User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
What your office says to patients
The article “A PEARL of wisdom about ‘Pearls,’” (Pearls, Current Psychiatry, September 2009) inspired me to share my own. After 35 years of clinical practice, my office décor has changed many times. In my early psychoanalytic days, it was simple and devoid of any personal references in order to be a “blank screen.” Later I learned that Freud had archeological pieces in this office, some of which he would fondle during his sessions.
In my community psychiatry days, my office contained cultural artifacts, such as Mexican yarn paintings and Hmong story cloth, to display my interest in the ethnic backgrounds of my patients.
When I was medical director of a private psychiatric hospital, my office was large with plush carpeting and fancy furniture. That arrangement seemed to give me extra respect but also created envy.
I now work part-time in a prison. The office is bare and spare, and my seat is closest to the door. In this setting, instead of looking around the office patients look at what I’m wearing.
In my academic office, untidy stacks of books are prominent and pictures of my grandchildren are on view. The computer is usually on.
There’s not much literature and no double-blind studies on office décor. Here are my thoughts:
- Patients pay attention to your office, especially when you are diverted by writing a prescription. Your office needs to be a sanctuary and feel comfortable and safe. Flexibility in where a patient may sit can help.
- Decorate your office to convey ideas or concepts that you think will be most helpful to your patients.
- Patients will view the office as an extension of you and it can affect the therapeutic alliance. Plants that are thriving may symbolize your healing abilities. Watch for unnecessary countertransference, such as a prominent clock that conveys your frustration with 15-minute med checks.
H. Steven Moffic, MD
Professor of psychiatry
Medical College of Wisconsin
Milwaukee, WI
The article “A PEARL of wisdom about ‘Pearls,’” (Pearls, Current Psychiatry, September 2009) inspired me to share my own. After 35 years of clinical practice, my office décor has changed many times. In my early psychoanalytic days, it was simple and devoid of any personal references in order to be a “blank screen.” Later I learned that Freud had archeological pieces in this office, some of which he would fondle during his sessions.
In my community psychiatry days, my office contained cultural artifacts, such as Mexican yarn paintings and Hmong story cloth, to display my interest in the ethnic backgrounds of my patients.
When I was medical director of a private psychiatric hospital, my office was large with plush carpeting and fancy furniture. That arrangement seemed to give me extra respect but also created envy.
I now work part-time in a prison. The office is bare and spare, and my seat is closest to the door. In this setting, instead of looking around the office patients look at what I’m wearing.
In my academic office, untidy stacks of books are prominent and pictures of my grandchildren are on view. The computer is usually on.
There’s not much literature and no double-blind studies on office décor. Here are my thoughts:
- Patients pay attention to your office, especially when you are diverted by writing a prescription. Your office needs to be a sanctuary and feel comfortable and safe. Flexibility in where a patient may sit can help.
- Decorate your office to convey ideas or concepts that you think will be most helpful to your patients.
- Patients will view the office as an extension of you and it can affect the therapeutic alliance. Plants that are thriving may symbolize your healing abilities. Watch for unnecessary countertransference, such as a prominent clock that conveys your frustration with 15-minute med checks.
H. Steven Moffic, MD
Professor of psychiatry
Medical College of Wisconsin
Milwaukee, WI
The article “A PEARL of wisdom about ‘Pearls,’” (Pearls, Current Psychiatry, September 2009) inspired me to share my own. After 35 years of clinical practice, my office décor has changed many times. In my early psychoanalytic days, it was simple and devoid of any personal references in order to be a “blank screen.” Later I learned that Freud had archeological pieces in this office, some of which he would fondle during his sessions.
In my community psychiatry days, my office contained cultural artifacts, such as Mexican yarn paintings and Hmong story cloth, to display my interest in the ethnic backgrounds of my patients.
When I was medical director of a private psychiatric hospital, my office was large with plush carpeting and fancy furniture. That arrangement seemed to give me extra respect but also created envy.
I now work part-time in a prison. The office is bare and spare, and my seat is closest to the door. In this setting, instead of looking around the office patients look at what I’m wearing.
In my academic office, untidy stacks of books are prominent and pictures of my grandchildren are on view. The computer is usually on.
There’s not much literature and no double-blind studies on office décor. Here are my thoughts:
- Patients pay attention to your office, especially when you are diverted by writing a prescription. Your office needs to be a sanctuary and feel comfortable and safe. Flexibility in where a patient may sit can help.
- Decorate your office to convey ideas or concepts that you think will be most helpful to your patients.
- Patients will view the office as an extension of you and it can affect the therapeutic alliance. Plants that are thriving may symbolize your healing abilities. Watch for unnecessary countertransference, such as a prominent clock that conveys your frustration with 15-minute med checks.
H. Steven Moffic, MD
Professor of psychiatry
Medical College of Wisconsin
Milwaukee, WI
Clinical trials support new algorithm for treating pediatric bipolar mania
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
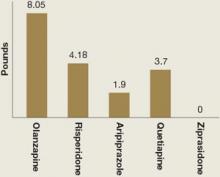
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
Five recent randomized controlled trials (RCTs) have demonstrated the efficacy of atypical antipsychotics for treating bipolar disorder in children and adolescents, but 4 of these 5 trials remain unpublished. The lag time between the completion of these trials and publication of their results—typically 4 to 5 years1—leaves psychiatrists without important evidence to explain to families and critics2 why they might recommend using these powerful medications in children with mental illness.
This article previews the preliminary results of these 5 RCTs of atypical antipsychotics, offers a treatment algorithm supported by this evidence, and discusses how to manage potentially serious risks when using antipsychotics to treat children and adolescents with bipolar disorder (BPD).
Where do atypical antipsychotics fit in?
Details of the 5 industry-sponsored RCTs of atypical antipsychotics in children and adolescents with bipolar I manic or mixed episodes are summarized in Table 1.3-7 Only the olanzapine study4 has been published; data from the other 4 trials were presented at medical meetings in 2007 and 2008.
Change in Young Mania Rating Scale (YMRS) score was the primary outcome measure in these 5 trials, and each compound was more effective than placebo. The trials demonstrated statistically significant and clinically relevant differences between each antipsychotic and placebo. The number needed to treat (NNT)—how many patients need to be treated for 1 to benefit in a controlled clinical trial—ranged from 2 to 4. For comparison, the NNT for statins in the prevention of coronary events is 12 to 22,8 and the NNT in an analysis of trials of selective serotonin reuptake inhibitors for pediatric major depressive disorder was 9.9 Thus, an NNT of ≤4 represents a clinically significant effect.
Risperidone is FDA-approved for short-term treatment of acute bipolar I manic or mixed episodes in patients age 10 to 17. Aripiprazole is approved for acute and maintenance treatment of bipolar I manic or mixed episodes (with or without psychosis) as monotherapy or with lithium or valproate in patients age 10 to 17. In June, an FDA advisory committee recommended pediatric bipolar indications for olanzapine, quetiapine, and ziprasidone.
‘Mood stabilizers’ such as lithium, valproate, and carbamazepine have been used for years to treat bipolar mania in adults, adolescents, and children, despite limited supporting evidence. Preliminary results of a National Institute of Mental Health-funded double-blind RCT provide insights on their efficacy.10
The 153 outpatients age 7 to 17 in a bipolar I manic or mixed episode were randomly assigned to lithium, divalproex, or placebo for 8 weeks. Response rates—based on a Clinical Global Impressions-Improvement score of 1 or 2 (very much or much improved)—were divalproex, 54%; lithium, 42%; and placebo, 29%. Lithium showed a trend toward efficacy but did not clearly separate from placebo on the primary outcome measures. Effect sizes for lithium and divalproex were moderate.10
Only 1 study has compared a mood stabilizer with an atypical antipsychotic for treating mania in adolescents. In a double-blind trial, DelBello et al11 randomly assigned 50 patients age 12 to 18 with a bipolar I manic or mixed episode to quetiapine, 400 to 600 mg/d, or divalproex, serum level 80 to 120 μg/mL, for 28 days. Manic symptoms resolved more rapidly, and remission rates measured by the YMRS were higher with quetiapine than with divalproex. Both medications were well tolerated.
Combination therapy. BPD as it presents in children and adolescents is often difficult to treat because of the disorder’s various phases (manic, depressed, mixed), frequent psychotic symptoms, and high rate of comorbidity. Pediatric BPD patients frequently require several psychotropics, including mood stabilizers and atypical antipsychotics.
