User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Getting to the heart of his ‘shocking’ trauma
CASE: ‘Like a sledgehammer’
Mr. J, age 54, is admitted to the cardiac critical care unit after repeated tachycardia episodes over 3 years. He also has depressive symptoms including social isolation, passive suicidal thoughts, lack of interest in sex, weight loss, difficulty sleeping, sadness, and decreased appetite, energy, and ability to concentrate. The psychiatry consult team subsequently evaluates him.
Shortly after retiring as a police officer, Mr. J started having 10-second episodes of loss of consciousness and suffered 30 episodes within 1 year. After diagnosing chronic idiopathic ventricular tachycardia, a cardiologist ablated an aberrant left ventricular pathway and inserted a single-lead implantable cardioverter-defibrillator (ICD). He also prescribed the antiarrhythmic amiodarone, but Mr. J could not tolerate the medication’s side effects.
Mr. J’s tachycardia persisted, and repeated episodes triggered an estimated 13 electrical shocks from the ICD over 5 months. At this point, the cardiologist performed a second ablation, removed the single-lead ICD, and implanted a two-lead ICD, which he hoped would more accurately discern between lethal and nonlethal fast heart rhythms.
In addition, the cardiologist prescribed the antiarrhythmic sotalol—which did not suppress the arrhythmia—before switching to flecainide, 100 mg bid, which did. However, Mr. J still suffered fatigue, exercise intolerance, near-syncope, and chest heaviness.
One week after receiving the first ICD, Mr. J recalls, he felt his first shock while out for a walk. He said the shock lasted 5 to 10 seconds and “felt like somebody took a sledgehammer to my chest.” Another time, he suffered 6 successive shocks that threw him to the ground. Motorists pulled over to assist him, which made him feel ashamed.
Before long, Mr. J became increasingly afraid of repeat discharges. As soon as he began a task, he would feel a “thumping” in the back of his neck and start panicking, fearful that a heart rate increase would trigger another shock.
The stress forced Mr. J to abandon his favorite retirement hobbies—remodeling houses and yard work—and to spend his days lying around watching television. Fearing another discharge in public, he has stopped seeing friends and going to church. He has also stopped driving and depends on his female partner of 14 years for daily visits, grocery shopping, and rides to medical appointments. She feels frustrated by his debility.
The authors’ observations
By delivering electrical shocks when ventricles beat too quickly, an ICD shocks the heart back into a normal rhythm. Based on our observation, Mr. J probably had both anxiety-induced tachycardia and recurrent atrial fibrillation.
Although ICDs have prolonged survival for patients with potentially fatal ventricular arrhythmias,1,2 painful discharges can occur without warning. Patients liken the discharge to an electric shock or to being kicked or punched in the chest.3
Depending on the patient’s activity level, cardiologists routinely program ICDs to discharge at approximately 10 beats per minute above expected heart rates during typical activities. Because ICD leads cannot differentiate between ventricular and supraventricular rhythm disturbances, a rapid supraventricular rhythm might precipitate a discharge intended to treat a more serious ventricular rhythm disturbance.
Frequent ICD discharges could indicate:
- the patient needs a more effective antiarrhythmic
- the device needs to be set at a higher rate to avoid discharge during periods of anxiety/exertion
- or the device is defective.
ICD-induced psychopathology
Depression or tachycardia could have caused Mr. J’s fatigue. Either way, he showed numerous other depressive symptoms.
Fear of implant discharge or malfunction often induces psychiatric disorders, particularly in patients who have experienced discharge. As many as 87% of ICD patients suffer anxiety, depression, or other psychiatric symptoms after implantation,5 and 13% to 38% meet DSM-IV-TR criteria for an anxiety spectrum disorder.6
Multiple psychological theories explain iatrogenic anxiety disorders resulting from ICD firing. Behaviorally, ICD discharge represents an initially unconditioned stimulus that the patient associates with the activity he was engaging in when shocked. The shock discourages the patient from that activity—however benign—for fear it triggered the discharge and could cause future shocks.
ICD recipients often fear the device will malfunction or discharge while they are in public, driving, or operating machinery—leading some to become homebound and cease activities of daily living. The discharge’s unpredictability shatters a patient’s perception of control over his or her life and might induce a learned helplessness7 that can strain relationships, as it did with Mr. J and his partner. The patient also could develop anticipatory anxiety, mistaking benign body symptoms or increasing shock frequency for signs of a potentially fatal heart problem.8
Whether quality of life diminishes as ICD firings become more frequent is uncertain.9 The Canadian Implantable Defibrillator Study (N=317) found greater quality of life improvements with ICD therapy than with amiodarone—200 to 400 mg/d maintenance therapy—but the improvements were lost in patients who experienced ≥5 shocks over 12 months.10 Pauli et al7 found misinterpretation of the reason for increasing shocks to be more emotionally destructive than shock frequency, however.
Detecting ICD maladjustment
Patients with ICD maladjustment typically show anticipatory anxiety and negative cognitive attributions, and many engage in fruitless maneuvers to prevent device firing.5 Nervousness, dizziness, weakness, and fear are common responses to shock by ICD.11
Most patients with new-onset, post-ICD anxiety disorders have no pre-implant psychiatric history.12 Only one trial assessing state and trait anxiety before and after ICD placement reported increased trait anxiety in some patients before implantation.13
HISTORY: Nights in the cornfield
During psychiatric evaluation, Mr. J reveals that his parents physically and emotionally abused him as a child. He says his father frequently beat him with farm tools, and sometimes the beatings were so severe that his parents kept him home from school to prevent teachers from noticing his bruises. He never received medical treatment for his injuries.
For Mr. J, the inescapable threat of painful, unannounced ICD discharges has brought back the anticipatory terror and helplessness of his childhood. Just as he feared his father’s sudden rages, the specter of repeat ICD shocks now haunts him. He says he’d rather have the ICD removed and risk death from tachycardia than live another minute in fear.
The authors’ observations
Mr. J meets DSM-IV-TR criteria for PTSD. He associates ICD discharge with childhood abuse and experiences new-onset flashbacks, hyperarousal, and avoidance behavior.
To our knowledge, ICD shock-induced flashbacks to pre-implant trauma have not been reported, although some data associate ICDs with posttraumatic stress related to heart disease and treatment.14-16 In one case series,14 patients showed:
- cluster B re-experiencing symptoms (cognitive preoccupation with trauma or psychophysiologic reactivity to reminders of the ICD and heart disease)
- cluster C avoidance symptoms (avoiding activities they thought might activate the ICD)
- cluster D hyperarousal symptoms (insomnia, decreased concentration, hypervigilance, and irritability).
The authors’ observations
Treating comorbid anxiety or depression in ICD recipients is critical. A number of psychiatric interventions might alleviate behavioral and psychological effects of body-device interactions.
CBT. In a retrospective study17 of 36 ICD recipients, those who received 9 months of CBT reported decreased depression, anxiety, distress, and sexual problems compared with those who did not. Interestingly, more CBT-group patients (11 of 18) suffered ICD shocks than did controls (6 of 18).
Peer support groups. Out of 58 ICD recipients who answered a post-implant questionnaire, 23 (39%) attended a peer support group.18 Of these, 22 (96%) found the group helpful and were happier, less hostile, and more sociable after participating. Peer group participants also were more likely to return to work than nonparticipants.
How would you handle a patient’s request to deactivate an implantable cardioverter-defibrillator (ICD) or other life-preserving device that is causing debilitating mental anguish? Physicians dealing with such requests will find themselves in an ethical wilderness.
Pinski22 offers guidelines in line with withdrawal of other life-extending technologies in terminally ill patients. “Deactivation of an ICD is appropriate when the device is believed to be prolonging patient suffering,” he writes, adding that preventing ICD shocks induced by frequent or agonal arrhythmias “will not only hasten but also permit a peaceful death.” Disabling the ICD function that responds to bradycardia will prevent agonal pacing and—as a result—shocks.
The literature, however, offers little guidance on responding to patient requests for ICD deactivation and few precedents on which to base such decisions for the terminally ill.
Even less guidance exists when mental illness resulting from ICD complications induces unbearable suffering. The underlying psychiatric condition should be optimally treated before clinicians entertain ICD removal. Mr. J, for example, decided to keep the implant once his crippling anxiety resolved and he was assured that his tachycardia finally was under control.
12 attributes reduction of ICD-induced anxiety to combination individual psychotherapy and unspecified dosages of benzodiazepines. Two patients also received adjunctive fluoxetine or paroxetine, dosages unspecified.
In a double-blind, placebo-controlled crossover study, implantable atrial defibrillator recipients reported decreased pain and anxiety while taking the short-acting benzodiazepine triazolam, 0.375 mg, before patient-activated shock.19
We recommend trying a combination regimen that acts acutely and subacutely. A long-acting benzodiazepine such as clonazepam can calm acute, overwhelming anxiety, and a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine or paroxetine can help manage chronic depressive and generalized anxiety symptoms.
SSRIs are relatively benign but more research on their cardiac safety is needed.20,21 Tricyclic antidepressants, which prolong cardiac conduction, should be avoided.
In addition to psychotropics, concomitant psychotherapy can reduce chronic symptoms.
The authors’ observations
Preparing patients for ICD problems. Anxiety after an ICD shock and the dread of repeat shocks are normal; the goal is to prevent that anxiety from destroying quality of life.
As with Mr. J, many ICD recipients are emotionally unprepared for device-related complications. Most cardiologists do not screen patients for pre-existing anxiety before ICD placement, nor do many adequately address ICD-induced anxiety once the device has been placed.
Psychological screening before implantation can help detect and manage preexisting anxiety disorders. Small-scale evaluations have used anxiety scales to continuously measure anxiety before and after ICD placement.13,23
Increased patient education on how ICDs work can help patients decide whether to proceed with implantation and tolerate discharges should they occur. Psychological screening and brief, routine communication between providers and patients about psychosocial issues can help patients adjust and identify those who need extended psychological services.4
- develop a plan for how a shock would be handled
- perform relaxation exercises immediately after the shock
- resume activities they were involved with when the shock occurred to prevent avoidance.24
TREATMENT: Third attempt
The cardiology team discontinues flecainide and performs a third radioablation, which eradicates ectopic ventricular activity.
Acting on the psychiatry consult team’s advice, Mr. J is transferred to the inpatient mood disorders unit to aggressively treat his PTSD. He undergoes 4 days of intensive CBT designed to explore the connection between his response to the discharges and his father’s abuse. We prescribe clonazepam, 0.5 mg bid, to reduce Mr. J’s agitation and anxiety, and recommend outpatient counseling to help manage his stress—particularly his anxious response to stimuli that remind him of the ICD discharge.
Mr. J is discharged after 12 days in the cardiac and psychiatric units. He has no suicidal thoughts, his sadness has decreased, and his energy, concentration, sleep, and outlook on his future have improved. He also is resolving relationship issues with his partner.
As Mr. J’s anxiety declines and he is increasingly reassured that his arrhythmias are under control, he decides to keep the ICD. His function gradually improves with continued cardiac rehabilitation, although he does not continue psychotherapy.
Related resources
- Stutts LA, Cross NJ, Conti JB, Sears SF. Examination of research trends on patient factors in patients with implantable cardioverter defibrillators. Clin Cardiol 2007;30:64-8.
- Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111;380-2. http://circ.ahajournals.org/cgi/reprint/111/23/e380.
- Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76. www.psychosomaticmedicine.org/cgi/reprint/61/1/69.
- Amiodarone • Cordarone
- Clonazepam • Klonopin
- Flecainide • Tambocor
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sotalol • Betapace
- Triazolam • Halcion, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morris PL, Badger J, Chmielewski C, et al. Psychiatric morbidity following implantation of the automatic implantable cardioverter defibrillator. Psychosomatics 1991;32:58-64.
2. Conti JB, Sears SF, Jr. Understanding and managing the psychological impact of the ICD. Card Electrophysiol Rev 2001;5:128-32.
3. Pelletier D, Gallagher R, Mitten-Lewis S, et al. Australian implantable cardiac defibrillator recipients: quality-of-life issues. Int J Nursing Pract 2002;8:68-74.
4. Eads AS, Sears SF, Jr, Sotile WM, Conti JB. Supportive communication with implantable cardioverter defibrillator patients: seven principles to facilitate psychosocial adjustment. J Cardiopulm Rehab 2000;20:109-14.
5. Sola CL, Bostwick JM. Implantable cardioverter-defibrillators, induced anxiety, and quality of life. Mayo Clin Proc 2005;80:232-7.
6. Sears SF, Jr, Todaro JF, Lewis TS, et al. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clin Cardiol 1999;22:481-9.
7. Goodman M, Hess B. Could implantable cardioverter defibrillators provide a human model supporting the learned helplessness theory of depression? Gen Hosp Psychiatry 1999;21:382-5.
8. Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
9. Godemann F, Ahrens B, Behrens S, et al. Classic conditioning and dysfunctional cognitions in patients with panic disorder and agoraphobia treated with an implantable cardioverter/defibrillator. Psychosom Med 2001;63:231-8.
10. Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282-9.
11. Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung 1993;22:494-501.
12. Bourke JP, Turkington D, Thomas G, et al. Florid psychopathology in patients receiving shocks from implanted cardioverter-defibrillators. Heart 1997;78:581-3.
13. Vlay SC, Olson LC, Fricchione GL, Friedman R. Anxiety and anger in patients with ventricular tachyarrhythmias: responses after automatic internal cardioverter defibrillator implantation. Pacing Clin Electrophysiol 1989;12:366-73.
14. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1998;40:82-5.
15. Friccione GL, Vlay LC, Vlay SC. Cardiac psychiatry and the management of malignant ventricular arrhythmias with the internal cardioverter-defibrillator. Am Heart J 1994;128:1050-9.
16. Friccione GL, Vlay SC. Psychiatric aspects of the implantable cardioverter-defibrillator. In: Estes NAM, Menolis AS, Want PG, eds. Implantable cardioverter-defibrillators. A comprehensive textbook. New York: Marcel Dekker; 1994:405-23.
17. Kohn CS, Petrucci RJ, Baesser C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4 pt 1):450-6.
18. Heller SS, Ormont MA, Lidagoster L, et al. Psychosocial outcome after ICD implantation: a current perspective. Pacing Clin Electrophysiol 1998;21:1207-15.
19. Fabian TJ, Schwartzman DS, Ujhelyi MR, et al. Decreasing pain and anxiety associated with patient-activated atrial shock: a placebo-controlled study of adjunctive sedation with oral triazolam. J Cardivasc Electrophysiol 2006;17:391-5.
20. Sala M, Coppa F, Cappucciati C, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs 2006;7:256-63.
21. Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry 2006;51:923-9.
22. Pinski SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med 2000;28(10 suppl):N174-N180.
23. Kuhl EA, Dixit NK, Walker RL, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-18.
24. Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111:380-2.
CASE: ‘Like a sledgehammer’
Mr. J, age 54, is admitted to the cardiac critical care unit after repeated tachycardia episodes over 3 years. He also has depressive symptoms including social isolation, passive suicidal thoughts, lack of interest in sex, weight loss, difficulty sleeping, sadness, and decreased appetite, energy, and ability to concentrate. The psychiatry consult team subsequently evaluates him.
Shortly after retiring as a police officer, Mr. J started having 10-second episodes of loss of consciousness and suffered 30 episodes within 1 year. After diagnosing chronic idiopathic ventricular tachycardia, a cardiologist ablated an aberrant left ventricular pathway and inserted a single-lead implantable cardioverter-defibrillator (ICD). He also prescribed the antiarrhythmic amiodarone, but Mr. J could not tolerate the medication’s side effects.
Mr. J’s tachycardia persisted, and repeated episodes triggered an estimated 13 electrical shocks from the ICD over 5 months. At this point, the cardiologist performed a second ablation, removed the single-lead ICD, and implanted a two-lead ICD, which he hoped would more accurately discern between lethal and nonlethal fast heart rhythms.
In addition, the cardiologist prescribed the antiarrhythmic sotalol—which did not suppress the arrhythmia—before switching to flecainide, 100 mg bid, which did. However, Mr. J still suffered fatigue, exercise intolerance, near-syncope, and chest heaviness.
One week after receiving the first ICD, Mr. J recalls, he felt his first shock while out for a walk. He said the shock lasted 5 to 10 seconds and “felt like somebody took a sledgehammer to my chest.” Another time, he suffered 6 successive shocks that threw him to the ground. Motorists pulled over to assist him, which made him feel ashamed.
Before long, Mr. J became increasingly afraid of repeat discharges. As soon as he began a task, he would feel a “thumping” in the back of his neck and start panicking, fearful that a heart rate increase would trigger another shock.
The stress forced Mr. J to abandon his favorite retirement hobbies—remodeling houses and yard work—and to spend his days lying around watching television. Fearing another discharge in public, he has stopped seeing friends and going to church. He has also stopped driving and depends on his female partner of 14 years for daily visits, grocery shopping, and rides to medical appointments. She feels frustrated by his debility.
The authors’ observations
By delivering electrical shocks when ventricles beat too quickly, an ICD shocks the heart back into a normal rhythm. Based on our observation, Mr. J probably had both anxiety-induced tachycardia and recurrent atrial fibrillation.
Although ICDs have prolonged survival for patients with potentially fatal ventricular arrhythmias,1,2 painful discharges can occur without warning. Patients liken the discharge to an electric shock or to being kicked or punched in the chest.3
Depending on the patient’s activity level, cardiologists routinely program ICDs to discharge at approximately 10 beats per minute above expected heart rates during typical activities. Because ICD leads cannot differentiate between ventricular and supraventricular rhythm disturbances, a rapid supraventricular rhythm might precipitate a discharge intended to treat a more serious ventricular rhythm disturbance.
Frequent ICD discharges could indicate:
- the patient needs a more effective antiarrhythmic
- the device needs to be set at a higher rate to avoid discharge during periods of anxiety/exertion
- or the device is defective.
ICD-induced psychopathology
Depression or tachycardia could have caused Mr. J’s fatigue. Either way, he showed numerous other depressive symptoms.
Fear of implant discharge or malfunction often induces psychiatric disorders, particularly in patients who have experienced discharge. As many as 87% of ICD patients suffer anxiety, depression, or other psychiatric symptoms after implantation,5 and 13% to 38% meet DSM-IV-TR criteria for an anxiety spectrum disorder.6
Multiple psychological theories explain iatrogenic anxiety disorders resulting from ICD firing. Behaviorally, ICD discharge represents an initially unconditioned stimulus that the patient associates with the activity he was engaging in when shocked. The shock discourages the patient from that activity—however benign—for fear it triggered the discharge and could cause future shocks.
ICD recipients often fear the device will malfunction or discharge while they are in public, driving, or operating machinery—leading some to become homebound and cease activities of daily living. The discharge’s unpredictability shatters a patient’s perception of control over his or her life and might induce a learned helplessness7 that can strain relationships, as it did with Mr. J and his partner. The patient also could develop anticipatory anxiety, mistaking benign body symptoms or increasing shock frequency for signs of a potentially fatal heart problem.8
Whether quality of life diminishes as ICD firings become more frequent is uncertain.9 The Canadian Implantable Defibrillator Study (N=317) found greater quality of life improvements with ICD therapy than with amiodarone—200 to 400 mg/d maintenance therapy—but the improvements were lost in patients who experienced ≥5 shocks over 12 months.10 Pauli et al7 found misinterpretation of the reason for increasing shocks to be more emotionally destructive than shock frequency, however.
Detecting ICD maladjustment
Patients with ICD maladjustment typically show anticipatory anxiety and negative cognitive attributions, and many engage in fruitless maneuvers to prevent device firing.5 Nervousness, dizziness, weakness, and fear are common responses to shock by ICD.11
Most patients with new-onset, post-ICD anxiety disorders have no pre-implant psychiatric history.12 Only one trial assessing state and trait anxiety before and after ICD placement reported increased trait anxiety in some patients before implantation.13
HISTORY: Nights in the cornfield
During psychiatric evaluation, Mr. J reveals that his parents physically and emotionally abused him as a child. He says his father frequently beat him with farm tools, and sometimes the beatings were so severe that his parents kept him home from school to prevent teachers from noticing his bruises. He never received medical treatment for his injuries.
For Mr. J, the inescapable threat of painful, unannounced ICD discharges has brought back the anticipatory terror and helplessness of his childhood. Just as he feared his father’s sudden rages, the specter of repeat ICD shocks now haunts him. He says he’d rather have the ICD removed and risk death from tachycardia than live another minute in fear.
The authors’ observations
Mr. J meets DSM-IV-TR criteria for PTSD. He associates ICD discharge with childhood abuse and experiences new-onset flashbacks, hyperarousal, and avoidance behavior.
To our knowledge, ICD shock-induced flashbacks to pre-implant trauma have not been reported, although some data associate ICDs with posttraumatic stress related to heart disease and treatment.14-16 In one case series,14 patients showed:
- cluster B re-experiencing symptoms (cognitive preoccupation with trauma or psychophysiologic reactivity to reminders of the ICD and heart disease)
- cluster C avoidance symptoms (avoiding activities they thought might activate the ICD)
- cluster D hyperarousal symptoms (insomnia, decreased concentration, hypervigilance, and irritability).
The authors’ observations
Treating comorbid anxiety or depression in ICD recipients is critical. A number of psychiatric interventions might alleviate behavioral and psychological effects of body-device interactions.
CBT. In a retrospective study17 of 36 ICD recipients, those who received 9 months of CBT reported decreased depression, anxiety, distress, and sexual problems compared with those who did not. Interestingly, more CBT-group patients (11 of 18) suffered ICD shocks than did controls (6 of 18).
Peer support groups. Out of 58 ICD recipients who answered a post-implant questionnaire, 23 (39%) attended a peer support group.18 Of these, 22 (96%) found the group helpful and were happier, less hostile, and more sociable after participating. Peer group participants also were more likely to return to work than nonparticipants.
How would you handle a patient’s request to deactivate an implantable cardioverter-defibrillator (ICD) or other life-preserving device that is causing debilitating mental anguish? Physicians dealing with such requests will find themselves in an ethical wilderness.
Pinski22 offers guidelines in line with withdrawal of other life-extending technologies in terminally ill patients. “Deactivation of an ICD is appropriate when the device is believed to be prolonging patient suffering,” he writes, adding that preventing ICD shocks induced by frequent or agonal arrhythmias “will not only hasten but also permit a peaceful death.” Disabling the ICD function that responds to bradycardia will prevent agonal pacing and—as a result—shocks.
The literature, however, offers little guidance on responding to patient requests for ICD deactivation and few precedents on which to base such decisions for the terminally ill.
Even less guidance exists when mental illness resulting from ICD complications induces unbearable suffering. The underlying psychiatric condition should be optimally treated before clinicians entertain ICD removal. Mr. J, for example, decided to keep the implant once his crippling anxiety resolved and he was assured that his tachycardia finally was under control.
12 attributes reduction of ICD-induced anxiety to combination individual psychotherapy and unspecified dosages of benzodiazepines. Two patients also received adjunctive fluoxetine or paroxetine, dosages unspecified.
In a double-blind, placebo-controlled crossover study, implantable atrial defibrillator recipients reported decreased pain and anxiety while taking the short-acting benzodiazepine triazolam, 0.375 mg, before patient-activated shock.19
We recommend trying a combination regimen that acts acutely and subacutely. A long-acting benzodiazepine such as clonazepam can calm acute, overwhelming anxiety, and a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine or paroxetine can help manage chronic depressive and generalized anxiety symptoms.
SSRIs are relatively benign but more research on their cardiac safety is needed.20,21 Tricyclic antidepressants, which prolong cardiac conduction, should be avoided.
In addition to psychotropics, concomitant psychotherapy can reduce chronic symptoms.
The authors’ observations
Preparing patients for ICD problems. Anxiety after an ICD shock and the dread of repeat shocks are normal; the goal is to prevent that anxiety from destroying quality of life.
As with Mr. J, many ICD recipients are emotionally unprepared for device-related complications. Most cardiologists do not screen patients for pre-existing anxiety before ICD placement, nor do many adequately address ICD-induced anxiety once the device has been placed.
Psychological screening before implantation can help detect and manage preexisting anxiety disorders. Small-scale evaluations have used anxiety scales to continuously measure anxiety before and after ICD placement.13,23
Increased patient education on how ICDs work can help patients decide whether to proceed with implantation and tolerate discharges should they occur. Psychological screening and brief, routine communication between providers and patients about psychosocial issues can help patients adjust and identify those who need extended psychological services.4
- develop a plan for how a shock would be handled
- perform relaxation exercises immediately after the shock
- resume activities they were involved with when the shock occurred to prevent avoidance.24
TREATMENT: Third attempt
The cardiology team discontinues flecainide and performs a third radioablation, which eradicates ectopic ventricular activity.
Acting on the psychiatry consult team’s advice, Mr. J is transferred to the inpatient mood disorders unit to aggressively treat his PTSD. He undergoes 4 days of intensive CBT designed to explore the connection between his response to the discharges and his father’s abuse. We prescribe clonazepam, 0.5 mg bid, to reduce Mr. J’s agitation and anxiety, and recommend outpatient counseling to help manage his stress—particularly his anxious response to stimuli that remind him of the ICD discharge.
Mr. J is discharged after 12 days in the cardiac and psychiatric units. He has no suicidal thoughts, his sadness has decreased, and his energy, concentration, sleep, and outlook on his future have improved. He also is resolving relationship issues with his partner.
As Mr. J’s anxiety declines and he is increasingly reassured that his arrhythmias are under control, he decides to keep the ICD. His function gradually improves with continued cardiac rehabilitation, although he does not continue psychotherapy.
Related resources
- Stutts LA, Cross NJ, Conti JB, Sears SF. Examination of research trends on patient factors in patients with implantable cardioverter defibrillators. Clin Cardiol 2007;30:64-8.
- Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111;380-2. http://circ.ahajournals.org/cgi/reprint/111/23/e380.
- Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76. www.psychosomaticmedicine.org/cgi/reprint/61/1/69.
- Amiodarone • Cordarone
- Clonazepam • Klonopin
- Flecainide • Tambocor
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sotalol • Betapace
- Triazolam • Halcion, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: ‘Like a sledgehammer’
Mr. J, age 54, is admitted to the cardiac critical care unit after repeated tachycardia episodes over 3 years. He also has depressive symptoms including social isolation, passive suicidal thoughts, lack of interest in sex, weight loss, difficulty sleeping, sadness, and decreased appetite, energy, and ability to concentrate. The psychiatry consult team subsequently evaluates him.
Shortly after retiring as a police officer, Mr. J started having 10-second episodes of loss of consciousness and suffered 30 episodes within 1 year. After diagnosing chronic idiopathic ventricular tachycardia, a cardiologist ablated an aberrant left ventricular pathway and inserted a single-lead implantable cardioverter-defibrillator (ICD). He also prescribed the antiarrhythmic amiodarone, but Mr. J could not tolerate the medication’s side effects.
Mr. J’s tachycardia persisted, and repeated episodes triggered an estimated 13 electrical shocks from the ICD over 5 months. At this point, the cardiologist performed a second ablation, removed the single-lead ICD, and implanted a two-lead ICD, which he hoped would more accurately discern between lethal and nonlethal fast heart rhythms.
In addition, the cardiologist prescribed the antiarrhythmic sotalol—which did not suppress the arrhythmia—before switching to flecainide, 100 mg bid, which did. However, Mr. J still suffered fatigue, exercise intolerance, near-syncope, and chest heaviness.
One week after receiving the first ICD, Mr. J recalls, he felt his first shock while out for a walk. He said the shock lasted 5 to 10 seconds and “felt like somebody took a sledgehammer to my chest.” Another time, he suffered 6 successive shocks that threw him to the ground. Motorists pulled over to assist him, which made him feel ashamed.
Before long, Mr. J became increasingly afraid of repeat discharges. As soon as he began a task, he would feel a “thumping” in the back of his neck and start panicking, fearful that a heart rate increase would trigger another shock.
The stress forced Mr. J to abandon his favorite retirement hobbies—remodeling houses and yard work—and to spend his days lying around watching television. Fearing another discharge in public, he has stopped seeing friends and going to church. He has also stopped driving and depends on his female partner of 14 years for daily visits, grocery shopping, and rides to medical appointments. She feels frustrated by his debility.
The authors’ observations
By delivering electrical shocks when ventricles beat too quickly, an ICD shocks the heart back into a normal rhythm. Based on our observation, Mr. J probably had both anxiety-induced tachycardia and recurrent atrial fibrillation.
Although ICDs have prolonged survival for patients with potentially fatal ventricular arrhythmias,1,2 painful discharges can occur without warning. Patients liken the discharge to an electric shock or to being kicked or punched in the chest.3
Depending on the patient’s activity level, cardiologists routinely program ICDs to discharge at approximately 10 beats per minute above expected heart rates during typical activities. Because ICD leads cannot differentiate between ventricular and supraventricular rhythm disturbances, a rapid supraventricular rhythm might precipitate a discharge intended to treat a more serious ventricular rhythm disturbance.
Frequent ICD discharges could indicate:
- the patient needs a more effective antiarrhythmic
- the device needs to be set at a higher rate to avoid discharge during periods of anxiety/exertion
- or the device is defective.
ICD-induced psychopathology
Depression or tachycardia could have caused Mr. J’s fatigue. Either way, he showed numerous other depressive symptoms.
Fear of implant discharge or malfunction often induces psychiatric disorders, particularly in patients who have experienced discharge. As many as 87% of ICD patients suffer anxiety, depression, or other psychiatric symptoms after implantation,5 and 13% to 38% meet DSM-IV-TR criteria for an anxiety spectrum disorder.6
Multiple psychological theories explain iatrogenic anxiety disorders resulting from ICD firing. Behaviorally, ICD discharge represents an initially unconditioned stimulus that the patient associates with the activity he was engaging in when shocked. The shock discourages the patient from that activity—however benign—for fear it triggered the discharge and could cause future shocks.
ICD recipients often fear the device will malfunction or discharge while they are in public, driving, or operating machinery—leading some to become homebound and cease activities of daily living. The discharge’s unpredictability shatters a patient’s perception of control over his or her life and might induce a learned helplessness7 that can strain relationships, as it did with Mr. J and his partner. The patient also could develop anticipatory anxiety, mistaking benign body symptoms or increasing shock frequency for signs of a potentially fatal heart problem.8
Whether quality of life diminishes as ICD firings become more frequent is uncertain.9 The Canadian Implantable Defibrillator Study (N=317) found greater quality of life improvements with ICD therapy than with amiodarone—200 to 400 mg/d maintenance therapy—but the improvements were lost in patients who experienced ≥5 shocks over 12 months.10 Pauli et al7 found misinterpretation of the reason for increasing shocks to be more emotionally destructive than shock frequency, however.
Detecting ICD maladjustment
Patients with ICD maladjustment typically show anticipatory anxiety and negative cognitive attributions, and many engage in fruitless maneuvers to prevent device firing.5 Nervousness, dizziness, weakness, and fear are common responses to shock by ICD.11
Most patients with new-onset, post-ICD anxiety disorders have no pre-implant psychiatric history.12 Only one trial assessing state and trait anxiety before and after ICD placement reported increased trait anxiety in some patients before implantation.13
HISTORY: Nights in the cornfield
During psychiatric evaluation, Mr. J reveals that his parents physically and emotionally abused him as a child. He says his father frequently beat him with farm tools, and sometimes the beatings were so severe that his parents kept him home from school to prevent teachers from noticing his bruises. He never received medical treatment for his injuries.
For Mr. J, the inescapable threat of painful, unannounced ICD discharges has brought back the anticipatory terror and helplessness of his childhood. Just as he feared his father’s sudden rages, the specter of repeat ICD shocks now haunts him. He says he’d rather have the ICD removed and risk death from tachycardia than live another minute in fear.
The authors’ observations
Mr. J meets DSM-IV-TR criteria for PTSD. He associates ICD discharge with childhood abuse and experiences new-onset flashbacks, hyperarousal, and avoidance behavior.
To our knowledge, ICD shock-induced flashbacks to pre-implant trauma have not been reported, although some data associate ICDs with posttraumatic stress related to heart disease and treatment.14-16 In one case series,14 patients showed:
- cluster B re-experiencing symptoms (cognitive preoccupation with trauma or psychophysiologic reactivity to reminders of the ICD and heart disease)
- cluster C avoidance symptoms (avoiding activities they thought might activate the ICD)
- cluster D hyperarousal symptoms (insomnia, decreased concentration, hypervigilance, and irritability).
The authors’ observations
Treating comorbid anxiety or depression in ICD recipients is critical. A number of psychiatric interventions might alleviate behavioral and psychological effects of body-device interactions.
