User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Psychiatry ethics guidelines need to be revised
At a time when 28.4% of psychiatric practices provided no psychotherapy during a typical week,1 applying psychotherapy principles to ethical judgments involving psychiatrists is inappropriate and should be abandoned (“Psychiatrist/patient boundaries: When it’s OK to stretch the line” Current Psychiatry, August 2008).
The American Psychiatric Association should revamp its ethics annotations by removing psychoanalytic references and terms such as “identification” to make these guidelines relevant to all psychiatric practices, even those without psychotherapy.
H. Berryman Edwards, MD
Bellevue, WA
Reference
1. Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry 2008;65(8):962-70.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
At a time when 28.4% of psychiatric practices provided no psychotherapy during a typical week,1 applying psychotherapy principles to ethical judgments involving psychiatrists is inappropriate and should be abandoned (“Psychiatrist/patient boundaries: When it’s OK to stretch the line” Current Psychiatry, August 2008).
The American Psychiatric Association should revamp its ethics annotations by removing psychoanalytic references and terms such as “identification” to make these guidelines relevant to all psychiatric practices, even those without psychotherapy.
H. Berryman Edwards, MD
Bellevue, WA
At a time when 28.4% of psychiatric practices provided no psychotherapy during a typical week,1 applying psychotherapy principles to ethical judgments involving psychiatrists is inappropriate and should be abandoned (“Psychiatrist/patient boundaries: When it’s OK to stretch the line” Current Psychiatry, August 2008).
The American Psychiatric Association should revamp its ethics annotations by removing psychoanalytic references and terms such as “identification” to make these guidelines relevant to all psychiatric practices, even those without psychotherapy.
H. Berryman Edwards, MD
Bellevue, WA
Reference
1. Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry 2008;65(8):962-70.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
Reference
1. Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry 2008;65(8):962-70.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
Ask returning veterans about traumatic brain injury
The article “6 screening questions for military veterans” (Pearls, Current Psychiatry, September 2008) is a well written and thorough review for clinicians treating active duty personnel and military veterans. A very important and clinically relevant seventh question is to ask about traumatic brain injury (TBI) that may have occurred during training or deployment to Iraq or Afghanistan. One tool clinicians can use to screen these patients is the Brief Traumatic Brain Injury Screen developed by the Defense and Veterans Brain Injury Center.1 Coupled with a clinical interview, this 3-question survey will assist clinicians in identifying possible TBI patients. Referring TBI patients to a polytrauma rehabilitative center through the Department of Veterans Affairs is part of a comprehensive treatment plan.2
Timothy Berigan, MD
Psychiatrist
Raymond W. Bliss Army Health Center
Fort Huachuca, AZ
1. Schwab KA, Baker G, Ivins B, et al. The Brief Traumatic Brain Injury Screen (BTBIS): investigating the validity of a self-report instrument for detecting traumatic brain injury (TBI) in troops returning from deployment in Afghanistan and Iraq. Neurology 2006;66(5)(suppl 2):A235.
2. Friedmann-Sánchez G, Sayer NA, Pickett T. Provider perspectives on rehabilitation of patients with polytrauma. Arch Phys Med Rehabil 2008;89(1):171-8.
Dr. Barry responds
Mild traumatic brain injury (mTBI) is one of the signature diagnoses of the conflicts in Iraq and Afghanistan, and we’re learning more about the pathogenesis, manifestations, sequelae, and treatment of this syndrome. Most veterans have basic knowledge about this condition because the military educates and screens all personnel for mTBI and employs aggressive, multi-disciplinary treatment plans for those in need. Thus, clinical interview, physical examination, and screening for mTBI—as suggested by Dr. Berigan—might yield useful information for clinicians treating veterans.
Matthew James Barry, DO
Chief of psychiatric services
U.S. Army’s Medical Department Activity
Fort Huachuca, AZ
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
The article “6 screening questions for military veterans” (Pearls, Current Psychiatry, September 2008) is a well written and thorough review for clinicians treating active duty personnel and military veterans. A very important and clinically relevant seventh question is to ask about traumatic brain injury (TBI) that may have occurred during training or deployment to Iraq or Afghanistan. One tool clinicians can use to screen these patients is the Brief Traumatic Brain Injury Screen developed by the Defense and Veterans Brain Injury Center.1 Coupled with a clinical interview, this 3-question survey will assist clinicians in identifying possible TBI patients. Referring TBI patients to a polytrauma rehabilitative center through the Department of Veterans Affairs is part of a comprehensive treatment plan.2
Timothy Berigan, MD
Psychiatrist
Raymond W. Bliss Army Health Center
Fort Huachuca, AZ
1. Schwab KA, Baker G, Ivins B, et al. The Brief Traumatic Brain Injury Screen (BTBIS): investigating the validity of a self-report instrument for detecting traumatic brain injury (TBI) in troops returning from deployment in Afghanistan and Iraq. Neurology 2006;66(5)(suppl 2):A235.
2. Friedmann-Sánchez G, Sayer NA, Pickett T. Provider perspectives on rehabilitation of patients with polytrauma. Arch Phys Med Rehabil 2008;89(1):171-8.
Dr. Barry responds
Mild traumatic brain injury (mTBI) is one of the signature diagnoses of the conflicts in Iraq and Afghanistan, and we’re learning more about the pathogenesis, manifestations, sequelae, and treatment of this syndrome. Most veterans have basic knowledge about this condition because the military educates and screens all personnel for mTBI and employs aggressive, multi-disciplinary treatment plans for those in need. Thus, clinical interview, physical examination, and screening for mTBI—as suggested by Dr. Berigan—might yield useful information for clinicians treating veterans.
Matthew James Barry, DO
Chief of psychiatric services
U.S. Army’s Medical Department Activity
Fort Huachuca, AZ
The article “6 screening questions for military veterans” (Pearls, Current Psychiatry, September 2008) is a well written and thorough review for clinicians treating active duty personnel and military veterans. A very important and clinically relevant seventh question is to ask about traumatic brain injury (TBI) that may have occurred during training or deployment to Iraq or Afghanistan. One tool clinicians can use to screen these patients is the Brief Traumatic Brain Injury Screen developed by the Defense and Veterans Brain Injury Center.1 Coupled with a clinical interview, this 3-question survey will assist clinicians in identifying possible TBI patients. Referring TBI patients to a polytrauma rehabilitative center through the Department of Veterans Affairs is part of a comprehensive treatment plan.2
Timothy Berigan, MD
Psychiatrist
Raymond W. Bliss Army Health Center
Fort Huachuca, AZ
1. Schwab KA, Baker G, Ivins B, et al. The Brief Traumatic Brain Injury Screen (BTBIS): investigating the validity of a self-report instrument for detecting traumatic brain injury (TBI) in troops returning from deployment in Afghanistan and Iraq. Neurology 2006;66(5)(suppl 2):A235.
2. Friedmann-Sánchez G, Sayer NA, Pickett T. Provider perspectives on rehabilitation of patients with polytrauma. Arch Phys Med Rehabil 2008;89(1):171-8.
Dr. Barry responds
Mild traumatic brain injury (mTBI) is one of the signature diagnoses of the conflicts in Iraq and Afghanistan, and we’re learning more about the pathogenesis, manifestations, sequelae, and treatment of this syndrome. Most veterans have basic knowledge about this condition because the military educates and screens all personnel for mTBI and employs aggressive, multi-disciplinary treatment plans for those in need. Thus, clinical interview, physical examination, and screening for mTBI—as suggested by Dr. Berigan—might yield useful information for clinicians treating veterans.
Matthew James Barry, DO
Chief of psychiatric services
U.S. Army’s Medical Department Activity
Fort Huachuca, AZ
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
Measuring cognition: Essential in clinical practice
Ever since Kraepelin used the label “dementia praecox” for the disorder Bleuler later renamed “schizophrenia,” psychiatrists have recognized cognitive impairment as a central feature of schizophrenia.1 Cognitive deficits are an important component of many psychiatric disorders, but formal cognitive assessment still is not a part of standard clinical evaluation in psychiatric practice. It’s time that it becomes so.
Almost 2 decades ago, studies in my laboratory discovered that patients with bipolar disorder have significant deficits in cognition—including short-term memory and executive function—similar to those seen in schizophrenia.2 This finding has been replicated extensively, and a book on the subject was published recently.3 Cognitive dysfunction also has been reported in unipolar depression,4 obsessive-compulsive disorder,5 posttraumatic stress disorder (PTSD),6 attention-deficit/hyperactivity disorder,7 and borderline personality disorder.8
This should not be surprising. Cognition is a major brain function, and mental illnesses are neurobiologic disorders in which cognitive domains can be moderately or seriously disrupted. Neurocognitive studies have established that specific cognitive dysfunctions correlate with brain pathology in specific regions. For example, because the hippocampus is a key brain region for memory, memory deficits are observed in any disorder that disrupts hippocampal structure, including Alzheimer’s disease, alcoholism, PTSD, schizophrenia, and depression.
Why assess cognition?
Cognitive measurement using validated test batteries as part of a thorough and systematic mental status examination is becoming essential—even required—in psychiatric practice. Formal cognitive assessment is useful for many clinical reasons:
- for diagnosis (the upcoming fifth edition of the Diagnostic and statistical manual of mental disorders [DSM-V] is sure to include cognitive performance in schizophrenia’s diagnostic criteria)
- to assess illness severity
- to localize dysfunctional neural pathways
- to formulate a reasonable prognosis
- to rule out possible mental retardation
- to tailor a pharmacologic treatment plan that does not further impair cognition but may enhance it
- to monitor response to treatment
- to assess cognitive side effects of pharmacotherapy
- to develop social and vocational rehabilitation programs that build on patients’ cognitive abilities.
‘Cognition enhancers’
The pharmaceutical industry’s recent surge of interest in developing cognition-enhancing (“nootropic”) drugs is timely, welcome, and supported by the National Institute of Mental Health. Initial targets of nootropic drug development are Alzheimer’s dementia and schizophrenia, but research is likely to extend to other psychiatric disorders.
When effective cognition-enhancing agents are developed and approved for use in dementia and schizophrenia, they undoubtedly will be tested in other neuropsychiatric disorders as well. They will be used as “add-on” medications to target cognitive deficits in many psychopathologic states.
Getting started
The time to vigorously assess and treat cognitive dysfunction is here. You can start—if you haven’t already—by incorporating into your practice a brief cognitive battery that measures performance on several key domains. One example is the Brief Assessment of Cognition in Schizophrenia (BACS)9 that was used in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. You not only will be ahead of the curve, but your patients will benefit from increased attention to cognition in their diagnosis and treatment.
1. Sharma T, Harvey P. eds. Cognition in schizophrenia: impairments, importance and treatment strategies. New York, New York: Oxford University Press; 2000.
2. Coffman JA, Bornstein RA, Olson SC, et al. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 1990;27:1188-96.
3. Goldberg F, Burdick KE, eds. Cognitive dysfunction in bipolar disorder. A guide for clinicians. Washington, DC: American Psychiatric Publishing; 2008.
4. Sullivan B, Payne TW. Affective disorders and cognitive failures: a comparison of seasonal and nonseasonal depression. Am J Psychiatry 2007;164:1663-7.
5. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affec Disord 2007;104:15-23.
6. Hart J, Jr, Kimbrell T, Fauver P. Cognitive dysfunction associated with PTSD: evidence from World War II prisoners of war. J Neuropsychiatry Clin Neurosci 2008;20:309-16.
7. Engelhardt PE, Nigg JT, Carr LA, Ferreira F. Cognitive inhibition and working memory in attention-deficit/hyperactivity disorder. J Abnorm Psychol 2008;117:591-605.
8. Beblo T, Saavedra AS, Mensebach C, et al. Deficits in visual functions and neuropsychological inconsistency in borderline personality disorder. Psychiatry Res 2006;145:127-35.
9. Keefe R, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004;68:283-97.
Ever since Kraepelin used the label “dementia praecox” for the disorder Bleuler later renamed “schizophrenia,” psychiatrists have recognized cognitive impairment as a central feature of schizophrenia.1 Cognitive deficits are an important component of many psychiatric disorders, but formal cognitive assessment still is not a part of standard clinical evaluation in psychiatric practice. It’s time that it becomes so.
Almost 2 decades ago, studies in my laboratory discovered that patients with bipolar disorder have significant deficits in cognition—including short-term memory and executive function—similar to those seen in schizophrenia.2 This finding has been replicated extensively, and a book on the subject was published recently.3 Cognitive dysfunction also has been reported in unipolar depression,4 obsessive-compulsive disorder,5 posttraumatic stress disorder (PTSD),6 attention-deficit/hyperactivity disorder,7 and borderline personality disorder.8
This should not be surprising. Cognition is a major brain function, and mental illnesses are neurobiologic disorders in which cognitive domains can be moderately or seriously disrupted. Neurocognitive studies have established that specific cognitive dysfunctions correlate with brain pathology in specific regions. For example, because the hippocampus is a key brain region for memory, memory deficits are observed in any disorder that disrupts hippocampal structure, including Alzheimer’s disease, alcoholism, PTSD, schizophrenia, and depression.
Why assess cognition?
Cognitive measurement using validated test batteries as part of a thorough and systematic mental status examination is becoming essential—even required—in psychiatric practice. Formal cognitive assessment is useful for many clinical reasons:
- for diagnosis (the upcoming fifth edition of the Diagnostic and statistical manual of mental disorders [DSM-V] is sure to include cognitive performance in schizophrenia’s diagnostic criteria)
- to assess illness severity
- to localize dysfunctional neural pathways
- to formulate a reasonable prognosis
- to rule out possible mental retardation
- to tailor a pharmacologic treatment plan that does not further impair cognition but may enhance it
- to monitor response to treatment
- to assess cognitive side effects of pharmacotherapy
- to develop social and vocational rehabilitation programs that build on patients’ cognitive abilities.
‘Cognition enhancers’
The pharmaceutical industry’s recent surge of interest in developing cognition-enhancing (“nootropic”) drugs is timely, welcome, and supported by the National Institute of Mental Health. Initial targets of nootropic drug development are Alzheimer’s dementia and schizophrenia, but research is likely to extend to other psychiatric disorders.
When effective cognition-enhancing agents are developed and approved for use in dementia and schizophrenia, they undoubtedly will be tested in other neuropsychiatric disorders as well. They will be used as “add-on” medications to target cognitive deficits in many psychopathologic states.
Getting started
The time to vigorously assess and treat cognitive dysfunction is here. You can start—if you haven’t already—by incorporating into your practice a brief cognitive battery that measures performance on several key domains. One example is the Brief Assessment of Cognition in Schizophrenia (BACS)9 that was used in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. You not only will be ahead of the curve, but your patients will benefit from increased attention to cognition in their diagnosis and treatment.
Ever since Kraepelin used the label “dementia praecox” for the disorder Bleuler later renamed “schizophrenia,” psychiatrists have recognized cognitive impairment as a central feature of schizophrenia.1 Cognitive deficits are an important component of many psychiatric disorders, but formal cognitive assessment still is not a part of standard clinical evaluation in psychiatric practice. It’s time that it becomes so.
Almost 2 decades ago, studies in my laboratory discovered that patients with bipolar disorder have significant deficits in cognition—including short-term memory and executive function—similar to those seen in schizophrenia.2 This finding has been replicated extensively, and a book on the subject was published recently.3 Cognitive dysfunction also has been reported in unipolar depression,4 obsessive-compulsive disorder,5 posttraumatic stress disorder (PTSD),6 attention-deficit/hyperactivity disorder,7 and borderline personality disorder.8
This should not be surprising. Cognition is a major brain function, and mental illnesses are neurobiologic disorders in which cognitive domains can be moderately or seriously disrupted. Neurocognitive studies have established that specific cognitive dysfunctions correlate with brain pathology in specific regions. For example, because the hippocampus is a key brain region for memory, memory deficits are observed in any disorder that disrupts hippocampal structure, including Alzheimer’s disease, alcoholism, PTSD, schizophrenia, and depression.
Why assess cognition?
Cognitive measurement using validated test batteries as part of a thorough and systematic mental status examination is becoming essential—even required—in psychiatric practice. Formal cognitive assessment is useful for many clinical reasons:
- for diagnosis (the upcoming fifth edition of the Diagnostic and statistical manual of mental disorders [DSM-V] is sure to include cognitive performance in schizophrenia’s diagnostic criteria)
- to assess illness severity
- to localize dysfunctional neural pathways
- to formulate a reasonable prognosis
- to rule out possible mental retardation
- to tailor a pharmacologic treatment plan that does not further impair cognition but may enhance it
- to monitor response to treatment
- to assess cognitive side effects of pharmacotherapy
- to develop social and vocational rehabilitation programs that build on patients’ cognitive abilities.
‘Cognition enhancers’
The pharmaceutical industry’s recent surge of interest in developing cognition-enhancing (“nootropic”) drugs is timely, welcome, and supported by the National Institute of Mental Health. Initial targets of nootropic drug development are Alzheimer’s dementia and schizophrenia, but research is likely to extend to other psychiatric disorders.
When effective cognition-enhancing agents are developed and approved for use in dementia and schizophrenia, they undoubtedly will be tested in other neuropsychiatric disorders as well. They will be used as “add-on” medications to target cognitive deficits in many psychopathologic states.
Getting started
The time to vigorously assess and treat cognitive dysfunction is here. You can start—if you haven’t already—by incorporating into your practice a brief cognitive battery that measures performance on several key domains. One example is the Brief Assessment of Cognition in Schizophrenia (BACS)9 that was used in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. You not only will be ahead of the curve, but your patients will benefit from increased attention to cognition in their diagnosis and treatment.
1. Sharma T, Harvey P. eds. Cognition in schizophrenia: impairments, importance and treatment strategies. New York, New York: Oxford University Press; 2000.
2. Coffman JA, Bornstein RA, Olson SC, et al. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 1990;27:1188-96.
3. Goldberg F, Burdick KE, eds. Cognitive dysfunction in bipolar disorder. A guide for clinicians. Washington, DC: American Psychiatric Publishing; 2008.
4. Sullivan B, Payne TW. Affective disorders and cognitive failures: a comparison of seasonal and nonseasonal depression. Am J Psychiatry 2007;164:1663-7.
5. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affec Disord 2007;104:15-23.
6. Hart J, Jr, Kimbrell T, Fauver P. Cognitive dysfunction associated with PTSD: evidence from World War II prisoners of war. J Neuropsychiatry Clin Neurosci 2008;20:309-16.
7. Engelhardt PE, Nigg JT, Carr LA, Ferreira F. Cognitive inhibition and working memory in attention-deficit/hyperactivity disorder. J Abnorm Psychol 2008;117:591-605.
8. Beblo T, Saavedra AS, Mensebach C, et al. Deficits in visual functions and neuropsychological inconsistency in borderline personality disorder. Psychiatry Res 2006;145:127-35.
9. Keefe R, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004;68:283-97.
1. Sharma T, Harvey P. eds. Cognition in schizophrenia: impairments, importance and treatment strategies. New York, New York: Oxford University Press; 2000.
2. Coffman JA, Bornstein RA, Olson SC, et al. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biol Psychiatry 1990;27:1188-96.
3. Goldberg F, Burdick KE, eds. Cognitive dysfunction in bipolar disorder. A guide for clinicians. Washington, DC: American Psychiatric Publishing; 2008.
4. Sullivan B, Payne TW. Affective disorders and cognitive failures: a comparison of seasonal and nonseasonal depression. Am J Psychiatry 2007;164:1663-7.
5. Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive-compulsive disorder: a selective review. J Affec Disord 2007;104:15-23.
6. Hart J, Jr, Kimbrell T, Fauver P. Cognitive dysfunction associated with PTSD: evidence from World War II prisoners of war. J Neuropsychiatry Clin Neurosci 2008;20:309-16.
7. Engelhardt PE, Nigg JT, Carr LA, Ferreira F. Cognitive inhibition and working memory in attention-deficit/hyperactivity disorder. J Abnorm Psychol 2008;117:591-605.
8. Beblo T, Saavedra AS, Mensebach C, et al. Deficits in visual functions and neuropsychological inconsistency in borderline personality disorder. Psychiatry Res 2006;145:127-35.
9. Keefe R, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004;68:283-97.
The inexplicably suicidal patient
CASE: Confused and suicidal
Mr. A, age 39, becomes disoriented while walking and approaches a suspension bridge. He borrows a passerby’s cell phone and calls his sister. His sister later states that he was confused and expressed his final goodbyes, saying, “I will see Mom in heaven.” He gives back the phone and leaps of the bridge. A nearby boat rescues him almost immediately.
Mr. A is brought to the trauma unit, where he is treated for a lacerated liver. After he is stabilized, Mr. A is awake and answering questions appropriately. He is placed on suicide precautions and direct 24-hour, one-to-one supervision. Our psychiatric team evaluates him.
Mr. A reports no history of diabetes, hypertension, cardiac disorders, or neurologic disorders, but does have a history of cognitive developmental delay. He has no history of psychiatric illness, suicide attempts, or self-injurious behavior. He denies a psychiatric family history or using alcohol, tobacco, or illicit drugs; drug screen is negative. He is unemployed, collects disability, and lives with his sister.
The authors’ observations
In our initial evaluation, we find no obvious reason for Mr. A’s confusion or suicide attempt. We decide to closely review Mr. A’s history in the days leading up to his jumping off the bridge.
HISTORY: Otitis media treatment
Mr. A has a history of chronic otitis media and sought treatment for ear pain at a local emergency room (ER) 10 days before his suicide attempt. He was prescribed amoxicillin, 500 mg tid for 10 days, and meclizine, 25 mg every 8 hours as needed for dizziness.
Immediately after his first dose of both drugs, the patient told his family he was feeling “weird,” but denied being dizzy. Thinking the unusual feeling was from meclizine, Mr. A stopped taking it but continued amoxicillin. On the second day of amoxicillin, he noticed bouts of confusion. He could perform his daily activities, but with difficulty. Mr. A’s niece said he had to ask for help with minor tasks, such as opening a can of soup.
On day 3, Mr. A developed prominent auditory hallucinations. He described hearing unrecognizable male and female voices chattering and mumbling throughout the day. The voices and confusion progressively worsened, but Mr. A continued taking the antibiotic and did not mention the voices to his family.
Mr. A’s sister reports that in a phone conversation with her brother on day 7, “he wasn’t himself…he was talking about my sister and mother but what he said didn’t make sense.” She asked a neighbor to check on Mr. A; he reported that Mr. A was “OK.” On the final day of amoxicillin—day 10—Mr. A became increasingly agitated. He says us that shortly before wandering onto the bridge and jumping, he was having a difficult time dealing with the voices and confusion.
We suspect amoxicillin might have been responsible for Mr. A’s psychotic symptoms.
The authors’ observations
Treatment modalities and pharmaceutical approaches used to treat infectious diseases carry many potential adverse effects. When a patient presents with new-onset psychiatric symptoms, explore whether they are related to an underlying mood disorder or medication side effects. Three important considerations are to:
- determine whether the condition is reversible by discontinuing a drug
- identify and characterize previously unrecognized adverse drug effects
- avoid inaccurate diagnosis that leads to nonindicated psychiatric treatment.1
Antibiotic side effects vary, depending on the particular drug and its target bacteria. The most common are gastrointestinal, such as upset stomach and diarrhea. Antibiotics also can induce an anaphylactic reaction ranging from mild (pruritic rash or slight wheezing) to life-threatening (swelling of the throat, difficulty breathing, and hypotension).
Several classes of antibiotics have psychiatric side effects that range from minor confusion and irritability to severe encephalopathy and suicide (Table 1).2 Case reports have described psychotic symptoms associated with cotrimoxazole,3 trimethoprim/sulfamethoxazole,4 and ciprofloxacin.5 An older review found that amoxicillin is among the top 10 most commonly prescribed medications associated with psychiatric side effects.1
Table 1
Potential psychiatric effects of antibiotics
| Medication | Side effects |
|---|---|
| Antibacterials | |
| Penicillins | Encephalopathy, irritability, sedation, anxiety, hallucinations |
| Cephalosporins | Sleep disturbances, hallucinations |
| Cycloserine | Dose-dependent side effects, depression, irritability, psychosis |
| Quinolones | Sleep and mood disorders, psychosis |
| Nitrofurans | Euphoria, psychosis, sleep disturbances |
| Tetracyclines | Decreased concentration, mood and sleep disorders |
| Chloramphenicol | Depression |
| Trimethoprim, sulfonamides | Depression, psychosis |
| Antimycobacterials | |
| Isoniazid | Cognitive impairment, mood disorder, psychosis |
| Clofazimine | Major depression, suicide |
| Rifampin | Sedation |
| Ethionamide | Sedation, irritability, agitation, depression, psychosis |
| Ganciclovir | Sleep disturbances, anxiety, mood disorders, psychosis |
| Antifungals | |
| Amphotericin B | Delirium |
| Ketoconazole | Decreased libido, mood disorders, psychosis |
| Flucytosine | Sedation, hallucinations |
| Griseofulvin | Depression, psychosis, sleep disturbances |
| Source: Turjanski N, Lloyd GG. Psychiatric side effects of medications: recent developments. Advances in Psychiatric Treatment 2005;11:58-70. Reprinted with permission | |
Amoxicillin is a penicillin-based, broad-spectrum antibiotic (Box).1,6 Its potential psychiatric side effects include encephalopathy, irritability, sedation, anxiety, and hallucinations.2 These symptoms usually are managed by reducing the dosage or discontinuing the medication. In some cases, antipsychotics may be used to control the symptoms.
Beta-lactam compounds inhibit bacterial growth by interfering with cell wall synthesis. As a beta-lactam antibiotic, amoxicillin’s chemistry, mechanism of action, pharmacologic and clinical effects, and immunologic characteristics are similar to those of cephalosporins, monobactams, carbapenems, and beta-lactamase inhibitors.6
Amoxicillin is an aminopenicillin. These antibiotics retain the antibacterial spectrum of penicillin but have a broader spectrum against gram-negative organisms because of their enhanced ability to penetrate the gram-negative outer membrane. Amoxicillin causes less gastrointestinal (GI) irritation than penicillin and is stable in an acidic environment.
Amoxicillin is administered 250 to 500 mg every 8 hours for adults and 20 to 40 mg/kg of body weight every 24 hours for pediatric patients.1 Amoxicillin is more stable and better absorbed in the GI tract than most penicillins, so amoxicillin 3 times a day is as effective as 4 daily doses of other penicillins.
A literature search reveals 3 cases of amoxicillin-related psychosis (Table 2).7-9 A 30-year-old woman with a urinary tract infection (UTI) developed “confusional manic symptoms” after 10 days of amoxicillin.7 The patient’s family reported she’d had a similar reaction 14 years earlier following 9 days of ampicillin for a perforated appendix; since then she had received non-aminopenicillins without incident. In both incidents, her psychotic symptoms resolved.
A 55-year-old man developed auditory, visual, and tactile hallucinations within hours of his first dose of amoxicillin for presumed pneumonia. The patient “was able to describe what he had experienced clearly with evidence of subjective terror.”8
Most recently, a 63-year-old woman taking amoxicillin, 250 mg tid, for a UTI developed sleep disturbance after 1 day and auditory and visual hallucinations after 4 days. She had a similar episode that required hospitalization 5 years earlier. In both episodes, psychotic symptoms resolved within 3 days of antibiotic discontinuation, with no psychotropic drug treatment.9
Table 2
Amoxicillin-triggered psychosis: 3 case reports
| Study | Patient | Description |
|---|---|---|
| Beal et al7 | Woman, age 30 | Confusional manic symptoms after 10 days of treatment; symptoms resolved within 12 days of admission; patient had a similar reaction to ampicillin 14 years earlier |
| Stell et al8 | Man, age 55 | Auditory, visual, and tactile hallucinations within hours of first dose |
| Rao9 | Woman, age 63 | Auditory and visual hallucinations 1 week after taking 250 mg tid; patient had a similar reaction to amoxicillin 5 years earlier; in both cases symptoms resolved within 3 days of discontinuing amoxicillin |
Mechanism of psychiatric effects
The mechanisms of antibiotic-related neuropsychiatric sequelae are uncertain and vary with drug class and patient factors.
Hoigné’s syndrome—an acute psychotic reaction to intramuscular procaine penicillin first reported around 1950—is characterized by psychiatric symptoms, predominantly anxiety and hallucinations, almost immediately following injection. Anxiety is marked by a fear of imminent death as well as autonomic hyperactivity. This “pseudoanaphylactic reaction” persists for 5 to 30 minutes and has been noted for its resemblance to temporal lobe and limbic seizures (perceptual disturbance, sympathetic hyperactivity, and “doom anxiety”).
The underlying pathophysiology remains unclear; the reaction was originally attributed to microembolization of procaine crystals to the lungs and brain, later to direct procaine neurotoxicity, and most recently to temporolimbic kindling—the appearance of physiologic and behavioral responses to repetition of a stimulus (procaine) that initially is without effect.10
A potential mechanism for amoxicillin’s neuropsychiatric effects is less clear. Because amoxicillin is an oral medication, hypotheses regarding Hoigné’s syndrome seem inapplicable. In addition, amoxicillin is largely excreted unchanged by the kidneys; the lack of significant P450 metabolism argues against mechanisms mediated by polypharmacy or altered metabolite levels. Furthermore, penicillins are polar molecules with poor CNS penetration.6 Penicillins demonstrate known neurotoxicity, however, most often causing convulsions or myelopathy. Identified risk factors for penicillin neurotoxicity include:
- intravenous/thecal administration
- high doses
- CNS disease
- renal insufficiency
- advanced age
- use of drugs that block antibiotic export from the CNS
- conditions that increase blood-brain barrier permeability.
One hypothesis focuses on penicillins’ inhibition of both the GABAA receptor-chloride ionophore complex and the benzodiazepine receptor, yielding CNS disinhibition and decreasing the seizure threshold. Notably, GABA antagonism is considered a primary facilitator of CNS kindling. Penicillin also has been reported to cause delirium related to allergy-mediated cerebral edema.11 Beal et al7 argue for an immune-mediated cerebritis.
Psychiatric symptoms secondary to antibiotics—particularly penicillins—are likely multifactorial, suggesting certain individuals may be predisposed to “Hoigné’s syndrome” from amoxicillin. In the 3 case reports of amoxicillin-related psychosis, there is variation in duration of exposure until symptom onset, medical indication for the antibiotic, and patient age and gender. Any or all of these factors may be clinically significant. None of these patients, however, had a psychiatric history.
It is not clear whether a single 25-mg dose of meclizine—an H1-receptor antagonist—played a role in Mr. A’s psychotic symptoms. Meclizine overdose can cause extreme drowsiness, seizures, hallucinations, and decreased breathing. This anticholinergic has a half-life of only 6 hours and a duration of action of up to 24 hours, although anticholinergic toxicity from overdose can last for days.10 Mr. A ingested a single 25-mg dose of meclizine, however, and his auditory hallucinations persisted for 9 days. Furthermore, Mr. A’s previous well-tolerated meclizine use and lack of other signs and symptoms of anticholinergic toxicity do not support a substantial role for meclizine in his psychotic symptoms.
