User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Innovation deficit disorder: Psychiatry needs ‘disruptive’ new drugs
Clinical psychiatry, like other medical specialties, eagerly awaits treatment breakthroughs. Despite the availability of many pharmacotherapies, even common illnesses such as diabetes, hypertension, and bipolar disorder cannot be cured.
Until investigators unravel a disease’s pathophysiology, treatments tend to remain symptomatic. Molecular genetics research ultimately may revolutionize the treatment of serious body and mind disorders, but how long must we wait?
As naïve medical students in the 1970s, my friends and I believed cures certainly would be discovered before the new millennium for cancer, arthritis, Alzheimer’s dementia, schizophrenia, diabetes, major depression, and anxiety. Although progress has been made, the pace of developing effective new treatments has been much slower than we expected.
As a researcher, I understand why brave new treatments are elusive. Even so, I keep hoping to see “disruptive” discoveries that will bring solutions to our patients’ suffering. Psychiatry needs a surge in pharmacologic innovations to advance from decades-old, serendipitously discovered, partially effective drugs to highly specific and effective biologic interventions.
Where will these desired breakthroughs come from? The National Institute of Mental Health (NIMH) does not have the budget to develop new drugs for psychiatry. The pharmaceutical industry—on which psychiatry is almost entirely dependent for new medications—seems to have stalled. Drug companies are complex, for-profit enterprises that employ tens of thousands of researchers to discover, test, and develop new drugs under an increasingly stifling web of regulatory controls.
Although drug companies have made progress against disease, the public has a rather negative view of them. Americans seem to appreciate that drug discovery is arduous, time-consuming, and costly, but they hold pharmaceutical companies to a different standard than other corporations. Practically everyone applauds when a high-tech or apparel company makes huge profits, yet when a pharmaceutical company does so there is outrage. Like it or not, creating new medications requires massive investment, and—other than taxes—profits are the only way to provide the resources for psychopharmacology research and development (R&D).
Perhaps the lull in new psychopharmacologics reflects escalating pressures on drug companies:
- Patent life is limited.
- Product development takes years and significant financial risk (only 1 in 10,000 new molecular entities makes it to market).
- Liability costs are skyrocketing because of unrealistic public expectations for new medications: high efficacy and no side effects.
Drug companies compound their problems by overspending on marketing and lobbying, and they often merge with one another to create behemoths that stifle innovation. No wonder we see so many reformulations of existing products and “me-too” drugs instead of high-risk, high-cost novel approaches to disease management.
What practical solutions could rejuvenate drug discovery? The answers are not simple, but consider these ideas:
- Extend by several years the patents on breakthrough (novel mechanism, first-in-class) drugs, and during this extension earmark a good chunk of profits for R&D.
- Limit punitive damages from lawsuits related to breakthrough medications to remove this impediment to innovation.
- Create incentives for drug companies to collaborate with NIMH researchers to translate neurobiologic and molecular genetics discoveries into innovative, biologically specific agents for psychiatric brain diseases.
Forging private-public collaborations may be a winning formula for all, with seriously afflicted patients as the ultimate beneficiaries of new drugs for their unmet needs.
Clinical psychiatry, like other medical specialties, eagerly awaits treatment breakthroughs. Despite the availability of many pharmacotherapies, even common illnesses such as diabetes, hypertension, and bipolar disorder cannot be cured.
Until investigators unravel a disease’s pathophysiology, treatments tend to remain symptomatic. Molecular genetics research ultimately may revolutionize the treatment of serious body and mind disorders, but how long must we wait?
As naïve medical students in the 1970s, my friends and I believed cures certainly would be discovered before the new millennium for cancer, arthritis, Alzheimer’s dementia, schizophrenia, diabetes, major depression, and anxiety. Although progress has been made, the pace of developing effective new treatments has been much slower than we expected.
As a researcher, I understand why brave new treatments are elusive. Even so, I keep hoping to see “disruptive” discoveries that will bring solutions to our patients’ suffering. Psychiatry needs a surge in pharmacologic innovations to advance from decades-old, serendipitously discovered, partially effective drugs to highly specific and effective biologic interventions.
