User login
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
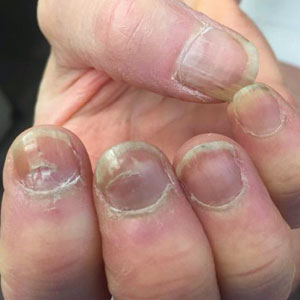
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).
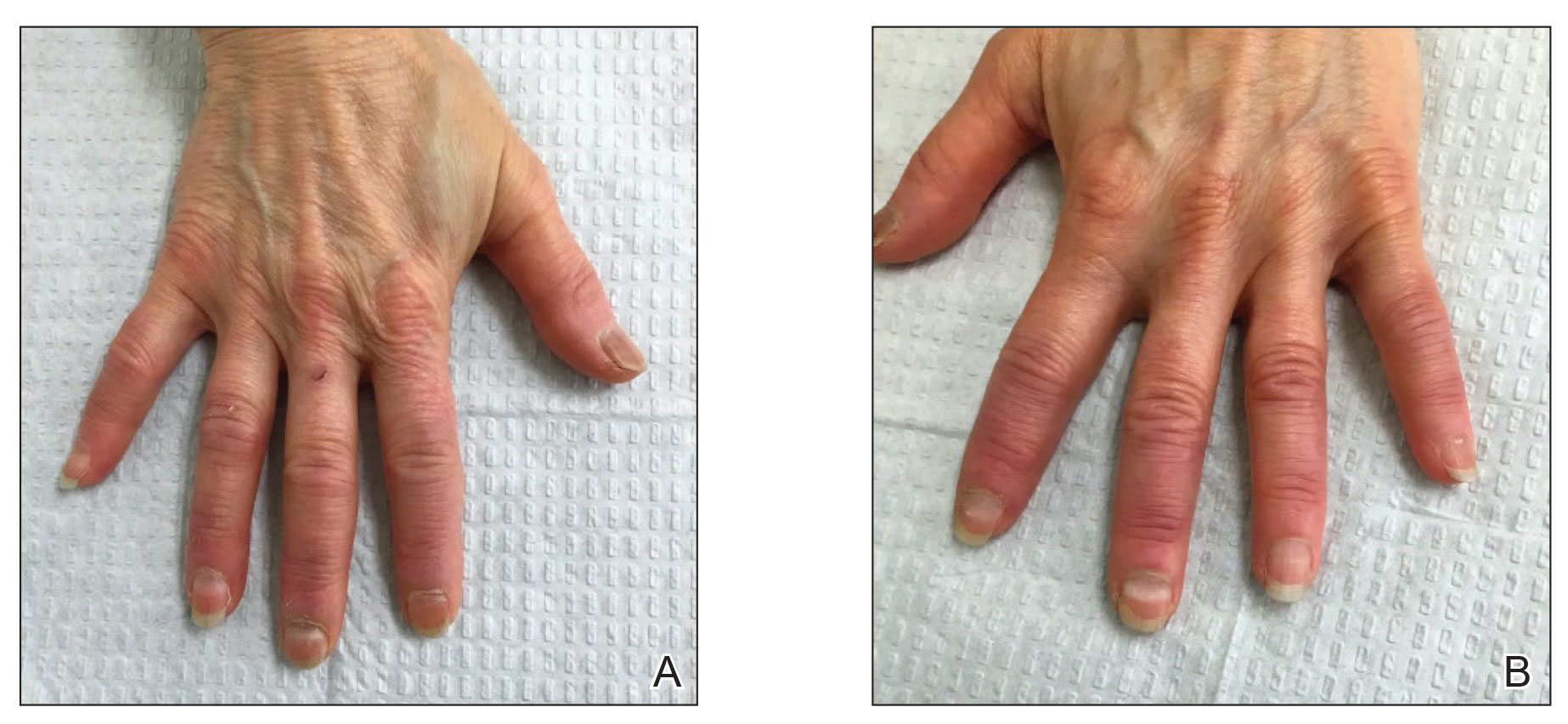
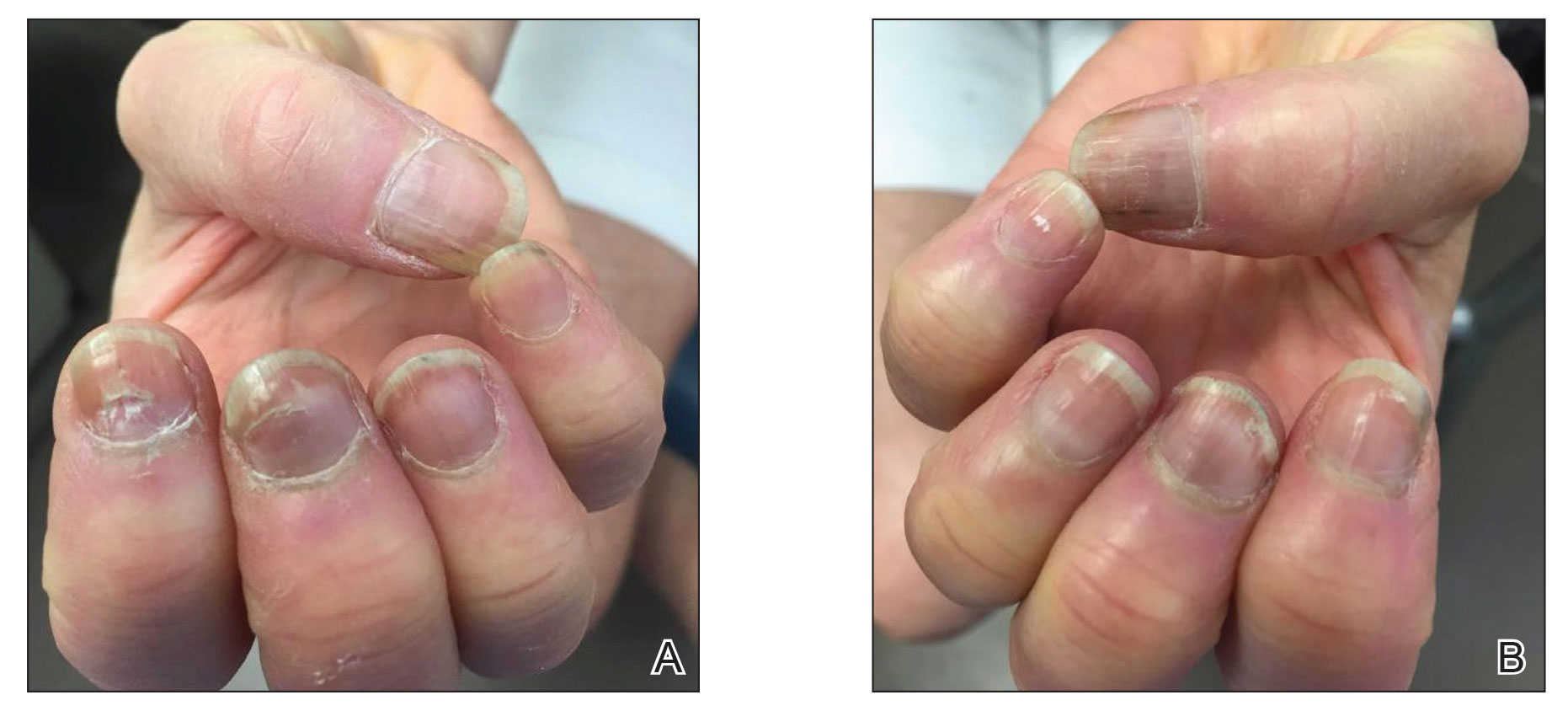
Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.
