Also, we are very familiar with this class of drugs and know how to treat their side effects. Similar drugs but different populations and different trials, so it’s very hard to judge.”
Dr. Garassino speculated that with the “super expensive” price tags on the new checkpoint inhibitors, having additional agents that could provide choice and drive prices down would be welcome.
ORIENT-3 was funded by Innovent Biologics and Eli Lilly. Dr. Shi disclosed consultancy for Innovent Biologics. Dr. Garassino disclosed relationships with Eli Lilly, AstraZeneca, Novartis, and several other companies, not including Innovent Biologics.
Sintilimab improved both overall survival (OS) and progression-free survival (PFS), according to Yuankai Shi, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College in Beijing.
Dr. Shi presented these findings, from the ORIENT-3 study, at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT041).
ORIENT-3 enrolled and randomized 290 patients with stage IIIB/IIIC or IV sqNSCLC and disease progression during or after first-line platimum-based chemotherapy. They were randomized 1:1 to receive sintilimab at 200 mg or docetaxel at 75 mg/m2intravenously every 3 weeks until disease progression or intolerable toxicity.
The median age was 60 years in the sintilimab arm and 61 years in the docetaxel arm. A majority of patients were men (94% in the sintilimab arm and 90% in the docetaxel arm), most were current or former smokers (90% and 80%, respectively), and more than three-quarters had an ECOG performance status of 1 (76% and 77%, respectively). More than half of patients had a PD-L1 tumor proportion score (TPS) of 1% or greater (57% and 47%, respectively), and 81% of patients in both arms had stage IV disease.
Results: Survival and safety
Patients in the sintilimab arm received a median of 8.0 cycles of therapy (range, 1-45), and those in the docetaxel arm received a median of 2.0 cycles of therapy (range, 1-15).
At a median follow-up of 23.56 months, the median OS was significantly longer in the sintilimab arm than in the docetaxel arm – 11.79 months and 8.25 months, respectively (hazard ratio, 0.74; P = .02489). OS benefits were generally consistent across subgroups.
The secondary endpoints of PFS and objective response rate also favored sintilimab, Dr. Shi reported.
The median PFS was 4.30 months in the sintilimab arm and 2.79 months in the docetaxel arm (HR, 0.52; P < .00001). Confirmed objective response rates were 25.5% and 2.2%, respectively; the median duration of response was 12.45 months and 4.14 months, respectively; and disease control rates were 65.5% and 37.8%, respectively.
“Sintilimab had a favorable safety profile over docetaxel, with a lower frequency of grade 3 or higher treatment-related adverse events, with no new safety signals observed,” Dr. Shi said.
Treatment-related adverse events (TRAEs) occurred in 84.7% of patients receiving sintilimab and 83.1% of those receiving docetaxel. Hypothyroidism was the most common TRAE in the sintilimab arm (18.1%), and alopecia was the most common TRAE in the docetaxel arm (34.6%).
Grade 3 or higher TRAEs were less frequent in the sintilimab arm than in the docetaxel arm (18.1% vs. 36.2%). Rates of discontinuation because of TRAEs were 12.5% and 5.4% in the sintilimab and docetaxel arms, respectively. TRAEs leading to death occurred in five patients in the sintilimab arm and one in the docetaxel arm.
Use in the real world
Noting sintilimab’s significant OS and PFS benefits as well as superior response rate and duration of response, Dr. Shi concluded, “Sintilimab might provide an alternative second-line treatment option for advanced and metastatic sqNSCLC.”
AACR moderator Marina Garassino, MD, of the University of Chicago, commented on the potential utility of sintilimab and tislelizumab, another checkpoint inhibitor that was evaluated in NSCLC in the RATIONALE 303 trial (AACR 2021, Abstract CT039). Dr. Garassino observed that both drugs have demonstrated superiority to docetaxel as second-line therapy in NSCLC.
Although there have been no head-to-head trials, sintilimab and tislelizumab appear to be very similar to the already approved immune checkpoint inhibitors, which are currently being used as first-line treatment.
“That similarity would make them inappropriate for second-line treatment, except in countries where immune checkpoint inhibitors are not yet approved for first-line therapy,” Dr. Garassino noted.
When asked to comment on the higher treatment-related death rate observed with sintilimab, Dr. Garassino said, “We need to remember that these drugs were developed in China with a population that may have a side effect profile differing from that of a Western population. Also, we are very familiar with this class of drugs and know how to treat their side effects. Similar drugs but different populations and different trials, so it’s very hard to judge.”
Dr. Garassino speculated that with the “super expensive” price tags on the new checkpoint inhibitors, having additional agents that could provide choice and drive prices down would be welcome.
ORIENT-3 was funded by Innovent Biologics and Eli Lilly. Dr. Shi disclosed consultancy for Innovent Biologics. Dr. Garassino disclosed relationships with Eli Lilly, AstraZeneca, Novartis, and several other companies, not including Innovent Biologics.
Sintilimab improved both overall survival (OS) and progression-free survival (PFS), according to Yuankai Shi, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College in Beijing.
Dr. Shi presented these findings, from the ORIENT-3 study, at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT041).
ORIENT-3 enrolled and randomized 290 patients with stage IIIB/IIIC or IV sqNSCLC and disease progression during or after first-line platimum-based chemotherapy. They were randomized 1:1 to receive sintilimab at 200 mg or docetaxel at 75 mg/m2intravenously every 3 weeks until disease progression or intolerable toxicity.
The median age was 60 years in the sintilimab arm and 61 years in the docetaxel arm. A majority of patients were men (94% in the sintilimab arm and 90% in the docetaxel arm), most were current or former smokers (90% and 80%, respectively), and more than three-quarters had an ECOG performance status of 1 (76% and 77%, respectively). More than half of patients had a PD-L1 tumor proportion score (TPS) of 1% or greater (57% and 47%, respectively), and 81% of patients in both arms had stage IV disease.
