User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Interoceptive cues: When ‘gut feelings’ point to anxiety
CASE: 'I don't know how I feel'
Ms. N, age 48, is seen in an outpatient clinic for episodic, impulsive aggression and evaluation of possible bipolar disorder. When you ask her to describe one of her episodes—which always involve a conflict with her partner or another loved one—Ms. N says, “I just lose control… I go blank.” You observe Ms. N’s deep, sighing respirations, trembling hands, and restless, fidgety leg movements. When you ask about her awareness of her physical state while she was recalling the incident, she immediately calms, looks at you quizzically, and states, “I don’t know how I feel.”
When assessing a patient who might have an anxiety disorder, don’t overlook the body. In addition to worry and avoidance, body-centered feelings are a vital component of anxiety and an important treatment target.1
This article:
- highlights clinically relevant neurobiology of anxious feelings
- discusses interoception—awareness of the physiologic state of one’s body—and its connection with anxiety
- explains the use of interoceptive cues as an aid to diagnosing and treating anxiety.
Affective neuroscience and fear
Interoceptive cues are questions directed toward the somatic manifestations of anxiety. Because these questions encourage patients to consciously experience the physical symptoms of anxiety, using interoceptive cues essentially is an exposure-based intervention that may feel counterintuitive to practitioners who are more accustomed to trying to relieve anxiety.
Emotions are thought to be grounded in brain areas that receive and regulate somatic signals, such as the amygdala and insula.2 A feeling-focused approach to anxiety weds affective neuroscience—the study of emotions—with clinical assessment and treatment of anxiety disorders, and conceptualizes that fear is a core component of many anxiety-related disorders.3,4
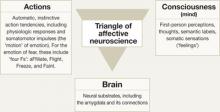
Although the DSM-IV-TR views anxiety disorders as clinically heterogeneous, affective neuroscience emphasizes what these disorders have in common.5 This unifying perspective allows clinicians to anchor anxiety disorders and anxiety-related disorders—such as hypochondriasis—in core emotional systems that have 3 clinically important aspects—actions (behavior and body), brain, and consciousness (mind) (Figure).4 Two emotional systems related to anxiety disorders are fear (anxious anticipation) and panic (evolutionarily related to separation anxiety and suffocation alarm signals). Viewing anxiety disorders as rooted in core emotion systems allows you to incorporate recent advances in emotional neuroscience, including interoception, into your clinical practice.
Figure: A,B,C model for understanding emotions
Affective neuroscience is a broad-based scientific discipline that explores emotions from 3 vantages: actions (behaviors and bodily responses), brain bases, and conscious manifestations. Two core emotional systems related to anxiety disorders are fear and panic.
Source: Adapted with permission from Panksepp J. Affective neuroscience. New York: Oxford University Press; 1998:31.
Detecting ‘hidden’ anxiety
Conscious symptoms. Activity in the brain’s fear system can generate conscious experiences, including worry, heightened arousal, attentional biases, and body-based feelings of fear. Anxious feelings—by definition, sensory experiences—are an important component of an anxiety assessment and relatively easy to identify.
Kroenke et al6 evaluated a 2-item screening tool, the Generalized Anxiety Disorder scale (GAD-2) that highlights both cognitive (worry) and somatic (feeling) sides of anxiety. Researchers asked 965 randomly sampled primary care patients, “Over the past 2 weeks, how often have you been bothered by the following problems:
- feeling nervous, anxious, or on edge
- not being able to stop or control worrying.
Possible responses ranged from 0 (not at all) to 3 (nearly every day). The GAD-2 was as specific for detecting anxiety disorders as a 7-item scale, the GAD-7, (88%), though less sensitive (65% vs 77%).
Nonconscious symptoms. A challenge arises, however, when patients demonstrate signs of anxiety (stress-related physical symptoms such as stomach pains or avoidance-related behaviors) without conscious awareness of anxious feelings. Though patients may intellectually understand the concept of body-based “gut feelings,” these sensations are often reflexively ignored, avoided, or mislabeled. Patients may use terms such as “stressed,” “distressed,” or “tense,” focus on the external source of the fear (rather than their response to it), or reflexively engage in behaviors (avoidance, impulsive behaviors) without being aware of their internal responses.
Anxiety symptoms that occur without corresponding awareness can be called occult, nonconscious, or unconscious anxiety. These symptoms, unique to each patient, can be used as:
- cues to the patient that he or she is anxious
- stimuli to be desensitized (via exposure-based interventions)
- markers of treatment progress.
Patients who experience occult anxiety often have a deficit in interoception (Box).2,7-11 Using interoceptive cues to foster awareness of these unrecognized body-based symptoms can provide insight into formerly unrecognized manifestations of anxiety.
Neurobiology of anxiety
The fear system. Dynamic changes in stimulus-specific physical sensations—anxious feelings—are linked to activity of the brain’s fear system. This system, which detects and rapidly learns to anticipate danger or distress, can exhibit low-level tonic activity (chronic, generalized anxiety), phasic high-amplitude reactivity (spikes of anxiety), and combinations of the 2.4,12
This precognitive, primary-process alarm system can generate:
- behaviors, often centered around avoidance—though other types (such as impulsive) can occur
- physiologic responses, which may or may not become conscious
- states of mind, including attention (hypervigilance, dissociation), cognitive contents (specific worries), and viscerosomatic awareness (“feelings”).
Through learning—and under the influence of temperamental/genetic predispositions—the fear system can be linked to internal and external stimuli, yielding a spectrum of clinical disorders that includes anxiety disorders.5
Consciously experiencing an emotion, attending to an emotionally arousing external stimulus, and remembering an emotionally arousing event all involve overlapping mental and neurobiologic processes in brain areas that process and regulate sensations from the body.2,7 Therefore, one does not need to remember “how one felt in the past” to elicit similar neurobiologic and physiologic responses in the present. These responses are recreated in the present when one consciously activates the memory. This understanding underlies the use of interoceptive cues.
Interoception is intentional, mindful awareness of the physiologic state of one’s body. Consciously directing attention to one’s internal state actively unifies the activity of the attending mind and brain with ongoing visceromotor sensations from the body.8,9 These body-based somatic markers often lie at the border of consciousness and can be brought into awareness via interoceptive cues.9 Awareness of and exposure to these often private, physiologic symptoms is an important part of many evidence-based therapies for anxiety disorders.10,11
Brain basis of fear. The amygdala and insula are 2 key components of the brain basis of fearful feelings.
The amygdala processes internal or external stimuli, alerts other brain areas that a threat is present, and triggers a fear or anxiety response (Table 1).13-16 Early, nonconscious threat detection by the amygdala may be a core component of the brain basis of many anxiety disorders.17
Amygdala activity has been associated with automatic fear perception, associative fear learning, trauma,18 and (on the treatment side) extinction of learned fears via active coping.19 The amygdala provides an extremely rapid response to fearful stimuli—within milliseconds—and can be active without conscious awareness of the stimuli (which may take several hundred milliseconds to develop).20,21
Inputs into the amygdala can come from:
- inside the brain (memories, images, emotions, predictions of the future)
- or outside (contemporary stimuli).
In treatment, the amygdala may be one site of activity of serotonergic medications.22 It is partially regulated by orbitofrontal and medial prefrontal areas that may be target sites of “top-down” psychotherapeutic interventions.23
The insula—a sector of cortex tucked beneath the fissure between the temporal and parietal lobes—is involved in interoception, modulation of emotional processing, and emotional learning, especially as related to aversive internal states.24,25
Paulus24 proposes that in anxiety-prone individuals the insula may create a negatively valenced, preattentive, body-centered warning of negative things to come—in a sense, a somatic semaphore that signals danger ahead. In a related study, Stein et al26 presented college students with emotion-provoking faces. Students prone to anxiety had elevated activity in the amygdala and insula compared with normal controls.
The insula also may respond to mindful mental exercise. Lazar et al27 found increased cortical thickness in prefrontal and anterior insula in 20 subjects with extensive experience in insight meditation, which involves focusing attention on internal states.
Table 1
Amygdala output pathways that result in anxiety symptoms
| Link to specific brain area | Clinically important responses |
|---|---|
| Hypothalamus | Sympathetic activation: increased heart rate, sweating, dilated pupils, striated muscle tension, strained breathing |
| Dorsal motor nucleus of vagus | Parasympathetic activation: slowed heart rate, bladder and bowel symptoms—frequent urination, diarrhea—via smooth muscle activity, gastric acid secretion |
| Parabrachial nucleus | Increased respiratory rate: sighing respirations |
| Ventral tegmental area/locus ceruleus | Generalized arousal, perceptual vigilance (excessive stimulation leads to disruption of attention/dissociation, via prefrontal cortical connections) |
| Nucleus reticularis pontis caudalis | Startle response, jumpiness |
| Periaqueductal gray matter | Automatic coping patterns, from passive (freeze, collapse) to active (confrontation, fight) |
| Trigeminal facial motor nuclei | Jaw tension, facial expressions of fear |
| Source: Adapted with permission from references 13 with additional information from references 14-16 | |
CASE CONTINUED: Using focused interoception
You help Ms. N become aware of her somatic symptoms of anxiety by using a series of questions to direct her attention to her physical responses in a “head-to-toe” approach: “Do you notice the tension in your jaw?” “Is your neck tense?” “How is your breathing now?” Though Ms. N had been unaware of these symptoms, she easily agrees: “Yes, now that you mention it, I am aware of that, but I never knew it was anxiety. I thought it was just stress.”
This exercise reveals marked generalized muscle tension, sweating, and a brief period of going “blank” in her mind when she recalled one of her impulsive, aggressive episodes. You explain that these physical reactions are part of the normal biologic fear response. Apart from these symptoms, Ms. N denies any prototypical manic symptoms and does not meet bipolar disorder criteria.
Using interoceptive cues
To frame an interoceptive inquiry, discuss with patients how the brain’s fear system is connected to the body, and explain that investigating these physical symptoms can assist diagnosis and treatment. For example, you might ask, “Could we look into your physical responses in these situations to help us better understand your difficulties?”
To actively explore somatic markers of anxiety (anxious feelings), encourage the patient to describe a specific stressful or avoided situation in detail. While he or she does this, direct the patient’s attention to objective physiologic markers of anxiety, such as strained breathing or increased heart rate. Use body-directed questions (interoceptive cues) to foreground these sensory experiences in the patient’s mind. For example:
- “As we are discussing this issue, I notice your breathing becomes more strained. Do you notice it?”
- “As you picture this incident in your mind, are you aware of what happens in your body?”
- “When you perceive her in that way, what do you notice about your physical response?”
You can further inquire into these somatic symptoms and their effect on the patient by asking, “How long have you been having these particular symptoms?” “How frequently do they occur?” or “How distressing are these symptoms?” These questions can separate transient physiologic arousal (normal) from pathologic (recurrent, disabling) responses that may respond to treatment. These cues and their responses can be used as person-specific biomarkers to assay a patient’s:
- ability to attend to his or her somatic state
- baseline level of autonomic arousal
- internal state before problematic behaviors (such as impulsive or self-harming behaviors, substance use)
- tendency toward anxiety-related perceptual disturbances (such as dissociation).
When the patient actively attends to and carefully describes his or her somatic sensations, the immediate outcome typically is anxiolytic. A shared awareness of the anxiolytic nature of this exercise—“It’s interesting that paying attention to these feelings actually reduces anxiety”—creates a positive first step toward further exploration. Patients can feel the power of the mind to regulate distress.
Overcoming barriers to interoception
Many patients—including those with dissociative disorders, impulse control disorders, or disorders with significant obsessive features—have difficulty using their attention to bring physical symptoms to mind. Some develop automatic, phobic patterns of disattention to contemporaneous somatic feelings of anxiety. This experiential avoidance is the fear of fear itself—fear of the conscious experience of fearful feelings. Their typical responses to interoceptive cues include:
- lack of awareness (“I don’t know,” “I wasn’t aware of anything”)
- perceptions, phrased as feelings (“I feel as if he doesn’t like me”)
- action tendencies or impulses, phrased as feelings (“I feel like I want to get out of there”)
- a verbal explanation of why they are anxious (“I’m worried about what might happen”).
Depending on the context of your inquiry, if the patient does not respond to an interoceptive cue with actual body-centered feelings, you can:
- reframe the question: “OK, but when you perceive him in that way, if you focus your mind on your physical reactions, what do you notice?”
- point out observable symptoms: “Did you notice as we were talking about this issue that your breathing got very shallow, and your hands got tense?”
Some patients may look transiently “spacey” or report “checking out” during the exercise. Inquire specifically about this because they may be demonstrating dissociative symptoms: “Does this sometimes happen when you are stressed, that you lose touch with your sense of your body, you go numb or your mind goes blank?” These symptoms warrant attention, as they may preclude effective retention of the exercise.
Explaining occult anxiety
Regardless of how far you choose to pursue an interoceptive inquiry, uncovering an interoceptive deficit—an inability to describe one’s somatic experience—may be diagnostically helpful. Doing so identifies a potentially modifiable component of self-awareness. So-called mindfulness-based and emotion-focused therapies assist patients in developing a more robust awareness and understanding of their emotions, including the somatic sensations of emotion (see Related Resources).
With appropriate psychoeducation, an interoceptive exploration makes anxiety a real, physical event anchored in brain-body function, and facilitates a nonshaming, organ-based explanation of anxiety. Psychoeducation about fear grounds physical symptoms of anxiety in a brain-based, evolutionarily selected neural system whose activity has a variety of inputs and outputs (Table 2).
An organ-based, body-centered discussion also may reduce defensiveness in patients who feel (or have been told) that anxiety is “not real” or signals personality weakness. This model may help trainees and medical colleagues avoid outdated distinctions between real/organic problems and functional/emotional problems and find a more conciliatory construct based in emotional neuroscience.
Serotonergic medications and psychotherapy—both of which work on the brain—have demonstrated broad efficacy for anxiety disorders.5 Several national organizations offer information about evidence-based psychotherapeutic treatments grounded in emotional awareness and neuroscience (see Related Resources).
Table 2
Activity of the fear system
| Inputs |
| Contemporary situations |
| Memories (visual and sensory) |
| Anticipated future situations |
| Other nonconscious body and brain processes (including the physiologic symptoms of emotions and anxiety—a ‘fear of fear’ or ‘fear of feelings’) |
| Outputs |
| Physical symptoms |
| Thoughts |
| Perceptions |
| Behaviors |
| States of attention |
CASE CONTINUED: Putting interoception to work
Your psychotherapeutic work with Ms. N focuses on attending to and consciously modulating her newly labeled anxiety For example, after an inquiry into a “stressful” situation, you help her use careful interoceptive attention—and when necessary, mindful relaxation and breathing—to regulate her fear symptoms.
She finds that these simple “exposure/regulation” exercises are enough to rapidly resolve her impulsive behaviors. In distressing situations, she can now be aware of her reactions and make a conscious choice of how to react. Your psychotherapeutic work now proceeds toward more effective interpersonal expression of other emotions.
- Barrett LF, Mesquita B, Ochsner K, Gross JJ. The experience of emotion. Annu Rev Psychol 2007;58:373-403.
- Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt; 1999.
- International Experiential Dynamic Therapy Association. www.iedta.net.
- International Center for Excellence in Emotionally Focused Therapy. www.eft.ca.
Disclosure
Dr. MacDonald reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007;29(2):147-55.
2. Niedenthal PM. Embodying emotion. Science 2007;316(5827):1002-5.
3. McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007;64(1):97-106.
4. Panksepp J. Affective neuroscience. New York: Oxford University Press; 1998:31.
5. Stein DJ. Advances in understanding the anxiety disorders: the cognitive-affective neuroscience of ‘false alarms’. Ann Clin Psychiatry 2006;18(3):173-82.
6. Kroenke K, Spitzer RL, Williams JBW, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146(5):317-25.
7. Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt; 1999.
8. Wiens S. Interoception in emotional experience. Curr Opin Neurol 2005;18(4):442-7.
9. Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosom Med 2001;63(5):697-710.
10. Wald J, Taylor S. Interoceptive exposure therapy combined with trauma-related exposure therapy for post-traumatic stress disorder: a case report. Cognit Behav Ther 2005;34(1):34-40.
11. Wells A, Papageorgiou C. Social phobic interoception: effects of bodily information on anxiety, beliefs and self-processing. Behav Res Ther 2001;39(1):1-11.
12. Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 2002;51(1):68-80.
13. Kandel ER, Squire LR. Memory: from mind to molecules. New York: Henry Holt and Company, 1999.
14. Fokkema DS. The psychobiology of strained breathing and its cardiovascular implications: a functional system review. Psychophysiology 1999;36(2):164-75.
15. Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry 2006;67 (suppl 8):7-12.
16. Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 2001;25(7-8):669-78.
17. Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med 2006;73(7):941-9.
18. Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol 2006;57:27-53.
19. LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry 2001;158(12):1953-5.
20. LeDoux JE. The emotional brain. New York: Simon and Schuster, 1996.
21. Liddell BJ, Brown KJ, Kemp AH, et al. A direct brainstemamygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage 2005;24(1):235-43.
22. Harmer CJ, Mackay CE, Reid CB, et al. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 2006;59(9):816-20.
23. Hariri AR, Mattay VS, Tessitore A, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 2003;53(6):494-501.
24. Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006;60(4):383-7.
25. Simmons A, Strigo I, Matthews SC, et al. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry 2006;60(4):402-9.
26. Stein MB, Simmons AN, Feinstein JS, et al. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007;164(2):318-27.
27. Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005;16(17):1893-7.
CASE: 'I don't know how I feel'
Ms. N, age 48, is seen in an outpatient clinic for episodic, impulsive aggression and evaluation of possible bipolar disorder. When you ask her to describe one of her episodes—which always involve a conflict with her partner or another loved one—Ms. N says, “I just lose control… I go blank.” You observe Ms. N’s deep, sighing respirations, trembling hands, and restless, fidgety leg movements. When you ask about her awareness of her physical state while she was recalling the incident, she immediately calms, looks at you quizzically, and states, “I don’t know how I feel.”
When assessing a patient who might have an anxiety disorder, don’t overlook the body. In addition to worry and avoidance, body-centered feelings are a vital component of anxiety and an important treatment target.1
This article:
- highlights clinically relevant neurobiology of anxious feelings
- discusses interoception—awareness of the physiologic state of one’s body—and its connection with anxiety
- explains the use of interoceptive cues as an aid to diagnosing and treating anxiety.
Affective neuroscience and fear
Interoceptive cues are questions directed toward the somatic manifestations of anxiety. Because these questions encourage patients to consciously experience the physical symptoms of anxiety, using interoceptive cues essentially is an exposure-based intervention that may feel counterintuitive to practitioners who are more accustomed to trying to relieve anxiety.
Emotions are thought to be grounded in brain areas that receive and regulate somatic signals, such as the amygdala and insula.2 A feeling-focused approach to anxiety weds affective neuroscience—the study of emotions—with clinical assessment and treatment of anxiety disorders, and conceptualizes that fear is a core component of many anxiety-related disorders.3,4
Although the DSM-IV-TR views anxiety disorders as clinically heterogeneous, affective neuroscience emphasizes what these disorders have in common.5 This unifying perspective allows clinicians to anchor anxiety disorders and anxiety-related disorders—such as hypochondriasis—in core emotional systems that have 3 clinically important aspects—actions (behavior and body), brain, and consciousness (mind) (Figure).4 Two emotional systems related to anxiety disorders are fear (anxious anticipation) and panic (evolutionarily related to separation anxiety and suffocation alarm signals). Viewing anxiety disorders as rooted in core emotion systems allows you to incorporate recent advances in emotional neuroscience, including interoception, into your clinical practice.
Figure: A,B,C model for understanding emotions
Affective neuroscience is a broad-based scientific discipline that explores emotions from 3 vantages: actions (behaviors and bodily responses), brain bases, and conscious manifestations. Two core emotional systems related to anxiety disorders are fear and panic.
Source: Adapted with permission from Panksepp J. Affective neuroscience. New York: Oxford University Press; 1998:31.
Detecting ‘hidden’ anxiety
Conscious symptoms. Activity in the brain’s fear system can generate conscious experiences, including worry, heightened arousal, attentional biases, and body-based feelings of fear. Anxious feelings—by definition, sensory experiences—are an important component of an anxiety assessment and relatively easy to identify.
Kroenke et al6 evaluated a 2-item screening tool, the Generalized Anxiety Disorder scale (GAD-2) that highlights both cognitive (worry) and somatic (feeling) sides of anxiety. Researchers asked 965 randomly sampled primary care patients, “Over the past 2 weeks, how often have you been bothered by the following problems:
- feeling nervous, anxious, or on edge
- not being able to stop or control worrying.
Possible responses ranged from 0 (not at all) to 3 (nearly every day). The GAD-2 was as specific for detecting anxiety disorders as a 7-item scale, the GAD-7, (88%), though less sensitive (65% vs 77%).
Nonconscious symptoms. A challenge arises, however, when patients demonstrate signs of anxiety (stress-related physical symptoms such as stomach pains or avoidance-related behaviors) without conscious awareness of anxious feelings. Though patients may intellectually understand the concept of body-based “gut feelings,” these sensations are often reflexively ignored, avoided, or mislabeled. Patients may use terms such as “stressed,” “distressed,” or “tense,” focus on the external source of the fear (rather than their response to it), or reflexively engage in behaviors (avoidance, impulsive behaviors) without being aware of their internal responses.
Anxiety symptoms that occur without corresponding awareness can be called occult, nonconscious, or unconscious anxiety. These symptoms, unique to each patient, can be used as:
- cues to the patient that he or she is anxious
- stimuli to be desensitized (via exposure-based interventions)
- markers of treatment progress.
Patients who experience occult anxiety often have a deficit in interoception (Box).2,7-11 Using interoceptive cues to foster awareness of these unrecognized body-based symptoms can provide insight into formerly unrecognized manifestations of anxiety.
Neurobiology of anxiety
The fear system. Dynamic changes in stimulus-specific physical sensations—anxious feelings—are linked to activity of the brain’s fear system. This system, which detects and rapidly learns to anticipate danger or distress, can exhibit low-level tonic activity (chronic, generalized anxiety), phasic high-amplitude reactivity (spikes of anxiety), and combinations of the 2.4,12
This precognitive, primary-process alarm system can generate:
- behaviors, often centered around avoidance—though other types (such as impulsive) can occur
- physiologic responses, which may or may not become conscious
- states of mind, including attention (hypervigilance, dissociation), cognitive contents (specific worries), and viscerosomatic awareness (“feelings”).
Through learning—and under the influence of temperamental/genetic predispositions—the fear system can be linked to internal and external stimuli, yielding a spectrum of clinical disorders that includes anxiety disorders.5
Consciously experiencing an emotion, attending to an emotionally arousing external stimulus, and remembering an emotionally arousing event all involve overlapping mental and neurobiologic processes in brain areas that process and regulate sensations from the body.2,7 Therefore, one does not need to remember “how one felt in the past” to elicit similar neurobiologic and physiologic responses in the present. These responses are recreated in the present when one consciously activates the memory. This understanding underlies the use of interoceptive cues.
Interoception is intentional, mindful awareness of the physiologic state of one’s body. Consciously directing attention to one’s internal state actively unifies the activity of the attending mind and brain with ongoing visceromotor sensations from the body.8,9 These body-based somatic markers often lie at the border of consciousness and can be brought into awareness via interoceptive cues.9 Awareness of and exposure to these often private, physiologic symptoms is an important part of many evidence-based therapies for anxiety disorders.10,11
Brain basis of fear. The amygdala and insula are 2 key components of the brain basis of fearful feelings.
The amygdala processes internal or external stimuli, alerts other brain areas that a threat is present, and triggers a fear or anxiety response (Table 1).13-16 Early, nonconscious threat detection by the amygdala may be a core component of the brain basis of many anxiety disorders.17
Amygdala activity has been associated with automatic fear perception, associative fear learning, trauma,18 and (on the treatment side) extinction of learned fears via active coping.19 The amygdala provides an extremely rapid response to fearful stimuli—within milliseconds—and can be active without conscious awareness of the stimuli (which may take several hundred milliseconds to develop).20,21
Inputs into the amygdala can come from:
- inside the brain (memories, images, emotions, predictions of the future)
- or outside (contemporary stimuli).
In treatment, the amygdala may be one site of activity of serotonergic medications.22 It is partially regulated by orbitofrontal and medial prefrontal areas that may be target sites of “top-down” psychotherapeutic interventions.23
The insula—a sector of cortex tucked beneath the fissure between the temporal and parietal lobes—is involved in interoception, modulation of emotional processing, and emotional learning, especially as related to aversive internal states.24,25
Paulus24 proposes that in anxiety-prone individuals the insula may create a negatively valenced, preattentive, body-centered warning of negative things to come—in a sense, a somatic semaphore that signals danger ahead. In a related study, Stein et al26 presented college students with emotion-provoking faces. Students prone to anxiety had elevated activity in the amygdala and insula compared with normal controls.
The insula also may respond to mindful mental exercise. Lazar et al27 found increased cortical thickness in prefrontal and anterior insula in 20 subjects with extensive experience in insight meditation, which involves focusing attention on internal states.
