User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
A mysterious case of mania
CASE: First-episode mania
Mrs. P, age 47, is brought to the emergency department (ED) because her family is concerned about her behavioral changes over the last week. Her husband reports that Mrs. P has become hyper-religious and talkative. She has been perseverating on numbers and dates and incessantly calling people. Mrs. P reports increased energy and decreased need for sleep. On examination, she has pressured speech. She has no psychiatric history; however, for the past year, she has been taking sertraline, 100 mg/d, and desipramine, 25 mg/d, which her primary care physician prescribed for unknown reasons.
Mrs. P has struggled with chronic back pain for years, but an MRI of her spine is negative. Her family strongly believes that for the past 3 years Mrs. P has been receiving too many medications from her pain management specialist. Six weeks before her current presentation, she was receiving methadone, 40 mg/d, hydrocodone, at least 20 mg/d, and tramadol, 400 mg/d in divided doses. She also was taking an unknown dose of at least 1 benzodiazepine.
Mrs. P’s husband notes she stopped taking methadone abruptly approximately 5 weeks ago. However, about 3 weeks ago, Mrs. P accidentally overdosed on opioids and was hospitalized for several days. Urine drug screen at the time was positive for acetaminophen, salicylate, propoxyphene, opiate, benzodiazepine, and tricyclic antidepressant.
Mrs. P’s medical history includes auditory nerve loss from birth; her mother had German measles (rubella). Mrs. P never learned American Sign Language. She underwent cochlear implant surgery 1 year ago and now has only mild difficulties speaking.
The authors’ observations
Manic symptoms are common in patients with comorbid medical disorders and present a diagnostic challenge. Obtaining an accurate history from the patient may be difficult. Such evaluations often require extensive investigation and collection of data from multiple sources, including:
- medical records
- family members
- patient observation.
Mrs. P’s history is marked by contradicting data from these sources. For example, her family says she stopped taking “pain medications” 5 weeks ago, but 2 weeks later her urine drug screen showed opioids.
Both illicit drugs and prescribed medications can precipitate manic symptoms. From medical records and drug testing, it was evident that Mrs. P had a history of medication abuse/overdose/misuse.
Mania also has been associated with substance withdrawal. Mrs. P allegedly stopped taking methadone 4 weeks before the onset of manic symptoms. Methadone is a synthetic opioid with a pharmacokinetic and pharmacodynamic profile that presents clinical challenges, including:
- large interindividual variability in methadone pharmacokinetics
- lack of reliable equianalgesic conversion ratio to and from other opioids
- potential for multiple drug interactions and complex pharmacodynamics.
An opioid’s half-life determines the onset and duration of withdrawal syndrome symptoms.1 Methadone metabolism is predominantly mediated by CYP3A4, CYP2B6, CYP2D6, and to some extent by CYP2C19.1 We performed genetic testing to help evaluate how Mrs. P metabolized medications. Mrs. P had a normal genotype for CYP2D6, which meant that she should process opioids at a normal rate; however, she was heterozygous for CYP2C19*2 polymorphism, so it is possible that methadone stayed in her system longer than average.
Evidence documenting methadone drug interactions is limited (Table 1).1 Mrs. P was taking sertraline and desipramine; both have potent effects via 2D6 inhibition that could increase plasma methadone concentration. Other evidence indicates that benzodiazepines and methadone may have synergistic interactions that could increase opioid sedation or respiratory depression.1
Table 1
Is a drug interaction with methadone causing Mrs. P’s mania?
| Medication class/agent | Effect on methadone level | Effect on methadone metabolism | Additional effects of interaction |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Fluvoxamine | Increase | Inhibition | Opioid toxicity |
| Fluoxetine | Increase | Inhibition | Torsades de pointes |
| Paroxetine | Increase | Inhibition | Decreased hepatic metabolism |
| Sertraline* | Increase | Autoinduction | Torsades de pointes |
| Citalopram | — | — | Torsades de pointes |
| Tricyclic antidepressants | |||
| Desipramine* | — | Inhibition | Increased desipramine levels/inhibition of desipramine metabolism |
| Amitriptyline | Increase methadone clearance | — | Torsades de pointes/prolonged QT interval |
| Anti-inflammatory drugs | |||
| NSAIDs* | — | — | Enhanced analgesia/opioid-sparing effect |
| Aspirin* | — | — | Paradoxical activation of platelet receptors |
| Benzodiazepines | |||
| Alprazolam | — | — | CNS depression/sedation/overdose |
| Diazepam* | — | Inhibition | Additive depressant effects |
| Opioid agonists | |||
| Dextromethorphan | — | Inhibition (not significant) | Increased side effects, especially sleepiness and drowsiness |
| Tramadol* | — | — | Well tolerated |
| Nicotine | Decrease | Can increase smoking rate | |
| *Medications taken by Mrs. P | |||
| NSAIDs: nonsteroidal anti-inflammatory drugs | |||
| Source: Reference 1 | |||
EVALUATION: Few clues
In our ED, Mrs. P’s urine drug abuse screen is positive for salicylate and benzodiazepine only. Findings from physical examination, vital signs, ECG, and chest radiography are within normal limits. Internal medicine consultation is unremarkable. Mrs. P’s laboratory investigation is notable for an elevated white blood cell count, but this normalizes over a week.
Mrs. P shows no evidence of infection and is normoglycemic. B12 and folate are within normal limits. Serum electrolytes, liver function testing, sensitive thyroid stimulating hormone, and C-reactive protein are within normal limits. Urinalysis is negative except for a small amount of hemoglobin. Her creatine kinase (CK) is in the upper normal range. Human immunodeficiency virus (HIV) and syphilis testing is negative. Ceruloplasmin level also is normal. Heavy metal screen is negative. Head MRI and CT from previous hospitalizations were unremarkable.
The authors’ observations
Our first step was to clarify Mrs. P’s diagnosis. In reviewing differential diagnoses, we considered:
- serotonin syndrome
- benzodiazepine withdrawal syndrome
- antidepressant-induced mania
- adrenergic toxicity
- malignant hyperthermia
- heat stroke
- infectious causes.
Our index of suspicion for serotonin syndrome was low because Mrs. P didn’t meet criteria required for diagnosis. Relevant signs and symptoms included confusion, elevated mood (major) and agitation, nervousness, insomnia, and low blood pressure (minor).
Based on concerns about medication interactions, we discontinued sertraline and desipramine. According to the patient’s sister, Mrs. P’s manic symptoms markedly responded to PRN doses of lorazepam. We prescribed lorazepam, 1 mg every 6 hours, and observed Mrs. P for signs and symptoms of benzodiazepine withdrawal.
HISTORY: OTC drug use
According to Mrs. P’s mother, after her daughter abruptly discontinued methadone, she began to have very strong headaches, which she treated with Excedrin or Excedrin Sinus. The mother said that 4 days before Mrs. P came to the ED, she found her daughter holding 4 tablets of Excedrin and an empty bottle. Unfortunately her mother was unable to say what type of Excedrin it was. When the treatment team asks Mrs. P how many pills she usually takes, she says she doesn’t know but usually until the pain stops.
The authors’ observations
Management of secondary mania should focus on treating the underlying condition (Algorithm). Neurology categorizes mania into 3 categories:2
- confusional-delirious states
- manic symptoms associated with focal or multifocal cerebral lesions
- affective disorders (manic-depressive and depressive psychoses).
Medical workup ruled out common secondary causes of psychosis. Collaborative information from relatives revealed no family history of mental illness.
Patients with hearing loss and deafness have been shown to be at increased risk for psychotic disorders compared with the general population. Severe sensory deficits early in Mrs. P’s life may have influenced the orderly development of neural connections in her sensory cortex and association areas.3 Mrs. P was deaf for the first 45 years of life. It could be hypothesized that her sensory deficits significantly influenced her ability to reality test. After receiving a cochlear implant, Mrs. P rapidly went from no auditory stimulation to marked improvement. This stressor might precipitate psychotic symptoms. However, her presentation seemed to be characterized more by manic symptoms or an agitated delirium. It also did not fit temporally with her presentation.
We begin to suspect that Mrs. P’s mania is substance-induced. Excedrin, an over-the-counter medication, contains aspirin and caffeine. Excedrin Sinus also contains phenylephrine. Amphetamines, caffeine, ephedrine, pseudoephedrine, and phenylpropanolamine have all been linked to manic-like psychotic episodes.
Concerns about the illicit conversion of pseudoephedrine into methamphetamine obliged pharmaceutical companies in the United States to switch product formulations to phenylephrine in 2005,4 although some “behind-the-counter” medications may contain pseudoephedrine. Phenylephrine is a relatively selective α1 agonist with weak α2 adrenoceptor agonist activity and low β agonist activity. It is very similar to pseudo-ephedrine, which is known to be implicated in the development of manic symptoms.5,6
Pseudoephedrine can raise CK levels and cause rhabdomyolysis.7,8 Mrs. P’s CK level was 176 (normal range 36 to 176 U/L) 4 days after her initial presentation, and she had a moderate amount of myoglobin in her urine. Her creatinine was normal. The patient was taking excessive amounts of caffeine and—if she was using Excedrin Sinus—pseudoephedrine or phenylephrine. We were unable to determine whether her Excedrin contained pseudoephedrine or phenylephrine. In addition, she was going through opioid withdrawal and reported problems with her sleep. There was also a question of Mrs. P’s unknown methadone use combined with its decreased clearance secondary to medication interactions.
While previously hospitalized for overdose, Mrs. P tested positive for propoxyphene. Excessive use of propoxyphene also can cause numerous adverse reactions. Some of that could have explained why Mrs. P’s presentation includes nervousness, CNS stimulation, excitement, insomnia, and restlessness.5
Based on multiple factors, we believe Mrs. P meets DSM-IV-TR criteria for substance-induced mood disorder (Table 2).9 This diagnosis is supported by Mrs. P’s history of complex polypharmacy, excessive caffeine use, sleep deprivation, and possible opioid withdrawal.
Algorithm: Managing substance-induced manic disorder
CK: creatine kinase; CRP: C-reactive protein; CT: computed tomography; EEG: electroencephalogram; HIV: human immunodeficiency virus; MRI: magnetic resonance imaging; VDRL: venereal disease research laboratoryTable 2
DSM-IV-TR criteria for substance-induced mood disorder*
| A. A prominent and persistent disturbance in mood predominates in the clinical picture and is characterized by either (or both) of the following: 1. depressed mood or markedly diminished interest or pleasure in all, or almost all, activities 2. elevated, expansive, or irritable mood |
| B. There is evidence from the history, physical examination, or laboratory findings of: 1. the symptoms in Criterion A developed during, or within 1 month of, substance intoxication or withdrawal, or 2. medication use is etiologically related to the disturbance |
| C. The disturbance is not better accounted for by a mood disorder that is not substance-induced |
| D. The disturbance does not occur exclusively during the course of a delirium |
| E. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Minimal criteria are A plus B plus E |
| *Make this diagnosis only when mood symptoms are in excess of those usually associated with substance intoxication or substance withdrawal syndrome and when symptoms are sufficiently severe to warrant independent clinical attention |
| Source: Reference 9 |
TREATMENT: Escalating symptoms
While hospitalized, Mrs. P focuses solely on receiving pain medication. She does not know why she is in the hospital. She is easily distractible, intermittently intrusive, and disorganized and tangential in her thought process.
Two days after admission, her uncontrolled behavior escalates and she has marked psychomotor agitation. She is confused but remains oriented to time, place, and person. We start treatment with risperidone, 0.5 mg each morning and 1 mg at bedtime, because this agent is well tolerated, efficacious, and easily titrated to symptom response. Mrs. P’s symptoms improve, but she does not return to her reported baseline. Two days later, we increase risperidone to 1 mg every morning and 2 mg at bedtime. On the 6th day of hospitalization, Mrs. P is more organized and able to follow simple commands. She denies auditory or visual hallucinations. On the 10th day, she improves markedly and is back to her baseline level of functioning.
We perform psychological testing, including the Wechsler Adult Intelligence Scale (WAIS III) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Form A). The results show global neurocognitive deficits. Mrs. P’s intellectual skill is significantly below average, with verbal abilities reflecting functioning in the mildly retarded range. Nonverbal skills were stronger but still below average. Mrs. P’s capacity to learn and retain new information and to understand even modestly complex concepts is quite limited.
Because of Mrs. P’s long history of poly-substance abuse, inability to process information, and chronic back pain, we judge her to be at high risk for relapse. However, Mrs. P and her family are not interested in chemical dependence treatment.
This left us facing a difficult clinical situation. Mrs. P had a pattern of presenting to multiple physicians and eventually receiving narcotics. Her family provided transportation for her to these appointments but also was concerned about her drug use. With the patient and her family, we carefully outline Mrs. P’s treatment needs, including:
- medication monitoring by a psychiatrist after discharge
- a single, consistent primary care physician to manage her care
- a treatment plan shared by all clinicians involved in her care.
We review with Mrs. P and her family the benefits of behavioral approaches to chronic pain management. They agree to our recommendation that the family control Mrs. P’s medication supply. We discharge her on risperidone, 0.5 mg each morning and 1 mg at bedtime, and she is scheduled for follow-up with a local psychiatrist.
Related resource
- Krauthammer C, Klerman GL. Manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Citalopram • Celexa
- Desipramine • Norpramin
- Diazepam • Valium
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Vicodin, Lortab, others
- Lorazepam • Ativan
- Methadone • Dolophine, Methadose
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Propoxyphene • Darvon, Darvocet, others
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. De Fazio S, Gallelli L, De Siena A, et al. Role of CYP3A5 in abnormal clearance methadone. Ann Pharmacother. 2008;42(6):893-897.
2. Ropper AH, Brown RH. Adams and Victor’s principles of neurology. 8th ed. New York, NY: McGraw-Hill Professional; 2005.
3. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005;76(1):99-103.
4. Eccles R. Substitution of phenylephrine for pseudoephedrine as a nasal decongeststant. An illogical way to control methamphetamine abuse. Br J Clin Pharmacol. 2007;63(1):10-14.
5. Wilson H, Woods D. Pseudoephedrine causing mania-like symptoms. N Z Med J. 2002;115(1148):86.-
6. Dalton R. Mixed bipolar disorder precipitated by pseudoephedrine hydrochloride. South Med J. 1990;83(1):64-65.
7. Mansi IA, Huang J. Rhabdomyolysis in response to weight-loss herbal medicine. Am J Med Sci. 2004;327:356-357.
8. Sandhu RS, Como JJ, Scalea TS. Renal failure and exercise-induced rhabdomyolysis in patients taking performance-enhancing compounds. J Trauma. 2002;53:761-764.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
CASE: First-episode mania
Mrs. P, age 47, is brought to the emergency department (ED) because her family is concerned about her behavioral changes over the last week. Her husband reports that Mrs. P has become hyper-religious and talkative. She has been perseverating on numbers and dates and incessantly calling people. Mrs. P reports increased energy and decreased need for sleep. On examination, she has pressured speech. She has no psychiatric history; however, for the past year, she has been taking sertraline, 100 mg/d, and desipramine, 25 mg/d, which her primary care physician prescribed for unknown reasons.
Mrs. P has struggled with chronic back pain for years, but an MRI of her spine is negative. Her family strongly believes that for the past 3 years Mrs. P has been receiving too many medications from her pain management specialist. Six weeks before her current presentation, she was receiving methadone, 40 mg/d, hydrocodone, at least 20 mg/d, and tramadol, 400 mg/d in divided doses. She also was taking an unknown dose of at least 1 benzodiazepine.
Mrs. P’s husband notes she stopped taking methadone abruptly approximately 5 weeks ago. However, about 3 weeks ago, Mrs. P accidentally overdosed on opioids and was hospitalized for several days. Urine drug screen at the time was positive for acetaminophen, salicylate, propoxyphene, opiate, benzodiazepine, and tricyclic antidepressant.
Mrs. P’s medical history includes auditory nerve loss from birth; her mother had German measles (rubella). Mrs. P never learned American Sign Language. She underwent cochlear implant surgery 1 year ago and now has only mild difficulties speaking.
The authors’ observations
Manic symptoms are common in patients with comorbid medical disorders and present a diagnostic challenge. Obtaining an accurate history from the patient may be difficult. Such evaluations often require extensive investigation and collection of data from multiple sources, including:
- medical records
- family members
- patient observation.
Mrs. P’s history is marked by contradicting data from these sources. For example, her family says she stopped taking “pain medications” 5 weeks ago, but 2 weeks later her urine drug screen showed opioids.
Both illicit drugs and prescribed medications can precipitate manic symptoms. From medical records and drug testing, it was evident that Mrs. P had a history of medication abuse/overdose/misuse.
Mania also has been associated with substance withdrawal. Mrs. P allegedly stopped taking methadone 4 weeks before the onset of manic symptoms. Methadone is a synthetic opioid with a pharmacokinetic and pharmacodynamic profile that presents clinical challenges, including:
- large interindividual variability in methadone pharmacokinetics
- lack of reliable equianalgesic conversion ratio to and from other opioids
- potential for multiple drug interactions and complex pharmacodynamics.
An opioid’s half-life determines the onset and duration of withdrawal syndrome symptoms.1 Methadone metabolism is predominantly mediated by CYP3A4, CYP2B6, CYP2D6, and to some extent by CYP2C19.1 We performed genetic testing to help evaluate how Mrs. P metabolized medications. Mrs. P had a normal genotype for CYP2D6, which meant that she should process opioids at a normal rate; however, she was heterozygous for CYP2C19*2 polymorphism, so it is possible that methadone stayed in her system longer than average.
Evidence documenting methadone drug interactions is limited (Table 1).1 Mrs. P was taking sertraline and desipramine; both have potent effects via 2D6 inhibition that could increase plasma methadone concentration. Other evidence indicates that benzodiazepines and methadone may have synergistic interactions that could increase opioid sedation or respiratory depression.1
Table 1
Is a drug interaction with methadone causing Mrs. P’s mania?
| Medication class/agent | Effect on methadone level | Effect on methadone metabolism | Additional effects of interaction |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Fluvoxamine | Increase | Inhibition | Opioid toxicity |
| Fluoxetine | Increase | Inhibition | Torsades de pointes |
| Paroxetine | Increase | Inhibition | Decreased hepatic metabolism |
| Sertraline* | Increase | Autoinduction | Torsades de pointes |
| Citalopram | — | — | Torsades de pointes |
| Tricyclic antidepressants | |||
| Desipramine* | — | Inhibition | Increased desipramine levels/inhibition of desipramine metabolism |
| Amitriptyline | Increase methadone clearance | — | Torsades de pointes/prolonged QT interval |
| Anti-inflammatory drugs | |||
| NSAIDs* | — | — | Enhanced analgesia/opioid-sparing effect |
| Aspirin* | — | — | Paradoxical activation of platelet receptors |
| Benzodiazepines | |||
| Alprazolam | — | — | CNS depression/sedation/overdose |
| Diazepam* | — | Inhibition | Additive depressant effects |
| Opioid agonists | |||
| Dextromethorphan | — | Inhibition (not significant) | Increased side effects, especially sleepiness and drowsiness |
| Tramadol* | — | — | Well tolerated |
| Nicotine | Decrease | Can increase smoking rate | |
| *Medications taken by Mrs. P | |||
| NSAIDs: nonsteroidal anti-inflammatory drugs | |||
| Source: Reference 1 | |||
EVALUATION: Few clues
In our ED, Mrs. P’s urine drug abuse screen is positive for salicylate and benzodiazepine only. Findings from physical examination, vital signs, ECG, and chest radiography are within normal limits. Internal medicine consultation is unremarkable. Mrs. P’s laboratory investigation is notable for an elevated white blood cell count, but this normalizes over a week.
Mrs. P shows no evidence of infection and is normoglycemic. B12 and folate are within normal limits. Serum electrolytes, liver function testing, sensitive thyroid stimulating hormone, and C-reactive protein are within normal limits. Urinalysis is negative except for a small amount of hemoglobin. Her creatine kinase (CK) is in the upper normal range. Human immunodeficiency virus (HIV) and syphilis testing is negative. Ceruloplasmin level also is normal. Heavy metal screen is negative. Head MRI and CT from previous hospitalizations were unremarkable.
The authors’ observations
Our first step was to clarify Mrs. P’s diagnosis. In reviewing differential diagnoses, we considered:
- serotonin syndrome
- benzodiazepine withdrawal syndrome
- antidepressant-induced mania
- adrenergic toxicity
- malignant hyperthermia
- heat stroke
- infectious causes.
Our index of suspicion for serotonin syndrome was low because Mrs. P didn’t meet criteria required for diagnosis. Relevant signs and symptoms included confusion, elevated mood (major) and agitation, nervousness, insomnia, and low blood pressure (minor).
Based on concerns about medication interactions, we discontinued sertraline and desipramine. According to the patient’s sister, Mrs. P’s manic symptoms markedly responded to PRN doses of lorazepam. We prescribed lorazepam, 1 mg every 6 hours, and observed Mrs. P for signs and symptoms of benzodiazepine withdrawal.
HISTORY: OTC drug use
According to Mrs. P’s mother, after her daughter abruptly discontinued methadone, she began to have very strong headaches, which she treated with Excedrin or Excedrin Sinus. The mother said that 4 days before Mrs. P came to the ED, she found her daughter holding 4 tablets of Excedrin and an empty bottle. Unfortunately her mother was unable to say what type of Excedrin it was. When the treatment team asks Mrs. P how many pills she usually takes, she says she doesn’t know but usually until the pain stops.
The authors’ observations
Management of secondary mania should focus on treating the underlying condition (Algorithm). Neurology categorizes mania into 3 categories:2
- confusional-delirious states
- manic symptoms associated with focal or multifocal cerebral lesions
- affective disorders (manic-depressive and depressive psychoses).
Medical workup ruled out common secondary causes of psychosis. Collaborative information from relatives revealed no family history of mental illness.
Patients with hearing loss and deafness have been shown to be at increased risk for psychotic disorders compared with the general population. Severe sensory deficits early in Mrs. P’s life may have influenced the orderly development of neural connections in her sensory cortex and association areas.3 Mrs. P was deaf for the first 45 years of life. It could be hypothesized that her sensory deficits significantly influenced her ability to reality test. After receiving a cochlear implant, Mrs. P rapidly went from no auditory stimulation to marked improvement. This stressor might precipitate psychotic symptoms. However, her presentation seemed to be characterized more by manic symptoms or an agitated delirium. It also did not fit temporally with her presentation.
We begin to suspect that Mrs. P’s mania is substance-induced. Excedrin, an over-the-counter medication, contains aspirin and caffeine. Excedrin Sinus also contains phenylephrine. Amphetamines, caffeine, ephedrine, pseudoephedrine, and phenylpropanolamine have all been linked to manic-like psychotic episodes.
Concerns about the illicit conversion of pseudoephedrine into methamphetamine obliged pharmaceutical companies in the United States to switch product formulations to phenylephrine in 2005,4 although some “behind-the-counter” medications may contain pseudoephedrine. Phenylephrine is a relatively selective α1 agonist with weak α2 adrenoceptor agonist activity and low β agonist activity. It is very similar to pseudo-ephedrine, which is known to be implicated in the development of manic symptoms.5,6
Pseudoephedrine can raise CK levels and cause rhabdomyolysis.7,8 Mrs. P’s CK level was 176 (normal range 36 to 176 U/L) 4 days after her initial presentation, and she had a moderate amount of myoglobin in her urine. Her creatinine was normal. The patient was taking excessive amounts of caffeine and—if she was using Excedrin Sinus—pseudoephedrine or phenylephrine. We were unable to determine whether her Excedrin contained pseudoephedrine or phenylephrine. In addition, she was going through opioid withdrawal and reported problems with her sleep. There was also a question of Mrs. P’s unknown methadone use combined with its decreased clearance secondary to medication interactions.
While previously hospitalized for overdose, Mrs. P tested positive for propoxyphene. Excessive use of propoxyphene also can cause numerous adverse reactions. Some of that could have explained why Mrs. P’s presentation includes nervousness, CNS stimulation, excitement, insomnia, and restlessness.5
Based on multiple factors, we believe Mrs. P meets DSM-IV-TR criteria for substance-induced mood disorder (Table 2).9 This diagnosis is supported by Mrs. P’s history of complex polypharmacy, excessive caffeine use, sleep deprivation, and possible opioid withdrawal.
Algorithm: Managing substance-induced manic disorder
CK: creatine kinase; CRP: C-reactive protein; CT: computed tomography; EEG: electroencephalogram; HIV: human immunodeficiency virus; MRI: magnetic resonance imaging; VDRL: venereal disease research laboratoryTable 2
DSM-IV-TR criteria for substance-induced mood disorder*
| A. A prominent and persistent disturbance in mood predominates in the clinical picture and is characterized by either (or both) of the following: 1. depressed mood or markedly diminished interest or pleasure in all, or almost all, activities 2. elevated, expansive, or irritable mood |
| B. There is evidence from the history, physical examination, or laboratory findings of: 1. the symptoms in Criterion A developed during, or within 1 month of, substance intoxication or withdrawal, or 2. medication use is etiologically related to the disturbance |
| C. The disturbance is not better accounted for by a mood disorder that is not substance-induced |
| D. The disturbance does not occur exclusively during the course of a delirium |
| E. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Minimal criteria are A plus B plus E |
| *Make this diagnosis only when mood symptoms are in excess of those usually associated with substance intoxication or substance withdrawal syndrome and when symptoms are sufficiently severe to warrant independent clinical attention |
| Source: Reference 9 |
TREATMENT: Escalating symptoms
While hospitalized, Mrs. P focuses solely on receiving pain medication. She does not know why she is in the hospital. She is easily distractible, intermittently intrusive, and disorganized and tangential in her thought process.
Two days after admission, her uncontrolled behavior escalates and she has marked psychomotor agitation. She is confused but remains oriented to time, place, and person. We start treatment with risperidone, 0.5 mg each morning and 1 mg at bedtime, because this agent is well tolerated, efficacious, and easily titrated to symptom response. Mrs. P’s symptoms improve, but she does not return to her reported baseline. Two days later, we increase risperidone to 1 mg every morning and 2 mg at bedtime. On the 6th day of hospitalization, Mrs. P is more organized and able to follow simple commands. She denies auditory or visual hallucinations. On the 10th day, she improves markedly and is back to her baseline level of functioning.
We perform psychological testing, including the Wechsler Adult Intelligence Scale (WAIS III) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Form A). The results show global neurocognitive deficits. Mrs. P’s intellectual skill is significantly below average, with verbal abilities reflecting functioning in the mildly retarded range. Nonverbal skills were stronger but still below average. Mrs. P’s capacity to learn and retain new information and to understand even modestly complex concepts is quite limited.
Because of Mrs. P’s long history of poly-substance abuse, inability to process information, and chronic back pain, we judge her to be at high risk for relapse. However, Mrs. P and her family are not interested in chemical dependence treatment.
This left us facing a difficult clinical situation. Mrs. P had a pattern of presenting to multiple physicians and eventually receiving narcotics. Her family provided transportation for her to these appointments but also was concerned about her drug use. With the patient and her family, we carefully outline Mrs. P’s treatment needs, including:
- medication monitoring by a psychiatrist after discharge
- a single, consistent primary care physician to manage her care
- a treatment plan shared by all clinicians involved in her care.
We review with Mrs. P and her family the benefits of behavioral approaches to chronic pain management. They agree to our recommendation that the family control Mrs. P’s medication supply. We discharge her on risperidone, 0.5 mg each morning and 1 mg at bedtime, and she is scheduled for follow-up with a local psychiatrist.
Related resource
- Krauthammer C, Klerman GL. Manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Citalopram • Celexa
- Desipramine • Norpramin
- Diazepam • Valium
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Vicodin, Lortab, others
- Lorazepam • Ativan
- Methadone • Dolophine, Methadose
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Propoxyphene • Darvon, Darvocet, others
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: First-episode mania
Mrs. P, age 47, is brought to the emergency department (ED) because her family is concerned about her behavioral changes over the last week. Her husband reports that Mrs. P has become hyper-religious and talkative. She has been perseverating on numbers and dates and incessantly calling people. Mrs. P reports increased energy and decreased need for sleep. On examination, she has pressured speech. She has no psychiatric history; however, for the past year, she has been taking sertraline, 100 mg/d, and desipramine, 25 mg/d, which her primary care physician prescribed for unknown reasons.
Mrs. P has struggled with chronic back pain for years, but an MRI of her spine is negative. Her family strongly believes that for the past 3 years Mrs. P has been receiving too many medications from her pain management specialist. Six weeks before her current presentation, she was receiving methadone, 40 mg/d, hydrocodone, at least 20 mg/d, and tramadol, 400 mg/d in divided doses. She also was taking an unknown dose of at least 1 benzodiazepine.
Mrs. P’s husband notes she stopped taking methadone abruptly approximately 5 weeks ago. However, about 3 weeks ago, Mrs. P accidentally overdosed on opioids and was hospitalized for several days. Urine drug screen at the time was positive for acetaminophen, salicylate, propoxyphene, opiate, benzodiazepine, and tricyclic antidepressant.
Mrs. P’s medical history includes auditory nerve loss from birth; her mother had German measles (rubella). Mrs. P never learned American Sign Language. She underwent cochlear implant surgery 1 year ago and now has only mild difficulties speaking.
The authors’ observations
Manic symptoms are common in patients with comorbid medical disorders and present a diagnostic challenge. Obtaining an accurate history from the patient may be difficult. Such evaluations often require extensive investigation and collection of data from multiple sources, including:
- medical records
- family members
- patient observation.
Mrs. P’s history is marked by contradicting data from these sources. For example, her family says she stopped taking “pain medications” 5 weeks ago, but 2 weeks later her urine drug screen showed opioids.