OUTCOME: Symptoms resolve
Mr. A’s confusion and auditory hallucinations resolve approximately 36 hours after he completed amoxicillin treatment. When transferred to the psychiatric unit, he denies auditory hallucinations or suicidal ideation. He also denies ear pain, tinnitus, vertigo, or ear tenderness; physical examination of the ear is unremarkable. Throughout the hospital admission, Mr. A experiences no confusion or changes in mental status and he continues to adamantly deny suicidal ideation.
He does not require treatment with anti-psychotics or other psychotropic medications and is discharged in stable condition.
Related resources
- Levenson JL, Schneider RK. Infectious diseases. In: Levenson JL, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005:577-98.
Drug brand names
- Amoxicillin • Amoxil, Trimox, others
- Amphotericin B • Amphocin, Abelcet
- Ampicillin • Principen
- Chloramphenicol • Chloromycetin
- Ciprofloxacin • Cipro
- Clofazimine • Lamprene
- Cycloserine • Seromycin
- Ethionamide • Trecator
- Flucytosine • Ancobon
- Ganciclovir • Cytovene
- Griseofulvin • Fulvicin U/F, Grifulvin V
- Isoniazid • Nydrazid
- Ketoconazole • Nizoral
- Meclizine • Antivert, Bonine, others
- Rifampin • Rifadin, Rimactane
- Trimethoprim/sulfamethoxazole • Bactrim, Septra
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hubbard JR, Levenson JL, Patrick GA. Psychiatric side effects associated with the ten most commonly dispensed prescription drugs: a review. J Fam Pract 1991;33(2):177-86.
2. Turjanski N, Lloyd GG. Psychiatric side effects of medications: recent developments. Advances in Psychiatric Treatment 2005;11:58-70.
3. Weis S, Karagülle D, Kornhuber J, Bayerlein K. Cotrimoxazole-induced psychosis: a case report and review of literature. Pharmacopsychiatry 2006;39:236-7.
4. Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprimsulfamethoxazole administration. Pediatrics 2005;115(6):e739-41.
5. Grimm O, Alm B, Für Seelische Z. A case of ciprofloxacin-induced acute polymorphic psychosis with a distinct deficit in executive functions. Psychosomatics 2007;48(3):269.-
6. Katzung BG. Basic and clinical pharmacology. 7th ed. Stamford, CT: Appleton & Lange; 1998;726-32.
7. Beal DM, Hudson B, Zaiac M. Amoxacillin-induced psychosis? Am J Psychiatry 1986;143(2):255-6.
8. Stell IM, Ojo OA. Amoxycillin-induced hallucinations—a variant of Hoigne’s syndrome? Br J Clin Pract 1996;50(5):279.-
9. Rao R. Penicillin psychosis in later life: Hoigne’s syndrome revisited. J Neuropsychiatry Clin Neurosci 1999;11(4):517-8.
10. Araszkiewicz A, Rybakowski JK. Hoigne’s syndrome, kindling, and panic disorder. Depress Anxiety 1996-1997;4(3):139-43.
11. Sternbach H, State R. Antibiotics: neuropsychiatric effects and psychotropic interactions. Harv Rev Psychiatry 1997;5(4):214-26.
CASE: Confused and suicidal
Mr. A, age 39, becomes disoriented while walking and approaches a suspension bridge. He borrows a passerby’s cell phone and calls his sister. His sister later states that he was confused and expressed his final goodbyes, saying, “I will see Mom in heaven.” He gives back the phone and leaps of the bridge. A nearby boat rescues him almost immediately.
Mr. A is brought to the trauma unit, where he is treated for a lacerated liver. After he is stabilized, Mr. A is awake and answering questions appropriately. He is placed on suicide precautions and direct 24-hour, one-to-one supervision. Our psychiatric team evaluates him.
Mr. A reports no history of diabetes, hypertension, cardiac disorders, or neurologic disorders, but does have a history of cognitive developmental delay. He has no history of psychiatric illness, suicide attempts, or self-injurious behavior. He denies a psychiatric family history or using alcohol, tobacco, or illicit drugs; drug screen is negative. He is unemployed, collects disability, and lives with his sister.
The authors’ observations
In our initial evaluation, we find no obvious reason for Mr. A’s confusion or suicide attempt. We decide to closely review Mr. A’s history in the days leading up to his jumping off the bridge.
HISTORY: Otitis media treatment
Mr. A has a history of chronic otitis media and sought treatment for ear pain at a local emergency room (ER) 10 days before his suicide attempt. He was prescribed amoxicillin, 500 mg tid for 10 days, and meclizine, 25 mg every 8 hours as needed for dizziness.
Immediately after his first dose of both drugs, the patient told his family he was feeling “weird,” but denied being dizzy. Thinking the unusual feeling was from meclizine, Mr. A stopped taking it but continued amoxicillin. On the second day of amoxicillin, he noticed bouts of confusion. He could perform his daily activities, but with difficulty. Mr. A’s niece said he had to ask for help with minor tasks, such as opening a can of soup.
On day 3, Mr. A developed prominent auditory hallucinations. He described hearing unrecognizable male and female voices chattering and mumbling throughout the day. The voices and confusion progressively worsened, but Mr. A continued taking the antibiotic and did not mention the voices to his family.
Mr. A’s sister reports that in a phone conversation with her brother on day 7, “he wasn’t himself…he was talking about my sister and mother but what he said didn’t make sense.” She asked a neighbor to check on Mr. A; he reported that Mr. A was “OK.” On the final day of amoxicillin—day 10—Mr. A became increasingly agitated. He says us that shortly before wandering onto the bridge and jumping, he was having a difficult time dealing with the voices and confusion.
We suspect amoxicillin might have been responsible for Mr. A’s psychotic symptoms.
The authors’ observations
Treatment modalities and pharmaceutical approaches used to treat infectious diseases carry many potential adverse effects. When a patient presents with new-onset psychiatric symptoms, explore whether they are related to an underlying mood disorder or medication side effects. Three important considerations are to:
- determine whether the condition is reversible by discontinuing a drug
- identify and characterize previously unrecognized adverse drug effects
- avoid inaccurate diagnosis that leads to nonindicated psychiatric treatment.1
Antibiotic side effects vary, depending on the particular drug and its target bacteria. The most common are gastrointestinal, such as upset stomach and diarrhea. Antibiotics also can induce an anaphylactic reaction ranging from mild (pruritic rash or slight wheezing) to life-threatening (swelling of the throat, difficulty breathing, and hypotension).
Several classes of antibiotics have psychiatric side effects that range from minor confusion and irritability to severe encephalopathy and suicide (Table 1).2 Case reports have described psychotic symptoms associated with cotrimoxazole,3 trimethoprim/sulfamethoxazole,4 and ciprofloxacin.5 An older review found that amoxicillin is among the top 10 most commonly prescribed medications associated with psychiatric side effects.1
Table 1
Potential psychiatric effects of antibiotics
| Medication | Side effects |
|---|---|
| Antibacterials | |
| Penicillins | Encephalopathy, irritability, sedation, anxiety, hallucinations |
| Cephalosporins | Sleep disturbances, hallucinations |
| Cycloserine | Dose-dependent side effects, depression, irritability, psychosis |
| Quinolones | Sleep and mood disorders, psychosis |
| Nitrofurans | Euphoria, psychosis, sleep disturbances |
| Tetracyclines | Decreased concentration, mood and sleep disorders |
| Chloramphenicol | Depression |
| Trimethoprim, sulfonamides | Depression, psychosis |
| Antimycobacterials | |
| Isoniazid | Cognitive impairment, mood disorder, psychosis |
| Clofazimine | Major depression, suicide |
| Rifampin | Sedation |
| Ethionamide | Sedation, irritability, agitation, depression, psychosis |
| Ganciclovir | Sleep disturbances, anxiety, mood disorders, psychosis |
| Antifungals | |
| Amphotericin B | Delirium |
| Ketoconazole | Decreased libido, mood disorders, psychosis |
| Flucytosine | Sedation, hallucinations |
| Griseofulvin | Depression, psychosis, sleep disturbances |
| Source: Turjanski N, Lloyd GG. Psychiatric side effects of medications: recent developments. Advances in Psychiatric Treatment 2005;11:58-70. Reprinted with permission | |
Amoxicillin is a penicillin-based, broad-spectrum antibiotic (Box).1,6 Its potential psychiatric side effects include encephalopathy, irritability, sedation, anxiety, and hallucinations.2 These symptoms usually are managed by reducing the dosage or discontinuing the medication. In some cases, antipsychotics may be used to control the symptoms.
Beta-lactam compounds inhibit bacterial growth by interfering with cell wall synthesis. As a beta-lactam antibiotic, amoxicillin’s chemistry, mechanism of action, pharmacologic and clinical effects, and immunologic characteristics are similar to those of cephalosporins, monobactams, carbapenems, and beta-lactamase inhibitors.6
Amoxicillin is an aminopenicillin. These antibiotics retain the antibacterial spectrum of penicillin but have a broader spectrum against gram-negative organisms because of their enhanced ability to penetrate the gram-negative outer membrane. Amoxicillin causes less gastrointestinal (GI) irritation than penicillin and is stable in an acidic environment.
Amoxicillin is administered 250 to 500 mg every 8 hours for adults and 20 to 40 mg/kg of body weight every 24 hours for pediatric patients.1 Amoxicillin is more stable and better absorbed in the GI tract than most penicillins, so amoxicillin 3 times a day is as effective as 4 daily doses of other penicillins.
A literature search reveals 3 cases of amoxicillin-related psychosis (Table 2).7-9 A 30-year-old woman with a urinary tract infection (UTI) developed “confusional manic symptoms” after 10 days of amoxicillin.7 The patient’s family reported she’d had a similar reaction 14 years earlier following 9 days of ampicillin for a perforated appendix; since then she had received non-aminopenicillins without incident. In both incidents, her psychotic symptoms resolved.
A 55-year-old man developed auditory, visual, and tactile hallucinations within hours of his first dose of amoxicillin for presumed pneumonia. The patient “was able to describe what he had experienced clearly with evidence of subjective terror.”8
Most recently, a 63-year-old woman taking amoxicillin, 250 mg tid, for a UTI developed sleep disturbance after 1 day and auditory and visual hallucinations after 4 days. She had a similar episode that required hospitalization 5 years earlier. In both episodes, psychotic symptoms resolved within 3 days of antibiotic discontinuation, with no psychotropic drug treatment.9
Table 2
Amoxicillin-triggered psychosis: 3 case reports
| Study | Patient | Description |
|---|---|---|
| Beal et al7 | Woman, age 30 | Confusional manic symptoms after 10 days of treatment; symptoms resolved within 12 days of admission; patient had a similar reaction to ampicillin 14 years earlier |
| Stell et al8 | Man, age 55 | Auditory, visual, and tactile hallucinations within hours of first dose |
| Rao9 | Woman, age 63 | Auditory and visual hallucinations 1 week after taking 250 mg tid; patient had a similar reaction to amoxicillin 5 years earlier; in both cases symptoms resolved within 3 days of discontinuing amoxicillin |
Mechanism of psychiatric effects
The mechanisms of antibiotic-related neuropsychiatric sequelae are uncertain and vary with drug class and patient factors.
Hoigné’s syndrome—an acute psychotic reaction to intramuscular procaine penicillin first reported around 1950—is characterized by psychiatric symptoms, predominantly anxiety and hallucinations, almost immediately following injection. Anxiety is marked by a fear of imminent death as well as autonomic hyperactivity. This “pseudoanaphylactic reaction” persists for 5 to 30 minutes and has been noted for its resemblance to temporal lobe and limbic seizures (perceptual disturbance, sympathetic hyperactivity, and “doom anxiety”).
The underlying pathophysiology remains unclear; the reaction was originally attributed to microembolization of procaine crystals to the lungs and brain, later to direct procaine neurotoxicity, and most recently to temporolimbic kindling—the appearance of physiologic and behavioral responses to repetition of a stimulus (procaine) that initially is without effect.10
A potential mechanism for amoxicillin’s neuropsychiatric effects is less clear. Because amoxicillin is an oral medication, hypotheses regarding Hoigné’s syndrome seem inapplicable. In addition, amoxicillin is largely excreted unchanged by the kidneys; the lack of significant P450 metabolism argues against mechanisms mediated by polypharmacy or altered metabolite levels. Furthermore, penicillins are polar molecules with poor CNS penetration.6 Penicillins demonstrate known neurotoxicity, however, most often causing convulsions or myelopathy. Identified risk factors for penicillin neurotoxicity include:
- intravenous/thecal administration
- high doses
- CNS disease
- renal insufficiency
- advanced age
- use of drugs that block antibiotic export from the CNS
- conditions that increase blood-brain barrier permeability.
One hypothesis focuses on penicillins’ inhibition of both the GABAA receptor-chloride ionophore complex and the benzodiazepine receptor, yielding CNS disinhibition and decreasing the seizure threshold. Notably, GABA antagonism is considered a primary facilitator of CNS kindling. Penicillin also has been reported to cause delirium related to allergy-mediated cerebral edema.11 Beal et al7 argue for an immune-mediated cerebritis.
Psychiatric symptoms secondary to antibiotics—particularly penicillins—are likely multifactorial, suggesting certain individuals may be predisposed to “Hoigné’s syndrome” from amoxicillin. In the 3 case reports of amoxicillin-related psychosis, there is variation in duration of exposure until symptom onset, medical indication for the antibiotic, and patient age and gender. Any or all of these factors may be clinically significant. None of these patients, however, had a psychiatric history.
It is not clear whether a single 25-mg dose of meclizine—an H1-receptor antagonist—played a role in Mr. A’s psychotic symptoms. Meclizine overdose can cause extreme drowsiness, seizures, hallucinations, and decreased breathing. This anticholinergic has a half-life of only 6 hours and a duration of action of up to 24 hours, although anticholinergic toxicity from overdose can last for days.10 Mr. A ingested a single 25-mg dose of meclizine, however, and his auditory hallucinations persisted for 9 days. Furthermore, Mr. A’s previous well-tolerated meclizine use and lack of other signs and symptoms of anticholinergic toxicity do not support a substantial role for meclizine in his psychotic symptoms.
OUTCOME: Symptoms resolve
Mr. A’s confusion and auditory hallucinations resolve approximately 36 hours after he completed amoxicillin treatment. When transferred to the psychiatric unit, he denies auditory hallucinations or suicidal ideation. He also denies ear pain, tinnitus, vertigo, or ear tenderness; physical examination of the ear is unremarkable. Throughout the hospital admission, Mr. A experiences no confusion or changes in mental status and he continues to adamantly deny suicidal ideation.
He does not require treatment with anti-psychotics or other psychotropic medications and is discharged in stable condition.
Related resources
- Levenson JL, Schneider RK. Infectious diseases. In: Levenson JL, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005:577-98.
Drug brand names
- Amoxicillin • Amoxil, Trimox, others
- Amphotericin B • Amphocin, Abelcet
- Ampicillin • Principen
- Chloramphenicol • Chloromycetin
- Ciprofloxacin • Cipro
- Clofazimine • Lamprene
- Cycloserine • Seromycin
- Ethionamide • Trecator
- Flucytosine • Ancobon
- Ganciclovir • Cytovene
- Griseofulvin • Fulvicin U/F, Grifulvin V
- Isoniazid • Nydrazid
- Ketoconazole • Nizoral
- Meclizine • Antivert, Bonine, others
- Rifampin • Rifadin, Rimactane
- Trimethoprim/sulfamethoxazole • Bactrim, Septra
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Confused and suicidal
Mr. A, age 39, becomes disoriented while walking and approaches a suspension bridge. He borrows a passerby’s cell phone and calls his sister. His sister later states that he was confused and expressed his final goodbyes, saying, “I will see Mom in heaven.” He gives back the phone and leaps of the bridge. A nearby boat rescues him almost immediately.
Mr. A is brought to the trauma unit, where he is treated for a lacerated liver. After he is stabilized, Mr. A is awake and answering questions appropriately. He is placed on suicide precautions and direct 24-hour, one-to-one supervision. Our psychiatric team evaluates him.
Mr. A reports no history of diabetes, hypertension, cardiac disorders, or neurologic disorders, but does have a history of cognitive developmental delay. He has no history of psychiatric illness, suicide attempts, or self-injurious behavior. He denies a psychiatric family history or using alcohol, tobacco, or illicit drugs; drug screen is negative. He is unemployed, collects disability, and lives with his sister.
The authors’ observations
In our initial evaluation, we find no obvious reason for Mr. A’s confusion or suicide attempt. We decide to closely review Mr. A’s history in the days leading up to his jumping off the bridge.
HISTORY: Otitis media treatment
Mr. A has a history of chronic otitis media and sought treatment for ear pain at a local emergency room (ER) 10 days before his suicide attempt. He was prescribed amoxicillin, 500 mg tid for 10 days, and meclizine, 25 mg every 8 hours as needed for dizziness.
Immediately after his first dose of both drugs, the patient told his family he was feeling “weird,” but denied being dizzy. Thinking the unusual feeling was from meclizine, Mr. A stopped taking it but continued amoxicillin. On the second day of amoxicillin, he noticed bouts of confusion. He could perform his daily activities, but with difficulty. Mr. A’s niece said he had to ask for help with minor tasks, such as opening a can of soup.
On day 3, Mr. A developed prominent auditory hallucinations. He described hearing unrecognizable male and female voices chattering and mumbling throughout the day. The voices and confusion progressively worsened, but Mr. A continued taking the antibiotic and did not mention the voices to his family.
Mr. A’s sister reports that in a phone conversation with her brother on day 7, “he wasn’t himself…he was talking about my sister and mother but what he said didn’t make sense.” She asked a neighbor to check on Mr. A; he reported that Mr. A was “OK.” On the final day of amoxicillin—day 10—Mr. A became increasingly agitated. He says us that shortly before wandering onto the bridge and jumping, he was having a difficult time dealing with the voices and confusion.
We suspect amoxicillin might have been responsible for Mr. A’s psychotic symptoms.
The authors’ observations
Treatment modalities and pharmaceutical approaches used to treat infectious diseases carry many potential adverse effects. When a patient presents with new-onset psychiatric symptoms, explore whether they are related to an underlying mood disorder or medication side effects. Three important considerations are to:
- determine whether the condition is reversible by discontinuing a drug
- identify and characterize previously unrecognized adverse drug effects
- avoid inaccurate diagnosis that leads to nonindicated psychiatric treatment.1
Antibiotic side effects vary, depending on the particular drug and its target bacteria. The most common are gastrointestinal, such as upset stomach and diarrhea. Antibiotics also can induce an anaphylactic reaction ranging from mild (pruritic rash or slight wheezing) to life-threatening (swelling of the throat, difficulty breathing, and hypotension).
Several classes of antibiotics have psychiatric side effects that range from minor confusion and irritability to severe encephalopathy and suicide (Table 1).2 Case reports have described psychotic symptoms associated with cotrimoxazole,3 trimethoprim/sulfamethoxazole,4 and ciprofloxacin.5 An older review found that amoxicillin is among the top 10 most commonly prescribed medications associated with psychiatric side effects.1
Table 1
Potential psychiatric effects of antibiotics
| Medication | Side effects |
|---|---|
| Antibacterials | |
| Penicillins | Encephalopathy, irritability, sedation, anxiety, hallucinations |
| Cephalosporins | Sleep disturbances, hallucinations |
| Cycloserine | Dose-dependent side effects, depression, irritability, psychosis |
| Quinolones | Sleep and mood disorders, psychosis |
| Nitrofurans | Euphoria, psychosis, sleep disturbances |
| Tetracyclines | Decreased concentration, mood and sleep disorders |
| Chloramphenicol | Depression |
| Trimethoprim, sulfonamides | Depression, psychosis |
| Antimycobacterials | |
| Isoniazid | Cognitive impairment, mood disorder, psychosis |
| Clofazimine | Major depression, suicide |
| Rifampin | Sedation |
| Ethionamide | Sedation, irritability, agitation, depression, psychosis |
| Ganciclovir | Sleep disturbances, anxiety, mood disorders, psychosis |
| Antifungals | |
| Amphotericin B | Delirium |
| Ketoconazole | Decreased libido, mood disorders, psychosis |
| Flucytosine | Sedation, hallucinations |
| Griseofulvin | Depression, psychosis, sleep disturbances |
| Source: Turjanski N, Lloyd GG. Psychiatric side effects of medications: recent developments. Advances in Psychiatric Treatment 2005;11:58-70. Reprinted with permission | |
Amoxicillin is a penicillin-based, broad-spectrum antibiotic (Box).1,6 Its potential psychiatric side effects include encephalopathy, irritability, sedation, anxiety, and hallucinations.2 These symptoms usually are managed by reducing the dosage or discontinuing the medication. In some cases, antipsychotics may be used to control the symptoms.
Beta-lactam compounds inhibit bacterial growth by interfering with cell wall synthesis. As a beta-lactam antibiotic, amoxicillin’s chemistry, mechanism of action, pharmacologic and clinical effects, and immunologic characteristics are similar to those of cephalosporins, monobactams, carbapenems, and beta-lactamase inhibitors.6
Amoxicillin is an aminopenicillin. These antibiotics retain the antibacterial spectrum of penicillin but have a broader spectrum against gram-negative organisms because of their enhanced ability to penetrate the gram-negative outer membrane. Amoxicillin causes less gastrointestinal (GI) irritation than penicillin and is stable in an acidic environment.
Amoxicillin is administered 250 to 500 mg every 8 hours for adults and 20 to 40 mg/kg of body weight every 24 hours for pediatric patients.1 Amoxicillin is more stable and better absorbed in the GI tract than most penicillins, so amoxicillin 3 times a day is as effective as 4 daily doses of other penicillins.
A literature search reveals 3 cases of amoxicillin-related psychosis (Table 2).7-9 A 30-year-old woman with a urinary tract infection (UTI) developed “confusional manic symptoms” after 10 days of amoxicillin.7 The patient’s family reported she’d had a similar reaction 14 years earlier following 9 days of ampicillin for a perforated appendix; since then she had received non-aminopenicillins without incident. In both incidents, her psychotic symptoms resolved.
A 55-year-old man developed auditory, visual, and tactile hallucinations within hours of his first dose of amoxicillin for presumed pneumonia. The patient “was able to describe what he had experienced clearly with evidence of subjective terror.”8
Most recently, a 63-year-old woman taking amoxicillin, 250 mg tid, for a UTI developed sleep disturbance after 1 day and auditory and visual hallucinations after 4 days. She had a similar episode that required hospitalization 5 years earlier. In both episodes, psychotic symptoms resolved within 3 days of antibiotic discontinuation, with no psychotropic drug treatment.9
Table 2
Amoxicillin-triggered psychosis: 3 case reports
| Study | Patient | Description |
|---|---|---|
| Beal et al7 | Woman, age 30 | Confusional manic symptoms after 10 days of treatment; symptoms resolved within 12 days of admission; patient had a similar reaction to ampicillin 14 years earlier |
| Stell et al8 | Man, age 55 | Auditory, visual, and tactile hallucinations within hours of first dose |
| Rao9 | Woman, age 63 | Auditory and visual hallucinations 1 week after taking 250 mg tid; patient had a similar reaction to amoxicillin 5 years earlier; in both cases symptoms resolved within 3 days of discontinuing amoxicillin |
Mechanism of psychiatric effects
The mechanisms of antibiotic-related neuropsychiatric sequelae are uncertain and vary with drug class and patient factors.
Hoigné’s syndrome—an acute psychotic reaction to intramuscular procaine penicillin first reported around 1950—is characterized by psychiatric symptoms, predominantly anxiety and hallucinations, almost immediately following injection. Anxiety is marked by a fear of imminent death as well as autonomic hyperactivity. This “pseudoanaphylactic reaction” persists for 5 to 30 minutes and has been noted for its resemblance to temporal lobe and limbic seizures (perceptual disturbance, sympathetic hyperactivity, and “doom anxiety”).
The underlying pathophysiology remains unclear; the reaction was originally attributed to microembolization of procaine crystals to the lungs and brain, later to direct procaine neurotoxicity, and most recently to temporolimbic kindling—the appearance of physiologic and behavioral responses to repetition of a stimulus (procaine) that initially is without effect.10
A potential mechanism for amoxicillin’s neuropsychiatric effects is less clear. Because amoxicillin is an oral medication, hypotheses regarding Hoigné’s syndrome seem inapplicable. In addition, amoxicillin is largely excreted unchanged by the kidneys; the lack of significant P450 metabolism argues against mechanisms mediated by polypharmacy or altered metabolite levels. Furthermore, penicillins are polar molecules with poor CNS penetration.6 Penicillins demonstrate known neurotoxicity, however, most often causing convulsions or myelopathy. Identified risk factors for penicillin neurotoxicity include:
- intravenous/thecal administration
- high doses
- CNS disease
- renal insufficiency
- advanced age
- use of drugs that block antibiotic export from the CNS
- conditions that increase blood-brain barrier permeability.
One hypothesis focuses on penicillins’ inhibition of both the GABAA receptor-chloride ionophore complex and the benzodiazepine receptor, yielding CNS disinhibition and decreasing the seizure threshold. Notably, GABA antagonism is considered a primary facilitator of CNS kindling. Penicillin also has been reported to cause delirium related to allergy-mediated cerebral edema.11 Beal et al7 argue for an immune-mediated cerebritis.
Psychiatric symptoms secondary to antibiotics—particularly penicillins—are likely multifactorial, suggesting certain individuals may be predisposed to “Hoigné’s syndrome” from amoxicillin. In the 3 case reports of amoxicillin-related psychosis, there is variation in duration of exposure until symptom onset, medical indication for the antibiotic, and patient age and gender. Any or all of these factors may be clinically significant. None of these patients, however, had a psychiatric history.
It is not clear whether a single 25-mg dose of meclizine—an H1-receptor antagonist—played a role in Mr. A’s psychotic symptoms. Meclizine overdose can cause extreme drowsiness, seizures, hallucinations, and decreased breathing. This anticholinergic has a half-life of only 6 hours and a duration of action of up to 24 hours, although anticholinergic toxicity from overdose can last for days.10 Mr. A ingested a single 25-mg dose of meclizine, however, and his auditory hallucinations persisted for 9 days. Furthermore, Mr. A’s previous well-tolerated meclizine use and lack of other signs and symptoms of anticholinergic toxicity do not support a substantial role for meclizine in his psychotic symptoms.
OUTCOME: Symptoms resolve
Mr. A’s confusion and auditory hallucinations resolve approximately 36 hours after he completed amoxicillin treatment. When transferred to the psychiatric unit, he denies auditory hallucinations or suicidal ideation. He also denies ear pain, tinnitus, vertigo, or ear tenderness; physical examination of the ear is unremarkable. Throughout the hospital admission, Mr. A experiences no confusion or changes in mental status and he continues to adamantly deny suicidal ideation.
He does not require treatment with anti-psychotics or other psychotropic medications and is discharged in stable condition.
Related resources
- Levenson JL, Schneider RK. Infectious diseases. In: Levenson JL, ed. The American Psychiatric Publishing textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005:577-98.
Drug brand names
- Amoxicillin • Amoxil, Trimox, others
- Amphotericin B • Amphocin, Abelcet
- Ampicillin • Principen
- Chloramphenicol • Chloromycetin
- Ciprofloxacin • Cipro
- Clofazimine • Lamprene
- Cycloserine • Seromycin
- Ethionamide • Trecator
- Flucytosine • Ancobon
- Ganciclovir • Cytovene
- Griseofulvin • Fulvicin U/F, Grifulvin V
- Isoniazid • Nydrazid
- Ketoconazole • Nizoral
- Meclizine • Antivert, Bonine, others
- Rifampin • Rifadin, Rimactane
- Trimethoprim/sulfamethoxazole • Bactrim, Septra
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hubbard JR, Levenson JL, Patrick GA. Psychiatric side effects associated with the ten most commonly dispensed prescription drugs: a review. J Fam Pract 1991;33(2):177-86.
2. Turjanski N, Lloyd GG. Psychiatric side effects of medications: recent developments. Advances in Psychiatric Treatment 2005;11:58-70.
3. Weis S, Karagülle D, Kornhuber J, Bayerlein K. Cotrimoxazole-induced psychosis: a case report and review of literature. Pharmacopsychiatry 2006;39:236-7.
4. Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprimsulfamethoxazole administration. Pediatrics 2005;115(6):e739-41.
5. Grimm O, Alm B, Für Seelische Z. A case of ciprofloxacin-induced acute polymorphic psychosis with a distinct deficit in executive functions. Psychosomatics 2007;48(3):269.-
6. Katzung BG. Basic and clinical pharmacology. 7th ed. Stamford, CT: Appleton & Lange; 1998;726-32.
7. Beal DM, Hudson B, Zaiac M. Amoxacillin-induced psychosis? Am J Psychiatry 1986;143(2):255-6.
8. Stell IM, Ojo OA. Amoxycillin-induced hallucinations—a variant of Hoigne’s syndrome? Br J Clin Pract 1996;50(5):279.-
9. Rao R. Penicillin psychosis in later life: Hoigne’s syndrome revisited. J Neuropsychiatry Clin Neurosci 1999;11(4):517-8.
10. Araszkiewicz A, Rybakowski JK. Hoigne’s syndrome, kindling, and panic disorder. Depress Anxiety 1996-1997;4(3):139-43.
11. Sternbach H, State R. Antibiotics: neuropsychiatric effects and psychotropic interactions. Harv Rev Psychiatry 1997;5(4):214-26.
1. Hubbard JR, Levenson JL, Patrick GA. Psychiatric side effects associated with the ten most commonly dispensed prescription drugs: a review. J Fam Pract 1991;33(2):177-86.
2. Turjanski N, Lloyd GG. Psychiatric side effects of medications: recent developments. Advances in Psychiatric Treatment 2005;11:58-70.
3. Weis S, Karagülle D, Kornhuber J, Bayerlein K. Cotrimoxazole-induced psychosis: a case report and review of literature. Pharmacopsychiatry 2006;39:236-7.
4. Saidinejad M, Ewald MB, Shannon MW. Transient psychosis in an immune-competent patient after oral trimethoprimsulfamethoxazole administration. Pediatrics 2005;115(6):e739-41.
5. Grimm O, Alm B, Für Seelische Z. A case of ciprofloxacin-induced acute polymorphic psychosis with a distinct deficit in executive functions. Psychosomatics 2007;48(3):269.-
6. Katzung BG. Basic and clinical pharmacology. 7th ed. Stamford, CT: Appleton & Lange; 1998;726-32.
7. Beal DM, Hudson B, Zaiac M. Amoxacillin-induced psychosis? Am J Psychiatry 1986;143(2):255-6.
8. Stell IM, Ojo OA. Amoxycillin-induced hallucinations—a variant of Hoigne’s syndrome? Br J Clin Pract 1996;50(5):279.-
9. Rao R. Penicillin psychosis in later life: Hoigne’s syndrome revisited. J Neuropsychiatry Clin Neurosci 1999;11(4):517-8.
10. Araszkiewicz A, Rybakowski JK. Hoigne’s syndrome, kindling, and panic disorder. Depress Anxiety 1996-1997;4(3):139-43.
11. Sternbach H, State R. Antibiotics: neuropsychiatric effects and psychotropic interactions. Harv Rev Psychiatry 1997;5(4):214-26.