Table 1
Amygdala output pathways that result in anxiety symptoms
| Link to specific brain area | Clinically important responses |
|---|---|
| Hypothalamus | Sympathetic activation: increased heart rate, sweating, dilated pupils, striated muscle tension, strained breathing |
| Dorsal motor nucleus of vagus | Parasympathetic activation: slowed heart rate, bladder and bowel symptoms—frequent urination, diarrhea—via smooth muscle activity, gastric acid secretion |
| Parabrachial nucleus | Increased respiratory rate: sighing respirations |
| Ventral tegmental area/locus ceruleus | Generalized arousal, perceptual vigilance (excessive stimulation leads to disruption of attention/dissociation, via prefrontal cortical connections) |
| Nucleus reticularis pontis caudalis | Startle response, jumpiness |
| Periaqueductal gray matter | Automatic coping patterns, from passive (freeze, collapse) to active (confrontation, fight) |
| Trigeminal facial motor nuclei | Jaw tension, facial expressions of fear |
| Source: Adapted with permission from references 13 with additional information from references 14-16 | |
CASE CONTINUED: Using focused interoception
You help Ms. N become aware of her somatic symptoms of anxiety by using a series of questions to direct her attention to her physical responses in a “head-to-toe” approach: “Do you notice the tension in your jaw?” “Is your neck tense?” “How is your breathing now?” Though Ms. N had been unaware of these symptoms, she easily agrees: “Yes, now that you mention it, I am aware of that, but I never knew it was anxiety. I thought it was just stress.”
This exercise reveals marked generalized muscle tension, sweating, and a brief period of going “blank” in her mind when she recalled one of her impulsive, aggressive episodes. You explain that these physical reactions are part of the normal biologic fear response. Apart from these symptoms, Ms. N denies any prototypical manic symptoms and does not meet bipolar disorder criteria.
Using interoceptive cues
To frame an interoceptive inquiry, discuss with patients how the brain’s fear system is connected to the body, and explain that investigating these physical symptoms can assist diagnosis and treatment. For example, you might ask, “Could we look into your physical responses in these situations to help us better understand your difficulties?”
To actively explore somatic markers of anxiety (anxious feelings), encourage the patient to describe a specific stressful or avoided situation in detail. While he or she does this, direct the patient’s attention to objective physiologic markers of anxiety, such as strained breathing or increased heart rate. Use body-directed questions (interoceptive cues) to foreground these sensory experiences in the patient’s mind. For example:
- “As we are discussing this issue, I notice your breathing becomes more strained. Do you notice it?”
- “As you picture this incident in your mind, are you aware of what happens in your body?”
- “When you perceive her in that way, what do you notice about your physical response?”
You can further inquire into these somatic symptoms and their effect on the patient by asking, “How long have you been having these particular symptoms?” “How frequently do they occur?” or “How distressing are these symptoms?” These questions can separate transient physiologic arousal (normal) from pathologic (recurrent, disabling) responses that may respond to treatment. These cues and their responses can be used as person-specific biomarkers to assay a patient’s:
- ability to attend to his or her somatic state
- baseline level of autonomic arousal
- internal state before problematic behaviors (such as impulsive or self-harming behaviors, substance use)
- tendency toward anxiety-related perceptual disturbances (such as dissociation).
When the patient actively attends to and carefully describes his or her somatic sensations, the immediate outcome typically is anxiolytic. A shared awareness of the anxiolytic nature of this exercise—“It’s interesting that paying attention to these feelings actually reduces anxiety”—creates a positive first step toward further exploration. Patients can feel the power of the mind to regulate distress.
Overcoming barriers to interoception
Many patients—including those with dissociative disorders, impulse control disorders, or disorders with significant obsessive features—have difficulty using their attention to bring physical symptoms to mind. Some develop automatic, phobic patterns of disattention to contemporaneous somatic feelings of anxiety. This experiential avoidance is the fear of fear itself—fear of the conscious experience of fearful feelings. Their typical responses to interoceptive cues include:
- lack of awareness (“I don’t know,” “I wasn’t aware of anything”)
- perceptions, phrased as feelings (“I feel as if he doesn’t like me”)
- action tendencies or impulses, phrased as feelings (“I feel like I want to get out of there”)
- a verbal explanation of why they are anxious (“I’m worried about what might happen”).
Depending on the context of your inquiry, if the patient does not respond to an interoceptive cue with actual body-centered feelings, you can:
- reframe the question: “OK, but when you perceive him in that way, if you focus your mind on your physical reactions, what do you notice?”
- point out observable symptoms: “Did you notice as we were talking about this issue that your breathing got very shallow, and your hands got tense?”
Some patients may look transiently “spacey” or report “checking out” during the exercise. Inquire specifically about this because they may be demonstrating dissociative symptoms: “Does this sometimes happen when you are stressed, that you lose touch with your sense of your body, you go numb or your mind goes blank?” These symptoms warrant attention, as they may preclude effective retention of the exercise.
Explaining occult anxiety
Regardless of how far you choose to pursue an interoceptive inquiry, uncovering an interoceptive deficit—an inability to describe one’s somatic experience—may be diagnostically helpful. Doing so identifies a potentially modifiable component of self-awareness. So-called mindfulness-based and emotion-focused therapies assist patients in developing a more robust awareness and understanding of their emotions, including the somatic sensations of emotion (see Related Resources).
With appropriate psychoeducation, an interoceptive exploration makes anxiety a real, physical event anchored in brain-body function, and facilitates a nonshaming, organ-based explanation of anxiety. Psychoeducation about fear grounds physical symptoms of anxiety in a brain-based, evolutionarily selected neural system whose activity has a variety of inputs and outputs (Table 2).
An organ-based, body-centered discussion also may reduce defensiveness in patients who feel (or have been told) that anxiety is “not real” or signals personality weakness. This model may help trainees and medical colleagues avoid outdated distinctions between real/organic problems and functional/emotional problems and find a more conciliatory construct based in emotional neuroscience.
Serotonergic medications and psychotherapy—both of which work on the brain—have demonstrated broad efficacy for anxiety disorders.5 Several national organizations offer information about evidence-based psychotherapeutic treatments grounded in emotional awareness and neuroscience (see Related Resources).
Table 2
Activity of the fear system
| Inputs |
| Contemporary situations |
| Memories (visual and sensory) |
| Anticipated future situations |
| Other nonconscious body and brain processes (including the physiologic symptoms of emotions and anxiety—a ‘fear of fear’ or ‘fear of feelings’) |
| Outputs |
| Physical symptoms |
| Thoughts |
| Perceptions |
| Behaviors |
| States of attention |
CASE CONTINUED: Putting interoception to work
Your psychotherapeutic work with Ms. N focuses on attending to and consciously modulating her newly labeled anxiety For example, after an inquiry into a “stressful” situation, you help her use careful interoceptive attention—and when necessary, mindful relaxation and breathing—to regulate her fear symptoms.
She finds that these simple “exposure/regulation” exercises are enough to rapidly resolve her impulsive behaviors. In distressing situations, she can now be aware of her reactions and make a conscious choice of how to react. Your psychotherapeutic work now proceeds toward more effective interpersonal expression of other emotions.
- Barrett LF, Mesquita B, Ochsner K, Gross JJ. The experience of emotion. Annu Rev Psychol 2007;58:373-403.
- Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt; 1999.
- International Experiential Dynamic Therapy Association. www.iedta.net.
- International Center for Excellence in Emotionally Focused Therapy. www.eft.ca.
Disclosure
Dr. MacDonald reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: 'I don't know how I feel'
Ms. N, age 48, is seen in an outpatient clinic for episodic, impulsive aggression and evaluation of possible bipolar disorder. When you ask her to describe one of her episodes—which always involve a conflict with her partner or another loved one—Ms. N says, “I just lose control… I go blank.” You observe Ms. N’s deep, sighing respirations, trembling hands, and restless, fidgety leg movements. When you ask about her awareness of her physical state while she was recalling the incident, she immediately calms, looks at you quizzically, and states, “I don’t know how I feel.”
When assessing a patient who might have an anxiety disorder, don’t overlook the body. In addition to worry and avoidance, body-centered feelings are a vital component of anxiety and an important treatment target.1
This article:
- highlights clinically relevant neurobiology of anxious feelings
- discusses interoception—awareness of the physiologic state of one’s body—and its connection with anxiety
- explains the use of interoceptive cues as an aid to diagnosing and treating anxiety.
Affective neuroscience and fear
Interoceptive cues are questions directed toward the somatic manifestations of anxiety. Because these questions encourage patients to consciously experience the physical symptoms of anxiety, using interoceptive cues essentially is an exposure-based intervention that may feel counterintuitive to practitioners who are more accustomed to trying to relieve anxiety.
Emotions are thought to be grounded in brain areas that receive and regulate somatic signals, such as the amygdala and insula.2 A feeling-focused approach to anxiety weds affective neuroscience—the study of emotions—with clinical assessment and treatment of anxiety disorders, and conceptualizes that fear is a core component of many anxiety-related disorders.3,4
Although the DSM-IV-TR views anxiety disorders as clinically heterogeneous, affective neuroscience emphasizes what these disorders have in common.5 This unifying perspective allows clinicians to anchor anxiety disorders and anxiety-related disorders—such as hypochondriasis—in core emotional systems that have 3 clinically important aspects—actions (behavior and body), brain, and consciousness (mind) (Figure).4 Two emotional systems related to anxiety disorders are fear (anxious anticipation) and panic (evolutionarily related to separation anxiety and suffocation alarm signals). Viewing anxiety disorders as rooted in core emotion systems allows you to incorporate recent advances in emotional neuroscience, including interoception, into your clinical practice.
Figure: A,B,C model for understanding emotions
Affective neuroscience is a broad-based scientific discipline that explores emotions from 3 vantages: actions (behaviors and bodily responses), brain bases, and conscious manifestations. Two core emotional systems related to anxiety disorders are fear and panic.
Source: Adapted with permission from Panksepp J. Affective neuroscience. New York: Oxford University Press; 1998:31.
Detecting ‘hidden’ anxiety
Conscious symptoms. Activity in the brain’s fear system can generate conscious experiences, including worry, heightened arousal, attentional biases, and body-based feelings of fear. Anxious feelings—by definition, sensory experiences—are an important component of an anxiety assessment and relatively easy to identify.
Kroenke et al6 evaluated a 2-item screening tool, the Generalized Anxiety Disorder scale (GAD-2) that highlights both cognitive (worry) and somatic (feeling) sides of anxiety. Researchers asked 965 randomly sampled primary care patients, “Over the past 2 weeks, how often have you been bothered by the following problems:
- feeling nervous, anxious, or on edge
- not being able to stop or control worrying.
Possible responses ranged from 0 (not at all) to 3 (nearly every day). The GAD-2 was as specific for detecting anxiety disorders as a 7-item scale, the GAD-7, (88%), though less sensitive (65% vs 77%).
Nonconscious symptoms. A challenge arises, however, when patients demonstrate signs of anxiety (stress-related physical symptoms such as stomach pains or avoidance-related behaviors) without conscious awareness of anxious feelings. Though patients may intellectually understand the concept of body-based “gut feelings,” these sensations are often reflexively ignored, avoided, or mislabeled. Patients may use terms such as “stressed,” “distressed,” or “tense,” focus on the external source of the fear (rather than their response to it), or reflexively engage in behaviors (avoidance, impulsive behaviors) without being aware of their internal responses.
Anxiety symptoms that occur without corresponding awareness can be called occult, nonconscious, or unconscious anxiety. These symptoms, unique to each patient, can be used as:
- cues to the patient that he or she is anxious
- stimuli to be desensitized (via exposure-based interventions)
- markers of treatment progress.
Patients who experience occult anxiety often have a deficit in interoception (Box).2,7-11 Using interoceptive cues to foster awareness of these unrecognized body-based symptoms can provide insight into formerly unrecognized manifestations of anxiety.
Neurobiology of anxiety
The fear system. Dynamic changes in stimulus-specific physical sensations—anxious feelings—are linked to activity of the brain’s fear system. This system, which detects and rapidly learns to anticipate danger or distress, can exhibit low-level tonic activity (chronic, generalized anxiety), phasic high-amplitude reactivity (spikes of anxiety), and combinations of the 2.4,12
This precognitive, primary-process alarm system can generate:
- behaviors, often centered around avoidance—though other types (such as impulsive) can occur
- physiologic responses, which may or may not become conscious
- states of mind, including attention (hypervigilance, dissociation), cognitive contents (specific worries), and viscerosomatic awareness (“feelings”).
Through learning—and under the influence of temperamental/genetic predispositions—the fear system can be linked to internal and external stimuli, yielding a spectrum of clinical disorders that includes anxiety disorders.5
Consciously experiencing an emotion, attending to an emotionally arousing external stimulus, and remembering an emotionally arousing event all involve overlapping mental and neurobiologic processes in brain areas that process and regulate sensations from the body.2,7 Therefore, one does not need to remember “how one felt in the past” to elicit similar neurobiologic and physiologic responses in the present. These responses are recreated in the present when one consciously activates the memory. This understanding underlies the use of interoceptive cues.
Interoception is intentional, mindful awareness of the physiologic state of one’s body. Consciously directing attention to one’s internal state actively unifies the activity of the attending mind and brain with ongoing visceromotor sensations from the body.8,9 These body-based somatic markers often lie at the border of consciousness and can be brought into awareness via interoceptive cues.9 Awareness of and exposure to these often private, physiologic symptoms is an important part of many evidence-based therapies for anxiety disorders.10,11
Brain basis of fear. The amygdala and insula are 2 key components of the brain basis of fearful feelings.
The amygdala processes internal or external stimuli, alerts other brain areas that a threat is present, and triggers a fear or anxiety response (Table 1).13-16 Early, nonconscious threat detection by the amygdala may be a core component of the brain basis of many anxiety disorders.17
Amygdala activity has been associated with automatic fear perception, associative fear learning, trauma,18 and (on the treatment side) extinction of learned fears via active coping.19 The amygdala provides an extremely rapid response to fearful stimuli—within milliseconds—and can be active without conscious awareness of the stimuli (which may take several hundred milliseconds to develop).20,21
Inputs into the amygdala can come from:
- inside the brain (memories, images, emotions, predictions of the future)
- or outside (contemporary stimuli).
In treatment, the amygdala may be one site of activity of serotonergic medications.22 It is partially regulated by orbitofrontal and medial prefrontal areas that may be target sites of “top-down” psychotherapeutic interventions.23
The insula—a sector of cortex tucked beneath the fissure between the temporal and parietal lobes—is involved in interoception, modulation of emotional processing, and emotional learning, especially as related to aversive internal states.24,25
Paulus24 proposes that in anxiety-prone individuals the insula may create a negatively valenced, preattentive, body-centered warning of negative things to come—in a sense, a somatic semaphore that signals danger ahead. In a related study, Stein et al26 presented college students with emotion-provoking faces. Students prone to anxiety had elevated activity in the amygdala and insula compared with normal controls.
The insula also may respond to mindful mental exercise. Lazar et al27 found increased cortical thickness in prefrontal and anterior insula in 20 subjects with extensive experience in insight meditation, which involves focusing attention on internal states.
Table 1
Amygdala output pathways that result in anxiety symptoms
| Link to specific brain area | Clinically important responses |
|---|---|
| Hypothalamus | Sympathetic activation: increased heart rate, sweating, dilated pupils, striated muscle tension, strained breathing |
| Dorsal motor nucleus of vagus | Parasympathetic activation: slowed heart rate, bladder and bowel symptoms—frequent urination, diarrhea—via smooth muscle activity, gastric acid secretion |
| Parabrachial nucleus | Increased respiratory rate: sighing respirations |
| Ventral tegmental area/locus ceruleus | Generalized arousal, perceptual vigilance (excessive stimulation leads to disruption of attention/dissociation, via prefrontal cortical connections) |
| Nucleus reticularis pontis caudalis | Startle response, jumpiness |
| Periaqueductal gray matter | Automatic coping patterns, from passive (freeze, collapse) to active (confrontation, fight) |
| Trigeminal facial motor nuclei | Jaw tension, facial expressions of fear |
| Source: Adapted with permission from references 13 with additional information from references 14-16 | |
CASE CONTINUED: Using focused interoception
You help Ms. N become aware of her somatic symptoms of anxiety by using a series of questions to direct her attention to her physical responses in a “head-to-toe” approach: “Do you notice the tension in your jaw?” “Is your neck tense?” “How is your breathing now?” Though Ms. N had been unaware of these symptoms, she easily agrees: “Yes, now that you mention it, I am aware of that, but I never knew it was anxiety. I thought it was just stress.”
This exercise reveals marked generalized muscle tension, sweating, and a brief period of going “blank” in her mind when she recalled one of her impulsive, aggressive episodes. You explain that these physical reactions are part of the normal biologic fear response. Apart from these symptoms, Ms. N denies any prototypical manic symptoms and does not meet bipolar disorder criteria.
Using interoceptive cues
To frame an interoceptive inquiry, discuss with patients how the brain’s fear system is connected to the body, and explain that investigating these physical symptoms can assist diagnosis and treatment. For example, you might ask, “Could we look into your physical responses in these situations to help us better understand your difficulties?”
To actively explore somatic markers of anxiety (anxious feelings), encourage the patient to describe a specific stressful or avoided situation in detail. While he or she does this, direct the patient’s attention to objective physiologic markers of anxiety, such as strained breathing or increased heart rate. Use body-directed questions (interoceptive cues) to foreground these sensory experiences in the patient’s mind. For example:
- “As we are discussing this issue, I notice your breathing becomes more strained. Do you notice it?”
- “As you picture this incident in your mind, are you aware of what happens in your body?”
- “When you perceive her in that way, what do you notice about your physical response?”
You can further inquire into these somatic symptoms and their effect on the patient by asking, “How long have you been having these particular symptoms?” “How frequently do they occur?” or “How distressing are these symptoms?” These questions can separate transient physiologic arousal (normal) from pathologic (recurrent, disabling) responses that may respond to treatment. These cues and their responses can be used as person-specific biomarkers to assay a patient’s:
- ability to attend to his or her somatic state
- baseline level of autonomic arousal
- internal state before problematic behaviors (such as impulsive or self-harming behaviors, substance use)
- tendency toward anxiety-related perceptual disturbances (such as dissociation).
When the patient actively attends to and carefully describes his or her somatic sensations, the immediate outcome typically is anxiolytic. A shared awareness of the anxiolytic nature of this exercise—“It’s interesting that paying attention to these feelings actually reduces anxiety”—creates a positive first step toward further exploration. Patients can feel the power of the mind to regulate distress.
Overcoming barriers to interoception
Many patients—including those with dissociative disorders, impulse control disorders, or disorders with significant obsessive features—have difficulty using their attention to bring physical symptoms to mind. Some develop automatic, phobic patterns of disattention to contemporaneous somatic feelings of anxiety. This experiential avoidance is the fear of fear itself—fear of the conscious experience of fearful feelings. Their typical responses to interoceptive cues include:
- lack of awareness (“I don’t know,” “I wasn’t aware of anything”)
- perceptions, phrased as feelings (“I feel as if he doesn’t like me”)
- action tendencies or impulses, phrased as feelings (“I feel like I want to get out of there”)
- a verbal explanation of why they are anxious (“I’m worried about what might happen”).
Depending on the context of your inquiry, if the patient does not respond to an interoceptive cue with actual body-centered feelings, you can:
- reframe the question: “OK, but when you perceive him in that way, if you focus your mind on your physical reactions, what do you notice?”
- point out observable symptoms: “Did you notice as we were talking about this issue that your breathing got very shallow, and your hands got tense?”
Some patients may look transiently “spacey” or report “checking out” during the exercise. Inquire specifically about this because they may be demonstrating dissociative symptoms: “Does this sometimes happen when you are stressed, that you lose touch with your sense of your body, you go numb or your mind goes blank?” These symptoms warrant attention, as they may preclude effective retention of the exercise.
Explaining occult anxiety
Regardless of how far you choose to pursue an interoceptive inquiry, uncovering an interoceptive deficit—an inability to describe one’s somatic experience—may be diagnostically helpful. Doing so identifies a potentially modifiable component of self-awareness. So-called mindfulness-based and emotion-focused therapies assist patients in developing a more robust awareness and understanding of their emotions, including the somatic sensations of emotion (see Related Resources).
With appropriate psychoeducation, an interoceptive exploration makes anxiety a real, physical event anchored in brain-body function, and facilitates a nonshaming, organ-based explanation of anxiety. Psychoeducation about fear grounds physical symptoms of anxiety in a brain-based, evolutionarily selected neural system whose activity has a variety of inputs and outputs (Table 2).
An organ-based, body-centered discussion also may reduce defensiveness in patients who feel (or have been told) that anxiety is “not real” or signals personality weakness. This model may help trainees and medical colleagues avoid outdated distinctions between real/organic problems and functional/emotional problems and find a more conciliatory construct based in emotional neuroscience.
Serotonergic medications and psychotherapy—both of which work on the brain—have demonstrated broad efficacy for anxiety disorders.5 Several national organizations offer information about evidence-based psychotherapeutic treatments grounded in emotional awareness and neuroscience (see Related Resources).
Table 2
Activity of the fear system
| Inputs |
| Contemporary situations |
| Memories (visual and sensory) |
| Anticipated future situations |
| Other nonconscious body and brain processes (including the physiologic symptoms of emotions and anxiety—a ‘fear of fear’ or ‘fear of feelings’) |
| Outputs |
| Physical symptoms |
| Thoughts |
| Perceptions |
| Behaviors |
| States of attention |
CASE CONTINUED: Putting interoception to work
Your psychotherapeutic work with Ms. N focuses on attending to and consciously modulating her newly labeled anxiety For example, after an inquiry into a “stressful” situation, you help her use careful interoceptive attention—and when necessary, mindful relaxation and breathing—to regulate her fear symptoms.
She finds that these simple “exposure/regulation” exercises are enough to rapidly resolve her impulsive behaviors. In distressing situations, she can now be aware of her reactions and make a conscious choice of how to react. Your psychotherapeutic work now proceeds toward more effective interpersonal expression of other emotions.
- Barrett LF, Mesquita B, Ochsner K, Gross JJ. The experience of emotion. Annu Rev Psychol 2007;58:373-403.
- Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt; 1999.
- International Experiential Dynamic Therapy Association. www.iedta.net.
- International Center for Excellence in Emotionally Focused Therapy. www.eft.ca.
Disclosure
Dr. MacDonald reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007;29(2):147-55.
2. Niedenthal PM. Embodying emotion. Science 2007;316(5827):1002-5.
3. McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007;64(1):97-106.
4. Panksepp J. Affective neuroscience. New York: Oxford University Press; 1998:31.
5. Stein DJ. Advances in understanding the anxiety disorders: the cognitive-affective neuroscience of ‘false alarms’. Ann Clin Psychiatry 2006;18(3):173-82.
6. Kroenke K, Spitzer RL, Williams JBW, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146(5):317-25.
7. Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt; 1999.
8. Wiens S. Interoception in emotional experience. Curr Opin Neurol 2005;18(4):442-7.
9. Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosom Med 2001;63(5):697-710.
10. Wald J, Taylor S. Interoceptive exposure therapy combined with trauma-related exposure therapy for post-traumatic stress disorder: a case report. Cognit Behav Ther 2005;34(1):34-40.
11. Wells A, Papageorgiou C. Social phobic interoception: effects of bodily information on anxiety, beliefs and self-processing. Behav Res Ther 2001;39(1):1-11.
12. Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 2002;51(1):68-80.
13. Kandel ER, Squire LR. Memory: from mind to molecules. New York: Henry Holt and Company, 1999.
14. Fokkema DS. The psychobiology of strained breathing and its cardiovascular implications: a functional system review. Psychophysiology 1999;36(2):164-75.
15. Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry 2006;67 (suppl 8):7-12.
16. Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 2001;25(7-8):669-78.
17. Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med 2006;73(7):941-9.
18. Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol 2006;57:27-53.
19. LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry 2001;158(12):1953-5.
20. LeDoux JE. The emotional brain. New York: Simon and Schuster, 1996.
21. Liddell BJ, Brown KJ, Kemp AH, et al. A direct brainstemamygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage 2005;24(1):235-43.
22. Harmer CJ, Mackay CE, Reid CB, et al. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 2006;59(9):816-20.
23. Hariri AR, Mattay VS, Tessitore A, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 2003;53(6):494-501.
24. Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006;60(4):383-7.
25. Simmons A, Strigo I, Matthews SC, et al. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry 2006;60(4):402-9.
26. Stein MB, Simmons AN, Feinstein JS, et al. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007;164(2):318-27.
27. Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005;16(17):1893-7.
1. Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007;29(2):147-55.
2. Niedenthal PM. Embodying emotion. Science 2007;316(5827):1002-5.
3. McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007;64(1):97-106.
4. Panksepp J. Affective neuroscience. New York: Oxford University Press; 1998:31.
5. Stein DJ. Advances in understanding the anxiety disorders: the cognitive-affective neuroscience of ‘false alarms’. Ann Clin Psychiatry 2006;18(3):173-82.
6. Kroenke K, Spitzer RL, Williams JBW, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146(5):317-25.
7. Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt; 1999.
8. Wiens S. Interoception in emotional experience. Curr Opin Neurol 2005;18(4):442-7.
9. Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosom Med 2001;63(5):697-710.
10. Wald J, Taylor S. Interoceptive exposure therapy combined with trauma-related exposure therapy for post-traumatic stress disorder: a case report. Cognit Behav Ther 2005;34(1):34-40.
11. Wells A, Papageorgiou C. Social phobic interoception: effects of bodily information on anxiety, beliefs and self-processing. Behav Res Ther 2001;39(1):1-11.
12. Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 2002;51(1):68-80.
