User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
What’s that rash? Recognize community-acquired MRSA
Some patients at high risk for mental illness—intravenous drug users, prisoners, human immunodeficiency virus-positive patients, and the homeless—also are at risk of community-acquired, methicillin-resistant Staphylococcus aureus (CA-MRSA) infections.1 Because your patients may present with CA-MRSA symptoms, you need a basic understanding of this infection’s risk factors and clinical features to initiate necessary referrals (Table).2
Table
Features of community-acquired MRSA infections
| At-risk populations | HIV infection, IV drug users, homeless, men who have sex with men, tattoo recipients, individuals living in close quarters such as group homes or prisons May affect healthy individuals without risk factors |
| Clinical presentation | Small, hard, red, painful lesions that resemble a spider bite Most common: skin infections such as a boil, abscess, or cellulitis Less common: bone and joint infections, pneumonia |
| Transmission | Skin-to-skin contact with infected persons Sharing personal hygiene items, such as towels, with infected persons Skin breaks |
| Symptoms requiring emergent referral | Fever, shortness of breath, hypotension, or other systemic symptoms Rapidly spreading lesion |
| Source: Reference 2 | |
Risk factors and transmission
CA-MRSA accounts for 78% of skin and soft tissue infections in emergency rooms.3 Patients typically have no known risk factors for infection or health-related exposures, such as recent hospitalization or employment in a healthcare setting. Persons who have taken antibiotics in the past 12 months are at increased risk.1,3,4
Infection spreads by person-to-person contact. In the community, crowding and sharing personal items also facilitate transmission, which accounts for increased risk among military personnel and athletes in contact sports.1 Therefore, caution psychiatric patients against sharing personal hygiene items, such as towels, and instruct infected patients to keep abscess sites covered at all times. Stress the importance of consistent handwashing.
Infection also may be acquired through a skin abrasion, although many infected patients do not remember having local skin trauma.
Clinical presentation. Unlike diffuse drug eruptions associated with psychotropic hypersensitivity reactions, skin involvement caused by CA-MRSA typically is limited. Patients generally present with a warm, swollen, and erythematous area of skin or a circumscribed abscess involving a hair follicle.1 Often patients attribute symptoms to a recent spider bite or report that a family member or friend has a similar rash or lesion.3
Single lesions on the extremities are common, although multiple “boils” are possible. Fluctuance—a wavelike motion beneath the lesion when pressure is applied—may be present. Fever and chills usually are absent unless the infection is invasive or systemic (Photo). Serious forms of infection—such as impetigo and necrotizing fasciitis—are less common, although the latter has been reported more frequently among IV drug users.1
© 2001-2007 DermAtlas
Warm, swollen, erythematous skin with red papules and plaques with central pustules often on the extremities. Treatment. Although the prognosis for most CA-MRSA skin and soft tissue infections is favorable, serious and potentially life-threatening complications can emerge.1 Most infections can be treated successfully with antibiotics and—when an abscess is present—incision and drainage performed in a primary care physician’s office. Trimethoprim-sulfamethoxazole—a commonly used antibiotic—can decrease serum levels of tricyclic antidepressants and prolong the QT interval. Be aware of this interaction in patients receiving antipsychotics, which also can prolong the QT interval.
- A single boil, abscess, or small, red, painful lesion suggests a community-acquired, methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infection.
- Be aware of the clinical presentation of CA-MRSA infections to facilitate necessary referrals to a primary care physician or ER.
- Educate your patients at risk for CA-MRSA skin infections to protect themselves and avoid transmitting infection to others.
Referral to a primary care physician for further management is appropriate for afebrile patients without a history of immunosuppression who present with localized rash involving 1 extremity. Severe infection with bacteremia or other systemic involvement is possible, especially in patients age ≥65.5 Consider ER referral for patients with:
- compromised immune systems
- high fever and/or chills
- rapidly progressing symptoms
- signs and symptoms consistent with systemic illness, such as shortness of breath or low blood pressure
- disease involving >1 extremity or multiple abscesses.
Related resources
- Centers for Disease Control and Prevention. Overview of community-associated MRSA. www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html.
- Zeller JL, Burke AE, Glass RM. JAMA patient page. MRSA infections. JAMA 2007;298(15):1826. Available at: http://jama.ama-assn.org/cgi/content/full/298/15/1826.
Drug brand name
- Trimethoprim-sulfamethoxazole • Bactrim, Septra
Disclosures
Dr. Hebert reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rado receives grant/research support from Neuronetics, Eli Lilly and Company, and Janssen Pharmaceutica.
1. Stryjewski ME, Chambers HF. Skin and soft tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46:S368-77.
2. Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46:S344-9.
3. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-74.
4. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468-75.
5. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007;298(15):1763-7
Some patients at high risk for mental illness—intravenous drug users, prisoners, human immunodeficiency virus-positive patients, and the homeless—also are at risk of community-acquired, methicillin-resistant Staphylococcus aureus (CA-MRSA) infections.1 Because your patients may present with CA-MRSA symptoms, you need a basic understanding of this infection’s risk factors and clinical features to initiate necessary referrals (Table).2
Table
Features of community-acquired MRSA infections
| At-risk populations | HIV infection, IV drug users, homeless, men who have sex with men, tattoo recipients, individuals living in close quarters such as group homes or prisons May affect healthy individuals without risk factors |
| Clinical presentation | Small, hard, red, painful lesions that resemble a spider bite Most common: skin infections such as a boil, abscess, or cellulitis Less common: bone and joint infections, pneumonia |
| Transmission | Skin-to-skin contact with infected persons Sharing personal hygiene items, such as towels, with infected persons Skin breaks |
| Symptoms requiring emergent referral | Fever, shortness of breath, hypotension, or other systemic symptoms Rapidly spreading lesion |
| Source: Reference 2 | |
Risk factors and transmission
CA-MRSA accounts for 78% of skin and soft tissue infections in emergency rooms.3 Patients typically have no known risk factors for infection or health-related exposures, such as recent hospitalization or employment in a healthcare setting. Persons who have taken antibiotics in the past 12 months are at increased risk.1,3,4
Infection spreads by person-to-person contact. In the community, crowding and sharing personal items also facilitate transmission, which accounts for increased risk among military personnel and athletes in contact sports.1 Therefore, caution psychiatric patients against sharing personal hygiene items, such as towels, and instruct infected patients to keep abscess sites covered at all times. Stress the importance of consistent handwashing.
Infection also may be acquired through a skin abrasion, although many infected patients do not remember having local skin trauma.
Clinical presentation. Unlike diffuse drug eruptions associated with psychotropic hypersensitivity reactions, skin involvement caused by CA-MRSA typically is limited. Patients generally present with a warm, swollen, and erythematous area of skin or a circumscribed abscess involving a hair follicle.1 Often patients attribute symptoms to a recent spider bite or report that a family member or friend has a similar rash or lesion.3
Single lesions on the extremities are common, although multiple “boils” are possible. Fluctuance—a wavelike motion beneath the lesion when pressure is applied—may be present. Fever and chills usually are absent unless the infection is invasive or systemic (Photo). Serious forms of infection—such as impetigo and necrotizing fasciitis—are less common, although the latter has been reported more frequently among IV drug users.1
© 2001-2007 DermAtlas
Warm, swollen, erythematous skin with red papules and plaques with central pustules often on the extremities. Treatment. Although the prognosis for most CA-MRSA skin and soft tissue infections is favorable, serious and potentially life-threatening complications can emerge.1 Most infections can be treated successfully with antibiotics and—when an abscess is present—incision and drainage performed in a primary care physician’s office. Trimethoprim-sulfamethoxazole—a commonly used antibiotic—can decrease serum levels of tricyclic antidepressants and prolong the QT interval. Be aware of this interaction in patients receiving antipsychotics, which also can prolong the QT interval.
- A single boil, abscess, or small, red, painful lesion suggests a community-acquired, methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infection.
- Be aware of the clinical presentation of CA-MRSA infections to facilitate necessary referrals to a primary care physician or ER.
- Educate your patients at risk for CA-MRSA skin infections to protect themselves and avoid transmitting infection to others.
Referral to a primary care physician for further management is appropriate for afebrile patients without a history of immunosuppression who present with localized rash involving 1 extremity. Severe infection with bacteremia or other systemic involvement is possible, especially in patients age ≥65.5 Consider ER referral for patients with:
- compromised immune systems
- high fever and/or chills
- rapidly progressing symptoms
- signs and symptoms consistent with systemic illness, such as shortness of breath or low blood pressure
- disease involving >1 extremity or multiple abscesses.
Related resources
- Centers for Disease Control and Prevention. Overview of community-associated MRSA. www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html.
- Zeller JL, Burke AE, Glass RM. JAMA patient page. MRSA infections. JAMA 2007;298(15):1826. Available at: http://jama.ama-assn.org/cgi/content/full/298/15/1826.
Drug brand name
- Trimethoprim-sulfamethoxazole • Bactrim, Septra
Disclosures
Dr. Hebert reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rado receives grant/research support from Neuronetics, Eli Lilly and Company, and Janssen Pharmaceutica.
Some patients at high risk for mental illness—intravenous drug users, prisoners, human immunodeficiency virus-positive patients, and the homeless—also are at risk of community-acquired, methicillin-resistant Staphylococcus aureus (CA-MRSA) infections.1 Because your patients may present with CA-MRSA symptoms, you need a basic understanding of this infection’s risk factors and clinical features to initiate necessary referrals (Table).2
Table
Features of community-acquired MRSA infections
| At-risk populations | HIV infection, IV drug users, homeless, men who have sex with men, tattoo recipients, individuals living in close quarters such as group homes or prisons May affect healthy individuals without risk factors |
| Clinical presentation | Small, hard, red, painful lesions that resemble a spider bite Most common: skin infections such as a boil, abscess, or cellulitis Less common: bone and joint infections, pneumonia |
| Transmission | Skin-to-skin contact with infected persons Sharing personal hygiene items, such as towels, with infected persons Skin breaks |
| Symptoms requiring emergent referral | Fever, shortness of breath, hypotension, or other systemic symptoms Rapidly spreading lesion |
| Source: Reference 2 | |
Risk factors and transmission
CA-MRSA accounts for 78% of skin and soft tissue infections in emergency rooms.3 Patients typically have no known risk factors for infection or health-related exposures, such as recent hospitalization or employment in a healthcare setting. Persons who have taken antibiotics in the past 12 months are at increased risk.1,3,4
Infection spreads by person-to-person contact. In the community, crowding and sharing personal items also facilitate transmission, which accounts for increased risk among military personnel and athletes in contact sports.1 Therefore, caution psychiatric patients against sharing personal hygiene items, such as towels, and instruct infected patients to keep abscess sites covered at all times. Stress the importance of consistent handwashing.
Infection also may be acquired through a skin abrasion, although many infected patients do not remember having local skin trauma.
Clinical presentation. Unlike diffuse drug eruptions associated with psychotropic hypersensitivity reactions, skin involvement caused by CA-MRSA typically is limited. Patients generally present with a warm, swollen, and erythematous area of skin or a circumscribed abscess involving a hair follicle.1 Often patients attribute symptoms to a recent spider bite or report that a family member or friend has a similar rash or lesion.3
Single lesions on the extremities are common, although multiple “boils” are possible. Fluctuance—a wavelike motion beneath the lesion when pressure is applied—may be present. Fever and chills usually are absent unless the infection is invasive or systemic (Photo). Serious forms of infection—such as impetigo and necrotizing fasciitis—are less common, although the latter has been reported more frequently among IV drug users.1
© 2001-2007 DermAtlas
Warm, swollen, erythematous skin with red papules and plaques with central pustules often on the extremities. Treatment. Although the prognosis for most CA-MRSA skin and soft tissue infections is favorable, serious and potentially life-threatening complications can emerge.1 Most infections can be treated successfully with antibiotics and—when an abscess is present—incision and drainage performed in a primary care physician’s office. Trimethoprim-sulfamethoxazole—a commonly used antibiotic—can decrease serum levels of tricyclic antidepressants and prolong the QT interval. Be aware of this interaction in patients receiving antipsychotics, which also can prolong the QT interval.
- A single boil, abscess, or small, red, painful lesion suggests a community-acquired, methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infection.
- Be aware of the clinical presentation of CA-MRSA infections to facilitate necessary referrals to a primary care physician or ER.
- Educate your patients at risk for CA-MRSA skin infections to protect themselves and avoid transmitting infection to others.
Referral to a primary care physician for further management is appropriate for afebrile patients without a history of immunosuppression who present with localized rash involving 1 extremity. Severe infection with bacteremia or other systemic involvement is possible, especially in patients age ≥65.5 Consider ER referral for patients with:
- compromised immune systems
- high fever and/or chills
- rapidly progressing symptoms
- signs and symptoms consistent with systemic illness, such as shortness of breath or low blood pressure
- disease involving >1 extremity or multiple abscesses.
Related resources
- Centers for Disease Control and Prevention. Overview of community-associated MRSA. www.cdc.gov/ncidod/dhqp/ar_mrsa_ca.html.
- Zeller JL, Burke AE, Glass RM. JAMA patient page. MRSA infections. JAMA 2007;298(15):1826. Available at: http://jama.ama-assn.org/cgi/content/full/298/15/1826.
Drug brand name
- Trimethoprim-sulfamethoxazole • Bactrim, Septra
Disclosures
Dr. Hebert reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rado receives grant/research support from Neuronetics, Eli Lilly and Company, and Janssen Pharmaceutica.
1. Stryjewski ME, Chambers HF. Skin and soft tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46:S368-77.
2. Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46:S344-9.
3. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-74.
4. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468-75.
5. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007;298(15):1763-7
1. Stryjewski ME, Chambers HF. Skin and soft tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46:S368-77.
2. Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008;46:S344-9.
3. Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-74.
4. Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med 2005;352:468-75.
5. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007;298(15):1763-7
Self-deception and changing roles
Another way of thinking about self-deception as noted by Dr. Henry Nasrallah in his editorial “Self-deception: A double-edged trait,” (From the Editor, Current Psychiatry, July 2008) relates to the psychology of personal constructs. Dr. George Kelly, a psychologist at Ohio State University, stated that each of us has several selves—child to our parents, parent to our children, mate, friend, citizen, professional, etc.—and it is important to keep it all straight and be the appropriate self in various settings. In Behavior in Public Places, sociologist Dr. Erving Goffman noted that nobody changes behavior more rapidly than a waiter—out in the dining room obsequiously fawning over the diner, and then in a few steps through the doors in the kitchen fighting for his food. Goffman asks, which is the real waiter?
It makes sense that there is not one self to deceive but many, and to be a highly functional person one must keep the cast of characters straight.
David C. Tinling, MD
Kingsboro Psychiatric Center
Brooklyn, NY
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
Another way of thinking about self-deception as noted by Dr. Henry Nasrallah in his editorial “Self-deception: A double-edged trait,” (From the Editor, Current Psychiatry, July 2008) relates to the psychology of personal constructs. Dr. George Kelly, a psychologist at Ohio State University, stated that each of us has several selves—child to our parents, parent to our children, mate, friend, citizen, professional, etc.—and it is important to keep it all straight and be the appropriate self in various settings. In Behavior in Public Places, sociologist Dr. Erving Goffman noted that nobody changes behavior more rapidly than a waiter—out in the dining room obsequiously fawning over the diner, and then in a few steps through the doors in the kitchen fighting for his food. Goffman asks, which is the real waiter?
It makes sense that there is not one self to deceive but many, and to be a highly functional person one must keep the cast of characters straight.
David C. Tinling, MD
Kingsboro Psychiatric Center
Brooklyn, NY
Another way of thinking about self-deception as noted by Dr. Henry Nasrallah in his editorial “Self-deception: A double-edged trait,” (From the Editor, Current Psychiatry, July 2008) relates to the psychology of personal constructs. Dr. George Kelly, a psychologist at Ohio State University, stated that each of us has several selves—child to our parents, parent to our children, mate, friend, citizen, professional, etc.—and it is important to keep it all straight and be the appropriate self in various settings. In Behavior in Public Places, sociologist Dr. Erving Goffman noted that nobody changes behavior more rapidly than a waiter—out in the dining room obsequiously fawning over the diner, and then in a few steps through the doors in the kitchen fighting for his food. Goffman asks, which is the real waiter?
It makes sense that there is not one self to deceive but many, and to be a highly functional person one must keep the cast of characters straight.
David C. Tinling, MD
Kingsboro Psychiatric Center
Brooklyn, NY
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
Is chlorpromazine safe?
I am concerned about a recommended treatment for serotonin syndrome in “Did Internet-purchased diet pills cause serotonin syndrome?” (Current Psychiatry, July 2008) by Drs. Kyoung Bin Im and Jess G. Fiedorowicz. The authors suggest administering the antipsychotic chlorpromazine and cite a case by Gillman1 supporting this indication.
This seems like a particularly risky option because of the overlap in symptomatology between neuroleptic malignant syndrome (NMS) and serotonin syndrome as described by the authors and the fact that administering an additional antipsychotic to a patient with NMS could be fatal. Further, most reports indicate that serotonin syndrome typically is self-limited and best treated with supportive measures and withdrawal of 5-HT active compounds.
Mark Beale, MD
Charleston Psychiatric Associates
Charleston, SC
1. Gillman PK. Serotonin syndrome treated with chlorpromazine. J Clin Psychopharmacol 1997;17(2):128-9.
Drs. Fiedorowicz and Im respond
In 1997, Gillman reported a case of a woman with serotonin syndrome whose condition did not improve with supportive management, cyproheptadine, and propranolol. The patient improved 2 hours after receiving intramuscular chlorpromazine, 50 mg. Chlorpromazine was selected because of its antagonism at both 5-HT1A and 5-HT2A receptors, nearly equipotent to cyproheptadine.1 In 1999 Gillman reviewed case reports for the treatment of serotonin syndrome with chlorpromazine vs cyproheptadine and concluded these 5-HT2 antagonists may be required as a lifesaving measure.2 Since then chlorpromazine has been suggested as part of serotonin syndrome treatment.3,4 High doses of chlorpromazine and cyproheptadine have been shown to reduce death in animal models of serotonin syndrome, an effect mediated by 5-HT2A antagonism.5
We briefly mentioned chlorpromazine as a medical management option for serotonin syndrome, though we did not recommend it in the case presented. We stated that antipsychotics are contraindicated in NMS and in Table 2 (p. 77) illustrated a common treatment strategy that included avoiding antipsychotics.
We share the writer’s concern and hope to reinforce this point. When diagnosis of NMS or serotonin syndrome is unclear, it is advisable to avoid antipsychotics such as chlorpromazine or serotonergic medications such as bromocriptine.
Jess G. Fiedorowicz, MD
Associate in psychiatry
Kyoung Bin Im, MD
Chief resident
Departments of internal medicine and psychiatry
Roy J. and Lucille A. Carver College of Medicine
University of Iowa
Iowa City
1. Gillman PK. Serotonin syndrome treated with chlorpromazine. J Clin Psychopharmacol 1997;17(2):128-9.
2. Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999;13(1):100-9.
3. Ener RA, Meglathery SB, Van Decker WA, Gallagher RM. Serotonin syndrome and other serotonergic disorders. Pain Med 2003;4(1):63-74.
4. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005;352(11):1112-20.
5. Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001;890(1):23-31.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
I am concerned about a recommended treatment for serotonin syndrome in “Did Internet-purchased diet pills cause serotonin syndrome?” (Current Psychiatry, July 2008) by Drs. Kyoung Bin Im and Jess G. Fiedorowicz. The authors suggest administering the antipsychotic chlorpromazine and cite a case by Gillman1 supporting this indication.
This seems like a particularly risky option because of the overlap in symptomatology between neuroleptic malignant syndrome (NMS) and serotonin syndrome as described by the authors and the fact that administering an additional antipsychotic to a patient with NMS could be fatal. Further, most reports indicate that serotonin syndrome typically is self-limited and best treated with supportive measures and withdrawal of 5-HT active compounds.
Mark Beale, MD
Charleston Psychiatric Associates
Charleston, SC
1. Gillman PK. Serotonin syndrome treated with chlorpromazine. J Clin Psychopharmacol 1997;17(2):128-9.
Drs. Fiedorowicz and Im respond
In 1997, Gillman reported a case of a woman with serotonin syndrome whose condition did not improve with supportive management, cyproheptadine, and propranolol. The patient improved 2 hours after receiving intramuscular chlorpromazine, 50 mg. Chlorpromazine was selected because of its antagonism at both 5-HT1A and 5-HT2A receptors, nearly equipotent to cyproheptadine.1 In 1999 Gillman reviewed case reports for the treatment of serotonin syndrome with chlorpromazine vs cyproheptadine and concluded these 5-HT2 antagonists may be required as a lifesaving measure.2 Since then chlorpromazine has been suggested as part of serotonin syndrome treatment.3,4 High doses of chlorpromazine and cyproheptadine have been shown to reduce death in animal models of serotonin syndrome, an effect mediated by 5-HT2A antagonism.5
We briefly mentioned chlorpromazine as a medical management option for serotonin syndrome, though we did not recommend it in the case presented. We stated that antipsychotics are contraindicated in NMS and in Table 2 (p. 77) illustrated a common treatment strategy that included avoiding antipsychotics.
We share the writer’s concern and hope to reinforce this point. When diagnosis of NMS or serotonin syndrome is unclear, it is advisable to avoid antipsychotics such as chlorpromazine or serotonergic medications such as bromocriptine.
Jess G. Fiedorowicz, MD
Associate in psychiatry
Kyoung Bin Im, MD
Chief resident
Departments of internal medicine and psychiatry
Roy J. and Lucille A. Carver College of Medicine
University of Iowa
Iowa City
I am concerned about a recommended treatment for serotonin syndrome in “Did Internet-purchased diet pills cause serotonin syndrome?” (Current Psychiatry, July 2008) by Drs. Kyoung Bin Im and Jess G. Fiedorowicz. The authors suggest administering the antipsychotic chlorpromazine and cite a case by Gillman1 supporting this indication.
This seems like a particularly risky option because of the overlap in symptomatology between neuroleptic malignant syndrome (NMS) and serotonin syndrome as described by the authors and the fact that administering an additional antipsychotic to a patient with NMS could be fatal. Further, most reports indicate that serotonin syndrome typically is self-limited and best treated with supportive measures and withdrawal of 5-HT active compounds.
Mark Beale, MD
Charleston Psychiatric Associates
Charleston, SC
1. Gillman PK. Serotonin syndrome treated with chlorpromazine. J Clin Psychopharmacol 1997;17(2):128-9.
Drs. Fiedorowicz and Im respond
In 1997, Gillman reported a case of a woman with serotonin syndrome whose condition did not improve with supportive management, cyproheptadine, and propranolol. The patient improved 2 hours after receiving intramuscular chlorpromazine, 50 mg. Chlorpromazine was selected because of its antagonism at both 5-HT1A and 5-HT2A receptors, nearly equipotent to cyproheptadine.1 In 1999 Gillman reviewed case reports for the treatment of serotonin syndrome with chlorpromazine vs cyproheptadine and concluded these 5-HT2 antagonists may be required as a lifesaving measure.2 Since then chlorpromazine has been suggested as part of serotonin syndrome treatment.3,4 High doses of chlorpromazine and cyproheptadine have been shown to reduce death in animal models of serotonin syndrome, an effect mediated by 5-HT2A antagonism.5
We briefly mentioned chlorpromazine as a medical management option for serotonin syndrome, though we did not recommend it in the case presented. We stated that antipsychotics are contraindicated in NMS and in Table 2 (p. 77) illustrated a common treatment strategy that included avoiding antipsychotics.
We share the writer’s concern and hope to reinforce this point. When diagnosis of NMS or serotonin syndrome is unclear, it is advisable to avoid antipsychotics such as chlorpromazine or serotonergic medications such as bromocriptine.
Jess G. Fiedorowicz, MD
Associate in psychiatry
Kyoung Bin Im, MD
Chief resident
Departments of internal medicine and psychiatry
Roy J. and Lucille A. Carver College of Medicine
University of Iowa
Iowa City
1. Gillman PK. Serotonin syndrome treated with chlorpromazine. J Clin Psychopharmacol 1997;17(2):128-9.
2. Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999;13(1):100-9.
