User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Dementia risk is 2-fold in type 1 diabetes mellitus patients
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Mind your ABCDs, and your Es, when caring for a ‘difficult patient’
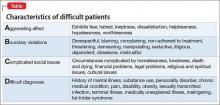
Much has been written about “the difficult patient” in the medical literature.1,2 Also labeled as a “heartsink patient,” “hateful patient,” and “black hole,” they possess characteristics that evoke powerful, often negative, emotional responses in providers that can be counter-therapeutic. “The difficult provider” also is thought to contribute to the failure of the patient encounter,3 and providers may have limited awareness of these patient–provider characteristics that can lead to such interactions. Early identification of these characteristics is essential to implementing effective interventions for the care of a difficult patient.
The mnemonic ABCD highlights patient characteristics that suggest you are dealing with a difficult patient (Table).
7 Negatives that affect the provider–patient relationship
The 7 Es highlight negative provider-related variables that contribute to perceived and actual difficulty providing care. As a psychiatrist doing consultation-liaison work, this memory device also can be a tool to educate physician–colleagues, nursing staff, and other members of the treatment team.
Expertise. Lack of basic knowledge or experience with your patient’s condition and circumstances, or not being familiar with available resources, could limit your confidence, be counter-productive, and lead to inappropriate care.
Experiences. Current and past life experiences could negatively color a provider’s feelings, thoughts, and interactions with the patient. Negative interpersonal experiences could manifest as countertransference.
Empathy. The inability to empathize makes it difficult to understand the patient, creating distance between you and the patient.
Engagement level. A lack of rapport and ineffective communication leads to a patient feeling misunderstood and unsatisfied with the clinical interaction.
Emotions. Feeling tired, angry, or resentful harms the provider–patient interaction.
Environment. A stressful, loud, pressured environment filled with distractions can undermine the provider–patient relationship.
Extra help. Limited access to, and the unavailability of, social services, housing, and similar resources could make an already difficult situation seem impossible to solve. Working without such help can lead to feelings of helplessness and hopelessness for you and your patient.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The views expressed in this publication/presentation are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
1. Strous RD, Ulman A, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Smith S. Dealing with the difficult patient. Postgrad Med J. 1995;71(841):653-657.
3. Hawken SJ. Strategies for dealing with the challenging patient. N Z Fam Physician. 2005;32(4):266-269.
Much has been written about “the difficult patient” in the medical literature.1,2 Also labeled as a “heartsink patient,” “hateful patient,” and “black hole,” they possess characteristics that evoke powerful, often negative, emotional responses in providers that can be counter-therapeutic. “The difficult provider” also is thought to contribute to the failure of the patient encounter,3 and providers may have limited awareness of these patient–provider characteristics that can lead to such interactions. Early identification of these characteristics is essential to implementing effective interventions for the care of a difficult patient.
The mnemonic ABCD highlights patient characteristics that suggest you are dealing with a difficult patient (Table).
7 Negatives that affect the provider–patient relationship
The 7 Es highlight negative provider-related variables that contribute to perceived and actual difficulty providing care. As a psychiatrist doing consultation-liaison work, this memory device also can be a tool to educate physician–colleagues, nursing staff, and other members of the treatment team.
Expertise. Lack of basic knowledge or experience with your patient’s condition and circumstances, or not being familiar with available resources, could limit your confidence, be counter-productive, and lead to inappropriate care.
Experiences. Current and past life experiences could negatively color a provider’s feelings, thoughts, and interactions with the patient. Negative interpersonal experiences could manifest as countertransference.
Empathy. The inability to empathize makes it difficult to understand the patient, creating distance between you and the patient.
Engagement level. A lack of rapport and ineffective communication leads to a patient feeling misunderstood and unsatisfied with the clinical interaction.
Emotions. Feeling tired, angry, or resentful harms the provider–patient interaction.
Environment. A stressful, loud, pressured environment filled with distractions can undermine the provider–patient relationship.
Extra help. Limited access to, and the unavailability of, social services, housing, and similar resources could make an already difficult situation seem impossible to solve. Working without such help can lead to feelings of helplessness and hopelessness for you and your patient.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The views expressed in this publication/presentation are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Much has been written about “the difficult patient” in the medical literature.1,2 Also labeled as a “heartsink patient,” “hateful patient,” and “black hole,” they possess characteristics that evoke powerful, often negative, emotional responses in providers that can be counter-therapeutic. “The difficult provider” also is thought to contribute to the failure of the patient encounter,3 and providers may have limited awareness of these patient–provider characteristics that can lead to such interactions. Early identification of these characteristics is essential to implementing effective interventions for the care of a difficult patient.
The mnemonic ABCD highlights patient characteristics that suggest you are dealing with a difficult patient (Table).
7 Negatives that affect the provider–patient relationship
The 7 Es highlight negative provider-related variables that contribute to perceived and actual difficulty providing care. As a psychiatrist doing consultation-liaison work, this memory device also can be a tool to educate physician–colleagues, nursing staff, and other members of the treatment team.
Expertise. Lack of basic knowledge or experience with your patient’s condition and circumstances, or not being familiar with available resources, could limit your confidence, be counter-productive, and lead to inappropriate care.
Experiences. Current and past life experiences could negatively color a provider’s feelings, thoughts, and interactions with the patient. Negative interpersonal experiences could manifest as countertransference.
Empathy. The inability to empathize makes it difficult to understand the patient, creating distance between you and the patient.
Engagement level. A lack of rapport and ineffective communication leads to a patient feeling misunderstood and unsatisfied with the clinical interaction.
Emotions. Feeling tired, angry, or resentful harms the provider–patient interaction.
Environment. A stressful, loud, pressured environment filled with distractions can undermine the provider–patient relationship.
Extra help. Limited access to, and the unavailability of, social services, housing, and similar resources could make an already difficult situation seem impossible to solve. Working without such help can lead to feelings of helplessness and hopelessness for you and your patient.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. The views expressed in this publication/presentation are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
1. Strous RD, Ulman A, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Smith S. Dealing with the difficult patient. Postgrad Med J. 1995;71(841):653-657.
3. Hawken SJ. Strategies for dealing with the challenging patient. N Z Fam Physician. 2005;32(4):266-269.
1. Strous RD, Ulman A, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
2. Smith S. Dealing with the difficult patient. Postgrad Med J. 1995;71(841):653-657.
3. Hawken SJ. Strategies for dealing with the challenging patient. N Z Fam Physician. 2005;32(4):266-269.
Mastering finance for your practice—without an MBA
Being a caring, knowledgeable clinician is vital for patient care, but having such skill does not necessarily mean that running a medical practice comes easy— especially if you do not have a basic background in business. Financial fundamentals are rarely taught in residency and, with administrative burdens increasingly placed on physicians in solo and small practices, it isn’t surprising that many practitioners feel underprepared.
Fortunately, it doesn’t take a master’s degree in business administration to conquer these challenges. You just need some understanding of key operating principles.
Accounting basics
It isn’t personal; it’s only business. Delineate the point at which personal finances stop and business finances begin. Make sure that you have a business checking account and credit card, and run all your business expenses through those accounts—never through your personal accounts. That policy will save you time if your practice is audited and, more important, will help you be efficient by guiding your focus to the right set of numbers by which to manage the practice.
Set up a system to track transactions. Many businesses use the accounting software QuickBooks; the program can generate sophisticated reports, and many banks can export data to it automatically. But QuickBooks might be more complicated than what you need to get started; a simple spreadsheet program, such as Excel, might suffice. By working through the numbers yourself, you gain a more intimate knowledge of the state of your finances.
Assemble a team of experts to assist you, at least in the beginning, with building a core knowledge base and good habits. Don’t think that this absolves you of responsibility, however: Ultimately, you sign off on what your advisors recommend. For example, an accountant can prepare your tax return, but you review and approve it, and a financial advisor might recommend certain investments, but only you can authorize them. You might work with a banker for a business loan or a bookkeeper to help you with your day-to-day record-keeping, but no one can give you the critical thinking you need to maximize your financial success.
The devil is in those details
Delve into your practice’s profit/loss statement, or create one if it doesn’t exist. Understanding these data is critical for maintaining financial health. Without knowing how much money you are taking in and where it is going, you cannot be confident that your business model is viable.
Revenue is easier to digest because it typically derives from only a few sources: professional fees and interest and, perhaps, speaking engagements, consultation to trainees, teaching, and rental income.
Expenses. Getting a grasp of where the money goes is more challenging. Common examples of costs of running a practice include, but aren’t limited to, the list in the Table.
By doing this basic profit/loss math, you will see how much money should be left over (profit) at the end of the month. To confirm, reconcile your numbers with your monthly business checking account statement; QuickBooks does this semi-automatically, or you can do it by hand. Reconciliation might feel uncomfortable if you are a novice to accounting, but spending a few moments to catch an error now is far less onerous than remedying what began as a small mistake and compounded to a big one over the years.
Other financial reports, such as a balance sheet and a statement of cash flow, are useful for giving you a sense of your practice’s long-term financial health. Typically, however, they are unnecessary during early stages of establishing a practice—and the work they require can be overwhelming.
After you’re done with the math
Based on your financial analysis of the practice, you will be able to pay yourself a salary based on the profit (that is, revenue minus expenses). Before you take your salary, however:
• Consider keeping enough in your business checking account to pay next month’s bills.
• Remember to adjust your monthly compensation downward by 20% to 50% to withhold for payroll and estimated federal and state taxes.
• Look into tax-advantaged business benefit plans. A retirement account, certain savings plans (eg, flexible spending accounts for dependent care or health care), a commuter plan, and life insurance paid for by the business can make your income go further. Some of these benefits are available only to employees of corporations; crunch the numbers, however, and discuss with your accountant whether the cost of incorporating is worthwhile.
• Determine whether hiring an assistant, or adding an additional one, will increase your bottom line. You incur significant expenses by hiring an employee—salary, payroll taxes, and time spent training, to name a few—but doing so might be worth it if the time that he (she) saves you opens up billable hours for seeing patients.
Good care requires a solid foundation
Caring for people who are suffering, while being financially successful, are not contradictory goals. Although you deal with a person’s private, intense feelings when you provide care, you also have an obligation to ensure the financial health of your practice.
Disclosure
Dr. Braslow is the founder of Luminello.com.
Being a caring, knowledgeable clinician is vital for patient care, but having such skill does not necessarily mean that running a medical practice comes easy— especially if you do not have a basic background in business. Financial fundamentals are rarely taught in residency and, with administrative burdens increasingly placed on physicians in solo and small practices, it isn’t surprising that many practitioners feel underprepared.
Fortunately, it doesn’t take a master’s degree in business administration to conquer these challenges. You just need some understanding of key operating principles.
Accounting basics
It isn’t personal; it’s only business. Delineate the point at which personal finances stop and business finances begin. Make sure that you have a business checking account and credit card, and run all your business expenses through those accounts—never through your personal accounts. That policy will save you time if your practice is audited and, more important, will help you be efficient by guiding your focus to the right set of numbers by which to manage the practice.
Set up a system to track transactions. Many businesses use the accounting software QuickBooks; the program can generate sophisticated reports, and many banks can export data to it automatically. But QuickBooks might be more complicated than what you need to get started; a simple spreadsheet program, such as Excel, might suffice. By working through the numbers yourself, you gain a more intimate knowledge of the state of your finances.
Assemble a team of experts to assist you, at least in the beginning, with building a core knowledge base and good habits. Don’t think that this absolves you of responsibility, however: Ultimately, you sign off on what your advisors recommend. For example, an accountant can prepare your tax return, but you review and approve it, and a financial advisor might recommend certain investments, but only you can authorize them. You might work with a banker for a business loan or a bookkeeper to help you with your day-to-day record-keeping, but no one can give you the critical thinking you need to maximize your financial success.
The devil is in those details
Delve into your practice’s profit/loss statement, or create one if it doesn’t exist. Understanding these data is critical for maintaining financial health. Without knowing how much money you are taking in and where it is going, you cannot be confident that your business model is viable.
Revenue is easier to digest because it typically derives from only a few sources: professional fees and interest and, perhaps, speaking engagements, consultation to trainees, teaching, and rental income.
Expenses. Getting a grasp of where the money goes is more challenging. Common examples of costs of running a practice include, but aren’t limited to, the list in the Table.
By doing this basic profit/loss math, you will see how much money should be left over (profit) at the end of the month. To confirm, reconcile your numbers with your monthly business checking account statement; QuickBooks does this semi-automatically, or you can do it by hand. Reconciliation might feel uncomfortable if you are a novice to accounting, but spending a few moments to catch an error now is far less onerous than remedying what began as a small mistake and compounded to a big one over the years.
Other financial reports, such as a balance sheet and a statement of cash flow, are useful for giving you a sense of your practice’s long-term financial health. Typically, however, they are unnecessary during early stages of establishing a practice—and the work they require can be overwhelming.
After you’re done with the math
Based on your financial analysis of the practice, you will be able to pay yourself a salary based on the profit (that is, revenue minus expenses). Before you take your salary, however:
• Consider keeping enough in your business checking account to pay next month’s bills.
• Remember to adjust your monthly compensation downward by 20% to 50% to withhold for payroll and estimated federal and state taxes.
• Look into tax-advantaged business benefit plans. A retirement account, certain savings plans (eg, flexible spending accounts for dependent care or health care), a commuter plan, and life insurance paid for by the business can make your income go further. Some of these benefits are available only to employees of corporations; crunch the numbers, however, and discuss with your accountant whether the cost of incorporating is worthwhile.
• Determine whether hiring an assistant, or adding an additional one, will increase your bottom line. You incur significant expenses by hiring an employee—salary, payroll taxes, and time spent training, to name a few—but doing so might be worth it if the time that he (she) saves you opens up billable hours for seeing patients.
Good care requires a solid foundation
Caring for people who are suffering, while being financially successful, are not contradictory goals. Although you deal with a person’s private, intense feelings when you provide care, you also have an obligation to ensure the financial health of your practice.
Disclosure
Dr. Braslow is the founder of Luminello.com.
Being a caring, knowledgeable clinician is vital for patient care, but having such skill does not necessarily mean that running a medical practice comes easy— especially if you do not have a basic background in business. Financial fundamentals are rarely taught in residency and, with administrative burdens increasingly placed on physicians in solo and small practices, it isn’t surprising that many practitioners feel underprepared.
Fortunately, it doesn’t take a master’s degree in business administration to conquer these challenges. You just need some understanding of key operating principles.
Accounting basics
It isn’t personal; it’s only business. Delineate the point at which personal finances stop and business finances begin. Make sure that you have a business checking account and credit card, and run all your business expenses through those accounts—never through your personal accounts. That policy will save you time if your practice is audited and, more important, will help you be efficient by guiding your focus to the right set of numbers by which to manage the practice.
Set up a system to track transactions. Many businesses use the accounting software QuickBooks; the program can generate sophisticated reports, and many banks can export data to it automatically. But QuickBooks might be more complicated than what you need to get started; a simple spreadsheet program, such as Excel, might suffice. By working through the numbers yourself, you gain a more intimate knowledge of the state of your finances.
Assemble a team of experts to assist you, at least in the beginning, with building a core knowledge base and good habits. Don’t think that this absolves you of responsibility, however: Ultimately, you sign off on what your advisors recommend. For example, an accountant can prepare your tax return, but you review and approve it, and a financial advisor might recommend certain investments, but only you can authorize them. You might work with a banker for a business loan or a bookkeeper to help you with your day-to-day record-keeping, but no one can give you the critical thinking you need to maximize your financial success.
The devil is in those details
Delve into your practice’s profit/loss statement, or create one if it doesn’t exist. Understanding these data is critical for maintaining financial health. Without knowing how much money you are taking in and where it is going, you cannot be confident that your business model is viable.
Revenue is easier to digest because it typically derives from only a few sources: professional fees and interest and, perhaps, speaking engagements, consultation to trainees, teaching, and rental income.
Expenses. Getting a grasp of where the money goes is more challenging. Common examples of costs of running a practice include, but aren’t limited to, the list in the Table.
By doing this basic profit/loss math, you will see how much money should be left over (profit) at the end of the month. To confirm, reconcile your numbers with your monthly business checking account statement; QuickBooks does this semi-automatically, or you can do it by hand. Reconciliation might feel uncomfortable if you are a novice to accounting, but spending a few moments to catch an error now is far less onerous than remedying what began as a small mistake and compounded to a big one over the years.
Other financial reports, such as a balance sheet and a statement of cash flow, are useful for giving you a sense of your practice’s long-term financial health. Typically, however, they are unnecessary during early stages of establishing a practice—and the work they require can be overwhelming.
After you’re done with the math
Based on your financial analysis of the practice, you will be able to pay yourself a salary based on the profit (that is, revenue minus expenses). Before you take your salary, however:
• Consider keeping enough in your business checking account to pay next month’s bills.
• Remember to adjust your monthly compensation downward by 20% to 50% to withhold for payroll and estimated federal and state taxes.
• Look into tax-advantaged business benefit plans. A retirement account, certain savings plans (eg, flexible spending accounts for dependent care or health care), a commuter plan, and life insurance paid for by the business can make your income go further. Some of these benefits are available only to employees of corporations; crunch the numbers, however, and discuss with your accountant whether the cost of incorporating is worthwhile.
• Determine whether hiring an assistant, or adding an additional one, will increase your bottom line. You incur significant expenses by hiring an employee—salary, payroll taxes, and time spent training, to name a few—but doing so might be worth it if the time that he (she) saves you opens up billable hours for seeing patients.
Good care requires a solid foundation
Caring for people who are suffering, while being financially successful, are not contradictory goals. Although you deal with a person’s private, intense feelings when you provide care, you also have an obligation to ensure the financial health of your practice.
Disclosure
Dr. Braslow is the founder of Luminello.com.
How coffee and cigarettes can affect the response to psychopharmacotherapy
When a patient who smokes enters a tobacco-free medical facility and has access to caffeinated beverages, he (she) might experience toxicity to many pharmaceuticals and caffeine. Similarly, if a patient is discharged from a smoke-free environment with a newly adjusted medication regimen and resumes smoking or caffeine consumption, alterations in enzyme activity might reduce therapeutic efficacy of prescribed medicines. These effects are a result of alterations in the hepatic cytochrome P450 (CYP) enzyme system.
Taking a careful history of tobacco and caffeine use, and knowing the effects that these substances will have on specific medications, will help guide treatment and management decisions.
The role of CYP enzymes
CYP hepatic enzymes detoxify a variety of environmental agents into water-soluble compounds that are excreted in urine. CYP1A2 metabolizes 20% of drugs handled by the CYP system and comprises 13% of all the CYP enzymes expressed in the liver. The wide interindividual variation in CYP1A2 enzyme activity is influenced by a combination of genetic, epigenetic, ethnic, and environmental variables.1
Influence of tobacco on CYP
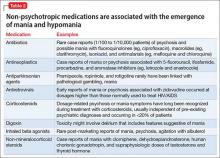
The polycyclic aromatic hydrocarbons in tobacco smoke induce CYP1A2 and CYP2B6 hepatic enzymes.2 Smokers exhibit increased activity of these enzymes, which results in faster clearance of many drugs, lower concentrations in blood, and diminished clinical response. The Table lists psychotropic medicines that are metabolized by CYP1A2 and CYP2B6. Co-administration of these substrates could decrease the elimination rate of other drugs also metabolized by CYP1A2. Nicotine in tobacco or in nicotine replacement therapies does not play a role in inducing CYP enzymes.
Psychiatric patients smoke at a higher rate than the general population.2 One study found that approximately 70% of patients with schizophrenia and as many as 45% of those with bipolar disorder smoke enough cigarettes (7 to 20 a day) to induce CYP1A2 and CYP2B6 activity.2 Patients who smoke and are given clozapine, haloperidol, or olanzapine show a lower serum concentration than non-smokers; in fact, the clozapine level can be reduced as much as 2.4-fold.2-5 Subsequently, patients can experience diminished clinical response to these 3 psychotropics.3
The turnover time for CYP1A2 is rapid— approximately 3 days—and a new CYP1A2 steady state activity is reached after approximately 1 week,4 which is important to remember when managing inpatients in a smoke-free facility. During acute hospitalization, patients could experience drug toxicity if the outpatient dosage is maintained.5
When they resume smoking after being discharged on a stabilized dosage of any of the medications listed in the Table, previous enzyme activity rebounds and might reduce the drug level, potentially leading to inadequate clinical response.
Caffeine and other substances
Asking about the patient’s caffeine intake is necessary because consumption of coffee is prevalent among smokers, and caffeine is metabolized by CYP1A2. Smokers need to consume as much as 4 times the amount of caffeine as non-smokers to achieve a similar caffeine serum concentration.2 Caffeine can form an insoluble precipitate with antipsychotic medication in the gut, which decreases absorption. The interaction between smoking-related induction of CYP1A2 enzymes and forced smoking cessation during hospitalization, with ongoing caffeine consumption, could lead to caffeine toxicity.4,5
Other common inducers of CYP1A2 are insulin, cabbage, cauliflower, broccoli, and charcoal-grilled meat. Also, cumin and turmeric inhibit CYP1A2 activity, which might explain an ethnic difference in drug tolerance across population groups. Additionally, certain genetic polymorphisms, in specific ethnic distributions, alter the potential for tobacco smoke to induce CYP1A2.6
Some of these polymorphisms can be genotyped for clinical application.3
Asking about a patient’s tobacco and caffeine use and understanding their interactions with specific medications provides guidance when prescribing antipsychotic medications and adjusting dosage for inpatients and during clinical follow-up care.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wang B, Zhou SF. Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16(31):4066-4218.