Both illicit drugs and prescribed medications can precipitate manic symptoms. From medical records and drug testing, it was evident that Mrs. P had a history of medication abuse/overdose/misuse.
Mania also has been associated with substance withdrawal. Mrs. P allegedly stopped taking methadone 4 weeks before the onset of manic symptoms. Methadone is a synthetic opioid with a pharmacokinetic and pharmacodynamic profile that presents clinical challenges, including:
- large interindividual variability in methadone pharmacokinetics
- lack of reliable equianalgesic conversion ratio to and from other opioids
- potential for multiple drug interactions and complex pharmacodynamics.
An opioid’s half-life determines the onset and duration of withdrawal syndrome symptoms.1 Methadone metabolism is predominantly mediated by CYP3A4, CYP2B6, CYP2D6, and to some extent by CYP2C19.1 We performed genetic testing to help evaluate how Mrs. P metabolized medications. Mrs. P had a normal genotype for CYP2D6, which meant that she should process opioids at a normal rate; however, she was heterozygous for CYP2C19*2 polymorphism, so it is possible that methadone stayed in her system longer than average.
Evidence documenting methadone drug interactions is limited (Table 1).1 Mrs. P was taking sertraline and desipramine; both have potent effects via 2D6 inhibition that could increase plasma methadone concentration. Other evidence indicates that benzodiazepines and methadone may have synergistic interactions that could increase opioid sedation or respiratory depression.1
Table 1
Is a drug interaction with methadone causing Mrs. P’s mania?
| Medication class/agent | Effect on methadone level | Effect on methadone metabolism | Additional effects of interaction |
|---|---|---|---|
| Selective serotonin reuptake inhibitors | |||
| Fluvoxamine | Increase | Inhibition | Opioid toxicity |
| Fluoxetine | Increase | Inhibition | Torsades de pointes |
| Paroxetine | Increase | Inhibition | Decreased hepatic metabolism |
| Sertraline* | Increase | Autoinduction | Torsades de pointes |
| Citalopram | — | — | Torsades de pointes |
| Tricyclic antidepressants | |||
| Desipramine* | — | Inhibition | Increased desipramine levels/inhibition of desipramine metabolism |
| Amitriptyline | Increase methadone clearance | — | Torsades de pointes/prolonged QT interval |
| Anti-inflammatory drugs | |||
| NSAIDs* | — | — | Enhanced analgesia/opioid-sparing effect |
| Aspirin* | — | — | Paradoxical activation of platelet receptors |
| Benzodiazepines | |||
| Alprazolam | — | — | CNS depression/sedation/overdose |
| Diazepam* | — | Inhibition | Additive depressant effects |
| Opioid agonists | |||
| Dextromethorphan | — | Inhibition (not significant) | Increased side effects, especially sleepiness and drowsiness |
| Tramadol* | — | — | Well tolerated |
| Nicotine | Decrease | Can increase smoking rate | |
| *Medications taken by Mrs. P | |||
| NSAIDs: nonsteroidal anti-inflammatory drugs | |||
| Source: Reference 1 | |||
EVALUATION: Few clues
In our ED, Mrs. P’s urine drug abuse screen is positive for salicylate and benzodiazepine only. Findings from physical examination, vital signs, ECG, and chest radiography are within normal limits. Internal medicine consultation is unremarkable. Mrs. P’s laboratory investigation is notable for an elevated white blood cell count, but this normalizes over a week.
Mrs. P shows no evidence of infection and is normoglycemic. B12 and folate are within normal limits. Serum electrolytes, liver function testing, sensitive thyroid stimulating hormone, and C-reactive protein are within normal limits. Urinalysis is negative except for a small amount of hemoglobin. Her creatine kinase (CK) is in the upper normal range. Human immunodeficiency virus (HIV) and syphilis testing is negative. Ceruloplasmin level also is normal. Heavy metal screen is negative. Head MRI and CT from previous hospitalizations were unremarkable.
The authors’ observations
Our first step was to clarify Mrs. P’s diagnosis. In reviewing differential diagnoses, we considered:
- serotonin syndrome
- benzodiazepine withdrawal syndrome
- antidepressant-induced mania
- adrenergic toxicity
- malignant hyperthermia
- heat stroke
- infectious causes.
Our index of suspicion for serotonin syndrome was low because Mrs. P didn’t meet criteria required for diagnosis. Relevant signs and symptoms included confusion, elevated mood (major) and agitation, nervousness, insomnia, and low blood pressure (minor).
Based on concerns about medication interactions, we discontinued sertraline and desipramine. According to the patient’s sister, Mrs. P’s manic symptoms markedly responded to PRN doses of lorazepam. We prescribed lorazepam, 1 mg every 6 hours, and observed Mrs. P for signs and symptoms of benzodiazepine withdrawal.
HISTORY: OTC drug use
According to Mrs. P’s mother, after her daughter abruptly discontinued methadone, she began to have very strong headaches, which she treated with Excedrin or Excedrin Sinus. The mother said that 4 days before Mrs. P came to the ED, she found her daughter holding 4 tablets of Excedrin and an empty bottle. Unfortunately her mother was unable to say what type of Excedrin it was. When the treatment team asks Mrs. P how many pills she usually takes, she says she doesn’t know but usually until the pain stops.
The authors’ observations
Management of secondary mania should focus on treating the underlying condition (Algorithm). Neurology categorizes mania into 3 categories:2
- confusional-delirious states
- manic symptoms associated with focal or multifocal cerebral lesions
- affective disorders (manic-depressive and depressive psychoses).
Medical workup ruled out common secondary causes of psychosis. Collaborative information from relatives revealed no family history of mental illness.
Patients with hearing loss and deafness have been shown to be at increased risk for psychotic disorders compared with the general population. Severe sensory deficits early in Mrs. P’s life may have influenced the orderly development of neural connections in her sensory cortex and association areas.3 Mrs. P was deaf for the first 45 years of life. It could be hypothesized that her sensory deficits significantly influenced her ability to reality test. After receiving a cochlear implant, Mrs. P rapidly went from no auditory stimulation to marked improvement. This stressor might precipitate psychotic symptoms. However, her presentation seemed to be characterized more by manic symptoms or an agitated delirium. It also did not fit temporally with her presentation.
We begin to suspect that Mrs. P’s mania is substance-induced. Excedrin, an over-the-counter medication, contains aspirin and caffeine. Excedrin Sinus also contains phenylephrine. Amphetamines, caffeine, ephedrine, pseudoephedrine, and phenylpropanolamine have all been linked to manic-like psychotic episodes.
Concerns about the illicit conversion of pseudoephedrine into methamphetamine obliged pharmaceutical companies in the United States to switch product formulations to phenylephrine in 2005,4 although some “behind-the-counter” medications may contain pseudoephedrine. Phenylephrine is a relatively selective α1 agonist with weak α2 adrenoceptor agonist activity and low β agonist activity. It is very similar to pseudo-ephedrine, which is known to be implicated in the development of manic symptoms.5,6
Pseudoephedrine can raise CK levels and cause rhabdomyolysis.7,8 Mrs. P’s CK level was 176 (normal range 36 to 176 U/L) 4 days after her initial presentation, and she had a moderate amount of myoglobin in her urine. Her creatinine was normal. The patient was taking excessive amounts of caffeine and—if she was using Excedrin Sinus—pseudoephedrine or phenylephrine. We were unable to determine whether her Excedrin contained pseudoephedrine or phenylephrine. In addition, she was going through opioid withdrawal and reported problems with her sleep. There was also a question of Mrs. P’s unknown methadone use combined with its decreased clearance secondary to medication interactions.
While previously hospitalized for overdose, Mrs. P tested positive for propoxyphene. Excessive use of propoxyphene also can cause numerous adverse reactions. Some of that could have explained why Mrs. P’s presentation includes nervousness, CNS stimulation, excitement, insomnia, and restlessness.5
Based on multiple factors, we believe Mrs. P meets DSM-IV-TR criteria for substance-induced mood disorder (Table 2).9 This diagnosis is supported by Mrs. P’s history of complex polypharmacy, excessive caffeine use, sleep deprivation, and possible opioid withdrawal.
Algorithm: Managing substance-induced manic disorder
CK: creatine kinase; CRP: C-reactive protein; CT: computed tomography; EEG: electroencephalogram; HIV: human immunodeficiency virus; MRI: magnetic resonance imaging; VDRL: venereal disease research laboratoryTable 2
DSM-IV-TR criteria for substance-induced mood disorder*
| A. A prominent and persistent disturbance in mood predominates in the clinical picture and is characterized by either (or both) of the following: 1. depressed mood or markedly diminished interest or pleasure in all, or almost all, activities 2. elevated, expansive, or irritable mood |
| B. There is evidence from the history, physical examination, or laboratory findings of: 1. the symptoms in Criterion A developed during, or within 1 month of, substance intoxication or withdrawal, or 2. medication use is etiologically related to the disturbance |
| C. The disturbance is not better accounted for by a mood disorder that is not substance-induced |
| D. The disturbance does not occur exclusively during the course of a delirium |
| E. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| Minimal criteria are A plus B plus E |
| *Make this diagnosis only when mood symptoms are in excess of those usually associated with substance intoxication or substance withdrawal syndrome and when symptoms are sufficiently severe to warrant independent clinical attention |
| Source: Reference 9 |
TREATMENT: Escalating symptoms
While hospitalized, Mrs. P focuses solely on receiving pain medication. She does not know why she is in the hospital. She is easily distractible, intermittently intrusive, and disorganized and tangential in her thought process.
Two days after admission, her uncontrolled behavior escalates and she has marked psychomotor agitation. She is confused but remains oriented to time, place, and person. We start treatment with risperidone, 0.5 mg each morning and 1 mg at bedtime, because this agent is well tolerated, efficacious, and easily titrated to symptom response. Mrs. P’s symptoms improve, but she does not return to her reported baseline. Two days later, we increase risperidone to 1 mg every morning and 2 mg at bedtime. On the 6th day of hospitalization, Mrs. P is more organized and able to follow simple commands. She denies auditory or visual hallucinations. On the 10th day, she improves markedly and is back to her baseline level of functioning.
We perform psychological testing, including the Wechsler Adult Intelligence Scale (WAIS III) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Form A). The results show global neurocognitive deficits. Mrs. P’s intellectual skill is significantly below average, with verbal abilities reflecting functioning in the mildly retarded range. Nonverbal skills were stronger but still below average. Mrs. P’s capacity to learn and retain new information and to understand even modestly complex concepts is quite limited.
Because of Mrs. P’s long history of poly-substance abuse, inability to process information, and chronic back pain, we judge her to be at high risk for relapse. However, Mrs. P and her family are not interested in chemical dependence treatment.
This left us facing a difficult clinical situation. Mrs. P had a pattern of presenting to multiple physicians and eventually receiving narcotics. Her family provided transportation for her to these appointments but also was concerned about her drug use. With the patient and her family, we carefully outline Mrs. P’s treatment needs, including:
- medication monitoring by a psychiatrist after discharge
- a single, consistent primary care physician to manage her care
- a treatment plan shared by all clinicians involved in her care.
We review with Mrs. P and her family the benefits of behavioral approaches to chronic pain management. They agree to our recommendation that the family control Mrs. P’s medication supply. We discharge her on risperidone, 0.5 mg each morning and 1 mg at bedtime, and she is scheduled for follow-up with a local psychiatrist.
Related resource
- Krauthammer C, Klerman GL. Manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Citalopram • Celexa
- Desipramine • Norpramin
- Diazepam • Valium
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Vicodin, Lortab, others
- Lorazepam • Ativan
- Methadone • Dolophine, Methadose
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Propoxyphene • Darvon, Darvocet, others
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. De Fazio S, Gallelli L, De Siena A, et al. Role of CYP3A5 in abnormal clearance methadone. Ann Pharmacother. 2008;42(6):893-897.
2. Ropper AH, Brown RH. Adams and Victor’s principles of neurology. 8th ed. New York, NY: McGraw-Hill Professional; 2005.
3. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005;76(1):99-103.
4. Eccles R. Substitution of phenylephrine for pseudoephedrine as a nasal decongeststant. An illogical way to control methamphetamine abuse. Br J Clin Pharmacol. 2007;63(1):10-14.
5. Wilson H, Woods D. Pseudoephedrine causing mania-like symptoms. N Z Med J. 2002;115(1148):86.-
6. Dalton R. Mixed bipolar disorder precipitated by pseudoephedrine hydrochloride. South Med J. 1990;83(1):64-65.
7. Mansi IA, Huang J. Rhabdomyolysis in response to weight-loss herbal medicine. Am J Med Sci. 2004;327:356-357.
8. Sandhu RS, Como JJ, Scalea TS. Renal failure and exercise-induced rhabdomyolysis in patients taking performance-enhancing compounds. J Trauma. 2002;53:761-764.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
1. De Fazio S, Gallelli L, De Siena A, et al. Role of CYP3A5 in abnormal clearance methadone. Ann Pharmacother. 2008;42(6):893-897.
2. Ropper AH, Brown RH. Adams and Victor’s principles of neurology. 8th ed. New York, NY: McGraw-Hill Professional; 2005.
3. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005;76(1):99-103.
4. Eccles R. Substitution of phenylephrine for pseudoephedrine as a nasal decongeststant. An illogical way to control methamphetamine abuse. Br J Clin Pharmacol. 2007;63(1):10-14.
5. Wilson H, Woods D. Pseudoephedrine causing mania-like symptoms. N Z Med J. 2002;115(1148):86.-
6. Dalton R. Mixed bipolar disorder precipitated by pseudoephedrine hydrochloride. South Med J. 1990;83(1):64-65.
7. Mansi IA, Huang J. Rhabdomyolysis in response to weight-loss herbal medicine. Am J Med Sci. 2004;327:356-357.
8. Sandhu RS, Como JJ, Scalea TS. Renal failure and exercise-induced rhabdomyolysis in patients taking performance-enhancing compounds. J Trauma. 2002;53:761-764.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
How to select pharmacologic treatments to manage recidivism risk in sex offenders
Sex offenders traditionally are managed by the criminal justice system, but psychiatrists are frequently called on to assess and treat these individuals. Part of the reason is the overlap of paraphilias (disorders of sexual preference) and sexual offending. Many sexual offenders do not meet DSM criteria for paraphilias,1 however, and individuals with paraphilias do not necessarily commit offenses or come into contact with the legal system.
As clinicians, we may need to assess and treat a wide range of sexual issues, from persons with paraphilias who are self-referred and have no legal involvement, to recurrent sexual offenders who are at a high risk of repeat offending. Successfully managing sex offenders includes psychological and pharmacologic interventions and possibly incarceration and post-incarceration surveillance. This article focuses on pharmacologic interventions for male sexual offenders.
Reducing sexual drive
Sex offending likely is the result of a complex interplay of environment and psychological and biologic factors. The biology of sexual function provides numerous targets for pharmacologic intervention, including:2
- endocrine factors, such as testosterone
- neurotransmitters, such as serotonin.
The use of pharmacologic treatments for sex offenders is off-label, and evidence is limited. In general, pharmacologic treatments are geared toward reducing sexual drive through nonhormonal or hormonal means (Table 1).3-5
Table 1: Pharmacologic treatment of male sex offenders: A risk-based approach
*Not available in the United States
†Some authors suggest administering this dosage once every 2 weeks
CPA: cyproterone acetate; GNRH: gonadotropin-releasing hormone; IM: intramuscular; MPA: medroxyprogesterone acetate; SSRI: selective serotonin reuptake inhibitor
Source: References 3-5
Nonhormonal treatments
SSRIs. Selective serotonin reuptake inhibitors act by blocking serotonin reuptake in the synaptic cleft. Soon after the first SSRIs were approved in 1988, reports appeared of SSRIs interfering with sexual functioning.6 This side effect quickly was exploited to assist the treatment of sexual offenders.7
The mechanism of action may include:8
- direct effects, such as general inhibition of sexual activity, reduced impulsiveness, and an effect on the hypothesized “obsessive-compulsive” nature of paraphilias9
- indirect reduction of testosterone.
A growing body of literature supports SSRIs’ effectiveness in treating paraphilias and sexual offenders. Greenberg7 reviewed case studies and open drug trials of nearly 200 patients receiving fluoxetine, fluvoxamine, or sertraline. Most studies showed response rates of 50% to 90%.10 Positive effects included decreases in:
- paraphiliac fantasies, urges, and sexual acts
- masturbation
- hypersexual activity.
Some studies reported a preferential decrease in paraphiliac interests with an increase in conventional sexual interests, although this may be related to placebo or halo effects—patients may have reported an increase in conventional interests because they noticed a decrease in paraphiliac interests. Negative side effects included decreased sexual desires, delayed ejaculation, decreased libido, and anorgasmia.
Adi et al11 completed a more rigorous literature evaluation that included 9 studies with a total of 225 patients receiving fluoxetine, fluvoxamine, sertraline, or paroxetine. Eight studies showed benefits; however, Adi noted that this preliminary evidence was “far from conclusive.”
SSRIs generally are well tolerated and may be more appealing to patients than the “chemical castration” of hormonal treatments. Dosing is similar to that used in depression or obsessive-compulsive disorder. Although most patients notice beneficial effects in 2 to 4 weeks, some notice the effect nearly immediately.
Naltrexone. An opioid antagonist thought to affect the CNS processes of pleasure and pain, naltrexone has been used to treat alcohol dependence and pathologic gambling. A few case studies12-14 and 1 study of 21 adolescent sex offenders15 have shown benefits in treating sexual offenders or paraphiliacs. Benefits were seen at 50 mg/d, with suggested dosing of 100 to 200 mg/d. Because data are very limited, consider naltrexone only on an individual basis or as a possible adjunctive treatment.
Psychostimulants. Methylphenidate was added to augment SSRI treatment in a study of 26 men with paraphilias or paraphilia-related disorders.16 Results included further significant decreases in total sexual outlets (orgasms per week) and average time spent per day in paraphilia and paraphilia-related behavior. These gains appeared to be independent of the presence of attention-deficit/hyperactivity disorder.
Again, because data are very limited, consider this strategy only on an individual basis or as a possible adjunctive treatment. Because sexual offenders have high rates of substance abuse,17 consider the potential for stimulant abuse.
Hormonal treatments
Because testosterone is required for healthy bone metabolism, the antiandrogen medications used in hormonal treatment can cause osteoporosis.18,19 Therefore, long-term antiandrogen treatment should include bone scans to monitor for osteopenia and osteoporosis. Some authors have suggested that monthly doses of 25 to 50 mg of testosterone could minimize this risk.20 Bisphosphonates, vitamin D, and calcium supplements at osteoporosis treatment levels might be helpful.18 Other common side effects of antiandrogen medications are listed in Table 2.
Finasteride is approved for treating benign prostatic hyperplasia and androgenetic alopecia. It works by preventing conversion of testosterone to dihydrotestosterone (DHT) by the type II isoenzyme.21 Serum DHT contributes to male sexual behavior and predicts frequency of orgasms in healthy volunteers.22 Although there have been no studies of finasteride in sex offenders, it may be more acceptable to patients than other hormonal treatments and have a theoretical benefit in reducing sexual drive. Clinically, some patients describe increased control over urges without substantial side effects. Because there is no evidence supporting finasteride use in sex offenders, consider this medication only on an individual basis or as a possible adjunctive treatment.
Cyproterone acetate (CPA) is a synthetic steroid that blocks androgen receptors.3,23 Some evidence supports its use in treating sex offenders,24 although this agent is not available in the United States. For more information about CPA, see Box.
Medroxyprogesterone acetate (MPA), a derivative of progesterone, lowers serum testosterone by inhibiting its production through reducing pituitary luteinizing hormone (LH).24 The typical dose range for use in sex offenders is 100 to 600 mg/d orally or 100 to 700 mg IM every week,3 although some authors suggest giving similar doses every 2 weeks.4
Side effects of MPA include hypersomnia; neurasthenia; weight gain; hot flashes; gynecomastia; increased scalp hair; and decreased erections, ejaculate volume, spermatogenesis, and body and facial hair. The drug decreases testosterone levels by about 50%. Positive effects include reduced interest in and energy spent on pursuing sexual goals, but preservation of nondeviant sexual arousal.4
MPA has been shown to effectively decrease deviant sexual arousal and recidivism. In a study of 100 patients receiving MPA (average 250 mg IM every 2 weeks) for an average of 3 years, only 1 re-offended while taking MPA.4
In a 5-year follow-up study25 of 275 men, subjects were classified into high risk/treatment with MPA, 200 to 400 mg IM every 2 weeks, and low risk/nontreatment groups. A portion of the high risk/treatment group did not receive MPA. No sexual re-offenses occurred among high-risk subjects who received MPA, whereas the recidivism rate was 18% among high-risk subjects who did not receive MPA. Subjects in the low risk/nontreatment group had a recidivism rate of 15%, which suggests the need for more liberal use of antiandrogens. One major confounding factor was that subjects in the high risk/treatment group had to report every 2 weeks for injections; this may have resulted in closer follow-up, monitoring, and support, which may have contributed to lower recidivism.
Gonadotropin-releasing hormone (GNRH) agonists. The terms gonadotropin-releasing hormone and luteinizing-releasing hormone are used interchangeably. Most body testosterone is produced and released by Leydig cells in the testes in response to stimulation by LH released by the pituitary gland. LH release is controlled by the pulsatile release of GNRH from the hypothalamus. GNRH agonists are high-potency analogs of GNRH that work by causing an initial surge of LH followed by down-regulation of gonadotroph cells, a drop in LH, and a drop in testosterone to castration levels.
The GNRH analogs leuprolide, goserelin, and triptorelin are used to treat paraphiliacs and sexual offenders.20 Leuprolide typically is dosed at 7.5 mg IM every month, 22.5 mg IM every 3 months, or 30 mg IM every 4 months. Goserelin is provided as a subcutaneous implant/depot injected as 3.6 mg every month or 10.8 mg every 3 months.
Triptorelin is FDA-approved as treatment for advanced prostate cancer. Triptorelin is given in depot formulation as 3.75 mg IM every month or in a long-acting form as 11.25 mg IM every 3 months.
When starting these medications, an initial surge of LH and testosterone can last up to 3 weeks.26 Theoretically, this could worsen paraphiliac interests. Many practitioners will use a testosterone blocker such as flutamide, 250 mg tid, for the initial weeks of treatment.
Side effects of the GNRH agonists are similar. Most patients initially experience hot flashes. A systemic literature review27 reported:
- weight gain
- perspiration
- gynecomastia
- urinary incontinence
- hot flashes
- decreased growth of facial and body hair
- asthenia
- erectile failure
- muscle tenderness
- frequent bone demineralization.
Rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported, possibly related to an underlying pituitary adenoma.28
In a literature review that totalled 118 patients,27 GNRH agonists significantly decreased erections, ejaculations, paraphiliac fantasies, and paraphiliac behavior. Patients also reported feeling more relaxed, and recidivism rates were low. Some patients who failed to respond to CPA and MPA responded to GNRH agonists. Subsequent studies found similar results.29,30
Cyproterone acetate (CPA), which is available in Canada and Europe, is a synthetic steroid with structure similar to that of progesterone. CPA blocks androgen receptors, which makes it antiandrogenic, progestational, and antigonadotrophic.a
Dosages for paraphilias range from 100 to 500 mg/d orally or 100 to 600 mg intramuscularly every 1 or 2 weeks. b
Once stabilized, some individuals can be maintained on very low doses, such as 12.5 to 50 mg/d. a Lower doses may be appropriate for individuals who are self-motivated for treatment and who reliably report their sexual interests.
CPA reduces testosterone by approximately 50%. Side effects include decreases in:
- erections
- ejaculate volume
- spermatogenesis.
Some patients experience hypersomnia, neurasthenia, weight gain, hot flashes, decreased body and facial hair, and increased scalp hair. About 20% of patients may experience gynecomastia, particularly at higher doses.
Evidence shows CPA reduces sexual arousal, activity, fantasy, and masturbation.a In a systematic review of 7 studies that included 127 patients, the re-offense rate averaged 6%.c This is significantly lower than the expected recidivism of approximately 13.4%.d
References
a. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
b. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
c. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
d. Hanson R, Bussiere MT. Predicting relapse: A meta-analysis of sexual offender recidivism studies. J Consult Clin Psychology. 1998;66:348-362.
Table 2
Common side effects of antiandrogen therapy
| Depression |
| Erectile dysfunction |
| Fatigue |
| Gynecomastia |
| Hot flashes |
| Hypertension |
| Low libido |
| Myalgia |
| Osteopenia |
| Osteoporosis |
| Sweating |
| Thromboembolism |
| Weight gain |
Monitoring
Laboratory investigations are recommended to monitor for side effects of antiandrogen medications (Table 3).19,27,31 Medical contraindications to rule out before initiating antiandrogen medications include:
- thromboembolic diseases
- liver disease
- bone demineralization disorders
- hypersensitivity to the drug.
Measure prolactin to rule out pituitary adenomas. Monitor serum testosterone because some patients will not experience testosterone suppression from GNRH agonists or other antiandrogens. Noncompliant patients could potentially reverse the effects of MPA and GNRH agonists by taking exogenous testosterone.
Table 3
Monitoring patients receiving antiandrogen medications
| Pre-therapy workup | Periodic monitoring |
|---|---|
| Endocrinology or internist consultation Bone scan Weight Blood pressure Electrocardiogram CBC, renal function, liver function, fasting glucose, and lipids LH, FSH, testosterone, prolactin | Monthly: testosterone for the first 6 months Every 6 months: testosterone, LH, FSH, prolactin, CBC, renal function, liver function, fasting glucose and lipids, weight, blood pressure Yearly: bone scan |
| CBC: complete blood count; FSH: follicle-stimulating hormone; LH: luteinizing hormone | |
| Source: References 19,27,31 | |
Medication selection
The goals of pharmacologic treatment of sex offenders are to:
- reduce sexual offending and victimization
- suppress sexual drive to a controllable level
- possibly preferentially eliminate deviant arousal/thoughts
- allow normal sexual relationships.
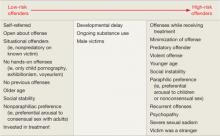
Gauging risk. In determining which pharmacologic treatment to offer a patient, first evaluate the individual’s risk for recidivism. Actuarial scales32,33 suggest that recidivism risk can be categorized, based on clinical factors (Table 4).4,25,34
In addition to statistical risk factors, several other factors affect medication selection. Self-referred individuals may be more reliable in taking oral medications than those referred by the courts. A developmentally delayed individual may be a poor candidate for oral medication, unless he resides in a group home setting where compliance can be assured. Efficacy also guides medication choice. Finally, some patients will be legally required to provide proof of compliance, which only IM medications provide.
Treatment. Based on clinical experience and available literature, Bradford5 created an algorithm to help clinicians select appropriate pharmacologic interventions. Although it has not been validated, this algorithm provides a reasonable starting place.
In general, start treatment with an SSRI for low-risk individuals (Table 1).3-5 If this strategy is insufficient, consider augmentation with methylphenidate, naltrexone, or finasteride.
The next step would be to add oral MPA or CPA, 50 mg/d, which would partially inhibit testosterone and may allow some normal sexual functioning.4,23 Higher-dose oral MPA or CPA would be tried next. For higher-risk individuals or treatment failures, IM MPA or CPA would be offered next, followed by a GNRH agonist. For individuals at highest risk for re-offending, combinations of agents may be indicated.
This simple strategy is appealing, but in reality, treatment should be individualized. Choose medications based on the patient’s risk, wishes, and the previously mentioned clinical factors.
Table 4: Will my patient commit another sexual offense? Evaluating risk
Source: References 4,25,34Related resources
- Krueger RB, Kaplan MS. The paraphilic and hypersexual disorders: an overview. J Psychiatr Pract. 2001;7:391-403.
- Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Pract. 2002;8:21-32.
- Association for the Treatment of Sexual Abusers. www.atsa.com.
Drug brand names
- Cyproterone acetate • Androcur
- Finasteride • Propecia, Proscar
- Fluoxetine • Prozac
- Flutamide • Eulexin
- Fluvoxamine • Luvox
- Goserelin • Zoladex
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone acetate • Depo-Provera, Provera
- Methylphenidate • Ritalin
- Naltrexone • ReVia
- Paroxetine • Paxil
- Sertraline • Zoloft
- Triptorelin • Trelstar Depot
Disclosure
Dr. Booth reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. First MB, Halon RL. Use of DSM paraphilia diagnoses in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
2. Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012-1030.
3. Bezchlibnyk-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs. 16th ed. Seattle, WA: Hogrefe & Huber Publishers; 2006:222–225.
4. Maletzky B. The use of medroxyprogesterone acetate to assist in the treatment of sexual offenders. Annals of Sex Research. 1991;4:117-129.
5. Bradford JM. The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behaviour. Can J Psychiatry. 2001;46(1):26-34.
6. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470.
7. Greenberg DM, Bradford JMW. Treatment of the paraphilic disorders: a review of the role of the selective serotonin reuptake inhibitors. Sex Abuse. 1997;9(4):349-360.
8. Hill A, Briken P, Kraus C, et al. Differential pharmacological treatment of paraphilias and sex offenders. Int J Offender Ther Comp Criminol. 2003;47(4):407-421.
9. Bradford JMW. The paraphilias, obsessive compulsive spectrum disorder, and the treatment of sexually deviant behaviour. Psychiatr Q. 1999;70(3):209-219.
10. Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Prac. 2002;8(1):21-32.
11. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost-consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
12. Raymond NC, Grant JE, Kim SW, et al. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharm. 2002;17(4):201-205.
13. Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Ann Clin Psychiatry. 2001;13(4):229-231.
14. Sandyk R. Naltrexone suppresses abnormal sexual behavior in Tourette’s syndrome. Int J Neurosci. 1988;43(1-2):107-110.
15. Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982-986.
