User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
‘Appreciate the editorials’
I appreciate Dr. Nasrallah’s editorial on the so-called abdominal brain (Current Psychiatry. 2015;14(5):10- 11 [http://bit.ly/1PcxFNP]). Current Psychiatry is a journal with useful reports of advances, reviews, and opinion of research and treatment in our specialty. The selections and editing are always pertinent and thoughtful.
I appreciate Dr. Nasrallah’s editorial on the so-called abdominal brain (Current Psychiatry. 2015;14(5):10- 11 [http://bit.ly/1PcxFNP]). Current Psychiatry is a journal with useful reports of advances, reviews, and opinion of research and treatment in our specialty. The selections and editing are always pertinent and thoughtful.
I appreciate Dr. Nasrallah’s editorial on the so-called abdominal brain (Current Psychiatry. 2015;14(5):10- 11 [http://bit.ly/1PcxFNP]). Current Psychiatry is a journal with useful reports of advances, reviews, and opinion of research and treatment in our specialty. The selections and editing are always pertinent and thoughtful.
Practical approaches to promoting brain health
More than once in his Current Psychiatry essays, Henry A. Nasrallah, MD, has stressed the seismic paradigmatic shifts in our understanding of mental illness and brain disease. He has highlighted the critical significance of processes of neurogenesis and neuroinflammation, yet little has been offered to practitioners in terms of practical approaches to promoting the brain health that he encourages.
Two of the most potent modalities for maintaining brain wellness and facilitating ongoing neurogenesis and synaptogenesis are exercise and nutrition—specifically, high-intensity interval training and a diet heavily, if not entirely, plant-based. The neuroprotective capabilities of mindfulness practice and its impact on prefrontal cortical regions also are relevant.
In society at large, it strikes me that physicians have not fared any better than the general population when it comes to maintaining a healthy diet and engaging in physical exercise. I encourage Dr. Nasrallah to continue addressing these themes, and to remind his audience of physicians to “heal thyself.”
More than once in his Current Psychiatry essays, Henry A. Nasrallah, MD, has stressed the seismic paradigmatic shifts in our understanding of mental illness and brain disease. He has highlighted the critical significance of processes of neurogenesis and neuroinflammation, yet little has been offered to practitioners in terms of practical approaches to promoting the brain health that he encourages.
Two of the most potent modalities for maintaining brain wellness and facilitating ongoing neurogenesis and synaptogenesis are exercise and nutrition—specifically, high-intensity interval training and a diet heavily, if not entirely, plant-based. The neuroprotective capabilities of mindfulness practice and its impact on prefrontal cortical regions also are relevant.
In society at large, it strikes me that physicians have not fared any better than the general population when it comes to maintaining a healthy diet and engaging in physical exercise. I encourage Dr. Nasrallah to continue addressing these themes, and to remind his audience of physicians to “heal thyself.”
More than once in his Current Psychiatry essays, Henry A. Nasrallah, MD, has stressed the seismic paradigmatic shifts in our understanding of mental illness and brain disease. He has highlighted the critical significance of processes of neurogenesis and neuroinflammation, yet little has been offered to practitioners in terms of practical approaches to promoting the brain health that he encourages.
Two of the most potent modalities for maintaining brain wellness and facilitating ongoing neurogenesis and synaptogenesis are exercise and nutrition—specifically, high-intensity interval training and a diet heavily, if not entirely, plant-based. The neuroprotective capabilities of mindfulness practice and its impact on prefrontal cortical regions also are relevant.
In society at large, it strikes me that physicians have not fared any better than the general population when it comes to maintaining a healthy diet and engaging in physical exercise. I encourage Dr. Nasrallah to continue addressing these themes, and to remind his audience of physicians to “heal thyself.”
Urine drug screens: When might a test result be false-positive?
Mr. L, age 35, has an appointment at a mental health clinic for ongoing treatment of depression. His medication list includes atorvastatin, bupropion, lisinopril, and cranberry capsules for non-descriptive urinary issues. He has been treated for some time at a different outpatient facility; however he recently moved and changed clinics.
At this visit, his first, Mr. L receives a full physical exam, including a urine drug screen point-of-care (POC) test. He informs the nurse that he has an extensive history of drug abuse: “You name it, I’ve done it.” Although he experimented with many illicit substances, he acknowledges that “downers” were his favorite. He believes that his drug abuse could have caused his depression, but is proud to declare that he has been “clean” for 12 months and his depression is approaching remission.
However, the urine drug screen is positive for amphetamines. Mr. L vehemently swears that the test must be wrong, restating that he has been clean for 12 months. “Besides, I don’t even like ‘uppers’!” Because of Mr. L’s insistence, the clinician does a brief literature search about false-positive results in urine drug screening, which shows that, rarely, bupropion can trigger a false positive in the amphetamine immunoassay.
Could this be a false-positive result? Or is Mr. L not telling the truth?
Because no clinical lab test is perfect, any clinician who runs urine drug screens will encounter a false-positive result. (See the Box,1-3 for discussion of false negatives.) Understanding how each test works—and potential sources of error— can help you evaluate test results and determine the best course of action.
There are 2 main methods involved in urine drug testing: in-office (POC) urine testing and laboratory-based testing. This article describes the differences between these tests and summarizes the potential for false-positive results.
In-office urine testing
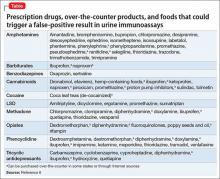
POC tests in urine drug screens use a technique called “immunoassay,” which is quantitative and generally will detect the agent in urine for only 3 to 7 days after ingestion.4 This test relies on the principle of competitive binding: If a parent drug or metabolite is present in urine, it will bind to a specific antibody site on the test strip and produce a positive result.5 Other compounds that are similarly “shaped” on a molecular level also can bind to these antibody sites when present in sufficient quantity, producing a “cross reaction,” also called a “false-positive” result. The Table6 lists agents that can cross-react with immunoassay tests. In addition to the cross-reaction, false positives also can occur because of technician or clerical error— making it important to review the process by which the specimen was obtained and tested if a false-positive result is suspected, as in the case described here.7
Different POC tests can have varying cross-reactivity patterns, based on the antibody used.8 In general, false positives in immunoassays are rare, but amphetamine and opiate false positives are more common than cocaine metabolite and cannabinoid false positives.9 The odds of a false positive vary, depending on the specificity of the immunoassay used and the substance under detection.6
A study that analyzed 10,000 POC urine drug screens found that 362 specimens tested positive for amphetamines, but that 128 of those did not test positive for amphetamines using more sensitive tests.10 Of these 128 false positives reported, 53 patients were taking bupropion at the time of the test.10 Therefore, clinicians should do a thorough patient medication review at the time of POC urine drug testing. In addition, consider identifying which type of test you are using at your practice site, and ask the manufacturer or lab to provide a list of known possible false positives.
Laboratory-based GC–MS testing
If a false positive is suspected on a POC immunoassay-based urine drug screen, results can be confirmed using gas chromatography–mass spectrometry (GC–MS). Although GC–MS is more accurate than an immunoassay, it also is more expensive and time-consuming.9
GC–MS breaks down a specimen into ionized fragments and separates them based on their mass–charge ratio. Because of this, GC–MS is able to identify the presence of a specific drug (eg, oxycodone) instead of a broad class (eg, opioid). The GC–MS method is a good tool to confirm initial positive screens when their integrity is in question because, unlike POC tests used during an office visit, GC–MS is not influenced by cross-reacting compounds.11-13
GC–MS is not error-free, however. For example, heroin and hydrocodone are metabolized into morphine and hydromorphone, respectively. Depending on when the specimen was collected, the metabolites, not the parents, might be the compounds identified, which might produce confusing results.
Clinical recommendations
When a POC drug screen is positive, confirming the result with GC–MS is good clinical practice. False positives can strain the relationship between patient and provider, thus compromising care. Examining the procedures that were used to obtain the specimen, as well as double-checking POC test results, is, when appropriate, good medicine.
CASE CONTINUED
Because Mr. L is adamant about his sobriety and the fact that his drugs of choice were sedatives, not stimulants, the clinician orders a second drug screen by GC–MS. The second screen is negative for substances of abuse; Mr. L’s clinician concludes that bupropion produced a false-positive result on the POC urine drug screen, confirming Mr. L’s assertions.
Related Resources
• Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
• Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
Drug Brand Names
Amantadine • Symadine, Symmetrel
Amitriptyline • Elavil
Atorvastatin • Lipitor
Brompheniramine • Dimetane
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clomipramine • Anafranil
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Desipramine • Nopramin
Desoxyephedrine • Desoxyn
Dextromethorphan • Delsym, Robitussin
Dicyclomine • Bentyl, Dicyclocot
Diphenhydramine • Benadryl, Unisom
Doxylamine • Robitussin, NyQuil
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Mistol, Va-Tro-Nol
Ergotamine • Ergomar, Cafergot
Hydrocodone • Vicodin
Hydromophone • Dilaudid, Palladone
Hydroxyzine • Atarax, Vistaril
Isometheptene • Amidrine, Migrend
Isoxsuprine • Vasodilan, Vasoprine
Ketoprofen • Orudis, Oruvail
Labetalol • Normodyne, Trandate
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Naproxen • Aleve, Naprosyn
Oxaprozin • Daypro
Oxycodone • Oxycontin, Percocet, Percodan, Roxicodone
Phentermine • Adipex, Phentrol
Phenylephrine • Sudafed PE, Neo-Synephrine
Piroxicam • Feldene
Promethazine • Phenergan
Pseudoephedrine • Sudafed, Dimetapp
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampin • Rifadin, Rimactane
Selegiline • EMSAM
Sertraline • Zoloft
Sulindac • Clinoril
Sumatriptan • Imitrex
Thioridazine • Mellaril
Tolmetin • Tolectin
Trazodone • Desyrel, Oleptro
Trimethobenzamide • Benzacot, Tigan
Trimipramine • Surmontil
Verapamil • Calan, Isoptin
1. Cobaugh DJ, Gainor C, Gaston CL, et al. The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm. 2014;71(18):1539-1554.
2. Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent medicare policy changes in Kentucky. Pain Physician. 2010;13(2):167-186.
3. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
4. U.S. Department of Justice. Fact sheet: drug testing in the criminal justice system. https://www.ncjrs.gov/pdffiles/dtest. pdf. Published March 1992. Accessed July 29, 2015.
5. Australian Diagnostic Services. Technical information: testing principle’s. http://www.australiandrugtesting. com/#!technical-info/c14h4. Accessed November 5, 2014.
6. University of Illinois at Chicago College of Pharmacy. What drugs are likely to interfere with urine drug screens? http://dig.pharm.uic.edu/faq/2011/Feb/faq1.aspx. Accessed November 5, 2014.
7. Wolff K, Farrell M, Marsden J, et al. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94(9):1279-1298.
8. Gourlay D, Heit H, Caplan YH. Urine drug testing in primary care – dispelling the myths & designing strategies. PharmaCom Group. http://www.mc.uky.edu/equip-4-pcps/documents/ section8/urine%20drug%20testing%20in%20clinical%20 practice.pdf. Accessed August 6, 2015.
9. Standridge JB, Adams SM, Zotos AP. Urine drug screen: a valuable office procedure. Am Fam Physician. 2010;81(5): 635-640.
10. Casey ER, Scott MG, Tang S, et al. Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay. J Med Toxicol. 2011;7(2):105-108.
11. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin N Am. 2002;49(2):317-327.
