User login
The Diagnosis: Cutaneous B-Cell Lymphoma, Follicle Center Subtype
A 4-mm punch biopsy through the center of the largest lesion on the right posterior shoulder demonstrated a superficial and deep dermal atypical lymphoid infiltrate composed predominantly of small mature lymphocytes with interspersed intermediate-sized cells with irregular to cleaved nuclei, dispersed chromatin, one or more distinct nucleoli, occasional mitoses, and small amounts of cytoplasm (Figure, A). Immunoperoxidase studies showed the infiltrate to be a mixture of CD3+ T cells and CD20+ B cells (Figure, B). The B cells coexpressed B-cell lymphoma (Bcl) 6 protein (Figure, C) but were negative for multiple myeloma 1/interferon regulatory factor 4 and CD10; Bcl2 protein was positive in T cells but inconclusive for staining in B cells. Very few plasma cells were seen with CD138 stain. Fluorescence in situ hybridization studies were negative for IgH and BCL2 gene rearrangement. Molecular diagnostic studies for IgH and κ light chain gene rearrangement were positive for a clonal population. A clonal T-cell receptor γ chain gene rearrangement was not identified. The overall morphologic, immunophenotypic, and molecular findings were consistent with cutaneous involvement by a B-cell lymphoproliferative disorder, favoring primary cutaneous follicle center lymphoma (PCFCL).

The patient was referred to our cancer center for further workup consisting of a complete blood cell count with differential; comprehensive metabolic panel; lactate dehydrogenase; serum protein electrophoresis; peripheral blood flow cytometry; and computed tomography of the chest, abdomen, and pelvis. The analysis was unremarkable, supporting primary cutaneous disease. Additional studies suggested in the National Comprehensive Cancer Network (NCCN) Guidelines for primary cutaneous B-cell lymphomas include hepatitis B testing if the patient is being considered for immunotherapy and/or chemotherapy due to risk of reactivation, pregnancy testing in women of childbearing age, and human immunodeficiency virus testing.1 These tests were not performed in our patient because he did not have any risk factors for hepatitis B or human immunodeficiency virus.
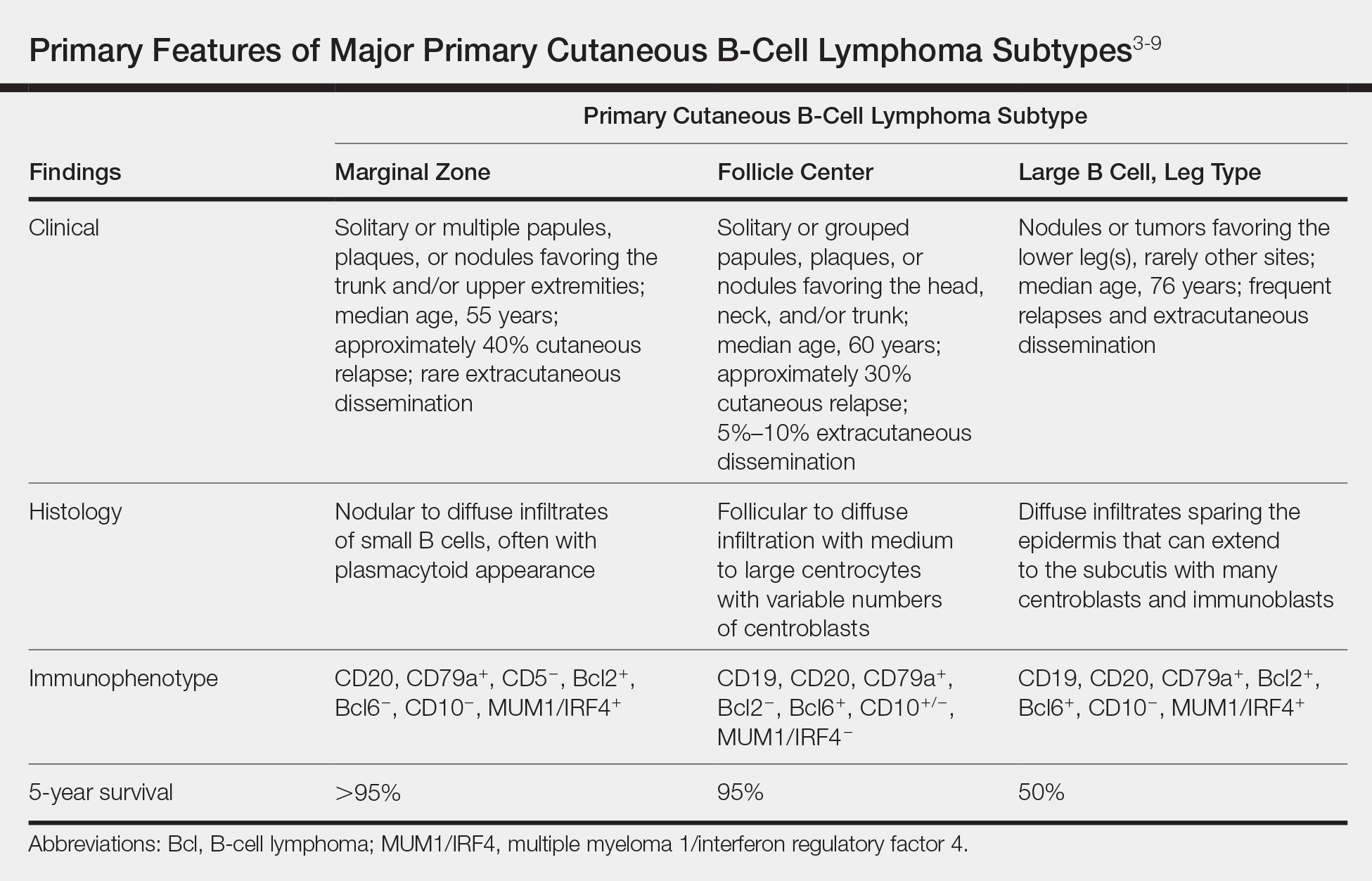