Results: Survival and safety
Patients in the sintilimab arm received a median of 8.0 cycles of therapy (range, 1-45), and those in the docetaxel arm received a median of 2.0 cycles of therapy (range, 1-15).
At a median follow-up of 23.56 months, the median OS was significantly longer in the sintilimab arm than in the docetaxel arm – 11.79 months and 8.25 months, respectively (hazard ratio, 0.74; P = .02489). OS benefits were generally consistent across subgroups.
The secondary endpoints of PFS and objective response rate also favored sintilimab, Dr. Shi reported.
The median PFS was 4.30 months in the sintilimab arm and 2.79 months in the docetaxel arm (HR, 0.52; P < .00001). Confirmed objective response rates were 25.5% and 2.2%, respectively; the median duration of response was 12.45 months and 4.14 months, respectively; and disease control rates were 65.5% and 37.8%, respectively.
“Sintilimab had a favorable safety profile over docetaxel, with a lower frequency of grade 3 or higher treatment-related adverse events, with no new safety signals observed,” Dr. Shi said.
Treatment-related adverse events (TRAEs) occurred in 84.7% of patients receiving sintilimab and 83.1% of those receiving docetaxel. Hypothyroidism was the most common TRAE in the sintilimab arm (18.1%), and alopecia was the most common TRAE in the docetaxel arm (34.6%).
Grade 3 or higher TRAEs were less frequent in the sintilimab arm than in the docetaxel arm (18.1% vs. 36.2%). Rates of discontinuation because of TRAEs were 12.5% and 5.4% in the sintilimab and docetaxel arms, respectively. TRAEs leading to death occurred in five patients in the sintilimab arm and one in the docetaxel arm.
Use in the real world
Noting sintilimab’s significant OS and PFS benefits as well as superior response rate and duration of response, Dr. Shi concluded, “Sintilimab might provide an alternative second-line treatment option for advanced and metastatic sqNSCLC.”
AACR moderator Marina Garassino, MD, of the University of Chicago, commented on the potential utility of sintilimab and tislelizumab, another checkpoint inhibitor that was evaluated in NSCLC in the RATIONALE 303 trial (AACR 2021, Abstract CT039). Dr. Garassino observed that both drugs have demonstrated superiority to docetaxel as second-line therapy in NSCLC.
Although there have been no head-to-head trials, sintilimab and tislelizumab appear to be very similar to the already approved immune checkpoint inhibitors, which are currently being used as first-line treatment.
“That similarity would make them inappropriate for second-line treatment, except in countries where immune checkpoint inhibitors are not yet approved for first-line therapy,” Dr. Garassino noted.
When asked to comment on the higher treatment-related death rate observed with sintilimab, Dr. Garassino said, “We need to remember that these drugs were developed in China with a population that may have a side effect profile differing from that of a Western population. Also, we are very familiar with this class of drugs and know how to treat their side effects. Similar drugs but different populations and different trials, so it’s very hard to judge.”
Dr. Garassino speculated that with the “super expensive” price tags on the new checkpoint inhibitors, having additional agents that could provide choice and drive prices down would be welcome.
ORIENT-3 was funded by Innovent Biologics and Eli Lilly. Dr. Shi disclosed consultancy for Innovent Biologics. Dr. Garassino disclosed relationships with Eli Lilly, AstraZeneca, Novartis, and several other companies, not including Innovent Biologics.
FROM AACR 2021
Tislelizumab bests docetaxel in NSCLC
The results were presented at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT039).
Tislelizumab is an anti–PD-1 antibody engineered to minimize Fc-gamma receptor binding on macrophages, a mechanism of T-cell clearance and potential anti–PD-1 resistance, according to investigator Caicun Zhou, MD, PhD, of Shanghai (China) Pulmonary Hospital.
Tislelizumab is approved for the treatment of relapsed/refractory classical Hodgkin lymphoma, the second-line treatment of locally advanced or metastatic urothelial carcinoma, and first-line treatment of advanced squamous NSCLC in China.
In patients with locally advanced or metastatic NSCLC whose disease has progressed after initial platinum-based chemotherapy, anti–PD-1/PD-L1 therapies have been shown to improve OS by 2-4 months versus docetaxel, Dr. Zhou said. A phase 1/2 study of second-line tislelizumab demonstrated antitumor activity in multiple advanced solid tumors, including NSCLC.
The phase 3 RATIONALE 303 study (NCT3358875) was designed to investigate the efficacy and safety of tislelizumab, compared with docetaxel in patients with locally advanced or metastatic NSCLC whose disease had progressed during or after platinum-containing doublet chemotherapy.
Study details
RATIONALE 303 enrolled 805 patients who had received up to two prior lines of systemic therapy and had no known EGFR mutations or ALK fusions.
The patients’ median age was 61 years, about 77% were male, about 80% were Asian, and about 70% were current or former smokers. Roughly 46% of patients had squamous histology, and about 43% had PD-L1 expression of 25% or greater.
Patients were stratified according to histology (squamous vs. nonsquamous), lines of prior therapy (second vs. third), and PD-L1 status (<25% vs. ≥25%).
Patients were randomized 2:1 to receive IV tislelizumab at 200 mg every 3 weeks (n = 535) or IV docetaxel at 75 mg/m2 every 3 weeks (n = 270) until unacceptable toxicity or disease progression.
The dual primary endpoints were OS in the intention-to-treat (ITT) population and in patients with PD-L1 expression of 25% or higher.
Survival and safety
In the ITT population, the 1-year OS rate was 61.9% in the tislelizumab arm and 49.8% in the docetaxel arm. At 2 years, the OS rates were 39.4% and 25.0%, respectively.