2. Lucas C, Martin J. Smoking and drug interactions. Australian Prescriber. 2013;36(3):102-104.
3. Eap CB, Bender S, Jaquenoud Sirot E, et al. Nonresponse to clozapine and ultrarapid CYP1A2 activity: clinical data and analysis of CYP1A2 gene. J Clin Psychopharmacol. 2004; 24(2):214-209.
4. Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
5. Berk M, Ng F, Wang WV, et al. Going up in smoke: tobacco smoking is associated with worse treatment outcomes in mania. J Affect Disord. 2008;110(1-2):126-134.
6. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS. 2009;11(3):481-494.
When a patient who smokes enters a tobacco-free medical facility and has access to caffeinated beverages, he (she) might experience toxicity to many pharmaceuticals and caffeine. Similarly, if a patient is discharged from a smoke-free environment with a newly adjusted medication regimen and resumes smoking or caffeine consumption, alterations in enzyme activity might reduce therapeutic efficacy of prescribed medicines. These effects are a result of alterations in the hepatic cytochrome P450 (CYP) enzyme system.
Taking a careful history of tobacco and caffeine use, and knowing the effects that these substances will have on specific medications, will help guide treatment and management decisions.
The role of CYP enzymes
CYP hepatic enzymes detoxify a variety of environmental agents into water-soluble compounds that are excreted in urine. CYP1A2 metabolizes 20% of drugs handled by the CYP system and comprises 13% of all the CYP enzymes expressed in the liver. The wide interindividual variation in CYP1A2 enzyme activity is influenced by a combination of genetic, epigenetic, ethnic, and environmental variables.1
Influence of tobacco on CYP
The polycyclic aromatic hydrocarbons in tobacco smoke induce CYP1A2 and CYP2B6 hepatic enzymes.2 Smokers exhibit increased activity of these enzymes, which results in faster clearance of many drugs, lower concentrations in blood, and diminished clinical response. The Table lists psychotropic medicines that are metabolized by CYP1A2 and CYP2B6. Co-administration of these substrates could decrease the elimination rate of other drugs also metabolized by CYP1A2. Nicotine in tobacco or in nicotine replacement therapies does not play a role in inducing CYP enzymes.
Psychiatric patients smoke at a higher rate than the general population.2 One study found that approximately 70% of patients with schizophrenia and as many as 45% of those with bipolar disorder smoke enough cigarettes (7 to 20 a day) to induce CYP1A2 and CYP2B6 activity.2 Patients who smoke and are given clozapine, haloperidol, or olanzapine show a lower serum concentration than non-smokers; in fact, the clozapine level can be reduced as much as 2.4-fold.2-5 Subsequently, patients can experience diminished clinical response to these 3 psychotropics.3
The turnover time for CYP1A2 is rapid— approximately 3 days—and a new CYP1A2 steady state activity is reached after approximately 1 week,4 which is important to remember when managing inpatients in a smoke-free facility. During acute hospitalization, patients could experience drug toxicity if the outpatient dosage is maintained.5
When they resume smoking after being discharged on a stabilized dosage of any of the medications listed in the Table, previous enzyme activity rebounds and might reduce the drug level, potentially leading to inadequate clinical response.
Caffeine and other substances
Asking about the patient’s caffeine intake is necessary because consumption of coffee is prevalent among smokers, and caffeine is metabolized by CYP1A2. Smokers need to consume as much as 4 times the amount of caffeine as non-smokers to achieve a similar caffeine serum concentration.2 Caffeine can form an insoluble precipitate with antipsychotic medication in the gut, which decreases absorption. The interaction between smoking-related induction of CYP1A2 enzymes and forced smoking cessation during hospitalization, with ongoing caffeine consumption, could lead to caffeine toxicity.4,5
Other common inducers of CYP1A2 are insulin, cabbage, cauliflower, broccoli, and charcoal-grilled meat. Also, cumin and turmeric inhibit CYP1A2 activity, which might explain an ethnic difference in drug tolerance across population groups. Additionally, certain genetic polymorphisms, in specific ethnic distributions, alter the potential for tobacco smoke to induce CYP1A2.6
Some of these polymorphisms can be genotyped for clinical application.3
Asking about a patient’s tobacco and caffeine use and understanding their interactions with specific medications provides guidance when prescribing antipsychotic medications and adjusting dosage for inpatients and during clinical follow-up care.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
When a patient who smokes enters a tobacco-free medical facility and has access to caffeinated beverages, he (she) might experience toxicity to many pharmaceuticals and caffeine. Similarly, if a patient is discharged from a smoke-free environment with a newly adjusted medication regimen and resumes smoking or caffeine consumption, alterations in enzyme activity might reduce therapeutic efficacy of prescribed medicines. These effects are a result of alterations in the hepatic cytochrome P450 (CYP) enzyme system.
Taking a careful history of tobacco and caffeine use, and knowing the effects that these substances will have on specific medications, will help guide treatment and management decisions.
The role of CYP enzymes
CYP hepatic enzymes detoxify a variety of environmental agents into water-soluble compounds that are excreted in urine. CYP1A2 metabolizes 20% of drugs handled by the CYP system and comprises 13% of all the CYP enzymes expressed in the liver. The wide interindividual variation in CYP1A2 enzyme activity is influenced by a combination of genetic, epigenetic, ethnic, and environmental variables.1
Influence of tobacco on CYP
The polycyclic aromatic hydrocarbons in tobacco smoke induce CYP1A2 and CYP2B6 hepatic enzymes.2 Smokers exhibit increased activity of these enzymes, which results in faster clearance of many drugs, lower concentrations in blood, and diminished clinical response. The Table lists psychotropic medicines that are metabolized by CYP1A2 and CYP2B6. Co-administration of these substrates could decrease the elimination rate of other drugs also metabolized by CYP1A2. Nicotine in tobacco or in nicotine replacement therapies does not play a role in inducing CYP enzymes.
Psychiatric patients smoke at a higher rate than the general population.2 One study found that approximately 70% of patients with schizophrenia and as many as 45% of those with bipolar disorder smoke enough cigarettes (7 to 20 a day) to induce CYP1A2 and CYP2B6 activity.2 Patients who smoke and are given clozapine, haloperidol, or olanzapine show a lower serum concentration than non-smokers; in fact, the clozapine level can be reduced as much as 2.4-fold.2-5 Subsequently, patients can experience diminished clinical response to these 3 psychotropics.3
The turnover time for CYP1A2 is rapid— approximately 3 days—and a new CYP1A2 steady state activity is reached after approximately 1 week,4 which is important to remember when managing inpatients in a smoke-free facility. During acute hospitalization, patients could experience drug toxicity if the outpatient dosage is maintained.5
When they resume smoking after being discharged on a stabilized dosage of any of the medications listed in the Table, previous enzyme activity rebounds and might reduce the drug level, potentially leading to inadequate clinical response.
Caffeine and other substances
Asking about the patient’s caffeine intake is necessary because consumption of coffee is prevalent among smokers, and caffeine is metabolized by CYP1A2. Smokers need to consume as much as 4 times the amount of caffeine as non-smokers to achieve a similar caffeine serum concentration.2 Caffeine can form an insoluble precipitate with antipsychotic medication in the gut, which decreases absorption. The interaction between smoking-related induction of CYP1A2 enzymes and forced smoking cessation during hospitalization, with ongoing caffeine consumption, could lead to caffeine toxicity.4,5
Other common inducers of CYP1A2 are insulin, cabbage, cauliflower, broccoli, and charcoal-grilled meat. Also, cumin and turmeric inhibit CYP1A2 activity, which might explain an ethnic difference in drug tolerance across population groups. Additionally, certain genetic polymorphisms, in specific ethnic distributions, alter the potential for tobacco smoke to induce CYP1A2.6
Some of these polymorphisms can be genotyped for clinical application.3
Asking about a patient’s tobacco and caffeine use and understanding their interactions with specific medications provides guidance when prescribing antipsychotic medications and adjusting dosage for inpatients and during clinical follow-up care.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Wang B, Zhou SF. Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16(31):4066-4218.
2. Lucas C, Martin J. Smoking and drug interactions. Australian Prescriber. 2013;36(3):102-104.
3. Eap CB, Bender S, Jaquenoud Sirot E, et al. Nonresponse to clozapine and ultrarapid CYP1A2 activity: clinical data and analysis of CYP1A2 gene. J Clin Psychopharmacol. 2004; 24(2):214-209.
4. Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
5. Berk M, Ng F, Wang WV, et al. Going up in smoke: tobacco smoking is associated with worse treatment outcomes in mania. J Affect Disord. 2008;110(1-2):126-134.
6. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS. 2009;11(3):481-494.
1. Wang B, Zhou SF. Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16(31):4066-4218.
2. Lucas C, Martin J. Smoking and drug interactions. Australian Prescriber. 2013;36(3):102-104.
3. Eap CB, Bender S, Jaquenoud Sirot E, et al. Nonresponse to clozapine and ultrarapid CYP1A2 activity: clinical data and analysis of CYP1A2 gene. J Clin Psychopharmacol. 2004; 24(2):214-209.
4. Faber MS, Fuhr U. Time response of cytochrome P450 1A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
5. Berk M, Ng F, Wang WV, et al. Going up in smoke: tobacco smoking is associated with worse treatment outcomes in mania. J Affect Disord. 2008;110(1-2):126-134.
6. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS. 2009;11(3):481-494.
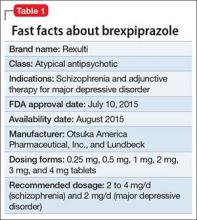
Brexpiprazole for schizophrenia and as adjunct for major depressive disorder
Brexpiprazole, FDA-approved in July 2015 to treat schizophrenia and as an adjunct for major depressive disorder (MDD) (Table 1), has shown efficacy in 2 phase-III acute trials for each indication.1-6 Although brexpiprazole is a dopamine D2 partial agonist, it differs from aripiprazole, the other available D2 partial agonist, because it is more potent at serotonin 5-HT1A and 5-HT2A receptors and displays less intrinsic activity at D2 receptors,7 which could mean better tolerability.
Clinical implications
Schizophrenia is heterogeneous, and individual response and tolerability to antipsychotics vary greatly8; therefore, new drug options are useful. For MDD, before the availability of brexpiprazole, only 3 antipsychotics were FDA-approved for adjunctive use with antidepressant therapy9; brexpiprazole represents another agent for patients whose depressive symptoms persist after standard antidepressant treatment.
Variables that limit the use of antipsychotics include extrapyramidal symptoms (EPS), akathisia, sedation/somnolence, weight gain, metabolic abnormalities, and hyperprolactinemia. If post-marketing studies and clinical experience confirm that brexpiprazole has an overall favorable side-effect profile regarding these tolerability obstacles, brexpiprazole would potentially have advantages over some other available agents, including aripiprazole.
How it works
In addition to a subnanomolar binding affinity (Ki < 1 nM) to dopamine D2 receptors as a partial agonist, brexpiprazole also exhibits similar binding affinities for serotonin 5-HT1A (partial agonist), 5-HT2A (antagonist), and adrenergic α1B (antagonist) and α2C (antagonist) receptors.7
Brexpiprazole also has high affinity (Ki < 5 nM) for dopamine D3 (partial ago nist), serotonin 5-HT2B (antagonist), and 5-HT7 (antagonist), and at adrenergic α1A (antagonist) and α1D (antagonist) receptors. Brexpiprazole has moderate affinity for histamine H1 receptors (Ki = 19 nM, antagonist), and low affinity for muscarinic M1 receptors (Ki > 1000 nM, antagonist).
Brexpiprazole’s pharmacodynamic profile differs from other available antipsychotics, including aripiprazole. Whether this translates to meaningful differences in efficacy and tolerability will depend on the outcomes of specifically designed clinical trials as well as “real-world” experience. Animal models have suggested amelioration of schizophrenia-like behavior, depression-like behavior, and anxiety-like behavior with brexipiprazole.6
Pharmacokinetics
At 91 hours, brexpiprazole’s half-life is relatively long; a steady-state concentration therefore is attained in approximately 2 weeks.1 In the phase-III clinical trials, brexpiprazole was titrated to target dosages, and therefore the product label recommends the same. Brexpiprazole can be administered with or without food.
In a study of brexpiprazole excretion, after a single oral dose of [14C]-labeled brexpiprazole, approximately 25% and 46% of the administered radioactivity was recovered in urine and feces, respectively. Less than 1% of unchanged brexpiprazole was excreted in the urine, and approximately 14% of the oral dose was recovered unchanged in the feces.
Exposure, as measured by maximum concentration and area under the concentration curve, is dose proportional.
Metabolism of brexpiprazole is mediated principally by cytochrome P450 (CYP) 3A4 and CYP2D6. Based on in vitro data, brexpiprazole shows little or no inhibition of CYP450 isozymes.
Efficacy
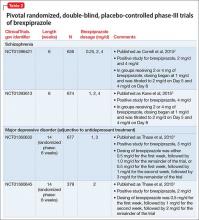
FDA approval for brexpiprazole for schizophrenia and for adjunctive use in MDD was based on 4 phase-III pivotal acute clinical trials conducted in adults, 2 studies each for each disorder.1-6 These studies are described in Table 2.2-5
Schizophrenia. The primary outcome measure for the acute schizophrenia trials was change on the Positive and Negative Syndrome Scale (PANSS) total scores from baseline to 6-week endpoint. Statistically significant reductions in PANSS total score were observed for brexpiprazole dosages of 2 mg/d and 4 mg/d in one study,2 and 4 mg/d in another study.3 Responder rates also were measured, with response defined as a reduction of ≥30% from baseline in PANSS total score or a Clinical Global Impressions-Improvement score of 1 (very much improved) or 2 (much improved).2,3 Pooling together the available data for the recommended target dosage of brexpiprazole for schizophrenia (2 to 4 mg/d) from the 2 phase-III studies, 45.5% of patients responded to the drug, compared with 31% for the pooled placebo groups, yielding a number needed to treat (NNT) of 7 (95% CI, 5-12).6
Although not described in product labeling, a phase-III 52-week maintenance study demonstrated brexpiprazole’s efficacy in preventing exacerbation of psychotic symptoms and impending relapse in patients with schizophrenia.10 Time from randomization to exacerbation of psychotic symptoms or impending relapse showed a beneficial effect with brexpiprazole compared with placebo (log-rank test: hazard ratio = 0.292, P < .0001). Significantly fewer patients in the brexpiprazole group relapsed compared with placebo (13.5% vs 38.5%, P < .0001), resulting in a NNT of 4 (95% CI, 3-8).
Major depressive disorder. The primary outcome measure for the acute MDD studies was change in Montgomery-Åsberg Depression Rating Scale (MADRS) scores from baseline to 6-week endpoint of the randomized treatment phase. All patients were required to have a history of inadequate response to 1 to 3 treatment trials of standard antidepressants for their current depressive episode. In addition, patients entered the randomized phase only if they had an inadequate response to antidepressant therapy during an 8-week prospective treatment trial of standard antidepressant treatment plus single-blind placebo.
Participants who responded adequately to the antidepressant in the prospective single-blind phase were not randomized, but instead continued on antidepressant treatment plus single-blind placebo for 6 weeks.
The phase-III studies showed positive results for brexpiprazole, 2 mg/d and 3 mg/d, with change in MADRS from baseline to endpoint superior to that observed with placebo.4,5
When examining treatment response, defined as a reduction of ≥50% in MADRS total score from baseline, NNT vs placebo for response were 12 at all dosages tested, however, NNT vs placebo for remission (defined as MADRS total score ≤10 and ≥50% improvement from baseline) ranged from 17 to 31 and were not statistically significant.6 When the results for brexpiprazole, 1 mg/d, 2 mg/d, and 3 mg/d, from the 2 phase-III trials are pooled together, 23.2% of the patients receiving brexpiprazole were responders, vs 14.5% for placebo, yielding a NNT of 12 (95% CI, 8-26); 14.4% of the brexpiprazole-treated patients met remission criteria, vs 9.6% for placebo, resulting in a NNT of 21 (95% CI, 12-138).6
Tolerability
Overall tolerability can be evaluated by examining the percentage of patients who discontinued the clinical trials because of an adverse event (AE). In the acute schizophrenia double-blind trials for the recommended dosage range of 2 to 4 mg, the discontinuation rates were lower overall for patients receiving brexpiprazole compared with placebo.2,3 In the acute MDD trials, 32.6% of brexpiprazole-treated patients and 10.7% of placebo-treated patients discontinued because of AEs,4,5 yielding a number needed to harm (NNH) of 53 (95% CI, 30-235).6
The most commonly encountered AEs for MDD (incidence ≥5% and at least twice the rate for placebo) were akathisia (8.6% vs 1.7% for brexpiprazole vs placebo, and dose-related) and weight gain (6.7% vs 1.9%),1 with NNH values of 15 (95% CI, 11-23), and 22 (95% CI, 15-42), respectively.6 The most commonly encountered AE for schizophrenia (incidence ≥4% and at least twice the rate for placebo) was weight gain (4% vs 2%),1 with a NNH of 50 (95% CI, 26-1773).6
Of note, rates of akathisia in the schizophrenia trials were 5.5% for brexpiprazole and 4.6% for placebo,1 yielding a non-statistically significant NNH of 112.6 In a 6-week exploratory study,11 the incidence of EPS-related AEs including akathisia was lower for brexpiprazole-treated patients (14.1%) compared with those receiving aripiprazole (30.3%), for a NNT advantage for brexpiprazole of 7 (not statistically significant).
Short-term weight gain appears modest; however, outliers with an increase of ≥7% of body weight were evident in open-label long-term safety studies.1,6 Effects on glucose and lipids were small. Minimal effects on prolactin were observed, and no clinically relevant effects on the QT interval were evident.
Contraindications
The only absolute contraindication for brexpiprazole is known hypersensitivity to brexpiprazole or any of its components. Reactions have included rash, facial swelling, urticaria, and anaphylaxis.1
As with all antipsychotics and antipsychotics with an indication for a depressive disorder:
• there is a bolded boxed warning in the product label regarding increased mortality in geriatric patients with dementia-related psychosis. Brexpiprazole is not approved for treating patients with dementia-related psychosis
• there is a bolded boxed warning in the product label about suicidal thoughts and behaviors in patients age ≤24. The safety and efficacy of brexpiprazole have not been established in pediatric patients.
Dosing
Schizophrenia. The recommended starting dosage for brexpiprazole for schizophrenia is 1 mg/d on Days 1 to 4. Brexpiprazole can be titrated to 2 mg/d on Day 5 through Day 7, then to 4 mg/d on Day 8 based on the patient’s response and ability to tolerate the medication. The recommended target dosage is 2 to 4 mg/d with a maximum recommended daily dosage of 4 mg.
Major depressive disorder. The recommended starting dosage for brexpiprazole as adjunctive treatment for MDD is 0.5 mg or 1 mg/d. Brexpiprazole can be titrated to 1 mg/d, then up to the target dosage of 2 mg/d, with dosage increases occurring at weekly intervals based on the patient’s clinical response and ability to tolerate the agent, with a maximum recommended dosage of 3 mg/d.
Other considerations. For patients with moderate to severe hepatic impairment, or moderate, severe, or end-stage renal impairment, the maximum recommended dosage is 3 mg/d for patients with schizophrenia, and 2 mg/d for patients with MDD.
In general, dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in those taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers1:
• for strong CYP2D6 or CYP3A4 inhibitors, administer one-half the usual dosage
• for strong/moderate CYP2D6 with strong/moderate CYP3A4 inhibitors, administer a one-quarter of the usual dosage
• for known CYP2D6 poor metabolizers taking strong/moderate CYP3A4 inhibitors, also administer a one-quarter of the usual dosage
• for strong CYP3A4 inducers, double the usual dosage and further adjust based on clinical response.
In clinical trials for MDD, brexpiprazole dosage was not adjusted for strong CYP2D6 inhibitors (eg, paroxetine, fluoxetine). Therefore, CYP considerations are already factored into general dosing recommendations and brexpiprazole could be administered without dosage adjustment in patients with MDD; however, under these circumstances, it would be prudent to start brexpiprazole at 0.5 mg, which, although “on-label,” represents a low starting dosage. (Whenever 2 drugs are co-administered and 1 agent has the ability to disturb the metabolism of the other, using smaller increments to the target dosage and possibly waiting longer between dosage adjustments could help avoid potential drug–drug interactions.)
No dosage adjustment for brexpiprazole is required on the basis of sex, race or ethnicity, or smoking status. Although clinical studies did not include patients age ≥65, the product label recommends that in general, dose selection for a geriatric patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, and cardiac function, concomitant diseases, and other drug therapy.
Bottom Line
Brexpiprazole, an atypical antipsychotic, is FDA-approved for schizophrenia and as an adjunct to antidepressants in major depressive disorder. For both indications, brexpiprazole demonstrated positive results compared with placebo in phase-III trials. Brexpiprazole is more potent at serotonin 5-HT1A and 5-HT2A receptors and displays less intrinsic activity at D2 receptors than aripiprazole, which could mean that the drug may be better-tolerated.