CBT. In a retrospective study17 of 36 ICD recipients, those who received 9 months of CBT reported decreased depression, anxiety, distress, and sexual problems compared with those who did not. Interestingly, more CBT-group patients (11 of 18) suffered ICD shocks than did controls (6 of 18).
Peer support groups. Out of 58 ICD recipients who answered a post-implant questionnaire, 23 (39%) attended a peer support group.18 Of these, 22 (96%) found the group helpful and were happier, less hostile, and more sociable after participating. Peer group participants also were more likely to return to work than nonparticipants.
How would you handle a patient’s request to deactivate an implantable cardioverter-defibrillator (ICD) or other life-preserving device that is causing debilitating mental anguish? Physicians dealing with such requests will find themselves in an ethical wilderness.
Pinski22 offers guidelines in line with withdrawal of other life-extending technologies in terminally ill patients. “Deactivation of an ICD is appropriate when the device is believed to be prolonging patient suffering,” he writes, adding that preventing ICD shocks induced by frequent or agonal arrhythmias “will not only hasten but also permit a peaceful death.” Disabling the ICD function that responds to bradycardia will prevent agonal pacing and—as a result—shocks.
The literature, however, offers little guidance on responding to patient requests for ICD deactivation and few precedents on which to base such decisions for the terminally ill.
Even less guidance exists when mental illness resulting from ICD complications induces unbearable suffering. The underlying psychiatric condition should be optimally treated before clinicians entertain ICD removal. Mr. J, for example, decided to keep the implant once his crippling anxiety resolved and he was assured that his tachycardia finally was under control.
12 attributes reduction of ICD-induced anxiety to combination individual psychotherapy and unspecified dosages of benzodiazepines. Two patients also received adjunctive fluoxetine or paroxetine, dosages unspecified.
In a double-blind, placebo-controlled crossover study, implantable atrial defibrillator recipients reported decreased pain and anxiety while taking the short-acting benzodiazepine triazolam, 0.375 mg, before patient-activated shock.19
We recommend trying a combination regimen that acts acutely and subacutely. A long-acting benzodiazepine such as clonazepam can calm acute, overwhelming anxiety, and a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine or paroxetine can help manage chronic depressive and generalized anxiety symptoms.
SSRIs are relatively benign but more research on their cardiac safety is needed.20,21 Tricyclic antidepressants, which prolong cardiac conduction, should be avoided.
In addition to psychotropics, concomitant psychotherapy can reduce chronic symptoms.
The authors’ observations
Preparing patients for ICD problems. Anxiety after an ICD shock and the dread of repeat shocks are normal; the goal is to prevent that anxiety from destroying quality of life.
As with Mr. J, many ICD recipients are emotionally unprepared for device-related complications. Most cardiologists do not screen patients for pre-existing anxiety before ICD placement, nor do many adequately address ICD-induced anxiety once the device has been placed.
Psychological screening before implantation can help detect and manage preexisting anxiety disorders. Small-scale evaluations have used anxiety scales to continuously measure anxiety before and after ICD placement.13,23
Increased patient education on how ICDs work can help patients decide whether to proceed with implantation and tolerate discharges should they occur. Psychological screening and brief, routine communication between providers and patients about psychosocial issues can help patients adjust and identify those who need extended psychological services.4
- develop a plan for how a shock would be handled
- perform relaxation exercises immediately after the shock
- resume activities they were involved with when the shock occurred to prevent avoidance.24
TREATMENT: Third attempt
The cardiology team discontinues flecainide and performs a third radioablation, which eradicates ectopic ventricular activity.
Acting on the psychiatry consult team’s advice, Mr. J is transferred to the inpatient mood disorders unit to aggressively treat his PTSD. He undergoes 4 days of intensive CBT designed to explore the connection between his response to the discharges and his father’s abuse. We prescribe clonazepam, 0.5 mg bid, to reduce Mr. J’s agitation and anxiety, and recommend outpatient counseling to help manage his stress—particularly his anxious response to stimuli that remind him of the ICD discharge.
Mr. J is discharged after 12 days in the cardiac and psychiatric units. He has no suicidal thoughts, his sadness has decreased, and his energy, concentration, sleep, and outlook on his future have improved. He also is resolving relationship issues with his partner.
As Mr. J’s anxiety declines and he is increasingly reassured that his arrhythmias are under control, he decides to keep the ICD. His function gradually improves with continued cardiac rehabilitation, although he does not continue psychotherapy.
Related resources
- Stutts LA, Cross NJ, Conti JB, Sears SF. Examination of research trends on patient factors in patients with implantable cardioverter defibrillators. Clin Cardiol 2007;30:64-8.
- Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111;380-2. http://circ.ahajournals.org/cgi/reprint/111/23/e380.
- Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76. www.psychosomaticmedicine.org/cgi/reprint/61/1/69.
- Amiodarone • Cordarone
- Clonazepam • Klonopin
- Flecainide • Tambocor
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sotalol • Betapace
- Triazolam • Halcion, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morris PL, Badger J, Chmielewski C, et al. Psychiatric morbidity following implantation of the automatic implantable cardioverter defibrillator. Psychosomatics 1991;32:58-64.
2. Conti JB, Sears SF, Jr. Understanding and managing the psychological impact of the ICD. Card Electrophysiol Rev 2001;5:128-32.
3. Pelletier D, Gallagher R, Mitten-Lewis S, et al. Australian implantable cardiac defibrillator recipients: quality-of-life issues. Int J Nursing Pract 2002;8:68-74.
4. Eads AS, Sears SF, Jr, Sotile WM, Conti JB. Supportive communication with implantable cardioverter defibrillator patients: seven principles to facilitate psychosocial adjustment. J Cardiopulm Rehab 2000;20:109-14.
5. Sola CL, Bostwick JM. Implantable cardioverter-defibrillators, induced anxiety, and quality of life. Mayo Clin Proc 2005;80:232-7.
6. Sears SF, Jr, Todaro JF, Lewis TS, et al. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clin Cardiol 1999;22:481-9.
7. Goodman M, Hess B. Could implantable cardioverter defibrillators provide a human model supporting the learned helplessness theory of depression? Gen Hosp Psychiatry 1999;21:382-5.
8. Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
9. Godemann F, Ahrens B, Behrens S, et al. Classic conditioning and dysfunctional cognitions in patients with panic disorder and agoraphobia treated with an implantable cardioverter/defibrillator. Psychosom Med 2001;63:231-8.
10. Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282-9.
11. Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung 1993;22:494-501.
12. Bourke JP, Turkington D, Thomas G, et al. Florid psychopathology in patients receiving shocks from implanted cardioverter-defibrillators. Heart 1997;78:581-3.
13. Vlay SC, Olson LC, Fricchione GL, Friedman R. Anxiety and anger in patients with ventricular tachyarrhythmias: responses after automatic internal cardioverter defibrillator implantation. Pacing Clin Electrophysiol 1989;12:366-73.
14. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1998;40:82-5.
15. Friccione GL, Vlay LC, Vlay SC. Cardiac psychiatry and the management of malignant ventricular arrhythmias with the internal cardioverter-defibrillator. Am Heart J 1994;128:1050-9.
16. Friccione GL, Vlay SC. Psychiatric aspects of the implantable cardioverter-defibrillator. In: Estes NAM, Menolis AS, Want PG, eds. Implantable cardioverter-defibrillators. A comprehensive textbook. New York: Marcel Dekker; 1994:405-23.
17. Kohn CS, Petrucci RJ, Baesser C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4 pt 1):450-6.
18. Heller SS, Ormont MA, Lidagoster L, et al. Psychosocial outcome after ICD implantation: a current perspective. Pacing Clin Electrophysiol 1998;21:1207-15.
19. Fabian TJ, Schwartzman DS, Ujhelyi MR, et al. Decreasing pain and anxiety associated with patient-activated atrial shock: a placebo-controlled study of adjunctive sedation with oral triazolam. J Cardivasc Electrophysiol 2006;17:391-5.
20. Sala M, Coppa F, Cappucciati C, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs 2006;7:256-63.
21. Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry 2006;51:923-9.
22. Pinski SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med 2000;28(10 suppl):N174-N180.
23. Kuhl EA, Dixit NK, Walker RL, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-18.
24. Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111:380-2.
1. Morris PL, Badger J, Chmielewski C, et al. Psychiatric morbidity following implantation of the automatic implantable cardioverter defibrillator. Psychosomatics 1991;32:58-64.
2. Conti JB, Sears SF, Jr. Understanding and managing the psychological impact of the ICD. Card Electrophysiol Rev 2001;5:128-32.
3. Pelletier D, Gallagher R, Mitten-Lewis S, et al. Australian implantable cardiac defibrillator recipients: quality-of-life issues. Int J Nursing Pract 2002;8:68-74.
4. Eads AS, Sears SF, Jr, Sotile WM, Conti JB. Supportive communication with implantable cardioverter defibrillator patients: seven principles to facilitate psychosocial adjustment. J Cardiopulm Rehab 2000;20:109-14.
5. Sola CL, Bostwick JM. Implantable cardioverter-defibrillators, induced anxiety, and quality of life. Mayo Clin Proc 2005;80:232-7.
6. Sears SF, Jr, Todaro JF, Lewis TS, et al. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clin Cardiol 1999;22:481-9.
7. Goodman M, Hess B. Could implantable cardioverter defibrillators provide a human model supporting the learned helplessness theory of depression? Gen Hosp Psychiatry 1999;21:382-5.
8. Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
9. Godemann F, Ahrens B, Behrens S, et al. Classic conditioning and dysfunctional cognitions in patients with panic disorder and agoraphobia treated with an implantable cardioverter/defibrillator. Psychosom Med 2001;63:231-8.
10. Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282-9.
11. Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung 1993;22:494-501.
12. Bourke JP, Turkington D, Thomas G, et al. Florid psychopathology in patients receiving shocks from implanted cardioverter-defibrillators. Heart 1997;78:581-3.
13. Vlay SC, Olson LC, Fricchione GL, Friedman R. Anxiety and anger in patients with ventricular tachyarrhythmias: responses after automatic internal cardioverter defibrillator implantation. Pacing Clin Electrophysiol 1989;12:366-73.
14. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1998;40:82-5.
15. Friccione GL, Vlay LC, Vlay SC. Cardiac psychiatry and the management of malignant ventricular arrhythmias with the internal cardioverter-defibrillator. Am Heart J 1994;128:1050-9.
16. Friccione GL, Vlay SC. Psychiatric aspects of the implantable cardioverter-defibrillator. In: Estes NAM, Menolis AS, Want PG, eds. Implantable cardioverter-defibrillators. A comprehensive textbook. New York: Marcel Dekker; 1994:405-23.
17. Kohn CS, Petrucci RJ, Baesser C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4 pt 1):450-6.
18. Heller SS, Ormont MA, Lidagoster L, et al. Psychosocial outcome after ICD implantation: a current perspective. Pacing Clin Electrophysiol 1998;21:1207-15.
19. Fabian TJ, Schwartzman DS, Ujhelyi MR, et al. Decreasing pain and anxiety associated with patient-activated atrial shock: a placebo-controlled study of adjunctive sedation with oral triazolam. J Cardivasc Electrophysiol 2006;17:391-5.
20. Sala M, Coppa F, Cappucciati C, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs 2006;7:256-63.
21. Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry 2006;51:923-9.
22. Pinski SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med 2000;28(10 suppl):N174-N180.
23. Kuhl EA, Dixit NK, Walker RL, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-18.
24. Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111:380-2.
Disaster ethics: What are the ground rules?
Bioethics of clinical practice change during disasters, as our staff learned when providing emergency care to Hurricane Katrina evacuees. During crises such as severe weather, terrorist acts, and epidemics, physicians can be torn between advocating for individual patients’ needs or the public good.1
As the storm’s 2-year anniversary approaches (Box),2,3 we share our experiences to help you prepare for disasters in your community and to contribute to the limited data on ethics in disaster psychiatry. This article describes 3 cases to show how mental health clinicians balanced issues such as conflict, consequences, patient rights, physician virtues, and justice when making treatment decisions in the Houston Astrodome clinic.
CASE 1: Benzodiazepines for anxiety?
Mr. R, age 23, presented to the Astrodome mental health clinic requesting “Xanax for my nerves.” He said he had been taking 6 mg/d “for years and years, and it’s the only thing that helps.” Mr. R claimed he had been without his medicines at least 48 hours.
The assessing psychiatrist found no evidence of benzodiazepine withdrawal or other psychiatric emergency. The dilemma: How to provide appropriate acute treatment of a chronic problem, without continuity of care and follow-up.
As a hurricane survivor, Mr. R experienced a traumatic event that could have exacerbated an underlying anxiety disorder. But patients’ use of and physicians’ prescription of benzodiazepines can have adverse short- and long-term consequences. Mr. R’s case highlights the conflict between establishing patient-physician trust vs enabling a patient’s suspected misuse of prescription medication.
Hurricane Katrina struck August 29, 2005, causing more than 1,000 deaths and displacing several hundred thousand Gulf Coast residents. Nearly 25,000 New Orleans evacuees were bused to the Houston Astrodome, where the medical clinic logged 11,000 patient visits in 15 days (including more than 1,000 to the mental health clinic).2,3
I joined a mental health team that met the first evacuees, who arrived disheveled, exhausted, and hungry at 5am. Many had chronic psychiatric disorders and had lost their medications in the flood. Mental health teams from Houston and elsewhere staffed the clinic around the clock to address the patients’ issues, including schizophrenia, depression, and anxiety.
Limited resources and privacy
Patients streamed through the clinic 24 hours a day, the vinyl sheets between “exam rooms” providing a modicum of privacy. Resources were limited, and we performed assessments much more rapidly than my usual 1-hour initial evaluation. I worked 12-hour shifts for 10 days until I developed the fever (104 °F) and infectious diarrhea that spread among patients and clinic workers.
Some patients arrived requesting “little round white pills” that had quieted their hallucinations, but we had no way to retrieve records destroyed in New Orleans pharmacies. Sometimes we carried backpacks filled with medicines and made “rounds” to patients who were afraid to leave their cots for fear of losing their beds.
Missing neonate
In one case, our team helped a distressed couple find a newborn who had been evacuated from a Louisiana hospital ICU to an unknown location. After several hours, we located the baby in a Texas hospital. In appreciation, the baby’s mother returned the next day to volunteer with us.
Managing patient care during a disaster was a powerful experience. I think about the evacuees often and hope I made a difference in their new beginnings.—Jennifer E. Pate, MD
Recommended postdisaster treatment now integrates 4 elements:
- providing for basic needs (food, shelter, clothing, and safety)
- psychological first aid
- needs assessment
- psychoeducation about normal responses to disasters.8
To make its decisions, the Astrodome clinic team considered the potential problems of prescribing benzodiazepines to patients such as Mr. R:
- Large numbers of traumatized victims might visit the clinic to request benzodiazepines, addictive drugs that for many would be inappropriate and potentially harmful.
- Resources such as medications, information, and time were limited. The team could not contact each patient’s health care provider or pharmacy to verify prescription records.
- Using benzodiazepines to manage anxiety in the acute aftermath of a traumatic event is not supported by the literature.10
In general, patients were not given benzodiazepines for acute anxiety or acute stress disorder. Evacuees who presented to the clinic were educated about normal responses to trauma, received supportive care, and were referred to on-site social service agencies for help finding housing and lost family members.
CASE 2: Urgent care for chronic illness?
Ms. J, age 46, presented to the mental health clinic for evaluation and treatment of chronic depression and anxiety. When asked how she was coping with the storm, she replied, “I wasn’t in the storm. I live in Houston, and I’ve been waiting 6 months to see doctors at the public hospital. I decided to come here and see everyone I needed to see.”
Because of news coverage, Houston residents were well-informed about the hurricane and the Astrodome clinics. Ms. J was resourceful in seeking needed treatment.
The Astrodome clinics were intended to provide acute care to evacuees who lacked alternate resources. Ms. J had chronic mental health problems, but her symptoms could have been exacerbated by graphic media reports of the storm’s devastation.
A challenge in treating chronic health problems in an acute setting is the inability to provide follow-up and continuity of care. An “emergency” clinic is meant to serve as a bridge to later care providers.
Four principles guide ethical decision-making: respect for autonomy, beneficence, nonmaleficence, and justice (Table 1). Would it be an injustice to allocate scarce resources—number of personnel, physician time, space, and medication—to a patient with chronic rather than acute needs?
One could argue that a patient-physician relationship and duty to treat began when Ms. J presented herself as a patient in need and began a dialogue with a physician. The treating physician felt Ms. J’s interest would be served best by continuing the evaluation and acutely managing her symptoms while trying to help her obtain treatment in a more stable setting.
The staff correctly anticipated that this case was unique; no other patients who were not evacuees are known to have requested treatment at the Astrodome clinic.
Table 1
Ethical principles that guide disaster psychiatry
| Principle | Definition | Example |
|---|---|---|
| Respect for autonomy | Promotion of and respect for the patient with capacity to make informed, voluntary decisions about his or her healthcare | A competent patient must provide voluntary informed consent to be admitted to an inpatient psychiatric facility |
| Beneficence | The commitment to act in a manner that brings about benefit or a good outcome | During an emergency, a physician overrides a patient’s confidentiality to inform his mother of his location |
| Nonmaleficence | An obligation to avoid doing harm | Physician refuses to prescribe potentially harmful medication to a patient with an addiction |
| Justice | “Fair” distribution of healthcare resources | Each patient receives care according to need or as resources are available |
| Source: Adapted from reference 11 | ||
CASE 3: Compassion vs confidentiality
Mrs. C, age 67, came to the mental health clinic in tears because she had been separated from her son when she boarded a bus to evacuate from New Orleans. Her son has schizophrenia, and she asked if we had seen him at our clinic. In fact, he had visited our clinic shortly before she arrived.
As healthcare professionals, we value compassion but also are bound by tenets of the physician-patient relationship—in this case, maintaining confidentiality. Physicians are ethically and legally obligated to refrain from disclosing information obtained from a patient without the patient’s permission.11
“Health care providers can share patient information as necessary to provide treatment. Health care providers can share patient information as necessary to identify, locate, and notify family members, guardians, or anyone else responsible for the individual’s care of the individual’s location, general condition, or death.”12
Based on these arguments, the treatment team believed that working with Mrs. C and, if necessary, informing her of her son’s location outweighed the conflicting need to maintain his right to confidentiality.
Therapeutic resources
Catastrophes evoke powerful emotions that can blur responders’ therapeutic boundaries and interfere with how we care for individuals in need (Table 2).13 Some Web-based resources to help you prepare for disasters are available from:
- American Psychiatric Association. www.psych.org/disasterpsych.
- Centers for Disease Control and Prevention. www.bt.cdc.gov/mentalhealth.
- Duke University. http://psychiatry.mc.duke.edu/clinical/disastermentalhealth.html.
Emotional dynamics that motivate disaster response
| Altruism |
| Courage |
| Empathy |
| Compassion |
| Confrontation with mortality |
| Loss of personal sense of invulnerability |
| Identification with those affected |
| Relief at survival |
| Reminders of past experiences |
| Wish to undo harm and “do good” |
| Guilt about being unaffected |
| Feelings of affiliation |
| Source: Reference 13 |
1. Lo B, Katz MH. Clinical decision making during public health emergencies: ethical considerations. Ann Intern Med 2005;143:493-8.
2. Gavagan TF, Smart K, Palacio H, et al. Hurricane Katrina: medical response at the Houston Astrodome/Reliant Center Complex. South Med J 2006;99:933-9.
3. Coker AL, Hanks JS, Eggleston KS, et al. Social and mental health needs assessment of Katrina evacuees. Disaster Manage Response 2006;4:88-94.
4. American Psychiatric Association. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder (2004). Available at: http://www.psych.org/disasterpsych. Accessed February 26, 2007.
5. Veterans Health Administration, Department of Defense. VA/DoD clinical practice guideline for the management of post-traumatic stress. Version 1.0. 2004. Available at: http://www.guideline.gov/summary/summary.aspx?doc_id=5187&nbr=003569&string=disaster+AND+response. Accessed February 26, 2007.
6. Rose S, Bisson J, Churchill R, Wessely S. Psychological debriefing for preventing posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev 2002;(2).-
7. Litz BT, Gray MJ, Bryant RA, et al. Early intervention for trauma: current status and future directions. Clinical Psychology: Science and Practice 2002;9:112-34.
8. National Institute of Mental Health. Mental health and mass violence: evidence-based early psychological intervention for victims/survivors of mass violence: a workshop to reach consensus on best practices, NIH Publication No. 02-5138. Washington, DC: U.S. Government Printing Office, 2002.
9. Gerbarg PL, Brown RP. Yoga: a breath of relief for Hurricane Katrina refugees. Current Psychiatry 2005;4(10):55-67.
10. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57(9):390-4.
11. Beauchamp T, Childress J. Principles of biomedical ethics, 5th ed. Oxford, UK: Oxford University Press, 2001.
12. U.S. Department of Health and Human Services. Hurricane Katrina Bulletin: HIPAA privacy and disclosures in emergency situations, 2005. Available at: http://privacyruleandresearch.nih.gov/pdf/HurricaneKatrina.pdf. Accessed February 26, 2007.
13. Raphael B. Early intervention and the debriefing debate. In: Ursano RJ, Fullerton CS, Norwood AE. Terrorism and disaster: individual and community mental health interventions. Cambridge, UK: Cambridge University Press, 2003.
Bioethics of clinical practice change during disasters, as our staff learned when providing emergency care to Hurricane Katrina evacuees. During crises such as severe weather, terrorist acts, and epidemics, physicians can be torn between advocating for individual patients’ needs or the public good.1
As the storm’s 2-year anniversary approaches (Box),2,3 we share our experiences to help you prepare for disasters in your community and to contribute to the limited data on ethics in disaster psychiatry. This article describes 3 cases to show how mental health clinicians balanced issues such as conflict, consequences, patient rights, physician virtues, and justice when making treatment decisions in the Houston Astrodome clinic.
CASE 1: Benzodiazepines for anxiety?
Mr. R, age 23, presented to the Astrodome mental health clinic requesting “Xanax for my nerves.” He said he had been taking 6 mg/d “for years and years, and it’s the only thing that helps.” Mr. R claimed he had been without his medicines at least 48 hours.
The assessing psychiatrist found no evidence of benzodiazepine withdrawal or other psychiatric emergency. The dilemma: How to provide appropriate acute treatment of a chronic problem, without continuity of care and follow-up.
As a hurricane survivor, Mr. R experienced a traumatic event that could have exacerbated an underlying anxiety disorder. But patients’ use of and physicians’ prescription of benzodiazepines can have adverse short- and long-term consequences. Mr. R’s case highlights the conflict between establishing patient-physician trust vs enabling a patient’s suspected misuse of prescription medication.
Hurricane Katrina struck August 29, 2005, causing more than 1,000 deaths and displacing several hundred thousand Gulf Coast residents. Nearly 25,000 New Orleans evacuees were bused to the Houston Astrodome, where the medical clinic logged 11,000 patient visits in 15 days (including more than 1,000 to the mental health clinic).2,3
I joined a mental health team that met the first evacuees, who arrived disheveled, exhausted, and hungry at 5am. Many had chronic psychiatric disorders and had lost their medications in the flood. Mental health teams from Houston and elsewhere staffed the clinic around the clock to address the patients’ issues, including schizophrenia, depression, and anxiety.
Limited resources and privacy
Patients streamed through the clinic 24 hours a day, the vinyl sheets between “exam rooms” providing a modicum of privacy. Resources were limited, and we performed assessments much more rapidly than my usual 1-hour initial evaluation. I worked 12-hour shifts for 10 days until I developed the fever (104 °F) and infectious diarrhea that spread among patients and clinic workers.
Some patients arrived requesting “little round white pills” that had quieted their hallucinations, but we had no way to retrieve records destroyed in New Orleans pharmacies. Sometimes we carried backpacks filled with medicines and made “rounds” to patients who were afraid to leave their cots for fear of losing their beds.
Missing neonate
In one case, our team helped a distressed couple find a newborn who had been evacuated from a Louisiana hospital ICU to an unknown location. After several hours, we located the baby in a Texas hospital. In appreciation, the baby’s mother returned the next day to volunteer with us.
Managing patient care during a disaster was a powerful experience. I think about the evacuees often and hope I made a difference in their new beginnings.—Jennifer E. Pate, MD
Recommended postdisaster treatment now integrates 4 elements:
- providing for basic needs (food, shelter, clothing, and safety)
- psychological first aid
- needs assessment
- psychoeducation about normal responses to disasters.8
To make its decisions, the Astrodome clinic team considered the potential problems of prescribing benzodiazepines to patients such as Mr. R:
- Large numbers of traumatized victims might visit the clinic to request benzodiazepines, addictive drugs that for many would be inappropriate and potentially harmful.
- Resources such as medications, information, and time were limited. The team could not contact each patient’s health care provider or pharmacy to verify prescription records.
- Using benzodiazepines to manage anxiety in the acute aftermath of a traumatic event is not supported by the literature.10
In general, patients were not given benzodiazepines for acute anxiety or acute stress disorder. Evacuees who presented to the clinic were educated about normal responses to trauma, received supportive care, and were referred to on-site social service agencies for help finding housing and lost family members.
CASE 2: Urgent care for chronic illness?
Ms. J, age 46, presented to the mental health clinic for evaluation and treatment of chronic depression and anxiety. When asked how she was coping with the storm, she replied, “I wasn’t in the storm. I live in Houston, and I’ve been waiting 6 months to see doctors at the public hospital. I decided to come here and see everyone I needed to see.”
Because of news coverage, Houston residents were well-informed about the hurricane and the Astrodome clinics. Ms. J was resourceful in seeking needed treatment.
The Astrodome clinics were intended to provide acute care to evacuees who lacked alternate resources. Ms. J had chronic mental health problems, but her symptoms could have been exacerbated by graphic media reports of the storm’s devastation.
A challenge in treating chronic health problems in an acute setting is the inability to provide follow-up and continuity of care. An “emergency” clinic is meant to serve as a bridge to later care providers.
Four principles guide ethical decision-making: respect for autonomy, beneficence, nonmaleficence, and justice (Table 1). Would it be an injustice to allocate scarce resources—number of personnel, physician time, space, and medication—to a patient with chronic rather than acute needs?
One could argue that a patient-physician relationship and duty to treat began when Ms. J presented herself as a patient in need and began a dialogue with a physician. The treating physician felt Ms. J’s interest would be served best by continuing the evaluation and acutely managing her symptoms while trying to help her obtain treatment in a more stable setting.
The staff correctly anticipated that this case was unique; no other patients who were not evacuees are known to have requested treatment at the Astrodome clinic.
Table 1
Ethical principles that guide disaster psychiatry
| Principle | Definition | Example |
|---|---|---|
| Respect for autonomy | Promotion of and respect for the patient with capacity to make informed, voluntary decisions about his or her healthcare | A competent patient must provide voluntary informed consent to be admitted to an inpatient psychiatric facility |
| Beneficence | The commitment to act in a manner that brings about benefit or a good outcome | During an emergency, a physician overrides a patient’s confidentiality to inform his mother of his location |
| Nonmaleficence | An obligation to avoid doing harm | Physician refuses to prescribe potentially harmful medication to a patient with an addiction |
| Justice | “Fair” distribution of healthcare resources | Each patient receives care according to need or as resources are available |
| Source: Adapted from reference 11 | ||
CASE 3: Compassion vs confidentiality
Mrs. C, age 67, came to the mental health clinic in tears because she had been separated from her son when she boarded a bus to evacuate from New Orleans. Her son has schizophrenia, and she asked if we had seen him at our clinic. In fact, he had visited our clinic shortly before she arrived.
As healthcare professionals, we value compassion but also are bound by tenets of the physician-patient relationship—in this case, maintaining confidentiality. Physicians are ethically and legally obligated to refrain from disclosing information obtained from a patient without the patient’s permission.11
“Health care providers can share patient information as necessary to provide treatment. Health care providers can share patient information as necessary to identify, locate, and notify family members, guardians, or anyone else responsible for the individual’s care of the individual’s location, general condition, or death.”12
Based on these arguments, the treatment team believed that working with Mrs. C and, if necessary, informing her of her son’s location outweighed the conflicting need to maintain his right to confidentiality.
Therapeutic resources
Catastrophes evoke powerful emotions that can blur responders’ therapeutic boundaries and interfere with how we care for individuals in need (Table 2).13 Some Web-based resources to help you prepare for disasters are available from:
- American Psychiatric Association. www.psych.org/disasterpsych.
- Centers for Disease Control and Prevention. www.bt.cdc.gov/mentalhealth.
- Duke University. http://psychiatry.mc.duke.edu/clinical/disastermentalhealth.html.
Emotional dynamics that motivate disaster response
| Altruism |
| Courage |
| Empathy |
| Compassion |
| Confrontation with mortality |
| Loss of personal sense of invulnerability |
| Identification with those affected |
| Relief at survival |
| Reminders of past experiences |
| Wish to undo harm and “do good” |
| Guilt about being unaffected |
| Feelings of affiliation |
| Source: Reference 13 |
Bioethics of clinical practice change during disasters, as our staff learned when providing emergency care to Hurricane Katrina evacuees. During crises such as severe weather, terrorist acts, and epidemics, physicians can be torn between advocating for individual patients’ needs or the public good.1
As the storm’s 2-year anniversary approaches (Box),2,3 we share our experiences to help you prepare for disasters in your community and to contribute to the limited data on ethics in disaster psychiatry. This article describes 3 cases to show how mental health clinicians balanced issues such as conflict, consequences, patient rights, physician virtues, and justice when making treatment decisions in the Houston Astrodome clinic.
CASE 1: Benzodiazepines for anxiety?
Mr. R, age 23, presented to the Astrodome mental health clinic requesting “Xanax for my nerves.” He said he had been taking 6 mg/d “for years and years, and it’s the only thing that helps.” Mr. R claimed he had been without his medicines at least 48 hours.
The assessing psychiatrist found no evidence of benzodiazepine withdrawal or other psychiatric emergency. The dilemma: How to provide appropriate acute treatment of a chronic problem, without continuity of care and follow-up.
As a hurricane survivor, Mr. R experienced a traumatic event that could have exacerbated an underlying anxiety disorder. But patients’ use of and physicians’ prescription of benzodiazepines can have adverse short- and long-term consequences. Mr. R’s case highlights the conflict between establishing patient-physician trust vs enabling a patient’s suspected misuse of prescription medication.
Hurricane Katrina struck August 29, 2005, causing more than 1,000 deaths and displacing several hundred thousand Gulf Coast residents. Nearly 25,000 New Orleans evacuees were bused to the Houston Astrodome, where the medical clinic logged 11,000 patient visits in 15 days (including more than 1,000 to the mental health clinic).2,3
I joined a mental health team that met the first evacuees, who arrived disheveled, exhausted, and hungry at 5am. Many had chronic psychiatric disorders and had lost their medications in the flood. Mental health teams from Houston and elsewhere staffed the clinic around the clock to address the patients’ issues, including schizophrenia, depression, and anxiety.
Limited resources and privacy
Patients streamed through the clinic 24 hours a day, the vinyl sheets between “exam rooms” providing a modicum of privacy. Resources were limited, and we performed assessments much more rapidly than my usual 1-hour initial evaluation. I worked 12-hour shifts for 10 days until I developed the fever (104 °F) and infectious diarrhea that spread among patients and clinic workers.
Some patients arrived requesting “little round white pills” that had quieted their hallucinations, but we had no way to retrieve records destroyed in New Orleans pharmacies. Sometimes we carried backpacks filled with medicines and made “rounds” to patients who were afraid to leave their cots for fear of losing their beds.
Missing neonate
In one case, our team helped a distressed couple find a newborn who had been evacuated from a Louisiana hospital ICU to an unknown location. After several hours, we located the baby in a Texas hospital. In appreciation, the baby’s mother returned the next day to volunteer with us.
Managing patient care during a disaster was a powerful experience. I think about the evacuees often and hope I made a difference in their new beginnings.—Jennifer E. Pate, MD
Recommended postdisaster treatment now integrates 4 elements:
- providing for basic needs (food, shelter, clothing, and safety)
- psychological first aid
- needs assessment
- psychoeducation about normal responses to disasters.8
To make its decisions, the Astrodome clinic team considered the potential problems of prescribing benzodiazepines to patients such as Mr. R:
- Large numbers of traumatized victims might visit the clinic to request benzodiazepines, addictive drugs that for many would be inappropriate and potentially harmful.
- Resources such as medications, information, and time were limited. The team could not contact each patient’s health care provider or pharmacy to verify prescription records.
