User login
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
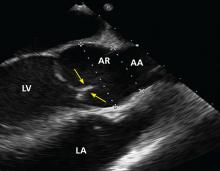
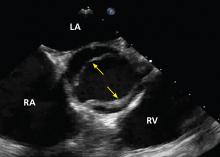
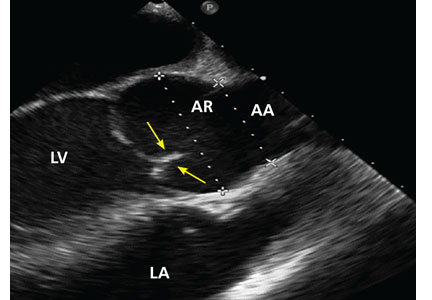
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
Bicuspid aortic valve may initially be asymptomatic, but it is associated with progressive valvular and aortic abnormalities that can lead to chronic heart failure and sudden death. Regular monitoring is required with an eye toward surgery when indicated.
This article reviews inheritance patterns and conditions associated with bicuspid aortic valve. We discuss diagnosis, management, and monitoring, and offer surgical recommendations. Special guidance for dental procedures, pregnancy, and athletes is also provided.
A YOUNG MAN WITH PALPITATIONS AND A MURMUR
A 34-year-old man presented to an outpatient clinic with occasional palpitations over the past several months. He reported that he had been diagnosed with a murmur as a child but had received no further testing.
Physical examination at this time revealed a faint systolic crescendo-decrescendo murmur along the right sternal border without radiation to the carotid arteries or to the apex. Transthoracic echocardiography (TTE) showed a bicuspid aortic valve with fusion of the right and left coronary cusps, with no aortic valve stenosis or insufficiency. There was mild dilation of the aortic root, but the mid-ascending aorta could not be evaluated because of limited acoustic windows.
Is further diagnostic testing needed, and if so, what? May he participate in exertional physical activity? Does his newborn son need evaluation?
ABNORMALITIES OCCUR DURING EMBRYOGENESIS
Bicuspid aortic valve develops because of abnormal valvulogenesis. Adjacent cusps fail to separate from each other, resulting in only 2 cusps, with 1 usually larger than the other. Morphology varies according to which commissures are fused.1
Bicuspid aortic valve is associated with abnormalities in the coronary artery anatomy in about 2% of patients, including anomalous origins of the coronary arteries and upwardly displaced coronary ostia.2 Such features need to be considered before surgical intervention.
Bicuspid aortic valve can be found in 1% to 2% of the general population, with a male-to-female predominance of 3:1.1,3,4 It is one of the most common congenital cardiac malformations and is the leading congenital cause of aortic valve stenosis.1,3 However, routine screening of newborns for the condition is not recommended, and most cases are diagnosed incidentally.
GENETIC FACTORS PROMINENT
Bicuspid aortic valve is thought to be primarily inherited in an autosomal-dominant pattern, but there is evidence of genetic heterogeneity, and the pattern may be variable.5,6
No single gene responsible for bicuspid aortic valve has been identified. The condition may occur as a component of different pleiotropic genetic syndromes such as Loeys-Dietz, DiGeorge, and Marfan syndromes,7,8 as well as in patients with Turner syndrome and Williams syndrome.8–11 It also commonly coexists with other congenital heart diseases, including ventricular septal defect, isolated aortic arch obstruction, and patent ductus arteriosus.9
Studies have found a 15% rate of familial clustering.6,12 In a study of 142 patients with bicuspid aortic valve, 20% of first-degree relatives had some cardiac abnormality found by screening, of whom 68% had bicuspid aortic valve. Of these, 71% were newly detected abnormalities.13
CHARACTERISTIC CLICK AND MURMUR
Physical examination findings of a functionally normal bicuspid aortic valve include a systolic ejection click followed by an early peaking systolic murmur at the apex or left lower sternal border. With progression of aortic stenosis, the ejection murmur has a harsher sound, with later peaking, and the S2 sound diminishes or becomes inaudible.14 If aortic regurgitation is present, a diastolic decrescendo murmur is heard best at the left lower sternal border.
DISEASE PROGRESSION
Although bicuspid aortic valve is typically asymptomatic at first, it is commonly associated with progressive valvulopathy and thoracic aortic disease.1,3,4,15 It can lead to chronic heart failure and increase the risk of acute aortic syndromes and sudden cardiac death.15
Michelena et al16 studied 212 cases of asymptomatic bicuspid aortic valve. Although the survival rate 20 years after diagnosis was the same as for an age-matched cohort in the general population, the frequency of adverse cardiovascular events and surgical interventions was higher.
