User login
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
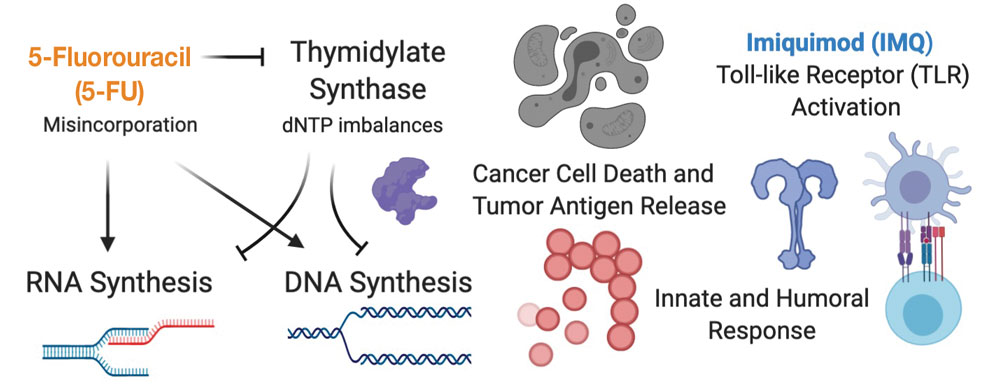
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.
Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea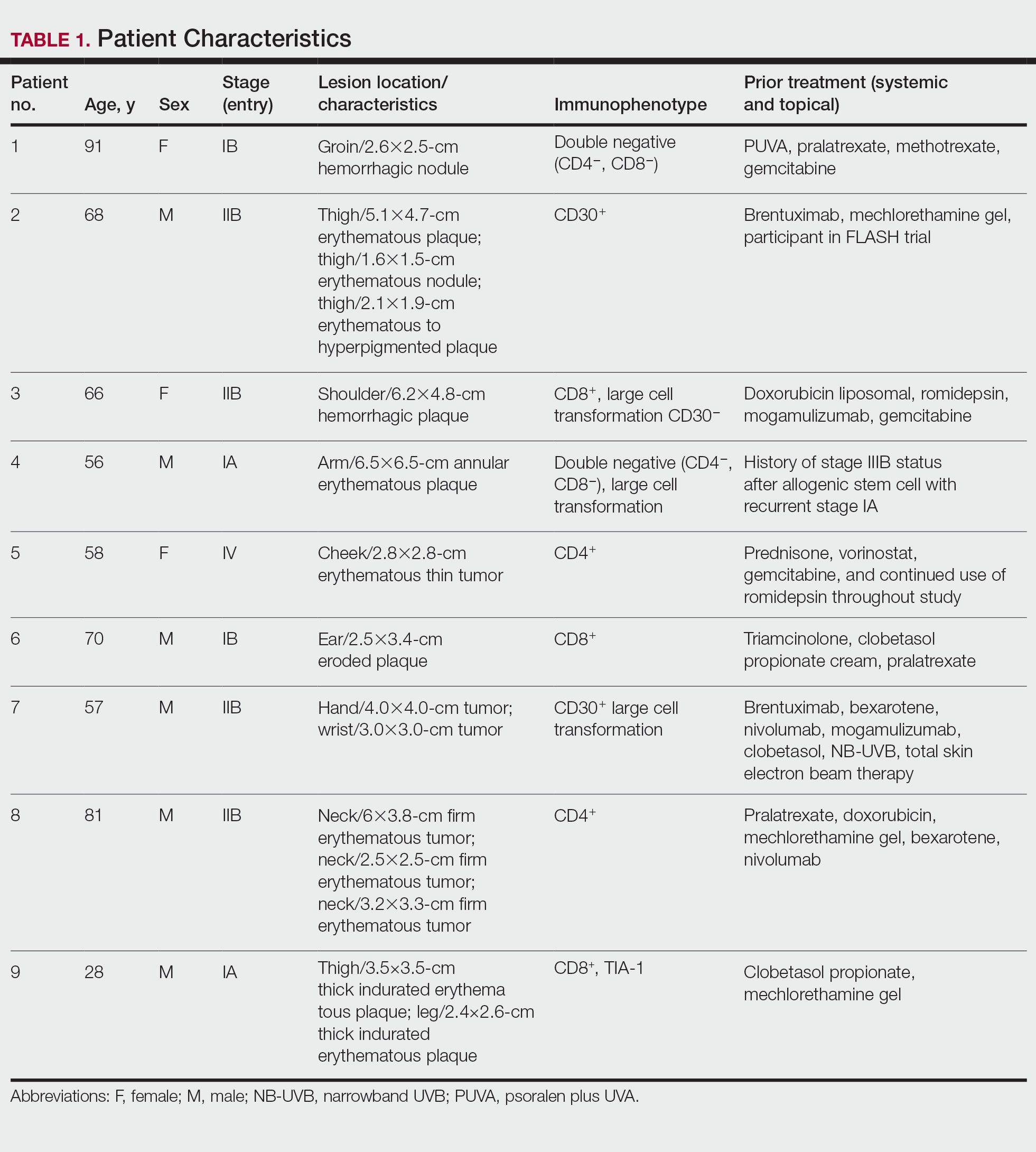
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.