3. Ener RA, Meglathery SB, Van Decker WA, Gallagher RM. Serotonin syndrome and other serotonergic disorders. Pain Med 2003;4(1):63-74.
4. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005;352(11):1112-20.
5. Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001;890(1):23-31.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
1. Gillman PK. Serotonin syndrome treated with chlorpromazine. J Clin Psychopharmacol 1997;17(2):128-9.
2. Gillman PK. The serotonin syndrome and its treatment. J Psychopharmacol 1999;13(1):100-9.
3. Ener RA, Meglathery SB, Van Decker WA, Gallagher RM. Serotonin syndrome and other serotonergic disorders. Pain Med 2003;4(1):63-74.
4. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005;352(11):1112-20.
5. Nisijima K, Yoshino T, Yui K, Katoh S. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001;890(1):23-31.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
CP + AACP > The sum of its parts
Current Psychiatry has built its reputation as the clinical journal that provides psychiatrists with peer-reviewed, practical advice by leading authorities, emphasizing up-to-date solutions to common clinical problems. As Editor-in-Chief, I’m pleased to announce that Current Psychiatry is expanding its mission to you and your patients through an agreement with the American Academy of Clinical Psychiatrists (AACP).
Quadrant HealthCom Inc., publisher of Current Psychiatry, will collaborate with the AACP by:
- publishing its official journal, the Annals of Clinical Psychiatry
- managing and co-sponsoring its annual conference, beginning in 2009
- developing and managing www.aacp.com, the official Web site of the AACP and the Annals.
This agreement provides excellent synergy. As a past president of the AACP and a founding editorial board member of the Annals, I believe it offers exciting and important benefits for psychiatry professionals.
Emphasis: Clinical psychiatry
The AACP is a unique professional society that encourages the exchange of information between clinicians interested in evidence-based practice and academicians involved in clinical research. It is the type of psychiatric association that Current Psychiatry readers should seriously consider joining. Its founder, the late George Winokur, MD, played a leading role in developing the first diagnostic criteria for use in psychiatric research—a prototype for the Diagnostic and Statistical Manual of Mental Disorders.1 A cherished mentor, Dr. Winokur trained a generation of outstanding psychiatrists during 24 years as professor and head of the University of Iowa’s department of psychiatry. He was my role model when I was on the faculty at Iowa in the 1980s.
Donald W. Black, MD, professor of psychiatry at the University of Iowa College of Medicine, has served as Editor-in-Chief of the Annals since 2004 and will continue in this role. Dr. Black also trained with Dr. Winokur and is recognized as an outstanding educator, researcher, and clinician.
The AACP launched the Annals in 1989 to provide clinical psychiatrists with up-to-date information on the symptoms, diagnosis, and treatment of mental disorders. The quarterly journal, which emphasizes the results of controlled clinical studies, is abstracted/indexed in Index Medicus, Excerpta Medica, Current Contents, EMBASE, PsychINFO, and other databases.
The AACP will continue to select and appoint the Annals’ Editor-in-Chief, set the journal’s editorial mission, recruit the editorial board, invite authors, and implement peer review. Quadrant HealthCom Inc. will be responsible for production, mailing, and other publishing duties. Continuing medical education (CME) supplements to the Annals will be distributed to Current Psychiatry’s circulation as well.
The AACP’s annual meetings provide educational updates that Current Psychiatry readers will find useful and relevant to clinical practice. “Bipolar disorder and ADHD: Solving clinical challenges, improving patient care” is the theme of the April 2009 conference, which will be held in Chicago. An outstanding faculty is onboard for this CME opportunity, which is open to AACP members and nonmembers alike.
Quadrant HealthCom Inc. will develop and relaunch the AACP Web site. Its content will be expanded to include materials from presentations at AACP annual meetings and industry-sponsored programs.
Benefits for all
I am very enthusiastic about this agreement. Current Psychiatry—a clinical journal that reaches more than 39,000 psychiatrists and psychiatric nurse practitioners—is joining forces with the Annals of Clinical Psychiatry, a well-established scientific journal that focuses on the latest clinical research. As a result:
- Readers of both journals will benefit from expanded CME opportunities as funding becomes available for industry-sponsored programs and supplements.
- Members of the AACP gain Quadrant HealthCom Inc.’s publishing expertise for their respected scientific publication and meeting planning experience to grow their annual meeting.
- The AACP and Current Psychiatry Web sites will complement each other in serving the needs of clinical practitioners with expanded content and educational offerings.
I predict that a vibrant community of clinical psychiatrists will emerge from this collaboration and energize psychiatric education, both online and in print.
1. Tsuang MT. Images in psychiatry: George Winokur, MD. 1925-1996. Am J Psychiatry 1999;156:465-6.
Current Psychiatry has built its reputation as the clinical journal that provides psychiatrists with peer-reviewed, practical advice by leading authorities, emphasizing up-to-date solutions to common clinical problems. As Editor-in-Chief, I’m pleased to announce that Current Psychiatry is expanding its mission to you and your patients through an agreement with the American Academy of Clinical Psychiatrists (AACP).
Quadrant HealthCom Inc., publisher of Current Psychiatry, will collaborate with the AACP by:
- publishing its official journal, the Annals of Clinical Psychiatry
- managing and co-sponsoring its annual conference, beginning in 2009
- developing and managing www.aacp.com, the official Web site of the AACP and the Annals.
This agreement provides excellent synergy. As a past president of the AACP and a founding editorial board member of the Annals, I believe it offers exciting and important benefits for psychiatry professionals.
Emphasis: Clinical psychiatry
The AACP is a unique professional society that encourages the exchange of information between clinicians interested in evidence-based practice and academicians involved in clinical research. It is the type of psychiatric association that Current Psychiatry readers should seriously consider joining. Its founder, the late George Winokur, MD, played a leading role in developing the first diagnostic criteria for use in psychiatric research—a prototype for the Diagnostic and Statistical Manual of Mental Disorders.1 A cherished mentor, Dr. Winokur trained a generation of outstanding psychiatrists during 24 years as professor and head of the University of Iowa’s department of psychiatry. He was my role model when I was on the faculty at Iowa in the 1980s.
Donald W. Black, MD, professor of psychiatry at the University of Iowa College of Medicine, has served as Editor-in-Chief of the Annals since 2004 and will continue in this role. Dr. Black also trained with Dr. Winokur and is recognized as an outstanding educator, researcher, and clinician.
The AACP launched the Annals in 1989 to provide clinical psychiatrists with up-to-date information on the symptoms, diagnosis, and treatment of mental disorders. The quarterly journal, which emphasizes the results of controlled clinical studies, is abstracted/indexed in Index Medicus, Excerpta Medica, Current Contents, EMBASE, PsychINFO, and other databases.
The AACP will continue to select and appoint the Annals’ Editor-in-Chief, set the journal’s editorial mission, recruit the editorial board, invite authors, and implement peer review. Quadrant HealthCom Inc. will be responsible for production, mailing, and other publishing duties. Continuing medical education (CME) supplements to the Annals will be distributed to Current Psychiatry’s circulation as well.
The AACP’s annual meetings provide educational updates that Current Psychiatry readers will find useful and relevant to clinical practice. “Bipolar disorder and ADHD: Solving clinical challenges, improving patient care” is the theme of the April 2009 conference, which will be held in Chicago. An outstanding faculty is onboard for this CME opportunity, which is open to AACP members and nonmembers alike.
Quadrant HealthCom Inc. will develop and relaunch the AACP Web site. Its content will be expanded to include materials from presentations at AACP annual meetings and industry-sponsored programs.
Benefits for all
I am very enthusiastic about this agreement. Current Psychiatry—a clinical journal that reaches more than 39,000 psychiatrists and psychiatric nurse practitioners—is joining forces with the Annals of Clinical Psychiatry, a well-established scientific journal that focuses on the latest clinical research. As a result:
- Readers of both journals will benefit from expanded CME opportunities as funding becomes available for industry-sponsored programs and supplements.
- Members of the AACP gain Quadrant HealthCom Inc.’s publishing expertise for their respected scientific publication and meeting planning experience to grow their annual meeting.
- The AACP and Current Psychiatry Web sites will complement each other in serving the needs of clinical practitioners with expanded content and educational offerings.
I predict that a vibrant community of clinical psychiatrists will emerge from this collaboration and energize psychiatric education, both online and in print.
Current Psychiatry has built its reputation as the clinical journal that provides psychiatrists with peer-reviewed, practical advice by leading authorities, emphasizing up-to-date solutions to common clinical problems. As Editor-in-Chief, I’m pleased to announce that Current Psychiatry is expanding its mission to you and your patients through an agreement with the American Academy of Clinical Psychiatrists (AACP).
Quadrant HealthCom Inc., publisher of Current Psychiatry, will collaborate with the AACP by:
- publishing its official journal, the Annals of Clinical Psychiatry
- managing and co-sponsoring its annual conference, beginning in 2009
- developing and managing www.aacp.com, the official Web site of the AACP and the Annals.
This agreement provides excellent synergy. As a past president of the AACP and a founding editorial board member of the Annals, I believe it offers exciting and important benefits for psychiatry professionals.
Emphasis: Clinical psychiatry
The AACP is a unique professional society that encourages the exchange of information between clinicians interested in evidence-based practice and academicians involved in clinical research. It is the type of psychiatric association that Current Psychiatry readers should seriously consider joining. Its founder, the late George Winokur, MD, played a leading role in developing the first diagnostic criteria for use in psychiatric research—a prototype for the Diagnostic and Statistical Manual of Mental Disorders.1 A cherished mentor, Dr. Winokur trained a generation of outstanding psychiatrists during 24 years as professor and head of the University of Iowa’s department of psychiatry. He was my role model when I was on the faculty at Iowa in the 1980s.
Donald W. Black, MD, professor of psychiatry at the University of Iowa College of Medicine, has served as Editor-in-Chief of the Annals since 2004 and will continue in this role. Dr. Black also trained with Dr. Winokur and is recognized as an outstanding educator, researcher, and clinician.
The AACP launched the Annals in 1989 to provide clinical psychiatrists with up-to-date information on the symptoms, diagnosis, and treatment of mental disorders. The quarterly journal, which emphasizes the results of controlled clinical studies, is abstracted/indexed in Index Medicus, Excerpta Medica, Current Contents, EMBASE, PsychINFO, and other databases.
The AACP will continue to select and appoint the Annals’ Editor-in-Chief, set the journal’s editorial mission, recruit the editorial board, invite authors, and implement peer review. Quadrant HealthCom Inc. will be responsible for production, mailing, and other publishing duties. Continuing medical education (CME) supplements to the Annals will be distributed to Current Psychiatry’s circulation as well.
The AACP’s annual meetings provide educational updates that Current Psychiatry readers will find useful and relevant to clinical practice. “Bipolar disorder and ADHD: Solving clinical challenges, improving patient care” is the theme of the April 2009 conference, which will be held in Chicago. An outstanding faculty is onboard for this CME opportunity, which is open to AACP members and nonmembers alike.
Quadrant HealthCom Inc. will develop and relaunch the AACP Web site. Its content will be expanded to include materials from presentations at AACP annual meetings and industry-sponsored programs.
Benefits for all
I am very enthusiastic about this agreement. Current Psychiatry—a clinical journal that reaches more than 39,000 psychiatrists and psychiatric nurse practitioners—is joining forces with the Annals of Clinical Psychiatry, a well-established scientific journal that focuses on the latest clinical research. As a result:
- Readers of both journals will benefit from expanded CME opportunities as funding becomes available for industry-sponsored programs and supplements.
- Members of the AACP gain Quadrant HealthCom Inc.’s publishing expertise for their respected scientific publication and meeting planning experience to grow their annual meeting.
- The AACP and Current Psychiatry Web sites will complement each other in serving the needs of clinical practitioners with expanded content and educational offerings.
I predict that a vibrant community of clinical psychiatrists will emerge from this collaboration and energize psychiatric education, both online and in print.
1. Tsuang MT. Images in psychiatry: George Winokur, MD. 1925-1996. Am J Psychiatry 1999;156:465-6.
1. Tsuang MT. Images in psychiatry: George Winokur, MD. 1925-1996. Am J Psychiatry 1999;156:465-6.
The puzzling self-poisoner
CASE: Unusual suicide attempt
After a friend calls 911, Ms. M, age 20, is brought to an emergency room (ER) complaining of severe leg and abdominal pain. The ER physician finds she is bleeding from her vagina and nose and has severe ecchymosis anemia. After Ms. M is admitted, clinicians discover these conditions are secondary to a suicide attempt—she ingested 15 to 16 pellets of rat poison daily for approximately 2 months.
While hospitalized, Ms. M is treated with several transfusions of fresh frozen plasma, packed red blood cells, and phytonadione (vitamin K). A consultation-liaison psychiatrist diagnoses bipolar disorder and starts Ms. M on lamotrigine, 25 mg once daily. (The justification for this diagnosis was not documented.) After physicians judge her to be medically stable, Ms. M is involuntarily committed to a short-term psychiatric care facility. Her vital signs and coagulation values are stable.
At the psychiatric facility, our team determines that her symptoms and history are consistent with major depressive disorder, recurrent. For 5 months, Ms. M had depressed mood for most of the day, diminished interest in activities, and feelings of worthlessness. She also experienced weight loss—10 lbs in 2 months—with decreased appetite and low energy for most of the day. She denies past symptoms of mania or psychosis. She says she does not know why she was diagnosed with bipolar disorder. She admits to multiple previous suicide attempts via hanging and ingesting cleaning fluid or rat poison.
We place Ms. M on suicide precautions and prescribe escitalopram, 10 mg/d, in addition to lamotrigine, 50 mg once daily. We continue lamotrigine despite a lack of documentation for Ms. M’s bipolar diagnosis because evidence suggests the drug may be an effective augmentation to antidepressants in patients with treatment-resistant depression.1
The author’s observations
Any patient transferred from a medical floor to a psychiatric inpatient unit should have documentation that clarifies any need for further medical treatment. Ms. M’s physicians told us that she was medically stable and should require little if any further treatment for her ingestion of rat poison.
TREATMENT: Coagulation concerns
We request a medical consult to monitor possible complications from the rat poison. The physician advises that rat poison essentially is the same as the anticoagulant warfarin and its effects should steadily decrease over time because its half-life is 20 to 60 hours. However, for safety reasons, we closely follow Ms. M’s coagulation values and order daily vitamin K injections, 5 mg SC.
Further medical investigation shows no evidence of complications, but Ms. M continues to request medication for pain in her left leg. The physician prescribes acetaminophen, 650 mg every 6 hours as needed for pain, which the patient takes at almost every opportunity, often 4 times a day. The physician does not choose a nonsteroidal anti-inflammatory drug (NSAID) for pain to avoid the possibility of gastrointestinal (GI) irritation that could lead to bleeding.
In the psychiatric facility, the patient’s international normalized ratio (INR) is found to be rising, indicating a lack of clotting and a risk of uncontrolled bleeding. The physician states that given the half-life of warfarin, Ms. M’s INR should be decreasing. Liver function testing does not show that liver dysfunction is contributing to the increasing INR.
Because we assume the vitamin K the patient received has been absorbed, we hypothesize that Ms. M has continued to surreptitiously ingest rat poison or another anticoagulant, which she denies. We search Ms. M and her room. She is placed on 1-to-1 observation 24 hours a day. Ms. M’s visitors also are searched, and visits are observed. We find no evidence of an anticoagulant agent.
Ms. M’s INR continues to rise. We search the facility to rule out the possibility that the patient had hidden a supply of anticoagulant outside her room. The search finds nothing. At this point we consider performing an abdominal x-ray to rule out the possibility that Ms. M may have a supply of medication hidden in her gastrointestinal tract.
The author’s observations
Patients hiding and using contraband is a common problem in involuntary inpatient units. It seemed that Ms. M was secretly ingesting rat poison. Her history showed she was determined to end her life, and she ingested rat poison daily for months. However, because an exhaustive search for contraband and 1-to-1 observation yielded no positive results, the evidence did not support this theory. Some team members thought we were not searching hard enough. I decided we needed to pursue other theories.
I was skeptical that escitalopram could be contributing to Ms. M’s rising anticoagulation values. Selective serotonin reuptake inhibitors have antiplatelet effects, but platelet function does not affect INR to the degree we were observing.
‘Superwarfarins’
Physicians had advised us that Ms. M’s INR should decrease under the assumption that rat poison is for all practical purposes the same as warfarin, but we had not investigated distinctions between the 2 compounds. A literature search revealed that several rat poisons are not simply warfarin repackaged as a pesticide. Most are “superwarfarins”—chemicals similar to warfarin but more potent and with a much longer half-life.2 Case report data suggest the plasma half-life of these chemicals is 20 to 62 days.3
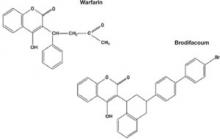
Most commercial rat poisons are made of brodifacoum, which has a chemical structure similar to warfarin but with an additional long polycyclic hydrocarbon side chain (Figure 1). The potency of brodifacoum compared with warfarin is approximately 100 to 1.4-6 The chemical is highly lipophilic and can stay in the body for an extended period.4-6 Lab tests can measure serum brodifacoum levels.3
After Ms. M identifies the brand name of the rat poison she ingested, we contact the American Association of Poison Control Centers and verify the agent she used was brodifacoum. This explains why her INR was not decreasing—but does not explain the increase.
A drug interaction? Because Ms. M’s liver function is within normal limits, the next theory to investigate is if brodifacoum is interacting with any medications she is taking. I could not find any medical journal articles, programs, or Web sites describing brodifacoum’s interactions with medications. After all, brodifacoum is a pesticide, not a medication.
I considered that because brodifacoum and warfarin have a similar structure and function, they may interact with medications in a similar manner. After another literature search, only acetaminophen had evidence of interaction with warfarin that could explain her rising INR.
Documentation of interactions between warfarin and acetaminophen are sparse. In one case, a 74-year-old man receiving warfarin for atrial fibrillation experienced an abrupt increase in INR after taking acetaminophen.7 In a double-blind, placebo controlled, randomized trial of patients taking warfarin, INR rose rapidly after the start of acetaminophen and was significantly increased within 1 week compared with patients receiving placebo.8
Figure 1 Chemical structures of warfarin and rat poison
Most commercial rat poisons are made of brodifacoum, which is chemically similar to warfarin but has an additional long polycyclic hydrocarbon side chain.
FOLLOW-UP: Analgesic substitution
We suggest to the physician that Ms. M’s INR may be increasing because of an interaction between brodifacoum and acetaminophen, which she took several times a day. On day 8 of Ms. M’s hospitalization, the physician discontinues acetaminophen and prescribes ibuprofen, 400 mg tid as needed for pain, and pantoprazole, 40 mg/d, to prevent GI bleeding from possible irritation caused by ibuprofen. The team continues to monitor Ms. M’s coagulation values.
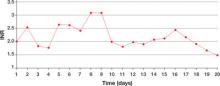
Within a day of discontinuing acetaminophen, Ms. M’s INR decreased as expected (Figure 2). The rest of her medication regimen is continued, and her INR levels continued to decrease.
One-to-one observation is discontinued. However, because of the patient’s continued determination to end her life and no significant improvement in her depression, Ms. M is considered a danger to herself and involuntary inpatient hospitalization is continued.
Figure 2 Ms. M’s INR values during hospitalization
The patient’s INR values began to rise mysteriously after she was transferred to the inpatient psychiatric unit. Acetaminophen was discontinued on day 8, and within a day her INR values began to drop.
INR: International normalized ratio
The author’s observations
Poisoning is a common method of attempting suicide, patients may use substances that clinicians rarely encounter. For most toxic, nonmedication substances, data on interactions with medications are sparse. if you suspect your patient has ingested a toxic substance with which the treatment team has little experience, contact the American Association of Poison Control Centers at 800-222-1222.
Suspect superwarfarin poisoning in suicidal patients with coagulopathy, prolonged prothrombin time, and elevated INR that does not respond to large amounts of vitamin K.9,10 These patients are at high risk of successfully completing suicide because of superwarfarins’ long half-life and daily maintenance required to keep coagulation levels within a safe range for at least several weeks.
The most serious complication these patients face is intracranial hemorrhage, which occurs in 2% of patients with excessive warfarin-based coagulation and is associated with a 77% mortality rate.11 GI bleeding occurs in 67% of these patients.2
Also take into account medical conditions—such as hypertension or hepatic disease—and medication side effects that can increase bleeding risk. When treating pain in these patients, consider avoiding acetaminophen but be aware of the risks of NSAIDs, such as gastritis or GI bleeding.
Related resource
- The American Association of Poison Control Centers. 800-222-1222; www.aapcc.org.
Drug brand names
- Escitalopram • Lexapro
- Lamotrigine • Lamictal
- Pantoprazole • Protonix
- Warfarin • Coumadin
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sharma V, Khan M, Corpse C. Role of lamotrigine in the management of treatment-resistant bipolar II depression: a chart review. J Affect Disord Epub 2008 Mar 1.
2. Su M, Hoffman R. Anticoagulants. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s toxicologic emergencies. 8th ed. New York, NY: McGraw-Hill Medical Publishing; 2006:891-4.
3. Chua JD, Friedenberg WR. Superwarfarin poisoning. Anaesth Intensive Care 1997;25:707-9.
4. Leck JB, Park BK. A comparative study of the effect of warfarin and brodifacoum on the relationship between vitamin K1 metabolism and clotting factor activity in warfarin susceptible and warfarin resistant rats. Biochem Pharmacol 1981;30:123-9.
5. Lund M. Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg 1981;87:101-7.
6. Park BK, Scott AK, Wilson AC, et al. Plasma disposition of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol 1982;31:3535-639.
7. Gebauer MG, Nyfort-Hansen K, Henschke PJ, Gallus AS. Warfarin and acetaminophen interaction. Pharmacotherapy 2003;23(1):109-12.
8. Mahe I, Bertrand N, Drouet L, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica 2006;91(12):1621-7.
9. Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet 2005;365:552-3.
10. Scully M. Warfarin therapy: rat poison and the prevention of thrombosis. Biochemist 2002;24:15-7.
11. Mathiesen T, Benediktsdottir K, Johnsson H, Lindqvist M. Intracranial traumatic and nontraumatic haemorrhagic complications of warfarin treatment. Acta Neurol Scan 1995;91:208-14.
CASE: Unusual suicide attempt
After a friend calls 911, Ms. M, age 20, is brought to an emergency room (ER) complaining of severe leg and abdominal pain. The ER physician finds she is bleeding from her vagina and nose and has severe ecchymosis anemia. After Ms. M is admitted, clinicians discover these conditions are secondary to a suicide attempt—she ingested 15 to 16 pellets of rat poison daily for approximately 2 months.
While hospitalized, Ms. M is treated with several transfusions of fresh frozen plasma, packed red blood cells, and phytonadione (vitamin K). A consultation-liaison psychiatrist diagnoses bipolar disorder and starts Ms. M on lamotrigine, 25 mg once daily. (The justification for this diagnosis was not documented.) After physicians judge her to be medically stable, Ms. M is involuntarily committed to a short-term psychiatric care facility. Her vital signs and coagulation values are stable.
At the psychiatric facility, our team determines that her symptoms and history are consistent with major depressive disorder, recurrent. For 5 months, Ms. M had depressed mood for most of the day, diminished interest in activities, and feelings of worthlessness. She also experienced weight loss—10 lbs in 2 months—with decreased appetite and low energy for most of the day. She denies past symptoms of mania or psychosis. She says she does not know why she was diagnosed with bipolar disorder. She admits to multiple previous suicide attempts via hanging and ingesting cleaning fluid or rat poison.