In a double-blind, placebo-controlled study, 30 adolescents in a bipolar I manic or mixed episode initially received divalproex, 20 mg/kg/d, then were randomly assigned to 6 weeks of adjunctive quetiapine, titrated to 450 mg/d in 7 days (n=15), or placebo (n=15). Those receiving divalproex plus quetiapine showed a statistically significant greater reduction in manic symptoms (P=.03) and a higher response rate (87% vs 53%, P=.05), compared with those receiving divalproex and placebo. This suggests that a mood stabilizer plus an atypical antipsychotic is more effective than a mood stabilizer alone for adolescent mania. Quetiapine was well tolerated.12
Treatment. The American Psychiatric Association’s outdated 2002 practice guideline for acute bipolar I manic or mixed episodes in adults recommends lithium, valproate, and/or an antipsychotic.13 The more recent Texas Medication Algorithm Project (TMAP) guidelines recommend monotherapy with lithium, valproate, aripiprazole, quetiapine, risperidone, or ziprasidone for adults with euphoric or irritable manic or hypomanic symptoms.14
Based on the TMAP algorithm, recent clinical trial evidence, and our experience in treating pediatric BPD, we offer an approach for treating mania/hypomania in patients age 10 to 17 (see Proposed Algorithm). For dosing and precautions when using atypical antipsychotics in children and adolescents with BPD, see Table 2.15-17
Comorbid psychiatric illnesses (such as anxiety disorders) are prevalent in adolescents with BPD. Evidence in adults and adolescents suggests that some atypical antipsychotics may provide additional benefit for these conditions as well. Thus, consider comorbid conditions and symptoms when choosing antimanic agents.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in children with BPD, and stimulant medications are most often prescribed to treat inattentiveness and hyperactivity. Caution is imperative when treating bipolar youth with stimulants, which can exacerbate manic symptoms. Treat the patient’s mania before adding or reintroducing stimulant medication. Research and clinical experience suggest that if you first stabilize these patients on a mood stabilizer or atypical antipsychotic, adding a stimulant can be very helpful in treating comorbid ADHD symptoms. Start with low stimulant doses, and increase slowly.
Table 1
RCTs of atypical antipsychotics in patients age 10 to 17
with bipolar I disorder*
| Antipsychotic and source | Bipolar I episode (# of subjects) | Trial duration (days) | Dosage (mg/d) | Response rate or YMRS score change | NNT | Mean weight gain (kg) |
|---|---|---|---|---|---|---|
| Risperidone Pandina et al3 AACAP 2007 | Manic, mixed (169) | 21 | 0.5 to 2.5 3 to 6 | 59% 63% | 3.3 3.5 | 1.9 1.4 |
| Olanzapine Tohen et al4 | Manic, mixed (161) | 21 | 10.4 ± 4.5 | 49% | 4.1 | 3.7 ± 2.2 |
| Quetiapine DelBello et al5 AACAP 2007 | Manic (284) | 21 | 400 600 | 64% 58% | 4.4 4.2 | 1.7 1.7 |
| Aripiprazole Wagner et al6 ACNP 2007 | Manic, mixed (296) | 28 | 10 30 | 45% 64% | 4.1 2.4 | 0.9 0.54 |
| Ziprasidone DelBello et al7 APA 2008 | Manic, mixed (238) | 28 | 80 to 160 | –13.83 with ziprasidone, –8.61 with placebo | 3.7 | None |
| *Each trial included a 6-month open extension phase; results are pending | ||||||
| AACAP: American Academy of Child and Adolescent Psychiatry; ACNP: American College of Neuropsychopharmacology; APA: American Psychiatric Association; NNT: number needed to treat; RCT: randomized controlled trial; YMRS: Young Mania Rating Scale | ||||||
Table 2
Recommended antipsychotic use in pediatric bipolar disorder
| Drug | Starting dosage (mg) | Target dosage (mg/d) | Precautions |
|---|---|---|---|
| Aripiprazole | 2.5 to 5 at bedtime | 10 to 30 | Monitor for CYP 3A4 and 2D6 interactions, weight, BMI, cholesterol, lipids, and glucose |
| Olanzapine | 2.5 bid | 10 to 20 | Monitor for CYP 2D6 interactions, weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Quetiapine | 50 bid | 400 to 1,200 | Monitor for weight, BMI, cholesterol, lipids, and glucose |
| Risperidone | 0.25 bid | 1 to 2.5 | Monitor for EPS, hyperprolactinemia (and associated sexual side effects, including galactorrhea), weight, BMI, cholesterol, lipids, glucose, and prolactin levels |
| Ziprasidone | 20 bid | 80 to 160 | Check baseline ECG and as dose increases or with reason for high level of concern; monitor prolactin levels |
| BMI: body mass index; CYP: cytochrome P450; ECG: electrocardiography; EPS: extrapyramidal symptoms | |||
| Source: References 15-17 | |||
Proposed Algorithm: Treating a bipolar mixed/manic episode in patients age 10 to 17
Stage 1. Consider patient’s experience with antipsychotics, body weight, and family history when choosing first-line monotherapy (1A). Quetiapine poses low risk for extrapyramidal symptoms and tardive dyskinesia. Aripiprazole and ziprasidone pose relatively low risk of weight gain. Risperidone is potent at low doses but increases prolactin levels (long-term effect unknown).
Second-line choices (1B) are mood stabilizers lithium and valproate (because of lower potency than atypical antipsychotics), and olanzapine (which—although potent—causes substantial weight gain). In case of lack of response or intolerable side effects with initial agent, select an alternate from 1A or 1B. If this is not effective, move to Stage 2.
Stage 2. Consider augmentation for patients who show partial response to monotherapy (in your clinical judgment “mild to moderately improved” but not “much or very much improved”).
Stage 3. Combination therapy could include 2 mood stabilizers (such as lithium and valproate) plus an atypical antipsychotic; 2 atypical antipsychotics; or other combinations based on patient’s past responses. No research has shown these combinations to be efficacious in bipolar children and adolescents, but we find they sometimes help those with treatment-resistant symptoms.
Duration. Maintain psychotropics 12 to 18 months. When patient is euthymic, slowly taper 1 medication across several months. If symptoms recur, reintroduce the mood-stabilizing agent(s).
Source: Adapted and reprinted with permission from Kowatch RA, Fristad MA, Findling R, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008
Managing adverse effects
Although clinically effective, atypical antipsychotics may cause serious side effects that must be recognized and managed. These include extrapyramidal symptoms (EPS), tardive dyskinesia (TD), weight gain and obesity, hyperlipidemia, increased prolactin levels, and QTc changes. Counsel patients and families about the risks and benefits of antipsychotics when you consider them for children and adolescents with BPD (Table 3).
EPS. Drug-induced parkinsonism and akathisia are the most common EPS in children and adolescents with BPD treated with atypical antipsychotics.18
Correll et al19 reported a 10% rate of EPS in patients treated with aripiprazole. Treatment-emergent EPS also was observed in the RCT of risperidone.20 EPS-related adverse events were associated with higher doses of risperidone, although none of the akathisia/EPS measures were thought to be “clinically significant.”
EPS frequency was relatively low and similar to placebo in the 3-week quetiapine trial,21 and no changes in movement disorder scale scores were observed during the olanzapine or ziprasidone RCTs.4,7
Recommendations. If your pediatric patient develops EPS, first try an antipsychotic dose reduction. Because anticholinergics can contribute to antipsychotic-induced weight gain, reserve them until after a dosage reduction has been unsuccessful.
Benztropine (0.25 to 0.5 mg given 2 to 3 times daily, not to exceed 3 mg/d) or diphenhydramine (25 to 50 mg given 3 to 4 times daily; maximum dosage 5 mg/kg/d) can be effective in treating EPS. Avoid anticholinergics in children with narrow-angle glaucoma or age <3.
Akathisia may be managed with propranolol (20 to 120 mg/d in divided doses). Multiple doses (typically 3 times daily) are needed to prevent interdose withdrawal symptoms. Use this beta blocker with caution in children with asthma because of the possibility of bronchospasm.
TD. Short-term trials and a meta-analysis of atypical antipsychotic trials (>11 months’ duration, subject age <18) suggest a low annual risk for TD (0.4%).22 Large, prospective, long-term trials of atypical antipsychotics are necessary to more accurately define the risk of TD in the pediatric population, however. Retrospective analyses of adolescents treated with antipsychotics suggest 3 TD risk factors:
- early age of antipsychotic use
- medication nonadherence
- concomitant use of antiparkinsonian agents.23
Kumra et al24 identified lower premorbid functioning and greater positive symptoms at baseline as factors associated with “withdrawal dyskinesia/tardive dyskinesia” in children and adolescents with early-onset psychotic-spectrum disorders treated with typical or atypical antipsychotics.
Recommendations. To minimize TD risk, use the lowest effective antipsychotic dose, monitor for abnormal involuntary movements with standardized assessments (such as the Abnormal Involuntary Movement Scale), review risks and benefits with parents and patients, and regularly evaluate the indication and need for antipsychotic therapy. It is reasonable to attempt to lower the antipsychotic dose after the patient has attained remission and been stable for 1 year.
Neuroleptic malignant syndrome (NMS). This complication of dopamine-blocking medications:
- is among the most serious adverse effects of antipsychotic treatment
- continues to be associated with a mortality rate of 10%.25
Recommendation. At least 1 recent review of pediatric NMS cases suggests that essential features (hyperthermia and severe muscular rigidity) are retained in children.26 Nonetheless, monitor for variant presentations; hyper thermia or muscle rigidity may be absent or develop slowly over several days in patients treated with atypical antipsychotics.27
Weight gain and glucose metabolism. A major adverse effect of most atypical antipsychotics is increased appetite, weight gain, and possible obesity.28 In children, “obesity” refers to a body mass index (BMI) >95th percentile for age and sex; “over-weight” refers to BMI between the 85th and 95th percentile. Mean weight gain in the 5 atypical antipsychotic pediatric bipolar trials ranged from 0 to 8 lbs across 3 to 4 weeks of treatment (Figure).3-7
Recommendations. Emphasize diet and exercise, with restriction of high-carbohydrate food, “fast foods,” and soft drinks. Another option is a trial of metformin, which decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization.
