User login
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
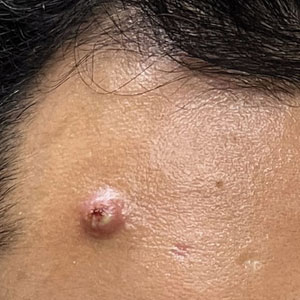
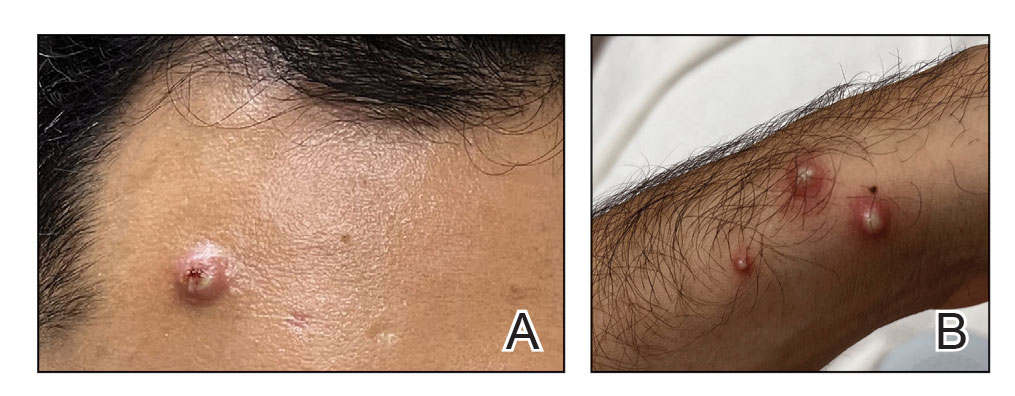
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13
A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745