- Using benzodiazepines to manage anxiety in the acute aftermath of a traumatic event is not supported by the literature.10
In general, patients were not given benzodiazepines for acute anxiety or acute stress disorder. Evacuees who presented to the clinic were educated about normal responses to trauma, received supportive care, and were referred to on-site social service agencies for help finding housing and lost family members.
CASE 2: Urgent care for chronic illness?
Ms. J, age 46, presented to the mental health clinic for evaluation and treatment of chronic depression and anxiety. When asked how she was coping with the storm, she replied, “I wasn’t in the storm. I live in Houston, and I’ve been waiting 6 months to see doctors at the public hospital. I decided to come here and see everyone I needed to see.”
Because of news coverage, Houston residents were well-informed about the hurricane and the Astrodome clinics. Ms. J was resourceful in seeking needed treatment.
The Astrodome clinics were intended to provide acute care to evacuees who lacked alternate resources. Ms. J had chronic mental health problems, but her symptoms could have been exacerbated by graphic media reports of the storm’s devastation.
A challenge in treating chronic health problems in an acute setting is the inability to provide follow-up and continuity of care. An “emergency” clinic is meant to serve as a bridge to later care providers.
Four principles guide ethical decision-making: respect for autonomy, beneficence, nonmaleficence, and justice (Table 1). Would it be an injustice to allocate scarce resources—number of personnel, physician time, space, and medication—to a patient with chronic rather than acute needs?
One could argue that a patient-physician relationship and duty to treat began when Ms. J presented herself as a patient in need and began a dialogue with a physician. The treating physician felt Ms. J’s interest would be served best by continuing the evaluation and acutely managing her symptoms while trying to help her obtain treatment in a more stable setting.
The staff correctly anticipated that this case was unique; no other patients who were not evacuees are known to have requested treatment at the Astrodome clinic.
Table 1
Ethical principles that guide disaster psychiatry
| Principle | Definition | Example |
|---|---|---|
| Respect for autonomy | Promotion of and respect for the patient with capacity to make informed, voluntary decisions about his or her healthcare | A competent patient must provide voluntary informed consent to be admitted to an inpatient psychiatric facility |
| Beneficence | The commitment to act in a manner that brings about benefit or a good outcome | During an emergency, a physician overrides a patient’s confidentiality to inform his mother of his location |
| Nonmaleficence | An obligation to avoid doing harm | Physician refuses to prescribe potentially harmful medication to a patient with an addiction |
| Justice | “Fair” distribution of healthcare resources | Each patient receives care according to need or as resources are available |
| Source: Adapted from reference 11 | ||
CASE 3: Compassion vs confidentiality
Mrs. C, age 67, came to the mental health clinic in tears because she had been separated from her son when she boarded a bus to evacuate from New Orleans. Her son has schizophrenia, and she asked if we had seen him at our clinic. In fact, he had visited our clinic shortly before she arrived.
As healthcare professionals, we value compassion but also are bound by tenets of the physician-patient relationship—in this case, maintaining confidentiality. Physicians are ethically and legally obligated to refrain from disclosing information obtained from a patient without the patient’s permission.11
“Health care providers can share patient information as necessary to provide treatment. Health care providers can share patient information as necessary to identify, locate, and notify family members, guardians, or anyone else responsible for the individual’s care of the individual’s location, general condition, or death.”12
Based on these arguments, the treatment team believed that working with Mrs. C and, if necessary, informing her of her son’s location outweighed the conflicting need to maintain his right to confidentiality.
Therapeutic resources
Catastrophes evoke powerful emotions that can blur responders’ therapeutic boundaries and interfere with how we care for individuals in need (Table 2).13 Some Web-based resources to help you prepare for disasters are available from:
- American Psychiatric Association. www.psych.org/disasterpsych.
- Centers for Disease Control and Prevention. www.bt.cdc.gov/mentalhealth.
- Duke University. http://psychiatry.mc.duke.edu/clinical/disastermentalhealth.html.
Emotional dynamics that motivate disaster response
| Altruism |
| Courage |
| Empathy |
| Compassion |
| Confrontation with mortality |
| Loss of personal sense of invulnerability |
| Identification with those affected |
| Relief at survival |
| Reminders of past experiences |
| Wish to undo harm and “do good” |
| Guilt about being unaffected |
| Feelings of affiliation |
| Source: Reference 13 |
1. Lo B, Katz MH. Clinical decision making during public health emergencies: ethical considerations. Ann Intern Med 2005;143:493-8.
2. Gavagan TF, Smart K, Palacio H, et al. Hurricane Katrina: medical response at the Houston Astrodome/Reliant Center Complex. South Med J 2006;99:933-9.
3. Coker AL, Hanks JS, Eggleston KS, et al. Social and mental health needs assessment of Katrina evacuees. Disaster Manage Response 2006;4:88-94.
4. American Psychiatric Association. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder (2004). Available at: http://www.psych.org/disasterpsych. Accessed February 26, 2007.
5. Veterans Health Administration, Department of Defense. VA/DoD clinical practice guideline for the management of post-traumatic stress. Version 1.0. 2004. Available at: http://www.guideline.gov/summary/summary.aspx?doc_id=5187&nbr=003569&string=disaster+AND+response. Accessed February 26, 2007.
6. Rose S, Bisson J, Churchill R, Wessely S. Psychological debriefing for preventing posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev 2002;(2).-
7. Litz BT, Gray MJ, Bryant RA, et al. Early intervention for trauma: current status and future directions. Clinical Psychology: Science and Practice 2002;9:112-34.
8. National Institute of Mental Health. Mental health and mass violence: evidence-based early psychological intervention for victims/survivors of mass violence: a workshop to reach consensus on best practices, NIH Publication No. 02-5138. Washington, DC: U.S. Government Printing Office, 2002.
9. Gerbarg PL, Brown RP. Yoga: a breath of relief for Hurricane Katrina refugees. Current Psychiatry 2005;4(10):55-67.
10. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57(9):390-4.
11. Beauchamp T, Childress J. Principles of biomedical ethics, 5th ed. Oxford, UK: Oxford University Press, 2001.
12. U.S. Department of Health and Human Services. Hurricane Katrina Bulletin: HIPAA privacy and disclosures in emergency situations, 2005. Available at: http://privacyruleandresearch.nih.gov/pdf/HurricaneKatrina.pdf. Accessed February 26, 2007.
13. Raphael B. Early intervention and the debriefing debate. In: Ursano RJ, Fullerton CS, Norwood AE. Terrorism and disaster: individual and community mental health interventions. Cambridge, UK: Cambridge University Press, 2003.
1. Lo B, Katz MH. Clinical decision making during public health emergencies: ethical considerations. Ann Intern Med 2005;143:493-8.
2. Gavagan TF, Smart K, Palacio H, et al. Hurricane Katrina: medical response at the Houston Astrodome/Reliant Center Complex. South Med J 2006;99:933-9.
3. Coker AL, Hanks JS, Eggleston KS, et al. Social and mental health needs assessment of Katrina evacuees. Disaster Manage Response 2006;4:88-94.
4. American Psychiatric Association. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder (2004). Available at: http://www.psych.org/disasterpsych. Accessed February 26, 2007.
5. Veterans Health Administration, Department of Defense. VA/DoD clinical practice guideline for the management of post-traumatic stress. Version 1.0. 2004. Available at: http://www.guideline.gov/summary/summary.aspx?doc_id=5187&nbr=003569&string=disaster+AND+response. Accessed February 26, 2007.
6. Rose S, Bisson J, Churchill R, Wessely S. Psychological debriefing for preventing posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev 2002;(2).-
7. Litz BT, Gray MJ, Bryant RA, et al. Early intervention for trauma: current status and future directions. Clinical Psychology: Science and Practice 2002;9:112-34.
8. National Institute of Mental Health. Mental health and mass violence: evidence-based early psychological intervention for victims/survivors of mass violence: a workshop to reach consensus on best practices, NIH Publication No. 02-5138. Washington, DC: U.S. Government Printing Office, 2002.
9. Gerbarg PL, Brown RP. Yoga: a breath of relief for Hurricane Katrina refugees. Current Psychiatry 2005;4(10):55-67.
10. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57(9):390-4.
11. Beauchamp T, Childress J. Principles of biomedical ethics, 5th ed. Oxford, UK: Oxford University Press, 2001.
12. U.S. Department of Health and Human Services. Hurricane Katrina Bulletin: HIPAA privacy and disclosures in emergency situations, 2005. Available at: http://privacyruleandresearch.nih.gov/pdf/HurricaneKatrina.pdf. Accessed February 26, 2007.
13. Raphael B. Early intervention and the debriefing debate. In: Ursano RJ, Fullerton CS, Norwood AE. Terrorism and disaster: individual and community mental health interventions. Cambridge, UK: Cambridge University Press, 2003.
ADHD: Only half the diagnosis in an adult with inattention?
Overlapping symptoms may obscure comorbid bipolar illness
An adult with function-impairing inattention could have attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), or both. Comorbid ADHD and BD often is unrecognized, however, because patients are more likely to report ADHD-related symptoms than manic symptoms.1
To help you recognize comorbid ADHD/BD—and protect adults who might switch into mania if given stimulants or antidepressants—this article describes a hierarchy to diagnose and treat this comorbidity. Based on the evidence and our experience, we:
- discuss how to differentiate between these disorders with overlapping symptoms
- provide tools and suggestions to screen for BD and adult ADHD
- offer 3 algorithms to guide your diagnosis and choice of medications.
Clinical challenges
Prevalence is unclear. Adult ADHD—with an estimated prevalence of 4.4%2—is more common than BD. Lifetime prevalences of BD types I and II are 1.6% and 0.5%, respectively.3 Studies of ADHD/BD comorbidity suggest wide-ranging prevalence rates:
Underdiagnosis. Adult ADHD/BD is a more severe illness than ADHD or BD alone and is highly comorbid with agoraphobia, social phobia, posttraumatic stress disorder, and alcohol or drug addiction. Adults with ADHD/BD have more frequent affective episodes, suicide attempts, violence, and legal problems.4 Diagnosing this comorbidity remains a challenge, however, because:
- identifying which symptoms are caused by which disorder can be difficult
- BD tends to be underdiagnosed9
- patients often misidentify, underreport, or deny manic symptoms1,10,11
- if a patient presents with active bipolar symptoms, DSM-IV-TR criteria require that ADHD not be diagnosed until mood symptoms are resolved.
Overlapping symptoms. ADHD and bipolar mania share some DSM-IV-TR diagnostic criteria, including talkativeness, distractibility, increased activity or physical restlessness, and loss of social inhibitions (Table 1).12 Overlapping symptoms also are notable within ADHD diagnostic criteria (Table 2). In the inattention category, for example, “easily distracted by extraneous stimuli,” “difficulty sustaining attention in tasks,” and “fails to give close attention to details” are considered 3 separate symptoms. In the hyperactivity category, “often leaves seat,” “often runs about or climbs excessively,” and “often on the go, or often acts as if driven by a motor” are 3 separate symptoms.
Given ADHD’s relatively loose diagnostic criteria and high comorbidity in adults with mood disorders, the question of whether adult ADHD/BD represents comorbidity or diagnostic overlap remains unresolved. For the clinician, the disorders’ nonoverlapping features (Table 1) can assist with the differential diagnosis. For example:
- ADHD symptoms tend to be chronic and BD symptoms episodic.
- ADHD patients may have high energy but lack increased productivity seen in BD patients.
- ADHD patients do not need less sleep or have inflated self-esteem like symptomatic BD patients.
- Psychotic symptoms such as hallucinations or delusions might be present in severe BD but are absent in ADHD.
Table 1
Overlap between DSM-IV-TR diagnostic criteria for ADHD and bipolar mania
| Overlapping symptoms | |
|---|---|
| ADHD | Bipolar mania |
| Talks excessively | More talkative than usual |
| Easily distracted/jumps from one activity to the next | Distractibility or constant changes in activity or plans |
| Fidgets Difficulty remaining seated Runs or climbs about inappropriately Difficulty playing quietly On the go as if driven by a motor | Increased activity or physical restlessness |
| Interrupts or butts in uninvited Blurts out answers | Loss of normal social inhibitions |
| Nonoverlapping symptoms | |
| ADHD Forgetful in daily activities Difficulty awaiting turn Difficulty organizing self Loses things Avoids sustained mental effort Does not seem to listen Difficulty following through on instructions/fails to finish work Difficulty sustaining attention Fails to give close attention to details/makes careless mistakes | |
| Bipolar mania Inflated self-esteem/grandiosity Increase in goal-directed activity Flight of ideas Decreased need for sleep Excessive involvement in pleasurable activities with disregard for potential adverse consequences Marked sexual energy or sexual indiscretions | |
| ADHD: attention-deficit/hyperactivity disorder | |
| Source: Adapted and reprinted with permission from reference 12 | |
Table 2
DSM-IV-TR diagnostic criteria for attention-deficit/ hyperactivity disorder
| Inattention |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Hyperactivity/impulsivity |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Diagnosis requires evidence of inattention or hyperactivity/impulsivity or both |
| Some hyperactive/impulsive or inattentive symptoms that caused impairment were present before age 7 |
| Some impairment from symptoms is present in ≥2 settings (such as at school, work, or home) |
| Symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder (mood disorder, anxiety disorder, dissociative disorder, or a personality disorder) |
| Source: DSM-IV-TR |
Mood symptoms first
A diagnostic hierarchy is implicit in DSM-IV-TR; anxiety disorders are not diagnosed during an active major depressive or manic episode, and schizophrenia is not diagnosed on the basis of psychotic symptoms during an active major depressive or manic episode. Mood disorders sit atop this implied diagnostic hierarchy and must be ruled out before psychotic or anxiety disorders are diagnosed. Similarly, most personality disorders are not diagnosed during an active mood or psychotic episode.
Diagnosing adult ADHD when a patient is actively depressed or manic is inconsistent with this hierarchy and conflicts with extensive nosologic literature.13 We suggest that ADHD—a cognitive-behavioral problem—not be diagnosed solely on symptoms observed when a patient is experiencing a mood episode or psychotic illness.
Bipolar disorder. Two useful mnemonics (Table 3) assist in screening for DSM-IV-TR symptoms of BD type I:
- Pure mania consists of euphoric mood and ≥3 of 7 DIGFAST criteria, or irritable mood and ≥4 of 7 DIGFAST criteria
- Mixed mania consists of depressed mood with ≥4 of 7 DIGFAST criteria and ≥4 of 8 SIGECAPS criteria.
To be diagnostic, these symptoms must cause substantial social or occupational dysfunction and be present at least 1 week. Diagnose BD type I if a patient has experienced a single pure or mixed manic episode at any time, unless the episode had a medical cause such as hyperthyroidism or antidepressant use. Because patients with mixed episodes experience depressed mood, assess any patient with clinical depression for manic symptoms. Otherwise, a patient with a mixed episode could be misdiagnosed as having unipolar depression instead of BD type I.14
BD type II also has been observed in patients with comorbid adult ADHD/BD.4,6 The main difference between BD types I and II is that manic symptoms in type II are not severe enough to cause functional impairment or psychotic symptoms.15
Adult ADHD. The clinical interview seeking evidence of inattention and hyperactivity/impulsivity remains the basis of adult ADHD diagnosis (Table 2). Key areas are:
- the patient’s past and current functional impairment
- whether substantial impairment occurs in at least 2 areas of life (such as school, work, or home).
Take medical, educational, social, psychological, and vocational histories, and rule out other conditions before concluding that adult ADHD is the appropriate diagnosis.16 In adult ADHD, inattentive symptoms become far more prominent, about twice as common as hyperactive symptoms.17 Inattentive symptoms may manifest as neglect, poor time management, motivational deficits, or poor concentration that results in forgetfulness, distractibility, item misplacement, or excessive mistakes in paperwork.18 When impulsive symptoms persist in adults, they may manifest as automobile accidents or low tolerance for frustration, which may lead to frequent job changes and unstable, interrupted interpersonal relationships.18
Neuropsychological testing is not required to make an adult ADHD diagnosis but can help establish the breadth of symptoms or comorbidity.17 Rating scales can screen, gather data (including presence and severity of symptoms), and measure treatment response.16 Commonly used rating scales include:
- Conners’ Adult ADHD Rating Scales19
- Brown Attention Deficit Disorder Rating Scale for Adults20
- Adult ADHD Self-Report Scale.21
When using rating scales, remember that adult psychopathology can distort perceptions, and some self-report scales have questionable reliability.16
Table 3
Mnemonics for diagnostic symptoms of pure and mixed bipolar mania
| DIGFAST* for bipolar mania symptoms | SIGECAPS† bipolar depression symptoms |
|---|---|
| Distractibility Insomnia Grandiosity Flight of ideas Activities Speech Thoughtlessness | Sleep Interest Guilt Energy Concentration Appetite Psychomotor Suicide |
| Pure mania: Euphoric mood with ≥3 DIGFAST criteria or irritable mood with ≥4 DIGFAST criteria. | |
| Mixed mania: Depressed mood with ≥4 DIGFAST criteria and ≥4 SIGECAPS criteria. | |
| * Developed by William Falk, MD | |
| †Developed by Carey Gross, MD | |
| Source: Adapted from Ghaemi SN. Mood disorders. New York: Lippincott, Williams, & Wilkins; 2003 | |
Treatment recommendations
Limited data. We found only 1 study on adult ADHD/BD treatment. In this open trial,22 36 adults with comorbid ADHD and BD received bupropion SR, up to 200 mg bid, for ADHD symptoms while maintained on mood stabilizers, antipsychotics, or both. Improvement was defined as ≥30% reduction in ADHD Symptom Checklist Scale scores, without concurrent mania. After 6 weeks, 82% of patients had improved; 1 dropped out at week 2 because of hypomanic activation. Methodologic limitations included trial design (non-randomized, nonblinded, short duration) and patient selection (90% of subjects had BD type II).
In the absence of adequate data on adult ADHD/BD, studies in children suggest:
- stimulants may not be effective for ADHD symptoms in patients with active manic or depressive symptoms
- mood stabilization is a prerequisite for successful pharmacologic treatment of ADHD in patients with both ADHD and manic or depressive symptoms.23,24
Follow the hierarchy. First treat acute mood symptoms, then reevaluate and possibly treat ADHD symptoms if they persist during euthymia (Algorithm 1). When a patient meets criteria for adult ADHD/BD, first stabilize bipolar manic or depressive symptoms (Algorithm 2). For acute mania, treat with standard mood stabilizers (lithium, valproate, lamotrigine, or carbamazepine) with or without a second-generation antipsychotic.25 Starting stimulants for ADHD when patients have active mood symptoms is sub-optimal and potentially harmful because of the risk of inducing mania. For acute bipolar depression, adjunctive antidepressant treatment has been found to be no more effective than a mood stabilizer alone.26
After bipolar symptoms respond or remit, reassess for adult ADHD. If ADHD symptoms persist during euthymia, additional treatment may be indicated.
Very little evidence exists on treating adult ADHD/BD; as mentioned, bupropion is the only medication studied in this population. For adult ADHD alone, clinical trials have showed varying efficacy with bupropion,27,28 atomoxetine,29 venlafaxine,30,31 desipramine,32 methylphenidate,33 mixed amphetamine salts,34 and guanfacine.35 Whether these treatments can be generalized as safe and efficacious for comorbid adult ADHD/BD is unclear. Nonetheless, we suggest using bupropion first, followed by atomoxetine or guanfacine before you consider amphetamine stimulants (Algorithm 3).
Algorithm 1
Hierarchy for diagnosis and treatment of adult ADHD/BD
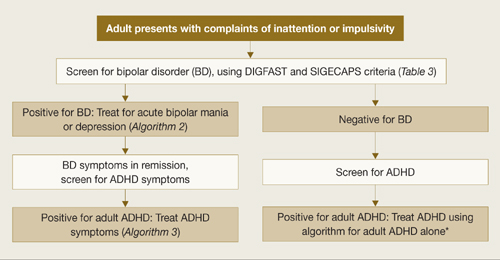
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
*Adler LA, Chua HC. Management of ADHD in adults. J Clin Psychiatry 2002;63(suppl 12):29-35.
Algorithm 2
Treating acute episodes of bipolar disorder
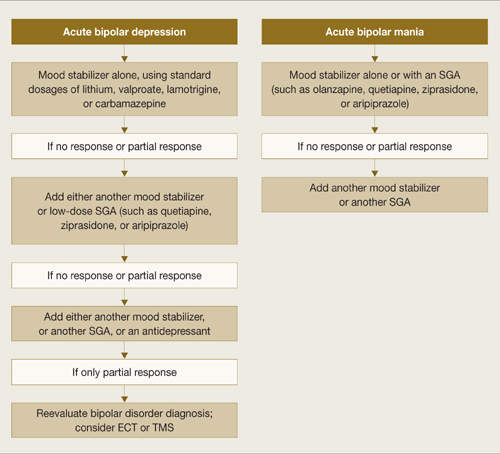
ECT: electroconvulsive therapy; SGA: second-generation antipsychotic; TMS: transcranial magnetic stimulation
Algorithm 3
Suggested approach to adult ADHD with comorbid BD*
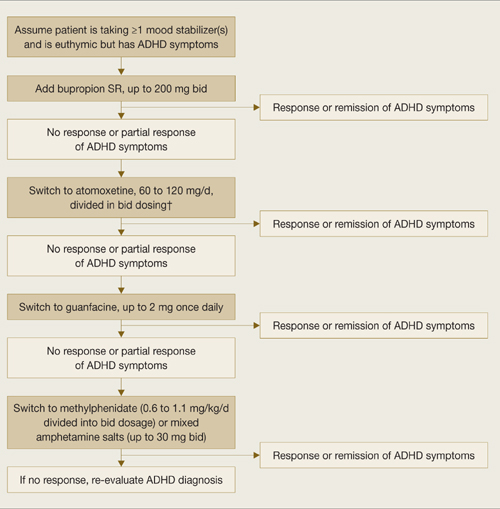
* Based on data extrapolated from samples of patients with ADHD alone because of very limited data in ADHD/BD samples.
† We recommend against combining antidepressants and stimulants because of additive risks of mania in BD. Discontinue stimulant or antidepressant if manic symptoms appear or rapid cycling emerges.
Reducing mania risk
Antidepressants and stimulants may help adults with ADHD alone, but risks of mania and rapid cycling limit their use in adults with ADHD/BD.
Stimulants and mania. One study found a 17% manic switch rate when methylphenidate (≤10 mg bid) was given to 14 bipolar depressed adults (10 BD type I, 2 BD type II, and 2 with secondary mania) taking mood stabilizers.36 A chart review of 82 bipolar children not taking mood stabilizers found an 18% switch rate with methylphenidate or amphetamine.37 Another chart review of 80 children with BD type I found that past amphetamine treatment (but not history of ADHD diagnosis or antidepressant treatment) was associated with more severe bipolar illness.38
No studies have examined predictors of amphetamine-induced mania. In our clinical experience, triggers are similar to those that can cause antidepressant-induced mania, such as:
- recent manic episodes
- current rapid cycling
- past antidepressant-induced mania.
Antidepressants and mania. When 64 patients with acute bipolar depression received both antidepressants and mood stabilizers in a randomized, double-blind trial, switch rates into mania or hypomania were 10% for bupropion, 9% for sertraline, and 29% for venlafaxine.39 In a meta analysis of clinical trials using selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs), the manic switch rate was threefold higher with TCAs than SSRIs.40 Antidepressant use in bipolar patients was associated with rapid cycling in the only randomized study of this topic.41
Insufficient data exist to clarify whether mania induction with antidepressants is dose-dependent.42 Factors associated with antidepressant-induced mania include:
- previous antidepressant-induced mania
- family history of BD
- exposure to multiple antidepressant trials42
- history of substance abuse and/or dependence.43
Related resources
- Bipolar disorder information and resources. www.psycheducation.org.
- ADHD Information and resources. www.adhdnews.com.
- Phelps J. Why am I still depressed? Recognizing and managing the ups and downs of bipolar II and soft bipolar disorder. New York: McGraw-Hill; 2006.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Wingo reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Ghaemi receives research grants from GlaxoSmithKline and Pfizer and is a speaker for GlaxoSmithKline, AstraZeneca, Pfizer, and Abbott Laboratories. Neither he nor his family hold equity positions in pharmaceutical companies.
1. Ghaemi SN, Stoll AL, Pope HG, Jr, et al. Lack of insight in bipolar disorder. The acute manic episode. J Nerv Ment Dis 1995;183(7):464-7.
2. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
3. Sadock BJ, Sadock VA, eds. Synopsis of psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.
4. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
5. Tamam L, Tuglu C, Karatas G, et al. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
6. Wilens TE, Biederman J, Wozniak J, et al. Can adults with attention-deficit/hyperactivity disorder be distinguished from those with comorbid bipolar disorder? Findings from a sample of clinically referred adults. Biol Psychiatry 2003;54(1):1-8.
7. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry 2005;162(9):1621-7.
8. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006;163(10):1720-9.
9. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999;52(1-3):135-44.
10. Keitner GI, Solomon DA, Ryan CE, et al. Prodromal and residual symptoms in bipolar I disorder. Compr Psychiatry 1996;37(5):362-7.
11. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv 2001;52(1):51-5.
12. Kent L, Craddock N. Is there a relationship between attention deficit hyperactivity disorder and bipolar disorder? J Affect Disord 2003;73(3):211-21.
13. Surtees PG, Kendell RE. The hierarchy model of psychiatric symptomatology: an investigation based on present state examination ratings. Br J Psychiatry 1979;135:438-43.
14. Benazzi F. Symptoms of depression as possible markers of bipolar II disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):471-7.
15. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
16. Murphy KR, Adler LA. Assessing attention-deficit hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry 2004;65(suppl 3):12-17.
17. Adler LA. Diagnosing adult attention deficit hyperactivity disorder. Primary Psychiatry 2006;13(suppl 3):9-10.
18. Montano B. Diagnosis and treatment of ADHD in adults in primary care. J Clin Psychiatry 2004;65(suppl 3):18-21.
19. Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS). North Tonawanda, NY: Multi-Health Systems; 1999.
20. Brown TE. Brown Attention Deficit Disorder Scales. San Antonio, TX: The Psychological Corporation; 1996.
21. Adler LA, Kessler RC, Spencer T. Adult ADHD Self-report Scale v1.1 (ASRS-v1.1) Symptom Checklist. World Health Organization. Available at: http://www.med.nyu.edu/psych/assets/adhdscreen18.pdf. Accessed May 7, 2007.
22. Wilens TE, Prince JB, Spencer T, et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry 2003;54(1):9-16.
23. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
24. Daviss WB, Bentivoglio P, Racusin R, et al. Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry 2001;40(3):307-14.
25. Scherk H, Pajonk FG, Leucht SL. Second-generation antipsychotic agents in the treatment of acute mania. Arch Gen Psychiatry 2007;64:442-55.
26. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007:356:(17):1711-22.
27. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention deficit hyperactivity disorder: a randomized, placebo controlled study. Biol Psychiatry 2005;57:793-801.
28. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
29. Michelson D, Adler LA, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo controlled studies. Biol Psychiatry 2003;53:112-20.
30. Adler LA, Resnick S, Kunz M, Devinsky O. Open-label trial of venlafaxine in adults with attention deficit disorder. Psychopharmacol Bull 1995;31(4):785-8.
31. Hedges D, Reimherr FW, Rogers A, et al. An open trial of venlafaxine in adult patients with attention deficit hyperactivity disorder. Psychopharmacol Bull 1995;31(4):779-83.
32. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153(9):1147-53.
33. Faraone SV, Spencer T, Aleardi M, et al. Meta analysis of the efficacy of methylphenidate for treating adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2004;24(1):24-8.
34. Spencer T, Biederman J, Wilens TE, et al. Efficacy of a mixed amphetamine salts compound in adults with attention deficit hyperactivity disorder. Arch Gen Psychiatry 2001;58:775-82.
35. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2000;21(2):223-8.
36. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
37. Faedda GL, Baldessarini RJ, Glovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord 2004;82(1):149-58.
38. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70(3):323-7.
39. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006;189:124-31.
40. Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994;164(4):549-50.
41. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145(2):179-84.
42. Goldberg JF. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract. 2003;9(3):181-94.
43. Goldberg JF, Whiteside JE. The association between substance abuse and antidepressant-induced mania in bipolar disorder: a preliminary study. J Clin Psychiatry 2002;63(9):791-5.
Overlapping symptoms may obscure comorbid bipolar illness
An adult with function-impairing inattention could have attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), or both. Comorbid ADHD and BD often is unrecognized, however, because patients are more likely to report ADHD-related symptoms than manic symptoms.1
To help you recognize comorbid ADHD/BD—and protect adults who might switch into mania if given stimulants or antidepressants—this article describes a hierarchy to diagnose and treat this comorbidity. Based on the evidence and our experience, we:
- discuss how to differentiate between these disorders with overlapping symptoms
- provide tools and suggestions to screen for BD and adult ADHD
- offer 3 algorithms to guide your diagnosis and choice of medications.
Clinical challenges
Prevalence is unclear. Adult ADHD—with an estimated prevalence of 4.4%2—is more common than BD. Lifetime prevalences of BD types I and II are 1.6% and 0.5%, respectively.3 Studies of ADHD/BD comorbidity suggest wide-ranging prevalence rates:
Underdiagnosis. Adult ADHD/BD is a more severe illness than ADHD or BD alone and is highly comorbid with agoraphobia, social phobia, posttraumatic stress disorder, and alcohol or drug addiction. Adults with ADHD/BD have more frequent affective episodes, suicide attempts, violence, and legal problems.4 Diagnosing this comorbidity remains a challenge, however, because:
- identifying which symptoms are caused by which disorder can be difficult
- BD tends to be underdiagnosed9
- patients often misidentify, underreport, or deny manic symptoms1,10,11
- if a patient presents with active bipolar symptoms, DSM-IV-TR criteria require that ADHD not be diagnosed until mood symptoms are resolved.
Overlapping symptoms. ADHD and bipolar mania share some DSM-IV-TR diagnostic criteria, including talkativeness, distractibility, increased activity or physical restlessness, and loss of social inhibitions (Table 1).12 Overlapping symptoms also are notable within ADHD diagnostic criteria (Table 2). In the inattention category, for example, “easily distracted by extraneous stimuli,” “difficulty sustaining attention in tasks,” and “fails to give close attention to details” are considered 3 separate symptoms. In the hyperactivity category, “often leaves seat,” “often runs about or climbs excessively,” and “often on the go, or often acts as if driven by a motor” are 3 separate symptoms.
Given ADHD’s relatively loose diagnostic criteria and high comorbidity in adults with mood disorders, the question of whether adult ADHD/BD represents comorbidity or diagnostic overlap remains unresolved. For the clinician, the disorders’ nonoverlapping features (Table 1) can assist with the differential diagnosis. For example:
- ADHD symptoms tend to be chronic and BD symptoms episodic.
- ADHD patients may have high energy but lack increased productivity seen in BD patients.
- ADHD patients do not need less sleep or have inflated self-esteem like symptomatic BD patients.
- Psychotic symptoms such as hallucinations or delusions might be present in severe BD but are absent in ADHD.
Table 1
Overlap between DSM-IV-TR diagnostic criteria for ADHD and bipolar mania
| Overlapping symptoms | |
|---|---|
| ADHD | Bipolar mania |
| Talks excessively | More talkative than usual |
| Easily distracted/jumps from one activity to the next | Distractibility or constant changes in activity or plans |
| Fidgets Difficulty remaining seated Runs or climbs about inappropriately Difficulty playing quietly On the go as if driven by a motor | Increased activity or physical restlessness |
| Interrupts or butts in uninvited Blurts out answers | Loss of normal social inhibitions |
| Nonoverlapping symptoms | |
| ADHD Forgetful in daily activities Difficulty awaiting turn Difficulty organizing self Loses things Avoids sustained mental effort Does not seem to listen Difficulty following through on instructions/fails to finish work Difficulty sustaining attention Fails to give close attention to details/makes careless mistakes | |
| Bipolar mania Inflated self-esteem/grandiosity Increase in goal-directed activity Flight of ideas Decreased need for sleep Excessive involvement in pleasurable activities with disregard for potential adverse consequences Marked sexual energy or sexual indiscretions | |
| ADHD: attention-deficit/hyperactivity disorder | |
| Source: Adapted and reprinted with permission from reference 12 | |
Table 2
DSM-IV-TR diagnostic criteria for attention-deficit/ hyperactivity disorder
| Inattention |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Hyperactivity/impulsivity |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Diagnosis requires evidence of inattention or hyperactivity/impulsivity or both |
| Some hyperactive/impulsive or inattentive symptoms that caused impairment were present before age 7 |
| Some impairment from symptoms is present in ≥2 settings (such as at school, work, or home) |
| Symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder (mood disorder, anxiety disorder, dissociative disorder, or a personality disorder) |
| Source: DSM-IV-TR |
Mood symptoms first
A diagnostic hierarchy is implicit in DSM-IV-TR; anxiety disorders are not diagnosed during an active major depressive or manic episode, and schizophrenia is not diagnosed on the basis of psychotic symptoms during an active major depressive or manic episode. Mood disorders sit atop this implied diagnostic hierarchy and must be ruled out before psychotic or anxiety disorders are diagnosed. Similarly, most personality disorders are not diagnosed during an active mood or psychotic episode.
