User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Lawsuit Targets Publishers: Is Peer Review Flawed?
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Is Metformin An Unexpected Ally Against Long COVID?
TOPLINE:
METHODOLOGY:
- Previous studies have shown that metformin use before and during SARS-CoV-2 infection reduces severe COVID-19 and postacute sequelae of SARS-CoV-2 (PASC), also referred to as long COVID, in adults.
- A retrospective cohort analysis was conducted to evaluate the association between metformin use before and during SARS-CoV-2 infection and the subsequent incidence of PASC.
- Researchers used data from the National COVID Cohort Collaborative (N3C) and National Patient-Centered Clinical Research Network (PCORnet) electronic health record (EHR) databases to identify adults (age, ≥ 21 years) with T2D prescribed a diabetes medication within the past 12 months.
- Participants were categorized into those using metformin (metformin group) and those using other noninsulin diabetes medications such as sulfonylureas, dipeptidyl peptidase-4 inhibitors, or thiazolidinediones (the comparator group); those who used glucagon-like peptide 1 receptor agonists or sodium-glucose cotransporter-2 inhibitors were excluded.
- The primary outcome was the incidence of PASC or death within 180 days after SARS-CoV-2 infection, defined using International Classification of Diseases U09.9 diagnosis code and/or computable phenotype defined by a predicted probability of > 75% for PASC using a machine learning model trained on patients diagnosed using U09.9 (PASC computable phenotype).
TAKEAWAY:
- Researchers identified 51,385 and 37,947 participants from the N3C and PCORnet datasets, respectively.
- Metformin use was associated with a 21% lower risk for death or PASC using the U09.9 diagnosis code (P < .001) and a 15% lower risk using the PASC computable phenotype (P < .001) in the N3C dataset than non-metformin use.
- In the PCORnet dataset, the risk for death or PASC was 13% lower using the U09.9 diagnosis code (P = .08) with metformin use vs non-metformin use, whereas the risk did not differ significantly between the groups when using the PASC computable phenotype (P = .58).
- The incidence of PASC using the U09.9 diagnosis code for the metformin and comparator groups was similar between the two datasets (1.6% and 2.0% in N3C and 2.2 and 2.6% in PCORnet, respectively).
- However, when using the computable phenotype, the incidence rates of PASC for the metformin and comparator groups were 4.8% and 5.2% in N3C and 25.2% and 24.2% in PCORnet, respectively.
IN PRACTICE:
“The incidence of PASC was lower when defined by [International Classification of Diseases] code, compared with a computable phenotype in both databases,” the authors wrote. “This may reflect the challenges of clinical care for adults needing chronic medication management and the likelihood of those adults receiving a formal PASC diagnosis.”
SOURCE:
The study was led by Steven G. Johnson, PhD, Institute for Health Informatics, University of Minnesota, Minneapolis. It was published online in Diabetes Care.
LIMITATIONS:
The use of EHR data had several limitations, including the inability to examine a dose-dependent relationship and the lack of information on whether medications were taken before, during, or after the acute infection. The outcome definition involved the need for a medical encounter and, thus, may not capture data on all patients experiencing symptoms of PASC. The analysis focused on the prevalent use of chronic medications, limiting the assessment of initiating metformin in those diagnosed with COVID-19.
DISCLOSURES:
The study was supported by the National Institutes of Health Agreement as part of the RECOVER research program. One author reported receiving salary support from the Center for Pharmacoepidemiology and owning stock options in various pharmaceutical and biopharmaceutical companies. Another author reported receiving grant support and consulting contracts, being involved in expert witness engagement, and owning stock options in various pharmaceutical, biopharmaceutical, diabetes management, and medical device companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Previous studies have shown that metformin use before and during SARS-CoV-2 infection reduces severe COVID-19 and postacute sequelae of SARS-CoV-2 (PASC), also referred to as long COVID, in adults.
- A retrospective cohort analysis was conducted to evaluate the association between metformin use before and during SARS-CoV-2 infection and the subsequent incidence of PASC.
- Researchers used data from the National COVID Cohort Collaborative (N3C) and National Patient-Centered Clinical Research Network (PCORnet) electronic health record (EHR) databases to identify adults (age, ≥ 21 years) with T2D prescribed a diabetes medication within the past 12 months.
- Participants were categorized into those using metformin (metformin group) and those using other noninsulin diabetes medications such as sulfonylureas, dipeptidyl peptidase-4 inhibitors, or thiazolidinediones (the comparator group); those who used glucagon-like peptide 1 receptor agonists or sodium-glucose cotransporter-2 inhibitors were excluded.
- The primary outcome was the incidence of PASC or death within 180 days after SARS-CoV-2 infection, defined using International Classification of Diseases U09.9 diagnosis code and/or computable phenotype defined by a predicted probability of > 75% for PASC using a machine learning model trained on patients diagnosed using U09.9 (PASC computable phenotype).
TAKEAWAY:
- Researchers identified 51,385 and 37,947 participants from the N3C and PCORnet datasets, respectively.
- Metformin use was associated with a 21% lower risk for death or PASC using the U09.9 diagnosis code (P < .001) and a 15% lower risk using the PASC computable phenotype (P < .001) in the N3C dataset than non-metformin use.
- In the PCORnet dataset, the risk for death or PASC was 13% lower using the U09.9 diagnosis code (P = .08) with metformin use vs non-metformin use, whereas the risk did not differ significantly between the groups when using the PASC computable phenotype (P = .58).
- The incidence of PASC using the U09.9 diagnosis code for the metformin and comparator groups was similar between the two datasets (1.6% and 2.0% in N3C and 2.2 and 2.6% in PCORnet, respectively).
- However, when using the computable phenotype, the incidence rates of PASC for the metformin and comparator groups were 4.8% and 5.2% in N3C and 25.2% and 24.2% in PCORnet, respectively.
IN PRACTICE:
“The incidence of PASC was lower when defined by [International Classification of Diseases] code, compared with a computable phenotype in both databases,” the authors wrote. “This may reflect the challenges of clinical care for adults needing chronic medication management and the likelihood of those adults receiving a formal PASC diagnosis.”
SOURCE:
The study was led by Steven G. Johnson, PhD, Institute for Health Informatics, University of Minnesota, Minneapolis. It was published online in Diabetes Care.
LIMITATIONS:
The use of EHR data had several limitations, including the inability to examine a dose-dependent relationship and the lack of information on whether medications were taken before, during, or after the acute infection. The outcome definition involved the need for a medical encounter and, thus, may not capture data on all patients experiencing symptoms of PASC. The analysis focused on the prevalent use of chronic medications, limiting the assessment of initiating metformin in those diagnosed with COVID-19.
DISCLOSURES:
The study was supported by the National Institutes of Health Agreement as part of the RECOVER research program. One author reported receiving salary support from the Center for Pharmacoepidemiology and owning stock options in various pharmaceutical and biopharmaceutical companies. Another author reported receiving grant support and consulting contracts, being involved in expert witness engagement, and owning stock options in various pharmaceutical, biopharmaceutical, diabetes management, and medical device companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Previous studies have shown that metformin use before and during SARS-CoV-2 infection reduces severe COVID-19 and postacute sequelae of SARS-CoV-2 (PASC), also referred to as long COVID, in adults.
- A retrospective cohort analysis was conducted to evaluate the association between metformin use before and during SARS-CoV-2 infection and the subsequent incidence of PASC.
- Researchers used data from the National COVID Cohort Collaborative (N3C) and National Patient-Centered Clinical Research Network (PCORnet) electronic health record (EHR) databases to identify adults (age, ≥ 21 years) with T2D prescribed a diabetes medication within the past 12 months.
- Participants were categorized into those using metformin (metformin group) and those using other noninsulin diabetes medications such as sulfonylureas, dipeptidyl peptidase-4 inhibitors, or thiazolidinediones (the comparator group); those who used glucagon-like peptide 1 receptor agonists or sodium-glucose cotransporter-2 inhibitors were excluded.
