User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
How to collaborate effectively with psychiatric nurse practitioners
Discuss this article at www.facebook.com/CurrentPsychiatry
Psychiatrists who are accustomed to working with “med/surg” or psychiatric nurses may be less familiar with how to collaborate with more specialized psychiatric-mental health nurse practitioners (PMHNPs). These clinicians play an important role in delivering mental health services, which is likely to continue because of the physician shortage in the United States1 and increasing mental health care needs from passage of the Affordable Health Care Act and the Mental Health Parity Act.2 These specialty trained, master’s level nurses work with psychiatrists in outpatient clinics, hospital consultation and liaison services, psychiatric emergency services, inpatient units, and geropsychiatric consultation.3-5 PMHNPs can fill gaps of coverage in underserved areas, supplement and complement busy and overburdened psychiatrists, and add an important dimension of holistic care.
This article reviews issues related to a successful psychiatrist-PMHNP collaboration, including:
- PMHNP’s training and scope of practice
- their skill and competency development in inpatient and outpatient settings
- the principles and dynamics of collaboration, hindrances to cooperation, and keys to relationship-building for PMHNPs and psychiatrists.
Rigorous requirements
PMHNPs enroll in an accredited graduate nursing program that takes 16 to 24 months to complete and builds on the competencies of their undergraduate nursing education and clinical experience. All programs meet standards set by national nursing accrediting agencies. The typical graduate-level curriculum for a PMHNP includes core bio-behavioral theory, research courses, advanced physiology and pathophysiology, advanced physical and psychiatric health assessment, pharmacologic and nonpharmacologic interventions, and managing health care delivery systems. For graduation and certification, PMHNPs must complete 500 supervised clinical hours focused on psychiatric and mental health care.
- comprehensive psychiatric evaluation
- formulation of a differential diagnosis
- ordering and interpreting diagnostic tests
- prescribing pharmacologic agents
- conducting individual, couple, group, or family psychotherapy using evidence-based approaches.
PMHNPs also are responsible for recognizing the limits of their knowledge and experience, planning for situations beyond their expertise, and providing appropriate referral to other health care providers when indicated.8
Successful collaborative practice requires a clear definition and understanding of roles.9 This is particularly important for collaborating psychiatrists and PMHNPs because there has been confusion among physicians and the general public related to the nurse practitioner’s role. Psychiatrists who work with PMHNPs need to be familiar with state regulations that govern levels of physician supervision and prescriptive authority for nurse practitioners. Eleven states and the District of Columbia allow nurse practitioners to prescribe independently, including controlled substances. Most states require physician collaboration for prescribing medications, but the language can be ambiguous, with restrictions on certain formularies or drug schedules—eg, Michigan nurse practitioners may prescribe schedule II through V controlled substances, but schedule II medications are limited to nurse practitioners who work in hospitals, surgical outpatient settings, or hospices.10
Competencies and development
New PMHNPs see patients and prescribe medication, but their work needs close supervision. Postgraduate clinical experience combined with supervision gradually allows the PMHNP greater independence. A PMHNP who provides care in a busy outpatient clinic, inpatient unit, or psychiatric emergency department is likely to master the treatment philosophy and ancillary competencies related to that particular clinical site—including favored pharmacologic approaches, electronic documentation and ordering functions, and admission and discharge facilitation—at a level exceeding that of psychiatric residents, who rotate on and off a service as part of their training.
It’s helpful for new PMHNPs to have a time frame for their development over several years. The Table11 outlines general graded competency areas PMHNPs may focus on in their development. See this article at CurrentPsychiatry.com for Tables that provide examples of detailed competencies for third-year PMHNPs in inpatient and outpatient settings.
Table
PMHNP development: General graded competency areas
| Psychiatric evaluation and diagnosis |
| Psychiatric treatments, including medications and psychotherapies |
| Maintenance of the therapeutic alliance, including monitoring the PMHNP’s emotional responses to patients |
| Participation in an interdisciplinary team |
| Understanding comorbid medical conditions, integrating laboratory and other tests into the treatment plan, and recognizing the need for consultation with the medical team |
| Documentation, such as initial evaluations, progress notes, and discharge summaries |
| Assessment for suicide and violence potential |
| Teaching |
| Patient and family psychoeducation |
| Use of feedback and supervision |
| PMHNP: psychiatric-mental health nurse practitioner Source: Reference 11 |
Table 1
Competencies for third-year PMHNPs in an outpatient clinic
| Recognize clinical presentations of complex psychiatric disorders, variants, and comorbidities |
| Firm knowledge of diagnostic criteria, and skills for independent comprehensive assessment and diagnosis |
| Firm knowledge of evidence-based outpatient treatments for disorders, with mastery of ≥1 nonpharmacologic modality in addition to prescribing and managing medications |
| Use and provide feedback in comprehensive case formulations and treatment plans |
| Assist in clinical education of trainees in psychiatric nursing, social work, psychiatric residency, and psychology |
| Participate and collaborate in educational events and initiatives |
| Knowledge of internal and external health system and resources, and facilitating patient access to these networks |
| Incorporate mental health and behavioral and psychiatric nursing research into patient care |
| PMHNP: psychiatric-mental health nurse practitioner |
Competencies for third-year PMHNPs on an inpatient psychiatric unit
| Refinement of assessment section in evaluations, progress notes, and discharge summaries |
| Understanding indications for neuropsychological testing, and integrating findings into the treatment plan |
| Assessment of readiness for discharge in patients with a history of suicidality or violence |
| Developing a sophisticated and detailed discharge or follow-up plan |
| Understanding treatment resistance in mood and psychotic disorders, and implementing treatment |
| More detailed knowledge of types of illness treated on an inpatient unit |
| Ability to orient and train PMHNPs and other inpatient unit trainees |
| Ability to gather and use articles and other literature pertaining to inpatient care |
| Increasing competence in short-term, crisis-based therapeutic techniques, including familiarity with DBT, CBT, and IPT |
| Understanding family systems and impact on patient care |
| CBT: cognitive-behavioral therapy; DBT: dialectical behavior therapy; IPT: interpersonal therapy; PMHNP: psychiatric-mental health nurse practitioner |
Principles of practice
Studies have demonstrated the importance of understanding how to effectively implement collaborative care across medical disciplines.12 See the Box12 for a discussion of 3 key determinants for successful clinical collaborations.
San Martín-Rodríguez et al12 recognized 3 key factors that may help develop successful collaborative clinical relationships.
Interactional factors include a mutual willingness to collaborate, a commitment to collaborate, a belief in the benefits of collaborating, and sharing common objectives. Trust in the partnering clinician’s competency contributes to a successful collaboration. Strong communication skills—including the ability to convey what each clinician can contribute to achieving goals—also strengthens collaboration. Learning and understanding skills in conflict management and dialogue are key. Mutual respect also is essential.
Organizational factors include a shift from a traditional hierarchical structure to a more horizontal structure, and a work climate that supports openness, risk taking—ie, a willingness to disagree with a colleague if it is in a patient’s best interest or to develop a new and innovative method of providing care—integrity, and trust. Administrative structures and supports that convey the importance of collaboration also are key components of a strong collaborative environment. Teamwork and shared decision-making are important elements; teamwork should include time to discuss patient issues and develop strong interpersonal relationships. A commitment to professional development is another key factor.
Systemic factors include a social system that supports collegial relationships and professionalism that respects and accepts other professions. This includes decreased focus on protecting professional territory and increased recognition of overlaps among professions.
Enhancing collaboration
Psychiatrists who work with PMHNPs develop trust based on observing each PMHNP’s work, including their relationship with patients, ability to conceptualize a case and develop a treatment plan, and the skill with which they function within a team. The psychiatrist’s comfort level also is related to his or her awareness of the comprehensiveness of the PMHNP’s training and the competencies gained from clinical experience. Respect for the PMHNP’s educational and professional background is the foundation for what is often—at least in the collaborative relationship’s initial stages—a combined cooperative and supervisory relationship with the PMHNP. As such, the PMHNP gradually will absorb certain “intangibles” to supplement the training and work experiences that preceded his or her position. This may include assimilating the psychiatrist’s or clinic’s philosophy and treatment practice, including expertise in dealing with specialized psychiatric populations (eg, developmental disabilities, acute psychosis, or treatment-resistant depression).
The patient’s comfort level
Collaborating PMHNPs and psychiatrists need to be prepared for a patient who expresses disappointment with being treated by a PMHNP or a preference to see “a doctor.” Psychiatrists who have not worked through their own ambivalence about the collaboration or who lack confidence in the PMHNP’s abilities may find themselves consciously or unconsciously aligning with the patient’s stance. They may neglect to explore the basis and meaning of the patient’s preference, which may be related to the patient’s lack of knowledge about the PMHNP’s role and training. The PMHNP who encounters such a patient has a more challenging task—namely, how to calmly address the patient’s concern while the patient is challenging the PMHNP’s competence. Both the PMHNP and psychiatrist need to be alert to the possibility of “splitting” in the treatment of axis II-disordered patients.
Barriers to collaboration
From the PMHNP perspective, barriers to a collaborative relationship include referring to PMHNPs by a less preferred term or title, instead of a nurse practitioner or APN, which can hinder the relationship. Although physician assistants and NPs have been grouped together under the term “mid-level providers,” the American Academy of Nurse Practitioners notes that this term suggests a lower level of care or service is being provided.18 “Physician extender” is another term that fails to recognize the PMHNP’s separate and unique role and the PMHNP’s view of their role as complementary to medicine, rather than an extension of a physician’s practice.
Territorial issues can impede collaborative relationships. Psychiatrists who resist collaborating will be less effective than those who welcome a PMHNP and readily delegate specific tasks and portions of the workload, whereas psychiatrists who value the help will be more likely to build a collaborative partnership, leading to better patient care.
Autonomy is a critical determinant of professional satisfaction for PMHNPs. A PMHNP’s autonomy can be impeded by organizational constraints and physician perceptions.19 PMHNPs require autonomy to self-direct patient diagnosis and treatment within the scope of their practice, and many find this relative independence essential to delivering high quality patient care. Lack of autonomy can lead to breaks in workflow in the outpatient setting and increased length of stay for hospitalized patients. In addition, an autonomously functioning, experienced PMHNP can increase efficiency in hospital settings where psychiatrists can be in short supply, preoccupied with administrative matters, or require help on weekends.
Related Resources
- American Psychiatric Nurses Association. www.apna.org.
- International Society of Psychiatric-Mental Health Nurses. www.ispn-psych.org.
- American Nurses Association. www.nursingworld.org.
Dr. Casher is a speaker for Sunovion Pharmaceuticals and receives royalties from Cambridge University Press.
Ms. Kuebler, Ms. Bastida, and Ms. Chipps report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sataline S, Wang SS. Medical schools can’t keep up. Wall Street Journal. April 12 2010. http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed August 21, 2012.
2. U.S. Department of Health and Human Services. The health care law & you. http://www.healthcare.gov/law/index.html. Accessed August 21, 2012.
3. Wand T, Fisher J. The mental health nurse practitioner in the emergency department: an Australian experience. Int J Ment Health Nurs. 2006;15(3):201-208.
4. Eisch JS, Brozovic B, Colling K, et al. Nurse practitioner geropsychiatric consultation service to nursing homes. Geriatr Nurs. 2000;21(3):150-155.
5. Baker N. Exploring the mental health nurse practitioner scope of practice in youth early psychosis: an anecdotal account. Contemp Nurse. 2010;34(2):211-220.
6. International Society of Psychiatric-Mental Health Nurses. Psychiatric mental health nursing scope & standards. http://www.ispn-psych.org/docs/standards/scope-standards-draft.pdf. Updated 2006. Accessed August 21, 2012.
7. Centers for Medicare and Medicaid Services. HHS finalizes new rules to cut regulations for hospitals and health care providers saving more than $5 billion. http://www.cms.gov/apps/media/press/release.asp?Counter=4362. Published May 9, 2012. Accessed August 21, 2012.
8. APRN Consensus Work Group, National Council of State Boards of Nursing APRN Advisory Committee. Consensus model for regulation: licensure accreditation, certification & education. https://www.ncsbn.org/Consensus_Model_for_APRN_Regulation_July_2008.pdf. Published July 7, 2008. Accessed August 21, 2012.
9. Legault F, Humbert J, Amos S, et al. Difficulties encountered in collaborative care: logistics trumps desire. J Am Board Fam Med. 2012;25(2):168-176.
10. Michigan Council of Nurse Practitioners. Michigan’s rules and regulations for prescriptive authority. http://micnp.org/displaycommon.cfm?an=1&subarticlenbr=109. Accessed August 21, 2012.
11. Wheeler K, Haber J. Development of psychiatric-mental health nurse practitioner competencies: opportunities for the 21st century. J Am Psychiatr Nurses Assoc. 2004;10(3):129-138.
12. San Martín-Rodríguez L, Beaulieu MD, D’Amour D, et al. The determinants of successful collaboration: a review of theoretical and empirical studies. J Interprof Care. 2005;19(suppl 1):132-147.
13. Suter E, Arndt J, Arthur N, et al. Role understanding and effective communication as core competencies for collaborative practice. J Interprof Care. 2009;23(1):41-51.
14. Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ. 2002;324(7341):819-823.
15. Byrne G, Richardson M, Brunsdon J, et al. Patient satisfaction with emergency nurse practitioners in A & E. J Clin Nurs. 2000;9(1):83-92.
16. McCann TV, Clark E. Attitudes of patients towards mental health nurse prescribing of antipsychotic agents. Int J Nurs Pract. 2008;14(2):115-121.
17. Wortans J, Happell B, Johnstone H. The role of the nurse practitioner in psychiatric/mental health nursing: exploring consumer satisfaction. J Psychiatr Ment Health Nurs. 2006;13(1):78-84.
18. Frellick M. The nurse practitioner will see you now. Advanced practice providers fill the physician gap. Hosp Health Netw. 2011;85(7):44-46, 48–49.
19. Maylone MM, Ranieri L, Quinn Griffin MT, et al. Collaboration and autonomy: perceptions among nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):51-57.
Discuss this article at www.facebook.com/CurrentPsychiatry
Psychiatrists who are accustomed to working with “med/surg” or psychiatric nurses may be less familiar with how to collaborate with more specialized psychiatric-mental health nurse practitioners (PMHNPs). These clinicians play an important role in delivering mental health services, which is likely to continue because of the physician shortage in the United States1 and increasing mental health care needs from passage of the Affordable Health Care Act and the Mental Health Parity Act.2 These specialty trained, master’s level nurses work with psychiatrists in outpatient clinics, hospital consultation and liaison services, psychiatric emergency services, inpatient units, and geropsychiatric consultation.3-5 PMHNPs can fill gaps of coverage in underserved areas, supplement and complement busy and overburdened psychiatrists, and add an important dimension of holistic care.
This article reviews issues related to a successful psychiatrist-PMHNP collaboration, including:
- PMHNP’s training and scope of practice
- their skill and competency development in inpatient and outpatient settings
- the principles and dynamics of collaboration, hindrances to cooperation, and keys to relationship-building for PMHNPs and psychiatrists.
Rigorous requirements
PMHNPs enroll in an accredited graduate nursing program that takes 16 to 24 months to complete and builds on the competencies of their undergraduate nursing education and clinical experience. All programs meet standards set by national nursing accrediting agencies. The typical graduate-level curriculum for a PMHNP includes core bio-behavioral theory, research courses, advanced physiology and pathophysiology, advanced physical and psychiatric health assessment, pharmacologic and nonpharmacologic interventions, and managing health care delivery systems. For graduation and certification, PMHNPs must complete 500 supervised clinical hours focused on psychiatric and mental health care.
- comprehensive psychiatric evaluation
- formulation of a differential diagnosis
- ordering and interpreting diagnostic tests
- prescribing pharmacologic agents
- conducting individual, couple, group, or family psychotherapy using evidence-based approaches.
PMHNPs also are responsible for recognizing the limits of their knowledge and experience, planning for situations beyond their expertise, and providing appropriate referral to other health care providers when indicated.8
Successful collaborative practice requires a clear definition and understanding of roles.9 This is particularly important for collaborating psychiatrists and PMHNPs because there has been confusion among physicians and the general public related to the nurse practitioner’s role. Psychiatrists who work with PMHNPs need to be familiar with state regulations that govern levels of physician supervision and prescriptive authority for nurse practitioners. Eleven states and the District of Columbia allow nurse practitioners to prescribe independently, including controlled substances. Most states require physician collaboration for prescribing medications, but the language can be ambiguous, with restrictions on certain formularies or drug schedules—eg, Michigan nurse practitioners may prescribe schedule II through V controlled substances, but schedule II medications are limited to nurse practitioners who work in hospitals, surgical outpatient settings, or hospices.10
Competencies and development
New PMHNPs see patients and prescribe medication, but their work needs close supervision. Postgraduate clinical experience combined with supervision gradually allows the PMHNP greater independence. A PMHNP who provides care in a busy outpatient clinic, inpatient unit, or psychiatric emergency department is likely to master the treatment philosophy and ancillary competencies related to that particular clinical site—including favored pharmacologic approaches, electronic documentation and ordering functions, and admission and discharge facilitation—at a level exceeding that of psychiatric residents, who rotate on and off a service as part of their training.
It’s helpful for new PMHNPs to have a time frame for their development over several years. The Table11 outlines general graded competency areas PMHNPs may focus on in their development. See this article at CurrentPsychiatry.com for Tables that provide examples of detailed competencies for third-year PMHNPs in inpatient and outpatient settings.
Table
PMHNP development: General graded competency areas
| Psychiatric evaluation and diagnosis |
| Psychiatric treatments, including medications and psychotherapies |
| Maintenance of the therapeutic alliance, including monitoring the PMHNP’s emotional responses to patients |
| Participation in an interdisciplinary team |
| Understanding comorbid medical conditions, integrating laboratory and other tests into the treatment plan, and recognizing the need for consultation with the medical team |
| Documentation, such as initial evaluations, progress notes, and discharge summaries |
| Assessment for suicide and violence potential |
| Teaching |
| Patient and family psychoeducation |
| Use of feedback and supervision |
| PMHNP: psychiatric-mental health nurse practitioner Source: Reference 11 |
Table 1
Competencies for third-year PMHNPs in an outpatient clinic
| Recognize clinical presentations of complex psychiatric disorders, variants, and comorbidities |
| Firm knowledge of diagnostic criteria, and skills for independent comprehensive assessment and diagnosis |
| Firm knowledge of evidence-based outpatient treatments for disorders, with mastery of ≥1 nonpharmacologic modality in addition to prescribing and managing medications |
| Use and provide feedback in comprehensive case formulations and treatment plans |
| Assist in clinical education of trainees in psychiatric nursing, social work, psychiatric residency, and psychology |
| Participate and collaborate in educational events and initiatives |
| Knowledge of internal and external health system and resources, and facilitating patient access to these networks |
| Incorporate mental health and behavioral and psychiatric nursing research into patient care |
| PMHNP: psychiatric-mental health nurse practitioner |
Competencies for third-year PMHNPs on an inpatient psychiatric unit
| Refinement of assessment section in evaluations, progress notes, and discharge summaries |
| Understanding indications for neuropsychological testing, and integrating findings into the treatment plan |
| Assessment of readiness for discharge in patients with a history of suicidality or violence |
| Developing a sophisticated and detailed discharge or follow-up plan |
| Understanding treatment resistance in mood and psychotic disorders, and implementing treatment |
| More detailed knowledge of types of illness treated on an inpatient unit |
| Ability to orient and train PMHNPs and other inpatient unit trainees |
| Ability to gather and use articles and other literature pertaining to inpatient care |
| Increasing competence in short-term, crisis-based therapeutic techniques, including familiarity with DBT, CBT, and IPT |
| Understanding family systems and impact on patient care |
| CBT: cognitive-behavioral therapy; DBT: dialectical behavior therapy; IPT: interpersonal therapy; PMHNP: psychiatric-mental health nurse practitioner |
Principles of practice
Studies have demonstrated the importance of understanding how to effectively implement collaborative care across medical disciplines.12 See the Box12 for a discussion of 3 key determinants for successful clinical collaborations.
San Martín-Rodríguez et al12 recognized 3 key factors that may help develop successful collaborative clinical relationships.
Interactional factors include a mutual willingness to collaborate, a commitment to collaborate, a belief in the benefits of collaborating, and sharing common objectives. Trust in the partnering clinician’s competency contributes to a successful collaboration. Strong communication skills—including the ability to convey what each clinician can contribute to achieving goals—also strengthens collaboration. Learning and understanding skills in conflict management and dialogue are key. Mutual respect also is essential.
Organizational factors include a shift from a traditional hierarchical structure to a more horizontal structure, and a work climate that supports openness, risk taking—ie, a willingness to disagree with a colleague if it is in a patient’s best interest or to develop a new and innovative method of providing care—integrity, and trust. Administrative structures and supports that convey the importance of collaboration also are key components of a strong collaborative environment. Teamwork and shared decision-making are important elements; teamwork should include time to discuss patient issues and develop strong interpersonal relationships. A commitment to professional development is another key factor.
Systemic factors include a social system that supports collegial relationships and professionalism that respects and accepts other professions. This includes decreased focus on protecting professional territory and increased recognition of overlaps among professions.
Enhancing collaboration
Psychiatrists who work with PMHNPs develop trust based on observing each PMHNP’s work, including their relationship with patients, ability to conceptualize a case and develop a treatment plan, and the skill with which they function within a team. The psychiatrist’s comfort level also is related to his or her awareness of the comprehensiveness of the PMHNP’s training and the competencies gained from clinical experience. Respect for the PMHNP’s educational and professional background is the foundation for what is often—at least in the collaborative relationship’s initial stages—a combined cooperative and supervisory relationship with the PMHNP. As such, the PMHNP gradually will absorb certain “intangibles” to supplement the training and work experiences that preceded his or her position. This may include assimilating the psychiatrist’s or clinic’s philosophy and treatment practice, including expertise in dealing with specialized psychiatric populations (eg, developmental disabilities, acute psychosis, or treatment-resistant depression).
The patient’s comfort level
Collaborating PMHNPs and psychiatrists need to be prepared for a patient who expresses disappointment with being treated by a PMHNP or a preference to see “a doctor.” Psychiatrists who have not worked through their own ambivalence about the collaboration or who lack confidence in the PMHNP’s abilities may find themselves consciously or unconsciously aligning with the patient’s stance. They may neglect to explore the basis and meaning of the patient’s preference, which may be related to the patient’s lack of knowledge about the PMHNP’s role and training. The PMHNP who encounters such a patient has a more challenging task—namely, how to calmly address the patient’s concern while the patient is challenging the PMHNP’s competence. Both the PMHNP and psychiatrist need to be alert to the possibility of “splitting” in the treatment of axis II-disordered patients.
Barriers to collaboration
From the PMHNP perspective, barriers to a collaborative relationship include referring to PMHNPs by a less preferred term or title, instead of a nurse practitioner or APN, which can hinder the relationship. Although physician assistants and NPs have been grouped together under the term “mid-level providers,” the American Academy of Nurse Practitioners notes that this term suggests a lower level of care or service is being provided.18 “Physician extender” is another term that fails to recognize the PMHNP’s separate and unique role and the PMHNP’s view of their role as complementary to medicine, rather than an extension of a physician’s practice.
Territorial issues can impede collaborative relationships. Psychiatrists who resist collaborating will be less effective than those who welcome a PMHNP and readily delegate specific tasks and portions of the workload, whereas psychiatrists who value the help will be more likely to build a collaborative partnership, leading to better patient care.
Autonomy is a critical determinant of professional satisfaction for PMHNPs. A PMHNP’s autonomy can be impeded by organizational constraints and physician perceptions.19 PMHNPs require autonomy to self-direct patient diagnosis and treatment within the scope of their practice, and many find this relative independence essential to delivering high quality patient care. Lack of autonomy can lead to breaks in workflow in the outpatient setting and increased length of stay for hospitalized patients. In addition, an autonomously functioning, experienced PMHNP can increase efficiency in hospital settings where psychiatrists can be in short supply, preoccupied with administrative matters, or require help on weekends.
Related Resources
- American Psychiatric Nurses Association. www.apna.org.
- International Society of Psychiatric-Mental Health Nurses. www.ispn-psych.org.
- American Nurses Association. www.nursingworld.org.
Dr. Casher is a speaker for Sunovion Pharmaceuticals and receives royalties from Cambridge University Press.
Ms. Kuebler, Ms. Bastida, and Ms. Chipps report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Psychiatrists who are accustomed to working with “med/surg” or psychiatric nurses may be less familiar with how to collaborate with more specialized psychiatric-mental health nurse practitioners (PMHNPs). These clinicians play an important role in delivering mental health services, which is likely to continue because of the physician shortage in the United States1 and increasing mental health care needs from passage of the Affordable Health Care Act and the Mental Health Parity Act.2 These specialty trained, master’s level nurses work with psychiatrists in outpatient clinics, hospital consultation and liaison services, psychiatric emergency services, inpatient units, and geropsychiatric consultation.3-5 PMHNPs can fill gaps of coverage in underserved areas, supplement and complement busy and overburdened psychiatrists, and add an important dimension of holistic care.
This article reviews issues related to a successful psychiatrist-PMHNP collaboration, including:
- PMHNP’s training and scope of practice
- their skill and competency development in inpatient and outpatient settings
- the principles and dynamics of collaboration, hindrances to cooperation, and keys to relationship-building for PMHNPs and psychiatrists.
Rigorous requirements
PMHNPs enroll in an accredited graduate nursing program that takes 16 to 24 months to complete and builds on the competencies of their undergraduate nursing education and clinical experience. All programs meet standards set by national nursing accrediting agencies. The typical graduate-level curriculum for a PMHNP includes core bio-behavioral theory, research courses, advanced physiology and pathophysiology, advanced physical and psychiatric health assessment, pharmacologic and nonpharmacologic interventions, and managing health care delivery systems. For graduation and certification, PMHNPs must complete 500 supervised clinical hours focused on psychiatric and mental health care.
- comprehensive psychiatric evaluation
- formulation of a differential diagnosis
- ordering and interpreting diagnostic tests
- prescribing pharmacologic agents
- conducting individual, couple, group, or family psychotherapy using evidence-based approaches.
PMHNPs also are responsible for recognizing the limits of their knowledge and experience, planning for situations beyond their expertise, and providing appropriate referral to other health care providers when indicated.8
Successful collaborative practice requires a clear definition and understanding of roles.9 This is particularly important for collaborating psychiatrists and PMHNPs because there has been confusion among physicians and the general public related to the nurse practitioner’s role. Psychiatrists who work with PMHNPs need to be familiar with state regulations that govern levels of physician supervision and prescriptive authority for nurse practitioners. Eleven states and the District of Columbia allow nurse practitioners to prescribe independently, including controlled substances. Most states require physician collaboration for prescribing medications, but the language can be ambiguous, with restrictions on certain formularies or drug schedules—eg, Michigan nurse practitioners may prescribe schedule II through V controlled substances, but schedule II medications are limited to nurse practitioners who work in hospitals, surgical outpatient settings, or hospices.10
Competencies and development
New PMHNPs see patients and prescribe medication, but their work needs close supervision. Postgraduate clinical experience combined with supervision gradually allows the PMHNP greater independence. A PMHNP who provides care in a busy outpatient clinic, inpatient unit, or psychiatric emergency department is likely to master the treatment philosophy and ancillary competencies related to that particular clinical site—including favored pharmacologic approaches, electronic documentation and ordering functions, and admission and discharge facilitation—at a level exceeding that of psychiatric residents, who rotate on and off a service as part of their training.
It’s helpful for new PMHNPs to have a time frame for their development over several years. The Table11 outlines general graded competency areas PMHNPs may focus on in their development. See this article at CurrentPsychiatry.com for Tables that provide examples of detailed competencies for third-year PMHNPs in inpatient and outpatient settings.
