User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Testosterone Increases Metabolic Syndrome Risk in Trans Men
TOPLINE:
Long-term gender-affirming hormone treatment with testosterone increases the risk for metabolic syndromes in transmasculine individuals, whereas transfeminine individuals receiving estradiol have a lower risk.
METHODOLOGY:
- Many transgender individuals receive exogenous sex hormone therapy to reduce gender dysphoria and improve quality of life. These treatments, however, may influence the development of metabolic syndrome.
- This retrospective, longitudinal cohort study investigated the association between gender-affirming hormone treatment and metabolic syndrome scores in transfeminine and transmasculine individuals compared with cisgender men and women not receiving the treatment.
- Overall, 645 transgender participants (mean age at index date, 41.3 years; 494 transfeminine and 151 transmasculine) were matched with 645 cisgender participants (280 women and 365 men) from the Veterans Health Administration.
- Metabolic syndrome scores were calculated based on blood pressure; body mass index (BMI); and levels of high-density lipoprotein (HDL) cholesterol, triglycerides, and blood glucose.
- Changes in metabolic syndrome scores before and after hormonal transition were compared among transgender and cisgender individuals for the corresponding dates.
TAKEAWAY:
- After hormonal transition, all measured metabolic syndrome components significantly worsened in the transmasculine group (P < .05 for all).
- In contrast, the systolic blood pressure and triglyceride levels decreased, HDL cholesterol levels increased, and BMI showed no significant change in the transfeminine group after hormonal transition.
- The increase in metabolic syndrome scores after vs before the date of hormonal transition was the highest for transmasculine individuals (298.0%; P < .001), followed by cisgender women (108.3%; P < .001), cisgender men (49.3%; P = .02), and transfeminine individuals (3.0%; P = .77).
IN PRACTICE:
“This is relevant for the management of metabolic syndrome risk factors in cisgender and transgender individuals and to potentially predict the risk of atherosclerotic cardiovascular disease, type 2 diabetes, systolic hypertension, insulin resistance, and nonalcoholic fatty liver disease,” the authors wrote.
SOURCE:
Leila Hashemi, MD, MS, of the Department of General Internal Medicine, David Geffen School of Medicine, University of California, Los Angeles, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
Causal inferences could not be drawn because of the study’s observational nature. The transmasculine and cisgender female groups were limited in size, and military veterans have special circumstances not representative of the general population. Minority stress among the transgender veterans was also not considered, which may have affected the health and well-being outcomes.
DISCLOSURES:
This study was supported by the National Institutes of Health and Office of Research on Women’s Health grants. One author received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term gender-affirming hormone treatment with testosterone increases the risk for metabolic syndromes in transmasculine individuals, whereas transfeminine individuals receiving estradiol have a lower risk.
METHODOLOGY:
- Many transgender individuals receive exogenous sex hormone therapy to reduce gender dysphoria and improve quality of life. These treatments, however, may influence the development of metabolic syndrome.
- This retrospective, longitudinal cohort study investigated the association between gender-affirming hormone treatment and metabolic syndrome scores in transfeminine and transmasculine individuals compared with cisgender men and women not receiving the treatment.
- Overall, 645 transgender participants (mean age at index date, 41.3 years; 494 transfeminine and 151 transmasculine) were matched with 645 cisgender participants (280 women and 365 men) from the Veterans Health Administration.
- Metabolic syndrome scores were calculated based on blood pressure; body mass index (BMI); and levels of high-density lipoprotein (HDL) cholesterol, triglycerides, and blood glucose.
- Changes in metabolic syndrome scores before and after hormonal transition were compared among transgender and cisgender individuals for the corresponding dates.
TAKEAWAY:
- After hormonal transition, all measured metabolic syndrome components significantly worsened in the transmasculine group (P < .05 for all).
- In contrast, the systolic blood pressure and triglyceride levels decreased, HDL cholesterol levels increased, and BMI showed no significant change in the transfeminine group after hormonal transition.
- The increase in metabolic syndrome scores after vs before the date of hormonal transition was the highest for transmasculine individuals (298.0%; P < .001), followed by cisgender women (108.3%; P < .001), cisgender men (49.3%; P = .02), and transfeminine individuals (3.0%; P = .77).
IN PRACTICE:
“This is relevant for the management of metabolic syndrome risk factors in cisgender and transgender individuals and to potentially predict the risk of atherosclerotic cardiovascular disease, type 2 diabetes, systolic hypertension, insulin resistance, and nonalcoholic fatty liver disease,” the authors wrote.
SOURCE:
Leila Hashemi, MD, MS, of the Department of General Internal Medicine, David Geffen School of Medicine, University of California, Los Angeles, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
Causal inferences could not be drawn because of the study’s observational nature. The transmasculine and cisgender female groups were limited in size, and military veterans have special circumstances not representative of the general population. Minority stress among the transgender veterans was also not considered, which may have affected the health and well-being outcomes.
DISCLOSURES:
This study was supported by the National Institutes of Health and Office of Research on Women’s Health grants. One author received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
Long-term gender-affirming hormone treatment with testosterone increases the risk for metabolic syndromes in transmasculine individuals, whereas transfeminine individuals receiving estradiol have a lower risk.
METHODOLOGY:
- Many transgender individuals receive exogenous sex hormone therapy to reduce gender dysphoria and improve quality of life. These treatments, however, may influence the development of metabolic syndrome.
- This retrospective, longitudinal cohort study investigated the association between gender-affirming hormone treatment and metabolic syndrome scores in transfeminine and transmasculine individuals compared with cisgender men and women not receiving the treatment.
- Overall, 645 transgender participants (mean age at index date, 41.3 years; 494 transfeminine and 151 transmasculine) were matched with 645 cisgender participants (280 women and 365 men) from the Veterans Health Administration.
- Metabolic syndrome scores were calculated based on blood pressure; body mass index (BMI); and levels of high-density lipoprotein (HDL) cholesterol, triglycerides, and blood glucose.
- Changes in metabolic syndrome scores before and after hormonal transition were compared among transgender and cisgender individuals for the corresponding dates.
TAKEAWAY:
- After hormonal transition, all measured metabolic syndrome components significantly worsened in the transmasculine group (P < .05 for all).
- In contrast, the systolic blood pressure and triglyceride levels decreased, HDL cholesterol levels increased, and BMI showed no significant change in the transfeminine group after hormonal transition.
- The increase in metabolic syndrome scores after vs before the date of hormonal transition was the highest for transmasculine individuals (298.0%; P < .001), followed by cisgender women (108.3%; P < .001), cisgender men (49.3%; P = .02), and transfeminine individuals (3.0%; P = .77).
IN PRACTICE:
“This is relevant for the management of metabolic syndrome risk factors in cisgender and transgender individuals and to potentially predict the risk of atherosclerotic cardiovascular disease, type 2 diabetes, systolic hypertension, insulin resistance, and nonalcoholic fatty liver disease,” the authors wrote.
SOURCE:
Leila Hashemi, MD, MS, of the Department of General Internal Medicine, David Geffen School of Medicine, University of California, Los Angeles, led this study, which was published online in JAMA Network Open.
LIMITATIONS:
Causal inferences could not be drawn because of the study’s observational nature. The transmasculine and cisgender female groups were limited in size, and military veterans have special circumstances not representative of the general population. Minority stress among the transgender veterans was also not considered, which may have affected the health and well-being outcomes.
DISCLOSURES:
This study was supported by the National Institutes of Health and Office of Research on Women’s Health grants. One author received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Revamping Resident Schedules to Reduce Burnout
It’s the difference between running a marathon and taking a leisurely stroll. That’s how recent pediatrics resident Joey Whelihan, MD, compared an 11-hour inpatient hospital day with an 8-hour outpatient shift where residents see patients in a clinic.
With inpatient training, “you are lucky if you have time to cook dinner, go to bed, and get ready for the next day,” said Dr. Whelihan, who recently started his adolescent medicine fellowship at Children’s Hospital of Philadelphia after 3 years of residency there. Some residents have call every fourth day during inpatient rotations, working 24-28 hours at a time. They come in one morning and go home the next, he told this news organization.
“Outpatient blocks give you more time to catch your breath and feel somewhat refreshed and ready to take care of patients.”
Longer stretches of inpatient rotations are not sustainable, Dr. Whelihan added, and residents are likely to become exhausted. Fatigue is a leading cause of burnout, a mental, physical, and emotional challenge that residency programs and national medical organizations have been struggling to address.
In recent years, there has been a movement to reduce the maximum consecutive duration of resident duty hours in residency programs across the country. Fueled by resident health and patient safety concerns, the movement is a shift from the previous 24- to 36-hour call duty schedules.
Improved Call Systems = Better Residents
The connection between burnout, well-being, and work schedules appears regularly in national program standards. “Residents and faculty members are at risk for burnout and depression,” according to the current Accreditation Council for Graduate Medical Education’s standard residency program requirements.
“Programs, in partnership with their sponsoring institutions, have the same responsibility to address well-being as other aspects of resident competence,” the guidelines state. That charge includes “attention to scheduling, work intensity, and work compression that impacts resident well-being.”
In Medscape’s Residents Lifestyle & Happiness Report 2023, a third of residents surveyed rarely or never paid attention to their well-being, which closely mirrors the 31% who rarely or never had time for a social life. Slightly more residents (37%) said their work-life balance was “somewhat worse” or “much worse” than they expected.
“I think everyone has burnout as a resident, regardless of the type of program they are in,” Dr. Whelihan said. He described the experience as when you lack fulfillment and empathy and feel exhausted, callous, and removed from interactions with colleagues and patients.
The American Medical Association’s recently released report on the state of residency well-being in 2023 also found that about 43% of residents and fellows had at least one symptom of burnout, about a 2% increase from 2022.
Efforts to Combat Burnout
One residency program found a way to reduce burnout by changing its block scheduling from 4 inpatient weeks followed by 1 outpatient week (4 + 1) to 4 inpatient call-based weeks and 4 outpatient ambulatory, non-call weeks (4 + 4), according to a survey study published recently in JAMA Network Open. The initiative drew praise from some residents and a med school professor who studies wellness issues.
In the survey of postgraduate year (PGY) 1 and PGY-2 hospitalist and primary care residents from the University of Colorado’s Internal Medicine Residency Program, Aurora, between June 2019 and June 2021, the schedule change resulted in improved burnout scores and self-reported professional, educational, and health benefits.
As part of the survey, residents rated symptoms on a 7-point scale on the basis of how frequently they experienced emotional exhaustion, depersonalization, and personal accomplishment.
Investigators also used a questionnaire to evaluate how participants perceived the rotation structure with various outcomes, including the ability to acquire clinical skills, access educational and scholarly opportunities, job satisfaction, and health.
The study concluded that the schedule change improved burnout, health, wellness, and professional development without weakening residents perceived clinical skills or standardized exam scores.
Still, the study authors acknowledged that several factors, including the pandemic, may have limited the findings. During that time, the study transitioned from in-person to electronic submissions, resulting in reduced response rates because of changes in staffing needs and fewer research and scholarly activities.
“One of the things we worried about was that the pandemic would make [burnout findings] look worse,” said lead author Dan Heppe, MD, a hospitalist and associate director of the CU Internal Medicine Residency Program. “Anecdotally, residents may have had more support in our program than perhaps some other programs. Though they had long hours with very sick patients, we tried to keep going in a positive direction.”
Dr. Heppe said in an interview that the purpose of the schedule change was to space out more intense rotations and build in more time for research, leadership, teaching, and professional development. He suggested the new schedule could help with other aspects of residents’ careers, exposing them to alternate avenues earlier in their training and in a more structured way.
Like most of the study authors, Dr. Heppe is a graduate of the residency program. He recalled how the program changed from multiple inpatient months in a row with clinic half days during those rotations to a 4 + 1 schedule. But the 1 week between inpatient rotations wasn’t enough time to recover or catch up on clinical work, said Dr. Heppe, who is also an associate professor of medicine at CU.
“It was too erratic,” he said of his former residency schedule. “There was a month of research here or there and clinic and then right back to the ICU for a couple of months without a break, and it was less predictable.”
Dr. Heppe said other residency programs have expressed interest in duplicating CU’s schedule change. He admits it may be difficult because of intensive schedule coordination, and some hospitals may not want to reduce clinical services.
The Yale Internal Medicine Traditional Residency Program also recently ended its 28-hour call, during which residents worked 24 hours with an additional 4 hours to transfer the patient to the incoming team. The move was made in response to residents’ requests, saying that the grueling call rotation’s time had come. The reaction has been overwhelmingly positive.
Proponents of alternate scheduling blocks [4 + 4 or 6 + 2] say that they improve residents’ educational experience, patient care, and continuity of care, reduce burnout, and guarantee residents time off.
Advancing Resident Well-Being
“The premise of looking at scheduling in a more intentional way is a sound one in the process of trying to support and advance resident well-being,” said Mark Greenawald, MD, vice chair of academic affairs, well-being, and professional development for the Virginia Tech Carilion School of Medicine’s Department of Family and Community Medicine in Roanoke.
He said it’s up to residency program directors or graduate medical education departments within a specialty to determine whether such scheduling changes fit their requirements for inpatient and outpatient care and training electives. Requirements may limit some scheduling changes, but within the specialty, there’s some flexibility to be creative with rotations. The CU study considered how to create a residency rhythm without stacking inpatient rotations so there’s recovery time.
“Human beings need a break. If residents work 80 hours continually, they will start to experience greater distress, which for many leads to burnout,” he said
Still, the study includes design flaws because it doesn’t explain how call times and hours differ between inpatient and outpatient rotations. “My own [family medicine] program also does outpatient clinics when we have inpatient service. We have half days in the clinic, which ensures better continuity care with the patient.”
Dr. Greenawald has yet to see much research published about the impact of resident schedule changes. By taking an experimental approach, the CU study showed that their particular change positively affected burnout. If the study leads to improvements in rotation schedules or encourages other programs to experiment with their schedules, it will be a step in the right direction.
How Residents Respond
Haidn Foster, MD, a third-year internal medicine resident at Penn State Health Milton S. Hershey Medical Center, Hershey, remembered experiencing burnout as an intern. At that time, he occasionally dealt with poor patient outcomes and sick patients while working long hours with only 1 day off each week. During a particularly challenging rotation, he felt overwhelmed and numb, which was exacerbated if a patient’s condition worsened or they passed away, he said.
His program follows a schedule of 6 weeks of inpatient training and 2 weeks of outpatient rotations (6 + 2). He said that restructuring residents’ schedules may be more effective than commonly used individual wellness modules, referring to the CU study. “The authors tried out a novel systematic way to tackle the epidemic of physician burnout overwhelming people in the medical community.”
Although the study found that schedule changes don’t affect standardized exam scores, Dr. Foster wondered about preceptor ratings, another marker for clinical competency.
He said future studies should attempt to change the structure of medical training delivery by evaluating models that best reduce burnout, are consistent with residents’ career goals, and produce competent physicians. “Burnout plagues our medical system and leads to too many physicians and physicians-in-training leaving the field or taking their lives. I’m not sure this particular mechanism gets us there, but it’s a step, and so that’s very important.”
Like Dr. Foster, Dr. Whelihan follows a 6 + 2 schedule. He said he would have welcomed a schedule that included more outpatient and less inpatient training and can see how changes in scheduling could reduce burnout. “More outpatient time gives you an opportunity to breathe. You get a little more time off working in clinic with less sick people at a slower pace.”
Ally Fuher, MD, said she chose CU’s Internal Medicine Residency Program 4 years ago largely because of its innovative schedule. Now the program’s chief medical resident, she knew the structure would give her more time to pursue other nonclinical interests including research and medical education, meet regularly with mentors, visit family in another state, and attend important life events.
She acknowledged that the alternative would have meant a more irregular schedule with the possibility of working as many as 80 hours a week on back-to-back inpatient rotations with only 1 day off a week, leaving minimal time to plan other activities, let alone rest and recover.
Dr. Fuher said a balanced schedule made her a more well-rounded person excited to engage in her profession. While she hasn’t personally experienced burnout, she realizes a schedule change may not completely solve the issue for others. However, it shows what progress programs can make when they create systemic structural change.
A version of this article first appeared on Medscape.com.
It’s the difference between running a marathon and taking a leisurely stroll. That’s how recent pediatrics resident Joey Whelihan, MD, compared an 11-hour inpatient hospital day with an 8-hour outpatient shift where residents see patients in a clinic.
With inpatient training, “you are lucky if you have time to cook dinner, go to bed, and get ready for the next day,” said Dr. Whelihan, who recently started his adolescent medicine fellowship at Children’s Hospital of Philadelphia after 3 years of residency there. Some residents have call every fourth day during inpatient rotations, working 24-28 hours at a time. They come in one morning and go home the next, he told this news organization.
“Outpatient blocks give you more time to catch your breath and feel somewhat refreshed and ready to take care of patients.”
Longer stretches of inpatient rotations are not sustainable, Dr. Whelihan added, and residents are likely to become exhausted. Fatigue is a leading cause of burnout, a mental, physical, and emotional challenge that residency programs and national medical organizations have been struggling to address.
In recent years, there has been a movement to reduce the maximum consecutive duration of resident duty hours in residency programs across the country. Fueled by resident health and patient safety concerns, the movement is a shift from the previous 24- to 36-hour call duty schedules.
Improved Call Systems = Better Residents
The connection between burnout, well-being, and work schedules appears regularly in national program standards. “Residents and faculty members are at risk for burnout and depression,” according to the current Accreditation Council for Graduate Medical Education’s standard residency program requirements.
“Programs, in partnership with their sponsoring institutions, have the same responsibility to address well-being as other aspects of resident competence,” the guidelines state. That charge includes “attention to scheduling, work intensity, and work compression that impacts resident well-being.”
In Medscape’s Residents Lifestyle & Happiness Report 2023, a third of residents surveyed rarely or never paid attention to their well-being, which closely mirrors the 31% who rarely or never had time for a social life. Slightly more residents (37%) said their work-life balance was “somewhat worse” or “much worse” than they expected.
“I think everyone has burnout as a resident, regardless of the type of program they are in,” Dr. Whelihan said. He described the experience as when you lack fulfillment and empathy and feel exhausted, callous, and removed from interactions with colleagues and patients.
The American Medical Association’s recently released report on the state of residency well-being in 2023 also found that about 43% of residents and fellows had at least one symptom of burnout, about a 2% increase from 2022.
Efforts to Combat Burnout
One residency program found a way to reduce burnout by changing its block scheduling from 4 inpatient weeks followed by 1 outpatient week (4 + 1) to 4 inpatient call-based weeks and 4 outpatient ambulatory, non-call weeks (4 + 4), according to a survey study published recently in JAMA Network Open. The initiative drew praise from some residents and a med school professor who studies wellness issues.
In the survey of postgraduate year (PGY) 1 and PGY-2 hospitalist and primary care residents from the University of Colorado’s Internal Medicine Residency Program, Aurora, between June 2019 and June 2021, the schedule change resulted in improved burnout scores and self-reported professional, educational, and health benefits.
