User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Considerations for CAM use
I thoroughly enjoyed the article on complementary and alternative medicine (CAM) for depression (“CAM for your depressed patient: 6 recommended options,” Current Psychiatry, October 2009) and have seen patients benefit from these treatments. I wish the authors had included information about the use of valerian root for anxiety, as this is common among some CAM users.
I think our use or discussion of CAM can show our patients we are flexible and will consider various treatments. I believe if you dismiss all CAM treatments as not as effective as prescription medications—which may be true—you will lose patients. We know how popular CAM is with the American public, despite lack of evidence and poor oversight.
In depression treatment, exercise is as effective as sertraline in some studies,1,2 but I would think the high dropout rate for exercise would make sertraline more likely to be effective in the long run. In 1 study, participants received a phone call if they missed an exercise session. This doesn’t mimic real life at all. Also St. John’s wort is administered 300 mg tid, while many antidepressants are once a day. Efficacy aside, we can guess that compliance with a medication taken 3 times a day will be less than 1 taken once daily.
I believe we need to examine our patients’ thoughts about CAM vs traditional treatment. Do they feel CAM is safer because it is natural? Do they feel less stigma if they use CAM? What are their “automatic thoughts” about this?
I disagree with the conclusion that bibliotherapy can’t hurt. Bibliotherapy does have a cost: the cost of the book, the time spent reading it, and minimal benefit. There are people making millions of dollars on self-help books that may be having little, if any, impact on our patients’ lives.
Corey Yilmaz, MD
Southwest Behavioral Health Rural Services
Tolleson, AZ
References
1. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
2. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
Dr. Saeed responds
Despite the common belief that valerian root is effective in reducing stress and anxiety, it has not been tested for depressive disorders and is not supported by studies on anxiety disorders. Our paper did not review CAM treatments for anxiety disorders, so we did not point out that a recent Cochrane review1 of valerian for anxiety disorders included only 1 randomized controlled trial2 and found no differences between valerian and placebo.
We also agree that treatment discontinuation is a serious problem, but this is a universal concern for treating many chronic disorders. There is evidence that patient reports of treatment adherence can be unreliable. Research has shown that periodic monitoring,3 even by automated systems, can maintain compliance longer.
We disagree with Dr. Yilmaz’ comments about bibliotherapy. A meta-analysis of 29 bibliotherapy studies found bibliotherapy using cognitive and behavioral techniques superior to wait-list comparison groups.4 We feel there is ample evidence supporting bibliotherapy as a low-risk, low-cost alternative or complementary treatment for mild-to-moderate depressive disorder.
Sy Atezaz Saeed, MD
Department of psychiatric medicine
Brody School of Medicine at
East Carolina University
1. Miyasaka LS, Atallah AN, Soares BG. Valerian for anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD004515.-
2. Andreatini R, Sartori VA, Seabra ML, et al. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytother Res. 2002;16(7):650-654.
3. Gensichen J, von Korff M, Peitz M, et al. Case management for depression by health care assistants in small primary care practices: a cluster randomized trial. Ann Intern Med. 2009;151(6):369-378.
4. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
I thoroughly enjoyed the article on complementary and alternative medicine (CAM) for depression (“CAM for your depressed patient: 6 recommended options,” Current Psychiatry, October 2009) and have seen patients benefit from these treatments. I wish the authors had included information about the use of valerian root for anxiety, as this is common among some CAM users.
I think our use or discussion of CAM can show our patients we are flexible and will consider various treatments. I believe if you dismiss all CAM treatments as not as effective as prescription medications—which may be true—you will lose patients. We know how popular CAM is with the American public, despite lack of evidence and poor oversight.
In depression treatment, exercise is as effective as sertraline in some studies,1,2 but I would think the high dropout rate for exercise would make sertraline more likely to be effective in the long run. In 1 study, participants received a phone call if they missed an exercise session. This doesn’t mimic real life at all. Also St. John’s wort is administered 300 mg tid, while many antidepressants are once a day. Efficacy aside, we can guess that compliance with a medication taken 3 times a day will be less than 1 taken once daily.
I believe we need to examine our patients’ thoughts about CAM vs traditional treatment. Do they feel CAM is safer because it is natural? Do they feel less stigma if they use CAM? What are their “automatic thoughts” about this?
I disagree with the conclusion that bibliotherapy can’t hurt. Bibliotherapy does have a cost: the cost of the book, the time spent reading it, and minimal benefit. There are people making millions of dollars on self-help books that may be having little, if any, impact on our patients’ lives.
Corey Yilmaz, MD
Southwest Behavioral Health Rural Services
Tolleson, AZ
References
1. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
2. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
Dr. Saeed responds
Despite the common belief that valerian root is effective in reducing stress and anxiety, it has not been tested for depressive disorders and is not supported by studies on anxiety disorders. Our paper did not review CAM treatments for anxiety disorders, so we did not point out that a recent Cochrane review1 of valerian for anxiety disorders included only 1 randomized controlled trial2 and found no differences between valerian and placebo.
We also agree that treatment discontinuation is a serious problem, but this is a universal concern for treating many chronic disorders. There is evidence that patient reports of treatment adherence can be unreliable. Research has shown that periodic monitoring,3 even by automated systems, can maintain compliance longer.
We disagree with Dr. Yilmaz’ comments about bibliotherapy. A meta-analysis of 29 bibliotherapy studies found bibliotherapy using cognitive and behavioral techniques superior to wait-list comparison groups.4 We feel there is ample evidence supporting bibliotherapy as a low-risk, low-cost alternative or complementary treatment for mild-to-moderate depressive disorder.
Sy Atezaz Saeed, MD
Department of psychiatric medicine
Brody School of Medicine at
East Carolina University
I thoroughly enjoyed the article on complementary and alternative medicine (CAM) for depression (“CAM for your depressed patient: 6 recommended options,” Current Psychiatry, October 2009) and have seen patients benefit from these treatments. I wish the authors had included information about the use of valerian root for anxiety, as this is common among some CAM users.
I think our use or discussion of CAM can show our patients we are flexible and will consider various treatments. I believe if you dismiss all CAM treatments as not as effective as prescription medications—which may be true—you will lose patients. We know how popular CAM is with the American public, despite lack of evidence and poor oversight.
In depression treatment, exercise is as effective as sertraline in some studies,1,2 but I would think the high dropout rate for exercise would make sertraline more likely to be effective in the long run. In 1 study, participants received a phone call if they missed an exercise session. This doesn’t mimic real life at all. Also St. John’s wort is administered 300 mg tid, while many antidepressants are once a day. Efficacy aside, we can guess that compliance with a medication taken 3 times a day will be less than 1 taken once daily.
I believe we need to examine our patients’ thoughts about CAM vs traditional treatment. Do they feel CAM is safer because it is natural? Do they feel less stigma if they use CAM? What are their “automatic thoughts” about this?
I disagree with the conclusion that bibliotherapy can’t hurt. Bibliotherapy does have a cost: the cost of the book, the time spent reading it, and minimal benefit. There are people making millions of dollars on self-help books that may be having little, if any, impact on our patients’ lives.
Corey Yilmaz, MD
Southwest Behavioral Health Rural Services
Tolleson, AZ
References
1. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349-2356.
2. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633-638.
Dr. Saeed responds
Despite the common belief that valerian root is effective in reducing stress and anxiety, it has not been tested for depressive disorders and is not supported by studies on anxiety disorders. Our paper did not review CAM treatments for anxiety disorders, so we did not point out that a recent Cochrane review1 of valerian for anxiety disorders included only 1 randomized controlled trial2 and found no differences between valerian and placebo.
We also agree that treatment discontinuation is a serious problem, but this is a universal concern for treating many chronic disorders. There is evidence that patient reports of treatment adherence can be unreliable. Research has shown that periodic monitoring,3 even by automated systems, can maintain compliance longer.
We disagree with Dr. Yilmaz’ comments about bibliotherapy. A meta-analysis of 29 bibliotherapy studies found bibliotherapy using cognitive and behavioral techniques superior to wait-list comparison groups.4 We feel there is ample evidence supporting bibliotherapy as a low-risk, low-cost alternative or complementary treatment for mild-to-moderate depressive disorder.
Sy Atezaz Saeed, MD
Department of psychiatric medicine
Brody School of Medicine at
East Carolina University
1. Miyasaka LS, Atallah AN, Soares BG. Valerian for anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD004515.-
2. Andreatini R, Sartori VA, Seabra ML, et al. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytother Res. 2002;16(7):650-654.
3. Gensichen J, von Korff M, Peitz M, et al. Case management for depression by health care assistants in small primary care practices: a cluster randomized trial. Ann Intern Med. 2009;151(6):369-378.
4. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
1. Miyasaka LS, Atallah AN, Soares BG. Valerian for anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD004515.-
2. Andreatini R, Sartori VA, Seabra ML, et al. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytother Res. 2002;16(7):650-654.
3. Gensichen J, von Korff M, Peitz M, et al. Case management for depression by health care assistants in small primary care practices: a cluster randomized trial. Ann Intern Med. 2009;151(6):369-378.
4. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
Are psychiatrists more evidence-based than psychologists?
A recent psychology journal article lambasted clinical psychologists for not using evidence-based psychotherapeutic modalities when treating their patients.1 The authors pointed out that many psychologists were ignoring efficacious and cost-effective psychotherapy interventions or using approaches that lack sufficient evidence.
An accompanying editorial2 was equally scathing—calling the disconnect between clinical psychology practice and advances in psychological science “an unconscionable embarrassment”—and warned that the profession “will increasingly discredit and marginalize itself” if it persists in neglecting evidence-based practices. The author quoted the respected late psychologist Paul Meehl as saying “most clinical psychologists select their methods like kids make choices in a candy store” and added that the comment is heart-breaking because it is true. A Newsweek column—“Ignoring the evidence: Why do psychologists reject science?”3—elicited little agreement and mostly howls of protest from psychologists.
So, are psychiatrists more evidence-based than psychologists? We manage patients who are more severely ill than those seen by psychologists, and we use both pharmacotherapy and psychotherapy to stabilize neurobiologic disorders. Because only 15% of DSM-IV-TR diagnostic categories have an evidence-based, FDA-approved drug treatment,4 we practice by necessity a substantial amount of non-evidenced-based (off-label) pharmacotherapy. But what about psychiatric conditions for which evidence-based treatments exist? Do studies show that we follow the evidence?
Psychiatrists’ track record
The Schizophrenia Patient Outcomes Research Team5 assessed how the treatment of 719 patients with schizophrenia conformed to 12 evidence-based treatment recommendations. Overall, <50% of treatments conformed to the recommendations, with higher conformance rates seen for rural than urban patients and for Caucasian patients than minorities.
A study using data from the National Comorbidity Survey6 found that only 40% of respondents with serious psychiatric disorders had received treatment in the previous 12 months, and only 15% received care considered at least minimally adequate. Four predictors of not receiving minimally adequate treatment included being a young adult or African-American, living in the South, suffering from a psychotic disorder, and being treated by physicians other than psychiatrists.
Finally, a recent survey of psychiatrists’ adherence to evidence-based antipsychotic treatment in schizophrenia7 showed: 1) mid-career psychiatrists more adherent than early or late-career counterparts; 2) male psychiatrists more adherent than female; 3) those carrying a large workload of schizophrenia patients more likely to adhere to scientific literature.
Who is evidence-based: A self-assessment
Are YOU an evidence-based psychiatric clinician? Ask yourself:
- Can I correctly define evidence-based psychopharmacology?
- Do I regularly read systematic reviews (such as Cochrane reviews) or meta-analytic articles about the medications I prescribe?
- Can I cite at least 1 randomized controlled trial supporting my use of each medication I prescribe?
- Do I know what “effect size” means?
- Do I usually or sometimes select a psychotherapeutic agent based on number needed to treat (NNT) or number needed to harm (NNH)?
- Do I routinely use clinical rating scales employed in FDA controlled trials to quantify the severity of my patients’ illness and determine whether they achieve “remission” or just a “response”?
Psychiatric practice should be evidence-based and continuously adapt to incorporate the wealth of evidence being generated. Psychiatrists who do not keep up with the evidence run the risk of practicing psychopharmacology of the previous millennium.
1. Baker TB, McFall RM, Shoham V. Current status and future prospects of clinical psychology: toward a scientifically principled approach to mental and behavioral health care. Psychological Science in the Public Interest. 2009;9(2):67-103. Available at: http://www.psychologicalscience.org/journals/pspi/inpress/baker.pdf. Accessed November 10, 2009.
2. Mischel W. Connecting clinical practice with scientific progress. Psychological Science in the Public Interest. 2009;9(2). Available at: http://www.psychologicalscience.org/journals/pspi/inpress/baker.pdf. Accessed November 10, 2009.
3. Begley S. Ignoring the evidence: why do psychologists reject science? Newsweek. October 12, 2009. Available at: http://www.newsweek.com/id/216506. Accessed November 10, 2009.
4. Devalupalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian Journal of Psychiatry. 2009;2(1):29-36.
5. Lehman AF, Steinwachs DM. Patterns of usual care for schizophrenia: initial results from the Schizophrenia Patient Outcomes Research Team (PORT) client survey. Schizophr Bull. 1998;24(1):11-20.
6. Wang PS, Delmer O, Kessler RC. Adequacy of treatment for serious mental illness in the United States. Am J Public Health. 2002;92(1):92-98.
7. Young GJ, Mohr DC, Meterko M, et al. Psychiatrists’ self-reported adherence to evidence-based prescribing practices in the treatment of schizophrenia. Psychiatr Serv. 2006;57(1):130-132.
A recent psychology journal article lambasted clinical psychologists for not using evidence-based psychotherapeutic modalities when treating their patients.1 The authors pointed out that many psychologists were ignoring efficacious and cost-effective psychotherapy interventions or using approaches that lack sufficient evidence.
An accompanying editorial2 was equally scathing—calling the disconnect between clinical psychology practice and advances in psychological science “an unconscionable embarrassment”—and warned that the profession “will increasingly discredit and marginalize itself” if it persists in neglecting evidence-based practices. The author quoted the respected late psychologist Paul Meehl as saying “most clinical psychologists select their methods like kids make choices in a candy store” and added that the comment is heart-breaking because it is true. A Newsweek column—“Ignoring the evidence: Why do psychologists reject science?”3—elicited little agreement and mostly howls of protest from psychologists.
So, are psychiatrists more evidence-based than psychologists? We manage patients who are more severely ill than those seen by psychologists, and we use both pharmacotherapy and psychotherapy to stabilize neurobiologic disorders. Because only 15% of DSM-IV-TR diagnostic categories have an evidence-based, FDA-approved drug treatment,4 we practice by necessity a substantial amount of non-evidenced-based (off-label) pharmacotherapy. But what about psychiatric conditions for which evidence-based treatments exist? Do studies show that we follow the evidence?
Psychiatrists’ track record
The Schizophrenia Patient Outcomes Research Team5 assessed how the treatment of 719 patients with schizophrenia conformed to 12 evidence-based treatment recommendations. Overall, <50% of treatments conformed to the recommendations, with higher conformance rates seen for rural than urban patients and for Caucasian patients than minorities.
A study using data from the National Comorbidity Survey6 found that only 40% of respondents with serious psychiatric disorders had received treatment in the previous 12 months, and only 15% received care considered at least minimally adequate. Four predictors of not receiving minimally adequate treatment included being a young adult or African-American, living in the South, suffering from a psychotic disorder, and being treated by physicians other than psychiatrists.
Finally, a recent survey of psychiatrists’ adherence to evidence-based antipsychotic treatment in schizophrenia7 showed: 1) mid-career psychiatrists more adherent than early or late-career counterparts; 2) male psychiatrists more adherent than female; 3) those carrying a large workload of schizophrenia patients more likely to adhere to scientific literature.
Who is evidence-based: A self-assessment
Are YOU an evidence-based psychiatric clinician? Ask yourself:
- Can I correctly define evidence-based psychopharmacology?
- Do I regularly read systematic reviews (such as Cochrane reviews) or meta-analytic articles about the medications I prescribe?
- Can I cite at least 1 randomized controlled trial supporting my use of each medication I prescribe?
- Do I know what “effect size” means?
- Do I usually or sometimes select a psychotherapeutic agent based on number needed to treat (NNT) or number needed to harm (NNH)?
- Do I routinely use clinical rating scales employed in FDA controlled trials to quantify the severity of my patients’ illness and determine whether they achieve “remission” or just a “response”?
Psychiatric practice should be evidence-based and continuously adapt to incorporate the wealth of evidence being generated. Psychiatrists who do not keep up with the evidence run the risk of practicing psychopharmacology of the previous millennium.
A recent psychology journal article lambasted clinical psychologists for not using evidence-based psychotherapeutic modalities when treating their patients.1 The authors pointed out that many psychologists were ignoring efficacious and cost-effective psychotherapy interventions or using approaches that lack sufficient evidence.
An accompanying editorial2 was equally scathing—calling the disconnect between clinical psychology practice and advances in psychological science “an unconscionable embarrassment”—and warned that the profession “will increasingly discredit and marginalize itself” if it persists in neglecting evidence-based practices. The author quoted the respected late psychologist Paul Meehl as saying “most clinical psychologists select their methods like kids make choices in a candy store” and added that the comment is heart-breaking because it is true. A Newsweek column—“Ignoring the evidence: Why do psychologists reject science?”3—elicited little agreement and mostly howls of protest from psychologists.
So, are psychiatrists more evidence-based than psychologists? We manage patients who are more severely ill than those seen by psychologists, and we use both pharmacotherapy and psychotherapy to stabilize neurobiologic disorders. Because only 15% of DSM-IV-TR diagnostic categories have an evidence-based, FDA-approved drug treatment,4 we practice by necessity a substantial amount of non-evidenced-based (off-label) pharmacotherapy. But what about psychiatric conditions for which evidence-based treatments exist? Do studies show that we follow the evidence?
Psychiatrists’ track record
The Schizophrenia Patient Outcomes Research Team5 assessed how the treatment of 719 patients with schizophrenia conformed to 12 evidence-based treatment recommendations. Overall, <50% of treatments conformed to the recommendations, with higher conformance rates seen for rural than urban patients and for Caucasian patients than minorities.
A study using data from the National Comorbidity Survey6 found that only 40% of respondents with serious psychiatric disorders had received treatment in the previous 12 months, and only 15% received care considered at least minimally adequate. Four predictors of not receiving minimally adequate treatment included being a young adult or African-American, living in the South, suffering from a psychotic disorder, and being treated by physicians other than psychiatrists.
Finally, a recent survey of psychiatrists’ adherence to evidence-based antipsychotic treatment in schizophrenia7 showed: 1) mid-career psychiatrists more adherent than early or late-career counterparts; 2) male psychiatrists more adherent than female; 3) those carrying a large workload of schizophrenia patients more likely to adhere to scientific literature.
Who is evidence-based: A self-assessment
Are YOU an evidence-based psychiatric clinician? Ask yourself:
- Can I correctly define evidence-based psychopharmacology?
- Do I regularly read systematic reviews (such as Cochrane reviews) or meta-analytic articles about the medications I prescribe?
- Can I cite at least 1 randomized controlled trial supporting my use of each medication I prescribe?
- Do I know what “effect size” means?
- Do I usually or sometimes select a psychotherapeutic agent based on number needed to treat (NNT) or number needed to harm (NNH)?
- Do I routinely use clinical rating scales employed in FDA controlled trials to quantify the severity of my patients’ illness and determine whether they achieve “remission” or just a “response”?
Psychiatric practice should be evidence-based and continuously adapt to incorporate the wealth of evidence being generated. Psychiatrists who do not keep up with the evidence run the risk of practicing psychopharmacology of the previous millennium.
1. Baker TB, McFall RM, Shoham V. Current status and future prospects of clinical psychology: toward a scientifically principled approach to mental and behavioral health care. Psychological Science in the Public Interest. 2009;9(2):67-103. Available at: http://www.psychologicalscience.org/journals/pspi/inpress/baker.pdf. Accessed November 10, 2009.
2. Mischel W. Connecting clinical practice with scientific progress. Psychological Science in the Public Interest. 2009;9(2). Available at: http://www.psychologicalscience.org/journals/pspi/inpress/baker.pdf. Accessed November 10, 2009.
3. Begley S. Ignoring the evidence: why do psychologists reject science? Newsweek. October 12, 2009. Available at: http://www.newsweek.com/id/216506. Accessed November 10, 2009.
4. Devalupalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian Journal of Psychiatry. 2009;2(1):29-36.
5. Lehman AF, Steinwachs DM. Patterns of usual care for schizophrenia: initial results from the Schizophrenia Patient Outcomes Research Team (PORT) client survey. Schizophr Bull. 1998;24(1):11-20.
6. Wang PS, Delmer O, Kessler RC. Adequacy of treatment for serious mental illness in the United States. Am J Public Health. 2002;92(1):92-98.
7. Young GJ, Mohr DC, Meterko M, et al. Psychiatrists’ self-reported adherence to evidence-based prescribing practices in the treatment of schizophrenia. Psychiatr Serv. 2006;57(1):130-132.
1. Baker TB, McFall RM, Shoham V. Current status and future prospects of clinical psychology: toward a scientifically principled approach to mental and behavioral health care. Psychological Science in the Public Interest. 2009;9(2):67-103. Available at: http://www.psychologicalscience.org/journals/pspi/inpress/baker.pdf. Accessed November 10, 2009.
2. Mischel W. Connecting clinical practice with scientific progress. Psychological Science in the Public Interest. 2009;9(2). Available at: http://www.psychologicalscience.org/journals/pspi/inpress/baker.pdf. Accessed November 10, 2009.
3. Begley S. Ignoring the evidence: why do psychologists reject science? Newsweek. October 12, 2009. Available at: http://www.newsweek.com/id/216506. Accessed November 10, 2009.
4. Devalupalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian Journal of Psychiatry. 2009;2(1):29-36.
5. Lehman AF, Steinwachs DM. Patterns of usual care for schizophrenia: initial results from the Schizophrenia Patient Outcomes Research Team (PORT) client survey. Schizophr Bull. 1998;24(1):11-20.
6. Wang PS, Delmer O, Kessler RC. Adequacy of treatment for serious mental illness in the United States. Am J Public Health. 2002;92(1):92-98.
7. Young GJ, Mohr DC, Meterko M, et al. Psychiatrists’ self-reported adherence to evidence-based prescribing practices in the treatment of schizophrenia. Psychiatr Serv. 2006;57(1):130-132.
The bedtime solution
CASE: Refractory depression
Ms. W, age 38, is brought to the emergency department after her son finds her unresponsive and calls 911. Suffering from worsening depression, she wrote a note telling her children goodbye, and overdosed on zolpidem from an old prescription and her daughter’s opioids. After being evaluated and medically cleared in the emergency department, Ms. W was admitted to the psychiatric unit.
Ms. W has a history of recurrent major depressive disorder that developed after she was sexually abused by a relative as a teen. She also has bulimia nervosa, alcohol dependence, and posttraumatic stress disorder. She was hospitalized twice for depression and suicidality but had not previously attempted suicide. In the mid-to-late 1990s, she had trials of paroxetine, clomipramine, lithium, and bupropion.
She was seen regularly in our outpatient psychiatry clinic for medication management and supportive psychotherapy. Since being followed in our clinic starting in early 2005, she has had the following medication trials:
- fluoxetine, citalopram, venlafaxine XR, and duloxetine for depression
- atomoxetine, buspirone, liothyronine, risperidone, and aripiprazole for antidepressant augmentation
- lorazepam, clonazepam, and gabapentin for anxiety
- zolpidem and trazodone for insomnia
- nortriptyline for migraine headache prophylaxis.
Some medications were not tolerated, primarily because of increased anxiety. Those that were tolerated were adequate trials in terms of dose titration and length. High-dose fluoxetine (80 mg/d) augmented by risperidone (0.375 to 0.5 mg/d) produced the most reliable and significant improvement.
Ms. W had 2 courses of electroconvulsive therapy (ECT) totaling 30 treatments—most recently in 2007—that resulted in significant memory loss with limited benefit. Premenstrual worsening of depression and suicidality were noted. In collaboration with her gynecologist, Ms. W was treated with a 3-month trial of leuprolide to suppress her ovarian axis, which was helpful. In 2008 she underwent bilateral oophorectomy. She has not had symptoms of mood elevation or psychosis. Family history includes schizophrenia, depression, anxiety, and alcoholism.
In the months before hospitalization, Ms. W had been increasingly depressed and intermittently suicidal, although she did not endorse a specific plan or intention to harm herself because she was concerned about the impact suicide would have on her children. Weight gain with risperidone had reactivated body image issues, so Ms. W stopped taking this medication 2 weeks before hospitalization. Her depression became worse, and she began using her husband’s hydrocodone/acetaminophen prescription.
The authors’ observations
Approximately 40% of patients with major depression fail to respond to an initial antidepressant trial.1 An additional 50% of these patients will be treatment-resistant to a subsequent antidepressant.1 Patients may be progressively less likely to respond to additional medication trials.2
One of the most rapid-acting and effective treatments for unipolar and bipolar depression is sleep deprivation. Wirz-Justice et al3 found total or partial sleep deprivation during the second half of the night induced rapid depression remission. Response rates range from 40% to 60% over hours to days.4 Sleep deprivation also can reduce suicidality in patients with seasonal depression.5 This treatment has not been widely employed, however, because up to 80% of patients who undergo sleep deprivation experience rapid and significant depressive relapse.4
Sleep deprivation usually is well tolerated. Potential side effects include:
- headache
- gastrointestinal upset
- fatigue
- cognitive impairment.