Klein et al29 studied 39 patients age 10 to 17 with mood and psychotic disorders whose weight increased by >10% during <1 year of olanzapine, risperidone, or quetiapine therapy. In this 16-week, double-blind, placebo-controlled trial, weight was stabilized in subjects receiving metformin, whereas those receiving placebo continued to gain weight (0.31 kg [0.68 lb]/week).
The usual starting metformin dose is 500 mg bid with meals. Increase in increments of 500 mg weekly, up to a maximum of 2,000 mg/d in divided doses. Potential side effects include diarrhea, nausea/vomiting, flatulence, and headache.
Hyperlipidemia. Patients who gain weight with atypical antipsychotics also may develop hyperlipidemia. Fasting serum triglycerides >150 mg/dL (1.70 mmol/L) in obese children are considered to be elevated and an early sign of metabolic syndrome.30 Fasting total cholesterol >200 mg/dL (5.18 mmol/L) or low-density lipoprotein cholesterol >130 mg/dL (3.38 mmol/L) is consistent with hyperlipidemia.
Recommendation. Monitor and treat hyperlipidemia, which increases the risk of atherosclerosis as obese children grow older.31
Prolactin. Elevated prolactin concentrations may have deleterious effects in the developing child or adolescent, including gynecomastia, oligomenorrhea, and amenorrhea.17 Long-term effects on growth and sexual maturation have not been fully evaluated.
The relative tendency of atypical antipsychotics to cause hyperprolactinemia is roughly: risperidone/paliperidone > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.18 In the risperidone RCT, mean changes in baseline prolactin levels were 41 ng/mL for boys and 59 ng/mL in girls.3 Results of the olanzapine RCT suggest a high incidence of hyperprolactinemia (26% of girls, 63% of boys).4 Decreases in serum prolactin were observed in bipolar children and adolescents treated with aripiprazole for 30 weeks.19
Recommendations. For any pediatric patient treated with an atypical antipsychotic that increases prolactin levels:
- Obtain a baseline prolactin level.
- Repeat after 6 months of treatment or with the emergence of elevated prolactin symptoms, such as gynecomastia in boys. Ask about increases in breast size, galactorrhea, changes in menstruation, sexual functioning, and pubertal development.
Switch patients who develop any of these side effects to another atypical agent that does not increase serum prolactin.32
QTc interval prolongation. All atypical antipsychotics can cause QTc prolongation. Several cases of significant QTc prolongation have been reported in children and adolescents treated with ziprasidone.33,34 In the RCT of ziprasidone, QTc prolongation was not clinically significant in most of the patients in which it was reported, and it did not lead to adverse events.34 Mean QTc change was 8.1 msec at study termination.7
Patients enrolled in clinical trails are screened very carefully, however, and those with preexisting medical abnormalities typically are excluded. Thus, these findings may have limited usefulness for “real-world” patients.
Recommendations. Until additional information is known about the cardiac effects of atypical antipsychotics in children and adolescents:
- Perform a careful history, review of symptoms, and physical exam looking for any history of palpitations, shortness of breath, or syncope.
- Query specifically about any family history of sudden cardiac death.
- Perform a baseline resting ECG for patients starting ziprasidone or clozapine, or for other atypicals if indicated by history, review of systems, physical exam, etc.
- For patients treated with ziprasidone or clozapine, repeat ECG as the dose increases or if the patient has cardiac symptoms (unexplained shortness of breath, palpitations, skipped beats, etc.).
Table 3
Talking to families about using antipsychotics
in children with bipolar disorder
| Effectiveness. Large, placebo-controlled studies have shown that atypical antipsychotics can significantly reduce manic symptoms in children and adolescents with bipolar disorder |
| Safety data. Additional 6-month safety data indicate that atypical antipsychotics continue to be effective in children and adolescents, without dramatic changes in side effects |
| Precautions. Antipsychotics are powerful medications and must be used carefully in pediatric patients |
| Potential side effects. All antipsychotics have serious potential side effects that must be recognized, monitored, and managed |
| Potential benefits from using atypical antipsychotics include mood stabilization, treatment of psychotic symptoms, and lower risk of extrapyramidal symptoms compared with typical antipsychotics |
| Risk vs benefit. On balance, the potential benefit of these agents outweighs the potential risk for children and adolescents with bipolar disorder |
Figure: Mean weight gain with atypical antipsychotics in pediatric bipolar trials
Weight gain in children and adolescents with bipolar disorder varied among atypical antipsychotics used in 5 recent randomized controlled trials. Treatment duration was 3 weeks with olanzapine, risperidone, and quetiapine and 4 weeks with aripiprazole and ziprasidone. Dosages were olanzapine, 10.4 ± 4.5 mg/d; risperidone, 0.5 to 2.5 mg/d or 3 to 6 mg/d; aripiprazole, 10 or 30 mg/d; quetiapine, 400 or 600 mg/d; and ziprasidone, 80 to 160 mg/d.
Source: References 3-7Related resources
- Child and Adolescent Bipolar Foundation. www.bpkids.org.
- University of Illinois at Chicago Pediatric Mood Disorders Clinic. www.psych.uic.edu/pmdc.
- Ryan Licht Sang Bipolar Foundation. www.ryanlichtsangbipolarfoundation.org.
Drug brand names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Carbamazepine • Carbatrol
- Clozapine • Clozaril
- Diphenhydramine • Benadryl
- Divalproex sodium • Depakote
- Lithium • Lithobid, others
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate • Depacon
- Ziprasidone • Geodon
Disclosures
Dr. Kowatch is a consultant to and speaker for AstraZeneca and a consultant to Forest Pharmaceuticals. He receives research support from the National Alliance for Research on Schizophrenia and Depression, the National Institute of Child Health and Human Development, the National Institute of Mental Health, and the Stanley Foundation.
Dr. Strawn has received research support from the American Academy of Child and Adolescent Psychiatry (Lilly Pilot Research Award).
Dr. Sorter receives research support from the National Institute of Mental Health and the Health Foundation of Greater Cincinnati.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
1. Hopewell S, Clarke M, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2007;(2):MR000011.-
2. Carey B. Risks found for youths in new antipsychotics. The New York Times. September 15, 2008:A17.
3. Pandina G, DelBello M, Kushner S, et al. Risperidone for the treatment of acute mania in bipolar youth. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
4. Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164(10):1547-1556.
5. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescents with bipolar mania: a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 23-28, 2007; Boston, MA.
6. Wagner K, Nyilas M, Forbes R, et al. Acute efficacy of aripiprazole for the treatment of bipolar I disorder, mixed or manic, in pediatric patients. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology, December 9-13, 2007; Boca Raton, FL.
7. DelBello M, Findling RL, Wang P, et al. Safety and efficacy of ziprasidone in pediatric bipolar disorder. Paper presented at: Annual Meeting of the American Psychiatric Association, May 3-8, 2008; Washington, DC.
8. McElduff P, Jaefarnezhad M, Durrington PN. American, British and European recommendations for statins in the primary prevention of cardiovascular disease applied to British men studied prospectively. Heart. 2006;92(9):1213-1218.
9. Tsapakis EM, Soldani F, Tondo L, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193(1):10-17.
10. Kowatch R, Findling R, Scheffer R, et al. Placebo controlled trial of divalproex versus lithium for bipolar disorder. Paper presented at: Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 23-28, 2007; Boston, MA.
11. DelBello MP, Kowatch RA, Adler CM, et al. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45(3):305-313.
12. DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1216-1223.
13. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
14. Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66(7):870-886.
15. Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc Psychiatr Clin N Am. 2006;15(1):207-220.
16. Correll CU, Penzner JB, Parikh UH, et al. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15(1):177-206.
17. Correll CU. Effect of hyperprolactinemia during development in children and adolescents. J Clin Psychiatry. 2008;69(8):e24.-
18. Correll CU. Antipsychotic use in children and adolescents: minimizing adverse effects to maximize outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(1):9-20.
19. Correll CU, Nyilas M, Ashfaque S, et al. Long-term safety and tolerability of aripiprazole in children (10-17 years) with bipolar disorder. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
20. Pandina GJ, Bossie CA, Youssef E, et al. Risperidone improves behavioral symptoms in children with autism in a improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37(2):367-373.
21. DelBello M, Findling RL, Earley W, et al. Efficacy of quetiapine in children and adolescent with bipolar mania; a 3-week, double-blind, randomized, placebo-controlled trial. Paper presented at: Annual Meeting of the American College of Neuropsychopharmacology; December 9-13, 2007; Boca Raton, FL.
22. Correll CU, Kane JM. One-year incidence rates of tardive dyskinesia in children and adolescents treated with second-generation antipsychotics: a systematic review. J Child Adolesc Psychopharmacol. 2007;17(5):647-656.
23. McDermid SA, Hood J, Bockus S, et al. Adolescents on neuroleptic medication: is this population at risk for tardive dyskinesia? Can J Psychiatry. 1998;43(6):629-631.
24. Kumra S, Jacobsen LK, Lenane M, et al. Case series: spectrum of neuroleptic-induced movement disorders and extrapyramidal side effects in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1998;37(2):221-227.
25. Strawn JR, Keck PE, Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
26. Croarkin PE, Emslie GJ, Mayes TL. Neuroleptic malignant syndrome associated with atypical antipsychotics in pediatric patients: a review of published cases. J Clin Psychiatry. 2008;69(7):1157-1165.
27. Picard LS, Lindsay S, Strawn JR, et al. Atypical neuroleptic malignant syndrome: diagnostic controversies and considerations. Pharmacotherapy. 2008;28(4):530-535.