Table
PMHNP development: General graded competency areas
| Psychiatric evaluation and diagnosis |
| Psychiatric treatments, including medications and psychotherapies |
| Maintenance of the therapeutic alliance, including monitoring the PMHNP’s emotional responses to patients |
| Participation in an interdisciplinary team |
| Understanding comorbid medical conditions, integrating laboratory and other tests into the treatment plan, and recognizing the need for consultation with the medical team |
| Documentation, such as initial evaluations, progress notes, and discharge summaries |
| Assessment for suicide and violence potential |
| Teaching |
| Patient and family psychoeducation |
| Use of feedback and supervision |
| PMHNP: psychiatric-mental health nurse practitioner Source: Reference 11 |
Table 1
Competencies for third-year PMHNPs in an outpatient clinic
| Recognize clinical presentations of complex psychiatric disorders, variants, and comorbidities |
| Firm knowledge of diagnostic criteria, and skills for independent comprehensive assessment and diagnosis |
| Firm knowledge of evidence-based outpatient treatments for disorders, with mastery of ≥1 nonpharmacologic modality in addition to prescribing and managing medications |
| Use and provide feedback in comprehensive case formulations and treatment plans |
| Assist in clinical education of trainees in psychiatric nursing, social work, psychiatric residency, and psychology |
| Participate and collaborate in educational events and initiatives |
| Knowledge of internal and external health system and resources, and facilitating patient access to these networks |
| Incorporate mental health and behavioral and psychiatric nursing research into patient care |
| PMHNP: psychiatric-mental health nurse practitioner |
Competencies for third-year PMHNPs on an inpatient psychiatric unit
| Refinement of assessment section in evaluations, progress notes, and discharge summaries |
| Understanding indications for neuropsychological testing, and integrating findings into the treatment plan |
| Assessment of readiness for discharge in patients with a history of suicidality or violence |
| Developing a sophisticated and detailed discharge or follow-up plan |
| Understanding treatment resistance in mood and psychotic disorders, and implementing treatment |
| More detailed knowledge of types of illness treated on an inpatient unit |
| Ability to orient and train PMHNPs and other inpatient unit trainees |
| Ability to gather and use articles and other literature pertaining to inpatient care |
| Increasing competence in short-term, crisis-based therapeutic techniques, including familiarity with DBT, CBT, and IPT |
| Understanding family systems and impact on patient care |
| CBT: cognitive-behavioral therapy; DBT: dialectical behavior therapy; IPT: interpersonal therapy; PMHNP: psychiatric-mental health nurse practitioner |
Principles of practice
Studies have demonstrated the importance of understanding how to effectively implement collaborative care across medical disciplines.12 See the Box12 for a discussion of 3 key determinants for successful clinical collaborations.
San Martín-Rodríguez et al12 recognized 3 key factors that may help develop successful collaborative clinical relationships.
Interactional factors include a mutual willingness to collaborate, a commitment to collaborate, a belief in the benefits of collaborating, and sharing common objectives. Trust in the partnering clinician’s competency contributes to a successful collaboration. Strong communication skills—including the ability to convey what each clinician can contribute to achieving goals—also strengthens collaboration. Learning and understanding skills in conflict management and dialogue are key. Mutual respect also is essential.
Organizational factors include a shift from a traditional hierarchical structure to a more horizontal structure, and a work climate that supports openness, risk taking—ie, a willingness to disagree with a colleague if it is in a patient’s best interest or to develop a new and innovative method of providing care—integrity, and trust. Administrative structures and supports that convey the importance of collaboration also are key components of a strong collaborative environment. Teamwork and shared decision-making are important elements; teamwork should include time to discuss patient issues and develop strong interpersonal relationships. A commitment to professional development is another key factor.
Systemic factors include a social system that supports collegial relationships and professionalism that respects and accepts other professions. This includes decreased focus on protecting professional territory and increased recognition of overlaps among professions.
Enhancing collaboration
Psychiatrists who work with PMHNPs develop trust based on observing each PMHNP’s work, including their relationship with patients, ability to conceptualize a case and develop a treatment plan, and the skill with which they function within a team. The psychiatrist’s comfort level also is related to his or her awareness of the comprehensiveness of the PMHNP’s training and the competencies gained from clinical experience. Respect for the PMHNP’s educational and professional background is the foundation for what is often—at least in the collaborative relationship’s initial stages—a combined cooperative and supervisory relationship with the PMHNP. As such, the PMHNP gradually will absorb certain “intangibles” to supplement the training and work experiences that preceded his or her position. This may include assimilating the psychiatrist’s or clinic’s philosophy and treatment practice, including expertise in dealing with specialized psychiatric populations (eg, developmental disabilities, acute psychosis, or treatment-resistant depression).
The patient’s comfort level
Collaborating PMHNPs and psychiatrists need to be prepared for a patient who expresses disappointment with being treated by a PMHNP or a preference to see “a doctor.” Psychiatrists who have not worked through their own ambivalence about the collaboration or who lack confidence in the PMHNP’s abilities may find themselves consciously or unconsciously aligning with the patient’s stance. They may neglect to explore the basis and meaning of the patient’s preference, which may be related to the patient’s lack of knowledge about the PMHNP’s role and training. The PMHNP who encounters such a patient has a more challenging task—namely, how to calmly address the patient’s concern while the patient is challenging the PMHNP’s competence. Both the PMHNP and psychiatrist need to be alert to the possibility of “splitting” in the treatment of axis II-disordered patients.
Barriers to collaboration
From the PMHNP perspective, barriers to a collaborative relationship include referring to PMHNPs by a less preferred term or title, instead of a nurse practitioner or APN, which can hinder the relationship. Although physician assistants and NPs have been grouped together under the term “mid-level providers,” the American Academy of Nurse Practitioners notes that this term suggests a lower level of care or service is being provided.18 “Physician extender” is another term that fails to recognize the PMHNP’s separate and unique role and the PMHNP’s view of their role as complementary to medicine, rather than an extension of a physician’s practice.
Territorial issues can impede collaborative relationships. Psychiatrists who resist collaborating will be less effective than those who welcome a PMHNP and readily delegate specific tasks and portions of the workload, whereas psychiatrists who value the help will be more likely to build a collaborative partnership, leading to better patient care.
Autonomy is a critical determinant of professional satisfaction for PMHNPs. A PMHNP’s autonomy can be impeded by organizational constraints and physician perceptions.19 PMHNPs require autonomy to self-direct patient diagnosis and treatment within the scope of their practice, and many find this relative independence essential to delivering high quality patient care. Lack of autonomy can lead to breaks in workflow in the outpatient setting and increased length of stay for hospitalized patients. In addition, an autonomously functioning, experienced PMHNP can increase efficiency in hospital settings where psychiatrists can be in short supply, preoccupied with administrative matters, or require help on weekends.
Related Resources
- American Psychiatric Nurses Association. www.apna.org.
- International Society of Psychiatric-Mental Health Nurses. www.ispn-psych.org.
- American Nurses Association. www.nursingworld.org.
Dr. Casher is a speaker for Sunovion Pharmaceuticals and receives royalties from Cambridge University Press.
Ms. Kuebler, Ms. Bastida, and Ms. Chipps report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sataline S, Wang SS. Medical schools can’t keep up. Wall Street Journal. April 12 2010. http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed August 21, 2012.
2. U.S. Department of Health and Human Services. The health care law & you. http://www.healthcare.gov/law/index.html. Accessed August 21, 2012.
3. Wand T, Fisher J. The mental health nurse practitioner in the emergency department: an Australian experience. Int J Ment Health Nurs. 2006;15(3):201-208.
4. Eisch JS, Brozovic B, Colling K, et al. Nurse practitioner geropsychiatric consultation service to nursing homes. Geriatr Nurs. 2000;21(3):150-155.
5. Baker N. Exploring the mental health nurse practitioner scope of practice in youth early psychosis: an anecdotal account. Contemp Nurse. 2010;34(2):211-220.
6. International Society of Psychiatric-Mental Health Nurses. Psychiatric mental health nursing scope & standards. http://www.ispn-psych.org/docs/standards/scope-standards-draft.pdf. Updated 2006. Accessed August 21, 2012.
7. Centers for Medicare and Medicaid Services. HHS finalizes new rules to cut regulations for hospitals and health care providers saving more than $5 billion. http://www.cms.gov/apps/media/press/release.asp?Counter=4362. Published May 9, 2012. Accessed August 21, 2012.
8. APRN Consensus Work Group, National Council of State Boards of Nursing APRN Advisory Committee. Consensus model for regulation: licensure accreditation, certification & education. https://www.ncsbn.org/Consensus_Model_for_APRN_Regulation_July_2008.pdf. Published July 7, 2008. Accessed August 21, 2012.
9. Legault F, Humbert J, Amos S, et al. Difficulties encountered in collaborative care: logistics trumps desire. J Am Board Fam Med. 2012;25(2):168-176.
10. Michigan Council of Nurse Practitioners. Michigan’s rules and regulations for prescriptive authority. http://micnp.org/displaycommon.cfm?an=1&subarticlenbr=109. Accessed August 21, 2012.
11. Wheeler K, Haber J. Development of psychiatric-mental health nurse practitioner competencies: opportunities for the 21st century. J Am Psychiatr Nurses Assoc. 2004;10(3):129-138.
12. San Martín-Rodríguez L, Beaulieu MD, D’Amour D, et al. The determinants of successful collaboration: a review of theoretical and empirical studies. J Interprof Care. 2005;19(suppl 1):132-147.
13. Suter E, Arndt J, Arthur N, et al. Role understanding and effective communication as core competencies for collaborative practice. J Interprof Care. 2009;23(1):41-51.
14. Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ. 2002;324(7341):819-823.
15. Byrne G, Richardson M, Brunsdon J, et al. Patient satisfaction with emergency nurse practitioners in A & E. J Clin Nurs. 2000;9(1):83-92.
16. McCann TV, Clark E. Attitudes of patients towards mental health nurse prescribing of antipsychotic agents. Int J Nurs Pract. 2008;14(2):115-121.
17. Wortans J, Happell B, Johnstone H. The role of the nurse practitioner in psychiatric/mental health nursing: exploring consumer satisfaction. J Psychiatr Ment Health Nurs. 2006;13(1):78-84.
18. Frellick M. The nurse practitioner will see you now. Advanced practice providers fill the physician gap. Hosp Health Netw. 2011;85(7):44-46, 48–49.
19. Maylone MM, Ranieri L, Quinn Griffin MT, et al. Collaboration and autonomy: perceptions among nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):51-57.
1. Sataline S, Wang SS. Medical schools can’t keep up. Wall Street Journal. April 12 2010. http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed August 21, 2012.
2. U.S. Department of Health and Human Services. The health care law & you. http://www.healthcare.gov/law/index.html. Accessed August 21, 2012.
3. Wand T, Fisher J. The mental health nurse practitioner in the emergency department: an Australian experience. Int J Ment Health Nurs. 2006;15(3):201-208.
4. Eisch JS, Brozovic B, Colling K, et al. Nurse practitioner geropsychiatric consultation service to nursing homes. Geriatr Nurs. 2000;21(3):150-155.
5. Baker N. Exploring the mental health nurse practitioner scope of practice in youth early psychosis: an anecdotal account. Contemp Nurse. 2010;34(2):211-220.
6. International Society of Psychiatric-Mental Health Nurses. Psychiatric mental health nursing scope & standards. http://www.ispn-psych.org/docs/standards/scope-standards-draft.pdf. Updated 2006. Accessed August 21, 2012.
7. Centers for Medicare and Medicaid Services. HHS finalizes new rules to cut regulations for hospitals and health care providers saving more than $5 billion. http://www.cms.gov/apps/media/press/release.asp?Counter=4362. Published May 9, 2012. Accessed August 21, 2012.
8. APRN Consensus Work Group, National Council of State Boards of Nursing APRN Advisory Committee. Consensus model for regulation: licensure accreditation, certification & education. https://www.ncsbn.org/Consensus_Model_for_APRN_Regulation_July_2008.pdf. Published July 7, 2008. Accessed August 21, 2012.
9. Legault F, Humbert J, Amos S, et al. Difficulties encountered in collaborative care: logistics trumps desire. J Am Board Fam Med. 2012;25(2):168-176.
10. Michigan Council of Nurse Practitioners. Michigan’s rules and regulations for prescriptive authority. http://micnp.org/displaycommon.cfm?an=1&subarticlenbr=109. Accessed August 21, 2012.
11. Wheeler K, Haber J. Development of psychiatric-mental health nurse practitioner competencies: opportunities for the 21st century. J Am Psychiatr Nurses Assoc. 2004;10(3):129-138.
12. San Martín-Rodríguez L, Beaulieu MD, D’Amour D, et al. The determinants of successful collaboration: a review of theoretical and empirical studies. J Interprof Care. 2005;19(suppl 1):132-147.
13. Suter E, Arndt J, Arthur N, et al. Role understanding and effective communication as core competencies for collaborative practice. J Interprof Care. 2009;23(1):41-51.
14. Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ. 2002;324(7341):819-823.
15. Byrne G, Richardson M, Brunsdon J, et al. Patient satisfaction with emergency nurse practitioners in A & E. J Clin Nurs. 2000;9(1):83-92.
16. McCann TV, Clark E. Attitudes of patients towards mental health nurse prescribing of antipsychotic agents. Int J Nurs Pract. 2008;14(2):115-121.
17. Wortans J, Happell B, Johnstone H. The role of the nurse practitioner in psychiatric/mental health nursing: exploring consumer satisfaction. J Psychiatr Ment Health Nurs. 2006;13(1):78-84.
18. Frellick M. The nurse practitioner will see you now. Advanced practice providers fill the physician gap. Hosp Health Netw. 2011;85(7):44-46, 48–49.
19. Maylone MM, Ranieri L, Quinn Griffin MT, et al. Collaboration and autonomy: perceptions among nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):51-57.
Differentiating Alzheimer’s disease from dementia with Lewy bodies
Discuss this article at www.facebook.com/CurrentPsychiatry
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are the first and second most common causes of neurodegenerative dementia, respectively.“New Alzheimer’s disease guidelines: Implications for clinicians,” Current Psychiatry, March 2012, p. 15-20; http://bit.ly/UNYikk.
The 2005 report of the DLB Consortium5 recognizes central, core, suggestive, and supportive features of DLB (Table 1).5,10 These features are considered in the context of other confounding clinical conditions and the timing of cognitive and motor symptoms. The revised DLB criteria5 require a central feature of progressive cognitive decline. “Probable DLB” is when a patient presents with 2 core features or 1 core feature and ≥1 suggestive features. A diagnosis of “possible DLB” requires 1 core feature or 1 suggestive feature in the presence of progressive cognitive decline.
Table 1
Diagnostic criteria for AD and DLB
| NIA-AA criteria for AD (2011)10 |
| Possible AD: Clinical and cognitive criteria (DSM-IV-TR) for AD are met and there is an absence of biomarkers to support the diagnosis or there is evidence of a secondary disorder that can cause dementia |
| Probable AD: Clinical and cognitive criteria for AD are met and there is documented progressive cognitive decline or abnormal biomarker(s) suggestive of AD or evidence of proven AD autosomal dominant genetic mutation (presenilin-1, presenilin-2, amyloid-β precursor protein) |
| Definite AD: Clinical criteria for probable AD are met and there is histopathologic evidence of the disorder |
| Revised clinical diagnostic criteria for DLB (2005)5 |
| Core features: Fluctuating cognition, recurrent visual hallucinations, soft motor features of parkinsonism |
| Suggestive features: REM sleep behavior disorder, severe antipsychotic sensitivity, decreased tracer uptake in striatum on SPECT dopamine transporter imaging or on myocardial scintigraphy with MIBG |
| Supportive features (common but lacking diagnostic specificity): repeated falls and syncope; transient, unexplained loss of consciousness; systematized delusions; hallucinations other than visual; relative preservation of medial temporal lobe on CT or MRI scan; decreased tracer uptake on SPECT or PET imaging in occipital regions; prominent slow waves on EEG with temporal lobe transient sharp waves |
| AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; MIBG: metaiodobenzylguanidine; NIA-AA: National Institute on Aging and the Alzheimer’s Association; PET: positron emission tomography; REM: rapid eye movement; SPECT: single photon emission computed tomography |
Biomarkers for AD, but not DLB
The 2011 diagnostic criteria for AD incorporate biomarkers that can be measured in vivo and reflect speci?c features of disease-related pathophysiologic processes. Biomarkers for AD are divided into 2 categories:11
- amyloid-beta (Aβ) accumulation: abnormal tracer retention on amyloid positron emission topography (PET) imaging and low cerebrospinal fluid (CSF) Aβ42
- neuronal degeneration or injury: elevated CSF tau (total and phosphorylated tau), decreased ?uorodeoxyglucose uptake on PET in temporo-parietal cortices, and atrophy on structural MRI in the hippocampal and temporo-parietal regions.
No clinically applicable genotypic or CSF markers exist to support a DLB diagnosis, but there are many promising candidates, including elevated levels of CSF p-tau 181, CSF levels of alpha- and beta-synuclein,12 and CSF beta-glucocerebrosidase levels.13 PET mapping of brain acetylcholinesterase activity,14 123I-2β-carbomethoxy-3β- (4-iodophenyl)-N-(3-fluoropropyl)nortropane single photon emission computed tomography (SPECT) dopamine transporter (DaT) imaging15 and metaiodobenzylguanidine (MIBG) scintigraphy also are promising methods. DaT scan SPECT is FDA-approved for detecting loss of functional dopaminergic neuron terminals in the striatum and can differentiate between AD and DLB with a sensitivity and specificity of 78% to 88% and 94% to 100%, respectively.16 This test is covered by Medicare for differentiating AD and DLB.
Differences in presentation
Cognitive impairment. Contrary to the early memory impairment that characterizes AD, memory deficits in DLB usually appear later in the disease course.5 Patients with DLB manifest greater attentional, visuospatial, and executive impairments than those with AD, whereas AD causes more profound episodic (declarative) memory impairment than DLB. DLB patients show more preserved consolidation and storage of verbal information than AD patients because of less neuroanatomical and cholinergic compromise in the medial temporal lobe. There is no evidence of significant differences in remote memory, semantic memory, and language (naming and fluency).
Compromised attention in DLB may be the basis for fluctuating cognition, a characteristic of the disease. The greater attentional impairment and reaction time variability in DLB compared with AD is evident during complex tasks for attention and may be a function of the executive and visuospatial demands of the tasks.17
Executive functions critical to adaptive, goal-directed behavior are more impaired in DLB than AD. DLB patients are more susceptible to distraction and have difficulty engaging in a task and shifting from 1 task to another. This, together with a tendency for confabulation and perseveration, are signs of executive dysfunction.
Neuropsychiatric features. DLB patients are more likely than AD patients to exhibit psychiatric symptoms and have more functional impairment.18 In an analysis of autopsy-confirmed cases, hallucinations and delusions were more frequent with Lewy body pathology (75%) than AD (21%) at initial clinical evaluation.18 By the end stages of both illnesses, the degree of psychotic symptoms is comparable.19 Depression is common in DLB; whether base rates of depressed mood and major depression differ between DLB and AD is uncertain.20
Psychosis in AD can be induced by medication or delirium, or triggered by poor sensory perceptions. Psychotic symptoms occur more frequently during the moderate and advanced stages of AD, when patients present with visual hallucinations, delusions, or delusional misidentifications. As many as 10% to 20% of patients with AD experience hallucinations, typically visual. Delusions occur in 30% to 50% of AD patients, usually in the later stages of the disease. The most common delusional themes are infidelity, theft, and paranoia. Female sex is a risk factor for psychosis in AD. Delusions co-occur with aggression, anxiety, and aberrant motor behavior.
Visual hallucinations—mostly vivid, well-formed, false perceptions of insects, animals, or people—are the defining feature of DLB.21 Many patients recognize that they are experiencing visual hallucinations and can ignore them. DLB patients also may experience visual illusions, such as misperceiving household objects as living beings. Delusions—typically paranoid—are common among DLB patients, as are depression and anxiety.1 Agitation or aggressive behavior tends to occur late in the illness, if at all.
The causes of psychotic symptoms in DLB are not fully understood, but dopamine dysfunction likely is involved in hallucinations, delusions, and agitation, and serotonin dysfunction may be associated with depression and anxiety. Rapid eye movement (REM) sleep/wakefulness dysregulation, in which the dream imagery of REM sleep may occur during wakefulness, also has been proposed as a mechanism for visual hallucinations in DLB.22 In DLB, psychotic symptoms occur early and are a hallmark of this illness, whereas in AD they usually occur in the middle to late stages of the disease.
Motor symptoms. In AD, extrapyramidal symptoms (EPS) are common later in the disease, are strongly correlated with disease severity, and are a strong, independent predictor of depression severity.23 EPS are more common in DLB than in AD24 and DLB patients are at higher risk of developing EPS even with low doses of typical antipsychotics, compared with AD patients.25
Other symptoms. REM sleep behavior disorder (RBD) is characterized by enacting dreams—often violent—during REM sleep. RBD is common in DLB and many patients also have excessive daytime somnolence. Other sleep disorders in DLB include insomnia, obstructive sleep apnea, central sleep apnea, restless legs syndrome, and periodic limb movements during sleep.
In AD patients, common sleep behaviors include confusion in the early evening (“sundowning”) and frequent nighttime awakenings, often accompanied by wandering.26 Orthostatic hypotension, impotence, urinary incontinence, and constipation are common in DLB. Lack of insight concerning personal cognitive, mood, and behavioral state is highly prevalent in AD patients and more common than in DLB.
Diagnostic evaluation
Because there are no definitive clinical markers for DLB, diagnosis is based on a detailed clinical and family history from the patient and a reliable informant, as well as a physical, neurologic, and mental status examination that looks for associated noncognitive symptoms, and neuropsychological evaluation. Reasons DLB may be misdiagnosed include:
- Some “core” clinical features of DLB may not appear or may overlap with AD.
- Presence and severity of concurrent AD pathology in DLB may modify the clinical presentation, with decreased rates of hallucinations and parkinsonism and increased neurofibrillary tangles.
- Failure to reliably identify fluctuations—variations in cognition and arousal, such as periods of unresponsiveness while awake (“zoning out”), excessive daytime somnolence, and disorganized speech.27
Detecting and characterizing cognitive deficits in dementia patients using neuropsychological testing is important in establishing a clinical diagnosis, determining baseline levels of impairment, forming a prognosis, and initiating disease-specific treatments. Differences in neuropsychological findings in AD and DLB are outlined in Table 2.16,28-33 Several studies have suggested using these measures to differentiate patients with DLB from those with AD.20
Table 2
Diagnostic testing for Alzheimer’s disease and dementia with Lewy bodies
| Alzheimer’s disease | Dementia with Lewy bodies |
|---|---|
| Neuropsychological testing findings | |
| Relatively more impairment on verbal memory tasks, delayed recall, delayed recognition, and encoding and storing information.28 Dysfunction of episodic memory function | Relatively more impairment on attention or concentration, verbal fluency, visuoperceptual, visuoconstructive, visual memory tests, and frontal executive functions.28 Relatively preserved confrontation naming and verbal memory |
| MRI findings | |
| Diffuse cortical atrophy, relatively greater volume loss in hippocampus and medial temporal lobe structures (strong correlation with severity)29 | Mild generalized cerebral cortical atrophy with minimal hippocampal atrophy and relative preservation of medial temporal lobe structures30 |
| [18F]FDG PET | |
| Widespread metabolic deficits in neocortical association areas, with sparing of the basal ganglia, thalamus, cerebellum, primary sensory motor cortex, and visual cortex | Widespread cortical hypometabolism, more marked in primary visual and occipital association areas, and less severe in parietal, frontal, and anterior cingulate cortices.31 Severe cholinergic deafferentation of the neocortex, particularly in posterior cortical regions32 |
| Single photon emission computed tomography | |
| Parietotemporal hypoperfusion | Occipital hypoperfusion |
| 123I-FP-CIT SPECT (DaT scan) | |
| No significant loss of DaT | Low nigrostriatal terminal density of DaT caused by severe nigrostriatal degeneration16 |
| Myocardial scintigraphy with MIBG | |
| No significant change in MIBG uptake | Decreased MIBG uptake33 |
| 123I-FP-CIT: 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane; DaT: dopamine transporter; FDG PET: [18F]-fluoro-d-glucose positron emission tomography; MIBG: metaiodobenzylguanidine; SPECT: single photon emission computed tomography | |
Evidence is insufficient to support using electroencephalographic and polysomnographic studies when initially evaluating patients with dementia. Brain CT or MRI are recommended as part of the initial evaluation of dementia patients to exclude treatable causes of dementia and help clarify the differential diagnosis. Occipital hypometabolism and hypoperfusion demonstrated on PET and SPECT imaging have high sensitivity and specificity for differentiating AD from DLB.
To diagnose DLB more consistently, look for core features of the disease, RBD, antipsychotic hypersensitivity, and decreased striatal binding at presynaptic DaT sites.15 Abnormal (low binding) DaT activity is the most reliable diagnostic marker for DLB.34 Myocardial scintigraphy with MIBG is sensitive to pathologic changes of DLB before clinical expression and could overcome the difficulties of using clinical criteria alone to identify patients with DLB.35 MIBG scintigraphy may be preferred to DaT scan because it is less expensive and its sensitivity and specificity to DLB are independent of the presence of parkinsonism.35
For an overview of pharmacotherapy options for patients with AD or DLB, see Box 2.
Pharmacotherapy options for patients with Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) include cholinesterase inhibitors, memantine, antipsychotics, and other agents.
Cholinesterase inhibitors. Donepezil, rivastigmine, and galantamine are FDA-approved for treating AD. Their efficacy appears to be similar, so the choice of agent is based largely on cost, patient tolerability, and physician experience.
No medications are FDA-approved for treating DLB. Neocortical cholinergic activity assessed by choline acetyltransferase levels is more severely depleted in DLB than in AD, and this deficit is correlated with the presence of visual hallucinations and global severity of cognitive impairment.a Therefore, drugs that enhance central cholinergic function offer a therapeutic approach for DLB; cognitive and hallucinatory symptoms are the anticipated targets. Multiple anecdotal reports, open-label studies,b,c and 1 randomized, placebo-controlled triald suggest that cholinesterase inhibitors are efficacious in DLB, with reported benefits in cognition, fluctuations, psychotic symptoms, and parkinsonian symptoms. A 20-week randomized, double-blind, placebo-controlled multicenter studyd of patients with DLB found rivastigmine, 6 to 12 mg/d, was superior to placebo. Patients receiving rivastigmine exhibited significantly reduced anxiety, delusions, and hallucinations and significantly better performance on a computerized battery of neuropsychological tests, especially tasks that required sustained attention. Differences between rivastigmine and placebo disappeared after drug discontinuation.
Memantine is a noncompetitive antagonist of N-methyl-d-aspartate receptors that is effective in AD.e The possible involvement of glutamate in DLB has provided a rationale for treating DLB with memantine. Two randomized controlled trials in DLB found that patients treated with memantine for 24 weeks performed better on Clinical Global Impression of Change, but not on most other secondary outcome measures.f,g In both studies, memantine was well tolerated. However, other studies have noted worsening of delusions and hallucinations with memantine in DLB patients.h
Antipsychotics. Agitation is common in moderate and advanced AD. Atypical antipsychotics have been used with variable efficacy to treat agitation, but their use is associated with excess mortality. DLB patients pose a considerable therapeutic challenge because antipsychotics—the mainstay of treatment of psychosis and behavioral problems in most other disorders—can provoke severe, irreversible, and often fatal sensitivity reactions in this type of dementia.i A 2- to 3-fold increased mortality risk associated with antipsychotic sensitivity reactions in DLB is partly mediated via acute blockade of postsynaptic dopamine D2 receptors in the striatum. For severe and disabling psychosis, a trial of a cholinesterase inhibitor and/or lowering the dose of antiparkinsonian medication should be considered first. In urgent situations, small doses of an atypical antipsychotic that is least associated with parkinsonism side effects—such as quetiapine or aripiprazole—should be used.
Other treatments. Treatment of parkinsonian symptoms in DLB patients is similar to that for Parkinson’s disease, but the risk of psychotic symptoms in DLB warrants a conservative approach. Levodopa seems to be more effective than dopamine agonists and produces fewer side effects.j Rapid eye movement sleep behavior disorder often responds to low doses of clonazepam (0.25 to 1.5 mg). Depression and anxiety disorders are common in AD at all stages and their treatment is not fundamentally different than in geriatric patients without dementia. Selective serotonin reuptake inhibitors and electroconvulsive therapy have been used successfully in depressed patients with AD or DLB.k,l
Disease-modifying treatments. Researchers are evaluating an array of antiamyloid and neuroprotective therapeutic approaches for AD based on the hypothesis that amyloid-beta protein plays a pivotal role in disease onset and progression. Interventions that reduce amyloid production, limit aggregation, or increase clearance may block the cascade of events comprising AD pathogenesis. Reducing tau hyperphosphorylation, limiting oxidation and excitotoxicity, and controlling inflammation also may be beneficial strategies. Potentially neuroprotective and restorative treatments such as neurotrophins, neurotrophic factor enhancers, and stem cell-related approaches also are being investigated.
There are no large-scale studies of disease-modifying treatments for DLB. Potential areas of research include the relationship between proteasome function and a-synuclein pathology, the role of beta-synuclein, and the impact of alterations to alpha-synuclein on its propensity to aggregate.
References
a. Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931-1934.
b. Beversdorf DQ, Warner JL, Davis RA, et al. Donepezil in the treatment of dementia with lewy bodies. Am J Geriatr Psychiatry. 2004;12(5):542-544.
c. Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401-405.
d. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
e. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
f. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
g. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
h. Ridha BH, Josephs KA, Rossor MN. Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology. 2005;65(3):481-482.
i. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
j. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
k. Swartz M, Barak Y, Mirecki I, et al. Treating depression in Alzheimer’s disease: integration of differing guidelines. Int Psychogeriatr. 2000;12(3):353-358.
l. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
Related Resources
- Hanyu H, Sato T, Hirao K, et al. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16(2):212-217.