Where will these desired breakthroughs come from? The National Institute of Mental Health (NIMH) does not have the budget to develop new drugs for psychiatry. The pharmaceutical industry—on which psychiatry is almost entirely dependent for new medications—seems to have stalled. Drug companies are complex, for-profit enterprises that employ tens of thousands of researchers to discover, test, and develop new drugs under an increasingly stifling web of regulatory controls.
Although drug companies have made progress against disease, the public has a rather negative view of them. Americans seem to appreciate that drug discovery is arduous, time-consuming, and costly, but they hold pharmaceutical companies to a different standard than other corporations. Practically everyone applauds when a high-tech or apparel company makes huge profits, yet when a pharmaceutical company does so there is outrage. Like it or not, creating new medications requires massive investment, and—other than taxes—profits are the only way to provide the resources for psychopharmacology research and development (R&D).
Perhaps the lull in new psychopharmacologics reflects escalating pressures on drug companies:
- Patent life is limited.
- Product development takes years and significant financial risk (only 1 in 10,000 new molecular entities makes it to market).
- Liability costs are skyrocketing because of unrealistic public expectations for new medications: high efficacy and no side effects.
Drug companies compound their problems by overspending on marketing and lobbying, and they often merge with one another to create behemoths that stifle innovation. No wonder we see so many reformulations of existing products and “me-too” drugs instead of high-risk, high-cost novel approaches to disease management.
What practical solutions could rejuvenate drug discovery? The answers are not simple, but consider these ideas:
- Extend by several years the patents on breakthrough (novel mechanism, first-in-class) drugs, and during this extension earmark a good chunk of profits for R&D.
- Limit punitive damages from lawsuits related to breakthrough medications to remove this impediment to innovation.
- Create incentives for drug companies to collaborate with NIMH researchers to translate neurobiologic and molecular genetics discoveries into innovative, biologically specific agents for psychiatric brain diseases.
Forging private-public collaborations may be a winning formula for all, with seriously afflicted patients as the ultimate beneficiaries of new drugs for their unmet needs.
Clinical psychiatry, like other medical specialties, eagerly awaits treatment breakthroughs. Despite the availability of many pharmacotherapies, even common illnesses such as diabetes, hypertension, and bipolar disorder cannot be cured.
Until investigators unravel a disease’s pathophysiology, treatments tend to remain symptomatic. Molecular genetics research ultimately may revolutionize the treatment of serious body and mind disorders, but how long must we wait?
As naïve medical students in the 1970s, my friends and I believed cures certainly would be discovered before the new millennium for cancer, arthritis, Alzheimer’s dementia, schizophrenia, diabetes, major depression, and anxiety. Although progress has been made, the pace of developing effective new treatments has been much slower than we expected.
As a researcher, I understand why brave new treatments are elusive. Even so, I keep hoping to see “disruptive” discoveries that will bring solutions to our patients’ suffering. Psychiatry needs a surge in pharmacologic innovations to advance from decades-old, serendipitously discovered, partially effective drugs to highly specific and effective biologic interventions.
Where will these desired breakthroughs come from? The National Institute of Mental Health (NIMH) does not have the budget to develop new drugs for psychiatry. The pharmaceutical industry—on which psychiatry is almost entirely dependent for new medications—seems to have stalled. Drug companies are complex, for-profit enterprises that employ tens of thousands of researchers to discover, test, and develop new drugs under an increasingly stifling web of regulatory controls.
Although drug companies have made progress against disease, the public has a rather negative view of them. Americans seem to appreciate that drug discovery is arduous, time-consuming, and costly, but they hold pharmaceutical companies to a different standard than other corporations. Practically everyone applauds when a high-tech or apparel company makes huge profits, yet when a pharmaceutical company does so there is outrage. Like it or not, creating new medications requires massive investment, and—other than taxes—profits are the only way to provide the resources for psychopharmacology research and development (R&D).
Perhaps the lull in new psychopharmacologics reflects escalating pressures on drug companies:
- Patent life is limited.
- Product development takes years and significant financial risk (only 1 in 10,000 new molecular entities makes it to market).
- Liability costs are skyrocketing because of unrealistic public expectations for new medications: high efficacy and no side effects.
Drug companies compound their problems by overspending on marketing and lobbying, and they often merge with one another to create behemoths that stifle innovation. No wonder we see so many reformulations of existing products and “me-too” drugs instead of high-risk, high-cost novel approaches to disease management.