16. Kafka M, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
17. Langstrom N, Sjostedt G, Grann M. Psychiatric disorders and recidivism in sexual offenders. Sex Abuse. 2004;16(2):139-150.
18. Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 suppl 1):79-85.
19. Grasswick LJ, Bradford JM. Osteoporosis associated with the treatment of paraphilias: a clinical review of seven case reports. J Forensic Sci. 2003;48:849-855.
20. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
21. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
22. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
23. Bradford JMW, Pawlak A. Double-blind placebo crossover study of cyproterone acetate in the treatment of the paraphilias. Arch Sex Behav. 1993;22(5):383-402.
24. Meyer WJ, Cole CM. Physical and chemical castration of sex offenders: a review. J Offender Rehab. 1997;25(3/4):1-18.
25. Maletzky BM, Tolan A, McFarland B. The Oregon depoProvera program: a five-year follow-up. Sex Abuse. 2006;18:303-316.
26. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001-1006.
27. Briken P, Hill A, Berner W. Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone–releasing hormone: a systematic review. J Clin Psychiatry. 2003;64:890-897.
28. Blaut K, Winiewski P, Syrenicz A, et al. Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett. 2006;27(5):569-572.
29. Schober JM, Kuhn PJ, Kovacs P, et al. Leuprolide acetate suppresses pedophilic urges and arousability. Arch Sex Behav. 2005;34(6):691-705.
30. Ösler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338:416-422.
31. Reilly DR, Delva NJ, Hudson RW. Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia. Can J Psychiatry. 2000;45:559-563.
32. Quinsey VL, Harris GT, Rice ME, et al. Violent offenders: appraising and managing risk. Washington, DC: American Psychological Association; 1998.
33. Epperson DL, Kaul JD, Huot SJ, et al. Minnesota Sex Offender Screening Tool–revised (MnSOST-R). St. Paul, MN: Minnesota Department of Corrections; 1998.
34. Bradford JMW. The treatment of sexual deviation using a pharmacological approach. J Sex Res. 2000;37(3):248-257.
Sex offenders traditionally are managed by the criminal justice system, but psychiatrists are frequently called on to assess and treat these individuals. Part of the reason is the overlap of paraphilias (disorders of sexual preference) and sexual offending. Many sexual offenders do not meet DSM criteria for paraphilias,1 however, and individuals with paraphilias do not necessarily commit offenses or come into contact with the legal system.
As clinicians, we may need to assess and treat a wide range of sexual issues, from persons with paraphilias who are self-referred and have no legal involvement, to recurrent sexual offenders who are at a high risk of repeat offending. Successfully managing sex offenders includes psychological and pharmacologic interventions and possibly incarceration and post-incarceration surveillance. This article focuses on pharmacologic interventions for male sexual offenders.
Reducing sexual drive
Sex offending likely is the result of a complex interplay of environment and psychological and biologic factors. The biology of sexual function provides numerous targets for pharmacologic intervention, including:2
- endocrine factors, such as testosterone
- neurotransmitters, such as serotonin.
The use of pharmacologic treatments for sex offenders is off-label, and evidence is limited. In general, pharmacologic treatments are geared toward reducing sexual drive through nonhormonal or hormonal means (Table 1).3-5
Table 1: Pharmacologic treatment of male sex offenders: A risk-based approach
*Not available in the United States
†Some authors suggest administering this dosage once every 2 weeks
CPA: cyproterone acetate; GNRH: gonadotropin-releasing hormone; IM: intramuscular; MPA: medroxyprogesterone acetate; SSRI: selective serotonin reuptake inhibitor
Source: References 3-5
Nonhormonal treatments
SSRIs. Selective serotonin reuptake inhibitors act by blocking serotonin reuptake in the synaptic cleft. Soon after the first SSRIs were approved in 1988, reports appeared of SSRIs interfering with sexual functioning.6 This side effect quickly was exploited to assist the treatment of sexual offenders.7
The mechanism of action may include:8
- direct effects, such as general inhibition of sexual activity, reduced impulsiveness, and an effect on the hypothesized “obsessive-compulsive” nature of paraphilias9
- indirect reduction of testosterone.
A growing body of literature supports SSRIs’ effectiveness in treating paraphilias and sexual offenders. Greenberg7 reviewed case studies and open drug trials of nearly 200 patients receiving fluoxetine, fluvoxamine, or sertraline. Most studies showed response rates of 50% to 90%.10 Positive effects included decreases in:
- paraphiliac fantasies, urges, and sexual acts
- masturbation
- hypersexual activity.
Some studies reported a preferential decrease in paraphiliac interests with an increase in conventional sexual interests, although this may be related to placebo or halo effects—patients may have reported an increase in conventional interests because they noticed a decrease in paraphiliac interests. Negative side effects included decreased sexual desires, delayed ejaculation, decreased libido, and anorgasmia.
Adi et al11 completed a more rigorous literature evaluation that included 9 studies with a total of 225 patients receiving fluoxetine, fluvoxamine, sertraline, or paroxetine. Eight studies showed benefits; however, Adi noted that this preliminary evidence was “far from conclusive.”
SSRIs generally are well tolerated and may be more appealing to patients than the “chemical castration” of hormonal treatments. Dosing is similar to that used in depression or obsessive-compulsive disorder. Although most patients notice beneficial effects in 2 to 4 weeks, some notice the effect nearly immediately.
Naltrexone. An opioid antagonist thought to affect the CNS processes of pleasure and pain, naltrexone has been used to treat alcohol dependence and pathologic gambling. A few case studies12-14 and 1 study of 21 adolescent sex offenders15 have shown benefits in treating sexual offenders or paraphiliacs. Benefits were seen at 50 mg/d, with suggested dosing of 100 to 200 mg/d. Because data are very limited, consider naltrexone only on an individual basis or as a possible adjunctive treatment.
Psychostimulants. Methylphenidate was added to augment SSRI treatment in a study of 26 men with paraphilias or paraphilia-related disorders.16 Results included further significant decreases in total sexual outlets (orgasms per week) and average time spent per day in paraphilia and paraphilia-related behavior. These gains appeared to be independent of the presence of attention-deficit/hyperactivity disorder.
Again, because data are very limited, consider this strategy only on an individual basis or as a possible adjunctive treatment. Because sexual offenders have high rates of substance abuse,17 consider the potential for stimulant abuse.
Hormonal treatments
Because testosterone is required for healthy bone metabolism, the antiandrogen medications used in hormonal treatment can cause osteoporosis.18,19 Therefore, long-term antiandrogen treatment should include bone scans to monitor for osteopenia and osteoporosis. Some authors have suggested that monthly doses of 25 to 50 mg of testosterone could minimize this risk.20 Bisphosphonates, vitamin D, and calcium supplements at osteoporosis treatment levels might be helpful.18 Other common side effects of antiandrogen medications are listed in Table 2.
Finasteride is approved for treating benign prostatic hyperplasia and androgenetic alopecia. It works by preventing conversion of testosterone to dihydrotestosterone (DHT) by the type II isoenzyme.21 Serum DHT contributes to male sexual behavior and predicts frequency of orgasms in healthy volunteers.22 Although there have been no studies of finasteride in sex offenders, it may be more acceptable to patients than other hormonal treatments and have a theoretical benefit in reducing sexual drive. Clinically, some patients describe increased control over urges without substantial side effects. Because there is no evidence supporting finasteride use in sex offenders, consider this medication only on an individual basis or as a possible adjunctive treatment.
Cyproterone acetate (CPA) is a synthetic steroid that blocks androgen receptors.3,23 Some evidence supports its use in treating sex offenders,24 although this agent is not available in the United States. For more information about CPA, see Box.
Medroxyprogesterone acetate (MPA), a derivative of progesterone, lowers serum testosterone by inhibiting its production through reducing pituitary luteinizing hormone (LH).24 The typical dose range for use in sex offenders is 100 to 600 mg/d orally or 100 to 700 mg IM every week,3 although some authors suggest giving similar doses every 2 weeks.4
Side effects of MPA include hypersomnia; neurasthenia; weight gain; hot flashes; gynecomastia; increased scalp hair; and decreased erections, ejaculate volume, spermatogenesis, and body and facial hair. The drug decreases testosterone levels by about 50%. Positive effects include reduced interest in and energy spent on pursuing sexual goals, but preservation of nondeviant sexual arousal.4
MPA has been shown to effectively decrease deviant sexual arousal and recidivism. In a study of 100 patients receiving MPA (average 250 mg IM every 2 weeks) for an average of 3 years, only 1 re-offended while taking MPA.4
In a 5-year follow-up study25 of 275 men, subjects were classified into high risk/treatment with MPA, 200 to 400 mg IM every 2 weeks, and low risk/nontreatment groups. A portion of the high risk/treatment group did not receive MPA. No sexual re-offenses occurred among high-risk subjects who received MPA, whereas the recidivism rate was 18% among high-risk subjects who did not receive MPA. Subjects in the low risk/nontreatment group had a recidivism rate of 15%, which suggests the need for more liberal use of antiandrogens. One major confounding factor was that subjects in the high risk/treatment group had to report every 2 weeks for injections; this may have resulted in closer follow-up, monitoring, and support, which may have contributed to lower recidivism.
Gonadotropin-releasing hormone (GNRH) agonists. The terms gonadotropin-releasing hormone and luteinizing-releasing hormone are used interchangeably. Most body testosterone is produced and released by Leydig cells in the testes in response to stimulation by LH released by the pituitary gland. LH release is controlled by the pulsatile release of GNRH from the hypothalamus. GNRH agonists are high-potency analogs of GNRH that work by causing an initial surge of LH followed by down-regulation of gonadotroph cells, a drop in LH, and a drop in testosterone to castration levels.
The GNRH analogs leuprolide, goserelin, and triptorelin are used to treat paraphiliacs and sexual offenders.20 Leuprolide typically is dosed at 7.5 mg IM every month, 22.5 mg IM every 3 months, or 30 mg IM every 4 months. Goserelin is provided as a subcutaneous implant/depot injected as 3.6 mg every month or 10.8 mg every 3 months.
Triptorelin is FDA-approved as treatment for advanced prostate cancer. Triptorelin is given in depot formulation as 3.75 mg IM every month or in a long-acting form as 11.25 mg IM every 3 months.
When starting these medications, an initial surge of LH and testosterone can last up to 3 weeks.26 Theoretically, this could worsen paraphiliac interests. Many practitioners will use a testosterone blocker such as flutamide, 250 mg tid, for the initial weeks of treatment.
Side effects of the GNRH agonists are similar. Most patients initially experience hot flashes. A systemic literature review27 reported:
- weight gain
- perspiration
- gynecomastia
- urinary incontinence
- hot flashes
- decreased growth of facial and body hair
- asthenia
- erectile failure
- muscle tenderness
- frequent bone demineralization.
Rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported, possibly related to an underlying pituitary adenoma.28
In a literature review that totalled 118 patients,27 GNRH agonists significantly decreased erections, ejaculations, paraphiliac fantasies, and paraphiliac behavior. Patients also reported feeling more relaxed, and recidivism rates were low. Some patients who failed to respond to CPA and MPA responded to GNRH agonists. Subsequent studies found similar results.29,30
Cyproterone acetate (CPA), which is available in Canada and Europe, is a synthetic steroid with structure similar to that of progesterone. CPA blocks androgen receptors, which makes it antiandrogenic, progestational, and antigonadotrophic.a
Dosages for paraphilias range from 100 to 500 mg/d orally or 100 to 600 mg intramuscularly every 1 or 2 weeks. b
Once stabilized, some individuals can be maintained on very low doses, such as 12.5 to 50 mg/d. a Lower doses may be appropriate for individuals who are self-motivated for treatment and who reliably report their sexual interests.
CPA reduces testosterone by approximately 50%. Side effects include decreases in:
- erections
- ejaculate volume
- spermatogenesis.
Some patients experience hypersomnia, neurasthenia, weight gain, hot flashes, decreased body and facial hair, and increased scalp hair. About 20% of patients may experience gynecomastia, particularly at higher doses.
Evidence shows CPA reduces sexual arousal, activity, fantasy, and masturbation.a In a systematic review of 7 studies that included 127 patients, the re-offense rate averaged 6%.c This is significantly lower than the expected recidivism of approximately 13.4%.d
References
a. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
b. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
c. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
d. Hanson R, Bussiere MT. Predicting relapse: A meta-analysis of sexual offender recidivism studies. J Consult Clin Psychology. 1998;66:348-362.
Table 2
Common side effects of antiandrogen therapy
| Depression |
| Erectile dysfunction |
| Fatigue |
| Gynecomastia |
| Hot flashes |
| Hypertension |
| Low libido |
| Myalgia |
| Osteopenia |
| Osteoporosis |
| Sweating |
| Thromboembolism |
| Weight gain |
Monitoring
Laboratory investigations are recommended to monitor for side effects of antiandrogen medications (Table 3).19,27,31 Medical contraindications to rule out before initiating antiandrogen medications include:
- thromboembolic diseases
- liver disease
- bone demineralization disorders
- hypersensitivity to the drug.
Measure prolactin to rule out pituitary adenomas. Monitor serum testosterone because some patients will not experience testosterone suppression from GNRH agonists or other antiandrogens. Noncompliant patients could potentially reverse the effects of MPA and GNRH agonists by taking exogenous testosterone.
Table 3
Monitoring patients receiving antiandrogen medications
| Pre-therapy workup | Periodic monitoring |
|---|---|
| Endocrinology or internist consultation Bone scan Weight Blood pressure Electrocardiogram CBC, renal function, liver function, fasting glucose, and lipids LH, FSH, testosterone, prolactin | Monthly: testosterone for the first 6 months Every 6 months: testosterone, LH, FSH, prolactin, CBC, renal function, liver function, fasting glucose and lipids, weight, blood pressure Yearly: bone scan |
| CBC: complete blood count; FSH: follicle-stimulating hormone; LH: luteinizing hormone | |
| Source: References 19,27,31 | |
Medication selection
The goals of pharmacologic treatment of sex offenders are to:
- reduce sexual offending and victimization
- suppress sexual drive to a controllable level
- possibly preferentially eliminate deviant arousal/thoughts
- allow normal sexual relationships.
Gauging risk. In determining which pharmacologic treatment to offer a patient, first evaluate the individual’s risk for recidivism. Actuarial scales32,33 suggest that recidivism risk can be categorized, based on clinical factors (Table 4).4,25,34
In addition to statistical risk factors, several other factors affect medication selection. Self-referred individuals may be more reliable in taking oral medications than those referred by the courts. A developmentally delayed individual may be a poor candidate for oral medication, unless he resides in a group home setting where compliance can be assured. Efficacy also guides medication choice. Finally, some patients will be legally required to provide proof of compliance, which only IM medications provide.
Treatment. Based on clinical experience and available literature, Bradford5 created an algorithm to help clinicians select appropriate pharmacologic interventions. Although it has not been validated, this algorithm provides a reasonable starting place.
In general, start treatment with an SSRI for low-risk individuals (Table 1).3-5 If this strategy is insufficient, consider augmentation with methylphenidate, naltrexone, or finasteride.
The next step would be to add oral MPA or CPA, 50 mg/d, which would partially inhibit testosterone and may allow some normal sexual functioning.4,23 Higher-dose oral MPA or CPA would be tried next. For higher-risk individuals or treatment failures, IM MPA or CPA would be offered next, followed by a GNRH agonist. For individuals at highest risk for re-offending, combinations of agents may be indicated.
This simple strategy is appealing, but in reality, treatment should be individualized. Choose medications based on the patient’s risk, wishes, and the previously mentioned clinical factors.
Table 4: Will my patient commit another sexual offense? Evaluating risk
Source: References 4,25,34Related resources
- Krueger RB, Kaplan MS. The paraphilic and hypersexual disorders: an overview. J Psychiatr Pract. 2001;7:391-403.
- Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Pract. 2002;8:21-32.
- Association for the Treatment of Sexual Abusers. www.atsa.com.
Drug brand names
- Cyproterone acetate • Androcur
- Finasteride • Propecia, Proscar
- Fluoxetine • Prozac
- Flutamide • Eulexin
- Fluvoxamine • Luvox
- Goserelin • Zoladex
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone acetate • Depo-Provera, Provera
- Methylphenidate • Ritalin
- Naltrexone • ReVia
- Paroxetine • Paxil
- Sertraline • Zoloft
- Triptorelin • Trelstar Depot
Disclosure
Dr. Booth reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
Sex offenders traditionally are managed by the criminal justice system, but psychiatrists are frequently called on to assess and treat these individuals. Part of the reason is the overlap of paraphilias (disorders of sexual preference) and sexual offending. Many sexual offenders do not meet DSM criteria for paraphilias,1 however, and individuals with paraphilias do not necessarily commit offenses or come into contact with the legal system.
As clinicians, we may need to assess and treat a wide range of sexual issues, from persons with paraphilias who are self-referred and have no legal involvement, to recurrent sexual offenders who are at a high risk of repeat offending. Successfully managing sex offenders includes psychological and pharmacologic interventions and possibly incarceration and post-incarceration surveillance. This article focuses on pharmacologic interventions for male sexual offenders.
Reducing sexual drive
Sex offending likely is the result of a complex interplay of environment and psychological and biologic factors. The biology of sexual function provides numerous targets for pharmacologic intervention, including:2
- endocrine factors, such as testosterone
- neurotransmitters, such as serotonin.
The use of pharmacologic treatments for sex offenders is off-label, and evidence is limited. In general, pharmacologic treatments are geared toward reducing sexual drive through nonhormonal or hormonal means (Table 1).3-5
Table 1: Pharmacologic treatment of male sex offenders: A risk-based approach
*Not available in the United States
†Some authors suggest administering this dosage once every 2 weeks
CPA: cyproterone acetate; GNRH: gonadotropin-releasing hormone; IM: intramuscular; MPA: medroxyprogesterone acetate; SSRI: selective serotonin reuptake inhibitor
Source: References 3-5
Nonhormonal treatments
SSRIs. Selective serotonin reuptake inhibitors act by blocking serotonin reuptake in the synaptic cleft. Soon after the first SSRIs were approved in 1988, reports appeared of SSRIs interfering with sexual functioning.6 This side effect quickly was exploited to assist the treatment of sexual offenders.7
The mechanism of action may include:8
- direct effects, such as general inhibition of sexual activity, reduced impulsiveness, and an effect on the hypothesized “obsessive-compulsive” nature of paraphilias9
- indirect reduction of testosterone.
A growing body of literature supports SSRIs’ effectiveness in treating paraphilias and sexual offenders. Greenberg7 reviewed case studies and open drug trials of nearly 200 patients receiving fluoxetine, fluvoxamine, or sertraline. Most studies showed response rates of 50% to 90%.10 Positive effects included decreases in:
- paraphiliac fantasies, urges, and sexual acts
- masturbation
- hypersexual activity.
Some studies reported a preferential decrease in paraphiliac interests with an increase in conventional sexual interests, although this may be related to placebo or halo effects—patients may have reported an increase in conventional interests because they noticed a decrease in paraphiliac interests. Negative side effects included decreased sexual desires, delayed ejaculation, decreased libido, and anorgasmia.
Adi et al11 completed a more rigorous literature evaluation that included 9 studies with a total of 225 patients receiving fluoxetine, fluvoxamine, sertraline, or paroxetine. Eight studies showed benefits; however, Adi noted that this preliminary evidence was “far from conclusive.”
SSRIs generally are well tolerated and may be more appealing to patients than the “chemical castration” of hormonal treatments. Dosing is similar to that used in depression or obsessive-compulsive disorder. Although most patients notice beneficial effects in 2 to 4 weeks, some notice the effect nearly immediately.
Naltrexone. An opioid antagonist thought to affect the CNS processes of pleasure and pain, naltrexone has been used to treat alcohol dependence and pathologic gambling. A few case studies12-14 and 1 study of 21 adolescent sex offenders15 have shown benefits in treating sexual offenders or paraphiliacs. Benefits were seen at 50 mg/d, with suggested dosing of 100 to 200 mg/d. Because data are very limited, consider naltrexone only on an individual basis or as a possible adjunctive treatment.
Psychostimulants. Methylphenidate was added to augment SSRI treatment in a study of 26 men with paraphilias or paraphilia-related disorders.16 Results included further significant decreases in total sexual outlets (orgasms per week) and average time spent per day in paraphilia and paraphilia-related behavior. These gains appeared to be independent of the presence of attention-deficit/hyperactivity disorder.
Again, because data are very limited, consider this strategy only on an individual basis or as a possible adjunctive treatment. Because sexual offenders have high rates of substance abuse,17 consider the potential for stimulant abuse.
Hormonal treatments
Because testosterone is required for healthy bone metabolism, the antiandrogen medications used in hormonal treatment can cause osteoporosis.18,19 Therefore, long-term antiandrogen treatment should include bone scans to monitor for osteopenia and osteoporosis. Some authors have suggested that monthly doses of 25 to 50 mg of testosterone could minimize this risk.20 Bisphosphonates, vitamin D, and calcium supplements at osteoporosis treatment levels might be helpful.18 Other common side effects of antiandrogen medications are listed in Table 2.
Finasteride is approved for treating benign prostatic hyperplasia and androgenetic alopecia. It works by preventing conversion of testosterone to dihydrotestosterone (DHT) by the type II isoenzyme.21 Serum DHT contributes to male sexual behavior and predicts frequency of orgasms in healthy volunteers.22 Although there have been no studies of finasteride in sex offenders, it may be more acceptable to patients than other hormonal treatments and have a theoretical benefit in reducing sexual drive. Clinically, some patients describe increased control over urges without substantial side effects. Because there is no evidence supporting finasteride use in sex offenders, consider this medication only on an individual basis or as a possible adjunctive treatment.
Cyproterone acetate (CPA) is a synthetic steroid that blocks androgen receptors.3,23 Some evidence supports its use in treating sex offenders,24 although this agent is not available in the United States. For more information about CPA, see Box.
Medroxyprogesterone acetate (MPA), a derivative of progesterone, lowers serum testosterone by inhibiting its production through reducing pituitary luteinizing hormone (LH).24 The typical dose range for use in sex offenders is 100 to 600 mg/d orally or 100 to 700 mg IM every week,3 although some authors suggest giving similar doses every 2 weeks.4
Side effects of MPA include hypersomnia; neurasthenia; weight gain; hot flashes; gynecomastia; increased scalp hair; and decreased erections, ejaculate volume, spermatogenesis, and body and facial hair. The drug decreases testosterone levels by about 50%. Positive effects include reduced interest in and energy spent on pursuing sexual goals, but preservation of nondeviant sexual arousal.4
MPA has been shown to effectively decrease deviant sexual arousal and recidivism. In a study of 100 patients receiving MPA (average 250 mg IM every 2 weeks) for an average of 3 years, only 1 re-offended while taking MPA.4
In a 5-year follow-up study25 of 275 men, subjects were classified into high risk/treatment with MPA, 200 to 400 mg IM every 2 weeks, and low risk/nontreatment groups. A portion of the high risk/treatment group did not receive MPA. No sexual re-offenses occurred among high-risk subjects who received MPA, whereas the recidivism rate was 18% among high-risk subjects who did not receive MPA. Subjects in the low risk/nontreatment group had a recidivism rate of 15%, which suggests the need for more liberal use of antiandrogens. One major confounding factor was that subjects in the high risk/treatment group had to report every 2 weeks for injections; this may have resulted in closer follow-up, monitoring, and support, which may have contributed to lower recidivism.
Gonadotropin-releasing hormone (GNRH) agonists. The terms gonadotropin-releasing hormone and luteinizing-releasing hormone are used interchangeably. Most body testosterone is produced and released by Leydig cells in the testes in response to stimulation by LH released by the pituitary gland. LH release is controlled by the pulsatile release of GNRH from the hypothalamus. GNRH agonists are high-potency analogs of GNRH that work by causing an initial surge of LH followed by down-regulation of gonadotroph cells, a drop in LH, and a drop in testosterone to castration levels.
The GNRH analogs leuprolide, goserelin, and triptorelin are used to treat paraphiliacs and sexual offenders.20 Leuprolide typically is dosed at 7.5 mg IM every month, 22.5 mg IM every 3 months, or 30 mg IM every 4 months. Goserelin is provided as a subcutaneous implant/depot injected as 3.6 mg every month or 10.8 mg every 3 months.
Triptorelin is FDA-approved as treatment for advanced prostate cancer. Triptorelin is given in depot formulation as 3.75 mg IM every month or in a long-acting form as 11.25 mg IM every 3 months.
When starting these medications, an initial surge of LH and testosterone can last up to 3 weeks.26 Theoretically, this could worsen paraphiliac interests. Many practitioners will use a testosterone blocker such as flutamide, 250 mg tid, for the initial weeks of treatment.
Side effects of the GNRH agonists are similar. Most patients initially experience hot flashes. A systemic literature review27 reported:
- weight gain
- perspiration
- gynecomastia
- urinary incontinence
- hot flashes
- decreased growth of facial and body hair
- asthenia
- erectile failure
- muscle tenderness
- frequent bone demineralization.
Rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported, possibly related to an underlying pituitary adenoma.28
In a literature review that totalled 118 patients,27 GNRH agonists significantly decreased erections, ejaculations, paraphiliac fantasies, and paraphiliac behavior. Patients also reported feeling more relaxed, and recidivism rates were low. Some patients who failed to respond to CPA and MPA responded to GNRH agonists. Subsequent studies found similar results.29,30
Cyproterone acetate (CPA), which is available in Canada and Europe, is a synthetic steroid with structure similar to that of progesterone. CPA blocks androgen receptors, which makes it antiandrogenic, progestational, and antigonadotrophic.a
Dosages for paraphilias range from 100 to 500 mg/d orally or 100 to 600 mg intramuscularly every 1 or 2 weeks. b
Once stabilized, some individuals can be maintained on very low doses, such as 12.5 to 50 mg/d. a Lower doses may be appropriate for individuals who are self-motivated for treatment and who reliably report their sexual interests.
CPA reduces testosterone by approximately 50%. Side effects include decreases in:
- erections
- ejaculate volume
- spermatogenesis.
Some patients experience hypersomnia, neurasthenia, weight gain, hot flashes, decreased body and facial hair, and increased scalp hair. About 20% of patients may experience gynecomastia, particularly at higher doses.
Evidence shows CPA reduces sexual arousal, activity, fantasy, and masturbation.a In a systematic review of 7 studies that included 127 patients, the re-offense rate averaged 6%.c This is significantly lower than the expected recidivism of approximately 13.4%.d
References
a. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
b. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
c. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
d. Hanson R, Bussiere MT. Predicting relapse: A meta-analysis of sexual offender recidivism studies. J Consult Clin Psychology. 1998;66:348-362.
Table 2
Common side effects of antiandrogen therapy
| Depression |
| Erectile dysfunction |
| Fatigue |
| Gynecomastia |
| Hot flashes |
| Hypertension |
| Low libido |
| Myalgia |
| Osteopenia |
| Osteoporosis |
| Sweating |
| Thromboembolism |
| Weight gain |
Monitoring
Laboratory investigations are recommended to monitor for side effects of antiandrogen medications (Table 3).19,27,31 Medical contraindications to rule out before initiating antiandrogen medications include:
- thromboembolic diseases
- liver disease
- bone demineralization disorders
- hypersensitivity to the drug.
Measure prolactin to rule out pituitary adenomas. Monitor serum testosterone because some patients will not experience testosterone suppression from GNRH agonists or other antiandrogens. Noncompliant patients could potentially reverse the effects of MPA and GNRH agonists by taking exogenous testosterone.
Table 3
Monitoring patients receiving antiandrogen medications
| Pre-therapy workup | Periodic monitoring |
|---|---|
| Endocrinology or internist consultation Bone scan Weight Blood pressure Electrocardiogram CBC, renal function, liver function, fasting glucose, and lipids LH, FSH, testosterone, prolactin | Monthly: testosterone for the first 6 months Every 6 months: testosterone, LH, FSH, prolactin, CBC, renal function, liver function, fasting glucose and lipids, weight, blood pressure Yearly: bone scan |
| CBC: complete blood count; FSH: follicle-stimulating hormone; LH: luteinizing hormone | |
| Source: References 19,27,31 | |
Medication selection
The goals of pharmacologic treatment of sex offenders are to:
- reduce sexual offending and victimization
- suppress sexual drive to a controllable level
- possibly preferentially eliminate deviant arousal/thoughts
- allow normal sexual relationships.
Gauging risk. In determining which pharmacologic treatment to offer a patient, first evaluate the individual’s risk for recidivism. Actuarial scales32,33 suggest that recidivism risk can be categorized, based on clinical factors (Table 4).4,25,34
In addition to statistical risk factors, several other factors affect medication selection. Self-referred individuals may be more reliable in taking oral medications than those referred by the courts. A developmentally delayed individual may be a poor candidate for oral medication, unless he resides in a group home setting where compliance can be assured. Efficacy also guides medication choice. Finally, some patients will be legally required to provide proof of compliance, which only IM medications provide.
Treatment. Based on clinical experience and available literature, Bradford5 created an algorithm to help clinicians select appropriate pharmacologic interventions. Although it has not been validated, this algorithm provides a reasonable starting place.
In general, start treatment with an SSRI for low-risk individuals (Table 1).3-5 If this strategy is insufficient, consider augmentation with methylphenidate, naltrexone, or finasteride.
The next step would be to add oral MPA or CPA, 50 mg/d, which would partially inhibit testosterone and may allow some normal sexual functioning.4,23 Higher-dose oral MPA or CPA would be tried next. For higher-risk individuals or treatment failures, IM MPA or CPA would be offered next, followed by a GNRH agonist. For individuals at highest risk for re-offending, combinations of agents may be indicated.
This simple strategy is appealing, but in reality, treatment should be individualized. Choose medications based on the patient’s risk, wishes, and the previously mentioned clinical factors.
