User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Teaching trainees how to discern professional boundaries
Psychiatrists often serve as risk-management consultants for our medical colleagues. As part of this role, psychiatrists working with trainees— including resident physicians, medical students, and physician assistant students— have an opportunity to emphasize the importance of professional boundaries.1 Discussing appropriate professional boundaries and describing what might represent a violation of these boundaries is meaningful because a good understanding of these concepts promotes high-quality treatment and minimizes professional liability.2
Physical boundaries
Psychiatric patients might be agitated or display potentially dangerous behaviors; discussing the importance of body language and contact between physicians and their patients is, therefore, first and foremost, a matter of safety. Students who can recognize the signs and symptoms of agitation and maintain a safe distance between themselves and their patients are less likely to be injured.
Addressing romantic and sexual relationships between patients and their health care providers also is necessary. One study reported that 21% of medical students surveyed might not regard sexual contact with a patient as inappropriate.3 An adequate discussion of this topic is necessary to protect trainees and patients from a catastrophic misstep.
Emotional boundaries
Maintaining appropriate emotional boundaries is necessary in psychiatry. Given the prevalence of mental illness and substance abuse, many trainees have personal experience with psychiatric illness outside of their training. Discussing issues of transference and countertransference with students will prepare them for intense emotional reactions they will experience while working in psychiatry. Students who feel comfortable recognizing their own countertransference feelings and discussing them in supervision with their attending psychiatrist will be more successful in addressing the complex interpersonal challenges that their patients face.
Personal and informational boundaries
Discussing personal and informational boundaries can protect trainees from uncomfortable experiences in their non-clinical lives. Although, in previous decades, we needed to discourage students only from sharing their home address and telephone number with patients, the Internet and social media have made it easier for patients to discover personal information about their treatment team. Addressing issues related to social networks and instructing students on how to appropriately address and decline requests for personal information can prevent unwanted boundary crossings.
Psychiatrists are well suited to discuss these issues with trainees. In doing so, we can help them become knowledgeable health care providers—no matter which medical discipline they specialize in.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Duckworth KS, Kahn MW, Gutheil TG. Roles, quandaries, and remedies: teaching professional boundaries to medical students. Harv Rev Psychiatry. 1994;1(5):266-270.
2. Gutheil TG, Gabbard GO. The concept of boundaries in clinical practice: theoretical and risk-management dimensions. Am J Psychiatry. 1993;150(2):188-196.
3. White GE. Medical students’ learning needs about setting and maintaining social and sexual boundaries: a report. Med Educ. 2003;37(11):1017-1019.
Psychiatrists often serve as risk-management consultants for our medical colleagues. As part of this role, psychiatrists working with trainees— including resident physicians, medical students, and physician assistant students— have an opportunity to emphasize the importance of professional boundaries.1 Discussing appropriate professional boundaries and describing what might represent a violation of these boundaries is meaningful because a good understanding of these concepts promotes high-quality treatment and minimizes professional liability.2
Physical boundaries
Psychiatric patients might be agitated or display potentially dangerous behaviors; discussing the importance of body language and contact between physicians and their patients is, therefore, first and foremost, a matter of safety. Students who can recognize the signs and symptoms of agitation and maintain a safe distance between themselves and their patients are less likely to be injured.
Addressing romantic and sexual relationships between patients and their health care providers also is necessary. One study reported that 21% of medical students surveyed might not regard sexual contact with a patient as inappropriate.3 An adequate discussion of this topic is necessary to protect trainees and patients from a catastrophic misstep.
Emotional boundaries
Maintaining appropriate emotional boundaries is necessary in psychiatry. Given the prevalence of mental illness and substance abuse, many trainees have personal experience with psychiatric illness outside of their training. Discussing issues of transference and countertransference with students will prepare them for intense emotional reactions they will experience while working in psychiatry. Students who feel comfortable recognizing their own countertransference feelings and discussing them in supervision with their attending psychiatrist will be more successful in addressing the complex interpersonal challenges that their patients face.
Personal and informational boundaries
Discussing personal and informational boundaries can protect trainees from uncomfortable experiences in their non-clinical lives. Although, in previous decades, we needed to discourage students only from sharing their home address and telephone number with patients, the Internet and social media have made it easier for patients to discover personal information about their treatment team. Addressing issues related to social networks and instructing students on how to appropriately address and decline requests for personal information can prevent unwanted boundary crossings.
Psychiatrists are well suited to discuss these issues with trainees. In doing so, we can help them become knowledgeable health care providers—no matter which medical discipline they specialize in.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatrists often serve as risk-management consultants for our medical colleagues. As part of this role, psychiatrists working with trainees— including resident physicians, medical students, and physician assistant students— have an opportunity to emphasize the importance of professional boundaries.1 Discussing appropriate professional boundaries and describing what might represent a violation of these boundaries is meaningful because a good understanding of these concepts promotes high-quality treatment and minimizes professional liability.2
Physical boundaries
Psychiatric patients might be agitated or display potentially dangerous behaviors; discussing the importance of body language and contact between physicians and their patients is, therefore, first and foremost, a matter of safety. Students who can recognize the signs and symptoms of agitation and maintain a safe distance between themselves and their patients are less likely to be injured.
Addressing romantic and sexual relationships between patients and their health care providers also is necessary. One study reported that 21% of medical students surveyed might not regard sexual contact with a patient as inappropriate.3 An adequate discussion of this topic is necessary to protect trainees and patients from a catastrophic misstep.
Emotional boundaries
Maintaining appropriate emotional boundaries is necessary in psychiatry. Given the prevalence of mental illness and substance abuse, many trainees have personal experience with psychiatric illness outside of their training. Discussing issues of transference and countertransference with students will prepare them for intense emotional reactions they will experience while working in psychiatry. Students who feel comfortable recognizing their own countertransference feelings and discussing them in supervision with their attending psychiatrist will be more successful in addressing the complex interpersonal challenges that their patients face.
Personal and informational boundaries
Discussing personal and informational boundaries can protect trainees from uncomfortable experiences in their non-clinical lives. Although, in previous decades, we needed to discourage students only from sharing their home address and telephone number with patients, the Internet and social media have made it easier for patients to discover personal information about their treatment team. Addressing issues related to social networks and instructing students on how to appropriately address and decline requests for personal information can prevent unwanted boundary crossings.
Psychiatrists are well suited to discuss these issues with trainees. In doing so, we can help them become knowledgeable health care providers—no matter which medical discipline they specialize in.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Duckworth KS, Kahn MW, Gutheil TG. Roles, quandaries, and remedies: teaching professional boundaries to medical students. Harv Rev Psychiatry. 1994;1(5):266-270.
2. Gutheil TG, Gabbard GO. The concept of boundaries in clinical practice: theoretical and risk-management dimensions. Am J Psychiatry. 1993;150(2):188-196.
3. White GE. Medical students’ learning needs about setting and maintaining social and sexual boundaries: a report. Med Educ. 2003;37(11):1017-1019.
1. Duckworth KS, Kahn MW, Gutheil TG. Roles, quandaries, and remedies: teaching professional boundaries to medical students. Harv Rev Psychiatry. 1994;1(5):266-270.
2. Gutheil TG, Gabbard GO. The concept of boundaries in clinical practice: theoretical and risk-management dimensions. Am J Psychiatry. 1993;150(2):188-196.
3. White GE. Medical students’ learning needs about setting and maintaining social and sexual boundaries: a report. Med Educ. 2003;37(11):1017-1019.
A physician who feels hopeless and worthless and complains of pain
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Feeling hopeless
Dr. D, age 33, a white, male physician, presents with worsening depression, suicidal ideation, and somatic complaints. Dr. D says his personal life has become increasingly unhappy. He describes the pressures of a busy practice and conflict with his wife about his availability to her. He is feeling financial pressure and general disappointment about practicing medicine. Lack of recreational activities and close friends and absent spiritual life has led to feelings of isolation and depression.
Dr. D reports difficulty falling asleep, waking up early, and feeling fatigued. He describes obsessive, negative thoughts about his work and his personal life; he is anxious and tense. Dissatisfied and exhausted, he says he feels hopeless and empty and has become preoccupied with thoughts of death.
Dr. D describes musculoskeletal tension in the neck, shoulders, and face, with pain in the back of the neck. When the depressive symptoms or pain are particularly severe, he admits that his attention to critical information lapses. When interacting with his patients, he has missed important nuances about medication side effects, for example, frustrating his patients and himself.
Dr. D and his wife do not have children. His mother and paternal grandfather had depression, but Dr. D has no family history of suicide or drug or alcohol abuse. He has no significant medical conditions, and is not taking any medications. Dr. D drinks 1 or 2 cups of caffeinated coffee a day. He does not smoke, use recreational drugs, or drink alcohol regularly.
What would be your next step in treating Dr. D?
a) alert the state medical board about his suicidal ideation
b) recommend inpatient treatment
c) refer Dr. D to a clinician who has experience treating physicians
d) formulate a suicide risk assessment
The authors’ observation
Assessment of the suicidal physician is complex. It requires patience and ability to understand the source and the extent of the physician’s desperation and suffering. Not all psychiatrists are well suited to working with patients who also are peers. An experienced clinician, who has confronted the challenges of practice and treated individuals from many professions, could be better equipped than a recent graduate. Physician− patients might not be forthcoming about the extent of their suicidal thinking, because they fear involuntary hospitalization and jeopardizing their career.1
The evaluating clinician must be thorough and clear, and able to facilitate a trusting relationship. The ill physician should be encouraged to express suicidal ideation freely—without judgments, restrictions, or threats—to a trusted psychiatrist. Questions should be clear without possibility of misinterpretation. Ask:
• “Do you have thoughts of death, dying, or wanting to be dead?”
• “Do you think about suicide?”
• “Do you feel you might act on those thoughts?”
• “What keeps you safe?”
Physicians and other health professional have a higher relative risk of suicide (Table 1).2 Hospitalization should be considered and the decision based on the severity of the illness and the associated risk. Dr. D has several risk factors for suicide, including marital discord, pain, professional demands, and access to lethal means (Table 2).1,3,4
HISTORY Pain and disappointment
After medical school, Dr. D completed residency and joined a large clinic with outpatient and inpatient services. His supervisor was pleased with his work and encouraged him to take on more responsibility. However, within the first years of practice, his mood slowly deteriorated; he came to realize that he was deeply sad and, likely, clinically depressed.
Dr. D describes his parents as detached and emotionally unavailable to him. His mother’s depression sometimes was severe enough that she stayed in her bedroom, isolating herself from her son. Dr. D did not feel close to either of his parents; his mother continued to work despite the depression, which meant that both parents were away from home for long hours. Dr. D became interested in service to others and found that those he served responded to him in a positive way. Service to others became a way to feel recognized, appreciated, respected, and even loved.
Dr. D’s depressive symptoms became worse when he discovered his wife was having an affair. The depression became so debilitating that he requested, and was granted, an 8-week medical leave. Once away from the daily pressures of work, his depression improved somewhat, but conflict with his wife intensified and thoughts of suicide became more frequent. Soon afterward, Dr. D and his wife separated and he moved out. His supervisor recommended that Dr. D obtain treatment, but it was only after the separation that Dr. D decided to seek psychiatric care.
What type of psychotherapy is recommended for physicians with suicidal ideation?
a) psychodynamic psychotherapy
b) person-centered therapy
c) cognitive-behavioral therapy (CBT)
d) dialectical behavior therapy (DBT)
The authors’ observation
Reassure your physician−patients that it is safe and reasonable to take personal time off from work to recover from any illness, whether physical or mental. Consider the best treatment approaches to ensure patient’s safety, comfort, and rapid recovery. A critical part of treatment is exploring and identifying changes needed to achieve a life that is compatible with the ideal self, the patient’s view of himself, his beliefs, goals, and life’s meaning.
Physicians are at particular risk of losing the ideal self.5 Loss of the ideal self is common, and can be life threatening. Person-centered psychotherapy, CBT, supportive psychotherapy, DBT, and pharmacotherapy are used to lessen emotional distress and promote adaptive coping strategies, but approaches are different. Short-term counseling can reduce the effects of job stress,6 but a longer-term intervention likely is necessary for a mood disorder with thoughts of self-harm.
CBT emphasizes helping physicians recognize cognitive distortions and finding solutions. The behavioral aspects of CBT promote physical and mental relaxation, which is helpful in easing muscle tension, lowering heart rate, and decreasing the tendency to hyperventilate during stress.7 Mindfulness-based stress reduction programs can provide physical and mental benefits.8 DBT, a type of behavioral therapy, combines mindfulness, acceptance of the current state, skills to regulate emotion, and positive interpersonal relationship strategies.9
Pharmacotherapy should be focused on improving sleep, anxiety, appetite, and mood. Your patient may have other symptoms that need to be addressed: Ask what symptom bothers your patient the most, then work to provide solutions. Some interventions could promote adaptive coping strategies to identify ways to increase perceived control over the work day.10
TREATMENT Self-exploration
The treatment team instructs Dr. D to take a personal inventory of the elements of his ideal self, along the lines suggested in person-centered therapy.11,12 How did Dr. D envision his practice when he was in residency? What other domains of life were important to him? When Dr. D comes back with his list, the need for change is discussed and the process for incorporating these elements into his life begins. He begins to realize that returning to the elements of his ideal self brought opportunities, friendship, love, and faith back into his life.13,14
Maintaining balance between work responsibilities and pleasurable activities is part of achieving the ideal self. Recreation, social support, and exercise decrease the experience of stress and promote wellness.15,16
An important discussion centers on Dr. D’s risk of losing meaning in life after distancing himself from his original motivation to help people though practicing medicine. Dr. D understands that the distance between his expectations and dreams as a student and his current reality contributed to his depression.17 These conversations and changes in behavior brings Dr. D’s actual life closer to this ideal self, reducing self-discrepancy and lessening negative mood.18
The treating psychiatrist is aware of the reporting requirements to the state medical board, which are discussed with Dr. D. No report is deemed necessary.
The authors’ observation
Dr. D’s treatment course was challenging and required a multi-component approach. Establishing trust, while defining the limits of confidentiality, formed the foundation for the therapeutic relationship. The treatment provider asked for names of colleagues or friends to be contacted in case of an emergency. Dr. D chose his physician supervisor and agreed that the psychiatrist could contact the supervisor and vice versa.
Medication was prescribed at the end of the first session to begin to address anxiety and sleep problems. The initial medication was fluvoxamine, 50 mg/d, for anxiety and depression, clonazepam, 0.5 mg/d for anxiety, and zolpidem, 10 mg/d, for sleep. Adjustments were made in the dosage of antidepressant and responses monitored closely until the therapeutic dosage was reached with minimal side effects. Sleep improved, irritability lessened, and Dr. D’s obsessive, negative thinking and depression improved. Deeper, restorative sleep also began to reduce physical tension and pain. Improved sleep and decreased measures of depression are associated with significantly reduced risk of suicide.19
A treating psychiatrist should be aware of the state medical board requirements. In Ohio, where this case unfolded, reporting is required when the physician−patient is deemed unable to practice medicine according to acceptable and prevailing standards of care.20
Relieving tension and somatic complaints
An important part of the treatment plan consisted of managing chronic muscle tension and pain. We decided to front-load treatment, addressing the severe depression, anxiety, and pain simultaneously. Even moderate pain relief would give Dr. D a greater sense of control and improve his mood.
Dr. D understood that a return to normal biorhythms was necessary to form the foundation for the next step of therapy.21 The treatment team introduced mindful breathing, but Dr. D questioned how something so simple could lift severe depression. Focused, mindful breathing was not a cure, but a first step in regaining control over the current disarray of physical and emotional variations. We encouraged daily practice and he agreed to 5 practice sessions per week.
Next, the treatment team introduced progressive relaxation. Again, the simplicity of this process of tensing and relaxing groups of muscles was met with disbelief. Our therapist explained that voluntarily producing muscle tension facilitates the relaxation response of both the mind and the body. The mind first commands the muscles to do what it does easily— “tense”; then is asked to elicit what is more difficult—“relax.” Repetition of the simple commands “tense—relax” in the arms, legs, back, abdomen, shoulders, neck, and face establishes a calming rhythm, again increasing the sense of control.22 We strongly encouraged daily practice of this exercise and Dr. D committed to the mindful breathing and relaxation exercise.
OUTCOME Recovery, maintenance
Dr. D and his psychotherapist address his anger, all-or-nothing thinking, and loneliness. Grief over his failed marriage was identified, giving them an opportunity to explore this loss and past, perceived losses of his parents’ affection in the context of the therapeutic relationship. Supportive therapy promoted ways to fulfill his ideal self.
Treatment lasted 2 years. Dr. D’s prior depressive episode indicates a need for maintenance medication. The antidepressant is continued and, with help from supportive psychotherapy, stress management, 8 weeks away from work, and the life changes mentioned above, our patient has not had a relapse.
Bottom Line
Depression and thoughts of suicide are common among physicians. Grant time off from work and reassure the physician that he (she) is entitled to seek medical treatment without repercussions. Adapt the type of psychotherapy to the physician’s specific concerns. Because physicians are at particular risk for loss of the ideal self, first consider person-centered therapy.
Related Resources
• Vanderbilt Center for Professional Health. www.mc.vanderbilt.edu/cph.
• Federation of State Physician Health Programs, Inc. www.fsphp.org.
Drug Brand Names
Clonazepam • Klonopin Fluvoxamine • Luvox Zolpidem • Ambien
AcknowledgementThe authors wish to acknowledge the contribution of Rachel Sieke, BS, Research Assistant, Department of Psychiatry, University of Toledo Medical Center, Toledo, Ohio.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
1. Bright RP, Krahn L. Depression and suicide among physicians. Current Psychiatry. 2011;10(4):16-17,25-26,30.
2. Burnett C, Maurer J, Dosemecl M. Mortality by occupation, industry, and cause of death: 24 reporting states (1984-1988). Centers for Disease Control and Prevention. http://www. cdc.gov/niosh/docs/97-114. Published June 1997. Accessed October 3, 2014.
3. Silverman MM. Physicians and suicide. In: Goldman LS, Myers M, Dickstein LJ, eds. The handbook of physician health: essential guide to understanding the health care needs of physicians. Chicago, IL: American Medical Association; 2000:95-117.
4. Lindeman S, Laara E, Hakko H, et al. A systematic review on gender-specific suicide mortality in medical doctors. Br J Psychiatry. 1996;168(3):274-279.
5. Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97(1):90-113.
6. Rø KE, Gude T, Tyssen R, et al. Counselling for burnout in Norwegian doctors: one year cohort study. BMJ. 2008;337:a2004. doi: 10.1136/bmj.a2004.
7. Broquet KE, Rockey PH. Teaching residents and program directors about physician impairment. Acad Psychiatry. 2004;28(3):221-225.
8. Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: a review of empirical studies of mindfulness-based stress reduction (MBSR). Complement Ther Clin Pract. 2009;15(2):61-66.
9. Robins C, Schmidt H, Linehan MM. Dialectical behavior therapy synthesizing radical acceptance with skillful means. In: Hayes S, Follette V, Linehan M, eds. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York, NY: Guilford Press; 2004:30-44.
10. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
11. Nevid JS, Rathus SA, Greene B. Abnormal psychology in a changing world, 7th ed. Upper Saddle River, NJ: Prentice- Hall; 2008:111-112.
12. Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951.
13. Selimbegovic´ L, Chatard A. The mirror effect: self-awareness alone increases suicide thought accessibility. Conscious Cogn. 2013;22(3):756-764.
14. Cornette M. Staff perspective: self-discrepancy and suicidal ideation. Center for Deployment Psychology. http:// www.deploymentpsych.org/blog/staff-perspective-self-discrepancy-and-suicidal-ideation. Published February 19, 2014. Accessed August 7, 2014.
15. Shanafelt TD, Novotny P, Johnson ME, et al. The well-being and personal wellness promotion strategies of medical oncologists in the North Central Cancer Treatment Group. Oncology. 2005;68(1):23-32.
16. Meldrum H. Exemplary physicians’ strategies for avoiding burnout. Health Care Manag (Frederick). 2010;29(4):324-331.
17. Orbach I, Mikulincer M, Stein D, et al. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107(3):435-439.
18. Higgins ET. Self-discrepancy: a theory related self and affect. Psychol Rev. 1987;94(3):319-340.
19. Christensen H, Batterham PJ, Mackinnon AJ, et al. Predictors of the risk factors for suicide identified by the interpersonal-psychological theory of suicidal behaviour. Psychiatry Res. 2014;219(2):290-297.
20. Ohio State Medical Board. Section 4731.22 (B), Rule 4731-18- 01. 2014.
21. McGrady A, Moss D. Pathways to illness, pathways to health. New York, NY: Springer; 2013.
22. Davis M, Eshelman ER, McKay M. The relaxation and stress reduction workbook, 6th ed. Oakland, CA: New Harbinger Publications, Inc; 2008.
Grapefruit juice and psychotropics: How to avoid potential interactions
Ms. H, age 42, was given a diagnosis of bipolar disorder 10 years ago and has been taking carbamazepine, 1,200 mg/d, and olanzapine, 10 mg/d, for the past 2 years. She has not experienced a mood episode while on this regimen, and her carbamazepine level was 9.2 μg/mL 6 months ago. The only adverse effect she experienced was weight gain of approximately 10 lb. Ms. H takes a calcium supplement, but no other medications.
Ms. H reports to her psychiatrist that, for the past few days, she has been feeling nauseated, fatigued, and dizzy, but has continued taking her medications as prescribed. Her carbamazepine level is found to be 13.1 μg/mL. Ms. H states she has not started any new medications or supplements; her serum creatinine and liver function test results are within normal limits.
Upon further questioning, Ms. H says that an upper respiratory infection has been “going around her office,” so she increased her vitamin C intake by drinking 2 glasses of grapefruit juice a day (she doesn’t like orange juice). She has heard grapefruit juice can cause problems with some drugs so she is careful not to drink it at the same time she takes her medications. Her psychiatrist recognizes there may be a drug interaction involved, and recommends Ms. H hold her carbamazepine for 1 day and not consume any more grapefruit juice. A few days later, she reports feeling much better during a follow-up call and she makes an appointment to have her carbamazepine level rechecked in a we
Although grapefruit products are high in vitamins and low in calories, they can be associated with potentially serious drug interactions. The interaction between grapefruit juice and the calcium channel blocker felodipine was discovered inadvertently >20 years ago; since that time, possible interactions with >85 medications have been identified.1 Interactions with grapefruit products are complicated because, although most result in increased drug exposure, reduced exposure of the medication also can occur. Additionally, the degree and clinical significance of the interaction varies among individuals and from one drug to another.
Mechanism of action
Most interactions with grapefruit products are thought to result from the inhibition of intestinal cytochrome P450 3A4 (CYP3A4). CYP3A4 is involved in the metabolism of numerous drugs, and is the most abundant cytochrome P450 enzyme in the liver and epithelial cells lining the intestine.2 Although hepatic CYP3A4 is thought to be minimally affected by grapefruit, inhibition of intestinal CYP3A4 can result in an overall increase in bioavailability of medications that are substrates and raise the risk of potential toxicity.3 Grapefruit contains various chemicals collectively known as furanocoumarins, which are largely responsible for inhibition of intestinal CYP3A4.4 Additionally, Seville oranges and the pomelo (a large, sweet grapefruit-like citrus fruit) also contain furanocoumarins and could have a similar effect, warranting caution with certain medications.5
Inhibition of CYP3A4 by furanocoumarins cannot be reversed, and new enzymes must be synthesized to return to the previous level of function.6 Therefore, drug interactions resulting from CYP3A4 inhibition can last for as long as 72 hours after ingesting grapefruit products.7 Separating consumption of grapefruit products and medication administration will not help manage this interaction.
Grapefruit products also could affect drug disposition through effects on various drug transporters. Decreased systemic exposure to certain medications could occur through grapefruit’s inhibition of organic anion-transporting polypeptides (OATPs). OATPs form a family of drug uptake transporters found in the intestine, liver, kidney, and brain.8 For drugs that are substrates of OATPs, grapefruit’s inhibition of this transporter can result in decreased absorption and a resulting decrease in efficacy. Flavanoids in grapefruit, such as naringin, inhibit OATPs, which is competitive in nature.9 Unlike the irreversible inhibition of CYP3A4 by furanocoumarins, flavanoids effects on OATPs have been shown to decrease within 4 hours.10
No psychotropic medications have been identified as being susceptible to this interaction, but for those medications affected—including fexofenadine and levothyroxine—separating consumption of grapefruit and medication administration by 4 hours could avoid this interaction.11 Additional data indicate that orange juice and apple juice could have similar effects on OATPs.12
Perhaps the most well-known drug transporter, P-glycoprotein is part of the multidrug-resistant subfamily of transporters. It is located throughout the body, including in the intestine, kidneys, liver, and blood-brain barrier. P-glycoprotein acts as an export pump to decrease the cellular concentration of many different drug substrates, and many agents can alter P-glycoprotein’s expression or function.
Small changes in P-glycoprotein’s activity can result in substantial changes in the disposition of substrates, which can include certain antineoplastics and antiretrovirals. Most reports have found grapefruit juice inhibits P-glycoprotein-mediated efflux; however, there also are reports of transporter activation.6 Additionally, P-glycoprotein and CYP3A4 share many substrates, so it can be difficult to isolate the contribution of P-glycoprotein to grapefruit−drug interactions.13 The effect of grapefruit on P-glycoprotein activity has been difficult to fully elucidate; more studies are needed.
Grapefruit consumption and its effect
Drug interactions can occur by consuming commercially produced grapefruit juice and juice from concentrate, as well as freshly squeezed juice and grapefruit segments.14 CYP3A4-inhibiting furanocoumarins also have been isolated in grapefruit peel; it is not known, however, whether items made from peel (marmalade, candied peel) contain concentrations high enough to pose a risk of a drug interaction.14 Contributing to the unpredictability of grapefruit-drug interactions, the amount or concentration of furanocoumarins can vary among grapefruit products and brands.15 This variability can be influenced by the variety or maturity of the fruit and the fruit’s exposure to environmental stress.4
The frequency of consuming a grapefruit product can influence the degree of a drug interaction. In general, consuming one 8-oz glass of grapefruit juice or the segments from a whole grapefruit is enough to alter a susceptible drug’s pharmacokinetics.14 Regular grapefruit product consumption, however, can result in an overall greater effect.16,17
Lilja et al16 conducted a randomized, 4-phase, crossover study to look at the effect of grapefruit juice dose on kinetics of triazolam. Grapefruit juice was found to increase the mean area under the concentration-time curve (AUC) of triazolam compared with water, but no difference was found between single glasses of normal-strength and double-strength grapefruit juice. However, repeated consumption of double-strength grapefruit juice (200 mL, 3 times/d for 3 days) increased triazolam’s mean AUC by 143%, compared with an increase of 49% with just a single 200-mL glass of double-strength juice.16 Recurrent consumption of grapefruit juice (8 oz, 3 times/d for 6 days) also was found to increase the kinetics of the antihypertensive felodipine more than a single glass of grapefruit juice.17
Clinical consequences of an interaction between a drug and grapefruit can be difficult to predict. Drug concentration changes caused by a grapefruit interaction could vary based on interindividual differences. The amount and activity of intestinal CYP3A4 can vary from person to person, and can be influenced by genetic polymorphisms in addition to race, age, and environmental variables.18 Interindividual sensitivity to a change in a drug’s concentration also will differ, and patient-specific factors, such as concomitant drugs or diseases, could influence the likelihood of harm.
Interactions with grapefruit products are not necessarily a “class effect,” and specific drugs within a therapeutic category can be affected (although others might not). Several drug-specific characteristics can help gauge the risk of a clinically relevant interaction with grapefruit, including:
• metabolism through CYP3A4
• low bioavailability
• oral administration
• a narrow therapeutic index.1
For drugs with low bioavailability because of first-pass metabolism, grapefruit’s inhibition of intestinal CYP3A4 can result in a greater relative increase in plasma concentrations compared with a drug with high bioavailability.19
For example, an increase in bioavailability from 5% to 10% will result in a much larger increase in AUC and overall clinical exposure compared with an increase from 85% to 90% even though both represent an absolute increase of 5%. Although a drug does not have to have low oral bioavailability for an interaction to occur, lower bioavailability means that a drug has a higher likelihood of causing a significant interaction because of altered pharmacokinetics. Of note, injectable medications will not interact with grapefruit because metabolism through intestinal CYP3A4 is bypassed and grapefruit does not significantly inhibit hepatic CYP3A4.
Although grapefruit products could alter the pharmacokinetics of susceptible drugs, those changes might not be associated with adverse effects. Therefore, a factor to consider in evaluating a potential interaction with grapefruit is the drug’s therapeutic index and its risk of serious adverse effects. Drugs with a narrow therapeutic index are of particular concern because a significant increase in therapeutic or adverse effects could result from a relatively small increase in the drug’s concentration.7
Which medications are affected?
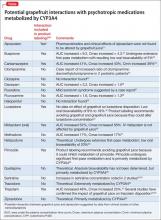
Among medications identified as interacting with grapefruit, some cardiovascular agents and several of the HMG-CoA reductase inhibitors (statins) have garnered the most attention. However, grapefruit also can affect the metabolism of several psychotropic medications through inhibition of intestinal CYP3A4 (Table).16,20-35 Prescribing information for some drugs warns against consuming grapefruit while using the medication. Among CNS agents, buspirone, carbamazepine, lurasidone, pimozide, triazolam, and oral midazolam all have such warnings in their product labeling.
Buspirone currently is not recommended with “large quantities of grapefruit juice.”20 A randomized, 2-phase crossover study looking at the effects of grapefruit juice on buspirone’s pharmacokinetics found that double-strength grapefruit juice (200 mL, administered 3 times/d for 3 days) resulted in a 9.2-fold increase in mean AUC and a 4.3-fold increase in mean Cmax after a single 10-mg buspirone dose.22 Highlighting the wide interindividual variability seen with drug-grapefruit interactions, the increase found in buspirone’s AUC ranged from 3-fold to 20-fold among study participants.22
Carbamazepine product labeling lists grapefruit juice as a CYP3A4 inhibitor that is expected to or has been found to increase plasma levels of the drug.20 Carbamazepine’s bioavailability is influenced by intestinal CYP3A4 activity; in a randomized, 2-phase crossover study of 10 patients with epilepsy, grapefruit juice was found to increase AUC of carbamazepine by 41% and Cmax by 40%.23,36
Lurasidone and pimozide, although not specifically studied, have product labels that recommend avoiding grapefruit juice because it could inhibit metabolism of these agents by CYP3A4.20 Of particular concern is the potential for elevated levels of pimozide to increase the risk of adverse cardiovascular effects including QT interval prolongation.19
Midazolam. Although grapefruit juice does not affect the disposition of IV midazolam, pretreatment with grapefruit juice was found to increase the AUC and Cmax of oral midazolam by 52% and 56%, respectively.30
Other considerations in drug-grapefruit interactions
Cautionary statements about a possible interaction with grapefruit juice for many other psychotropics can be found in commonly used drug information references or online sources. If you are concerned about a possible interaction and avoiding grapefruit products is not feasible, consider a different medication in the same class.
However, you also should consider the level of evidence supporting any purported interaction. Several psychotropic agents do have studies or case reports supporting an interaction with grapefruit, but cautionary statements could be based on theoretical concerns because of a medication’s bioavailability, metabolic pathway, and concern for increased adverse events related to higher drug concentrations. Adding to the confusion, cautionary statements can be found about medications, such as clozapine, that have not been shown to have an interaction with grapefruit juice when studied.