Related Resources
• Citrome L. Brexpiprazole: a new dopamine D2 receptor partial agonist for the treatment of schizophrenia and major depressive disorder. Drugs Today (Barc). 2015;51(7):397-414.
• Citrome L, Stensbøl TB, Maeda K. The preclinical profile of brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother. In press.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Fluoxetine • Prozac
Paroxetine • Paxil
Disclosure
Dr. Citrome is a consultant to Alexza Pharmaceuticals, Alkermes, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, Forum Pharmaceuticals, Genentech, Janssen, Jazz Pharmaceuticals, Lundbeck, Merck, Medivation, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, and Valeant Pharmaceuticals; and is a speaker for Allergan, AstraZeneca, Janssen, Jazz Pharmaceuticals, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Shire, Sunovion, Takeda, and Teva.
1. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
2. Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870-880.
3. Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1-3):127-135.
4. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study [published online August 4, 2015]. J Clin Psychiatry. doi: 10.4088/ JCP.14m09689.
5. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants [published online August 4, 2015]. J Clin Psychiatry. doi: 10.4088/JCP.14m09688.
6. Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978-997.
7. Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604.
8. Volavka J, Citrome L. Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother. 2009;10(12):1917-1928.
9. Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010;122(4):39-48.
10. Hobart M, Ouyang J, Forbes A, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. Poster presented at: the American Society of Clinical Psychopharmacology Annual Meeting; June 22 to 25, 2015; Miami, FL.
11. Citrome L, Ota A, Nagamizu K, Perry P, et al. The effect of brexpiprazole (OPC‐34712) versus aripiprazole in adult patients with acute schizophrenia: an exploratory study. Poster presented at: the Society of Biological Psychiatry Annual Scientific Meeting and Convention; May 15, 2015; Toronto, Ontario, Canada.
Brexpiprazole, FDA-approved in July 2015 to treat schizophrenia and as an adjunct for major depressive disorder (MDD) (Table 1), has shown efficacy in 2 phase-III acute trials for each indication.1-6 Although brexpiprazole is a dopamine D2 partial agonist, it differs from aripiprazole, the other available D2 partial agonist, because it is more potent at serotonin 5-HT1A and 5-HT2A receptors and displays less intrinsic activity at D2 receptors,7 which could mean better tolerability.
Clinical implications
Schizophrenia is heterogeneous, and individual response and tolerability to antipsychotics vary greatly8; therefore, new drug options are useful. For MDD, before the availability of brexpiprazole, only 3 antipsychotics were FDA-approved for adjunctive use with antidepressant therapy9; brexpiprazole represents another agent for patients whose depressive symptoms persist after standard antidepressant treatment.
Variables that limit the use of antipsychotics include extrapyramidal symptoms (EPS), akathisia, sedation/somnolence, weight gain, metabolic abnormalities, and hyperprolactinemia. If post-marketing studies and clinical experience confirm that brexpiprazole has an overall favorable side-effect profile regarding these tolerability obstacles, brexpiprazole would potentially have advantages over some other available agents, including aripiprazole.
How it works
In addition to a subnanomolar binding affinity (Ki < 1 nM) to dopamine D2 receptors as a partial agonist, brexpiprazole also exhibits similar binding affinities for serotonin 5-HT1A (partial agonist), 5-HT2A (antagonist), and adrenergic α1B (antagonist) and α2C (antagonist) receptors.7
Brexpiprazole also has high affinity (Ki < 5 nM) for dopamine D3 (partial ago nist), serotonin 5-HT2B (antagonist), and 5-HT7 (antagonist), and at adrenergic α1A (antagonist) and α1D (antagonist) receptors. Brexpiprazole has moderate affinity for histamine H1 receptors (Ki = 19 nM, antagonist), and low affinity for muscarinic M1 receptors (Ki > 1000 nM, antagonist).
Brexpiprazole’s pharmacodynamic profile differs from other available antipsychotics, including aripiprazole. Whether this translates to meaningful differences in efficacy and tolerability will depend on the outcomes of specifically designed clinical trials as well as “real-world” experience. Animal models have suggested amelioration of schizophrenia-like behavior, depression-like behavior, and anxiety-like behavior with brexipiprazole.6
Pharmacokinetics
At 91 hours, brexpiprazole’s half-life is relatively long; a steady-state concentration therefore is attained in approximately 2 weeks.1 In the phase-III clinical trials, brexpiprazole was titrated to target dosages, and therefore the product label recommends the same. Brexpiprazole can be administered with or without food.
In a study of brexpiprazole excretion, after a single oral dose of [14C]-labeled brexpiprazole, approximately 25% and 46% of the administered radioactivity was recovered in urine and feces, respectively. Less than 1% of unchanged brexpiprazole was excreted in the urine, and approximately 14% of the oral dose was recovered unchanged in the feces.
Exposure, as measured by maximum concentration and area under the concentration curve, is dose proportional.
Metabolism of brexpiprazole is mediated principally by cytochrome P450 (CYP) 3A4 and CYP2D6. Based on in vitro data, brexpiprazole shows little or no inhibition of CYP450 isozymes.
Efficacy
FDA approval for brexpiprazole for schizophrenia and for adjunctive use in MDD was based on 4 phase-III pivotal acute clinical trials conducted in adults, 2 studies each for each disorder.1-6 These studies are described in Table 2.2-5
Schizophrenia. The primary outcome measure for the acute schizophrenia trials was change on the Positive and Negative Syndrome Scale (PANSS) total scores from baseline to 6-week endpoint. Statistically significant reductions in PANSS total score were observed for brexpiprazole dosages of 2 mg/d and 4 mg/d in one study,2 and 4 mg/d in another study.3 Responder rates also were measured, with response defined as a reduction of ≥30% from baseline in PANSS total score or a Clinical Global Impressions-Improvement score of 1 (very much improved) or 2 (much improved).2,3 Pooling together the available data for the recommended target dosage of brexpiprazole for schizophrenia (2 to 4 mg/d) from the 2 phase-III studies, 45.5% of patients responded to the drug, compared with 31% for the pooled placebo groups, yielding a number needed to treat (NNT) of 7 (95% CI, 5-12).6
Although not described in product labeling, a phase-III 52-week maintenance study demonstrated brexpiprazole’s efficacy in preventing exacerbation of psychotic symptoms and impending relapse in patients with schizophrenia.10 Time from randomization to exacerbation of psychotic symptoms or impending relapse showed a beneficial effect with brexpiprazole compared with placebo (log-rank test: hazard ratio = 0.292, P < .0001). Significantly fewer patients in the brexpiprazole group relapsed compared with placebo (13.5% vs 38.5%, P < .0001), resulting in a NNT of 4 (95% CI, 3-8).
Major depressive disorder. The primary outcome measure for the acute MDD studies was change in Montgomery-Åsberg Depression Rating Scale (MADRS) scores from baseline to 6-week endpoint of the randomized treatment phase. All patients were required to have a history of inadequate response to 1 to 3 treatment trials of standard antidepressants for their current depressive episode. In addition, patients entered the randomized phase only if they had an inadequate response to antidepressant therapy during an 8-week prospective treatment trial of standard antidepressant treatment plus single-blind placebo.
Participants who responded adequately to the antidepressant in the prospective single-blind phase were not randomized, but instead continued on antidepressant treatment plus single-blind placebo for 6 weeks.
The phase-III studies showed positive results for brexpiprazole, 2 mg/d and 3 mg/d, with change in MADRS from baseline to endpoint superior to that observed with placebo.4,5
When examining treatment response, defined as a reduction of ≥50% in MADRS total score from baseline, NNT vs placebo for response were 12 at all dosages tested, however, NNT vs placebo for remission (defined as MADRS total score ≤10 and ≥50% improvement from baseline) ranged from 17 to 31 and were not statistically significant.6 When the results for brexpiprazole, 1 mg/d, 2 mg/d, and 3 mg/d, from the 2 phase-III trials are pooled together, 23.2% of the patients receiving brexpiprazole were responders, vs 14.5% for placebo, yielding a NNT of 12 (95% CI, 8-26); 14.4% of the brexpiprazole-treated patients met remission criteria, vs 9.6% for placebo, resulting in a NNT of 21 (95% CI, 12-138).6
Tolerability
Overall tolerability can be evaluated by examining the percentage of patients who discontinued the clinical trials because of an adverse event (AE). In the acute schizophrenia double-blind trials for the recommended dosage range of 2 to 4 mg, the discontinuation rates were lower overall for patients receiving brexpiprazole compared with placebo.2,3 In the acute MDD trials, 32.6% of brexpiprazole-treated patients and 10.7% of placebo-treated patients discontinued because of AEs,4,5 yielding a number needed to harm (NNH) of 53 (95% CI, 30-235).6
The most commonly encountered AEs for MDD (incidence ≥5% and at least twice the rate for placebo) were akathisia (8.6% vs 1.7% for brexpiprazole vs placebo, and dose-related) and weight gain (6.7% vs 1.9%),1 with NNH values of 15 (95% CI, 11-23), and 22 (95% CI, 15-42), respectively.6 The most commonly encountered AE for schizophrenia (incidence ≥4% and at least twice the rate for placebo) was weight gain (4% vs 2%),1 with a NNH of 50 (95% CI, 26-1773).6
Of note, rates of akathisia in the schizophrenia trials were 5.5% for brexpiprazole and 4.6% for placebo,1 yielding a non-statistically significant NNH of 112.6 In a 6-week exploratory study,11 the incidence of EPS-related AEs including akathisia was lower for brexpiprazole-treated patients (14.1%) compared with those receiving aripiprazole (30.3%), for a NNT advantage for brexpiprazole of 7 (not statistically significant).
Short-term weight gain appears modest; however, outliers with an increase of ≥7% of body weight were evident in open-label long-term safety studies.1,6 Effects on glucose and lipids were small. Minimal effects on prolactin were observed, and no clinically relevant effects on the QT interval were evident.
Contraindications
The only absolute contraindication for brexpiprazole is known hypersensitivity to brexpiprazole or any of its components. Reactions have included rash, facial swelling, urticaria, and anaphylaxis.1
As with all antipsychotics and antipsychotics with an indication for a depressive disorder:
• there is a bolded boxed warning in the product label regarding increased mortality in geriatric patients with dementia-related psychosis. Brexpiprazole is not approved for treating patients with dementia-related psychosis
• there is a bolded boxed warning in the product label about suicidal thoughts and behaviors in patients age ≤24. The safety and efficacy of brexpiprazole have not been established in pediatric patients.
Dosing
Schizophrenia. The recommended starting dosage for brexpiprazole for schizophrenia is 1 mg/d on Days 1 to 4. Brexpiprazole can be titrated to 2 mg/d on Day 5 through Day 7, then to 4 mg/d on Day 8 based on the patient’s response and ability to tolerate the medication. The recommended target dosage is 2 to 4 mg/d with a maximum recommended daily dosage of 4 mg.
Major depressive disorder. The recommended starting dosage for brexpiprazole as adjunctive treatment for MDD is 0.5 mg or 1 mg/d. Brexpiprazole can be titrated to 1 mg/d, then up to the target dosage of 2 mg/d, with dosage increases occurring at weekly intervals based on the patient’s clinical response and ability to tolerate the agent, with a maximum recommended dosage of 3 mg/d.
Other considerations. For patients with moderate to severe hepatic impairment, or moderate, severe, or end-stage renal impairment, the maximum recommended dosage is 3 mg/d for patients with schizophrenia, and 2 mg/d for patients with MDD.
In general, dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in those taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers1:
• for strong CYP2D6 or CYP3A4 inhibitors, administer one-half the usual dosage
• for strong/moderate CYP2D6 with strong/moderate CYP3A4 inhibitors, administer a one-quarter of the usual dosage
• for known CYP2D6 poor metabolizers taking strong/moderate CYP3A4 inhibitors, also administer a one-quarter of the usual dosage
• for strong CYP3A4 inducers, double the usual dosage and further adjust based on clinical response.
In clinical trials for MDD, brexpiprazole dosage was not adjusted for strong CYP2D6 inhibitors (eg, paroxetine, fluoxetine). Therefore, CYP considerations are already factored into general dosing recommendations and brexpiprazole could be administered without dosage adjustment in patients with MDD; however, under these circumstances, it would be prudent to start brexpiprazole at 0.5 mg, which, although “on-label,” represents a low starting dosage. (Whenever 2 drugs are co-administered and 1 agent has the ability to disturb the metabolism of the other, using smaller increments to the target dosage and possibly waiting longer between dosage adjustments could help avoid potential drug–drug interactions.)
No dosage adjustment for brexpiprazole is required on the basis of sex, race or ethnicity, or smoking status. Although clinical studies did not include patients age ≥65, the product label recommends that in general, dose selection for a geriatric patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, and cardiac function, concomitant diseases, and other drug therapy.
Bottom Line
Brexpiprazole, an atypical antipsychotic, is FDA-approved for schizophrenia and as an adjunct to antidepressants in major depressive disorder. For both indications, brexpiprazole demonstrated positive results compared with placebo in phase-III trials. Brexpiprazole is more potent at serotonin 5-HT1A and 5-HT2A receptors and displays less intrinsic activity at D2 receptors than aripiprazole, which could mean that the drug may be better-tolerated.
Related Resources
• Citrome L. Brexpiprazole: a new dopamine D2 receptor partial agonist for the treatment of schizophrenia and major depressive disorder. Drugs Today (Barc). 2015;51(7):397-414.
• Citrome L, Stensbøl TB, Maeda K. The preclinical profile of brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother. In press.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Fluoxetine • Prozac
Paroxetine • Paxil
Disclosure
Dr. Citrome is a consultant to Alexza Pharmaceuticals, Alkermes, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, Forum Pharmaceuticals, Genentech, Janssen, Jazz Pharmaceuticals, Lundbeck, Merck, Medivation, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, and Valeant Pharmaceuticals; and is a speaker for Allergan, AstraZeneca, Janssen, Jazz Pharmaceuticals, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Shire, Sunovion, Takeda, and Teva.
Brexpiprazole, FDA-approved in July 2015 to treat schizophrenia and as an adjunct for major depressive disorder (MDD) (Table 1), has shown efficacy in 2 phase-III acute trials for each indication.1-6 Although brexpiprazole is a dopamine D2 partial agonist, it differs from aripiprazole, the other available D2 partial agonist, because it is more potent at serotonin 5-HT1A and 5-HT2A receptors and displays less intrinsic activity at D2 receptors,7 which could mean better tolerability.
Clinical implications
Schizophrenia is heterogeneous, and individual response and tolerability to antipsychotics vary greatly8; therefore, new drug options are useful. For MDD, before the availability of brexpiprazole, only 3 antipsychotics were FDA-approved for adjunctive use with antidepressant therapy9; brexpiprazole represents another agent for patients whose depressive symptoms persist after standard antidepressant treatment.
Variables that limit the use of antipsychotics include extrapyramidal symptoms (EPS), akathisia, sedation/somnolence, weight gain, metabolic abnormalities, and hyperprolactinemia. If post-marketing studies and clinical experience confirm that brexpiprazole has an overall favorable side-effect profile regarding these tolerability obstacles, brexpiprazole would potentially have advantages over some other available agents, including aripiprazole.
How it works
In addition to a subnanomolar binding affinity (Ki < 1 nM) to dopamine D2 receptors as a partial agonist, brexpiprazole also exhibits similar binding affinities for serotonin 5-HT1A (partial agonist), 5-HT2A (antagonist), and adrenergic α1B (antagonist) and α2C (antagonist) receptors.7
Brexpiprazole also has high affinity (Ki < 5 nM) for dopamine D3 (partial ago nist), serotonin 5-HT2B (antagonist), and 5-HT7 (antagonist), and at adrenergic α1A (antagonist) and α1D (antagonist) receptors. Brexpiprazole has moderate affinity for histamine H1 receptors (Ki = 19 nM, antagonist), and low affinity for muscarinic M1 receptors (Ki > 1000 nM, antagonist).
Brexpiprazole’s pharmacodynamic profile differs from other available antipsychotics, including aripiprazole. Whether this translates to meaningful differences in efficacy and tolerability will depend on the outcomes of specifically designed clinical trials as well as “real-world” experience. Animal models have suggested amelioration of schizophrenia-like behavior, depression-like behavior, and anxiety-like behavior with brexipiprazole.6
Pharmacokinetics
At 91 hours, brexpiprazole’s half-life is relatively long; a steady-state concentration therefore is attained in approximately 2 weeks.1 In the phase-III clinical trials, brexpiprazole was titrated to target dosages, and therefore the product label recommends the same. Brexpiprazole can be administered with or without food.
In a study of brexpiprazole excretion, after a single oral dose of [14C]-labeled brexpiprazole, approximately 25% and 46% of the administered radioactivity was recovered in urine and feces, respectively. Less than 1% of unchanged brexpiprazole was excreted in the urine, and approximately 14% of the oral dose was recovered unchanged in the feces.
Exposure, as measured by maximum concentration and area under the concentration curve, is dose proportional.
Metabolism of brexpiprazole is mediated principally by cytochrome P450 (CYP) 3A4 and CYP2D6. Based on in vitro data, brexpiprazole shows little or no inhibition of CYP450 isozymes.
Efficacy
FDA approval for brexpiprazole for schizophrenia and for adjunctive use in MDD was based on 4 phase-III pivotal acute clinical trials conducted in adults, 2 studies each for each disorder.1-6 These studies are described in Table 2.2-5
Schizophrenia. The primary outcome measure for the acute schizophrenia trials was change on the Positive and Negative Syndrome Scale (PANSS) total scores from baseline to 6-week endpoint. Statistically significant reductions in PANSS total score were observed for brexpiprazole dosages of 2 mg/d and 4 mg/d in one study,2 and 4 mg/d in another study.3 Responder rates also were measured, with response defined as a reduction of ≥30% from baseline in PANSS total score or a Clinical Global Impressions-Improvement score of 1 (very much improved) or 2 (much improved).2,3 Pooling together the available data for the recommended target dosage of brexpiprazole for schizophrenia (2 to 4 mg/d) from the 2 phase-III studies, 45.5% of patients responded to the drug, compared with 31% for the pooled placebo groups, yielding a number needed to treat (NNT) of 7 (95% CI, 5-12).6
Although not described in product labeling, a phase-III 52-week maintenance study demonstrated brexpiprazole’s efficacy in preventing exacerbation of psychotic symptoms and impending relapse in patients with schizophrenia.10 Time from randomization to exacerbation of psychotic symptoms or impending relapse showed a beneficial effect with brexpiprazole compared with placebo (log-rank test: hazard ratio = 0.292, P < .0001). Significantly fewer patients in the brexpiprazole group relapsed compared with placebo (13.5% vs 38.5%, P < .0001), resulting in a NNT of 4 (95% CI, 3-8).
Major depressive disorder. The primary outcome measure for the acute MDD studies was change in Montgomery-Åsberg Depression Rating Scale (MADRS) scores from baseline to 6-week endpoint of the randomized treatment phase. All patients were required to have a history of inadequate response to 1 to 3 treatment trials of standard antidepressants for their current depressive episode. In addition, patients entered the randomized phase only if they had an inadequate response to antidepressant therapy during an 8-week prospective treatment trial of standard antidepressant treatment plus single-blind placebo.
Participants who responded adequately to the antidepressant in the prospective single-blind phase were not randomized, but instead continued on antidepressant treatment plus single-blind placebo for 6 weeks.
The phase-III studies showed positive results for brexpiprazole, 2 mg/d and 3 mg/d, with change in MADRS from baseline to endpoint superior to that observed with placebo.4,5
When examining treatment response, defined as a reduction of ≥50% in MADRS total score from baseline, NNT vs placebo for response were 12 at all dosages tested, however, NNT vs placebo for remission (defined as MADRS total score ≤10 and ≥50% improvement from baseline) ranged from 17 to 31 and were not statistically significant.6 When the results for brexpiprazole, 1 mg/d, 2 mg/d, and 3 mg/d, from the 2 phase-III trials are pooled together, 23.2% of the patients receiving brexpiprazole were responders, vs 14.5% for placebo, yielding a NNT of 12 (95% CI, 8-26); 14.4% of the brexpiprazole-treated patients met remission criteria, vs 9.6% for placebo, resulting in a NNT of 21 (95% CI, 12-138).6
Tolerability
Overall tolerability can be evaluated by examining the percentage of patients who discontinued the clinical trials because of an adverse event (AE). In the acute schizophrenia double-blind trials for the recommended dosage range of 2 to 4 mg, the discontinuation rates were lower overall for patients receiving brexpiprazole compared with placebo.2,3 In the acute MDD trials, 32.6% of brexpiprazole-treated patients and 10.7% of placebo-treated patients discontinued because of AEs,4,5 yielding a number needed to harm (NNH) of 53 (95% CI, 30-235).6
The most commonly encountered AEs for MDD (incidence ≥5% and at least twice the rate for placebo) were akathisia (8.6% vs 1.7% for brexpiprazole vs placebo, and dose-related) and weight gain (6.7% vs 1.9%),1 with NNH values of 15 (95% CI, 11-23), and 22 (95% CI, 15-42), respectively.6 The most commonly encountered AE for schizophrenia (incidence ≥4% and at least twice the rate for placebo) was weight gain (4% vs 2%),1 with a NNH of 50 (95% CI, 26-1773).6
Of note, rates of akathisia in the schizophrenia trials were 5.5% for brexpiprazole and 4.6% for placebo,1 yielding a non-statistically significant NNH of 112.6 In a 6-week exploratory study,11 the incidence of EPS-related AEs including akathisia was lower for brexpiprazole-treated patients (14.1%) compared with those receiving aripiprazole (30.3%), for a NNT advantage for brexpiprazole of 7 (not statistically significant).