Table 4: Will my patient commit another sexual offense? Evaluating risk
Source: References 4,25,34Related resources
- Krueger RB, Kaplan MS. The paraphilic and hypersexual disorders: an overview. J Psychiatr Pract. 2001;7:391-403.
- Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Pract. 2002;8:21-32.
- Association for the Treatment of Sexual Abusers. www.atsa.com.
Drug brand names
- Cyproterone acetate • Androcur
- Finasteride • Propecia, Proscar
- Fluoxetine • Prozac
- Flutamide • Eulexin
- Fluvoxamine • Luvox
- Goserelin • Zoladex
- Leuprolide • Eligard, Lupron
- Medroxyprogesterone acetate • Depo-Provera, Provera
- Methylphenidate • Ritalin
- Naltrexone • ReVia
- Paroxetine • Paxil
- Sertraline • Zoloft
- Triptorelin • Trelstar Depot
Disclosure
Dr. Booth reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products.
1. First MB, Halon RL. Use of DSM paraphilia diagnoses in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
2. Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012-1030.
3. Bezchlibnyk-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs. 16th ed. Seattle, WA: Hogrefe & Huber Publishers; 2006:222–225.
4. Maletzky B. The use of medroxyprogesterone acetate to assist in the treatment of sexual offenders. Annals of Sex Research. 1991;4:117-129.
5. Bradford JM. The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behaviour. Can J Psychiatry. 2001;46(1):26-34.
6. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470.
7. Greenberg DM, Bradford JMW. Treatment of the paraphilic disorders: a review of the role of the selective serotonin reuptake inhibitors. Sex Abuse. 1997;9(4):349-360.
8. Hill A, Briken P, Kraus C, et al. Differential pharmacological treatment of paraphilias and sex offenders. Int J Offender Ther Comp Criminol. 2003;47(4):407-421.
9. Bradford JMW. The paraphilias, obsessive compulsive spectrum disorder, and the treatment of sexually deviant behaviour. Psychiatr Q. 1999;70(3):209-219.
10. Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Prac. 2002;8(1):21-32.
11. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost-consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
12. Raymond NC, Grant JE, Kim SW, et al. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharm. 2002;17(4):201-205.
13. Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Ann Clin Psychiatry. 2001;13(4):229-231.
14. Sandyk R. Naltrexone suppresses abnormal sexual behavior in Tourette’s syndrome. Int J Neurosci. 1988;43(1-2):107-110.
15. Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982-986.
16. Kafka M, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
17. Langstrom N, Sjostedt G, Grann M. Psychiatric disorders and recidivism in sexual offenders. Sex Abuse. 2004;16(2):139-150.
18. Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 suppl 1):79-85.
19. Grasswick LJ, Bradford JM. Osteoporosis associated with the treatment of paraphilias: a clinical review of seven case reports. J Forensic Sci. 2003;48:849-855.
20. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
21. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
22. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
23. Bradford JMW, Pawlak A. Double-blind placebo crossover study of cyproterone acetate in the treatment of the paraphilias. Arch Sex Behav. 1993;22(5):383-402.
24. Meyer WJ, Cole CM. Physical and chemical castration of sex offenders: a review. J Offender Rehab. 1997;25(3/4):1-18.
25. Maletzky BM, Tolan A, McFarland B. The Oregon depoProvera program: a five-year follow-up. Sex Abuse. 2006;18:303-316.
26. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001-1006.
27. Briken P, Hill A, Berner W. Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone–releasing hormone: a systematic review. J Clin Psychiatry. 2003;64:890-897.
28. Blaut K, Winiewski P, Syrenicz A, et al. Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett. 2006;27(5):569-572.
29. Schober JM, Kuhn PJ, Kovacs P, et al. Leuprolide acetate suppresses pedophilic urges and arousability. Arch Sex Behav. 2005;34(6):691-705.
30. Ösler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338:416-422.
31. Reilly DR, Delva NJ, Hudson RW. Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia. Can J Psychiatry. 2000;45:559-563.
32. Quinsey VL, Harris GT, Rice ME, et al. Violent offenders: appraising and managing risk. Washington, DC: American Psychological Association; 1998.
33. Epperson DL, Kaul JD, Huot SJ, et al. Minnesota Sex Offender Screening Tool–revised (MnSOST-R). St. Paul, MN: Minnesota Department of Corrections; 1998.
34. Bradford JMW. The treatment of sexual deviation using a pharmacological approach. J Sex Res. 2000;37(3):248-257.
1. First MB, Halon RL. Use of DSM paraphilia diagnoses in sexually violent predator commitment cases. J Am Acad Psychiatry Law. 2008;36(4):443-454.
2. Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012-1030.
3. Bezchlibnyk-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs. 16th ed. Seattle, WA: Hogrefe & Huber Publishers; 2006:222–225.
4. Maletzky B. The use of medroxyprogesterone acetate to assist in the treatment of sexual offenders. Annals of Sex Research. 1991;4:117-129.
5. Bradford JM. The neurobiology, neuropharmacology, and pharmacological treatment of the paraphilias and compulsive sexual behaviour. Can J Psychiatry. 2001;46(1):26-34.
6. Baldwin DS. Sexual dysfunction associated with antidepressant drugs. Expert Opin Drug Saf. 2004;3(5):457-470.
7. Greenberg DM, Bradford JMW. Treatment of the paraphilic disorders: a review of the role of the selective serotonin reuptake inhibitors. Sex Abuse. 1997;9(4):349-360.
8. Hill A, Briken P, Kraus C, et al. Differential pharmacological treatment of paraphilias and sex offenders. Int J Offender Ther Comp Criminol. 2003;47(4):407-421.
9. Bradford JMW. The paraphilias, obsessive compulsive spectrum disorder, and the treatment of sexually deviant behaviour. Psychiatr Q. 1999;70(3):209-219.
10. Krueger RB, Kaplan MS. Behavioral and psychopharmacological treatment of the paraphilic and hypersexual disorders. J Psychiatr Prac. 2002;8(1):21-32.
11. Adi Y, Ashcroft D, Browne K, et al. Clinical effectiveness and cost-consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders. Health Technol Assess. 2002;6(28):1-66.
12. Raymond NC, Grant JE, Kim SW, et al. Treatment of compulsive sexual behaviour with naltrexone and serotonin reuptake inhibitors: two case studies. Int Clin Psychopharm. 2002;17(4):201-205.
13. Grant JE, Kim SW. A case of kleptomania and compulsive sexual behavior treated with naltrexone. Ann Clin Psychiatry. 2001;13(4):229-231.
14. Sandyk R. Naltrexone suppresses abnormal sexual behavior in Tourette’s syndrome. Int J Neurosci. 1988;43(1-2):107-110.
15. Ryback RS. Naltrexone in the treatment of adolescent sexual offenders. J Clin Psychiatry. 2004;65(7):982-986.
16. Kafka M, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
17. Langstrom N, Sjostedt G, Grann M. Psychiatric disorders and recidivism in sexual offenders. Sex Abuse. 2004;16(2):139-150.
18. Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60(3 suppl 1):79-85.
19. Grasswick LJ, Bradford JM. Osteoporosis associated with the treatment of paraphilias: a clinical review of seven case reports. J Forensic Sci. 2003;48:849-855.
20. Rösler A, Witztum E. Pharmacotherapy of paraphilias in the next millennium. Behav Sci Law. 2000;18:43-56.
21. Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5-alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76.
22. Mantzoros CS, Georgiadis EI, Trichopoulos D. Contribution of dihydrotestosterone to male sexual behaviour. BMJ. 1995;310(6990):1289-1291.
23. Bradford JMW, Pawlak A. Double-blind placebo crossover study of cyproterone acetate in the treatment of the paraphilias. Arch Sex Behav. 1993;22(5):383-402.
24. Meyer WJ, Cole CM. Physical and chemical castration of sex offenders: a review. J Offender Rehab. 1997;25(3/4):1-18.
25. Maletzky BM, Tolan A, McFarland B. The Oregon depoProvera program: a five-year follow-up. Sex Abuse. 2006;18:303-316.
26. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001-1006.
27. Briken P, Hill A, Berner W. Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone–releasing hormone: a systematic review. J Clin Psychiatry. 2003;64:890-897.
28. Blaut K, Winiewski P, Syrenicz A, et al. Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett. 2006;27(5):569-572.
29. Schober JM, Kuhn PJ, Kovacs P, et al. Leuprolide acetate suppresses pedophilic urges and arousability. Arch Sex Behav. 2005;34(6):691-705.
30. Ösler A, Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N Engl J Med. 1998;338:416-422.
31. Reilly DR, Delva NJ, Hudson RW. Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia. Can J Psychiatry. 2000;45:559-563.
32. Quinsey VL, Harris GT, Rice ME, et al. Violent offenders: appraising and managing risk. Washington, DC: American Psychological Association; 1998.
33. Epperson DL, Kaul JD, Huot SJ, et al. Minnesota Sex Offender Screening Tool–revised (MnSOST-R). St. Paul, MN: Minnesota Department of Corrections; 1998.
34. Bradford JMW. The treatment of sexual deviation using a pharmacological approach. J Sex Res. 2000;37(3):248-257.
Borderline personality disorder: STEPPS is practical, evidence-based, easier to use
Treatment of borderline personality disorder (BPD) often is viewed as challenging and the results so discouraging that some clinicians avoid referrals of BPD patients.1-3 Psychotherapy has been the treatment mainstay for decades, and supportive approaches are probably the most widely employed.4 Psychodynamic therapy often has been recommended.
This article introduces a new evidence-based group treatment program that we developed for BPD patients. Systems Training for Emotional Predictability and Problem Solving (STEPPS) is founded on the successes of better known psychoeducational models but is easier for practicing psychiatrists to implement.
A different approach to BPD
Linehan5 introduced dialectical behavior therapy (DBT)—a manualized, time-limited, cognitive-behavioral approach in which patients learn to regulate their emotions and behaviors rather than change their personality structure. Other evidence-based BPD treatments include transference-focused psychotherapy,6 schema-focused psychotherapy,7 and Bateman and Fonagy’s mentalization program.8 For a description of the unique challenges presented by BPD patients, see Box.
In the mid-1990s, we set out to create a treatment program for our BPD patients in response to managed care directives to lower the cost of care, decrease length of inpatient treatment, and reduce rehospitalization rates. Despite DBT’s many appealing features, we felt this model was too lengthy and labor-intensive for our treatment setting. We concluded that modifying a program developed by Bartels and Crotty9 would serve our needs. This 12-week psychoeducational program:
- employs established cognitive-behavioral techniques in group treatment intended to supplement but not replace patients’ ongoing treatment
- incorporates a “systems” component that recognizes the importance of the patient’s family and friends.
We eventually renamed the program Systems Training for Emotional Predictability and Problem Solving (STEPPS)10 and created a new manual (see Related Resources) to simplify group leader training and ensure fidelity to the model. Data from 5 studies, including 2 randomized controlled trials (Table 1), show that STEPPS has a robust antidepressant effect and leads to broad-based improvements in the affective, cognitive, impulsive, and disturbed relationship domains of BPD.11-15
Borderline personality disorder (BPD) is 1 of the most challenging mental health conditions. BPD is surprisingly common, with prevalence rates of 0.5% to 1% in the community, 10% in outpatient mental health settings, and up to 20% in inpatient psychiatric settings.a-c Patients with BPD experience substantial functional impairment in several areas (eg, difficulty maintaining employment, disturbed interpersonal relationships, and disrupted family relationships).a,d,e
Many borderline patients have childhood histories of abuse and continue to be victims of domestic and other violence through adulthood.f High utilization of medical and psychiatric health care services is common and costly.g BPD also is associated with substantial psychiatric comorbidity, particularly mood and anxiety disorders, substance use disorders, eating disorders, and other Axis II disorders.h,i
Persons with BPD experience intense dysphoria and intrapsychic pain. Characteristic features include affective intensity, reactivity, and lability; a pervasive pattern of unstable interpersonal relationships; marked behavioral impulsivity; unstable self-identity; intense anger; and extreme fear of abandonment.j
The symptom that probably makes the greatest demand on mental health resources is recurrent suicidal threats/attempts or episodes of self-mutilation, many prompted by disappointment in a relationship.k Two-thirds to three-quarters of BPD patients will attempt suicide, with up to 10% eventually completing suicide, often following multiple failed treatments.l
References
a. Gunderson J. Borderline personality disorder: A clinical guide. 2nd ed. Washington, DC: American Psychiatric Publishing; 2008.
b. Widiger TA, Frances AJ. Epidemiology, diagnosis, and co-morbidity of borderline personality disorder. In: Tasman A, Hales RE, Frances AJ (eds). American Psychiatric Press Review of Psychiatry, vol. 8. Washington, DC: American Psychiatric Press; 1989:8-24.
c. Swartz MS, Blazer D, George L, et al. Estimating the prevalence of borderline personality disorder in the community. J Personal Disord. 1990;4:257-272.
d. Nakao K, Gunderson JG, Phillips KA, et al. Functional impairment in personality disorders. J Personal Disord. 1992;6:24-31.
e. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159:276-283.
f. Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am. 2000;23:89-101.
g. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
h. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I co-morbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733-1739.
i. Zimmerman M, Coryell W. DSM-III personality disorder diagnoses in a non-patient sample: demographic correlates and co-morbidity. Arch Gen Psychiatry. 1989;46:682-689.
j. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 1994.
k. Paris J. Social factors in personality disorders— a biopsychosocial approach to etiology and treatment. New York, NY: Cambridge University Press; 1996.
l. Soloff PH, Lynch KG, Kelly TM. Characteristics of suicide attempts of patients with major depressive episode and borderline personality disorder: a comparative study. Am J Psychiatry. 2000;157:601-608.
Table 1
STEPPS: Trials show improvement across BPD domains
| Study | Patients | Results |
|---|---|---|
| Uncontrolled trials | ||
| Blum et al, 200211 | 52 outpatients; 94% female; mean age 33 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Black et al, 200812 | 12 incarcerated women; mean age 35 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Freije et al, 200213 | 85 patients; 91% female; mean age 32 | Significant improvement in score on a Dutch version of BEST; significant improvement on SCL-90 subscales, especially those rating anxiety, depression, and interpersonal sensitivity |
| Randomized controlled trials | ||
| Blum et al, 200814 | 165 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usual | Patients receiving STEPPS plus treatment as usual experienced greater improvements in ZAN-BPD total score, impulsivity, negative affect, mood, and global functioning |
| van Wel, 200715 | 79 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usuals | Patients receiving STEPPS plus treatment as usual had greater improvements in global psychiatric symptoms using the SCL-90, BPD symptoms, and quality of life measures at the end of treatment and at 6-month follow-up |
| BDI: Beck Depression Inventory; BEST: Borderline Evaluation of Severity Over Time; BPD: borderline personality disorder; PANAS: Positive and Negative Affect Scale; SCL-90: Symptoms Checklist-90; STEPPS: Systems Training for Emotional Predictability and Problem Solving; ZAN-BPD: Zanarini Rating Scale for Borderline Personality Disorder | ||
STEPPS’ theoretical foundation
- does not disrupt the patient’s present regimen, and
- potentially enhances relationship skills by encouraging the patient to remain in longer relationships with professional and non-professional support.
STEPPS employs cognitive-behavioral methods, including identifying and challenging distorted thoughts and specific behavioral change, combined with psycho-education and skills training.11,12 The addition of a systems component that enlists the help of the patient’s family and friends is unique to STEPPS (Box 1).
Emotional intensity disorder. Many clinicians assume that the core deficit in BPD is inability to manage emotional intensity. In STEPPS, therapists reframe BPD as emotional intensity disorder (EID), a term patients find easier to understand and accept. Patients tend to “see themselves as driven by the disorder to seek relief from a painful illness through desperate behaviors that are reinforced by negative and distorted thinking.”16 Starting with the first session, STEPPS therapists validate the patients’ experience of BPD and provide hope by teaching that patients can acquire skills to manage the disorder.
In the first Systems Training for Emotional Predictability and Problem Solving (STEPPS) session, patients identify and utilize a “reinforcement team” that consists of any person or persons—family members, professionals, friends, coworkers, etc.—who agree to assist the patient in reinforcing STEPPS skills. The systems perspective emphasizes patients’ responsibility for responding to their system more effectively by using their skills and helps patients develop more realistic expectations of—and more helpful interactions with—their support system. Patients are:
- expected to become STEPPS experts and to teach their reinforcement team how to respond with the STEPPS “language”
- encouraged to share what they are learning in group sessions, including relevant handouts
- given “reinforcement team” cards that explain how team members should respond when the patient contacts them.
The cards also list the skills taught in STEPPS and provide questions for team members to ask when contacted by the participant (ie, “Where are you on the Emotional Intensity Continuum?” “Have you used your notebook?” “What skill can you use in this situation?” “How will you use it?”). The cards provide a common language and consistent interaction between patients and their support systems. Patients are instructed to give the cards to their reinforcement team members when they request their assistance.
After the first 4 to 6 STEPPS sessions, a 2-hour meeting is arranged for reinforcement team members, during which the facilitators describe diagnostic criteria and clinical symptoms of borderline personality disorder and discuss the STEPPS language and format. Team members are taught that their role is to reinforce and support the use of skills taught in STEPPS. They are shown how to use the reinforcement team cards.
Group format
STEPPS consists of 20 consecutive weekly, 2-hour sessions led by 2 therapists (we prefer the term “facilitators”). Sessions take place in a classroom setting and are highly structured, with specific facilitator guidelines for each session.
When they arrive at each session, patients fill out the Borderline Evaluation of Severity Over Time (BEST) scale (Box 2)11,17 and record the results on a graph to measure their progress. Each session has a specific handout, including an agenda, followed by the homework assignment for the next week. Participants read the handout material aloud during the group session and start the homework assignment to be sure they understand it.
Handouts also include poems, essays, drawings, and examples created by previous STEPPS participants; these provide a sense of ownership among participants past, present, and future. Participants are encouraged to share their own writings and drawings, as well as other resources they have found helpful to illustrate the skills being taught.
Skills training. Facilitators introduce a new skill at each session, and each skill builds on previously taught skills. A recurring theme in STEPPS is that “most of the work is done between sessions”—during the week, patients are expected to practice the skill taught at the previous session. Using the STEPPS skills is framed as “change from the outside in.” As patients challenge maladaptive filters and distorted cognitions, they find that negative feelings and dysfunctional behaviors change.
Patients identify their use of specific skills by completing a 5-point Emotional Intensity Continuum (EIC) scale. This abstract concept is made concrete with drawings of pots on a burner. At level 1 (baseline), there is no heat under the burner; at level 5, the pot is boiling over.
We developed the Borderline Evaluation of Severity Over Time (BEST) to rate severity and change in patients with borderline personality disorder (BPD).11,17 The self-rated scale has 15 items for which patients rate themselves on a 5-point scale; scores can range from 12 to 72.
The BEST shows evidence for good internal consistency and for both face and content validity because the items were constructed to assess behaviors relevant to BPD. We recently assessed the BEST in subjects who had participated in our randomized controlled trials and concluded that the scale is reliable, valid, and sensitive to clinical change as early as week 4.17 To obtain a copy of this scale, contact the authors.
3 components of STEPPS
Awareness of illness. The first step is for patients to replace misconceptions about BPD with awareness of the behaviors and feelings that define the disorder. They are provided with a printed handout listing DSM-IV criteria for BPD and given time to acknowledge examples in their own behavior. This is called “owning” the illness.
The second step is to introduce the concept of schemas, referred to as cognitive filters. With the author’s permission, we extracted 64 items from Young’s Schema Questionnaire,18 which helps patients identify their early maladaptive schemas. We encourage patients to understand the relationship among these filters, DSM-IV criteria, and their subsequent pattern of feelings, thoughts, and behaviors.
Emotion management skills taught in STEPPS are distancing, communicating, challenging, distracting, and managing problems (Table 2). Using these skills, participants learn to:
- predict the course of emotional states
- anticipate stressful situations
- develop functional coping strategies.
Table 2
Patient skills taught in STEPPS
| Awareness of illness |
| Understand what BPD is |
| Reframe BPD as ‘emotional intensity disorder’ |
| ‘Own’ the illness |
| Identify and challenge dysfunctional schemas |
| Emotion management skills |
| Distancing: Provide distance from emotional intensity |
| Communicating: Describe and define feelings, physical sensations, thoughts, filters, action urges, and behaviors |
| Challenging: Identify distorted thinking and develop alternate ways of thinking |
| Distracting: Identify and engage in behaviors that lower emotional intensity or assist in getting through an episode without resorting to damaging behaviors |
| Managing problems: Identify and define problems, then plan and carry out action steps |
| Behavior management skills |
| Setting goals: Identify specific goals and develop strategies to manage specific problematic behaviors |
| Eating: Balanced diet |
| Sleeping: Good sleep hygiene |
| Exercising: Regular and balanced exercise |
| Leisure: Regular leisure activities |
| Physical health: Manage medical problems |
| Abuse avoidance: Develop strategies to replace abusive behaviors (self-harm, substance abuse, gambling, etc.) |
| Relationship management: Identify and determine strategies to develop healthy relationships. Understand and implement healthy boundaries |
| BPD: borderline personality disorder; STEPPS: Systems Training for Emotional Predictability and Problem Solving |
Patient characteristics
Although some patients learn of STEPPS from previous participants, at our facility we usually request a formal professional referral. We then send potential participants a letter inviting them to attend the group, along with a brochure describing STEPPS and a dated syllabus. We generally begin with 12 to 15 patients but typically have 7 to 10 by the fifth session.
Patients with strong narcissistic or antisocial traits may have difficulty in group settings, probably because they prefer to be the center of attention. That said, we have successfully implemented STEPPS in Iowa prisons and have not experienced difficulties.12 Patients who are abusing substances or have active eating disorders (primarily anorexia nervosa) may not be cognitively able to benefit from STEPPS until these behaviors are better controlled. We recommend that patients seek treatment for these behaviors before—or concurrent with—STEPPS participation.
Persons who deal with conflict by physical threats or intimidation are potential threats to group integrity and are removed immediately. We avoid forming groups with a lone male participant because:
- he may come to represent all men to the rest of the group
- he may have difficulty identifying with problems unique to women with BPD.
In a recent study we found that patients who were rated as more symptomatic at baseline experienced the greatest improvement. Apart from this finding, there were few response predictors, but it was reassuring that both men and women improved.19 Members are cautiously encouraged to use each other as reinforcement team members between sessions, once they feel safe in the group. They are instructed to follow the reinforcement team guidelines.
The facilitators’ role
STEPPS groups are led by 2 facilitators with graduate level training in social sciences and psychotherapy experience. Therapists may be trained in STEPPS during a 1- to 2-day on-site workshop or by attending a 20-week group. These trainees are identified as professionals and do not participate in the sessions.
Using 1 male and 1 female facilitator for a STEPPS group allows modeling of relationship behaviors between genders, projects a healthy male role, and provides support for male participants, who in most groups are in the minority. Initially the facilitators’ stance is active and directive, although this tends to decrease as patients gradually are given increasing leadership responsibilities (such as leading brief reviews of homework assignments).
The therapists’ main tasks include:
- maintaining the psychoeducational format
- adhering to the guidelines
- avoiding involvement in individual issues and past traumas (providing individual psychotherapy in a group setting)
- maintaining focus on skills acquisition
- encouraging group cohesion through identification
- facilitating participants’ change of perspective from victims of EID to experts on managing EID.
Follow-up: STAIRWAYS
STAIRWAYS is a 1-year follow-up group that meets twice a month after the 20-week STEPPS program and consists of stand-alone modules addressing:
- Setting goals
- Trying new things (oriented toward long-term goals, such as obtaining a degree, employment, etc.)
- Anger management
- Impulsivity control
- Relationship management (emphasis on conflict management)
- Writing a script (identifying and preparing for future stressors)
- Assertiveness training
- Your choices (making healthy choices)
- Staying on track (relapse prevention).
- The STEPPS Model for Borderline Personality Disorder Manual. www.steppsforbpd.com.
- van Wel B, Kockmann I, Blum N, et al. STEPPS group treatment for borderline personality disorder in The Netherlands. Ann Clin Psychiatry. 2006;18(1):63-67.
Disclosures
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Ms. Blum and Mr. St. John receive royalties from Blums’ Books LLC, publisher of the Systems Training for Emotional Predictability and Problem Solving (STEPPS) manual.
1. American Psychiatric Association. Practice guidelines for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 1):1-52.
2. Soloff PH. Psychopharmacology of borderline personality disorder. Psychiatr Clin North Am. 2000;23:169-191.
3. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6:66-70.
4. Paris J. Borderline personality disorder—a multidimensional approach. Washington, DC: American Psychiatric Press; 1994.
5. Linehan MM. Cognitive-behavioral treatment for borderline personality disorder. New York, NY: Guilford Press; 1993.
6. Clarkin JF, Levy KN, Lenzenweger MF, et al. Evaluating three treatments for borderline personality disorder: a multiwave study. Am J Psychiatry. 2007;164:922-928.
7. Giesen-Bloo J, van Dyck R, Spinhoven P, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused therapy. Arch Gen Psychiatry. 2006;63:649-658.
8. Bateman A, Fonagy P. Effectiveness of partial hospitalization in the treatment of borderline personality disorder: a randomized controlled trial. Am J Psychiatry. 1999;156:1563-1569.
9. Bartels N, Crotty T. A systems approach to treatment: the borderline personality disorder skill training manual. Winfield, IL: EID Treatment Systems, Inc; 1992.
10. Blum N, Bartels N, St. John D, et al. STEPPS: Systems Training for Emotional Predictability and Problem Solving—group treatment for borderline personality disorder. Coralville, IA: Blum’s Books; 2002.
11. Blum N, Pfohl B, St. John D, et al. STEPPS: a cognitive behavioral systems based group treatment for outpatients with borderline personality disorder—a preliminary report. Compr Psychiatry. 2002;43:301-310.
12. Black DW, Blum N, Eichinger L, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) in women offenders with borderline personality disorder in prison: a pilot study. CNS Spectr. 2008;13(10):881-886.
13. Freije H, Dietz B, Appelo M. Behandling van de borderline persoonlijk heidsstoornis met de VERS: de Vaardigheidstraining emotionele regulatiestoornis. Directive Therapies. 2002;4:367-378.
14. Blum N, Pfohl B, St. John D, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165:468-478.
15. van Wel B. VERS: RCT on Dutch STEPPS. Presented at: Annual Meeting of the International Society for the Study of Personality Disorders; September 21, 2007; Den Haag, The Netherlands.
16. Black DW, Blum N, Pfohl B, et al. The STEPPS group treatment program for outpatients with borderline personality disorder. Journal of Contemporary Psychotherapy. 2004;34:193-210.
17. Pfohl B, Blum N, St. John D, et al. Reliability and validity of the Borderline Evaluation of Severity over Time (BEST): a self-rated scale to measure severity and change in persons with borderline personality disorder. J Pers Disord. 2009;23(3):281-293.
18. Young J. Cognitive therapy for personality disorders: a schema-focused approach. Sarasota, FL: Professional Resource Press; 1994.
19. Black DW, Blum N, Pfohl B, et al. Predictors of response to Systems Training to Emotional Predictability and Problem Solving (STEPPS) for borderline personality disorder: an exploratory study. Acta Psychiatr Scand. 2009;120:53-61.
Treatment of borderline personality disorder (BPD) often is viewed as challenging and the results so discouraging that some clinicians avoid referrals of BPD patients.1-3 Psychotherapy has been the treatment mainstay for decades, and supportive approaches are probably the most widely employed.4 Psychodynamic therapy often has been recommended.
This article introduces a new evidence-based group treatment program that we developed for BPD patients. Systems Training for Emotional Predictability and Problem Solving (STEPPS) is founded on the successes of better known psychoeducational models but is easier for practicing psychiatrists to implement.
A different approach to BPD
Linehan5 introduced dialectical behavior therapy (DBT)—a manualized, time-limited, cognitive-behavioral approach in which patients learn to regulate their emotions and behaviors rather than change their personality structure. Other evidence-based BPD treatments include transference-focused psychotherapy,6 schema-focused psychotherapy,7 and Bateman and Fonagy’s mentalization program.8 For a description of the unique challenges presented by BPD patients, see Box.
In the mid-1990s, we set out to create a treatment program for our BPD patients in response to managed care directives to lower the cost of care, decrease length of inpatient treatment, and reduce rehospitalization rates. Despite DBT’s many appealing features, we felt this model was too lengthy and labor-intensive for our treatment setting. We concluded that modifying a program developed by Bartels and Crotty9 would serve our needs. This 12-week psychoeducational program:
- employs established cognitive-behavioral techniques in group treatment intended to supplement but not replace patients’ ongoing treatment
- incorporates a “systems” component that recognizes the importance of the patient’s family and friends.
