User login
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
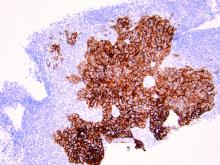
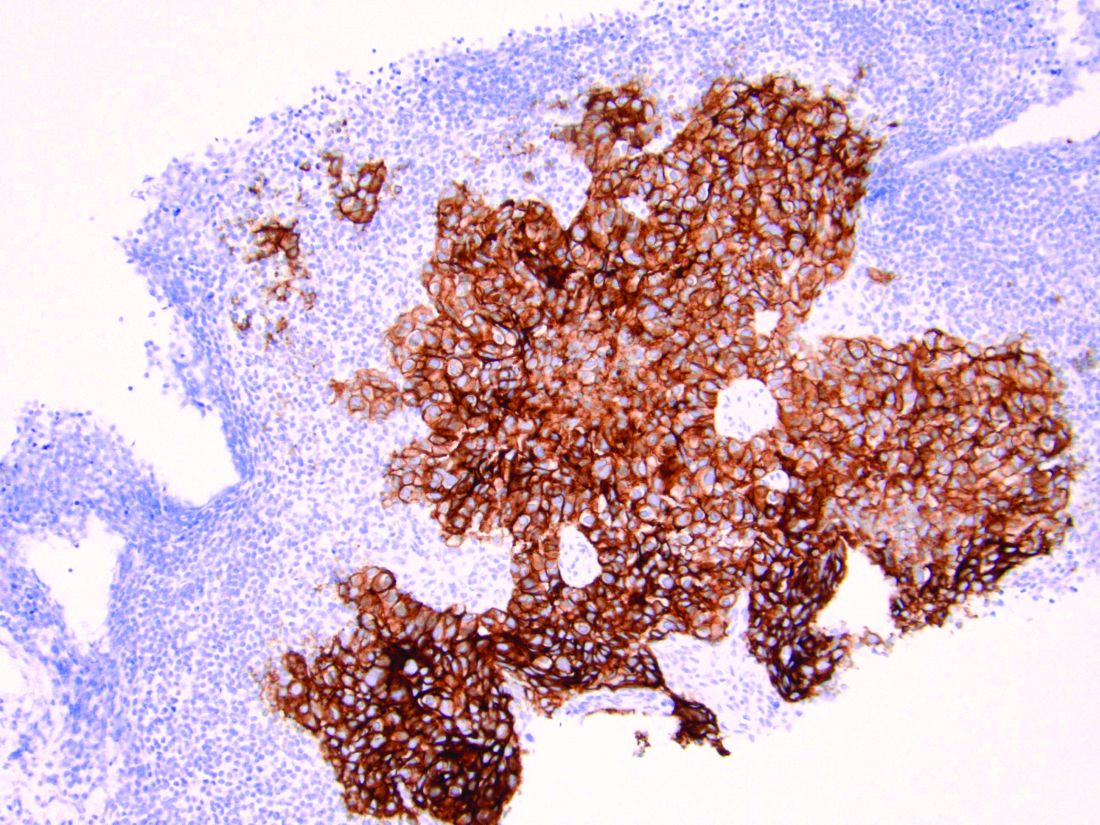
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.