We place Ms. M on suicide precautions and prescribe escitalopram, 10 mg/d, in addition to lamotrigine, 50 mg once daily. We continue lamotrigine despite a lack of documentation for Ms. M’s bipolar diagnosis because evidence suggests the drug may be an effective augmentation to antidepressants in patients with treatment-resistant depression.1
The author’s observations
Any patient transferred from a medical floor to a psychiatric inpatient unit should have documentation that clarifies any need for further medical treatment. Ms. M’s physicians told us that she was medically stable and should require little if any further treatment for her ingestion of rat poison.
TREATMENT: Coagulation concerns
We request a medical consult to monitor possible complications from the rat poison. The physician advises that rat poison essentially is the same as the anticoagulant warfarin and its effects should steadily decrease over time because its half-life is 20 to 60 hours. However, for safety reasons, we closely follow Ms. M’s coagulation values and order daily vitamin K injections, 5 mg SC.
Further medical investigation shows no evidence of complications, but Ms. M continues to request medication for pain in her left leg. The physician prescribes acetaminophen, 650 mg every 6 hours as needed for pain, which the patient takes at almost every opportunity, often 4 times a day. The physician does not choose a nonsteroidal anti-inflammatory drug (NSAID) for pain to avoid the possibility of gastrointestinal (GI) irritation that could lead to bleeding.
In the psychiatric facility, the patient’s international normalized ratio (INR) is found to be rising, indicating a lack of clotting and a risk of uncontrolled bleeding. The physician states that given the half-life of warfarin, Ms. M’s INR should be decreasing. Liver function testing does not show that liver dysfunction is contributing to the increasing INR.
Because we assume the vitamin K the patient received has been absorbed, we hypothesize that Ms. M has continued to surreptitiously ingest rat poison or another anticoagulant, which she denies. We search Ms. M and her room. She is placed on 1-to-1 observation 24 hours a day. Ms. M’s visitors also are searched, and visits are observed. We find no evidence of an anticoagulant agent.
Ms. M’s INR continues to rise. We search the facility to rule out the possibility that the patient had hidden a supply of anticoagulant outside her room. The search finds nothing. At this point we consider performing an abdominal x-ray to rule out the possibility that Ms. M may have a supply of medication hidden in her gastrointestinal tract.
The author’s observations
Patients hiding and using contraband is a common problem in involuntary inpatient units. It seemed that Ms. M was secretly ingesting rat poison. Her history showed she was determined to end her life, and she ingested rat poison daily for months. However, because an exhaustive search for contraband and 1-to-1 observation yielded no positive results, the evidence did not support this theory. Some team members thought we were not searching hard enough. I decided we needed to pursue other theories.
I was skeptical that escitalopram could be contributing to Ms. M’s rising anticoagulation values. Selective serotonin reuptake inhibitors have antiplatelet effects, but platelet function does not affect INR to the degree we were observing.
‘Superwarfarins’
Physicians had advised us that Ms. M’s INR should decrease under the assumption that rat poison is for all practical purposes the same as warfarin, but we had not investigated distinctions between the 2 compounds. A literature search revealed that several rat poisons are not simply warfarin repackaged as a pesticide. Most are “superwarfarins”—chemicals similar to warfarin but more potent and with a much longer half-life.2 Case report data suggest the plasma half-life of these chemicals is 20 to 62 days.3
Most commercial rat poisons are made of brodifacoum, which has a chemical structure similar to warfarin but with an additional long polycyclic hydrocarbon side chain (Figure 1). The potency of brodifacoum compared with warfarin is approximately 100 to 1.4-6 The chemical is highly lipophilic and can stay in the body for an extended period.4-6 Lab tests can measure serum brodifacoum levels.3
After Ms. M identifies the brand name of the rat poison she ingested, we contact the American Association of Poison Control Centers and verify the agent she used was brodifacoum. This explains why her INR was not decreasing—but does not explain the increase.
A drug interaction? Because Ms. M’s liver function is within normal limits, the next theory to investigate is if brodifacoum is interacting with any medications she is taking. I could not find any medical journal articles, programs, or Web sites describing brodifacoum’s interactions with medications. After all, brodifacoum is a pesticide, not a medication.
I considered that because brodifacoum and warfarin have a similar structure and function, they may interact with medications in a similar manner. After another literature search, only acetaminophen had evidence of interaction with warfarin that could explain her rising INR.
Documentation of interactions between warfarin and acetaminophen are sparse. In one case, a 74-year-old man receiving warfarin for atrial fibrillation experienced an abrupt increase in INR after taking acetaminophen.7 In a double-blind, placebo controlled, randomized trial of patients taking warfarin, INR rose rapidly after the start of acetaminophen and was significantly increased within 1 week compared with patients receiving placebo.8
Figure 1 Chemical structures of warfarin and rat poison
Most commercial rat poisons are made of brodifacoum, which is chemically similar to warfarin but has an additional long polycyclic hydrocarbon side chain.
FOLLOW-UP: Analgesic substitution
We suggest to the physician that Ms. M’s INR may be increasing because of an interaction between brodifacoum and acetaminophen, which she took several times a day. On day 8 of Ms. M’s hospitalization, the physician discontinues acetaminophen and prescribes ibuprofen, 400 mg tid as needed for pain, and pantoprazole, 40 mg/d, to prevent GI bleeding from possible irritation caused by ibuprofen. The team continues to monitor Ms. M’s coagulation values.
Within a day of discontinuing acetaminophen, Ms. M’s INR decreased as expected (Figure 2). The rest of her medication regimen is continued, and her INR levels continued to decrease.
One-to-one observation is discontinued. However, because of the patient’s continued determination to end her life and no significant improvement in her depression, Ms. M is considered a danger to herself and involuntary inpatient hospitalization is continued.
Figure 2 Ms. M’s INR values during hospitalization
The patient’s INR values began to rise mysteriously after she was transferred to the inpatient psychiatric unit. Acetaminophen was discontinued on day 8, and within a day her INR values began to drop.
INR: International normalized ratio
The author’s observations
Poisoning is a common method of attempting suicide, patients may use substances that clinicians rarely encounter. For most toxic, nonmedication substances, data on interactions with medications are sparse. if you suspect your patient has ingested a toxic substance with which the treatment team has little experience, contact the American Association of Poison Control Centers at 800-222-1222.
Suspect superwarfarin poisoning in suicidal patients with coagulopathy, prolonged prothrombin time, and elevated INR that does not respond to large amounts of vitamin K.9,10 These patients are at high risk of successfully completing suicide because of superwarfarins’ long half-life and daily maintenance required to keep coagulation levels within a safe range for at least several weeks.
The most serious complication these patients face is intracranial hemorrhage, which occurs in 2% of patients with excessive warfarin-based coagulation and is associated with a 77% mortality rate.11 GI bleeding occurs in 67% of these patients.2
Also take into account medical conditions—such as hypertension or hepatic disease—and medication side effects that can increase bleeding risk. When treating pain in these patients, consider avoiding acetaminophen but be aware of the risks of NSAIDs, such as gastritis or GI bleeding.
Related resource
- The American Association of Poison Control Centers. 800-222-1222; www.aapcc.org.
Drug brand names
- Escitalopram • Lexapro
- Lamotrigine • Lamictal
- Pantoprazole • Protonix
- Warfarin • Coumadin
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Unusual suicide attempt
After a friend calls 911, Ms. M, age 20, is brought to an emergency room (ER) complaining of severe leg and abdominal pain. The ER physician finds she is bleeding from her vagina and nose and has severe ecchymosis anemia. After Ms. M is admitted, clinicians discover these conditions are secondary to a suicide attempt—she ingested 15 to 16 pellets of rat poison daily for approximately 2 months.
While hospitalized, Ms. M is treated with several transfusions of fresh frozen plasma, packed red blood cells, and phytonadione (vitamin K). A consultation-liaison psychiatrist diagnoses bipolar disorder and starts Ms. M on lamotrigine, 25 mg once daily. (The justification for this diagnosis was not documented.) After physicians judge her to be medically stable, Ms. M is involuntarily committed to a short-term psychiatric care facility. Her vital signs and coagulation values are stable.
At the psychiatric facility, our team determines that her symptoms and history are consistent with major depressive disorder, recurrent. For 5 months, Ms. M had depressed mood for most of the day, diminished interest in activities, and feelings of worthlessness. She also experienced weight loss—10 lbs in 2 months—with decreased appetite and low energy for most of the day. She denies past symptoms of mania or psychosis. She says she does not know why she was diagnosed with bipolar disorder. She admits to multiple previous suicide attempts via hanging and ingesting cleaning fluid or rat poison.
We place Ms. M on suicide precautions and prescribe escitalopram, 10 mg/d, in addition to lamotrigine, 50 mg once daily. We continue lamotrigine despite a lack of documentation for Ms. M’s bipolar diagnosis because evidence suggests the drug may be an effective augmentation to antidepressants in patients with treatment-resistant depression.1
The author’s observations
Any patient transferred from a medical floor to a psychiatric inpatient unit should have documentation that clarifies any need for further medical treatment. Ms. M’s physicians told us that she was medically stable and should require little if any further treatment for her ingestion of rat poison.
TREATMENT: Coagulation concerns
We request a medical consult to monitor possible complications from the rat poison. The physician advises that rat poison essentially is the same as the anticoagulant warfarin and its effects should steadily decrease over time because its half-life is 20 to 60 hours. However, for safety reasons, we closely follow Ms. M’s coagulation values and order daily vitamin K injections, 5 mg SC.
Further medical investigation shows no evidence of complications, but Ms. M continues to request medication for pain in her left leg. The physician prescribes acetaminophen, 650 mg every 6 hours as needed for pain, which the patient takes at almost every opportunity, often 4 times a day. The physician does not choose a nonsteroidal anti-inflammatory drug (NSAID) for pain to avoid the possibility of gastrointestinal (GI) irritation that could lead to bleeding.
In the psychiatric facility, the patient’s international normalized ratio (INR) is found to be rising, indicating a lack of clotting and a risk of uncontrolled bleeding. The physician states that given the half-life of warfarin, Ms. M’s INR should be decreasing. Liver function testing does not show that liver dysfunction is contributing to the increasing INR.
Because we assume the vitamin K the patient received has been absorbed, we hypothesize that Ms. M has continued to surreptitiously ingest rat poison or another anticoagulant, which she denies. We search Ms. M and her room. She is placed on 1-to-1 observation 24 hours a day. Ms. M’s visitors also are searched, and visits are observed. We find no evidence of an anticoagulant agent.
Ms. M’s INR continues to rise. We search the facility to rule out the possibility that the patient had hidden a supply of anticoagulant outside her room. The search finds nothing. At this point we consider performing an abdominal x-ray to rule out the possibility that Ms. M may have a supply of medication hidden in her gastrointestinal tract.
The author’s observations
Patients hiding and using contraband is a common problem in involuntary inpatient units. It seemed that Ms. M was secretly ingesting rat poison. Her history showed she was determined to end her life, and she ingested rat poison daily for months. However, because an exhaustive search for contraband and 1-to-1 observation yielded no positive results, the evidence did not support this theory. Some team members thought we were not searching hard enough. I decided we needed to pursue other theories.
I was skeptical that escitalopram could be contributing to Ms. M’s rising anticoagulation values. Selective serotonin reuptake inhibitors have antiplatelet effects, but platelet function does not affect INR to the degree we were observing.
‘Superwarfarins’
Physicians had advised us that Ms. M’s INR should decrease under the assumption that rat poison is for all practical purposes the same as warfarin, but we had not investigated distinctions between the 2 compounds. A literature search revealed that several rat poisons are not simply warfarin repackaged as a pesticide. Most are “superwarfarins”—chemicals similar to warfarin but more potent and with a much longer half-life.2 Case report data suggest the plasma half-life of these chemicals is 20 to 62 days.3
Most commercial rat poisons are made of brodifacoum, which has a chemical structure similar to warfarin but with an additional long polycyclic hydrocarbon side chain (Figure 1). The potency of brodifacoum compared with warfarin is approximately 100 to 1.4-6 The chemical is highly lipophilic and can stay in the body for an extended period.4-6 Lab tests can measure serum brodifacoum levels.3
After Ms. M identifies the brand name of the rat poison she ingested, we contact the American Association of Poison Control Centers and verify the agent she used was brodifacoum. This explains why her INR was not decreasing—but does not explain the increase.
A drug interaction? Because Ms. M’s liver function is within normal limits, the next theory to investigate is if brodifacoum is interacting with any medications she is taking. I could not find any medical journal articles, programs, or Web sites describing brodifacoum’s interactions with medications. After all, brodifacoum is a pesticide, not a medication.
I considered that because brodifacoum and warfarin have a similar structure and function, they may interact with medications in a similar manner. After another literature search, only acetaminophen had evidence of interaction with warfarin that could explain her rising INR.
Documentation of interactions between warfarin and acetaminophen are sparse. In one case, a 74-year-old man receiving warfarin for atrial fibrillation experienced an abrupt increase in INR after taking acetaminophen.7 In a double-blind, placebo controlled, randomized trial of patients taking warfarin, INR rose rapidly after the start of acetaminophen and was significantly increased within 1 week compared with patients receiving placebo.8
Figure 1 Chemical structures of warfarin and rat poison
Most commercial rat poisons are made of brodifacoum, which is chemically similar to warfarin but has an additional long polycyclic hydrocarbon side chain.
FOLLOW-UP: Analgesic substitution
We suggest to the physician that Ms. M’s INR may be increasing because of an interaction between brodifacoum and acetaminophen, which she took several times a day. On day 8 of Ms. M’s hospitalization, the physician discontinues acetaminophen and prescribes ibuprofen, 400 mg tid as needed for pain, and pantoprazole, 40 mg/d, to prevent GI bleeding from possible irritation caused by ibuprofen. The team continues to monitor Ms. M’s coagulation values.
Within a day of discontinuing acetaminophen, Ms. M’s INR decreased as expected (Figure 2). The rest of her medication regimen is continued, and her INR levels continued to decrease.
One-to-one observation is discontinued. However, because of the patient’s continued determination to end her life and no significant improvement in her depression, Ms. M is considered a danger to herself and involuntary inpatient hospitalization is continued.
Figure 2 Ms. M’s INR values during hospitalization
The patient’s INR values began to rise mysteriously after she was transferred to the inpatient psychiatric unit. Acetaminophen was discontinued on day 8, and within a day her INR values began to drop.
INR: International normalized ratio
The author’s observations
Poisoning is a common method of attempting suicide, patients may use substances that clinicians rarely encounter. For most toxic, nonmedication substances, data on interactions with medications are sparse. if you suspect your patient has ingested a toxic substance with which the treatment team has little experience, contact the American Association of Poison Control Centers at 800-222-1222.
Suspect superwarfarin poisoning in suicidal patients with coagulopathy, prolonged prothrombin time, and elevated INR that does not respond to large amounts of vitamin K.9,10 These patients are at high risk of successfully completing suicide because of superwarfarins’ long half-life and daily maintenance required to keep coagulation levels within a safe range for at least several weeks.
The most serious complication these patients face is intracranial hemorrhage, which occurs in 2% of patients with excessive warfarin-based coagulation and is associated with a 77% mortality rate.11 GI bleeding occurs in 67% of these patients.2
Also take into account medical conditions—such as hypertension or hepatic disease—and medication side effects that can increase bleeding risk. When treating pain in these patients, consider avoiding acetaminophen but be aware of the risks of NSAIDs, such as gastritis or GI bleeding.
Related resource
- The American Association of Poison Control Centers. 800-222-1222; www.aapcc.org.
Drug brand names
- Escitalopram • Lexapro
- Lamotrigine • Lamictal
- Pantoprazole • Protonix
- Warfarin • Coumadin
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sharma V, Khan M, Corpse C. Role of lamotrigine in the management of treatment-resistant bipolar II depression: a chart review. J Affect Disord Epub 2008 Mar 1.
2. Su M, Hoffman R. Anticoagulants. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s toxicologic emergencies. 8th ed. New York, NY: McGraw-Hill Medical Publishing; 2006:891-4.
3. Chua JD, Friedenberg WR. Superwarfarin poisoning. Anaesth Intensive Care 1997;25:707-9.
4. Leck JB, Park BK. A comparative study of the effect of warfarin and brodifacoum on the relationship between vitamin K1 metabolism and clotting factor activity in warfarin susceptible and warfarin resistant rats. Biochem Pharmacol 1981;30:123-9.
5. Lund M. Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg 1981;87:101-7.
6. Park BK, Scott AK, Wilson AC, et al. Plasma disposition of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol 1982;31:3535-639.
7. Gebauer MG, Nyfort-Hansen K, Henschke PJ, Gallus AS. Warfarin and acetaminophen interaction. Pharmacotherapy 2003;23(1):109-12.
8. Mahe I, Bertrand N, Drouet L, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica 2006;91(12):1621-7.
9. Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet 2005;365:552-3.
10. Scully M. Warfarin therapy: rat poison and the prevention of thrombosis. Biochemist 2002;24:15-7.
11. Mathiesen T, Benediktsdottir K, Johnsson H, Lindqvist M. Intracranial traumatic and nontraumatic haemorrhagic complications of warfarin treatment. Acta Neurol Scan 1995;91:208-14.
1. Sharma V, Khan M, Corpse C. Role of lamotrigine in the management of treatment-resistant bipolar II depression: a chart review. J Affect Disord Epub 2008 Mar 1.
2. Su M, Hoffman R. Anticoagulants. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s toxicologic emergencies. 8th ed. New York, NY: McGraw-Hill Medical Publishing; 2006:891-4.
3. Chua JD, Friedenberg WR. Superwarfarin poisoning. Anaesth Intensive Care 1997;25:707-9.
4. Leck JB, Park BK. A comparative study of the effect of warfarin and brodifacoum on the relationship between vitamin K1 metabolism and clotting factor activity in warfarin susceptible and warfarin resistant rats. Biochem Pharmacol 1981;30:123-9.
5. Lund M. Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg 1981;87:101-7.
6. Park BK, Scott AK, Wilson AC, et al. Plasma disposition of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol 1982;31:3535-639.
7. Gebauer MG, Nyfort-Hansen K, Henschke PJ, Gallus AS. Warfarin and acetaminophen interaction. Pharmacotherapy 2003;23(1):109-12.
8. Mahe I, Bertrand N, Drouet L, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica 2006;91(12):1621-7.
9. Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet 2005;365:552-3.
10. Scully M. Warfarin therapy: rat poison and the prevention of thrombosis. Biochemist 2002;24:15-7.
11. Mathiesen T, Benediktsdottir K, Johnsson H, Lindqvist M. Intracranial traumatic and nontraumatic haemorrhagic complications of warfarin treatment. Acta Neurol Scan 1995;91:208-14.
ADHD in adults: Matching therapies with patients’ needs
Mr. Z, age 42, is referred by his primary care physician with symptoms suggesting attention-deficit/hyperactivity disorder (ADHD). Mr. Z has seen his physician sporadically for 10 years and acknowledges not following dietary and exercise advice. He has had intermittent “minor” depression, is overweight, and is a smoker with a family history of cardiovascular disease and diabetes.
A salesman, Mr. Z recently was promoted to an administrative position that substantially increased his paperwork. He is having difficulty performing his job because of longstanding forgetfulness and disorganization. He says he feels “like I’m in grade school again, lost in paperwork.” He also describes a recent educational assessment for his son, age 7, who may have ADHD. Similarities between Mr. Z’s and his son’s early childhood academic struggles are striking.
Like Mr. Z, adults with ADHD commonly seek treatment when increasing stressors and demands overwhelm their cognitive-attentional abilities. Some may be “healthy” men and women without psychiatric histories, whose disorganization, forgetfulness, or impulsivity contributes to functional impairment, including nonadherence with medical advice. For others, such as those with known psychiatric disorders, ADHD may be a hidden comorbidity contributing to seemingly refractory depression or anxiety disorder.
Despite growing evidence related to adult ADHD, individualizing and maintaining treatment over time can be challenging for clinicians and patients. Fortunately, new tools and multiple stimulant and nonstimulant medications can help you screen for, assess, and treat adult ADHD.
ADHD diagnosis
To diagnose ADHD in an adult patient, first establish that symptoms have existed from childhood to adulthood. One approach is to review DSM-IV-TR criteria for ADHD with your patient and ask him or her to reflect on childhood symptoms and dysfunction. Begin with orienting questions, such as “Do you remember your first grade teacher, your school, where you lived?” ADHD symptoms might have been present even if the patient maintained acceptable grades, particularly in elementary school, as dedicated parents or teachers might have contributed to early academic success.
Next, turn to diagnostic language that captures ADHD symptoms in adults. For example, the 18-item World Health Organization Adult ADHD Self-Report Scale (ASRS-v1.1) prompts individuals to self-report DSM-IV ADHD symptoms, and a 6-item subset (Table 1) is a highly specific screener (see Related Resources). The ASRS is most reliable in adults with limited psychiatric comorbidity.1
Adults often describe fluctuations in symptom severity over time. Symptoms may have less impact with more physically demanding work—such as sales—and greater impact with organizationally demanding work—such as administration.
Base your summary ADHD diagnosis on DSM-IV-TR criteria, including:
- lifetime persistence of symptoms, beginning before age 7
- functional impairment in ≥2 life settings, such as work, school, or home
- lack of another medical or psychiatric condition sufficient to explain the symptoms.
Table 1
Adult Self-Report Scale-v1.1 WHO 6-question screening tool for ADHD*
| Check the box that best describes how you have felt and conducted yourself over the past 6 months. Please give the completed questionnaire to your healthcare professional during your next appointment to discuss the results | Never | Rarely | Sometimes | Often | Very often |
|---|---|---|---|---|---|
| 1. How often do you have trouble wrapping up the final details of a project, once the challenging parts have been done? | |||||
| 2. How often do you have difficulty getting things in order when you have to do a task that requires organization? | |||||
| 3. How often do you have problems remembering appointments or obligations? | |||||
| 4. When you have a task that requires a lot of thought, how often do you avoid or delay getting started? | |||||
| 5. How often do you fidget or squirm with your hands or feet when you have to sit down for a long time? | |||||
| 6. How often do you feel overly active and compelled to do things, like you were driven by a motor? | |||||
| Add the number of checkmarks that appear in the darkly shaded area. Four (4) or more checkmarks indicate that your symptoms may be consistent with adult ADHD. It may be beneficial for you to talk with your healthcare provider about an evaluation. | |||||
| * Intended for use by persons age 18 and older ADHD: attention-deficit/hyperactivity disorder; WHO: World Health Organization | |||||
| Source: Reprinted with permission. World Health Organization Copyright 2003. All rights reserved | |||||
CASE CONTINUED: ‘All the time, every day’
Mr. Z completes the ASRS self-report symptom checklist and brings his wife to the next appointment. He rated all 6 screening symptoms and most others as occurring “often” or “very often.” He describes functional impairments “essentially all the time, basically every day” at work, home, and socially. His wife confirms these symptoms and the frustrations and conflicts they have caused.
Mr. Z describes ADHD symptoms from early elementary school to college. He was held back in kindergarten for being “immature,” his academic performance was inconsistent, and he “just got by…by cramming” in high school and college. His school performance pattern does not suggest a learning disability; he did not need special help in 1 subject more than others, and under pressure he could achieve average grades.
Medical review excludes explanations other than ADHD for his inattention, restlessness, and impulsivity. You conclude that Mr. Z meets criteria for ADHD, combined subtype, and discuss medication treatment.
FDA-approved medications
Medication for ADHD is appropriate only if symptoms are impairing. Five effective and generally well-tolerated medications are FDA-approved for adults with ADHD (Table 2):
- extended-release mixed amphetamine (Adderall XR)
- extended-release OROS methylphenidate (Concerta)
- extended-release dexmethylphenidate (Focalin XR)
- atomoxetine (Strattera)
- lisdexamfetamine (Vyvanse).