Diagnosing adult ADHD when a patient is actively depressed or manic is inconsistent with this hierarchy and conflicts with extensive nosologic literature.13 We suggest that ADHD—a cognitive-behavioral problem—not be diagnosed solely on symptoms observed when a patient is experiencing a mood episode or psychotic illness.
Bipolar disorder. Two useful mnemonics (Table 3) assist in screening for DSM-IV-TR symptoms of BD type I:
- Pure mania consists of euphoric mood and ≥3 of 7 DIGFAST criteria, or irritable mood and ≥4 of 7 DIGFAST criteria
- Mixed mania consists of depressed mood with ≥4 of 7 DIGFAST criteria and ≥4 of 8 SIGECAPS criteria.
To be diagnostic, these symptoms must cause substantial social or occupational dysfunction and be present at least 1 week. Diagnose BD type I if a patient has experienced a single pure or mixed manic episode at any time, unless the episode had a medical cause such as hyperthyroidism or antidepressant use. Because patients with mixed episodes experience depressed mood, assess any patient with clinical depression for manic symptoms. Otherwise, a patient with a mixed episode could be misdiagnosed as having unipolar depression instead of BD type I.14
BD type II also has been observed in patients with comorbid adult ADHD/BD.4,6 The main difference between BD types I and II is that manic symptoms in type II are not severe enough to cause functional impairment or psychotic symptoms.15
Adult ADHD. The clinical interview seeking evidence of inattention and hyperactivity/impulsivity remains the basis of adult ADHD diagnosis (Table 2). Key areas are:
- the patient’s past and current functional impairment
- whether substantial impairment occurs in at least 2 areas of life (such as school, work, or home).
Take medical, educational, social, psychological, and vocational histories, and rule out other conditions before concluding that adult ADHD is the appropriate diagnosis.16 In adult ADHD, inattentive symptoms become far more prominent, about twice as common as hyperactive symptoms.17 Inattentive symptoms may manifest as neglect, poor time management, motivational deficits, or poor concentration that results in forgetfulness, distractibility, item misplacement, or excessive mistakes in paperwork.18 When impulsive symptoms persist in adults, they may manifest as automobile accidents or low tolerance for frustration, which may lead to frequent job changes and unstable, interrupted interpersonal relationships.18
Neuropsychological testing is not required to make an adult ADHD diagnosis but can help establish the breadth of symptoms or comorbidity.17 Rating scales can screen, gather data (including presence and severity of symptoms), and measure treatment response.16 Commonly used rating scales include:
- Conners’ Adult ADHD Rating Scales19
- Brown Attention Deficit Disorder Rating Scale for Adults20
- Adult ADHD Self-Report Scale.21
When using rating scales, remember that adult psychopathology can distort perceptions, and some self-report scales have questionable reliability.16
Table 3
Mnemonics for diagnostic symptoms of pure and mixed bipolar mania
| DIGFAST* for bipolar mania symptoms | SIGECAPS† bipolar depression symptoms |
|---|---|
| Distractibility Insomnia Grandiosity Flight of ideas Activities Speech Thoughtlessness | Sleep Interest Guilt Energy Concentration Appetite Psychomotor Suicide |
| Pure mania: Euphoric mood with ≥3 DIGFAST criteria or irritable mood with ≥4 DIGFAST criteria. | |
| Mixed mania: Depressed mood with ≥4 DIGFAST criteria and ≥4 SIGECAPS criteria. | |
| * Developed by William Falk, MD | |
| †Developed by Carey Gross, MD | |
| Source: Adapted from Ghaemi SN. Mood disorders. New York: Lippincott, Williams, & Wilkins; 2003 | |
Treatment recommendations
Limited data. We found only 1 study on adult ADHD/BD treatment. In this open trial,22 36 adults with comorbid ADHD and BD received bupropion SR, up to 200 mg bid, for ADHD symptoms while maintained on mood stabilizers, antipsychotics, or both. Improvement was defined as ≥30% reduction in ADHD Symptom Checklist Scale scores, without concurrent mania. After 6 weeks, 82% of patients had improved; 1 dropped out at week 2 because of hypomanic activation. Methodologic limitations included trial design (non-randomized, nonblinded, short duration) and patient selection (90% of subjects had BD type II).
In the absence of adequate data on adult ADHD/BD, studies in children suggest:
- stimulants may not be effective for ADHD symptoms in patients with active manic or depressive symptoms
- mood stabilization is a prerequisite for successful pharmacologic treatment of ADHD in patients with both ADHD and manic or depressive symptoms.23,24
Follow the hierarchy. First treat acute mood symptoms, then reevaluate and possibly treat ADHD symptoms if they persist during euthymia (Algorithm 1). When a patient meets criteria for adult ADHD/BD, first stabilize bipolar manic or depressive symptoms (Algorithm 2). For acute mania, treat with standard mood stabilizers (lithium, valproate, lamotrigine, or carbamazepine) with or without a second-generation antipsychotic.25 Starting stimulants for ADHD when patients have active mood symptoms is sub-optimal and potentially harmful because of the risk of inducing mania. For acute bipolar depression, adjunctive antidepressant treatment has been found to be no more effective than a mood stabilizer alone.26
After bipolar symptoms respond or remit, reassess for adult ADHD. If ADHD symptoms persist during euthymia, additional treatment may be indicated.
Very little evidence exists on treating adult ADHD/BD; as mentioned, bupropion is the only medication studied in this population. For adult ADHD alone, clinical trials have showed varying efficacy with bupropion,27,28 atomoxetine,29 venlafaxine,30,31 desipramine,32 methylphenidate,33 mixed amphetamine salts,34 and guanfacine.35 Whether these treatments can be generalized as safe and efficacious for comorbid adult ADHD/BD is unclear. Nonetheless, we suggest using bupropion first, followed by atomoxetine or guanfacine before you consider amphetamine stimulants (Algorithm 3).
Algorithm 1
Hierarchy for diagnosis and treatment of adult ADHD/BD

ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
*Adler LA, Chua HC. Management of ADHD in adults. J Clin Psychiatry 2002;63(suppl 12):29-35.
Algorithm 2
Treating acute episodes of bipolar disorder

ECT: electroconvulsive therapy; SGA: second-generation antipsychotic; TMS: transcranial magnetic stimulation
Algorithm 3
Suggested approach to adult ADHD with comorbid BD*

* Based on data extrapolated from samples of patients with ADHD alone because of very limited data in ADHD/BD samples.
† We recommend against combining antidepressants and stimulants because of additive risks of mania in BD. Discontinue stimulant or antidepressant if manic symptoms appear or rapid cycling emerges.
Reducing mania risk
Antidepressants and stimulants may help adults with ADHD alone, but risks of mania and rapid cycling limit their use in adults with ADHD/BD.
Stimulants and mania. One study found a 17% manic switch rate when methylphenidate (≤10 mg bid) was given to 14 bipolar depressed adults (10 BD type I, 2 BD type II, and 2 with secondary mania) taking mood stabilizers.36 A chart review of 82 bipolar children not taking mood stabilizers found an 18% switch rate with methylphenidate or amphetamine.37 Another chart review of 80 children with BD type I found that past amphetamine treatment (but not history of ADHD diagnosis or antidepressant treatment) was associated with more severe bipolar illness.38
No studies have examined predictors of amphetamine-induced mania. In our clinical experience, triggers are similar to those that can cause antidepressant-induced mania, such as:
- recent manic episodes
- current rapid cycling
- past antidepressant-induced mania.
Antidepressants and mania. When 64 patients with acute bipolar depression received both antidepressants and mood stabilizers in a randomized, double-blind trial, switch rates into mania or hypomania were 10% for bupropion, 9% for sertraline, and 29% for venlafaxine.39 In a meta analysis of clinical trials using selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs), the manic switch rate was threefold higher with TCAs than SSRIs.40 Antidepressant use in bipolar patients was associated with rapid cycling in the only randomized study of this topic.41
Insufficient data exist to clarify whether mania induction with antidepressants is dose-dependent.42 Factors associated with antidepressant-induced mania include:
- previous antidepressant-induced mania
- family history of BD
- exposure to multiple antidepressant trials42
- history of substance abuse and/or dependence.43
Related resources
- Bipolar disorder information and resources. www.psycheducation.org.
- ADHD Information and resources. www.adhdnews.com.
- Phelps J. Why am I still depressed? Recognizing and managing the ups and downs of bipolar II and soft bipolar disorder. New York: McGraw-Hill; 2006.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Wingo reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Ghaemi receives research grants from GlaxoSmithKline and Pfizer and is a speaker for GlaxoSmithKline, AstraZeneca, Pfizer, and Abbott Laboratories. Neither he nor his family hold equity positions in pharmaceutical companies.
Overlapping symptoms may obscure comorbid bipolar illness
An adult with function-impairing inattention could have attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), or both. Comorbid ADHD and BD often is unrecognized, however, because patients are more likely to report ADHD-related symptoms than manic symptoms.1
To help you recognize comorbid ADHD/BD—and protect adults who might switch into mania if given stimulants or antidepressants—this article describes a hierarchy to diagnose and treat this comorbidity. Based on the evidence and our experience, we:
- discuss how to differentiate between these disorders with overlapping symptoms
- provide tools and suggestions to screen for BD and adult ADHD
- offer 3 algorithms to guide your diagnosis and choice of medications.
Clinical challenges
Prevalence is unclear. Adult ADHD—with an estimated prevalence of 4.4%2—is more common than BD. Lifetime prevalences of BD types I and II are 1.6% and 0.5%, respectively.3 Studies of ADHD/BD comorbidity suggest wide-ranging prevalence rates:
Underdiagnosis. Adult ADHD/BD is a more severe illness than ADHD or BD alone and is highly comorbid with agoraphobia, social phobia, posttraumatic stress disorder, and alcohol or drug addiction. Adults with ADHD/BD have more frequent affective episodes, suicide attempts, violence, and legal problems.4 Diagnosing this comorbidity remains a challenge, however, because:
- identifying which symptoms are caused by which disorder can be difficult
- BD tends to be underdiagnosed9
- patients often misidentify, underreport, or deny manic symptoms1,10,11
- if a patient presents with active bipolar symptoms, DSM-IV-TR criteria require that ADHD not be diagnosed until mood symptoms are resolved.
Overlapping symptoms. ADHD and bipolar mania share some DSM-IV-TR diagnostic criteria, including talkativeness, distractibility, increased activity or physical restlessness, and loss of social inhibitions (Table 1).12 Overlapping symptoms also are notable within ADHD diagnostic criteria (Table 2). In the inattention category, for example, “easily distracted by extraneous stimuli,” “difficulty sustaining attention in tasks,” and “fails to give close attention to details” are considered 3 separate symptoms. In the hyperactivity category, “often leaves seat,” “often runs about or climbs excessively,” and “often on the go, or often acts as if driven by a motor” are 3 separate symptoms.
Given ADHD’s relatively loose diagnostic criteria and high comorbidity in adults with mood disorders, the question of whether adult ADHD/BD represents comorbidity or diagnostic overlap remains unresolved. For the clinician, the disorders’ nonoverlapping features (Table 1) can assist with the differential diagnosis. For example:
- ADHD symptoms tend to be chronic and BD symptoms episodic.
- ADHD patients may have high energy but lack increased productivity seen in BD patients.
- ADHD patients do not need less sleep or have inflated self-esteem like symptomatic BD patients.
- Psychotic symptoms such as hallucinations or delusions might be present in severe BD but are absent in ADHD.
Table 1
Overlap between DSM-IV-TR diagnostic criteria for ADHD and bipolar mania
| Overlapping symptoms | |
|---|---|
| ADHD | Bipolar mania |
| Talks excessively | More talkative than usual |
| Easily distracted/jumps from one activity to the next | Distractibility or constant changes in activity or plans |
| Fidgets Difficulty remaining seated Runs or climbs about inappropriately Difficulty playing quietly On the go as if driven by a motor | Increased activity or physical restlessness |
| Interrupts or butts in uninvited Blurts out answers | Loss of normal social inhibitions |
| Nonoverlapping symptoms | |
| ADHD Forgetful in daily activities Difficulty awaiting turn Difficulty organizing self Loses things Avoids sustained mental effort Does not seem to listen Difficulty following through on instructions/fails to finish work Difficulty sustaining attention Fails to give close attention to details/makes careless mistakes | |
| Bipolar mania Inflated self-esteem/grandiosity Increase in goal-directed activity Flight of ideas Decreased need for sleep Excessive involvement in pleasurable activities with disregard for potential adverse consequences Marked sexual energy or sexual indiscretions | |
| ADHD: attention-deficit/hyperactivity disorder | |
| Source: Adapted and reprinted with permission from reference 12 | |
Table 2
DSM-IV-TR diagnostic criteria for attention-deficit/ hyperactivity disorder
| Inattention |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Hyperactivity/impulsivity |
≥6 symptoms have persisted ≥6 months to a degree that is maladaptive and inconsistent with developmental level. The patient often:
|
| Diagnosis requires evidence of inattention or hyperactivity/impulsivity or both |
| Some hyperactive/impulsive or inattentive symptoms that caused impairment were present before age 7 |
| Some impairment from symptoms is present in ≥2 settings (such as at school, work, or home) |
| Symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder (mood disorder, anxiety disorder, dissociative disorder, or a personality disorder) |
| Source: DSM-IV-TR |
Mood symptoms first
A diagnostic hierarchy is implicit in DSM-IV-TR; anxiety disorders are not diagnosed during an active major depressive or manic episode, and schizophrenia is not diagnosed on the basis of psychotic symptoms during an active major depressive or manic episode. Mood disorders sit atop this implied diagnostic hierarchy and must be ruled out before psychotic or anxiety disorders are diagnosed. Similarly, most personality disorders are not diagnosed during an active mood or psychotic episode.
Diagnosing adult ADHD when a patient is actively depressed or manic is inconsistent with this hierarchy and conflicts with extensive nosologic literature.13 We suggest that ADHD—a cognitive-behavioral problem—not be diagnosed solely on symptoms observed when a patient is experiencing a mood episode or psychotic illness.
Bipolar disorder. Two useful mnemonics (Table 3) assist in screening for DSM-IV-TR symptoms of BD type I:
- Pure mania consists of euphoric mood and ≥3 of 7 DIGFAST criteria, or irritable mood and ≥4 of 7 DIGFAST criteria
- Mixed mania consists of depressed mood with ≥4 of 7 DIGFAST criteria and ≥4 of 8 SIGECAPS criteria.
To be diagnostic, these symptoms must cause substantial social or occupational dysfunction and be present at least 1 week. Diagnose BD type I if a patient has experienced a single pure or mixed manic episode at any time, unless the episode had a medical cause such as hyperthyroidism or antidepressant use. Because patients with mixed episodes experience depressed mood, assess any patient with clinical depression for manic symptoms. Otherwise, a patient with a mixed episode could be misdiagnosed as having unipolar depression instead of BD type I.14
BD type II also has been observed in patients with comorbid adult ADHD/BD.4,6 The main difference between BD types I and II is that manic symptoms in type II are not severe enough to cause functional impairment or psychotic symptoms.15
Adult ADHD. The clinical interview seeking evidence of inattention and hyperactivity/impulsivity remains the basis of adult ADHD diagnosis (Table 2). Key areas are:
- the patient’s past and current functional impairment
- whether substantial impairment occurs in at least 2 areas of life (such as school, work, or home).
Take medical, educational, social, psychological, and vocational histories, and rule out other conditions before concluding that adult ADHD is the appropriate diagnosis.16 In adult ADHD, inattentive symptoms become far more prominent, about twice as common as hyperactive symptoms.17 Inattentive symptoms may manifest as neglect, poor time management, motivational deficits, or poor concentration that results in forgetfulness, distractibility, item misplacement, or excessive mistakes in paperwork.18 When impulsive symptoms persist in adults, they may manifest as automobile accidents or low tolerance for frustration, which may lead to frequent job changes and unstable, interrupted interpersonal relationships.18
Neuropsychological testing is not required to make an adult ADHD diagnosis but can help establish the breadth of symptoms or comorbidity.17 Rating scales can screen, gather data (including presence and severity of symptoms), and measure treatment response.16 Commonly used rating scales include:
- Conners’ Adult ADHD Rating Scales19
- Brown Attention Deficit Disorder Rating Scale for Adults20
- Adult ADHD Self-Report Scale.21
When using rating scales, remember that adult psychopathology can distort perceptions, and some self-report scales have questionable reliability.16
Table 3
Mnemonics for diagnostic symptoms of pure and mixed bipolar mania
| DIGFAST* for bipolar mania symptoms | SIGECAPS† bipolar depression symptoms |
|---|---|
| Distractibility Insomnia Grandiosity Flight of ideas Activities Speech Thoughtlessness | Sleep Interest Guilt Energy Concentration Appetite Psychomotor Suicide |
| Pure mania: Euphoric mood with ≥3 DIGFAST criteria or irritable mood with ≥4 DIGFAST criteria. | |
| Mixed mania: Depressed mood with ≥4 DIGFAST criteria and ≥4 SIGECAPS criteria. | |
| * Developed by William Falk, MD | |
| †Developed by Carey Gross, MD | |
| Source: Adapted from Ghaemi SN. Mood disorders. New York: Lippincott, Williams, & Wilkins; 2003 | |
Treatment recommendations
Limited data. We found only 1 study on adult ADHD/BD treatment. In this open trial,22 36 adults with comorbid ADHD and BD received bupropion SR, up to 200 mg bid, for ADHD symptoms while maintained on mood stabilizers, antipsychotics, or both. Improvement was defined as ≥30% reduction in ADHD Symptom Checklist Scale scores, without concurrent mania. After 6 weeks, 82% of patients had improved; 1 dropped out at week 2 because of hypomanic activation. Methodologic limitations included trial design (non-randomized, nonblinded, short duration) and patient selection (90% of subjects had BD type II).
In the absence of adequate data on adult ADHD/BD, studies in children suggest:
- stimulants may not be effective for ADHD symptoms in patients with active manic or depressive symptoms
- mood stabilization is a prerequisite for successful pharmacologic treatment of ADHD in patients with both ADHD and manic or depressive symptoms.23,24
Follow the hierarchy. First treat acute mood symptoms, then reevaluate and possibly treat ADHD symptoms if they persist during euthymia (Algorithm 1). When a patient meets criteria for adult ADHD/BD, first stabilize bipolar manic or depressive symptoms (Algorithm 2). For acute mania, treat with standard mood stabilizers (lithium, valproate, lamotrigine, or carbamazepine) with or without a second-generation antipsychotic.25 Starting stimulants for ADHD when patients have active mood symptoms is sub-optimal and potentially harmful because of the risk of inducing mania. For acute bipolar depression, adjunctive antidepressant treatment has been found to be no more effective than a mood stabilizer alone.26
After bipolar symptoms respond or remit, reassess for adult ADHD. If ADHD symptoms persist during euthymia, additional treatment may be indicated.
Very little evidence exists on treating adult ADHD/BD; as mentioned, bupropion is the only medication studied in this population. For adult ADHD alone, clinical trials have showed varying efficacy with bupropion,27,28 atomoxetine,29 venlafaxine,30,31 desipramine,32 methylphenidate,33 mixed amphetamine salts,34 and guanfacine.35 Whether these treatments can be generalized as safe and efficacious for comorbid adult ADHD/BD is unclear. Nonetheless, we suggest using bupropion first, followed by atomoxetine or guanfacine before you consider amphetamine stimulants (Algorithm 3).
Algorithm 1
Hierarchy for diagnosis and treatment of adult ADHD/BD

ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
*Adler LA, Chua HC. Management of ADHD in adults. J Clin Psychiatry 2002;63(suppl 12):29-35.
Algorithm 2
Treating acute episodes of bipolar disorder

ECT: electroconvulsive therapy; SGA: second-generation antipsychotic; TMS: transcranial magnetic stimulation
Algorithm 3
Suggested approach to adult ADHD with comorbid BD*

* Based on data extrapolated from samples of patients with ADHD alone because of very limited data in ADHD/BD samples.
† We recommend against combining antidepressants and stimulants because of additive risks of mania in BD. Discontinue stimulant or antidepressant if manic symptoms appear or rapid cycling emerges.
Reducing mania risk
Antidepressants and stimulants may help adults with ADHD alone, but risks of mania and rapid cycling limit their use in adults with ADHD/BD.
Stimulants and mania. One study found a 17% manic switch rate when methylphenidate (≤10 mg bid) was given to 14 bipolar depressed adults (10 BD type I, 2 BD type II, and 2 with secondary mania) taking mood stabilizers.36 A chart review of 82 bipolar children not taking mood stabilizers found an 18% switch rate with methylphenidate or amphetamine.37 Another chart review of 80 children with BD type I found that past amphetamine treatment (but not history of ADHD diagnosis or antidepressant treatment) was associated with more severe bipolar illness.38
No studies have examined predictors of amphetamine-induced mania. In our clinical experience, triggers are similar to those that can cause antidepressant-induced mania, such as:
- recent manic episodes
- current rapid cycling
- past antidepressant-induced mania.
Antidepressants and mania. When 64 patients with acute bipolar depression received both antidepressants and mood stabilizers in a randomized, double-blind trial, switch rates into mania or hypomania were 10% for bupropion, 9% for sertraline, and 29% for venlafaxine.39 In a meta analysis of clinical trials using selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs), the manic switch rate was threefold higher with TCAs than SSRIs.40 Antidepressant use in bipolar patients was associated with rapid cycling in the only randomized study of this topic.41
Insufficient data exist to clarify whether mania induction with antidepressants is dose-dependent.42 Factors associated with antidepressant-induced mania include:
- previous antidepressant-induced mania
- family history of BD
- exposure to multiple antidepressant trials42
- history of substance abuse and/or dependence.43
Related resources
- Bipolar disorder information and resources. www.psycheducation.org.
- ADHD Information and resources. www.adhdnews.com.
- Phelps J. Why am I still depressed? Recognizing and managing the ups and downs of bipolar II and soft bipolar disorder. New York: McGraw-Hill; 2006.
Drug brand names
- Amphetamine/Dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Desipramine • Norpramin
- Dextroamphetamine • Dexedrine
- Guanfacine • Tenex
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Methylphenidate • Ritalin
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosures
Dr. Wingo reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Ghaemi receives research grants from GlaxoSmithKline and Pfizer and is a speaker for GlaxoSmithKline, AstraZeneca, Pfizer, and Abbott Laboratories. Neither he nor his family hold equity positions in pharmaceutical companies.
1. Ghaemi SN, Stoll AL, Pope HG, Jr, et al. Lack of insight in bipolar disorder. The acute manic episode. J Nerv Ment Dis 1995;183(7):464-7.
2. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
3. Sadock BJ, Sadock VA, eds. Synopsis of psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.
4. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
5. Tamam L, Tuglu C, Karatas G, et al. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
6. Wilens TE, Biederman J, Wozniak J, et al. Can adults with attention-deficit/hyperactivity disorder be distinguished from those with comorbid bipolar disorder? Findings from a sample of clinically referred adults. Biol Psychiatry 2003;54(1):1-8.
7. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry 2005;162(9):1621-7.
8. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006;163(10):1720-9.
9. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999;52(1-3):135-44.
10. Keitner GI, Solomon DA, Ryan CE, et al. Prodromal and residual symptoms in bipolar I disorder. Compr Psychiatry 1996;37(5):362-7.
11. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv 2001;52(1):51-5.
12. Kent L, Craddock N. Is there a relationship between attention deficit hyperactivity disorder and bipolar disorder? J Affect Disord 2003;73(3):211-21.
13. Surtees PG, Kendell RE. The hierarchy model of psychiatric symptomatology: an investigation based on present state examination ratings. Br J Psychiatry 1979;135:438-43.
14. Benazzi F. Symptoms of depression as possible markers of bipolar II disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):471-7.
15. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
16. Murphy KR, Adler LA. Assessing attention-deficit hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry 2004;65(suppl 3):12-17.
17. Adler LA. Diagnosing adult attention deficit hyperactivity disorder. Primary Psychiatry 2006;13(suppl 3):9-10.
18. Montano B. Diagnosis and treatment of ADHD in adults in primary care. J Clin Psychiatry 2004;65(suppl 3):18-21.
19. Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS). North Tonawanda, NY: Multi-Health Systems; 1999.
20. Brown TE. Brown Attention Deficit Disorder Scales. San Antonio, TX: The Psychological Corporation; 1996.
21. Adler LA, Kessler RC, Spencer T. Adult ADHD Self-report Scale v1.1 (ASRS-v1.1) Symptom Checklist. World Health Organization. Available at: http://www.med.nyu.edu/psych/assets/adhdscreen18.pdf. Accessed May 7, 2007.
22. Wilens TE, Prince JB, Spencer T, et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry 2003;54(1):9-16.
23. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
24. Daviss WB, Bentivoglio P, Racusin R, et al. Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry 2001;40(3):307-14.
25. Scherk H, Pajonk FG, Leucht SL. Second-generation antipsychotic agents in the treatment of acute mania. Arch Gen Psychiatry 2007;64:442-55.
26. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007:356:(17):1711-22.
27. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention deficit hyperactivity disorder: a randomized, placebo controlled study. Biol Psychiatry 2005;57:793-801.
28. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
29. Michelson D, Adler LA, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo controlled studies. Biol Psychiatry 2003;53:112-20.
30. Adler LA, Resnick S, Kunz M, Devinsky O. Open-label trial of venlafaxine in adults with attention deficit disorder. Psychopharmacol Bull 1995;31(4):785-8.
31. Hedges D, Reimherr FW, Rogers A, et al. An open trial of venlafaxine in adult patients with attention deficit hyperactivity disorder. Psychopharmacol Bull 1995;31(4):779-83.
32. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153(9):1147-53.
33. Faraone SV, Spencer T, Aleardi M, et al. Meta analysis of the efficacy of methylphenidate for treating adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2004;24(1):24-8.
34. Spencer T, Biederman J, Wilens TE, et al. Efficacy of a mixed amphetamine salts compound in adults with attention deficit hyperactivity disorder. Arch Gen Psychiatry 2001;58:775-82.
35. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2000;21(2):223-8.
36. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
37. Faedda GL, Baldessarini RJ, Glovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord 2004;82(1):149-58.
38. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70(3):323-7.
39. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006;189:124-31.
40. Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994;164(4):549-50.
41. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145(2):179-84.
42. Goldberg JF. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract. 2003;9(3):181-94.
43. Goldberg JF, Whiteside JE. The association between substance abuse and antidepressant-induced mania in bipolar disorder: a preliminary study. J Clin Psychiatry 2002;63(9):791-5.
1. Ghaemi SN, Stoll AL, Pope HG, Jr, et al. Lack of insight in bipolar disorder. The acute manic episode. J Nerv Ment Dis 1995;183(7):464-7.
2. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
3. Sadock BJ, Sadock VA, eds. Synopsis of psychiatry, 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2003.
4. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
5. Tamam L, Tuglu C, Karatas G, et al. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
6. Wilens TE, Biederman J, Wozniak J, et al. Can adults with attention-deficit/hyperactivity disorder be distinguished from those with comorbid bipolar disorder? Findings from a sample of clinically referred adults. Biol Psychiatry 2003;54(1):1-8.
7. McGough JJ, Smalley SL, McCracken JT, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry 2005;162(9):1621-7.
8. Faraone SV, Biederman J, Spencer T, et al. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006;163(10):1720-9.
9. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999;52(1-3):135-44.
10. Keitner GI, Solomon DA, Ryan CE, et al. Prodromal and residual symptoms in bipolar I disorder. Compr Psychiatry 1996;37(5):362-7.
11. Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv 2001;52(1):51-5.
12. Kent L, Craddock N. Is there a relationship between attention deficit hyperactivity disorder and bipolar disorder? J Affect Disord 2003;73(3):211-21.
13. Surtees PG, Kendell RE. The hierarchy model of psychiatric symptomatology: an investigation based on present state examination ratings. Br J Psychiatry 1979;135:438-43.
14. Benazzi F. Symptoms of depression as possible markers of bipolar II disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):471-7.
15. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association; 2000.
16. Murphy KR, Adler LA. Assessing attention-deficit hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry 2004;65(suppl 3):12-17.
17. Adler LA. Diagnosing adult attention deficit hyperactivity disorder. Primary Psychiatry 2006;13(suppl 3):9-10.
18. Montano B. Diagnosis and treatment of ADHD in adults in primary care. J Clin Psychiatry 2004;65(suppl 3):18-21.
19. Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS). North Tonawanda, NY: Multi-Health Systems; 1999.
20. Brown TE. Brown Attention Deficit Disorder Scales. San Antonio, TX: The Psychological Corporation; 1996.
21. Adler LA, Kessler RC, Spencer T. Adult ADHD Self-report Scale v1.1 (ASRS-v1.1) Symptom Checklist. World Health Organization. Available at: http://www.med.nyu.edu/psych/assets/adhdscreen18.pdf. Accessed May 7, 2007.
22. Wilens TE, Prince JB, Spencer T, et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry 2003;54(1):9-16.
23. Biederman J, Mick E, Prince J, et al. Systematic chart review of the pharmacologic treatment of comorbid attention deficit hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol 1999;9(4):247-56.
24. Daviss WB, Bentivoglio P, Racusin R, et al. Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry 2001;40(3):307-14.
25. Scherk H, Pajonk FG, Leucht SL. Second-generation antipsychotic agents in the treatment of acute mania. Arch Gen Psychiatry 2007;64:442-55.
26. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007:356:(17):1711-22.
27. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention deficit hyperactivity disorder: a randomized, placebo controlled study. Biol Psychiatry 2005;57:793-801.
28. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001;158(2):282-8.
29. Michelson D, Adler LA, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo controlled studies. Biol Psychiatry 2003;53:112-20.
30. Adler LA, Resnick S, Kunz M, Devinsky O. Open-label trial of venlafaxine in adults with attention deficit disorder. Psychopharmacol Bull 1995;31(4):785-8.
31. Hedges D, Reimherr FW, Rogers A, et al. An open trial of venlafaxine in adult patients with attention deficit hyperactivity disorder. Psychopharmacol Bull 1995;31(4):779-83.
32. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153(9):1147-53.
33. Faraone SV, Spencer T, Aleardi M, et al. Meta analysis of the efficacy of methylphenidate for treating adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2004;24(1):24-8.
34. Spencer T, Biederman J, Wilens TE, et al. Efficacy of a mixed amphetamine salts compound in adults with attention deficit hyperactivity disorder. Arch Gen Psychiatry 2001;58:775-82.
35. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit hyperactivity disorder. J Clin Psychopharmacol 2000;21(2):223-8.
36. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
37. Faedda GL, Baldessarini RJ, Glovinsky IP, et al. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord 2004;82(1):149-58.
38. Soutullo CA, DelBello MP, Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord 2002;70(3):323-7.
39. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006;189:124-31.
40. Peet M. Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994;164(4):549-50.
41. Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 1988;145(2):179-84.
42. Goldberg JF. When do antidepressants worsen the course of bipolar disorder? J Psychiatr Pract. 2003;9(3):181-94.
43. Goldberg JF, Whiteside JE. The association between substance abuse and antidepressant-induced mania in bipolar disorder: a preliminary study. J Clin Psychiatry 2002;63(9):791-5.
5 keys to good results with supportive psychotherapy
Supportive psychotherapy began as a second-class treatment whose only operating principle was “being friendly” with the patient (Box).1 Critics called it “simple-minded”2 and sniffed, “if it is supportive, it is not therapy…if it is therapy, it is not supportive.”3
Since its lowly beginning, however, supportive psychotherapy has been proven highly effective, and clinicians have developed operating principles that distinguish it from expressive psychotherapy (Table 1).4
To help you make good use of supportive psychotherapy, this article describes its evolution and:
- evidence that demonstrates its effectiveness
- 5 key components for clinical practice
- how to use it when treating challenging patients.