12. Shults TF. The medical review officer handbook. 7th ed. Chapel Hill, NC: Quadrangle Research; 1999.
13. Baden LR, Horowitz G, Jacoby H, et al. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286(24):3115-3119.
Mr. L, age 35, has an appointment at a mental health clinic for ongoing treatment of depression. His medication list includes atorvastatin, bupropion, lisinopril, and cranberry capsules for non-descriptive urinary issues. He has been treated for some time at a different outpatient facility; however he recently moved and changed clinics.
At this visit, his first, Mr. L receives a full physical exam, including a urine drug screen point-of-care (POC) test. He informs the nurse that he has an extensive history of drug abuse: “You name it, I’ve done it.” Although he experimented with many illicit substances, he acknowledges that “downers” were his favorite. He believes that his drug abuse could have caused his depression, but is proud to declare that he has been “clean” for 12 months and his depression is approaching remission.
However, the urine drug screen is positive for amphetamines. Mr. L vehemently swears that the test must be wrong, restating that he has been clean for 12 months. “Besides, I don’t even like ‘uppers’!” Because of Mr. L’s insistence, the clinician does a brief literature search about false-positive results in urine drug screening, which shows that, rarely, bupropion can trigger a false positive in the amphetamine immunoassay.
Could this be a false-positive result? Or is Mr. L not telling the truth?
Because no clinical lab test is perfect, any clinician who runs urine drug screens will encounter a false-positive result. (See the Box,1-3 for discussion of false negatives.) Understanding how each test works—and potential sources of error— can help you evaluate test results and determine the best course of action.
There are 2 main methods involved in urine drug testing: in-office (POC) urine testing and laboratory-based testing. This article describes the differences between these tests and summarizes the potential for false-positive results.
In-office urine testing
POC tests in urine drug screens use a technique called “immunoassay,” which is quantitative and generally will detect the agent in urine for only 3 to 7 days after ingestion.4 This test relies on the principle of competitive binding: If a parent drug or metabolite is present in urine, it will bind to a specific antibody site on the test strip and produce a positive result.5 Other compounds that are similarly “shaped” on a molecular level also can bind to these antibody sites when present in sufficient quantity, producing a “cross reaction,” also called a “false-positive” result. The Table6 lists agents that can cross-react with immunoassay tests. In addition to the cross-reaction, false positives also can occur because of technician or clerical error— making it important to review the process by which the specimen was obtained and tested if a false-positive result is suspected, as in the case described here.7
Different POC tests can have varying cross-reactivity patterns, based on the antibody used.8 In general, false positives in immunoassays are rare, but amphetamine and opiate false positives are more common than cocaine metabolite and cannabinoid false positives.9 The odds of a false positive vary, depending on the specificity of the immunoassay used and the substance under detection.6
A study that analyzed 10,000 POC urine drug screens found that 362 specimens tested positive for amphetamines, but that 128 of those did not test positive for amphetamines using more sensitive tests.10 Of these 128 false positives reported, 53 patients were taking bupropion at the time of the test.10 Therefore, clinicians should do a thorough patient medication review at the time of POC urine drug testing. In addition, consider identifying which type of test you are using at your practice site, and ask the manufacturer or lab to provide a list of known possible false positives.
Laboratory-based GC–MS testing
If a false positive is suspected on a POC immunoassay-based urine drug screen, results can be confirmed using gas chromatography–mass spectrometry (GC–MS). Although GC–MS is more accurate than an immunoassay, it also is more expensive and time-consuming.9
GC–MS breaks down a specimen into ionized fragments and separates them based on their mass–charge ratio. Because of this, GC–MS is able to identify the presence of a specific drug (eg, oxycodone) instead of a broad class (eg, opioid). The GC–MS method is a good tool to confirm initial positive screens when their integrity is in question because, unlike POC tests used during an office visit, GC–MS is not influenced by cross-reacting compounds.11-13
GC–MS is not error-free, however. For example, heroin and hydrocodone are metabolized into morphine and hydromorphone, respectively. Depending on when the specimen was collected, the metabolites, not the parents, might be the compounds identified, which might produce confusing results.
Clinical recommendations
When a POC drug screen is positive, confirming the result with GC–MS is good clinical practice. False positives can strain the relationship between patient and provider, thus compromising care. Examining the procedures that were used to obtain the specimen, as well as double-checking POC test results, is, when appropriate, good medicine.
CASE CONTINUED
Because Mr. L is adamant about his sobriety and the fact that his drugs of choice were sedatives, not stimulants, the clinician orders a second drug screen by GC–MS. The second screen is negative for substances of abuse; Mr. L’s clinician concludes that bupropion produced a false-positive result on the POC urine drug screen, confirming Mr. L’s assertions.
Related Resources
• Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
• Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
Drug Brand Names
Amantadine • Symadine, Symmetrel
Amitriptyline • Elavil
Atorvastatin • Lipitor
Brompheniramine • Dimetane
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clomipramine • Anafranil
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Desipramine • Nopramin
Desoxyephedrine • Desoxyn
Dextromethorphan • Delsym, Robitussin
Dicyclomine • Bentyl, Dicyclocot
Diphenhydramine • Benadryl, Unisom
Doxylamine • Robitussin, NyQuil
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Mistol, Va-Tro-Nol
Ergotamine • Ergomar, Cafergot
Hydrocodone • Vicodin
Hydromophone • Dilaudid, Palladone
Hydroxyzine • Atarax, Vistaril
Isometheptene • Amidrine, Migrend
Isoxsuprine • Vasodilan, Vasoprine
Ketoprofen • Orudis, Oruvail
Labetalol • Normodyne, Trandate
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Naproxen • Aleve, Naprosyn
Oxaprozin • Daypro
Oxycodone • Oxycontin, Percocet, Percodan, Roxicodone
Phentermine • Adipex, Phentrol
Phenylephrine • Sudafed PE, Neo-Synephrine
Piroxicam • Feldene
Promethazine • Phenergan
Pseudoephedrine • Sudafed, Dimetapp
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampin • Rifadin, Rimactane
Selegiline • EMSAM
Sertraline • Zoloft
Sulindac • Clinoril
Sumatriptan • Imitrex
Thioridazine • Mellaril
Tolmetin • Tolectin
Trazodone • Desyrel, Oleptro
Trimethobenzamide • Benzacot, Tigan
Trimipramine • Surmontil
Verapamil • Calan, Isoptin
Mr. L, age 35, has an appointment at a mental health clinic for ongoing treatment of depression. His medication list includes atorvastatin, bupropion, lisinopril, and cranberry capsules for non-descriptive urinary issues. He has been treated for some time at a different outpatient facility; however he recently moved and changed clinics.
At this visit, his first, Mr. L receives a full physical exam, including a urine drug screen point-of-care (POC) test. He informs the nurse that he has an extensive history of drug abuse: “You name it, I’ve done it.” Although he experimented with many illicit substances, he acknowledges that “downers” were his favorite. He believes that his drug abuse could have caused his depression, but is proud to declare that he has been “clean” for 12 months and his depression is approaching remission.
However, the urine drug screen is positive for amphetamines. Mr. L vehemently swears that the test must be wrong, restating that he has been clean for 12 months. “Besides, I don’t even like ‘uppers’!” Because of Mr. L’s insistence, the clinician does a brief literature search about false-positive results in urine drug screening, which shows that, rarely, bupropion can trigger a false positive in the amphetamine immunoassay.
Could this be a false-positive result? Or is Mr. L not telling the truth?
Because no clinical lab test is perfect, any clinician who runs urine drug screens will encounter a false-positive result. (See the Box,1-3 for discussion of false negatives.) Understanding how each test works—and potential sources of error— can help you evaluate test results and determine the best course of action.
There are 2 main methods involved in urine drug testing: in-office (POC) urine testing and laboratory-based testing. This article describes the differences between these tests and summarizes the potential for false-positive results.
In-office urine testing
POC tests in urine drug screens use a technique called “immunoassay,” which is quantitative and generally will detect the agent in urine for only 3 to 7 days after ingestion.4 This test relies on the principle of competitive binding: If a parent drug or metabolite is present in urine, it will bind to a specific antibody site on the test strip and produce a positive result.5 Other compounds that are similarly “shaped” on a molecular level also can bind to these antibody sites when present in sufficient quantity, producing a “cross reaction,” also called a “false-positive” result. The Table6 lists agents that can cross-react with immunoassay tests. In addition to the cross-reaction, false positives also can occur because of technician or clerical error— making it important to review the process by which the specimen was obtained and tested if a false-positive result is suspected, as in the case described here.7
Different POC tests can have varying cross-reactivity patterns, based on the antibody used.8 In general, false positives in immunoassays are rare, but amphetamine and opiate false positives are more common than cocaine metabolite and cannabinoid false positives.9 The odds of a false positive vary, depending on the specificity of the immunoassay used and the substance under detection.6
A study that analyzed 10,000 POC urine drug screens found that 362 specimens tested positive for amphetamines, but that 128 of those did not test positive for amphetamines using more sensitive tests.10 Of these 128 false positives reported, 53 patients were taking bupropion at the time of the test.10 Therefore, clinicians should do a thorough patient medication review at the time of POC urine drug testing. In addition, consider identifying which type of test you are using at your practice site, and ask the manufacturer or lab to provide a list of known possible false positives.
Laboratory-based GC–MS testing
If a false positive is suspected on a POC immunoassay-based urine drug screen, results can be confirmed using gas chromatography–mass spectrometry (GC–MS). Although GC–MS is more accurate than an immunoassay, it also is more expensive and time-consuming.9
GC–MS breaks down a specimen into ionized fragments and separates them based on their mass–charge ratio. Because of this, GC–MS is able to identify the presence of a specific drug (eg, oxycodone) instead of a broad class (eg, opioid). The GC–MS method is a good tool to confirm initial positive screens when their integrity is in question because, unlike POC tests used during an office visit, GC–MS is not influenced by cross-reacting compounds.11-13
GC–MS is not error-free, however. For example, heroin and hydrocodone are metabolized into morphine and hydromorphone, respectively. Depending on when the specimen was collected, the metabolites, not the parents, might be the compounds identified, which might produce confusing results.
Clinical recommendations
When a POC drug screen is positive, confirming the result with GC–MS is good clinical practice. False positives can strain the relationship between patient and provider, thus compromising care. Examining the procedures that were used to obtain the specimen, as well as double-checking POC test results, is, when appropriate, good medicine.
CASE CONTINUED
Because Mr. L is adamant about his sobriety and the fact that his drugs of choice were sedatives, not stimulants, the clinician orders a second drug screen by GC–MS. The second screen is negative for substances of abuse; Mr. L’s clinician concludes that bupropion produced a false-positive result on the POC urine drug screen, confirming Mr. L’s assertions.
Related Resources
• Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
• Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
Drug Brand Names
Amantadine • Symadine, Symmetrel
Amitriptyline • Elavil
Atorvastatin • Lipitor
Brompheniramine • Dimetane
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Clomipramine • Anafranil
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Desipramine • Nopramin
Desoxyephedrine • Desoxyn
Dextromethorphan • Delsym, Robitussin
Dicyclomine • Bentyl, Dicyclocot
Diphenhydramine • Benadryl, Unisom
Doxylamine • Robitussin, NyQuil
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Mistol, Va-Tro-Nol
Ergotamine • Ergomar, Cafergot
Hydrocodone • Vicodin
Hydromophone • Dilaudid, Palladone
Hydroxyzine • Atarax, Vistaril
Isometheptene • Amidrine, Migrend
Isoxsuprine • Vasodilan, Vasoprine
Ketoprofen • Orudis, Oruvail
Labetalol • Normodyne, Trandate
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Naproxen • Aleve, Naprosyn
Oxaprozin • Daypro
Oxycodone • Oxycontin, Percocet, Percodan, Roxicodone
Phentermine • Adipex, Phentrol
Phenylephrine • Sudafed PE, Neo-Synephrine
Piroxicam • Feldene
Promethazine • Phenergan
Pseudoephedrine • Sudafed, Dimetapp
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampin • Rifadin, Rimactane
Selegiline • EMSAM
Sertraline • Zoloft
Sulindac • Clinoril
Sumatriptan • Imitrex
Thioridazine • Mellaril
Tolmetin • Tolectin
Trazodone • Desyrel, Oleptro
Trimethobenzamide • Benzacot, Tigan
Trimipramine • Surmontil
Verapamil • Calan, Isoptin
1. Cobaugh DJ, Gainor C, Gaston CL, et al. The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm. 2014;71(18):1539-1554.
2. Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent medicare policy changes in Kentucky. Pain Physician. 2010;13(2):167-186.
3. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
4. U.S. Department of Justice. Fact sheet: drug testing in the criminal justice system. https://www.ncjrs.gov/pdffiles/dtest. pdf. Published March 1992. Accessed July 29, 2015.
5. Australian Diagnostic Services. Technical information: testing principle’s. http://www.australiandrugtesting. com/#!technical-info/c14h4. Accessed November 5, 2014.
6. University of Illinois at Chicago College of Pharmacy. What drugs are likely to interfere with urine drug screens? http://dig.pharm.uic.edu/faq/2011/Feb/faq1.aspx. Accessed November 5, 2014.
7. Wolff K, Farrell M, Marsden J, et al. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94(9):1279-1298.
8. Gourlay D, Heit H, Caplan YH. Urine drug testing in primary care – dispelling the myths & designing strategies. PharmaCom Group. http://www.mc.uky.edu/equip-4-pcps/documents/ section8/urine%20drug%20testing%20in%20clinical%20 practice.pdf. Accessed August 6, 2015.
9. Standridge JB, Adams SM, Zotos AP. Urine drug screen: a valuable office procedure. Am Fam Physician. 2010;81(5): 635-640.
10. Casey ER, Scott MG, Tang S, et al. Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay. J Med Toxicol. 2011;7(2):105-108.
11. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin N Am. 2002;49(2):317-327.
12. Shults TF. The medical review officer handbook. 7th ed. Chapel Hill, NC: Quadrangle Research; 1999.
13. Baden LR, Horowitz G, Jacoby H, et al. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286(24):3115-3119.
1. Cobaugh DJ, Gainor C, Gaston CL, et al. The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm. 2014;71(18):1539-1554.
2. Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: implications of recent medicare policy changes in Kentucky. Pain Physician. 2010;13(2):167-186.
3. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
4. U.S. Department of Justice. Fact sheet: drug testing in the criminal justice system. https://www.ncjrs.gov/pdffiles/dtest. pdf. Published March 1992. Accessed July 29, 2015.
5. Australian Diagnostic Services. Technical information: testing principle’s. http://www.australiandrugtesting. com/#!technical-info/c14h4. Accessed November 5, 2014.
6. University of Illinois at Chicago College of Pharmacy. What drugs are likely to interfere with urine drug screens? http://dig.pharm.uic.edu/faq/2011/Feb/faq1.aspx. Accessed November 5, 2014.
7. Wolff K, Farrell M, Marsden J, et al. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94(9):1279-1298.
8. Gourlay D, Heit H, Caplan YH. Urine drug testing in primary care – dispelling the myths & designing strategies. PharmaCom Group. http://www.mc.uky.edu/equip-4-pcps/documents/ section8/urine%20drug%20testing%20in%20clinical%20 practice.pdf. Accessed August 6, 2015.
9. Standridge JB, Adams SM, Zotos AP. Urine drug screen: a valuable office procedure. Am Fam Physician. 2010;81(5): 635-640.
10. Casey ER, Scott MG, Tang S, et al. Frequency of false positive amphetamine screens due to bupropion using the Syva EMIT II immunoassay. J Med Toxicol. 2011;7(2):105-108.
11. Casavant MJ. Urine drug screening in adolescents. Pediatr Clin N Am. 2002;49(2):317-327.
12. Shults TF. The medical review officer handbook. 7th ed. Chapel Hill, NC: Quadrangle Research; 1999.
13. Baden LR, Horowitz G, Jacoby H, et al. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286(24):3115-3119.
Needed: A biopsychosocial ‘therapeutic placenta’ for people with schizophrenia
Consider stroke. Guidelines for acute treatment, access, intervention, prevention of post-hospitalization relapse, and rehabilitation are extensively spelled out and implemented.1 (The Box outlines Mayo Clinic guidelines for stroke management, as a demonstration of the comprehensiveness of the approach.)
Schizophrenia and related severe mental illnesses (SMI) need a similar all-inclusive system that seamlessly provides the myriad components of care needed for this vulnerable population. I propose the term “therapeutic placenta” to describe what people with a disabling SMI brain disorder deserve, just as stroke patients do.
Closing asylums: Psychosocial abruptio placentae
In a past Editorial,2 I described the appalling consequences of eliminating the asylum, an entity that I believe must be a key component of the SMI therapeutic placenta. The asylum is to schizophrenia as the skilled nursing home is to stroke. SMI patients suffered extensively when asylums were shut down; they lost a medical refuge with psychiatric and primary care, nursing and social work support, occupational and recreational therapies, and work therapy (farming, carpentry shop, cafeteria, laundry, etc.). For SMI, these services are the psychosocial counterpart of various physical rehabilitation therapies for stroke patients that no one would ever dare to eliminate.
Persons with schizophrenia and other SMI have suffered tragically with rupture of the main components of the therapeutic placenta that existed for decades before the advent of medications. The massive homelessness, widespread incarceration, persistent poverty, rampant access to alcohol and drugs of abuse, early death due to lack of primary care, and absence of meaningful opportunities for vocational rehabilitation are all consequences of a neglectful society that refuses to fund a therapeutic placenta for the SMI population.
The public mental health system in charge of SMI patients is broken, disconnected, and failing to provide the necessary components of a therapeutic placenta. It should not be surprising to witness the terribly stressful life and premature mortality of SMI patients, who are modern-day les misérables.
The Table lists what I consider to be the necessary spectrum of health care services through the life of an SMI patient that an optimal therapeutic placenta must provide until an effective prevention or a cure for SMI is discovered.
Reasons to be hopeful
Admittedly, encouraging steps are being made toward establishing a therapeutic placenta for SMI:
The RAISE Study3and Navigate Program4 demonstrate that implementing a comprehensive program of acute treatment and psychosocial interventions and rehabilitation yields better outcomes in SMI.
The Institute of Medicine released a landmark report on psychosocial interventions for mental illness and substance abuse disorders. It outlines a new model for establishing the effectiveness of intervention and the implementation of psychosocial strategies in clinical practice.5
The 21st Century Cures Act, if passed by Congress and signed by the President, will increase funding for the National Institutes of Health, which in turn will bolster the budgets of the National Institute of Mental Health, National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism and enhance the chances of discovering better treatments and prevention of SMI.
The Helping Families in Mental Health Crisis Act, more directly relevant to mental health and psychiatry, proposes, if passed, to:
• enhance evidence-based and scientifically validated interventions in the public sector
• raise the profile of mental health within the federal government by creating a position of Assistant Secretary for Mental Health in the U.S. Department of Health and Human Services, who will have oversight of both research and mental health care within the federal government.
Unacceptable disparity must be remedied
Planning an effective therapeutic placenta is imperative if health care for SMI patients is to approach the comprehensive spectrum of treatment, rehabilitation, and prevention available to stroke patients. Although stroke is regarded as a sensory-motor brain disorder, it is also associated with mental symptoms, just as schizophrenia is associated with sensory-motor symptoms. Both are disabling brain disorders: one, physically and cognitively; the other, mentally and socially. Both require a therapeutic placenta: Stroke is supported by one; schizophrenia is not. This is an unacceptable disparity that must be addressed—soon.
1. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
2. Nasrallah HA. Bring back the asylums? Current Psychiatry. 2008;7(3):19-20.
3. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):240-246.
4. Mueser KT, Penn DL, Addington J, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66(7):680-690.
5. The National Academy of Sciences. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Washington, DC. http:// iom.nationalacademies.org/Reports/2015/ Psychosocial-Interventions-Mental-Substance- Abuse-Disorders.aspx. Published July 14, 2015. Accessed September 3, 2015.
Consider stroke. Guidelines for acute treatment, access, intervention, prevention of post-hospitalization relapse, and rehabilitation are extensively spelled out and implemented.1 (The Box outlines Mayo Clinic guidelines for stroke management, as a demonstration of the comprehensiveness of the approach.)
Schizophrenia and related severe mental illnesses (SMI) need a similar all-inclusive system that seamlessly provides the myriad components of care needed for this vulnerable population. I propose the term “therapeutic placenta” to describe what people with a disabling SMI brain disorder deserve, just as stroke patients do.
Closing asylums: Psychosocial abruptio placentae
In a past Editorial,2 I described the appalling consequences of eliminating the asylum, an entity that I believe must be a key component of the SMI therapeutic placenta. The asylum is to schizophrenia as the skilled nursing home is to stroke. SMI patients suffered extensively when asylums were shut down; they lost a medical refuge with psychiatric and primary care, nursing and social work support, occupational and recreational therapies, and work therapy (farming, carpentry shop, cafeteria, laundry, etc.). For SMI, these services are the psychosocial counterpart of various physical rehabilitation therapies for stroke patients that no one would ever dare to eliminate.
Persons with schizophrenia and other SMI have suffered tragically with rupture of the main components of the therapeutic placenta that existed for decades before the advent of medications. The massive homelessness, widespread incarceration, persistent poverty, rampant access to alcohol and drugs of abuse, early death due to lack of primary care, and absence of meaningful opportunities for vocational rehabilitation are all consequences of a neglectful society that refuses to fund a therapeutic placenta for the SMI population.
The public mental health system in charge of SMI patients is broken, disconnected, and failing to provide the necessary components of a therapeutic placenta. It should not be surprising to witness the terribly stressful life and premature mortality of SMI patients, who are modern-day les misérables.
The Table lists what I consider to be the necessary spectrum of health care services through the life of an SMI patient that an optimal therapeutic placenta must provide until an effective prevention or a cure for SMI is discovered.
Reasons to be hopeful
Admittedly, encouraging steps are being made toward establishing a therapeutic placenta for SMI:
The RAISE Study3and Navigate Program4 demonstrate that implementing a comprehensive program of acute treatment and psychosocial interventions and rehabilitation yields better outcomes in SMI.
The Institute of Medicine released a landmark report on psychosocial interventions for mental illness and substance abuse disorders. It outlines a new model for establishing the effectiveness of intervention and the implementation of psychosocial strategies in clinical practice.5
The 21st Century Cures Act, if passed by Congress and signed by the President, will increase funding for the National Institutes of Health, which in turn will bolster the budgets of the National Institute of Mental Health, National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism and enhance the chances of discovering better treatments and prevention of SMI.
The Helping Families in Mental Health Crisis Act, more directly relevant to mental health and psychiatry, proposes, if passed, to:
• enhance evidence-based and scientifically validated interventions in the public sector
• raise the profile of mental health within the federal government by creating a position of Assistant Secretary for Mental Health in the U.S. Department of Health and Human Services, who will have oversight of both research and mental health care within the federal government.
Unacceptable disparity must be remedied
Planning an effective therapeutic placenta is imperative if health care for SMI patients is to approach the comprehensive spectrum of treatment, rehabilitation, and prevention available to stroke patients. Although stroke is regarded as a sensory-motor brain disorder, it is also associated with mental symptoms, just as schizophrenia is associated with sensory-motor symptoms. Both are disabling brain disorders: one, physically and cognitively; the other, mentally and socially. Both require a therapeutic placenta: Stroke is supported by one; schizophrenia is not. This is an unacceptable disparity that must be addressed—soon.