- The primary outcome was the incidence of PASC or death within 180 days after SARS-CoV-2 infection, defined using International Classification of Diseases U09.9 diagnosis code and/or computable phenotype defined by a predicted probability of > 75% for PASC using a machine learning model trained on patients diagnosed using U09.9 (PASC computable phenotype).
TAKEAWAY:
- Researchers identified 51,385 and 37,947 participants from the N3C and PCORnet datasets, respectively.
- Metformin use was associated with a 21% lower risk for death or PASC using the U09.9 diagnosis code (P < .001) and a 15% lower risk using the PASC computable phenotype (P < .001) in the N3C dataset than non-metformin use.
- In the PCORnet dataset, the risk for death or PASC was 13% lower using the U09.9 diagnosis code (P = .08) with metformin use vs non-metformin use, whereas the risk did not differ significantly between the groups when using the PASC computable phenotype (P = .58).
- The incidence of PASC using the U09.9 diagnosis code for the metformin and comparator groups was similar between the two datasets (1.6% and 2.0% in N3C and 2.2 and 2.6% in PCORnet, respectively).
- However, when using the computable phenotype, the incidence rates of PASC for the metformin and comparator groups were 4.8% and 5.2% in N3C and 25.2% and 24.2% in PCORnet, respectively.
IN PRACTICE:
“The incidence of PASC was lower when defined by [International Classification of Diseases] code, compared with a computable phenotype in both databases,” the authors wrote. “This may reflect the challenges of clinical care for adults needing chronic medication management and the likelihood of those adults receiving a formal PASC diagnosis.”
SOURCE:
The study was led by Steven G. Johnson, PhD, Institute for Health Informatics, University of Minnesota, Minneapolis. It was published online in Diabetes Care.
LIMITATIONS:
The use of EHR data had several limitations, including the inability to examine a dose-dependent relationship and the lack of information on whether medications were taken before, during, or after the acute infection. The outcome definition involved the need for a medical encounter and, thus, may not capture data on all patients experiencing symptoms of PASC. The analysis focused on the prevalent use of chronic medications, limiting the assessment of initiating metformin in those diagnosed with COVID-19.
DISCLOSURES:
The study was supported by the National Institutes of Health Agreement as part of the RECOVER research program. One author reported receiving salary support from the Center for Pharmacoepidemiology and owning stock options in various pharmaceutical and biopharmaceutical companies. Another author reported receiving grant support and consulting contracts, being involved in expert witness engagement, and owning stock options in various pharmaceutical, biopharmaceutical, diabetes management, and medical device companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Hospitalized Patients With COPD and GERD Have Better Short-Term Outcomes
BOSTON — Gastroesophageal reflux disease (GERD) is associated with better in-hospital outcomes for patients hospitalized with chronic obstructive pulmonary disease (COPD).
“It was a very surprising result. We double-checked the analysis once we got it the first time because the whole expectation was that the outcomes will be worse. But because it’s a retrospective study and it’s based on a national database, there are some limitations,” said ABM Nasibul Alam, MD, who presented the study at the annual meeting of the American College of Chest Physicians (CHEST) . Alam is an internal medicine resident at Northwestern Medicine McHenry Hospital, McHenry, Illinois.
One possible conclusion is that acid reflux therapies received in hospital may be benefitting COPD. The retrospective nature of the study precludes establishing a causal relationship, but there are possible mechanisms that could account for a benefit, according to Alam.
“They might prevent micro-aspirations or silent aspirations in COPD patients. Sometimes you may not have a clinical diagnosis of GERD, but the patient might have silent micro-aspirations, so it might contribute to decreasing that,” said Alam.
The study was conducted to fill a gap in the literature. “Some studies have shown that the lung function in COPD patients gets moderately decreased if they have coexisting GERD, but there aren’t any studies that have looked into how it impacts COPD patients when they’re hospitalized, and especially acute complications,” said Alam.
The researchers retrospectively analyzed data from the Nationwide Readmissions Database from 2017 to 2020, utilizing ICD-10 codes to identify 3,798,952 hospitalized adults with a primary diagnosis of COPD, of which 26.97% also had GERD. Individuals without GERD were more likely to be male (47.72% vs 39.88%).
After multivariate adjustment, the presence of GERD was associated with a lower mortality rate (adjusted odds ratio [aOR], 0.717; P < .001) and reduced risks for acute respiratory failure (aOR, 0.915; P < .001), need for noninvasive mechanical ventilation (aOR, 0.907; P < .001), need for invasive ventilation for 24 hours or more (aOR, 0.727; P < .001), acute kidney injury (aOR, 0.877; P < .001), septic shock (aOR, 0.731; P < .001), and acute heart failure (aOR, 0.762; P < .001).
Despite these improved in-hospital outcomes, the researchers found that patients with GERD were at a higher risk for 30-day readmission (aOR, 1.08; P < .001). They also had slightly longer lengths of stay (+0.09 day; P < .001) and lower total charges (−$2824.5996; P < .001).
There have also been studies suggesting that GERD can directly lead to worse lung function among patients with COPD. “So it will be interesting to see if these medications have some kind of impact on the lung function as well. We need more robust studies [to determine that],” said Alam.
It is also important to keep in mind the long-term risk of proton pump inhibitors, especially in older patients. “We have to have good data before we start recommending this,” said Alam.
He suggested that physicians should begin to think more holistically about COPD management and consider the comorbidities. Alam has studied vitamin B12 deficiency in patients with COPD and found an association with cardiovascular comorbidities. “There are so many comorbidities with COPD. COPD itself puts patients at risk of cardiovascular comorbidity, for example. So when we have patients with COPD, we have to think about all those comorbidities and have to manage the patients comprehensively rather than just focusing on the specific targeted interventions,” said Alam.
The study should encourage further research, according to Kunal Deokar, MD, who moderated the session where the study was presented. “It does give us a signal that probably we should have more studies to look into whether patients hospitalized for COPD with GERD really have lower mortality rates, and what will be the effect of treatment on these patients,” said Deokar, who is an assistant professor of pulmonary medicine at the All India Institute of Medical Sciences, Delhi, India.
Alam and Deokar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON — Gastroesophageal reflux disease (GERD) is associated with better in-hospital outcomes for patients hospitalized with chronic obstructive pulmonary disease (COPD).
“It was a very surprising result. We double-checked the analysis once we got it the first time because the whole expectation was that the outcomes will be worse. But because it’s a retrospective study and it’s based on a national database, there are some limitations,” said ABM Nasibul Alam, MD, who presented the study at the annual meeting of the American College of Chest Physicians (CHEST) . Alam is an internal medicine resident at Northwestern Medicine McHenry Hospital, McHenry, Illinois.
One possible conclusion is that acid reflux therapies received in hospital may be benefitting COPD. The retrospective nature of the study precludes establishing a causal relationship, but there are possible mechanisms that could account for a benefit, according to Alam.
“They might prevent micro-aspirations or silent aspirations in COPD patients. Sometimes you may not have a clinical diagnosis of GERD, but the patient might have silent micro-aspirations, so it might contribute to decreasing that,” said Alam.
The study was conducted to fill a gap in the literature. “Some studies have shown that the lung function in COPD patients gets moderately decreased if they have coexisting GERD, but there aren’t any studies that have looked into how it impacts COPD patients when they’re hospitalized, and especially acute complications,” said Alam.
The researchers retrospectively analyzed data from the Nationwide Readmissions Database from 2017 to 2020, utilizing ICD-10 codes to identify 3,798,952 hospitalized adults with a primary diagnosis of COPD, of which 26.97% also had GERD. Individuals without GERD were more likely to be male (47.72% vs 39.88%).