Table 1
Differences between expressive and supportive psychotherapy
| Component | Expressive psychotherapy | Supportive psychotherapy |
|---|---|---|
| Treatment goal | Insight | Reduce Symptoms |
| Therapist style | Opaque | Conversational (“real”) |
| Transference | Examine | Nurture positive transference |
| Regression | Enhance | Minimize |
| Unconscious | Explore | Focus on conscious material |
| Defenses | Interpret | Reinforce mature defenses |
| Source: Reference 4 | ||
A proven treatment
Effective long-term therapy. Much research on supportive psychotherapy comes from studies in which supportive psychotherapy was included as a “treatment as usual” comparison. In an extensive longitudinal study, for example, the Meninger Psychotherapy Research Project examined 42 patients receiving psychoanalysis, psychodynamic psychotherapy, or supportive psychotherapy over 25 years.5
Despite the institutional expertise in psychoanalysis and expressive psychotherapy, patients in supportive psychotherapy did just as well as those receiving the other treatments. Researchers found that each therapy carried more supportive elements than was intended, and supportive elements accounted for many of the observed changes. They concluded that:
- thinking of change in terms of “structural” vs “behavioral” was not useful
- change did not occur in proportion to resolving unconscious conflict.
Early psychotherapy consisted of directive methods by which Charcot, Freud, and others “suggested” that patients rid themselves of symptoms while under hypnotic trance. Beneficial effects were sometimes immediate and dramatic but rarely lasted.
Dissatisfied with directive techniques, clinicians developed psychoanalytic principles and expressive psychotherapy, which emphasizes analyzing transference and uncovering unconscious thoughts, feelings, and motivations. Although expressive psychotherapy became popular, many patients—especially those with severe mental illness—were deemed unsuitable candidates or failed to improve.
These patients were relegated to supportive interventions, which initially were vaguely defined methods to reduce anxiety and provide encouragement. Therapists required little or no specialized training to provide supportive therapy and did not expect patients to make character (or structural) change. Surprisingly, many patients improved despite vague therapeutic guidelines.
Source: Reference 1
Therapists in the behavior therapy group used a manualized, highly structured treatment protocol that included in vivo desensitization and homework. Therapists who used supportive psychotherapy simply encouraged patients to ventilate their feelings and discuss problems. Supportive therapists were instructed to be nondirective and avoid confrontation unless the patient proposed it.
Improving personality disorders. Several studies examined a form of supportive psychotherapy that used a manualized, structured protocol for treating higher functioning patients who traditionally have been treated with expressive psychotherapy. The protocol used a conversation-based, dyadic style to improve self-esteem and adaptive skills through data-based praise, advice, education, appropriate reassurance, anticipatory guidance, clarification, and confrontation. Under these reproducible conditions, supportive psychotherapy showed good efficacy compared with dynamic therapies for patients with depressive, anxiety, and personality disorders.
A review of studies from 1986 to 1992 found that supportive psychotherapy was effective for a variety of psychiatric and medical conditions, including schizophrenia, bipolar disorder, depression, posttraumatic stress disorder, anxiety disorders, personality disorders, substance abuse, and stress associated with breast cancer and back pain.9
CASE STUDY: A negative experience
Mrs. S, a 32-year-old grant writer, is referred to a psychiatrist by an emergency department physician after she cut herself following an argument with her husband. She has chronic dysthymia, thoughts of harming herself, low self-esteem, and indecision about her marriage.
Mrs. S was not receiving mental health treatment because her first experience with a psychiatrist had a poor outcome: “He hardly ever said anything; in fact, sometimes I wondered if he was sleeping. I needed advice desperately, and I was hoping to get some help and direction for my life. Instead he answered every question with a question, and I ended up getting more confused. I felt guilty, like I wasn’t being a good patient because I couldn’t think for myself. I felt like he thought I was stupid. He gave me some antidepressants, but after a few months of feeling even worse I stopped going and vowed to never see a therapist again.”
5 key components
Although all psychotherapies have some elements of support, effective supportive psychotherapy has 5 key components (Table 2).
- asking directive questions
- allowing inflection in your voice
- making gestures
- discussing opinions.
Table 2
5 components of supportive psychotherapy
|
CASE CONTINUED: Learning to cope
Mrs. S’ new psychiatrist starts her on an antidepressant and once-weekly supportive psychotherapy. For the initial sessions, the psychiatrist helps Mrs. S explore options for her highly conflicted marriage and strategies for coping with panic symptoms.
Mrs. S develops a strong feeling of attachment to the psychiatrist, sometimes projecting anger onto him by declaring that he does not care enough. Instead of interpreting this transference, the psychiatrist uses it as an opportunity to explore coping options Mrs. S can try when she feels unloved or rejected.
Nurture positive transference. A positive relationship is essential for the therapeutic alliance. In most instances, a patient naturally develops good feelings toward the therapist over time as a result of repeated empathic interchange. In supportive psychotherapy, you may acknowledge these good feelings but do not interpret them for unconscious underpinnings.
Address transference only if it is negative. If the patient develops hostility or anger toward you, use techniques to improve the relationship, such as:
- acknowledging the validity of the patient’s angry feelings
- gaining an understanding of your role in the conflict and apologizing if sincere
- offering solutions to improve the conflict
- providing reassurance that working through the conflict will strengthen the therapeutic relationship.
Reduce anxiety. In supportive psychotherapy, the primary goal is to lessen the patient’s suffering. Although the patient often must talk about stressful or painful topics, you can help him or her do so in a tolerable manner. Focus on making it easier for the patient to talk.
Reducing anxiety means not only helping the patient talk about painful matters but also allowing him or her to avoid topics that are too uncomfortable to endure. You can always “earmark” areas of concern for later discussion. This modulation of anxiety is consistent with the object relations approach proposed by Kohut,10 in which emotional pain is addressed in “small, psychologically manageable portions.”
Enhance self-esteem. Virtually all patients in supportive psychotherapy suffer from low self-esteem, so it is beneficial to help them feel better about themselves. Take an active role by using positive comments and acknowledgements (“plussing”) as well as compliments when appropriate.
Most patients with low self-esteem have defects in the ability to nurture or forgive themselves (“self-soothe”). Work with patients to enhance this ability by:
- plussing where appropriate
- correcting negative self-distortions or self-reproach
- educating patients on how to both placate and reward themselves.
Strengthen coping mechanisms. In supportive psychotherapy the therapist acts as a coach, giving the patient suggestions on how to cope with difficult matters. As part of treatment, you might assign the patient homework and instruct him to practice specific coping strategies.
CASE CONTINUED: Feeling stronger
Eventually Mrs. S is able to talk in a limited fashion about childhood sexual abuse. With her psychiatrist’s encouragement, she begins to write about her feelings in a journal and exercising to help her “feel strong.” The psychiatrist often acknowledges her struggle and compliments her attempts at coping in healthy ways. After a year of supportive psychotherapy Mrs. S is better able to modulate her feelings and make decisions without feeling overwhelmed.
An option for challenging patients
Psychotic disorders. Although it may seem intuitive that psychotic conditions are a contraindication for psychotherapy, patients with schizophrenia and other psychotic disorders often benefit immensely from supportive psychotherapy. A supportive therapist’s guiding influence can help psychotic patients cope with fractured social and family life, struggles with independence, loneliness, frequent disturbances of reality, stigmatization from society, and difficulty with decision-making.
During a patient’s acute psychotic episodes, you can draw on the therapeutic relationship you have established, strongly advising the patient to accept treatment when he or she is paranoid and rejecting help. In such situations, you might say, “Joe, you know me. You know that in the past I have helped you get through some tough times. You are going to have to trust me that you need this medicine now, even if you don’t want to take it.”
Borderline personality disorder. Supportive psychotherapy’s emphasis on reducing anxiety and nurturing a therapeutic relationship makes it a good treatment for patients with borderline personality disorder. The focus on adaptive skills, self-esteem, and higher order defenses—such as repression, sublimation, rationalization, intellectualization, inhibition, displacement, and humor—is particularly suitable for self-injurious and suicidal patients.11
In addition, dialectical behavior therapy is congruent with supportive psychotherapy.12 I have found it useful to let patients know I am experienced and strong enough to undergo therapy with them and can live with the chaos of their lives. This often comforts patients with borderline personality disorder, as their internal state conveys a sense of destruction not only for them but anyone close to them. From a psychoanalytic perspective, conveying a sense of safety is a core healing component of supportive therapy.13
Substance abuse. A lack of treatment response and therapist burn-out are recurrent problems when treating patients with substance abuse.14 I have found it useful to “stretch” my treatment timeline—for example, by measuring change in years instead of months—so that I don’t continually feel unsuccessful. This allows me to focus not on the patient’s immediate sobriety but instead on the supportive relationship, especially on helping the patient address his or her sense of guilt and failure, which frequently underpins substance abuse.
Helping your patient to reframe his or her substance abuse as “bad choices” instead of the actions of a “bad person” is essential. Accompanying the patient to an Alcoholics Anonymous meeting—“I’ll go with you to the first one, after that it is up to you”—can be a powerful intervention with lasting benefits.
Related resources
- Werman DS. The practice of supportive psychotherapy. New York: Brunner/Mazel; 1984.
- Winston A, Rosenthal RN, Pinsker H. Introduction to supportive psychotherapy. Arlington, VA: American Psychiatric Publishing, Inc; 2004.
- Pinsker H. A primer of supportive psychotherapy. Hillsdale, NJ. The Analytic Press; 1997.
- Imipramine • Tofranil
Dr. Battaglia is a consultant to Eli Lilly and Company.
1. Stewart RL. Psychoanalysis and psychoanalytic psychotherapy. In: Kaplan HI, Sadock BJ, eds. Comprehensive textbook of psychiatry/IV. Baltimore, MD: Williams & Wilkins; 1985:1331-65.
2. Sullivan PR. Learning theories and supportive psychotherapy. Am J Psychiatry 1971;128:763-6.
3. Crown S. Supportive psychotherapy: a contradiction in terms? Br J Psychiatry 1988;152:266-9.
4. Dewald P. Principles of supportive psychotherapy. Am J Psychother 1994;48(4):505-18.
5. Wallerstein RS. Psychoanalysis and psychotherapy: an historical perspective. Int J Psychoanal 1989;70:563-91.
6. Klein DF, Zitrin CM, Woerner MG, Ross DC. Treatment of phobias II. Behavior therapy and supportive psychotherapy: are there any specific ingredients? Arch Gen Psychiatry 1983;(40):139-45.
7. Hellerstein DJ, Rosenthal RN, Pinsker H, et al. A randomized prospective study comparing supportive and dynamic therapies. J Psychother Pract Res 1998;(7):261-71.
8. Rosenthal RN, Muran JC, Pinsker H, et al. Interpersonal change in brief supportive psychotherapy. J Psychother Pract Res 1999;(8):55-63.
9. Rockland LH. A review of supportive psychotherapy, 1986–1992. Hosp Community Psychiatry 1993;(44):1053-60.
10. Kohut H. The analysis of the self. New York: International Universities Press; 1971:229.
11. Aviram RB, Hellerstein DJ, Gerson J, Stanley B. Adapting supportive psychotherapy for individuals with borderline personality disorder who self-injure or attempt suicide. J Psychiatr Pract 2004;(10):145-55.
12. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: The Guilford Press; 1993.
13. Werman DS. On the mode of therapeutic action of psychoanalytic supportive psychotherapy. In: Rothstein A, ed. How does treatment help?: On the modes of therapeutic action of psychoanalytic psychotherapy. Madison, CT: International Universities Press; 1988:157–67.
14. Knudsen HK, Ducharme LJ, Roman PM. Counselor emotional exhaustion and turnover intention on therapeutic communities. J Subst Abuse Treat 2006;31(2):173-80.
Supportive psychotherapy began as a second-class treatment whose only operating principle was “being friendly” with the patient (Box).1 Critics called it “simple-minded”2 and sniffed, “if it is supportive, it is not therapy…if it is therapy, it is not supportive.”3
Since its lowly beginning, however, supportive psychotherapy has been proven highly effective, and clinicians have developed operating principles that distinguish it from expressive psychotherapy (Table 1).4
To help you make good use of supportive psychotherapy, this article describes its evolution and:
- evidence that demonstrates its effectiveness
- 5 key components for clinical practice
- how to use it when treating challenging patients.
Table 1
Differences between expressive and supportive psychotherapy
| Component | Expressive psychotherapy | Supportive psychotherapy |
|---|---|---|
| Treatment goal | Insight | Reduce Symptoms |
| Therapist style | Opaque | Conversational (“real”) |
| Transference | Examine | Nurture positive transference |
| Regression | Enhance | Minimize |
| Unconscious | Explore | Focus on conscious material |
| Defenses | Interpret | Reinforce mature defenses |
| Source: Reference 4 | ||
A proven treatment
Effective long-term therapy. Much research on supportive psychotherapy comes from studies in which supportive psychotherapy was included as a “treatment as usual” comparison. In an extensive longitudinal study, for example, the Meninger Psychotherapy Research Project examined 42 patients receiving psychoanalysis, psychodynamic psychotherapy, or supportive psychotherapy over 25 years.5
Despite the institutional expertise in psychoanalysis and expressive psychotherapy, patients in supportive psychotherapy did just as well as those receiving the other treatments. Researchers found that each therapy carried more supportive elements than was intended, and supportive elements accounted for many of the observed changes. They concluded that:
- thinking of change in terms of “structural” vs “behavioral” was not useful
- change did not occur in proportion to resolving unconscious conflict.
Early psychotherapy consisted of directive methods by which Charcot, Freud, and others “suggested” that patients rid themselves of symptoms while under hypnotic trance. Beneficial effects were sometimes immediate and dramatic but rarely lasted.
Dissatisfied with directive techniques, clinicians developed psychoanalytic principles and expressive psychotherapy, which emphasizes analyzing transference and uncovering unconscious thoughts, feelings, and motivations. Although expressive psychotherapy became popular, many patients—especially those with severe mental illness—were deemed unsuitable candidates or failed to improve.
These patients were relegated to supportive interventions, which initially were vaguely defined methods to reduce anxiety and provide encouragement. Therapists required little or no specialized training to provide supportive therapy and did not expect patients to make character (or structural) change. Surprisingly, many patients improved despite vague therapeutic guidelines.
Source: Reference 1
Therapists in the behavior therapy group used a manualized, highly structured treatment protocol that included in vivo desensitization and homework. Therapists who used supportive psychotherapy simply encouraged patients to ventilate their feelings and discuss problems. Supportive therapists were instructed to be nondirective and avoid confrontation unless the patient proposed it.
Improving personality disorders. Several studies examined a form of supportive psychotherapy that used a manualized, structured protocol for treating higher functioning patients who traditionally have been treated with expressive psychotherapy. The protocol used a conversation-based, dyadic style to improve self-esteem and adaptive skills through data-based praise, advice, education, appropriate reassurance, anticipatory guidance, clarification, and confrontation. Under these reproducible conditions, supportive psychotherapy showed good efficacy compared with dynamic therapies for patients with depressive, anxiety, and personality disorders.
A review of studies from 1986 to 1992 found that supportive psychotherapy was effective for a variety of psychiatric and medical conditions, including schizophrenia, bipolar disorder, depression, posttraumatic stress disorder, anxiety disorders, personality disorders, substance abuse, and stress associated with breast cancer and back pain.9
CASE STUDY: A negative experience
Mrs. S, a 32-year-old grant writer, is referred to a psychiatrist by an emergency department physician after she cut herself following an argument with her husband. She has chronic dysthymia, thoughts of harming herself, low self-esteem, and indecision about her marriage.
Mrs. S was not receiving mental health treatment because her first experience with a psychiatrist had a poor outcome: “He hardly ever said anything; in fact, sometimes I wondered if he was sleeping. I needed advice desperately, and I was hoping to get some help and direction for my life. Instead he answered every question with a question, and I ended up getting more confused. I felt guilty, like I wasn’t being a good patient because I couldn’t think for myself. I felt like he thought I was stupid. He gave me some antidepressants, but after a few months of feeling even worse I stopped going and vowed to never see a therapist again.”
5 key components
Although all psychotherapies have some elements of support, effective supportive psychotherapy has 5 key components (Table 2).
- asking directive questions
- allowing inflection in your voice
- making gestures
- discussing opinions.
Table 2
5 components of supportive psychotherapy
|
CASE CONTINUED: Learning to cope
Mrs. S’ new psychiatrist starts her on an antidepressant and once-weekly supportive psychotherapy. For the initial sessions, the psychiatrist helps Mrs. S explore options for her highly conflicted marriage and strategies for coping with panic symptoms.
Mrs. S develops a strong feeling of attachment to the psychiatrist, sometimes projecting anger onto him by declaring that he does not care enough. Instead of interpreting this transference, the psychiatrist uses it as an opportunity to explore coping options Mrs. S can try when she feels unloved or rejected.
Nurture positive transference. A positive relationship is essential for the therapeutic alliance. In most instances, a patient naturally develops good feelings toward the therapist over time as a result of repeated empathic interchange. In supportive psychotherapy, you may acknowledge these good feelings but do not interpret them for unconscious underpinnings.
Address transference only if it is negative. If the patient develops hostility or anger toward you, use techniques to improve the relationship, such as:
- acknowledging the validity of the patient’s angry feelings
- gaining an understanding of your role in the conflict and apologizing if sincere
- offering solutions to improve the conflict
- providing reassurance that working through the conflict will strengthen the therapeutic relationship.
Reduce anxiety. In supportive psychotherapy, the primary goal is to lessen the patient’s suffering. Although the patient often must talk about stressful or painful topics, you can help him or her do so in a tolerable manner. Focus on making it easier for the patient to talk.
Reducing anxiety means not only helping the patient talk about painful matters but also allowing him or her to avoid topics that are too uncomfortable to endure. You can always “earmark” areas of concern for later discussion. This modulation of anxiety is consistent with the object relations approach proposed by Kohut,10 in which emotional pain is addressed in “small, psychologically manageable portions.”
Enhance self-esteem. Virtually all patients in supportive psychotherapy suffer from low self-esteem, so it is beneficial to help them feel better about themselves. Take an active role by using positive comments and acknowledgements (“plussing”) as well as compliments when appropriate.
Most patients with low self-esteem have defects in the ability to nurture or forgive themselves (“self-soothe”). Work with patients to enhance this ability by:
- plussing where appropriate
- correcting negative self-distortions or self-reproach
- educating patients on how to both placate and reward themselves.
Strengthen coping mechanisms. In supportive psychotherapy the therapist acts as a coach, giving the patient suggestions on how to cope with difficult matters. As part of treatment, you might assign the patient homework and instruct him to practice specific coping strategies.
CASE CONTINUED: Feeling stronger
Eventually Mrs. S is able to talk in a limited fashion about childhood sexual abuse. With her psychiatrist’s encouragement, she begins to write about her feelings in a journal and exercising to help her “feel strong.” The psychiatrist often acknowledges her struggle and compliments her attempts at coping in healthy ways. After a year of supportive psychotherapy Mrs. S is better able to modulate her feelings and make decisions without feeling overwhelmed.
An option for challenging patients
Psychotic disorders. Although it may seem intuitive that psychotic conditions are a contraindication for psychotherapy, patients with schizophrenia and other psychotic disorders often benefit immensely from supportive psychotherapy. A supportive therapist’s guiding influence can help psychotic patients cope with fractured social and family life, struggles with independence, loneliness, frequent disturbances of reality, stigmatization from society, and difficulty with decision-making.
During a patient’s acute psychotic episodes, you can draw on the therapeutic relationship you have established, strongly advising the patient to accept treatment when he or she is paranoid and rejecting help. In such situations, you might say, “Joe, you know me. You know that in the past I have helped you get through some tough times. You are going to have to trust me that you need this medicine now, even if you don’t want to take it.”
Borderline personality disorder. Supportive psychotherapy’s emphasis on reducing anxiety and nurturing a therapeutic relationship makes it a good treatment for patients with borderline personality disorder. The focus on adaptive skills, self-esteem, and higher order defenses—such as repression, sublimation, rationalization, intellectualization, inhibition, displacement, and humor—is particularly suitable for self-injurious and suicidal patients.11
In addition, dialectical behavior therapy is congruent with supportive psychotherapy.12 I have found it useful to let patients know I am experienced and strong enough to undergo therapy with them and can live with the chaos of their lives. This often comforts patients with borderline personality disorder, as their internal state conveys a sense of destruction not only for them but anyone close to them. From a psychoanalytic perspective, conveying a sense of safety is a core healing component of supportive therapy.13
Substance abuse. A lack of treatment response and therapist burn-out are recurrent problems when treating patients with substance abuse.14 I have found it useful to “stretch” my treatment timeline—for example, by measuring change in years instead of months—so that I don’t continually feel unsuccessful. This allows me to focus not on the patient’s immediate sobriety but instead on the supportive relationship, especially on helping the patient address his or her sense of guilt and failure, which frequently underpins substance abuse.
Helping your patient to reframe his or her substance abuse as “bad choices” instead of the actions of a “bad person” is essential. Accompanying the patient to an Alcoholics Anonymous meeting—“I’ll go with you to the first one, after that it is up to you”—can be a powerful intervention with lasting benefits.
Related resources
- Werman DS. The practice of supportive psychotherapy. New York: Brunner/Mazel; 1984.
- Winston A, Rosenthal RN, Pinsker H. Introduction to supportive psychotherapy. Arlington, VA: American Psychiatric Publishing, Inc; 2004.
- Pinsker H. A primer of supportive psychotherapy. Hillsdale, NJ. The Analytic Press; 1997.
- Imipramine • Tofranil
Dr. Battaglia is a consultant to Eli Lilly and Company.
Supportive psychotherapy began as a second-class treatment whose only operating principle was “being friendly” with the patient (Box).1 Critics called it “simple-minded”2 and sniffed, “if it is supportive, it is not therapy…if it is therapy, it is not supportive.”3
Since its lowly beginning, however, supportive psychotherapy has been proven highly effective, and clinicians have developed operating principles that distinguish it from expressive psychotherapy (Table 1).4
To help you make good use of supportive psychotherapy, this article describes its evolution and:
- evidence that demonstrates its effectiveness
- 5 key components for clinical practice
- how to use it when treating challenging patients.
Table 1
Differences between expressive and supportive psychotherapy
| Component | Expressive psychotherapy | Supportive psychotherapy |
|---|---|---|
| Treatment goal | Insight | Reduce Symptoms |
| Therapist style | Opaque | Conversational (“real”) |
| Transference | Examine | Nurture positive transference |
| Regression | Enhance | Minimize |
| Unconscious | Explore | Focus on conscious material |
| Defenses | Interpret | Reinforce mature defenses |
| Source: Reference 4 | ||
A proven treatment
Effective long-term therapy. Much research on supportive psychotherapy comes from studies in which supportive psychotherapy was included as a “treatment as usual” comparison. In an extensive longitudinal study, for example, the Meninger Psychotherapy Research Project examined 42 patients receiving psychoanalysis, psychodynamic psychotherapy, or supportive psychotherapy over 25 years.5
Despite the institutional expertise in psychoanalysis and expressive psychotherapy, patients in supportive psychotherapy did just as well as those receiving the other treatments. Researchers found that each therapy carried more supportive elements than was intended, and supportive elements accounted for many of the observed changes. They concluded that:
- thinking of change in terms of “structural” vs “behavioral” was not useful
- change did not occur in proportion to resolving unconscious conflict.
Early psychotherapy consisted of directive methods by which Charcot, Freud, and others “suggested” that patients rid themselves of symptoms while under hypnotic trance. Beneficial effects were sometimes immediate and dramatic but rarely lasted.
Dissatisfied with directive techniques, clinicians developed psychoanalytic principles and expressive psychotherapy, which emphasizes analyzing transference and uncovering unconscious thoughts, feelings, and motivations. Although expressive psychotherapy became popular, many patients—especially those with severe mental illness—were deemed unsuitable candidates or failed to improve.
These patients were relegated to supportive interventions, which initially were vaguely defined methods to reduce anxiety and provide encouragement. Therapists required little or no specialized training to provide supportive therapy and did not expect patients to make character (or structural) change. Surprisingly, many patients improved despite vague therapeutic guidelines.
Source: Reference 1
Therapists in the behavior therapy group used a manualized, highly structured treatment protocol that included in vivo desensitization and homework. Therapists who used supportive psychotherapy simply encouraged patients to ventilate their feelings and discuss problems. Supportive therapists were instructed to be nondirective and avoid confrontation unless the patient proposed it.
Improving personality disorders. Several studies examined a form of supportive psychotherapy that used a manualized, structured protocol for treating higher functioning patients who traditionally have been treated with expressive psychotherapy. The protocol used a conversation-based, dyadic style to improve self-esteem and adaptive skills through data-based praise, advice, education, appropriate reassurance, anticipatory guidance, clarification, and confrontation. Under these reproducible conditions, supportive psychotherapy showed good efficacy compared with dynamic therapies for patients with depressive, anxiety, and personality disorders.
A review of studies from 1986 to 1992 found that supportive psychotherapy was effective for a variety of psychiatric and medical conditions, including schizophrenia, bipolar disorder, depression, posttraumatic stress disorder, anxiety disorders, personality disorders, substance abuse, and stress associated with breast cancer and back pain.9
CASE STUDY: A negative experience
Mrs. S, a 32-year-old grant writer, is referred to a psychiatrist by an emergency department physician after she cut herself following an argument with her husband. She has chronic dysthymia, thoughts of harming herself, low self-esteem, and indecision about her marriage.
Mrs. S was not receiving mental health treatment because her first experience with a psychiatrist had a poor outcome: “He hardly ever said anything; in fact, sometimes I wondered if he was sleeping. I needed advice desperately, and I was hoping to get some help and direction for my life. Instead he answered every question with a question, and I ended up getting more confused. I felt guilty, like I wasn’t being a good patient because I couldn’t think for myself. I felt like he thought I was stupid. He gave me some antidepressants, but after a few months of feeling even worse I stopped going and vowed to never see a therapist again.”
5 key components
Although all psychotherapies have some elements of support, effective supportive psychotherapy has 5 key components (Table 2).
- asking directive questions
- allowing inflection in your voice
- making gestures
- discussing opinions.
Table 2
5 components of supportive psychotherapy
|
CASE CONTINUED: Learning to cope
Mrs. S’ new psychiatrist starts her on an antidepressant and once-weekly supportive psychotherapy. For the initial sessions, the psychiatrist helps Mrs. S explore options for her highly conflicted marriage and strategies for coping with panic symptoms.
Mrs. S develops a strong feeling of attachment to the psychiatrist, sometimes projecting anger onto him by declaring that he does not care enough. Instead of interpreting this transference, the psychiatrist uses it as an opportunity to explore coping options Mrs. S can try when she feels unloved or rejected.
Nurture positive transference. A positive relationship is essential for the therapeutic alliance. In most instances, a patient naturally develops good feelings toward the therapist over time as a result of repeated empathic interchange. In supportive psychotherapy, you may acknowledge these good feelings but do not interpret them for unconscious underpinnings.
Address transference only if it is negative. If the patient develops hostility or anger toward you, use techniques to improve the relationship, such as:
- acknowledging the validity of the patient’s angry feelings
- gaining an understanding of your role in the conflict and apologizing if sincere
- offering solutions to improve the conflict
- providing reassurance that working through the conflict will strengthen the therapeutic relationship.
Reduce anxiety. In supportive psychotherapy, the primary goal is to lessen the patient’s suffering. Although the patient often must talk about stressful or painful topics, you can help him or her do so in a tolerable manner. Focus on making it easier for the patient to talk.
Reducing anxiety means not only helping the patient talk about painful matters but also allowing him or her to avoid topics that are too uncomfortable to endure. You can always “earmark” areas of concern for later discussion. This modulation of anxiety is consistent with the object relations approach proposed by Kohut,10 in which emotional pain is addressed in “small, psychologically manageable portions.”
Enhance self-esteem. Virtually all patients in supportive psychotherapy suffer from low self-esteem, so it is beneficial to help them feel better about themselves. Take an active role by using positive comments and acknowledgements (“plussing”) as well as compliments when appropriate.
Most patients with low self-esteem have defects in the ability to nurture or forgive themselves (“self-soothe”). Work with patients to enhance this ability by:
- plussing where appropriate
- correcting negative self-distortions or self-reproach
- educating patients on how to both placate and reward themselves.
Strengthen coping mechanisms. In supportive psychotherapy the therapist acts as a coach, giving the patient suggestions on how to cope with difficult matters. As part of treatment, you might assign the patient homework and instruct him to practice specific coping strategies.
CASE CONTINUED: Feeling stronger
Eventually Mrs. S is able to talk in a limited fashion about childhood sexual abuse. With her psychiatrist’s encouragement, she begins to write about her feelings in a journal and exercising to help her “feel strong.” The psychiatrist often acknowledges her struggle and compliments her attempts at coping in healthy ways. After a year of supportive psychotherapy Mrs. S is better able to modulate her feelings and make decisions without feeling overwhelmed.
An option for challenging patients
Psychotic disorders. Although it may seem intuitive that psychotic conditions are a contraindication for psychotherapy, patients with schizophrenia and other psychotic disorders often benefit immensely from supportive psychotherapy. A supportive therapist’s guiding influence can help psychotic patients cope with fractured social and family life, struggles with independence, loneliness, frequent disturbances of reality, stigmatization from society, and difficulty with decision-making.
During a patient’s acute psychotic episodes, you can draw on the therapeutic relationship you have established, strongly advising the patient to accept treatment when he or she is paranoid and rejecting help. In such situations, you might say, “Joe, you know me. You know that in the past I have helped you get through some tough times. You are going to have to trust me that you need this medicine now, even if you don’t want to take it.”
Borderline personality disorder. Supportive psychotherapy’s emphasis on reducing anxiety and nurturing a therapeutic relationship makes it a good treatment for patients with borderline personality disorder. The focus on adaptive skills, self-esteem, and higher order defenses—such as repression, sublimation, rationalization, intellectualization, inhibition, displacement, and humor—is particularly suitable for self-injurious and suicidal patients.11
In addition, dialectical behavior therapy is congruent with supportive psychotherapy.12 I have found it useful to let patients know I am experienced and strong enough to undergo therapy with them and can live with the chaos of their lives. This often comforts patients with borderline personality disorder, as their internal state conveys a sense of destruction not only for them but anyone close to them. From a psychoanalytic perspective, conveying a sense of safety is a core healing component of supportive therapy.13
Substance abuse. A lack of treatment response and therapist burn-out are recurrent problems when treating patients with substance abuse.14 I have found it useful to “stretch” my treatment timeline—for example, by measuring change in years instead of months—so that I don’t continually feel unsuccessful. This allows me to focus not on the patient’s immediate sobriety but instead on the supportive relationship, especially on helping the patient address his or her sense of guilt and failure, which frequently underpins substance abuse.
Helping your patient to reframe his or her substance abuse as “bad choices” instead of the actions of a “bad person” is essential. Accompanying the patient to an Alcoholics Anonymous meeting—“I’ll go with you to the first one, after that it is up to you”—can be a powerful intervention with lasting benefits.
Related resources
- Werman DS. The practice of supportive psychotherapy. New York: Brunner/Mazel; 1984.
- Winston A, Rosenthal RN, Pinsker H. Introduction to supportive psychotherapy. Arlington, VA: American Psychiatric Publishing, Inc; 2004.
- Pinsker H. A primer of supportive psychotherapy. Hillsdale, NJ. The Analytic Press; 1997.
- Imipramine • Tofranil
Dr. Battaglia is a consultant to Eli Lilly and Company.
1. Stewart RL. Psychoanalysis and psychoanalytic psychotherapy. In: Kaplan HI, Sadock BJ, eds. Comprehensive textbook of psychiatry/IV. Baltimore, MD: Williams & Wilkins; 1985:1331-65.
2. Sullivan PR. Learning theories and supportive psychotherapy. Am J Psychiatry 1971;128:763-6.
3. Crown S. Supportive psychotherapy: a contradiction in terms? Br J Psychiatry 1988;152:266-9.
4. Dewald P. Principles of supportive psychotherapy. Am J Psychother 1994;48(4):505-18.
5. Wallerstein RS. Psychoanalysis and psychotherapy: an historical perspective. Int J Psychoanal 1989;70:563-91.
6. Klein DF, Zitrin CM, Woerner MG, Ross DC. Treatment of phobias II. Behavior therapy and supportive psychotherapy: are there any specific ingredients? Arch Gen Psychiatry 1983;(40):139-45.
7. Hellerstein DJ, Rosenthal RN, Pinsker H, et al. A randomized prospective study comparing supportive and dynamic therapies. J Psychother Pract Res 1998;(7):261-71.
8. Rosenthal RN, Muran JC, Pinsker H, et al. Interpersonal change in brief supportive psychotherapy. J Psychother Pract Res 1999;(8):55-63.