28. Correll CU. Metabolic side effects of second-generation antipsychotics in children and adolescents: a different story? J Clin Psychiatry. 2005;66(10):1331-1332.
29. Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072-2079.
30. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs. 2007;22(3):218-253.
31. O’Grady MJ, Brown AM, O’Neill MB. Cholesterol screening in an at-risk pediatric population. Pediatr Cardiol. 2008;29(3):609-613.
32. Ali J, Khemka M. Hyperprolactinemia: monitoring children on long-term risperidone. Current Psychiatry. 2008;7(11):64-72.
33. Blair J, Scahill L, State M, et al. Electrocardiographic changes in children and adolescents treated with ziprasidone: a prospective study. J Am Acad Child Adolesc Psychiatry. 2005;44(1):73-79.
34. Malone RP, Delaney MA, Hyman SB, et al. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779-790.
Discussing CAM options with your patients
CAM for your depressed patient: 6 recommended options
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Americans with depression turn to complementary and alternative medicine (CAM) more often than conventional psychotherapy or FDA-approved medication. In a nationally representative sample, 54% of respondents with self-reported “severe depression”—including two-thirds of those receiving conventional therapies—reported using CAM during the previous 12 months.1
Unfortunately, popular acceptance of CAM for depression is disproportionate to the evidence base, which—although growing—remains limited. As a result, your patients may be self-medicating with poorly supported treatments that are unlikely to help them recover from depression.
In reviewing CAM treatments for depression, we found some with enough evidence of positive effect that we feel comfortable recommending them as evidence-based options. These promising, short-term treatments are supported by level 1a or 1b evidence and at least 1 study that demonstrates an ability to induce remission ( Table 1 ).2
For patients seeking “natural” or nonprescription treatments—or when you wish to augment standard treatments that are not working adequately—you might recommend fatty acids, St. John’s wort, or S-adenosyl-L-methionine (SAMe). Similar recommendations can be made for yoga, exercise, and bibliotherapy, as we discuss here.
Table 1
Evidence these authors required to recommend a CAM treatment
| Minimum requirements | Level of evidence | Recommendation |
|---|---|---|
| Systematic review showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1a + | A |
| At least 2 RCTs showing superiority to placebo or standard treatment Plus 1 study showing CAM treatment can induce remission | 1b | A– |
| CAM: complementary and alternative medicine; RCT: randomized controlled trial | ||
| Source: Reference 2 | ||
Reviewing CAM evidence
This article refers to as “alternative” any treatment other than a form of psychotherapy or an FDA-approved medication that substitutes for a standard psychiatric treatment. When used to augment standard psychiatric treatments, these approaches are considered “complementary.”
Our search for evidence on CAM treatments for depressive disorders raised questions about what constitutes acceptable and convincing methodology:
- Studies often had problems with blinding and suitable placebos. Many were small, with short duration and no long-term follow-up.
- Comparisons with active treatments that showed no differences were assumed to imply comparability, even though the studies were powered to detect only large differences.
Applying the evidence. Because CAM use is widespread, be sure to ask psychiatric patients if they are using CAM treatments. If the answer is “yes,” a risk-benefit assessment is needed. Inform patients who are using poorly supported CAM approaches that they could consider better-supported CAM options as well as standard effective antidepressants.
Monitor patients for an adequately prompt positive response to an evidence-based CAM approach that has shown efficacy for their level of depression. As with any treatment, consider other evidence-based options when CAM treatments are inadequate or unsuccessful in achieving remission of depressive symptoms.
Sufficient evidence of efficacy
Yoga. In their systematic review of yoga’s effectiveness for depression, Pilkington et al3 analyzed 5 RCTs that met 3 criteria:
- participants had mild to severe depression or depressive disorders
- yoga or yoga-based exercises alone were used for treatment
- depression rating scales were used as outcome measures.
Conclusion. Yoga has been studied primarily as an alternative treatment, but its physical health and group participation benefits and lack of side effects make it a suitable complementary treatment as well.
- 45% with supervised exercise
- 40% with home-based exercise
- 47% with sertraline, 50 to 200 mg/d
- 31% with placebo.4
5 RCTs of yoga’s effectiveness in treating depression
| RCT | Interventions | Conclusion |
|---|---|---|
| Broota and Dhir, 1990 | Yoga and PMR vs control | Yoga and PMR were more effective than control, with yoga more effective than PMR |
| Khumar et al, 1993 | Shavasana yoga vs no intervention | College students with severe depression improved during and after yoga treatment |
| Janakiramaiah et al, 2000 | SKY vs ECT vs imipramine | Reductions in BDI scores for all 3 groups; ECT > SKY or imipramine, SKY=imipramine |
| Rohini et al, 2000 | Full SKY vs partial SKY | 30 individuals with MDD improved with either therapy, but results were not statistically significant |
| Woolery, 2004 | Iyengar yoga vs wait list | 28 mildly depressed individuals benefitted from yoga, as measured by BDI scores at midpoint and throughout treatment |
| BDI: Beck Depression Inventory; ECT: electroconvulsive therapy; MDD: major depressive disorder; PMR: progressive muscle relaxation; RCT: randomized controlled trial; SKY: Sudarshan Kriya yoga | ||
| Source: Broota A, Dhir R. Efficacy of two relaxation techniques in depression. Journal of Personality and Clinical Studies. 1990;6(1):83-90. Khumar SS, Kaur P, Kaur S. Effectiveness of Shavasana on depression among university students. Indian J Clin Psychol. 1993;20(2):82-87. Janakiramaiah N, Gangadhar BN, Naga Venkatesha Murthy PJ, et al. Antidepressant efficacy of Sudarshan Kriya yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. J Affect Disord. 2000;57(1-3):255-259. Rohini V, Pandey RS, Janakiramaiah N, et al. A comparative study of full and partial Sudarshan Kriya yoga (SKY) in major depressive disorder. NIMHANS Journal. 2000;18(1):53-57. Woolery A, Myers H, Sternlieb B, et al. A yoga intervention for young adults with elevated symptoms of depression. Altern Ther Health Med. 2004;10(2):60-63. | ||
Table 3
Evidence of the antidepressant effect of exercise
| Literature review | Methodology | Conclusion |
|---|---|---|
| Byrne and Byrne, 1993 | 13 studies, clinical samples, measured changes in depressed mood | 90% of studies reported beneficial effects, especially in clinical populations |
| Scully et al, 1998 | 4 literature reviews, 1 monograph, 1 study | Positive relationship of physical activity and depression in healthy and clinical samples, increased over time |
| Lawlor and Hopker, 2001 | 14 RCTs from 1966 to 1999 with depression as an outcome | Significant methodologic weaknesses, but exercise effect > no treatment and=cognitive therapy |
| Dunn et al, 2001 | Examined dose effect in 37 studies; subjects diagnosed with depressive disorders, mild-to-moderate symptoms, and no medical comorbidity | Only level B and C evidence; positive effects with exercise from light to heavy intensity; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| Brosse et al, 2002 | 12 RCTs from 1979 to 1999 | Significant methodologic limitations, but authors concluded evidence supports a positive effect of exercise in healthy and clinical populations |
| Larun et al, 2006 | 4 RCTs in children and youth age | Exercise effect same as no intervention, low-intensity relaxation, or psychosocial intervention |
| Barbour et al, 2007 | 2 meta-analyses, 1 RCT, 2 studies | Positive effect; high-energy was optimal dose; aerobic=nonaerobic; improvement may or may not be related to improved fitness |
| RCT: randomized controlled trial | ||
| Source: Byrne AE, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. J Psychosom Res. 1993;37(6):565-574. Scully D, Kremer J, Meade MM, et al. Physical exercise and psychological well being: a critical review. Br J Sports Med. 1998;32(2):111-120. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322(7289):763-767. Dunn AL, Trivedi MH, O’Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):S587. Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741-760. Larun L, Nordheim LV, Ekeland E, et al. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3:CD004691. Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: a review. J Cardiopulm Rehabil Prev. 2007;27(6):359-367. | ||
At least 2 studies suggest that high-energy exercise and aerobic or resistance training provide greater reductions in depressive symptoms than exercises such as walking.5,6 Yoga’s positive effects suggest, however, that an aerobic effect is not necessary for an antidepressant benefit.
Exercise has not been adequately tested as a complementary treatment but likely is safe for most psychiatric patients. Perspiration and dehydration might alter therapeutic blood levels of lithium or other medications. Advise patients to drink water before, during, and after exercise and to avoid outdoor exercise in extreme temperatures. More vigorous monitoring might be indicated in specific cases.
Tailor exercise programs to individual needs, considering the patient’s age and health status. Refer a patient with a known heart problem or increased cardiovascular risk to his or her physician for selective exercise testing.
Bibliotherapy—reading self-help books, usually about cognitive-behavioral approaches to depressive disorders—has been relatively well studied. A recent meta-analysis examined 29 studies with pre-post designs. Group differences in the 17 controlled studies yielded a large effect size of 0.77. Participants who read the materials benefitted similarly whether they met in groups or applied the information on their own. Older adults tended to be less depressed at baseline and made smaller treatment gains.7
Conclusion. Evidence supports bibliotherapy as an effective treatment for mild-to-moderate depression. No convincing data support its use as a complementary treatment, but it poses virtually no risk.