Consider stroke. Guidelines for acute treatment, access, intervention, prevention of post-hospitalization relapse, and rehabilitation are extensively spelled out and implemented.1 (The Box outlines Mayo Clinic guidelines for stroke management, as a demonstration of the comprehensiveness of the approach.)
Schizophrenia and related severe mental illnesses (SMI) need a similar all-inclusive system that seamlessly provides the myriad components of care needed for this vulnerable population. I propose the term “therapeutic placenta” to describe what people with a disabling SMI brain disorder deserve, just as stroke patients do.
Closing asylums: Psychosocial abruptio placentae
In a past Editorial,2 I described the appalling consequences of eliminating the asylum, an entity that I believe must be a key component of the SMI therapeutic placenta. The asylum is to schizophrenia as the skilled nursing home is to stroke. SMI patients suffered extensively when asylums were shut down; they lost a medical refuge with psychiatric and primary care, nursing and social work support, occupational and recreational therapies, and work therapy (farming, carpentry shop, cafeteria, laundry, etc.). For SMI, these services are the psychosocial counterpart of various physical rehabilitation therapies for stroke patients that no one would ever dare to eliminate.
Persons with schizophrenia and other SMI have suffered tragically with rupture of the main components of the therapeutic placenta that existed for decades before the advent of medications. The massive homelessness, widespread incarceration, persistent poverty, rampant access to alcohol and drugs of abuse, early death due to lack of primary care, and absence of meaningful opportunities for vocational rehabilitation are all consequences of a neglectful society that refuses to fund a therapeutic placenta for the SMI population.
The public mental health system in charge of SMI patients is broken, disconnected, and failing to provide the necessary components of a therapeutic placenta. It should not be surprising to witness the terribly stressful life and premature mortality of SMI patients, who are modern-day les misérables.
The Table lists what I consider to be the necessary spectrum of health care services through the life of an SMI patient that an optimal therapeutic placenta must provide until an effective prevention or a cure for SMI is discovered.
Reasons to be hopeful
Admittedly, encouraging steps are being made toward establishing a therapeutic placenta for SMI:
The RAISE Study3and Navigate Program4 demonstrate that implementing a comprehensive program of acute treatment and psychosocial interventions and rehabilitation yields better outcomes in SMI.
The Institute of Medicine released a landmark report on psychosocial interventions for mental illness and substance abuse disorders. It outlines a new model for establishing the effectiveness of intervention and the implementation of psychosocial strategies in clinical practice.5
The 21st Century Cures Act, if passed by Congress and signed by the President, will increase funding for the National Institutes of Health, which in turn will bolster the budgets of the National Institute of Mental Health, National Institute on Drug Abuse, and the National Institute on Alcohol Abuse and Alcoholism and enhance the chances of discovering better treatments and prevention of SMI.
The Helping Families in Mental Health Crisis Act, more directly relevant to mental health and psychiatry, proposes, if passed, to:
• enhance evidence-based and scientifically validated interventions in the public sector
• raise the profile of mental health within the federal government by creating a position of Assistant Secretary for Mental Health in the U.S. Department of Health and Human Services, who will have oversight of both research and mental health care within the federal government.
Unacceptable disparity must be remedied
Planning an effective therapeutic placenta is imperative if health care for SMI patients is to approach the comprehensive spectrum of treatment, rehabilitation, and prevention available to stroke patients. Although stroke is regarded as a sensory-motor brain disorder, it is also associated with mental symptoms, just as schizophrenia is associated with sensory-motor symptoms. Both are disabling brain disorders: one, physically and cognitively; the other, mentally and socially. Both require a therapeutic placenta: Stroke is supported by one; schizophrenia is not. This is an unacceptable disparity that must be addressed—soon.
1. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
2. Nasrallah HA. Bring back the asylums? Current Psychiatry. 2008;7(3):19-20.
3. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):240-246.
4. Mueser KT, Penn DL, Addington J, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66(7):680-690.
5. The National Academy of Sciences. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Washington, DC. http:// iom.nationalacademies.org/Reports/2015/ Psychosocial-Interventions-Mental-Substance- Abuse-Disorders.aspx. Published July 14, 2015. Accessed September 3, 2015.
1. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
2. Nasrallah HA. Bring back the asylums? Current Psychiatry. 2008;7(3):19-20.
3. Kane JM, Schooler NR, Marcy P, et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76(3):240-246.
4. Mueser KT, Penn DL, Addington J, et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66(7):680-690.
5. The National Academy of Sciences. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Washington, DC. http:// iom.nationalacademies.org/Reports/2015/ Psychosocial-Interventions-Mental-Substance- Abuse-Disorders.aspx. Published July 14, 2015. Accessed September 3, 2015.
The value and veracity of psychiatric themes depicted in modern cinema
Perhaps more than any other medical specialty, psychiatry enjoys a longstanding and, at times, complicated relationship with cinema. Recent award-winning films, such as Still Alice, Silver Linings Playbook, and Birdman: Or (The Unexpected Virtue of Ignorance) continue traditions rooted in One Flew Over the Cuckoo’s Nest, Martha Marcy May Marlene, Spellbound, and dozens of other films. Through these films, psychiatry is afforded exposure unavailable to most medical specialties. This exposure has proven to be a double-edged sword, however.
Exposure vs accurate portrayal
Relative benefits and disadvantages of psychiatry’s position in film and popular media are difficult to calculate. A film such as Still Alice can provide a vivid, concrete personal narrative of a patient with Alzheimer’s disease, equipping millions of viewers with knowledge that might otherwise remain esoteric and inaccessible. Martha Marcy May Marlene offers a similar stage for posttraumatic stress disorder (PTSD), as does Spellbound for dissociative amnesia.
Such exposure comes at a cost, inevitably, because information about psychiatry is incorporated into a dramatic storyline assembled by filmmakers who are not medical professionals and who are bound by conflicting pressures. At times, those pressures outweigh the desire to accurately portray psychiatric illness.
‘Magical realism’
Two of last year’s celebrated films, Birdman and Still Alice, have continued the longstanding tradition of portraying mental illness in film. Medicine often is touted as art and science; film likewise sits at this intersection. However, filmmakers are artists, primarily, and the nature of storytelling is to emphasize art over scientific accuracy.
The main character in Birdman, for example, manifests psychosis, but many of his presenting signs and symptoms are incongruent with any diagnosable form of psychosis. To tell its story, the film employs magical realism, a celebrated literary and film technique. Although magical realism might detract from the accuracy of the condition portrayed, it adds cinematic appeal to the film, likely creates more entertainment value, and, in turn, garners appreciation from a broader audience.
Expansion of medical information, accurate and otherwise
As in the 1970’s, we are, today, in the midst of rapidly evolving societal norms. One of the most rapid changes is in how the public acquires information. We are in the midst of the “Googlification” of medical knowledge and the expansion of online medical resources. These resources can, simultaneously, inform and mislead the public.
Popular films behave in much the same way. There is no motion picture-guild requirement that mentally ill characters in films such as Birdman meet any set of psychiatric criteria, from DSM-5 or elsewhere. Similarly, the fact that psychiatrists do not control the information in films that portray mental illness comes as no surprise.
‘One flew East, one flew West…’
The tension between engaging storytelling and medical accuracy certainly is not a new phenomenon, extending not only to representations of disease but to representations of treatment. Consider director Miloš Forman’s seminal 1975 film, One Flew Over the Cuckoo’s Nest, whose chief importance for psychiatry rests not in its individualized representations of patients but in a portrayal of the environment in which they are treated. Louise Fletcher’s Academy Award-winning portrayal of cruel Nurse Ratched has lingered in the public consciousness, remaining a prominent image for many Americans when they think of a psychiatric institution.
When Cuckoo’s Nest was released, it was considered by critics to be an “exploration of society’s enforcement of conformism” that “almost willfully overlooked the realities of mental illness”1 so that it could vivify its protagonist’s struggle against tyrannical Nurse Ratched. The film’s primary intent might not have been to make a statement about the injustices of the time, but it has certainly had a lasting effect on the public’s perception of psychiatric illness and treatment.
Films offer an opportunity for discussion
Films on the theme of psychiatry and mental illness have long held a distinctive position in the canon of Western cinema. In this vein, films from the past year have made timely contributions to the genre. Although Still Alice and Birdman might prove to be ground-breaking in changing societal views over time, we must not expect them to do so.
Nevertheless, psychiatry ought to take advantage of popular films’ wide exposure and ability to destigmatize mental illness—rather than lament medical inaccuracies in these films.
Cinema is, first and foremost, an art. Although patients and the public might pick up misconceptions about psychosis, Alzheimer’s disease, or PTSD because popular films take artistic liberty about mental illness, psychiatrists are available to set the record straight. After all, psychiatry has long been about managing perceptions, and patient education is at the core of our specialty.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Ebert R. “One Flew Over The Cuckoo’s Nest (review). http://www.rogerebert.com/reviews/great-movie-one-flew-overthe-cuckoos-nest-1975. February 2, 2003. Accessed September 9, 2015.
Perhaps more than any other medical specialty, psychiatry enjoys a longstanding and, at times, complicated relationship with cinema. Recent award-winning films, such as Still Alice, Silver Linings Playbook, and Birdman: Or (The Unexpected Virtue of Ignorance) continue traditions rooted in One Flew Over the Cuckoo’s Nest, Martha Marcy May Marlene, Spellbound, and dozens of other films. Through these films, psychiatry is afforded exposure unavailable to most medical specialties. This exposure has proven to be a double-edged sword, however.
Exposure vs accurate portrayal
Relative benefits and disadvantages of psychiatry’s position in film and popular media are difficult to calculate. A film such as Still Alice can provide a vivid, concrete personal narrative of a patient with Alzheimer’s disease, equipping millions of viewers with knowledge that might otherwise remain esoteric and inaccessible. Martha Marcy May Marlene offers a similar stage for posttraumatic stress disorder (PTSD), as does Spellbound for dissociative amnesia.
Such exposure comes at a cost, inevitably, because information about psychiatry is incorporated into a dramatic storyline assembled by filmmakers who are not medical professionals and who are bound by conflicting pressures. At times, those pressures outweigh the desire to accurately portray psychiatric illness.
‘Magical realism’
Two of last year’s celebrated films, Birdman and Still Alice, have continued the longstanding tradition of portraying mental illness in film. Medicine often is touted as art and science; film likewise sits at this intersection. However, filmmakers are artists, primarily, and the nature of storytelling is to emphasize art over scientific accuracy.
The main character in Birdman, for example, manifests psychosis, but many of his presenting signs and symptoms are incongruent with any diagnosable form of psychosis. To tell its story, the film employs magical realism, a celebrated literary and film technique. Although magical realism might detract from the accuracy of the condition portrayed, it adds cinematic appeal to the film, likely creates more entertainment value, and, in turn, garners appreciation from a broader audience.
Expansion of medical information, accurate and otherwise
As in the 1970’s, we are, today, in the midst of rapidly evolving societal norms. One of the most rapid changes is in how the public acquires information. We are in the midst of the “Googlification” of medical knowledge and the expansion of online medical resources. These resources can, simultaneously, inform and mislead the public.
Popular films behave in much the same way. There is no motion picture-guild requirement that mentally ill characters in films such as Birdman meet any set of psychiatric criteria, from DSM-5 or elsewhere. Similarly, the fact that psychiatrists do not control the information in films that portray mental illness comes as no surprise.