We eventually renamed the program Systems Training for Emotional Predictability and Problem Solving (STEPPS)10 and created a new manual (see Related Resources) to simplify group leader training and ensure fidelity to the model. Data from 5 studies, including 2 randomized controlled trials (Table 1), show that STEPPS has a robust antidepressant effect and leads to broad-based improvements in the affective, cognitive, impulsive, and disturbed relationship domains of BPD.11-15
Borderline personality disorder (BPD) is 1 of the most challenging mental health conditions. BPD is surprisingly common, with prevalence rates of 0.5% to 1% in the community, 10% in outpatient mental health settings, and up to 20% in inpatient psychiatric settings.a-c Patients with BPD experience substantial functional impairment in several areas (eg, difficulty maintaining employment, disturbed interpersonal relationships, and disrupted family relationships).a,d,e
Many borderline patients have childhood histories of abuse and continue to be victims of domestic and other violence through adulthood.f High utilization of medical and psychiatric health care services is common and costly.g BPD also is associated with substantial psychiatric comorbidity, particularly mood and anxiety disorders, substance use disorders, eating disorders, and other Axis II disorders.h,i
Persons with BPD experience intense dysphoria and intrapsychic pain. Characteristic features include affective intensity, reactivity, and lability; a pervasive pattern of unstable interpersonal relationships; marked behavioral impulsivity; unstable self-identity; intense anger; and extreme fear of abandonment.j
The symptom that probably makes the greatest demand on mental health resources is recurrent suicidal threats/attempts or episodes of self-mutilation, many prompted by disappointment in a relationship.k Two-thirds to three-quarters of BPD patients will attempt suicide, with up to 10% eventually completing suicide, often following multiple failed treatments.l
References
a. Gunderson J. Borderline personality disorder: A clinical guide. 2nd ed. Washington, DC: American Psychiatric Publishing; 2008.
b. Widiger TA, Frances AJ. Epidemiology, diagnosis, and co-morbidity of borderline personality disorder. In: Tasman A, Hales RE, Frances AJ (eds). American Psychiatric Press Review of Psychiatry, vol. 8. Washington, DC: American Psychiatric Press; 1989:8-24.
c. Swartz MS, Blazer D, George L, et al. Estimating the prevalence of borderline personality disorder in the community. J Personal Disord. 1990;4:257-272.
d. Nakao K, Gunderson JG, Phillips KA, et al. Functional impairment in personality disorders. J Personal Disord. 1992;6:24-31.
e. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159:276-283.
f. Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am. 2000;23:89-101.
g. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
h. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I co-morbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733-1739.
i. Zimmerman M, Coryell W. DSM-III personality disorder diagnoses in a non-patient sample: demographic correlates and co-morbidity. Arch Gen Psychiatry. 1989;46:682-689.
j. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 1994.
k. Paris J. Social factors in personality disorders— a biopsychosocial approach to etiology and treatment. New York, NY: Cambridge University Press; 1996.
l. Soloff PH, Lynch KG, Kelly TM. Characteristics of suicide attempts of patients with major depressive episode and borderline personality disorder: a comparative study. Am J Psychiatry. 2000;157:601-608.
Table 1
STEPPS: Trials show improvement across BPD domains
| Study | Patients | Results |
|---|---|---|
| Uncontrolled trials | ||
| Blum et al, 200211 | 52 outpatients; 94% female; mean age 33 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Black et al, 200812 | 12 incarcerated women; mean age 35 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Freije et al, 200213 | 85 patients; 91% female; mean age 32 | Significant improvement in score on a Dutch version of BEST; significant improvement on SCL-90 subscales, especially those rating anxiety, depression, and interpersonal sensitivity |
| Randomized controlled trials | ||
| Blum et al, 200814 | 165 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usual | Patients receiving STEPPS plus treatment as usual experienced greater improvements in ZAN-BPD total score, impulsivity, negative affect, mood, and global functioning |
| van Wel, 200715 | 79 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usuals | Patients receiving STEPPS plus treatment as usual had greater improvements in global psychiatric symptoms using the SCL-90, BPD symptoms, and quality of life measures at the end of treatment and at 6-month follow-up |
| BDI: Beck Depression Inventory; BEST: Borderline Evaluation of Severity Over Time; BPD: borderline personality disorder; PANAS: Positive and Negative Affect Scale; SCL-90: Symptoms Checklist-90; STEPPS: Systems Training for Emotional Predictability and Problem Solving; ZAN-BPD: Zanarini Rating Scale for Borderline Personality Disorder | ||
STEPPS’ theoretical foundation
- does not disrupt the patient’s present regimen, and
- potentially enhances relationship skills by encouraging the patient to remain in longer relationships with professional and non-professional support.
STEPPS employs cognitive-behavioral methods, including identifying and challenging distorted thoughts and specific behavioral change, combined with psycho-education and skills training.11,12 The addition of a systems component that enlists the help of the patient’s family and friends is unique to STEPPS (Box 1).
Emotional intensity disorder. Many clinicians assume that the core deficit in BPD is inability to manage emotional intensity. In STEPPS, therapists reframe BPD as emotional intensity disorder (EID), a term patients find easier to understand and accept. Patients tend to “see themselves as driven by the disorder to seek relief from a painful illness through desperate behaviors that are reinforced by negative and distorted thinking.”16 Starting with the first session, STEPPS therapists validate the patients’ experience of BPD and provide hope by teaching that patients can acquire skills to manage the disorder.
In the first Systems Training for Emotional Predictability and Problem Solving (STEPPS) session, patients identify and utilize a “reinforcement team” that consists of any person or persons—family members, professionals, friends, coworkers, etc.—who agree to assist the patient in reinforcing STEPPS skills. The systems perspective emphasizes patients’ responsibility for responding to their system more effectively by using their skills and helps patients develop more realistic expectations of—and more helpful interactions with—their support system. Patients are:
- expected to become STEPPS experts and to teach their reinforcement team how to respond with the STEPPS “language”
- encouraged to share what they are learning in group sessions, including relevant handouts
- given “reinforcement team” cards that explain how team members should respond when the patient contacts them.
The cards also list the skills taught in STEPPS and provide questions for team members to ask when contacted by the participant (ie, “Where are you on the Emotional Intensity Continuum?” “Have you used your notebook?” “What skill can you use in this situation?” “How will you use it?”). The cards provide a common language and consistent interaction between patients and their support systems. Patients are instructed to give the cards to their reinforcement team members when they request their assistance.
After the first 4 to 6 STEPPS sessions, a 2-hour meeting is arranged for reinforcement team members, during which the facilitators describe diagnostic criteria and clinical symptoms of borderline personality disorder and discuss the STEPPS language and format. Team members are taught that their role is to reinforce and support the use of skills taught in STEPPS. They are shown how to use the reinforcement team cards.
Group format
STEPPS consists of 20 consecutive weekly, 2-hour sessions led by 2 therapists (we prefer the term “facilitators”). Sessions take place in a classroom setting and are highly structured, with specific facilitator guidelines for each session.
When they arrive at each session, patients fill out the Borderline Evaluation of Severity Over Time (BEST) scale (Box 2)11,17 and record the results on a graph to measure their progress. Each session has a specific handout, including an agenda, followed by the homework assignment for the next week. Participants read the handout material aloud during the group session and start the homework assignment to be sure they understand it.
Handouts also include poems, essays, drawings, and examples created by previous STEPPS participants; these provide a sense of ownership among participants past, present, and future. Participants are encouraged to share their own writings and drawings, as well as other resources they have found helpful to illustrate the skills being taught.
Skills training. Facilitators introduce a new skill at each session, and each skill builds on previously taught skills. A recurring theme in STEPPS is that “most of the work is done between sessions”—during the week, patients are expected to practice the skill taught at the previous session. Using the STEPPS skills is framed as “change from the outside in.” As patients challenge maladaptive filters and distorted cognitions, they find that negative feelings and dysfunctional behaviors change.
Patients identify their use of specific skills by completing a 5-point Emotional Intensity Continuum (EIC) scale. This abstract concept is made concrete with drawings of pots on a burner. At level 1 (baseline), there is no heat under the burner; at level 5, the pot is boiling over.
We developed the Borderline Evaluation of Severity Over Time (BEST) to rate severity and change in patients with borderline personality disorder (BPD).11,17 The self-rated scale has 15 items for which patients rate themselves on a 5-point scale; scores can range from 12 to 72.
The BEST shows evidence for good internal consistency and for both face and content validity because the items were constructed to assess behaviors relevant to BPD. We recently assessed the BEST in subjects who had participated in our randomized controlled trials and concluded that the scale is reliable, valid, and sensitive to clinical change as early as week 4.17 To obtain a copy of this scale, contact the authors.
3 components of STEPPS
Awareness of illness. The first step is for patients to replace misconceptions about BPD with awareness of the behaviors and feelings that define the disorder. They are provided with a printed handout listing DSM-IV criteria for BPD and given time to acknowledge examples in their own behavior. This is called “owning” the illness.
The second step is to introduce the concept of schemas, referred to as cognitive filters. With the author’s permission, we extracted 64 items from Young’s Schema Questionnaire,18 which helps patients identify their early maladaptive schemas. We encourage patients to understand the relationship among these filters, DSM-IV criteria, and their subsequent pattern of feelings, thoughts, and behaviors.
Emotion management skills taught in STEPPS are distancing, communicating, challenging, distracting, and managing problems (Table 2). Using these skills, participants learn to:
- predict the course of emotional states
- anticipate stressful situations
- develop functional coping strategies.
Table 2
Patient skills taught in STEPPS
| Awareness of illness |
| Understand what BPD is |
| Reframe BPD as ‘emotional intensity disorder’ |
| ‘Own’ the illness |
| Identify and challenge dysfunctional schemas |
| Emotion management skills |
| Distancing: Provide distance from emotional intensity |
| Communicating: Describe and define feelings, physical sensations, thoughts, filters, action urges, and behaviors |
| Challenging: Identify distorted thinking and develop alternate ways of thinking |
| Distracting: Identify and engage in behaviors that lower emotional intensity or assist in getting through an episode without resorting to damaging behaviors |
| Managing problems: Identify and define problems, then plan and carry out action steps |
| Behavior management skills |
| Setting goals: Identify specific goals and develop strategies to manage specific problematic behaviors |
| Eating: Balanced diet |
| Sleeping: Good sleep hygiene |
| Exercising: Regular and balanced exercise |
| Leisure: Regular leisure activities |
| Physical health: Manage medical problems |
| Abuse avoidance: Develop strategies to replace abusive behaviors (self-harm, substance abuse, gambling, etc.) |
| Relationship management: Identify and determine strategies to develop healthy relationships. Understand and implement healthy boundaries |
| BPD: borderline personality disorder; STEPPS: Systems Training for Emotional Predictability and Problem Solving |
Patient characteristics
Although some patients learn of STEPPS from previous participants, at our facility we usually request a formal professional referral. We then send potential participants a letter inviting them to attend the group, along with a brochure describing STEPPS and a dated syllabus. We generally begin with 12 to 15 patients but typically have 7 to 10 by the fifth session.
Patients with strong narcissistic or antisocial traits may have difficulty in group settings, probably because they prefer to be the center of attention. That said, we have successfully implemented STEPPS in Iowa prisons and have not experienced difficulties.12 Patients who are abusing substances or have active eating disorders (primarily anorexia nervosa) may not be cognitively able to benefit from STEPPS until these behaviors are better controlled. We recommend that patients seek treatment for these behaviors before—or concurrent with—STEPPS participation.
Persons who deal with conflict by physical threats or intimidation are potential threats to group integrity and are removed immediately. We avoid forming groups with a lone male participant because:
- he may come to represent all men to the rest of the group
- he may have difficulty identifying with problems unique to women with BPD.
In a recent study we found that patients who were rated as more symptomatic at baseline experienced the greatest improvement. Apart from this finding, there were few response predictors, but it was reassuring that both men and women improved.19 Members are cautiously encouraged to use each other as reinforcement team members between sessions, once they feel safe in the group. They are instructed to follow the reinforcement team guidelines.
The facilitators’ role
STEPPS groups are led by 2 facilitators with graduate level training in social sciences and psychotherapy experience. Therapists may be trained in STEPPS during a 1- to 2-day on-site workshop or by attending a 20-week group. These trainees are identified as professionals and do not participate in the sessions.
Using 1 male and 1 female facilitator for a STEPPS group allows modeling of relationship behaviors between genders, projects a healthy male role, and provides support for male participants, who in most groups are in the minority. Initially the facilitators’ stance is active and directive, although this tends to decrease as patients gradually are given increasing leadership responsibilities (such as leading brief reviews of homework assignments).
The therapists’ main tasks include:
- maintaining the psychoeducational format
- adhering to the guidelines
- avoiding involvement in individual issues and past traumas (providing individual psychotherapy in a group setting)
- maintaining focus on skills acquisition
- encouraging group cohesion through identification
- facilitating participants’ change of perspective from victims of EID to experts on managing EID.
Follow-up: STAIRWAYS
STAIRWAYS is a 1-year follow-up group that meets twice a month after the 20-week STEPPS program and consists of stand-alone modules addressing:
- Setting goals
- Trying new things (oriented toward long-term goals, such as obtaining a degree, employment, etc.)
- Anger management
- Impulsivity control
- Relationship management (emphasis on conflict management)
- Writing a script (identifying and preparing for future stressors)
- Assertiveness training
- Your choices (making healthy choices)
- Staying on track (relapse prevention).
- The STEPPS Model for Borderline Personality Disorder Manual. www.steppsforbpd.com.
- van Wel B, Kockmann I, Blum N, et al. STEPPS group treatment for borderline personality disorder in The Netherlands. Ann Clin Psychiatry. 2006;18(1):63-67.
Disclosures
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Ms. Blum and Mr. St. John receive royalties from Blums’ Books LLC, publisher of the Systems Training for Emotional Predictability and Problem Solving (STEPPS) manual.
Treatment of borderline personality disorder (BPD) often is viewed as challenging and the results so discouraging that some clinicians avoid referrals of BPD patients.1-3 Psychotherapy has been the treatment mainstay for decades, and supportive approaches are probably the most widely employed.4 Psychodynamic therapy often has been recommended.
This article introduces a new evidence-based group treatment program that we developed for BPD patients. Systems Training for Emotional Predictability and Problem Solving (STEPPS) is founded on the successes of better known psychoeducational models but is easier for practicing psychiatrists to implement.
A different approach to BPD
Linehan5 introduced dialectical behavior therapy (DBT)—a manualized, time-limited, cognitive-behavioral approach in which patients learn to regulate their emotions and behaviors rather than change their personality structure. Other evidence-based BPD treatments include transference-focused psychotherapy,6 schema-focused psychotherapy,7 and Bateman and Fonagy’s mentalization program.8 For a description of the unique challenges presented by BPD patients, see Box.
In the mid-1990s, we set out to create a treatment program for our BPD patients in response to managed care directives to lower the cost of care, decrease length of inpatient treatment, and reduce rehospitalization rates. Despite DBT’s many appealing features, we felt this model was too lengthy and labor-intensive for our treatment setting. We concluded that modifying a program developed by Bartels and Crotty9 would serve our needs. This 12-week psychoeducational program:
- employs established cognitive-behavioral techniques in group treatment intended to supplement but not replace patients’ ongoing treatment
- incorporates a “systems” component that recognizes the importance of the patient’s family and friends.
We eventually renamed the program Systems Training for Emotional Predictability and Problem Solving (STEPPS)10 and created a new manual (see Related Resources) to simplify group leader training and ensure fidelity to the model. Data from 5 studies, including 2 randomized controlled trials (Table 1), show that STEPPS has a robust antidepressant effect and leads to broad-based improvements in the affective, cognitive, impulsive, and disturbed relationship domains of BPD.11-15
Borderline personality disorder (BPD) is 1 of the most challenging mental health conditions. BPD is surprisingly common, with prevalence rates of 0.5% to 1% in the community, 10% in outpatient mental health settings, and up to 20% in inpatient psychiatric settings.a-c Patients with BPD experience substantial functional impairment in several areas (eg, difficulty maintaining employment, disturbed interpersonal relationships, and disrupted family relationships).a,d,e
Many borderline patients have childhood histories of abuse and continue to be victims of domestic and other violence through adulthood.f High utilization of medical and psychiatric health care services is common and costly.g BPD also is associated with substantial psychiatric comorbidity, particularly mood and anxiety disorders, substance use disorders, eating disorders, and other Axis II disorders.h,i
Persons with BPD experience intense dysphoria and intrapsychic pain. Characteristic features include affective intensity, reactivity, and lability; a pervasive pattern of unstable interpersonal relationships; marked behavioral impulsivity; unstable self-identity; intense anger; and extreme fear of abandonment.j
The symptom that probably makes the greatest demand on mental health resources is recurrent suicidal threats/attempts or episodes of self-mutilation, many prompted by disappointment in a relationship.k Two-thirds to three-quarters of BPD patients will attempt suicide, with up to 10% eventually completing suicide, often following multiple failed treatments.l
References
a. Gunderson J. Borderline personality disorder: A clinical guide. 2nd ed. Washington, DC: American Psychiatric Publishing; 2008.
b. Widiger TA, Frances AJ. Epidemiology, diagnosis, and co-morbidity of borderline personality disorder. In: Tasman A, Hales RE, Frances AJ (eds). American Psychiatric Press Review of Psychiatry, vol. 8. Washington, DC: American Psychiatric Press; 1989:8-24.
c. Swartz MS, Blazer D, George L, et al. Estimating the prevalence of borderline personality disorder in the community. J Personal Disord. 1990;4:257-272.
d. Nakao K, Gunderson JG, Phillips KA, et al. Functional impairment in personality disorders. J Personal Disord. 1992;6:24-31.
e. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159:276-283.
f. Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am. 2000;23:89-101.
g. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158:295-302.
h. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I co-morbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733-1739.
i. Zimmerman M, Coryell W. DSM-III personality disorder diagnoses in a non-patient sample: demographic correlates and co-morbidity. Arch Gen Psychiatry. 1989;46:682-689.
j. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 1994.
k. Paris J. Social factors in personality disorders— a biopsychosocial approach to etiology and treatment. New York, NY: Cambridge University Press; 1996.
l. Soloff PH, Lynch KG, Kelly TM. Characteristics of suicide attempts of patients with major depressive episode and borderline personality disorder: a comparative study. Am J Psychiatry. 2000;157:601-608.
Table 1
STEPPS: Trials show improvement across BPD domains
| Study | Patients | Results |
|---|---|---|
| Uncontrolled trials | ||
| Blum et al, 200211 | 52 outpatients; 94% female; mean age 33 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Black et al, 200812 | 12 incarcerated women; mean age 35 | Significant improvement in BEST score; significant drop in BDI score and the PANAS negative affect scale |
| Freije et al, 200213 | 85 patients; 91% female; mean age 32 | Significant improvement in score on a Dutch version of BEST; significant improvement on SCL-90 subscales, especially those rating anxiety, depression, and interpersonal sensitivity |
| Randomized controlled trials | ||
| Blum et al, 200814 | 165 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usual | Patients receiving STEPPS plus treatment as usual experienced greater improvements in ZAN-BPD total score, impulsivity, negative affect, mood, and global functioning |
| van Wel, 200715 | 79 adults with BPD assigned to STEPPS plus treatment as usual or only treatment as usuals | Patients receiving STEPPS plus treatment as usual had greater improvements in global psychiatric symptoms using the SCL-90, BPD symptoms, and quality of life measures at the end of treatment and at 6-month follow-up |
| BDI: Beck Depression Inventory; BEST: Borderline Evaluation of Severity Over Time; BPD: borderline personality disorder; PANAS: Positive and Negative Affect Scale; SCL-90: Symptoms Checklist-90; STEPPS: Systems Training for Emotional Predictability and Problem Solving; ZAN-BPD: Zanarini Rating Scale for Borderline Personality Disorder | ||
STEPPS’ theoretical foundation
- does not disrupt the patient’s present regimen, and
- potentially enhances relationship skills by encouraging the patient to remain in longer relationships with professional and non-professional support.
STEPPS employs cognitive-behavioral methods, including identifying and challenging distorted thoughts and specific behavioral change, combined with psycho-education and skills training.11,12 The addition of a systems component that enlists the help of the patient’s family and friends is unique to STEPPS (Box 1).
Emotional intensity disorder. Many clinicians assume that the core deficit in BPD is inability to manage emotional intensity. In STEPPS, therapists reframe BPD as emotional intensity disorder (EID), a term patients find easier to understand and accept. Patients tend to “see themselves as driven by the disorder to seek relief from a painful illness through desperate behaviors that are reinforced by negative and distorted thinking.”16 Starting with the first session, STEPPS therapists validate the patients’ experience of BPD and provide hope by teaching that patients can acquire skills to manage the disorder.
In the first Systems Training for Emotional Predictability and Problem Solving (STEPPS) session, patients identify and utilize a “reinforcement team” that consists of any person or persons—family members, professionals, friends, coworkers, etc.—who agree to assist the patient in reinforcing STEPPS skills. The systems perspective emphasizes patients’ responsibility for responding to their system more effectively by using their skills and helps patients develop more realistic expectations of—and more helpful interactions with—their support system. Patients are:
- expected to become STEPPS experts and to teach their reinforcement team how to respond with the STEPPS “language”
- encouraged to share what they are learning in group sessions, including relevant handouts
- given “reinforcement team” cards that explain how team members should respond when the patient contacts them.
The cards also list the skills taught in STEPPS and provide questions for team members to ask when contacted by the participant (ie, “Where are you on the Emotional Intensity Continuum?” “Have you used your notebook?” “What skill can you use in this situation?” “How will you use it?”). The cards provide a common language and consistent interaction between patients and their support systems. Patients are instructed to give the cards to their reinforcement team members when they request their assistance.
After the first 4 to 6 STEPPS sessions, a 2-hour meeting is arranged for reinforcement team members, during which the facilitators describe diagnostic criteria and clinical symptoms of borderline personality disorder and discuss the STEPPS language and format. Team members are taught that their role is to reinforce and support the use of skills taught in STEPPS. They are shown how to use the reinforcement team cards.
Group format
STEPPS consists of 20 consecutive weekly, 2-hour sessions led by 2 therapists (we prefer the term “facilitators”). Sessions take place in a classroom setting and are highly structured, with specific facilitator guidelines for each session.
When they arrive at each session, patients fill out the Borderline Evaluation of Severity Over Time (BEST) scale (Box 2)11,17 and record the results on a graph to measure their progress. Each session has a specific handout, including an agenda, followed by the homework assignment for the next week. Participants read the handout material aloud during the group session and start the homework assignment to be sure they understand it.
Handouts also include poems, essays, drawings, and examples created by previous STEPPS participants; these provide a sense of ownership among participants past, present, and future. Participants are encouraged to share their own writings and drawings, as well as other resources they have found helpful to illustrate the skills being taught.
Skills training. Facilitators introduce a new skill at each session, and each skill builds on previously taught skills. A recurring theme in STEPPS is that “most of the work is done between sessions”—during the week, patients are expected to practice the skill taught at the previous session. Using the STEPPS skills is framed as “change from the outside in.” As patients challenge maladaptive filters and distorted cognitions, they find that negative feelings and dysfunctional behaviors change.
Patients identify their use of specific skills by completing a 5-point Emotional Intensity Continuum (EIC) scale. This abstract concept is made concrete with drawings of pots on a burner. At level 1 (baseline), there is no heat under the burner; at level 5, the pot is boiling over.
We developed the Borderline Evaluation of Severity Over Time (BEST) to rate severity and change in patients with borderline personality disorder (BPD).11,17 The self-rated scale has 15 items for which patients rate themselves on a 5-point scale; scores can range from 12 to 72.
The BEST shows evidence for good internal consistency and for both face and content validity because the items were constructed to assess behaviors relevant to BPD. We recently assessed the BEST in subjects who had participated in our randomized controlled trials and concluded that the scale is reliable, valid, and sensitive to clinical change as early as week 4.17 To obtain a copy of this scale, contact the authors.
3 components of STEPPS
Awareness of illness. The first step is for patients to replace misconceptions about BPD with awareness of the behaviors and feelings that define the disorder. They are provided with a printed handout listing DSM-IV criteria for BPD and given time to acknowledge examples in their own behavior. This is called “owning” the illness.
The second step is to introduce the concept of schemas, referred to as cognitive filters. With the author’s permission, we extracted 64 items from Young’s Schema Questionnaire,18 which helps patients identify their early maladaptive schemas. We encourage patients to understand the relationship among these filters, DSM-IV criteria, and their subsequent pattern of feelings, thoughts, and behaviors.
Emotion management skills taught in STEPPS are distancing, communicating, challenging, distracting, and managing problems (Table 2). Using these skills, participants learn to:
- predict the course of emotional states
- anticipate stressful situations
- develop functional coping strategies.
Table 2
Patient skills taught in STEPPS
| Awareness of illness |
| Understand what BPD is |
| Reframe BPD as ‘emotional intensity disorder’ |
| ‘Own’ the illness |
| Identify and challenge dysfunctional schemas |
| Emotion management skills |
| Distancing: Provide distance from emotional intensity |
| Communicating: Describe and define feelings, physical sensations, thoughts, filters, action urges, and behaviors |
| Challenging: Identify distorted thinking and develop alternate ways of thinking |
| Distracting: Identify and engage in behaviors that lower emotional intensity or assist in getting through an episode without resorting to damaging behaviors |
| Managing problems: Identify and define problems, then plan and carry out action steps |
| Behavior management skills |
| Setting goals: Identify specific goals and develop strategies to manage specific problematic behaviors |
| Eating: Balanced diet |
| Sleeping: Good sleep hygiene |
| Exercising: Regular and balanced exercise |
| Leisure: Regular leisure activities |
| Physical health: Manage medical problems |
| Abuse avoidance: Develop strategies to replace abusive behaviors (self-harm, substance abuse, gambling, etc.) |
| Relationship management: Identify and determine strategies to develop healthy relationships. Understand and implement healthy boundaries |
| BPD: borderline personality disorder; STEPPS: Systems Training for Emotional Predictability and Problem Solving |
Patient characteristics
Although some patients learn of STEPPS from previous participants, at our facility we usually request a formal professional referral. We then send potential participants a letter inviting them to attend the group, along with a brochure describing STEPPS and a dated syllabus. We generally begin with 12 to 15 patients but typically have 7 to 10 by the fifth session.
Patients with strong narcissistic or antisocial traits may have difficulty in group settings, probably because they prefer to be the center of attention. That said, we have successfully implemented STEPPS in Iowa prisons and have not experienced difficulties.12 Patients who are abusing substances or have active eating disorders (primarily anorexia nervosa) may not be cognitively able to benefit from STEPPS until these behaviors are better controlled. We recommend that patients seek treatment for these behaviors before—or concurrent with—STEPPS participation.
Persons who deal with conflict by physical threats or intimidation are potential threats to group integrity and are removed immediately. We avoid forming groups with a lone male participant because:
- he may come to represent all men to the rest of the group
- he may have difficulty identifying with problems unique to women with BPD.
In a recent study we found that patients who were rated as more symptomatic at baseline experienced the greatest improvement. Apart from this finding, there were few response predictors, but it was reassuring that both men and women improved.19 Members are cautiously encouraged to use each other as reinforcement team members between sessions, once they feel safe in the group. They are instructed to follow the reinforcement team guidelines.
The facilitators’ role
STEPPS groups are led by 2 facilitators with graduate level training in social sciences and psychotherapy experience. Therapists may be trained in STEPPS during a 1- to 2-day on-site workshop or by attending a 20-week group. These trainees are identified as professionals and do not participate in the sessions.
Using 1 male and 1 female facilitator for a STEPPS group allows modeling of relationship behaviors between genders, projects a healthy male role, and provides support for male participants, who in most groups are in the minority. Initially the facilitators’ stance is active and directive, although this tends to decrease as patients gradually are given increasing leadership responsibilities (such as leading brief reviews of homework assignments).
The therapists’ main tasks include:
- maintaining the psychoeducational format
- adhering to the guidelines
- avoiding involvement in individual issues and past traumas (providing individual psychotherapy in a group setting)
- maintaining focus on skills acquisition
- encouraging group cohesion through identification
- facilitating participants’ change of perspective from victims of EID to experts on managing EID.
Follow-up: STAIRWAYS
STAIRWAYS is a 1-year follow-up group that meets twice a month after the 20-week STEPPS program and consists of stand-alone modules addressing:
- Setting goals
- Trying new things (oriented toward long-term goals, such as obtaining a degree, employment, etc.)
- Anger management
- Impulsivity control
- Relationship management (emphasis on conflict management)
- Writing a script (identifying and preparing for future stressors)
- Assertiveness training
- Your choices (making healthy choices)
- Staying on track (relapse prevention).
- The STEPPS Model for Borderline Personality Disorder Manual. www.steppsforbpd.com.
- van Wel B, Kockmann I, Blum N, et al. STEPPS group treatment for borderline personality disorder in The Netherlands. Ann Clin Psychiatry. 2006;18(1):63-67.
Disclosures
Dr. Black receives research/grant support from AstraZeneca and Forest Laboratories and is a consultant to Jazz Pharmaceuticals.
Ms. Blum and Mr. St. John receive royalties from Blums’ Books LLC, publisher of the Systems Training for Emotional Predictability and Problem Solving (STEPPS) manual.
1. American Psychiatric Association. Practice guidelines for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 1):1-52.
2. Soloff PH. Psychopharmacology of borderline personality disorder. Psychiatr Clin North Am. 2000;23:169-191.
3. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6:66-70.
4. Paris J. Borderline personality disorder—a multidimensional approach. Washington, DC: American Psychiatric Press; 1994.
5. Linehan MM. Cognitive-behavioral treatment for borderline personality disorder. New York, NY: Guilford Press; 1993.
6. Clarkin JF, Levy KN, Lenzenweger MF, et al. Evaluating three treatments for borderline personality disorder: a multiwave study. Am J Psychiatry. 2007;164:922-928.
7. Giesen-Bloo J, van Dyck R, Spinhoven P, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused therapy. Arch Gen Psychiatry. 2006;63:649-658.
8. Bateman A, Fonagy P. Effectiveness of partial hospitalization in the treatment of borderline personality disorder: a randomized controlled trial. Am J Psychiatry. 1999;156:1563-1569.
9. Bartels N, Crotty T. A systems approach to treatment: the borderline personality disorder skill training manual. Winfield, IL: EID Treatment Systems, Inc; 1992.
10. Blum N, Bartels N, St. John D, et al. STEPPS: Systems Training for Emotional Predictability and Problem Solving—group treatment for borderline personality disorder. Coralville, IA: Blum’s Books; 2002.