Aortic stenosis progresses rapidly
Aortic stenosis associated with a bicuspid aortic valve tends to affect younger patients and progress more rapidly than when associated with a tricuspid valve.17
In a study of 542 patients with congenital bicuspid aortic valve undergoing aortic valve replacement,3 75% had isolated aortic stenosis, 10% had aortic stenosis with some degree of aortic insufficiency, and 13% had isolated aortic insufficiency. Given the tendency of aortic stenosis to progress rapidly, early surgery is often pursued.17,18
Aneurysmal disease is common
The thoracic aorta is at increased risk of aneurysmal disease, coarctation, and dissection in patients with a bicuspid aortic valve.1,6,15
Michelena et al16 reported that in patients without an aneurysm at the time of bicuspid aortic valve diagnosis, the 25-year risk of aneurysm formation was approximately 26%. In patients with an aneurysm at the time of diagnosis, the 15-year risk of aortic surgery after the diagnosis of aneurysm was about 46% and the risk of aortic dissection after aneurysm diagnosis was 7%.15 Compared with the general population, the age-adjusted relative risk of aortic aneurysm in patients with bicuspid aortic valve was 86.2, and that of aortic dissection was 8.4. Although the absolute incidence of dissection is low in these patients, it is markedly higher than in the general population, particularly in older patients (age > 50) and those with an aneurysm at the time of diagnosis.15
The risk of infective endocarditis
Patients with bicuspid aortic valve are highly prone to infective endocarditis for reasons that remain poorly understood. The pathogens in most cases are staphylococci or viridans streptococci.19 Patients with infective endocarditis typically require emergency surgery. Complications including valvular abscess, myocardial abscess, and overt heart failure are common.19
Lamas and Eykyn20 studied 408 cases of native valve endocarditis; in 12.3%, the patient had a bicuspid aortic valve. In this subset, all were male, the mean age was 39 at diagnosis, 82% needed surgery, and the death rate was 14%.
Patients with bicuspid aortic valve do not routinely need antibiotics before dental and surgical procedures, but if they have had endocarditis in the past, they need antibiotics to prevent a recurrence.21
REGULAR MONITORING NEEDED
Because complications may be life-threatening, early detection of progressive disease by regular screening is critical. Echocardiographic evaluation of valvular function, ventricular dimensions and function, and diameter of the aortic root and ascending aorta should be performed in every patient with bicuspid aortic valve. If initial imaging is normal and there is no aortic dilation, imaging should be repeated every 5 to 10 years. If any abnormality is found, repeat imaging is needed every year.22
Magnetic resonance imaging (MRI) or computed tomographic (CT) angiography may be required to better assess the aorta for patients requiring a surgical intervention, or when aortic dimensions are not clearly visualized on TTE. MRI has 2 advantages over CT angiography: it poses no radiation risk, and it provides more information on left ventricular function and dimensions, in addition to valve assessment.23,24
No published study has compared MRI or CT angiography and transesophageal echocardiography (TEE), but in a study of 174 patients with dilated aortic root, combined TTE and TEE detected aortic valve morphology accurately in 98% of cases. As TEE is more invasive, it is not recommended for regular surveillance (Figures 1 and 2).25
FAMILY SCREENING RECOMMENDED
Close relatives should be evaluated for aortic valve and thoracic aortic disease.12,13,23,26
The American College of Cardiology (ACC) and the American Heart Association (AHA), backed by radiologic and cardiovascular associations, concur in recommending echocardiographic screening and routine screening of the thoracic aorta for aortic root dilation in first-degree relatives (ie, siblings, parents, and children) of patients with bicuspid aortic valve (class I recommendation).22,27,28
A comprehensive physical examination is recommended for family members in addition to TTE, with careful assessment of the aortic valve in short and long axes, and of the aortic root.14 If the aorta cannot be adequately evaluated with TTE, further assessment should be pursued with CT angiography or MRI.
EXERCISE RESTRICTIONS
The 2015 ACC/AHA guidelines for competitive athletes with cardiovascular abnormalities recommend annual screening with TTE or MRI angiography for athletes with bicuspid aortic valve and coexisting dilation of the ascending aorta (aortic diameter 40–42 mm in men and 36–39 mm in women) (class I recommendation, level of evidence C).29
Athletes with a bicuspid aortic valve and a normal aortic root and ascending aorta may participate in all competitive activities.29 However, those with a dilated aorta should avoid strenuous activities because of the increased risk of rupture.30 The ACC/AHA recommendations29 depend on the diameter of the ascending aorta and the nature of the sport:
- For an aortic diameter 40 to 42 mm in men or 36 to 39 mm in women, and no features of connective tissue disease or familial thoracic ascending aortic syndrome, low- and moderate-intensity sports with a low likelihood of significant body contact may be considered; consider avoiding intense weight training (class IIb, level of evidence C)
- For an aortic diameter 43 to 45 mm, low-intensity sports with a low likelihood of body contact may be considered (class IIb, level of evidence C)
- For an aortic diameter greater than 43 mm in men or greater than 40 mm in women, sports involving body collision should be avoided (class III, level of evidence C)
- For an aortic diameter greater than 45 mm, sports activities should be avoided (class III, level of evidence C).