With many of the drugs that have a reported or theoretical interaction with grapefruit, data are inconsistent as to whether the resulting interaction will be clinically relevant. A number of variables relating to the individual patient, grapefruit product, or particular drug can play a role in the significance of an interaction. Additionally, effects on drug disposition can last for a few days after consuming a grapefruit product.
Keep alert to situations of increased risk
Recall that the case patient, Ms. H, presented with an elevated carbamazepine level and suffered resulting adverse effects because of an interaction between the drug and grapefruit juice. Although Ms. H was careful to separate intake of grapefruit juice from carbamazepine administration, grapefruit’s inhibition of intestinal CYP3A4 still was present, leading to the interaction.
It is important for health care professionals to recognize this potential risk and to advise patients regarding possible interactions between medications and grapefruit products.
Related Resources
• U.S. Food and Drug Administration. Grapefruit juice and medicine may not mix. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm292276.htm.
• Hanley MJ, Cancalon P, Widmer WW, et al. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7(3):267-286.
• Andrade C. Fruit juice, organic anion transporting polypeptides, and drug interactions in psychiatry. J Clin Psychiatry. 2014;75(11):e1323-e1325.
Drug Brand Names
Alprazolam • Xanax Lurasidone • Latuda
Buspirone • BuSpar Midazolam • Versed
Carbamazepine • Tegretol Methadone • Dolophine
Clomipramine • Anafranil Nefazodone • Serzone
Clozapine • Clozaril Olanzapine • Zyprexa
Diazepam • Valium Pimozide • Orap
Felodipine • Plendil Quetiapine • Seroquel
Fexofenadine • Allegra Sertraline • Zoloft
Fluoxetine • Prozac Trazodone • Desyrel
Fluvoxamine • Luvox Triazolam • Halcion
Haloperidol • Haldol Ziprasidone • Geodon
Levothyroxine • Levoxyl, Synthroid
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185(4):309-316.
2. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21): 2211-2221.
3. Saito M, Hirata-Koizumi M, Matsumoto M, et al. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28(8):677- 694.
4. Cancalon PF, Barros SM, Haun C, et al. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J Food Sci. 2011;76(4):C543-C548.
5. Pirmohamed M. Drug-grapefruit juice interactions: two mechanisms are clear but individual responses vary. BMJ. 2013;346:f1. doi: 10.1136/bmj.f1.
6. Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability–mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1-9.
7. Stump AL, Mayo T, Blum A. Management of grapefruit-drug interactions. Am Fam Physician. 2006;74(4):605-608.
8. Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(suppl 2):1-5.
9. Bailey DG, Dressker GK, Leak BF, et al. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81(4):495-502.
10. Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362-370.
11. Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70(5): 645-655.
12. Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11-20.
13. Seden K, Dickinson L, Khoo S, et al. Grapefruit-drug interactions. Drugs. 2010;70(18):2373-2407.
14. Bailey DG, Dresser GK, Kreeft JH, et al. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68(5):468-477.
15. De Castro WV, Mertens-Talcott S, Rubner A, et al. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54(1):249-255.
16. Lilja JJ, Kivistö KT, Backman JT, et al. Effect of grapefruit juice on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000;56(5):411-415.
17. Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545-2553.
18. Lin JH, Lu AY. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol. 2001;41:535-567.
19. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000; 38(1):41-57.
20. U.S. Food and Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed July 14, 2014.
21. Yasui, N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;15(2):185-190.
22. Lilja JJ, Kivistö KT, Backman JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64(6):655-660.
23. Garg SK, Kumar N, Bhargava VK, et al. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64(3):286-288.
24. Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: shifting metabolic ratios. J Clin Psychopharm. 1997;17(1):62-63.
25. Lane HY, Jann MW, Chang YC, et al. Repeated ingestion of grapefruit juice does not alter clozapine’s steady-state plasma levels, effectiveness, and tolerability. J Clin Psychiatry. 2001;62(10):812-817.
26. Ozdemir M, Aktan Y, Boydag BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59.
27. DeSilva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281-1285.
28. Hori H, Yoshimura R, Ueda N, et al. Grapefruit juice-fluvoxamine interaction—is it risky or not? J Clin Psychopharmacol. 2003;23(4):422-424.
29. Yasui N, Kondo T, Suzuki A, et al. Lack of significant pharmacokinetic interaction between haloperidol and grapefruit juice. Int Clin Psychopharmacol. 1999;142(2):113-118.
30. Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58(1):20-28.
31. Benmebarek M, Cevaud C, Gex-Fabry M, et al. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004;76(1):55-63.
32. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509-522.
33. Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10(4 pt 3):832-835.
34. Lee AJ, Chan WK, Harralson AF, et al. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999;21(11):1890-1899.
35. Sugimoto K, Araki N, Ohmori M, et al. Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006;62(3):209-215.
36. Fagiolino P, Vazquez M, Olano I, et al. Systemic and presystemic conversion of carbamazepine to carbamazepine- 10-11-epoxide during long term treatment. Journal of Epilepsy and Clinical Neurophysiology. 2006;12(1):13-16.
Ms. H, age 42, was given a diagnosis of bipolar disorder 10 years ago and has been taking carbamazepine, 1,200 mg/d, and olanzapine, 10 mg/d, for the past 2 years. She has not experienced a mood episode while on this regimen, and her carbamazepine level was 9.2 μg/mL 6 months ago. The only adverse effect she experienced was weight gain of approximately 10 lb. Ms. H takes a calcium supplement, but no other medications.
Ms. H reports to her psychiatrist that, for the past few days, she has been feeling nauseated, fatigued, and dizzy, but has continued taking her medications as prescribed. Her carbamazepine level is found to be 13.1 μg/mL. Ms. H states she has not started any new medications or supplements; her serum creatinine and liver function test results are within normal limits.
Upon further questioning, Ms. H says that an upper respiratory infection has been “going around her office,” so she increased her vitamin C intake by drinking 2 glasses of grapefruit juice a day (she doesn’t like orange juice). She has heard grapefruit juice can cause problems with some drugs so she is careful not to drink it at the same time she takes her medications. Her psychiatrist recognizes there may be a drug interaction involved, and recommends Ms. H hold her carbamazepine for 1 day and not consume any more grapefruit juice. A few days later, she reports feeling much better during a follow-up call and she makes an appointment to have her carbamazepine level rechecked in a we
Although grapefruit products are high in vitamins and low in calories, they can be associated with potentially serious drug interactions. The interaction between grapefruit juice and the calcium channel blocker felodipine was discovered inadvertently >20 years ago; since that time, possible interactions with >85 medications have been identified.1 Interactions with grapefruit products are complicated because, although most result in increased drug exposure, reduced exposure of the medication also can occur. Additionally, the degree and clinical significance of the interaction varies among individuals and from one drug to another.
Mechanism of action
Most interactions with grapefruit products are thought to result from the inhibition of intestinal cytochrome P450 3A4 (CYP3A4). CYP3A4 is involved in the metabolism of numerous drugs, and is the most abundant cytochrome P450 enzyme in the liver and epithelial cells lining the intestine.2 Although hepatic CYP3A4 is thought to be minimally affected by grapefruit, inhibition of intestinal CYP3A4 can result in an overall increase in bioavailability of medications that are substrates and raise the risk of potential toxicity.3 Grapefruit contains various chemicals collectively known as furanocoumarins, which are largely responsible for inhibition of intestinal CYP3A4.4 Additionally, Seville oranges and the pomelo (a large, sweet grapefruit-like citrus fruit) also contain furanocoumarins and could have a similar effect, warranting caution with certain medications.5
Inhibition of CYP3A4 by furanocoumarins cannot be reversed, and new enzymes must be synthesized to return to the previous level of function.6 Therefore, drug interactions resulting from CYP3A4 inhibition can last for as long as 72 hours after ingesting grapefruit products.7 Separating consumption of grapefruit products and medication administration will not help manage this interaction.
Grapefruit products also could affect drug disposition through effects on various drug transporters. Decreased systemic exposure to certain medications could occur through grapefruit’s inhibition of organic anion-transporting polypeptides (OATPs). OATPs form a family of drug uptake transporters found in the intestine, liver, kidney, and brain.8 For drugs that are substrates of OATPs, grapefruit’s inhibition of this transporter can result in decreased absorption and a resulting decrease in efficacy. Flavanoids in grapefruit, such as naringin, inhibit OATPs, which is competitive in nature.9 Unlike the irreversible inhibition of CYP3A4 by furanocoumarins, flavanoids effects on OATPs have been shown to decrease within 4 hours.10
No psychotropic medications have been identified as being susceptible to this interaction, but for those medications affected—including fexofenadine and levothyroxine—separating consumption of grapefruit and medication administration by 4 hours could avoid this interaction.11 Additional data indicate that orange juice and apple juice could have similar effects on OATPs.12
Perhaps the most well-known drug transporter, P-glycoprotein is part of the multidrug-resistant subfamily of transporters. It is located throughout the body, including in the intestine, kidneys, liver, and blood-brain barrier. P-glycoprotein acts as an export pump to decrease the cellular concentration of many different drug substrates, and many agents can alter P-glycoprotein’s expression or function.
Small changes in P-glycoprotein’s activity can result in substantial changes in the disposition of substrates, which can include certain antineoplastics and antiretrovirals. Most reports have found grapefruit juice inhibits P-glycoprotein-mediated efflux; however, there also are reports of transporter activation.6 Additionally, P-glycoprotein and CYP3A4 share many substrates, so it can be difficult to isolate the contribution of P-glycoprotein to grapefruit−drug interactions.13 The effect of grapefruit on P-glycoprotein activity has been difficult to fully elucidate; more studies are needed.
Grapefruit consumption and its effect
Drug interactions can occur by consuming commercially produced grapefruit juice and juice from concentrate, as well as freshly squeezed juice and grapefruit segments.14 CYP3A4-inhibiting furanocoumarins also have been isolated in grapefruit peel; it is not known, however, whether items made from peel (marmalade, candied peel) contain concentrations high enough to pose a risk of a drug interaction.14 Contributing to the unpredictability of grapefruit-drug interactions, the amount or concentration of furanocoumarins can vary among grapefruit products and brands.15 This variability can be influenced by the variety or maturity of the fruit and the fruit’s exposure to environmental stress.4
The frequency of consuming a grapefruit product can influence the degree of a drug interaction. In general, consuming one 8-oz glass of grapefruit juice or the segments from a whole grapefruit is enough to alter a susceptible drug’s pharmacokinetics.14 Regular grapefruit product consumption, however, can result in an overall greater effect.16,17
Lilja et al16 conducted a randomized, 4-phase, crossover study to look at the effect of grapefruit juice dose on kinetics of triazolam. Grapefruit juice was found to increase the mean area under the concentration-time curve (AUC) of triazolam compared with water, but no difference was found between single glasses of normal-strength and double-strength grapefruit juice. However, repeated consumption of double-strength grapefruit juice (200 mL, 3 times/d for 3 days) increased triazolam’s mean AUC by 143%, compared with an increase of 49% with just a single 200-mL glass of double-strength juice.16 Recurrent consumption of grapefruit juice (8 oz, 3 times/d for 6 days) also was found to increase the kinetics of the antihypertensive felodipine more than a single glass of grapefruit juice.17
Clinical consequences of an interaction between a drug and grapefruit can be difficult to predict. Drug concentration changes caused by a grapefruit interaction could vary based on interindividual differences. The amount and activity of intestinal CYP3A4 can vary from person to person, and can be influenced by genetic polymorphisms in addition to race, age, and environmental variables.18 Interindividual sensitivity to a change in a drug’s concentration also will differ, and patient-specific factors, such as concomitant drugs or diseases, could influence the likelihood of harm.
Interactions with grapefruit products are not necessarily a “class effect,” and specific drugs within a therapeutic category can be affected (although others might not). Several drug-specific characteristics can help gauge the risk of a clinically relevant interaction with grapefruit, including:
• metabolism through CYP3A4
• low bioavailability
• oral administration
• a narrow therapeutic index.1
For drugs with low bioavailability because of first-pass metabolism, grapefruit’s inhibition of intestinal CYP3A4 can result in a greater relative increase in plasma concentrations compared with a drug with high bioavailability.19
For example, an increase in bioavailability from 5% to 10% will result in a much larger increase in AUC and overall clinical exposure compared with an increase from 85% to 90% even though both represent an absolute increase of 5%. Although a drug does not have to have low oral bioavailability for an interaction to occur, lower bioavailability means that a drug has a higher likelihood of causing a significant interaction because of altered pharmacokinetics. Of note, injectable medications will not interact with grapefruit because metabolism through intestinal CYP3A4 is bypassed and grapefruit does not significantly inhibit hepatic CYP3A4.
Although grapefruit products could alter the pharmacokinetics of susceptible drugs, those changes might not be associated with adverse effects. Therefore, a factor to consider in evaluating a potential interaction with grapefruit is the drug’s therapeutic index and its risk of serious adverse effects. Drugs with a narrow therapeutic index are of particular concern because a significant increase in therapeutic or adverse effects could result from a relatively small increase in the drug’s concentration.7
Which medications are affected?
Among medications identified as interacting with grapefruit, some cardiovascular agents and several of the HMG-CoA reductase inhibitors (statins) have garnered the most attention. However, grapefruit also can affect the metabolism of several psychotropic medications through inhibition of intestinal CYP3A4 (Table).16,20-35 Prescribing information for some drugs warns against consuming grapefruit while using the medication. Among CNS agents, buspirone, carbamazepine, lurasidone, pimozide, triazolam, and oral midazolam all have such warnings in their product labeling.
Buspirone currently is not recommended with “large quantities of grapefruit juice.”20 A randomized, 2-phase crossover study looking at the effects of grapefruit juice on buspirone’s pharmacokinetics found that double-strength grapefruit juice (200 mL, administered 3 times/d for 3 days) resulted in a 9.2-fold increase in mean AUC and a 4.3-fold increase in mean Cmax after a single 10-mg buspirone dose.22 Highlighting the wide interindividual variability seen with drug-grapefruit interactions, the increase found in buspirone’s AUC ranged from 3-fold to 20-fold among study participants.22
Carbamazepine product labeling lists grapefruit juice as a CYP3A4 inhibitor that is expected to or has been found to increase plasma levels of the drug.20 Carbamazepine’s bioavailability is influenced by intestinal CYP3A4 activity; in a randomized, 2-phase crossover study of 10 patients with epilepsy, grapefruit juice was found to increase AUC of carbamazepine by 41% and Cmax by 40%.23,36
Lurasidone and pimozide, although not specifically studied, have product labels that recommend avoiding grapefruit juice because it could inhibit metabolism of these agents by CYP3A4.20 Of particular concern is the potential for elevated levels of pimozide to increase the risk of adverse cardiovascular effects including QT interval prolongation.19
Midazolam. Although grapefruit juice does not affect the disposition of IV midazolam, pretreatment with grapefruit juice was found to increase the AUC and Cmax of oral midazolam by 52% and 56%, respectively.30
Other considerations in drug-grapefruit interactions
Cautionary statements about a possible interaction with grapefruit juice for many other psychotropics can be found in commonly used drug information references or online sources. If you are concerned about a possible interaction and avoiding grapefruit products is not feasible, consider a different medication in the same class.
However, you also should consider the level of evidence supporting any purported interaction. Several psychotropic agents do have studies or case reports supporting an interaction with grapefruit, but cautionary statements could be based on theoretical concerns because of a medication’s bioavailability, metabolic pathway, and concern for increased adverse events related to higher drug concentrations. Adding to the confusion, cautionary statements can be found about medications, such as clozapine, that have not been shown to have an interaction with grapefruit juice when studied.
With many of the drugs that have a reported or theoretical interaction with grapefruit, data are inconsistent as to whether the resulting interaction will be clinically relevant. A number of variables relating to the individual patient, grapefruit product, or particular drug can play a role in the significance of an interaction. Additionally, effects on drug disposition can last for a few days after consuming a grapefruit product.
Keep alert to situations of increased risk
Recall that the case patient, Ms. H, presented with an elevated carbamazepine level and suffered resulting adverse effects because of an interaction between the drug and grapefruit juice. Although Ms. H was careful to separate intake of grapefruit juice from carbamazepine administration, grapefruit’s inhibition of intestinal CYP3A4 still was present, leading to the interaction.
It is important for health care professionals to recognize this potential risk and to advise patients regarding possible interactions between medications and grapefruit products.
Related Resources
• U.S. Food and Drug Administration. Grapefruit juice and medicine may not mix. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm292276.htm.
• Hanley MJ, Cancalon P, Widmer WW, et al. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7(3):267-286.
• Andrade C. Fruit juice, organic anion transporting polypeptides, and drug interactions in psychiatry. J Clin Psychiatry. 2014;75(11):e1323-e1325.
Drug Brand Names
Alprazolam • Xanax Lurasidone • Latuda
Buspirone • BuSpar Midazolam • Versed
Carbamazepine • Tegretol Methadone • Dolophine
Clomipramine • Anafranil Nefazodone • Serzone
Clozapine • Clozaril Olanzapine • Zyprexa
Diazepam • Valium Pimozide • Orap
Felodipine • Plendil Quetiapine • Seroquel
Fexofenadine • Allegra Sertraline • Zoloft
Fluoxetine • Prozac Trazodone • Desyrel
Fluvoxamine • Luvox Triazolam • Halcion
Haloperidol • Haldol Ziprasidone • Geodon
Levothyroxine • Levoxyl, Synthroid
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. H, age 42, was given a diagnosis of bipolar disorder 10 years ago and has been taking carbamazepine, 1,200 mg/d, and olanzapine, 10 mg/d, for the past 2 years. She has not experienced a mood episode while on this regimen, and her carbamazepine level was 9.2 μg/mL 6 months ago. The only adverse effect she experienced was weight gain of approximately 10 lb. Ms. H takes a calcium supplement, but no other medications.
Ms. H reports to her psychiatrist that, for the past few days, she has been feeling nauseated, fatigued, and dizzy, but has continued taking her medications as prescribed. Her carbamazepine level is found to be 13.1 μg/mL. Ms. H states she has not started any new medications or supplements; her serum creatinine and liver function test results are within normal limits.
Upon further questioning, Ms. H says that an upper respiratory infection has been “going around her office,” so she increased her vitamin C intake by drinking 2 glasses of grapefruit juice a day (she doesn’t like orange juice). She has heard grapefruit juice can cause problems with some drugs so she is careful not to drink it at the same time she takes her medications. Her psychiatrist recognizes there may be a drug interaction involved, and recommends Ms. H hold her carbamazepine for 1 day and not consume any more grapefruit juice. A few days later, she reports feeling much better during a follow-up call and she makes an appointment to have her carbamazepine level rechecked in a we
Although grapefruit products are high in vitamins and low in calories, they can be associated with potentially serious drug interactions. The interaction between grapefruit juice and the calcium channel blocker felodipine was discovered inadvertently >20 years ago; since that time, possible interactions with >85 medications have been identified.1 Interactions with grapefruit products are complicated because, although most result in increased drug exposure, reduced exposure of the medication also can occur. Additionally, the degree and clinical significance of the interaction varies among individuals and from one drug to another.
Mechanism of action
Most interactions with grapefruit products are thought to result from the inhibition of intestinal cytochrome P450 3A4 (CYP3A4). CYP3A4 is involved in the metabolism of numerous drugs, and is the most abundant cytochrome P450 enzyme in the liver and epithelial cells lining the intestine.2 Although hepatic CYP3A4 is thought to be minimally affected by grapefruit, inhibition of intestinal CYP3A4 can result in an overall increase in bioavailability of medications that are substrates and raise the risk of potential toxicity.3 Grapefruit contains various chemicals collectively known as furanocoumarins, which are largely responsible for inhibition of intestinal CYP3A4.4 Additionally, Seville oranges and the pomelo (a large, sweet grapefruit-like citrus fruit) also contain furanocoumarins and could have a similar effect, warranting caution with certain medications.5
Inhibition of CYP3A4 by furanocoumarins cannot be reversed, and new enzymes must be synthesized to return to the previous level of function.6 Therefore, drug interactions resulting from CYP3A4 inhibition can last for as long as 72 hours after ingesting grapefruit products.7 Separating consumption of grapefruit products and medication administration will not help manage this interaction.
Grapefruit products also could affect drug disposition through effects on various drug transporters. Decreased systemic exposure to certain medications could occur through grapefruit’s inhibition of organic anion-transporting polypeptides (OATPs). OATPs form a family of drug uptake transporters found in the intestine, liver, kidney, and brain.8 For drugs that are substrates of OATPs, grapefruit’s inhibition of this transporter can result in decreased absorption and a resulting decrease in efficacy. Flavanoids in grapefruit, such as naringin, inhibit OATPs, which is competitive in nature.9 Unlike the irreversible inhibition of CYP3A4 by furanocoumarins, flavanoids effects on OATPs have been shown to decrease within 4 hours.10
No psychotropic medications have been identified as being susceptible to this interaction, but for those medications affected—including fexofenadine and levothyroxine—separating consumption of grapefruit and medication administration by 4 hours could avoid this interaction.11 Additional data indicate that orange juice and apple juice could have similar effects on OATPs.12
Perhaps the most well-known drug transporter, P-glycoprotein is part of the multidrug-resistant subfamily of transporters. It is located throughout the body, including in the intestine, kidneys, liver, and blood-brain barrier. P-glycoprotein acts as an export pump to decrease the cellular concentration of many different drug substrates, and many agents can alter P-glycoprotein’s expression or function.
Small changes in P-glycoprotein’s activity can result in substantial changes in the disposition of substrates, which can include certain antineoplastics and antiretrovirals. Most reports have found grapefruit juice inhibits P-glycoprotein-mediated efflux; however, there also are reports of transporter activation.6 Additionally, P-glycoprotein and CYP3A4 share many substrates, so it can be difficult to isolate the contribution of P-glycoprotein to grapefruit−drug interactions.13 The effect of grapefruit on P-glycoprotein activity has been difficult to fully elucidate; more studies are needed.
Grapefruit consumption and its effect
Drug interactions can occur by consuming commercially produced grapefruit juice and juice from concentrate, as well as freshly squeezed juice and grapefruit segments.14 CYP3A4-inhibiting furanocoumarins also have been isolated in grapefruit peel; it is not known, however, whether items made from peel (marmalade, candied peel) contain concentrations high enough to pose a risk of a drug interaction.14 Contributing to the unpredictability of grapefruit-drug interactions, the amount or concentration of furanocoumarins can vary among grapefruit products and brands.15 This variability can be influenced by the variety or maturity of the fruit and the fruit’s exposure to environmental stress.4
The frequency of consuming a grapefruit product can influence the degree of a drug interaction. In general, consuming one 8-oz glass of grapefruit juice or the segments from a whole grapefruit is enough to alter a susceptible drug’s pharmacokinetics.14 Regular grapefruit product consumption, however, can result in an overall greater effect.16,17
Lilja et al16 conducted a randomized, 4-phase, crossover study to look at the effect of grapefruit juice dose on kinetics of triazolam. Grapefruit juice was found to increase the mean area under the concentration-time curve (AUC) of triazolam compared with water, but no difference was found between single glasses of normal-strength and double-strength grapefruit juice. However, repeated consumption of double-strength grapefruit juice (200 mL, 3 times/d for 3 days) increased triazolam’s mean AUC by 143%, compared with an increase of 49% with just a single 200-mL glass of double-strength juice.16 Recurrent consumption of grapefruit juice (8 oz, 3 times/d for 6 days) also was found to increase the kinetics of the antihypertensive felodipine more than a single glass of grapefruit juice.17
Clinical consequences of an interaction between a drug and grapefruit can be difficult to predict. Drug concentration changes caused by a grapefruit interaction could vary based on interindividual differences. The amount and activity of intestinal CYP3A4 can vary from person to person, and can be influenced by genetic polymorphisms in addition to race, age, and environmental variables.18 Interindividual sensitivity to a change in a drug’s concentration also will differ, and patient-specific factors, such as concomitant drugs or diseases, could influence the likelihood of harm.
Interactions with grapefruit products are not necessarily a “class effect,” and specific drugs within a therapeutic category can be affected (although others might not). Several drug-specific characteristics can help gauge the risk of a clinically relevant interaction with grapefruit, including:
• metabolism through CYP3A4
• low bioavailability
• oral administration
• a narrow therapeutic index.1
For drugs with low bioavailability because of first-pass metabolism, grapefruit’s inhibition of intestinal CYP3A4 can result in a greater relative increase in plasma concentrations compared with a drug with high bioavailability.19
For example, an increase in bioavailability from 5% to 10% will result in a much larger increase in AUC and overall clinical exposure compared with an increase from 85% to 90% even though both represent an absolute increase of 5%. Although a drug does not have to have low oral bioavailability for an interaction to occur, lower bioavailability means that a drug has a higher likelihood of causing a significant interaction because of altered pharmacokinetics. Of note, injectable medications will not interact with grapefruit because metabolism through intestinal CYP3A4 is bypassed and grapefruit does not significantly inhibit hepatic CYP3A4.
Although grapefruit products could alter the pharmacokinetics of susceptible drugs, those changes might not be associated with adverse effects. Therefore, a factor to consider in evaluating a potential interaction with grapefruit is the drug’s therapeutic index and its risk of serious adverse effects. Drugs with a narrow therapeutic index are of particular concern because a significant increase in therapeutic or adverse effects could result from a relatively small increase in the drug’s concentration.7
Which medications are affected?
Among medications identified as interacting with grapefruit, some cardiovascular agents and several of the HMG-CoA reductase inhibitors (statins) have garnered the most attention. However, grapefruit also can affect the metabolism of several psychotropic medications through inhibition of intestinal CYP3A4 (Table).16,20-35 Prescribing information for some drugs warns against consuming grapefruit while using the medication. Among CNS agents, buspirone, carbamazepine, lurasidone, pimozide, triazolam, and oral midazolam all have such warnings in their product labeling.
Buspirone currently is not recommended with “large quantities of grapefruit juice.”20 A randomized, 2-phase crossover study looking at the effects of grapefruit juice on buspirone’s pharmacokinetics found that double-strength grapefruit juice (200 mL, administered 3 times/d for 3 days) resulted in a 9.2-fold increase in mean AUC and a 4.3-fold increase in mean Cmax after a single 10-mg buspirone dose.22 Highlighting the wide interindividual variability seen with drug-grapefruit interactions, the increase found in buspirone’s AUC ranged from 3-fold to 20-fold among study participants.22
Carbamazepine product labeling lists grapefruit juice as a CYP3A4 inhibitor that is expected to or has been found to increase plasma levels of the drug.20 Carbamazepine’s bioavailability is influenced by intestinal CYP3A4 activity; in a randomized, 2-phase crossover study of 10 patients with epilepsy, grapefruit juice was found to increase AUC of carbamazepine by 41% and Cmax by 40%.23,36
Lurasidone and pimozide, although not specifically studied, have product labels that recommend avoiding grapefruit juice because it could inhibit metabolism of these agents by CYP3A4.20 Of particular concern is the potential for elevated levels of pimozide to increase the risk of adverse cardiovascular effects including QT interval prolongation.19
Midazolam. Although grapefruit juice does not affect the disposition of IV midazolam, pretreatment with grapefruit juice was found to increase the AUC and Cmax of oral midazolam by 52% and 56%, respectively.30
Other considerations in drug-grapefruit interactions
Cautionary statements about a possible interaction with grapefruit juice for many other psychotropics can be found in commonly used drug information references or online sources. If you are concerned about a possible interaction and avoiding grapefruit products is not feasible, consider a different medication in the same class.
However, you also should consider the level of evidence supporting any purported interaction. Several psychotropic agents do have studies or case reports supporting an interaction with grapefruit, but cautionary statements could be based on theoretical concerns because of a medication’s bioavailability, metabolic pathway, and concern for increased adverse events related to higher drug concentrations. Adding to the confusion, cautionary statements can be found about medications, such as clozapine, that have not been shown to have an interaction with grapefruit juice when studied.
With many of the drugs that have a reported or theoretical interaction with grapefruit, data are inconsistent as to whether the resulting interaction will be clinically relevant. A number of variables relating to the individual patient, grapefruit product, or particular drug can play a role in the significance of an interaction. Additionally, effects on drug disposition can last for a few days after consuming a grapefruit product.
Keep alert to situations of increased risk
Recall that the case patient, Ms. H, presented with an elevated carbamazepine level and suffered resulting adverse effects because of an interaction between the drug and grapefruit juice. Although Ms. H was careful to separate intake of grapefruit juice from carbamazepine administration, grapefruit’s inhibition of intestinal CYP3A4 still was present, leading to the interaction.
It is important for health care professionals to recognize this potential risk and to advise patients regarding possible interactions between medications and grapefruit products.
Related Resources
• U.S. Food and Drug Administration. Grapefruit juice and medicine may not mix. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm292276.htm.
• Hanley MJ, Cancalon P, Widmer WW, et al. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7(3):267-286.
• Andrade C. Fruit juice, organic anion transporting polypeptides, and drug interactions in psychiatry. J Clin Psychiatry. 2014;75(11):e1323-e1325.
Drug Brand Names
Alprazolam • Xanax Lurasidone • Latuda
Buspirone • BuSpar Midazolam • Versed
Carbamazepine • Tegretol Methadone • Dolophine
Clomipramine • Anafranil Nefazodone • Serzone
Clozapine • Clozaril Olanzapine • Zyprexa
Diazepam • Valium Pimozide • Orap
Felodipine • Plendil Quetiapine • Seroquel
Fexofenadine • Allegra Sertraline • Zoloft
Fluoxetine • Prozac Trazodone • Desyrel
Fluvoxamine • Luvox Triazolam • Halcion
Haloperidol • Haldol Ziprasidone • Geodon
Levothyroxine • Levoxyl, Synthroid
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185(4):309-316.
2. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21): 2211-2221.
3. Saito M, Hirata-Koizumi M, Matsumoto M, et al. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28(8):677- 694.
4. Cancalon PF, Barros SM, Haun C, et al. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J Food Sci. 2011;76(4):C543-C548.
5. Pirmohamed M. Drug-grapefruit juice interactions: two mechanisms are clear but individual responses vary. BMJ. 2013;346:f1. doi: 10.1136/bmj.f1.
6. Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability–mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1-9.
7. Stump AL, Mayo T, Blum A. Management of grapefruit-drug interactions. Am Fam Physician. 2006;74(4):605-608.
8. Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(suppl 2):1-5.
9. Bailey DG, Dressker GK, Leak BF, et al. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81(4):495-502.
10. Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362-370.
11. Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70(5): 645-655.
12. Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11-20.
13. Seden K, Dickinson L, Khoo S, et al. Grapefruit-drug interactions. Drugs. 2010;70(18):2373-2407.
14. Bailey DG, Dresser GK, Kreeft JH, et al. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68(5):468-477.
15. De Castro WV, Mertens-Talcott S, Rubner A, et al. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54(1):249-255.
16. Lilja JJ, Kivistö KT, Backman JT, et al. Effect of grapefruit juice on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000;56(5):411-415.
17. Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545-2553.
18. Lin JH, Lu AY. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol. 2001;41:535-567.
19. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000; 38(1):41-57.
20. U.S. Food and Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed July 14, 2014.
21. Yasui, N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;15(2):185-190.
22. Lilja JJ, Kivistö KT, Backman JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64(6):655-660.
23. Garg SK, Kumar N, Bhargava VK, et al. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64(3):286-288.
24. Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: shifting metabolic ratios. J Clin Psychopharm. 1997;17(1):62-63.
