User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
What are ‘normal’ feelings?
The article “Subsyndromal depression: Help your bipolar patients feel better” (Current Psychiatry, August 2008) brought to mind what may seem like a hypothetical question but is one I struggle with every day. What emotions are bipolar patients allowed to feel without necessarily leading to medical intervention?
When a loved one dies is the bipolar patient permitted to feel sad, have no sense of pleasure, become tearful, and have difficulty sleeping and concentrating? An individual who experiences these feelings but is not bipolar would be considered to be grieving—a very natural human emotion. What should we do for a bipolar patient?
I tell my patients that with medication I hope to restore the normal range of emotions that an average, healthy person would feel under similar circumstances, and I would address emotional reactions that clearly are beyond that range, such as suicidality or delusional guilt. Am I misinforming my patient?
Mohamed Dattu, MD
Presto, PA
Dr. Ostacher responds
Dr. Dattu brings up what can be a lifelong dilemma for patients with bipolar disorder and their families and suggests a well-conceived response to those in his care. After suffering through a number of mood episodes, patients often find it hard to judge whether they are experiencing bipolar symptoms or merely having emotions and feelings that are normal and expected. Many of my patients report being traumatized in some way by their episodes, so the appearance of any symptoms—sadness, anxiety, excitement, or insomnia—may make them worried that they will relapse to a new mood episode. Just because a patient has bipolar disorder doesn’t mean that these experiences are pathologic.
Psychiatrists use clinical judgment to decide when to intervene. If functioning becomes significantly impaired, thoughts and risk of self-harm appear, and symptoms persist for months, then intervention—whether changes in medical or psychosocial treatment—probably is necessary.
Michael J. Ostacher, MD, MPH
Assistant professor of psychiatry
Harvard Medical School
Boston
The article “Subsyndromal depression: Help your bipolar patients feel better” (Current Psychiatry, August 2008) brought to mind what may seem like a hypothetical question but is one I struggle with every day. What emotions are bipolar patients allowed to feel without necessarily leading to medical intervention?
When a loved one dies is the bipolar patient permitted to feel sad, have no sense of pleasure, become tearful, and have difficulty sleeping and concentrating? An individual who experiences these feelings but is not bipolar would be considered to be grieving—a very natural human emotion. What should we do for a bipolar patient?
I tell my patients that with medication I hope to restore the normal range of emotions that an average, healthy person would feel under similar circumstances, and I would address emotional reactions that clearly are beyond that range, such as suicidality or delusional guilt. Am I misinforming my patient?
Mohamed Dattu, MD
Presto, PA
Dr. Ostacher responds
Dr. Dattu brings up what can be a lifelong dilemma for patients with bipolar disorder and their families and suggests a well-conceived response to those in his care. After suffering through a number of mood episodes, patients often find it hard to judge whether they are experiencing bipolar symptoms or merely having emotions and feelings that are normal and expected. Many of my patients report being traumatized in some way by their episodes, so the appearance of any symptoms—sadness, anxiety, excitement, or insomnia—may make them worried that they will relapse to a new mood episode. Just because a patient has bipolar disorder doesn’t mean that these experiences are pathologic.
Psychiatrists use clinical judgment to decide when to intervene. If functioning becomes significantly impaired, thoughts and risk of self-harm appear, and symptoms persist for months, then intervention—whether changes in medical or psychosocial treatment—probably is necessary.
Michael J. Ostacher, MD, MPH
Assistant professor of psychiatry
Harvard Medical School
Boston
The article “Subsyndromal depression: Help your bipolar patients feel better” (Current Psychiatry, August 2008) brought to mind what may seem like a hypothetical question but is one I struggle with every day. What emotions are bipolar patients allowed to feel without necessarily leading to medical intervention?
When a loved one dies is the bipolar patient permitted to feel sad, have no sense of pleasure, become tearful, and have difficulty sleeping and concentrating? An individual who experiences these feelings but is not bipolar would be considered to be grieving—a very natural human emotion. What should we do for a bipolar patient?
I tell my patients that with medication I hope to restore the normal range of emotions that an average, healthy person would feel under similar circumstances, and I would address emotional reactions that clearly are beyond that range, such as suicidality or delusional guilt. Am I misinforming my patient?
Mohamed Dattu, MD
Presto, PA
Dr. Ostacher responds
Dr. Dattu brings up what can be a lifelong dilemma for patients with bipolar disorder and their families and suggests a well-conceived response to those in his care. After suffering through a number of mood episodes, patients often find it hard to judge whether they are experiencing bipolar symptoms or merely having emotions and feelings that are normal and expected. Many of my patients report being traumatized in some way by their episodes, so the appearance of any symptoms—sadness, anxiety, excitement, or insomnia—may make them worried that they will relapse to a new mood episode. Just because a patient has bipolar disorder doesn’t mean that these experiences are pathologic.
Psychiatrists use clinical judgment to decide when to intervene. If functioning becomes significantly impaired, thoughts and risk of self-harm appear, and symptoms persist for months, then intervention—whether changes in medical or psychosocial treatment—probably is necessary.
Michael J. Ostacher, MD, MPH
Assistant professor of psychiatry
Harvard Medical School
Boston
Adverse drug effects: An upside to the downside?
Medication side effects are generally regarded as the Achilles’ heel of pharmacologic treatment. And who can argue with that? Adverse effects are the downside of drug treatment of psychiatric disorders and are blamed for tolerability and adherence problems. Patients dread side effects, physicians feel uncomfortable or even guilty about them, and litigation lawyers thrive on them.
Psychotropics’ package inserts are loaded with side-effect descriptions. The precautions, warnings, and black boxes frequently make patients anxious about taking medications, even when the diseases they suffer from pose far greater risk to their lives and health.
Are side effects entirely bad, or is there a possible upside lurking within them? Psychiatrists know, for example, that a common adverse effect of selective serotonin reuptake inhibitor (SSRI) antidepressants/anxiolytics is delayed orgasm. But because of this side effect, SSRIs can be dramatically helpful for treating premature ejaculation in nondepressed men.
Let’s consider some effects of atypical antipsychotics that usually are considered a downside of antipsychotic treatment yet appear to be associated with advantages for the same patients.
Therapeutic sedation
Sedation was considered a major adverse effect of quetiapine when this antipsychotic was approved for treating schizophrenia. Yet with time—and as the drug received additional indications for bipolar mania and bipolar depression—it became apparent to psychiatrists that quetiapine’s sedative effects could be useful for treating insomnia in individuals with psychotic and mood disorders. Thus, practitioners saw an advantage in using a sedating antipsychotic, instead of adding a sedative/hypnotic, for psychotic or manic patients suffering from agitation, anxiety, and insomnia. Three advantages of this approach for patients are:
- fewer medications to manage
- lower costs without an additional sedative/hypnotic
- avoiding the potential addictive effects of a sedative/hypnotic.
Hyperprolactinemia and myelin repair
Some patients receiving risperidone or paliperidone for psychotic symptoms develop sexual dysfunction because of the side effect of increased serum prolactin. On the other hand, recent research indicates that prolactin enhances the synthesis of oligodendrocytes, which are critical for myelin (white matter) integrity in brain tissue.
In a recent study, researchers used a toxin to destroy patches of white matter in the brains of nonpregnant mice. Subsequent pregnancy and prolactin elevation repaired the myelin lesions completely, whereas no changes occurred in the white matter lesions in the brains of control mice that remained nonpregnant.1
This suggested myelin-repairing property of prolactin is highly relevant to the development of potential therapies, not only for demyelinating disorders such as multiple sclerosis but also for schizophrenia. Numerous studies have demonstrated that schizophrenia is associated with serious white-matter pathology,2 which may account for many of the disorder’s thought, emotional, and cognitive impairments.
Weight gain as marker for antipsychotic efficacy
Some weight increase is observed with all antipsychotics, although certain atypicals such as olanzapine and clozapine are associated with more weight gain than others. No clinician would see anything except a downside to weight gain, which may lead to metabolic complications such as diabetes, hyperlipidemia, and hypertension.
However, some weight gain appears to be related to the efficacy of all antipsychotics (first- and second-generation), according to the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)3 and previous sporadic observations in the literature. Even Kraepelin noted in the 1920s—long before the era of antipsychotics—that patients with psychosis gained weight when they spontaneously improved.
Thus, some weight gain appears to be a necessary correlate of improvement in persons treated with antipsychotics. The reasons are still unclear, but weight gain may be a modulator, mediator, or marker of antipsychotic efficacy.
In summary, there appears to be at least some upside to the downside of certain drug side effects. In other words, every dark cloud has a silver lining, and it might be helpful for patients and physicians to see more than just the cloud.
1. Gregg C, Shikar V, Larsen P, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci 2007;27:1812-23.
2. Kubicki M, McCarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr Opin Psychiatry 2005;18:121-34.
3. Nasrallah, et al. Biol Psychiatry 2007;61(suppl 1):12S.-
Medication side effects are generally regarded as the Achilles’ heel of pharmacologic treatment. And who can argue with that? Adverse effects are the downside of drug treatment of psychiatric disorders and are blamed for tolerability and adherence problems. Patients dread side effects, physicians feel uncomfortable or even guilty about them, and litigation lawyers thrive on them.
Psychotropics’ package inserts are loaded with side-effect descriptions. The precautions, warnings, and black boxes frequently make patients anxious about taking medications, even when the diseases they suffer from pose far greater risk to their lives and health.
Are side effects entirely bad, or is there a possible upside lurking within them? Psychiatrists know, for example, that a common adverse effect of selective serotonin reuptake inhibitor (SSRI) antidepressants/anxiolytics is delayed orgasm. But because of this side effect, SSRIs can be dramatically helpful for treating premature ejaculation in nondepressed men.
Let’s consider some effects of atypical antipsychotics that usually are considered a downside of antipsychotic treatment yet appear to be associated with advantages for the same patients.
Therapeutic sedation
Sedation was considered a major adverse effect of quetiapine when this antipsychotic was approved for treating schizophrenia. Yet with time—and as the drug received additional indications for bipolar mania and bipolar depression—it became apparent to psychiatrists that quetiapine’s sedative effects could be useful for treating insomnia in individuals with psychotic and mood disorders. Thus, practitioners saw an advantage in using a sedating antipsychotic, instead of adding a sedative/hypnotic, for psychotic or manic patients suffering from agitation, anxiety, and insomnia. Three advantages of this approach for patients are:
- fewer medications to manage
- lower costs without an additional sedative/hypnotic
- avoiding the potential addictive effects of a sedative/hypnotic.
Hyperprolactinemia and myelin repair
Some patients receiving risperidone or paliperidone for psychotic symptoms develop sexual dysfunction because of the side effect of increased serum prolactin. On the other hand, recent research indicates that prolactin enhances the synthesis of oligodendrocytes, which are critical for myelin (white matter) integrity in brain tissue.
In a recent study, researchers used a toxin to destroy patches of white matter in the brains of nonpregnant mice. Subsequent pregnancy and prolactin elevation repaired the myelin lesions completely, whereas no changes occurred in the white matter lesions in the brains of control mice that remained nonpregnant.1
This suggested myelin-repairing property of prolactin is highly relevant to the development of potential therapies, not only for demyelinating disorders such as multiple sclerosis but also for schizophrenia. Numerous studies have demonstrated that schizophrenia is associated with serious white-matter pathology,2 which may account for many of the disorder’s thought, emotional, and cognitive impairments.
Weight gain as marker for antipsychotic efficacy
Some weight increase is observed with all antipsychotics, although certain atypicals such as olanzapine and clozapine are associated with more weight gain than others. No clinician would see anything except a downside to weight gain, which may lead to metabolic complications such as diabetes, hyperlipidemia, and hypertension.
However, some weight gain appears to be related to the efficacy of all antipsychotics (first- and second-generation), according to the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)3 and previous sporadic observations in the literature. Even Kraepelin noted in the 1920s—long before the era of antipsychotics—that patients with psychosis gained weight when they spontaneously improved.
Thus, some weight gain appears to be a necessary correlate of improvement in persons treated with antipsychotics. The reasons are still unclear, but weight gain may be a modulator, mediator, or marker of antipsychotic efficacy.
In summary, there appears to be at least some upside to the downside of certain drug side effects. In other words, every dark cloud has a silver lining, and it might be helpful for patients and physicians to see more than just the cloud.
Medication side effects are generally regarded as the Achilles’ heel of pharmacologic treatment. And who can argue with that? Adverse effects are the downside of drug treatment of psychiatric disorders and are blamed for tolerability and adherence problems. Patients dread side effects, physicians feel uncomfortable or even guilty about them, and litigation lawyers thrive on them.
Psychotropics’ package inserts are loaded with side-effect descriptions. The precautions, warnings, and black boxes frequently make patients anxious about taking medications, even when the diseases they suffer from pose far greater risk to their lives and health.
Are side effects entirely bad, or is there a possible upside lurking within them? Psychiatrists know, for example, that a common adverse effect of selective serotonin reuptake inhibitor (SSRI) antidepressants/anxiolytics is delayed orgasm. But because of this side effect, SSRIs can be dramatically helpful for treating premature ejaculation in nondepressed men.
Let’s consider some effects of atypical antipsychotics that usually are considered a downside of antipsychotic treatment yet appear to be associated with advantages for the same patients.
Therapeutic sedation
Sedation was considered a major adverse effect of quetiapine when this antipsychotic was approved for treating schizophrenia. Yet with time—and as the drug received additional indications for bipolar mania and bipolar depression—it became apparent to psychiatrists that quetiapine’s sedative effects could be useful for treating insomnia in individuals with psychotic and mood disorders. Thus, practitioners saw an advantage in using a sedating antipsychotic, instead of adding a sedative/hypnotic, for psychotic or manic patients suffering from agitation, anxiety, and insomnia. Three advantages of this approach for patients are:
- fewer medications to manage
- lower costs without an additional sedative/hypnotic
- avoiding the potential addictive effects of a sedative/hypnotic.
Hyperprolactinemia and myelin repair
Some patients receiving risperidone or paliperidone for psychotic symptoms develop sexual dysfunction because of the side effect of increased serum prolactin. On the other hand, recent research indicates that prolactin enhances the synthesis of oligodendrocytes, which are critical for myelin (white matter) integrity in brain tissue.
In a recent study, researchers used a toxin to destroy patches of white matter in the brains of nonpregnant mice. Subsequent pregnancy and prolactin elevation repaired the myelin lesions completely, whereas no changes occurred in the white matter lesions in the brains of control mice that remained nonpregnant.1
This suggested myelin-repairing property of prolactin is highly relevant to the development of potential therapies, not only for demyelinating disorders such as multiple sclerosis but also for schizophrenia. Numerous studies have demonstrated that schizophrenia is associated with serious white-matter pathology,2 which may account for many of the disorder’s thought, emotional, and cognitive impairments.
Weight gain as marker for antipsychotic efficacy
Some weight increase is observed with all antipsychotics, although certain atypicals such as olanzapine and clozapine are associated with more weight gain than others. No clinician would see anything except a downside to weight gain, which may lead to metabolic complications such as diabetes, hyperlipidemia, and hypertension.
However, some weight gain appears to be related to the efficacy of all antipsychotics (first- and second-generation), according to the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)3 and previous sporadic observations in the literature. Even Kraepelin noted in the 1920s—long before the era of antipsychotics—that patients with psychosis gained weight when they spontaneously improved.
Thus, some weight gain appears to be a necessary correlate of improvement in persons treated with antipsychotics. The reasons are still unclear, but weight gain may be a modulator, mediator, or marker of antipsychotic efficacy.
In summary, there appears to be at least some upside to the downside of certain drug side effects. In other words, every dark cloud has a silver lining, and it might be helpful for patients and physicians to see more than just the cloud.
1. Gregg C, Shikar V, Larsen P, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci 2007;27:1812-23.
2. Kubicki M, McCarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr Opin Psychiatry 2005;18:121-34.
3. Nasrallah, et al. Biol Psychiatry 2007;61(suppl 1):12S.-
1. Gregg C, Shikar V, Larsen P, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci 2007;27:1812-23.
2. Kubicki M, McCarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr Opin Psychiatry 2005;18:121-34.
3. Nasrallah, et al. Biol Psychiatry 2007;61(suppl 1):12S.-
The sailor who won’t follow orders
CASE: An unlikable patient
Mr. L, age 56, is admitted to the psychiatric unit at our Veterans Affairs Medical Center for active suicidal ideation; he has a history of self-injurious behaviors that include mutilation and overdose. He also has a history of alcohol dependence and multiple inpatient psychiatric admissions. He has never married and conflicts with his siblings—in whose home he has been staying—have led to frequent homelessness.
His affect is silly and shallow. He also shows signs of haughtiness, disinhibition, grandiosity, and confabulation. For example, he says that while in the Navy he had 82 sexual exploits and developed a drug that cured herpes.
We start Mr. L on divalproex, 1,500 mg/d, and quetiapine, titrated to 200 mg/d. After 3 days he is discharged, but this begins a cycle of repeated suicide gestures and readmissions—9 within the next 3 months. Each time he is discharged, Mr. L fails to follow through on treatment recommendations and is indifferent to our staff’s annoyed reactions.
The author’s observations
Some of our staff members regard Mr. L’s suicidal gestures as manipulative and feel angry and demoralized by his poor adherence to outpatient treatment plans. Their negative countertransference might have impacted how they evaluated Mr. L through repeated admissions and discharges. During Mr. L’s ninth admission, we decide to reevaluate his longitudinal history for clues to his noncompliant behavior.
History: Undocumented injury
Mr. L says he began drinking alcohol at age 16. He reports that he has grown marijuana but has not smoked it since 1991. He denies using heroin or other drugs.
Mr. L states that he suffered a head injury in 1975 after falling off a ladder on a Navy ship. He describes losing consciousness for a brief but uncertain duration. He reports that he has developed a seizure disorder since this fall and a history of amnesia secondary to past seizures. His medical records contain no witnessed seizures. Mr. L also says he was hospitalized a few years ago and placed on a ventilator for 7 days for an undetermined reason.
The authors’ observations
Based on Mr. L’s report of a possible traumatic brain injury (TBI), we order a neurologic evaluation. A year earlier, MRI of the brain without contrast demonstrated minimal, nonspecific periventricular and subcortical, punctuate hyperintensities on flair and T2 weighted sequences that are nonspecific. Overall, the impression was “diffuse involutional changes and mild nonspecific periventricular and subcortical white matter hyperintensities,” which might reflect covert vascular brain injury.
Our frustration over Mr. L’s repeated readmissions for suicidal gestures led us to seek outside evaluation and consultation from a senior psychiatrist for assistance with discharge and treatment planning. Unlike our staff, the consulting psychiatrist did not harbor strong negative feelings toward the patient.
Mr. L’s history of deterioration in psychosocial functioning prompted this psychiatrist to perform a thorough mental status examination that focused on cognitive elements and request formal neuropsychological testing.
Evalutation: Cognitive Deficits
During mental status examination, Mr. L has difficulty recalling 3 items and uses a memory strategy to assist himself. He fails to recollect in reverse order the last 5 U.S. presidents. He spells “world” backward, but has difficulty repeating 6 digits forward and 4 backward. He is unable to do serial 7 subtractions from 93 to 65 correctly. He adequately copies interlocking pentagons and draws a clock with the correct time. He achieves a score of 28/30 on the Folstein Mini Mental State Exam, missing the date by 4 days and recalling 2 of 3 words.
These results suggest Mr. L has difficulty with attention and working memory, short-term memory, fund of general information and long-term memory, and ability to perform simple calculations. Most important, they indicate the need for further study, especially a neuropsychological test battery.
Mr. L’s abnormal neuropsychological test results are summarized in the Table. He manifests concretization of thought. His loss of conceptual fluidity is documented formally by measures of perseverative errors and categories completed on the Wisconsin Card Sorting Test (WCST). These findings support a diagnosis of acquired dementia.
Table
Abnormal findings on Mr. L’s neuropsychological testing
| Cognitive domain | Test | Score | Interpretation |
|---|---|---|---|
| Mental status and effort | |||
| Mental status | MMSE total score | 28/30 | 2 of 3 items recalled after delay |
| Orientation | MMSE orientation questions | 9/10 | Date off by 4 days |
| Premorbid IQ estimate | WRAT-4 Reading Standard | 66th percentile | Within normal limits. Inconsistent with educational attainment, but could be impacted by temporal lobe findings |
| Verbal memory | |||
| Immediate memory | RBANS Immediate Memory Index (List and Story Learning) | 1st percentile | Severe impairment |
| Delayed memory | RBANS Delayed Memory Index | 1st percentile | Severe impairment |
| Recognition memory | List Learning | Severe impairment | |
| Visuospatial memory | |||
| Delayed memory | RBANS Figure Recall | 3rd percentile | Severe impairment |
| Executive functioning | |||
| Cognitive flexibility | Trails B | 10th percentile | Severe impairment based on educational attainment |
| WCST | Low scores: Nonperseverative errors, perseverative errors, and categories completed | ||
| * Tests of mental status effort, visuomotor processing speed, confrontation naming, visuospatial function, attention, and executive functioning fluency/initiation were within normal limits | |||
| MMSE: Mini Mental State Exam; WRAT: Wide Range Achievement Test; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; WCST: Wisconsin Card Sorting Test | |||
The authors’ observations
Mr. L’s history, cognitive testing, head imaging, and behavioral observations suggest that several pathogenic factors contribute to his impaired functioning. First, he describes a TBI of unknown severity occurring in 1975. Although brain scans did not show evidence of midline shift or encephalomalacia, a direct blow to the head after falling from a height combined with possible post-injury seizures suggests a TBI of at least moderate severity.
Second, Mr. L describes an incident in which he required inpatient respiratory assistance. Although the precipitating medical event was unclear, anoxia or hypoxia is likely. A recent CT revealed low attenuation in the left temporal region that could represent an infarct.
Mr. L’s severe memory impairment and moderate to severe impairment in cognitive flexibility are commonly reported after a TBI of moderate severity. If an ischemic incident were the primary contributor, a lateralized pattern of cognitive dysfunction—which Mr. L does not exhibit—would be expected.
Although Mr. L likely has vascular dementia, his MRI findings do not indicate sufficient disease to account for his memory scores. Vascular dementia is associated with slow, stepwise cognitive deterioration, which is not consistent with severely impaired memory in a 56-year-old patient.
Finally, alcoholism is associated with cognitive difficulty in memory, visuospatial functioning, and abstract reasoning. Mr. L demonstrated significant difficulty in memory and abstract reasoning, but his visuospatial functioning was largely intact. In the absence of Wernicke’s encephalopathy, chronic alcoholics generally do not show memory decrements in line with Mr. L’s. His MRI results indicated only minimal ventricular and sulcal enlargement. Because atrophy is present in approximately 60% of chronic alcoholics, this finding provides evidence of a contribution, but the other contributory factors are associated with more definitive medical outcomes. Thus, alcoholism must be viewed as a secondary contributor to Mr. L’s impaired functioning.
Taking into account all known contributors, TBI emerges as the primary diagnosis.
Consider neurologic injury
Recognizing and characterizing personality changes related to neurologic injury and disease is often problematic and unreliable, even when psychometrically validated instruments and structured diagnostic interviews are used (Box 1).1-5 Mr. L’s presentation differed from the more commonly reported “impulsive aggression” associated with closed head injury. Sequelae from TBI were contributing to his clinical presentation but was obscured by his shallow and silly affect, inability to accurately assess social cues, and lack of empathy.
Mr. L reported suffering a head injury from falling off a ladder. Personality changes that result from traumatic brain injury (TBI) of the sudden deceleration type—even when mild—are frequently referable to the frontal lobe, especially focal orbital and/or ventromedial damage of the prefrontal cortex.1-5 This is because of the physical proximity of the sphenoid wing to the orbitofrontal region and effects of shearing.
As a result of this damage, patients lack insight into their accompanying cognitive and behavioral abnormalities, such as the egocentricity and impaired empathy shown by Mr. L. These changes might not be detected in clinical interviews and over brief periods.2 Appreciating an acquired personality disturbance may require evaluating the patient’s behavior over months or years.2
In retrospect, Mr. L’s seeking repeated inpatient psychiatric hospitalizations is consistent with poor planning and problem-solving skills. He has a limited repertoire of adaptive behaviors and has learned that suicidal gestures lead to admission and caretaking. These are important to him because he is frequently homeless. His lack of insight is seen in his unrealistic plans for employment in jobs requiring specialized technical skills.
Mr. L’s case emphasizes the importance of considering brain injury as an etiologic factor in personality changes. It also highlights the complex—and seemingly nonoverlapping—functions and dysfunctions of the frontal lobe, including:
- source memory
- working memory
- sustained attention
- conceptual fluidity
- imaginative thinking
- impulse regulation
- planning and problem-solving skills.
Documenting Mr. L’s cognitive deficits and acquired dementia diagnosis changed our staff’s perception of his behavior, enabling us to overcome negative countertransference (Box 2). We no longer regarded him as deliberately manipulative and refer him for appropriate treatment.
Countertransference can interfere with optimal workup and treatment of patients with character changes related to traumatic brain injury and neurodegenerative processes. When we interpreted Mr. L’s suicidal gestures and hospitalizations as manipulative and deliberate, we failed to appreciate the limited number of things he could do to obtain a safe and protective environment. We also failed to recognize that his poor planning and problem-solving skills—as well as lack of insight into his illness—prevented him from adhering to outpatient treatment.
Originally, we attributed Mr. L’s egocentricity, lack of empathy, and lack of adherence to axis II pathology.
Our staff’s hostile feelings toward Mr. L led us to insufficiently consider his history—which is consistent with cognitive decline—during biopsychosocial evaluation and treatment planning. Mr. L’s status as a frequently homeless, unemployed person reflects a sharp decline for a highly educated person who served as a Navy officer and performed radiation inspections on nuclear-powered vessels.
Outome: Residential placement
We realize Mr. L needs cognitive rehabilitation—including assistance with planning and problem solving—and arrange for his placement in a residential facility for this specialized rehabilitation. Mr. L receives supportive psychotherapy and cognitive remediation from a psychologist. He also is involved in incentive work therapy with a vocational rehabilitation specialist.
Related resource
- Silver, JM, McAllister TW, Yudofsky SC, eds. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing; 2005.
- Divalproex • Depakote
- Quetiapine • Seroquel
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tranel D. Functional neuroanatomy: neuropsychological correlates of cortical and subcortical damage. In: Yudofsky SC, Hales RE, eds. The American Psychiatric Publishing textbook of neuropsychiatry and clinical neurosciences. 4th ed. Washington, DC: American Psychiatric Publishing; 2002:71-113.
2. Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol 2000;18(3):355-81.
3. Silver JM, Hales RE, Yudofsky SC. Neuropsychiatric aspects of traumatic brain injury. In: Yudofsky SC, Hales RE, eds. The American Psychiatric Publishing textbook of neuropsychiatry and clinical neurosciences. 4th ed. Washington, DC: American Psychiatric Publishing; 2002:625-72.
4. Damasio AR, Tranel D, Damasio HC. Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, eds. Frontal lobe function and dysfunction. Oxford, UK: Oxford University Press; 1991:217-29.
5. Prigatano GP. The relationship of frontal lobe damage to diminished awareness: studies in rehabilitation. In: Levin HS, Eisenberg HM, Benton AL, eds. Frontal lobe function and dysfunction. Oxford, UK: Oxford University Press; 1991:381-97.
CASE: An unlikable patient
Mr. L, age 56, is admitted to the psychiatric unit at our Veterans Affairs Medical Center for active suicidal ideation; he has a history of self-injurious behaviors that include mutilation and overdose. He also has a history of alcohol dependence and multiple inpatient psychiatric admissions. He has never married and conflicts with his siblings—in whose home he has been staying—have led to frequent homelessness.
His affect is silly and shallow. He also shows signs of haughtiness, disinhibition, grandiosity, and confabulation. For example, he says that while in the Navy he had 82 sexual exploits and developed a drug that cured herpes.
We start Mr. L on divalproex, 1,500 mg/d, and quetiapine, titrated to 200 mg/d. After 3 days he is discharged, but this begins a cycle of repeated suicide gestures and readmissions—9 within the next 3 months. Each time he is discharged, Mr. L fails to follow through on treatment recommendations and is indifferent to our staff’s annoyed reactions.
The author’s observations
Some of our staff members regard Mr. L’s suicidal gestures as manipulative and feel angry and demoralized by his poor adherence to outpatient treatment plans. Their negative countertransference might have impacted how they evaluated Mr. L through repeated admissions and discharges. During Mr. L’s ninth admission, we decide to reevaluate his longitudinal history for clues to his noncompliant behavior.
History: Undocumented injury
Mr. L says he began drinking alcohol at age 16. He reports that he has grown marijuana but has not smoked it since 1991. He denies using heroin or other drugs.
Mr. L states that he suffered a head injury in 1975 after falling off a ladder on a Navy ship. He describes losing consciousness for a brief but uncertain duration. He reports that he has developed a seizure disorder since this fall and a history of amnesia secondary to past seizures. His medical records contain no witnessed seizures. Mr. L also says he was hospitalized a few years ago and placed on a ventilator for 7 days for an undetermined reason.
The authors’ observations
Based on Mr. L’s report of a possible traumatic brain injury (TBI), we order a neurologic evaluation. A year earlier, MRI of the brain without contrast demonstrated minimal, nonspecific periventricular and subcortical, punctuate hyperintensities on flair and T2 weighted sequences that are nonspecific. Overall, the impression was “diffuse involutional changes and mild nonspecific periventricular and subcortical white matter hyperintensities,” which might reflect covert vascular brain injury.
Our frustration over Mr. L’s repeated readmissions for suicidal gestures led us to seek outside evaluation and consultation from a senior psychiatrist for assistance with discharge and treatment planning. Unlike our staff, the consulting psychiatrist did not harbor strong negative feelings toward the patient.
Mr. L’s history of deterioration in psychosocial functioning prompted this psychiatrist to perform a thorough mental status examination that focused on cognitive elements and request formal neuropsychological testing.
Evalutation: Cognitive Deficits
During mental status examination, Mr. L has difficulty recalling 3 items and uses a memory strategy to assist himself. He fails to recollect in reverse order the last 5 U.S. presidents. He spells “world” backward, but has difficulty repeating 6 digits forward and 4 backward. He is unable to do serial 7 subtractions from 93 to 65 correctly. He adequately copies interlocking pentagons and draws a clock with the correct time. He achieves a score of 28/30 on the Folstein Mini Mental State Exam, missing the date by 4 days and recalling 2 of 3 words.
These results suggest Mr. L has difficulty with attention and working memory, short-term memory, fund of general information and long-term memory, and ability to perform simple calculations. Most important, they indicate the need for further study, especially a neuropsychological test battery.
Mr. L’s abnormal neuropsychological test results are summarized in the Table. He manifests concretization of thought. His loss of conceptual fluidity is documented formally by measures of perseverative errors and categories completed on the Wisconsin Card Sorting Test (WCST). These findings support a diagnosis of acquired dementia.
Table
Abnormal findings on Mr. L’s neuropsychological testing
| Cognitive domain | Test | Score | Interpretation |
|---|---|---|---|
| Mental status and effort | |||
| Mental status | MMSE total score | 28/30 | 2 of 3 items recalled after delay |
| Orientation | MMSE orientation questions | 9/10 | Date off by 4 days |
| Premorbid IQ estimate | WRAT-4 Reading Standard | 66th percentile | Within normal limits. Inconsistent with educational attainment, but could be impacted by temporal lobe findings |
| Verbal memory | |||
| Immediate memory | RBANS Immediate Memory Index (List and Story Learning) | 1st percentile | Severe impairment |
| Delayed memory | RBANS Delayed Memory Index | 1st percentile | Severe impairment |
| Recognition memory | List Learning | Severe impairment | |
| Visuospatial memory | |||
| Delayed memory | RBANS Figure Recall | 3rd percentile | Severe impairment |
| Executive functioning | |||
| Cognitive flexibility | Trails B | 10th percentile | Severe impairment based on educational attainment |
| WCST | Low scores: Nonperseverative errors, perseverative errors, and categories completed | ||
| * Tests of mental status effort, visuomotor processing speed, confrontation naming, visuospatial function, attention, and executive functioning fluency/initiation were within normal limits | |||
| MMSE: Mini Mental State Exam; WRAT: Wide Range Achievement Test; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; WCST: Wisconsin Card Sorting Test | |||
The authors’ observations
Mr. L’s history, cognitive testing, head imaging, and behavioral observations suggest that several pathogenic factors contribute to his impaired functioning. First, he describes a TBI of unknown severity occurring in 1975. Although brain scans did not show evidence of midline shift or encephalomalacia, a direct blow to the head after falling from a height combined with possible post-injury seizures suggests a TBI of at least moderate severity.
Second, Mr. L describes an incident in which he required inpatient respiratory assistance. Although the precipitating medical event was unclear, anoxia or hypoxia is likely. A recent CT revealed low attenuation in the left temporal region that could represent an infarct.
Mr. L’s severe memory impairment and moderate to severe impairment in cognitive flexibility are commonly reported after a TBI of moderate severity. If an ischemic incident were the primary contributor, a lateralized pattern of cognitive dysfunction—which Mr. L does not exhibit—would be expected.
Although Mr. L likely has vascular dementia, his MRI findings do not indicate sufficient disease to account for his memory scores. Vascular dementia is associated with slow, stepwise cognitive deterioration, which is not consistent with severely impaired memory in a 56-year-old patient.
Finally, alcoholism is associated with cognitive difficulty in memory, visuospatial functioning, and abstract reasoning. Mr. L demonstrated significant difficulty in memory and abstract reasoning, but his visuospatial functioning was largely intact. In the absence of Wernicke’s encephalopathy, chronic alcoholics generally do not show memory decrements in line with Mr. L’s. His MRI results indicated only minimal ventricular and sulcal enlargement. Because atrophy is present in approximately 60% of chronic alcoholics, this finding provides evidence of a contribution, but the other contributory factors are associated with more definitive medical outcomes. Thus, alcoholism must be viewed as a secondary contributor to Mr. L’s impaired functioning.
Taking into account all known contributors, TBI emerges as the primary diagnosis.
Consider neurologic injury
Recognizing and characterizing personality changes related to neurologic injury and disease is often problematic and unreliable, even when psychometrically validated instruments and structured diagnostic interviews are used (Box 1).1-5 Mr. L’s presentation differed from the more commonly reported “impulsive aggression” associated with closed head injury. Sequelae from TBI were contributing to his clinical presentation but was obscured by his shallow and silly affect, inability to accurately assess social cues, and lack of empathy.