As part of the survey, residents rated symptoms on a 7-point scale on the basis of how frequently they experienced emotional exhaustion, depersonalization, and personal accomplishment.
Investigators also used a questionnaire to evaluate how participants perceived the rotation structure with various outcomes, including the ability to acquire clinical skills, access educational and scholarly opportunities, job satisfaction, and health.
The study concluded that the schedule change improved burnout, health, wellness, and professional development without weakening residents perceived clinical skills or standardized exam scores.
Still, the study authors acknowledged that several factors, including the pandemic, may have limited the findings. During that time, the study transitioned from in-person to electronic submissions, resulting in reduced response rates because of changes in staffing needs and fewer research and scholarly activities.
“One of the things we worried about was that the pandemic would make [burnout findings] look worse,” said lead author Dan Heppe, MD, a hospitalist and associate director of the CU Internal Medicine Residency Program. “Anecdotally, residents may have had more support in our program than perhaps some other programs. Though they had long hours with very sick patients, we tried to keep going in a positive direction.”
Dr. Heppe said in an interview that the purpose of the schedule change was to space out more intense rotations and build in more time for research, leadership, teaching, and professional development. He suggested the new schedule could help with other aspects of residents’ careers, exposing them to alternate avenues earlier in their training and in a more structured way.
Like most of the study authors, Dr. Heppe is a graduate of the residency program. He recalled how the program changed from multiple inpatient months in a row with clinic half days during those rotations to a 4 + 1 schedule. But the 1 week between inpatient rotations wasn’t enough time to recover or catch up on clinical work, said Dr. Heppe, who is also an associate professor of medicine at CU.
“It was too erratic,” he said of his former residency schedule. “There was a month of research here or there and clinic and then right back to the ICU for a couple of months without a break, and it was less predictable.”
Dr. Heppe said other residency programs have expressed interest in duplicating CU’s schedule change. He admits it may be difficult because of intensive schedule coordination, and some hospitals may not want to reduce clinical services.
The Yale Internal Medicine Traditional Residency Program also recently ended its 28-hour call, during which residents worked 24 hours with an additional 4 hours to transfer the patient to the incoming team. The move was made in response to residents’ requests, saying that the grueling call rotation’s time had come. The reaction has been overwhelmingly positive.
Proponents of alternate scheduling blocks [4 + 4 or 6 + 2] say that they improve residents’ educational experience, patient care, and continuity of care, reduce burnout, and guarantee residents time off.
Advancing Resident Well-Being
“The premise of looking at scheduling in a more intentional way is a sound one in the process of trying to support and advance resident well-being,” said Mark Greenawald, MD, vice chair of academic affairs, well-being, and professional development for the Virginia Tech Carilion School of Medicine’s Department of Family and Community Medicine in Roanoke.
He said it’s up to residency program directors or graduate medical education departments within a specialty to determine whether such scheduling changes fit their requirements for inpatient and outpatient care and training electives. Requirements may limit some scheduling changes, but within the specialty, there’s some flexibility to be creative with rotations. The CU study considered how to create a residency rhythm without stacking inpatient rotations so there’s recovery time.
“Human beings need a break. If residents work 80 hours continually, they will start to experience greater distress, which for many leads to burnout,” he said
Still, the study includes design flaws because it doesn’t explain how call times and hours differ between inpatient and outpatient rotations. “My own [family medicine] program also does outpatient clinics when we have inpatient service. We have half days in the clinic, which ensures better continuity care with the patient.”
Dr. Greenawald has yet to see much research published about the impact of resident schedule changes. By taking an experimental approach, the CU study showed that their particular change positively affected burnout. If the study leads to improvements in rotation schedules or encourages other programs to experiment with their schedules, it will be a step in the right direction.
How Residents Respond
Haidn Foster, MD, a third-year internal medicine resident at Penn State Health Milton S. Hershey Medical Center, Hershey, remembered experiencing burnout as an intern. At that time, he occasionally dealt with poor patient outcomes and sick patients while working long hours with only 1 day off each week. During a particularly challenging rotation, he felt overwhelmed and numb, which was exacerbated if a patient’s condition worsened or they passed away, he said.
His program follows a schedule of 6 weeks of inpatient training and 2 weeks of outpatient rotations (6 + 2). He said that restructuring residents’ schedules may be more effective than commonly used individual wellness modules, referring to the CU study. “The authors tried out a novel systematic way to tackle the epidemic of physician burnout overwhelming people in the medical community.”
Although the study found that schedule changes don’t affect standardized exam scores, Dr. Foster wondered about preceptor ratings, another marker for clinical competency.
He said future studies should attempt to change the structure of medical training delivery by evaluating models that best reduce burnout, are consistent with residents’ career goals, and produce competent physicians. “Burnout plagues our medical system and leads to too many physicians and physicians-in-training leaving the field or taking their lives. I’m not sure this particular mechanism gets us there, but it’s a step, and so that’s very important.”
Like Dr. Foster, Dr. Whelihan follows a 6 + 2 schedule. He said he would have welcomed a schedule that included more outpatient and less inpatient training and can see how changes in scheduling could reduce burnout. “More outpatient time gives you an opportunity to breathe. You get a little more time off working in clinic with less sick people at a slower pace.”
Ally Fuher, MD, said she chose CU’s Internal Medicine Residency Program 4 years ago largely because of its innovative schedule. Now the program’s chief medical resident, she knew the structure would give her more time to pursue other nonclinical interests including research and medical education, meet regularly with mentors, visit family in another state, and attend important life events.
She acknowledged that the alternative would have meant a more irregular schedule with the possibility of working as many as 80 hours a week on back-to-back inpatient rotations with only 1 day off a week, leaving minimal time to plan other activities, let alone rest and recover.
Dr. Fuher said a balanced schedule made her a more well-rounded person excited to engage in her profession. While she hasn’t personally experienced burnout, she realizes a schedule change may not completely solve the issue for others. However, it shows what progress programs can make when they create systemic structural change.
A version of this article first appeared on Medscape.com.
It’s the difference between running a marathon and taking a leisurely stroll. That’s how recent pediatrics resident Joey Whelihan, MD, compared an 11-hour inpatient hospital day with an 8-hour outpatient shift where residents see patients in a clinic.
With inpatient training, “you are lucky if you have time to cook dinner, go to bed, and get ready for the next day,” said Dr. Whelihan, who recently started his adolescent medicine fellowship at Children’s Hospital of Philadelphia after 3 years of residency there. Some residents have call every fourth day during inpatient rotations, working 24-28 hours at a time. They come in one morning and go home the next, he told this news organization.
“Outpatient blocks give you more time to catch your breath and feel somewhat refreshed and ready to take care of patients.”
Longer stretches of inpatient rotations are not sustainable, Dr. Whelihan added, and residents are likely to become exhausted. Fatigue is a leading cause of burnout, a mental, physical, and emotional challenge that residency programs and national medical organizations have been struggling to address.
In recent years, there has been a movement to reduce the maximum consecutive duration of resident duty hours in residency programs across the country. Fueled by resident health and patient safety concerns, the movement is a shift from the previous 24- to 36-hour call duty schedules.
Improved Call Systems = Better Residents
The connection between burnout, well-being, and work schedules appears regularly in national program standards. “Residents and faculty members are at risk for burnout and depression,” according to the current Accreditation Council for Graduate Medical Education’s standard residency program requirements.
“Programs, in partnership with their sponsoring institutions, have the same responsibility to address well-being as other aspects of resident competence,” the guidelines state. That charge includes “attention to scheduling, work intensity, and work compression that impacts resident well-being.”
In Medscape’s Residents Lifestyle & Happiness Report 2023, a third of residents surveyed rarely or never paid attention to their well-being, which closely mirrors the 31% who rarely or never had time for a social life. Slightly more residents (37%) said their work-life balance was “somewhat worse” or “much worse” than they expected.
“I think everyone has burnout as a resident, regardless of the type of program they are in,” Dr. Whelihan said. He described the experience as when you lack fulfillment and empathy and feel exhausted, callous, and removed from interactions with colleagues and patients.
The American Medical Association’s recently released report on the state of residency well-being in 2023 also found that about 43% of residents and fellows had at least one symptom of burnout, about a 2% increase from 2022.
Efforts to Combat Burnout
One residency program found a way to reduce burnout by changing its block scheduling from 4 inpatient weeks followed by 1 outpatient week (4 + 1) to 4 inpatient call-based weeks and 4 outpatient ambulatory, non-call weeks (4 + 4), according to a survey study published recently in JAMA Network Open. The initiative drew praise from some residents and a med school professor who studies wellness issues.
In the survey of postgraduate year (PGY) 1 and PGY-2 hospitalist and primary care residents from the University of Colorado’s Internal Medicine Residency Program, Aurora, between June 2019 and June 2021, the schedule change resulted in improved burnout scores and self-reported professional, educational, and health benefits.
As part of the survey, residents rated symptoms on a 7-point scale on the basis of how frequently they experienced emotional exhaustion, depersonalization, and personal accomplishment.
Investigators also used a questionnaire to evaluate how participants perceived the rotation structure with various outcomes, including the ability to acquire clinical skills, access educational and scholarly opportunities, job satisfaction, and health.
The study concluded that the schedule change improved burnout, health, wellness, and professional development without weakening residents perceived clinical skills or standardized exam scores.
Still, the study authors acknowledged that several factors, including the pandemic, may have limited the findings. During that time, the study transitioned from in-person to electronic submissions, resulting in reduced response rates because of changes in staffing needs and fewer research and scholarly activities.
“One of the things we worried about was that the pandemic would make [burnout findings] look worse,” said lead author Dan Heppe, MD, a hospitalist and associate director of the CU Internal Medicine Residency Program. “Anecdotally, residents may have had more support in our program than perhaps some other programs. Though they had long hours with very sick patients, we tried to keep going in a positive direction.”
Dr. Heppe said in an interview that the purpose of the schedule change was to space out more intense rotations and build in more time for research, leadership, teaching, and professional development. He suggested the new schedule could help with other aspects of residents’ careers, exposing them to alternate avenues earlier in their training and in a more structured way.
Like most of the study authors, Dr. Heppe is a graduate of the residency program. He recalled how the program changed from multiple inpatient months in a row with clinic half days during those rotations to a 4 + 1 schedule. But the 1 week between inpatient rotations wasn’t enough time to recover or catch up on clinical work, said Dr. Heppe, who is also an associate professor of medicine at CU.
“It was too erratic,” he said of his former residency schedule. “There was a month of research here or there and clinic and then right back to the ICU for a couple of months without a break, and it was less predictable.”
Dr. Heppe said other residency programs have expressed interest in duplicating CU’s schedule change. He admits it may be difficult because of intensive schedule coordination, and some hospitals may not want to reduce clinical services.
The Yale Internal Medicine Traditional Residency Program also recently ended its 28-hour call, during which residents worked 24 hours with an additional 4 hours to transfer the patient to the incoming team. The move was made in response to residents’ requests, saying that the grueling call rotation’s time had come. The reaction has been overwhelmingly positive.
Proponents of alternate scheduling blocks [4 + 4 or 6 + 2] say that they improve residents’ educational experience, patient care, and continuity of care, reduce burnout, and guarantee residents time off.
Advancing Resident Well-Being
“The premise of looking at scheduling in a more intentional way is a sound one in the process of trying to support and advance resident well-being,” said Mark Greenawald, MD, vice chair of academic affairs, well-being, and professional development for the Virginia Tech Carilion School of Medicine’s Department of Family and Community Medicine in Roanoke.
He said it’s up to residency program directors or graduate medical education departments within a specialty to determine whether such scheduling changes fit their requirements for inpatient and outpatient care and training electives. Requirements may limit some scheduling changes, but within the specialty, there’s some flexibility to be creative with rotations. The CU study considered how to create a residency rhythm without stacking inpatient rotations so there’s recovery time.
“Human beings need a break. If residents work 80 hours continually, they will start to experience greater distress, which for many leads to burnout,” he said
Still, the study includes design flaws because it doesn’t explain how call times and hours differ between inpatient and outpatient rotations. “My own [family medicine] program also does outpatient clinics when we have inpatient service. We have half days in the clinic, which ensures better continuity care with the patient.”
Dr. Greenawald has yet to see much research published about the impact of resident schedule changes. By taking an experimental approach, the CU study showed that their particular change positively affected burnout. If the study leads to improvements in rotation schedules or encourages other programs to experiment with their schedules, it will be a step in the right direction.
How Residents Respond
Haidn Foster, MD, a third-year internal medicine resident at Penn State Health Milton S. Hershey Medical Center, Hershey, remembered experiencing burnout as an intern. At that time, he occasionally dealt with poor patient outcomes and sick patients while working long hours with only 1 day off each week. During a particularly challenging rotation, he felt overwhelmed and numb, which was exacerbated if a patient’s condition worsened or they passed away, he said.
His program follows a schedule of 6 weeks of inpatient training and 2 weeks of outpatient rotations (6 + 2). He said that restructuring residents’ schedules may be more effective than commonly used individual wellness modules, referring to the CU study. “The authors tried out a novel systematic way to tackle the epidemic of physician burnout overwhelming people in the medical community.”
Although the study found that schedule changes don’t affect standardized exam scores, Dr. Foster wondered about preceptor ratings, another marker for clinical competency.
He said future studies should attempt to change the structure of medical training delivery by evaluating models that best reduce burnout, are consistent with residents’ career goals, and produce competent physicians. “Burnout plagues our medical system and leads to too many physicians and physicians-in-training leaving the field or taking their lives. I’m not sure this particular mechanism gets us there, but it’s a step, and so that’s very important.”
Like Dr. Foster, Dr. Whelihan follows a 6 + 2 schedule. He said he would have welcomed a schedule that included more outpatient and less inpatient training and can see how changes in scheduling could reduce burnout. “More outpatient time gives you an opportunity to breathe. You get a little more time off working in clinic with less sick people at a slower pace.”
Ally Fuher, MD, said she chose CU’s Internal Medicine Residency Program 4 years ago largely because of its innovative schedule. Now the program’s chief medical resident, she knew the structure would give her more time to pursue other nonclinical interests including research and medical education, meet regularly with mentors, visit family in another state, and attend important life events.
She acknowledged that the alternative would have meant a more irregular schedule with the possibility of working as many as 80 hours a week on back-to-back inpatient rotations with only 1 day off a week, leaving minimal time to plan other activities, let alone rest and recover.
Dr. Fuher said a balanced schedule made her a more well-rounded person excited to engage in her profession. While she hasn’t personally experienced burnout, she realizes a schedule change may not completely solve the issue for others. However, it shows what progress programs can make when they create systemic structural change.
A version of this article first appeared on Medscape.com.
The SOPHIA Project Conceives of Obesity Beyond BMI
During a lecture at the 2024 International Congress on Obesity in São Paulo, Brazil, Dr. Carel Le Roux, a South African researcher, reflected on the Stratification of Obesity Phenotypes to Optimize Future Therapy (SOPHIA) project. The effort, of which Dr. Le Roux is a leader, involves using federated data and reframing obesity as a set of diseases, each with its own peculiarities and treatment needs.
A collaborative research initiative led by the European Union, the SOPHIA project is a public-private partnership that brings together healthcare professionals, universities, industry leaders, and patient organizations to rethink how we understand and treat obesity, considering factors beyond body mass index (BMI).
“We need to ask ourselves, ‘Is obesity a disease? Or, in fact, does ‘obesity’ refer to multiple diseases that lead to excess adipose tissue?’ ” Dr. Le Roux asked at the beginning of his presentation.
The researcher, who is also the director of the Obesity and Metabolic Medicine Group, stated that obesity can no longer be seen as a single homogeneous pathology but rather should be viewed as clinical conditions affecting various subpopulations that respond differently to treatments.
Patients are currently diagnosed with obesity based on BMI value or waist measurement, as recommended by current clinical guidelines, but this method contributes to treating obesity subtypes as if they were identical.
“By taking into account the patient’s specificities, we can identify individuals who are likely to progress rapidly with the disease and those who will respond well to targeted interventions,” said Dr. Le Roux, emphasizing that this approach also contributes to reducing public health system costs.
Researchers proposed creating a map that allows the visualization of the distinct characteristics of patients with obesity, such as the presence of associated diseases like hypertension and diabetes. One of the main challenges of the project was finding a way to share sensitive data among SOPHIA partners without compromising individual privacy. The solution was the creation of a federated database.
In practice, this system allows academic and industry partners to send data to a central server, which keeps them protected. “We wanted to reach the optimal point, where we can have maximum utility and maximum privacy protection using technology. Researchers can then obtain statistics, enabling the analysis of large data sets without compromising security,” Dr. Le Roux explained.
Most patients analyzed in the project fall into the main group, where “the higher the weight, the greater the risk” for associated diseases, he added. However, the project allows for specifically visualizing patients with alterations related to high blood pressure, liver function, lipid profile, blood glucose, and inflammation.
“Subclassifying diseases helps us better understand the various mechanisms by which these pathologies arise and why some individuals exhibit unexpected phenotypic patterns of increased susceptibility or resilience. For example, patients with inflammation changes have a much higher risk for developing type 2 diabetes, rheumatoid arthritis, and liver failure,” said Dr. Le Roux.
In addition to visualizing the associated diseases of each participant, SOPHIA, in which 30 partners in Europe, the Middle East, and the United States participate, also features treatment overlap, which allows researchers to track individual responses to the treatment.
“With this overlap, we confirm something that many know: When treating people with type 2 diabetes, whether through lifestyle changes, medication, or surgery, weight loss is lower. But, to our surprise, we found that patients with inflammation-related changes had greater weight loss. This finding tells us that some groups benefit more, and others less,” said Dr. Le Roux.
This analysis is particularly interesting when it comes to bariatric surgery, he continued. “Often, the surgeon performs an incredibly well-done gastric bypass, and the response is not as expected. In this case, we can say that it is purely biology,” said Dr. Le Roux, who concluded the presentation by discussing the benefits of this approach for good patient counseling.
“When we talk about ‘obesities’ and not ‘obesity,’ we can also conduct our consultations more carefully by explaining to our patients that if they do not respond to treatment, it is not their fault, not because they did something wrong, but because of something that is not usually taken into account, such as the presence of comorbidities, or even personal characteristics and lifestyle, such as age and smoking,” said Dr. Le Roux.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
During a lecture at the 2024 International Congress on Obesity in São Paulo, Brazil, Dr. Carel Le Roux, a South African researcher, reflected on the Stratification of Obesity Phenotypes to Optimize Future Therapy (SOPHIA) project. The effort, of which Dr. Le Roux is a leader, involves using federated data and reframing obesity as a set of diseases, each with its own peculiarities and treatment needs.
A collaborative research initiative led by the European Union, the SOPHIA project is a public-private partnership that brings together healthcare professionals, universities, industry leaders, and patient organizations to rethink how we understand and treat obesity, considering factors beyond body mass index (BMI).