PREGNANCY CONSIDERATIONS
Bicuspid aortic valve is associated with aortic dissection, mainly in the third trimester.31 Patients should ideally undergo echocardiographic screening before conception. The 2010 ACC/AHA guidelines for managing thoracic aortic disease recommend monthly or bimonthly echocardiography until delivery in pregnant women with a dilated thoracic aorta.22
Patients with bicuspid aortic valve and aortic root enlargement of more than 40 mm should have preconception counseling about surgery for aortic root replacement before becoming pregnant. If the diagnosis of enlarged aortic root is made during pregnancy, echocardiographic surveillance at 4- to 6-week intervals is indicated.32
SURGICAL MANAGEMENT
In the past, beta-blockers and angiotensin-converting enzyme inhibitors were recommended to minimize shear stress, with the goal of slowing progression of aortic dilation. However, evidence to support their use is inadequate.33,34
The only definitive treatment is surgery, with various procedures that lower the risk of death or dissection.24,35
The dimensions of the aortic root or ascending aorta should be examined vigilantly, according to the 2014 ACC/AHA guidelines27:
- Repairing the aortic sinuses or replacing the ascending aorta is indicated if the diameter of the aortic sinuses or ascending aorta is greater than 5.5 cm (class I, level of evidence B)
- Repairing the aortic sinuses or replacing the ascending aorta is reasonable if the diameter of the aortic sinuses or ascending aorta is greater than 5.0 cm and the patient has a risk factor for dissection such as a family history of aortic dissection or an increase in diameter of 0.5 cm or greater per year (class IIa, level of evidence C)
- Replacement of the ascending aorta is reasonable if the diameter of the ascending aorta is greater than 4.5 cm and the patient is undergoing aortic valve surgery for severe aortic stenosis or regurgitation.
Valve repair or replacement
Aortic valve repair or replacement is sometimes done separately from aortic root repair.
The value of aortic valve repair is debatable, but a series of 728 patients at Cleveland Clinic showed a very low mortality rate (0.41%) and an annual reoperation rate of 2.6% during up to 15 years of follow-up.36
Aortic valve replacement is usually considered for patients with severe valve dysfunction, abnormal left ventricular dimensions, or symptoms. It is important to determine if the patient is a good surgical candidate and to refer early for surgical evaluation to avoid the higher risk of death associated with emergency surgery.36
Transcatheter aortic valve replacement has been studied in patients deemed to be at too high a risk for surgical replacement. Short- and intermediate-term outcomes have been good in these patients, but long-term data are lacking.37
Surveillance after surgery
The type of operation determines postoperative surveillance.
After isolated aortic valve repair or replacement, patients should continue with surveillance at least annually to monitor for progressive aortopathy, as they remain at increased risk of dissection or rupture after isolated valve surgery, especially if they had aortic insufficiency preoperatively.38
After definitive surgery with replacement or repair of the ascending aorta, no clear recommendations have been established for continued surveillance. However, it is reasonable to image these patients with either MRI or CT angiography 3 to 5 years after their surgery to monitor for anastomotic complications.
CASE QUESTIONS ANSWERED
Our patient should undergo repeat TTE in 1 year. He should also undergo CT angiography of the ascending aorta if it is not seen by TTE. He can participate in low-intensity sports but should avoid intense weight training. His parents, siblings, and children should be screened for bicuspid aortic valve or associated aortopathies.
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol 1970; 26(1):72–83. pmid:5427836
- Michalowska IM, Hryniewiecki T, Kwiatek P, Stoklosa P, Swoboda-Rydz U, Szymanski P. Coronary artery variants and anomalies in patients with bicuspid aortic valve. J Thorac Imaging 2016; 31(3):156–162. doi:10.1097/RTI.0000000000000205
- Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999; 74(1):14–26. doi:10.4065/74.1.14
- Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005; 150(3):513–515. doi:10.1016/j.ahj.2004.10.036
- Benson DW. The genetics of congenital heart disease: a point in the revolution. Cardiol Clin 2002; 20(3):385–394. pmid:12371007
- Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978; 40(12):1402–1407. pmid:737099
- Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: the contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol 2017; 8:612. doi:10.3389/fphys.2017.00612
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008; 51(19):1904–1909. doi:10.1016/j.jacc.2008.02.035
- Duran AC, Frescura C, Sans-Coma V, Angelini A, Basso C, Thiene G. Bicuspid aortic valves in hearts with other congenital heart disease. J Heart Valve Dis 1995; 4(6):581–590. pmid:8611973
- De Rubens Figueroa J, Rodríguez LM, Hach JL, Del Castillo Ruíz V, Martínez HO. Cardiovascular spectrum in Williams-Beuren syndrome: the Mexican experience in 40 patients. Tex Heart Inst J 2008; 35(3):279–285. pmid:18941598
- Yuan SM, Jing H. The bicuspid aortic valve and related disorders. Sao Paulo Med J 2010; 128(5):296–301. pmid:21181071
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004; 44(1):138–143. doi:10.1016/j.jacc.2004.03.050
- Kerstjens-Frederikse WS, Sarvaas GJ, Ruiter JS, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? Heart 2011; 97(15):1228–1232. doi:10.1136/hrt.2010.211433
- Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol 2010; 55(25):2789–2800. doi:10.1016/j.jacc.2009.12.068
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112.