After multivariate adjustment, the presence of GERD was associated with a lower mortality rate (adjusted odds ratio [aOR], 0.717; P < .001) and reduced risks for acute respiratory failure (aOR, 0.915; P < .001), need for noninvasive mechanical ventilation (aOR, 0.907; P < .001), need for invasive ventilation for 24 hours or more (aOR, 0.727; P < .001), acute kidney injury (aOR, 0.877; P < .001), septic shock (aOR, 0.731; P < .001), and acute heart failure (aOR, 0.762; P < .001).
Despite these improved in-hospital outcomes, the researchers found that patients with GERD were at a higher risk for 30-day readmission (aOR, 1.08; P < .001). They also had slightly longer lengths of stay (+0.09 day; P < .001) and lower total charges (−$2824.5996; P < .001).
There have also been studies suggesting that GERD can directly lead to worse lung function among patients with COPD. “So it will be interesting to see if these medications have some kind of impact on the lung function as well. We need more robust studies [to determine that],” said Alam.
It is also important to keep in mind the long-term risk of proton pump inhibitors, especially in older patients. “We have to have good data before we start recommending this,” said Alam.
He suggested that physicians should begin to think more holistically about COPD management and consider the comorbidities. Alam has studied vitamin B12 deficiency in patients with COPD and found an association with cardiovascular comorbidities. “There are so many comorbidities with COPD. COPD itself puts patients at risk of cardiovascular comorbidity, for example. So when we have patients with COPD, we have to think about all those comorbidities and have to manage the patients comprehensively rather than just focusing on the specific targeted interventions,” said Alam.
The study should encourage further research, according to Kunal Deokar, MD, who moderated the session where the study was presented. “It does give us a signal that probably we should have more studies to look into whether patients hospitalized for COPD with GERD really have lower mortality rates, and what will be the effect of treatment on these patients,” said Deokar, who is an assistant professor of pulmonary medicine at the All India Institute of Medical Sciences, Delhi, India.
Alam and Deokar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON — Gastroesophageal reflux disease (GERD) is associated with better in-hospital outcomes for patients hospitalized with chronic obstructive pulmonary disease (COPD).
“It was a very surprising result. We double-checked the analysis once we got it the first time because the whole expectation was that the outcomes will be worse. But because it’s a retrospective study and it’s based on a national database, there are some limitations,” said ABM Nasibul Alam, MD, who presented the study at the annual meeting of the American College of Chest Physicians (CHEST) . Alam is an internal medicine resident at Northwestern Medicine McHenry Hospital, McHenry, Illinois.
One possible conclusion is that acid reflux therapies received in hospital may be benefitting COPD. The retrospective nature of the study precludes establishing a causal relationship, but there are possible mechanisms that could account for a benefit, according to Alam.
“They might prevent micro-aspirations or silent aspirations in COPD patients. Sometimes you may not have a clinical diagnosis of GERD, but the patient might have silent micro-aspirations, so it might contribute to decreasing that,” said Alam.
The study was conducted to fill a gap in the literature. “Some studies have shown that the lung function in COPD patients gets moderately decreased if they have coexisting GERD, but there aren’t any studies that have looked into how it impacts COPD patients when they’re hospitalized, and especially acute complications,” said Alam.
The researchers retrospectively analyzed data from the Nationwide Readmissions Database from 2017 to 2020, utilizing ICD-10 codes to identify 3,798,952 hospitalized adults with a primary diagnosis of COPD, of which 26.97% also had GERD. Individuals without GERD were more likely to be male (47.72% vs 39.88%).
After multivariate adjustment, the presence of GERD was associated with a lower mortality rate (adjusted odds ratio [aOR], 0.717; P < .001) and reduced risks for acute respiratory failure (aOR, 0.915; P < .001), need for noninvasive mechanical ventilation (aOR, 0.907; P < .001), need for invasive ventilation for 24 hours or more (aOR, 0.727; P < .001), acute kidney injury (aOR, 0.877; P < .001), septic shock (aOR, 0.731; P < .001), and acute heart failure (aOR, 0.762; P < .001).
Despite these improved in-hospital outcomes, the researchers found that patients with GERD were at a higher risk for 30-day readmission (aOR, 1.08; P < .001). They also had slightly longer lengths of stay (+0.09 day; P < .001) and lower total charges (−$2824.5996; P < .001).
There have also been studies suggesting that GERD can directly lead to worse lung function among patients with COPD. “So it will be interesting to see if these medications have some kind of impact on the lung function as well. We need more robust studies [to determine that],” said Alam.
It is also important to keep in mind the long-term risk of proton pump inhibitors, especially in older patients. “We have to have good data before we start recommending this,” said Alam.
He suggested that physicians should begin to think more holistically about COPD management and consider the comorbidities. Alam has studied vitamin B12 deficiency in patients with COPD and found an association with cardiovascular comorbidities. “There are so many comorbidities with COPD. COPD itself puts patients at risk of cardiovascular comorbidity, for example. So when we have patients with COPD, we have to think about all those comorbidities and have to manage the patients comprehensively rather than just focusing on the specific targeted interventions,” said Alam.
The study should encourage further research, according to Kunal Deokar, MD, who moderated the session where the study was presented. “It does give us a signal that probably we should have more studies to look into whether patients hospitalized for COPD with GERD really have lower mortality rates, and what will be the effect of treatment on these patients,” said Deokar, who is an assistant professor of pulmonary medicine at the All India Institute of Medical Sciences, Delhi, India.
Alam and Deokar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CHEST 2024
Why Residents Are Joining Unions in Droves
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Clozapine and Respiratory Infection Risk: What to Know
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Anticipated Effects of Pneumococcal Vaccines on Otitis
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).
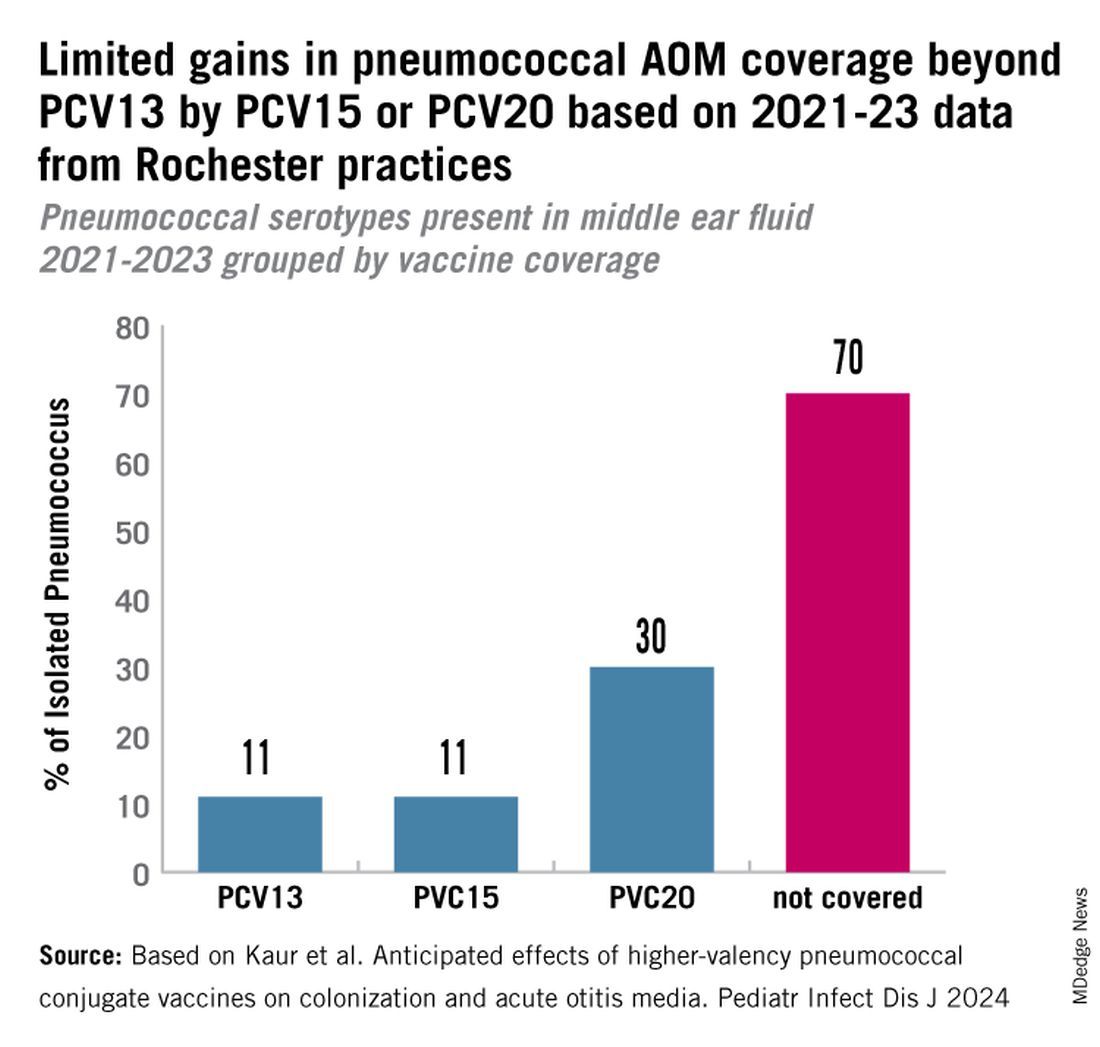
Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Surgical Center Wins $421 Million Verdict Against Blue Cross
Insurance specialists told this news organization that the September 20 verdict is unusual. If upheld on appeal, one said, it could give out-of-network providers more power to decide how much insurers must pay them.