The median OS was 17.2 months in the tislelizumab arm and 11.9 months in the docetaxel arm (hazard ratio, 0.64; 95% CI, 0.53-0.78; P < .0001).
In the PD-L1–high subgroup, the median OS was 19.1 months with tislelizumab and 11.9 months with docetaxel (HR, 0.52; 95% CI, 0.38-0.71; P < .0001). The 1-year OS rates in this group were 67.5% and 49.1%, respectively, and the 2-year OS rates were 44.7% and 24.5%, respectively.
The OS benefit with tislelizumab was observed across nearly all subgroups, Dr. Zhou noted.
In the ITT population, benefits were seen with tislelizumab over docetaxel for progression-free survival (4.1 months vs. 2.6 months, P < .0001), objective response rate (21.9% vs. 7.1%, P < .0001), and median duration of response (13.5 months vs. 6.2 months, P < .0001).
The rate of treatment-related adverse events (TRAEs) was 73.0% in the tislelizumab arm and 93.8% in the docetaxel arm. Rates of grade 3 or higher TRAEs were 14.4% and 66.3%, respectively. Rates of TRAEs leading to permanent discontinuation of treatment were 6.0% and 9.7%, respectively, and rates of TRAEs leading to death were 1.5% and 1.6%, respectively.
The most common treatment-emergent adverse events were anemia in the tislelizumab arm (28.5%) and alopecia in the docetaxel arm (47.3%). The most common grade 3 or higher treatment-emergent adverse event was neutropenia in the docetaxel arm (27.9% vs. 0.6% with tislelizumab).
‘Very important trial’
“RATIONALE 303 demonstrated that, as second- or third-line therapy in patients with advanced NSCLC, tislelizumab was tolerable and prolonged overall survival by 5-7 months. It also improved progression-free survival and objective response rate versus docetaxel, regardless of histology or PD-L1 expression,” Dr. Zhou concluded.
Session moderator Marina Chiara Garassino, MD, of the University of Chicago called RATIONALE 303 a “very important trial.”
Citing the range of immunotherapies available for NSCLC, Dr. Garassino said, “We have a very crowded space in the treatment of NSCLC. ... It is difficult to do a direct comparison [of immunotherapy trials] because we know that populations can be different and other factors can play a role. In the near future, we have to understand if they are all the same and interchangeable or if they are different.”
RATIONALE 303 was funded by BeiGene. Dr. Zhou disclosed relationships with Lily China, Sanofi, Roche, and several other companies, not including BeiGene. Dr. Garassino disclosed relationships with AstraZeneca, Novartis, Bristol-Myers Squibb, and several other companies, not including BeiGene.
The results were presented at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT039).
Tislelizumab is an anti–PD-1 antibody engineered to minimize Fc-gamma receptor binding on macrophages, a mechanism of T-cell clearance and potential anti–PD-1 resistance, according to investigator Caicun Zhou, MD, PhD, of Shanghai (China) Pulmonary Hospital.
Tislelizumab is approved for the treatment of relapsed/refractory classical Hodgkin lymphoma, the second-line treatment of locally advanced or metastatic urothelial carcinoma, and first-line treatment of advanced squamous NSCLC in China.
In patients with locally advanced or metastatic NSCLC whose disease has progressed after initial platinum-based chemotherapy, anti–PD-1/PD-L1 therapies have been shown to improve OS by 2-4 months versus docetaxel, Dr. Zhou said. A phase 1/2 study of second-line tislelizumab demonstrated antitumor activity in multiple advanced solid tumors, including NSCLC.
The phase 3 RATIONALE 303 study (NCT3358875) was designed to investigate the efficacy and safety of tislelizumab, compared with docetaxel in patients with locally advanced or metastatic NSCLC whose disease had progressed during or after platinum-containing doublet chemotherapy.
Study details
RATIONALE 303 enrolled 805 patients who had received up to two prior lines of systemic therapy and had no known EGFR mutations or ALK fusions.
The patients’ median age was 61 years, about 77% were male, about 80% were Asian, and about 70% were current or former smokers. Roughly 46% of patients had squamous histology, and about 43% had PD-L1 expression of 25% or greater.
Patients were stratified according to histology (squamous vs. nonsquamous), lines of prior therapy (second vs. third), and PD-L1 status (<25% vs. ≥25%).
Patients were randomized 2:1 to receive IV tislelizumab at 200 mg every 3 weeks (n = 535) or IV docetaxel at 75 mg/m2 every 3 weeks (n = 270) until unacceptable toxicity or disease progression.
The dual primary endpoints were OS in the intention-to-treat (ITT) population and in patients with PD-L1 expression of 25% or higher.
Survival and safety
In the ITT population, the 1-year OS rate was 61.9% in the tislelizumab arm and 49.8% in the docetaxel arm. At 2 years, the OS rates were 39.4% and 25.0%, respectively.
The median OS was 17.2 months in the tislelizumab arm and 11.9 months in the docetaxel arm (hazard ratio, 0.64; 95% CI, 0.53-0.78; P < .0001).
In the PD-L1–high subgroup, the median OS was 19.1 months with tislelizumab and 11.9 months with docetaxel (HR, 0.52; 95% CI, 0.38-0.71; P < .0001). The 1-year OS rates in this group were 67.5% and 49.1%, respectively, and the 2-year OS rates were 44.7% and 24.5%, respectively.
The OS benefit with tislelizumab was observed across nearly all subgroups, Dr. Zhou noted.
In the ITT population, benefits were seen with tislelizumab over docetaxel for progression-free survival (4.1 months vs. 2.6 months, P < .0001), objective response rate (21.9% vs. 7.1%, P < .0001), and median duration of response (13.5 months vs. 6.2 months, P < .0001).