What practical solutions could rejuvenate drug discovery? The answers are not simple, but consider these ideas:
- Extend by several years the patents on breakthrough (novel mechanism, first-in-class) drugs, and during this extension earmark a good chunk of profits for R&D.
- Limit punitive damages from lawsuits related to breakthrough medications to remove this impediment to innovation.
- Create incentives for drug companies to collaborate with NIMH researchers to translate neurobiologic and molecular genetics discoveries into innovative, biologically specific agents for psychiatric brain diseases.
Forging private-public collaborations may be a winning formula for all, with seriously afflicted patients as the ultimate beneficiaries of new drugs for their unmet needs.
Depression, medication, and ‘bad blood’
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
Teen girl brain: High drama, high risk for depression
Kate, age 14, is referred for follow-up treatment of depression after she impulsively swallowed a bottle of acetaminophen. She says she is in academic trouble and has no friends. Kate describes her childhood as mostly happy except for her parents’ arguments. Her medical history indicates she began developing breasts at age 10 and had her first menstrual period at age 12.
Her father is largely absent, traveling and working long hours. Her mother developed postpartum depression and stopped working after Kate’s younger brother was born.
Girls and boys show similar depression risks during childhood, but girls are twice as likely as boys to become clinically depressed after puberty. The key to treating depression in teen girls is to recognize that brain development and fluctuating hormones can influence behavior in ways that confuse them and the people around them. Successfully treating teen girls’ depression may require a gender-specific approach.
3 stages of brain development
Fetal differentiation. All brains start out with female-type brain circuits. At 8 weeks of fetal life, however, tiny testicles in the male begin to produce large amounts of testosterone, which changes the brain and body to male. Thus, sex-specific genes and hormones guide aspects of the first phase of brain development.1
Table 1
Female hormonal development: Gestation to puberty
| Stage/age | Hormonal events | Effect on female brain |
|---|---|---|
| Gestation | Components of reproductive axis form in early embryonic development; at 8 weeks, testosterone from fetal testicles begins to change female-type brain areas to male | Unperturbed by testosterone, brain continues to develop along female lines |
| Birth to age 24 months | Hormone-secreting placenta detaches at birth, dramatically increasing GnRH and LH/FSH and driving infant gonads to produce estrogen in girls or testosterone in boys (“infantile puberty”) | Abundant ovarian estrogen secretion enhances development of brain circuits, such as those associated with reproduction, maternal behavior, and social relatedness |
| Age 24 months to prepuberty | “Brakes” put on GnRH and LH/FSH pulsatile brain cells | “Juvenile pause” begins, with constant low estrogen secretion in girls by 24 months (in boys, “brakes” are on by 12 months) |
| Puberty | “Brakes” released on GnRH and LH/FSH neurons, reactivating reproductive axis | Ovary resumes estrogen production (“adolescent puberty”); increase in estrogen, progesterone, and testosterone stimulates brain circuit development; unipolar depression rates increase to 2:1 (female to male) by age 15 |
| GnRH: gonadotropin-releasing hormone; LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Source: References 4,5 | ||
Infantile puberty and the second phase of brain development begin in early childhood, as the ovaries and testicles start to produce large amounts of estrogen and testosterone soon after birth.
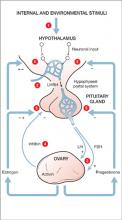
Puberty launches the final brain development phase. Up to 2 years before menstruation begins, pulsatile gonadotropin-releasing hormone cells in the hypothalamus wake up and start stimulating the ovaries to produce estrogen, thrusting the girl brain into puberty (Figure). The teen girl brain begins to experience not only estrogen surges from the ovary but progesterone and testosterone surges as well.
Although brain size and basic circuitry are mostly set by age 5, puberty stimulates new brain cells and increases myelin production.2 Faster myelinated connections between emotionally impulsive limbic brain areas such as the amygdala and sensible, cognitive areas such as the prefrontal cortex are not finished until the early 20s.3
Hormonal changes at puberty
The female brain is remodeled during puberty, leading to sexually dimorphic brain activation and development that further differentiates it from the male brain.4
Estrogen surges are associated with increased production of neurohormones and neurochemicals, such as:
- oxytocin, which reinforces social bonding and intimacy
- dopamine, which stimulates motivation and pleasure circuits in the brain.