13. Kandel ER, Squire LR. Memory: from mind to molecules. New York: Henry Holt and Company, 1999.
14. Fokkema DS. The psychobiology of strained breathing and its cardiovascular implications: a functional system review. Psychophysiology 1999;36(2):164-75.
15. Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry 2006;67 (suppl 8):7-12.
16. Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 2001;25(7-8):669-78.
17. Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med 2006;73(7):941-9.
18. Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol 2006;57:27-53.
19. LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry 2001;158(12):1953-5.
20. LeDoux JE. The emotional brain. New York: Simon and Schuster, 1996.
21. Liddell BJ, Brown KJ, Kemp AH, et al. A direct brainstemamygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage 2005;24(1):235-43.
22. Harmer CJ, Mackay CE, Reid CB, et al. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 2006;59(9):816-20.
23. Hariri AR, Mattay VS, Tessitore A, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 2003;53(6):494-501.
24. Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006;60(4):383-7.
25. Simmons A, Strigo I, Matthews SC, et al. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry 2006;60(4):402-9.
26. Stein MB, Simmons AN, Feinstein JS, et al. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 2007;164(2):318-27.
27. Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005;16(17):1893-7.
The #1 question to ask inpatients
When consulting on a medical or surgical ward, consider asking the patient, “How are they treating you here in the hospital?” The response to this straightforward question often clarifies the reason for the consultation and helps establish the patient’s psychiatric diagnosis.
Asking about the patient’s experience in the hospital can reveal the dynamics of his or her interpersonal relationships. In a well-functioning ward, healthy answers are, “Everybody is really nice,” or “The staff is great, but I can’t wait to go home.” Any other answer should be investigated.
Questioning reveals disorders
Patients with borderline personality disorder (BPD) will describe a hospital staff split into idealized and rejected components and try to enlist you in their fight. However, most BPD patients won’t need encouragement to discuss their conflicts with the staff.
Unhappy narcissistic patients will com-plain about assaults on their dignity—often housekeeping issues such as poor-quality food and linens, indifferent cleanliness, or delayed response when they use the call button. Happier narcissistic patients will celebrate their doctors’ outstanding credentials and clinical brilliance.
Patients with substance abuse disorders will respond by discussing the timing and adequacy of their opioid and benzodiazepine prescriptions.
Depressed patients may guiltily apologize for wasting everybody’s time.
When patients hint that they are enjoying the hospital experience or would like to prolong their stay, inquire into their situations outside the hospital. They may be homeless, abused, or destitute. Malingerers and patients with factitious disorder typically will insist on their desire to be cured and discharged.
Proper phrasing is essential
As phrased, the question is a “counterprojective” maneuver.1 It distances you from patients’ suspicions, resentments, and presuppositions. By referring to hospital personnel as “they,” you signal that you are distinct and neutral if the patient is feuding with the staff. By comparison, asking “How are my friends on the medical staff treating you?” would invalidate this counterprojective effect, align you with the hospital staff, and subtly encourage the patient to keep his problems to himself.
If the question elicits a complaint, try to stay neutral as long as possible. Guard against perceived defensiveness and the patient’s projections by saying, “I’m sorry to hear things aren’t going well. Tell me more.” Offer to help if there is a concrete and reasonable solution.
Patients might not tell you about problems with their care in the hospital unless you ask. Some patients are too polite to say anything. Others are afraid to complain because they recognize that their comfort and perhaps even survival are in the hands of hospital staff.
Reference
1. Havens L. Making contact: uses of language in psychotherapy Cambridge MA: Harvard University Press; 1988;29:126-9.
Dr. Lakritz is a psychiatrist at the Lahey Clinic Medical Center in Burlington, MA.
When consulting on a medical or surgical ward, consider asking the patient, “How are they treating you here in the hospital?” The response to this straightforward question often clarifies the reason for the consultation and helps establish the patient’s psychiatric diagnosis.
Asking about the patient’s experience in the hospital can reveal the dynamics of his or her interpersonal relationships. In a well-functioning ward, healthy answers are, “Everybody is really nice,” or “The staff is great, but I can’t wait to go home.” Any other answer should be investigated.
Questioning reveals disorders
Patients with borderline personality disorder (BPD) will describe a hospital staff split into idealized and rejected components and try to enlist you in their fight. However, most BPD patients won’t need encouragement to discuss their conflicts with the staff.
Unhappy narcissistic patients will com-plain about assaults on their dignity—often housekeeping issues such as poor-quality food and linens, indifferent cleanliness, or delayed response when they use the call button. Happier narcissistic patients will celebrate their doctors’ outstanding credentials and clinical brilliance.
Patients with substance abuse disorders will respond by discussing the timing and adequacy of their opioid and benzodiazepine prescriptions.
Depressed patients may guiltily apologize for wasting everybody’s time.
When patients hint that they are enjoying the hospital experience or would like to prolong their stay, inquire into their situations outside the hospital. They may be homeless, abused, or destitute. Malingerers and patients with factitious disorder typically will insist on their desire to be cured and discharged.
Proper phrasing is essential
As phrased, the question is a “counterprojective” maneuver.1 It distances you from patients’ suspicions, resentments, and presuppositions. By referring to hospital personnel as “they,” you signal that you are distinct and neutral if the patient is feuding with the staff. By comparison, asking “How are my friends on the medical staff treating you?” would invalidate this counterprojective effect, align you with the hospital staff, and subtly encourage the patient to keep his problems to himself.
If the question elicits a complaint, try to stay neutral as long as possible. Guard against perceived defensiveness and the patient’s projections by saying, “I’m sorry to hear things aren’t going well. Tell me more.” Offer to help if there is a concrete and reasonable solution.
Patients might not tell you about problems with their care in the hospital unless you ask. Some patients are too polite to say anything. Others are afraid to complain because they recognize that their comfort and perhaps even survival are in the hands of hospital staff.
When consulting on a medical or surgical ward, consider asking the patient, “How are they treating you here in the hospital?” The response to this straightforward question often clarifies the reason for the consultation and helps establish the patient’s psychiatric diagnosis.
Asking about the patient’s experience in the hospital can reveal the dynamics of his or her interpersonal relationships. In a well-functioning ward, healthy answers are, “Everybody is really nice,” or “The staff is great, but I can’t wait to go home.” Any other answer should be investigated.
Questioning reveals disorders
Patients with borderline personality disorder (BPD) will describe a hospital staff split into idealized and rejected components and try to enlist you in their fight. However, most BPD patients won’t need encouragement to discuss their conflicts with the staff.
Unhappy narcissistic patients will com-plain about assaults on their dignity—often housekeeping issues such as poor-quality food and linens, indifferent cleanliness, or delayed response when they use the call button. Happier narcissistic patients will celebrate their doctors’ outstanding credentials and clinical brilliance.
Patients with substance abuse disorders will respond by discussing the timing and adequacy of their opioid and benzodiazepine prescriptions.
Depressed patients may guiltily apologize for wasting everybody’s time.
When patients hint that they are enjoying the hospital experience or would like to prolong their stay, inquire into their situations outside the hospital. They may be homeless, abused, or destitute. Malingerers and patients with factitious disorder typically will insist on their desire to be cured and discharged.
Proper phrasing is essential
As phrased, the question is a “counterprojective” maneuver.1 It distances you from patients’ suspicions, resentments, and presuppositions. By referring to hospital personnel as “they,” you signal that you are distinct and neutral if the patient is feuding with the staff. By comparison, asking “How are my friends on the medical staff treating you?” would invalidate this counterprojective effect, align you with the hospital staff, and subtly encourage the patient to keep his problems to himself.
If the question elicits a complaint, try to stay neutral as long as possible. Guard against perceived defensiveness and the patient’s projections by saying, “I’m sorry to hear things aren’t going well. Tell me more.” Offer to help if there is a concrete and reasonable solution.
Patients might not tell you about problems with their care in the hospital unless you ask. Some patients are too polite to say anything. Others are afraid to complain because they recognize that their comfort and perhaps even survival are in the hands of hospital staff.
Reference
1. Havens L. Making contact: uses of language in psychotherapy Cambridge MA: Harvard University Press; 1988;29:126-9.
Dr. Lakritz is a psychiatrist at the Lahey Clinic Medical Center in Burlington, MA.
Reference
1. Havens L. Making contact: uses of language in psychotherapy Cambridge MA: Harvard University Press; 1988;29:126-9.
Dr. Lakritz is a psychiatrist at the Lahey Clinic Medical Center in Burlington, MA.
Boundary crossings: Guard against inappropriate contact
Woman claims improper contact during treatment
Fairfax County (VA) Circuit Court
A 23-year-old woman who received treatment from a psychiatrist for approximately 2½ years claimed that he sexually abused her during that time. She alleged that the inappropriate sexual relationship included holding, hugging, kissing, fondling, and watching pornography. The patient claimed that the relationship led to emotional distress and caused her to attempt suicide.
The psychiatrist admitted that a sexual relationship occurred but contended that the patient suffered no harm.
A $400,000 verdict was returned
Did inappropriate contact cause agoraphobia, anorexia?
Suffolk County (MA) Superior Court
A patient in her 20s had a history of emotional problems and sexual assaults against her. A psychiatrist treated her for obsessive-compulsive disorder for 4 years. He acknowledged giving the patient stuffed animals, cards, and letters and visiting her home several times when she was unable to go to his office. During sessions he touched her hand for comfort and hugged her. The patient claimed they had regular sexual contact.
The patient alleged that the psychiatrist was negligent for engaging in inappropriate sexual conduct, which she claims caused ongoing emotional distress. She claimed she was unable to work and suffered from agoraphobia, intimate relationships difficulties, and anorexia as a result of his actions. The psychiatrist denied any inappropriate sexual conduct.
The psychiatrist’s license was suspended indefinitely, but the suspension was stayed under an agreement that he attend medical education courses.
A $750,000 settlement was reached
Dr. Grant’s observations
Although most physicians would agree that sexual relations with a patient are inappropriate,1 the fact that cases continue to occur suggests a need to emphasize treatment boundaries. Establishing clear boundaries in the doctor-patient relationship creates an atmosphere of safety and predictability that allows treatment to thrive.2
Boundary problems are one of the most frequent reasons for malpractice actions against mental health providers.3 Although much of the literature discusses boundary violations during psychotherapy, issues may arise in all treatment settings, including psychopharmacologic management.
One-half of all psychiatrists will treat at least 1 victim of physician sexual misconduct during their careers.4 One study5 examining sex-related offenses committed by U.S. physicians in all specialties found:
- The number of physicians disciplined for sex-related offenses increased each year from 1989 to 1996.
- 22% of disciplined physicians had sexual intercourse with patients, 15% had sexual contact or touching, 37% committed other sexual abuse that did not fit in either of these 2 categories, and 25% involved nonpatients.
- 28% of disciplined physicians were psychiatrists, the most represented specialty in the study.
The American Medical Association’s Principles of Medical Ethics with Annotations Especially Applicable to Psychiatry states: A psychiatrist shall not gratify his or her own needs by exploiting the patient. The psychiatrist shall be ever vigilant about the impact that his or her conduct has upon the boundaries of the doctor-patient relationship, and thus upon the well-being of the patient. These requirements become particularly important because of the essentially private, highly personal, and sometimes intensely emotional nature of the relationship established with the psychiatrist.
“Further, the necessary intensity of the treatment relationship may tend to activate sexual and other needs and fantasies on the part of both patient and psychiatrist, while weakening the objectivity necessary for control. Additionally the inherent inequality in the doctor-patient relationship may lead to exploitation of the patient. Sexual activity with a current or former patient is unethical.”
Source: Reference 9
In a 1986 survey of psychiatrists, 7% of male and 3% of female clinicians reported having sexual contact with their patients.6 A 1988 survey of fourth-year psychiatry residents found that 1% of respondents acknowledged having sexual relations with a patient.7 In a 1992 study, 9% of physicians across specialties reported engaging in sexual contact with 1 or more current or former patients.8 In that study, 19% of female physicians and 40% of male physicians reported that they did not think physician-patient sexual misconduct was always harmful to patients.8 These views and behaviors are in violation of medical codes of ethics (Box).9
How misconduct harms patients
Trust is essential to establishing a secure therapeutic relationship. Boundary violations may result in missed diagnoses, inappropriate treatment, and/or worsened psychiatric symptoms. Patients might develop complex posttraumatic stress disorder, depression, anxiety, dissociation, sexual dysfunction, somatoform disorders, eating disorders, sleep disorders, or substance use disorders.4 They could lose faith in their treatment providers, have difficulties expressing anger, feel guilty, develop poor self-concept, experience a loss of confidence, and develop problems establishing trusting relationships.4 For these reasons, clinicians can be sued for negligent treatment and sexual misconduct.10
Boundary violations
Although sexual activities with patients are clear boundary violations, what about the second case when the therapist gave the patient stuffed animals and cards and hugged her? Progressive boundary violations often precede and accompany sexual misconduct.10
Five risk factors have been associated with therapist boundary violations:3
- life crises—effects of aging, career disappointments, unfulfilled hopes, or marital conflicts
- transitions—job changes or job loss
- medical illness
- arrogance—the belief that a boundary violation couldn’t happen to him or her and not recognizing the need for consultation
- common stress points with the patient
Although the list is not exhaustive, these factors may be associated with a psychiatrist turning to the patient for solace, gratification, or excitement.
Drawing boundary lines
Not all boundary issues are the same, and Gutheil et al2 suggest 2 categories:
- Boundary crossings—a benign variant where the deviation may advance therapy in a constructive way that does not harm the patient, such as discussion of countertransference.
- Boundary violations—the transgression harms or exploits the patient.
Although some boundary issues may appear benign, even theoretically harmless boundary crossings can be misrepresented or misconstrued by the patient.11 Also, boundary transgressions that do not involve erotic touch might harm the treatment process and the patient.2
When examining “minor” boundary issues that may seem innocuous, ask yourself if the action is for your benefit rather than to advance the patient’s therapy. Also, determine if the intervention is part of a series of progressive boundary violations. If the answer to either question is “yes,” desist immediately and take corrective action.10
The psychiatrist has a professional code of ethics to follow and can be held responsible for failing to set or adhere to boundaries.11 If a patient initiates a boundary violation, you must refuse and then explore the patient’s underlying psychological issues, perhaps aided by consultation with a peer or mentor (Table). Repeated patient demands to breach boundaries requires prompt consultation to determine if you can continue treating the patient or if you should transfer the patient to another clinician. Document the patient’s demands to breach boundaries and your actions when seeking consultation.3
Table 1
How to maintain integrity of the treatment process
| Maintain relative therapist neutrality |
| Foster psychological separateness of the patient |
| Protect confidentiality |
| Obtain informed consent for treatments and procedures |
| Interact verbally with patients |
| Ensure that you do not have any previous, current, or future personal relationships with the patient |
| Minimize physical contact |
| Preserve the therapist’s relative anonymity |
| Establish a stable fee policy |
| Provide a consistent, private, and professional setting |
| Define the time and length of sessions |
| Source: Reference 10 |
1. Herman J, Gartrell N, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, II: psychiatrists’ attitudes. Am J Psychiatry 1987;144:164-9.
2. Gutheil TG, Gabbard GO. Misuses and misunderstandings of boundary theory in clinical and regulatory settings. Am J Psychiatry 1998;155:409-14.
3. Norris DM, Gutheil TG, Strasburger LH. This couldn’t happen to me: boundary problems and sexual misconduct in the psychotherapy relationship. Psychiatr Serv 2003;54:517-22.
4. Roman B, Kay J. Residency education on the prevention of physician-patient sexual misconduct. Acad Psychiatry 1997;21:26-34.
5. Dehlendorf CE, Wolfe SM. Physicians disciplined for sexrelated offenses. JAMA 1998;279:1883-8.
6. Gartrell NK, Herman J, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, 1: prevalence. Am J Psychiatry 1986;143:1126-31.
7. Gartrell NK, Herman J, Olarte S, et al. Psychiatric residents’ sexual contact with educators and patients: results of a national survey. Am J Psychiatry 1988;145:690-4.
8. Gartrell NK, Milliken M, Goodsen WH, et al. Physicianpatient sexual contact. West J Med 1992;157:139-43.
9. The principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed August 28, 2007.
10. Simon RI. Boundary violations in psychotherapy. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:195-215.
11. Gutheil TG. Boundaries, blackmail, and double binds: a pattern observed in malpractice consultation. J Am Acad Psychiatry Law 2005;33:476-81.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Woman claims improper contact during treatment
Fairfax County (VA) Circuit Court
A 23-year-old woman who received treatment from a psychiatrist for approximately 2½ years claimed that he sexually abused her during that time. She alleged that the inappropriate sexual relationship included holding, hugging, kissing, fondling, and watching pornography. The patient claimed that the relationship led to emotional distress and caused her to attempt suicide.
The psychiatrist admitted that a sexual relationship occurred but contended that the patient suffered no harm.
A $400,000 verdict was returned
Did inappropriate contact cause agoraphobia, anorexia?
Suffolk County (MA) Superior Court
A patient in her 20s had a history of emotional problems and sexual assaults against her. A psychiatrist treated her for obsessive-compulsive disorder for 4 years. He acknowledged giving the patient stuffed animals, cards, and letters and visiting her home several times when she was unable to go to his office. During sessions he touched her hand for comfort and hugged her. The patient claimed they had regular sexual contact.
The patient alleged that the psychiatrist was negligent for engaging in inappropriate sexual conduct, which she claims caused ongoing emotional distress. She claimed she was unable to work and suffered from agoraphobia, intimate relationships difficulties, and anorexia as a result of his actions. The psychiatrist denied any inappropriate sexual conduct.
The psychiatrist’s license was suspended indefinitely, but the suspension was stayed under an agreement that he attend medical education courses.
A $750,000 settlement was reached
Dr. Grant’s observations
Although most physicians would agree that sexual relations with a patient are inappropriate,1 the fact that cases continue to occur suggests a need to emphasize treatment boundaries. Establishing clear boundaries in the doctor-patient relationship creates an atmosphere of safety and predictability that allows treatment to thrive.2
Boundary problems are one of the most frequent reasons for malpractice actions against mental health providers.3 Although much of the literature discusses boundary violations during psychotherapy, issues may arise in all treatment settings, including psychopharmacologic management.
One-half of all psychiatrists will treat at least 1 victim of physician sexual misconduct during their careers.4 One study5 examining sex-related offenses committed by U.S. physicians in all specialties found:
- The number of physicians disciplined for sex-related offenses increased each year from 1989 to 1996.
- 22% of disciplined physicians had sexual intercourse with patients, 15% had sexual contact or touching, 37% committed other sexual abuse that did not fit in either of these 2 categories, and 25% involved nonpatients.
- 28% of disciplined physicians were psychiatrists, the most represented specialty in the study.
The American Medical Association’s Principles of Medical Ethics with Annotations Especially Applicable to Psychiatry states: A psychiatrist shall not gratify his or her own needs by exploiting the patient. The psychiatrist shall be ever vigilant about the impact that his or her conduct has upon the boundaries of the doctor-patient relationship, and thus upon the well-being of the patient. These requirements become particularly important because of the essentially private, highly personal, and sometimes intensely emotional nature of the relationship established with the psychiatrist.
“Further, the necessary intensity of the treatment relationship may tend to activate sexual and other needs and fantasies on the part of both patient and psychiatrist, while weakening the objectivity necessary for control. Additionally the inherent inequality in the doctor-patient relationship may lead to exploitation of the patient. Sexual activity with a current or former patient is unethical.”
Source: Reference 9
In a 1986 survey of psychiatrists, 7% of male and 3% of female clinicians reported having sexual contact with their patients.6 A 1988 survey of fourth-year psychiatry residents found that 1% of respondents acknowledged having sexual relations with a patient.7 In a 1992 study, 9% of physicians across specialties reported engaging in sexual contact with 1 or more current or former patients.8 In that study, 19% of female physicians and 40% of male physicians reported that they did not think physician-patient sexual misconduct was always harmful to patients.8 These views and behaviors are in violation of medical codes of ethics (Box).9
How misconduct harms patients
Trust is essential to establishing a secure therapeutic relationship. Boundary violations may result in missed diagnoses, inappropriate treatment, and/or worsened psychiatric symptoms. Patients might develop complex posttraumatic stress disorder, depression, anxiety, dissociation, sexual dysfunction, somatoform disorders, eating disorders, sleep disorders, or substance use disorders.4 They could lose faith in their treatment providers, have difficulties expressing anger, feel guilty, develop poor self-concept, experience a loss of confidence, and develop problems establishing trusting relationships.4 For these reasons, clinicians can be sued for negligent treatment and sexual misconduct.10
Boundary violations
Although sexual activities with patients are clear boundary violations, what about the second case when the therapist gave the patient stuffed animals and cards and hugged her? Progressive boundary violations often precede and accompany sexual misconduct.10
Five risk factors have been associated with therapist boundary violations:3
- life crises—effects of aging, career disappointments, unfulfilled hopes, or marital conflicts
- transitions—job changes or job loss
- medical illness
- arrogance—the belief that a boundary violation couldn’t happen to him or her and not recognizing the need for consultation
- common stress points with the patient
Although the list is not exhaustive, these factors may be associated with a psychiatrist turning to the patient for solace, gratification, or excitement.
Drawing boundary lines
Not all boundary issues are the same, and Gutheil et al2 suggest 2 categories:
- Boundary crossings—a benign variant where the deviation may advance therapy in a constructive way that does not harm the patient, such as discussion of countertransference.
- Boundary violations—the transgression harms or exploits the patient.
Although some boundary issues may appear benign, even theoretically harmless boundary crossings can be misrepresented or misconstrued by the patient.11 Also, boundary transgressions that do not involve erotic touch might harm the treatment process and the patient.2
When examining “minor” boundary issues that may seem innocuous, ask yourself if the action is for your benefit rather than to advance the patient’s therapy. Also, determine if the intervention is part of a series of progressive boundary violations. If the answer to either question is “yes,” desist immediately and take corrective action.10
The psychiatrist has a professional code of ethics to follow and can be held responsible for failing to set or adhere to boundaries.11 If a patient initiates a boundary violation, you must refuse and then explore the patient’s underlying psychological issues, perhaps aided by consultation with a peer or mentor (Table). Repeated patient demands to breach boundaries requires prompt consultation to determine if you can continue treating the patient or if you should transfer the patient to another clinician. Document the patient’s demands to breach boundaries and your actions when seeking consultation.3
Table 1
How to maintain integrity of the treatment process
| Maintain relative therapist neutrality |
| Foster psychological separateness of the patient |
| Protect confidentiality |
| Obtain informed consent for treatments and procedures |
| Interact verbally with patients |
| Ensure that you do not have any previous, current, or future personal relationships with the patient |
| Minimize physical contact |
| Preserve the therapist’s relative anonymity |
| Establish a stable fee policy |
| Provide a consistent, private, and professional setting |
| Define the time and length of sessions |
| Source: Reference 10 |
Woman claims improper contact during treatment
Fairfax County (VA) Circuit Court
A 23-year-old woman who received treatment from a psychiatrist for approximately 2½ years claimed that he sexually abused her during that time. She alleged that the inappropriate sexual relationship included holding, hugging, kissing, fondling, and watching pornography. The patient claimed that the relationship led to emotional distress and caused her to attempt suicide.
The psychiatrist admitted that a sexual relationship occurred but contended that the patient suffered no harm.
A $400,000 verdict was returned
Did inappropriate contact cause agoraphobia, anorexia?
Suffolk County (MA) Superior Court
A patient in her 20s had a history of emotional problems and sexual assaults against her. A psychiatrist treated her for obsessive-compulsive disorder for 4 years. He acknowledged giving the patient stuffed animals, cards, and letters and visiting her home several times when she was unable to go to his office. During sessions he touched her hand for comfort and hugged her. The patient claimed they had regular sexual contact.
The patient alleged that the psychiatrist was negligent for engaging in inappropriate sexual conduct, which she claims caused ongoing emotional distress. She claimed she was unable to work and suffered from agoraphobia, intimate relationships difficulties, and anorexia as a result of his actions. The psychiatrist denied any inappropriate sexual conduct.
The psychiatrist’s license was suspended indefinitely, but the suspension was stayed under an agreement that he attend medical education courses.
A $750,000 settlement was reached
Dr. Grant’s observations
Although most physicians would agree that sexual relations with a patient are inappropriate,1 the fact that cases continue to occur suggests a need to emphasize treatment boundaries. Establishing clear boundaries in the doctor-patient relationship creates an atmosphere of safety and predictability that allows treatment to thrive.2
Boundary problems are one of the most frequent reasons for malpractice actions against mental health providers.3 Although much of the literature discusses boundary violations during psychotherapy, issues may arise in all treatment settings, including psychopharmacologic management.
One-half of all psychiatrists will treat at least 1 victim of physician sexual misconduct during their careers.4 One study5 examining sex-related offenses committed by U.S. physicians in all specialties found:
- The number of physicians disciplined for sex-related offenses increased each year from 1989 to 1996.
- 22% of disciplined physicians had sexual intercourse with patients, 15% had sexual contact or touching, 37% committed other sexual abuse that did not fit in either of these 2 categories, and 25% involved nonpatients.
- 28% of disciplined physicians were psychiatrists, the most represented specialty in the study.
The American Medical Association’s Principles of Medical Ethics with Annotations Especially Applicable to Psychiatry states: A psychiatrist shall not gratify his or her own needs by exploiting the patient. The psychiatrist shall be ever vigilant about the impact that his or her conduct has upon the boundaries of the doctor-patient relationship, and thus upon the well-being of the patient. These requirements become particularly important because of the essentially private, highly personal, and sometimes intensely emotional nature of the relationship established with the psychiatrist.