The rate of treatment-related adverse events (TRAEs) was 73.0% in the tislelizumab arm and 93.8% in the docetaxel arm. Rates of grade 3 or higher TRAEs were 14.4% and 66.3%, respectively. Rates of TRAEs leading to permanent discontinuation of treatment were 6.0% and 9.7%, respectively, and rates of TRAEs leading to death were 1.5% and 1.6%, respectively.
The most common treatment-emergent adverse events were anemia in the tislelizumab arm (28.5%) and alopecia in the docetaxel arm (47.3%). The most common grade 3 or higher treatment-emergent adverse event was neutropenia in the docetaxel arm (27.9% vs. 0.6% with tislelizumab).
‘Very important trial’
“RATIONALE 303 demonstrated that, as second- or third-line therapy in patients with advanced NSCLC, tislelizumab was tolerable and prolonged overall survival by 5-7 months. It also improved progression-free survival and objective response rate versus docetaxel, regardless of histology or PD-L1 expression,” Dr. Zhou concluded.
Session moderator Marina Chiara Garassino, MD, of the University of Chicago called RATIONALE 303 a “very important trial.”
Citing the range of immunotherapies available for NSCLC, Dr. Garassino said, “We have a very crowded space in the treatment of NSCLC. ... It is difficult to do a direct comparison [of immunotherapy trials] because we know that populations can be different and other factors can play a role. In the near future, we have to understand if they are all the same and interchangeable or if they are different.”
RATIONALE 303 was funded by BeiGene. Dr. Zhou disclosed relationships with Lily China, Sanofi, Roche, and several other companies, not including BeiGene. Dr. Garassino disclosed relationships with AstraZeneca, Novartis, Bristol-Myers Squibb, and several other companies, not including BeiGene.
The results were presented at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT039).
Tislelizumab is an anti–PD-1 antibody engineered to minimize Fc-gamma receptor binding on macrophages, a mechanism of T-cell clearance and potential anti–PD-1 resistance, according to investigator Caicun Zhou, MD, PhD, of Shanghai (China) Pulmonary Hospital.
Tislelizumab is approved for the treatment of relapsed/refractory classical Hodgkin lymphoma, the second-line treatment of locally advanced or metastatic urothelial carcinoma, and first-line treatment of advanced squamous NSCLC in China.
In patients with locally advanced or metastatic NSCLC whose disease has progressed after initial platinum-based chemotherapy, anti–PD-1/PD-L1 therapies have been shown to improve OS by 2-4 months versus docetaxel, Dr. Zhou said. A phase 1/2 study of second-line tislelizumab demonstrated antitumor activity in multiple advanced solid tumors, including NSCLC.
The phase 3 RATIONALE 303 study (NCT3358875) was designed to investigate the efficacy and safety of tislelizumab, compared with docetaxel in patients with locally advanced or metastatic NSCLC whose disease had progressed during or after platinum-containing doublet chemotherapy.
Study details
RATIONALE 303 enrolled 805 patients who had received up to two prior lines of systemic therapy and had no known EGFR mutations or ALK fusions.
The patients’ median age was 61 years, about 77% were male, about 80% were Asian, and about 70% were current or former smokers. Roughly 46% of patients had squamous histology, and about 43% had PD-L1 expression of 25% or greater.
Patients were stratified according to histology (squamous vs. nonsquamous), lines of prior therapy (second vs. third), and PD-L1 status (<25% vs. ≥25%).
Patients were randomized 2:1 to receive IV tislelizumab at 200 mg every 3 weeks (n = 535) or IV docetaxel at 75 mg/m2 every 3 weeks (n = 270) until unacceptable toxicity or disease progression.
The dual primary endpoints were OS in the intention-to-treat (ITT) population and in patients with PD-L1 expression of 25% or higher.
Survival and safety
In the ITT population, the 1-year OS rate was 61.9% in the tislelizumab arm and 49.8% in the docetaxel arm. At 2 years, the OS rates were 39.4% and 25.0%, respectively.
The median OS was 17.2 months in the tislelizumab arm and 11.9 months in the docetaxel arm (hazard ratio, 0.64; 95% CI, 0.53-0.78; P < .0001).
In the PD-L1–high subgroup, the median OS was 19.1 months with tislelizumab and 11.9 months with docetaxel (HR, 0.52; 95% CI, 0.38-0.71; P < .0001). The 1-year OS rates in this group were 67.5% and 49.1%, respectively, and the 2-year OS rates were 44.7% and 24.5%, respectively.
The OS benefit with tislelizumab was observed across nearly all subgroups, Dr. Zhou noted.
In the ITT population, benefits were seen with tislelizumab over docetaxel for progression-free survival (4.1 months vs. 2.6 months, P < .0001), objective response rate (21.9% vs. 7.1%, P < .0001), and median duration of response (13.5 months vs. 6.2 months, P < .0001).
The rate of treatment-related adverse events (TRAEs) was 73.0% in the tislelizumab arm and 93.8% in the docetaxel arm. Rates of grade 3 or higher TRAEs were 14.4% and 66.3%, respectively. Rates of TRAEs leading to permanent discontinuation of treatment were 6.0% and 9.7%, respectively, and rates of TRAEs leading to death were 1.5% and 1.6%, respectively.
The most common treatment-emergent adverse events were anemia in the tislelizumab arm (28.5%) and alopecia in the docetaxel arm (47.3%). The most common grade 3 or higher treatment-emergent adverse event was neutropenia in the docetaxel arm (27.9% vs. 0.6% with tislelizumab).