Less often, patients report worsening of depressive symptoms and, rarely, suicidal ideation or psychosis.4 Mania or hypomania are potential complications of sleep loss for patients with bipolar or unipolar depression. In a review, Oliwenstein6 suggested that rates of total sleep deprivation-induced mania are likely to be similar to or less than those reported for antidepressants. Because sleep deprivation can induce seizures, this therapy is contraindicated for patients with epilepsy or those at risk for seizures.4
Researchers have successfully explored strategies to reduce the rate of depressive relapse after sleep deprivation, including coadministering light therapy, antidepressants, lithium (particularly for bipolar depression), and sleep-phase advance.4 Sleep-phase advance involves shifting the sleep-wake schedule to a very early sleep time and wake-up time (such as 5 PM to midnight) for 1 day, and then pushing back this schedule by 1 or 2 hours each day until the patient is returned to a “normal” sleep schedule (such as 10 PM to 5 AM). Researchers have demonstrated that sleep-phase advance can have antidepressant effects.7
TREATMENT: Sleep manipulation
Ms. W is continued on fluoxetine, 80 mg/d. We opt for a trial of partial sleep deprivation and sleep-phase advance for Ms. W because of the severity of her depression, her multiple ineffective or poorly tolerated medication trials, and limited benefit from ECT. This treatment involves instituting partial sleep deprivation the first night and subsequently advancing her sleep phase over the next several days (Table 1).
Although she is sleepy the morning after partial sleep deprivation, Ms. W reports a marked improvement in her mood, decline in hopelessness, and absence of suicidal ideation. She continues the sleep-phase advance protocol for the next 3 nights and participates in cognitive-behavioral therapy groups and ward activities. Psychiatric unit staff support her continued wakefulness during sleep manipulation. Because Ms. W had previously responded to antidepressant augmentation with an atypical antipsychotic we add aripiprazole and titrate the dosage to 7.5 mg/d. We also continue fluoxetine, 80 mg/d, and add trazodone, 100 mg at bedtime, and hydroxyzine, 25 mg as needed.
Table 1
Ms. W’s chronotherapy protocol: Hours permitted for sleep*
| Day number | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sleep deprivation | 9 PM to2 AM | ||||
| Sleep-phase advance | 5 PM to midnight | 7 PM to 2 AM | 9 PM to 4 AM | 10 PM to 5 AM | |
| *Treatment was implemented while Ms. W was hospitalized | |||||
The authors’ observations
Chronotherapy incorporates manipulations of the sleep/wake cycle such as sleep deprivation and dark or light therapy. It may use combinations of interventions to generate and sustain a response in patients with depression. In a 4-week pilot study, Moscovici et al8 employed a regimen of late partial sleep deprivation, light, and sleep-phase advance to generate and maintain an anti depressant response in 12 patients. Benedetti et al9 used a similar regimen plus lithium to successfully treat bipolar depression and sleep-phase advance to continue that response in 50% of patients for 3 months.
Circadian rhythms affect the function of serotonin (5-HT), norepinephrine, and dopamine.9,10 In a manner similar to antidepressant medications, sleep deprivation may up-regulate or otherwise alter these neurotransmitters’ function. In animals, sleep deprivation increases serotonin function.11 Several hypothetical mechanisms of action for sleep deprivation and other types of chronotherapies have been suggested (Table 2).11-14
Chronotherapies may affect function in brain pathways, as demonstrated by neuroimaging with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Depression has been associated with increased or decreased brain activity measured by PET or fMRI in regions of the limbic cortex (cingulate and anterior cingulate) and frontal cortex.12
Wu et al13 examined patients treated for depression with medication and total sleep deprivation therapy. Response to treatment was associated with increased function in the cingulate, anterior cingulate, and medial prefrontal cortex as measured by PET. In contrast, mood improvement was associated with reduced baseline activity in the left medial prefrontal cortex, left frontal pole, and right lateral prefrontal cortex.
Researchers have noted the convergence of sleep-wake rhythms and abnormalities seen in depression and the subsequent link with improved sleep-wake cycles related to depression remission. Bunney and Potkin14 note the powerful effect of zeitgebers—environmental agents that reset the body’s internal clock. They suggested that sleep deprivation may affect the function of “master clock” genes involved in controlling the biological clock. These effects on the suprachiasmatic nucleus hypothalamic pacemaker may improve mood by altering control of genetic expression through chromatin remodeling of this master clock circuit.
Certain factors may increase the likelihood that a patient may respond to chronotherapy (Table 3).9,15-17
Table 2
Sleep deprivation for depression: Possible mechanisms
| Mechanism | Components |
|---|---|
| Alterations to neurotransmitter function | Serotonin, norepinephrine, dopamine11 |
| Alterations to endogenous circadian pacemaker function | Increased gene expression14 |
| Changes in perfusion/activity of brain regions | Anterior cingulate, frontal cortex regions12,13 |
Table 3
Factors that suggest a patient might respond to chronotherapy
| Diurnal mood variation15 |
| Endogenous depression including insomnia and anorexia16 |
| Abnormal dexamethasone suppression17 |
| High motivation for treatment |
| Bipolar depression (possibly)9 |
OUTCOME: Lasting improvement
Ms. W’s mood improvement is sustained during her week-long hospitalization. At discharge she is hopeful about the future and does not have thoughts of suicide.
At subsequent outpatient visits up to 4 months after discharge, her depressive symptoms remain improved. Patient Health Questionnaire scores indicate mild depression, but Ms. W is not suicidal. She maintains a sleep schedule of 10 PM to 6:30 AM and undergoes 10,000 lux bright light therapy, which she began shortly after discharge, for 30 minutes every morning. She works more productively in psychotherapy, focusing on her eating disorder and anxiety.
Related resource
- Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009; 66(3): 298-301.
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone/APAP • Vicodin
- Hydroxyzine • Atarax, Vistaril
- Leuprolide • Lupron
- Liothyronine • Cytomel
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Risperidone • Risperdal, Risperdal Consta
- Trazodone • Desyrel
- Venlafaxine XR • Effexor XR
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. AHCPR Depression Guideline Panel. Clinical practice guideline number 5. Depression in primary care. Volume 2: Treatment of major depression. Rockville, MD: Agency for Health Care Policy and Research, Public Health Services, U.S. Department of Health and Human Services; 1993. AHCPR publication 93-0550.
2. Fava M, Rush JA, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161-1172.
3. Wirz-Justice A, Benedetti F, Berger M. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
4. Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361-377.
5. Lam RW, Tam EM, Shiah IS, et al. Effects of light therapy on suicidal ideation in patients with winter depression. J Clin Psychiatry. 2000;61(1):30-32.
6. Oliwenstein L. Lifting moods by losing sleep: an adjunct therapy for treating depression. Alternative and Complementary Therapies. 2006;12(2):66-70.
7. Wehr TA, Wirz-Justice A, Goodwin FK, et al. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710-713.
8. Moscovici L, Kotler M. A multistage chronobiologic intervention for the treatment of depression: a pilot study. J Affect Disord. 2009;116(3):201-217.
9. Benedetti F, Colombo C, Barbini B, et al. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62(3):221-223.
10. Benedetti F, Barbini B, Colombo C, et al. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509-522.
11. Lopez-Rodriguez F, Wilson CL, Maidment NT, et al. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience. 2003;121(2):523-530.
12. Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729-730.
13. Wu JC, Gillin JC, Buchsbaum MS, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1-3):181-186.
14. Bunney JN, Potkin SG. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull. 2008;86:23-32.
15. Benedetti F, Barbini B, Lucca A, et al. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci. 1997;247(2):100-103.
16. Vogel GW, Thurmond A, Gibbons P, et al. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32(6):765-777.
17. King D, Dowdy S, Jack R, et al. The dexamethasone suppression test as a predictor of sleep deprivation antidepressant effect. Psychiatry Res. 1982;7(1):93-99.
CASE: Refractory depression
Ms. W, age 38, is brought to the emergency department after her son finds her unresponsive and calls 911. Suffering from worsening depression, she wrote a note telling her children goodbye, and overdosed on zolpidem from an old prescription and her daughter’s opioids. After being evaluated and medically cleared in the emergency department, Ms. W was admitted to the psychiatric unit.
Ms. W has a history of recurrent major depressive disorder that developed after she was sexually abused by a relative as a teen. She also has bulimia nervosa, alcohol dependence, and posttraumatic stress disorder. She was hospitalized twice for depression and suicidality but had not previously attempted suicide. In the mid-to-late 1990s, she had trials of paroxetine, clomipramine, lithium, and bupropion.
She was seen regularly in our outpatient psychiatry clinic for medication management and supportive psychotherapy. Since being followed in our clinic starting in early 2005, she has had the following medication trials:
- fluoxetine, citalopram, venlafaxine XR, and duloxetine for depression
- atomoxetine, buspirone, liothyronine, risperidone, and aripiprazole for antidepressant augmentation
- lorazepam, clonazepam, and gabapentin for anxiety
- zolpidem and trazodone for insomnia
- nortriptyline for migraine headache prophylaxis.
Some medications were not tolerated, primarily because of increased anxiety. Those that were tolerated were adequate trials in terms of dose titration and length. High-dose fluoxetine (80 mg/d) augmented by risperidone (0.375 to 0.5 mg/d) produced the most reliable and significant improvement.
Ms. W had 2 courses of electroconvulsive therapy (ECT) totaling 30 treatments—most recently in 2007—that resulted in significant memory loss with limited benefit. Premenstrual worsening of depression and suicidality were noted. In collaboration with her gynecologist, Ms. W was treated with a 3-month trial of leuprolide to suppress her ovarian axis, which was helpful. In 2008 she underwent bilateral oophorectomy. She has not had symptoms of mood elevation or psychosis. Family history includes schizophrenia, depression, anxiety, and alcoholism.
In the months before hospitalization, Ms. W had been increasingly depressed and intermittently suicidal, although she did not endorse a specific plan or intention to harm herself because she was concerned about the impact suicide would have on her children. Weight gain with risperidone had reactivated body image issues, so Ms. W stopped taking this medication 2 weeks before hospitalization. Her depression became worse, and she began using her husband’s hydrocodone/acetaminophen prescription.
The authors’ observations
Approximately 40% of patients with major depression fail to respond to an initial antidepressant trial.1 An additional 50% of these patients will be treatment-resistant to a subsequent antidepressant.1 Patients may be progressively less likely to respond to additional medication trials.2
One of the most rapid-acting and effective treatments for unipolar and bipolar depression is sleep deprivation. Wirz-Justice et al3 found total or partial sleep deprivation during the second half of the night induced rapid depression remission. Response rates range from 40% to 60% over hours to days.4 Sleep deprivation also can reduce suicidality in patients with seasonal depression.5 This treatment has not been widely employed, however, because up to 80% of patients who undergo sleep deprivation experience rapid and significant depressive relapse.4
Sleep deprivation usually is well tolerated. Potential side effects include:
- headache
- gastrointestinal upset
- fatigue
- cognitive impairment.
Less often, patients report worsening of depressive symptoms and, rarely, suicidal ideation or psychosis.4 Mania or hypomania are potential complications of sleep loss for patients with bipolar or unipolar depression. In a review, Oliwenstein6 suggested that rates of total sleep deprivation-induced mania are likely to be similar to or less than those reported for antidepressants. Because sleep deprivation can induce seizures, this therapy is contraindicated for patients with epilepsy or those at risk for seizures.4
Researchers have successfully explored strategies to reduce the rate of depressive relapse after sleep deprivation, including coadministering light therapy, antidepressants, lithium (particularly for bipolar depression), and sleep-phase advance.4 Sleep-phase advance involves shifting the sleep-wake schedule to a very early sleep time and wake-up time (such as 5 PM to midnight) for 1 day, and then pushing back this schedule by 1 or 2 hours each day until the patient is returned to a “normal” sleep schedule (such as 10 PM to 5 AM). Researchers have demonstrated that sleep-phase advance can have antidepressant effects.7
TREATMENT: Sleep manipulation
Ms. W is continued on fluoxetine, 80 mg/d. We opt for a trial of partial sleep deprivation and sleep-phase advance for Ms. W because of the severity of her depression, her multiple ineffective or poorly tolerated medication trials, and limited benefit from ECT. This treatment involves instituting partial sleep deprivation the first night and subsequently advancing her sleep phase over the next several days (Table 1).
Although she is sleepy the morning after partial sleep deprivation, Ms. W reports a marked improvement in her mood, decline in hopelessness, and absence of suicidal ideation. She continues the sleep-phase advance protocol for the next 3 nights and participates in cognitive-behavioral therapy groups and ward activities. Psychiatric unit staff support her continued wakefulness during sleep manipulation. Because Ms. W had previously responded to antidepressant augmentation with an atypical antipsychotic we add aripiprazole and titrate the dosage to 7.5 mg/d. We also continue fluoxetine, 80 mg/d, and add trazodone, 100 mg at bedtime, and hydroxyzine, 25 mg as needed.
Table 1
Ms. W’s chronotherapy protocol: Hours permitted for sleep*
| Day number | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sleep deprivation | 9 PM to2 AM | ||||
| Sleep-phase advance | 5 PM to midnight | 7 PM to 2 AM | 9 PM to 4 AM | 10 PM to 5 AM | |
| *Treatment was implemented while Ms. W was hospitalized | |||||
The authors’ observations
Chronotherapy incorporates manipulations of the sleep/wake cycle such as sleep deprivation and dark or light therapy. It may use combinations of interventions to generate and sustain a response in patients with depression. In a 4-week pilot study, Moscovici et al8 employed a regimen of late partial sleep deprivation, light, and sleep-phase advance to generate and maintain an anti depressant response in 12 patients. Benedetti et al9 used a similar regimen plus lithium to successfully treat bipolar depression and sleep-phase advance to continue that response in 50% of patients for 3 months.
Circadian rhythms affect the function of serotonin (5-HT), norepinephrine, and dopamine.9,10 In a manner similar to antidepressant medications, sleep deprivation may up-regulate or otherwise alter these neurotransmitters’ function. In animals, sleep deprivation increases serotonin function.11 Several hypothetical mechanisms of action for sleep deprivation and other types of chronotherapies have been suggested (Table 2).11-14
Chronotherapies may affect function in brain pathways, as demonstrated by neuroimaging with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Depression has been associated with increased or decreased brain activity measured by PET or fMRI in regions of the limbic cortex (cingulate and anterior cingulate) and frontal cortex.12
Wu et al13 examined patients treated for depression with medication and total sleep deprivation therapy. Response to treatment was associated with increased function in the cingulate, anterior cingulate, and medial prefrontal cortex as measured by PET. In contrast, mood improvement was associated with reduced baseline activity in the left medial prefrontal cortex, left frontal pole, and right lateral prefrontal cortex.
Researchers have noted the convergence of sleep-wake rhythms and abnormalities seen in depression and the subsequent link with improved sleep-wake cycles related to depression remission. Bunney and Potkin14 note the powerful effect of zeitgebers—environmental agents that reset the body’s internal clock. They suggested that sleep deprivation may affect the function of “master clock” genes involved in controlling the biological clock. These effects on the suprachiasmatic nucleus hypothalamic pacemaker may improve mood by altering control of genetic expression through chromatin remodeling of this master clock circuit.
Certain factors may increase the likelihood that a patient may respond to chronotherapy (Table 3).9,15-17
Table 2
Sleep deprivation for depression: Possible mechanisms
| Mechanism | Components |
|---|---|
| Alterations to neurotransmitter function | Serotonin, norepinephrine, dopamine11 |
| Alterations to endogenous circadian pacemaker function | Increased gene expression14 |
| Changes in perfusion/activity of brain regions | Anterior cingulate, frontal cortex regions12,13 |
Table 3
Factors that suggest a patient might respond to chronotherapy
| Diurnal mood variation15 |
| Endogenous depression including insomnia and anorexia16 |
| Abnormal dexamethasone suppression17 |
| High motivation for treatment |
| Bipolar depression (possibly)9 |
OUTCOME: Lasting improvement
Ms. W’s mood improvement is sustained during her week-long hospitalization. At discharge she is hopeful about the future and does not have thoughts of suicide.
At subsequent outpatient visits up to 4 months after discharge, her depressive symptoms remain improved. Patient Health Questionnaire scores indicate mild depression, but Ms. W is not suicidal. She maintains a sleep schedule of 10 PM to 6:30 AM and undergoes 10,000 lux bright light therapy, which she began shortly after discharge, for 30 minutes every morning. She works more productively in psychotherapy, focusing on her eating disorder and anxiety.
Related resource
- Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009; 66(3): 298-301.
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone/APAP • Vicodin
- Hydroxyzine • Atarax, Vistaril
- Leuprolide • Lupron
- Liothyronine • Cytomel
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Risperidone • Risperdal, Risperdal Consta
- Trazodone • Desyrel
- Venlafaxine XR • Effexor XR
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Refractory depression
Ms. W, age 38, is brought to the emergency department after her son finds her unresponsive and calls 911. Suffering from worsening depression, she wrote a note telling her children goodbye, and overdosed on zolpidem from an old prescription and her daughter’s opioids. After being evaluated and medically cleared in the emergency department, Ms. W was admitted to the psychiatric unit.
Ms. W has a history of recurrent major depressive disorder that developed after she was sexually abused by a relative as a teen. She also has bulimia nervosa, alcohol dependence, and posttraumatic stress disorder. She was hospitalized twice for depression and suicidality but had not previously attempted suicide. In the mid-to-late 1990s, she had trials of paroxetine, clomipramine, lithium, and bupropion.
She was seen regularly in our outpatient psychiatry clinic for medication management and supportive psychotherapy. Since being followed in our clinic starting in early 2005, she has had the following medication trials:
- fluoxetine, citalopram, venlafaxine XR, and duloxetine for depression
- atomoxetine, buspirone, liothyronine, risperidone, and aripiprazole for antidepressant augmentation
- lorazepam, clonazepam, and gabapentin for anxiety
- zolpidem and trazodone for insomnia
- nortriptyline for migraine headache prophylaxis.
Some medications were not tolerated, primarily because of increased anxiety. Those that were tolerated were adequate trials in terms of dose titration and length. High-dose fluoxetine (80 mg/d) augmented by risperidone (0.375 to 0.5 mg/d) produced the most reliable and significant improvement.
Ms. W had 2 courses of electroconvulsive therapy (ECT) totaling 30 treatments—most recently in 2007—that resulted in significant memory loss with limited benefit. Premenstrual worsening of depression and suicidality were noted. In collaboration with her gynecologist, Ms. W was treated with a 3-month trial of leuprolide to suppress her ovarian axis, which was helpful. In 2008 she underwent bilateral oophorectomy. She has not had symptoms of mood elevation or psychosis. Family history includes schizophrenia, depression, anxiety, and alcoholism.
In the months before hospitalization, Ms. W had been increasingly depressed and intermittently suicidal, although she did not endorse a specific plan or intention to harm herself because she was concerned about the impact suicide would have on her children. Weight gain with risperidone had reactivated body image issues, so Ms. W stopped taking this medication 2 weeks before hospitalization. Her depression became worse, and she began using her husband’s hydrocodone/acetaminophen prescription.
The authors’ observations
Approximately 40% of patients with major depression fail to respond to an initial antidepressant trial.1 An additional 50% of these patients will be treatment-resistant to a subsequent antidepressant.1 Patients may be progressively less likely to respond to additional medication trials.2
One of the most rapid-acting and effective treatments for unipolar and bipolar depression is sleep deprivation. Wirz-Justice et al3 found total or partial sleep deprivation during the second half of the night induced rapid depression remission. Response rates range from 40% to 60% over hours to days.4 Sleep deprivation also can reduce suicidality in patients with seasonal depression.5 This treatment has not been widely employed, however, because up to 80% of patients who undergo sleep deprivation experience rapid and significant depressive relapse.4
Sleep deprivation usually is well tolerated. Potential side effects include:
- headache
- gastrointestinal upset
- fatigue
- cognitive impairment.
Less often, patients report worsening of depressive symptoms and, rarely, suicidal ideation or psychosis.4 Mania or hypomania are potential complications of sleep loss for patients with bipolar or unipolar depression. In a review, Oliwenstein6 suggested that rates of total sleep deprivation-induced mania are likely to be similar to or less than those reported for antidepressants. Because sleep deprivation can induce seizures, this therapy is contraindicated for patients with epilepsy or those at risk for seizures.4
Researchers have successfully explored strategies to reduce the rate of depressive relapse after sleep deprivation, including coadministering light therapy, antidepressants, lithium (particularly for bipolar depression), and sleep-phase advance.4 Sleep-phase advance involves shifting the sleep-wake schedule to a very early sleep time and wake-up time (such as 5 PM to midnight) for 1 day, and then pushing back this schedule by 1 or 2 hours each day until the patient is returned to a “normal” sleep schedule (such as 10 PM to 5 AM). Researchers have demonstrated that sleep-phase advance can have antidepressant effects.7
TREATMENT: Sleep manipulation
Ms. W is continued on fluoxetine, 80 mg/d. We opt for a trial of partial sleep deprivation and sleep-phase advance for Ms. W because of the severity of her depression, her multiple ineffective or poorly tolerated medication trials, and limited benefit from ECT. This treatment involves instituting partial sleep deprivation the first night and subsequently advancing her sleep phase over the next several days (Table 1).
Although she is sleepy the morning after partial sleep deprivation, Ms. W reports a marked improvement in her mood, decline in hopelessness, and absence of suicidal ideation. She continues the sleep-phase advance protocol for the next 3 nights and participates in cognitive-behavioral therapy groups and ward activities. Psychiatric unit staff support her continued wakefulness during sleep manipulation. Because Ms. W had previously responded to antidepressant augmentation with an atypical antipsychotic we add aripiprazole and titrate the dosage to 7.5 mg/d. We also continue fluoxetine, 80 mg/d, and add trazodone, 100 mg at bedtime, and hydroxyzine, 25 mg as needed.
Table 1
Ms. W’s chronotherapy protocol: Hours permitted for sleep*
| Day number | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sleep deprivation | 9 PM to2 AM | ||||
| Sleep-phase advance | 5 PM to midnight | 7 PM to 2 AM | 9 PM to 4 AM | 10 PM to 5 AM | |
| *Treatment was implemented while Ms. W was hospitalized | |||||
The authors’ observations
Chronotherapy incorporates manipulations of the sleep/wake cycle such as sleep deprivation and dark or light therapy. It may use combinations of interventions to generate and sustain a response in patients with depression. In a 4-week pilot study, Moscovici et al8 employed a regimen of late partial sleep deprivation, light, and sleep-phase advance to generate and maintain an anti depressant response in 12 patients. Benedetti et al9 used a similar regimen plus lithium to successfully treat bipolar depression and sleep-phase advance to continue that response in 50% of patients for 3 months.
Circadian rhythms affect the function of serotonin (5-HT), norepinephrine, and dopamine.9,10 In a manner similar to antidepressant medications, sleep deprivation may up-regulate or otherwise alter these neurotransmitters’ function. In animals, sleep deprivation increases serotonin function.11 Several hypothetical mechanisms of action for sleep deprivation and other types of chronotherapies have been suggested (Table 2).11-14
Chronotherapies may affect function in brain pathways, as demonstrated by neuroimaging with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). Depression has been associated with increased or decreased brain activity measured by PET or fMRI in regions of the limbic cortex (cingulate and anterior cingulate) and frontal cortex.12
Wu et al13 examined patients treated for depression with medication and total sleep deprivation therapy. Response to treatment was associated with increased function in the cingulate, anterior cingulate, and medial prefrontal cortex as measured by PET. In contrast, mood improvement was associated with reduced baseline activity in the left medial prefrontal cortex, left frontal pole, and right lateral prefrontal cortex.
Researchers have noted the convergence of sleep-wake rhythms and abnormalities seen in depression and the subsequent link with improved sleep-wake cycles related to depression remission. Bunney and Potkin14 note the powerful effect of zeitgebers—environmental agents that reset the body’s internal clock. They suggested that sleep deprivation may affect the function of “master clock” genes involved in controlling the biological clock. These effects on the suprachiasmatic nucleus hypothalamic pacemaker may improve mood by altering control of genetic expression through chromatin remodeling of this master clock circuit.
Certain factors may increase the likelihood that a patient may respond to chronotherapy (Table 3).9,15-17
Table 2
Sleep deprivation for depression: Possible mechanisms
| Mechanism | Components |
|---|---|
| Alterations to neurotransmitter function | Serotonin, norepinephrine, dopamine11 |
| Alterations to endogenous circadian pacemaker function | Increased gene expression14 |
| Changes in perfusion/activity of brain regions | Anterior cingulate, frontal cortex regions12,13 |
Table 3
Factors that suggest a patient might respond to chronotherapy
| Diurnal mood variation15 |
| Endogenous depression including insomnia and anorexia16 |
| Abnormal dexamethasone suppression17 |
| High motivation for treatment |
| Bipolar depression (possibly)9 |
OUTCOME: Lasting improvement
Ms. W’s mood improvement is sustained during her week-long hospitalization. At discharge she is hopeful about the future and does not have thoughts of suicide.
At subsequent outpatient visits up to 4 months after discharge, her depressive symptoms remain improved. Patient Health Questionnaire scores indicate mild depression, but Ms. W is not suicidal. She maintains a sleep schedule of 10 PM to 6:30 AM and undergoes 10,000 lux bright light therapy, which she began shortly after discharge, for 30 minutes every morning. She works more productively in psychotherapy, focusing on her eating disorder and anxiety.
Related resource
- Wu JC, Kelsoe JR, Schachat C, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009; 66(3): 298-301.
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone/APAP • Vicodin
- Hydroxyzine • Atarax, Vistaril
- Leuprolide • Lupron
- Liothyronine • Cytomel
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Nortriptyline • Aventyl
- Paroxetine • Paxil
- Risperidone • Risperdal, Risperdal Consta
- Trazodone • Desyrel
- Venlafaxine XR • Effexor XR
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. AHCPR Depression Guideline Panel. Clinical practice guideline number 5. Depression in primary care. Volume 2: Treatment of major depression. Rockville, MD: Agency for Health Care Policy and Research, Public Health Services, U.S. Department of Health and Human Services; 1993. AHCPR publication 93-0550.
2. Fava M, Rush JA, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161-1172.
3. Wirz-Justice A, Benedetti F, Berger M. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
4. Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361-377.
5. Lam RW, Tam EM, Shiah IS, et al. Effects of light therapy on suicidal ideation in patients with winter depression. J Clin Psychiatry. 2000;61(1):30-32.
6. Oliwenstein L. Lifting moods by losing sleep: an adjunct therapy for treating depression. Alternative and Complementary Therapies. 2006;12(2):66-70.
7. Wehr TA, Wirz-Justice A, Goodwin FK, et al. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710-713.
8. Moscovici L, Kotler M. A multistage chronobiologic intervention for the treatment of depression: a pilot study. J Affect Disord. 2009;116(3):201-217.