25. Lane HY, Jann MW, Chang YC, et al. Repeated ingestion of grapefruit juice does not alter clozapine’s steady-state plasma levels, effectiveness, and tolerability. J Clin Psychiatry. 2001;62(10):812-817.
26. Ozdemir M, Aktan Y, Boydag BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59.
27. DeSilva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281-1285.
28. Hori H, Yoshimura R, Ueda N, et al. Grapefruit juice-fluvoxamine interaction—is it risky or not? J Clin Psychopharmacol. 2003;23(4):422-424.
29. Yasui N, Kondo T, Suzuki A, et al. Lack of significant pharmacokinetic interaction between haloperidol and grapefruit juice. Int Clin Psychopharmacol. 1999;142(2):113-118.
30. Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58(1):20-28.
31. Benmebarek M, Cevaud C, Gex-Fabry M, et al. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004;76(1):55-63.
32. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509-522.
33. Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10(4 pt 3):832-835.
34. Lee AJ, Chan WK, Harralson AF, et al. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999;21(11):1890-1899.
35. Sugimoto K, Araki N, Ohmori M, et al. Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006;62(3):209-215.
36. Fagiolino P, Vazquez M, Olano I, et al. Systemic and presystemic conversion of carbamazepine to carbamazepine- 10-11-epoxide during long term treatment. Journal of Epilepsy and Clinical Neurophysiology. 2006;12(1):13-16.
1. Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185(4):309-316.
2. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21): 2211-2221.
3. Saito M, Hirata-Koizumi M, Matsumoto M, et al. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28(8):677- 694.
4. Cancalon PF, Barros SM, Haun C, et al. Effect of maturity, processing, and storage on the furanocoumarin composition of grapefruit and grapefruit juice. J Food Sci. 2011;76(4):C543-C548.
5. Pirmohamed M. Drug-grapefruit juice interactions: two mechanisms are clear but individual responses vary. BMJ. 2013;346:f1. doi: 10.1136/bmj.f1.
6. Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability–mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1-9.
7. Stump AL, Mayo T, Blum A. Management of grapefruit-drug interactions. Am Fam Physician. 2006;74(4):605-608.
8. Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(suppl 2):1-5.
9. Bailey DG, Dressker GK, Leak BF, et al. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81(4):495-502.
10. Glaeser H, Bailey DG, Dresser GK, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81(3):362-370.
11. Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70(5): 645-655.
12. Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11-20.
13. Seden K, Dickinson L, Khoo S, et al. Grapefruit-drug interactions. Drugs. 2010;70(18):2373-2407.
14. Bailey DG, Dresser GK, Kreeft JH, et al. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68(5):468-477.
15. De Castro WV, Mertens-Talcott S, Rubner A, et al. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54(1):249-255.
16. Lilja JJ, Kivistö KT, Backman JT, et al. Effect of grapefruit juice on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000;56(5):411-415.
17. Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99(10):2545-2553.
18. Lin JH, Lu AY. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu Rev Pharmacol Toxicol. 2001;41:535-567.
19. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000; 38(1):41-57.
20. U.S. Food and Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed July 14, 2014.
21. Yasui, N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;15(2):185-190.
22. Lilja JJ, Kivistö KT, Backman JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64(6):655-660.
23. Garg SK, Kumar N, Bhargava VK, et al. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64(3):286-288.
24. Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: shifting metabolic ratios. J Clin Psychopharm. 1997;17(1):62-63.
25. Lane HY, Jann MW, Chang YC, et al. Repeated ingestion of grapefruit juice does not alter clozapine’s steady-state plasma levels, effectiveness, and tolerability. J Clin Psychiatry. 2001;62(10):812-817.
26. Ozdemir M, Aktan Y, Boydag BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59.
27. DeSilva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281-1285.
28. Hori H, Yoshimura R, Ueda N, et al. Grapefruit juice-fluvoxamine interaction—is it risky or not? J Clin Psychopharmacol. 2003;23(4):422-424.
29. Yasui N, Kondo T, Suzuki A, et al. Lack of significant pharmacokinetic interaction between haloperidol and grapefruit juice. Int Clin Psychopharmacol. 1999;142(2):113-118.
30. Kupferschmidt HH, Ha HR, Ziegler WH, et al. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58(1):20-28.
31. Benmebarek M, Cevaud C, Gex-Fabry M, et al. Effects of grapefruit juice on the pharmacokinetics of the enantiomers of methadone. Clin Pharmacol Ther. 2004;76(1):55-63.
32. DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40(7):509-522.
33. Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10(4 pt 3):832-835.
34. Lee AJ, Chan WK, Harralson AF, et al. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999;21(11):1890-1899.
35. Sugimoto K, Araki N, Ohmori M, et al. Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006;62(3):209-215.
36. Fagiolino P, Vazquez M, Olano I, et al. Systemic and presystemic conversion of carbamazepine to carbamazepine- 10-11-epoxide during long term treatment. Journal of Epilepsy and Clinical Neurophysiology. 2006;12(1):13-16.
PSYCHIATRY UPDATE 2015
Current Psychiatry welcomed more than 650 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, April 16-18, 2015, at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us next year in Chicago, March 10-12, 2016.
THURSDAY, APRIL 16, 2015
MORNING SESSION
Attention-deficit/hyperactivity disorder (ADHD) is a lifespan disorder that is “everywhere,” Anthony L. Rostain, MD, MA, University of Pennsylvania Perelman School of Medicine, began—including in adults and even “seniors.” This means that the disorder “is not a diagnosis of exclusion,” and that “comorbidity is the rule,” including learning difficulties. Among adults, the focus of symptoms and management is on executive dysfunction and its characteristics: difficulty multitasking, problems keeping commitments, and excessive reliance on help from others. Inattention and disorganization are hallmarks of adult ADHD, and become worse as environmental demands (work, home) increase; hyperactivity decreases with age. Dr. Rostain recommends ruling out other causes of a patient’s symptoms when an adult self-reports ADHD, including transient stressors, medical conditions, psychiatric disorders, and malingering.
Donald W. Black, MD, University of Iowa, reviewed DSM-5 criteria for borderline personality disorder (BPD) and offered tips for avoiding misdiagnosis, including obtaining collateral information and using rating scales. Co-occuring disorders, such as depression and substance abuse, are common. Treatment for BPD patients includes psychotherapy (individual or group), medication, and lifestyle changes. Psychotropics treat symptoms of depression, anxiety, hostility, and impulsivity of BPD but not the fundamental nature of the disorder. When establishing a patient’s treatment plan, consider the stage of illness, evaluate for any co-occurring disorders, and ask the patient what he (she) wants from treatment.
Dr. Rostain began by discussing the neurobiological basis of ADHD, which guides pharmacotherapy. He reviewed the response rate of FDA-approved agents for adults with ADHD, including stimulants, atomoxetine, and alpha-adrenergic agonists. Best response is seen with stimulants, but some patients improve with bupropion and tricyclic antidepressants (TCAs). Employ a multimodal treatment approach, Dr. Rostain recommended, which should include psychoeducation and environmental restructuring, because, as he says, “Pills don’t teach skills.” He also reviewed strategies for treating ADHD in patients who have a comorbid disorder, such as bipolar disorder, major depressive disorder, or substance abuse.
Patients with psychotic depression meet criteria for major depressive disorder but also have delusions or hallucinations. Diagnostic issues include increased guilt, cognitive impairment, paranoia, and increased hopelessness. Anthony J. Rothschild, MD, University of Massachusetts Medical School, reviewed methods for differentiating psychotic depression from schizophrenia, posttraumatic stress disorder, obsessive-compulsive disorder, and body dysmorphic disorder. There are no FDA-approved medications for psychotic depression, Dr. Rothschild explained; however, evidence shows that the combination of an antidepressant and an antipsychotic is superior to monotherapy with an agent from either class. In addition, he noted, studies show a high response rate with electroconvulsive therapy (ECT).
AFTERNOON SESSION
Return of symptoms after initial remission— while the patient is still taking an antidepressant—is considered tachyphylaxis, or “poop out.” Residual depressive symptoms, when a patient meets criteria for remission but still has troubling symptoms, is a different phenomenon, although symptoms can overlap. First, Dr. Rothschild advised, ensure that patients are given an adequate trial of an antidepressant. Options are similar when tachyphylaxis or residual symptoms are present: switch drugs or add augmentation therapy, such as lithium, thyroid hormone, or an atypical antipsychotic. Data on the efficacy for bupropion and buspirone are not strong. For treatment-resistant depression when a patient does not respond to 3 adequate antidepressant trials—consider ECT or rTMS, if available, or a monoamine oxidase inhibitor or a TCA.
Dr. Black defines antisocial personality disorder (ASPD) as a disorder of lifelong serial misbehavior, one characterized by impaired relationships, aggressive behavior, non-aggressive delinquent behavior, manipulation, and a disturbing lack of conscience. There is no standard treatment for ASPD, and no FDA-approved medications; however, potential treatments have not been adequately studied, he pointed out. Cognitive-behavioral therapy might be appropriate in mild cases; some patients benefit from specific programs— for example, ones that address drug or alcohol addiction or anger, although evidence is limited. When treating ASPD patients, Dr. Black concluded, be mindful of high attrition, possible misuse of prescribed medications, and drug-drug or drug-alcohol interactions.
Bipolar disorder is associated with the highest risk of suicide and increased lethality among all psychiatric disorders. Lithium has evidence of an anti-suicidality effect and may reduce suicide by decreasing relapse, aggression, and impulsivity. An FDA advisory on increased risk of suicidality with anticonvulsants was based on data about patients with epilepsy, not bipolar disorder. Second-generation antipsychotics, including olanzapine, quetiapine, and lurasidone, have been shown to be effective for bipolar depression. Avoid antidepressants if possible, Philip G. Janicak, MD, Northwestern University Feinberg School of Medicine, advised; if you must prescribe one, reassess the need for the drug often. Several psychotherapy modalities have evidence supporting their use in bipolar disorder.
FRIDAY, APRIL 17, 2015
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University School of Medicine, offered enlightening historical touch-points on how psychiatry’s understanding of, and its approach to, schizophrenia have changed in the past 50 years. His goal? To challenge practitioners to rethink ideas about the disorder and how they care for affected patients. From a laundry list of comparative shifts, here are a few of Dr. Nasrallah’s “then” and “now” observations:
• The old paradigm was: Clinical and functional deterioration are inevitable in schizophrenia. The new paradigm is: Complete remission and restoration of function are feasible in many patients when they are fully adherent to the treatment plan.
• The old: Long-acting injectable (LAI) antipsychotics are a last-resort treatment, to be prescribed after a patient is stabilized. The new: Use LAI antipsychotics early in the course.
• Old: Begin treatment when psychosis appears. New: Work to prevent conversion to psychosis.
• Old: The disorder is considered a consequence of neurochemical dysregulation. New: Impaired neuroplasticity is to blame.
• Old: Treatment is a matter of trial and error. New: We can apply pharmaco-genomics to predict a patient’s response to various drugs and thus increase the likelihood of therapeutic success.
In his second presentation, Dr. Nasrallah described the many pathways to psychosis and several psychotic disorders other than schizophrenia, including schizoaffective, delusional disorder, and psychotic disorder caused by a general medical condition. He listed symptom clusters in psychosis beyond positive and negative symptoms, including neuromotor symptoms, mood symptoms, and neurocognitive deficits. Development of schizophrenia is multifactorial and involves risk genes and environmental factors seen before conception, during birth, and in early childhood; good prenatal care is the best way to prevent schizophrenia, Dr. Nasrallah noted. Several general medical conditions can produce schizophrenia-like psychosis, including some CNS disorders, toxins, autoimmune diseases, infectious diseases, and chromosomal abnormalities. The session concluded with a live interview with one of Dr. Nasrallah’s patients, whose schizophrenia is in remission with clozapine.
Drug abuse can mask signs and symptoms of bipolar disorder, which can delay diagnosis. Commonly abused substances are nicotine, alcohol, Cannabis, and cocaine; polysubstance abuse is the rule. Bipolar disorder and substance abuse share common mechanisms: impulsivity, poor modulation of motivation and response to reward, and behavioral sensitization. Treatment approaches should be flexible. Dr. Janicak reviewed the evidence for using anticonvulsants, antipsychotics, and bupropion for alcohol, Cannabis, and cocaine abuse; there are no data on treating opioid abuse. He also discussed the evidence for using naltrexone, acamprosate, disulfiram, and varenicline, as well as psychotherapeutic options, to treat substance abuse. Dr. Janicak encouraged clinicians in the audience to treat substance abuse in bipolar disorder patients themselves, instead of referring them to a subspecialist.
Untreated psychiatric disorders increase obstetrical complications, possibly through decreased self-care or increased stress. For mild or moderate depression, psychotherapy might be sufficient treatment; but for severe cases, medication is the first-line approach. In her presentation on mood disorders during pregnancy, Marlene P. Freeman, MD, Massachusetts General Hospital, advises that clinicians select medications based on known safety information, patient preference, and the previous course of illness. Results of studies that lasted 4 to 5 years do not show major long-term adverse effects of antidepressant exposure on neurodevelopment or neurobehavior. When treating patients for bipolar disorder, valproate is associated with an increased risk of adverse cognitive and neurodevelopmental effects in infants compared with other anticonvulsants; evidence suggests that lamotrigine is a safer option. The research does not show an increased risk of major malformations with second-generation antipsychotics.
AFTERNOON SESSION
Most women have premenstrual symptoms; a minority have a full-blown syndrome, now known as premenstrual dysphoric disorder (PMDD). This is not an existing mood disorder that becomes worse premenstrually. Clinician and patients should track the temporal relationship of symptoms on a calendar for a few months. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine have been well studied and are effective compared with placebo, but don’t help all patients with PMDD. Consider flexible dosing strategies with SSRIs—perhaps daily use, a higher dosage premenstrually, and as-needed administration. Start with an oral contraceptive or SSRI; if symptoms don’t respond, add the other. Serotonergic antidepressants have been shown helpful for hot flashes and depressive symptoms in perimenopause. Dr. Freeman reviewed the evidence for using complementary and alternative therapies for menopausal symptoms and hot flushes.
Smoking contributes to excess mortality in seriously mentally ill patients as a result of such tobacco-related illnesses as heart disease, lung disease, and cancer. Overall improvement in mental health as well as physical health is seen when a patient stops smoking. All nicotine replacement products are effective, but patients often don’t use them long enough or correctly. Robert M. Anthenelli, MD, University of California, San Diego, said to begin sustained-release bupropion 1 or 2 weeks before quit date; maintain the dosage for 1 to 12 weeks after quit date and consider maintenance therapy for as long as 6 months. Varenicline is superior to placebo and bupropion, but is known to have gastrointestinal (GI) and sleep disturbance adverse effects. Quitting smoking can increase the blood level of some psychotropics, meaning that you might need to reduce their dosage. It is best to begin smoking cessation when patients are mentally stable, when motivated, and stable on their medications.
In discussing trends in substance abuse, Dr. Anthenelli
faddish. Fentanyl and fentanyl analogues are 100 times more powerful than morphine; ingestion of even a minuscule dose can be fatal. Synthetic cannabinoids primarily are a problem among adolescents; they are more dangerous than marijuana and are associated with aggressive and suicidal behaviors. A standard toxicology screen will not detect synthetic cannabinoids.
E-cigarettes are considered by users to be safer than tobacco cigarettes—and probably are—but they still put patients at risk of nicotine addiction. There are no safety data on e-cigarettes; the devices might contain potentially harmful chemicals and potentially toxic nicotine levels. Dr. Anthenelli reported that topiramate is “the best medication I’ve used” for alcohol abuse disorder. The drug is not FDA-approved for this use, but has been used in a number of studies with positive outcomes.
SATURDAY, APRIL 18, 2015
MORNING SESSION
Psychiatrists are well positioned to help patients with mental illness lose weight because of their psychotherapeutic background. Best treatment strategy is diet plus exercise plus behavioral modification. Robert M. McCarron, DO, University of California, Davis, recommends keeping it simple and telling patients to only consider calories of foods, and not to worry about sodium or fat content. Ask patients “How many minutes a day of exercise can you do?” but recommend that patients walk for 30 minutes a day at 4 mph, 5 days per week, which will help patients lose 1% to 3% of body weight. For treatment-refractory obese patients, consider medications such as bupropion, orlistat, lorcaserin, topiramate, or metformin; for those with a BMI ≥40, recommend bariatric surgery.
George T. Grossberg, MD, Saint Louis University School of Medicine, reviewed the evidence for anxiety disorders in older adults, including generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Older patients with cardiovascular disease, cancer, Parkinson’s disease, diabetes, GI disorders, or chronic obstructive pulmonary disease are at high risk of anxiety symptoms. In a study of centenarians, predictors of anxiety are worse health perception, financial concerns related to medical expenses, higher number of medical conditions, and loneliness. Secondary anxiety is prevalent in Alzheimer’s disease; the condition can present as fidgeting, pacing, anger, or agitation, and can be prompted by a change in routine. Acute, new-onset anxiety symptoms should trigger a complete medical evaluation, including a review of medications, supplements, and substance use. In geriatric patients, minimize use of benzodiazepines and avoid anticholinergics.
Overall, psychiatry patients do not receive optimal preventive and primary medical care, leading to decreased life expectancy, often as a result of cardiovascular disease. Psychiatric patients have a high rate of dyslipidemia, hypertension, smoking, and obesity. Psychiatrists often don’t treat these conditions, but they need to be aware of changing standard practices in preventive medicine; be able to recognize a potential problem; and make referrals when appropriate. Dr. McCarron reviewed age-based screening recommendations for hypertension, dyslipidemia, and diabetes from the book Preventive Medical Care in Psychiatry, which he co-edited. He recommends using online cardiovascular risk calculators to determine which patients need to be screened.
AFTERNOON SESSION
Some older patients who abuse substances took drugs as young adults and never gave them up; others have rediscovered drugs in later life. Potential indicators of alcohol abuse in older patients are changes in cognition, mood, memory, hygiene, or sleep. Substance abuse in older adults frequently is comorbid with depression or bereavement, anxiety, and adjustment disorders. Dr. Grossberg recommends addressing the topic directly with patients. Although there are few data to guide treatment, prompt detection and appropriate treatment can improve the quality of life of older adults and their family.
SPONSORS AND SUPPORTERS
• American Professional Agency
• American Psychiatric Publishing
• Arbor Pharmaceuticals
• AstraZeneca
• Banner Health
• Bassett Healthcare Network
• Ministry Health Care
• Pine Rest Christian Mental Health Services
• PRMS
• Sinai Health System
• Sunovion
• Takeda Pharmaceuticals
• U.S. Army Healthcare
• Wexford Health Sources
• Wolters Kluwer Health
The meeting organizers acknowledge the support provided by the sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-directors. Sponsors and supporters did not have input in these areas.
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
Current Psychiatry welcomed more than 650 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, April 16-18, 2015, at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us next year in Chicago, March 10-12, 2016.
THURSDAY, APRIL 16, 2015
MORNING SESSION
Attention-deficit/hyperactivity disorder (ADHD) is a lifespan disorder that is “everywhere,” Anthony L. Rostain, MD, MA, University of Pennsylvania Perelman School of Medicine, began—including in adults and even “seniors.” This means that the disorder “is not a diagnosis of exclusion,” and that “comorbidity is the rule,” including learning difficulties. Among adults, the focus of symptoms and management is on executive dysfunction and its characteristics: difficulty multitasking, problems keeping commitments, and excessive reliance on help from others. Inattention and disorganization are hallmarks of adult ADHD, and become worse as environmental demands (work, home) increase; hyperactivity decreases with age. Dr. Rostain recommends ruling out other causes of a patient’s symptoms when an adult self-reports ADHD, including transient stressors, medical conditions, psychiatric disorders, and malingering.
Donald W. Black, MD, University of Iowa, reviewed DSM-5 criteria for borderline personality disorder (BPD) and offered tips for avoiding misdiagnosis, including obtaining collateral information and using rating scales. Co-occuring disorders, such as depression and substance abuse, are common. Treatment for BPD patients includes psychotherapy (individual or group), medication, and lifestyle changes. Psychotropics treat symptoms of depression, anxiety, hostility, and impulsivity of BPD but not the fundamental nature of the disorder. When establishing a patient’s treatment plan, consider the stage of illness, evaluate for any co-occurring disorders, and ask the patient what he (she) wants from treatment.
Dr. Rostain began by discussing the neurobiological basis of ADHD, which guides pharmacotherapy. He reviewed the response rate of FDA-approved agents for adults with ADHD, including stimulants, atomoxetine, and alpha-adrenergic agonists. Best response is seen with stimulants, but some patients improve with bupropion and tricyclic antidepressants (TCAs). Employ a multimodal treatment approach, Dr. Rostain recommended, which should include psychoeducation and environmental restructuring, because, as he says, “Pills don’t teach skills.” He also reviewed strategies for treating ADHD in patients who have a comorbid disorder, such as bipolar disorder, major depressive disorder, or substance abuse.
Patients with psychotic depression meet criteria for major depressive disorder but also have delusions or hallucinations. Diagnostic issues include increased guilt, cognitive impairment, paranoia, and increased hopelessness. Anthony J. Rothschild, MD, University of Massachusetts Medical School, reviewed methods for differentiating psychotic depression from schizophrenia, posttraumatic stress disorder, obsessive-compulsive disorder, and body dysmorphic disorder. There are no FDA-approved medications for psychotic depression, Dr. Rothschild explained; however, evidence shows that the combination of an antidepressant and an antipsychotic is superior to monotherapy with an agent from either class. In addition, he noted, studies show a high response rate with electroconvulsive therapy (ECT).
AFTERNOON SESSION
Return of symptoms after initial remission— while the patient is still taking an antidepressant—is considered tachyphylaxis, or “poop out.” Residual depressive symptoms, when a patient meets criteria for remission but still has troubling symptoms, is a different phenomenon, although symptoms can overlap. First, Dr. Rothschild advised, ensure that patients are given an adequate trial of an antidepressant. Options are similar when tachyphylaxis or residual symptoms are present: switch drugs or add augmentation therapy, such as lithium, thyroid hormone, or an atypical antipsychotic. Data on the efficacy for bupropion and buspirone are not strong. For treatment-resistant depression when a patient does not respond to 3 adequate antidepressant trials—consider ECT or rTMS, if available, or a monoamine oxidase inhibitor or a TCA.
Dr. Black defines antisocial personality disorder (ASPD) as a disorder of lifelong serial misbehavior, one characterized by impaired relationships, aggressive behavior, non-aggressive delinquent behavior, manipulation, and a disturbing lack of conscience. There is no standard treatment for ASPD, and no FDA-approved medications; however, potential treatments have not been adequately studied, he pointed out. Cognitive-behavioral therapy might be appropriate in mild cases; some patients benefit from specific programs— for example, ones that address drug or alcohol addiction or anger, although evidence is limited. When treating ASPD patients, Dr. Black concluded, be mindful of high attrition, possible misuse of prescribed medications, and drug-drug or drug-alcohol interactions.
Bipolar disorder is associated with the highest risk of suicide and increased lethality among all psychiatric disorders. Lithium has evidence of an anti-suicidality effect and may reduce suicide by decreasing relapse, aggression, and impulsivity. An FDA advisory on increased risk of suicidality with anticonvulsants was based on data about patients with epilepsy, not bipolar disorder. Second-generation antipsychotics, including olanzapine, quetiapine, and lurasidone, have been shown to be effective for bipolar depression. Avoid antidepressants if possible, Philip G. Janicak, MD, Northwestern University Feinberg School of Medicine, advised; if you must prescribe one, reassess the need for the drug often. Several psychotherapy modalities have evidence supporting their use in bipolar disorder.
FRIDAY, APRIL 17, 2015
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University School of Medicine, offered enlightening historical touch-points on how psychiatry’s understanding of, and its approach to, schizophrenia have changed in the past 50 years. His goal? To challenge practitioners to rethink ideas about the disorder and how they care for affected patients. From a laundry list of comparative shifts, here are a few of Dr. Nasrallah’s “then” and “now” observations:
• The old paradigm was: Clinical and functional deterioration are inevitable in schizophrenia. The new paradigm is: Complete remission and restoration of function are feasible in many patients when they are fully adherent to the treatment plan.
• The old: Long-acting injectable (LAI) antipsychotics are a last-resort treatment, to be prescribed after a patient is stabilized. The new: Use LAI antipsychotics early in the course.
• Old: Begin treatment when psychosis appears. New: Work to prevent conversion to psychosis.
• Old: The disorder is considered a consequence of neurochemical dysregulation. New: Impaired neuroplasticity is to blame.
• Old: Treatment is a matter of trial and error. New: We can apply pharmaco-genomics to predict a patient’s response to various drugs and thus increase the likelihood of therapeutic success.
In his second presentation, Dr. Nasrallah described the many pathways to psychosis and several psychotic disorders other than schizophrenia, including schizoaffective, delusional disorder, and psychotic disorder caused by a general medical condition. He listed symptom clusters in psychosis beyond positive and negative symptoms, including neuromotor symptoms, mood symptoms, and neurocognitive deficits. Development of schizophrenia is multifactorial and involves risk genes and environmental factors seen before conception, during birth, and in early childhood; good prenatal care is the best way to prevent schizophrenia, Dr. Nasrallah noted. Several general medical conditions can produce schizophrenia-like psychosis, including some CNS disorders, toxins, autoimmune diseases, infectious diseases, and chromosomal abnormalities. The session concluded with a live interview with one of Dr. Nasrallah’s patients, whose schizophrenia is in remission with clozapine.
Drug abuse can mask signs and symptoms of bipolar disorder, which can delay diagnosis. Commonly abused substances are nicotine, alcohol, Cannabis, and cocaine; polysubstance abuse is the rule. Bipolar disorder and substance abuse share common mechanisms: impulsivity, poor modulation of motivation and response to reward, and behavioral sensitization. Treatment approaches should be flexible. Dr. Janicak reviewed the evidence for using anticonvulsants, antipsychotics, and bupropion for alcohol, Cannabis, and cocaine abuse; there are no data on treating opioid abuse. He also discussed the evidence for using naltrexone, acamprosate, disulfiram, and varenicline, as well as psychotherapeutic options, to treat substance abuse. Dr. Janicak encouraged clinicians in the audience to treat substance abuse in bipolar disorder patients themselves, instead of referring them to a subspecialist.
Untreated psychiatric disorders increase obstetrical complications, possibly through decreased self-care or increased stress. For mild or moderate depression, psychotherapy might be sufficient treatment; but for severe cases, medication is the first-line approach. In her presentation on mood disorders during pregnancy, Marlene P. Freeman, MD, Massachusetts General Hospital, advises that clinicians select medications based on known safety information, patient preference, and the previous course of illness. Results of studies that lasted 4 to 5 years do not show major long-term adverse effects of antidepressant exposure on neurodevelopment or neurobehavior. When treating patients for bipolar disorder, valproate is associated with an increased risk of adverse cognitive and neurodevelopmental effects in infants compared with other anticonvulsants; evidence suggests that lamotrigine is a safer option. The research does not show an increased risk of major malformations with second-generation antipsychotics.
AFTERNOON SESSION
Most women have premenstrual symptoms; a minority have a full-blown syndrome, now known as premenstrual dysphoric disorder (PMDD). This is not an existing mood disorder that becomes worse premenstrually. Clinician and patients should track the temporal relationship of symptoms on a calendar for a few months. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine have been well studied and are effective compared with placebo, but don’t help all patients with PMDD. Consider flexible dosing strategies with SSRIs—perhaps daily use, a higher dosage premenstrually, and as-needed administration. Start with an oral contraceptive or SSRI; if symptoms don’t respond, add the other. Serotonergic antidepressants have been shown helpful for hot flashes and depressive symptoms in perimenopause. Dr. Freeman reviewed the evidence for using complementary and alternative therapies for menopausal symptoms and hot flushes.
Smoking contributes to excess mortality in seriously mentally ill patients as a result of such tobacco-related illnesses as heart disease, lung disease, and cancer. Overall improvement in mental health as well as physical health is seen when a patient stops smoking. All nicotine replacement products are effective, but patients often don’t use them long enough or correctly. Robert M. Anthenelli, MD, University of California, San Diego, said to begin sustained-release bupropion 1 or 2 weeks before quit date; maintain the dosage for 1 to 12 weeks after quit date and consider maintenance therapy for as long as 6 months. Varenicline is superior to placebo and bupropion, but is known to have gastrointestinal (GI) and sleep disturbance adverse effects. Quitting smoking can increase the blood level of some psychotropics, meaning that you might need to reduce their dosage. It is best to begin smoking cessation when patients are mentally stable, when motivated, and stable on their medications.
In discussing trends in substance abuse, Dr. Anthenelli
faddish. Fentanyl and fentanyl analogues are 100 times more powerful than morphine; ingestion of even a minuscule dose can be fatal. Synthetic cannabinoids primarily are a problem among adolescents; they are more dangerous than marijuana and are associated with aggressive and suicidal behaviors. A standard toxicology screen will not detect synthetic cannabinoids.
E-cigarettes are considered by users to be safer than tobacco cigarettes—and probably are—but they still put patients at risk of nicotine addiction. There are no safety data on e-cigarettes; the devices might contain potentially harmful chemicals and potentially toxic nicotine levels. Dr. Anthenelli reported that topiramate is “the best medication I’ve used” for alcohol abuse disorder. The drug is not FDA-approved for this use, but has been used in a number of studies with positive outcomes.
SATURDAY, APRIL 18, 2015
MORNING SESSION
Psychiatrists are well positioned to help patients with mental illness lose weight because of their psychotherapeutic background. Best treatment strategy is diet plus exercise plus behavioral modification. Robert M. McCarron, DO, University of California, Davis, recommends keeping it simple and telling patients to only consider calories of foods, and not to worry about sodium or fat content. Ask patients “How many minutes a day of exercise can you do?” but recommend that patients walk for 30 minutes a day at 4 mph, 5 days per week, which will help patients lose 1% to 3% of body weight. For treatment-refractory obese patients, consider medications such as bupropion, orlistat, lorcaserin, topiramate, or metformin; for those with a BMI ≥40, recommend bariatric surgery.
George T. Grossberg, MD, Saint Louis University School of Medicine, reviewed the evidence for anxiety disorders in older adults, including generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Older patients with cardiovascular disease, cancer, Parkinson’s disease, diabetes, GI disorders, or chronic obstructive pulmonary disease are at high risk of anxiety symptoms. In a study of centenarians, predictors of anxiety are worse health perception, financial concerns related to medical expenses, higher number of medical conditions, and loneliness. Secondary anxiety is prevalent in Alzheimer’s disease; the condition can present as fidgeting, pacing, anger, or agitation, and can be prompted by a change in routine. Acute, new-onset anxiety symptoms should trigger a complete medical evaluation, including a review of medications, supplements, and substance use. In geriatric patients, minimize use of benzodiazepines and avoid anticholinergics.
Overall, psychiatry patients do not receive optimal preventive and primary medical care, leading to decreased life expectancy, often as a result of cardiovascular disease. Psychiatric patients have a high rate of dyslipidemia, hypertension, smoking, and obesity. Psychiatrists often don’t treat these conditions, but they need to be aware of changing standard practices in preventive medicine; be able to recognize a potential problem; and make referrals when appropriate. Dr. McCarron reviewed age-based screening recommendations for hypertension, dyslipidemia, and diabetes from the book Preventive Medical Care in Psychiatry, which he co-edited. He recommends using online cardiovascular risk calculators to determine which patients need to be screened.