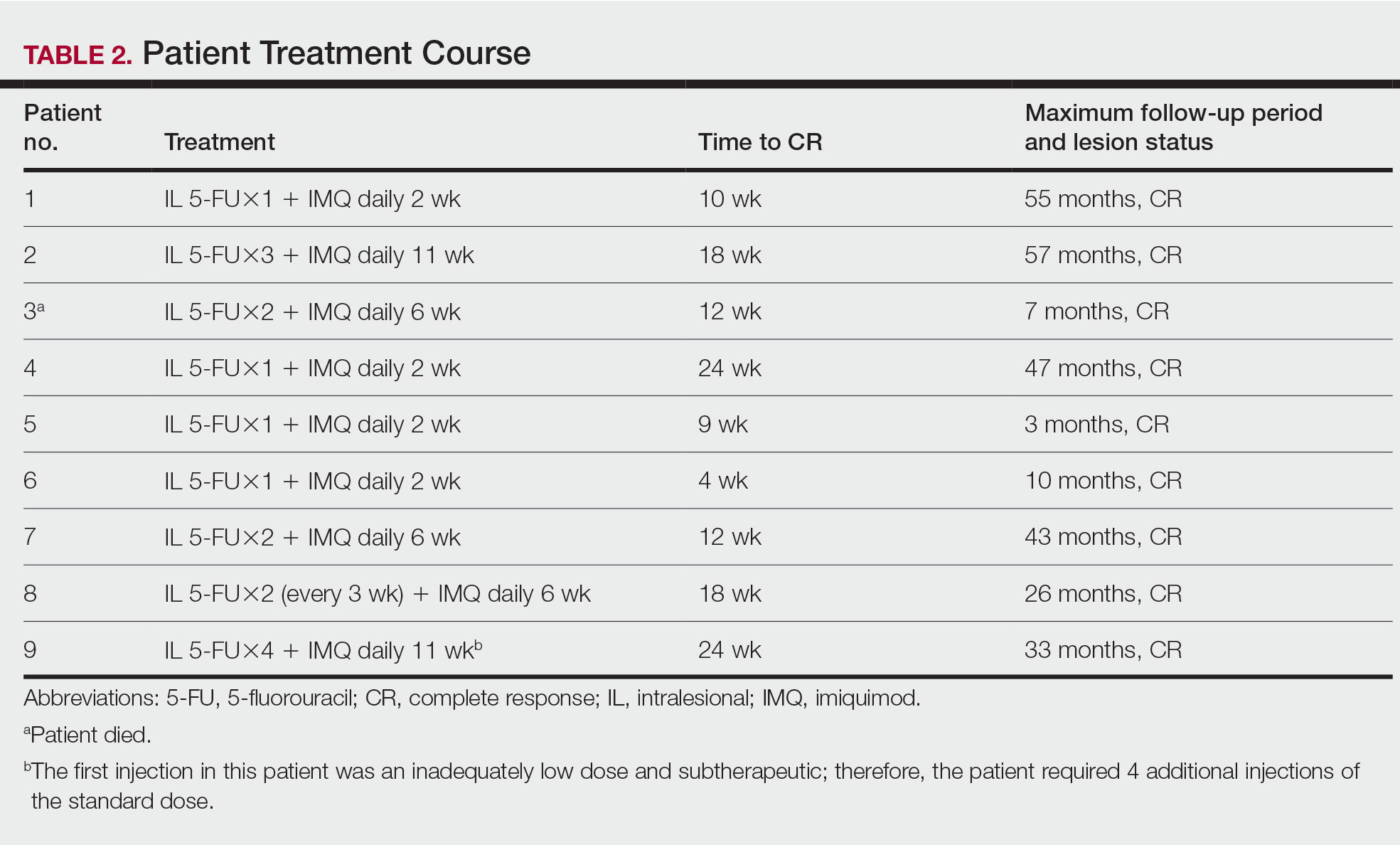
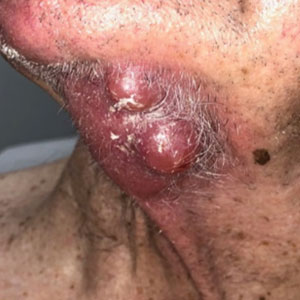
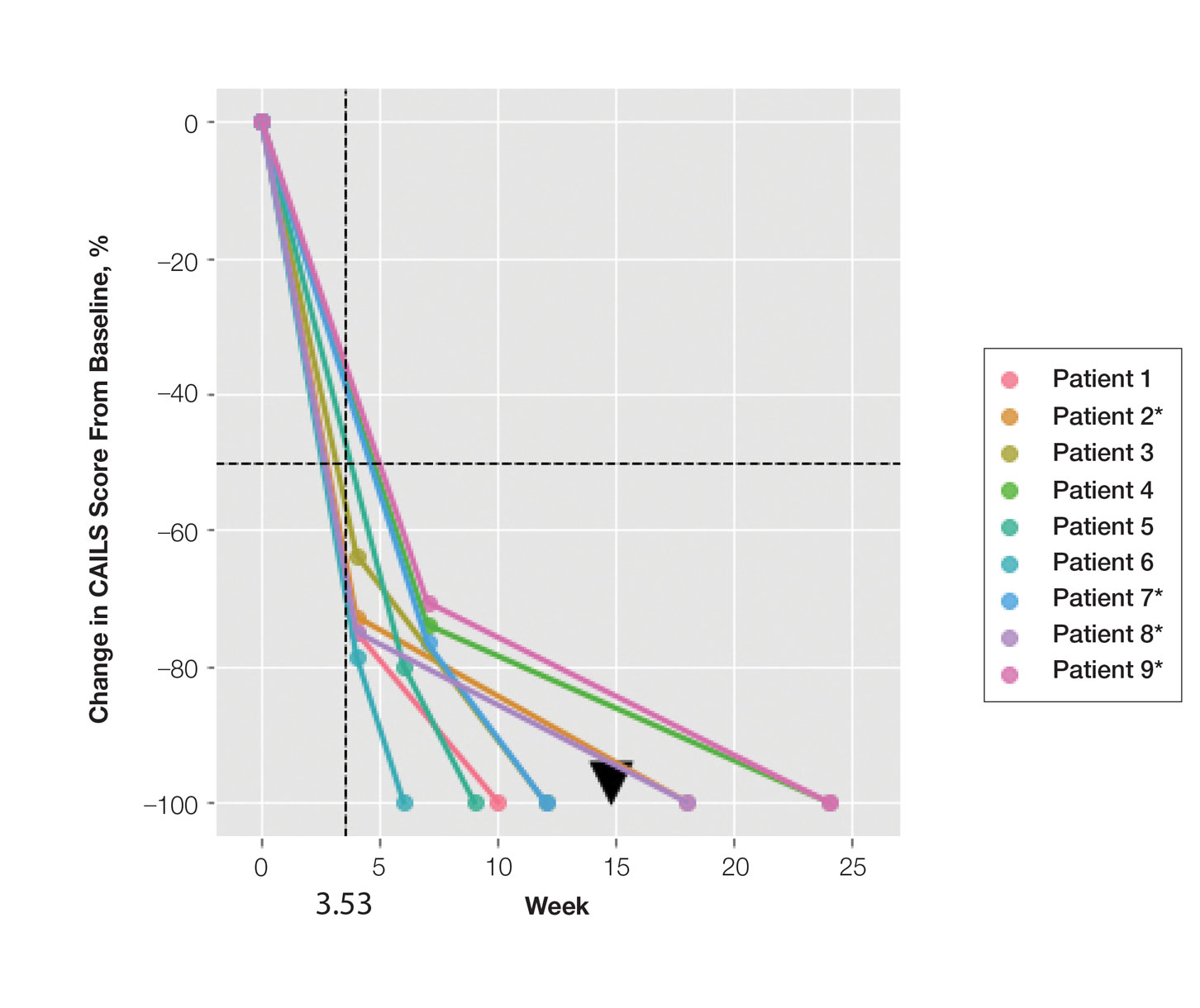
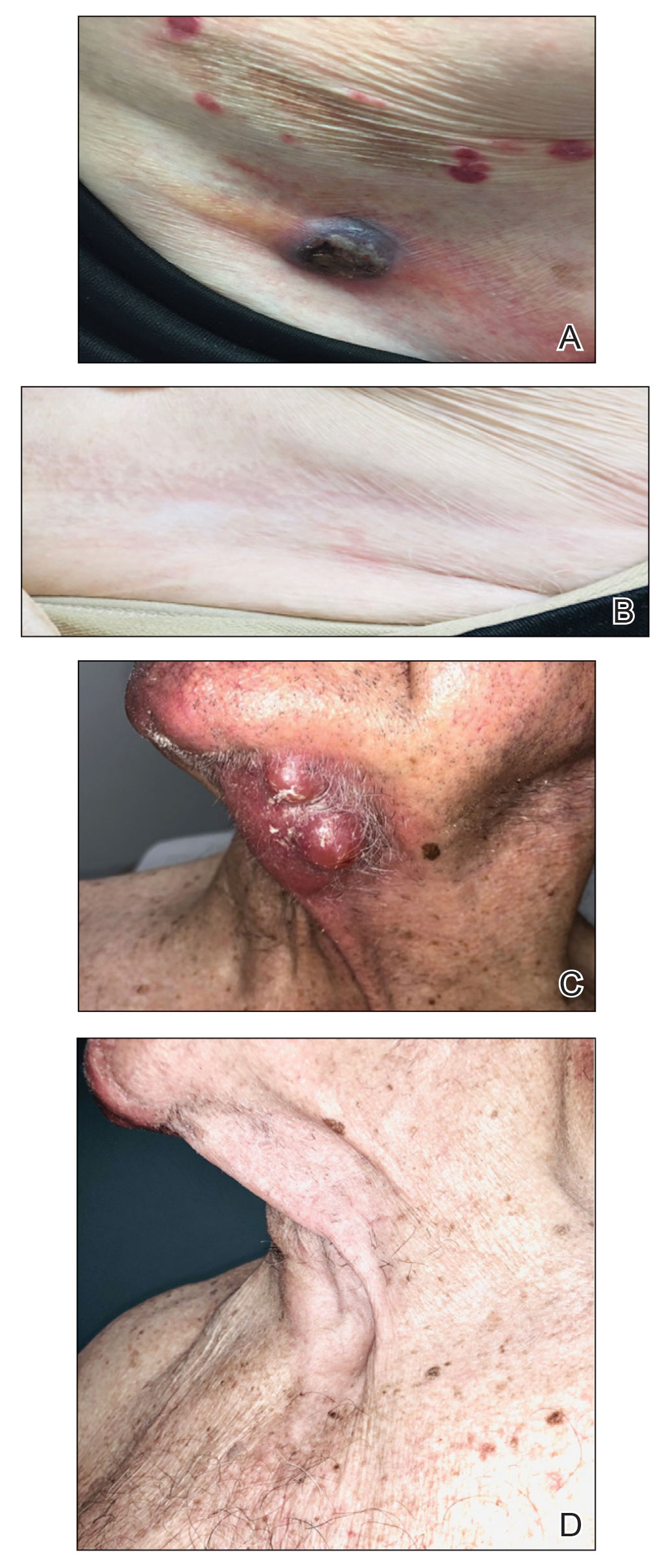
Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.
Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.
Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
PRACTICE POINTS
- Cutaneous T-cell lymphoma (CTCL) is a chronic lymphoma affecting the skin with limited durable effective skin-directed therapies.
- Combination intralesional 5-fluorouracil and topical imiquimod is a well-tolerated, fast, convenient, and durable therapy for recalcitrant thick plaques and tumors of CTCL.
- This regimen may be utilized as monotherapy or as the skin-directed component of combination therapy based on disease stage.