User login
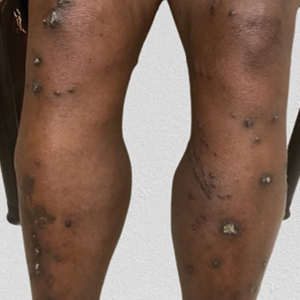
Diffuse Pruritic Keratotic Papules
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).
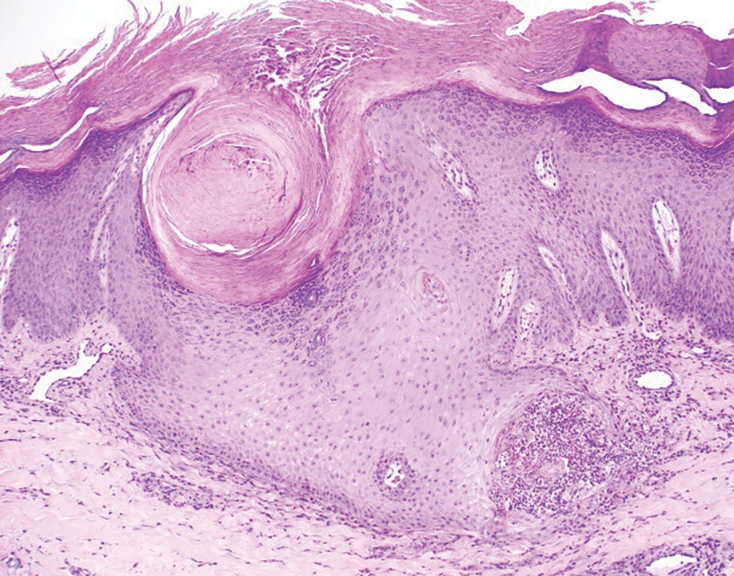
Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
THE DIAGNOSIS: Reactive Perforating Collagenosis
Histopathology revealed invagination of the epidermis with hyperkeratosis; prominent epidermal hyperplasia; and a central basophilic plug of keratin, collagen, and inflammatory debris. Transepidermal elimination of bright eosinophilic altered collagen fibers was seen (Figure). The findings were consistent with a diagnosis of reactive perforating collagenosis (RPC).

Reactive perforating collagenosis, a subtype of perforating dermatosis, is a rare skin condition in which altered collagen is eliminated through the epidermis.1 There are 2 forms of RPC: the inherited form, which is very rare and manifests in childhood, and the acquired form, which manifests in adulthood and is associated with systemic diseases, most notably diabetes and/or chronic renal failure, both of which our patient had been diagnosed with.1,2 The clinical presentation of RPC includes erythematous papules or nodules that evolve into umbilicated 4- to 10-mm craterlike ulcerations with a central keratotic plug. The lesions favor a linear distribution along the extensor surfaces of the arms and legs, trunk, and gluteal area. Involvement of the head, neck, and scalp has been reported less commonly, which makes our case particularly unique.3 Histopathologically, RPC is characterized by a cup-shaped depression of the epidermis with an overlying keratin plug containing inflammatory cells, keratinous debris, and collagen fibers. Vertically oriented collagen fibers are seen extruded through the epidermis.4,5
While the pathogenesis of RPC remains unknown, it is believed that superficial trauma due to chronic scratching results in transepithelial elimination of collagen. Due to the association of acquired RPC (ARPC) with diabetes, it also has been proposed that scratching can cause microtrauma and necrosis of the dermal structures, potentially due to diabetic microangiopathy.3 Additionally, RPC is associated with overexpression of transforming growth factor beta 3 in lesional skin, suggesting that transforming growth factor beta 3 is involved with tissue repair and extracellular remodeling in this condition.6
Treatment of ARPC should include the management of underlying disease. While no definitive treatment has been reported to date, topical corticosteroids, retinoids, keratolytics, emollients, antihistamines, narrow-band UVB phototherapy, and psoralen plus UVA phototherapy have been used with varying degrees of improvement. Typically, the lesions self-resolve within 6 to 8 weeks; however, they often recur and usually leave scarring with or without hyperpigmentation.2,7-10
Acquired RPC can be misdiagnosed initially, as it mimics several other conditions and commonly is associated with systemic diseases. While biopsy is necessary for diagnosis, if it cannot be performed or the results are indeterminate, dermoscopy can serve as a helpful diagnostic tool. The most common dermoscopic patterns seen in RPC include a yellow-brown structureless area in the center of the lesion with a peripheral surface crust and surrounding white rim—thought to represent epidermal invagination or keratinous debris. Additionally, inflammation with visible vessels both centrally and peripherally is represented by an outer pink circle on dermoscopy.5,11
The differential diagnoses for RPC include perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), prurigo nodularis, and keratoacanthomas. The primary perforating dermatoses (PF, EPS, and RPC) are similarly characterized by elimination of altered dermal material through the epidermis. As these conditions manifest with similar features on clinical examination, differentiation is made by the type of epidermal damage and the features of elimination material, making histopathologic examination paramount for definitive diagnosis.