‘One flew East, one flew West…’
The tension between engaging storytelling and medical accuracy certainly is not a new phenomenon, extending not only to representations of disease but to representations of treatment. Consider director Miloš Forman’s seminal 1975 film, One Flew Over the Cuckoo’s Nest, whose chief importance for psychiatry rests not in its individualized representations of patients but in a portrayal of the environment in which they are treated. Louise Fletcher’s Academy Award-winning portrayal of cruel Nurse Ratched has lingered in the public consciousness, remaining a prominent image for many Americans when they think of a psychiatric institution.
When Cuckoo’s Nest was released, it was considered by critics to be an “exploration of society’s enforcement of conformism” that “almost willfully overlooked the realities of mental illness”1 so that it could vivify its protagonist’s struggle against tyrannical Nurse Ratched. The film’s primary intent might not have been to make a statement about the injustices of the time, but it has certainly had a lasting effect on the public’s perception of psychiatric illness and treatment.
Films offer an opportunity for discussion
Films on the theme of psychiatry and mental illness have long held a distinctive position in the canon of Western cinema. In this vein, films from the past year have made timely contributions to the genre. Although Still Alice and Birdman might prove to be ground-breaking in changing societal views over time, we must not expect them to do so.
Nevertheless, psychiatry ought to take advantage of popular films’ wide exposure and ability to destigmatize mental illness—rather than lament medical inaccuracies in these films.
Cinema is, first and foremost, an art. Although patients and the public might pick up misconceptions about psychosis, Alzheimer’s disease, or PTSD because popular films take artistic liberty about mental illness, psychiatrists are available to set the record straight. After all, psychiatry has long been about managing perceptions, and patient education is at the core of our specialty.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Perhaps more than any other medical specialty, psychiatry enjoys a longstanding and, at times, complicated relationship with cinema. Recent award-winning films, such as Still Alice, Silver Linings Playbook, and Birdman: Or (The Unexpected Virtue of Ignorance) continue traditions rooted in One Flew Over the Cuckoo’s Nest, Martha Marcy May Marlene, Spellbound, and dozens of other films. Through these films, psychiatry is afforded exposure unavailable to most medical specialties. This exposure has proven to be a double-edged sword, however.
Exposure vs accurate portrayal
Relative benefits and disadvantages of psychiatry’s position in film and popular media are difficult to calculate. A film such as Still Alice can provide a vivid, concrete personal narrative of a patient with Alzheimer’s disease, equipping millions of viewers with knowledge that might otherwise remain esoteric and inaccessible. Martha Marcy May Marlene offers a similar stage for posttraumatic stress disorder (PTSD), as does Spellbound for dissociative amnesia.
Such exposure comes at a cost, inevitably, because information about psychiatry is incorporated into a dramatic storyline assembled by filmmakers who are not medical professionals and who are bound by conflicting pressures. At times, those pressures outweigh the desire to accurately portray psychiatric illness.
‘Magical realism’
Two of last year’s celebrated films, Birdman and Still Alice, have continued the longstanding tradition of portraying mental illness in film. Medicine often is touted as art and science; film likewise sits at this intersection. However, filmmakers are artists, primarily, and the nature of storytelling is to emphasize art over scientific accuracy.
The main character in Birdman, for example, manifests psychosis, but many of his presenting signs and symptoms are incongruent with any diagnosable form of psychosis. To tell its story, the film employs magical realism, a celebrated literary and film technique. Although magical realism might detract from the accuracy of the condition portrayed, it adds cinematic appeal to the film, likely creates more entertainment value, and, in turn, garners appreciation from a broader audience.
Expansion of medical information, accurate and otherwise
As in the 1970’s, we are, today, in the midst of rapidly evolving societal norms. One of the most rapid changes is in how the public acquires information. We are in the midst of the “Googlification” of medical knowledge and the expansion of online medical resources. These resources can, simultaneously, inform and mislead the public.
Popular films behave in much the same way. There is no motion picture-guild requirement that mentally ill characters in films such as Birdman meet any set of psychiatric criteria, from DSM-5 or elsewhere. Similarly, the fact that psychiatrists do not control the information in films that portray mental illness comes as no surprise.
‘One flew East, one flew West…’
The tension between engaging storytelling and medical accuracy certainly is not a new phenomenon, extending not only to representations of disease but to representations of treatment. Consider director Miloš Forman’s seminal 1975 film, One Flew Over the Cuckoo’s Nest, whose chief importance for psychiatry rests not in its individualized representations of patients but in a portrayal of the environment in which they are treated. Louise Fletcher’s Academy Award-winning portrayal of cruel Nurse Ratched has lingered in the public consciousness, remaining a prominent image for many Americans when they think of a psychiatric institution.
When Cuckoo’s Nest was released, it was considered by critics to be an “exploration of society’s enforcement of conformism” that “almost willfully overlooked the realities of mental illness”1 so that it could vivify its protagonist’s struggle against tyrannical Nurse Ratched. The film’s primary intent might not have been to make a statement about the injustices of the time, but it has certainly had a lasting effect on the public’s perception of psychiatric illness and treatment.
Films offer an opportunity for discussion
Films on the theme of psychiatry and mental illness have long held a distinctive position in the canon of Western cinema. In this vein, films from the past year have made timely contributions to the genre. Although Still Alice and Birdman might prove to be ground-breaking in changing societal views over time, we must not expect them to do so.
Nevertheless, psychiatry ought to take advantage of popular films’ wide exposure and ability to destigmatize mental illness—rather than lament medical inaccuracies in these films.
Cinema is, first and foremost, an art. Although patients and the public might pick up misconceptions about psychosis, Alzheimer’s disease, or PTSD because popular films take artistic liberty about mental illness, psychiatrists are available to set the record straight. After all, psychiatry has long been about managing perceptions, and patient education is at the core of our specialty.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Ebert R. “One Flew Over The Cuckoo’s Nest (review). http://www.rogerebert.com/reviews/great-movie-one-flew-overthe-cuckoos-nest-1975. February 2, 2003. Accessed September 9, 2015.
Reference
1. Ebert R. “One Flew Over The Cuckoo’s Nest (review). http://www.rogerebert.com/reviews/great-movie-one-flew-overthe-cuckoos-nest-1975. February 2, 2003. Accessed September 9, 2015.
Managing borderline personality disorder
Assessing head pain
CUT DOWNTIME: The Lean way for a busy practitioner to improve efficiency
The mnemonic CUT DOWNTIME, which I have adapted and modified from the book Lean Healthcare Deployment and Sustainability,1 breaks down waste in health care—an activity that adds no value to a service—into 11 major categories (Table). This mnemonic provides the busy practitioner a simple framework for improving quality and efficiency of services by identifying and eliminating wastes the Lean way.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Dean ML. Lean healthcare deployment and sustainability. New York, NY: McGraw-Hill; 2013.
The mnemonic CUT DOWNTIME, which I have adapted and modified from the book Lean Healthcare Deployment and Sustainability,1 breaks down waste in health care—an activity that adds no value to a service—into 11 major categories (Table). This mnemonic provides the busy practitioner a simple framework for improving quality and efficiency of services by identifying and eliminating wastes the Lean way.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The mnemonic CUT DOWNTIME, which I have adapted and modified from the book Lean Healthcare Deployment and Sustainability,1 breaks down waste in health care—an activity that adds no value to a service—into 11 major categories (Table). This mnemonic provides the busy practitioner a simple framework for improving quality and efficiency of services by identifying and eliminating wastes the Lean way.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Dean ML. Lean healthcare deployment and sustainability. New York, NY: McGraw-Hill; 2013.
Reference
1. Dean ML. Lean healthcare deployment and sustainability. New York, NY: McGraw-Hill; 2013.
‘It’s my money, and I want it now!’ Clinical variables related to payeeship under Social Security
The Social Security Administration (SSA) does not provide much guidance on the contentious issue of determining payeeship for disability beneficiaries. The only description available is stated on the “Physician/medical officer’s statement of patient’s capability to manage benefits” (form SSA-787): “By capable we mean that the patient: Is able to understand and act on the ordinary affairs of life, such as providing for own adequate food, housing, etc., and is able, in spite of physical impairments, to manage funds or direct others how to manage them.”
Physicians will be asked to make a capability statement if they are performing a consultative examination for SSA or if their patient:
• is applying for benefits
• needs to have a payee.
Regrettably, the published literature on capability is scant.1,2 Based on decades of personal experience, here is the approach I have adopted to determine capability.
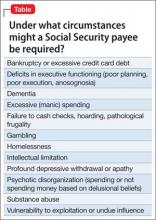
Diagnoses, circumstances, and clinical syndromes that strongly suggest the need for a payee include those listed in the Table.
The psychiatric rehabilitation agency I work at adheres to a recovery model. I consult with caseworkers on the issue of capability, but generally endorse a “team” recommendation for initiating or terminating payeeship. A number of factors are involved:
Adherence to recovery means that we encourage autonomy; we do not attempt to prevent every bad decision.
Demands for money from the patient and demands to terminate payeeship can be strident and potentially violent.
Confrontations over payeeship can be a safety risk for family or staff who have been acting as the payee.
Guardianship (or conservatorship) is a judicially determined restriction of financial decision-making.
Payeeship is an extrajudicial restriction of financial decision-making. Treating physicians, understandably, may feel uneasy restricting the rights of a patient. Additionally, there is ethical stress when a physician does anything that might compromise the primacy of the treatment relationship.
If all parties agree that payeeship should be terminated, I recommend the payee (whether the family or an institutional payee) begin a 3-month trial, during which the payee does not pay bills or keep a budget. The patient receives his (her) money in a lump sum at the beginning of the month, which begins a naturalistic trial of the patient’s capability to pay rent and budget adequately for all other necessities. If the patient demonstrates capability, I sign the SSA-787 form.
Offering a structured plan for restoring a patient’s benefits could defuse hostile demands.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marson DC, Savage R, Phillips J. Financial capacity in persons with schizophrenia and serious mental illness: clinical and research ethics aspects. Schizophr Bull. 2006; 32(1):81-91.
2. Rosen MI. The ‘check effect’ reconsidered. Addiction. 2011;106(6):1071-1077.
The Social Security Administration (SSA) does not provide much guidance on the contentious issue of determining payeeship for disability beneficiaries. The only description available is stated on the “Physician/medical officer’s statement of patient’s capability to manage benefits” (form SSA-787): “By capable we mean that the patient: Is able to understand and act on the ordinary affairs of life, such as providing for own adequate food, housing, etc., and is able, in spite of physical impairments, to manage funds or direct others how to manage them.”
Physicians will be asked to make a capability statement if they are performing a consultative examination for SSA or if their patient:
• is applying for benefits
• needs to have a payee.
Regrettably, the published literature on capability is scant.1,2 Based on decades of personal experience, here is the approach I have adopted to determine capability.
Diagnoses, circumstances, and clinical syndromes that strongly suggest the need for a payee include those listed in the Table.
The psychiatric rehabilitation agency I work at adheres to a recovery model. I consult with caseworkers on the issue of capability, but generally endorse a “team” recommendation for initiating or terminating payeeship. A number of factors are involved:
Adherence to recovery means that we encourage autonomy; we do not attempt to prevent every bad decision.
Demands for money from the patient and demands to terminate payeeship can be strident and potentially violent.
Confrontations over payeeship can be a safety risk for family or staff who have been acting as the payee.
Guardianship (or conservatorship) is a judicially determined restriction of financial decision-making.
Payeeship is an extrajudicial restriction of financial decision-making. Treating physicians, understandably, may feel uneasy restricting the rights of a patient. Additionally, there is ethical stress when a physician does anything that might compromise the primacy of the treatment relationship.
If all parties agree that payeeship should be terminated, I recommend the payee (whether the family or an institutional payee) begin a 3-month trial, during which the payee does not pay bills or keep a budget. The patient receives his (her) money in a lump sum at the beginning of the month, which begins a naturalistic trial of the patient’s capability to pay rent and budget adequately for all other necessities. If the patient demonstrates capability, I sign the SSA-787 form.