- Walker Z, McKeith I, Rodda J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer’s disease: a cohort study. BMJ Open. 2012;2:e000380.
Drug Brand Names
- Aripiprazole • Abilify
- Clonazepam • Klonopin
- Donepezil • Aricept
- Galantamine • Razadyne, Reminyl
- Levodopa • Dopar, Larodopa
- Memantine • Namenda
- Quetiapine • Seroquel
- Rivastigmine • Exelon
Disclosure
Drs. Bishnoi and Manepalli report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg serves as a consultant to Forest, Janssen, Novartis, and Pfizer. His department receives research funding from Novartis, Janssen, and Pfizer.
1. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
2. Buracchio T, Arvanitakis Z, Gorbien M. Dementia with Lewy bodies: current concepts. Dement Geriatr Cogn Disord. 2005;20(5):306-320.
3. Fujishiro H, Iseki E, Higashi S, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19-23.
4. Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(suppl):S73-S78.
5. McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
6. Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287(18):2335-2338.
7. Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561-566.
8. Bradshaw J, Saling M, Hopwood M, et al. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 2004;75(3):382-387.
9. Singleton AB, Wharton A, O’Brien KK, et al. Clinical and neuropathological correlates of apolipoprotein E genotype in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(4):167-175.
10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
11. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
12. Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315-325.
13. Parnetti L, Balducci C, Pierguidi L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in dementia with Lewy bodies. Neurobiol Dis. 2009;34(3):484-486.
14. Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273-278.
15. McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305-313.
16. Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176-1181.
17. Bradshaw JM, Saling M, Anderson V, et al. Higher cortical deficits influence attentional processing in dementia with Lewy bodies, relative to patients with dementia of the Alzheimer’s type and controls. J Neurol Neurosurg Psychiatry. 2006;77(10):1129-1135.
18. Weiner MF, Hynan LS, Parikh B, et al. Can Alzheimer’s disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245-250.
19. Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63(10):1450-1456.
20. Ferman TJ, Smith GE, Boeve BF, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clin Neuropsychol. 2006;20(4):623-636.
21. McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902-905.
22. Boeve BF, Silber MH, Ferman TJ, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622-630.
23. Portet F, Scarmeas N, Cosentino S, et al. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120-1126.
24. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
25. Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer’s disease. Expert Rev Neurother. 2007;7(11):1499-1516.
26. Ancoli-Israel S, Klauber MR, Gillin JC, et al. Sleep in non-institutionalized Alzheimer’s disease patients. Aging (Milano). 1994;6(6):451-458.
27. Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181-187.
28. Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148-165.
29. Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484-489.
30. Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(pt 1):195-203.
31. Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48(5):704-711.
32. Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885-892.
33. Fujishiro H, Nakamura S, Kitazawa M, et al. Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy. J Neurol Sci. 2012;315(1-2):115-119.
34. O’Brien JT, McKeith IG, Walker Z, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194:34-39.
35. Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66(12):1850-1854.
Discuss this article at www.facebook.com/CurrentPsychiatry
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are the first and second most common causes of neurodegenerative dementia, respectively.“New Alzheimer’s disease guidelines: Implications for clinicians,” Current Psychiatry, March 2012, p. 15-20; http://bit.ly/UNYikk.
The 2005 report of the DLB Consortium5 recognizes central, core, suggestive, and supportive features of DLB (Table 1).5,10 These features are considered in the context of other confounding clinical conditions and the timing of cognitive and motor symptoms. The revised DLB criteria5 require a central feature of progressive cognitive decline. “Probable DLB” is when a patient presents with 2 core features or 1 core feature and ≥1 suggestive features. A diagnosis of “possible DLB” requires 1 core feature or 1 suggestive feature in the presence of progressive cognitive decline.
Table 1
Diagnostic criteria for AD and DLB
| NIA-AA criteria for AD (2011)10 |
| Possible AD: Clinical and cognitive criteria (DSM-IV-TR) for AD are met and there is an absence of biomarkers to support the diagnosis or there is evidence of a secondary disorder that can cause dementia |
| Probable AD: Clinical and cognitive criteria for AD are met and there is documented progressive cognitive decline or abnormal biomarker(s) suggestive of AD or evidence of proven AD autosomal dominant genetic mutation (presenilin-1, presenilin-2, amyloid-β precursor protein) |
| Definite AD: Clinical criteria for probable AD are met and there is histopathologic evidence of the disorder |
| Revised clinical diagnostic criteria for DLB (2005)5 |
| Core features: Fluctuating cognition, recurrent visual hallucinations, soft motor features of parkinsonism |
| Suggestive features: REM sleep behavior disorder, severe antipsychotic sensitivity, decreased tracer uptake in striatum on SPECT dopamine transporter imaging or on myocardial scintigraphy with MIBG |
| Supportive features (common but lacking diagnostic specificity): repeated falls and syncope; transient, unexplained loss of consciousness; systematized delusions; hallucinations other than visual; relative preservation of medial temporal lobe on CT or MRI scan; decreased tracer uptake on SPECT or PET imaging in occipital regions; prominent slow waves on EEG with temporal lobe transient sharp waves |
| AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; MIBG: metaiodobenzylguanidine; NIA-AA: National Institute on Aging and the Alzheimer’s Association; PET: positron emission tomography; REM: rapid eye movement; SPECT: single photon emission computed tomography |
Biomarkers for AD, but not DLB
The 2011 diagnostic criteria for AD incorporate biomarkers that can be measured in vivo and reflect speci?c features of disease-related pathophysiologic processes. Biomarkers for AD are divided into 2 categories:11
- amyloid-beta (Aβ) accumulation: abnormal tracer retention on amyloid positron emission topography (PET) imaging and low cerebrospinal fluid (CSF) Aβ42
- neuronal degeneration or injury: elevated CSF tau (total and phosphorylated tau), decreased ?uorodeoxyglucose uptake on PET in temporo-parietal cortices, and atrophy on structural MRI in the hippocampal and temporo-parietal regions.
No clinically applicable genotypic or CSF markers exist to support a DLB diagnosis, but there are many promising candidates, including elevated levels of CSF p-tau 181, CSF levels of alpha- and beta-synuclein,12 and CSF beta-glucocerebrosidase levels.13 PET mapping of brain acetylcholinesterase activity,14 123I-2β-carbomethoxy-3β- (4-iodophenyl)-N-(3-fluoropropyl)nortropane single photon emission computed tomography (SPECT) dopamine transporter (DaT) imaging15 and metaiodobenzylguanidine (MIBG) scintigraphy also are promising methods. DaT scan SPECT is FDA-approved for detecting loss of functional dopaminergic neuron terminals in the striatum and can differentiate between AD and DLB with a sensitivity and specificity of 78% to 88% and 94% to 100%, respectively.16 This test is covered by Medicare for differentiating AD and DLB.
Differences in presentation
Cognitive impairment. Contrary to the early memory impairment that characterizes AD, memory deficits in DLB usually appear later in the disease course.5 Patients with DLB manifest greater attentional, visuospatial, and executive impairments than those with AD, whereas AD causes more profound episodic (declarative) memory impairment than DLB. DLB patients show more preserved consolidation and storage of verbal information than AD patients because of less neuroanatomical and cholinergic compromise in the medial temporal lobe. There is no evidence of significant differences in remote memory, semantic memory, and language (naming and fluency).
Compromised attention in DLB may be the basis for fluctuating cognition, a characteristic of the disease. The greater attentional impairment and reaction time variability in DLB compared with AD is evident during complex tasks for attention and may be a function of the executive and visuospatial demands of the tasks.17
Executive functions critical to adaptive, goal-directed behavior are more impaired in DLB than AD. DLB patients are more susceptible to distraction and have difficulty engaging in a task and shifting from 1 task to another. This, together with a tendency for confabulation and perseveration, are signs of executive dysfunction.
Neuropsychiatric features. DLB patients are more likely than AD patients to exhibit psychiatric symptoms and have more functional impairment.18 In an analysis of autopsy-confirmed cases, hallucinations and delusions were more frequent with Lewy body pathology (75%) than AD (21%) at initial clinical evaluation.18 By the end stages of both illnesses, the degree of psychotic symptoms is comparable.19 Depression is common in DLB; whether base rates of depressed mood and major depression differ between DLB and AD is uncertain.20
Psychosis in AD can be induced by medication or delirium, or triggered by poor sensory perceptions. Psychotic symptoms occur more frequently during the moderate and advanced stages of AD, when patients present with visual hallucinations, delusions, or delusional misidentifications. As many as 10% to 20% of patients with AD experience hallucinations, typically visual. Delusions occur in 30% to 50% of AD patients, usually in the later stages of the disease. The most common delusional themes are infidelity, theft, and paranoia. Female sex is a risk factor for psychosis in AD. Delusions co-occur with aggression, anxiety, and aberrant motor behavior.
Visual hallucinations—mostly vivid, well-formed, false perceptions of insects, animals, or people—are the defining feature of DLB.21 Many patients recognize that they are experiencing visual hallucinations and can ignore them. DLB patients also may experience visual illusions, such as misperceiving household objects as living beings. Delusions—typically paranoid—are common among DLB patients, as are depression and anxiety.1 Agitation or aggressive behavior tends to occur late in the illness, if at all.
The causes of psychotic symptoms in DLB are not fully understood, but dopamine dysfunction likely is involved in hallucinations, delusions, and agitation, and serotonin dysfunction may be associated with depression and anxiety. Rapid eye movement (REM) sleep/wakefulness dysregulation, in which the dream imagery of REM sleep may occur during wakefulness, also has been proposed as a mechanism for visual hallucinations in DLB.22 In DLB, psychotic symptoms occur early and are a hallmark of this illness, whereas in AD they usually occur in the middle to late stages of the disease.
Motor symptoms. In AD, extrapyramidal symptoms (EPS) are common later in the disease, are strongly correlated with disease severity, and are a strong, independent predictor of depression severity.23 EPS are more common in DLB than in AD24 and DLB patients are at higher risk of developing EPS even with low doses of typical antipsychotics, compared with AD patients.25
Other symptoms. REM sleep behavior disorder (RBD) is characterized by enacting dreams—often violent—during REM sleep. RBD is common in DLB and many patients also have excessive daytime somnolence. Other sleep disorders in DLB include insomnia, obstructive sleep apnea, central sleep apnea, restless legs syndrome, and periodic limb movements during sleep.
In AD patients, common sleep behaviors include confusion in the early evening (“sundowning”) and frequent nighttime awakenings, often accompanied by wandering.26 Orthostatic hypotension, impotence, urinary incontinence, and constipation are common in DLB. Lack of insight concerning personal cognitive, mood, and behavioral state is highly prevalent in AD patients and more common than in DLB.
Diagnostic evaluation
Because there are no definitive clinical markers for DLB, diagnosis is based on a detailed clinical and family history from the patient and a reliable informant, as well as a physical, neurologic, and mental status examination that looks for associated noncognitive symptoms, and neuropsychological evaluation. Reasons DLB may be misdiagnosed include:
- Some “core” clinical features of DLB may not appear or may overlap with AD.
- Presence and severity of concurrent AD pathology in DLB may modify the clinical presentation, with decreased rates of hallucinations and parkinsonism and increased neurofibrillary tangles.
- Failure to reliably identify fluctuations—variations in cognition and arousal, such as periods of unresponsiveness while awake (“zoning out”), excessive daytime somnolence, and disorganized speech.27
Detecting and characterizing cognitive deficits in dementia patients using neuropsychological testing is important in establishing a clinical diagnosis, determining baseline levels of impairment, forming a prognosis, and initiating disease-specific treatments. Differences in neuropsychological findings in AD and DLB are outlined in Table 2.16,28-33 Several studies have suggested using these measures to differentiate patients with DLB from those with AD.20
Table 2
Diagnostic testing for Alzheimer’s disease and dementia with Lewy bodies
| Alzheimer’s disease | Dementia with Lewy bodies |
|---|---|
| Neuropsychological testing findings | |
| Relatively more impairment on verbal memory tasks, delayed recall, delayed recognition, and encoding and storing information.28 Dysfunction of episodic memory function | Relatively more impairment on attention or concentration, verbal fluency, visuoperceptual, visuoconstructive, visual memory tests, and frontal executive functions.28 Relatively preserved confrontation naming and verbal memory |
| MRI findings | |
| Diffuse cortical atrophy, relatively greater volume loss in hippocampus and medial temporal lobe structures (strong correlation with severity)29 | Mild generalized cerebral cortical atrophy with minimal hippocampal atrophy and relative preservation of medial temporal lobe structures30 |
| [18F]FDG PET | |
| Widespread metabolic deficits in neocortical association areas, with sparing of the basal ganglia, thalamus, cerebellum, primary sensory motor cortex, and visual cortex | Widespread cortical hypometabolism, more marked in primary visual and occipital association areas, and less severe in parietal, frontal, and anterior cingulate cortices.31 Severe cholinergic deafferentation of the neocortex, particularly in posterior cortical regions32 |
| Single photon emission computed tomography | |
| Parietotemporal hypoperfusion | Occipital hypoperfusion |
| 123I-FP-CIT SPECT (DaT scan) | |
| No significant loss of DaT | Low nigrostriatal terminal density of DaT caused by severe nigrostriatal degeneration16 |
| Myocardial scintigraphy with MIBG | |
| No significant change in MIBG uptake | Decreased MIBG uptake33 |
| 123I-FP-CIT: 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane; DaT: dopamine transporter; FDG PET: [18F]-fluoro-d-glucose positron emission tomography; MIBG: metaiodobenzylguanidine; SPECT: single photon emission computed tomography | |
Evidence is insufficient to support using electroencephalographic and polysomnographic studies when initially evaluating patients with dementia. Brain CT or MRI are recommended as part of the initial evaluation of dementia patients to exclude treatable causes of dementia and help clarify the differential diagnosis. Occipital hypometabolism and hypoperfusion demonstrated on PET and SPECT imaging have high sensitivity and specificity for differentiating AD from DLB.
To diagnose DLB more consistently, look for core features of the disease, RBD, antipsychotic hypersensitivity, and decreased striatal binding at presynaptic DaT sites.15 Abnormal (low binding) DaT activity is the most reliable diagnostic marker for DLB.34 Myocardial scintigraphy with MIBG is sensitive to pathologic changes of DLB before clinical expression and could overcome the difficulties of using clinical criteria alone to identify patients with DLB.35 MIBG scintigraphy may be preferred to DaT scan because it is less expensive and its sensitivity and specificity to DLB are independent of the presence of parkinsonism.35
For an overview of pharmacotherapy options for patients with AD or DLB, see Box 2.
Pharmacotherapy options for patients with Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) include cholinesterase inhibitors, memantine, antipsychotics, and other agents.
Cholinesterase inhibitors. Donepezil, rivastigmine, and galantamine are FDA-approved for treating AD. Their efficacy appears to be similar, so the choice of agent is based largely on cost, patient tolerability, and physician experience.
No medications are FDA-approved for treating DLB. Neocortical cholinergic activity assessed by choline acetyltransferase levels is more severely depleted in DLB than in AD, and this deficit is correlated with the presence of visual hallucinations and global severity of cognitive impairment.a Therefore, drugs that enhance central cholinergic function offer a therapeutic approach for DLB; cognitive and hallucinatory symptoms are the anticipated targets. Multiple anecdotal reports, open-label studies,b,c and 1 randomized, placebo-controlled triald suggest that cholinesterase inhibitors are efficacious in DLB, with reported benefits in cognition, fluctuations, psychotic symptoms, and parkinsonian symptoms. A 20-week randomized, double-blind, placebo-controlled multicenter studyd of patients with DLB found rivastigmine, 6 to 12 mg/d, was superior to placebo. Patients receiving rivastigmine exhibited significantly reduced anxiety, delusions, and hallucinations and significantly better performance on a computerized battery of neuropsychological tests, especially tasks that required sustained attention. Differences between rivastigmine and placebo disappeared after drug discontinuation.
Memantine is a noncompetitive antagonist of N-methyl-d-aspartate receptors that is effective in AD.e The possible involvement of glutamate in DLB has provided a rationale for treating DLB with memantine. Two randomized controlled trials in DLB found that patients treated with memantine for 24 weeks performed better on Clinical Global Impression of Change, but not on most other secondary outcome measures.f,g In both studies, memantine was well tolerated. However, other studies have noted worsening of delusions and hallucinations with memantine in DLB patients.h
Antipsychotics. Agitation is common in moderate and advanced AD. Atypical antipsychotics have been used with variable efficacy to treat agitation, but their use is associated with excess mortality. DLB patients pose a considerable therapeutic challenge because antipsychotics—the mainstay of treatment of psychosis and behavioral problems in most other disorders—can provoke severe, irreversible, and often fatal sensitivity reactions in this type of dementia.i A 2- to 3-fold increased mortality risk associated with antipsychotic sensitivity reactions in DLB is partly mediated via acute blockade of postsynaptic dopamine D2 receptors in the striatum. For severe and disabling psychosis, a trial of a cholinesterase inhibitor and/or lowering the dose of antiparkinsonian medication should be considered first. In urgent situations, small doses of an atypical antipsychotic that is least associated with parkinsonism side effects—such as quetiapine or aripiprazole—should be used.
Other treatments. Treatment of parkinsonian symptoms in DLB patients is similar to that for Parkinson’s disease, but the risk of psychotic symptoms in DLB warrants a conservative approach. Levodopa seems to be more effective than dopamine agonists and produces fewer side effects.j Rapid eye movement sleep behavior disorder often responds to low doses of clonazepam (0.25 to 1.5 mg). Depression and anxiety disorders are common in AD at all stages and their treatment is not fundamentally different than in geriatric patients without dementia. Selective serotonin reuptake inhibitors and electroconvulsive therapy have been used successfully in depressed patients with AD or DLB.k,l
Disease-modifying treatments. Researchers are evaluating an array of antiamyloid and neuroprotective therapeutic approaches for AD based on the hypothesis that amyloid-beta protein plays a pivotal role in disease onset and progression. Interventions that reduce amyloid production, limit aggregation, or increase clearance may block the cascade of events comprising AD pathogenesis. Reducing tau hyperphosphorylation, limiting oxidation and excitotoxicity, and controlling inflammation also may be beneficial strategies. Potentially neuroprotective and restorative treatments such as neurotrophins, neurotrophic factor enhancers, and stem cell-related approaches also are being investigated.
There are no large-scale studies of disease-modifying treatments for DLB. Potential areas of research include the relationship between proteasome function and a-synuclein pathology, the role of beta-synuclein, and the impact of alterations to alpha-synuclein on its propensity to aggregate.
References
a. Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931-1934.
b. Beversdorf DQ, Warner JL, Davis RA, et al. Donepezil in the treatment of dementia with lewy bodies. Am J Geriatr Psychiatry. 2004;12(5):542-544.
c. Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401-405.
d. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
e. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
f. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
g. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
h. Ridha BH, Josephs KA, Rossor MN. Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology. 2005;65(3):481-482.
i. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
j. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
k. Swartz M, Barak Y, Mirecki I, et al. Treating depression in Alzheimer’s disease: integration of differing guidelines. Int Psychogeriatr. 2000;12(3):353-358.
l. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
Related Resources
- Hanyu H, Sato T, Hirao K, et al. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16(2):212-217.
- Walker Z, McKeith I, Rodda J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer’s disease: a cohort study. BMJ Open. 2012;2:e000380.
Drug Brand Names
- Aripiprazole • Abilify
- Clonazepam • Klonopin
- Donepezil • Aricept
- Galantamine • Razadyne, Reminyl
- Levodopa • Dopar, Larodopa
- Memantine • Namenda
- Quetiapine • Seroquel
- Rivastigmine • Exelon
Disclosure
Drs. Bishnoi and Manepalli report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg serves as a consultant to Forest, Janssen, Novartis, and Pfizer. His department receives research funding from Novartis, Janssen, and Pfizer.
Discuss this article at www.facebook.com/CurrentPsychiatry
Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are the first and second most common causes of neurodegenerative dementia, respectively.“New Alzheimer’s disease guidelines: Implications for clinicians,” Current Psychiatry, March 2012, p. 15-20; http://bit.ly/UNYikk.
The 2005 report of the DLB Consortium5 recognizes central, core, suggestive, and supportive features of DLB (Table 1).5,10 These features are considered in the context of other confounding clinical conditions and the timing of cognitive and motor symptoms. The revised DLB criteria5 require a central feature of progressive cognitive decline. “Probable DLB” is when a patient presents with 2 core features or 1 core feature and ≥1 suggestive features. A diagnosis of “possible DLB” requires 1 core feature or 1 suggestive feature in the presence of progressive cognitive decline.
Table 1
Diagnostic criteria for AD and DLB
| NIA-AA criteria for AD (2011)10 |
| Possible AD: Clinical and cognitive criteria (DSM-IV-TR) for AD are met and there is an absence of biomarkers to support the diagnosis or there is evidence of a secondary disorder that can cause dementia |
| Probable AD: Clinical and cognitive criteria for AD are met and there is documented progressive cognitive decline or abnormal biomarker(s) suggestive of AD or evidence of proven AD autosomal dominant genetic mutation (presenilin-1, presenilin-2, amyloid-β precursor protein) |
| Definite AD: Clinical criteria for probable AD are met and there is histopathologic evidence of the disorder |
| Revised clinical diagnostic criteria for DLB (2005)5 |
| Core features: Fluctuating cognition, recurrent visual hallucinations, soft motor features of parkinsonism |
| Suggestive features: REM sleep behavior disorder, severe antipsychotic sensitivity, decreased tracer uptake in striatum on SPECT dopamine transporter imaging or on myocardial scintigraphy with MIBG |
| Supportive features (common but lacking diagnostic specificity): repeated falls and syncope; transient, unexplained loss of consciousness; systematized delusions; hallucinations other than visual; relative preservation of medial temporal lobe on CT or MRI scan; decreased tracer uptake on SPECT or PET imaging in occipital regions; prominent slow waves on EEG with temporal lobe transient sharp waves |
| AD: Alzheimer’s disease; DLB: dementia with Lewy bodies; MIBG: metaiodobenzylguanidine; NIA-AA: National Institute on Aging and the Alzheimer’s Association; PET: positron emission tomography; REM: rapid eye movement; SPECT: single photon emission computed tomography |
Biomarkers for AD, but not DLB
The 2011 diagnostic criteria for AD incorporate biomarkers that can be measured in vivo and reflect speci?c features of disease-related pathophysiologic processes. Biomarkers for AD are divided into 2 categories:11
- amyloid-beta (Aβ) accumulation: abnormal tracer retention on amyloid positron emission topography (PET) imaging and low cerebrospinal fluid (CSF) Aβ42
- neuronal degeneration or injury: elevated CSF tau (total and phosphorylated tau), decreased ?uorodeoxyglucose uptake on PET in temporo-parietal cortices, and atrophy on structural MRI in the hippocampal and temporo-parietal regions.
No clinically applicable genotypic or CSF markers exist to support a DLB diagnosis, but there are many promising candidates, including elevated levels of CSF p-tau 181, CSF levels of alpha- and beta-synuclein,12 and CSF beta-glucocerebrosidase levels.13 PET mapping of brain acetylcholinesterase activity,14 123I-2β-carbomethoxy-3β- (4-iodophenyl)-N-(3-fluoropropyl)nortropane single photon emission computed tomography (SPECT) dopamine transporter (DaT) imaging15 and metaiodobenzylguanidine (MIBG) scintigraphy also are promising methods. DaT scan SPECT is FDA-approved for detecting loss of functional dopaminergic neuron terminals in the striatum and can differentiate between AD and DLB with a sensitivity and specificity of 78% to 88% and 94% to 100%, respectively.16 This test is covered by Medicare for differentiating AD and DLB.
Differences in presentation
Cognitive impairment. Contrary to the early memory impairment that characterizes AD, memory deficits in DLB usually appear later in the disease course.5 Patients with DLB manifest greater attentional, visuospatial, and executive impairments than those with AD, whereas AD causes more profound episodic (declarative) memory impairment than DLB. DLB patients show more preserved consolidation and storage of verbal information than AD patients because of less neuroanatomical and cholinergic compromise in the medial temporal lobe. There is no evidence of significant differences in remote memory, semantic memory, and language (naming and fluency).
Compromised attention in DLB may be the basis for fluctuating cognition, a characteristic of the disease. The greater attentional impairment and reaction time variability in DLB compared with AD is evident during complex tasks for attention and may be a function of the executive and visuospatial demands of the tasks.17
Executive functions critical to adaptive, goal-directed behavior are more impaired in DLB than AD. DLB patients are more susceptible to distraction and have difficulty engaging in a task and shifting from 1 task to another. This, together with a tendency for confabulation and perseveration, are signs of executive dysfunction.
Neuropsychiatric features. DLB patients are more likely than AD patients to exhibit psychiatric symptoms and have more functional impairment.18 In an analysis of autopsy-confirmed cases, hallucinations and delusions were more frequent with Lewy body pathology (75%) than AD (21%) at initial clinical evaluation.18 By the end stages of both illnesses, the degree of psychotic symptoms is comparable.19 Depression is common in DLB; whether base rates of depressed mood and major depression differ between DLB and AD is uncertain.20
Psychosis in AD can be induced by medication or delirium, or triggered by poor sensory perceptions. Psychotic symptoms occur more frequently during the moderate and advanced stages of AD, when patients present with visual hallucinations, delusions, or delusional misidentifications. As many as 10% to 20% of patients with AD experience hallucinations, typically visual. Delusions occur in 30% to 50% of AD patients, usually in the later stages of the disease. The most common delusional themes are infidelity, theft, and paranoia. Female sex is a risk factor for psychosis in AD. Delusions co-occur with aggression, anxiety, and aberrant motor behavior.
Visual hallucinations—mostly vivid, well-formed, false perceptions of insects, animals, or people—are the defining feature of DLB.21 Many patients recognize that they are experiencing visual hallucinations and can ignore them. DLB patients also may experience visual illusions, such as misperceiving household objects as living beings. Delusions—typically paranoid—are common among DLB patients, as are depression and anxiety.1 Agitation or aggressive behavior tends to occur late in the illness, if at all.
The causes of psychotic symptoms in DLB are not fully understood, but dopamine dysfunction likely is involved in hallucinations, delusions, and agitation, and serotonin dysfunction may be associated with depression and anxiety. Rapid eye movement (REM) sleep/wakefulness dysregulation, in which the dream imagery of REM sleep may occur during wakefulness, also has been proposed as a mechanism for visual hallucinations in DLB.22 In DLB, psychotic symptoms occur early and are a hallmark of this illness, whereas in AD they usually occur in the middle to late stages of the disease.
Motor symptoms. In AD, extrapyramidal symptoms (EPS) are common later in the disease, are strongly correlated with disease severity, and are a strong, independent predictor of depression severity.23 EPS are more common in DLB than in AD24 and DLB patients are at higher risk of developing EPS even with low doses of typical antipsychotics, compared with AD patients.25
Other symptoms. REM sleep behavior disorder (RBD) is characterized by enacting dreams—often violent—during REM sleep. RBD is common in DLB and many patients also have excessive daytime somnolence. Other sleep disorders in DLB include insomnia, obstructive sleep apnea, central sleep apnea, restless legs syndrome, and periodic limb movements during sleep.
In AD patients, common sleep behaviors include confusion in the early evening (“sundowning”) and frequent nighttime awakenings, often accompanied by wandering.26 Orthostatic hypotension, impotence, urinary incontinence, and constipation are common in DLB. Lack of insight concerning personal cognitive, mood, and behavioral state is highly prevalent in AD patients and more common than in DLB.
Diagnostic evaluation
Because there are no definitive clinical markers for DLB, diagnosis is based on a detailed clinical and family history from the patient and a reliable informant, as well as a physical, neurologic, and mental status examination that looks for associated noncognitive symptoms, and neuropsychological evaluation. Reasons DLB may be misdiagnosed include:
- Some “core” clinical features of DLB may not appear or may overlap with AD.
- Presence and severity of concurrent AD pathology in DLB may modify the clinical presentation, with decreased rates of hallucinations and parkinsonism and increased neurofibrillary tangles.