9. Benedetti F, Colombo C, Barbini B, et al. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62(3):221-223.
10. Benedetti F, Barbini B, Colombo C, et al. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509-522.
11. Lopez-Rodriguez F, Wilson CL, Maidment NT, et al. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience. 2003;121(2):523-530.
12. Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729-730.
13. Wu JC, Gillin JC, Buchsbaum MS, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1-3):181-186.
14. Bunney JN, Potkin SG. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull. 2008;86:23-32.
15. Benedetti F, Barbini B, Lucca A, et al. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci. 1997;247(2):100-103.
16. Vogel GW, Thurmond A, Gibbons P, et al. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32(6):765-777.
17. King D, Dowdy S, Jack R, et al. The dexamethasone suppression test as a predictor of sleep deprivation antidepressant effect. Psychiatry Res. 1982;7(1):93-99.
1. AHCPR Depression Guideline Panel. Clinical practice guideline number 5. Depression in primary care. Volume 2: Treatment of major depression. Rockville, MD: Agency for Health Care Policy and Research, Public Health Services, U.S. Department of Health and Human Services; 1993. AHCPR publication 93-0550.
2. Fava M, Rush JA, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161-1172.
3. Wirz-Justice A, Benedetti F, Berger M. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35(7):939-944.
4. Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6(5):361-377.
5. Lam RW, Tam EM, Shiah IS, et al. Effects of light therapy on suicidal ideation in patients with winter depression. J Clin Psychiatry. 2000;61(1):30-32.
6. Oliwenstein L. Lifting moods by losing sleep: an adjunct therapy for treating depression. Alternative and Complementary Therapies. 2006;12(2):66-70.
7. Wehr TA, Wirz-Justice A, Goodwin FK, et al. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710-713.
8. Moscovici L, Kotler M. A multistage chronobiologic intervention for the treatment of depression: a pilot study. J Affect Disord. 2009;116(3):201-217.
9. Benedetti F, Colombo C, Barbini B, et al. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62(3):221-223.
10. Benedetti F, Barbini B, Colombo C, et al. Chronotherapeutics in a psychiatric ward. Sleep Med Rev. 2007;11(6):509-522.
11. Lopez-Rodriguez F, Wilson CL, Maidment NT, et al. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience. 2003;121(2):523-530.
12. Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729-730.
13. Wu JC, Gillin JC, Buchsbaum MS, et al. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107(1-3):181-186.
14. Bunney JN, Potkin SG. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull. 2008;86:23-32.
15. Benedetti F, Barbini B, Lucca A, et al. Sleep deprivation hastens the antidepressant action of fluoxetine. Eur Arch Psychiatry Clin Neurosci. 1997;247(2):100-103.
16. Vogel GW, Thurmond A, Gibbons P, et al. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32(6):765-777.
17. King D, Dowdy S, Jack R, et al. The dexamethasone suppression test as a predictor of sleep deprivation antidepressant effect. Psychiatry Res. 1982;7(1):93-99.
Late-life depression: Managing mood in patients with vascular disease
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
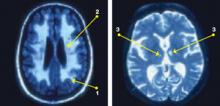
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
Are you ready to testify? 3 ways to prepare
A case of sudden psychosis
CASE: New-onset psychosis
Ms. T, age 26, presents to the psychiatric emergency room after a 1-week change in behavior. According to her family, Ms. T began to experience hyperactivity, increased rate of speech, and decreased sleep after her mother passed away 1 week ago. On the day of presentation, Ms. T had returned to work after a week’s hiatus. Coworkers brought her to the hospital when Ms. T threw herself on the floor and flailed about. Family members report that Ms. T had been complaining of headache that day and during the preceding week. In the emergency room, the patient is intrusive and easily distractible, although able to give a history.
Ms. T has no psychiatric history. Her family history is positive for bipolar spectrum illness. Our initial consideration is that Ms. T is experiencing mania or psychotic symptoms triggered by the recent loss of her mother. Ms. T is evaluated in the medical emergency room to rule out a primary medical illness. Standard labs and head CT are normal, so she is returned to the psychiatric emergency room. She becomes severely agitated and requires multiple IM antipsychotics—2 courses of haloperidol, 10 mg; 2 courses of ziprasidone, 20 mg; and olanzapine, 10 mg. She is admitted to the inpatient psychiatric service with a diagnosis of psychosis not otherwise specified.
Soon after admission, Ms. T suffers a witnessed generalized tonic-clonic seizure and is transferred to the internal medicine service. After the seizure she is awake but minimally responsive. She does not display purposeful movements, opens her eyes but can follow the examiner only on occasion, and displays periodic facial grimacing. In addition, Ms. T is intermittently hypoxic—requiring supplemental oxygen via nasal cannula—and febrile, with persistent tachycardia. Electroencephalography (EEG) shows nonconvulsive status epilepticus involving the bilateral temporal regions.
Ms. T is transferred to the neurosurgical intensive care unit for monitoring and IV anticonvulsants. Subsequent EEGs demonstrate generalized slowing but no epileptiform activity. An infectious workup is negative. Head MRI shows bilateral cerebellar T2/FLAIR increased signal, which is a nonspecific finding. Cerebrospinal fluid (CSF) studies show lymphocytic pleocytosis and oligoclonal bands. These findings suggest a CSF humoral immune response; an extensive laboratory workup is otherwise largely unremarkable ( Table 1 ).
The authors’ observations
We consider that Ms. T may have schizophrenia. Schizophrenia onset is insidious, often with prodromal symptoms occurring months to years before diagnosis.1,2 In Ms. T, the onset of the disturbance was brief; her family noted a change in behavior for only 1 week before presentation. Given this history, brief psychotic disorder remains high on the differential diagnosis because Ms. T’s disorganized speech and behavior occurred seeming in relation to her mother’s death.
Bipolar disorder is characterized by strong heritability, with risks increasing if there is a first-degree relative with the illness. The hallmark of bipolar I disorder is a manic episode, which presents as:
- decreased need for sleep
- grandiosity
- flight of ideas
- reckless or thoughtless behaviors
- increased energy
- increased productivity
- expansive or irritable mood.
Psychiatric symptoms secondary to seizure disorder are well documented. Cognitive, mood, anxiety, and psychotic phenomena may occur in up to 50% of patients with seizures.3 Typically, these symptoms are categorized as occurring during a seizure, after a seizure (post-ictal), or between seizures (interictal).
Manic syndromes secondary to seizure disorders present in an atypical manner with irritability and hyperactivity. Psychotic syndromes, on the other hand, appear with more classic schizophrenia-type symptoms:
- paranoia and persecutory delusions
- auditory and visual hallucinations
- amotivation
- apathy
- flattened affect
- disorganization.3
Paraneoplastic syndromes may be associated with mood changes and other psychiatric symptoms.4-6 Diagnosis is contingent on discovering the primary neoplasm, with or without specific paraneoplastic antibodies. Treatment is tailored to the oncologic process.
Table 1
Ms. T’s laboratory workup*
| Test | Result |
|---|---|
| C-reactive protein | 0.7 |
| Erythrocyte sedimentation rate | 5 |
| Cryptococcal antigen (serum) | Negative |
| Antinuclear antibody | Negative |
| CSF lymphocytes | 88 |
| CSF nucleated cells | 200 |
| CSF RBC | 33 |
| CSF glucose | 44 |
| CSF protein | 45 |
| CSF igG index | 1.2 |
| CSF oligoclonal bands | Present |
| CSF: cerebrospinal fluid; igG: immunoglobulin G; RBC: red blood cell | |
| *Results were negative for gonorrhea, chlamydia, lupus, human immunodeficiency virus, syphilis, Lyme disease, varicella zoster virus, West Nile virus, herpes simplex virus, Epstein-Barr virus, cytomegalovirus, tuberculosis, and California, St. Louis, eastern equine, and western equine encephalitis | |
EVALUATION: A medical cause
The psychiatry consultation-liaison service is asked to further evaluate Ms. T for psychiatric contributions to her continued altered mental status. Ms. T remains in restraints and receives fosphenytoin, 200 mg bid; levetiracetam, 1,000 mg bid; and lorazepam as needed for agitation. Following consultation, the team considers a working diagnosis of an autoimmune encephalopathy based on the negative infectious workup, the patient’s demographics, and the clinical picture (psychiatric symptoms, seizure, and encephalopathy). Ms. T undergoes 5 courses of plasma exchange with no effect. Catatonia is considered, but the patient does not demonstrate significant change with numerous doses of lorazepam.
Because Ms. T does not improve, the team starts a more specific paraneoplastic workup. MRI reveals a 9-mm lesion on her right ovary. Corticosteroids, including IV methylprednisolone, 1 g/d, are started. Ms. T’s clinical presentation improves; soon after scheduled corticosteroid dosing, she is taken to the operating room for right salpingo-oophorectomy. Surgical pathology later confirms the lesion as a mature teratoma. A standard paraneoplastic panel is negative; a separate test for anti-NMDA (N-methyl-D-aspartate) receptor antibodies is positive, however, and confirms the diagnosis of ovarian mass-associated anti-NMDA receptor limbic encephalitis.
Paraneoplastic syndromes
This case represents the interface between a complicated medical phenomenon and psychiatric symptomatology. Mood changes (typically depression), memory problems, paranoia, hypersomnolence, aggressive behavior, agitation, and catatonia have been associated with paraneoplastic syndromes.4-6
Common malignant associations include small cell lung carcinoma (most common) and breast, stomach, colon, renal, bladder, ovarian, uterine, testicular, cell line, and thymic cancers. Research strongly suggests an autoimmune mechanism: tumor-related antibodies cross-react with similar antigens in the neurologic system. Paraneoplastic symptoms often precede symptoms of the malignancy, and the diagnosis is suggested by positive imaging and a paraneoplastic panel.
Anti-NMDA receptor limbic encephalitis is a paraneoplastic syndrome associated with ovarian teratomas and antibodies specific to the glutamate receptor. It is thought to be an autoimmune phenomenon whereby tumor-related antibodies elicit an immune response within certain parts of the neurologic system. Ms. T represents a typical clinical presentation of this syndrome—she is a young, otherwise healthy woman with:
- preceding headache
- new-onset psychotic symptoms
- seizure activity (particularly in the temporal lobes)
- central hypoventilation
- hyperthermia and tachycardia
- dyskinesia and catatonia-like symptoms.5
TREATMENT: Rapid improvement
After removal of the dermoid lesion and IV corticosteroids, Ms. T exhibits rapid improvement. She begins acknowledging others in the room, making eye contact for nearly the first time during this hospitalization, and starts recognizing family members. She also begins verbalizing, responding appropriately to questions in 1 or 2 words. After a 34-day hospital stay, Ms. T is transferred to another facility for rehabilitation; her medication list consists of a corticosteroid taper from prednisone, 20 mg/d, over 2 weeks; fosphenytoin, 200 mg bid; and levetiracetam, 1,000 mg bid.
She eventually is discharged from the rehabilitation facility with noted improvement in multiple domains: she demonstrates cognitive improvement and can walk short distances. She continues to require 24-hour care and exhibits intermittent agitation.
The authors’ observations
We present the case of a patient with a specific paraneoplastic disorder—anti-NMDA receptor limbic encephalitis—with symptoms mimicking those seen in psychiatric disorders such as schizophrenia and bipolar disorder. These similarities complicate recognition and treatment of the underlying disorder.
Ms. T had a complicated yet typical presentation of anti-NMDA receptor limbic encephalitis ( Table 2 ) that was initially mistaken for a manic episode with psychotic features. The diagnosis was made more complex by the death of her mother 1 week before presentation, which could have precipitated her symptom onset. Similar case reports have appeared in neurologic and—less frequently—psychiatric literature ( Box ).5,7-10
5 Ms. T spent time on psychiatric, internal medicine, and neurologic services before her team established a definitive diagnosis.
Because neurobehavioral symptoms predominate early in the course of paraneoplastic illness,5 psychiatrists should prepare to be the first medical point of contact for these patients.
Table 2
Anti-NMDA receptor encephalitis: Symptoms, findings, and treatment
| Typical presentation |
| Young female Prodromal symptoms New onset psychosis, anxiety, or mood symptoms Catatonia Coma Seizure activity (typically bilateral temporal lobe activity on EEG) Hypoventilation Autonomic instability Dyskinesia |
| Laboratory and radiologic findings |
| CSF or serum antibodies CSF pleocytosis and elevated protein, normal glucose Background slowing or sharp-wave activity on EEG Temporal lobe abnormalities |
| Treatment |
| Tumor resection Immunosuppressants (typically corticosteroids) Intravenous immunoglobulin Plasmapheresis |
| CSF: cerebrospinal fluid; EEG: electroencephalography; NMDA: N-methyl-D-aspartate |
Box
Neurology. Several case reports in neurologic literature describe presentations similar to Ms. T’s.
Sansing et al8 described a 34-year-old woman with prominent psychiatric symptoms who had an immature ovarian teratoma with positive anti-NMDA (N-methyl-D-aspartate) receptor antibody. She was treated with tumor resection, plasmapheresis, and corticosteroids and experienced significant improvement.
Nasky et al7 describe a 23-year-old woman with paranoia, agitation, and delusions. A neoplasm was not identified, but she was anti-NMDA receptor antibody positive and improved with IV corticosteroids and IV immunoglobulin.
Dalmau et al5 compiled a case series analysis of 100 cases of anti-NMDA receptor encephalitis. Tumor removal with IV corticosteroids, IV immunoglobulin, and plasma exchange were the most common treatments. Patients with tumors that were identified and resected had better functional recovery than those without tumor resection.5
Psychiatry. A search of psychiatric literature yielded only 2 pertinent case reports. Lee et al9 described an 11-year-old girl with acute confusion, agitation, paranoia, hallucinations, and later malignant catatonia that improved after removal of an ovarian teratoma. Seki et al10 reported on an 18-year-old woman who presented with schizophrenia-like symptoms of disorganization and loss of self awareness. This patient’s symptoms resolved almost completely after unilateral salpingo-oophorectomy, corticosteroid administration, and plasma exchange.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327-340.
- Fosphenytoin • Cerebyx
- Haloperidol • Haldol
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Methylprednisolone • Medrol, Depo-Medrol, others
- Olanzapine • Zyprexa
- Prednisone • Deltasone, Meticorten
- Ziprasidone • Geodon
Drs. Cavalieri and Southammakosane report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. White is a consultant for Pfizer Inc.
1. Buckley PF. Update on the etiology and treatment of schizophrenia and bipolar disorder. CNS Spectr. 2008;13 (2 suppl 1):1-12.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Lyketsos CG, Kozauer N, Rabins PV. Psychiatric manifestations of neurologic disease: where are we headed? Dialogues Clin Neurosci. 2007;9:111-124.
4. Foster AR, Caplan JP. Paraneoplastic limbic encephalitis. Psychosomatics. 2009;50:108-113.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091-1098.
6. Dalmau J, Tuzun E, Hai-Yan W, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36.
7. Nasky KM, Knittel D, Manos GH. Psychosis associated with anti-N-methyl-D-aspartate receptor antibodies. CNS Spectr. 2008;13(8):699-703.
8. Sansing LH, Tuzun E, Ko MW, et al. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3(5):291-296.
9. Lee A, Glick DB, Dinwiddie SH. Electroconvulsive therapy in a pediatric patient with malignant catatonia and paraneoplastic limbic encephalitis. J ECT. 2006;22:267-270.
10. Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:324-326.
CASE: New-onset psychosis
Ms. T, age 26, presents to the psychiatric emergency room after a 1-week change in behavior. According to her family, Ms. T began to experience hyperactivity, increased rate of speech, and decreased sleep after her mother passed away 1 week ago. On the day of presentation, Ms. T had returned to work after a week’s hiatus. Coworkers brought her to the hospital when Ms. T threw herself on the floor and flailed about. Family members report that Ms. T had been complaining of headache that day and during the preceding week. In the emergency room, the patient is intrusive and easily distractible, although able to give a history.
Ms. T has no psychiatric history. Her family history is positive for bipolar spectrum illness. Our initial consideration is that Ms. T is experiencing mania or psychotic symptoms triggered by the recent loss of her mother. Ms. T is evaluated in the medical emergency room to rule out a primary medical illness. Standard labs and head CT are normal, so she is returned to the psychiatric emergency room. She becomes severely agitated and requires multiple IM antipsychotics—2 courses of haloperidol, 10 mg; 2 courses of ziprasidone, 20 mg; and olanzapine, 10 mg. She is admitted to the inpatient psychiatric service with a diagnosis of psychosis not otherwise specified.
Soon after admission, Ms. T suffers a witnessed generalized tonic-clonic seizure and is transferred to the internal medicine service. After the seizure she is awake but minimally responsive. She does not display purposeful movements, opens her eyes but can follow the examiner only on occasion, and displays periodic facial grimacing. In addition, Ms. T is intermittently hypoxic—requiring supplemental oxygen via nasal cannula—and febrile, with persistent tachycardia. Electroencephalography (EEG) shows nonconvulsive status epilepticus involving the bilateral temporal regions.
Ms. T is transferred to the neurosurgical intensive care unit for monitoring and IV anticonvulsants. Subsequent EEGs demonstrate generalized slowing but no epileptiform activity. An infectious workup is negative. Head MRI shows bilateral cerebellar T2/FLAIR increased signal, which is a nonspecific finding. Cerebrospinal fluid (CSF) studies show lymphocytic pleocytosis and oligoclonal bands. These findings suggest a CSF humoral immune response; an extensive laboratory workup is otherwise largely unremarkable ( Table 1 ).
The authors’ observations
We consider that Ms. T may have schizophrenia. Schizophrenia onset is insidious, often with prodromal symptoms occurring months to years before diagnosis.1,2 In Ms. T, the onset of the disturbance was brief; her family noted a change in behavior for only 1 week before presentation. Given this history, brief psychotic disorder remains high on the differential diagnosis because Ms. T’s disorganized speech and behavior occurred seeming in relation to her mother’s death.
Bipolar disorder is characterized by strong heritability, with risks increasing if there is a first-degree relative with the illness. The hallmark of bipolar I disorder is a manic episode, which presents as:
- decreased need for sleep
- grandiosity
- flight of ideas
- reckless or thoughtless behaviors
- increased energy
- increased productivity
- expansive or irritable mood.
Psychiatric symptoms secondary to seizure disorder are well documented. Cognitive, mood, anxiety, and psychotic phenomena may occur in up to 50% of patients with seizures.3 Typically, these symptoms are categorized as occurring during a seizure, after a seizure (post-ictal), or between seizures (interictal).
Manic syndromes secondary to seizure disorders present in an atypical manner with irritability and hyperactivity. Psychotic syndromes, on the other hand, appear with more classic schizophrenia-type symptoms:
- paranoia and persecutory delusions
- auditory and visual hallucinations
- amotivation
- apathy
- flattened affect
- disorganization.3
Paraneoplastic syndromes may be associated with mood changes and other psychiatric symptoms.4-6 Diagnosis is contingent on discovering the primary neoplasm, with or without specific paraneoplastic antibodies. Treatment is tailored to the oncologic process.
Table 1
Ms. T’s laboratory workup*
| Test | Result |
|---|---|
| C-reactive protein | 0.7 |
| Erythrocyte sedimentation rate | 5 |
| Cryptococcal antigen (serum) | Negative |
| Antinuclear antibody | Negative |
| CSF lymphocytes | 88 |
| CSF nucleated cells | 200 |
| CSF RBC | 33 |
| CSF glucose | 44 |
| CSF protein | 45 |
| CSF igG index | 1.2 |
| CSF oligoclonal bands | Present |
| CSF: cerebrospinal fluid; igG: immunoglobulin G; RBC: red blood cell | |
| *Results were negative for gonorrhea, chlamydia, lupus, human immunodeficiency virus, syphilis, Lyme disease, varicella zoster virus, West Nile virus, herpes simplex virus, Epstein-Barr virus, cytomegalovirus, tuberculosis, and California, St. Louis, eastern equine, and western equine encephalitis | |
EVALUATION: A medical cause
The psychiatry consultation-liaison service is asked to further evaluate Ms. T for psychiatric contributions to her continued altered mental status. Ms. T remains in restraints and receives fosphenytoin, 200 mg bid; levetiracetam, 1,000 mg bid; and lorazepam as needed for agitation. Following consultation, the team considers a working diagnosis of an autoimmune encephalopathy based on the negative infectious workup, the patient’s demographics, and the clinical picture (psychiatric symptoms, seizure, and encephalopathy). Ms. T undergoes 5 courses of plasma exchange with no effect. Catatonia is considered, but the patient does not demonstrate significant change with numerous doses of lorazepam.
Because Ms. T does not improve, the team starts a more specific paraneoplastic workup. MRI reveals a 9-mm lesion on her right ovary. Corticosteroids, including IV methylprednisolone, 1 g/d, are started. Ms. T’s clinical presentation improves; soon after scheduled corticosteroid dosing, she is taken to the operating room for right salpingo-oophorectomy. Surgical pathology later confirms the lesion as a mature teratoma. A standard paraneoplastic panel is negative; a separate test for anti-NMDA (N-methyl-D-aspartate) receptor antibodies is positive, however, and confirms the diagnosis of ovarian mass-associated anti-NMDA receptor limbic encephalitis.
Paraneoplastic syndromes
This case represents the interface between a complicated medical phenomenon and psychiatric symptomatology. Mood changes (typically depression), memory problems, paranoia, hypersomnolence, aggressive behavior, agitation, and catatonia have been associated with paraneoplastic syndromes.4-6
Common malignant associations include small cell lung carcinoma (most common) and breast, stomach, colon, renal, bladder, ovarian, uterine, testicular, cell line, and thymic cancers. Research strongly suggests an autoimmune mechanism: tumor-related antibodies cross-react with similar antigens in the neurologic system. Paraneoplastic symptoms often precede symptoms of the malignancy, and the diagnosis is suggested by positive imaging and a paraneoplastic panel.
Anti-NMDA receptor limbic encephalitis is a paraneoplastic syndrome associated with ovarian teratomas and antibodies specific to the glutamate receptor. It is thought to be an autoimmune phenomenon whereby tumor-related antibodies elicit an immune response within certain parts of the neurologic system. Ms. T represents a typical clinical presentation of this syndrome—she is a young, otherwise healthy woman with:
- preceding headache
- new-onset psychotic symptoms
- seizure activity (particularly in the temporal lobes)
- central hypoventilation
- hyperthermia and tachycardia
- dyskinesia and catatonia-like symptoms.5
TREATMENT: Rapid improvement
After removal of the dermoid lesion and IV corticosteroids, Ms. T exhibits rapid improvement. She begins acknowledging others in the room, making eye contact for nearly the first time during this hospitalization, and starts recognizing family members. She also begins verbalizing, responding appropriately to questions in 1 or 2 words. After a 34-day hospital stay, Ms. T is transferred to another facility for rehabilitation; her medication list consists of a corticosteroid taper from prednisone, 20 mg/d, over 2 weeks; fosphenytoin, 200 mg bid; and levetiracetam, 1,000 mg bid.
She eventually is discharged from the rehabilitation facility with noted improvement in multiple domains: she demonstrates cognitive improvement and can walk short distances. She continues to require 24-hour care and exhibits intermittent agitation.
The authors’ observations
We present the case of a patient with a specific paraneoplastic disorder—anti-NMDA receptor limbic encephalitis—with symptoms mimicking those seen in psychiatric disorders such as schizophrenia and bipolar disorder. These similarities complicate recognition and treatment of the underlying disorder.
Ms. T had a complicated yet typical presentation of anti-NMDA receptor limbic encephalitis ( Table 2 ) that was initially mistaken for a manic episode with psychotic features. The diagnosis was made more complex by the death of her mother 1 week before presentation, which could have precipitated her symptom onset. Similar case reports have appeared in neurologic and—less frequently—psychiatric literature ( Box ).5,7-10
5 Ms. T spent time on psychiatric, internal medicine, and neurologic services before her team established a definitive diagnosis.
Because neurobehavioral symptoms predominate early in the course of paraneoplastic illness,5 psychiatrists should prepare to be the first medical point of contact for these patients.
Table 2
Anti-NMDA receptor encephalitis: Symptoms, findings, and treatment
| Typical presentation |
| Young female Prodromal symptoms New onset psychosis, anxiety, or mood symptoms Catatonia Coma Seizure activity (typically bilateral temporal lobe activity on EEG) Hypoventilation Autonomic instability Dyskinesia |
| Laboratory and radiologic findings |
| CSF or serum antibodies CSF pleocytosis and elevated protein, normal glucose Background slowing or sharp-wave activity on EEG Temporal lobe abnormalities |
| Treatment |
| Tumor resection Immunosuppressants (typically corticosteroids) Intravenous immunoglobulin Plasmapheresis |
| CSF: cerebrospinal fluid; EEG: electroencephalography; NMDA: N-methyl-D-aspartate |
Box
Neurology. Several case reports in neurologic literature describe presentations similar to Ms. T’s.
Sansing et al8 described a 34-year-old woman with prominent psychiatric symptoms who had an immature ovarian teratoma with positive anti-NMDA (N-methyl-D-aspartate) receptor antibody. She was treated with tumor resection, plasmapheresis, and corticosteroids and experienced significant improvement.
Nasky et al7 describe a 23-year-old woman with paranoia, agitation, and delusions. A neoplasm was not identified, but she was anti-NMDA receptor antibody positive and improved with IV corticosteroids and IV immunoglobulin.
Dalmau et al5 compiled a case series analysis of 100 cases of anti-NMDA receptor encephalitis. Tumor removal with IV corticosteroids, IV immunoglobulin, and plasma exchange were the most common treatments. Patients with tumors that were identified and resected had better functional recovery than those without tumor resection.5
Psychiatry. A search of psychiatric literature yielded only 2 pertinent case reports. Lee et al9 described an 11-year-old girl with acute confusion, agitation, paranoia, hallucinations, and later malignant catatonia that improved after removal of an ovarian teratoma. Seki et al10 reported on an 18-year-old woman who presented with schizophrenia-like symptoms of disorganization and loss of self awareness. This patient’s symptoms resolved almost completely after unilateral salpingo-oophorectomy, corticosteroid administration, and plasma exchange.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327-340.