9. Rockland LH. A review of supportive psychotherapy, 1986–1992. Hosp Community Psychiatry 1993;(44):1053-60.
10. Kohut H. The analysis of the self. New York: International Universities Press; 1971:229.
11. Aviram RB, Hellerstein DJ, Gerson J, Stanley B. Adapting supportive psychotherapy for individuals with borderline personality disorder who self-injure or attempt suicide. J Psychiatr Pract 2004;(10):145-55.
12. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: The Guilford Press; 1993.
13. Werman DS. On the mode of therapeutic action of psychoanalytic supportive psychotherapy. In: Rothstein A, ed. How does treatment help?: On the modes of therapeutic action of psychoanalytic psychotherapy. Madison, CT: International Universities Press; 1988:157–67.
14. Knudsen HK, Ducharme LJ, Roman PM. Counselor emotional exhaustion and turnover intention on therapeutic communities. J Subst Abuse Treat 2006;31(2):173-80.
1. Stewart RL. Psychoanalysis and psychoanalytic psychotherapy. In: Kaplan HI, Sadock BJ, eds. Comprehensive textbook of psychiatry/IV. Baltimore, MD: Williams & Wilkins; 1985:1331-65.
2. Sullivan PR. Learning theories and supportive psychotherapy. Am J Psychiatry 1971;128:763-6.
3. Crown S. Supportive psychotherapy: a contradiction in terms? Br J Psychiatry 1988;152:266-9.
4. Dewald P. Principles of supportive psychotherapy. Am J Psychother 1994;48(4):505-18.
5. Wallerstein RS. Psychoanalysis and psychotherapy: an historical perspective. Int J Psychoanal 1989;70:563-91.
6. Klein DF, Zitrin CM, Woerner MG, Ross DC. Treatment of phobias II. Behavior therapy and supportive psychotherapy: are there any specific ingredients? Arch Gen Psychiatry 1983;(40):139-45.
7. Hellerstein DJ, Rosenthal RN, Pinsker H, et al. A randomized prospective study comparing supportive and dynamic therapies. J Psychother Pract Res 1998;(7):261-71.
8. Rosenthal RN, Muran JC, Pinsker H, et al. Interpersonal change in brief supportive psychotherapy. J Psychother Pract Res 1999;(8):55-63.
9. Rockland LH. A review of supportive psychotherapy, 1986–1992. Hosp Community Psychiatry 1993;(44):1053-60.
10. Kohut H. The analysis of the self. New York: International Universities Press; 1971:229.
11. Aviram RB, Hellerstein DJ, Gerson J, Stanley B. Adapting supportive psychotherapy for individuals with borderline personality disorder who self-injure or attempt suicide. J Psychiatr Pract 2004;(10):145-55.
12. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York: The Guilford Press; 1993.
13. Werman DS. On the mode of therapeutic action of psychoanalytic supportive psychotherapy. In: Rothstein A, ed. How does treatment help?: On the modes of therapeutic action of psychoanalytic psychotherapy. Madison, CT: International Universities Press; 1988:157–67.
14. Knudsen HK, Ducharme LJ, Roman PM. Counselor emotional exhaustion and turnover intention on therapeutic communities. J Subst Abuse Treat 2006;31(2):173-80.
‘I want to leave now’: Handling discharge against medical advice
Patients voluntarily admitted to locked psychiatric intensive care units sometimes ask to leave against medical advice. They may minimize the severity of their acute illness or deny psychiatric symptoms to obtain a discharge.
Patient characteristics and provider procedures contribute to patients’ decisions to request a discharge against medical advice (DAMA) (Table).1
Table
Risk factors for discharge against medical advice
| Patient characteristics |
| Young (age 20 to 29) |
| Single marital status |
| Male |
| Comorbid personality or substance use disorder |
| Pessimistic attitude toward treatment |
| Antisocial, aggressive, or disruptive behavior |
| Numerous hospitalizations |
| History of discharge against medical advice |
| Provider characteristics |
| Failure to orient patient to hospitalization |
| Lack of a supportive provider-patient relationship |
| Discharge during evening or night shifts |
| Source: Reference 1 |
Not for everyone. Acutely psychotic, delusional, delirious, or demented patients or those with suicidal and homicidal ideation are not candidates for DAMA. For others, approach the patient calmly, explain with empathy what DAMA entails, and support the reasons for admission. Emphasize that following the treatment plan will alleviate psychiatric symptoms sooner and may shorten their stay.
Know the involuntary commitment procedures for your jurisdiction, and be prepared to discuss them with the patient. Assess the patient’s decision-making capacity, including awareness of the severity of his or her psychiatric illness and potential consequences of leaving against medical advice.
Arrange follow-up care for DAMA patients:
- Provide the patient with a brief summary of diagnosis, medications, and follow-up plans.
- Arrange the next available office or telephone appointment.
- Obtain contact information of those responsible for the patient’s safety.
- Provide the patient with emergency room and other phone numbers for crisis intervention.
DAMA does not absolve the physician of responsibility for poor outcomes. Carefully document the DAMA process because these patients are at increased risk of harm.2 Make sure the patient signs, dates, and notes the time on the DAMA request.
1. Brook M, Hilty DM, Liu W, et al. Discharge against medical advice from inpatient psychiatric treatment: a literature review. Psychiatr Serv 2006;57(8):1192-8.
2. Pages KP, Russo JE, Wingerson DK, et al. Predictors and outcome of discharge against medical advice from the psychiatric units of a general hospital. Psychiatr Serv 1998;49(9):1187-92.
Ms. Vanega is a nurse, Mental Health and Behavioral Science Department, Veterans Affairs Medical Center, Omaha, NE.
Dr. Ramakrishnan is a resident at Creighton University department of psychiatry, Omaha.
Dr. Ramaswamy is a director of psychopharmacology research, Creighton University, Omaha.
Patients voluntarily admitted to locked psychiatric intensive care units sometimes ask to leave against medical advice. They may minimize the severity of their acute illness or deny psychiatric symptoms to obtain a discharge.
Patient characteristics and provider procedures contribute to patients’ decisions to request a discharge against medical advice (DAMA) (Table).1
Table
Risk factors for discharge against medical advice
| Patient characteristics |
| Young (age 20 to 29) |
| Single marital status |
| Male |
| Comorbid personality or substance use disorder |
| Pessimistic attitude toward treatment |
| Antisocial, aggressive, or disruptive behavior |
| Numerous hospitalizations |
| History of discharge against medical advice |
| Provider characteristics |
| Failure to orient patient to hospitalization |
| Lack of a supportive provider-patient relationship |
| Discharge during evening or night shifts |
| Source: Reference 1 |
Not for everyone. Acutely psychotic, delusional, delirious, or demented patients or those with suicidal and homicidal ideation are not candidates for DAMA. For others, approach the patient calmly, explain with empathy what DAMA entails, and support the reasons for admission. Emphasize that following the treatment plan will alleviate psychiatric symptoms sooner and may shorten their stay.
Know the involuntary commitment procedures for your jurisdiction, and be prepared to discuss them with the patient. Assess the patient’s decision-making capacity, including awareness of the severity of his or her psychiatric illness and potential consequences of leaving against medical advice.
Arrange follow-up care for DAMA patients:
- Provide the patient with a brief summary of diagnosis, medications, and follow-up plans.
- Arrange the next available office or telephone appointment.
- Obtain contact information of those responsible for the patient’s safety.
- Provide the patient with emergency room and other phone numbers for crisis intervention.
DAMA does not absolve the physician of responsibility for poor outcomes. Carefully document the DAMA process because these patients are at increased risk of harm.2 Make sure the patient signs, dates, and notes the time on the DAMA request.
Patients voluntarily admitted to locked psychiatric intensive care units sometimes ask to leave against medical advice. They may minimize the severity of their acute illness or deny psychiatric symptoms to obtain a discharge.
Patient characteristics and provider procedures contribute to patients’ decisions to request a discharge against medical advice (DAMA) (Table).1
Table
Risk factors for discharge against medical advice
| Patient characteristics |
| Young (age 20 to 29) |
| Single marital status |
| Male |
| Comorbid personality or substance use disorder |
| Pessimistic attitude toward treatment |
| Antisocial, aggressive, or disruptive behavior |
| Numerous hospitalizations |
| History of discharge against medical advice |
| Provider characteristics |
| Failure to orient patient to hospitalization |
| Lack of a supportive provider-patient relationship |
| Discharge during evening or night shifts |
| Source: Reference 1 |
Not for everyone. Acutely psychotic, delusional, delirious, or demented patients or those with suicidal and homicidal ideation are not candidates for DAMA. For others, approach the patient calmly, explain with empathy what DAMA entails, and support the reasons for admission. Emphasize that following the treatment plan will alleviate psychiatric symptoms sooner and may shorten their stay.
Know the involuntary commitment procedures for your jurisdiction, and be prepared to discuss them with the patient. Assess the patient’s decision-making capacity, including awareness of the severity of his or her psychiatric illness and potential consequences of leaving against medical advice.
Arrange follow-up care for DAMA patients:
- Provide the patient with a brief summary of diagnosis, medications, and follow-up plans.
- Arrange the next available office or telephone appointment.
- Obtain contact information of those responsible for the patient’s safety.
- Provide the patient with emergency room and other phone numbers for crisis intervention.
DAMA does not absolve the physician of responsibility for poor outcomes. Carefully document the DAMA process because these patients are at increased risk of harm.2 Make sure the patient signs, dates, and notes the time on the DAMA request.
1. Brook M, Hilty DM, Liu W, et al. Discharge against medical advice from inpatient psychiatric treatment: a literature review. Psychiatr Serv 2006;57(8):1192-8.
2. Pages KP, Russo JE, Wingerson DK, et al. Predictors and outcome of discharge against medical advice from the psychiatric units of a general hospital. Psychiatr Serv 1998;49(9):1187-92.
Ms. Vanega is a nurse, Mental Health and Behavioral Science Department, Veterans Affairs Medical Center, Omaha, NE.
Dr. Ramakrishnan is a resident at Creighton University department of psychiatry, Omaha.
Dr. Ramaswamy is a director of psychopharmacology research, Creighton University, Omaha.
1. Brook M, Hilty DM, Liu W, et al. Discharge against medical advice from inpatient psychiatric treatment: a literature review. Psychiatr Serv 2006;57(8):1192-8.
2. Pages KP, Russo JE, Wingerson DK, et al. Predictors and outcome of discharge against medical advice from the psychiatric units of a general hospital. Psychiatr Serv 1998;49(9):1187-92.
Ms. Vanega is a nurse, Mental Health and Behavioral Science Department, Veterans Affairs Medical Center, Omaha, NE.
Dr. Ramakrishnan is a resident at Creighton University department of psychiatry, Omaha.
Dr. Ramaswamy is a director of psychopharmacology research, Creighton University, Omaha.
Do neural disconnects cause schizophrenia?
Advances in neuroimaging, cell biology, and post mortem analysis are starting to explain what happens in the brain of a person who develops schizophrenia. Schizophrenia appears to be a developmental disorder of disrupted neural connection within and between regions of the brain. These disruptions seem to result from genetic predispositions interacting with negative environmental events.
A matter of gray and white
Individuals with schizophrenia have deficits in gray matter and white matter, as illustrated by studies linking auditory hallucinations with brain regions associated with normal hearing (Box).
Gray matter. Magnetic resonance imaging (MRI) indicates that gray matter volume peaks in early adolescence and declines with age. The normal adolescent brain shrinks as inefficient neural connections are pruned away, a process that refines and matures gray matter. In individuals with schizophrenia, this reduction is more aggressive—perhaps because of excessive pruning—and occurs in the time frame when schizophrenia symptoms typically emerge.
Rapoport et al1 documented this process through sequential MRI scans in children with early-onset schizophrenia (mean age 14.5). Compared with age-matched healthy controls, youths with schizophrenia show greater and more rapid gray matter loss during late adolescence (Figure 1).2
Increased density. Reduced neuronal branching and spine formation also likely causes subtle reductions in gray matter volume (Figure 2). The resulting lack of dendritic connectivity may produce cognitive impairments and negative symptoms seen in schizophrenia.
Postmortem studies of gray matter cells show increased neuron density in patients with schizophrenia when compared with controls.3 Patients with schizophrenia have the same number of neurons as controls, but the neurons are more tightly packed because of reduced cell size, branching, and synapse formation.4
Research over the past decade has revealed schizophrenia to be a neurodegenerative disorder characterized by substantial brain tissue loss during first and subsequent psychotic episodes.5 Neuroimaging studies show that clinical and functional deterioration accompanies progressive loss of cortical gray matter volume and enlargement of cerebral ventricles. Thus, preventing relapses has come to be regarded as critical to long-term schizophrenia management.
Auditory hallucinations appear to emanate from the temporal lobe, the same brain region that processes external sound. Thus, it may be that patients experiencing hallucinations are misidentifying inner speech as coming from an outside source.
Using functional MRI to differentiate brain activity signals associated with hallucinating and nonhallucinating states, Dierks et al21 documented increased activity in auditory cortical gray matter during hallucinations in schizophrenia patients.
Auditory signals make synaptic connections in the thalamus (left) before reaching the auditory cortex. White matter fiber tracts called the arcuate fasciculus (right) connect the auditory cortex in the temporal lobe with Broca’s area in the frontal cortex.
Source: Adapted from reference 2
Using MR diffusion tensor imaging, Hubl et al22 identified white matter changes in the arcuate fasciculus of schizophrenia patients prone to hallucinations, compared with healthy controls and patients who had schizophrenia but not hallucinations.
These findings support the understanding that auditory hallucinations originate from altered connectivity of the same regions that process normal hearing and speech. The schizophrenia patient may perceive external voices from aberrant internal signals.
Figure 1 Rates of gray matter volume loss during adolescence
Youths with early-onset schizophrenia show greater gray matter volume loss during adolescence, compared with normal controls.
Source: Adapted from reference 2
Figure 2 Structural differences between neurons
in patients with schizophrenia and controls
Schizophrenic neurons show reduced soma size, spine formation, and dendritic branching
Source: Adapted from reference 2White matter. Recent research suggests that white matter deficits also may be involved in schizophrenia’s pathophysiology. Studies using diffusion tensor imaging (DTI)—which measures the sum of vectors of water diffusion along axons—have documented white matter impairments in patients with schizophrenia.6
White matter tracks—myelinated axons that transport electrical signals among neurons—connect regions within the cortex and between the cortex and deeper brain structures. Disruption of white matter tracks may degrade signals and confuse neuronal communication.
Myelination. Genetic studies in patients with schizophrenia also have suggested that decreased neuron myelination may play a role in white matter deficits. Hakak et al8 examined more than 6,000 genes using microarray analysis and found only 17 genes were significantly down-regulated in patients with schizophrenia. Of those 17 genes, 6 were related to myelin and 11 showed no pattern.
Oligodendrocytes are glial cells that insulate axons with myelin and allow faster transmission of electrical impulses in the brain. In a postmortem study, Hof et al7 found 7 patients schizophrenia had 28% fewer oligodendrocytes per section of the superior frontal gyrus and 27% less white matter compared with 7 age-matched controls (Figure 3).
Figure 3 Reduced neuron myelination possible in schizophrenia
In a postmortem analysis, stained white matter sections taken from schizophrenia patients had fewer oligodendrocytes, cells that insulate axons with myelin and facilitate electrical transmission.
Source: Adapted from reference 2
Genes and the environment
Schizophrenia’s heritability is among the most repeated research findings in psychiatry.9 Other mechanisms besides genetics must be involved, however, as studies consistently show that monozygotic twins have a concordance rate of approximately 50% for the development of schizophrenia.
Environmental factors. Adverse environmental events may act in conjuction with genetic predisposition to trigger schizophrenia development. Ischemia or an impoverished diet, for example, have the potential to change DNA methylation.
Environmental factors associated with increased risk for schizophrenia include:
- maternal starvation during pregnancy10
- prenatal exposure to influenza11
- obstetrical complications with hypoxia12
- being born and raised in an urban environment13
- using marijuana during adolescence.14
Gene expression. Important genes may be silenced in individuals with increased DNA methylation and a susceptible genetic profile. Alterations in gene expression are the fundamental mechanism of behavioral change. Research shows that environmental events can alter gene expression without changing the genetic code, such as by adding methyl groups to DNA.15,16 The silencing of important developmental genes in this way can have devastating effects on development.
One explanation for the development of schizophrenia is that environmental events in susceptible individuals silence the production of proteins essential for maintaining neuronal connections through methylation of DNA. Postmortem analysis of brains of patients with schizophrenia show reduced mRNA of reelin,17 a protein produced in gamma-aminobutyric acid neurons involved in neuronal migration, axon branching, and synapse formation during brain development. Lowered production of proteins such as reelin may reduce connections between neurons and cause schizophrenia symptoms. Two research groups also have reported increased methylation of reelin DNA in postmortem studies of the brains of patients with schizophrenia.18,19 Increased methylation of DNA would silence production of this important protein.
Preventing neural disconnects? If schizophrenia is a developmental disorder resulting from failures in brain connectivity, then the ultimate treatment may be prevention. Recent research suggests that intervening with second-generation antipsychotics during the prodromal stage can prevent or delay the emergence of the disorder.20 Further research is needed to establish whether early intervention can prevent schizophrenia’s neuronal disruption.
1. Gogtay N, Sporn A, Rapoport J. Structural brain MRI studies in childhood-onset schizophrenia and childhood atypical psychosis. In: Lawrie S, Johnstone E, Weinberger D, eds. Schizophrenia: from neuroimaging to neuroscience. New York, NY: Oxford University Press; 2004.
2. Higgins ES, George MS. The neuroscience of clinical psychiatry. Philadelphia: Lippincott, Williams, and Wilkins; 2007.
3. Selemon LD. Increased cortical neuronal density in schizophrenia. Am J Psychiatry 2004;161(9):1564.-
4. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000;57(1):65-73.
5. Csernansky JG. Neurodegeneration in schizophrenia: evidence from in vivo neuroimaging studies. Scientific World Journal 2007;7:135-43.
6. Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 2007;41(1-2):15-30.
7. Hof PR, Haroutunian V, Friedrich VL, Jr, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 2003;53(12):1075-85.
8. Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 2001;98(8):4746-51.
9. Shih RA, Belmonte PL, Zandi PP. A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. Int Rev Psychiatr 2004;16(4):260-83.
10. McClellan JM, Susser E, King MC. Maternal famine, de novo mutations, and schizophrenia. JAMA 2006;296(5):582-4.
11. Limosin F, Rouillon F, Payan C, et al. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr Scand 2003;107(5):331-5.
12. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 2002;159(7):1080-92.
13. Pedersen CB, Mortensen PB. Urbanization and traffic related exposures as risk factors for schizophrenia. BMC Psychiatry 2006;6:2.-
14. Arendt M, Rosenberg R, Foldager L, et al. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry 2005;187:510-5.
15. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics 2003;33(suppl):245-54.
16. Abdolmaleky HM, Smith CL, Faraone SV, et al. Methylomics in psychiatry: modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet B Neuropsychiatr Genet 2004;127(1):51-9.
17. Fatemi SH, Stary JM, Earle JA, et al. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res 2005;72(2-3):109-22.
18. Abdolmaleky HM, Cheng KH, Russo A, et al. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet 2005;134(1):60-6.
19. Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA 2005;102(26):9341-6.
20. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry 2006;163(5):790-799.
21. Dierks T, Linden DE, Jandl M, et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron 1999;22(3):615-21.
22. Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry 2004;61(7):658-68.
Adapted from The neuroscience of clinical psychiatry, by Edmund S. Higgins and Mark S. George. Philadelphia: Lippincott, Williams, and Wilkins; 2007:251-63.
Advances in neuroimaging, cell biology, and post mortem analysis are starting to explain what happens in the brain of a person who develops schizophrenia. Schizophrenia appears to be a developmental disorder of disrupted neural connection within and between regions of the brain. These disruptions seem to result from genetic predispositions interacting with negative environmental events.
A matter of gray and white
Individuals with schizophrenia have deficits in gray matter and white matter, as illustrated by studies linking auditory hallucinations with brain regions associated with normal hearing (Box).
Gray matter. Magnetic resonance imaging (MRI) indicates that gray matter volume peaks in early adolescence and declines with age. The normal adolescent brain shrinks as inefficient neural connections are pruned away, a process that refines and matures gray matter. In individuals with schizophrenia, this reduction is more aggressive—perhaps because of excessive pruning—and occurs in the time frame when schizophrenia symptoms typically emerge.
Rapoport et al1 documented this process through sequential MRI scans in children with early-onset schizophrenia (mean age 14.5). Compared with age-matched healthy controls, youths with schizophrenia show greater and more rapid gray matter loss during late adolescence (Figure 1).2
Increased density. Reduced neuronal branching and spine formation also likely causes subtle reductions in gray matter volume (Figure 2). The resulting lack of dendritic connectivity may produce cognitive impairments and negative symptoms seen in schizophrenia.
Postmortem studies of gray matter cells show increased neuron density in patients with schizophrenia when compared with controls.3 Patients with schizophrenia have the same number of neurons as controls, but the neurons are more tightly packed because of reduced cell size, branching, and synapse formation.4
Research over the past decade has revealed schizophrenia to be a neurodegenerative disorder characterized by substantial brain tissue loss during first and subsequent psychotic episodes.5 Neuroimaging studies show that clinical and functional deterioration accompanies progressive loss of cortical gray matter volume and enlargement of cerebral ventricles. Thus, preventing relapses has come to be regarded as critical to long-term schizophrenia management.
Auditory hallucinations appear to emanate from the temporal lobe, the same brain region that processes external sound. Thus, it may be that patients experiencing hallucinations are misidentifying inner speech as coming from an outside source.
Using functional MRI to differentiate brain activity signals associated with hallucinating and nonhallucinating states, Dierks et al21 documented increased activity in auditory cortical gray matter during hallucinations in schizophrenia patients.
Auditory signals make synaptic connections in the thalamus (left) before reaching the auditory cortex. White matter fiber tracts called the arcuate fasciculus (right) connect the auditory cortex in the temporal lobe with Broca’s area in the frontal cortex.
Source: Adapted from reference 2
Using MR diffusion tensor imaging, Hubl et al22 identified white matter changes in the arcuate fasciculus of schizophrenia patients prone to hallucinations, compared with healthy controls and patients who had schizophrenia but not hallucinations.
These findings support the understanding that auditory hallucinations originate from altered connectivity of the same regions that process normal hearing and speech. The schizophrenia patient may perceive external voices from aberrant internal signals.
Figure 1 Rates of gray matter volume loss during adolescence
Youths with early-onset schizophrenia show greater gray matter volume loss during adolescence, compared with normal controls.
Source: Adapted from reference 2
Figure 2 Structural differences between neurons
in patients with schizophrenia and controls
Schizophrenic neurons show reduced soma size, spine formation, and dendritic branching
Source: Adapted from reference 2White matter. Recent research suggests that white matter deficits also may be involved in schizophrenia’s pathophysiology. Studies using diffusion tensor imaging (DTI)—which measures the sum of vectors of water diffusion along axons—have documented white matter impairments in patients with schizophrenia.6
White matter tracks—myelinated axons that transport electrical signals among neurons—connect regions within the cortex and between the cortex and deeper brain structures. Disruption of white matter tracks may degrade signals and confuse neuronal communication.
Myelination. Genetic studies in patients with schizophrenia also have suggested that decreased neuron myelination may play a role in white matter deficits. Hakak et al8 examined more than 6,000 genes using microarray analysis and found only 17 genes were significantly down-regulated in patients with schizophrenia. Of those 17 genes, 6 were related to myelin and 11 showed no pattern.
Oligodendrocytes are glial cells that insulate axons with myelin and allow faster transmission of electrical impulses in the brain. In a postmortem study, Hof et al7 found 7 patients schizophrenia had 28% fewer oligodendrocytes per section of the superior frontal gyrus and 27% less white matter compared with 7 age-matched controls (Figure 3).
Figure 3 Reduced neuron myelination possible in schizophrenia
In a postmortem analysis, stained white matter sections taken from schizophrenia patients had fewer oligodendrocytes, cells that insulate axons with myelin and facilitate electrical transmission.
Source: Adapted from reference 2
Genes and the environment
Schizophrenia’s heritability is among the most repeated research findings in psychiatry.9 Other mechanisms besides genetics must be involved, however, as studies consistently show that monozygotic twins have a concordance rate of approximately 50% for the development of schizophrenia.
Environmental factors. Adverse environmental events may act in conjuction with genetic predisposition to trigger schizophrenia development. Ischemia or an impoverished diet, for example, have the potential to change DNA methylation.
Environmental factors associated with increased risk for schizophrenia include:
- maternal starvation during pregnancy10
- prenatal exposure to influenza11
- obstetrical complications with hypoxia12
- being born and raised in an urban environment13
- using marijuana during adolescence.14
Gene expression. Important genes may be silenced in individuals with increased DNA methylation and a susceptible genetic profile. Alterations in gene expression are the fundamental mechanism of behavioral change. Research shows that environmental events can alter gene expression without changing the genetic code, such as by adding methyl groups to DNA.15,16 The silencing of important developmental genes in this way can have devastating effects on development.
One explanation for the development of schizophrenia is that environmental events in susceptible individuals silence the production of proteins essential for maintaining neuronal connections through methylation of DNA. Postmortem analysis of brains of patients with schizophrenia show reduced mRNA of reelin,17 a protein produced in gamma-aminobutyric acid neurons involved in neuronal migration, axon branching, and synapse formation during brain development. Lowered production of proteins such as reelin may reduce connections between neurons and cause schizophrenia symptoms. Two research groups also have reported increased methylation of reelin DNA in postmortem studies of the brains of patients with schizophrenia.18,19 Increased methylation of DNA would silence production of this important protein.
Preventing neural disconnects? If schizophrenia is a developmental disorder resulting from failures in brain connectivity, then the ultimate treatment may be prevention. Recent research suggests that intervening with second-generation antipsychotics during the prodromal stage can prevent or delay the emergence of the disorder.20 Further research is needed to establish whether early intervention can prevent schizophrenia’s neuronal disruption.
Advances in neuroimaging, cell biology, and post mortem analysis are starting to explain what happens in the brain of a person who develops schizophrenia. Schizophrenia appears to be a developmental disorder of disrupted neural connection within and between regions of the brain. These disruptions seem to result from genetic predispositions interacting with negative environmental events.
A matter of gray and white
Individuals with schizophrenia have deficits in gray matter and white matter, as illustrated by studies linking auditory hallucinations with brain regions associated with normal hearing (Box).
Gray matter. Magnetic resonance imaging (MRI) indicates that gray matter volume peaks in early adolescence and declines with age. The normal adolescent brain shrinks as inefficient neural connections are pruned away, a process that refines and matures gray matter. In individuals with schizophrenia, this reduction is more aggressive—perhaps because of excessive pruning—and occurs in the time frame when schizophrenia symptoms typically emerge.
Rapoport et al1 documented this process through sequential MRI scans in children with early-onset schizophrenia (mean age 14.5). Compared with age-matched healthy controls, youths with schizophrenia show greater and more rapid gray matter loss during late adolescence (Figure 1).2
Increased density. Reduced neuronal branching and spine formation also likely causes subtle reductions in gray matter volume (Figure 2). The resulting lack of dendritic connectivity may produce cognitive impairments and negative symptoms seen in schizophrenia.
Postmortem studies of gray matter cells show increased neuron density in patients with schizophrenia when compared with controls.3 Patients with schizophrenia have the same number of neurons as controls, but the neurons are more tightly packed because of reduced cell size, branching, and synapse formation.4
Research over the past decade has revealed schizophrenia to be a neurodegenerative disorder characterized by substantial brain tissue loss during first and subsequent psychotic episodes.5 Neuroimaging studies show that clinical and functional deterioration accompanies progressive loss of cortical gray matter volume and enlargement of cerebral ventricles. Thus, preventing relapses has come to be regarded as critical to long-term schizophrenia management.
Auditory hallucinations appear to emanate from the temporal lobe, the same brain region that processes external sound. Thus, it may be that patients experiencing hallucinations are misidentifying inner speech as coming from an outside source.
Using functional MRI to differentiate brain activity signals associated with hallucinating and nonhallucinating states, Dierks et al21 documented increased activity in auditory cortical gray matter during hallucinations in schizophrenia patients.
Auditory signals make synaptic connections in the thalamus (left) before reaching the auditory cortex. White matter fiber tracts called the arcuate fasciculus (right) connect the auditory cortex in the temporal lobe with Broca’s area in the frontal cortex.
Source: Adapted from reference 2
Using MR diffusion tensor imaging, Hubl et al22 identified white matter changes in the arcuate fasciculus of schizophrenia patients prone to hallucinations, compared with healthy controls and patients who had schizophrenia but not hallucinations.
These findings support the understanding that auditory hallucinations originate from altered connectivity of the same regions that process normal hearing and speech. The schizophrenia patient may perceive external voices from aberrant internal signals.
Figure 1 Rates of gray matter volume loss during adolescence
Youths with early-onset schizophrenia show greater gray matter volume loss during adolescence, compared with normal controls.
Source: Adapted from reference 2
Figure 2 Structural differences between neurons
in patients with schizophrenia and controls
Schizophrenic neurons show reduced soma size, spine formation, and dendritic branching
Source: Adapted from reference 2White matter. Recent research suggests that white matter deficits also may be involved in schizophrenia’s pathophysiology. Studies using diffusion tensor imaging (DTI)—which measures the sum of vectors of water diffusion along axons—have documented white matter impairments in patients with schizophrenia.6
White matter tracks—myelinated axons that transport electrical signals among neurons—connect regions within the cortex and between the cortex and deeper brain structures. Disruption of white matter tracks may degrade signals and confuse neuronal communication.
Myelination. Genetic studies in patients with schizophrenia also have suggested that decreased neuron myelination may play a role in white matter deficits. Hakak et al8 examined more than 6,000 genes using microarray analysis and found only 17 genes were significantly down-regulated in patients with schizophrenia. Of those 17 genes, 6 were related to myelin and 11 showed no pattern.
Oligodendrocytes are glial cells that insulate axons with myelin and allow faster transmission of electrical impulses in the brain. In a postmortem study, Hof et al7 found 7 patients schizophrenia had 28% fewer oligodendrocytes per section of the superior frontal gyrus and 27% less white matter compared with 7 age-matched controls (Figure 3).
Figure 3 Reduced neuron myelination possible in schizophrenia
In a postmortem analysis, stained white matter sections taken from schizophrenia patients had fewer oligodendrocytes, cells that insulate axons with myelin and facilitate electrical transmission.
Source: Adapted from reference 2
Genes and the environment
Schizophrenia’s heritability is among the most repeated research findings in psychiatry.9 Other mechanisms besides genetics must be involved, however, as studies consistently show that monozygotic twins have a concordance rate of approximately 50% for the development of schizophrenia.
Environmental factors. Adverse environmental events may act in conjuction with genetic predisposition to trigger schizophrenia development. Ischemia or an impoverished diet, for example, have the potential to change DNA methylation.
Environmental factors associated with increased risk for schizophrenia include:
- maternal starvation during pregnancy10
- prenatal exposure to influenza11
- obstetrical complications with hypoxia12
- being born and raised in an urban environment13
- using marijuana during adolescence.14
Gene expression. Important genes may be silenced in individuals with increased DNA methylation and a susceptible genetic profile. Alterations in gene expression are the fundamental mechanism of behavioral change. Research shows that environmental events can alter gene expression without changing the genetic code, such as by adding methyl groups to DNA.15,16 The silencing of important developmental genes in this way can have devastating effects on development.
One explanation for the development of schizophrenia is that environmental events in susceptible individuals silence the production of proteins essential for maintaining neuronal connections through methylation of DNA. Postmortem analysis of brains of patients with schizophrenia show reduced mRNA of reelin,17 a protein produced in gamma-aminobutyric acid neurons involved in neuronal migration, axon branching, and synapse formation during brain development. Lowered production of proteins such as reelin may reduce connections between neurons and cause schizophrenia symptoms. Two research groups also have reported increased methylation of reelin DNA in postmortem studies of the brains of patients with schizophrenia.18,19 Increased methylation of DNA would silence production of this important protein.
Preventing neural disconnects? If schizophrenia is a developmental disorder resulting from failures in brain connectivity, then the ultimate treatment may be prevention. Recent research suggests that intervening with second-generation antipsychotics during the prodromal stage can prevent or delay the emergence of the disorder.20 Further research is needed to establish whether early intervention can prevent schizophrenia’s neuronal disruption.