St. John’s wort (Hypericum perforatum) has been extensively studied for depressive disorders, with 29 RCTs in a meta-analysis of MDD trials through July 2008.10 Another meta-analysis compared St. John’s wort with selective serotonin reuptake inhibitors (SSRIs) in 13 studies through June 2008.11 These and most RCTs have found St. John’s wort significantly more effective than placebo in reducing depressive symptoms.
Data selected from double-blind RCTs totaling 217 patients with mild depression [Hamilton Depression Rating Scale (HDRS) scores 12 Studies routinely show that treating MDD with St. John’s wort is comparable to using tricyclic or SSRI antidepressants.
Side effects with St. John’s wort generally are no different than with placebo and significantly less than with comparison treatments. Even so, using St. John’s wort instead of SSRIs for MDD remains controversial.
Studies vs SSRIs. Many of the favorable St. John’s wort trials were conducted in Europe, particularly in Germany. Two large RCTs conducted in the United States reported that the St. John’s wort standardized extract LI-160 was not more effective than placebo, but neither could be clearly interpreted as negative for St. John’s wort:
- In an 8-week trial, St. John’s wort and placebo groups improved significantly but at unusually low rates. The remission rate with St. John’s wort was small but significantly higher than with placebo.13
- A study sponsored by the National Institute of Mental Health compared St. John’s wort, 900 to 1,500 mg/d; sertraline, 50 to 100 mg/d; and placebo in 340 adults with MDD. No positive effects were found for St. John’s wort or sertraline.14
Conclusion. Standardized extracts of St. John’s wort—particularly WS5570, 300 mg tid, and ZE117, 250 mg bid—appear to be effective treatments, especially for mild-to-moderate MDD. Because St. John’s wort is available without prescription and can interact with SSRIs or other antidepressants:
- care is required for its complementary use
- it is important to ask if patients are using St. John’s wort on their own.
- for first-line use only when you can adequately gauge its effects on your patient’s other medications
- especially for depressed patients who cannot tolerate SSRIs.
SAMe has become a popular alternative treatment for depression since its introduction to the United States in the late 1990s, but it has been studied in only 2 U.S. open trials. One showed SAMe to be very effective in reducing depressive symptoms in patients with HIV/AIDS.17 The other found a 50% response rate and 43% remission rate with adjunctive SAMe, 800 to 1,600 mg/d for 6 weeks, in 30 adults with MDD who failed to respond adequately to SSRIs or the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine. The most common side effects were gastrointestinal (GI) symptoms and headaches.18 This open trial led to an ongoing National Institutes of Health-sponsored RCT on the safety and efficacy of SAMe for patients with treatment-resistant depression.
Conclusion. SAMe appears to have a faster onset of antidepressant effect than standard SSRIs or SNRIs and a favorable side-effect profile, which make the lack of rigorous trials in the United States striking. We recommend that you consider SAMe:
- as an adjunct in patients with incomplete response to standard treatments
- as a complementary treatment to speed onset of antidepressant effects.
Four meta-analyses independently looked at largely the same dozen RCTs through 2006 and found that 1 to 2 grams daily of omega-3 PUFAs was significantly more effective at reducing depressive symptoms than placebo.19-22 Other data suggest that omega-3 PUFAs can induce depression remission in depressed Parkinson’s disease patients23 and depressed pregnant women.24 Since 2006, however, findings have been inconsistent. Several trials have found PUFAs no more effective than placebo.25-27
An 8-week double-blind study compared EPA, 1 gram daily; fluoxetine, 20 mg/d; or both agents in 60 outpatients with MDD. Response rates—as measured by ≥50% reduction in baseline HDRS scores—were 50% with fluoxetine, 56% with EPA, and 81% with combination therapy.28
Insufficient evidence
L-tryptophan. It seems reasonable to expect a serotonin precursor to increase serotonin in the CNS and improve depressive symptoms. Of 111 trials on L-tryptophan for depression, however, only 2 met the quality criteria for inclusion in a recent meta-analysis.29 Combining the 2 trials showed L-tryptophan alone and in combination with a tricyclic antidepressant was more effective than placebo for treating depressive disorders in adults.
Conclusion. Very little research continues to test L-tryptophan as a viable CAM for depressive disorder. Its serious side effect of eosinophilia-myalgia syndrome makes clinical use of this agent unlikely.
Acupuncture. Numerous small studies with questionable controls, different needling placements, and poor allocation concealment and blinding limit the ability to draw conclusions about acupuncture for treating depression ( Table 4 ). A recent meta-analysis by Wang et al30 added 2 Chinese trials not included in an earlier review31 and found acupuncture significantly reduced depressive symptoms. No consistent differences were detected in response or remission rates, however.
Conclusion. Evidence is methodologically weak, and the use of acupuncture as an alternative or complementary treatment of depression is questionable.
Table 4
Acupuncture: Insufficient evidence of antidepressant effect
| Literature review | Methodology | Conclusion |
|---|---|---|
| Mukaino et al, 2005 | Systematic review of 7 RCTs including 509 patients; compared either manual or electroacupuncture with any control procedure | Inconsistent evidence of manual acupuncture’s effectiveness vs sham; electroacupuncture’s effect may be similar to that of antidepressant medication and merits further study |
| Leo and Ligot, 2007 | Systematic review of 9 RCTs, 5 considered low quality; some focused on very specific populations (ie, hospitalized stroke patients or pregnant depressed patients) | Evidence inconclusive because of study designs and methodologies |
| Smith and Hay, 2005 | Meta-analysis of 7 trials including 517 adults with mild-to-moderate depression; 5 trials (409 participants) compared acupuncture with medication; 2 trials compared acupuncture with wait list or sham acupuncture | No difference between acupuncture and medication; study quality too poor to support acupuncture’s efficacy |
| Wang et al, 2008 | Meta-analysis of 8 small RCTs totalling 477 subjects (256 received active acupuncture, remainder received sham acupuncture); sham acupuncture design, number of acupuncture sessions, and duration varied among studies | Significant reduction in HRSD or BDI scores for acupuncture vs sham, but no significant effect of acupuncture on response or remission rates |
| BDI: Beck Depression Inventory; HRSD: Hamilton Rating Scale for Depression; RCT: randomized controlled trial | ||
| Source: Mukaino Y, Park J, White A, et al. The effectiveness of acupuncture for depression—a systematic review of randomised controlled trials. Acupunct Med. 2005;23(2):70-76. Leo RJ, Ligot JS Jr. A systematic review of randomized controlled trials of acupuncture in the treatment of depression. J Affect Disord. 2007;97(1-3):13-22. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression? A meta-analysis of 8 randomized controlled trials. J Affect Disord. 2008;111(2-3):125-134. | ||
- National Center for Complementary and Alternative Medicine, National Institutes of Health. http://nccam.nih.gov.
- Journal of Alternative and Complementary Medicine. www.liebertpub.com/products/product.aspx?pid=26.
- Complementary and alternative medicine. www.nlm.nih.gov/medlineplus/complementaryandalternativemedicine.html.
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Lithium • Eskalith, Lithobid
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
1. Kessler RC, Soukup J, Davis RB, et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am J Psychiatry. 2001;158(2):289-294.
2. Phillips B, Ball C, Sackett D, et al. Oxford University Centre for Evidence Based Medicine levels of evidence and grades of recommendation (March 2009). Available at: http://www.cebm.net/index.aspx?o=1025#levels. Accessed August 19, 2009.
3. Pilkington K, Kirkwood G, Rampes H, et al. Yoga for depression: the research evidence. J Affect Disord. 2005;89(1-3):13-24.
4. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587-596.
5. Dunn AL, Trivedi MH, Kampert JB, et al. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1-8.
6. Legrand F, Heuze JP. Antidepressant effects associated with different exercise conditions in participants with depression: a pilot study. J Sport Exercise Psychol. 2007;29(3):348-364.
7. Gregory RJ, Schwer Canning S, Lee TW, et al. Cognitive bibliotherapy for depression: a meta-analysis. Professional Psychology: Research and Practice. 2004;35(3):275-280.
8. Floyd M, Scogin F, McKendree-Smith NL, et al. Cognitive therapy for depression: a comparison of individual psychotherapy and bibliotherapy for depressed older adults. Behav Modif. 2004;28(2):297-318.
9. Burns DD. Feeling good: the new mood therapy. New York, NY: HarperCollins; 1980.
10. Linde K, Berner MM, Kriston L. St John’s Wort for major depression. [update of Cochrane Database Syst Rev. 2005;(2):CD000448; PMID: 15846605]. Cochrane Database Syst Rev. 2008(4):000448.
11. Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
12. Kasper S, Gastpar M, Müller WE, et al. Efficacy of St. John’s wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008;258(1):59-63.
13. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285(15):1978-1986.
14. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807-1814.
15. Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76(suppl):1158S-1161S.
16. Hardy ML, Coulter I, Morton SC, et al. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease [comment in ACP J Club. 2003;139(1):20]. Evid Rep Technol Assess (Summ). 2003;Aug(64):1-3.
17. Shippy RA, Mendez D, Jones K, et al. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38.-
18. Alpert JE, Papakostas G, Mischoulon D, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24(6):661-664.
19. Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056-1061.
20. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [published correction appears in J Clin Psychiatry. 2007;68(2):338]. J Clin Psychiatry. 2006;67(12):1954-1967.
21. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21.-
22. Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials [comment in Am J Clin Nutr. 2007;85(6):1665-1666; author reply 1666]. Am J Clin Nutr. 2006;84(6):1308-1316.