11. Blum N, Pfohl B, St. John D, et al. STEPPS: a cognitive behavioral systems based group treatment for outpatients with borderline personality disorder—a preliminary report. Compr Psychiatry. 2002;43:301-310.
12. Black DW, Blum N, Eichinger L, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) in women offenders with borderline personality disorder in prison: a pilot study. CNS Spectr. 2008;13(10):881-886.
13. Freije H, Dietz B, Appelo M. Behandling van de borderline persoonlijk heidsstoornis met de VERS: de Vaardigheidstraining emotionele regulatiestoornis. Directive Therapies. 2002;4:367-378.
14. Blum N, Pfohl B, St. John D, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165:468-478.
15. van Wel B. VERS: RCT on Dutch STEPPS. Presented at: Annual Meeting of the International Society for the Study of Personality Disorders; September 21, 2007; Den Haag, The Netherlands.
16. Black DW, Blum N, Pfohl B, et al. The STEPPS group treatment program for outpatients with borderline personality disorder. Journal of Contemporary Psychotherapy. 2004;34:193-210.
17. Pfohl B, Blum N, St. John D, et al. Reliability and validity of the Borderline Evaluation of Severity over Time (BEST): a self-rated scale to measure severity and change in persons with borderline personality disorder. J Pers Disord. 2009;23(3):281-293.
18. Young J. Cognitive therapy for personality disorders: a schema-focused approach. Sarasota, FL: Professional Resource Press; 1994.
19. Black DW, Blum N, Pfohl B, et al. Predictors of response to Systems Training to Emotional Predictability and Problem Solving (STEPPS) for borderline personality disorder: an exploratory study. Acta Psychiatr Scand. 2009;120:53-61.
1. American Psychiatric Association. Practice guidelines for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 1):1-52.
2. Soloff PH. Psychopharmacology of borderline personality disorder. Psychiatr Clin North Am. 2000;23:169-191.
3. Zanarini MC. Update on pharmacotherapy of borderline personality disorder. Curr Psychiatry Rep. 2004;6:66-70.
4. Paris J. Borderline personality disorder—a multidimensional approach. Washington, DC: American Psychiatric Press; 1994.
5. Linehan MM. Cognitive-behavioral treatment for borderline personality disorder. New York, NY: Guilford Press; 1993.
6. Clarkin JF, Levy KN, Lenzenweger MF, et al. Evaluating three treatments for borderline personality disorder: a multiwave study. Am J Psychiatry. 2007;164:922-928.
7. Giesen-Bloo J, van Dyck R, Spinhoven P, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs transference-focused therapy. Arch Gen Psychiatry. 2006;63:649-658.
8. Bateman A, Fonagy P. Effectiveness of partial hospitalization in the treatment of borderline personality disorder: a randomized controlled trial. Am J Psychiatry. 1999;156:1563-1569.
9. Bartels N, Crotty T. A systems approach to treatment: the borderline personality disorder skill training manual. Winfield, IL: EID Treatment Systems, Inc; 1992.
10. Blum N, Bartels N, St. John D, et al. STEPPS: Systems Training for Emotional Predictability and Problem Solving—group treatment for borderline personality disorder. Coralville, IA: Blum’s Books; 2002.
11. Blum N, Pfohl B, St. John D, et al. STEPPS: a cognitive behavioral systems based group treatment for outpatients with borderline personality disorder—a preliminary report. Compr Psychiatry. 2002;43:301-310.
12. Black DW, Blum N, Eichinger L, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) in women offenders with borderline personality disorder in prison: a pilot study. CNS Spectr. 2008;13(10):881-886.
13. Freije H, Dietz B, Appelo M. Behandling van de borderline persoonlijk heidsstoornis met de VERS: de Vaardigheidstraining emotionele regulatiestoornis. Directive Therapies. 2002;4:367-378.
14. Blum N, Pfohl B, St. John D, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry. 2008;165:468-478.
15. van Wel B. VERS: RCT on Dutch STEPPS. Presented at: Annual Meeting of the International Society for the Study of Personality Disorders; September 21, 2007; Den Haag, The Netherlands.
16. Black DW, Blum N, Pfohl B, et al. The STEPPS group treatment program for outpatients with borderline personality disorder. Journal of Contemporary Psychotherapy. 2004;34:193-210.
17. Pfohl B, Blum N, St. John D, et al. Reliability and validity of the Borderline Evaluation of Severity over Time (BEST): a self-rated scale to measure severity and change in persons with borderline personality disorder. J Pers Disord. 2009;23(3):281-293.
18. Young J. Cognitive therapy for personality disorders: a schema-focused approach. Sarasota, FL: Professional Resource Press; 1994.
19. Black DW, Blum N, Pfohl B, et al. Predictors of response to Systems Training to Emotional Predictability and Problem Solving (STEPPS) for borderline personality disorder: an exploratory study. Acta Psychiatr Scand. 2009;120:53-61.
How and why it pays to genuinely praise a difficult patient
Iloperidone for schizophrenia
Iloperidone is a second-generation (atypical) antipsychotic the FDA approved in May 2009 for treating acute schizophrenia in adults (Table 1). Iloperidone is not a derivative (metabolite, isomer, or different formulation) of any other antipsychotic. Clinical trials have shown that iloperidone is efficacious and suggest that for some patients its side-effect profile may be more favorable than that of other antipsychotics.
Table 1
Iloperidone: Fast facts
| Brand name: Fanapt |
| Class: Atypical antipsychotic (serotonin/dopamine antagonist) |
| Indication: Acute schizophrenia in adults |
| Approval date: May 2009 |
| Availability date: Late 2009 |
| Manufacturer: Vanda Pharmaceuticals, Inc. |
| Dosing forms: 1-, 2-, 4-, 6-, 8-, 10-, and 12-mg tablets (nonscored); titration pack of 2×1-mg, 2×2-mg, 2×4-mg, and 2×6-mg tablets |
| Starting dose: 1 mg bid (2 mg total daily dose) |
| Target dose: 12 to 24 mg total daily dose |
Clinical implications
Iloperidone’s binding profile is similar to that of other antipsychotics with relatively stronger affinity for serotonin (5-HT2A) than dopamine (D2) receptors, and its efficacy is roughly comparable to that of other non-clozapine antipsychotics.
Individual patients may respond differently to specific antipsychotics, even when those agents have shown equivalent efficacy in clinical trials. Therefore, a key therapeutic question is the degree of differential efficacy—differences in response at an individual level—among iloperidone and other antipsychotics.
The differential efficacy among iloperidone and other antipsychotics is unknown. Our clinical experience and iloperidone’s unique structure suggest, however, that this agent might be helpful for certain patients who do not fully respond to or are unable to tolerate other antipsychotics.
How iloperidone works
Like other antipsychotics, iloperidone’s efficacy presumably is based on its ability to block [antagonize] dopamine D2 receptors. Its chemical structure is most similar to risperidone, paliperidone, and ziprasidone, but its receptor binding profile is distinguished by a relatively lower affinity for serotonin receptors 5-HT1A and 5-HT2C than ziprasidone, and a relative lack of muscarinic and histaminic antagonist properties (Table 2).
The relatively higher affinity of iloperidone (and its metabolite P95) for the NEα1 receptor correlates with the drug’s propensity to cause orthostatic hypotension during initial up-titration.1 Differences in iloperidone’s receptor binding profile compared with other antipsychotics likely are responsible for its different side-effect profile.2,3
Table 2
Relative receptor binding affinities of 3 atypical antipsychotics*
| Binding affinity | |||
|---|---|---|---|
| Receptor | Risperidone | Ziprasidone | Iloperidone |
| Dopamine D2 | High | High | High |
| Serotonin 5-HT1A | Low | High | Low |
| Serotonin 5-HT2A | High | High | High |
| Serotonin 5-HT2C | Moderate | High | Moderate† |
| Norepinephrine NEα1 | High | Moderate | Moderate‡ |
| Histamine H1 | Moderate | Moderate | Low |
| Muscarinic M1 | Negligible | Negligible | Negligible |
| * Cross-comparison of binding strengths reflects the subjective judgment of the authors. The goal is to demonstrate differences in overall binding patterns, and these estimates should not be considered an exact cross-comparison | |||
| † Published reports of binding affinity of iloperidone show considerable variation for the 5HT2C site | |||
| ‡ One metabolite of iloperidone [P95] does not have CNS activity but has potent alpha-1 antagonism and may contribute to the initial orthostatic hypotension seen in clinical trials | |||
Pharmacokinetics
Iloperidone is administered twice daily and can be taken with or without food. The bioavailability of iloperidone tablets is 96%, and peak plasma concentrations are achieved 2 to 4 hours after ingestion.
Like all antipsychotics except paliperidone, iloperidone is metabolized by the liver’s cytochrome P450 (CYP) system. The enzyme pathways CYP3A4 and CYP2D6 transform iloperidone into 2 metabolites: one with CNS activity (P88) and one that does not cross the blood-brain barrier and is not active in the CNS (P95) but likely has peripheral effects.
Genetic variations in CYP2D6 activity can substantially alter how individual patients metabolize iloperidone. The half-life of iloperidone and its active metabolites differs depending on whether someone is a poor metabolizer (no functional CYP2D6 activity), intermediate metabolizer (reduced CYP2D6 activity), or extensive metabolizer (“normal” CYP2D6 activity). The usual half-life of iloperidone (approximately 18 hours in extensive metabolizers) can be almost 50% longer (>24 hours) in CYP2D6 poor metabolizers.
There are no recommendations to test patients for genetic variants that result in poor metabolism from CYP2D6. Rather, clinicians simply need to be aware that this could be the source of interindividual differences they see in iloperidone tolerability, just as it is for any other medication that is a substrate for the CYP2D6 enzyme system.
Interactions. Medications that inhibit the CYP3A4 or CYP2D6 systems can increase iloperidone plasma level when taken concurrently with iloperidone, even if intrinsic liver metabolism activity is normal. Fluoxetine and paroxetine are potent CYP2D6 inhibitors. Concurrent treatment with either of these selective serotonin reuptake inhibitors could increase iloperidone plasma concentration by 100% or more.4
Similarly, cotreatment with a potent CYP3A4 inhibitor such as ketoconazole (or drinking grapefruit juice) will decrease metabolism and increase plasma concentrations of iloperidone and its active metabolites by about 50%. Smoking status should not influence iloperidone plasma concentration because this drug is not a primary substrate for CYP1A2, the enzyme induced by cigarette smoking.
The bottom line: reduce iloperidone dosage by 50% for patients who are taking a strong CYP2D6 and/or CYP3A4 inhibitor (see Dosing below).
Efficacy
In clinical trials, iloperidone was shown to be efficacious in treating positive and negative symptoms and general psychopathology in acute episodes of schizophrenia. It is important to consider the efficacy studies of iloperidone within the context of the history of its development plan.
Early clinical trials. Most of iloperidone’s phase II and III studies were conducted by Novartis between 1998 and 2002. Initial phase III studies included three 6-week, double-blind, placebo-controlled acute trials comparing a range of iloperidone doses with placebo and an active comparator:5,6
- The first trial compared iloperidone, 4, 8, or 12 mg/d, with placebo or haloperidol, 15 mg/d.
- The second compared iloperidone, 4 to 8 mg/d or 10 to 16 mg/d, with placebo and risperidone, 4 to 8 mg/d.
- The third compared iloperidone, 12 to 16 mg/d or 20 to 24 mg/d, with placebo or risperidone, 6 to 8 mg/d.
These studies totaled 1,066 patients in the iloperidone treatment arms, with target dosages for iloperidone ranging from 4 to 24 mg/d. Iloperidone was more efficacious than placebo for positive, negative, and overall total symptoms on the Positive and Negative Syndrome Scale (PANSS), albeit 4 mg/d and 8 mg/d dosages narrowly missed the .05 significance level.
The haloperidol and risperidone active controls appeared more effective than iloperidone in the original analyses, but these studies were not designed for analysis of comparative efficacy. The protocols for all of these studies used an up-titration schedule for the iloperidone groups that took 1 week to reach steady-state levels, whereas the haloperidol and risperidone groups had a briefer up-titration to target dose.
The interpretation of these studies is complex and a detailed discussion is beyond the scope of this article. However, a post-hoc analysis that included subjects who remained in the study after 2 weeks of double-blind medication showed that iloperidone performed comparably to risperidone7 and haloperidol.8
A new phase III trial. The question remained whether iloperidone was as efficacious as other first-line antipsychotics but had been “penalized” by its slower up-titration schedule and clinical trial design flaws. After acquiring the development rights to iloperidone from Novartis and reviewing prior study designs and results, Vanda Pharmaceuticals designed another phase III study comparing iloperidone with placebo and ziprasidone. Its purpose was to correct for possible design flaws in the previous studies.
Ziprasidone was selected as the active control because of its established efficacy, safety, and twice-daily dosing. In this trial, researchers attempted to match the 2 drugs’ up-titration schedules. Twice-daily doses were given with food as follows:
- iloperidone, 1, 2, 4, 6, 8, 10, and 12 mg (days 1 to 7, respectively)
- ziprasidone, 20 mg (days 1 to 2), 40 mg (days 3 to 4), 60 mg (days 5 to 6), and 80 mg (day 7).
By day 7, target dosages were reached: iloperidone, 24 mg/d, and ziprasidone, 160 mg/d.9
Patients receiving iloperidone showed significantly greater improvement in PANSS total scores at 4 weeks vs those receiving placebo (–12.0, iloperidone; –7.1, placebo; P < .01).9 Patients receiving ziprasidone also achieved significantly greater improvement vs those receiving placebo (–12.3; P < .05 vs placebo).
The iloperidone and ziprasidone groups showed significantly greater improvement from baseline vs placebo in PANSS positive (P) and negative (N) subscale scores. Significantly more patients receiving iloperidone (72%) than placebo (52%) experienced improvement (≥20% reduction from baseline) in PANSS-P scores (P = .005).
Patients receiving iloperidone had a significantly greater reduction in Clinical Global Impression-Severity scale score vs placebo (–0.65 and –0.39, respectively; P = .007), as did patients receiving ziprasidone (–0.67; P = .013).
Iloperidone met all predefined protocol criteria for efficacy vs placebo and had efficacy equal to the highest approved dose of ziprasidone. These results demonstrated that iloperidone has comparable efficacy to ziprasidone and support the validity of the re-analysis of earlier studies showing comparable efficacy between iloperidone and risperidone7 or haloperidol.8 In July 2008 the FDA issued a not approvable letter for iloperidone, requesting further clinical trials because of concerns about the drug’s efficacy compared with risperidone. The FDA approved iloperidone in May 2009 after the manufacturer provided additional data from existing trials that demonstrated comparable efficacy to risperidone.
Long-term efficacy. A double-blind extension study compared patients remaining on blinded iloperidone (4 to 16 mg/d) or haloperidol (5 to 20 mg/d) after completing a 6-week efficacy study.10 The drugs showed equivalent efficacy in preventing relapse over 46 weeks follow-up. Because this study included no placebo group, the FDA does not consider it to be an interpretable relapse prevention study.
Tolerability
Clinicians might consider iloperidone when seeking to switch a patient to an antipsychotic with a potentially lower side-effect burden.6,11 Compared with risperidone, iloperidone has a lower liability for extrapyramidal symptoms (EPS) and does not cause clinically significant prolactin elevation (Table 3).5,7-9 Compared with ziprasidone, iloperidone has a lower EPS and akathisia liability. Somewhat greater weight gain was seen with iloperidone when compared with ziprasidone in a 4-week study (iloperidone, +2.8 kg; ziprasidone, +1.1 kg; placebo, +0.5 kg) but the 2 drugs’ effects on triglycerides and cholesterol were comparable.9
Iloperidone has a similar degree of QTc prolongation as ziprasidone (mean 9 msec at the highest dosage of 12 mg bid). Safety studies including administration of maximal doses of iloperidone with CYP3A4 and CYP2D6 inhibitors showed a mean QTc prolongation of 19 msec without clinically significant problems, and iloperidone has not been associated with serious arrhythmia.4 Iloperidone should not be prescribed to patients with significant cardiac problems or electrolyte disturbances, however, or those taking drugs known to have clinically significant QTc/proarrhythmic properties, such as thioridazine, droperidol, pimozide, or methadone.4
Iloperidone has the same safety concerns associated with other atypical antipsychotics, including tardive dyskinesia and neuroleptic malignant syndrome. Like other atypical antipsychotics, iloperidone carries a warning of increased mortality risk in elderly patients with dementia-related psychosis.
Dose-related side effects include dizziness, orthostatic hypotension, and tachycardia. Dizziness occurred more often at higher doses (20% at 20 to 24 mg/d vs 10% at 10 to 16 mg/d vs 7% in placebo groups). Presumably these side effects are related to NEα1 antagonism and are the basis for the recommended dose-titration schedule described below. Clinical trials do not seem to demonstrate a dose-response relationship for acute EPS or akathisia.
Table 3
Common side effects: Iloperidone vs other antipsychotics*
| Other antipsychotic | Less likely or less severe with iloperidone | More likely or more severe with iloperidone |
|---|---|---|
| Haloperidol4,8 | EPS Akathisia Prolactin elevation | Weight gain Orthostasis |
| Olanzapine | Dyslipidemia Weight gain Sedation | Orthostasis |
| Quetiapine | Dyslipidemia Sedation | EPS |
| Risperidone4,6 | EPS Prolactin elevation Akathisia | None |
| Ziprasidone7 | EPS Akathisia | Weight gain Orthostasis |
| Aripiprazole | Akathisia | Weight gain Orthostasis |
| * Iloperidone has been compared head-to-head with haloperidol, risperidone, and ziprasidone in clinical trials. Other suggested antipsychotic side effect liabilities are based on indirect comparisons | ||
| EPS: extrapyramidal symptoms | ||
The approved dosage range for iloperidone is 12 to 24 mg/d, given as 6 to 12 mg bid. Some trials suggest a dose-response relationship, with 24 mg being more effective than lower target doses.5 Reduce the target dosage of iloperidone by one-half when administering it concomitantly with medications that are strong CYP2D6 or CYP3A4 inhibitors.
Titration schedule. Because iloperidone’s relatively strong NEα1 antagonism creates risk for initial orthostatic hypotension, the drug needs to be titrated to the target dose over 4 to 7 days:
- 1 mg bid at day 1
- 2 mg bid at day 2
- 4 mg bid at day 3
- 6 mg bid at day 4 (for a target dosage of 12 mg/d)
- 8 mg bid at day 5
- 10 mg bid at day 6
- 12 mg bid at day 7 (for a target dosage of 24 mg/d).
Monitor patients for dose adjustments based on clinical status, concomitant use of other medications (including antipsychotics), and tolerability of up-titration.
The need to titrate iloperidone slowly to reach efficacious acute antipsychotic doses may lead to delayed effectiveness compared with antipsychotics that can be started at therapeutic doses. This problem also may be seen with up-titration of other agents with strong NEα1 properties, including chlorpromazine, clozapine, quetiapine, and risperidone, and should be considered when treating patients who need rapid dose escalation.
Related resource
- Iloperidone (Fanapt) prescribing information. www.fanapt.com/fanapt-pi-may09.pdf.
Drug brand names
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Droperidol • Inapsine
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Ketoconazole • Nizoral
- Methadone • Dolophine, Methadose
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosures
Dr. Weiden receives research support from the National Institute of Mental Health and Ortho-McNeil Janssen. He is a consultant to AstraZeneca, Bristol-Myers Squibb/Otsuka America Pharmaceutical, Eli Lilly and Company, Forest, Ortho-McNeil Janssen, Pfizer Inc., Schering-Plough, Vanda, and Wyeth, and a speaker for Ortho-McNeil Janssen and Pfizer Inc.
Dr. Bishop receives research/grant support from the National Institute of Mental Health, NARSAD, and Ortho-McNeil Janssen.
1. Subramanian N, Kalkman HO. Receptor profile of P88-8991 and P95-12113, metabolites of the novel antipsychotic iloperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):553-560.
2. Kalkman HO, Feuerbach D, Lötscher E. Functional characterization of the novel antipsychotic iloperidone at human D2, D3, alpha 2C, 5-HT6, and 5-HT1A receptors. Life Sci. 2003;73(9):1151-1159.
3. Kalkman HO, Subramanian N, Hoyer D. Extended radioligand binding profile of iloperidone: a broad spectrum dopamine/serotonin/norepinephrine receptor antagonist for the management of psychotic disorders. Neuropsychopharmacology. 2001;25(6):904-914.
4. Fanapt [package insert]. Rockville, MD: Vanda Pharmaceuticals Inc; 2009.
5. Potkin SG, Litman RE, Torres R. Efficacy of iloperidone in the treatment of schizophrenia: initial phase 3 studies. J Clin Psychopharmacol. 2008;28(2 suppl 1):S4-S11.
6. Weiden PJ, Cutler AJ, Polymeropoulos MH. Safety profile of iloperidone: a pooled analysis of 6-week acute-phase pivotal trials. J Clin Psychopharmacol. 2008;28(2 suppl 1):S12-19.
7. Hamilton J, Wolfgang C, Feeney J, et al. Efficacy of iloperidone is comparable to risperidone in analyses of a placebo- and risperidone-controlled clinical trial for schizophrenia (pp. NR1-033). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
8. Feeney J, Wolfgang C, Polymeropoulos M, et al. The comparative efficacy of iloperidone and haloperidol across four short-term controlled trials (pp. NR1-026). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
9. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-28.
10. Torres R, Nasrallah H, Baroldi P. Iloperidone versus haloperidol as long-term maintenance treatment for patients with schizophrenia or schizoaffective disorder (pp. NR4-093). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
11. Kane JM, Lauriello J, Laska E, et al. Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S29-S35.
Iloperidone is a second-generation (atypical) antipsychotic the FDA approved in May 2009 for treating acute schizophrenia in adults (Table 1). Iloperidone is not a derivative (metabolite, isomer, or different formulation) of any other antipsychotic. Clinical trials have shown that iloperidone is efficacious and suggest that for some patients its side-effect profile may be more favorable than that of other antipsychotics.
Table 1
Iloperidone: Fast facts
| Brand name: Fanapt |
| Class: Atypical antipsychotic (serotonin/dopamine antagonist) |
| Indication: Acute schizophrenia in adults |
| Approval date: May 2009 |
| Availability date: Late 2009 |
| Manufacturer: Vanda Pharmaceuticals, Inc. |
| Dosing forms: 1-, 2-, 4-, 6-, 8-, 10-, and 12-mg tablets (nonscored); titration pack of 2×1-mg, 2×2-mg, 2×4-mg, and 2×6-mg tablets |
| Starting dose: 1 mg bid (2 mg total daily dose) |
| Target dose: 12 to 24 mg total daily dose |
Clinical implications
Iloperidone’s binding profile is similar to that of other antipsychotics with relatively stronger affinity for serotonin (5-HT2A) than dopamine (D2) receptors, and its efficacy is roughly comparable to that of other non-clozapine antipsychotics.
Individual patients may respond differently to specific antipsychotics, even when those agents have shown equivalent efficacy in clinical trials. Therefore, a key therapeutic question is the degree of differential efficacy—differences in response at an individual level—among iloperidone and other antipsychotics.
The differential efficacy among iloperidone and other antipsychotics is unknown. Our clinical experience and iloperidone’s unique structure suggest, however, that this agent might be helpful for certain patients who do not fully respond to or are unable to tolerate other antipsychotics.
How iloperidone works
Like other antipsychotics, iloperidone’s efficacy presumably is based on its ability to block [antagonize] dopamine D2 receptors. Its chemical structure is most similar to risperidone, paliperidone, and ziprasidone, but its receptor binding profile is distinguished by a relatively lower affinity for serotonin receptors 5-HT1A and 5-HT2C than ziprasidone, and a relative lack of muscarinic and histaminic antagonist properties (Table 2).
The relatively higher affinity of iloperidone (and its metabolite P95) for the NEα1 receptor correlates with the drug’s propensity to cause orthostatic hypotension during initial up-titration.1 Differences in iloperidone’s receptor binding profile compared with other antipsychotics likely are responsible for its different side-effect profile.2,3
Table 2
Relative receptor binding affinities of 3 atypical antipsychotics*
| Binding affinity | |||
|---|---|---|---|
| Receptor | Risperidone | Ziprasidone | Iloperidone |
| Dopamine D2 | High | High | High |
| Serotonin 5-HT1A | Low | High | Low |
| Serotonin 5-HT2A | High | High | High |
| Serotonin 5-HT2C | Moderate | High | Moderate† |
| Norepinephrine NEα1 | High | Moderate | Moderate‡ |
| Histamine H1 | Moderate | Moderate | Low |
| Muscarinic M1 | Negligible | Negligible | Negligible |
| * Cross-comparison of binding strengths reflects the subjective judgment of the authors. The goal is to demonstrate differences in overall binding patterns, and these estimates should not be considered an exact cross-comparison | |||
| † Published reports of binding affinity of iloperidone show considerable variation for the 5HT2C site | |||
| ‡ One metabolite of iloperidone [P95] does not have CNS activity but has potent alpha-1 antagonism and may contribute to the initial orthostatic hypotension seen in clinical trials | |||
Pharmacokinetics
Iloperidone is administered twice daily and can be taken with or without food. The bioavailability of iloperidone tablets is 96%, and peak plasma concentrations are achieved 2 to 4 hours after ingestion.
Like all antipsychotics except paliperidone, iloperidone is metabolized by the liver’s cytochrome P450 (CYP) system. The enzyme pathways CYP3A4 and CYP2D6 transform iloperidone into 2 metabolites: one with CNS activity (P88) and one that does not cross the blood-brain barrier and is not active in the CNS (P95) but likely has peripheral effects.
Genetic variations in CYP2D6 activity can substantially alter how individual patients metabolize iloperidone. The half-life of iloperidone and its active metabolites differs depending on whether someone is a poor metabolizer (no functional CYP2D6 activity), intermediate metabolizer (reduced CYP2D6 activity), or extensive metabolizer (“normal” CYP2D6 activity). The usual half-life of iloperidone (approximately 18 hours in extensive metabolizers) can be almost 50% longer (>24 hours) in CYP2D6 poor metabolizers.
There are no recommendations to test patients for genetic variants that result in poor metabolism from CYP2D6. Rather, clinicians simply need to be aware that this could be the source of interindividual differences they see in iloperidone tolerability, just as it is for any other medication that is a substrate for the CYP2D6 enzyme system.
Interactions. Medications that inhibit the CYP3A4 or CYP2D6 systems can increase iloperidone plasma level when taken concurrently with iloperidone, even if intrinsic liver metabolism activity is normal. Fluoxetine and paroxetine are potent CYP2D6 inhibitors. Concurrent treatment with either of these selective serotonin reuptake inhibitors could increase iloperidone plasma concentration by 100% or more.4
Similarly, cotreatment with a potent CYP3A4 inhibitor such as ketoconazole (or drinking grapefruit juice) will decrease metabolism and increase plasma concentrations of iloperidone and its active metabolites by about 50%. Smoking status should not influence iloperidone plasma concentration because this drug is not a primary substrate for CYP1A2, the enzyme induced by cigarette smoking.
The bottom line: reduce iloperidone dosage by 50% for patients who are taking a strong CYP2D6 and/or CYP3A4 inhibitor (see Dosing below).
Efficacy
In clinical trials, iloperidone was shown to be efficacious in treating positive and negative symptoms and general psychopathology in acute episodes of schizophrenia. It is important to consider the efficacy studies of iloperidone within the context of the history of its development plan.
Early clinical trials. Most of iloperidone’s phase II and III studies were conducted by Novartis between 1998 and 2002. Initial phase III studies included three 6-week, double-blind, placebo-controlled acute trials comparing a range of iloperidone doses with placebo and an active comparator:5,6
- The first trial compared iloperidone, 4, 8, or 12 mg/d, with placebo or haloperidol, 15 mg/d.
- The second compared iloperidone, 4 to 8 mg/d or 10 to 16 mg/d, with placebo and risperidone, 4 to 8 mg/d.
- The third compared iloperidone, 12 to 16 mg/d or 20 to 24 mg/d, with placebo or risperidone, 6 to 8 mg/d.
These studies totaled 1,066 patients in the iloperidone treatment arms, with target dosages for iloperidone ranging from 4 to 24 mg/d. Iloperidone was more efficacious than placebo for positive, negative, and overall total symptoms on the Positive and Negative Syndrome Scale (PANSS), albeit 4 mg/d and 8 mg/d dosages narrowly missed the .05 significance level.
The haloperidol and risperidone active controls appeared more effective than iloperidone in the original analyses, but these studies were not designed for analysis of comparative efficacy. The protocols for all of these studies used an up-titration schedule for the iloperidone groups that took 1 week to reach steady-state levels, whereas the haloperidol and risperidone groups had a briefer up-titration to target dose.
The interpretation of these studies is complex and a detailed discussion is beyond the scope of this article. However, a post-hoc analysis that included subjects who remained in the study after 2 weeks of double-blind medication showed that iloperidone performed comparably to risperidone7 and haloperidol.8
A new phase III trial. The question remained whether iloperidone was as efficacious as other first-line antipsychotics but had been “penalized” by its slower up-titration schedule and clinical trial design flaws. After acquiring the development rights to iloperidone from Novartis and reviewing prior study designs and results, Vanda Pharmaceuticals designed another phase III study comparing iloperidone with placebo and ziprasidone. Its purpose was to correct for possible design flaws in the previous studies.
Ziprasidone was selected as the active control because of its established efficacy, safety, and twice-daily dosing. In this trial, researchers attempted to match the 2 drugs’ up-titration schedules. Twice-daily doses were given with food as follows:
- iloperidone, 1, 2, 4, 6, 8, 10, and 12 mg (days 1 to 7, respectively)
- ziprasidone, 20 mg (days 1 to 2), 40 mg (days 3 to 4), 60 mg (days 5 to 6), and 80 mg (day 7).
