User login
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
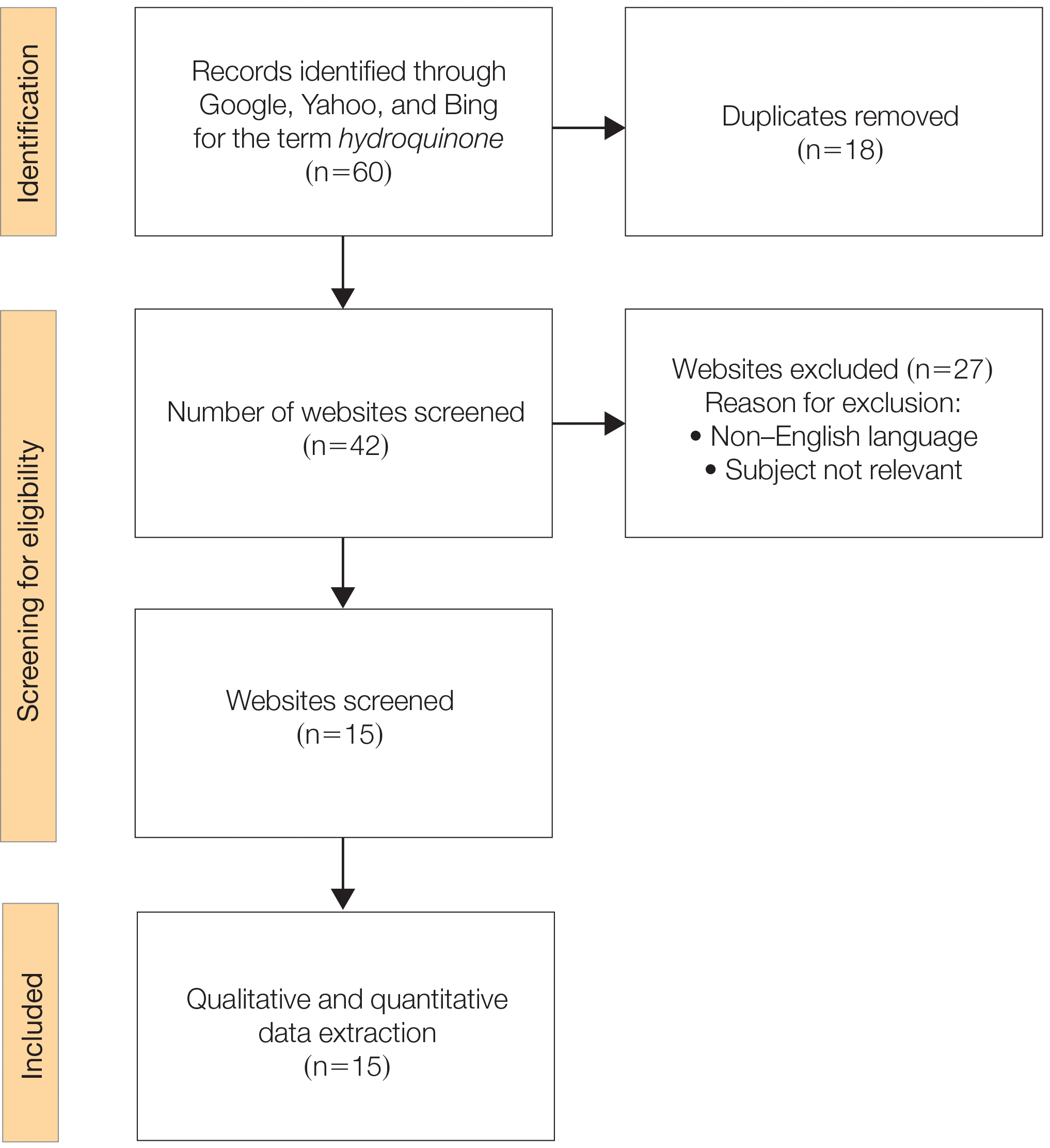
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
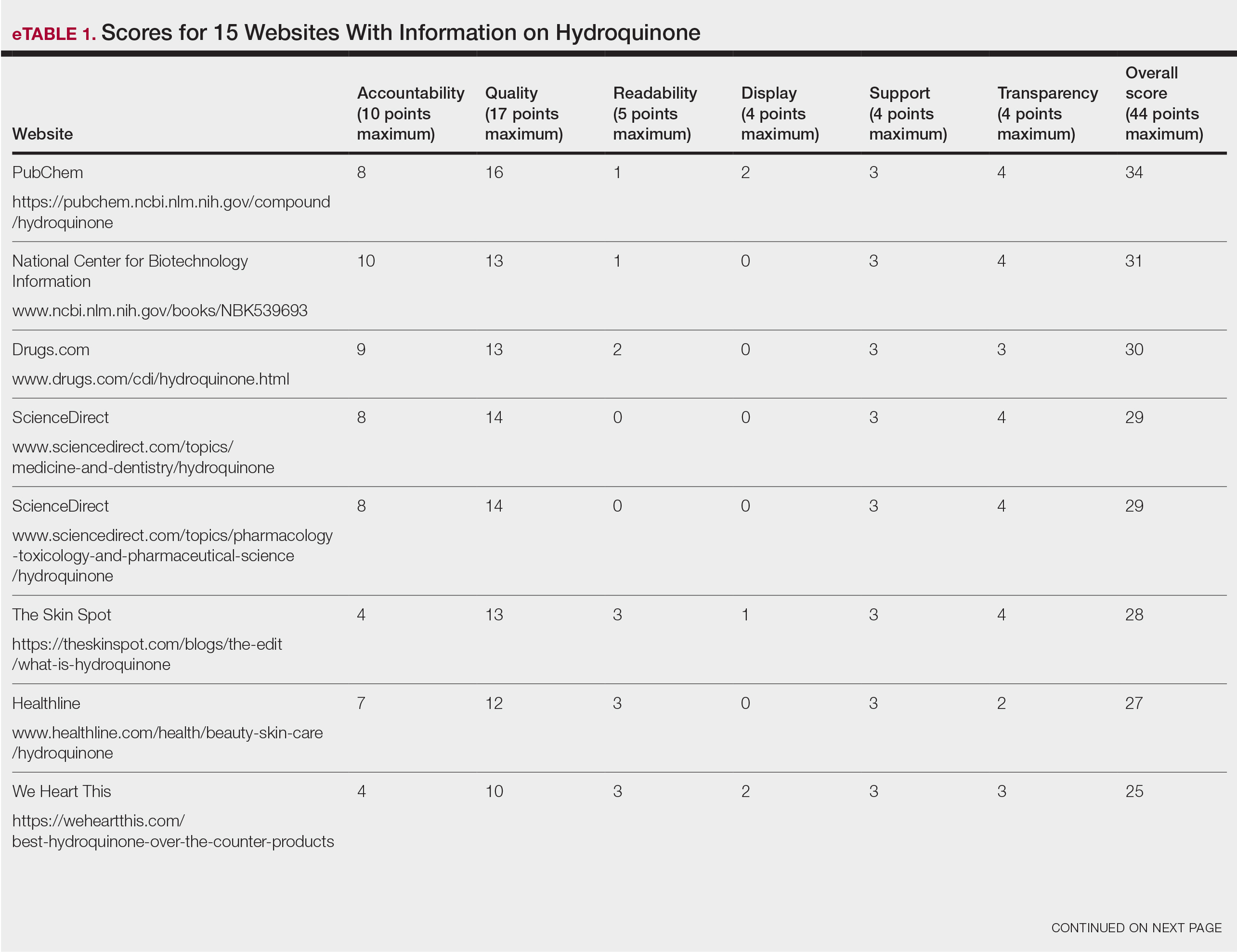
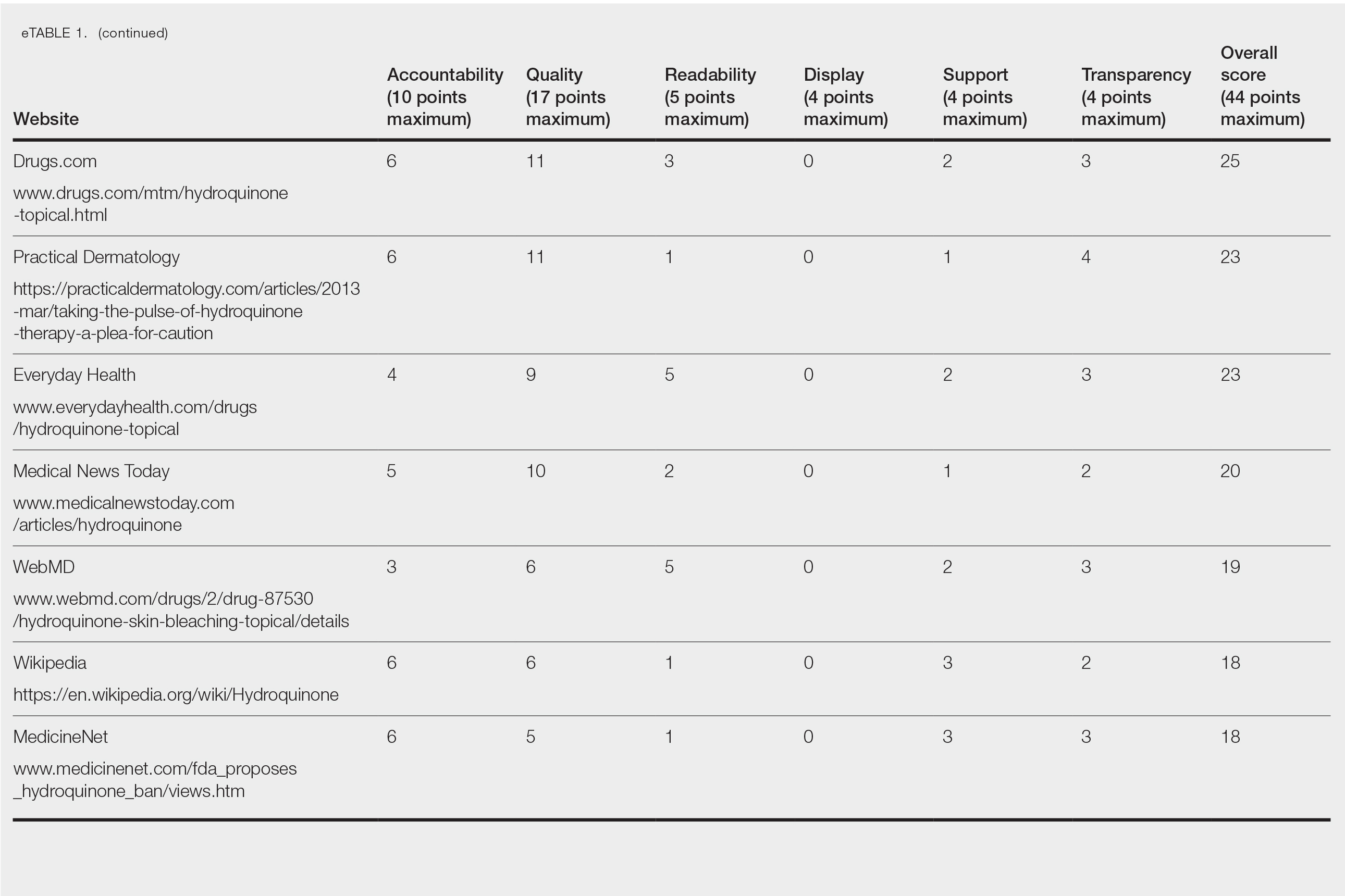