Mr. L reported suffering a head injury from falling off a ladder. Personality changes that result from traumatic brain injury (TBI) of the sudden deceleration type—even when mild—are frequently referable to the frontal lobe, especially focal orbital and/or ventromedial damage of the prefrontal cortex.1-5 This is because of the physical proximity of the sphenoid wing to the orbitofrontal region and effects of shearing.
As a result of this damage, patients lack insight into their accompanying cognitive and behavioral abnormalities, such as the egocentricity and impaired empathy shown by Mr. L. These changes might not be detected in clinical interviews and over brief periods.2 Appreciating an acquired personality disturbance may require evaluating the patient’s behavior over months or years.2
In retrospect, Mr. L’s seeking repeated inpatient psychiatric hospitalizations is consistent with poor planning and problem-solving skills. He has a limited repertoire of adaptive behaviors and has learned that suicidal gestures lead to admission and caretaking. These are important to him because he is frequently homeless. His lack of insight is seen in his unrealistic plans for employment in jobs requiring specialized technical skills.
Mr. L’s case emphasizes the importance of considering brain injury as an etiologic factor in personality changes. It also highlights the complex—and seemingly nonoverlapping—functions and dysfunctions of the frontal lobe, including:
- source memory
- working memory
- sustained attention
- conceptual fluidity
- imaginative thinking
- impulse regulation
- planning and problem-solving skills.
Documenting Mr. L’s cognitive deficits and acquired dementia diagnosis changed our staff’s perception of his behavior, enabling us to overcome negative countertransference (Box 2). We no longer regarded him as deliberately manipulative and refer him for appropriate treatment.
Countertransference can interfere with optimal workup and treatment of patients with character changes related to traumatic brain injury and neurodegenerative processes. When we interpreted Mr. L’s suicidal gestures and hospitalizations as manipulative and deliberate, we failed to appreciate the limited number of things he could do to obtain a safe and protective environment. We also failed to recognize that his poor planning and problem-solving skills—as well as lack of insight into his illness—prevented him from adhering to outpatient treatment.
Originally, we attributed Mr. L’s egocentricity, lack of empathy, and lack of adherence to axis II pathology.
Our staff’s hostile feelings toward Mr. L led us to insufficiently consider his history—which is consistent with cognitive decline—during biopsychosocial evaluation and treatment planning. Mr. L’s status as a frequently homeless, unemployed person reflects a sharp decline for a highly educated person who served as a Navy officer and performed radiation inspections on nuclear-powered vessels.
Outome: Residential placement
We realize Mr. L needs cognitive rehabilitation—including assistance with planning and problem solving—and arrange for his placement in a residential facility for this specialized rehabilitation. Mr. L receives supportive psychotherapy and cognitive remediation from a psychologist. He also is involved in incentive work therapy with a vocational rehabilitation specialist.
Related resource
- Silver, JM, McAllister TW, Yudofsky SC, eds. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing; 2005.
- Divalproex • Depakote
- Quetiapine • Seroquel
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: An unlikable patient
Mr. L, age 56, is admitted to the psychiatric unit at our Veterans Affairs Medical Center for active suicidal ideation; he has a history of self-injurious behaviors that include mutilation and overdose. He also has a history of alcohol dependence and multiple inpatient psychiatric admissions. He has never married and conflicts with his siblings—in whose home he has been staying—have led to frequent homelessness.
His affect is silly and shallow. He also shows signs of haughtiness, disinhibition, grandiosity, and confabulation. For example, he says that while in the Navy he had 82 sexual exploits and developed a drug that cured herpes.
We start Mr. L on divalproex, 1,500 mg/d, and quetiapine, titrated to 200 mg/d. After 3 days he is discharged, but this begins a cycle of repeated suicide gestures and readmissions—9 within the next 3 months. Each time he is discharged, Mr. L fails to follow through on treatment recommendations and is indifferent to our staff’s annoyed reactions.
The author’s observations
Some of our staff members regard Mr. L’s suicidal gestures as manipulative and feel angry and demoralized by his poor adherence to outpatient treatment plans. Their negative countertransference might have impacted how they evaluated Mr. L through repeated admissions and discharges. During Mr. L’s ninth admission, we decide to reevaluate his longitudinal history for clues to his noncompliant behavior.
History: Undocumented injury
Mr. L says he began drinking alcohol at age 16. He reports that he has grown marijuana but has not smoked it since 1991. He denies using heroin or other drugs.
Mr. L states that he suffered a head injury in 1975 after falling off a ladder on a Navy ship. He describes losing consciousness for a brief but uncertain duration. He reports that he has developed a seizure disorder since this fall and a history of amnesia secondary to past seizures. His medical records contain no witnessed seizures. Mr. L also says he was hospitalized a few years ago and placed on a ventilator for 7 days for an undetermined reason.
The authors’ observations
Based on Mr. L’s report of a possible traumatic brain injury (TBI), we order a neurologic evaluation. A year earlier, MRI of the brain without contrast demonstrated minimal, nonspecific periventricular and subcortical, punctuate hyperintensities on flair and T2 weighted sequences that are nonspecific. Overall, the impression was “diffuse involutional changes and mild nonspecific periventricular and subcortical white matter hyperintensities,” which might reflect covert vascular brain injury.
Our frustration over Mr. L’s repeated readmissions for suicidal gestures led us to seek outside evaluation and consultation from a senior psychiatrist for assistance with discharge and treatment planning. Unlike our staff, the consulting psychiatrist did not harbor strong negative feelings toward the patient.
Mr. L’s history of deterioration in psychosocial functioning prompted this psychiatrist to perform a thorough mental status examination that focused on cognitive elements and request formal neuropsychological testing.
Evalutation: Cognitive Deficits
During mental status examination, Mr. L has difficulty recalling 3 items and uses a memory strategy to assist himself. He fails to recollect in reverse order the last 5 U.S. presidents. He spells “world” backward, but has difficulty repeating 6 digits forward and 4 backward. He is unable to do serial 7 subtractions from 93 to 65 correctly. He adequately copies interlocking pentagons and draws a clock with the correct time. He achieves a score of 28/30 on the Folstein Mini Mental State Exam, missing the date by 4 days and recalling 2 of 3 words.
These results suggest Mr. L has difficulty with attention and working memory, short-term memory, fund of general information and long-term memory, and ability to perform simple calculations. Most important, they indicate the need for further study, especially a neuropsychological test battery.
Mr. L’s abnormal neuropsychological test results are summarized in the Table. He manifests concretization of thought. His loss of conceptual fluidity is documented formally by measures of perseverative errors and categories completed on the Wisconsin Card Sorting Test (WCST). These findings support a diagnosis of acquired dementia.
Table
Abnormal findings on Mr. L’s neuropsychological testing
| Cognitive domain | Test | Score | Interpretation |
|---|---|---|---|
| Mental status and effort | |||
| Mental status | MMSE total score | 28/30 | 2 of 3 items recalled after delay |
| Orientation | MMSE orientation questions | 9/10 | Date off by 4 days |
| Premorbid IQ estimate | WRAT-4 Reading Standard | 66th percentile | Within normal limits. Inconsistent with educational attainment, but could be impacted by temporal lobe findings |
| Verbal memory | |||
| Immediate memory | RBANS Immediate Memory Index (List and Story Learning) | 1st percentile | Severe impairment |
| Delayed memory | RBANS Delayed Memory Index | 1st percentile | Severe impairment |
| Recognition memory | List Learning | Severe impairment | |
| Visuospatial memory | |||
| Delayed memory | RBANS Figure Recall | 3rd percentile | Severe impairment |
| Executive functioning | |||
| Cognitive flexibility | Trails B | 10th percentile | Severe impairment based on educational attainment |
| WCST | Low scores: Nonperseverative errors, perseverative errors, and categories completed | ||
| * Tests of mental status effort, visuomotor processing speed, confrontation naming, visuospatial function, attention, and executive functioning fluency/initiation were within normal limits | |||
| MMSE: Mini Mental State Exam; WRAT: Wide Range Achievement Test; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; WCST: Wisconsin Card Sorting Test | |||
The authors’ observations
Mr. L’s history, cognitive testing, head imaging, and behavioral observations suggest that several pathogenic factors contribute to his impaired functioning. First, he describes a TBI of unknown severity occurring in 1975. Although brain scans did not show evidence of midline shift or encephalomalacia, a direct blow to the head after falling from a height combined with possible post-injury seizures suggests a TBI of at least moderate severity.
Second, Mr. L describes an incident in which he required inpatient respiratory assistance. Although the precipitating medical event was unclear, anoxia or hypoxia is likely. A recent CT revealed low attenuation in the left temporal region that could represent an infarct.
Mr. L’s severe memory impairment and moderate to severe impairment in cognitive flexibility are commonly reported after a TBI of moderate severity. If an ischemic incident were the primary contributor, a lateralized pattern of cognitive dysfunction—which Mr. L does not exhibit—would be expected.
Although Mr. L likely has vascular dementia, his MRI findings do not indicate sufficient disease to account for his memory scores. Vascular dementia is associated with slow, stepwise cognitive deterioration, which is not consistent with severely impaired memory in a 56-year-old patient.
Finally, alcoholism is associated with cognitive difficulty in memory, visuospatial functioning, and abstract reasoning. Mr. L demonstrated significant difficulty in memory and abstract reasoning, but his visuospatial functioning was largely intact. In the absence of Wernicke’s encephalopathy, chronic alcoholics generally do not show memory decrements in line with Mr. L’s. His MRI results indicated only minimal ventricular and sulcal enlargement. Because atrophy is present in approximately 60% of chronic alcoholics, this finding provides evidence of a contribution, but the other contributory factors are associated with more definitive medical outcomes. Thus, alcoholism must be viewed as a secondary contributor to Mr. L’s impaired functioning.
Taking into account all known contributors, TBI emerges as the primary diagnosis.
Consider neurologic injury
Recognizing and characterizing personality changes related to neurologic injury and disease is often problematic and unreliable, even when psychometrically validated instruments and structured diagnostic interviews are used (Box 1).1-5 Mr. L’s presentation differed from the more commonly reported “impulsive aggression” associated with closed head injury. Sequelae from TBI were contributing to his clinical presentation but was obscured by his shallow and silly affect, inability to accurately assess social cues, and lack of empathy.
Mr. L reported suffering a head injury from falling off a ladder. Personality changes that result from traumatic brain injury (TBI) of the sudden deceleration type—even when mild—are frequently referable to the frontal lobe, especially focal orbital and/or ventromedial damage of the prefrontal cortex.1-5 This is because of the physical proximity of the sphenoid wing to the orbitofrontal region and effects of shearing.
As a result of this damage, patients lack insight into their accompanying cognitive and behavioral abnormalities, such as the egocentricity and impaired empathy shown by Mr. L. These changes might not be detected in clinical interviews and over brief periods.2 Appreciating an acquired personality disturbance may require evaluating the patient’s behavior over months or years.2
In retrospect, Mr. L’s seeking repeated inpatient psychiatric hospitalizations is consistent with poor planning and problem-solving skills. He has a limited repertoire of adaptive behaviors and has learned that suicidal gestures lead to admission and caretaking. These are important to him because he is frequently homeless. His lack of insight is seen in his unrealistic plans for employment in jobs requiring specialized technical skills.
Mr. L’s case emphasizes the importance of considering brain injury as an etiologic factor in personality changes. It also highlights the complex—and seemingly nonoverlapping—functions and dysfunctions of the frontal lobe, including:
- source memory
- working memory
- sustained attention
- conceptual fluidity
- imaginative thinking
- impulse regulation
- planning and problem-solving skills.
Documenting Mr. L’s cognitive deficits and acquired dementia diagnosis changed our staff’s perception of his behavior, enabling us to overcome negative countertransference (Box 2). We no longer regarded him as deliberately manipulative and refer him for appropriate treatment.
Countertransference can interfere with optimal workup and treatment of patients with character changes related to traumatic brain injury and neurodegenerative processes. When we interpreted Mr. L’s suicidal gestures and hospitalizations as manipulative and deliberate, we failed to appreciate the limited number of things he could do to obtain a safe and protective environment. We also failed to recognize that his poor planning and problem-solving skills—as well as lack of insight into his illness—prevented him from adhering to outpatient treatment.
Originally, we attributed Mr. L’s egocentricity, lack of empathy, and lack of adherence to axis II pathology.
Our staff’s hostile feelings toward Mr. L led us to insufficiently consider his history—which is consistent with cognitive decline—during biopsychosocial evaluation and treatment planning. Mr. L’s status as a frequently homeless, unemployed person reflects a sharp decline for a highly educated person who served as a Navy officer and performed radiation inspections on nuclear-powered vessels.
Outome: Residential placement
We realize Mr. L needs cognitive rehabilitation—including assistance with planning and problem solving—and arrange for his placement in a residential facility for this specialized rehabilitation. Mr. L receives supportive psychotherapy and cognitive remediation from a psychologist. He also is involved in incentive work therapy with a vocational rehabilitation specialist.
Related resource
- Silver, JM, McAllister TW, Yudofsky SC, eds. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing; 2005.
- Divalproex • Depakote
- Quetiapine • Seroquel
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tranel D. Functional neuroanatomy: neuropsychological correlates of cortical and subcortical damage. In: Yudofsky SC, Hales RE, eds. The American Psychiatric Publishing textbook of neuropsychiatry and clinical neurosciences. 4th ed. Washington, DC: American Psychiatric Publishing; 2002:71-113.
2. Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol 2000;18(3):355-81.
3. Silver JM, Hales RE, Yudofsky SC. Neuropsychiatric aspects of traumatic brain injury. In: Yudofsky SC, Hales RE, eds. The American Psychiatric Publishing textbook of neuropsychiatry and clinical neurosciences. 4th ed. Washington, DC: American Psychiatric Publishing; 2002:625-72.
4. Damasio AR, Tranel D, Damasio HC. Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, eds. Frontal lobe function and dysfunction. Oxford, UK: Oxford University Press; 1991:217-29.
5. Prigatano GP. The relationship of frontal lobe damage to diminished awareness: studies in rehabilitation. In: Levin HS, Eisenberg HM, Benton AL, eds. Frontal lobe function and dysfunction. Oxford, UK: Oxford University Press; 1991:381-97.
1. Tranel D. Functional neuroanatomy: neuropsychological correlates of cortical and subcortical damage. In: Yudofsky SC, Hales RE, eds. The American Psychiatric Publishing textbook of neuropsychiatry and clinical neurosciences. 4th ed. Washington, DC: American Psychiatric Publishing; 2002:71-113.
2. Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol 2000;18(3):355-81.
3. Silver JM, Hales RE, Yudofsky SC. Neuropsychiatric aspects of traumatic brain injury. In: Yudofsky SC, Hales RE, eds. The American Psychiatric Publishing textbook of neuropsychiatry and clinical neurosciences. 4th ed. Washington, DC: American Psychiatric Publishing; 2002:625-72.
4. Damasio AR, Tranel D, Damasio HC. Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, eds. Frontal lobe function and dysfunction. Oxford, UK: Oxford University Press; 1991:217-29.
5. Prigatano GP. The relationship of frontal lobe damage to diminished awareness: studies in rehabilitation. In: Levin HS, Eisenberg HM, Benton AL, eds. Frontal lobe function and dysfunction. Oxford, UK: Oxford University Press; 1991:381-97.
Perimenopausal depression: Covering mood and vasomotor symptoms
Symptoms of perimenopausal depression are not inherently different from those of depression diagnosed at any other time in life, but they present in a unique context:
- Hormonal fluctuations may persist for a long duration.
- Women experiencing hormonal fluctuations may be vulnerable to mood problems.
- Psychosocial/psychodynamic stressors often complicate this life transition.
Managing perimenopausal depression has become more complicated since the Women’s Health Initiative (WHI) studies found fewer benefits and greater risks with hormone replacement therapy (HRT) than had been perceived. This article discusses the clinical presentation of perimenopausal depression, its risk factors, and treatment options in post-WHI psychiatric practice.
Who is at risk?
Perimenopausal depression is diagnosed when onset of major depressive disorder (MDD) is associated with menstrual cycle irregularity and/or somatic symptoms of the menopausal transition.1 Diagnosis is based on the overall clinical picture, and treatment requires a thoughtful exploration of the complex relationship between hormonal function and mood regulation.
Presentation. For many women, perimenopause is characterized by mild to severe vasomotor, cognitive, and mood symptoms (Table 1). Thus, in your workup of depression in midlife women, document somatic symptoms—such as hot flushes, vaginal dryness, and incontinence—and affective/behavioral symp toms such as mood and sleep disturbances.
Table 1
Vasomotor, cognitive, and mood symptoms of perimenopause
| Vasomotor | Cognitive and mood |
|---|---|
| Hot flushes | Decreased concentration |
| Sweating | Anxiety |
| Heart palpitations | Irritability |
| Painful intercourse | Mood lability |
| Vaginal dryness and discomfort | Memory difficulty |
| Sleep disruption | |
| Headache |
Explore psychiatric and medical histories of your patient and her close relatives. Ask about depression, dysthymia, hypomania, or mood fluctuations around hormonal events such as menses, pregnancy, postpartum, or starting/stopping oral contraceptives. In the differential diagnosis, consider:
- Is low mood temporally connected with hot flushes and disturbed sleep?
- Is low mood secondary to stressful life events?
- Does the patient have another medical illness (such as thyroid disorder) with symptoms similar to depression?
- Is low mood secondary to anxiety or another psychiatric disorder?
Screening. Menopause is considered to have been reached after 12 months of amenorrhea not due to another cause. Median ages for this transition in the United States are 47.5 for perimenopause and 51 for menopause, with an average of 8 years between regular cycles and amenorrhea.2 Therefore, begin talking with women about perimenopausal symptoms when they turn 40.
Evidence supports screening perimenopausal women for depressive symptoms even when their primary complaints are vasomotor. The Greene Climacteric Scale3 is convenient for quantifying and monitoring perimenopausal symptoms. It includes depressive symptoms plus physical and cognitive markers. The Quick Inventory of Depressive Symptomatology—Self Report (QIDS-SR)4 questionnaire:
- takes minutes to complete
- is easy to score
- quantitates the number and severity of depressive symptoms (see Related Resources).
Psychosocial factors can predict depression at any time in life, but some are specific to the menopausal transition (Table 2).5 The “empty nest syndrome,” for example, is often used to explain depressive symptoms in midlife mothers, but no evidence links mood lability with the maturation and departure of children. What may be more stressing for women is supporting adolescents/young adults in their exit to independence while caring for aging parents.
Table 2
Risk factors for depression in women
| Predictive over lifetime | High risk during menopausal transition |
|---|---|
| History of depression | History of PMS, perinatal depression, mood symptoms associated with contraceptives |
| Family history of affective disorders | Premature or surgical menopause |
| Insomnia | Lengthy menopausal transition (≥27 months) |
| Reduced physical activities | Persistent and/or severe vasomotor symptoms |
| Weight gain | Negative attitudes toward menopause and aging |
| Less education | |
| Perceived lower economic status | |
| Perceived lower social support | |
| Perceived lower health status | |
| Smoking | |
| Stressful life events | |
| History of trauma | |
| Marital dissatisfaction | |
| PMS: Premenstrual syndrome | |
Sociocultural beliefs about sexuality and menopause may play a role in how your patient experiences and reports her symptoms. In some cultures, menopause elevates a woman’s social status and is associated with increased respect and authority. In others, such as Western societies that emphasize youth and beauty, women may view menopause and its physical changes in a negative light.6
Therefore, give careful attention to the psychosocial context of menopause to your patient and the social resources available to her. Questions to ask include:
- Has your lifestyle changed recently?
- Have your husband, family members, or close friends noticed any changes in your functioning?
- Is there anyone in your life that you feel comfortable confiding in?
Explaining the complexity of this life transition may ease her anxiety by normalizing her experience, helping her understand her symptoms, and validating her distress.
What might be the cause?
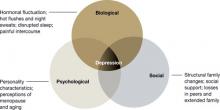
Although the exact pathophysiology of perimenopausal depression is unknown, hormonal changes,7 general health, the experience of menopause,8 and the psychosocial context2 likely work together to increase vulnerability for depressive symptoms (Figure).
Figure Biopsychosocial milieu of depression during perimenopauseHormonal fluctuation. The estrogen withdrawal theory7 explains depressive symptoms as resulting from a sustained decline in ovarian estrogen in tandem with spiking secretions of follicle-stimulating hormone by the pituitary. The finding that women with surgical menopause have a higher incidence of depressive symptoms than women with natural menopause supports this hypothesis.
Mood disorders occur across various female reproductive events, and increased risk appears to be associated with fluctuating gonadal hormones. Thus, declining estrogen may be less causative of perimenopausal depression than extreme fluctuations in estradiol activity.9,10
Estrogen interacts with dopamine, norepinephrine, beta-endorphin, and serotonin metabolism. In particular, estrogen facilitates serotonin delivery to neurons across the brain. These findings—and the success of selective serotonin reuptake inhibitors (SSRIs) in treating mood disorders—support the theory that fluctuating estrogen affects the serotonergic system and may cause depressive symptoms.
‘Domino theory.’ Others have hypothesized that depressive symptoms are the secondhand result of somatic symptoms of perimenopause. In a “domino effect,” hot flushes and night sweats disrupt women’s sleep, bringing fatigue and impaired daytime concentration, which lead to irritability and feelings of being overwhelmed.8
This theory, which incorporates perimenopausal hormone changes, is supported by elevated levels of depression in women who report frequent and intense vasomotor symptoms persisting >27 months.2
The psychosocial theory suggests that depression results from increased stress or adverse events.2 Midlife women with depressive symptoms report many possible sources of stress:
- demanding jobs
- family responsibilities
- dual demands of career and family
- little time for self
- poverty or employment stressors
- not enough sleep
- changing social relationships.
Negative interpretations of aging or the menopausal transition also have been implicated in cross-cultural studies.6 The predictive nature of psychosocial issues for depression during perimenopause supports this theory.
Evidence-based treatment
HRT. Research and clinical reports suggest that estrogen may have antidepressant effects, either alone or as an adjunct to antidepressant medication.11 Before the WHI studies, expert consensus guidelines on treating depression in women recommended HRT as first-line treatment for patients experiencing a first lifetime onset of mild to moderate depression during perimenopause.12 WHI findings since 2002 that associated HRT with increased risk of stroke, deep vein thrombosis, and pulmonary embolism—without clear protection against coronary heart disease or cognitive decline—have left HRT a controversial option for treating perimenopausal depression. In the WHI trials:
- 10,739 postmenopausal women age 50 to 79 without a uterus received unopposed conjugated equine estrogens, 0.625 mg/d, or placebo for an average 6.8 years.13
- 16,608 postmenopausal women age 50 to 79 with an intact uterus received combination HRT (conjugated equine estrogens, 0.625 mg/d, plus 2.5 mg of medroxyprogesterone), or placebo for an average 5.6 years.14
The study using combination HRT found increased risks of breast cancer, ischemic stroke, blood clots, and coronary heart disease.15 A follow-up study showed that vasomotor symptoms returned in more than one-half the women after they stopped using combination HRT.15
A companion WHI trial found that estrogen, 0.625 mg/d—given unopposed or with a progestin—did not prevent cognitive decline in women age 65 to 79 and may have been associated with a slightly greater risk of probable dementia.16,17
The FDA recommends that women who want to use HRT to control menopausal symptoms use the lowest effective dose for the shortest time necessary.18
Antidepressants. SSRIs may be more useful than estrogen for producing MDD remission in perimenopausal women.19 SSRIs and other psychotropics may reduce perimenopausal vasomotor symptoms in addition to addressing depressive symptoms (Table 3). When choosing antidepressant therapy, consider the patient’s dominant presenting perimenopausal symptoms and side effects associated with treatment.20
Table 3
Nonhormone medications for perimenopausal depression: Evidence-based dosages and target symptoms
| Medication | Dosage effective for perimenopausal depression | Symptoms assessed |
|---|---|---|
| SSRIs | ||
| Citaloprama | 40 to 60 mg | Depressive and vasomotor |
| Escitalopramb,c | 5 to 20 mg | Depressive and vasomotor |
| Fluoxetined | 20 to 40 mg | Depressive and vasomotor |
| Paroxetinee,f | 12.5 or 25 mg | Depressive and vasomotor |
| Sertralineg | 100 mg | Depressive and vasomotor |
| Other antidepressants | ||
| Duloxetineh | 60 to 120 mg | Depressive and vasomotor |
| Venlafaxinei | 75 to 225 mg | Depressive and vasomotor |
| Mirtazapinej | 30 to 60 mg | Severe depressive symptoms; used as an adjunct to estrogen |
| Hypnotics | ||
| Eszopiclonek | 3 mg | Depressive and vasomotor; insomnia |
| Zolpideml | 5 to 10 mg | Insomnia |
| Anticonvulsant | ||
| Gabapentinm | 300 to 900 mg | Vasomotor |
| SSRIs: selective serotonin reuptake inhibitors | ||
| Source: Reference Citations | ||
Nonpharmacologic interventions are viable options for women who are reluctant to begin HRT or psychotropics.
Psychotherapy. Interpersonal psychotherapy (IPT) and cognitive-behavioral therapy (CBT) have been recommended to address psychosocial elements of perimenopausal mood lability.21 For women with climacteric depression, IPT focuses on role transitions, loss, and interpersonal support, whereas CBT focuses on identifying and altering negative thoughts and beliefs.
Although no randomized trials have examined psychotherapies for perimenopausal depression, a pilot open trial provided group CBT—psychoeducation, group discussion, and coping skills training—to 30 women with climacteric symptoms. Anxiety, depression, partnership relations, overall sexuality, hot flushes, and cardiac complaints improved significantly, based on pre- and post-intervention surveys. Sexual satisfaction and the stressfulness of menopausal symptoms did not change.22
Integrative medicine. Plant-based substances and herbal remedies such as phytoestrogens, red-clover isoflavones, black cohosh, and evening primrose oil have been included in a few research investigations, and the evidence is equivocal. Because of potential interactions between alternative therapies and medications, inquire about their use. Although a comprehensive review of integrative medicine for perimenopausal symptoms is beyond the scope of this article, see suggested readings (Box).
- Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric 2007;10(suppl 2):115-20.
- Blair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause 2008;15:32-43.
- Freeman MP, Helgason C, Hill RA. Selected integrative medicine treatments for depression: considerations for women. J Am Med Womens Assoc 2004;59(3):216-24.
- Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Psychiatr Clin North Am 2007;30:51-68.
- Thachil AF, Mohan R, Bhugra D. The evidence base of complementary and alternative therapies in depression. J Affect Disord 2007;97:23-35.
- Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. J North Am Menopause Soc 2008;15:193-202.
Clinical recommendations
Explore options with your patient; discuss side effects, risks, and expected minimum duration of treatment. Antidepressants, hormonal therapies, psychotherapy, and complementary and alternative treatments each might have a role in managing perimenopausal depression. A patient’s preferences, psychiatric history, and depression severity help determine which options to consider and in what order. How she responded to past treatments also can help you individualize a plan.
HRT may be appropriate for women who express a preference for HRT, have responded well to past hormone therapy, and have no personal history or high-risk factors for breast cancer. Based on the WHI findings, we consider a history of breast cancer in the patient or a first- or second-degree relative a contraindication to HRT.
Estrogen can be used alone or with an antidepressant. Studies support 17β-estradiol, 0.1 to 0.3 mg/d, for 8 to 12 weeks.11,23 Concomitant progesterone may be indicated to offset the effects of unopposed estrogen in women with an intact uterus. This option calls for an informed discussion with the patient about risks and benefits.
No data support long-term use of estrogen for recurrent or chronic depression. Because HRT’s risks and benefits vary with the length of exposure, individualize the extended use of estrogen solely to augment treatment for depression. Because vasomotor symptoms may recur when HRT is discontinued,15 we recommend that women make an informed decision in consultation with a gynecologist or primary care physician.
Antidepressants that have serotonergic activity—such as SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)—appear most promising for treating comorbid depressive and vasomotor symptoms. If a patient has had a good response to an antidepressant in the past, consider starting with that medication.
Common antidepressant side effects are difficult to assess in perimenopausal patients with MDD because the symptoms attributed to antidepressant side effects—such as low libido, sleep disturbance, and weight changes—also can be caused by mood disorders and hormonal changes. Therefore, inquire about these symptoms when you initiate antidepressant therapy and at follow-up assessments.
Psychotherapy. We recommend that all women who present with perimenopausal depression receive information about psychotherapy. Psychotherapy alone often is adequate for mild depression, and adding psychotherapy to antidepressant treatment usually enhances recovery from moderate and severe depression episodes. In addition, patients who engage in psychotherapy for depression may have a lower rate of relapse.24
Individual psychotherapy can help patients with perimenopausal depression:
- accept this life transition
- recognize the benefits of menopause, such as no need for contraception
- develop awareness of personal potential in the years ahead.
Because depression often occurs in an interpersonal context, consider including family members in psychotherapy to improve the patient’s interpersonal support.
Integrative therapies. A full evaluation and consideration of standard treatment options is indicated for all women with MDD. Integrative medicine appeals to many patients but has not been sufficiently studied for perimenopausal depression. Supplemental omega-3 fatty acids and folate are reasonable adjuncts to the treatment of MDD25-27 and deserve study in perimenopausal MDD.
- Greene Climacteric Scale. www.menopausematters.co.uk/greenescale.php.
- Quick Inventory of Depressive Symptomatology. www.idsqids.org.
- National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov.
Drug brand names
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Estradiol • various
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
Dr. Brandon and Dr. Shivakumar report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman receives research support from GlaxoSmithKline, Forest Pharmaceuticals, and Eli Lilly and Company. She was associate professor of psychiatry at the University of Texas Southwestern Medical Center at Dallas when this article was written and is now on the faculty at Harvard Medical School and Massachusetts General Hospital, Boston.
1. Schmidt PJ, Rubinow DR. Reproductive ageing, sex steroids and depression. J Br Menopause Soc 2006;12(4):178-85.
2. Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr 2005;10(6):471-8.
3. Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29(1):25-31.
4. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54(5):573-83.Erratum in: Biol Psychiatry. 2003;54(5):585.
5. Feld J, Halbreich U, Karkun S. The association of perimenopausal mood disorders with other reproductive-related disorders. CNS Spectr 2005;10(6):461-70.
6. Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 2001;52:345-56.
7. Campbell S, Whitehead M. Oestrogen therapy and the menopause syndrome. Clin Obstet Gynecol 1977;4:31-47.
8. Schmidt PJ, Rubinow DR. Menopause-related affective disorders: a justification for further study. Am J Psychiatry 1991;48:844-52.
9. Soares CN. Menopausal transition and depression: who is at risk and how to treat it? Expert Rev Neurother 2007;7(10):1285-93.
10. Prior JC. The complex endocrinology of menopausal transition. Endocrinol Rev 1998;19:397-428.
11. Rasgon N, Altshuler LL, Fairbanks LA, et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry 2002;63(suppl):45-8.
12. Altshuler LL, Cohen LS, Moline ML, et al. The Expert Consensus Guideline Series. Treatment of depression in women. Postgrad Med 2001 Mar;(Spec No):1-107.
13. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701-12.
14. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
15. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA 2005;294:183-93.
16. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947-58.
17. Shumaker SA, Legault C, Rapp SR. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651-62.
18. FDA approves new labeling and provides new advice to postmenopausal women who use or who are considering using estrogen and estrogen with progestin [FDA Fact Sheet January 8, 2003]. Available at: http://www.fda.gov/oc/factsheets/WHI.html. Accessed September 11, 2008.
19. Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13(5):780-6.
20. Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med 2005;118(suppl 12B):93-7.
21. Kahn DA, Moline ML, Ross RW, et al. Depression during the transition to menopause: a guide for patients and families. Postgrad Med 2001 Mar;(Spec No):110-1.
22. Alder J, Besken KE, Armbruster U, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychother Psychosom 2006;75(5):298-303.
23. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
24. Otto MW, Smits JA, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science and Practice 2005;12:72-86.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomized, placebo controlled trial. J Affect Disord 2000;60:121-30.
26. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [American Psychiatric Association subcommittee report]. J Clin Psychiatry 2006;67:1954-67.
27. Otto MW, Church TS, Craft LL, et al. Exercise for mood and anxiety disorders. J Clin Psychiatry 2007;68:669-76.
Symptoms of perimenopausal depression are not inherently different from those of depression diagnosed at any other time in life, but they present in a unique context:
- Hormonal fluctuations may persist for a long duration.
- Women experiencing hormonal fluctuations may be vulnerable to mood problems.
- Psychosocial/psychodynamic stressors often complicate this life transition.
Managing perimenopausal depression has become more complicated since the Women’s Health Initiative (WHI) studies found fewer benefits and greater risks with hormone replacement therapy (HRT) than had been perceived. This article discusses the clinical presentation of perimenopausal depression, its risk factors, and treatment options in post-WHI psychiatric practice.
Who is at risk?
Perimenopausal depression is diagnosed when onset of major depressive disorder (MDD) is associated with menstrual cycle irregularity and/or somatic symptoms of the menopausal transition.1 Diagnosis is based on the overall clinical picture, and treatment requires a thoughtful exploration of the complex relationship between hormonal function and mood regulation.
Presentation. For many women, perimenopause is characterized by mild to severe vasomotor, cognitive, and mood symptoms (Table 1). Thus, in your workup of depression in midlife women, document somatic symptoms—such as hot flushes, vaginal dryness, and incontinence—and affective/behavioral symp toms such as mood and sleep disturbances.
Table 1
Vasomotor, cognitive, and mood symptoms of perimenopause
| Vasomotor | Cognitive and mood |
|---|---|
| Hot flushes | Decreased concentration |
| Sweating | Anxiety |
| Heart palpitations | Irritability |
| Painful intercourse | Mood lability |
| Vaginal dryness and discomfort | Memory difficulty |
| Sleep disruption | |
| Headache |
Explore psychiatric and medical histories of your patient and her close relatives. Ask about depression, dysthymia, hypomania, or mood fluctuations around hormonal events such as menses, pregnancy, postpartum, or starting/stopping oral contraceptives. In the differential diagnosis, consider:
- Is low mood temporally connected with hot flushes and disturbed sleep?
- Is low mood secondary to stressful life events?
- Does the patient have another medical illness (such as thyroid disorder) with symptoms similar to depression?
- Is low mood secondary to anxiety or another psychiatric disorder?
Screening. Menopause is considered to have been reached after 12 months of amenorrhea not due to another cause. Median ages for this transition in the United States are 47.5 for perimenopause and 51 for menopause, with an average of 8 years between regular cycles and amenorrhea.2 Therefore, begin talking with women about perimenopausal symptoms when they turn 40.