Primary cutaneous B-cell lymphomas originate in the skin without evidence of extracutaneous disease at presentation. They account for approximately 25% of primary cutaneous lymphomas in the United States, with primary cutaneous T-cell lymphoma being most common.2 The revised 2017 World Health Organization classification system defines 3 major subtypes of primary cutaneous B-cell lymphoma (Table).3-9 Primary cutaneous follicle center lymphoma is the most common subtype, accounting for approximately 60% of cases. In Europe, an association with Borrelia burgdorferi has been reported.10 The extent of skin involvement determines the T portion of TNM staging for PCFCL. It is based on the size and location of affected body regions that are delineated, such as the head and neck, chest, abdomen/genitalia, upper back, lower back/buttocks, each upper arm, each lower arm/hand, each upper leg, and each lower leg/foot. T1 is for solitary skin involvement in which the lesion is 5 cm or less in diameter (T1a) or greater than 5 cm (T1b). T2 is for regional skin involvement limited to 1 or 2 contiguous body regions, whereas T2a has all lesions confined to an area 15 cm or less in diameter, T2b has lesions confined to an area greater than 15 cm up to 30 cm in diameter, and the area for T2c is greater than 30 cm in diameter. Finally, T3 is generalized skin involvement, whereas T3a has multiple lesions in 2 noncontiguous body regions, and T3b has multiple lesions on 3 or more regions.11 At presentation, our patient was considered T2cN0M0, as his lesions were present on only 2 contiguous regions extending beyond 30 cm without any evidence of lymph node involvement or metastasis.
Treatment of PCFCL is tailored to each case, as there is a paucity of randomized data in this rare entity. It is guided by the number and location of cutaneous lesions, associated skin symptoms, age of the patient, and performance status. Local disease can be treated with intralesional corticosteroids, excision, or close monitoring if the patient is asymptomatic. Low-dose radiation therapy may be used as primary treatment or for local recurrence.12 Patients with more extensive skin lesions can relapse after clearing; those with refractory disease can be managed with single-agent rituximab.13 Our patient underwent low-dose radiation therapy with good response and has not experienced recurrence.