“We need to ask ourselves, ‘Is obesity a disease? Or, in fact, does ‘obesity’ refer to multiple diseases that lead to excess adipose tissue?’ ” Dr. Le Roux asked at the beginning of his presentation.
The researcher, who is also the director of the Obesity and Metabolic Medicine Group, stated that obesity can no longer be seen as a single homogeneous pathology but rather should be viewed as clinical conditions affecting various subpopulations that respond differently to treatments.
Patients are currently diagnosed with obesity based on BMI value or waist measurement, as recommended by current clinical guidelines, but this method contributes to treating obesity subtypes as if they were identical.
“By taking into account the patient’s specificities, we can identify individuals who are likely to progress rapidly with the disease and those who will respond well to targeted interventions,” said Dr. Le Roux, emphasizing that this approach also contributes to reducing public health system costs.
Researchers proposed creating a map that allows the visualization of the distinct characteristics of patients with obesity, such as the presence of associated diseases like hypertension and diabetes. One of the main challenges of the project was finding a way to share sensitive data among SOPHIA partners without compromising individual privacy. The solution was the creation of a federated database.
In practice, this system allows academic and industry partners to send data to a central server, which keeps them protected. “We wanted to reach the optimal point, where we can have maximum utility and maximum privacy protection using technology. Researchers can then obtain statistics, enabling the analysis of large data sets without compromising security,” Dr. Le Roux explained.
Most patients analyzed in the project fall into the main group, where “the higher the weight, the greater the risk” for associated diseases, he added. However, the project allows for specifically visualizing patients with alterations related to high blood pressure, liver function, lipid profile, blood glucose, and inflammation.
“Subclassifying diseases helps us better understand the various mechanisms by which these pathologies arise and why some individuals exhibit unexpected phenotypic patterns of increased susceptibility or resilience. For example, patients with inflammation changes have a much higher risk for developing type 2 diabetes, rheumatoid arthritis, and liver failure,” said Dr. Le Roux.
In addition to visualizing the associated diseases of each participant, SOPHIA, in which 30 partners in Europe, the Middle East, and the United States participate, also features treatment overlap, which allows researchers to track individual responses to the treatment.
“With this overlap, we confirm something that many know: When treating people with type 2 diabetes, whether through lifestyle changes, medication, or surgery, weight loss is lower. But, to our surprise, we found that patients with inflammation-related changes had greater weight loss. This finding tells us that some groups benefit more, and others less,” said Dr. Le Roux.
This analysis is particularly interesting when it comes to bariatric surgery, he continued. “Often, the surgeon performs an incredibly well-done gastric bypass, and the response is not as expected. In this case, we can say that it is purely biology,” said Dr. Le Roux, who concluded the presentation by discussing the benefits of this approach for good patient counseling.
“When we talk about ‘obesities’ and not ‘obesity,’ we can also conduct our consultations more carefully by explaining to our patients that if they do not respond to treatment, it is not their fault, not because they did something wrong, but because of something that is not usually taken into account, such as the presence of comorbidities, or even personal characteristics and lifestyle, such as age and smoking,” said Dr. Le Roux.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
During a lecture at the 2024 International Congress on Obesity in São Paulo, Brazil, Dr. Carel Le Roux, a South African researcher, reflected on the Stratification of Obesity Phenotypes to Optimize Future Therapy (SOPHIA) project. The effort, of which Dr. Le Roux is a leader, involves using federated data and reframing obesity as a set of diseases, each with its own peculiarities and treatment needs.
A collaborative research initiative led by the European Union, the SOPHIA project is a public-private partnership that brings together healthcare professionals, universities, industry leaders, and patient organizations to rethink how we understand and treat obesity, considering factors beyond body mass index (BMI).
“We need to ask ourselves, ‘Is obesity a disease? Or, in fact, does ‘obesity’ refer to multiple diseases that lead to excess adipose tissue?’ ” Dr. Le Roux asked at the beginning of his presentation.
The researcher, who is also the director of the Obesity and Metabolic Medicine Group, stated that obesity can no longer be seen as a single homogeneous pathology but rather should be viewed as clinical conditions affecting various subpopulations that respond differently to treatments.
Patients are currently diagnosed with obesity based on BMI value or waist measurement, as recommended by current clinical guidelines, but this method contributes to treating obesity subtypes as if they were identical.
“By taking into account the patient’s specificities, we can identify individuals who are likely to progress rapidly with the disease and those who will respond well to targeted interventions,” said Dr. Le Roux, emphasizing that this approach also contributes to reducing public health system costs.
Researchers proposed creating a map that allows the visualization of the distinct characteristics of patients with obesity, such as the presence of associated diseases like hypertension and diabetes. One of the main challenges of the project was finding a way to share sensitive data among SOPHIA partners without compromising individual privacy. The solution was the creation of a federated database.
In practice, this system allows academic and industry partners to send data to a central server, which keeps them protected. “We wanted to reach the optimal point, where we can have maximum utility and maximum privacy protection using technology. Researchers can then obtain statistics, enabling the analysis of large data sets without compromising security,” Dr. Le Roux explained.
Most patients analyzed in the project fall into the main group, where “the higher the weight, the greater the risk” for associated diseases, he added. However, the project allows for specifically visualizing patients with alterations related to high blood pressure, liver function, lipid profile, blood glucose, and inflammation.
“Subclassifying diseases helps us better understand the various mechanisms by which these pathologies arise and why some individuals exhibit unexpected phenotypic patterns of increased susceptibility or resilience. For example, patients with inflammation changes have a much higher risk for developing type 2 diabetes, rheumatoid arthritis, and liver failure,” said Dr. Le Roux.
In addition to visualizing the associated diseases of each participant, SOPHIA, in which 30 partners in Europe, the Middle East, and the United States participate, also features treatment overlap, which allows researchers to track individual responses to the treatment.
“With this overlap, we confirm something that many know: When treating people with type 2 diabetes, whether through lifestyle changes, medication, or surgery, weight loss is lower. But, to our surprise, we found that patients with inflammation-related changes had greater weight loss. This finding tells us that some groups benefit more, and others less,” said Dr. Le Roux.
This analysis is particularly interesting when it comes to bariatric surgery, he continued. “Often, the surgeon performs an incredibly well-done gastric bypass, and the response is not as expected. In this case, we can say that it is purely biology,” said Dr. Le Roux, who concluded the presentation by discussing the benefits of this approach for good patient counseling.
“When we talk about ‘obesities’ and not ‘obesity,’ we can also conduct our consultations more carefully by explaining to our patients that if they do not respond to treatment, it is not their fault, not because they did something wrong, but because of something that is not usually taken into account, such as the presence of comorbidities, or even personal characteristics and lifestyle, such as age and smoking,” said Dr. Le Roux.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Can Diabetes Lead to a False-Positive Alcohol Test?
This transcript has been edited for clarity.
I’m going to tell you the story of two patients with diabetes who had false-positive alcohol tests.
The first patient is a patient of mine with type 1 diabetes. He was in a car accident. He hit the car in front of him that hit the car in front of them. Because the cars were quite damaged, the police were summoned.
At the scene, he had a breathalyzer test. He flunked the breathalyzer test, and he was charged with a DUI. The woman in the middle car got out of her car and said her neck hurt. This then rose this to the level of a DUI with injury to one of the other people, and my patient was charged with a felony. He was taken to jail.
He told them at the scene and at the jail that he had type 1 diabetes. The reason this is so important is because type 1 diabetes can cause a false-positive breathalyzer test. In particular, this patient of mine had not been eating all day long. He’d been getting his basal insulin through the pump, but he had not given any bolus doses of insulin. He was actually quite ketotic.
When he was put in jail, they took away his cell phone so he could no longer see his glucose levels, and they took away the controller for his Omnipod system. He basically had no way to give bolus doses of insulin. Fortunately, the Omnipod system lasted for a day and a half just by giving him basal. The jail physicians did not give him insulin until he’d been in jail for 3 days.
This is someone with type 1 diabetes, and their protocol for insulin has something to do with high glucose levels and giving something like a sliding scale of insulin. They were not really prepared for managing somebody with type 1 diabetes who was on an automated insulin delivery system.
I, along with my patient’s parents, worked very hard to get the jail doctors to finally give him Lantus. Inherent in all of this, it made me aware of a number of different issues. The first is that breathalyzer tests can be falsely positive in people with type 1 diabetes if they are ketotic; therefore, people with type 1 diabetes should ask for a blood test to test their alcohol levels if they think it could be a false positive.
Second, we need to actually figure out a way to help people with type 1 diabetes who happen to be in jail or in prison because if they don’t have access to a smartphone, they’re not going to be able to run their devices. We need to make sure that devices have receivers that can be used, particularly continuous glucose monitors (CGMs), because CGM is the standard of care for patients and should be so for people who are incarcerated.
The second case is much shorter and isn’t mine, but it was a letter in The New England Journal of Medicine about a man who was on probation, who was having urine tests to show that he had not been consuming alcohol. He was started on empagliflozin, which, interestingly, made his urine test become falsely positive.
Why? Well, it’s thought it’s because it caused fermentation of the sugar with the bacteria that was in his urine because the people who were processing the sample hadn’t done it correctly, and they kept it out at room temperature for a prolonged period of time before testing it. The urine samples should be kept refrigerated to prevent this from happening.
These are two people who had false-positive tests because they had diabetes. I think it’s important that we realize that this can happen, and we need to help our patients deal with these situations.
Dr. Peters is professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles. She reported conflicts of interest with Abbott Diabetes Care, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, Lexicon Pharmaceuticals, Livongo, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to tell you the story of two patients with diabetes who had false-positive alcohol tests.
The first patient is a patient of mine with type 1 diabetes. He was in a car accident. He hit the car in front of him that hit the car in front of them. Because the cars were quite damaged, the police were summoned.
At the scene, he had a breathalyzer test. He flunked the breathalyzer test, and he was charged with a DUI. The woman in the middle car got out of her car and said her neck hurt. This then rose this to the level of a DUI with injury to one of the other people, and my patient was charged with a felony. He was taken to jail.
He told them at the scene and at the jail that he had type 1 diabetes. The reason this is so important is because type 1 diabetes can cause a false-positive breathalyzer test. In particular, this patient of mine had not been eating all day long. He’d been getting his basal insulin through the pump, but he had not given any bolus doses of insulin. He was actually quite ketotic.
When he was put in jail, they took away his cell phone so he could no longer see his glucose levels, and they took away the controller for his Omnipod system. He basically had no way to give bolus doses of insulin. Fortunately, the Omnipod system lasted for a day and a half just by giving him basal. The jail physicians did not give him insulin until he’d been in jail for 3 days.
This is someone with type 1 diabetes, and their protocol for insulin has something to do with high glucose levels and giving something like a sliding scale of insulin. They were not really prepared for managing somebody with type 1 diabetes who was on an automated insulin delivery system.
I, along with my patient’s parents, worked very hard to get the jail doctors to finally give him Lantus. Inherent in all of this, it made me aware of a number of different issues. The first is that breathalyzer tests can be falsely positive in people with type 1 diabetes if they are ketotic; therefore, people with type 1 diabetes should ask for a blood test to test their alcohol levels if they think it could be a false positive.
Second, we need to actually figure out a way to help people with type 1 diabetes who happen to be in jail or in prison because if they don’t have access to a smartphone, they’re not going to be able to run their devices. We need to make sure that devices have receivers that can be used, particularly continuous glucose monitors (CGMs), because CGM is the standard of care for patients and should be so for people who are incarcerated.
The second case is much shorter and isn’t mine, but it was a letter in The New England Journal of Medicine about a man who was on probation, who was having urine tests to show that he had not been consuming alcohol. He was started on empagliflozin, which, interestingly, made his urine test become falsely positive.
Why? Well, it’s thought it’s because it caused fermentation of the sugar with the bacteria that was in his urine because the people who were processing the sample hadn’t done it correctly, and they kept it out at room temperature for a prolonged period of time before testing it. The urine samples should be kept refrigerated to prevent this from happening.
These are two people who had false-positive tests because they had diabetes. I think it’s important that we realize that this can happen, and we need to help our patients deal with these situations.
Dr. Peters is professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles. She reported conflicts of interest with Abbott Diabetes Care, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, Lexicon Pharmaceuticals, Livongo, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to tell you the story of two patients with diabetes who had false-positive alcohol tests.
The first patient is a patient of mine with type 1 diabetes. He was in a car accident. He hit the car in front of him that hit the car in front of them. Because the cars were quite damaged, the police were summoned.
At the scene, he had a breathalyzer test. He flunked the breathalyzer test, and he was charged with a DUI. The woman in the middle car got out of her car and said her neck hurt. This then rose this to the level of a DUI with injury to one of the other people, and my patient was charged with a felony. He was taken to jail.
He told them at the scene and at the jail that he had type 1 diabetes. The reason this is so important is because type 1 diabetes can cause a false-positive breathalyzer test. In particular, this patient of mine had not been eating all day long. He’d been getting his basal insulin through the pump, but he had not given any bolus doses of insulin. He was actually quite ketotic.
When he was put in jail, they took away his cell phone so he could no longer see his glucose levels, and they took away the controller for his Omnipod system. He basically had no way to give bolus doses of insulin. Fortunately, the Omnipod system lasted for a day and a half just by giving him basal. The jail physicians did not give him insulin until he’d been in jail for 3 days.
This is someone with type 1 diabetes, and their protocol for insulin has something to do with high glucose levels and giving something like a sliding scale of insulin. They were not really prepared for managing somebody with type 1 diabetes who was on an automated insulin delivery system.
I, along with my patient’s parents, worked very hard to get the jail doctors to finally give him Lantus. Inherent in all of this, it made me aware of a number of different issues. The first is that breathalyzer tests can be falsely positive in people with type 1 diabetes if they are ketotic; therefore, people with type 1 diabetes should ask for a blood test to test their alcohol levels if they think it could be a false positive.
Second, we need to actually figure out a way to help people with type 1 diabetes who happen to be in jail or in prison because if they don’t have access to a smartphone, they’re not going to be able to run their devices. We need to make sure that devices have receivers that can be used, particularly continuous glucose monitors (CGMs), because CGM is the standard of care for patients and should be so for people who are incarcerated.
The second case is much shorter and isn’t mine, but it was a letter in The New England Journal of Medicine about a man who was on probation, who was having urine tests to show that he had not been consuming alcohol. He was started on empagliflozin, which, interestingly, made his urine test become falsely positive.
Why? Well, it’s thought it’s because it caused fermentation of the sugar with the bacteria that was in his urine because the people who were processing the sample hadn’t done it correctly, and they kept it out at room temperature for a prolonged period of time before testing it. The urine samples should be kept refrigerated to prevent this from happening.
These are two people who had false-positive tests because they had diabetes. I think it’s important that we realize that this can happen, and we need to help our patients deal with these situations.
Dr. Peters is professor, Department of Clinical Medicine, Keck School of Medicine; Director, University of Southern California Westside Center for Diabetes, University of Southern California, Los Angeles. She reported conflicts of interest with Abbott Diabetes Care, Becton Dickinson, Boehringer Ingelheim, Eli Lilly, Lexicon Pharmaceuticals, Livongo, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, Zafgen, Dexcom, MannKind, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Meet the Pregnancy Challenges of Women With Chronic Conditions
Preconception and prenatal care are more complicated in women with chronic health conditions but attention to disease management and promoting the adoption of a healthier lifestyle can improve outcomes for mothers and infants, according to a growing body of research.
The latest version of the International Federation of Gynecology and Obstetrics Preconception Checklist, published in the International Journal of Gynecology & Obstetrics, highlights preexisting chronic medical conditions such as diabetes, lupus, and obesity as key factors to address in preconception care through disease management. A growing number of studies support the impact of these strategies on short- and long-term outcomes for mothers and babies, according to the authors.
Meet Glycemic Control Goals Prior to Pregnancy
“Women with diabetes can have healthy pregnancies but need to prepare for pregnancy in advance,” Ellen W. Seely, MD, professor of medicine at Harvard Medical School and director of clinical research in the endocrinology, diabetes, and hypertension division of Brigham and Women’s Hospital, Boston, said in an interview.
“If glucose levels are running high in the first trimester, this is associated with an increased risk of birth defects, some of which are very serious,” said Dr. Seely. Getting glucose levels under control reduces the risk of birth defects in women with diabetes close to that of the general population, she said.
The American Diabetes Association has set a goal for women to attain an HbA1c of less than 6.5% before conception, Dr. Seely said. “In addition, some women with diabetes may be on medications that should be changed to another class prior to pregnancy,” she noted. Women with type 1 or type 2 diabetes often have hypertension as well, but ACE inhibitors are associated with an increased risk of fetal renal damage that can result in neonatal death; therefore, these medications should be stopped prior to pregnancy, Dr. Seely emphasized.
“If a woman with type 2 diabetes is on medications other than insulin, recommendations from the ADA are to change to insulin prior to pregnancy, since we have the most data on the safety profile of insulin use in pregnancy,” she said.
To help women with diabetes improve glycemic control prior to pregnancy, Dr. Seely recommends home glucose monitoring, with checks of glucose four times a day, fasting, and 2 hours after each meal, and adjustment of insulin accordingly.
A healthy diet and physical activity remain important components of glycemic control as well. A barrier to proper preconception and prenatal care for women with diabetes is not knowing that a pregnancy should be planned, Dr. Seely said. Discussions about pregnancy should start at puberty for women with diabetes, according to the ADA, and the topic should be raised yearly so women can optimize their health and adjust medications prior to conception.
Although studies of drugs have been done to inform preconception care for women with diabetes, research is lacking in several areas, notably the safety of GLP-1 agonists in pregnancy, said Dr. Seely. “This class of drug is commonly used in type 2 diabetes and the current recommendation is to stop these agents 2 months prior to conception,” she said.
Conceive in Times of Lupus Remission
Advance planning also is important for a healthy pregnancy in women with systemic lupus erythematosus (SLE), Sayna Norouzi, MD, director of the glomerular disease clinic and polycystic kidney disease clinic of Loma Linda University Medical Center, California, said in an interview.
“Lupus mostly affects women of childbearing age and can create many challenges during pregnancy,” said Dr. Norouzi, the corresponding author of a recent review on managing lupus nephritis during pregnancy.
“Women with lupus face an increased risk of pregnancy complications such as preeclampsia, problems with fetal growth, stillbirth, and premature birth, and these risks increase based on factors such as disease activity, certain antibodies in the body, and other baseline existing conditions such as high blood pressure,” she said.
“It can be difficult to distinguish between a lupus flare and pregnancy-related issues, so proper management is important,” she noted. The Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus (PROMISSE) study findings indicated a lupus nephritis relapse rate of 7.8% of patients in complete remission and 21% of those in partial remission during pregnancy, said Dr. Norouzi. “Current evidence has shown that SLE patients without lupus nephritis flare in the preconception period have a small risk of relapse during pregnancy,” she said.
Before and during pregnancy, women with lupus should work with their treating physicians to adjust medications for safety, watch for signs of flare, and aim to conceive during a period of lupus remission.