Short-term weight gain appears modest; however, outliers with an increase of ≥7% of body weight were evident in open-label long-term safety studies.1,6 Effects on glucose and lipids were small. Minimal effects on prolactin were observed, and no clinically relevant effects on the QT interval were evident.
Contraindications
The only absolute contraindication for brexpiprazole is known hypersensitivity to brexpiprazole or any of its components. Reactions have included rash, facial swelling, urticaria, and anaphylaxis.1
As with all antipsychotics and antipsychotics with an indication for a depressive disorder:
• there is a bolded boxed warning in the product label regarding increased mortality in geriatric patients with dementia-related psychosis. Brexpiprazole is not approved for treating patients with dementia-related psychosis
• there is a bolded boxed warning in the product label about suicidal thoughts and behaviors in patients age ≤24. The safety and efficacy of brexpiprazole have not been established in pediatric patients.
Dosing
Schizophrenia. The recommended starting dosage for brexpiprazole for schizophrenia is 1 mg/d on Days 1 to 4. Brexpiprazole can be titrated to 2 mg/d on Day 5 through Day 7, then to 4 mg/d on Day 8 based on the patient’s response and ability to tolerate the medication. The recommended target dosage is 2 to 4 mg/d with a maximum recommended daily dosage of 4 mg.
Major depressive disorder. The recommended starting dosage for brexpiprazole as adjunctive treatment for MDD is 0.5 mg or 1 mg/d. Brexpiprazole can be titrated to 1 mg/d, then up to the target dosage of 2 mg/d, with dosage increases occurring at weekly intervals based on the patient’s clinical response and ability to tolerate the agent, with a maximum recommended dosage of 3 mg/d.
Other considerations. For patients with moderate to severe hepatic impairment, or moderate, severe, or end-stage renal impairment, the maximum recommended dosage is 3 mg/d for patients with schizophrenia, and 2 mg/d for patients with MDD.
In general, dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in those taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers1:
• for strong CYP2D6 or CYP3A4 inhibitors, administer one-half the usual dosage
• for strong/moderate CYP2D6 with strong/moderate CYP3A4 inhibitors, administer a one-quarter of the usual dosage
• for known CYP2D6 poor metabolizers taking strong/moderate CYP3A4 inhibitors, also administer a one-quarter of the usual dosage
• for strong CYP3A4 inducers, double the usual dosage and further adjust based on clinical response.
In clinical trials for MDD, brexpiprazole dosage was not adjusted for strong CYP2D6 inhibitors (eg, paroxetine, fluoxetine). Therefore, CYP considerations are already factored into general dosing recommendations and brexpiprazole could be administered without dosage adjustment in patients with MDD; however, under these circumstances, it would be prudent to start brexpiprazole at 0.5 mg, which, although “on-label,” represents a low starting dosage. (Whenever 2 drugs are co-administered and 1 agent has the ability to disturb the metabolism of the other, using smaller increments to the target dosage and possibly waiting longer between dosage adjustments could help avoid potential drug–drug interactions.)
No dosage adjustment for brexpiprazole is required on the basis of sex, race or ethnicity, or smoking status. Although clinical studies did not include patients age ≥65, the product label recommends that in general, dose selection for a geriatric patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, and cardiac function, concomitant diseases, and other drug therapy.
Bottom Line
Brexpiprazole, an atypical antipsychotic, is FDA-approved for schizophrenia and as an adjunct to antidepressants in major depressive disorder. For both indications, brexpiprazole demonstrated positive results compared with placebo in phase-III trials. Brexpiprazole is more potent at serotonin 5-HT1A and 5-HT2A receptors and displays less intrinsic activity at D2 receptors than aripiprazole, which could mean that the drug may be better-tolerated.
Related Resources
• Citrome L. Brexpiprazole: a new dopamine D2 receptor partial agonist for the treatment of schizophrenia and major depressive disorder. Drugs Today (Barc). 2015;51(7):397-414.
• Citrome L, Stensbøl TB, Maeda K. The preclinical profile of brexpiprazole: what is its clinical relevance for the treatment of psychiatric disorders? Expert Rev Neurother. In press.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Fluoxetine • Prozac
Paroxetine • Paxil
Disclosure
Dr. Citrome is a consultant to Alexza Pharmaceuticals, Alkermes, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, Forum Pharmaceuticals, Genentech, Janssen, Jazz Pharmaceuticals, Lundbeck, Merck, Medivation, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, and Valeant Pharmaceuticals; and is a speaker for Allergan, AstraZeneca, Janssen, Jazz Pharmaceuticals, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Shire, Sunovion, Takeda, and Teva.
1. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
2. Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870-880.
3. Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1-3):127-135.
4. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study [published online August 4, 2015]. J Clin Psychiatry. doi: 10.4088/ JCP.14m09689.
5. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants [published online August 4, 2015]. J Clin Psychiatry. doi: 10.4088/JCP.14m09688.
6. Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978-997.
7. Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604.
8. Volavka J, Citrome L. Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother. 2009;10(12):1917-1928.
9. Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010;122(4):39-48.
10. Hobart M, Ouyang J, Forbes A, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. Poster presented at: the American Society of Clinical Psychopharmacology Annual Meeting; June 22 to 25, 2015; Miami, FL.
11. Citrome L, Ota A, Nagamizu K, Perry P, et al. The effect of brexpiprazole (OPC‐34712) versus aripiprazole in adult patients with acute schizophrenia: an exploratory study. Poster presented at: the Society of Biological Psychiatry Annual Scientific Meeting and Convention; May 15, 2015; Toronto, Ontario, Canada.
1. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
2. Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870-880.
3. Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1-3):127-135.
4. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study [published online August 4, 2015]. J Clin Psychiatry. doi: 10.4088/ JCP.14m09689.
5. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants [published online August 4, 2015]. J Clin Psychiatry. doi: 10.4088/JCP.14m09688.
6. Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978-997.
7. Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604.
8. Volavka J, Citrome L. Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother. 2009;10(12):1917-1928.
9. Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010;122(4):39-48.
10. Hobart M, Ouyang J, Forbes A, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. Poster presented at: the American Society of Clinical Psychopharmacology Annual Meeting; June 22 to 25, 2015; Miami, FL.
11. Citrome L, Ota A, Nagamizu K, Perry P, et al. The effect of brexpiprazole (OPC‐34712) versus aripiprazole in adult patients with acute schizophrenia: an exploratory study. Poster presented at: the Society of Biological Psychiatry Annual Scientific Meeting and Convention; May 15, 2015; Toronto, Ontario, Canada.
Your significant role in modifying risk factors for coronary artery disease and managing problems subsequently
The problem is enormous: Heart disease is the leading cause of death in the United States, and coronary artery disease (CAD) is the most common form of heart disease—responsible for 385,000 deaths in the United States in 2009 (http://www.cdc.gov/heartdisease/facts. htm). Patients with psychiatric illness have higher rates of morbidity and mortality from CAD than the general population, and warrant consideration as a special population. You should be familiar with routine cardiac medications; your patients’ medical problems; potential cardiac-related interactions among their psychotropic medications; and interactions among illnesses in their mental health and medical health domains (Box).
CASE Type 2 diabetes mellitus plus a long history of heavy smoking
Ms. S, age 57, is an African American woman with chronic paranoid schizophrenia who has been seeing a psychiatrist for the past 10 years. Ms. S’s psychiatric symptoms have been well controlled on risperidone, 3 mg/d.
Ms. S has a family history of diabetes, hypertension, and early CAD (a brother died of a myocardial infarction [MI] in his late 40s). She continues to smoke 2 packs of unfiltered cigarettes daily, as she has done for the past 40 years.
The psychiatrist has been following American Diabetes Association/American Psychiatric Association guidelines for monitoring; he has noticed that Ms. S’s body mass index (BMI) has increased from 27 to 31 kg/m2 over the past year. She has developed type 2 diabetes mellitus (T2DM).
At today’s visit, Ms. S arrives a few minutes late and appears flustered and out of breath. She explains that she had to climb a flight of stairs to get to office because the elevator is broken.
During the visit, the psychiatrist notes that Ms. S occasionally winces and massages her left shoulder.
Questions to ponder
• What else could the psychiatrist do to modify Ms. S’s cardiac risk factors?
• What is Ms. S’s 10-year risk of an acute coronary event?
• What should her physician do now?
Overview: Cardiac risk in patients with mental illness
Modifiable risks for CAD include hypertension, hypercholesterolemia, T2DM, obesity (all of which, taken together, constitute the metabolic syndrome), smoking, and a sedentary lifestyle. Some risk factors, including sex, age, and family history, are not modifiable. Whether or not this modification leads to better outcomes, psychiatric comorbidity is associated with higher morbidity and mortality from CAD.
Whether a common underlying pathological process manifesting in both CAD and mental illness exists, or whether the association is causal, are not well understood. Symptoms characteristic of depression (apathy, amotivation) and schizophrenia (disorganization, paranoia) could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors.1,2
People with mental illness smoke at a higher rate than those who do not have mental illness.3 This finding is of particular relevance because smoking contributes to worse outcomes with respect to CAD, even when medications are prescribed to address metabolic risks.4
Lower socioeconomic status is associated with poorer prognosis from CAD5 and is a risk factor for depression.6 Depression is a strong independent predictor of worse survival in acute coronary syndromes.5 Some experts consider depression to be a stronger risk factor for MI than traditional medical risk factors such as obesity, hypertension, and second-hand smoke.7
Interventions used to treat certain mental illnesses can exacerbate, or predispose to, metabolic syndrome (which, in turn, increases the risk of CAD). Although some studies have demonstrated metabolic derangements in medication-naïve patients who have a new diagnosis of schizophrenia,8 there is a clearly established association between second-generation antipsychotic use and obesity, hypertension, hyperlipidemia, and T2DM. This association prompted development in 2004 of consensus recommendations for cardiovascular monitoring of patients who are taking an atypical antipsychotic.9
Some studies suggest that the stress of mental illness contributes to the pathogen esis of CAD.8 Hypothesized mechanisms include:
• sympathetic activation
• vagal deactivation
• platelet activation
• hypothalamic-pituitary-adrenocortical pathways
• anticholinergic mechanisms
• inflammatory mediators, including cytokines.
Mental stress itself has the capacity to induce coronary ischemia.10 The mental stress of psychiatric illness could have an important pathophysiologic role in CAD. It can be tempting to disregard chest pain in a patient who is known to have panic disorder, but that patient might in fact be experiencing stress-induced myocardial ischemia.11
As many as 30% to 40% of patients with CAD suffer from clinically significant symptoms of depression; as many as 20% of patients with CAD meet criteria for major depressive disorder, compared with 5% to 10% of people who do not have CAD.2 Depression post-MI has been associated with a higher rate of sudden cardiac death and worse outcomes.12
Anxiety also can portend worse outcomes from CAD,13 including higher all-cause mortality.14 There is some hope, but limited evidence, that treating depression and anxiety, whether with antidepressant medication or behavioral therapy, can improve CAD outcomes.10,15
Making a diagnosis of CAD
CAD can present in a variety of ways, ranging from unrecognized or so-called silent CAD (there is an association between T2DM and unrecognized CAD and between hypertension and unrecognized CAD) to stable angina, unstable angina, acute coronary syndrome, MI, and sudden cardiac death. A variety of abnormalities on resting and exercise electrocardiogram (ECG), including ST segment depression, ST elevation, Q waves, and other morphological changes are indicative of CAD.
Other modalities, including coronary calcification score on computed tomography and coronary angiography can confirm the presence of CAD. Some clinicians recommend periodic ECG treadmill testing in patients who have:
• a total cholesterol level is >240 mg/dL
• systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or both
• a family history of MI or sudden cardiac death in young (age <60) first-degree relatives
• a history of smoking
• diabetes.
Preventive guidelines
Risk stratification. A low (<10%), moderate (10% to 20%), or high (>20%) 10-year risk of CAD can be ascertained using a risk calculator, such as one that is available through the Framingham Heart Study (Figure) and the National Heart, Lung, and Blood Institute (http://cvdrisk.nhlbi. nih.gov). Because patients with risk factors for CAD should be offered interventions— including smoking cessation therapy, diet and exercise, aspirin, lipid-lowering therapy, and blood pressure modification strategies—whether or not they have evidence of CAD, the United States Preventive Services Task Force does not recommend for or against diagnostic screening in patients at moderate or elevated risk of CAD.16
There are guidelines in the literature recommending specific screening strategies for patients with mental illness, although the vetting and update process has been ill defined. Among patients with schizophrenia, though, regardless of antipsychotic prescription status, baseline and then regular monitoring of metabolic risk parameters is recommended.17
Primary prevention. Lifestyle modification and attention to modifiable coronary risk factors are important primary prevention strategies. Dietary modifications, exercise, not smoking, and maintenance of a normal BMI (<25 kg/m2) are associated with a lower risk of CAD.18,19
Lifestyle modifications can be challenging for patients with persistent mental illness, however: For example, patients with schizophrenia smoke more, eat less healthfully, and participate less in behavioral modification that targets risk factors than patients who do not have schizophrenia.20,21
According to 2012 evidence-based practice guidelines established by a collaboration that included the American College of Physicians and several cardiology and thoracic medicine societies, persons age >50 who do not have symptomatic CAD should take low-dose (75 to 100 mg/d) aspirin; the benefit of low-dose aspirin in persons at moderate or high risk of CAD is even greater. Other medications, including statins and fixed-dose combinations of antihypertensive medications in combination with a statin are not clearly beneficial as primary prevention strategies across the board, although selected high-risk populations might benefit.
Regrettably, the high-risk population of persons with mental illness and whose primary care is suboptimal has not been studied. It stands to reason that these patients would especially benefit from more attentive monitoring and intervention.
Collaborative care? Although many psychiatrists do not practice in such a model, a comprehensive approach to the care of their patients, using a collaborative care strategy that includes attention to the mental health diagnosis along with medical health, can result in improved health in both domains.22 However, enlisting patients with paranoia or an inherent distrust of medications and health care providers to adhere to either a medication regimen or lifestyle modification can be challenging.
Common-sense strategies, such as creating a multidisciplinary team with the psychiatrist coordinating care and optimizing antipsychotic treatment, might provide benefit.1 Data demonstrate that patients with severe mental illness who experience acute coronary events undergo revascularization at a lower rate than their mentally heathy counterparts, despite the fact that patients with severe mental illness die at a higher rate from their CAD than patients who do not have mental illness. An important role for the psychiatrist, even in the absence of a collaborative care program, is to be an advocate for appropriate guideline-based care.23
Secondary prevention. Once a patient develops CAD, ongoing risk factor modification is important. Adherence to a therapeutic regimen that variously combines a platelet inhibitor, beta blocker, statin, and angiotensin-converting enzyme (ACE) inhibitor is associated with improved outcomes in patients with CAD.24 Specific antiplatelet recommendations and a recommendation for single vs combination antiplatelet therapy depends on chronicity and type of revascularization in a setting of CAD.25
Summary of guideline-based recommendations
Treatment guidelines published in the National Guidelines Clearinghouse address depression, CAD screening, and specific cardiac therapies, including ACE inhibitors, angiotensin-receptor blockers, oral anticoagulants, platelet inhibitors, beta blockers, and lifestyle modification.
Primary prevention. Recommendations for treatment to prevent CAD are listed in Table 1.
Secondary prevention. Recommendations for treatment after a diagnosis of CAD are listed in Table 2.
Special considerations for psychiatric providers
You should be comfortable with patients’ use of antihypertensive therapies and familiar with the potential these agents have to interact with psychotropics; in addition, you can take a more active role in prescribing, and monitoring patients’ responses to, these medications. Provide appropriate monitoring of ACE inhibitors, statins, and beta blockers; also, provide appropriate monitoring of psychotropics in patients who take recommended cardioprotective medications.
In situations that prompt referral (such as recent MI, new symptoms of heart failure, any history of syncope or new identification of T2DM), ideally you should collaborate with the patient’s primary care provider to help enhance adherence to recommended treatment strategies. You also should employ motivational interviewing techniques and offer strategies by which patients can engage in meaningful lifestyle modification.
There are official recommendations for depression screening strategies26 and psychosocial risk screening for patients in whom CAD has been identified.27 Official screening strategies for CAD in patients with psychiatric illness have not, however, been spelled out.
Primary CAD prevention with medication is not routinely recommended for the general population, but the increased risk of CAD associated with psychiatric diagnoses (particularly schizophrenia, as well as the medications used to treat it) might warrant consideration of aggressive primary prevention strategies.28 For example, some experts recommend starting metformin to reduce the risk of T2DM in patients who have been started on olanzapine or clozapine, regardless of the baseline fasting blood glucose level.29
You should be fully informed and aware of patients’ underlying medical conditions and the medications that are recommended to treat their conditions. Ideally, an integrated care strategy or, at the least, clear communication between you and the patient’s primary care providers should be in place to avoid foreseeable problems.
Stimulants. Systematic reviews suggest an association between prescription stimulants and at least the 2 cardiovascular risk factors of elevated heart rate and blood pressure. Stimulants are not recommended, therefore, for routine use in patients who have known hypertension or CAD.30
Second-generation antipsychotics are associated with significant weight gain and development of metabolic syndrome.
Selective serotonin reuptake inhibitors are associated with an increased risk of gastrointestinal bleeding risk related to platelet inhibition and gastric effects. Risk increases with additional platelet inhibitors, such as aspirin or clopidogrel.31
Lithium is excreted solely by the kidney. Guidelines recommend ACE inhibitors and angiotensin receptor-blockers for patients with CAD or T2DM, and many patients with symptomatic congestive heart failure are prescribed a diuretic; all of these classes of medications impair excretion of lithium. In a nested case-control study, 3% of observed cases of lithium toxicity were attributable to a newly initiated ACE inhibitor or angiotensin receptor-blocker.32 It is essential that you, and your patients taking lithium, be aware of the need to monitor the drug level frequently and be vigilant for symptoms of mild toxicity.
Beta blockers. No prospectively collected data support a association between beta blockers and depression.33 Patients with CAD should be given a trial of a beta blocker to achieve optimal medical management; because they are at increased risk of depression in the first place, all patients with CAD should undergo monitoring for depressive symptoms.
Clopidogrel is activated through the cytochrome P450 2C19 isoenzyme; medications such as fluoxetine and fluvoxamine that inhibit the function of CYP2C19 can impair the effectiveness of clopidogrel.31
Other considerations. Patients taking a second-generation antipsychotic should have baseline and periodic (monthly for the first quarter, then quarterly) assessments of BMI and, after monitoring at 3 months after baseline, annual monitoring of blood pressure, the fasting glucose level, and abdominal waist circumference. Lipid levels should be monitored every 5 years9 (Table 3).
Baseline and periodic monitoring of hepatic enzymes is recommended for patients taking a statin. You, and the patient, should be alert to the possible development of muscle weakness or pain; establish a low threshold for screening for an elevated creatine kinase level, which signals rhabdomyolysis.
Case concluded
Ms. S’s psychiatrist measures her blood pressure and finds that it is 147/92 mm Hg. He uses the Pooled Cohort Equations to determine that her lifetime risk of cardiovascular event is 50% (compared with a 8% lifetime risk among a cohort in whom risk factors are optimized) and that her 10-year risk is 41% (compared with a 2.2% risk among optimized controls).
At this point, the psychiatrist starts metformin to prevent T2DM. He also starts Ms. S on a statin to prevent CAD in a setting of diagnosed T2DM.
Ms. S’s exertional dyspnea and shoulder discomfort could be associated with angina, and the physician wisely refers her for urgent evaluation. Because he is aware of the literature demonstrating decreased revascularization among patients with mental illness, he urges her other health care providers to provide her with guideline-based strategies to treat her cardiovascular disease.
Bottom Line
Patients with psychiatric illness have higher rates of morbidity and mortality from coronary artery disease (CAD) than the general population. Symptoms characteristic of depression and schizophrenia could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors. Institute strategies for primary and secondary prevention of CAD among your patients, based on published guidelines, and be aware of, and alert for, adverse cardiac effects and an increase in risk factors for CAD from the use of psychotropics.
Related Resources
• Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):511-523.
• Interactive cardiovascular risk calculator developed from the Framingham Heart Study. https://www.framingham heartstudy.org/risk-functions/cardiovascular-disease/ 10-year-risk.php.
• Pooled Cohort Equations calculator. To determine estimated cardiovascular risk in comparison with peers with optimized risk factors. http://clincalc.com/cardiology/ascvd/ pooledcohort.aspx.
• To learn more about traditional cardiovascular risk factors from the Framingham Heart Study. http://www.framinghamheart study.org/risk-functions/.
Drug Brand Names
Amlodipine • Norvasc
Clozapine • Clozaril
Clopidogrel • Plavix
Felodipine • Plendil
Fluoxetine • Prozac
Fluvoxamine • Luvox
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Risperidone • Risperdal
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Heald A, Montejo AL, Millar H, et al. Management of physical health in patients with schizophrenia: practical recommendations. Eur Psychiatry. 2010;25(suppl 2):S41-S45.