The case, which the St. Charles Surgical Hospital and Center for Restorative Breast Surgery first filed in 2017 in Louisiana state court, will be appealed and could ultimately land in federal court. The center has seen mixed results from a similar case it filed in federal court, legal documents show. Physicians from the center declined comment.
At issue: Did Blue Cross fail to fully pay the surgery center for about 7000 out-of-network procedures that it authorized?
The lawsuit claimed that the insurer’s online system confirmed that claims would be paid and noted the percentage of patient bills that would be reimbursed.
However, “Blue Cross and Blue Shield of Louisiana either slow-paid, low-paid, or no-paid all their bills over an eight-year period, hoping to pressure the doctors and hospital to either come into the network or fail and close down,” the surgery center’s attorney, James Williams, said in a statement.
Blue Cross denied that it acted fraudulently, “arguing that because the hospital is not a member of its provider network, it had no contractual obligation to pay anything,” the Times-Picayune newspaper reported. Authorization of a procedure doesn’t guarantee payment, the insurer argued in court.
In a statement to the media, Blue Cross said it disagrees with the verdict and will appeal.
Out-of-Network Free For All
Paul B. Ginsburg, PhD, professor of the Practice of Health Policy at the Price School of Public Policy, University of Southern California, Los Angeles, said out-of-network care doesn’t come with a contractual relationship.
Without a contract, he said, “providers can charge whatever they want, and the insurers will pay them whatever they want, and then it’s up to the provider to see how much additional balance bill they can collect from the patients.” (Some states and the federal government have laws partly protecting patients from balance billing when doctors and insurers conflict over payment.)
He added that “if insurance companies were on the hook to pay whatever any provider charges, nobody would ever belong to a network, and rates would be sky high. Many fewer people would buy insurance. Providers would [then] charge as much as they think they can get from the patients.”
What about the insurer’s apparent authorization of the out-of-network procedures? “They’re authorizing them because they believe the procedures are medically warranted,” Dr. Ginsburg said. “That’s totally separate from how much they’ll pay.”
Dr. Ginsburg added that juries in the South are known for imposing high penalties against companies. “They often come up with crazy verdicts.”
Mark V. Pauly, PhD, MA, professor emeritus of health care management at the Wharton School of the University of Pennsylvania, Philadelphia, questioned why the clinic kept accepting Blue Cross patients.
“Once it became apparent that Blue Cross wasn’t going to pay them well or would give them a lot of grief,” Dr. Pauly said, “the simplest thing would have been to tell patients that we’re going to go back to the old-fashioned way of doing things: You pay us up front, or assure us that you’re going to pay.”
Lawton Robert Burns, PhD, MBA, professor of health care management at the Wharton School, said the case and the verdict are unusual. He noted that insurer contracts with employers often state that out-of-network care will be covered at a specific rate, such as 70% of “reasonable charges.”
A 2020 analysis found that initial breast reconstruction surgeries in the United States cost a median of $24,600-$38,000 from 2009 to 2016. According to the Times-Picayune, the New Orleans clinic billed Blue Cross for $506.7 million, averaging more than $72,385 per procedure.
Dr. Ginsburg, Dr. Pauly, and Dr. Burns had no disclosures.
A version of this article first appeared on Medscape.com.
Insurance specialists told this news organization that the September 20 verdict is unusual. If upheld on appeal, one said, it could give out-of-network providers more power to decide how much insurers must pay them.
The case, which the St. Charles Surgical Hospital and Center for Restorative Breast Surgery first filed in 2017 in Louisiana state court, will be appealed and could ultimately land in federal court. The center has seen mixed results from a similar case it filed in federal court, legal documents show. Physicians from the center declined comment.
At issue: Did Blue Cross fail to fully pay the surgery center for about 7000 out-of-network procedures that it authorized?
The lawsuit claimed that the insurer’s online system confirmed that claims would be paid and noted the percentage of patient bills that would be reimbursed.
However, “Blue Cross and Blue Shield of Louisiana either slow-paid, low-paid, or no-paid all their bills over an eight-year period, hoping to pressure the doctors and hospital to either come into the network or fail and close down,” the surgery center’s attorney, James Williams, said in a statement.
Blue Cross denied that it acted fraudulently, “arguing that because the hospital is not a member of its provider network, it had no contractual obligation to pay anything,” the Times-Picayune newspaper reported. Authorization of a procedure doesn’t guarantee payment, the insurer argued in court.
In a statement to the media, Blue Cross said it disagrees with the verdict and will appeal.
Out-of-Network Free For All
Paul B. Ginsburg, PhD, professor of the Practice of Health Policy at the Price School of Public Policy, University of Southern California, Los Angeles, said out-of-network care doesn’t come with a contractual relationship.
Without a contract, he said, “providers can charge whatever they want, and the insurers will pay them whatever they want, and then it’s up to the provider to see how much additional balance bill they can collect from the patients.” (Some states and the federal government have laws partly protecting patients from balance billing when doctors and insurers conflict over payment.)
He added that “if insurance companies were on the hook to pay whatever any provider charges, nobody would ever belong to a network, and rates would be sky high. Many fewer people would buy insurance. Providers would [then] charge as much as they think they can get from the patients.”
What about the insurer’s apparent authorization of the out-of-network procedures? “They’re authorizing them because they believe the procedures are medically warranted,” Dr. Ginsburg said. “That’s totally separate from how much they’ll pay.”
Dr. Ginsburg added that juries in the South are known for imposing high penalties against companies. “They often come up with crazy verdicts.”
Mark V. Pauly, PhD, MA, professor emeritus of health care management at the Wharton School of the University of Pennsylvania, Philadelphia, questioned why the clinic kept accepting Blue Cross patients.
“Once it became apparent that Blue Cross wasn’t going to pay them well or would give them a lot of grief,” Dr. Pauly said, “the simplest thing would have been to tell patients that we’re going to go back to the old-fashioned way of doing things: You pay us up front, or assure us that you’re going to pay.”
Lawton Robert Burns, PhD, MBA, professor of health care management at the Wharton School, said the case and the verdict are unusual. He noted that insurer contracts with employers often state that out-of-network care will be covered at a specific rate, such as 70% of “reasonable charges.”