AFTERNOON SESSION
Some older patients who abuse substances took drugs as young adults and never gave them up; others have rediscovered drugs in later life. Potential indicators of alcohol abuse in older patients are changes in cognition, mood, memory, hygiene, or sleep. Substance abuse in older adults frequently is comorbid with depression or bereavement, anxiety, and adjustment disorders. Dr. Grossberg recommends addressing the topic directly with patients. Although there are few data to guide treatment, prompt detection and appropriate treatment can improve the quality of life of older adults and their family.
SPONSORS AND SUPPORTERS
• American Professional Agency
• American Psychiatric Publishing
• Arbor Pharmaceuticals
• AstraZeneca
• Banner Health
• Bassett Healthcare Network
• Ministry Health Care
• Pine Rest Christian Mental Health Services
• PRMS
• Sinai Health System
• Sunovion
• Takeda Pharmaceuticals
• U.S. Army Healthcare
• Wexford Health Sources
• Wolters Kluwer Health
The meeting organizers acknowledge the support provided by the sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-directors. Sponsors and supporters did not have input in these areas.
Current Psychiatry welcomed more than 650 psychiatric practitioners from across the United States and abroad to this annual conference, which was headed by Meeting Co-chairs Richard Balon, MD, and Donald W. Black, MD, April 16-18, 2015, at the Hilton Chicago in Chicago, Illinois. Attendees earned as many as 18 AMA PRA Category 1 Credits™. We welcome you to join us next year in Chicago, March 10-12, 2016.
THURSDAY, APRIL 16, 2015
MORNING SESSION
Attention-deficit/hyperactivity disorder (ADHD) is a lifespan disorder that is “everywhere,” Anthony L. Rostain, MD, MA, University of Pennsylvania Perelman School of Medicine, began—including in adults and even “seniors.” This means that the disorder “is not a diagnosis of exclusion,” and that “comorbidity is the rule,” including learning difficulties. Among adults, the focus of symptoms and management is on executive dysfunction and its characteristics: difficulty multitasking, problems keeping commitments, and excessive reliance on help from others. Inattention and disorganization are hallmarks of adult ADHD, and become worse as environmental demands (work, home) increase; hyperactivity decreases with age. Dr. Rostain recommends ruling out other causes of a patient’s symptoms when an adult self-reports ADHD, including transient stressors, medical conditions, psychiatric disorders, and malingering.
Donald W. Black, MD, University of Iowa, reviewed DSM-5 criteria for borderline personality disorder (BPD) and offered tips for avoiding misdiagnosis, including obtaining collateral information and using rating scales. Co-occuring disorders, such as depression and substance abuse, are common. Treatment for BPD patients includes psychotherapy (individual or group), medication, and lifestyle changes. Psychotropics treat symptoms of depression, anxiety, hostility, and impulsivity of BPD but not the fundamental nature of the disorder. When establishing a patient’s treatment plan, consider the stage of illness, evaluate for any co-occurring disorders, and ask the patient what he (she) wants from treatment.
Dr. Rostain began by discussing the neurobiological basis of ADHD, which guides pharmacotherapy. He reviewed the response rate of FDA-approved agents for adults with ADHD, including stimulants, atomoxetine, and alpha-adrenergic agonists. Best response is seen with stimulants, but some patients improve with bupropion and tricyclic antidepressants (TCAs). Employ a multimodal treatment approach, Dr. Rostain recommended, which should include psychoeducation and environmental restructuring, because, as he says, “Pills don’t teach skills.” He also reviewed strategies for treating ADHD in patients who have a comorbid disorder, such as bipolar disorder, major depressive disorder, or substance abuse.
Patients with psychotic depression meet criteria for major depressive disorder but also have delusions or hallucinations. Diagnostic issues include increased guilt, cognitive impairment, paranoia, and increased hopelessness. Anthony J. Rothschild, MD, University of Massachusetts Medical School, reviewed methods for differentiating psychotic depression from schizophrenia, posttraumatic stress disorder, obsessive-compulsive disorder, and body dysmorphic disorder. There are no FDA-approved medications for psychotic depression, Dr. Rothschild explained; however, evidence shows that the combination of an antidepressant and an antipsychotic is superior to monotherapy with an agent from either class. In addition, he noted, studies show a high response rate with electroconvulsive therapy (ECT).
AFTERNOON SESSION
Return of symptoms after initial remission— while the patient is still taking an antidepressant—is considered tachyphylaxis, or “poop out.” Residual depressive symptoms, when a patient meets criteria for remission but still has troubling symptoms, is a different phenomenon, although symptoms can overlap. First, Dr. Rothschild advised, ensure that patients are given an adequate trial of an antidepressant. Options are similar when tachyphylaxis or residual symptoms are present: switch drugs or add augmentation therapy, such as lithium, thyroid hormone, or an atypical antipsychotic. Data on the efficacy for bupropion and buspirone are not strong. For treatment-resistant depression when a patient does not respond to 3 adequate antidepressant trials—consider ECT or rTMS, if available, or a monoamine oxidase inhibitor or a TCA.
Dr. Black defines antisocial personality disorder (ASPD) as a disorder of lifelong serial misbehavior, one characterized by impaired relationships, aggressive behavior, non-aggressive delinquent behavior, manipulation, and a disturbing lack of conscience. There is no standard treatment for ASPD, and no FDA-approved medications; however, potential treatments have not been adequately studied, he pointed out. Cognitive-behavioral therapy might be appropriate in mild cases; some patients benefit from specific programs— for example, ones that address drug or alcohol addiction or anger, although evidence is limited. When treating ASPD patients, Dr. Black concluded, be mindful of high attrition, possible misuse of prescribed medications, and drug-drug or drug-alcohol interactions.
Bipolar disorder is associated with the highest risk of suicide and increased lethality among all psychiatric disorders. Lithium has evidence of an anti-suicidality effect and may reduce suicide by decreasing relapse, aggression, and impulsivity. An FDA advisory on increased risk of suicidality with anticonvulsants was based on data about patients with epilepsy, not bipolar disorder. Second-generation antipsychotics, including olanzapine, quetiapine, and lurasidone, have been shown to be effective for bipolar depression. Avoid antidepressants if possible, Philip G. Janicak, MD, Northwestern University Feinberg School of Medicine, advised; if you must prescribe one, reassess the need for the drug often. Several psychotherapy modalities have evidence supporting their use in bipolar disorder.
FRIDAY, APRIL 17, 2015
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University School of Medicine, offered enlightening historical touch-points on how psychiatry’s understanding of, and its approach to, schizophrenia have changed in the past 50 years. His goal? To challenge practitioners to rethink ideas about the disorder and how they care for affected patients. From a laundry list of comparative shifts, here are a few of Dr. Nasrallah’s “then” and “now” observations:
• The old paradigm was: Clinical and functional deterioration are inevitable in schizophrenia. The new paradigm is: Complete remission and restoration of function are feasible in many patients when they are fully adherent to the treatment plan.
• The old: Long-acting injectable (LAI) antipsychotics are a last-resort treatment, to be prescribed after a patient is stabilized. The new: Use LAI antipsychotics early in the course.
• Old: Begin treatment when psychosis appears. New: Work to prevent conversion to psychosis.
• Old: The disorder is considered a consequence of neurochemical dysregulation. New: Impaired neuroplasticity is to blame.
• Old: Treatment is a matter of trial and error. New: We can apply pharmaco-genomics to predict a patient’s response to various drugs and thus increase the likelihood of therapeutic success.
In his second presentation, Dr. Nasrallah described the many pathways to psychosis and several psychotic disorders other than schizophrenia, including schizoaffective, delusional disorder, and psychotic disorder caused by a general medical condition. He listed symptom clusters in psychosis beyond positive and negative symptoms, including neuromotor symptoms, mood symptoms, and neurocognitive deficits. Development of schizophrenia is multifactorial and involves risk genes and environmental factors seen before conception, during birth, and in early childhood; good prenatal care is the best way to prevent schizophrenia, Dr. Nasrallah noted. Several general medical conditions can produce schizophrenia-like psychosis, including some CNS disorders, toxins, autoimmune diseases, infectious diseases, and chromosomal abnormalities. The session concluded with a live interview with one of Dr. Nasrallah’s patients, whose schizophrenia is in remission with clozapine.
Drug abuse can mask signs and symptoms of bipolar disorder, which can delay diagnosis. Commonly abused substances are nicotine, alcohol, Cannabis, and cocaine; polysubstance abuse is the rule. Bipolar disorder and substance abuse share common mechanisms: impulsivity, poor modulation of motivation and response to reward, and behavioral sensitization. Treatment approaches should be flexible. Dr. Janicak reviewed the evidence for using anticonvulsants, antipsychotics, and bupropion for alcohol, Cannabis, and cocaine abuse; there are no data on treating opioid abuse. He also discussed the evidence for using naltrexone, acamprosate, disulfiram, and varenicline, as well as psychotherapeutic options, to treat substance abuse. Dr. Janicak encouraged clinicians in the audience to treat substance abuse in bipolar disorder patients themselves, instead of referring them to a subspecialist.
Untreated psychiatric disorders increase obstetrical complications, possibly through decreased self-care or increased stress. For mild or moderate depression, psychotherapy might be sufficient treatment; but for severe cases, medication is the first-line approach. In her presentation on mood disorders during pregnancy, Marlene P. Freeman, MD, Massachusetts General Hospital, advises that clinicians select medications based on known safety information, patient preference, and the previous course of illness. Results of studies that lasted 4 to 5 years do not show major long-term adverse effects of antidepressant exposure on neurodevelopment or neurobehavior. When treating patients for bipolar disorder, valproate is associated with an increased risk of adverse cognitive and neurodevelopmental effects in infants compared with other anticonvulsants; evidence suggests that lamotrigine is a safer option. The research does not show an increased risk of major malformations with second-generation antipsychotics.
AFTERNOON SESSION
Most women have premenstrual symptoms; a minority have a full-blown syndrome, now known as premenstrual dysphoric disorder (PMDD). This is not an existing mood disorder that becomes worse premenstrually. Clinician and patients should track the temporal relationship of symptoms on a calendar for a few months. Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine have been well studied and are effective compared with placebo, but don’t help all patients with PMDD. Consider flexible dosing strategies with SSRIs—perhaps daily use, a higher dosage premenstrually, and as-needed administration. Start with an oral contraceptive or SSRI; if symptoms don’t respond, add the other. Serotonergic antidepressants have been shown helpful for hot flashes and depressive symptoms in perimenopause. Dr. Freeman reviewed the evidence for using complementary and alternative therapies for menopausal symptoms and hot flushes.
Smoking contributes to excess mortality in seriously mentally ill patients as a result of such tobacco-related illnesses as heart disease, lung disease, and cancer. Overall improvement in mental health as well as physical health is seen when a patient stops smoking. All nicotine replacement products are effective, but patients often don’t use them long enough or correctly. Robert M. Anthenelli, MD, University of California, San Diego, said to begin sustained-release bupropion 1 or 2 weeks before quit date; maintain the dosage for 1 to 12 weeks after quit date and consider maintenance therapy for as long as 6 months. Varenicline is superior to placebo and bupropion, but is known to have gastrointestinal (GI) and sleep disturbance adverse effects. Quitting smoking can increase the blood level of some psychotropics, meaning that you might need to reduce their dosage. It is best to begin smoking cessation when patients are mentally stable, when motivated, and stable on their medications.
In discussing trends in substance abuse, Dr. Anthenelli
faddish. Fentanyl and fentanyl analogues are 100 times more powerful than morphine; ingestion of even a minuscule dose can be fatal. Synthetic cannabinoids primarily are a problem among adolescents; they are more dangerous than marijuana and are associated with aggressive and suicidal behaviors. A standard toxicology screen will not detect synthetic cannabinoids.
E-cigarettes are considered by users to be safer than tobacco cigarettes—and probably are—but they still put patients at risk of nicotine addiction. There are no safety data on e-cigarettes; the devices might contain potentially harmful chemicals and potentially toxic nicotine levels. Dr. Anthenelli reported that topiramate is “the best medication I’ve used” for alcohol abuse disorder. The drug is not FDA-approved for this use, but has been used in a number of studies with positive outcomes.
SATURDAY, APRIL 18, 2015
MORNING SESSION
Psychiatrists are well positioned to help patients with mental illness lose weight because of their psychotherapeutic background. Best treatment strategy is diet plus exercise plus behavioral modification. Robert M. McCarron, DO, University of California, Davis, recommends keeping it simple and telling patients to only consider calories of foods, and not to worry about sodium or fat content. Ask patients “How many minutes a day of exercise can you do?” but recommend that patients walk for 30 minutes a day at 4 mph, 5 days per week, which will help patients lose 1% to 3% of body weight. For treatment-refractory obese patients, consider medications such as bupropion, orlistat, lorcaserin, topiramate, or metformin; for those with a BMI ≥40, recommend bariatric surgery.
George T. Grossberg, MD, Saint Louis University School of Medicine, reviewed the evidence for anxiety disorders in older adults, including generalized anxiety disorder, obsessive-compulsive disorder, panic disorder, and posttraumatic stress disorder. Older patients with cardiovascular disease, cancer, Parkinson’s disease, diabetes, GI disorders, or chronic obstructive pulmonary disease are at high risk of anxiety symptoms. In a study of centenarians, predictors of anxiety are worse health perception, financial concerns related to medical expenses, higher number of medical conditions, and loneliness. Secondary anxiety is prevalent in Alzheimer’s disease; the condition can present as fidgeting, pacing, anger, or agitation, and can be prompted by a change in routine. Acute, new-onset anxiety symptoms should trigger a complete medical evaluation, including a review of medications, supplements, and substance use. In geriatric patients, minimize use of benzodiazepines and avoid anticholinergics.
Overall, psychiatry patients do not receive optimal preventive and primary medical care, leading to decreased life expectancy, often as a result of cardiovascular disease. Psychiatric patients have a high rate of dyslipidemia, hypertension, smoking, and obesity. Psychiatrists often don’t treat these conditions, but they need to be aware of changing standard practices in preventive medicine; be able to recognize a potential problem; and make referrals when appropriate. Dr. McCarron reviewed age-based screening recommendations for hypertension, dyslipidemia, and diabetes from the book Preventive Medical Care in Psychiatry, which he co-edited. He recommends using online cardiovascular risk calculators to determine which patients need to be screened.
AFTERNOON SESSION
Some older patients who abuse substances took drugs as young adults and never gave them up; others have rediscovered drugs in later life. Potential indicators of alcohol abuse in older patients are changes in cognition, mood, memory, hygiene, or sleep. Substance abuse in older adults frequently is comorbid with depression or bereavement, anxiety, and adjustment disorders. Dr. Grossberg recommends addressing the topic directly with patients. Although there are few data to guide treatment, prompt detection and appropriate treatment can improve the quality of life of older adults and their family.
SPONSORS AND SUPPORTERS
• American Professional Agency
• American Psychiatric Publishing
• Arbor Pharmaceuticals
• AstraZeneca
• Banner Health
• Bassett Healthcare Network
• Ministry Health Care
• Pine Rest Christian Mental Health Services
• PRMS
• Sinai Health System
• Sunovion
• Takeda Pharmaceuticals
• U.S. Army Healthcare
• Wexford Health Sources
• Wolters Kluwer Health
The meeting organizers acknowledge the support provided by the sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-directors. Sponsors and supporters did not have input in these areas.
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
What stalking victims need to restore their mental and somatic health
The obsessive pursuit of another has long been described in fiction and the scientific literature, but was conceptualized as “stalking” only relatively recently—first, under the guise of celebrity stalking and, later, as a public health issue recognized as affecting the general population. A useful working definition of stalking is “… the willful, malicious, and repeated following of and harassing of another person that threatens his/her safety.”1
Stalking victims report numerous, severe, life-changing effects from being stalked, including physical, social, and psychological harm. They typically experience mood, anxiety, and posttraumatic stress symptoms that require prompt evaluation and treatment.
Prevalence and other characteristics
Stalking and its subsequent victimization are common. Here are statistics:
• in the United States, approximately 1 million women and 370,000 men are stalked annually
• women are 3 times more likely to be stalked than raped2
• lifetime prevalence of stalking victimization is 20% (women, 23.5%; men, 10.5%)
• 75% of stalking victims are women
• 77% of stalking emerges from a prior acquaintance, including 49% that originated in a romantic relationship
• 33% of stalking encounters eventually lead to physical violence; slightly >10% of encounters lead to sexual violence
• stalking persists for an extended period; on average, almost 2 years.3
Penalties. Stalking can result in intervention by the criminal justice system. Legal sanctions levied on the perpetrator vary, depending on (among other variables) the severity of stalking; type of stalking; motive of the stalker; and the strength of incriminating evidence. Surprisingly, the outcome of the perpetrator’s prosecution (arrest, conviction, length of sentence) is unrelated to whether the victim reported continued stalking at follow-up.4,5
What are the symptoms and the damage? Given the intrusive nature of stalking behaviors and the extended period during which stalking persists, victims typically experience harmful psychological effects that range from subclinical symptoms to overt psychiatric disorders.
Stalking can have a profound impact on the victim and result in numerous psychological symptoms that become the focus of clinical attention. The typically chronic nature of stalking probably plays a significant role in its contributions to its victims’ psychological distress.6 Melton7 found that the most common adverse effect of stalking was related to the emotional impact of being stalked—with victims feeling scared, depressed, humiliated, embarrassed, distrustful of others, and angry or hateful.
Stalking victims report traumatic stress, hypervigilance, excessive fear, and anxiety coupled with disruptions in employment and social interactions.8 Many report having become highly distrustful or suspicious (44%); fearful (42%); nervous (31%); angry (27%); paranoid (36%); and depressed (21%). In general, victims have elevated scores on the Trauma Symptom Checklist.9
Stalking in the setting of intimate partner abuse is associated with harmful outcomes for the victim. These include repeat physical violence, psychological distress, and impaired physical or mental health, or both.3,7,10
Stalking victims who are female; had a prior relationship with the stalker; have experienced a greater variety of stalking behaviors; are divorced or separated; and have received government assistance were found to be more likely to experience multiple negative outcomes from stalking.11
Effects on mental health. Stalking victims have a higher incidence of mental disorders and comorbid illnesses compared with the general population,12 with the most robust associations identified between stalking victimization, major depressive disorder, and panic disorder. Stalking contributes to symptoms of posttraumatic stress disorder,13 and there is an association between posttraumatic stress and poor general health.14 Stalking victims report higher current use of psychotropic medications.12
Victims who blame themselves for being stalked report a significantly higher severity of depression, anxiety, and posttraumatic stress symptoms. Those who ruminate more about the stalking experience, or who explicitly emphasize the terror of stalking to a greater extent, also report a significantly higher severity of symptoms.15
Spitzberg3 reported that stalking victimization has several possible effects on victims (Table 1).
Coping by movement. Victims might attempt to cope with stalking through several means,2 including:
• moving away—trying to avoid contact with the stalker
• moving with—negotiating a more acceptable form of relationship with the stalker
• moving against—attempting to harm, constrain, or punish the stalker
• moving inward—seeking self-control or self-actualization
• moving outward—seeking the assistance of others.
The degree of a victim’s symptoms correlates partially with the severity of stalking. However, other variables play a crucial role in explaining the level of distress among stalking victims15; these include the types of coping strategies adopted by victims. Self-blame, catastrophizing, and rumination are significantly associated with maladjustment; on the other hand, positive reappraisal—thoughts of attaching a positive meaning to the event, in terms of personal growth—is associated with greater psychological adjustment.
The more stalking a victim experiences (and, presumably, experiences greater distress), the greater the variety of coping strategies she (he) employs.16
How should stalking victims be treated?
Stalking victims are an underserved population. Practitioners often are unsure how to address stalking; furthermore, available treatments can be ineffective.
There is a great deal of variability in what professionals who work with stalking victims believe is appropriate practice. Services provided to victims vary widely,17 and the field has not yet come to a consensus on best practices.16
Proceed case by case. Practitioners must understand the nuances of each case to consider what might work at a particular point in time, and information from victims can help guide decision-making.16 Evidence suggests that stalking victims can feel frustrated in their attempt to seek help, particularly from the criminal justice system; it is possible that such bad experiences may dissuade them from seeking help later.5,8,18 It is worth noting that, as the frequency of stalking decreases for any given victim, her (his) perception of safety increases and distress diminishes.16
Few communities have attempted to address systemically the problem of stalking. Existing anti-stalking programs have focused on the criminal justice aspects of intervention,8 with less emphasis on treating victims.
Some stalking victims rely on friends and family for support and assistance, but research shows that most reach out to agencies for assistance and, generally, seek help from multiple sources.18 Typically, stalking victims are served by 2 types of victim service organizations:
• specialized, small, private and nonprofit agencies (eg, domestic violence shelters, rape crisis centers, victims’ rights advocacy organizations)
• small units housed in police departments and prosecutors’ offices.17
Note: When victims seek services at criminal justice agencies, they may be feeling particularly unsafe and distressed. This underscores the importance of co-locating victim service providers and criminal justice agencies.16
Stalking victims might benefit from multi-disciplinary team consultation, including input from psychiatric, psychotherapeutic, and law enforcement or security professionals. Key priorities for practitioners to address with stalking victims are given in Table 2.19
Stalking behavior does not significantly decrease when victims are in contact with victim services.16 Practitioners can integrate this prospect into their understanding of stalking when they work with victims: That is, it is likely that the problem will not go away quickly, even with intervention.
Victims’ needs remain great and broad-based. Spence-Diehl et al17 conducted a survey of service providers for stalking victims, evaluating the needs of those victims and the response of their communities. Some of their recommendations for better meeting victims’ needs are in Table 3.16
Keeping victims at the center
Several authors have written about the need to return to a victim-centered model of care. This approach (1) puts the victim’s understanding of her (his) situation at the center of victim assistance work and (2) views service providers as consultants in the decision-making process.20,21 The victim-centered approach to treatment, in which the client has a greater voice and degree of control over interventions, is associated with positive outcomes.22,23
At the heart of a client-centered model of victim assistance is the provider’s ability to listen to a victim’s story and respond in a nonjudgmental manner. This approach honors the victim’s circumstances and her personal understanding of risk.21
Bottom Line
Stalking victims are a distinctive population, experiencing numerous emotional, physical, and social effects of their stalking over an extended period. Services to treat this underserved population need to be further developed. A multifaceted approach to treating victims incorporates psychological, somatic, and practical interventions, and a victim-centered approach is associated with better outcomes.
Related Resources
• Harmon RB, O’Connor M. Forcier A, et al. The impact of anti-stalking training on front line service providers: using the anti-stalking training evaluation protocol (ASTEP). J Forensic Science. 2004;49(5):1050-1055.
• Spitzberg BH, Cupach WR. The state of the art of stalking: taking stock of the emerging literature. Aggression and Violence Behavior. 2007;12(1):64-86.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Meloy JR, Gothard S. Demographic and clinical comparison of obsessional followers and offenders with mental disorders. Am J Psychiatry. 1995;152(2):258-263.
2. Tjaden P, Thoennes N. Stalking in America: findings from the National Violence Against Women Survey. National Institute of Justice and Centers for Disease Control and Prevention. https://www.ncjrs.gov/pdffiles/169592.pdf. Published April 1998. Accessed March 25, 2015.
3. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma, Violence, & Abuse. 2002;3(4):261-288.
4. McFarlane J, Willson P, Lemmey D, et al. Women filing assault charges on an intimate partner: criminal justice outcome and future violence experienced. Violence Against Women. 2000;6(4):396-408.
5. Melton HC. Stalking in the context of domestic violence: findings on the criminal justice system. Women & Criminal Justice. 2004;15:33-58.
6. Davies KE, Frieze IH. Research on stalking: what do we know and where do we go? Violence Vict. 2000;15(4):473-487.
7. Melton HC. Stalking in the context of intimate partner abuse: in the victims’ words. Feminist Criminology. 2007;2(4):346-363.
8. Spence-Diehl E. Intensive case management for victims of stalking: a pilot test evaluation. Brief Treatment Crisis Intervention. 2004;4(4):323-341.
9. Brewster MP. An exploration of the experiences and needs of former intimate stalking victims: final report submitted to the National Institute of Justice. West Chester, PA: West Chester University; 1997.
10. Logan TK, Shannon L, Cole J, et al. The impact of differential patterns of physical violence and stalking on mental health and help-seeking among women with protective orders. Violence Against Women. 2006;12(9):866-886.
11. Johnson MC, Kercher GA. Identifying predictors of negative psychological reactions to stalking victimization. J Interpers Violence. 2009;24(5):866-882.
12. Kuehner C, Gass P, Dressing H. Increased risk of mental disorders among lifetime victims of stalking—findings from a community study. Eur Psychiatry. 2007;22(3):142-145.
13. Basile KC, Arias I, Desai S, et al. The differential association of intimate partner physical, sexual, psychological, and stalking violence and post-traumatic stress symptoms in a nationally representative sample of women. J Traumatic Stress. 2004;17(5):413-421.
14. Kamphuis JH, Emmelkamp PM. Traumatic distress among support-seeking female victims of stalking. Am J Psychiatry. 2001;158(5):795-798.
15. Kraaij V, Arensman E, Garnefski N, et al. The role of cognitive coping in female victims of stalking. J Interpers Violence. 2007;22(12):1603-1612.
16. Bennett Cattaneo L, Cho S, Botuck S. Describing intimate partner stalking over time: an effort to inform victim-centered service provision. J Interpers Violence. 2011;26(17):3428-3454.
17. Spence-Diehl E, Potocky-Tripodi M. Victims of stalking: a study of service needs as perceived by victim services practitioners. J Interpers Violence. 2001;16(1):86-94.
18. Galeazzi GM, Buc˘ar-Ruc˘man A, DeFazio L, et al. Experiences of stalking victims and requests for help in three European countries. A survey. European Journal of Criminal Policy Research. 2009;15:243-260.
19. McEwan T, Purcell R. Assessing and surviving stalkers. Presented at: 45th Annual Meeting of American Academy of Psychiatry and the Law; October 2014; Chicago IL.
20. Cattaneo LB, Goodman LA. New directions in IPV risk assessment: an empowerment approach to risk management. In: Kendall-Tackett K, Giacomoni S, eds. Intimate partner violence. Kingston, NJ: Civic Research Institute; 2007:1-17.
21. Goodman LA, Epstein D. Listening to battered women: a survivor-centered approach to advocacy, mental health, and justice. Washington DC: American Psychological Association; 2008.
22. Cattaneo LB, Goodman LA. Through the lens of jurisprudence: the relationship between empowerment in the court system and well-being for intimate partner violence victims. J Interpers Violence. 2010;25(3):481-502.
23. Zweig JM, Burt MR. Predicting women’s perceptions of domestic violence and sexual assault agency helpfulness: what matters to program clients? Violence Against Women. 2007;13(11):1149-1178.
The obsessive pursuit of another has long been described in fiction and the scientific literature, but was conceptualized as “stalking” only relatively recently—first, under the guise of celebrity stalking and, later, as a public health issue recognized as affecting the general population. A useful working definition of stalking is “… the willful, malicious, and repeated following of and harassing of another person that threatens his/her safety.”1
Stalking victims report numerous, severe, life-changing effects from being stalked, including physical, social, and psychological harm. They typically experience mood, anxiety, and posttraumatic stress symptoms that require prompt evaluation and treatment.
Prevalence and other characteristics
Stalking and its subsequent victimization are common. Here are statistics:
• in the United States, approximately 1 million women and 370,000 men are stalked annually
• women are 3 times more likely to be stalked than raped2
• lifetime prevalence of stalking victimization is 20% (women, 23.5%; men, 10.5%)
• 75% of stalking victims are women
• 77% of stalking emerges from a prior acquaintance, including 49% that originated in a romantic relationship
• 33% of stalking encounters eventually lead to physical violence; slightly >10% of encounters lead to sexual violence
• stalking persists for an extended period; on average, almost 2 years.3
Penalties. Stalking can result in intervention by the criminal justice system. Legal sanctions levied on the perpetrator vary, depending on (among other variables) the severity of stalking; type of stalking; motive of the stalker; and the strength of incriminating evidence. Surprisingly, the outcome of the perpetrator’s prosecution (arrest, conviction, length of sentence) is unrelated to whether the victim reported continued stalking at follow-up.4,5
What are the symptoms and the damage? Given the intrusive nature of stalking behaviors and the extended period during which stalking persists, victims typically experience harmful psychological effects that range from subclinical symptoms to overt psychiatric disorders.
Stalking can have a profound impact on the victim and result in numerous psychological symptoms that become the focus of clinical attention. The typically chronic nature of stalking probably plays a significant role in its contributions to its victims’ psychological distress.6 Melton7 found that the most common adverse effect of stalking was related to the emotional impact of being stalked—with victims feeling scared, depressed, humiliated, embarrassed, distrustful of others, and angry or hateful.
Stalking victims report traumatic stress, hypervigilance, excessive fear, and anxiety coupled with disruptions in employment and social interactions.8 Many report having become highly distrustful or suspicious (44%); fearful (42%); nervous (31%); angry (27%); paranoid (36%); and depressed (21%). In general, victims have elevated scores on the Trauma Symptom Checklist.9
Stalking in the setting of intimate partner abuse is associated with harmful outcomes for the victim. These include repeat physical violence, psychological distress, and impaired physical or mental health, or both.3,7,10
Stalking victims who are female; had a prior relationship with the stalker; have experienced a greater variety of stalking behaviors; are divorced or separated; and have received government assistance were found to be more likely to experience multiple negative outcomes from stalking.11
Effects on mental health. Stalking victims have a higher incidence of mental disorders and comorbid illnesses compared with the general population,12 with the most robust associations identified between stalking victimization, major depressive disorder, and panic disorder. Stalking contributes to symptoms of posttraumatic stress disorder,13 and there is an association between posttraumatic stress and poor general health.14 Stalking victims report higher current use of psychotropic medications.12
Victims who blame themselves for being stalked report a significantly higher severity of depression, anxiety, and posttraumatic stress symptoms. Those who ruminate more about the stalking experience, or who explicitly emphasize the terror of stalking to a greater extent, also report a significantly higher severity of symptoms.15
Spitzberg3 reported that stalking victimization has several possible effects on victims (Table 1).
Coping by movement. Victims might attempt to cope with stalking through several means,2 including:
• moving away—trying to avoid contact with the stalker
• moving with—negotiating a more acceptable form of relationship with the stalker
• moving against—attempting to harm, constrain, or punish the stalker
• moving inward—seeking self-control or self-actualization
• moving outward—seeking the assistance of others.
The degree of a victim’s symptoms correlates partially with the severity of stalking. However, other variables play a crucial role in explaining the level of distress among stalking victims15; these include the types of coping strategies adopted by victims. Self-blame, catastrophizing, and rumination are significantly associated with maladjustment; on the other hand, positive reappraisal—thoughts of attaching a positive meaning to the event, in terms of personal growth—is associated with greater psychological adjustment.
The more stalking a victim experiences (and, presumably, experiences greater distress), the greater the variety of coping strategies she (he) employs.16
How should stalking victims be treated?
Stalking victims are an underserved population. Practitioners often are unsure how to address stalking; furthermore, available treatments can be ineffective.