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
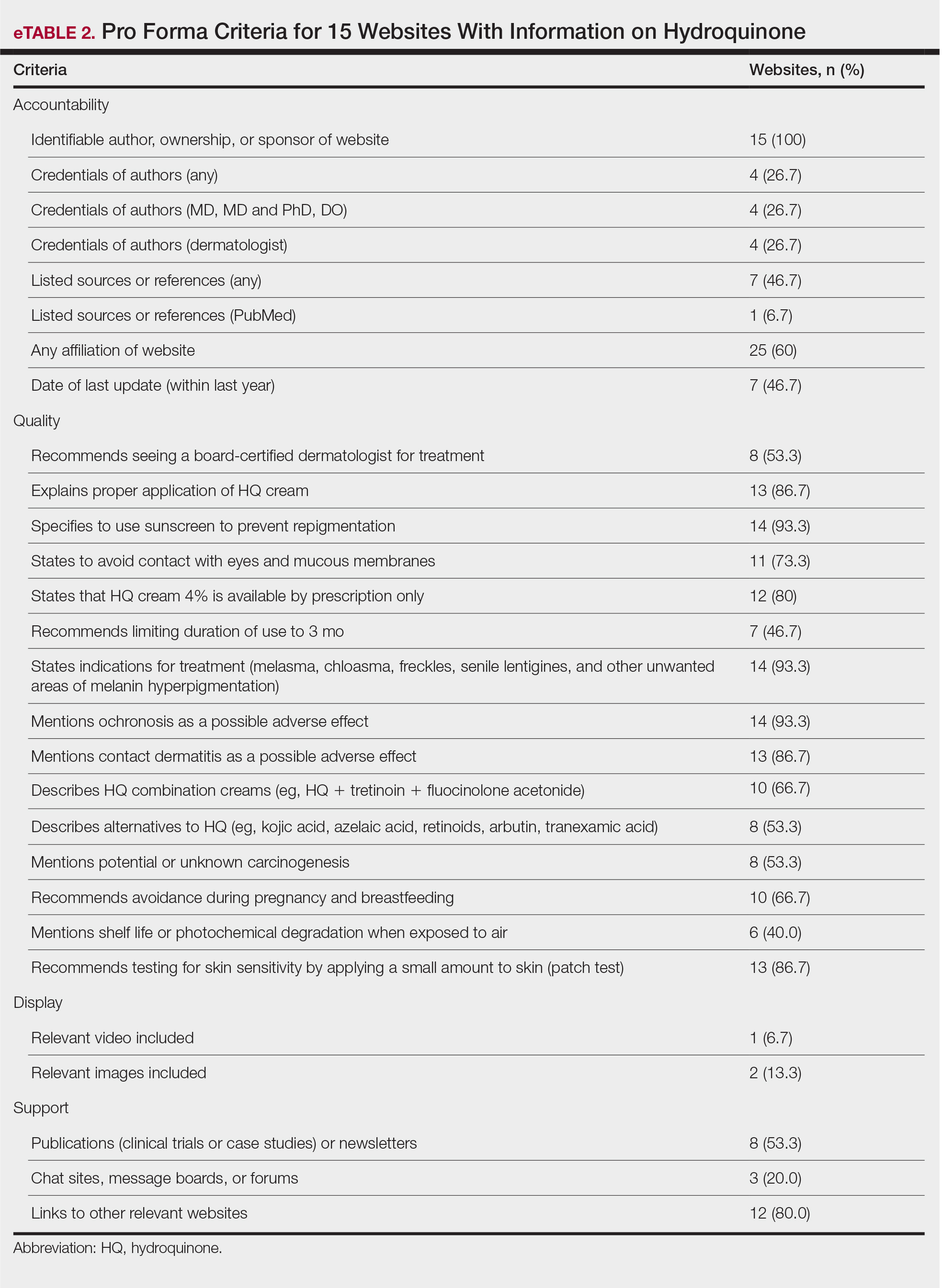
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
Practice Points
- Hydroquinone (HQ) 4% is US Food and Drug Administration (FDA) approved for skin hyperpigmentation including melasma.
- In September 2020, the FDA enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform, announcing that HQ is not classified as Category II/not generally recognized as safe and effective, thus prohibiting the distribution of OTC HQ products.
- Exogenous ochronosis is a potential side effect associated with HQ.
- There is a need for dermatologists to develop online patient education materials on HQ that are characterized by high-quality and up-to-date medical information.