Hormonal changes and brain development alter gene expression and affect neurodevelopment. These events may trigger a first depression in pubertal girls with a family history of mood disorder (Table 1).4,5 Although menarche has begun at an average age of 12 in the United States for decades, the most recent National Health and Examination Survey (NHANES) shows puberty onset in girls is occurring earlier (Table 2).6-9
Tanner stage—a measure of pubertal status—is a more accurate predictor of depression in teen girls than age.10 Pubertal transition to Tanner stage 3 (development of pubic and axillary hair and breast buds) is associated with a sharp increase in depression rates. Girls at stage 3 and higher are approximately 3 times more likely to be depressed than girls at stages 1 or 2.11
Pubic hair, breast development, and menstruation are markers for underlying hormonal changes (Table 3).4,5 The onset of estrogen, progesterone, and testosterone surges closely correlates with the difference in depression rates between pre- and postpubertal girls.12 After estrogen and progesterone surges begin at puberty, negative emotions exert an increased activating effect on the female brain,13 and social stressors more deeply affect girls than they do boys. This may explain why girls are more susceptible to depression when a friendship fails.14
CASE CONTINUED: Boy troubles
Kate tells you that in 9th grade she and her best friend, Ellen, would talk about boys for hours after school and try on sexually provocative outfits. They both liked Matt, a 10th grader, so when he asked Kate out, Ellen stopped speaking to her. Kate and Matt began some heavy petting, and Kate said she felt selfish and guilty about hurting Ellen. But when girls at school began spreading rumors that Kate was a “slut,” Kate blamed Ellen and told her, “I hate you!”
Soon after, Matt broke up with Kate. Distraught, she dreaded going to school and cried in her room at night for several weeks. She became chronically tired and had difficulty concentrating in class. She ruminated about losing Matt and worried that she was too fat, too ugly, or too flat-chested. She missed Ellen and felt no one liked her.
Table 2
Puberty’s developmental milestones in U.S. girls (averages)
| Correlate | African Americans | Whites | School grade* |
|---|---|---|---|
| Breast bud development | Age 9 | Age 10 | 4th to 5th |
| Girls with puberty onset by age 8 | 32% | 11% | 3rd |
| Girls with puberty onset by age 10 | 76% | 53% | 5th |
| Menarche onset | Age 12.1 | Age 12.6 | 7th |
| Tanner stage 5† onset | Age 13.9 | Age 15.5 | 8th to 9th |
| * Approximate grade level for age groups | |||
| † Pubic hair and breast development reach adult stage | |||
| Source: Data from references 6-9, including the Pediatric Research in Office Settings network and Third National Health and Nutrition Examination Survey, 1988-1994. | |||
Figure Hypothalamic-pituitary-ovarian axis: Turned on at puberty in girls
Puberty onset stimulates depression in genetically vulnerable girls; more likely after Tanner stage 3 (development of pubic and axillary hair and breast buds).
Male vs female teen brains
Depression after a relationship failure in teen girls often begins with ruminative thoughts about her flaws, mistakes, or appearance. These negative thoughts may preoccupy her day and night. Teen girls often feel confused by contradictory social pressures to look and dress provocatively but resist having sex. A sexual encounter can trigger shame and fear.
Although clinical and developmental studies indicate that teen girls respond more dramatically to relationship troubles than boys, the brain and hormone differences responsible for these effects remain unclear. Male hormones hugely increase in boys at puberty—up to 25-fold between ages 9 and 15—but do not cycle. Male brains do not have the same capacity as female brains to respond to cyclical hormonal activity because exposure to androgens during fetal development eliminates this ability. The fetal testosterone surge causes the area associated with sexual pursuit to double in the male brain.
Outside of fertility considerations, Baron-Cohen et al15 suggest that male brain circuits have been formed by fetal testosterone to focus more on systematization—which emphasizes figuring out how things work and performing tasks—rather than empathy and bonding in relationships. This difference has been shown in neuroimaging studies comparing the genders’ attentional systems.16,17 In contrast to the systematizing male brain, female brains are more likely to activate the mirror neuron system—the area required for empathizing.18
Female brains, of course, respond to cyclical hormonal activity. However, the regular monthly waves of estrogen and progesterone do not affect all female brains the same. A subset of women who experience premenstrual dysphoric disorder appear to have brains that trigger depressed moods and irritability during the last 2 weeks of the menstrual cycle.19 A genetic difference in these women is suspected as the culprit; these genes may affect the way their brains metabolize progesterone.