Hyperprolactinemia: Monitoring children on long-term risperidone
Serum prolactin increases in children and adolescents when risperidone therapy begins, then decreases over time in many patients. When prolactin levels remain elevated, evidence suggests that children may experience adverse effects such as delayed sexual maturation or reduced bone growth because of hypothalamic-pituitary-gonadal axis (HPG) dysfunction.
To help you make informed prescribing decisions, we discuss what the evidence says about the effects of elevated prolactin in children and adolescents. We then suggest clinical steps to help you manage hyperprolactinemia when prescribing risperidone.
Pediatric indications
Based on short-term clinical trials of efficacy and tolerability, risperidone is FDA-approved for 3 pediatric indications:
- short-term treatment of acute mania or mixed episodes associated with bipolar I disorder in patients age 10 to 17
- schizophrenia treatment in patients age 13 to 17
- treatment of irritability (including aggression, self-injury, temper tantrums, and mood swings) associated with autistic disorder in patients age 5 to 16.
Recommended risperidone dosages are lower for children and adolescents than for adults (Table 1). Off-label pediatric uses described in case reports include psychotic, mood, disruptive, movement, and pervasive developmental disorders.
Table 1
Recommended risperidone dosing for pediatric indications*
| Indication | Starting dose | Maximum dose |
|---|---|---|
| Acute mania or mixed episodes | 0.5 mg once daily in morning or evening | 2.5 mg/d |
| Irritability in autism | 0.25 mg/d for patients weighing <20 kg 0.5 mg/d for patients weighing ≥20 kg | 0.5 mg/d for patients weighing <20 kg 1 mg/d for patients weighing ≥20 kg |
| Schizophrenia | 0.5 mg once daily in morning or evening | 3 mg/d |
| * FDA-approved dosages; individualize based on response and tolerability | ||
| Source: Drug facts and comparisons. St. Louis, MO: Wolters Kluwer Health; 2008:949-50 | ||
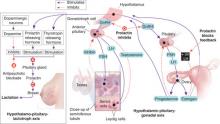
Prolactin physiology
Prolactin’s primary physiologic function is to cause breast enlargement during pregnancy and milk secretion during lactation.1 A polypeptide hormone, prolactin is secreted by lactotroph cells in the anterior pituitary, under the complex control of stimulatory and inhibitory factors (Table 2). Its pulsatile secretion peaks 13 to 14 times daily, with approximately 95 minutes between pulses.
Serum prolactin levels show marked circadian variation.2 The reference value for serum prolactin is 1 to 25 ng/mL for women and 1 to 20 ng/mL for men. The higher prolactin levels seen in women begin after puberty and presumably are caused by estrogen’s stimulatory effect.3 Age- and sex-specific normal prolactin ranges vary widely and from lab to lab (Table 3).
Risperidone is a strong dopamine D2 and serotonin 5HT-2A antagonist with low affinity for alpha-1 and alpha-2 adrenergic receptors and histamine H1 receptors.4 Antagonism of these receptors is thought to explain the drug’s therapeutic effects and many of its side effects, including hyperprolactinemia.5 Prolactin release is also influenced by thyrotropin-releasing hormone.6 A rare association between pituitary tumors and atypical antipsychotics has been proposed as a probable cause of sustained prolactin elevation.7
Pituitary prolactin secretion is regulated by neuroendocrine neurons in the hypothalamus, specifically in the tuberoinfundibular tract that extends from the arcuate nucleus of the mediobasal hypothalamus (tuberal region) and projects to the median eminence (infundibular region). Neurosecretory dopamine neurons of the arcuate nucleus inhibit prolactin secretion. Hence, prolactin secretion increases when antipsychotic therapy results in dopamine receptor blockade.
Antipsychotics vary in affinity for the D2 dopamine receptor, rate of dissociation from the receptor, and ability to act on the receptor as both a dopamine agonist (which lowers serum prolactin) and a dopamine antagonist (which increases serum prolactin). Based on adult and pediatric data, the relative potency of antipsychotic drugs in inducing hyperprolactinemia is roughly risperidone > haloperidol > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.8 Even though risperidone ranks highest in the hierarchy to cause hyperprolactinemia, it is accepted as the first-line antipsychotic in children and adolescents. This is probably because risperidone:
- has been in clinical use longer than other atypical antipsychotics except clozapine
- has received FDA approval for 3 pediatric indications.
Table 2
Factors that regulate prolactin secretion
| Effect | Factors | Mechanism |
|---|---|---|
| Inhibitory | Dopamine, gonadotropin-associated protein, acetylcholine | D2 receptor stimulation of lactotroph cells |
| Stimulatory | Serotonin, thyrotropin-releasing hormone, cholecystokinin | Through 5-HT1A and 5-HT2 |
Table 3
Sample age- and sex-specific reference ranges for serum prolactin (ng/mL)*
| Age | Males | Females |
|---|---|---|
| 0 to 1 month | 3.7 to 81.2 | 0.3 to 95.0 |
| 1 to 12 months | 0.3 to 28.9 | 0.2 to 29.9 |
| 1 to 3 years | 2.3 to 13.2 | 1.0 to 17.0 |
| 4 to 6 years | 0.8 to 16.9 | 1.6 to 13.1 |
| 7 to 9 years | 1.9 to 11.6 | 0.3 to 12.9 |
| 10 to 12 years | 0.9 to 12.9 | 1.9 to 9.6 |
| 13 to 15 years | 1.6 to 16.6 | 3.0 to 14.4 |
| Adult | 2.1 to 17.7 | 2.8 to 29.2 |
| Female: nonpregnant | 2.8 to 29.2 | |
| Female: pregnant | 9.7 to 208.5 | |
| Postmenopausal | 1.8 to 20.3 | |
| * Reference values may vary from lab to lab | ||
| Source: LabCorp, Birmingham, AL | ||
Prolactin and the HPG axis
Elevated serum prolactin inhibits the hypothalamus’ pulsatile release of gonadotrophin-releasing hormone (GnRH), which in turn decreases the pituitary’s secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In women, prolactin also blocks the feedback effect of estradiol on LH secretion (Figure). The prolactin level that triggers gonadal hypofunction appears to vary substantially among individuals.9
Symptoms of elevated prolactin can occur as a direct result of prolactin’s physiologic effect on breast tissue or indirectly through hypogonadism related to decreased FSH and LH. Symptoms of hyperprolactinemia—which can be seen more readily in sexually mature adolescents than in children—include:
- amenorrhea or oligorrhea
- breast enlargement or engorgement in females and males
- galactorrhea (females > males)
- decreased libido
- erectile dysfunction.
Although evidence is inconclusive, other problems may be associated with increased prolactin in children and adolescents. These include failure to enter or progress through puberty,8 increased risk of benign breast tumors,22 and reduced bone density.10
Bone changes. Decreased estrogen related to hyperprolactinemia may inhibit bone mineralization, causing osteopenia, osteoporosis, and increased fracture risk.10 The mechanism of bone density loss may be estrogen’s osteoclast activating and osteoblast inhibiting action. The level and duration of prolactin elevation that can hamper bone growth has not been defined, although evidence suggests a pervasive effect:
- 65% of a group of 38 premenstrual patients developed osteoporosis or osteopenia when taking risperidone or typical antipsychotics for schizophrenia for a mean of 8 years.11
- Bone loss has persisted 2 years after prolactin normalized in adolescents with prolactinomas.12
Prolactin secretion is controlled by stimulatory and inhibitory influences (A). Antipsychotic blockade of dopamine’s inhibitory influence (B) increases serum prolactin and its effect on mammary tissue (C). In the brain, hyperprolactinemia inhibits the release of gonadotropin-releasing hormone (GnRH) by the hypothalamus, which results in decreased follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion by the pituitary (D). FSH and LH are important determinants of male and female gonadal maturation by their direct action on testes and ovaries within the hypothalamic-pituitary-gonadal axis.
Source: Developed by Manpreet Khemka, MBBS, and Jeffrey Ali, MD, MSc. Current Psychiatry Illustration by Rob Flewell
Hyperprolactinemia in children and adolescents
We suggest that children and adolescents receiving prolonged risperidone treatment can present with symptoms similar to those associated with hyperprolactinemia secondary to other causes, including:
- prolactinomas (the most common cause)13
- thyrotropin-releasing hormone stimulation in primary hypothyroidism
- hypoglycemia
- inherited endocrine syndromes
- physiologic stress
- medications.
The most common presenting symptoms of prolactinomas are headache, amenorrhea, and galactorrhea. A few patients have delayed puberty.13 In a review of hyperprolactinemia in children, Massart and Saggese14 proposed a correlation between elevated serum prolactin and underlying pathology:
- >100 ng/mL usually suggests organic pathology and requires MRI or CT confirmation
- <100 ng/mL usually indicates functional pathology.
What the evidence says. Using the key words “risperidone and hyperprolactinemia in children and adolescents” in a PubMed search, we identified 7 prospective, cross-sectional, and retrospective studies.14-20 We then analyzed these studies in terms of subjects’ age, sex, primary psychiatric disorder, dosage of risperidone used, prolactin elevation pattern, reported clinical consequences, and interventions used to ameliorate asymptomatic or symptomatic hyperprolactinemia.
Prolactin and antipsychotics. In a cross-sectional study, Staller15 compared serum prolactin at baseline and after 6 months in 50 children treated with atypical antipsychotics. Patients taking risperidone showed greater increases in prolactin than those taking quetiapine or olanzapine. Saito et al16 reached a similar conclusion in a prospective study of 40 children treated with atypical antipsychotics for 4 to 15 weeks.
Ups and downs. Prolactin levels increase sharply in the first weeks of risperidone treatment, peak at around 6 to 8 weeks, and then trend downward toward normal.17 In a post hoc analysis of pooled data from 5 clinical trials totaling 700 patients age 5 to 15, Findling et al17 reported that mean serum prolactin:
- peaked in the first 1 to 2 months of patients’ starting risperidone, 0.02 to 0.06 mg/kg/d
- returned to within or close to normal range by 3 to 5 months.
No correlation was seen between prolactin elevation and side effects that could be attributed to prolactin.
In a 2-part study, Anderson et al18 examined the short- and long-term effects of risperidone treatment on prolactin in children age 5 to 17 with autism. In the initial double-blind, placebo-controlled trial, 101 children were randomly assigned to risperidone 1.8 mg/d, or placebo. After 8 weeks, 63 children continued with open-label risperidone, mean dose 1.96 mg/d, for up to a total 22 months. Serum prolactin was measured at baseline (9.3±7.5 ng/ mL) and then at 2, 6, and 22 months.
Serum prolactin increased sharply in children treated with risperidone in the placebo-controlled trial. After 8 weeks, prolactin levels were 4 times higher with risperidone treatment than with placebo (39.0±19.2 ng/mL vs 10.1±8.8 ng/mL [P< 0.0001]). In the open-label risperidone continuation trial, prolactin levels remained significantly higher than at baseline but decreased over time to:
- 32.4±17.8 ng/mL in 43 children who remained in the study at 6 months (P< 0.0001)
- 25.3±15.6 ng/mL in 30 children who remained at 22 months (P< 0.0001).
In this study, a sharp rise in serum prolactin in the first 2 months trended down to the upper limit of normal at 22 months. None of the children showed known clinical manifestations of elevated prolactin, including gynecomastia, galactorrhea, or menstrual disturbance.
A double-blind, placebo-controlled trial by Hellings et al18 examined risperidone’s effect on aggression and self-injury in children, adolescents, and adults with mental retardation and pervasive developmental disorders. In a subset of 10 children and adolescents whose serum prolactin was measured during the trial, prolactin remained elevated during at least 26 weeks of risperidone treatment. Mean prolactin levels were:
- 13.2±8.6 ng/mL at baseline
- 31.0±11.6 ng/mL during acute risperidone therapy
- 37.9±10.4 ng/mL during maintenance therapy.
Clinical features. Higher risperidone dosages—rather than longer duration of use—appear more likely to cause symptomatic elevated serum prolactin. A case series of 3 adolescents with symptomatic prolactin elevation showed:
- gynecomastia and galactorrhea in 2 adolescent males age 17 and 18, receiving risperidone, 4 mg/d and 5 mg/d, respectively
- amenorrhea within 2 to 6 weeks of starting treatment in a female patient age 15 receiving risperidone, 6 mg/d.20
Holzer et al21 described 5 adolescents who showed symptoms of elevated prolactin after 3 to 15 months while taking risperidone, 2 to 6 mg/d, as treatment for psychosis.
Long-term health risks?
Some evidence suggests an association between elevated serum prolactin and carcinogenesis and infertility in adults. No studies have examined these long-term risks in children and adolescents who develop hyperprolactinemia from risperidone treatment. A link may be possible, however, if prolactin elevation affects postpubertal and HPG axis development.
Breast cancer. Halbriech et al22 reviewed mammograms and charts of 275 female psychiatric hospital patients age >40 and 928 women of similar age at a hospital radiology clinic. The incidence of breast cancer among psychiatric patients was:
- >3.5 times higher than among radiology clinic patients
- 9.5 times higher than in the general population.
The authors speculated that the observed increased breast cancer incidence in psychiatric patients could be associated with medications, although high rates of cigarette smoking and alcohol consumption also might have played a role.
A case-control study by Hankinson et al23 of blood samples collected from women in the Nurses’ Health Study found a statistically significant association between hyperprolactinemia and breast cancer. This analysis included 306 postmenopausal women diagnosed with breast cancer and 448 controls matched for age, postmenopausal hormone use, and time of day and month when blood samples were drawn.
Several putative mechanisms have been proposed to explain a possible role of prolactin in breast carcinoma. Breast tissue—whether normal or cancerous—expresses the prolactin receptor, but the density of prolactin receptors is higher in tumor tissue. In several mouse models, prolactin induces tumor formation and increases tumor growth rates.23,24
Infertility. As noted, hyperprolactinemia can cause HPG axis dysfunction.9 A retrospective review by Sigman and Jarow25 linked endocrine disorders with infertility in 10% of 1,035 consecutive men attending 2 infertility centers. Hyperprolactinemia accounted for infertility in 0.4% of that population. No studies have associated hyperprolactinemia with female infertility.
Targeting hyperprolactinemia
When prescribing risperidone, consider obtaining a baseline serum prolactin level, especially in sexually mature patients. Repeat after 2 months, and ask the patient about menstruation, nipple discharge, sexual functioning, and pubertal development. Sexual side effects may be difficult to ascertain in patients receiving antipsychotics because of psychiatric comorbidities in this population.
Elevation without symptoms. If serum prolactin is elevated after 2 months but the patient has no clinical symptoms, repeat evaluation after another 2 months without altering the risperidone dosage. As discussed, serum prolactin tends to decline and may normalize with continued antipsychotic therapy in adults and children. A reasonable approach may be to wait 6 to 12 months for symptoms to resolve and hyperprolactinemia to diminish in patients who benefit from risperidone and have no or mild prolactin-related symptoms.8
Elevation with symptoms. Intervene if serum prolactin is elevated and your patient has clinical symptoms of hyperprolactinemia. Consider gradually tapering risperidone over 2 weeks and switching to a prolactin-sparing antipsychotic such as aripiprazole. If serum prolactin is >200 ng/mL or is persistently elevated despite switching to a prolactin-sparing antipsychotic, obtain an MRI of the sella turcica to look for a pituitary adenoma or parasellar tumor.
Use of dopamine agonists. Few studies have evaluated the safety and efficacy of using dopamine agonists such as cabergoline or amantadine to resolve the effects of hyperprolactinemia.26,27 Further research is warranted before this approach can be recommended.
Related resources
- Masi G, Cosenza A. Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. J Child Adolesc Psychopharmacol 2001;11:389-94.
- Cheng-Shannon J, McGough JJ, Pataki C, McCracken JT. Second-generation antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 2004;14:372-94.
Drug brand names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Cabergoline • Dostinex
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Freeman ME, Kanyicska B, Lerant A. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000;80:1523-1631.
2. Frantz AG. Prolactin. N Engl J Med 1978;198:201-7.
3. Guber HA, Farag AF. Evaluation of endocrine function. In: McPherson RA, Pincus MR, eds. Henry’s clinical diagnosis and management by laboratory methods. 21st ed. Philadelphia, PA: WB Saunders; 2006.
4. Melmed S, Jameson JL. Endocrinology and metabolism. In: Kasper DL, Braunwald E, Fauci AS, et al. eds. Harrison’s principles of internal medicine. 16th ed. New York, NY: McGraw-Hill; 2007.
5. Malone RP, Maislin G, Choudhary MS, et al. Risperidone treatment in children and adolescents with autism—short-and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry 2002;41:140-7.
6. Colao A, Loche S, Cappabianca P. Pituitary adenomas in children and adolescents. Endocrinologist 2000;10:314-27.
7. Szarfman A, Tonning JM, Levine JG, et al. Atypical antipsychotics and pituitary tumors—a pharmacovigilance study. Pharmacotherapy 2006;26:748-58.
8. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry 2006;45(7):771-91.
9. Haddad PM, Wieck A. Antipsychotic induced hyperprolactinemia: mechanisms, clinical features and management. Drugs 2004;64:2291-314.
10. Naidoo U, Goff DC, Klibanski A. Hyperprolactinemia and bone mineral density—the potential impact of antipsychotic agents. Psychoneuroendocrinol 2003;28:97-108.
11. O’Keane V, Meaney AM. Antipsychotic drugs: a new risk factor for osteoporosis in young women with schizophrenia? J Clin Psychopharmacol 2005;25:26-31.
12. Colao A, Di Somma C, Loche S, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf) 2000;52:319-27.
13. Parks JS, Felner EI. Hormones of the hypothalamus and pituitary gland. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson textbook of pediatrics. 17th ed. Philadelphia, PA: Elsevier Science; 2003.
14. Massart F, Saggese G. Hyperprolactinaemia in children—a common diagnostic dilemma. Eur Endocrine Rev January 2006. Available at: http://www.touchbriefings.com/download.cfm?fileID=7546. Accessed August 26, 2008.
15. Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol 2006;16:317-26.
16. Saito E, Correll CU, Gallelli K, et al. A prospective study of hyperprolactinemia in children and adolescents treated with atypical antipsychotic agents. J Child Adolesc Psychopharmacol 2004;14:350-8.
17. Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 2003;64:1362-9.
18. Anderson GM, Scahill L, McCracken JT, et al. Effects of short-and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry 2007;61:545-50.
19. Hellings JA, Zarcone JR, Valdovinos MG, et al. Risperidone-induced prolactin elevation in a prospective study of children, adolescents, and adults with mental retardation and pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005;15:885-92.
20. Madhusoodanan S, Moise D. Risperidone induced hyperprolactinemia in adolescents: a case series. J Clin Psychiatry 2006;67:1110-3.
21. Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinaemia in adolescents. J Clin Psychopharmacol 2006;26:167-71.
22. Halbreich U, Shen J, Panaro V. Are chronic psychiatric patients at increased risk for developing breast cancer? Am J Psychiatry 1996;153:59-60.
23. Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 1999;91:629-34.
24. Ginsburg E, Vonderhann B. Prolactin synthesis and secretion by human breast cells. Cancer Res 1995;55:2591-5.
25. Sigman M, Jarow JP. Endocrine evaluation of infertile men. Urology 1997;50:659-64.
26. Cavallaro R, Cocchi F, Angelone SM, et al. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry 2004;65(2):187-90.
27. Cohen LG, Biederman J. Treatment of risperidone-induced hyperprolactinemia with a dopamine agonist in children. J Child Adolesc Psychopharmacol 2001;11:435-40.
Serum prolactin increases in children and adolescents when risperidone therapy begins, then decreases over time in many patients. When prolactin levels remain elevated, evidence suggests that children may experience adverse effects such as delayed sexual maturation or reduced bone growth because of hypothalamic-pituitary-gonadal axis (HPG) dysfunction.
To help you make informed prescribing decisions, we discuss what the evidence says about the effects of elevated prolactin in children and adolescents. We then suggest clinical steps to help you manage hyperprolactinemia when prescribing risperidone.
Pediatric indications
Based on short-term clinical trials of efficacy and tolerability, risperidone is FDA-approved for 3 pediatric indications:
- short-term treatment of acute mania or mixed episodes associated with bipolar I disorder in patients age 10 to 17
- schizophrenia treatment in patients age 13 to 17
- treatment of irritability (including aggression, self-injury, temper tantrums, and mood swings) associated with autistic disorder in patients age 5 to 16.
Recommended risperidone dosages are lower for children and adolescents than for adults (Table 1). Off-label pediatric uses described in case reports include psychotic, mood, disruptive, movement, and pervasive developmental disorders.
Table 1
Recommended risperidone dosing for pediatric indications*
| Indication | Starting dose | Maximum dose |
|---|---|---|
| Acute mania or mixed episodes | 0.5 mg once daily in morning or evening | 2.5 mg/d |
| Irritability in autism | 0.25 mg/d for patients weighing <20 kg 0.5 mg/d for patients weighing ≥20 kg | 0.5 mg/d for patients weighing <20 kg 1 mg/d for patients weighing ≥20 kg |
| Schizophrenia | 0.5 mg once daily in morning or evening | 3 mg/d |
| * FDA-approved dosages; individualize based on response and tolerability | ||
| Source: Drug facts and comparisons. St. Louis, MO: Wolters Kluwer Health; 2008:949-50 | ||
Prolactin physiology
Prolactin’s primary physiologic function is to cause breast enlargement during pregnancy and milk secretion during lactation.1 A polypeptide hormone, prolactin is secreted by lactotroph cells in the anterior pituitary, under the complex control of stimulatory and inhibitory factors (Table 2). Its pulsatile secretion peaks 13 to 14 times daily, with approximately 95 minutes between pulses.
Serum prolactin levels show marked circadian variation.2 The reference value for serum prolactin is 1 to 25 ng/mL for women and 1 to 20 ng/mL for men. The higher prolactin levels seen in women begin after puberty and presumably are caused by estrogen’s stimulatory effect.3 Age- and sex-specific normal prolactin ranges vary widely and from lab to lab (Table 3).
Risperidone is a strong dopamine D2 and serotonin 5HT-2A antagonist with low affinity for alpha-1 and alpha-2 adrenergic receptors and histamine H1 receptors.4 Antagonism of these receptors is thought to explain the drug’s therapeutic effects and many of its side effects, including hyperprolactinemia.5 Prolactin release is also influenced by thyrotropin-releasing hormone.6 A rare association between pituitary tumors and atypical antipsychotics has been proposed as a probable cause of sustained prolactin elevation.7
Pituitary prolactin secretion is regulated by neuroendocrine neurons in the hypothalamus, specifically in the tuberoinfundibular tract that extends from the arcuate nucleus of the mediobasal hypothalamus (tuberal region) and projects to the median eminence (infundibular region). Neurosecretory dopamine neurons of the arcuate nucleus inhibit prolactin secretion. Hence, prolactin secretion increases when antipsychotic therapy results in dopamine receptor blockade.
Antipsychotics vary in affinity for the D2 dopamine receptor, rate of dissociation from the receptor, and ability to act on the receptor as both a dopamine agonist (which lowers serum prolactin) and a dopamine antagonist (which increases serum prolactin). Based on adult and pediatric data, the relative potency of antipsychotic drugs in inducing hyperprolactinemia is roughly risperidone > haloperidol > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.8 Even though risperidone ranks highest in the hierarchy to cause hyperprolactinemia, it is accepted as the first-line antipsychotic in children and adolescents. This is probably because risperidone:
- has been in clinical use longer than other atypical antipsychotics except clozapine
- has received FDA approval for 3 pediatric indications.
Table 2
Factors that regulate prolactin secretion
| Effect | Factors | Mechanism |
|---|---|---|
| Inhibitory | Dopamine, gonadotropin-associated protein, acetylcholine | D2 receptor stimulation of lactotroph cells |
| Stimulatory | Serotonin, thyrotropin-releasing hormone, cholecystokinin | Through 5-HT1A and 5-HT2 |
Table 3
Sample age- and sex-specific reference ranges for serum prolactin (ng/mL)*
| Age | Males | Females |
|---|---|---|
| 0 to 1 month | 3.7 to 81.2 | 0.3 to 95.0 |
| 1 to 12 months | 0.3 to 28.9 | 0.2 to 29.9 |
| 1 to 3 years | 2.3 to 13.2 | 1.0 to 17.0 |
| 4 to 6 years | 0.8 to 16.9 | 1.6 to 13.1 |
| 7 to 9 years | 1.9 to 11.6 | 0.3 to 12.9 |
| 10 to 12 years | 0.9 to 12.9 | 1.9 to 9.6 |
| 13 to 15 years | 1.6 to 16.6 | 3.0 to 14.4 |
| Adult | 2.1 to 17.7 | 2.8 to 29.2 |
| Female: nonpregnant | 2.8 to 29.2 | |
| Female: pregnant | 9.7 to 208.5 | |
| Postmenopausal | 1.8 to 20.3 | |
| * Reference values may vary from lab to lab | ||
| Source: LabCorp, Birmingham, AL | ||
Prolactin and the HPG axis
Elevated serum prolactin inhibits the hypothalamus’ pulsatile release of gonadotrophin-releasing hormone (GnRH), which in turn decreases the pituitary’s secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In women, prolactin also blocks the feedback effect of estradiol on LH secretion (Figure). The prolactin level that triggers gonadal hypofunction appears to vary substantially among individuals.9
Symptoms of elevated prolactin can occur as a direct result of prolactin’s physiologic effect on breast tissue or indirectly through hypogonadism related to decreased FSH and LH. Symptoms of hyperprolactinemia—which can be seen more readily in sexually mature adolescents than in children—include:
- amenorrhea or oligorrhea
- breast enlargement or engorgement in females and males
- galactorrhea (females > males)
- decreased libido
- erectile dysfunction.
Although evidence is inconclusive, other problems may be associated with increased prolactin in children and adolescents. These include failure to enter or progress through puberty,8 increased risk of benign breast tumors,22 and reduced bone density.10
Bone changes. Decreased estrogen related to hyperprolactinemia may inhibit bone mineralization, causing osteopenia, osteoporosis, and increased fracture risk.10 The mechanism of bone density loss may be estrogen’s osteoclast activating and osteoblast inhibiting action. The level and duration of prolactin elevation that can hamper bone growth has not been defined, although evidence suggests a pervasive effect:
- 65% of a group of 38 premenstrual patients developed osteoporosis or osteopenia when taking risperidone or typical antipsychotics for schizophrenia for a mean of 8 years.11
- Bone loss has persisted 2 years after prolactin normalized in adolescents with prolactinomas.12
Prolactin secretion is controlled by stimulatory and inhibitory influences (A). Antipsychotic blockade of dopamine’s inhibitory influence (B) increases serum prolactin and its effect on mammary tissue (C). In the brain, hyperprolactinemia inhibits the release of gonadotropin-releasing hormone (GnRH) by the hypothalamus, which results in decreased follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion by the pituitary (D). FSH and LH are important determinants of male and female gonadal maturation by their direct action on testes and ovaries within the hypothalamic-pituitary-gonadal axis.
Source: Developed by Manpreet Khemka, MBBS, and Jeffrey Ali, MD, MSc. Current Psychiatry Illustration by Rob Flewell
Hyperprolactinemia in children and adolescents
We suggest that children and adolescents receiving prolonged risperidone treatment can present with symptoms similar to those associated with hyperprolactinemia secondary to other causes, including:
- prolactinomas (the most common cause)13
- thyrotropin-releasing hormone stimulation in primary hypothyroidism
- hypoglycemia
- inherited endocrine syndromes
- physiologic stress
- medications.
The most common presenting symptoms of prolactinomas are headache, amenorrhea, and galactorrhea. A few patients have delayed puberty.13 In a review of hyperprolactinemia in children, Massart and Saggese14 proposed a correlation between elevated serum prolactin and underlying pathology:
- >100 ng/mL usually suggests organic pathology and requires MRI or CT confirmation
- <100 ng/mL usually indicates functional pathology.
What the evidence says. Using the key words “risperidone and hyperprolactinemia in children and adolescents” in a PubMed search, we identified 7 prospective, cross-sectional, and retrospective studies.14-20 We then analyzed these studies in terms of subjects’ age, sex, primary psychiatric disorder, dosage of risperidone used, prolactin elevation pattern, reported clinical consequences, and interventions used to ameliorate asymptomatic or symptomatic hyperprolactinemia.
Prolactin and antipsychotics. In a cross-sectional study, Staller15 compared serum prolactin at baseline and after 6 months in 50 children treated with atypical antipsychotics. Patients taking risperidone showed greater increases in prolactin than those taking quetiapine or olanzapine. Saito et al16 reached a similar conclusion in a prospective study of 40 children treated with atypical antipsychotics for 4 to 15 weeks.
Ups and downs. Prolactin levels increase sharply in the first weeks of risperidone treatment, peak at around 6 to 8 weeks, and then trend downward toward normal.17 In a post hoc analysis of pooled data from 5 clinical trials totaling 700 patients age 5 to 15, Findling et al17 reported that mean serum prolactin:
- peaked in the first 1 to 2 months of patients’ starting risperidone, 0.02 to 0.06 mg/kg/d
- returned to within or close to normal range by 3 to 5 months.
No correlation was seen between prolactin elevation and side effects that could be attributed to prolactin.
In a 2-part study, Anderson et al18 examined the short- and long-term effects of risperidone treatment on prolactin in children age 5 to 17 with autism. In the initial double-blind, placebo-controlled trial, 101 children were randomly assigned to risperidone 1.8 mg/d, or placebo. After 8 weeks, 63 children continued with open-label risperidone, mean dose 1.96 mg/d, for up to a total 22 months. Serum prolactin was measured at baseline (9.3±7.5 ng/ mL) and then at 2, 6, and 22 months.
Serum prolactin increased sharply in children treated with risperidone in the placebo-controlled trial. After 8 weeks, prolactin levels were 4 times higher with risperidone treatment than with placebo (39.0±19.2 ng/mL vs 10.1±8.8 ng/mL [P< 0.0001]). In the open-label risperidone continuation trial, prolactin levels remained significantly higher than at baseline but decreased over time to:
- 32.4±17.8 ng/mL in 43 children who remained in the study at 6 months (P< 0.0001)
- 25.3±15.6 ng/mL in 30 children who remained at 22 months (P< 0.0001).
In this study, a sharp rise in serum prolactin in the first 2 months trended down to the upper limit of normal at 22 months. None of the children showed known clinical manifestations of elevated prolactin, including gynecomastia, galactorrhea, or menstrual disturbance.
A double-blind, placebo-controlled trial by Hellings et al18 examined risperidone’s effect on aggression and self-injury in children, adolescents, and adults with mental retardation and pervasive developmental disorders. In a subset of 10 children and adolescents whose serum prolactin was measured during the trial, prolactin remained elevated during at least 26 weeks of risperidone treatment. Mean prolactin levels were:
- 13.2±8.6 ng/mL at baseline
- 31.0±11.6 ng/mL during acute risperidone therapy
- 37.9±10.4 ng/mL during maintenance therapy.
