User login
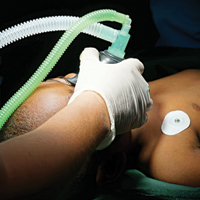
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
Clinicians who have attempted to perform an in-office procedure on infants or young children will recognize the difficulties that arise from the developmental inability to cooperate with procedures.1 Potential problems mentioned in the literature include but are not limited to anxiety, which is identified in all age groups of patients undergoing dermatologic procedures2; limitation of pain control3; and poor outcomes due to movement by the patient.1 In one author’s experience (N.B.S.), anxious and scared children can potentially cause injury to themselves, parents/guardians, and health care professionals by flailing and kicking; children are flexible and can wriggle out of even fine grips, and some children, especially toddlers, can be strong.
The usage of topical anesthetics can only give superficial anesthesia. They can ostensibly reduce pain and are useful for anesthesia of curettage, but their use is limited in infants and young children by the minimal amount of drug that is safe for application, as risks of absorption include methemoglobinemia and seizure
General anesthesia seems to be the best alternative due to associated amnesia of the events occurring including pain; immobilization and ability to produce more accurate biopsy sampling; better immobilization leading to superior cosmetic results; and reduced risk to patients, parents/guardians, and health care professionals from a flailing child. In the field of pediatric dermatology, general anesthesia often is used for excision of larger lesions and cosmetic repairs. Operating room privileges are not always easy to obtain, but many pediatric dermatologists take advantage of outpatient surgical centers associated with their medical center. A retrospective review of 226 children receiving 681 procedures at a single institution documented low rates of complications.1
If it was that easy, most children would be anesthetized with general anesthesia. However, there are risks associated with general anesthesia. Parents/guardians often will do what they can to avoid risk and may therefore refuse general anesthesia, but it is not completely avoidable in complicated skin disease. Despite the risks, the benefit is present in a major anomaly correction such as a cleft palate in a 6-month-old but may not be there for the treatment of a wart. When procedures are nonessential or may be conducted without anesthesia, avoidance of general anesthesia is reasonable and a combination of topical and local infiltrative anesthesia can help. In the American Academy of Dermatology guidelines on in-office anesthesia, Kouba et al5 states: “Topical agents are recommended as a first-line method of anesthesia for the repair of dermal lacerations in children and for other minor dermatologic procedures, including curettage. For skin biopsy, excision, or other cases where topical agents alone are insufficient, adjunctive use of topical anesthesia to lessen the discomfort of infiltrative anesthetic should be considered.”
A new controversy recently has emerged concerning the potential risks of anesthesia on neurocognitive development in infants and young children. These concerns regardingthe labeling changes of anesthetic and sedation drugs by the US Food and Drug Administration (FDA) in December 2016 specifically focused on these risks in children younger than 3 years with prolonged (>3 hours) and repeated exposures; however, this kind of exposure is unlikely with standard pediatric dermatologic procedures.6-9
There is compelling evidence from animal studies that exposure to all anesthetic agents in clinical use induces neurotoxicity and long-term adverse neurobehavioral deficits; however, whether these findings are applicable in human infants is unknown.6-9 Most of the studies in humans showing adverse outcomes have been retrospective observational studies subject to multiple sources of bias. Two recent large clinical studies—the GAS (General Anaesthesia compared to Spinal anaesthesia) trial10 and the PANDA (Pediatric Anesthesia and Neurodevelopment Assessment) study11—have shown no evidence of abnormal neurocognitive effects with a single brief exposure before 3 years of age (PANDA) or during infancy (GAS) in otherwise-healthy children.10,11
It is important to note that the FDA labeling change warning specifically stated that “[c]onsistent with animal studies, recent human data suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” Moreover, the FDA emphasized that “Surgeries or procedures in children younger than 3 years should not be delayed or avoided when medically necessary.”12 Taking these points into consideration, we should offer our patients in-office care when possible and postpone elective procedures when advisable but proceed when necessary for our patients’ physical and emotional health.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.
- Juern AM, Cassidy LD, Lyon VB. More evidence confirming the safety of general anesthesia in pediatric dermatologic surgery. Pediatr Dermatol. 2010;27:355-360.
- Gerwels JW, Bezzant JL, Le Maire L, et al. Oral transmucosal fentanyl citrate premedication in patients undergoing outpatient dermatologic procedures. J Dermatol Surg Oncol. 1994;20:823-826.
- D’Acunto C, Raone B, Neri I, et al. Outpatient pediatric dermatologic surgery: experience in 296 patients. Pediatr Dermatol. 2015;32:424-426.
- Gunter JB. Benefit and risks of local anesthetics in infants and children. Paediatr Drugs. 2002;4:649-672.
- Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery [published online March 4, 2016]. J Am Acad Dermatol. 2016;74:1201-1219.
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
- Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834-841.
- Raper J, Alvarado MC, Murphy KL, et al. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084-1092.
- Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatric Anaesthesia. 2011;21:716-721.
- Davidson AJ, Disma N, de Graaff JC, et al; GAS consortium. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239-250.
- Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312-2320.
- General anesthetic and sedation drugs: drug safety communication—new warnings for young children and pregnant women. US Food and Drug Administration website. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm533195.htm. Published December 14, 2016. Accessed July 25, 2017.