Preconception care for women with lupus nephritis involves a careful review of the medications used to control the disease and protect the kidneys and other organs, said Dr. Norouzi.
“Adjustments,” she said, “should be personalized, taking into account the mother’s health and the safety of the baby. Managing the disease actively during pregnancy may require changes to the treatment plan while minimizing risks,” she noted. However, changing medications can cause challenges for patients, as medications that are safer for pregnancy may lead to new symptoms and side effects, and patients will need to work closely with their healthcare providers to overcome new issues that arise, she added.
Preconception lifestyle changes such as increasing exercise and adopting a healthier diet can help with blood pressure control for kidney disease patients, said Dr. Norouzi.
In the review article, Dr. Norouzi and colleagues noted that preconception counseling for patients with lupus should address common comorbidities such as hypertension, diabetes, obesity, and dyslipidemia, and the risk for immediate and long-term cardiovascular complications.
Benefits of Preconception Obesity Care Extend to Infants
Current guidelines from the American College of Obstetricians and Gynecologists and the Institute of Medicine advise lifestyle interventions to reduce excessive weight gain during pregnancy and reduce the risk of inflammation, oxidative stress, insulin resistance, and lipotoxicity that can promote complications in the mother and fetus during pregnancy.
In addition, a growing number of studies suggest that women with obesity who make healthy lifestyle changes prior to conception can reduce obesity-associated risks to their infants.
Adults born to women with obesity are at increased risk of cardiovascular disease and early signs of heart remodeling are identifiable in newborns, Samuel J. Burden, PhD, a research associate in the department of women and children’s health, Kings’ College, London, said in an interview. “It is therefore important to investigate whether intervening either before or during pregnancy by promoting a healthy lifestyle can reduce this adverse impact on the heart and blood vessels,” he said.
In a recent study published in the International Journal of Obesity, Dr. Burden and colleagues examined data from eight studies based on data from five randomized, controlled trials including children of mothers with obesity who engaged in healthy lifestyle interventions of improved diet and increased physical activity prior to and during pregnancy. The study population included children ranging in age from less than 2 months to 3-7 years.
Lifestyle interventions for mothers both before conception and during pregnancy were associated with significant changes in cardiac remodeling in the children, notably reduced interventricular septal wall thickness. Additionally, five studies of cardiac systolic function and three studies of diastolic function showed improvement in blood pressure in children of mothers who took part in the interventions.
Dr. Burden acknowledged that lifestyle changes in women with obesity before conception and during pregnancy can be challenging, but should be encouraged. “During pregnancy, it may also seem unnatural to increase daily physical activity or change the way you are eating.” He emphasized that patients should consult their physicians and follow an established program. More randomized, controlled trials are needed from the preconception period to examine whether the health benefits are greater if the intervention begins prior to pregnancy, said Dr. Burden. However, “the current findings indeed indicate that women with obesity who lead a healthy lifestyle before and during their pregnancy can reduce the degree of unhealthy heart remodeling in their children,” he said.
Dr. Seely, Dr. Norouzi, and Dr. Burden had no financial conflicts to disclose.
Preconception and prenatal care are more complicated in women with chronic health conditions but attention to disease management and promoting the adoption of a healthier lifestyle can improve outcomes for mothers and infants, according to a growing body of research.
The latest version of the International Federation of Gynecology and Obstetrics Preconception Checklist, published in the International Journal of Gynecology & Obstetrics, highlights preexisting chronic medical conditions such as diabetes, lupus, and obesity as key factors to address in preconception care through disease management. A growing number of studies support the impact of these strategies on short- and long-term outcomes for mothers and babies, according to the authors.
Meet Glycemic Control Goals Prior to Pregnancy
“Women with diabetes can have healthy pregnancies but need to prepare for pregnancy in advance,” Ellen W. Seely, MD, professor of medicine at Harvard Medical School and director of clinical research in the endocrinology, diabetes, and hypertension division of Brigham and Women’s Hospital, Boston, said in an interview.
“If glucose levels are running high in the first trimester, this is associated with an increased risk of birth defects, some of which are very serious,” said Dr. Seely. Getting glucose levels under control reduces the risk of birth defects in women with diabetes close to that of the general population, she said.
The American Diabetes Association has set a goal for women to attain an HbA1c of less than 6.5% before conception, Dr. Seely said. “In addition, some women with diabetes may be on medications that should be changed to another class prior to pregnancy,” she noted. Women with type 1 or type 2 diabetes often have hypertension as well, but ACE inhibitors are associated with an increased risk of fetal renal damage that can result in neonatal death; therefore, these medications should be stopped prior to pregnancy, Dr. Seely emphasized.
“If a woman with type 2 diabetes is on medications other than insulin, recommendations from the ADA are to change to insulin prior to pregnancy, since we have the most data on the safety profile of insulin use in pregnancy,” she said.
To help women with diabetes improve glycemic control prior to pregnancy, Dr. Seely recommends home glucose monitoring, with checks of glucose four times a day, fasting, and 2 hours after each meal, and adjustment of insulin accordingly.
A healthy diet and physical activity remain important components of glycemic control as well. A barrier to proper preconception and prenatal care for women with diabetes is not knowing that a pregnancy should be planned, Dr. Seely said. Discussions about pregnancy should start at puberty for women with diabetes, according to the ADA, and the topic should be raised yearly so women can optimize their health and adjust medications prior to conception.
Although studies of drugs have been done to inform preconception care for women with diabetes, research is lacking in several areas, notably the safety of GLP-1 agonists in pregnancy, said Dr. Seely. “This class of drug is commonly used in type 2 diabetes and the current recommendation is to stop these agents 2 months prior to conception,” she said.
Conceive in Times of Lupus Remission
Advance planning also is important for a healthy pregnancy in women with systemic lupus erythematosus (SLE), Sayna Norouzi, MD, director of the glomerular disease clinic and polycystic kidney disease clinic of Loma Linda University Medical Center, California, said in an interview.
“Lupus mostly affects women of childbearing age and can create many challenges during pregnancy,” said Dr. Norouzi, the corresponding author of a recent review on managing lupus nephritis during pregnancy.
“Women with lupus face an increased risk of pregnancy complications such as preeclampsia, problems with fetal growth, stillbirth, and premature birth, and these risks increase based on factors such as disease activity, certain antibodies in the body, and other baseline existing conditions such as high blood pressure,” she said.
“It can be difficult to distinguish between a lupus flare and pregnancy-related issues, so proper management is important,” she noted. The Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus (PROMISSE) study findings indicated a lupus nephritis relapse rate of 7.8% of patients in complete remission and 21% of those in partial remission during pregnancy, said Dr. Norouzi. “Current evidence has shown that SLE patients without lupus nephritis flare in the preconception period have a small risk of relapse during pregnancy,” she said.
Before and during pregnancy, women with lupus should work with their treating physicians to adjust medications for safety, watch for signs of flare, and aim to conceive during a period of lupus remission.
Preconception care for women with lupus nephritis involves a careful review of the medications used to control the disease and protect the kidneys and other organs, said Dr. Norouzi.
“Adjustments,” she said, “should be personalized, taking into account the mother’s health and the safety of the baby. Managing the disease actively during pregnancy may require changes to the treatment plan while minimizing risks,” she noted. However, changing medications can cause challenges for patients, as medications that are safer for pregnancy may lead to new symptoms and side effects, and patients will need to work closely with their healthcare providers to overcome new issues that arise, she added.
Preconception lifestyle changes such as increasing exercise and adopting a healthier diet can help with blood pressure control for kidney disease patients, said Dr. Norouzi.
In the review article, Dr. Norouzi and colleagues noted that preconception counseling for patients with lupus should address common comorbidities such as hypertension, diabetes, obesity, and dyslipidemia, and the risk for immediate and long-term cardiovascular complications.
Benefits of Preconception Obesity Care Extend to Infants
Current guidelines from the American College of Obstetricians and Gynecologists and the Institute of Medicine advise lifestyle interventions to reduce excessive weight gain during pregnancy and reduce the risk of inflammation, oxidative stress, insulin resistance, and lipotoxicity that can promote complications in the mother and fetus during pregnancy.
In addition, a growing number of studies suggest that women with obesity who make healthy lifestyle changes prior to conception can reduce obesity-associated risks to their infants.
Adults born to women with obesity are at increased risk of cardiovascular disease and early signs of heart remodeling are identifiable in newborns, Samuel J. Burden, PhD, a research associate in the department of women and children’s health, Kings’ College, London, said in an interview. “It is therefore important to investigate whether intervening either before or during pregnancy by promoting a healthy lifestyle can reduce this adverse impact on the heart and blood vessels,” he said.
In a recent study published in the International Journal of Obesity, Dr. Burden and colleagues examined data from eight studies based on data from five randomized, controlled trials including children of mothers with obesity who engaged in healthy lifestyle interventions of improved diet and increased physical activity prior to and during pregnancy. The study population included children ranging in age from less than 2 months to 3-7 years.
Lifestyle interventions for mothers both before conception and during pregnancy were associated with significant changes in cardiac remodeling in the children, notably reduced interventricular septal wall thickness. Additionally, five studies of cardiac systolic function and three studies of diastolic function showed improvement in blood pressure in children of mothers who took part in the interventions.
Dr. Burden acknowledged that lifestyle changes in women with obesity before conception and during pregnancy can be challenging, but should be encouraged. “During pregnancy, it may also seem unnatural to increase daily physical activity or change the way you are eating.” He emphasized that patients should consult their physicians and follow an established program. More randomized, controlled trials are needed from the preconception period to examine whether the health benefits are greater if the intervention begins prior to pregnancy, said Dr. Burden. However, “the current findings indeed indicate that women with obesity who lead a healthy lifestyle before and during their pregnancy can reduce the degree of unhealthy heart remodeling in their children,” he said.
Dr. Seely, Dr. Norouzi, and Dr. Burden had no financial conflicts to disclose.
Preconception and prenatal care are more complicated in women with chronic health conditions but attention to disease management and promoting the adoption of a healthier lifestyle can improve outcomes for mothers and infants, according to a growing body of research.
The latest version of the International Federation of Gynecology and Obstetrics Preconception Checklist, published in the International Journal of Gynecology & Obstetrics, highlights preexisting chronic medical conditions such as diabetes, lupus, and obesity as key factors to address in preconception care through disease management. A growing number of studies support the impact of these strategies on short- and long-term outcomes for mothers and babies, according to the authors.
Meet Glycemic Control Goals Prior to Pregnancy
“Women with diabetes can have healthy pregnancies but need to prepare for pregnancy in advance,” Ellen W. Seely, MD, professor of medicine at Harvard Medical School and director of clinical research in the endocrinology, diabetes, and hypertension division of Brigham and Women’s Hospital, Boston, said in an interview.
“If glucose levels are running high in the first trimester, this is associated with an increased risk of birth defects, some of which are very serious,” said Dr. Seely. Getting glucose levels under control reduces the risk of birth defects in women with diabetes close to that of the general population, she said.
The American Diabetes Association has set a goal for women to attain an HbA1c of less than 6.5% before conception, Dr. Seely said. “In addition, some women with diabetes may be on medications that should be changed to another class prior to pregnancy,” she noted. Women with type 1 or type 2 diabetes often have hypertension as well, but ACE inhibitors are associated with an increased risk of fetal renal damage that can result in neonatal death; therefore, these medications should be stopped prior to pregnancy, Dr. Seely emphasized.
“If a woman with type 2 diabetes is on medications other than insulin, recommendations from the ADA are to change to insulin prior to pregnancy, since we have the most data on the safety profile of insulin use in pregnancy,” she said.
To help women with diabetes improve glycemic control prior to pregnancy, Dr. Seely recommends home glucose monitoring, with checks of glucose four times a day, fasting, and 2 hours after each meal, and adjustment of insulin accordingly.
A healthy diet and physical activity remain important components of glycemic control as well. A barrier to proper preconception and prenatal care for women with diabetes is not knowing that a pregnancy should be planned, Dr. Seely said. Discussions about pregnancy should start at puberty for women with diabetes, according to the ADA, and the topic should be raised yearly so women can optimize their health and adjust medications prior to conception.
Although studies of drugs have been done to inform preconception care for women with diabetes, research is lacking in several areas, notably the safety of GLP-1 agonists in pregnancy, said Dr. Seely. “This class of drug is commonly used in type 2 diabetes and the current recommendation is to stop these agents 2 months prior to conception,” she said.
Conceive in Times of Lupus Remission
Advance planning also is important for a healthy pregnancy in women with systemic lupus erythematosus (SLE), Sayna Norouzi, MD, director of the glomerular disease clinic and polycystic kidney disease clinic of Loma Linda University Medical Center, California, said in an interview.
“Lupus mostly affects women of childbearing age and can create many challenges during pregnancy,” said Dr. Norouzi, the corresponding author of a recent review on managing lupus nephritis during pregnancy.
“Women with lupus face an increased risk of pregnancy complications such as preeclampsia, problems with fetal growth, stillbirth, and premature birth, and these risks increase based on factors such as disease activity, certain antibodies in the body, and other baseline existing conditions such as high blood pressure,” she said.
“It can be difficult to distinguish between a lupus flare and pregnancy-related issues, so proper management is important,” she noted. The Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus (PROMISSE) study findings indicated a lupus nephritis relapse rate of 7.8% of patients in complete remission and 21% of those in partial remission during pregnancy, said Dr. Norouzi. “Current evidence has shown that SLE patients without lupus nephritis flare in the preconception period have a small risk of relapse during pregnancy,” she said.
Before and during pregnancy, women with lupus should work with their treating physicians to adjust medications for safety, watch for signs of flare, and aim to conceive during a period of lupus remission.
Preconception care for women with lupus nephritis involves a careful review of the medications used to control the disease and protect the kidneys and other organs, said Dr. Norouzi.
“Adjustments,” she said, “should be personalized, taking into account the mother’s health and the safety of the baby. Managing the disease actively during pregnancy may require changes to the treatment plan while minimizing risks,” she noted. However, changing medications can cause challenges for patients, as medications that are safer for pregnancy may lead to new symptoms and side effects, and patients will need to work closely with their healthcare providers to overcome new issues that arise, she added.
Preconception lifestyle changes such as increasing exercise and adopting a healthier diet can help with blood pressure control for kidney disease patients, said Dr. Norouzi.
In the review article, Dr. Norouzi and colleagues noted that preconception counseling for patients with lupus should address common comorbidities such as hypertension, diabetes, obesity, and dyslipidemia, and the risk for immediate and long-term cardiovascular complications.
Benefits of Preconception Obesity Care Extend to Infants
Current guidelines from the American College of Obstetricians and Gynecologists and the Institute of Medicine advise lifestyle interventions to reduce excessive weight gain during pregnancy and reduce the risk of inflammation, oxidative stress, insulin resistance, and lipotoxicity that can promote complications in the mother and fetus during pregnancy.
In addition, a growing number of studies suggest that women with obesity who make healthy lifestyle changes prior to conception can reduce obesity-associated risks to their infants.
Adults born to women with obesity are at increased risk of cardiovascular disease and early signs of heart remodeling are identifiable in newborns, Samuel J. Burden, PhD, a research associate in the department of women and children’s health, Kings’ College, London, said in an interview. “It is therefore important to investigate whether intervening either before or during pregnancy by promoting a healthy lifestyle can reduce this adverse impact on the heart and blood vessels,” he said.
In a recent study published in the International Journal of Obesity, Dr. Burden and colleagues examined data from eight studies based on data from five randomized, controlled trials including children of mothers with obesity who engaged in healthy lifestyle interventions of improved diet and increased physical activity prior to and during pregnancy. The study population included children ranging in age from less than 2 months to 3-7 years.
Lifestyle interventions for mothers both before conception and during pregnancy were associated with significant changes in cardiac remodeling in the children, notably reduced interventricular septal wall thickness. Additionally, five studies of cardiac systolic function and three studies of diastolic function showed improvement in blood pressure in children of mothers who took part in the interventions.
Dr. Burden acknowledged that lifestyle changes in women with obesity before conception and during pregnancy can be challenging, but should be encouraged. “During pregnancy, it may also seem unnatural to increase daily physical activity or change the way you are eating.” He emphasized that patients should consult their physicians and follow an established program. More randomized, controlled trials are needed from the preconception period to examine whether the health benefits are greater if the intervention begins prior to pregnancy, said Dr. Burden. However, “the current findings indeed indicate that women with obesity who lead a healthy lifestyle before and during their pregnancy can reduce the degree of unhealthy heart remodeling in their children,” he said.
Dr. Seely, Dr. Norouzi, and Dr. Burden had no financial conflicts to disclose.
Expanding Use of GLP-1 RAs for Weight Management
To discuss issues related to counseling patients about weight loss with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), I recently posted a case from my own practice. This was a 44-year-old woman with hyperlipidemia, hypertension, and obesity who wanted to try to lose weight with a GLP-1 RA, having been unsuccessful in maintaining a normal weight with lifestyle change alone.
I am very happy to see a high number of favorable responses to this article, and I also recognize that it was very focused on GLP-1 RA therapy while not addressing the multivariate treatment of obesity.
A healthy lifestyle remains foundational for the management of obesity, and clinicians should guide patients to make constructive choices regarding their diet, physical activity, mental health, and sleep. However, like for our patient introduced in that article, lifestyle changes are rarely sufficient to obtain a goal of sustained weight loss that promotes better health outcomes. A meta-analysis of clinical trials testing lifestyle interventions to lose weight among adults with overweight and obesity found that the relative reduction in body weight in the intervention vs control cohorts was −3.63 kg at 1 year and −2.45 kg at 3 years. More intensive programs with at least 28 interventions per year were associated with slightly more weight loss than less intensive programs.
That is why clinicians and patients have been reaching for effective pharmacotherapy to create better outcomes among adults with obesity. In a national survey of 1479 US adults, 12% reported having used a GLP-1 RA. Diabetes was the most common indication (43%), followed by heart disease (26%) and overweight/obesity (22%).
The high cost of GLP-1 RA therapy was a major barrier to even wider use. Some 54% of participants said that it was difficult to afford GLP-1 RA therapy, and an additional 22% found it very difficult to pay for the drugs. Having health insurance did not alter these figures substantially.
While cost and access remain some of the greatest challenges with the use of GLP-1 RAs, there is hope for change there. In March 2024, the US Food and Drug Administration approved semaglutide to reduce the risk for cardiovascular events among patients with overweight and obesity and existing cardiovascular disease. It appears that Medicare will cover semaglutide for that indication, which bucks a trend of more than 20 years during which Medicare Part D would not cover pharmacotherapy for weight loss.
There is bipartisan support in the US Congress to further increase coverage of GLP-1 RAs for obesity, which makes sense. GLP-1 RAs are associated with greater average weight loss than either lifestyle interventions alone or that associated with previous anti-obesity medications. While there are no safety data for these drugs stretching back for 50 or 100 years, clinicians should bear in mind that exenatide was approved for the management of type 2 diabetes in 2005. So, we are approaching two decades of practical experience with these drugs, and it appears clear that the benefits of GLP-1 RAs outweigh any known harms. For the right patient, and with the right kind of guidance by clinicians, GLP-1 RA therapy can have a profound effect on individual and public health.