Evidence supports screening perimenopausal women for depressive symptoms even when their primary complaints are vasomotor. The Greene Climacteric Scale3 is convenient for quantifying and monitoring perimenopausal symptoms. It includes depressive symptoms plus physical and cognitive markers. The Quick Inventory of Depressive Symptomatology—Self Report (QIDS-SR)4 questionnaire:
- takes minutes to complete
- is easy to score
- quantitates the number and severity of depressive symptoms (see Related Resources).
Psychosocial factors can predict depression at any time in life, but some are specific to the menopausal transition (Table 2).5 The “empty nest syndrome,” for example, is often used to explain depressive symptoms in midlife mothers, but no evidence links mood lability with the maturation and departure of children. What may be more stressing for women is supporting adolescents/young adults in their exit to independence while caring for aging parents.
Table 2
Risk factors for depression in women
| Predictive over lifetime | High risk during menopausal transition |
|---|---|
| History of depression | History of PMS, perinatal depression, mood symptoms associated with contraceptives |
| Family history of affective disorders | Premature or surgical menopause |
| Insomnia | Lengthy menopausal transition (≥27 months) |
| Reduced physical activities | Persistent and/or severe vasomotor symptoms |
| Weight gain | Negative attitudes toward menopause and aging |
| Less education | |
| Perceived lower economic status | |
| Perceived lower social support | |
| Perceived lower health status | |
| Smoking | |
| Stressful life events | |
| History of trauma | |
| Marital dissatisfaction | |
| PMS: Premenstrual syndrome | |
Sociocultural beliefs about sexuality and menopause may play a role in how your patient experiences and reports her symptoms. In some cultures, menopause elevates a woman’s social status and is associated with increased respect and authority. In others, such as Western societies that emphasize youth and beauty, women may view menopause and its physical changes in a negative light.6
Therefore, give careful attention to the psychosocial context of menopause to your patient and the social resources available to her. Questions to ask include:
- Has your lifestyle changed recently?
- Have your husband, family members, or close friends noticed any changes in your functioning?
- Is there anyone in your life that you feel comfortable confiding in?
Explaining the complexity of this life transition may ease her anxiety by normalizing her experience, helping her understand her symptoms, and validating her distress.
What might be the cause?
Although the exact pathophysiology of perimenopausal depression is unknown, hormonal changes,7 general health, the experience of menopause,8 and the psychosocial context2 likely work together to increase vulnerability for depressive symptoms (Figure).
Figure Biopsychosocial milieu of depression during perimenopauseHormonal fluctuation. The estrogen withdrawal theory7 explains depressive symptoms as resulting from a sustained decline in ovarian estrogen in tandem with spiking secretions of follicle-stimulating hormone by the pituitary. The finding that women with surgical menopause have a higher incidence of depressive symptoms than women with natural menopause supports this hypothesis.
Mood disorders occur across various female reproductive events, and increased risk appears to be associated with fluctuating gonadal hormones. Thus, declining estrogen may be less causative of perimenopausal depression than extreme fluctuations in estradiol activity.9,10
Estrogen interacts with dopamine, norepinephrine, beta-endorphin, and serotonin metabolism. In particular, estrogen facilitates serotonin delivery to neurons across the brain. These findings—and the success of selective serotonin reuptake inhibitors (SSRIs) in treating mood disorders—support the theory that fluctuating estrogen affects the serotonergic system and may cause depressive symptoms.
‘Domino theory.’ Others have hypothesized that depressive symptoms are the secondhand result of somatic symptoms of perimenopause. In a “domino effect,” hot flushes and night sweats disrupt women’s sleep, bringing fatigue and impaired daytime concentration, which lead to irritability and feelings of being overwhelmed.8
This theory, which incorporates perimenopausal hormone changes, is supported by elevated levels of depression in women who report frequent and intense vasomotor symptoms persisting >27 months.2
The psychosocial theory suggests that depression results from increased stress or adverse events.2 Midlife women with depressive symptoms report many possible sources of stress:
- demanding jobs
- family responsibilities
- dual demands of career and family
- little time for self
- poverty or employment stressors
- not enough sleep
- changing social relationships.
Negative interpretations of aging or the menopausal transition also have been implicated in cross-cultural studies.6 The predictive nature of psychosocial issues for depression during perimenopause supports this theory.
Evidence-based treatment
HRT. Research and clinical reports suggest that estrogen may have antidepressant effects, either alone or as an adjunct to antidepressant medication.11 Before the WHI studies, expert consensus guidelines on treating depression in women recommended HRT as first-line treatment for patients experiencing a first lifetime onset of mild to moderate depression during perimenopause.12 WHI findings since 2002 that associated HRT with increased risk of stroke, deep vein thrombosis, and pulmonary embolism—without clear protection against coronary heart disease or cognitive decline—have left HRT a controversial option for treating perimenopausal depression. In the WHI trials:
- 10,739 postmenopausal women age 50 to 79 without a uterus received unopposed conjugated equine estrogens, 0.625 mg/d, or placebo for an average 6.8 years.13
- 16,608 postmenopausal women age 50 to 79 with an intact uterus received combination HRT (conjugated equine estrogens, 0.625 mg/d, plus 2.5 mg of medroxyprogesterone), or placebo for an average 5.6 years.14
The study using combination HRT found increased risks of breast cancer, ischemic stroke, blood clots, and coronary heart disease.15 A follow-up study showed that vasomotor symptoms returned in more than one-half the women after they stopped using combination HRT.15
A companion WHI trial found that estrogen, 0.625 mg/d—given unopposed or with a progestin—did not prevent cognitive decline in women age 65 to 79 and may have been associated with a slightly greater risk of probable dementia.16,17
The FDA recommends that women who want to use HRT to control menopausal symptoms use the lowest effective dose for the shortest time necessary.18
Antidepressants. SSRIs may be more useful than estrogen for producing MDD remission in perimenopausal women.19 SSRIs and other psychotropics may reduce perimenopausal vasomotor symptoms in addition to addressing depressive symptoms (Table 3). When choosing antidepressant therapy, consider the patient’s dominant presenting perimenopausal symptoms and side effects associated with treatment.20
Table 3
Nonhormone medications for perimenopausal depression: Evidence-based dosages and target symptoms
| Medication | Dosage effective for perimenopausal depression | Symptoms assessed |
|---|---|---|
| SSRIs | ||
| Citaloprama | 40 to 60 mg | Depressive and vasomotor |
| Escitalopramb,c | 5 to 20 mg | Depressive and vasomotor |
| Fluoxetined | 20 to 40 mg | Depressive and vasomotor |
| Paroxetinee,f | 12.5 or 25 mg | Depressive and vasomotor |
| Sertralineg | 100 mg | Depressive and vasomotor |
| Other antidepressants | ||
| Duloxetineh | 60 to 120 mg | Depressive and vasomotor |
| Venlafaxinei | 75 to 225 mg | Depressive and vasomotor |
| Mirtazapinej | 30 to 60 mg | Severe depressive symptoms; used as an adjunct to estrogen |
| Hypnotics | ||
| Eszopiclonek | 3 mg | Depressive and vasomotor; insomnia |
| Zolpideml | 5 to 10 mg | Insomnia |
| Anticonvulsant | ||
| Gabapentinm | 300 to 900 mg | Vasomotor |
| SSRIs: selective serotonin reuptake inhibitors | ||
| Source: Reference Citations | ||
Nonpharmacologic interventions are viable options for women who are reluctant to begin HRT or psychotropics.
Psychotherapy. Interpersonal psychotherapy (IPT) and cognitive-behavioral therapy (CBT) have been recommended to address psychosocial elements of perimenopausal mood lability.21 For women with climacteric depression, IPT focuses on role transitions, loss, and interpersonal support, whereas CBT focuses on identifying and altering negative thoughts and beliefs.
Although no randomized trials have examined psychotherapies for perimenopausal depression, a pilot open trial provided group CBT—psychoeducation, group discussion, and coping skills training—to 30 women with climacteric symptoms. Anxiety, depression, partnership relations, overall sexuality, hot flushes, and cardiac complaints improved significantly, based on pre- and post-intervention surveys. Sexual satisfaction and the stressfulness of menopausal symptoms did not change.22
Integrative medicine. Plant-based substances and herbal remedies such as phytoestrogens, red-clover isoflavones, black cohosh, and evening primrose oil have been included in a few research investigations, and the evidence is equivocal. Because of potential interactions between alternative therapies and medications, inquire about their use. Although a comprehensive review of integrative medicine for perimenopausal symptoms is beyond the scope of this article, see suggested readings (Box).
- Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric 2007;10(suppl 2):115-20.
- Blair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause 2008;15:32-43.
- Freeman MP, Helgason C, Hill RA. Selected integrative medicine treatments for depression: considerations for women. J Am Med Womens Assoc 2004;59(3):216-24.
- Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Psychiatr Clin North Am 2007;30:51-68.
- Thachil AF, Mohan R, Bhugra D. The evidence base of complementary and alternative therapies in depression. J Affect Disord 2007;97:23-35.
- Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. J North Am Menopause Soc 2008;15:193-202.
Clinical recommendations
Explore options with your patient; discuss side effects, risks, and expected minimum duration of treatment. Antidepressants, hormonal therapies, psychotherapy, and complementary and alternative treatments each might have a role in managing perimenopausal depression. A patient’s preferences, psychiatric history, and depression severity help determine which options to consider and in what order. How she responded to past treatments also can help you individualize a plan.
HRT may be appropriate for women who express a preference for HRT, have responded well to past hormone therapy, and have no personal history or high-risk factors for breast cancer. Based on the WHI findings, we consider a history of breast cancer in the patient or a first- or second-degree relative a contraindication to HRT.
Estrogen can be used alone or with an antidepressant. Studies support 17β-estradiol, 0.1 to 0.3 mg/d, for 8 to 12 weeks.11,23 Concomitant progesterone may be indicated to offset the effects of unopposed estrogen in women with an intact uterus. This option calls for an informed discussion with the patient about risks and benefits.
No data support long-term use of estrogen for recurrent or chronic depression. Because HRT’s risks and benefits vary with the length of exposure, individualize the extended use of estrogen solely to augment treatment for depression. Because vasomotor symptoms may recur when HRT is discontinued,15 we recommend that women make an informed decision in consultation with a gynecologist or primary care physician.
Antidepressants that have serotonergic activity—such as SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)—appear most promising for treating comorbid depressive and vasomotor symptoms. If a patient has had a good response to an antidepressant in the past, consider starting with that medication.
Common antidepressant side effects are difficult to assess in perimenopausal patients with MDD because the symptoms attributed to antidepressant side effects—such as low libido, sleep disturbance, and weight changes—also can be caused by mood disorders and hormonal changes. Therefore, inquire about these symptoms when you initiate antidepressant therapy and at follow-up assessments.
Psychotherapy. We recommend that all women who present with perimenopausal depression receive information about psychotherapy. Psychotherapy alone often is adequate for mild depression, and adding psychotherapy to antidepressant treatment usually enhances recovery from moderate and severe depression episodes. In addition, patients who engage in psychotherapy for depression may have a lower rate of relapse.24
Individual psychotherapy can help patients with perimenopausal depression:
- accept this life transition
- recognize the benefits of menopause, such as no need for contraception
- develop awareness of personal potential in the years ahead.
Because depression often occurs in an interpersonal context, consider including family members in psychotherapy to improve the patient’s interpersonal support.
Integrative therapies. A full evaluation and consideration of standard treatment options is indicated for all women with MDD. Integrative medicine appeals to many patients but has not been sufficiently studied for perimenopausal depression. Supplemental omega-3 fatty acids and folate are reasonable adjuncts to the treatment of MDD25-27 and deserve study in perimenopausal MDD.
- Greene Climacteric Scale. www.menopausematters.co.uk/greenescale.php.
- Quick Inventory of Depressive Symptomatology. www.idsqids.org.
- National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov.
Drug brand names
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Estradiol • various
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
Dr. Brandon and Dr. Shivakumar report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman receives research support from GlaxoSmithKline, Forest Pharmaceuticals, and Eli Lilly and Company. She was associate professor of psychiatry at the University of Texas Southwestern Medical Center at Dallas when this article was written and is now on the faculty at Harvard Medical School and Massachusetts General Hospital, Boston.
Symptoms of perimenopausal depression are not inherently different from those of depression diagnosed at any other time in life, but they present in a unique context:
- Hormonal fluctuations may persist for a long duration.
- Women experiencing hormonal fluctuations may be vulnerable to mood problems.
- Psychosocial/psychodynamic stressors often complicate this life transition.
Managing perimenopausal depression has become more complicated since the Women’s Health Initiative (WHI) studies found fewer benefits and greater risks with hormone replacement therapy (HRT) than had been perceived. This article discusses the clinical presentation of perimenopausal depression, its risk factors, and treatment options in post-WHI psychiatric practice.
Who is at risk?
Perimenopausal depression is diagnosed when onset of major depressive disorder (MDD) is associated with menstrual cycle irregularity and/or somatic symptoms of the menopausal transition.1 Diagnosis is based on the overall clinical picture, and treatment requires a thoughtful exploration of the complex relationship between hormonal function and mood regulation.
Presentation. For many women, perimenopause is characterized by mild to severe vasomotor, cognitive, and mood symptoms (Table 1). Thus, in your workup of depression in midlife women, document somatic symptoms—such as hot flushes, vaginal dryness, and incontinence—and affective/behavioral symp toms such as mood and sleep disturbances.
Table 1
Vasomotor, cognitive, and mood symptoms of perimenopause
| Vasomotor | Cognitive and mood |
|---|---|
| Hot flushes | Decreased concentration |
| Sweating | Anxiety |
| Heart palpitations | Irritability |
| Painful intercourse | Mood lability |
| Vaginal dryness and discomfort | Memory difficulty |
| Sleep disruption | |
| Headache |
Explore psychiatric and medical histories of your patient and her close relatives. Ask about depression, dysthymia, hypomania, or mood fluctuations around hormonal events such as menses, pregnancy, postpartum, or starting/stopping oral contraceptives. In the differential diagnosis, consider:
- Is low mood temporally connected with hot flushes and disturbed sleep?
- Is low mood secondary to stressful life events?
- Does the patient have another medical illness (such as thyroid disorder) with symptoms similar to depression?
- Is low mood secondary to anxiety or another psychiatric disorder?
Screening. Menopause is considered to have been reached after 12 months of amenorrhea not due to another cause. Median ages for this transition in the United States are 47.5 for perimenopause and 51 for menopause, with an average of 8 years between regular cycles and amenorrhea.2 Therefore, begin talking with women about perimenopausal symptoms when they turn 40.
Evidence supports screening perimenopausal women for depressive symptoms even when their primary complaints are vasomotor. The Greene Climacteric Scale3 is convenient for quantifying and monitoring perimenopausal symptoms. It includes depressive symptoms plus physical and cognitive markers. The Quick Inventory of Depressive Symptomatology—Self Report (QIDS-SR)4 questionnaire:
- takes minutes to complete
- is easy to score
- quantitates the number and severity of depressive symptoms (see Related Resources).
Psychosocial factors can predict depression at any time in life, but some are specific to the menopausal transition (Table 2).5 The “empty nest syndrome,” for example, is often used to explain depressive symptoms in midlife mothers, but no evidence links mood lability with the maturation and departure of children. What may be more stressing for women is supporting adolescents/young adults in their exit to independence while caring for aging parents.
Table 2
Risk factors for depression in women
| Predictive over lifetime | High risk during menopausal transition |
|---|---|
| History of depression | History of PMS, perinatal depression, mood symptoms associated with contraceptives |
| Family history of affective disorders | Premature or surgical menopause |
| Insomnia | Lengthy menopausal transition (≥27 months) |
| Reduced physical activities | Persistent and/or severe vasomotor symptoms |
| Weight gain | Negative attitudes toward menopause and aging |
| Less education | |
| Perceived lower economic status | |
| Perceived lower social support | |
| Perceived lower health status | |
| Smoking | |
| Stressful life events | |
| History of trauma | |
| Marital dissatisfaction | |
| PMS: Premenstrual syndrome | |
Sociocultural beliefs about sexuality and menopause may play a role in how your patient experiences and reports her symptoms. In some cultures, menopause elevates a woman’s social status and is associated with increased respect and authority. In others, such as Western societies that emphasize youth and beauty, women may view menopause and its physical changes in a negative light.6
Therefore, give careful attention to the psychosocial context of menopause to your patient and the social resources available to her. Questions to ask include:
- Has your lifestyle changed recently?
- Have your husband, family members, or close friends noticed any changes in your functioning?
- Is there anyone in your life that you feel comfortable confiding in?
Explaining the complexity of this life transition may ease her anxiety by normalizing her experience, helping her understand her symptoms, and validating her distress.
What might be the cause?
Although the exact pathophysiology of perimenopausal depression is unknown, hormonal changes,7 general health, the experience of menopause,8 and the psychosocial context2 likely work together to increase vulnerability for depressive symptoms (Figure).
Figure Biopsychosocial milieu of depression during perimenopauseHormonal fluctuation. The estrogen withdrawal theory7 explains depressive symptoms as resulting from a sustained decline in ovarian estrogen in tandem with spiking secretions of follicle-stimulating hormone by the pituitary. The finding that women with surgical menopause have a higher incidence of depressive symptoms than women with natural menopause supports this hypothesis.
Mood disorders occur across various female reproductive events, and increased risk appears to be associated with fluctuating gonadal hormones. Thus, declining estrogen may be less causative of perimenopausal depression than extreme fluctuations in estradiol activity.9,10
Estrogen interacts with dopamine, norepinephrine, beta-endorphin, and serotonin metabolism. In particular, estrogen facilitates serotonin delivery to neurons across the brain. These findings—and the success of selective serotonin reuptake inhibitors (SSRIs) in treating mood disorders—support the theory that fluctuating estrogen affects the serotonergic system and may cause depressive symptoms.
‘Domino theory.’ Others have hypothesized that depressive symptoms are the secondhand result of somatic symptoms of perimenopause. In a “domino effect,” hot flushes and night sweats disrupt women’s sleep, bringing fatigue and impaired daytime concentration, which lead to irritability and feelings of being overwhelmed.8
This theory, which incorporates perimenopausal hormone changes, is supported by elevated levels of depression in women who report frequent and intense vasomotor symptoms persisting >27 months.2
The psychosocial theory suggests that depression results from increased stress or adverse events.2 Midlife women with depressive symptoms report many possible sources of stress:
- demanding jobs
- family responsibilities
- dual demands of career and family
- little time for self
- poverty or employment stressors
- not enough sleep
- changing social relationships.
Negative interpretations of aging or the menopausal transition also have been implicated in cross-cultural studies.6 The predictive nature of psychosocial issues for depression during perimenopause supports this theory.
Evidence-based treatment
HRT. Research and clinical reports suggest that estrogen may have antidepressant effects, either alone or as an adjunct to antidepressant medication.11 Before the WHI studies, expert consensus guidelines on treating depression in women recommended HRT as first-line treatment for patients experiencing a first lifetime onset of mild to moderate depression during perimenopause.12 WHI findings since 2002 that associated HRT with increased risk of stroke, deep vein thrombosis, and pulmonary embolism—without clear protection against coronary heart disease or cognitive decline—have left HRT a controversial option for treating perimenopausal depression. In the WHI trials:
- 10,739 postmenopausal women age 50 to 79 without a uterus received unopposed conjugated equine estrogens, 0.625 mg/d, or placebo for an average 6.8 years.13
- 16,608 postmenopausal women age 50 to 79 with an intact uterus received combination HRT (conjugated equine estrogens, 0.625 mg/d, plus 2.5 mg of medroxyprogesterone), or placebo for an average 5.6 years.14
The study using combination HRT found increased risks of breast cancer, ischemic stroke, blood clots, and coronary heart disease.15 A follow-up study showed that vasomotor symptoms returned in more than one-half the women after they stopped using combination HRT.15
A companion WHI trial found that estrogen, 0.625 mg/d—given unopposed or with a progestin—did not prevent cognitive decline in women age 65 to 79 and may have been associated with a slightly greater risk of probable dementia.16,17
The FDA recommends that women who want to use HRT to control menopausal symptoms use the lowest effective dose for the shortest time necessary.18
Antidepressants. SSRIs may be more useful than estrogen for producing MDD remission in perimenopausal women.19 SSRIs and other psychotropics may reduce perimenopausal vasomotor symptoms in addition to addressing depressive symptoms (Table 3). When choosing antidepressant therapy, consider the patient’s dominant presenting perimenopausal symptoms and side effects associated with treatment.20
Table 3
Nonhormone medications for perimenopausal depression: Evidence-based dosages and target symptoms
| Medication | Dosage effective for perimenopausal depression | Symptoms assessed |
|---|---|---|
| SSRIs | ||
| Citaloprama | 40 to 60 mg | Depressive and vasomotor |
| Escitalopramb,c | 5 to 20 mg | Depressive and vasomotor |
| Fluoxetined | 20 to 40 mg | Depressive and vasomotor |
| Paroxetinee,f | 12.5 or 25 mg | Depressive and vasomotor |
| Sertralineg | 100 mg | Depressive and vasomotor |
| Other antidepressants | ||
| Duloxetineh | 60 to 120 mg | Depressive and vasomotor |
| Venlafaxinei | 75 to 225 mg | Depressive and vasomotor |
| Mirtazapinej | 30 to 60 mg | Severe depressive symptoms; used as an adjunct to estrogen |
| Hypnotics | ||
| Eszopiclonek | 3 mg | Depressive and vasomotor; insomnia |
| Zolpideml | 5 to 10 mg | Insomnia |
| Anticonvulsant | ||
| Gabapentinm | 300 to 900 mg | Vasomotor |
| SSRIs: selective serotonin reuptake inhibitors | ||
| Source: Reference Citations | ||
Nonpharmacologic interventions are viable options for women who are reluctant to begin HRT or psychotropics.
Psychotherapy. Interpersonal psychotherapy (IPT) and cognitive-behavioral therapy (CBT) have been recommended to address psychosocial elements of perimenopausal mood lability.21 For women with climacteric depression, IPT focuses on role transitions, loss, and interpersonal support, whereas CBT focuses on identifying and altering negative thoughts and beliefs.
Although no randomized trials have examined psychotherapies for perimenopausal depression, a pilot open trial provided group CBT—psychoeducation, group discussion, and coping skills training—to 30 women with climacteric symptoms. Anxiety, depression, partnership relations, overall sexuality, hot flushes, and cardiac complaints improved significantly, based on pre- and post-intervention surveys. Sexual satisfaction and the stressfulness of menopausal symptoms did not change.22
Integrative medicine. Plant-based substances and herbal remedies such as phytoestrogens, red-clover isoflavones, black cohosh, and evening primrose oil have been included in a few research investigations, and the evidence is equivocal. Because of potential interactions between alternative therapies and medications, inquire about their use. Although a comprehensive review of integrative medicine for perimenopausal symptoms is beyond the scope of this article, see suggested readings (Box).
- Albertazzi P. Non-estrogenic approaches for the treatment of climacteric symptoms. Climacteric 2007;10(suppl 2):115-20.
- Blair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause 2008;15:32-43.
- Freeman MP, Helgason C, Hill RA. Selected integrative medicine treatments for depression: considerations for women. J Am Med Womens Assoc 2004;59(3):216-24.
- Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Psychiatr Clin North Am 2007;30:51-68.
- Thachil AF, Mohan R, Bhugra D. The evidence base of complementary and alternative therapies in depression. J Affect Disord 2007;97:23-35.
- Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. J North Am Menopause Soc 2008;15:193-202.
Clinical recommendations
Explore options with your patient; discuss side effects, risks, and expected minimum duration of treatment. Antidepressants, hormonal therapies, psychotherapy, and complementary and alternative treatments each might have a role in managing perimenopausal depression. A patient’s preferences, psychiatric history, and depression severity help determine which options to consider and in what order. How she responded to past treatments also can help you individualize a plan.
HRT may be appropriate for women who express a preference for HRT, have responded well to past hormone therapy, and have no personal history or high-risk factors for breast cancer. Based on the WHI findings, we consider a history of breast cancer in the patient or a first- or second-degree relative a contraindication to HRT.
Estrogen can be used alone or with an antidepressant. Studies support 17β-estradiol, 0.1 to 0.3 mg/d, for 8 to 12 weeks.11,23 Concomitant progesterone may be indicated to offset the effects of unopposed estrogen in women with an intact uterus. This option calls for an informed discussion with the patient about risks and benefits.
No data support long-term use of estrogen for recurrent or chronic depression. Because HRT’s risks and benefits vary with the length of exposure, individualize the extended use of estrogen solely to augment treatment for depression. Because vasomotor symptoms may recur when HRT is discontinued,15 we recommend that women make an informed decision in consultation with a gynecologist or primary care physician.
Antidepressants that have serotonergic activity—such as SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)—appear most promising for treating comorbid depressive and vasomotor symptoms. If a patient has had a good response to an antidepressant in the past, consider starting with that medication.
Common antidepressant side effects are difficult to assess in perimenopausal patients with MDD because the symptoms attributed to antidepressant side effects—such as low libido, sleep disturbance, and weight changes—also can be caused by mood disorders and hormonal changes. Therefore, inquire about these symptoms when you initiate antidepressant therapy and at follow-up assessments.
Psychotherapy. We recommend that all women who present with perimenopausal depression receive information about psychotherapy. Psychotherapy alone often is adequate for mild depression, and adding psychotherapy to antidepressant treatment usually enhances recovery from moderate and severe depression episodes. In addition, patients who engage in psychotherapy for depression may have a lower rate of relapse.24
Individual psychotherapy can help patients with perimenopausal depression:
- accept this life transition
- recognize the benefits of menopause, such as no need for contraception
- develop awareness of personal potential in the years ahead.
Because depression often occurs in an interpersonal context, consider including family members in psychotherapy to improve the patient’s interpersonal support.
Integrative therapies. A full evaluation and consideration of standard treatment options is indicated for all women with MDD. Integrative medicine appeals to many patients but has not been sufficiently studied for perimenopausal depression. Supplemental omega-3 fatty acids and folate are reasonable adjuncts to the treatment of MDD25-27 and deserve study in perimenopausal MDD.
- Greene Climacteric Scale. www.menopausematters.co.uk/greenescale.php.
- Quick Inventory of Depressive Symptomatology. www.idsqids.org.
- National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov.
Drug brand names
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Estradiol • various
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
Dr. Brandon and Dr. Shivakumar report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman receives research support from GlaxoSmithKline, Forest Pharmaceuticals, and Eli Lilly and Company. She was associate professor of psychiatry at the University of Texas Southwestern Medical Center at Dallas when this article was written and is now on the faculty at Harvard Medical School and Massachusetts General Hospital, Boston.
1. Schmidt PJ, Rubinow DR. Reproductive ageing, sex steroids and depression. J Br Menopause Soc 2006;12(4):178-85.
2. Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr 2005;10(6):471-8.
3. Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29(1):25-31.
4. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54(5):573-83.Erratum in: Biol Psychiatry. 2003;54(5):585.
5. Feld J, Halbreich U, Karkun S. The association of perimenopausal mood disorders with other reproductive-related disorders. CNS Spectr 2005;10(6):461-70.
6. Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 2001;52:345-56.
7. Campbell S, Whitehead M. Oestrogen therapy and the menopause syndrome. Clin Obstet Gynecol 1977;4:31-47.
8. Schmidt PJ, Rubinow DR. Menopause-related affective disorders: a justification for further study. Am J Psychiatry 1991;48:844-52.
9. Soares CN. Menopausal transition and depression: who is at risk and how to treat it? Expert Rev Neurother 2007;7(10):1285-93.
10. Prior JC. The complex endocrinology of menopausal transition. Endocrinol Rev 1998;19:397-428.
11. Rasgon N, Altshuler LL, Fairbanks LA, et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry 2002;63(suppl):45-8.
12. Altshuler LL, Cohen LS, Moline ML, et al. The Expert Consensus Guideline Series. Treatment of depression in women. Postgrad Med 2001 Mar;(Spec No):1-107.
13. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701-12.
14. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
15. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA 2005;294:183-93.
16. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947-58.
17. Shumaker SA, Legault C, Rapp SR. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651-62.
18. FDA approves new labeling and provides new advice to postmenopausal women who use or who are considering using estrogen and estrogen with progestin [FDA Fact Sheet January 8, 2003]. Available at: http://www.fda.gov/oc/factsheets/WHI.html. Accessed September 11, 2008.
19. Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13(5):780-6.
20. Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med 2005;118(suppl 12B):93-7.
21. Kahn DA, Moline ML, Ross RW, et al. Depression during the transition to menopause: a guide for patients and families. Postgrad Med 2001 Mar;(Spec No):110-1.
22. Alder J, Besken KE, Armbruster U, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychother Psychosom 2006;75(5):298-303.
23. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
24. Otto MW, Smits JA, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science and Practice 2005;12:72-86.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomized, placebo controlled trial. J Affect Disord 2000;60:121-30.
26. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [American Psychiatric Association subcommittee report]. J Clin Psychiatry 2006;67:1954-67.
27. Otto MW, Church TS, Craft LL, et al. Exercise for mood and anxiety disorders. J Clin Psychiatry 2007;68:669-76.
1. Schmidt PJ, Rubinow DR. Reproductive ageing, sex steroids and depression. J Br Menopause Soc 2006;12(4):178-85.
2. Rasgon N, Shelton S, Halbreich U. Perimenopausal mental disorders: epidemiology and phenomenology. CNS Spectr 2005;10(6):471-8.
3. Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29(1):25-31.
4. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54(5):573-83.Erratum in: Biol Psychiatry. 2003;54(5):585.
5. Feld J, Halbreich U, Karkun S. The association of perimenopausal mood disorders with other reproductive-related disorders. CNS Spectr 2005;10(6):461-70.
6. Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 2001;52:345-56.
7. Campbell S, Whitehead M. Oestrogen therapy and the menopause syndrome. Clin Obstet Gynecol 1977;4:31-47.
8. Schmidt PJ, Rubinow DR. Menopause-related affective disorders: a justification for further study. Am J Psychiatry 1991;48:844-52.
9. Soares CN. Menopausal transition and depression: who is at risk and how to treat it? Expert Rev Neurother 2007;7(10):1285-93.
10. Prior JC. The complex endocrinology of menopausal transition. Endocrinol Rev 1998;19:397-428.
11. Rasgon N, Altshuler LL, Fairbanks LA, et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry 2002;63(suppl):45-8.
12. Altshuler LL, Cohen LS, Moline ML, et al. The Expert Consensus Guideline Series. Treatment of depression in women. Postgrad Med 2001 Mar;(Spec No):1-107.
13. The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701-12.
14. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
15. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA 2005;294:183-93.
16. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947-58.
17. Shumaker SA, Legault C, Rapp SR. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651-62.
18. FDA approves new labeling and provides new advice to postmenopausal women who use or who are considering using estrogen and estrogen with progestin [FDA Fact Sheet January 8, 2003]. Available at: http://www.fda.gov/oc/factsheets/WHI.html. Accessed September 11, 2008.
19. Soares CN, Arsenio H, Joffe H, et al. Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006;13(5):780-6.
20. Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med 2005;118(suppl 12B):93-7.
21. Kahn DA, Moline ML, Ross RW, et al. Depression during the transition to menopause: a guide for patients and families. Postgrad Med 2001 Mar;(Spec No):110-1.
22. Alder J, Besken KE, Armbruster U, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychother Psychosom 2006;75(5):298-303.
23. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
24. Otto MW, Smits JA, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science and Practice 2005;12:72-86.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomized, placebo controlled trial. J Affect Disord 2000;60:121-30.
26. Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry [American Psychiatric Association subcommittee report]. J Clin Psychiatry 2006;67:1954-67.
27. Otto MW, Church TS, Craft LL, et al. Exercise for mood and anxiety disorders. J Clin Psychiatry 2007;68:669-76.
Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis
From SIG: E CAPS to CAGE and WWHHHHIMPS, mnemonics help practitioners and trainees recall important lists (such as criteria for depression, screening questions for alcoholism, or life-threatening causes of delirium, respectively). Mnemonics’ efficacy rests on the principle that grouped information is easier to remember than individual points of data.
Not everyone loves mnemonics, but recollecting diagnostic criteria is useful in clinical practice and research, on board examinations, and for insurance reimbursement. Thus, tools that assist in recalling diagnostic criteria have a role in psychiatric practice and teaching.
In this article, we present 32 mnemonics to help clinicians diagnose:
- affective disorders (Box 1)1,2
- anxiety disorders (Box 2)3-6
- medication adverse effects (Box 3)7,8
- personality disorders (Box 4)9-11
- addiction disorders (Box 5)12,13
- causes of delirium (Box 6).14
We also discuss how mnemonics improve one’s memory, based on the principles of learning theory.
How mnemonics work
A mnemonic—from the Greek word “mnemonikos” (“of memory”)—links new data with previously learned information. Mnemonics assist in learning by reducing the amount of information (“cognitive load”) that needs to be stored for long-term processing and retrieval.15
Memory, defined as the “persistence of learning in a state that can be revealed at a later time,”16 can be divided into 2 types:
- declarative (a conscious recollection of facts, such as remembering a relative’s birthday)
- procedural (skills-based learning, such as riding a bicycle).
Declarative memory has a conscious component and may be mediated by the medial temporal lobe and cortical association structures. Procedural memory has less of a conscious component; it may involve the basal ganglia, cerebellum, and a variety of cortical sensory-perceptive regions.17
| Depression SIG: E CAPS* Suicidal thoughts Interests decreased Guilt Energy decreased Concentration decreased Appetite disturbance (increased or decreased) Psychomotor changes (agitation or retardation) Sleep disturbance (increased or decreased) * Created by Carey Gross, MD | Dysthymia HE’S 2 SAD2 Hopelessness Energy loss or fatigue Self-esteem is low 2 years minimum of depressed mood most of the day, for more days than not Sleep is increased or decreased Appetite is increased or decreased Decision-making or concentration is impaired | Mania DIG FAST Distractibility Indiscretion Grandiosity Flight of ideas Activity increase Sleep deficit Talkativeness |
| Depression C GASP DIE1 Concentration decreased Guilt Appetite Sleep disturbance Psychomotor agitation or retardation Death or suicide (thoughts or acts of) Interests decreased Energy decreased | Hypomania TAD HIGH Talkative Attention deficit Decreased need for sleep High self-esteem/grandiosity Ideas that race Goal-directed activity increased High-risk activity | Mania DeTeR the HIGH* Distractibility Talkativeness Reckless behavior Hyposomnia Ideas that race Grandiosity Hypersexuality * Created by Carey Gross, MD |
Declarative memory can be subdivided into working memory and long-term memory.
With working memory, new items of information are held briefly so that encoding and eventual storage can take place.