Efficacy. A meta-analysis of 29 pediatric ADHD trials across 30 years demonstrated greater effect size for stimulant class medications (immediate- and long-acting), compared with nonstimulant medications (including bupropion, atomoxetine, and modafinil).2 This finding is consistent with the American Academy of Child and Adolescent Psychiatry’s recommendation of stimulant medications as first-line agents for pediatric ADHD.3 A similar meta-analysis of 6 controlled studies of methylphenidate-class medications in adults found a large mean effect size (0.9), with greater effects associated with higher doses.4
Atomoxetine, a norepinephrine reuptake inhibitor, is the only nonstimulant medication FDA-approved for ADHD in adults. More than 6,000 children, adolescents, and adults have taken atomoxetine in clinical trials for ADHD (Lilly, prescribing information), with 4 years of open treatment data showing benefit being maintained over time.5
Tolerability. Although ADHD medications are generally well-tolerated by healthy adults, assess for a history of potential contraindications:
- unstable medical condition, hyperthyroidism, glaucoma
- treatment with a monoamine oxidase inhibitor or other pressor agents because of possible effects on blood pressure and heart rate
- use of cytochrome P450 2D6 inhibitors, which may increase atomoxetine steady-state plasma concentrations
- cardiovascular disease or family history of early cardiac disease (Box 1)6,7
- history of or active substance use disorder, such as alcohol dependence, cocaine or heroin abuse
- history of psychosis, bipolar disorder, or an active clinically significant psychiatric comorbidity (major depression, agitated state, suicidality).
Clinically, some patients appear to tolerate 1 class of stimulant (such as methylphenidate or amphetamine) over another. Consider switching to an alternate stimulant if your patient has bothersome side effects—mild low appetite, insomnia, tension, or jitteriness—or has received limited or partial benefit during an initial stimulant trial.
Serious cardiovascular events and sudden death have occurred in adults and children treated with stimulants.6 Agents used for attention-deficit/hyperactivity disorder (ADHD) have not been shown to cause sudden cardiac death, but the FDA requires stimulants’ labeling to warn about this risk in patients with structural cardiac abnormalities. The warning advises against using stimulants in adults with cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease.
When treating adults with ADHD, look to advisories about cardiovascular monitoring in children with ADHD. Before initiating medications, do a physical exam focused on cardiovascular disease risk factors and obtain a patient and family health history of:
- fainting or dizziness
- sudden or unexplained death in someone young
- sudden cardiac death or “heart attack” in family members age <35 years.
The American Academy of Pediatrics, American Academy of Child and Adolescent Psychiatry, and American Heart Association concur that electrocardiography (ECG) is not mandatory in cardiovascular assessment and monitoring during ADHD pharmacotherapy.7 This author (PH) refers cardiovascular questions to a primary care physician or cardiologist.
During ADHD treatment, monitor vital signs and refer patients with emergent cardiac symptoms or concerns to a cardiologist. Expect increases in blood pressure (1 to 4 mm Hg) and heart rate (2 to 6 bpm) during treatment with methylphenidate and amphetamine-class stimulants as well as with atomoxetine. Do not expect significant changes in ECG parameters (PR, QRS, and QTC intervals).
Extended-release formulations. Early adult studies demonstrated the efficacy of immediate-release stimulants, but adults with ADHD’s inherent deficits in organization and memory may have higher adherence rates and greater success with once-daily, extended-release formulations.8-11 Unless your patient wants to begin with small, short-acting dosages (5 to 10 mg) or desires to target treatment to specific times of day (such as in the morning for administrative work only), many appreciate once-daily formulations. Extended-release formulations also may be the simplest stimulants with which to begin ADHD treatment.
Over time, patients may benefit from an immediate-release form:
- added for certain times of day—such as in late afternoon, when the morning extended-release dose has worn off (Box 2)12,13
- to use as an alternative to extended-release formulations when more or less flexibly is desired, such as on weekends.
Table 2
Administering medications approved for adult ADHD
| Drug | Recommended dosage* | Comments |
|---|---|---|
| Stimulants | ||
| Extended-release mixed amphetamine (Adderall XR) | 20 mg | Initial prescription of 10-mg XR capsules allows gradual titration |
| Extended-release OROS methylphenidate (Concerta) | 18 to 72 mg/d | Initial prescription of 18-mg OROS MPH capsules allows gradual titration |
| Extended-release dexmethylphenidate (Focalin XR) | 10 mg/d; maximum 20 mg/d | Dosing is one-half the typical dosing of racemic MPH |
| Lisdexamfetamine (Vyvanse) | 30 mg/d; maximum 70 mg/d | May be adjusted weekly in 10-mg or 20-mg increments |
| Nonstimulant | ||
| Atomoxetine (Strattera) | 80 mg/d; maximum 100 mg/d | Initial dosage of 40 mg/d can be increased to target dosage after a minimum of 3 days; can be given as a morning dose or divided evenly between morning and evening doses |
| * FDA-approved dosages as listed in the package inserts of these medications ADHD: attention-deficit/hyperactivity disorder; MPH: methylphenidate; OROS: osmotic release oral system; XR: extended-release formulation | ||
CASE CONTINUED: Feeling ‘calm, less frenetic’
During the next 6 months, you start Mr. Z on stimulant treatment at robust dosing consistent with his weight (90 kg). He complains that extended-duration methylphenidate (MPH)—titrated to 90 mg/d—doesn’t last into the late afternoon, and he feels mildly tense with a low appetite. Because of an apparent partial response and relatively mild adverse effects, you discontinue MPH and try an extended-duration amphetamine, titrated to 60 mg.
Mr. Z’s blood pressure and heart rate remain stable. He begins to exercise regularly and reduce his use of tobacco and caffeine drinks, as you recommend. He says he feels “calm, less frenetic.” He reports no tension on this medication and only mild reduced appetite. With a plan to continue taking the stimulant medication with regular monitoring, he then disappears from treatment.
Promoting adherence
Treatment nonadherence is an issue throughout medicine, and individuals with disorganization, forgetfulness, and impulsivity may be at higher-than-usual risk of not following through on medication regimens.
Combining short- and long-acting stimulants may cover hours when attention-deficit/hyperactivity (ADHD) symptoms emerge despite therapy with a long-acting agent.12,13 Ask patients who report lack of full-day coverage if the once-daily, extended-duration formulation they are taking works well until a certain time of day. Then consider adding a similar-class immediate-release stimulant at this time to cover the later hours.
If a patient reports partial response throughout the day—such as early in treatment—begin by optimizing the long-acting agent’s dosage. Keep a target daily dose in mind, based on FDA recommendations and clinical trial data. For example, an adult weighing 80 kg may respond optimally to a combination of 60 mg of a long-acting methylphenidate (MPH) in the morning, followed by 10 to 20 mg of an immediate-release MPH in mid-afternoon.
The later stimulants are taken in the day, the more likely insomnia may emerge as an adverse effect. Some patients adjust to this problem within the first weeks of treatment. If insomnia remains impairing, reduce the stimulant dose or consider switching to a shorter duration medication or to the nonstimulant atomoxetine.
In addition, restrictions on stimulant-class medications do not permit multiple-month prescribing (refills), as is allowed with non-scheduled medications such as atomoxetine. Discuss with patients how they will obtain stimulant medications on a regular, monthly or bimonthly basis. In our experience, the practical challenges of remaining in treatment at times may limit patients’ adherence to ADHD medications more than a lack of response or tolerability concerns.
Explain to patients early in treatment that they might need to try several different medications before settling on 1 that is optimally tolerated and efficacious. Because stimulants are generally quite effective for ADHD symptoms, set your goal to identify adverse effects and aim for a patient response of “this works well, and I don’t feel any different on it.”
CASE CONTINUED: Ready to try again
Three years later, Mr. Z returns and reports gradually discontinuing the stimulant because he “wanted to go it on my own.” He functioned relatively well at first, but errors and conflicts at his job led to his dismissal.
Since then, he has been unemployed. He is increasingly depressed and reports drinking and smoking “more heavily than in college.” He asks about resuming ADHD treatment.
Mr. Z does not meet DSM-IV-TR criteria for major depressive disorder or alcohol abuse/dependence. His depressed mood appears to be linked to his marked ADHD symptoms. Mr. Z agrees to a new treatment plan that includes starting atomoxetine at 25 mg to allow for flexible titration and psychotherapy to monitor his mood and achieve sobriety.
ADHD and substance abuse
Clinical judgment determines whether an adult with ADHD and a history of substance use disorders may safely benefit from treatment with a stimulant. The relationship between ADHD and substance use disorders is of clinical concern, but ADHD medications have not been shown to increase risk for later substance use disorders in children.14 Conversely, effective ADHD treatment appears to reduce later cigarette and substance use.15
Consider using a nonstimulant-class medication in adults with ADHD and active substance use disorders. In a 12-week, double-blind, controlled trial, atomoxetine improved ADHD symptoms significantly more than placebo in adults meeting DS-MIV-TR criteria for comorbid alcohol use disorders. After 4 to 30 days of alcohol abstinence, 72 patients were randomly assigned to atomoxetine, 25 to 100 mg/d (mean final dose 90 mg/d), and 75 patients to placebo. Although estimated times to initial relapse to heavy drinking did not differ:
- atomoxetine-treated subjects had 26% fewer cumulative heavy drinking days than placebo-treated subjects (P=0.023)
- the difference in cumulative heavy drinking days between the atomoxetine and placebo groups became statistically significant after 55 days of treatment.16
- World Health Organization Adult Self-Report Scale (ASRS) 18-item instrument and 6-item screener. www.med.nyu.edu/psych/psychiatrist/adhd.html.
- Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 2008;165:553-5.
- Adler LA, Spencer TJ, Levine LR, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord 2008; 11:720-7.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Extended-release mixed amphetamine • Adderall XR
- Extended duration OROS methylphenidate • Concerta
- Extended-release dexmethylphenidate • Focalin XR
- Lisdexamfetamine • Vyvanse
- Modafinil • Provigil
Disclosure
Dr. Hammerness has received research support from and is on the speakers bureau for Shire Pharmaceuticals. He has received support for CME activities and talks from Shire Pharmaceuticals, Ortho-McNeil, and Abbott Laboratories.
Dr. Surman receives research support and/or is a speaker for Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, Ortho-McNeil, Merck, New River Pharmaceuticals, Novartis, Pfizer Inc., Shire Pharmaceuticals, and Takeda Pharmaceutical Company.
Dr. Sassi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Clinical research assistant Katherine Miller, BA, contributed to the literature review for this article and assisted in preparing the manuscript.
1. Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005;35:245-56.
2. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed 2006;8(4):4.-
3. Greenhill L, Pliszka S, Dulcan M, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2001;40(11):1352-5.
4. Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2004;24:24-9.
5. Adler LA, Spencer TJ, Williams DW, et al. Long-term, open-label safety and efficacy of atomoxetine in adults with ADHD: final report of a 4-year study. J Atten Disord Epub 2008 April 30.
6. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med 2006;354:1445-8.
7. American Academy of Pediatrics/American Heart Association clarification of statement on cardiovascular evaluation and monitoring of children and adolescents with heart disease receiving medications for ADHD May 16, 2008. Available at: http://www.aap.org/pressroom/aap-ahastatement.htm. Accessed August 14, 2008.
8. Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2006;59(9):829-35.
9. Biederman J, Mick E, Surman C, et al. Comparative acute efficacy and tolerability of OROS and immediate release formulations of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2007;7:49.-
10. Mick E, Spencer TJ, Surman C, et al. Randomized single-blind substitution study of methylphenidate in ADHD adults receiving immediate-release methylphenidate. NR357. Poster presented at: Annual Meeting of the American Psychiatric Association; May 19-24, 2007; San Diego, CA.
11. Capone N, McDonnel T. Medication persistence among agents used to treat attention-deficit/hyperactivity disorder, diabetes, and elevated serum cholesterol. NR 639. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
12. Adler L, Morrill M, Reingold B. d-methylphenidate augmentation of extended-release stimulant therapy in ADHD. NR 619. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
13. Adler L, Reingold LS, Morrill MS, Wilens TE. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep 2006;8:409-15.
14. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry 2008;165:597-603.
15. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 2007;68(suppl 11):15-22.
16. Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 2008;96:145-54.
Mr. Z, age 42, is referred by his primary care physician with symptoms suggesting attention-deficit/hyperactivity disorder (ADHD). Mr. Z has seen his physician sporadically for 10 years and acknowledges not following dietary and exercise advice. He has had intermittent “minor” depression, is overweight, and is a smoker with a family history of cardiovascular disease and diabetes.
A salesman, Mr. Z recently was promoted to an administrative position that substantially increased his paperwork. He is having difficulty performing his job because of longstanding forgetfulness and disorganization. He says he feels “like I’m in grade school again, lost in paperwork.” He also describes a recent educational assessment for his son, age 7, who may have ADHD. Similarities between Mr. Z’s and his son’s early childhood academic struggles are striking.
Like Mr. Z, adults with ADHD commonly seek treatment when increasing stressors and demands overwhelm their cognitive-attentional abilities. Some may be “healthy” men and women without psychiatric histories, whose disorganization, forgetfulness, or impulsivity contributes to functional impairment, including nonadherence with medical advice. For others, such as those with known psychiatric disorders, ADHD may be a hidden comorbidity contributing to seemingly refractory depression or anxiety disorder.
Despite growing evidence related to adult ADHD, individualizing and maintaining treatment over time can be challenging for clinicians and patients. Fortunately, new tools and multiple stimulant and nonstimulant medications can help you screen for, assess, and treat adult ADHD.
ADHD diagnosis
To diagnose ADHD in an adult patient, first establish that symptoms have existed from childhood to adulthood. One approach is to review DSM-IV-TR criteria for ADHD with your patient and ask him or her to reflect on childhood symptoms and dysfunction. Begin with orienting questions, such as “Do you remember your first grade teacher, your school, where you lived?” ADHD symptoms might have been present even if the patient maintained acceptable grades, particularly in elementary school, as dedicated parents or teachers might have contributed to early academic success.
Next, turn to diagnostic language that captures ADHD symptoms in adults. For example, the 18-item World Health Organization Adult ADHD Self-Report Scale (ASRS-v1.1) prompts individuals to self-report DSM-IV ADHD symptoms, and a 6-item subset (Table 1) is a highly specific screener (see Related Resources). The ASRS is most reliable in adults with limited psychiatric comorbidity.1
Adults often describe fluctuations in symptom severity over time. Symptoms may have less impact with more physically demanding work—such as sales—and greater impact with organizationally demanding work—such as administration.
Base your summary ADHD diagnosis on DSM-IV-TR criteria, including:
- lifetime persistence of symptoms, beginning before age 7
- functional impairment in ≥2 life settings, such as work, school, or home
- lack of another medical or psychiatric condition sufficient to explain the symptoms.
Table 1
Adult Self-Report Scale-v1.1 WHO 6-question screening tool for ADHD*
| Check the box that best describes how you have felt and conducted yourself over the past 6 months. Please give the completed questionnaire to your healthcare professional during your next appointment to discuss the results | Never | Rarely | Sometimes | Often | Very often |
|---|---|---|---|---|---|
| 1. How often do you have trouble wrapping up the final details of a project, once the challenging parts have been done? | |||||
| 2. How often do you have difficulty getting things in order when you have to do a task that requires organization? | |||||
| 3. How often do you have problems remembering appointments or obligations? | |||||
| 4. When you have a task that requires a lot of thought, how often do you avoid or delay getting started? | |||||
| 5. How often do you fidget or squirm with your hands or feet when you have to sit down for a long time? | |||||
| 6. How often do you feel overly active and compelled to do things, like you were driven by a motor? | |||||
| Add the number of checkmarks that appear in the darkly shaded area. Four (4) or more checkmarks indicate that your symptoms may be consistent with adult ADHD. It may be beneficial for you to talk with your healthcare provider about an evaluation. | |||||
| * Intended for use by persons age 18 and older ADHD: attention-deficit/hyperactivity disorder; WHO: World Health Organization | |||||
| Source: Reprinted with permission. World Health Organization Copyright 2003. All rights reserved | |||||
CASE CONTINUED: ‘All the time, every day’
Mr. Z completes the ASRS self-report symptom checklist and brings his wife to the next appointment. He rated all 6 screening symptoms and most others as occurring “often” or “very often.” He describes functional impairments “essentially all the time, basically every day” at work, home, and socially. His wife confirms these symptoms and the frustrations and conflicts they have caused.
Mr. Z describes ADHD symptoms from early elementary school to college. He was held back in kindergarten for being “immature,” his academic performance was inconsistent, and he “just got by…by cramming” in high school and college. His school performance pattern does not suggest a learning disability; he did not need special help in 1 subject more than others, and under pressure he could achieve average grades.
Medical review excludes explanations other than ADHD for his inattention, restlessness, and impulsivity. You conclude that Mr. Z meets criteria for ADHD, combined subtype, and discuss medication treatment.
FDA-approved medications
Medication for ADHD is appropriate only if symptoms are impairing. Five effective and generally well-tolerated medications are FDA-approved for adults with ADHD (Table 2):
- extended-release mixed amphetamine (Adderall XR)
- extended-release OROS methylphenidate (Concerta)
- extended-release dexmethylphenidate (Focalin XR)
- atomoxetine (Strattera)
- lisdexamfetamine (Vyvanse).
Efficacy. A meta-analysis of 29 pediatric ADHD trials across 30 years demonstrated greater effect size for stimulant class medications (immediate- and long-acting), compared with nonstimulant medications (including bupropion, atomoxetine, and modafinil).2 This finding is consistent with the American Academy of Child and Adolescent Psychiatry’s recommendation of stimulant medications as first-line agents for pediatric ADHD.3 A similar meta-analysis of 6 controlled studies of methylphenidate-class medications in adults found a large mean effect size (0.9), with greater effects associated with higher doses.4
Atomoxetine, a norepinephrine reuptake inhibitor, is the only nonstimulant medication FDA-approved for ADHD in adults. More than 6,000 children, adolescents, and adults have taken atomoxetine in clinical trials for ADHD (Lilly, prescribing information), with 4 years of open treatment data showing benefit being maintained over time.5
Tolerability. Although ADHD medications are generally well-tolerated by healthy adults, assess for a history of potential contraindications:
- unstable medical condition, hyperthyroidism, glaucoma
- treatment with a monoamine oxidase inhibitor or other pressor agents because of possible effects on blood pressure and heart rate
- use of cytochrome P450 2D6 inhibitors, which may increase atomoxetine steady-state plasma concentrations
- cardiovascular disease or family history of early cardiac disease (Box 1)6,7
- history of or active substance use disorder, such as alcohol dependence, cocaine or heroin abuse
- history of psychosis, bipolar disorder, or an active clinically significant psychiatric comorbidity (major depression, agitated state, suicidality).
Clinically, some patients appear to tolerate 1 class of stimulant (such as methylphenidate or amphetamine) over another. Consider switching to an alternate stimulant if your patient has bothersome side effects—mild low appetite, insomnia, tension, or jitteriness—or has received limited or partial benefit during an initial stimulant trial.
Serious cardiovascular events and sudden death have occurred in adults and children treated with stimulants.6 Agents used for attention-deficit/hyperactivity disorder (ADHD) have not been shown to cause sudden cardiac death, but the FDA requires stimulants’ labeling to warn about this risk in patients with structural cardiac abnormalities. The warning advises against using stimulants in adults with cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease.
When treating adults with ADHD, look to advisories about cardiovascular monitoring in children with ADHD. Before initiating medications, do a physical exam focused on cardiovascular disease risk factors and obtain a patient and family health history of:
- fainting or dizziness
- sudden or unexplained death in someone young
- sudden cardiac death or “heart attack” in family members age <35 years.
The American Academy of Pediatrics, American Academy of Child and Adolescent Psychiatry, and American Heart Association concur that electrocardiography (ECG) is not mandatory in cardiovascular assessment and monitoring during ADHD pharmacotherapy.7 This author (PH) refers cardiovascular questions to a primary care physician or cardiologist.
During ADHD treatment, monitor vital signs and refer patients with emergent cardiac symptoms or concerns to a cardiologist. Expect increases in blood pressure (1 to 4 mm Hg) and heart rate (2 to 6 bpm) during treatment with methylphenidate and amphetamine-class stimulants as well as with atomoxetine. Do not expect significant changes in ECG parameters (PR, QRS, and QTC intervals).
Extended-release formulations. Early adult studies demonstrated the efficacy of immediate-release stimulants, but adults with ADHD’s inherent deficits in organization and memory may have higher adherence rates and greater success with once-daily, extended-release formulations.8-11 Unless your patient wants to begin with small, short-acting dosages (5 to 10 mg) or desires to target treatment to specific times of day (such as in the morning for administrative work only), many appreciate once-daily formulations. Extended-release formulations also may be the simplest stimulants with which to begin ADHD treatment.
Over time, patients may benefit from an immediate-release form:
- added for certain times of day—such as in late afternoon, when the morning extended-release dose has worn off (Box 2)12,13
- to use as an alternative to extended-release formulations when more or less flexibly is desired, such as on weekends.
Table 2
Administering medications approved for adult ADHD
| Drug | Recommended dosage* | Comments |
|---|---|---|
| Stimulants | ||
| Extended-release mixed amphetamine (Adderall XR) | 20 mg | Initial prescription of 10-mg XR capsules allows gradual titration |
| Extended-release OROS methylphenidate (Concerta) | 18 to 72 mg/d | Initial prescription of 18-mg OROS MPH capsules allows gradual titration |
| Extended-release dexmethylphenidate (Focalin XR) | 10 mg/d; maximum 20 mg/d | Dosing is one-half the typical dosing of racemic MPH |
| Lisdexamfetamine (Vyvanse) | 30 mg/d; maximum 70 mg/d | May be adjusted weekly in 10-mg or 20-mg increments |
| Nonstimulant | ||
| Atomoxetine (Strattera) | 80 mg/d; maximum 100 mg/d | Initial dosage of 40 mg/d can be increased to target dosage after a minimum of 3 days; can be given as a morning dose or divided evenly between morning and evening doses |
| * FDA-approved dosages as listed in the package inserts of these medications ADHD: attention-deficit/hyperactivity disorder; MPH: methylphenidate; OROS: osmotic release oral system; XR: extended-release formulation | ||
CASE CONTINUED: Feeling ‘calm, less frenetic’
During the next 6 months, you start Mr. Z on stimulant treatment at robust dosing consistent with his weight (90 kg). He complains that extended-duration methylphenidate (MPH)—titrated to 90 mg/d—doesn’t last into the late afternoon, and he feels mildly tense with a low appetite. Because of an apparent partial response and relatively mild adverse effects, you discontinue MPH and try an extended-duration amphetamine, titrated to 60 mg.
Mr. Z’s blood pressure and heart rate remain stable. He begins to exercise regularly and reduce his use of tobacco and caffeine drinks, as you recommend. He says he feels “calm, less frenetic.” He reports no tension on this medication and only mild reduced appetite. With a plan to continue taking the stimulant medication with regular monitoring, he then disappears from treatment.
Promoting adherence
Treatment nonadherence is an issue throughout medicine, and individuals with disorganization, forgetfulness, and impulsivity may be at higher-than-usual risk of not following through on medication regimens.
Combining short- and long-acting stimulants may cover hours when attention-deficit/hyperactivity (ADHD) symptoms emerge despite therapy with a long-acting agent.12,13 Ask patients who report lack of full-day coverage if the once-daily, extended-duration formulation they are taking works well until a certain time of day. Then consider adding a similar-class immediate-release stimulant at this time to cover the later hours.
If a patient reports partial response throughout the day—such as early in treatment—begin by optimizing the long-acting agent’s dosage. Keep a target daily dose in mind, based on FDA recommendations and clinical trial data. For example, an adult weighing 80 kg may respond optimally to a combination of 60 mg of a long-acting methylphenidate (MPH) in the morning, followed by 10 to 20 mg of an immediate-release MPH in mid-afternoon.
The later stimulants are taken in the day, the more likely insomnia may emerge as an adverse effect. Some patients adjust to this problem within the first weeks of treatment. If insomnia remains impairing, reduce the stimulant dose or consider switching to a shorter duration medication or to the nonstimulant atomoxetine.
In addition, restrictions on stimulant-class medications do not permit multiple-month prescribing (refills), as is allowed with non-scheduled medications such as atomoxetine. Discuss with patients how they will obtain stimulant medications on a regular, monthly or bimonthly basis. In our experience, the practical challenges of remaining in treatment at times may limit patients’ adherence to ADHD medications more than a lack of response or tolerability concerns.