She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
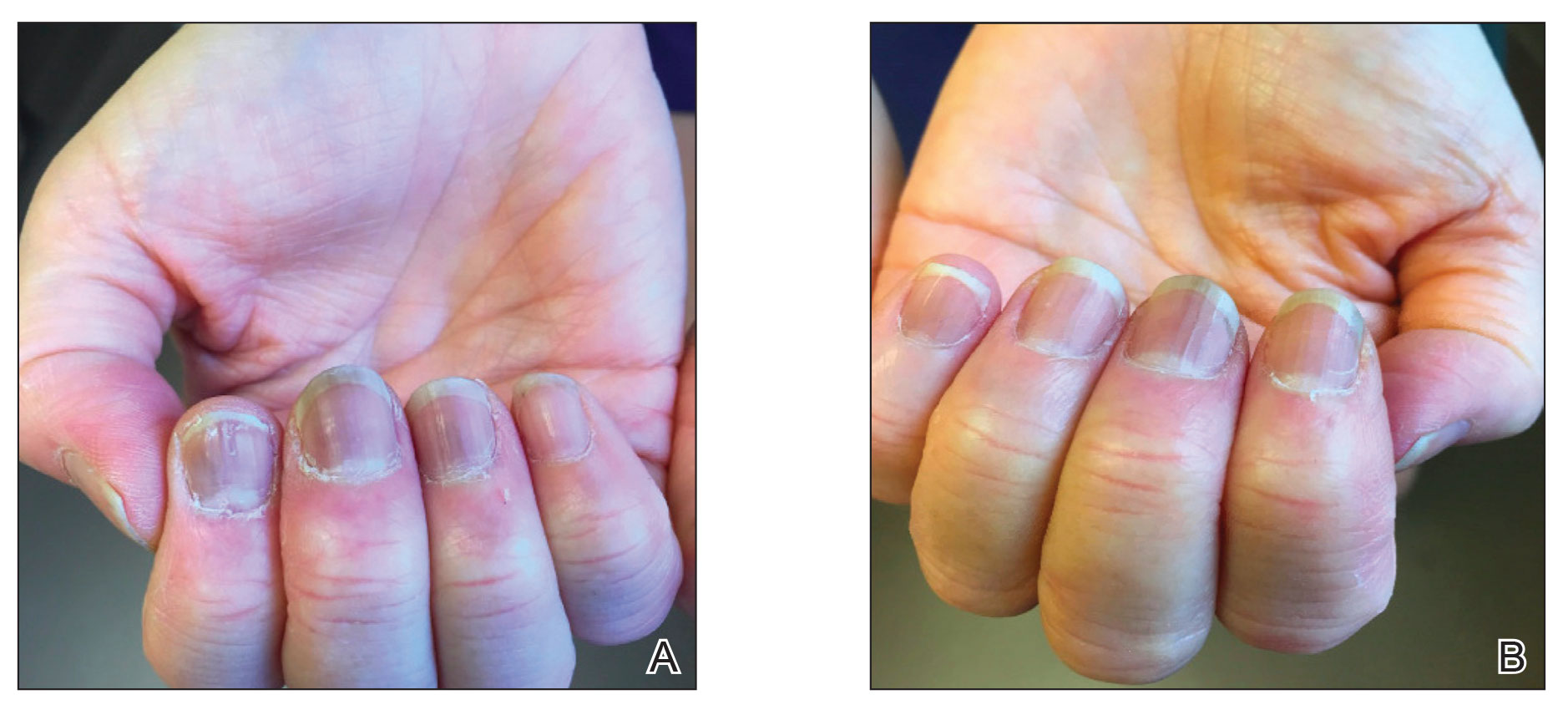
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.
Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
Practice Points
- Given accelerated global vaccination efforts to control the COVID-19 pandemic, cases of nail changes associated with COVID-19 vaccines are expected.
- Nail abnormalities are a potential general, temporary, and self-limiting adverse effect of COVID-19 vaccines that should not discourage patients from getting vaccinated.