Working memory guides decision-making and future planning and is intricately related to attention.18-21 Functional MRI and positron emission tomography as well as neurocognitive testing have shown that working memory tasks activate the prefrontal cortex and brain regions specific to language and visuospatial memory.
The hippocampus is thought to rapidly absorb new information, and this data is consolidated and permanently stored via the prefrontal cortex.22-26 Given the hippocampus’ limited storage capacity, new information (such as what you ate for breakfast 3 weeks ago) will disappear if it is not repeated regularly.17
Long-term memory, on the other hand, is encoded knowledge that is linked to facts learned in the past; it is consolidated in the brain and can be readily retrieved. Neuroimaging studies have demonstrated opposing patterns of activation in the hippocampus and prefrontal cortex, depending on whether the memory being recalled is:
- new (high hippocampal activity, low prefrontal cortex activity)
- old (low hippocampal activity, high prefrontal cortex activity).27
Mnemonics are thought to affect working memory by reducing the introduced cognitive load and increasing the efficiency of memory acquisition and encoding. They reduce cognitive load by grouping objects into a single verbal or visual cue that can be introduced into working memory. Learning is optimized when the load on working memory is minimized, enabling long-term memory to be facilitated.28
| Generalized anxiety disorder Worry WARTS3 Wound up Worn-out Absentminded Restless Touchy Sleepless | Posttraumatic stress disorder TRAUMA5 Traumatic event Re-experience Avoidance Unable to function Month or more of symptoms Arousal increased | Anxiety disorder due to a general medical condition Physical Diseases That Have Commonly Appeared Anxious: Pheochromocytoma Diabetes mellitus Temporal lobe epilepsy Hyperthyroidism Carcinoid Alcohol withdrawal Arrhythmias |
| Generalized anxiety disorder WATCHERS4 Worry Anxiety Tension in muscles Concentration difficulty Hyperarousal (or irritability) Energy loss Restlessness Sleep disturbance | Posttraumatic stress disorder DREAMS6 Disinterest in usual activities Re-experience Event preceding symptoms Avoidance Month or more of symptoms Sympathetic arousal |
| Antidepressant discontinuation syndrome FINISH7 Flu-like symptoms Insomnia Nausea Imbalance Sensory disturbances Hyperarousal (anxiety/agitation) | Neuroleptic malignant syndrome FEVER8 Fever Encephalopathy Vital sign instability Elevated WBC/CPK Rigidity WBC: white blood cell count CPK: creatine phosphokinase | Serotonin syndrome HARMED Hyperthermia Autonomic instability Rigidity Myoclonus Encephalopathy Diaphoresis |
Mnemonics may use rhyme, music, or visual cues to enhance memory. Most mnemonics used in medical practice and education are word-based, including:
- Acronyms—words, each letter of which stands for a particular piece of information to be recalled (such as RICE for treatment of a sprained joint: rest, ice, compression, elevation).
- Acrostics—sentences with the first letter of each word prompting the desired recollection (such as “To Zanzibar by motor car” for the branches of the facial nerve: temporal, zygomatic, buccal, mandibular, cervical).
- Alphabetical sequences (such as ABCDE of trauma assessment: airway, breathing, circulation, disability, exposure).29
An appropriate teaching tool?
Dozens of mnemonics addressing psychiatric diagnosis and treatment have been published, but relatively few are widely used. Psychiatric educators may resist teaching with mnemonics, believing they might erode a humanistic approach to patients by reducing psychopathology to “a laundry list” of symptoms and the art of psychiatric diagnosis to a “check-box” endeavor. Mnemonics that use humor may be rejected as irreverent or unprofessional.30 Publishing a novel mnemonic may be viewed with disdain by some as an “easy” way of padding a curriculum vitae.
| Paranoid personality disorder SUSPECT9 Spousal infidelity suspected Unforgiving (bears grudges) Suspicious Perceives attacks (and reacts quickly) Enemy or friend? (suspects associates and friends) Confiding in others is feared Threats perceived in benign events | Schizotypal personality disorder ME PECULIAR9 Magical thinking Experiences unusual perceptions Paranoid ideation Eccentric behavior or appearance Constricted or inappropriate affect Unusual thinking or speech Lacks close friends Ideas of reference Anxiety in social situations Rule out psychotic or pervasive developmental disorders | Borderline personality disorder IMPULSIVE10 Impulsive Moodiness Paranoia or dissociation under stress Unstable self-image Labile intense relationships Suicidal gestures Inappropriate anger Vulnerability to abandonment Emptiness (feelings of) | Histrionic personality disorder PRAISE ME9 Provocative or seductive behavior Relationships considered more intimate than they are Attention (need to be the center of) Influenced easily Style of speech (impressionistic, lacking detail) Emotions (rapidly shifting, shallow) Make up (physical appearance used to draw attention to self) Emotions exaggerated | Narcissistic personality disorder GRANDIOSE11 Grandiose Requires attention Arrogant Need to be special Dreams of success and power Interpersonally exploitative Others (unable to recognize feelings/needs of) Sense of entitlement Envious | Dependent personality disorder RELIANCE9 Reassurance required Expressing disagreement difficult Life responsibilities assumed by others Initiating projects difficult Alone (feels helpless and uncomfortable when alone) Nurturance (goes to excessive lengths to obtain) Companionship sought urgently when a relationship ends Exaggerated fears of being left to care for self |
| Schizoid personality disorder DISTANT9 Detached or flattened affect Indifferent to criticism or praise Sexual experiences of little interest Tasks done solitarily Absence of close friends Neither desires nor enjoys close relationships Takes pleasure in few activities | Antisocial personality disorder CORRUPT9 Cannot conform to law Obligations ignored Reckless disregard for safety Remorseless Underhanded (deceitful) Planning insufficient (impulsive) Temper (irritable and aggressive) | Borderline personality disorder DESPAIRER* Disturbance of identity Emotionally labile Suicidal behavior Paranoia or dissociation Abandonment (fear of) Impulsive Relationships unstable Emptiness (feelings of) Rage (inappropriate) * Created by Jason P. Caplan, MD | Histrionic personality disorder ACTRESSS* Appearance focused Center of attention Theatrical Relationships (believed to be more intimate than they are) Easily influenced Seductive behavior Shallow emotions Speech (impressionistic and vague) * Created by Jason P. Caplan, MD | Avoidant personality disorder CRINGES9 Criticism or rejection preoccupies thoughts in social situations Restraint in relationships due to fear of shame Inhibited in new relationships Needs to be sure of being liked before engaging socially Gets around occupational activities with need for interpersonal contact Embarrassment prevents new activity or taking risks Self viewed as unappealing or inferior | Obsessive-compulsive personality disorder SCRIMPER* Stubborn Cannot discard worthless objects Rule obsessed Inflexible Miserly Perfectionistic Excludes leisure due to devotion to work Reluctant to delegate to others * Created by Jason P. Caplan, MD |
Entire Web sites exist to share mnemonics for medical education (see Related Resources). Thus it is likely that trainees are using them with or without their teachers’ supervision. Psychiatric educators need to be aware of the mnemonics their trainees are using and to:
- screen these tools for factual errors (such as incomplete diagnostic criteria)
- remind trainees that although mnemonics are useful, psychiatrists should approach patients as individuals without the prejudice of a potentially pejorative label.
Our methodology
In preparing this article, we gathered numerous mnemonics (some published and some novel) designed to capture the learner’s attention and impart information pertinent to psychiatric diagnosis and treatment. Whenever possible, we credited each mnemonic to its creator, but—given the difficulty in confirming authorship of (what in many cases has become) oral history—we’ve listed some mnemonics without citation.
Our list is far from complete because we likely are unaware of many mnemonics, and we have excluded some that seemed obscure, unwieldy, or redundant. We have not excluded mnemonics that some may view as pejorative but merely report their existence. Including them does not mean that we endorse them.
This article lists 32 mnemonics related to psychiatric diagnosis. Thus, it seems odd that an informal survey of >60 residents at the Massachusetts General Hospital (MGH)/McLean Residency Training Program in Psychiatry revealed that most were aware of only 2 or 3 psychiatric mnemonics, typically:
- SIG: E CAPS (a tool to recall the criteria for depression)
- DIG FAST (a list of criteria for diagnosing mania)
- WWHHHHIMPS (a tool for recalling life-threatening causes of delirium).
Although this unscientific survey may be biased because faculty or trainees at MGH created the above 3 mnemonics, it nonetheless begs the question of what qualities make a mnemonic memorable.
Learning theory provides several clues. George Miller’s classic 1956 paper, “The magical number seven, plus or minus two: some limits on our capacity for processing information,” discussed the finding that 7 seems to be the upper limit of individual pieces of data that can be easily remembered.31 Research also has shown that recruiting the limbic system (potentially through the use of humor) aids in the recall of otherwise dry, cortical information.32,33
Intuitively, it would seem that nonrepeating letters would facilitate the recall of the linked data, allowing each letter to provide a distinct cue, without any clouding by redundancy. Of the 3 most popular psychiatric mnemonics, however, only DIG FAST fits the learning theory. It contains 7 letters, repeats no letters, and has the limbic cue of allowing the learner to imagine a person with mania digging furiously.
| Substance dependence ADDICTeD12 Activities are given up or reduced Dependence, physical: tolerance Dependence, physical: withdrawal Intrapersonal (Internal) consequences, physical or psychological Can’t cut down or control use Time-consuming Duration or amount of use is greater than intended | Substance abuse WILD12 Work, school, or home role obligation failures Interpersonal or social consequences Legal problems Dangerous use | Alcohol abuse CAGE13 Have you ever felt you should CUT DOWN your drinking? Have people ANNOYED you by criticizing your drinking? Have you ever felt bad or GUILTY about your drinking? Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover (EYE-OPENER)? |
SIG: E CAPS falls within the range of 7 plus or minus 2, includes a limbic cue (although often forgotten, it refers to the prescription of energy capsules for depression), but repeats the letter S.
WWHHHHIMPS, with 10 letters, exceeds the recommended range, repeats the W (appearing twice) and the H (appearing 4 times), and provides no clear limbic cue.
| Causes I WATCH DEATH Infection Withdrawal Acute metabolic Trauma CNS pathology Hypoxia Deficiencies Endocrinopathies Acute vascular Toxins or drugs Heavy metals | Life-threatening causes WWHHHHIMPS* Wernicke’s encephalopathy Withdrawal Hypertensive crisis Hypoperfusion/hypoxia of the brain Hypoglycemia Hyper/hypothermia Intracranial process/infection Metabolic/meningitis Poisons Status epilepticus * Created by Gary W. Small, MD | Deliriogenic medications ACUTE CHANGE IN MS14 Antibiotics Cardiac drugs Urinary incontinence drugs Theophylline Ethanol Corticosteroids H2 blockers Antiparkinsonian drugs Narcotics Geriatric psychiatric drugs ENT drugs Insomnia drugs NSAIDs Muscle relaxants Seizure medicines |
It may be that recruiting the limbic system provides the greatest likelihood of recall. Recruiting this system may add increased valence to a particular mnemonic for a specific individual, but this same limbic valence may limit its usefulness in a professional context.
- Free searchable database of medical mnemonics. www.medicalmnemonics.com.
- Robinson DJ. Mnemonics and more for psychiatry. Port Huron, MI: Rapid Psychler Press, 2001.
1. Abraham PF, Shirley ER. New mnemonic for depressive symptoms. Am J Psychiatry 2006;163(2):329-30.
2. Christman DS. “HE’S 2 SAD” detects dysthymic disorder. Current Psychiatry 2008;7(3):120.-
3. Coupland NJ. Worry WARTS have generalized anxiety disorder. Can J Psychiatry 2002;47(2):197.-
4. Berber MJ. WATCHERS: recognizing generalized anxiety disorder. J Clin Psychiatry 2000;61(6):447.-
5. Khouzam HR. A simple mnemonic for the diagnostic criteria for post-traumatic stress disorder. West J Med 2001;174(6):424.-
6. Short DD, Workman EA, Morse JH, Turner RL. Mnemonics for eight DSM-III-R disorders. Hosp Community Psychiatry 1992;43(6):642-4.
7. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, Insomnia, Nausea, Imbalance, Sensory disturbances, and Hyperarousal (anxiety/agitation). J Clin Psychiatry 1998;59(5):255.-
8. Christensen RC. Identify neuroleptic malignant syndrome with FEVER. Current Psychiatry 2005;4(7):102.-
9. Pinkofsky HB. Mnemonics for DSM-IV personality disorders. Psychiatr Serv 1997;48(9):1197-8.
10. Senger HL. Borderline mnemonic. Am J Psychiatry 1997;154(9):1321.-
11. Kim SI, Swanson TA, Caplan JP, eds. Underground clinical vignettes step 2: psychiatry. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:130.
12. Bogenschutz MP, Quinn DK. Acronyms for substance use disorders. J Clin Psychiatry 2001;62(6):474-5.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252(14):1905-7.
14. Flaherty JH. Psychotherapeutic agents in older adults. Commonly prescribed and over-the-counter remedies: causes of confusion. Clin Geriatr Med 1998;14:101-27.
15. Sweller J. Cognitive load theory, learning difficulty, and instructional design. Learn Instr 1994;4:295-312.
16. Squire LR. Memory and brain. New York, NY: Oxford University Press; 1987.
17. DeLuca J, Lengenfelder J, Eslinger P. Memory and learning. In: Rizzo M, Eslinger P, eds. Principles and practice of behavioral neurology and neuropsychology. Philadelphia, PA: Saunders; 2004:251.
18. Dash PK, Moore AN, Kobori N, et al. Molecular activity underlying working memory. Learn Mem 2007;14:554-63.
19. Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience 2006;139:201-8.
20. Knudson EI. Fundamental components of attention. Ann Rev Neurosci 2007;30:57-78.
21. Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience 2006;139:23-36.
22. Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain 2001;124:849-81.
23. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann Rev Neurosci 2001;24:167-202.
24. Schumacher EH, Lauber E, Awh E, et al. PET evidence for a modal verbal working memory system. Neuroimage 1996;3:79-88.
25. Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex 1996;6:11-20.
26. Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 2003;3(4):255-74.
27. Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 2005;6:119-30.
28. Sweller J. Cognitive load during problem solving: effects on learning. Cogn Sci 1988;12(1):257-85.
29. Beitz JM. Unleashing the power of memory: the mighty mnemonic. Nurse Educ 1997;22(2):25-9.
30. Larson EW. Criticism of mnemonic device. Am J Psychiatry 1990;147(7):963-4.
31. Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev 1956;63:81-97.
32. Schmidt SR. Effects of humor on sentence memory. J Exp Psychol Learn Mem Cogn 1994;20(4):953-67.
33. Lippman LG, Dunn ML. Contextual connections within puns: effects on perceived humor and memory. J Gen Psychol 2000;127(2):185-97.
From SIG: E CAPS to CAGE and WWHHHHIMPS, mnemonics help practitioners and trainees recall important lists (such as criteria for depression, screening questions for alcoholism, or life-threatening causes of delirium, respectively). Mnemonics’ efficacy rests on the principle that grouped information is easier to remember than individual points of data.
Not everyone loves mnemonics, but recollecting diagnostic criteria is useful in clinical practice and research, on board examinations, and for insurance reimbursement. Thus, tools that assist in recalling diagnostic criteria have a role in psychiatric practice and teaching.
In this article, we present 32 mnemonics to help clinicians diagnose:
- affective disorders (Box 1)1,2
- anxiety disorders (Box 2)3-6
- medication adverse effects (Box 3)7,8
- personality disorders (Box 4)9-11
- addiction disorders (Box 5)12,13
- causes of delirium (Box 6).14
We also discuss how mnemonics improve one’s memory, based on the principles of learning theory.
How mnemonics work
A mnemonic—from the Greek word “mnemonikos” (“of memory”)—links new data with previously learned information. Mnemonics assist in learning by reducing the amount of information (“cognitive load”) that needs to be stored for long-term processing and retrieval.15
Memory, defined as the “persistence of learning in a state that can be revealed at a later time,”16 can be divided into 2 types:
- declarative (a conscious recollection of facts, such as remembering a relative’s birthday)
- procedural (skills-based learning, such as riding a bicycle).
Declarative memory has a conscious component and may be mediated by the medial temporal lobe and cortical association structures. Procedural memory has less of a conscious component; it may involve the basal ganglia, cerebellum, and a variety of cortical sensory-perceptive regions.17
| Depression SIG: E CAPS* Suicidal thoughts Interests decreased Guilt Energy decreased Concentration decreased Appetite disturbance (increased or decreased) Psychomotor changes (agitation or retardation) Sleep disturbance (increased or decreased) * Created by Carey Gross, MD | Dysthymia HE’S 2 SAD2 Hopelessness Energy loss or fatigue Self-esteem is low 2 years minimum of depressed mood most of the day, for more days than not Sleep is increased or decreased Appetite is increased or decreased Decision-making or concentration is impaired | Mania DIG FAST Distractibility Indiscretion Grandiosity Flight of ideas Activity increase Sleep deficit Talkativeness |
| Depression C GASP DIE1 Concentration decreased Guilt Appetite Sleep disturbance Psychomotor agitation or retardation Death or suicide (thoughts or acts of) Interests decreased Energy decreased | Hypomania TAD HIGH Talkative Attention deficit Decreased need for sleep High self-esteem/grandiosity Ideas that race Goal-directed activity increased High-risk activity | Mania DeTeR the HIGH* Distractibility Talkativeness Reckless behavior Hyposomnia Ideas that race Grandiosity Hypersexuality * Created by Carey Gross, MD |
Declarative memory can be subdivided into working memory and long-term memory.
With working memory, new items of information are held briefly so that encoding and eventual storage can take place.
Working memory guides decision-making and future planning and is intricately related to attention.18-21 Functional MRI and positron emission tomography as well as neurocognitive testing have shown that working memory tasks activate the prefrontal cortex and brain regions specific to language and visuospatial memory.
The hippocampus is thought to rapidly absorb new information, and this data is consolidated and permanently stored via the prefrontal cortex.22-26 Given the hippocampus’ limited storage capacity, new information (such as what you ate for breakfast 3 weeks ago) will disappear if it is not repeated regularly.17
Long-term memory, on the other hand, is encoded knowledge that is linked to facts learned in the past; it is consolidated in the brain and can be readily retrieved. Neuroimaging studies have demonstrated opposing patterns of activation in the hippocampus and prefrontal cortex, depending on whether the memory being recalled is:
- new (high hippocampal activity, low prefrontal cortex activity)
- old (low hippocampal activity, high prefrontal cortex activity).27
Mnemonics are thought to affect working memory by reducing the introduced cognitive load and increasing the efficiency of memory acquisition and encoding. They reduce cognitive load by grouping objects into a single verbal or visual cue that can be introduced into working memory. Learning is optimized when the load on working memory is minimized, enabling long-term memory to be facilitated.28
| Generalized anxiety disorder Worry WARTS3 Wound up Worn-out Absentminded Restless Touchy Sleepless | Posttraumatic stress disorder TRAUMA5 Traumatic event Re-experience Avoidance Unable to function Month or more of symptoms Arousal increased | Anxiety disorder due to a general medical condition Physical Diseases That Have Commonly Appeared Anxious: Pheochromocytoma Diabetes mellitus Temporal lobe epilepsy Hyperthyroidism Carcinoid Alcohol withdrawal Arrhythmias |
| Generalized anxiety disorder WATCHERS4 Worry Anxiety Tension in muscles Concentration difficulty Hyperarousal (or irritability) Energy loss Restlessness Sleep disturbance | Posttraumatic stress disorder DREAMS6 Disinterest in usual activities Re-experience Event preceding symptoms Avoidance Month or more of symptoms Sympathetic arousal |
| Antidepressant discontinuation syndrome FINISH7 Flu-like symptoms Insomnia Nausea Imbalance Sensory disturbances Hyperarousal (anxiety/agitation) | Neuroleptic malignant syndrome FEVER8 Fever Encephalopathy Vital sign instability Elevated WBC/CPK Rigidity WBC: white blood cell count CPK: creatine phosphokinase | Serotonin syndrome HARMED Hyperthermia Autonomic instability Rigidity Myoclonus Encephalopathy Diaphoresis |
Mnemonics may use rhyme, music, or visual cues to enhance memory. Most mnemonics used in medical practice and education are word-based, including:
- Acronyms—words, each letter of which stands for a particular piece of information to be recalled (such as RICE for treatment of a sprained joint: rest, ice, compression, elevation).
- Acrostics—sentences with the first letter of each word prompting the desired recollection (such as “To Zanzibar by motor car” for the branches of the facial nerve: temporal, zygomatic, buccal, mandibular, cervical).
- Alphabetical sequences (such as ABCDE of trauma assessment: airway, breathing, circulation, disability, exposure).29
An appropriate teaching tool?
Dozens of mnemonics addressing psychiatric diagnosis and treatment have been published, but relatively few are widely used. Psychiatric educators may resist teaching with mnemonics, believing they might erode a humanistic approach to patients by reducing psychopathology to “a laundry list” of symptoms and the art of psychiatric diagnosis to a “check-box” endeavor. Mnemonics that use humor may be rejected as irreverent or unprofessional.30 Publishing a novel mnemonic may be viewed with disdain by some as an “easy” way of padding a curriculum vitae.
| Paranoid personality disorder SUSPECT9 Spousal infidelity suspected Unforgiving (bears grudges) Suspicious Perceives attacks (and reacts quickly) Enemy or friend? (suspects associates and friends) Confiding in others is feared Threats perceived in benign events | Schizotypal personality disorder ME PECULIAR9 Magical thinking Experiences unusual perceptions Paranoid ideation Eccentric behavior or appearance Constricted or inappropriate affect Unusual thinking or speech Lacks close friends Ideas of reference Anxiety in social situations Rule out psychotic or pervasive developmental disorders | Borderline personality disorder IMPULSIVE10 Impulsive Moodiness Paranoia or dissociation under stress Unstable self-image Labile intense relationships Suicidal gestures Inappropriate anger Vulnerability to abandonment Emptiness (feelings of) | Histrionic personality disorder PRAISE ME9 Provocative or seductive behavior Relationships considered more intimate than they are Attention (need to be the center of) Influenced easily Style of speech (impressionistic, lacking detail) Emotions (rapidly shifting, shallow) Make up (physical appearance used to draw attention to self) Emotions exaggerated | Narcissistic personality disorder GRANDIOSE11 Grandiose Requires attention Arrogant Need to be special Dreams of success and power Interpersonally exploitative Others (unable to recognize feelings/needs of) Sense of entitlement Envious | Dependent personality disorder RELIANCE9 Reassurance required Expressing disagreement difficult Life responsibilities assumed by others Initiating projects difficult Alone (feels helpless and uncomfortable when alone) Nurturance (goes to excessive lengths to obtain) Companionship sought urgently when a relationship ends Exaggerated fears of being left to care for self |
| Schizoid personality disorder DISTANT9 Detached or flattened affect Indifferent to criticism or praise Sexual experiences of little interest Tasks done solitarily Absence of close friends Neither desires nor enjoys close relationships Takes pleasure in few activities | Antisocial personality disorder CORRUPT9 Cannot conform to law Obligations ignored Reckless disregard for safety Remorseless Underhanded (deceitful) Planning insufficient (impulsive) Temper (irritable and aggressive) | Borderline personality disorder DESPAIRER* Disturbance of identity Emotionally labile Suicidal behavior Paranoia or dissociation Abandonment (fear of) Impulsive Relationships unstable Emptiness (feelings of) Rage (inappropriate) * Created by Jason P. Caplan, MD | Histrionic personality disorder ACTRESSS* Appearance focused Center of attention Theatrical Relationships (believed to be more intimate than they are) Easily influenced Seductive behavior Shallow emotions Speech (impressionistic and vague) * Created by Jason P. Caplan, MD | Avoidant personality disorder CRINGES9 Criticism or rejection preoccupies thoughts in social situations Restraint in relationships due to fear of shame Inhibited in new relationships Needs to be sure of being liked before engaging socially Gets around occupational activities with need for interpersonal contact Embarrassment prevents new activity or taking risks Self viewed as unappealing or inferior | Obsessive-compulsive personality disorder SCRIMPER* Stubborn Cannot discard worthless objects Rule obsessed Inflexible Miserly Perfectionistic Excludes leisure due to devotion to work Reluctant to delegate to others * Created by Jason P. Caplan, MD |
Entire Web sites exist to share mnemonics for medical education (see Related Resources). Thus it is likely that trainees are using them with or without their teachers’ supervision. Psychiatric educators need to be aware of the mnemonics their trainees are using and to:
- screen these tools for factual errors (such as incomplete diagnostic criteria)
- remind trainees that although mnemonics are useful, psychiatrists should approach patients as individuals without the prejudice of a potentially pejorative label.
Our methodology
In preparing this article, we gathered numerous mnemonics (some published and some novel) designed to capture the learner’s attention and impart information pertinent to psychiatric diagnosis and treatment. Whenever possible, we credited each mnemonic to its creator, but—given the difficulty in confirming authorship of (what in many cases has become) oral history—we’ve listed some mnemonics without citation.
Our list is far from complete because we likely are unaware of many mnemonics, and we have excluded some that seemed obscure, unwieldy, or redundant. We have not excluded mnemonics that some may view as pejorative but merely report their existence. Including them does not mean that we endorse them.
This article lists 32 mnemonics related to psychiatric diagnosis. Thus, it seems odd that an informal survey of >60 residents at the Massachusetts General Hospital (MGH)/McLean Residency Training Program in Psychiatry revealed that most were aware of only 2 or 3 psychiatric mnemonics, typically:
- SIG: E CAPS (a tool to recall the criteria for depression)
- DIG FAST (a list of criteria for diagnosing mania)
- WWHHHHIMPS (a tool for recalling life-threatening causes of delirium).
Although this unscientific survey may be biased because faculty or trainees at MGH created the above 3 mnemonics, it nonetheless begs the question of what qualities make a mnemonic memorable.
Learning theory provides several clues. George Miller’s classic 1956 paper, “The magical number seven, plus or minus two: some limits on our capacity for processing information,” discussed the finding that 7 seems to be the upper limit of individual pieces of data that can be easily remembered.31 Research also has shown that recruiting the limbic system (potentially through the use of humor) aids in the recall of otherwise dry, cortical information.32,33
Intuitively, it would seem that nonrepeating letters would facilitate the recall of the linked data, allowing each letter to provide a distinct cue, without any clouding by redundancy. Of the 3 most popular psychiatric mnemonics, however, only DIG FAST fits the learning theory. It contains 7 letters, repeats no letters, and has the limbic cue of allowing the learner to imagine a person with mania digging furiously.
| Substance dependence ADDICTeD12 Activities are given up or reduced Dependence, physical: tolerance Dependence, physical: withdrawal Intrapersonal (Internal) consequences, physical or psychological Can’t cut down or control use Time-consuming Duration or amount of use is greater than intended | Substance abuse WILD12 Work, school, or home role obligation failures Interpersonal or social consequences Legal problems Dangerous use | Alcohol abuse CAGE13 Have you ever felt you should CUT DOWN your drinking? Have people ANNOYED you by criticizing your drinking? Have you ever felt bad or GUILTY about your drinking? Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover (EYE-OPENER)? |
SIG: E CAPS falls within the range of 7 plus or minus 2, includes a limbic cue (although often forgotten, it refers to the prescription of energy capsules for depression), but repeats the letter S.
WWHHHHIMPS, with 10 letters, exceeds the recommended range, repeats the W (appearing twice) and the H (appearing 4 times), and provides no clear limbic cue.
| Causes I WATCH DEATH Infection Withdrawal Acute metabolic Trauma CNS pathology Hypoxia Deficiencies Endocrinopathies Acute vascular Toxins or drugs Heavy metals | Life-threatening causes WWHHHHIMPS* Wernicke’s encephalopathy Withdrawal Hypertensive crisis Hypoperfusion/hypoxia of the brain Hypoglycemia Hyper/hypothermia Intracranial process/infection Metabolic/meningitis Poisons Status epilepticus * Created by Gary W. Small, MD | Deliriogenic medications ACUTE CHANGE IN MS14 Antibiotics Cardiac drugs Urinary incontinence drugs Theophylline Ethanol Corticosteroids H2 blockers Antiparkinsonian drugs Narcotics Geriatric psychiatric drugs ENT drugs Insomnia drugs NSAIDs Muscle relaxants Seizure medicines |
It may be that recruiting the limbic system provides the greatest likelihood of recall. Recruiting this system may add increased valence to a particular mnemonic for a specific individual, but this same limbic valence may limit its usefulness in a professional context.
- Free searchable database of medical mnemonics. www.medicalmnemonics.com.
- Robinson DJ. Mnemonics and more for psychiatry. Port Huron, MI: Rapid Psychler Press, 2001.
From SIG: E CAPS to CAGE and WWHHHHIMPS, mnemonics help practitioners and trainees recall important lists (such as criteria for depression, screening questions for alcoholism, or life-threatening causes of delirium, respectively). Mnemonics’ efficacy rests on the principle that grouped information is easier to remember than individual points of data.
Not everyone loves mnemonics, but recollecting diagnostic criteria is useful in clinical practice and research, on board examinations, and for insurance reimbursement. Thus, tools that assist in recalling diagnostic criteria have a role in psychiatric practice and teaching.
In this article, we present 32 mnemonics to help clinicians diagnose:
- affective disorders (Box 1)1,2
- anxiety disorders (Box 2)3-6
- medication adverse effects (Box 3)7,8
- personality disorders (Box 4)9-11
- addiction disorders (Box 5)12,13
- causes of delirium (Box 6).14
We also discuss how mnemonics improve one’s memory, based on the principles of learning theory.
How mnemonics work
A mnemonic—from the Greek word “mnemonikos” (“of memory”)—links new data with previously learned information. Mnemonics assist in learning by reducing the amount of information (“cognitive load”) that needs to be stored for long-term processing and retrieval.15
Memory, defined as the “persistence of learning in a state that can be revealed at a later time,”16 can be divided into 2 types:
- declarative (a conscious recollection of facts, such as remembering a relative’s birthday)
- procedural (skills-based learning, such as riding a bicycle).
Declarative memory has a conscious component and may be mediated by the medial temporal lobe and cortical association structures. Procedural memory has less of a conscious component; it may involve the basal ganglia, cerebellum, and a variety of cortical sensory-perceptive regions.17
| Depression SIG: E CAPS* Suicidal thoughts Interests decreased Guilt Energy decreased Concentration decreased Appetite disturbance (increased or decreased) Psychomotor changes (agitation or retardation) Sleep disturbance (increased or decreased) * Created by Carey Gross, MD | Dysthymia HE’S 2 SAD2 Hopelessness Energy loss or fatigue Self-esteem is low 2 years minimum of depressed mood most of the day, for more days than not Sleep is increased or decreased Appetite is increased or decreased Decision-making or concentration is impaired | Mania DIG FAST Distractibility Indiscretion Grandiosity Flight of ideas Activity increase Sleep deficit Talkativeness |
| Depression C GASP DIE1 Concentration decreased Guilt Appetite Sleep disturbance Psychomotor agitation or retardation Death or suicide (thoughts or acts of) Interests decreased Energy decreased | Hypomania TAD HIGH Talkative Attention deficit Decreased need for sleep High self-esteem/grandiosity Ideas that race Goal-directed activity increased High-risk activity | Mania DeTeR the HIGH* Distractibility Talkativeness Reckless behavior Hyposomnia Ideas that race Grandiosity Hypersexuality * Created by Carey Gross, MD |
Declarative memory can be subdivided into working memory and long-term memory.
With working memory, new items of information are held briefly so that encoding and eventual storage can take place.
Working memory guides decision-making and future planning and is intricately related to attention.18-21 Functional MRI and positron emission tomography as well as neurocognitive testing have shown that working memory tasks activate the prefrontal cortex and brain regions specific to language and visuospatial memory.
The hippocampus is thought to rapidly absorb new information, and this data is consolidated and permanently stored via the prefrontal cortex.22-26 Given the hippocampus’ limited storage capacity, new information (such as what you ate for breakfast 3 weeks ago) will disappear if it is not repeated regularly.17
Long-term memory, on the other hand, is encoded knowledge that is linked to facts learned in the past; it is consolidated in the brain and can be readily retrieved. Neuroimaging studies have demonstrated opposing patterns of activation in the hippocampus and prefrontal cortex, depending on whether the memory being recalled is:
- new (high hippocampal activity, low prefrontal cortex activity)
- old (low hippocampal activity, high prefrontal cortex activity).27
Mnemonics are thought to affect working memory by reducing the introduced cognitive load and increasing the efficiency of memory acquisition and encoding. They reduce cognitive load by grouping objects into a single verbal or visual cue that can be introduced into working memory. Learning is optimized when the load on working memory is minimized, enabling long-term memory to be facilitated.28
| Generalized anxiety disorder Worry WARTS3 Wound up Worn-out Absentminded Restless Touchy Sleepless | Posttraumatic stress disorder TRAUMA5 Traumatic event Re-experience Avoidance Unable to function Month or more of symptoms Arousal increased | Anxiety disorder due to a general medical condition Physical Diseases That Have Commonly Appeared Anxious: Pheochromocytoma Diabetes mellitus Temporal lobe epilepsy Hyperthyroidism Carcinoid Alcohol withdrawal Arrhythmias |
| Generalized anxiety disorder WATCHERS4 Worry Anxiety Tension in muscles Concentration difficulty Hyperarousal (or irritability) Energy loss Restlessness Sleep disturbance | Posttraumatic stress disorder DREAMS6 Disinterest in usual activities Re-experience Event preceding symptoms Avoidance Month or more of symptoms Sympathetic arousal |
| Antidepressant discontinuation syndrome FINISH7 Flu-like symptoms Insomnia Nausea Imbalance Sensory disturbances Hyperarousal (anxiety/agitation) | Neuroleptic malignant syndrome FEVER8 Fever Encephalopathy Vital sign instability Elevated WBC/CPK Rigidity WBC: white blood cell count CPK: creatine phosphokinase | Serotonin syndrome HARMED Hyperthermia Autonomic instability Rigidity Myoclonus Encephalopathy Diaphoresis |
Mnemonics may use rhyme, music, or visual cues to enhance memory. Most mnemonics used in medical practice and education are word-based, including:
- Acronyms—words, each letter of which stands for a particular piece of information to be recalled (such as RICE for treatment of a sprained joint: rest, ice, compression, elevation).
- Acrostics—sentences with the first letter of each word prompting the desired recollection (such as “To Zanzibar by motor car” for the branches of the facial nerve: temporal, zygomatic, buccal, mandibular, cervical).
- Alphabetical sequences (such as ABCDE of trauma assessment: airway, breathing, circulation, disability, exposure).29
An appropriate teaching tool?