Explain to patients early in treatment that they might need to try several different medications before settling on 1 that is optimally tolerated and efficacious. Because stimulants are generally quite effective for ADHD symptoms, set your goal to identify adverse effects and aim for a patient response of “this works well, and I don’t feel any different on it.”
CASE CONTINUED: Ready to try again
Three years later, Mr. Z returns and reports gradually discontinuing the stimulant because he “wanted to go it on my own.” He functioned relatively well at first, but errors and conflicts at his job led to his dismissal.
Since then, he has been unemployed. He is increasingly depressed and reports drinking and smoking “more heavily than in college.” He asks about resuming ADHD treatment.
Mr. Z does not meet DSM-IV-TR criteria for major depressive disorder or alcohol abuse/dependence. His depressed mood appears to be linked to his marked ADHD symptoms. Mr. Z agrees to a new treatment plan that includes starting atomoxetine at 25 mg to allow for flexible titration and psychotherapy to monitor his mood and achieve sobriety.
ADHD and substance abuse
Clinical judgment determines whether an adult with ADHD and a history of substance use disorders may safely benefit from treatment with a stimulant. The relationship between ADHD and substance use disorders is of clinical concern, but ADHD medications have not been shown to increase risk for later substance use disorders in children.14 Conversely, effective ADHD treatment appears to reduce later cigarette and substance use.15
Consider using a nonstimulant-class medication in adults with ADHD and active substance use disorders. In a 12-week, double-blind, controlled trial, atomoxetine improved ADHD symptoms significantly more than placebo in adults meeting DS-MIV-TR criteria for comorbid alcohol use disorders. After 4 to 30 days of alcohol abstinence, 72 patients were randomly assigned to atomoxetine, 25 to 100 mg/d (mean final dose 90 mg/d), and 75 patients to placebo. Although estimated times to initial relapse to heavy drinking did not differ:
- atomoxetine-treated subjects had 26% fewer cumulative heavy drinking days than placebo-treated subjects (P=0.023)
- the difference in cumulative heavy drinking days between the atomoxetine and placebo groups became statistically significant after 55 days of treatment.16
- World Health Organization Adult Self-Report Scale (ASRS) 18-item instrument and 6-item screener. www.med.nyu.edu/psych/psychiatrist/adhd.html.
- Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 2008;165:553-5.
- Adler LA, Spencer TJ, Levine LR, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord 2008; 11:720-7.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Extended-release mixed amphetamine • Adderall XR
- Extended duration OROS methylphenidate • Concerta
- Extended-release dexmethylphenidate • Focalin XR
- Lisdexamfetamine • Vyvanse
- Modafinil • Provigil
Disclosure
Dr. Hammerness has received research support from and is on the speakers bureau for Shire Pharmaceuticals. He has received support for CME activities and talks from Shire Pharmaceuticals, Ortho-McNeil, and Abbott Laboratories.
Dr. Surman receives research support and/or is a speaker for Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, Ortho-McNeil, Merck, New River Pharmaceuticals, Novartis, Pfizer Inc., Shire Pharmaceuticals, and Takeda Pharmaceutical Company.
Dr. Sassi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Clinical research assistant Katherine Miller, BA, contributed to the literature review for this article and assisted in preparing the manuscript.
Mr. Z, age 42, is referred by his primary care physician with symptoms suggesting attention-deficit/hyperactivity disorder (ADHD). Mr. Z has seen his physician sporadically for 10 years and acknowledges not following dietary and exercise advice. He has had intermittent “minor” depression, is overweight, and is a smoker with a family history of cardiovascular disease and diabetes.
A salesman, Mr. Z recently was promoted to an administrative position that substantially increased his paperwork. He is having difficulty performing his job because of longstanding forgetfulness and disorganization. He says he feels “like I’m in grade school again, lost in paperwork.” He also describes a recent educational assessment for his son, age 7, who may have ADHD. Similarities between Mr. Z’s and his son’s early childhood academic struggles are striking.
Like Mr. Z, adults with ADHD commonly seek treatment when increasing stressors and demands overwhelm their cognitive-attentional abilities. Some may be “healthy” men and women without psychiatric histories, whose disorganization, forgetfulness, or impulsivity contributes to functional impairment, including nonadherence with medical advice. For others, such as those with known psychiatric disorders, ADHD may be a hidden comorbidity contributing to seemingly refractory depression or anxiety disorder.
Despite growing evidence related to adult ADHD, individualizing and maintaining treatment over time can be challenging for clinicians and patients. Fortunately, new tools and multiple stimulant and nonstimulant medications can help you screen for, assess, and treat adult ADHD.
ADHD diagnosis
To diagnose ADHD in an adult patient, first establish that symptoms have existed from childhood to adulthood. One approach is to review DSM-IV-TR criteria for ADHD with your patient and ask him or her to reflect on childhood symptoms and dysfunction. Begin with orienting questions, such as “Do you remember your first grade teacher, your school, where you lived?” ADHD symptoms might have been present even if the patient maintained acceptable grades, particularly in elementary school, as dedicated parents or teachers might have contributed to early academic success.
Next, turn to diagnostic language that captures ADHD symptoms in adults. For example, the 18-item World Health Organization Adult ADHD Self-Report Scale (ASRS-v1.1) prompts individuals to self-report DSM-IV ADHD symptoms, and a 6-item subset (Table 1) is a highly specific screener (see Related Resources). The ASRS is most reliable in adults with limited psychiatric comorbidity.1
Adults often describe fluctuations in symptom severity over time. Symptoms may have less impact with more physically demanding work—such as sales—and greater impact with organizationally demanding work—such as administration.
Base your summary ADHD diagnosis on DSM-IV-TR criteria, including:
- lifetime persistence of symptoms, beginning before age 7
- functional impairment in ≥2 life settings, such as work, school, or home
- lack of another medical or psychiatric condition sufficient to explain the symptoms.
Table 1
Adult Self-Report Scale-v1.1 WHO 6-question screening tool for ADHD*
| Check the box that best describes how you have felt and conducted yourself over the past 6 months. Please give the completed questionnaire to your healthcare professional during your next appointment to discuss the results | Never | Rarely | Sometimes | Often | Very often |
|---|---|---|---|---|---|
| 1. How often do you have trouble wrapping up the final details of a project, once the challenging parts have been done? | |||||
| 2. How often do you have difficulty getting things in order when you have to do a task that requires organization? | |||||
| 3. How often do you have problems remembering appointments or obligations? | |||||
| 4. When you have a task that requires a lot of thought, how often do you avoid or delay getting started? | |||||
| 5. How often do you fidget or squirm with your hands or feet when you have to sit down for a long time? | |||||
| 6. How often do you feel overly active and compelled to do things, like you were driven by a motor? | |||||
| Add the number of checkmarks that appear in the darkly shaded area. Four (4) or more checkmarks indicate that your symptoms may be consistent with adult ADHD. It may be beneficial for you to talk with your healthcare provider about an evaluation. | |||||
| * Intended for use by persons age 18 and older ADHD: attention-deficit/hyperactivity disorder; WHO: World Health Organization | |||||
| Source: Reprinted with permission. World Health Organization Copyright 2003. All rights reserved | |||||
CASE CONTINUED: ‘All the time, every day’
Mr. Z completes the ASRS self-report symptom checklist and brings his wife to the next appointment. He rated all 6 screening symptoms and most others as occurring “often” or “very often.” He describes functional impairments “essentially all the time, basically every day” at work, home, and socially. His wife confirms these symptoms and the frustrations and conflicts they have caused.
Mr. Z describes ADHD symptoms from early elementary school to college. He was held back in kindergarten for being “immature,” his academic performance was inconsistent, and he “just got by…by cramming” in high school and college. His school performance pattern does not suggest a learning disability; he did not need special help in 1 subject more than others, and under pressure he could achieve average grades.
Medical review excludes explanations other than ADHD for his inattention, restlessness, and impulsivity. You conclude that Mr. Z meets criteria for ADHD, combined subtype, and discuss medication treatment.
FDA-approved medications
Medication for ADHD is appropriate only if symptoms are impairing. Five effective and generally well-tolerated medications are FDA-approved for adults with ADHD (Table 2):
- extended-release mixed amphetamine (Adderall XR)
- extended-release OROS methylphenidate (Concerta)
- extended-release dexmethylphenidate (Focalin XR)
- atomoxetine (Strattera)
- lisdexamfetamine (Vyvanse).
Efficacy. A meta-analysis of 29 pediatric ADHD trials across 30 years demonstrated greater effect size for stimulant class medications (immediate- and long-acting), compared with nonstimulant medications (including bupropion, atomoxetine, and modafinil).2 This finding is consistent with the American Academy of Child and Adolescent Psychiatry’s recommendation of stimulant medications as first-line agents for pediatric ADHD.3 A similar meta-analysis of 6 controlled studies of methylphenidate-class medications in adults found a large mean effect size (0.9), with greater effects associated with higher doses.4
Atomoxetine, a norepinephrine reuptake inhibitor, is the only nonstimulant medication FDA-approved for ADHD in adults. More than 6,000 children, adolescents, and adults have taken atomoxetine in clinical trials for ADHD (Lilly, prescribing information), with 4 years of open treatment data showing benefit being maintained over time.5
Tolerability. Although ADHD medications are generally well-tolerated by healthy adults, assess for a history of potential contraindications:
- unstable medical condition, hyperthyroidism, glaucoma
- treatment with a monoamine oxidase inhibitor or other pressor agents because of possible effects on blood pressure and heart rate
- use of cytochrome P450 2D6 inhibitors, which may increase atomoxetine steady-state plasma concentrations
- cardiovascular disease or family history of early cardiac disease (Box 1)6,7
- history of or active substance use disorder, such as alcohol dependence, cocaine or heroin abuse
- history of psychosis, bipolar disorder, or an active clinically significant psychiatric comorbidity (major depression, agitated state, suicidality).
Clinically, some patients appear to tolerate 1 class of stimulant (such as methylphenidate or amphetamine) over another. Consider switching to an alternate stimulant if your patient has bothersome side effects—mild low appetite, insomnia, tension, or jitteriness—or has received limited or partial benefit during an initial stimulant trial.
Serious cardiovascular events and sudden death have occurred in adults and children treated with stimulants.6 Agents used for attention-deficit/hyperactivity disorder (ADHD) have not been shown to cause sudden cardiac death, but the FDA requires stimulants’ labeling to warn about this risk in patients with structural cardiac abnormalities. The warning advises against using stimulants in adults with cardiomyopathy, serious heart rhythm abnormalities, or coronary artery disease.
When treating adults with ADHD, look to advisories about cardiovascular monitoring in children with ADHD. Before initiating medications, do a physical exam focused on cardiovascular disease risk factors and obtain a patient and family health history of:
- fainting or dizziness
- sudden or unexplained death in someone young
- sudden cardiac death or “heart attack” in family members age <35 years.
The American Academy of Pediatrics, American Academy of Child and Adolescent Psychiatry, and American Heart Association concur that electrocardiography (ECG) is not mandatory in cardiovascular assessment and monitoring during ADHD pharmacotherapy.7 This author (PH) refers cardiovascular questions to a primary care physician or cardiologist.
During ADHD treatment, monitor vital signs and refer patients with emergent cardiac symptoms or concerns to a cardiologist. Expect increases in blood pressure (1 to 4 mm Hg) and heart rate (2 to 6 bpm) during treatment with methylphenidate and amphetamine-class stimulants as well as with atomoxetine. Do not expect significant changes in ECG parameters (PR, QRS, and QTC intervals).
Extended-release formulations. Early adult studies demonstrated the efficacy of immediate-release stimulants, but adults with ADHD’s inherent deficits in organization and memory may have higher adherence rates and greater success with once-daily, extended-release formulations.8-11 Unless your patient wants to begin with small, short-acting dosages (5 to 10 mg) or desires to target treatment to specific times of day (such as in the morning for administrative work only), many appreciate once-daily formulations. Extended-release formulations also may be the simplest stimulants with which to begin ADHD treatment.
Over time, patients may benefit from an immediate-release form:
- added for certain times of day—such as in late afternoon, when the morning extended-release dose has worn off (Box 2)12,13
- to use as an alternative to extended-release formulations when more or less flexibly is desired, such as on weekends.
Table 2
Administering medications approved for adult ADHD
| Drug | Recommended dosage* | Comments |
|---|---|---|
| Stimulants | ||
| Extended-release mixed amphetamine (Adderall XR) | 20 mg | Initial prescription of 10-mg XR capsules allows gradual titration |
| Extended-release OROS methylphenidate (Concerta) | 18 to 72 mg/d | Initial prescription of 18-mg OROS MPH capsules allows gradual titration |
| Extended-release dexmethylphenidate (Focalin XR) | 10 mg/d; maximum 20 mg/d | Dosing is one-half the typical dosing of racemic MPH |
| Lisdexamfetamine (Vyvanse) | 30 mg/d; maximum 70 mg/d | May be adjusted weekly in 10-mg or 20-mg increments |
| Nonstimulant | ||
| Atomoxetine (Strattera) | 80 mg/d; maximum 100 mg/d | Initial dosage of 40 mg/d can be increased to target dosage after a minimum of 3 days; can be given as a morning dose or divided evenly between morning and evening doses |
| * FDA-approved dosages as listed in the package inserts of these medications ADHD: attention-deficit/hyperactivity disorder; MPH: methylphenidate; OROS: osmotic release oral system; XR: extended-release formulation | ||
CASE CONTINUED: Feeling ‘calm, less frenetic’
During the next 6 months, you start Mr. Z on stimulant treatment at robust dosing consistent with his weight (90 kg). He complains that extended-duration methylphenidate (MPH)—titrated to 90 mg/d—doesn’t last into the late afternoon, and he feels mildly tense with a low appetite. Because of an apparent partial response and relatively mild adverse effects, you discontinue MPH and try an extended-duration amphetamine, titrated to 60 mg.
Mr. Z’s blood pressure and heart rate remain stable. He begins to exercise regularly and reduce his use of tobacco and caffeine drinks, as you recommend. He says he feels “calm, less frenetic.” He reports no tension on this medication and only mild reduced appetite. With a plan to continue taking the stimulant medication with regular monitoring, he then disappears from treatment.
Promoting adherence
Treatment nonadherence is an issue throughout medicine, and individuals with disorganization, forgetfulness, and impulsivity may be at higher-than-usual risk of not following through on medication regimens.
Combining short- and long-acting stimulants may cover hours when attention-deficit/hyperactivity (ADHD) symptoms emerge despite therapy with a long-acting agent.12,13 Ask patients who report lack of full-day coverage if the once-daily, extended-duration formulation they are taking works well until a certain time of day. Then consider adding a similar-class immediate-release stimulant at this time to cover the later hours.
If a patient reports partial response throughout the day—such as early in treatment—begin by optimizing the long-acting agent’s dosage. Keep a target daily dose in mind, based on FDA recommendations and clinical trial data. For example, an adult weighing 80 kg may respond optimally to a combination of 60 mg of a long-acting methylphenidate (MPH) in the morning, followed by 10 to 20 mg of an immediate-release MPH in mid-afternoon.
The later stimulants are taken in the day, the more likely insomnia may emerge as an adverse effect. Some patients adjust to this problem within the first weeks of treatment. If insomnia remains impairing, reduce the stimulant dose or consider switching to a shorter duration medication or to the nonstimulant atomoxetine.
In addition, restrictions on stimulant-class medications do not permit multiple-month prescribing (refills), as is allowed with non-scheduled medications such as atomoxetine. Discuss with patients how they will obtain stimulant medications on a regular, monthly or bimonthly basis. In our experience, the practical challenges of remaining in treatment at times may limit patients’ adherence to ADHD medications more than a lack of response or tolerability concerns.
Explain to patients early in treatment that they might need to try several different medications before settling on 1 that is optimally tolerated and efficacious. Because stimulants are generally quite effective for ADHD symptoms, set your goal to identify adverse effects and aim for a patient response of “this works well, and I don’t feel any different on it.”
CASE CONTINUED: Ready to try again
Three years later, Mr. Z returns and reports gradually discontinuing the stimulant because he “wanted to go it on my own.” He functioned relatively well at first, but errors and conflicts at his job led to his dismissal.
Since then, he has been unemployed. He is increasingly depressed and reports drinking and smoking “more heavily than in college.” He asks about resuming ADHD treatment.
Mr. Z does not meet DSM-IV-TR criteria for major depressive disorder or alcohol abuse/dependence. His depressed mood appears to be linked to his marked ADHD symptoms. Mr. Z agrees to a new treatment plan that includes starting atomoxetine at 25 mg to allow for flexible titration and psychotherapy to monitor his mood and achieve sobriety.
ADHD and substance abuse
Clinical judgment determines whether an adult with ADHD and a history of substance use disorders may safely benefit from treatment with a stimulant. The relationship between ADHD and substance use disorders is of clinical concern, but ADHD medications have not been shown to increase risk for later substance use disorders in children.14 Conversely, effective ADHD treatment appears to reduce later cigarette and substance use.15
Consider using a nonstimulant-class medication in adults with ADHD and active substance use disorders. In a 12-week, double-blind, controlled trial, atomoxetine improved ADHD symptoms significantly more than placebo in adults meeting DS-MIV-TR criteria for comorbid alcohol use disorders. After 4 to 30 days of alcohol abstinence, 72 patients were randomly assigned to atomoxetine, 25 to 100 mg/d (mean final dose 90 mg/d), and 75 patients to placebo. Although estimated times to initial relapse to heavy drinking did not differ:
- atomoxetine-treated subjects had 26% fewer cumulative heavy drinking days than placebo-treated subjects (P=0.023)
- the difference in cumulative heavy drinking days between the atomoxetine and placebo groups became statistically significant after 55 days of treatment.16
- World Health Organization Adult Self-Report Scale (ASRS) 18-item instrument and 6-item screener. www.med.nyu.edu/psych/psychiatrist/adhd.html.
- Volkow ND, Swanson JM. Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 2008;165:553-5.
- Adler LA, Spencer TJ, Levine LR, et al. Functional outcomes in the treatment of adults with ADHD. J Atten Disord 2008; 11:720-7.
Drug brand names
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Extended-release mixed amphetamine • Adderall XR
- Extended duration OROS methylphenidate • Concerta
- Extended-release dexmethylphenidate • Focalin XR
- Lisdexamfetamine • Vyvanse
- Modafinil • Provigil
Disclosure
Dr. Hammerness has received research support from and is on the speakers bureau for Shire Pharmaceuticals. He has received support for CME activities and talks from Shire Pharmaceuticals, Ortho-McNeil, and Abbott Laboratories.
Dr. Surman receives research support and/or is a speaker for Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, Ortho-McNeil, Merck, New River Pharmaceuticals, Novartis, Pfizer Inc., Shire Pharmaceuticals, and Takeda Pharmaceutical Company.
Dr. Sassi reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgement
Clinical research assistant Katherine Miller, BA, contributed to the literature review for this article and assisted in preparing the manuscript.
1. Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005;35:245-56.
2. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed 2006;8(4):4.-
3. Greenhill L, Pliszka S, Dulcan M, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2001;40(11):1352-5.
4. Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2004;24:24-9.
5. Adler LA, Spencer TJ, Williams DW, et al. Long-term, open-label safety and efficacy of atomoxetine in adults with ADHD: final report of a 4-year study. J Atten Disord Epub 2008 April 30.
6. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med 2006;354:1445-8.
7. American Academy of Pediatrics/American Heart Association clarification of statement on cardiovascular evaluation and monitoring of children and adolescents with heart disease receiving medications for ADHD May 16, 2008. Available at: http://www.aap.org/pressroom/aap-ahastatement.htm. Accessed August 14, 2008.
8. Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2006;59(9):829-35.
9. Biederman J, Mick E, Surman C, et al. Comparative acute efficacy and tolerability of OROS and immediate release formulations of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2007;7:49.-
10. Mick E, Spencer TJ, Surman C, et al. Randomized single-blind substitution study of methylphenidate in ADHD adults receiving immediate-release methylphenidate. NR357. Poster presented at: Annual Meeting of the American Psychiatric Association; May 19-24, 2007; San Diego, CA.
11. Capone N, McDonnel T. Medication persistence among agents used to treat attention-deficit/hyperactivity disorder, diabetes, and elevated serum cholesterol. NR 639. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
12. Adler L, Morrill M, Reingold B. d-methylphenidate augmentation of extended-release stimulant therapy in ADHD. NR 619. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
13. Adler L, Reingold LS, Morrill MS, Wilens TE. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep 2006;8:409-15.
14. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry 2008;165:597-603.
15. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 2007;68(suppl 11):15-22.
16. Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 2008;96:145-54.
1. Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 2005;35:245-56.
2. Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed 2006;8(4):4.-
3. Greenhill L, Pliszka S, Dulcan M, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2001;40(11):1352-5.
4. Faraone SV, Spencer T, Aleardi M, et al. Meta-analysis of the efficacy of methylphenidate for treating adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2004;24:24-9.
5. Adler LA, Spencer TJ, Williams DW, et al. Long-term, open-label safety and efficacy of atomoxetine in adults with ADHD: final report of a 4-year study. J Atten Disord Epub 2008 April 30.
6. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med 2006;354:1445-8.
7. American Academy of Pediatrics/American Heart Association clarification of statement on cardiovascular evaluation and monitoring of children and adolescents with heart disease receiving medications for ADHD May 16, 2008. Available at: http://www.aap.org/pressroom/aap-ahastatement.htm. Accessed August 14, 2008.
8. Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2006;59(9):829-35.
9. Biederman J, Mick E, Surman C, et al. Comparative acute efficacy and tolerability of OROS and immediate release formulations of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. BMC Psychiatry 2007;7:49.-
10. Mick E, Spencer TJ, Surman C, et al. Randomized single-blind substitution study of methylphenidate in ADHD adults receiving immediate-release methylphenidate. NR357. Poster presented at: Annual Meeting of the American Psychiatric Association; May 19-24, 2007; San Diego, CA.
11. Capone N, McDonnel T. Medication persistence among agents used to treat attention-deficit/hyperactivity disorder, diabetes, and elevated serum cholesterol. NR 639. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
12. Adler L, Morrill M, Reingold B. d-methylphenidate augmentation of extended-release stimulant therapy in ADHD. NR 619. Poster presented at: Annual Meeting of the American Psychiatric Association; May 20-25, 2006; Toronto, Ontario, Canada.
13. Adler L, Reingold LS, Morrill MS, Wilens TE. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep 2006;8:409-15.
14. Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry 2008;165:597-603.
15. Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 2007;68(suppl 11):15-22.
16. Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 2008;96:145-54.
‘I’m sober, Doctor, really’: Best biomarkers for underreported alcohol use
Hospitalized patients who are not truthful about their alcohol consumption may be at risk for an unplanned withdrawal. Self-reports of alcohol use—such as CAGE and the Alcohol Use Disorders Identification Test (AUDIT)—are valid, inexpensive, and noninvasive, but patients easily can feign results.1 Biochemical measures are more objective, and combinations of markers are an effective tool to detect recent heavy drinking in the 10% to 25% of patients who underreport alcohol use.2
Biochemical measures can detect acute alcohol intoxication and recent prolonged drinking. Because marker levels return to normal after long-term abstinence, ongoing monitoring can help detect a relapse before a patient admits to it.3
This article presents 3 cases in which biochemical markers helped prevent alcohol withdrawal in patients who denied alcohol abuse. We discuss why we ordered biochemical tests and which combinations provided highly sensitive results.
CASE 1: Depression and substance abuse
Ms. C, age 39, presents with bleeding gums due to excessive warfarin, which she takes prophylactically for a history of deep vein thrombosis. She is seen by the psychiatric consultation service for depression—which she says she has experienced since “the day I was born”—and substance abuse that includes a history binge drinking. Ms. C says she has stopped drinking and remained abstinent for the past year because she is fearful of further damaging her kidneys. She also denies psychosis. She does not have a history or symptoms of hepatobiliary or hematologic disease.