CASE CONTINUED: An overdose of stress
Kate’s poor concentration lingered, and her grades continued to drop. She tells you her parents were having marital problems and she did not want to bother them with her difficulties. Two days before her period was due, she learned she had failed 2 classes. That night, as she got some acetaminophen for a headache, she impulsively took the rest of the bottle.
After swallowing the pills, Kate panicked. She forced herself to vomit and tearfully told her parents what she had done. They took her to the emergency room, where she was medically stabilized, evaluated by a psychiatrist, and referred to you for outpatient treatment.
Treatment recommendations
A combination of factors—genetic, hormonal, and neurodevelopmental—probably contributed to Kate’s acute depressed mood and overdose. Thus, to treat depression in adolescent girls, emerging evidence supports:
- stabilizing hormonal fluctuations such as rapidly falling progesterone just before the start of menstrual periods with an extended-cycle contraceptive (we would try an ethinyl estradiol/levonorgestrel combination such as Seasonale®)
- treating depressive symptoms with a selective serotonin reuptake inhibitor such as citalopram, 10 mg once daily, with careful monitoring for suicidal thoughts or behavior
- providing tools to manage stress and impulsive behavior through weekly psychotherapy (such as cognitive-behavioral therapy, dialectical behavioral therapy, or supportive therapy).
Genetic factors. Kate’s mother’s history of postpartum depression suggests genetic risk for Kate. Studies have found that the expression of particular genes—such as the serotonin transporter (5-HTT) gene—may be associated with depression. Staley et al20 found that depressed women show a significantly greater decrease in 5-HTT availability in the diencephalon (forebrain region containing the thalamus, hypothalamus, and part of the pituitary gland) when compared with healthy women and depressed men.
Table 3
3 stages of girls’ gonadal development
| Stage | Timing | Developmental events |
|---|---|---|
| Adrenarche | Onset around age 6, peaks by age 20 | Rise in weak androgens (DHEA and DHEAS) from adrenal gland results in pubic and axillary hair and increases likelihood of acne |
| Gonadarche | Usually ~2 years before menarche | Pulses of GnRH, LH/FSH lead to increased estrogen, which stimulates breast development, widening of hips, and increased subcutaneous fat deposition |
| Menarche | Relatively late in puberty (usually not before Tanner stage 4) | “Monthly” cycle established; ovarian estrogen pulses in response to GnRH and FSH, the LH surge, and ovulation; progesterone produced after ovulation |
| DHEA: dehydroepiandrosterone; DHEAS: dehydroepiandrosterone sulfate; GnRH: gonadotropin-releasing hormone; | ||
| LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Tanner stage 4: pubic hair and breast development typical of middle to late adolescence (ages 12 to 17) | ||
| Source: References 4,5 | ||
Although men and women have the same 5-HTT gene, women may possess a gender-specific factor—such as estrogen or progesterone—that differentially alters this and other genes’ expression in women with depression. Individuals who carry a short version of the gene may be at particular risk of becoming depressed when exposed to stressful life events.
Caspi et al21 found a polymorphism in the 5-HTT gene on chromosome 17 that can manifest differentially based on environmental factors. In this study, individuals with 2 copies of the long version of this gene were relatively resistant to stressful life events, whereas those with 1 or 2 copies of the short version were highly sensitive to stressful life events. The depression rate in short-gene individuals was:
- 9% in those who had not experienced stressful life events
- nearly 40% in those who had experienced ≥4 stressful life events.
Hormonal and stress factors. Stress responsiveness becomes sexually dimorphic at puberty. Compared with men, women are:
- at greater risk after puberty for heightened stress responsiveness, which is associated with major depressive disorder
- 3 times more likely to develop depression after a stressful life event.22
Women’s and men’s different biological responses to stress might be related to the gender-specific hormones that emerge during puberty. Kate could be at increased risk for depression—especially immediately before her period—if she inherited a stress-sensitive gene and now has increased stress sensitivity triggered by the hormones of puberty.23
Neurodevelopmental factors. Dorsolateral prefrontal cortex circuits associated with making good decisions and weighing the consequences of actions are immature in the adolescent and the last part of the brain to undergo myelination.24-26 Teens are well-known for erratic, emotionally driven behaviors.27,28 Kate’s impulsive overdose exemplifies the consequences of emotional reactivity without the benefit of inhibitory mature brain connections.