Lymphocytoma cutis, also known as benign reactive lymphoid hyperplasia, can be idiopathic or can arise after arthropod assault, penetrative skin trauma, drugs, or infections. In granuloma annulare, small dermal papules may present in isolation or coalesce to form annular plaques. It is a benign inflammatory disorder of unknown cause, can have mild pruritus, and usually is self-limited. Pyogenic granuloma is a benign vascular proliferation of unknown etiology. Sarcoidosis is an immune-mediated systemic disorder with granuloma formation that has a predilection for the lungs and the skin.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Primary Cutaneous B-Cell Lymphomas. Version 2.2018. https://oncolife.com.ua/doc/nccn/Primary_Cutaneous_B-Cell_Lymphomas.pdf. Published January 10, 2018. Accessed June 21, 2019.
- Dores GM, Anderson WF, Devesa SS. Cutaneous lymphomas reported to the National Cancer Institute's surveillance, epidemiology, and end results program: applying the new WHO-European Organisation for Research and Treatment of Cancer classification system. J Clin Oncol. 2005;23:7246-7248.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2017.
- Surveillance, Epidemiology, and End Results Program. National Cancer Institute website. https://seer.cancer.gov/. Accessed June 26, 2019.
- Cerroni L. B-cell lymphomas of the skin. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2113-2126.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous follicle center lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-follicle-center-lymphoma. Updated February 7, 2018. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous marginal zone lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-marginal-zone-lymphoma. Updated March 6, 2019. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous large B cell lymphoma, leg type. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-large-b-cell-lymphoma-leg-type. Updated July 3, 2017. Accessed June 26, 2019.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 241-342.
- Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotand. Am J Surg Pathol. 2000;24:1279-1285.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Wilcon RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Morales AV, Advani R, Horwitz SM, et al. Indolent primary cutaneous B-cell lymphoma: experience using systemic rituximab. J Am Acad Dermatol. 2008;59:953-957.
The Diagnosis: Cutaneous B-Cell Lymphoma, Follicle Center Subtype
A 4-mm punch biopsy through the center of the largest lesion on the right posterior shoulder demonstrated a superficial and deep dermal atypical lymphoid infiltrate composed predominantly of small mature lymphocytes with interspersed intermediate-sized cells with irregular to cleaved nuclei, dispersed chromatin, one or more distinct nucleoli, occasional mitoses, and small amounts of cytoplasm (Figure, A). Immunoperoxidase studies showed the infiltrate to be a mixture of CD3+ T cells and CD20+ B cells (Figure, B). The B cells coexpressed B-cell lymphoma (Bcl) 6 protein (Figure, C) but were negative for multiple myeloma 1/interferon regulatory factor 4 and CD10; Bcl2 protein was positive in T cells but inconclusive for staining in B cells. Very few plasma cells were seen with CD138 stain. Fluorescence in situ hybridization studies were negative for IgH and BCL2 gene rearrangement. Molecular diagnostic studies for IgH and κ light chain gene rearrangement were positive for a clonal population. A clonal T-cell receptor γ chain gene rearrangement was not identified. The overall morphologic, immunophenotypic, and molecular findings were consistent with cutaneous involvement by a B-cell lymphoproliferative disorder, favoring primary cutaneous follicle center lymphoma (PCFCL).

The patient was referred to our cancer center for further workup consisting of a complete blood cell count with differential; comprehensive metabolic panel; lactate dehydrogenase; serum protein electrophoresis; peripheral blood flow cytometry; and computed tomography of the chest, abdomen, and pelvis. The analysis was unremarkable, supporting primary cutaneous disease. Additional studies suggested in the National Comprehensive Cancer Network (NCCN) Guidelines for primary cutaneous B-cell lymphomas include hepatitis B testing if the patient is being considered for immunotherapy and/or chemotherapy due to risk of reactivation, pregnancy testing in women of childbearing age, and human immunodeficiency virus testing.1 These tests were not performed in our patient because he did not have any risk factors for hepatitis B or human immunodeficiency virus.