Dozens of mnemonics addressing psychiatric diagnosis and treatment have been published, but relatively few are widely used. Psychiatric educators may resist teaching with mnemonics, believing they might erode a humanistic approach to patients by reducing psychopathology to “a laundry list” of symptoms and the art of psychiatric diagnosis to a “check-box” endeavor. Mnemonics that use humor may be rejected as irreverent or unprofessional.30 Publishing a novel mnemonic may be viewed with disdain by some as an “easy” way of padding a curriculum vitae.
| Paranoid personality disorder SUSPECT9 Spousal infidelity suspected Unforgiving (bears grudges) Suspicious Perceives attacks (and reacts quickly) Enemy or friend? (suspects associates and friends) Confiding in others is feared Threats perceived in benign events | Schizotypal personality disorder ME PECULIAR9 Magical thinking Experiences unusual perceptions Paranoid ideation Eccentric behavior or appearance Constricted or inappropriate affect Unusual thinking or speech Lacks close friends Ideas of reference Anxiety in social situations Rule out psychotic or pervasive developmental disorders | Borderline personality disorder IMPULSIVE10 Impulsive Moodiness Paranoia or dissociation under stress Unstable self-image Labile intense relationships Suicidal gestures Inappropriate anger Vulnerability to abandonment Emptiness (feelings of) | Histrionic personality disorder PRAISE ME9 Provocative or seductive behavior Relationships considered more intimate than they are Attention (need to be the center of) Influenced easily Style of speech (impressionistic, lacking detail) Emotions (rapidly shifting, shallow) Make up (physical appearance used to draw attention to self) Emotions exaggerated | Narcissistic personality disorder GRANDIOSE11 Grandiose Requires attention Arrogant Need to be special Dreams of success and power Interpersonally exploitative Others (unable to recognize feelings/needs of) Sense of entitlement Envious | Dependent personality disorder RELIANCE9 Reassurance required Expressing disagreement difficult Life responsibilities assumed by others Initiating projects difficult Alone (feels helpless and uncomfortable when alone) Nurturance (goes to excessive lengths to obtain) Companionship sought urgently when a relationship ends Exaggerated fears of being left to care for self |
| Schizoid personality disorder DISTANT9 Detached or flattened affect Indifferent to criticism or praise Sexual experiences of little interest Tasks done solitarily Absence of close friends Neither desires nor enjoys close relationships Takes pleasure in few activities | Antisocial personality disorder CORRUPT9 Cannot conform to law Obligations ignored Reckless disregard for safety Remorseless Underhanded (deceitful) Planning insufficient (impulsive) Temper (irritable and aggressive) | Borderline personality disorder DESPAIRER* Disturbance of identity Emotionally labile Suicidal behavior Paranoia or dissociation Abandonment (fear of) Impulsive Relationships unstable Emptiness (feelings of) Rage (inappropriate) * Created by Jason P. Caplan, MD | Histrionic personality disorder ACTRESSS* Appearance focused Center of attention Theatrical Relationships (believed to be more intimate than they are) Easily influenced Seductive behavior Shallow emotions Speech (impressionistic and vague) * Created by Jason P. Caplan, MD | Avoidant personality disorder CRINGES9 Criticism or rejection preoccupies thoughts in social situations Restraint in relationships due to fear of shame Inhibited in new relationships Needs to be sure of being liked before engaging socially Gets around occupational activities with need for interpersonal contact Embarrassment prevents new activity or taking risks Self viewed as unappealing or inferior | Obsessive-compulsive personality disorder SCRIMPER* Stubborn Cannot discard worthless objects Rule obsessed Inflexible Miserly Perfectionistic Excludes leisure due to devotion to work Reluctant to delegate to others * Created by Jason P. Caplan, MD |
Entire Web sites exist to share mnemonics for medical education (see Related Resources). Thus it is likely that trainees are using them with or without their teachers’ supervision. Psychiatric educators need to be aware of the mnemonics their trainees are using and to:
- screen these tools for factual errors (such as incomplete diagnostic criteria)
- remind trainees that although mnemonics are useful, psychiatrists should approach patients as individuals without the prejudice of a potentially pejorative label.
Our methodology
In preparing this article, we gathered numerous mnemonics (some published and some novel) designed to capture the learner’s attention and impart information pertinent to psychiatric diagnosis and treatment. Whenever possible, we credited each mnemonic to its creator, but—given the difficulty in confirming authorship of (what in many cases has become) oral history—we’ve listed some mnemonics without citation.
Our list is far from complete because we likely are unaware of many mnemonics, and we have excluded some that seemed obscure, unwieldy, or redundant. We have not excluded mnemonics that some may view as pejorative but merely report their existence. Including them does not mean that we endorse them.
This article lists 32 mnemonics related to psychiatric diagnosis. Thus, it seems odd that an informal survey of >60 residents at the Massachusetts General Hospital (MGH)/McLean Residency Training Program in Psychiatry revealed that most were aware of only 2 or 3 psychiatric mnemonics, typically:
- SIG: E CAPS (a tool to recall the criteria for depression)
- DIG FAST (a list of criteria for diagnosing mania)
- WWHHHHIMPS (a tool for recalling life-threatening causes of delirium).
Although this unscientific survey may be biased because faculty or trainees at MGH created the above 3 mnemonics, it nonetheless begs the question of what qualities make a mnemonic memorable.
Learning theory provides several clues. George Miller’s classic 1956 paper, “The magical number seven, plus or minus two: some limits on our capacity for processing information,” discussed the finding that 7 seems to be the upper limit of individual pieces of data that can be easily remembered.31 Research also has shown that recruiting the limbic system (potentially through the use of humor) aids in the recall of otherwise dry, cortical information.32,33
Intuitively, it would seem that nonrepeating letters would facilitate the recall of the linked data, allowing each letter to provide a distinct cue, without any clouding by redundancy. Of the 3 most popular psychiatric mnemonics, however, only DIG FAST fits the learning theory. It contains 7 letters, repeats no letters, and has the limbic cue of allowing the learner to imagine a person with mania digging furiously.
| Substance dependence ADDICTeD12 Activities are given up or reduced Dependence, physical: tolerance Dependence, physical: withdrawal Intrapersonal (Internal) consequences, physical or psychological Can’t cut down or control use Time-consuming Duration or amount of use is greater than intended | Substance abuse WILD12 Work, school, or home role obligation failures Interpersonal or social consequences Legal problems Dangerous use | Alcohol abuse CAGE13 Have you ever felt you should CUT DOWN your drinking? Have people ANNOYED you by criticizing your drinking? Have you ever felt bad or GUILTY about your drinking? Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover (EYE-OPENER)? |
SIG: E CAPS falls within the range of 7 plus or minus 2, includes a limbic cue (although often forgotten, it refers to the prescription of energy capsules for depression), but repeats the letter S.
WWHHHHIMPS, with 10 letters, exceeds the recommended range, repeats the W (appearing twice) and the H (appearing 4 times), and provides no clear limbic cue.
| Causes I WATCH DEATH Infection Withdrawal Acute metabolic Trauma CNS pathology Hypoxia Deficiencies Endocrinopathies Acute vascular Toxins or drugs Heavy metals | Life-threatening causes WWHHHHIMPS* Wernicke’s encephalopathy Withdrawal Hypertensive crisis Hypoperfusion/hypoxia of the brain Hypoglycemia Hyper/hypothermia Intracranial process/infection Metabolic/meningitis Poisons Status epilepticus * Created by Gary W. Small, MD | Deliriogenic medications ACUTE CHANGE IN MS14 Antibiotics Cardiac drugs Urinary incontinence drugs Theophylline Ethanol Corticosteroids H2 blockers Antiparkinsonian drugs Narcotics Geriatric psychiatric drugs ENT drugs Insomnia drugs NSAIDs Muscle relaxants Seizure medicines |
It may be that recruiting the limbic system provides the greatest likelihood of recall. Recruiting this system may add increased valence to a particular mnemonic for a specific individual, but this same limbic valence may limit its usefulness in a professional context.
- Free searchable database of medical mnemonics. www.medicalmnemonics.com.
- Robinson DJ. Mnemonics and more for psychiatry. Port Huron, MI: Rapid Psychler Press, 2001.
1. Abraham PF, Shirley ER. New mnemonic for depressive symptoms. Am J Psychiatry 2006;163(2):329-30.
2. Christman DS. “HE’S 2 SAD” detects dysthymic disorder. Current Psychiatry 2008;7(3):120.-
3. Coupland NJ. Worry WARTS have generalized anxiety disorder. Can J Psychiatry 2002;47(2):197.-
4. Berber MJ. WATCHERS: recognizing generalized anxiety disorder. J Clin Psychiatry 2000;61(6):447.-
5. Khouzam HR. A simple mnemonic for the diagnostic criteria for post-traumatic stress disorder. West J Med 2001;174(6):424.-
6. Short DD, Workman EA, Morse JH, Turner RL. Mnemonics for eight DSM-III-R disorders. Hosp Community Psychiatry 1992;43(6):642-4.
7. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, Insomnia, Nausea, Imbalance, Sensory disturbances, and Hyperarousal (anxiety/agitation). J Clin Psychiatry 1998;59(5):255.-
8. Christensen RC. Identify neuroleptic malignant syndrome with FEVER. Current Psychiatry 2005;4(7):102.-
9. Pinkofsky HB. Mnemonics for DSM-IV personality disorders. Psychiatr Serv 1997;48(9):1197-8.
10. Senger HL. Borderline mnemonic. Am J Psychiatry 1997;154(9):1321.-
11. Kim SI, Swanson TA, Caplan JP, eds. Underground clinical vignettes step 2: psychiatry. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:130.
12. Bogenschutz MP, Quinn DK. Acronyms for substance use disorders. J Clin Psychiatry 2001;62(6):474-5.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252(14):1905-7.
14. Flaherty JH. Psychotherapeutic agents in older adults. Commonly prescribed and over-the-counter remedies: causes of confusion. Clin Geriatr Med 1998;14:101-27.
15. Sweller J. Cognitive load theory, learning difficulty, and instructional design. Learn Instr 1994;4:295-312.
16. Squire LR. Memory and brain. New York, NY: Oxford University Press; 1987.
17. DeLuca J, Lengenfelder J, Eslinger P. Memory and learning. In: Rizzo M, Eslinger P, eds. Principles and practice of behavioral neurology and neuropsychology. Philadelphia, PA: Saunders; 2004:251.
18. Dash PK, Moore AN, Kobori N, et al. Molecular activity underlying working memory. Learn Mem 2007;14:554-63.
19. Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience 2006;139:201-8.
20. Knudson EI. Fundamental components of attention. Ann Rev Neurosci 2007;30:57-78.
21. Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience 2006;139:23-36.
22. Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain 2001;124:849-81.
23. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann Rev Neurosci 2001;24:167-202.
24. Schumacher EH, Lauber E, Awh E, et al. PET evidence for a modal verbal working memory system. Neuroimage 1996;3:79-88.
25. Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex 1996;6:11-20.
26. Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 2003;3(4):255-74.
27. Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 2005;6:119-30.
28. Sweller J. Cognitive load during problem solving: effects on learning. Cogn Sci 1988;12(1):257-85.
29. Beitz JM. Unleashing the power of memory: the mighty mnemonic. Nurse Educ 1997;22(2):25-9.
30. Larson EW. Criticism of mnemonic device. Am J Psychiatry 1990;147(7):963-4.
31. Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev 1956;63:81-97.
32. Schmidt SR. Effects of humor on sentence memory. J Exp Psychol Learn Mem Cogn 1994;20(4):953-67.
33. Lippman LG, Dunn ML. Contextual connections within puns: effects on perceived humor and memory. J Gen Psychol 2000;127(2):185-97.
1. Abraham PF, Shirley ER. New mnemonic for depressive symptoms. Am J Psychiatry 2006;163(2):329-30.
2. Christman DS. “HE’S 2 SAD” detects dysthymic disorder. Current Psychiatry 2008;7(3):120.-
3. Coupland NJ. Worry WARTS have generalized anxiety disorder. Can J Psychiatry 2002;47(2):197.-
4. Berber MJ. WATCHERS: recognizing generalized anxiety disorder. J Clin Psychiatry 2000;61(6):447.-
5. Khouzam HR. A simple mnemonic for the diagnostic criteria for post-traumatic stress disorder. West J Med 2001;174(6):424.-
6. Short DD, Workman EA, Morse JH, Turner RL. Mnemonics for eight DSM-III-R disorders. Hosp Community Psychiatry 1992;43(6):642-4.
7. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, Insomnia, Nausea, Imbalance, Sensory disturbances, and Hyperarousal (anxiety/agitation). J Clin Psychiatry 1998;59(5):255.-
8. Christensen RC. Identify neuroleptic malignant syndrome with FEVER. Current Psychiatry 2005;4(7):102.-
9. Pinkofsky HB. Mnemonics for DSM-IV personality disorders. Psychiatr Serv 1997;48(9):1197-8.
10. Senger HL. Borderline mnemonic. Am J Psychiatry 1997;154(9):1321.-
11. Kim SI, Swanson TA, Caplan JP, eds. Underground clinical vignettes step 2: psychiatry. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:130.
12. Bogenschutz MP, Quinn DK. Acronyms for substance use disorders. J Clin Psychiatry 2001;62(6):474-5.
13. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252(14):1905-7.
14. Flaherty JH. Psychotherapeutic agents in older adults. Commonly prescribed and over-the-counter remedies: causes of confusion. Clin Geriatr Med 1998;14:101-27.
15. Sweller J. Cognitive load theory, learning difficulty, and instructional design. Learn Instr 1994;4:295-312.
16. Squire LR. Memory and brain. New York, NY: Oxford University Press; 1987.
17. DeLuca J, Lengenfelder J, Eslinger P. Memory and learning. In: Rizzo M, Eslinger P, eds. Principles and practice of behavioral neurology and neuropsychology. Philadelphia, PA: Saunders; 2004:251.
18. Dash PK, Moore AN, Kobori N, et al. Molecular activity underlying working memory. Learn Mem 2007;14:554-63.
19. Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience 2006;139:201-8.
20. Knudson EI. Fundamental components of attention. Ann Rev Neurosci 2007;30:57-78.
21. Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience 2006;139:23-36.
22. Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain 2001;124:849-81.
23. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann Rev Neurosci 2001;24:167-202.
24. Schumacher EH, Lauber E, Awh E, et al. PET evidence for a modal verbal working memory system. Neuroimage 1996;3:79-88.
25. Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex 1996;6:11-20.
26. Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 2003;3(4):255-74.
27. Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 2005;6:119-30.
28. Sweller J. Cognitive load during problem solving: effects on learning. Cogn Sci 1988;12(1):257-85.
29. Beitz JM. Unleashing the power of memory: the mighty mnemonic. Nurse Educ 1997;22(2):25-9.
30. Larson EW. Criticism of mnemonic device. Am J Psychiatry 1990;147(7):963-4.
31. Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev 1956;63:81-97.
32. Schmidt SR. Effects of humor on sentence memory. J Exp Psychol Learn Mem Cogn 1994;20(4):953-67.
33. Lippman LG, Dunn ML. Contextual connections within puns: effects on perceived humor and memory. J Gen Psychol 2000;127(2):185-97.
Put your patients to sleep: Useful nondrug strategies for chronic insomnia
Ms. H, age 53, has a 20-year history of recurrent major depressive disorder. She seeks treatment for insomnia; her primary complaint is that “no medicine has really ever helped me to sleep for very long.” She reports that every night she experiences a 2-hour sleep onset delay and an average of 5 awakenings that last 10 to 60 minutes each. Her mood is stable.
After failed trials of zolpidem, mirtazapine, amitriptyline, and sertraline plus trazodone, she improves with quetiapine, 50 mg at bedtime, plus sertraline, 150 mg at bedtime. Unfortunately, over the next 6 months Ms. H gains 20 pounds and her physician becomes concerned about her fasting serum glucose levels, which suggest borderline diabetes.
After Ms. H discontinues quetiapine, onset and maintenance insomnia remain clinically significant. Polysomnography reveals moderately loud snoring, a normal respiratory disturbance index of 4.5 per hour, no periodic leg movements of sleep, 32-minute sleep onset, total sleep time of 389 minutes (6.5 hours), and a sleep efficiency of 72%. Ms. H estimates that it took her 120 minutes to fall asleep and that she slept only 270 minutes (4.5 hours) of the 540 minutes (9 hours) in bed. The sleep specialist recommends cognitive-behavioral therapy for insomnia.
For some chronic insomnia patients—such as Ms. H—pharmacotherapy is ineffective or causes intolerable side effects. In any year, >50% of adults in the general population report experiencing difficulty falling asleep, staying asleep, early awakening, or poorly restorative sleep, but these symptoms are usually time-limited and have only a small impact on daytime alertness and function. Chronic insomnia, on the other hand, lasts ≥1 month and has substantial impact on daytime alertness and attention, cognitive function, depressed and anxious mood, and focused performance (Box).1
Medications used to treat insomnia include FDA-approved drugs such as eszopiclone and zolpidem and off-label agents such as mirtazapine and trazodone. The cognitive, behavioral, and other nonpharmacologic therapies described below can be effective options, either alone or in combination with medication.
One in 10 adults in industrialized nations experiences chronic insomnia. Women are affected twice as often as men, with higher rates also reported in older patients and those in lower socioeconomic groups.
Among adults with chronic insomnia, 35% to 45% have psychiatric comorbidities, such as anxiety or mood disorders, and 15% have primary insomnia—sleep disturbance with no identifiable cause, which traditional medical literature described as conditioned or psychophysiologic insomnia.
In the remaining cases, chronic insomnia is associated with:
- medical and sleep disorders (restless legs syndrome, periodic leg movements of sleep, and sleep apnea)
- general medical disorders, particularly those that cause pain
- use of medications that disrupt normal CNS sleep mechanisms.
Source: Reference 1
Assessing insomnia
Start by performing a thorough assessment and history. I have described this process in previous reviews,1,2 as has Neubauer in Current Psychiatry.3
Before initiating therapy for insomnia, assess and address the following:
- significant ongoing depression, mania, hypomania, generalized anxiety, panic, or obsessive-compulsive symptoms that impact sleep
- primary medical disorders of sleep, including restless legs syndrome, increased motor activity during sleep such as periodic leg movements of sleep, and the snoring/snorting of sleep apnea
- prescribed or self-administered medications or substances that can disrupt sleep, such as alcohol, caffeine, stimulants, corticosteroids, or beta blockers.
Recommended nondrug therapies
In 2006, the Standards of Practice Committee of the American Academy of Sleep Medicine (AASM) updated a comprehensive literature review of psychological and behavioral treatments of primary and secondary insomnia. On the basis of this peer-reviewed, graded evidence, the AASM recommended:
- stimulus control therapy
- relaxation training
- cognitive-behavioral therapy for insomnia (CBTi).4
The AASM also offered guidelines for sleep restriction therapy, multi-component therapy without cognitive therapy, paradoxical intention, and biofeedback. Evidence for sleep hygiene, imaging training, or cognitive therapy alone was insufficient, and the AASM neither recommended nor excluded these methods. Psychological and behavioral interventions were considered effective for treating insomnia in older adults and patients withdrawing from hypnotics.
Stimulus control therapy. Bootzin et al5 first evaluated stimulus control therapy for conditioned insomnia (subsequently identified as primary insomnia). This therapy’s goal is to interrupt the conditioned activation that occurs at bedtime. Patients are instructed to:
- go to bed when sleepy
- remain in bed for no more than 10 minutes (20 minutes if elderly) without sleeping
- if unable to sleep, get up, do something boring, and return to bed only when sleepy
- repeat getting up and returning as frequently as necessary until sleep onset.
For the first 2 weeks of stimulus control therapy, patients are required to self-monitor their sleep behaviors using a sleep diary. Stimulus control therapy is beneficial for primary insomnia and insomnia related to anxious preoccupation. About 70% of patients with conditioned insomnia will improve using stimulus control therapy,4 but it is not clear whether the primary effective intervention is:
- patients dissociating conditioned responses at bedtime, or
- the inevitable sleep restriction caused by getting out of bed.
Relaxation training. Progressive muscle relaxation is a common behavioral treatment of insomnia. Patients learn to tense and then relax individual muscles, beginning at the feet or head and working their way up or down the body. Patients are taught the difference between tension and relaxation to facilitate a relaxation response at bedtime. Another method is the body scanning technique, in which the patient “talks” to each body part, telling it to “relax… relax… relax.”
Relaxation training is predicated on the belief that insomnia is caused by somatized tension and psychophysiologic arousal. The greatest challenge to effective relaxation training is that patients need extensive daytime practice before they can bring the method to the bedroom.
Remind patients that “practice makes perfect.” Therapists often instruct patients to start practicing their relaxation method during the day while self-monitoring by sleep diary and restricting time in bed at night.2
CBTi is the most extensively investigated nonpharmacologic therapy for insomnia.6 It has been used to effectively manage comorbid insomnia in patients with psychiatric disorders,7,8 such as depression,9 generalized anxiety,10 and alcohol dependence,11 as well as those with breast cancer,12 traumatic brain injury,13 and fibromyalgia.14 Age does not appear to be a limitation; research trials show the technique is effective in elderly patients.15
CBTi incorporates cognitive strategies and behavioral interventions to improve sleep quality. Patient self-monitoring with sleep diaries and worksheets is essential.
CBTi commonly is provided in 5 to 8 sessions over 8 to 12 weeks, although studies have described abbreviated practices that used 2 sessions16 and CBTi delivered over the Internet.17 Highly trained clinical psychologists are at the forefront of therapy, but counselors and nurses in primary care settings have administered CBTi.18 For primary insomnia, CBTi is superior in efficacy to pharmacotherapy:
- as initial treatment19
- for long-term management4
- in assisting discontinuation of hypnotic medication.20
CASE CONTINUED: An effective approach
You refer Ms. H to a clinical psychologist who specializes in CBTi. Ms. H begins self-monitoring with a sleep diary and has 5 CBTi sessions over 8 weeks. Initial interventions reduce time in bed from 9 hours to 7 hours per night. Ms. H learns simple relaxation methods that she practices for 2 weeks before attempting to use them to sleep. The psychologist addresses her dysfunctional beliefs about sleep.
During the last 2 weeks of therapy, Ms. H’s sleep diary reveals a sleep efficiency of 92% and improvements in well being, energy, and perceived work efficiency. At a 3-month booster visit, Ms. H has sustained these gains in sleep and daytime function.
Implementing nondrug therapy
I recommend the following steps when offering psychological and behavioral treatment of chronic insomnia, such as CBTi.
Initial visit. Determine whether your patient needs treatment for depressive or anxiety symptoms. Assess the need for polysomnography. Does the patient have a history of an urge to move the legs (restless legs syndrome), increased kicking behavior at night (periodic leg movements of sleep), or loud, disruptive snoring (obstructive sleep apnea)? It is often helpful to have patients think back to when they were consistently sleeping well to identify factors that might be exacerbating poor sleep.
Session 1 (Week 0). Teach patients about normal sleep, how it changes over the life cycle, and common dysfunctional beliefs and behaviors that worsen sleep. Tell patients that every morning when they wake up they should complete a sleep diary (Table 1); you can download a sample sleep diary by visiting this article on CurrentPsychiatry.com.
Table 1
Insomnia: What to document on a sleep diary
| Daytime fatigue |
| Minutes spent napping |
| Medication use |
| Time the patient first tried to fall asleep |
| How long it took to fall asleep |
| How many times the patient woke up |
| Final waking time |
| Hours slept |
| Sleep quality rating |
| How refreshed the patient feels on awakening |
Session 2 (Week 1). Review the sleep diary. Address infractions of sleep hygiene, such as working until bedtime, using caffeine or alcohol in the evening, excessive smoking, or eating in bed. Discuss and specify mutual therapeutic goals for:
- minutes to sleep onset
- minutes of nighttime wakefulness
- number of awakenings
- improvements in sleep efficiency, morning refreshment/alertness, and daytime functioning.
Therapeutic intervention: Instruct patients to reduce their total time in bed (TIB) to their estimated total sleep time, unless they report <6 hours. Insomnia patients commonly overestimate their amount of wakefulness. Because research indicates daytime performance is adversely affected when sleep falls below 6 hours per night,21 I initially limit TIB to 6 hours and further restrict TIB in future sessions as needed to improve sleep efficiency.
Session 3 (Week 2). Review the sleep diary, and calculate the average time to sleep onset and sleep efficiency (divide total minutes of reported sleep by the total minutes spent in bed). Typical goals include an average onset of 10 to 20 minutes and an average efficiency of >90%.
Therapeutic intervention: If sleep efficiency falls below 80%, further restrict TIB by 15 minutes; if sleep efficiency is >90%, increase TIB by 15 minutes (no TIB change is needed with efficiencies between 80% and 90%). Identify dysfunctional beliefs about sleep, and provide strategies to interrupt cognitive overactivation—the pressured “talking to oneself” in hopes of falling asleep.
Session 4 (Week 3). Review the sleep diary, and calculate the average time to sleep onset and sleep efficiency. Increase or decrease TIB based on sleep efficiency as described above. Determine if the patient has dysfunctional beliefs regarding sleep.
Therapeutic intervention: Reframe the patient’s dysfunctional beliefs/concepts by comparing sleep diary entries with dysfunctional beliefs (Table 2). Remind patients about strategies to address cognitive overactivation, and have them practice daily to apply the appropriate reframe response from Table 2 that improves sleep. Review progressive muscular relaxation to address somatized tension and arousal, but instruct patients to practice relaxation only during the day at this point.
Table 2
Correcting patients’ dysfunctional sleep beliefs/concepts
| Belief/concept | Reframe responses |
|---|---|
| ‘I need 8 hours sleep per night’ | 1. Nightly sleep need varies among individuals from 5 to 9 hours, particularly with aging 2. Employed adults sleep 6.5 to 7 hours per workweek night 3. For the ‘average’ person, it takes <6 hours of sleep to reduce performance |
| ‘If I don’t sleep, I can’t _____ (work, socialize, take care of the kids, etc.) or ‘If I don’t sleep tonight, I won’t be able to ____’ | 1. Every day one-third of Americans sleep <6.5 hours and yet work, socialize, and live their lives 2. ‘You told me that on ____ you had a terrible night, yet you did ____ (that presentation, meeting, activity with family, etc.)’ |
| ‘If I don’t sleep, I feel _____’ | Explore situations where the person has felt tired, irritable, angry, anxious, etc. independent from lack of sleep |
| ‘If X happens, I won’t sleep’ | Explore situations where X or something like it happened, yet sleep occurred |
| ‘I don’t sleep at all’ | 1. Explore whether a bed partner reports the patient was sleeping or snoring when the person was convinced he or she was awake 2. Tell patients that if they remain in bed for >30 minutes, it is likely they slept, particularly if anxious or frustrated (older depressed patients may be an exception) 3. Teach patients that ‘don’t at all’ statements often represent an excessive focus on wakefulness, and that self-monitoring by sleep diary is helpful |
Session 5 (Week 4). Review the sleep diary. Adjust TIB as necessary. Emphasize the patient’s mastery of dysfunctional beliefs, and highlight progress on the sleep diary. Spend much of this session helping patients improve their relaxation practice and preparing them to bring it to bedtime.
Therapeutic intervention: Tell the patient to apply the relaxation training to bedtime and nocturnal awakenings.
Session 6 (Week 6). Review the sleep diary. Emphasize progress. Address any problem areas regarding dysfunctional beliefs, maladaptive behaviors, or relaxation methods.
Therapeutic intervention: Prepare patients to maintain sleep gains on their own.
Session 7 (Week 8). Review the sleep diary. Have patients identify areas of mastery. Discuss scenarios that might be expected to result in a temporary return of insomnia—such as difficulties with work or home life, stress of job change, or medical illness—and strategies they could apply to improve sleep. Such strategies might include a “safety net” of a sedative/hypnotic agent to use after ≥2 nights of poor sleep.
‘Booster’ session. Three months later, schedule a booster session to determine whether the patient has maintained mastery of improved sleep. Patients who are doing well often cancel this session because they are satisfied with their progress.
Related resource
- American Academy of Sleep Medicine. www.aasmnet.org.
Drug brand names
- Amitriptyline • Elavil, Endep
- Eszopiclone • Lunesta
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Becker receives research/grant support from sanofi-aventis and is a speaker for Sepracor Inc. and Takeda Pharmaceutical.
1. Becker PM. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Psychiatr Clin North Am 2006;29(4):855-70.
2. Becker PM. Pharmacologic and nonpharmacologic treatments of insomnia. Neurol Clin 2005;23(4):1149-63.
3. Neubauer DN. Treatment resistant-insomnia: ask yourself 8 questions. Current Psychiatry 2007;6(12):46-54.
4. Morgenthaler T, Kramer M, Alessi C, et al. American Academy of Sleep Medicine. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep 2006;29(11):1415-9.
5. Bootzin RR, Perlis ML. Nonpharmacologic treatments of insomnia. J Clin Psychiatry 1992;53(suppl):37-41.
6. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29(11):1398-414.
7. Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005;25(5):559-92.
8. Dopke CA, Lehner RK, Wells AM. Cognitive-behavioral group therapy for insomnia in individuals with serious mental illnesses: a preliminary evaluation. Psychiatr Rehabil J 2004;27(3):235-42.
9. Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry 2007;68(2):254-60.
10. Bélanger L, Morin CM, Langlois F, Ladouceur R. Insomnia and generalized anxiety disorder: effects of cognitive behavior therapy for GAD on insomnia symptoms. J Anxiety Disord 2004;18(4):561-71.
11. Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction 2004;99(9):1121-32.
12. Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum 2007;34(5):E51-9.
13. Ouellet MC, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Arch Phys Med Rehabil 2007;88(12):1581-92.
14. Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med 2005;165(21):2527-35.
15. Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol 2006;25(1):3-14.
16. Edinger JD, Wohlgemuth WK, Radtke RA, et al. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep 2007;30(2):203-12.
17. Ström L, Pettersson R, Andersson G. Internet-based treatment for insomnia: a controlled evaluation. J Consult Clin Psychol 2004;72(1):113-20.
18. Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep 2007;30(5):574-84.
19. Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med 2004;164(17):1888-96.
20. Morin CM, Bélanger L, Bastien C, Vallières A. Long-term outcome after discontinuation of benzodiazepines for insomnia: a survival analysis of relapse. Behav Res Ther 2005;43(1):1-14.
21. Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci 2008;1129:305-22.
Ms. H, age 53, has a 20-year history of recurrent major depressive disorder. She seeks treatment for insomnia; her primary complaint is that “no medicine has really ever helped me to sleep for very long.” She reports that every night she experiences a 2-hour sleep onset delay and an average of 5 awakenings that last 10 to 60 minutes each. Her mood is stable.
After failed trials of zolpidem, mirtazapine, amitriptyline, and sertraline plus trazodone, she improves with quetiapine, 50 mg at bedtime, plus sertraline, 150 mg at bedtime. Unfortunately, over the next 6 months Ms. H gains 20 pounds and her physician becomes concerned about her fasting serum glucose levels, which suggest borderline diabetes.
After Ms. H discontinues quetiapine, onset and maintenance insomnia remain clinically significant. Polysomnography reveals moderately loud snoring, a normal respiratory disturbance index of 4.5 per hour, no periodic leg movements of sleep, 32-minute sleep onset, total sleep time of 389 minutes (6.5 hours), and a sleep efficiency of 72%. Ms. H estimates that it took her 120 minutes to fall asleep and that she slept only 270 minutes (4.5 hours) of the 540 minutes (9 hours) in bed. The sleep specialist recommends cognitive-behavioral therapy for insomnia.
For some chronic insomnia patients—such as Ms. H—pharmacotherapy is ineffective or causes intolerable side effects. In any year, >50% of adults in the general population report experiencing difficulty falling asleep, staying asleep, early awakening, or poorly restorative sleep, but these symptoms are usually time-limited and have only a small impact on daytime alertness and function. Chronic insomnia, on the other hand, lasts ≥1 month and has substantial impact on daytime alertness and attention, cognitive function, depressed and anxious mood, and focused performance (Box).1
Medications used to treat insomnia include FDA-approved drugs such as eszopiclone and zolpidem and off-label agents such as mirtazapine and trazodone. The cognitive, behavioral, and other nonpharmacologic therapies described below can be effective options, either alone or in combination with medication.
One in 10 adults in industrialized nations experiences chronic insomnia. Women are affected twice as often as men, with higher rates also reported in older patients and those in lower socioeconomic groups.
Among adults with chronic insomnia, 35% to 45% have psychiatric comorbidities, such as anxiety or mood disorders, and 15% have primary insomnia—sleep disturbance with no identifiable cause, which traditional medical literature described as conditioned or psychophysiologic insomnia.
In the remaining cases, chronic insomnia is associated with:
- medical and sleep disorders (restless legs syndrome, periodic leg movements of sleep, and sleep apnea)
- general medical disorders, particularly those that cause pain
- use of medications that disrupt normal CNS sleep mechanisms.
Source: Reference 1
Assessing insomnia
Start by performing a thorough assessment and history. I have described this process in previous reviews,1,2 as has Neubauer in Current Psychiatry.3
Before initiating therapy for insomnia, assess and address the following:
- significant ongoing depression, mania, hypomania, generalized anxiety, panic, or obsessive-compulsive symptoms that impact sleep
- primary medical disorders of sleep, including restless legs syndrome, increased motor activity during sleep such as periodic leg movements of sleep, and the snoring/snorting of sleep apnea
- prescribed or self-administered medications or substances that can disrupt sleep, such as alcohol, caffeine, stimulants, corticosteroids, or beta blockers.
Recommended nondrug therapies
In 2006, the Standards of Practice Committee of the American Academy of Sleep Medicine (AASM) updated a comprehensive literature review of psychological and behavioral treatments of primary and secondary insomnia. On the basis of this peer-reviewed, graded evidence, the AASM recommended:
- stimulus control therapy
- relaxation training
- cognitive-behavioral therapy for insomnia (CBTi).4
The AASM also offered guidelines for sleep restriction therapy, multi-component therapy without cognitive therapy, paradoxical intention, and biofeedback. Evidence for sleep hygiene, imaging training, or cognitive therapy alone was insufficient, and the AASM neither recommended nor excluded these methods. Psychological and behavioral interventions were considered effective for treating insomnia in older adults and patients withdrawing from hypnotics.
Stimulus control therapy. Bootzin et al5 first evaluated stimulus control therapy for conditioned insomnia (subsequently identified as primary insomnia). This therapy’s goal is to interrupt the conditioned activation that occurs at bedtime. Patients are instructed to:
- go to bed when sleepy
- remain in bed for no more than 10 minutes (20 minutes if elderly) without sleeping
- if unable to sleep, get up, do something boring, and return to bed only when sleepy
- repeat getting up and returning as frequently as necessary until sleep onset.
