User login
The Diagnosis: Chromoblastomycosis
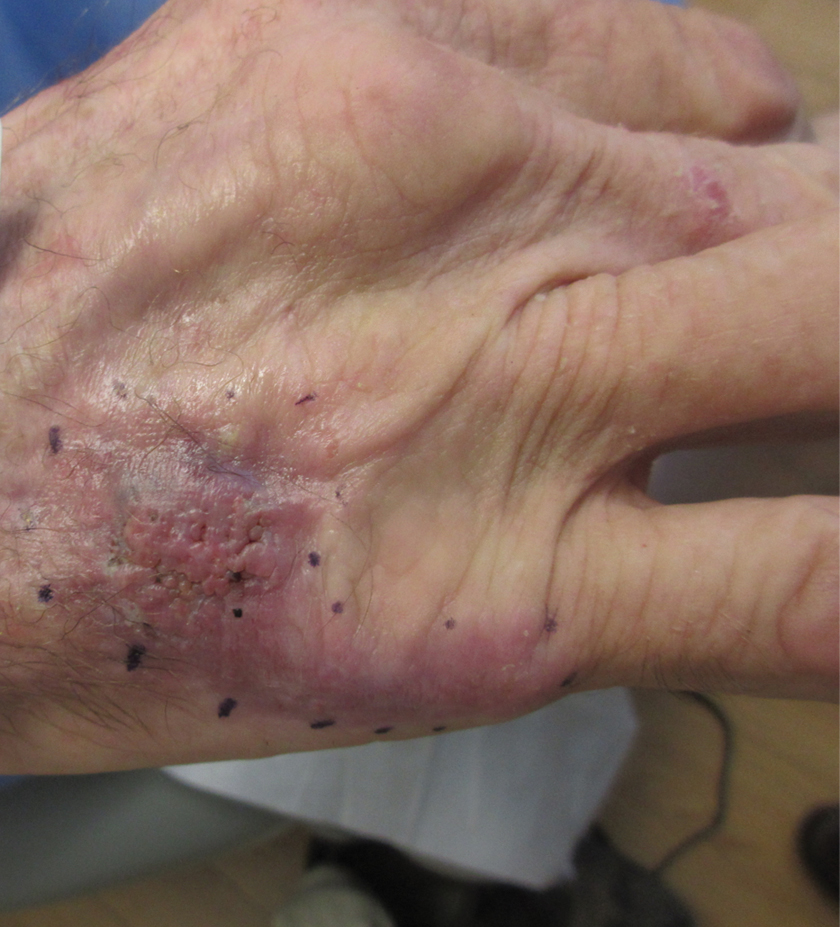
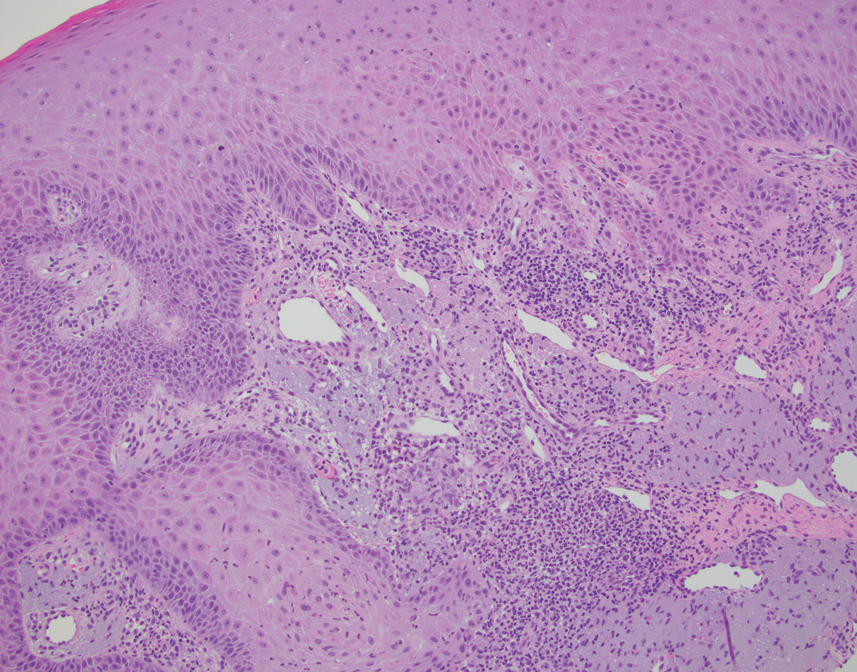
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.
Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.

Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.

Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
A 70-year-old immunocompetent man presented to the dermatology department with a progressive asymptomatic hand wound of 2 years’ duration following a splinter injury in Belize. Prior treatment included oral antibiotics without improvement. Physical examination revealed a 5.1×3.0 cm, pink to violaceous, nonpurulent plaque with a cobblestonelike appearance on the dorsal aspect of the right hand. Both the initial and a repeat skin biopsy revealed nonspecific changes, including hyperkeratosis, hypergranulosis, acute and chronic inflammation, and vascular ectasia. Grocott-Gomori methenamine-silver staining was negative for fungal organisms. One month after the repeat biopsy, a tissue culture returned positive for the rare Fonsecaea pedrosoi.