Clinical features. Higher risperidone dosages—rather than longer duration of use—appear more likely to cause symptomatic elevated serum prolactin. A case series of 3 adolescents with symptomatic prolactin elevation showed:
- gynecomastia and galactorrhea in 2 adolescent males age 17 and 18, receiving risperidone, 4 mg/d and 5 mg/d, respectively
- amenorrhea within 2 to 6 weeks of starting treatment in a female patient age 15 receiving risperidone, 6 mg/d.20
Holzer et al21 described 5 adolescents who showed symptoms of elevated prolactin after 3 to 15 months while taking risperidone, 2 to 6 mg/d, as treatment for psychosis.
Long-term health risks?
Some evidence suggests an association between elevated serum prolactin and carcinogenesis and infertility in adults. No studies have examined these long-term risks in children and adolescents who develop hyperprolactinemia from risperidone treatment. A link may be possible, however, if prolactin elevation affects postpubertal and HPG axis development.
Breast cancer. Halbriech et al22 reviewed mammograms and charts of 275 female psychiatric hospital patients age >40 and 928 women of similar age at a hospital radiology clinic. The incidence of breast cancer among psychiatric patients was:
- >3.5 times higher than among radiology clinic patients
- 9.5 times higher than in the general population.
The authors speculated that the observed increased breast cancer incidence in psychiatric patients could be associated with medications, although high rates of cigarette smoking and alcohol consumption also might have played a role.
A case-control study by Hankinson et al23 of blood samples collected from women in the Nurses’ Health Study found a statistically significant association between hyperprolactinemia and breast cancer. This analysis included 306 postmenopausal women diagnosed with breast cancer and 448 controls matched for age, postmenopausal hormone use, and time of day and month when blood samples were drawn.
Several putative mechanisms have been proposed to explain a possible role of prolactin in breast carcinoma. Breast tissue—whether normal or cancerous—expresses the prolactin receptor, but the density of prolactin receptors is higher in tumor tissue. In several mouse models, prolactin induces tumor formation and increases tumor growth rates.23,24
Infertility. As noted, hyperprolactinemia can cause HPG axis dysfunction.9 A retrospective review by Sigman and Jarow25 linked endocrine disorders with infertility in 10% of 1,035 consecutive men attending 2 infertility centers. Hyperprolactinemia accounted for infertility in 0.4% of that population. No studies have associated hyperprolactinemia with female infertility.
Targeting hyperprolactinemia
When prescribing risperidone, consider obtaining a baseline serum prolactin level, especially in sexually mature patients. Repeat after 2 months, and ask the patient about menstruation, nipple discharge, sexual functioning, and pubertal development. Sexual side effects may be difficult to ascertain in patients receiving antipsychotics because of psychiatric comorbidities in this population.
Elevation without symptoms. If serum prolactin is elevated after 2 months but the patient has no clinical symptoms, repeat evaluation after another 2 months without altering the risperidone dosage. As discussed, serum prolactin tends to decline and may normalize with continued antipsychotic therapy in adults and children. A reasonable approach may be to wait 6 to 12 months for symptoms to resolve and hyperprolactinemia to diminish in patients who benefit from risperidone and have no or mild prolactin-related symptoms.8
Elevation with symptoms. Intervene if serum prolactin is elevated and your patient has clinical symptoms of hyperprolactinemia. Consider gradually tapering risperidone over 2 weeks and switching to a prolactin-sparing antipsychotic such as aripiprazole. If serum prolactin is >200 ng/mL or is persistently elevated despite switching to a prolactin-sparing antipsychotic, obtain an MRI of the sella turcica to look for a pituitary adenoma or parasellar tumor.
Use of dopamine agonists. Few studies have evaluated the safety and efficacy of using dopamine agonists such as cabergoline or amantadine to resolve the effects of hyperprolactinemia.26,27 Further research is warranted before this approach can be recommended.
Related resources
- Masi G, Cosenza A. Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. J Child Adolesc Psychopharmacol 2001;11:389-94.
- Cheng-Shannon J, McGough JJ, Pataki C, McCracken JT. Second-generation antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 2004;14:372-94.
Drug brand names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Cabergoline • Dostinex
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Serum prolactin increases in children and adolescents when risperidone therapy begins, then decreases over time in many patients. When prolactin levels remain elevated, evidence suggests that children may experience adverse effects such as delayed sexual maturation or reduced bone growth because of hypothalamic-pituitary-gonadal axis (HPG) dysfunction.
To help you make informed prescribing decisions, we discuss what the evidence says about the effects of elevated prolactin in children and adolescents. We then suggest clinical steps to help you manage hyperprolactinemia when prescribing risperidone.
Pediatric indications
Based on short-term clinical trials of efficacy and tolerability, risperidone is FDA-approved for 3 pediatric indications:
- short-term treatment of acute mania or mixed episodes associated with bipolar I disorder in patients age 10 to 17
- schizophrenia treatment in patients age 13 to 17
- treatment of irritability (including aggression, self-injury, temper tantrums, and mood swings) associated with autistic disorder in patients age 5 to 16.
Recommended risperidone dosages are lower for children and adolescents than for adults (Table 1). Off-label pediatric uses described in case reports include psychotic, mood, disruptive, movement, and pervasive developmental disorders.
Table 1
Recommended risperidone dosing for pediatric indications*
| Indication | Starting dose | Maximum dose |
|---|---|---|
| Acute mania or mixed episodes | 0.5 mg once daily in morning or evening | 2.5 mg/d |
| Irritability in autism | 0.25 mg/d for patients weighing <20 kg 0.5 mg/d for patients weighing ≥20 kg | 0.5 mg/d for patients weighing <20 kg 1 mg/d for patients weighing ≥20 kg |
| Schizophrenia | 0.5 mg once daily in morning or evening | 3 mg/d |
| * FDA-approved dosages; individualize based on response and tolerability | ||
| Source: Drug facts and comparisons. St. Louis, MO: Wolters Kluwer Health; 2008:949-50 | ||
Prolactin physiology
Prolactin’s primary physiologic function is to cause breast enlargement during pregnancy and milk secretion during lactation.1 A polypeptide hormone, prolactin is secreted by lactotroph cells in the anterior pituitary, under the complex control of stimulatory and inhibitory factors (Table 2). Its pulsatile secretion peaks 13 to 14 times daily, with approximately 95 minutes between pulses.
Serum prolactin levels show marked circadian variation.2 The reference value for serum prolactin is 1 to 25 ng/mL for women and 1 to 20 ng/mL for men. The higher prolactin levels seen in women begin after puberty and presumably are caused by estrogen’s stimulatory effect.3 Age- and sex-specific normal prolactin ranges vary widely and from lab to lab (Table 3).
Risperidone is a strong dopamine D2 and serotonin 5HT-2A antagonist with low affinity for alpha-1 and alpha-2 adrenergic receptors and histamine H1 receptors.4 Antagonism of these receptors is thought to explain the drug’s therapeutic effects and many of its side effects, including hyperprolactinemia.5 Prolactin release is also influenced by thyrotropin-releasing hormone.6 A rare association between pituitary tumors and atypical antipsychotics has been proposed as a probable cause of sustained prolactin elevation.7
Pituitary prolactin secretion is regulated by neuroendocrine neurons in the hypothalamus, specifically in the tuberoinfundibular tract that extends from the arcuate nucleus of the mediobasal hypothalamus (tuberal region) and projects to the median eminence (infundibular region). Neurosecretory dopamine neurons of the arcuate nucleus inhibit prolactin secretion. Hence, prolactin secretion increases when antipsychotic therapy results in dopamine receptor blockade.
Antipsychotics vary in affinity for the D2 dopamine receptor, rate of dissociation from the receptor, and ability to act on the receptor as both a dopamine agonist (which lowers serum prolactin) and a dopamine antagonist (which increases serum prolactin). Based on adult and pediatric data, the relative potency of antipsychotic drugs in inducing hyperprolactinemia is roughly risperidone > haloperidol > olanzapine > ziprasidone > quetiapine > clozapine > aripiprazole.8 Even though risperidone ranks highest in the hierarchy to cause hyperprolactinemia, it is accepted as the first-line antipsychotic in children and adolescents. This is probably because risperidone:
- has been in clinical use longer than other atypical antipsychotics except clozapine
- has received FDA approval for 3 pediatric indications.
Table 2
Factors that regulate prolactin secretion
| Effect | Factors | Mechanism |
|---|---|---|
| Inhibitory | Dopamine, gonadotropin-associated protein, acetylcholine | D2 receptor stimulation of lactotroph cells |
| Stimulatory | Serotonin, thyrotropin-releasing hormone, cholecystokinin | Through 5-HT1A and 5-HT2 |
Table 3
Sample age- and sex-specific reference ranges for serum prolactin (ng/mL)*
| Age | Males | Females |
|---|---|---|
| 0 to 1 month | 3.7 to 81.2 | 0.3 to 95.0 |
| 1 to 12 months | 0.3 to 28.9 | 0.2 to 29.9 |
| 1 to 3 years | 2.3 to 13.2 | 1.0 to 17.0 |
| 4 to 6 years | 0.8 to 16.9 | 1.6 to 13.1 |
| 7 to 9 years | 1.9 to 11.6 | 0.3 to 12.9 |
| 10 to 12 years | 0.9 to 12.9 | 1.9 to 9.6 |
| 13 to 15 years | 1.6 to 16.6 | 3.0 to 14.4 |
| Adult | 2.1 to 17.7 | 2.8 to 29.2 |
| Female: nonpregnant | 2.8 to 29.2 | |
| Female: pregnant | 9.7 to 208.5 | |
| Postmenopausal | 1.8 to 20.3 | |
| * Reference values may vary from lab to lab | ||
| Source: LabCorp, Birmingham, AL | ||
Prolactin and the HPG axis
Elevated serum prolactin inhibits the hypothalamus’ pulsatile release of gonadotrophin-releasing hormone (GnRH), which in turn decreases the pituitary’s secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In women, prolactin also blocks the feedback effect of estradiol on LH secretion (Figure). The prolactin level that triggers gonadal hypofunction appears to vary substantially among individuals.9
Symptoms of elevated prolactin can occur as a direct result of prolactin’s physiologic effect on breast tissue or indirectly through hypogonadism related to decreased FSH and LH. Symptoms of hyperprolactinemia—which can be seen more readily in sexually mature adolescents than in children—include:
- amenorrhea or oligorrhea
- breast enlargement or engorgement in females and males
- galactorrhea (females > males)
- decreased libido
- erectile dysfunction.
Although evidence is inconclusive, other problems may be associated with increased prolactin in children and adolescents. These include failure to enter or progress through puberty,8 increased risk of benign breast tumors,22 and reduced bone density.10
Bone changes. Decreased estrogen related to hyperprolactinemia may inhibit bone mineralization, causing osteopenia, osteoporosis, and increased fracture risk.10 The mechanism of bone density loss may be estrogen’s osteoclast activating and osteoblast inhibiting action. The level and duration of prolactin elevation that can hamper bone growth has not been defined, although evidence suggests a pervasive effect:
- 65% of a group of 38 premenstrual patients developed osteoporosis or osteopenia when taking risperidone or typical antipsychotics for schizophrenia for a mean of 8 years.11
- Bone loss has persisted 2 years after prolactin normalized in adolescents with prolactinomas.12
Prolactin secretion is controlled by stimulatory and inhibitory influences (A). Antipsychotic blockade of dopamine’s inhibitory influence (B) increases serum prolactin and its effect on mammary tissue (C). In the brain, hyperprolactinemia inhibits the release of gonadotropin-releasing hormone (GnRH) by the hypothalamus, which results in decreased follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion by the pituitary (D). FSH and LH are important determinants of male and female gonadal maturation by their direct action on testes and ovaries within the hypothalamic-pituitary-gonadal axis.
Source: Developed by Manpreet Khemka, MBBS, and Jeffrey Ali, MD, MSc. Current Psychiatry Illustration by Rob Flewell
Hyperprolactinemia in children and adolescents
We suggest that children and adolescents receiving prolonged risperidone treatment can present with symptoms similar to those associated with hyperprolactinemia secondary to other causes, including:
- prolactinomas (the most common cause)13
- thyrotropin-releasing hormone stimulation in primary hypothyroidism
- hypoglycemia
- inherited endocrine syndromes
- physiologic stress
- medications.
The most common presenting symptoms of prolactinomas are headache, amenorrhea, and galactorrhea. A few patients have delayed puberty.13 In a review of hyperprolactinemia in children, Massart and Saggese14 proposed a correlation between elevated serum prolactin and underlying pathology:
- >100 ng/mL usually suggests organic pathology and requires MRI or CT confirmation
- <100 ng/mL usually indicates functional pathology.
What the evidence says. Using the key words “risperidone and hyperprolactinemia in children and adolescents” in a PubMed search, we identified 7 prospective, cross-sectional, and retrospective studies.14-20 We then analyzed these studies in terms of subjects’ age, sex, primary psychiatric disorder, dosage of risperidone used, prolactin elevation pattern, reported clinical consequences, and interventions used to ameliorate asymptomatic or symptomatic hyperprolactinemia.
Prolactin and antipsychotics. In a cross-sectional study, Staller15 compared serum prolactin at baseline and after 6 months in 50 children treated with atypical antipsychotics. Patients taking risperidone showed greater increases in prolactin than those taking quetiapine or olanzapine. Saito et al16 reached a similar conclusion in a prospective study of 40 children treated with atypical antipsychotics for 4 to 15 weeks.
Ups and downs. Prolactin levels increase sharply in the first weeks of risperidone treatment, peak at around 6 to 8 weeks, and then trend downward toward normal.17 In a post hoc analysis of pooled data from 5 clinical trials totaling 700 patients age 5 to 15, Findling et al17 reported that mean serum prolactin:
- peaked in the first 1 to 2 months of patients’ starting risperidone, 0.02 to 0.06 mg/kg/d
- returned to within or close to normal range by 3 to 5 months.
No correlation was seen between prolactin elevation and side effects that could be attributed to prolactin.
In a 2-part study, Anderson et al18 examined the short- and long-term effects of risperidone treatment on prolactin in children age 5 to 17 with autism. In the initial double-blind, placebo-controlled trial, 101 children were randomly assigned to risperidone 1.8 mg/d, or placebo. After 8 weeks, 63 children continued with open-label risperidone, mean dose 1.96 mg/d, for up to a total 22 months. Serum prolactin was measured at baseline (9.3±7.5 ng/ mL) and then at 2, 6, and 22 months.
Serum prolactin increased sharply in children treated with risperidone in the placebo-controlled trial. After 8 weeks, prolactin levels were 4 times higher with risperidone treatment than with placebo (39.0±19.2 ng/mL vs 10.1±8.8 ng/mL [P< 0.0001]). In the open-label risperidone continuation trial, prolactin levels remained significantly higher than at baseline but decreased over time to:
- 32.4±17.8 ng/mL in 43 children who remained in the study at 6 months (P< 0.0001)
- 25.3±15.6 ng/mL in 30 children who remained at 22 months (P< 0.0001).
In this study, a sharp rise in serum prolactin in the first 2 months trended down to the upper limit of normal at 22 months. None of the children showed known clinical manifestations of elevated prolactin, including gynecomastia, galactorrhea, or menstrual disturbance.
A double-blind, placebo-controlled trial by Hellings et al18 examined risperidone’s effect on aggression and self-injury in children, adolescents, and adults with mental retardation and pervasive developmental disorders. In a subset of 10 children and adolescents whose serum prolactin was measured during the trial, prolactin remained elevated during at least 26 weeks of risperidone treatment. Mean prolactin levels were:
- 13.2±8.6 ng/mL at baseline
- 31.0±11.6 ng/mL during acute risperidone therapy
- 37.9±10.4 ng/mL during maintenance therapy.
Clinical features. Higher risperidone dosages—rather than longer duration of use—appear more likely to cause symptomatic elevated serum prolactin. A case series of 3 adolescents with symptomatic prolactin elevation showed:
- gynecomastia and galactorrhea in 2 adolescent males age 17 and 18, receiving risperidone, 4 mg/d and 5 mg/d, respectively
- amenorrhea within 2 to 6 weeks of starting treatment in a female patient age 15 receiving risperidone, 6 mg/d.20
Holzer et al21 described 5 adolescents who showed symptoms of elevated prolactin after 3 to 15 months while taking risperidone, 2 to 6 mg/d, as treatment for psychosis.
Long-term health risks?
Some evidence suggests an association between elevated serum prolactin and carcinogenesis and infertility in adults. No studies have examined these long-term risks in children and adolescents who develop hyperprolactinemia from risperidone treatment. A link may be possible, however, if prolactin elevation affects postpubertal and HPG axis development.
Breast cancer. Halbriech et al22 reviewed mammograms and charts of 275 female psychiatric hospital patients age >40 and 928 women of similar age at a hospital radiology clinic. The incidence of breast cancer among psychiatric patients was:
- >3.5 times higher than among radiology clinic patients
- 9.5 times higher than in the general population.
The authors speculated that the observed increased breast cancer incidence in psychiatric patients could be associated with medications, although high rates of cigarette smoking and alcohol consumption also might have played a role.
A case-control study by Hankinson et al23 of blood samples collected from women in the Nurses’ Health Study found a statistically significant association between hyperprolactinemia and breast cancer. This analysis included 306 postmenopausal women diagnosed with breast cancer and 448 controls matched for age, postmenopausal hormone use, and time of day and month when blood samples were drawn.
Several putative mechanisms have been proposed to explain a possible role of prolactin in breast carcinoma. Breast tissue—whether normal or cancerous—expresses the prolactin receptor, but the density of prolactin receptors is higher in tumor tissue. In several mouse models, prolactin induces tumor formation and increases tumor growth rates.23,24
Infertility. As noted, hyperprolactinemia can cause HPG axis dysfunction.9 A retrospective review by Sigman and Jarow25 linked endocrine disorders with infertility in 10% of 1,035 consecutive men attending 2 infertility centers. Hyperprolactinemia accounted for infertility in 0.4% of that population. No studies have associated hyperprolactinemia with female infertility.
Targeting hyperprolactinemia
When prescribing risperidone, consider obtaining a baseline serum prolactin level, especially in sexually mature patients. Repeat after 2 months, and ask the patient about menstruation, nipple discharge, sexual functioning, and pubertal development. Sexual side effects may be difficult to ascertain in patients receiving antipsychotics because of psychiatric comorbidities in this population.
Elevation without symptoms. If serum prolactin is elevated after 2 months but the patient has no clinical symptoms, repeat evaluation after another 2 months without altering the risperidone dosage. As discussed, serum prolactin tends to decline and may normalize with continued antipsychotic therapy in adults and children. A reasonable approach may be to wait 6 to 12 months for symptoms to resolve and hyperprolactinemia to diminish in patients who benefit from risperidone and have no or mild prolactin-related symptoms.8
Elevation with symptoms. Intervene if serum prolactin is elevated and your patient has clinical symptoms of hyperprolactinemia. Consider gradually tapering risperidone over 2 weeks and switching to a prolactin-sparing antipsychotic such as aripiprazole. If serum prolactin is >200 ng/mL or is persistently elevated despite switching to a prolactin-sparing antipsychotic, obtain an MRI of the sella turcica to look for a pituitary adenoma or parasellar tumor.
Use of dopamine agonists. Few studies have evaluated the safety and efficacy of using dopamine agonists such as cabergoline or amantadine to resolve the effects of hyperprolactinemia.26,27 Further research is warranted before this approach can be recommended.
Related resources
- Masi G, Cosenza A. Prolactin levels in young children with pervasive developmental disorders during risperidone treatment. J Child Adolesc Psychopharmacol 2001;11:389-94.
- Cheng-Shannon J, McGough JJ, Pataki C, McCracken JT. Second-generation antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 2004;14:372-94.
Drug brand names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Cabergoline • Dostinex
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Freeman ME, Kanyicska B, Lerant A. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000;80:1523-1631.
2. Frantz AG. Prolactin. N Engl J Med 1978;198:201-7.
3. Guber HA, Farag AF. Evaluation of endocrine function. In: McPherson RA, Pincus MR, eds. Henry’s clinical diagnosis and management by laboratory methods. 21st ed. Philadelphia, PA: WB Saunders; 2006.
4. Melmed S, Jameson JL. Endocrinology and metabolism. In: Kasper DL, Braunwald E, Fauci AS, et al. eds. Harrison’s principles of internal medicine. 16th ed. New York, NY: McGraw-Hill; 2007.
5. Malone RP, Maislin G, Choudhary MS, et al. Risperidone treatment in children and adolescents with autism—short-and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry 2002;41:140-7.
6. Colao A, Loche S, Cappabianca P. Pituitary adenomas in children and adolescents. Endocrinologist 2000;10:314-27.
7. Szarfman A, Tonning JM, Levine JG, et al. Atypical antipsychotics and pituitary tumors—a pharmacovigilance study. Pharmacotherapy 2006;26:748-58.
8. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry 2006;45(7):771-91.
9. Haddad PM, Wieck A. Antipsychotic induced hyperprolactinemia: mechanisms, clinical features and management. Drugs 2004;64:2291-314.
10. Naidoo U, Goff DC, Klibanski A. Hyperprolactinemia and bone mineral density—the potential impact of antipsychotic agents. Psychoneuroendocrinol 2003;28:97-108.
11. O’Keane V, Meaney AM. Antipsychotic drugs: a new risk factor for osteoporosis in young women with schizophrenia? J Clin Psychopharmacol 2005;25:26-31.
12. Colao A, Di Somma C, Loche S, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf) 2000;52:319-27.
13. Parks JS, Felner EI. Hormones of the hypothalamus and pituitary gland. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson textbook of pediatrics. 17th ed. Philadelphia, PA: Elsevier Science; 2003.
14. Massart F, Saggese G. Hyperprolactinaemia in children—a common diagnostic dilemma. Eur Endocrine Rev January 2006. Available at: http://www.touchbriefings.com/download.cfm?fileID=7546. Accessed August 26, 2008.
15. Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol 2006;16:317-26.
16. Saito E, Correll CU, Gallelli K, et al. A prospective study of hyperprolactinemia in children and adolescents treated with atypical antipsychotic agents. J Child Adolesc Psychopharmacol 2004;14:350-8.
17. Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 2003;64:1362-9.
18. Anderson GM, Scahill L, McCracken JT, et al. Effects of short-and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry 2007;61:545-50.
19. Hellings JA, Zarcone JR, Valdovinos MG, et al. Risperidone-induced prolactin elevation in a prospective study of children, adolescents, and adults with mental retardation and pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005;15:885-92.
20. Madhusoodanan S, Moise D. Risperidone induced hyperprolactinemia in adolescents: a case series. J Clin Psychiatry 2006;67:1110-3.
21. Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinaemia in adolescents. J Clin Psychopharmacol 2006;26:167-71.
22. Halbreich U, Shen J, Panaro V. Are chronic psychiatric patients at increased risk for developing breast cancer? Am J Psychiatry 1996;153:59-60.
23. Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 1999;91:629-34.
24. Ginsburg E, Vonderhann B. Prolactin synthesis and secretion by human breast cells. Cancer Res 1995;55:2591-5.
25. Sigman M, Jarow JP. Endocrine evaluation of infertile men. Urology 1997;50:659-64.
26. Cavallaro R, Cocchi F, Angelone SM, et al. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry 2004;65(2):187-90.
27. Cohen LG, Biederman J. Treatment of risperidone-induced hyperprolactinemia with a dopamine agonist in children. J Child Adolesc Psychopharmacol 2001;11:435-40.
1. Freeman ME, Kanyicska B, Lerant A. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000;80:1523-1631.
2. Frantz AG. Prolactin. N Engl J Med 1978;198:201-7.
3. Guber HA, Farag AF. Evaluation of endocrine function. In: McPherson RA, Pincus MR, eds. Henry’s clinical diagnosis and management by laboratory methods. 21st ed. Philadelphia, PA: WB Saunders; 2006.
4. Melmed S, Jameson JL. Endocrinology and metabolism. In: Kasper DL, Braunwald E, Fauci AS, et al. eds. Harrison’s principles of internal medicine. 16th ed. New York, NY: McGraw-Hill; 2007.
5. Malone RP, Maislin G, Choudhary MS, et al. Risperidone treatment in children and adolescents with autism—short-and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry 2002;41:140-7.
6. Colao A, Loche S, Cappabianca P. Pituitary adenomas in children and adolescents. Endocrinologist 2000;10:314-27.
7. Szarfman A, Tonning JM, Levine JG, et al. Atypical antipsychotics and pituitary tumors—a pharmacovigilance study. Pharmacotherapy 2006;26:748-58.
8. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry 2006;45(7):771-91.
9. Haddad PM, Wieck A. Antipsychotic induced hyperprolactinemia: mechanisms, clinical features and management. Drugs 2004;64:2291-314.
10. Naidoo U, Goff DC, Klibanski A. Hyperprolactinemia and bone mineral density—the potential impact of antipsychotic agents. Psychoneuroendocrinol 2003;28:97-108.
11. O’Keane V, Meaney AM. Antipsychotic drugs: a new risk factor for osteoporosis in young women with schizophrenia? J Clin Psychopharmacol 2005;25:26-31.
12. Colao A, Di Somma C, Loche S, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf) 2000;52:319-27.
13. Parks JS, Felner EI. Hormones of the hypothalamus and pituitary gland. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson textbook of pediatrics. 17th ed. Philadelphia, PA: Elsevier Science; 2003.
14. Massart F, Saggese G. Hyperprolactinaemia in children—a common diagnostic dilemma. Eur Endocrine Rev January 2006. Available at: http://www.touchbriefings.com/download.cfm?fileID=7546. Accessed August 26, 2008.
15. Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol 2006;16:317-26.
16. Saito E, Correll CU, Gallelli K, et al. A prospective study of hyperprolactinemia in children and adolescents treated with atypical antipsychotic agents. J Child Adolesc Psychopharmacol 2004;14:350-8.
17. Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 2003;64:1362-9.
18. Anderson GM, Scahill L, McCracken JT, et al. Effects of short-and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry 2007;61:545-50.
19. Hellings JA, Zarcone JR, Valdovinos MG, et al. Risperidone-induced prolactin elevation in a prospective study of children, adolescents, and adults with mental retardation and pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005;15:885-92.
20. Madhusoodanan S, Moise D. Risperidone induced hyperprolactinemia in adolescents: a case series. J Clin Psychiatry 2006;67:1110-3.
21. Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinaemia in adolescents. J Clin Psychopharmacol 2006;26:167-71.
22. Halbreich U, Shen J, Panaro V. Are chronic psychiatric patients at increased risk for developing breast cancer? Am J Psychiatry 1996;153:59-60.
23. Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 1999;91:629-34.
24. Ginsburg E, Vonderhann B. Prolactin synthesis and secretion by human breast cells. Cancer Res 1995;55:2591-5.
25. Sigman M, Jarow JP. Endocrine evaluation of infertile men. Urology 1997;50:659-64.
26. Cavallaro R, Cocchi F, Angelone SM, et al. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry 2004;65(2):187-90.
27. Cohen LG, Biederman J. Treatment of risperidone-induced hyperprolactinemia with a dopamine agonist in children. J Child Adolesc Psychopharmacol 2001;11:435-40.
Stimulants for adult bipolar disorder?
Patients with bipolar disorder show an unpredictable range of responses to stimulants, from virtually no ill effects to emerging manic-like symptoms.1 Thus, although stimulants may be beneficial to some bipolar patients, there is a great deal of concern about using stimulants in this population. Even so, stimulants may be a rational adjunct for treating certain aspects of bipolar illness, particularly resistant depression, iatrogenic sedation, and comorbid attention-deficit/hyperactivity disorder (ADHD).
To help you decide if and when your patient might be a candidate for stimulant therapy, this article:
- reviews the evidence on stimulants’ safety and tolerability for patients with bipolar disorder
- weighs potential benefits and risks of using stimulants in this population
- addresses stimulants’ possible adverse effects on illness course and from interactions with other psychotropics
- discusses treatment options based on the limited evidence and our clinical experience.
Limited support
We are aware that using stimulants to treat patients with bipolar disorder is not an uncommon clinical practice, but supportive evidence is limited (Table 1). In searching the literature, we found only 2 randomized controlled studies—Frye et al2 and Scheffer et al3—that addressed this practice. (One author of this review [TS] participated as a coinvestigator with Frye et al.2) Other evidence that suggests a role for stimulants in bipolar disorder comes from case reports, retrospective case series, and open-label studies.4-11
- “traditional” stimulants (including amphetamine-based compounds such as dextroamphetamine, methylphenidate, dexmethylphenidate, and lisdexamfetamine) thought to affect the dopamine transporter, resulting in increased dopamine in nerve terminals
- the “novel” psychostimulant modafinil, thought to affect multiple neurotransmitter systems (dopamine, GABA, serotonin, histamine, and glutamate), although its mechanism of action is unclear.
Table 1
Clinical studies of stimulant use in patients with bipolar disorder
| Stimulant(s) studied | Study design | Patients studied | Clinical outcomes |
|---|---|---|---|
| Traditional stimulants | |||
| Adjunctive methylphenidate | Chart review, naturalistic12 | 16 adults (5 with comorbid ADHD, 11 with bipolar depression) | Improvements in depression, overall functioning, and ability to concentrate; sleep disturbance, irritability/agitation reported |
| Adjunctive methylphenidate or racemic mixture of AMPH salts | Chart review of sedation and depressive symptoms13 | 8 adults (BD II) | Improved clinical impression of bipolar illness; no manic switches, changes in cycling patterns, or substance abuse noted |
| Adjunctive methylphenidate | 12-week open study, bipolar depression14 | 12 adults (10 BD I, 2 BD II) | Significant clinical improvements in depressive symptoms; no change in manic symptoms; anxiety, agitation, and hypomania reported |
| Multiple stimulants | Chart review, history of stimulants and bipolar illness course25 | 34 hospitalized adolescents | Prior stimulant treatment associated with earlier age of illness onset |
| Adjunctive mixed amphetamine salts | Randomized, placebo-controlled; comorbid BD and ADHD3 | 30 children with ADHD symptoms stabilized on divalproex sodium | Decrease in ADHD symptoms with adjunctive amphetamine treatment but not with divalproex sodium alone; 1 case of mania |
| Novel stimulant | |||
| Adjunctive modafinil | Case series15 | Mixed sample of depressed adults (4 unipolar, 3 bipolar) | Significant improvement in depressive symptoms |
| Adjunctive modafinil | Randomized, double-blind, placebo-controlled2 | 85 adults with bipolar depression | Treatment group showed greater response and remission of depressive symptoms compared with placebo group; no difference in development of manic symptoms |
| ADHD: attention-deficit/hyperactivity disorder; AMPH: amphetamine; BD: bipolar disorder; NOS: not otherwise specified | |||
Depression and iatrogenic sedation
Small, uncontrolled trials have reported some benefit and tolerability in bipolar disorder patients when stimulants are used to treat residual depressive symptoms or iatrogenic sedation associated with mood stabilizers.