2. Huffman JC, Celano CM, Beach SR, et al. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925.
3. Lawrence D, Mitrou F, Zubrick ZR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Athyros VG, Tziomalos K, Katsiki N, et al; GREACE Study Collaborative Group. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: a post hoc analysis of the GREACE study. Curr Vasc Pharmacol. 2013;11(5):779-784.
5. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 AACF/ AHA/ACP/AATS/PCNA/SCAI/STS Guidelines for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164.
6. Gilman SE, Kawachi I, Fitzmaurice GM, et al. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359-367.
7. Pozuelo L, Tesar G, Zhang J, et al. Depression and heart disease: what do we know, and where are we headed? Cleve Clin J Med. 2009;76(1):59-70.
8. Osborn DP, Wright CA, Levy G, et al. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry. 2008;8:84.
9. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
10. Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139-2049.
11. Soares-Filho GL, Mesquita CT, Mesquita ET, et al. Panic attack triggering myocardial ischemia documented by myocardial perfusion imaging study. A case report. Int Arch Med. 2012;5(1):24.
12. Khawaja IS, Westermeyer JJ, Gajwani P, et al. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6(1):38-51.
13. Wang G, Cui J, Wang Y, et al. Anxiety and adverse coronary artery disease outcomes in Chinese patients. Psychosom Med. 2013;75(6):530-536.
14. Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2):e000068. doi: 10.1161/JAHA.112.000068.
15. Chiavarino C, Rabellino D, Ardito RB, et al. Emotional coping is a better predictor of cardiac prognosis than depression and anxiety. J Psychosom Res. 2012;73(6):473-475.
16. Moyer VA; U.S. Preventive Services Task Force. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(7):512-518.
17. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2012;199(2):99-105.
18. Hartley L, Igbinedion E, Holmes J, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6:CD009874. doi: 10.1002/14651858.CD009874.pub2.
19. Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62-69.
20. Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. J Clin Psychiatry. 2002;63(suppl 9):5-11.
21. Dipasquale S, Pariante CM, Dazzan P, et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47(2):197-207.
22. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illness. N Engl J Med. 2010;363(27):2611-2620.
23. Manderbacka K, Arffman M, Sund R, et al. How does a history of psychiatric hospital care influence access to coronary care: a cohort study. BMJ Open. 2012;2(2):e000831. doi: 10.1136/bmjopen-2012-000831.
24. Kumbhani DJ, Steg PG, Cannon CP, et al; REduction of Atherothrombosis for Continued Health Registry Investigators. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126(8):693-700.
25. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e637S-e668S.
26. Lichtman JH, Bigger T, Blumenthal JA, et al; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768-1775.
27. Albus C, Jordan J, Herrmann-Lingen C. Screening for psychosocial risk factors in patients with coronary heart disease-recommendations for clinical practice. Eur J Cardiovasc Prev Rehabil. 2004;11(1):75-79.
28. Srihari VH, Phutane VH, Ozkan B, et al. Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophr Res. 2013;146(1-3):64-68.
29. Brooks JO 3rd, Chang HS, Krasnykh O. Metabolic risks in older adults receiving second-generation antipsychotic medication. Curr Psychiatry Rep. 2009;11(1):33-40.
30. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
31. Andrade C. Drug interactions in the treatment of depression in patients with ischemic heart disease. J Clin Psychiatry. 2012;73(12):e1475-e1477.
32. Juurlink DN, Mamdani MM, Kopp A, et al. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52(5):794-798.
33. Muzyk AJ, Gagliardi JP. Do beta blockers cause depression? Current Psychiatry. 2010;9(5):50,51,55.
The problem is enormous: Heart disease is the leading cause of death in the United States, and coronary artery disease (CAD) is the most common form of heart disease—responsible for 385,000 deaths in the United States in 2009 (http://www.cdc.gov/heartdisease/facts. htm). Patients with psychiatric illness have higher rates of morbidity and mortality from CAD than the general population, and warrant consideration as a special population. You should be familiar with routine cardiac medications; your patients’ medical problems; potential cardiac-related interactions among their psychotropic medications; and interactions among illnesses in their mental health and medical health domains (Box).
CASE Type 2 diabetes mellitus plus a long history of heavy smoking
Ms. S, age 57, is an African American woman with chronic paranoid schizophrenia who has been seeing a psychiatrist for the past 10 years. Ms. S’s psychiatric symptoms have been well controlled on risperidone, 3 mg/d.
Ms. S has a family history of diabetes, hypertension, and early CAD (a brother died of a myocardial infarction [MI] in his late 40s). She continues to smoke 2 packs of unfiltered cigarettes daily, as she has done for the past 40 years.
The psychiatrist has been following American Diabetes Association/American Psychiatric Association guidelines for monitoring; he has noticed that Ms. S’s body mass index (BMI) has increased from 27 to 31 kg/m2 over the past year. She has developed type 2 diabetes mellitus (T2DM).
At today’s visit, Ms. S arrives a few minutes late and appears flustered and out of breath. She explains that she had to climb a flight of stairs to get to office because the elevator is broken.
During the visit, the psychiatrist notes that Ms. S occasionally winces and massages her left shoulder.
Questions to ponder
• What else could the psychiatrist do to modify Ms. S’s cardiac risk factors?
• What is Ms. S’s 10-year risk of an acute coronary event?
• What should her physician do now?
Overview: Cardiac risk in patients with mental illness
Modifiable risks for CAD include hypertension, hypercholesterolemia, T2DM, obesity (all of which, taken together, constitute the metabolic syndrome), smoking, and a sedentary lifestyle. Some risk factors, including sex, age, and family history, are not modifiable. Whether or not this modification leads to better outcomes, psychiatric comorbidity is associated with higher morbidity and mortality from CAD.
Whether a common underlying pathological process manifesting in both CAD and mental illness exists, or whether the association is causal, are not well understood. Symptoms characteristic of depression (apathy, amotivation) and schizophrenia (disorganization, paranoia) could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors.1,2
People with mental illness smoke at a higher rate than those who do not have mental illness.3 This finding is of particular relevance because smoking contributes to worse outcomes with respect to CAD, even when medications are prescribed to address metabolic risks.4
Lower socioeconomic status is associated with poorer prognosis from CAD5 and is a risk factor for depression.6 Depression is a strong independent predictor of worse survival in acute coronary syndromes.5 Some experts consider depression to be a stronger risk factor for MI than traditional medical risk factors such as obesity, hypertension, and second-hand smoke.7
Interventions used to treat certain mental illnesses can exacerbate, or predispose to, metabolic syndrome (which, in turn, increases the risk of CAD). Although some studies have demonstrated metabolic derangements in medication-naïve patients who have a new diagnosis of schizophrenia,8 there is a clearly established association between second-generation antipsychotic use and obesity, hypertension, hyperlipidemia, and T2DM. This association prompted development in 2004 of consensus recommendations for cardiovascular monitoring of patients who are taking an atypical antipsychotic.9
Some studies suggest that the stress of mental illness contributes to the pathogen esis of CAD.8 Hypothesized mechanisms include:
• sympathetic activation
• vagal deactivation
• platelet activation
• hypothalamic-pituitary-adrenocortical pathways
• anticholinergic mechanisms
• inflammatory mediators, including cytokines.
Mental stress itself has the capacity to induce coronary ischemia.10 The mental stress of psychiatric illness could have an important pathophysiologic role in CAD. It can be tempting to disregard chest pain in a patient who is known to have panic disorder, but that patient might in fact be experiencing stress-induced myocardial ischemia.11
As many as 30% to 40% of patients with CAD suffer from clinically significant symptoms of depression; as many as 20% of patients with CAD meet criteria for major depressive disorder, compared with 5% to 10% of people who do not have CAD.2 Depression post-MI has been associated with a higher rate of sudden cardiac death and worse outcomes.12
Anxiety also can portend worse outcomes from CAD,13 including higher all-cause mortality.14 There is some hope, but limited evidence, that treating depression and anxiety, whether with antidepressant medication or behavioral therapy, can improve CAD outcomes.10,15
Making a diagnosis of CAD
CAD can present in a variety of ways, ranging from unrecognized or so-called silent CAD (there is an association between T2DM and unrecognized CAD and between hypertension and unrecognized CAD) to stable angina, unstable angina, acute coronary syndrome, MI, and sudden cardiac death. A variety of abnormalities on resting and exercise electrocardiogram (ECG), including ST segment depression, ST elevation, Q waves, and other morphological changes are indicative of CAD.
Other modalities, including coronary calcification score on computed tomography and coronary angiography can confirm the presence of CAD. Some clinicians recommend periodic ECG treadmill testing in patients who have:
• a total cholesterol level is >240 mg/dL
• systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or both
• a family history of MI or sudden cardiac death in young (age <60) first-degree relatives
• a history of smoking
• diabetes.
Preventive guidelines
Risk stratification. A low (<10%), moderate (10% to 20%), or high (>20%) 10-year risk of CAD can be ascertained using a risk calculator, such as one that is available through the Framingham Heart Study (Figure) and the National Heart, Lung, and Blood Institute (http://cvdrisk.nhlbi. nih.gov). Because patients with risk factors for CAD should be offered interventions— including smoking cessation therapy, diet and exercise, aspirin, lipid-lowering therapy, and blood pressure modification strategies—whether or not they have evidence of CAD, the United States Preventive Services Task Force does not recommend for or against diagnostic screening in patients at moderate or elevated risk of CAD.16
There are guidelines in the literature recommending specific screening strategies for patients with mental illness, although the vetting and update process has been ill defined. Among patients with schizophrenia, though, regardless of antipsychotic prescription status, baseline and then regular monitoring of metabolic risk parameters is recommended.17
Primary prevention. Lifestyle modification and attention to modifiable coronary risk factors are important primary prevention strategies. Dietary modifications, exercise, not smoking, and maintenance of a normal BMI (<25 kg/m2) are associated with a lower risk of CAD.18,19
Lifestyle modifications can be challenging for patients with persistent mental illness, however: For example, patients with schizophrenia smoke more, eat less healthfully, and participate less in behavioral modification that targets risk factors than patients who do not have schizophrenia.20,21
According to 2012 evidence-based practice guidelines established by a collaboration that included the American College of Physicians and several cardiology and thoracic medicine societies, persons age >50 who do not have symptomatic CAD should take low-dose (75 to 100 mg/d) aspirin; the benefit of low-dose aspirin in persons at moderate or high risk of CAD is even greater. Other medications, including statins and fixed-dose combinations of antihypertensive medications in combination with a statin are not clearly beneficial as primary prevention strategies across the board, although selected high-risk populations might benefit.
Regrettably, the high-risk population of persons with mental illness and whose primary care is suboptimal has not been studied. It stands to reason that these patients would especially benefit from more attentive monitoring and intervention.
Collaborative care? Although many psychiatrists do not practice in such a model, a comprehensive approach to the care of their patients, using a collaborative care strategy that includes attention to the mental health diagnosis along with medical health, can result in improved health in both domains.22 However, enlisting patients with paranoia or an inherent distrust of medications and health care providers to adhere to either a medication regimen or lifestyle modification can be challenging.
Common-sense strategies, such as creating a multidisciplinary team with the psychiatrist coordinating care and optimizing antipsychotic treatment, might provide benefit.1 Data demonstrate that patients with severe mental illness who experience acute coronary events undergo revascularization at a lower rate than their mentally heathy counterparts, despite the fact that patients with severe mental illness die at a higher rate from their CAD than patients who do not have mental illness. An important role for the psychiatrist, even in the absence of a collaborative care program, is to be an advocate for appropriate guideline-based care.23
Secondary prevention. Once a patient develops CAD, ongoing risk factor modification is important. Adherence to a therapeutic regimen that variously combines a platelet inhibitor, beta blocker, statin, and angiotensin-converting enzyme (ACE) inhibitor is associated with improved outcomes in patients with CAD.24 Specific antiplatelet recommendations and a recommendation for single vs combination antiplatelet therapy depends on chronicity and type of revascularization in a setting of CAD.25
Summary of guideline-based recommendations
Treatment guidelines published in the National Guidelines Clearinghouse address depression, CAD screening, and specific cardiac therapies, including ACE inhibitors, angiotensin-receptor blockers, oral anticoagulants, platelet inhibitors, beta blockers, and lifestyle modification.
Primary prevention. Recommendations for treatment to prevent CAD are listed in Table 1.
Secondary prevention. Recommendations for treatment after a diagnosis of CAD are listed in Table 2.
Special considerations for psychiatric providers
You should be comfortable with patients’ use of antihypertensive therapies and familiar with the potential these agents have to interact with psychotropics; in addition, you can take a more active role in prescribing, and monitoring patients’ responses to, these medications. Provide appropriate monitoring of ACE inhibitors, statins, and beta blockers; also, provide appropriate monitoring of psychotropics in patients who take recommended cardioprotective medications.
In situations that prompt referral (such as recent MI, new symptoms of heart failure, any history of syncope or new identification of T2DM), ideally you should collaborate with the patient’s primary care provider to help enhance adherence to recommended treatment strategies. You also should employ motivational interviewing techniques and offer strategies by which patients can engage in meaningful lifestyle modification.
There are official recommendations for depression screening strategies26 and psychosocial risk screening for patients in whom CAD has been identified.27 Official screening strategies for CAD in patients with psychiatric illness have not, however, been spelled out.
Primary CAD prevention with medication is not routinely recommended for the general population, but the increased risk of CAD associated with psychiatric diagnoses (particularly schizophrenia, as well as the medications used to treat it) might warrant consideration of aggressive primary prevention strategies.28 For example, some experts recommend starting metformin to reduce the risk of T2DM in patients who have been started on olanzapine or clozapine, regardless of the baseline fasting blood glucose level.29
You should be fully informed and aware of patients’ underlying medical conditions and the medications that are recommended to treat their conditions. Ideally, an integrated care strategy or, at the least, clear communication between you and the patient’s primary care providers should be in place to avoid foreseeable problems.
Stimulants. Systematic reviews suggest an association between prescription stimulants and at least the 2 cardiovascular risk factors of elevated heart rate and blood pressure. Stimulants are not recommended, therefore, for routine use in patients who have known hypertension or CAD.30
Second-generation antipsychotics are associated with significant weight gain and development of metabolic syndrome.
Selective serotonin reuptake inhibitors are associated with an increased risk of gastrointestinal bleeding risk related to platelet inhibition and gastric effects. Risk increases with additional platelet inhibitors, such as aspirin or clopidogrel.31
Lithium is excreted solely by the kidney. Guidelines recommend ACE inhibitors and angiotensin receptor-blockers for patients with CAD or T2DM, and many patients with symptomatic congestive heart failure are prescribed a diuretic; all of these classes of medications impair excretion of lithium. In a nested case-control study, 3% of observed cases of lithium toxicity were attributable to a newly initiated ACE inhibitor or angiotensin receptor-blocker.32 It is essential that you, and your patients taking lithium, be aware of the need to monitor the drug level frequently and be vigilant for symptoms of mild toxicity.
Beta blockers. No prospectively collected data support a association between beta blockers and depression.33 Patients with CAD should be given a trial of a beta blocker to achieve optimal medical management; because they are at increased risk of depression in the first place, all patients with CAD should undergo monitoring for depressive symptoms.
Clopidogrel is activated through the cytochrome P450 2C19 isoenzyme; medications such as fluoxetine and fluvoxamine that inhibit the function of CYP2C19 can impair the effectiveness of clopidogrel.31
Other considerations. Patients taking a second-generation antipsychotic should have baseline and periodic (monthly for the first quarter, then quarterly) assessments of BMI and, after monitoring at 3 months after baseline, annual monitoring of blood pressure, the fasting glucose level, and abdominal waist circumference. Lipid levels should be monitored every 5 years9 (Table 3).
Baseline and periodic monitoring of hepatic enzymes is recommended for patients taking a statin. You, and the patient, should be alert to the possible development of muscle weakness or pain; establish a low threshold for screening for an elevated creatine kinase level, which signals rhabdomyolysis.
Case concluded
Ms. S’s psychiatrist measures her blood pressure and finds that it is 147/92 mm Hg. He uses the Pooled Cohort Equations to determine that her lifetime risk of cardiovascular event is 50% (compared with a 8% lifetime risk among a cohort in whom risk factors are optimized) and that her 10-year risk is 41% (compared with a 2.2% risk among optimized controls).
At this point, the psychiatrist starts metformin to prevent T2DM. He also starts Ms. S on a statin to prevent CAD in a setting of diagnosed T2DM.
Ms. S’s exertional dyspnea and shoulder discomfort could be associated with angina, and the physician wisely refers her for urgent evaluation. Because he is aware of the literature demonstrating decreased revascularization among patients with mental illness, he urges her other health care providers to provide her with guideline-based strategies to treat her cardiovascular disease.
Bottom Line
Patients with psychiatric illness have higher rates of morbidity and mortality from coronary artery disease (CAD) than the general population. Symptoms characteristic of depression and schizophrenia could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors. Institute strategies for primary and secondary prevention of CAD among your patients, based on published guidelines, and be aware of, and alert for, adverse cardiac effects and an increase in risk factors for CAD from the use of psychotropics.
Related Resources
• Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):511-523.
• Interactive cardiovascular risk calculator developed from the Framingham Heart Study. https://www.framingham heartstudy.org/risk-functions/cardiovascular-disease/ 10-year-risk.php.
• Pooled Cohort Equations calculator. To determine estimated cardiovascular risk in comparison with peers with optimized risk factors. http://clincalc.com/cardiology/ascvd/ pooledcohort.aspx.
• To learn more about traditional cardiovascular risk factors from the Framingham Heart Study. http://www.framinghamheart study.org/risk-functions/.
Drug Brand Names
Amlodipine • Norvasc
Clozapine • Clozaril
Clopidogrel • Plavix
Felodipine • Plendil
Fluoxetine • Prozac
Fluvoxamine • Luvox
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Risperidone • Risperdal
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The problem is enormous: Heart disease is the leading cause of death in the United States, and coronary artery disease (CAD) is the most common form of heart disease—responsible for 385,000 deaths in the United States in 2009 (http://www.cdc.gov/heartdisease/facts. htm). Patients with psychiatric illness have higher rates of morbidity and mortality from CAD than the general population, and warrant consideration as a special population. You should be familiar with routine cardiac medications; your patients’ medical problems; potential cardiac-related interactions among their psychotropic medications; and interactions among illnesses in their mental health and medical health domains (Box).
CASE Type 2 diabetes mellitus plus a long history of heavy smoking
Ms. S, age 57, is an African American woman with chronic paranoid schizophrenia who has been seeing a psychiatrist for the past 10 years. Ms. S’s psychiatric symptoms have been well controlled on risperidone, 3 mg/d.
Ms. S has a family history of diabetes, hypertension, and early CAD (a brother died of a myocardial infarction [MI] in his late 40s). She continues to smoke 2 packs of unfiltered cigarettes daily, as she has done for the past 40 years.
The psychiatrist has been following American Diabetes Association/American Psychiatric Association guidelines for monitoring; he has noticed that Ms. S’s body mass index (BMI) has increased from 27 to 31 kg/m2 over the past year. She has developed type 2 diabetes mellitus (T2DM).
At today’s visit, Ms. S arrives a few minutes late and appears flustered and out of breath. She explains that she had to climb a flight of stairs to get to office because the elevator is broken.
During the visit, the psychiatrist notes that Ms. S occasionally winces and massages her left shoulder.
Questions to ponder
• What else could the psychiatrist do to modify Ms. S’s cardiac risk factors?
• What is Ms. S’s 10-year risk of an acute coronary event?
• What should her physician do now?
Overview: Cardiac risk in patients with mental illness
Modifiable risks for CAD include hypertension, hypercholesterolemia, T2DM, obesity (all of which, taken together, constitute the metabolic syndrome), smoking, and a sedentary lifestyle. Some risk factors, including sex, age, and family history, are not modifiable. Whether or not this modification leads to better outcomes, psychiatric comorbidity is associated with higher morbidity and mortality from CAD.
Whether a common underlying pathological process manifesting in both CAD and mental illness exists, or whether the association is causal, are not well understood. Symptoms characteristic of depression (apathy, amotivation) and schizophrenia (disorganization, paranoia) could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors.1,2
People with mental illness smoke at a higher rate than those who do not have mental illness.3 This finding is of particular relevance because smoking contributes to worse outcomes with respect to CAD, even when medications are prescribed to address metabolic risks.4
Lower socioeconomic status is associated with poorer prognosis from CAD5 and is a risk factor for depression.6 Depression is a strong independent predictor of worse survival in acute coronary syndromes.5 Some experts consider depression to be a stronger risk factor for MI than traditional medical risk factors such as obesity, hypertension, and second-hand smoke.7
Interventions used to treat certain mental illnesses can exacerbate, or predispose to, metabolic syndrome (which, in turn, increases the risk of CAD). Although some studies have demonstrated metabolic derangements in medication-naïve patients who have a new diagnosis of schizophrenia,8 there is a clearly established association between second-generation antipsychotic use and obesity, hypertension, hyperlipidemia, and T2DM. This association prompted development in 2004 of consensus recommendations for cardiovascular monitoring of patients who are taking an atypical antipsychotic.9
Some studies suggest that the stress of mental illness contributes to the pathogen esis of CAD.8 Hypothesized mechanisms include:
• sympathetic activation
• vagal deactivation
• platelet activation
• hypothalamic-pituitary-adrenocortical pathways
• anticholinergic mechanisms
• inflammatory mediators, including cytokines.