‘Very important trial’
“RATIONALE 303 demonstrated that, as second- or third-line therapy in patients with advanced NSCLC, tislelizumab was tolerable and prolonged overall survival by 5-7 months. It also improved progression-free survival and objective response rate versus docetaxel, regardless of histology or PD-L1 expression,” Dr. Zhou concluded.
Session moderator Marina Chiara Garassino, MD, of the University of Chicago called RATIONALE 303 a “very important trial.”
Citing the range of immunotherapies available for NSCLC, Dr. Garassino said, “We have a very crowded space in the treatment of NSCLC. ... It is difficult to do a direct comparison [of immunotherapy trials] because we know that populations can be different and other factors can play a role. In the near future, we have to understand if they are all the same and interchangeable or if they are different.”
RATIONALE 303 was funded by BeiGene. Dr. Zhou disclosed relationships with Lily China, Sanofi, Roche, and several other companies, not including BeiGene. Dr. Garassino disclosed relationships with AstraZeneca, Novartis, Bristol-Myers Squibb, and several other companies, not including BeiGene.
FROM AACR 2021
Antibiotics may prolong PFS in HCC patients on immunotherapy
Response rates and overall survival (OS), on the other hand, did not seem to be affected by antibiotic administration, according to investigator Petros Fessas, MD, of Imperial College London.
Dr. Fessas and colleagues presented these findings in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract 485).
Dr. Fessas noted that, in other cancers, antibiotics have been shown to reduce both response and survival rates after ICI.
To assess the impact of early antibiotic exposure on ICI efficacy in HCC, Dr. Fessas and colleagues examined data from 449 patients treated at 12 centers in the United States, Europe, and Asia.
The patients’ median age was 65 years, and 79.1% were men. Nearly three-quarters (73.3%) were cirrhotic (60.4% because of viral hepatitis), 79.9% were Child-Pugh class A, 72.4% were Barcelona Clinic Liver Cancer stage C, and 79% had an Eastern Cooperative Oncology Group performance status of 0-1.
Response and survival results
The investigators compared outcomes between patients with and without antibiotic exposure in the early immunotherapy period (EIOP) 30 days before and after ICI initiation.
In all, 170 patients (37.9%) received antibiotics in the EIOP. There were no differences in response rates, disease control rates, or median OS between patients who received antibiotics and those who did not.
The objective response rate was 20.2% in patients who received antibiotics in the EIOP and 16.1% in patients who did not (P = .2808). Disease control rates were 63.1% and 55.4%, respectively (P = .1144). The median OS was 15.3 months and 15.4 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.72-1.21; P = .6275).
The median PFS, however, was significantly longer in patients who received antibiotics than in patients who did not – 6.1 months and 3.7 months, respectively (HR, 0.74; 95% CI, 0.60-0.93; P = .0135).
To overcome possible bias introduced by misclassification of patients who received antibiotics but discontinued immunotherapy within 30 days of initiation, the investigators conducted a landmark selection analysis among only those patients with a median follow-up for PFS of 30 days or longer (n = 402). This analysis confirmed the prior findings.
“Antibiotic exposure in the 30 days before or after immune checkpoint initiation in hepatocellular carcinoma is associated with prolonged progression-free survival,” Dr. Fessas concluded.
He added that a key question for future research is to discover the immune-microbiologic determinants of response to initiation of ICIs.
Positive effect surprising
“My group has shown that antibiotic therapy is normally detrimental in patients with cancer,” investigator David J. Pinato, MD, PhD, of Imperial College London, said in an interview. “So we were very surprised to see a positive effect on PFS.”
He added that the new findings should be interpreted with caution.
“My feeling is that, unlike with many other malignancies, the gut microbiome is heavily involved in the progression of the cirrhosis that pre-dates HCC onset,” he said.
That would suggest the relationship between antibiotics and perturbation of the gut microbiome is dictated by something more than changes in antitumor immune tolerance, he added.
“Overall, I think the interplay is more complex in HCC: cirrhosis/cancer/microbiome, not just microbiome/cancer as in many other tumors,” Dr. Pinato said. “So we are looking at microbial determinants of response in HCC patients undergoing ICI therapy, and we are hopeful to see some more mechanistic evidence behind this association.”
Dr. Pinato disclosed relationships with ViiV Healthcare, Bayer Healthcare, Bristol Myers Squibb, Mina Therapeutics, Eisai, Roche, and AstraZeneca. Dr. Fessas reported having no conflicts of interest. No funding source for the study was disclosed.
Response rates and overall survival (OS), on the other hand, did not seem to be affected by antibiotic administration, according to investigator Petros Fessas, MD, of Imperial College London.
Dr. Fessas and colleagues presented these findings in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract 485).
Dr. Fessas noted that, in other cancers, antibiotics have been shown to reduce both response and survival rates after ICI.
To assess the impact of early antibiotic exposure on ICI efficacy in HCC, Dr. Fessas and colleagues examined data from 449 patients treated at 12 centers in the United States, Europe, and Asia.
The patients’ median age was 65 years, and 79.1% were men. Nearly three-quarters (73.3%) were cirrhotic (60.4% because of viral hepatitis), 79.9% were Child-Pugh class A, 72.4% were Barcelona Clinic Liver Cancer stage C, and 79% had an Eastern Cooperative Oncology Group performance status of 0-1.
Response and survival results
The investigators compared outcomes between patients with and without antibiotic exposure in the early immunotherapy period (EIOP) 30 days before and after ICI initiation.
In all, 170 patients (37.9%) received antibiotics in the EIOP. There were no differences in response rates, disease control rates, or median OS between patients who received antibiotics and those who did not.
The objective response rate was 20.2% in patients who received antibiotics in the EIOP and 16.1% in patients who did not (P = .2808). Disease control rates were 63.1% and 55.4%, respectively (P = .1144). The median OS was 15.3 months and 15.4 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.72-1.21; P = .6275).