Perforating folliculitis manifests as erythematous, follicular papules with a small central keratotic core or a central hair. Histopathologically, PF reveals a widely dilated follicle containing keratin, necrotic debris, and degenerated inflammatory cells. Elastosis perforans serpiginosa manifests clinically as hyperkeratotic papules in serpiginous patterns rather than the linear pattern commonly seen with ARPC. Histopathologically, EPS reveals thickened elastic fibers, rather than collagen fibers as seen in ARPC, extruded through the epidermis. Prurigo nodularis manifests clinically as dome-shaped papules with possible excoriation and crusting. Histopathologic examination reveals epidermal hyperplasia and hyperkeratosis; however, the characteristic features of transepithelial elimination of collagen and invaginations of epidermis differentiate ARPC from prurigo nodularis.12,13 Keratoacanthomas manifest clinically as an eruption of small, round, pink papules that rapidly grow and evolve into 1- to 2-cm dome-shaped nodules with central keratinaceous plugs, mimicking a crateriform appearance. Histopathologic examination reveals a circumscribed proliferation of well-differentiated keratinocytes. Multilobular exophytic or endophytic cystlike invaginations of the epidermis also are noted. The expulsion of collagen from the epidermis is more consistent with ARPC.14
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
- Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989;20(2 pt 1):287-289. doi:10.1016/s0190 -9622(89)80059-3
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Gontijo JRV, Júnior FF, Pereira LB, et al. Trauma-induced acquired reactive perforating collagenosis. An Bras Dermatol. 2021;96:392-393. doi:10.1016/j.abd.2020.06.022
- Ambalathinkal JJ, Phiske MM, Someshwar SJ. Acquired reactive perforating collagenosis, a rare entity at uncommon site. Indian J Pathol Microbiol. 2022;65:895-897. doi:10.4103/ijpm.ijpm_333_21
- Ormerod E, Atwan A, Intzedy L, et al. Dermoscopy features of acquired reactive perforating collagenosis: a case series. Dermatol Pract Concept. 2018;8:303-305. doi:10.5826/dpc.0804a11