Offering a structured plan for restoring a patient’s benefits could defuse hostile demands.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The Social Security Administration (SSA) does not provide much guidance on the contentious issue of determining payeeship for disability beneficiaries. The only description available is stated on the “Physician/medical officer’s statement of patient’s capability to manage benefits” (form SSA-787): “By capable we mean that the patient: Is able to understand and act on the ordinary affairs of life, such as providing for own adequate food, housing, etc., and is able, in spite of physical impairments, to manage funds or direct others how to manage them.”
Physicians will be asked to make a capability statement if they are performing a consultative examination for SSA or if their patient:
• is applying for benefits
• needs to have a payee.
Regrettably, the published literature on capability is scant.1,2 Based on decades of personal experience, here is the approach I have adopted to determine capability.
Diagnoses, circumstances, and clinical syndromes that strongly suggest the need for a payee include those listed in the Table.
The psychiatric rehabilitation agency I work at adheres to a recovery model. I consult with caseworkers on the issue of capability, but generally endorse a “team” recommendation for initiating or terminating payeeship. A number of factors are involved:
Adherence to recovery means that we encourage autonomy; we do not attempt to prevent every bad decision.
Demands for money from the patient and demands to terminate payeeship can be strident and potentially violent.
Confrontations over payeeship can be a safety risk for family or staff who have been acting as the payee.
Guardianship (or conservatorship) is a judicially determined restriction of financial decision-making.
Payeeship is an extrajudicial restriction of financial decision-making. Treating physicians, understandably, may feel uneasy restricting the rights of a patient. Additionally, there is ethical stress when a physician does anything that might compromise the primacy of the treatment relationship.
If all parties agree that payeeship should be terminated, I recommend the payee (whether the family or an institutional payee) begin a 3-month trial, during which the payee does not pay bills or keep a budget. The patient receives his (her) money in a lump sum at the beginning of the month, which begins a naturalistic trial of the patient’s capability to pay rent and budget adequately for all other necessities. If the patient demonstrates capability, I sign the SSA-787 form.
Offering a structured plan for restoring a patient’s benefits could defuse hostile demands.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marson DC, Savage R, Phillips J. Financial capacity in persons with schizophrenia and serious mental illness: clinical and research ethics aspects. Schizophr Bull. 2006; 32(1):81-91.
2. Rosen MI. The ‘check effect’ reconsidered. Addiction. 2011;106(6):1071-1077.
1. Marson DC, Savage R, Phillips J. Financial capacity in persons with schizophrenia and serious mental illness: clinical and research ethics aspects. Schizophr Bull. 2006; 32(1):81-91.
2. Rosen MI. The ‘check effect’ reconsidered. Addiction. 2011;106(6):1071-1077.
Peaceful feeling, or up in smoke? Medical marijuana in medicolegal context
Dear Dr. Mossman,
I practice in a state that allows medical marijuana use. A few of my patients have asked me to help them obtain marijuana for their conditions. How risky would it be to oblige?
Submitted by “Dr. J”
In recent years, public debate about marijuana has acquired 2 new dimensions: (1) the wishes and medical needs of people who seek marijuana for its purported health benefits, and (2) the role of physicians who practice where “medical marijuana” is legal. This article, the authors’ joint effort to address Dr. J’s concerns, hits 3 topics:
• the intersection of marijuana policy and health care in the United States
• the risks and possible benefits of marijuana use
• the medicolegal problems faced by physicians who might advise patients to use marijuana.
Legal haze
Two cannabinoids—dronabinol and nabilone—have received FDA approval as appetite enhancers and anti-nausea agents. Third-party payors usually cover these types of medications, but no insurer pays for medical marijuana.1 The Controlled Substances Act of 19702 classified marijuana as a Schedule I drug because of its abuse potential, lack of accepted medical applications, and uncertain safety. The FDA has not approved marijuana use for any medical condition.
Although people commonly speak of “prescribing” marijuana, physicians cannot legally do this in the United States. What physicians may do, in the 23 states that allow medical marijuana, is recommend or certify a patient’s marijuana use—an action that has constitutional protection under the First Amendment’s freedom of speech clause.3,4
A physician may complete documentation that a patient has one of the qualifying medical conditions for which the jurisdiction has legalized medical marijuana. Either the patient or the physician then submits that documentation to the appropriate government agency (eg, the state’s department of health).
If the documentation receives approval, the agency will issue the patient a registration card that allows possession of medical marijuana, with which the patient can obtain or grow a small amount of marijuana. The cannabinoid content of marijuana products varies considerably,5 and physicians who certify marijuana typically defer dosage recommendations to the patient or the dispensary.1
In states that allow medical marijuana, users may assert an affirmative defense of medical necessity if they face criminal prosecution.3,6 Possession of marijuana remains illegal under federal law, however, regardless of one’s reason for having it.7,8 Since October 2009, the Attorney General’s office has discouraged federal prosecutions of persons “whose actions are in clear and unambiguous compliance with existing state laws providing for the medical use of marijuana.”9 But given the remaining conflicts between state and federal laws, “the legal implications of certifying patients for medical marijuana remain unclear.”10
Physicians have few resources to instruct them on the legal risks of certifying medical marijuana. When Canada legalized medical marijuana, the organization that provides malpractice insurance to Canadian physicians told its members that “prescribing medical marijuana cannot be compared to prescribing prescription drugs” and recommended that physicians obtain signed release forms documenting that they have discussed the risks of medical marijuana with patients.11 For some risky approved drugs, the FDA has established a risk evaluation and mitigation strategy, but no such guidance is available for marijuana.
Highlighting the benefits and risks
Proponents of medical marijuana claim that Cannabis can help patients, and dispassionate experts acknowledge that at least modest evidence supports the benefits of using “marijuana for nausea and vomiting related to chemotherapy, specific pain syndromes, and spasticity from multiple sclerosis.”10 For several other conditions— HIV/AIDS, depression, anxiety disorders, sleep disorders, psychosis, Tourette syndrome—evidence of benefit is poor.12 Rigorous evaluation of medical marijuana is difficult because the plant contains hundreds of active chemical compounds. The chemical content of marijuana is highly variable, depending on its preparation and administration,10,13—one reason why only a few good randomized controlled trials of marijuana have been conducted.
Marijuana has several side effects and carries many health risks (Table 1).4,14-20
On the highway: Marijuana and driving
Marijuana use impairs driving ability.14 Following enactment of more lenient marijuana laws, several states have reported higher numbers of fatally injured drivers who tested positive for Cannabis21-23 and had a positive screen of tetrahydrocannabinol (THC) in driving under the influence cases.24,25 One study showed that a blood THC concentration >5 ng/mL (comparable to a blood alcohol concentration of 0.15%) increased the crash odds ratio to 6.6.25,26
Marijuana impairs reaction time, information processing, motor performance, attention, and visual processing.14,16,27,28 Drivers who are under the influence of marijuana make more driving errors, despite being cautious about how they react to traffic.29 Even after weeks of abstinence, previous daily users of marijuana display some cognitive processing and driving-related impairments.30,31
Courts have found physicians negligent if their patients’ treatment-induced driving impairments injured others when the risk of driving-related injury was foreseeable.32 The Massachusetts case of Coombes v Florio33 likened the physician’s duty to that of a liquor store that sells alcohol to a minor who subsequently crashes, or to a father who did not lock his firearms away from his violent adult son.
Three variables influence a court’s judgment about whether risk is “foreseeable”: “the relative knowledge of the risk as between lay persons and physicians, whether the patient has previously used the medication and/or experienced the adverse effect, and whether a warning would otherwise have been futile.”34 A physician who certified a patient to use marijuana without adequately explaining the risks of driving might be vulnerable to a lawsuit if the patient’s driving accident occurred while the patient was under the influence of the drug. Recommending marijuana as a treatment also could lead to a malpractice action if a patient experienced and was harmed by the drug’s adverse effects.
Other drags
Another malpractice risk stems from marijuana’s addiction potential. Although many people think Cannabis isn’t addictive, nearly 10% of all marijuana users develop dependence.10,17 Regular Cannabis users are more likely to use alcohol, tobacco, and “recreational” drugs,17,35 and using alcohol and marijuana together greatly heightens the risk of driving accidents.14,15 Although we know of no case that relates directly to marijuana, physicians have faced lawsuits for injuries stemming from a patient’s addiction to prescription drugs,36 particularly when the patient’s behavior should have led the physician to suspect abuse or overuse.37
When certifying marijuana use, physicians have the same obligations that apply to more conventional medical treatment:
• establishing a proper physician–patient relationship
• taking an appropriate history
• conducting a proper examination
• reviewing records
• developing a comprehensive treatment plan
• weighing risks and alternatives
• providing follow-up care.
Neglecting these steps could lead to medical board sanctions and suspension or revocation of a medical license.13
The blunt reality
We advise against recommending marijuana for your patients. But if you have exhausted the alternatives, see marijuana as the last resort, and believe that taking the risk is worth the potential benefit, you can take some steps to reduce your legal risk (Table 2,1,32,37,38 and Table 313).
Bottom LinE
Medical marijuana is a controversial topic that demands more rigorous research and regulatory consideration. In the present climate, cautious physicians will avoid recommending marijuana to their patients. If you think that a patient has a medical indication, with no treatment option better than medical marijuana, be sure to understand the medical and legal ramifications before you authorize its use.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474-2483.
2. Controlled Substances Act title 21, §801.
3. Frezza C. Medical marijuana: a drug without a medical model. Georgetown Law J. 2013;101:1117-1145.
4. Conant v Walters, 309 F3d 629, 637 (9th Cir 2002).
5. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2493.
6. Thompson AE. JAMA patient page. Medical marijuana. JAMA. 2015;313(24):2508.
7. United States v Oakland Cannabis Buyers’ Cooperative, 532 U.S. 483 (2001).
8. Gonzales v Raich, 545 U.S. 1 (2005).
9. Ogden DW. Memorandum for selected United States Attorneys on investigations and prosecutions in states authorizing the medical use of marijuana. http://www. justice.gov/opa/blog/memorandum-selected-united-state-attorneys-investigations-and-prosecutions-states. Published October 19, 2009. Accessed July 11, 2015.
10. D’Souza DC, Ranganathan M. Medical marijuana: is the cart before the horse? JAMA. 2015;313(24):2431-2432.
11. Picard A. Pot-prescribing doctors warned. The Globe and Mail. http://www.theglobeandmail.com/news/national/ pot-prescribing-doctors-warned/article22506373. Published October 19, 2005. Accessed July 21, 2015.
12. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
13. Barthwell AG, Baxter LE, Cermak T, et al. The role of the physician in “medical” marijuana: American Society of Addiction Medicine. http://www.aoaam.org/usr/ ASAM_Med_Marijuana_White_Paper_Final.pdf. Published September 2010. Accessed July 11, 2015.
14. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109-119.
15. Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478-492.
16. Kondrad E, Reid A. Colorado family physicians’ attitudes toward medical marijuana. J Am Board Fam Med. 2013;26(1):52-60.
17. Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19-35.
18. Huang YH, Zhang ZF, Tashkin DP, et al. An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev. 2015;24(1):15-31.
19. Delforterie MJ, Lynskey MT, Huizink AC, et al. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug Alcohol Depend. 2015;150:98-104.
20. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot-a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
21. Masten SV, Guenzburger GV. Changes in driver cannabinoid prevalence in 12 U.S. states after implementing medical marijuana laws. J Safety Res. 2014;50:35-52.
22. Pollini RA, Romano E, Johnson MB, et al. The impact of marijuana decriminalization on California drivers. Drug Alcohol Depend. 2015;150:135-140.