“Further, the necessary intensity of the treatment relationship may tend to activate sexual and other needs and fantasies on the part of both patient and psychiatrist, while weakening the objectivity necessary for control. Additionally the inherent inequality in the doctor-patient relationship may lead to exploitation of the patient. Sexual activity with a current or former patient is unethical.”
Source: Reference 9
In a 1986 survey of psychiatrists, 7% of male and 3% of female clinicians reported having sexual contact with their patients.6 A 1988 survey of fourth-year psychiatry residents found that 1% of respondents acknowledged having sexual relations with a patient.7 In a 1992 study, 9% of physicians across specialties reported engaging in sexual contact with 1 or more current or former patients.8 In that study, 19% of female physicians and 40% of male physicians reported that they did not think physician-patient sexual misconduct was always harmful to patients.8 These views and behaviors are in violation of medical codes of ethics (Box).9
How misconduct harms patients
Trust is essential to establishing a secure therapeutic relationship. Boundary violations may result in missed diagnoses, inappropriate treatment, and/or worsened psychiatric symptoms. Patients might develop complex posttraumatic stress disorder, depression, anxiety, dissociation, sexual dysfunction, somatoform disorders, eating disorders, sleep disorders, or substance use disorders.4 They could lose faith in their treatment providers, have difficulties expressing anger, feel guilty, develop poor self-concept, experience a loss of confidence, and develop problems establishing trusting relationships.4 For these reasons, clinicians can be sued for negligent treatment and sexual misconduct.10
Boundary violations
Although sexual activities with patients are clear boundary violations, what about the second case when the therapist gave the patient stuffed animals and cards and hugged her? Progressive boundary violations often precede and accompany sexual misconduct.10
Five risk factors have been associated with therapist boundary violations:3
- life crises—effects of aging, career disappointments, unfulfilled hopes, or marital conflicts
- transitions—job changes or job loss
- medical illness
- arrogance—the belief that a boundary violation couldn’t happen to him or her and not recognizing the need for consultation
- common stress points with the patient
Although the list is not exhaustive, these factors may be associated with a psychiatrist turning to the patient for solace, gratification, or excitement.
Drawing boundary lines
Not all boundary issues are the same, and Gutheil et al2 suggest 2 categories:
- Boundary crossings—a benign variant where the deviation may advance therapy in a constructive way that does not harm the patient, such as discussion of countertransference.
- Boundary violations—the transgression harms or exploits the patient.
Although some boundary issues may appear benign, even theoretically harmless boundary crossings can be misrepresented or misconstrued by the patient.11 Also, boundary transgressions that do not involve erotic touch might harm the treatment process and the patient.2
When examining “minor” boundary issues that may seem innocuous, ask yourself if the action is for your benefit rather than to advance the patient’s therapy. Also, determine if the intervention is part of a series of progressive boundary violations. If the answer to either question is “yes,” desist immediately and take corrective action.10
The psychiatrist has a professional code of ethics to follow and can be held responsible for failing to set or adhere to boundaries.11 If a patient initiates a boundary violation, you must refuse and then explore the patient’s underlying psychological issues, perhaps aided by consultation with a peer or mentor (Table). Repeated patient demands to breach boundaries requires prompt consultation to determine if you can continue treating the patient or if you should transfer the patient to another clinician. Document the patient’s demands to breach boundaries and your actions when seeking consultation.3
Table 1
How to maintain integrity of the treatment process
| Maintain relative therapist neutrality |
| Foster psychological separateness of the patient |
| Protect confidentiality |
| Obtain informed consent for treatments and procedures |
| Interact verbally with patients |
| Ensure that you do not have any previous, current, or future personal relationships with the patient |
| Minimize physical contact |
| Preserve the therapist’s relative anonymity |
| Establish a stable fee policy |
| Provide a consistent, private, and professional setting |
| Define the time and length of sessions |
| Source: Reference 10 |
1. Herman J, Gartrell N, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, II: psychiatrists’ attitudes. Am J Psychiatry 1987;144:164-9.
2. Gutheil TG, Gabbard GO. Misuses and misunderstandings of boundary theory in clinical and regulatory settings. Am J Psychiatry 1998;155:409-14.
3. Norris DM, Gutheil TG, Strasburger LH. This couldn’t happen to me: boundary problems and sexual misconduct in the psychotherapy relationship. Psychiatr Serv 2003;54:517-22.
4. Roman B, Kay J. Residency education on the prevention of physician-patient sexual misconduct. Acad Psychiatry 1997;21:26-34.
5. Dehlendorf CE, Wolfe SM. Physicians disciplined for sexrelated offenses. JAMA 1998;279:1883-8.
6. Gartrell NK, Herman J, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, 1: prevalence. Am J Psychiatry 1986;143:1126-31.
7. Gartrell NK, Herman J, Olarte S, et al. Psychiatric residents’ sexual contact with educators and patients: results of a national survey. Am J Psychiatry 1988;145:690-4.
8. Gartrell NK, Milliken M, Goodsen WH, et al. Physicianpatient sexual contact. West J Med 1992;157:139-43.
9. The principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed August 28, 2007.
10. Simon RI. Boundary violations in psychotherapy. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:195-215.
11. Gutheil TG. Boundaries, blackmail, and double binds: a pattern observed in malpractice consultation. J Am Acad Psychiatry Law 2005;33:476-81.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
1. Herman J, Gartrell N, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, II: psychiatrists’ attitudes. Am J Psychiatry 1987;144:164-9.
2. Gutheil TG, Gabbard GO. Misuses and misunderstandings of boundary theory in clinical and regulatory settings. Am J Psychiatry 1998;155:409-14.
3. Norris DM, Gutheil TG, Strasburger LH. This couldn’t happen to me: boundary problems and sexual misconduct in the psychotherapy relationship. Psychiatr Serv 2003;54:517-22.
4. Roman B, Kay J. Residency education on the prevention of physician-patient sexual misconduct. Acad Psychiatry 1997;21:26-34.
5. Dehlendorf CE, Wolfe SM. Physicians disciplined for sexrelated offenses. JAMA 1998;279:1883-8.
6. Gartrell NK, Herman J, Olarte S, et al. Psychiatrist-patient sexual contact: results of a national survey, 1: prevalence. Am J Psychiatry 1986;143:1126-31.
7. Gartrell NK, Herman J, Olarte S, et al. Psychiatric residents’ sexual contact with educators and patients: results of a national survey. Am J Psychiatry 1988;145:690-4.
8. Gartrell NK, Milliken M, Goodsen WH, et al. Physicianpatient sexual contact. West J Med 1992;157:139-43.
9. The principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC: American Psychiatric Association. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.pdf. Accessed August 28, 2007.
10. Simon RI. Boundary violations in psychotherapy. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law. Cambridge, MA: Harvard University Press; 1998:195-215.
11. Gutheil TG. Boundaries, blackmail, and double binds: a pattern observed in malpractice consultation. J Am Acad Psychiatry Law 2005;33:476-81.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Correction
An illustration of the heart was mislabeled in “Managing anxiety in patients with implantable cardiac defibrillators” (Current Psychiatry, September 2007). The error has been corrected in the article at CurrentPsychiatry.com.
An illustration of the heart was mislabeled in “Managing anxiety in patients with implantable cardiac defibrillators” (Current Psychiatry, September 2007). The error has been corrected in the article at CurrentPsychiatry.com.
An illustration of the heart was mislabeled in “Managing anxiety in patients with implantable cardiac defibrillators” (Current Psychiatry, September 2007). The error has been corrected in the article at CurrentPsychiatry.com.
Treat the head and the heart
Dr. Henry Nasrallah’s editorial on mortality in schizophrenia (“Dying too young: Cardiovascular neglect of the mentally ill”, Current Psychiatry, January 2007) to my knowledge could be the first article on the subject written by a psychiatrist. It is time that psychiatrists remember that the specialty is a branch of medicine and its practitioners are physicians.
Because of psychiatry’s unique understanding of the effects of the mind and emotions on the body—particularly on the heart—we have an opportunity to make a major contribution to medicine in terms of understanding and treating heart disease, the world’s leading cause of death.
I practiced family medicine for many years and later devoted my career to adult and child psychiatry. I employ an integrative approach to psychiatric illness because of my background in medicine and psychoanalysis. I focus on the physiology of mood and its link to the development of heart disease and diabetes.
Based on the literature, the causes of premature death in schizophrenia—such as heart disease and diabetes—result from disturbances in underlying physiology including activation of the hypothalamic-pituitary-adrenal axis and sympathetic adrenal-medullary system, autonomic dysfunction, low heart rate variability, and platelet activation. These are the same physiologic aberrations that increase the risk of depression in patients with heart disease.
R. Claire Friend, MD
Assistant clinical professor,
University of California, Los Angeles
Harbor General Hospital Medical Center
Pasadena, CA
Dr. Henry Nasrallah’s editorial on mortality in schizophrenia (“Dying too young: Cardiovascular neglect of the mentally ill”, Current Psychiatry, January 2007) to my knowledge could be the first article on the subject written by a psychiatrist. It is time that psychiatrists remember that the specialty is a branch of medicine and its practitioners are physicians.
Because of psychiatry’s unique understanding of the effects of the mind and emotions on the body—particularly on the heart—we have an opportunity to make a major contribution to medicine in terms of understanding and treating heart disease, the world’s leading cause of death.
I practiced family medicine for many years and later devoted my career to adult and child psychiatry. I employ an integrative approach to psychiatric illness because of my background in medicine and psychoanalysis. I focus on the physiology of mood and its link to the development of heart disease and diabetes.
Based on the literature, the causes of premature death in schizophrenia—such as heart disease and diabetes—result from disturbances in underlying physiology including activation of the hypothalamic-pituitary-adrenal axis and sympathetic adrenal-medullary system, autonomic dysfunction, low heart rate variability, and platelet activation. These are the same physiologic aberrations that increase the risk of depression in patients with heart disease.
R. Claire Friend, MD
Assistant clinical professor,
University of California, Los Angeles
Harbor General Hospital Medical Center
Pasadena, CA
Dr. Henry Nasrallah’s editorial on mortality in schizophrenia (“Dying too young: Cardiovascular neglect of the mentally ill”, Current Psychiatry, January 2007) to my knowledge could be the first article on the subject written by a psychiatrist. It is time that psychiatrists remember that the specialty is a branch of medicine and its practitioners are physicians.
Because of psychiatry’s unique understanding of the effects of the mind and emotions on the body—particularly on the heart—we have an opportunity to make a major contribution to medicine in terms of understanding and treating heart disease, the world’s leading cause of death.
I practiced family medicine for many years and later devoted my career to adult and child psychiatry. I employ an integrative approach to psychiatric illness because of my background in medicine and psychoanalysis. I focus on the physiology of mood and its link to the development of heart disease and diabetes.
Based on the literature, the causes of premature death in schizophrenia—such as heart disease and diabetes—result from disturbances in underlying physiology including activation of the hypothalamic-pituitary-adrenal axis and sympathetic adrenal-medullary system, autonomic dysfunction, low heart rate variability, and platelet activation. These are the same physiologic aberrations that increase the risk of depression in patients with heart disease.
R. Claire Friend, MD
Assistant clinical professor,
University of California, Los Angeles
Harbor General Hospital Medical Center
Pasadena, CA
Opiates calm addicts’ anger
I have treated several opiate addicts whose family members have reported them as being “angry” without opiates (“A life of drugs and ‘downtime’”, Current Psychiatry, August 2007). When these individuals are asked if they feel angry without opiates, their response has been “yes, how did you know?”
These patients also said they used opiates not to get high but to avoid being angry and impossible to be around. In these select few—who also had not responded to antidepressants or mood stabilizers—I have found thiothixene to be especially helpful to rapidly reduce anger. None of these patients were psychotic, and all had good work histories.
I recall a patient who was suicidal because she couldn’t stand how angry she was without opiates but knew that staying on the drugs wasn’t an acceptable option.
Sheridan Tucker, MD
Whitfield, MS
I have treated several opiate addicts whose family members have reported them as being “angry” without opiates (“A life of drugs and ‘downtime’”, Current Psychiatry, August 2007). When these individuals are asked if they feel angry without opiates, their response has been “yes, how did you know?”
These patients also said they used opiates not to get high but to avoid being angry and impossible to be around. In these select few—who also had not responded to antidepressants or mood stabilizers—I have found thiothixene to be especially helpful to rapidly reduce anger. None of these patients were psychotic, and all had good work histories.
I recall a patient who was suicidal because she couldn’t stand how angry she was without opiates but knew that staying on the drugs wasn’t an acceptable option.
Sheridan Tucker, MD
Whitfield, MS
I have treated several opiate addicts whose family members have reported them as being “angry” without opiates (“A life of drugs and ‘downtime’”, Current Psychiatry, August 2007). When these individuals are asked if they feel angry without opiates, their response has been “yes, how did you know?”
These patients also said they used opiates not to get high but to avoid being angry and impossible to be around. In these select few—who also had not responded to antidepressants or mood stabilizers—I have found thiothixene to be especially helpful to rapidly reduce anger. None of these patients were psychotic, and all had good work histories.
I recall a patient who was suicidal because she couldn’t stand how angry she was without opiates but knew that staying on the drugs wasn’t an acceptable option.
Sheridan Tucker, MD
Whitfield, MS
CME 'conflicts of interest'
In his editorial “Sponsored CME: Do drug companies influence the content?” (Current Psychiatry, August 2007), Dr. Henry Nasrallah argues that most CME speakers in psychopharmacology have financial relationships with more than one pharmaceutical company. Consequently Dr. Nasrallah says, “it would be difficult for speakers to assume a conflict of interest.” There are several reasons why this assumption is not reassuring.
Sponsor drugs often are compared against placebo, generic medications, or nonpharmacologic interventions, none of which can offer the presenter competing funding. Support from multiple sponsors may create a bias toward the mean, and differences among drugs may be minimized. A speaker might not receive equal support from all sponsors, and therefore one company may have a greater financial relationship with the speaker. Many speakers are supported by several, but not all, companies.
No matter how we rationalize it, when speakers receive pharmaceutical support there will be conflicts of interest. The medical academy must acknowledge this fact and decide if presentations by sponsored speakers merit CME status or if they are too similar to infomercials.
Carl I. Cohen, MD
Professor and director,
Division of geriatric psychiatry,
SUNY Downstate Medical Center
Brooklyn, NY
Dr. Henry Nasrallah is almost convincing in his defense of drug company sponsorship of CME programs. However, I believe that closer scrutiny reveals problems with his reasoning.
Dr. Nasrallah advances several arguments for maintaining the status quo. He notes that teaching institutions generally lack funds to cover the costs of CME programs, and existing regulatory CME oversight is sufficient to ensure neutrality. He also contends that CME speakers’ financial relationships with multiple, competing pharmaceutical companies help prevent “a conflict of interest.”
Many nonmedical professionals must engage in ongoing education, and they manage to fulfill these mandates without funding from the pharmaceutical industry. The nature of CME would change without corporate sponsorship. Indeed, Dr. Nasrallah lists “refreshments and meals” as costs associated with sponsoring CME, but we don’t need free food to learn. Perhaps we should pay for our own meals for the sake of neutrality.
With regard to Dr. Nasrallah’s contention that speakers’ multiple financial relationships help prevent bias, I feel that his argument exposes one of the greatest sources of bias, that “most CME speakers are experts in psychopharmacology.” The concern isn’t that speakers will favor one drug over another, rather that they will overstate medications’ efficacy in general, downplay side effects, and give short shrift to nonpharmacologic interventions.
Robert Hierholzer, MD
Fresno, CA
In his editorial, Dr. Nasrallah discusses the relationship between drug companies and CME forums and imagines that science—not special interests— best determines physicians’ prescribing choices. The issue is not whether a speaker participating at a drug company sponsored CME event endorses a medication but, more fundamentally, the codependent relationship between the pharmaceutical industry and the medical establishment.
Physicians and patients are the offspring of this codependent relationship and often assume that the only response to illness or disease is prescribing one or more drugs. Advertising’s ubiquity fuels this belief thoroughly and subtly, similar to how parents bequeath their values and communication style to their children. Advertising has become so pervasive in all areas of professional and consumer life that it blares non-stop from the background.
The choice of Seroquel vs Geodon or Lexapro vs Effexor XR increasingly becomes our only choice in a society of ever-deepening disintegration and desperation, in which the easy and quick becomes the unquestioned norm and psychiatrist-diagnosed mental illness is more common.
If psychiatrists don’t work to expose the way the marriage between medicine and the pharmaceutical industry influences our clinical decisions as well as our thought processes, physicians and patients simply perpetuate the same unquestioned codependency inherited from our mega-industry parents. This dysfunctional system consumes more of our choices until it alone determines our decisions. While doctors, the pharmaceutical industry, and insurers get richer—priding ourselves on our good work—society grows more impoverished and seeks solutions to expanding malaise from the system that needs illness to continue making profits.
P.S. The first draft of this letter was written with a pen commissioned by AstraZeneca on a note pad paid for by Eli Lilly.
Jeff Berger, MD
Sedro-Woolley, WA
Dr. Nasrallah Responds
Despite the perceptions of a “collusion” between the continuing medical education establishment and pharmaceutical sponsors, the fact is that CME audiences are sophisticated enough not to tolerate biased presentations. Further, CME providers are adhering ever so strictly to both the letter and the spirit of the CME guidelines. Potential conflict of interest is being more vigorously disclosed, addressed, and resolved by CME accreditation planners and by speakers themselves.
The CME process has seen unprecedented improvements over the past 2 years, but old perceptions of "scratching the sponsor’s back"—which admittedly occurred in the past—persist. Thus, practitioners must actively participate in providing feedback about CME offerings regarding the extremes: blatant bias or exemplary, evidenced-based neutrality.
Finally, although psychopharmacologic advances represent a large proportion of the new knowledge in psychiatry, it is my hope that pharmaceutical companies would support CME programs that update practitioners about progress in psychosocial interventions as well.
Henry A. Nasrallah, MD
Editor-in-Chief
In his editorial “Sponsored CME: Do drug companies influence the content?” (Current Psychiatry, August 2007), Dr. Henry Nasrallah argues that most CME speakers in psychopharmacology have financial relationships with more than one pharmaceutical company. Consequently Dr. Nasrallah says, “it would be difficult for speakers to assume a conflict of interest.” There are several reasons why this assumption is not reassuring.
Sponsor drugs often are compared against placebo, generic medications, or nonpharmacologic interventions, none of which can offer the presenter competing funding. Support from multiple sponsors may create a bias toward the mean, and differences among drugs may be minimized. A speaker might not receive equal support from all sponsors, and therefore one company may have a greater financial relationship with the speaker. Many speakers are supported by several, but not all, companies.
No matter how we rationalize it, when speakers receive pharmaceutical support there will be conflicts of interest. The medical academy must acknowledge this fact and decide if presentations by sponsored speakers merit CME status or if they are too similar to infomercials.
Carl I. Cohen, MD
Professor and director,
Division of geriatric psychiatry,
SUNY Downstate Medical Center
Brooklyn, NY
Dr. Henry Nasrallah is almost convincing in his defense of drug company sponsorship of CME programs. However, I believe that closer scrutiny reveals problems with his reasoning.
Dr. Nasrallah advances several arguments for maintaining the status quo. He notes that teaching institutions generally lack funds to cover the costs of CME programs, and existing regulatory CME oversight is sufficient to ensure neutrality. He also contends that CME speakers’ financial relationships with multiple, competing pharmaceutical companies help prevent “a conflict of interest.”
Many nonmedical professionals must engage in ongoing education, and they manage to fulfill these mandates without funding from the pharmaceutical industry. The nature of CME would change without corporate sponsorship. Indeed, Dr. Nasrallah lists “refreshments and meals” as costs associated with sponsoring CME, but we don’t need free food to learn. Perhaps we should pay for our own meals for the sake of neutrality.
With regard to Dr. Nasrallah’s contention that speakers’ multiple financial relationships help prevent bias, I feel that his argument exposes one of the greatest sources of bias, that “most CME speakers are experts in psychopharmacology.” The concern isn’t that speakers will favor one drug over another, rather that they will overstate medications’ efficacy in general, downplay side effects, and give short shrift to nonpharmacologic interventions.
Robert Hierholzer, MD
Fresno, CA
In his editorial, Dr. Nasrallah discusses the relationship between drug companies and CME forums and imagines that science—not special interests— best determines physicians’ prescribing choices. The issue is not whether a speaker participating at a drug company sponsored CME event endorses a medication but, more fundamentally, the codependent relationship between the pharmaceutical industry and the medical establishment.
Physicians and patients are the offspring of this codependent relationship and often assume that the only response to illness or disease is prescribing one or more drugs. Advertising’s ubiquity fuels this belief thoroughly and subtly, similar to how parents bequeath their values and communication style to their children. Advertising has become so pervasive in all areas of professional and consumer life that it blares non-stop from the background.
The choice of Seroquel vs Geodon or Lexapro vs Effexor XR increasingly becomes our only choice in a society of ever-deepening disintegration and desperation, in which the easy and quick becomes the unquestioned norm and psychiatrist-diagnosed mental illness is more common.
If psychiatrists don’t work to expose the way the marriage between medicine and the pharmaceutical industry influences our clinical decisions as well as our thought processes, physicians and patients simply perpetuate the same unquestioned codependency inherited from our mega-industry parents. This dysfunctional system consumes more of our choices until it alone determines our decisions. While doctors, the pharmaceutical industry, and insurers get richer—priding ourselves on our good work—society grows more impoverished and seeks solutions to expanding malaise from the system that needs illness to continue making profits.
P.S. The first draft of this letter was written with a pen commissioned by AstraZeneca on a note pad paid for by Eli Lilly.
Jeff Berger, MD
Sedro-Woolley, WA
Dr. Nasrallah Responds
Despite the perceptions of a “collusion” between the continuing medical education establishment and pharmaceutical sponsors, the fact is that CME audiences are sophisticated enough not to tolerate biased presentations. Further, CME providers are adhering ever so strictly to both the letter and the spirit of the CME guidelines. Potential conflict of interest is being more vigorously disclosed, addressed, and resolved by CME accreditation planners and by speakers themselves.
The CME process has seen unprecedented improvements over the past 2 years, but old perceptions of "scratching the sponsor’s back"—which admittedly occurred in the past—persist. Thus, practitioners must actively participate in providing feedback about CME offerings regarding the extremes: blatant bias or exemplary, evidenced-based neutrality.
Finally, although psychopharmacologic advances represent a large proportion of the new knowledge in psychiatry, it is my hope that pharmaceutical companies would support CME programs that update practitioners about progress in psychosocial interventions as well.
Henry A. Nasrallah, MD
Editor-in-Chief
In his editorial “Sponsored CME: Do drug companies influence the content?” (Current Psychiatry, August 2007), Dr. Henry Nasrallah argues that most CME speakers in psychopharmacology have financial relationships with more than one pharmaceutical company. Consequently Dr. Nasrallah says, “it would be difficult for speakers to assume a conflict of interest.” There are several reasons why this assumption is not reassuring.
Sponsor drugs often are compared against placebo, generic medications, or nonpharmacologic interventions, none of which can offer the presenter competing funding. Support from multiple sponsors may create a bias toward the mean, and differences among drugs may be minimized. A speaker might not receive equal support from all sponsors, and therefore one company may have a greater financial relationship with the speaker. Many speakers are supported by several, but not all, companies.
No matter how we rationalize it, when speakers receive pharmaceutical support there will be conflicts of interest. The medical academy must acknowledge this fact and decide if presentations by sponsored speakers merit CME status or if they are too similar to infomercials.
Carl I. Cohen, MD
Professor and director,
Division of geriatric psychiatry,
SUNY Downstate Medical Center
Brooklyn, NY
Dr. Henry Nasrallah is almost convincing in his defense of drug company sponsorship of CME programs. However, I believe that closer scrutiny reveals problems with his reasoning.
Dr. Nasrallah advances several arguments for maintaining the status quo. He notes that teaching institutions generally lack funds to cover the costs of CME programs, and existing regulatory CME oversight is sufficient to ensure neutrality. He also contends that CME speakers’ financial relationships with multiple, competing pharmaceutical companies help prevent “a conflict of interest.”
Many nonmedical professionals must engage in ongoing education, and they manage to fulfill these mandates without funding from the pharmaceutical industry. The nature of CME would change without corporate sponsorship. Indeed, Dr. Nasrallah lists “refreshments and meals” as costs associated with sponsoring CME, but we don’t need free food to learn. Perhaps we should pay for our own meals for the sake of neutrality.
With regard to Dr. Nasrallah’s contention that speakers’ multiple financial relationships help prevent bias, I feel that his argument exposes one of the greatest sources of bias, that “most CME speakers are experts in psychopharmacology.” The concern isn’t that speakers will favor one drug over another, rather that they will overstate medications’ efficacy in general, downplay side effects, and give short shrift to nonpharmacologic interventions.
Robert Hierholzer, MD
Fresno, CA
In his editorial, Dr. Nasrallah discusses the relationship between drug companies and CME forums and imagines that science—not special interests— best determines physicians’ prescribing choices. The issue is not whether a speaker participating at a drug company sponsored CME event endorses a medication but, more fundamentally, the codependent relationship between the pharmaceutical industry and the medical establishment.