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Beppu S, Suzuki S, Matsuda H, Ohmori F, Nagata S, Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol 1993; 71(4):322–327. pmid:8427176
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111(7):920–925. doi:10.1161/01.CIR.0000155623.48408.C5
- Yener N, Oktar GL, Erer D, Yardimci MM, Yener A. Bicuspid aortic valve. Ann Thorac Cardiovasc Surg 2002; 8(5):264–267. pmid:12472407
- Lamas CC, Eykyn SJ. Bicuspid aortic valve—a silent danger: analysis of 50 cases of infective endocarditis. Clin Infect Dis 2000; 30(2):336–341. doi:10.1086/313646
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 2007; 116(15):1736–1754. doi:10.1161/CIRCULATIONAHA.106.183095
- Hiratzka L, Bakris G, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation 2010; 121(13):e266–e369. doi:10.1161/CIR.0b013e3181d4739e
- Chun EJ, Choi SI, Lim C, et al. Aortic stenosis: evaluation with multidetector CT angiography and MR imaging. Korean J Radiol 2008; 9(5):439–448. doi:10.3348/kjr.2008.9.5.439
- Kiefer TL, Wang A, Hughes GC, Bashore TM. Management of patients with bicuspid aortic valve disease. Curr Treat Options Cardiovasc Med 2011; 13(6):489–505. doi:10.1007/s11936-011-0152-7
- Alegret JM, Palazon O, Duran I, Vernis JM. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int J Cardiovasc Imaging 2005; 21(2-3):213–217. doi:10.1007/s10554-004-3901-9
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol 2009; 53(24):2288–2295. doi:10.1016/j.jacc.2009.03.027
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Thorac Cardiovasc Surg 2014; 148(1):e1-e132. doi:10.1016/j.jtcvs.2014.05.014
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23):e143–e263. doi:10.1016/j.jacc.2008.10.001
- Braverman AC, Harris KM, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 7: aortic diseases, including Marfan syndrome. Circulation 2015; 132(22):e303–e309. doi:10.1161/CIR.0000000000000243
- De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull 2008; 85:63–85. doi:10.1093/bmb/ldn002
- Thorne SA. Pregnancy in heart disease. Heart 2004; 90(4):450–456. pmid:15020530
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg 2003; 76(1):309–314. pmid:12842575
- Allen BD, Markl M, Barker AJ, et al. Influence of beta-blocker therapy on aortic blood flow in patients with bicuspid aortic valve. Int J Cardiovasc Imaging 2016; 32(4):621–628. doi:10.1007/s10554-015-0819-3
- Ohnemus D, Oster ME, Gatlin S, Jokhadar M, Mahle WT. The effect of angiotensin-converting enzyme inhibitors on the rate of ascending aorta dilation in patients with bicuspid aortic valve. Congenit Heart Dis 2015; 10(1):E1–E5. doi:10.1111/chd.12184
- Masri A, Kalahasti V, Alkharabsheh S, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016; 151(6):1650–1659.e1. doi:10.1016/j.jtcvs.2015.12.019
- Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014; 97(5):1539–1548. doi:10.1016/j.athoracsur.2013.11.036
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012; 42(5):832–838. doi:10.1093/ejcts/ezs137
KEY POINTS
- Associated aortopathies such as aortic root dilation, aneurysm, dissection, and coarctation may initially be asymptomatic.
- Regular surveillance with transthoracic echocardiography (TTE) is required.
- Transesophageal echocardiography should be performed if TTE does not clearly show the aorta and aortic root. Magnetic resonance imaging or computed tomographic angiography may also be needed to measure the aortic root and ascending thoracic aorta.
- If initial imaging is normal and there is no aortic dilation, repeat imaging should be done every 5 to 10 years. If any abnormality is found, annual surveillance is needed.
- Women with a bicuspid aortic valve who are contemplating pregnancy should undergo echocardiography first, and some may need to undergo surgery.