There is a great deal of variability in what professionals who work with stalking victims believe is appropriate practice. Services provided to victims vary widely,17 and the field has not yet come to a consensus on best practices.16
Proceed case by case. Practitioners must understand the nuances of each case to consider what might work at a particular point in time, and information from victims can help guide decision-making.16 Evidence suggests that stalking victims can feel frustrated in their attempt to seek help, particularly from the criminal justice system; it is possible that such bad experiences may dissuade them from seeking help later.5,8,18 It is worth noting that, as the frequency of stalking decreases for any given victim, her (his) perception of safety increases and distress diminishes.16
Few communities have attempted to address systemically the problem of stalking. Existing anti-stalking programs have focused on the criminal justice aspects of intervention,8 with less emphasis on treating victims.
Some stalking victims rely on friends and family for support and assistance, but research shows that most reach out to agencies for assistance and, generally, seek help from multiple sources.18 Typically, stalking victims are served by 2 types of victim service organizations:
• specialized, small, private and nonprofit agencies (eg, domestic violence shelters, rape crisis centers, victims’ rights advocacy organizations)
• small units housed in police departments and prosecutors’ offices.17
Note: When victims seek services at criminal justice agencies, they may be feeling particularly unsafe and distressed. This underscores the importance of co-locating victim service providers and criminal justice agencies.16
Stalking victims might benefit from multi-disciplinary team consultation, including input from psychiatric, psychotherapeutic, and law enforcement or security professionals. Key priorities for practitioners to address with stalking victims are given in Table 2.19
Stalking behavior does not significantly decrease when victims are in contact with victim services.16 Practitioners can integrate this prospect into their understanding of stalking when they work with victims: That is, it is likely that the problem will not go away quickly, even with intervention.
Victims’ needs remain great and broad-based. Spence-Diehl et al17 conducted a survey of service providers for stalking victims, evaluating the needs of those victims and the response of their communities. Some of their recommendations for better meeting victims’ needs are in Table 3.16
Keeping victims at the center
Several authors have written about the need to return to a victim-centered model of care. This approach (1) puts the victim’s understanding of her (his) situation at the center of victim assistance work and (2) views service providers as consultants in the decision-making process.20,21 The victim-centered approach to treatment, in which the client has a greater voice and degree of control over interventions, is associated with positive outcomes.22,23
At the heart of a client-centered model of victim assistance is the provider’s ability to listen to a victim’s story and respond in a nonjudgmental manner. This approach honors the victim’s circumstances and her personal understanding of risk.21
Bottom Line
Stalking victims are a distinctive population, experiencing numerous emotional, physical, and social effects of their stalking over an extended period. Services to treat this underserved population need to be further developed. A multifaceted approach to treating victims incorporates psychological, somatic, and practical interventions, and a victim-centered approach is associated with better outcomes.
Related Resources
• Harmon RB, O’Connor M. Forcier A, et al. The impact of anti-stalking training on front line service providers: using the anti-stalking training evaluation protocol (ASTEP). J Forensic Science. 2004;49(5):1050-1055.
• Spitzberg BH, Cupach WR. The state of the art of stalking: taking stock of the emerging literature. Aggression and Violence Behavior. 2007;12(1):64-86.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The obsessive pursuit of another has long been described in fiction and the scientific literature, but was conceptualized as “stalking” only relatively recently—first, under the guise of celebrity stalking and, later, as a public health issue recognized as affecting the general population. A useful working definition of stalking is “… the willful, malicious, and repeated following of and harassing of another person that threatens his/her safety.”1
Stalking victims report numerous, severe, life-changing effects from being stalked, including physical, social, and psychological harm. They typically experience mood, anxiety, and posttraumatic stress symptoms that require prompt evaluation and treatment.
Prevalence and other characteristics
Stalking and its subsequent victimization are common. Here are statistics:
• in the United States, approximately 1 million women and 370,000 men are stalked annually
• women are 3 times more likely to be stalked than raped2
• lifetime prevalence of stalking victimization is 20% (women, 23.5%; men, 10.5%)
• 75% of stalking victims are women
• 77% of stalking emerges from a prior acquaintance, including 49% that originated in a romantic relationship
• 33% of stalking encounters eventually lead to physical violence; slightly >10% of encounters lead to sexual violence
• stalking persists for an extended period; on average, almost 2 years.3
Penalties. Stalking can result in intervention by the criminal justice system. Legal sanctions levied on the perpetrator vary, depending on (among other variables) the severity of stalking; type of stalking; motive of the stalker; and the strength of incriminating evidence. Surprisingly, the outcome of the perpetrator’s prosecution (arrest, conviction, length of sentence) is unrelated to whether the victim reported continued stalking at follow-up.4,5
What are the symptoms and the damage? Given the intrusive nature of stalking behaviors and the extended period during which stalking persists, victims typically experience harmful psychological effects that range from subclinical symptoms to overt psychiatric disorders.
Stalking can have a profound impact on the victim and result in numerous psychological symptoms that become the focus of clinical attention. The typically chronic nature of stalking probably plays a significant role in its contributions to its victims’ psychological distress.6 Melton7 found that the most common adverse effect of stalking was related to the emotional impact of being stalked—with victims feeling scared, depressed, humiliated, embarrassed, distrustful of others, and angry or hateful.
Stalking victims report traumatic stress, hypervigilance, excessive fear, and anxiety coupled with disruptions in employment and social interactions.8 Many report having become highly distrustful or suspicious (44%); fearful (42%); nervous (31%); angry (27%); paranoid (36%); and depressed (21%). In general, victims have elevated scores on the Trauma Symptom Checklist.9
Stalking in the setting of intimate partner abuse is associated with harmful outcomes for the victim. These include repeat physical violence, psychological distress, and impaired physical or mental health, or both.3,7,10
Stalking victims who are female; had a prior relationship with the stalker; have experienced a greater variety of stalking behaviors; are divorced or separated; and have received government assistance were found to be more likely to experience multiple negative outcomes from stalking.11
Effects on mental health. Stalking victims have a higher incidence of mental disorders and comorbid illnesses compared with the general population,12 with the most robust associations identified between stalking victimization, major depressive disorder, and panic disorder. Stalking contributes to symptoms of posttraumatic stress disorder,13 and there is an association between posttraumatic stress and poor general health.14 Stalking victims report higher current use of psychotropic medications.12
Victims who blame themselves for being stalked report a significantly higher severity of depression, anxiety, and posttraumatic stress symptoms. Those who ruminate more about the stalking experience, or who explicitly emphasize the terror of stalking to a greater extent, also report a significantly higher severity of symptoms.15
Spitzberg3 reported that stalking victimization has several possible effects on victims (Table 1).
Coping by movement. Victims might attempt to cope with stalking through several means,2 including:
• moving away—trying to avoid contact with the stalker
• moving with—negotiating a more acceptable form of relationship with the stalker
• moving against—attempting to harm, constrain, or punish the stalker
• moving inward—seeking self-control or self-actualization
• moving outward—seeking the assistance of others.
The degree of a victim’s symptoms correlates partially with the severity of stalking. However, other variables play a crucial role in explaining the level of distress among stalking victims15; these include the types of coping strategies adopted by victims. Self-blame, catastrophizing, and rumination are significantly associated with maladjustment; on the other hand, positive reappraisal—thoughts of attaching a positive meaning to the event, in terms of personal growth—is associated with greater psychological adjustment.
The more stalking a victim experiences (and, presumably, experiences greater distress), the greater the variety of coping strategies she (he) employs.16
How should stalking victims be treated?
Stalking victims are an underserved population. Practitioners often are unsure how to address stalking; furthermore, available treatments can be ineffective.
There is a great deal of variability in what professionals who work with stalking victims believe is appropriate practice. Services provided to victims vary widely,17 and the field has not yet come to a consensus on best practices.16
Proceed case by case. Practitioners must understand the nuances of each case to consider what might work at a particular point in time, and information from victims can help guide decision-making.16 Evidence suggests that stalking victims can feel frustrated in their attempt to seek help, particularly from the criminal justice system; it is possible that such bad experiences may dissuade them from seeking help later.5,8,18 It is worth noting that, as the frequency of stalking decreases for any given victim, her (his) perception of safety increases and distress diminishes.16
Few communities have attempted to address systemically the problem of stalking. Existing anti-stalking programs have focused on the criminal justice aspects of intervention,8 with less emphasis on treating victims.
Some stalking victims rely on friends and family for support and assistance, but research shows that most reach out to agencies for assistance and, generally, seek help from multiple sources.18 Typically, stalking victims are served by 2 types of victim service organizations:
• specialized, small, private and nonprofit agencies (eg, domestic violence shelters, rape crisis centers, victims’ rights advocacy organizations)
• small units housed in police departments and prosecutors’ offices.17
Note: When victims seek services at criminal justice agencies, they may be feeling particularly unsafe and distressed. This underscores the importance of co-locating victim service providers and criminal justice agencies.16
Stalking victims might benefit from multi-disciplinary team consultation, including input from psychiatric, psychotherapeutic, and law enforcement or security professionals. Key priorities for practitioners to address with stalking victims are given in Table 2.19
Stalking behavior does not significantly decrease when victims are in contact with victim services.16 Practitioners can integrate this prospect into their understanding of stalking when they work with victims: That is, it is likely that the problem will not go away quickly, even with intervention.
Victims’ needs remain great and broad-based. Spence-Diehl et al17 conducted a survey of service providers for stalking victims, evaluating the needs of those victims and the response of their communities. Some of their recommendations for better meeting victims’ needs are in Table 3.16
Keeping victims at the center
Several authors have written about the need to return to a victim-centered model of care. This approach (1) puts the victim’s understanding of her (his) situation at the center of victim assistance work and (2) views service providers as consultants in the decision-making process.20,21 The victim-centered approach to treatment, in which the client has a greater voice and degree of control over interventions, is associated with positive outcomes.22,23
At the heart of a client-centered model of victim assistance is the provider’s ability to listen to a victim’s story and respond in a nonjudgmental manner. This approach honors the victim’s circumstances and her personal understanding of risk.21
Bottom Line
Stalking victims are a distinctive population, experiencing numerous emotional, physical, and social effects of their stalking over an extended period. Services to treat this underserved population need to be further developed. A multifaceted approach to treating victims incorporates psychological, somatic, and practical interventions, and a victim-centered approach is associated with better outcomes.
Related Resources
• Harmon RB, O’Connor M. Forcier A, et al. The impact of anti-stalking training on front line service providers: using the anti-stalking training evaluation protocol (ASTEP). J Forensic Science. 2004;49(5):1050-1055.
• Spitzberg BH, Cupach WR. The state of the art of stalking: taking stock of the emerging literature. Aggression and Violence Behavior. 2007;12(1):64-86.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Meloy JR, Gothard S. Demographic and clinical comparison of obsessional followers and offenders with mental disorders. Am J Psychiatry. 1995;152(2):258-263.
2. Tjaden P, Thoennes N. Stalking in America: findings from the National Violence Against Women Survey. National Institute of Justice and Centers for Disease Control and Prevention. https://www.ncjrs.gov/pdffiles/169592.pdf. Published April 1998. Accessed March 25, 2015.
3. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma, Violence, & Abuse. 2002;3(4):261-288.
4. McFarlane J, Willson P, Lemmey D, et al. Women filing assault charges on an intimate partner: criminal justice outcome and future violence experienced. Violence Against Women. 2000;6(4):396-408.
5. Melton HC. Stalking in the context of domestic violence: findings on the criminal justice system. Women & Criminal Justice. 2004;15:33-58.
6. Davies KE, Frieze IH. Research on stalking: what do we know and where do we go? Violence Vict. 2000;15(4):473-487.
7. Melton HC. Stalking in the context of intimate partner abuse: in the victims’ words. Feminist Criminology. 2007;2(4):346-363.
8. Spence-Diehl E. Intensive case management for victims of stalking: a pilot test evaluation. Brief Treatment Crisis Intervention. 2004;4(4):323-341.
9. Brewster MP. An exploration of the experiences and needs of former intimate stalking victims: final report submitted to the National Institute of Justice. West Chester, PA: West Chester University; 1997.
10. Logan TK, Shannon L, Cole J, et al. The impact of differential patterns of physical violence and stalking on mental health and help-seeking among women with protective orders. Violence Against Women. 2006;12(9):866-886.
11. Johnson MC, Kercher GA. Identifying predictors of negative psychological reactions to stalking victimization. J Interpers Violence. 2009;24(5):866-882.
12. Kuehner C, Gass P, Dressing H. Increased risk of mental disorders among lifetime victims of stalking—findings from a community study. Eur Psychiatry. 2007;22(3):142-145.
13. Basile KC, Arias I, Desai S, et al. The differential association of intimate partner physical, sexual, psychological, and stalking violence and post-traumatic stress symptoms in a nationally representative sample of women. J Traumatic Stress. 2004;17(5):413-421.
14. Kamphuis JH, Emmelkamp PM. Traumatic distress among support-seeking female victims of stalking. Am J Psychiatry. 2001;158(5):795-798.
15. Kraaij V, Arensman E, Garnefski N, et al. The role of cognitive coping in female victims of stalking. J Interpers Violence. 2007;22(12):1603-1612.
16. Bennett Cattaneo L, Cho S, Botuck S. Describing intimate partner stalking over time: an effort to inform victim-centered service provision. J Interpers Violence. 2011;26(17):3428-3454.
17. Spence-Diehl E, Potocky-Tripodi M. Victims of stalking: a study of service needs as perceived by victim services practitioners. J Interpers Violence. 2001;16(1):86-94.
18. Galeazzi GM, Buc˘ar-Ruc˘man A, DeFazio L, et al. Experiences of stalking victims and requests for help in three European countries. A survey. European Journal of Criminal Policy Research. 2009;15:243-260.
19. McEwan T, Purcell R. Assessing and surviving stalkers. Presented at: 45th Annual Meeting of American Academy of Psychiatry and the Law; October 2014; Chicago IL.
20. Cattaneo LB, Goodman LA. New directions in IPV risk assessment: an empowerment approach to risk management. In: Kendall-Tackett K, Giacomoni S, eds. Intimate partner violence. Kingston, NJ: Civic Research Institute; 2007:1-17.
21. Goodman LA, Epstein D. Listening to battered women: a survivor-centered approach to advocacy, mental health, and justice. Washington DC: American Psychological Association; 2008.
22. Cattaneo LB, Goodman LA. Through the lens of jurisprudence: the relationship between empowerment in the court system and well-being for intimate partner violence victims. J Interpers Violence. 2010;25(3):481-502.
23. Zweig JM, Burt MR. Predicting women’s perceptions of domestic violence and sexual assault agency helpfulness: what matters to program clients? Violence Against Women. 2007;13(11):1149-1178.
1. Meloy JR, Gothard S. Demographic and clinical comparison of obsessional followers and offenders with mental disorders. Am J Psychiatry. 1995;152(2):258-263.
2. Tjaden P, Thoennes N. Stalking in America: findings from the National Violence Against Women Survey. National Institute of Justice and Centers for Disease Control and Prevention. https://www.ncjrs.gov/pdffiles/169592.pdf. Published April 1998. Accessed March 25, 2015.
3. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma, Violence, & Abuse. 2002;3(4):261-288.
4. McFarlane J, Willson P, Lemmey D, et al. Women filing assault charges on an intimate partner: criminal justice outcome and future violence experienced. Violence Against Women. 2000;6(4):396-408.
5. Melton HC. Stalking in the context of domestic violence: findings on the criminal justice system. Women & Criminal Justice. 2004;15:33-58.
6. Davies KE, Frieze IH. Research on stalking: what do we know and where do we go? Violence Vict. 2000;15(4):473-487.
7. Melton HC. Stalking in the context of intimate partner abuse: in the victims’ words. Feminist Criminology. 2007;2(4):346-363.
8. Spence-Diehl E. Intensive case management for victims of stalking: a pilot test evaluation. Brief Treatment Crisis Intervention. 2004;4(4):323-341.
9. Brewster MP. An exploration of the experiences and needs of former intimate stalking victims: final report submitted to the National Institute of Justice. West Chester, PA: West Chester University; 1997.
10. Logan TK, Shannon L, Cole J, et al. The impact of differential patterns of physical violence and stalking on mental health and help-seeking among women with protective orders. Violence Against Women. 2006;12(9):866-886.
11. Johnson MC, Kercher GA. Identifying predictors of negative psychological reactions to stalking victimization. J Interpers Violence. 2009;24(5):866-882.
12. Kuehner C, Gass P, Dressing H. Increased risk of mental disorders among lifetime victims of stalking—findings from a community study. Eur Psychiatry. 2007;22(3):142-145.
13. Basile KC, Arias I, Desai S, et al. The differential association of intimate partner physical, sexual, psychological, and stalking violence and post-traumatic stress symptoms in a nationally representative sample of women. J Traumatic Stress. 2004;17(5):413-421.
14. Kamphuis JH, Emmelkamp PM. Traumatic distress among support-seeking female victims of stalking. Am J Psychiatry. 2001;158(5):795-798.
15. Kraaij V, Arensman E, Garnefski N, et al. The role of cognitive coping in female victims of stalking. J Interpers Violence. 2007;22(12):1603-1612.
16. Bennett Cattaneo L, Cho S, Botuck S. Describing intimate partner stalking over time: an effort to inform victim-centered service provision. J Interpers Violence. 2011;26(17):3428-3454.
17. Spence-Diehl E, Potocky-Tripodi M. Victims of stalking: a study of service needs as perceived by victim services practitioners. J Interpers Violence. 2001;16(1):86-94.
18. Galeazzi GM, Buc˘ar-Ruc˘man A, DeFazio L, et al. Experiences of stalking victims and requests for help in three European countries. A survey. European Journal of Criminal Policy Research. 2009;15:243-260.
19. McEwan T, Purcell R. Assessing and surviving stalkers. Presented at: 45th Annual Meeting of American Academy of Psychiatry and the Law; October 2014; Chicago IL.
20. Cattaneo LB, Goodman LA. New directions in IPV risk assessment: an empowerment approach to risk management. In: Kendall-Tackett K, Giacomoni S, eds. Intimate partner violence. Kingston, NJ: Civic Research Institute; 2007:1-17.
21. Goodman LA, Epstein D. Listening to battered women: a survivor-centered approach to advocacy, mental health, and justice. Washington DC: American Psychological Association; 2008.
22. Cattaneo LB, Goodman LA. Through the lens of jurisprudence: the relationship between empowerment in the court system and well-being for intimate partner violence victims. J Interpers Violence. 2010;25(3):481-502.
23. Zweig JM, Burt MR. Predicting women’s perceptions of domestic violence and sexual assault agency helpfulness: what matters to program clients? Violence Against Women. 2007;13(11):1149-1178.
Before you hit 'send': Will an e-mail to your patient put you at legal risk?
Dear Dr. Mossman,
Some of my patients e-mail me questions about their prescriptions, test results, treatment, appointments, etc. I’m often unsure about the best way to respond. If I use e-mail to communicate with patients, what step(s) should I take to minimize medicolegal risks?
Submitted by “Dr. V”
Medicine adopts new communication technologies cautiously. Calling patients seems unremarkable to us now, but it took decades after the invention of the telephone for doctors to feel comfortable talking to patients other than in face-to-face meetings.1,2
Patients want to communicate with their physicians via electronic mail,3 but concerns about security, confidentiality, and liability stop many physicians from using e-mail in their practice. Yet many medical organizations, including the Institute of Medicine,4 the American Medical Association,5 and the American Psychiatric Association,6 recognize that e-mail can facilitate care, if used properly.
Although e-mailing patients may feel awkward, a growing minority of clinicians regularly use e-mail for patient communication.2,7 In this article, we discuss ways to help safeguard your patients and their communications and to protect yourself from legal headaches.8
As you’re reading, please remember that we’re discussing communications to patients through standard e-mail, not secure portals (such as MyChart) that allow patients to contact physicians confidentially through their electronic medical records.
Privacy and security
Doctor-patient e-mails implicate the same professional, ethical, and legal responsibilities that govern any communication with patients.2,9,10 If handled improperly, outside-the-office doctor-patient communication can breach traditional duties to protect confidentiality, or they can violate provisions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA).11 Confidentiality breaches can lead to malpractice litigation, and HIPAA infractions can result in civil and criminal penalties levied by federal agencies.12 Further, e-mails that breach ethical standards (Table 15) can generate complaints to your state’s medical licensure board.
E-mail appeals to many patients, if for no other reason than to save time or avoid the inconvenience of playing “phone tag” with the doctor’s office. But e-mail has drawbacks. Patients may think or behave as though online communications are intimate and confidential, but they usually aren’t. If e-mail programs are left open or aren’t password protected, friends and family members might look at messages and even act upon them. For this reason, doctors often cannot be sure whether they are communicating with the patient or with someone else who has gained access to the patient’s e-mail account.
Parties outside the treatment relationship could have access to e-mail data stored on servers.6 Also, it’s easy to misread or mistype an e-mail address and send confidential information to the wrong person. A truly “secure” e-mail exchange uses encryption software that protects messages during transmission and storage and requires users to authenticate who they are through actions that link their identity to the e-mail address.13 But some patients and physicians do not know about the availability of such security measures, and implementing them can feel cumbersome to those who are not computer savvy. Not surprisingly, then, recent studies have shown that such measures are used infrequently by physicians and patients.14
Topics for e-mail communication
One way to minimize potential privacy problems is to limit the topics and types of communication dealt with by e-mail. Several experts and organizations have published suggestions, recommendations, and resources for doing this with common practices (Table 2).6,7,15
Receiving e-mail permission
Many patients e-mail their physicians without the physicians’ prior agreement. But physicians who plan to use e-mail in their practice should get patients’ explicit consent. This can be done verbally, with the content of the discussion documented in the medical record. But it’s better to have patients authorize e-mail communications in writing by means of a permission form that also sets out your office’s e-mail policies, expected response times, and privacy limitations.
Commonly recommended contents of such forms5-7,9,15,16 include:
• discussing security mechanisms and limits of security
• e-mail encryption requirements (or waiving them, if the patient prefers)
• providing an expected response time
• indemnifying you or your institution for information loss caused by technical failure
• identifying who reads e-mails (eg, office staff members, a nurse, physician [only])
• asking patients to put their name and other identifying information in the body of the message, not the subject line
• asking patients to put the type of question in the subject line (eg, “prescription,” “appointment,” “billing”)
• asking patients to use the “auto reply” feature to acknowledge receipt of your messages.
In addition to using patient consent forms, other suggestions and recommendations for physicians include:
• Do not use e-mail to establish patient-physician relationships, only to supplement personal encounters.
• If you work for an agency or institution, know and follow its guidelines and policies.
• If a rule or “boundary” is breached (eg, a patient sends you a detailed e-mail on a topic beyond the scope of your previous agreement), address this directly in a treatment session.
• File e-mail correspondence, including your reply, in the patient’s medical record.
• Use encryption technology if it is available, practical, and user-friendly.
• Use a practice-dedicated e-mail address with an automatic response that explains when e-mail will be answered and reminds patients to seek immediate help for urgent matters.
Real legal risk
Earlier, we described conceivable legal risks that e-mail might create. But has e-mail caused legal problems for physicians? At least 3 recent published decisions answer: “Yes.” And, remember, only a fraction of legal cases lead to published decisions.
• Huffine v Department of Health17 concerns a psychiatrist who was censured by the Washington state medical quality assurance commission for several boundary crossings, including sending his adolescent patient overly intimate e-mails.
• Wheeler v Kron18 lists a variety of legal claims—intentional infliction of emotional distress, negligent infliction of emotional distress, general negligence, and medical malpractice—that arose from a psychiatrist’s e-mailed concerns about visitation arrangements in a divorcing couple’s custody dispute. Although the court dismissed the last 3 claims, it allowed the intentional infliction of emotional distress claim to proceed.
• Ortegoza v Kho19 includes excerpts of e-mails between a primary care physician and his married patient, with whom the physician had affair that led to a medical malpractice lawsuit.
Bottom Line
Most patients want to e-mail their physicians, and many psychiatrists find e-mail helpful in caring for patients. If you are using e-mail in your practice or are contemplating doing so, get the patient’s permission (preferably in writing), and follow the recommendations and guidelines cited in this article’s references.
Related Resources
• Kane B, Sands DZ. Guidelines for the clinical use of electronic mail with patients. http://jamia.oxfordjournals.org/content/5/1/104.long.
• Professional Risk Management Services, Inc. Sample email consent and guide to email use. www.psychprogram.com/currentpsychiatry.html.
1. Wieczorek SM. From telegraph to e-mail: preserving the doctor-patient relationship in a high-tech environment. ETC: A Review of General Semantics. 2010;67(3):311-327.
2. Spielberg AR. Online without a net: physician-patient communication by electronic mail. Am J Law Med. 1999;25(2-3):267-295.
3. Pelletier AL, Sutton GR, Walker RR. Are your patients ready for electronic communication? Fam Pract Manag. 2007;14(9):25-26.
4. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001.
5. American Medical Association. AMA Code of Medical Ethics. Opinion 5.026 - the use of electronic mail. http:// www.ama-assn.org/ama/pub/physician-resources/ medical-ethics/code-medical-ethics/opinion5026.page. Published June 2013. Accessed March 8, 2015.
6. American Psychiatric Association, Council on Psychiatry & Law. Resource document on telepsychiatry and related technologies in clinical psychiatry. http://www.psychiatry. org/learn/library--archives/resource-documents. Published January 2014. Accessed March 25, 2015.
7. Koh S, Cattell GM, Cochran DM, et al. Psychiatrists’ use of electronic communication and social media and a proposed framework for future guidelines. J Psychiatr Pract. 2013;19(3):254-263.
8. Sands DZ. Help for physicians contemplating use of e-mail with patients. J Am Med Inform Assoc. 2004;11(4):268-269.
9. Bovi AM; Council on Ethical and Judicial Affairs of the American Medical Association. Ethical guidelines for use of electronic mail between patients and physicians. Am J Bioeth. 2003;3(3):W-IF2.
10. Kuszler PC. A question of duty: common law legal issues resulting from physician response to unsolicited patient email inquiries. J Med Internet Res. 2000;2(3):E17.
11. 45 CFR Parts 160 and 164.
12. Vanderpool D. Hippa-should I be worried? Innov Clin Neurosci. 2012;9(11-12):51-55.
13. Tjora A, Tran T, Faxvaag A. Privacy vs. usability: a qualitative exploration of patients’ experiences with secure internet communication with their general practitioner. J Med Internet Res. 2005;7(2):e15.
14. Menachemi N, Prickett CT, Brooks RG. The use of physician-patient email: a follow-up examination of adoption and best-practice adherence 2005-2008. J Med Internet Res. 2011;13(1):e23.
15. Kane B, Sands DZ. Guidelines for the clinical use of electronic mail with patients. The AMIA Internet Working Group, Task Force on guidelines for the use of clinic-patient electronic mail. J Am Med Inform Assoc. 1998;5(1):104-111.
16. Car J, Sheikh A. Email consultations in health care: 2–acceptability and safe application. BMJ. 2004; 329(7463):439-442.
17. Huffine v Department of Health, 148 Wn App 1015 (Wash Ct App 2009).
18. Wheeler v Akron (NY Misc LEXIS 942, 2011) NY Slip Op 30530(U) (NY Misc 2011).
19. Ortegoza v Kho, 2013 U.S. Dist .LEXIS 69999 (SD Cal 2013).
Dear Dr. Mossman,
Some of my patients e-mail me questions about their prescriptions, test results, treatment, appointments, etc. I’m often unsure about the best way to respond. If I use e-mail to communicate with patients, what step(s) should I take to minimize medicolegal risks?
Submitted by “Dr. V”
Medicine adopts new communication technologies cautiously. Calling patients seems unremarkable to us now, but it took decades after the invention of the telephone for doctors to feel comfortable talking to patients other than in face-to-face meetings.1,2
Patients want to communicate with their physicians via electronic mail,3 but concerns about security, confidentiality, and liability stop many physicians from using e-mail in their practice. Yet many medical organizations, including the Institute of Medicine,4 the American Medical Association,5 and the American Psychiatric Association,6 recognize that e-mail can facilitate care, if used properly.
Although e-mailing patients may feel awkward, a growing minority of clinicians regularly use e-mail for patient communication.2,7 In this article, we discuss ways to help safeguard your patients and their communications and to protect yourself from legal headaches.8
As you’re reading, please remember that we’re discussing communications to patients through standard e-mail, not secure portals (such as MyChart) that allow patients to contact physicians confidentially through their electronic medical records.
Privacy and security
Doctor-patient e-mails implicate the same professional, ethical, and legal responsibilities that govern any communication with patients.2,9,10 If handled improperly, outside-the-office doctor-patient communication can breach traditional duties to protect confidentiality, or they can violate provisions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA).11 Confidentiality breaches can lead to malpractice litigation, and HIPAA infractions can result in civil and criminal penalties levied by federal agencies.12 Further, e-mails that breach ethical standards (Table 15) can generate complaints to your state’s medical licensure board.
E-mail appeals to many patients, if for no other reason than to save time or avoid the inconvenience of playing “phone tag” with the doctor’s office. But e-mail has drawbacks. Patients may think or behave as though online communications are intimate and confidential, but they usually aren’t. If e-mail programs are left open or aren’t password protected, friends and family members might look at messages and even act upon them. For this reason, doctors often cannot be sure whether they are communicating with the patient or with someone else who has gained access to the patient’s e-mail account.
Parties outside the treatment relationship could have access to e-mail data stored on servers.6 Also, it’s easy to misread or mistype an e-mail address and send confidential information to the wrong person. A truly “secure” e-mail exchange uses encryption software that protects messages during transmission and storage and requires users to authenticate who they are through actions that link their identity to the e-mail address.13 But some patients and physicians do not know about the availability of such security measures, and implementing them can feel cumbersome to those who are not computer savvy. Not surprisingly, then, recent studies have shown that such measures are used infrequently by physicians and patients.14
Topics for e-mail communication
One way to minimize potential privacy problems is to limit the topics and types of communication dealt with by e-mail. Several experts and organizations have published suggestions, recommendations, and resources for doing this with common practices (Table 2).6,7,15
Receiving e-mail permission
Many patients e-mail their physicians without the physicians’ prior agreement. But physicians who plan to use e-mail in their practice should get patients’ explicit consent. This can be done verbally, with the content of the discussion documented in the medical record. But it’s better to have patients authorize e-mail communications in writing by means of a permission form that also sets out your office’s e-mail policies, expected response times, and privacy limitations.
Commonly recommended contents of such forms5-7,9,15,16 include:
• discussing security mechanisms and limits of security
• e-mail encryption requirements (or waiving them, if the patient prefers)
• providing an expected response time
• indemnifying you or your institution for information loss caused by technical failure
• identifying who reads e-mails (eg, office staff members, a nurse, physician [only])
• asking patients to put their name and other identifying information in the body of the message, not the subject line
• asking patients to put the type of question in the subject line (eg, “prescription,” “appointment,” “billing”)
• asking patients to use the “auto reply” feature to acknowledge receipt of your messages.
In addition to using patient consent forms, other suggestions and recommendations for physicians include:
• Do not use e-mail to establish patient-physician relationships, only to supplement personal encounters.
• If you work for an agency or institution, know and follow its guidelines and policies.
• If a rule or “boundary” is breached (eg, a patient sends you a detailed e-mail on a topic beyond the scope of your previous agreement), address this directly in a treatment session.
• File e-mail correspondence, including your reply, in the patient’s medical record.
• Use encryption technology if it is available, practical, and user-friendly.