- Fosphenytoin • Cerebyx
- Haloperidol • Haldol
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Methylprednisolone • Medrol, Depo-Medrol, others
- Olanzapine • Zyprexa
- Prednisone • Deltasone, Meticorten
- Ziprasidone • Geodon
Drs. Cavalieri and Southammakosane report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. White is a consultant for Pfizer Inc.
CASE: New-onset psychosis
Ms. T, age 26, presents to the psychiatric emergency room after a 1-week change in behavior. According to her family, Ms. T began to experience hyperactivity, increased rate of speech, and decreased sleep after her mother passed away 1 week ago. On the day of presentation, Ms. T had returned to work after a week’s hiatus. Coworkers brought her to the hospital when Ms. T threw herself on the floor and flailed about. Family members report that Ms. T had been complaining of headache that day and during the preceding week. In the emergency room, the patient is intrusive and easily distractible, although able to give a history.
Ms. T has no psychiatric history. Her family history is positive for bipolar spectrum illness. Our initial consideration is that Ms. T is experiencing mania or psychotic symptoms triggered by the recent loss of her mother. Ms. T is evaluated in the medical emergency room to rule out a primary medical illness. Standard labs and head CT are normal, so she is returned to the psychiatric emergency room. She becomes severely agitated and requires multiple IM antipsychotics—2 courses of haloperidol, 10 mg; 2 courses of ziprasidone, 20 mg; and olanzapine, 10 mg. She is admitted to the inpatient psychiatric service with a diagnosis of psychosis not otherwise specified.
Soon after admission, Ms. T suffers a witnessed generalized tonic-clonic seizure and is transferred to the internal medicine service. After the seizure she is awake but minimally responsive. She does not display purposeful movements, opens her eyes but can follow the examiner only on occasion, and displays periodic facial grimacing. In addition, Ms. T is intermittently hypoxic—requiring supplemental oxygen via nasal cannula—and febrile, with persistent tachycardia. Electroencephalography (EEG) shows nonconvulsive status epilepticus involving the bilateral temporal regions.
Ms. T is transferred to the neurosurgical intensive care unit for monitoring and IV anticonvulsants. Subsequent EEGs demonstrate generalized slowing but no epileptiform activity. An infectious workup is negative. Head MRI shows bilateral cerebellar T2/FLAIR increased signal, which is a nonspecific finding. Cerebrospinal fluid (CSF) studies show lymphocytic pleocytosis and oligoclonal bands. These findings suggest a CSF humoral immune response; an extensive laboratory workup is otherwise largely unremarkable ( Table 1 ).
The authors’ observations
We consider that Ms. T may have schizophrenia. Schizophrenia onset is insidious, often with prodromal symptoms occurring months to years before diagnosis.1,2 In Ms. T, the onset of the disturbance was brief; her family noted a change in behavior for only 1 week before presentation. Given this history, brief psychotic disorder remains high on the differential diagnosis because Ms. T’s disorganized speech and behavior occurred seeming in relation to her mother’s death.
Bipolar disorder is characterized by strong heritability, with risks increasing if there is a first-degree relative with the illness. The hallmark of bipolar I disorder is a manic episode, which presents as:
- decreased need for sleep
- grandiosity
- flight of ideas
- reckless or thoughtless behaviors
- increased energy
- increased productivity
- expansive or irritable mood.
Psychiatric symptoms secondary to seizure disorder are well documented. Cognitive, mood, anxiety, and psychotic phenomena may occur in up to 50% of patients with seizures.3 Typically, these symptoms are categorized as occurring during a seizure, after a seizure (post-ictal), or between seizures (interictal).
Manic syndromes secondary to seizure disorders present in an atypical manner with irritability and hyperactivity. Psychotic syndromes, on the other hand, appear with more classic schizophrenia-type symptoms:
- paranoia and persecutory delusions
- auditory and visual hallucinations
- amotivation
- apathy
- flattened affect
- disorganization.3
Paraneoplastic syndromes may be associated with mood changes and other psychiatric symptoms.4-6 Diagnosis is contingent on discovering the primary neoplasm, with or without specific paraneoplastic antibodies. Treatment is tailored to the oncologic process.
Table 1
Ms. T’s laboratory workup*
| Test | Result |
|---|---|
| C-reactive protein | 0.7 |
| Erythrocyte sedimentation rate | 5 |
| Cryptococcal antigen (serum) | Negative |
| Antinuclear antibody | Negative |
| CSF lymphocytes | 88 |
| CSF nucleated cells | 200 |
| CSF RBC | 33 |
| CSF glucose | 44 |
| CSF protein | 45 |
| CSF igG index | 1.2 |
| CSF oligoclonal bands | Present |
| CSF: cerebrospinal fluid; igG: immunoglobulin G; RBC: red blood cell | |
| *Results were negative for gonorrhea, chlamydia, lupus, human immunodeficiency virus, syphilis, Lyme disease, varicella zoster virus, West Nile virus, herpes simplex virus, Epstein-Barr virus, cytomegalovirus, tuberculosis, and California, St. Louis, eastern equine, and western equine encephalitis | |
EVALUATION: A medical cause
The psychiatry consultation-liaison service is asked to further evaluate Ms. T for psychiatric contributions to her continued altered mental status. Ms. T remains in restraints and receives fosphenytoin, 200 mg bid; levetiracetam, 1,000 mg bid; and lorazepam as needed for agitation. Following consultation, the team considers a working diagnosis of an autoimmune encephalopathy based on the negative infectious workup, the patient’s demographics, and the clinical picture (psychiatric symptoms, seizure, and encephalopathy). Ms. T undergoes 5 courses of plasma exchange with no effect. Catatonia is considered, but the patient does not demonstrate significant change with numerous doses of lorazepam.
Because Ms. T does not improve, the team starts a more specific paraneoplastic workup. MRI reveals a 9-mm lesion on her right ovary. Corticosteroids, including IV methylprednisolone, 1 g/d, are started. Ms. T’s clinical presentation improves; soon after scheduled corticosteroid dosing, she is taken to the operating room for right salpingo-oophorectomy. Surgical pathology later confirms the lesion as a mature teratoma. A standard paraneoplastic panel is negative; a separate test for anti-NMDA (N-methyl-D-aspartate) receptor antibodies is positive, however, and confirms the diagnosis of ovarian mass-associated anti-NMDA receptor limbic encephalitis.
Paraneoplastic syndromes
This case represents the interface between a complicated medical phenomenon and psychiatric symptomatology. Mood changes (typically depression), memory problems, paranoia, hypersomnolence, aggressive behavior, agitation, and catatonia have been associated with paraneoplastic syndromes.4-6
Common malignant associations include small cell lung carcinoma (most common) and breast, stomach, colon, renal, bladder, ovarian, uterine, testicular, cell line, and thymic cancers. Research strongly suggests an autoimmune mechanism: tumor-related antibodies cross-react with similar antigens in the neurologic system. Paraneoplastic symptoms often precede symptoms of the malignancy, and the diagnosis is suggested by positive imaging and a paraneoplastic panel.
Anti-NMDA receptor limbic encephalitis is a paraneoplastic syndrome associated with ovarian teratomas and antibodies specific to the glutamate receptor. It is thought to be an autoimmune phenomenon whereby tumor-related antibodies elicit an immune response within certain parts of the neurologic system. Ms. T represents a typical clinical presentation of this syndrome—she is a young, otherwise healthy woman with:
- preceding headache
- new-onset psychotic symptoms
- seizure activity (particularly in the temporal lobes)
- central hypoventilation
- hyperthermia and tachycardia
- dyskinesia and catatonia-like symptoms.5
TREATMENT: Rapid improvement
After removal of the dermoid lesion and IV corticosteroids, Ms. T exhibits rapid improvement. She begins acknowledging others in the room, making eye contact for nearly the first time during this hospitalization, and starts recognizing family members. She also begins verbalizing, responding appropriately to questions in 1 or 2 words. After a 34-day hospital stay, Ms. T is transferred to another facility for rehabilitation; her medication list consists of a corticosteroid taper from prednisone, 20 mg/d, over 2 weeks; fosphenytoin, 200 mg bid; and levetiracetam, 1,000 mg bid.
She eventually is discharged from the rehabilitation facility with noted improvement in multiple domains: she demonstrates cognitive improvement and can walk short distances. She continues to require 24-hour care and exhibits intermittent agitation.
The authors’ observations
We present the case of a patient with a specific paraneoplastic disorder—anti-NMDA receptor limbic encephalitis—with symptoms mimicking those seen in psychiatric disorders such as schizophrenia and bipolar disorder. These similarities complicate recognition and treatment of the underlying disorder.
Ms. T had a complicated yet typical presentation of anti-NMDA receptor limbic encephalitis ( Table 2 ) that was initially mistaken for a manic episode with psychotic features. The diagnosis was made more complex by the death of her mother 1 week before presentation, which could have precipitated her symptom onset. Similar case reports have appeared in neurologic and—less frequently—psychiatric literature ( Box ).5,7-10
5 Ms. T spent time on psychiatric, internal medicine, and neurologic services before her team established a definitive diagnosis.
Because neurobehavioral symptoms predominate early in the course of paraneoplastic illness,5 psychiatrists should prepare to be the first medical point of contact for these patients.
Table 2
Anti-NMDA receptor encephalitis: Symptoms, findings, and treatment
| Typical presentation |
| Young female Prodromal symptoms New onset psychosis, anxiety, or mood symptoms Catatonia Coma Seizure activity (typically bilateral temporal lobe activity on EEG) Hypoventilation Autonomic instability Dyskinesia |
| Laboratory and radiologic findings |
| CSF or serum antibodies CSF pleocytosis and elevated protein, normal glucose Background slowing or sharp-wave activity on EEG Temporal lobe abnormalities |
| Treatment |
| Tumor resection Immunosuppressants (typically corticosteroids) Intravenous immunoglobulin Plasmapheresis |
| CSF: cerebrospinal fluid; EEG: electroencephalography; NMDA: N-methyl-D-aspartate |
Box
Neurology. Several case reports in neurologic literature describe presentations similar to Ms. T’s.
Sansing et al8 described a 34-year-old woman with prominent psychiatric symptoms who had an immature ovarian teratoma with positive anti-NMDA (N-methyl-D-aspartate) receptor antibody. She was treated with tumor resection, plasmapheresis, and corticosteroids and experienced significant improvement.
Nasky et al7 describe a 23-year-old woman with paranoia, agitation, and delusions. A neoplasm was not identified, but she was anti-NMDA receptor antibody positive and improved with IV corticosteroids and IV immunoglobulin.
Dalmau et al5 compiled a case series analysis of 100 cases of anti-NMDA receptor encephalitis. Tumor removal with IV corticosteroids, IV immunoglobulin, and plasma exchange were the most common treatments. Patients with tumors that were identified and resected had better functional recovery than those without tumor resection.5
Psychiatry. A search of psychiatric literature yielded only 2 pertinent case reports. Lee et al9 described an 11-year-old girl with acute confusion, agitation, paranoia, hallucinations, and later malignant catatonia that improved after removal of an ovarian teratoma. Seki et al10 reported on an 18-year-old woman who presented with schizophrenia-like symptoms of disorganization and loss of self awareness. This patient’s symptoms resolved almost completely after unilateral salpingo-oophorectomy, corticosteroid administration, and plasma exchange.
- Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327-340.
- Fosphenytoin • Cerebyx
- Haloperidol • Haldol
- Levetiracetam • Keppra
- Lorazepam • Ativan
- Methylprednisolone • Medrol, Depo-Medrol, others
- Olanzapine • Zyprexa
- Prednisone • Deltasone, Meticorten
- Ziprasidone • Geodon
Drs. Cavalieri and Southammakosane report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. White is a consultant for Pfizer Inc.
1. Buckley PF. Update on the etiology and treatment of schizophrenia and bipolar disorder. CNS Spectr. 2008;13 (2 suppl 1):1-12.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Lyketsos CG, Kozauer N, Rabins PV. Psychiatric manifestations of neurologic disease: where are we headed? Dialogues Clin Neurosci. 2007;9:111-124.
4. Foster AR, Caplan JP. Paraneoplastic limbic encephalitis. Psychosomatics. 2009;50:108-113.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091-1098.
6. Dalmau J, Tuzun E, Hai-Yan W, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36.
7. Nasky KM, Knittel D, Manos GH. Psychosis associated with anti-N-methyl-D-aspartate receptor antibodies. CNS Spectr. 2008;13(8):699-703.
8. Sansing LH, Tuzun E, Ko MW, et al. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3(5):291-296.
9. Lee A, Glick DB, Dinwiddie SH. Electroconvulsive therapy in a pediatric patient with malignant catatonia and paraneoplastic limbic encephalitis. J ECT. 2006;22:267-270.
10. Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:324-326.
1. Buckley PF. Update on the etiology and treatment of schizophrenia and bipolar disorder. CNS Spectr. 2008;13 (2 suppl 1):1-12.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Lyketsos CG, Kozauer N, Rabins PV. Psychiatric manifestations of neurologic disease: where are we headed? Dialogues Clin Neurosci. 2007;9:111-124.
4. Foster AR, Caplan JP. Paraneoplastic limbic encephalitis. Psychosomatics. 2009;50:108-113.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091-1098.
6. Dalmau J, Tuzun E, Hai-Yan W, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36.
7. Nasky KM, Knittel D, Manos GH. Psychosis associated with anti-N-methyl-D-aspartate receptor antibodies. CNS Spectr. 2008;13(8):699-703.
8. Sansing LH, Tuzun E, Ko MW, et al. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3(5):291-296.
9. Lee A, Glick DB, Dinwiddie SH. Electroconvulsive therapy in a pediatric patient with malignant catatonia and paraneoplastic limbic encephalitis. J ECT. 2006;22:267-270.
10. Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:324-326.
Help ‘sensitive’ patients tolerate medication
Some psychiatric patients report intolerable side effects with almost every psychotropic one reasonably could prescribe. Their psychiatric disorders remain undertreated as treatment-emergent side effects lead again and again to medication nonadherence. These patients often are frustrated by their lack of progress and in turn exasperate practitioners, creating strong transference and countertransference reactions.
To keep the therapeutic relationship on track, consider that your patient’s physiologic response to medication may be the result of genetic or psychodynamic factors you can overcome.
Start low and go slow
One option is to initiate medication far below the recommended starting dose. For example, try starting a patient on 1, 2, or 5 mg/d of fluoxetine instead of the typical 20 mg/d. These small doses can be achieved with a pill cutter or by using a liquid formulation with a measuring spoon that allows for 1-mg increments.
Some patients may report significant amelioration of symptoms even if they do not reach what is considered a therapeutic dose. These clinical observations have been confirmed by pharmacogenetic research that demonstrates metabolic variation across the population.1 Improving patients’ well-being rather than arriving at a predetermined therapeutic dose should guide treatment.
Patients who experience multiple side effects may need extra time to acclimate to a new medication. Try initiating medication changes or increases at longer intervals, such as over months rather than weeks.
Examine psychodynamic factors
Seek to understand dynamic factors that contribute to your patient’s pattern of intolerable side effects. For example, patients’ disappointing early experiences with parents may result in angry feelings toward authority figures and a desire to frustrate them. In traumatized patients, internalized object relations consisting of pain-inflicting authority figures may be acted out through medication matters.
By understanding patients’ dynamics, we can better understand our own countertransference reactions and devise interventions that are more likely to help patients tolerate medication.
Accept patients’ sensitivity
I often tell patients, “You happen to be sensitive to side effects of medication. We might have to try a number of different medications before we find one that works and is tolerable. Furthermore, we need to start at a very low dose and take things very slowly.” This statement:
- recognizes and accepts patients’ sensitivity to psychotropics
- allows for externalization of some responsibility for troublesome side effects to the medication
- conveys a sense of therapeutic uncertainty
- allows patients to undertake treatment at a comfortable pace.
Reference
1. DeVane CL. Principles of pharmacokinetics and pharmacodynamics. In: Schatzberg AF, Nemeroff CB, eds. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
Some psychiatric patients report intolerable side effects with almost every psychotropic one reasonably could prescribe. Their psychiatric disorders remain undertreated as treatment-emergent side effects lead again and again to medication nonadherence. These patients often are frustrated by their lack of progress and in turn exasperate practitioners, creating strong transference and countertransference reactions.
To keep the therapeutic relationship on track, consider that your patient’s physiologic response to medication may be the result of genetic or psychodynamic factors you can overcome.
Start low and go slow
One option is to initiate medication far below the recommended starting dose. For example, try starting a patient on 1, 2, or 5 mg/d of fluoxetine instead of the typical 20 mg/d. These small doses can be achieved with a pill cutter or by using a liquid formulation with a measuring spoon that allows for 1-mg increments.
Some patients may report significant amelioration of symptoms even if they do not reach what is considered a therapeutic dose. These clinical observations have been confirmed by pharmacogenetic research that demonstrates metabolic variation across the population.1 Improving patients’ well-being rather than arriving at a predetermined therapeutic dose should guide treatment.
Patients who experience multiple side effects may need extra time to acclimate to a new medication. Try initiating medication changes or increases at longer intervals, such as over months rather than weeks.
Examine psychodynamic factors
Seek to understand dynamic factors that contribute to your patient’s pattern of intolerable side effects. For example, patients’ disappointing early experiences with parents may result in angry feelings toward authority figures and a desire to frustrate them. In traumatized patients, internalized object relations consisting of pain-inflicting authority figures may be acted out through medication matters.
By understanding patients’ dynamics, we can better understand our own countertransference reactions and devise interventions that are more likely to help patients tolerate medication.
Accept patients’ sensitivity
I often tell patients, “You happen to be sensitive to side effects of medication. We might have to try a number of different medications before we find one that works and is tolerable. Furthermore, we need to start at a very low dose and take things very slowly.” This statement:
- recognizes and accepts patients’ sensitivity to psychotropics
- allows for externalization of some responsibility for troublesome side effects to the medication
- conveys a sense of therapeutic uncertainty
- allows patients to undertake treatment at a comfortable pace.
Some psychiatric patients report intolerable side effects with almost every psychotropic one reasonably could prescribe. Their psychiatric disorders remain undertreated as treatment-emergent side effects lead again and again to medication nonadherence. These patients often are frustrated by their lack of progress and in turn exasperate practitioners, creating strong transference and countertransference reactions.
To keep the therapeutic relationship on track, consider that your patient’s physiologic response to medication may be the result of genetic or psychodynamic factors you can overcome.
Start low and go slow
One option is to initiate medication far below the recommended starting dose. For example, try starting a patient on 1, 2, or 5 mg/d of fluoxetine instead of the typical 20 mg/d. These small doses can be achieved with a pill cutter or by using a liquid formulation with a measuring spoon that allows for 1-mg increments.
Some patients may report significant amelioration of symptoms even if they do not reach what is considered a therapeutic dose. These clinical observations have been confirmed by pharmacogenetic research that demonstrates metabolic variation across the population.1 Improving patients’ well-being rather than arriving at a predetermined therapeutic dose should guide treatment.
Patients who experience multiple side effects may need extra time to acclimate to a new medication. Try initiating medication changes or increases at longer intervals, such as over months rather than weeks.
Examine psychodynamic factors
Seek to understand dynamic factors that contribute to your patient’s pattern of intolerable side effects. For example, patients’ disappointing early experiences with parents may result in angry feelings toward authority figures and a desire to frustrate them. In traumatized patients, internalized object relations consisting of pain-inflicting authority figures may be acted out through medication matters.
By understanding patients’ dynamics, we can better understand our own countertransference reactions and devise interventions that are more likely to help patients tolerate medication.
Accept patients’ sensitivity
I often tell patients, “You happen to be sensitive to side effects of medication. We might have to try a number of different medications before we find one that works and is tolerable. Furthermore, we need to start at a very low dose and take things very slowly.” This statement:
- recognizes and accepts patients’ sensitivity to psychotropics
- allows for externalization of some responsibility for troublesome side effects to the medication
- conveys a sense of therapeutic uncertainty
- allows patients to undertake treatment at a comfortable pace.
Reference
1. DeVane CL. Principles of pharmacokinetics and pharmacodynamics. In: Schatzberg AF, Nemeroff CB, eds. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
Reference
1. DeVane CL. Principles of pharmacokinetics and pharmacodynamics. In: Schatzberg AF, Nemeroff CB, eds. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
Health care debate: Do psychiatrists support the public option?
Like everyone else, I could not avoid being swept up by the national debate about how to reform our health care system. The debate has been highly politicized, with the liberal left strongly supporting and the conservative right vehemently opposing a single-payer government-run public option (but keeping Medicare and Medicaid). Independents seem to waver between the major overhaul of a public option and making the system more competitive and less expensive.
Developing an idea
Recently I was speaking to psychiatry colleagues in the United Kingdom as their National Health Service (NHS) celebrated its 60th anniversary. Some raved about the NHS, others damned it with faint praise, and a few were scathingly critical. One psychiatrist said her father developed a brain tumor and was placed on a waiting list of several weeks before surgery would be performed. She paid a private neurosurgeon several thousand pounds to remove his tumor and save his life! She says this happens often, and only the wealthy can afford to bypass the NHS for prompt medical care.
Later, I was lecturing to psychiatrists in Canada and decided to poll them about their government-run national health service. I asked my audience to raise their hands if they believed their health care system is working well and if they would encourage the United States to adopt a similar model. Ninety percent raised their hands!
So I started thinking: where do U.S. psychiatrists stand on a public health care option? I decided to formulate a hypothesis and test it by polling a sample of Current Psychiatry readers. My hypothesis : A substantial proportion (>60%) of practicing U.S. psychiatrists favor a single-payer public option. My rationale: My hunch was that what we psychiatrists deal with in clinical practice may shape and predict how we think about health care, irrespective of our politics. For example:
- Empathy and caring about our patients are basic tenets of psychiatric practice.
- Many of our patients are uninsured, underinsured, or belong to a government-run health plan.
- Chronic psychiatric patients tend to be indigent or poor and can barely afford to see a health care provider, were it not for government programs designed to help them.
- Psychiatrists are trained to work in teams including other mental health disciplines, and many of us work in community settings. In some ways, we are “community organizers” for our patients.
- Psychiatrists are passionately opposed to discrimination based on age, gender, race, religion, or sexual orientation. We serve as advocates for our patients who are “disabled” and depend on the government for food, shelter, and medical care.
- Psychiatrists are keenly aware of the importance of a “social placenta” to help nurture our patients and provide for their personal, social, vocational, and health needs.
- Psychiatrists believe in individual responsibility but in sharing the burden when an individual cannot cope because of biological, psychological, or social reasons.
- Psychiatrists suffer along with our patients. The stigma of mental illness is social and economic. Even so-called “good insurance” rarely offers parity for psychiatric disorders, leaving patients with high co-payments, ridiculous annual and lifetime caps, and arbitrary limits on hospital stays and outpatient visits.
Poll results. I wrote this editorial before I learned the results of an online poll of 5,000 Current Psychiatry readers conducted from September 24 to October 7, 2009 (320 responses [6% response rate]). To the statement, “If you could reform the nation’s health care system, you would favor a single government-run system to cover every American,” the responses were as follows:
- 32.5% for “strongly agree”
- 23.4% for “agree”
- 17.2% for “disagree”
- 26.9% for “strongly disagree.”
Thus, my hypothesis was confirmed by this poll but by a smaller margin than I predicted (56% of respondents favored a single-payer public option, instead of >60%). These colleagues sent in many insightful comments—agreeing and disagreeing—which you can read by clicking here.
It appears that politics may be more potent in shaping psychiatrists’ attitudes about the government’s role in health care than I postulated. I would be happy to hear your thoughts.
Like everyone else, I could not avoid being swept up by the national debate about how to reform our health care system. The debate has been highly politicized, with the liberal left strongly supporting and the conservative right vehemently opposing a single-payer government-run public option (but keeping Medicare and Medicaid). Independents seem to waver between the major overhaul of a public option and making the system more competitive and less expensive.
Developing an idea
Recently I was speaking to psychiatry colleagues in the United Kingdom as their National Health Service (NHS) celebrated its 60th anniversary. Some raved about the NHS, others damned it with faint praise, and a few were scathingly critical. One psychiatrist said her father developed a brain tumor and was placed on a waiting list of several weeks before surgery would be performed. She paid a private neurosurgeon several thousand pounds to remove his tumor and save his life! She says this happens often, and only the wealthy can afford to bypass the NHS for prompt medical care.
Later, I was lecturing to psychiatrists in Canada and decided to poll them about their government-run national health service. I asked my audience to raise their hands if they believed their health care system is working well and if they would encourage the United States to adopt a similar model. Ninety percent raised their hands!
So I started thinking: where do U.S. psychiatrists stand on a public health care option? I decided to formulate a hypothesis and test it by polling a sample of Current Psychiatry readers. My hypothesis : A substantial proportion (>60%) of practicing U.S. psychiatrists favor a single-payer public option. My rationale: My hunch was that what we psychiatrists deal with in clinical practice may shape and predict how we think about health care, irrespective of our politics. For example:
- Empathy and caring about our patients are basic tenets of psychiatric practice.
- Many of our patients are uninsured, underinsured, or belong to a government-run health plan.
- Chronic psychiatric patients tend to be indigent or poor and can barely afford to see a health care provider, were it not for government programs designed to help them.
- Psychiatrists are trained to work in teams including other mental health disciplines, and many of us work in community settings. In some ways, we are “community organizers” for our patients.
- Psychiatrists are passionately opposed to discrimination based on age, gender, race, religion, or sexual orientation. We serve as advocates for our patients who are “disabled” and depend on the government for food, shelter, and medical care.
- Psychiatrists are keenly aware of the importance of a “social placenta” to help nurture our patients and provide for their personal, social, vocational, and health needs.
- Psychiatrists believe in individual responsibility but in sharing the burden when an individual cannot cope because of biological, psychological, or social reasons.
- Psychiatrists suffer along with our patients. The stigma of mental illness is social and economic. Even so-called “good insurance” rarely offers parity for psychiatric disorders, leaving patients with high co-payments, ridiculous annual and lifetime caps, and arbitrary limits on hospital stays and outpatient visits.