- Failure to reliably identify fluctuations—variations in cognition and arousal, such as periods of unresponsiveness while awake (“zoning out”), excessive daytime somnolence, and disorganized speech.27
Detecting and characterizing cognitive deficits in dementia patients using neuropsychological testing is important in establishing a clinical diagnosis, determining baseline levels of impairment, forming a prognosis, and initiating disease-specific treatments. Differences in neuropsychological findings in AD and DLB are outlined in Table 2.16,28-33 Several studies have suggested using these measures to differentiate patients with DLB from those with AD.20
Table 2
Diagnostic testing for Alzheimer’s disease and dementia with Lewy bodies
| Alzheimer’s disease | Dementia with Lewy bodies |
|---|---|
| Neuropsychological testing findings | |
| Relatively more impairment on verbal memory tasks, delayed recall, delayed recognition, and encoding and storing information.28 Dysfunction of episodic memory function | Relatively more impairment on attention or concentration, verbal fluency, visuoperceptual, visuoconstructive, visual memory tests, and frontal executive functions.28 Relatively preserved confrontation naming and verbal memory |
| MRI findings | |
| Diffuse cortical atrophy, relatively greater volume loss in hippocampus and medial temporal lobe structures (strong correlation with severity)29 | Mild generalized cerebral cortical atrophy with minimal hippocampal atrophy and relative preservation of medial temporal lobe structures30 |
| [18F]FDG PET | |
| Widespread metabolic deficits in neocortical association areas, with sparing of the basal ganglia, thalamus, cerebellum, primary sensory motor cortex, and visual cortex | Widespread cortical hypometabolism, more marked in primary visual and occipital association areas, and less severe in parietal, frontal, and anterior cingulate cortices.31 Severe cholinergic deafferentation of the neocortex, particularly in posterior cortical regions32 |
| Single photon emission computed tomography | |
| Parietotemporal hypoperfusion | Occipital hypoperfusion |
| 123I-FP-CIT SPECT (DaT scan) | |
| No significant loss of DaT | Low nigrostriatal terminal density of DaT caused by severe nigrostriatal degeneration16 |
| Myocardial scintigraphy with MIBG | |
| No significant change in MIBG uptake | Decreased MIBG uptake33 |
| 123I-FP-CIT: 123I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane; DaT: dopamine transporter; FDG PET: [18F]-fluoro-d-glucose positron emission tomography; MIBG: metaiodobenzylguanidine; SPECT: single photon emission computed tomography | |
Evidence is insufficient to support using electroencephalographic and polysomnographic studies when initially evaluating patients with dementia. Brain CT or MRI are recommended as part of the initial evaluation of dementia patients to exclude treatable causes of dementia and help clarify the differential diagnosis. Occipital hypometabolism and hypoperfusion demonstrated on PET and SPECT imaging have high sensitivity and specificity for differentiating AD from DLB.
To diagnose DLB more consistently, look for core features of the disease, RBD, antipsychotic hypersensitivity, and decreased striatal binding at presynaptic DaT sites.15 Abnormal (low binding) DaT activity is the most reliable diagnostic marker for DLB.34 Myocardial scintigraphy with MIBG is sensitive to pathologic changes of DLB before clinical expression and could overcome the difficulties of using clinical criteria alone to identify patients with DLB.35 MIBG scintigraphy may be preferred to DaT scan because it is less expensive and its sensitivity and specificity to DLB are independent of the presence of parkinsonism.35
For an overview of pharmacotherapy options for patients with AD or DLB, see Box 2.
Pharmacotherapy options for patients with Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) include cholinesterase inhibitors, memantine, antipsychotics, and other agents.
Cholinesterase inhibitors. Donepezil, rivastigmine, and galantamine are FDA-approved for treating AD. Their efficacy appears to be similar, so the choice of agent is based largely on cost, patient tolerability, and physician experience.
No medications are FDA-approved for treating DLB. Neocortical cholinergic activity assessed by choline acetyltransferase levels is more severely depleted in DLB than in AD, and this deficit is correlated with the presence of visual hallucinations and global severity of cognitive impairment.a Therefore, drugs that enhance central cholinergic function offer a therapeutic approach for DLB; cognitive and hallucinatory symptoms are the anticipated targets. Multiple anecdotal reports, open-label studies,b,c and 1 randomized, placebo-controlled triald suggest that cholinesterase inhibitors are efficacious in DLB, with reported benefits in cognition, fluctuations, psychotic symptoms, and parkinsonian symptoms. A 20-week randomized, double-blind, placebo-controlled multicenter studyd of patients with DLB found rivastigmine, 6 to 12 mg/d, was superior to placebo. Patients receiving rivastigmine exhibited significantly reduced anxiety, delusions, and hallucinations and significantly better performance on a computerized battery of neuropsychological tests, especially tasks that required sustained attention. Differences between rivastigmine and placebo disappeared after drug discontinuation.
Memantine is a noncompetitive antagonist of N-methyl-d-aspartate receptors that is effective in AD.e The possible involvement of glutamate in DLB has provided a rationale for treating DLB with memantine. Two randomized controlled trials in DLB found that patients treated with memantine for 24 weeks performed better on Clinical Global Impression of Change, but not on most other secondary outcome measures.f,g In both studies, memantine was well tolerated. However, other studies have noted worsening of delusions and hallucinations with memantine in DLB patients.h
Antipsychotics. Agitation is common in moderate and advanced AD. Atypical antipsychotics have been used with variable efficacy to treat agitation, but their use is associated with excess mortality. DLB patients pose a considerable therapeutic challenge because antipsychotics—the mainstay of treatment of psychosis and behavioral problems in most other disorders—can provoke severe, irreversible, and often fatal sensitivity reactions in this type of dementia.i A 2- to 3-fold increased mortality risk associated with antipsychotic sensitivity reactions in DLB is partly mediated via acute blockade of postsynaptic dopamine D2 receptors in the striatum. For severe and disabling psychosis, a trial of a cholinesterase inhibitor and/or lowering the dose of antiparkinsonian medication should be considered first. In urgent situations, small doses of an atypical antipsychotic that is least associated with parkinsonism side effects—such as quetiapine or aripiprazole—should be used.
Other treatments. Treatment of parkinsonian symptoms in DLB patients is similar to that for Parkinson’s disease, but the risk of psychotic symptoms in DLB warrants a conservative approach. Levodopa seems to be more effective than dopamine agonists and produces fewer side effects.j Rapid eye movement sleep behavior disorder often responds to low doses of clonazepam (0.25 to 1.5 mg). Depression and anxiety disorders are common in AD at all stages and their treatment is not fundamentally different than in geriatric patients without dementia. Selective serotonin reuptake inhibitors and electroconvulsive therapy have been used successfully in depressed patients with AD or DLB.k,l
Disease-modifying treatments. Researchers are evaluating an array of antiamyloid and neuroprotective therapeutic approaches for AD based on the hypothesis that amyloid-beta protein plays a pivotal role in disease onset and progression. Interventions that reduce amyloid production, limit aggregation, or increase clearance may block the cascade of events comprising AD pathogenesis. Reducing tau hyperphosphorylation, limiting oxidation and excitotoxicity, and controlling inflammation also may be beneficial strategies. Potentially neuroprotective and restorative treatments such as neurotrophins, neurotrophic factor enhancers, and stem cell-related approaches also are being investigated.
There are no large-scale studies of disease-modifying treatments for DLB. Potential areas of research include the relationship between proteasome function and a-synuclein pathology, the role of beta-synuclein, and the impact of alterations to alpha-synuclein on its propensity to aggregate.
References
a. Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931-1934.
b. Beversdorf DQ, Warner JL, Davis RA, et al. Donepezil in the treatment of dementia with lewy bodies. Am J Geriatr Psychiatry. 2004;12(5):542-544.
c. Edwards K, Royall D, Hershey L, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401-405.
d. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
e. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324.
f. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
g. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969-977.
h. Ridha BH, Josephs KA, Rossor MN. Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology. 2005;65(3):481-482.
i. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
j. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
k. Swartz M, Barak Y, Mirecki I, et al. Treating depression in Alzheimer’s disease: integration of differing guidelines. Int Psychogeriatr. 2000;12(3):353-358.
l. Takahashi S, Mizukami K, Yasuno F, et al. Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics. 2009;9(2):56-61.
Related Resources
- Hanyu H, Sato T, Hirao K, et al. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16(2):212-217.
- Walker Z, McKeith I, Rodda J, et al. Comparison of cognitive decline between dementia with Lewy bodies and Alzheimer’s disease: a cohort study. BMJ Open. 2012;2:e000380.
Drug Brand Names
- Aripiprazole • Abilify
- Clonazepam • Klonopin
- Donepezil • Aricept
- Galantamine • Razadyne, Reminyl
- Levodopa • Dopar, Larodopa
- Memantine • Namenda
- Quetiapine • Seroquel
- Rivastigmine • Exelon
Disclosure
Drs. Bishnoi and Manepalli report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg serves as a consultant to Forest, Janssen, Novartis, and Pfizer. His department receives research funding from Novartis, Janssen, and Pfizer.
1. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
2. Buracchio T, Arvanitakis Z, Gorbien M. Dementia with Lewy bodies: current concepts. Dement Geriatr Cogn Disord. 2005;20(5):306-320.
3. Fujishiro H, Iseki E, Higashi S, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19-23.
4. Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(suppl):S73-S78.
5. McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
6. Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287(18):2335-2338.
7. Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561-566.
8. Bradshaw J, Saling M, Hopwood M, et al. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 2004;75(3):382-387.
9. Singleton AB, Wharton A, O’Brien KK, et al. Clinical and neuropathological correlates of apolipoprotein E genotype in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(4):167-175.
10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
11. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
12. Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315-325.
13. Parnetti L, Balducci C, Pierguidi L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in dementia with Lewy bodies. Neurobiol Dis. 2009;34(3):484-486.
14. Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273-278.
15. McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305-313.
16. Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176-1181.
17. Bradshaw JM, Saling M, Anderson V, et al. Higher cortical deficits influence attentional processing in dementia with Lewy bodies, relative to patients with dementia of the Alzheimer’s type and controls. J Neurol Neurosurg Psychiatry. 2006;77(10):1129-1135.
18. Weiner MF, Hynan LS, Parikh B, et al. Can Alzheimer’s disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245-250.
19. Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63(10):1450-1456.
20. Ferman TJ, Smith GE, Boeve BF, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clin Neuropsychol. 2006;20(4):623-636.
21. McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902-905.
22. Boeve BF, Silber MH, Ferman TJ, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622-630.
23. Portet F, Scarmeas N, Cosentino S, et al. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120-1126.
24. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
25. Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer’s disease. Expert Rev Neurother. 2007;7(11):1499-1516.
26. Ancoli-Israel S, Klauber MR, Gillin JC, et al. Sleep in non-institutionalized Alzheimer’s disease patients. Aging (Milano). 1994;6(6):451-458.
27. Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181-187.
28. Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148-165.
29. Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484-489.
30. Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(pt 1):195-203.
31. Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48(5):704-711.
32. Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885-892.
33. Fujishiro H, Nakamura S, Kitazawa M, et al. Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy. J Neurol Sci. 2012;315(1-2):115-119.
34. O’Brien JT, McKeith IG, Walker Z, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194:34-39.
35. Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66(12):1850-1854.
1. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124.
2. Buracchio T, Arvanitakis Z, Gorbien M. Dementia with Lewy bodies: current concepts. Dement Geriatr Cogn Disord. 2005;20(5):306-320.
3. Fujishiro H, Iseki E, Higashi S, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19-23.
4. Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(suppl):S73-S78.
5. McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872.
6. Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287(18):2335-2338.
7. Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561-566.
8. Bradshaw J, Saling M, Hopwood M, et al. Fluctuating cognition in dementia with Lewy bodies and Alzheimer’s disease is qualitatively distinct. J Neurol Neurosurg Psychiatry. 2004;75(3):382-387.
9. Singleton AB, Wharton A, O’Brien KK, et al. Clinical and neuropathological correlates of apolipoprotein E genotype in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(4):167-175.
10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
11. Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
12. Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315-325.
13. Parnetti L, Balducci C, Pierguidi L, et al. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in dementia with Lewy bodies. Neurobiol Dis. 2009;34(3):484-486.
14. Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273-278.
15. McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305-313.
16. Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78(11):1176-1181.
17. Bradshaw JM, Saling M, Anderson V, et al. Higher cortical deficits influence attentional processing in dementia with Lewy bodies, relative to patients with dementia of the Alzheimer’s type and controls. J Neurol Neurosurg Psychiatry. 2006;77(10):1129-1135.
18. Weiner MF, Hynan LS, Parikh B, et al. Can Alzheimer’s disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16(4):245-250.
19. Stavitsky K, Brickman AM, Scarmeas N, et al. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63(10):1450-1456.
20. Ferman TJ, Smith GE, Boeve BF, et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clin Neuropsychol. 2006;20(4):623-636.
21. McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902-905.
22. Boeve BF, Silber MH, Ferman TJ, et al. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622-630.
23. Portet F, Scarmeas N, Cosentino S, et al. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120-1126.
24. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ. 1992;305(6855):673-678.
25. Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer’s disease. Expert Rev Neurother. 2007;7(11):1499-1516.
26. Ancoli-Israel S, Klauber MR, Gillin JC, et al. Sleep in non-institutionalized Alzheimer’s disease patients. Aging (Milano). 1994;6(6):451-458.
27. Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181-187.
28. Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31(2):148-165.
29. Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484-489.
30. Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(pt 1):195-203.
31. Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48(5):704-711.
32. Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885-892.
33. Fujishiro H, Nakamura S, Kitazawa M, et al. Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy. J Neurol Sci. 2012;315(1-2):115-119.
34. O’Brien JT, McKeith IG, Walker Z, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry. 2009;194:34-39.
35. Yoshita M, Taki J, Yokoyama K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66(12):1850-1854.
Therapeutic neuromodulation
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Alcohol withdrawal
Stalked by a ‘patient’
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Delusions and threats
For over 20 months, Ms. I, age 48, sends a psychiatric resident letters and postcards that total approximately 3,000 pages and come from dozens of return addresses. Ms. I expresses romantic feelings toward the resident and believes that he was her physician and prescribed medications, including “mood stabilizers.” The resident never treated Ms. I; to his knowledge, he has never interacted with her.
Ms. I describes the resident’s refusal to continue treating her as “abandonment” and states that she is contemplating self-harm because of this rejection. In her letters, Ms. I admits that she was a long-term patient in a state psychiatric hospital in her home state and suffers from persistent auditory hallucinations. She also wants a romantic relationship with the resident and repeatedly threatens the resident’s female acquaintances and former romantic partners whose relationships she had surmised from news articles available on the Internet. Ms. I also threatens to strangle the resident. The resident sends her multiple written requests that she cease contact, but they are not acknowledged.
The authors’ observations
Stalking—repeated, unwanted attention or communication that would cause a reasonable person fear—is a serious threat for many psychiatric clinicians.1 Prevalence rates among mental health care providers range from 3% to 21%.2,3 Most stalkers have engaged in previous stalking behavior.3
Being stalked is highly distressing,4 and mental health professionals often do not reveal such experiences to colleagues.5 Irrational feelings of guilt or embarrassment, such as being thought to have poorly managed interactions with the stalker, often motivate a self-imposed silence (Table 1).6 This isolation may foster anxiety, interfere with receiving problem-solving advice, and increase physical vulnerability. In the case involving Ms. I, the psychiatric resident’s primary responsibility is safeguarding his own physical and psychological welfare.
Clinicians who work in a hospital or other institutional setting who are being stalked should inform their supervisors and the facility’s security personnel. Security personnel may be able to gather data about the stalker, decrease the stalker’s ability to communicate with the victim, and reduce unwanted physical access to the victim by distributing a photo of the stalker or installing a camera or receptionist-controlled door lock in patient entryways. Security personnel also may collaborate with local law enforcement. Having a third party respond to a stalker’s aggressive behavior—rather than the victim responding directly—avoids rewarding the stalker, which may generate further unwanted contact.7 Any intervention by the victim may increase the risk of violence, creating an “intervention dilemma.” Resnick8 argues that before deciding how best to address the stalker’s behavior, a stalking victim must “first separate the risk of continued stalking from the risk that the stalker will commit a violent act.”
Mental health professionals in private practice who are being stalked should consider retaining an attorney. An attorney often can maintain privacy of communications regarding the stalker via the attorney-client and attorney-work product privileges, which may help during legal proceedings.
Table 1
Factors that can impede psychiatrists from reporting stalking
| Fear of being perceived as a failure |
| Embarrassment |
| High professional tolerance for antisocial and threatening behavior |
| Misplaced sense of duty |
| Source: Reference 6 |
RESPONSE: Involving police
Over 2 months, Ms. I phones the resident’s home 105 times (the resident screens the calls). During 1 call, she states that she is hidden in a closet in her home and will hurt herself unless the resident “resumes” her psychiatric care. The resident contacts police in his city and Ms. I’s community, but authorities are reluctant to act when he acknowledges that he is not Ms. I’s psychiatrist and does not know her. Police officers in Ms. I’s hometown tell the resident no one answered the door when they visited her home. They state that they would enter the residence forcibly only if Ms. I’s physician or a family member asked them to do so, and because the resident admits that he is not her psychiatrist, they cannot take further action. Ms. I leaves the resident a phone message several hours later to inform him she is safe.
The authors’ observations
Stalking-induced countertransference responses may lead a psychiatrist to unwittingly place himself in harm’s way. For example, intense rage at a stalker’s request for treatment may generate guilt that motivates the psychiatrist to agree to treat the stalker. Feelings of helplessness may produce a frantic desire to do something even when such activity is ill-advised. Psychiatrists may develop a tolerance for antisocial or threatening behavior—which is common in mental health settings—and could accept unnecessary risks.
A psychiatrist who is being stalked may be able to assist a mentally ill stalker in a way that does not create a duty to treat and does not expose the psychiatrist to harm, such as contacting a mobile crisis intervention team, a mental health professional who recently treated the stalker, a family member of the stalker, or law enforcement personnel. A psychiatrist who is thrust from the role of helper to victim and must protect his or her own well-being instead of attending to a patient’s welfare is prone to suffer substantial countertransference distress.
The situation with Ms. I was particularly challenging because the resident did not know her complete history and therefore had little information to gauge how likely she was to act on her aggressive threats. Factors that predict future violence include:
- a history of violence
- significant prior criminality
- young age at first arrest
- concomitant substance abuse
- male sex.9
Unfortunately, other than sex, this data regarding Ms. I could not be readily obtained.
A psychiatrist’s duty
Although sympathetic to his stalker’s distress, the resident did not want to treat this woman, nor was he ethically or legally obligated to do so. An individual’s wish to be treated by a particular psychiatrist does not create a duty for the psychiatrist to satisfy this wish.10 State-based “Good Samaritan” laws encourage physicians to assist those in acute need by shielding them from liability, as long as they reasonably act within the scope of their expertise.11 However, they do not require a physician to care for an individual in acute need. A delusional wish for treatment or a false belief of already being in treatment does not create a duty to care for a person.
OUTCOME: Seeking help
Ms. I’s phone calls and letters continue. The resident discusses the situation with his associate residency director, who refers him to the hospital’s legal and investigative staffs. Based on advice from the hospital’s private investigator, the resident sends Ms. I a formal “cease and desist” letter that threatens her with legal action and possible jail time. The staff at the front desk of the clinic where the resident works and the hospital’s security department are instructed to watch for a visitor with Ms. I’s name and description, although the hospital’s investigator is unable to obtain a photograph of her. Shortly after the resident sends the letter, Ms. I ceases communication.
The authors’ observations
This case is unusual because most stalking victims know their stalkers. Identifying a stalker’s motivation can be helpful in formulating a risk assessment. One classification system recognizes 5 categories of stalkers: rejected, intimacy seeking, incompetent, resentful, and predatory (Table 2).1 Rejected stalkers appear to pose the greatest risk of violence and homicide.8 However, all stalkers may pose a risk of violence and therefore all stalking behavior should be treated seriously.
Table 2
Classification of stalkers
| Category | Common features |
|---|---|
| Rejected | Most have a personality disorder; often seeking reconciliation and revenge; most frequent victims are ex-romantic partners, but also target estranged relatives, former friends |
| Intimacy seeking | Erotomania; “morbid infatuation” |
| Incompetent | Lacking social skills; often have stalked others |
| Resentful | Pursuing a vendetta; generally feeling aggrieved |
| Predatory | Often comorbid with paraphilias; may have past convictions for sex offenses |
| Source: Adapted from reference 1 | |
Responding to a stalker
The approach should be tailored to the stalker’s characteristics.12 Silence—ie, lack of acknowledgement of a stalker’s intrusions—is one tactic.13 Consistent and persistent lack of engagement may bore the stalker, but also may provoke frustration or narcissistic or paranoia-fueled rage, and increased efforts to interact with the mental health professional. Other responses include:
- obtaining a protection or restraining order
- promoting the stalker’s participation in adversarial civil litigation, such as a lawsuit
- issuing verbal counterthreats.
Restraining orders are controversial and assessments of their effectiveness vary.14 How well a restraining order works may depend on the stalker’s:
- ability to appreciate reality, and how likely he or she is to experience anxiety when confronted with adverse consequences of his or her actions
- how consistently, rapidly, and harshly the criminal justice system responds to violations of restraining orders.
Restraining orders also may provide the victim a false sense of security.15 One of her letters revealed that Ms. I violated a criminal plea arrangement years earlier, which suggests she was capable of violating a restraining order.
Litigation. A stalker may initiate civil litigation against the victim to feel that he or she has an impact on the victim, which may reduce the stalker’s risk of violence if he or she is emotionally engaged in the litigation. Based on the authors’ experience, as long as the stalker is talking, he or she generally is less likely to act out violently and terminate a satisfying process. Adversarial civil litigation could give a stalker the opportunity to be “close” to the victim and a means of expressing aggressive wishes. The benefit of litigation lasts only as long as the case persists and the stalker believes he or she may prevail. In one of her letters, Ms. I bragged that she had represented herself as a pro se litigant in a complex civil matter, suggesting that she might be constructively channeled into litigation.
Promoting litigation carries significant risk.16 Being a defendant in pro se litigation may be emotionally and financially stressful. This approach may be desirable if the psychiatrist’s institution is willing to offer substantial support. For example, an institution may provide legal assistance—including helping to defray the cost of litigation—and litigation-related scheduling flexibility. An attorney may serve as a boundary between the victim and the pro se litigant’s sometimes ceaseless, time-devouring, anxiety-inducing legal maneuvers.
Counterthreats. Warning a stalker that he or she will face severe civil and criminal consequences if his or her behavior continues can make clear that his or her conduct is unacceptable.17 Such warnings may be delivered verbally or in writing by a legal representative, law enforcement personnel, a private security agent, or the victim.
Issuing a counterthreat can be risky. Stalkers with antisocial or narcissistic personality features may perceive a counterthreat as narcissistically diminishing, and to save face will escalate their stalking in retaliation. Avoid counterthreats if you believe the stalker might be psychotic because destabilizing such an individual—such as by precipitating a short psychotic episode—may increase unpredictability and diminish their responsive to interventions.
Ms. I’s contact with the resident lasted approximately 20 months, slightly less than the average 26 months reported in a survey of mental health professionals.3 Because stalkers are unpredictable, the psychiatric resident remains cautious.
Related Resources
- National Center for Victims of Crime. Stalking resource center. www.victimsofcrime.org/our-programs/stalking-resource-center.
- Mullen PE, Pathé M, Purcell R. Stalkers and their victims. New York, NY: Cambridge University Press; 2009.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
1. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
2. Sandberg DA, McNiel DE, Binder RL. Stalking threatening, and harassing behavior by psychiatric patients toward clinicians. J Am Acad Psychiatry Law. 2002;30(2):221-229.
3. McIvor R, Potter L, Davies L. Stalking behavior by patients towards psychiatrists in a large mental health organization. Int J Soc Psychiatry. 2008;54(4):350-357.
4. Mullen PE, Pathé M. Stalking. Crime and Justice. 2002;29:273-318.
5. Bird S. Strategies for managing and minimizing the impact of harassment and stalking by patients. ANZ J Surg. 2009;79(7-8):537-538.
6. Sinwelski SA, Vinton L. Stalking: the constant threat of violence. Affilia. 2001;16(1):46-65.
7. Meloy JR. Commentary: stalking threatening, and harassing behavior by patients—the risk-management response. J Am Acad Psychiatry Law. 2002;30(2):230-231.
8. Resnick PJ. Stalking risk assessment. In: Pinals DA, ed. Stalking: psychiatric perspectives and practical approaches. New York, NY: Oxford University Press; 2007:61–84.
9. Dietz PE. Defenses against dangerous people when arrest and commitment fail. In: Simon RI, ed. American Psychiatric Press review of clinical psychiatry and the law. 1st ed. Washington, DC: American Psychiatric Press; 1989:205–219.
10. Hilliard J. Termination of treatment with troublesome patients. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:216–224.
11. Paterick TJ, Paterick BB, Paterick TE. Implications of Good Samaritan laws for physicians. J Med Pract Manage. 2008;23(6):372-375.
12. MacKenzie RD, James DV. Management and treatment of stalkers: problems options, and solutions. Behav Sci Law. 2011;29(2):220-239.
13. Fremouw WJ, Westrup D, Pennypacker J. Stalking on campus: the prevalence and strategies for coping with stalking. J Forensic Sci. 1997;42(4):666-669.
14. Nicastro AM, Cousins AV, Spitzberg BH. The tactical face of stalking. Journal of Criminal Justice. 2000;28(1):69-82.
15. Spitzberg BH. The tactical topography of stalking victimization and management. Trauma Violence Abuse. 2002;3(4):261-288.
16. Pathé M, MacKenzie R, Mullen PE. Stalking by law: damaging victims and rewarding offenders. J Law Med. 2004;12(1):103-111.
17. Lion JR, Herschler JA. The stalking of physicians by their patients. In: Meloy JR. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:163–173.
Safer use of benzodiazepines for alcohol detoxification
Clinicians often use the symptom-triggered Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised (CIWA-Ar)1 to assess patients’ risk for alcohol withdrawal because it has well-documented reliability, reproducibility, and validity based on comparison with ratings by expert clinicians.2,3 The CIWA-Ar commonly is used to determine when to administer lorazepam to limit or prevent morbidity and mortality in patients who are at risk of or are experiencing alcohol withdrawal. Refined to a list of 10 signs and symptoms, the CIWA-Ar is easy to administer and useful in a variety of clinical settings. The maximum score is 67, and patients with a score >15 are at increased risk for severe alcohol withdrawal.1 For a downloadable copy of the CIWA-Ar, click here.
Despite the benefits of using the CIWA-Ar, qualitative description of certain alcohol withdrawal symptoms is prone to subjective misinterpretation and can result in falsely elevated scores, excessive benzodiazepine administration, and associated sequelae.4 This article describes such a scenario, and examines factors that can contribute to a falsely elevated CIWA-Ar score.
CASE REPORT: Resistant alcohol withdrawal
Mr. J, age 24, is referred to the consultation-liaison service at our teaching hospital for “overall psychiatric assessment and help with alcohol withdrawal.” When brought to the hospital, Mr. J was experiencing diaphoresis and tachycardia. During the interview, he says he “experiences withdrawal symptoms all the time, so I am familiar with the signs.”
Mr. J is cooperative with the interview. Psychomotor agitation or retardation is not noted. His speech is goal-directed, his mood is “calm,” and his affect is within normal range. His thought content is devoid of psychoses or lethal ideations. On Mini-Mental State Examination, Mr. J scores 28 out of 30, which indicates normal cognitive functioning. He reports drinking eight 40-oz bottles of beer daily for the past 3 months. He started drinking alcohol at age 14 and has had only one 1-year period of sobriety. He denies using illicit drugs and his urine drug screen is unremarkable. Mr. J has a history of delirium tremens (DTs), no significant medical history, and was not taking any medications when admitted. His psychiatric history includes generalized anxiety disorder (GAD) and antisocial personality disorder and his family history is significant for alcohol dependence.
Laboratory workup is unremarkable except for a blood alcohol level of 0.23%. Review of systems is significant for mild tremor but no other symptoms of alcohol withdrawal. Physical examination is within normal limits.
Mr. J is started on a symptom-trigger alcohol detoxification protocol using the CIWA-Ar. Based on an elevated CIWA-Ar score of 33, he receives lorazepam IV, 11 mg on his first day of hospitalization and 8 mg on the second day. On the third day, Mr. J is agitated and pulls his IV lines in an attempt to leave. Over the next 24 hours, his blood pressure ranges from 136/90 mm Hg to 169/92 mm Hg and his pulse ranges from 94 to 115 beats per minute. He is given lorazepam, 30 mg, and is transferred to the intensive care unit (ICU).
At this time, Mr. J’s Delirium Rating Scale (DRS) score is 20 (maximum: 32). He remains in the ICU on lorazepam, 25 mg/hr. After 3 days in the ICU, lorazepam is titrated and stopped 2 days later. After lorazepam is stopped, Mr. J’s DRS score is 0, his vital signs are stable, and he no longer demonstrates signs or symptoms of DTs or alcohol withdrawal. He is discharged 1 day later.
Symptom-triggered treatment
Alcohol withdrawal symptoms mainly are caused by the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems; benzodiazepines are the standard of care (Box).5,6 Mr. J had a history of DTs, which is a risk factor for more severe alcohol withdrawal symptoms and recurrence of DTs.7 Some authors report that fixed dosing intervals are the “gold standard therapy” for alcohol withdrawal, and may be preferable for patients with a history of DTs.8 However, Mr. J was placed on a symptom-triggered protocol, which is standard at our hospital. The decision to implement this protocol was based on concerns of oversedation and possible respiratory suppression. Clinical trials have demonstrated that compared with fixed scheduled therapy for alcohol withdrawal, symptom-triggered protocols result in a reduced need for benzodiazepines (Table).