Dr. Vega, health sciences clinical professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
To discuss issues related to counseling patients about weight loss with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), I recently posted a case from my own practice. This was a 44-year-old woman with hyperlipidemia, hypertension, and obesity who wanted to try to lose weight with a GLP-1 RA, having been unsuccessful in maintaining a normal weight with lifestyle change alone.
I am very happy to see a high number of favorable responses to this article, and I also recognize that it was very focused on GLP-1 RA therapy while not addressing the multivariate treatment of obesity.
A healthy lifestyle remains foundational for the management of obesity, and clinicians should guide patients to make constructive choices regarding their diet, physical activity, mental health, and sleep. However, like for our patient introduced in that article, lifestyle changes are rarely sufficient to obtain a goal of sustained weight loss that promotes better health outcomes. A meta-analysis of clinical trials testing lifestyle interventions to lose weight among adults with overweight and obesity found that the relative reduction in body weight in the intervention vs control cohorts was −3.63 kg at 1 year and −2.45 kg at 3 years. More intensive programs with at least 28 interventions per year were associated with slightly more weight loss than less intensive programs.
That is why clinicians and patients have been reaching for effective pharmacotherapy to create better outcomes among adults with obesity. In a national survey of 1479 US adults, 12% reported having used a GLP-1 RA. Diabetes was the most common indication (43%), followed by heart disease (26%) and overweight/obesity (22%).
The high cost of GLP-1 RA therapy was a major barrier to even wider use. Some 54% of participants said that it was difficult to afford GLP-1 RA therapy, and an additional 22% found it very difficult to pay for the drugs. Having health insurance did not alter these figures substantially.
While cost and access remain some of the greatest challenges with the use of GLP-1 RAs, there is hope for change there. In March 2024, the US Food and Drug Administration approved semaglutide to reduce the risk for cardiovascular events among patients with overweight and obesity and existing cardiovascular disease. It appears that Medicare will cover semaglutide for that indication, which bucks a trend of more than 20 years during which Medicare Part D would not cover pharmacotherapy for weight loss.
There is bipartisan support in the US Congress to further increase coverage of GLP-1 RAs for obesity, which makes sense. GLP-1 RAs are associated with greater average weight loss than either lifestyle interventions alone or that associated with previous anti-obesity medications. While there are no safety data for these drugs stretching back for 50 or 100 years, clinicians should bear in mind that exenatide was approved for the management of type 2 diabetes in 2005. So, we are approaching two decades of practical experience with these drugs, and it appears clear that the benefits of GLP-1 RAs outweigh any known harms. For the right patient, and with the right kind of guidance by clinicians, GLP-1 RA therapy can have a profound effect on individual and public health.
Dr. Vega, health sciences clinical professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
To discuss issues related to counseling patients about weight loss with glucagon-like peptide 1 receptor agonists (GLP-1 RAs), I recently posted a case from my own practice. This was a 44-year-old woman with hyperlipidemia, hypertension, and obesity who wanted to try to lose weight with a GLP-1 RA, having been unsuccessful in maintaining a normal weight with lifestyle change alone.
I am very happy to see a high number of favorable responses to this article, and I also recognize that it was very focused on GLP-1 RA therapy while not addressing the multivariate treatment of obesity.
A healthy lifestyle remains foundational for the management of obesity, and clinicians should guide patients to make constructive choices regarding their diet, physical activity, mental health, and sleep. However, like for our patient introduced in that article, lifestyle changes are rarely sufficient to obtain a goal of sustained weight loss that promotes better health outcomes. A meta-analysis of clinical trials testing lifestyle interventions to lose weight among adults with overweight and obesity found that the relative reduction in body weight in the intervention vs control cohorts was −3.63 kg at 1 year and −2.45 kg at 3 years. More intensive programs with at least 28 interventions per year were associated with slightly more weight loss than less intensive programs.
That is why clinicians and patients have been reaching for effective pharmacotherapy to create better outcomes among adults with obesity. In a national survey of 1479 US adults, 12% reported having used a GLP-1 RA. Diabetes was the most common indication (43%), followed by heart disease (26%) and overweight/obesity (22%).
The high cost of GLP-1 RA therapy was a major barrier to even wider use. Some 54% of participants said that it was difficult to afford GLP-1 RA therapy, and an additional 22% found it very difficult to pay for the drugs. Having health insurance did not alter these figures substantially.
While cost and access remain some of the greatest challenges with the use of GLP-1 RAs, there is hope for change there. In March 2024, the US Food and Drug Administration approved semaglutide to reduce the risk for cardiovascular events among patients with overweight and obesity and existing cardiovascular disease. It appears that Medicare will cover semaglutide for that indication, which bucks a trend of more than 20 years during which Medicare Part D would not cover pharmacotherapy for weight loss.
There is bipartisan support in the US Congress to further increase coverage of GLP-1 RAs for obesity, which makes sense. GLP-1 RAs are associated with greater average weight loss than either lifestyle interventions alone or that associated with previous anti-obesity medications. While there are no safety data for these drugs stretching back for 50 or 100 years, clinicians should bear in mind that exenatide was approved for the management of type 2 diabetes in 2005. So, we are approaching two decades of practical experience with these drugs, and it appears clear that the benefits of GLP-1 RAs outweigh any known harms. For the right patient, and with the right kind of guidance by clinicians, GLP-1 RA therapy can have a profound effect on individual and public health.
Dr. Vega, health sciences clinical professor, Family Medicine, University of California, Irvine, disclosed ties with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Uproar Over Vitamin D Disease-Prevention Guideline
A recent report by this news organization of a vitamin D clinical practice guideline released by the Endocrine Society in June triggered an outpouring of objections in the comments section from doctors and other readers.
A society press release listed the key new recommendations on the use of vitamin D supplementation and screening to reduce disease risks in individuals without established indications for such treatment or testing:
- For healthy adults younger than 75, no supplementation at doses above the recommended dietary intakes.
- Populations that may benefit from higher doses include: children and adolescents 18 and younger to prevent rickets and to reduce risk for respiratory infection, individuals 75 and older to possibly lower mortality risk, “pregnant people” to potentially reduce various risks, and people with prediabetes to potentially reduce risk of progression.
- No routine testing for 25-hydroxyvitamin D levels because outcome-specific benefits based on those levels have not been identified (including screening in people with dark complexion or obesity).
- Based on insufficient evidence, the panel could not determine specific blood-level thresholds for 25-hydroxyvitamin D for adequacy or for target levels for disease prevention.
This news organization covered the guideline release and simultaneous presentation at the Endocrine Society annual meeting. In response to the coverage, more than 200 doctors and other readers expressed concerns about the guideline, and some said outright that they would not follow it (readers quoted below are identified by the usernames they registered with on the website).
One reader who posted as Dr. Joseph Destefano went so far as to call the guideline “dangerous” and “almost ... evil.” Ironically, some readers attacked this news organization, thinking that the coverage implied an endorsement, rather than a news report.
Ignores Potential Benefits
“They address issues dealing only with endocrinology and bone health for the most part,” Dr. Emilio Gonzalez wrote. “However, vitamin D insufficiency and deficiency are not rare, and they impact the treatment of autoimmune disorders, chronic pain control, immunosuppression, cancer prevention, cardiovascular health, etc. There is plenty of literature in this regard.”
“They make these claims as if quality studies contradicting their guidelines have not been out there for years,” Dr. Brian Batcheldor said. “What about the huge demographic with diseases that impact intestinal absorption, eg, Crohn’s and celiac disease, cystic fibrosis, and ulcerative colitis? What about the one in nine that now have autoimmune diseases still awaiting diagnosis? What about night workers or anyone with more restricted access to sun exposure? How about those whose cultural or religious dress code limit skin exposure?”
The latter group was also mentioned in a post from Dr. Eve Finkelstein who said, “They don’t take into account women who are totally covered for religious reasons. They have no skin other than part of their face exposed. It does not make sense not to supplement them. Ignoring women’s health needs seems to be the norm.”
“I don’t think they considered the oral health effects of vitamin D deficiency,” pointed out commenter Corie Lewis. “Excess dental calculus (tartar) from excess calcium/phosphate in saliva significantly increases an individual’s periodontal disease risks (gum disease), and low saliva calcium/phosphate increases dental caries (cavities) risks, which generally indicates an imbalance of the oral microbiome. Vitamin D can help create balance and reduce those oral health risks.”
Noted Kimberley Morris-Windisch, “Having worked in rheumatology and pain for most of my career, I have seen too many people benefit from correcting deficiency of vitamin D. To ignore this is to miss opportunities to improve patient health.” Furthermore, “I find it unlikely that it would only improve mortality after age 75. That makes no sense.”
“Also,” she added, “what is the number [needed] to harm? In my 25 years, I have seen vitamin D toxicity once and an excessively high level without symptoms one other time.”
“WHY? Just WHY?” lamented Anne Kinchen. “Low levels in pregnant women have long-term effects on the developing fetus — higher and earlier rates of osteopenia in female children, weaker immune systems overall. There are just SO many reasons to test. These guidelines for no testing are absurd!”
No Screening, No Need for Decision-Making?
Several readers questioned the society’s rationale for not screening, as expressed by session moderator Clifford J. Rosen, MD, director of Clinical and Translational Research and senior scientist at Maine Medical Center Research Institute, Scarborough, Maine.
“When clinicians measure vitamin D, then they’re forced to make a decision what to do about it,” Dr. Rosen said. “That’s where questions about the levels come in. And that’s a big problem. So what the panel’s saying is, don’t screen. ... This really gets to the heart of the issue, because we have no data that there’s anything about screening that allows us to improve quality of life. ... Screening is probably not worthwhile in any age group.”
Among the reader comments in this regard:
“So misguided. Don’t look because we don’t know what do to with data. That’s the message this article exposes. The recommendation is do nothing. But, doing nothing IS an action — not a default.” (Lisa Tracy)
“So now, you will not screen for vitamin D because you do not know what to do next? See a naturopathic doctor — we know what to do next!” (Dr. Joyce Roberson)
“Gee, how do we treat it? ... What to do? Sounds incompetent at minimum. I suspect it’s vital, easy, and inexpensive ... so hide it.” (Holly Kohley)
“Just because we do not know is not a rationale for not testing. The opposite should be done.” (Dr. JJ Gold)
Caters to Industry?
Many commentators intimated that pharma and/or insurance company considerations played a role in the recommendations. Their comments included the following:
“I have been under the impression people do routine checkups to verify there are no hidden problems. If only some testing is done, the probability of not finding a problem is huge. ... Preventive healthcare should be looking for something to prevent instead of waiting until they can cure it. Of course, it might come back to ‘follow the money.’ It is much more profitable to diagnose and treat than it is to prevent.” (Grace Kyser)
“The current irrational ‘recommendation’ gives insurance companies an excuse to deny ALL tests of vitamin D — even if the proper code is supplied. The result is — people suffer. This recommendation does harm!” (Dr JJ Gold)
“Essentially, they are saying let’s not screen ‘healthy’ individuals and ignore it altogether. Better to wait till they’re old, pregnant, or already sick and diagnosed with a disease. This is the problem with the healthcare in this country.” (Brittney Lesher)
“Until allopathic medicine stops waiting for severe symptoms to develop before even screening for potential health problems, the most expensive healthcare (aka, sick care) system in the world will continue to be content to focus on medical emergencies and ignore prevention. ...” (Dean Raffelock)
“Don’t test? Are you kidding me? Especially when people are supplementing? That is akin to taking a blood pressure medication without measuring blood pressures! ... Don’t test? Don’t supplement? ... I have only one explanation for such nonsense: Pharma lives off sick people, not healthy ones.” (Georg Schlomka)
On a somewhat conciliatory and pointed note, Dr Francesca Luna-Rudin commented, “I would like to remind all of my fellow physicians that recommendations should be regarded as just that, a ‘recommendation.’ As doctors, we can use guidelines and recommendations in our practice, but if a new one is presented that does not make sense or would lead to harm based on our education and training, then we are not bound to follow it!”
A version of this article first appeared on Medscape.com.
A recent report by this news organization of a vitamin D clinical practice guideline released by the Endocrine Society in June triggered an outpouring of objections in the comments section from doctors and other readers.
A society press release listed the key new recommendations on the use of vitamin D supplementation and screening to reduce disease risks in individuals without established indications for such treatment or testing:
- For healthy adults younger than 75, no supplementation at doses above the recommended dietary intakes.
- Populations that may benefit from higher doses include: children and adolescents 18 and younger to prevent rickets and to reduce risk for respiratory infection, individuals 75 and older to possibly lower mortality risk, “pregnant people” to potentially reduce various risks, and people with prediabetes to potentially reduce risk of progression.
- No routine testing for 25-hydroxyvitamin D levels because outcome-specific benefits based on those levels have not been identified (including screening in people with dark complexion or obesity).
- Based on insufficient evidence, the panel could not determine specific blood-level thresholds for 25-hydroxyvitamin D for adequacy or for target levels for disease prevention.
This news organization covered the guideline release and simultaneous presentation at the Endocrine Society annual meeting. In response to the coverage, more than 200 doctors and other readers expressed concerns about the guideline, and some said outright that they would not follow it (readers quoted below are identified by the usernames they registered with on the website).
One reader who posted as Dr. Joseph Destefano went so far as to call the guideline “dangerous” and “almost ... evil.” Ironically, some readers attacked this news organization, thinking that the coverage implied an endorsement, rather than a news report.
Ignores Potential Benefits
“They address issues dealing only with endocrinology and bone health for the most part,” Dr. Emilio Gonzalez wrote. “However, vitamin D insufficiency and deficiency are not rare, and they impact the treatment of autoimmune disorders, chronic pain control, immunosuppression, cancer prevention, cardiovascular health, etc. There is plenty of literature in this regard.”
“They make these claims as if quality studies contradicting their guidelines have not been out there for years,” Dr. Brian Batcheldor said. “What about the huge demographic with diseases that impact intestinal absorption, eg, Crohn’s and celiac disease, cystic fibrosis, and ulcerative colitis? What about the one in nine that now have autoimmune diseases still awaiting diagnosis? What about night workers or anyone with more restricted access to sun exposure? How about those whose cultural or religious dress code limit skin exposure?”
The latter group was also mentioned in a post from Dr. Eve Finkelstein who said, “They don’t take into account women who are totally covered for religious reasons. They have no skin other than part of their face exposed. It does not make sense not to supplement them. Ignoring women’s health needs seems to be the norm.”
“I don’t think they considered the oral health effects of vitamin D deficiency,” pointed out commenter Corie Lewis. “Excess dental calculus (tartar) from excess calcium/phosphate in saliva significantly increases an individual’s periodontal disease risks (gum disease), and low saliva calcium/phosphate increases dental caries (cavities) risks, which generally indicates an imbalance of the oral microbiome. Vitamin D can help create balance and reduce those oral health risks.”
Noted Kimberley Morris-Windisch, “Having worked in rheumatology and pain for most of my career, I have seen too many people benefit from correcting deficiency of vitamin D. To ignore this is to miss opportunities to improve patient health.” Furthermore, “I find it unlikely that it would only improve mortality after age 75. That makes no sense.”
“Also,” she added, “what is the number [needed] to harm? In my 25 years, I have seen vitamin D toxicity once and an excessively high level without symptoms one other time.”
“WHY? Just WHY?” lamented Anne Kinchen. “Low levels in pregnant women have long-term effects on the developing fetus — higher and earlier rates of osteopenia in female children, weaker immune systems overall. There are just SO many reasons to test. These guidelines for no testing are absurd!”
No Screening, No Need for Decision-Making?
Several readers questioned the society’s rationale for not screening, as expressed by session moderator Clifford J. Rosen, MD, director of Clinical and Translational Research and senior scientist at Maine Medical Center Research Institute, Scarborough, Maine.
“When clinicians measure vitamin D, then they’re forced to make a decision what to do about it,” Dr. Rosen said. “That’s where questions about the levels come in. And that’s a big problem. So what the panel’s saying is, don’t screen. ... This really gets to the heart of the issue, because we have no data that there’s anything about screening that allows us to improve quality of life. ... Screening is probably not worthwhile in any age group.”
Among the reader comments in this regard:
“So misguided. Don’t look because we don’t know what do to with data. That’s the message this article exposes. The recommendation is do nothing. But, doing nothing IS an action — not a default.” (Lisa Tracy)
“So now, you will not screen for vitamin D because you do not know what to do next? See a naturopathic doctor — we know what to do next!” (Dr. Joyce Roberson)
“Gee, how do we treat it? ... What to do? Sounds incompetent at minimum. I suspect it’s vital, easy, and inexpensive ... so hide it.” (Holly Kohley)
“Just because we do not know is not a rationale for not testing. The opposite should be done.” (Dr. JJ Gold)
Caters to Industry?
Many commentators intimated that pharma and/or insurance company considerations played a role in the recommendations. Their comments included the following:
“I have been under the impression people do routine checkups to verify there are no hidden problems. If only some testing is done, the probability of not finding a problem is huge. ... Preventive healthcare should be looking for something to prevent instead of waiting until they can cure it. Of course, it might come back to ‘follow the money.’ It is much more profitable to diagnose and treat than it is to prevent.” (Grace Kyser)
“The current irrational ‘recommendation’ gives insurance companies an excuse to deny ALL tests of vitamin D — even if the proper code is supplied. The result is — people suffer. This recommendation does harm!” (Dr JJ Gold)
“Essentially, they are saying let’s not screen ‘healthy’ individuals and ignore it altogether. Better to wait till they’re old, pregnant, or already sick and diagnosed with a disease. This is the problem with the healthcare in this country.” (Brittney Lesher)
“Until allopathic medicine stops waiting for severe symptoms to develop before even screening for potential health problems, the most expensive healthcare (aka, sick care) system in the world will continue to be content to focus on medical emergencies and ignore prevention. ...” (Dean Raffelock)
“Don’t test? Are you kidding me? Especially when people are supplementing? That is akin to taking a blood pressure medication without measuring blood pressures! ... Don’t test? Don’t supplement? ... I have only one explanation for such nonsense: Pharma lives off sick people, not healthy ones.” (Georg Schlomka)
On a somewhat conciliatory and pointed note, Dr Francesca Luna-Rudin commented, “I would like to remind all of my fellow physicians that recommendations should be regarded as just that, a ‘recommendation.’ As doctors, we can use guidelines and recommendations in our practice, but if a new one is presented that does not make sense or would lead to harm based on our education and training, then we are not bound to follow it!”
A version of this article first appeared on Medscape.com.
A recent report by this news organization of a vitamin D clinical practice guideline released by the Endocrine Society in June triggered an outpouring of objections in the comments section from doctors and other readers.
A society press release listed the key new recommendations on the use of vitamin D supplementation and screening to reduce disease risks in individuals without established indications for such treatment or testing:
- For healthy adults younger than 75, no supplementation at doses above the recommended dietary intakes.