Primary cutaneous B-cell lymphomas originate in the skin without evidence of extracutaneous disease at presentation. They account for approximately 25% of primary cutaneous lymphomas in the United States, with primary cutaneous T-cell lymphoma being most common.2 The revised 2017 World Health Organization classification system defines 3 major subtypes of primary cutaneous B-cell lymphoma (Table).3-9 Primary cutaneous follicle center lymphoma is the most common subtype, accounting for approximately 60% of cases. In Europe, an association with Borrelia burgdorferi has been reported.10 The extent of skin involvement determines the T portion of TNM staging for PCFCL. It is based on the size and location of affected body regions that are delineated, such as the head and neck, chest, abdomen/genitalia, upper back, lower back/buttocks, each upper arm, each lower arm/hand, each upper leg, and each lower leg/foot. T1 is for solitary skin involvement in which the lesion is 5 cm or less in diameter (T1a) or greater than 5 cm (T1b). T2 is for regional skin involvement limited to 1 or 2 contiguous body regions, whereas T2a has all lesions confined to an area 15 cm or less in diameter, T2b has lesions confined to an area greater than 15 cm up to 30 cm in diameter, and the area for T2c is greater than 30 cm in diameter. Finally, T3 is generalized skin involvement, whereas T3a has multiple lesions in 2 noncontiguous body regions, and T3b has multiple lesions on 3 or more regions.11 At presentation, our patient was considered T2cN0M0, as his lesions were present on only 2 contiguous regions extending beyond 30 cm without any evidence of lymph node involvement or metastasis.

Treatment of PCFCL is tailored to each case, as there is a paucity of randomized data in this rare entity. It is guided by the number and location of cutaneous lesions, associated skin symptoms, age of the patient, and performance status. Local disease can be treated with intralesional corticosteroids, excision, or close monitoring if the patient is asymptomatic. Low-dose radiation therapy may be used as primary treatment or for local recurrence.12 Patients with more extensive skin lesions can relapse after clearing; those with refractory disease can be managed with single-agent rituximab.13 Our patient underwent low-dose radiation therapy with good response and has not experienced recurrence.
Lymphocytoma cutis, also known as benign reactive lymphoid hyperplasia, can be idiopathic or can arise after arthropod assault, penetrative skin trauma, drugs, or infections. In granuloma annulare, small dermal papules may present in isolation or coalesce to form annular plaques. It is a benign inflammatory disorder of unknown cause, can have mild pruritus, and usually is self-limited. Pyogenic granuloma is a benign vascular proliferation of unknown etiology. Sarcoidosis is an immune-mediated systemic disorder with granuloma formation that has a predilection for the lungs and the skin.
The Diagnosis: Cutaneous B-Cell Lymphoma, Follicle Center Subtype
A 4-mm punch biopsy through the center of the largest lesion on the right posterior shoulder demonstrated a superficial and deep dermal atypical lymphoid infiltrate composed predominantly of small mature lymphocytes with interspersed intermediate-sized cells with irregular to cleaved nuclei, dispersed chromatin, one or more distinct nucleoli, occasional mitoses, and small amounts of cytoplasm (Figure, A). Immunoperoxidase studies showed the infiltrate to be a mixture of CD3+ T cells and CD20+ B cells (Figure, B). The B cells coexpressed B-cell lymphoma (Bcl) 6 protein (Figure, C) but were negative for multiple myeloma 1/interferon regulatory factor 4 and CD10; Bcl2 protein was positive in T cells but inconclusive for staining in B cells. Very few plasma cells were seen with CD138 stain. Fluorescence in situ hybridization studies were negative for IgH and BCL2 gene rearrangement. Molecular diagnostic studies for IgH and κ light chain gene rearrangement were positive for a clonal population. A clonal T-cell receptor γ chain gene rearrangement was not identified. The overall morphologic, immunophenotypic, and molecular findings were consistent with cutaneous involvement by a B-cell lymphoproliferative disorder, favoring primary cutaneous follicle center lymphoma (PCFCL).