A 2020 analysis found that initial breast reconstruction surgeries in the United States cost a median of $24,600-$38,000 from 2009 to 2016. According to the Times-Picayune, the New Orleans clinic billed Blue Cross for $506.7 million, averaging more than $72,385 per procedure.
Dr. Ginsburg, Dr. Pauly, and Dr. Burns had no disclosures.
A version of this article first appeared on Medscape.com.
Insurance specialists told this news organization that the September 20 verdict is unusual. If upheld on appeal, one said, it could give out-of-network providers more power to decide how much insurers must pay them.
The case, which the St. Charles Surgical Hospital and Center for Restorative Breast Surgery first filed in 2017 in Louisiana state court, will be appealed and could ultimately land in federal court. The center has seen mixed results from a similar case it filed in federal court, legal documents show. Physicians from the center declined comment.
At issue: Did Blue Cross fail to fully pay the surgery center for about 7000 out-of-network procedures that it authorized?
The lawsuit claimed that the insurer’s online system confirmed that claims would be paid and noted the percentage of patient bills that would be reimbursed.
However, “Blue Cross and Blue Shield of Louisiana either slow-paid, low-paid, or no-paid all their bills over an eight-year period, hoping to pressure the doctors and hospital to either come into the network or fail and close down,” the surgery center’s attorney, James Williams, said in a statement.
Blue Cross denied that it acted fraudulently, “arguing that because the hospital is not a member of its provider network, it had no contractual obligation to pay anything,” the Times-Picayune newspaper reported. Authorization of a procedure doesn’t guarantee payment, the insurer argued in court.
In a statement to the media, Blue Cross said it disagrees with the verdict and will appeal.
Out-of-Network Free For All
Paul B. Ginsburg, PhD, professor of the Practice of Health Policy at the Price School of Public Policy, University of Southern California, Los Angeles, said out-of-network care doesn’t come with a contractual relationship.
Without a contract, he said, “providers can charge whatever they want, and the insurers will pay them whatever they want, and then it’s up to the provider to see how much additional balance bill they can collect from the patients.” (Some states and the federal government have laws partly protecting patients from balance billing when doctors and insurers conflict over payment.)
He added that “if insurance companies were on the hook to pay whatever any provider charges, nobody would ever belong to a network, and rates would be sky high. Many fewer people would buy insurance. Providers would [then] charge as much as they think they can get from the patients.”
What about the insurer’s apparent authorization of the out-of-network procedures? “They’re authorizing them because they believe the procedures are medically warranted,” Dr. Ginsburg said. “That’s totally separate from how much they’ll pay.”
Dr. Ginsburg added that juries in the South are known for imposing high penalties against companies. “They often come up with crazy verdicts.”
Mark V. Pauly, PhD, MA, professor emeritus of health care management at the Wharton School of the University of Pennsylvania, Philadelphia, questioned why the clinic kept accepting Blue Cross patients.
“Once it became apparent that Blue Cross wasn’t going to pay them well or would give them a lot of grief,” Dr. Pauly said, “the simplest thing would have been to tell patients that we’re going to go back to the old-fashioned way of doing things: You pay us up front, or assure us that you’re going to pay.”
Lawton Robert Burns, PhD, MBA, professor of health care management at the Wharton School, said the case and the verdict are unusual. He noted that insurer contracts with employers often state that out-of-network care will be covered at a specific rate, such as 70% of “reasonable charges.”
A 2020 analysis found that initial breast reconstruction surgeries in the United States cost a median of $24,600-$38,000 from 2009 to 2016. According to the Times-Picayune, the New Orleans clinic billed Blue Cross for $506.7 million, averaging more than $72,385 per procedure.
Dr. Ginsburg, Dr. Pauly, and Dr. Burns had no disclosures.
A version of this article first appeared on Medscape.com.
ART Linked With Congenital Heart Defects in Newborns
The rate of congenital heart defects is higher in newborns conceived using assisted reproductive technologies (ART) than in newborns conceived without assistance. This finding comes from a population-based cohort study led by Dr. Nona Sargisian, a gynecologist at the University of Gothenburg, Sweden, and colleagues, which was published in the European Heart Journal.
The researchers analyzed more than 7 million results of all live-born children in Denmark, Finland, Sweden, and Norway between 1984 and 2015. They found that congenital heart defects occurred more frequently in the ART newborn group (1.85%) than in naturally conceived newborns (1.15%).
The study also revealed that the risk for congenital heart defects in multiple births is higher than in single births, with and without the use of ART. However, the result that congenital heart defects occur more often in ART newborns remained significant when comparing single births from both groups (1.62% vs 1.11%).
Relatively Low Prevalence
Barbara Sonntag, MD, PhD, a gynecologist at Amedes Fertility Center in Hamburg, Germany, referred to a “clinically relevant risk increase” with a relatively low prevalence of the condition.
“When 1000 children are born, an abnormality occurs in 18 children after ART, compared with 11 children born after natural conception,” she told the Science Media Center.
Dr. Sonntag emphasized that the risk is particularly increased by a multiple pregnancy. A statement about causality is not possible based on the study, but multiple pregnancies are generally associated with increased risks during pregnancy and for the children.
The large and robust dataset confirms long-known findings, said Georg Griesinger, MD, PhD, medical director of the fertility centers of the University Medical Center Schleswig-Holstein in Lübeck and Manhagen, Germany.
The key figures can be found in single births, he explained. “Among single births conceived by ART, the rate of severe congenital heart defects was 1.62% compared with 1.11% in spontaneously conceived single births, an increase in risk by 1.19 times. For severe heart defects, the rate was 0.31% in ART single births, compared with 0.25% in spontaneously conceived single births.”
The increased risks are consistent with existing literature. Therefore, the current study does not reveal any new risk signals, said Dr. Griesinger.
Single Embryo Transfer
The “risks are small but present,” according to Michael von Wolff, MD, head of gynecological endocrinology and reproductive medicine at Bern University Hospital in Switzerland. “Therefore, ART therapy should only be carried out after exhausting conservative treatments,” he recommended. For example, ovarian stimulation with low-dose hormone preparations could be an option.
Dr. Griesinger pointed out that, in absolute numbers, all maternal and fetal or neonatal risks are significantly increased in twins and higher-order multiples, compared with the estimated risk association within the actual ART treatment.
“For this reason, reproductive medicine specialists have been advocating for single-embryo transfer for years to promote the occurrence of single pregnancies through ART,” said Dr. Griesinger.
The study “emphasizes the importance of single embryo transfer to avoid the higher risks associated with multiple pregnancies,” according to Rocío Núñez Calonge, PhD, scientific director of the International Reproduction Unit in Alicante, Spain.
Dr. Sonntag also sees a “strong additional call to avoid multiple pregnancies through a predominant strategy of single-embryo transfer in the data. The increased rate of childhood birth defects is already part of the information provided before assisted reproduction.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The rate of congenital heart defects is higher in newborns conceived using assisted reproductive technologies (ART) than in newborns conceived without assistance. This finding comes from a population-based cohort study led by Dr. Nona Sargisian, a gynecologist at the University of Gothenburg, Sweden, and colleagues, which was published in the European Heart Journal.
The researchers analyzed more than 7 million results of all live-born children in Denmark, Finland, Sweden, and Norway between 1984 and 2015. They found that congenital heart defects occurred more frequently in the ART newborn group (1.85%) than in naturally conceived newborns (1.15%).
The study also revealed that the risk for congenital heart defects in multiple births is higher than in single births, with and without the use of ART. However, the result that congenital heart defects occur more often in ART newborns remained significant when comparing single births from both groups (1.62% vs 1.11%).
Relatively Low Prevalence
Barbara Sonntag, MD, PhD, a gynecologist at Amedes Fertility Center in Hamburg, Germany, referred to a “clinically relevant risk increase” with a relatively low prevalence of the condition.