By day 7, target dosages were reached: iloperidone, 24 mg/d, and ziprasidone, 160 mg/d.9
Patients receiving iloperidone showed significantly greater improvement in PANSS total scores at 4 weeks vs those receiving placebo (–12.0, iloperidone; –7.1, placebo; P < .01).9 Patients receiving ziprasidone also achieved significantly greater improvement vs those receiving placebo (–12.3; P < .05 vs placebo).
The iloperidone and ziprasidone groups showed significantly greater improvement from baseline vs placebo in PANSS positive (P) and negative (N) subscale scores. Significantly more patients receiving iloperidone (72%) than placebo (52%) experienced improvement (≥20% reduction from baseline) in PANSS-P scores (P = .005).
Patients receiving iloperidone had a significantly greater reduction in Clinical Global Impression-Severity scale score vs placebo (–0.65 and –0.39, respectively; P = .007), as did patients receiving ziprasidone (–0.67; P = .013).
Iloperidone met all predefined protocol criteria for efficacy vs placebo and had efficacy equal to the highest approved dose of ziprasidone. These results demonstrated that iloperidone has comparable efficacy to ziprasidone and support the validity of the re-analysis of earlier studies showing comparable efficacy between iloperidone and risperidone7 or haloperidol.8 In July 2008 the FDA issued a not approvable letter for iloperidone, requesting further clinical trials because of concerns about the drug’s efficacy compared with risperidone. The FDA approved iloperidone in May 2009 after the manufacturer provided additional data from existing trials that demonstrated comparable efficacy to risperidone.
Long-term efficacy. A double-blind extension study compared patients remaining on blinded iloperidone (4 to 16 mg/d) or haloperidol (5 to 20 mg/d) after completing a 6-week efficacy study.10 The drugs showed equivalent efficacy in preventing relapse over 46 weeks follow-up. Because this study included no placebo group, the FDA does not consider it to be an interpretable relapse prevention study.
Tolerability
Clinicians might consider iloperidone when seeking to switch a patient to an antipsychotic with a potentially lower side-effect burden.6,11 Compared with risperidone, iloperidone has a lower liability for extrapyramidal symptoms (EPS) and does not cause clinically significant prolactin elevation (Table 3).5,7-9 Compared with ziprasidone, iloperidone has a lower EPS and akathisia liability. Somewhat greater weight gain was seen with iloperidone when compared with ziprasidone in a 4-week study (iloperidone, +2.8 kg; ziprasidone, +1.1 kg; placebo, +0.5 kg) but the 2 drugs’ effects on triglycerides and cholesterol were comparable.9
Iloperidone has a similar degree of QTc prolongation as ziprasidone (mean 9 msec at the highest dosage of 12 mg bid). Safety studies including administration of maximal doses of iloperidone with CYP3A4 and CYP2D6 inhibitors showed a mean QTc prolongation of 19 msec without clinically significant problems, and iloperidone has not been associated with serious arrhythmia.4 Iloperidone should not be prescribed to patients with significant cardiac problems or electrolyte disturbances, however, or those taking drugs known to have clinically significant QTc/proarrhythmic properties, such as thioridazine, droperidol, pimozide, or methadone.4
Iloperidone has the same safety concerns associated with other atypical antipsychotics, including tardive dyskinesia and neuroleptic malignant syndrome. Like other atypical antipsychotics, iloperidone carries a warning of increased mortality risk in elderly patients with dementia-related psychosis.
Dose-related side effects include dizziness, orthostatic hypotension, and tachycardia. Dizziness occurred more often at higher doses (20% at 20 to 24 mg/d vs 10% at 10 to 16 mg/d vs 7% in placebo groups). Presumably these side effects are related to NEα1 antagonism and are the basis for the recommended dose-titration schedule described below. Clinical trials do not seem to demonstrate a dose-response relationship for acute EPS or akathisia.
Table 3
Common side effects: Iloperidone vs other antipsychotics*
| Other antipsychotic | Less likely or less severe with iloperidone | More likely or more severe with iloperidone |
|---|---|---|
| Haloperidol4,8 | EPS Akathisia Prolactin elevation | Weight gain Orthostasis |
| Olanzapine | Dyslipidemia Weight gain Sedation | Orthostasis |
| Quetiapine | Dyslipidemia Sedation | EPS |
| Risperidone4,6 | EPS Prolactin elevation Akathisia | None |
| Ziprasidone7 | EPS Akathisia | Weight gain Orthostasis |
| Aripiprazole | Akathisia | Weight gain Orthostasis |
| * Iloperidone has been compared head-to-head with haloperidol, risperidone, and ziprasidone in clinical trials. Other suggested antipsychotic side effect liabilities are based on indirect comparisons | ||
| EPS: extrapyramidal symptoms | ||
The approved dosage range for iloperidone is 12 to 24 mg/d, given as 6 to 12 mg bid. Some trials suggest a dose-response relationship, with 24 mg being more effective than lower target doses.5 Reduce the target dosage of iloperidone by one-half when administering it concomitantly with medications that are strong CYP2D6 or CYP3A4 inhibitors.
Titration schedule. Because iloperidone’s relatively strong NEα1 antagonism creates risk for initial orthostatic hypotension, the drug needs to be titrated to the target dose over 4 to 7 days:
- 1 mg bid at day 1
- 2 mg bid at day 2
- 4 mg bid at day 3
- 6 mg bid at day 4 (for a target dosage of 12 mg/d)
- 8 mg bid at day 5
- 10 mg bid at day 6
- 12 mg bid at day 7 (for a target dosage of 24 mg/d).
Monitor patients for dose adjustments based on clinical status, concomitant use of other medications (including antipsychotics), and tolerability of up-titration.
The need to titrate iloperidone slowly to reach efficacious acute antipsychotic doses may lead to delayed effectiveness compared with antipsychotics that can be started at therapeutic doses. This problem also may be seen with up-titration of other agents with strong NEα1 properties, including chlorpromazine, clozapine, quetiapine, and risperidone, and should be considered when treating patients who need rapid dose escalation.
Related resource
- Iloperidone (Fanapt) prescribing information. www.fanapt.com/fanapt-pi-may09.pdf.
Drug brand names
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Droperidol • Inapsine
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Ketoconazole • Nizoral
- Methadone • Dolophine, Methadose
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosures
Dr. Weiden receives research support from the National Institute of Mental Health and Ortho-McNeil Janssen. He is a consultant to AstraZeneca, Bristol-Myers Squibb/Otsuka America Pharmaceutical, Eli Lilly and Company, Forest, Ortho-McNeil Janssen, Pfizer Inc., Schering-Plough, Vanda, and Wyeth, and a speaker for Ortho-McNeil Janssen and Pfizer Inc.
Dr. Bishop receives research/grant support from the National Institute of Mental Health, NARSAD, and Ortho-McNeil Janssen.
Iloperidone is a second-generation (atypical) antipsychotic the FDA approved in May 2009 for treating acute schizophrenia in adults (Table 1). Iloperidone is not a derivative (metabolite, isomer, or different formulation) of any other antipsychotic. Clinical trials have shown that iloperidone is efficacious and suggest that for some patients its side-effect profile may be more favorable than that of other antipsychotics.
Table 1
Iloperidone: Fast facts
| Brand name: Fanapt |
| Class: Atypical antipsychotic (serotonin/dopamine antagonist) |
| Indication: Acute schizophrenia in adults |
| Approval date: May 2009 |
| Availability date: Late 2009 |
| Manufacturer: Vanda Pharmaceuticals, Inc. |
| Dosing forms: 1-, 2-, 4-, 6-, 8-, 10-, and 12-mg tablets (nonscored); titration pack of 2×1-mg, 2×2-mg, 2×4-mg, and 2×6-mg tablets |
| Starting dose: 1 mg bid (2 mg total daily dose) |
| Target dose: 12 to 24 mg total daily dose |
Clinical implications
Iloperidone’s binding profile is similar to that of other antipsychotics with relatively stronger affinity for serotonin (5-HT2A) than dopamine (D2) receptors, and its efficacy is roughly comparable to that of other non-clozapine antipsychotics.
Individual patients may respond differently to specific antipsychotics, even when those agents have shown equivalent efficacy in clinical trials. Therefore, a key therapeutic question is the degree of differential efficacy—differences in response at an individual level—among iloperidone and other antipsychotics.
The differential efficacy among iloperidone and other antipsychotics is unknown. Our clinical experience and iloperidone’s unique structure suggest, however, that this agent might be helpful for certain patients who do not fully respond to or are unable to tolerate other antipsychotics.
How iloperidone works
Like other antipsychotics, iloperidone’s efficacy presumably is based on its ability to block [antagonize] dopamine D2 receptors. Its chemical structure is most similar to risperidone, paliperidone, and ziprasidone, but its receptor binding profile is distinguished by a relatively lower affinity for serotonin receptors 5-HT1A and 5-HT2C than ziprasidone, and a relative lack of muscarinic and histaminic antagonist properties (Table 2).
The relatively higher affinity of iloperidone (and its metabolite P95) for the NEα1 receptor correlates with the drug’s propensity to cause orthostatic hypotension during initial up-titration.1 Differences in iloperidone’s receptor binding profile compared with other antipsychotics likely are responsible for its different side-effect profile.2,3
Table 2
Relative receptor binding affinities of 3 atypical antipsychotics*
| Binding affinity | |||
|---|---|---|---|
| Receptor | Risperidone | Ziprasidone | Iloperidone |
| Dopamine D2 | High | High | High |
| Serotonin 5-HT1A | Low | High | Low |
| Serotonin 5-HT2A | High | High | High |
| Serotonin 5-HT2C | Moderate | High | Moderate† |
| Norepinephrine NEα1 | High | Moderate | Moderate‡ |
| Histamine H1 | Moderate | Moderate | Low |
| Muscarinic M1 | Negligible | Negligible | Negligible |
| * Cross-comparison of binding strengths reflects the subjective judgment of the authors. The goal is to demonstrate differences in overall binding patterns, and these estimates should not be considered an exact cross-comparison | |||
| † Published reports of binding affinity of iloperidone show considerable variation for the 5HT2C site | |||
| ‡ One metabolite of iloperidone [P95] does not have CNS activity but has potent alpha-1 antagonism and may contribute to the initial orthostatic hypotension seen in clinical trials | |||
Pharmacokinetics
Iloperidone is administered twice daily and can be taken with or without food. The bioavailability of iloperidone tablets is 96%, and peak plasma concentrations are achieved 2 to 4 hours after ingestion.
Like all antipsychotics except paliperidone, iloperidone is metabolized by the liver’s cytochrome P450 (CYP) system. The enzyme pathways CYP3A4 and CYP2D6 transform iloperidone into 2 metabolites: one with CNS activity (P88) and one that does not cross the blood-brain barrier and is not active in the CNS (P95) but likely has peripheral effects.
Genetic variations in CYP2D6 activity can substantially alter how individual patients metabolize iloperidone. The half-life of iloperidone and its active metabolites differs depending on whether someone is a poor metabolizer (no functional CYP2D6 activity), intermediate metabolizer (reduced CYP2D6 activity), or extensive metabolizer (“normal” CYP2D6 activity). The usual half-life of iloperidone (approximately 18 hours in extensive metabolizers) can be almost 50% longer (>24 hours) in CYP2D6 poor metabolizers.
There are no recommendations to test patients for genetic variants that result in poor metabolism from CYP2D6. Rather, clinicians simply need to be aware that this could be the source of interindividual differences they see in iloperidone tolerability, just as it is for any other medication that is a substrate for the CYP2D6 enzyme system.
Interactions. Medications that inhibit the CYP3A4 or CYP2D6 systems can increase iloperidone plasma level when taken concurrently with iloperidone, even if intrinsic liver metabolism activity is normal. Fluoxetine and paroxetine are potent CYP2D6 inhibitors. Concurrent treatment with either of these selective serotonin reuptake inhibitors could increase iloperidone plasma concentration by 100% or more.4
Similarly, cotreatment with a potent CYP3A4 inhibitor such as ketoconazole (or drinking grapefruit juice) will decrease metabolism and increase plasma concentrations of iloperidone and its active metabolites by about 50%. Smoking status should not influence iloperidone plasma concentration because this drug is not a primary substrate for CYP1A2, the enzyme induced by cigarette smoking.
The bottom line: reduce iloperidone dosage by 50% for patients who are taking a strong CYP2D6 and/or CYP3A4 inhibitor (see Dosing below).
Efficacy
In clinical trials, iloperidone was shown to be efficacious in treating positive and negative symptoms and general psychopathology in acute episodes of schizophrenia. It is important to consider the efficacy studies of iloperidone within the context of the history of its development plan.
Early clinical trials. Most of iloperidone’s phase II and III studies were conducted by Novartis between 1998 and 2002. Initial phase III studies included three 6-week, double-blind, placebo-controlled acute trials comparing a range of iloperidone doses with placebo and an active comparator:5,6
- The first trial compared iloperidone, 4, 8, or 12 mg/d, with placebo or haloperidol, 15 mg/d.
- The second compared iloperidone, 4 to 8 mg/d or 10 to 16 mg/d, with placebo and risperidone, 4 to 8 mg/d.
- The third compared iloperidone, 12 to 16 mg/d or 20 to 24 mg/d, with placebo or risperidone, 6 to 8 mg/d.
These studies totaled 1,066 patients in the iloperidone treatment arms, with target dosages for iloperidone ranging from 4 to 24 mg/d. Iloperidone was more efficacious than placebo for positive, negative, and overall total symptoms on the Positive and Negative Syndrome Scale (PANSS), albeit 4 mg/d and 8 mg/d dosages narrowly missed the .05 significance level.
The haloperidol and risperidone active controls appeared more effective than iloperidone in the original analyses, but these studies were not designed for analysis of comparative efficacy. The protocols for all of these studies used an up-titration schedule for the iloperidone groups that took 1 week to reach steady-state levels, whereas the haloperidol and risperidone groups had a briefer up-titration to target dose.
The interpretation of these studies is complex and a detailed discussion is beyond the scope of this article. However, a post-hoc analysis that included subjects who remained in the study after 2 weeks of double-blind medication showed that iloperidone performed comparably to risperidone7 and haloperidol.8
A new phase III trial. The question remained whether iloperidone was as efficacious as other first-line antipsychotics but had been “penalized” by its slower up-titration schedule and clinical trial design flaws. After acquiring the development rights to iloperidone from Novartis and reviewing prior study designs and results, Vanda Pharmaceuticals designed another phase III study comparing iloperidone with placebo and ziprasidone. Its purpose was to correct for possible design flaws in the previous studies.
Ziprasidone was selected as the active control because of its established efficacy, safety, and twice-daily dosing. In this trial, researchers attempted to match the 2 drugs’ up-titration schedules. Twice-daily doses were given with food as follows:
- iloperidone, 1, 2, 4, 6, 8, 10, and 12 mg (days 1 to 7, respectively)
- ziprasidone, 20 mg (days 1 to 2), 40 mg (days 3 to 4), 60 mg (days 5 to 6), and 80 mg (day 7).
By day 7, target dosages were reached: iloperidone, 24 mg/d, and ziprasidone, 160 mg/d.9
Patients receiving iloperidone showed significantly greater improvement in PANSS total scores at 4 weeks vs those receiving placebo (–12.0, iloperidone; –7.1, placebo; P < .01).9 Patients receiving ziprasidone also achieved significantly greater improvement vs those receiving placebo (–12.3; P < .05 vs placebo).
The iloperidone and ziprasidone groups showed significantly greater improvement from baseline vs placebo in PANSS positive (P) and negative (N) subscale scores. Significantly more patients receiving iloperidone (72%) than placebo (52%) experienced improvement (≥20% reduction from baseline) in PANSS-P scores (P = .005).
Patients receiving iloperidone had a significantly greater reduction in Clinical Global Impression-Severity scale score vs placebo (–0.65 and –0.39, respectively; P = .007), as did patients receiving ziprasidone (–0.67; P = .013).
Iloperidone met all predefined protocol criteria for efficacy vs placebo and had efficacy equal to the highest approved dose of ziprasidone. These results demonstrated that iloperidone has comparable efficacy to ziprasidone and support the validity of the re-analysis of earlier studies showing comparable efficacy between iloperidone and risperidone7 or haloperidol.8 In July 2008 the FDA issued a not approvable letter for iloperidone, requesting further clinical trials because of concerns about the drug’s efficacy compared with risperidone. The FDA approved iloperidone in May 2009 after the manufacturer provided additional data from existing trials that demonstrated comparable efficacy to risperidone.
Long-term efficacy. A double-blind extension study compared patients remaining on blinded iloperidone (4 to 16 mg/d) or haloperidol (5 to 20 mg/d) after completing a 6-week efficacy study.10 The drugs showed equivalent efficacy in preventing relapse over 46 weeks follow-up. Because this study included no placebo group, the FDA does not consider it to be an interpretable relapse prevention study.
Tolerability
Clinicians might consider iloperidone when seeking to switch a patient to an antipsychotic with a potentially lower side-effect burden.6,11 Compared with risperidone, iloperidone has a lower liability for extrapyramidal symptoms (EPS) and does not cause clinically significant prolactin elevation (Table 3).5,7-9 Compared with ziprasidone, iloperidone has a lower EPS and akathisia liability. Somewhat greater weight gain was seen with iloperidone when compared with ziprasidone in a 4-week study (iloperidone, +2.8 kg; ziprasidone, +1.1 kg; placebo, +0.5 kg) but the 2 drugs’ effects on triglycerides and cholesterol were comparable.9
Iloperidone has a similar degree of QTc prolongation as ziprasidone (mean 9 msec at the highest dosage of 12 mg bid). Safety studies including administration of maximal doses of iloperidone with CYP3A4 and CYP2D6 inhibitors showed a mean QTc prolongation of 19 msec without clinically significant problems, and iloperidone has not been associated with serious arrhythmia.4 Iloperidone should not be prescribed to patients with significant cardiac problems or electrolyte disturbances, however, or those taking drugs known to have clinically significant QTc/proarrhythmic properties, such as thioridazine, droperidol, pimozide, or methadone.4
Iloperidone has the same safety concerns associated with other atypical antipsychotics, including tardive dyskinesia and neuroleptic malignant syndrome. Like other atypical antipsychotics, iloperidone carries a warning of increased mortality risk in elderly patients with dementia-related psychosis.
Dose-related side effects include dizziness, orthostatic hypotension, and tachycardia. Dizziness occurred more often at higher doses (20% at 20 to 24 mg/d vs 10% at 10 to 16 mg/d vs 7% in placebo groups). Presumably these side effects are related to NEα1 antagonism and are the basis for the recommended dose-titration schedule described below. Clinical trials do not seem to demonstrate a dose-response relationship for acute EPS or akathisia.
Table 3
Common side effects: Iloperidone vs other antipsychotics*
| Other antipsychotic | Less likely or less severe with iloperidone | More likely or more severe with iloperidone |
|---|---|---|
| Haloperidol4,8 | EPS Akathisia Prolactin elevation | Weight gain Orthostasis |
| Olanzapine | Dyslipidemia Weight gain Sedation | Orthostasis |
| Quetiapine | Dyslipidemia Sedation | EPS |
| Risperidone4,6 | EPS Prolactin elevation Akathisia | None |
| Ziprasidone7 | EPS Akathisia | Weight gain Orthostasis |
| Aripiprazole | Akathisia | Weight gain Orthostasis |
| * Iloperidone has been compared head-to-head with haloperidol, risperidone, and ziprasidone in clinical trials. Other suggested antipsychotic side effect liabilities are based on indirect comparisons | ||
| EPS: extrapyramidal symptoms | ||
The approved dosage range for iloperidone is 12 to 24 mg/d, given as 6 to 12 mg bid. Some trials suggest a dose-response relationship, with 24 mg being more effective than lower target doses.5 Reduce the target dosage of iloperidone by one-half when administering it concomitantly with medications that are strong CYP2D6 or CYP3A4 inhibitors.
Titration schedule. Because iloperidone’s relatively strong NEα1 antagonism creates risk for initial orthostatic hypotension, the drug needs to be titrated to the target dose over 4 to 7 days:
- 1 mg bid at day 1
- 2 mg bid at day 2
- 4 mg bid at day 3
- 6 mg bid at day 4 (for a target dosage of 12 mg/d)
- 8 mg bid at day 5
- 10 mg bid at day 6
- 12 mg bid at day 7 (for a target dosage of 24 mg/d).
Monitor patients for dose adjustments based on clinical status, concomitant use of other medications (including antipsychotics), and tolerability of up-titration.
The need to titrate iloperidone slowly to reach efficacious acute antipsychotic doses may lead to delayed effectiveness compared with antipsychotics that can be started at therapeutic doses. This problem also may be seen with up-titration of other agents with strong NEα1 properties, including chlorpromazine, clozapine, quetiapine, and risperidone, and should be considered when treating patients who need rapid dose escalation.
Related resource
- Iloperidone (Fanapt) prescribing information. www.fanapt.com/fanapt-pi-may09.pdf.
Drug brand names
- Aripiprazole • Abilify
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Droperidol • Inapsine
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Iloperidone • Fanapt
- Ketoconazole • Nizoral
- Methadone • Dolophine, Methadose
- Olanzapine • Zyprexa
- Paliperidone • Invega
- Paroxetine • Paxil
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosures
Dr. Weiden receives research support from the National Institute of Mental Health and Ortho-McNeil Janssen. He is a consultant to AstraZeneca, Bristol-Myers Squibb/Otsuka America Pharmaceutical, Eli Lilly and Company, Forest, Ortho-McNeil Janssen, Pfizer Inc., Schering-Plough, Vanda, and Wyeth, and a speaker for Ortho-McNeil Janssen and Pfizer Inc.
Dr. Bishop receives research/grant support from the National Institute of Mental Health, NARSAD, and Ortho-McNeil Janssen.
1. Subramanian N, Kalkman HO. Receptor profile of P88-8991 and P95-12113, metabolites of the novel antipsychotic iloperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):553-560.
2. Kalkman HO, Feuerbach D, Lötscher E. Functional characterization of the novel antipsychotic iloperidone at human D2, D3, alpha 2C, 5-HT6, and 5-HT1A receptors. Life Sci. 2003;73(9):1151-1159.
3. Kalkman HO, Subramanian N, Hoyer D. Extended radioligand binding profile of iloperidone: a broad spectrum dopamine/serotonin/norepinephrine receptor antagonist for the management of psychotic disorders. Neuropsychopharmacology. 2001;25(6):904-914.
4. Fanapt [package insert]. Rockville, MD: Vanda Pharmaceuticals Inc; 2009.
5. Potkin SG, Litman RE, Torres R. Efficacy of iloperidone in the treatment of schizophrenia: initial phase 3 studies. J Clin Psychopharmacol. 2008;28(2 suppl 1):S4-S11.
6. Weiden PJ, Cutler AJ, Polymeropoulos MH. Safety profile of iloperidone: a pooled analysis of 6-week acute-phase pivotal trials. J Clin Psychopharmacol. 2008;28(2 suppl 1):S12-19.
7. Hamilton J, Wolfgang C, Feeney J, et al. Efficacy of iloperidone is comparable to risperidone in analyses of a placebo- and risperidone-controlled clinical trial for schizophrenia (pp. NR1-033). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
8. Feeney J, Wolfgang C, Polymeropoulos M, et al. The comparative efficacy of iloperidone and haloperidol across four short-term controlled trials (pp. NR1-026). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
9. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-28.
10. Torres R, Nasrallah H, Baroldi P. Iloperidone versus haloperidol as long-term maintenance treatment for patients with schizophrenia or schizoaffective disorder (pp. NR4-093). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
11. Kane JM, Lauriello J, Laska E, et al. Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S29-S35.
1. Subramanian N, Kalkman HO. Receptor profile of P88-8991 and P95-12113, metabolites of the novel antipsychotic iloperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):553-560.
2. Kalkman HO, Feuerbach D, Lötscher E. Functional characterization of the novel antipsychotic iloperidone at human D2, D3, alpha 2C, 5-HT6, and 5-HT1A receptors. Life Sci. 2003;73(9):1151-1159.
3. Kalkman HO, Subramanian N, Hoyer D. Extended radioligand binding profile of iloperidone: a broad spectrum dopamine/serotonin/norepinephrine receptor antagonist for the management of psychotic disorders. Neuropsychopharmacology. 2001;25(6):904-914.
4. Fanapt [package insert]. Rockville, MD: Vanda Pharmaceuticals Inc; 2009.
5. Potkin SG, Litman RE, Torres R. Efficacy of iloperidone in the treatment of schizophrenia: initial phase 3 studies. J Clin Psychopharmacol. 2008;28(2 suppl 1):S4-S11.
6. Weiden PJ, Cutler AJ, Polymeropoulos MH. Safety profile of iloperidone: a pooled analysis of 6-week acute-phase pivotal trials. J Clin Psychopharmacol. 2008;28(2 suppl 1):S12-19.
7. Hamilton J, Wolfgang C, Feeney J, et al. Efficacy of iloperidone is comparable to risperidone in analyses of a placebo- and risperidone-controlled clinical trial for schizophrenia (pp. NR1-033). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
8. Feeney J, Wolfgang C, Polymeropoulos M, et al. The comparative efficacy of iloperidone and haloperidol across four short-term controlled trials (pp. NR1-026). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
9. Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S20-28.
10. Torres R, Nasrallah H, Baroldi P. Iloperidone versus haloperidol as long-term maintenance treatment for patients with schizophrenia or schizoaffective disorder (pp. NR4-093). Presented at: American Psychiatric Association Annual Meeting; May 16-21, 2009; San Francisco, CA.
11. Kane JM, Lauriello J, Laska E, et al. Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol. 2008;28(2 suppl 1):S29-S35.
A PEARL of wisdom about ‘Pearls’
Since 2005, I’ve had the opportunity to peer review Pearls articles submitted for publication in Current Psychiatry. In that time, I have read many worthwhile papers written by authors who may not be entirely clear about what constitutes a Pearl. The mnemonic PEARL could help authors:
- decide if their article or idea is appropriate for Pearls
- construct the article to conform to the Pearls format.
Precise. A Pearls article should make an accurate and concise statement. It should not be an elaborate or generalized idea based on either limited or copious information.
Easy to remember. Lengthy, highly detailed articles may be helpful and informative but are not consistent with the purpose of Pearls.
Alert. A Pearl should alert a physician to identify a problem, diagnosis, or adverse effect that they might otherwise miss or take unnecessary time to identify. Classic examples are the “handshake diagnosis” of hyperthyroidism,1 or the “3 little words that can diagnose mild cognitive impairment.”2
References. A professional article of any length should include references. References add immediate credibility to the information presented. For a Pearl, even one reference is acceptable. A writer can easily search PubMed and the Internet to find references to confirm or support their ideas.
Less is more. Architect Mies van der Rohe’s minimalist concept applies to Pearls. A Pearl—like its namesake—is small, polished, and valuable. Simplicity is its essence.
I hope this mnemonic is useful for clinicians interested in sharing their ideas or experiences to help others in the field. I look forward to reviewing many more Pearls in the future.
Since 2005, I’ve had the opportunity to peer review Pearls articles submitted for publication in Current Psychiatry. In that time, I have read many worthwhile papers written by authors who may not be entirely clear about what constitutes a Pearl. The mnemonic PEARL could help authors:
- decide if their article or idea is appropriate for Pearls
- construct the article to conform to the Pearls format.
Precise. A Pearls article should make an accurate and concise statement. It should not be an elaborate or generalized idea based on either limited or copious information.
Easy to remember. Lengthy, highly detailed articles may be helpful and informative but are not consistent with the purpose of Pearls.
Alert. A Pearl should alert a physician to identify a problem, diagnosis, or adverse effect that they might otherwise miss or take unnecessary time to identify. Classic examples are the “handshake diagnosis” of hyperthyroidism,1 or the “3 little words that can diagnose mild cognitive impairment.”2
References. A professional article of any length should include references. References add immediate credibility to the information presented. For a Pearl, even one reference is acceptable. A writer can easily search PubMed and the Internet to find references to confirm or support their ideas.
Less is more. Architect Mies van der Rohe’s minimalist concept applies to Pearls. A Pearl—like its namesake—is small, polished, and valuable. Simplicity is its essence.
I hope this mnemonic is useful for clinicians interested in sharing their ideas or experiences to help others in the field. I look forward to reviewing many more Pearls in the future.
Since 2005, I’ve had the opportunity to peer review Pearls articles submitted for publication in Current Psychiatry. In that time, I have read many worthwhile papers written by authors who may not be entirely clear about what constitutes a Pearl. The mnemonic PEARL could help authors:
- decide if their article or idea is appropriate for Pearls
- construct the article to conform to the Pearls format.
Precise. A Pearls article should make an accurate and concise statement. It should not be an elaborate or generalized idea based on either limited or copious information.
Easy to remember. Lengthy, highly detailed articles may be helpful and informative but are not consistent with the purpose of Pearls.
Alert. A Pearl should alert a physician to identify a problem, diagnosis, or adverse effect that they might otherwise miss or take unnecessary time to identify. Classic examples are the “handshake diagnosis” of hyperthyroidism,1 or the “3 little words that can diagnose mild cognitive impairment.”2
References. A professional article of any length should include references. References add immediate credibility to the information presented. For a Pearl, even one reference is acceptable. A writer can easily search PubMed and the Internet to find references to confirm or support their ideas.
Less is more. Architect Mies van der Rohe’s minimalist concept applies to Pearls. A Pearl—like its namesake—is small, polished, and valuable. Simplicity is its essence.
I hope this mnemonic is useful for clinicians interested in sharing their ideas or experiences to help others in the field. I look forward to reviewing many more Pearls in the future.
