User login
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
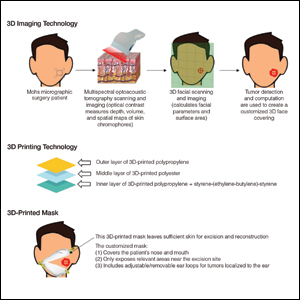
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
Practice Points
- Coronavirus disease 19 has overwhelmed our health care system and affected all specialties, including dermatology.
- There are concerns about shortages of personal protective equipment to safely care for patients.
- 3-Dimensional imaging and printing technologies can be harnessed to create face coverings and face shields for the dermatology outpatient setting.