This treatment strategy requires frequent patient reevaluations—particularly early on—with attention to signs and symptoms of alcohol withdrawal and excessive sedation from medications. Additionally, although most patients with alcohol withdrawal respond to standard treatment that includes benzodiazepines, optimal nutrition, and good supportive care, a subgroup may resist therapy (resistant alcohol withdrawal). Therefore, Mr. J—and others with resistant alcohol withdrawal—may require large doses of benzodiazepines and additional sedatives and undergo complicated hospitalizations.9 Nonetheless, as exemplified by Mr. J, symptom-triggered protocols for alcohol withdrawal can result in potential morbidity and mortality.
Common symptoms of alcohol withdrawal include autonomic hyperactivity, tremor, insomnia, nausea, vomiting, agitation, anxiety, grand mal seizures, and transient visual, tactile, or auditory hallucinations.5 These symptoms result, in part, from the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems. Alcohol acutely enhances presynaptic GABA release through allosteric modulation at GABAA receptors and inhibits glutamate function through antagonism of N-methyl-d-aspartate (NMDA) receptors. Chronic alcohol exposure elicits compensatory downregulated GABAA and upregulated NMDA expression.
When alcohol intake abruptly stops and its acute effects dissipate, the sudden reduction in GABAergic tone and increase in glutamatergic tone cause alcohol withdrawal symptoms.6 Benzodiazepines, which bind at the benzodiazepine site on the GABAA receptor and, similar to alcohol, acutely enhance GABA and inhibit glutamate signaling, are the standard of care for alcohol withdrawal because they reduce anxiety and the risk of seizures and delirium tremens, which is a severe form of alcohol withdrawal characterized by disturbance in consciousness and cognition and hallucinations.5,6
Table
Benefits of symptom-triggered vs fixed scheduled therapy for alcohol withdrawal
| ST | FS | Benefits of ST | |
|---|---|---|---|
| Efficacy in alcohol withdrawal | Yes | Yes | |
| Flexibility in dosing with fluctuations in CIWA-Ar score | Yes | No | Less medication can be given overall if alcohol withdrawal signs resolve rapidly |
| Lower total benzodiazepine doses | + | – | Smaller chance of side effects such as oversedation, paradoxical agitation, delirium due to benzodiazepine intoxication, or respiratory depression |
| Fewer complications of higher benzodiazepine doses | + | – | Reduced risk of prolonged hospitalization, morbidity from aspiration pneumonia, or need to administer a reversal agent such as flumazenil |
| + = more likely; – = less likely CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; FS: fixed scheduled; ST: symptom-triggered Bibliography Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063. Cassidy EM, O’Sullivan I, Bradshaw P, et al. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen [published online ahead of print October 19, 2011]. Emerg Med J. doi: 10.1136/emermed-2011-200509. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. DeCarolis DD, Rice KL, Ho L, et al. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27(4):510-518. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701. Weaver MF, Hoffman HJ, Johnson RE, et al. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis. 2006;25(2):17-24. | |||
Factors influencing CIWA-Ar score
Vital signs monitoring. One limitation of the CIWA-Ar is that vital signs—an objective measurement of alcohol withdrawal— are not used to determine the score. Indeed, Mr. J presented with vital sign dysregulation. However, research suggests that the best predictor of high withdrawal scores includes groups of symptoms rather than individual symptoms.10 In that study, pulse and blood pressure did not correlate with withdrawal severity. Pulse and blood pressure elevations occur in alcohol withdrawal, but other signs and symptoms are more reliable in assessing withdrawal severity. This is clinically important because physicians often prescribe medications for alcohol withdrawal treatment based on pulse and blood pressure measures.1 This needs to be balanced against research that found a systolic blood pressure >150 mm Hg and axillary temperature >38°C can predict development of DTs in patients experiencing alcohol withdrawal.7
Lorazepam-induced disinhibition. Benzodiazepines affect functions associated with processing within the orbital prefrontal cortex,11 including response inhibition and socially acceptable behavior, and impairment in this functioning can result in behavioral disinhibition.12 This effect could account for the apparent paradoxical clinical observation of aggression in benzodiazepine-sedated patients.13 Because agitation is scored on the CIWA-Ar,1,10 falsely elevated scores caused by interpreting benzodiazepine-induced aggression as agitation could result in patients (such as Mr. J) receiving more lorazepam, therefore perpetuating this cycle.
Comorbid anxiety disorders also could falsely accentuate CIWA-Ar scores. For example, the odds of an alcohol dependence diagnosis are 2 to 3 times greater among patients with an anxiety disorder.14 Additionally, the lifetime prevalence of comorbid alcohol dependence for patients with GAD—such as Mr. J— is 30% to 35%.14,15
Alcohol withdrawal can be more severe in patients with alcohol dependence and anxiety disorders because evidence suggests the neurochemical processes underlying both are similar and potentially additive. Studies have shown that these dual diagnosis patients experience more severe symptoms of alcohol withdrawal as assessed by total CIWA-Ar score than those without an anxiety disorder.15 Although such patients may require more aggressive pharmacologic treatment, the dangers of higher benzodiazepine dosages may be even greater.
Benzodiazepine-induced delirium. A recent meta-analysis suggested that benzodiazepines may be associated with an increased risk of delirium.16 Longer-acting benzodiazepines may be associated with increased risk of delirium compared with short-acting agents, and higher doses during a 24-hour period may be associated with increased risk of delirium compared with lower doses. However, wide confidence intervals imply significant uncertainty with these results, and not all patients in the studies reviewed were undergoing alcohol detoxification.16 Benzodiazepines have been reported to accentuate delirium when used to treat DTs.17
We postulate that although Mr. J received lorazepam—a short- to moderate-acting benzodiazepine with a half-life of 12 to 16 hours18—the cumulative dose was high enough to have accentuated—rather than attenuated—delirium.16
Personality disorders. Comorbid alcohol use disorders (AUDs) and personality disorders are well documented. One study found the prevalence of personality disorders in AUDs ranged from 22% to 78%.19 Psychologically, drinking to cope with negative subjective states and emotions (coping motives) and drinking to enhance positive emotions (enhancement motives) may explain the relation between Cluster B personality disorders and AUDs.20
Research on prefrontal functioning in alcoholics and individuals with antisocial personality disorder symptoms has suggested that both groups may be impaired on tasks sensitive to compromised orbitofrontal functioning.21 The orbitofrontal system is essential for maintaining normal inhibitory influences on behavior.22 Benzodiazepines can increase the likelihood of developing disinhibition or impulsivity, which are symptoms of antisocial personality disorder. Because Mr. J had antisocial personality disorder, treating his alcohol withdrawal with a benzodiazepine could have accentuated these symptoms, which were subsequently “treated” with additional lorazepam, therefore worsening the cycle.
Medical comorbidities. The CIWA-Ar relies on autonomic signs and subjective symptoms and was not designed for use in nonverbal patients in the ICU. It is possible that the presence of other acute illnesses may contribute to increased CIWA-Ar scores, but we are unaware of any studies that have evaluated such factors.23
However, tremor, which is scored on the CIWA-Ar, can falsely elevate scores if it is caused by something other than acute alcohol withdrawal. Although essential tremors attenuate with acute alcohol use, chronic alcohol use can result in parkinsonism with a resting tremor, and cerebellar degeneration, which can include an action tremor and cerebellar 3-Hz leg tremor.24 Finally, hepatic encephalopathy—a neuropsychiatric syndrome characterized by disturbances in consciousness, mood, behavior, and cognition—can occur in patients with advanced liver disease, which may be precipitated by alcohol use. The clinical presentation and symptom severity of hepatic encephalopathy varies from minor cognitive impairment to gross disorientation, confusion, and agitation,25 all of which can elevate CIWA-Ar scores.
The role of disinhibition
Disinhibition could serve as the “final common pathway” through which CIWA-Ar scores can be falsely elevated.11 For a Figure that illustrates this, see below. Mr. J presented with several variables that could have elevated his CIWA-Ar score; additional potential factors include other psychiatric diagnoses such as bipolar disorder, opiate withdrawal, dementia, drug-seeking behavior, or malingering.26,27
Treating disinhibition in patients with alcohol withdrawal. Continuing to administer escalating doses of benzodiazepines is counterintuitive for benzodiazepine-induced disinhibition. In a study of alcohol withdrawal in rats, antipsychotics evaluated had some beneficial effects on alcohol withdrawal signs.28 In this study, the comparative effectiveness of atypical antipsychotics was as follows: risperidone = quetiapine > ziprasidone > clozapine > olanzapine.
The American Society of Addiction Medicine’s practice guideline advises against using antipsychotics as the sole agent for DTs because these agents are associated with a longer duration of delirium, higher complication rates, and higher mortality.28 However, antipsychotics have a role as an adjunct to benzodiazepines when benzodiazepines don’t sufficiently control agitation, thought disorder, or perceptual disturbances. Although haloperidol use is well established in this scenario, chlorpromazine is contraindicated because it is epileptogenic, and little information is available on atypical antipsychotics.29 If Mr. J had not responded to tapering lorazepam, evidence would support using haloperidol.
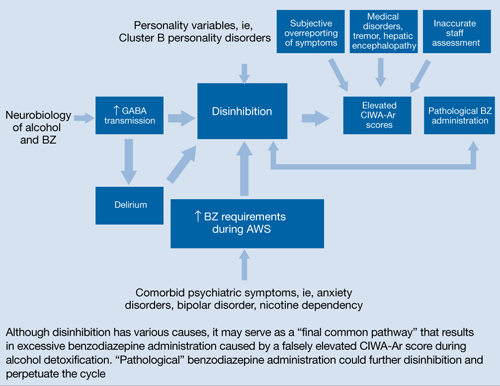
Figure: Unifying concept for pathological BZ administration during alcohol withdrawal syndrome: Disinhibition
AWS: alcohol withdrawal syndrome; BZ: benzodiazepine; CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; GABA: γ-aminobutyric acid
Source: Reference 11Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health & Research World. 1998;22(1):38-43. http://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf.
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537.
Drug Brand Names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Flumazenil • Romazicon
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Spiegel is on the speaker’s bureau of Sunovion Pharmaceuticals.
Drs. Kumari and Petri report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Amy Herndon for her help in preparing this article.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knott DH, Lerner WD, Davis-Knott T, et al. Decision for alcohol detoxication: a method to standardize patient evaluation. Postgrad Med. 1981;69(5):65-69, 72-75, 78.
3. Wiehl WO, Hayner G, Galloway G. Haight Ashbury Free Clinics’ drug detoxification protocols—Part 4: alcohol. J Psychoactive Drugs. 1994;26(1):57-59.
4. Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256-261.
5. Diagnostic and statistical manual of mental disorders, 4th ed text rev. Washington DC: American Psychiatric Association; 2000.
6. Schacht JP, Randall PK, Waid LR, et al. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011;35(11):2030-2038.
7. Monte R, Rabuñal R, Casariego E, et al. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20(7):690-694.
8. Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
9. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60.
10. Pittman B, Gueorguieva R, Krupitsky E, et al. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31(4):612-618.
11. Deakin JB, Aitken MR, Dowson JH, et al. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (Berl). 2004;173(1-2):88-97.
12. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(pt 9):2502-2512.
13. Jones KA, Nielsen S, Bruno R, et al. Benzodiazepines - their role in aggression and why GPs should prescribe with caution. Aust Fam Physician. 2011;40(11):862-865.
14. Scott EL, Hulvershorn L. Anxiety disorders with comorbid substance abuse. Psychiatric Times. 2011; 28(9).
15. Faingold CL, Knapp DJ, Chester JA, et al. Integrative neurobiology of the alcohol withdrawal syndrome—from anxiety to seizures. Alcohol Clin Exp Res. 2004;28(2):268-278.
16. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23-29.
17. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
18. Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106(12):2086-2109.
19. Mellos E, Liappas I, Paparrigopoulos T. Comorbidity of personality disorders with alcohol abuse. In Vivo. 2010;24(5):761-769.
20. Tragesser SL, Sher KJ, Trull TJ, et al. Personality disorder symptoms, drinking motives, and alcohol use and consequences: cross-sectional and prospective mediation. Exp Clin Psychopharmacol. 2007;15(3):282-292.
21. Oscar-Berman M, Valmas MM, Sawyer KS, et al. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309-326.
22. Dom G, De Wilde B, Hulstijn W, et al. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol Alcohol. 2006;41(4):412-420.
23. de Wit M, Jones DG, Sessler CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994-1003.
24. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25(14):2274-2284.
25. Crone CC, Gabriel GM, DiMartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics. 2006;47(3):188-205.
26. Reoux JP, Oreskovich MR. A comparison of two versions of the clinical institute withdrawal assessment for alcohol: the CIWA-Ar and CIWA-AD. Am J Addict. 2006;15(1):85-93.
27. Gray S, Borgundvaag B, Sirvastava A, et al. Feasibility and reliability of the SHOT: a short scale for measuring pretreatment severity of alcohol withdrawal in the emergency department. Acad Emerg Med. 2010;17(10):1048-1054.
28. Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47(1):33-41.
29. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862.
Clinicians often use the symptom-triggered Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised (CIWA-Ar)1 to assess patients’ risk for alcohol withdrawal because it has well-documented reliability, reproducibility, and validity based on comparison with ratings by expert clinicians.2,3 The CIWA-Ar commonly is used to determine when to administer lorazepam to limit or prevent morbidity and mortality in patients who are at risk of or are experiencing alcohol withdrawal. Refined to a list of 10 signs and symptoms, the CIWA-Ar is easy to administer and useful in a variety of clinical settings. The maximum score is 67, and patients with a score >15 are at increased risk for severe alcohol withdrawal.1 For a downloadable copy of the CIWA-Ar, click here.
Despite the benefits of using the CIWA-Ar, qualitative description of certain alcohol withdrawal symptoms is prone to subjective misinterpretation and can result in falsely elevated scores, excessive benzodiazepine administration, and associated sequelae.4 This article describes such a scenario, and examines factors that can contribute to a falsely elevated CIWA-Ar score.
CASE REPORT: Resistant alcohol withdrawal
Mr. J, age 24, is referred to the consultation-liaison service at our teaching hospital for “overall psychiatric assessment and help with alcohol withdrawal.” When brought to the hospital, Mr. J was experiencing diaphoresis and tachycardia. During the interview, he says he “experiences withdrawal symptoms all the time, so I am familiar with the signs.”
Mr. J is cooperative with the interview. Psychomotor agitation or retardation is not noted. His speech is goal-directed, his mood is “calm,” and his affect is within normal range. His thought content is devoid of psychoses or lethal ideations. On Mini-Mental State Examination, Mr. J scores 28 out of 30, which indicates normal cognitive functioning. He reports drinking eight 40-oz bottles of beer daily for the past 3 months. He started drinking alcohol at age 14 and has had only one 1-year period of sobriety. He denies using illicit drugs and his urine drug screen is unremarkable. Mr. J has a history of delirium tremens (DTs), no significant medical history, and was not taking any medications when admitted. His psychiatric history includes generalized anxiety disorder (GAD) and antisocial personality disorder and his family history is significant for alcohol dependence.
Laboratory workup is unremarkable except for a blood alcohol level of 0.23%. Review of systems is significant for mild tremor but no other symptoms of alcohol withdrawal. Physical examination is within normal limits.
Mr. J is started on a symptom-trigger alcohol detoxification protocol using the CIWA-Ar. Based on an elevated CIWA-Ar score of 33, he receives lorazepam IV, 11 mg on his first day of hospitalization and 8 mg on the second day. On the third day, Mr. J is agitated and pulls his IV lines in an attempt to leave. Over the next 24 hours, his blood pressure ranges from 136/90 mm Hg to 169/92 mm Hg and his pulse ranges from 94 to 115 beats per minute. He is given lorazepam, 30 mg, and is transferred to the intensive care unit (ICU).
At this time, Mr. J’s Delirium Rating Scale (DRS) score is 20 (maximum: 32). He remains in the ICU on lorazepam, 25 mg/hr. After 3 days in the ICU, lorazepam is titrated and stopped 2 days later. After lorazepam is stopped, Mr. J’s DRS score is 0, his vital signs are stable, and he no longer demonstrates signs or symptoms of DTs or alcohol withdrawal. He is discharged 1 day later.
Symptom-triggered treatment
Alcohol withdrawal symptoms mainly are caused by the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems; benzodiazepines are the standard of care (Box).5,6 Mr. J had a history of DTs, which is a risk factor for more severe alcohol withdrawal symptoms and recurrence of DTs.7 Some authors report that fixed dosing intervals are the “gold standard therapy” for alcohol withdrawal, and may be preferable for patients with a history of DTs.8 However, Mr. J was placed on a symptom-triggered protocol, which is standard at our hospital. The decision to implement this protocol was based on concerns of oversedation and possible respiratory suppression. Clinical trials have demonstrated that compared with fixed scheduled therapy for alcohol withdrawal, symptom-triggered protocols result in a reduced need for benzodiazepines (Table).
This treatment strategy requires frequent patient reevaluations—particularly early on—with attention to signs and symptoms of alcohol withdrawal and excessive sedation from medications. Additionally, although most patients with alcohol withdrawal respond to standard treatment that includes benzodiazepines, optimal nutrition, and good supportive care, a subgroup may resist therapy (resistant alcohol withdrawal). Therefore, Mr. J—and others with resistant alcohol withdrawal—may require large doses of benzodiazepines and additional sedatives and undergo complicated hospitalizations.9 Nonetheless, as exemplified by Mr. J, symptom-triggered protocols for alcohol withdrawal can result in potential morbidity and mortality.
Common symptoms of alcohol withdrawal include autonomic hyperactivity, tremor, insomnia, nausea, vomiting, agitation, anxiety, grand mal seizures, and transient visual, tactile, or auditory hallucinations.5 These symptoms result, in part, from the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems. Alcohol acutely enhances presynaptic GABA release through allosteric modulation at GABAA receptors and inhibits glutamate function through antagonism of N-methyl-d-aspartate (NMDA) receptors. Chronic alcohol exposure elicits compensatory downregulated GABAA and upregulated NMDA expression.
When alcohol intake abruptly stops and its acute effects dissipate, the sudden reduction in GABAergic tone and increase in glutamatergic tone cause alcohol withdrawal symptoms.6 Benzodiazepines, which bind at the benzodiazepine site on the GABAA receptor and, similar to alcohol, acutely enhance GABA and inhibit glutamate signaling, are the standard of care for alcohol withdrawal because they reduce anxiety and the risk of seizures and delirium tremens, which is a severe form of alcohol withdrawal characterized by disturbance in consciousness and cognition and hallucinations.5,6
Table
Benefits of symptom-triggered vs fixed scheduled therapy for alcohol withdrawal
| ST | FS | Benefits of ST | |
|---|---|---|---|
| Efficacy in alcohol withdrawal | Yes | Yes | |
| Flexibility in dosing with fluctuations in CIWA-Ar score | Yes | No | Less medication can be given overall if alcohol withdrawal signs resolve rapidly |
| Lower total benzodiazepine doses | + | – | Smaller chance of side effects such as oversedation, paradoxical agitation, delirium due to benzodiazepine intoxication, or respiratory depression |
| Fewer complications of higher benzodiazepine doses | + | – | Reduced risk of prolonged hospitalization, morbidity from aspiration pneumonia, or need to administer a reversal agent such as flumazenil |
| + = more likely; – = less likely CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; FS: fixed scheduled; ST: symptom-triggered Bibliography Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063. Cassidy EM, O’Sullivan I, Bradshaw P, et al. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen [published online ahead of print October 19, 2011]. Emerg Med J. doi: 10.1136/emermed-2011-200509. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. DeCarolis DD, Rice KL, Ho L, et al. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27(4):510-518. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701. Weaver MF, Hoffman HJ, Johnson RE, et al. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis. 2006;25(2):17-24. | |||
Factors influencing CIWA-Ar score
Vital signs monitoring. One limitation of the CIWA-Ar is that vital signs—an objective measurement of alcohol withdrawal— are not used to determine the score. Indeed, Mr. J presented with vital sign dysregulation. However, research suggests that the best predictor of high withdrawal scores includes groups of symptoms rather than individual symptoms.10 In that study, pulse and blood pressure did not correlate with withdrawal severity. Pulse and blood pressure elevations occur in alcohol withdrawal, but other signs and symptoms are more reliable in assessing withdrawal severity. This is clinically important because physicians often prescribe medications for alcohol withdrawal treatment based on pulse and blood pressure measures.1 This needs to be balanced against research that found a systolic blood pressure >150 mm Hg and axillary temperature >38°C can predict development of DTs in patients experiencing alcohol withdrawal.7
Lorazepam-induced disinhibition. Benzodiazepines affect functions associated with processing within the orbital prefrontal cortex,11 including response inhibition and socially acceptable behavior, and impairment in this functioning can result in behavioral disinhibition.12 This effect could account for the apparent paradoxical clinical observation of aggression in benzodiazepine-sedated patients.13 Because agitation is scored on the CIWA-Ar,1,10 falsely elevated scores caused by interpreting benzodiazepine-induced aggression as agitation could result in patients (such as Mr. J) receiving more lorazepam, therefore perpetuating this cycle.
Comorbid anxiety disorders also could falsely accentuate CIWA-Ar scores. For example, the odds of an alcohol dependence diagnosis are 2 to 3 times greater among patients with an anxiety disorder.14 Additionally, the lifetime prevalence of comorbid alcohol dependence for patients with GAD—such as Mr. J— is 30% to 35%.14,15
Alcohol withdrawal can be more severe in patients with alcohol dependence and anxiety disorders because evidence suggests the neurochemical processes underlying both are similar and potentially additive. Studies have shown that these dual diagnosis patients experience more severe symptoms of alcohol withdrawal as assessed by total CIWA-Ar score than those without an anxiety disorder.15 Although such patients may require more aggressive pharmacologic treatment, the dangers of higher benzodiazepine dosages may be even greater.
Benzodiazepine-induced delirium. A recent meta-analysis suggested that benzodiazepines may be associated with an increased risk of delirium.16 Longer-acting benzodiazepines may be associated with increased risk of delirium compared with short-acting agents, and higher doses during a 24-hour period may be associated with increased risk of delirium compared with lower doses. However, wide confidence intervals imply significant uncertainty with these results, and not all patients in the studies reviewed were undergoing alcohol detoxification.16 Benzodiazepines have been reported to accentuate delirium when used to treat DTs.17
We postulate that although Mr. J received lorazepam—a short- to moderate-acting benzodiazepine with a half-life of 12 to 16 hours18—the cumulative dose was high enough to have accentuated—rather than attenuated—delirium.16
Personality disorders. Comorbid alcohol use disorders (AUDs) and personality disorders are well documented. One study found the prevalence of personality disorders in AUDs ranged from 22% to 78%.19 Psychologically, drinking to cope with negative subjective states and emotions (coping motives) and drinking to enhance positive emotions (enhancement motives) may explain the relation between Cluster B personality disorders and AUDs.20
Research on prefrontal functioning in alcoholics and individuals with antisocial personality disorder symptoms has suggested that both groups may be impaired on tasks sensitive to compromised orbitofrontal functioning.21 The orbitofrontal system is essential for maintaining normal inhibitory influences on behavior.22 Benzodiazepines can increase the likelihood of developing disinhibition or impulsivity, which are symptoms of antisocial personality disorder. Because Mr. J had antisocial personality disorder, treating his alcohol withdrawal with a benzodiazepine could have accentuated these symptoms, which were subsequently “treated” with additional lorazepam, therefore worsening the cycle.
Medical comorbidities. The CIWA-Ar relies on autonomic signs and subjective symptoms and was not designed for use in nonverbal patients in the ICU. It is possible that the presence of other acute illnesses may contribute to increased CIWA-Ar scores, but we are unaware of any studies that have evaluated such factors.23
However, tremor, which is scored on the CIWA-Ar, can falsely elevate scores if it is caused by something other than acute alcohol withdrawal. Although essential tremors attenuate with acute alcohol use, chronic alcohol use can result in parkinsonism with a resting tremor, and cerebellar degeneration, which can include an action tremor and cerebellar 3-Hz leg tremor.24 Finally, hepatic encephalopathy—a neuropsychiatric syndrome characterized by disturbances in consciousness, mood, behavior, and cognition—can occur in patients with advanced liver disease, which may be precipitated by alcohol use. The clinical presentation and symptom severity of hepatic encephalopathy varies from minor cognitive impairment to gross disorientation, confusion, and agitation,25 all of which can elevate CIWA-Ar scores.
The role of disinhibition
Disinhibition could serve as the “final common pathway” through which CIWA-Ar scores can be falsely elevated.11 For a Figure that illustrates this, see below. Mr. J presented with several variables that could have elevated his CIWA-Ar score; additional potential factors include other psychiatric diagnoses such as bipolar disorder, opiate withdrawal, dementia, drug-seeking behavior, or malingering.26,27
Treating disinhibition in patients with alcohol withdrawal. Continuing to administer escalating doses of benzodiazepines is counterintuitive for benzodiazepine-induced disinhibition. In a study of alcohol withdrawal in rats, antipsychotics evaluated had some beneficial effects on alcohol withdrawal signs.28 In this study, the comparative effectiveness of atypical antipsychotics was as follows: risperidone = quetiapine > ziprasidone > clozapine > olanzapine.
The American Society of Addiction Medicine’s practice guideline advises against using antipsychotics as the sole agent for DTs because these agents are associated with a longer duration of delirium, higher complication rates, and higher mortality.28 However, antipsychotics have a role as an adjunct to benzodiazepines when benzodiazepines don’t sufficiently control agitation, thought disorder, or perceptual disturbances. Although haloperidol use is well established in this scenario, chlorpromazine is contraindicated because it is epileptogenic, and little information is available on atypical antipsychotics.29 If Mr. J had not responded to tapering lorazepam, evidence would support using haloperidol.

Figure: Unifying concept for pathological BZ administration during alcohol withdrawal syndrome: Disinhibition
AWS: alcohol withdrawal syndrome; BZ: benzodiazepine; CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; GABA: γ-aminobutyric acid
Source: Reference 11Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health & Research World. 1998;22(1):38-43. http://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf.
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537.
Drug Brand Names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Flumazenil • Romazicon
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Spiegel is on the speaker’s bureau of Sunovion Pharmaceuticals.
Drs. Kumari and Petri report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Amy Herndon for her help in preparing this article.
Clinicians often use the symptom-triggered Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised (CIWA-Ar)1 to assess patients’ risk for alcohol withdrawal because it has well-documented reliability, reproducibility, and validity based on comparison with ratings by expert clinicians.2,3 The CIWA-Ar commonly is used to determine when to administer lorazepam to limit or prevent morbidity and mortality in patients who are at risk of or are experiencing alcohol withdrawal. Refined to a list of 10 signs and symptoms, the CIWA-Ar is easy to administer and useful in a variety of clinical settings. The maximum score is 67, and patients with a score >15 are at increased risk for severe alcohol withdrawal.1 For a downloadable copy of the CIWA-Ar, click here.
Despite the benefits of using the CIWA-Ar, qualitative description of certain alcohol withdrawal symptoms is prone to subjective misinterpretation and can result in falsely elevated scores, excessive benzodiazepine administration, and associated sequelae.4 This article describes such a scenario, and examines factors that can contribute to a falsely elevated CIWA-Ar score.
CASE REPORT: Resistant alcohol withdrawal
Mr. J, age 24, is referred to the consultation-liaison service at our teaching hospital for “overall psychiatric assessment and help with alcohol withdrawal.” When brought to the hospital, Mr. J was experiencing diaphoresis and tachycardia. During the interview, he says he “experiences withdrawal symptoms all the time, so I am familiar with the signs.”
Mr. J is cooperative with the interview. Psychomotor agitation or retardation is not noted. His speech is goal-directed, his mood is “calm,” and his affect is within normal range. His thought content is devoid of psychoses or lethal ideations. On Mini-Mental State Examination, Mr. J scores 28 out of 30, which indicates normal cognitive functioning. He reports drinking eight 40-oz bottles of beer daily for the past 3 months. He started drinking alcohol at age 14 and has had only one 1-year period of sobriety. He denies using illicit drugs and his urine drug screen is unremarkable. Mr. J has a history of delirium tremens (DTs), no significant medical history, and was not taking any medications when admitted. His psychiatric history includes generalized anxiety disorder (GAD) and antisocial personality disorder and his family history is significant for alcohol dependence.
Laboratory workup is unremarkable except for a blood alcohol level of 0.23%. Review of systems is significant for mild tremor but no other symptoms of alcohol withdrawal. Physical examination is within normal limits.