Mental stress itself has the capacity to induce coronary ischemia.10 The mental stress of psychiatric illness could have an important pathophysiologic role in CAD. It can be tempting to disregard chest pain in a patient who is known to have panic disorder, but that patient might in fact be experiencing stress-induced myocardial ischemia.11
As many as 30% to 40% of patients with CAD suffer from clinically significant symptoms of depression; as many as 20% of patients with CAD meet criteria for major depressive disorder, compared with 5% to 10% of people who do not have CAD.2 Depression post-MI has been associated with a higher rate of sudden cardiac death and worse outcomes.12
Anxiety also can portend worse outcomes from CAD,13 including higher all-cause mortality.14 There is some hope, but limited evidence, that treating depression and anxiety, whether with antidepressant medication or behavioral therapy, can improve CAD outcomes.10,15
Making a diagnosis of CAD
CAD can present in a variety of ways, ranging from unrecognized or so-called silent CAD (there is an association between T2DM and unrecognized CAD and between hypertension and unrecognized CAD) to stable angina, unstable angina, acute coronary syndrome, MI, and sudden cardiac death. A variety of abnormalities on resting and exercise electrocardiogram (ECG), including ST segment depression, ST elevation, Q waves, and other morphological changes are indicative of CAD.
Other modalities, including coronary calcification score on computed tomography and coronary angiography can confirm the presence of CAD. Some clinicians recommend periodic ECG treadmill testing in patients who have:
• a total cholesterol level is >240 mg/dL
• systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or both
• a family history of MI or sudden cardiac death in young (age <60) first-degree relatives
• a history of smoking
• diabetes.
Preventive guidelines
Risk stratification. A low (<10%), moderate (10% to 20%), or high (>20%) 10-year risk of CAD can be ascertained using a risk calculator, such as one that is available through the Framingham Heart Study (Figure) and the National Heart, Lung, and Blood Institute (http://cvdrisk.nhlbi. nih.gov). Because patients with risk factors for CAD should be offered interventions— including smoking cessation therapy, diet and exercise, aspirin, lipid-lowering therapy, and blood pressure modification strategies—whether or not they have evidence of CAD, the United States Preventive Services Task Force does not recommend for or against diagnostic screening in patients at moderate or elevated risk of CAD.16
There are guidelines in the literature recommending specific screening strategies for patients with mental illness, although the vetting and update process has been ill defined. Among patients with schizophrenia, though, regardless of antipsychotic prescription status, baseline and then regular monitoring of metabolic risk parameters is recommended.17
Primary prevention. Lifestyle modification and attention to modifiable coronary risk factors are important primary prevention strategies. Dietary modifications, exercise, not smoking, and maintenance of a normal BMI (<25 kg/m2) are associated with a lower risk of CAD.18,19
Lifestyle modifications can be challenging for patients with persistent mental illness, however: For example, patients with schizophrenia smoke more, eat less healthfully, and participate less in behavioral modification that targets risk factors than patients who do not have schizophrenia.20,21
According to 2012 evidence-based practice guidelines established by a collaboration that included the American College of Physicians and several cardiology and thoracic medicine societies, persons age >50 who do not have symptomatic CAD should take low-dose (75 to 100 mg/d) aspirin; the benefit of low-dose aspirin in persons at moderate or high risk of CAD is even greater. Other medications, including statins and fixed-dose combinations of antihypertensive medications in combination with a statin are not clearly beneficial as primary prevention strategies across the board, although selected high-risk populations might benefit.
Regrettably, the high-risk population of persons with mental illness and whose primary care is suboptimal has not been studied. It stands to reason that these patients would especially benefit from more attentive monitoring and intervention.
Collaborative care? Although many psychiatrists do not practice in such a model, a comprehensive approach to the care of their patients, using a collaborative care strategy that includes attention to the mental health diagnosis along with medical health, can result in improved health in both domains.22 However, enlisting patients with paranoia or an inherent distrust of medications and health care providers to adhere to either a medication regimen or lifestyle modification can be challenging.
Common-sense strategies, such as creating a multidisciplinary team with the psychiatrist coordinating care and optimizing antipsychotic treatment, might provide benefit.1 Data demonstrate that patients with severe mental illness who experience acute coronary events undergo revascularization at a lower rate than their mentally heathy counterparts, despite the fact that patients with severe mental illness die at a higher rate from their CAD than patients who do not have mental illness. An important role for the psychiatrist, even in the absence of a collaborative care program, is to be an advocate for appropriate guideline-based care.23
Secondary prevention. Once a patient develops CAD, ongoing risk factor modification is important. Adherence to a therapeutic regimen that variously combines a platelet inhibitor, beta blocker, statin, and angiotensin-converting enzyme (ACE) inhibitor is associated with improved outcomes in patients with CAD.24 Specific antiplatelet recommendations and a recommendation for single vs combination antiplatelet therapy depends on chronicity and type of revascularization in a setting of CAD.25
Summary of guideline-based recommendations
Treatment guidelines published in the National Guidelines Clearinghouse address depression, CAD screening, and specific cardiac therapies, including ACE inhibitors, angiotensin-receptor blockers, oral anticoagulants, platelet inhibitors, beta blockers, and lifestyle modification.
Primary prevention. Recommendations for treatment to prevent CAD are listed in Table 1.
Secondary prevention. Recommendations for treatment after a diagnosis of CAD are listed in Table 2.
Special considerations for psychiatric providers
You should be comfortable with patients’ use of antihypertensive therapies and familiar with the potential these agents have to interact with psychotropics; in addition, you can take a more active role in prescribing, and monitoring patients’ responses to, these medications. Provide appropriate monitoring of ACE inhibitors, statins, and beta blockers; also, provide appropriate monitoring of psychotropics in patients who take recommended cardioprotective medications.
In situations that prompt referral (such as recent MI, new symptoms of heart failure, any history of syncope or new identification of T2DM), ideally you should collaborate with the patient’s primary care provider to help enhance adherence to recommended treatment strategies. You also should employ motivational interviewing techniques and offer strategies by which patients can engage in meaningful lifestyle modification.
There are official recommendations for depression screening strategies26 and psychosocial risk screening for patients in whom CAD has been identified.27 Official screening strategies for CAD in patients with psychiatric illness have not, however, been spelled out.
Primary CAD prevention with medication is not routinely recommended for the general population, but the increased risk of CAD associated with psychiatric diagnoses (particularly schizophrenia, as well as the medications used to treat it) might warrant consideration of aggressive primary prevention strategies.28 For example, some experts recommend starting metformin to reduce the risk of T2DM in patients who have been started on olanzapine or clozapine, regardless of the baseline fasting blood glucose level.29
You should be fully informed and aware of patients’ underlying medical conditions and the medications that are recommended to treat their conditions. Ideally, an integrated care strategy or, at the least, clear communication between you and the patient’s primary care providers should be in place to avoid foreseeable problems.
Stimulants. Systematic reviews suggest an association between prescription stimulants and at least the 2 cardiovascular risk factors of elevated heart rate and blood pressure. Stimulants are not recommended, therefore, for routine use in patients who have known hypertension or CAD.30
Second-generation antipsychotics are associated with significant weight gain and development of metabolic syndrome.
Selective serotonin reuptake inhibitors are associated with an increased risk of gastrointestinal bleeding risk related to platelet inhibition and gastric effects. Risk increases with additional platelet inhibitors, such as aspirin or clopidogrel.31
Lithium is excreted solely by the kidney. Guidelines recommend ACE inhibitors and angiotensin receptor-blockers for patients with CAD or T2DM, and many patients with symptomatic congestive heart failure are prescribed a diuretic; all of these classes of medications impair excretion of lithium. In a nested case-control study, 3% of observed cases of lithium toxicity were attributable to a newly initiated ACE inhibitor or angiotensin receptor-blocker.32 It is essential that you, and your patients taking lithium, be aware of the need to monitor the drug level frequently and be vigilant for symptoms of mild toxicity.
Beta blockers. No prospectively collected data support a association between beta blockers and depression.33 Patients with CAD should be given a trial of a beta blocker to achieve optimal medical management; because they are at increased risk of depression in the first place, all patients with CAD should undergo monitoring for depressive symptoms.
Clopidogrel is activated through the cytochrome P450 2C19 isoenzyme; medications such as fluoxetine and fluvoxamine that inhibit the function of CYP2C19 can impair the effectiveness of clopidogrel.31
Other considerations. Patients taking a second-generation antipsychotic should have baseline and periodic (monthly for the first quarter, then quarterly) assessments of BMI and, after monitoring at 3 months after baseline, annual monitoring of blood pressure, the fasting glucose level, and abdominal waist circumference. Lipid levels should be monitored every 5 years9 (Table 3).
Baseline and periodic monitoring of hepatic enzymes is recommended for patients taking a statin. You, and the patient, should be alert to the possible development of muscle weakness or pain; establish a low threshold for screening for an elevated creatine kinase level, which signals rhabdomyolysis.
Case concluded
Ms. S’s psychiatrist measures her blood pressure and finds that it is 147/92 mm Hg. He uses the Pooled Cohort Equations to determine that her lifetime risk of cardiovascular event is 50% (compared with a 8% lifetime risk among a cohort in whom risk factors are optimized) and that her 10-year risk is 41% (compared with a 2.2% risk among optimized controls).
At this point, the psychiatrist starts metformin to prevent T2DM. He also starts Ms. S on a statin to prevent CAD in a setting of diagnosed T2DM.
Ms. S’s exertional dyspnea and shoulder discomfort could be associated with angina, and the physician wisely refers her for urgent evaluation. Because he is aware of the literature demonstrating decreased revascularization among patients with mental illness, he urges her other health care providers to provide her with guideline-based strategies to treat her cardiovascular disease.
Bottom Line
Patients with psychiatric illness have higher rates of morbidity and mortality from coronary artery disease (CAD) than the general population. Symptoms characteristic of depression and schizophrenia could lead to poor self-care or impaired adherence to programs designed to lower CAD risk factors. Institute strategies for primary and secondary prevention of CAD among your patients, based on published guidelines, and be aware of, and alert for, adverse cardiac effects and an increase in risk factors for CAD from the use of psychotropics.
Related Resources
• Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):511-523.
• Interactive cardiovascular risk calculator developed from the Framingham Heart Study. https://www.framingham heartstudy.org/risk-functions/cardiovascular-disease/ 10-year-risk.php.
• Pooled Cohort Equations calculator. To determine estimated cardiovascular risk in comparison with peers with optimized risk factors. http://clincalc.com/cardiology/ascvd/ pooledcohort.aspx.
• To learn more about traditional cardiovascular risk factors from the Framingham Heart Study. http://www.framinghamheart study.org/risk-functions/.
Drug Brand Names
Amlodipine • Norvasc
Clozapine • Clozaril
Clopidogrel • Plavix
Felodipine • Plendil
Fluoxetine • Prozac
Fluvoxamine • Luvox
Lithium • Eskalith, Lithobid
Metformin • Glucophage
Olanzapine • Zyprexa
Risperidone • Risperdal
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Heald A, Montejo AL, Millar H, et al. Management of physical health in patients with schizophrenia: practical recommendations. Eur Psychiatry. 2010;25(suppl 2):S41-S45.
2. Huffman JC, Celano CM, Beach SR, et al. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925.
3. Lawrence D, Mitrou F, Zubrick ZR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Athyros VG, Tziomalos K, Katsiki N, et al; GREACE Study Collaborative Group. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: a post hoc analysis of the GREACE study. Curr Vasc Pharmacol. 2013;11(5):779-784.
5. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 AACF/ AHA/ACP/AATS/PCNA/SCAI/STS Guidelines for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164.
6. Gilman SE, Kawachi I, Fitzmaurice GM, et al. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359-367.
7. Pozuelo L, Tesar G, Zhang J, et al. Depression and heart disease: what do we know, and where are we headed? Cleve Clin J Med. 2009;76(1):59-70.
8. Osborn DP, Wright CA, Levy G, et al. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry. 2008;8:84.
9. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
10. Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139-2049.
11. Soares-Filho GL, Mesquita CT, Mesquita ET, et al. Panic attack triggering myocardial ischemia documented by myocardial perfusion imaging study. A case report. Int Arch Med. 2012;5(1):24.
12. Khawaja IS, Westermeyer JJ, Gajwani P, et al. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6(1):38-51.
13. Wang G, Cui J, Wang Y, et al. Anxiety and adverse coronary artery disease outcomes in Chinese patients. Psychosom Med. 2013;75(6):530-536.
14. Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2):e000068. doi: 10.1161/JAHA.112.000068.
15. Chiavarino C, Rabellino D, Ardito RB, et al. Emotional coping is a better predictor of cardiac prognosis than depression and anxiety. J Psychosom Res. 2012;73(6):473-475.
16. Moyer VA; U.S. Preventive Services Task Force. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(7):512-518.
17. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2012;199(2):99-105.
18. Hartley L, Igbinedion E, Holmes J, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6:CD009874. doi: 10.1002/14651858.CD009874.pub2.
19. Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62-69.
20. Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. J Clin Psychiatry. 2002;63(suppl 9):5-11.
21. Dipasquale S, Pariante CM, Dazzan P, et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47(2):197-207.
22. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illness. N Engl J Med. 2010;363(27):2611-2620.
23. Manderbacka K, Arffman M, Sund R, et al. How does a history of psychiatric hospital care influence access to coronary care: a cohort study. BMJ Open. 2012;2(2):e000831. doi: 10.1136/bmjopen-2012-000831.
24. Kumbhani DJ, Steg PG, Cannon CP, et al; REduction of Atherothrombosis for Continued Health Registry Investigators. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126(8):693-700.
25. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e637S-e668S.
26. Lichtman JH, Bigger T, Blumenthal JA, et al; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768-1775.
27. Albus C, Jordan J, Herrmann-Lingen C. Screening for psychosocial risk factors in patients with coronary heart disease-recommendations for clinical practice. Eur J Cardiovasc Prev Rehabil. 2004;11(1):75-79.
28. Srihari VH, Phutane VH, Ozkan B, et al. Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophr Res. 2013;146(1-3):64-68.
29. Brooks JO 3rd, Chang HS, Krasnykh O. Metabolic risks in older adults receiving second-generation antipsychotic medication. Curr Psychiatry Rep. 2009;11(1):33-40.
30. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
31. Andrade C. Drug interactions in the treatment of depression in patients with ischemic heart disease. J Clin Psychiatry. 2012;73(12):e1475-e1477.
32. Juurlink DN, Mamdani MM, Kopp A, et al. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52(5):794-798.
33. Muzyk AJ, Gagliardi JP. Do beta blockers cause depression? Current Psychiatry. 2010;9(5):50,51,55.
1. Heald A, Montejo AL, Millar H, et al. Management of physical health in patients with schizophrenia: practical recommendations. Eur Psychiatry. 2010;25(suppl 2):S41-S45.
2. Huffman JC, Celano CM, Beach SR, et al. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013:695925. doi: 10.1155/2013/695925.
3. Lawrence D, Mitrou F, Zubrick ZR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285.
4. Athyros VG, Tziomalos K, Katsiki N, et al; GREACE Study Collaborative Group. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: a post hoc analysis of the GREACE study. Curr Vasc Pharmacol. 2013;11(5):779-784.
5. Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2012 AACF/ AHA/ACP/AATS/PCNA/SCAI/STS Guidelines for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164.
6. Gilman SE, Kawachi I, Fitzmaurice GM, et al. Socioeconomic status in childhood and the lifetime risk of major depression. Int J Epidemiol. 2002;31(2):359-367.
7. Pozuelo L, Tesar G, Zhang J, et al. Depression and heart disease: what do we know, and where are we headed? Cleve Clin J Med. 2009;76(1):59-70.
8. Osborn DP, Wright CA, Levy G, et al. Relative risk of diabetes, dyslipidaemia, hypertension and the metabolic syndrome in people with severe mental illnesses: systematic review and metaanalysis. BMC Psychiatry. 2008;8:84.
9. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
10. Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309(20):2139-2049.
11. Soares-Filho GL, Mesquita CT, Mesquita ET, et al. Panic attack triggering myocardial ischemia documented by myocardial perfusion imaging study. A case report. Int Arch Med. 2012;5(1):24.
12. Khawaja IS, Westermeyer JJ, Gajwani P, et al. Depression and coronary artery disease: the association, mechanisms, and therapeutic implications. Psychiatry (Edgmont). 2009;6(1):38-51.
13. Wang G, Cui J, Wang Y, et al. Anxiety and adverse coronary artery disease outcomes in Chinese patients. Psychosom Med. 2013;75(6):530-536.
14. Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. J Am Heart Assoc. 2013;2(2):e000068. doi: 10.1161/JAHA.112.000068.
15. Chiavarino C, Rabellino D, Ardito RB, et al. Emotional coping is a better predictor of cardiac prognosis than depression and anxiety. J Psychosom Res. 2012;73(6):473-475.
16. Moyer VA; U.S. Preventive Services Task Force. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(7):512-518.
17. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2012;199(2):99-105.
18. Hartley L, Igbinedion E, Holmes J, et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6:CD009874. doi: 10.1002/14651858.CD009874.pub2.
19. Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62-69.
20. Davidson M. Risk of cardiovascular disease and sudden death in schizophrenia. J Clin Psychiatry. 2002;63(suppl 9):5-11.
21. Dipasquale S, Pariante CM, Dazzan P, et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47(2):197-207.
22. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illness. N Engl J Med. 2010;363(27):2611-2620.
23. Manderbacka K, Arffman M, Sund R, et al. How does a history of psychiatric hospital care influence access to coronary care: a cohort study. BMJ Open. 2012;2(2):e000831. doi: 10.1136/bmjopen-2012-000831.
24. Kumbhani DJ, Steg PG, Cannon CP, et al; REduction of Atherothrombosis for Continued Health Registry Investigators. Adherence to secondary prevention medications and four-year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126(8):693-700.
25. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e637S-e668S.
26. Lichtman JH, Bigger T, Blumenthal JA, et al; American Heart Association Prevention Committee of the Council on Cardiovascular Nursing; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Epidemiology and Prevention; American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research; American Psychiatric Association. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768-1775.
27. Albus C, Jordan J, Herrmann-Lingen C. Screening for psychosocial risk factors in patients with coronary heart disease-recommendations for clinical practice. Eur J Cardiovasc Prev Rehabil. 2004;11(1):75-79.
28. Srihari VH, Phutane VH, Ozkan B, et al. Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophr Res. 2013;146(1-3):64-68.
29. Brooks JO 3rd, Chang HS, Krasnykh O. Metabolic risks in older adults receiving second-generation antipsychotic medication. Curr Psychiatry Rep. 2009;11(1):33-40.
30. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
31. Andrade C. Drug interactions in the treatment of depression in patients with ischemic heart disease. J Clin Psychiatry. 2012;73(12):e1475-e1477.
32. Juurlink DN, Mamdani MM, Kopp A, et al. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc. 2004;52(5):794-798.
33. Muzyk AJ, Gagliardi JP. Do beta blockers cause depression? Current Psychiatry. 2010;9(5):50,51,55.
Malnourished and psychotic, and found incompetent to stand trial
Mr. N, age 48, has chronic mental illness and has been in and out of psychiatric hospitals for 30 years, with diagnoses of bipolar disorder, not otherwise specified, without psychotic features and schizophrenia. He often is delusional and disorganized and does not adhere to treatment. Since age 18, his psychiatric care has been sporadic; during his last admission 3 years ago, he refused treatment and left the hospital against medical advice. Mr. N is homeless and often eats out of a dumpster.
Recently, Mr. N was arrested for cocaine possession, for which he was held in custody. His mental status continued to deteriorate while in jail, where he was evaluated by a forensics examiner.
Mr. N was declared incompetent to stand trial and was transferred to a state psychiatric hospital.
In the hospital, the treatment team finds that Mr. N is disorganized and preoccupied with thoughts of not wanting to “lose control” to the physicians. He shows no evidence of suicidal or homicidal ideation or perceptual disturbance. Mr. N has difficulty grasping concepts, making plans, and following through with them. He has poor insight and impulse control and impaired judgment.
Mr. N’s past and present diagnoses include bipolar disorder without psychotic features, schizophrenia, obsessive-compulsive personality disorder, paranoid personality traits, borderline intelligence, cellulitis of both legs, and chronic venous stasis. Although he was arrested for cocaine possession, we are not able to obtain much information about his history of substance abuse because of his poor mental status.
What could be causing Mr. N’s deteriorating mental status?
a) substance withdrawal
b) malnutrition
c) worsening schizophrenia
d) untreated infection due to cellulitis
HISTORY Sporadic care
Mr. N can provide few details of his early life. He was adopted as a child. He spent time in juvenile detention center. He completed 10th grade but did not graduate from high school. Symptoms of mental illness emerged at age 18. His employment history is consistent with chronic mental illness: His longest job, at a grocery store, lasted only 6 months. He has had multiple admissions to psychiatric hospitals. Over the years his treatment has included divalproex sodium, risperidone, paroxetine, chlorpromazine, thioridazine, amitriptyline, methylphenidate, and a multivitamin; however, he often is noncompliant with treatment and was not taking any medications when he arrived at the hospital.