For the first 2 weeks of stimulus control therapy, patients are required to self-monitor their sleep behaviors using a sleep diary. Stimulus control therapy is beneficial for primary insomnia and insomnia related to anxious preoccupation. About 70% of patients with conditioned insomnia will improve using stimulus control therapy,4 but it is not clear whether the primary effective intervention is:
- patients dissociating conditioned responses at bedtime, or
- the inevitable sleep restriction caused by getting out of bed.
Relaxation training. Progressive muscle relaxation is a common behavioral treatment of insomnia. Patients learn to tense and then relax individual muscles, beginning at the feet or head and working their way up or down the body. Patients are taught the difference between tension and relaxation to facilitate a relaxation response at bedtime. Another method is the body scanning technique, in which the patient “talks” to each body part, telling it to “relax… relax… relax.”
Relaxation training is predicated on the belief that insomnia is caused by somatized tension and psychophysiologic arousal. The greatest challenge to effective relaxation training is that patients need extensive daytime practice before they can bring the method to the bedroom.
Remind patients that “practice makes perfect.” Therapists often instruct patients to start practicing their relaxation method during the day while self-monitoring by sleep diary and restricting time in bed at night.2
CBTi is the most extensively investigated nonpharmacologic therapy for insomnia.6 It has been used to effectively manage comorbid insomnia in patients with psychiatric disorders,7,8 such as depression,9 generalized anxiety,10 and alcohol dependence,11 as well as those with breast cancer,12 traumatic brain injury,13 and fibromyalgia.14 Age does not appear to be a limitation; research trials show the technique is effective in elderly patients.15
CBTi incorporates cognitive strategies and behavioral interventions to improve sleep quality. Patient self-monitoring with sleep diaries and worksheets is essential.
CBTi commonly is provided in 5 to 8 sessions over 8 to 12 weeks, although studies have described abbreviated practices that used 2 sessions16 and CBTi delivered over the Internet.17 Highly trained clinical psychologists are at the forefront of therapy, but counselors and nurses in primary care settings have administered CBTi.18 For primary insomnia, CBTi is superior in efficacy to pharmacotherapy:
- as initial treatment19
- for long-term management4
- in assisting discontinuation of hypnotic medication.20
CASE CONTINUED: An effective approach
You refer Ms. H to a clinical psychologist who specializes in CBTi. Ms. H begins self-monitoring with a sleep diary and has 5 CBTi sessions over 8 weeks. Initial interventions reduce time in bed from 9 hours to 7 hours per night. Ms. H learns simple relaxation methods that she practices for 2 weeks before attempting to use them to sleep. The psychologist addresses her dysfunctional beliefs about sleep.
During the last 2 weeks of therapy, Ms. H’s sleep diary reveals a sleep efficiency of 92% and improvements in well being, energy, and perceived work efficiency. At a 3-month booster visit, Ms. H has sustained these gains in sleep and daytime function.
Implementing nondrug therapy
I recommend the following steps when offering psychological and behavioral treatment of chronic insomnia, such as CBTi.
Initial visit. Determine whether your patient needs treatment for depressive or anxiety symptoms. Assess the need for polysomnography. Does the patient have a history of an urge to move the legs (restless legs syndrome), increased kicking behavior at night (periodic leg movements of sleep), or loud, disruptive snoring (obstructive sleep apnea)? It is often helpful to have patients think back to when they were consistently sleeping well to identify factors that might be exacerbating poor sleep.
Session 1 (Week 0). Teach patients about normal sleep, how it changes over the life cycle, and common dysfunctional beliefs and behaviors that worsen sleep. Tell patients that every morning when they wake up they should complete a sleep diary (Table 1); you can download a sample sleep diary by visiting this article on CurrentPsychiatry.com.
Table 1
Insomnia: What to document on a sleep diary
| Daytime fatigue |
| Minutes spent napping |
| Medication use |
| Time the patient first tried to fall asleep |
| How long it took to fall asleep |
| How many times the patient woke up |
| Final waking time |
| Hours slept |
| Sleep quality rating |
| How refreshed the patient feels on awakening |
Session 2 (Week 1). Review the sleep diary. Address infractions of sleep hygiene, such as working until bedtime, using caffeine or alcohol in the evening, excessive smoking, or eating in bed. Discuss and specify mutual therapeutic goals for:
- minutes to sleep onset
- minutes of nighttime wakefulness
- number of awakenings
- improvements in sleep efficiency, morning refreshment/alertness, and daytime functioning.
Therapeutic intervention: Instruct patients to reduce their total time in bed (TIB) to their estimated total sleep time, unless they report <6 hours. Insomnia patients commonly overestimate their amount of wakefulness. Because research indicates daytime performance is adversely affected when sleep falls below 6 hours per night,21 I initially limit TIB to 6 hours and further restrict TIB in future sessions as needed to improve sleep efficiency.
Session 3 (Week 2). Review the sleep diary, and calculate the average time to sleep onset and sleep efficiency (divide total minutes of reported sleep by the total minutes spent in bed). Typical goals include an average onset of 10 to 20 minutes and an average efficiency of >90%.
Therapeutic intervention: If sleep efficiency falls below 80%, further restrict TIB by 15 minutes; if sleep efficiency is >90%, increase TIB by 15 minutes (no TIB change is needed with efficiencies between 80% and 90%). Identify dysfunctional beliefs about sleep, and provide strategies to interrupt cognitive overactivation—the pressured “talking to oneself” in hopes of falling asleep.
Session 4 (Week 3). Review the sleep diary, and calculate the average time to sleep onset and sleep efficiency. Increase or decrease TIB based on sleep efficiency as described above. Determine if the patient has dysfunctional beliefs regarding sleep.
Therapeutic intervention: Reframe the patient’s dysfunctional beliefs/concepts by comparing sleep diary entries with dysfunctional beliefs (Table 2). Remind patients about strategies to address cognitive overactivation, and have them practice daily to apply the appropriate reframe response from Table 2 that improves sleep. Review progressive muscular relaxation to address somatized tension and arousal, but instruct patients to practice relaxation only during the day at this point.
Table 2
Correcting patients’ dysfunctional sleep beliefs/concepts
| Belief/concept | Reframe responses |
|---|---|
| ‘I need 8 hours sleep per night’ | 1. Nightly sleep need varies among individuals from 5 to 9 hours, particularly with aging 2. Employed adults sleep 6.5 to 7 hours per workweek night 3. For the ‘average’ person, it takes <6 hours of sleep to reduce performance |
| ‘If I don’t sleep, I can’t _____ (work, socialize, take care of the kids, etc.) or ‘If I don’t sleep tonight, I won’t be able to ____’ | 1. Every day one-third of Americans sleep <6.5 hours and yet work, socialize, and live their lives 2. ‘You told me that on ____ you had a terrible night, yet you did ____ (that presentation, meeting, activity with family, etc.)’ |
| ‘If I don’t sleep, I feel _____’ | Explore situations where the person has felt tired, irritable, angry, anxious, etc. independent from lack of sleep |
| ‘If X happens, I won’t sleep’ | Explore situations where X or something like it happened, yet sleep occurred |
| ‘I don’t sleep at all’ | 1. Explore whether a bed partner reports the patient was sleeping or snoring when the person was convinced he or she was awake 2. Tell patients that if they remain in bed for >30 minutes, it is likely they slept, particularly if anxious or frustrated (older depressed patients may be an exception) 3. Teach patients that ‘don’t at all’ statements often represent an excessive focus on wakefulness, and that self-monitoring by sleep diary is helpful |
Session 5 (Week 4). Review the sleep diary. Adjust TIB as necessary. Emphasize the patient’s mastery of dysfunctional beliefs, and highlight progress on the sleep diary. Spend much of this session helping patients improve their relaxation practice and preparing them to bring it to bedtime.
Therapeutic intervention: Tell the patient to apply the relaxation training to bedtime and nocturnal awakenings.
Session 6 (Week 6). Review the sleep diary. Emphasize progress. Address any problem areas regarding dysfunctional beliefs, maladaptive behaviors, or relaxation methods.
Therapeutic intervention: Prepare patients to maintain sleep gains on their own.
Session 7 (Week 8). Review the sleep diary. Have patients identify areas of mastery. Discuss scenarios that might be expected to result in a temporary return of insomnia—such as difficulties with work or home life, stress of job change, or medical illness—and strategies they could apply to improve sleep. Such strategies might include a “safety net” of a sedative/hypnotic agent to use after ≥2 nights of poor sleep.
‘Booster’ session. Three months later, schedule a booster session to determine whether the patient has maintained mastery of improved sleep. Patients who are doing well often cancel this session because they are satisfied with their progress.
Related resource
- American Academy of Sleep Medicine. www.aasmnet.org.
Drug brand names
- Amitriptyline • Elavil, Endep
- Eszopiclone • Lunesta
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Becker receives research/grant support from sanofi-aventis and is a speaker for Sepracor Inc. and Takeda Pharmaceutical.
Ms. H, age 53, has a 20-year history of recurrent major depressive disorder. She seeks treatment for insomnia; her primary complaint is that “no medicine has really ever helped me to sleep for very long.” She reports that every night she experiences a 2-hour sleep onset delay and an average of 5 awakenings that last 10 to 60 minutes each. Her mood is stable.
After failed trials of zolpidem, mirtazapine, amitriptyline, and sertraline plus trazodone, she improves with quetiapine, 50 mg at bedtime, plus sertraline, 150 mg at bedtime. Unfortunately, over the next 6 months Ms. H gains 20 pounds and her physician becomes concerned about her fasting serum glucose levels, which suggest borderline diabetes.
After Ms. H discontinues quetiapine, onset and maintenance insomnia remain clinically significant. Polysomnography reveals moderately loud snoring, a normal respiratory disturbance index of 4.5 per hour, no periodic leg movements of sleep, 32-minute sleep onset, total sleep time of 389 minutes (6.5 hours), and a sleep efficiency of 72%. Ms. H estimates that it took her 120 minutes to fall asleep and that she slept only 270 minutes (4.5 hours) of the 540 minutes (9 hours) in bed. The sleep specialist recommends cognitive-behavioral therapy for insomnia.
For some chronic insomnia patients—such as Ms. H—pharmacotherapy is ineffective or causes intolerable side effects. In any year, >50% of adults in the general population report experiencing difficulty falling asleep, staying asleep, early awakening, or poorly restorative sleep, but these symptoms are usually time-limited and have only a small impact on daytime alertness and function. Chronic insomnia, on the other hand, lasts ≥1 month and has substantial impact on daytime alertness and attention, cognitive function, depressed and anxious mood, and focused performance (Box).1
Medications used to treat insomnia include FDA-approved drugs such as eszopiclone and zolpidem and off-label agents such as mirtazapine and trazodone. The cognitive, behavioral, and other nonpharmacologic therapies described below can be effective options, either alone or in combination with medication.
One in 10 adults in industrialized nations experiences chronic insomnia. Women are affected twice as often as men, with higher rates also reported in older patients and those in lower socioeconomic groups.
Among adults with chronic insomnia, 35% to 45% have psychiatric comorbidities, such as anxiety or mood disorders, and 15% have primary insomnia—sleep disturbance with no identifiable cause, which traditional medical literature described as conditioned or psychophysiologic insomnia.
In the remaining cases, chronic insomnia is associated with:
- medical and sleep disorders (restless legs syndrome, periodic leg movements of sleep, and sleep apnea)
- general medical disorders, particularly those that cause pain
- use of medications that disrupt normal CNS sleep mechanisms.
Source: Reference 1
Assessing insomnia
Start by performing a thorough assessment and history. I have described this process in previous reviews,1,2 as has Neubauer in Current Psychiatry.3
Before initiating therapy for insomnia, assess and address the following:
- significant ongoing depression, mania, hypomania, generalized anxiety, panic, or obsessive-compulsive symptoms that impact sleep
- primary medical disorders of sleep, including restless legs syndrome, increased motor activity during sleep such as periodic leg movements of sleep, and the snoring/snorting of sleep apnea
- prescribed or self-administered medications or substances that can disrupt sleep, such as alcohol, caffeine, stimulants, corticosteroids, or beta blockers.
Recommended nondrug therapies
In 2006, the Standards of Practice Committee of the American Academy of Sleep Medicine (AASM) updated a comprehensive literature review of psychological and behavioral treatments of primary and secondary insomnia. On the basis of this peer-reviewed, graded evidence, the AASM recommended:
- stimulus control therapy
- relaxation training
- cognitive-behavioral therapy for insomnia (CBTi).4
The AASM also offered guidelines for sleep restriction therapy, multi-component therapy without cognitive therapy, paradoxical intention, and biofeedback. Evidence for sleep hygiene, imaging training, or cognitive therapy alone was insufficient, and the AASM neither recommended nor excluded these methods. Psychological and behavioral interventions were considered effective for treating insomnia in older adults and patients withdrawing from hypnotics.
Stimulus control therapy. Bootzin et al5 first evaluated stimulus control therapy for conditioned insomnia (subsequently identified as primary insomnia). This therapy’s goal is to interrupt the conditioned activation that occurs at bedtime. Patients are instructed to:
- go to bed when sleepy
- remain in bed for no more than 10 minutes (20 minutes if elderly) without sleeping
- if unable to sleep, get up, do something boring, and return to bed only when sleepy
- repeat getting up and returning as frequently as necessary until sleep onset.
For the first 2 weeks of stimulus control therapy, patients are required to self-monitor their sleep behaviors using a sleep diary. Stimulus control therapy is beneficial for primary insomnia and insomnia related to anxious preoccupation. About 70% of patients with conditioned insomnia will improve using stimulus control therapy,4 but it is not clear whether the primary effective intervention is:
- patients dissociating conditioned responses at bedtime, or
- the inevitable sleep restriction caused by getting out of bed.
Relaxation training. Progressive muscle relaxation is a common behavioral treatment of insomnia. Patients learn to tense and then relax individual muscles, beginning at the feet or head and working their way up or down the body. Patients are taught the difference between tension and relaxation to facilitate a relaxation response at bedtime. Another method is the body scanning technique, in which the patient “talks” to each body part, telling it to “relax… relax… relax.”
Relaxation training is predicated on the belief that insomnia is caused by somatized tension and psychophysiologic arousal. The greatest challenge to effective relaxation training is that patients need extensive daytime practice before they can bring the method to the bedroom.
Remind patients that “practice makes perfect.” Therapists often instruct patients to start practicing their relaxation method during the day while self-monitoring by sleep diary and restricting time in bed at night.2
CBTi is the most extensively investigated nonpharmacologic therapy for insomnia.6 It has been used to effectively manage comorbid insomnia in patients with psychiatric disorders,7,8 such as depression,9 generalized anxiety,10 and alcohol dependence,11 as well as those with breast cancer,12 traumatic brain injury,13 and fibromyalgia.14 Age does not appear to be a limitation; research trials show the technique is effective in elderly patients.15
CBTi incorporates cognitive strategies and behavioral interventions to improve sleep quality. Patient self-monitoring with sleep diaries and worksheets is essential.
CBTi commonly is provided in 5 to 8 sessions over 8 to 12 weeks, although studies have described abbreviated practices that used 2 sessions16 and CBTi delivered over the Internet.17 Highly trained clinical psychologists are at the forefront of therapy, but counselors and nurses in primary care settings have administered CBTi.18 For primary insomnia, CBTi is superior in efficacy to pharmacotherapy:
- as initial treatment19
- for long-term management4
- in assisting discontinuation of hypnotic medication.20
CASE CONTINUED: An effective approach
You refer Ms. H to a clinical psychologist who specializes in CBTi. Ms. H begins self-monitoring with a sleep diary and has 5 CBTi sessions over 8 weeks. Initial interventions reduce time in bed from 9 hours to 7 hours per night. Ms. H learns simple relaxation methods that she practices for 2 weeks before attempting to use them to sleep. The psychologist addresses her dysfunctional beliefs about sleep.
During the last 2 weeks of therapy, Ms. H’s sleep diary reveals a sleep efficiency of 92% and improvements in well being, energy, and perceived work efficiency. At a 3-month booster visit, Ms. H has sustained these gains in sleep and daytime function.
Implementing nondrug therapy
I recommend the following steps when offering psychological and behavioral treatment of chronic insomnia, such as CBTi.
Initial visit. Determine whether your patient needs treatment for depressive or anxiety symptoms. Assess the need for polysomnography. Does the patient have a history of an urge to move the legs (restless legs syndrome), increased kicking behavior at night (periodic leg movements of sleep), or loud, disruptive snoring (obstructive sleep apnea)? It is often helpful to have patients think back to when they were consistently sleeping well to identify factors that might be exacerbating poor sleep.
Session 1 (Week 0). Teach patients about normal sleep, how it changes over the life cycle, and common dysfunctional beliefs and behaviors that worsen sleep. Tell patients that every morning when they wake up they should complete a sleep diary (Table 1); you can download a sample sleep diary by visiting this article on CurrentPsychiatry.com.
Table 1
Insomnia: What to document on a sleep diary
| Daytime fatigue |
| Minutes spent napping |
| Medication use |
| Time the patient first tried to fall asleep |
| How long it took to fall asleep |
| How many times the patient woke up |
| Final waking time |
| Hours slept |
| Sleep quality rating |
| How refreshed the patient feels on awakening |
Session 2 (Week 1). Review the sleep diary. Address infractions of sleep hygiene, such as working until bedtime, using caffeine or alcohol in the evening, excessive smoking, or eating in bed. Discuss and specify mutual therapeutic goals for:
- minutes to sleep onset
- minutes of nighttime wakefulness
- number of awakenings
- improvements in sleep efficiency, morning refreshment/alertness, and daytime functioning.
Therapeutic intervention: Instruct patients to reduce their total time in bed (TIB) to their estimated total sleep time, unless they report <6 hours. Insomnia patients commonly overestimate their amount of wakefulness. Because research indicates daytime performance is adversely affected when sleep falls below 6 hours per night,21 I initially limit TIB to 6 hours and further restrict TIB in future sessions as needed to improve sleep efficiency.
Session 3 (Week 2). Review the sleep diary, and calculate the average time to sleep onset and sleep efficiency (divide total minutes of reported sleep by the total minutes spent in bed). Typical goals include an average onset of 10 to 20 minutes and an average efficiency of >90%.
Therapeutic intervention: If sleep efficiency falls below 80%, further restrict TIB by 15 minutes; if sleep efficiency is >90%, increase TIB by 15 minutes (no TIB change is needed with efficiencies between 80% and 90%). Identify dysfunctional beliefs about sleep, and provide strategies to interrupt cognitive overactivation—the pressured “talking to oneself” in hopes of falling asleep.
Session 4 (Week 3). Review the sleep diary, and calculate the average time to sleep onset and sleep efficiency. Increase or decrease TIB based on sleep efficiency as described above. Determine if the patient has dysfunctional beliefs regarding sleep.
Therapeutic intervention: Reframe the patient’s dysfunctional beliefs/concepts by comparing sleep diary entries with dysfunctional beliefs (Table 2). Remind patients about strategies to address cognitive overactivation, and have them practice daily to apply the appropriate reframe response from Table 2 that improves sleep. Review progressive muscular relaxation to address somatized tension and arousal, but instruct patients to practice relaxation only during the day at this point.
Table 2
Correcting patients’ dysfunctional sleep beliefs/concepts
| Belief/concept | Reframe responses |
|---|---|
| ‘I need 8 hours sleep per night’ | 1. Nightly sleep need varies among individuals from 5 to 9 hours, particularly with aging 2. Employed adults sleep 6.5 to 7 hours per workweek night 3. For the ‘average’ person, it takes <6 hours of sleep to reduce performance |
| ‘If I don’t sleep, I can’t _____ (work, socialize, take care of the kids, etc.) or ‘If I don’t sleep tonight, I won’t be able to ____’ | 1. Every day one-third of Americans sleep <6.5 hours and yet work, socialize, and live their lives 2. ‘You told me that on ____ you had a terrible night, yet you did ____ (that presentation, meeting, activity with family, etc.)’ |
| ‘If I don’t sleep, I feel _____’ | Explore situations where the person has felt tired, irritable, angry, anxious, etc. independent from lack of sleep |
| ‘If X happens, I won’t sleep’ | Explore situations where X or something like it happened, yet sleep occurred |
| ‘I don’t sleep at all’ | 1. Explore whether a bed partner reports the patient was sleeping or snoring when the person was convinced he or she was awake 2. Tell patients that if they remain in bed for >30 minutes, it is likely they slept, particularly if anxious or frustrated (older depressed patients may be an exception) 3. Teach patients that ‘don’t at all’ statements often represent an excessive focus on wakefulness, and that self-monitoring by sleep diary is helpful |
Session 5 (Week 4). Review the sleep diary. Adjust TIB as necessary. Emphasize the patient’s mastery of dysfunctional beliefs, and highlight progress on the sleep diary. Spend much of this session helping patients improve their relaxation practice and preparing them to bring it to bedtime.
Therapeutic intervention: Tell the patient to apply the relaxation training to bedtime and nocturnal awakenings.
Session 6 (Week 6). Review the sleep diary. Emphasize progress. Address any problem areas regarding dysfunctional beliefs, maladaptive behaviors, or relaxation methods.
Therapeutic intervention: Prepare patients to maintain sleep gains on their own.
Session 7 (Week 8). Review the sleep diary. Have patients identify areas of mastery. Discuss scenarios that might be expected to result in a temporary return of insomnia—such as difficulties with work or home life, stress of job change, or medical illness—and strategies they could apply to improve sleep. Such strategies might include a “safety net” of a sedative/hypnotic agent to use after ≥2 nights of poor sleep.
‘Booster’ session. Three months later, schedule a booster session to determine whether the patient has maintained mastery of improved sleep. Patients who are doing well often cancel this session because they are satisfied with their progress.
Related resource
- American Academy of Sleep Medicine. www.aasmnet.org.
Drug brand names
- Amitriptyline • Elavil, Endep
- Eszopiclone • Lunesta
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Becker receives research/grant support from sanofi-aventis and is a speaker for Sepracor Inc. and Takeda Pharmaceutical.
1. Becker PM. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Psychiatr Clin North Am 2006;29(4):855-70.
2. Becker PM. Pharmacologic and nonpharmacologic treatments of insomnia. Neurol Clin 2005;23(4):1149-63.
3. Neubauer DN. Treatment resistant-insomnia: ask yourself 8 questions. Current Psychiatry 2007;6(12):46-54.
4. Morgenthaler T, Kramer M, Alessi C, et al. American Academy of Sleep Medicine. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep 2006;29(11):1415-9.
5. Bootzin RR, Perlis ML. Nonpharmacologic treatments of insomnia. J Clin Psychiatry 1992;53(suppl):37-41.
6. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29(11):1398-414.
7. Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005;25(5):559-92.
8. Dopke CA, Lehner RK, Wells AM. Cognitive-behavioral group therapy for insomnia in individuals with serious mental illnesses: a preliminary evaluation. Psychiatr Rehabil J 2004;27(3):235-42.
9. Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry 2007;68(2):254-60.
10. Bélanger L, Morin CM, Langlois F, Ladouceur R. Insomnia and generalized anxiety disorder: effects of cognitive behavior therapy for GAD on insomnia symptoms. J Anxiety Disord 2004;18(4):561-71.
11. Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction 2004;99(9):1121-32.
12. Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum 2007;34(5):E51-9.
13. Ouellet MC, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Arch Phys Med Rehabil 2007;88(12):1581-92.
14. Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med 2005;165(21):2527-35.
15. Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol 2006;25(1):3-14.
16. Edinger JD, Wohlgemuth WK, Radtke RA, et al. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep 2007;30(2):203-12.
17. Ström L, Pettersson R, Andersson G. Internet-based treatment for insomnia: a controlled evaluation. J Consult Clin Psychol 2004;72(1):113-20.
18. Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep 2007;30(5):574-84.
19. Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med 2004;164(17):1888-96.
20. Morin CM, Bélanger L, Bastien C, Vallières A. Long-term outcome after discontinuation of benzodiazepines for insomnia: a survival analysis of relapse. Behav Res Ther 2005;43(1):1-14.
21. Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci 2008;1129:305-22.
1. Becker PM. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Psychiatr Clin North Am 2006;29(4):855-70.
2. Becker PM. Pharmacologic and nonpharmacologic treatments of insomnia. Neurol Clin 2005;23(4):1149-63.
3. Neubauer DN. Treatment resistant-insomnia: ask yourself 8 questions. Current Psychiatry 2007;6(12):46-54.
4. Morgenthaler T, Kramer M, Alessi C, et al. American Academy of Sleep Medicine. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep 2006;29(11):1415-9.
5. Bootzin RR, Perlis ML. Nonpharmacologic treatments of insomnia. J Clin Psychiatry 1992;53(suppl):37-41.
6. Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep 2006;29(11):1398-414.
7. Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev 2005;25(5):559-92.
8. Dopke CA, Lehner RK, Wells AM. Cognitive-behavioral group therapy for insomnia in individuals with serious mental illnesses: a preliminary evaluation. Psychiatr Rehabil J 2004;27(3):235-42.
9. Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry 2007;68(2):254-60.
10. Bélanger L, Morin CM, Langlois F, Ladouceur R. Insomnia and generalized anxiety disorder: effects of cognitive behavior therapy for GAD on insomnia symptoms. J Anxiety Disord 2004;18(4):561-71.
11. Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Addiction 2004;99(9):1121-32.
12. Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum 2007;34(5):E51-9.
13. Ouellet MC, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Arch Phys Med Rehabil 2007;88(12):1581-92.
14. Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med 2005;165(21):2527-35.
15. Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol 2006;25(1):3-14.
16. Edinger JD, Wohlgemuth WK, Radtke RA, et al. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep 2007;30(2):203-12.
17. Ström L, Pettersson R, Andersson G. Internet-based treatment for insomnia: a controlled evaluation. J Consult Clin Psychol 2004;72(1):113-20.
18. Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep 2007;30(5):574-84.
19. Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med 2004;164(17):1888-96.
20. Morin CM, Bélanger L, Bastien C, Vallières A. Long-term outcome after discontinuation of benzodiazepines for insomnia: a survival analysis of relapse. Behav Res Ther 2005;43(1):1-14.
21. Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci 2008;1129:305-22.
Know your patient’s mental health benefits
Many Americans do not have health care insurance, and those who have mental health benefits are subject to limits on inpatient days and outpatient visits during the policy period (Table). Accordingly, it is important to review a patient’s mental health benefits before you formulate a treatment plan. Otherwise, you and your patient may find yourselves in a predicament.
If, for example, a patient with a major mental disorder requires close follow-up and you have not inquired about his or her mental health coverage, the benefits may run out before the need for monitoring ends. Abrupt transfer to another provider who is willing to accept a lower reimbursement or to a different mental health system could result in clinical decompensation.
Table
Common limitations in mental health insurance
| Mental health benefit | Coverage limitation |
|---|---|
| Outpatient care | Plans typically limit the number of outpatient visits per year, including partial hospitalization and intensive outpatient programs; copayments or coinsurance costs may be prohibitive |
| Emergency department | Copayments may be prohibitive; some states limit these amounts |
| Inpatient care | Insurance plans often limit the number of inpatient days per year; concurrent reviews by managed care organizations pressure the provider/hospital to discharge patients as soon as they no longer represent an imminent risk of harm to themselves or others |
| Psychosocial and drug rehabilitation programs | Most mental health care plans do not cover these programs |
| Pharmacy | Copayments may be prohibitive, especially if the prescriber writes numerous prescriptions when titrating a new medication |
Employ a proactive approach and inquire about your patients’ benefits during the initial evaluation.1 This information can guide the treatment plan and enable a thoughtful use of the patient’s resources. Knowing that a patient has a limited number of outpatient visits, for example, allows time for creative scheduling. You might spread outpatient visits over a longer period of time by incorporating telephone check-ins between appointments.
Other suggestions for maximizing your patient’s benefits include:
- Review pharmacy benefits. Often the greatest barrier to a drug’s bioavailability is the patient’s inability to obtain a prescribed medication. When appropriate, consider prescribing generic formulations.
- If you work part-time at a community mental health center or agency that has a sliding payment scale, suggest that a patient begin treatment with you at this location.
1. Campbell WH, Rohrbaugh RM. The biopsychosocial formulation manual: a guide for mental health professionals. New York, NY: Routledge; 2006.
Many Americans do not have health care insurance, and those who have mental health benefits are subject to limits on inpatient days and outpatient visits during the policy period (Table). Accordingly, it is important to review a patient’s mental health benefits before you formulate a treatment plan. Otherwise, you and your patient may find yourselves in a predicament.
If, for example, a patient with a major mental disorder requires close follow-up and you have not inquired about his or her mental health coverage, the benefits may run out before the need for monitoring ends. Abrupt transfer to another provider who is willing to accept a lower reimbursement or to a different mental health system could result in clinical decompensation.
Table
Common limitations in mental health insurance
| Mental health benefit | Coverage limitation |
|---|---|
| Outpatient care | Plans typically limit the number of outpatient visits per year, including partial hospitalization and intensive outpatient programs; copayments or coinsurance costs may be prohibitive |
| Emergency department | Copayments may be prohibitive; some states limit these amounts |
| Inpatient care | Insurance plans often limit the number of inpatient days per year; concurrent reviews by managed care organizations pressure the provider/hospital to discharge patients as soon as they no longer represent an imminent risk of harm to themselves or others |
| Psychosocial and drug rehabilitation programs | Most mental health care plans do not cover these programs |
| Pharmacy | Copayments may be prohibitive, especially if the prescriber writes numerous prescriptions when titrating a new medication |
Employ a proactive approach and inquire about your patients’ benefits during the initial evaluation.1 This information can guide the treatment plan and enable a thoughtful use of the patient’s resources. Knowing that a patient has a limited number of outpatient visits, for example, allows time for creative scheduling. You might spread outpatient visits over a longer period of time by incorporating telephone check-ins between appointments.
Other suggestions for maximizing your patient’s benefits include:
- Review pharmacy benefits. Often the greatest barrier to a drug’s bioavailability is the patient’s inability to obtain a prescribed medication. When appropriate, consider prescribing generic formulations.
- If you work part-time at a community mental health center or agency that has a sliding payment scale, suggest that a patient begin treatment with you at this location.
Many Americans do not have health care insurance, and those who have mental health benefits are subject to limits on inpatient days and outpatient visits during the policy period (Table). Accordingly, it is important to review a patient’s mental health benefits before you formulate a treatment plan. Otherwise, you and your patient may find yourselves in a predicament.
If, for example, a patient with a major mental disorder requires close follow-up and you have not inquired about his or her mental health coverage, the benefits may run out before the need for monitoring ends. Abrupt transfer to another provider who is willing to accept a lower reimbursement or to a different mental health system could result in clinical decompensation.
Table
Common limitations in mental health insurance
| Mental health benefit | Coverage limitation |
|---|---|
| Outpatient care | Plans typically limit the number of outpatient visits per year, including partial hospitalization and intensive outpatient programs; copayments or coinsurance costs may be prohibitive |
| Emergency department | Copayments may be prohibitive; some states limit these amounts |
| Inpatient care | Insurance plans often limit the number of inpatient days per year; concurrent reviews by managed care organizations pressure the provider/hospital to discharge patients as soon as they no longer represent an imminent risk of harm to themselves or others |
| Psychosocial and drug rehabilitation programs | Most mental health care plans do not cover these programs |
| Pharmacy | Copayments may be prohibitive, especially if the prescriber writes numerous prescriptions when titrating a new medication |
Employ a proactive approach and inquire about your patients’ benefits during the initial evaluation.1 This information can guide the treatment plan and enable a thoughtful use of the patient’s resources. Knowing that a patient has a limited number of outpatient visits, for example, allows time for creative scheduling. You might spread outpatient visits over a longer period of time by incorporating telephone check-ins between appointments.
Other suggestions for maximizing your patient’s benefits include:
- Review pharmacy benefits. Often the greatest barrier to a drug’s bioavailability is the patient’s inability to obtain a prescribed medication. When appropriate, consider prescribing generic formulations.
- If you work part-time at a community mental health center or agency that has a sliding payment scale, suggest that a patient begin treatment with you at this location.
1. Campbell WH, Rohrbaugh RM. The biopsychosocial formulation manual: a guide for mental health professionals. New York, NY: Routledge; 2006.
1. Campbell WH, Rohrbaugh RM. The biopsychosocial formulation manual: a guide for mental health professionals. New York, NY: Routledge; 2006.
6 screening questions for military veterans
Of the >1.6 million military personnel deployed to Iraq and Afghanistan since 2001, an estimated 300,000 have experienced major depression or post-traumatic stress disorder.1 Consequently, psychiatrists and mental health providers outside the Veterans Administration (VA) and Department of Defense likely will encounter veterans with psychiatric symptoms related to military service.
These 6 questions can help you:
- take a thorough mental health history
- demonstrate a basic familiarity with common military terminology and issues when treating veterans or veterans’ family members.
1 Did you experience traumatic events while deployed?
War without front lines or a clearly identified opposing force is referred to as a “low intensity conflict on an asymmetric battlefield.” This description epitomizes military operations in Iraq and Afghanistan, where random warfare with improvised explosive devices, sporadic firefights, suicide bombings, and rocket attacks are the norm. This type of warfare can put every deployed individual—not just combat soldiers—in harm’s way.
2 What was your job in the military?
“Military occupational specialty” (MOS) refers to an individual’s job in the military. In the Army, for example, an 11B is an infantryman, 88M is a truck driver, 68W is a medic, and 60W is a psychiatrist. The code itself is unimportant, but recognizing the term MOS shows familiarity with the military and provides potentially valuable information. An infantryman who was assigned to security and engaged the enemy regularly while on patrol is more likely to have experienced traumatic events than a soldier supporting the fight from an air-conditioned office in a fairly secure area.
3 Were you stop-lossed?
Stop-loss—a program created by Congress after the Vietnam War—is the involuntary extension of a service member’s active duty to retain the individual beyond the initial expiration of term of service (ETS) date. At a certain time before a unit departs for deployment—usually 90 days—the roster is “locked-in.” If an individual is deemed essential and his or her ETS date occurs after the lock-in date, that person can be stop-lossed and required to deploy—an involuntary prolonging of military service.
Most military personnel accept this practice, but it can cause disenchantment, especially when individuals who were looking forward to leaving the military think they will get stop-lossed and begrudgingly choose to re-enlist to receive financial and/ or occupational perks.
4 Did you receive mental health care downrange?
The term “downrange” is commonly used in the military and is synonymous with “theater of operations,” “Iraq,” or “Afghanistan.” Mental health teams of psychiatrists, psychologists, social workers, nurse practitioners, and mental health technicians have been deployed with fighting forces since the conflicts began in Afghanistan and Iraq. These teams have a well-established doctrine, concepts of operation, and access to a formulary of somatic interventions to meet clinical demand.