Poll results. I wrote this editorial before I learned the results of an online poll of 5,000 Current Psychiatry readers conducted from September 24 to October 7, 2009 (320 responses [6% response rate]). To the statement, “If you could reform the nation’s health care system, you would favor a single government-run system to cover every American,” the responses were as follows:
- 32.5% for “strongly agree”
- 23.4% for “agree”
- 17.2% for “disagree”
- 26.9% for “strongly disagree.”
Thus, my hypothesis was confirmed by this poll but by a smaller margin than I predicted (56% of respondents favored a single-payer public option, instead of >60%). These colleagues sent in many insightful comments—agreeing and disagreeing—which you can read by clicking here.
It appears that politics may be more potent in shaping psychiatrists’ attitudes about the government’s role in health care than I postulated. I would be happy to hear your thoughts.
Like everyone else, I could not avoid being swept up by the national debate about how to reform our health care system. The debate has been highly politicized, with the liberal left strongly supporting and the conservative right vehemently opposing a single-payer government-run public option (but keeping Medicare and Medicaid). Independents seem to waver between the major overhaul of a public option and making the system more competitive and less expensive.
Developing an idea
Recently I was speaking to psychiatry colleagues in the United Kingdom as their National Health Service (NHS) celebrated its 60th anniversary. Some raved about the NHS, others damned it with faint praise, and a few were scathingly critical. One psychiatrist said her father developed a brain tumor and was placed on a waiting list of several weeks before surgery would be performed. She paid a private neurosurgeon several thousand pounds to remove his tumor and save his life! She says this happens often, and only the wealthy can afford to bypass the NHS for prompt medical care.
Later, I was lecturing to psychiatrists in Canada and decided to poll them about their government-run national health service. I asked my audience to raise their hands if they believed their health care system is working well and if they would encourage the United States to adopt a similar model. Ninety percent raised their hands!
So I started thinking: where do U.S. psychiatrists stand on a public health care option? I decided to formulate a hypothesis and test it by polling a sample of Current Psychiatry readers. My hypothesis : A substantial proportion (>60%) of practicing U.S. psychiatrists favor a single-payer public option. My rationale: My hunch was that what we psychiatrists deal with in clinical practice may shape and predict how we think about health care, irrespective of our politics. For example:
- Empathy and caring about our patients are basic tenets of psychiatric practice.
- Many of our patients are uninsured, underinsured, or belong to a government-run health plan.
- Chronic psychiatric patients tend to be indigent or poor and can barely afford to see a health care provider, were it not for government programs designed to help them.
- Psychiatrists are trained to work in teams including other mental health disciplines, and many of us work in community settings. In some ways, we are “community organizers” for our patients.
- Psychiatrists are passionately opposed to discrimination based on age, gender, race, religion, or sexual orientation. We serve as advocates for our patients who are “disabled” and depend on the government for food, shelter, and medical care.
- Psychiatrists are keenly aware of the importance of a “social placenta” to help nurture our patients and provide for their personal, social, vocational, and health needs.
- Psychiatrists believe in individual responsibility but in sharing the burden when an individual cannot cope because of biological, psychological, or social reasons.
- Psychiatrists suffer along with our patients. The stigma of mental illness is social and economic. Even so-called “good insurance” rarely offers parity for psychiatric disorders, leaving patients with high co-payments, ridiculous annual and lifetime caps, and arbitrary limits on hospital stays and outpatient visits.
Poll results. I wrote this editorial before I learned the results of an online poll of 5,000 Current Psychiatry readers conducted from September 24 to October 7, 2009 (320 responses [6% response rate]). To the statement, “If you could reform the nation’s health care system, you would favor a single government-run system to cover every American,” the responses were as follows:
- 32.5% for “strongly agree”
- 23.4% for “agree”
- 17.2% for “disagree”
- 26.9% for “strongly disagree.”
Thus, my hypothesis was confirmed by this poll but by a smaller margin than I predicted (56% of respondents favored a single-payer public option, instead of >60%). These colleagues sent in many insightful comments—agreeing and disagreeing—which you can read by clicking here.
It appears that politics may be more potent in shaping psychiatrists’ attitudes about the government’s role in health care than I postulated. I would be happy to hear your thoughts.
Testifying for civil commitment
Testifying in civil commitment proceedings sometimes is the only way to make sure dangerous patients get the hospital care they need. But for many psychiatrists, providing courtroom testimony can be a nerve-wracking experience because they:
- lack formal training about how to testify
- lack familiarity with laws and court procedures
- fear cross-examination.
Training programs are required to teach psychiatry residents about civil commitment but not about how to testify.1,2 Residents who get to take the stand during training usually do not receive any instruction.2 Knowing some fundamentals of testifying can reduce your anxiety and reluctance to take the stand3 and help you to perform better in court.
Court procedures
A doctor may not force a patient to stay in a hospital, no matter how much the patient needs treatment. Only courts have legal authority to order involuntary psychiatric hospitalization, and courts may do this only after receiving proof that civil commitment is legally justified. Statutory criteria for civil commitment vary across jurisdictions, but typically, the court must hear evidence proving that a person:
- exhibits clear signs of a mental illness
- and because of the mental illness recently did something that placed himself or others in physical danger.
Courts usually rely on testimony from patients’ caregivers for this evidence. Thus, testifying is a skill psychiatrists must exercise to care for seriously ill patients who need treatment but don’t want it.
Testifying and playing basketball have a lot in common. To score points in basketball, a player must put the ball through the hoop and stay in bounds.
To be effective in a civil commitment hearing, a psychiatrist needs a similar game plan. The ball is your testimony, the hoop is the law’s exact wording in your state, and the bounds are recent dangerous behavior.
Completing the Civil Commitment Checklist ( Figure ) will help you determine if you are ready to go to court. Download a PDF of this checklist and a worksheet to compile information you will need to provide accurate and relevant testimony.
Figure: Are you ready to testify in a civil commitment hearing?
*If you answer “yes” to all questions, you are ready to testify about the need for civil commitment. If you answer “no” to any bulleted items, civil commitment may be inappropriate. If you answer “no” to any of the other questions, you’re not ready to go to court
Shoot the ball through the hoop
As early as the mid-19th century, attorneys and physicians realized that “no physician or surgeon could be a satisfactory expert witness without some knowledge of the law.”4 You may have the best basketball shooting technique in the world, but it won’t help if you don’t know where the hoop is. Likewise, you’ll be shooting blind if you come to court without knowing your state’s requirements for civil commitment—which many psychiatrists don’t know.5
Your skills at diagnosis and verbal persuasiveness are critical to good testimony, but if you don’t directly address the requirements for involuntary hospitalization in your state, your testimony may be irrelevant. A court cannot authorize civil commitment unless your testimony clearly and convincingly shows that a patient is mentally ill and dangerous—as defined by the law in your state.
In most states, you can look up your state’s commitment statute on the Internet, and you can take a printed copy of the statute to the witness stand if you wish. Using the law’s actual wording, you can give the court examples of behavior that show why your patient needs hospitalization.
For example, Ohio law defines a “mental disorder” for purposes of involuntary hospitalization as “a substantial disorder of thought, mood, perception, orientation, or memory that grossly impairs judgment, behavior, capacity to recognize reality, or ability to meet the ordinary demands of life.”6 In Ohio and many other states, an official psychiatric diagnosis is neither necessary nor sufficient for civil commitment. Of course, psychiatrists should formulate diagnostic opinions using well-established criteria. But in court, the diagnosis is like the backboard—it is not the hoop that the ball must pass through. The court needs to know whether a patient’s recent actions are manifestations of impairments listed in the statute.
Here’s an example of testimony that makes the basketball hit the backboard but doesn’t put the ball through the hoop:
Doctor: “Your honor, my patient has schizophrenia, paranoid type, which he’s had for quite a number of years. Patients with paranoid schizophrenia have a hard time because they think people are after them. Based on my experience, I don’t see how my patient can survive outside the hospital right now. He’s too paranoid, and his thinking is messed up.”
Though this testimony may be medically sound, the psychiatrist has not told the court specifically how the patient’s illness impairs his present functioning. Here’s an example of a ball going through the hoop after bouncing off the backboard:
Doctor: “Your honor, my patient has schizophrenia, paranoid type. He hears voices saying that the government is trying to assassinate him by poisoning his food and medications. As a result, he stopped taking medications 5 days ago and left the safety of his group home. He has been living under an overpass and refusing to eat. He has become malnourished and dehydrated. This shows he has a substantial disorder of thought and judgment that keeps him from recognizing reality and meeting the ordinary demands of life.”
Staying in bounds
As a witness, your role is to tell the court the truth about your patient’s situation so that justice can be served—if necessary, by allowing the state to override your patient’s liberty interests through involuntary hospitalization.7 To justify involuntary hospitalization in most states, the court must find that your patient is both mentally ill and has recently done something dangerous.
In basketball, the referee stops the game when the ball goes out of bounds. Similarly, the court may stop you if your testimony goes out of bounds by invoking non-recent evidence to demonstrate current dangerousness. For example:
Doctor: “Your honor, this patient has been hospitalized twice in the last 10 months after intentionally overdosing on medications. He told me that last month he tried to kill himself with pills. I know this patient well, and I fear he’s going to overdose again.”
Patient’s attorney: “Objection, your honor. What my client said 1 month ago has nothing to do with his alleged dangerousness today.”
The judge may sustain the objection and disregard your testimony because the events you have recounted fall outside your jurisdiction’s time frame for a “recent” event. What your patient said last month may well make you think your patient is at risk now, but the law establishes sometimes-arbitrary boundaries to protect patients’ liberty. The legal justification is that placing time limits on dangerous behavior minimizes the risk of an erroneous civil commitment; evidence of actual, recent behavior increases the likelihood of real, current danger and reduces the risk of involuntarily hospitalizing someone who would not do harm.8
The definition of “recent” varies from state to state. Pennsylvania looks at behavior within the last 30 days;9 Utah uses 7 days.10 Often, the law is not clear; rather than set firm time restrictions, some states consider whether the actions in question are “material and relevant to the person’s present condition.”11
Know what your jurisdiction considers “recent” behavior. If your state’s statute is not clear, ask a judge or attorney. As you think about your testimony, make sure that information describes dangerous behavior within the time frame that the court will accept as “recent.”
Cross-examination
Civil commitment hearings are supposed to be adversarial. The “proponent” of involuntary hospitalization (the State) puts on its best case, hoping to convince the judge (or occasionally, a jury) that commitment is justified. The “respondent” (the patient facing potential commitment) has many of the rights that criminal defendants have, including the right to an attorney and the right to challenge witnesses—including psychiatrists—through cross-examination.12
Psychiatrists aren’t accustomed to having their clinical reasoning forcefully challenged. When they disagree, psychiatrists usually seek to persuade each other and reach consensus rather than openly criticize colleagues and point out flaws in their opinions about patients. Even when insurance companies and patients disagree with you, they usually don’t try to discredit you.
Testifying is different.13 Expect to have your conclusions bluntly challenged in civil commitment hearings. But remember: as in a basketball game, attorneys’ cross-examination challenges are not personal—they’re intended only to win the game. Also, you can practice. Just as practicing against opponents improves skills needed to win basketball games, practicing against real or anticipated cross-examination can help you respond when you’re testifying. Be prepared to answer commonly asked questions ( Table 1 ), such as:
“Doctor, you’ve testified that Mr. Jones has bipolar disorder. Aren’t all psychiatric diagnoses just a guess?”
“Doctor, how can you be certain that Mr. Smith’s psychosis, as you call it, was the result of schizophrenia, not alcohol intoxication?”
“Well, doctor, if you’re saying that Mrs. Clark’s psychosis was caused by a urinary tract infection, isn’t that a medical problem and not a psychiatric problem that we can lock her up for?”
Other ways to prepare:
- Have a colleague play the part of an opposing attorney who is trying to find fault with your clinical reasoning.
- Imagine you are retained by an attorney who wants to find holes in your own testimony.
- Watch other psychiatrists testify, and learn from their triumphs or mistakes.
Table 1
Questions you’re likely to face when testifying
| Question | Importance |
|---|---|
| What is your diagnosis? | In all states, a mental illness leading to dangerous behavior is required for involuntary hospitalization. Courts are less interested in the name of the disorder than in knowing how its symptoms affect the respondent and lead to danger |
| Why is the hospital the least restrictive environment? | The “least restrictive” legal standard14 is a safeguard against unwarranted hospitalization. Be ready to explain why other treatment options (outpatient, day hospitals, etc.) are not appropriate |
| What medications is the patient taking? | Be prepared to tell the court whether the patient was taking medications before and since admission. Know which medications are being prescribed for the patient in the hospital |
| Why does your diagnosis differ from the diagnosis of another clinician in the chart? | Cross-examining attorneys will often try to discredit your opinion by pointing out that other clinicians diagnosed something different. You can explain that different disorders belong within common diagnostic categories (for example, schizophrenia and schizoaffective disorder are both psychoses). Explaining your answer this way enhances its credibility by demonstrating agreement with the other clinician |
| What is the patient’s response to the allegations of dangerousness listed in the chart? | Don’t neglect patients’ views. Their answers allow you to testify about the patient from direct knowledge and often provide evidence of thought, mood, or judgment problems |
Good and bad shots
Good basketball players avoid fouls and taking shots that opponents can block easily. A good psychiatric witness knows how to avoid committing legal fouls and having testimony blocked.
The hearsay block. In clinical practice, psychiatrists should use and often rely on information about their patients that they obtain from other persons. But in court, testifying about such information could be disallowed on grounds that it is hearsay—testimony about what someone else saw or heard.
In civil commitment hearings, rules against allowing hearsay protect patients from accusations that may be false or misleading and that they cannot challenge through cross-examination. Although many states have a “hearsay exception” for civil commitment hearings—meaning that doctors may testify about what others have told them—not all do. If you practice in a state without this exception, you’ll need to gather information and plan your testimony carefully to avoid having the basis for your opinion excluded.
To avoid this “block,” testify only about events you saw or heard. To be fair to patients, always ask them about their side of the story. You can then testify about your clinical findings—what you saw and heard—instead of what someone else said. For example:
Doctor: “Your wife told me you wanted to kill yourself. Is that true?”
Patient: “It wasn’t my wife. I told my brother I wanted to kill myself.”
Doctor: “How about now? Do you still want to kill yourself?”
Patient: “Yes, I do.”
The doctor now can testify about first-hand experience with the patient:
Doctor: “Your honor, I asked the patient whether he told anyone that he wanted to kill himself. He told me he had told his brother he wanted to kill himself, and that he still felt that way.”
Play only your position
Basketball teams get into trouble if players try to do things others are supposed to do. Players are not supposed to give orders to the coach. In court, your role is to provide expert testimony about your patient and psychiatry. Stay in that role:
- Don’t address the ultimate legal question, as in saying, “My patient meets this state’s commitment criteria.” That’s for the judge to decide.
- Don’t opine about the moral virtues or shortcomings of the courts or hearings: “My patient desperately needs treatment, but you’re just asking about whether he fits narrow legal rules.”
- Don’t testify about topics on which you’re not an expert: “I think the police used too much force when they handcuffed my patient.”
Table 2
Dos and don’ts of testifying in a civil commitment hearing
| Do… | Don’t… |
|---|---|
| Wear conservative business attire, which shows that you take your work seriously | Dress casually. Though casual dress is OK in many workplaces, lawyers wear suits in court |
| Remain calm, professional, and respectful | Make jokes or sarcastic remarks; a cross-examining attorney will easily discredit you by pointing out that this is a ‘serious matter’ |
| Use recent examples of the patient’s dangerousness | Testify about your patient’s childhood or remote events |
| Describe behaviors or statements you witnessed or obtained from the patient | Testify about information obtained only from other people |
| Pause before answering each question. Doing this allows time for you to think and for an attorney to object to the question | Lose your cool or argue with the attorneys or judge |
| Source: Adapted from Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2007;7(3):25-40 | |
Related resources
- Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007.
- Perlin ML. Mental disability law—civil and criminal. 2nd ed. Charlottesville, VA: Lexis Law Publishing; 1998.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. Barr NI, Suarez JM. The teaching of forensic psychiatry in law schools, medical schools and psychiatric residencies in the United States. Am J Psychiatry. 1965;122(6):612-616.
2. Lewis CF. Teaching forensic psychiatry to general psychiatry residents. Acad Psychiatry. 2004;28(1):40-46.
3. Sata LS, Goldenberg EE. A study of involuntary patients in Seattle. Hosp Comm Psychiatry. 1977;28(11):834-837.
4. Curran WJ. Titles in the medicolegal field: a proposal for reform. Am J Law Med. 1975;1:1-11.
5. Brooks RA. Psychiatrist’s opinions about involuntary civil commitment: results of a national survey. J Am Acad Psychiatry Law. 2007;35:219-228.
6. Ohio Rev Code § 5122.01(A).
7. Rotter M, Preven D. Commentary: general residency training—the first forensic stage. J Am Acad Psychiatry Law. 2005;33:324-327.
8. Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007:337.
9. 50 PS § 7301(b).
10. Utah Code Ann § 62A-15-631(a).
11. Matter of D.D., 920 P2d 973, 975 (Mont 1996).
12. Perlin ML. Mental disability law—civil and criminal. 2nd ed, vol. 1, § 2B-3.1. Charlottesville, VA: Lexis Law Publishing; 1998.
13. Gutheil T. The psychiatrist as expert witness. Arlington, VA: American Psychiatric Publishing, Inc.; 1998:11–18.
14. Lake v Cameron, 364 F2d 657 (DC Cir 1967).
Testifying in civil commitment proceedings sometimes is the only way to make sure dangerous patients get the hospital care they need. But for many psychiatrists, providing courtroom testimony can be a nerve-wracking experience because they:
- lack formal training about how to testify
- lack familiarity with laws and court procedures
- fear cross-examination.
Training programs are required to teach psychiatry residents about civil commitment but not about how to testify.1,2 Residents who get to take the stand during training usually do not receive any instruction.2 Knowing some fundamentals of testifying can reduce your anxiety and reluctance to take the stand3 and help you to perform better in court.
Court procedures
A doctor may not force a patient to stay in a hospital, no matter how much the patient needs treatment. Only courts have legal authority to order involuntary psychiatric hospitalization, and courts may do this only after receiving proof that civil commitment is legally justified. Statutory criteria for civil commitment vary across jurisdictions, but typically, the court must hear evidence proving that a person:
- exhibits clear signs of a mental illness
- and because of the mental illness recently did something that placed himself or others in physical danger.
Courts usually rely on testimony from patients’ caregivers for this evidence. Thus, testifying is a skill psychiatrists must exercise to care for seriously ill patients who need treatment but don’t want it.
Testifying and playing basketball have a lot in common. To score points in basketball, a player must put the ball through the hoop and stay in bounds.
To be effective in a civil commitment hearing, a psychiatrist needs a similar game plan. The ball is your testimony, the hoop is the law’s exact wording in your state, and the bounds are recent dangerous behavior.
Completing the Civil Commitment Checklist ( Figure ) will help you determine if you are ready to go to court. Download a PDF of this checklist and a worksheet to compile information you will need to provide accurate and relevant testimony.
Figure: Are you ready to testify in a civil commitment hearing?
*If you answer “yes” to all questions, you are ready to testify about the need for civil commitment. If you answer “no” to any bulleted items, civil commitment may be inappropriate. If you answer “no” to any of the other questions, you’re not ready to go to court
Shoot the ball through the hoop
As early as the mid-19th century, attorneys and physicians realized that “no physician or surgeon could be a satisfactory expert witness without some knowledge of the law.”4 You may have the best basketball shooting technique in the world, but it won’t help if you don’t know where the hoop is. Likewise, you’ll be shooting blind if you come to court without knowing your state’s requirements for civil commitment—which many psychiatrists don’t know.5
Your skills at diagnosis and verbal persuasiveness are critical to good testimony, but if you don’t directly address the requirements for involuntary hospitalization in your state, your testimony may be irrelevant. A court cannot authorize civil commitment unless your testimony clearly and convincingly shows that a patient is mentally ill and dangerous—as defined by the law in your state.
In most states, you can look up your state’s commitment statute on the Internet, and you can take a printed copy of the statute to the witness stand if you wish. Using the law’s actual wording, you can give the court examples of behavior that show why your patient needs hospitalization.
For example, Ohio law defines a “mental disorder” for purposes of involuntary hospitalization as “a substantial disorder of thought, mood, perception, orientation, or memory that grossly impairs judgment, behavior, capacity to recognize reality, or ability to meet the ordinary demands of life.”6 In Ohio and many other states, an official psychiatric diagnosis is neither necessary nor sufficient for civil commitment. Of course, psychiatrists should formulate diagnostic opinions using well-established criteria. But in court, the diagnosis is like the backboard—it is not the hoop that the ball must pass through. The court needs to know whether a patient’s recent actions are manifestations of impairments listed in the statute.
Here’s an example of testimony that makes the basketball hit the backboard but doesn’t put the ball through the hoop:
Doctor: “Your honor, my patient has schizophrenia, paranoid type, which he’s had for quite a number of years. Patients with paranoid schizophrenia have a hard time because they think people are after them. Based on my experience, I don’t see how my patient can survive outside the hospital right now. He’s too paranoid, and his thinking is messed up.”
Though this testimony may be medically sound, the psychiatrist has not told the court specifically how the patient’s illness impairs his present functioning. Here’s an example of a ball going through the hoop after bouncing off the backboard:
Doctor: “Your honor, my patient has schizophrenia, paranoid type. He hears voices saying that the government is trying to assassinate him by poisoning his food and medications. As a result, he stopped taking medications 5 days ago and left the safety of his group home. He has been living under an overpass and refusing to eat. He has become malnourished and dehydrated. This shows he has a substantial disorder of thought and judgment that keeps him from recognizing reality and meeting the ordinary demands of life.”
Staying in bounds
As a witness, your role is to tell the court the truth about your patient’s situation so that justice can be served—if necessary, by allowing the state to override your patient’s liberty interests through involuntary hospitalization.7 To justify involuntary hospitalization in most states, the court must find that your patient is both mentally ill and has recently done something dangerous.
In basketball, the referee stops the game when the ball goes out of bounds. Similarly, the court may stop you if your testimony goes out of bounds by invoking non-recent evidence to demonstrate current dangerousness. For example:
Doctor: “Your honor, this patient has been hospitalized twice in the last 10 months after intentionally overdosing on medications. He told me that last month he tried to kill himself with pills. I know this patient well, and I fear he’s going to overdose again.”
Patient’s attorney: “Objection, your honor. What my client said 1 month ago has nothing to do with his alleged dangerousness today.”
The judge may sustain the objection and disregard your testimony because the events you have recounted fall outside your jurisdiction’s time frame for a “recent” event. What your patient said last month may well make you think your patient is at risk now, but the law establishes sometimes-arbitrary boundaries to protect patients’ liberty. The legal justification is that placing time limits on dangerous behavior minimizes the risk of an erroneous civil commitment; evidence of actual, recent behavior increases the likelihood of real, current danger and reduces the risk of involuntarily hospitalizing someone who would not do harm.8
The definition of “recent” varies from state to state. Pennsylvania looks at behavior within the last 30 days;9 Utah uses 7 days.10 Often, the law is not clear; rather than set firm time restrictions, some states consider whether the actions in question are “material and relevant to the person’s present condition.”11
Know what your jurisdiction considers “recent” behavior. If your state’s statute is not clear, ask a judge or attorney. As you think about your testimony, make sure that information describes dangerous behavior within the time frame that the court will accept as “recent.”
Cross-examination
Civil commitment hearings are supposed to be adversarial. The “proponent” of involuntary hospitalization (the State) puts on its best case, hoping to convince the judge (or occasionally, a jury) that commitment is justified. The “respondent” (the patient facing potential commitment) has many of the rights that criminal defendants have, including the right to an attorney and the right to challenge witnesses—including psychiatrists—through cross-examination.12
Psychiatrists aren’t accustomed to having their clinical reasoning forcefully challenged. When they disagree, psychiatrists usually seek to persuade each other and reach consensus rather than openly criticize colleagues and point out flaws in their opinions about patients. Even when insurance companies and patients disagree with you, they usually don’t try to discredit you.
Testifying is different.13 Expect to have your conclusions bluntly challenged in civil commitment hearings. But remember: as in a basketball game, attorneys’ cross-examination challenges are not personal—they’re intended only to win the game. Also, you can practice. Just as practicing against opponents improves skills needed to win basketball games, practicing against real or anticipated cross-examination can help you respond when you’re testifying. Be prepared to answer commonly asked questions ( Table 1 ), such as:
“Doctor, you’ve testified that Mr. Jones has bipolar disorder. Aren’t all psychiatric diagnoses just a guess?”
“Doctor, how can you be certain that Mr. Smith’s psychosis, as you call it, was the result of schizophrenia, not alcohol intoxication?”
“Well, doctor, if you’re saying that Mrs. Clark’s psychosis was caused by a urinary tract infection, isn’t that a medical problem and not a psychiatric problem that we can lock her up for?”
Other ways to prepare:
- Have a colleague play the part of an opposing attorney who is trying to find fault with your clinical reasoning.
- Imagine you are retained by an attorney who wants to find holes in your own testimony.
- Watch other psychiatrists testify, and learn from their triumphs or mistakes.
Table 1
Questions you’re likely to face when testifying
| Question | Importance |
|---|---|
| What is your diagnosis? | In all states, a mental illness leading to dangerous behavior is required for involuntary hospitalization. Courts are less interested in the name of the disorder than in knowing how its symptoms affect the respondent and lead to danger |
| Why is the hospital the least restrictive environment? | The “least restrictive” legal standard14 is a safeguard against unwarranted hospitalization. Be ready to explain why other treatment options (outpatient, day hospitals, etc.) are not appropriate |
| What medications is the patient taking? | Be prepared to tell the court whether the patient was taking medications before and since admission. Know which medications are being prescribed for the patient in the hospital |
| Why does your diagnosis differ from the diagnosis of another clinician in the chart? | Cross-examining attorneys will often try to discredit your opinion by pointing out that other clinicians diagnosed something different. You can explain that different disorders belong within common diagnostic categories (for example, schizophrenia and schizoaffective disorder are both psychoses). Explaining your answer this way enhances its credibility by demonstrating agreement with the other clinician |
| What is the patient’s response to the allegations of dangerousness listed in the chart? | Don’t neglect patients’ views. Their answers allow you to testify about the patient from direct knowledge and often provide evidence of thought, mood, or judgment problems |
Good and bad shots
Good basketball players avoid fouls and taking shots that opponents can block easily. A good psychiatric witness knows how to avoid committing legal fouls and having testimony blocked.