EVALUATION Possible deficiency
The treatment team discusses guardianship, but the public administrator’s office provides little support because of Mr. N’s refusal to stay in one place. He was evicted from his last apartment because of hoarding behavior, which created a fire hazard. He has been homeless most of his adult life, which might have significantly restricted his diet.
A routine laboratory workup—complete blood count, basic metabolic panel, liver function test, thyroid-stimulating hormone, and lipids—is ordered, revealing an absolute neutrophil count (ANC) in the low range at 1,200/μL (normal range, 1,500 to 8,000/μL). Mr. N is offered treatment with a long-acting IM injection of risperidone because of his history of noncompliance, but he refuses the medication. Instead, he is started on oral risperidone, 2 mg/d.
The cellulitis of both lower limbs and chronic venous stasis are of concern; the medical team is consulted. Review of Mr. N’s medical records from an affiliated hospital reveals a history of vitamin B12 deficiency. Further tests show that the vitamin B12 level is low at <50 pg/mL (normal range, 160 to 950 pg/mL). Pernicious anemia had been ruled out after Mr. N tested negative for antibodies to intrinsic factor (a glycoprotein secreted in the stomach that is necessary for absorption of vitamin B12). Suspicion is that vitamin B12 deficiency is caused by Mr. N’s restricted diet in the context of chronic homelessness.
The authors’ observations
A review of the literature on vitamin B12 deficiency describes tingling or numbness, ataxia, and dementia; however, in rare cases, vitamin B12 deficiency presents with psychiatric symptoms, such as depression, mania, psychosis, dementia, and catatonia.1-13
We suspected that Mr. N’s vitamin B12 deficiency could have been affecting his mental status; consequently, we ordered routine laboratory work-up that included a complete blood count with differential and peripheral smear, which showed macrocytic anemia and ovalocytes. We also tested his vitamin B12 level, which was very low at 55 pg/mL. These results, combined with his previously recorded vitamin B12 level (Table 1), suggested deficiency.
TREATMENT Oral medication
Two months after starting risperidone, the medical team recommends IM vitamin B12 as first-line treatment, but Mr. N refuses. We considered guardianship ex parte for involuntary administration of IM B12 injection to prevent life-threatening consequences of a non-healing ulcer on his leg that was related to his cellulitis. Meanwhile, we reviewed the literature on vitamin B12 therapy, including route, dosage, and outcome.14-23 Mr. N agrees to oral vitamin B12, 1,000 μg/d,21 and we no longer consider guardianship ex parte. Mr. N’s vitamin B12 level and clinical picture improve 1 month after oral vitamin B12 is added to oral risperidone. His thought process is more organized, he is no longer paranoid, and he shows improved insight and judgement. ANC and neutrophil count improve as well (Table 2). Mr. N’s ulcer begins to heal despite his noncompliance with wound care.
The forensic examiner sees Mr. N after 3 months of continued therapy. His thought pattern is more organized and he is able to comprehend the criminal charges against him and to work with his attorney. He is determined competent by the forensic examiner; in a court hearing, the judge finds Mr. N competent to stand trial.
The authors’ observations
Based on our experience treating Mr. N, we think that it is important to establish an association between vitamin B12 deficiency and psychosis. Vitamin B12 deficiency is uncommon; however, serum levels do not need to be significantly low to produce severe neuropsychiatric morbidity, which has been reported with serum levels ≤457 pg/mL.2-5,24,25 It is more frequent than the other organic causes of psychosis5,10,24 and Mr. N’s improvement further strengthened the correlation.
Parenteral vitamin B12 therapy is the first-line treatment for a deficiency, but oral or sublingual vitamin B12 can be given to patients who are disabled, geriatric, or refuse parenteral administration.21 Only approximately 1% of oral vitamin B12 is absorbed in patients who do not have intrinsic factor. The daily requirement of vitamin B12 is 1.0 to 2.5 μg/d; large oral dosages of 1,000 to 5,000 μg/d therefore seem to be effective in correcting deficiency, even in the presence of intrinsic factor deficiency.15,20,21 Large oral dosages also benefit other hematological abnormalities, such as a low white blood cell count and neutropenia.
How vitamin B12 deficiency affects neuropsychiatric illness
Vitamin B12 is essential for methylation, a process crucial for the formation of neurotransmitters such as serotonin, dopamine, and epinephrine. A low level of vitamin B12 can interrupt methylation and cause accumulation of homocysteine and impaired metabolism of serotonin, dopamine, and epinephrine. Hyperhomocysteinemia can contribute to cerebral dysfunction by causing vascular injury.26
Vitamin B12 also is involved in tetrahydrobiopterin synthesis in the brain, which is pivotal for synthesis of monoamine neurotransmitters. Vitamin B12 deficiency can lead to accumulation of methyltetrahydrofolate, an excitatory neurotoxin. All of these can contribute to development of psychosis. Therefore, a defect in the methylation process could be responsible for the neuropsychiatric manifestations of vitamin B12 deficiency.
What did we learn from Mr. N?
In most people, vitamin B12 levels are normal, however, we recommend that clinicians consider vitamin B12 deficiency when a patient has new-onset or unresponsive psychosis,27 particularly in a homeless person or one who has a restricted diet.28 It is important to rule out vitamin B12 deficiency in a patient with a low serum folate level because folic acid therapy could exacerbate neurologic manifestations of underlying vitamin B12 deficiency and increase the risk of permanent nerve damage and cognitive decline.
We were intrigued to see improvement in Mr. N after we added vitamin B12 to his ongoing treatment with an antipsychotic. We did not believe that vitamin B12 supplementation was the sole reason his mental status improved enough to be found competent to stand trial, although we believe that initiating oral vitamin B12 was beneficial for Mr. N.
Last, this case supports the need for research to further explore the role of vitamin B12 in refractory psychosis, depression, and mania.
Bottom Line
Vitamin B12 deficiency can contribute to psychosis and other psychiatric disorders, especially in patients with a restricted diet, such as those who are homeless. Parenteral vitamin B12 therapy is the first-line treatment, but oral supplementation can be used if the patient refuses therapy. Large oral dosages of 1,000 to 5,000 μg/d seem to be effective in correcting vitamin B12 deficiency.
Related Resources
• Ramsey D, Muskin PR. Vitamin deficiencies and mental health: How are they linked? Current Psychiatry. 2013;12(1):37-43.
• Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
Drug Brand Names
Amitriptyline • Elavil
Chlorpromazine • Thorazine
Divalproex sodium • Depakote
Methylphenidate • Ritalin
Paroxetine • Paxil
Risperidone • Risperdal
Thioridazine • Mellaril
Acknowledgements
The authors thank Jan Jill-Jordan, PhD, for her help preparing the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dogan M, Ozdemir O, Sal EA, et al. Psychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiency. J Trop Pediatr. 2009;55(3):205-207.
2. Levine J, Stahl Z, Sela BA, et al. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159(10):1790-1792.
3. Jauhar S, Blackett A, Srireddy P, et al. Pernicious anaemia presenting as catatonia without signs of anaemia or macrocytosis. Br J Psychiatry. 2010;197(3):244-245.
4. de Carvalho Abi-Abib R, Milech A, Ramalho FV, et al. Psychosis as the initial manifestation of pernicious anemia in a type 1 diabetes mellitus patient. Endocrinologist. 2010;20(5):224-225.
5. Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108(2):156-159.
6. Zucker DK, Livingston RL, Nakra R, et al. B12 deficiency and psychiatric disorders: case report and literature review. Biol Psychiatry. 1981;16(2):197-205.
7. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393-1412.
8. Roze E, Gervais D, Demeret S, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60(10):1457-1462.
9. Lewis AL, Pelic C, Kahn DA. Malignant catatonia in a patient with bipolar disorder, B12 deficiency, and neuroleptic malignant syndrome: one cause or three? J Psychiatr Pract. 2009;15(5):415-422.
10. Rajkumar AP, Jebaraj P. Chronic psychosis associated with vitamin B12 deficiency. J Assoc Physicians India. 2008;56:115-116.
11. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Assoc J. 2001;3(9):701-703.
12. Smith R, Oliver RA. Sudden onset of psychosis in association with vitamin-B12 deficiency. Br Med J. 1967;3(5556):34.
13. Russell RM, Baik HW. Clinical implications of vitamin B12 deficiency in the elderly. Nutrition in Clinical Care. 2001;4(4):214-220.
14. Sharabi A, Cohen E, Sulkes J, et al. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003; 56(6):635-638.
15. Chalmers RA, Bain MD, Costello I. Oral cobalamin therapy. Lancet. 2000;355(9198):148.
16. Borchardt J, Malnick S. Sublingual cobalamin for pernicious anaemia. Lancet. 1999;354(9195):2081.
17. Seal EC, Metz J, Flicker L, et al. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc. 2002;50(1):146-151.
18. Erkurt MA, Aydogdu I, Dikilitas M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135.
19. Andrès E, Kaltenbach G, Noel E, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: a study of 30 patients. Clin Lab Haematol. 2003;25(3):161-166.
20. Wellmer J, Sturm KU, Herrmann W, et al. Oral treatment of vitamin B12 deficiency in subacute combined degeneration [in German]. Nervenarzt. 2006;77(10):1228-1231.
21. Lederle FA. Oral cobalamin for pernicious anemia. Medicine‘s best kept secret? JAMA. 1991;265(1):94-95.
22. Chalouhi C, Faesch S, Anthoine-Milhomme MC, et al. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care. 2008;24(8):538-541.
23. Andrès E, Federici L, Affenberger S, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract. 2007;56(7):537-542.
24. Aaron S, Kumar S, Vijayan J, et al. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency related neurological syndromes. Neurol India. 2005;53(1):55-58.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60(9):1296-1301.
26. Tsai AC, Morel CF, Scharer G, et al. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A(20):2430-2434.
27. Brett AS, Roberts MS. Screening for vitamin B12 deficiency in psychiatric patients. J Gen Intern Med. 1994;9(9):522-524.
28. Kaltenbach G, Noblet-Dick M, Barnier-Figue G, et al. Early normalization of low vitamin B12 levels by oral cobalamin therapy in three older patients with pernicious anemia. J Am Geriatr Soc. 2002;50(11):1914-1915.
Mr. N, age 48, has chronic mental illness and has been in and out of psychiatric hospitals for 30 years, with diagnoses of bipolar disorder, not otherwise specified, without psychotic features and schizophrenia. He often is delusional and disorganized and does not adhere to treatment. Since age 18, his psychiatric care has been sporadic; during his last admission 3 years ago, he refused treatment and left the hospital against medical advice. Mr. N is homeless and often eats out of a dumpster.
Recently, Mr. N was arrested for cocaine possession, for which he was held in custody. His mental status continued to deteriorate while in jail, where he was evaluated by a forensics examiner.
Mr. N was declared incompetent to stand trial and was transferred to a state psychiatric hospital.
In the hospital, the treatment team finds that Mr. N is disorganized and preoccupied with thoughts of not wanting to “lose control” to the physicians. He shows no evidence of suicidal or homicidal ideation or perceptual disturbance. Mr. N has difficulty grasping concepts, making plans, and following through with them. He has poor insight and impulse control and impaired judgment.
Mr. N’s past and present diagnoses include bipolar disorder without psychotic features, schizophrenia, obsessive-compulsive personality disorder, paranoid personality traits, borderline intelligence, cellulitis of both legs, and chronic venous stasis. Although he was arrested for cocaine possession, we are not able to obtain much information about his history of substance abuse because of his poor mental status.
What could be causing Mr. N’s deteriorating mental status?
a) substance withdrawal
b) malnutrition
c) worsening schizophrenia
d) untreated infection due to cellulitis
HISTORY Sporadic care
Mr. N can provide few details of his early life. He was adopted as a child. He spent time in juvenile detention center. He completed 10th grade but did not graduate from high school. Symptoms of mental illness emerged at age 18. His employment history is consistent with chronic mental illness: His longest job, at a grocery store, lasted only 6 months. He has had multiple admissions to psychiatric hospitals. Over the years his treatment has included divalproex sodium, risperidone, paroxetine, chlorpromazine, thioridazine, amitriptyline, methylphenidate, and a multivitamin; however, he often is noncompliant with treatment and was not taking any medications when he arrived at the hospital.
EVALUATION Possible deficiency
The treatment team discusses guardianship, but the public administrator’s office provides little support because of Mr. N’s refusal to stay in one place. He was evicted from his last apartment because of hoarding behavior, which created a fire hazard. He has been homeless most of his adult life, which might have significantly restricted his diet.
A routine laboratory workup—complete blood count, basic metabolic panel, liver function test, thyroid-stimulating hormone, and lipids—is ordered, revealing an absolute neutrophil count (ANC) in the low range at 1,200/μL (normal range, 1,500 to 8,000/μL). Mr. N is offered treatment with a long-acting IM injection of risperidone because of his history of noncompliance, but he refuses the medication. Instead, he is started on oral risperidone, 2 mg/d.
The cellulitis of both lower limbs and chronic venous stasis are of concern; the medical team is consulted. Review of Mr. N’s medical records from an affiliated hospital reveals a history of vitamin B12 deficiency. Further tests show that the vitamin B12 level is low at <50 pg/mL (normal range, 160 to 950 pg/mL). Pernicious anemia had been ruled out after Mr. N tested negative for antibodies to intrinsic factor (a glycoprotein secreted in the stomach that is necessary for absorption of vitamin B12). Suspicion is that vitamin B12 deficiency is caused by Mr. N’s restricted diet in the context of chronic homelessness.
The authors’ observations
A review of the literature on vitamin B12 deficiency describes tingling or numbness, ataxia, and dementia; however, in rare cases, vitamin B12 deficiency presents with psychiatric symptoms, such as depression, mania, psychosis, dementia, and catatonia.1-13
We suspected that Mr. N’s vitamin B12 deficiency could have been affecting his mental status; consequently, we ordered routine laboratory work-up that included a complete blood count with differential and peripheral smear, which showed macrocytic anemia and ovalocytes. We also tested his vitamin B12 level, which was very low at 55 pg/mL. These results, combined with his previously recorded vitamin B12 level (Table 1), suggested deficiency.
TREATMENT Oral medication
Two months after starting risperidone, the medical team recommends IM vitamin B12 as first-line treatment, but Mr. N refuses. We considered guardianship ex parte for involuntary administration of IM B12 injection to prevent life-threatening consequences of a non-healing ulcer on his leg that was related to his cellulitis. Meanwhile, we reviewed the literature on vitamin B12 therapy, including route, dosage, and outcome.14-23 Mr. N agrees to oral vitamin B12, 1,000 μg/d,21 and we no longer consider guardianship ex parte. Mr. N’s vitamin B12 level and clinical picture improve 1 month after oral vitamin B12 is added to oral risperidone. His thought process is more organized, he is no longer paranoid, and he shows improved insight and judgement. ANC and neutrophil count improve as well (Table 2). Mr. N’s ulcer begins to heal despite his noncompliance with wound care.
The forensic examiner sees Mr. N after 3 months of continued therapy. His thought pattern is more organized and he is able to comprehend the criminal charges against him and to work with his attorney. He is determined competent by the forensic examiner; in a court hearing, the judge finds Mr. N competent to stand trial.
The authors’ observations
Based on our experience treating Mr. N, we think that it is important to establish an association between vitamin B12 deficiency and psychosis. Vitamin B12 deficiency is uncommon; however, serum levels do not need to be significantly low to produce severe neuropsychiatric morbidity, which has been reported with serum levels ≤457 pg/mL.2-5,24,25 It is more frequent than the other organic causes of psychosis5,10,24 and Mr. N’s improvement further strengthened the correlation.
Parenteral vitamin B12 therapy is the first-line treatment for a deficiency, but oral or sublingual vitamin B12 can be given to patients who are disabled, geriatric, or refuse parenteral administration.21 Only approximately 1% of oral vitamin B12 is absorbed in patients who do not have intrinsic factor. The daily requirement of vitamin B12 is 1.0 to 2.5 μg/d; large oral dosages of 1,000 to 5,000 μg/d therefore seem to be effective in correcting deficiency, even in the presence of intrinsic factor deficiency.15,20,21 Large oral dosages also benefit other hematological abnormalities, such as a low white blood cell count and neutropenia.
How vitamin B12 deficiency affects neuropsychiatric illness
Vitamin B12 is essential for methylation, a process crucial for the formation of neurotransmitters such as serotonin, dopamine, and epinephrine. A low level of vitamin B12 can interrupt methylation and cause accumulation of homocysteine and impaired metabolism of serotonin, dopamine, and epinephrine. Hyperhomocysteinemia can contribute to cerebral dysfunction by causing vascular injury.26
Vitamin B12 also is involved in tetrahydrobiopterin synthesis in the brain, which is pivotal for synthesis of monoamine neurotransmitters. Vitamin B12 deficiency can lead to accumulation of methyltetrahydrofolate, an excitatory neurotoxin. All of these can contribute to development of psychosis. Therefore, a defect in the methylation process could be responsible for the neuropsychiatric manifestations of vitamin B12 deficiency.
What did we learn from Mr. N?
In most people, vitamin B12 levels are normal, however, we recommend that clinicians consider vitamin B12 deficiency when a patient has new-onset or unresponsive psychosis,27 particularly in a homeless person or one who has a restricted diet.28 It is important to rule out vitamin B12 deficiency in a patient with a low serum folate level because folic acid therapy could exacerbate neurologic manifestations of underlying vitamin B12 deficiency and increase the risk of permanent nerve damage and cognitive decline.
We were intrigued to see improvement in Mr. N after we added vitamin B12 to his ongoing treatment with an antipsychotic. We did not believe that vitamin B12 supplementation was the sole reason his mental status improved enough to be found competent to stand trial, although we believe that initiating oral vitamin B12 was beneficial for Mr. N.
Last, this case supports the need for research to further explore the role of vitamin B12 in refractory psychosis, depression, and mania.
Bottom Line
Vitamin B12 deficiency can contribute to psychosis and other psychiatric disorders, especially in patients with a restricted diet, such as those who are homeless. Parenteral vitamin B12 therapy is the first-line treatment, but oral supplementation can be used if the patient refuses therapy. Large oral dosages of 1,000 to 5,000 μg/d seem to be effective in correcting vitamin B12 deficiency.
Related Resources
• Ramsey D, Muskin PR. Vitamin deficiencies and mental health: How are they linked? Current Psychiatry. 2013;12(1):37-43.
• Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
Drug Brand Names
Amitriptyline • Elavil
Chlorpromazine • Thorazine
Divalproex sodium • Depakote
Methylphenidate • Ritalin
Paroxetine • Paxil
Risperidone • Risperdal
Thioridazine • Mellaril
Acknowledgements
The authors thank Jan Jill-Jordan, PhD, for her help preparing the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. N, age 48, has chronic mental illness and has been in and out of psychiatric hospitals for 30 years, with diagnoses of bipolar disorder, not otherwise specified, without psychotic features and schizophrenia. He often is delusional and disorganized and does not adhere to treatment. Since age 18, his psychiatric care has been sporadic; during his last admission 3 years ago, he refused treatment and left the hospital against medical advice. Mr. N is homeless and often eats out of a dumpster.
Recently, Mr. N was arrested for cocaine possession, for which he was held in custody. His mental status continued to deteriorate while in jail, where he was evaluated by a forensics examiner.
Mr. N was declared incompetent to stand trial and was transferred to a state psychiatric hospital.
In the hospital, the treatment team finds that Mr. N is disorganized and preoccupied with thoughts of not wanting to “lose control” to the physicians. He shows no evidence of suicidal or homicidal ideation or perceptual disturbance. Mr. N has difficulty grasping concepts, making plans, and following through with them. He has poor insight and impulse control and impaired judgment.
Mr. N’s past and present diagnoses include bipolar disorder without psychotic features, schizophrenia, obsessive-compulsive personality disorder, paranoid personality traits, borderline intelligence, cellulitis of both legs, and chronic venous stasis. Although he was arrested for cocaine possession, we are not able to obtain much information about his history of substance abuse because of his poor mental status.
What could be causing Mr. N’s deteriorating mental status?
a) substance withdrawal
b) malnutrition
c) worsening schizophrenia
d) untreated infection due to cellulitis
HISTORY Sporadic care
Mr. N can provide few details of his early life. He was adopted as a child. He spent time in juvenile detention center. He completed 10th grade but did not graduate from high school. Symptoms of mental illness emerged at age 18. His employment history is consistent with chronic mental illness: His longest job, at a grocery store, lasted only 6 months. He has had multiple admissions to psychiatric hospitals. Over the years his treatment has included divalproex sodium, risperidone, paroxetine, chlorpromazine, thioridazine, amitriptyline, methylphenidate, and a multivitamin; however, he often is noncompliant with treatment and was not taking any medications when he arrived at the hospital.
EVALUATION Possible deficiency
The treatment team discusses guardianship, but the public administrator’s office provides little support because of Mr. N’s refusal to stay in one place. He was evicted from his last apartment because of hoarding behavior, which created a fire hazard. He has been homeless most of his adult life, which might have significantly restricted his diet.