Mr. J is started on a symptom-trigger alcohol detoxification protocol using the CIWA-Ar. Based on an elevated CIWA-Ar score of 33, he receives lorazepam IV, 11 mg on his first day of hospitalization and 8 mg on the second day. On the third day, Mr. J is agitated and pulls his IV lines in an attempt to leave. Over the next 24 hours, his blood pressure ranges from 136/90 mm Hg to 169/92 mm Hg and his pulse ranges from 94 to 115 beats per minute. He is given lorazepam, 30 mg, and is transferred to the intensive care unit (ICU).
At this time, Mr. J’s Delirium Rating Scale (DRS) score is 20 (maximum: 32). He remains in the ICU on lorazepam, 25 mg/hr. After 3 days in the ICU, lorazepam is titrated and stopped 2 days later. After lorazepam is stopped, Mr. J’s DRS score is 0, his vital signs are stable, and he no longer demonstrates signs or symptoms of DTs or alcohol withdrawal. He is discharged 1 day later.
Symptom-triggered treatment
Alcohol withdrawal symptoms mainly are caused by the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems; benzodiazepines are the standard of care (Box).5,6 Mr. J had a history of DTs, which is a risk factor for more severe alcohol withdrawal symptoms and recurrence of DTs.7 Some authors report that fixed dosing intervals are the “gold standard therapy” for alcohol withdrawal, and may be preferable for patients with a history of DTs.8 However, Mr. J was placed on a symptom-triggered protocol, which is standard at our hospital. The decision to implement this protocol was based on concerns of oversedation and possible respiratory suppression. Clinical trials have demonstrated that compared with fixed scheduled therapy for alcohol withdrawal, symptom-triggered protocols result in a reduced need for benzodiazepines (Table).
This treatment strategy requires frequent patient reevaluations—particularly early on—with attention to signs and symptoms of alcohol withdrawal and excessive sedation from medications. Additionally, although most patients with alcohol withdrawal respond to standard treatment that includes benzodiazepines, optimal nutrition, and good supportive care, a subgroup may resist therapy (resistant alcohol withdrawal). Therefore, Mr. J—and others with resistant alcohol withdrawal—may require large doses of benzodiazepines and additional sedatives and undergo complicated hospitalizations.9 Nonetheless, as exemplified by Mr. J, symptom-triggered protocols for alcohol withdrawal can result in potential morbidity and mortality.
Common symptoms of alcohol withdrawal include autonomic hyperactivity, tremor, insomnia, nausea, vomiting, agitation, anxiety, grand mal seizures, and transient visual, tactile, or auditory hallucinations.5 These symptoms result, in part, from the effects of chronic alcohol exposure on brain γ–aminobutyric acid (GABA) and glutamate systems. Alcohol acutely enhances presynaptic GABA release through allosteric modulation at GABAA receptors and inhibits glutamate function through antagonism of N-methyl-d-aspartate (NMDA) receptors. Chronic alcohol exposure elicits compensatory downregulated GABAA and upregulated NMDA expression.
When alcohol intake abruptly stops and its acute effects dissipate, the sudden reduction in GABAergic tone and increase in glutamatergic tone cause alcohol withdrawal symptoms.6 Benzodiazepines, which bind at the benzodiazepine site on the GABAA receptor and, similar to alcohol, acutely enhance GABA and inhibit glutamate signaling, are the standard of care for alcohol withdrawal because they reduce anxiety and the risk of seizures and delirium tremens, which is a severe form of alcohol withdrawal characterized by disturbance in consciousness and cognition and hallucinations.5,6
Table
Benefits of symptom-triggered vs fixed scheduled therapy for alcohol withdrawal
| ST | FS | Benefits of ST | |
|---|---|---|---|
| Efficacy in alcohol withdrawal | Yes | Yes | |
| Flexibility in dosing with fluctuations in CIWA-Ar score | Yes | No | Less medication can be given overall if alcohol withdrawal signs resolve rapidly |
| Lower total benzodiazepine doses | + | – | Smaller chance of side effects such as oversedation, paradoxical agitation, delirium due to benzodiazepine intoxication, or respiratory depression |
| Fewer complications of higher benzodiazepine doses | + | – | Reduced risk of prolonged hospitalization, morbidity from aspiration pneumonia, or need to administer a reversal agent such as flumazenil |
| + = more likely; – = less likely CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; FS: fixed scheduled; ST: symptom-triggered Bibliography Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063. Cassidy EM, O’Sullivan I, Bradshaw P, et al. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen [published online ahead of print October 19, 2011]. Emerg Med J. doi: 10.1136/emermed-2011-200509. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. DeCarolis DD, Rice KL, Ho L, et al. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27(4):510-518. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701. Weaver MF, Hoffman HJ, Johnson RE, et al. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis. 2006;25(2):17-24. | |||
Factors influencing CIWA-Ar score
Vital signs monitoring. One limitation of the CIWA-Ar is that vital signs—an objective measurement of alcohol withdrawal— are not used to determine the score. Indeed, Mr. J presented with vital sign dysregulation. However, research suggests that the best predictor of high withdrawal scores includes groups of symptoms rather than individual symptoms.10 In that study, pulse and blood pressure did not correlate with withdrawal severity. Pulse and blood pressure elevations occur in alcohol withdrawal, but other signs and symptoms are more reliable in assessing withdrawal severity. This is clinically important because physicians often prescribe medications for alcohol withdrawal treatment based on pulse and blood pressure measures.1 This needs to be balanced against research that found a systolic blood pressure >150 mm Hg and axillary temperature >38°C can predict development of DTs in patients experiencing alcohol withdrawal.7
Lorazepam-induced disinhibition. Benzodiazepines affect functions associated with processing within the orbital prefrontal cortex,11 including response inhibition and socially acceptable behavior, and impairment in this functioning can result in behavioral disinhibition.12 This effect could account for the apparent paradoxical clinical observation of aggression in benzodiazepine-sedated patients.13 Because agitation is scored on the CIWA-Ar,1,10 falsely elevated scores caused by interpreting benzodiazepine-induced aggression as agitation could result in patients (such as Mr. J) receiving more lorazepam, therefore perpetuating this cycle.
Comorbid anxiety disorders also could falsely accentuate CIWA-Ar scores. For example, the odds of an alcohol dependence diagnosis are 2 to 3 times greater among patients with an anxiety disorder.14 Additionally, the lifetime prevalence of comorbid alcohol dependence for patients with GAD—such as Mr. J— is 30% to 35%.14,15
Alcohol withdrawal can be more severe in patients with alcohol dependence and anxiety disorders because evidence suggests the neurochemical processes underlying both are similar and potentially additive. Studies have shown that these dual diagnosis patients experience more severe symptoms of alcohol withdrawal as assessed by total CIWA-Ar score than those without an anxiety disorder.15 Although such patients may require more aggressive pharmacologic treatment, the dangers of higher benzodiazepine dosages may be even greater.
Benzodiazepine-induced delirium. A recent meta-analysis suggested that benzodiazepines may be associated with an increased risk of delirium.16 Longer-acting benzodiazepines may be associated with increased risk of delirium compared with short-acting agents, and higher doses during a 24-hour period may be associated with increased risk of delirium compared with lower doses. However, wide confidence intervals imply significant uncertainty with these results, and not all patients in the studies reviewed were undergoing alcohol detoxification.16 Benzodiazepines have been reported to accentuate delirium when used to treat DTs.17
We postulate that although Mr. J received lorazepam—a short- to moderate-acting benzodiazepine with a half-life of 12 to 16 hours18—the cumulative dose was high enough to have accentuated—rather than attenuated—delirium.16
Personality disorders. Comorbid alcohol use disorders (AUDs) and personality disorders are well documented. One study found the prevalence of personality disorders in AUDs ranged from 22% to 78%.19 Psychologically, drinking to cope with negative subjective states and emotions (coping motives) and drinking to enhance positive emotions (enhancement motives) may explain the relation between Cluster B personality disorders and AUDs.20
Research on prefrontal functioning in alcoholics and individuals with antisocial personality disorder symptoms has suggested that both groups may be impaired on tasks sensitive to compromised orbitofrontal functioning.21 The orbitofrontal system is essential for maintaining normal inhibitory influences on behavior.22 Benzodiazepines can increase the likelihood of developing disinhibition or impulsivity, which are symptoms of antisocial personality disorder. Because Mr. J had antisocial personality disorder, treating his alcohol withdrawal with a benzodiazepine could have accentuated these symptoms, which were subsequently “treated” with additional lorazepam, therefore worsening the cycle.
Medical comorbidities. The CIWA-Ar relies on autonomic signs and subjective symptoms and was not designed for use in nonverbal patients in the ICU. It is possible that the presence of other acute illnesses may contribute to increased CIWA-Ar scores, but we are unaware of any studies that have evaluated such factors.23
However, tremor, which is scored on the CIWA-Ar, can falsely elevate scores if it is caused by something other than acute alcohol withdrawal. Although essential tremors attenuate with acute alcohol use, chronic alcohol use can result in parkinsonism with a resting tremor, and cerebellar degeneration, which can include an action tremor and cerebellar 3-Hz leg tremor.24 Finally, hepatic encephalopathy—a neuropsychiatric syndrome characterized by disturbances in consciousness, mood, behavior, and cognition—can occur in patients with advanced liver disease, which may be precipitated by alcohol use. The clinical presentation and symptom severity of hepatic encephalopathy varies from minor cognitive impairment to gross disorientation, confusion, and agitation,25 all of which can elevate CIWA-Ar scores.
The role of disinhibition
Disinhibition could serve as the “final common pathway” through which CIWA-Ar scores can be falsely elevated.11 For a Figure that illustrates this, see below. Mr. J presented with several variables that could have elevated his CIWA-Ar score; additional potential factors include other psychiatric diagnoses such as bipolar disorder, opiate withdrawal, dementia, drug-seeking behavior, or malingering.26,27
Treating disinhibition in patients with alcohol withdrawal. Continuing to administer escalating doses of benzodiazepines is counterintuitive for benzodiazepine-induced disinhibition. In a study of alcohol withdrawal in rats, antipsychotics evaluated had some beneficial effects on alcohol withdrawal signs.28 In this study, the comparative effectiveness of atypical antipsychotics was as follows: risperidone = quetiapine > ziprasidone > clozapine > olanzapine.
The American Society of Addiction Medicine’s practice guideline advises against using antipsychotics as the sole agent for DTs because these agents are associated with a longer duration of delirium, higher complication rates, and higher mortality.28 However, antipsychotics have a role as an adjunct to benzodiazepines when benzodiazepines don’t sufficiently control agitation, thought disorder, or perceptual disturbances. Although haloperidol use is well established in this scenario, chlorpromazine is contraindicated because it is epileptogenic, and little information is available on atypical antipsychotics.29 If Mr. J had not responded to tapering lorazepam, evidence would support using haloperidol.

Figure: Unifying concept for pathological BZ administration during alcohol withdrawal syndrome: Disinhibition
AWS: alcohol withdrawal syndrome; BZ: benzodiazepine; CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised; GABA: γ-aminobutyric acid
Source: Reference 11Related Resources
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health & Research World. 1998;22(1):38-43. http://pubs.niaaa.nih.gov/publications/arh22-1/38-43.pdf.
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537.
Drug Brand Names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Flumazenil • Romazicon
- Haloperidol • Haldol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Spiegel is on the speaker’s bureau of Sunovion Pharmaceuticals.
Drs. Kumari and Petri report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
The authors thank Amy Herndon for her help in preparing this article.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knott DH, Lerner WD, Davis-Knott T, et al. Decision for alcohol detoxication: a method to standardize patient evaluation. Postgrad Med. 1981;69(5):65-69, 72-75, 78.
3. Wiehl WO, Hayner G, Galloway G. Haight Ashbury Free Clinics’ drug detoxification protocols—Part 4: alcohol. J Psychoactive Drugs. 1994;26(1):57-59.
4. Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256-261.
5. Diagnostic and statistical manual of mental disorders, 4th ed text rev. Washington DC: American Psychiatric Association; 2000.
6. Schacht JP, Randall PK, Waid LR, et al. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011;35(11):2030-2038.
7. Monte R, Rabuñal R, Casariego E, et al. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20(7):690-694.
8. Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
9. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60.
10. Pittman B, Gueorguieva R, Krupitsky E, et al. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31(4):612-618.
11. Deakin JB, Aitken MR, Dowson JH, et al. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (Berl). 2004;173(1-2):88-97.
12. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(pt 9):2502-2512.
13. Jones KA, Nielsen S, Bruno R, et al. Benzodiazepines - their role in aggression and why GPs should prescribe with caution. Aust Fam Physician. 2011;40(11):862-865.
14. Scott EL, Hulvershorn L. Anxiety disorders with comorbid substance abuse. Psychiatric Times. 2011; 28(9).
15. Faingold CL, Knapp DJ, Chester JA, et al. Integrative neurobiology of the alcohol withdrawal syndrome—from anxiety to seizures. Alcohol Clin Exp Res. 2004;28(2):268-278.
16. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23-29.
17. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
18. Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106(12):2086-2109.
19. Mellos E, Liappas I, Paparrigopoulos T. Comorbidity of personality disorders with alcohol abuse. In Vivo. 2010;24(5):761-769.
20. Tragesser SL, Sher KJ, Trull TJ, et al. Personality disorder symptoms, drinking motives, and alcohol use and consequences: cross-sectional and prospective mediation. Exp Clin Psychopharmacol. 2007;15(3):282-292.
21. Oscar-Berman M, Valmas MM, Sawyer KS, et al. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309-326.
22. Dom G, De Wilde B, Hulstijn W, et al. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol Alcohol. 2006;41(4):412-420.
23. de Wit M, Jones DG, Sessler CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994-1003.
24. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25(14):2274-2284.
25. Crone CC, Gabriel GM, DiMartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics. 2006;47(3):188-205.
26. Reoux JP, Oreskovich MR. A comparison of two versions of the clinical institute withdrawal assessment for alcohol: the CIWA-Ar and CIWA-AD. Am J Addict. 2006;15(1):85-93.
27. Gray S, Borgundvaag B, Sirvastava A, et al. Feasibility and reliability of the SHOT: a short scale for measuring pretreatment severity of alcohol withdrawal in the emergency department. Acad Emerg Med. 2010;17(10):1048-1054.
28. Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47(1):33-41.
29. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knott DH, Lerner WD, Davis-Knott T, et al. Decision for alcohol detoxication: a method to standardize patient evaluation. Postgrad Med. 1981;69(5):65-69, 72-75, 78.
3. Wiehl WO, Hayner G, Galloway G. Haight Ashbury Free Clinics’ drug detoxification protocols—Part 4: alcohol. J Psychoactive Drugs. 1994;26(1):57-59.
4. Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics. 2004;45(3):256-261.
5. Diagnostic and statistical manual of mental disorders, 4th ed text rev. Washington DC: American Psychiatric Association; 2000.
6. Schacht JP, Randall PK, Waid LR, et al. Neurocognitive performance, alcohol withdrawal, and effects of a combination of flumazenil and gabapentin in alcohol dependence. Alcohol Clin Exp Res. 2011;35(11):2030-2038.
7. Monte R, Rabuñal R, Casariego E, et al. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20(7):690-694.
8. Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
9. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60.
10. Pittman B, Gueorguieva R, Krupitsky E, et al. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31(4):612-618.
11. Deakin JB, Aitken MR, Dowson JH, et al. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology (Berl). 2004;173(1-2):88-97.
12. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(pt 9):2502-2512.
13. Jones KA, Nielsen S, Bruno R, et al. Benzodiazepines - their role in aggression and why GPs should prescribe with caution. Aust Fam Physician. 2011;40(11):862-865.
14. Scott EL, Hulvershorn L. Anxiety disorders with comorbid substance abuse. Psychiatric Times. 2011; 28(9).
15. Faingold CL, Knapp DJ, Chester JA, et al. Integrative neurobiology of the alcohol withdrawal syndrome—from anxiety to seizures. Alcohol Clin Exp Res. 2004;28(2):268-278.
16. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23-29.
17. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
18. Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106(12):2086-2109.
19. Mellos E, Liappas I, Paparrigopoulos T. Comorbidity of personality disorders with alcohol abuse. In Vivo. 2010;24(5):761-769.
20. Tragesser SL, Sher KJ, Trull TJ, et al. Personality disorder symptoms, drinking motives, and alcohol use and consequences: cross-sectional and prospective mediation. Exp Clin Psychopharmacol. 2007;15(3):282-292.
21. Oscar-Berman M, Valmas MM, Sawyer KS, et al. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309-326.
22. Dom G, De Wilde B, Hulstijn W, et al. Behavioural aspects of impulsivity in alcoholics with and without a cluster-B personality disorder. Alcohol Alcohol. 2006;41(4):412-420.
23. de Wit M, Jones DG, Sessler CN, et al. Alcohol-use disorders in the critically ill patient. Chest. 2010;138(4):994-1003.
24. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25(14):2274-2284.
25. Crone CC, Gabriel GM, DiMartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics. 2006;47(3):188-205.
26. Reoux JP, Oreskovich MR. A comparison of two versions of the clinical institute withdrawal assessment for alcohol: the CIWA-Ar and CIWA-AD. Am J Addict. 2006;15(1):85-93.
27. Gray S, Borgundvaag B, Sirvastava A, et al. Feasibility and reliability of the SHOT: a short scale for measuring pretreatment severity of alcohol withdrawal in the emergency department. Acad Emerg Med. 2010;17(10):1048-1054.
28. Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47(1):33-41.
29. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862.
Which psychotropics carry the greatest risk of QTc prolongation?
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
- Screen patients for risk factors for prolonged QTc interval, such as congenital long QT syndrome, family history of cardiac conduction abnormalities, and previous occurrences of medication-mediated QTc prolongation.
- Obtain baseline and steady state ECG when initiating high-risk agents, particularly when administering combination therapy.
- Use the lowest effective dose of antidepressants and antipsychotics and monitor symptoms closely.
Mrs. A, age 68, has a 40-year history of schizoaffective disorder with comorbid anxiety disorder not otherwise specified, type 2 diabetes mellitus, and hypertension. She takes furosemide, 40 mg/d, lisinopril, 20 mg/d, and metformin, 2,000 mg/d, for hypertension and diabetes; lorazepam, 1.5 mg/d, and paroxetine, 40 mg/d, for anxiety; and quetiapine extended release, 800 mg/d, for psychotic features and mood dysregulation with schizoaffective disorder. Mrs. A’s husband died 5 years ago and she lives alone in a senior care facility. Mrs. A uses a weekly pill reminder box because her residential facility does not monitor medication adherence. She sees her psychiatrist once a month and her primary care provider every 3 months. She has no history of illicit drug, alcohol, or tobacco use.
Two weeks ago, Mrs. A was found leaning against the wall in a hallway, complaining of dizziness and disorientation, and unable to find her way back to her apartment. In the emergency department, her serum potassium is low (3.0 mEq/L; normal range: 3.5 to 5.0), fasting glucose is elevated (110 mg/dL; range: 65 to 99), and ECG reveals a prolonged QTc interval of 530 milliseconds. Before this episode, Mrs. A had been medically stable without mood or psychotic symptoms, although her daughter reported medication self-administration was becoming difficult.
Exposure to psychotropics carries a risk of QTc prolongation. The QT interval is an ECG measure of ventricular depolarization and repolarization. The QTc designation indicates a correction for heart rate with increasing heart rate correlating with a shorter QT interval. Readings of 440 milliseconds are considered normal.1 QTc prolongation is defined as >450 milliseconds for men and >470 milliseconds for women.2 An increase in the QT interval is a predictor of serious cardiac events.3
Antidepressants and antipsychotics have been associated with QTc prolongation. When identifying agents that could disrupt cardiac conduction, clinicians need to consider whether the drug’s molecular structure, receptor affinity, or pharmacologic effects are most critical.2 Although these may be important, patient-specific variables that increase the risk of QTc prolongation may have greater impact. These include:
- age >65
- female sex
- electrolyte imbalances (specifically low serum potassium and magnesium levels)
- high or toxic serum levels of the suspected drug
- preexisting cardiovascular impairment, such as bradycardia.4,5
Other risk factors include concurrent use of an agent with similar cardiovascular effects or one that competes for metabolism (either enzymatic or at the binding site), physiologic limitations such as renal insufficiency, and medication changes that may increase or decrease psychotropic clearance.4,6 Geriatric patients with dementia have an increased risk for cardiovascular-related death.7,8
Antidepressants
Among tricyclic antidepressants, most reports of QTc prolongation involve amitriptyline and maprotiline.9 Risk factors include demographics (eg, female sex, age), personal or family history (congenital long QT syndrome, cardiovascular disease), and concurrent conditions or drug use, particularly those associated with QTc prolongation.3 Desipramine and nortriptyline also have been identified as high-risk agents.10
QTc prolongation has been reported with all selective serotonin reuptake inhibitors at plasma concentrations above the therapeutic level.11 Fluoxetine-associated QTc prolongation was limited to cases of overdose or when additional risk factors were reported.4 QTc prolongation from psychotropics could increase the risk of torsades de pointes, according to an analysis of the FDA Adverse Event Reporting System.12 In 2011, the FDA reported an increased risk of abnormal heart rhythms—including QTc prolongation—with citalopram doses >40 mg/d.13 Although cases of QTc prolongation with paroxetine have not been reported,11 the Arizona Center for Education and Research on Therapeutics lists paroxetine with other agents that may increase the risk for QTc prolongation with concurrent use of medications that may prolong QTc interval.14 Venlafaxine doses >300 mg/d may require additional cardiac monitoring.5,12 Data from venlafaxine poisoning case reports found a positive correlation between dose and QTc prolongation.15 In a review of toxicology database information, Wenzel-Seifert et al4 found extended QT interval with citalopram, fluoxetine, and venlafaxine at toxic doses or in the presence of additional risk factors such as sex, older age, or personal or family history of congenital long QT syndrome or cardiovascular disease.
Antipsychotics
Case reports, case series, and research trials have evaluated the risk of QTc prolongation with antipsychotics (Table).1,2,4,16,17 The first-generation antipsychotics thioridazine,4,16,18 mesoridazine,16,18 chlorpromazine,19 and haloperidol3 warrant cardiac monitoring. The QTc prolongation effects of thioridazine and its active metabolite mesoridazine are well-documented and thioridazine-mediated QTc prolongation increases are dose-dependent.4,18 ECG monitoring is recommended with IV haloperidol, which is used for delirium in adults.20 QTc prolongation has been associated with long-term ziprasidone use more often than with risperidone, olanzapine, or quetiapine.19 Ziprasidone prolongs the QTc interval an average of 20 milliseconds,21 which could represent a clinically significant change. QTc prolongation for iloperidone is comparable to ziprasidone and haloperidol.22 There is some evidence that aripiprazole may shorten, rather than prolong, the QTc interval.4,17
Cardiovascular adverse effects associated with clozapine—including QTc prolongation—are dose-dependent.3 Olanzapine prolongs QTc interval, although the mean change is less than with other agents unless other variables were present, such as:
- concomitant use of medications that may prolong QTc interval (ie, amantadine, hydroxyzine, or tamoxifen2)
- preexisting cardiovascular conduction disorders
- higher doses (>40 mg/d).3,23
In 17 case reports of cardiac changes associated with quetiapine use, doses ranged from 100 mg/d24 to an overdose of 36 g/d.25 Only 1 patient death was reported secondary to overdose and preexisting dysrhythmia and hypertension.26 QTc prolongation associated with risperidone was minor1 based on oral doses in the normal therapeutic range and incidences of overdose.10 Paliperidone27 and lurasidone28 are associated with clinically insignificant QTc prolongation. Changes in QTc interval were positively correlated with asenapine dose, although at the highest dose of 40 mg/d, the increase was <5 milliseconds.29
Mrs. A presents with a number of risk factors for QTc prolongation, including older age, female sex, and psychiatric and medical comorbidities that require medication. A pill count revealed that she was taking more than the prescribed daily doses of her medications. During the interview, Mrs. A said that if she missed her medication time, she would take them when she remembered. If she could not remember if she took her pills, she would take them again. Her physicians will explore strategies to increase medication adherence.
Table
Examples of QTc prolongation associated with select antipsychoticsa
| Antipsychotic | Approximate QTc interval prolongation in millisecondsb |
|---|---|
| Aripiprazole4,17 | -1 to -4 |
| Clozapine4 | 10 |
| Haloperidol1,2 | 7 to 15 |
| Mesoridazine16 | 39 to 53 |
| Olanzapine1 | 2 to 6.5 |
| Paliperidone4 | 2 to 4 |
| Pimozide2 | 19 |
| Quetiapine1,2 | 6 to 15 |
| Risperidone1,2 | 3.5 to 10 |
| Sertindole1 | 30 |
| Thioridazine2,16 | 33 to 41 |
| Ziprasidone1,2 | 16 to 21 |
| aList is not comprehensive. Other antipsychotics may be associated with QTc prolongation bQTc prolongation interval may depend on the route of administration | |
Related Resources
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
- Vieweg WV, Wood MA, Fernandez A, et al. Proarrhythmic risk with antipsychotic and antidepressant drugs: implications in the elderly. Drugs Aging. 2009;26(12):997-1012.
- Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46(5):464-494.
Drug Brand Names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Asenapine • Saphris
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Furosemide • Lasix
- Haloperidol • Haldol
- Hydroxyzine • Atarax, Vistaril
- Iloperidone • Fanapt
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Lurasidone • Latuda
- Maprotiline • Ludiomil
- Mesoridazine • Serentil
- Metformin • Glucophage
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Tamoxifen • Nolvadex, Soltamox
- Thioridazine • Mellaril
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. No similar work by the authors is under review or in press. No funding was requested or received in conjunction with this manuscript.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
1. Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Devel Ther. 2010;4:187-201.
2. Taylor DM. Antipsychotics and QT prolongation. Acta Psychiatr Scand. 2003;107(2):85-95.
3. Alvarez PA, Pahissa J. QT alterations in psychopharmacology: proven candidates and suspects. Curr Drug Saf. 2010;5(1):97-104.
4. Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687-693.
5. Vieweg WV. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205-215.
6. Nielsen J, Graff C, Kanters JK, et al. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473-490.
7. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
8. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
9. Vieweg WV, Wood MA. Tricyclic antidepressants QT interval prolongation, and torsade de pointes. Psychosomatics. 2004;45(5):371-377.
10. Jeon SH, Jaekal J, Lee SH, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649-1656.
11. Wenzel-Seifert K, Wittmann M, Haen E. Torsade de pointes episodes under treatment with selective serotonin reuptake inhibitors. Pharmacopsychiatry. 2010;43(7):279-281.
12. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512-518.
13. U.S. Food and Drug Administration. FDA drug safety communication: revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm. Published March 28, 2012. Accessed June 26, 2012.
14. Arizona CERT-QT Center for Education and Research on Therapeutics. QT drug lists by risk groups. http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm. Accessed June 26 2012.
15. Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64(2):192-197.
16. Salih IS, Thanacoody RH, McKay GA, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharmacol Ther. 2007;82(5):548-554.
17. Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opin Pharmacother. 2002;3(5):479-498.
18. Dallaire S. Thioridazine (Mellaril) and mesoridazine (Serentil): prolongation of the QTc interval. CMAJ. 2001;164(1):91,95.-
19. Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation torsade de pointes and sudden death. Drugs. 2002;62(11):1649-1671.
20. Shapiro BA, Warren J, Egol AB, et al. Practice parameters for intravenous analgesia and sedation for adult patients in the intensive care unit: an executive summary. Crit Care Med. 1995;23(9):1596-1600.
21. Vieweg WV, Hasnain M. Question regarding ziprasidone and QTc interval prolongation in the ZODIAC Study. Am J Psychiatry. 2011;168(6):650-651.
22. Caccia S, Pasina L, Nobili A. New atypical antipsychotics for schizophrenia: iloperidone. Drug Des Devel Ther. 2010;4:33-48.
23. Dineen S, Withrow K, Voronovitch L, et al. QTc prolongation and high-dose olanzapine. Psychosomatics. 2003;44(2):174-175.
24. Vieweg WV, Schneider RK, Wood MA. Torsade de pointes in a patient with complex medical and psychiatric conditions receiving low-dose quetiapine. Acta Psychiatr Scand. 2005;112(4):318-322.
25. Capuano A, Ruggiero S, Vestini F, et al. Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol. 2011;34(4):475-477.
26. Fernandes PP, Marcil WA. Death associated with quetiapine overdose. Am J Psychiatry. 2002;159(12):2114.-
27. Sedky K, Nazir R, Lindenmayer JP, et al. Paliperidone palmitate: once-monthly treatment option for schizophrenia. Current Psychiatry. 2010;9(3):48-50.
28. Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189-210.
29. Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308.
How to talk to patients about religion and spirituality
Discuss this article at www.facebook.com/CurrentPsychiatry
In the midst of turmoil and suffering, patients often search for meaning and interpret their circumstances in the context of their religious or spiritual (R/S) beliefs. National polling data show that most Americans identify themselves as R/S, and inpatient and outpatient studies demonstrate that patients want clinicians to inquire about their R/S beliefs.1 In addition, R/S beliefs can benefit patients as a source of well-being, hope, purpose, higher self-esteem, coping, and social support.2 Given the importance of R/S to patients, psychiatrists should seek to understand their patient’s distress in the context of their beliefs.