The hearsay block. In clinical practice, psychiatrists should use and often rely on information about their patients that they obtain from other persons. But in court, testifying about such information could be disallowed on grounds that it is hearsay—testimony about what someone else saw or heard.
In civil commitment hearings, rules against allowing hearsay protect patients from accusations that may be false or misleading and that they cannot challenge through cross-examination. Although many states have a “hearsay exception” for civil commitment hearings—meaning that doctors may testify about what others have told them—not all do. If you practice in a state without this exception, you’ll need to gather information and plan your testimony carefully to avoid having the basis for your opinion excluded.
To avoid this “block,” testify only about events you saw or heard. To be fair to patients, always ask them about their side of the story. You can then testify about your clinical findings—what you saw and heard—instead of what someone else said. For example:
Doctor: “Your wife told me you wanted to kill yourself. Is that true?”
Patient: “It wasn’t my wife. I told my brother I wanted to kill myself.”
Doctor: “How about now? Do you still want to kill yourself?”
Patient: “Yes, I do.”
The doctor now can testify about first-hand experience with the patient:
Doctor: “Your honor, I asked the patient whether he told anyone that he wanted to kill himself. He told me he had told his brother he wanted to kill himself, and that he still felt that way.”
Play only your position
Basketball teams get into trouble if players try to do things others are supposed to do. Players are not supposed to give orders to the coach. In court, your role is to provide expert testimony about your patient and psychiatry. Stay in that role:
- Don’t address the ultimate legal question, as in saying, “My patient meets this state’s commitment criteria.” That’s for the judge to decide.
- Don’t opine about the moral virtues or shortcomings of the courts or hearings: “My patient desperately needs treatment, but you’re just asking about whether he fits narrow legal rules.”
- Don’t testify about topics on which you’re not an expert: “I think the police used too much force when they handcuffed my patient.”
Table 2
Dos and don’ts of testifying in a civil commitment hearing
| Do… | Don’t… |
|---|---|
| Wear conservative business attire, which shows that you take your work seriously | Dress casually. Though casual dress is OK in many workplaces, lawyers wear suits in court |
| Remain calm, professional, and respectful | Make jokes or sarcastic remarks; a cross-examining attorney will easily discredit you by pointing out that this is a ‘serious matter’ |
| Use recent examples of the patient’s dangerousness | Testify about your patient’s childhood or remote events |
| Describe behaviors or statements you witnessed or obtained from the patient | Testify about information obtained only from other people |
| Pause before answering each question. Doing this allows time for you to think and for an attorney to object to the question | Lose your cool or argue with the attorneys or judge |
| Source: Adapted from Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2007;7(3):25-40 | |
Related resources
- Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007.
- Perlin ML. Mental disability law—civil and criminal. 2nd ed. Charlottesville, VA: Lexis Law Publishing; 1998.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Testifying in civil commitment proceedings sometimes is the only way to make sure dangerous patients get the hospital care they need. But for many psychiatrists, providing courtroom testimony can be a nerve-wracking experience because they:
- lack formal training about how to testify
- lack familiarity with laws and court procedures
- fear cross-examination.
Training programs are required to teach psychiatry residents about civil commitment but not about how to testify.1,2 Residents who get to take the stand during training usually do not receive any instruction.2 Knowing some fundamentals of testifying can reduce your anxiety and reluctance to take the stand3 and help you to perform better in court.
Court procedures
A doctor may not force a patient to stay in a hospital, no matter how much the patient needs treatment. Only courts have legal authority to order involuntary psychiatric hospitalization, and courts may do this only after receiving proof that civil commitment is legally justified. Statutory criteria for civil commitment vary across jurisdictions, but typically, the court must hear evidence proving that a person:
- exhibits clear signs of a mental illness
- and because of the mental illness recently did something that placed himself or others in physical danger.
Courts usually rely on testimony from patients’ caregivers for this evidence. Thus, testifying is a skill psychiatrists must exercise to care for seriously ill patients who need treatment but don’t want it.
Testifying and playing basketball have a lot in common. To score points in basketball, a player must put the ball through the hoop and stay in bounds.
To be effective in a civil commitment hearing, a psychiatrist needs a similar game plan. The ball is your testimony, the hoop is the law’s exact wording in your state, and the bounds are recent dangerous behavior.
Completing the Civil Commitment Checklist ( Figure ) will help you determine if you are ready to go to court. Download a PDF of this checklist and a worksheet to compile information you will need to provide accurate and relevant testimony.
Figure: Are you ready to testify in a civil commitment hearing?
*If you answer “yes” to all questions, you are ready to testify about the need for civil commitment. If you answer “no” to any bulleted items, civil commitment may be inappropriate. If you answer “no” to any of the other questions, you’re not ready to go to court
Shoot the ball through the hoop
As early as the mid-19th century, attorneys and physicians realized that “no physician or surgeon could be a satisfactory expert witness without some knowledge of the law.”4 You may have the best basketball shooting technique in the world, but it won’t help if you don’t know where the hoop is. Likewise, you’ll be shooting blind if you come to court without knowing your state’s requirements for civil commitment—which many psychiatrists don’t know.5
Your skills at diagnosis and verbal persuasiveness are critical to good testimony, but if you don’t directly address the requirements for involuntary hospitalization in your state, your testimony may be irrelevant. A court cannot authorize civil commitment unless your testimony clearly and convincingly shows that a patient is mentally ill and dangerous—as defined by the law in your state.
In most states, you can look up your state’s commitment statute on the Internet, and you can take a printed copy of the statute to the witness stand if you wish. Using the law’s actual wording, you can give the court examples of behavior that show why your patient needs hospitalization.
For example, Ohio law defines a “mental disorder” for purposes of involuntary hospitalization as “a substantial disorder of thought, mood, perception, orientation, or memory that grossly impairs judgment, behavior, capacity to recognize reality, or ability to meet the ordinary demands of life.”6 In Ohio and many other states, an official psychiatric diagnosis is neither necessary nor sufficient for civil commitment. Of course, psychiatrists should formulate diagnostic opinions using well-established criteria. But in court, the diagnosis is like the backboard—it is not the hoop that the ball must pass through. The court needs to know whether a patient’s recent actions are manifestations of impairments listed in the statute.
Here’s an example of testimony that makes the basketball hit the backboard but doesn’t put the ball through the hoop:
Doctor: “Your honor, my patient has schizophrenia, paranoid type, which he’s had for quite a number of years. Patients with paranoid schizophrenia have a hard time because they think people are after them. Based on my experience, I don’t see how my patient can survive outside the hospital right now. He’s too paranoid, and his thinking is messed up.”
Though this testimony may be medically sound, the psychiatrist has not told the court specifically how the patient’s illness impairs his present functioning. Here’s an example of a ball going through the hoop after bouncing off the backboard:
Doctor: “Your honor, my patient has schizophrenia, paranoid type. He hears voices saying that the government is trying to assassinate him by poisoning his food and medications. As a result, he stopped taking medications 5 days ago and left the safety of his group home. He has been living under an overpass and refusing to eat. He has become malnourished and dehydrated. This shows he has a substantial disorder of thought and judgment that keeps him from recognizing reality and meeting the ordinary demands of life.”
Staying in bounds
As a witness, your role is to tell the court the truth about your patient’s situation so that justice can be served—if necessary, by allowing the state to override your patient’s liberty interests through involuntary hospitalization.7 To justify involuntary hospitalization in most states, the court must find that your patient is both mentally ill and has recently done something dangerous.
In basketball, the referee stops the game when the ball goes out of bounds. Similarly, the court may stop you if your testimony goes out of bounds by invoking non-recent evidence to demonstrate current dangerousness. For example:
Doctor: “Your honor, this patient has been hospitalized twice in the last 10 months after intentionally overdosing on medications. He told me that last month he tried to kill himself with pills. I know this patient well, and I fear he’s going to overdose again.”
Patient’s attorney: “Objection, your honor. What my client said 1 month ago has nothing to do with his alleged dangerousness today.”
The judge may sustain the objection and disregard your testimony because the events you have recounted fall outside your jurisdiction’s time frame for a “recent” event. What your patient said last month may well make you think your patient is at risk now, but the law establishes sometimes-arbitrary boundaries to protect patients’ liberty. The legal justification is that placing time limits on dangerous behavior minimizes the risk of an erroneous civil commitment; evidence of actual, recent behavior increases the likelihood of real, current danger and reduces the risk of involuntarily hospitalizing someone who would not do harm.8
The definition of “recent” varies from state to state. Pennsylvania looks at behavior within the last 30 days;9 Utah uses 7 days.10 Often, the law is not clear; rather than set firm time restrictions, some states consider whether the actions in question are “material and relevant to the person’s present condition.”11
Know what your jurisdiction considers “recent” behavior. If your state’s statute is not clear, ask a judge or attorney. As you think about your testimony, make sure that information describes dangerous behavior within the time frame that the court will accept as “recent.”
Cross-examination
Civil commitment hearings are supposed to be adversarial. The “proponent” of involuntary hospitalization (the State) puts on its best case, hoping to convince the judge (or occasionally, a jury) that commitment is justified. The “respondent” (the patient facing potential commitment) has many of the rights that criminal defendants have, including the right to an attorney and the right to challenge witnesses—including psychiatrists—through cross-examination.12
Psychiatrists aren’t accustomed to having their clinical reasoning forcefully challenged. When they disagree, psychiatrists usually seek to persuade each other and reach consensus rather than openly criticize colleagues and point out flaws in their opinions about patients. Even when insurance companies and patients disagree with you, they usually don’t try to discredit you.
Testifying is different.13 Expect to have your conclusions bluntly challenged in civil commitment hearings. But remember: as in a basketball game, attorneys’ cross-examination challenges are not personal—they’re intended only to win the game. Also, you can practice. Just as practicing against opponents improves skills needed to win basketball games, practicing against real or anticipated cross-examination can help you respond when you’re testifying. Be prepared to answer commonly asked questions ( Table 1 ), such as:
“Doctor, you’ve testified that Mr. Jones has bipolar disorder. Aren’t all psychiatric diagnoses just a guess?”
“Doctor, how can you be certain that Mr. Smith’s psychosis, as you call it, was the result of schizophrenia, not alcohol intoxication?”
“Well, doctor, if you’re saying that Mrs. Clark’s psychosis was caused by a urinary tract infection, isn’t that a medical problem and not a psychiatric problem that we can lock her up for?”
Other ways to prepare:
- Have a colleague play the part of an opposing attorney who is trying to find fault with your clinical reasoning.
- Imagine you are retained by an attorney who wants to find holes in your own testimony.
- Watch other psychiatrists testify, and learn from their triumphs or mistakes.
Table 1
Questions you’re likely to face when testifying
| Question | Importance |
|---|---|
| What is your diagnosis? | In all states, a mental illness leading to dangerous behavior is required for involuntary hospitalization. Courts are less interested in the name of the disorder than in knowing how its symptoms affect the respondent and lead to danger |
| Why is the hospital the least restrictive environment? | The “least restrictive” legal standard14 is a safeguard against unwarranted hospitalization. Be ready to explain why other treatment options (outpatient, day hospitals, etc.) are not appropriate |
| What medications is the patient taking? | Be prepared to tell the court whether the patient was taking medications before and since admission. Know which medications are being prescribed for the patient in the hospital |
| Why does your diagnosis differ from the diagnosis of another clinician in the chart? | Cross-examining attorneys will often try to discredit your opinion by pointing out that other clinicians diagnosed something different. You can explain that different disorders belong within common diagnostic categories (for example, schizophrenia and schizoaffective disorder are both psychoses). Explaining your answer this way enhances its credibility by demonstrating agreement with the other clinician |
| What is the patient’s response to the allegations of dangerousness listed in the chart? | Don’t neglect patients’ views. Their answers allow you to testify about the patient from direct knowledge and often provide evidence of thought, mood, or judgment problems |
Good and bad shots
Good basketball players avoid fouls and taking shots that opponents can block easily. A good psychiatric witness knows how to avoid committing legal fouls and having testimony blocked.
The hearsay block. In clinical practice, psychiatrists should use and often rely on information about their patients that they obtain from other persons. But in court, testifying about such information could be disallowed on grounds that it is hearsay—testimony about what someone else saw or heard.
In civil commitment hearings, rules against allowing hearsay protect patients from accusations that may be false or misleading and that they cannot challenge through cross-examination. Although many states have a “hearsay exception” for civil commitment hearings—meaning that doctors may testify about what others have told them—not all do. If you practice in a state without this exception, you’ll need to gather information and plan your testimony carefully to avoid having the basis for your opinion excluded.
To avoid this “block,” testify only about events you saw or heard. To be fair to patients, always ask them about their side of the story. You can then testify about your clinical findings—what you saw and heard—instead of what someone else said. For example:
Doctor: “Your wife told me you wanted to kill yourself. Is that true?”
Patient: “It wasn’t my wife. I told my brother I wanted to kill myself.”
Doctor: “How about now? Do you still want to kill yourself?”
Patient: “Yes, I do.”
The doctor now can testify about first-hand experience with the patient:
Doctor: “Your honor, I asked the patient whether he told anyone that he wanted to kill himself. He told me he had told his brother he wanted to kill himself, and that he still felt that way.”
Play only your position
Basketball teams get into trouble if players try to do things others are supposed to do. Players are not supposed to give orders to the coach. In court, your role is to provide expert testimony about your patient and psychiatry. Stay in that role:
- Don’t address the ultimate legal question, as in saying, “My patient meets this state’s commitment criteria.” That’s for the judge to decide.
- Don’t opine about the moral virtues or shortcomings of the courts or hearings: “My patient desperately needs treatment, but you’re just asking about whether he fits narrow legal rules.”
- Don’t testify about topics on which you’re not an expert: “I think the police used too much force when they handcuffed my patient.”
Table 2
Dos and don’ts of testifying in a civil commitment hearing
| Do… | Don’t… |
|---|---|
| Wear conservative business attire, which shows that you take your work seriously | Dress casually. Though casual dress is OK in many workplaces, lawyers wear suits in court |
| Remain calm, professional, and respectful | Make jokes or sarcastic remarks; a cross-examining attorney will easily discredit you by pointing out that this is a ‘serious matter’ |
| Use recent examples of the patient’s dangerousness | Testify about your patient’s childhood or remote events |
| Describe behaviors or statements you witnessed or obtained from the patient | Testify about information obtained only from other people |
| Pause before answering each question. Doing this allows time for you to think and for an attorney to object to the question | Lose your cool or argue with the attorneys or judge |
| Source: Adapted from Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2007;7(3):25-40 | |
Related resources
- Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007.
- Perlin ML. Mental disability law—civil and criminal. 2nd ed. Charlottesville, VA: Lexis Law Publishing; 1998.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. Barr NI, Suarez JM. The teaching of forensic psychiatry in law schools, medical schools and psychiatric residencies in the United States. Am J Psychiatry. 1965;122(6):612-616.
2. Lewis CF. Teaching forensic psychiatry to general psychiatry residents. Acad Psychiatry. 2004;28(1):40-46.
3. Sata LS, Goldenberg EE. A study of involuntary patients in Seattle. Hosp Comm Psychiatry. 1977;28(11):834-837.
4. Curran WJ. Titles in the medicolegal field: a proposal for reform. Am J Law Med. 1975;1:1-11.
5. Brooks RA. Psychiatrist’s opinions about involuntary civil commitment: results of a national survey. J Am Acad Psychiatry Law. 2007;35:219-228.
6. Ohio Rev Code § 5122.01(A).
7. Rotter M, Preven D. Commentary: general residency training—the first forensic stage. J Am Acad Psychiatry Law. 2005;33:324-327.
8. Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007:337.
9. 50 PS § 7301(b).
10. Utah Code Ann § 62A-15-631(a).
11. Matter of D.D., 920 P2d 973, 975 (Mont 1996).
12. Perlin ML. Mental disability law—civil and criminal. 2nd ed, vol. 1, § 2B-3.1. Charlottesville, VA: Lexis Law Publishing; 1998.
13. Gutheil T. The psychiatrist as expert witness. Arlington, VA: American Psychiatric Publishing, Inc.; 1998:11–18.
14. Lake v Cameron, 364 F2d 657 (DC Cir 1967).
1. Barr NI, Suarez JM. The teaching of forensic psychiatry in law schools, medical schools and psychiatric residencies in the United States. Am J Psychiatry. 1965;122(6):612-616.
2. Lewis CF. Teaching forensic psychiatry to general psychiatry residents. Acad Psychiatry. 2004;28(1):40-46.
3. Sata LS, Goldenberg EE. A study of involuntary patients in Seattle. Hosp Comm Psychiatry. 1977;28(11):834-837.
4. Curran WJ. Titles in the medicolegal field: a proposal for reform. Am J Law Med. 1975;1:1-11.
5. Brooks RA. Psychiatrist’s opinions about involuntary civil commitment: results of a national survey. J Am Acad Psychiatry Law. 2007;35:219-228.
6. Ohio Rev Code § 5122.01(A).
7. Rotter M, Preven D. Commentary: general residency training—the first forensic stage. J Am Acad Psychiatry Law. 2005;33:324-327.
8. Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. New York, NY: Guilford Press; 2007:337.
9. 50 PS § 7301(b).
10. Utah Code Ann § 62A-15-631(a).
11. Matter of D.D., 920 P2d 973, 975 (Mont 1996).
12. Perlin ML. Mental disability law—civil and criminal. 2nd ed, vol. 1, § 2B-3.1. Charlottesville, VA: Lexis Law Publishing; 1998.
13. Gutheil T. The psychiatrist as expert witness. Arlington, VA: American Psychiatric Publishing, Inc.; 1998:11–18.
14. Lake v Cameron, 364 F2d 657 (DC Cir 1967).
How to manage medical complications of the 5 most abused substances
Individuals who abuse substances often have comorbid psychiatric disorders—80% of alcoholics have another axis I disorder1—and the reverse also is true. More than one-half of schizophrenia patients and 30% of anxiety and affective disorder patients abuse substances.1
In addition to worsening psychiatric illnesses and interfering with proper treatment, alcohol and other substances can lead to serious cardiac, neurologic, pulmonary, or gastrointestinal complications that can linger even after your patient stops abusing drugs. This article provides an overview of common medical complications related to using alcohol, marijuana, cocaine, methamphetamines, and opioids.
Alcohol
Because some consequences of alcohol abuse (Table 1) are thought to be dose-dependent, ask about your patient’s alcohol consumption. Moderate drinking is defined as up to 2 drinks/day for men and 1 drink/day for women.2 Heavy drinking is ≥5 drinks/day (or ≥15 drinks/week) for men and ≥4/day (or ≥8/week) for women.3 A drink contains 12.5 grams of ethanol and is defined as:
- 12 oz (360 mL) of beer or wine cooler
- 5 oz (150 mL) of wine
- 1.5 oz (45 mL) of 80-proof distilled spirits.3
Gastrointestinal effects. Chronic heavy alcohol consumption can lead to fatty liver (steatosis), alcoholic hepatitis, and cirrhosis. Steatosis—the first stage of alcoholic liver disease—can occur from heavy drinking for just a few days but can be reversed with abstinence from alcohol. Prolonged use can lead to alcoholic hepatitis. Symptoms include nausea, lack of appetite, vomiting, fatigue, abdominal pain and tenderness, spider-like blood vessels, and increased bleeding times.
Abstinence might not reverse liver damage from alcoholic hepatitis, and cirrhosis can still develop. Up to 70% of patients with alcoholic hepatitis will develop cirrhosis.4,5 Common physical manifestations of cirrhosis include generalized weakness, fatigue, malaise, anorexia with signs of malnutrition, and increased bleeding.
Laboratory findings of elevated aspartate aminotransferase/alanine aminotransferase, gamma-glutamyltransferase, and carbohydrate-deficient transferrin also point to heavy alcohol use.6
Acute pancreatitis—the most common cause of hospitalization from alcohol-related GI complications—is seen more often than liver disease.7
Cardiovascular effects. Light to moderate drinking may be cardioprotective, but heavy alcohol consumption increases the risk of hypertension and ischemic heart disease.8 Incidence of hypertension is two-fold greater in individuals who have >2 drinks/day and highest in those who have >5 drinks/day.9
Prolonged excessive alcohol consumption is the leading cause of nonischemic dilated cardiomyopathy. Symptoms of alcoholic cardiomyopathy include fatigue; dyspnea, including paroxysmal nocturnal dyspnea and orthopnea; loss of appetite; irregular pulse; productive cough with pink/frothy material; lower extremity edema; and nocturia.10 Cardiac function can recover with early diagnosis and alcohol abstinence.11
Cognitive decline. The effects of light drinking on cognitive function are controversial, but heavy consumption—especially at ≥30 drinks/week—is known to cause impairment.12 Alcohol-dependent individuals have been shown to have impaired verbal fluency, working memory, and frontal function as is seen in Alzheimer’s disease.13 One possible factor contributing to cognitive dysfunction is cortical volume loss in chronic alcoholics.12
To read how nicotine plus alcohol increases the risk of heart disease and brain atrophy, click here.
To read about the medical complications of nicotine, click here.
Table 1
Medical complications of alcohol abuse
| Cardiovascular: Cardiomyopathy; hypertension; ischemic heart disease; acute myocardial infarction |
| Gastrointestinal: Alcohol hepatitis; cirrhosis of the liver; pancreatitis; cancer of the mouth, larynx, pharynx, esophagus, liver, and colon/rectum/appendix |
| Neurologic: Wernicke’s encephalopathy; Korsakoff’s syndrome; decline in cognitive abilities; decreased gray and white matter; increased ventricular and sulcal volume; peripheral neuropathy |
| Other: Renal dysfunction; osteoporosis; breast cancer |
Marijuana
Marijuana is the most commonly abused illicit substance worldwide, and data show an increasing prevalence of marijuana abuse and dependence (32% of U.S. 12th graders endorsed its use in 2007).14
In many populations marijuana use seems to precede use of cocaine, opioids, or other substances.15 Although the concept of marijuana as a “gateway drug” is still debated, consider the possibility that your patients who use marijuana also are using other illicit substances. In a 2004 survey, 19% of marijuana users admitted to use of other illicit drugs.16 Although many people consider marijuana a “safe” drug, it can cause adverse effects (Table 2).
Pulmonary complications. Even infrequent marijuana use can lead to burning and stinging of the mouth and throat, usually accompanied by a heavy cough. Regular users may develop complications similar to chronic tobacco use: daily cough, chronic phlegm production, susceptibility to lung infections (such as acute bronchitis), and potential for airway obstruction.17,18
Marijuana use can double or triple the risk of cancer of the respiratory tract and lungs.19 Tetrahydrocannabinol—the active chemical in marijuana—might contribute to this risk because it can augment oxidative stress, lead to mitochondrial dysfunction, and inhibit apoptosis.19
Cardiac complications. Acute marijuana use causes tachycardia, increases supine blood pressure, and decreases standing blood pressure, resulting in dizziness, syncope, falls, and possible injuries.20,21 Increased cardiac output and cardiac work—coupled with a decreased capacity to carry oxygen—can lead to angina or acute coronary syndrome, especially in older adults with preexisting cardiovascular disease.21 Growing evidence shows that marijuana use could lead to cardiac arrhythmias, such as atrial fibrillation.20 Long-term heavy users seem to develop tolerance to some cardiovascular effects, but blood volume overall increases, heart rate slows, and circulatory responses to exercise are diminished.18
Cognitive impairment. Chronic marijuana users might experience cognitive impairment—particularly on memory of word lists and attention tasks22—but there is debate as to whether these deficits are stable or temporary. Some studies show persistent cognitive impairments in longer-term cannabis users, even after 2 years of abstinence.22 However, most studies suggest that marijuana-associated cognitive deficits are reversible and related to recent exposure.18
Table 2
Medical complications of marijuana use
| Cardiovascular: Tachycardia; increased supine blood pressure; increased risk of myocardial infarction; atrial fibrillation |
| Pulmonary: Stinging of mouth/throat; chronic/heavy cough; increased lung infections; obstructed airways; lung cancer |
| Neurologic: Decreased performance on cognitive tasks (word lists, attention); diminished reaction times |
| Other: Decreased serum testosterone, sperm count, and sperm motility; shorter menstrual cycles; increased prolactin; suppressed activity of macrophages and natural killer cell |
Cocaine
Cocaine is the most frequent cause of drug-related death, particularly when combined with alcohol.23
Chronic nasal insufflations can cause loss of sense of smell, nosebleeds, dysphagia, hoarseness, and overall irritation of the nasal septum, which in turn can lead to chronic mucosal inflammation and rhinorrhea.24 Intravenous users often have puncture marks or “tracks,” usually on the forearms, and are predisposed to infectious diseases such as human immunodeficiency virus (HIV) and other blood-borne infections.24,25 Regular cocaine ingestion can lead to bowel gangrene because of reduced blood flow and orofacial complications.24 Asking about how your patient ingests cocaine will guide your evaluation of possible medical complications (Table 3).
Cardiac complications. Recent cocaine use is a common cause of chest pain. A 2002 survey reported that 25% of patients in urban hospitals and 13% in rural settings presenting with nontraumatic chest pain tested positive for cocaine use.26 Although cocaine can lead to ventricular fibrillation, tachycardia, and increased blood pressure, its main mechanism for inducing chest pain and myocardial infarction (MI) is coronary vasospasm, especially of diseased vessels. The acute risk of MI is increased by a factor of 24 in the first 60 minutes after cocaine use.23 Chronic use promotes thrombus formation, leading to atherosclerotic disease.23 Recurrent chest pain in a young, otherwise healthy individual could indicate cocaine abuse.