• Use a practice-dedicated e-mail address with an automatic response that explains when e-mail will be answered and reminds patients to seek immediate help for urgent matters.
Real legal risk
Earlier, we described conceivable legal risks that e-mail might create. But has e-mail caused legal problems for physicians? At least 3 recent published decisions answer: “Yes.” And, remember, only a fraction of legal cases lead to published decisions.
• Huffine v Department of Health17 concerns a psychiatrist who was censured by the Washington state medical quality assurance commission for several boundary crossings, including sending his adolescent patient overly intimate e-mails.
• Wheeler v Kron18 lists a variety of legal claims—intentional infliction of emotional distress, negligent infliction of emotional distress, general negligence, and medical malpractice—that arose from a psychiatrist’s e-mailed concerns about visitation arrangements in a divorcing couple’s custody dispute. Although the court dismissed the last 3 claims, it allowed the intentional infliction of emotional distress claim to proceed.
• Ortegoza v Kho19 includes excerpts of e-mails between a primary care physician and his married patient, with whom the physician had affair that led to a medical malpractice lawsuit.
Bottom Line
Most patients want to e-mail their physicians, and many psychiatrists find e-mail helpful in caring for patients. If you are using e-mail in your practice or are contemplating doing so, get the patient’s permission (preferably in writing), and follow the recommendations and guidelines cited in this article’s references.
Related Resources
• Kane B, Sands DZ. Guidelines for the clinical use of electronic mail with patients. http://jamia.oxfordjournals.org/content/5/1/104.long.
• Professional Risk Management Services, Inc. Sample email consent and guide to email use. www.psychprogram.com/currentpsychiatry.html.
Dear Dr. Mossman,
Some of my patients e-mail me questions about their prescriptions, test results, treatment, appointments, etc. I’m often unsure about the best way to respond. If I use e-mail to communicate with patients, what step(s) should I take to minimize medicolegal risks?
Submitted by “Dr. V”
Medicine adopts new communication technologies cautiously. Calling patients seems unremarkable to us now, but it took decades after the invention of the telephone for doctors to feel comfortable talking to patients other than in face-to-face meetings.1,2
Patients want to communicate with their physicians via electronic mail,3 but concerns about security, confidentiality, and liability stop many physicians from using e-mail in their practice. Yet many medical organizations, including the Institute of Medicine,4 the American Medical Association,5 and the American Psychiatric Association,6 recognize that e-mail can facilitate care, if used properly.
Although e-mailing patients may feel awkward, a growing minority of clinicians regularly use e-mail for patient communication.2,7 In this article, we discuss ways to help safeguard your patients and their communications and to protect yourself from legal headaches.8
As you’re reading, please remember that we’re discussing communications to patients through standard e-mail, not secure portals (such as MyChart) that allow patients to contact physicians confidentially through their electronic medical records.
Privacy and security
Doctor-patient e-mails implicate the same professional, ethical, and legal responsibilities that govern any communication with patients.2,9,10 If handled improperly, outside-the-office doctor-patient communication can breach traditional duties to protect confidentiality, or they can violate provisions of the Health Insurance Portability and Accountability Act of 1996 (HIPAA).11 Confidentiality breaches can lead to malpractice litigation, and HIPAA infractions can result in civil and criminal penalties levied by federal agencies.12 Further, e-mails that breach ethical standards (Table 15) can generate complaints to your state’s medical licensure board.
E-mail appeals to many patients, if for no other reason than to save time or avoid the inconvenience of playing “phone tag” with the doctor’s office. But e-mail has drawbacks. Patients may think or behave as though online communications are intimate and confidential, but they usually aren’t. If e-mail programs are left open or aren’t password protected, friends and family members might look at messages and even act upon them. For this reason, doctors often cannot be sure whether they are communicating with the patient or with someone else who has gained access to the patient’s e-mail account.
Parties outside the treatment relationship could have access to e-mail data stored on servers.6 Also, it’s easy to misread or mistype an e-mail address and send confidential information to the wrong person. A truly “secure” e-mail exchange uses encryption software that protects messages during transmission and storage and requires users to authenticate who they are through actions that link their identity to the e-mail address.13 But some patients and physicians do not know about the availability of such security measures, and implementing them can feel cumbersome to those who are not computer savvy. Not surprisingly, then, recent studies have shown that such measures are used infrequently by physicians and patients.14
Topics for e-mail communication
One way to minimize potential privacy problems is to limit the topics and types of communication dealt with by e-mail. Several experts and organizations have published suggestions, recommendations, and resources for doing this with common practices (Table 2).6,7,15
Receiving e-mail permission
Many patients e-mail their physicians without the physicians’ prior agreement. But physicians who plan to use e-mail in their practice should get patients’ explicit consent. This can be done verbally, with the content of the discussion documented in the medical record. But it’s better to have patients authorize e-mail communications in writing by means of a permission form that also sets out your office’s e-mail policies, expected response times, and privacy limitations.
Commonly recommended contents of such forms5-7,9,15,16 include:
• discussing security mechanisms and limits of security
• e-mail encryption requirements (or waiving them, if the patient prefers)
• providing an expected response time
• indemnifying you or your institution for information loss caused by technical failure
• identifying who reads e-mails (eg, office staff members, a nurse, physician [only])
• asking patients to put their name and other identifying information in the body of the message, not the subject line
• asking patients to put the type of question in the subject line (eg, “prescription,” “appointment,” “billing”)
• asking patients to use the “auto reply” feature to acknowledge receipt of your messages.
In addition to using patient consent forms, other suggestions and recommendations for physicians include:
• Do not use e-mail to establish patient-physician relationships, only to supplement personal encounters.
• If you work for an agency or institution, know and follow its guidelines and policies.
• If a rule or “boundary” is breached (eg, a patient sends you a detailed e-mail on a topic beyond the scope of your previous agreement), address this directly in a treatment session.
• File e-mail correspondence, including your reply, in the patient’s medical record.
• Use encryption technology if it is available, practical, and user-friendly.
• Use a practice-dedicated e-mail address with an automatic response that explains when e-mail will be answered and reminds patients to seek immediate help for urgent matters.
Real legal risk
Earlier, we described conceivable legal risks that e-mail might create. But has e-mail caused legal problems for physicians? At least 3 recent published decisions answer: “Yes.” And, remember, only a fraction of legal cases lead to published decisions.
• Huffine v Department of Health17 concerns a psychiatrist who was censured by the Washington state medical quality assurance commission for several boundary crossings, including sending his adolescent patient overly intimate e-mails.
• Wheeler v Kron18 lists a variety of legal claims—intentional infliction of emotional distress, negligent infliction of emotional distress, general negligence, and medical malpractice—that arose from a psychiatrist’s e-mailed concerns about visitation arrangements in a divorcing couple’s custody dispute. Although the court dismissed the last 3 claims, it allowed the intentional infliction of emotional distress claim to proceed.
• Ortegoza v Kho19 includes excerpts of e-mails between a primary care physician and his married patient, with whom the physician had affair that led to a medical malpractice lawsuit.
Bottom Line
Most patients want to e-mail their physicians, and many psychiatrists find e-mail helpful in caring for patients. If you are using e-mail in your practice or are contemplating doing so, get the patient’s permission (preferably in writing), and follow the recommendations and guidelines cited in this article’s references.
Related Resources
• Kane B, Sands DZ. Guidelines for the clinical use of electronic mail with patients. http://jamia.oxfordjournals.org/content/5/1/104.long.
• Professional Risk Management Services, Inc. Sample email consent and guide to email use. www.psychprogram.com/currentpsychiatry.html.
1. Wieczorek SM. From telegraph to e-mail: preserving the doctor-patient relationship in a high-tech environment. ETC: A Review of General Semantics. 2010;67(3):311-327.
2. Spielberg AR. Online without a net: physician-patient communication by electronic mail. Am J Law Med. 1999;25(2-3):267-295.
3. Pelletier AL, Sutton GR, Walker RR. Are your patients ready for electronic communication? Fam Pract Manag. 2007;14(9):25-26.
4. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001.
5. American Medical Association. AMA Code of Medical Ethics. Opinion 5.026 - the use of electronic mail. http:// www.ama-assn.org/ama/pub/physician-resources/ medical-ethics/code-medical-ethics/opinion5026.page. Published June 2013. Accessed March 8, 2015.
6. American Psychiatric Association, Council on Psychiatry & Law. Resource document on telepsychiatry and related technologies in clinical psychiatry. http://www.psychiatry. org/learn/library--archives/resource-documents. Published January 2014. Accessed March 25, 2015.
7. Koh S, Cattell GM, Cochran DM, et al. Psychiatrists’ use of electronic communication and social media and a proposed framework for future guidelines. J Psychiatr Pract. 2013;19(3):254-263.
8. Sands DZ. Help for physicians contemplating use of e-mail with patients. J Am Med Inform Assoc. 2004;11(4):268-269.
9. Bovi AM; Council on Ethical and Judicial Affairs of the American Medical Association. Ethical guidelines for use of electronic mail between patients and physicians. Am J Bioeth. 2003;3(3):W-IF2.
10. Kuszler PC. A question of duty: common law legal issues resulting from physician response to unsolicited patient email inquiries. J Med Internet Res. 2000;2(3):E17.
11. 45 CFR Parts 160 and 164.
12. Vanderpool D. Hippa-should I be worried? Innov Clin Neurosci. 2012;9(11-12):51-55.
13. Tjora A, Tran T, Faxvaag A. Privacy vs. usability: a qualitative exploration of patients’ experiences with secure internet communication with their general practitioner. J Med Internet Res. 2005;7(2):e15.
14. Menachemi N, Prickett CT, Brooks RG. The use of physician-patient email: a follow-up examination of adoption and best-practice adherence 2005-2008. J Med Internet Res. 2011;13(1):e23.
15. Kane B, Sands DZ. Guidelines for the clinical use of electronic mail with patients. The AMIA Internet Working Group, Task Force on guidelines for the use of clinic-patient electronic mail. J Am Med Inform Assoc. 1998;5(1):104-111.
16. Car J, Sheikh A. Email consultations in health care: 2–acceptability and safe application. BMJ. 2004; 329(7463):439-442.
17. Huffine v Department of Health, 148 Wn App 1015 (Wash Ct App 2009).
18. Wheeler v Akron (NY Misc LEXIS 942, 2011) NY Slip Op 30530(U) (NY Misc 2011).
19. Ortegoza v Kho, 2013 U.S. Dist .LEXIS 69999 (SD Cal 2013).
1. Wieczorek SM. From telegraph to e-mail: preserving the doctor-patient relationship in a high-tech environment. ETC: A Review of General Semantics. 2010;67(3):311-327.
2. Spielberg AR. Online without a net: physician-patient communication by electronic mail. Am J Law Med. 1999;25(2-3):267-295.
3. Pelletier AL, Sutton GR, Walker RR. Are your patients ready for electronic communication? Fam Pract Manag. 2007;14(9):25-26.
4. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001.
5. American Medical Association. AMA Code of Medical Ethics. Opinion 5.026 - the use of electronic mail. http:// www.ama-assn.org/ama/pub/physician-resources/ medical-ethics/code-medical-ethics/opinion5026.page. Published June 2013. Accessed March 8, 2015.
6. American Psychiatric Association, Council on Psychiatry & Law. Resource document on telepsychiatry and related technologies in clinical psychiatry. http://www.psychiatry. org/learn/library--archives/resource-documents. Published January 2014. Accessed March 25, 2015.
7. Koh S, Cattell GM, Cochran DM, et al. Psychiatrists’ use of electronic communication and social media and a proposed framework for future guidelines. J Psychiatr Pract. 2013;19(3):254-263.
8. Sands DZ. Help for physicians contemplating use of e-mail with patients. J Am Med Inform Assoc. 2004;11(4):268-269.
9. Bovi AM; Council on Ethical and Judicial Affairs of the American Medical Association. Ethical guidelines for use of electronic mail between patients and physicians. Am J Bioeth. 2003;3(3):W-IF2.
10. Kuszler PC. A question of duty: common law legal issues resulting from physician response to unsolicited patient email inquiries. J Med Internet Res. 2000;2(3):E17.
11. 45 CFR Parts 160 and 164.
12. Vanderpool D. Hippa-should I be worried? Innov Clin Neurosci. 2012;9(11-12):51-55.
13. Tjora A, Tran T, Faxvaag A. Privacy vs. usability: a qualitative exploration of patients’ experiences with secure internet communication with their general practitioner. J Med Internet Res. 2005;7(2):e15.
14. Menachemi N, Prickett CT, Brooks RG. The use of physician-patient email: a follow-up examination of adoption and best-practice adherence 2005-2008. J Med Internet Res. 2011;13(1):e23.
15. Kane B, Sands DZ. Guidelines for the clinical use of electronic mail with patients. The AMIA Internet Working Group, Task Force on guidelines for the use of clinic-patient electronic mail. J Am Med Inform Assoc. 1998;5(1):104-111.
16. Car J, Sheikh A. Email consultations in health care: 2–acceptability and safe application. BMJ. 2004; 329(7463):439-442.
17. Huffine v Department of Health, 148 Wn App 1015 (Wash Ct App 2009).
18. Wheeler v Akron (NY Misc LEXIS 942, 2011) NY Slip Op 30530(U) (NY Misc 2011).
19. Ortegoza v Kho, 2013 U.S. Dist .LEXIS 69999 (SD Cal 2013).
Fatigue after depression responds to therapy. What are the next steps?
Fatigue and depression can be viewed as a “vicious cycle”: Fatigue can be a symptom of major depression, and fatigue can be a risk factor for depression.1 For example, fatigue associated with a general medical condition or traumatic brain injury can be a risk factor for developing major depressive disorder (MDD).1-3 It isn’t surprising that fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD.
Despite the observed association between fatigue and depression, their underlying relationship often is unclear. The literature does not differentiate among fatigue associated with depression, fatigue as a treatment-emergent adverse effect, and fatigue as a residual symptom of depression that is partially responsive to treatment.4,5 To complicate the situation, many medications used to treat MDD can cause fatigue.
Patients often describe fatigue as (1) feeling tired, exhausted, or drained and (2) lacking energy and motivation. Fatigue can be related to impaired wakefulness but is believed to be a different entity than sleepiness.6 Residual fatigue can affect social, cognitive, emotional, and physical health.
We reviewed the literature about fatigue as a symptom of MDD by conducting a search of Medline, PubMed, and Google Scholar, using keywords depression, fatigue, residual symptoms, and treatment. We chose the papers cited in this article based on our consensus and because these publications represent expert opinion or the highest quality evidence available.
Residual fatigue has an effect on prognosis
Fatigue is a common symptom of MDD that persists in 20% to 30% of patients whose symptoms of depression otherwise remit.4,7-9 Several studies have linked residual fatigue with the overall prognosis of MDD.5 Data from a prospective study demonstrate that depressed patients have a higher risk of relapse when they continue to report symptoms of fatigue after their symptoms of depression have otherwise entered partial remission.10 Another study demonstrated that the severity of residual symptoms of depression is a strong predictor of another major depressive episode.11
In a large-scale study, the prevalence of residual fatigue after adequate treatment of MDD in both partial responders and remitters was 84.6%.12 The same study showed that one-third of patients who had been treated for MDD had persistent and clinically significant fatigue, which could suggest a relationship between fatigue and selective serotonin reuptake inhibitors (SSRIs) and other antidepressants.
Another study demonstrated that 64.6% of patients who responded to antidepressant treatment and who had baseline fatigue continued to exhibit symptoms of fatigue after an adequate trial of an antidepressant.13
Neurobiological considerations
Studies have shown that the neuronal circuits that malfunction in fatigue are different from those that malfunction in depression.14 Although the neurobiology of fatigue has not been determined, decreased neuronal activity in the prefrontal circuits has been associated with symptoms of fatigue.15
In addition, evidence from the literature shows a decrease in hormone secretion16 and cognitive abilities in patients exhibiting symptoms of fatigue.17 These findings have led some experts to hypothesize that symptoms of fatigue associated with depression could be the result of (1) immune dysregulation18 and (2) an inability of available antidepressants to target the underlying biology of the disorder.2
Despite the hypothesis that fatigue associated with depression might be biologically related to immune dysregulation, some authors continue to point to an imbalance in neurotransmitters—norepinephrine, histamine, dopamine, acetylcholine—as being associated with fatigue.14 For example, a study demonstrated that drugs targeting noradrenergic reuptake inhibition were more effective at preventing a relapse of fatigue compared with serotonergic drugs.19 Another study showed improvement in energy with an increase in the plasma level of desipramine, which affects noradrenergic neurotransmission.20
Inflammatory cytokines also have been explored in the search for an understanding of the etiology of fatigue and depression.21 Physical and mental stress promote the release of cytokines, which activate the immune system by inducing an inflammatory response; this response has been etiologically linked to depressive disorders.22 Furthermore, studies have demonstrated an elevated level of inflammatory cytokines in patients who have MDD— suggesting that MDD is associated with a chronic low level of inflammation that crosses the blood−brain barrier.23
Clinical considerations: A role for rating scales?
Despite the significance of residual fatigue on the quality of life of patients who have MDD, most common rating scales, such as the Hamilton Depression Rating Scale24 and the Montgomery-Åsberg Depression Rating Scale,25 have limited sensitivity for measuring fatigue.26 The Fatigue Associated with Depression (FAsD)27 questionnaire, designed according to FDA guidelines,28 is used to assess fatigue associated with depression. The final version of the FAsD includes 13 items: a 6-item experience subscale and a 7-item impact subscale.
Is the FAsD helpful? The experience subscale of the FAsD assesses how often the patient experiences different aspects of fatigue (tiredness, exhaustion, lack of energy, physical weakness, and a feeling that everything requires too much effort). The impact subscale of the FAsD assesses the effect of fatigue on daily life.
The overall FAsD score is calculated by taking the mean of each subscale; a change of 0.67 on the experience subscale and 0.57 on the impact subscale are considered clinically meaningful.27 The measurement properties of the questionnaire showed internal consistency, reliability, and validity in testing. Researchers note, however, that FAsD does not include items to assess the impact of fatigue on cognition. This means that the FAsD might not distinguish between physical and mental aspects of fatigue.
Treatment
It isn’t surprising that residual depression can increase health care utilization and economic burden, including such indirect costs as lost productivity and wages.29 Despite these impacts, there is a paucity of studies evaluating the relationship between residual symptoms, such as fatigue, and work productivity. It has been established that improving a depressed patient’s level of energy correlates with improved performance at work.
Treating fatigue as a residual symptom of MDD can be complicated because symptoms of fatigue might be:
• a discrete symptom of MDD
• a prodromal symptom of another disorder
• an adverse effect of an antidepressant.2,30
It is a major clinical problem, therefore, that antidepressants can alleviate and cause symptoms of fatigue.31 Treatment strategy should focus on identifying antidepressants that are less likely to cause fatigue (ie, noradrenergic or dopaminergic drugs, or both). Adjunctive treatments to target residual fatigue also can be used.32
There are limited published data on the effective treatment of residual fatigue in patients with MDD. Given the absence of sufficient evidence, agents that promote noradrenergic and dopaminergic neurotransmission have been the treatment of choice when targeting fatigue in depressed patients.2,14,21,33
The Table34-37 lists potential treatment options often used to treat fatigue associated with depression.
SSRIs. Treatment with SSRIs has been associated with a low probability of achieving remission when targeting fatigue as a symptom of MDD.21
One study reported that, after 8 weeks of treatment with an SSRI, treatment-emergent adverse events, such as worsening fatigue and weakness, were observed—along with an overall lack of efficacy in targeting all symptoms of depression.38
Another study demonstrated positive effects when a noradrenergic agent was added to an SSRI in partial responders who continued to complain of residual fatigue.33
However, studies that compared the effects of SSRIs with those of antidepressants that have pronoradrenergic effects showed that the 2 mechanisms of action were not significantly different from each other in their ability to resolve residual symptoms of fatigue.21 A limiting factor might be that these studies were retrospective and did not analyze the efficacy of a noradrenergic agent as an adjunct for alleviating symptoms of fatigue.39
Bupropion. This commonly used medication for fatigue is believed to cause a significantly lower level of fatigue compared with SSRIs.40 The potential utility of bupropion in this area could be a reflection of its mechanism of action—ie, the drug targets both noradrenergic and dopaminergic neurotransmission.41
A study comparing bupropion with SSRIs in targeting somatic symptoms of depression reported a small but statistically significant difference in favor of the bupropion-treated group. However, this finding was confounded by the small effect size and difficulty quantifying somatic symptoms.40
Stimulants and modafinil. Psycho-stimulants have been shown to be efficacious for depression and fatigue, both as monotherapy and adjunctively.39,42
Modafinil has demonstrated efficacy in open-label trials for improving residual fatigue, but failed to separate from placebo in controlled trials.43 At least 1 other failed study has been published examining modafinil as a treatment for fatigue associated with depression.43
Adjunctive therapy with CNS stimulants, such as amphetamine/dextroamphetamine and methylphenidate, has been used to treat fatigue, with positive results.16 Modafinil and stimulants also could be tried as an augmentation strategy to other antidepressants; such use is off-label and should be attempted only after careful consideration.16
Exercise might be a nonpharmacotherapeutic modality that targets the underlying physiology associated with fatigue. Exercise releases endorphins, which can affect overall brain chemistry and which have been theorized to diminish symptoms of fatigue and depression.44 Consider exercise in addition to treatment with an antidepressant in selected patients.45
To sum up
In general, the literature does not recommend one medication as superior to any other for treating fatigue that is a residual symptom of depression. Such hesitation suggests that more empirical studies are needed to determine what is the best and proper management of treating fatigue associated with depression.
Bottom LinE
Fatigue can be a symptom of major depressive disorder (MDD) or a risk factor for depression. Fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD. Residual fatigue can affect social, cognitive, emotional, and physical health and can result in increased utilization of health care services. A number of treatment options are available; none has been shown to be superior to the others.
Related Resources
• Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry. 2010;197(2):86-87.
• Targum SD, Fava M. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 2011;8(10):40-43.
• Illiades C. How to fight depression fatigue. Everyday Health. http://www.everydayhealth.com/health-report/major-depression-living-well/fight-depression-fatigue.aspx.
• Kerr M. Depression and fatigue: a vicious cycle. Healthline. http://www.healthline.com/health/depression/fatigue.
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Bupropion • Wellbutrin
Desipramine • Norpramin
Methylphenidate • Ritalin
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Disclosures
Dr. Sohail reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Macaluso has conducted clinical trials research as principal investigator for the following pharmaceutical manufacturers in the past 12 months: AbbVie, Inc.; Alkermes; AssureRx Health, Inc.; Eisai Co., Ltd.; FORUM Pharmaceuticals, Inc.; Janssen Pharmaceuticals, Inc.; and Naurex Inc. All clinical trial and study contracts were with, and payments were made to, University of Kansas Medical Center Research Institute, Kansas City, Kansas, a research institute affiliated with University of Kansas School of Medicine−Wichita.
1. Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427-431.
2. Demyttenaere K, De Fruyt J, Stahl, SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93-105.
3. Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosom Med. 2004;66(3):330-335.
4. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50.
5. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80(2-3):135-144.
6. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63-76.
7. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225.
8. Tylee A, Gastpar M, Lépine JP, et al. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14(3):139-151.
9. Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2-3):141-150.
10. Paykel ES, Ramana, R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180.
11. Bockting CL, Spinhoven P, Koeter MW, et al; Depression Evaluation Longitudinal Therapy Assessment Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67(5):747-755.
12. Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med. 2004;19(8):813-818.
13. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180-186.
14. Stahl SM, Zhang L, Damatarca C, et al. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(suppl 14):6-17.
15. MacHale SM, Law´rie SM, Cavanagh JT, et al. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550-556.
16. Paykel ES. Achieving gains beyond response. Acta Psychiatrica Scandinavica Suppl. 2002;(415):12-17.
17. van den Heuvel OA, Groenewegen HJ, Barkhof F, et al. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18(2):367-374.
18. Jaremka LM, Fagundes CP, Glaser R, et al. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310-1317.
19. Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47(5):411-418.
20. Nelson JC, Mazure C, Quinlan DM, et al. Drug-responsive symptoms in melancholia. Arch Gen Psychiatry. 1984;41(7):663-668.
21. Fava M, Ball S, Nelson, JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250-257.
22. Anisman H, Merali Z, Poulter MO, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963-972.
23. Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230-233.
24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
26. Matza LS, Phillips GA, Revicki DA, et al. Development and validation of a patient-report measure of fatigue associated with depression. J Affect Disord. 2011;134(1-3):294-303.
27. Matza LS, Wyrwich KW, Phillips GA, et al. The Fatigue Associated with Depression Questionnaire (FAsD): responsiveness and responder definition. Qual Life Res. 2013;22(2):351-360.
28. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Food and Drug Administration. http://www.fda. gov/downloads/Drugs/Guidances/UCM193282.pdf. Published December 2009. Accessed May 7, 2015.
29. Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188-e196.
30. Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64(suppl 14):30-34.
31. Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971-975.
32. Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry. 2006;67(suppl 6):9-15.
33. Ball SG, Dellva MA, D’Souza D, et al. A double-blind, placebo-controlled study of augmentation with LY2216684 for major depressive disorder patients who are partial responders to selective serotonin reuptake inhibitors [abstract P 05]. Int J Psych Clin Pract. 2010;14(suppl 1):19.
34. Stahl SM. Using secondary binding properties to select a not so elective serotonin reuptake inhibitor. J Clin Psychiatry. 1998;59(12):642-643.
35. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 2nd ed. New York, NY: Cambridge University Press; 2000.
36. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
37. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620-8628.
38. Daly EJ, Trivedi MH, Fava M, et al. The relationship between adverse events during selective serotonin reuptake inhibitor treatment for major depressive disorder and nonremission in the suicide assessment methodology study. J Clin Psychopharmacol. 2011;31(1):31-38.
39. Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry. 1999;46(9):1301-1308.
40. Papakostas GI, Nutt DJ, Hallett LA, et al. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350-1355.
41. Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113.
42. Candy M, Jones CB, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;(2):CD006722. doi: 10.1002/14651858.CD006722.pub2.
43. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother. 2007;41(6):1005-1012.
44. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychol Rev. 2001;21(1):33-61.
45. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12(4):205-213.
Fatigue and depression can be viewed as a “vicious cycle”: Fatigue can be a symptom of major depression, and fatigue can be a risk factor for depression.1 For example, fatigue associated with a general medical condition or traumatic brain injury can be a risk factor for developing major depressive disorder (MDD).1-3 It isn’t surprising that fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD.
Despite the observed association between fatigue and depression, their underlying relationship often is unclear. The literature does not differentiate among fatigue associated with depression, fatigue as a treatment-emergent adverse effect, and fatigue as a residual symptom of depression that is partially responsive to treatment.4,5 To complicate the situation, many medications used to treat MDD can cause fatigue.
Patients often describe fatigue as (1) feeling tired, exhausted, or drained and (2) lacking energy and motivation. Fatigue can be related to impaired wakefulness but is believed to be a different entity than sleepiness.6 Residual fatigue can affect social, cognitive, emotional, and physical health.
We reviewed the literature about fatigue as a symptom of MDD by conducting a search of Medline, PubMed, and Google Scholar, using keywords depression, fatigue, residual symptoms, and treatment. We chose the papers cited in this article based on our consensus and because these publications represent expert opinion or the highest quality evidence available.
Residual fatigue has an effect on prognosis
Fatigue is a common symptom of MDD that persists in 20% to 30% of patients whose symptoms of depression otherwise remit.4,7-9 Several studies have linked residual fatigue with the overall prognosis of MDD.5 Data from a prospective study demonstrate that depressed patients have a higher risk of relapse when they continue to report symptoms of fatigue after their symptoms of depression have otherwise entered partial remission.10 Another study demonstrated that the severity of residual symptoms of depression is a strong predictor of another major depressive episode.11
In a large-scale study, the prevalence of residual fatigue after adequate treatment of MDD in both partial responders and remitters was 84.6%.12 The same study showed that one-third of patients who had been treated for MDD had persistent and clinically significant fatigue, which could suggest a relationship between fatigue and selective serotonin reuptake inhibitors (SSRIs) and other antidepressants.
Another study demonstrated that 64.6% of patients who responded to antidepressant treatment and who had baseline fatigue continued to exhibit symptoms of fatigue after an adequate trial of an antidepressant.13
Neurobiological considerations
Studies have shown that the neuronal circuits that malfunction in fatigue are different from those that malfunction in depression.14 Although the neurobiology of fatigue has not been determined, decreased neuronal activity in the prefrontal circuits has been associated with symptoms of fatigue.15
In addition, evidence from the literature shows a decrease in hormone secretion16 and cognitive abilities in patients exhibiting symptoms of fatigue.17 These findings have led some experts to hypothesize that symptoms of fatigue associated with depression could be the result of (1) immune dysregulation18 and (2) an inability of available antidepressants to target the underlying biology of the disorder.2
Despite the hypothesis that fatigue associated with depression might be biologically related to immune dysregulation, some authors continue to point to an imbalance in neurotransmitters—norepinephrine, histamine, dopamine, acetylcholine—as being associated with fatigue.14 For example, a study demonstrated that drugs targeting noradrenergic reuptake inhibition were more effective at preventing a relapse of fatigue compared with serotonergic drugs.19 Another study showed improvement in energy with an increase in the plasma level of desipramine, which affects noradrenergic neurotransmission.20
Inflammatory cytokines also have been explored in the search for an understanding of the etiology of fatigue and depression.21 Physical and mental stress promote the release of cytokines, which activate the immune system by inducing an inflammatory response; this response has been etiologically linked to depressive disorders.22 Furthermore, studies have demonstrated an elevated level of inflammatory cytokines in patients who have MDD— suggesting that MDD is associated with a chronic low level of inflammation that crosses the blood−brain barrier.23
Clinical considerations: A role for rating scales?
Despite the significance of residual fatigue on the quality of life of patients who have MDD, most common rating scales, such as the Hamilton Depression Rating Scale24 and the Montgomery-Åsberg Depression Rating Scale,25 have limited sensitivity for measuring fatigue.26 The Fatigue Associated with Depression (FAsD)27 questionnaire, designed according to FDA guidelines,28 is used to assess fatigue associated with depression. The final version of the FAsD includes 13 items: a 6-item experience subscale and a 7-item impact subscale.
Is the FAsD helpful? The experience subscale of the FAsD assesses how often the patient experiences different aspects of fatigue (tiredness, exhaustion, lack of energy, physical weakness, and a feeling that everything requires too much effort). The impact subscale of the FAsD assesses the effect of fatigue on daily life.
The overall FAsD score is calculated by taking the mean of each subscale; a change of 0.67 on the experience subscale and 0.57 on the impact subscale are considered clinically meaningful.27 The measurement properties of the questionnaire showed internal consistency, reliability, and validity in testing. Researchers note, however, that FAsD does not include items to assess the impact of fatigue on cognition. This means that the FAsD might not distinguish between physical and mental aspects of fatigue.
Treatment
It isn’t surprising that residual depression can increase health care utilization and economic burden, including such indirect costs as lost productivity and wages.29 Despite these impacts, there is a paucity of studies evaluating the relationship between residual symptoms, such as fatigue, and work productivity. It has been established that improving a depressed patient’s level of energy correlates with improved performance at work.