Traditional stimulants. A retrospective chart review of 16 patients treated with adjunctive methylphenidate noted improved functioning, as measured by the Global Assessment of Functioning scale. Some patients’ depressive symptoms and concentration also appeared to improve, but how these parameters were assessed is not clear. Some patients tolerated stimulants well, whereas others experienced irritability, agitation, and sleep disturbances.12
Another retrospective chart review described 8 patients with iatrogenic sedation or depression who received adjunctive methylphenidate, mean 20 to 40 mg/d, or a racemic mixture of amphetamine salts, mean 20 to 40 mg/d. Overall bipolar symptoms decreased in severity, as measured by Clinical Global Impression (CGI) scores, but the authors did not directly measure sedation or depression. The stimulants were well-tolerated, with no evidence of stimulant-induced mania.13
In a 12-week open-label trial of methylphenidate in 14 patients with bipolar disorder, depressive symptoms improved as measured by the Hamilton Depression Rating Scale (HAM-D). Mean doses were 10 mg/d for the 3 patients who discontinued because of anxiety, agitation, or hypomania and 16.6 mg/d for those who completed the trial.14
Modafinil may have some efficacy in treating bipolar depression. In a case series of 7 depressed patients (4 unipolar and 3 bipolar), 5 patients showed a 50% decrease in HAM-D scores with adjunctive modafinil. Dosages ranged from 100 to 200 mg/d, although most patients took 200 mg/d. In this series, modafinil was added to a variety of treatments, including bupropion, nefazodone, paroxetine, venlafaxine, an unspecified tricyclic antidepressant (TCA), divalproex sodium, lamotrigine, lithium, electroconvulsive therapy, olanzapine, and gabapentin.15
The only randomized, double-blind, placebo-controlled trial of adjunctive modafinil for bipolar depression enrolled 85 patients with moderate or more severe depression. In this 6-week trial by Frye et al,2 41 patients received modafinil, 100 to 200 mg/d (mean dose 174.2 mg/d), and 44 received placebo.
Bipolar disorder plus ADHD
An estimated 10% to 21% of bipolar patients meet criteria for ADHD,16-19 although at times the line differentiating these 2 disorders is unclear. Co-occurring ADHD worsens the course of bipolar illness,20-22 and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial suggest that only 2% of dual-diagnosis patients are receiving treatment specifically for ADHD symptoms.23
Theoretically, overlapping symptoms such as talkativeness, distractibility, and physical activity remain relatively constant in ADHD but wax and wane with bipolar disorder’s manic and depressive phases. Recent evidence suggests, however, that many bipolar patients experience prodromal symptoms that may resemble ADHD, including cognitive impairment, distractibility, and increased psychomotor activity.24 In addition, medications used to treat bipolar disorder may impair cognitive function, making ADHD diagnosis difficult in this population.
We are not aware of any clinical trials that examined stimulants’ safety and efficacy in adult bipolar patients with co-occurring ADHD. One of the only studies to examine stimulant treatment of ADHD symptoms in a bipolar population was a retrospective chart review of 34 adolescents hospitalized with bipolar mania. An earlier age of bipolar illness onset was reported in adolescents who had been exposed to stimulants, whether or not they also had ADHD.25
Possible adverse events
Some bipolar disorder patients tolerate stimulants well, whereas others experience serious side effects, toxicities, and illness destabilization (Table 2). Because mood-stabilizer treatment may attenuate stimulants’ undesirable effects in bipolar disorder patients,26,27 be sure to use adequate dosing of a mood stabilizer if you determine a stimulant trial is warranted in your patient.
Destabilization. Stimulants can have a direct negative effect on mood; they can cause restlessness, irritability, anxiety, and mood lability. Some bipolar patients may be more sensitive to these adverse effects than others. Particularly concerning is the possibility of switching to mania or worsening of manic symptoms.28,29 Other potential destabilizing effects include:
- changing cycling patterns, such as inducing rapid cycling
- sleep disturbance because stimulants promote wakefulness.
If you are considering stimulant treatment for a bipolar disorder patient in whom substance abuse is a concern, modafinil or lisdexamfetamine may have a lower abuse potential compared with immediate-release psychostimulants. Lisdexamfetamine is metabolized in the GI tract and does not produce high d-amphetamine blood levels or cause reinforcing effects if injected or snorted.34
Table 2
Possible stimulant side effects, signs of toxicity, and contraindications
| Stimulant class | Possible side effects | Signs of toxicity/overdose | Contraindications/cautions |
|---|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | Restlessness, insomnia, mood lability, anxiety | Agitation, confusion, tremor, tachycardia, hyperreflexia, hypertension, sweating, psychomotor agitation, seizure, arrhythmia, coma, psychosis | Cardiovascular disease, hypertension, hyperthyroidism, glaucoma, Tourette’s syndrome/motor tics, history of seizure disorder, hypersensitivity to medication class |
| Novel (modafinil) | Restlessness, insomnia, mood lability, anxiety | Agitation, tremor, nausea, diarrhea, confusion | Cardiovascular disease, hepatic impairment, psychosis |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | |||
Drug-drug interactions
Polypharmacy is the rule in treating bipolar disorder, and stimulants can interact with many other psychotropics (Table 3).
Antidepressants. Never use traditional stimulants with monoamine oxidase inhibitors, as this combination may precipitate a hypertensive crisis. Coadministered stimulants also may decrease the metabolism of serotonergic agents—such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs)—and cause side effects associated with increased serotonin neurotransmission, including serotonin syndrome.
Combining traditional stimulants with TCAs may increase TCA concentrations. When coadministered with bupropion, stimulants can increase the risk of seizures.
Modafinil is both an inducer and inhibitor of cytochrome P450 isoenzymes. Because it induces CYP3A4 and inhibits CYP2C19 and CYP2C9, modafinil interacts with many other psychopharmacologic agents:
- Its induction of CYP3A4 may increase the metabolism of commonly used medications such as carbamazepine, aripiprazole, and triazolam.
- Its inhibition of CYP2C19 may decrease the metabolism of many SSRIs, TCAs, diazepam, and clozapine, increasing these drugs’ effects and adverse events.
Possible stimulant interactions with other psychotropics
| Stimulant class | Psychotropic medication | Possible adverse effects |
|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | MAOIs | Hypertensive crisis |
| CBZ | Reduced methylphenidate levels; abruptly stopping CBZ increases methylphenidate’s effect | |
| TCAs | Increased TCA concentration | |
| SSRIs, SNRIs | Possible decreased metabolism of antidepressants; potential for serotonin syndrome or NMS-like syndrome | |
| Typical and atypical antipsychotics | Each may interfere with the other’s therapeutic action | |
| Novel (modafinil) | CBZ | Decreased modafinil efficacy; decreased CBZ levels |
| Triazolam | Decreased triazolam efficacy; increased effects of triazolam with modafinil discontinuation | |
| Fluoxetine, fluvoxamine | Decreased modafinil clearance | |
| Citalopram, escitalopram, sertraline | Prolonged elimination and increased levels of antidepressant | |
| MAOIs | Hypertensive crisis(?); not recommended | |
| Diazepam | Prolonged elimination and increased levels of diazepam | |
| TCAs | Prolonged elimination and increased levels of TCAs | |
| Clozapine | Increased clozapine concentration (case report) | |
| Aripiprazole | Decreased levels of aripiprazole | |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | ||
| CBZ: carbamazepine; MAOIs: monoamine oxidase inhibitors; NMS: neuroleptic malignant syndrome; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | ||
Treatment considerations
Without evidence to support stimulants’ safety and efficacy in patients with bipolar disorder, we cannot make specific recommendations. We would, however, like to offer some general recommendations if you decide to use stimulants when treating patients with bipolar disorder (Table 4).
Carefully assess and—in many cases—reassess the patient’s symptoms to clarify the diagnosis. As mentioned, ADHD and bipolar disorder share many symptoms, particularly in the manic phase of bipolar illness. Overlapping symptoms include decreased ability to concentrate and focus, distractibility, hyperactivity and psychomotor agitation, racing thoughts, and impulsivity.
Substance abuse can negatively impact bipolar illness and present as clinical scenarios in which stimulants are used (such as treatment-resistant depression, impulsivity, somnolence, or fatigue).
Treat medical conditions such as thyroid disease, diabetes, and sleep apnea, which may worsen depression, cause somnolence and sedation, and present with symptoms similar to those of ADHD.
When possible, use lifestyle techniques to help patients manage the course of bipolar illness. Encourage good sleep hygiene, exercise, stable social rhythms, and limited use of alcohol and caffeine (both of which can impair sleep quality, which affects illness stability).
The next step. When you have explored all medication options and ruled out all other causes for the patient’s symptoms, stimulant treatment may be an appropriate next step. In these cases:
Encourage patients to participate in treatment, particularly in monitoring mood changes (as with life charts), symptoms associated with mood episodes, and emergence of side effects. When possible, involve family members in monitoring for adverse events.
Administration. Start stimulants only when bipolar illness is well-stabilized, especially regarding manic symptoms. We highly caution against using stimulants in patients with manic or hypomanic symptoms, including mixed states. We recommend not using stimulants in patients with:
- clinically significant insomnia or sleep fragmentation
- active suicidal ideation or psychotic symptoms, particularly if associated with manic symptoms.
Schedule frequent office visits when prescribing stimulants. At least initially, see patients every other week to assess for the emergence of adverse events.
Table 4
6 recommendations when using stimulants in bipolar disorder
| Carefully assess patient’s symptoms | Manic symptoms vs ADHD; medical conditions such as thyroid disorders, diabetes, or sleep apnea |
| Review possible iatrogenic causes of symptoms | Somnolence, decreased energy/fatigue, sedation, difficulty with concentration/focus |
| Engage patient in the therapeutic process | Discuss risks and benefits; monitor mood with life charts; enlist help of family, significant others when appropriate |
| Use caution in clinical scenarios that may herald adverse response to stimulants | Manic/hypomanic symptoms; sleep disturbances; psychosis; history of substance abuse |
| Administer stimulants with caution | Start low and go slow; always use stimulants in conjunction with a mood-stabilizing agent; be aware of possible interactions with patient’s other medications; schedule more frequent visits when starting stimulants |
| Monitor for adverse events associated with stimulant administration | Manic symptoms, changes in cycling patterns, sleep disturbances, substance abuse |
| ADHD: attention-deficit/hyperactivity disorder | |
- The Texas Medication Algorithm Project. Texas Department of State Health Services. www.dshs.state.tx.us/mhprograms/tmapover.shtm.
- The Cochrane Collaboration. www.cochrane.org.
- Amphetamine and dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine, DextroStat
- Diazepam • Valium
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Lisdexamfetamine • Vyvanse
- Lithium • various
- Methylphenidate • Ritalin, Concerta, others
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Triazolam • Halcion
- Valproic acid • Depakene
- Venlafaxine • Effexor
Dr. Gonzalez reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products. He is a recipient of a T32 Ruth L. Kirschstein National Research Service Awards training fellowship sponsored by the National Institutes of Health.
Dr. Suppes receives grants/research support from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, JDS Pharmaceuticals, Janssen Pharmaceutica, National Institute of Mental Health, Novartis, Pfizer Inc., and the Stanley Medical Research Institute.
1. Silberman EK, Reus VI, Jimerson DC, et al. Heterogeneity of amphetamine response in depressed patients. Am J Psychiatry 1981;138(10):1302-7.
2. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164(8):1242-9.
3. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
4. Meyers B. Treatment of imipramine-resistant depression and lithium-refractory mania through drug interactions. Am J Psychiatry 1978;135(11):1420-1.
5. Bannet J, Ebstein RP, Belmaker RH. Clinical aspects of the interaction of lithium and stimulants. Br J Psychiatry 1980;136:204.-
6. Drimmer EJ, Gitlin MJ, Gwirtsman HE. Desipramine and methylphenidate combination treatment for depression: case report. Am J Psychiatry 1983;140(2):241-2.
7. Fernandes PP, Petty F. Modafinil for remitted bipolar depression with hypersomnia. Ann Pharmacother 2003;37(12):1807-9.
8. Berigan TR. Augmentation with modafinil to achieve remission in depression: a case report. Prim Care Companion J Clin Psychiatry 2001;3(1):32.-
9. Berigan TR. Modafinil treatment of excessive daytime sedation and fatigue associated with topiramate. Prim Care Companion J Clin Psychiatry 2002;4(6):249-50.
10. Berigan T. Modafinil treatment of excessive sedation associated with divalproex sodium. Can J Psychiatry 2004;49(1):72-3.
11. Even C, Thuile J, Santos J, Bourgin P. Modafinil as an adjunctive treatment to sleep deprivation in depression. J Psychiatry Neurosci 2005;30(6):432-3.
12. Lydon E, El-Mallakh RS. Naturalistic long-term use of methylphenidate in bipolar disorder. J Clin Psychopharmacol 2006;26(5):516-8.
13. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6(5):416-20.
14. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
15. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry 2000;61(5):378-81.
16. Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry 2007;68(11):1776-84.
17. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
18. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
19. Tamam L, Tuglu C, Karatas G, Ozcan S. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
20. Faraone SV, Biederman J, Mennin D, et al. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997;36(10):1378-87; discussion 1387-90.
21. Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001;64(1):19-26.
22. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatriconset bipolar disorder. Biol Psychiatry 2003;53(11):970-7.
23. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol 2004;24(5):512-20.
24. Calabrese JR. Overview of patient care issues and treatment in bipolar spectrum and bipolar II disorder. J Clin Psychiatry 2008;69(6):e18.-
25. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3(2):53-7.
26. Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975;44(3):215-24.
27. Huey LY, Janowsky DS, Judd LL, et al. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73(2):161-4.
28. Gerner RH, Post RM, Bunney WE, Jr. A dopaminergic mechanism in mania. Am J Psychiatry 1976;133(10):1177-80.
29. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidateinduced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
30. Brady KT, Sonne SC. The relationship between substance abuse and bipolar disorder. J Clin Psychiatry 1995;56(suppl 3):19-24.
31. Sonne SC, Brady KT. Substance abuse and bipolar comorbidity. Psychiatr Clin North Am 1999;22(3):609-27,ix.
32. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264(19):2511-8.
33. Estroff TW, Dackis CA, Gold MS, Pottash AL. Drug abuse and bipolar disorders. Int J Psychiatry Med 1985-1986;15(1):37-40.
34. Faraone SV. Lisdexamfetamine dimesylate: the first longacting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother 2008;9(9):1565-74.
Patients with bipolar disorder show an unpredictable range of responses to stimulants, from virtually no ill effects to emerging manic-like symptoms.1 Thus, although stimulants may be beneficial to some bipolar patients, there is a great deal of concern about using stimulants in this population. Even so, stimulants may be a rational adjunct for treating certain aspects of bipolar illness, particularly resistant depression, iatrogenic sedation, and comorbid attention-deficit/hyperactivity disorder (ADHD).
To help you decide if and when your patient might be a candidate for stimulant therapy, this article:
- reviews the evidence on stimulants’ safety and tolerability for patients with bipolar disorder
- weighs potential benefits and risks of using stimulants in this population
- addresses stimulants’ possible adverse effects on illness course and from interactions with other psychotropics
- discusses treatment options based on the limited evidence and our clinical experience.
Limited support
We are aware that using stimulants to treat patients with bipolar disorder is not an uncommon clinical practice, but supportive evidence is limited (Table 1). In searching the literature, we found only 2 randomized controlled studies—Frye et al2 and Scheffer et al3—that addressed this practice. (One author of this review [TS] participated as a coinvestigator with Frye et al.2) Other evidence that suggests a role for stimulants in bipolar disorder comes from case reports, retrospective case series, and open-label studies.4-11
- “traditional” stimulants (including amphetamine-based compounds such as dextroamphetamine, methylphenidate, dexmethylphenidate, and lisdexamfetamine) thought to affect the dopamine transporter, resulting in increased dopamine in nerve terminals
- the “novel” psychostimulant modafinil, thought to affect multiple neurotransmitter systems (dopamine, GABA, serotonin, histamine, and glutamate), although its mechanism of action is unclear.
Table 1
Clinical studies of stimulant use in patients with bipolar disorder
| Stimulant(s) studied | Study design | Patients studied | Clinical outcomes |
|---|---|---|---|
| Traditional stimulants | |||
| Adjunctive methylphenidate | Chart review, naturalistic12 | 16 adults (5 with comorbid ADHD, 11 with bipolar depression) | Improvements in depression, overall functioning, and ability to concentrate; sleep disturbance, irritability/agitation reported |
| Adjunctive methylphenidate or racemic mixture of AMPH salts | Chart review of sedation and depressive symptoms13 | 8 adults (BD II) | Improved clinical impression of bipolar illness; no manic switches, changes in cycling patterns, or substance abuse noted |
| Adjunctive methylphenidate | 12-week open study, bipolar depression14 | 12 adults (10 BD I, 2 BD II) | Significant clinical improvements in depressive symptoms; no change in manic symptoms; anxiety, agitation, and hypomania reported |
| Multiple stimulants | Chart review, history of stimulants and bipolar illness course25 | 34 hospitalized adolescents | Prior stimulant treatment associated with earlier age of illness onset |
| Adjunctive mixed amphetamine salts | Randomized, placebo-controlled; comorbid BD and ADHD3 | 30 children with ADHD symptoms stabilized on divalproex sodium | Decrease in ADHD symptoms with adjunctive amphetamine treatment but not with divalproex sodium alone; 1 case of mania |
| Novel stimulant | |||
| Adjunctive modafinil | Case series15 | Mixed sample of depressed adults (4 unipolar, 3 bipolar) | Significant improvement in depressive symptoms |
| Adjunctive modafinil | Randomized, double-blind, placebo-controlled2 | 85 adults with bipolar depression | Treatment group showed greater response and remission of depressive symptoms compared with placebo group; no difference in development of manic symptoms |
| ADHD: attention-deficit/hyperactivity disorder; AMPH: amphetamine; BD: bipolar disorder; NOS: not otherwise specified | |||
Depression and iatrogenic sedation
Small, uncontrolled trials have reported some benefit and tolerability in bipolar disorder patients when stimulants are used to treat residual depressive symptoms or iatrogenic sedation associated with mood stabilizers.
Traditional stimulants. A retrospective chart review of 16 patients treated with adjunctive methylphenidate noted improved functioning, as measured by the Global Assessment of Functioning scale. Some patients’ depressive symptoms and concentration also appeared to improve, but how these parameters were assessed is not clear. Some patients tolerated stimulants well, whereas others experienced irritability, agitation, and sleep disturbances.12
Another retrospective chart review described 8 patients with iatrogenic sedation or depression who received adjunctive methylphenidate, mean 20 to 40 mg/d, or a racemic mixture of amphetamine salts, mean 20 to 40 mg/d. Overall bipolar symptoms decreased in severity, as measured by Clinical Global Impression (CGI) scores, but the authors did not directly measure sedation or depression. The stimulants were well-tolerated, with no evidence of stimulant-induced mania.13
In a 12-week open-label trial of methylphenidate in 14 patients with bipolar disorder, depressive symptoms improved as measured by the Hamilton Depression Rating Scale (HAM-D). Mean doses were 10 mg/d for the 3 patients who discontinued because of anxiety, agitation, or hypomania and 16.6 mg/d for those who completed the trial.14
Modafinil may have some efficacy in treating bipolar depression. In a case series of 7 depressed patients (4 unipolar and 3 bipolar), 5 patients showed a 50% decrease in HAM-D scores with adjunctive modafinil. Dosages ranged from 100 to 200 mg/d, although most patients took 200 mg/d. In this series, modafinil was added to a variety of treatments, including bupropion, nefazodone, paroxetine, venlafaxine, an unspecified tricyclic antidepressant (TCA), divalproex sodium, lamotrigine, lithium, electroconvulsive therapy, olanzapine, and gabapentin.15
The only randomized, double-blind, placebo-controlled trial of adjunctive modafinil for bipolar depression enrolled 85 patients with moderate or more severe depression. In this 6-week trial by Frye et al,2 41 patients received modafinil, 100 to 200 mg/d (mean dose 174.2 mg/d), and 44 received placebo.
Bipolar disorder plus ADHD
An estimated 10% to 21% of bipolar patients meet criteria for ADHD,16-19 although at times the line differentiating these 2 disorders is unclear. Co-occurring ADHD worsens the course of bipolar illness,20-22 and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial suggest that only 2% of dual-diagnosis patients are receiving treatment specifically for ADHD symptoms.23
Theoretically, overlapping symptoms such as talkativeness, distractibility, and physical activity remain relatively constant in ADHD but wax and wane with bipolar disorder’s manic and depressive phases. Recent evidence suggests, however, that many bipolar patients experience prodromal symptoms that may resemble ADHD, including cognitive impairment, distractibility, and increased psychomotor activity.24 In addition, medications used to treat bipolar disorder may impair cognitive function, making ADHD diagnosis difficult in this population.
We are not aware of any clinical trials that examined stimulants’ safety and efficacy in adult bipolar patients with co-occurring ADHD. One of the only studies to examine stimulant treatment of ADHD symptoms in a bipolar population was a retrospective chart review of 34 adolescents hospitalized with bipolar mania. An earlier age of bipolar illness onset was reported in adolescents who had been exposed to stimulants, whether or not they also had ADHD.25
Possible adverse events
Some bipolar disorder patients tolerate stimulants well, whereas others experience serious side effects, toxicities, and illness destabilization (Table 2). Because mood-stabilizer treatment may attenuate stimulants’ undesirable effects in bipolar disorder patients,26,27 be sure to use adequate dosing of a mood stabilizer if you determine a stimulant trial is warranted in your patient.
Destabilization. Stimulants can have a direct negative effect on mood; they can cause restlessness, irritability, anxiety, and mood lability. Some bipolar patients may be more sensitive to these adverse effects than others. Particularly concerning is the possibility of switching to mania or worsening of manic symptoms.28,29 Other potential destabilizing effects include:
- changing cycling patterns, such as inducing rapid cycling
- sleep disturbance because stimulants promote wakefulness.
If you are considering stimulant treatment for a bipolar disorder patient in whom substance abuse is a concern, modafinil or lisdexamfetamine may have a lower abuse potential compared with immediate-release psychostimulants. Lisdexamfetamine is metabolized in the GI tract and does not produce high d-amphetamine blood levels or cause reinforcing effects if injected or snorted.34
Table 2
Possible stimulant side effects, signs of toxicity, and contraindications
| Stimulant class | Possible side effects | Signs of toxicity/overdose | Contraindications/cautions |
|---|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | Restlessness, insomnia, mood lability, anxiety | Agitation, confusion, tremor, tachycardia, hyperreflexia, hypertension, sweating, psychomotor agitation, seizure, arrhythmia, coma, psychosis | Cardiovascular disease, hypertension, hyperthyroidism, glaucoma, Tourette’s syndrome/motor tics, history of seizure disorder, hypersensitivity to medication class |
| Novel (modafinil) | Restlessness, insomnia, mood lability, anxiety | Agitation, tremor, nausea, diarrhea, confusion | Cardiovascular disease, hepatic impairment, psychosis |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | |||
Drug-drug interactions
Polypharmacy is the rule in treating bipolar disorder, and stimulants can interact with many other psychotropics (Table 3).
Antidepressants. Never use traditional stimulants with monoamine oxidase inhibitors, as this combination may precipitate a hypertensive crisis. Coadministered stimulants also may decrease the metabolism of serotonergic agents—such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs)—and cause side effects associated with increased serotonin neurotransmission, including serotonin syndrome.
Combining traditional stimulants with TCAs may increase TCA concentrations. When coadministered with bupropion, stimulants can increase the risk of seizures.
Modafinil is both an inducer and inhibitor of cytochrome P450 isoenzymes. Because it induces CYP3A4 and inhibits CYP2C19 and CYP2C9, modafinil interacts with many other psychopharmacologic agents:
- Its induction of CYP3A4 may increase the metabolism of commonly used medications such as carbamazepine, aripiprazole, and triazolam.
- Its inhibition of CYP2C19 may decrease the metabolism of many SSRIs, TCAs, diazepam, and clozapine, increasing these drugs’ effects and adverse events.
Possible stimulant interactions with other psychotropics
| Stimulant class | Psychotropic medication | Possible adverse effects |
|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | MAOIs | Hypertensive crisis |
| CBZ | Reduced methylphenidate levels; abruptly stopping CBZ increases methylphenidate’s effect | |
| TCAs | Increased TCA concentration | |
| SSRIs, SNRIs | Possible decreased metabolism of antidepressants; potential for serotonin syndrome or NMS-like syndrome | |
| Typical and atypical antipsychotics | Each may interfere with the other’s therapeutic action | |
| Novel (modafinil) | CBZ | Decreased modafinil efficacy; decreased CBZ levels |
| Triazolam | Decreased triazolam efficacy; increased effects of triazolam with modafinil discontinuation | |
| Fluoxetine, fluvoxamine | Decreased modafinil clearance | |
| Citalopram, escitalopram, sertraline | Prolonged elimination and increased levels of antidepressant | |
| MAOIs | Hypertensive crisis(?); not recommended | |
| Diazepam | Prolonged elimination and increased levels of diazepam | |
| TCAs | Prolonged elimination and increased levels of TCAs | |
| Clozapine | Increased clozapine concentration (case report) | |
| Aripiprazole | Decreased levels of aripiprazole | |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | ||
| CBZ: carbamazepine; MAOIs: monoamine oxidase inhibitors; NMS: neuroleptic malignant syndrome; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | ||
Treatment considerations
Without evidence to support stimulants’ safety and efficacy in patients with bipolar disorder, we cannot make specific recommendations. We would, however, like to offer some general recommendations if you decide to use stimulants when treating patients with bipolar disorder (Table 4).
Carefully assess and—in many cases—reassess the patient’s symptoms to clarify the diagnosis. As mentioned, ADHD and bipolar disorder share many symptoms, particularly in the manic phase of bipolar illness. Overlapping symptoms include decreased ability to concentrate and focus, distractibility, hyperactivity and psychomotor agitation, racing thoughts, and impulsivity.
Substance abuse can negatively impact bipolar illness and present as clinical scenarios in which stimulants are used (such as treatment-resistant depression, impulsivity, somnolence, or fatigue).
Treat medical conditions such as thyroid disease, diabetes, and sleep apnea, which may worsen depression, cause somnolence and sedation, and present with symptoms similar to those of ADHD.
When possible, use lifestyle techniques to help patients manage the course of bipolar illness. Encourage good sleep hygiene, exercise, stable social rhythms, and limited use of alcohol and caffeine (both of which can impair sleep quality, which affects illness stability).
The next step. When you have explored all medication options and ruled out all other causes for the patient’s symptoms, stimulant treatment may be an appropriate next step. In these cases:
Encourage patients to participate in treatment, particularly in monitoring mood changes (as with life charts), symptoms associated with mood episodes, and emergence of side effects. When possible, involve family members in monitoring for adverse events.
Administration. Start stimulants only when bipolar illness is well-stabilized, especially regarding manic symptoms. We highly caution against using stimulants in patients with manic or hypomanic symptoms, including mixed states. We recommend not using stimulants in patients with:
- clinically significant insomnia or sleep fragmentation
- active suicidal ideation or psychotic symptoms, particularly if associated with manic symptoms.
Schedule frequent office visits when prescribing stimulants. At least initially, see patients every other week to assess for the emergence of adverse events.
Table 4
6 recommendations when using stimulants in bipolar disorder
| Carefully assess patient’s symptoms | Manic symptoms vs ADHD; medical conditions such as thyroid disorders, diabetes, or sleep apnea |
| Review possible iatrogenic causes of symptoms | Somnolence, decreased energy/fatigue, sedation, difficulty with concentration/focus |
| Engage patient in the therapeutic process | Discuss risks and benefits; monitor mood with life charts; enlist help of family, significant others when appropriate |
| Use caution in clinical scenarios that may herald adverse response to stimulants | Manic/hypomanic symptoms; sleep disturbances; psychosis; history of substance abuse |
| Administer stimulants with caution | Start low and go slow; always use stimulants in conjunction with a mood-stabilizing agent; be aware of possible interactions with patient’s other medications; schedule more frequent visits when starting stimulants |
| Monitor for adverse events associated with stimulant administration | Manic symptoms, changes in cycling patterns, sleep disturbances, substance abuse |
| ADHD: attention-deficit/hyperactivity disorder | |
- The Texas Medication Algorithm Project. Texas Department of State Health Services. www.dshs.state.tx.us/mhprograms/tmapover.shtm.
- The Cochrane Collaboration. www.cochrane.org.
- Amphetamine and dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine, DextroStat
- Diazepam • Valium
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Lisdexamfetamine • Vyvanse
- Lithium • various
- Methylphenidate • Ritalin, Concerta, others
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Triazolam • Halcion
- Valproic acid • Depakene
- Venlafaxine • Effexor
Dr. Gonzalez reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products. He is a recipient of a T32 Ruth L. Kirschstein National Research Service Awards training fellowship sponsored by the National Institutes of Health.
Dr. Suppes receives grants/research support from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, JDS Pharmaceuticals, Janssen Pharmaceutica, National Institute of Mental Health, Novartis, Pfizer Inc., and the Stanley Medical Research Institute.
Patients with bipolar disorder show an unpredictable range of responses to stimulants, from virtually no ill effects to emerging manic-like symptoms.1 Thus, although stimulants may be beneficial to some bipolar patients, there is a great deal of concern about using stimulants in this population. Even so, stimulants may be a rational adjunct for treating certain aspects of bipolar illness, particularly resistant depression, iatrogenic sedation, and comorbid attention-deficit/hyperactivity disorder (ADHD).
To help you decide if and when your patient might be a candidate for stimulant therapy, this article:
- reviews the evidence on stimulants’ safety and tolerability for patients with bipolar disorder
- weighs potential benefits and risks of using stimulants in this population
- addresses stimulants’ possible adverse effects on illness course and from interactions with other psychotropics
- discusses treatment options based on the limited evidence and our clinical experience.
Limited support
We are aware that using stimulants to treat patients with bipolar disorder is not an uncommon clinical practice, but supportive evidence is limited (Table 1). In searching the literature, we found only 2 randomized controlled studies—Frye et al2 and Scheffer et al3—that addressed this practice. (One author of this review [TS] participated as a coinvestigator with Frye et al.2) Other evidence that suggests a role for stimulants in bipolar disorder comes from case reports, retrospective case series, and open-label studies.4-11
- “traditional” stimulants (including amphetamine-based compounds such as dextroamphetamine, methylphenidate, dexmethylphenidate, and lisdexamfetamine) thought to affect the dopamine transporter, resulting in increased dopamine in nerve terminals
- the “novel” psychostimulant modafinil, thought to affect multiple neurotransmitter systems (dopamine, GABA, serotonin, histamine, and glutamate), although its mechanism of action is unclear.