23. Salomonsen-Sautel S, Min SJ, Sakai JT, et al. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend. 2014;140:137-144.
24. Urfer S, Morton J, Beall V, et al. Analysis of Δ9- tetrahydrocannabinol driving under the influence of drug cases in Colorado from January 2011 to February 2014. J Anal Toxicol. 2014;38(8):575-581.
25. Couper FJ, Peterson BL. The prevalence of marijuana in suspected impaired driving cases in Washington state. J Anal Toxicol. 2014;38(8):569-574.
26. Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36(2):239-248.
27. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106.
28. Schwitzer T, Schwan R, Angioi-Duprez K, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25(1):100-112.
29. Neavyn MJ, Blohm E, Babu KM, et al. Medical marijuana and driving: a review. J Med Toxicol. 2014;10(3):269-279.
30. Bosker WM, Karschner EL, Lee D, et al. Sustained abstinence improves psychomotor function in chronic daily cannabis smokers. Paper presented at: SOFT 2012: Society of Forensic Toxicologists 2012 Annual Meeting; July 1-6, 2012; Boston, MA.
31. Fabritius M, Augsburger M, Chtioui H, et al. Fitness to drive and cannabis: validation of two blood THCCOOH thresholds to distinguish occasional users from heavy users. Forensic Sci Int. 2014;242:1-8.
32. Annas GJ. Doctors, drugs, and driving—tort liability for patient-caused accidents. New Engl J Med. 2008;359(5):521-525.
33. Coombes v Florio, 877 NE2d 567 (Mass 2007).
34. McKenzie v Hawaii Permanente Medical Group, Inc. 47 P3d 209 (Haw 2002).
35. Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132(3):654-659.
36. Osborne v United States, 166 F Supp 2d 479 (SDW Va 2001).
37. Conrad-Hutsell v Colturi, 2002 Ohio App. LEXIS 2740 (2002).
38. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
Dear Dr. Mossman,
I practice in a state that allows medical marijuana use. A few of my patients have asked me to help them obtain marijuana for their conditions. How risky would it be to oblige?
Submitted by “Dr. J”
In recent years, public debate about marijuana has acquired 2 new dimensions: (1) the wishes and medical needs of people who seek marijuana for its purported health benefits, and (2) the role of physicians who practice where “medical marijuana” is legal. This article, the authors’ joint effort to address Dr. J’s concerns, hits 3 topics:
• the intersection of marijuana policy and health care in the United States
• the risks and possible benefits of marijuana use
• the medicolegal problems faced by physicians who might advise patients to use marijuana.
Legal haze
Two cannabinoids—dronabinol and nabilone—have received FDA approval as appetite enhancers and anti-nausea agents. Third-party payors usually cover these types of medications, but no insurer pays for medical marijuana.1 The Controlled Substances Act of 19702 classified marijuana as a Schedule I drug because of its abuse potential, lack of accepted medical applications, and uncertain safety. The FDA has not approved marijuana use for any medical condition.
Although people commonly speak of “prescribing” marijuana, physicians cannot legally do this in the United States. What physicians may do, in the 23 states that allow medical marijuana, is recommend or certify a patient’s marijuana use—an action that has constitutional protection under the First Amendment’s freedom of speech clause.3,4
A physician may complete documentation that a patient has one of the qualifying medical conditions for which the jurisdiction has legalized medical marijuana. Either the patient or the physician then submits that documentation to the appropriate government agency (eg, the state’s department of health).
If the documentation receives approval, the agency will issue the patient a registration card that allows possession of medical marijuana, with which the patient can obtain or grow a small amount of marijuana. The cannabinoid content of marijuana products varies considerably,5 and physicians who certify marijuana typically defer dosage recommendations to the patient or the dispensary.1
In states that allow medical marijuana, users may assert an affirmative defense of medical necessity if they face criminal prosecution.3,6 Possession of marijuana remains illegal under federal law, however, regardless of one’s reason for having it.7,8 Since October 2009, the Attorney General’s office has discouraged federal prosecutions of persons “whose actions are in clear and unambiguous compliance with existing state laws providing for the medical use of marijuana.”9 But given the remaining conflicts between state and federal laws, “the legal implications of certifying patients for medical marijuana remain unclear.”10
Physicians have few resources to instruct them on the legal risks of certifying medical marijuana. When Canada legalized medical marijuana, the organization that provides malpractice insurance to Canadian physicians told its members that “prescribing medical marijuana cannot be compared to prescribing prescription drugs” and recommended that physicians obtain signed release forms documenting that they have discussed the risks of medical marijuana with patients.11 For some risky approved drugs, the FDA has established a risk evaluation and mitigation strategy, but no such guidance is available for marijuana.
Highlighting the benefits and risks
Proponents of medical marijuana claim that Cannabis can help patients, and dispassionate experts acknowledge that at least modest evidence supports the benefits of using “marijuana for nausea and vomiting related to chemotherapy, specific pain syndromes, and spasticity from multiple sclerosis.”10 For several other conditions— HIV/AIDS, depression, anxiety disorders, sleep disorders, psychosis, Tourette syndrome—evidence of benefit is poor.12 Rigorous evaluation of medical marijuana is difficult because the plant contains hundreds of active chemical compounds. The chemical content of marijuana is highly variable, depending on its preparation and administration,10,13—one reason why only a few good randomized controlled trials of marijuana have been conducted.
Marijuana has several side effects and carries many health risks (Table 1).4,14-20
On the highway: Marijuana and driving
Marijuana use impairs driving ability.14 Following enactment of more lenient marijuana laws, several states have reported higher numbers of fatally injured drivers who tested positive for Cannabis21-23 and had a positive screen of tetrahydrocannabinol (THC) in driving under the influence cases.24,25 One study showed that a blood THC concentration >5 ng/mL (comparable to a blood alcohol concentration of 0.15%) increased the crash odds ratio to 6.6.25,26
Marijuana impairs reaction time, information processing, motor performance, attention, and visual processing.14,16,27,28 Drivers who are under the influence of marijuana make more driving errors, despite being cautious about how they react to traffic.29 Even after weeks of abstinence, previous daily users of marijuana display some cognitive processing and driving-related impairments.30,31
Courts have found physicians negligent if their patients’ treatment-induced driving impairments injured others when the risk of driving-related injury was foreseeable.32 The Massachusetts case of Coombes v Florio33 likened the physician’s duty to that of a liquor store that sells alcohol to a minor who subsequently crashes, or to a father who did not lock his firearms away from his violent adult son.
Three variables influence a court’s judgment about whether risk is “foreseeable”: “the relative knowledge of the risk as between lay persons and physicians, whether the patient has previously used the medication and/or experienced the adverse effect, and whether a warning would otherwise have been futile.”34 A physician who certified a patient to use marijuana without adequately explaining the risks of driving might be vulnerable to a lawsuit if the patient’s driving accident occurred while the patient was under the influence of the drug. Recommending marijuana as a treatment also could lead to a malpractice action if a patient experienced and was harmed by the drug’s adverse effects.
Other drags
Another malpractice risk stems from marijuana’s addiction potential. Although many people think Cannabis isn’t addictive, nearly 10% of all marijuana users develop dependence.10,17 Regular Cannabis users are more likely to use alcohol, tobacco, and “recreational” drugs,17,35 and using alcohol and marijuana together greatly heightens the risk of driving accidents.14,15 Although we know of no case that relates directly to marijuana, physicians have faced lawsuits for injuries stemming from a patient’s addiction to prescription drugs,36 particularly when the patient’s behavior should have led the physician to suspect abuse or overuse.37
When certifying marijuana use, physicians have the same obligations that apply to more conventional medical treatment:
• establishing a proper physician–patient relationship
• taking an appropriate history
• conducting a proper examination
• reviewing records
• developing a comprehensive treatment plan
• weighing risks and alternatives
• providing follow-up care.
Neglecting these steps could lead to medical board sanctions and suspension or revocation of a medical license.13
The blunt reality
We advise against recommending marijuana for your patients. But if you have exhausted the alternatives, see marijuana as the last resort, and believe that taking the risk is worth the potential benefit, you can take some steps to reduce your legal risk (Table 2,1,32,37,38 and Table 313).
Bottom LinE
Medical marijuana is a controversial topic that demands more rigorous research and regulatory consideration. In the present climate, cautious physicians will avoid recommending marijuana to their patients. If you think that a patient has a medical indication, with no treatment option better than medical marijuana, be sure to understand the medical and legal ramifications before you authorize its use.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dear Dr. Mossman,
I practice in a state that allows medical marijuana use. A few of my patients have asked me to help them obtain marijuana for their conditions. How risky would it be to oblige?
Submitted by “Dr. J”
In recent years, public debate about marijuana has acquired 2 new dimensions: (1) the wishes and medical needs of people who seek marijuana for its purported health benefits, and (2) the role of physicians who practice where “medical marijuana” is legal. This article, the authors’ joint effort to address Dr. J’s concerns, hits 3 topics:
• the intersection of marijuana policy and health care in the United States
• the risks and possible benefits of marijuana use
• the medicolegal problems faced by physicians who might advise patients to use marijuana.
Legal haze
Two cannabinoids—dronabinol and nabilone—have received FDA approval as appetite enhancers and anti-nausea agents. Third-party payors usually cover these types of medications, but no insurer pays for medical marijuana.1 The Controlled Substances Act of 19702 classified marijuana as a Schedule I drug because of its abuse potential, lack of accepted medical applications, and uncertain safety. The FDA has not approved marijuana use for any medical condition.
Although people commonly speak of “prescribing” marijuana, physicians cannot legally do this in the United States. What physicians may do, in the 23 states that allow medical marijuana, is recommend or certify a patient’s marijuana use—an action that has constitutional protection under the First Amendment’s freedom of speech clause.3,4
A physician may complete documentation that a patient has one of the qualifying medical conditions for which the jurisdiction has legalized medical marijuana. Either the patient or the physician then submits that documentation to the appropriate government agency (eg, the state’s department of health).
If the documentation receives approval, the agency will issue the patient a registration card that allows possession of medical marijuana, with which the patient can obtain or grow a small amount of marijuana. The cannabinoid content of marijuana products varies considerably,5 and physicians who certify marijuana typically defer dosage recommendations to the patient or the dispensary.1
In states that allow medical marijuana, users may assert an affirmative defense of medical necessity if they face criminal prosecution.3,6 Possession of marijuana remains illegal under federal law, however, regardless of one’s reason for having it.7,8 Since October 2009, the Attorney General’s office has discouraged federal prosecutions of persons “whose actions are in clear and unambiguous compliance with existing state laws providing for the medical use of marijuana.”9 But given the remaining conflicts between state and federal laws, “the legal implications of certifying patients for medical marijuana remain unclear.”10
Physicians have few resources to instruct them on the legal risks of certifying medical marijuana. When Canada legalized medical marijuana, the organization that provides malpractice insurance to Canadian physicians told its members that “prescribing medical marijuana cannot be compared to prescribing prescription drugs” and recommended that physicians obtain signed release forms documenting that they have discussed the risks of medical marijuana with patients.11 For some risky approved drugs, the FDA has established a risk evaluation and mitigation strategy, but no such guidance is available for marijuana.
Highlighting the benefits and risks
Proponents of medical marijuana claim that Cannabis can help patients, and dispassionate experts acknowledge that at least modest evidence supports the benefits of using “marijuana for nausea and vomiting related to chemotherapy, specific pain syndromes, and spasticity from multiple sclerosis.”10 For several other conditions— HIV/AIDS, depression, anxiety disorders, sleep disorders, psychosis, Tourette syndrome—evidence of benefit is poor.12 Rigorous evaluation of medical marijuana is difficult because the plant contains hundreds of active chemical compounds. The chemical content of marijuana is highly variable, depending on its preparation and administration,10,13—one reason why only a few good randomized controlled trials of marijuana have been conducted.