The median PFS, however, was significantly longer in patients who received antibiotics than in patients who did not – 6.1 months and 3.7 months, respectively (HR, 0.74; 95% CI, 0.60-0.93; P = .0135).
To overcome possible bias introduced by misclassification of patients who received antibiotics but discontinued immunotherapy within 30 days of initiation, the investigators conducted a landmark selection analysis among only those patients with a median follow-up for PFS of 30 days or longer (n = 402). This analysis confirmed the prior findings.
“Antibiotic exposure in the 30 days before or after immune checkpoint initiation in hepatocellular carcinoma is associated with prolonged progression-free survival,” Dr. Fessas concluded.
He added that a key question for future research is to discover the immune-microbiologic determinants of response to initiation of ICIs.
Positive effect surprising
“My group has shown that antibiotic therapy is normally detrimental in patients with cancer,” investigator David J. Pinato, MD, PhD, of Imperial College London, said in an interview. “So we were very surprised to see a positive effect on PFS.”
He added that the new findings should be interpreted with caution.
“My feeling is that, unlike with many other malignancies, the gut microbiome is heavily involved in the progression of the cirrhosis that pre-dates HCC onset,” he said.
That would suggest the relationship between antibiotics and perturbation of the gut microbiome is dictated by something more than changes in antitumor immune tolerance, he added.
“Overall, I think the interplay is more complex in HCC: cirrhosis/cancer/microbiome, not just microbiome/cancer as in many other tumors,” Dr. Pinato said. “So we are looking at microbial determinants of response in HCC patients undergoing ICI therapy, and we are hopeful to see some more mechanistic evidence behind this association.”
Dr. Pinato disclosed relationships with ViiV Healthcare, Bayer Healthcare, Bristol Myers Squibb, Mina Therapeutics, Eisai, Roche, and AstraZeneca. Dr. Fessas reported having no conflicts of interest. No funding source for the study was disclosed.
Response rates and overall survival (OS), on the other hand, did not seem to be affected by antibiotic administration, according to investigator Petros Fessas, MD, of Imperial College London.
Dr. Fessas and colleagues presented these findings in a poster at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract 485).
Dr. Fessas noted that, in other cancers, antibiotics have been shown to reduce both response and survival rates after ICI.
To assess the impact of early antibiotic exposure on ICI efficacy in HCC, Dr. Fessas and colleagues examined data from 449 patients treated at 12 centers in the United States, Europe, and Asia.
The patients’ median age was 65 years, and 79.1% were men. Nearly three-quarters (73.3%) were cirrhotic (60.4% because of viral hepatitis), 79.9% were Child-Pugh class A, 72.4% were Barcelona Clinic Liver Cancer stage C, and 79% had an Eastern Cooperative Oncology Group performance status of 0-1.
Response and survival results
The investigators compared outcomes between patients with and without antibiotic exposure in the early immunotherapy period (EIOP) 30 days before and after ICI initiation.
In all, 170 patients (37.9%) received antibiotics in the EIOP. There were no differences in response rates, disease control rates, or median OS between patients who received antibiotics and those who did not.
The objective response rate was 20.2% in patients who received antibiotics in the EIOP and 16.1% in patients who did not (P = .2808). Disease control rates were 63.1% and 55.4%, respectively (P = .1144). The median OS was 15.3 months and 15.4 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.72-1.21; P = .6275).
The median PFS, however, was significantly longer in patients who received antibiotics than in patients who did not – 6.1 months and 3.7 months, respectively (HR, 0.74; 95% CI, 0.60-0.93; P = .0135).
To overcome possible bias introduced by misclassification of patients who received antibiotics but discontinued immunotherapy within 30 days of initiation, the investigators conducted a landmark selection analysis among only those patients with a median follow-up for PFS of 30 days or longer (n = 402). This analysis confirmed the prior findings.
“Antibiotic exposure in the 30 days before or after immune checkpoint initiation in hepatocellular carcinoma is associated with prolonged progression-free survival,” Dr. Fessas concluded.
He added that a key question for future research is to discover the immune-microbiologic determinants of response to initiation of ICIs.
Positive effect surprising
“My group has shown that antibiotic therapy is normally detrimental in patients with cancer,” investigator David J. Pinato, MD, PhD, of Imperial College London, said in an interview. “So we were very surprised to see a positive effect on PFS.”
He added that the new findings should be interpreted with caution.
“My feeling is that, unlike with many other malignancies, the gut microbiome is heavily involved in the progression of the cirrhosis that pre-dates HCC onset,” he said.
That would suggest the relationship between antibiotics and perturbation of the gut microbiome is dictated by something more than changes in antitumor immune tolerance, he added.
“Overall, I think the interplay is more complex in HCC: cirrhosis/cancer/microbiome, not just microbiome/cancer as in many other tumors,” Dr. Pinato said. “So we are looking at microbial determinants of response in HCC patients undergoing ICI therapy, and we are hopeful to see some more mechanistic evidence behind this association.”
Dr. Pinato disclosed relationships with ViiV Healthcare, Bayer Healthcare, Bristol Myers Squibb, Mina Therapeutics, Eisai, Roche, and AstraZeneca. Dr. Fessas reported having no conflicts of interest. No funding source for the study was disclosed.
FROM AACR 2021
Tebentafusp improves OS: A first in metastatic uveal melanoma
Tebentafusp is the first investigational therapy in a phase 3 trial to improve OS in metastatic uveal melanoma, said Jessica Hassel, MD, of University Hospital Heidelberg in Germany, when presenting the results at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT002).