Consider PTSD subtypes in patient workup
Posttraumatic stress disorder (PTSD) is a confusing diagnostic category because it includes victims of trauma as well as individuals exposed to trauma. Also, PTSD encompasses exposure to different types of trauma, which can have significant implications for symptom development and treatment.
Consider the treatment history of a male combat veteran who exhibits multiple PTSD symptoms, including nightmares, flashbacks, social isolation, anger, and guilt related to his war experiences. Several psychiatrists saw the patient, which resulted in multiple medication changes but little benefit. On further assessment, the practitioners noted that the veteran’s war experiences were minimally problematic; the prominent nightmares, ruminations, flashbacks, and guilt were related to his witnessing a civilian female being sexually assaulted. The veteran’s guilt about not intervening was the basis of his PTSD. This led to a change in treatment from pharmacotherapy to a focus on supportive therapy.
Conceptualizing subtypes of PTSD—similar to many DSM-IV-TR diagnoses such as phobias or delusional disorders—might help better define the diagnosis. Each sub-type, as conceptualized below, might have its own prognosis and treatment. Our hope is that this strategy will benefit the patient by improving research and evidence-based practice.
PTSD subtypes
Victim-related trauma. Related to witnessing a criminal act or being a victim of a criminal act such as rape or assault. The patient is in a passive role.
Natural disasters, such as a tornado, earthquake, or hurricane.
Survivor guilt. The patient is not a perpetrator and might have been exposed to trauma, but symptoms are related to surviving while others close to the patient did not.
Perpetrator guilt. It is debatable whether this should be a PTSD subtype but our experience suggests that this pattern severely complicates PTSD diagnosis and treatment. It often is not initially disclosed by patients but surfaces when treatment is not working despite a strong therapeutic alliance.
PTSD not otherwise specified. This subtype is typical in patients who were not directly involved in a traumatic event but experienced symptoms related to it. Examples include picking up dead bodies, cleaning up a tornado site, or observing siblings being beaten. This category also may reflect an unclear picture if no primary subtype accounts for the majority of symptoms.
Qualifiers
Individuals who previously have been exposed to trauma are more vulnerable to subsequent trauma. Experiencing ongoing multiple traumatic events—such as in military combat—can have a cumulative effect. Thus, identifying episodes of trauma also should be part of the PTSD assessment.
Single event. The patient is exposed to a single traumatic episode, such as being the victim of a crime.
Multiple events/single episode. The patient is exposed to repeated, related traumatic events. Examples include ongoing military combat or child abuse.
Multiple events. The patient is exposed to ≥2 separate traumatic events. A combination such as this might include a serious motor vehicle accident followed by a natural disaster.
As the diagnosis of PTSD evolves, utilizing subtypes and qualifiers might clarify treatment strategies because some subtypes might be more amenable to certain psychopharmacologic or psychotherapeutic treatment regimens.
Diagnostic confusion
Some researchers question whether traumatic stress causes PTSD syndrome,1 whereas others recommend “tightening” the diagnostic criteria.2 Concerns regarding PTSD diagnosis are multiple and include:
- the importance of ruling out malingering3
- the effects of different diagnostic criteria resulting in disparate prevalence rates
- emphasizing the importance of dysfunction as a criterion for PTSD.4
Conceptual inconsistencies in DSM-IV-TR diagnostic criteria also can lead to confusion. Although there is a category of arousal symptoms, Criterion B4 (intense psychological distress) and Criterion B5 (physiological reactivity) are listed as re-experiencing symptoms rather than arousal symptoms. Finally, the criteria presented do not follow a logical progression. Research suggests that re-experiencing symptoms do not lead to avoidance but result in arousal symptoms, which in turn trigger avoidance.5
1. Bodkin JA, Pope HG, Detke MJ, et al. Is PTSD caused by traumatic stress? J Anxiety Disord. 2007;21:176-182.
2. Spitzer RL, First MB, Wakefield JC. Saving PTSD from itself in DSM-V. J Anxiety Disord. 2007;21:233-241.
3. Rosen GM, Taylor S. Pseudo-PTSD. J Anxiety Disord 2007;21:201-210.
4. Zahava S, Horesh D. Changes in diagnostic criteria for PTSD: implications from two prospective longitudinal studies. Am J Orthopsychiatry. 2007;77:182-188.
5. Resick PA, Monson CM, Chard KM. Cognitive processing therapy: veteran/military version. Washington, DC: Department of Veterans Affairs; 2007.
Posttraumatic stress disorder (PTSD) is a confusing diagnostic category because it includes victims of trauma as well as individuals exposed to trauma. Also, PTSD encompasses exposure to different types of trauma, which can have significant implications for symptom development and treatment.
Consider the treatment history of a male combat veteran who exhibits multiple PTSD symptoms, including nightmares, flashbacks, social isolation, anger, and guilt related to his war experiences. Several psychiatrists saw the patient, which resulted in multiple medication changes but little benefit. On further assessment, the practitioners noted that the veteran’s war experiences were minimally problematic; the prominent nightmares, ruminations, flashbacks, and guilt were related to his witnessing a civilian female being sexually assaulted. The veteran’s guilt about not intervening was the basis of his PTSD. This led to a change in treatment from pharmacotherapy to a focus on supportive therapy.
Conceptualizing subtypes of PTSD—similar to many DSM-IV-TR diagnoses such as phobias or delusional disorders—might help better define the diagnosis. Each sub-type, as conceptualized below, might have its own prognosis and treatment. Our hope is that this strategy will benefit the patient by improving research and evidence-based practice.
PTSD subtypes
Victim-related trauma. Related to witnessing a criminal act or being a victim of a criminal act such as rape or assault. The patient is in a passive role.
Natural disasters, such as a tornado, earthquake, or hurricane.
Survivor guilt. The patient is not a perpetrator and might have been exposed to trauma, but symptoms are related to surviving while others close to the patient did not.
Perpetrator guilt. It is debatable whether this should be a PTSD subtype but our experience suggests that this pattern severely complicates PTSD diagnosis and treatment. It often is not initially disclosed by patients but surfaces when treatment is not working despite a strong therapeutic alliance.
PTSD not otherwise specified. This subtype is typical in patients who were not directly involved in a traumatic event but experienced symptoms related to it. Examples include picking up dead bodies, cleaning up a tornado site, or observing siblings being beaten. This category also may reflect an unclear picture if no primary subtype accounts for the majority of symptoms.
Qualifiers
Individuals who previously have been exposed to trauma are more vulnerable to subsequent trauma. Experiencing ongoing multiple traumatic events—such as in military combat—can have a cumulative effect. Thus, identifying episodes of trauma also should be part of the PTSD assessment.
Single event. The patient is exposed to a single traumatic episode, such as being the victim of a crime.
Multiple events/single episode. The patient is exposed to repeated, related traumatic events. Examples include ongoing military combat or child abuse.
Multiple events. The patient is exposed to ≥2 separate traumatic events. A combination such as this might include a serious motor vehicle accident followed by a natural disaster.
As the diagnosis of PTSD evolves, utilizing subtypes and qualifiers might clarify treatment strategies because some subtypes might be more amenable to certain psychopharmacologic or psychotherapeutic treatment regimens.
Diagnostic confusion
Some researchers question whether traumatic stress causes PTSD syndrome,1 whereas others recommend “tightening” the diagnostic criteria.2 Concerns regarding PTSD diagnosis are multiple and include:
- the importance of ruling out malingering3
- the effects of different diagnostic criteria resulting in disparate prevalence rates
- emphasizing the importance of dysfunction as a criterion for PTSD.4
Conceptual inconsistencies in DSM-IV-TR diagnostic criteria also can lead to confusion. Although there is a category of arousal symptoms, Criterion B4 (intense psychological distress) and Criterion B5 (physiological reactivity) are listed as re-experiencing symptoms rather than arousal symptoms. Finally, the criteria presented do not follow a logical progression. Research suggests that re-experiencing symptoms do not lead to avoidance but result in arousal symptoms, which in turn trigger avoidance.5
Posttraumatic stress disorder (PTSD) is a confusing diagnostic category because it includes victims of trauma as well as individuals exposed to trauma. Also, PTSD encompasses exposure to different types of trauma, which can have significant implications for symptom development and treatment.
Consider the treatment history of a male combat veteran who exhibits multiple PTSD symptoms, including nightmares, flashbacks, social isolation, anger, and guilt related to his war experiences. Several psychiatrists saw the patient, which resulted in multiple medication changes but little benefit. On further assessment, the practitioners noted that the veteran’s war experiences were minimally problematic; the prominent nightmares, ruminations, flashbacks, and guilt were related to his witnessing a civilian female being sexually assaulted. The veteran’s guilt about not intervening was the basis of his PTSD. This led to a change in treatment from pharmacotherapy to a focus on supportive therapy.
Conceptualizing subtypes of PTSD—similar to many DSM-IV-TR diagnoses such as phobias or delusional disorders—might help better define the diagnosis. Each sub-type, as conceptualized below, might have its own prognosis and treatment. Our hope is that this strategy will benefit the patient by improving research and evidence-based practice.
PTSD subtypes
Victim-related trauma. Related to witnessing a criminal act or being a victim of a criminal act such as rape or assault. The patient is in a passive role.
Natural disasters, such as a tornado, earthquake, or hurricane.
Survivor guilt. The patient is not a perpetrator and might have been exposed to trauma, but symptoms are related to surviving while others close to the patient did not.
Perpetrator guilt. It is debatable whether this should be a PTSD subtype but our experience suggests that this pattern severely complicates PTSD diagnosis and treatment. It often is not initially disclosed by patients but surfaces when treatment is not working despite a strong therapeutic alliance.
PTSD not otherwise specified. This subtype is typical in patients who were not directly involved in a traumatic event but experienced symptoms related to it. Examples include picking up dead bodies, cleaning up a tornado site, or observing siblings being beaten. This category also may reflect an unclear picture if no primary subtype accounts for the majority of symptoms.
Qualifiers
Individuals who previously have been exposed to trauma are more vulnerable to subsequent trauma. Experiencing ongoing multiple traumatic events—such as in military combat—can have a cumulative effect. Thus, identifying episodes of trauma also should be part of the PTSD assessment.
Single event. The patient is exposed to a single traumatic episode, such as being the victim of a crime.
Multiple events/single episode. The patient is exposed to repeated, related traumatic events. Examples include ongoing military combat or child abuse.
Multiple events. The patient is exposed to ≥2 separate traumatic events. A combination such as this might include a serious motor vehicle accident followed by a natural disaster.
As the diagnosis of PTSD evolves, utilizing subtypes and qualifiers might clarify treatment strategies because some subtypes might be more amenable to certain psychopharmacologic or psychotherapeutic treatment regimens.
Diagnostic confusion
Some researchers question whether traumatic stress causes PTSD syndrome,1 whereas others recommend “tightening” the diagnostic criteria.2 Concerns regarding PTSD diagnosis are multiple and include:
- the importance of ruling out malingering3
- the effects of different diagnostic criteria resulting in disparate prevalence rates
- emphasizing the importance of dysfunction as a criterion for PTSD.4
Conceptual inconsistencies in DSM-IV-TR diagnostic criteria also can lead to confusion. Although there is a category of arousal symptoms, Criterion B4 (intense psychological distress) and Criterion B5 (physiological reactivity) are listed as re-experiencing symptoms rather than arousal symptoms. Finally, the criteria presented do not follow a logical progression. Research suggests that re-experiencing symptoms do not lead to avoidance but result in arousal symptoms, which in turn trigger avoidance.5
1. Bodkin JA, Pope HG, Detke MJ, et al. Is PTSD caused by traumatic stress? J Anxiety Disord. 2007;21:176-182.
2. Spitzer RL, First MB, Wakefield JC. Saving PTSD from itself in DSM-V. J Anxiety Disord. 2007;21:233-241.
3. Rosen GM, Taylor S. Pseudo-PTSD. J Anxiety Disord 2007;21:201-210.
4. Zahava S, Horesh D. Changes in diagnostic criteria for PTSD: implications from two prospective longitudinal studies. Am J Orthopsychiatry. 2007;77:182-188.
5. Resick PA, Monson CM, Chard KM. Cognitive processing therapy: veteran/military version. Washington, DC: Department of Veterans Affairs; 2007.
1. Bodkin JA, Pope HG, Detke MJ, et al. Is PTSD caused by traumatic stress? J Anxiety Disord. 2007;21:176-182.
2. Spitzer RL, First MB, Wakefield JC. Saving PTSD from itself in DSM-V. J Anxiety Disord. 2007;21:233-241.
3. Rosen GM, Taylor S. Pseudo-PTSD. J Anxiety Disord 2007;21:201-210.
4. Zahava S, Horesh D. Changes in diagnostic criteria for PTSD: implications from two prospective longitudinal studies. Am J Orthopsychiatry. 2007;77:182-188.
5. Resick PA, Monson CM, Chard KM. Cognitive processing therapy: veteran/military version. Washington, DC: Department of Veterans Affairs; 2007.
Antipsychotics and bones
Antipsychotics were not mentioned in Drs. Sarah K. Rivelli and Andrew J. Muzyk’s list of psychiatric medications that could increase the risk of osteoporosis (“Protect patients’ bones when prescribing,” Medicine in Brief, Current Psychiatry, June 2009). Data show that hyperprolactinemia associated with antipsychotics can increase osteoporosis risk.1
Antipsychotics often are given on a long-term basis, which creates concern for all patients taking these medications, especially because obtaining prolactin levels typically is not the standard of care. For medical professionals, linking hyperprolactinemia with osteoporosis may seem like common sense, as is linking hyperprolactinemia with antipsychotics, but we rarely correlate antipsychotics with osteoporosis. We should make that connection in consideration of the long-term health effects antipsychotics have on our patients.
James Cho, MD
Forensic psychiatry fellow
University of Cincinnati
Cincinnati, OH
Reference
1. Meaney AM, Smith S, Howes OD, et al. Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. Br J Psychiatry. 2004;184:503-508.
Drs. Rivelli and Muzyk respond
We agree with Dr. Cho about the need to be aware of deleterious effects of antipsychotics on bone density. Hyperprolactinemia from antipsychotics results from antagonism of D2 receptors on pituitary lactotroph cells. Blockade prevents dopamine stimulation, which normally inhibits prolactin release. Stimulation of serotonin-2A (5-HT2A) receptors on pituitary lactotroph cells also contributes to prolactin release. Second-generation antipsychotics (SGAs) strongly inhibit 5-HT2A receptors in the tuberoinfundibular pathway, which means these agents may have a lower risk of hyperprolactinemia compared with first-generation antipsychotics (FGAs). Osteoporosis is caused by prolonged dysregulation of the HPA axis and hypogonadism.1
Other factors—including a schizophrenia diagnosis, sedentary lifestyle, smoking, substance abuse, and malnutrition—also may contribute to osteoporosis.2 This condition may be highly prevalent and underdiagnosed in male schizophrenics.3 We would consider patients on chronic antipsychotic therapy—particularly those receiving higher doses or FGAs—at higher risk of osteoporosis.
Sarah K. Rivelli, MD
Associate program director, internal medicine-psychiatry residency
Andrew J. Muzyk, PharmD
Clinical pharmacist
Duke University Medical Center
Durham, NC
1. Byerly M, Suppes T, Tran QV, et al. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorder. J Clin Psychopharmacol. 2007;27(6):639-661.
2. Halbreich U. Osteoporosis, schizophrenia and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS Drugs. 2007;21(8):641-657.
3. Meyer JM, Lehman D. Bone mineral density in male schizophrenia patients: a review. Ann Clin Psychiatry. 2006;18(10):43-48.
Antipsychotics were not mentioned in Drs. Sarah K. Rivelli and Andrew J. Muzyk’s list of psychiatric medications that could increase the risk of osteoporosis (“Protect patients’ bones when prescribing,” Medicine in Brief, Current Psychiatry, June 2009). Data show that hyperprolactinemia associated with antipsychotics can increase osteoporosis risk.1
Antipsychotics often are given on a long-term basis, which creates concern for all patients taking these medications, especially because obtaining prolactin levels typically is not the standard of care. For medical professionals, linking hyperprolactinemia with osteoporosis may seem like common sense, as is linking hyperprolactinemia with antipsychotics, but we rarely correlate antipsychotics with osteoporosis. We should make that connection in consideration of the long-term health effects antipsychotics have on our patients.
James Cho, MD
Forensic psychiatry fellow
University of Cincinnati
Cincinnati, OH
Reference
1. Meaney AM, Smith S, Howes OD, et al. Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. Br J Psychiatry. 2004;184:503-508.
Drs. Rivelli and Muzyk respond
We agree with Dr. Cho about the need to be aware of deleterious effects of antipsychotics on bone density. Hyperprolactinemia from antipsychotics results from antagonism of D2 receptors on pituitary lactotroph cells. Blockade prevents dopamine stimulation, which normally inhibits prolactin release. Stimulation of serotonin-2A (5-HT2A) receptors on pituitary lactotroph cells also contributes to prolactin release. Second-generation antipsychotics (SGAs) strongly inhibit 5-HT2A receptors in the tuberoinfundibular pathway, which means these agents may have a lower risk of hyperprolactinemia compared with first-generation antipsychotics (FGAs). Osteoporosis is caused by prolonged dysregulation of the HPA axis and hypogonadism.1
Other factors—including a schizophrenia diagnosis, sedentary lifestyle, smoking, substance abuse, and malnutrition—also may contribute to osteoporosis.2 This condition may be highly prevalent and underdiagnosed in male schizophrenics.3 We would consider patients on chronic antipsychotic therapy—particularly those receiving higher doses or FGAs—at higher risk of osteoporosis.
Sarah K. Rivelli, MD
Associate program director, internal medicine-psychiatry residency
Andrew J. Muzyk, PharmD
Clinical pharmacist
Duke University Medical Center
Durham, NC
Antipsychotics were not mentioned in Drs. Sarah K. Rivelli and Andrew J. Muzyk’s list of psychiatric medications that could increase the risk of osteoporosis (“Protect patients’ bones when prescribing,” Medicine in Brief, Current Psychiatry, June 2009). Data show that hyperprolactinemia associated with antipsychotics can increase osteoporosis risk.1
Antipsychotics often are given on a long-term basis, which creates concern for all patients taking these medications, especially because obtaining prolactin levels typically is not the standard of care. For medical professionals, linking hyperprolactinemia with osteoporosis may seem like common sense, as is linking hyperprolactinemia with antipsychotics, but we rarely correlate antipsychotics with osteoporosis. We should make that connection in consideration of the long-term health effects antipsychotics have on our patients.
James Cho, MD
Forensic psychiatry fellow
University of Cincinnati
Cincinnati, OH
Reference
1. Meaney AM, Smith S, Howes OD, et al. Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. Br J Psychiatry. 2004;184:503-508.
Drs. Rivelli and Muzyk respond
We agree with Dr. Cho about the need to be aware of deleterious effects of antipsychotics on bone density. Hyperprolactinemia from antipsychotics results from antagonism of D2 receptors on pituitary lactotroph cells. Blockade prevents dopamine stimulation, which normally inhibits prolactin release. Stimulation of serotonin-2A (5-HT2A) receptors on pituitary lactotroph cells also contributes to prolactin release. Second-generation antipsychotics (SGAs) strongly inhibit 5-HT2A receptors in the tuberoinfundibular pathway, which means these agents may have a lower risk of hyperprolactinemia compared with first-generation antipsychotics (FGAs). Osteoporosis is caused by prolonged dysregulation of the HPA axis and hypogonadism.1
Other factors—including a schizophrenia diagnosis, sedentary lifestyle, smoking, substance abuse, and malnutrition—also may contribute to osteoporosis.2 This condition may be highly prevalent and underdiagnosed in male schizophrenics.3 We would consider patients on chronic antipsychotic therapy—particularly those receiving higher doses or FGAs—at higher risk of osteoporosis.
Sarah K. Rivelli, MD
Associate program director, internal medicine-psychiatry residency
Andrew J. Muzyk, PharmD
Clinical pharmacist
Duke University Medical Center
Durham, NC
1. Byerly M, Suppes T, Tran QV, et al. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorder. J Clin Psychopharmacol. 2007;27(6):639-661.
2. Halbreich U. Osteoporosis, schizophrenia and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS Drugs. 2007;21(8):641-657.
3. Meyer JM, Lehman D. Bone mineral density in male schizophrenia patients: a review. Ann Clin Psychiatry. 2006;18(10):43-48.
1. Byerly M, Suppes T, Tran QV, et al. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorder. J Clin Psychopharmacol. 2007;27(6):639-661.
2. Halbreich U. Osteoporosis, schizophrenia and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS Drugs. 2007;21(8):641-657.
3. Meyer JM, Lehman D. Bone mineral density in male schizophrenia patients: a review. Ann Clin Psychiatry. 2006;18(10):43-48.
No need to soften criteria
I am concerned about the article on “soft bipolarity” and easing the diagnostic criteria for bipolar disorder (BP) II (“Soft bipolarity: How to recognize and treat bipolar II disorder,” Current Psychiatry, July 2009).
I’ve found no issue as vexing as that of dealing with the “soft” end of the so-called “bipolar spectrum.” At that end of the spectrum, it can be very difficult to determine whether my patient’s symptoms are most properly attributed to 1 or more of several other DSM-IV conditions, most notably attention-deficit/hyperactivity disorder (ADHD), posttraumatic stress disorder (PTSD), and borderline personality disorder. Including overactivity would sweep in a multitude of patients with other diagnoses, most notably ADHD. A number of psychiatric conditions can cause at least 1 night of not sleeping. Softening the diagnostic criteria for hypomania to include only 1 night of sleeplessness would capture a number of patients who do not have BP.
In my clinical practice, I routinely encounter patients who I believe have been misdiagnosed with BP II or BP not otherwise specified by clinicians who are using “soft” criteria such as those promoted by Dr. Daniel J. Smith. These patients often have been exposed to a number of psychiatric medications that have caused adverse effects and have not lead to significant benefits. Instead of using “soft” criteria for BP, I adhere to the “hard criteria” for BP II and other conditions in the DSM-IV when making diagnoses, and I utilize evidence-based treatments for these conditions. Supporting my skepticism is the fact that patients who would meet soft BP II criteria often experience excellent responses to treatments for conditions such as PTSD or ADHD, and ultimately never require treatment for BP.
I believe there is real potential for harm to our patients in softening current criteria:
- Overdiagnosis of bipolar disorder in my experience leads to underdiagnosis and undertreatment of other psychiatric conditions.
- Diagnosis naturally leads to treatment, often with drugs that do not have good data supporting their use for BP II, as Dr. Smith states in his article.
- Medications for bipolar disorder are among the most toxic medications used in psychiatry, with serious side effects, including renal failure, weight gain, Stevens-Johnson syndrome, and hypercholesterolemia.
Exposing more patients to these treatments without clear evidence that softening the diagnostic criteria identifies those with true bipolar disorder is a frightening prospect.
Joseph Lasek, MD
Associate medical director
University of Vermont College of Medicine
Burlington, VT
I am concerned about the article on “soft bipolarity” and easing the diagnostic criteria for bipolar disorder (BP) II (“Soft bipolarity: How to recognize and treat bipolar II disorder,” Current Psychiatry, July 2009).
I’ve found no issue as vexing as that of dealing with the “soft” end of the so-called “bipolar spectrum.” At that end of the spectrum, it can be very difficult to determine whether my patient’s symptoms are most properly attributed to 1 or more of several other DSM-IV conditions, most notably attention-deficit/hyperactivity disorder (ADHD), posttraumatic stress disorder (PTSD), and borderline personality disorder. Including overactivity would sweep in a multitude of patients with other diagnoses, most notably ADHD. A number of psychiatric conditions can cause at least 1 night of not sleeping. Softening the diagnostic criteria for hypomania to include only 1 night of sleeplessness would capture a number of patients who do not have BP.
In my clinical practice, I routinely encounter patients who I believe have been misdiagnosed with BP II or BP not otherwise specified by clinicians who are using “soft” criteria such as those promoted by Dr. Daniel J. Smith. These patients often have been exposed to a number of psychiatric medications that have caused adverse effects and have not lead to significant benefits. Instead of using “soft” criteria for BP, I adhere to the “hard criteria” for BP II and other conditions in the DSM-IV when making diagnoses, and I utilize evidence-based treatments for these conditions. Supporting my skepticism is the fact that patients who would meet soft BP II criteria often experience excellent responses to treatments for conditions such as PTSD or ADHD, and ultimately never require treatment for BP.
I believe there is real potential for harm to our patients in softening current criteria:
- Overdiagnosis of bipolar disorder in my experience leads to underdiagnosis and undertreatment of other psychiatric conditions.
- Diagnosis naturally leads to treatment, often with drugs that do not have good data supporting their use for BP II, as Dr. Smith states in his article.
- Medications for bipolar disorder are among the most toxic medications used in psychiatry, with serious side effects, including renal failure, weight gain, Stevens-Johnson syndrome, and hypercholesterolemia.
Exposing more patients to these treatments without clear evidence that softening the diagnostic criteria identifies those with true bipolar disorder is a frightening prospect.
Joseph Lasek, MD
Associate medical director
University of Vermont College of Medicine
Burlington, VT
I am concerned about the article on “soft bipolarity” and easing the diagnostic criteria for bipolar disorder (BP) II (“Soft bipolarity: How to recognize and treat bipolar II disorder,” Current Psychiatry, July 2009).
I’ve found no issue as vexing as that of dealing with the “soft” end of the so-called “bipolar spectrum.” At that end of the spectrum, it can be very difficult to determine whether my patient’s symptoms are most properly attributed to 1 or more of several other DSM-IV conditions, most notably attention-deficit/hyperactivity disorder (ADHD), posttraumatic stress disorder (PTSD), and borderline personality disorder. Including overactivity would sweep in a multitude of patients with other diagnoses, most notably ADHD. A number of psychiatric conditions can cause at least 1 night of not sleeping. Softening the diagnostic criteria for hypomania to include only 1 night of sleeplessness would capture a number of patients who do not have BP.
In my clinical practice, I routinely encounter patients who I believe have been misdiagnosed with BP II or BP not otherwise specified by clinicians who are using “soft” criteria such as those promoted by Dr. Daniel J. Smith. These patients often have been exposed to a number of psychiatric medications that have caused adverse effects and have not lead to significant benefits. Instead of using “soft” criteria for BP, I adhere to the “hard criteria” for BP II and other conditions in the DSM-IV when making diagnoses, and I utilize evidence-based treatments for these conditions. Supporting my skepticism is the fact that patients who would meet soft BP II criteria often experience excellent responses to treatments for conditions such as PTSD or ADHD, and ultimately never require treatment for BP.
I believe there is real potential for harm to our patients in softening current criteria:
- Overdiagnosis of bipolar disorder in my experience leads to underdiagnosis and undertreatment of other psychiatric conditions.
- Diagnosis naturally leads to treatment, often with drugs that do not have good data supporting their use for BP II, as Dr. Smith states in his article.
- Medications for bipolar disorder are among the most toxic medications used in psychiatry, with serious side effects, including renal failure, weight gain, Stevens-Johnson syndrome, and hypercholesterolemia.
Exposing more patients to these treatments without clear evidence that softening the diagnostic criteria identifies those with true bipolar disorder is a frightening prospect.
Joseph Lasek, MD
Associate medical director
University of Vermont College of Medicine
Burlington, VT
Reducing potential for harm
I enjoyed the review of assessing harm to self and others by Drs. Charles Scott and Phillip J. Resnick (“Assessing potential for harm: Would your patient injure himself or others?” Current Psychiatry, July 2009). All too often mental health professionals rely on “gut instinct” and neglect evidence-based strategies when assessing for dangerousness. Generally, I believe this to be an issue of complacency rather than willful neglect or lack of training.
I would like to add a few points that I believe are key to conducting a proper assessment of suicide risk. First, schedule and document a firm follow-up appointment with the patient after evaluation. Second, I believe religious and spiritual beliefs function as a protective factor for many individuals, although this varies from person to person. And last, inquiring about the patient’s immediate future orientation (eg, “What are your plans for tomorrow?”) is crucial when conducting a comprehensive risk assessment.
Bret A. Moore, PsyD, ABPP
Poplar, MT
I enjoyed the review of assessing harm to self and others by Drs. Charles Scott and Phillip J. Resnick (“Assessing potential for harm: Would your patient injure himself or others?” Current Psychiatry, July 2009). All too often mental health professionals rely on “gut instinct” and neglect evidence-based strategies when assessing for dangerousness. Generally, I believe this to be an issue of complacency rather than willful neglect or lack of training.
I would like to add a few points that I believe are key to conducting a proper assessment of suicide risk. First, schedule and document a firm follow-up appointment with the patient after evaluation. Second, I believe religious and spiritual beliefs function as a protective factor for many individuals, although this varies from person to person. And last, inquiring about the patient’s immediate future orientation (eg, “What are your plans for tomorrow?”) is crucial when conducting a comprehensive risk assessment.
Bret A. Moore, PsyD, ABPP
Poplar, MT
I enjoyed the review of assessing harm to self and others by Drs. Charles Scott and Phillip J. Resnick (“Assessing potential for harm: Would your patient injure himself or others?” Current Psychiatry, July 2009). All too often mental health professionals rely on “gut instinct” and neglect evidence-based strategies when assessing for dangerousness. Generally, I believe this to be an issue of complacency rather than willful neglect or lack of training.
I would like to add a few points that I believe are key to conducting a proper assessment of suicide risk. First, schedule and document a firm follow-up appointment with the patient after evaluation. Second, I believe religious and spiritual beliefs function as a protective factor for many individuals, although this varies from person to person. And last, inquiring about the patient’s immediate future orientation (eg, “What are your plans for tomorrow?”) is crucial when conducting a comprehensive risk assessment.
Bret A. Moore, PsyD, ABPP
Poplar, MT