User login
The Diagnosis: Lichen Sclerosus
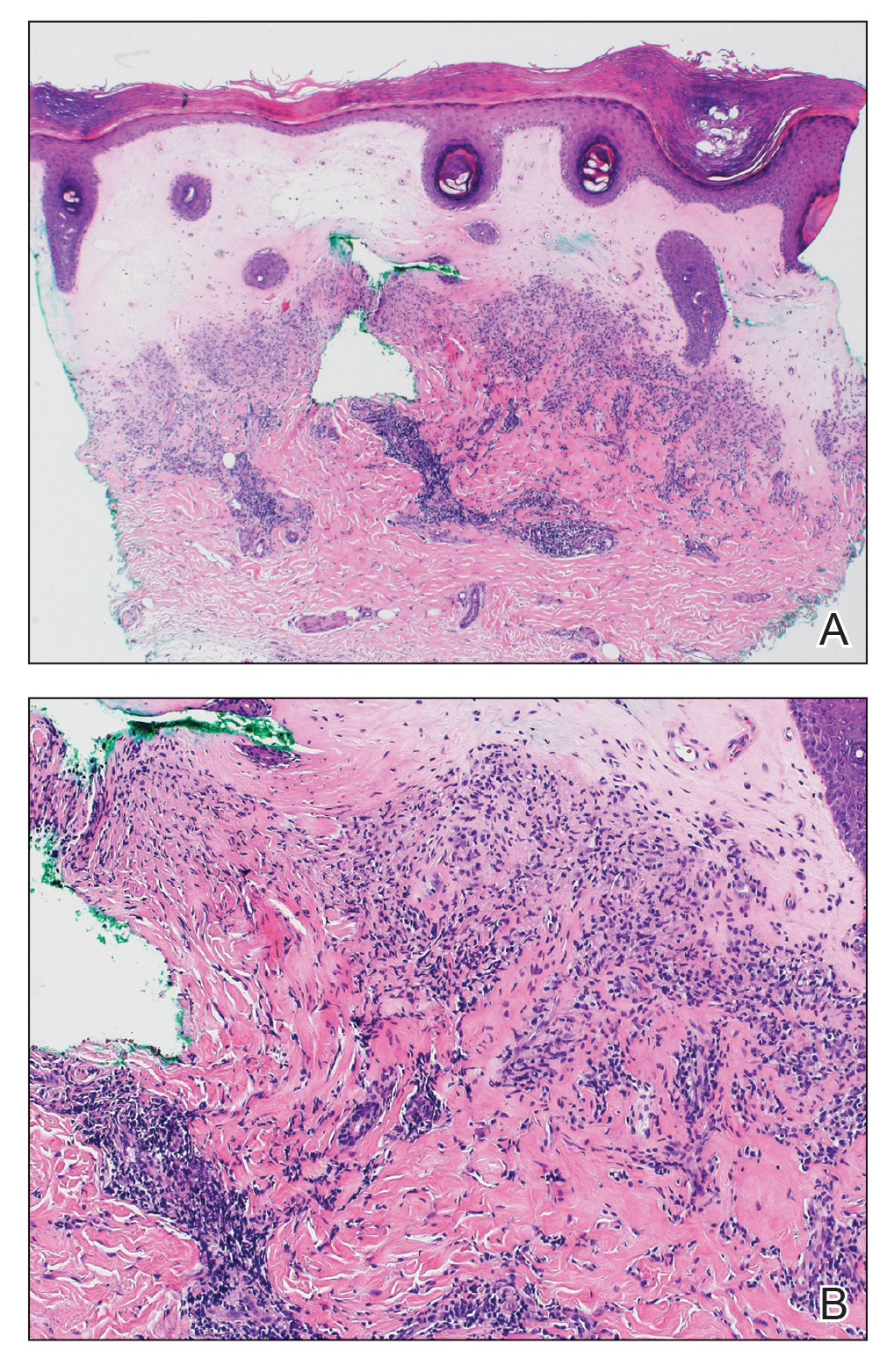
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).
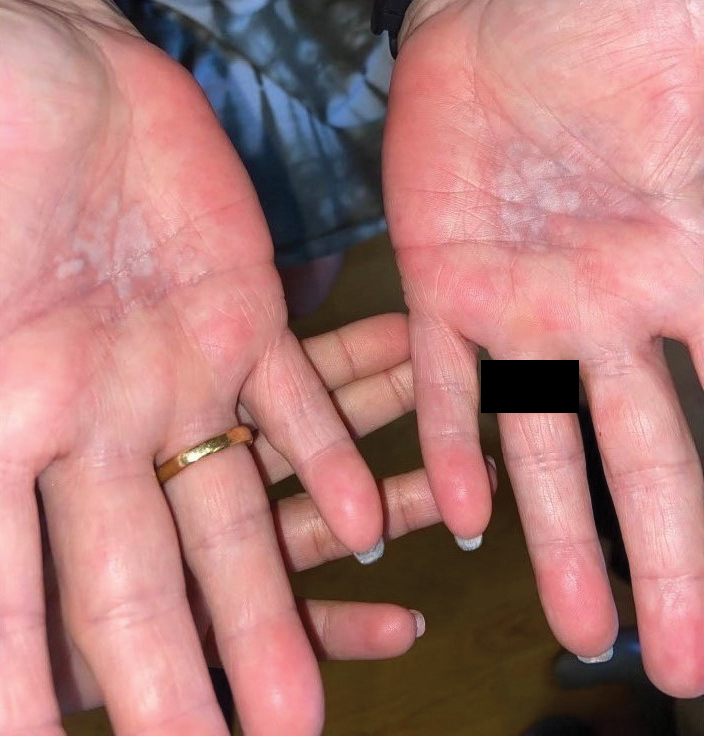
Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).

Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).

Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
A 59-year-old woman presented with atrophic, hypopigmented, ivory papules and plaques localized to the central palms and soles of 3 years’ duration. The lesions were associated with burning that was most notable after extended periods of ambulation. The lesions initially were diagnosed as plaque psoriasis by an external dermatology clinic. At the time of presentation to our clinic, treatment with several highpotency topical steroids and biologics approved for plaque psoriasis had failed. Her medical history and concurrent medical workup were notable for type 2 diabetes mellitus, liver dysfunction, thyroid nodules overseen by an endocrinologist, vitamin B12 and vitamin D deficiencies managed with supplementation, and diffuse androgenic alopecia with suspected telogen effluvium. Physical examination revealed no plaque fissuring, pruritus, or scaling. She had no history of radiation therapy or organ transplantation. A punch biopsy of the left palm was performed.