1. Gogtay N, Sporn A, Rapoport J. Structural brain MRI studies in childhood-onset schizophrenia and childhood atypical psychosis. In: Lawrie S, Johnstone E, Weinberger D, eds. Schizophrenia: from neuroimaging to neuroscience. New York, NY: Oxford University Press; 2004.
2. Higgins ES, George MS. The neuroscience of clinical psychiatry. Philadelphia: Lippincott, Williams, and Wilkins; 2007.
3. Selemon LD. Increased cortical neuronal density in schizophrenia. Am J Psychiatry 2004;161(9):1564.-
4. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000;57(1):65-73.
5. Csernansky JG. Neurodegeneration in schizophrenia: evidence from in vivo neuroimaging studies. Scientific World Journal 2007;7:135-43.
6. Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 2007;41(1-2):15-30.
7. Hof PR, Haroutunian V, Friedrich VL, Jr, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 2003;53(12):1075-85.
8. Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 2001;98(8):4746-51.
9. Shih RA, Belmonte PL, Zandi PP. A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. Int Rev Psychiatr 2004;16(4):260-83.
10. McClellan JM, Susser E, King MC. Maternal famine, de novo mutations, and schizophrenia. JAMA 2006;296(5):582-4.
11. Limosin F, Rouillon F, Payan C, et al. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr Scand 2003;107(5):331-5.
12. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 2002;159(7):1080-92.
13. Pedersen CB, Mortensen PB. Urbanization and traffic related exposures as risk factors for schizophrenia. BMC Psychiatry 2006;6:2.-
14. Arendt M, Rosenberg R, Foldager L, et al. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry 2005;187:510-5.
15. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics 2003;33(suppl):245-54.
16. Abdolmaleky HM, Smith CL, Faraone SV, et al. Methylomics in psychiatry: modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet B Neuropsychiatr Genet 2004;127(1):51-9.
17. Fatemi SH, Stary JM, Earle JA, et al. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res 2005;72(2-3):109-22.
18. Abdolmaleky HM, Cheng KH, Russo A, et al. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet 2005;134(1):60-6.
19. Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA 2005;102(26):9341-6.
20. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry 2006;163(5):790-799.
21. Dierks T, Linden DE, Jandl M, et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron 1999;22(3):615-21.
22. Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry 2004;61(7):658-68.
Adapted from The neuroscience of clinical psychiatry, by Edmund S. Higgins and Mark S. George. Philadelphia: Lippincott, Williams, and Wilkins; 2007:251-63.
1. Gogtay N, Sporn A, Rapoport J. Structural brain MRI studies in childhood-onset schizophrenia and childhood atypical psychosis. In: Lawrie S, Johnstone E, Weinberger D, eds. Schizophrenia: from neuroimaging to neuroscience. New York, NY: Oxford University Press; 2004.
2. Higgins ES, George MS. The neuroscience of clinical psychiatry. Philadelphia: Lippincott, Williams, and Wilkins; 2007.
3. Selemon LD. Increased cortical neuronal density in schizophrenia. Am J Psychiatry 2004;161(9):1564.-
4. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000;57(1):65-73.
5. Csernansky JG. Neurodegeneration in schizophrenia: evidence from in vivo neuroimaging studies. Scientific World Journal 2007;7:135-43.
6. Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 2007;41(1-2):15-30.
7. Hof PR, Haroutunian V, Friedrich VL, Jr, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry 2003;53(12):1075-85.
8. Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 2001;98(8):4746-51.
9. Shih RA, Belmonte PL, Zandi PP. A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. Int Rev Psychiatr 2004;16(4):260-83.
10. McClellan JM, Susser E, King MC. Maternal famine, de novo mutations, and schizophrenia. JAMA 2006;296(5):582-4.
11. Limosin F, Rouillon F, Payan C, et al. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr Scand 2003;107(5):331-5.
12. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry 2002;159(7):1080-92.
13. Pedersen CB, Mortensen PB. Urbanization and traffic related exposures as risk factors for schizophrenia. BMC Psychiatry 2006;6:2.-
14. Arendt M, Rosenberg R, Foldager L, et al. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry 2005;187:510-5.
15. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics 2003;33(suppl):245-54.
16. Abdolmaleky HM, Smith CL, Faraone SV, et al. Methylomics in psychiatry: modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet B Neuropsychiatr Genet 2004;127(1):51-9.
17. Fatemi SH, Stary JM, Earle JA, et al. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res 2005;72(2-3):109-22.
18. Abdolmaleky HM, Cheng KH, Russo A, et al. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet 2005;134(1):60-6.
19. Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA 2005;102(26):9341-6.
20. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry 2006;163(5):790-799.
21. Dierks T, Linden DE, Jandl M, et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron 1999;22(3):615-21.
22. Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry 2004;61(7):658-68.
Adapted from The neuroscience of clinical psychiatry, by Edmund S. Higgins and Mark S. George. Philadelphia: Lippincott, Williams, and Wilkins; 2007:251-63.
Interpreting liver function tests
Mrs. W, age 53, is referred by her primary provider for consultation on depressive symptoms, including worsening depressed mood, anhedonia, anxiety, and suicidal thoughts for 2 months. She reports at least 2 similar episodes in the past 15 years. Mrs. W has a remote history of IV drug use and history of alcohol abuse, but she attends Alcoholics Anonymous and has 10 years of sobriety. She has no history of hospitalizations for medical illness and denies any medical problems.
Mrs. W is taking amitriptyline, 50 mg, for insomnia. She has no history of manic or psychotic symptoms, and the mental status examination is consistent with major depression. Her past depressive episodes were treated successfully with a medication that she does not recall.
The psychiatrist diagnoses recurrent and severe major depression and prescribes cognitive-behavioral therapy and sertraline, 25 mg/d, titrated to 50 mg/d over the next 2 weeks. Amitriptyline is discontinued.
When the psychiatrist receives Mrs. W’s medical records, electrolytes, complete blood count, thyroid stimulating hormone level, and fasting glucose are within normal limits, but alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are greatly elevated at 250 U/L and 150 U/L, respectively. Progress notes contain no references to liver disease.
Interpreting psychiatric patients’ liver function tests (LFTs) can be challenging, especially in those with polypharmacy, co-occurring substance abuse, or risk factors for viral hepatitis. You can improve collaboration with primary care providers by understanding:
- what an LFT measures
- how to interpret abnormal results
- which conditions to suspect, based on the results.
A standard LFT usually measures several enzymes and proteins, typically ALT, AST, alkaline phosphatase (ALP), total bilirubin (TBIL), albumin (ALB), and total protein (TP). Measures of gamma-glutamyl transpeptidase (GGT) and prothrombin time (PT) are often requested with an LFT. Table 1 provides normal ranges and ranges that indicate liver damage for several of these parameters.1,2
“Liver function test” is a misnomer because LFTs do not directly measure liver function. Rather, they reflect hepatocyte injury or cholestasis (blockage or damage in the biliary system). ALB and PT measure liver synthetic function, but are nonspecific. ALB levels can be altered by nutritional status, protein-losing enteropathies, or nephropathies, whereas PT may be modified by warfarin, vitamin K deficiency, or consumptive coagulopathy.
Table 1
Test results: what’s normal, what suggests liver damage
| Parameter | Description | Normal range | Range indicating liver damage |
|---|---|---|---|
| Alanine aminotransferase (ALT) | Enzyme highly concentrated in the liver | 3 to 30 U/L | >3 times upper limit of normal |
| Alkaline phosphatase (ALP) | Enzyme highly concentrated in the liver, bile ducts, placenta, and bone | 35 to 150 U/L | >2 times upper limit of normal |
| Aspartate aminotransferase (AST) | Enzyme highly concentrated in heart muscle, liver cells, skeletal muscle cells, and (to a lesser degree) other tissues | 11 to 32 U/L | Used to evaluate elevations in other serum enzyme level |
| Gamma-glutamyl transpeptidase (GGT) | Enzyme highly concentrated in the liver, bile ducts, and kidneys | 5 to 40 U/L | Used to evaluate elevations in other serum enzyme levels |
| Total bilirubin (TBIL) | Yellow bile pigment produced when liver processes waste products | 0.3 to 1.1 mg/dL | >2 times upper limit of normal if associated with elevation in ALT or ALP |
| Sources: References 1,2 | |||
CASE CONTINUED: Spotting a pattern of injury
Mrs. W’s elevated ALT and AST levels are of unknown duration. Her AST:ALT ratio is approximately 2:1, suggesting hepatocellular injury.
Interpreting abnormal LFT results
To properly interpret LFTs, consider the patient’s symptoms, physical exam findings, medical history, medical illnesses, potential substance use, risk factors for HIV and viral hepatitis, and medication list. Collaborate with the patient’s primary care provider or facilitate primary care (Figure 1).
ALT and AST are highly concentrated in the liver, but ALT is a more specific indicator of liver injury. For both, levels may vary according to age, sex, and ethnicity but in general, levels <30 U/L are considered normal.1,2
ALP originates predominately from the liver and from bone. Persistently elevated ALP levels in the liver may indicate chronic cholestasis or infiltrative liver disease.
GGT is best used to evaluate the meaning of elevations in other serum enzymes.3 Elevated GGT can help confirm hepatic origin of elevated ALP or support a suspicion of alcohol use in patients with an AST: ALT ratio >2:1.
If an asymptomatic patient has elevated LFT results, first repeat the test. If repeat results are normal, perform the test again in 3-6 months. Keep in mind, however, that normal LFT results do not always indicate the absence of disease. For example, up to 16% of patients with hepatitis C and 13% of patients with nonalcoholic steatohepatitis (NASH) have normal LFT results despite histologic abnormalities.4
If repeat results are abnormal, obtain the patient’s consent to inform the primary care provider. Then take a thorough history and perform a focused physical exam. In the history, focus on use of prescription and nonprescription medications, including over-the-counter and herbal therapies, alcohol, and drugs of abuse, such as MDMA (“ecstasy”), phencyclidine (“angel dust”), and glues or solvents. Also assess for risk factors for infectious hepatitis, such as IV drug use, work-related blood exposure, and tattoos. Ask about a family history of liver disease. Focus your physical exam on visible stigmata of chronic liver disease, such as jaundice, temporal wasting, ascites, and palmar erythema.
Next, analyze the severity and pattern of the LFT abnormality. Liver injury is defined as:
- ALT >3 times the upper limit of normal
- ALP >2 times the upper limit of normal
- or total bilirubin >2 times the upper limit of normal if associated with any elevation of ALT or ALP.5
If ALT elevations predominate, consider hepatocellular injury. If ALP elevations predominate, suspect cholestatic injury. Elevations of both ALT and ALP suggest a mixed pattern of hepatocellular and cholestatic injury.
Figure 1 Interpreting liver function test results
ALB: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transpeptidase; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; LFT: liver function test; PCP: primary care provider; TBIL: total bilirubin; TP: total protein
CASE CONTINUED: Pinpointing a diagnosis
Mrs. W undergoes repeat LFTs with GGT testing, screening tests for hepatitis B and C, and a comprehensive physical exam. The psychiatrist screens for alcohol use, asks the patient about her use of herbal therapies and substance abuse relapse, and evaluates cognitive mental status for symptoms of encephalopathy. Results reveal that Mrs. W’s ALT and AST are elevated because of chronic active hepatitis C.
Causes of hepatocellular injury
Further evaluation of your patient’s test results can help narrow down potential causes of liver damage. If your patient’s ALT is disproportionately elevated, estimate the:
- severity of aminotransferase elevation
- ratio of AST:ALT
- rate of change over multiple LFTs.
If AST or ALT is >10 times normal, consider toxin-induced or ischemic injury.6 An AST:ALT ratio of 2:1 or 3:1, especially when associated with elevated GGT, strongly suggests alcohol-induced injury. With acute mild transaminase elevations—ALT>AST, 2 to 3 times normal—suspect medication-related injury.
A variety of factors and conditions can result in hepatocellular damage:
Common causes
Medications. Many drugs, including common psychotropics, can cause elevated liver enzymes (Table 2).5 As little as 4 grams per day of acetaminophen can cause mild transaminitis.4 Antidepressants, second-generation antipsychotics (SGAs), and anticonvulsants can cause increases in AST and ALT.7 If liver enzymes rise after a patient starts a new medication, drug-related liver toxicity is likely. Remember to consider a patient’s use of drugs of abuse and herbal therapies.
Discontinuing the suspect agent usually produces steady (although sometimes slow) improvement in LFTs. Use serial LFT testing and focused history and physical examinations to confirm improvement.
Alcohol. Screen for alcohol abuse using the CAGE questionnaire, the Alcohol Use Disorders Identification Test, or a similar tool. More than 90% of patients with an AST:ALT ratio of 2:1 have alcoholic liver disease; this percentage increases to >96% when the ratio is 3:1.1 A 2-fold increase in GGT in a patient with an AST:ALT ratio >2:1 further supports the diagnosis. In patients with alcohol abuse, AST rarely exceeds 10 times normal.5
Hepatitis C. The prevalence of hepatitis C is increasing among patients with severe mental illness, especially a dual diagnosis.8 Hepatitis C rarely causes acute symptoms.
Offer a hepatitis C antibody screening to test patients with even a remote history of IV drug use or comorbid substance abuse. Patients with a positive hepatitis C antibody test or a negative hepatitis C antibody test but a high risk for the disease should receive further testing.9
Hepatitis B. Risk factors include exposure to blood, sexual transmission, and emigration from endemic areas in Southeast Asia and sub-Saharan Africa. Initial screening panels include tests for hepatitis B surface antigen, hepatitis B surface antibody, and hepatitis B core antibody. Positive B surface antigen and core antibody tests indicate infection.
NASH. Nonalcoholic steatohepatitis (NASH) is the most common cause of mild transaminitis in the Western world (Box).4,10,11
Nonalcoholic steatohepatitis (NASH) is inflammatory liver disease of uncertain pathogenesis that commonly occurs with metabolic syndrome. It affects up to 5% of Americans, most often those who are middle-aged and overweight or obese, hyperlipidemic, or diabetic. NASH resembles alcoholic liver disease but occurs in people who drink little or no alcohol. In addition to inflammation, it is characterized by accumulation of fat and fibrous tissue in the liver. Typically patients are asymptomatic, but NASH can lead to cirrhosis.
NASH is a common cause of mild transaminitis. Aminotransferase levels are usually <4 times the normal value.10 Thirteen percent of patients with NASH have normal LFT results despite histologic abnormalities.4 NASH is a diagnosis of exclusion that is confirmed by liver biopsy.
NASH has no specific therapies or cure. Treatment focuses on controlling associated conditions such as diabetes, obesity, and hyperlipidemia. In obese patients, weight loss is the cornerstone of treatment. If you prescribe a second-generation antipsychotic (SGA) for a patient who has NASH, be aware that SGAs increase the risk of hyperglycemia and dyslipidemia, which can exacerbate NASH.11
Table 2
Medications that affect liver function test (LFT) results
| Medication class | Hepatocellular injury (↑ALT) | Cholestatic injury (↑ALP and ↑ALT) | Mixed injury (↑ALP and ↑TBIL) |
|---|---|---|---|
| Psychotropic | Bupropion, fluoxetine, paroxetine, risperidone, sertraline, trazodone, valproic acid | Chlorpromazine, mirtazapine, tricyclic antidepressants | Amitriptyline, Amitriptyline, phenobarbital, phenytoin, trazodone |
| Cardiovascular | Amiodarone, lisinopril, losartan, statins | Clopidogrel, irbesartan | Captopril, enalapril, verapamil |
| Endocrine | Acarbose, allopurinol | – | – |
| Gastrointestinal | Omeprazole | – | – |
| Herbal remedies | Germander, kava | – | – |
| Anti-infectives | HAART drugs, isoniazid, ketoconazole, pyrazinamide, rifampin, tetracycline, trovafloxacin | Amoxicillin/clavulanic acid, erythromycins, terbinafine | Clindamycin, nitrofurantoin, trimethoprim-sulfamethoxazole |
| Rheumatologic | Acetaminophen, baclofen, methotrexate, NSAIDs | Anabolic steroids | Azathioprine, sulfonamides |
| Others | – | Oral contraceptives, estrogens, phenothiazines | Cyproheptadine, flutamide |
| ALP: alkaline phosphatase; ALT: alanine aminotransferase; HAART: highly active antiretroviral therapy; NSAID: nonsteroidal anti-inflammatory drug; TBIL: total bilirubin | |||
| Source: Reference 5 | |||
Less common causes
Hemochromatosis is an autosomal recessive disease that causes pathologic deposition of iron in the liver, pancreas, and heart and leads to cirrhosis, diabetes, and heart disease. Suspect it in patients with a clinical syndrome and transferrin saturation index >45%.12 A hemochromatosis gene mutation analysis confirms the diagnosis.
Autoimmune hepatitis occurs primarily in women ages 20 to 50 years.13 Because >80% of patients with autoimmune hepatitis have hypergammaglobulinemia, serum protein electrophores is a useful screening test.5
Wilson’s disease is a genetic disorder of biliary copper excretion classically diagnosed in young people with concomitant neurologic or psychiatric conditions. Those affected have low serum ceruloplasmin. Neuropsychiatric symptoms include parkinsonian-like tremor, rigidity, clumsiness of gait, slurred speech, drooling, and inappropriate and uncontrollable grinning (risus sardonicus).8 Psychosis and suicidality also are common in patients with Wilson’s disease.
Alpha-1 antitrypsin deficiency. Alpha-1 antitrypsin is a protein produced primarily in the liver that protects the lungs from neutrophil elastase. Suspect alpha-1 antitrypsin deficiency in patients with abnormal LFTs and emphysema. Low serum alpha-1 antitrypsin confirms the diagnosis.
Celiac disease. Consider celiac disease in patients with chronic diarrhea or abdominal distension and abnormal LFTs. Small bowel biopsy and elevated tissue transglutaminase antibodies and anti-endomysial antibodies confirm the diagnosis.
Causes of cholestatic injury
If your patient has a disproportionate ALP elevation, identify the source of the ALP by testing GGT. GGT levels are elevated in liver disease but not in bone disease. Partial bile duct obstruction is a common cause of ALP elevation. For initial testing, include ultrasonography of the right upper quadrant.
Medications. Many medications can cause biliary stasis and cholestatic LFTs, including mirtazapine, tricyclic antidepressants, anabolic steroids, phenytoin, and estrogens.
Primary sclerosing cholangitis is inflammatory disease of the bile ducts that can lead to cholestasis (blockage of bile transport blockage). Consider it in patients with inflammatory bowel disease. Endoscopic retrograde cholangiopancreatography and magnetic resonance cholangiopancreatography aid diagnosis.
Primary biliary cirrhosis generally presents in middle-aged women with other autoimmune processes, cholestasis, and pruritus. Consider testing serum antimitochondrial antibodies.
Infiltrative liver diseases such as sarcoidosis, metastatic disease, or lymphoma can also present with cholestasis. Liver imaging is required for these diagnoses.
Causes of mixed injury
If LFT results suggest a mixed injury pattern, focus on the predominant pattern and evaluate the causes listed above. Certain medications typically result in a mixed injury pattern.
Related resources
- American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. www.guideline.gov/summary/summary.aspx?ss=15&doc_id=3492&nbr=002718.
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician 1999;59(8):2223-30.
Drug brand names
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Chlorpromazine • Thorazine
- Fluoxetine • Prozac
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Warfarin • Coumadin
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137(1):1-10.
2. Gopal DV, Rosen HR. Abnormal findings on liver function tests. Interpreting results to narrow the diagnosis and establish a prognosis. Postgrad Med 2000;107(2):100-14.
3. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342(17):1266-71.
4. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol 2006;101(1):76-82.
5. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006;354(7):731-9.
6. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172(3):367-79.
7. Bezchilibny-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs, 15th ed. Cambridge Hogrefe & Huber; 2005.
8. Crone CC, Gabriel GM, Dimartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics 2006;47(3):188-205.
9. Kaplan MA. Approach to the patient with abnormal liver function tests. Available at: http://www.uptodate.com. Accessed December 12, 2006.
10. Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107(4):1103-9.
11. Lieberman JA, Stroup SS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
12. Yen AW, Fancher TL, Bowlus CL. Revisiting hereditary hemochromatosis: current concepts and progress. Am J Med 2006;119(5):391-9.
13. Krawitt EL. Autoimmune hepatitis. N Engl J Med 1996;334(14):897-903.
Mrs. W, age 53, is referred by her primary provider for consultation on depressive symptoms, including worsening depressed mood, anhedonia, anxiety, and suicidal thoughts for 2 months. She reports at least 2 similar episodes in the past 15 years. Mrs. W has a remote history of IV drug use and history of alcohol abuse, but she attends Alcoholics Anonymous and has 10 years of sobriety. She has no history of hospitalizations for medical illness and denies any medical problems.
Mrs. W is taking amitriptyline, 50 mg, for insomnia. She has no history of manic or psychotic symptoms, and the mental status examination is consistent with major depression. Her past depressive episodes were treated successfully with a medication that she does not recall.
The psychiatrist diagnoses recurrent and severe major depression and prescribes cognitive-behavioral therapy and sertraline, 25 mg/d, titrated to 50 mg/d over the next 2 weeks. Amitriptyline is discontinued.
When the psychiatrist receives Mrs. W’s medical records, electrolytes, complete blood count, thyroid stimulating hormone level, and fasting glucose are within normal limits, but alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are greatly elevated at 250 U/L and 150 U/L, respectively. Progress notes contain no references to liver disease.
Interpreting psychiatric patients’ liver function tests (LFTs) can be challenging, especially in those with polypharmacy, co-occurring substance abuse, or risk factors for viral hepatitis. You can improve collaboration with primary care providers by understanding:
- what an LFT measures
- how to interpret abnormal results
- which conditions to suspect, based on the results.
A standard LFT usually measures several enzymes and proteins, typically ALT, AST, alkaline phosphatase (ALP), total bilirubin (TBIL), albumin (ALB), and total protein (TP). Measures of gamma-glutamyl transpeptidase (GGT) and prothrombin time (PT) are often requested with an LFT. Table 1 provides normal ranges and ranges that indicate liver damage for several of these parameters.1,2
“Liver function test” is a misnomer because LFTs do not directly measure liver function. Rather, they reflect hepatocyte injury or cholestasis (blockage or damage in the biliary system). ALB and PT measure liver synthetic function, but are nonspecific. ALB levels can be altered by nutritional status, protein-losing enteropathies, or nephropathies, whereas PT may be modified by warfarin, vitamin K deficiency, or consumptive coagulopathy.
Table 1
Test results: what’s normal, what suggests liver damage
| Parameter | Description | Normal range | Range indicating liver damage |
|---|---|---|---|
| Alanine aminotransferase (ALT) | Enzyme highly concentrated in the liver | 3 to 30 U/L | >3 times upper limit of normal |
| Alkaline phosphatase (ALP) | Enzyme highly concentrated in the liver, bile ducts, placenta, and bone | 35 to 150 U/L | >2 times upper limit of normal |
| Aspartate aminotransferase (AST) | Enzyme highly concentrated in heart muscle, liver cells, skeletal muscle cells, and (to a lesser degree) other tissues | 11 to 32 U/L | Used to evaluate elevations in other serum enzyme level |
| Gamma-glutamyl transpeptidase (GGT) | Enzyme highly concentrated in the liver, bile ducts, and kidneys | 5 to 40 U/L | Used to evaluate elevations in other serum enzyme levels |
| Total bilirubin (TBIL) | Yellow bile pigment produced when liver processes waste products | 0.3 to 1.1 mg/dL | >2 times upper limit of normal if associated with elevation in ALT or ALP |
| Sources: References 1,2 | |||
CASE CONTINUED: Spotting a pattern of injury
Mrs. W’s elevated ALT and AST levels are of unknown duration. Her AST:ALT ratio is approximately 2:1, suggesting hepatocellular injury.
Interpreting abnormal LFT results
To properly interpret LFTs, consider the patient’s symptoms, physical exam findings, medical history, medical illnesses, potential substance use, risk factors for HIV and viral hepatitis, and medication list. Collaborate with the patient’s primary care provider or facilitate primary care (Figure 1).
ALT and AST are highly concentrated in the liver, but ALT is a more specific indicator of liver injury. For both, levels may vary according to age, sex, and ethnicity but in general, levels <30 U/L are considered normal.1,2
ALP originates predominately from the liver and from bone. Persistently elevated ALP levels in the liver may indicate chronic cholestasis or infiltrative liver disease.
GGT is best used to evaluate the meaning of elevations in other serum enzymes.3 Elevated GGT can help confirm hepatic origin of elevated ALP or support a suspicion of alcohol use in patients with an AST: ALT ratio >2:1.
If an asymptomatic patient has elevated LFT results, first repeat the test. If repeat results are normal, perform the test again in 3-6 months. Keep in mind, however, that normal LFT results do not always indicate the absence of disease. For example, up to 16% of patients with hepatitis C and 13% of patients with nonalcoholic steatohepatitis (NASH) have normal LFT results despite histologic abnormalities.4
If repeat results are abnormal, obtain the patient’s consent to inform the primary care provider. Then take a thorough history and perform a focused physical exam. In the history, focus on use of prescription and nonprescription medications, including over-the-counter and herbal therapies, alcohol, and drugs of abuse, such as MDMA (“ecstasy”), phencyclidine (“angel dust”), and glues or solvents. Also assess for risk factors for infectious hepatitis, such as IV drug use, work-related blood exposure, and tattoos. Ask about a family history of liver disease. Focus your physical exam on visible stigmata of chronic liver disease, such as jaundice, temporal wasting, ascites, and palmar erythema.
Next, analyze the severity and pattern of the LFT abnormality. Liver injury is defined as:
- ALT >3 times the upper limit of normal
- ALP >2 times the upper limit of normal
- or total bilirubin >2 times the upper limit of normal if associated with any elevation of ALT or ALP.5
If ALT elevations predominate, consider hepatocellular injury. If ALP elevations predominate, suspect cholestatic injury. Elevations of both ALT and ALP suggest a mixed pattern of hepatocellular and cholestatic injury.
Figure 1 Interpreting liver function test results
ALB: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transpeptidase; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; LFT: liver function test; PCP: primary care provider; TBIL: total bilirubin; TP: total protein
CASE CONTINUED: Pinpointing a diagnosis
Mrs. W undergoes repeat LFTs with GGT testing, screening tests for hepatitis B and C, and a comprehensive physical exam. The psychiatrist screens for alcohol use, asks the patient about her use of herbal therapies and substance abuse relapse, and evaluates cognitive mental status for symptoms of encephalopathy. Results reveal that Mrs. W’s ALT and AST are elevated because of chronic active hepatitis C.
Causes of hepatocellular injury
Further evaluation of your patient’s test results can help narrow down potential causes of liver damage. If your patient’s ALT is disproportionately elevated, estimate the:
- severity of aminotransferase elevation
- ratio of AST:ALT
- rate of change over multiple LFTs.
If AST or ALT is >10 times normal, consider toxin-induced or ischemic injury.6 An AST:ALT ratio of 2:1 or 3:1, especially when associated with elevated GGT, strongly suggests alcohol-induced injury. With acute mild transaminase elevations—ALT>AST, 2 to 3 times normal—suspect medication-related injury.
A variety of factors and conditions can result in hepatocellular damage:
Common causes
Medications. Many drugs, including common psychotropics, can cause elevated liver enzymes (Table 2).5 As little as 4 grams per day of acetaminophen can cause mild transaminitis.4 Antidepressants, second-generation antipsychotics (SGAs), and anticonvulsants can cause increases in AST and ALT.7 If liver enzymes rise after a patient starts a new medication, drug-related liver toxicity is likely. Remember to consider a patient’s use of drugs of abuse and herbal therapies.
Discontinuing the suspect agent usually produces steady (although sometimes slow) improvement in LFTs. Use serial LFT testing and focused history and physical examinations to confirm improvement.
Alcohol. Screen for alcohol abuse using the CAGE questionnaire, the Alcohol Use Disorders Identification Test, or a similar tool. More than 90% of patients with an AST:ALT ratio of 2:1 have alcoholic liver disease; this percentage increases to >96% when the ratio is 3:1.1 A 2-fold increase in GGT in a patient with an AST:ALT ratio >2:1 further supports the diagnosis. In patients with alcohol abuse, AST rarely exceeds 10 times normal.5
Hepatitis C. The prevalence of hepatitis C is increasing among patients with severe mental illness, especially a dual diagnosis.8 Hepatitis C rarely causes acute symptoms.
Offer a hepatitis C antibody screening to test patients with even a remote history of IV drug use or comorbid substance abuse. Patients with a positive hepatitis C antibody test or a negative hepatitis C antibody test but a high risk for the disease should receive further testing.9
Hepatitis B. Risk factors include exposure to blood, sexual transmission, and emigration from endemic areas in Southeast Asia and sub-Saharan Africa. Initial screening panels include tests for hepatitis B surface antigen, hepatitis B surface antibody, and hepatitis B core antibody. Positive B surface antigen and core antibody tests indicate infection.
NASH. Nonalcoholic steatohepatitis (NASH) is the most common cause of mild transaminitis in the Western world (Box).4,10,11
Nonalcoholic steatohepatitis (NASH) is inflammatory liver disease of uncertain pathogenesis that commonly occurs with metabolic syndrome. It affects up to 5% of Americans, most often those who are middle-aged and overweight or obese, hyperlipidemic, or diabetic. NASH resembles alcoholic liver disease but occurs in people who drink little or no alcohol. In addition to inflammation, it is characterized by accumulation of fat and fibrous tissue in the liver. Typically patients are asymptomatic, but NASH can lead to cirrhosis.
NASH is a common cause of mild transaminitis. Aminotransferase levels are usually <4 times the normal value.10 Thirteen percent of patients with NASH have normal LFT results despite histologic abnormalities.4 NASH is a diagnosis of exclusion that is confirmed by liver biopsy.
NASH has no specific therapies or cure. Treatment focuses on controlling associated conditions such as diabetes, obesity, and hyperlipidemia. In obese patients, weight loss is the cornerstone of treatment. If you prescribe a second-generation antipsychotic (SGA) for a patient who has NASH, be aware that SGAs increase the risk of hyperglycemia and dyslipidemia, which can exacerbate NASH.11
Table 2
Medications that affect liver function test (LFT) results
| Medication class | Hepatocellular injury (↑ALT) | Cholestatic injury (↑ALP and ↑ALT) | Mixed injury (↑ALP and ↑TBIL) |
|---|---|---|---|
| Psychotropic | Bupropion, fluoxetine, paroxetine, risperidone, sertraline, trazodone, valproic acid | Chlorpromazine, mirtazapine, tricyclic antidepressants | Amitriptyline, Amitriptyline, phenobarbital, phenytoin, trazodone |
| Cardiovascular | Amiodarone, lisinopril, losartan, statins | Clopidogrel, irbesartan | Captopril, enalapril, verapamil |
| Endocrine | Acarbose, allopurinol | – | – |
| Gastrointestinal | Omeprazole | – | – |
| Herbal remedies | Germander, kava | – | – |
| Anti-infectives | HAART drugs, isoniazid, ketoconazole, pyrazinamide, rifampin, tetracycline, trovafloxacin | Amoxicillin/clavulanic acid, erythromycins, terbinafine | Clindamycin, nitrofurantoin, trimethoprim-sulfamethoxazole |
| Rheumatologic | Acetaminophen, baclofen, methotrexate, NSAIDs | Anabolic steroids | Azathioprine, sulfonamides |
| Others | – | Oral contraceptives, estrogens, phenothiazines | Cyproheptadine, flutamide |
| ALP: alkaline phosphatase; ALT: alanine aminotransferase; HAART: highly active antiretroviral therapy; NSAID: nonsteroidal anti-inflammatory drug; TBIL: total bilirubin | |||
| Source: Reference 5 | |||
Less common causes
Hemochromatosis is an autosomal recessive disease that causes pathologic deposition of iron in the liver, pancreas, and heart and leads to cirrhosis, diabetes, and heart disease. Suspect it in patients with a clinical syndrome and transferrin saturation index >45%.12 A hemochromatosis gene mutation analysis confirms the diagnosis.
Autoimmune hepatitis occurs primarily in women ages 20 to 50 years.13 Because >80% of patients with autoimmune hepatitis have hypergammaglobulinemia, serum protein electrophores is a useful screening test.5
Wilson’s disease is a genetic disorder of biliary copper excretion classically diagnosed in young people with concomitant neurologic or psychiatric conditions. Those affected have low serum ceruloplasmin. Neuropsychiatric symptoms include parkinsonian-like tremor, rigidity, clumsiness of gait, slurred speech, drooling, and inappropriate and uncontrollable grinning (risus sardonicus).8 Psychosis and suicidality also are common in patients with Wilson’s disease.