The patient was referred to our cancer center for further workup consisting of a complete blood cell count with differential; comprehensive metabolic panel; lactate dehydrogenase; serum protein electrophoresis; peripheral blood flow cytometry; and computed tomography of the chest, abdomen, and pelvis. The analysis was unremarkable, supporting primary cutaneous disease. Additional studies suggested in the National Comprehensive Cancer Network (NCCN) Guidelines for primary cutaneous B-cell lymphomas include hepatitis B testing if the patient is being considered for immunotherapy and/or chemotherapy due to risk of reactivation, pregnancy testing in women of childbearing age, and human immunodeficiency virus testing.1 These tests were not performed in our patient because he did not have any risk factors for hepatitis B or human immunodeficiency virus.
Primary cutaneous B-cell lymphomas originate in the skin without evidence of extracutaneous disease at presentation. They account for approximately 25% of primary cutaneous lymphomas in the United States, with primary cutaneous T-cell lymphoma being most common.2 The revised 2017 World Health Organization classification system defines 3 major subtypes of primary cutaneous B-cell lymphoma (Table).3-9 Primary cutaneous follicle center lymphoma is the most common subtype, accounting for approximately 60% of cases. In Europe, an association with Borrelia burgdorferi has been reported.10 The extent of skin involvement determines the T portion of TNM staging for PCFCL. It is based on the size and location of affected body regions that are delineated, such as the head and neck, chest, abdomen/genitalia, upper back, lower back/buttocks, each upper arm, each lower arm/hand, each upper leg, and each lower leg/foot. T1 is for solitary skin involvement in which the lesion is 5 cm or less in diameter (T1a) or greater than 5 cm (T1b). T2 is for regional skin involvement limited to 1 or 2 contiguous body regions, whereas T2a has all lesions confined to an area 15 cm or less in diameter, T2b has lesions confined to an area greater than 15 cm up to 30 cm in diameter, and the area for T2c is greater than 30 cm in diameter. Finally, T3 is generalized skin involvement, whereas T3a has multiple lesions in 2 noncontiguous body regions, and T3b has multiple lesions on 3 or more regions.11 At presentation, our patient was considered T2cN0M0, as his lesions were present on only 2 contiguous regions extending beyond 30 cm without any evidence of lymph node involvement or metastasis.

Treatment of PCFCL is tailored to each case, as there is a paucity of randomized data in this rare entity. It is guided by the number and location of cutaneous lesions, associated skin symptoms, age of the patient, and performance status. Local disease can be treated with intralesional corticosteroids, excision, or close monitoring if the patient is asymptomatic. Low-dose radiation therapy may be used as primary treatment or for local recurrence.12 Patients with more extensive skin lesions can relapse after clearing; those with refractory disease can be managed with single-agent rituximab.13 Our patient underwent low-dose radiation therapy with good response and has not experienced recurrence.
Lymphocytoma cutis, also known as benign reactive lymphoid hyperplasia, can be idiopathic or can arise after arthropod assault, penetrative skin trauma, drugs, or infections. In granuloma annulare, small dermal papules may present in isolation or coalesce to form annular plaques. It is a benign inflammatory disorder of unknown cause, can have mild pruritus, and usually is self-limited. Pyogenic granuloma is a benign vascular proliferation of unknown etiology. Sarcoidosis is an immune-mediated systemic disorder with granuloma formation that has a predilection for the lungs and the skin.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Primary Cutaneous B-Cell Lymphomas. Version 2.2018. https://oncolife.com.ua/doc/nccn/Primary_Cutaneous_B-Cell_Lymphomas.pdf. Published January 10, 2018. Accessed June 21, 2019.
- Dores GM, Anderson WF, Devesa SS. Cutaneous lymphomas reported to the National Cancer Institute's surveillance, epidemiology, and end results program: applying the new WHO-European Organisation for Research and Treatment of Cancer classification system. J Clin Oncol. 2005;23:7246-7248.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2017.
- Surveillance, Epidemiology, and End Results Program. National Cancer Institute website. https://seer.cancer.gov/. Accessed June 26, 2019.