Dr. Hassel explained that tebentafusp is a bispecific fusion protein designed to target gp100 through a high affinity T-cell receptor binding domain and an anti-CD3 T-cell engaging domain, which redirects T cells to kill gp100-expressing tumor cells. Because the T-cell receptor binding domain only recognizes a specific gp100-derived peptide presented on HLA-A*02:01, tebentafusp can only be used to treat patients with this HLA type.
In the phase 3 trial, investigators enrolled 378 treatment-naive HLA-A*02:01-positive patients with metastatic uveal melanoma. Their median age was 65 years, and 50% were men.
Patients were assigned 2:1 to receive tebentafusp (n = 252) or investigator’s choice of pembrolizumab (n = 103), ipilimumab (n = 16), or dacarbazine (n = 7).
Prolonged OS despite low response rate
At a median follow-up of 14.1 months, patients receiving tebentafusp had significantly longer OS than that of patients in the investigator’s choice arm – 21.7 months and 16.0 months, respectively. The estimated 1-year OS rate was 73.2% in the tebentafusp arm and 58.5% in the standard therapy arm (hazard ratio, 0.51; 95% confidence interval, 0.37-0.71; P < .0001). The OS benefit was consistent across subgroups, Dr. Hassel said.
At a median follow-up of 11.4 months, the median progression-free survival was 3.3 months in the tebentafusp arm and 2.9 months in the investigator’s choice arm (HR, 0.73; 95% CI, 0.58-0.94; P = .0139).
The objective response rate was 9% in the tebentafusp arm and 5% in the investigator’s choice arm. There was only one complete response, and it was in the tebentafusp arm.
The disease control rate, defined as response or stable disease for 12 or more weeks, was 46% in the tebentafusp arm and 27% in the investigator’s choice arm. Rates of progressive disease were 52% and 62%, respectively.
Dr. Hassel pointed out that a landmark analysis of OS in patients with a best response of progressive disease, with patients continuing to receive treatment after progression, showed a hazard ratio of 0.4 (95% CI, 0.248-0.642) for those receiving tebentafusp vs. investigator’s choice. The OS benefit, despite low response rates, suggests that patients progress but are then stabilized with tebentafusp treatment.
“So this drug is slowing down developing disease,” she said.
‘Manageable’ adverse events
Target-mediated or cytokine-mediated adverse events were the most common side effects with tebentafusp. These included pyrexia (76%), pruritus (69%), and rash (83%), which decreased in frequency and severity after the first three to four doses.
While cytokine release syndrome was common (89%), the rate of grade 3-4 cytokine release syndrome was very low (1%). Adverse events were generally manageable with standard interventions, Dr. Hassel said.
The discontinuation rate was lower in the tebentafusp arm than in the investigator’s choice arm – 2% and 4.5%, respectively. There were no tebentafusp-related deaths.
‘Practice-changing’ results
“This is the first randomized controlled trial to be positive for overall survival in uveal melanoma. These are seminal and practice-changing results,” said AACR discussant Caroline Robert, MD, PhD, of Gustave Roussy and Paris-Saclay University in France.
She observed that the biology of uveal melanoma is distinct from that of cutaneous melanoma, and future research will have to address why tebentafusp doesn’t work as well in cutaneous melanoma. Tebentafusp will be evaluated in combination with immune checkpoint inhibitors as well, she added.
The major limitation of tebentafusp, Dr. Hassel observed, is that it can be used only in HLA-A*02:01-positive patients. “There still remains an unmet need for patients who do not have this particular surface protein,” she said.
The study was sponsored by Immunocore. Dr. Hassel disclosed relationships with Immunocore and other companies. Dr. Robert disclosed relationships with Bristol Myers Squibb, Pierre Fabre, Novartis, and other companies.
Tebentafusp is the first investigational therapy in a phase 3 trial to improve OS in metastatic uveal melanoma, said Jessica Hassel, MD, of University Hospital Heidelberg in Germany, when presenting the results at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT002).
Dr. Hassel explained that tebentafusp is a bispecific fusion protein designed to target gp100 through a high affinity T-cell receptor binding domain and an anti-CD3 T-cell engaging domain, which redirects T cells to kill gp100-expressing tumor cells. Because the T-cell receptor binding domain only recognizes a specific gp100-derived peptide presented on HLA-A*02:01, tebentafusp can only be used to treat patients with this HLA type.
In the phase 3 trial, investigators enrolled 378 treatment-naive HLA-A*02:01-positive patients with metastatic uveal melanoma. Their median age was 65 years, and 50% were men.
Patients were assigned 2:1 to receive tebentafusp (n = 252) or investigator’s choice of pembrolizumab (n = 103), ipilimumab (n = 16), or dacarbazine (n = 7).
Prolonged OS despite low response rate
At a median follow-up of 14.1 months, patients receiving tebentafusp had significantly longer OS than that of patients in the investigator’s choice arm – 21.7 months and 16.0 months, respectively. The estimated 1-year OS rate was 73.2% in the tebentafusp arm and 58.5% in the standard therapy arm (hazard ratio, 0.51; 95% confidence interval, 0.37-0.71; P < .0001). The OS benefit was consistent across subgroups, Dr. Hassel said.
At a median follow-up of 11.4 months, the median progression-free survival was 3.3 months in the tebentafusp arm and 2.9 months in the investigator’s choice arm (HR, 0.73; 95% CI, 0.58-0.94; P = .0139).
The objective response rate was 9% in the tebentafusp arm and 5% in the investigator’s choice arm. There was only one complete response, and it was in the tebentafusp arm.
The disease control rate, defined as response or stable disease for 12 or more weeks, was 46% in the tebentafusp arm and 27% in the investigator’s choice arm. Rates of progressive disease were 52% and 62%, respectively.