23. da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2-3):351-359.
24. Su KP, Huang SY, Chiu TH, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(4):644-651.
25. Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393-1396.
26. Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust N Z J Psychiatry. 2008;42(3):199-205.
27. Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421-431.
28. Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
29. Shaw K, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database Syst Rev. 2002;(1):CD003198.-
30. Wang H, Qi H, Wang BS, et al. Is acupuncture beneficial in depression: a meta-analysis of 8 randomized controlled trials? J Affect Disord. 2008;111(2-3):125-134.
31. Smith CA, Hay PPJ. Acupuncture for depression. Cochrane Database Syst Rev. 2005;(2):CD004046.-
Regimen promotes orlistat efficacy in teens
Orlistat is an FDA-approved anorexic agent for adults and adolescents that interferes with gastrointestinal lipase and prevents the absorption of 30% of dietary fat. Some clinical trials have failed to show significant weight reduction with orlistat,1,2 but in our adolescent residential psychiatric treatment center:
- 15 patients (13 girls and 2 boys) lost an average 17.9 lbs over 4 months
- one patient lost 100 lbs over 8 months.
In a naturalistic observation, we offered orlistat plus dietary counseling to all teenage patients with a body mass index (BMI) >25 kg/m2. Gastrointestinal (GI) side effects, such as odoriferous loose stools and fat-stained and occasionally soiled underwear, are common.
Factors contributing to weight loss
Motivation. Most of our patients gained weight while being treated with psychiatric medications, particularly mood stabilizers and atypical antipsychotics. These were continued during orlistat use.
Larger waist sizes and exaggerated facial and body features diminished our overweight patients’ self-respect and made them targets of bullying and rejection. Weight loss helped reverse these negative factors. Peers often supported these changes by including the teens in their circle of friends. Self-esteem, maturity, and motivation for personal growth were reflected in the teens’ social and psychotherapeutic encounters.
Overweight. Weight loss was statistically significant in teens with BMI >25 kg/m2. Average pre-orlistat weight was 193 lbs and BMI 33 kg/m2. Average BMI loss was 4.3 kg/m2 (median loss 3.7 kg/m2, standard deviation ±4.08). Although we did not treat patients with initial BMI
Diet, dosing, and activity. Our patients began a low-fat, 1,800-calorie diet in 3 meals and 2 snacks per day. They also received a daily supplement with vitamins A, D, E, and K to prevent deficiencies from the loss of fat-soluble vitamins.
Orlistat, 120 mg tid, was dispensed before meals, which enhanced awareness of healthy food choices because high-fat foods caused GI side effects.
As patients lost weight, they became more interested in exercise, and tolerance for physical activity improved.
Monitoring and support. Nursing staff weighed the teenagers weekly. Patients also had twice-monthly psychiatric assessments of orlistat’s impact on their medical, psychiatric, and psychopharmacologic status. The teens’ primary concerns included the amount of weight loss and GI side effects.
Summary. Under these conditions, orlistat may be an effective weight loss medication that can enhance self-esteem in motivated, overweight teenagers with psychiatric comorbidities.
1. Chanoine JP, Hampl S, Jensen C, et al. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873-2883.
2. Maahs D, Gonzalez de Serna D, Kolotkin RL, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12:18-28.
Orlistat is an FDA-approved anorexic agent for adults and adolescents that interferes with gastrointestinal lipase and prevents the absorption of 30% of dietary fat. Some clinical trials have failed to show significant weight reduction with orlistat,1,2 but in our adolescent residential psychiatric treatment center:
- 15 patients (13 girls and 2 boys) lost an average 17.9 lbs over 4 months
- one patient lost 100 lbs over 8 months.
In a naturalistic observation, we offered orlistat plus dietary counseling to all teenage patients with a body mass index (BMI) >25 kg/m2. Gastrointestinal (GI) side effects, such as odoriferous loose stools and fat-stained and occasionally soiled underwear, are common.
Factors contributing to weight loss
Motivation. Most of our patients gained weight while being treated with psychiatric medications, particularly mood stabilizers and atypical antipsychotics. These were continued during orlistat use.
Larger waist sizes and exaggerated facial and body features diminished our overweight patients’ self-respect and made them targets of bullying and rejection. Weight loss helped reverse these negative factors. Peers often supported these changes by including the teens in their circle of friends. Self-esteem, maturity, and motivation for personal growth were reflected in the teens’ social and psychotherapeutic encounters.
Overweight. Weight loss was statistically significant in teens with BMI >25 kg/m2. Average pre-orlistat weight was 193 lbs and BMI 33 kg/m2. Average BMI loss was 4.3 kg/m2 (median loss 3.7 kg/m2, standard deviation ±4.08). Although we did not treat patients with initial BMI
Diet, dosing, and activity. Our patients began a low-fat, 1,800-calorie diet in 3 meals and 2 snacks per day. They also received a daily supplement with vitamins A, D, E, and K to prevent deficiencies from the loss of fat-soluble vitamins.
Orlistat, 120 mg tid, was dispensed before meals, which enhanced awareness of healthy food choices because high-fat foods caused GI side effects.
As patients lost weight, they became more interested in exercise, and tolerance for physical activity improved.
Monitoring and support. Nursing staff weighed the teenagers weekly. Patients also had twice-monthly psychiatric assessments of orlistat’s impact on their medical, psychiatric, and psychopharmacologic status. The teens’ primary concerns included the amount of weight loss and GI side effects.
Summary. Under these conditions, orlistat may be an effective weight loss medication that can enhance self-esteem in motivated, overweight teenagers with psychiatric comorbidities.
Orlistat is an FDA-approved anorexic agent for adults and adolescents that interferes with gastrointestinal lipase and prevents the absorption of 30% of dietary fat. Some clinical trials have failed to show significant weight reduction with orlistat,1,2 but in our adolescent residential psychiatric treatment center:
- 15 patients (13 girls and 2 boys) lost an average 17.9 lbs over 4 months
- one patient lost 100 lbs over 8 months.
In a naturalistic observation, we offered orlistat plus dietary counseling to all teenage patients with a body mass index (BMI) >25 kg/m2. Gastrointestinal (GI) side effects, such as odoriferous loose stools and fat-stained and occasionally soiled underwear, are common.
Factors contributing to weight loss
Motivation. Most of our patients gained weight while being treated with psychiatric medications, particularly mood stabilizers and atypical antipsychotics. These were continued during orlistat use.
Larger waist sizes and exaggerated facial and body features diminished our overweight patients’ self-respect and made them targets of bullying and rejection. Weight loss helped reverse these negative factors. Peers often supported these changes by including the teens in their circle of friends. Self-esteem, maturity, and motivation for personal growth were reflected in the teens’ social and psychotherapeutic encounters.
Overweight. Weight loss was statistically significant in teens with BMI >25 kg/m2. Average pre-orlistat weight was 193 lbs and BMI 33 kg/m2. Average BMI loss was 4.3 kg/m2 (median loss 3.7 kg/m2, standard deviation ±4.08). Although we did not treat patients with initial BMI
Diet, dosing, and activity. Our patients began a low-fat, 1,800-calorie diet in 3 meals and 2 snacks per day. They also received a daily supplement with vitamins A, D, E, and K to prevent deficiencies from the loss of fat-soluble vitamins.
Orlistat, 120 mg tid, was dispensed before meals, which enhanced awareness of healthy food choices because high-fat foods caused GI side effects.
As patients lost weight, they became more interested in exercise, and tolerance for physical activity improved.
Monitoring and support. Nursing staff weighed the teenagers weekly. Patients also had twice-monthly psychiatric assessments of orlistat’s impact on their medical, psychiatric, and psychopharmacologic status. The teens’ primary concerns included the amount of weight loss and GI side effects.
Summary. Under these conditions, orlistat may be an effective weight loss medication that can enhance self-esteem in motivated, overweight teenagers with psychiatric comorbidities.
1. Chanoine JP, Hampl S, Jensen C, et al. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873-2883.
2. Maahs D, Gonzalez de Serna D, Kolotkin RL, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12:18-28.
1. Chanoine JP, Hampl S, Jensen C, et al. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873-2883.
2. Maahs D, Gonzalez de Serna D, Kolotkin RL, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12:18-28.
How independent can a CME program be?
I appreciate Dr. Henry A. Nasrallah’s editorial examining funding of continuing medical education (CME) programs (“The $1.2 billion CME crisis,” From the Editor, Current Psychiatry, July 2009). CME programs are critical to keep doctors informed about the latest advances and enhance their skills at frequent intervals. In addition, CME programs are important support for primary care physicians who practice in rural areas and work with traditionally underserved populations.
The majority of physicians agree that CME programs meet their educational needs. Pharmaceutical firms often view sponsored CME programs as opportunities to promote their product. It is well known that these companies try to influence the selection of topics and speakers as well as content of CME programs.
I strongly agree with Dr. Nasrallah’s proposal to pool funding from multiple pharmaceutical companies that is then allocated to applicants by a third party. In this manner, CME programs could guarantee the highest level of transparency and scrutiny.
Gurprit Lamba, MD
Third-year resident
St. Elizabeth’s Medical Center
Boston, MA
I appreciate Dr. Henry A. Nasrallah’s editorial examining funding of continuing medical education (CME) programs (“The $1.2 billion CME crisis,” From the Editor, Current Psychiatry, July 2009). CME programs are critical to keep doctors informed about the latest advances and enhance their skills at frequent intervals. In addition, CME programs are important support for primary care physicians who practice in rural areas and work with traditionally underserved populations.