Why is it hard for psychiatrists to bring up the subject? Psychiatrists might be hesitant to discuss R/S beliefs with patients because of personal discomfort, limited training opportunities during residency and in clinical practice, or time or economic constraints.3 Psychiatrists tend to be less R/S than the general population4 and may fear that they are being perceived as overly intrusive or offensive.
When should we inquire about spirituality and religion? Take an R/S history during each new patient evaluation and when admitting a patient for hospitalization, and include this information in the social history.5 Doing so could lead to a chaplain referral when appropriate. Questions about R/S beliefs usually are not perceived as intrusive if asked along with other questions that focus on patients’ social support system and may help identify barriers to self-harm or harm to others.
How do we start the conversation? There are several ways to start the discussion about R/S that are engaging, efficient, respectful, and caring. Start with simple questions, such as “Is R/S an important part of your life?” or “Do you rely on your faith during a difficult time like this?”
If your patient answers yes to these questions, consider exploring:
- How does your patient use R/S? Does he or she use it to cope with mental illness, or is it a source of distress? Is it both?
- How would your patient like you to address R/S in your work together?
- Is your patient a member of an R/S community, and if so, is it a source of support for him or her?
- Is your patient interested in working collaboratively with an R/S provider—eg, clergy, pastoral counselor?
If your patients say R/S is not important to them or they do not rely on faith, ask if R/S has been important to them in the past. Also, have them consider what gives their life meaning and hope, what is sacred to them, and who or what will help them cope during a difficult time.
Disclosure
Dr. Clark and the Reverend Doctor King report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Harrison is a consultant to the Samaritan Center of Puget Sound, Seattle, WA.
Acknowledgement
The authors thank J. Gary Trantham, MD, for his assistance with this article.
1. Puchalski C. Spiritual assessment in clinical practice. Psychiatr Ann. 2006;36(3):150-155.
2. Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28(3):242-250.
3. Griffith JL. Managing religious countertransference in clinical settings. Psychiatr Ann. 2006;36(3):196-204.
4. Curlin FA, Lawrence RE, Odell S, et al. Religion, spirituality, and medicine: psychiatrists’ and other physicians’ differing observations, interpretations, and clinical approaches. Am J Psychiatry. 2007;164(12):1825-1831.
5. Koenig HG. Spirituality in patient care: why how, when, and what. West Conshohocken, PA: Templeton Press; 2007.
Discuss this article at www.facebook.com/CurrentPsychiatry
In the midst of turmoil and suffering, patients often search for meaning and interpret their circumstances in the context of their religious or spiritual (R/S) beliefs. National polling data show that most Americans identify themselves as R/S, and inpatient and outpatient studies demonstrate that patients want clinicians to inquire about their R/S beliefs.1 In addition, R/S beliefs can benefit patients as a source of well-being, hope, purpose, higher self-esteem, coping, and social support.2 Given the importance of R/S to patients, psychiatrists should seek to understand their patient’s distress in the context of their beliefs.
Why is it hard for psychiatrists to bring up the subject? Psychiatrists might be hesitant to discuss R/S beliefs with patients because of personal discomfort, limited training opportunities during residency and in clinical practice, or time or economic constraints.3 Psychiatrists tend to be less R/S than the general population4 and may fear that they are being perceived as overly intrusive or offensive.
When should we inquire about spirituality and religion? Take an R/S history during each new patient evaluation and when admitting a patient for hospitalization, and include this information in the social history.5 Doing so could lead to a chaplain referral when appropriate. Questions about R/S beliefs usually are not perceived as intrusive if asked along with other questions that focus on patients’ social support system and may help identify barriers to self-harm or harm to others.
How do we start the conversation? There are several ways to start the discussion about R/S that are engaging, efficient, respectful, and caring. Start with simple questions, such as “Is R/S an important part of your life?” or “Do you rely on your faith during a difficult time like this?”
If your patient answers yes to these questions, consider exploring:
- How does your patient use R/S? Does he or she use it to cope with mental illness, or is it a source of distress? Is it both?
- How would your patient like you to address R/S in your work together?
- Is your patient a member of an R/S community, and if so, is it a source of support for him or her?
- Is your patient interested in working collaboratively with an R/S provider—eg, clergy, pastoral counselor?
If your patients say R/S is not important to them or they do not rely on faith, ask if R/S has been important to them in the past. Also, have them consider what gives their life meaning and hope, what is sacred to them, and who or what will help them cope during a difficult time.
Disclosure
Dr. Clark and the Reverend Doctor King report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Harrison is a consultant to the Samaritan Center of Puget Sound, Seattle, WA.
Acknowledgement
The authors thank J. Gary Trantham, MD, for his assistance with this article.
Discuss this article at www.facebook.com/CurrentPsychiatry
In the midst of turmoil and suffering, patients often search for meaning and interpret their circumstances in the context of their religious or spiritual (R/S) beliefs. National polling data show that most Americans identify themselves as R/S, and inpatient and outpatient studies demonstrate that patients want clinicians to inquire about their R/S beliefs.1 In addition, R/S beliefs can benefit patients as a source of well-being, hope, purpose, higher self-esteem, coping, and social support.2 Given the importance of R/S to patients, psychiatrists should seek to understand their patient’s distress in the context of their beliefs.
Why is it hard for psychiatrists to bring up the subject? Psychiatrists might be hesitant to discuss R/S beliefs with patients because of personal discomfort, limited training opportunities during residency and in clinical practice, or time or economic constraints.3 Psychiatrists tend to be less R/S than the general population4 and may fear that they are being perceived as overly intrusive or offensive.
When should we inquire about spirituality and religion? Take an R/S history during each new patient evaluation and when admitting a patient for hospitalization, and include this information in the social history.5 Doing so could lead to a chaplain referral when appropriate. Questions about R/S beliefs usually are not perceived as intrusive if asked along with other questions that focus on patients’ social support system and may help identify barriers to self-harm or harm to others.
How do we start the conversation? There are several ways to start the discussion about R/S that are engaging, efficient, respectful, and caring. Start with simple questions, such as “Is R/S an important part of your life?” or “Do you rely on your faith during a difficult time like this?”
If your patient answers yes to these questions, consider exploring:
- How does your patient use R/S? Does he or she use it to cope with mental illness, or is it a source of distress? Is it both?
- How would your patient like you to address R/S in your work together?
- Is your patient a member of an R/S community, and if so, is it a source of support for him or her?
- Is your patient interested in working collaboratively with an R/S provider—eg, clergy, pastoral counselor?
If your patients say R/S is not important to them or they do not rely on faith, ask if R/S has been important to them in the past. Also, have them consider what gives their life meaning and hope, what is sacred to them, and who or what will help them cope during a difficult time.
Disclosure
Dr. Clark and the Reverend Doctor King report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Harrison is a consultant to the Samaritan Center of Puget Sound, Seattle, WA.
Acknowledgement
The authors thank J. Gary Trantham, MD, for his assistance with this article.
1. Puchalski C. Spiritual assessment in clinical practice. Psychiatr Ann. 2006;36(3):150-155.
2. Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28(3):242-250.
3. Griffith JL. Managing religious countertransference in clinical settings. Psychiatr Ann. 2006;36(3):196-204.
4. Curlin FA, Lawrence RE, Odell S, et al. Religion, spirituality, and medicine: psychiatrists’ and other physicians’ differing observations, interpretations, and clinical approaches. Am J Psychiatry. 2007;164(12):1825-1831.
5. Koenig HG. Spirituality in patient care: why how, when, and what. West Conshohocken, PA: Templeton Press; 2007.
1. Puchalski C. Spiritual assessment in clinical practice. Psychiatr Ann. 2006;36(3):150-155.
2. Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28(3):242-250.
3. Griffith JL. Managing religious countertransference in clinical settings. Psychiatr Ann. 2006;36(3):196-204.
4. Curlin FA, Lawrence RE, Odell S, et al. Religion, spirituality, and medicine: psychiatrists’ and other physicians’ differing observations, interpretations, and clinical approaches. Am J Psychiatry. 2007;164(12):1825-1831.
5. Koenig HG. Spirituality in patient care: why how, when, and what. West Conshohocken, PA: Templeton Press; 2007.
Electroconvulsive therapy: How modern techniques improve patient outcomes
Discuss this article at www.facebook.com/CurrentPsychiatry
Electroconvulsive therapy (ECT) has remained one of the most effective treatments for major depressive disorder (MDD) since it was introduced >70 years ago.1 ECT’s primary indication is severe, treatment-resistant MDD but sometimes it is used to treat other disorders, including bipolar mania and schizophrenia. In ECT, electrical current is delivered to a patient’s brain via electrodes placed on the scalp to induce a seizure while the patient is under anesthesia and a muscle relaxant. ECT’s exact mechanism of action for MDD is unknown, but researchers believe it may relieve depressive symptoms by regulating functional disturbances in relevant neural circuits.2
Research has shown that 64% to 87% of patients with severe MDD respond to ECT, with response rates as high as 95% for patients with MDD with psychotic features.3-5 Although patients may respond more quickly, 6 to 12 sessions typically are required to resolve a severe depressive episode.2
Despite ECT’s proven effectiveness, several factors have limited its widespread use, including limited access and expertise, adverse cognitive effects such as memory impairment, and negative public perception based on how ECT was administered decades ago.2 This article describes current methods of administering ECT, and how these changes have helped minimize these concerns while retaining efficacy.
Modern ECT practices
Since ECT was first used in the 1930s, clinicians have made many modifications to improve its efficacy and safety. Refinements to how ECT is administered include changing waveform parameters, individualizing dosing to seizure threshold, and altering electrode placement.6,7
Pulse width. Most ECT devices used today feature a constant-current output stimulator8 that allows continuous current regulation.7 Total charge, in millicoulombs (mC), is the common metric.7 Pulse width is a commonly altered waveform parameter in ECT delivery. Most research supports administering repeated brief or ultra-brief pulses (0.5 to 2 milliseconds), which is associated with greater charge efficiency and fewer side effects than traditional sine wave ECT dosing.8,9 Using a brief or ultra-brief pulse width increases clinical efficiency and decreases side effects because it focuses the stimulus on brain regions that regulate mood while limiting stimulation of brain regions involved in cognitive functioning.7 With brief-pulse stimulus, a patient’s cognitive performance may return to baseline levels within 3 days of treatment.6 Increasing evidence demonstrates that using a larger number of pulses with a brief pulse width and amplitude enhances ECT’s antidepressant effects while reducing unwanted neurocognitive side effects.7
Acute therapy patients typically receive 2 to 3 treatments each week,11,12 culminating in 12 to 18 treatments.8,12 The optimum number of sessions administered is determined by the ratio of clinical improvement to the severity of cognitive adverse effects.3
Electrode placement. Spatial targeting of stimulus is crucial to maximize therapeutic benefits and minimize side effects. Concerns about cognitive side effects have led to variations in electrode placement to minimize the amount of brain parenchyma affected by electrical discharge (Table).1,7,8 The most commonly used placements are:
- bitemporal (BT)—electrodes are placed midline between the eye and ear on both sides of the head
- right unilateral (RUL)—1 electrode is positioned just lateral to the vertex and the other at the right temple.7
Table
ECT electrodes: Bitemporal vs right unilateral placement
| Placement | Location | Comments |
|---|---|---|
| BT | Electrodes are placed midline between the eye and ear on both sides of the head | Stimulus is administered at 1.5 times a patient’s seizure threshold. Often used for patients who do not respond to several seizures with RUL |
| RUL | 1 electrode positioned just lateral to the vertex and the other at the right temple | When stimulus is administered in doses 6 times a patient’s seizure threshold, RUL is as effective as BT but avoids cognitive disruption. Offers only modest effects when stimulus is administered in doses close to a patient’s seizure threshold |
| BT: bitemporal; ECT: electroconvulsive therapy; RUL: right unilateral Source: References 1,7,8 | ||
Addressing safety concerns
In addition to changes to waveforms, dosing, and electrode placement, using anesthesia, muscle relaxants, and other medications has dramatically reduced adverse effects of ECT.8,10,13 See the Box10,14,15 for the specific agents used and their purposes. Before these medications and electroencephalography and electrocardiography (ECG) monitoring were used during ECT, the mortality rate was approximately 0.1%.13 Today, ECT is considered a low-risk medical intervention, with a mortality rate of approximately 0.002%.1,16 Before beginning an acute course of ECT, patients undergo laboratory testing, including a complete blood count, basic metabolic panel, and ECG. Spinal radiography and neuroimaging studies can be obtained to rule out preexisting vertebral injuries or neurologic disorders.1,8
Hemodynamic changes in response to ECT-induced seizures can exacerbate preexisting cardiac conditions. Normal physiologic response to ECT consists of a brief parasympathetic outflow, inducing bradycardia for 10 to 15 seconds, followed by a prominent sympathetic response characterized by hypertension and tachycardia for approximately 5 minutes. Although these changes can induce myocardial ischemia or infarction,14 the most common cardiac disturbances caused by ECT are arrhythmias, primarily in patients with preexisting cardiac abnormalities.17
Memory impairment. The most prevalent adverse reaction to ECT is memory loss, although not all aspects of recall are impaired to the same degree.18 Memory impairment varies based on factors such as electrode placement,9 stimulus waveform,19 site of seizure initiation, and pattern of activation.20 The risk of experiencing memory loss or other cognitive side effects following ECT can be decreased by using RUL electrode placement, brief pulses, and lower stimulus charge relative to seizure threshold.21 Memory deficits incurred by ECT usually are transient. In a study of 21 patients who received BT ECT for severe MDD, Meeter et al22 found that memory was stable and possibly improved at 3-month follow-up.
Procedural memory—memories of learned motor skills or mechanical tasks—often are left intact compared with semantic memory, which is general, declarative information recalled without context.18 The subsets of memory collectively regarded as declarative memory—the recollection of facts and events—may be most severely affected because this type of memory relies upon median temporal lobe structures, which are affected by ECT.21
Anterograde amnesia—the inability to form new memories—often is limited to the immediate posttreatment period and has been shown to become less pronounced at follow-up visits.22 Many clinicians and patients consider retrograde amnesia—forgetting memories that were formed before ECT—to be the most serious adverse effect of ECT. However, Mini-Mental State Examination scores tend to improve for patients who undergo ECT.1,16 Retrograde amnesia usually improves within weeks to months after ECT.12 Evidence suggests that retrograde amnesia mostly lifts during the recovery period and typically is not evident after 3 months.22 The best indicators of possible retrograde amnesic effects are preexisting cognitive deficits12 and duration of disorientation after ECT.1 Therefore, retrograde amnesia is more common among older adults, in whom age-related changes predispose patients to ECT’s adverse effects.24
The conventionally accepted mechanism for memory deficits after ECT is excitotoxic damage in the pyramidal cell layer of neurons in the hippocampus that induces calcium influx, which damages cells and causes neuronal atrophy.12 However, in animal studies, Dwork et al25 found an absence of neuronal or glial loss in regions subserving memory or cognitive functions (ie, the hippocampus or frontal cortex). Even in regions exquisitely sensitive to neuronal damage—such as CA1 of the hippocampus—neither cell number or volume or density of neuronal or glial cells were detected at statistically significant levels.25 Therefore, it is unlikely that ECT causes cell damage or atrophy in hippocampal neurons.
Anesthesia increases patients’ comfort during electroconvulsive therapy (ECT) by making them unaware of and unable to recall the procedure. The most commonly used anesthetic for ECT is methohexital, 0.5 to 1 mg/kg.14 Etomidate can be used in patients with contraindications to methohexital15; however, this medication can lengthen ictal duration.14 After the initial ECT treatment, clinicians can adjust the anesthetic dose based on the patient’s previous response.14
Using muscle relaxants during ECT has virtually eliminated bone fractures resulting from the procedure.10 The most common muscle relaxant is succinylcholine,15 which also reduces delirium in patients with post-ECT agitation.14 Mask ventilation and standard, noninvasive monitoring of cardiac parameters and oxygen saturation are necessary.14
Tachycardia and hypertension associated with ECT can be countered with beta blockers such as esmolol or labetalol as well as calcium channel blockers such as nicardipine.14 In addition, most patients are treated with the anticholinergic glycopyrrolate before the procedure to avoid bradycardia14 and reduce secretions, which may cause aspiration.15 Patients who experience headache or muscle pain after ECT can be treated with ibuprofen or acetaminophen before ECT sessions; patients with more severe complaints can be treated with IV ketorolac, 15 to 30 mg, before stimulus administration.15
Related Resources
- Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. 2012;2(3):283-344.
- Manka MV, Beyer JL, Weiner RD, et al. Clinical manual of electroconvulsive therapy. Arlington, VA: American Psychiatric Publishing; 2010.
- Esmolol • Brevibloc
- Etomidate • Amidate
- Glycopyrrolate • Robinul
- Ketorolac • Toradol
- Labetalol • Normodyne, Trandate
- Methohexital • Brevital
- Nicardipine • Cardene
- Succinylcholine • Anectine
Dr. Husain receives grant or research support from Brainsway, Cyberonics, MagStim, NARSAD, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Institute on Drug Abuse, NeoSync, Neuronetics, St. Jude Medical, and the Stanley Foundation.
Drs. Raza, Tirmizi, and Trevino report no relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatr Psychiatry. 2005;13(4):268-281.
2. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):67-74.
3. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the consortium for research in electroconvulsive therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337-1344.
4. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a consortium for research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491.
5. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244-253.
6. Semkovska M, Keane D, Babalola O, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res. 2011;45(6):770-780.
7. Peterchev AV, Rosa MA, Deng ZD, et al. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159-174.
8. Swartz CM. Electroconvulsive and neuromodulation therapies. New York, NY: Cambridge University Press; 2009.
9. Weiner RD, Rogers HJ, Davidson JR, et al. Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci. 1986;462:315-325.
10. Isenberg KE, Zorumski CF. Electroconvulsive therapy. In: Sadock BJ Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. Vol 2. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:2503–2515.
11. Trevino K, McClintock SM, Husain MM. A review of continuation electroconvulsive therapy: application safety, and efficacy. J ECT. 2010;26(3):186-195.
12. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
13. McDonald WM, McCall WV, Epstein CM. Electroconvulsive therapy: sixty years of progress and a comparison with transcranial magnetic stimulation and vagal nerve stimulation. In: Davis KL Charney D, Coyle JT, et al, eds. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:1097-1108.
14. Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94(5):1351-1364.
15. Kalinowsky LB. History of convulsive therapy. Ann N Y Acad Sci. 1986;462:1-4.
16. Ghaziuddin N, Dumas S, Hodges E. Use of continuation or maintenance electroconvulsive therapy in adolescents with severe treatment-resistant depression. J ECT. 2011;27(2):168-174.
17. Nuttall GA, Bowersox MR, Douglass SB, et al. Morbidity and mortality in the use of electroconvulsive therapy. J ECT. 2004;20(4):237-241.
18. Hihn H, Baune BT, Michael N, et al. Memory performance in severely depressed patients treated by electroconvulsive therapy. J ECT. 2006;22(3):189-195.
19. Prudic J, Peyser S, Sackeim HA. Subjective memory complaints: a review of patient self-assessment of memory after electroconvulsive therapy. J ECT. 2000;16(2):121-132.
20. Cycowicz YM, Luber B, Spellman T, et al. Neuro-physiological characterization of high-dose magnetic seizure therapy: comparisons with electroconvulsive shock and cognitive outcomes. J ECT. 2009;25(3):157-164.
21. Rami-Gonzalez L, Bernardo M, Boget T, et al. Subtypes of memory dysfunction associated with ECT: characteristics and neurobiological bases. J ECT. 2001;17(2):129-135.
22. Meeter M, Murre JM, Janssen SM, et al. Retrograde amnesia after electroconvulsive therapy: a temporary effect? J Affect Disord. 2011;132(1-2):216-222.
23. Kayser S, Bewernick BH, Grubert C, et al. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45(5):569-576.
24. van Schaik AM, Comijs HC, Sonnenberg CM, et al. Efficacy and safety of continuation and maintenance electroconvulsive therapy in depressed elderly patients: a systematic review. Am J Geriatr Psychiatry. 2012;20(1):5-17.
25. Dwork AJ, Christensen JR, Larsen KB, et al. Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience. 2009;164(4):1557-1564.
Discuss this article at www.facebook.com/CurrentPsychiatry
Electroconvulsive therapy (ECT) has remained one of the most effective treatments for major depressive disorder (MDD) since it was introduced >70 years ago.1 ECT’s primary indication is severe, treatment-resistant MDD but sometimes it is used to treat other disorders, including bipolar mania and schizophrenia. In ECT, electrical current is delivered to a patient’s brain via electrodes placed on the scalp to induce a seizure while the patient is under anesthesia and a muscle relaxant. ECT’s exact mechanism of action for MDD is unknown, but researchers believe it may relieve depressive symptoms by regulating functional disturbances in relevant neural circuits.2
Research has shown that 64% to 87% of patients with severe MDD respond to ECT, with response rates as high as 95% for patients with MDD with psychotic features.3-5 Although patients may respond more quickly, 6 to 12 sessions typically are required to resolve a severe depressive episode.2
Despite ECT’s proven effectiveness, several factors have limited its widespread use, including limited access and expertise, adverse cognitive effects such as memory impairment, and negative public perception based on how ECT was administered decades ago.2 This article describes current methods of administering ECT, and how these changes have helped minimize these concerns while retaining efficacy.
Modern ECT practices
Since ECT was first used in the 1930s, clinicians have made many modifications to improve its efficacy and safety. Refinements to how ECT is administered include changing waveform parameters, individualizing dosing to seizure threshold, and altering electrode placement.6,7
Pulse width. Most ECT devices used today feature a constant-current output stimulator8 that allows continuous current regulation.7 Total charge, in millicoulombs (mC), is the common metric.7 Pulse width is a commonly altered waveform parameter in ECT delivery. Most research supports administering repeated brief or ultra-brief pulses (0.5 to 2 milliseconds), which is associated with greater charge efficiency and fewer side effects than traditional sine wave ECT dosing.8,9 Using a brief or ultra-brief pulse width increases clinical efficiency and decreases side effects because it focuses the stimulus on brain regions that regulate mood while limiting stimulation of brain regions involved in cognitive functioning.7 With brief-pulse stimulus, a patient’s cognitive performance may return to baseline levels within 3 days of treatment.6 Increasing evidence demonstrates that using a larger number of pulses with a brief pulse width and amplitude enhances ECT’s antidepressant effects while reducing unwanted neurocognitive side effects.7
Acute therapy patients typically receive 2 to 3 treatments each week,11,12 culminating in 12 to 18 treatments.8,12 The optimum number of sessions administered is determined by the ratio of clinical improvement to the severity of cognitive adverse effects.3
Electrode placement. Spatial targeting of stimulus is crucial to maximize therapeutic benefits and minimize side effects. Concerns about cognitive side effects have led to variations in electrode placement to minimize the amount of brain parenchyma affected by electrical discharge (Table).1,7,8 The most commonly used placements are:
- bitemporal (BT)—electrodes are placed midline between the eye and ear on both sides of the head
- right unilateral (RUL)—1 electrode is positioned just lateral to the vertex and the other at the right temple.7
Table
ECT electrodes: Bitemporal vs right unilateral placement
| Placement | Location | Comments |
|---|---|---|
| BT | Electrodes are placed midline between the eye and ear on both sides of the head | Stimulus is administered at 1.5 times a patient’s seizure threshold. Often used for patients who do not respond to several seizures with RUL |
| RUL | 1 electrode positioned just lateral to the vertex and the other at the right temple | When stimulus is administered in doses 6 times a patient’s seizure threshold, RUL is as effective as BT but avoids cognitive disruption. Offers only modest effects when stimulus is administered in doses close to a patient’s seizure threshold |
| BT: bitemporal; ECT: electroconvulsive therapy; RUL: right unilateral Source: References 1,7,8 | ||
Addressing safety concerns
In addition to changes to waveforms, dosing, and electrode placement, using anesthesia, muscle relaxants, and other medications has dramatically reduced adverse effects of ECT.8,10,13 See the Box10,14,15 for the specific agents used and their purposes. Before these medications and electroencephalography and electrocardiography (ECG) monitoring were used during ECT, the mortality rate was approximately 0.1%.13 Today, ECT is considered a low-risk medical intervention, with a mortality rate of approximately 0.002%.1,16 Before beginning an acute course of ECT, patients undergo laboratory testing, including a complete blood count, basic metabolic panel, and ECG. Spinal radiography and neuroimaging studies can be obtained to rule out preexisting vertebral injuries or neurologic disorders.1,8
Hemodynamic changes in response to ECT-induced seizures can exacerbate preexisting cardiac conditions. Normal physiologic response to ECT consists of a brief parasympathetic outflow, inducing bradycardia for 10 to 15 seconds, followed by a prominent sympathetic response characterized by hypertension and tachycardia for approximately 5 minutes. Although these changes can induce myocardial ischemia or infarction,14 the most common cardiac disturbances caused by ECT are arrhythmias, primarily in patients with preexisting cardiac abnormalities.17
Memory impairment. The most prevalent adverse reaction to ECT is memory loss, although not all aspects of recall are impaired to the same degree.18 Memory impairment varies based on factors such as electrode placement,9 stimulus waveform,19 site of seizure initiation, and pattern of activation.20 The risk of experiencing memory loss or other cognitive side effects following ECT can be decreased by using RUL electrode placement, brief pulses, and lower stimulus charge relative to seizure threshold.21 Memory deficits incurred by ECT usually are transient. In a study of 21 patients who received BT ECT for severe MDD, Meeter et al22 found that memory was stable and possibly improved at 3-month follow-up.
Procedural memory—memories of learned motor skills or mechanical tasks—often are left intact compared with semantic memory, which is general, declarative information recalled without context.18 The subsets of memory collectively regarded as declarative memory—the recollection of facts and events—may be most severely affected because this type of memory relies upon median temporal lobe structures, which are affected by ECT.21
Anterograde amnesia—the inability to form new memories—often is limited to the immediate posttreatment period and has been shown to become less pronounced at follow-up visits.22 Many clinicians and patients consider retrograde amnesia—forgetting memories that were formed before ECT—to be the most serious adverse effect of ECT. However, Mini-Mental State Examination scores tend to improve for patients who undergo ECT.1,16 Retrograde amnesia usually improves within weeks to months after ECT.12 Evidence suggests that retrograde amnesia mostly lifts during the recovery period and typically is not evident after 3 months.22 The best indicators of possible retrograde amnesic effects are preexisting cognitive deficits12 and duration of disorientation after ECT.1 Therefore, retrograde amnesia is more common among older adults, in whom age-related changes predispose patients to ECT’s adverse effects.24
The conventionally accepted mechanism for memory deficits after ECT is excitotoxic damage in the pyramidal cell layer of neurons in the hippocampus that induces calcium influx, which damages cells and causes neuronal atrophy.12 However, in animal studies, Dwork et al25 found an absence of neuronal or glial loss in regions subserving memory or cognitive functions (ie, the hippocampus or frontal cortex). Even in regions exquisitely sensitive to neuronal damage—such as CA1 of the hippocampus—neither cell number or volume or density of neuronal or glial cells were detected at statistically significant levels.25 Therefore, it is unlikely that ECT causes cell damage or atrophy in hippocampal neurons.
Anesthesia increases patients’ comfort during electroconvulsive therapy (ECT) by making them unaware of and unable to recall the procedure. The most commonly used anesthetic for ECT is methohexital, 0.5 to 1 mg/kg.14 Etomidate can be used in patients with contraindications to methohexital15; however, this medication can lengthen ictal duration.14 After the initial ECT treatment, clinicians can adjust the anesthetic dose based on the patient’s previous response.14
Using muscle relaxants during ECT has virtually eliminated bone fractures resulting from the procedure.10 The most common muscle relaxant is succinylcholine,15 which also reduces delirium in patients with post-ECT agitation.14 Mask ventilation and standard, noninvasive monitoring of cardiac parameters and oxygen saturation are necessary.14
Tachycardia and hypertension associated with ECT can be countered with beta blockers such as esmolol or labetalol as well as calcium channel blockers such as nicardipine.14 In addition, most patients are treated with the anticholinergic glycopyrrolate before the procedure to avoid bradycardia14 and reduce secretions, which may cause aspiration.15 Patients who experience headache or muscle pain after ECT can be treated with ibuprofen or acetaminophen before ECT sessions; patients with more severe complaints can be treated with IV ketorolac, 15 to 30 mg, before stimulus administration.15
Related Resources
- Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. 2012;2(3):283-344.
- Manka MV, Beyer JL, Weiner RD, et al. Clinical manual of electroconvulsive therapy. Arlington, VA: American Psychiatric Publishing; 2010.
