User login
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
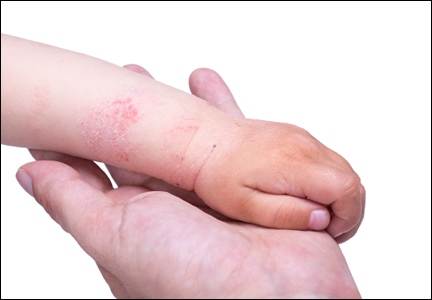
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
Practice Points
- Atopic dermatitis (AD) has a variety of comorbidities including psychosocial disorders, obesity, and infection.
- A variety of skin conditions can mimic AD.
- Atopic dermatitis can be complicated by coinfections.