User login
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
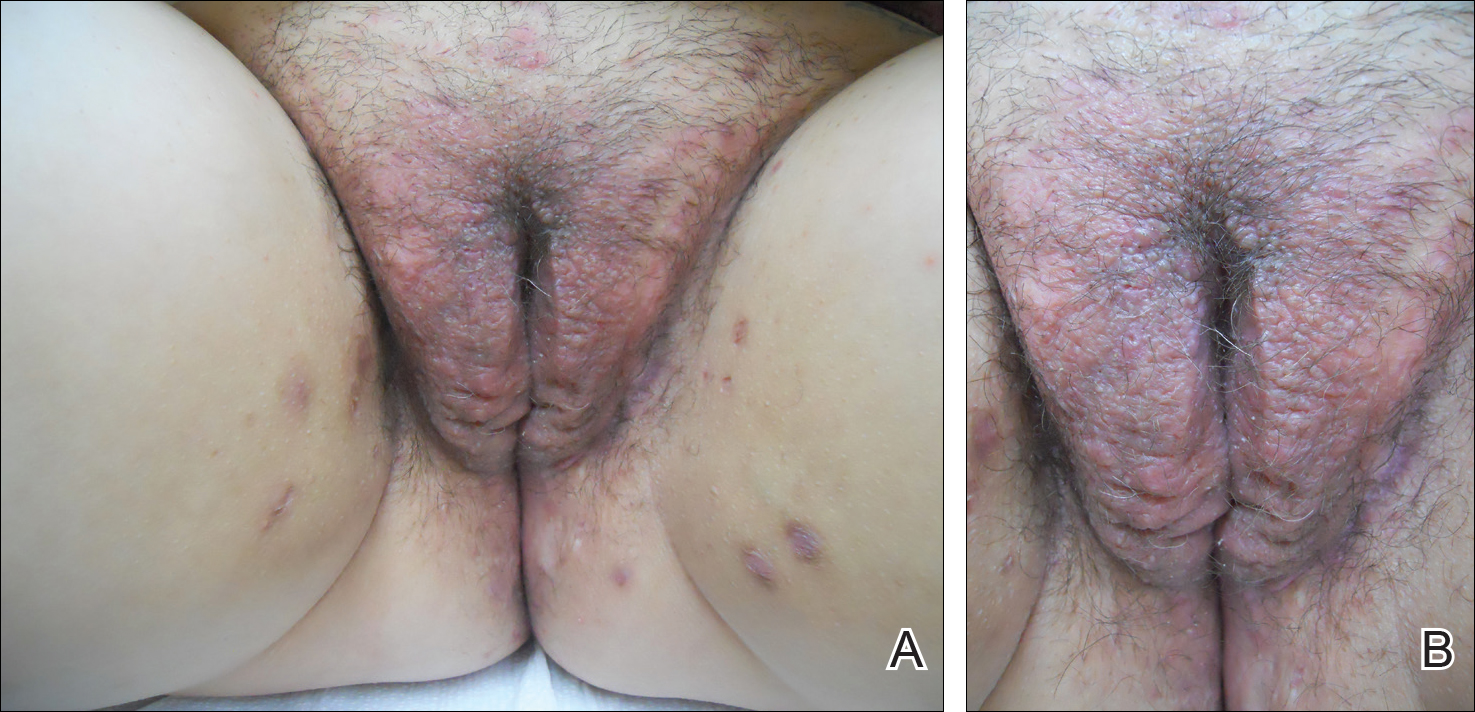
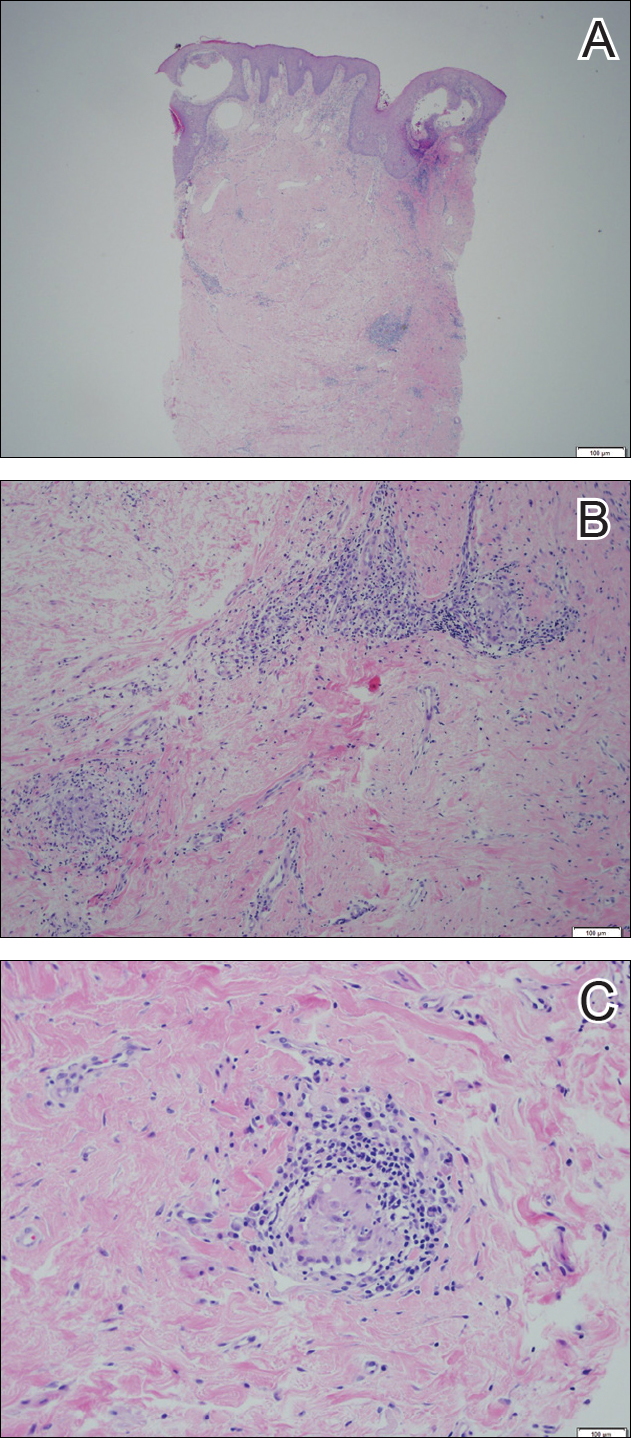
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.
Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.


Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.


Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