A routine laboratory workup—complete blood count, basic metabolic panel, liver function test, thyroid-stimulating hormone, and lipids—is ordered, revealing an absolute neutrophil count (ANC) in the low range at 1,200/μL (normal range, 1,500 to 8,000/μL). Mr. N is offered treatment with a long-acting IM injection of risperidone because of his history of noncompliance, but he refuses the medication. Instead, he is started on oral risperidone, 2 mg/d.
The cellulitis of both lower limbs and chronic venous stasis are of concern; the medical team is consulted. Review of Mr. N’s medical records from an affiliated hospital reveals a history of vitamin B12 deficiency. Further tests show that the vitamin B12 level is low at <50 pg/mL (normal range, 160 to 950 pg/mL). Pernicious anemia had been ruled out after Mr. N tested negative for antibodies to intrinsic factor (a glycoprotein secreted in the stomach that is necessary for absorption of vitamin B12). Suspicion is that vitamin B12 deficiency is caused by Mr. N’s restricted diet in the context of chronic homelessness.
The authors’ observations
A review of the literature on vitamin B12 deficiency describes tingling or numbness, ataxia, and dementia; however, in rare cases, vitamin B12 deficiency presents with psychiatric symptoms, such as depression, mania, psychosis, dementia, and catatonia.1-13
We suspected that Mr. N’s vitamin B12 deficiency could have been affecting his mental status; consequently, we ordered routine laboratory work-up that included a complete blood count with differential and peripheral smear, which showed macrocytic anemia and ovalocytes. We also tested his vitamin B12 level, which was very low at 55 pg/mL. These results, combined with his previously recorded vitamin B12 level (Table 1), suggested deficiency.
TREATMENT Oral medication
Two months after starting risperidone, the medical team recommends IM vitamin B12 as first-line treatment, but Mr. N refuses. We considered guardianship ex parte for involuntary administration of IM B12 injection to prevent life-threatening consequences of a non-healing ulcer on his leg that was related to his cellulitis. Meanwhile, we reviewed the literature on vitamin B12 therapy, including route, dosage, and outcome.14-23 Mr. N agrees to oral vitamin B12, 1,000 μg/d,21 and we no longer consider guardianship ex parte. Mr. N’s vitamin B12 level and clinical picture improve 1 month after oral vitamin B12 is added to oral risperidone. His thought process is more organized, he is no longer paranoid, and he shows improved insight and judgement. ANC and neutrophil count improve as well (Table 2). Mr. N’s ulcer begins to heal despite his noncompliance with wound care.
The forensic examiner sees Mr. N after 3 months of continued therapy. His thought pattern is more organized and he is able to comprehend the criminal charges against him and to work with his attorney. He is determined competent by the forensic examiner; in a court hearing, the judge finds Mr. N competent to stand trial.
The authors’ observations
Based on our experience treating Mr. N, we think that it is important to establish an association between vitamin B12 deficiency and psychosis. Vitamin B12 deficiency is uncommon; however, serum levels do not need to be significantly low to produce severe neuropsychiatric morbidity, which has been reported with serum levels ≤457 pg/mL.2-5,24,25 It is more frequent than the other organic causes of psychosis5,10,24 and Mr. N’s improvement further strengthened the correlation.
Parenteral vitamin B12 therapy is the first-line treatment for a deficiency, but oral or sublingual vitamin B12 can be given to patients who are disabled, geriatric, or refuse parenteral administration.21 Only approximately 1% of oral vitamin B12 is absorbed in patients who do not have intrinsic factor. The daily requirement of vitamin B12 is 1.0 to 2.5 μg/d; large oral dosages of 1,000 to 5,000 μg/d therefore seem to be effective in correcting deficiency, even in the presence of intrinsic factor deficiency.15,20,21 Large oral dosages also benefit other hematological abnormalities, such as a low white blood cell count and neutropenia.
How vitamin B12 deficiency affects neuropsychiatric illness
Vitamin B12 is essential for methylation, a process crucial for the formation of neurotransmitters such as serotonin, dopamine, and epinephrine. A low level of vitamin B12 can interrupt methylation and cause accumulation of homocysteine and impaired metabolism of serotonin, dopamine, and epinephrine. Hyperhomocysteinemia can contribute to cerebral dysfunction by causing vascular injury.26
Vitamin B12 also is involved in tetrahydrobiopterin synthesis in the brain, which is pivotal for synthesis of monoamine neurotransmitters. Vitamin B12 deficiency can lead to accumulation of methyltetrahydrofolate, an excitatory neurotoxin. All of these can contribute to development of psychosis. Therefore, a defect in the methylation process could be responsible for the neuropsychiatric manifestations of vitamin B12 deficiency.
What did we learn from Mr. N?
In most people, vitamin B12 levels are normal, however, we recommend that clinicians consider vitamin B12 deficiency when a patient has new-onset or unresponsive psychosis,27 particularly in a homeless person or one who has a restricted diet.28 It is important to rule out vitamin B12 deficiency in a patient with a low serum folate level because folic acid therapy could exacerbate neurologic manifestations of underlying vitamin B12 deficiency and increase the risk of permanent nerve damage and cognitive decline.
We were intrigued to see improvement in Mr. N after we added vitamin B12 to his ongoing treatment with an antipsychotic. We did not believe that vitamin B12 supplementation was the sole reason his mental status improved enough to be found competent to stand trial, although we believe that initiating oral vitamin B12 was beneficial for Mr. N.
Last, this case supports the need for research to further explore the role of vitamin B12 in refractory psychosis, depression, and mania.
Bottom Line
Vitamin B12 deficiency can contribute to psychosis and other psychiatric disorders, especially in patients with a restricted diet, such as those who are homeless. Parenteral vitamin B12 therapy is the first-line treatment, but oral supplementation can be used if the patient refuses therapy. Large oral dosages of 1,000 to 5,000 μg/d seem to be effective in correcting vitamin B12 deficiency.
Related Resources
• Ramsey D, Muskin PR. Vitamin deficiencies and mental health: How are they linked? Current Psychiatry. 2013;12(1):37-43.
• Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
Drug Brand Names
Amitriptyline • Elavil
Chlorpromazine • Thorazine
Divalproex sodium • Depakote
Methylphenidate • Ritalin
Paroxetine • Paxil
Risperidone • Risperdal
Thioridazine • Mellaril
Acknowledgements
The authors thank Jan Jill-Jordan, PhD, for her help preparing the manuscript of this article.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dogan M, Ozdemir O, Sal EA, et al. Psychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiency. J Trop Pediatr. 2009;55(3):205-207.
2. Levine J, Stahl Z, Sela BA, et al. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159(10):1790-1792.
3. Jauhar S, Blackett A, Srireddy P, et al. Pernicious anaemia presenting as catatonia without signs of anaemia or macrocytosis. Br J Psychiatry. 2010;197(3):244-245.
4. de Carvalho Abi-Abib R, Milech A, Ramalho FV, et al. Psychosis as the initial manifestation of pernicious anemia in a type 1 diabetes mellitus patient. Endocrinologist. 2010;20(5):224-225.
5. Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108(2):156-159.
6. Zucker DK, Livingston RL, Nakra R, et al. B12 deficiency and psychiatric disorders: case report and literature review. Biol Psychiatry. 1981;16(2):197-205.
7. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393-1412.
8. Roze E, Gervais D, Demeret S, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60(10):1457-1462.
9. Lewis AL, Pelic C, Kahn DA. Malignant catatonia in a patient with bipolar disorder, B12 deficiency, and neuroleptic malignant syndrome: one cause or three? J Psychiatr Pract. 2009;15(5):415-422.
10. Rajkumar AP, Jebaraj P. Chronic psychosis associated with vitamin B12 deficiency. J Assoc Physicians India. 2008;56:115-116.
11. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Assoc J. 2001;3(9):701-703.
12. Smith R, Oliver RA. Sudden onset of psychosis in association with vitamin-B12 deficiency. Br Med J. 1967;3(5556):34.
13. Russell RM, Baik HW. Clinical implications of vitamin B12 deficiency in the elderly. Nutrition in Clinical Care. 2001;4(4):214-220.
14. Sharabi A, Cohen E, Sulkes J, et al. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003; 56(6):635-638.
15. Chalmers RA, Bain MD, Costello I. Oral cobalamin therapy. Lancet. 2000;355(9198):148.
16. Borchardt J, Malnick S. Sublingual cobalamin for pernicious anaemia. Lancet. 1999;354(9195):2081.
17. Seal EC, Metz J, Flicker L, et al. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc. 2002;50(1):146-151.
18. Erkurt MA, Aydogdu I, Dikilitas M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135.
19. Andrès E, Kaltenbach G, Noel E, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: a study of 30 patients. Clin Lab Haematol. 2003;25(3):161-166.
20. Wellmer J, Sturm KU, Herrmann W, et al. Oral treatment of vitamin B12 deficiency in subacute combined degeneration [in German]. Nervenarzt. 2006;77(10):1228-1231.
21. Lederle FA. Oral cobalamin for pernicious anemia. Medicine‘s best kept secret? JAMA. 1991;265(1):94-95.
22. Chalouhi C, Faesch S, Anthoine-Milhomme MC, et al. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care. 2008;24(8):538-541.
23. Andrès E, Federici L, Affenberger S, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract. 2007;56(7):537-542.
24. Aaron S, Kumar S, Vijayan J, et al. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency related neurological syndromes. Neurol India. 2005;53(1):55-58.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60(9):1296-1301.
26. Tsai AC, Morel CF, Scharer G, et al. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A(20):2430-2434.
27. Brett AS, Roberts MS. Screening for vitamin B12 deficiency in psychiatric patients. J Gen Intern Med. 1994;9(9):522-524.
28. Kaltenbach G, Noblet-Dick M, Barnier-Figue G, et al. Early normalization of low vitamin B12 levels by oral cobalamin therapy in three older patients with pernicious anemia. J Am Geriatr Soc. 2002;50(11):1914-1915.
1. Dogan M, Ozdemir O, Sal EA, et al. Psychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiency. J Trop Pediatr. 2009;55(3):205-207.
2. Levine J, Stahl Z, Sela BA, et al. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159(10):1790-1792.
3. Jauhar S, Blackett A, Srireddy P, et al. Pernicious anaemia presenting as catatonia without signs of anaemia or macrocytosis. Br J Psychiatry. 2010;197(3):244-245.
4. de Carvalho Abi-Abib R, Milech A, Ramalho FV, et al. Psychosis as the initial manifestation of pernicious anemia in a type 1 diabetes mellitus patient. Endocrinologist. 2010;20(5):224-225.
5. Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108(2):156-159.
6. Zucker DK, Livingston RL, Nakra R, et al. B12 deficiency and psychiatric disorders: case report and literature review. Biol Psychiatry. 1981;16(2):197-205.
7. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9(9):1393-1412.
8. Roze E, Gervais D, Demeret S, et al. Neuropsychiatric disturbances in presumed late-onset cobalamin C disease. Arch Neurol. 2003;60(10):1457-1462.
9. Lewis AL, Pelic C, Kahn DA. Malignant catatonia in a patient with bipolar disorder, B12 deficiency, and neuroleptic malignant syndrome: one cause or three? J Psychiatr Pract. 2009;15(5):415-422.
10. Rajkumar AP, Jebaraj P. Chronic psychosis associated with vitamin B12 deficiency. J Assoc Physicians India. 2008;56:115-116.
11. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Assoc J. 2001;3(9):701-703.
12. Smith R, Oliver RA. Sudden onset of psychosis in association with vitamin-B12 deficiency. Br Med J. 1967;3(5556):34.
13. Russell RM, Baik HW. Clinical implications of vitamin B12 deficiency in the elderly. Nutrition in Clinical Care. 2001;4(4):214-220.
14. Sharabi A, Cohen E, Sulkes J, et al. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003; 56(6):635-638.
15. Chalmers RA, Bain MD, Costello I. Oral cobalamin therapy. Lancet. 2000;355(9198):148.
16. Borchardt J, Malnick S. Sublingual cobalamin for pernicious anaemia. Lancet. 1999;354(9195):2081.
17. Seal EC, Metz J, Flicker L, et al. A randomized, double-blind, placebo-controlled study of oral vitamin B12 supplementation in older patients with subnormal or borderline serum vitamin B12 concentrations. J Am Geriatr Soc. 2002;50(1):146-151.
18. Erkurt MA, Aydogdu I, Dikilitas M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135.
19. Andrès E, Kaltenbach G, Noel E, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption: a study of 30 patients. Clin Lab Haematol. 2003;25(3):161-166.
20. Wellmer J, Sturm KU, Herrmann W, et al. Oral treatment of vitamin B12 deficiency in subacute combined degeneration [in German]. Nervenarzt. 2006;77(10):1228-1231.
21. Lederle FA. Oral cobalamin for pernicious anemia. Medicine‘s best kept secret? JAMA. 1991;265(1):94-95.
22. Chalouhi C, Faesch S, Anthoine-Milhomme MC, et al. Neurological consequences of vitamin B12 deficiency and its treatment. Pediatr Emerg Care. 2008;24(8):538-541.
23. Andrès E, Federici L, Affenberger S, et al. B12 deficiency: a look beyond pernicious anemia. J Fam Pract. 2007;56(7):537-542.
24. Aaron S, Kumar S, Vijayan J, et al. Clinical and laboratory features and response to treatment in patients presenting with vitamin B12 deficiency related neurological syndromes. Neurol India. 2005;53(1):55-58.
25. Saperstein DS, Wolfe GI, Gronseth GS, et al. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60(9):1296-1301.
26. Tsai AC, Morel CF, Scharer G, et al. Late-onset combined homocystinuria and methylmalonic aciduria (cblC) and neuropsychiatric disturbance. Am J Med Genet A. 2007;143A(20):2430-2434.
27. Brett AS, Roberts MS. Screening for vitamin B12 deficiency in psychiatric patients. J Gen Intern Med. 1994;9(9):522-524.
28. Kaltenbach G, Noblet-Dick M, Barnier-Figue G, et al. Early normalization of low vitamin B12 levels by oral cobalamin therapy in three older patients with pernicious anemia. J Am Geriatr Soc. 2002;50(11):1914-1915.
What to do when your depressed patient develops mania
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
Big changes in practice, but none for the better
Dr. Nasrallah’s approach to psychiatry, especially to the real problems confronting us, is both professionally astute and realistic.
I have been in practice for more than 60 years and have seen tremendous changes—none for the better— in the intrusion of insurance tyranny and government overregulation. Dr. Nasrallah’s comments about obstacles created from the system, regardless of so-called “parity,” hit the mark. The insurance companies control everything by setting fees so low and documentation requirements so high that there is little time to focus on the dynamics of the patient population. Unqualified people are deciding whether or not the medications we prescribe will be paid for.
To illustrate what I think of some of the changes, here is what I have observed:
• Electroconvulsive therapy for severe depression has been “rediscovered,” but for decades those of us who used it selectively, with good results, were considered to be practicing quackery.
• Dr. Nasrallah’s recent article on development of immediate treatment plans for a patient given a diagnosis of schizophrenia (Current Psychiatry. 2015;14(5):32-34,36-40,42 [http://bit.ly/1GLGtrZ]) reminds me of a time when it was thought that you couldn’t diagnose schizophrenia until symptoms had been present for 6 months. (Had they ever heard of the work of Eugen Bleuler?)
• At a national meeting 20 years ago, I raised the question of why every other specialty of medicine stressed early diagnosis and treatment but psychiatry did not—and, instead, was doing the opposite. The speaker did not have the courtesy to answer my question.
Keep up the good work! We need people such as Dr. Nasrallah to educate the public about how government and insurance companies are destroying our specialty.
Dr. Nasrallah’s approach to psychiatry, especially to the real problems confronting us, is both professionally astute and realistic.
I have been in practice for more than 60 years and have seen tremendous changes—none for the better— in the intrusion of insurance tyranny and government overregulation. Dr. Nasrallah’s comments about obstacles created from the system, regardless of so-called “parity,” hit the mark. The insurance companies control everything by setting fees so low and documentation requirements so high that there is little time to focus on the dynamics of the patient population. Unqualified people are deciding whether or not the medications we prescribe will be paid for.
To illustrate what I think of some of the changes, here is what I have observed:
• Electroconvulsive therapy for severe depression has been “rediscovered,” but for decades those of us who used it selectively, with good results, were considered to be practicing quackery.
• Dr. Nasrallah’s recent article on development of immediate treatment plans for a patient given a diagnosis of schizophrenia (Current Psychiatry. 2015;14(5):32-34,36-40,42 [http://bit.ly/1GLGtrZ]) reminds me of a time when it was thought that you couldn’t diagnose schizophrenia until symptoms had been present for 6 months. (Had they ever heard of the work of Eugen Bleuler?)
• At a national meeting 20 years ago, I raised the question of why every other specialty of medicine stressed early diagnosis and treatment but psychiatry did not—and, instead, was doing the opposite. The speaker did not have the courtesy to answer my question.
Keep up the good work! We need people such as Dr. Nasrallah to educate the public about how government and insurance companies are destroying our specialty.
Dr. Nasrallah’s approach to psychiatry, especially to the real problems confronting us, is both professionally astute and realistic.
I have been in practice for more than 60 years and have seen tremendous changes—none for the better— in the intrusion of insurance tyranny and government overregulation. Dr. Nasrallah’s comments about obstacles created from the system, regardless of so-called “parity,” hit the mark. The insurance companies control everything by setting fees so low and documentation requirements so high that there is little time to focus on the dynamics of the patient population. Unqualified people are deciding whether or not the medications we prescribe will be paid for.
To illustrate what I think of some of the changes, here is what I have observed:
• Electroconvulsive therapy for severe depression has been “rediscovered,” but for decades those of us who used it selectively, with good results, were considered to be practicing quackery.
• Dr. Nasrallah’s recent article on development of immediate treatment plans for a patient given a diagnosis of schizophrenia (Current Psychiatry. 2015;14(5):32-34,36-40,42 [http://bit.ly/1GLGtrZ]) reminds me of a time when it was thought that you couldn’t diagnose schizophrenia until symptoms had been present for 6 months. (Had they ever heard of the work of Eugen Bleuler?)
• At a national meeting 20 years ago, I raised the question of why every other specialty of medicine stressed early diagnosis and treatment but psychiatry did not—and, instead, was doing the opposite. The speaker did not have the courtesy to answer my question.
Keep up the good work! We need people such as Dr. Nasrallah to educate the public about how government and insurance companies are destroying our specialty.
A nod to a ‘psychiatry great’
In his recent editorial, “Is there only 1 neurobiologic psychiatric disorder, with different clinical expressions?” (Current Psychiatry. 2015;14(7):10-12 [http://bit.ly/1INCvxw]), Dr. Nasrallah presents convincing evidence for such a conclusion. However, he did not mention that a somewhat similar concept was established by one of psychiatry’s greats, Karl Menninger, MD, more than 50 years ago. I’m referring to Menninger’s unitary concept of mental illness, espoused in his book, The Vital Balance.1 Dr. Menninger was, of course, founder of the Menninger Clinic in Topeka, Kansas, which now thrives in Houston, Texas, at Baylor College of Medicine. Like Freud, who predicted that advances in viewing the brain will someday help us understand it better than he could at the time, Dr. Menninger perhaps was ahead of his time in his perspective on mental illness.
We should appreciate Dr. Nasrallah for putting together the research that provides an updated, somewhat revised view of Dr. Menninger’s theory.
Reference
1. Menninger K. The vital balance. New York, NY: Viking; 1963.
In his recent editorial, “Is there only 1 neurobiologic psychiatric disorder, with different clinical expressions?” (Current Psychiatry. 2015;14(7):10-12 [http://bit.ly/1INCvxw]), Dr. Nasrallah presents convincing evidence for such a conclusion. However, he did not mention that a somewhat similar concept was established by one of psychiatry’s greats, Karl Menninger, MD, more than 50 years ago. I’m referring to Menninger’s unitary concept of mental illness, espoused in his book, The Vital Balance.1 Dr. Menninger was, of course, founder of the Menninger Clinic in Topeka, Kansas, which now thrives in Houston, Texas, at Baylor College of Medicine. Like Freud, who predicted that advances in viewing the brain will someday help us understand it better than he could at the time, Dr. Menninger perhaps was ahead of his time in his perspective on mental illness.
We should appreciate Dr. Nasrallah for putting together the research that provides an updated, somewhat revised view of Dr. Menninger’s theory.
In his recent editorial, “Is there only 1 neurobiologic psychiatric disorder, with different clinical expressions?” (Current Psychiatry. 2015;14(7):10-12 [http://bit.ly/1INCvxw]), Dr. Nasrallah presents convincing evidence for such a conclusion. However, he did not mention that a somewhat similar concept was established by one of psychiatry’s greats, Karl Menninger, MD, more than 50 years ago. I’m referring to Menninger’s unitary concept of mental illness, espoused in his book, The Vital Balance.1 Dr. Menninger was, of course, founder of the Menninger Clinic in Topeka, Kansas, which now thrives in Houston, Texas, at Baylor College of Medicine. Like Freud, who predicted that advances in viewing the brain will someday help us understand it better than he could at the time, Dr. Menninger perhaps was ahead of his time in his perspective on mental illness.
We should appreciate Dr. Nasrallah for putting together the research that provides an updated, somewhat revised view of Dr. Menninger’s theory.
Reference
1. Menninger K. The vital balance. New York, NY: Viking; 1963.
Reference
1. Menninger K. The vital balance. New York, NY: Viking; 1963.