Alpha-1 antitrypsin deficiency. Alpha-1 antitrypsin is a protein produced primarily in the liver that protects the lungs from neutrophil elastase. Suspect alpha-1 antitrypsin deficiency in patients with abnormal LFTs and emphysema. Low serum alpha-1 antitrypsin confirms the diagnosis.
Celiac disease. Consider celiac disease in patients with chronic diarrhea or abdominal distension and abnormal LFTs. Small bowel biopsy and elevated tissue transglutaminase antibodies and anti-endomysial antibodies confirm the diagnosis.
Causes of cholestatic injury
If your patient has a disproportionate ALP elevation, identify the source of the ALP by testing GGT. GGT levels are elevated in liver disease but not in bone disease. Partial bile duct obstruction is a common cause of ALP elevation. For initial testing, include ultrasonography of the right upper quadrant.
Medications. Many medications can cause biliary stasis and cholestatic LFTs, including mirtazapine, tricyclic antidepressants, anabolic steroids, phenytoin, and estrogens.
Primary sclerosing cholangitis is inflammatory disease of the bile ducts that can lead to cholestasis (blockage of bile transport blockage). Consider it in patients with inflammatory bowel disease. Endoscopic retrograde cholangiopancreatography and magnetic resonance cholangiopancreatography aid diagnosis.
Primary biliary cirrhosis generally presents in middle-aged women with other autoimmune processes, cholestasis, and pruritus. Consider testing serum antimitochondrial antibodies.
Infiltrative liver diseases such as sarcoidosis, metastatic disease, or lymphoma can also present with cholestasis. Liver imaging is required for these diagnoses.
Causes of mixed injury
If LFT results suggest a mixed injury pattern, focus on the predominant pattern and evaluate the causes listed above. Certain medications typically result in a mixed injury pattern.
Related resources
- American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. www.guideline.gov/summary/summary.aspx?ss=15&doc_id=3492&nbr=002718.
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician 1999;59(8):2223-30.
Drug brand names
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Chlorpromazine • Thorazine
- Fluoxetine • Prozac
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Warfarin • Coumadin
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mrs. W, age 53, is referred by her primary provider for consultation on depressive symptoms, including worsening depressed mood, anhedonia, anxiety, and suicidal thoughts for 2 months. She reports at least 2 similar episodes in the past 15 years. Mrs. W has a remote history of IV drug use and history of alcohol abuse, but she attends Alcoholics Anonymous and has 10 years of sobriety. She has no history of hospitalizations for medical illness and denies any medical problems.
Mrs. W is taking amitriptyline, 50 mg, for insomnia. She has no history of manic or psychotic symptoms, and the mental status examination is consistent with major depression. Her past depressive episodes were treated successfully with a medication that she does not recall.
The psychiatrist diagnoses recurrent and severe major depression and prescribes cognitive-behavioral therapy and sertraline, 25 mg/d, titrated to 50 mg/d over the next 2 weeks. Amitriptyline is discontinued.
When the psychiatrist receives Mrs. W’s medical records, electrolytes, complete blood count, thyroid stimulating hormone level, and fasting glucose are within normal limits, but alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are greatly elevated at 250 U/L and 150 U/L, respectively. Progress notes contain no references to liver disease.
Interpreting psychiatric patients’ liver function tests (LFTs) can be challenging, especially in those with polypharmacy, co-occurring substance abuse, or risk factors for viral hepatitis. You can improve collaboration with primary care providers by understanding:
- what an LFT measures
- how to interpret abnormal results
- which conditions to suspect, based on the results.
A standard LFT usually measures several enzymes and proteins, typically ALT, AST, alkaline phosphatase (ALP), total bilirubin (TBIL), albumin (ALB), and total protein (TP). Measures of gamma-glutamyl transpeptidase (GGT) and prothrombin time (PT) are often requested with an LFT. Table 1 provides normal ranges and ranges that indicate liver damage for several of these parameters.1,2
“Liver function test” is a misnomer because LFTs do not directly measure liver function. Rather, they reflect hepatocyte injury or cholestasis (blockage or damage in the biliary system). ALB and PT measure liver synthetic function, but are nonspecific. ALB levels can be altered by nutritional status, protein-losing enteropathies, or nephropathies, whereas PT may be modified by warfarin, vitamin K deficiency, or consumptive coagulopathy.
Table 1
Test results: what’s normal, what suggests liver damage
| Parameter | Description | Normal range | Range indicating liver damage |
|---|---|---|---|
| Alanine aminotransferase (ALT) | Enzyme highly concentrated in the liver | 3 to 30 U/L | >3 times upper limit of normal |
| Alkaline phosphatase (ALP) | Enzyme highly concentrated in the liver, bile ducts, placenta, and bone | 35 to 150 U/L | >2 times upper limit of normal |
| Aspartate aminotransferase (AST) | Enzyme highly concentrated in heart muscle, liver cells, skeletal muscle cells, and (to a lesser degree) other tissues | 11 to 32 U/L | Used to evaluate elevations in other serum enzyme level |
| Gamma-glutamyl transpeptidase (GGT) | Enzyme highly concentrated in the liver, bile ducts, and kidneys | 5 to 40 U/L | Used to evaluate elevations in other serum enzyme levels |
| Total bilirubin (TBIL) | Yellow bile pigment produced when liver processes waste products | 0.3 to 1.1 mg/dL | >2 times upper limit of normal if associated with elevation in ALT or ALP |
| Sources: References 1,2 | |||
CASE CONTINUED: Spotting a pattern of injury
Mrs. W’s elevated ALT and AST levels are of unknown duration. Her AST:ALT ratio is approximately 2:1, suggesting hepatocellular injury.
Interpreting abnormal LFT results
To properly interpret LFTs, consider the patient’s symptoms, physical exam findings, medical history, medical illnesses, potential substance use, risk factors for HIV and viral hepatitis, and medication list. Collaborate with the patient’s primary care provider or facilitate primary care (Figure 1).
ALT and AST are highly concentrated in the liver, but ALT is a more specific indicator of liver injury. For both, levels may vary according to age, sex, and ethnicity but in general, levels <30 U/L are considered normal.1,2
ALP originates predominately from the liver and from bone. Persistently elevated ALP levels in the liver may indicate chronic cholestasis or infiltrative liver disease.
GGT is best used to evaluate the meaning of elevations in other serum enzymes.3 Elevated GGT can help confirm hepatic origin of elevated ALP or support a suspicion of alcohol use in patients with an AST: ALT ratio >2:1.
If an asymptomatic patient has elevated LFT results, first repeat the test. If repeat results are normal, perform the test again in 3-6 months. Keep in mind, however, that normal LFT results do not always indicate the absence of disease. For example, up to 16% of patients with hepatitis C and 13% of patients with nonalcoholic steatohepatitis (NASH) have normal LFT results despite histologic abnormalities.4
If repeat results are abnormal, obtain the patient’s consent to inform the primary care provider. Then take a thorough history and perform a focused physical exam. In the history, focus on use of prescription and nonprescription medications, including over-the-counter and herbal therapies, alcohol, and drugs of abuse, such as MDMA (“ecstasy”), phencyclidine (“angel dust”), and glues or solvents. Also assess for risk factors for infectious hepatitis, such as IV drug use, work-related blood exposure, and tattoos. Ask about a family history of liver disease. Focus your physical exam on visible stigmata of chronic liver disease, such as jaundice, temporal wasting, ascites, and palmar erythema.
Next, analyze the severity and pattern of the LFT abnormality. Liver injury is defined as:
- ALT >3 times the upper limit of normal
- ALP >2 times the upper limit of normal
- or total bilirubin >2 times the upper limit of normal if associated with any elevation of ALT or ALP.5
If ALT elevations predominate, consider hepatocellular injury. If ALP elevations predominate, suspect cholestatic injury. Elevations of both ALT and ALP suggest a mixed pattern of hepatocellular and cholestatic injury.
Figure 1 Interpreting liver function test results
ALB: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transpeptidase; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; LFT: liver function test; PCP: primary care provider; TBIL: total bilirubin; TP: total protein
CASE CONTINUED: Pinpointing a diagnosis
Mrs. W undergoes repeat LFTs with GGT testing, screening tests for hepatitis B and C, and a comprehensive physical exam. The psychiatrist screens for alcohol use, asks the patient about her use of herbal therapies and substance abuse relapse, and evaluates cognitive mental status for symptoms of encephalopathy. Results reveal that Mrs. W’s ALT and AST are elevated because of chronic active hepatitis C.
Causes of hepatocellular injury
Further evaluation of your patient’s test results can help narrow down potential causes of liver damage. If your patient’s ALT is disproportionately elevated, estimate the:
- severity of aminotransferase elevation
- ratio of AST:ALT
- rate of change over multiple LFTs.
If AST or ALT is >10 times normal, consider toxin-induced or ischemic injury.6 An AST:ALT ratio of 2:1 or 3:1, especially when associated with elevated GGT, strongly suggests alcohol-induced injury. With acute mild transaminase elevations—ALT>AST, 2 to 3 times normal—suspect medication-related injury.
A variety of factors and conditions can result in hepatocellular damage:
Common causes
Medications. Many drugs, including common psychotropics, can cause elevated liver enzymes (Table 2).5 As little as 4 grams per day of acetaminophen can cause mild transaminitis.4 Antidepressants, second-generation antipsychotics (SGAs), and anticonvulsants can cause increases in AST and ALT.7 If liver enzymes rise after a patient starts a new medication, drug-related liver toxicity is likely. Remember to consider a patient’s use of drugs of abuse and herbal therapies.
Discontinuing the suspect agent usually produces steady (although sometimes slow) improvement in LFTs. Use serial LFT testing and focused history and physical examinations to confirm improvement.
Alcohol. Screen for alcohol abuse using the CAGE questionnaire, the Alcohol Use Disorders Identification Test, or a similar tool. More than 90% of patients with an AST:ALT ratio of 2:1 have alcoholic liver disease; this percentage increases to >96% when the ratio is 3:1.1 A 2-fold increase in GGT in a patient with an AST:ALT ratio >2:1 further supports the diagnosis. In patients with alcohol abuse, AST rarely exceeds 10 times normal.5
Hepatitis C. The prevalence of hepatitis C is increasing among patients with severe mental illness, especially a dual diagnosis.8 Hepatitis C rarely causes acute symptoms.
Offer a hepatitis C antibody screening to test patients with even a remote history of IV drug use or comorbid substance abuse. Patients with a positive hepatitis C antibody test or a negative hepatitis C antibody test but a high risk for the disease should receive further testing.9
Hepatitis B. Risk factors include exposure to blood, sexual transmission, and emigration from endemic areas in Southeast Asia and sub-Saharan Africa. Initial screening panels include tests for hepatitis B surface antigen, hepatitis B surface antibody, and hepatitis B core antibody. Positive B surface antigen and core antibody tests indicate infection.
NASH. Nonalcoholic steatohepatitis (NASH) is the most common cause of mild transaminitis in the Western world (Box).4,10,11
Nonalcoholic steatohepatitis (NASH) is inflammatory liver disease of uncertain pathogenesis that commonly occurs with metabolic syndrome. It affects up to 5% of Americans, most often those who are middle-aged and overweight or obese, hyperlipidemic, or diabetic. NASH resembles alcoholic liver disease but occurs in people who drink little or no alcohol. In addition to inflammation, it is characterized by accumulation of fat and fibrous tissue in the liver. Typically patients are asymptomatic, but NASH can lead to cirrhosis.
NASH is a common cause of mild transaminitis. Aminotransferase levels are usually <4 times the normal value.10 Thirteen percent of patients with NASH have normal LFT results despite histologic abnormalities.4 NASH is a diagnosis of exclusion that is confirmed by liver biopsy.
NASH has no specific therapies or cure. Treatment focuses on controlling associated conditions such as diabetes, obesity, and hyperlipidemia. In obese patients, weight loss is the cornerstone of treatment. If you prescribe a second-generation antipsychotic (SGA) for a patient who has NASH, be aware that SGAs increase the risk of hyperglycemia and dyslipidemia, which can exacerbate NASH.11
Table 2
Medications that affect liver function test (LFT) results
| Medication class | Hepatocellular injury (↑ALT) | Cholestatic injury (↑ALP and ↑ALT) | Mixed injury (↑ALP and ↑TBIL) |
|---|---|---|---|
| Psychotropic | Bupropion, fluoxetine, paroxetine, risperidone, sertraline, trazodone, valproic acid | Chlorpromazine, mirtazapine, tricyclic antidepressants | Amitriptyline, Amitriptyline, phenobarbital, phenytoin, trazodone |
| Cardiovascular | Amiodarone, lisinopril, losartan, statins | Clopidogrel, irbesartan | Captopril, enalapril, verapamil |
| Endocrine | Acarbose, allopurinol | – | – |
| Gastrointestinal | Omeprazole | – | – |
| Herbal remedies | Germander, kava | – | – |
| Anti-infectives | HAART drugs, isoniazid, ketoconazole, pyrazinamide, rifampin, tetracycline, trovafloxacin | Amoxicillin/clavulanic acid, erythromycins, terbinafine | Clindamycin, nitrofurantoin, trimethoprim-sulfamethoxazole |
| Rheumatologic | Acetaminophen, baclofen, methotrexate, NSAIDs | Anabolic steroids | Azathioprine, sulfonamides |
| Others | – | Oral contraceptives, estrogens, phenothiazines | Cyproheptadine, flutamide |
| ALP: alkaline phosphatase; ALT: alanine aminotransferase; HAART: highly active antiretroviral therapy; NSAID: nonsteroidal anti-inflammatory drug; TBIL: total bilirubin | |||
| Source: Reference 5 | |||
Less common causes
Hemochromatosis is an autosomal recessive disease that causes pathologic deposition of iron in the liver, pancreas, and heart and leads to cirrhosis, diabetes, and heart disease. Suspect it in patients with a clinical syndrome and transferrin saturation index >45%.12 A hemochromatosis gene mutation analysis confirms the diagnosis.
Autoimmune hepatitis occurs primarily in women ages 20 to 50 years.13 Because >80% of patients with autoimmune hepatitis have hypergammaglobulinemia, serum protein electrophores is a useful screening test.5
Wilson’s disease is a genetic disorder of biliary copper excretion classically diagnosed in young people with concomitant neurologic or psychiatric conditions. Those affected have low serum ceruloplasmin. Neuropsychiatric symptoms include parkinsonian-like tremor, rigidity, clumsiness of gait, slurred speech, drooling, and inappropriate and uncontrollable grinning (risus sardonicus).8 Psychosis and suicidality also are common in patients with Wilson’s disease.
Alpha-1 antitrypsin deficiency. Alpha-1 antitrypsin is a protein produced primarily in the liver that protects the lungs from neutrophil elastase. Suspect alpha-1 antitrypsin deficiency in patients with abnormal LFTs and emphysema. Low serum alpha-1 antitrypsin confirms the diagnosis.
Celiac disease. Consider celiac disease in patients with chronic diarrhea or abdominal distension and abnormal LFTs. Small bowel biopsy and elevated tissue transglutaminase antibodies and anti-endomysial antibodies confirm the diagnosis.
Causes of cholestatic injury
If your patient has a disproportionate ALP elevation, identify the source of the ALP by testing GGT. GGT levels are elevated in liver disease but not in bone disease. Partial bile duct obstruction is a common cause of ALP elevation. For initial testing, include ultrasonography of the right upper quadrant.
Medications. Many medications can cause biliary stasis and cholestatic LFTs, including mirtazapine, tricyclic antidepressants, anabolic steroids, phenytoin, and estrogens.
Primary sclerosing cholangitis is inflammatory disease of the bile ducts that can lead to cholestasis (blockage of bile transport blockage). Consider it in patients with inflammatory bowel disease. Endoscopic retrograde cholangiopancreatography and magnetic resonance cholangiopancreatography aid diagnosis.
Primary biliary cirrhosis generally presents in middle-aged women with other autoimmune processes, cholestasis, and pruritus. Consider testing serum antimitochondrial antibodies.
Infiltrative liver diseases such as sarcoidosis, metastatic disease, or lymphoma can also present with cholestasis. Liver imaging is required for these diagnoses.
Causes of mixed injury
If LFT results suggest a mixed injury pattern, focus on the predominant pattern and evaluate the causes listed above. Certain medications typically result in a mixed injury pattern.
Related resources
- American Gastroenterological Association medical position statement: evaluation of liver chemistry tests. www.guideline.gov/summary/summary.aspx?ss=15&doc_id=3492&nbr=002718.
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician 1999;59(8):2223-30.
Drug brand names
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Chlorpromazine • Thorazine
- Fluoxetine • Prozac
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Phenobarbital • Luminal
- Phenytoin • Dilantin
- Risperidone • Risperdal
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Warfarin • Coumadin
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137(1):1-10.
2. Gopal DV, Rosen HR. Abnormal findings on liver function tests. Interpreting results to narrow the diagnosis and establish a prognosis. Postgrad Med 2000;107(2):100-14.
3. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342(17):1266-71.
4. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol 2006;101(1):76-82.
5. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006;354(7):731-9.
6. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172(3):367-79.
7. Bezchilibny-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs, 15th ed. Cambridge Hogrefe & Huber; 2005.
8. Crone CC, Gabriel GM, Dimartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics 2006;47(3):188-205.
9. Kaplan MA. Approach to the patient with abnormal liver function tests. Available at: http://www.uptodate.com. Accessed December 12, 2006.
10. Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107(4):1103-9.
11. Lieberman JA, Stroup SS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
12. Yen AW, Fancher TL, Bowlus CL. Revisiting hereditary hemochromatosis: current concepts and progress. Am J Med 2006;119(5):391-9.
13. Krawitt EL. Autoimmune hepatitis. N Engl J Med 1996;334(14):897-903.
1. Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137(1):1-10.
2. Gopal DV, Rosen HR. Abnormal findings on liver function tests. Interpreting results to narrow the diagnosis and establish a prognosis. Postgrad Med 2000;107(2):100-14.
3. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342(17):1266-71.
4. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol 2006;101(1):76-82.
5. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006;354(7):731-9.
6. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ 2005;172(3):367-79.
7. Bezchilibny-Butler KZ, Jeffries JJ, eds. Clinical handbook of psychotropic drugs, 15th ed. Cambridge Hogrefe & Huber; 2005.
8. Crone CC, Gabriel GM, Dimartini A. An overview of psychiatric issues in liver disease for the consultation-liaison psychiatrist. Psychosomatics 2006;47(3):188-205.
9. Kaplan MA. Approach to the patient with abnormal liver function tests. Available at: http://www.uptodate.com. Accessed December 12, 2006.
10. Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107(4):1103-9.
11. Lieberman JA, Stroup SS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
12. Yen AW, Fancher TL, Bowlus CL. Revisiting hereditary hemochromatosis: current concepts and progress. Am J Med 2006;119(5):391-9.
13. Krawitt EL. Autoimmune hepatitis. N Engl J Med 1996;334(14):897-903.
The meaning of gender
In “Transsexualism: Clinical guide to gender identity disorder” (Current Psychiatry, February 2007), the author states, “Persons with GID transgress the traditional binary gender system.” This statement implies that gender is a social convention. If gender is just a social convention, isn’t the term “disorder” pejorative to those seeking to actualize the fluidness of sexual differentiation? Don’t persons with GID transgress their own “unambiguous genotype and phenotype,” which actually is a diagnostic criterion? Could the considerable psychiatric comorbidity reflect more than social discrimination, but also this rare, complex, subjective disorientation towards one’s body?
Underlying the treatment of GID are several tenuous philosophical assumptions, including the notion one can “reassign” through phenotypic alterations the genetic reality of gender. There also is an implied dualism in which the person is the conscious subject of experience, while the human body is sub-personal, a possession devoid of inherent meaning with only instrumental value. This position reduces masculinity and femininity to psychological phenomena.1
Clinicians should be wary of colluding with patient pathology by cooperating in mutilation of a healthy human body, a violation of our ethical duty to preserve bodily integrity.
Thomas K. Nelson, MD
Assistant professor of psychiatry
Mayo Clinic College of Medicine
1. Ryan PF. Secularist and Christian views of human nature and its fulfillment. Implications for bioethics and environmentalism. In: Robinson DN, Sweeney GM, Gill K, eds. Human nature in its wholeness. Washington, D.C.: The Catholic University of America Press; 2006:57-79.
In “Transsexualism: Clinical guide to gender identity disorder” (Current Psychiatry, February 2007), the author states, “Persons with GID transgress the traditional binary gender system.” This statement implies that gender is a social convention. If gender is just a social convention, isn’t the term “disorder” pejorative to those seeking to actualize the fluidness of sexual differentiation? Don’t persons with GID transgress their own “unambiguous genotype and phenotype,” which actually is a diagnostic criterion? Could the considerable psychiatric comorbidity reflect more than social discrimination, but also this rare, complex, subjective disorientation towards one’s body?
Underlying the treatment of GID are several tenuous philosophical assumptions, including the notion one can “reassign” through phenotypic alterations the genetic reality of gender. There also is an implied dualism in which the person is the conscious subject of experience, while the human body is sub-personal, a possession devoid of inherent meaning with only instrumental value. This position reduces masculinity and femininity to psychological phenomena.1
Clinicians should be wary of colluding with patient pathology by cooperating in mutilation of a healthy human body, a violation of our ethical duty to preserve bodily integrity.
Thomas K. Nelson, MD
Assistant professor of psychiatry
Mayo Clinic College of Medicine
In “Transsexualism: Clinical guide to gender identity disorder” (Current Psychiatry, February 2007), the author states, “Persons with GID transgress the traditional binary gender system.” This statement implies that gender is a social convention. If gender is just a social convention, isn’t the term “disorder” pejorative to those seeking to actualize the fluidness of sexual differentiation? Don’t persons with GID transgress their own “unambiguous genotype and phenotype,” which actually is a diagnostic criterion? Could the considerable psychiatric comorbidity reflect more than social discrimination, but also this rare, complex, subjective disorientation towards one’s body?
Underlying the treatment of GID are several tenuous philosophical assumptions, including the notion one can “reassign” through phenotypic alterations the genetic reality of gender. There also is an implied dualism in which the person is the conscious subject of experience, while the human body is sub-personal, a possession devoid of inherent meaning with only instrumental value. This position reduces masculinity and femininity to psychological phenomena.1
Clinicians should be wary of colluding with patient pathology by cooperating in mutilation of a healthy human body, a violation of our ethical duty to preserve bodily integrity.
Thomas K. Nelson, MD
Assistant professor of psychiatry
Mayo Clinic College of Medicine
1. Ryan PF. Secularist and Christian views of human nature and its fulfillment. Implications for bioethics and environmentalism. In: Robinson DN, Sweeney GM, Gill K, eds. Human nature in its wholeness. Washington, D.C.: The Catholic University of America Press; 2006:57-79.
1. Ryan PF. Secularist and Christian views of human nature and its fulfillment. Implications for bioethics and environmentalism. In: Robinson DN, Sweeney GM, Gill K, eds. Human nature in its wholeness. Washington, D.C.: The Catholic University of America Press; 2006:57-79.
Target symptoms, not studies
I will go further than Dr. Nasrallah’s editorial did to endorse off-label use. Our diagnostic categories are 19th century superstitions. Only individual target symptoms have concrete reality. FDA approval studies have parametric statistics based on the bell-shaped curve. These studies represent the population at large. The consecutive series of single-case, on-off experiments have nonparametric distributions. These FDA parametric studies have no relevance to individual care. The FDA serves as a screening tool for potentially useful medications. When applied to individual patients, FDA approval studies are irresponsible, garbage science.
HMO or clinician prescribing that is limited to FDA-approved drugs represents gross malpractice. A better indicator of the standard of care is clinician prescribing patterns recorded by prescription databases. Any use of medication by more than 10% of clinicians at least is a minority standard of care.
Physicians are motivated by patient success. Repeat and widespread prescription is the best indicator of clinical validity.
David Behar, MD
Bethlehem, PA
I will go further than Dr. Nasrallah’s editorial did to endorse off-label use. Our diagnostic categories are 19th century superstitions. Only individual target symptoms have concrete reality. FDA approval studies have parametric statistics based on the bell-shaped curve. These studies represent the population at large. The consecutive series of single-case, on-off experiments have nonparametric distributions. These FDA parametric studies have no relevance to individual care. The FDA serves as a screening tool for potentially useful medications. When applied to individual patients, FDA approval studies are irresponsible, garbage science.
HMO or clinician prescribing that is limited to FDA-approved drugs represents gross malpractice. A better indicator of the standard of care is clinician prescribing patterns recorded by prescription databases. Any use of medication by more than 10% of clinicians at least is a minority standard of care.
Physicians are motivated by patient success. Repeat and widespread prescription is the best indicator of clinical validity.
David Behar, MD
Bethlehem, PA
I will go further than Dr. Nasrallah’s editorial did to endorse off-label use. Our diagnostic categories are 19th century superstitions. Only individual target symptoms have concrete reality. FDA approval studies have parametric statistics based on the bell-shaped curve. These studies represent the population at large. The consecutive series of single-case, on-off experiments have nonparametric distributions. These FDA parametric studies have no relevance to individual care. The FDA serves as a screening tool for potentially useful medications. When applied to individual patients, FDA approval studies are irresponsible, garbage science.
HMO or clinician prescribing that is limited to FDA-approved drugs represents gross malpractice. A better indicator of the standard of care is clinician prescribing patterns recorded by prescription databases. Any use of medication by more than 10% of clinicians at least is a minority standard of care.
Physicians are motivated by patient success. Repeat and widespread prescription is the best indicator of clinical validity.
David Behar, MD
Bethlehem, PA
Off-label ‘experiments’?
If I am correct, your editorial (“Off-label prescribing,” Current Psychiatry, March 2007) appears to invite those without adequate experience and knowledge—myself included—to experiment, come up with their own combinations, and engage in off-label prescribing. Although off-label prescribing is important, it is not justified by your statement that “compassionate practitioners use whatever is available to alleviate suffering.”
I think it is best to warn clinicians that off-label prescribing should be based on reason, knowledge, and good clinical judgment after conventional and accepted treatments have proven ineffective—in which case it would be advisable to review the initial diagnosis and obtain opinions from more experienced colleagues or well-established experts.
I say enough with the current status of our profession, which allows for permanent gross deficiencies in diagnosing techniques, outrageous treatments, and many skeptical and contemptuous looks from colleagues in other specialties. Your editorial seems to sanction encouragement of primary care physicians to detect and treat depression, with the sad result that only 20% of depressed patients in primary care receive adequate pharmacological treatment, according to a recent study.1
Daniel Pistone, MD
Rock Hill, SC
Dr. Nasrallah Responds
In my editorial I was not encouraging off-label use but suggesting that the practice is inevitable due to the pervasive lack of approved drugs or controlled studies for many psychiatric diagnoses. Experienced psychiatrists sometimes focus on severe target symptoms rather than a syndrome and may choose to use a drug approved for another disorder that has a clear therapeutic effect. For example, various psychiatric disorders share symptoms such as anxiety, depression, impulsivity, agitation, aggression, or hyperactivity. In the absence of an approved agent, clinicians are likely to use medications approved for bipolar disorder for a different psychiatric illness, such as a traumatic brain injury, that may share some but not all the symptoms of bipolar disorder. The patient gets some relief from symptoms and, until a drug is officially approved for that condition, off-label use arguably is a reasonable mode of action for a patient who may harm himself or others.
My editorial did not encourage primary care colleagues to use psychotropic drugs off-label. Primary care providers usually diagnose and treat anxiety and depression, which have several approved therapeutic agents. These physicians should refer patients with serious psychiatric conditions that have no approved drugs to a psychiatrist for diagnosis and management.
Off-label practices will continue as long as there are DSM-IV-TR diagnostic categories that do not have an approved drug. Medical practitioners can generate important observations and data during this process that can be leveraged into future treatment indications.
Henry A. Nasrallah, MD
Editor-In-Chief
1. Lecrubier Y. Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J Clin Psychiatry 2007;68(suppl 2):36-41.
If I am correct, your editorial (“Off-label prescribing,” Current Psychiatry, March 2007) appears to invite those without adequate experience and knowledge—myself included—to experiment, come up with their own combinations, and engage in off-label prescribing. Although off-label prescribing is important, it is not justified by your statement that “compassionate practitioners use whatever is available to alleviate suffering.”
I think it is best to warn clinicians that off-label prescribing should be based on reason, knowledge, and good clinical judgment after conventional and accepted treatments have proven ineffective—in which case it would be advisable to review the initial diagnosis and obtain opinions from more experienced colleagues or well-established experts.
I say enough with the current status of our profession, which allows for permanent gross deficiencies in diagnosing techniques, outrageous treatments, and many skeptical and contemptuous looks from colleagues in other specialties. Your editorial seems to sanction encouragement of primary care physicians to detect and treat depression, with the sad result that only 20% of depressed patients in primary care receive adequate pharmacological treatment, according to a recent study.1
Daniel Pistone, MD
Rock Hill, SC
Dr. Nasrallah Responds
In my editorial I was not encouraging off-label use but suggesting that the practice is inevitable due to the pervasive lack of approved drugs or controlled studies for many psychiatric diagnoses. Experienced psychiatrists sometimes focus on severe target symptoms rather than a syndrome and may choose to use a drug approved for another disorder that has a clear therapeutic effect. For example, various psychiatric disorders share symptoms such as anxiety, depression, impulsivity, agitation, aggression, or hyperactivity. In the absence of an approved agent, clinicians are likely to use medications approved for bipolar disorder for a different psychiatric illness, such as a traumatic brain injury, that may share some but not all the symptoms of bipolar disorder. The patient gets some relief from symptoms and, until a drug is officially approved for that condition, off-label use arguably is a reasonable mode of action for a patient who may harm himself or others.
My editorial did not encourage primary care colleagues to use psychotropic drugs off-label. Primary care providers usually diagnose and treat anxiety and depression, which have several approved therapeutic agents. These physicians should refer patients with serious psychiatric conditions that have no approved drugs to a psychiatrist for diagnosis and management.
Off-label practices will continue as long as there are DSM-IV-TR diagnostic categories that do not have an approved drug. Medical practitioners can generate important observations and data during this process that can be leveraged into future treatment indications.
Henry A. Nasrallah, MD
Editor-In-Chief
If I am correct, your editorial (“Off-label prescribing,” Current Psychiatry, March 2007) appears to invite those without adequate experience and knowledge—myself included—to experiment, come up with their own combinations, and engage in off-label prescribing. Although off-label prescribing is important, it is not justified by your statement that “compassionate practitioners use whatever is available to alleviate suffering.”
I think it is best to warn clinicians that off-label prescribing should be based on reason, knowledge, and good clinical judgment after conventional and accepted treatments have proven ineffective—in which case it would be advisable to review the initial diagnosis and obtain opinions from more experienced colleagues or well-established experts.
I say enough with the current status of our profession, which allows for permanent gross deficiencies in diagnosing techniques, outrageous treatments, and many skeptical and contemptuous looks from colleagues in other specialties. Your editorial seems to sanction encouragement of primary care physicians to detect and treat depression, with the sad result that only 20% of depressed patients in primary care receive adequate pharmacological treatment, according to a recent study.1
Daniel Pistone, MD
Rock Hill, SC
Dr. Nasrallah Responds
In my editorial I was not encouraging off-label use but suggesting that the practice is inevitable due to the pervasive lack of approved drugs or controlled studies for many psychiatric diagnoses. Experienced psychiatrists sometimes focus on severe target symptoms rather than a syndrome and may choose to use a drug approved for another disorder that has a clear therapeutic effect. For example, various psychiatric disorders share symptoms such as anxiety, depression, impulsivity, agitation, aggression, or hyperactivity. In the absence of an approved agent, clinicians are likely to use medications approved for bipolar disorder for a different psychiatric illness, such as a traumatic brain injury, that may share some but not all the symptoms of bipolar disorder. The patient gets some relief from symptoms and, until a drug is officially approved for that condition, off-label use arguably is a reasonable mode of action for a patient who may harm himself or others.
My editorial did not encourage primary care colleagues to use psychotropic drugs off-label. Primary care providers usually diagnose and treat anxiety and depression, which have several approved therapeutic agents. These physicians should refer patients with serious psychiatric conditions that have no approved drugs to a psychiatrist for diagnosis and management.
Off-label practices will continue as long as there are DSM-IV-TR diagnostic categories that do not have an approved drug. Medical practitioners can generate important observations and data during this process that can be leveraged into future treatment indications.
Henry A. Nasrallah, MD
Editor-In-Chief
1. Lecrubier Y. Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J Clin Psychiatry 2007;68(suppl 2):36-41.
1. Lecrubier Y. Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J Clin Psychiatry 2007;68(suppl 2):36-41.