The majority of physicians agree that CME programs meet their educational needs. Pharmaceutical firms often view sponsored CME programs as opportunities to promote their product. It is well known that these companies try to influence the selection of topics and speakers as well as content of CME programs.
I strongly agree with Dr. Nasrallah’s proposal to pool funding from multiple pharmaceutical companies that is then allocated to applicants by a third party. In this manner, CME programs could guarantee the highest level of transparency and scrutiny.
Gurprit Lamba, MD
Third-year resident
St. Elizabeth’s Medical Center
Boston, MA
I appreciate Dr. Henry A. Nasrallah’s editorial examining funding of continuing medical education (CME) programs (“The $1.2 billion CME crisis,” From the Editor, Current Psychiatry, July 2009). CME programs are critical to keep doctors informed about the latest advances and enhance their skills at frequent intervals. In addition, CME programs are important support for primary care physicians who practice in rural areas and work with traditionally underserved populations.
The majority of physicians agree that CME programs meet their educational needs. Pharmaceutical firms often view sponsored CME programs as opportunities to promote their product. It is well known that these companies try to influence the selection of topics and speakers as well as content of CME programs.
I strongly agree with Dr. Nasrallah’s proposal to pool funding from multiple pharmaceutical companies that is then allocated to applicants by a third party. In this manner, CME programs could guarantee the highest level of transparency and scrutiny.
Gurprit Lamba, MD
Third-year resident
St. Elizabeth’s Medical Center
Boston, MA
How I really feel
I find Dr. Nasrallah’s ideas to be consonant with my own (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009).
I believe insurance companies have discriminated against psychiatrists and our patients for many years. I believe the legal profession has made us what we are today. The oath to tell the truth in the courtroom should include lawyers. We need court-ordered outpatient treatment to help control our patients’ psychiatric illnesses and noncompliance. The worst development in psychiatry has been our relegation to being “pill-pushers,” off limits to psychotherapy.
Howard W. Fisher, MD
Psychiatrist
Colorado Mental Health Institute at Pueblo
Pueblo, CO
I find Dr. Nasrallah’s ideas to be consonant with my own (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009).
I believe insurance companies have discriminated against psychiatrists and our patients for many years. I believe the legal profession has made us what we are today. The oath to tell the truth in the courtroom should include lawyers. We need court-ordered outpatient treatment to help control our patients’ psychiatric illnesses and noncompliance. The worst development in psychiatry has been our relegation to being “pill-pushers,” off limits to psychotherapy.
Howard W. Fisher, MD
Psychiatrist
Colorado Mental Health Institute at Pueblo
Pueblo, CO
I find Dr. Nasrallah’s ideas to be consonant with my own (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009).
I believe insurance companies have discriminated against psychiatrists and our patients for many years. I believe the legal profession has made us what we are today. The oath to tell the truth in the courtroom should include lawyers. We need court-ordered outpatient treatment to help control our patients’ psychiatric illnesses and noncompliance. The worst development in psychiatry has been our relegation to being “pill-pushers,” off limits to psychotherapy.
Howard W. Fisher, MD
Psychiatrist
Colorado Mental Health Institute at Pueblo
Pueblo, CO
There are no ‘clients’ in the doctor-patient relationship
I read with amusement Dr. Nasrallah’s August editorial, and certainly agree with most of his points of “ventilation” (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009). In regard to relabeling patients as “clients,” I think this is an encroachment on our profession by therapists (eg, MSW, LISWs, RNs, PhDs, and PsyDs).
I do not feel that anyone that I prescribe medications to is a “client.” These individuals are patients and always will be. I suspect the term “client” comes from those without prescribing privileges and only serves to erode our profession, much like wearing suits or street clothes instead of white lab coats, which is Dr. Nasrallah’s third point.
I also am a practicing pediatrician, and I can assure you that there is a slow attempt by nurses and practice managers to turn pediatric patients into “clients” as well. The same trend away from white lab coats is now being seen in primary care, which I feel is eroding the doctor-patient relationship.
In my opinion, you can be my client if I never prescribe you medicine. Until then, you’re my patient. To date, I have only 2 “clients,” but a slew of patients.
Paul Trombley, MD, FAAP
Cleveland Heights, OH
I read with amusement Dr. Nasrallah’s August editorial, and certainly agree with most of his points of “ventilation” (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009). In regard to relabeling patients as “clients,” I think this is an encroachment on our profession by therapists (eg, MSW, LISWs, RNs, PhDs, and PsyDs).
I do not feel that anyone that I prescribe medications to is a “client.” These individuals are patients and always will be. I suspect the term “client” comes from those without prescribing privileges and only serves to erode our profession, much like wearing suits or street clothes instead of white lab coats, which is Dr. Nasrallah’s third point.
I also am a practicing pediatrician, and I can assure you that there is a slow attempt by nurses and practice managers to turn pediatric patients into “clients” as well. The same trend away from white lab coats is now being seen in primary care, which I feel is eroding the doctor-patient relationship.
In my opinion, you can be my client if I never prescribe you medicine. Until then, you’re my patient. To date, I have only 2 “clients,” but a slew of patients.
Paul Trombley, MD, FAAP
Cleveland Heights, OH
I read with amusement Dr. Nasrallah’s August editorial, and certainly agree with most of his points of “ventilation” (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009). In regard to relabeling patients as “clients,” I think this is an encroachment on our profession by therapists (eg, MSW, LISWs, RNs, PhDs, and PsyDs).
I do not feel that anyone that I prescribe medications to is a “client.” These individuals are patients and always will be. I suspect the term “client” comes from those without prescribing privileges and only serves to erode our profession, much like wearing suits or street clothes instead of white lab coats, which is Dr. Nasrallah’s third point.
I also am a practicing pediatrician, and I can assure you that there is a slow attempt by nurses and practice managers to turn pediatric patients into “clients” as well. The same trend away from white lab coats is now being seen in primary care, which I feel is eroding the doctor-patient relationship.
In my opinion, you can be my client if I never prescribe you medicine. Until then, you’re my patient. To date, I have only 2 “clients,” but a slew of patients.
Paul Trombley, MD, FAAP
Cleveland Heights, OH
We treat patients, not clients
All of Dr. Nasrallah’s comprehensive list of “nags” are noteworthy (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009) however, I will focus on calling patients “clients.” I feel that this practice strikes at psychiatrists’ identity as physicians. I have noted with increasing frequency and alarm that psychiatrists and nurses are falling into this trap that strikes at the core of our profession. It is as if words are not important.
Roslyn Seligman, MD
Professor of psychiatry
University of Cincinnati
Cincinnati, OH
All of Dr. Nasrallah’s comprehensive list of “nags” are noteworthy (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009) however, I will focus on calling patients “clients.” I feel that this practice strikes at psychiatrists’ identity as physicians. I have noted with increasing frequency and alarm that psychiatrists and nurses are falling into this trap that strikes at the core of our profession. It is as if words are not important.
Roslyn Seligman, MD
Professor of psychiatry
University of Cincinnati
Cincinnati, OH
All of Dr. Nasrallah’s comprehensive list of “nags” are noteworthy (“Let me tell you how I feel…” From the Editor, Current Psychiatry, August 2009) however, I will focus on calling patients “clients.” I feel that this practice strikes at psychiatrists’ identity as physicians. I have noted with increasing frequency and alarm that psychiatrists and nurses are falling into this trap that strikes at the core of our profession. It is as if words are not important.
Roslyn Seligman, MD
Professor of psychiatry
University of Cincinnati
Cincinnati, OH
Does psychiatric practice make us wise?
At a recent morning rounds, a resident presented a case of a do-not-resuscitate decision for an elderly patient, which our psychiatry consultation service received overnight from an internal medicine ward. Another resident casually mentioned how physicians from other services at our hospital habitually call on psychiatrists to “make the difficult ethical decisions for them.”
That got me thinking. Psychiatrists are expected to analyze conflicts, resolve dilemmas, exercise good judgment, provide advice to colleagues and patients, and display a transcendent and objective perspective about the complexities of life. Psychiatric training and practice prompt us to be thoughtful, tolerant of ambiguity, and willing to tackle the multilayered meanings and consequences of human behavior. Indeed, developing attributes related to the most advanced functions of the human mind is at the core of our professional training and clinical practice.
Medical specialties develop different skills
Consider the training consequences of other medical specialties: surgeons become adept at navigating structural anatomy with superb dexterity to extricate lesions, repair wounds, or transplant organs; radiologists excel at scanning complex black and white patterns in radiographic images to detect the subtlest pathologies or anomalies; pathologists pinpoint cause of death with autopsies and elegant tissue examinations; and obstetricians become virtuosos of birthing or repairing intricate reproductive structures.
We psychiatrists go well beyond the standard medical history, physical exam, and laboratory findings. Our major skills are detecting gross and minute deviations in the mental status exam and the range and nuances of patients’ behaviors, insight, judgment, cognition, coping skills, internal conflicts, drives, compulsions, thought processes, personality traits, decision-making, resilience, social skills, interpersonal adroitness, truthfulness, emotiveness, impulsivity, ambition, perceptions, perceptiveness, verbal and nonverbal communications, defense mechanisms, and outlook on life.