“When 1000 children are born, an abnormality occurs in 18 children after ART, compared with 11 children born after natural conception,” she told the Science Media Center.
Dr. Sonntag emphasized that the risk is particularly increased by a multiple pregnancy. A statement about causality is not possible based on the study, but multiple pregnancies are generally associated with increased risks during pregnancy and for the children.
The large and robust dataset confirms long-known findings, said Georg Griesinger, MD, PhD, medical director of the fertility centers of the University Medical Center Schleswig-Holstein in Lübeck and Manhagen, Germany.
The key figures can be found in single births, he explained. “Among single births conceived by ART, the rate of severe congenital heart defects was 1.62% compared with 1.11% in spontaneously conceived single births, an increase in risk by 1.19 times. For severe heart defects, the rate was 0.31% in ART single births, compared with 0.25% in spontaneously conceived single births.”
The increased risks are consistent with existing literature. Therefore, the current study does not reveal any new risk signals, said Dr. Griesinger.
Single Embryo Transfer
The “risks are small but present,” according to Michael von Wolff, MD, head of gynecological endocrinology and reproductive medicine at Bern University Hospital in Switzerland. “Therefore, ART therapy should only be carried out after exhausting conservative treatments,” he recommended. For example, ovarian stimulation with low-dose hormone preparations could be an option.
Dr. Griesinger pointed out that, in absolute numbers, all maternal and fetal or neonatal risks are significantly increased in twins and higher-order multiples, compared with the estimated risk association within the actual ART treatment.
“For this reason, reproductive medicine specialists have been advocating for single-embryo transfer for years to promote the occurrence of single pregnancies through ART,” said Dr. Griesinger.
The study “emphasizes the importance of single embryo transfer to avoid the higher risks associated with multiple pregnancies,” according to Rocío Núñez Calonge, PhD, scientific director of the International Reproduction Unit in Alicante, Spain.
Dr. Sonntag also sees a “strong additional call to avoid multiple pregnancies through a predominant strategy of single-embryo transfer in the data. The increased rate of childhood birth defects is already part of the information provided before assisted reproduction.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The rate of congenital heart defects is higher in newborns conceived using assisted reproductive technologies (ART) than in newborns conceived without assistance. This finding comes from a population-based cohort study led by Dr. Nona Sargisian, a gynecologist at the University of Gothenburg, Sweden, and colleagues, which was published in the European Heart Journal.
The researchers analyzed more than 7 million results of all live-born children in Denmark, Finland, Sweden, and Norway between 1984 and 2015. They found that congenital heart defects occurred more frequently in the ART newborn group (1.85%) than in naturally conceived newborns (1.15%).
The study also revealed that the risk for congenital heart defects in multiple births is higher than in single births, with and without the use of ART. However, the result that congenital heart defects occur more often in ART newborns remained significant when comparing single births from both groups (1.62% vs 1.11%).
Relatively Low Prevalence
Barbara Sonntag, MD, PhD, a gynecologist at Amedes Fertility Center in Hamburg, Germany, referred to a “clinically relevant risk increase” with a relatively low prevalence of the condition.
“When 1000 children are born, an abnormality occurs in 18 children after ART, compared with 11 children born after natural conception,” she told the Science Media Center.
Dr. Sonntag emphasized that the risk is particularly increased by a multiple pregnancy. A statement about causality is not possible based on the study, but multiple pregnancies are generally associated with increased risks during pregnancy and for the children.
The large and robust dataset confirms long-known findings, said Georg Griesinger, MD, PhD, medical director of the fertility centers of the University Medical Center Schleswig-Holstein in Lübeck and Manhagen, Germany.
The key figures can be found in single births, he explained. “Among single births conceived by ART, the rate of severe congenital heart defects was 1.62% compared with 1.11% in spontaneously conceived single births, an increase in risk by 1.19 times. For severe heart defects, the rate was 0.31% in ART single births, compared with 0.25% in spontaneously conceived single births.”
The increased risks are consistent with existing literature. Therefore, the current study does not reveal any new risk signals, said Dr. Griesinger.
Single Embryo Transfer
The “risks are small but present,” according to Michael von Wolff, MD, head of gynecological endocrinology and reproductive medicine at Bern University Hospital in Switzerland. “Therefore, ART therapy should only be carried out after exhausting conservative treatments,” he recommended. For example, ovarian stimulation with low-dose hormone preparations could be an option.
Dr. Griesinger pointed out that, in absolute numbers, all maternal and fetal or neonatal risks are significantly increased in twins and higher-order multiples, compared with the estimated risk association within the actual ART treatment.
“For this reason, reproductive medicine specialists have been advocating for single-embryo transfer for years to promote the occurrence of single pregnancies through ART,” said Dr. Griesinger.
The study “emphasizes the importance of single embryo transfer to avoid the higher risks associated with multiple pregnancies,” according to Rocío Núñez Calonge, PhD, scientific director of the International Reproduction Unit in Alicante, Spain.
Dr. Sonntag also sees a “strong additional call to avoid multiple pregnancies through a predominant strategy of single-embryo transfer in the data. The increased rate of childhood birth defects is already part of the information provided before assisted reproduction.”
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
FROM EUROPEAN HEART JOURNAL
Is Wildfire Smoke More Toxic Than General Air Pollution?
Wildfire-related air pollution in Europe kills more than non-wildfire air pollution. As climate change exacerbates the frequency and violence of wildfires, researchers are studying the health implications of mitigation methods such as prescribed fires.
Presenting at the annual congress of the European Respiratory Society (ERS), Cathryn Tonne, PhD, an environmental epidemiologist at the Instituto de Salud Global de Barcelona, Spain, said wildfire-related PM2.5 is more toxic than general PM2.5, leading to significantly higher mortality rates.
Prescribed, controlled fires have been employed worldwide to reduce the chance of uncontrolled, catastrophic fires. However, researchers wonder whether the techniques reduce the overall fire-related PM2.5 or add up to it. “Prescribed fire increases ecosystem resilience and can reduce the risk of catastrophic wildfire,” said Jason Sacks, MPH, an epidemiologist in the Center for Public Health and Environmental Assessment in the Office of Research and Development at the Environmental Protection Agency (EPA), at the congress. “But it also leads to poorer air quality and health impacts, and we still don’t know what this means at a regional scale.”
Wildfire Pollution Kills More Than Other Air Pollution
Researchers at the Instituto de Salud Global de Barcelona used a large dataset of daily mortality data from 32 European countries collected through the EARLY-ADAPT project. They utilized the SILAM model to derive daily average concentrations of wildfire-related PM2.5, non-fire PM2.5, and total PM2.5 levels. They also employed GEOSTAT population grids at a 1-km resolution to calculate the attributable number of deaths across different regions, specifically focusing on data from 2006, 2011, and 2018.
The data analysis indicated that the relative risk per unit of PM2.5 is substantially larger for wildfire-related PM2.5, compared with non-fire PM2.5. “We essentially assume that wildfire smoke PM2.5 has the same toxicity as total PM2.5, but it’s increasingly clear that’s likely not the case,” Dr. Tonne said, presenting the study.
When employing exposure-response functions (ERFs) specific to wildfire smoke, researchers found that the attributable deaths from all causes of wildfire PM2.5 were approximately 10 times larger than those calculated using total PM2.5 exposure estimates. Dr. Tonne explained that this stark difference highlights the critical need for tailored ERFs that accurately reflect the unique health risks posed by wildfire smoke.
“Respiratory mortality usually has the strongest relative risks, and we’re seeing that in this study as well,” Dr. Tonne said. “Wildfire smoke seems to operate through quite immediate mechanisms, likely through inflammation and oxidative stress.”
