User login
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
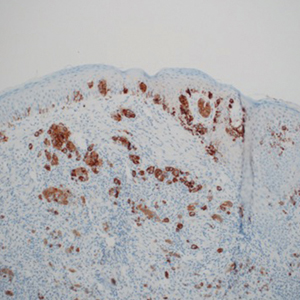
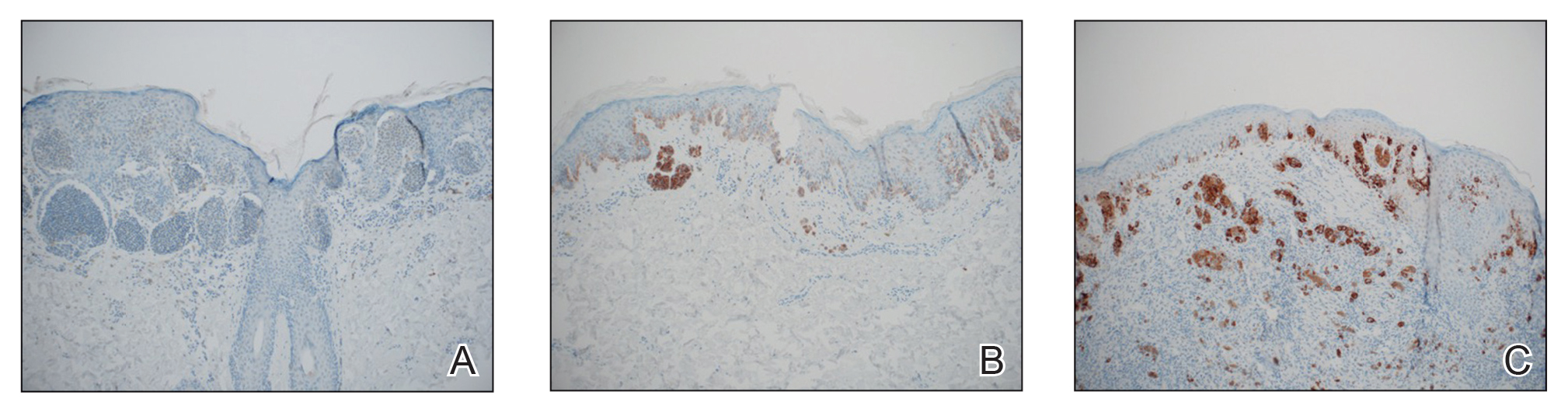
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.
Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
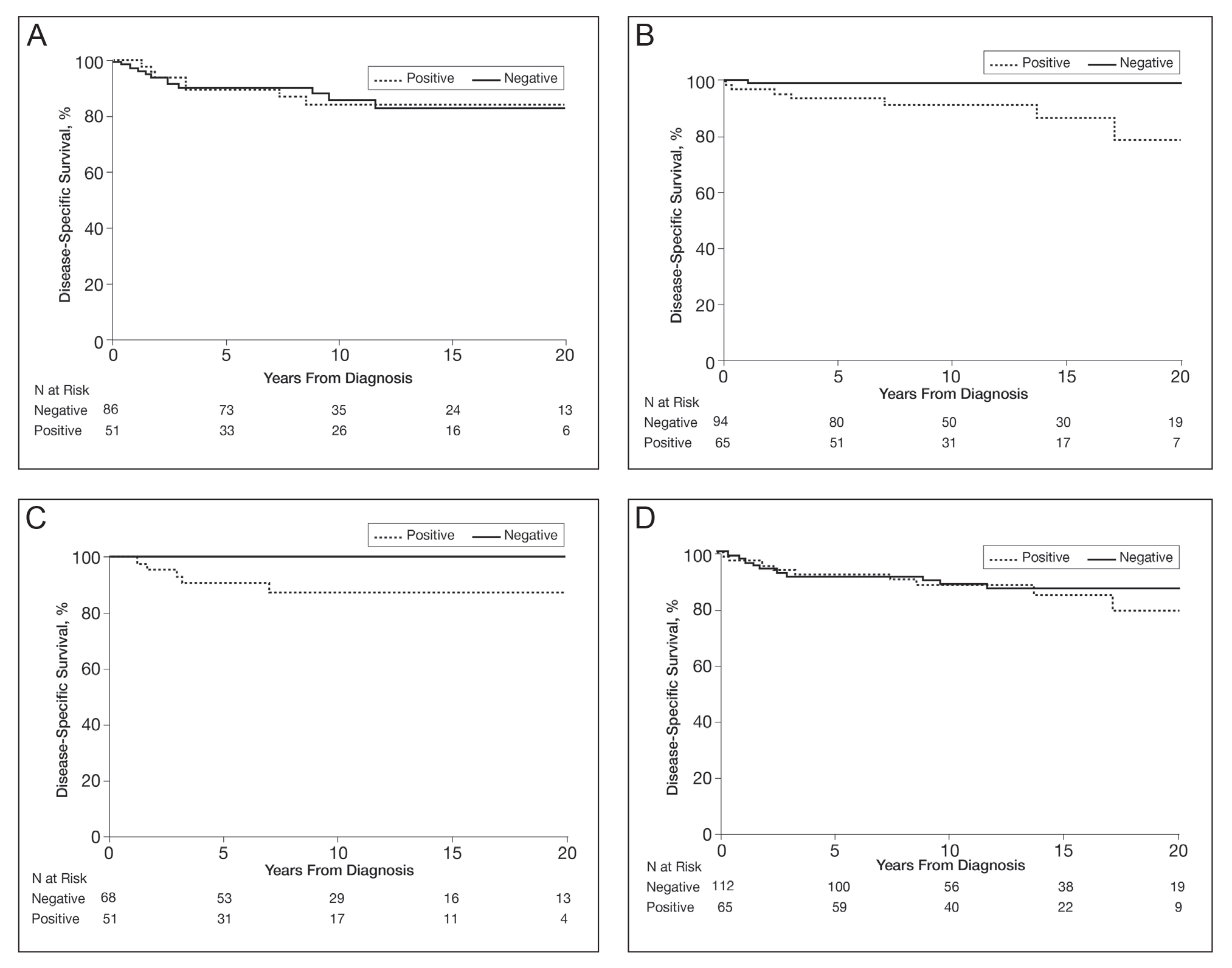
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).
BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.

Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).

BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.

Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).

BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Practice Points
- Approximately 50% of melanomas contain BRAF mutations; the effects on survival are unclear.
- Women with BRAF-mutated melanoma are at increased risk for death from melanoma.
- BRAF expression is associated with death of any cause for adults aged 18 to 39 years.