Marijuana has several side effects and carries many health risks (Table 1).4,14-20
On the highway: Marijuana and driving
Marijuana use impairs driving ability.14 Following enactment of more lenient marijuana laws, several states have reported higher numbers of fatally injured drivers who tested positive for Cannabis21-23 and had a positive screen of tetrahydrocannabinol (THC) in driving under the influence cases.24,25 One study showed that a blood THC concentration >5 ng/mL (comparable to a blood alcohol concentration of 0.15%) increased the crash odds ratio to 6.6.25,26
Marijuana impairs reaction time, information processing, motor performance, attention, and visual processing.14,16,27,28 Drivers who are under the influence of marijuana make more driving errors, despite being cautious about how they react to traffic.29 Even after weeks of abstinence, previous daily users of marijuana display some cognitive processing and driving-related impairments.30,31
Courts have found physicians negligent if their patients’ treatment-induced driving impairments injured others when the risk of driving-related injury was foreseeable.32 The Massachusetts case of Coombes v Florio33 likened the physician’s duty to that of a liquor store that sells alcohol to a minor who subsequently crashes, or to a father who did not lock his firearms away from his violent adult son.
Three variables influence a court’s judgment about whether risk is “foreseeable”: “the relative knowledge of the risk as between lay persons and physicians, whether the patient has previously used the medication and/or experienced the adverse effect, and whether a warning would otherwise have been futile.”34 A physician who certified a patient to use marijuana without adequately explaining the risks of driving might be vulnerable to a lawsuit if the patient’s driving accident occurred while the patient was under the influence of the drug. Recommending marijuana as a treatment also could lead to a malpractice action if a patient experienced and was harmed by the drug’s adverse effects.
Other drags
Another malpractice risk stems from marijuana’s addiction potential. Although many people think Cannabis isn’t addictive, nearly 10% of all marijuana users develop dependence.10,17 Regular Cannabis users are more likely to use alcohol, tobacco, and “recreational” drugs,17,35 and using alcohol and marijuana together greatly heightens the risk of driving accidents.14,15 Although we know of no case that relates directly to marijuana, physicians have faced lawsuits for injuries stemming from a patient’s addiction to prescription drugs,36 particularly when the patient’s behavior should have led the physician to suspect abuse or overuse.37
When certifying marijuana use, physicians have the same obligations that apply to more conventional medical treatment:
• establishing a proper physician–patient relationship
• taking an appropriate history
• conducting a proper examination
• reviewing records
• developing a comprehensive treatment plan
• weighing risks and alternatives
• providing follow-up care.
Neglecting these steps could lead to medical board sanctions and suspension or revocation of a medical license.13
The blunt reality
We advise against recommending marijuana for your patients. But if you have exhausted the alternatives, see marijuana as the last resort, and believe that taking the risk is worth the potential benefit, you can take some steps to reduce your legal risk (Table 2,1,32,37,38 and Table 313).
Bottom LinE
Medical marijuana is a controversial topic that demands more rigorous research and regulatory consideration. In the present climate, cautious physicians will avoid recommending marijuana to their patients. If you think that a patient has a medical indication, with no treatment option better than medical marijuana, be sure to understand the medical and legal ramifications before you authorize its use.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474-2483.
2. Controlled Substances Act title 21, §801.
3. Frezza C. Medical marijuana: a drug without a medical model. Georgetown Law J. 2013;101:1117-1145.
4. Conant v Walters, 309 F3d 629, 637 (9th Cir 2002).
5. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2493.
6. Thompson AE. JAMA patient page. Medical marijuana. JAMA. 2015;313(24):2508.
7. United States v Oakland Cannabis Buyers’ Cooperative, 532 U.S. 483 (2001).
8. Gonzales v Raich, 545 U.S. 1 (2005).
9. Ogden DW. Memorandum for selected United States Attorneys on investigations and prosecutions in states authorizing the medical use of marijuana. http://www. justice.gov/opa/blog/memorandum-selected-united-state-attorneys-investigations-and-prosecutions-states. Published October 19, 2009. Accessed July 11, 2015.
10. D’Souza DC, Ranganathan M. Medical marijuana: is the cart before the horse? JAMA. 2015;313(24):2431-2432.
11. Picard A. Pot-prescribing doctors warned. The Globe and Mail. http://www.theglobeandmail.com/news/national/ pot-prescribing-doctors-warned/article22506373. Published October 19, 2005. Accessed July 21, 2015.
12. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
13. Barthwell AG, Baxter LE, Cermak T, et al. The role of the physician in “medical” marijuana: American Society of Addiction Medicine. http://www.aoaam.org/usr/ ASAM_Med_Marijuana_White_Paper_Final.pdf. Published September 2010. Accessed July 11, 2015.
14. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109-119.
15. Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478-492.
16. Kondrad E, Reid A. Colorado family physicians’ attitudes toward medical marijuana. J Am Board Fam Med. 2013;26(1):52-60.
17. Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19-35.
18. Huang YH, Zhang ZF, Tashkin DP, et al. An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev. 2015;24(1):15-31.
19. Delforterie MJ, Lynskey MT, Huizink AC, et al. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug Alcohol Depend. 2015;150:98-104.
20. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot-a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
21. Masten SV, Guenzburger GV. Changes in driver cannabinoid prevalence in 12 U.S. states after implementing medical marijuana laws. J Safety Res. 2014;50:35-52.
22. Pollini RA, Romano E, Johnson MB, et al. The impact of marijuana decriminalization on California drivers. Drug Alcohol Depend. 2015;150:135-140.
23. Salomonsen-Sautel S, Min SJ, Sakai JT, et al. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend. 2014;140:137-144.
24. Urfer S, Morton J, Beall V, et al. Analysis of Δ9- tetrahydrocannabinol driving under the influence of drug cases in Colorado from January 2011 to February 2014. J Anal Toxicol. 2014;38(8):575-581.
25. Couper FJ, Peterson BL. The prevalence of marijuana in suspected impaired driving cases in Washington state. J Anal Toxicol. 2014;38(8):569-574.
26. Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36(2):239-248.
27. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106.
28. Schwitzer T, Schwan R, Angioi-Duprez K, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25(1):100-112.
29. Neavyn MJ, Blohm E, Babu KM, et al. Medical marijuana and driving: a review. J Med Toxicol. 2014;10(3):269-279.
30. Bosker WM, Karschner EL, Lee D, et al. Sustained abstinence improves psychomotor function in chronic daily cannabis smokers. Paper presented at: SOFT 2012: Society of Forensic Toxicologists 2012 Annual Meeting; July 1-6, 2012; Boston, MA.
31. Fabritius M, Augsburger M, Chtioui H, et al. Fitness to drive and cannabis: validation of two blood THCCOOH thresholds to distinguish occasional users from heavy users. Forensic Sci Int. 2014;242:1-8.
32. Annas GJ. Doctors, drugs, and driving—tort liability for patient-caused accidents. New Engl J Med. 2008;359(5):521-525.
33. Coombes v Florio, 877 NE2d 567 (Mass 2007).
34. McKenzie v Hawaii Permanente Medical Group, Inc. 47 P3d 209 (Haw 2002).
35. Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132(3):654-659.
36. Osborne v United States, 166 F Supp 2d 479 (SDW Va 2001).
37. Conrad-Hutsell v Colturi, 2002 Ohio App. LEXIS 2740 (2002).
38. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
1. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474-2483.
2. Controlled Substances Act title 21, §801.
3. Frezza C. Medical marijuana: a drug without a medical model. Georgetown Law J. 2013;101:1117-1145.
4. Conant v Walters, 309 F3d 629, 637 (9th Cir 2002).
5. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2493.
6. Thompson AE. JAMA patient page. Medical marijuana. JAMA. 2015;313(24):2508.
7. United States v Oakland Cannabis Buyers’ Cooperative, 532 U.S. 483 (2001).
8. Gonzales v Raich, 545 U.S. 1 (2005).
9. Ogden DW. Memorandum for selected United States Attorneys on investigations and prosecutions in states authorizing the medical use of marijuana. http://www. justice.gov/opa/blog/memorandum-selected-united-state-attorneys-investigations-and-prosecutions-states. Published October 19, 2009. Accessed July 11, 2015.
10. D’Souza DC, Ranganathan M. Medical marijuana: is the cart before the horse? JAMA. 2015;313(24):2431-2432.
11. Picard A. Pot-prescribing doctors warned. The Globe and Mail. http://www.theglobeandmail.com/news/national/ pot-prescribing-doctors-warned/article22506373. Published October 19, 2005. Accessed July 21, 2015.
12. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
13. Barthwell AG, Baxter LE, Cermak T, et al. The role of the physician in “medical” marijuana: American Society of Addiction Medicine. http://www.aoaam.org/usr/ ASAM_Med_Marijuana_White_Paper_Final.pdf. Published September 2010. Accessed July 11, 2015.
14. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109-119.
15. Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478-492.
16. Kondrad E, Reid A. Colorado family physicians’ attitudes toward medical marijuana. J Am Board Fam Med. 2013;26(1):52-60.
17. Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19-35.
18. Huang YH, Zhang ZF, Tashkin DP, et al. An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev. 2015;24(1):15-31.
19. Delforterie MJ, Lynskey MT, Huizink AC, et al. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug Alcohol Depend. 2015;150:98-104.
20. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot-a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
21. Masten SV, Guenzburger GV. Changes in driver cannabinoid prevalence in 12 U.S. states after implementing medical marijuana laws. J Safety Res. 2014;50:35-52.
22. Pollini RA, Romano E, Johnson MB, et al. The impact of marijuana decriminalization on California drivers. Drug Alcohol Depend. 2015;150:135-140.
23. Salomonsen-Sautel S, Min SJ, Sakai JT, et al. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend. 2014;140:137-144.
24. Urfer S, Morton J, Beall V, et al. Analysis of Δ9- tetrahydrocannabinol driving under the influence of drug cases in Colorado from January 2011 to February 2014. J Anal Toxicol. 2014;38(8):575-581.
25. Couper FJ, Peterson BL. The prevalence of marijuana in suspected impaired driving cases in Washington state. J Anal Toxicol. 2014;38(8):569-574.
26. Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36(2):239-248.
27. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106.
28. Schwitzer T, Schwan R, Angioi-Duprez K, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25(1):100-112.
29. Neavyn MJ, Blohm E, Babu KM, et al. Medical marijuana and driving: a review. J Med Toxicol. 2014;10(3):269-279.
30. Bosker WM, Karschner EL, Lee D, et al. Sustained abstinence improves psychomotor function in chronic daily cannabis smokers. Paper presented at: SOFT 2012: Society of Forensic Toxicologists 2012 Annual Meeting; July 1-6, 2012; Boston, MA.
31. Fabritius M, Augsburger M, Chtioui H, et al. Fitness to drive and cannabis: validation of two blood THCCOOH thresholds to distinguish occasional users from heavy users. Forensic Sci Int. 2014;242:1-8.
32. Annas GJ. Doctors, drugs, and driving—tort liability for patient-caused accidents. New Engl J Med. 2008;359(5):521-525.
33. Coombes v Florio, 877 NE2d 567 (Mass 2007).
34. McKenzie v Hawaii Permanente Medical Group, Inc. 47 P3d 209 (Haw 2002).
35. Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132(3):654-659.
36. Osborne v United States, 166 F Supp 2d 479 (SDW Va 2001).
37. Conrad-Hutsell v Colturi, 2002 Ohio App. LEXIS 2740 (2002).
38. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.