One significant challenge of the study was the lack of uniform spatial resolution across all countries involved in the analysis. This inconsistency may affect how accurately mortality estimates can be attributed to specific PM2.5 sources. Additionally, the study had limited statistical power for generating age- and sex-specific mortality estimates, which could obscure important demographic differences in vulnerability to wildfire smoke exposure. The analysis was also constrained to data available only up to 2020, thereby excluding critical wildfire events from subsequent years, such as those in 2022 and 2023, which may have further elucidated the health impacts of wildfire smoke in Europe.
Fires Prescription
Prescribed fires or controlled burns are intentional fires set by land managers under carefully managed conditions.
Historically, many forested areas have been subjected to fire suppression practices, which allow combustible materials like dry leaves, twigs, and shrubs to accumulate over time. This buildup leads to a higher likelihood of severe, uncontrollable wildfires. Prescribed fires can reduce these fuel loads and improve the health and resilience of ecosystems.
They release fewer pollutants and emissions than the large-scale, unmanageable wildfires they help prevent because they happen at lower temperatures. But they still introduce pollutants in the air that can negatively affect nearby communities’ health.
People with preexisting respiratory conditions, such as asthma or chronic obstructive pulmonary disease (COPD), are particularly vulnerable to smoke, which can trigger health issues like breathing difficulties, coughing, and eye irritation. The cumulative impact of increased burns raises concerns about long-term air quality, especially in densely populated areas. “We need to understand if we’re actually tipping the scale to having less wildfire smoke or just increasing the total amount of smoke.”
Mitigation strategies include accurately picking the right timing and weather conditions to determine when and where to conduct controlled burns and effective and timely communication to inform local communities about upcoming burns, the potential for smoke exposure, and how to protect themselves.
There is a growing need to improve public messaging around prescribed fires, Mr. Sacks said, because often the message communicated is oversimplified, such as “there will be smoke, but don’t worry. But that’s not the message we want to convey, especially for people with asthma or COPD.”
Instead, he said public health agencies should provide clearer, science-based guidance on the risks for smoke exposure and practical steps people can take to reduce their risk.
What Can Doctors Do?
Chris Carlsten, MD, director of the Centre for Lung Health and professor and head of the Respiratory Medicine Division at the University of British Columbia, Vancouver, Canada, told this news organization that determining whether an exacerbation of a respiratory condition is caused by fire exposure or other factors, such as viral infections, is complex because both can trigger similar responses and may complement each other. “It’s very difficult for any individual to know whether, when they’re having an exacerbation of asthma or COPD, that’s due to the fire,” he said. Fire smoke also increases infection risks, further complicating diagnosis.
Dr. Carlsten suggested that physicians could recommend preventative use of inhalers for at-risk patients when wildfires occur rather than waiting for symptoms to worsen. “That is a really interesting idea that could be practical.” Still, he advises caution, stressing that patients should consult their providers because not all may react well to increased inhaler use.
He also highlighted a significant shift in the healthcare landscape, noting that traditionally, the focus has been on the cardiovascular impacts of pollution, particularly traffic-related pollution. However, as wildfire smoke becomes a growing issue, the focus is shifting back to respiratory problems, with profound implications for healthcare resources, budgets, and drug approvals based on the burden of respiratory disease. “Fire smoke is becoming more of a problem. This swing back to respiratory has huge implications for healthcare systems and respiratory disease burden.”
Mr. Sacks and Dr. Carlsten reported no relevant financial relationships. The study presented by Dr. Tonne received funding from the European Union’s Horizon Europe research and innovation programme under Grant Agreement No. 101057131.
A version of this article first appeared on Medscape.com.
Wildfire-related air pollution in Europe kills more than non-wildfire air pollution. As climate change exacerbates the frequency and violence of wildfires, researchers are studying the health implications of mitigation methods such as prescribed fires.
Presenting at the annual congress of the European Respiratory Society (ERS), Cathryn Tonne, PhD, an environmental epidemiologist at the Instituto de Salud Global de Barcelona, Spain, said wildfire-related PM2.5 is more toxic than general PM2.5, leading to significantly higher mortality rates.
Prescribed, controlled fires have been employed worldwide to reduce the chance of uncontrolled, catastrophic fires. However, researchers wonder whether the techniques reduce the overall fire-related PM2.5 or add up to it. “Prescribed fire increases ecosystem resilience and can reduce the risk of catastrophic wildfire,” said Jason Sacks, MPH, an epidemiologist in the Center for Public Health and Environmental Assessment in the Office of Research and Development at the Environmental Protection Agency (EPA), at the congress. “But it also leads to poorer air quality and health impacts, and we still don’t know what this means at a regional scale.”
Wildfire Pollution Kills More Than Other Air Pollution
Researchers at the Instituto de Salud Global de Barcelona used a large dataset of daily mortality data from 32 European countries collected through the EARLY-ADAPT project. They utilized the SILAM model to derive daily average concentrations of wildfire-related PM2.5, non-fire PM2.5, and total PM2.5 levels. They also employed GEOSTAT population grids at a 1-km resolution to calculate the attributable number of deaths across different regions, specifically focusing on data from 2006, 2011, and 2018.
The data analysis indicated that the relative risk per unit of PM2.5 is substantially larger for wildfire-related PM2.5, compared with non-fire PM2.5. “We essentially assume that wildfire smoke PM2.5 has the same toxicity as total PM2.5, but it’s increasingly clear that’s likely not the case,” Dr. Tonne said, presenting the study.
When employing exposure-response functions (ERFs) specific to wildfire smoke, researchers found that the attributable deaths from all causes of wildfire PM2.5 were approximately 10 times larger than those calculated using total PM2.5 exposure estimates. Dr. Tonne explained that this stark difference highlights the critical need for tailored ERFs that accurately reflect the unique health risks posed by wildfire smoke.
“Respiratory mortality usually has the strongest relative risks, and we’re seeing that in this study as well,” Dr. Tonne said. “Wildfire smoke seems to operate through quite immediate mechanisms, likely through inflammation and oxidative stress.”
One significant challenge of the study was the lack of uniform spatial resolution across all countries involved in the analysis. This inconsistency may affect how accurately mortality estimates can be attributed to specific PM2.5 sources. Additionally, the study had limited statistical power for generating age- and sex-specific mortality estimates, which could obscure important demographic differences in vulnerability to wildfire smoke exposure. The analysis was also constrained to data available only up to 2020, thereby excluding critical wildfire events from subsequent years, such as those in 2022 and 2023, which may have further elucidated the health impacts of wildfire smoke in Europe.
Fires Prescription
Prescribed fires or controlled burns are intentional fires set by land managers under carefully managed conditions.
Historically, many forested areas have been subjected to fire suppression practices, which allow combustible materials like dry leaves, twigs, and shrubs to accumulate over time. This buildup leads to a higher likelihood of severe, uncontrollable wildfires. Prescribed fires can reduce these fuel loads and improve the health and resilience of ecosystems.
They release fewer pollutants and emissions than the large-scale, unmanageable wildfires they help prevent because they happen at lower temperatures. But they still introduce pollutants in the air that can negatively affect nearby communities’ health.
People with preexisting respiratory conditions, such as asthma or chronic obstructive pulmonary disease (COPD), are particularly vulnerable to smoke, which can trigger health issues like breathing difficulties, coughing, and eye irritation. The cumulative impact of increased burns raises concerns about long-term air quality, especially in densely populated areas. “We need to understand if we’re actually tipping the scale to having less wildfire smoke or just increasing the total amount of smoke.”
Mitigation strategies include accurately picking the right timing and weather conditions to determine when and where to conduct controlled burns and effective and timely communication to inform local communities about upcoming burns, the potential for smoke exposure, and how to protect themselves.
There is a growing need to improve public messaging around prescribed fires, Mr. Sacks said, because often the message communicated is oversimplified, such as “there will be smoke, but don’t worry. But that’s not the message we want to convey, especially for people with asthma or COPD.”