We also integrate our complex observations and findings with the rich collage of each patient’s unique cultural, religious, and educational background. We strive to find hidden or higher meaning in patients’ symptoms, words, and actions. We assess their potential lethality toward themselves or others and examine the often tortuous course of their existence. And, unlike other physicians, we observe their transference toward us and simultaneously examine our own conscious or subconscious countertransference—channeled via thoughts, emotions, and behavior—and we scrutinize potential or real boundary violations by patients and ourselves and act judiciously. No other specialty has as wide or deep a view as psychiatry of the totality of people’s lives.
Neurobiology of wisdom
The wonder of psychiatric practice is that we somehow navigate each patient’s unique jungle of thoughts, emotions, behaviors, and cognitions and skillfully weave a biopsychosocial diagnosis and treatment plan. By doing so, we develop different regions or circuits in our brains than surgeons, radiologists, or internists do. Meeks and Jeste’s wonderful article about the neurobiology of wisdom suggests that psychiatrists’ brains probably develop “wisdom circuitry” via advanced neuroplastic connectivity in the:
- prefrontal cortex (for emotional regulation, decision-making, and value relativism)
- lateral prefrontal cortex (to facilitate calculated reason-based decision-making)
- medial prefrontal cortex (for emotional valence and prosocial attitudes and behavior).1
Is it possible that just as the finger-related motor cortex grows in pianists’ brains, psychiatrists’ prefrontal pathways undergo hypertrophy as we repeatedly assess and integrate mental observations, conceptualize a diagnosis, develop a strategic treatment plan, then counsel patient after patient on how to deal with stressors and develop more adaptive living skills and attitudes? Could those well-developed pathways enhance good judgment, decision-making, insightfulness, and wisdom?
Perhaps our medical/surgical colleagues consult with us because they have noted our ability—although by no means perfect—to assess and manage conflicting or ambiguous situations and develop wise solutions for the knotty and often painful human condition. However, they also should know that, human as we are, developing wisdom does not immunize us from making unwise decisions now and then.
1. Meeks TW, Jeste DV. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 2009;66(4):355-365.
At a recent morning rounds, a resident presented a case of a do-not-resuscitate decision for an elderly patient, which our psychiatry consultation service received overnight from an internal medicine ward. Another resident casually mentioned how physicians from other services at our hospital habitually call on psychiatrists to “make the difficult ethical decisions for them.”
That got me thinking. Psychiatrists are expected to analyze conflicts, resolve dilemmas, exercise good judgment, provide advice to colleagues and patients, and display a transcendent and objective perspective about the complexities of life. Psychiatric training and practice prompt us to be thoughtful, tolerant of ambiguity, and willing to tackle the multilayered meanings and consequences of human behavior. Indeed, developing attributes related to the most advanced functions of the human mind is at the core of our professional training and clinical practice.
Medical specialties develop different skills
Consider the training consequences of other medical specialties: surgeons become adept at navigating structural anatomy with superb dexterity to extricate lesions, repair wounds, or transplant organs; radiologists excel at scanning complex black and white patterns in radiographic images to detect the subtlest pathologies or anomalies; pathologists pinpoint cause of death with autopsies and elegant tissue examinations; and obstetricians become virtuosos of birthing or repairing intricate reproductive structures.
We psychiatrists go well beyond the standard medical history, physical exam, and laboratory findings. Our major skills are detecting gross and minute deviations in the mental status exam and the range and nuances of patients’ behaviors, insight, judgment, cognition, coping skills, internal conflicts, drives, compulsions, thought processes, personality traits, decision-making, resilience, social skills, interpersonal adroitness, truthfulness, emotiveness, impulsivity, ambition, perceptions, perceptiveness, verbal and nonverbal communications, defense mechanisms, and outlook on life.
We also integrate our complex observations and findings with the rich collage of each patient’s unique cultural, religious, and educational background. We strive to find hidden or higher meaning in patients’ symptoms, words, and actions. We assess their potential lethality toward themselves or others and examine the often tortuous course of their existence. And, unlike other physicians, we observe their transference toward us and simultaneously examine our own conscious or subconscious countertransference—channeled via thoughts, emotions, and behavior—and we scrutinize potential or real boundary violations by patients and ourselves and act judiciously. No other specialty has as wide or deep a view as psychiatry of the totality of people’s lives.
Neurobiology of wisdom
The wonder of psychiatric practice is that we somehow navigate each patient’s unique jungle of thoughts, emotions, behaviors, and cognitions and skillfully weave a biopsychosocial diagnosis and treatment plan. By doing so, we develop different regions or circuits in our brains than surgeons, radiologists, or internists do. Meeks and Jeste’s wonderful article about the neurobiology of wisdom suggests that psychiatrists’ brains probably develop “wisdom circuitry” via advanced neuroplastic connectivity in the:
- prefrontal cortex (for emotional regulation, decision-making, and value relativism)
- lateral prefrontal cortex (to facilitate calculated reason-based decision-making)
- medial prefrontal cortex (for emotional valence and prosocial attitudes and behavior).1
Is it possible that just as the finger-related motor cortex grows in pianists’ brains, psychiatrists’ prefrontal pathways undergo hypertrophy as we repeatedly assess and integrate mental observations, conceptualize a diagnosis, develop a strategic treatment plan, then counsel patient after patient on how to deal with stressors and develop more adaptive living skills and attitudes? Could those well-developed pathways enhance good judgment, decision-making, insightfulness, and wisdom?
Perhaps our medical/surgical colleagues consult with us because they have noted our ability—although by no means perfect—to assess and manage conflicting or ambiguous situations and develop wise solutions for the knotty and often painful human condition. However, they also should know that, human as we are, developing wisdom does not immunize us from making unwise decisions now and then.
At a recent morning rounds, a resident presented a case of a do-not-resuscitate decision for an elderly patient, which our psychiatry consultation service received overnight from an internal medicine ward. Another resident casually mentioned how physicians from other services at our hospital habitually call on psychiatrists to “make the difficult ethical decisions for them.”
That got me thinking. Psychiatrists are expected to analyze conflicts, resolve dilemmas, exercise good judgment, provide advice to colleagues and patients, and display a transcendent and objective perspective about the complexities of life. Psychiatric training and practice prompt us to be thoughtful, tolerant of ambiguity, and willing to tackle the multilayered meanings and consequences of human behavior. Indeed, developing attributes related to the most advanced functions of the human mind is at the core of our professional training and clinical practice.
Medical specialties develop different skills
Consider the training consequences of other medical specialties: surgeons become adept at navigating structural anatomy with superb dexterity to extricate lesions, repair wounds, or transplant organs; radiologists excel at scanning complex black and white patterns in radiographic images to detect the subtlest pathologies or anomalies; pathologists pinpoint cause of death with autopsies and elegant tissue examinations; and obstetricians become virtuosos of birthing or repairing intricate reproductive structures.
We psychiatrists go well beyond the standard medical history, physical exam, and laboratory findings. Our major skills are detecting gross and minute deviations in the mental status exam and the range and nuances of patients’ behaviors, insight, judgment, cognition, coping skills, internal conflicts, drives, compulsions, thought processes, personality traits, decision-making, resilience, social skills, interpersonal adroitness, truthfulness, emotiveness, impulsivity, ambition, perceptions, perceptiveness, verbal and nonverbal communications, defense mechanisms, and outlook on life.
We also integrate our complex observations and findings with the rich collage of each patient’s unique cultural, religious, and educational background. We strive to find hidden or higher meaning in patients’ symptoms, words, and actions. We assess their potential lethality toward themselves or others and examine the often tortuous course of their existence. And, unlike other physicians, we observe their transference toward us and simultaneously examine our own conscious or subconscious countertransference—channeled via thoughts, emotions, and behavior—and we scrutinize potential or real boundary violations by patients and ourselves and act judiciously. No other specialty has as wide or deep a view as psychiatry of the totality of people’s lives.
Neurobiology of wisdom
The wonder of psychiatric practice is that we somehow navigate each patient’s unique jungle of thoughts, emotions, behaviors, and cognitions and skillfully weave a biopsychosocial diagnosis and treatment plan. By doing so, we develop different regions or circuits in our brains than surgeons, radiologists, or internists do. Meeks and Jeste’s wonderful article about the neurobiology of wisdom suggests that psychiatrists’ brains probably develop “wisdom circuitry” via advanced neuroplastic connectivity in the:
- prefrontal cortex (for emotional regulation, decision-making, and value relativism)
- lateral prefrontal cortex (to facilitate calculated reason-based decision-making)
- medial prefrontal cortex (for emotional valence and prosocial attitudes and behavior).1
Is it possible that just as the finger-related motor cortex grows in pianists’ brains, psychiatrists’ prefrontal pathways undergo hypertrophy as we repeatedly assess and integrate mental observations, conceptualize a diagnosis, develop a strategic treatment plan, then counsel patient after patient on how to deal with stressors and develop more adaptive living skills and attitudes? Could those well-developed pathways enhance good judgment, decision-making, insightfulness, and wisdom?
Perhaps our medical/surgical colleagues consult with us because they have noted our ability—although by no means perfect—to assess and manage conflicting or ambiguous situations and develop wise solutions for the knotty and often painful human condition. However, they also should know that, human as we are, developing wisdom does not immunize us from making unwise decisions now and then.
1. Meeks TW, Jeste DV. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 2009;66(4):355-365.
1. Meeks TW, Jeste DV. Neurobiology of wisdom: a literature overview. Arch Gen Psychiatry. 2009;66(4):355-365.