Challenge. Despite Ms. C’s self-reported 1 year of sobriety, her history of binge drinking and depression calls for evaluating her alcohol withdrawal risk. Laboratory markers of alcohol abuse are the only means to assess her recent drinking behavior.
Discussion. Lab results include serum albumin of 3.4 g/dL, total bilirubin of 0.3 mg/dL, total protein of 6.3 g/dL, aspartate aminotransferase (AST) of 13 U/L, alanine aminotransferase (ALT) of 19 U/L, alkaline phosphatase of 136 U/L, and blood ammonia level of 37 μg/dL. Gamma-glutamyl transferase (GGT) is elevated at 104 U/L (normal range for women: 0 to 45 U/L). Mean corpuscular volume (MCV) is elevated at 101 fL (normal range 80 to 100 fL).
The combination of elevated MCV and GGT has a 95% sensitivity for alcohol abuse.4 GGT levels become elevated after 24 hours to 2 weeks of heavy alcohol consumption and return to normal within 2 to 6 weeks of abstinence, which allows them to detect binge drinking. MCV takes 6 to 8 weeks of heavy drinking—we which we define as consuming ≥40 grams of alcohol/day5—to become elevated and returns to normal within 3 months of abstinence.
These data provide evidence that Ms. C recently consumed substantial amounts of alcohol. As a result, we start her on alcohol withdrawal precautions (AWP).
Markers of alcohol abuse
Biochemical markers commonly used to detect alcohol abuse (Table 1) include:
- blood alcohol level (BAL)
- MCV
- liver function tests (LFTs) such as ALT, AST, and GGT
- carbohydrate deficient transferrin (CDT).
Table 1
By the numbers: Biomarkers of excessive alcohol consumption
| Biomarker | |||||
|---|---|---|---|---|---|
| CDT | GGT | AST | ALT | MCV | |
| Blood test normal range | Women: 0 to 45 U/L Men: 0 to 53 U/L | 10 to 34 U/L | 8 to 37 U/L | 80 to 100 fL | |
| Blood test abnormal range | >1.3% of total transferrin concentration | Women: >45 U/L Men: >53 U/L | Levels rarely exceed 500 U/L | Levels rarely exceed 300 U/L | >100 fL |
| Time to elevation | 2 to 3 weeks | 24 hours to 2 weeks | 3 to 7 days | 3 to 7 days | After 6 weeks |
| Time to descent to normal levels | 2 to 4 weeks of abstinence | 2 to 6 weeks of abstinence | Half-life 12 to 24 hours | Half-life 37 to 57 hours | 3 months |
| Dose-response of alcohol | 60 g/d | 80 to 200 g/d | ≥40 g/d | ≥40 g/d | ≥40 g/d |
| Sensitivity | 55% to 90%a-e | 37% to 85%b, f, g | AST:ALT ratio >2:1 has a 70% sensitivity and 92% to 100% specificity for alcoholic-induced liver diseaseh-j | 20% to 70%b,k | |
| Relapse sensitivity | 55% to 76%a,l,m | 50%a,e | 20%a,n | ||
| Specificity | 92% to 97%a,b | 18% to 93%a,b,e | 64% to 66%b,k,n | ||
| Positive predictive value | 46% to 75%c,g | 41%g | 36%g | ||
| Negative predictive value | 72% to 98%a,c,g | 69% to 92%a,e,g | 67%g | ||
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | |||||
| Source: Reference Citations: click here | |||||
BAL can document acute alcohol intoxication, but its use is limited because alcohol has a 4-hour half-life and an elimination rate of 7 grams/hour—equivalent to 1 drink/hour.6 (A “drink” typically is defined as a 12-ounce bottle of beer or wine cooler, a 5-ounce glass of wine, or 1.5 ounces of 80-proof distilled spirits.) Therefore, BAL will identify as false negatives alcohol-dependent patients who abstain from alcohol within 24 hours of testing.
MCV is an index of the average volume of erythrocytes. Macrocytosis occurs when the volume exceeds 100 fL. Elevated MCV is the most typical morphologic abnormality associated with excessive alcohol consumption7,8 and macrocytosis—sometimes without associated anemia—is often evident in persons with alcoholism. MCV elevates after 6 weeks of alcohol misuse and may remain elevated for up to 3 months after a person has stopped drinking.9
Because patients with disorders unrelated to alcohol use can have elevated MCV, alone it is not a useful screening marker for alcohol abuse.10 Additionally, because macrocytosis can persist under strictly controlled alcohol abstinence, MCV is not a reliable clinical indicator of relapse.11
LFTs measure enzymes and proteins. ALT, AST, and GGT are the most relevant for detecting heavy drinking. An AST:ALT ratio >2:1 supports a suspicion of alcohol abuse.12 More than 90% of patients with an AST:ALT ratio of 2:1 have alcoholic liver disease. This increases to more than 96% if the ratio is 3:1.13
GGT is an enzyme concentrated in the liver, bile ducts, and kidneys; normal range is 0 to 45 U/L (for females) or 53 U/L (for males).14 GGT levels >30 U/L correlate with alcohol consumption of >4 drinks per day.15 GGT has a half-life of 14 to 26 days and remains elevated for 4 to 6 weeks after drinking cessation, which make it useful for monitoring abstinence in treatment programs.16 Sensitivity ranges from 37% to 85% and specificity is as high as 93% in nonmedical populations.17 Although nonalcoholic liver disease can elevate GGT in persons who do not abuse alcohol, 50% to 72% of GGT elevations can be explained by excessive alcohol consumption.18
CDT is a newer biomarker used to monitor alcohol consumption. The most accurate way to express CDT level is as a percentage of total transferrin concentration. This method accounts for individual variations in transferrin levels, thus minimizing false positives.18 In persons who consume >4 or 5 drinks per day for 2 weeks or more, CDT is >1.3% of total transferrin.19 Unfortunately, because it is expensive and requires sophisticated test methodology, CDT testing is not available at most hospitals.20
Combinations improve detection
Each biochemical measure has strengths and weaknesses as a marker for determining patients’ alcohol consumption (Table 2). CDT and GGT show the highest sensitivity for heavy drinking, and CDT has a higher specificity than GGT (Table 3).21,22 Relapse to alcohol use after abstinence may be best identified by a simultaneous 30% increase in CDT and GGT.5
Because GGT has a longer half-life than CDT, its diagnostic efficiency in detecting alcohol relapse may not develop until 4 weeks after alcohol detoxification, whereas CDT may become clinically useful for detecting relapse as early as 1 week after detoxification.23
Table 2
Biomarkers of alcohol use: Strengths and weaknesses
| Biomarker | Strengths | Weaknesses |
|---|---|---|
| CDT | High specificity for alcohol use; few factors cause false positives High sensitivity in distinguishing alcoholics from social drinkers Marker of relapse and abstinence from drinking Confirmatory test for patients suspected of alcohol abuse | Low sensitivity; more valuable to confirm than exclude heavy drinking Cost (average $30/assay) and low availability of testing Likely less sensitive for women and younger patients compared with men Poor screening tool for alcohol use in general population |
| GGT | Elevations precede alcohol-induced liver damage High specificity in patients with suspected alcohol abuse Effective marker for patients suspected of binge drinking Inexpensive ( | Can be falsely elevated by liver and biliary disease, smoking, obesity, and medications that induce microsomal enzymes Low sensitivity makes it a poor screening tool in general population Poor marker of relapse |
| AST:ALT >2:1 | Highly sensitive and specific for alcohol-induced liver damage | Enzyme elevations can be detected only after periods of heavy drinking Elevations secondary to liver damage at the hepatocellular level (after fatty changes) |
| MCV | Accuracy similar in male and female patients Elevations in suspected cases of alcohol use indicate chronicity of drinking Routine laboratory test | Poor biomarker for relapse False positives caused by liver disease, hemolysis, bleeding disorders, anemia, folate deficiency, and medications that reduce folate Low sensitivity and specificity for alcohol use make it a poor screening tool for alcohol abuse |
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | ||
Table 3
Interpreting diagnostic test performance
| Term | Definition | Applicability |
|---|---|---|
| Sensitivity | Percent of persons with disease who test positive | High value is desirable for ruling out disease (low false-negative rate) |
| Specificity | Percent of persons without disease who test negative | High value is desirable for ruling in disease (low false-positive rate) |
| Positive predictive value | Percent of positive test results that are true positives | Probability that a person with a positive test result has the disease |
| Negative predictive value | Percent of negative test results that are true negatives | Probability that a person with a negative test result is disease-free |
| Source: References 21,22 | ||
There is evidence that combining tests can improve alcohol use detection.24 For example, Dolman et al25 found that the ability of the AUDIT questionnaire to correctly predict which patients would experience alcohol withdrawal increases when it is used in combination with biochemical markers. Specifically, the positive predictive value of an AUDIT score ≥8 increased from 17% to 47% when found in combination with ≥2 abnormal biochemical marker levels; the study looked at GGT, ALT, AST, and MCV. Sensitivity was 94% and specificity was 98%.
Similarly, combinations of biochemical markers—especially CDT and GGT—have improved detection of alcohol use and subsequent risk of withdrawal.26Table 4 provides a summary of studies that evaluated using combinations of biochemical markers.4,5,27-31
Table 4
Combining biomarker tests: An effective approach
| Combination | Study | Sensitivity* |
|---|---|---|
| GGT + MCV | Morgan et al4 | 95% |
| GGT + CDT | Hietala et al5 | 90% |
| Mundle et al29 | 90% | |
| Bell et al30 | 90% | |
| Sillanaukee et al31 | 95% | |
| GGT + AST:ALT >2:1 | Gluud et al27 | 92% |
| Morgan et al4 | 100% | |
| MCV + AST:ALT >2:1 | Kawachi et al28 | 97% |
| Morgan et al4 | 95% | |
| GGT + MCV + AST:ALT >2:1 | Morgan et al4 | 100% |
| GGT + MCV + CDT | Sillanaukee et al31 | 70% |
| * Sensitivity for detecting excessive alcohol consumption | ||
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | ||
Consider patients’ comorbidities
Patients at risk for underreporting alcohol use include those with unemployment histories, previous alcohol treatment, and higher scores on the Alcohol Dependence Scale (18.5, SD=8.1).2 Interpret biochemical testing results in the context of a patient’s overall clinical picture.
The following 2 case patients denied or underreported recent alcohol use but we determined they were at high risk for an alcohol disorder because of their medical and/or psychiatric histories. Analysis of biochemical markers helped assess the risk of alcohol withdrawal.
CASE 2: Altered mental status
Family members bring Mr. N, age 44, to the hospital because of his odd behavior. He presents with paranoid delusions and an inappropriate elated mood. His medical history includes acquired immune deficiency syndrome (AIDS). After cerebrospinal fluid analysis, computed tomography of the head, electroencephalogram, and metabolic workup are within normal limits, the patient is diagnosed with human immunodeficiency virus (HIV) mania and is admitted.
On admission, Mr. N denies alcohol use. A blood alcohol/urine toxicity screen is negative. One day after admission, Mr. M develops elevated blood pressure and tachycardia and reports headache and nausea.
Challenge. Gathering a valid history of Mr. N’s alcohol use is difficult because of his acutely altered mental status and manic-like state. We use laboratory data to assess his risk of alcohol withdrawal. His liver function tests include an AST of 33 U/L, ALT of 30 U/L, and an alkaline phosphatase of 94 U/L. MCV is normal at 90 fL. Interestingly, the GGT level is elevated almost 4 times normal at 164 U/L.
Discussion. Although Mr. N denied alcohol use and presented with a negative BAL, laboratory data support alcohol dependence. His GGT was elevated well beyond normal limits, without evidence of hepatobiliary disease. GGT has a sensitivity as high as 85%32 and limited specificity for alcohol abuse. Because of his high probability of recent alcohol consumption, we place Mr. N on AWP.
We postulate that our patient’s autonomic instability, headache, and nausea are related to alcohol withdrawal. We are aware that delirium occurs frequently in patients with HIV infection, and although Mr. N’s medical workup is negative, HIV infection can produce an acute encephalopathy that could resemble our patient’s clinical picture.33
Mr. N’s autonomic instability, headache, and nausea abated after treatment for alcohol withdrawal.
CASE 3: Suicide attempt?
Mr. S, age 28, presents to the trauma service with a self-inflicted gunshot wound to the face. He reports feeling depressed for the last year but denies a history of psychotic symptoms or heroin withdrawal symptoms. He also denies recent or past alcohol abuse and does not have a history of biliary tract disease or megaloblastic anemia. His mother tells us Mr. S has had a history of depression since childhood.
Challenge. Based on Mr. S’ apparent suicide attempt and history, we feel he is at high risk for alcohol abuse. We use laboratory markers to assess the likelihood of alcohol consumption and possibly decrease his risk of alcohol withdrawal.
Discussion. Mr. S’ lab data show an MCV of 91 fL, AST of 95 U/L, alanine ALT of 156 U/L, and alkaline phosphatase of 160 U/L. GGT was elevated at 122 U/L.
Although Mr. S’ MCV is within the normal range, his GGT is elevated, and the combination of an elevated GGT and MCV has a 95% sensitivity for the diagnosis of alcohol abuse. We place Mr. S on alcohol withdrawal precautions and discuss with him the potential life-threatening complications of alcohol withdrawal. Confronted with this information and the possible implication of his elevated LFTs, the patient admits his alcohol history—which consists of drinking 12 beers/day for at least the past 2 years. He admits this despite exhibiting no signs or symptoms of alcohol withdrawal.
Related Resources
- National Institute on Alcohol Abuse and Alcoholism Data/Statistical Tables. www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts.
- Maisto SA, Connors GJ, Allen JP. Contrasting self-report screens for alcohol problems: a review. Alcohol Clin Exp Res 1995;19(6):1510-6.
- Coulton S, Drummond C, James D, et al. Opportunistic screening for alcohol use disorders in primary care: comparative study. BMJ 2006;332:511-7.
Bottom line
Because CDT—the most accurate biomarker—is not available at most hospitals, we recommend using combinations of other measures to detect unreported recent alcohol consumption. If GGT and MCV are elevated, GGT is elevated and AST:ALT is >2:1, or MCV is elevated and AST:ALT is >2:1, consider initiating alcohol withdrawal precautions.
Acknowledgement
The authors acknowledge Daiana Radac, BA, a third-year medical student at Eastern Virginia Medical School, for her contributions to this article.
1. Allen JP, Anthenelli RM. Getting to the bottom of problem drinking: the case for routine screening. Current Psychiatry 2003;2(6):26-44.
2. Killeen TK, Brady KT, Gold PB, et al. Comparison of self-report versus agency records of service utilization in a community sample of individuals with alcohol use disorders. Drug Alcohol Depend 2004;73(2):141-7.
3. Alcohol withdrawal syndrome: how to predict, prevent, diagnose and treat it. Prescrire Int 2007;16(87):24-31.
4. Morgan MY, Colman JC, Sherlock S. The use of a combination of peripheral markers for diagnosing alcoholism and monitoring for continued abuse. Alcohol Alcohol 1981;16:167-77.
5. Hietala J, Koivisto H, Anttila P, et al. Comparison of the combined marker GGT-CDT and the conventional laboratory markers of alcohol abuse in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol 2006;41(5):528-33.
6. Swift R. Direct measurement of alcohol and its metabolites. Addiction 2003;98:73-80.
7. Koivisto H, Hietala J, Anttila P, et al. Long-term ethanol consumption and macrocytosis: diagnostic and pathogenic implications. J Lab Clin Med 2005;147(4):191-6.
8. Savage DG, Ogundipe A, Allen RH, et al. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci 2000;319(6):343-52.
9. Gordon H. Detection of alcoholic liver disease. World J Gastroenterol 2001;7(3):297-302.
10. Bernadt M, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet 1982;1:325-8.
11. Hasselblatt M, Martin F, Maul O, et al. Persistent macrocytosis following abstinence from chronic alcohol use. JAMA 2001;286:2946.-
12. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1-9.
13. Fancher T, Kamboj A, Onate J. Interpreting liver function tests. Current Psychiatry 2007;6(5):61-8.
14. Puukka K, Hietala J, Koivisto H, et al. Obesity and the clinical use of serum GGT activity as a marker of heavy drinking. Scand J Clin Lab Invest 2007;67(5):480-8.
15. Litten RZ, Allen JP, Fertig JB. y-glutamyl transpeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
16. National Institute on Alcohol Abuse and Alcoholism. Screening for alcohol problems—an update. Alcohol Alert No 56 Available at: http://pubs.niaaa.nih.gov/publications/aa56.htm. Accessed May 5, 2007.
17. DiMartini A. A clinical guide to assessing alcohol use and problems. Available at: http://www.alcoholmedicalscholars.org/clin-asmt.ppt. Accessed June 30, 2008.
18. Wolff K, Marshall E. Biological markers of alcohol use. Psychiatry 2006;5(12):437-8.
19. ARUP Laboratories. Carbohydrate-deficient transferrin (CDT) for alcohol use. 2006. Available at: http://www.aruplab.com/TestDirectory/resources/TechnicalBulletins/Carbohydrate-Deficient%20Transferrin%20(CDT)%20Mar%202006.pdf. Accessed July 30, 2008.
20. Allen JP, Litten RZ. The role of laboratory testing in alcoholism treatment. J Subst Abuse Treat 2001;20:81-5.
21. Bhushan V, Le T, Ozturk A, et al. Behavioral Science. In: Le T, Bhushan V, Rao DA, eds. First aid for the USMLE step 1: a student to student guide. New York, NY: McGraw Hill Medical Publishing Division; 2007.
22. Miller PM, Anton RF. Biochemical alcohol screening in primary care. Addict Behav 2004;29(7):1427-37.
23. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
24. Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999;19(3):261-71.
25. Dolman JM, Hawkes ND. Combining the AUDIT questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical patients. Alcohol Alcohol 2005;40(6):515-9.
26. Helander A, Carlsson AV, Borg S. Longitudinal comparison of carbohydrate-deficient transferrin and gamma-glutamyl transferase: complementary markers of excessive alcohol consumption. Alcohol Alcohol 1996;31(1):101-7.
27. Gluud C, Andersen I, Dietrichson O, et al. Gamma-glutamyltransferase, aspartate aminotransferase and alkaline phosphatase as markers of alcohol consumption in out-patient alcoholics. Eur J Clin Invest 1981;11(3):171-6.
28. Kawachi I, Robinson GM, Stace NH. A combination of raised serum AST: ALT ratio and erythrocyte mean cell volume level detects excessive alcohol consumption. N Z Med J 1990;103(887):145-8.
29. Mundle G, Ackerman K, Mann K. Biological markers as indicators for relapse in alcohol-dependent patients. Addict Biol 1999;4(2):209-14.
30. Bell H, Tallaksen C, Sjåheim T, et al. Serum carbohydrate-deficient transferrin as a marker of alcohol consumption in patients with chronic liver diseases. Alcohol Clin Exp Res 1993;17(2):246-52.
31. Sillanaukee P, Aalto M, Seppa K. Carbohydrate-deficient transferrin and conventional alcohol markers as indicators for brief intervention among heavy drinkers in primary health care. Alcohol Clin Exp Res 1998;22(4):892-6.
32. Salaspuro S. Conventional and coming laboratory markers of alcoholism and heavy drinking. Alcohol Clin Exp Res 1986;10(6 suppl):5-12.
33. Della Penna ND, Treisman GJ. HIV/AIDS. In: Levenson J, ed. Essentials of psychosomatic medicine. Washington, DC: American Psychiatric Publishing, Inc; 2007.
Hospitalized patients who are not truthful about their alcohol consumption may be at risk for an unplanned withdrawal. Self-reports of alcohol use—such as CAGE and the Alcohol Use Disorders Identification Test (AUDIT)—are valid, inexpensive, and noninvasive, but patients easily can feign results.1 Biochemical measures are more objective, and combinations of markers are an effective tool to detect recent heavy drinking in the 10% to 25% of patients who underreport alcohol use.2
Biochemical measures can detect acute alcohol intoxication and recent prolonged drinking. Because marker levels return to normal after long-term abstinence, ongoing monitoring can help detect a relapse before a patient admits to it.3
This article presents 3 cases in which biochemical markers helped prevent alcohol withdrawal in patients who denied alcohol abuse. We discuss why we ordered biochemical tests and which combinations provided highly sensitive results.
CASE 1: Depression and substance abuse
Ms. C, age 39, presents with bleeding gums due to excessive warfarin, which she takes prophylactically for a history of deep vein thrombosis. She is seen by the psychiatric consultation service for depression—which she says she has experienced since “the day I was born”—and substance abuse that includes a history binge drinking. Ms. C says she has stopped drinking and remained abstinent for the past year because she is fearful of further damaging her kidneys. She also denies psychosis. She does not have a history or symptoms of hepatobiliary or hematologic disease.
Challenge. Despite Ms. C’s self-reported 1 year of sobriety, her history of binge drinking and depression calls for evaluating her alcohol withdrawal risk. Laboratory markers of alcohol abuse are the only means to assess her recent drinking behavior.
Discussion. Lab results include serum albumin of 3.4 g/dL, total bilirubin of 0.3 mg/dL, total protein of 6.3 g/dL, aspartate aminotransferase (AST) of 13 U/L, alanine aminotransferase (ALT) of 19 U/L, alkaline phosphatase of 136 U/L, and blood ammonia level of 37 μg/dL. Gamma-glutamyl transferase (GGT) is elevated at 104 U/L (normal range for women: 0 to 45 U/L). Mean corpuscular volume (MCV) is elevated at 101 fL (normal range 80 to 100 fL).
The combination of elevated MCV and GGT has a 95% sensitivity for alcohol abuse.4 GGT levels become elevated after 24 hours to 2 weeks of heavy alcohol consumption and return to normal within 2 to 6 weeks of abstinence, which allows them to detect binge drinking. MCV takes 6 to 8 weeks of heavy drinking—we which we define as consuming ≥40 grams of alcohol/day5—to become elevated and returns to normal within 3 months of abstinence.
These data provide evidence that Ms. C recently consumed substantial amounts of alcohol. As a result, we start her on alcohol withdrawal precautions (AWP).
Markers of alcohol abuse
Biochemical markers commonly used to detect alcohol abuse (Table 1) include:
- blood alcohol level (BAL)
- MCV
- liver function tests (LFTs) such as ALT, AST, and GGT
- carbohydrate deficient transferrin (CDT).
Table 1
By the numbers: Biomarkers of excessive alcohol consumption
| Biomarker | |||||
|---|---|---|---|---|---|
| CDT | GGT | AST | ALT | MCV | |
| Blood test normal range | Women: 0 to 45 U/L Men: 0 to 53 U/L | 10 to 34 U/L | 8 to 37 U/L | 80 to 100 fL | |
| Blood test abnormal range | >1.3% of total transferrin concentration | Women: >45 U/L Men: >53 U/L | Levels rarely exceed 500 U/L | Levels rarely exceed 300 U/L | >100 fL |
| Time to elevation | 2 to 3 weeks | 24 hours to 2 weeks | 3 to 7 days | 3 to 7 days | After 6 weeks |
| Time to descent to normal levels | 2 to 4 weeks of abstinence | 2 to 6 weeks of abstinence | Half-life 12 to 24 hours | Half-life 37 to 57 hours | 3 months |
| Dose-response of alcohol | 60 g/d | 80 to 200 g/d | ≥40 g/d | ≥40 g/d | ≥40 g/d |
| Sensitivity | 55% to 90%a-e | 37% to 85%b, f, g | AST:ALT ratio >2:1 has a 70% sensitivity and 92% to 100% specificity for alcoholic-induced liver diseaseh-j | 20% to 70%b,k | |
| Relapse sensitivity | 55% to 76%a,l,m | 50%a,e | 20%a,n | ||
| Specificity | 92% to 97%a,b | 18% to 93%a,b,e | 64% to 66%b,k,n | ||
| Positive predictive value | 46% to 75%c,g | 41%g | 36%g | ||
| Negative predictive value | 72% to 98%a,c,g | 69% to 92%a,e,g | 67%g | ||
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | |||||
| Source: Reference Citations: click here | |||||
BAL can document acute alcohol intoxication, but its use is limited because alcohol has a 4-hour half-life and an elimination rate of 7 grams/hour—equivalent to 1 drink/hour.6 (A “drink” typically is defined as a 12-ounce bottle of beer or wine cooler, a 5-ounce glass of wine, or 1.5 ounces of 80-proof distilled spirits.) Therefore, BAL will identify as false negatives alcohol-dependent patients who abstain from alcohol within 24 hours of testing.