Dr. Hassel pointed out that a landmark analysis of OS in patients with a best response of progressive disease, with patients continuing to receive treatment after progression, showed a hazard ratio of 0.4 (95% CI, 0.248-0.642) for those receiving tebentafusp vs. investigator’s choice. The OS benefit, despite low response rates, suggests that patients progress but are then stabilized with tebentafusp treatment.
“So this drug is slowing down developing disease,” she said.
‘Manageable’ adverse events
Target-mediated or cytokine-mediated adverse events were the most common side effects with tebentafusp. These included pyrexia (76%), pruritus (69%), and rash (83%), which decreased in frequency and severity after the first three to four doses.
While cytokine release syndrome was common (89%), the rate of grade 3-4 cytokine release syndrome was very low (1%). Adverse events were generally manageable with standard interventions, Dr. Hassel said.
The discontinuation rate was lower in the tebentafusp arm than in the investigator’s choice arm – 2% and 4.5%, respectively. There were no tebentafusp-related deaths.
‘Practice-changing’ results
“This is the first randomized controlled trial to be positive for overall survival in uveal melanoma. These are seminal and practice-changing results,” said AACR discussant Caroline Robert, MD, PhD, of Gustave Roussy and Paris-Saclay University in France.
She observed that the biology of uveal melanoma is distinct from that of cutaneous melanoma, and future research will have to address why tebentafusp doesn’t work as well in cutaneous melanoma. Tebentafusp will be evaluated in combination with immune checkpoint inhibitors as well, she added.
The major limitation of tebentafusp, Dr. Hassel observed, is that it can be used only in HLA-A*02:01-positive patients. “There still remains an unmet need for patients who do not have this particular surface protein,” she said.
The study was sponsored by Immunocore. Dr. Hassel disclosed relationships with Immunocore and other companies. Dr. Robert disclosed relationships with Bristol Myers Squibb, Pierre Fabre, Novartis, and other companies.
Tebentafusp is the first investigational therapy in a phase 3 trial to improve OS in metastatic uveal melanoma, said Jessica Hassel, MD, of University Hospital Heidelberg in Germany, when presenting the results at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract CT002).
Dr. Hassel explained that tebentafusp is a bispecific fusion protein designed to target gp100 through a high affinity T-cell receptor binding domain and an anti-CD3 T-cell engaging domain, which redirects T cells to kill gp100-expressing tumor cells. Because the T-cell receptor binding domain only recognizes a specific gp100-derived peptide presented on HLA-A*02:01, tebentafusp can only be used to treat patients with this HLA type.
In the phase 3 trial, investigators enrolled 378 treatment-naive HLA-A*02:01-positive patients with metastatic uveal melanoma. Their median age was 65 years, and 50% were men.
Patients were assigned 2:1 to receive tebentafusp (n = 252) or investigator’s choice of pembrolizumab (n = 103), ipilimumab (n = 16), or dacarbazine (n = 7).
Prolonged OS despite low response rate
At a median follow-up of 14.1 months, patients receiving tebentafusp had significantly longer OS than that of patients in the investigator’s choice arm – 21.7 months and 16.0 months, respectively. The estimated 1-year OS rate was 73.2% in the tebentafusp arm and 58.5% in the standard therapy arm (hazard ratio, 0.51; 95% confidence interval, 0.37-0.71; P < .0001). The OS benefit was consistent across subgroups, Dr. Hassel said.
At a median follow-up of 11.4 months, the median progression-free survival was 3.3 months in the tebentafusp arm and 2.9 months in the investigator’s choice arm (HR, 0.73; 95% CI, 0.58-0.94; P = .0139).
The objective response rate was 9% in the tebentafusp arm and 5% in the investigator’s choice arm. There was only one complete response, and it was in the tebentafusp arm.
The disease control rate, defined as response or stable disease for 12 or more weeks, was 46% in the tebentafusp arm and 27% in the investigator’s choice arm. Rates of progressive disease were 52% and 62%, respectively.
Dr. Hassel pointed out that a landmark analysis of OS in patients with a best response of progressive disease, with patients continuing to receive treatment after progression, showed a hazard ratio of 0.4 (95% CI, 0.248-0.642) for those receiving tebentafusp vs. investigator’s choice. The OS benefit, despite low response rates, suggests that patients progress but are then stabilized with tebentafusp treatment.
“So this drug is slowing down developing disease,” she said.
‘Manageable’ adverse events
Target-mediated or cytokine-mediated adverse events were the most common side effects with tebentafusp. These included pyrexia (76%), pruritus (69%), and rash (83%), which decreased in frequency and severity after the first three to four doses.
While cytokine release syndrome was common (89%), the rate of grade 3-4 cytokine release syndrome was very low (1%). Adverse events were generally manageable with standard interventions, Dr. Hassel said.
The discontinuation rate was lower in the tebentafusp arm than in the investigator’s choice arm – 2% and 4.5%, respectively. There were no tebentafusp-related deaths.
‘Practice-changing’ results
“This is the first randomized controlled trial to be positive for overall survival in uveal melanoma. These are seminal and practice-changing results,” said AACR discussant Caroline Robert, MD, PhD, of Gustave Roussy and Paris-Saclay University in France.
She observed that the biology of uveal melanoma is distinct from that of cutaneous melanoma, and future research will have to address why tebentafusp doesn’t work as well in cutaneous melanoma. Tebentafusp will be evaluated in combination with immune checkpoint inhibitors as well, she added.
The major limitation of tebentafusp, Dr. Hassel observed, is that it can be used only in HLA-A*02:01-positive patients. “There still remains an unmet need for patients who do not have this particular surface protein,” she said.
The study was sponsored by Immunocore. Dr. Hassel disclosed relationships with Immunocore and other companies. Dr. Robert disclosed relationships with Bristol Myers Squibb, Pierre Fabre, Novartis, and other companies.
FROM AACR 2021