Treating fatigue as a residual symptom of MDD can be complicated because symptoms of fatigue might be:
• a discrete symptom of MDD
• a prodromal symptom of another disorder
• an adverse effect of an antidepressant.2,30
It is a major clinical problem, therefore, that antidepressants can alleviate and cause symptoms of fatigue.31 Treatment strategy should focus on identifying antidepressants that are less likely to cause fatigue (ie, noradrenergic or dopaminergic drugs, or both). Adjunctive treatments to target residual fatigue also can be used.32
There are limited published data on the effective treatment of residual fatigue in patients with MDD. Given the absence of sufficient evidence, agents that promote noradrenergic and dopaminergic neurotransmission have been the treatment of choice when targeting fatigue in depressed patients.2,14,21,33
The Table34-37 lists potential treatment options often used to treat fatigue associated with depression.
SSRIs. Treatment with SSRIs has been associated with a low probability of achieving remission when targeting fatigue as a symptom of MDD.21
One study reported that, after 8 weeks of treatment with an SSRI, treatment-emergent adverse events, such as worsening fatigue and weakness, were observed—along with an overall lack of efficacy in targeting all symptoms of depression.38
Another study demonstrated positive effects when a noradrenergic agent was added to an SSRI in partial responders who continued to complain of residual fatigue.33
However, studies that compared the effects of SSRIs with those of antidepressants that have pronoradrenergic effects showed that the 2 mechanisms of action were not significantly different from each other in their ability to resolve residual symptoms of fatigue.21 A limiting factor might be that these studies were retrospective and did not analyze the efficacy of a noradrenergic agent as an adjunct for alleviating symptoms of fatigue.39
Bupropion. This commonly used medication for fatigue is believed to cause a significantly lower level of fatigue compared with SSRIs.40 The potential utility of bupropion in this area could be a reflection of its mechanism of action—ie, the drug targets both noradrenergic and dopaminergic neurotransmission.41
A study comparing bupropion with SSRIs in targeting somatic symptoms of depression reported a small but statistically significant difference in favor of the bupropion-treated group. However, this finding was confounded by the small effect size and difficulty quantifying somatic symptoms.40
Stimulants and modafinil. Psycho-stimulants have been shown to be efficacious for depression and fatigue, both as monotherapy and adjunctively.39,42
Modafinil has demonstrated efficacy in open-label trials for improving residual fatigue, but failed to separate from placebo in controlled trials.43 At least 1 other failed study has been published examining modafinil as a treatment for fatigue associated with depression.43
Adjunctive therapy with CNS stimulants, such as amphetamine/dextroamphetamine and methylphenidate, has been used to treat fatigue, with positive results.16 Modafinil and stimulants also could be tried as an augmentation strategy to other antidepressants; such use is off-label and should be attempted only after careful consideration.16
Exercise might be a nonpharmacotherapeutic modality that targets the underlying physiology associated with fatigue. Exercise releases endorphins, which can affect overall brain chemistry and which have been theorized to diminish symptoms of fatigue and depression.44 Consider exercise in addition to treatment with an antidepressant in selected patients.45
To sum up
In general, the literature does not recommend one medication as superior to any other for treating fatigue that is a residual symptom of depression. Such hesitation suggests that more empirical studies are needed to determine what is the best and proper management of treating fatigue associated with depression.
Bottom LinE
Fatigue can be a symptom of major depressive disorder (MDD) or a risk factor for depression. Fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD. Residual fatigue can affect social, cognitive, emotional, and physical health and can result in increased utilization of health care services. A number of treatment options are available; none has been shown to be superior to the others.
Related Resources
• Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry. 2010;197(2):86-87.
• Targum SD, Fava M. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 2011;8(10):40-43.
• Illiades C. How to fight depression fatigue. Everyday Health. http://www.everydayhealth.com/health-report/major-depression-living-well/fight-depression-fatigue.aspx.
• Kerr M. Depression and fatigue: a vicious cycle. Healthline. http://www.healthline.com/health/depression/fatigue.
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Bupropion • Wellbutrin
Desipramine • Norpramin
Methylphenidate • Ritalin
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Disclosures
Dr. Sohail reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Macaluso has conducted clinical trials research as principal investigator for the following pharmaceutical manufacturers in the past 12 months: AbbVie, Inc.; Alkermes; AssureRx Health, Inc.; Eisai Co., Ltd.; FORUM Pharmaceuticals, Inc.; Janssen Pharmaceuticals, Inc.; and Naurex Inc. All clinical trial and study contracts were with, and payments were made to, University of Kansas Medical Center Research Institute, Kansas City, Kansas, a research institute affiliated with University of Kansas School of Medicine−Wichita.
Fatigue and depression can be viewed as a “vicious cycle”: Fatigue can be a symptom of major depression, and fatigue can be a risk factor for depression.1 For example, fatigue associated with a general medical condition or traumatic brain injury can be a risk factor for developing major depressive disorder (MDD).1-3 It isn’t surprising that fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD.
Despite the observed association between fatigue and depression, their underlying relationship often is unclear. The literature does not differentiate among fatigue associated with depression, fatigue as a treatment-emergent adverse effect, and fatigue as a residual symptom of depression that is partially responsive to treatment.4,5 To complicate the situation, many medications used to treat MDD can cause fatigue.
Patients often describe fatigue as (1) feeling tired, exhausted, or drained and (2) lacking energy and motivation. Fatigue can be related to impaired wakefulness but is believed to be a different entity than sleepiness.6 Residual fatigue can affect social, cognitive, emotional, and physical health.
We reviewed the literature about fatigue as a symptom of MDD by conducting a search of Medline, PubMed, and Google Scholar, using keywords depression, fatigue, residual symptoms, and treatment. We chose the papers cited in this article based on our consensus and because these publications represent expert opinion or the highest quality evidence available.
Residual fatigue has an effect on prognosis
Fatigue is a common symptom of MDD that persists in 20% to 30% of patients whose symptoms of depression otherwise remit.4,7-9 Several studies have linked residual fatigue with the overall prognosis of MDD.5 Data from a prospective study demonstrate that depressed patients have a higher risk of relapse when they continue to report symptoms of fatigue after their symptoms of depression have otherwise entered partial remission.10 Another study demonstrated that the severity of residual symptoms of depression is a strong predictor of another major depressive episode.11
In a large-scale study, the prevalence of residual fatigue after adequate treatment of MDD in both partial responders and remitters was 84.6%.12 The same study showed that one-third of patients who had been treated for MDD had persistent and clinically significant fatigue, which could suggest a relationship between fatigue and selective serotonin reuptake inhibitors (SSRIs) and other antidepressants.
Another study demonstrated that 64.6% of patients who responded to antidepressant treatment and who had baseline fatigue continued to exhibit symptoms of fatigue after an adequate trial of an antidepressant.13
Neurobiological considerations
Studies have shown that the neuronal circuits that malfunction in fatigue are different from those that malfunction in depression.14 Although the neurobiology of fatigue has not been determined, decreased neuronal activity in the prefrontal circuits has been associated with symptoms of fatigue.15
In addition, evidence from the literature shows a decrease in hormone secretion16 and cognitive abilities in patients exhibiting symptoms of fatigue.17 These findings have led some experts to hypothesize that symptoms of fatigue associated with depression could be the result of (1) immune dysregulation18 and (2) an inability of available antidepressants to target the underlying biology of the disorder.2
Despite the hypothesis that fatigue associated with depression might be biologically related to immune dysregulation, some authors continue to point to an imbalance in neurotransmitters—norepinephrine, histamine, dopamine, acetylcholine—as being associated with fatigue.14 For example, a study demonstrated that drugs targeting noradrenergic reuptake inhibition were more effective at preventing a relapse of fatigue compared with serotonergic drugs.19 Another study showed improvement in energy with an increase in the plasma level of desipramine, which affects noradrenergic neurotransmission.20
Inflammatory cytokines also have been explored in the search for an understanding of the etiology of fatigue and depression.21 Physical and mental stress promote the release of cytokines, which activate the immune system by inducing an inflammatory response; this response has been etiologically linked to depressive disorders.22 Furthermore, studies have demonstrated an elevated level of inflammatory cytokines in patients who have MDD— suggesting that MDD is associated with a chronic low level of inflammation that crosses the blood−brain barrier.23
Clinical considerations: A role for rating scales?
Despite the significance of residual fatigue on the quality of life of patients who have MDD, most common rating scales, such as the Hamilton Depression Rating Scale24 and the Montgomery-Åsberg Depression Rating Scale,25 have limited sensitivity for measuring fatigue.26 The Fatigue Associated with Depression (FAsD)27 questionnaire, designed according to FDA guidelines,28 is used to assess fatigue associated with depression. The final version of the FAsD includes 13 items: a 6-item experience subscale and a 7-item impact subscale.
Is the FAsD helpful? The experience subscale of the FAsD assesses how often the patient experiences different aspects of fatigue (tiredness, exhaustion, lack of energy, physical weakness, and a feeling that everything requires too much effort). The impact subscale of the FAsD assesses the effect of fatigue on daily life.
The overall FAsD score is calculated by taking the mean of each subscale; a change of 0.67 on the experience subscale and 0.57 on the impact subscale are considered clinically meaningful.27 The measurement properties of the questionnaire showed internal consistency, reliability, and validity in testing. Researchers note, however, that FAsD does not include items to assess the impact of fatigue on cognition. This means that the FAsD might not distinguish between physical and mental aspects of fatigue.
Treatment
It isn’t surprising that residual depression can increase health care utilization and economic burden, including such indirect costs as lost productivity and wages.29 Despite these impacts, there is a paucity of studies evaluating the relationship between residual symptoms, such as fatigue, and work productivity. It has been established that improving a depressed patient’s level of energy correlates with improved performance at work.
Treating fatigue as a residual symptom of MDD can be complicated because symptoms of fatigue might be:
• a discrete symptom of MDD
• a prodromal symptom of another disorder
• an adverse effect of an antidepressant.2,30
It is a major clinical problem, therefore, that antidepressants can alleviate and cause symptoms of fatigue.31 Treatment strategy should focus on identifying antidepressants that are less likely to cause fatigue (ie, noradrenergic or dopaminergic drugs, or both). Adjunctive treatments to target residual fatigue also can be used.32
There are limited published data on the effective treatment of residual fatigue in patients with MDD. Given the absence of sufficient evidence, agents that promote noradrenergic and dopaminergic neurotransmission have been the treatment of choice when targeting fatigue in depressed patients.2,14,21,33
The Table34-37 lists potential treatment options often used to treat fatigue associated with depression.
SSRIs. Treatment with SSRIs has been associated with a low probability of achieving remission when targeting fatigue as a symptom of MDD.21
One study reported that, after 8 weeks of treatment with an SSRI, treatment-emergent adverse events, such as worsening fatigue and weakness, were observed—along with an overall lack of efficacy in targeting all symptoms of depression.38
Another study demonstrated positive effects when a noradrenergic agent was added to an SSRI in partial responders who continued to complain of residual fatigue.33
However, studies that compared the effects of SSRIs with those of antidepressants that have pronoradrenergic effects showed that the 2 mechanisms of action were not significantly different from each other in their ability to resolve residual symptoms of fatigue.21 A limiting factor might be that these studies were retrospective and did not analyze the efficacy of a noradrenergic agent as an adjunct for alleviating symptoms of fatigue.39
Bupropion. This commonly used medication for fatigue is believed to cause a significantly lower level of fatigue compared with SSRIs.40 The potential utility of bupropion in this area could be a reflection of its mechanism of action—ie, the drug targets both noradrenergic and dopaminergic neurotransmission.41
A study comparing bupropion with SSRIs in targeting somatic symptoms of depression reported a small but statistically significant difference in favor of the bupropion-treated group. However, this finding was confounded by the small effect size and difficulty quantifying somatic symptoms.40
Stimulants and modafinil. Psycho-stimulants have been shown to be efficacious for depression and fatigue, both as monotherapy and adjunctively.39,42
Modafinil has demonstrated efficacy in open-label trials for improving residual fatigue, but failed to separate from placebo in controlled trials.43 At least 1 other failed study has been published examining modafinil as a treatment for fatigue associated with depression.43
Adjunctive therapy with CNS stimulants, such as amphetamine/dextroamphetamine and methylphenidate, has been used to treat fatigue, with positive results.16 Modafinil and stimulants also could be tried as an augmentation strategy to other antidepressants; such use is off-label and should be attempted only after careful consideration.16
Exercise might be a nonpharmacotherapeutic modality that targets the underlying physiology associated with fatigue. Exercise releases endorphins, which can affect overall brain chemistry and which have been theorized to diminish symptoms of fatigue and depression.44 Consider exercise in addition to treatment with an antidepressant in selected patients.45
To sum up
In general, the literature does not recommend one medication as superior to any other for treating fatigue that is a residual symptom of depression. Such hesitation suggests that more empirical studies are needed to determine what is the best and proper management of treating fatigue associated with depression.
Bottom LinE
Fatigue can be a symptom of major depressive disorder (MDD) or a risk factor for depression. Fatigue has been studied as a predictor of relapse after previous response to treatment in patients with MDD. Residual fatigue can affect social, cognitive, emotional, and physical health and can result in increased utilization of health care services. A number of treatment options are available; none has been shown to be superior to the others.
Related Resources
• Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry. 2010;197(2):86-87.
• Targum SD, Fava M. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 2011;8(10):40-43.
• Illiades C. How to fight depression fatigue. Everyday Health. http://www.everydayhealth.com/health-report/major-depression-living-well/fight-depression-fatigue.aspx.
• Kerr M. Depression and fatigue: a vicious cycle. Healthline. http://www.healthline.com/health/depression/fatigue.
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Bupropion • Wellbutrin
Desipramine • Norpramin
Methylphenidate • Ritalin
Modafinil • Provigil
Sertraline • Zoloft
Venlafaxine • Effexor
Disclosures
Dr. Sohail reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Macaluso has conducted clinical trials research as principal investigator for the following pharmaceutical manufacturers in the past 12 months: AbbVie, Inc.; Alkermes; AssureRx Health, Inc.; Eisai Co., Ltd.; FORUM Pharmaceuticals, Inc.; Janssen Pharmaceuticals, Inc.; and Naurex Inc. All clinical trial and study contracts were with, and payments were made to, University of Kansas Medical Center Research Institute, Kansas City, Kansas, a research institute affiliated with University of Kansas School of Medicine−Wichita.
1. Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427-431.
2. Demyttenaere K, De Fruyt J, Stahl, SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93-105.
3. Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosom Med. 2004;66(3):330-335.
4. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50.
5. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80(2-3):135-144.
6. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63-76.
7. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225.
8. Tylee A, Gastpar M, Lépine JP, et al. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14(3):139-151.
9. Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2-3):141-150.
10. Paykel ES, Ramana, R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180.
11. Bockting CL, Spinhoven P, Koeter MW, et al; Depression Evaluation Longitudinal Therapy Assessment Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67(5):747-755.
12. Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med. 2004;19(8):813-818.
13. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180-186.
14. Stahl SM, Zhang L, Damatarca C, et al. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(suppl 14):6-17.
15. MacHale SM, Law´rie SM, Cavanagh JT, et al. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550-556.
16. Paykel ES. Achieving gains beyond response. Acta Psychiatrica Scandinavica Suppl. 2002;(415):12-17.
17. van den Heuvel OA, Groenewegen HJ, Barkhof F, et al. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18(2):367-374.
18. Jaremka LM, Fagundes CP, Glaser R, et al. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310-1317.
19. Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47(5):411-418.
20. Nelson JC, Mazure C, Quinlan DM, et al. Drug-responsive symptoms in melancholia. Arch Gen Psychiatry. 1984;41(7):663-668.
21. Fava M, Ball S, Nelson, JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250-257.
22. Anisman H, Merali Z, Poulter MO, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963-972.
23. Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230-233.
24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
26. Matza LS, Phillips GA, Revicki DA, et al. Development and validation of a patient-report measure of fatigue associated with depression. J Affect Disord. 2011;134(1-3):294-303.
27. Matza LS, Wyrwich KW, Phillips GA, et al. The Fatigue Associated with Depression Questionnaire (FAsD): responsiveness and responder definition. Qual Life Res. 2013;22(2):351-360.
28. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Food and Drug Administration. http://www.fda. gov/downloads/Drugs/Guidances/UCM193282.pdf. Published December 2009. Accessed May 7, 2015.
29. Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188-e196.
30. Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64(suppl 14):30-34.
31. Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971-975.
32. Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry. 2006;67(suppl 6):9-15.
33. Ball SG, Dellva MA, D’Souza D, et al. A double-blind, placebo-controlled study of augmentation with LY2216684 for major depressive disorder patients who are partial responders to selective serotonin reuptake inhibitors [abstract P 05]. Int J Psych Clin Pract. 2010;14(suppl 1):19.
34. Stahl SM. Using secondary binding properties to select a not so elective serotonin reuptake inhibitor. J Clin Psychiatry. 1998;59(12):642-643.
35. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 2nd ed. New York, NY: Cambridge University Press; 2000.
36. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
37. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620-8628.
38. Daly EJ, Trivedi MH, Fava M, et al. The relationship between adverse events during selective serotonin reuptake inhibitor treatment for major depressive disorder and nonremission in the suicide assessment methodology study. J Clin Psychopharmacol. 2011;31(1):31-38.
39. Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry. 1999;46(9):1301-1308.
40. Papakostas GI, Nutt DJ, Hallett LA, et al. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350-1355.
41. Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113.
42. Candy M, Jones CB, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;(2):CD006722. doi: 10.1002/14651858.CD006722.pub2.
43. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother. 2007;41(6):1005-1012.
44. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychol Rev. 2001;21(1):33-61.
45. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12(4):205-213.
1. Schönberger M, Herrberg M, Ponsford J. Fatigue as a cause, not a consequence of depression and daytime sleepiness: a cross-lagged analysis. J Head Trauma Rehabil. 2014;29(5):427-431.
2. Demyttenaere K, De Fruyt J, Stahl, SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93-105.
3. Skapinakis P, Lewis G, Mavreas V. Temporal relations between unexplained fatigue and depression: longitudinal data from an international study in primary care. Psychosom Med. 2004;66(3):330-335.
4. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40(1):41-50.
5. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80(2-3):135-144.
6. Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63-76.
7. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225.
8. Tylee A, Gastpar M, Lépine JP, et al. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14(3):139-151.
9. Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2-3):141-150.
10. Paykel ES, Ramana, R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180.
11. Bockting CL, Spinhoven P, Koeter MW, et al; Depression Evaluation Longitudinal Therapy Assessment Study Group. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67(5):747-755.
12. Greco T, Eckert G, Kroenke K. The outcome of physical symptoms with treatment of depression. J Gen Intern Med. 2004;19(8):813-818.
13. McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31(2):180-186.
14. Stahl SM, Zhang L, Damatarca C, et al. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. J Clin Psychiatry. 2003;64(suppl 14):6-17.
15. MacHale SM, Law´rie SM, Cavanagh JT, et al. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550-556.
16. Paykel ES. Achieving gains beyond response. Acta Psychiatrica Scandinavica Suppl. 2002;(415):12-17.
17. van den Heuvel OA, Groenewegen HJ, Barkhof F, et al. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of Tower of London task. Neuroimage. 2003;18(2):367-374.
18. Jaremka LM, Fagundes CP, Glaser R, et al. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310-1317.
19. Delgado PL, Charney DS, Price LH, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47(5):411-418.
20. Nelson JC, Mazure C, Quinlan DM, et al. Drug-responsive symptoms in melancholia. Arch Gen Psychiatry. 1984;41(7):663-668.
21. Fava M, Ball S, Nelson, JC, et al. Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety. 2014;31(3):250-257.
22. Anisman H, Merali Z, Poulter MO, et al. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963-972.
23. Simon NM, McNamara K, Chow CW, et al. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18(3):230-233.
24. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
26. Matza LS, Phillips GA, Revicki DA, et al. Development and validation of a patient-report measure of fatigue associated with depression. J Affect Disord. 2011;134(1-3):294-303.
27. Matza LS, Wyrwich KW, Phillips GA, et al. The Fatigue Associated with Depression Questionnaire (FAsD): responsiveness and responder definition. Qual Life Res. 2013;22(2):351-360.
28. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Food and Drug Administration. http://www.fda. gov/downloads/Drugs/Guidances/UCM193282.pdf. Published December 2009. Accessed May 7, 2015.
29. Knoth RL, Bolge SC, Kim E, et al. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188-e196.
30. Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64(suppl 14):30-34.
31. Chang T, Fava M. The future of psychopharmacology of depression. J Clin Psychiatry. 2010;71(8):971-975.
32. Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry. 2006;67(suppl 6):9-15.
33. Ball SG, Dellva MA, D’Souza D, et al. A double-blind, placebo-controlled study of augmentation with LY2216684 for major depressive disorder patients who are partial responders to selective serotonin reuptake inhibitors [abstract P 05]. Int J Psych Clin Pract. 2010;14(suppl 1):19.
34. Stahl SM. Using secondary binding properties to select a not so elective serotonin reuptake inhibitor. J Clin Psychiatry. 1998;59(12):642-643.
35. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical applications. 2nd ed. New York, NY: Cambridge University Press; 2000.
36. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
37. Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620-8628.
38. Daly EJ, Trivedi MH, Fava M, et al. The relationship between adverse events during selective serotonin reuptake inhibitor treatment for major depressive disorder and nonremission in the suicide assessment methodology study. J Clin Psychopharmacol. 2011;31(1):31-38.
39. Nelson JC. A review of the efficacy of serotonergic and noradrenergic reuptake inhibitors for treatment of major depression. Biol Psychiatry. 1999;46(9):1301-1308.
40. Papakostas GI, Nutt DJ, Hallett LA, et al. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350-1355.
41. Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113.
42. Candy M, Jones CB, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;(2):CD006722. doi: 10.1002/14651858.CD006722.pub2.
43. Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants. Ann Pharmacother. 2007;41(6):1005-1012.
44. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychol Rev. 2001;21(1):33-61.
45. Trivedi MH, Greer TL, Grannemann BD, et al. Exercise as an augmentation strategy for treatment of major depression. J Psychiatr Pract. 2006;12(4):205-213.
Beyond dopamine: Brain repair tactics in schizophrenia
For the past 60 years, the standard of care has remained one-dimensional in this brain syndrome, even though the clinical and neurobiological complexities of schizophrenia are multidimensional. Dopamine D2 receptor antagonists, discovered serendipitously in the 1950s, have remained the mainstay of treatment, despite momentous insights about the neurodevelopmental and neurodegenerative processes of schizophrenia.
Why do we ignore abundant evidence that the brain in schizophrenia needs extensive structural repair, not simply a reduction in the activity of a single neurotransmitter in the mesolimbic dopamine tract? Perhaps the age-old dogmatic pessimism that neurodegeneration cannot be reversed has inhibited the field from attempting to escape the dopamine box, so to speak, and from developing innovative, even radical, approaches to repair of the brain of persons with schizophrenia.
But radical thinking is justified when dealing with a cruel brain syndrome that disables young adults in the prime of life.
We should exploit neuroprotective tactics
Several neuroprotective approaches to preventing or reversing the degenerative changes across brain regions in schizophrenia are now recognized. Indirect evidence exists for such interventions in animal models, but the results of few controlled human studies have been published.
Here are my proposals for using neuroprotective tactics to address the unmet need to repair the brain of patients ravaged by neurotoxic psychotic relapses.
Promote 100% adherence to antipsychotic therapy. The simplest tactic to protect the brain from atrophy in patients with schizophrenia is to use long-acting injectable antipsychotic agents immediately after the first psychotic episode. The risk of a psychotic relapse is far lower (7-fold lower, according to a study performed at the University of California, Los Angeles, that soon will be published) with an injectable medication than with oral medication in first-episode patients. Preventing psychotic episodes is, logically, the most important neuroprotective tactic.
Enhance neurogenesis. The brain has 2 neurogenic regions that produce progenitor cells (stem cells) that gradually mature and differentiate into neurons and glia. That is how the brain naturally replenishes itself throughout life. This adult neurogenesis process, carried out in the dentate gyrus of the hippocampus and in the subventricular zone, stops during psychosis but resumes when psychosis remits.
Second-generation antipsychotics (but not first-generation agents) stimulate neurogenesis in animals.1 Haloperidol, in fact, does the opposite—suppressing neurogenesis and causing neuronal death via 15 different molecular mechanisms (see my editorial, “Haloperidol clearly is neurotoxic. Should it be banned?,” in the July 2013 issue).
Other psychotropics also induce neurogenesis, including selective serotonin reuptake inhibitors (SSRIs), which increase hippocampal neurogenesis (atypical antipsychotics appear to increase neurogenesis in the subventricular zone).2 SSRIs often have been used in schizophrenia patients for 2 common comorbid conditions: depression and anxiety. These agents can help regenerate brain tissue, in addition to providing their approved therapeutic indications.
Lithium and valproate have been shown to be neuroprotective3 and to stimulate neurogenesis. Both are often used in schizoaffective disorder, bipolar type; they can exert a neuroprotective effect in addition to their clinical usefulness. The combination of an SSRI or lithium with a second-generation antipsychotic could be synergistic in turbocharging neurogenesis. This sounds like polypharmacy—but it is a rational approach that deserves to be put to the test.
Increase neurotrophins, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). When neurotrophin levels decline, the brain starts shrinking because of apoptosis. Psychosis lowers neurotrophins drastically—by approximately 60%. Atypical antipsychotics have been reported to increase the level of neurotrophins; haloperidol actually lowers those levels.4
Decrease inflammation. Psychosis has been shown to be associated with neuro-inflammation, as reflected in a surge of pro-inflammatory cytokines (released from activated microglia).5 A rise in interleukin-6, tumor necrosis factor-alpha, interferon-gamma, and other pro-inflammatory markers has been extensively documented in many studies.
With that observed rise in mind, several controlled studies have shown that adding an anti-inflammatory agent (aspirin, a nonsteroidal anti-inflammatory drug, a COX-2 inhibitor, or minocycline) to an antipsychotic can accentuate the therapeutic response, especially during a first episode of psychosis.6 Note also that second-generation antipsychotics have anti-inflammatory effects7 as well that might be part of their efficacy beyond blocking dopamine D2 receptors.
Decrease free radicals. Microglia are activated by psychosis to release free radicals, also known as reactive oxygen species; these include nitric oxide, superoxide, and peroxynitrate. All these species are destructive to brain tissue. Using an adjunctive strong antioxidant, such as N-acetyl cysteine,8 with an antipsychotic might help neutralize destructive effects of free radicals and protect the brain from tissue loss during a psychotic episode.
Avoid apoptosis inducers. Several substances can initiate programmed cell death (apoptosis), which is triggered during psychosis (believed to be caused by increased dopamine and, possibly, glutamate, activity) and which leads to brain atrophy. Patients with schizophrenia must be protected from these apoptosis inducers:
• amphetamine
• cocaine
• Cannabis
• lipid peroxidation products
• inflammatory cytokines.
Apoptosis can be inhibited by maintaining high levels of neurotrophic factors. Atypical, but not typical, antipsychotics increase levels of neurotrophins, such as NGF and BDNF.4 In addition, the Bcl-2 family of proteins inhibits apoptosis,9 and drugs such as lithium and valproate can induce Bcl-2 and protect against apoptosis and neuronal loss.3
Restore white-matter integrity. Numerous studies using diffusion tensor imaging have revealed that myelin is reduced or lacks integrity in schizophrenia. This results in loss of critical connectivity among brain regions, which might explain psychotic and cognitive symptoms. One possible way to repair white matter, which becomes more damaged after multiple psychotic episodes, is to use drugs indicated to treat the demyelinating disorder multiple sclerosis. Antagonists of LINGO-1, a negative regulator of axonal myelination, are a prominent possibility; a recent study reported altered signaling of LINGO-1 in schizophrenia.10
Decrease excessive glutamate. Because glutamate is neurotoxic and might contribute to brain-tissue loss during psychosis, it is important to reduce glutamate activity in schizophrenia. Lamotrigine and valproate are both known to do that.11 Several studies indicate that adjunctive lamotrigine might be helpful in schizophrenia.12
Inhibit caspase-3, also known as the “death cascade,” which is involved in brain-tissue loss. Eicosapentaenoic acid is an omega-3 fatty acid that inhibits caspase-3. Interestingly, omega-3 levels in patients with schizophrenia are significantly lower than in healthy subjects.13 Lithium also can inhibit caspase-3.
Do these proposals sound radical?
Most of the recommendations I’ve made here are not employed in the clinical practice of psychiatry. These ideas must be put to the test in controlled clinical trials.
The crux of my argument is that we need to think outside the “dopamine box” and focus on brain repair if we are to make progress in reversing, even preventing, neurodegeneration and clinical deterioration in this disabling brain syndrome. Just as cancer often is treated with rational polypharmacy, schizophrenia might need a similar approach. To vanquish schizophrenia—a goal that has eluded us—it is imperative to pursue radically novel and disruptive therapeutic strategies. The ideas I’ve listed here sound the call that the quest to repair the brain in schizophrenia must begin, and soon.
1. Agius N, Nandra, KS. Do atypical antipsychotics promote neurogenesis as a class effect? Psychiatr Danub. 2012;24(suppl 1):S191-S193.
2. Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res. 2010;1354:23-29.
3. Chiu CT, Wang Z, Hunsberger JG, et al. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev. 2013;65(1):105-142.
4. Parikh V, Khan MM, Terry A, et al. Differential effects of typical and atypical antipsychotics on nerve growth factor and choline acetyltransferase expression in the cortex and nucleus basalis of rats. J Psychiatr Res. 2004;38(5):521-529.
5. Monji A, Kato TA, Mizoguchi Y, et al. Neuro-inflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
6. Sommer IE, deWitte L, Begemann M, et al. Nonsteriodal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
7. Bian Q, Kato T, Monji A, et al. The effect of atypical anti-psychotics perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):42-48.
8. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64(5):361-368.
9. Huang J, Fairbrother W, Reed JC, et al. Therapeutic targeting of Bcl-2 family for treatment of B-cell malignancies. Expert Rev Hematol. 2015;8(3):283-297.
10. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014; 4:e348.
11. Zink M, Correll CU. Glutamatergic agents for schizophrenia: current evidence and perspectives. Expert Rev Clin Pharmacol. 2015;8(3):335-352.
12. Kremer I, Vass A, Gorelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry. 2004;56(6):444-446.
13. McEvoy J, Baillie RA, Zhu H, et al. Lipidomics reveals early metabolic changes in subjects with schizophrenia: effects of atypical antipsychotics. PLoS One. 2013;8(7):e68717.
For the past 60 years, the standard of care has remained one-dimensional in this brain syndrome, even though the clinical and neurobiological complexities of schizophrenia are multidimensional. Dopamine D2 receptor antagonists, discovered serendipitously in the 1950s, have remained the mainstay of treatment, despite momentous insights about the neurodevelopmental and neurodegenerative processes of schizophrenia.
Why do we ignore abundant evidence that the brain in schizophrenia needs extensive structural repair, not simply a reduction in the activity of a single neurotransmitter in the mesolimbic dopamine tract? Perhaps the age-old dogmatic pessimism that neurodegeneration cannot be reversed has inhibited the field from attempting to escape the dopamine box, so to speak, and from developing innovative, even radical, approaches to repair of the brain of persons with schizophrenia.
But radical thinking is justified when dealing with a cruel brain syndrome that disables young adults in the prime of life.
We should exploit neuroprotective tactics
Several neuroprotective approaches to preventing or reversing the degenerative changes across brain regions in schizophrenia are now recognized. Indirect evidence exists for such interventions in animal models, but the results of few controlled human studies have been published.