MCV is an index of the average volume of erythrocytes. Macrocytosis occurs when the volume exceeds 100 fL. Elevated MCV is the most typical morphologic abnormality associated with excessive alcohol consumption7,8 and macrocytosis—sometimes without associated anemia—is often evident in persons with alcoholism. MCV elevates after 6 weeks of alcohol misuse and may remain elevated for up to 3 months after a person has stopped drinking.9
Because patients with disorders unrelated to alcohol use can have elevated MCV, alone it is not a useful screening marker for alcohol abuse.10 Additionally, because macrocytosis can persist under strictly controlled alcohol abstinence, MCV is not a reliable clinical indicator of relapse.11
LFTs measure enzymes and proteins. ALT, AST, and GGT are the most relevant for detecting heavy drinking. An AST:ALT ratio >2:1 supports a suspicion of alcohol abuse.12 More than 90% of patients with an AST:ALT ratio of 2:1 have alcoholic liver disease. This increases to more than 96% if the ratio is 3:1.13
GGT is an enzyme concentrated in the liver, bile ducts, and kidneys; normal range is 0 to 45 U/L (for females) or 53 U/L (for males).14 GGT levels >30 U/L correlate with alcohol consumption of >4 drinks per day.15 GGT has a half-life of 14 to 26 days and remains elevated for 4 to 6 weeks after drinking cessation, which make it useful for monitoring abstinence in treatment programs.16 Sensitivity ranges from 37% to 85% and specificity is as high as 93% in nonmedical populations.17 Although nonalcoholic liver disease can elevate GGT in persons who do not abuse alcohol, 50% to 72% of GGT elevations can be explained by excessive alcohol consumption.18
CDT is a newer biomarker used to monitor alcohol consumption. The most accurate way to express CDT level is as a percentage of total transferrin concentration. This method accounts for individual variations in transferrin levels, thus minimizing false positives.18 In persons who consume >4 or 5 drinks per day for 2 weeks or more, CDT is >1.3% of total transferrin.19 Unfortunately, because it is expensive and requires sophisticated test methodology, CDT testing is not available at most hospitals.20
Combinations improve detection
Each biochemical measure has strengths and weaknesses as a marker for determining patients’ alcohol consumption (Table 2). CDT and GGT show the highest sensitivity for heavy drinking, and CDT has a higher specificity than GGT (Table 3).21,22 Relapse to alcohol use after abstinence may be best identified by a simultaneous 30% increase in CDT and GGT.5
Because GGT has a longer half-life than CDT, its diagnostic efficiency in detecting alcohol relapse may not develop until 4 weeks after alcohol detoxification, whereas CDT may become clinically useful for detecting relapse as early as 1 week after detoxification.23
Table 2
Biomarkers of alcohol use: Strengths and weaknesses
| Biomarker | Strengths | Weaknesses |
|---|---|---|
| CDT | High specificity for alcohol use; few factors cause false positives High sensitivity in distinguishing alcoholics from social drinkers Marker of relapse and abstinence from drinking Confirmatory test for patients suspected of alcohol abuse | Low sensitivity; more valuable to confirm than exclude heavy drinking Cost (average $30/assay) and low availability of testing Likely less sensitive for women and younger patients compared with men Poor screening tool for alcohol use in general population |
| GGT | Elevations precede alcohol-induced liver damage High specificity in patients with suspected alcohol abuse Effective marker for patients suspected of binge drinking Inexpensive ( | Can be falsely elevated by liver and biliary disease, smoking, obesity, and medications that induce microsomal enzymes Low sensitivity makes it a poor screening tool in general population Poor marker of relapse |
| AST:ALT >2:1 | Highly sensitive and specific for alcohol-induced liver damage | Enzyme elevations can be detected only after periods of heavy drinking Elevations secondary to liver damage at the hepatocellular level (after fatty changes) |
| MCV | Accuracy similar in male and female patients Elevations in suspected cases of alcohol use indicate chronicity of drinking Routine laboratory test | Poor biomarker for relapse False positives caused by liver disease, hemolysis, bleeding disorders, anemia, folate deficiency, and medications that reduce folate Low sensitivity and specificity for alcohol use make it a poor screening tool for alcohol abuse |
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | ||
Table 3
Interpreting diagnostic test performance
| Term | Definition | Applicability |
|---|---|---|
| Sensitivity | Percent of persons with disease who test positive | High value is desirable for ruling out disease (low false-negative rate) |
| Specificity | Percent of persons without disease who test negative | High value is desirable for ruling in disease (low false-positive rate) |
| Positive predictive value | Percent of positive test results that are true positives | Probability that a person with a positive test result has the disease |
| Negative predictive value | Percent of negative test results that are true negatives | Probability that a person with a negative test result is disease-free |
| Source: References 21,22 | ||
There is evidence that combining tests can improve alcohol use detection.24 For example, Dolman et al25 found that the ability of the AUDIT questionnaire to correctly predict which patients would experience alcohol withdrawal increases when it is used in combination with biochemical markers. Specifically, the positive predictive value of an AUDIT score ≥8 increased from 17% to 47% when found in combination with ≥2 abnormal biochemical marker levels; the study looked at GGT, ALT, AST, and MCV. Sensitivity was 94% and specificity was 98%.
Similarly, combinations of biochemical markers—especially CDT and GGT—have improved detection of alcohol use and subsequent risk of withdrawal.26Table 4 provides a summary of studies that evaluated using combinations of biochemical markers.4,5,27-31
Table 4
Combining biomarker tests: An effective approach
| Combination | Study | Sensitivity* |
|---|---|---|
| GGT + MCV | Morgan et al4 | 95% |
| GGT + CDT | Hietala et al5 | 90% |
| Mundle et al29 | 90% | |
| Bell et al30 | 90% | |
| Sillanaukee et al31 | 95% | |
| GGT + AST:ALT >2:1 | Gluud et al27 | 92% |
| Morgan et al4 | 100% | |
| MCV + AST:ALT >2:1 | Kawachi et al28 | 97% |
| Morgan et al4 | 95% | |
| GGT + MCV + AST:ALT >2:1 | Morgan et al4 | 100% |
| GGT + MCV + CDT | Sillanaukee et al31 | 70% |
| * Sensitivity for detecting excessive alcohol consumption | ||
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | ||
Consider patients’ comorbidities
Patients at risk for underreporting alcohol use include those with unemployment histories, previous alcohol treatment, and higher scores on the Alcohol Dependence Scale (18.5, SD=8.1).2 Interpret biochemical testing results in the context of a patient’s overall clinical picture.
The following 2 case patients denied or underreported recent alcohol use but we determined they were at high risk for an alcohol disorder because of their medical and/or psychiatric histories. Analysis of biochemical markers helped assess the risk of alcohol withdrawal.
CASE 2: Altered mental status
Family members bring Mr. N, age 44, to the hospital because of his odd behavior. He presents with paranoid delusions and an inappropriate elated mood. His medical history includes acquired immune deficiency syndrome (AIDS). After cerebrospinal fluid analysis, computed tomography of the head, electroencephalogram, and metabolic workup are within normal limits, the patient is diagnosed with human immunodeficiency virus (HIV) mania and is admitted.
On admission, Mr. N denies alcohol use. A blood alcohol/urine toxicity screen is negative. One day after admission, Mr. M develops elevated blood pressure and tachycardia and reports headache and nausea.
Challenge. Gathering a valid history of Mr. N’s alcohol use is difficult because of his acutely altered mental status and manic-like state. We use laboratory data to assess his risk of alcohol withdrawal. His liver function tests include an AST of 33 U/L, ALT of 30 U/L, and an alkaline phosphatase of 94 U/L. MCV is normal at 90 fL. Interestingly, the GGT level is elevated almost 4 times normal at 164 U/L.
Discussion. Although Mr. N denied alcohol use and presented with a negative BAL, laboratory data support alcohol dependence. His GGT was elevated well beyond normal limits, without evidence of hepatobiliary disease. GGT has a sensitivity as high as 85%32 and limited specificity for alcohol abuse. Because of his high probability of recent alcohol consumption, we place Mr. N on AWP.
We postulate that our patient’s autonomic instability, headache, and nausea are related to alcohol withdrawal. We are aware that delirium occurs frequently in patients with HIV infection, and although Mr. N’s medical workup is negative, HIV infection can produce an acute encephalopathy that could resemble our patient’s clinical picture.33
Mr. N’s autonomic instability, headache, and nausea abated after treatment for alcohol withdrawal.
CASE 3: Suicide attempt?
Mr. S, age 28, presents to the trauma service with a self-inflicted gunshot wound to the face. He reports feeling depressed for the last year but denies a history of psychotic symptoms or heroin withdrawal symptoms. He also denies recent or past alcohol abuse and does not have a history of biliary tract disease or megaloblastic anemia. His mother tells us Mr. S has had a history of depression since childhood.
Challenge. Based on Mr. S’ apparent suicide attempt and history, we feel he is at high risk for alcohol abuse. We use laboratory markers to assess the likelihood of alcohol consumption and possibly decrease his risk of alcohol withdrawal.
Discussion. Mr. S’ lab data show an MCV of 91 fL, AST of 95 U/L, alanine ALT of 156 U/L, and alkaline phosphatase of 160 U/L. GGT was elevated at 122 U/L.
Although Mr. S’ MCV is within the normal range, his GGT is elevated, and the combination of an elevated GGT and MCV has a 95% sensitivity for the diagnosis of alcohol abuse. We place Mr. S on alcohol withdrawal precautions and discuss with him the potential life-threatening complications of alcohol withdrawal. Confronted with this information and the possible implication of his elevated LFTs, the patient admits his alcohol history—which consists of drinking 12 beers/day for at least the past 2 years. He admits this despite exhibiting no signs or symptoms of alcohol withdrawal.
Related Resources
- National Institute on Alcohol Abuse and Alcoholism Data/Statistical Tables. www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts.
- Maisto SA, Connors GJ, Allen JP. Contrasting self-report screens for alcohol problems: a review. Alcohol Clin Exp Res 1995;19(6):1510-6.
- Coulton S, Drummond C, James D, et al. Opportunistic screening for alcohol use disorders in primary care: comparative study. BMJ 2006;332:511-7.
Bottom line
Because CDT—the most accurate biomarker—is not available at most hospitals, we recommend using combinations of other measures to detect unreported recent alcohol consumption. If GGT and MCV are elevated, GGT is elevated and AST:ALT is >2:1, or MCV is elevated and AST:ALT is >2:1, consider initiating alcohol withdrawal precautions.
Acknowledgement
The authors acknowledge Daiana Radac, BA, a third-year medical student at Eastern Virginia Medical School, for her contributions to this article.
Hospitalized patients who are not truthful about their alcohol consumption may be at risk for an unplanned withdrawal. Self-reports of alcohol use—such as CAGE and the Alcohol Use Disorders Identification Test (AUDIT)—are valid, inexpensive, and noninvasive, but patients easily can feign results.1 Biochemical measures are more objective, and combinations of markers are an effective tool to detect recent heavy drinking in the 10% to 25% of patients who underreport alcohol use.2
Biochemical measures can detect acute alcohol intoxication and recent prolonged drinking. Because marker levels return to normal after long-term abstinence, ongoing monitoring can help detect a relapse before a patient admits to it.3
This article presents 3 cases in which biochemical markers helped prevent alcohol withdrawal in patients who denied alcohol abuse. We discuss why we ordered biochemical tests and which combinations provided highly sensitive results.
CASE 1: Depression and substance abuse
Ms. C, age 39, presents with bleeding gums due to excessive warfarin, which she takes prophylactically for a history of deep vein thrombosis. She is seen by the psychiatric consultation service for depression—which she says she has experienced since “the day I was born”—and substance abuse that includes a history binge drinking. Ms. C says she has stopped drinking and remained abstinent for the past year because she is fearful of further damaging her kidneys. She also denies psychosis. She does not have a history or symptoms of hepatobiliary or hematologic disease.
Challenge. Despite Ms. C’s self-reported 1 year of sobriety, her history of binge drinking and depression calls for evaluating her alcohol withdrawal risk. Laboratory markers of alcohol abuse are the only means to assess her recent drinking behavior.
Discussion. Lab results include serum albumin of 3.4 g/dL, total bilirubin of 0.3 mg/dL, total protein of 6.3 g/dL, aspartate aminotransferase (AST) of 13 U/L, alanine aminotransferase (ALT) of 19 U/L, alkaline phosphatase of 136 U/L, and blood ammonia level of 37 μg/dL. Gamma-glutamyl transferase (GGT) is elevated at 104 U/L (normal range for women: 0 to 45 U/L). Mean corpuscular volume (MCV) is elevated at 101 fL (normal range 80 to 100 fL).
The combination of elevated MCV and GGT has a 95% sensitivity for alcohol abuse.4 GGT levels become elevated after 24 hours to 2 weeks of heavy alcohol consumption and return to normal within 2 to 6 weeks of abstinence, which allows them to detect binge drinking. MCV takes 6 to 8 weeks of heavy drinking—we which we define as consuming ≥40 grams of alcohol/day5—to become elevated and returns to normal within 3 months of abstinence.
These data provide evidence that Ms. C recently consumed substantial amounts of alcohol. As a result, we start her on alcohol withdrawal precautions (AWP).
Markers of alcohol abuse
Biochemical markers commonly used to detect alcohol abuse (Table 1) include:
- blood alcohol level (BAL)
- MCV
- liver function tests (LFTs) such as ALT, AST, and GGT
- carbohydrate deficient transferrin (CDT).
Table 1
By the numbers: Biomarkers of excessive alcohol consumption
| Biomarker | |||||
|---|---|---|---|---|---|
| CDT | GGT | AST | ALT | MCV | |
| Blood test normal range | Women: 0 to 45 U/L Men: 0 to 53 U/L | 10 to 34 U/L | 8 to 37 U/L | 80 to 100 fL | |
| Blood test abnormal range | >1.3% of total transferrin concentration | Women: >45 U/L Men: >53 U/L | Levels rarely exceed 500 U/L | Levels rarely exceed 300 U/L | >100 fL |
| Time to elevation | 2 to 3 weeks | 24 hours to 2 weeks | 3 to 7 days | 3 to 7 days | After 6 weeks |
| Time to descent to normal levels | 2 to 4 weeks of abstinence | 2 to 6 weeks of abstinence | Half-life 12 to 24 hours | Half-life 37 to 57 hours | 3 months |
| Dose-response of alcohol | 60 g/d | 80 to 200 g/d | ≥40 g/d | ≥40 g/d | ≥40 g/d |
| Sensitivity | 55% to 90%a-e | 37% to 85%b, f, g | AST:ALT ratio >2:1 has a 70% sensitivity and 92% to 100% specificity for alcoholic-induced liver diseaseh-j | 20% to 70%b,k | |
| Relapse sensitivity | 55% to 76%a,l,m | 50%a,e | 20%a,n | ||
| Specificity | 92% to 97%a,b | 18% to 93%a,b,e | 64% to 66%b,k,n | ||
| Positive predictive value | 46% to 75%c,g | 41%g | 36%g | ||
| Negative predictive value | 72% to 98%a,c,g | 69% to 92%a,e,g | 67%g | ||
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | |||||
| Source: Reference Citations: click here | |||||
BAL can document acute alcohol intoxication, but its use is limited because alcohol has a 4-hour half-life and an elimination rate of 7 grams/hour—equivalent to 1 drink/hour.6 (A “drink” typically is defined as a 12-ounce bottle of beer or wine cooler, a 5-ounce glass of wine, or 1.5 ounces of 80-proof distilled spirits.) Therefore, BAL will identify as false negatives alcohol-dependent patients who abstain from alcohol within 24 hours of testing.
MCV is an index of the average volume of erythrocytes. Macrocytosis occurs when the volume exceeds 100 fL. Elevated MCV is the most typical morphologic abnormality associated with excessive alcohol consumption7,8 and macrocytosis—sometimes without associated anemia—is often evident in persons with alcoholism. MCV elevates after 6 weeks of alcohol misuse and may remain elevated for up to 3 months after a person has stopped drinking.9
Because patients with disorders unrelated to alcohol use can have elevated MCV, alone it is not a useful screening marker for alcohol abuse.10 Additionally, because macrocytosis can persist under strictly controlled alcohol abstinence, MCV is not a reliable clinical indicator of relapse.11
LFTs measure enzymes and proteins. ALT, AST, and GGT are the most relevant for detecting heavy drinking. An AST:ALT ratio >2:1 supports a suspicion of alcohol abuse.12 More than 90% of patients with an AST:ALT ratio of 2:1 have alcoholic liver disease. This increases to more than 96% if the ratio is 3:1.13
GGT is an enzyme concentrated in the liver, bile ducts, and kidneys; normal range is 0 to 45 U/L (for females) or 53 U/L (for males).14 GGT levels >30 U/L correlate with alcohol consumption of >4 drinks per day.15 GGT has a half-life of 14 to 26 days and remains elevated for 4 to 6 weeks after drinking cessation, which make it useful for monitoring abstinence in treatment programs.16 Sensitivity ranges from 37% to 85% and specificity is as high as 93% in nonmedical populations.17 Although nonalcoholic liver disease can elevate GGT in persons who do not abuse alcohol, 50% to 72% of GGT elevations can be explained by excessive alcohol consumption.18
CDT is a newer biomarker used to monitor alcohol consumption. The most accurate way to express CDT level is as a percentage of total transferrin concentration. This method accounts for individual variations in transferrin levels, thus minimizing false positives.18 In persons who consume >4 or 5 drinks per day for 2 weeks or more, CDT is >1.3% of total transferrin.19 Unfortunately, because it is expensive and requires sophisticated test methodology, CDT testing is not available at most hospitals.20
Combinations improve detection
Each biochemical measure has strengths and weaknesses as a marker for determining patients’ alcohol consumption (Table 2). CDT and GGT show the highest sensitivity for heavy drinking, and CDT has a higher specificity than GGT (Table 3).21,22 Relapse to alcohol use after abstinence may be best identified by a simultaneous 30% increase in CDT and GGT.5
Because GGT has a longer half-life than CDT, its diagnostic efficiency in detecting alcohol relapse may not develop until 4 weeks after alcohol detoxification, whereas CDT may become clinically useful for detecting relapse as early as 1 week after detoxification.23
Table 2
Biomarkers of alcohol use: Strengths and weaknesses
| Biomarker | Strengths | Weaknesses |
|---|---|---|
| CDT | High specificity for alcohol use; few factors cause false positives High sensitivity in distinguishing alcoholics from social drinkers Marker of relapse and abstinence from drinking Confirmatory test for patients suspected of alcohol abuse | Low sensitivity; more valuable to confirm than exclude heavy drinking Cost (average $30/assay) and low availability of testing Likely less sensitive for women and younger patients compared with men Poor screening tool for alcohol use in general population |
| GGT | Elevations precede alcohol-induced liver damage High specificity in patients with suspected alcohol abuse Effective marker for patients suspected of binge drinking Inexpensive ( | Can be falsely elevated by liver and biliary disease, smoking, obesity, and medications that induce microsomal enzymes Low sensitivity makes it a poor screening tool in general population Poor marker of relapse |
| AST:ALT >2:1 | Highly sensitive and specific for alcohol-induced liver damage | Enzyme elevations can be detected only after periods of heavy drinking Elevations secondary to liver damage at the hepatocellular level (after fatty changes) |
| MCV | Accuracy similar in male and female patients Elevations in suspected cases of alcohol use indicate chronicity of drinking Routine laboratory test | Poor biomarker for relapse False positives caused by liver disease, hemolysis, bleeding disorders, anemia, folate deficiency, and medications that reduce folate Low sensitivity and specificity for alcohol use make it a poor screening tool for alcohol abuse |
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | ||
Table 3
Interpreting diagnostic test performance
| Term | Definition | Applicability |
|---|---|---|
| Sensitivity | Percent of persons with disease who test positive | High value is desirable for ruling out disease (low false-negative rate) |
| Specificity | Percent of persons without disease who test negative | High value is desirable for ruling in disease (low false-positive rate) |
| Positive predictive value | Percent of positive test results that are true positives | Probability that a person with a positive test result has the disease |
| Negative predictive value | Percent of negative test results that are true negatives | Probability that a person with a negative test result is disease-free |
| Source: References 21,22 | ||
There is evidence that combining tests can improve alcohol use detection.24 For example, Dolman et al25 found that the ability of the AUDIT questionnaire to correctly predict which patients would experience alcohol withdrawal increases when it is used in combination with biochemical markers. Specifically, the positive predictive value of an AUDIT score ≥8 increased from 17% to 47% when found in combination with ≥2 abnormal biochemical marker levels; the study looked at GGT, ALT, AST, and MCV. Sensitivity was 94% and specificity was 98%.
Similarly, combinations of biochemical markers—especially CDT and GGT—have improved detection of alcohol use and subsequent risk of withdrawal.26Table 4 provides a summary of studies that evaluated using combinations of biochemical markers.4,5,27-31
Table 4
Combining biomarker tests: An effective approach
| Combination | Study | Sensitivity* |
|---|---|---|
| GGT + MCV | Morgan et al4 | 95% |
| GGT + CDT | Hietala et al5 | 90% |
| Mundle et al29 | 90% | |
| Bell et al30 | 90% | |
| Sillanaukee et al31 | 95% | |
| GGT + AST:ALT >2:1 | Gluud et al27 | 92% |
| Morgan et al4 | 100% | |
| MCV + AST:ALT >2:1 | Kawachi et al28 | 97% |
| Morgan et al4 | 95% | |
| GGT + MCV + AST:ALT >2:1 | Morgan et al4 | 100% |
| GGT + MCV + CDT | Sillanaukee et al31 | 70% |
| * Sensitivity for detecting excessive alcohol consumption | ||
| AST: aspartate aminotransferase; ALT: alanine aminotransferase; CDT: carbohydrate deficient transferrin; GGT: gamma-glutamyl transferase; MCV: mean corpuscular volume | ||
Consider patients’ comorbidities
Patients at risk for underreporting alcohol use include those with unemployment histories, previous alcohol treatment, and higher scores on the Alcohol Dependence Scale (18.5, SD=8.1).2 Interpret biochemical testing results in the context of a patient’s overall clinical picture.
The following 2 case patients denied or underreported recent alcohol use but we determined they were at high risk for an alcohol disorder because of their medical and/or psychiatric histories. Analysis of biochemical markers helped assess the risk of alcohol withdrawal.
CASE 2: Altered mental status
Family members bring Mr. N, age 44, to the hospital because of his odd behavior. He presents with paranoid delusions and an inappropriate elated mood. His medical history includes acquired immune deficiency syndrome (AIDS). After cerebrospinal fluid analysis, computed tomography of the head, electroencephalogram, and metabolic workup are within normal limits, the patient is diagnosed with human immunodeficiency virus (HIV) mania and is admitted.
On admission, Mr. N denies alcohol use. A blood alcohol/urine toxicity screen is negative. One day after admission, Mr. M develops elevated blood pressure and tachycardia and reports headache and nausea.
Challenge. Gathering a valid history of Mr. N’s alcohol use is difficult because of his acutely altered mental status and manic-like state. We use laboratory data to assess his risk of alcohol withdrawal. His liver function tests include an AST of 33 U/L, ALT of 30 U/L, and an alkaline phosphatase of 94 U/L. MCV is normal at 90 fL. Interestingly, the GGT level is elevated almost 4 times normal at 164 U/L.
