User login
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
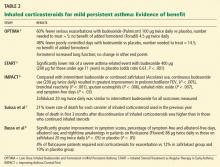
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.