Neurologic complications. Headache is the most common neurologic complication of cocaine use. Although usually associated with intoxication or withdrawal, headaches can become chronic with chronic use.25 Reduced seizure threshold also has been reported with cocaine use, particularly in patients with cerebral lesions, and most seizures occur with first-time use. Isolated events might not require anticonvulsant therapy, although referral to a neurologist is recommended.27
Cocaine use puts individuals at higher risk for subarachnoid hemorrhage, intracerebral bleed, ischemic stroke, and transient ischemic attacks. The route of cocaine ingestion seems to influence the type of stroke: IV and intranasal use are associated with hemorrhagic stroke, and inhalation with ischemic stroke.25
Table 3
Medical complications of cocaine use
| Cardiovascular: Chest pain; 24-fold increased risk of myocardial infarction; coronary vasospasm; ventricular fibrillation; tachycardia; hypertension |
| Pulmonary: Pleuritic chest pain; chronic cough; wheezing; hemoptysis; melanoptysis (black sputum); ‘crack lung’ (fever, cough, difficulty breathing, and chest pain) |
| Gastrointestinal: Xerostomia; bruxism; decreased gastric motility; ischemic colitis; bowel ulceration, infarction, and perforation |
| Neurologic: Seizures; headaches; cerebral vasoconstriction; hemorrhagic/ischemic stroke; cerebral gray matter atrophy (especially frontotemporal lobes); dystonic reactions; akathisia; choreoathetosis (‘crack dancers’) |
| Other: Acute renal failure via rhabdomyolysis; nephrosclerosis; impaired sexual function (chronic use) |
Methamphetamine
Like many illicit substances, methamphetamine can be taken in many forms.
- “Speed,” a powder form, can be snorted or injected.
- “Base” is a powder with higher purity.
- “Ice,” also known as “crystal,” has very high purity and can be smoked, “chased” (cooked on aluminum foil and smoked), mixed with marijuana, or injected.28
Evaluate meth-abusing patients for many of the same medical complications associated with cocaine and other stimulants. Acute effects include hypertension, tachycardia, and arrhythmias; chronic effects include stroke and cardiac valve sclerosis. Pulmonary hypertension can occur when the drug is smoked (Table 4).28
Dental complications. Originally believed to result from the acidity of methamphetamine, advanced tooth decay or “meth mouth” is thought to be caused by decreased production of saliva—a consequence of increased sympathetic activity—combined with overall decreased oral intake, sugar and soft drink consumption, and poor oral hygiene. Methamphetamine abusers often experience bruxism, which exacerbates tooth decay.29
Neurologic changes. Chronic methamphetamine use is characterized by poor cognitive functioning and emotional changes such as paranoia and depression.29 These are believed to be caused by neuropathologic changes in the cortex, striatum, and hippocampus.
Table 4
Medical complications of methamphetamine abuse
| Cardiovascular: Arrhythmias; hypertensive crisis; myocardial infarction; cardiomyopathy; tachycardia |
| Pulmonary: Pneumomediastinum respiratory failure |
| Gastrointestinal: Tooth decay (‘Meth mouth’); xerostomia; bruxism; hepatitis infection; hepatotoxicity |
| Neurologic: Cerebral infarct; seizures; blurred vision; obtundation |
| Other: Jaw clenching; excessive sweating; aplastic anemia; hyperthermia; muscle cramping |
Opioids
Prescriptions of opioid analgesics for chronic pain—and their subsequent diversion—are the main conduit to nonmedical use.30 IV heroin use is the most common cause of illicit drug overdose.31 Opioids are used by:
- ingestion, usually of synthetic analgesics (prescription drugs)
- parenteral administration, often IV heroin
- inhalation, a pure form that is heated and burned.
Infectious complications. Injection drug use—especially with unsterilized shared needles—is an efficient vector for blood-borne infections. Needle sharing is the most common cause of new HIV and viral hepatitis infections.32 All IV drug users should be routinely tested for these viral infections. Chronic IV drug use can cause vein sclerosis, leading to visible “track marks” and, rarely, thromboembolic events. Be alert for integumentary infections—especially in patients who “skin pop” drugs by injecting them under the skin—or systemic infectious diseases, such as skin abscesses, cellulitis, septicemia, botulism, or bacterial endocarditis (Table 5).33
Pulmonary complications. Overstimulation of opioid receptors in the brainstem and carotid bodies can cause slow and irregular respiration and decreased gag and coughing reflex during acute intoxication. The rate of opioid intake appears to play a role; a gradual increase in opioid blood levels leads to progressive respiratory depression by causing gradual hypercapnia, and a quick rise in receptor occupancy can lead to rapid apnea. Therefore opioids with slow receptor binding, such as buprenorphine, may be safer than those that bind more quickly, such as fentanyl. However, all opioids can cause this dangerous side effect.34 Inhaled forms of heroin have also been shown to lead to status asthmaticus.35
Table 5
Medical complications of opioid abuse
| Cardiovascular: Prolonged QTc interval (methadone) |
| Pulmonary: Respiratory suppression |
| Gastrointestinal: Hepatitis C infection; hepatotoxicity; nausea; constipation |
| Neurologic: Drowsiness; lightheadedness; confusion; myoclonus; hyperalgesia; miosis |
| Other: Urinary retention; pruritus |
Cardiac and neurologic complications. Methadone use could prolong the QTc interval, leading to dysrhythmias such as torsades de pointes. Higher doses increase the incidence of syncope.36 Ongoing monitoring of the QTc interval is warranted for all patients on methadone.
Neurologic effects of opioids include:
- delayed leukoencephalopathy with IV overdose and inhaled preheated heroin, known as ”chasing the dragon”
- widespread cortical dysfunction (abulia, lack of volition, hemineglect,37 and deficits in executive functioning and emotional processing) leading to impaired decision-making.38
Related resources
- National Institute on Drug Abuse. www.nida.nih.gov.
- Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the Future national survey results on drug use, 1975-2008. Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2009. NIH Publication No. 09-7402.
Drug brand names
- Buprenorphine • Subutex
- Fentanyl • Actiq, Duragesic, others
- Methadone • Dolophine, Methadose
Disclosures
Drs. Khan and Morrow report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCarron is a consultant to Eli Lilly and Company.
1. Brady KT. Comorbidity of substance use and Axis I psychiatric disorders. Medscape Psychiatry and Mental Health eJournal [serial online]. March 25, 2002. Available at: http://www.medscape.com/viewarticle/430610. Accessed September 28, 2009.
2. Dietary guidelines for Americans, 2005. Chapter 9 alcoholic beverages. Washington, DC: United States Department of Agriculture; 2005. Available at: http://www.health.gov/dietaryguidelines/dga2005/document/html/chapter9.htm. Accessed September 15, 2008.
3. National Institute on Alcohol Abuse and Alcoholism. How to screen for heavy drinking. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2005. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/PocketGuide/pocket_guide5.htm. Accessed September 15, 2008.
4. Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032-2039.
5. National Institute on Alcohol Abuse and Alcoholism. Alcohol alert. Alcoholic liver disease. Washington, DC: US Department of Health and Human Services; 2005. Available at: http://pubs.niaaa.nih.gov/publications/aa64/aa64.htm. Accessed August 23, 2009.
6. Spiegel DR, Dhadwal N, Gill F. ‘I’m sober, doctor, really’: best biomarkers for underreported alcohol use. Current Psychiatry. 2008;7(9):15-27.
7. Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168(6):649-656.
8. Hvidtfeldt UA, Frederiksen ME, Thysesen LC, et al. Incidence of cardiovascular and cerebrovascular disease in Danish men and women with a prolonged heavy alcohol intake. Alcohol Clin Exp Res. 2008;32(11):1920-1924.
9. Fuchs FD, Chambless LE, Whelton PK, et al. Alcohol consumption and the incidence of hypertension: the Athersclerosis Risk in Communities Study. Hypertension. 2001;37(5):1242-1250.
10. Alcoholic cardiomyopathy. The New York Times Health Guide. Available at: http://health.nytimes.com/health/guides/disease/alcoholic-cardiomyopathy/overview.html. Accessed September 28, 2009.
11. McKenna CJ, Codd MB, McCann HA, et al. Alcohol consumption and idiopathic dilated cardiomyopathy: a case control study. Am Heart J. 1998;135(5 pt 1):833-837.
12. Meyerhoff DJ, Bode C, Nixon SJ, et al. Health risks of chronic moderate and heavy alcohol consumption: how much is too much? Alcohol Clin Exp Res. 2005;29(7):1334-1340.
13. Liappas I, Theotoka I, Kapaki E, et al. Neuropsychological assessment of cognitive function in chronic alcohol-dependent patients and patients with Alzheimer’s disease. In Vivo. 2007;21(6):1115-1118.
14. Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the Future. National Results on Adolescent Drug Use. Overview of Key Findings, 2007. Bethesda, MD: National Institute on Drug Abuse; 2008. Available at: http://www.monitoringthefuture.org/pubs/monographs/overview2007.pdf. Accessed August 20, 2008.
15. Hales RE, Yudofsky SC, Gabbard GO. The American Psychiatric Publishing textbook of psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
16. Substance Abuse and Mental Health Services Administration. Results from the 2004 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. NSDUH Series H-28, DHHS Publication No. SMA 05-4062.
17. National Institute on Drug Abuse. Research report series—marijuana abuse. Bethesda, MD: National Institute on Drug Abuse; 2005. Available at: http://www.nida.nih.gov/ResearchReports/Marijuana. Accessed September 1, 2008.
18. Khalsa JH, Genser S, Francis H, et al. Clinical consequences of marijuana. J Clin Pharmacol. 2002;42(suppl 11):7S-10S.
19. Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. 2005;63(2):93-100.
20. Korantzopoulos P, Liou T, Papaioannides D, et al. Atrial fibrillation and marijuana smoking. Int J Clin Pract. 2008;62(2):308-313.
21. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(suppl 11):58S-63S.
22. Harrison GP, Jr, Gruber AJ, Hudson JI, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42(suppl 11):41S-47S.
23. Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345(5):351-358.
24. National Institute on Drug Abuse. What are the long-term effects of cocaine use? Bethesda, MD: National Institute on Drug Abuse; 1999. Available at: http://www.nida.nih.gov/PDF/RRCocaine.pdf Accessed September 1, 2008.
25. Wang CM, Huang CL, Hu CTS, et al. Medical complications of cocaine abuse. Medical Update for Psychiatrists. 1997;2(2):34-38.
26. Hollander JE, Todd KH, Green G, et al. Chest pain associated with cocaine: an assessment of prevalence in suburban and urban emergency departments. Ann Emerg Med. 1995;26:671-676.
27. Agarwal P, Sen S. Cocaine. e-medicine [serial online]. February 21, 2007. Available at: http://www.emedicine.com/neuro/TOPIC72.HTM. Accessed September 27, 2008.
28. Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18(3):235-242.
29. Goodchild JH, Donaldson M, Mangini DJ. Methamphetamine abuse and the impact on dental health. Dent Today. 2007;26(5):124-131.
30. Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration. Available at: http://www.oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.cfm#TOC. Accessed January 21, 2009.
31. Schuckit MA. Drug and alcohol abuse. 5th ed. New York, NY: Kluwer Academic/Plenum Publishers; 2000:114-119.
32. National Institute for Drug Addiction. Research on the nature and extent of drug use in the United States. Bethesda, MD: National Institute for Drug Addiction; 1999. Available at: http://www.drugabuse.gov/STRC/Data.html. Accessed January 21, 2009.
33. Galanter M, Kleber H. The American Psychiatric Publishing textbook of substance abuse treatment. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
34. Pattinson KT. Opioids and the control of respiration. Br J Anesth. 2008;100(6):747-758.
35. Cygan J, Trunsky M, Corbridge T. Inhaled heroin-induced status asthmaticus: five cases and a review of the literature. Chest. 2000;117(1):272-275.
36. Fanoe S, Hvidt C, Ege P, et al. Syncope and QT prolongation among patients treated with methadone for heroin dependence in the city of Copenhagen. Heart. 2006;93(9):1051-1055.
37. Barnett MH, Miller LA, Reddel SW, et al. Reversible delayed leukoencephalopathy following intravenous heroin overdose. J Clin Neurosci. 2001;8(2):165-167.
38. Brand M, Roth-Bauer M, Driessen M, et al. Executive functions and risky decision-making in patients with opiate dependence. Drug Alcohol Depend. 2008;97(1-2):64-72.
Individuals who abuse substances often have comorbid psychiatric disorders—80% of alcoholics have another axis I disorder1—and the reverse also is true. More than one-half of schizophrenia patients and 30% of anxiety and affective disorder patients abuse substances.1
In addition to worsening psychiatric illnesses and interfering with proper treatment, alcohol and other substances can lead to serious cardiac, neurologic, pulmonary, or gastrointestinal complications that can linger even after your patient stops abusing drugs. This article provides an overview of common medical complications related to using alcohol, marijuana, cocaine, methamphetamines, and opioids.
Alcohol
Because some consequences of alcohol abuse (Table 1) are thought to be dose-dependent, ask about your patient’s alcohol consumption. Moderate drinking is defined as up to 2 drinks/day for men and 1 drink/day for women.2 Heavy drinking is ≥5 drinks/day (or ≥15 drinks/week) for men and ≥4/day (or ≥8/week) for women.3 A drink contains 12.5 grams of ethanol and is defined as:
- 12 oz (360 mL) of beer or wine cooler
- 5 oz (150 mL) of wine
- 1.5 oz (45 mL) of 80-proof distilled spirits.3
Gastrointestinal effects. Chronic heavy alcohol consumption can lead to fatty liver (steatosis), alcoholic hepatitis, and cirrhosis. Steatosis—the first stage of alcoholic liver disease—can occur from heavy drinking for just a few days but can be reversed with abstinence from alcohol. Prolonged use can lead to alcoholic hepatitis. Symptoms include nausea, lack of appetite, vomiting, fatigue, abdominal pain and tenderness, spider-like blood vessels, and increased bleeding times.
Abstinence might not reverse liver damage from alcoholic hepatitis, and cirrhosis can still develop. Up to 70% of patients with alcoholic hepatitis will develop cirrhosis.4,5 Common physical manifestations of cirrhosis include generalized weakness, fatigue, malaise, anorexia with signs of malnutrition, and increased bleeding.
Laboratory findings of elevated aspartate aminotransferase/alanine aminotransferase, gamma-glutamyltransferase, and carbohydrate-deficient transferrin also point to heavy alcohol use.6
Acute pancreatitis—the most common cause of hospitalization from alcohol-related GI complications—is seen more often than liver disease.7
Cardiovascular effects. Light to moderate drinking may be cardioprotective, but heavy alcohol consumption increases the risk of hypertension and ischemic heart disease.8 Incidence of hypertension is two-fold greater in individuals who have >2 drinks/day and highest in those who have >5 drinks/day.9
Prolonged excessive alcohol consumption is the leading cause of nonischemic dilated cardiomyopathy. Symptoms of alcoholic cardiomyopathy include fatigue; dyspnea, including paroxysmal nocturnal dyspnea and orthopnea; loss of appetite; irregular pulse; productive cough with pink/frothy material; lower extremity edema; and nocturia.10 Cardiac function can recover with early diagnosis and alcohol abstinence.11
Cognitive decline. The effects of light drinking on cognitive function are controversial, but heavy consumption—especially at ≥30 drinks/week—is known to cause impairment.12 Alcohol-dependent individuals have been shown to have impaired verbal fluency, working memory, and frontal function as is seen in Alzheimer’s disease.13 One possible factor contributing to cognitive dysfunction is cortical volume loss in chronic alcoholics.12
To read how nicotine plus alcohol increases the risk of heart disease and brain atrophy, click here.
To read about the medical complications of nicotine, click here.
Table 1
Medical complications of alcohol abuse
| Cardiovascular: Cardiomyopathy; hypertension; ischemic heart disease; acute myocardial infarction |
| Gastrointestinal: Alcohol hepatitis; cirrhosis of the liver; pancreatitis; cancer of the mouth, larynx, pharynx, esophagus, liver, and colon/rectum/appendix |
| Neurologic: Wernicke’s encephalopathy; Korsakoff’s syndrome; decline in cognitive abilities; decreased gray and white matter; increased ventricular and sulcal volume; peripheral neuropathy |
| Other: Renal dysfunction; osteoporosis; breast cancer |
Marijuana
Marijuana is the most commonly abused illicit substance worldwide, and data show an increasing prevalence of marijuana abuse and dependence (32% of U.S. 12th graders endorsed its use in 2007).14
In many populations marijuana use seems to precede use of cocaine, opioids, or other substances.15 Although the concept of marijuana as a “gateway drug” is still debated, consider the possibility that your patients who use marijuana also are using other illicit substances. In a 2004 survey, 19% of marijuana users admitted to use of other illicit drugs.16 Although many people consider marijuana a “safe” drug, it can cause adverse effects (Table 2).
Pulmonary complications. Even infrequent marijuana use can lead to burning and stinging of the mouth and throat, usually accompanied by a heavy cough. Regular users may develop complications similar to chronic tobacco use: daily cough, chronic phlegm production, susceptibility to lung infections (such as acute bronchitis), and potential for airway obstruction.17,18
Marijuana use can double or triple the risk of cancer of the respiratory tract and lungs.19 Tetrahydrocannabinol—the active chemical in marijuana—might contribute to this risk because it can augment oxidative stress, lead to mitochondrial dysfunction, and inhibit apoptosis.19
Cardiac complications. Acute marijuana use causes tachycardia, increases supine blood pressure, and decreases standing blood pressure, resulting in dizziness, syncope, falls, and possible injuries.20,21 Increased cardiac output and cardiac work—coupled with a decreased capacity to carry oxygen—can lead to angina or acute coronary syndrome, especially in older adults with preexisting cardiovascular disease.21 Growing evidence shows that marijuana use could lead to cardiac arrhythmias, such as atrial fibrillation.20 Long-term heavy users seem to develop tolerance to some cardiovascular effects, but blood volume overall increases, heart rate slows, and circulatory responses to exercise are diminished.18
Cognitive impairment. Chronic marijuana users might experience cognitive impairment—particularly on memory of word lists and attention tasks22—but there is debate as to whether these deficits are stable or temporary. Some studies show persistent cognitive impairments in longer-term cannabis users, even after 2 years of abstinence.22 However, most studies suggest that marijuana-associated cognitive deficits are reversible and related to recent exposure.18
Table 2
Medical complications of marijuana use
| Cardiovascular: Tachycardia; increased supine blood pressure; increased risk of myocardial infarction; atrial fibrillation |
| Pulmonary: Stinging of mouth/throat; chronic/heavy cough; increased lung infections; obstructed airways; lung cancer |
| Neurologic: Decreased performance on cognitive tasks (word lists, attention); diminished reaction times |
| Other: Decreased serum testosterone, sperm count, and sperm motility; shorter menstrual cycles; increased prolactin; suppressed activity of macrophages and natural killer cell |
Cocaine
Cocaine is the most frequent cause of drug-related death, particularly when combined with alcohol.23
Chronic nasal insufflations can cause loss of sense of smell, nosebleeds, dysphagia, hoarseness, and overall irritation of the nasal septum, which in turn can lead to chronic mucosal inflammation and rhinorrhea.24 Intravenous users often have puncture marks or “tracks,” usually on the forearms, and are predisposed to infectious diseases such as human immunodeficiency virus (HIV) and other blood-borne infections.24,25 Regular cocaine ingestion can lead to bowel gangrene because of reduced blood flow and orofacial complications.24 Asking about how your patient ingests cocaine will guide your evaluation of possible medical complications (Table 3).
Cardiac complications. Recent cocaine use is a common cause of chest pain. A 2002 survey reported that 25% of patients in urban hospitals and 13% in rural settings presenting with nontraumatic chest pain tested positive for cocaine use.26 Although cocaine can lead to ventricular fibrillation, tachycardia, and increased blood pressure, its main mechanism for inducing chest pain and myocardial infarction (MI) is coronary vasospasm, especially of diseased vessels. The acute risk of MI is increased by a factor of 24 in the first 60 minutes after cocaine use.23 Chronic use promotes thrombus formation, leading to atherosclerotic disease.23 Recurrent chest pain in a young, otherwise healthy individual could indicate cocaine abuse.
Neurologic complications. Headache is the most common neurologic complication of cocaine use. Although usually associated with intoxication or withdrawal, headaches can become chronic with chronic use.25 Reduced seizure threshold also has been reported with cocaine use, particularly in patients with cerebral lesions, and most seizures occur with first-time use. Isolated events might not require anticonvulsant therapy, although referral to a neurologist is recommended.27
Cocaine use puts individuals at higher risk for subarachnoid hemorrhage, intracerebral bleed, ischemic stroke, and transient ischemic attacks. The route of cocaine ingestion seems to influence the type of stroke: IV and intranasal use are associated with hemorrhagic stroke, and inhalation with ischemic stroke.25
Table 3
Medical complications of cocaine use
| Cardiovascular: Chest pain; 24-fold increased risk of myocardial infarction; coronary vasospasm; ventricular fibrillation; tachycardia; hypertension |
| Pulmonary: Pleuritic chest pain; chronic cough; wheezing; hemoptysis; melanoptysis (black sputum); ‘crack lung’ (fever, cough, difficulty breathing, and chest pain) |
| Gastrointestinal: Xerostomia; bruxism; decreased gastric motility; ischemic colitis; bowel ulceration, infarction, and perforation |
| Neurologic: Seizures; headaches; cerebral vasoconstriction; hemorrhagic/ischemic stroke; cerebral gray matter atrophy (especially frontotemporal lobes); dystonic reactions; akathisia; choreoathetosis (‘crack dancers’) |
| Other: Acute renal failure via rhabdomyolysis; nephrosclerosis; impaired sexual function (chronic use) |
Methamphetamine
Like many illicit substances, methamphetamine can be taken in many forms.
- “Speed,” a powder form, can be snorted or injected.
- “Base” is a powder with higher purity.
- “Ice,” also known as “crystal,” has very high purity and can be smoked, “chased” (cooked on aluminum foil and smoked), mixed with marijuana, or injected.28
Evaluate meth-abusing patients for many of the same medical complications associated with cocaine and other stimulants. Acute effects include hypertension, tachycardia, and arrhythmias; chronic effects include stroke and cardiac valve sclerosis. Pulmonary hypertension can occur when the drug is smoked (Table 4).28
Dental complications. Originally believed to result from the acidity of methamphetamine, advanced tooth decay or “meth mouth” is thought to be caused by decreased production of saliva—a consequence of increased sympathetic activity—combined with overall decreased oral intake, sugar and soft drink consumption, and poor oral hygiene. Methamphetamine abusers often experience bruxism, which exacerbates tooth decay.29
Neurologic changes. Chronic methamphetamine use is characterized by poor cognitive functioning and emotional changes such as paranoia and depression.29 These are believed to be caused by neuropathologic changes in the cortex, striatum, and hippocampus.
Table 4
Medical complications of methamphetamine abuse
| Cardiovascular: Arrhythmias; hypertensive crisis; myocardial infarction; cardiomyopathy; tachycardia |
| Pulmonary: Pneumomediastinum respiratory failure |
| Gastrointestinal: Tooth decay (‘Meth mouth’); xerostomia; bruxism; hepatitis infection; hepatotoxicity |
| Neurologic: Cerebral infarct; seizures; blurred vision; obtundation |
| Other: Jaw clenching; excessive sweating; aplastic anemia; hyperthermia; muscle cramping |
Opioids
Prescriptions of opioid analgesics for chronic pain—and their subsequent diversion—are the main conduit to nonmedical use.30 IV heroin use is the most common cause of illicit drug overdose.31 Opioids are used by:
- ingestion, usually of synthetic analgesics (prescription drugs)
- parenteral administration, often IV heroin
- inhalation, a pure form that is heated and burned.
Infectious complications. Injection drug use—especially with unsterilized shared needles—is an efficient vector for blood-borne infections. Needle sharing is the most common cause of new HIV and viral hepatitis infections.32 All IV drug users should be routinely tested for these viral infections. Chronic IV drug use can cause vein sclerosis, leading to visible “track marks” and, rarely, thromboembolic events. Be alert for integumentary infections—especially in patients who “skin pop” drugs by injecting them under the skin—or systemic infectious diseases, such as skin abscesses, cellulitis, septicemia, botulism, or bacterial endocarditis (Table 5).33
Pulmonary complications. Overstimulation of opioid receptors in the brainstem and carotid bodies can cause slow and irregular respiration and decreased gag and coughing reflex during acute intoxication. The rate of opioid intake appears to play a role; a gradual increase in opioid blood levels leads to progressive respiratory depression by causing gradual hypercapnia, and a quick rise in receptor occupancy can lead to rapid apnea. Therefore opioids with slow receptor binding, such as buprenorphine, may be safer than those that bind more quickly, such as fentanyl. However, all opioids can cause this dangerous side effect.34 Inhaled forms of heroin have also been shown to lead to status asthmaticus.35
Table 5
Medical complications of opioid abuse
| Cardiovascular: Prolonged QTc interval (methadone) |
| Pulmonary: Respiratory suppression |
| Gastrointestinal: Hepatitis C infection; hepatotoxicity; nausea; constipation |
| Neurologic: Drowsiness; lightheadedness; confusion; myoclonus; hyperalgesia; miosis |
| Other: Urinary retention; pruritus |
Cardiac and neurologic complications. Methadone use could prolong the QTc interval, leading to dysrhythmias such as torsades de pointes. Higher doses increase the incidence of syncope.36 Ongoing monitoring of the QTc interval is warranted for all patients on methadone.
Neurologic effects of opioids include:
- delayed leukoencephalopathy with IV overdose and inhaled preheated heroin, known as ”chasing the dragon”
- widespread cortical dysfunction (abulia, lack of volition, hemineglect,37 and deficits in executive functioning and emotional processing) leading to impaired decision-making.38
Related resources
- National Institute on Drug Abuse. www.nida.nih.gov.
- Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the Future national survey results on drug use, 1975-2008. Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2009. NIH Publication No. 09-7402.
Drug brand names
- Buprenorphine • Subutex
- Fentanyl • Actiq, Duragesic, others
- Methadone • Dolophine, Methadose
Disclosures
Drs. Khan and Morrow report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCarron is a consultant to Eli Lilly and Company.
Individuals who abuse substances often have comorbid psychiatric disorders—80% of alcoholics have another axis I disorder1—and the reverse also is true. More than one-half of schizophrenia patients and 30% of anxiety and affective disorder patients abuse substances.1
In addition to worsening psychiatric illnesses and interfering with proper treatment, alcohol and other substances can lead to serious cardiac, neurologic, pulmonary, or gastrointestinal complications that can linger even after your patient stops abusing drugs. This article provides an overview of common medical complications related to using alcohol, marijuana, cocaine, methamphetamines, and opioids.