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305. doi:10.1097/md.0000000000004305
- Bartling SJ, Naff JL, Canevari MM, et al. Pruritic rash in an elderly patient with uncontrolled diabetes mellitus. AACE Clin Case Rep. 2018;5:E146-E149. doi:10.4158/ACCR-2018-0388
- Kollipara H, Satya RS, Rao GR, et al. Acquired reactive perforating collagenosis: case series. Indian Dermatol Online J. 2023;14:72-76. doi:10.4103/idoj.idoj_373_22
- Wang C, Liu YH, Wang YX, et al. Acquired reactive perforating collagenosis. Chin Med J (Engl). 2020;133:2119-2120. doi:10.1097 /cm9.0000000000000906
- Harbaoui S, Litaiem N. Acquired perforating dermatosis. StatPearls [Internet]. Updated February 13, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539715/
- Elmas ÖF, Kilitci A, Uyar B. Dermoscopic patterns of acquired reactive perforating collagenosis. Dermatol Pract Concept. 2021;11:E2020085. doi:10.5826/dpc.1101a85
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Zito PM, Scharf R. Keratoacanthoma. StatPearls [Internet]. Updated August 8, 2023. Accessed August 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK499931/
Diffuse Pruritic Keratotic Papules
Diffuse Pruritic Keratotic Papules
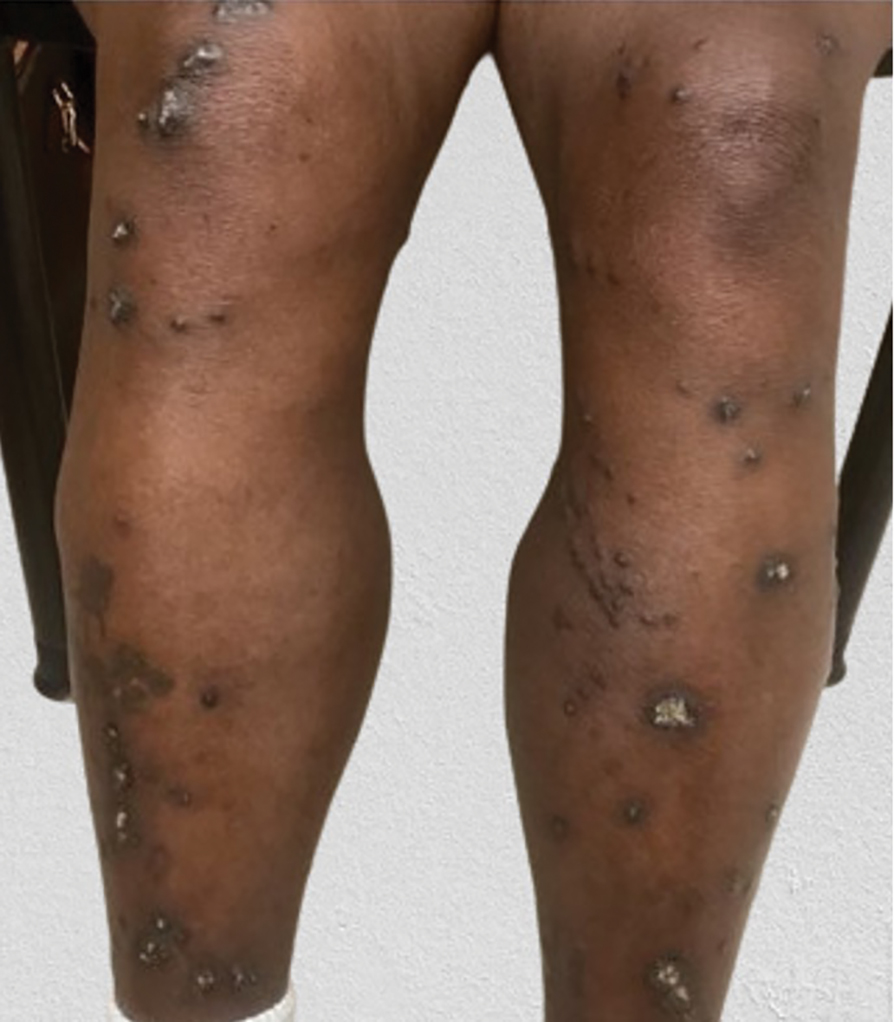
A 65-year-old woman presented to dermatology with an intensely pruritic rash on the arms, legs, neck, and face of several months’ duration. The patient reported scratching the lesions but denied any recent trauma to the affected areas. She previously had been evaluated by her primary care provider, who prescribed cephalexin with no improvement. Her medical history was remarkable for chronic renal failure on dialysis, diabetes, hypertension, and congestive heart failure. Physical examination of the skin revealed hard white cutaneous nodules distributed on the proximal posterior upper arms, bilateral proximal pretibial regions, right elbow, and left knee. Two shave biopsies from the right elbow and left knee were obtained for histopathology.