Discussion. Although Mr. N denied alcohol use and presented with a negative BAL, laboratory data support alcohol dependence. His GGT was elevated well beyond normal limits, without evidence of hepatobiliary disease. GGT has a sensitivity as high as 85%32 and limited specificity for alcohol abuse. Because of his high probability of recent alcohol consumption, we place Mr. N on AWP.
We postulate that our patient’s autonomic instability, headache, and nausea are related to alcohol withdrawal. We are aware that delirium occurs frequently in patients with HIV infection, and although Mr. N’s medical workup is negative, HIV infection can produce an acute encephalopathy that could resemble our patient’s clinical picture.33
Mr. N’s autonomic instability, headache, and nausea abated after treatment for alcohol withdrawal.
CASE 3: Suicide attempt?
Mr. S, age 28, presents to the trauma service with a self-inflicted gunshot wound to the face. He reports feeling depressed for the last year but denies a history of psychotic symptoms or heroin withdrawal symptoms. He also denies recent or past alcohol abuse and does not have a history of biliary tract disease or megaloblastic anemia. His mother tells us Mr. S has had a history of depression since childhood.
Challenge. Based on Mr. S’ apparent suicide attempt and history, we feel he is at high risk for alcohol abuse. We use laboratory markers to assess the likelihood of alcohol consumption and possibly decrease his risk of alcohol withdrawal.
Discussion. Mr. S’ lab data show an MCV of 91 fL, AST of 95 U/L, alanine ALT of 156 U/L, and alkaline phosphatase of 160 U/L. GGT was elevated at 122 U/L.
Although Mr. S’ MCV is within the normal range, his GGT is elevated, and the combination of an elevated GGT and MCV has a 95% sensitivity for the diagnosis of alcohol abuse. We place Mr. S on alcohol withdrawal precautions and discuss with him the potential life-threatening complications of alcohol withdrawal. Confronted with this information and the possible implication of his elevated LFTs, the patient admits his alcohol history—which consists of drinking 12 beers/day for at least the past 2 years. He admits this despite exhibiting no signs or symptoms of alcohol withdrawal.
Related Resources
- National Institute on Alcohol Abuse and Alcoholism Data/Statistical Tables. www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts.
- Maisto SA, Connors GJ, Allen JP. Contrasting self-report screens for alcohol problems: a review. Alcohol Clin Exp Res 1995;19(6):1510-6.
- Coulton S, Drummond C, James D, et al. Opportunistic screening for alcohol use disorders in primary care: comparative study. BMJ 2006;332:511-7.
Bottom line
Because CDT—the most accurate biomarker—is not available at most hospitals, we recommend using combinations of other measures to detect unreported recent alcohol consumption. If GGT and MCV are elevated, GGT is elevated and AST:ALT is >2:1, or MCV is elevated and AST:ALT is >2:1, consider initiating alcohol withdrawal precautions.
Acknowledgement
The authors acknowledge Daiana Radac, BA, a third-year medical student at Eastern Virginia Medical School, for her contributions to this article.
1. Allen JP, Anthenelli RM. Getting to the bottom of problem drinking: the case for routine screening. Current Psychiatry 2003;2(6):26-44.
2. Killeen TK, Brady KT, Gold PB, et al. Comparison of self-report versus agency records of service utilization in a community sample of individuals with alcohol use disorders. Drug Alcohol Depend 2004;73(2):141-7.
3. Alcohol withdrawal syndrome: how to predict, prevent, diagnose and treat it. Prescrire Int 2007;16(87):24-31.
4. Morgan MY, Colman JC, Sherlock S. The use of a combination of peripheral markers for diagnosing alcoholism and monitoring for continued abuse. Alcohol Alcohol 1981;16:167-77.
5. Hietala J, Koivisto H, Anttila P, et al. Comparison of the combined marker GGT-CDT and the conventional laboratory markers of alcohol abuse in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol 2006;41(5):528-33.
6. Swift R. Direct measurement of alcohol and its metabolites. Addiction 2003;98:73-80.
7. Koivisto H, Hietala J, Anttila P, et al. Long-term ethanol consumption and macrocytosis: diagnostic and pathogenic implications. J Lab Clin Med 2005;147(4):191-6.
8. Savage DG, Ogundipe A, Allen RH, et al. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci 2000;319(6):343-52.
9. Gordon H. Detection of alcoholic liver disease. World J Gastroenterol 2001;7(3):297-302.
10. Bernadt M, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet 1982;1:325-8.
11. Hasselblatt M, Martin F, Maul O, et al. Persistent macrocytosis following abstinence from chronic alcohol use. JAMA 2001;286:2946.-
12. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1-9.
13. Fancher T, Kamboj A, Onate J. Interpreting liver function tests. Current Psychiatry 2007;6(5):61-8.
14. Puukka K, Hietala J, Koivisto H, et al. Obesity and the clinical use of serum GGT activity as a marker of heavy drinking. Scand J Clin Lab Invest 2007;67(5):480-8.
15. Litten RZ, Allen JP, Fertig JB. y-glutamyl transpeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
16. National Institute on Alcohol Abuse and Alcoholism. Screening for alcohol problems—an update. Alcohol Alert No 56 Available at: http://pubs.niaaa.nih.gov/publications/aa56.htm. Accessed May 5, 2007.
17. DiMartini A. A clinical guide to assessing alcohol use and problems. Available at: http://www.alcoholmedicalscholars.org/clin-asmt.ppt. Accessed June 30, 2008.
18. Wolff K, Marshall E. Biological markers of alcohol use. Psychiatry 2006;5(12):437-8.
19. ARUP Laboratories. Carbohydrate-deficient transferrin (CDT) for alcohol use. 2006. Available at: http://www.aruplab.com/TestDirectory/resources/TechnicalBulletins/Carbohydrate-Deficient%20Transferrin%20(CDT)%20Mar%202006.pdf. Accessed July 30, 2008.
20. Allen JP, Litten RZ. The role of laboratory testing in alcoholism treatment. J Subst Abuse Treat 2001;20:81-5.
21. Bhushan V, Le T, Ozturk A, et al. Behavioral Science. In: Le T, Bhushan V, Rao DA, eds. First aid for the USMLE step 1: a student to student guide. New York, NY: McGraw Hill Medical Publishing Division; 2007.
22. Miller PM, Anton RF. Biochemical alcohol screening in primary care. Addict Behav 2004;29(7):1427-37.
23. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
24. Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999;19(3):261-71.
25. Dolman JM, Hawkes ND. Combining the AUDIT questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical patients. Alcohol Alcohol 2005;40(6):515-9.
26. Helander A, Carlsson AV, Borg S. Longitudinal comparison of carbohydrate-deficient transferrin and gamma-glutamyl transferase: complementary markers of excessive alcohol consumption. Alcohol Alcohol 1996;31(1):101-7.
27. Gluud C, Andersen I, Dietrichson O, et al. Gamma-glutamyltransferase, aspartate aminotransferase and alkaline phosphatase as markers of alcohol consumption in out-patient alcoholics. Eur J Clin Invest 1981;11(3):171-6.
28. Kawachi I, Robinson GM, Stace NH. A combination of raised serum AST: ALT ratio and erythrocyte mean cell volume level detects excessive alcohol consumption. N Z Med J 1990;103(887):145-8.
29. Mundle G, Ackerman K, Mann K. Biological markers as indicators for relapse in alcohol-dependent patients. Addict Biol 1999;4(2):209-14.
30. Bell H, Tallaksen C, Sjåheim T, et al. Serum carbohydrate-deficient transferrin as a marker of alcohol consumption in patients with chronic liver diseases. Alcohol Clin Exp Res 1993;17(2):246-52.
31. Sillanaukee P, Aalto M, Seppa K. Carbohydrate-deficient transferrin and conventional alcohol markers as indicators for brief intervention among heavy drinkers in primary health care. Alcohol Clin Exp Res 1998;22(4):892-6.
32. Salaspuro S. Conventional and coming laboratory markers of alcoholism and heavy drinking. Alcohol Clin Exp Res 1986;10(6 suppl):5-12.
33. Della Penna ND, Treisman GJ. HIV/AIDS. In: Levenson J, ed. Essentials of psychosomatic medicine. Washington, DC: American Psychiatric Publishing, Inc; 2007.
1. Allen JP, Anthenelli RM. Getting to the bottom of problem drinking: the case for routine screening. Current Psychiatry 2003;2(6):26-44.
2. Killeen TK, Brady KT, Gold PB, et al. Comparison of self-report versus agency records of service utilization in a community sample of individuals with alcohol use disorders. Drug Alcohol Depend 2004;73(2):141-7.
3. Alcohol withdrawal syndrome: how to predict, prevent, diagnose and treat it. Prescrire Int 2007;16(87):24-31.
4. Morgan MY, Colman JC, Sherlock S. The use of a combination of peripheral markers for diagnosing alcoholism and monitoring for continued abuse. Alcohol Alcohol 1981;16:167-77.
5. Hietala J, Koivisto H, Anttila P, et al. Comparison of the combined marker GGT-CDT and the conventional laboratory markers of alcohol abuse in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol 2006;41(5):528-33.
6. Swift R. Direct measurement of alcohol and its metabolites. Addiction 2003;98:73-80.
7. Koivisto H, Hietala J, Anttila P, et al. Long-term ethanol consumption and macrocytosis: diagnostic and pathogenic implications. J Lab Clin Med 2005;147(4):191-6.
8. Savage DG, Ogundipe A, Allen RH, et al. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci 2000;319(6):343-52.
9. Gordon H. Detection of alcoholic liver disease. World J Gastroenterol 2001;7(3):297-302.
10. Bernadt M, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet 1982;1:325-8.
11. Hasselblatt M, Martin F, Maul O, et al. Persistent macrocytosis following abstinence from chronic alcohol use. JAMA 2001;286:2946.-
12. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1-9.
13. Fancher T, Kamboj A, Onate J. Interpreting liver function tests. Current Psychiatry 2007;6(5):61-8.
14. Puukka K, Hietala J, Koivisto H, et al. Obesity and the clinical use of serum GGT activity as a marker of heavy drinking. Scand J Clin Lab Invest 2007;67(5):480-8.
15. Litten RZ, Allen JP, Fertig JB. y-glutamyl transpeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
16. National Institute on Alcohol Abuse and Alcoholism. Screening for alcohol problems—an update. Alcohol Alert No 56 Available at: http://pubs.niaaa.nih.gov/publications/aa56.htm. Accessed May 5, 2007.
17. DiMartini A. A clinical guide to assessing alcohol use and problems. Available at: http://www.alcoholmedicalscholars.org/clin-asmt.ppt. Accessed June 30, 2008.
18. Wolff K, Marshall E. Biological markers of alcohol use. Psychiatry 2006;5(12):437-8.
19. ARUP Laboratories. Carbohydrate-deficient transferrin (CDT) for alcohol use. 2006. Available at: http://www.aruplab.com/TestDirectory/resources/TechnicalBulletins/Carbohydrate-Deficient%20Transferrin%20(CDT)%20Mar%202006.pdf. Accessed July 30, 2008.
20. Allen JP, Litten RZ. The role of laboratory testing in alcoholism treatment. J Subst Abuse Treat 2001;20:81-5.
21. Bhushan V, Le T, Ozturk A, et al. Behavioral Science. In: Le T, Bhushan V, Rao DA, eds. First aid for the USMLE step 1: a student to student guide. New York, NY: McGraw Hill Medical Publishing Division; 2007.
22. Miller PM, Anton RF. Biochemical alcohol screening in primary care. Addict Behav 2004;29(7):1427-37.
23. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
24. Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999;19(3):261-71.
25. Dolman JM, Hawkes ND. Combining the AUDIT questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical patients. Alcohol Alcohol 2005;40(6):515-9.
26. Helander A, Carlsson AV, Borg S. Longitudinal comparison of carbohydrate-deficient transferrin and gamma-glutamyl transferase: complementary markers of excessive alcohol consumption. Alcohol Alcohol 1996;31(1):101-7.
27. Gluud C, Andersen I, Dietrichson O, et al. Gamma-glutamyltransferase, aspartate aminotransferase and alkaline phosphatase as markers of alcohol consumption in out-patient alcoholics. Eur J Clin Invest 1981;11(3):171-6.
28. Kawachi I, Robinson GM, Stace NH. A combination of raised serum AST: ALT ratio and erythrocyte mean cell volume level detects excessive alcohol consumption. N Z Med J 1990;103(887):145-8.
29. Mundle G, Ackerman K, Mann K. Biological markers as indicators for relapse in alcohol-dependent patients. Addict Biol 1999;4(2):209-14.
30. Bell H, Tallaksen C, Sjåheim T, et al. Serum carbohydrate-deficient transferrin as a marker of alcohol consumption in patients with chronic liver diseases. Alcohol Clin Exp Res 1993;17(2):246-52.
31. Sillanaukee P, Aalto M, Seppa K. Carbohydrate-deficient transferrin and conventional alcohol markers as indicators for brief intervention among heavy drinkers in primary health care. Alcohol Clin Exp Res 1998;22(4):892-6.
32. Salaspuro S. Conventional and coming laboratory markers of alcoholism and heavy drinking. Alcohol Clin Exp Res 1986;10(6 suppl):5-12.
33. Della Penna ND, Treisman GJ. HIV/AIDS. In: Levenson J, ed. Essentials of psychosomatic medicine. Washington, DC: American Psychiatric Publishing, Inc; 2007.
Malpractice Minute
Did the medication cause a young girl's mood disorder?
THE PATIENT. A young girl was prescribed paroxetine after complaining of stomachaches and headaches.
CASE FACTS. The patient saw many healthcare providers and received several different medications until a psychiatrist diagnosed the girl with bipolar disorder with psychotic features, prescribed numerous medications, and hospitalized the patient. The girl was released then readmitted to another hospital, where a different psychiatrist tapered several medications and left her on low doses of clonazepam and topiramate. The patient improved and returned home. Later she stopped taking her medications, became psychotic, and was rehospitalized. The patient was then tapered off all medications and her condition returned to normal.
THE PATIENT’S CLAIM. She was not bipolar and had a substance-induced mood disorder caused by the medications she had been prescribed.
THE PSYCHIATRISTS’ DEFENSE. The patient was bipolar.
Submit your verdict and find out how the court ruled. To offer additional feedback, use the ‘Enter comments’ field above.
Cases are selected by current psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Did the medication cause a young girl's mood disorder?
THE PATIENT. A young girl was prescribed paroxetine after complaining of stomachaches and headaches.
CASE FACTS. The patient saw many healthcare providers and received several different medications until a psychiatrist diagnosed the girl with bipolar disorder with psychotic features, prescribed numerous medications, and hospitalized the patient. The girl was released then readmitted to another hospital, where a different psychiatrist tapered several medications and left her on low doses of clonazepam and topiramate. The patient improved and returned home. Later she stopped taking her medications, became psychotic, and was rehospitalized. The patient was then tapered off all medications and her condition returned to normal.
THE PATIENT’S CLAIM. She was not bipolar and had a substance-induced mood disorder caused by the medications she had been prescribed.
THE PSYCHIATRISTS’ DEFENSE. The patient was bipolar.
Submit your verdict and find out how the court ruled. To offer additional feedback, use the ‘Enter comments’ field above.
Did the medication cause a young girl's mood disorder?
THE PATIENT. A young girl was prescribed paroxetine after complaining of stomachaches and headaches.
CASE FACTS. The patient saw many healthcare providers and received several different medications until a psychiatrist diagnosed the girl with bipolar disorder with psychotic features, prescribed numerous medications, and hospitalized the patient. The girl was released then readmitted to another hospital, where a different psychiatrist tapered several medications and left her on low doses of clonazepam and topiramate. The patient improved and returned home. Later she stopped taking her medications, became psychotic, and was rehospitalized. The patient was then tapered off all medications and her condition returned to normal.
THE PATIENT’S CLAIM. She was not bipolar and had a substance-induced mood disorder caused by the medications she had been prescribed.
THE PSYCHIATRISTS’ DEFENSE. The patient was bipolar.
Submit your verdict and find out how the court ruled. To offer additional feedback, use the ‘Enter comments’ field above.
Cases are selected by current psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by current psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Dosing units help avoid medication errors
Many medications are available in numerous dosage forms, which increases the risk of medication errors. To reduce dosing errors and avoid unnecessarily complex dosing, I suggest employing a “clinical reference dosing unit” (CRDU)—a basic reference dose expressed in milligrams that covers the typical dose range if administered as 1 to 4 pills.
CRDUs can help you and your patients remember a typical starting dose (1 pill), a target dose (2 or 3 pills), a high dose (4 pills), and a safe dose to make changes (1 pill). CRDUs also can help you track your prescribing because you can easily spot doses outside the usual range. For example, 8 pills indicate an unusually high dosage and a half pill might be too low.
Implementing CRDUs
Develop a list of CRDUs for the psychotropics you frequently prescribe. Note that the appropriate CRDU for a medication might vary among different clinical populations (Table). For any given medication use only 1 formulation, such as immediate-release or extended-release.
Monitor dosing by asking patients how many pills they take and when they take them.
Table
Sample CRDU prescribing of risperidone
| Patient population | CRDU (1 pill) | Dose range (1 to 4 pills) |
|---|---|---|
| First-episode psychosis patients | 1 mg | 1 to 4 mg |
| Chronic patients | 2 mg | 2 to 8 mg |
| Geriatric patients | 0.5 mg | 0.5 to 2 mg |
| CRDU: clinical reference dosing unit | ||
Patient education
Instruct your patients to initiate or change doses based on the number of pills, with 1 pill corresponding to the medication’s CRDU. For example, you might tell your patient, “Start with 1 pill at night for 1 week, then go up to 2 pills at night until you see me again.” Patients are more likely to correctly implement changes when instructions are based on the number of pills rather than on milligrams. Change the dosing to reach desired efficacy or increase tolerability by in-creasing or decreasing the number of pills or shifting the timing of the dosage, such as going from 1 pill twice daily to 2 pills at night.
Although CRDUs can be used for many antipsychotics, antidepressants, and anxiolytics, this method is not appropriate for medications that:
- are administered based on plasma levels or body weight, such as lithium or valproate
- do not have linear pharmacokinetics, such as phenytoin
- require a slower titration, such as clozapine.
Many medications are available in numerous dosage forms, which increases the risk of medication errors. To reduce dosing errors and avoid unnecessarily complex dosing, I suggest employing a “clinical reference dosing unit” (CRDU)—a basic reference dose expressed in milligrams that covers the typical dose range if administered as 1 to 4 pills.
CRDUs can help you and your patients remember a typical starting dose (1 pill), a target dose (2 or 3 pills), a high dose (4 pills), and a safe dose to make changes (1 pill). CRDUs also can help you track your prescribing because you can easily spot doses outside the usual range. For example, 8 pills indicate an unusually high dosage and a half pill might be too low.
Implementing CRDUs
Develop a list of CRDUs for the psychotropics you frequently prescribe. Note that the appropriate CRDU for a medication might vary among different clinical populations (Table). For any given medication use only 1 formulation, such as immediate-release or extended-release.
Monitor dosing by asking patients how many pills they take and when they take them.
Table
Sample CRDU prescribing of risperidone
| Patient population | CRDU (1 pill) | Dose range (1 to 4 pills) |
|---|---|---|
| First-episode psychosis patients | 1 mg | 1 to 4 mg |
| Chronic patients | 2 mg | 2 to 8 mg |
| Geriatric patients | 0.5 mg | 0.5 to 2 mg |
| CRDU: clinical reference dosing unit | ||
Patient education
Instruct your patients to initiate or change doses based on the number of pills, with 1 pill corresponding to the medication’s CRDU. For example, you might tell your patient, “Start with 1 pill at night for 1 week, then go up to 2 pills at night until you see me again.” Patients are more likely to correctly implement changes when instructions are based on the number of pills rather than on milligrams. Change the dosing to reach desired efficacy or increase tolerability by in-creasing or decreasing the number of pills or shifting the timing of the dosage, such as going from 1 pill twice daily to 2 pills at night.
Although CRDUs can be used for many antipsychotics, antidepressants, and anxiolytics, this method is not appropriate for medications that:
- are administered based on plasma levels or body weight, such as lithium or valproate
- do not have linear pharmacokinetics, such as phenytoin
- require a slower titration, such as clozapine.
Many medications are available in numerous dosage forms, which increases the risk of medication errors. To reduce dosing errors and avoid unnecessarily complex dosing, I suggest employing a “clinical reference dosing unit” (CRDU)—a basic reference dose expressed in milligrams that covers the typical dose range if administered as 1 to 4 pills.
CRDUs can help you and your patients remember a typical starting dose (1 pill), a target dose (2 or 3 pills), a high dose (4 pills), and a safe dose to make changes (1 pill). CRDUs also can help you track your prescribing because you can easily spot doses outside the usual range. For example, 8 pills indicate an unusually high dosage and a half pill might be too low.
Implementing CRDUs
Develop a list of CRDUs for the psychotropics you frequently prescribe. Note that the appropriate CRDU for a medication might vary among different clinical populations (Table). For any given medication use only 1 formulation, such as immediate-release or extended-release.
Monitor dosing by asking patients how many pills they take and when they take them.
Table
Sample CRDU prescribing of risperidone
| Patient population | CRDU (1 pill) | Dose range (1 to 4 pills) |
|---|---|---|
| First-episode psychosis patients | 1 mg | 1 to 4 mg |
| Chronic patients | 2 mg | 2 to 8 mg |
| Geriatric patients | 0.5 mg | 0.5 to 2 mg |
| CRDU: clinical reference dosing unit | ||
Patient education
Instruct your patients to initiate or change doses based on the number of pills, with 1 pill corresponding to the medication’s CRDU. For example, you might tell your patient, “Start with 1 pill at night for 1 week, then go up to 2 pills at night until you see me again.” Patients are more likely to correctly implement changes when instructions are based on the number of pills rather than on milligrams. Change the dosing to reach desired efficacy or increase tolerability by in-creasing or decreasing the number of pills or shifting the timing of the dosage, such as going from 1 pill twice daily to 2 pills at night.
Although CRDUs can be used for many antipsychotics, antidepressants, and anxiolytics, this method is not appropriate for medications that:
- are administered based on plasma levels or body weight, such as lithium or valproate
- do not have linear pharmacokinetics, such as phenytoin
- require a slower titration, such as clozapine.
Malpractice minute: June POLL RESULTS
Could a patient’s violent act have been prevented?
A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type. He killed his developmentally disabled niece, age 26. The niece’s family claimed the death could have been prevented if the man was civilly committed or heavily medicated. Was the behavioral health authority liable?
⋥ LIABLE: 11% ⋥ NOT LIABLE: 89%
What did the court decide?
The mother was found to be 39% at fault, the patient 11% at fault, and the behavioral health authority 50% at fault for the woman’s death and paid half of the verdict amount to the parents. A $101,740 verdict was returned for the niece’s mother and a $100,625 verdict was returned for the father.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Could a patient’s violent act have been prevented?
A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type. He killed his developmentally disabled niece, age 26. The niece’s family claimed the death could have been prevented if the man was civilly committed or heavily medicated. Was the behavioral health authority liable?
⋥ LIABLE: 11% ⋥ NOT LIABLE: 89%
What did the court decide?
The mother was found to be 39% at fault, the patient 11% at fault, and the behavioral health authority 50% at fault for the woman’s death and paid half of the verdict amount to the parents. A $101,740 verdict was returned for the niece’s mother and a $100,625 verdict was returned for the father.
Could a patient’s violent act have been prevented?
A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type. He killed his developmentally disabled niece, age 26. The niece’s family claimed the death could have been prevented if the man was civilly committed or heavily medicated. Was the behavioral health authority liable?
⋥ LIABLE: 11% ⋥ NOT LIABLE: 89%
What did the court decide?
The mother was found to be 39% at fault, the patient 11% at fault, and the behavioral health authority 50% at fault for the woman’s death and paid half of the verdict amount to the parents. A $101,740 verdict was returned for the niece’s mother and a $100,625 verdict was returned for the father.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.