Table 1
Clinical studies of stimulant use in patients with bipolar disorder
| Stimulant(s) studied | Study design | Patients studied | Clinical outcomes |
|---|---|---|---|
| Traditional stimulants | |||
| Adjunctive methylphenidate | Chart review, naturalistic12 | 16 adults (5 with comorbid ADHD, 11 with bipolar depression) | Improvements in depression, overall functioning, and ability to concentrate; sleep disturbance, irritability/agitation reported |
| Adjunctive methylphenidate or racemic mixture of AMPH salts | Chart review of sedation and depressive symptoms13 | 8 adults (BD II) | Improved clinical impression of bipolar illness; no manic switches, changes in cycling patterns, or substance abuse noted |
| Adjunctive methylphenidate | 12-week open study, bipolar depression14 | 12 adults (10 BD I, 2 BD II) | Significant clinical improvements in depressive symptoms; no change in manic symptoms; anxiety, agitation, and hypomania reported |
| Multiple stimulants | Chart review, history of stimulants and bipolar illness course25 | 34 hospitalized adolescents | Prior stimulant treatment associated with earlier age of illness onset |
| Adjunctive mixed amphetamine salts | Randomized, placebo-controlled; comorbid BD and ADHD3 | 30 children with ADHD symptoms stabilized on divalproex sodium | Decrease in ADHD symptoms with adjunctive amphetamine treatment but not with divalproex sodium alone; 1 case of mania |
| Novel stimulant | |||
| Adjunctive modafinil | Case series15 | Mixed sample of depressed adults (4 unipolar, 3 bipolar) | Significant improvement in depressive symptoms |
| Adjunctive modafinil | Randomized, double-blind, placebo-controlled2 | 85 adults with bipolar depression | Treatment group showed greater response and remission of depressive symptoms compared with placebo group; no difference in development of manic symptoms |
| ADHD: attention-deficit/hyperactivity disorder; AMPH: amphetamine; BD: bipolar disorder; NOS: not otherwise specified | |||
Depression and iatrogenic sedation
Small, uncontrolled trials have reported some benefit and tolerability in bipolar disorder patients when stimulants are used to treat residual depressive symptoms or iatrogenic sedation associated with mood stabilizers.
Traditional stimulants. A retrospective chart review of 16 patients treated with adjunctive methylphenidate noted improved functioning, as measured by the Global Assessment of Functioning scale. Some patients’ depressive symptoms and concentration also appeared to improve, but how these parameters were assessed is not clear. Some patients tolerated stimulants well, whereas others experienced irritability, agitation, and sleep disturbances.12
Another retrospective chart review described 8 patients with iatrogenic sedation or depression who received adjunctive methylphenidate, mean 20 to 40 mg/d, or a racemic mixture of amphetamine salts, mean 20 to 40 mg/d. Overall bipolar symptoms decreased in severity, as measured by Clinical Global Impression (CGI) scores, but the authors did not directly measure sedation or depression. The stimulants were well-tolerated, with no evidence of stimulant-induced mania.13
In a 12-week open-label trial of methylphenidate in 14 patients with bipolar disorder, depressive symptoms improved as measured by the Hamilton Depression Rating Scale (HAM-D). Mean doses were 10 mg/d for the 3 patients who discontinued because of anxiety, agitation, or hypomania and 16.6 mg/d for those who completed the trial.14
Modafinil may have some efficacy in treating bipolar depression. In a case series of 7 depressed patients (4 unipolar and 3 bipolar), 5 patients showed a 50% decrease in HAM-D scores with adjunctive modafinil. Dosages ranged from 100 to 200 mg/d, although most patients took 200 mg/d. In this series, modafinil was added to a variety of treatments, including bupropion, nefazodone, paroxetine, venlafaxine, an unspecified tricyclic antidepressant (TCA), divalproex sodium, lamotrigine, lithium, electroconvulsive therapy, olanzapine, and gabapentin.15
The only randomized, double-blind, placebo-controlled trial of adjunctive modafinil for bipolar depression enrolled 85 patients with moderate or more severe depression. In this 6-week trial by Frye et al,2 41 patients received modafinil, 100 to 200 mg/d (mean dose 174.2 mg/d), and 44 received placebo.
Bipolar disorder plus ADHD
An estimated 10% to 21% of bipolar patients meet criteria for ADHD,16-19 although at times the line differentiating these 2 disorders is unclear. Co-occurring ADHD worsens the course of bipolar illness,20-22 and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial suggest that only 2% of dual-diagnosis patients are receiving treatment specifically for ADHD symptoms.23
Theoretically, overlapping symptoms such as talkativeness, distractibility, and physical activity remain relatively constant in ADHD but wax and wane with bipolar disorder’s manic and depressive phases. Recent evidence suggests, however, that many bipolar patients experience prodromal symptoms that may resemble ADHD, including cognitive impairment, distractibility, and increased psychomotor activity.24 In addition, medications used to treat bipolar disorder may impair cognitive function, making ADHD diagnosis difficult in this population.
We are not aware of any clinical trials that examined stimulants’ safety and efficacy in adult bipolar patients with co-occurring ADHD. One of the only studies to examine stimulant treatment of ADHD symptoms in a bipolar population was a retrospective chart review of 34 adolescents hospitalized with bipolar mania. An earlier age of bipolar illness onset was reported in adolescents who had been exposed to stimulants, whether or not they also had ADHD.25
Possible adverse events
Some bipolar disorder patients tolerate stimulants well, whereas others experience serious side effects, toxicities, and illness destabilization (Table 2). Because mood-stabilizer treatment may attenuate stimulants’ undesirable effects in bipolar disorder patients,26,27 be sure to use adequate dosing of a mood stabilizer if you determine a stimulant trial is warranted in your patient.
Destabilization. Stimulants can have a direct negative effect on mood; they can cause restlessness, irritability, anxiety, and mood lability. Some bipolar patients may be more sensitive to these adverse effects than others. Particularly concerning is the possibility of switching to mania or worsening of manic symptoms.28,29 Other potential destabilizing effects include:
- changing cycling patterns, such as inducing rapid cycling
- sleep disturbance because stimulants promote wakefulness.
If you are considering stimulant treatment for a bipolar disorder patient in whom substance abuse is a concern, modafinil or lisdexamfetamine may have a lower abuse potential compared with immediate-release psychostimulants. Lisdexamfetamine is metabolized in the GI tract and does not produce high d-amphetamine blood levels or cause reinforcing effects if injected or snorted.34
Table 2
Possible stimulant side effects, signs of toxicity, and contraindications
| Stimulant class | Possible side effects | Signs of toxicity/overdose | Contraindications/cautions |
|---|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | Restlessness, insomnia, mood lability, anxiety | Agitation, confusion, tremor, tachycardia, hyperreflexia, hypertension, sweating, psychomotor agitation, seizure, arrhythmia, coma, psychosis | Cardiovascular disease, hypertension, hyperthyroidism, glaucoma, Tourette’s syndrome/motor tics, history of seizure disorder, hypersensitivity to medication class |
| Novel (modafinil) | Restlessness, insomnia, mood lability, anxiety | Agitation, tremor, nausea, diarrhea, confusion | Cardiovascular disease, hepatic impairment, psychosis |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | |||
Drug-drug interactions
Polypharmacy is the rule in treating bipolar disorder, and stimulants can interact with many other psychotropics (Table 3).
Antidepressants. Never use traditional stimulants with monoamine oxidase inhibitors, as this combination may precipitate a hypertensive crisis. Coadministered stimulants also may decrease the metabolism of serotonergic agents—such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs)—and cause side effects associated with increased serotonin neurotransmission, including serotonin syndrome.
Combining traditional stimulants with TCAs may increase TCA concentrations. When coadministered with bupropion, stimulants can increase the risk of seizures.
Modafinil is both an inducer and inhibitor of cytochrome P450 isoenzymes. Because it induces CYP3A4 and inhibits CYP2C19 and CYP2C9, modafinil interacts with many other psychopharmacologic agents:
- Its induction of CYP3A4 may increase the metabolism of commonly used medications such as carbamazepine, aripiprazole, and triazolam.
- Its inhibition of CYP2C19 may decrease the metabolism of many SSRIs, TCAs, diazepam, and clozapine, increasing these drugs’ effects and adverse events.
Possible stimulant interactions with other psychotropics
| Stimulant class | Psychotropic medication | Possible adverse effects |
|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | MAOIs | Hypertensive crisis |
| CBZ | Reduced methylphenidate levels; abruptly stopping CBZ increases methylphenidate’s effect | |
| TCAs | Increased TCA concentration | |
| SSRIs, SNRIs | Possible decreased metabolism of antidepressants; potential for serotonin syndrome or NMS-like syndrome | |
| Typical and atypical antipsychotics | Each may interfere with the other’s therapeutic action | |
| Novel (modafinil) | CBZ | Decreased modafinil efficacy; decreased CBZ levels |
| Triazolam | Decreased triazolam efficacy; increased effects of triazolam with modafinil discontinuation | |
| Fluoxetine, fluvoxamine | Decreased modafinil clearance | |
| Citalopram, escitalopram, sertraline | Prolonged elimination and increased levels of antidepressant | |
| MAOIs | Hypertensive crisis(?); not recommended | |
| Diazepam | Prolonged elimination and increased levels of diazepam | |
| TCAs | Prolonged elimination and increased levels of TCAs | |
| Clozapine | Increased clozapine concentration (case report) | |
| Aripiprazole | Decreased levels of aripiprazole | |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | ||
| CBZ: carbamazepine; MAOIs: monoamine oxidase inhibitors; NMS: neuroleptic malignant syndrome; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | ||
Treatment considerations
Without evidence to support stimulants’ safety and efficacy in patients with bipolar disorder, we cannot make specific recommendations. We would, however, like to offer some general recommendations if you decide to use stimulants when treating patients with bipolar disorder (Table 4).
Carefully assess and—in many cases—reassess the patient’s symptoms to clarify the diagnosis. As mentioned, ADHD and bipolar disorder share many symptoms, particularly in the manic phase of bipolar illness. Overlapping symptoms include decreased ability to concentrate and focus, distractibility, hyperactivity and psychomotor agitation, racing thoughts, and impulsivity.
Substance abuse can negatively impact bipolar illness and present as clinical scenarios in which stimulants are used (such as treatment-resistant depression, impulsivity, somnolence, or fatigue).
Treat medical conditions such as thyroid disease, diabetes, and sleep apnea, which may worsen depression, cause somnolence and sedation, and present with symptoms similar to those of ADHD.
When possible, use lifestyle techniques to help patients manage the course of bipolar illness. Encourage good sleep hygiene, exercise, stable social rhythms, and limited use of alcohol and caffeine (both of which can impair sleep quality, which affects illness stability).
The next step. When you have explored all medication options and ruled out all other causes for the patient’s symptoms, stimulant treatment may be an appropriate next step. In these cases:
Encourage patients to participate in treatment, particularly in monitoring mood changes (as with life charts), symptoms associated with mood episodes, and emergence of side effects. When possible, involve family members in monitoring for adverse events.
Administration. Start stimulants only when bipolar illness is well-stabilized, especially regarding manic symptoms. We highly caution against using stimulants in patients with manic or hypomanic symptoms, including mixed states. We recommend not using stimulants in patients with:
- clinically significant insomnia or sleep fragmentation
- active suicidal ideation or psychotic symptoms, particularly if associated with manic symptoms.
Schedule frequent office visits when prescribing stimulants. At least initially, see patients every other week to assess for the emergence of adverse events.
Table 4
6 recommendations when using stimulants in bipolar disorder
| Carefully assess patient’s symptoms | Manic symptoms vs ADHD; medical conditions such as thyroid disorders, diabetes, or sleep apnea |
| Review possible iatrogenic causes of symptoms | Somnolence, decreased energy/fatigue, sedation, difficulty with concentration/focus |
| Engage patient in the therapeutic process | Discuss risks and benefits; monitor mood with life charts; enlist help of family, significant others when appropriate |
| Use caution in clinical scenarios that may herald adverse response to stimulants | Manic/hypomanic symptoms; sleep disturbances; psychosis; history of substance abuse |
| Administer stimulants with caution | Start low and go slow; always use stimulants in conjunction with a mood-stabilizing agent; be aware of possible interactions with patient’s other medications; schedule more frequent visits when starting stimulants |
| Monitor for adverse events associated with stimulant administration | Manic symptoms, changes in cycling patterns, sleep disturbances, substance abuse |
| ADHD: attention-deficit/hyperactivity disorder | |
- The Texas Medication Algorithm Project. Texas Department of State Health Services. www.dshs.state.tx.us/mhprograms/tmapover.shtm.
- The Cochrane Collaboration. www.cochrane.org.
- Amphetamine and dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine, DextroStat
- Diazepam • Valium
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Lisdexamfetamine • Vyvanse
- Lithium • various
- Methylphenidate • Ritalin, Concerta, others
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Triazolam • Halcion
- Valproic acid • Depakene
- Venlafaxine • Effexor
Dr. Gonzalez reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products. He is a recipient of a T32 Ruth L. Kirschstein National Research Service Awards training fellowship sponsored by the National Institutes of Health.
Dr. Suppes receives grants/research support from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, JDS Pharmaceuticals, Janssen Pharmaceutica, National Institute of Mental Health, Novartis, Pfizer Inc., and the Stanley Medical Research Institute.
1. Silberman EK, Reus VI, Jimerson DC, et al. Heterogeneity of amphetamine response in depressed patients. Am J Psychiatry 1981;138(10):1302-7.
2. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164(8):1242-9.
3. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
4. Meyers B. Treatment of imipramine-resistant depression and lithium-refractory mania through drug interactions. Am J Psychiatry 1978;135(11):1420-1.
5. Bannet J, Ebstein RP, Belmaker RH. Clinical aspects of the interaction of lithium and stimulants. Br J Psychiatry 1980;136:204.-
6. Drimmer EJ, Gitlin MJ, Gwirtsman HE. Desipramine and methylphenidate combination treatment for depression: case report. Am J Psychiatry 1983;140(2):241-2.
7. Fernandes PP, Petty F. Modafinil for remitted bipolar depression with hypersomnia. Ann Pharmacother 2003;37(12):1807-9.
8. Berigan TR. Augmentation with modafinil to achieve remission in depression: a case report. Prim Care Companion J Clin Psychiatry 2001;3(1):32.-
9. Berigan TR. Modafinil treatment of excessive daytime sedation and fatigue associated with topiramate. Prim Care Companion J Clin Psychiatry 2002;4(6):249-50.
10. Berigan T. Modafinil treatment of excessive sedation associated with divalproex sodium. Can J Psychiatry 2004;49(1):72-3.
11. Even C, Thuile J, Santos J, Bourgin P. Modafinil as an adjunctive treatment to sleep deprivation in depression. J Psychiatry Neurosci 2005;30(6):432-3.
12. Lydon E, El-Mallakh RS. Naturalistic long-term use of methylphenidate in bipolar disorder. J Clin Psychopharmacol 2006;26(5):516-8.
13. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6(5):416-20.
14. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
15. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry 2000;61(5):378-81.
16. Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry 2007;68(11):1776-84.
17. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
18. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
19. Tamam L, Tuglu C, Karatas G, Ozcan S. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
20. Faraone SV, Biederman J, Mennin D, et al. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997;36(10):1378-87; discussion 1387-90.
21. Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001;64(1):19-26.
22. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatriconset bipolar disorder. Biol Psychiatry 2003;53(11):970-7.
23. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol 2004;24(5):512-20.
24. Calabrese JR. Overview of patient care issues and treatment in bipolar spectrum and bipolar II disorder. J Clin Psychiatry 2008;69(6):e18.-
25. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3(2):53-7.
26. Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975;44(3):215-24.
27. Huey LY, Janowsky DS, Judd LL, et al. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73(2):161-4.
28. Gerner RH, Post RM, Bunney WE, Jr. A dopaminergic mechanism in mania. Am J Psychiatry 1976;133(10):1177-80.
29. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidateinduced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
30. Brady KT, Sonne SC. The relationship between substance abuse and bipolar disorder. J Clin Psychiatry 1995;56(suppl 3):19-24.
31. Sonne SC, Brady KT. Substance abuse and bipolar comorbidity. Psychiatr Clin North Am 1999;22(3):609-27,ix.
32. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264(19):2511-8.
33. Estroff TW, Dackis CA, Gold MS, Pottash AL. Drug abuse and bipolar disorders. Int J Psychiatry Med 1985-1986;15(1):37-40.
34. Faraone SV. Lisdexamfetamine dimesylate: the first longacting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother 2008;9(9):1565-74.
1. Silberman EK, Reus VI, Jimerson DC, et al. Heterogeneity of amphetamine response in depressed patients. Am J Psychiatry 1981;138(10):1302-7.
2. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164(8):1242-9.
3. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
4. Meyers B. Treatment of imipramine-resistant depression and lithium-refractory mania through drug interactions. Am J Psychiatry 1978;135(11):1420-1.
5. Bannet J, Ebstein RP, Belmaker RH. Clinical aspects of the interaction of lithium and stimulants. Br J Psychiatry 1980;136:204.-
6. Drimmer EJ, Gitlin MJ, Gwirtsman HE. Desipramine and methylphenidate combination treatment for depression: case report. Am J Psychiatry 1983;140(2):241-2.
7. Fernandes PP, Petty F. Modafinil for remitted bipolar depression with hypersomnia. Ann Pharmacother 2003;37(12):1807-9.
8. Berigan TR. Augmentation with modafinil to achieve remission in depression: a case report. Prim Care Companion J Clin Psychiatry 2001;3(1):32.-
9. Berigan TR. Modafinil treatment of excessive daytime sedation and fatigue associated with topiramate. Prim Care Companion J Clin Psychiatry 2002;4(6):249-50.
10. Berigan T. Modafinil treatment of excessive sedation associated with divalproex sodium. Can J Psychiatry 2004;49(1):72-3.
11. Even C, Thuile J, Santos J, Bourgin P. Modafinil as an adjunctive treatment to sleep deprivation in depression. J Psychiatry Neurosci 2005;30(6):432-3.
12. Lydon E, El-Mallakh RS. Naturalistic long-term use of methylphenidate in bipolar disorder. J Clin Psychopharmacol 2006;26(5):516-8.
13. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6(5):416-20.
14. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
15. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry 2000;61(5):378-81.
16. Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry 2007;68(11):1776-84.
17. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
18. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
19. Tamam L, Tuglu C, Karatas G, Ozcan S. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
20. Faraone SV, Biederman J, Mennin D, et al. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997;36(10):1378-87; discussion 1387-90.
21. Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001;64(1):19-26.
22. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatriconset bipolar disorder. Biol Psychiatry 2003;53(11):970-7.
23. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol 2004;24(5):512-20.
24. Calabrese JR. Overview of patient care issues and treatment in bipolar spectrum and bipolar II disorder. J Clin Psychiatry 2008;69(6):e18.-
25. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3(2):53-7.
26. Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975;44(3):215-24.
27. Huey LY, Janowsky DS, Judd LL, et al. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73(2):161-4.
28. Gerner RH, Post RM, Bunney WE, Jr. A dopaminergic mechanism in mania. Am J Psychiatry 1976;133(10):1177-80.
29. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidateinduced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
30. Brady KT, Sonne SC. The relationship between substance abuse and bipolar disorder. J Clin Psychiatry 1995;56(suppl 3):19-24.
31. Sonne SC, Brady KT. Substance abuse and bipolar comorbidity. Psychiatr Clin North Am 1999;22(3):609-27,ix.
32. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264(19):2511-8.
33. Estroff TW, Dackis CA, Gold MS, Pottash AL. Drug abuse and bipolar disorders. Int J Psychiatry Med 1985-1986;15(1):37-40.
34. Faraone SV. Lisdexamfetamine dimesylate: the first longacting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother 2008;9(9):1565-74.
Will my patient attempt suicide again?
Ms. J, age 32, comes to our mental health clinic seeking treatment for depression and anxiety. She reports she has attempted suicide 3 times. Ms. J describes the first 2 attempts—both of which occurred when she was in her 20s after the end of a relationship—as “cries for attention” that were relatively innocuous. Her third suicide attempt, however, was an acetaminophen overdose approximately 1 year ago that resulted in hospitalization and irreversible liver damage.
Ms. J acknowledges that over the last several weeks she has been thinking about suicide almost constantly, especially as the anniversary of her fiancé’s death approaches. She says she has a nearly full bottle of zolpidem in her medicine cabinet and fantasizes about taking all of them and just “going to sleep.”
Many patients—especially those with depression—experience recurrent thoughts of death or a wish to die, but only about 10% attempt suicide.1 To identify those who are at highest risk and warrant hospitalization, it is vital to assess how a history of suicidal behavior and other factors impact the risk for future suicide attempts. This article:
- examines research on patients who have attempted suicide and risk factors for repeat suicide attempts
- describes characteristics of patients with multiple attempts
- explores the link between a history of self-injurious behavior and suicide attempts.
A strong predictor
A previous suicide attempt is among the strongest predictors of future suicide attempts.2-4 In a sample of clinically referred European adolescents, those who had attempted suicide were 3 times more likely to try again during the 1-year follow-up compared with those who had never attempted suicide.5 In addition, Harris et al6 found that patients with a previous suicide attempt were 38 times more likely to eventually commit suicide than those with no past attempts.
Other risk factors
Other factors might help predict which individuals will continue to engage in suicidal behavior after a first attempt (Table 1).7,8 Spirito et al7 followed 58 adolescent suicide attempters for 3 months after their initial attempt. Seven (12%) made a subsequent attempt, and 26 (45%) reported continued suicidal ideation. Depressed mood was the strongest predictor of subsequent suicidal behavior, followed by poor family functioning, affect regulation difficulty, and hopelessness.
Hopelessness. Beck et al9 found that patients who scored ≥9 on the Beck Hopelessness Scale (BHS)—the most common self-report measure of hopelessness—were approximately 11 times more likely to commit suicide than patients who scored ≤8. A study of hospitalized suicide attempters found that BHS scores were unique predictors of future suicide attempts.10 Several studies have found that persons who remain consistently hopeless are more likely to kill themselves compared with those who have fluctuating hopelessness levels.11,12
History of abuse—specifically sexual abuse—is associated with suicidal behavior. A study of depressed women age >50 found that among those who were sexually abused before age 18, 83% reported 1 suicide attempt and 67% made multiple attempts.13 Among women who had not experienced childhood sexual abuse, 58% reported a past suicide attempt and 27% made multiple attempts.13
In a separate study of psychiatric inpatients, those who had been physically or sexually abused were more likely to have made a suicide attempt than patients with no such history.14 This study did not find a difference in reported abuse between single and multiple suicide attempters.
Stressors. In many cases suicide attempts are precipitated by acute or chronic stressors, including:
- job stress
- chronic illness
- financial problems
- relationship discord
- retirement and declining physical health (especially for older men)
- death of a loved one.15
Risk is not necessarily cumulative—and not all risk factors are weighted equally. In general, however, the more risk factors a patient has, the greater the likelihood that he or she may attempt suicide.17
Table 1
Repeated suicidal behavior: Factors that increase risk
| History of ≥1 suicide attempts |
| Feelings of hopelessness |
| Presence of an Axis I or II disorder |
| High levels of perceived stress |
| History of physical or sexual abuse |
| Source: References 7,8 |
Red flag: Multiple attempts
When assessing a patient’s suicide history, ask about the number of attempts. A person who makes >1 suicide attempt—a multiple attempter—has a significantly higher chance of making subsequent attempts compared with those with 1 or no attempts.18,19
Persons who make multiple attempts share certain characteristics (Table 2).19-21 Rudd et al19 compared 68 multiple attempters with 128 single attempters and found that multiple attempters had higher levels of:
- suicide ideation
- depression
- hopelessness
- perceived stress.
Similarly, Foreman et al20 found that compared with single suicide attempters, multiple attempters had higher levels of depression, hopelessness, and suicidal ideation and met criteria for more Axis I diagnoses. Multiple attempters also were more likely to be:
- diagnosed with substance use disorders, psychotic disorder, or borderline personality disorder
- unemployed and have relationship difficulties, a history of emotional abuse, and a family history of psychiatric problems and suicide.
Among 326 individuals in a military medical setting treated for suicidal behavior or severe suicidal ideation, multiple suicide attempters reported higher levels of ongoing distress that was unrelated to specific life stressors.22 This suggests these patients may not respond well to psychological interventions that focus on problem-solving.
Table 2
Common characteristics of multiple suicide attempters
| History of Axis I disorder (major depressive disorder, bipolar disorder, schizophrenia, substance use disorders, eating disorders) |
| High levels of perceived stress |
| High levels of depression |
| Symptoms of borderline personality disorder |
| Poor problem-solving skills |
| Family history of psychiatric illness |
| Source: References 19-21 |
Self-harm and suicidal behavior
Patients who engage in nonsuicidal self harm—also called self-injurious behavior (SIB)—may be mistaken for suicide attempters. Although differences exist between suicide attempters and those who engage in SIB, evidence suggests that a history of SIB increases risk for suicidal behavior.23,24 In a retrospective study of 4,167 self-harmers, females who engaged in ≥4 acts of SIB were more likely to die from suicide than those who engaged in ≤3 acts.25 A cross-sectional analysis of data from 3,069 students responding to a random Web-based survey found that an increased incidence of SIB significantly increased the odds of suicidal behavior.26
Although the link between SIB and suicide attempts remains unclear, evidence suggests SIB is a risk factor for suicidal behavior and therefore should be assessed when evaluating a patient’s suicide risk.
CASE CONTINUED: At high risk
Ms. J has several risk factors for making another suicide attempt. She has 3 previous attempts, and because her last attempt caused liver damage we know she is capable of lethal behavior. In addition, the anniversary of the death of her fiancé is approaching. Ms. J also reports almost constant suicidal ideation, with a specific plan (to overdose). Her fantasies of taking pills could be interpreted as mental rehearsal and desensitization to the behavior.
Because we believe Ms. J is at high risk for a serious, if not lethal, suicide attempt we conduct a 4-question suicide inquiry. It is clear that Ms. J had suicidal thoughts and a plan. Her answer to “How likely is it that once you leave my office you will do something to hurt yourself?” is the key to determining whether or not she requires hospitalization. Ms. J states that she is “pretty certain she will hurt herself” once she leaves the office, so we hospitalize her.
To determine if a patient requires immediate hospitalization, perform a specific suicide inquiry. Although there is no surefire way to determine if a patient will kill himself or herself, asking specific questions can help you gauge risk. Based on evidence28 and my clinical experience, I focus on patients’ answers to 4 questions (Table 3). Affirmative answers to these questions are a strong indication that a patient requires hospitalization.
Occasionally, patients are not truthful when asked about their suicidal intent. If you suspect a patient is lying, clinical judgment and the patient’s history guide the decision on hospitalization.
Table 3
Hospitalize? 4 questions to guide your decision
| Are you having thoughts of hurting or killing yourself? If yes: What are you thinking/planning to do? |
| Do you have access to lethal means? |
| What is the likelihood that you will hurt yourself? |
| Have you ever done something to hurt yourself (either suicide attempt or self-injurious behavior)? If yes: How many times? |
- Joiner TE. Why people die by suicide. Cambridge, MA: Harvard University Press; 2005:46-93,203-22.
- American Foundation for Suicide Prevention. www.afsp.org.
- SAVE: Suicide Awareness Voices of Education. www.save.org.
- Zolpidem • Ambien
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Suicidal thoughts, suicide attempts, major depressive episode, and substance use among adults. Rockville, MD: US Department of Health and Human Services; 2006.
2. Pfeffer CR, Klerman GL, Hurt SW, et al. Suicidal children grow up: rates and psychosocial risk factors for suicide attempts during follow-up. J Am Acad Child Adolesc Psychiatry 1993;32:106-13.
3. Lewinsohn PM, Rohde P, Seeley JR. Psychosocial risk factors for future adolescent suicide attempts. J Consult Clin Psychol 1994;62:297-305.
4. Brown GK, Beck AT, Steer RA, et al. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol 2000;68:371-7.
5. Hultén A, Jiang GX, Wasserman D, et al. Repetition of attempted suicide among teenagers in Europe: frequency, timing, and risk factors. Eur Child Adolesc Psychiatry 2001;10:161-9.
6. Harris EC, Barraclough B. Suicide as an outcome for mental disorders: a metaanalysis. Br J Psychiatr 1997;170:205-28.
7. Spirito A, Valeri S, Boergers J, et al. Predictors of continued suicidal behavior in adolescents following a suicide attempt. J Clin Child Adolesc Psychol 2003;32(2):284-9.
8. Moscicki EK. Epidemiology of completed and attempted suicide: toward a framework for prevention. Clin Neurosci Res 2001;1:310-23.
9. Beck AT, Steer RA. Clinical predictors of eventual suicide: a 5- to 10-year prospective study of suicide attempters. J Affec Disord 1989;17:203-9.
10. Petrie K, Chamberlain K, Clarke D. Psychological predictors of future suicidal behaviour in hospitalized suicide attempters. Br J Clin Psychol 1988;27:247-57.
11. Dahlsgaard KK, Beck AT, Brown GK. Inadequate response to therapy as a predictor of suicide. Suicide Life Threat Behav 1998;28:197-204.
12. Young MA, Fogg LF, Scheftner W, et al. Stable trait components of hopelessness: baseline and sensitivity to depression. J Abnorm Psychol 1996;105(2):155-65.
13. Talbot NL, Duberstein PR, Cox C, et al. Preliminary report on childhood sexual abuse, suicidal ideation, and suicide attempts among middle-aged and older depressed women. Am J Geriatr Psychiatry 2004;12:536-8.
14. Andover MS, Zlotnick C, Miller IW. Childhood physical and sexual abuse in depressed patients with single and multiple suicide attempts. Suicide Life Threat Behav 2007;37(4):467-74.
15. Heikkinen M, Aro H, Lönnqvist J. The partners’ views on precipitant stressors in suicide. Acta Psychiatr Scand 1992;85(5):380-4.
16. Bunch J, Barraclough B. The influence of parental death anniversaries upon suicide dates. Br J Psychiatry 1971;118:621-6.
17. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry 1999;56(7):617-26.
18. Goldston DB, Daniel SS, Reboussin DM, et al. Suicide attempts among formerly hospitalized adolescents: a prospective naturalistic study of risk during the first 5 years after discharge. J Am Acad Child Adolesc Psychiatry 1999;38:660-71.
19. Rudd MD, Joiner T, Rajab MH. Relationships among suicide ideators, attempters, and multiple attempters in a young-adult sample. J Abnorm Psychol 1996;105(4):541-50.
20. Foreman EM, Berk MS, Henriques GR, et al. History of multiple suicide attempts as a behavioral marker of severe psychopathology. Am J Psychiatry 2004;161(3):437-43.
21. Miranda R, Scott M, Roger H, et al. Suicide attempt characteristics, diagnoses, and future attempts: comparing multiple attempters to single attempters and ideators. J Am Acad Child Adolesc Psychiatry 2008;47:32-40.
22. Joiner TE, Rudd MD. Intensity and duration of suicidal crises vary as a function of previous suicide attempts and negative life events. J Consult Clin Psychol 2000;68(5):909-16.
23. Suominen K, Isometsä E, Suokas J, et al. Completed suicide after a suicide attempt: a 37-year follow-up study. Am J Psychiatry 2004;161:562-3.
24. Owens D, Wood C, Greenwood D, et al. Mortality and suicide after non-fatal self-poisoning: a 16-year outcome study of patients attending accident and emergency. Br J Psychiatry 2005;187:470-5.
25. Haw C, Bergen H, Casey D, Hawton K. Repetition of deliberate self-harm: a study of the characteristics and subsequent deaths in patients presenting to a general hospital according to extent of repetition. Suicide Life Threat Behav 2007;37(4):379-96.