- Esmolol • Brevibloc
- Etomidate • Amidate
- Glycopyrrolate • Robinul
- Ketorolac • Toradol
- Labetalol • Normodyne, Trandate
- Methohexital • Brevital
- Nicardipine • Cardene
- Succinylcholine • Anectine
Dr. Husain receives grant or research support from Brainsway, Cyberonics, MagStim, NARSAD, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Institute on Drug Abuse, NeoSync, Neuronetics, St. Jude Medical, and the Stanley Foundation.
Drs. Raza, Tirmizi, and Trevino report no relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Electroconvulsive therapy (ECT) has remained one of the most effective treatments for major depressive disorder (MDD) since it was introduced >70 years ago.1 ECT’s primary indication is severe, treatment-resistant MDD but sometimes it is used to treat other disorders, including bipolar mania and schizophrenia. In ECT, electrical current is delivered to a patient’s brain via electrodes placed on the scalp to induce a seizure while the patient is under anesthesia and a muscle relaxant. ECT’s exact mechanism of action for MDD is unknown, but researchers believe it may relieve depressive symptoms by regulating functional disturbances in relevant neural circuits.2
Research has shown that 64% to 87% of patients with severe MDD respond to ECT, with response rates as high as 95% for patients with MDD with psychotic features.3-5 Although patients may respond more quickly, 6 to 12 sessions typically are required to resolve a severe depressive episode.2
Despite ECT’s proven effectiveness, several factors have limited its widespread use, including limited access and expertise, adverse cognitive effects such as memory impairment, and negative public perception based on how ECT was administered decades ago.2 This article describes current methods of administering ECT, and how these changes have helped minimize these concerns while retaining efficacy.
Modern ECT practices
Since ECT was first used in the 1930s, clinicians have made many modifications to improve its efficacy and safety. Refinements to how ECT is administered include changing waveform parameters, individualizing dosing to seizure threshold, and altering electrode placement.6,7
Pulse width. Most ECT devices used today feature a constant-current output stimulator8 that allows continuous current regulation.7 Total charge, in millicoulombs (mC), is the common metric.7 Pulse width is a commonly altered waveform parameter in ECT delivery. Most research supports administering repeated brief or ultra-brief pulses (0.5 to 2 milliseconds), which is associated with greater charge efficiency and fewer side effects than traditional sine wave ECT dosing.8,9 Using a brief or ultra-brief pulse width increases clinical efficiency and decreases side effects because it focuses the stimulus on brain regions that regulate mood while limiting stimulation of brain regions involved in cognitive functioning.7 With brief-pulse stimulus, a patient’s cognitive performance may return to baseline levels within 3 days of treatment.6 Increasing evidence demonstrates that using a larger number of pulses with a brief pulse width and amplitude enhances ECT’s antidepressant effects while reducing unwanted neurocognitive side effects.7
Acute therapy patients typically receive 2 to 3 treatments each week,11,12 culminating in 12 to 18 treatments.8,12 The optimum number of sessions administered is determined by the ratio of clinical improvement to the severity of cognitive adverse effects.3
Electrode placement. Spatial targeting of stimulus is crucial to maximize therapeutic benefits and minimize side effects. Concerns about cognitive side effects have led to variations in electrode placement to minimize the amount of brain parenchyma affected by electrical discharge (Table).1,7,8 The most commonly used placements are:
- bitemporal (BT)—electrodes are placed midline between the eye and ear on both sides of the head
- right unilateral (RUL)—1 electrode is positioned just lateral to the vertex and the other at the right temple.7
Table
ECT electrodes: Bitemporal vs right unilateral placement
| Placement | Location | Comments |
|---|---|---|
| BT | Electrodes are placed midline between the eye and ear on both sides of the head | Stimulus is administered at 1.5 times a patient’s seizure threshold. Often used for patients who do not respond to several seizures with RUL |
| RUL | 1 electrode positioned just lateral to the vertex and the other at the right temple | When stimulus is administered in doses 6 times a patient’s seizure threshold, RUL is as effective as BT but avoids cognitive disruption. Offers only modest effects when stimulus is administered in doses close to a patient’s seizure threshold |
| BT: bitemporal; ECT: electroconvulsive therapy; RUL: right unilateral Source: References 1,7,8 | ||
Addressing safety concerns
In addition to changes to waveforms, dosing, and electrode placement, using anesthesia, muscle relaxants, and other medications has dramatically reduced adverse effects of ECT.8,10,13 See the Box10,14,15 for the specific agents used and their purposes. Before these medications and electroencephalography and electrocardiography (ECG) monitoring were used during ECT, the mortality rate was approximately 0.1%.13 Today, ECT is considered a low-risk medical intervention, with a mortality rate of approximately 0.002%.1,16 Before beginning an acute course of ECT, patients undergo laboratory testing, including a complete blood count, basic metabolic panel, and ECG. Spinal radiography and neuroimaging studies can be obtained to rule out preexisting vertebral injuries or neurologic disorders.1,8
Hemodynamic changes in response to ECT-induced seizures can exacerbate preexisting cardiac conditions. Normal physiologic response to ECT consists of a brief parasympathetic outflow, inducing bradycardia for 10 to 15 seconds, followed by a prominent sympathetic response characterized by hypertension and tachycardia for approximately 5 minutes. Although these changes can induce myocardial ischemia or infarction,14 the most common cardiac disturbances caused by ECT are arrhythmias, primarily in patients with preexisting cardiac abnormalities.17
Memory impairment. The most prevalent adverse reaction to ECT is memory loss, although not all aspects of recall are impaired to the same degree.18 Memory impairment varies based on factors such as electrode placement,9 stimulus waveform,19 site of seizure initiation, and pattern of activation.20 The risk of experiencing memory loss or other cognitive side effects following ECT can be decreased by using RUL electrode placement, brief pulses, and lower stimulus charge relative to seizure threshold.21 Memory deficits incurred by ECT usually are transient. In a study of 21 patients who received BT ECT for severe MDD, Meeter et al22 found that memory was stable and possibly improved at 3-month follow-up.
Procedural memory—memories of learned motor skills or mechanical tasks—often are left intact compared with semantic memory, which is general, declarative information recalled without context.18 The subsets of memory collectively regarded as declarative memory—the recollection of facts and events—may be most severely affected because this type of memory relies upon median temporal lobe structures, which are affected by ECT.21
Anterograde amnesia—the inability to form new memories—often is limited to the immediate posttreatment period and has been shown to become less pronounced at follow-up visits.22 Many clinicians and patients consider retrograde amnesia—forgetting memories that were formed before ECT—to be the most serious adverse effect of ECT. However, Mini-Mental State Examination scores tend to improve for patients who undergo ECT.1,16 Retrograde amnesia usually improves within weeks to months after ECT.12 Evidence suggests that retrograde amnesia mostly lifts during the recovery period and typically is not evident after 3 months.22 The best indicators of possible retrograde amnesic effects are preexisting cognitive deficits12 and duration of disorientation after ECT.1 Therefore, retrograde amnesia is more common among older adults, in whom age-related changes predispose patients to ECT’s adverse effects.24
The conventionally accepted mechanism for memory deficits after ECT is excitotoxic damage in the pyramidal cell layer of neurons in the hippocampus that induces calcium influx, which damages cells and causes neuronal atrophy.12 However, in animal studies, Dwork et al25 found an absence of neuronal or glial loss in regions subserving memory or cognitive functions (ie, the hippocampus or frontal cortex). Even in regions exquisitely sensitive to neuronal damage—such as CA1 of the hippocampus—neither cell number or volume or density of neuronal or glial cells were detected at statistically significant levels.25 Therefore, it is unlikely that ECT causes cell damage or atrophy in hippocampal neurons.
Anesthesia increases patients’ comfort during electroconvulsive therapy (ECT) by making them unaware of and unable to recall the procedure. The most commonly used anesthetic for ECT is methohexital, 0.5 to 1 mg/kg.14 Etomidate can be used in patients with contraindications to methohexital15; however, this medication can lengthen ictal duration.14 After the initial ECT treatment, clinicians can adjust the anesthetic dose based on the patient’s previous response.14
Using muscle relaxants during ECT has virtually eliminated bone fractures resulting from the procedure.10 The most common muscle relaxant is succinylcholine,15 which also reduces delirium in patients with post-ECT agitation.14 Mask ventilation and standard, noninvasive monitoring of cardiac parameters and oxygen saturation are necessary.14
Tachycardia and hypertension associated with ECT can be countered with beta blockers such as esmolol or labetalol as well as calcium channel blockers such as nicardipine.14 In addition, most patients are treated with the anticholinergic glycopyrrolate before the procedure to avoid bradycardia14 and reduce secretions, which may cause aspiration.15 Patients who experience headache or muscle pain after ECT can be treated with ibuprofen or acetaminophen before ECT sessions; patients with more severe complaints can be treated with IV ketorolac, 15 to 30 mg, before stimulus administration.15
Related Resources
- Leiknes KA, Jarosh-von Schweder L, Høie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain Behav. 2012;2(3):283-344.
- Manka MV, Beyer JL, Weiner RD, et al. Clinical manual of electroconvulsive therapy. Arlington, VA: American Psychiatric Publishing; 2010.
- Esmolol • Brevibloc
- Etomidate • Amidate
- Glycopyrrolate • Robinul
- Ketorolac • Toradol
- Labetalol • Normodyne, Trandate
- Methohexital • Brevital
- Nicardipine • Cardene
- Succinylcholine • Anectine
Dr. Husain receives grant or research support from Brainsway, Cyberonics, MagStim, NARSAD, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Institute on Drug Abuse, NeoSync, Neuronetics, St. Jude Medical, and the Stanley Foundation.
Drs. Raza, Tirmizi, and Trevino report no relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatr Psychiatry. 2005;13(4):268-281.
2. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):67-74.
3. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the consortium for research in electroconvulsive therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337-1344.
4. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a consortium for research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491.
5. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244-253.
6. Semkovska M, Keane D, Babalola O, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res. 2011;45(6):770-780.
7. Peterchev AV, Rosa MA, Deng ZD, et al. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159-174.
8. Swartz CM. Electroconvulsive and neuromodulation therapies. New York, NY: Cambridge University Press; 2009.
9. Weiner RD, Rogers HJ, Davidson JR, et al. Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci. 1986;462:315-325.
10. Isenberg KE, Zorumski CF. Electroconvulsive therapy. In: Sadock BJ Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. Vol 2. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:2503–2515.
11. Trevino K, McClintock SM, Husain MM. A review of continuation electroconvulsive therapy: application safety, and efficacy. J ECT. 2010;26(3):186-195.
12. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
13. McDonald WM, McCall WV, Epstein CM. Electroconvulsive therapy: sixty years of progress and a comparison with transcranial magnetic stimulation and vagal nerve stimulation. In: Davis KL Charney D, Coyle JT, et al, eds. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:1097-1108.
14. Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94(5):1351-1364.
15. Kalinowsky LB. History of convulsive therapy. Ann N Y Acad Sci. 1986;462:1-4.
16. Ghaziuddin N, Dumas S, Hodges E. Use of continuation or maintenance electroconvulsive therapy in adolescents with severe treatment-resistant depression. J ECT. 2011;27(2):168-174.
17. Nuttall GA, Bowersox MR, Douglass SB, et al. Morbidity and mortality in the use of electroconvulsive therapy. J ECT. 2004;20(4):237-241.
18. Hihn H, Baune BT, Michael N, et al. Memory performance in severely depressed patients treated by electroconvulsive therapy. J ECT. 2006;22(3):189-195.
19. Prudic J, Peyser S, Sackeim HA. Subjective memory complaints: a review of patient self-assessment of memory after electroconvulsive therapy. J ECT. 2000;16(2):121-132.
20. Cycowicz YM, Luber B, Spellman T, et al. Neuro-physiological characterization of high-dose magnetic seizure therapy: comparisons with electroconvulsive shock and cognitive outcomes. J ECT. 2009;25(3):157-164.
21. Rami-Gonzalez L, Bernardo M, Boget T, et al. Subtypes of memory dysfunction associated with ECT: characteristics and neurobiological bases. J ECT. 2001;17(2):129-135.
22. Meeter M, Murre JM, Janssen SM, et al. Retrograde amnesia after electroconvulsive therapy: a temporary effect? J Affect Disord. 2011;132(1-2):216-222.
23. Kayser S, Bewernick BH, Grubert C, et al. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45(5):569-576.
24. van Schaik AM, Comijs HC, Sonnenberg CM, et al. Efficacy and safety of continuation and maintenance electroconvulsive therapy in depressed elderly patients: a systematic review. Am J Geriatr Psychiatry. 2012;20(1):5-17.
25. Dwork AJ, Christensen JR, Larsen KB, et al. Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience. 2009;164(4):1557-1564.
1. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatr Psychiatry. 2005;13(4):268-281.
2. Janicak PG, Dowd SM, Rado JT, et al. The re-emerging role of therapeutic neuromodulation. Current Psychiatry. 2010;9(11):67-74.
3. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the consortium for research in electroconvulsive therapy (CORE). Arch Gen Psychiatry. 2006;63(12):1337-1344.
4. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a consortium for research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491.
5. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244-253.
6. Semkovska M, Keane D, Babalola O, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: effects of electrode placement, stimulus dosage and time. J Psychiatr Res. 2011;45(6):770-780.
7. Peterchev AV, Rosa MA, Deng ZD, et al. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159-174.
8. Swartz CM. Electroconvulsive and neuromodulation therapies. New York, NY: Cambridge University Press; 2009.
9. Weiner RD, Rogers HJ, Davidson JR, et al. Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci. 1986;462:315-325.
10. Isenberg KE, Zorumski CF. Electroconvulsive therapy. In: Sadock BJ Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. Vol 2. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:2503–2515.
11. Trevino K, McClintock SM, Husain MM. A review of continuation electroconvulsive therapy: application safety, and efficacy. J ECT. 2010;26(3):186-195.
12. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
13. McDonald WM, McCall WV, Epstein CM. Electroconvulsive therapy: sixty years of progress and a comparison with transcranial magnetic stimulation and vagal nerve stimulation. In: Davis KL Charney D, Coyle JT, et al, eds. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002:1097-1108.
14. Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94(5):1351-1364.
15. Kalinowsky LB. History of convulsive therapy. Ann N Y Acad Sci. 1986;462:1-4.
16. Ghaziuddin N, Dumas S, Hodges E. Use of continuation or maintenance electroconvulsive therapy in adolescents with severe treatment-resistant depression. J ECT. 2011;27(2):168-174.
17. Nuttall GA, Bowersox MR, Douglass SB, et al. Morbidity and mortality in the use of electroconvulsive therapy. J ECT. 2004;20(4):237-241.
18. Hihn H, Baune BT, Michael N, et al. Memory performance in severely depressed patients treated by electroconvulsive therapy. J ECT. 2006;22(3):189-195.
19. Prudic J, Peyser S, Sackeim HA. Subjective memory complaints: a review of patient self-assessment of memory after electroconvulsive therapy. J ECT. 2000;16(2):121-132.
20. Cycowicz YM, Luber B, Spellman T, et al. Neuro-physiological characterization of high-dose magnetic seizure therapy: comparisons with electroconvulsive shock and cognitive outcomes. J ECT. 2009;25(3):157-164.
21. Rami-Gonzalez L, Bernardo M, Boget T, et al. Subtypes of memory dysfunction associated with ECT: characteristics and neurobiological bases. J ECT. 2001;17(2):129-135.
22. Meeter M, Murre JM, Janssen SM, et al. Retrograde amnesia after electroconvulsive therapy: a temporary effect? J Affect Disord. 2011;132(1-2):216-222.
23. Kayser S, Bewernick BH, Grubert C, et al. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45(5):569-576.
24. van Schaik AM, Comijs HC, Sonnenberg CM, et al. Efficacy and safety of continuation and maintenance electroconvulsive therapy in depressed elderly patients: a systematic review. Am J Geriatr Psychiatry. 2012;20(1):5-17.
25. Dwork AJ, Christensen JR, Larsen KB, et al. Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience. 2009;164(4):1557-1564.
Psychiatry and the politics of incarceration
I have always regarded the French saying “plus ça change, plus c’est la même chose” (the more things change, the more they are the same) to be a quote for the ages. Nowhere is this truism more evident than in the fluctuations in incarceration of individuals with serious mental illness (SMI) in the United States and persecution of real and faux patients in certain regimes around the world.
A truly jarring 2010 report by E. Fuller Torrey et al1 revealed the shocking deterioration and regression of the United States mental health system. In 2010, the percentage of persons with SMI in jails and prisons ballooned to the same as it was 170 years ago! The deplorable mistreatment of the mentally ill in 1840, due to pervasive ignorance, prompted legendary reformer Dorothea Dix to launch her historic campaign for a more humane (asylum-based) treatment of persons afflicted with severe mental disorders. How troubling it is that the iconic Dorothea Dix Hospital in Raleigh, NC was shuttered earlier this year! Built on >2,300 acres and eventually growing to 282 buildings (in 1974), housing approximately 3,000 patients cared for by >6,000 employees on 3 around-the-clock shifts, this institution was a revered symbol of the transition from unjust criminalization to humane medical treatment of the SMI population. All other states eventually established similar medical institutions to house, protect, and care for the severely mentally ill, even though no effective treatments were available until the serendipitous discovery that an anesthetic adjunctive agent, a phenothiazine called chlorpromazine, could miraculously suppress delusions, hallucinations, and bizarre behavior.
Throughout the 20th century, while patients with SMI in the United States were hospitalized instead of incarcerated, several despotic regimes abused the mentally ill or misused psychiatric institutions as proxies for prisons. The malevolent and criminal Nazi regime determined that mentally ill or mentally challenged individuals were “unworthy to live” and turned many psychiatric institutions into “killing centers” to “euthanize” persons with SMI with lethal injections, and later with carbon monoxide. Some psychiatrists and clergy raised objections but they were ignored or suppressed.
The totalitarian Soviet Union was notorious for abusing psychiatry by “diagnosing” political dissenters as “schizophrenic” and incarcerating them for life in psychiatric hospitals, which eventually were transformed into political prisons for those protesting the dictatorship of the Soviet regime. Other communist countries adopted a similar approach to silence dissenters and some reportedly still are doing this today. Regrettably, a regressive event took place in America, a paragon of freedom and social justice in the world. In 1983, 6.4% of prison inmates had SMI. This proportion almost tripled to 16% in 2010 and continues to grow steadily. This tragic deterioration is embodied in the following statistics from Torrey et al1: today there are 300% more patients with SMI in jails and prisons than in hospitals around the United States. Some states have truly scandalous figures: in Arizona and Nevada, there are 10 times more patients with SMI in prisons and jails than in hospitals!
There also is an alarming, even dangerous, shortage of psychiatric beds in the United States. Psychiatrists and other mental health professionals are painfully aware of how many inpatient units have closed in cities and towns across the country: In 1955, there was 1 psychiatric bed for every 300 citizens. In 2010, the ratio had fallen drastically to 1 bed for every 3,000 Americans. To make things worse, in most states most remaining beds are filled by court-ordered patients and are, in fact, not available for new patients.
Sadly, as was the case in 1840, the United States now incarcerates the majority of its seriously mentally ill citizens. So much has changed in the United States over the past 2 centuries, yet for patients with SMI, things are practically the same as in a medically primitive era of our past. How can we allow this virulent plague of widespread incarceration and criminalization that has afflicted the sickest and most vulnerable psychiatric patients, who are being denied the compassion and medical management that they deserve? When will we unite and strongly demand and lobby for a more just treatment of persons with psychiatric disorders and scream that they are medical conditions, not criminal cases? Why do we, mental health professionals, remain silent and go on with our daily work, implicitly accepting the awful status quo? Isn’t incarcerating, instead of hospitalizing, the truly mentally ill just as immoral and deplorable as forcibly hospitalizing mentally healthy political dissenters in the Soviet Union? Why was there universal condemnation of human rights violations by the Soviet dictatorship but a deafening silence about widespread incarceration of medically disabled persons in the land of the free? Why aren’t we adopting policies to expedite and facilitate psychiatric treatment, ensure adherence, and promote remission in those who suffer their first psychotic or manic episode? Why are we building more jails and prisons instead of therapeutic communities?
Although things have changed a lot in the United States since 1840, if a modern day Dorothea Dix does not emerge, then in many ways they will remain the same. And that’s a real shame.
Reference
1. Torrey EF, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. Arlington, VA: Treatment Advisory Center; 2010.
I have always regarded the French saying “plus ça change, plus c’est la même chose” (the more things change, the more they are the same) to be a quote for the ages. Nowhere is this truism more evident than in the fluctuations in incarceration of individuals with serious mental illness (SMI) in the United States and persecution of real and faux patients in certain regimes around the world.
A truly jarring 2010 report by E. Fuller Torrey et al1 revealed the shocking deterioration and regression of the United States mental health system. In 2010, the percentage of persons with SMI in jails and prisons ballooned to the same as it was 170 years ago! The deplorable mistreatment of the mentally ill in 1840, due to pervasive ignorance, prompted legendary reformer Dorothea Dix to launch her historic campaign for a more humane (asylum-based) treatment of persons afflicted with severe mental disorders. How troubling it is that the iconic Dorothea Dix Hospital in Raleigh, NC was shuttered earlier this year! Built on >2,300 acres and eventually growing to 282 buildings (in 1974), housing approximately 3,000 patients cared for by >6,000 employees on 3 around-the-clock shifts, this institution was a revered symbol of the transition from unjust criminalization to humane medical treatment of the SMI population. All other states eventually established similar medical institutions to house, protect, and care for the severely mentally ill, even though no effective treatments were available until the serendipitous discovery that an anesthetic adjunctive agent, a phenothiazine called chlorpromazine, could miraculously suppress delusions, hallucinations, and bizarre behavior.
Throughout the 20th century, while patients with SMI in the United States were hospitalized instead of incarcerated, several despotic regimes abused the mentally ill or misused psychiatric institutions as proxies for prisons. The malevolent and criminal Nazi regime determined that mentally ill or mentally challenged individuals were “unworthy to live” and turned many psychiatric institutions into “killing centers” to “euthanize” persons with SMI with lethal injections, and later with carbon monoxide. Some psychiatrists and clergy raised objections but they were ignored or suppressed.
The totalitarian Soviet Union was notorious for abusing psychiatry by “diagnosing” political dissenters as “schizophrenic” and incarcerating them for life in psychiatric hospitals, which eventually were transformed into political prisons for those protesting the dictatorship of the Soviet regime. Other communist countries adopted a similar approach to silence dissenters and some reportedly still are doing this today. Regrettably, a regressive event took place in America, a paragon of freedom and social justice in the world. In 1983, 6.4% of prison inmates had SMI. This proportion almost tripled to 16% in 2010 and continues to grow steadily. This tragic deterioration is embodied in the following statistics from Torrey et al1: today there are 300% more patients with SMI in jails and prisons than in hospitals around the United States. Some states have truly scandalous figures: in Arizona and Nevada, there are 10 times more patients with SMI in prisons and jails than in hospitals!
There also is an alarming, even dangerous, shortage of psychiatric beds in the United States. Psychiatrists and other mental health professionals are painfully aware of how many inpatient units have closed in cities and towns across the country: In 1955, there was 1 psychiatric bed for every 300 citizens. In 2010, the ratio had fallen drastically to 1 bed for every 3,000 Americans. To make things worse, in most states most remaining beds are filled by court-ordered patients and are, in fact, not available for new patients.
Sadly, as was the case in 1840, the United States now incarcerates the majority of its seriously mentally ill citizens. So much has changed in the United States over the past 2 centuries, yet for patients with SMI, things are practically the same as in a medically primitive era of our past. How can we allow this virulent plague of widespread incarceration and criminalization that has afflicted the sickest and most vulnerable psychiatric patients, who are being denied the compassion and medical management that they deserve? When will we unite and strongly demand and lobby for a more just treatment of persons with psychiatric disorders and scream that they are medical conditions, not criminal cases? Why do we, mental health professionals, remain silent and go on with our daily work, implicitly accepting the awful status quo? Isn’t incarcerating, instead of hospitalizing, the truly mentally ill just as immoral and deplorable as forcibly hospitalizing mentally healthy political dissenters in the Soviet Union? Why was there universal condemnation of human rights violations by the Soviet dictatorship but a deafening silence about widespread incarceration of medically disabled persons in the land of the free? Why aren’t we adopting policies to expedite and facilitate psychiatric treatment, ensure adherence, and promote remission in those who suffer their first psychotic or manic episode? Why are we building more jails and prisons instead of therapeutic communities?
Although things have changed a lot in the United States since 1840, if a modern day Dorothea Dix does not emerge, then in many ways they will remain the same. And that’s a real shame.
I have always regarded the French saying “plus ça change, plus c’est la même chose” (the more things change, the more they are the same) to be a quote for the ages. Nowhere is this truism more evident than in the fluctuations in incarceration of individuals with serious mental illness (SMI) in the United States and persecution of real and faux patients in certain regimes around the world.
A truly jarring 2010 report by E. Fuller Torrey et al1 revealed the shocking deterioration and regression of the United States mental health system. In 2010, the percentage of persons with SMI in jails and prisons ballooned to the same as it was 170 years ago! The deplorable mistreatment of the mentally ill in 1840, due to pervasive ignorance, prompted legendary reformer Dorothea Dix to launch her historic campaign for a more humane (asylum-based) treatment of persons afflicted with severe mental disorders. How troubling it is that the iconic Dorothea Dix Hospital in Raleigh, NC was shuttered earlier this year! Built on >2,300 acres and eventually growing to 282 buildings (in 1974), housing approximately 3,000 patients cared for by >6,000 employees on 3 around-the-clock shifts, this institution was a revered symbol of the transition from unjust criminalization to humane medical treatment of the SMI population. All other states eventually established similar medical institutions to house, protect, and care for the severely mentally ill, even though no effective treatments were available until the serendipitous discovery that an anesthetic adjunctive agent, a phenothiazine called chlorpromazine, could miraculously suppress delusions, hallucinations, and bizarre behavior.
Throughout the 20th century, while patients with SMI in the United States were hospitalized instead of incarcerated, several despotic regimes abused the mentally ill or misused psychiatric institutions as proxies for prisons. The malevolent and criminal Nazi regime determined that mentally ill or mentally challenged individuals were “unworthy to live” and turned many psychiatric institutions into “killing centers” to “euthanize” persons with SMI with lethal injections, and later with carbon monoxide. Some psychiatrists and clergy raised objections but they were ignored or suppressed.
The totalitarian Soviet Union was notorious for abusing psychiatry by “diagnosing” political dissenters as “schizophrenic” and incarcerating them for life in psychiatric hospitals, which eventually were transformed into political prisons for those protesting the dictatorship of the Soviet regime. Other communist countries adopted a similar approach to silence dissenters and some reportedly still are doing this today. Regrettably, a regressive event took place in America, a paragon of freedom and social justice in the world. In 1983, 6.4% of prison inmates had SMI. This proportion almost tripled to 16% in 2010 and continues to grow steadily. This tragic deterioration is embodied in the following statistics from Torrey et al1: today there are 300% more patients with SMI in jails and prisons than in hospitals around the United States. Some states have truly scandalous figures: in Arizona and Nevada, there are 10 times more patients with SMI in prisons and jails than in hospitals!
There also is an alarming, even dangerous, shortage of psychiatric beds in the United States. Psychiatrists and other mental health professionals are painfully aware of how many inpatient units have closed in cities and towns across the country: In 1955, there was 1 psychiatric bed for every 300 citizens. In 2010, the ratio had fallen drastically to 1 bed for every 3,000 Americans. To make things worse, in most states most remaining beds are filled by court-ordered patients and are, in fact, not available for new patients.
Sadly, as was the case in 1840, the United States now incarcerates the majority of its seriously mentally ill citizens. So much has changed in the United States over the past 2 centuries, yet for patients with SMI, things are practically the same as in a medically primitive era of our past. How can we allow this virulent plague of widespread incarceration and criminalization that has afflicted the sickest and most vulnerable psychiatric patients, who are being denied the compassion and medical management that they deserve? When will we unite and strongly demand and lobby for a more just treatment of persons with psychiatric disorders and scream that they are medical conditions, not criminal cases? Why do we, mental health professionals, remain silent and go on with our daily work, implicitly accepting the awful status quo? Isn’t incarcerating, instead of hospitalizing, the truly mentally ill just as immoral and deplorable as forcibly hospitalizing mentally healthy political dissenters in the Soviet Union? Why was there universal condemnation of human rights violations by the Soviet dictatorship but a deafening silence about widespread incarceration of medically disabled persons in the land of the free? Why aren’t we adopting policies to expedite and facilitate psychiatric treatment, ensure adherence, and promote remission in those who suffer their first psychotic or manic episode? Why are we building more jails and prisons instead of therapeutic communities?
Although things have changed a lot in the United States since 1840, if a modern day Dorothea Dix does not emerge, then in many ways they will remain the same. And that’s a real shame.
Reference
1. Torrey EF, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. Arlington, VA: Treatment Advisory Center; 2010.
Reference
1. Torrey EF, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. Arlington, VA: Treatment Advisory Center; 2010.