Military personnel can seek mental health services from the “CSH” (pronounced “cash” and stands for combat support hospital); “CSC team” (combat stress control, which are mobile outreach services); or “the BHO” (brigade behavioral health officer). Interventions include but are not limited to:
- time-limited psychotherapies using supportive, expressive, cognitive-behavioral, or psychoeducational methods
- medications, such as low-dose selective serotonin reuptake inhibitors or brief trials of zolpidem or trazodone for sleep, anxiety, and mood symptoms.
5 How did you exit the military?
Generally, there are 4 ways to leave the military:
Retirement. Military personnel in good standing are eligible to retire after 20 years of service and must obtain a waiver to serve for more than 30 years. A retiree receives a pension, health care, and other benefits.
Completion of service obligation is commonly referred to as “meeting ETS.” When an individual signs a contract to enlist for a specific number of years and chooses to leave the military after completing those years, that person has “ETS’d.” These individuals may be eligible for VA services and military alumni programs, such as the Montgomery GI Bill, but they are not retirees and do not receive the same benefits.
Administrative separation. Following regulations,2 a commander can separate individuals from the military for a variety reasons such as unsatisfactory performance, misconduct, pregnancy, and—with comprehensive input from mental health professionals—personality disorder.
Medical evaluation board (MEB) is a medical retirement from the military. A service member can get a MEB for physical and/or psychiatric conditions. If a soldier can no longer function in the military because of injuries or mental health disorders sustained while on active duty as defined by regulation,3 an “MEB packet” summarizing the case is prepared and sent to a review board. The board returns a rating that grants a severance package or permanent disability retirement and determines the final day of military service, often called the “final-out.” An individual who receives a MEB also can apply for a disability rating from the VA, regardless of the military’s decision.
6 Have you enrolled in the VA?
Every service member receives information on VA services during outprocessing from the military. Most—if not all—are eligible for some VA services. The individual is responsible for negotiating the process, which begins with an administrative visit and review of all of military documents at a local VA medical facility.
1. Tanielian TL, Jaycox LH. Invisible wounds of war. Santa Monica, CA: RAND Corporation; 2008.
2. Army Regulation [AR] 635-200. Active duty enlisted administrative separations, (Headquarters, Department of the Army [HQDA], Washington, DC, 6 June 2005).
3. Army Regulation [AR] 40-501. Standards of medical fitness, (Headquarters, Department of the Army [HQDA], Washington, DC, 14 December 2007).
Of the >1.6 million military personnel deployed to Iraq and Afghanistan since 2001, an estimated 300,000 have experienced major depression or post-traumatic stress disorder.1 Consequently, psychiatrists and mental health providers outside the Veterans Administration (VA) and Department of Defense likely will encounter veterans with psychiatric symptoms related to military service.
These 6 questions can help you:
- take a thorough mental health history
- demonstrate a basic familiarity with common military terminology and issues when treating veterans or veterans’ family members.
1 Did you experience traumatic events while deployed?
War without front lines or a clearly identified opposing force is referred to as a “low intensity conflict on an asymmetric battlefield.” This description epitomizes military operations in Iraq and Afghanistan, where random warfare with improvised explosive devices, sporadic firefights, suicide bombings, and rocket attacks are the norm. This type of warfare can put every deployed individual—not just combat soldiers—in harm’s way.
2 What was your job in the military?
“Military occupational specialty” (MOS) refers to an individual’s job in the military. In the Army, for example, an 11B is an infantryman, 88M is a truck driver, 68W is a medic, and 60W is a psychiatrist. The code itself is unimportant, but recognizing the term MOS shows familiarity with the military and provides potentially valuable information. An infantryman who was assigned to security and engaged the enemy regularly while on patrol is more likely to have experienced traumatic events than a soldier supporting the fight from an air-conditioned office in a fairly secure area.
3 Were you stop-lossed?
Stop-loss—a program created by Congress after the Vietnam War—is the involuntary extension of a service member’s active duty to retain the individual beyond the initial expiration of term of service (ETS) date. At a certain time before a unit departs for deployment—usually 90 days—the roster is “locked-in.” If an individual is deemed essential and his or her ETS date occurs after the lock-in date, that person can be stop-lossed and required to deploy—an involuntary prolonging of military service.
Most military personnel accept this practice, but it can cause disenchantment, especially when individuals who were looking forward to leaving the military think they will get stop-lossed and begrudgingly choose to re-enlist to receive financial and/ or occupational perks.
4 Did you receive mental health care downrange?
The term “downrange” is commonly used in the military and is synonymous with “theater of operations,” “Iraq,” or “Afghanistan.” Mental health teams of psychiatrists, psychologists, social workers, nurse practitioners, and mental health technicians have been deployed with fighting forces since the conflicts began in Afghanistan and Iraq. These teams have a well-established doctrine, concepts of operation, and access to a formulary of somatic interventions to meet clinical demand.
Military personnel can seek mental health services from the “CSH” (pronounced “cash” and stands for combat support hospital); “CSC team” (combat stress control, which are mobile outreach services); or “the BHO” (brigade behavioral health officer). Interventions include but are not limited to:
- time-limited psychotherapies using supportive, expressive, cognitive-behavioral, or psychoeducational methods
- medications, such as low-dose selective serotonin reuptake inhibitors or brief trials of zolpidem or trazodone for sleep, anxiety, and mood symptoms.
5 How did you exit the military?
Generally, there are 4 ways to leave the military:
Retirement. Military personnel in good standing are eligible to retire after 20 years of service and must obtain a waiver to serve for more than 30 years. A retiree receives a pension, health care, and other benefits.
Completion of service obligation is commonly referred to as “meeting ETS.” When an individual signs a contract to enlist for a specific number of years and chooses to leave the military after completing those years, that person has “ETS’d.” These individuals may be eligible for VA services and military alumni programs, such as the Montgomery GI Bill, but they are not retirees and do not receive the same benefits.
Administrative separation. Following regulations,2 a commander can separate individuals from the military for a variety reasons such as unsatisfactory performance, misconduct, pregnancy, and—with comprehensive input from mental health professionals—personality disorder.
Medical evaluation board (MEB) is a medical retirement from the military. A service member can get a MEB for physical and/or psychiatric conditions. If a soldier can no longer function in the military because of injuries or mental health disorders sustained while on active duty as defined by regulation,3 an “MEB packet” summarizing the case is prepared and sent to a review board. The board returns a rating that grants a severance package or permanent disability retirement and determines the final day of military service, often called the “final-out.” An individual who receives a MEB also can apply for a disability rating from the VA, regardless of the military’s decision.
6 Have you enrolled in the VA?
Every service member receives information on VA services during outprocessing from the military. Most—if not all—are eligible for some VA services. The individual is responsible for negotiating the process, which begins with an administrative visit and review of all of military documents at a local VA medical facility.
Of the >1.6 million military personnel deployed to Iraq and Afghanistan since 2001, an estimated 300,000 have experienced major depression or post-traumatic stress disorder.1 Consequently, psychiatrists and mental health providers outside the Veterans Administration (VA) and Department of Defense likely will encounter veterans with psychiatric symptoms related to military service.
These 6 questions can help you:
- take a thorough mental health history
- demonstrate a basic familiarity with common military terminology and issues when treating veterans or veterans’ family members.
1 Did you experience traumatic events while deployed?
War without front lines or a clearly identified opposing force is referred to as a “low intensity conflict on an asymmetric battlefield.” This description epitomizes military operations in Iraq and Afghanistan, where random warfare with improvised explosive devices, sporadic firefights, suicide bombings, and rocket attacks are the norm. This type of warfare can put every deployed individual—not just combat soldiers—in harm’s way.
2 What was your job in the military?
“Military occupational specialty” (MOS) refers to an individual’s job in the military. In the Army, for example, an 11B is an infantryman, 88M is a truck driver, 68W is a medic, and 60W is a psychiatrist. The code itself is unimportant, but recognizing the term MOS shows familiarity with the military and provides potentially valuable information. An infantryman who was assigned to security and engaged the enemy regularly while on patrol is more likely to have experienced traumatic events than a soldier supporting the fight from an air-conditioned office in a fairly secure area.
3 Were you stop-lossed?
Stop-loss—a program created by Congress after the Vietnam War—is the involuntary extension of a service member’s active duty to retain the individual beyond the initial expiration of term of service (ETS) date. At a certain time before a unit departs for deployment—usually 90 days—the roster is “locked-in.” If an individual is deemed essential and his or her ETS date occurs after the lock-in date, that person can be stop-lossed and required to deploy—an involuntary prolonging of military service.
Most military personnel accept this practice, but it can cause disenchantment, especially when individuals who were looking forward to leaving the military think they will get stop-lossed and begrudgingly choose to re-enlist to receive financial and/ or occupational perks.
4 Did you receive mental health care downrange?
The term “downrange” is commonly used in the military and is synonymous with “theater of operations,” “Iraq,” or “Afghanistan.” Mental health teams of psychiatrists, psychologists, social workers, nurse practitioners, and mental health technicians have been deployed with fighting forces since the conflicts began in Afghanistan and Iraq. These teams have a well-established doctrine, concepts of operation, and access to a formulary of somatic interventions to meet clinical demand.
Military personnel can seek mental health services from the “CSH” (pronounced “cash” and stands for combat support hospital); “CSC team” (combat stress control, which are mobile outreach services); or “the BHO” (brigade behavioral health officer). Interventions include but are not limited to:
- time-limited psychotherapies using supportive, expressive, cognitive-behavioral, or psychoeducational methods
- medications, such as low-dose selective serotonin reuptake inhibitors or brief trials of zolpidem or trazodone for sleep, anxiety, and mood symptoms.
5 How did you exit the military?
Generally, there are 4 ways to leave the military:
Retirement. Military personnel in good standing are eligible to retire after 20 years of service and must obtain a waiver to serve for more than 30 years. A retiree receives a pension, health care, and other benefits.
Completion of service obligation is commonly referred to as “meeting ETS.” When an individual signs a contract to enlist for a specific number of years and chooses to leave the military after completing those years, that person has “ETS’d.” These individuals may be eligible for VA services and military alumni programs, such as the Montgomery GI Bill, but they are not retirees and do not receive the same benefits.
Administrative separation. Following regulations,2 a commander can separate individuals from the military for a variety reasons such as unsatisfactory performance, misconduct, pregnancy, and—with comprehensive input from mental health professionals—personality disorder.
Medical evaluation board (MEB) is a medical retirement from the military. A service member can get a MEB for physical and/or psychiatric conditions. If a soldier can no longer function in the military because of injuries or mental health disorders sustained while on active duty as defined by regulation,3 an “MEB packet” summarizing the case is prepared and sent to a review board. The board returns a rating that grants a severance package or permanent disability retirement and determines the final day of military service, often called the “final-out.” An individual who receives a MEB also can apply for a disability rating from the VA, regardless of the military’s decision.
6 Have you enrolled in the VA?
Every service member receives information on VA services during outprocessing from the military. Most—if not all—are eligible for some VA services. The individual is responsible for negotiating the process, which begins with an administrative visit and review of all of military documents at a local VA medical facility.
1. Tanielian TL, Jaycox LH. Invisible wounds of war. Santa Monica, CA: RAND Corporation; 2008.
2. Army Regulation [AR] 635-200. Active duty enlisted administrative separations, (Headquarters, Department of the Army [HQDA], Washington, DC, 6 June 2005).
3. Army Regulation [AR] 40-501. Standards of medical fitness, (Headquarters, Department of the Army [HQDA], Washington, DC, 14 December 2007).
1. Tanielian TL, Jaycox LH. Invisible wounds of war. Santa Monica, CA: RAND Corporation; 2008.
2. Army Regulation [AR] 635-200. Active duty enlisted administrative separations, (Headquarters, Department of the Army [HQDA], Washington, DC, 6 June 2005).
3. Army Regulation [AR] 40-501. Standards of medical fitness, (Headquarters, Department of the Army [HQDA], Washington, DC, 14 December 2007).
8 lifestyle fixes to help patients lose weight
Psychiatric patients are at high risk of becoming obese—with rates up to 63% in schizophrenia and 68% in bipolar disorder.1 Moreover, weight gain from psychotropics is associated with medication nonadherence.
Psychiatrists can suggest and encourage lifestyle changes that will help patients lose weight. The 8 behaviors described below can help patients become more active and take steps toward a healthier lifestyle.
Keep a food diary. Ask patients to keep a written record of everything they eat or drink in a day. Encourage them to learn about healthy foods and look up the calories of common foods using food packaging, pocket books listing calorie counts, and online sources.
Start walking. Pedometers could motivate patients to exercise regularly and reach goals of taking a certain number of steps each day. A physically healthy individual should walk approximately 10,000 steps per day. Scheduling daily walks also provides structure for your patients.
Plan meals and eat mindfully. Advise your patients to schedule meals and eat mindfully. This means keeping your full attention on eating by noticing the smell, taste, and texture of food. Encourage patients to eat slowly, enjoy every bite, and avoid eating while watching television or when occupied by another activity.
Have a healthy snack before a meal. Eating a serving of boiled vegetables or a piece of fruit such as an apple before a meal can satisfy hunger and reduce food intake.
Increase fluid intake. Feeling hungry might be a signal that the body needs more fluid. Advise patients to drink water, avoid beverages that contain sugar, and limit fruit juice to 4 to 8 ounces per day.
Obtain support from family and friends. Loved ones can reinforce a patient’s weight loss efforts by not eating high-calorie food in front of the patient and buying only healthy snacks such as fruits and vegetables.
Improve nutrition. Advise patients to:
- eat at least 3 meals and 2 to 3 healthy snacks per day
- choose lean meats and whole grains
- eat 5 servings of fruits and vegetables daily
- avoid eating after 7 Pm or 3 to 4 hours before bedtime.
Monitor weight regularly. Digital scales give more precise measurements, which can prompt patients to reduce food intake when they notice weight gain. Frequent feedback can help facilitate behavior changes necessary for weight loss.
Patients often need help setting appropriate weight loss goals because achieving their ideal weight may not be possible. Losing 10% of body weight usually is a realistic goal that can improve their health.
1. Kolotkin RL, Corey-Lisle PK, Crosby RD, et al. Impact of obesity on health-related quality of life in schizophrenia and bipolar disorder. Obesity (Silver Spring) 2008;16:749-54.
Psychiatric patients are at high risk of becoming obese—with rates up to 63% in schizophrenia and 68% in bipolar disorder.1 Moreover, weight gain from psychotropics is associated with medication nonadherence.
Psychiatrists can suggest and encourage lifestyle changes that will help patients lose weight. The 8 behaviors described below can help patients become more active and take steps toward a healthier lifestyle.
Keep a food diary. Ask patients to keep a written record of everything they eat or drink in a day. Encourage them to learn about healthy foods and look up the calories of common foods using food packaging, pocket books listing calorie counts, and online sources.
Start walking. Pedometers could motivate patients to exercise regularly and reach goals of taking a certain number of steps each day. A physically healthy individual should walk approximately 10,000 steps per day. Scheduling daily walks also provides structure for your patients.
Plan meals and eat mindfully. Advise your patients to schedule meals and eat mindfully. This means keeping your full attention on eating by noticing the smell, taste, and texture of food. Encourage patients to eat slowly, enjoy every bite, and avoid eating while watching television or when occupied by another activity.
Have a healthy snack before a meal. Eating a serving of boiled vegetables or a piece of fruit such as an apple before a meal can satisfy hunger and reduce food intake.
Increase fluid intake. Feeling hungry might be a signal that the body needs more fluid. Advise patients to drink water, avoid beverages that contain sugar, and limit fruit juice to 4 to 8 ounces per day.
Obtain support from family and friends. Loved ones can reinforce a patient’s weight loss efforts by not eating high-calorie food in front of the patient and buying only healthy snacks such as fruits and vegetables.
Improve nutrition. Advise patients to:
- eat at least 3 meals and 2 to 3 healthy snacks per day
- choose lean meats and whole grains
- eat 5 servings of fruits and vegetables daily
- avoid eating after 7 Pm or 3 to 4 hours before bedtime.
Monitor weight regularly. Digital scales give more precise measurements, which can prompt patients to reduce food intake when they notice weight gain. Frequent feedback can help facilitate behavior changes necessary for weight loss.
Patients often need help setting appropriate weight loss goals because achieving their ideal weight may not be possible. Losing 10% of body weight usually is a realistic goal that can improve their health.
Psychiatric patients are at high risk of becoming obese—with rates up to 63% in schizophrenia and 68% in bipolar disorder.1 Moreover, weight gain from psychotropics is associated with medication nonadherence.
Psychiatrists can suggest and encourage lifestyle changes that will help patients lose weight. The 8 behaviors described below can help patients become more active and take steps toward a healthier lifestyle.
Keep a food diary. Ask patients to keep a written record of everything they eat or drink in a day. Encourage them to learn about healthy foods and look up the calories of common foods using food packaging, pocket books listing calorie counts, and online sources.
Start walking. Pedometers could motivate patients to exercise regularly and reach goals of taking a certain number of steps each day. A physically healthy individual should walk approximately 10,000 steps per day. Scheduling daily walks also provides structure for your patients.
Plan meals and eat mindfully. Advise your patients to schedule meals and eat mindfully. This means keeping your full attention on eating by noticing the smell, taste, and texture of food. Encourage patients to eat slowly, enjoy every bite, and avoid eating while watching television or when occupied by another activity.
Have a healthy snack before a meal. Eating a serving of boiled vegetables or a piece of fruit such as an apple before a meal can satisfy hunger and reduce food intake.
Increase fluid intake. Feeling hungry might be a signal that the body needs more fluid. Advise patients to drink water, avoid beverages that contain sugar, and limit fruit juice to 4 to 8 ounces per day.
Obtain support from family and friends. Loved ones can reinforce a patient’s weight loss efforts by not eating high-calorie food in front of the patient and buying only healthy snacks such as fruits and vegetables.
Improve nutrition. Advise patients to:
- eat at least 3 meals and 2 to 3 healthy snacks per day
- choose lean meats and whole grains
- eat 5 servings of fruits and vegetables daily
- avoid eating after 7 Pm or 3 to 4 hours before bedtime.
Monitor weight regularly. Digital scales give more precise measurements, which can prompt patients to reduce food intake when they notice weight gain. Frequent feedback can help facilitate behavior changes necessary for weight loss.
Patients often need help setting appropriate weight loss goals because achieving their ideal weight may not be possible. Losing 10% of body weight usually is a realistic goal that can improve their health.
1. Kolotkin RL, Corey-Lisle PK, Crosby RD, et al. Impact of obesity on health-related quality of life in schizophrenia and bipolar disorder. Obesity (Silver Spring) 2008;16:749-54.
1. Kolotkin RL, Corey-Lisle PK, Crosby RD, et al. Impact of obesity on health-related quality of life in schizophrenia and bipolar disorder. Obesity (Silver Spring) 2008;16:749-54.
How seizure disorders change depression treatment
Ms. A, age 29, has had depression for 6 years and has taken antidepressants with inconsistent response. For 3 weeks, while not taking any antidepressant, she reports loss of energy; feeling sad, subdued, and tearful; poor concentration; and reduced interest in enjoyable activities including sex, the same symptoms she ?rst reported 6 years ago. She has no appetite but has not lost weight.
Several times a month Ms. A “loses” short periods of time. For example she says sometimes she cannot remember what happens between parking her car and sitting at her desk at work. After these episodes, which began 9 years ago, her speech is slightly slurred, and coworkers tease her about being “hungover.” She feels fuzzy-headed, but her speech and thinking clear after a few hours. At other times she smells burning rubber and feels that everything she does repeats what she has done before. Sometimes she feels “out of body” and can watch herself from the ceiling.
Ms. A’s symptoms suggest a seizure disorder. Her depressive features appeared after these ictal episodes began 9 years ago.
Recognizing mood disorders in patients with epilepsy is important because these disorders can be successfully treated within the context of the medical condition.
Many cases of comorbid depression in epilepsy are undiagnosed. A study of 100 patients with refractory epilepsy and depression severe enough for pharmacotherapy found that referral for psychiatric treatment was delayed >1 year in 75% of patients with spontaneous mood disorders and 89% of patients with depression secondary to antiepileptic drugs (AEDs).1
Psychiatrists often are called on to evaluate and treat depression in epilepsy patients or to assess for nonadherence to AEDs. Successfully treating these patients requires understanding:
- the relationship between epilepsy and depression
- the etiology of depression in patients with seizure disorder
- how to treat depression in this population.
High comorbidity
Depression rates are higher in epilepsy patients than in the general population (1% to 3% of men, 2% to 9% of women).2 Depression can be diagnosed in:
- 20% to 30% of patients with recurrent seizures
- 6% to 9% of patients in remission
- 50% to 55% of patients attending hospital epilepsy clinics and video telemetry units.3
Temporal relationship. Depression can be preictal, ictal, postictal, or interictal.4 One-third of patients with partial seizures report premonitory symptoms, usually before secondary generalized tonic clonic seizures.5
- Preictal depression occurs hours to days before a seizure and often is relieved by the convulsion.
- Ictal depression—more common in TLE—occurs as an aura in approximately 1% of patients. Onset is sudden and ranges from mild sadness to profound helplessness and despair. Treating the seizures also treats the depression.
- Postictal depression in TLE patients lasts hours to days after a seizure.
- Interictal depression affects up to two-thirds of epilepsy patients, especially those with severe or frequent seizures. Treat interictal depression separately from the Seizures.
Differential diagnosis
Search for seizure cause. Although 70% of epilepsies are idiopathic, search for the cause of a patient’s seizures. Neuroimaging can rule out a stroke, cerebral tumor, or traumatic brain injury as the cause of both depression and epilepsy.4 Even after exhaustive study, 10% to 20% of epilepsy cases cannot be identified by electroencephalography (EEG).
Seizure type and location, severity, laterality of seizure focus, and frequency are important variables in the etiology of depression in patients with epilepsy.6 Similar changes in neurotransmitters—serotonin, noradrenaline, dopamine, and gamma-aminobutyric acid—are observed in both depression and epilepsy.5
Characterize depressive symptoms. Consider involving the patient’s spouse or partner in the discussion to validate and augment the patient’s report. Often patients describe depressive symptoms— such as sleep problems, changes in appetite, loss of libido, and impaired cognition—that could be side effects of AEDs or symptoms of epilepsy.
Depression associated with epilepsy has distinct features. Blumer7 coined the term interictal dysphoric disorder (IDD), characterized by these types of symptoms:
- somatoform (anergia, pain, and insomnia)
- affective (irritability, euphoric moods, fear, and anxiety).
Is it bipolar depression? Determine if your patient’s depression might be part of a bipolar disorder. This diagnosis may modify your treatment plan because antidepressants could trigger a manic or hypomanic episode. Also look for untreated psychotic features, which are associated with a suicide completion rate of 19% in bipolar epilepsy patients.6
Screen for suicidal behavior. Ask about suicidal ideation, plans, and attempts within the past month and in the patient’s lifetime.4 Compared with the general population, the risk of suicide is 4 to 5 times higher in depressed persons with epilepsy8 and 25 times higher in those with TLE (Table 1).9 Overdoses are used in 80% to 90% of suicide attempts among patients with epilepsy, perhaps because of the availability of AEDs.3
Perceived social stigma, inability to drive, AEDs’ effects on cognition, and interpersonal and psychosocial issues may contribute to the patient’s depression. Ask about social support, recent stressful events, and financial and vocational impact of the seizure disorder.6
Table 1
Risk factors for suicide among epilepsy patients
| Age 25 to 49 years |
| Male gender |
| Coexisting psychopathology, including personality disorders |
| Temporal lobe epilepsy |
| Personal difficulties such as social or workrelated problems |
| Prolonged duration of epilepsy |
| Poor seizure control |
| Source: Reference 3 |
CASE CONTINUED: Searching for a cause
Previously Ms. A worked steadily and led an active life. She does not have a history of mania or hypomania. She is alert, neat, and cooperative. She sits calmly, gestures appropriately when speaking, and has no abnormal movements. She is subdued but says she is a bit anxious.
Her workup includes routine hematologic and biochemical parameters, EEG, and brain MRI. All are negative.
Her episodes are sudden, brief, and involve altered awareness followed by amnesia. She describes déjà vu, perceptual changes, and motor speech problems.
Ms. A’s behavioral syndrome is consistent with complex partial seizure with frontal and temporal foci. Her dominant brain hemisphere likely is involved because speech is affected. We classify her depression as postictal because of the temporal relationship with the seizures. Thus, controlling the seizures should prevent the depression.
Depression as a side effect
Depression in epilepsy is multifactorial, and pharmacotherapy is one of many biologic and psychosocial risk factors.
AED’s negative effects such as depression and cognitive changes may be caused by polypharmacy, drug- induced folate deficiency, drug titration and dosage, or withdrawal.6 Patients receiving combination therapies are more likely to be depressed.4 AED polypharmacy might be a marker for refractory epilepsy, and thus depression can be caused by both the neurologic illness and its treatment. Shorvon et al10 reported improved alertness, concentration, drive, mood, and sociability after patients’ polytherapy was reduced to monotherapy, especially carbamazepine monotherapy.
Onset or worsening of depression could coincide with starting a new AED.6 Phenobarbital and topiramate are most closely associated with acute depression during initial treatment.3 One study noted that depressed patients taking barbiturates as part of polytherapy were significantly more depressed than those taking carbamazepine.8 Phenobarbital can produce depression, suicidal ideation, and suicidal and parasuicidal behavior.
Patients starting tiagabine might develop agitation, withdrawal, and mood disturbance that could suggest depression.3 With topiramate the rate of affective symptoms is dose-dependent, with an incidence of 9% with 200 mg/d and 19% with 1,000 mg/d.11
Some patients (11% to 15%) receiving polytherapy that includes liver enzyme -inducing AEDs (carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate) present with decreased serum, erythrocyte, and cerebrospinal fluid folate levels, which is associated with depression.4
Bipolar depression. AEDs such as carbamazepine, valproic acid, and lamotrigine are used for bipolar maintenance therapy in patients without a seizure disorder. Lamotrigine is used to treat bipolar depression in non-epilepsy patients. Therefore, these AEDs are first-line treatment for comorbid epilepsy and bipolar depression.12
Using antidepressants
Effectively treating depression in epilepsy patients (Algorithm) encompasses assessment of prescribed AEDs and the use of antidepressants, electroconvulsive therapy (ECT), and psychotherapy. No evidence indicates that any 1 antidepressant is more effective than others for treating depression in patients with epilepsy. When starting an antidepressant, consider the drug’s effect on seizure threshold, its efficacy, and drug-drug interactions.
Seizure risk. Seizures are a rare but serious adverse effect of most antidepressants (Table 2).13 Compared with the incidence of first seizures in the general population (4 Generalized tonic-clonic seizures are associated with increased mortality in tricyclic antidepressant (TCA) overdose, especially with amitriptyline, maprotiline, and clomipramine.
Desipramine, monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), and trazodone are preferred options for depressed epilepsy patients because these drugs lower the seizure threshold less than other antidepressants, with a 1% to 1.5% incidence of seizures during the first 2 years of treatment.14 Because most seizures reported with these medications are dose-related, blood level monitoring is helpful. Avoid bupropion, which has a seizure rate double that of other antidepressants.6
Clinical trial experience and EEG studies suggest that the SSRIs are less epileptogenic than TCAs. MAOIs also are less likely than TCAs to cause seizures but can cause excessive sedation when coadministered with barbiturates. The only double-blind trial of antidepressants (amitriptyline, nomifensine, and placebo) to treat comorbid depression and epilepsy found no significant differences among drugs or placebo.8
Drug-drug interactions. Consider the cytochrome P450 enzyme system when choosing an antidepressant for an epilepsy patient.6 Antidepressants can alter serum levels of phenobarbital and carbamazepine, and AEDs usually reduce antidepressant levels.8 For example, carbamazepine could lower TCA levels, valproic acid might elevate TCA levels,15 and imipramine and nortriptyline might increase phenytoin levels. Similarly, monitor TCA levels during AED withdrawal, as increased TCA concentrations can result in toxicity and concomitant behavioral effects.13
Start antidepressants at doses lower than used in patients without epilepsy, and gradually increase until depression remits. Periodically check AED levels during antidepressant treatment, and adjust dosages to maintain a therapeutic level.
Algorithm
Stepwise approach to treating
comorbid psychiatric disorders and epilepsy
| Step 1 |
| Determine the etiology of depression |
| ↓ |
| Step 2 |
| Assess AED regimen Avoid polytherapy Consider the adverse psychotropic effects with phenobarbital and primidone Consider changing to carbamazepine or valproic acid, if clinically appropriate; modified release preparations usually are better tolerated Monitor total plasma levels of AEDs Screen erythrocyte folate levels |
| ↓ |
| Step 3 |
| Start the antidepressant at a low dose and then increase slowly Start with drugs of choice such as selective serotonin reuptake inhibitors Bupropion, maprotiline, and clomipramine are contraindicated in patients with a history of seizures, brain injury, or EEG abnormality Assess for antidepressant-AED interactions Continue to monitor plasma levels of AEDs Remember that all antidepressants can lower the seizure threshold |
| ↓ |
| Step 4 |
| Consider ECT for refractory or severe depression |
| ↓ |
| Step 5 |
| Recommend support groups and cognitivebehavioral therapy Communicate regularly with patient’s neurologist, primary care physician, and other specialists involved in his or her care |
| AEDs: antiepileptic drugs; ECT: electroconvulsive therapy; |
| EEG: electroencephalography |
Antidepressants’ seizure potential in any patient
| Antidepressant | Risk of seizures (%) |
|---|---|
| High risk (not indicated for epilepsy patients) | |
| Bupropion 450 to 650 mg | 0.4 0.6 to2.19 |
| Clomipramine | 0.5 to 1.66 |
| Maprotiline | 0.4 to 15.6 |
| Intermediate risk | |
| Tricyclics | 0.1 to 15.6 |
| Amitriptyline | 0.4 to 0.5 |
| Imipramine | 0.6 to 0.9 |
| Low risk | |
| Citalopram | |
| Fluoxetine | |
| Fluvoxamine | 0.2 |
| Paroxetine | 0.1 |
| Sertraline | |
| Trazodone | |
| Venlafaxine | 0.1 to 0.2 |
| Mirtazapine | |
| Duloxetine | 0.2 |
| Source: References 6,3 | |
Nondrug therapies
ECT. Consider ECT for patients with refractory or severe depression. ECT can be lifesaving—especially in patients with psychotic depression—and is a viable and safe alternative to antidepressants for patients with epilepsy.3
ECT can raise a patient’s seizure threshold.13 Unilateral nondominant electrode placement is recommended to minimize the combined cognitive side effects of AEDs and ECT. Except for those at high risk of status epilepticus, advise patients not to take their AEDs the morning of ECT treatments.
Support groups. Epilepsy can affect many aspects of a patient’s life, including education, employment, family life, and selfesteem.13 Epilepsy support groups can provide emotional support by introducing patients to others with a seizure disorder. Patients often experience a sense of relief when they discover that they are not alone and other group members share similar dilemmas. Becu et al16 reported that self-help group intervention characterized by education, support, and socialization significantly reduced depression scores in epilepsy patients. These groups often offer education about the nature of the patient’s illness.
Psychotherapy. Because psychosocial factors can play a role in the expression of depression in patients with epilepsy, cognitive, behavioral, and interpersonal therapy may be useful.6 These treatment modalities provide an opportunity to educate patients and families about epilepsy, explore emotional reactions to the condition, and correct false beliefs about the illness. Goldstein17 showed that after 12 sessions of cognitive-behavioral therapy, 6 patients with chronic, poorly controlled epilepsy reported reduced depression scores. Their initial epilepsy-related problem had less impact on their daily lives and self-rated work and social adjustment improved significantly.
CASE CONTINUED: Seeing results
We treat Ms. A’s mood episodes with carbamazepine, titrated up to 400 mg bid. Her seizures decrease in frequency, and after 1 month her depressive symptoms subside. Her coworkers describe Ms. A as more outgoing and full of energy.
We refer Ms. A to an epilepsy support group that meets twice a month, and she is relieved to know other patients experience similar symptoms. She continues individual psychotherapy, which helps her adjust to epilepsy’s chronic nature and complications.
- Epilepsy Foundation. Epilepsy and mood disorders. www.epilepsyfoundation.org/programs/mood.
- Epilepsy Foundation. Neurological Disorders Depression Inventory for Epilepsy Screening Tool. www.epilepsyfoundation.org/about/related/mood/nddietool.cfm.
- Amitriptyline • Elavil
- Amoxapine • Asendin
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranill
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Nomifensine • Merital
- Maprotiline • Ludiomil
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Primidone • Mysoline
- Sertraline • Zoloft
- Tiagabine • Gabitril
- Topiramate • Topamax
- Trazodone • Desyrel
- Valproic acid • Depakote
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kanner AM. Should neurologists be trained to recognize and treat comorbid depression of neurologic disorders? Yes. Epilepsy Behav 2005;6(3):303-11.
2. Gilliam FG. Diagnosis and treatment of mood disorders in persons with epilepsy. Curr Opin Neurol 2005;18(2):129-33.
3. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry 2005;76(suppl 1):45-7.
4. Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999;40(suppl 10):S21-S47.
5. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand 2004;110(4):207-20.
6. Barry JJ. The recognition and management of mood disorders as a comorbidity of epilepsy. Epilepsia 2003;44(suppl 4):30-40.
7. Blumer D, Montouris G, Davies K. The interictal dysphoric disorder: recognition, pathogenesis, and treatment of the major psychiatric disorder of epilepsy. Epilepsy Behav 2004;5(6):826-40.
8. Robertson M. Mood disorders associated with epilepsy. In: McConnell HW, Snyder PJ, eds. Psychiatric comorbidity in epilepsy: basic mechanisms, diagnosis, and treatment. Washington DC: American Psychiatric Publishing; 1998:133-67.
9. Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry 1997;170:205-28.
10. Shorvon SD, Reynolds EH. Reduction in polypharmacy for epilepsy. Br Med J 1979;2(6197):1023-5.
11. Mula M, Trimle MR, Lhatoo SD, Sander JW. Topiramate and psychiatric adverse events in patients with epilepsy. Epilepsia 2003;44(5):659-63.
12. Selai C, Bannister D, Trimble M. Antiepileptic drugs and the regulation of mood and quality (QOL): the evidence from epilepsy. Epilepsia 2005;46(suppl 4):50-7.
13. McConnell H, Duncan D. Treatment of psychiatric comorbidity in epilepsy. In: McConnell HW, Snyder PJ, eds. Psychiatric comorbidity in epilepsy: basic mechanisms, diagnosis, and treatment. Washington DC: American Psychiatric Publishing; 1998:245-361.