Alcohol
Because some consequences of alcohol abuse (Table 1) are thought to be dose-dependent, ask about your patient’s alcohol consumption. Moderate drinking is defined as up to 2 drinks/day for men and 1 drink/day for women.2 Heavy drinking is ≥5 drinks/day (or ≥15 drinks/week) for men and ≥4/day (or ≥8/week) for women.3 A drink contains 12.5 grams of ethanol and is defined as:
- 12 oz (360 mL) of beer or wine cooler
- 5 oz (150 mL) of wine
- 1.5 oz (45 mL) of 80-proof distilled spirits.3
Gastrointestinal effects. Chronic heavy alcohol consumption can lead to fatty liver (steatosis), alcoholic hepatitis, and cirrhosis. Steatosis—the first stage of alcoholic liver disease—can occur from heavy drinking for just a few days but can be reversed with abstinence from alcohol. Prolonged use can lead to alcoholic hepatitis. Symptoms include nausea, lack of appetite, vomiting, fatigue, abdominal pain and tenderness, spider-like blood vessels, and increased bleeding times.
Abstinence might not reverse liver damage from alcoholic hepatitis, and cirrhosis can still develop. Up to 70% of patients with alcoholic hepatitis will develop cirrhosis.4,5 Common physical manifestations of cirrhosis include generalized weakness, fatigue, malaise, anorexia with signs of malnutrition, and increased bleeding.
Laboratory findings of elevated aspartate aminotransferase/alanine aminotransferase, gamma-glutamyltransferase, and carbohydrate-deficient transferrin also point to heavy alcohol use.6
Acute pancreatitis—the most common cause of hospitalization from alcohol-related GI complications—is seen more often than liver disease.7
Cardiovascular effects. Light to moderate drinking may be cardioprotective, but heavy alcohol consumption increases the risk of hypertension and ischemic heart disease.8 Incidence of hypertension is two-fold greater in individuals who have >2 drinks/day and highest in those who have >5 drinks/day.9
Prolonged excessive alcohol consumption is the leading cause of nonischemic dilated cardiomyopathy. Symptoms of alcoholic cardiomyopathy include fatigue; dyspnea, including paroxysmal nocturnal dyspnea and orthopnea; loss of appetite; irregular pulse; productive cough with pink/frothy material; lower extremity edema; and nocturia.10 Cardiac function can recover with early diagnosis and alcohol abstinence.11
Cognitive decline. The effects of light drinking on cognitive function are controversial, but heavy consumption—especially at ≥30 drinks/week—is known to cause impairment.12 Alcohol-dependent individuals have been shown to have impaired verbal fluency, working memory, and frontal function as is seen in Alzheimer’s disease.13 One possible factor contributing to cognitive dysfunction is cortical volume loss in chronic alcoholics.12
To read how nicotine plus alcohol increases the risk of heart disease and brain atrophy, click here.
To read about the medical complications of nicotine, click here.
Table 1
Medical complications of alcohol abuse
| Cardiovascular: Cardiomyopathy; hypertension; ischemic heart disease; acute myocardial infarction |
| Gastrointestinal: Alcohol hepatitis; cirrhosis of the liver; pancreatitis; cancer of the mouth, larynx, pharynx, esophagus, liver, and colon/rectum/appendix |
| Neurologic: Wernicke’s encephalopathy; Korsakoff’s syndrome; decline in cognitive abilities; decreased gray and white matter; increased ventricular and sulcal volume; peripheral neuropathy |
| Other: Renal dysfunction; osteoporosis; breast cancer |
Marijuana
Marijuana is the most commonly abused illicit substance worldwide, and data show an increasing prevalence of marijuana abuse and dependence (32% of U.S. 12th graders endorsed its use in 2007).14
In many populations marijuana use seems to precede use of cocaine, opioids, or other substances.15 Although the concept of marijuana as a “gateway drug” is still debated, consider the possibility that your patients who use marijuana also are using other illicit substances. In a 2004 survey, 19% of marijuana users admitted to use of other illicit drugs.16 Although many people consider marijuana a “safe” drug, it can cause adverse effects (Table 2).
Pulmonary complications. Even infrequent marijuana use can lead to burning and stinging of the mouth and throat, usually accompanied by a heavy cough. Regular users may develop complications similar to chronic tobacco use: daily cough, chronic phlegm production, susceptibility to lung infections (such as acute bronchitis), and potential for airway obstruction.17,18
Marijuana use can double or triple the risk of cancer of the respiratory tract and lungs.19 Tetrahydrocannabinol—the active chemical in marijuana—might contribute to this risk because it can augment oxidative stress, lead to mitochondrial dysfunction, and inhibit apoptosis.19
Cardiac complications. Acute marijuana use causes tachycardia, increases supine blood pressure, and decreases standing blood pressure, resulting in dizziness, syncope, falls, and possible injuries.20,21 Increased cardiac output and cardiac work—coupled with a decreased capacity to carry oxygen—can lead to angina or acute coronary syndrome, especially in older adults with preexisting cardiovascular disease.21 Growing evidence shows that marijuana use could lead to cardiac arrhythmias, such as atrial fibrillation.20 Long-term heavy users seem to develop tolerance to some cardiovascular effects, but blood volume overall increases, heart rate slows, and circulatory responses to exercise are diminished.18
Cognitive impairment. Chronic marijuana users might experience cognitive impairment—particularly on memory of word lists and attention tasks22—but there is debate as to whether these deficits are stable or temporary. Some studies show persistent cognitive impairments in longer-term cannabis users, even after 2 years of abstinence.22 However, most studies suggest that marijuana-associated cognitive deficits are reversible and related to recent exposure.18
Table 2
Medical complications of marijuana use
| Cardiovascular: Tachycardia; increased supine blood pressure; increased risk of myocardial infarction; atrial fibrillation |
| Pulmonary: Stinging of mouth/throat; chronic/heavy cough; increased lung infections; obstructed airways; lung cancer |
| Neurologic: Decreased performance on cognitive tasks (word lists, attention); diminished reaction times |
| Other: Decreased serum testosterone, sperm count, and sperm motility; shorter menstrual cycles; increased prolactin; suppressed activity of macrophages and natural killer cell |
Cocaine
Cocaine is the most frequent cause of drug-related death, particularly when combined with alcohol.23
Chronic nasal insufflations can cause loss of sense of smell, nosebleeds, dysphagia, hoarseness, and overall irritation of the nasal septum, which in turn can lead to chronic mucosal inflammation and rhinorrhea.24 Intravenous users often have puncture marks or “tracks,” usually on the forearms, and are predisposed to infectious diseases such as human immunodeficiency virus (HIV) and other blood-borne infections.24,25 Regular cocaine ingestion can lead to bowel gangrene because of reduced blood flow and orofacial complications.24 Asking about how your patient ingests cocaine will guide your evaluation of possible medical complications (Table 3).
Cardiac complications. Recent cocaine use is a common cause of chest pain. A 2002 survey reported that 25% of patients in urban hospitals and 13% in rural settings presenting with nontraumatic chest pain tested positive for cocaine use.26 Although cocaine can lead to ventricular fibrillation, tachycardia, and increased blood pressure, its main mechanism for inducing chest pain and myocardial infarction (MI) is coronary vasospasm, especially of diseased vessels. The acute risk of MI is increased by a factor of 24 in the first 60 minutes after cocaine use.23 Chronic use promotes thrombus formation, leading to atherosclerotic disease.23 Recurrent chest pain in a young, otherwise healthy individual could indicate cocaine abuse.
Neurologic complications. Headache is the most common neurologic complication of cocaine use. Although usually associated with intoxication or withdrawal, headaches can become chronic with chronic use.25 Reduced seizure threshold also has been reported with cocaine use, particularly in patients with cerebral lesions, and most seizures occur with first-time use. Isolated events might not require anticonvulsant therapy, although referral to a neurologist is recommended.27
Cocaine use puts individuals at higher risk for subarachnoid hemorrhage, intracerebral bleed, ischemic stroke, and transient ischemic attacks. The route of cocaine ingestion seems to influence the type of stroke: IV and intranasal use are associated with hemorrhagic stroke, and inhalation with ischemic stroke.25
Table 3
Medical complications of cocaine use
| Cardiovascular: Chest pain; 24-fold increased risk of myocardial infarction; coronary vasospasm; ventricular fibrillation; tachycardia; hypertension |
| Pulmonary: Pleuritic chest pain; chronic cough; wheezing; hemoptysis; melanoptysis (black sputum); ‘crack lung’ (fever, cough, difficulty breathing, and chest pain) |
| Gastrointestinal: Xerostomia; bruxism; decreased gastric motility; ischemic colitis; bowel ulceration, infarction, and perforation |
| Neurologic: Seizures; headaches; cerebral vasoconstriction; hemorrhagic/ischemic stroke; cerebral gray matter atrophy (especially frontotemporal lobes); dystonic reactions; akathisia; choreoathetosis (‘crack dancers’) |
| Other: Acute renal failure via rhabdomyolysis; nephrosclerosis; impaired sexual function (chronic use) |
Methamphetamine
Like many illicit substances, methamphetamine can be taken in many forms.
- “Speed,” a powder form, can be snorted or injected.
- “Base” is a powder with higher purity.
- “Ice,” also known as “crystal,” has very high purity and can be smoked, “chased” (cooked on aluminum foil and smoked), mixed with marijuana, or injected.28
Evaluate meth-abusing patients for many of the same medical complications associated with cocaine and other stimulants. Acute effects include hypertension, tachycardia, and arrhythmias; chronic effects include stroke and cardiac valve sclerosis. Pulmonary hypertension can occur when the drug is smoked (Table 4).28
Dental complications. Originally believed to result from the acidity of methamphetamine, advanced tooth decay or “meth mouth” is thought to be caused by decreased production of saliva—a consequence of increased sympathetic activity—combined with overall decreased oral intake, sugar and soft drink consumption, and poor oral hygiene. Methamphetamine abusers often experience bruxism, which exacerbates tooth decay.29
Neurologic changes. Chronic methamphetamine use is characterized by poor cognitive functioning and emotional changes such as paranoia and depression.29 These are believed to be caused by neuropathologic changes in the cortex, striatum, and hippocampus.
Table 4
Medical complications of methamphetamine abuse
| Cardiovascular: Arrhythmias; hypertensive crisis; myocardial infarction; cardiomyopathy; tachycardia |
| Pulmonary: Pneumomediastinum respiratory failure |
| Gastrointestinal: Tooth decay (‘Meth mouth’); xerostomia; bruxism; hepatitis infection; hepatotoxicity |
| Neurologic: Cerebral infarct; seizures; blurred vision; obtundation |
| Other: Jaw clenching; excessive sweating; aplastic anemia; hyperthermia; muscle cramping |
Opioids
Prescriptions of opioid analgesics for chronic pain—and their subsequent diversion—are the main conduit to nonmedical use.30 IV heroin use is the most common cause of illicit drug overdose.31 Opioids are used by:
- ingestion, usually of synthetic analgesics (prescription drugs)
- parenteral administration, often IV heroin
- inhalation, a pure form that is heated and burned.
Infectious complications. Injection drug use—especially with unsterilized shared needles—is an efficient vector for blood-borne infections. Needle sharing is the most common cause of new HIV and viral hepatitis infections.32 All IV drug users should be routinely tested for these viral infections. Chronic IV drug use can cause vein sclerosis, leading to visible “track marks” and, rarely, thromboembolic events. Be alert for integumentary infections—especially in patients who “skin pop” drugs by injecting them under the skin—or systemic infectious diseases, such as skin abscesses, cellulitis, septicemia, botulism, or bacterial endocarditis (Table 5).33
Pulmonary complications. Overstimulation of opioid receptors in the brainstem and carotid bodies can cause slow and irregular respiration and decreased gag and coughing reflex during acute intoxication. The rate of opioid intake appears to play a role; a gradual increase in opioid blood levels leads to progressive respiratory depression by causing gradual hypercapnia, and a quick rise in receptor occupancy can lead to rapid apnea. Therefore opioids with slow receptor binding, such as buprenorphine, may be safer than those that bind more quickly, such as fentanyl. However, all opioids can cause this dangerous side effect.34 Inhaled forms of heroin have also been shown to lead to status asthmaticus.35
Table 5
Medical complications of opioid abuse
| Cardiovascular: Prolonged QTc interval (methadone) |
| Pulmonary: Respiratory suppression |
| Gastrointestinal: Hepatitis C infection; hepatotoxicity; nausea; constipation |
| Neurologic: Drowsiness; lightheadedness; confusion; myoclonus; hyperalgesia; miosis |
| Other: Urinary retention; pruritus |
Cardiac and neurologic complications. Methadone use could prolong the QTc interval, leading to dysrhythmias such as torsades de pointes. Higher doses increase the incidence of syncope.36 Ongoing monitoring of the QTc interval is warranted for all patients on methadone.
Neurologic effects of opioids include:
- delayed leukoencephalopathy with IV overdose and inhaled preheated heroin, known as ”chasing the dragon”
- widespread cortical dysfunction (abulia, lack of volition, hemineglect,37 and deficits in executive functioning and emotional processing) leading to impaired decision-making.38
Related resources
- National Institute on Drug Abuse. www.nida.nih.gov.
- Substance Abuse and Mental Health Services Administration. www.samhsa.gov.
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the Future national survey results on drug use, 1975-2008. Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2009. NIH Publication No. 09-7402.
Drug brand names
- Buprenorphine • Subutex
- Fentanyl • Actiq, Duragesic, others
- Methadone • Dolophine, Methadose
Disclosures
Drs. Khan and Morrow report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCarron is a consultant to Eli Lilly and Company.
1. Brady KT. Comorbidity of substance use and Axis I psychiatric disorders. Medscape Psychiatry and Mental Health eJournal [serial online]. March 25, 2002. Available at: http://www.medscape.com/viewarticle/430610. Accessed September 28, 2009.
2. Dietary guidelines for Americans, 2005. Chapter 9 alcoholic beverages. Washington, DC: United States Department of Agriculture; 2005. Available at: http://www.health.gov/dietaryguidelines/dga2005/document/html/chapter9.htm. Accessed September 15, 2008.
3. National Institute on Alcohol Abuse and Alcoholism. How to screen for heavy drinking. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2005. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/PocketGuide/pocket_guide5.htm. Accessed September 15, 2008.
4. Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032-2039.
5. National Institute on Alcohol Abuse and Alcoholism. Alcohol alert. Alcoholic liver disease. Washington, DC: US Department of Health and Human Services; 2005. Available at: http://pubs.niaaa.nih.gov/publications/aa64/aa64.htm. Accessed August 23, 2009.
6. Spiegel DR, Dhadwal N, Gill F. ‘I’m sober, doctor, really’: best biomarkers for underreported alcohol use. Current Psychiatry. 2008;7(9):15-27.
7. Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168(6):649-656.
8. Hvidtfeldt UA, Frederiksen ME, Thysesen LC, et al. Incidence of cardiovascular and cerebrovascular disease in Danish men and women with a prolonged heavy alcohol intake. Alcohol Clin Exp Res. 2008;32(11):1920-1924.
9. Fuchs FD, Chambless LE, Whelton PK, et al. Alcohol consumption and the incidence of hypertension: the Athersclerosis Risk in Communities Study. Hypertension. 2001;37(5):1242-1250.
10. Alcoholic cardiomyopathy. The New York Times Health Guide. Available at: http://health.nytimes.com/health/guides/disease/alcoholic-cardiomyopathy/overview.html. Accessed September 28, 2009.
11. McKenna CJ, Codd MB, McCann HA, et al. Alcohol consumption and idiopathic dilated cardiomyopathy: a case control study. Am Heart J. 1998;135(5 pt 1):833-837.
12. Meyerhoff DJ, Bode C, Nixon SJ, et al. Health risks of chronic moderate and heavy alcohol consumption: how much is too much? Alcohol Clin Exp Res. 2005;29(7):1334-1340.
13. Liappas I, Theotoka I, Kapaki E, et al. Neuropsychological assessment of cognitive function in chronic alcohol-dependent patients and patients with Alzheimer’s disease. In Vivo. 2007;21(6):1115-1118.
14. Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the Future. National Results on Adolescent Drug Use. Overview of Key Findings, 2007. Bethesda, MD: National Institute on Drug Abuse; 2008. Available at: http://www.monitoringthefuture.org/pubs/monographs/overview2007.pdf. Accessed August 20, 2008.
15. Hales RE, Yudofsky SC, Gabbard GO. The American Psychiatric Publishing textbook of psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
16. Substance Abuse and Mental Health Services Administration. Results from the 2004 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. NSDUH Series H-28, DHHS Publication No. SMA 05-4062.
17. National Institute on Drug Abuse. Research report series—marijuana abuse. Bethesda, MD: National Institute on Drug Abuse; 2005. Available at: http://www.nida.nih.gov/ResearchReports/Marijuana. Accessed September 1, 2008.
18. Khalsa JH, Genser S, Francis H, et al. Clinical consequences of marijuana. J Clin Pharmacol. 2002;42(suppl 11):7S-10S.
19. Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. 2005;63(2):93-100.
20. Korantzopoulos P, Liou T, Papaioannides D, et al. Atrial fibrillation and marijuana smoking. Int J Clin Pract. 2008;62(2):308-313.
21. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(suppl 11):58S-63S.
22. Harrison GP, Jr, Gruber AJ, Hudson JI, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42(suppl 11):41S-47S.
23. Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345(5):351-358.
24. National Institute on Drug Abuse. What are the long-term effects of cocaine use? Bethesda, MD: National Institute on Drug Abuse; 1999. Available at: http://www.nida.nih.gov/PDF/RRCocaine.pdf Accessed September 1, 2008.
25. Wang CM, Huang CL, Hu CTS, et al. Medical complications of cocaine abuse. Medical Update for Psychiatrists. 1997;2(2):34-38.
26. Hollander JE, Todd KH, Green G, et al. Chest pain associated with cocaine: an assessment of prevalence in suburban and urban emergency departments. Ann Emerg Med. 1995;26:671-676.
27. Agarwal P, Sen S. Cocaine. e-medicine [serial online]. February 21, 2007. Available at: http://www.emedicine.com/neuro/TOPIC72.HTM. Accessed September 27, 2008.
28. Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18(3):235-242.
29. Goodchild JH, Donaldson M, Mangini DJ. Methamphetamine abuse and the impact on dental health. Dent Today. 2007;26(5):124-131.
30. Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration. Available at: http://www.oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.cfm#TOC. Accessed January 21, 2009.
31. Schuckit MA. Drug and alcohol abuse. 5th ed. New York, NY: Kluwer Academic/Plenum Publishers; 2000:114-119.
32. National Institute for Drug Addiction. Research on the nature and extent of drug use in the United States. Bethesda, MD: National Institute for Drug Addiction; 1999. Available at: http://www.drugabuse.gov/STRC/Data.html. Accessed January 21, 2009.
33. Galanter M, Kleber H. The American Psychiatric Publishing textbook of substance abuse treatment. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
34. Pattinson KT. Opioids and the control of respiration. Br J Anesth. 2008;100(6):747-758.
35. Cygan J, Trunsky M, Corbridge T. Inhaled heroin-induced status asthmaticus: five cases and a review of the literature. Chest. 2000;117(1):272-275.
36. Fanoe S, Hvidt C, Ege P, et al. Syncope and QT prolongation among patients treated with methadone for heroin dependence in the city of Copenhagen. Heart. 2006;93(9):1051-1055.
37. Barnett MH, Miller LA, Reddel SW, et al. Reversible delayed leukoencephalopathy following intravenous heroin overdose. J Clin Neurosci. 2001;8(2):165-167.
38. Brand M, Roth-Bauer M, Driessen M, et al. Executive functions and risky decision-making in patients with opiate dependence. Drug Alcohol Depend. 2008;97(1-2):64-72.
1. Brady KT. Comorbidity of substance use and Axis I psychiatric disorders. Medscape Psychiatry and Mental Health eJournal [serial online]. March 25, 2002. Available at: http://www.medscape.com/viewarticle/430610. Accessed September 28, 2009.
2. Dietary guidelines for Americans, 2005. Chapter 9 alcoholic beverages. Washington, DC: United States Department of Agriculture; 2005. Available at: http://www.health.gov/dietaryguidelines/dga2005/document/html/chapter9.htm. Accessed September 15, 2008.
3. National Institute on Alcohol Abuse and Alcoholism. How to screen for heavy drinking. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2005. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/PocketGuide/pocket_guide5.htm. Accessed September 15, 2008.
4. Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46(6):2032-2039.
5. National Institute on Alcohol Abuse and Alcoholism. Alcohol alert. Alcoholic liver disease. Washington, DC: US Department of Health and Human Services; 2005. Available at: http://pubs.niaaa.nih.gov/publications/aa64/aa64.htm. Accessed August 23, 2009.
6. Spiegel DR, Dhadwal N, Gill F. ‘I’m sober, doctor, really’: best biomarkers for underreported alcohol use. Current Psychiatry. 2008;7(9):15-27.
7. Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168(6):649-656.
8. Hvidtfeldt UA, Frederiksen ME, Thysesen LC, et al. Incidence of cardiovascular and cerebrovascular disease in Danish men and women with a prolonged heavy alcohol intake. Alcohol Clin Exp Res. 2008;32(11):1920-1924.
9. Fuchs FD, Chambless LE, Whelton PK, et al. Alcohol consumption and the incidence of hypertension: the Athersclerosis Risk in Communities Study. Hypertension. 2001;37(5):1242-1250.
10. Alcoholic cardiomyopathy. The New York Times Health Guide. Available at: http://health.nytimes.com/health/guides/disease/alcoholic-cardiomyopathy/overview.html. Accessed September 28, 2009.
11. McKenna CJ, Codd MB, McCann HA, et al. Alcohol consumption and idiopathic dilated cardiomyopathy: a case control study. Am Heart J. 1998;135(5 pt 1):833-837.
12. Meyerhoff DJ, Bode C, Nixon SJ, et al. Health risks of chronic moderate and heavy alcohol consumption: how much is too much? Alcohol Clin Exp Res. 2005;29(7):1334-1340.
13. Liappas I, Theotoka I, Kapaki E, et al. Neuropsychological assessment of cognitive function in chronic alcohol-dependent patients and patients with Alzheimer’s disease. In Vivo. 2007;21(6):1115-1118.
14. Johnston LD, O’Malley PM, Bachman JG, et al. Monitoring the Future. National Results on Adolescent Drug Use. Overview of Key Findings, 2007. Bethesda, MD: National Institute on Drug Abuse; 2008. Available at: http://www.monitoringthefuture.org/pubs/monographs/overview2007.pdf. Accessed August 20, 2008.
15. Hales RE, Yudofsky SC, Gabbard GO. The American Psychiatric Publishing textbook of psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
16. Substance Abuse and Mental Health Services Administration. Results from the 2004 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. NSDUH Series H-28, DHHS Publication No. SMA 05-4062.
17. National Institute on Drug Abuse. Research report series—marijuana abuse. Bethesda, MD: National Institute on Drug Abuse; 2005. Available at: http://www.nida.nih.gov/ResearchReports/Marijuana. Accessed September 1, 2008.
18. Khalsa JH, Genser S, Francis H, et al. Clinical consequences of marijuana. J Clin Pharmacol. 2002;42(suppl 11):7S-10S.
19. Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. 2005;63(2):93-100.
20. Korantzopoulos P, Liou T, Papaioannides D, et al. Atrial fibrillation and marijuana smoking. Int J Clin Pract. 2008;62(2):308-313.
21. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42(suppl 11):58S-63S.
22. Harrison GP, Jr, Gruber AJ, Hudson JI, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42(suppl 11):41S-47S.
23. Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345(5):351-358.
24. National Institute on Drug Abuse. What are the long-term effects of cocaine use? Bethesda, MD: National Institute on Drug Abuse; 1999. Available at: http://www.nida.nih.gov/PDF/RRCocaine.pdf Accessed September 1, 2008.
25. Wang CM, Huang CL, Hu CTS, et al. Medical complications of cocaine abuse. Medical Update for Psychiatrists. 1997;2(2):34-38.
26. Hollander JE, Todd KH, Green G, et al. Chest pain associated with cocaine: an assessment of prevalence in suburban and urban emergency departments. Ann Emerg Med. 1995;26:671-676.
27. Agarwal P, Sen S. Cocaine. e-medicine [serial online]. February 21, 2007. Available at: http://www.emedicine.com/neuro/TOPIC72.HTM. Accessed September 27, 2008.
28. Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18(3):235-242.
29. Goodchild JH, Donaldson M, Mangini DJ. Methamphetamine abuse and the impact on dental health. Dent Today. 2007;26(5):124-131.
30. Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration. Available at: http://www.oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.cfm#TOC. Accessed January 21, 2009.
31. Schuckit MA. Drug and alcohol abuse. 5th ed. New York, NY: Kluwer Academic/Plenum Publishers; 2000:114-119.
32. National Institute for Drug Addiction. Research on the nature and extent of drug use in the United States. Bethesda, MD: National Institute for Drug Addiction; 1999. Available at: http://www.drugabuse.gov/STRC/Data.html. Accessed January 21, 2009.
33. Galanter M, Kleber H. The American Psychiatric Publishing textbook of substance abuse treatment. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
34. Pattinson KT. Opioids and the control of respiration. Br J Anesth. 2008;100(6):747-758.
35. Cygan J, Trunsky M, Corbridge T. Inhaled heroin-induced status asthmaticus: five cases and a review of the literature. Chest. 2000;117(1):272-275.
36. Fanoe S, Hvidt C, Ege P, et al. Syncope and QT prolongation among patients treated with methadone for heroin dependence in the city of Copenhagen. Heart. 2006;93(9):1051-1055.
37. Barnett MH, Miller LA, Reddel SW, et al. Reversible delayed leukoencephalopathy following intravenous heroin overdose. J Clin Neurosci. 2001;8(2):165-167.
38. Brand M, Roth-Bauer M, Driessen M, et al. Executive functions and risky decision-making in patients with opiate dependence. Drug Alcohol Depend. 2008;97(1-2):64-72.