Instead, he said public health agencies should provide clearer, science-based guidance on the risks for smoke exposure and practical steps people can take to reduce their risk.
What Can Doctors Do?
Chris Carlsten, MD, director of the Centre for Lung Health and professor and head of the Respiratory Medicine Division at the University of British Columbia, Vancouver, Canada, told this news organization that determining whether an exacerbation of a respiratory condition is caused by fire exposure or other factors, such as viral infections, is complex because both can trigger similar responses and may complement each other. “It’s very difficult for any individual to know whether, when they’re having an exacerbation of asthma or COPD, that’s due to the fire,” he said. Fire smoke also increases infection risks, further complicating diagnosis.
Dr. Carlsten suggested that physicians could recommend preventative use of inhalers for at-risk patients when wildfires occur rather than waiting for symptoms to worsen. “That is a really interesting idea that could be practical.” Still, he advises caution, stressing that patients should consult their providers because not all may react well to increased inhaler use.
He also highlighted a significant shift in the healthcare landscape, noting that traditionally, the focus has been on the cardiovascular impacts of pollution, particularly traffic-related pollution. However, as wildfire smoke becomes a growing issue, the focus is shifting back to respiratory problems, with profound implications for healthcare resources, budgets, and drug approvals based on the burden of respiratory disease. “Fire smoke is becoming more of a problem. This swing back to respiratory has huge implications for healthcare systems and respiratory disease burden.”
Mr. Sacks and Dr. Carlsten reported no relevant financial relationships. The study presented by Dr. Tonne received funding from the European Union’s Horizon Europe research and innovation programme under Grant Agreement No. 101057131.
A version of this article first appeared on Medscape.com.
Wildfire-related air pollution in Europe kills more than non-wildfire air pollution. As climate change exacerbates the frequency and violence of wildfires, researchers are studying the health implications of mitigation methods such as prescribed fires.
Presenting at the annual congress of the European Respiratory Society (ERS), Cathryn Tonne, PhD, an environmental epidemiologist at the Instituto de Salud Global de Barcelona, Spain, said wildfire-related PM2.5 is more toxic than general PM2.5, leading to significantly higher mortality rates.
Prescribed, controlled fires have been employed worldwide to reduce the chance of uncontrolled, catastrophic fires. However, researchers wonder whether the techniques reduce the overall fire-related PM2.5 or add up to it. “Prescribed fire increases ecosystem resilience and can reduce the risk of catastrophic wildfire,” said Jason Sacks, MPH, an epidemiologist in the Center for Public Health and Environmental Assessment in the Office of Research and Development at the Environmental Protection Agency (EPA), at the congress. “But it also leads to poorer air quality and health impacts, and we still don’t know what this means at a regional scale.”
Wildfire Pollution Kills More Than Other Air Pollution
Researchers at the Instituto de Salud Global de Barcelona used a large dataset of daily mortality data from 32 European countries collected through the EARLY-ADAPT project. They utilized the SILAM model to derive daily average concentrations of wildfire-related PM2.5, non-fire PM2.5, and total PM2.5 levels. They also employed GEOSTAT population grids at a 1-km resolution to calculate the attributable number of deaths across different regions, specifically focusing on data from 2006, 2011, and 2018.
The data analysis indicated that the relative risk per unit of PM2.5 is substantially larger for wildfire-related PM2.5, compared with non-fire PM2.5. “We essentially assume that wildfire smoke PM2.5 has the same toxicity as total PM2.5, but it’s increasingly clear that’s likely not the case,” Dr. Tonne said, presenting the study.
When employing exposure-response functions (ERFs) specific to wildfire smoke, researchers found that the attributable deaths from all causes of wildfire PM2.5 were approximately 10 times larger than those calculated using total PM2.5 exposure estimates. Dr. Tonne explained that this stark difference highlights the critical need for tailored ERFs that accurately reflect the unique health risks posed by wildfire smoke.
“Respiratory mortality usually has the strongest relative risks, and we’re seeing that in this study as well,” Dr. Tonne said. “Wildfire smoke seems to operate through quite immediate mechanisms, likely through inflammation and oxidative stress.”
One significant challenge of the study was the lack of uniform spatial resolution across all countries involved in the analysis. This inconsistency may affect how accurately mortality estimates can be attributed to specific PM2.5 sources. Additionally, the study had limited statistical power for generating age- and sex-specific mortality estimates, which could obscure important demographic differences in vulnerability to wildfire smoke exposure. The analysis was also constrained to data available only up to 2020, thereby excluding critical wildfire events from subsequent years, such as those in 2022 and 2023, which may have further elucidated the health impacts of wildfire smoke in Europe.
Fires Prescription
Prescribed fires or controlled burns are intentional fires set by land managers under carefully managed conditions.
Historically, many forested areas have been subjected to fire suppression practices, which allow combustible materials like dry leaves, twigs, and shrubs to accumulate over time. This buildup leads to a higher likelihood of severe, uncontrollable wildfires. Prescribed fires can reduce these fuel loads and improve the health and resilience of ecosystems.
They release fewer pollutants and emissions than the large-scale, unmanageable wildfires they help prevent because they happen at lower temperatures. But they still introduce pollutants in the air that can negatively affect nearby communities’ health.
People with preexisting respiratory conditions, such as asthma or chronic obstructive pulmonary disease (COPD), are particularly vulnerable to smoke, which can trigger health issues like breathing difficulties, coughing, and eye irritation. The cumulative impact of increased burns raises concerns about long-term air quality, especially in densely populated areas. “We need to understand if we’re actually tipping the scale to having less wildfire smoke or just increasing the total amount of smoke.”
Mitigation strategies include accurately picking the right timing and weather conditions to determine when and where to conduct controlled burns and effective and timely communication to inform local communities about upcoming burns, the potential for smoke exposure, and how to protect themselves.
There is a growing need to improve public messaging around prescribed fires, Mr. Sacks said, because often the message communicated is oversimplified, such as “there will be smoke, but don’t worry. But that’s not the message we want to convey, especially for people with asthma or COPD.”
Instead, he said public health agencies should provide clearer, science-based guidance on the risks for smoke exposure and practical steps people can take to reduce their risk.
What Can Doctors Do?
Chris Carlsten, MD, director of the Centre for Lung Health and professor and head of the Respiratory Medicine Division at the University of British Columbia, Vancouver, Canada, told this news organization that determining whether an exacerbation of a respiratory condition is caused by fire exposure or other factors, such as viral infections, is complex because both can trigger similar responses and may complement each other. “It’s very difficult for any individual to know whether, when they’re having an exacerbation of asthma or COPD, that’s due to the fire,” he said. Fire smoke also increases infection risks, further complicating diagnosis.
Dr. Carlsten suggested that physicians could recommend preventative use of inhalers for at-risk patients when wildfires occur rather than waiting for symptoms to worsen. “That is a really interesting idea that could be practical.” Still, he advises caution, stressing that patients should consult their providers because not all may react well to increased inhaler use.
He also highlighted a significant shift in the healthcare landscape, noting that traditionally, the focus has been on the cardiovascular impacts of pollution, particularly traffic-related pollution. However, as wildfire smoke becomes a growing issue, the focus is shifting back to respiratory problems, with profound implications for healthcare resources, budgets, and drug approvals based on the burden of respiratory disease. “Fire smoke is becoming more of a problem. This swing back to respiratory has huge implications for healthcare systems and respiratory disease burden.”
Mr. Sacks and Dr. Carlsten reported no relevant financial relationships. The study presented by Dr. Tonne received funding from the European Union’s Horizon Europe research and innovation programme under Grant Agreement No. 101057131.
A version of this article first appeared on Medscape.com.
FROM ERS 2024
How Doctors Can Overcome Vaccine Hesitancy Through Empathy, Storytelling, and Patient-Centered Communication
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