- Populations that may benefit from higher doses include: children and adolescents 18 and younger to prevent rickets and to reduce risk for respiratory infection, individuals 75 and older to possibly lower mortality risk, “pregnant people” to potentially reduce various risks, and people with prediabetes to potentially reduce risk of progression.
- No routine testing for 25-hydroxyvitamin D levels because outcome-specific benefits based on those levels have not been identified (including screening in people with dark complexion or obesity).
- Based on insufficient evidence, the panel could not determine specific blood-level thresholds for 25-hydroxyvitamin D for adequacy or for target levels for disease prevention.
This news organization covered the guideline release and simultaneous presentation at the Endocrine Society annual meeting. In response to the coverage, more than 200 doctors and other readers expressed concerns about the guideline, and some said outright that they would not follow it (readers quoted below are identified by the usernames they registered with on the website).
One reader who posted as Dr. Joseph Destefano went so far as to call the guideline “dangerous” and “almost ... evil.” Ironically, some readers attacked this news organization, thinking that the coverage implied an endorsement, rather than a news report.
Ignores Potential Benefits
“They address issues dealing only with endocrinology and bone health for the most part,” Dr. Emilio Gonzalez wrote. “However, vitamin D insufficiency and deficiency are not rare, and they impact the treatment of autoimmune disorders, chronic pain control, immunosuppression, cancer prevention, cardiovascular health, etc. There is plenty of literature in this regard.”
“They make these claims as if quality studies contradicting their guidelines have not been out there for years,” Dr. Brian Batcheldor said. “What about the huge demographic with diseases that impact intestinal absorption, eg, Crohn’s and celiac disease, cystic fibrosis, and ulcerative colitis? What about the one in nine that now have autoimmune diseases still awaiting diagnosis? What about night workers or anyone with more restricted access to sun exposure? How about those whose cultural or religious dress code limit skin exposure?”
The latter group was also mentioned in a post from Dr. Eve Finkelstein who said, “They don’t take into account women who are totally covered for religious reasons. They have no skin other than part of their face exposed. It does not make sense not to supplement them. Ignoring women’s health needs seems to be the norm.”
“I don’t think they considered the oral health effects of vitamin D deficiency,” pointed out commenter Corie Lewis. “Excess dental calculus (tartar) from excess calcium/phosphate in saliva significantly increases an individual’s periodontal disease risks (gum disease), and low saliva calcium/phosphate increases dental caries (cavities) risks, which generally indicates an imbalance of the oral microbiome. Vitamin D can help create balance and reduce those oral health risks.”
Noted Kimberley Morris-Windisch, “Having worked in rheumatology and pain for most of my career, I have seen too many people benefit from correcting deficiency of vitamin D. To ignore this is to miss opportunities to improve patient health.” Furthermore, “I find it unlikely that it would only improve mortality after age 75. That makes no sense.”
“Also,” she added, “what is the number [needed] to harm? In my 25 years, I have seen vitamin D toxicity once and an excessively high level without symptoms one other time.”
“WHY? Just WHY?” lamented Anne Kinchen. “Low levels in pregnant women have long-term effects on the developing fetus — higher and earlier rates of osteopenia in female children, weaker immune systems overall. There are just SO many reasons to test. These guidelines for no testing are absurd!”
No Screening, No Need for Decision-Making?
Several readers questioned the society’s rationale for not screening, as expressed by session moderator Clifford J. Rosen, MD, director of Clinical and Translational Research and senior scientist at Maine Medical Center Research Institute, Scarborough, Maine.
“When clinicians measure vitamin D, then they’re forced to make a decision what to do about it,” Dr. Rosen said. “That’s where questions about the levels come in. And that’s a big problem. So what the panel’s saying is, don’t screen. ... This really gets to the heart of the issue, because we have no data that there’s anything about screening that allows us to improve quality of life. ... Screening is probably not worthwhile in any age group.”
Among the reader comments in this regard:
“So misguided. Don’t look because we don’t know what do to with data. That’s the message this article exposes. The recommendation is do nothing. But, doing nothing IS an action — not a default.” (Lisa Tracy)
“So now, you will not screen for vitamin D because you do not know what to do next? See a naturopathic doctor — we know what to do next!” (Dr. Joyce Roberson)
“Gee, how do we treat it? ... What to do? Sounds incompetent at minimum. I suspect it’s vital, easy, and inexpensive ... so hide it.” (Holly Kohley)
“Just because we do not know is not a rationale for not testing. The opposite should be done.” (Dr. JJ Gold)
Caters to Industry?
Many commentators intimated that pharma and/or insurance company considerations played a role in the recommendations. Their comments included the following:
“I have been under the impression people do routine checkups to verify there are no hidden problems. If only some testing is done, the probability of not finding a problem is huge. ... Preventive healthcare should be looking for something to prevent instead of waiting until they can cure it. Of course, it might come back to ‘follow the money.’ It is much more profitable to diagnose and treat than it is to prevent.” (Grace Kyser)
“The current irrational ‘recommendation’ gives insurance companies an excuse to deny ALL tests of vitamin D — even if the proper code is supplied. The result is — people suffer. This recommendation does harm!” (Dr JJ Gold)
“Essentially, they are saying let’s not screen ‘healthy’ individuals and ignore it altogether. Better to wait till they’re old, pregnant, or already sick and diagnosed with a disease. This is the problem with the healthcare in this country.” (Brittney Lesher)
“Until allopathic medicine stops waiting for severe symptoms to develop before even screening for potential health problems, the most expensive healthcare (aka, sick care) system in the world will continue to be content to focus on medical emergencies and ignore prevention. ...” (Dean Raffelock)
“Don’t test? Are you kidding me? Especially when people are supplementing? That is akin to taking a blood pressure medication without measuring blood pressures! ... Don’t test? Don’t supplement? ... I have only one explanation for such nonsense: Pharma lives off sick people, not healthy ones.” (Georg Schlomka)
On a somewhat conciliatory and pointed note, Dr Francesca Luna-Rudin commented, “I would like to remind all of my fellow physicians that recommendations should be regarded as just that, a ‘recommendation.’ As doctors, we can use guidelines and recommendations in our practice, but if a new one is presented that does not make sense or would lead to harm based on our education and training, then we are not bound to follow it!”
A version of this article first appeared on Medscape.com.
Mounjaro Beats Ozempic, So Why Isn’t It More Popular?
This transcript has been edited for clarity.
It’s July, which means our hospital is filled with new interns, residents, and fellows all eager to embark on a new stage of their career. It’s an exciting time — a bit of a scary time — but it’s also the time when the medical strategies I’ve been taking for granted get called into question. At this point in the year, I tend to get a lot of “why” questions. Why did you order that test? Why did you suspect that diagnosis? Why did you choose that medication?
Meds are the hardest, I find. Sure, I can explain that I prescribed a glucagon-like peptide 1 (GLP-1) receptor agonist because the patient had diabetes and was overweight, and multiple studies show that this class of drug leads to weight loss and reduced mortality risk. But then I get the follow-up: Sure, but why THAT GLP-1 drug? Why did you pick semaglutide (Ozempic) over tirzepatide (Mounjaro)?
Here’s where I run out of good answers. Sometimes I choose a drug because that’s what the patient’s insurance has on their formulary. Sometimes it’s because it’s cheaper in general. Sometimes, it’s just force of habit. I know the correct dose, I have experience with the side effects — it’s comfortable.
What I can’t say is that I have solid evidence that one drug is superior to another, say from a randomized trial of semaglutide vs tirzepatide. I don’t have that evidence because that trial has never happened and, as I’ll explain in a minute, may never happen at all.
But we might have the next best thing. And the results may surprise you.
Why don’t we see more head-to-head trials of competitor drugs? The answer is pretty simple, honestly: risk management. For drugs that are on patent, like the GLP-1s, conducting a trial without the buy-in of the pharmaceutical company is simply too expensive — we can’t run a trial unless someone provides the drug for free. That gives the companies a lot of say in what trials get done, and it seems that most pharma companies have reached the same conclusion: A head-to-head trial is too risky. Be happy with the market share you have, and try to nibble away at the edges through good old-fashioned marketing.
But if you look at the data that are out there, you might wonder why Ozempic is the market leader. I mean, sure, it’s a heck of a weight loss drug. But the weight loss in the trials of Mounjaro was actually a bit higher. It’s worth noting here that tirzepatide (Mounjaro) is not just a GLP-1 receptor agonist; it is also a gastric inhibitory polypeptide agonist. 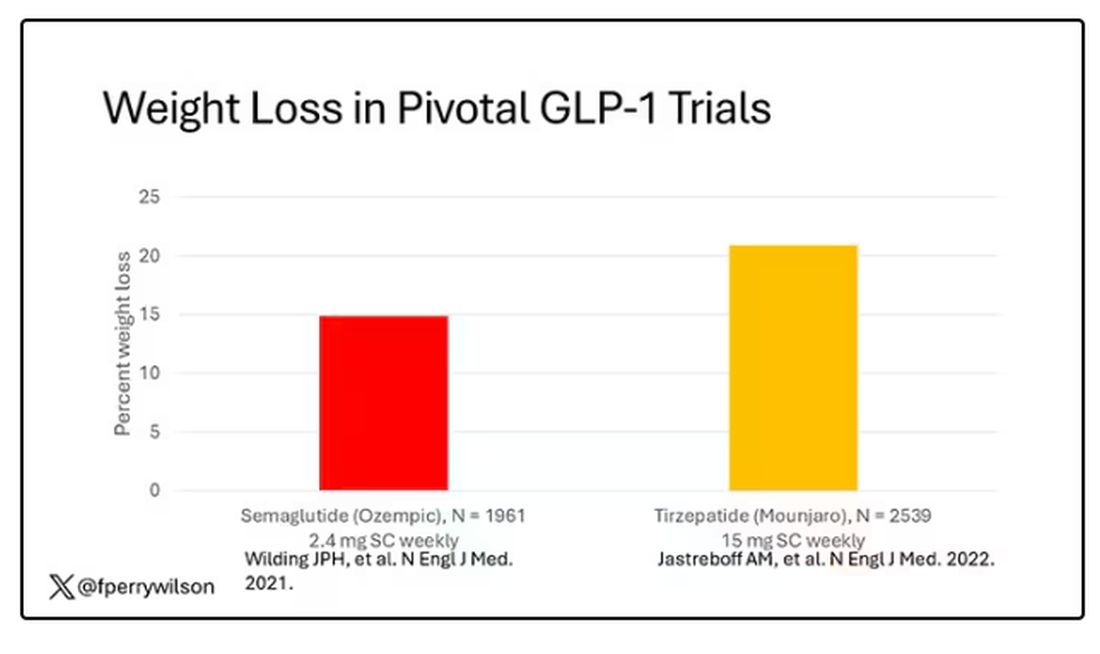
But it’s very hard to compare the results of a trial pitting Ozempic against placebo with a totally different trial pitting Mounjaro against placebo. You can always argue that the patients studied were just too different at baseline — an apples and oranges situation.
Newly published, a study appearing in JAMA Internal Medicine uses real-world data and propensity-score matching to turn oranges back into apples. I’ll walk you through it.
The data and analysis here come from Truveta, a collective of various US healthcare systems that share a broad swath of electronic health record data. Researchers identified 41,222 adults with overweight or obesity who were prescribed semaglutide or tirzepatide between May 2022 and September 2023.
You’d be tempted to just see which group lost more weight over time, but that is the apples and oranges problem. People prescribed Mounjaro were different from people who were prescribed Ozempic. There are a variety of factors to look at here, but the vibe is that the Mounjaro group seems healthier at baseline. They were younger and had less kidney disease, less hypertension, and less hyperlipidemia. They had higher incomes and were more likely to be White. They were also dramatically less likely to have diabetes. 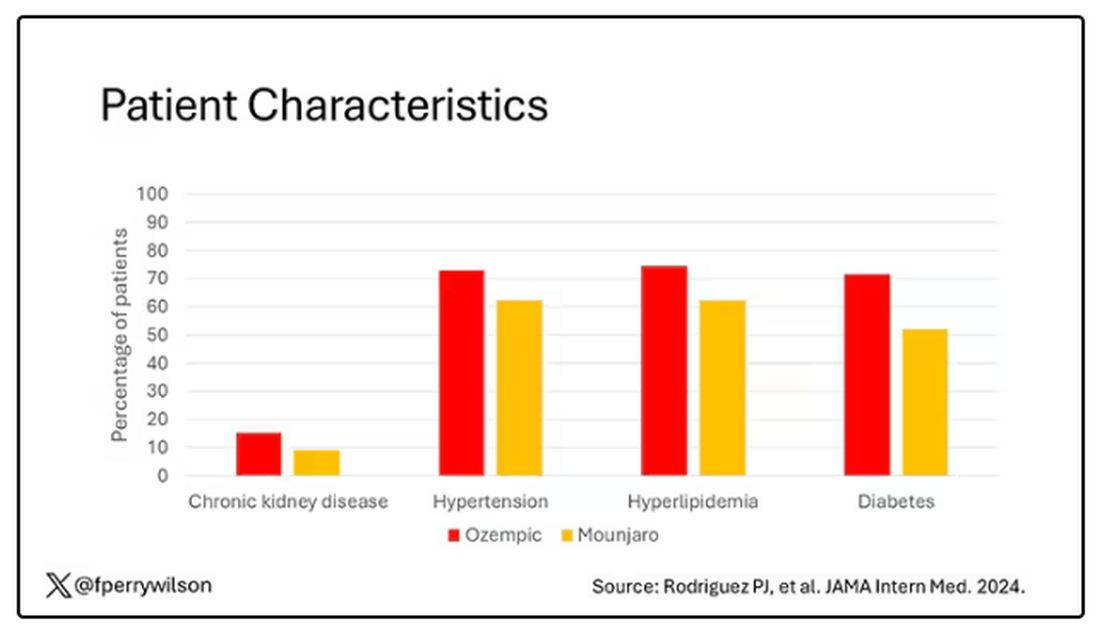
To account for this, the researchers used a statistical technique called propensity-score matching. Briefly, you create a model based on a variety of patient factors to predict who would be prescribed Ozempic and who would be prescribed Mounjaro. You then identify pairs of patients with similar probability (or propensity) of receiving, say, Ozempic, where one member of the pair got Ozempic and one got Mounjaro. Any unmatched individuals simply get dropped from the analysis.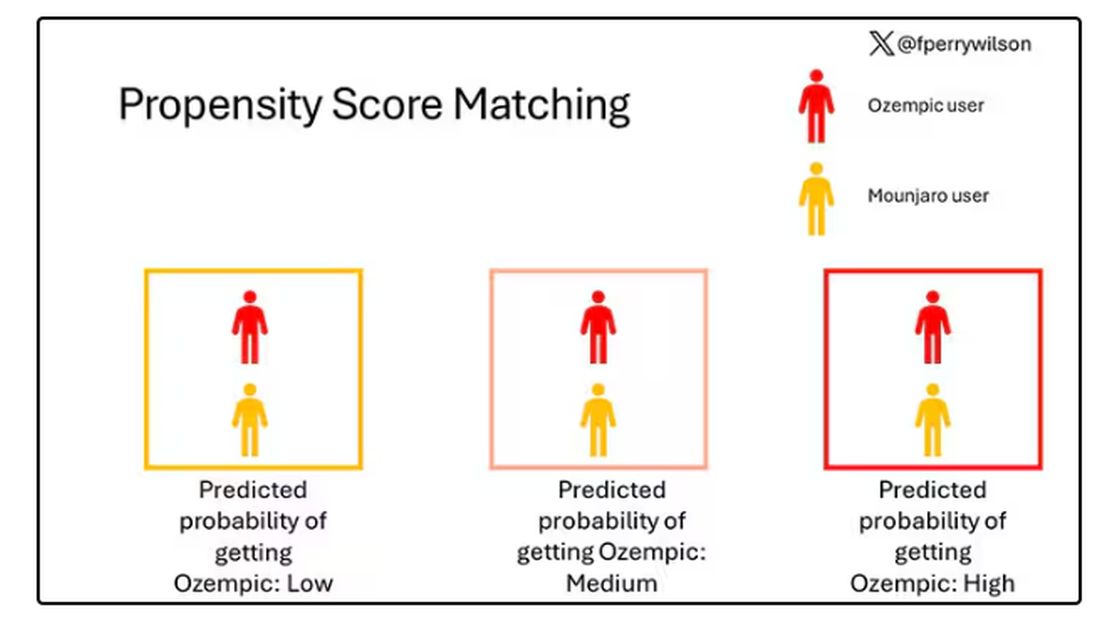
Thus, the researchers took the 41,222 individuals who started the analysis, of whom 9193 received Mounjaro, and identified the 9193 patients who got Ozempic that most closely matched the Mounjaro crowd. I know, it sounds confusing. But as an example, in the original dataset, 51.9% of those who got Mounjaro had diabetes compared with 71.5% of those who got Ozempic. Among the 9193 individuals who remained in the Ozempic group after matching, 52.1% had diabetes. By matching in this way, you balance your baseline characteristics. Turning apples into oranges. Or, maybe the better metaphor would be plucking the oranges out of a big pile of mostly apples.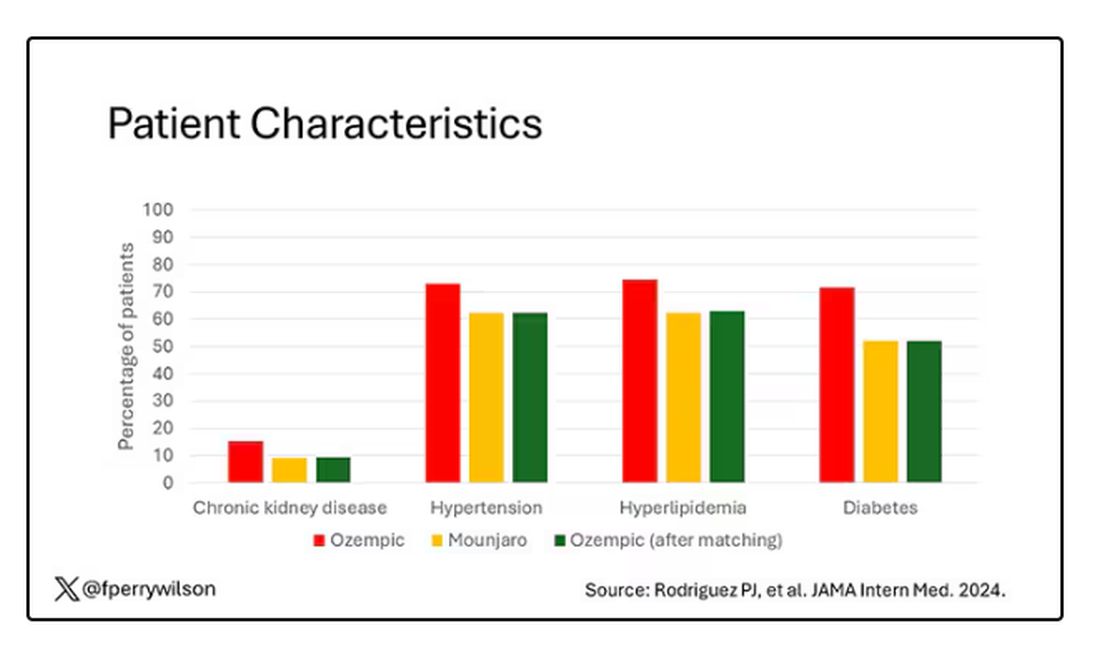
Once that’s done, we can go back to do what we wanted to do in the beginning, which is to look at the weight loss between the groups.