Here are my proposals for using neuroprotective tactics to address the unmet need to repair the brain of patients ravaged by neurotoxic psychotic relapses.
Promote 100% adherence to antipsychotic therapy. The simplest tactic to protect the brain from atrophy in patients with schizophrenia is to use long-acting injectable antipsychotic agents immediately after the first psychotic episode. The risk of a psychotic relapse is far lower (7-fold lower, according to a study performed at the University of California, Los Angeles, that soon will be published) with an injectable medication than with oral medication in first-episode patients. Preventing psychotic episodes is, logically, the most important neuroprotective tactic.
Enhance neurogenesis. The brain has 2 neurogenic regions that produce progenitor cells (stem cells) that gradually mature and differentiate into neurons and glia. That is how the brain naturally replenishes itself throughout life. This adult neurogenesis process, carried out in the dentate gyrus of the hippocampus and in the subventricular zone, stops during psychosis but resumes when psychosis remits.
Second-generation antipsychotics (but not first-generation agents) stimulate neurogenesis in animals.1 Haloperidol, in fact, does the opposite—suppressing neurogenesis and causing neuronal death via 15 different molecular mechanisms (see my editorial, “Haloperidol clearly is neurotoxic. Should it be banned?,” in the July 2013 issue).
Other psychotropics also induce neurogenesis, including selective serotonin reuptake inhibitors (SSRIs), which increase hippocampal neurogenesis (atypical antipsychotics appear to increase neurogenesis in the subventricular zone).2 SSRIs often have been used in schizophrenia patients for 2 common comorbid conditions: depression and anxiety. These agents can help regenerate brain tissue, in addition to providing their approved therapeutic indications.
Lithium and valproate have been shown to be neuroprotective3 and to stimulate neurogenesis. Both are often used in schizoaffective disorder, bipolar type; they can exert a neuroprotective effect in addition to their clinical usefulness. The combination of an SSRI or lithium with a second-generation antipsychotic could be synergistic in turbocharging neurogenesis. This sounds like polypharmacy—but it is a rational approach that deserves to be put to the test.
Increase neurotrophins, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). When neurotrophin levels decline, the brain starts shrinking because of apoptosis. Psychosis lowers neurotrophins drastically—by approximately 60%. Atypical antipsychotics have been reported to increase the level of neurotrophins; haloperidol actually lowers those levels.4
Decrease inflammation. Psychosis has been shown to be associated with neuro-inflammation, as reflected in a surge of pro-inflammatory cytokines (released from activated microglia).5 A rise in interleukin-6, tumor necrosis factor-alpha, interferon-gamma, and other pro-inflammatory markers has been extensively documented in many studies.
With that observed rise in mind, several controlled studies have shown that adding an anti-inflammatory agent (aspirin, a nonsteroidal anti-inflammatory drug, a COX-2 inhibitor, or minocycline) to an antipsychotic can accentuate the therapeutic response, especially during a first episode of psychosis.6 Note also that second-generation antipsychotics have anti-inflammatory effects7 as well that might be part of their efficacy beyond blocking dopamine D2 receptors.
Decrease free radicals. Microglia are activated by psychosis to release free radicals, also known as reactive oxygen species; these include nitric oxide, superoxide, and peroxynitrate. All these species are destructive to brain tissue. Using an adjunctive strong antioxidant, such as N-acetyl cysteine,8 with an antipsychotic might help neutralize destructive effects of free radicals and protect the brain from tissue loss during a psychotic episode.
Avoid apoptosis inducers. Several substances can initiate programmed cell death (apoptosis), which is triggered during psychosis (believed to be caused by increased dopamine and, possibly, glutamate, activity) and which leads to brain atrophy. Patients with schizophrenia must be protected from these apoptosis inducers:
• amphetamine
• cocaine
• Cannabis
• lipid peroxidation products
• inflammatory cytokines.
Apoptosis can be inhibited by maintaining high levels of neurotrophic factors. Atypical, but not typical, antipsychotics increase levels of neurotrophins, such as NGF and BDNF.4 In addition, the Bcl-2 family of proteins inhibits apoptosis,9 and drugs such as lithium and valproate can induce Bcl-2 and protect against apoptosis and neuronal loss.3
Restore white-matter integrity. Numerous studies using diffusion tensor imaging have revealed that myelin is reduced or lacks integrity in schizophrenia. This results in loss of critical connectivity among brain regions, which might explain psychotic and cognitive symptoms. One possible way to repair white matter, which becomes more damaged after multiple psychotic episodes, is to use drugs indicated to treat the demyelinating disorder multiple sclerosis. Antagonists of LINGO-1, a negative regulator of axonal myelination, are a prominent possibility; a recent study reported altered signaling of LINGO-1 in schizophrenia.10
Decrease excessive glutamate. Because glutamate is neurotoxic and might contribute to brain-tissue loss during psychosis, it is important to reduce glutamate activity in schizophrenia. Lamotrigine and valproate are both known to do that.11 Several studies indicate that adjunctive lamotrigine might be helpful in schizophrenia.12
Inhibit caspase-3, also known as the “death cascade,” which is involved in brain-tissue loss. Eicosapentaenoic acid is an omega-3 fatty acid that inhibits caspase-3. Interestingly, omega-3 levels in patients with schizophrenia are significantly lower than in healthy subjects.13 Lithium also can inhibit caspase-3.
Do these proposals sound radical?
Most of the recommendations I’ve made here are not employed in the clinical practice of psychiatry. These ideas must be put to the test in controlled clinical trials.
The crux of my argument is that we need to think outside the “dopamine box” and focus on brain repair if we are to make progress in reversing, even preventing, neurodegeneration and clinical deterioration in this disabling brain syndrome. Just as cancer often is treated with rational polypharmacy, schizophrenia might need a similar approach. To vanquish schizophrenia—a goal that has eluded us—it is imperative to pursue radically novel and disruptive therapeutic strategies. The ideas I’ve listed here sound the call that the quest to repair the brain in schizophrenia must begin, and soon.
For the past 60 years, the standard of care has remained one-dimensional in this brain syndrome, even though the clinical and neurobiological complexities of schizophrenia are multidimensional. Dopamine D2 receptor antagonists, discovered serendipitously in the 1950s, have remained the mainstay of treatment, despite momentous insights about the neurodevelopmental and neurodegenerative processes of schizophrenia.
Why do we ignore abundant evidence that the brain in schizophrenia needs extensive structural repair, not simply a reduction in the activity of a single neurotransmitter in the mesolimbic dopamine tract? Perhaps the age-old dogmatic pessimism that neurodegeneration cannot be reversed has inhibited the field from attempting to escape the dopamine box, so to speak, and from developing innovative, even radical, approaches to repair of the brain of persons with schizophrenia.
But radical thinking is justified when dealing with a cruel brain syndrome that disables young adults in the prime of life.
We should exploit neuroprotective tactics
Several neuroprotective approaches to preventing or reversing the degenerative changes across brain regions in schizophrenia are now recognized. Indirect evidence exists for such interventions in animal models, but the results of few controlled human studies have been published.
Here are my proposals for using neuroprotective tactics to address the unmet need to repair the brain of patients ravaged by neurotoxic psychotic relapses.
Promote 100% adherence to antipsychotic therapy. The simplest tactic to protect the brain from atrophy in patients with schizophrenia is to use long-acting injectable antipsychotic agents immediately after the first psychotic episode. The risk of a psychotic relapse is far lower (7-fold lower, according to a study performed at the University of California, Los Angeles, that soon will be published) with an injectable medication than with oral medication in first-episode patients. Preventing psychotic episodes is, logically, the most important neuroprotective tactic.
Enhance neurogenesis. The brain has 2 neurogenic regions that produce progenitor cells (stem cells) that gradually mature and differentiate into neurons and glia. That is how the brain naturally replenishes itself throughout life. This adult neurogenesis process, carried out in the dentate gyrus of the hippocampus and in the subventricular zone, stops during psychosis but resumes when psychosis remits.
Second-generation antipsychotics (but not first-generation agents) stimulate neurogenesis in animals.1 Haloperidol, in fact, does the opposite—suppressing neurogenesis and causing neuronal death via 15 different molecular mechanisms (see my editorial, “Haloperidol clearly is neurotoxic. Should it be banned?,” in the July 2013 issue).
Other psychotropics also induce neurogenesis, including selective serotonin reuptake inhibitors (SSRIs), which increase hippocampal neurogenesis (atypical antipsychotics appear to increase neurogenesis in the subventricular zone).2 SSRIs often have been used in schizophrenia patients for 2 common comorbid conditions: depression and anxiety. These agents can help regenerate brain tissue, in addition to providing their approved therapeutic indications.
Lithium and valproate have been shown to be neuroprotective3 and to stimulate neurogenesis. Both are often used in schizoaffective disorder, bipolar type; they can exert a neuroprotective effect in addition to their clinical usefulness. The combination of an SSRI or lithium with a second-generation antipsychotic could be synergistic in turbocharging neurogenesis. This sounds like polypharmacy—but it is a rational approach that deserves to be put to the test.
Increase neurotrophins, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). When neurotrophin levels decline, the brain starts shrinking because of apoptosis. Psychosis lowers neurotrophins drastically—by approximately 60%. Atypical antipsychotics have been reported to increase the level of neurotrophins; haloperidol actually lowers those levels.4
Decrease inflammation. Psychosis has been shown to be associated with neuro-inflammation, as reflected in a surge of pro-inflammatory cytokines (released from activated microglia).5 A rise in interleukin-6, tumor necrosis factor-alpha, interferon-gamma, and other pro-inflammatory markers has been extensively documented in many studies.
With that observed rise in mind, several controlled studies have shown that adding an anti-inflammatory agent (aspirin, a nonsteroidal anti-inflammatory drug, a COX-2 inhibitor, or minocycline) to an antipsychotic can accentuate the therapeutic response, especially during a first episode of psychosis.6 Note also that second-generation antipsychotics have anti-inflammatory effects7 as well that might be part of their efficacy beyond blocking dopamine D2 receptors.
Decrease free radicals. Microglia are activated by psychosis to release free radicals, also known as reactive oxygen species; these include nitric oxide, superoxide, and peroxynitrate. All these species are destructive to brain tissue. Using an adjunctive strong antioxidant, such as N-acetyl cysteine,8 with an antipsychotic might help neutralize destructive effects of free radicals and protect the brain from tissue loss during a psychotic episode.
Avoid apoptosis inducers. Several substances can initiate programmed cell death (apoptosis), which is triggered during psychosis (believed to be caused by increased dopamine and, possibly, glutamate, activity) and which leads to brain atrophy. Patients with schizophrenia must be protected from these apoptosis inducers:
• amphetamine
• cocaine
• Cannabis
• lipid peroxidation products
• inflammatory cytokines.
Apoptosis can be inhibited by maintaining high levels of neurotrophic factors. Atypical, but not typical, antipsychotics increase levels of neurotrophins, such as NGF and BDNF.4 In addition, the Bcl-2 family of proteins inhibits apoptosis,9 and drugs such as lithium and valproate can induce Bcl-2 and protect against apoptosis and neuronal loss.3
Restore white-matter integrity. Numerous studies using diffusion tensor imaging have revealed that myelin is reduced or lacks integrity in schizophrenia. This results in loss of critical connectivity among brain regions, which might explain psychotic and cognitive symptoms. One possible way to repair white matter, which becomes more damaged after multiple psychotic episodes, is to use drugs indicated to treat the demyelinating disorder multiple sclerosis. Antagonists of LINGO-1, a negative regulator of axonal myelination, are a prominent possibility; a recent study reported altered signaling of LINGO-1 in schizophrenia.10
Decrease excessive glutamate. Because glutamate is neurotoxic and might contribute to brain-tissue loss during psychosis, it is important to reduce glutamate activity in schizophrenia. Lamotrigine and valproate are both known to do that.11 Several studies indicate that adjunctive lamotrigine might be helpful in schizophrenia.12
Inhibit caspase-3, also known as the “death cascade,” which is involved in brain-tissue loss. Eicosapentaenoic acid is an omega-3 fatty acid that inhibits caspase-3. Interestingly, omega-3 levels in patients with schizophrenia are significantly lower than in healthy subjects.13 Lithium also can inhibit caspase-3.
Do these proposals sound radical?
Most of the recommendations I’ve made here are not employed in the clinical practice of psychiatry. These ideas must be put to the test in controlled clinical trials.
The crux of my argument is that we need to think outside the “dopamine box” and focus on brain repair if we are to make progress in reversing, even preventing, neurodegeneration and clinical deterioration in this disabling brain syndrome. Just as cancer often is treated with rational polypharmacy, schizophrenia might need a similar approach. To vanquish schizophrenia—a goal that has eluded us—it is imperative to pursue radically novel and disruptive therapeutic strategies. The ideas I’ve listed here sound the call that the quest to repair the brain in schizophrenia must begin, and soon.
1. Agius N, Nandra, KS. Do atypical antipsychotics promote neurogenesis as a class effect? Psychiatr Danub. 2012;24(suppl 1):S191-S193.
2. Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res. 2010;1354:23-29.
3. Chiu CT, Wang Z, Hunsberger JG, et al. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev. 2013;65(1):105-142.
4. Parikh V, Khan MM, Terry A, et al. Differential effects of typical and atypical antipsychotics on nerve growth factor and choline acetyltransferase expression in the cortex and nucleus basalis of rats. J Psychiatr Res. 2004;38(5):521-529.
5. Monji A, Kato TA, Mizoguchi Y, et al. Neuro-inflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
6. Sommer IE, deWitte L, Begemann M, et al. Nonsteriodal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
7. Bian Q, Kato T, Monji A, et al. The effect of atypical anti-psychotics perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):42-48.
8. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64(5):361-368.
9. Huang J, Fairbrother W, Reed JC, et al. Therapeutic targeting of Bcl-2 family for treatment of B-cell malignancies. Expert Rev Hematol. 2015;8(3):283-297.
10. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014; 4:e348.
11. Zink M, Correll CU. Glutamatergic agents for schizophrenia: current evidence and perspectives. Expert Rev Clin Pharmacol. 2015;8(3):335-352.
12. Kremer I, Vass A, Gorelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry. 2004;56(6):444-446.
13. McEvoy J, Baillie RA, Zhu H, et al. Lipidomics reveals early metabolic changes in subjects with schizophrenia: effects of atypical antipsychotics. PLoS One. 2013;8(7):e68717.
1. Agius N, Nandra, KS. Do atypical antipsychotics promote neurogenesis as a class effect? Psychiatr Danub. 2012;24(suppl 1):S191-S193.
2. Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res. 2010;1354:23-29.
3. Chiu CT, Wang Z, Hunsberger JG, et al. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev. 2013;65(1):105-142.
4. Parikh V, Khan MM, Terry A, et al. Differential effects of typical and atypical antipsychotics on nerve growth factor and choline acetyltransferase expression in the cortex and nucleus basalis of rats. J Psychiatr Res. 2004;38(5):521-529.
5. Monji A, Kato TA, Mizoguchi Y, et al. Neuro-inflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
6. Sommer IE, deWitte L, Begemann M, et al. Nonsteriodal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
7. Bian Q, Kato T, Monji A, et al. The effect of atypical anti-psychotics perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):42-48.
8. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64(5):361-368.
9. Huang J, Fairbrother W, Reed JC, et al. Therapeutic targeting of Bcl-2 family for treatment of B-cell malignancies. Expert Rev Hematol. 2015;8(3):283-297.
10. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014; 4:e348.
11. Zink M, Correll CU. Glutamatergic agents for schizophrenia: current evidence and perspectives. Expert Rev Clin Pharmacol. 2015;8(3):335-352.
12. Kremer I, Vass A, Gorelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry. 2004;56(6):444-446.
13. McEvoy J, Baillie RA, Zhu H, et al. Lipidomics reveals early metabolic changes in subjects with schizophrenia: effects of atypical antipsychotics. PLoS One. 2013;8(7):e68717.
Reducing the likelihood that a patient will be readmitted: A resident's perspective
Over the past year, as a part of a treatment team, I encountered many discharged patients who did not heed their physician’s instructions—be it rehabilitation advice, follow-up appointments, or adherence to a drug regimen. Consequently, these patients found themselves back in the inpatient unit a few days later. A cycle of admission−discharge−readmission began for them.
I have witnessed conflicting emotions on the part of the staff (nurses and residents) toward these patients. Some staff are empathetic to their needs; others see the recurrent admissions as a ruse to find food and shelter and get attention.
In this article, I explore several aspects of this behavioral pattern and possible reasons for it, and describe the staff’s reaction to a returning patient in one case.
CASE REPORT Depressed and heavily intoxicated
Mr. R, age 35, with a history of major depressive disorder and nonadherence to treatment, is brought to the emergency room (ER) by emergency medical services because he is heavily intoxicated (blood alcohol level, >200 mg/dL). Mr. R has had 4 admissions to the inpatient psychiatry unit in the past 6 months, including 2 in the past 30 days.
After a few hours’ stay in the ER, Mr. R’s blood alcohol level drops to <100 mg/dL. He is being prepared for discharge to follow-up with outpatient psychiatric services when he begins complaining of chest pain. A cardiac workup is negative; he is again prepared for discharge when he begins reporting suicidal ideation, with a plan to jump in front of traffic.
Mr. R is admitted to the inpatient psychiatry unit.
During Mr. R’s hospitalization, he admits that he lied about being suicidal because he recently lost his job and is homeless and in dire need of food and shelter. He stays in the inpatient unit for 6 days.
An unexpected ‘adverse reaction’
During the hospital stay, staff members, who initially were concerned about Mr. R’s condition, underwent a striking transformation in their attitude toward the patient once his suicidal ideation was exposed as a hoax: They became less receptive to his needs.
The staff’s experience with Mr. R also altered their approach to other patients, who were put under unnecessary scrutiny in response to heightened suspicion of feigned illness—a classic case of “once bitten, twice shy.” The staff felt betrayed by Mr. R’s false claim of being suicidal.
Furthermore, I noticed self-doubt creeping into the minds of the residents who had admitted Mr. R. Consequently, they advocated that he should be discharged patients of an acute care bed.
The attending physicians and other members of the staff remained compassionate toward the patient, however; instead of condemning him, they tried to understand the root cause of why he sought admission: Was it nonadherence with his medication regimen? Substance abuse? Social issues? These staff members were opposed to discharging Mr. R because they believed that forced discharge would encourage him to further manipulate the system—and he would be back in the ER.
CASE CONCLUDED
The medical team concludes that it is prudent to prepare a well-thought-out discharge plan for Mr. R. He is allowed to remain as an inpatient until his social issues are addressed; he is plugged into the rehabilitation program for his alcohol addiction, with a plan for close outpatient psychiatry follow-up.
One year later, Mr. R has not been admitted again.
How to tackle shortcomings of the system
Because of changing hospital policies, an acute shortage of psychiatry inpatient beds, and the reluctance of insurance companies to reimburse for an extended stay, these beds are often hurriedly evacuated and patients are discharged prematurely to make room for acutely ill patients.1 Such policies can lead to failure to reach a therapeutic medication dosage or establish an appropriate disposition plan. Patients might relapse and find their way back to the inpatient unit.
Even though this is a system—not a personal—shortcoming, these patients are viewed negatively and are unwelcome when they return to the hospital. Notably, longer hospital stays do not necessarily lead to better care or fewer readmissions. Patients who have a longer length of stay are, in fact, sicker and have inadequate community and social support.1,2
After a year’s experience as a psychiatry resident, I came to understand that, before discharging a patient from the inpatient unit, a resident should pose a few questions to himself (herself), including:
• What is the likelihood that the patient will adhere to his (her) medication regimen?
• Where is the patient going to get his medications? Will he (she) be able to pay for them?
• Does he have a substance use disorder?
• Have the patient’s personal circumstances changed since he was admitted? If so, how?
Finding answers to these questions and working on solutions can help minimize the readmission rate.
The post-discharge component of care has a significant role, too, including psycho-education of the patient and the family regarding:
• ongoing psychiatric disease
• potential side effects of medications
• post-discharge telephone calls
• timely follow-up (within 2 or 3 weeks)
• good communication with the outpatient provider, through telephone calls or a faxed discharge summary.2,3
I’ve learned that it isn’t uncommon for health care providers to give in to negative emotions and become frustrated. For residents and other members of the team alike, it is important to talk to one’s supervisor and colleagues about that frustration. It is the duty of every member of the treatment team to support each another and maintain a therapeutic posture on the unit.
At the end of every day, of course, what matters is the well-being of our patients.
Disclosure
Dr. Sharma reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Botha UA, Koen L, Joska JA, et al. The revolving door phenomenon in psychiatry: comparing low-frequency and high-frequency users in psychiatry inpatient services in a developing country. Soc Psychiatry Psychiatr Epidemiol. 2010;45(4):461-468.
2. Clary C, Dever A, Schweizer E. Psychiatric inpatient’s knowledge of medication at hospital discharge. Hosp Community Psychiatry. 1992;43(2):140-144.
3. Boyer CA, McAlpine DD, Pottick KJ, et al. Identifying risk factors and key strategies in linkage to outpatient psychiatric care. Am J Psychiatry. 2000;157(10):1592-1598.
Over the past year, as a part of a treatment team, I encountered many discharged patients who did not heed their physician’s instructions—be it rehabilitation advice, follow-up appointments, or adherence to a drug regimen. Consequently, these patients found themselves back in the inpatient unit a few days later. A cycle of admission−discharge−readmission began for them.
I have witnessed conflicting emotions on the part of the staff (nurses and residents) toward these patients. Some staff are empathetic to their needs; others see the recurrent admissions as a ruse to find food and shelter and get attention.
In this article, I explore several aspects of this behavioral pattern and possible reasons for it, and describe the staff’s reaction to a returning patient in one case.
CASE REPORT Depressed and heavily intoxicated
Mr. R, age 35, with a history of major depressive disorder and nonadherence to treatment, is brought to the emergency room (ER) by emergency medical services because he is heavily intoxicated (blood alcohol level, >200 mg/dL). Mr. R has had 4 admissions to the inpatient psychiatry unit in the past 6 months, including 2 in the past 30 days.
After a few hours’ stay in the ER, Mr. R’s blood alcohol level drops to <100 mg/dL. He is being prepared for discharge to follow-up with outpatient psychiatric services when he begins complaining of chest pain. A cardiac workup is negative; he is again prepared for discharge when he begins reporting suicidal ideation, with a plan to jump in front of traffic.
Mr. R is admitted to the inpatient psychiatry unit.
During Mr. R’s hospitalization, he admits that he lied about being suicidal because he recently lost his job and is homeless and in dire need of food and shelter. He stays in the inpatient unit for 6 days.
An unexpected ‘adverse reaction’
During the hospital stay, staff members, who initially were concerned about Mr. R’s condition, underwent a striking transformation in their attitude toward the patient once his suicidal ideation was exposed as a hoax: They became less receptive to his needs.
The staff’s experience with Mr. R also altered their approach to other patients, who were put under unnecessary scrutiny in response to heightened suspicion of feigned illness—a classic case of “once bitten, twice shy.” The staff felt betrayed by Mr. R’s false claim of being suicidal.
Furthermore, I noticed self-doubt creeping into the minds of the residents who had admitted Mr. R. Consequently, they advocated that he should be discharged patients of an acute care bed.
The attending physicians and other members of the staff remained compassionate toward the patient, however; instead of condemning him, they tried to understand the root cause of why he sought admission: Was it nonadherence with his medication regimen? Substance abuse? Social issues? These staff members were opposed to discharging Mr. R because they believed that forced discharge would encourage him to further manipulate the system—and he would be back in the ER.
CASE CONCLUDED
The medical team concludes that it is prudent to prepare a well-thought-out discharge plan for Mr. R. He is allowed to remain as an inpatient until his social issues are addressed; he is plugged into the rehabilitation program for his alcohol addiction, with a plan for close outpatient psychiatry follow-up.
One year later, Mr. R has not been admitted again.
How to tackle shortcomings of the system
Because of changing hospital policies, an acute shortage of psychiatry inpatient beds, and the reluctance of insurance companies to reimburse for an extended stay, these beds are often hurriedly evacuated and patients are discharged prematurely to make room for acutely ill patients.1 Such policies can lead to failure to reach a therapeutic medication dosage or establish an appropriate disposition plan. Patients might relapse and find their way back to the inpatient unit.
Even though this is a system—not a personal—shortcoming, these patients are viewed negatively and are unwelcome when they return to the hospital. Notably, longer hospital stays do not necessarily lead to better care or fewer readmissions. Patients who have a longer length of stay are, in fact, sicker and have inadequate community and social support.1,2
After a year’s experience as a psychiatry resident, I came to understand that, before discharging a patient from the inpatient unit, a resident should pose a few questions to himself (herself), including:
• What is the likelihood that the patient will adhere to his (her) medication regimen?
• Where is the patient going to get his medications? Will he (she) be able to pay for them?
• Does he have a substance use disorder?
• Have the patient’s personal circumstances changed since he was admitted? If so, how?
Finding answers to these questions and working on solutions can help minimize the readmission rate.
The post-discharge component of care has a significant role, too, including psycho-education of the patient and the family regarding:
• ongoing psychiatric disease
• potential side effects of medications
• post-discharge telephone calls
• timely follow-up (within 2 or 3 weeks)
• good communication with the outpatient provider, through telephone calls or a faxed discharge summary.2,3
I’ve learned that it isn’t uncommon for health care providers to give in to negative emotions and become frustrated. For residents and other members of the team alike, it is important to talk to one’s supervisor and colleagues about that frustration. It is the duty of every member of the treatment team to support each another and maintain a therapeutic posture on the unit.
At the end of every day, of course, what matters is the well-being of our patients.
Disclosure
Dr. Sharma reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Over the past year, as a part of a treatment team, I encountered many discharged patients who did not heed their physician’s instructions—be it rehabilitation advice, follow-up appointments, or adherence to a drug regimen. Consequently, these patients found themselves back in the inpatient unit a few days later. A cycle of admission−discharge−readmission began for them.
I have witnessed conflicting emotions on the part of the staff (nurses and residents) toward these patients. Some staff are empathetic to their needs; others see the recurrent admissions as a ruse to find food and shelter and get attention.
In this article, I explore several aspects of this behavioral pattern and possible reasons for it, and describe the staff’s reaction to a returning patient in one case.
CASE REPORT Depressed and heavily intoxicated
Mr. R, age 35, with a history of major depressive disorder and nonadherence to treatment, is brought to the emergency room (ER) by emergency medical services because he is heavily intoxicated (blood alcohol level, >200 mg/dL). Mr. R has had 4 admissions to the inpatient psychiatry unit in the past 6 months, including 2 in the past 30 days.
After a few hours’ stay in the ER, Mr. R’s blood alcohol level drops to <100 mg/dL. He is being prepared for discharge to follow-up with outpatient psychiatric services when he begins complaining of chest pain. A cardiac workup is negative; he is again prepared for discharge when he begins reporting suicidal ideation, with a plan to jump in front of traffic.
Mr. R is admitted to the inpatient psychiatry unit.
During Mr. R’s hospitalization, he admits that he lied about being suicidal because he recently lost his job and is homeless and in dire need of food and shelter. He stays in the inpatient unit for 6 days.
An unexpected ‘adverse reaction’
During the hospital stay, staff members, who initially were concerned about Mr. R’s condition, underwent a striking transformation in their attitude toward the patient once his suicidal ideation was exposed as a hoax: They became less receptive to his needs.
The staff’s experience with Mr. R also altered their approach to other patients, who were put under unnecessary scrutiny in response to heightened suspicion of feigned illness—a classic case of “once bitten, twice shy.” The staff felt betrayed by Mr. R’s false claim of being suicidal.
Furthermore, I noticed self-doubt creeping into the minds of the residents who had admitted Mr. R. Consequently, they advocated that he should be discharged patients of an acute care bed.
The attending physicians and other members of the staff remained compassionate toward the patient, however; instead of condemning him, they tried to understand the root cause of why he sought admission: Was it nonadherence with his medication regimen? Substance abuse? Social issues? These staff members were opposed to discharging Mr. R because they believed that forced discharge would encourage him to further manipulate the system—and he would be back in the ER.
CASE CONCLUDED
The medical team concludes that it is prudent to prepare a well-thought-out discharge plan for Mr. R. He is allowed to remain as an inpatient until his social issues are addressed; he is plugged into the rehabilitation program for his alcohol addiction, with a plan for close outpatient psychiatry follow-up.
One year later, Mr. R has not been admitted again.
How to tackle shortcomings of the system
Because of changing hospital policies, an acute shortage of psychiatry inpatient beds, and the reluctance of insurance companies to reimburse for an extended stay, these beds are often hurriedly evacuated and patients are discharged prematurely to make room for acutely ill patients.1 Such policies can lead to failure to reach a therapeutic medication dosage or establish an appropriate disposition plan. Patients might relapse and find their way back to the inpatient unit.
Even though this is a system—not a personal—shortcoming, these patients are viewed negatively and are unwelcome when they return to the hospital. Notably, longer hospital stays do not necessarily lead to better care or fewer readmissions. Patients who have a longer length of stay are, in fact, sicker and have inadequate community and social support.1,2
After a year’s experience as a psychiatry resident, I came to understand that, before discharging a patient from the inpatient unit, a resident should pose a few questions to himself (herself), including:
• What is the likelihood that the patient will adhere to his (her) medication regimen?
• Where is the patient going to get his medications? Will he (she) be able to pay for them?
• Does he have a substance use disorder?
• Have the patient’s personal circumstances changed since he was admitted? If so, how?
Finding answers to these questions and working on solutions can help minimize the readmission rate.
The post-discharge component of care has a significant role, too, including psycho-education of the patient and the family regarding:
• ongoing psychiatric disease
• potential side effects of medications
• post-discharge telephone calls
• timely follow-up (within 2 or 3 weeks)
• good communication with the outpatient provider, through telephone calls or a faxed discharge summary.2,3
I’ve learned that it isn’t uncommon for health care providers to give in to negative emotions and become frustrated. For residents and other members of the team alike, it is important to talk to one’s supervisor and colleagues about that frustration. It is the duty of every member of the treatment team to support each another and maintain a therapeutic posture on the unit.
At the end of every day, of course, what matters is the well-being of our patients.
Disclosure
Dr. Sharma reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Botha UA, Koen L, Joska JA, et al. The revolving door phenomenon in psychiatry: comparing low-frequency and high-frequency users in psychiatry inpatient services in a developing country. Soc Psychiatry Psychiatr Epidemiol. 2010;45(4):461-468.
2. Clary C, Dever A, Schweizer E. Psychiatric inpatient’s knowledge of medication at hospital discharge. Hosp Community Psychiatry. 1992;43(2):140-144.
3. Boyer CA, McAlpine DD, Pottick KJ, et al. Identifying risk factors and key strategies in linkage to outpatient psychiatric care. Am J Psychiatry. 2000;157(10):1592-1598.
1. Botha UA, Koen L, Joska JA, et al. The revolving door phenomenon in psychiatry: comparing low-frequency and high-frequency users in psychiatry inpatient services in a developing country. Soc Psychiatry Psychiatr Epidemiol. 2010;45(4):461-468.
2. Clary C, Dever A, Schweizer E. Psychiatric inpatient’s knowledge of medication at hospital discharge. Hosp Community Psychiatry. 1992;43(2):140-144.
3. Boyer CA, McAlpine DD, Pottick KJ, et al. Identifying risk factors and key strategies in linkage to outpatient psychiatric care. Am J Psychiatry. 2000;157(10):1592-1598.