Physicians and patients are the offspring of this codependent relationship and often assume that the only response to illness or disease is prescribing one or more drugs. Advertising’s ubiquity fuels this belief thoroughly and subtly, similar to how parents bequeath their values and communication style to their children. Advertising has become so pervasive in all areas of professional and consumer life that it blares non-stop from the background.
The choice of Seroquel vs Geodon or Lexapro vs Effexor XR increasingly becomes our only choice in a society of ever-deepening disintegration and desperation, in which the easy and quick becomes the unquestioned norm and psychiatrist-diagnosed mental illness is more common.
If psychiatrists don’t work to expose the way the marriage between medicine and the pharmaceutical industry influences our clinical decisions as well as our thought processes, physicians and patients simply perpetuate the same unquestioned codependency inherited from our mega-industry parents. This dysfunctional system consumes more of our choices until it alone determines our decisions. While doctors, the pharmaceutical industry, and insurers get richer—priding ourselves on our good work—society grows more impoverished and seeks solutions to expanding malaise from the system that needs illness to continue making profits.
P.S. The first draft of this letter was written with a pen commissioned by AstraZeneca on a note pad paid for by Eli Lilly.
Jeff Berger, MD
Sedro-Woolley, WA
Dr. Nasrallah Responds
Despite the perceptions of a “collusion” between the continuing medical education establishment and pharmaceutical sponsors, the fact is that CME audiences are sophisticated enough not to tolerate biased presentations. Further, CME providers are adhering ever so strictly to both the letter and the spirit of the CME guidelines. Potential conflict of interest is being more vigorously disclosed, addressed, and resolved by CME accreditation planners and by speakers themselves.
The CME process has seen unprecedented improvements over the past 2 years, but old perceptions of "scratching the sponsor’s back"—which admittedly occurred in the past—persist. Thus, practitioners must actively participate in providing feedback about CME offerings regarding the extremes: blatant bias or exemplary, evidenced-based neutrality.
Finally, although psychopharmacologic advances represent a large proportion of the new knowledge in psychiatry, it is my hope that pharmaceutical companies would support CME programs that update practitioners about progress in psychosocial interventions as well.
Henry A. Nasrallah, MD
Editor-in-Chief
Remember the hippocampus!: You can protect the brain’s ‘regeneration center’
What part of the brain incorporates our moment-to-moment experiences, weaves them into coherent and interconnected verbal, spatial, and emotional memories, and enables us to be aware of our entire ‘life story’?
It’s the hippocampus, of course. Damage to this portion of the brain—as in seriously mentally ill individuals—severely impairs the ability to form new memories, with subsequent social and vocational impairment.
Interestingly, the hippocampus also is the “regeneration center” of the brain, continuously producing progenitor cells that can differentiate into neurons and glia that migrate to brain regions that need replenishment.
What does that have to do with psychiatry? A lot. It is now well established that the hippocampus is structurally and functionally impaired in several severe neuropsychiatric disorders. The hippocampus:
- fails to develop adequately in schizophrenia
- shows progressive atrophy in persons with recurrent unipolar or bipolar depression
- shrivels in severe stress disorders such as posttraumatic stress disorder (PTSD)
- is damaged by the toxicity of alcohol addiction
- is rapidly devastated in Alzheimer’s dementia.
It’s no wonder that cognitive functions—especially memory and learning—are seriously impaired in persons suffering from these disorders.
Regeneration and repair
What can psychiatrists do about our patients’ hippocampal dysfunction? There is good news on that front.
Abstinence from alcohol will reverse hippocampal damage within 6 to 12 months. Antidepressants have been found to stimulate production of new brain cells (neurogenesis) and to gradually rebuild the structure of the hippocampus in depressed individuals. Ditto for atypical (but not conventional) antipsychotics, which induce neurotrophic growth factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). NGF and BDNF facilitate survival and maturation of new neurons produced in the hippocampus. Some atypicals have been shown to prevent or reverse stress-induced suppression of neurogenesis in the hippocampus and, theoretically, prevent PTSD.
Recent studies demonstrate that antidepressants lose their clinical efficacy if neurogenesis is inhibited. This suggests that hippocampal neurogenesis—rather than neurotransmitters—may be the mechanism by which depression is lifted. Only dementia still defies efforts to halt its ruthless destruction of the hippocampus, with severe cognitive decline and a faded sense of self and the world.
Flexing the memory center
Besides medication, other practical tools can keep the hippocampus healthy (prevention) or restore its health (intervention), whether in psychiatric patients or in mentally healthy but aging individuals. These include:
- physical exercise, which stimulates neurogenesis
- stress management to reduce the neurotoxic effects of cortisol on the hippocampus
- mental exercises—such as memorizing a poem or a list of words or numbers, reading, writing, or retrieving vocabulary—all activate the hippocampus
- deep breathing several times a day to oxygenate the brain adequately (the hippocampus is the most vascularized brain region and the first to suffer from low oxygen).
We clinicians also should keep our hippocampi healthy through prevention and intervention so we can take good care of our patients.
What part of the brain incorporates our moment-to-moment experiences, weaves them into coherent and interconnected verbal, spatial, and emotional memories, and enables us to be aware of our entire ‘life story’?
It’s the hippocampus, of course. Damage to this portion of the brain—as in seriously mentally ill individuals—severely impairs the ability to form new memories, with subsequent social and vocational impairment.
Interestingly, the hippocampus also is the “regeneration center” of the brain, continuously producing progenitor cells that can differentiate into neurons and glia that migrate to brain regions that need replenishment.
What does that have to do with psychiatry? A lot. It is now well established that the hippocampus is structurally and functionally impaired in several severe neuropsychiatric disorders. The hippocampus:
- fails to develop adequately in schizophrenia
- shows progressive atrophy in persons with recurrent unipolar or bipolar depression
- shrivels in severe stress disorders such as posttraumatic stress disorder (PTSD)
- is damaged by the toxicity of alcohol addiction
- is rapidly devastated in Alzheimer’s dementia.
It’s no wonder that cognitive functions—especially memory and learning—are seriously impaired in persons suffering from these disorders.
Regeneration and repair
What can psychiatrists do about our patients’ hippocampal dysfunction? There is good news on that front.
Abstinence from alcohol will reverse hippocampal damage within 6 to 12 months. Antidepressants have been found to stimulate production of new brain cells (neurogenesis) and to gradually rebuild the structure of the hippocampus in depressed individuals. Ditto for atypical (but not conventional) antipsychotics, which induce neurotrophic growth factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). NGF and BDNF facilitate survival and maturation of new neurons produced in the hippocampus. Some atypicals have been shown to prevent or reverse stress-induced suppression of neurogenesis in the hippocampus and, theoretically, prevent PTSD.
Recent studies demonstrate that antidepressants lose their clinical efficacy if neurogenesis is inhibited. This suggests that hippocampal neurogenesis—rather than neurotransmitters—may be the mechanism by which depression is lifted. Only dementia still defies efforts to halt its ruthless destruction of the hippocampus, with severe cognitive decline and a faded sense of self and the world.
Flexing the memory center
Besides medication, other practical tools can keep the hippocampus healthy (prevention) or restore its health (intervention), whether in psychiatric patients or in mentally healthy but aging individuals. These include:
- physical exercise, which stimulates neurogenesis
- stress management to reduce the neurotoxic effects of cortisol on the hippocampus
- mental exercises—such as memorizing a poem or a list of words or numbers, reading, writing, or retrieving vocabulary—all activate the hippocampus
- deep breathing several times a day to oxygenate the brain adequately (the hippocampus is the most vascularized brain region and the first to suffer from low oxygen).
We clinicians also should keep our hippocampi healthy through prevention and intervention so we can take good care of our patients.
What part of the brain incorporates our moment-to-moment experiences, weaves them into coherent and interconnected verbal, spatial, and emotional memories, and enables us to be aware of our entire ‘life story’?
It’s the hippocampus, of course. Damage to this portion of the brain—as in seriously mentally ill individuals—severely impairs the ability to form new memories, with subsequent social and vocational impairment.
Interestingly, the hippocampus also is the “regeneration center” of the brain, continuously producing progenitor cells that can differentiate into neurons and glia that migrate to brain regions that need replenishment.
What does that have to do with psychiatry? A lot. It is now well established that the hippocampus is structurally and functionally impaired in several severe neuropsychiatric disorders. The hippocampus:
- fails to develop adequately in schizophrenia
- shows progressive atrophy in persons with recurrent unipolar or bipolar depression
- shrivels in severe stress disorders such as posttraumatic stress disorder (PTSD)
- is damaged by the toxicity of alcohol addiction
- is rapidly devastated in Alzheimer’s dementia.
It’s no wonder that cognitive functions—especially memory and learning—are seriously impaired in persons suffering from these disorders.
Regeneration and repair
What can psychiatrists do about our patients’ hippocampal dysfunction? There is good news on that front.
Abstinence from alcohol will reverse hippocampal damage within 6 to 12 months. Antidepressants have been found to stimulate production of new brain cells (neurogenesis) and to gradually rebuild the structure of the hippocampus in depressed individuals. Ditto for atypical (but not conventional) antipsychotics, which induce neurotrophic growth factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). NGF and BDNF facilitate survival and maturation of new neurons produced in the hippocampus. Some atypicals have been shown to prevent or reverse stress-induced suppression of neurogenesis in the hippocampus and, theoretically, prevent PTSD.
Recent studies demonstrate that antidepressants lose their clinical efficacy if neurogenesis is inhibited. This suggests that hippocampal neurogenesis—rather than neurotransmitters—may be the mechanism by which depression is lifted. Only dementia still defies efforts to halt its ruthless destruction of the hippocampus, with severe cognitive decline and a faded sense of self and the world.
Flexing the memory center
Besides medication, other practical tools can keep the hippocampus healthy (prevention) or restore its health (intervention), whether in psychiatric patients or in mentally healthy but aging individuals. These include:
- physical exercise, which stimulates neurogenesis
- stress management to reduce the neurotoxic effects of cortisol on the hippocampus
- mental exercises—such as memorizing a poem or a list of words or numbers, reading, writing, or retrieving vocabulary—all activate the hippocampus
- deep breathing several times a day to oxygenate the brain adequately (the hippocampus is the most vascularized brain region and the first to suffer from low oxygen).
We clinicians also should keep our hippocampi healthy through prevention and intervention so we can take good care of our patients.
Unhappy feet: One woman’s severe akathisia
HISTORY: ‘Bizarre’ days
Ms. K, age 45, is brought to the ER by her brother, who reports she has been acting “bizarre and crazy” for 3 days. He says his sister—who has bipolar I disorder— has had trouble sleeping, is restless, hears voices, and is contemplating suicide. He adds she was discharged from a psychiatric hospital 2 weeks ago after a 3-month stay.
Risperidone, 2 mg nightly, was controlling Ms. K’s mania until this recent episode. According to her brother, she also has developed continuous involuntary leg and arm movements and cannot sit or stand still. When she tries to sleep, her feet sway back and forth in bed for hours.
We admit Ms. K to the psychiatric inpatient unit because of her suicidality and hallucinations. She is restless and agitated during initial evaluation, pacing around the room or rocking her feet while standing or sitting. Her speech is pressured and the “voices” are urging her to kill herself.
Ms. K is dysphoric and severely distraught about her “nervousness” and continuous urges to move. She says she would rather die than live with incessantly “jittery” legs and arms, yet she wants to be discharged and denies that she is mentally ill. She believes decreased sleep is causing her symptoms and requests a “sleeping pill.”
she habitually views her medications as useless and stops taking them.
The patient has been hospitalized at least 4 times with severe manic and psychotic symptoms. She does not use illicit drugs and is medically healthy.
The authors’ observations
Ms. K’s involuntary movements suggest akathisia, a common extrapyramidal side effect of antipsychotics and other psychotropics (Table).1
Akathisia is characterized by strong feelings of inner restlessness that manifest as excessive walking or pacing and difficulty remaining still. Ms. K’s movements met at least 2 of 5 DSM-IV-TR criteria for acute akathisia (Box). ,2
Akathisia is characterized by at least 5 subtypes: 3
- Acute akathisia begins hours or days after starting the offending medication and lasts
- Tardive is similar to acute akathisia but can arise within 3 to 4 months of starting the offending medication and persists for years.
- Chronic akathisia lasts ≥3 months and usually has no temporal correlation with antipsychotic initiation or dosage increase.4
- Withdrawal akathisia begins within 6 weeks of discontinuing a medication or significantly reducing the dosage.
- Pseudo akathisia consists of objective symptoms of movement without subjective awareness or distress. This subtype usually is seen in older patients.
history of medication nonadherence strongly suggest akathisia secondary to risperidone withdrawal.5 Several
cases of akathisia after risperidone cessation have been reported.5
We know risperidone is not causing acute akathisia because Ms. K responded well to the medication during her last hospitalization with no adverse effects. Also, her family confirmed that she stopped taking risperidone after her most recent discharge.
Mania also can fuel incessant movement and increase physical activity, but patients often do not realize they have a problem while in a manic phase. Also, swinging and rocking of legs is rarely seen in mania. By contrast, Ms. K was morbidly distraught over her akathisia.
Table
Drugs that can cause akathisia
|
The authors’ observations
Numerous treatments are available for akathisia:
Beta blockers such as propranolol are most widely used because of their rapid onset of action and overall effectiveness in akathisia.3 Researchers believe these drugs reduce extrapyramidal symptoms (EPS) by blocking the adrenergic system. Propranolol can be used at a maximum 120 mg/d in divided doses.
Beta blockers, however, can cause bradycardia, hypotension, or respiratory distress. Use beta blockers with caution, and monitor for these adverse effects.
Benzodiazepines. Clonazepam, which enhances the inhibitory effect of GABA in the brain, is commonly used for akathisia because of its effectiveness and long elimination half-life3 (30 to 40 hours), which decreases the risk of medication withdrawal.
and disinhibition-induced aggression in all patients.
Anticholinergics such as trihexyphenidyl are more commonly used for EPS associated with parkinsonian symptoms or side effects but can be partially effective for akathisia.3 Anticholinergics block the CNS cholinergic activity that causes parkinsonian symptoms.
Cyproheptadine, clonidine, and mianserin have shown some positive results against akathisia in clinical trials.6-8 Iron, nicotine patches, and amantadine have shown limited effectiveness against akathisia in research studies and case reports.3,9
Restarting risperidone at a lower dosage—rather than adding a medication— might have resolved Ms. K’s akathisia, but because she was morbidly despondent over her akathisia, we felt we had no time to experiment. We also believed Ms. K’s would respond well to a neuroleptic with a lower EPS risk—such as quetiapine.1,10
A. Subjective complaints of restlessness after exposure to neuroleptics
B. At least 1 of the following is observed:
- Fidgety movements or swinging of the legs
- Rocking from foot to foot while standing
- Pacing to relieve restlessness
- Inability to sit or stand still for at least several minutes
C. Symptoms develop within 4 weeks of starting or raising the dosage of a neuroleptic or after reducing a medication used to treat extrapyramidal symptoms
D. Criterion A symptoms are not better accounted for by a mental disorder
E. Criterion A symptoms are not caused by a nonneuroleptic or a general medical condition
Source: Adapted from reference 2 with permission
TREATMENT: Trying trials
We perform a complete medical workup to rule out an underlying medical problem. We then start valproic acid, 500 mg bid, for Ms. K’s mania; quetiapine, 50 mg bid, for psychosis and mania; and propranolol, 30 mg bid, for akathisia.
We titrate quetiapine by 100 mg/d every 2 days to 400 mg/d, but after 10 days her akathisia, irritable mood, decreased sleep, and suicidal thoughts persist. We cannot increase propranolol because her blood pressure is 90/60 mm Hg, and adding lorazepam, 0.5 mg tid, does not control her movements. Three days later, we add trihexyphenidyl, 5 mg bid.
Fifteen days after admission, Ms. K remains akathisic, dysphoric, and suicidal despite a 5-drug regimen. Her “nervousness” prevents her from attending groups or other unit activities, and her uncontrollable foot swaying still keeps her awake at night.
The authors’ observations
Neither propranolol, clonazepam, nor trihexyphenidyl alleviated Ms. K’s akathisia. Switching to another neuroleptic with a relatively low EPS risk—such as olanzapine—might help. Olanzapine reduced akathisia in 3 case reports,11 and we hope its strong anticholinergic and antiserotonergic action will help resolve Ms. K’s akathisia.
with patients receiving therapeutic dosages of risperidone.12 In another study, olanzapine showed anticholinergic activity at therapeutic doses but risperidone did not.13 Researchers believe these features reduce olanzapine’s EPS risk compared with other antipsychotics.
TREATMENT: Drug works, but …
Three weeks after Ms. K’s presentation, we stop all psychotropics, start olanzapine, 10 mg nightly, for psychosis and mania, and continue propranolol, 30 mg bid, for akathisia. Within 2 days, Ms. K’s akathisia improves significantly.
We also start lithium, 150 mg bid, for mania, and increase it 4 days later to 300 mg bid to maintain serum lithium at approximately 1 mEq/L. We check serum lithium every 3 days after dosage adjustment. Although lithium can induce akathisia, we thought it would most effectively control her mania.
Six days after we started the new medications, Ms. K’s mania and psychosis begin to improve and she becomes euthymic. She is able to sit calmly during group therapy and community meetings.
Ten days after we start olanzapine and lithium, Ms. K appears bloated. Weight check shows an approximate 5-lb weight gain since starting the medications, both of which can cause weight gain and other metabolic side effects.
At Ms. K’s request, we stop olanzapine and start aripiprazole, 5 mg/d, to try to control her weight gain. We continue lithium and propranolol, which have been controlling her mood and akathisia. The next day—after 1 dose of aripiprazole—her akathisia returns.
The authors’ observations
Because aripiprazole was started as soon as olanzapine was discontinued, it is unclear which action aggravated Ms. K’s akathisia or if both were to blame.
Akathisia’s underlying cause is uncertain. Researchers believe dopamine receptor blockade in the mesocortical dopamine system might be responsible.3 Positron-emission tomography studies suggest that D2 receptor occupancy in the striatum contributes to akathisia, and noradrenergic and serotonergic systems also play a role.3,14
Antipsychotics, antidepressants, and sympathomimetics all have been implicated in akathisia, but antipsychotics that are potent serotonin (5HT) receptor antagonists—such as olanzapine and clozapine—show a lower incidence compared with other psychotropic agents.3
Aripiprazole—a partial D2 and 5HT1A receptor agonist and 5HT2 receptor antagonist—could have caused Ms. K’s akathisia. In 1 study, 11% of patients receiving aripiprazole, 15 to 30 mg/d, for acute mania reported akathisia symptoms.15
Olanzapine cessation could have caused Ms. K’s akathisia, although no cases of akathisia secondary to olanzapine withdrawal have been reported. Alternatively, olanzapine could have interacted with lithium to block lithium’s ability to induce akathisia.
TREATMENT: Back to olanzapine
After we thoroughly discuss olanzapine’s risks and benefits with Ms. K, she consents to switch back to olanzapine, 10 mg/d. We also instruct her to exercise daily and strictly control her diet after discharge.
Ms. K’s akathisia improves dramatically within 1 to 2 days, and her psychosis and mania improve gradually. Her persistent delusions and hallucinations are less intense, although she is still concocting grandiose get-rich-quick schemes.
Ten days after this latest dosage change, we discharge Ms. K on olanzapine, 10 mg/d, and lithium, 300 mg bid. She has no akathisia symptoms, and we arrange placement in an adult home where a psychiatrist sees her regularly. Three years later, she has been lost to follow-up.
Related resource
- AkathisiaSupport.org. Online resource offers links to articles describing causes of and treatments for akathisia subtypes. www.akathisiasupport.org.
- Aripiprazole • Abilify
- Carbidopa/levodopa • Stalevo, Parcopa
- Clonazepam • Klonopin
- Clonidine • Catapres
- Cyproheptadine • Periactin
- Ethosuximide • Zarontin
- Lithium • Eskalith, others
- Lorazepam • Ativan
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Reserpine • various
- Risperidone • Risperdal
- Trihexyphenidyl • Artane
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sadock BJ, Sadock VA. Biological therapies (chapter 36). Kaplan & Sadock’s synopsis of psychiatry: behaviorial sciences/clinical psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003:1110.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
3. Nelson DE. Akathisia—a brief review. Scott Med J 2001;46:133-4.
4. Sachdev P. The epidemiology of drug-induced akathisia: part II. Chronic, tardive and withdrawal akathisias. Schizophr Bull 1995;21:451-60.
5. Bertolín Guillén JM, Martínez Franco L, Juni Anahuja J. Akathisia due to risperidone withdrawal: two clinical cases [in Spanish]. Actas Esp Psiquiatr 2002;30:195-7.
6. Weiss D, Aizenberg D, Hermesh H, et al. Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry 1995;167:483-6.
7. Poyurovsky M, Kreinin A, Modai I, Weizman A. Lithium-induced akathisia responds to low-dose mianserin: case report. Int Clin Psychopharmacol 1995;10:261-3.
8. Poyurovsky M, Shardorodsky M, Fuchs C, et al. Treatment of neuroleptic induced akathisia with the 5HT2 antagonist mianserin. Double-blind, placebo-controlled study. Br J Psychiatry 1999;174:238-42.
9. Anfang MK, Pope HG Jr. Treatment of neuroleptic-induced akathisia with nicotine patches. Psychopharmacology (Berl) 1997;134:153-6.
10. Hong WW, Arvanitis LA, Miller BG. ‘Seroquel’ (ICI 204,636): not different from placebo for EPS or prolactin. Biol Psychiatry 1996;39:598.-
11. Yousaf F, Fialho A, Warden M. Akathisia treated with olanzapine: three case reports. Int J Psychiatry Clin Pract 2004;8:123-5(3).
12. Lavalaye J, Booij J, Linszen DH, et al. Higher occupancy of muscarinic receptors by olanzapine than risperidone in patients with schizophrenia. A[123I]-IDEX SPECT study. Psychopharmacology (Berl) 2001;156:53-7.
13. Chew ML, Mulsant BH, Pollock BG, et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res 2006;88:63-72.
14. Chung WS, Chiu HP. Drug-induced akathisia revisited. Br J Clin Pract 1996;50:270-8.
15. Keck P, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripriprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160:1651-8.
HISTORY: ‘Bizarre’ days
Ms. K, age 45, is brought to the ER by her brother, who reports she has been acting “bizarre and crazy” for 3 days. He says his sister—who has bipolar I disorder— has had trouble sleeping, is restless, hears voices, and is contemplating suicide. He adds she was discharged from a psychiatric hospital 2 weeks ago after a 3-month stay.
Risperidone, 2 mg nightly, was controlling Ms. K’s mania until this recent episode. According to her brother, she also has developed continuous involuntary leg and arm movements and cannot sit or stand still. When she tries to sleep, her feet sway back and forth in bed for hours.
We admit Ms. K to the psychiatric inpatient unit because of her suicidality and hallucinations. She is restless and agitated during initial evaluation, pacing around the room or rocking her feet while standing or sitting. Her speech is pressured and the “voices” are urging her to kill herself.
Ms. K is dysphoric and severely distraught about her “nervousness” and continuous urges to move. She says she would rather die than live with incessantly “jittery” legs and arms, yet she wants to be discharged and denies that she is mentally ill. She believes decreased sleep is causing her symptoms and requests a “sleeping pill.”
she habitually views her medications as useless and stops taking them.
The patient has been hospitalized at least 4 times with severe manic and psychotic symptoms. She does not use illicit drugs and is medically healthy.
The authors’ observations
Ms. K’s involuntary movements suggest akathisia, a common extrapyramidal side effect of antipsychotics and other psychotropics (Table).1
Akathisia is characterized by strong feelings of inner restlessness that manifest as excessive walking or pacing and difficulty remaining still. Ms. K’s movements met at least 2 of 5 DSM-IV-TR criteria for acute akathisia (Box). ,2
Akathisia is characterized by at least 5 subtypes: 3
- Acute akathisia begins hours or days after starting the offending medication and lasts
- Tardive is similar to acute akathisia but can arise within 3 to 4 months of starting the offending medication and persists for years.
- Chronic akathisia lasts ≥3 months and usually has no temporal correlation with antipsychotic initiation or dosage increase.4
- Withdrawal akathisia begins within 6 weeks of discontinuing a medication or significantly reducing the dosage.
- Pseudo akathisia consists of objective symptoms of movement without subjective awareness or distress. This subtype usually is seen in older patients.
history of medication nonadherence strongly suggest akathisia secondary to risperidone withdrawal.5 Several
cases of akathisia after risperidone cessation have been reported.5
We know risperidone is not causing acute akathisia because Ms. K responded well to the medication during her last hospitalization with no adverse effects. Also, her family confirmed that she stopped taking risperidone after her most recent discharge.
Mania also can fuel incessant movement and increase physical activity, but patients often do not realize they have a problem while in a manic phase. Also, swinging and rocking of legs is rarely seen in mania. By contrast, Ms. K was morbidly distraught over her akathisia.
Table
Drugs that can cause akathisia
|
The authors’ observations
Numerous treatments are available for akathisia:
Beta blockers such as propranolol are most widely used because of their rapid onset of action and overall effectiveness in akathisia.3 Researchers believe these drugs reduce extrapyramidal symptoms (EPS) by blocking the adrenergic system. Propranolol can be used at a maximum 120 mg/d in divided doses.
Beta blockers, however, can cause bradycardia, hypotension, or respiratory distress. Use beta blockers with caution, and monitor for these adverse effects.