26. Whitlock J, Knox KL. The relationship between self-injurious behavior and suicide in a young adult population. Arch Pediatr Adolesc Med 2007;161:634-40.
27. Joiner TE. Why people die by suicide. Cambridge, MA: Harvard University Press; 2005:46-93,203-22.
28. Gliatto MF, Rai AK. Evaluation and treatment of patients with suicidal ideation. Am Fam Physician. 1999;59(6):1500-6.
Ms. J, age 32, comes to our mental health clinic seeking treatment for depression and anxiety. She reports she has attempted suicide 3 times. Ms. J describes the first 2 attempts—both of which occurred when she was in her 20s after the end of a relationship—as “cries for attention” that were relatively innocuous. Her third suicide attempt, however, was an acetaminophen overdose approximately 1 year ago that resulted in hospitalization and irreversible liver damage.
Ms. J acknowledges that over the last several weeks she has been thinking about suicide almost constantly, especially as the anniversary of her fiancé’s death approaches. She says she has a nearly full bottle of zolpidem in her medicine cabinet and fantasizes about taking all of them and just “going to sleep.”
Many patients—especially those with depression—experience recurrent thoughts of death or a wish to die, but only about 10% attempt suicide.1 To identify those who are at highest risk and warrant hospitalization, it is vital to assess how a history of suicidal behavior and other factors impact the risk for future suicide attempts. This article:
- examines research on patients who have attempted suicide and risk factors for repeat suicide attempts
- describes characteristics of patients with multiple attempts
- explores the link between a history of self-injurious behavior and suicide attempts.
A strong predictor
A previous suicide attempt is among the strongest predictors of future suicide attempts.2-4 In a sample of clinically referred European adolescents, those who had attempted suicide were 3 times more likely to try again during the 1-year follow-up compared with those who had never attempted suicide.5 In addition, Harris et al6 found that patients with a previous suicide attempt were 38 times more likely to eventually commit suicide than those with no past attempts.
Other risk factors
Other factors might help predict which individuals will continue to engage in suicidal behavior after a first attempt (Table 1).7,8 Spirito et al7 followed 58 adolescent suicide attempters for 3 months after their initial attempt. Seven (12%) made a subsequent attempt, and 26 (45%) reported continued suicidal ideation. Depressed mood was the strongest predictor of subsequent suicidal behavior, followed by poor family functioning, affect regulation difficulty, and hopelessness.
Hopelessness. Beck et al9 found that patients who scored ≥9 on the Beck Hopelessness Scale (BHS)—the most common self-report measure of hopelessness—were approximately 11 times more likely to commit suicide than patients who scored ≤8. A study of hospitalized suicide attempters found that BHS scores were unique predictors of future suicide attempts.10 Several studies have found that persons who remain consistently hopeless are more likely to kill themselves compared with those who have fluctuating hopelessness levels.11,12
History of abuse—specifically sexual abuse—is associated with suicidal behavior. A study of depressed women age >50 found that among those who were sexually abused before age 18, 83% reported 1 suicide attempt and 67% made multiple attempts.13 Among women who had not experienced childhood sexual abuse, 58% reported a past suicide attempt and 27% made multiple attempts.13
In a separate study of psychiatric inpatients, those who had been physically or sexually abused were more likely to have made a suicide attempt than patients with no such history.14 This study did not find a difference in reported abuse between single and multiple suicide attempters.
Stressors. In many cases suicide attempts are precipitated by acute or chronic stressors, including:
- job stress
- chronic illness
- financial problems
- relationship discord
- retirement and declining physical health (especially for older men)
- death of a loved one.15
Risk is not necessarily cumulative—and not all risk factors are weighted equally. In general, however, the more risk factors a patient has, the greater the likelihood that he or she may attempt suicide.17
Table 1
Repeated suicidal behavior: Factors that increase risk
| History of ≥1 suicide attempts |
| Feelings of hopelessness |
| Presence of an Axis I or II disorder |
| High levels of perceived stress |
| History of physical or sexual abuse |
| Source: References 7,8 |
Red flag: Multiple attempts
When assessing a patient’s suicide history, ask about the number of attempts. A person who makes >1 suicide attempt—a multiple attempter—has a significantly higher chance of making subsequent attempts compared with those with 1 or no attempts.18,19
Persons who make multiple attempts share certain characteristics (Table 2).19-21 Rudd et al19 compared 68 multiple attempters with 128 single attempters and found that multiple attempters had higher levels of:
- suicide ideation
- depression
- hopelessness
- perceived stress.
Similarly, Foreman et al20 found that compared with single suicide attempters, multiple attempters had higher levels of depression, hopelessness, and suicidal ideation and met criteria for more Axis I diagnoses. Multiple attempters also were more likely to be:
- diagnosed with substance use disorders, psychotic disorder, or borderline personality disorder
- unemployed and have relationship difficulties, a history of emotional abuse, and a family history of psychiatric problems and suicide.
Among 326 individuals in a military medical setting treated for suicidal behavior or severe suicidal ideation, multiple suicide attempters reported higher levels of ongoing distress that was unrelated to specific life stressors.22 This suggests these patients may not respond well to psychological interventions that focus on problem-solving.
Table 2
Common characteristics of multiple suicide attempters
| History of Axis I disorder (major depressive disorder, bipolar disorder, schizophrenia, substance use disorders, eating disorders) |
| High levels of perceived stress |
| High levels of depression |
| Symptoms of borderline personality disorder |
| Poor problem-solving skills |
| Family history of psychiatric illness |
| Source: References 19-21 |
Self-harm and suicidal behavior
Patients who engage in nonsuicidal self harm—also called self-injurious behavior (SIB)—may be mistaken for suicide attempters. Although differences exist between suicide attempters and those who engage in SIB, evidence suggests that a history of SIB increases risk for suicidal behavior.23,24 In a retrospective study of 4,167 self-harmers, females who engaged in ≥4 acts of SIB were more likely to die from suicide than those who engaged in ≤3 acts.25 A cross-sectional analysis of data from 3,069 students responding to a random Web-based survey found that an increased incidence of SIB significantly increased the odds of suicidal behavior.26
Although the link between SIB and suicide attempts remains unclear, evidence suggests SIB is a risk factor for suicidal behavior and therefore should be assessed when evaluating a patient’s suicide risk.
CASE CONTINUED: At high risk
Ms. J has several risk factors for making another suicide attempt. She has 3 previous attempts, and because her last attempt caused liver damage we know she is capable of lethal behavior. In addition, the anniversary of the death of her fiancé is approaching. Ms. J also reports almost constant suicidal ideation, with a specific plan (to overdose). Her fantasies of taking pills could be interpreted as mental rehearsal and desensitization to the behavior.
Because we believe Ms. J is at high risk for a serious, if not lethal, suicide attempt we conduct a 4-question suicide inquiry. It is clear that Ms. J had suicidal thoughts and a plan. Her answer to “How likely is it that once you leave my office you will do something to hurt yourself?” is the key to determining whether or not she requires hospitalization. Ms. J states that she is “pretty certain she will hurt herself” once she leaves the office, so we hospitalize her.
To determine if a patient requires immediate hospitalization, perform a specific suicide inquiry. Although there is no surefire way to determine if a patient will kill himself or herself, asking specific questions can help you gauge risk. Based on evidence28 and my clinical experience, I focus on patients’ answers to 4 questions (Table 3). Affirmative answers to these questions are a strong indication that a patient requires hospitalization.
Occasionally, patients are not truthful when asked about their suicidal intent. If you suspect a patient is lying, clinical judgment and the patient’s history guide the decision on hospitalization.
Table 3
Hospitalize? 4 questions to guide your decision
| Are you having thoughts of hurting or killing yourself? If yes: What are you thinking/planning to do? |
| Do you have access to lethal means? |
| What is the likelihood that you will hurt yourself? |
| Have you ever done something to hurt yourself (either suicide attempt or self-injurious behavior)? If yes: How many times? |
- Joiner TE. Why people die by suicide. Cambridge, MA: Harvard University Press; 2005:46-93,203-22.
- American Foundation for Suicide Prevention. www.afsp.org.
- SAVE: Suicide Awareness Voices of Education. www.save.org.
- Zolpidem • Ambien
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. J, age 32, comes to our mental health clinic seeking treatment for depression and anxiety. She reports she has attempted suicide 3 times. Ms. J describes the first 2 attempts—both of which occurred when she was in her 20s after the end of a relationship—as “cries for attention” that were relatively innocuous. Her third suicide attempt, however, was an acetaminophen overdose approximately 1 year ago that resulted in hospitalization and irreversible liver damage.
Ms. J acknowledges that over the last several weeks she has been thinking about suicide almost constantly, especially as the anniversary of her fiancé’s death approaches. She says she has a nearly full bottle of zolpidem in her medicine cabinet and fantasizes about taking all of them and just “going to sleep.”
Many patients—especially those with depression—experience recurrent thoughts of death or a wish to die, but only about 10% attempt suicide.1 To identify those who are at highest risk and warrant hospitalization, it is vital to assess how a history of suicidal behavior and other factors impact the risk for future suicide attempts. This article:
- examines research on patients who have attempted suicide and risk factors for repeat suicide attempts
- describes characteristics of patients with multiple attempts
- explores the link between a history of self-injurious behavior and suicide attempts.
A strong predictor
A previous suicide attempt is among the strongest predictors of future suicide attempts.2-4 In a sample of clinically referred European adolescents, those who had attempted suicide were 3 times more likely to try again during the 1-year follow-up compared with those who had never attempted suicide.5 In addition, Harris et al6 found that patients with a previous suicide attempt were 38 times more likely to eventually commit suicide than those with no past attempts.
Other risk factors
Other factors might help predict which individuals will continue to engage in suicidal behavior after a first attempt (Table 1).7,8 Spirito et al7 followed 58 adolescent suicide attempters for 3 months after their initial attempt. Seven (12%) made a subsequent attempt, and 26 (45%) reported continued suicidal ideation. Depressed mood was the strongest predictor of subsequent suicidal behavior, followed by poor family functioning, affect regulation difficulty, and hopelessness.
Hopelessness. Beck et al9 found that patients who scored ≥9 on the Beck Hopelessness Scale (BHS)—the most common self-report measure of hopelessness—were approximately 11 times more likely to commit suicide than patients who scored ≤8. A study of hospitalized suicide attempters found that BHS scores were unique predictors of future suicide attempts.10 Several studies have found that persons who remain consistently hopeless are more likely to kill themselves compared with those who have fluctuating hopelessness levels.11,12
History of abuse—specifically sexual abuse—is associated with suicidal behavior. A study of depressed women age >50 found that among those who were sexually abused before age 18, 83% reported 1 suicide attempt and 67% made multiple attempts.13 Among women who had not experienced childhood sexual abuse, 58% reported a past suicide attempt and 27% made multiple attempts.13
In a separate study of psychiatric inpatients, those who had been physically or sexually abused were more likely to have made a suicide attempt than patients with no such history.14 This study did not find a difference in reported abuse between single and multiple suicide attempters.
Stressors. In many cases suicide attempts are precipitated by acute or chronic stressors, including:
- job stress
- chronic illness
- financial problems
- relationship discord
- retirement and declining physical health (especially for older men)
- death of a loved one.15
Risk is not necessarily cumulative—and not all risk factors are weighted equally. In general, however, the more risk factors a patient has, the greater the likelihood that he or she may attempt suicide.17
Table 1
Repeated suicidal behavior: Factors that increase risk
| History of ≥1 suicide attempts |
| Feelings of hopelessness |
| Presence of an Axis I or II disorder |
| High levels of perceived stress |
| History of physical or sexual abuse |
| Source: References 7,8 |
Red flag: Multiple attempts
When assessing a patient’s suicide history, ask about the number of attempts. A person who makes >1 suicide attempt—a multiple attempter—has a significantly higher chance of making subsequent attempts compared with those with 1 or no attempts.18,19
Persons who make multiple attempts share certain characteristics (Table 2).19-21 Rudd et al19 compared 68 multiple attempters with 128 single attempters and found that multiple attempters had higher levels of:
- suicide ideation
- depression
- hopelessness
- perceived stress.
Similarly, Foreman et al20 found that compared with single suicide attempters, multiple attempters had higher levels of depression, hopelessness, and suicidal ideation and met criteria for more Axis I diagnoses. Multiple attempters also were more likely to be:
- diagnosed with substance use disorders, psychotic disorder, or borderline personality disorder
- unemployed and have relationship difficulties, a history of emotional abuse, and a family history of psychiatric problems and suicide.
Among 326 individuals in a military medical setting treated for suicidal behavior or severe suicidal ideation, multiple suicide attempters reported higher levels of ongoing distress that was unrelated to specific life stressors.22 This suggests these patients may not respond well to psychological interventions that focus on problem-solving.
Table 2
Common characteristics of multiple suicide attempters
| History of Axis I disorder (major depressive disorder, bipolar disorder, schizophrenia, substance use disorders, eating disorders) |
| High levels of perceived stress |
| High levels of depression |
| Symptoms of borderline personality disorder |
| Poor problem-solving skills |
| Family history of psychiatric illness |
| Source: References 19-21 |
Self-harm and suicidal behavior
Patients who engage in nonsuicidal self harm—also called self-injurious behavior (SIB)—may be mistaken for suicide attempters. Although differences exist between suicide attempters and those who engage in SIB, evidence suggests that a history of SIB increases risk for suicidal behavior.23,24 In a retrospective study of 4,167 self-harmers, females who engaged in ≥4 acts of SIB were more likely to die from suicide than those who engaged in ≤3 acts.25 A cross-sectional analysis of data from 3,069 students responding to a random Web-based survey found that an increased incidence of SIB significantly increased the odds of suicidal behavior.26
Although the link between SIB and suicide attempts remains unclear, evidence suggests SIB is a risk factor for suicidal behavior and therefore should be assessed when evaluating a patient’s suicide risk.
CASE CONTINUED: At high risk
Ms. J has several risk factors for making another suicide attempt. She has 3 previous attempts, and because her last attempt caused liver damage we know she is capable of lethal behavior. In addition, the anniversary of the death of her fiancé is approaching. Ms. J also reports almost constant suicidal ideation, with a specific plan (to overdose). Her fantasies of taking pills could be interpreted as mental rehearsal and desensitization to the behavior.
Because we believe Ms. J is at high risk for a serious, if not lethal, suicide attempt we conduct a 4-question suicide inquiry. It is clear that Ms. J had suicidal thoughts and a plan. Her answer to “How likely is it that once you leave my office you will do something to hurt yourself?” is the key to determining whether or not she requires hospitalization. Ms. J states that she is “pretty certain she will hurt herself” once she leaves the office, so we hospitalize her.
To determine if a patient requires immediate hospitalization, perform a specific suicide inquiry. Although there is no surefire way to determine if a patient will kill himself or herself, asking specific questions can help you gauge risk. Based on evidence28 and my clinical experience, I focus on patients’ answers to 4 questions (Table 3). Affirmative answers to these questions are a strong indication that a patient requires hospitalization.
Occasionally, patients are not truthful when asked about their suicidal intent. If you suspect a patient is lying, clinical judgment and the patient’s history guide the decision on hospitalization.
Table 3
Hospitalize? 4 questions to guide your decision
| Are you having thoughts of hurting or killing yourself? If yes: What are you thinking/planning to do? |
| Do you have access to lethal means? |
| What is the likelihood that you will hurt yourself? |
| Have you ever done something to hurt yourself (either suicide attempt or self-injurious behavior)? If yes: How many times? |
- Joiner TE. Why people die by suicide. Cambridge, MA: Harvard University Press; 2005:46-93,203-22.
- American Foundation for Suicide Prevention. www.afsp.org.
- SAVE: Suicide Awareness Voices of Education. www.save.org.
- Zolpidem • Ambien
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Suicidal thoughts, suicide attempts, major depressive episode, and substance use among adults. Rockville, MD: US Department of Health and Human Services; 2006.
2. Pfeffer CR, Klerman GL, Hurt SW, et al. Suicidal children grow up: rates and psychosocial risk factors for suicide attempts during follow-up. J Am Acad Child Adolesc Psychiatry 1993;32:106-13.
3. Lewinsohn PM, Rohde P, Seeley JR. Psychosocial risk factors for future adolescent suicide attempts. J Consult Clin Psychol 1994;62:297-305.
4. Brown GK, Beck AT, Steer RA, et al. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol 2000;68:371-7.
5. Hultén A, Jiang GX, Wasserman D, et al. Repetition of attempted suicide among teenagers in Europe: frequency, timing, and risk factors. Eur Child Adolesc Psychiatry 2001;10:161-9.
6. Harris EC, Barraclough B. Suicide as an outcome for mental disorders: a metaanalysis. Br J Psychiatr 1997;170:205-28.
7. Spirito A, Valeri S, Boergers J, et al. Predictors of continued suicidal behavior in adolescents following a suicide attempt. J Clin Child Adolesc Psychol 2003;32(2):284-9.
8. Moscicki EK. Epidemiology of completed and attempted suicide: toward a framework for prevention. Clin Neurosci Res 2001;1:310-23.
9. Beck AT, Steer RA. Clinical predictors of eventual suicide: a 5- to 10-year prospective study of suicide attempters. J Affec Disord 1989;17:203-9.
10. Petrie K, Chamberlain K, Clarke D. Psychological predictors of future suicidal behaviour in hospitalized suicide attempters. Br J Clin Psychol 1988;27:247-57.
11. Dahlsgaard KK, Beck AT, Brown GK. Inadequate response to therapy as a predictor of suicide. Suicide Life Threat Behav 1998;28:197-204.
12. Young MA, Fogg LF, Scheftner W, et al. Stable trait components of hopelessness: baseline and sensitivity to depression. J Abnorm Psychol 1996;105(2):155-65.
13. Talbot NL, Duberstein PR, Cox C, et al. Preliminary report on childhood sexual abuse, suicidal ideation, and suicide attempts among middle-aged and older depressed women. Am J Geriatr Psychiatry 2004;12:536-8.
14. Andover MS, Zlotnick C, Miller IW. Childhood physical and sexual abuse in depressed patients with single and multiple suicide attempts. Suicide Life Threat Behav 2007;37(4):467-74.
15. Heikkinen M, Aro H, Lönnqvist J. The partners’ views on precipitant stressors in suicide. Acta Psychiatr Scand 1992;85(5):380-4.
16. Bunch J, Barraclough B. The influence of parental death anniversaries upon suicide dates. Br J Psychiatry 1971;118:621-6.
17. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry 1999;56(7):617-26.
18. Goldston DB, Daniel SS, Reboussin DM, et al. Suicide attempts among formerly hospitalized adolescents: a prospective naturalistic study of risk during the first 5 years after discharge. J Am Acad Child Adolesc Psychiatry 1999;38:660-71.
19. Rudd MD, Joiner T, Rajab MH. Relationships among suicide ideators, attempters, and multiple attempters in a young-adult sample. J Abnorm Psychol 1996;105(4):541-50.
20. Foreman EM, Berk MS, Henriques GR, et al. History of multiple suicide attempts as a behavioral marker of severe psychopathology. Am J Psychiatry 2004;161(3):437-43.
21. Miranda R, Scott M, Roger H, et al. Suicide attempt characteristics, diagnoses, and future attempts: comparing multiple attempters to single attempters and ideators. J Am Acad Child Adolesc Psychiatry 2008;47:32-40.
22. Joiner TE, Rudd MD. Intensity and duration of suicidal crises vary as a function of previous suicide attempts and negative life events. J Consult Clin Psychol 2000;68(5):909-16.
23. Suominen K, Isometsä E, Suokas J, et al. Completed suicide after a suicide attempt: a 37-year follow-up study. Am J Psychiatry 2004;161:562-3.
24. Owens D, Wood C, Greenwood D, et al. Mortality and suicide after non-fatal self-poisoning: a 16-year outcome study of patients attending accident and emergency. Br J Psychiatry 2005;187:470-5.
25. Haw C, Bergen H, Casey D, Hawton K. Repetition of deliberate self-harm: a study of the characteristics and subsequent deaths in patients presenting to a general hospital according to extent of repetition. Suicide Life Threat Behav 2007;37(4):379-96.
26. Whitlock J, Knox KL. The relationship between self-injurious behavior and suicide in a young adult population. Arch Pediatr Adolesc Med 2007;161:634-40.
27. Joiner TE. Why people die by suicide. Cambridge, MA: Harvard University Press; 2005:46-93,203-22.
28. Gliatto MF, Rai AK. Evaluation and treatment of patients with suicidal ideation. Am Fam Physician. 1999;59(6):1500-6.
1. Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Suicidal thoughts, suicide attempts, major depressive episode, and substance use among adults. Rockville, MD: US Department of Health and Human Services; 2006.
2. Pfeffer CR, Klerman GL, Hurt SW, et al. Suicidal children grow up: rates and psychosocial risk factors for suicide attempts during follow-up. J Am Acad Child Adolesc Psychiatry 1993;32:106-13.
3. Lewinsohn PM, Rohde P, Seeley JR. Psychosocial risk factors for future adolescent suicide attempts. J Consult Clin Psychol 1994;62:297-305.
4. Brown GK, Beck AT, Steer RA, et al. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol 2000;68:371-7.
5. Hultén A, Jiang GX, Wasserman D, et al. Repetition of attempted suicide among teenagers in Europe: frequency, timing, and risk factors. Eur Child Adolesc Psychiatry 2001;10:161-9.
6. Harris EC, Barraclough B. Suicide as an outcome for mental disorders: a metaanalysis. Br J Psychiatr 1997;170:205-28.
7. Spirito A, Valeri S, Boergers J, et al. Predictors of continued suicidal behavior in adolescents following a suicide attempt. J Clin Child Adolesc Psychol 2003;32(2):284-9.
8. Moscicki EK. Epidemiology of completed and attempted suicide: toward a framework for prevention. Clin Neurosci Res 2001;1:310-23.
9. Beck AT, Steer RA. Clinical predictors of eventual suicide: a 5- to 10-year prospective study of suicide attempters. J Affec Disord 1989;17:203-9.
10. Petrie K, Chamberlain K, Clarke D. Psychological predictors of future suicidal behaviour in hospitalized suicide attempters. Br J Clin Psychol 1988;27:247-57.
11. Dahlsgaard KK, Beck AT, Brown GK. Inadequate response to therapy as a predictor of suicide. Suicide Life Threat Behav 1998;28:197-204.
12. Young MA, Fogg LF, Scheftner W, et al. Stable trait components of hopelessness: baseline and sensitivity to depression. J Abnorm Psychol 1996;105(2):155-65.
13. Talbot NL, Duberstein PR, Cox C, et al. Preliminary report on childhood sexual abuse, suicidal ideation, and suicide attempts among middle-aged and older depressed women. Am J Geriatr Psychiatry 2004;12:536-8.
14. Andover MS, Zlotnick C, Miller IW. Childhood physical and sexual abuse in depressed patients with single and multiple suicide attempts. Suicide Life Threat Behav 2007;37(4):467-74.
15. Heikkinen M, Aro H, Lönnqvist J. The partners’ views on precipitant stressors in suicide. Acta Psychiatr Scand 1992;85(5):380-4.
16. Bunch J, Barraclough B. The influence of parental death anniversaries upon suicide dates. Br J Psychiatry 1971;118:621-6.
17. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry 1999;56(7):617-26.
18. Goldston DB, Daniel SS, Reboussin DM, et al. Suicide attempts among formerly hospitalized adolescents: a prospective naturalistic study of risk during the first 5 years after discharge. J Am Acad Child Adolesc Psychiatry 1999;38:660-71.
19. Rudd MD, Joiner T, Rajab MH. Relationships among suicide ideators, attempters, and multiple attempters in a young-adult sample. J Abnorm Psychol 1996;105(4):541-50.
20. Foreman EM, Berk MS, Henriques GR, et al. History of multiple suicide attempts as a behavioral marker of severe psychopathology. Am J Psychiatry 2004;161(3):437-43.
21. Miranda R, Scott M, Roger H, et al. Suicide attempt characteristics, diagnoses, and future attempts: comparing multiple attempters to single attempters and ideators. J Am Acad Child Adolesc Psychiatry 2008;47:32-40.
22. Joiner TE, Rudd MD. Intensity and duration of suicidal crises vary as a function of previous suicide attempts and negative life events. J Consult Clin Psychol 2000;68(5):909-16.
23. Suominen K, Isometsä E, Suokas J, et al. Completed suicide after a suicide attempt: a 37-year follow-up study. Am J Psychiatry 2004;161:562-3.
24. Owens D, Wood C, Greenwood D, et al. Mortality and suicide after non-fatal self-poisoning: a 16-year outcome study of patients attending accident and emergency. Br J Psychiatry 2005;187:470-5.
25. Haw C, Bergen H, Casey D, Hawton K. Repetition of deliberate self-harm: a study of the characteristics and subsequent deaths in patients presenting to a general hospital according to extent of repetition. Suicide Life Threat Behav 2007;37(4):379-96.
26. Whitlock J, Knox KL. The relationship between self-injurious behavior and suicide in a young adult population. Arch Pediatr Adolesc Med 2007;161:634-40.
27. Joiner TE. Why people die by suicide. Cambridge, MA: Harvard University Press; 2005:46-93,203-22.
28. Gliatto MF, Rai AK. Evaluation and treatment of patients with suicidal ideation. Am Fam Physician. 1999;59(6):1500-6.
Malpractice minute
Could a child’s suicide have been prevented?
THE PATIENT. A 9-year-old boy was undergoing psychiatric treatment.
CASE FACTS. A psychiatrist prescribed bupropion. The child committed suicide.
THE PARENTS’ CLAIM. The psychiatrist was negligent because he did not diagnose suicidal behavior during the initial visit and prescribed bupropion without proper warnings and follow-up.
THE DOCTOR’S DEFENSE. He did not receive information from the patient’s family that would have indicated suicidal behavior, bupropion was an appropriate treatment and was unrelated to the suicide, the family received proper warnings about the drug, and the suicide was unforeseeable.
Submit your verdict and find out how the court ruled and see how your colleagues voted in August’s Malpractice Minute. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Could a child’s suicide have been prevented?
THE PATIENT. A 9-year-old boy was undergoing psychiatric treatment.
CASE FACTS. A psychiatrist prescribed bupropion. The child committed suicide.
THE PARENTS’ CLAIM. The psychiatrist was negligent because he did not diagnose suicidal behavior during the initial visit and prescribed bupropion without proper warnings and follow-up.
THE DOCTOR’S DEFENSE. He did not receive information from the patient’s family that would have indicated suicidal behavior, bupropion was an appropriate treatment and was unrelated to the suicide, the family received proper warnings about the drug, and the suicide was unforeseeable.
Submit your verdict and find out how the court ruled and see how your colleagues voted in August’s Malpractice Minute. Click on “Have more to say about this topic?” to comment.
Could a child’s suicide have been prevented?
THE PATIENT. A 9-year-old boy was undergoing psychiatric treatment.
CASE FACTS. A psychiatrist prescribed bupropion. The child committed suicide.
THE PARENTS’ CLAIM. The psychiatrist was negligent because he did not diagnose suicidal behavior during the initial visit and prescribed bupropion without proper warnings and follow-up.
THE DOCTOR’S DEFENSE. He did not receive information from the patient’s family that would have indicated suicidal behavior, bupropion was an appropriate treatment and was unrelated to the suicide, the family received proper warnings about the drug, and the suicide was unforeseeable.
Submit your verdict and find out how the court ruled and see how your colleagues voted in August’s Malpractice Minute. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
How to reduce overdose risk with ‘super benzodiazepine’ atypicals
A new pattern of morbidity and mortality in suicidal patients who overdose has emerged with the broader use of atypical antipsychotics.1 Although it is not known how often antipsychotics and benzodiazepines are combined in suicide attempts, clinicians need to prescribe atypicals carefully to prevent their use in self-poisoning.
We recently treated nonpsychotic patients whose most common clinical presentation after overdosing on some atypicals was near-fatal respiratory outcomes.
The FDA has warned of the risk of potentially fatal respiratory depression with concomitant administration of antipsychotics and benzodiazepines.2 Each atypical carries a different respiratory warning and precaution. This observation prompted us to review the package inserts of the 3 “super benzodiazepine” atypicals.
Clozapine is a dibenzodiazepine. Its black-box warning states, “Since collapse, respiratory arrest, and cardiac arrest during initial treatment has occurred in patients who were being administered benzodiazepines or other psychotropic drugs, caution is advised when clozapine is initiated in patients taking a benzodiazepine or any other psychotropic drug.”3
Olanzapine is a thienobenzodiazepine. Its precaution states, “co-administration of intramuscular lorazepam and intramuscular olanzapine for injection added to the somnolence observed with either drug alone. Concomitant administration of intramuscular olanzapine and parenteral benzodiazepine has not been studied and is therefore not recommended.”3
Quetiapine is a dibenzothiazepine. Its precaution states, “The mean oral clearance of lorazepam (2 mg, single dose) was reduced by 20% in the presence of quetiapine administered as 250 mg PO tid dosing.”3
Recommendations. Because psychiatric patients have higher respiratory mortality than the general population, monitor patients’ pulmonary status when administering these 3 atypicals as one might when prescribing benzodiazepines. Note:
- preexisting conditions that compromise respiratory function such as chronic obstructive pulmonary disease, sleep apnea, asthma, or pneumonia
- clinical indicators of changes in respiratory function, such as respiratory rate, dyspnea, hypoxemia, and acidosis.
Be cautious of adverse respiratory events when prescribing atypicals alone or with any traditional CNS depressants such as benzodiazepines, sedative/hypnotics, minor tranquilizers, sleep aids, opiates, methadone, and GABAminergic agents. Controlling the amount of antipsychotics dispensed could minimize the risk of overdose. Screen for depression before prescribing a combination of atypicals and CNS depressants. Consider prescribing other atypicals—not “super benzodiazepines”—to patients with possible suicide risk.
1. Viner MW, Chen Y, Bakshi I, et al. Low-dose risperidone augmentation of antidepressants in nonpsychotic depressive disorders with suicidal ideation. J Clin Psychopharmacol 2003;23:105-6.
2. Ativan prescribing information. April 2007. Available at: http://www.fda.gov/medwatch/SAFETY/2007/Apr_PI/Ativan_PI.pdf. Accessed September 7, 2007.
3. Physicians’ desk reference. 62nd ed. Montvale, NJ: Thomson Healthcare Inc.; 2007.
A new pattern of morbidity and mortality in suicidal patients who overdose has emerged with the broader use of atypical antipsychotics.1 Although it is not known how often antipsychotics and benzodiazepines are combined in suicide attempts, clinicians need to prescribe atypicals carefully to prevent their use in self-poisoning.
We recently treated nonpsychotic patients whose most common clinical presentation after overdosing on some atypicals was near-fatal respiratory outcomes.
The FDA has warned of the risk of potentially fatal respiratory depression with concomitant administration of antipsychotics and benzodiazepines.2 Each atypical carries a different respiratory warning and precaution. This observation prompted us to review the package inserts of the 3 “super benzodiazepine” atypicals.
