User login
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
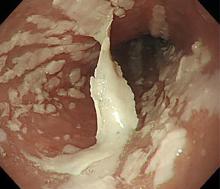
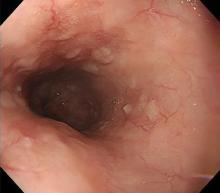
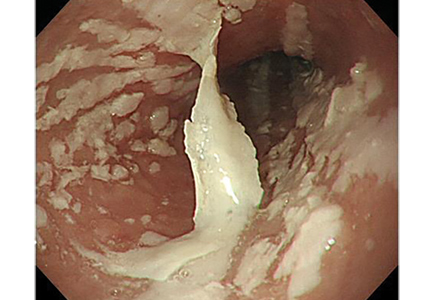
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
A 74-year-old man presented to the gastroenterology clinic with a 2-day history of retrosternal discomfort. His vital signs were normal, and laboratory testing showed a normal leukocyte count.
Esophagogastroduodenoscopy (EGD) revealed longitudinal sloughing mucosal casts in the middle and lower esophagus (Figure 1).
The patient had been taking dabigatran 110 mg twice daily for 2 years because of nonvalvular atrial fibrillation. He was also taking amlodipine 2.5 mg/day for hypertension.
DABIGATRAN-INDUCED ESOPHAGITIS
Although no study has investigated the overall prevalence of dabigatran-induced esophagitis, a retrospective database review of 91 patients taking dabigatran and undergoing upper-gastrointestinal endoscopy reported that 19 (20.9%) had endoscopic signs of dabigatran-induced esophagitis.2
Typical symptoms are the acute onset of chest pain, epigastralgia, odynophagia, and dysphagia. But patients can also have no symptoms or only mild symptoms.2,3
Despite dabigatran’s anticoagulant activity, there have been few reports of bleeding, perhaps because the lesions tend to be superficial on the surface of the esophageal mucosa.
Symptoms usually resolve within 1 week after stopping dabigatran and starting a proton pump inhibitor. To prevent mucosal injury, patients should be instructed to take dabigatran with sufficient water and to remain in an upright position for at least 30 minutes afterward.4
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010
- Baehr PH, McDonald GB. Esophageal infections: risk factors, presentation, diagnosis, and treatment. Gastroenterology 1994; 106(2):509–532. pmid:7980741
- Toya Y, Nakamura S, Tomita K, et al. Dabigatran-induced esophagitis: the prevalence and endoscopic characteristics. J Gastroenterol Hepatol 2016; 31(3):610–614. doi:10.1111/jgh.13024
- Ueta E, Fujikawa T, Imagawa A. A case of a slightly symptomatic exfoliative oesophagitis. BMJ Case Rep 2015; pii:bcr2015211925. doi:10.1136/bcr-2015-211925
- Ootani A, Hayashi Y, Miyagi Y. Dabigatran-induced esophagitis. Clin Gastroenterol Hepatol 2014; 12(7):e55–e56. doi:10.1016/j.cgh.2013.09.010