Benzodiazepines. Clonazepam, which enhances the inhibitory effect of GABA in the brain, is commonly used for akathisia because of its effectiveness and long elimination half-life3 (30 to 40 hours), which decreases the risk of medication withdrawal.
and disinhibition-induced aggression in all patients.
Anticholinergics such as trihexyphenidyl are more commonly used for EPS associated with parkinsonian symptoms or side effects but can be partially effective for akathisia.3 Anticholinergics block the CNS cholinergic activity that causes parkinsonian symptoms.
Cyproheptadine, clonidine, and mianserin have shown some positive results against akathisia in clinical trials.6-8 Iron, nicotine patches, and amantadine have shown limited effectiveness against akathisia in research studies and case reports.3,9
Restarting risperidone at a lower dosage—rather than adding a medication— might have resolved Ms. K’s akathisia, but because she was morbidly despondent over her akathisia, we felt we had no time to experiment. We also believed Ms. K’s would respond well to a neuroleptic with a lower EPS risk—such as quetiapine.1,10
A. Subjective complaints of restlessness after exposure to neuroleptics
B. At least 1 of the following is observed:
- Fidgety movements or swinging of the legs
- Rocking from foot to foot while standing
- Pacing to relieve restlessness
- Inability to sit or stand still for at least several minutes
C. Symptoms develop within 4 weeks of starting or raising the dosage of a neuroleptic or after reducing a medication used to treat extrapyramidal symptoms
D. Criterion A symptoms are not better accounted for by a mental disorder
E. Criterion A symptoms are not caused by a nonneuroleptic or a general medical condition
Source: Adapted from reference 2 with permission
TREATMENT: Trying trials
We perform a complete medical workup to rule out an underlying medical problem. We then start valproic acid, 500 mg bid, for Ms. K’s mania; quetiapine, 50 mg bid, for psychosis and mania; and propranolol, 30 mg bid, for akathisia.
We titrate quetiapine by 100 mg/d every 2 days to 400 mg/d, but after 10 days her akathisia, irritable mood, decreased sleep, and suicidal thoughts persist. We cannot increase propranolol because her blood pressure is 90/60 mm Hg, and adding lorazepam, 0.5 mg tid, does not control her movements. Three days later, we add trihexyphenidyl, 5 mg bid.
Fifteen days after admission, Ms. K remains akathisic, dysphoric, and suicidal despite a 5-drug regimen. Her “nervousness” prevents her from attending groups or other unit activities, and her uncontrollable foot swaying still keeps her awake at night.
The authors’ observations
Neither propranolol, clonazepam, nor trihexyphenidyl alleviated Ms. K’s akathisia. Switching to another neuroleptic with a relatively low EPS risk—such as olanzapine—might help. Olanzapine reduced akathisia in 3 case reports,11 and we hope its strong anticholinergic and antiserotonergic action will help resolve Ms. K’s akathisia.
with patients receiving therapeutic dosages of risperidone.12 In another study, olanzapine showed anticholinergic activity at therapeutic doses but risperidone did not.13 Researchers believe these features reduce olanzapine’s EPS risk compared with other antipsychotics.
TREATMENT: Drug works, but …
Three weeks after Ms. K’s presentation, we stop all psychotropics, start olanzapine, 10 mg nightly, for psychosis and mania, and continue propranolol, 30 mg bid, for akathisia. Within 2 days, Ms. K’s akathisia improves significantly.
We also start lithium, 150 mg bid, for mania, and increase it 4 days later to 300 mg bid to maintain serum lithium at approximately 1 mEq/L. We check serum lithium every 3 days after dosage adjustment. Although lithium can induce akathisia, we thought it would most effectively control her mania.
Six days after we started the new medications, Ms. K’s mania and psychosis begin to improve and she becomes euthymic. She is able to sit calmly during group therapy and community meetings.
Ten days after we start olanzapine and lithium, Ms. K appears bloated. Weight check shows an approximate 5-lb weight gain since starting the medications, both of which can cause weight gain and other metabolic side effects.
At Ms. K’s request, we stop olanzapine and start aripiprazole, 5 mg/d, to try to control her weight gain. We continue lithium and propranolol, which have been controlling her mood and akathisia. The next day—after 1 dose of aripiprazole—her akathisia returns.
The authors’ observations
Because aripiprazole was started as soon as olanzapine was discontinued, it is unclear which action aggravated Ms. K’s akathisia or if both were to blame.
Akathisia’s underlying cause is uncertain. Researchers believe dopamine receptor blockade in the mesocortical dopamine system might be responsible.3 Positron-emission tomography studies suggest that D2 receptor occupancy in the striatum contributes to akathisia, and noradrenergic and serotonergic systems also play a role.3,14
Antipsychotics, antidepressants, and sympathomimetics all have been implicated in akathisia, but antipsychotics that are potent serotonin (5HT) receptor antagonists—such as olanzapine and clozapine—show a lower incidence compared with other psychotropic agents.3
Aripiprazole—a partial D2 and 5HT1A receptor agonist and 5HT2 receptor antagonist—could have caused Ms. K’s akathisia. In 1 study, 11% of patients receiving aripiprazole, 15 to 30 mg/d, for acute mania reported akathisia symptoms.15
Olanzapine cessation could have caused Ms. K’s akathisia, although no cases of akathisia secondary to olanzapine withdrawal have been reported. Alternatively, olanzapine could have interacted with lithium to block lithium’s ability to induce akathisia.
TREATMENT: Back to olanzapine
After we thoroughly discuss olanzapine’s risks and benefits with Ms. K, she consents to switch back to olanzapine, 10 mg/d. We also instruct her to exercise daily and strictly control her diet after discharge.
Ms. K’s akathisia improves dramatically within 1 to 2 days, and her psychosis and mania improve gradually. Her persistent delusions and hallucinations are less intense, although she is still concocting grandiose get-rich-quick schemes.
Ten days after this latest dosage change, we discharge Ms. K on olanzapine, 10 mg/d, and lithium, 300 mg bid. She has no akathisia symptoms, and we arrange placement in an adult home where a psychiatrist sees her regularly. Three years later, she has been lost to follow-up.
Related resource
- AkathisiaSupport.org. Online resource offers links to articles describing causes of and treatments for akathisia subtypes. www.akathisiasupport.org.
- Aripiprazole • Abilify
- Carbidopa/levodopa • Stalevo, Parcopa
- Clonazepam • Klonopin
- Clonidine • Catapres
- Cyproheptadine • Periactin
- Ethosuximide • Zarontin
- Lithium • Eskalith, others
- Lorazepam • Ativan
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Reserpine • various
- Risperidone • Risperdal
- Trihexyphenidyl • Artane
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
HISTORY: ‘Bizarre’ days
Ms. K, age 45, is brought to the ER by her brother, who reports she has been acting “bizarre and crazy” for 3 days. He says his sister—who has bipolar I disorder— has had trouble sleeping, is restless, hears voices, and is contemplating suicide. He adds she was discharged from a psychiatric hospital 2 weeks ago after a 3-month stay.
Risperidone, 2 mg nightly, was controlling Ms. K’s mania until this recent episode. According to her brother, she also has developed continuous involuntary leg and arm movements and cannot sit or stand still. When she tries to sleep, her feet sway back and forth in bed for hours.
We admit Ms. K to the psychiatric inpatient unit because of her suicidality and hallucinations. She is restless and agitated during initial evaluation, pacing around the room or rocking her feet while standing or sitting. Her speech is pressured and the “voices” are urging her to kill herself.
Ms. K is dysphoric and severely distraught about her “nervousness” and continuous urges to move. She says she would rather die than live with incessantly “jittery” legs and arms, yet she wants to be discharged and denies that she is mentally ill. She believes decreased sleep is causing her symptoms and requests a “sleeping pill.”
she habitually views her medications as useless and stops taking them.
The patient has been hospitalized at least 4 times with severe manic and psychotic symptoms. She does not use illicit drugs and is medically healthy.
The authors’ observations
Ms. K’s involuntary movements suggest akathisia, a common extrapyramidal side effect of antipsychotics and other psychotropics (Table).1
Akathisia is characterized by strong feelings of inner restlessness that manifest as excessive walking or pacing and difficulty remaining still. Ms. K’s movements met at least 2 of 5 DSM-IV-TR criteria for acute akathisia (Box). ,2
Akathisia is characterized by at least 5 subtypes: 3
- Acute akathisia begins hours or days after starting the offending medication and lasts
- Tardive is similar to acute akathisia but can arise within 3 to 4 months of starting the offending medication and persists for years.
- Chronic akathisia lasts ≥3 months and usually has no temporal correlation with antipsychotic initiation or dosage increase.4
- Withdrawal akathisia begins within 6 weeks of discontinuing a medication or significantly reducing the dosage.
- Pseudo akathisia consists of objective symptoms of movement without subjective awareness or distress. This subtype usually is seen in older patients.
history of medication nonadherence strongly suggest akathisia secondary to risperidone withdrawal.5 Several
cases of akathisia after risperidone cessation have been reported.5
We know risperidone is not causing acute akathisia because Ms. K responded well to the medication during her last hospitalization with no adverse effects. Also, her family confirmed that she stopped taking risperidone after her most recent discharge.
Mania also can fuel incessant movement and increase physical activity, but patients often do not realize they have a problem while in a manic phase. Also, swinging and rocking of legs is rarely seen in mania. By contrast, Ms. K was morbidly distraught over her akathisia.
Table
Drugs that can cause akathisia
|
The authors’ observations
Numerous treatments are available for akathisia:
Beta blockers such as propranolol are most widely used because of their rapid onset of action and overall effectiveness in akathisia.3 Researchers believe these drugs reduce extrapyramidal symptoms (EPS) by blocking the adrenergic system. Propranolol can be used at a maximum 120 mg/d in divided doses.
Beta blockers, however, can cause bradycardia, hypotension, or respiratory distress. Use beta blockers with caution, and monitor for these adverse effects.
Benzodiazepines. Clonazepam, which enhances the inhibitory effect of GABA in the brain, is commonly used for akathisia because of its effectiveness and long elimination half-life3 (30 to 40 hours), which decreases the risk of medication withdrawal.
and disinhibition-induced aggression in all patients.
Anticholinergics such as trihexyphenidyl are more commonly used for EPS associated with parkinsonian symptoms or side effects but can be partially effective for akathisia.3 Anticholinergics block the CNS cholinergic activity that causes parkinsonian symptoms.
Cyproheptadine, clonidine, and mianserin have shown some positive results against akathisia in clinical trials.6-8 Iron, nicotine patches, and amantadine have shown limited effectiveness against akathisia in research studies and case reports.3,9
Restarting risperidone at a lower dosage—rather than adding a medication— might have resolved Ms. K’s akathisia, but because she was morbidly despondent over her akathisia, we felt we had no time to experiment. We also believed Ms. K’s would respond well to a neuroleptic with a lower EPS risk—such as quetiapine.1,10
A. Subjective complaints of restlessness after exposure to neuroleptics
B. At least 1 of the following is observed:
- Fidgety movements or swinging of the legs
- Rocking from foot to foot while standing
- Pacing to relieve restlessness
- Inability to sit or stand still for at least several minutes
C. Symptoms develop within 4 weeks of starting or raising the dosage of a neuroleptic or after reducing a medication used to treat extrapyramidal symptoms
D. Criterion A symptoms are not better accounted for by a mental disorder
E. Criterion A symptoms are not caused by a nonneuroleptic or a general medical condition
Source: Adapted from reference 2 with permission
TREATMENT: Trying trials
We perform a complete medical workup to rule out an underlying medical problem. We then start valproic acid, 500 mg bid, for Ms. K’s mania; quetiapine, 50 mg bid, for psychosis and mania; and propranolol, 30 mg bid, for akathisia.
We titrate quetiapine by 100 mg/d every 2 days to 400 mg/d, but after 10 days her akathisia, irritable mood, decreased sleep, and suicidal thoughts persist. We cannot increase propranolol because her blood pressure is 90/60 mm Hg, and adding lorazepam, 0.5 mg tid, does not control her movements. Three days later, we add trihexyphenidyl, 5 mg bid.
Fifteen days after admission, Ms. K remains akathisic, dysphoric, and suicidal despite a 5-drug regimen. Her “nervousness” prevents her from attending groups or other unit activities, and her uncontrollable foot swaying still keeps her awake at night.
The authors’ observations
Neither propranolol, clonazepam, nor trihexyphenidyl alleviated Ms. K’s akathisia. Switching to another neuroleptic with a relatively low EPS risk—such as olanzapine—might help. Olanzapine reduced akathisia in 3 case reports,11 and we hope its strong anticholinergic and antiserotonergic action will help resolve Ms. K’s akathisia.
with patients receiving therapeutic dosages of risperidone.12 In another study, olanzapine showed anticholinergic activity at therapeutic doses but risperidone did not.13 Researchers believe these features reduce olanzapine’s EPS risk compared with other antipsychotics.
TREATMENT: Drug works, but …
Three weeks after Ms. K’s presentation, we stop all psychotropics, start olanzapine, 10 mg nightly, for psychosis and mania, and continue propranolol, 30 mg bid, for akathisia. Within 2 days, Ms. K’s akathisia improves significantly.
We also start lithium, 150 mg bid, for mania, and increase it 4 days later to 300 mg bid to maintain serum lithium at approximately 1 mEq/L. We check serum lithium every 3 days after dosage adjustment. Although lithium can induce akathisia, we thought it would most effectively control her mania.
Six days after we started the new medications, Ms. K’s mania and psychosis begin to improve and she becomes euthymic. She is able to sit calmly during group therapy and community meetings.
Ten days after we start olanzapine and lithium, Ms. K appears bloated. Weight check shows an approximate 5-lb weight gain since starting the medications, both of which can cause weight gain and other metabolic side effects.
At Ms. K’s request, we stop olanzapine and start aripiprazole, 5 mg/d, to try to control her weight gain. We continue lithium and propranolol, which have been controlling her mood and akathisia. The next day—after 1 dose of aripiprazole—her akathisia returns.
The authors’ observations
Because aripiprazole was started as soon as olanzapine was discontinued, it is unclear which action aggravated Ms. K’s akathisia or if both were to blame.
Akathisia’s underlying cause is uncertain. Researchers believe dopamine receptor blockade in the mesocortical dopamine system might be responsible.3 Positron-emission tomography studies suggest that D2 receptor occupancy in the striatum contributes to akathisia, and noradrenergic and serotonergic systems also play a role.3,14
Antipsychotics, antidepressants, and sympathomimetics all have been implicated in akathisia, but antipsychotics that are potent serotonin (5HT) receptor antagonists—such as olanzapine and clozapine—show a lower incidence compared with other psychotropic agents.3
Aripiprazole—a partial D2 and 5HT1A receptor agonist and 5HT2 receptor antagonist—could have caused Ms. K’s akathisia. In 1 study, 11% of patients receiving aripiprazole, 15 to 30 mg/d, for acute mania reported akathisia symptoms.15
Olanzapine cessation could have caused Ms. K’s akathisia, although no cases of akathisia secondary to olanzapine withdrawal have been reported. Alternatively, olanzapine could have interacted with lithium to block lithium’s ability to induce akathisia.
TREATMENT: Back to olanzapine
After we thoroughly discuss olanzapine’s risks and benefits with Ms. K, she consents to switch back to olanzapine, 10 mg/d. We also instruct her to exercise daily and strictly control her diet after discharge.
Ms. K’s akathisia improves dramatically within 1 to 2 days, and her psychosis and mania improve gradually. Her persistent delusions and hallucinations are less intense, although she is still concocting grandiose get-rich-quick schemes.
Ten days after this latest dosage change, we discharge Ms. K on olanzapine, 10 mg/d, and lithium, 300 mg bid. She has no akathisia symptoms, and we arrange placement in an adult home where a psychiatrist sees her regularly. Three years later, she has been lost to follow-up.
Related resource
- AkathisiaSupport.org. Online resource offers links to articles describing causes of and treatments for akathisia subtypes. www.akathisiasupport.org.
- Aripiprazole • Abilify
- Carbidopa/levodopa • Stalevo, Parcopa
- Clonazepam • Klonopin
- Clonidine • Catapres
- Cyproheptadine • Periactin
- Ethosuximide • Zarontin
- Lithium • Eskalith, others
- Lorazepam • Ativan
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Reserpine • various
- Risperidone • Risperdal
- Trihexyphenidyl • Artane
- Valproic acid • Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sadock BJ, Sadock VA. Biological therapies (chapter 36). Kaplan & Sadock’s synopsis of psychiatry: behaviorial sciences/clinical psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003:1110.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
3. Nelson DE. Akathisia—a brief review. Scott Med J 2001;46:133-4.
4. Sachdev P. The epidemiology of drug-induced akathisia: part II. Chronic, tardive and withdrawal akathisias. Schizophr Bull 1995;21:451-60.
5. Bertolín Guillén JM, Martínez Franco L, Juni Anahuja J. Akathisia due to risperidone withdrawal: two clinical cases [in Spanish]. Actas Esp Psiquiatr 2002;30:195-7.
6. Weiss D, Aizenberg D, Hermesh H, et al. Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry 1995;167:483-6.
7. Poyurovsky M, Kreinin A, Modai I, Weizman A. Lithium-induced akathisia responds to low-dose mianserin: case report. Int Clin Psychopharmacol 1995;10:261-3.
8. Poyurovsky M, Shardorodsky M, Fuchs C, et al. Treatment of neuroleptic induced akathisia with the 5HT2 antagonist mianserin. Double-blind, placebo-controlled study. Br J Psychiatry 1999;174:238-42.
9. Anfang MK, Pope HG Jr. Treatment of neuroleptic-induced akathisia with nicotine patches. Psychopharmacology (Berl) 1997;134:153-6.
10. Hong WW, Arvanitis LA, Miller BG. ‘Seroquel’ (ICI 204,636): not different from placebo for EPS or prolactin. Biol Psychiatry 1996;39:598.-
11. Yousaf F, Fialho A, Warden M. Akathisia treated with olanzapine: three case reports. Int J Psychiatry Clin Pract 2004;8:123-5(3).
12. Lavalaye J, Booij J, Linszen DH, et al. Higher occupancy of muscarinic receptors by olanzapine than risperidone in patients with schizophrenia. A[123I]-IDEX SPECT study. Psychopharmacology (Berl) 2001;156:53-7.
13. Chew ML, Mulsant BH, Pollock BG, et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res 2006;88:63-72.
14. Chung WS, Chiu HP. Drug-induced akathisia revisited. Br J Clin Pract 1996;50:270-8.
15. Keck P, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripriprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160:1651-8.
1. Sadock BJ, Sadock VA. Biological therapies (chapter 36). Kaplan & Sadock’s synopsis of psychiatry: behaviorial sciences/clinical psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003:1110.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
3. Nelson DE. Akathisia—a brief review. Scott Med J 2001;46:133-4.
4. Sachdev P. The epidemiology of drug-induced akathisia: part II. Chronic, tardive and withdrawal akathisias. Schizophr Bull 1995;21:451-60.
5. Bertolín Guillén JM, Martínez Franco L, Juni Anahuja J. Akathisia due to risperidone withdrawal: two clinical cases [in Spanish]. Actas Esp Psiquiatr 2002;30:195-7.
6. Weiss D, Aizenberg D, Hermesh H, et al. Cyproheptadine treatment in neuroleptic-induced akathisia. Br J Psychiatry 1995;167:483-6.
7. Poyurovsky M, Kreinin A, Modai I, Weizman A. Lithium-induced akathisia responds to low-dose mianserin: case report. Int Clin Psychopharmacol 1995;10:261-3.
8. Poyurovsky M, Shardorodsky M, Fuchs C, et al. Treatment of neuroleptic induced akathisia with the 5HT2 antagonist mianserin. Double-blind, placebo-controlled study. Br J Psychiatry 1999;174:238-42.
9. Anfang MK, Pope HG Jr. Treatment of neuroleptic-induced akathisia with nicotine patches. Psychopharmacology (Berl) 1997;134:153-6.
10. Hong WW, Arvanitis LA, Miller BG. ‘Seroquel’ (ICI 204,636): not different from placebo for EPS or prolactin. Biol Psychiatry 1996;39:598.-
11. Yousaf F, Fialho A, Warden M. Akathisia treated with olanzapine: three case reports. Int J Psychiatry Clin Pract 2004;8:123-5(3).
12. Lavalaye J, Booij J, Linszen DH, et al. Higher occupancy of muscarinic receptors by olanzapine than risperidone in patients with schizophrenia. A[123I]-IDEX SPECT study. Psychopharmacology (Berl) 2001;156:53-7.
13. Chew ML, Mulsant BH, Pollock BG, et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res 2006;88:63-72.
14. Chung WS, Chiu HP. Drug-induced akathisia revisited. Br J Clin Pract 1996;50:270-8.
15. Keck P, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripriprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160:1651-8.
Beating nicotine: Medication algorithm helps teens quit
CASE: Depressed, irritable — and smoking
Michael, age 16, is admitted to a psychiatric unit for severe depressive symptoms and suicidal ideation. The next day, he is irritable and refuses to cooperate with the interview. During group therapy he is distractible and unable to focus. The treating psychiatrist learns that before admission Michael had been smoking 10 to 15 cigarettes per day and now feels a strong craving for cigarettes.
Unrecognized nicotine dependence can be problematic on inpatient psychiatric units, where adolescents such as Michael are not permitted to smoke and rarely are offered nicotine replacement therapy (NRT). Unfortunately, psychiatrists seldom diagnose and treat nicotine dependence—particularly in adolescents—whether in outpatient or inpatient settings.1,2
Do adolescent smokers need help quitting? Do they experience withdrawal symptoms when they stop smoking? Are pharmacologic interventions appropriate? For each question, the answer is a resounding yes.
To help you treat young smokers, this article offers:
- tools for assessing adolescent tobacco use and dependence
- evidence-based treatment options
- an algorithm to guide treatment choice.
Not just a ‘phase’
Early smoking—especially among those younger than age 13—is associated with adolescent psychopathology, including depressive disorders and other substance use disorders.3 Compared with nonsmoking teens, those who smoke at least monthly are significantly more likely to smoke as adults.4 Among high school seniors:
- >20% report smoking cigarettes in the past 30 days
- 12% smoke daily
- 6% smoke ≥10 cigarettes per day.5
Nicotine dependence can develop very rapidly: nearly 25% of adolescents have ≥1 symptom within 2 weeks of starting to smoke at least once a month.6
Role of parents. Early intervention for teen nicotine addiction is particularly important because of the long-term health risks associated with tobacco use.7 In our experience, however, teen smokers’ parents’ attitudes can make addressing adolescent nicotine dependence a therapeutic challenge.
Modified Fagerstrom Tolerance Questionnaire8 (7 items)
Stanford Dependence Inventory (SDI)9 (5 items)
Hooked on Nicotine Checklist (HONC)10 (10 items)
Nicotine Dependence Scale for Adolescents11 (6 items)
Cigarette Dependence Scale (CDS-5 and CDS-12)12 (5 or 12 items)
Parents may be unaware of their teens’ smoking, and those who are aware may:
- not know what help is available
- dismiss teen smoking as “just a phase”
- feel that smoking cigarettes is preferable to smoking marijuana or using other illicit drugs.
Other parents have no objections because they themselves smoke. Some permit their teens to smoke and may even give them cigarettes.
Parents who want their teens to stop smoking often believe erroneously that the best method is to quit “cold turkey.”
Assessing use and dependence
Teen smokers’ nicotine withdrawal symptoms—such as irritability, anxiety, and impaired concentration—can imitate or exacerbate other psychiatric symptoms, thus complicating diagnosis and treatment. Ask all adolescent patients about the quantity, frequency, pattern, and duration of use of all forms of tobacco, including:
- cigarettes
- cigars
- cigarillos (short, narrow cigars)
- bidis (thin, flavored South Asian cigarettes wrapped in leaf)
- smokeless tobacco.
Dependence. Establishing nicotine dependence in young smokers is more complicated than in adults because of teen smokers’ variable smoking patterns. Several self-rating scales have been developed to assess nicotine dependence in adolescents (Box 1).8-12 Although some of these tools have been used primarily in research, outpatient psychiatrists may find these scales useful for evaluating adolescents’ smoking.
Some DSM-IV-TR criteria for substance dependence may not apply to nicotine dependence or correlate with other validated measures of nicotine dependence. For example, “significant time spent obtaining, using, or recovering from the effects of a substance” might not apply to all adolescent smokers.13 Based on our clinical experience, daily smoking for an extended period of time (several months) is a marker of dependence for almost all adolescents.
The Timeline Follow Back method can help you capture a more complete picture of adolescent tobacco use over time.14 This involves asking teens about tobacco use over the past 30 or 90 days, beginning with the assessment day and working backward. Record tobacco use on a calendar, using holidays, weekends, and events as anchor points to help teens recall their smoking.
Biomarker tests can be used to measure nicotine use. The 2 most common are:
- expired carbon monoxide (CO) level (essentially a “breathalyzer” for smoking)
- cotinine level—a metabolite of nicotine.
Expired CO testing is simple to conduct but requires specialized equipment that costs approximately $1,000. Marijuana use may affect CO results, but NRT will not. Measuring CO levels provides information about cigarette smoking over the past several hours, compared with the past several days with cotinine.15
Cotinine can be tested in serum, saliva, or urine. Serum testing can be expensive and may require shipping samples to a specialized laboratory for processing. Testing saliva or urine is less expensive and may be conducted in an office. Cotinine testing in teens who use NRT may be unreliable because the nicotine in these products will be metabolized to cotinine and yield a positive result.