14. Charney DS, Berman RM, Miller HL. Treatment of depression. In: Schatzberg AF, Nemeroff CB, eds. Essentials of clinical psychopharmacology. Washington DC: American Psychiatric Publishing; 2001:359.
15. Tucker GJ. Neuropsychiatric aspects of seizure disorders. In: Yudofsky SC, Hales RE, eds. Essentials of neuropsychiatry and clinical neurosciences. Washington DC: American Psychiatric Publishing; 2004:293-313.
16. Becu M, Becu N, Manzur G, Kochen S. Self-help epilepsy groups: an evaluation of effect on depression and schizophrenia. Epilepsia 1993;34(5):841-5.
17. Goldstein LH, McAlpine M, Deale A, et al. Cognitive behaviour therapy with adults with intractable epilepsy and psychiatric co-morbidity: preliminary observations on changes in psychological state and seizure frequency. Behav Res Ther 2003;41(4):447-6.
Ms. A, age 29, has had depression for 6 years and has taken antidepressants with inconsistent response. For 3 weeks, while not taking any antidepressant, she reports loss of energy; feeling sad, subdued, and tearful; poor concentration; and reduced interest in enjoyable activities including sex, the same symptoms she ?rst reported 6 years ago. She has no appetite but has not lost weight.
Several times a month Ms. A “loses” short periods of time. For example she says sometimes she cannot remember what happens between parking her car and sitting at her desk at work. After these episodes, which began 9 years ago, her speech is slightly slurred, and coworkers tease her about being “hungover.” She feels fuzzy-headed, but her speech and thinking clear after a few hours. At other times she smells burning rubber and feels that everything she does repeats what she has done before. Sometimes she feels “out of body” and can watch herself from the ceiling.
Ms. A’s symptoms suggest a seizure disorder. Her depressive features appeared after these ictal episodes began 9 years ago.
Recognizing mood disorders in patients with epilepsy is important because these disorders can be successfully treated within the context of the medical condition.
Many cases of comorbid depression in epilepsy are undiagnosed. A study of 100 patients with refractory epilepsy and depression severe enough for pharmacotherapy found that referral for psychiatric treatment was delayed >1 year in 75% of patients with spontaneous mood disorders and 89% of patients with depression secondary to antiepileptic drugs (AEDs).1
Psychiatrists often are called on to evaluate and treat depression in epilepsy patients or to assess for nonadherence to AEDs. Successfully treating these patients requires understanding:
- the relationship between epilepsy and depression
- the etiology of depression in patients with seizure disorder
- how to treat depression in this population.
High comorbidity
Depression rates are higher in epilepsy patients than in the general population (1% to 3% of men, 2% to 9% of women).2 Depression can be diagnosed in:
- 20% to 30% of patients with recurrent seizures
- 6% to 9% of patients in remission
- 50% to 55% of patients attending hospital epilepsy clinics and video telemetry units.3
Temporal relationship. Depression can be preictal, ictal, postictal, or interictal.4 One-third of patients with partial seizures report premonitory symptoms, usually before secondary generalized tonic clonic seizures.5
- Preictal depression occurs hours to days before a seizure and often is relieved by the convulsion.
- Ictal depression—more common in TLE—occurs as an aura in approximately 1% of patients. Onset is sudden and ranges from mild sadness to profound helplessness and despair. Treating the seizures also treats the depression.
- Postictal depression in TLE patients lasts hours to days after a seizure.
- Interictal depression affects up to two-thirds of epilepsy patients, especially those with severe or frequent seizures. Treat interictal depression separately from the Seizures.
Differential diagnosis
Search for seizure cause. Although 70% of epilepsies are idiopathic, search for the cause of a patient’s seizures. Neuroimaging can rule out a stroke, cerebral tumor, or traumatic brain injury as the cause of both depression and epilepsy.4 Even after exhaustive study, 10% to 20% of epilepsy cases cannot be identified by electroencephalography (EEG).
Seizure type and location, severity, laterality of seizure focus, and frequency are important variables in the etiology of depression in patients with epilepsy.6 Similar changes in neurotransmitters—serotonin, noradrenaline, dopamine, and gamma-aminobutyric acid—are observed in both depression and epilepsy.5
Characterize depressive symptoms. Consider involving the patient’s spouse or partner in the discussion to validate and augment the patient’s report. Often patients describe depressive symptoms— such as sleep problems, changes in appetite, loss of libido, and impaired cognition—that could be side effects of AEDs or symptoms of epilepsy.
Depression associated with epilepsy has distinct features. Blumer7 coined the term interictal dysphoric disorder (IDD), characterized by these types of symptoms:
- somatoform (anergia, pain, and insomnia)
- affective (irritability, euphoric moods, fear, and anxiety).
Is it bipolar depression? Determine if your patient’s depression might be part of a bipolar disorder. This diagnosis may modify your treatment plan because antidepressants could trigger a manic or hypomanic episode. Also look for untreated psychotic features, which are associated with a suicide completion rate of 19% in bipolar epilepsy patients.6
Screen for suicidal behavior. Ask about suicidal ideation, plans, and attempts within the past month and in the patient’s lifetime.4 Compared with the general population, the risk of suicide is 4 to 5 times higher in depressed persons with epilepsy8 and 25 times higher in those with TLE (Table 1).9 Overdoses are used in 80% to 90% of suicide attempts among patients with epilepsy, perhaps because of the availability of AEDs.3
Perceived social stigma, inability to drive, AEDs’ effects on cognition, and interpersonal and psychosocial issues may contribute to the patient’s depression. Ask about social support, recent stressful events, and financial and vocational impact of the seizure disorder.6
Table 1
Risk factors for suicide among epilepsy patients
| Age 25 to 49 years |
| Male gender |
| Coexisting psychopathology, including personality disorders |
| Temporal lobe epilepsy |
| Personal difficulties such as social or workrelated problems |
| Prolonged duration of epilepsy |
| Poor seizure control |
| Source: Reference 3 |
CASE CONTINUED: Searching for a cause
Previously Ms. A worked steadily and led an active life. She does not have a history of mania or hypomania. She is alert, neat, and cooperative. She sits calmly, gestures appropriately when speaking, and has no abnormal movements. She is subdued but says she is a bit anxious.
Her workup includes routine hematologic and biochemical parameters, EEG, and brain MRI. All are negative.
Her episodes are sudden, brief, and involve altered awareness followed by amnesia. She describes déjà vu, perceptual changes, and motor speech problems.
Ms. A’s behavioral syndrome is consistent with complex partial seizure with frontal and temporal foci. Her dominant brain hemisphere likely is involved because speech is affected. We classify her depression as postictal because of the temporal relationship with the seizures. Thus, controlling the seizures should prevent the depression.
Depression as a side effect
Depression in epilepsy is multifactorial, and pharmacotherapy is one of many biologic and psychosocial risk factors.
AED’s negative effects such as depression and cognitive changes may be caused by polypharmacy, drug- induced folate deficiency, drug titration and dosage, or withdrawal.6 Patients receiving combination therapies are more likely to be depressed.4 AED polypharmacy might be a marker for refractory epilepsy, and thus depression can be caused by both the neurologic illness and its treatment. Shorvon et al10 reported improved alertness, concentration, drive, mood, and sociability after patients’ polytherapy was reduced to monotherapy, especially carbamazepine monotherapy.
Onset or worsening of depression could coincide with starting a new AED.6 Phenobarbital and topiramate are most closely associated with acute depression during initial treatment.3 One study noted that depressed patients taking barbiturates as part of polytherapy were significantly more depressed than those taking carbamazepine.8 Phenobarbital can produce depression, suicidal ideation, and suicidal and parasuicidal behavior.
Patients starting tiagabine might develop agitation, withdrawal, and mood disturbance that could suggest depression.3 With topiramate the rate of affective symptoms is dose-dependent, with an incidence of 9% with 200 mg/d and 19% with 1,000 mg/d.11
Some patients (11% to 15%) receiving polytherapy that includes liver enzyme -inducing AEDs (carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate) present with decreased serum, erythrocyte, and cerebrospinal fluid folate levels, which is associated with depression.4
Bipolar depression. AEDs such as carbamazepine, valproic acid, and lamotrigine are used for bipolar maintenance therapy in patients without a seizure disorder. Lamotrigine is used to treat bipolar depression in non-epilepsy patients. Therefore, these AEDs are first-line treatment for comorbid epilepsy and bipolar depression.12
Using antidepressants
Effectively treating depression in epilepsy patients (Algorithm) encompasses assessment of prescribed AEDs and the use of antidepressants, electroconvulsive therapy (ECT), and psychotherapy. No evidence indicates that any 1 antidepressant is more effective than others for treating depression in patients with epilepsy. When starting an antidepressant, consider the drug’s effect on seizure threshold, its efficacy, and drug-drug interactions.
Seizure risk. Seizures are a rare but serious adverse effect of most antidepressants (Table 2).13 Compared with the incidence of first seizures in the general population (4 Generalized tonic-clonic seizures are associated with increased mortality in tricyclic antidepressant (TCA) overdose, especially with amitriptyline, maprotiline, and clomipramine.
Desipramine, monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), and trazodone are preferred options for depressed epilepsy patients because these drugs lower the seizure threshold less than other antidepressants, with a 1% to 1.5% incidence of seizures during the first 2 years of treatment.14 Because most seizures reported with these medications are dose-related, blood level monitoring is helpful. Avoid bupropion, which has a seizure rate double that of other antidepressants.6
Clinical trial experience and EEG studies suggest that the SSRIs are less epileptogenic than TCAs. MAOIs also are less likely than TCAs to cause seizures but can cause excessive sedation when coadministered with barbiturates. The only double-blind trial of antidepressants (amitriptyline, nomifensine, and placebo) to treat comorbid depression and epilepsy found no significant differences among drugs or placebo.8
Drug-drug interactions. Consider the cytochrome P450 enzyme system when choosing an antidepressant for an epilepsy patient.6 Antidepressants can alter serum levels of phenobarbital and carbamazepine, and AEDs usually reduce antidepressant levels.8 For example, carbamazepine could lower TCA levels, valproic acid might elevate TCA levels,15 and imipramine and nortriptyline might increase phenytoin levels. Similarly, monitor TCA levels during AED withdrawal, as increased TCA concentrations can result in toxicity and concomitant behavioral effects.13
Start antidepressants at doses lower than used in patients without epilepsy, and gradually increase until depression remits. Periodically check AED levels during antidepressant treatment, and adjust dosages to maintain a therapeutic level.
Algorithm
Stepwise approach to treating
comorbid psychiatric disorders and epilepsy
| Step 1 |
| Determine the etiology of depression |
| ↓ |
| Step 2 |
| Assess AED regimen Avoid polytherapy Consider the adverse psychotropic effects with phenobarbital and primidone Consider changing to carbamazepine or valproic acid, if clinically appropriate; modified release preparations usually are better tolerated Monitor total plasma levels of AEDs Screen erythrocyte folate levels |
| ↓ |
| Step 3 |
| Start the antidepressant at a low dose and then increase slowly Start with drugs of choice such as selective serotonin reuptake inhibitors Bupropion, maprotiline, and clomipramine are contraindicated in patients with a history of seizures, brain injury, or EEG abnormality Assess for antidepressant-AED interactions Continue to monitor plasma levels of AEDs Remember that all antidepressants can lower the seizure threshold |
| ↓ |
| Step 4 |
| Consider ECT for refractory or severe depression |
| ↓ |
| Step 5 |
| Recommend support groups and cognitivebehavioral therapy Communicate regularly with patient’s neurologist, primary care physician, and other specialists involved in his or her care |
| AEDs: antiepileptic drugs; ECT: electroconvulsive therapy; |
| EEG: electroencephalography |
Antidepressants’ seizure potential in any patient
| Antidepressant | Risk of seizures (%) |
|---|---|
| High risk (not indicated for epilepsy patients) | |
| Bupropion 450 to 650 mg | 0.4 0.6 to2.19 |
| Clomipramine | 0.5 to 1.66 |
| Maprotiline | 0.4 to 15.6 |
| Intermediate risk | |
| Tricyclics | 0.1 to 15.6 |
| Amitriptyline | 0.4 to 0.5 |
| Imipramine | 0.6 to 0.9 |
| Low risk | |
| Citalopram | |
| Fluoxetine | |
| Fluvoxamine | 0.2 |
| Paroxetine | 0.1 |
| Sertraline | |
| Trazodone | |
| Venlafaxine | 0.1 to 0.2 |
| Mirtazapine | |
| Duloxetine | 0.2 |
| Source: References 6,3 | |
Nondrug therapies
ECT. Consider ECT for patients with refractory or severe depression. ECT can be lifesaving—especially in patients with psychotic depression—and is a viable and safe alternative to antidepressants for patients with epilepsy.3
ECT can raise a patient’s seizure threshold.13 Unilateral nondominant electrode placement is recommended to minimize the combined cognitive side effects of AEDs and ECT. Except for those at high risk of status epilepticus, advise patients not to take their AEDs the morning of ECT treatments.
Support groups. Epilepsy can affect many aspects of a patient’s life, including education, employment, family life, and selfesteem.13 Epilepsy support groups can provide emotional support by introducing patients to others with a seizure disorder. Patients often experience a sense of relief when they discover that they are not alone and other group members share similar dilemmas. Becu et al16 reported that self-help group intervention characterized by education, support, and socialization significantly reduced depression scores in epilepsy patients. These groups often offer education about the nature of the patient’s illness.
Psychotherapy. Because psychosocial factors can play a role in the expression of depression in patients with epilepsy, cognitive, behavioral, and interpersonal therapy may be useful.6 These treatment modalities provide an opportunity to educate patients and families about epilepsy, explore emotional reactions to the condition, and correct false beliefs about the illness. Goldstein17 showed that after 12 sessions of cognitive-behavioral therapy, 6 patients with chronic, poorly controlled epilepsy reported reduced depression scores. Their initial epilepsy-related problem had less impact on their daily lives and self-rated work and social adjustment improved significantly.
CASE CONTINUED: Seeing results
We treat Ms. A’s mood episodes with carbamazepine, titrated up to 400 mg bid. Her seizures decrease in frequency, and after 1 month her depressive symptoms subside. Her coworkers describe Ms. A as more outgoing and full of energy.
We refer Ms. A to an epilepsy support group that meets twice a month, and she is relieved to know other patients experience similar symptoms. She continues individual psychotherapy, which helps her adjust to epilepsy’s chronic nature and complications.
- Epilepsy Foundation. Epilepsy and mood disorders. www.epilepsyfoundation.org/programs/mood.
- Epilepsy Foundation. Neurological Disorders Depression Inventory for Epilepsy Screening Tool. www.epilepsyfoundation.org/about/related/mood/nddietool.cfm.
- Amitriptyline • Elavil
- Amoxapine • Asendin
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranill
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Nomifensine • Merital
- Maprotiline • Ludiomil
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Primidone • Mysoline
- Sertraline • Zoloft
- Tiagabine • Gabitril
- Topiramate • Topamax
- Trazodone • Desyrel
- Valproic acid • Depakote
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. A, age 29, has had depression for 6 years and has taken antidepressants with inconsistent response. For 3 weeks, while not taking any antidepressant, she reports loss of energy; feeling sad, subdued, and tearful; poor concentration; and reduced interest in enjoyable activities including sex, the same symptoms she ?rst reported 6 years ago. She has no appetite but has not lost weight.
Several times a month Ms. A “loses” short periods of time. For example she says sometimes she cannot remember what happens between parking her car and sitting at her desk at work. After these episodes, which began 9 years ago, her speech is slightly slurred, and coworkers tease her about being “hungover.” She feels fuzzy-headed, but her speech and thinking clear after a few hours. At other times she smells burning rubber and feels that everything she does repeats what she has done before. Sometimes she feels “out of body” and can watch herself from the ceiling.
Ms. A’s symptoms suggest a seizure disorder. Her depressive features appeared after these ictal episodes began 9 years ago.
Recognizing mood disorders in patients with epilepsy is important because these disorders can be successfully treated within the context of the medical condition.
Many cases of comorbid depression in epilepsy are undiagnosed. A study of 100 patients with refractory epilepsy and depression severe enough for pharmacotherapy found that referral for psychiatric treatment was delayed >1 year in 75% of patients with spontaneous mood disorders and 89% of patients with depression secondary to antiepileptic drugs (AEDs).1
Psychiatrists often are called on to evaluate and treat depression in epilepsy patients or to assess for nonadherence to AEDs. Successfully treating these patients requires understanding:
- the relationship between epilepsy and depression
- the etiology of depression in patients with seizure disorder
- how to treat depression in this population.
High comorbidity
Depression rates are higher in epilepsy patients than in the general population (1% to 3% of men, 2% to 9% of women).2 Depression can be diagnosed in:
- 20% to 30% of patients with recurrent seizures
- 6% to 9% of patients in remission
- 50% to 55% of patients attending hospital epilepsy clinics and video telemetry units.3
Temporal relationship. Depression can be preictal, ictal, postictal, or interictal.4 One-third of patients with partial seizures report premonitory symptoms, usually before secondary generalized tonic clonic seizures.5
- Preictal depression occurs hours to days before a seizure and often is relieved by the convulsion.
- Ictal depression—more common in TLE—occurs as an aura in approximately 1% of patients. Onset is sudden and ranges from mild sadness to profound helplessness and despair. Treating the seizures also treats the depression.
- Postictal depression in TLE patients lasts hours to days after a seizure.
- Interictal depression affects up to two-thirds of epilepsy patients, especially those with severe or frequent seizures. Treat interictal depression separately from the Seizures.
Differential diagnosis
Search for seizure cause. Although 70% of epilepsies are idiopathic, search for the cause of a patient’s seizures. Neuroimaging can rule out a stroke, cerebral tumor, or traumatic brain injury as the cause of both depression and epilepsy.4 Even after exhaustive study, 10% to 20% of epilepsy cases cannot be identified by electroencephalography (EEG).
Seizure type and location, severity, laterality of seizure focus, and frequency are important variables in the etiology of depression in patients with epilepsy.6 Similar changes in neurotransmitters—serotonin, noradrenaline, dopamine, and gamma-aminobutyric acid—are observed in both depression and epilepsy.5
Characterize depressive symptoms. Consider involving the patient’s spouse or partner in the discussion to validate and augment the patient’s report. Often patients describe depressive symptoms— such as sleep problems, changes in appetite, loss of libido, and impaired cognition—that could be side effects of AEDs or symptoms of epilepsy.
Depression associated with epilepsy has distinct features. Blumer7 coined the term interictal dysphoric disorder (IDD), characterized by these types of symptoms:
- somatoform (anergia, pain, and insomnia)
- affective (irritability, euphoric moods, fear, and anxiety).
Is it bipolar depression? Determine if your patient’s depression might be part of a bipolar disorder. This diagnosis may modify your treatment plan because antidepressants could trigger a manic or hypomanic episode. Also look for untreated psychotic features, which are associated with a suicide completion rate of 19% in bipolar epilepsy patients.6
Screen for suicidal behavior. Ask about suicidal ideation, plans, and attempts within the past month and in the patient’s lifetime.4 Compared with the general population, the risk of suicide is 4 to 5 times higher in depressed persons with epilepsy8 and 25 times higher in those with TLE (Table 1).9 Overdoses are used in 80% to 90% of suicide attempts among patients with epilepsy, perhaps because of the availability of AEDs.3
Perceived social stigma, inability to drive, AEDs’ effects on cognition, and interpersonal and psychosocial issues may contribute to the patient’s depression. Ask about social support, recent stressful events, and financial and vocational impact of the seizure disorder.6
Table 1
Risk factors for suicide among epilepsy patients
| Age 25 to 49 years |
| Male gender |
| Coexisting psychopathology, including personality disorders |
| Temporal lobe epilepsy |
| Personal difficulties such as social or workrelated problems |
| Prolonged duration of epilepsy |
| Poor seizure control |
| Source: Reference 3 |
CASE CONTINUED: Searching for a cause
Previously Ms. A worked steadily and led an active life. She does not have a history of mania or hypomania. She is alert, neat, and cooperative. She sits calmly, gestures appropriately when speaking, and has no abnormal movements. She is subdued but says she is a bit anxious.
Her workup includes routine hematologic and biochemical parameters, EEG, and brain MRI. All are negative.
Her episodes are sudden, brief, and involve altered awareness followed by amnesia. She describes déjà vu, perceptual changes, and motor speech problems.
Ms. A’s behavioral syndrome is consistent with complex partial seizure with frontal and temporal foci. Her dominant brain hemisphere likely is involved because speech is affected. We classify her depression as postictal because of the temporal relationship with the seizures. Thus, controlling the seizures should prevent the depression.
Depression as a side effect
Depression in epilepsy is multifactorial, and pharmacotherapy is one of many biologic and psychosocial risk factors.
AED’s negative effects such as depression and cognitive changes may be caused by polypharmacy, drug- induced folate deficiency, drug titration and dosage, or withdrawal.6 Patients receiving combination therapies are more likely to be depressed.4 AED polypharmacy might be a marker for refractory epilepsy, and thus depression can be caused by both the neurologic illness and its treatment. Shorvon et al10 reported improved alertness, concentration, drive, mood, and sociability after patients’ polytherapy was reduced to monotherapy, especially carbamazepine monotherapy.
Onset or worsening of depression could coincide with starting a new AED.6 Phenobarbital and topiramate are most closely associated with acute depression during initial treatment.3 One study noted that depressed patients taking barbiturates as part of polytherapy were significantly more depressed than those taking carbamazepine.8 Phenobarbital can produce depression, suicidal ideation, and suicidal and parasuicidal behavior.
Patients starting tiagabine might develop agitation, withdrawal, and mood disturbance that could suggest depression.3 With topiramate the rate of affective symptoms is dose-dependent, with an incidence of 9% with 200 mg/d and 19% with 1,000 mg/d.11
Some patients (11% to 15%) receiving polytherapy that includes liver enzyme -inducing AEDs (carbamazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and topiramate) present with decreased serum, erythrocyte, and cerebrospinal fluid folate levels, which is associated with depression.4
Bipolar depression. AEDs such as carbamazepine, valproic acid, and lamotrigine are used for bipolar maintenance therapy in patients without a seizure disorder. Lamotrigine is used to treat bipolar depression in non-epilepsy patients. Therefore, these AEDs are first-line treatment for comorbid epilepsy and bipolar depression.12
Using antidepressants
Effectively treating depression in epilepsy patients (Algorithm) encompasses assessment of prescribed AEDs and the use of antidepressants, electroconvulsive therapy (ECT), and psychotherapy. No evidence indicates that any 1 antidepressant is more effective than others for treating depression in patients with epilepsy. When starting an antidepressant, consider the drug’s effect on seizure threshold, its efficacy, and drug-drug interactions.
Seizure risk. Seizures are a rare but serious adverse effect of most antidepressants (Table 2).13 Compared with the incidence of first seizures in the general population (4 Generalized tonic-clonic seizures are associated with increased mortality in tricyclic antidepressant (TCA) overdose, especially with amitriptyline, maprotiline, and clomipramine.
Desipramine, monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), and trazodone are preferred options for depressed epilepsy patients because these drugs lower the seizure threshold less than other antidepressants, with a 1% to 1.5% incidence of seizures during the first 2 years of treatment.14 Because most seizures reported with these medications are dose-related, blood level monitoring is helpful. Avoid bupropion, which has a seizure rate double that of other antidepressants.6
Clinical trial experience and EEG studies suggest that the SSRIs are less epileptogenic than TCAs. MAOIs also are less likely than TCAs to cause seizures but can cause excessive sedation when coadministered with barbiturates. The only double-blind trial of antidepressants (amitriptyline, nomifensine, and placebo) to treat comorbid depression and epilepsy found no significant differences among drugs or placebo.8
Drug-drug interactions. Consider the cytochrome P450 enzyme system when choosing an antidepressant for an epilepsy patient.6 Antidepressants can alter serum levels of phenobarbital and carbamazepine, and AEDs usually reduce antidepressant levels.8 For example, carbamazepine could lower TCA levels, valproic acid might elevate TCA levels,15 and imipramine and nortriptyline might increase phenytoin levels. Similarly, monitor TCA levels during AED withdrawal, as increased TCA concentrations can result in toxicity and concomitant behavioral effects.13
Start antidepressants at doses lower than used in patients without epilepsy, and gradually increase until depression remits. Periodically check AED levels during antidepressant treatment, and adjust dosages to maintain a therapeutic level.
Algorithm
Stepwise approach to treating
comorbid psychiatric disorders and epilepsy
| Step 1 |
| Determine the etiology of depression |
| ↓ |
| Step 2 |
| Assess AED regimen Avoid polytherapy Consider the adverse psychotropic effects with phenobarbital and primidone Consider changing to carbamazepine or valproic acid, if clinically appropriate; modified release preparations usually are better tolerated Monitor total plasma levels of AEDs Screen erythrocyte folate levels |
| ↓ |
| Step 3 |
| Start the antidepressant at a low dose and then increase slowly Start with drugs of choice such as selective serotonin reuptake inhibitors Bupropion, maprotiline, and clomipramine are contraindicated in patients with a history of seizures, brain injury, or EEG abnormality Assess for antidepressant-AED interactions Continue to monitor plasma levels of AEDs Remember that all antidepressants can lower the seizure threshold |
| ↓ |
| Step 4 |
| Consider ECT for refractory or severe depression |
| ↓ |
| Step 5 |
| Recommend support groups and cognitivebehavioral therapy Communicate regularly with patient’s neurologist, primary care physician, and other specialists involved in his or her care |
| AEDs: antiepileptic drugs; ECT: electroconvulsive therapy; |
| EEG: electroencephalography |
Antidepressants’ seizure potential in any patient
| Antidepressant | Risk of seizures (%) |
|---|---|
| High risk (not indicated for epilepsy patients) | |
| Bupropion 450 to 650 mg | 0.4 0.6 to2.19 |
| Clomipramine | 0.5 to 1.66 |
| Maprotiline | 0.4 to 15.6 |
| Intermediate risk | |
| Tricyclics | 0.1 to 15.6 |
| Amitriptyline | 0.4 to 0.5 |
| Imipramine | 0.6 to 0.9 |
| Low risk | |
| Citalopram | |
| Fluoxetine | |
| Fluvoxamine | 0.2 |
| Paroxetine | 0.1 |
| Sertraline | |
| Trazodone | |
| Venlafaxine | 0.1 to 0.2 |
| Mirtazapine | |
| Duloxetine | 0.2 |
| Source: References 6,3 | |
Nondrug therapies
ECT. Consider ECT for patients with refractory or severe depression. ECT can be lifesaving—especially in patients with psychotic depression—and is a viable and safe alternative to antidepressants for patients with epilepsy.3
ECT can raise a patient’s seizure threshold.13 Unilateral nondominant electrode placement is recommended to minimize the combined cognitive side effects of AEDs and ECT. Except for those at high risk of status epilepticus, advise patients not to take their AEDs the morning of ECT treatments.
Support groups. Epilepsy can affect many aspects of a patient’s life, including education, employment, family life, and selfesteem.13 Epilepsy support groups can provide emotional support by introducing patients to others with a seizure disorder. Patients often experience a sense of relief when they discover that they are not alone and other group members share similar dilemmas. Becu et al16 reported that self-help group intervention characterized by education, support, and socialization significantly reduced depression scores in epilepsy patients. These groups often offer education about the nature of the patient’s illness.
Psychotherapy. Because psychosocial factors can play a role in the expression of depression in patients with epilepsy, cognitive, behavioral, and interpersonal therapy may be useful.6 These treatment modalities provide an opportunity to educate patients and families about epilepsy, explore emotional reactions to the condition, and correct false beliefs about the illness. Goldstein17 showed that after 12 sessions of cognitive-behavioral therapy, 6 patients with chronic, poorly controlled epilepsy reported reduced depression scores. Their initial epilepsy-related problem had less impact on their daily lives and self-rated work and social adjustment improved significantly.
CASE CONTINUED: Seeing results
We treat Ms. A’s mood episodes with carbamazepine, titrated up to 400 mg bid. Her seizures decrease in frequency, and after 1 month her depressive symptoms subside. Her coworkers describe Ms. A as more outgoing and full of energy.
We refer Ms. A to an epilepsy support group that meets twice a month, and she is relieved to know other patients experience similar symptoms. She continues individual psychotherapy, which helps her adjust to epilepsy’s chronic nature and complications.
- Epilepsy Foundation. Epilepsy and mood disorders. www.epilepsyfoundation.org/programs/mood.
- Epilepsy Foundation. Neurological Disorders Depression Inventory for Epilepsy Screening Tool. www.epilepsyfoundation.org/about/related/mood/nddietool.cfm.
- Amitriptyline • Elavil
- Amoxapine • Asendin
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranill
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Nomifensine • Merital
- Maprotiline • Ludiomil
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Aventyl, Pamelor
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Primidone • Mysoline
- Sertraline • Zoloft
- Tiagabine • Gabitril
- Topiramate • Topamax
- Trazodone • Desyrel
- Valproic acid • Depakote
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kanner AM. Should neurologists be trained to recognize and treat comorbid depression of neurologic disorders? Yes. Epilepsy Behav 2005;6(3):303-11.
2. Gilliam FG. Diagnosis and treatment of mood disorders in persons with epilepsy. Curr Opin Neurol 2005;18(2):129-33.
3. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry 2005;76(suppl 1):45-7.
4. Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999;40(suppl 10):S21-S47.
5. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand 2004;110(4):207-20.
6. Barry JJ. The recognition and management of mood disorders as a comorbidity of epilepsy. Epilepsia 2003;44(suppl 4):30-40.
7. Blumer D, Montouris G, Davies K. The interictal dysphoric disorder: recognition, pathogenesis, and treatment of the major psychiatric disorder of epilepsy. Epilepsy Behav 2004;5(6):826-40.
8. Robertson M. Mood disorders associated with epilepsy. In: McConnell HW, Snyder PJ, eds. Psychiatric comorbidity in epilepsy: basic mechanisms, diagnosis, and treatment. Washington DC: American Psychiatric Publishing; 1998:133-67.
9. Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry 1997;170:205-28.
10. Shorvon SD, Reynolds EH. Reduction in polypharmacy for epilepsy. Br Med J 1979;2(6197):1023-5.
11. Mula M, Trimle MR, Lhatoo SD, Sander JW. Topiramate and psychiatric adverse events in patients with epilepsy. Epilepsia 2003;44(5):659-63.
12. Selai C, Bannister D, Trimble M. Antiepileptic drugs and the regulation of mood and quality (QOL): the evidence from epilepsy. Epilepsia 2005;46(suppl 4):50-7.
13. McConnell H, Duncan D. Treatment of psychiatric comorbidity in epilepsy. In: McConnell HW, Snyder PJ, eds. Psychiatric comorbidity in epilepsy: basic mechanisms, diagnosis, and treatment. Washington DC: American Psychiatric Publishing; 1998:245-361.
14. Charney DS, Berman RM, Miller HL. Treatment of depression. In: Schatzberg AF, Nemeroff CB, eds. Essentials of clinical psychopharmacology. Washington DC: American Psychiatric Publishing; 2001:359.
15. Tucker GJ. Neuropsychiatric aspects of seizure disorders. In: Yudofsky SC, Hales RE, eds. Essentials of neuropsychiatry and clinical neurosciences. Washington DC: American Psychiatric Publishing; 2004:293-313.
16. Becu M, Becu N, Manzur G, Kochen S. Self-help epilepsy groups: an evaluation of effect on depression and schizophrenia. Epilepsia 1993;34(5):841-5.
17. Goldstein LH, McAlpine M, Deale A, et al. Cognitive behaviour therapy with adults with intractable epilepsy and psychiatric co-morbidity: preliminary observations on changes in psychological state and seizure frequency. Behav Res Ther 2003;41(4):447-6.
1. Kanner AM. Should neurologists be trained to recognize and treat comorbid depression of neurologic disorders? Yes. Epilepsy Behav 2005;6(3):303-11.
2. Gilliam FG. Diagnosis and treatment of mood disorders in persons with epilepsy. Curr Opin Neurol 2005;18(2):129-33.
3. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry 2005;76(suppl 1):45-7.
4. Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999;40(suppl 10):S21-S47.
5. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand 2004;110(4):207-20.
6. Barry JJ. The recognition and management of mood disorders as a comorbidity of epilepsy. Epilepsia 2003;44(suppl 4):30-40.
7. Blumer D, Montouris G, Davies K. The interictal dysphoric disorder: recognition, pathogenesis, and treatment of the major psychiatric disorder of epilepsy. Epilepsy Behav 2004;5(6):826-40.
8. Robertson M. Mood disorders associated with epilepsy. In: McConnell HW, Snyder PJ, eds. Psychiatric comorbidity in epilepsy: basic mechanisms, diagnosis, and treatment. Washington DC: American Psychiatric Publishing; 1998:133-67.
9. Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry 1997;170:205-28.
10. Shorvon SD, Reynolds EH. Reduction in polypharmacy for epilepsy. Br Med J 1979;2(6197):1023-5.
11. Mula M, Trimle MR, Lhatoo SD, Sander JW. Topiramate and psychiatric adverse events in patients with epilepsy. Epilepsia 2003;44(5):659-63.
12. Selai C, Bannister D, Trimble M. Antiepileptic drugs and the regulation of mood and quality (QOL): the evidence from epilepsy. Epilepsia 2005;46(suppl 4):50-7.
13. McConnell H, Duncan D. Treatment of psychiatric comorbidity in epilepsy. In: McConnell HW, Snyder PJ, eds. Psychiatric comorbidity in epilepsy: basic mechanisms, diagnosis, and treatment. Washington DC: American Psychiatric Publishing; 1998:245-361.
14. Charney DS, Berman RM, Miller HL. Treatment of depression. In: Schatzberg AF, Nemeroff CB, eds. Essentials of clinical psychopharmacology. Washington DC: American Psychiatric Publishing; 2001:359.
15. Tucker GJ. Neuropsychiatric aspects of seizure disorders. In: Yudofsky SC, Hales RE, eds. Essentials of neuropsychiatry and clinical neurosciences. Washington DC: American Psychiatric Publishing; 2004:293-313.
16. Becu M, Becu N, Manzur G, Kochen S. Self-help epilepsy groups: an evaluation of effect on depression and schizophrenia. Epilepsia 1993;34(5):841-5.
17. Goldstein LH, McAlpine M, Deale A, et al. Cognitive behaviour therapy with adults with intractable epilepsy and psychiatric co-morbidity: preliminary observations on changes in psychological state and seizure frequency. Behav Res Ther 2003;41(4):447-6.