Clozapine is a dibenzodiazepine. Its black-box warning states, “Since collapse, respiratory arrest, and cardiac arrest during initial treatment has occurred in patients who were being administered benzodiazepines or other psychotropic drugs, caution is advised when clozapine is initiated in patients taking a benzodiazepine or any other psychotropic drug.”3
Olanzapine is a thienobenzodiazepine. Its precaution states, “co-administration of intramuscular lorazepam and intramuscular olanzapine for injection added to the somnolence observed with either drug alone. Concomitant administration of intramuscular olanzapine and parenteral benzodiazepine has not been studied and is therefore not recommended.”3
Quetiapine is a dibenzothiazepine. Its precaution states, “The mean oral clearance of lorazepam (2 mg, single dose) was reduced by 20% in the presence of quetiapine administered as 250 mg PO tid dosing.”3
Recommendations. Because psychiatric patients have higher respiratory mortality than the general population, monitor patients’ pulmonary status when administering these 3 atypicals as one might when prescribing benzodiazepines. Note:
- preexisting conditions that compromise respiratory function such as chronic obstructive pulmonary disease, sleep apnea, asthma, or pneumonia
- clinical indicators of changes in respiratory function, such as respiratory rate, dyspnea, hypoxemia, and acidosis.
Be cautious of adverse respiratory events when prescribing atypicals alone or with any traditional CNS depressants such as benzodiazepines, sedative/hypnotics, minor tranquilizers, sleep aids, opiates, methadone, and GABAminergic agents. Controlling the amount of antipsychotics dispensed could minimize the risk of overdose. Screen for depression before prescribing a combination of atypicals and CNS depressants. Consider prescribing other atypicals—not “super benzodiazepines”—to patients with possible suicide risk.
A new pattern of morbidity and mortality in suicidal patients who overdose has emerged with the broader use of atypical antipsychotics.1 Although it is not known how often antipsychotics and benzodiazepines are combined in suicide attempts, clinicians need to prescribe atypicals carefully to prevent their use in self-poisoning.
We recently treated nonpsychotic patients whose most common clinical presentation after overdosing on some atypicals was near-fatal respiratory outcomes.
The FDA has warned of the risk of potentially fatal respiratory depression with concomitant administration of antipsychotics and benzodiazepines.2 Each atypical carries a different respiratory warning and precaution. This observation prompted us to review the package inserts of the 3 “super benzodiazepine” atypicals.
Clozapine is a dibenzodiazepine. Its black-box warning states, “Since collapse, respiratory arrest, and cardiac arrest during initial treatment has occurred in patients who were being administered benzodiazepines or other psychotropic drugs, caution is advised when clozapine is initiated in patients taking a benzodiazepine or any other psychotropic drug.”3
Olanzapine is a thienobenzodiazepine. Its precaution states, “co-administration of intramuscular lorazepam and intramuscular olanzapine for injection added to the somnolence observed with either drug alone. Concomitant administration of intramuscular olanzapine and parenteral benzodiazepine has not been studied and is therefore not recommended.”3
Quetiapine is a dibenzothiazepine. Its precaution states, “The mean oral clearance of lorazepam (2 mg, single dose) was reduced by 20% in the presence of quetiapine administered as 250 mg PO tid dosing.”3
Recommendations. Because psychiatric patients have higher respiratory mortality than the general population, monitor patients’ pulmonary status when administering these 3 atypicals as one might when prescribing benzodiazepines. Note:
- preexisting conditions that compromise respiratory function such as chronic obstructive pulmonary disease, sleep apnea, asthma, or pneumonia
- clinical indicators of changes in respiratory function, such as respiratory rate, dyspnea, hypoxemia, and acidosis.
Be cautious of adverse respiratory events when prescribing atypicals alone or with any traditional CNS depressants such as benzodiazepines, sedative/hypnotics, minor tranquilizers, sleep aids, opiates, methadone, and GABAminergic agents. Controlling the amount of antipsychotics dispensed could minimize the risk of overdose. Screen for depression before prescribing a combination of atypicals and CNS depressants. Consider prescribing other atypicals—not “super benzodiazepines”—to patients with possible suicide risk.
1. Viner MW, Chen Y, Bakshi I, et al. Low-dose risperidone augmentation of antidepressants in nonpsychotic depressive disorders with suicidal ideation. J Clin Psychopharmacol 2003;23:105-6.
2. Ativan prescribing information. April 2007. Available at: http://www.fda.gov/medwatch/SAFETY/2007/Apr_PI/Ativan_PI.pdf. Accessed September 7, 2007.
3. Physicians’ desk reference. 62nd ed. Montvale, NJ: Thomson Healthcare Inc.; 2007.
1. Viner MW, Chen Y, Bakshi I, et al. Low-dose risperidone augmentation of antidepressants in nonpsychotic depressive disorders with suicidal ideation. J Clin Psychopharmacol 2003;23:105-6.
2. Ativan prescribing information. April 2007. Available at: http://www.fda.gov/medwatch/SAFETY/2007/Apr_PI/Ativan_PI.pdf. Accessed September 7, 2007.
3. Physicians’ desk reference. 62nd ed. Montvale, NJ: Thomson Healthcare Inc.; 2007.
Going outside your area of expertise: How far is too far?
Dear Dr. Mossman:
I am an adult psychiatrist practicing in a geographically isolated area. I am working with the family of 10-year-old “Bobby” who is struggling with attention problems. Top notch neuropsychologic testing recommends a stimulant trial, but the local pediatrician is too busy to give Bobby adequate follow-up and attention.
I am an experienced psychopharmacologist but have not prescribed medication to children since residency. My relationship with the family is excellent, and the local pediatrician said that she would supervise me. If I choose to treat Bobby, what are the possible liability issues I should be aware of, and how can I address them?—Submitted by “Dr. F”
Dr. F’s question raises issues that come up whenever patients need treatment for conditions outside the few with which you are highly familiar. Although you can’t be an expert on every aspect of every patient’s treatment, psychiatrists shouldn’t practice outside their area of competence.
Thus, the main liability-related issue that Dr. F should ask herself is, “Can I treat Bobby competently?” Of course, whenever you decide to treat any patient, you should be able to answer “yes” to this question. When thinking about potential liability related to treating Bobby, Dr. F might also ask, “If a lawsuit occurred, how would my treatment of Bobby appear?” This article discusses key issues that arise when general psychiatrists treat children and the steps general psychiatrists can take to show that they are practicing prudently.
Problem: Not enough clinicians
Child and adolescent psychiatrists (CAPs) are in short supply.1,2 In 2001 the United States had 8.67 CAPs per 100,000 youths and 1.6 CAPs for every 1,000 youths with severe mental disorders.1 Studies suggest that the United States needs nearly twice that many CAPs.3 The shortage is especially severe in rural areas, but approximately one-half of metropolitan counties with populations of >250,000 have no CAPs.1 In much of the nation, finding CAPs who are accepting new patients is difficult, and child and adolescent psychiatric treatment often is delivered by pediatricians, family practitioners, psychiatric nurse practitioners, and general adult psychiatrists.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Children’s special medical issues
General psychiatrists know that children aren’t just little adults. CAPs develop skills and thinking styles during their 2 years of subspecialty fellowship training that are quite different from those used by their general psychiatric colleagues.
Communication. Children and adolescents who need psychiatric care often have limited verbal abilities. Working and communicating with these patients requires a different interactive style.
Information sources. CAPs learn to seek and assimilate clinically important information from many settings—especially a child’s home—where their patients interact with others.
Caution. Only a small subset of psychotropic medications that adult psychiatrists prescribe are FDA-approved for use in children (Table 1).4 Because we don’t know how psychotropic drugs affect brain development, CAPs sometimes are leery of giving kids the same medications that adult psychiatrists readily prescribe.
Table 1
FDA-approved drugs and dosages for ADHD in children and adults
| Brand name | Generic name | Drug class | Dosing forms (mg) | Dosage range | Age range |
|---|---|---|---|---|---|
| Adderall | Amphetamine-dextroamphetamine | IR stimulant | 5, 7.5, 10, 12.5, 15, 20, 30 | 5 to 40 mg | 3 to 18 years |
| Adderall XR | Amphetamine-dextroamphetamine | ER stimulant | 5, 10, 15, 20, 25, 30 | 5 to 30 mg | 3 years to adult |
| Concerta | Methylphenidate | ER stimulant | 18, 27, 36, 54 | 18 to 72 mg | 6 years to adult |
| Daytrana | Methylphenidate transdermal | Stimulant | 10, 15, 20, 30 (patch) | 10 to 30 mg | 6 to 18 years |
| Focalin | Dexmethylphenidate | IR stimulant | 2.5, 5, 10 | 2.5 to 10 mg bid | 6 to 17 years |
| Focalin XR | Dexmethylphenidate | ER stimulant | 5, 10, 15, 20 | 5 to 20 mg | 6 years to adult |
| Metadate CD | Methylphenidate | ER stimulant | 10, 20, 30, 50, 60 | 10 to 60 mg | 6 to 18 years |
| Ritalin | Methylphenidate | IR stimulant | 5, 10, 20 | 5 to 20 mg bid or tid | 6 to 18 years |
| Ritalin LA | Methylphenidate | ER stimulant | 10, 20, 30, 40 | 10 to 60 mg | 6 to 18 years |
| Strattera | Atomoxetine | SNRI | 10, 18, 25, 40, 60, 80, 100 | 10 to 100 mg | 6 years to adult |
| Vyvanse | Lisdexamfetamine dimesylate | ER stimulant (precursor) | 30, 50, 70 | 30 to 70 mg | 6 years to adult |
| LA: long acting; CD: controlled delivery; ER, XR: extended release; IR: immediate release; SNRI: selective norepinephrine reuptake inhibitor | |||||
| Source: Adapted from references 2,3 | |||||
Different drugs. Some medications commonly taken by children are not often prescribed for adults, although this is changing as attention-deficit/hyperactivity disorder (ADHD) is better recognized in adults.5,6
Dosages. Dosing psychotropics in adults is fairly standardized, but in children and adolescents dosages vary with age, body weight, and physical maturity.
Adverse effects. The side effects kids experience and the way they report them can differ markedly from adults and will vary with age and developmental maturity. Some issues related to monitoring children—such as appropriate cardiac screening before starting stimulants—are controversial and remain unsettled.7,8
Consider alternatives
Dr. F may be tempted to treat Bobby because of her preexisting, positive relationship with the child’s family and a laudable desire to help. But Dr. F needs to ask, “Is there really no other workable alternative for Bobby?” Some possibilities include:
- Refer Bobby to a CAP in another community for initiation of treatment. Dr. F or Bobby’s pediatrician might safely continue care once a CAP establishes an effective treatment regimen.
- Find another pediatrician who might have more time to provide the follow-up that Dr. F feels is necessary.
- Decline to treat Bobby. Before doing this, Dr. F should consider what effect this refusal might have on her relationship with the family and the consequences for Bobby if his problems go untreated.
- Consult a CAP from another community, describing the situation and clinical factors in detail without naming or identifying the patient, and then ask, “Is this really the best thing to do?”
From a liability standpoint, this last point may be crucial. If the CAP answers “yes,” Dr. F can document the alternatives she has considered and her consultation and discussion with the CAP colleague as evidence of prudent practice. Dr. F can also document any advice that she has received and her plans to follow it.
If you choose to treat
Presumably, Dr. F would not perform thoracic surgery or provide any treatment that is far outside a general psychiatrist’s competence except under the most dire circumstances. General psychiatrists receive child psychiatry training during residency, and treating children is within their scope of practice. Similarly, most elderly patients are treated by general psychiatrists, rather than graduates of geropsychiatry fellowships. Prescribing medication for Bobby is not grossly different from Dr. F’s other duties, and she might provide services that a pediatrician might not.
Ask yourself 4 questions to determine if you are competent to provide medical treatment outside your usual area of expertise (Table 2). In Bobby’s case, Dr. F can consider these additional questions:
- Am I comfortable doing this? Would I be comfortable with this scenario if Bobby were my child?
- How extensive was my general residency training in child psychiatry?
- How long ago was my last CAP experience?
- Have I treated ADHD in adults, and am I familiar with stimulant medications?
- What kind of supervision could I arrange, such as regular phone consultation with a CAP or pediatrician?
- How helpful are other information sources, such as recent texts, journals, and medical Web sites?
- What is my relationship with the family, and how would treating Bobby affect it?
Table 2
Should you provide treatment? 4 questions to ask yourself
| How sure am I that I know what I don’t know? |
| How will I know when I should ask for help? |
| Do I have colleagues readily available for consultation if I need help? |
| Do I have a good track record for seeking consultation when I need it? |
Advantages and benefits
So far, we’ve emphasized cautions, but Dr. F also should remember that she may offer patients services that general psychiatrists provide but that pediatricians might not do routinely. Among the possibilities:
- Performing a diagnostic assessment that incorporates biopsychosocial factors.
- Taking time to foster a strong doctor-patient relationship with the family.
- Reserving time for medication-related psychoeducation.
- Scheduling longer visits to discuss a child’s psychiatric problems and explore solutions.
- Utilizing knowledge of and existing relationships with nonphysician therapists who could provide additional psychotherapy.
1. Thomas CR, Holzer CE, III. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 2006;45:1023-31.
2. U.S. Department of Health and Human Services. Mental health: a report of the Surgeon General. Rockville, MD: National Institute of Mental Health; 1999. Available at: http://www.surgeongeneral.gov/library/mentalhealth/home.html. Accessed August 20, 2008.
3. Levin A. Rural counties suffer from child psychiatry shortage. Psychiatr News 2006;42(14):4-41.
4. National Institute of Mental Health. Treatment of children with mental disorders. 2004. Available at: http://www.nimh.nih.gov/publicat/childqa.cfm. Accessed August 2, 2008.
5. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med 2002;53:113-31.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S-49S.
7. Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics 2008;122:451-3.
8. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 2008;117:2407-23.
Dear Dr. Mossman:
I am an adult psychiatrist practicing in a geographically isolated area. I am working with the family of 10-year-old “Bobby” who is struggling with attention problems. Top notch neuropsychologic testing recommends a stimulant trial, but the local pediatrician is too busy to give Bobby adequate follow-up and attention.
I am an experienced psychopharmacologist but have not prescribed medication to children since residency. My relationship with the family is excellent, and the local pediatrician said that she would supervise me. If I choose to treat Bobby, what are the possible liability issues I should be aware of, and how can I address them?—Submitted by “Dr. F”
Dr. F’s question raises issues that come up whenever patients need treatment for conditions outside the few with which you are highly familiar. Although you can’t be an expert on every aspect of every patient’s treatment, psychiatrists shouldn’t practice outside their area of competence.
Thus, the main liability-related issue that Dr. F should ask herself is, “Can I treat Bobby competently?” Of course, whenever you decide to treat any patient, you should be able to answer “yes” to this question. When thinking about potential liability related to treating Bobby, Dr. F might also ask, “If a lawsuit occurred, how would my treatment of Bobby appear?” This article discusses key issues that arise when general psychiatrists treat children and the steps general psychiatrists can take to show that they are practicing prudently.
Problem: Not enough clinicians
Child and adolescent psychiatrists (CAPs) are in short supply.1,2 In 2001 the United States had 8.67 CAPs per 100,000 youths and 1.6 CAPs for every 1,000 youths with severe mental disorders.1 Studies suggest that the United States needs nearly twice that many CAPs.3 The shortage is especially severe in rural areas, but approximately one-half of metropolitan counties with populations of >250,000 have no CAPs.1 In much of the nation, finding CAPs who are accepting new patients is difficult, and child and adolescent psychiatric treatment often is delivered by pediatricians, family practitioners, psychiatric nurse practitioners, and general adult psychiatrists.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Children’s special medical issues
General psychiatrists know that children aren’t just little adults. CAPs develop skills and thinking styles during their 2 years of subspecialty fellowship training that are quite different from those used by their general psychiatric colleagues.
Communication. Children and adolescents who need psychiatric care often have limited verbal abilities. Working and communicating with these patients requires a different interactive style.
Information sources. CAPs learn to seek and assimilate clinically important information from many settings—especially a child’s home—where their patients interact with others.
Caution. Only a small subset of psychotropic medications that adult psychiatrists prescribe are FDA-approved for use in children (Table 1).4 Because we don’t know how psychotropic drugs affect brain development, CAPs sometimes are leery of giving kids the same medications that adult psychiatrists readily prescribe.
Table 1
FDA-approved drugs and dosages for ADHD in children and adults
| Brand name | Generic name | Drug class | Dosing forms (mg) | Dosage range | Age range |
|---|---|---|---|---|---|
| Adderall | Amphetamine-dextroamphetamine | IR stimulant | 5, 7.5, 10, 12.5, 15, 20, 30 | 5 to 40 mg | 3 to 18 years |
| Adderall XR | Amphetamine-dextroamphetamine | ER stimulant | 5, 10, 15, 20, 25, 30 | 5 to 30 mg | 3 years to adult |
| Concerta | Methylphenidate | ER stimulant | 18, 27, 36, 54 | 18 to 72 mg | 6 years to adult |
| Daytrana | Methylphenidate transdermal | Stimulant | 10, 15, 20, 30 (patch) | 10 to 30 mg | 6 to 18 years |
| Focalin | Dexmethylphenidate | IR stimulant | 2.5, 5, 10 | 2.5 to 10 mg bid | 6 to 17 years |
| Focalin XR | Dexmethylphenidate | ER stimulant | 5, 10, 15, 20 | 5 to 20 mg | 6 years to adult |
| Metadate CD | Methylphenidate | ER stimulant | 10, 20, 30, 50, 60 | 10 to 60 mg | 6 to 18 years |
| Ritalin | Methylphenidate | IR stimulant | 5, 10, 20 | 5 to 20 mg bid or tid | 6 to 18 years |
| Ritalin LA | Methylphenidate | ER stimulant | 10, 20, 30, 40 | 10 to 60 mg | 6 to 18 years |
| Strattera | Atomoxetine | SNRI | 10, 18, 25, 40, 60, 80, 100 | 10 to 100 mg | 6 years to adult |
| Vyvanse | Lisdexamfetamine dimesylate | ER stimulant (precursor) | 30, 50, 70 | 30 to 70 mg | 6 years to adult |
| LA: long acting; CD: controlled delivery; ER, XR: extended release; IR: immediate release; SNRI: selective norepinephrine reuptake inhibitor | |||||
| Source: Adapted from references 2,3 | |||||
Different drugs. Some medications commonly taken by children are not often prescribed for adults, although this is changing as attention-deficit/hyperactivity disorder (ADHD) is better recognized in adults.5,6
Dosages. Dosing psychotropics in adults is fairly standardized, but in children and adolescents dosages vary with age, body weight, and physical maturity.
Adverse effects. The side effects kids experience and the way they report them can differ markedly from adults and will vary with age and developmental maturity. Some issues related to monitoring children—such as appropriate cardiac screening before starting stimulants—are controversial and remain unsettled.7,8
Consider alternatives
Dr. F may be tempted to treat Bobby because of her preexisting, positive relationship with the child’s family and a laudable desire to help. But Dr. F needs to ask, “Is there really no other workable alternative for Bobby?” Some possibilities include:
- Refer Bobby to a CAP in another community for initiation of treatment. Dr. F or Bobby’s pediatrician might safely continue care once a CAP establishes an effective treatment regimen.
- Find another pediatrician who might have more time to provide the follow-up that Dr. F feels is necessary.
- Decline to treat Bobby. Before doing this, Dr. F should consider what effect this refusal might have on her relationship with the family and the consequences for Bobby if his problems go untreated.
- Consult a CAP from another community, describing the situation and clinical factors in detail without naming or identifying the patient, and then ask, “Is this really the best thing to do?”
From a liability standpoint, this last point may be crucial. If the CAP answers “yes,” Dr. F can document the alternatives she has considered and her consultation and discussion with the CAP colleague as evidence of prudent practice. Dr. F can also document any advice that she has received and her plans to follow it.
If you choose to treat
Presumably, Dr. F would not perform thoracic surgery or provide any treatment that is far outside a general psychiatrist’s competence except under the most dire circumstances. General psychiatrists receive child psychiatry training during residency, and treating children is within their scope of practice. Similarly, most elderly patients are treated by general psychiatrists, rather than graduates of geropsychiatry fellowships. Prescribing medication for Bobby is not grossly different from Dr. F’s other duties, and she might provide services that a pediatrician might not.
Ask yourself 4 questions to determine if you are competent to provide medical treatment outside your usual area of expertise (Table 2). In Bobby’s case, Dr. F can consider these additional questions:
- Am I comfortable doing this? Would I be comfortable with this scenario if Bobby were my child?
- How extensive was my general residency training in child psychiatry?
- How long ago was my last CAP experience?
- Have I treated ADHD in adults, and am I familiar with stimulant medications?
- What kind of supervision could I arrange, such as regular phone consultation with a CAP or pediatrician?
- How helpful are other information sources, such as recent texts, journals, and medical Web sites?
- What is my relationship with the family, and how would treating Bobby affect it?
Table 2
Should you provide treatment? 4 questions to ask yourself
| How sure am I that I know what I don’t know? |
| How will I know when I should ask for help? |
| Do I have colleagues readily available for consultation if I need help? |
| Do I have a good track record for seeking consultation when I need it? |
Advantages and benefits
So far, we’ve emphasized cautions, but Dr. F also should remember that she may offer patients services that general psychiatrists provide but that pediatricians might not do routinely. Among the possibilities:
- Performing a diagnostic assessment that incorporates biopsychosocial factors.
- Taking time to foster a strong doctor-patient relationship with the family.
- Reserving time for medication-related psychoeducation.
- Scheduling longer visits to discuss a child’s psychiatric problems and explore solutions.
- Utilizing knowledge of and existing relationships with nonphysician therapists who could provide additional psychotherapy.
Dear Dr. Mossman:
I am an adult psychiatrist practicing in a geographically isolated area. I am working with the family of 10-year-old “Bobby” who is struggling with attention problems. Top notch neuropsychologic testing recommends a stimulant trial, but the local pediatrician is too busy to give Bobby adequate follow-up and attention.
I am an experienced psychopharmacologist but have not prescribed medication to children since residency. My relationship with the family is excellent, and the local pediatrician said that she would supervise me. If I choose to treat Bobby, what are the possible liability issues I should be aware of, and how can I address them?—Submitted by “Dr. F”
Dr. F’s question raises issues that come up whenever patients need treatment for conditions outside the few with which you are highly familiar. Although you can’t be an expert on every aspect of every patient’s treatment, psychiatrists shouldn’t practice outside their area of competence.
Thus, the main liability-related issue that Dr. F should ask herself is, “Can I treat Bobby competently?” Of course, whenever you decide to treat any patient, you should be able to answer “yes” to this question. When thinking about potential liability related to treating Bobby, Dr. F might also ask, “If a lawsuit occurred, how would my treatment of Bobby appear?” This article discusses key issues that arise when general psychiatrists treat children and the steps general psychiatrists can take to show that they are practicing prudently.
Problem: Not enough clinicians
Child and adolescent psychiatrists (CAPs) are in short supply.1,2 In 2001 the United States had 8.67 CAPs per 100,000 youths and 1.6 CAPs for every 1,000 youths with severe mental disorders.1 Studies suggest that the United States needs nearly twice that many CAPs.3 The shortage is especially severe in rural areas, but approximately one-half of metropolitan counties with populations of >250,000 have no CAPs.1 In much of the nation, finding CAPs who are accepting new patients is difficult, and child and adolescent psychiatric treatment often is delivered by pediatricians, family practitioners, psychiatric nurse practitioners, and general adult psychiatrists.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
Children’s special medical issues
General psychiatrists know that children aren’t just little adults. CAPs develop skills and thinking styles during their 2 years of subspecialty fellowship training that are quite different from those used by their general psychiatric colleagues.
Communication. Children and adolescents who need psychiatric care often have limited verbal abilities. Working and communicating with these patients requires a different interactive style.
Information sources. CAPs learn to seek and assimilate clinically important information from many settings—especially a child’s home—where their patients interact with others.
Caution. Only a small subset of psychotropic medications that adult psychiatrists prescribe are FDA-approved for use in children (Table 1).4 Because we don’t know how psychotropic drugs affect brain development, CAPs sometimes are leery of giving kids the same medications that adult psychiatrists readily prescribe.
Table 1
FDA-approved drugs and dosages for ADHD in children and adults
| Brand name | Generic name | Drug class | Dosing forms (mg) | Dosage range | Age range |
|---|---|---|---|---|---|
| Adderall | Amphetamine-dextroamphetamine | IR stimulant | 5, 7.5, 10, 12.5, 15, 20, 30 | 5 to 40 mg | 3 to 18 years |
| Adderall XR | Amphetamine-dextroamphetamine | ER stimulant | 5, 10, 15, 20, 25, 30 | 5 to 30 mg | 3 years to adult |
| Concerta | Methylphenidate | ER stimulant | 18, 27, 36, 54 | 18 to 72 mg | 6 years to adult |
| Daytrana | Methylphenidate transdermal | Stimulant | 10, 15, 20, 30 (patch) | 10 to 30 mg | 6 to 18 years |
| Focalin | Dexmethylphenidate | IR stimulant | 2.5, 5, 10 | 2.5 to 10 mg bid | 6 to 17 years |
| Focalin XR | Dexmethylphenidate | ER stimulant | 5, 10, 15, 20 | 5 to 20 mg | 6 years to adult |
| Metadate CD | Methylphenidate | ER stimulant | 10, 20, 30, 50, 60 | 10 to 60 mg | 6 to 18 years |
| Ritalin | Methylphenidate | IR stimulant | 5, 10, 20 | 5 to 20 mg bid or tid | 6 to 18 years |
| Ritalin LA | Methylphenidate | ER stimulant | 10, 20, 30, 40 | 10 to 60 mg | 6 to 18 years |
| Strattera | Atomoxetine | SNRI | 10, 18, 25, 40, 60, 80, 100 | 10 to 100 mg | 6 years to adult |
| Vyvanse | Lisdexamfetamine dimesylate | ER stimulant (precursor) | 30, 50, 70 | 30 to 70 mg | 6 years to adult |
| LA: long acting; CD: controlled delivery; ER, XR: extended release; IR: immediate release; SNRI: selective norepinephrine reuptake inhibitor | |||||
| Source: Adapted from references 2,3 | |||||
Different drugs. Some medications commonly taken by children are not often prescribed for adults, although this is changing as attention-deficit/hyperactivity disorder (ADHD) is better recognized in adults.5,6
Dosages. Dosing psychotropics in adults is fairly standardized, but in children and adolescents dosages vary with age, body weight, and physical maturity.
Adverse effects. The side effects kids experience and the way they report them can differ markedly from adults and will vary with age and developmental maturity. Some issues related to monitoring children—such as appropriate cardiac screening before starting stimulants—are controversial and remain unsettled.7,8
Consider alternatives
Dr. F may be tempted to treat Bobby because of her preexisting, positive relationship with the child’s family and a laudable desire to help. But Dr. F needs to ask, “Is there really no other workable alternative for Bobby?” Some possibilities include:
- Refer Bobby to a CAP in another community for initiation of treatment. Dr. F or Bobby’s pediatrician might safely continue care once a CAP establishes an effective treatment regimen.
- Find another pediatrician who might have more time to provide the follow-up that Dr. F feels is necessary.
- Decline to treat Bobby. Before doing this, Dr. F should consider what effect this refusal might have on her relationship with the family and the consequences for Bobby if his problems go untreated.
- Consult a CAP from another community, describing the situation and clinical factors in detail without naming or identifying the patient, and then ask, “Is this really the best thing to do?”
From a liability standpoint, this last point may be crucial. If the CAP answers “yes,” Dr. F can document the alternatives she has considered and her consultation and discussion with the CAP colleague as evidence of prudent practice. Dr. F can also document any advice that she has received and her plans to follow it.
If you choose to treat
Presumably, Dr. F would not perform thoracic surgery or provide any treatment that is far outside a general psychiatrist’s competence except under the most dire circumstances. General psychiatrists receive child psychiatry training during residency, and treating children is within their scope of practice. Similarly, most elderly patients are treated by general psychiatrists, rather than graduates of geropsychiatry fellowships. Prescribing medication for Bobby is not grossly different from Dr. F’s other duties, and she might provide services that a pediatrician might not.
Ask yourself 4 questions to determine if you are competent to provide medical treatment outside your usual area of expertise (Table 2). In Bobby’s case, Dr. F can consider these additional questions:
- Am I comfortable doing this? Would I be comfortable with this scenario if Bobby were my child?
- How extensive was my general residency training in child psychiatry?
- How long ago was my last CAP experience?
- Have I treated ADHD in adults, and am I familiar with stimulant medications?
- What kind of supervision could I arrange, such as regular phone consultation with a CAP or pediatrician?
- How helpful are other information sources, such as recent texts, journals, and medical Web sites?
- What is my relationship with the family, and how would treating Bobby affect it?
Table 2
Should you provide treatment? 4 questions to ask yourself
| How sure am I that I know what I don’t know? |
| How will I know when I should ask for help? |
| Do I have colleagues readily available for consultation if I need help? |
| Do I have a good track record for seeking consultation when I need it? |
Advantages and benefits
So far, we’ve emphasized cautions, but Dr. F also should remember that she may offer patients services that general psychiatrists provide but that pediatricians might not do routinely. Among the possibilities:
- Performing a diagnostic assessment that incorporates biopsychosocial factors.
- Taking time to foster a strong doctor-patient relationship with the family.
- Reserving time for medication-related psychoeducation.
- Scheduling longer visits to discuss a child’s psychiatric problems and explore solutions.
- Utilizing knowledge of and existing relationships with nonphysician therapists who could provide additional psychotherapy.
1. Thomas CR, Holzer CE, III. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 2006;45:1023-31.
2. U.S. Department of Health and Human Services. Mental health: a report of the Surgeon General. Rockville, MD: National Institute of Mental Health; 1999. Available at: http://www.surgeongeneral.gov/library/mentalhealth/home.html. Accessed August 20, 2008.
3. Levin A. Rural counties suffer from child psychiatry shortage. Psychiatr News 2006;42(14):4-41.
4. National Institute of Mental Health. Treatment of children with mental disorders. 2004. Available at: http://www.nimh.nih.gov/publicat/childqa.cfm. Accessed August 2, 2008.
5. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med 2002;53:113-31.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S-49S.
7. Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics 2008;122:451-3.
8. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 2008;117:2407-23.
1. Thomas CR, Holzer CE, III. The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry 2006;45:1023-31.
2. U.S. Department of Health and Human Services. Mental health: a report of the Surgeon General. Rockville, MD: National Institute of Mental Health; 1999. Available at: http://www.surgeongeneral.gov/library/mentalhealth/home.html. Accessed August 20, 2008.
3. Levin A. Rural counties suffer from child psychiatry shortage. Psychiatr News 2006;42(14):4-41.
4. National Institute of Mental Health. Treatment of children with mental disorders. 2004. Available at: http://www.nimh.nih.gov/publicat/childqa.cfm. Accessed August 2, 2008.
5. Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annu Rev Med 2002;53:113-31.
6. Greenhill LL, Pliszka S, Dulcan MK, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S-49S.
7. Perrin JM, Friedman RA, Knilans TK. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics 2008;122:451-3.
8. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation 2008;117:2407-23.