CASE CONTINUED: Wanting to quit
Michael was placed on a 21-mg transdermal nicotine patch, which greatly reduced his craving and irritability. He expressed an interest in quitting smoking. Given Michael’s depressive symptoms, bupropion SR was initiated to treat his depression and assist with smoking cessation.
Treatment options
Optimal smoking cessation treatment includes a combination of medication and behavioral counseling.16
Pharmacologic treatments. FDA-approved medications for adult smoking cessation include NRT—available as a gum, inhaler, nasal spray, lozenge, or transdermal patch—bupropion SR, and varenicline. Although not FDA-approved for patients younger than age 18, NRT and bupropion SR have been evaluated for smoking in adolescents.
NRT helps smokers by reducing nicotine withdrawal symptoms during cessation. Only nicotine gum and transdermal nicotine patch have been studied in adolescents. Results are modest at best, although in some studies including behavioral treatments may have obscured any medication effect (Table 1).17-20
Bupropion SR. How bupropion SR helps patients stop smoking is not completely clear. Three studies have evaluated bupropion SR in adolescents; 2 had positive results, but all 3 had important limitations (Table 2).21-23
One of the 2 positive studies included only 16 patients and had an open-label design.22 The second—a larger randomized, placebo-controlled trial23—found that bupropion SR improved nicotine abstinence compared with placebo at 6 weeks, but this effect did not last after subjects stopped taking the drug.
The third bupropion SR study used 150 mg/d (the recommend adult dose is 300 mg/d) and had poor medication adherence.21 The difference in abstinence rate compared with placebo was not statistically significant.
Other medications. Varenicline—a partial nicotine receptor agonist recently approved for adult smoking cessation—has not been studied in adolescents. Nortriptyline, doxepin, selegiline, clonidine, and mecamylamine have shown promise in adult smokers but are not approved for smoking cessation and require further study, especially in young smokers.24-27
Pharmacotherapy risks. NRT can cause nicotine overdose symptoms, such as rapid heart rate or nausea, especially if used while smoking. Transdermal NRT can cause a local reaction at the application site and can cause burns if worn while undergoing magnetic resonance imaging.28
Adverse effects associated with bupropion SR include a small risk of seizure, weight loss, and insomnia. This drug is contraindicated for patients who:
- have a seizure disorder
- have ever been diagnosed with bulimia or anorexia nervosa
- are taking other bupropion formulations.
Patients should not take bupropion SR during abrupt discontinuation of alcohol or sedatives or within 14 days of taking a monoamine oxidase inhibitor.29
Table 1
Can the nicotine patch help teens quit smoking?
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Smith et al, 199617 | 13- to 17-year-olds (N=22) who smoked ≥20 cpd | 8 weeks of open-label treatment with transdermal NRT plus behavioral counseling and group support | 14% at 8 weeks, 4.5% at 3 and 6 months |
| Hurt et al, 200418 | 13- to 17-year-olds (N=101) who smoked ≥10 cpd | 6 weeks of open-label treatment with transdermal NRT plus self-help material and brief individual counseling if requested | 11% at 6 weeks, 5% at 6 months |
| Hanson et al, 200319 | 13- to 19-year-olds (N=100) who smoked ≥10 cpd | 10 weeks of double-blind treatment with transdermal NRT or placebo plus CBT and contingency management | 20% (active) vs 18% (placebo); not statistically significant |
| Moolchan et al, 200520 | 13- to 17-year-olds (N=120) who smoked ≥10 cpd | 12 weeks of double-blind treatment with:
| 17.7% (active transdermal NRT)* vs 6.5% (active gum) vs 2.5% (placebo only) |
| *P=0.04 for transdermal NRT vs placebo | |||
| CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
Table 2
Teen smoking cessation: Evidence for bupropion SR
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Upadhyaya et al, 200421 | 12- to 19-year-olds (N=16, 11 of whom had ADHD) who smoked ≥5 cpd | 7 weeks of open-label treatment with bupropion SR, 150 mg bid, with brief smoking cessation counseling | 31.3% after 4 weeks of medication |
| Killen et al, 200422 | 15- to 18-year-olds (N=211) who smoked ≥10 cpd | 9 weeks of double-blind treatment with bupropion SR, 150 mg/d, or placebo; subjects received 8 weeks of transdermal NRT and group skills training | 23% (active) vs 28% (placebo) at 10 weeks; 8% (active) vs 7% placebo) at 26 weeks; not statistically significant |
| Muramoto et al, 200523 | 14- to 17-year-olds (N=312) who smoked ≥6 cpd | 6 weeks of double-blind treatment with bupropion SR, 150 mg/d; 150 mg bid; or placebo, with CBT and motivational enhancement | 16.9% (150 mg bid) vs 10.3% (150 mg/d) vs 6.7% (placebo) at 6 weeks* No differences at 26 weeks |
| ADHD: attention-deficit/hyperactivity disorder; CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
| *P=0.019 for 150 mg bid vs placebo | |||
Behavioral therapy. Specialized treatments developed specifically for teen smoking cessation—such as Not On Tobacco (see Related Resources)—often are delivered in schools or other group settings. The most successful consist of ≥5 sessions and include motivational enhancement, cognitive-behavioral, and social influence-oriented approaches.30
Other behavioral treatments. Most psychiatrists are not equipped to deliver these specialized behavioral treatments. Instead, you can use simple yet effective behavioral treatments during routine office visits as adjuncts to pharmacotherapy. At the very least, we recommend the U.S. Public Health Service’s “5 As” strategy (Box 2).16
Educate patients about what to expect during withdrawal, how long withdrawal will last, and medication side effects. To help adolescents develop appropriate treatment expectations:
- discuss the difference between a “slip” (having 1 cigarette) and a “relapse” (returning to daily smoking)
- explain that many individuals need multiple attempts before they quit.
Encourage adolescent smokers to contact the National Network of Tobacco Cessation Quitlines (1-800-784-8669; www.smokefree.gov), which provides free access to telephone-based counseling services.
Ask every patient about tobacco use during every visit, and have a system for recording and tracking tobacco use in the chart
Advise patients clearly and unambiguously to stop smoking, and tailor that advice to each patient’s needs
Assess every patient’s readiness to quit
Assist patients who are ready to quit through self-help materials, referral, and/or smoking cessation treatment
Arrange follow-up visits for relapse prevention or to reassess readiness to quit
Source: Reference 16
Choosing treatment
Evidence guiding treatment choice for teen smoking cessation is limited but growing. Most studies examined daily cigarette smoking, with significantly less evidence to support treatment decisions for light (non-daily) smokers and teens who use other tobacco products.
Recommendations.We have developed a strategy to guide treatment of adolescent smokers (Algorithm). We recommend using pharmacologic interventions only for teens who smoke daily because:
- most studies have focused on daily smoking
- efficacy data are limited
- pharmacologic interventions carry potential risks.
Because of bupropion SR’s contraindications and potential side effect profile, we suggest NRT in combination with smoking cessation counseling as a first-line treatment for young smokers. We recommend beginning with transdermal NRT because of the low likelihood of underdosing with the patch’s once-daily application.20 With either NRT or bupropion SR, schedule follow-up appointments to target relapse prevention and solve any issues that arise.
Help your patient choose a “quit date,” preferably 1 to 2 weeks after your initial assessment. We recommend encouraging young smokers to reduce their smoking by 1 cigarette per day to help minimize withdrawal symptoms from “cold turkey” cessation.31
Some physicians have found it helpful to see the patient on the quit day—or 2 days after when withdrawal symptoms tend to be most robust—to provide support and encouragement. Ask the adolescent to bring and discard during the visit all smoking paraphernalia as a symbol of his or her new smoke-free status.
Step 1: NRT. Initiate smoking cessation counseling plus transdermal NRT using the dosing guidelines in Table 3,28,31 and adjust the dose depending on severity of withdrawal symptoms. Ideally, the patch delivery will be used for 12 weeks, with at least 3 and ideally 6 weeks on the initial dose, followed by a gradual taper.28 We strongly recommend using transdermal NRT on adolescent inpatient units, especially for daily smokers and those who exhibit nicotine withdrawal symptoms.
Step 2: Bupropion SR. If NRT fails, the next step is bupropion SR plus smoking cessation counseling, assuming the adolescent does not have contraindications to bupropion SR. Start the medication based on the dosing guidelines in Table 3,28,31 and set a quit date for 2 weeks after starting bupropion SR.
Again, encourage adolescents to reduce by 1 cigarette per day over the 2 weeks before the quit day to minimize withdrawal symptoms.31 Continue bupropion SR for at least 8 and optimally 12 weeks.31
Combination therapy? If teens are unable to successfully quit smoking with NRT or bupropion SR alone, experience with adults suggests combining the 2 therapies might be beneficial. However, no evidence supports combination therapy in adolescents. Instead, consider referring adolescents who can’t quit to a smoking cessation specialist.
AlgorithmA strategy to initiate smoking cessation in adolescents
CDC: Centers for Disease Control and Prevention; CM: contingency management; NIDA: National Institute of Drug Abuse; NRT: nicotine replacement therapy
Source: Adapted from Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6 with permission of Lippincott Williams & Wilkins.Table 3
Dosing guidelines for adolescent smoking cessation therapy
| Medication | Recommended regimen |
|---|---|
| Bupropion SR31 | Patient weight ≥90 lbs: 150 mg once in the morning for 3 to 6 days, then 150 mg bid for 12 weeks Patient weight <90 lbs: Maximum 150 mg once in the morning, if tolerated |
| Transdermal NRT28 | Patient smokes ≥10 cpd: 21 mg/d for 6 weeks, then 14 mg/d for 2 weeks, then 7 mg/d for 2 weeks, then discontinue Patient smokes <10 cpd: 14 mg/d for 6 weeks, then 7 mg/d for 2 weeks, then discontinue |
| cpd: cigarettes per day; NRT: nicotine replacement therapy | |
CASE CONTINUED: Staying smoke-free
Upon discharge, Michael discontinued NRT and followed up with his outpatient psychiatrist, who provided brief smoking cessation counseling in addition to bupropion SR, 150 mg bid. Michael’s depressive symptoms improved with the medication. He was able to stop smoking within 3 months with the combination of medication and behavioral therapy.
- Centers for Disease Control and Prevention, Youth Tobacco Prevention. www.cdc.gov/tobacco/youth/index.htm.
- Not On Tobacco model program. Substance Abuse and Mental Health Services Administration.www.modelprograms.samhsa.gov/pdfs/model/Not_On_Tobacco.pdf.
Drug brand names
- Bupropion SR • Zyban
- Clonidine • Catapres
- Doxepin • Sinequan
- Mecamylamine • Inversine
- Nicotine/inhalation system • Nicotrol Inhaler
- Nicotine/lozenge • Commit
- Nicotine/nasal spray • Nicotrol NS
- Nicotine/polacrilex • Nicorette
- Nicotine/transdermal • Nicotrol, Prostep
- Nortriptyline • Pamelor
- Selegiline • Eldepryl
- Varenicline • Chantix
Disclosures
Dr. Verduin has received research/grant support from the National Institute of Drug Abuse.
Dr. Upadhyaya is a consultant and speaker for Shire Pharmaceuticals and has received grant/research support from and is a consultant to Eli Lilly and Company.
1. Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol 1990;9(6):701-16.
2. Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings 2006. Bethesda, MD: National Institute on Drug Abuse; 2007. NIH publication no. 07-6202.
3. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescence. Tob Control 2000;9(3):313-9.
4. Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry 2002;41(11):1294-1305.
5. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):222-830.
7. Upadhyaya HP, Brady KT, Wharton M, Liao J. Psychiatric disorders and cigarette smoking among child and adolescent psychiatry inpatients. Am J Addict 2003;12(2):144-52.
8. Prokhorov AV, Pallonen UE, Fava JL, et al. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996;21(1):117-27.
9. Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 1998;152(2):151-6.
10. O’Loughlin J, DiFranza J, Tarasuk J, et al. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control 2002;11(4):354-60.
11. Nonnemaker J, Mowery P, Hersey J, et al. Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res 2004;6(2):295-301.
12. Etter JF, LeHouezec J, Perneger TV. A self-administered questionnaire to measure addiction to cigarettes: the Cigarette Dependence Scale. Neuropsychopharmacology 2003;28(2):359-70.
13. Hughes JR, Oliveto AH, Riggs R, et al. Concordance of different measures of nicotine dependence: two pilot studies. Addict Behav 2004;29(8):1527-39.
14. Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, eds. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: The Humana Press Inc.; 1992.
15. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4(2):149-59.
16. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Quick reference guide for clinicians. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
17. Smith TA, House RF, Croghan IT, et al. Nicotine patch therapy in adolescent smokers. Pediatrics 1996;98(4):659-67.
18. Hurt RD, Croghan GA, Beede SD, et al. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Arch Pediatr Adolesc Med 2000;154(1):31-7.
19. Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tob Res 2003;5(4):515-26.
20. Moolchan ET, Robinson ML, Ernst M, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407-14.
21. Upadhyaya HP, Brady KT, Wang W. Bupropion SR in adolescents with comorbid ADHD and nicotine dependence: a pilot study. J Am Acad Child Adolesc Psychiatry 2004;43(2):199-205.
22. Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol 2004;72(4):729-35.
23. Muramoto ML, Leischow SJ, Sherrill D. A randomized trial of the efficacy of bupropion for adolescent smoking cessation. Paper presented at: Annual Meeting of the Society for Research on Nicotine and Tobacco; March 20-23, 2005; Prague, Czech Republic.
24. Hall SM. Tricyclic antidepressants in the treatment of nicotine dependence. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:95-107.
25. Berlin I. Monoamine oxidase inhibitors for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:109-21.
26. Weinberger AH, Reutenauer EL, George TP. Other nonapproved agents for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:137-48.
27. Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev 2005;2:CD001009.-
28. Nicoderm CQ [package insert]. Bridgewater, NJ: Sanofi Aventis; 2006.
29. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
30. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006;25(5):549-57.
31. Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6.
CASE: Depressed, irritable — and smoking
Michael, age 16, is admitted to a psychiatric unit for severe depressive symptoms and suicidal ideation. The next day, he is irritable and refuses to cooperate with the interview. During group therapy he is distractible and unable to focus. The treating psychiatrist learns that before admission Michael had been smoking 10 to 15 cigarettes per day and now feels a strong craving for cigarettes.
Unrecognized nicotine dependence can be problematic on inpatient psychiatric units, where adolescents such as Michael are not permitted to smoke and rarely are offered nicotine replacement therapy (NRT). Unfortunately, psychiatrists seldom diagnose and treat nicotine dependence—particularly in adolescents—whether in outpatient or inpatient settings.1,2
Do adolescent smokers need help quitting? Do they experience withdrawal symptoms when they stop smoking? Are pharmacologic interventions appropriate? For each question, the answer is a resounding yes.
To help you treat young smokers, this article offers:
- tools for assessing adolescent tobacco use and dependence
- evidence-based treatment options
- an algorithm to guide treatment choice.
Not just a ‘phase’
Early smoking—especially among those younger than age 13—is associated with adolescent psychopathology, including depressive disorders and other substance use disorders.3 Compared with nonsmoking teens, those who smoke at least monthly are significantly more likely to smoke as adults.4 Among high school seniors:
- >20% report smoking cigarettes in the past 30 days
- 12% smoke daily
- 6% smoke ≥10 cigarettes per day.5
Nicotine dependence can develop very rapidly: nearly 25% of adolescents have ≥1 symptom within 2 weeks of starting to smoke at least once a month.6
Role of parents. Early intervention for teen nicotine addiction is particularly important because of the long-term health risks associated with tobacco use.7 In our experience, however, teen smokers’ parents’ attitudes can make addressing adolescent nicotine dependence a therapeutic challenge.
Modified Fagerstrom Tolerance Questionnaire8 (7 items)
Stanford Dependence Inventory (SDI)9 (5 items)
Hooked on Nicotine Checklist (HONC)10 (10 items)
Nicotine Dependence Scale for Adolescents11 (6 items)
Cigarette Dependence Scale (CDS-5 and CDS-12)12 (5 or 12 items)
Parents may be unaware of their teens’ smoking, and those who are aware may:
- not know what help is available
- dismiss teen smoking as “just a phase”
- feel that smoking cigarettes is preferable to smoking marijuana or using other illicit drugs.
Other parents have no objections because they themselves smoke. Some permit their teens to smoke and may even give them cigarettes.
Parents who want their teens to stop smoking often believe erroneously that the best method is to quit “cold turkey.”
Assessing use and dependence
Teen smokers’ nicotine withdrawal symptoms—such as irritability, anxiety, and impaired concentration—can imitate or exacerbate other psychiatric symptoms, thus complicating diagnosis and treatment. Ask all adolescent patients about the quantity, frequency, pattern, and duration of use of all forms of tobacco, including:
- cigarettes
- cigars
- cigarillos (short, narrow cigars)
- bidis (thin, flavored South Asian cigarettes wrapped in leaf)
- smokeless tobacco.
Dependence. Establishing nicotine dependence in young smokers is more complicated than in adults because of teen smokers’ variable smoking patterns. Several self-rating scales have been developed to assess nicotine dependence in adolescents (Box 1).8-12 Although some of these tools have been used primarily in research, outpatient psychiatrists may find these scales useful for evaluating adolescents’ smoking.
Some DSM-IV-TR criteria for substance dependence may not apply to nicotine dependence or correlate with other validated measures of nicotine dependence. For example, “significant time spent obtaining, using, or recovering from the effects of a substance” might not apply to all adolescent smokers.13 Based on our clinical experience, daily smoking for an extended period of time (several months) is a marker of dependence for almost all adolescents.
The Timeline Follow Back method can help you capture a more complete picture of adolescent tobacco use over time.14 This involves asking teens about tobacco use over the past 30 or 90 days, beginning with the assessment day and working backward. Record tobacco use on a calendar, using holidays, weekends, and events as anchor points to help teens recall their smoking.
Biomarker tests can be used to measure nicotine use. The 2 most common are:
- expired carbon monoxide (CO) level (essentially a “breathalyzer” for smoking)
- cotinine level—a metabolite of nicotine.
Expired CO testing is simple to conduct but requires specialized equipment that costs approximately $1,000. Marijuana use may affect CO results, but NRT will not. Measuring CO levels provides information about cigarette smoking over the past several hours, compared with the past several days with cotinine.15
Cotinine can be tested in serum, saliva, or urine. Serum testing can be expensive and may require shipping samples to a specialized laboratory for processing. Testing saliva or urine is less expensive and may be conducted in an office. Cotinine testing in teens who use NRT may be unreliable because the nicotine in these products will be metabolized to cotinine and yield a positive result.
CASE CONTINUED: Wanting to quit
Michael was placed on a 21-mg transdermal nicotine patch, which greatly reduced his craving and irritability. He expressed an interest in quitting smoking. Given Michael’s depressive symptoms, bupropion SR was initiated to treat his depression and assist with smoking cessation.
Treatment options
Optimal smoking cessation treatment includes a combination of medication and behavioral counseling.16
Pharmacologic treatments. FDA-approved medications for adult smoking cessation include NRT—available as a gum, inhaler, nasal spray, lozenge, or transdermal patch—bupropion SR, and varenicline. Although not FDA-approved for patients younger than age 18, NRT and bupropion SR have been evaluated for smoking in adolescents.
NRT helps smokers by reducing nicotine withdrawal symptoms during cessation. Only nicotine gum and transdermal nicotine patch have been studied in adolescents. Results are modest at best, although in some studies including behavioral treatments may have obscured any medication effect (Table 1).17-20
Bupropion SR. How bupropion SR helps patients stop smoking is not completely clear. Three studies have evaluated bupropion SR in adolescents; 2 had positive results, but all 3 had important limitations (Table 2).21-23
One of the 2 positive studies included only 16 patients and had an open-label design.22 The second—a larger randomized, placebo-controlled trial23—found that bupropion SR improved nicotine abstinence compared with placebo at 6 weeks, but this effect did not last after subjects stopped taking the drug.
The third bupropion SR study used 150 mg/d (the recommend adult dose is 300 mg/d) and had poor medication adherence.21 The difference in abstinence rate compared with placebo was not statistically significant.
Other medications. Varenicline—a partial nicotine receptor agonist recently approved for adult smoking cessation—has not been studied in adolescents. Nortriptyline, doxepin, selegiline, clonidine, and mecamylamine have shown promise in adult smokers but are not approved for smoking cessation and require further study, especially in young smokers.24-27
Pharmacotherapy risks. NRT can cause nicotine overdose symptoms, such as rapid heart rate or nausea, especially if used while smoking. Transdermal NRT can cause a local reaction at the application site and can cause burns if worn while undergoing magnetic resonance imaging.28
Adverse effects associated with bupropion SR include a small risk of seizure, weight loss, and insomnia. This drug is contraindicated for patients who:
- have a seizure disorder
- have ever been diagnosed with bulimia or anorexia nervosa
- are taking other bupropion formulations.
Patients should not take bupropion SR during abrupt discontinuation of alcohol or sedatives or within 14 days of taking a monoamine oxidase inhibitor.29
Table 1
Can the nicotine patch help teens quit smoking?
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Smith et al, 199617 | 13- to 17-year-olds (N=22) who smoked ≥20 cpd | 8 weeks of open-label treatment with transdermal NRT plus behavioral counseling and group support | 14% at 8 weeks, 4.5% at 3 and 6 months |
| Hurt et al, 200418 | 13- to 17-year-olds (N=101) who smoked ≥10 cpd | 6 weeks of open-label treatment with transdermal NRT plus self-help material and brief individual counseling if requested | 11% at 6 weeks, 5% at 6 months |
| Hanson et al, 200319 | 13- to 19-year-olds (N=100) who smoked ≥10 cpd | 10 weeks of double-blind treatment with transdermal NRT or placebo plus CBT and contingency management | 20% (active) vs 18% (placebo); not statistically significant |
| Moolchan et al, 200520 | 13- to 17-year-olds (N=120) who smoked ≥10 cpd | 12 weeks of double-blind treatment with:
| 17.7% (active transdermal NRT)* vs 6.5% (active gum) vs 2.5% (placebo only) |
| *P=0.04 for transdermal NRT vs placebo | |||
| CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
Table 2
Teen smoking cessation: Evidence for bupropion SR
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Upadhyaya et al, 200421 | 12- to 19-year-olds (N=16, 11 of whom had ADHD) who smoked ≥5 cpd | 7 weeks of open-label treatment with bupropion SR, 150 mg bid, with brief smoking cessation counseling | 31.3% after 4 weeks of medication |
| Killen et al, 200422 | 15- to 18-year-olds (N=211) who smoked ≥10 cpd | 9 weeks of double-blind treatment with bupropion SR, 150 mg/d, or placebo; subjects received 8 weeks of transdermal NRT and group skills training | 23% (active) vs 28% (placebo) at 10 weeks; 8% (active) vs 7% placebo) at 26 weeks; not statistically significant |
| Muramoto et al, 200523 | 14- to 17-year-olds (N=312) who smoked ≥6 cpd | 6 weeks of double-blind treatment with bupropion SR, 150 mg/d; 150 mg bid; or placebo, with CBT and motivational enhancement | 16.9% (150 mg bid) vs 10.3% (150 mg/d) vs 6.7% (placebo) at 6 weeks* No differences at 26 weeks |
| ADHD: attention-deficit/hyperactivity disorder; CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
| *P=0.019 for 150 mg bid vs placebo | |||
Behavioral therapy. Specialized treatments developed specifically for teen smoking cessation—such as Not On Tobacco (see Related Resources)—often are delivered in schools or other group settings. The most successful consist of ≥5 sessions and include motivational enhancement, cognitive-behavioral, and social influence-oriented approaches.30
Other behavioral treatments. Most psychiatrists are not equipped to deliver these specialized behavioral treatments. Instead, you can use simple yet effective behavioral treatments during routine office visits as adjuncts to pharmacotherapy. At the very least, we recommend the U.S. Public Health Service’s “5 As” strategy (Box 2).16
Educate patients about what to expect during withdrawal, how long withdrawal will last, and medication side effects. To help adolescents develop appropriate treatment expectations:
- discuss the difference between a “slip” (having 1 cigarette) and a “relapse” (returning to daily smoking)
- explain that many individuals need multiple attempts before they quit.
Encourage adolescent smokers to contact the National Network of Tobacco Cessation Quitlines (1-800-784-8669; www.smokefree.gov), which provides free access to telephone-based counseling services.
Ask every patient about tobacco use during every visit, and have a system for recording and tracking tobacco use in the chart
Advise patients clearly and unambiguously to stop smoking, and tailor that advice to each patient’s needs
Assess every patient’s readiness to quit
Assist patients who are ready to quit through self-help materials, referral, and/or smoking cessation treatment
Arrange follow-up visits for relapse prevention or to reassess readiness to quit
Source: Reference 16
Choosing treatment
Evidence guiding treatment choice for teen smoking cessation is limited but growing. Most studies examined daily cigarette smoking, with significantly less evidence to support treatment decisions for light (non-daily) smokers and teens who use other tobacco products.
Recommendations.We have developed a strategy to guide treatment of adolescent smokers (Algorithm). We recommend using pharmacologic interventions only for teens who smoke daily because:
- most studies have focused on daily smoking
- efficacy data are limited
- pharmacologic interventions carry potential risks.
Because of bupropion SR’s contraindications and potential side effect profile, we suggest NRT in combination with smoking cessation counseling as a first-line treatment for young smokers. We recommend beginning with transdermal NRT because of the low likelihood of underdosing with the patch’s once-daily application.20 With either NRT or bupropion SR, schedule follow-up appointments to target relapse prevention and solve any issues that arise.