- Cerroni L. B-cell lymphomas of the skin. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2113-2126.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous follicle center lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-follicle-center-lymphoma. Updated February 7, 2018. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous marginal zone lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-marginal-zone-lymphoma. Updated March 6, 2019. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous large B cell lymphoma, leg type. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-large-b-cell-lymphoma-leg-type. Updated July 3, 2017. Accessed June 26, 2019.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 241-342.
- Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotand. Am J Surg Pathol. 2000;24:1279-1285.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Wilcon RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Morales AV, Advani R, Horwitz SM, et al. Indolent primary cutaneous B-cell lymphoma: experience using systemic rituximab. J Am Acad Dermatol. 2008;59:953-957.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Primary Cutaneous B-Cell Lymphomas. Version 2.2018. https://oncolife.com.ua/doc/nccn/Primary_Cutaneous_B-Cell_Lymphomas.pdf. Published January 10, 2018. Accessed June 21, 2019.
- Dores GM, Anderson WF, Devesa SS. Cutaneous lymphomas reported to the National Cancer Institute's surveillance, epidemiology, and end results program: applying the new WHO-European Organisation for Research and Treatment of Cancer classification system. J Clin Oncol. 2005;23:7246-7248.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2017.
- Surveillance, Epidemiology, and End Results Program. National Cancer Institute website. https://seer.cancer.gov/. Accessed June 26, 2019.
- Cerroni L. B-cell lymphomas of the skin. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:2113-2126.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous follicle center lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-follicle-center-lymphoma. Updated February 7, 2018. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous marginal zone lymphoma. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-marginal-zone-lymphoma. Updated March 6, 2019. Accessed June 26, 2019.
- Jacobsen E, Freedman AS, Willemze R. Primary cutaneous large B cell lymphoma, leg type. UpToDate website. https://www.uptodate.com/contents/primary-cutaneous-large-b-cell-lymphoma-leg-type. Updated July 3, 2017. Accessed June 26, 2019.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 241-342.
- Goodlad JR, Davidson MM, Hollowood K, et al. Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in patients from the Highlands of Scotand. Am J Surg Pathol. 2000;24:1279-1285.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Wilcon RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Morales AV, Advani R, Horwitz SM, et al. Indolent primary cutaneous B-cell lymphoma: experience using systemic rituximab. J Am Acad Dermatol. 2008;59:953-957.
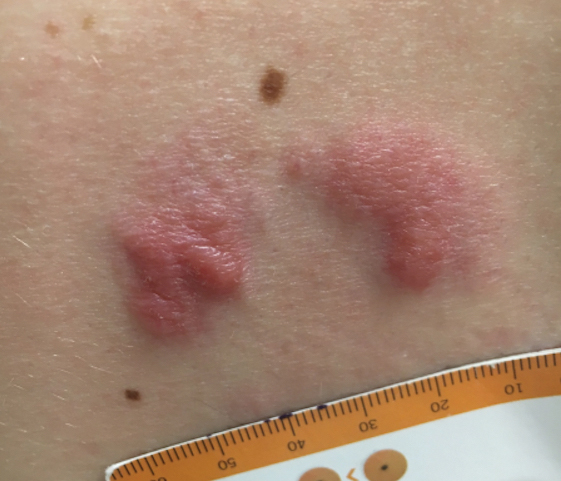
A 34-year-old man presented to the outpatient dermatology clinic with 3 groups of mildly pruritic, erythematous papules and plaques. The most prominent group appeared on the right posterior shoulder and had been slowly enlarging in size over the last 12 months (quiz image). A similar thinner group appeared on the left mid-back 6 months prior, and a third smaller group appeared over the left serratus anterior muscle 2 months prior. The patient reported having similar episodes dating back to his early 20s. In those instances, the lesions presented without an inciting incident, became more pronounced, and persisted for months to years before resolving. Previously affected areas included the upper and lateral back, flanks, and posterior upper arms. The patient used triamcinolone cream 0.1% up to 3 times daily on active lesions, which improved the pruritus and seemed to make the lesions resolve more quickly. He denied fever, chills, night sweats, anorexia, weight loss, fatigue, cough, and shortness of breath. His only medication was ranitidine 150 mg twice daily for gastroesophageal reflux disease. Physical examination revealed no palpable lymphadenopathy.