What I’m showing you here is the average percent change in body weight at 3, 6, and 12 months across the two drugs in the matched cohort. By a year out, you have basically 15% weight loss in the Mounjaro group compared with 8% or so in the Ozempic group. 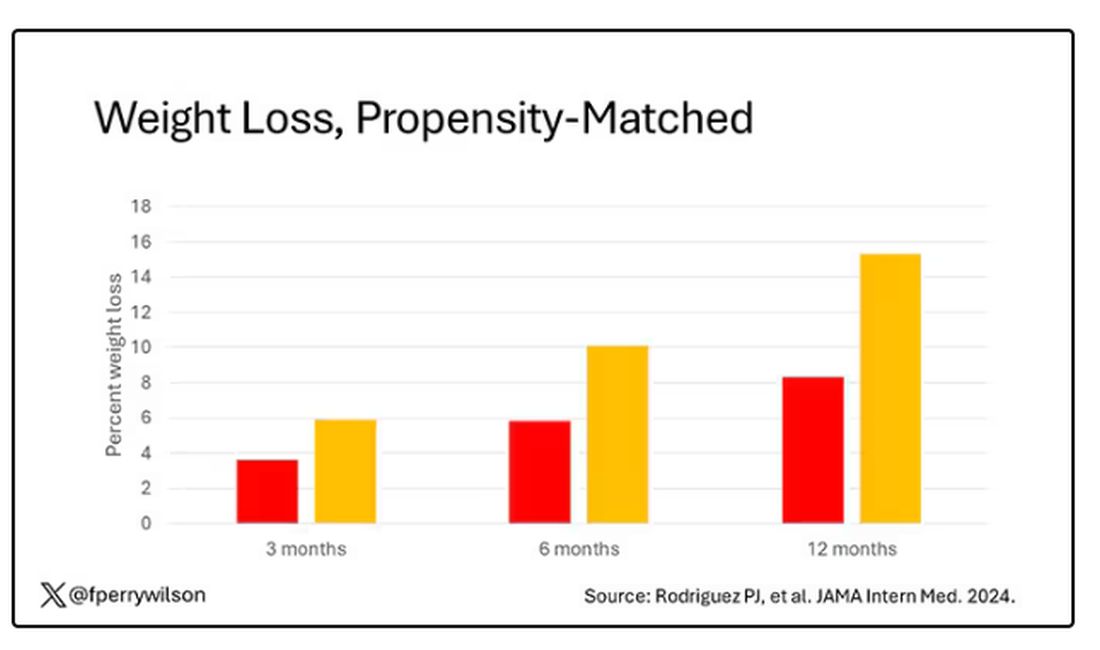
We can slice this a different way as well — asking what percent of people in each group achieve, say, 10% weight loss? This graph examines the percentage of each treatment group who hit that weight loss target over time. Mounjaro gets there faster.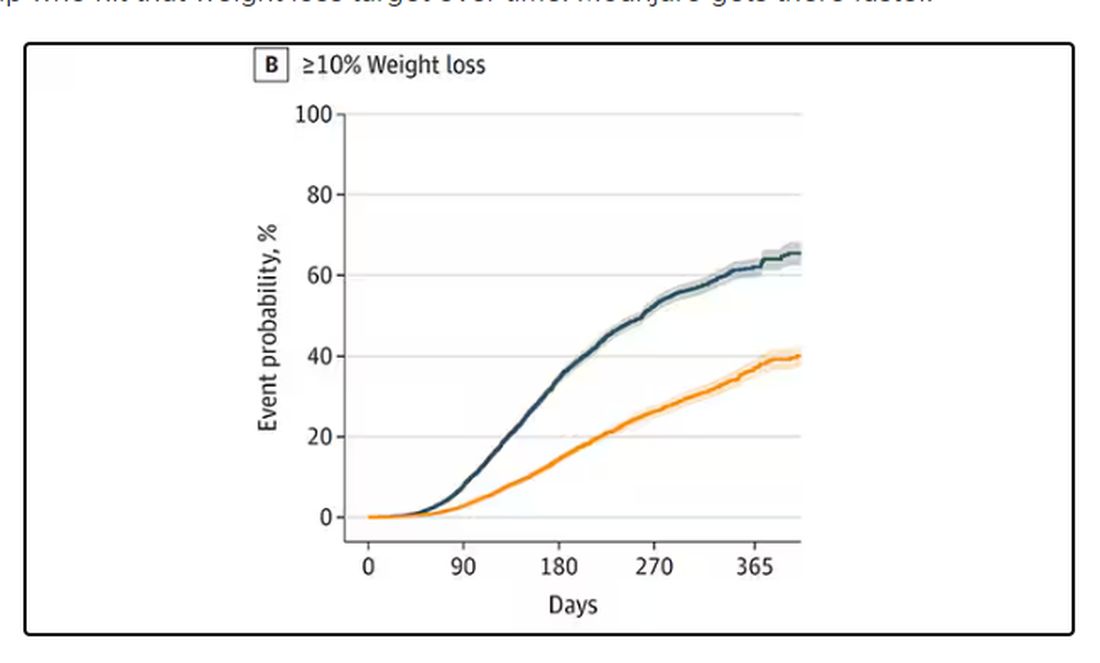
I should point out that this was a so-called “on treatment” analysis: If people stopped taking either of the drugs, they were no longer included in the study. That tends to make drugs like this appear better than they are because as time goes on, you may weed out the people who stop the drug owing to lack of efficacy or to side effects. But in a sensitivity analysis, the authors see what happens if they just treat people as if they were taking the drug for the entire year once they had it prescribed, and the results, while not as dramatic, were broadly similar. Mounjaro still came out on top.
Adverse events— stuff like gastroparesis and pancreatitis — were rare, but rates were similar between the two groups.
It’s great to see studies like this that leverage real world data and a solid statistical underpinning to give us providers actionable information. Is it 100% definitive? No. But, especially considering the clinical trial data, I don’t think I’m going out on a limb to say that Mounjaro seems to be the more effective weight loss agent. That said, we don’t actually live in a world where we can prescribe medications based on a silly little thing like which is the most effective. Especially given the cost of these agents — the patient’s insurance status is going to guide our prescription pen more than this study ever could. And of course, given the demand for this class of agents and the fact that both are actually quite effective, you may be best off prescribing whatever you can get your hands on.
But I’d like to see more of this. When I do have a choice of a medication, when costs and availability are similar, I’d like to be able to answer that question of “why did you choose that one?” with an evidence-based answer: “It’s better.”
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s July, which means our hospital is filled with new interns, residents, and fellows all eager to embark on a new stage of their career. It’s an exciting time — a bit of a scary time — but it’s also the time when the medical strategies I’ve been taking for granted get called into question. At this point in the year, I tend to get a lot of “why” questions. Why did you order that test? Why did you suspect that diagnosis? Why did you choose that medication?
Meds are the hardest, I find. Sure, I can explain that I prescribed a glucagon-like peptide 1 (GLP-1) receptor agonist because the patient had diabetes and was overweight, and multiple studies show that this class of drug leads to weight loss and reduced mortality risk. But then I get the follow-up: Sure, but why THAT GLP-1 drug? Why did you pick semaglutide (Ozempic) over tirzepatide (Mounjaro)?
Here’s where I run out of good answers. Sometimes I choose a drug because that’s what the patient’s insurance has on their formulary. Sometimes it’s because it’s cheaper in general. Sometimes, it’s just force of habit. I know the correct dose, I have experience with the side effects — it’s comfortable.
What I can’t say is that I have solid evidence that one drug is superior to another, say from a randomized trial of semaglutide vs tirzepatide. I don’t have that evidence because that trial has never happened and, as I’ll explain in a minute, may never happen at all.
But we might have the next best thing. And the results may surprise you.
Why don’t we see more head-to-head trials of competitor drugs? The answer is pretty simple, honestly: risk management. For drugs that are on patent, like the GLP-1s, conducting a trial without the buy-in of the pharmaceutical company is simply too expensive — we can’t run a trial unless someone provides the drug for free. That gives the companies a lot of say in what trials get done, and it seems that most pharma companies have reached the same conclusion: A head-to-head trial is too risky. Be happy with the market share you have, and try to nibble away at the edges through good old-fashioned marketing.
But if you look at the data that are out there, you might wonder why Ozempic is the market leader. I mean, sure, it’s a heck of a weight loss drug. But the weight loss in the trials of Mounjaro was actually a bit higher. It’s worth noting here that tirzepatide (Mounjaro) is not just a GLP-1 receptor agonist; it is also a gastric inhibitory polypeptide agonist. 
But it’s very hard to compare the results of a trial pitting Ozempic against placebo with a totally different trial pitting Mounjaro against placebo. You can always argue that the patients studied were just too different at baseline — an apples and oranges situation.
Newly published, a study appearing in JAMA Internal Medicine uses real-world data and propensity-score matching to turn oranges back into apples. I’ll walk you through it.
The data and analysis here come from Truveta, a collective of various US healthcare systems that share a broad swath of electronic health record data. Researchers identified 41,222 adults with overweight or obesity who were prescribed semaglutide or tirzepatide between May 2022 and September 2023.
You’d be tempted to just see which group lost more weight over time, but that is the apples and oranges problem. People prescribed Mounjaro were different from people who were prescribed Ozempic. There are a variety of factors to look at here, but the vibe is that the Mounjaro group seems healthier at baseline. They were younger and had less kidney disease, less hypertension, and less hyperlipidemia. They had higher incomes and were more likely to be White. They were also dramatically less likely to have diabetes. 
To account for this, the researchers used a statistical technique called propensity-score matching. Briefly, you create a model based on a variety of patient factors to predict who would be prescribed Ozempic and who would be prescribed Mounjaro. You then identify pairs of patients with similar probability (or propensity) of receiving, say, Ozempic, where one member of the pair got Ozempic and one got Mounjaro. Any unmatched individuals simply get dropped from the analysis.
Thus, the researchers took the 41,222 individuals who started the analysis, of whom 9193 received Mounjaro, and identified the 9193 patients who got Ozempic that most closely matched the Mounjaro crowd. I know, it sounds confusing. But as an example, in the original dataset, 51.9% of those who got Mounjaro had diabetes compared with 71.5% of those who got Ozempic. Among the 9193 individuals who remained in the Ozempic group after matching, 52.1% had diabetes. By matching in this way, you balance your baseline characteristics. Turning apples into oranges. Or, maybe the better metaphor would be plucking the oranges out of a big pile of mostly apples.
Once that’s done, we can go back to do what we wanted to do in the beginning, which is to look at the weight loss between the groups.
What I’m showing you here is the average percent change in body weight at 3, 6, and 12 months across the two drugs in the matched cohort. By a year out, you have basically 15% weight loss in the Mounjaro group compared with 8% or so in the Ozempic group. 
We can slice this a different way as well — asking what percent of people in each group achieve, say, 10% weight loss? This graph examines the percentage of each treatment group who hit that weight loss target over time. Mounjaro gets there faster.
I should point out that this was a so-called “on treatment” analysis: If people stopped taking either of the drugs, they were no longer included in the study. That tends to make drugs like this appear better than they are because as time goes on, you may weed out the people who stop the drug owing to lack of efficacy or to side effects. But in a sensitivity analysis, the authors see what happens if they just treat people as if they were taking the drug for the entire year once they had it prescribed, and the results, while not as dramatic, were broadly similar. Mounjaro still came out on top.
Adverse events— stuff like gastroparesis and pancreatitis — were rare, but rates were similar between the two groups.
It’s great to see studies like this that leverage real world data and a solid statistical underpinning to give us providers actionable information. Is it 100% definitive? No. But, especially considering the clinical trial data, I don’t think I’m going out on a limb to say that Mounjaro seems to be the more effective weight loss agent. That said, we don’t actually live in a world where we can prescribe medications based on a silly little thing like which is the most effective. Especially given the cost of these agents — the patient’s insurance status is going to guide our prescription pen more than this study ever could. And of course, given the demand for this class of agents and the fact that both are actually quite effective, you may be best off prescribing whatever you can get your hands on.
But I’d like to see more of this. When I do have a choice of a medication, when costs and availability are similar, I’d like to be able to answer that question of “why did you choose that one?” with an evidence-based answer: “It’s better.”
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s July, which means our hospital is filled with new interns, residents, and fellows all eager to embark on a new stage of their career. It’s an exciting time — a bit of a scary time — but it’s also the time when the medical strategies I’ve been taking for granted get called into question. At this point in the year, I tend to get a lot of “why” questions. Why did you order that test? Why did you suspect that diagnosis? Why did you choose that medication?
Meds are the hardest, I find. Sure, I can explain that I prescribed a glucagon-like peptide 1 (GLP-1) receptor agonist because the patient had diabetes and was overweight, and multiple studies show that this class of drug leads to weight loss and reduced mortality risk. But then I get the follow-up: Sure, but why THAT GLP-1 drug? Why did you pick semaglutide (Ozempic) over tirzepatide (Mounjaro)?
Here’s where I run out of good answers. Sometimes I choose a drug because that’s what the patient’s insurance has on their formulary. Sometimes it’s because it’s cheaper in general. Sometimes, it’s just force of habit. I know the correct dose, I have experience with the side effects — it’s comfortable.
What I can’t say is that I have solid evidence that one drug is superior to another, say from a randomized trial of semaglutide vs tirzepatide. I don’t have that evidence because that trial has never happened and, as I’ll explain in a minute, may never happen at all.
But we might have the next best thing. And the results may surprise you.
Why don’t we see more head-to-head trials of competitor drugs? The answer is pretty simple, honestly: risk management. For drugs that are on patent, like the GLP-1s, conducting a trial without the buy-in of the pharmaceutical company is simply too expensive — we can’t run a trial unless someone provides the drug for free. That gives the companies a lot of say in what trials get done, and it seems that most pharma companies have reached the same conclusion: A head-to-head trial is too risky. Be happy with the market share you have, and try to nibble away at the edges through good old-fashioned marketing.
But if you look at the data that are out there, you might wonder why Ozempic is the market leader. I mean, sure, it’s a heck of a weight loss drug. But the weight loss in the trials of Mounjaro was actually a bit higher. It’s worth noting here that tirzepatide (Mounjaro) is not just a GLP-1 receptor agonist; it is also a gastric inhibitory polypeptide agonist. 
But it’s very hard to compare the results of a trial pitting Ozempic against placebo with a totally different trial pitting Mounjaro against placebo. You can always argue that the patients studied were just too different at baseline — an apples and oranges situation.
Newly published, a study appearing in JAMA Internal Medicine uses real-world data and propensity-score matching to turn oranges back into apples. I’ll walk you through it.
The data and analysis here come from Truveta, a collective of various US healthcare systems that share a broad swath of electronic health record data. Researchers identified 41,222 adults with overweight or obesity who were prescribed semaglutide or tirzepatide between May 2022 and September 2023.
You’d be tempted to just see which group lost more weight over time, but that is the apples and oranges problem. People prescribed Mounjaro were different from people who were prescribed Ozempic. There are a variety of factors to look at here, but the vibe is that the Mounjaro group seems healthier at baseline. They were younger and had less kidney disease, less hypertension, and less hyperlipidemia. They had higher incomes and were more likely to be White. They were also dramatically less likely to have diabetes. 
To account for this, the researchers used a statistical technique called propensity-score matching. Briefly, you create a model based on a variety of patient factors to predict who would be prescribed Ozempic and who would be prescribed Mounjaro. You then identify pairs of patients with similar probability (or propensity) of receiving, say, Ozempic, where one member of the pair got Ozempic and one got Mounjaro. Any unmatched individuals simply get dropped from the analysis.
Thus, the researchers took the 41,222 individuals who started the analysis, of whom 9193 received Mounjaro, and identified the 9193 patients who got Ozempic that most closely matched the Mounjaro crowd. I know, it sounds confusing. But as an example, in the original dataset, 51.9% of those who got Mounjaro had diabetes compared with 71.5% of those who got Ozempic. Among the 9193 individuals who remained in the Ozempic group after matching, 52.1% had diabetes. By matching in this way, you balance your baseline characteristics. Turning apples into oranges. Or, maybe the better metaphor would be plucking the oranges out of a big pile of mostly apples.
Once that’s done, we can go back to do what we wanted to do in the beginning, which is to look at the weight loss between the groups.
What I’m showing you here is the average percent change in body weight at 3, 6, and 12 months across the two drugs in the matched cohort. By a year out, you have basically 15% weight loss in the Mounjaro group compared with 8% or so in the Ozempic group. 
We can slice this a different way as well — asking what percent of people in each group achieve, say, 10% weight loss? This graph examines the percentage of each treatment group who hit that weight loss target over time. Mounjaro gets there faster.
I should point out that this was a so-called “on treatment” analysis: If people stopped taking either of the drugs, they were no longer included in the study. That tends to make drugs like this appear better than they are because as time goes on, you may weed out the people who stop the drug owing to lack of efficacy or to side effects. But in a sensitivity analysis, the authors see what happens if they just treat people as if they were taking the drug for the entire year once they had it prescribed, and the results, while not as dramatic, were broadly similar. Mounjaro still came out on top.
Adverse events— stuff like gastroparesis and pancreatitis — were rare, but rates were similar between the two groups.
It’s great to see studies like this that leverage real world data and a solid statistical underpinning to give us providers actionable information. Is it 100% definitive? No. But, especially considering the clinical trial data, I don’t think I’m going out on a limb to say that Mounjaro seems to be the more effective weight loss agent. That said, we don’t actually live in a world where we can prescribe medications based on a silly little thing like which is the most effective. Especially given the cost of these agents — the patient’s insurance status is going to guide our prescription pen more than this study ever could. And of course, given the demand for this class of agents and the fact that both are actually quite effective, you may be best off prescribing whatever you can get your hands on.
But I’d like to see more of this. When I do have a choice of a medication, when costs and availability are similar, I’d like to be able to answer that question of “why did you choose that one?” with an evidence-based answer: “It’s better.”
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Does Medicare Enrollment Raise Diabetes Medication Costs?
TOPLINE:
Reaching age 65 years and enrolling in Medicare is associated with a $23 increase in quarterly out-of-pocket costs for type 2 diabetes (T2D) medications. Medication usage decreased by 5.3%, with a notable shift toward more expensive insulin use.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using 2012-2020 prescription drug claims data from the TriNetX Diamond Network.
- A total of 129,997 individuals diagnosed with T2D were included, with claims observed both before and after age 65 years.