Help your patient choose a “quit date,” preferably 1 to 2 weeks after your initial assessment. We recommend encouraging young smokers to reduce their smoking by 1 cigarette per day to help minimize withdrawal symptoms from “cold turkey” cessation.31
Some physicians have found it helpful to see the patient on the quit day—or 2 days after when withdrawal symptoms tend to be most robust—to provide support and encouragement. Ask the adolescent to bring and discard during the visit all smoking paraphernalia as a symbol of his or her new smoke-free status.
Step 1: NRT. Initiate smoking cessation counseling plus transdermal NRT using the dosing guidelines in Table 3,28,31 and adjust the dose depending on severity of withdrawal symptoms. Ideally, the patch delivery will be used for 12 weeks, with at least 3 and ideally 6 weeks on the initial dose, followed by a gradual taper.28 We strongly recommend using transdermal NRT on adolescent inpatient units, especially for daily smokers and those who exhibit nicotine withdrawal symptoms.
Step 2: Bupropion SR. If NRT fails, the next step is bupropion SR plus smoking cessation counseling, assuming the adolescent does not have contraindications to bupropion SR. Start the medication based on the dosing guidelines in Table 3,28,31 and set a quit date for 2 weeks after starting bupropion SR.
Again, encourage adolescents to reduce by 1 cigarette per day over the 2 weeks before the quit day to minimize withdrawal symptoms.31 Continue bupropion SR for at least 8 and optimally 12 weeks.31
Combination therapy? If teens are unable to successfully quit smoking with NRT or bupropion SR alone, experience with adults suggests combining the 2 therapies might be beneficial. However, no evidence supports combination therapy in adolescents. Instead, consider referring adolescents who can’t quit to a smoking cessation specialist.
AlgorithmA strategy to initiate smoking cessation in adolescents
CDC: Centers for Disease Control and Prevention; CM: contingency management; NIDA: National Institute of Drug Abuse; NRT: nicotine replacement therapy
Source: Adapted from Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6 with permission of Lippincott Williams & Wilkins.Table 3
Dosing guidelines for adolescent smoking cessation therapy
| Medication | Recommended regimen |
|---|---|
| Bupropion SR31 | Patient weight ≥90 lbs: 150 mg once in the morning for 3 to 6 days, then 150 mg bid for 12 weeks Patient weight <90 lbs: Maximum 150 mg once in the morning, if tolerated |
| Transdermal NRT28 | Patient smokes ≥10 cpd: 21 mg/d for 6 weeks, then 14 mg/d for 2 weeks, then 7 mg/d for 2 weeks, then discontinue Patient smokes <10 cpd: 14 mg/d for 6 weeks, then 7 mg/d for 2 weeks, then discontinue |
| cpd: cigarettes per day; NRT: nicotine replacement therapy | |
CASE CONTINUED: Staying smoke-free
Upon discharge, Michael discontinued NRT and followed up with his outpatient psychiatrist, who provided brief smoking cessation counseling in addition to bupropion SR, 150 mg bid. Michael’s depressive symptoms improved with the medication. He was able to stop smoking within 3 months with the combination of medication and behavioral therapy.
- Centers for Disease Control and Prevention, Youth Tobacco Prevention. www.cdc.gov/tobacco/youth/index.htm.
- Not On Tobacco model program. Substance Abuse and Mental Health Services Administration.www.modelprograms.samhsa.gov/pdfs/model/Not_On_Tobacco.pdf.
Drug brand names
- Bupropion SR • Zyban
- Clonidine • Catapres
- Doxepin • Sinequan
- Mecamylamine • Inversine
- Nicotine/inhalation system • Nicotrol Inhaler
- Nicotine/lozenge • Commit
- Nicotine/nasal spray • Nicotrol NS
- Nicotine/polacrilex • Nicorette
- Nicotine/transdermal • Nicotrol, Prostep
- Nortriptyline • Pamelor
- Selegiline • Eldepryl
- Varenicline • Chantix
Disclosures
Dr. Verduin has received research/grant support from the National Institute of Drug Abuse.
Dr. Upadhyaya is a consultant and speaker for Shire Pharmaceuticals and has received grant/research support from and is a consultant to Eli Lilly and Company.
CASE: Depressed, irritable — and smoking
Michael, age 16, is admitted to a psychiatric unit for severe depressive symptoms and suicidal ideation. The next day, he is irritable and refuses to cooperate with the interview. During group therapy he is distractible and unable to focus. The treating psychiatrist learns that before admission Michael had been smoking 10 to 15 cigarettes per day and now feels a strong craving for cigarettes.
Unrecognized nicotine dependence can be problematic on inpatient psychiatric units, where adolescents such as Michael are not permitted to smoke and rarely are offered nicotine replacement therapy (NRT). Unfortunately, psychiatrists seldom diagnose and treat nicotine dependence—particularly in adolescents—whether in outpatient or inpatient settings.1,2
Do adolescent smokers need help quitting? Do they experience withdrawal symptoms when they stop smoking? Are pharmacologic interventions appropriate? For each question, the answer is a resounding yes.
To help you treat young smokers, this article offers:
- tools for assessing adolescent tobacco use and dependence
- evidence-based treatment options
- an algorithm to guide treatment choice.
Not just a ‘phase’
Early smoking—especially among those younger than age 13—is associated with adolescent psychopathology, including depressive disorders and other substance use disorders.3 Compared with nonsmoking teens, those who smoke at least monthly are significantly more likely to smoke as adults.4 Among high school seniors:
- >20% report smoking cigarettes in the past 30 days
- 12% smoke daily
- 6% smoke ≥10 cigarettes per day.5
Nicotine dependence can develop very rapidly: nearly 25% of adolescents have ≥1 symptom within 2 weeks of starting to smoke at least once a month.6
Role of parents. Early intervention for teen nicotine addiction is particularly important because of the long-term health risks associated with tobacco use.7 In our experience, however, teen smokers’ parents’ attitudes can make addressing adolescent nicotine dependence a therapeutic challenge.
Modified Fagerstrom Tolerance Questionnaire8 (7 items)
Stanford Dependence Inventory (SDI)9 (5 items)
Hooked on Nicotine Checklist (HONC)10 (10 items)
Nicotine Dependence Scale for Adolescents11 (6 items)
Cigarette Dependence Scale (CDS-5 and CDS-12)12 (5 or 12 items)
Parents may be unaware of their teens’ smoking, and those who are aware may:
- not know what help is available
- dismiss teen smoking as “just a phase”
- feel that smoking cigarettes is preferable to smoking marijuana or using other illicit drugs.
Other parents have no objections because they themselves smoke. Some permit their teens to smoke and may even give them cigarettes.
Parents who want their teens to stop smoking often believe erroneously that the best method is to quit “cold turkey.”
Assessing use and dependence
Teen smokers’ nicotine withdrawal symptoms—such as irritability, anxiety, and impaired concentration—can imitate or exacerbate other psychiatric symptoms, thus complicating diagnosis and treatment. Ask all adolescent patients about the quantity, frequency, pattern, and duration of use of all forms of tobacco, including:
- cigarettes
- cigars
- cigarillos (short, narrow cigars)
- bidis (thin, flavored South Asian cigarettes wrapped in leaf)
- smokeless tobacco.
Dependence. Establishing nicotine dependence in young smokers is more complicated than in adults because of teen smokers’ variable smoking patterns. Several self-rating scales have been developed to assess nicotine dependence in adolescents (Box 1).8-12 Although some of these tools have been used primarily in research, outpatient psychiatrists may find these scales useful for evaluating adolescents’ smoking.
Some DSM-IV-TR criteria for substance dependence may not apply to nicotine dependence or correlate with other validated measures of nicotine dependence. For example, “significant time spent obtaining, using, or recovering from the effects of a substance” might not apply to all adolescent smokers.13 Based on our clinical experience, daily smoking for an extended period of time (several months) is a marker of dependence for almost all adolescents.
The Timeline Follow Back method can help you capture a more complete picture of adolescent tobacco use over time.14 This involves asking teens about tobacco use over the past 30 or 90 days, beginning with the assessment day and working backward. Record tobacco use on a calendar, using holidays, weekends, and events as anchor points to help teens recall their smoking.
Biomarker tests can be used to measure nicotine use. The 2 most common are:
- expired carbon monoxide (CO) level (essentially a “breathalyzer” for smoking)
- cotinine level—a metabolite of nicotine.
Expired CO testing is simple to conduct but requires specialized equipment that costs approximately $1,000. Marijuana use may affect CO results, but NRT will not. Measuring CO levels provides information about cigarette smoking over the past several hours, compared with the past several days with cotinine.15
Cotinine can be tested in serum, saliva, or urine. Serum testing can be expensive and may require shipping samples to a specialized laboratory for processing. Testing saliva or urine is less expensive and may be conducted in an office. Cotinine testing in teens who use NRT may be unreliable because the nicotine in these products will be metabolized to cotinine and yield a positive result.
CASE CONTINUED: Wanting to quit
Michael was placed on a 21-mg transdermal nicotine patch, which greatly reduced his craving and irritability. He expressed an interest in quitting smoking. Given Michael’s depressive symptoms, bupropion SR was initiated to treat his depression and assist with smoking cessation.
Treatment options
Optimal smoking cessation treatment includes a combination of medication and behavioral counseling.16
Pharmacologic treatments. FDA-approved medications for adult smoking cessation include NRT—available as a gum, inhaler, nasal spray, lozenge, or transdermal patch—bupropion SR, and varenicline. Although not FDA-approved for patients younger than age 18, NRT and bupropion SR have been evaluated for smoking in adolescents.
NRT helps smokers by reducing nicotine withdrawal symptoms during cessation. Only nicotine gum and transdermal nicotine patch have been studied in adolescents. Results are modest at best, although in some studies including behavioral treatments may have obscured any medication effect (Table 1).17-20
Bupropion SR. How bupropion SR helps patients stop smoking is not completely clear. Three studies have evaluated bupropion SR in adolescents; 2 had positive results, but all 3 had important limitations (Table 2).21-23
One of the 2 positive studies included only 16 patients and had an open-label design.22 The second—a larger randomized, placebo-controlled trial23—found that bupropion SR improved nicotine abstinence compared with placebo at 6 weeks, but this effect did not last after subjects stopped taking the drug.
The third bupropion SR study used 150 mg/d (the recommend adult dose is 300 mg/d) and had poor medication adherence.21 The difference in abstinence rate compared with placebo was not statistically significant.
Other medications. Varenicline—a partial nicotine receptor agonist recently approved for adult smoking cessation—has not been studied in adolescents. Nortriptyline, doxepin, selegiline, clonidine, and mecamylamine have shown promise in adult smokers but are not approved for smoking cessation and require further study, especially in young smokers.24-27
Pharmacotherapy risks. NRT can cause nicotine overdose symptoms, such as rapid heart rate or nausea, especially if used while smoking. Transdermal NRT can cause a local reaction at the application site and can cause burns if worn while undergoing magnetic resonance imaging.28
Adverse effects associated with bupropion SR include a small risk of seizure, weight loss, and insomnia. This drug is contraindicated for patients who:
- have a seizure disorder
- have ever been diagnosed with bulimia or anorexia nervosa
- are taking other bupropion formulations.
Patients should not take bupropion SR during abrupt discontinuation of alcohol or sedatives or within 14 days of taking a monoamine oxidase inhibitor.29
Table 1
Can the nicotine patch help teens quit smoking?
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Smith et al, 199617 | 13- to 17-year-olds (N=22) who smoked ≥20 cpd | 8 weeks of open-label treatment with transdermal NRT plus behavioral counseling and group support | 14% at 8 weeks, 4.5% at 3 and 6 months |
| Hurt et al, 200418 | 13- to 17-year-olds (N=101) who smoked ≥10 cpd | 6 weeks of open-label treatment with transdermal NRT plus self-help material and brief individual counseling if requested | 11% at 6 weeks, 5% at 6 months |
| Hanson et al, 200319 | 13- to 19-year-olds (N=100) who smoked ≥10 cpd | 10 weeks of double-blind treatment with transdermal NRT or placebo plus CBT and contingency management | 20% (active) vs 18% (placebo); not statistically significant |
| Moolchan et al, 200520 | 13- to 17-year-olds (N=120) who smoked ≥10 cpd | 12 weeks of double-blind treatment with:
| 17.7% (active transdermal NRT)* vs 6.5% (active gum) vs 2.5% (placebo only) |
| *P=0.04 for transdermal NRT vs placebo | |||
| CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
Table 2
Teen smoking cessation: Evidence for bupropion SR
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Upadhyaya et al, 200421 | 12- to 19-year-olds (N=16, 11 of whom had ADHD) who smoked ≥5 cpd | 7 weeks of open-label treatment with bupropion SR, 150 mg bid, with brief smoking cessation counseling | 31.3% after 4 weeks of medication |
| Killen et al, 200422 | 15- to 18-year-olds (N=211) who smoked ≥10 cpd | 9 weeks of double-blind treatment with bupropion SR, 150 mg/d, or placebo; subjects received 8 weeks of transdermal NRT and group skills training | 23% (active) vs 28% (placebo) at 10 weeks; 8% (active) vs 7% placebo) at 26 weeks; not statistically significant |
| Muramoto et al, 200523 | 14- to 17-year-olds (N=312) who smoked ≥6 cpd | 6 weeks of double-blind treatment with bupropion SR, 150 mg/d; 150 mg bid; or placebo, with CBT and motivational enhancement | 16.9% (150 mg bid) vs 10.3% (150 mg/d) vs 6.7% (placebo) at 6 weeks* No differences at 26 weeks |
| ADHD: attention-deficit/hyperactivity disorder; CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
| *P=0.019 for 150 mg bid vs placebo | |||
Behavioral therapy. Specialized treatments developed specifically for teen smoking cessation—such as Not On Tobacco (see Related Resources)—often are delivered in schools or other group settings. The most successful consist of ≥5 sessions and include motivational enhancement, cognitive-behavioral, and social influence-oriented approaches.30
Other behavioral treatments. Most psychiatrists are not equipped to deliver these specialized behavioral treatments. Instead, you can use simple yet effective behavioral treatments during routine office visits as adjuncts to pharmacotherapy. At the very least, we recommend the U.S. Public Health Service’s “5 As” strategy (Box 2).16
Educate patients about what to expect during withdrawal, how long withdrawal will last, and medication side effects. To help adolescents develop appropriate treatment expectations:
- discuss the difference between a “slip” (having 1 cigarette) and a “relapse” (returning to daily smoking)
- explain that many individuals need multiple attempts before they quit.
Encourage adolescent smokers to contact the National Network of Tobacco Cessation Quitlines (1-800-784-8669; www.smokefree.gov), which provides free access to telephone-based counseling services.
Ask every patient about tobacco use during every visit, and have a system for recording and tracking tobacco use in the chart
Advise patients clearly and unambiguously to stop smoking, and tailor that advice to each patient’s needs
Assess every patient’s readiness to quit
Assist patients who are ready to quit through self-help materials, referral, and/or smoking cessation treatment
Arrange follow-up visits for relapse prevention or to reassess readiness to quit
Source: Reference 16
Choosing treatment
Evidence guiding treatment choice for teen smoking cessation is limited but growing. Most studies examined daily cigarette smoking, with significantly less evidence to support treatment decisions for light (non-daily) smokers and teens who use other tobacco products.
Recommendations.We have developed a strategy to guide treatment of adolescent smokers (Algorithm). We recommend using pharmacologic interventions only for teens who smoke daily because:
- most studies have focused on daily smoking
- efficacy data are limited
- pharmacologic interventions carry potential risks.
Because of bupropion SR’s contraindications and potential side effect profile, we suggest NRT in combination with smoking cessation counseling as a first-line treatment for young smokers. We recommend beginning with transdermal NRT because of the low likelihood of underdosing with the patch’s once-daily application.20 With either NRT or bupropion SR, schedule follow-up appointments to target relapse prevention and solve any issues that arise.
Help your patient choose a “quit date,” preferably 1 to 2 weeks after your initial assessment. We recommend encouraging young smokers to reduce their smoking by 1 cigarette per day to help minimize withdrawal symptoms from “cold turkey” cessation.31
Some physicians have found it helpful to see the patient on the quit day—or 2 days after when withdrawal symptoms tend to be most robust—to provide support and encouragement. Ask the adolescent to bring and discard during the visit all smoking paraphernalia as a symbol of his or her new smoke-free status.
Step 1: NRT. Initiate smoking cessation counseling plus transdermal NRT using the dosing guidelines in Table 3,28,31 and adjust the dose depending on severity of withdrawal symptoms. Ideally, the patch delivery will be used for 12 weeks, with at least 3 and ideally 6 weeks on the initial dose, followed by a gradual taper.28 We strongly recommend using transdermal NRT on adolescent inpatient units, especially for daily smokers and those who exhibit nicotine withdrawal symptoms.
Step 2: Bupropion SR. If NRT fails, the next step is bupropion SR plus smoking cessation counseling, assuming the adolescent does not have contraindications to bupropion SR. Start the medication based on the dosing guidelines in Table 3,28,31 and set a quit date for 2 weeks after starting bupropion SR.
Again, encourage adolescents to reduce by 1 cigarette per day over the 2 weeks before the quit day to minimize withdrawal symptoms.31 Continue bupropion SR for at least 8 and optimally 12 weeks.31
Combination therapy? If teens are unable to successfully quit smoking with NRT or bupropion SR alone, experience with adults suggests combining the 2 therapies might be beneficial. However, no evidence supports combination therapy in adolescents. Instead, consider referring adolescents who can’t quit to a smoking cessation specialist.
AlgorithmA strategy to initiate smoking cessation in adolescents
CDC: Centers for Disease Control and Prevention; CM: contingency management; NIDA: National Institute of Drug Abuse; NRT: nicotine replacement therapy
Source: Adapted from Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6 with permission of Lippincott Williams & Wilkins.Table 3
Dosing guidelines for adolescent smoking cessation therapy
| Medication | Recommended regimen |
|---|---|
| Bupropion SR31 | Patient weight ≥90 lbs: 150 mg once in the morning for 3 to 6 days, then 150 mg bid for 12 weeks Patient weight <90 lbs: Maximum 150 mg once in the morning, if tolerated |
| Transdermal NRT28 | Patient smokes ≥10 cpd: 21 mg/d for 6 weeks, then 14 mg/d for 2 weeks, then 7 mg/d for 2 weeks, then discontinue Patient smokes <10 cpd: 14 mg/d for 6 weeks, then 7 mg/d for 2 weeks, then discontinue |
| cpd: cigarettes per day; NRT: nicotine replacement therapy | |
CASE CONTINUED: Staying smoke-free
Upon discharge, Michael discontinued NRT and followed up with his outpatient psychiatrist, who provided brief smoking cessation counseling in addition to bupropion SR, 150 mg bid. Michael’s depressive symptoms improved with the medication. He was able to stop smoking within 3 months with the combination of medication and behavioral therapy.
- Centers for Disease Control and Prevention, Youth Tobacco Prevention. www.cdc.gov/tobacco/youth/index.htm.
- Not On Tobacco model program. Substance Abuse and Mental Health Services Administration.www.modelprograms.samhsa.gov/pdfs/model/Not_On_Tobacco.pdf.
Drug brand names
- Bupropion SR • Zyban
- Clonidine • Catapres
- Doxepin • Sinequan
- Mecamylamine • Inversine
- Nicotine/inhalation system • Nicotrol Inhaler
- Nicotine/lozenge • Commit
- Nicotine/nasal spray • Nicotrol NS
- Nicotine/polacrilex • Nicorette
- Nicotine/transdermal • Nicotrol, Prostep
- Nortriptyline • Pamelor
- Selegiline • Eldepryl
- Varenicline • Chantix
Disclosures
Dr. Verduin has received research/grant support from the National Institute of Drug Abuse.
Dr. Upadhyaya is a consultant and speaker for Shire Pharmaceuticals and has received grant/research support from and is a consultant to Eli Lilly and Company.
1. Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol 1990;9(6):701-16.
2. Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings 2006. Bethesda, MD: National Institute on Drug Abuse; 2007. NIH publication no. 07-6202.
3. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescence. Tob Control 2000;9(3):313-9.
4. Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry 2002;41(11):1294-1305.
5. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):222-830.
7. Upadhyaya HP, Brady KT, Wharton M, Liao J. Psychiatric disorders and cigarette smoking among child and adolescent psychiatry inpatients. Am J Addict 2003;12(2):144-52.
8. Prokhorov AV, Pallonen UE, Fava JL, et al. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996;21(1):117-27.
9. Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 1998;152(2):151-6.
10. O’Loughlin J, DiFranza J, Tarasuk J, et al. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control 2002;11(4):354-60.
11. Nonnemaker J, Mowery P, Hersey J, et al. Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res 2004;6(2):295-301.
12. Etter JF, LeHouezec J, Perneger TV. A self-administered questionnaire to measure addiction to cigarettes: the Cigarette Dependence Scale. Neuropsychopharmacology 2003;28(2):359-70.
13. Hughes JR, Oliveto AH, Riggs R, et al. Concordance of different measures of nicotine dependence: two pilot studies. Addict Behav 2004;29(8):1527-39.
14. Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, eds. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: The Humana Press Inc.; 1992.
15. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4(2):149-59.
16. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Quick reference guide for clinicians. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
17. Smith TA, House RF, Croghan IT, et al. Nicotine patch therapy in adolescent smokers. Pediatrics 1996;98(4):659-67.
18. Hurt RD, Croghan GA, Beede SD, et al. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Arch Pediatr Adolesc Med 2000;154(1):31-7.
19. Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tob Res 2003;5(4):515-26.
20. Moolchan ET, Robinson ML, Ernst M, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407-14.
21. Upadhyaya HP, Brady KT, Wang W. Bupropion SR in adolescents with comorbid ADHD and nicotine dependence: a pilot study. J Am Acad Child Adolesc Psychiatry 2004;43(2):199-205.
22. Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol 2004;72(4):729-35.
23. Muramoto ML, Leischow SJ, Sherrill D. A randomized trial of the efficacy of bupropion for adolescent smoking cessation. Paper presented at: Annual Meeting of the Society for Research on Nicotine and Tobacco; March 20-23, 2005; Prague, Czech Republic.
24. Hall SM. Tricyclic antidepressants in the treatment of nicotine dependence. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:95-107.
25. Berlin I. Monoamine oxidase inhibitors for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:109-21.
26. Weinberger AH, Reutenauer EL, George TP. Other nonapproved agents for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:137-48.
27. Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev 2005;2:CD001009.-
28. Nicoderm CQ [package insert]. Bridgewater, NJ: Sanofi Aventis; 2006.
29. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
30. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006;25(5):549-57.
31. Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6.
1. Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol 1990;9(6):701-16.
2. Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings 2006. Bethesda, MD: National Institute on Drug Abuse; 2007. NIH publication no. 07-6202.
3. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescence. Tob Control 2000;9(3):313-9.
4. Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry 2002;41(11):1294-1305.
5. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):222-830.
7. Upadhyaya HP, Brady KT, Wharton M, Liao J. Psychiatric disorders and cigarette smoking among child and adolescent psychiatry inpatients. Am J Addict 2003;12(2):144-52.
8. Prokhorov AV, Pallonen UE, Fava JL, et al. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996;21(1):117-27.
9. Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 1998;152(2):151-6.
10. O’Loughlin J, DiFranza J, Tarasuk J, et al. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control 2002;11(4):354-60.
11. Nonnemaker J, Mowery P, Hersey J, et al. Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res 2004;6(2):295-301.
12. Etter JF, LeHouezec J, Perneger TV. A self-administered questionnaire to measure addiction to cigarettes: the Cigarette Dependence Scale. Neuropsychopharmacology 2003;28(2):359-70.
13. Hughes JR, Oliveto AH, Riggs R, et al. Concordance of different measures of nicotine dependence: two pilot studies. Addict Behav 2004;29(8):1527-39.
14. Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, eds. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: The Humana Press Inc.; 1992.
15. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4(2):149-59.
16. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Quick reference guide for clinicians. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
17. Smith TA, House RF, Croghan IT, et al. Nicotine patch therapy in adolescent smokers. Pediatrics 1996;98(4):659-67.
18. Hurt RD, Croghan GA, Beede SD, et al. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Arch Pediatr Adolesc Med 2000;154(1):31-7.
19. Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tob Res 2003;5(4):515-26.
20. Moolchan ET, Robinson ML, Ernst M, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407-14.
21. Upadhyaya HP, Brady KT, Wang W. Bupropion SR in adolescents with comorbid ADHD and nicotine dependence: a pilot study. J Am Acad Child Adolesc Psychiatry 2004;43(2):199-205.
22. Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol 2004;72(4):729-35.
23. Muramoto ML, Leischow SJ, Sherrill D. A randomized trial of the efficacy of bupropion for adolescent smoking cessation. Paper presented at: Annual Meeting of the Society for Research on Nicotine and Tobacco; March 20-23, 2005; Prague, Czech Republic.
24. Hall SM. Tricyclic antidepressants in the treatment of nicotine dependence. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:95-107.
25. Berlin I. Monoamine oxidase inhibitors for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:109-21.
26. Weinberger AH, Reutenauer EL, George TP. Other nonapproved agents for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:137-48.
27. Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev 2005;2:CD001009.-
28. Nicoderm CQ [package insert]. Bridgewater, NJ: Sanofi Aventis; 2006.
29. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
30. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006;25(5):549-57.
31. Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6.