- The primary outcome was patient out-of-pocket costs for T2D drugs per quarter, adjusted to 2020 dollars.
- Drugs measured included biguanides (metformin), sulfonylureas, thiazolidinediones, insulin, dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, sodium-glucose cotransporter 2 (SGLT-2 inhibitors), and amylin analogs, among others.
- Regression discontinuity design was used to examine the outcomes, adjusting for differential linear quarterly time trends, year fixed effects, and utilization composition and intensity.
TAKEAWAY:
- Reaching age 65 years was associated with an increase of $23.04 in mean quarterly out-of-pocket costs for T2D drugs (95% confidence interval [CI], $19.86-$26.22).
- The 95th percentile of out-of-pocket spending increased by $56.36 (95% CI, $51.48-$61.23) after utilization adjustment.
- T2D medication usage decreased by 5.3% at age 65 years, from 3.40 claims per quarter to 3.22 claims per quarter.
- Higher out-of-pockets were associated with insulin use, DPP-4 inhibitors, GLP-1s, and SGLT2 inhibitors.
IN PRACTICE:
“Our results have important implications for the provisions of the Inflation Reduction Act, many of which aim to reduce these costs. Reduced patient cost burden will improve adherence and the management of type 2 diabetes, likely leading to reductions in T2D complications,” wrote the authors of the study.
SOURCE:
The study was led by Douglas Barthold, PhD, Jing Li, MA, PhD, and Anirban Basu, MS, PhD, at the Comparative Health Outcomes, Policy, and Economics Institute, School of Pharmacy, University of Washington, Seattle. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s limitations include the possibility that not all claims of an individual were observed, as TriNetX claims data may not capture individuals who leave the healthcare system or have inaccurate or changing diagnoses. Additionally, the data lack individual-level insurance characteristics. The assumption that individuals transition to Medicare at age 65 years may not be true for all participants. The study also lacks clinical information regarding the severity of T2D, which could influence medication usage and out-of-pocket costs.
DISCLOSURES:
The study was supported by grants from the National Institute on Aging (NIA) and the University of Washington’s Population Health Initiative, Student Technology Fee program, and Provost’s office. Dr. Barthold and Dr. Li received grants from the NIA. Dr. Basu reported receiving personal fees from Salutis Consulting LLC outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Reaching age 65 years and enrolling in Medicare is associated with a $23 increase in quarterly out-of-pocket costs for type 2 diabetes (T2D) medications. Medication usage decreased by 5.3%, with a notable shift toward more expensive insulin use.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using 2012-2020 prescription drug claims data from the TriNetX Diamond Network.
- A total of 129,997 individuals diagnosed with T2D were included, with claims observed both before and after age 65 years.
- The primary outcome was patient out-of-pocket costs for T2D drugs per quarter, adjusted to 2020 dollars.
- Drugs measured included biguanides (metformin), sulfonylureas, thiazolidinediones, insulin, dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, sodium-glucose cotransporter 2 (SGLT-2 inhibitors), and amylin analogs, among others.
- Regression discontinuity design was used to examine the outcomes, adjusting for differential linear quarterly time trends, year fixed effects, and utilization composition and intensity.
TAKEAWAY:
- Reaching age 65 years was associated with an increase of $23.04 in mean quarterly out-of-pocket costs for T2D drugs (95% confidence interval [CI], $19.86-$26.22).
- The 95th percentile of out-of-pocket spending increased by $56.36 (95% CI, $51.48-$61.23) after utilization adjustment.
- T2D medication usage decreased by 5.3% at age 65 years, from 3.40 claims per quarter to 3.22 claims per quarter.
- Higher out-of-pockets were associated with insulin use, DPP-4 inhibitors, GLP-1s, and SGLT2 inhibitors.
IN PRACTICE:
“Our results have important implications for the provisions of the Inflation Reduction Act, many of which aim to reduce these costs. Reduced patient cost burden will improve adherence and the management of type 2 diabetes, likely leading to reductions in T2D complications,” wrote the authors of the study.
SOURCE:
The study was led by Douglas Barthold, PhD, Jing Li, MA, PhD, and Anirban Basu, MS, PhD, at the Comparative Health Outcomes, Policy, and Economics Institute, School of Pharmacy, University of Washington, Seattle. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s limitations include the possibility that not all claims of an individual were observed, as TriNetX claims data may not capture individuals who leave the healthcare system or have inaccurate or changing diagnoses. Additionally, the data lack individual-level insurance characteristics. The assumption that individuals transition to Medicare at age 65 years may not be true for all participants. The study also lacks clinical information regarding the severity of T2D, which could influence medication usage and out-of-pocket costs.
DISCLOSURES:
The study was supported by grants from the National Institute on Aging (NIA) and the University of Washington’s Population Health Initiative, Student Technology Fee program, and Provost’s office. Dr. Barthold and Dr. Li received grants from the NIA. Dr. Basu reported receiving personal fees from Salutis Consulting LLC outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Reaching age 65 years and enrolling in Medicare is associated with a $23 increase in quarterly out-of-pocket costs for type 2 diabetes (T2D) medications. Medication usage decreased by 5.3%, with a notable shift toward more expensive insulin use.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using 2012-2020 prescription drug claims data from the TriNetX Diamond Network.
- A total of 129,997 individuals diagnosed with T2D were included, with claims observed both before and after age 65 years.
- The primary outcome was patient out-of-pocket costs for T2D drugs per quarter, adjusted to 2020 dollars.
- Drugs measured included biguanides (metformin), sulfonylureas, thiazolidinediones, insulin, dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, sodium-glucose cotransporter 2 (SGLT-2 inhibitors), and amylin analogs, among others.
- Regression discontinuity design was used to examine the outcomes, adjusting for differential linear quarterly time trends, year fixed effects, and utilization composition and intensity.
TAKEAWAY:
- Reaching age 65 years was associated with an increase of $23.04 in mean quarterly out-of-pocket costs for T2D drugs (95% confidence interval [CI], $19.86-$26.22).
- The 95th percentile of out-of-pocket spending increased by $56.36 (95% CI, $51.48-$61.23) after utilization adjustment.
- T2D medication usage decreased by 5.3% at age 65 years, from 3.40 claims per quarter to 3.22 claims per quarter.
- Higher out-of-pockets were associated with insulin use, DPP-4 inhibitors, GLP-1s, and SGLT2 inhibitors.
IN PRACTICE:
“Our results have important implications for the provisions of the Inflation Reduction Act, many of which aim to reduce these costs. Reduced patient cost burden will improve adherence and the management of type 2 diabetes, likely leading to reductions in T2D complications,” wrote the authors of the study.
SOURCE:
The study was led by Douglas Barthold, PhD, Jing Li, MA, PhD, and Anirban Basu, MS, PhD, at the Comparative Health Outcomes, Policy, and Economics Institute, School of Pharmacy, University of Washington, Seattle. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s limitations include the possibility that not all claims of an individual were observed, as TriNetX claims data may not capture individuals who leave the healthcare system or have inaccurate or changing diagnoses. Additionally, the data lack individual-level insurance characteristics. The assumption that individuals transition to Medicare at age 65 years may not be true for all participants. The study also lacks clinical information regarding the severity of T2D, which could influence medication usage and out-of-pocket costs.
DISCLOSURES:
The study was supported by grants from the National Institute on Aging (NIA) and the University of Washington’s Population Health Initiative, Student Technology Fee program, and Provost’s office. Dr. Barthold and Dr. Li received grants from the NIA. Dr. Basu reported receiving personal fees from Salutis Consulting LLC outside the submitted work. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Light During Nighttime Linked to Diabetes Risk
Concerned about your patient’s type 2 diabetes risk? Along with the usual preventive strategies — like diet and exercise and, when appropriate, glucagon-like peptide 1 (GLP-1) agonists — there’s another simple, no-risk strategy that just might help: Turning off the light at night.
A study in The Lancet found that people who were exposed to the most light between 12:30 a.m. and 6 a.m. were 1.5 times more likely to develop diabetes than those who remained in darkness during that time frame.
The study builds on growing evidence linking nighttime light exposure to type 2 diabetes risk. But unlike previous large studies that relied on satellite data of outdoor light levels (an indirect measure of light exposure), the recent study looked at personal light exposure — that is, light measured directly on individuals — as recorded by a wrist-worn sensor.
“Those previous studies likely underestimated the effect,” said study author Andrew Phillips, PhD, professor of sleep health at Flinders University in Adelaide, Australia, “since they did not capture indoor light environments.”
Using data from 85,000 participants from the UK Biobank, the recent study is the largest to date linking diabetes risk to personal light exposure at night.
“This is really a phenomenal study,” said Courtney Peterson, PhD, a scientist at the University of Alabama at Birmingham’s Diabetes Research Center, who was not involved in the study. “This is the first large-scale study we have looking at people’s light exposure patterns and linking it to their long-term health.”
What the Study Showed
The participants wore the light sensors for a week, recording day and night light from all sources — whether from sunlight, lamps, streetlights, or digital screens. The researchers then tracked participants for 8 years.
“About half of the people that we looked at had very dim levels of light at night, so less than 1 lux — that basically means less than candlelight,” said Dr. Phillips. “They were the people who were protected against type 2 diabetes.”
Participants in the top 10% of light exposure — who were exposed to about 48 lux , or the equivalent of relatively dim overhead lighting — were 1.5 times more likely to develop diabetes than those in the dark. That’s about the risk increase you’d get from having a family history of type 2 diabetes, the researchers said.
Even when they controlled for factors like socioeconomic status, smoking, diet, exercise, and shift work, “we still found there was this very strong relationship between light exposure and risk of type 2 diabetes,” said Dr. Phillips.
How Light at Night May Increase Diabetes Risk
The results are not entirely surprising, said endocrinologist Susanne Miedlich, MD, a professor at the University of Rochester Medical Center, Rochester, New York, who was not involved in the study.
Light at night can disrupt the circadian rhythm, or your body’s internal 24-hour cycle. And scientists have long known that circadian rhythm is important for all kinds of biologic processes, including how the body manages blood sugar.
One’s internal clock regulates food intake, sugar absorption, and the release of insulin. Dysregulation in the circadian rhythm is associated with insulin resistance, a precursor to type 2 diabetes.
Dr. Phillips speculated that the sleep hormone melatonin also plays a role.
“Melatonin does a lot of things, but one of the things that it does is it manages our glucose and our insulin responses,” Dr. Phillips said. “So if you’re chronically getting light exposure at night, that’s reducing a level of melatonin that, in the long term, could lead to poor metabolic outcomes.”
Previous studies have explored melatonin supplementation to help manage diabetes. “However, while melatonin clearly regulates circadian rhythms, its utility as a drug to prevent diabetes has not really panned out thus far,” Dr. Miedlich said.
Takeaways
Interventional studies are needed to confirm whether strategies like powering down screens, turning off lights, or using blackout curtains could reduce diabetes risk.
That said, “there’s no reason not to tell people to get healthy light exposure patterns and sleep, especially in the context of diabetes,” said Dr. Phillips.
Other known strategies for reducing diabetes risk include intensive lifestyle programs, which reduce risk by up to 58%, and GLP-1 agonists.
“Probably a GLP-1 agonist is going to be more effective,” Dr. Peterson said. “But this is still a fairly large effect without having to go through the expense of buying a GLP-1 or losing a lot of weight or making a big lifestyle change.”
A version of this article first appeared on Medscape.com.
Concerned about your patient’s type 2 diabetes risk? Along with the usual preventive strategies — like diet and exercise and, when appropriate, glucagon-like peptide 1 (GLP-1) agonists — there’s another simple, no-risk strategy that just might help: Turning off the light at night.
A study in The Lancet found that people who were exposed to the most light between 12:30 a.m. and 6 a.m. were 1.5 times more likely to develop diabetes than those who remained in darkness during that time frame.
The study builds on growing evidence linking nighttime light exposure to type 2 diabetes risk. But unlike previous large studies that relied on satellite data of outdoor light levels (an indirect measure of light exposure), the recent study looked at personal light exposure — that is, light measured directly on individuals — as recorded by a wrist-worn sensor.
“Those previous studies likely underestimated the effect,” said study author Andrew Phillips, PhD, professor of sleep health at Flinders University in Adelaide, Australia, “since they did not capture indoor light environments.”
Using data from 85,000 participants from the UK Biobank, the recent study is the largest to date linking diabetes risk to personal light exposure at night.
“This is really a phenomenal study,” said Courtney Peterson, PhD, a scientist at the University of Alabama at Birmingham’s Diabetes Research Center, who was not involved in the study. “This is the first large-scale study we have looking at people’s light exposure patterns and linking it to their long-term health.”
What the Study Showed
The participants wore the light sensors for a week, recording day and night light from all sources — whether from sunlight, lamps, streetlights, or digital screens. The researchers then tracked participants for 8 years.
“About half of the people that we looked at had very dim levels of light at night, so less than 1 lux — that basically means less than candlelight,” said Dr. Phillips. “They were the people who were protected against type 2 diabetes.”
Participants in the top 10% of light exposure — who were exposed to about 48 lux , or the equivalent of relatively dim overhead lighting — were 1.5 times more likely to develop diabetes than those in the dark. That’s about the risk increase you’d get from having a family history of type 2 diabetes, the researchers said.
Even when they controlled for factors like socioeconomic status, smoking, diet, exercise, and shift work, “we still found there was this very strong relationship between light exposure and risk of type 2 diabetes,” said Dr. Phillips.
How Light at Night May Increase Diabetes Risk
The results are not entirely surprising, said endocrinologist Susanne Miedlich, MD, a professor at the University of Rochester Medical Center, Rochester, New York, who was not involved in the study.
Light at night can disrupt the circadian rhythm, or your body’s internal 24-hour cycle. And scientists have long known that circadian rhythm is important for all kinds of biologic processes, including how the body manages blood sugar.
One’s internal clock regulates food intake, sugar absorption, and the release of insulin. Dysregulation in the circadian rhythm is associated with insulin resistance, a precursor to type 2 diabetes.
Dr. Phillips speculated that the sleep hormone melatonin also plays a role.
“Melatonin does a lot of things, but one of the things that it does is it manages our glucose and our insulin responses,” Dr. Phillips said. “So if you’re chronically getting light exposure at night, that’s reducing a level of melatonin that, in the long term, could lead to poor metabolic outcomes.”
Previous studies have explored melatonin supplementation to help manage diabetes. “However, while melatonin clearly regulates circadian rhythms, its utility as a drug to prevent diabetes has not really panned out thus far,” Dr. Miedlich said.
Takeaways
Interventional studies are needed to confirm whether strategies like powering down screens, turning off lights, or using blackout curtains could reduce diabetes risk.
That said, “there’s no reason not to tell people to get healthy light exposure patterns and sleep, especially in the context of diabetes,” said Dr. Phillips.
Other known strategies for reducing diabetes risk include intensive lifestyle programs, which reduce risk by up to 58%, and GLP-1 agonists.
“Probably a GLP-1 agonist is going to be more effective,” Dr. Peterson said. “But this is still a fairly large effect without having to go through the expense of buying a GLP-1 or losing a lot of weight or making a big lifestyle change.”
A version of this article first appeared on Medscape.com.
Concerned about your patient’s type 2 diabetes risk? Along with the usual preventive strategies — like diet and exercise and, when appropriate, glucagon-like peptide 1 (GLP-1) agonists — there’s another simple, no-risk strategy that just might help: Turning off the light at night.
A study in The Lancet found that people who were exposed to the most light between 12:30 a.m. and 6 a.m. were 1.5 times more likely to develop diabetes than those who remained in darkness during that time frame.
The study builds on growing evidence linking nighttime light exposure to type 2 diabetes risk. But unlike previous large studies that relied on satellite data of outdoor light levels (an indirect measure of light exposure), the recent study looked at personal light exposure — that is, light measured directly on individuals — as recorded by a wrist-worn sensor.
“Those previous studies likely underestimated the effect,” said study author Andrew Phillips, PhD, professor of sleep health at Flinders University in Adelaide, Australia, “since they did not capture indoor light environments.”
Using data from 85,000 participants from the UK Biobank, the recent study is the largest to date linking diabetes risk to personal light exposure at night.
“This is really a phenomenal study,” said Courtney Peterson, PhD, a scientist at the University of Alabama at Birmingham’s Diabetes Research Center, who was not involved in the study. “This is the first large-scale study we have looking at people’s light exposure patterns and linking it to their long-term health.”
What the Study Showed
The participants wore the light sensors for a week, recording day and night light from all sources — whether from sunlight, lamps, streetlights, or digital screens. The researchers then tracked participants for 8 years.
“About half of the people that we looked at had very dim levels of light at night, so less than 1 lux — that basically means less than candlelight,” said Dr. Phillips. “They were the people who were protected against type 2 diabetes.”
Participants in the top 10% of light exposure — who were exposed to about 48 lux , or the equivalent of relatively dim overhead lighting — were 1.5 times more likely to develop diabetes than those in the dark. That’s about the risk increase you’d get from having a family history of type 2 diabetes, the researchers said.
Even when they controlled for factors like socioeconomic status, smoking, diet, exercise, and shift work, “we still found there was this very strong relationship between light exposure and risk of type 2 diabetes,” said Dr. Phillips.
How Light at Night May Increase Diabetes Risk
The results are not entirely surprising, said endocrinologist Susanne Miedlich, MD, a professor at the University of Rochester Medical Center, Rochester, New York, who was not involved in the study.
Light at night can disrupt the circadian rhythm, or your body’s internal 24-hour cycle. And scientists have long known that circadian rhythm is important for all kinds of biologic processes, including how the body manages blood sugar.
One’s internal clock regulates food intake, sugar absorption, and the release of insulin. Dysregulation in the circadian rhythm is associated with insulin resistance, a precursor to type 2 diabetes.
Dr. Phillips speculated that the sleep hormone melatonin also plays a role.
“Melatonin does a lot of things, but one of the things that it does is it manages our glucose and our insulin responses,” Dr. Phillips said. “So if you’re chronically getting light exposure at night, that’s reducing a level of melatonin that, in the long term, could lead to poor metabolic outcomes.”
Previous studies have explored melatonin supplementation to help manage diabetes. “However, while melatonin clearly regulates circadian rhythms, its utility as a drug to prevent diabetes has not really panned out thus far,” Dr. Miedlich said.
Takeaways
Interventional studies are needed to confirm whether strategies like powering down screens, turning off lights, or using blackout curtains could reduce diabetes risk.
That said, “there’s no reason not to tell people to get healthy light exposure patterns and sleep, especially in the context of diabetes,” said Dr. Phillips.
Other known strategies for reducing diabetes risk include intensive lifestyle programs, which reduce risk by up to 58%, and GLP-1 agonists.
“Probably a GLP-1 agonist is going to be more effective,” Dr. Peterson said. “But this is still a fairly large effect without having to go through the expense of buying a GLP-1 or losing a lot of weight or making a big lifestyle change.”
A version of this article first appeared on Medscape.com.
FROM THE LANCET