Related resources
- Brizendine L. Teen girl brain. In: The female brain. New York: Morgan Road Books; 2006:31-56. www.thefemalebrain.com.
- Strauch B. The primal teen: what discoveries about the teenage brain tell us about our kids. New York: Doubleday; 2003.
- Harter S. Self and identity development. In: Feldman S, Elliott G, eds. At the threshold: the developing adolescent. Cambridge, MA: Harvard University Press; 1990:352-87.
Drug brand names
- Ethinyl estradiol/levonorgestrel • Seasonale
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci 2004;5(9):701-8.
2. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 1996;6(4):551-60.
3. Yurgelun-Todd DA. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. In press.
4. Cameron J. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Ann NY Acad Sci 2004;1021:110-23.
5. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
6. Biro F, Huang B, Crawford P, et al. Pubertal correlates in black and white girls. J Pediatr 2006;148(2):234-40.
7. Herman-Giddens M, Kaplowitz P, Wasserman R. Navigating the recent articles on girls’ puberty in pediatrics: what do we know and where do we go from here? Pediatrics 2004;113(4):911-7.
8. Herman-Giddens M, Slora E, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997;99(4):505-12.
9. Wu T, Mendola P, Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics 2002;110(4):752-7.
10. Rapkin A, Tsao J, Turk N, et al. Relationships among self-rated Tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol 2006;19:181-7.
11. Angold A, Costello E, Worthman C. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 1998;28:51-61.
12. Angold A, Costello E, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol Med 1999;29:1043-53.
13. Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage 2006;32(2):854-62.
14. McClure EB, Parrish JM, Nelson EE, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol. In press.
15. Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci 2003;358(1430):361-74.
16. Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 2005;28(3):618-26.
17. Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept Mot Skills 2004;99(2):371-91.
18. Cheng YW, Tzeng OJ, Decety J, et al. Gender differences in the human mirror system: a magnetoencephalography study. Neuroreport 2006;17(11):1115-9.
19. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and those without premenstrual syndrome. N Engl J Med 1998;338(4):209-16.
20. Staley J, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59:40-7.
21. Caspi A, Sugden K, Moffitt T, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9.
22. Maciejewski P, Prigerson H, Mazure C. Sex differences in event-related risk for major depression. Psychol Med 2001;31(4):593-604.
23. Seeman M. Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997;154(12):1641-7.
24. Kupfer D, Woodward H. Adolescent development and the regulation of behavior and emotion. Ann NY Acad Sci 2004;1021:320-2.
25. Kelley A, Schochet T, Landry C. Risk taking and novelty seeking in adolescence. Ann NY Acad Sci 2004;1021:27-32.
26. Ellis L, Rothbart M, Posner M. Individual differences in executive attention predict self-regulation and adolescent psychosocial behaviors. Ann NY Acad Sci 2004;1021:337-40.
27. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
28. Pelkonen M, Marttunen M. Child and adolescent suicide: epidemiology, risk factors, and approaches to prevention. Paediatr Drugs 2003;5(4):243-65.
Kate, age 14, is referred for follow-up treatment of depression after she impulsively swallowed a bottle of acetaminophen. She says she is in academic trouble and has no friends. Kate describes her childhood as mostly happy except for her parents’ arguments. Her medical history indicates she began developing breasts at age 10 and had her first menstrual period at age 12.
Her father is largely absent, traveling and working long hours. Her mother developed postpartum depression and stopped working after Kate’s younger brother was born.
Girls and boys show similar depression risks during childhood, but girls are twice as likely as boys to become clinically depressed after puberty. The key to treating depression in teen girls is to recognize that brain development and fluctuating hormones can influence behavior in ways that confuse them and the people around them. Successfully treating teen girls’ depression may require a gender-specific approach.
3 stages of brain development
Fetal differentiation. All brains start out with female-type brain circuits. At 8 weeks of fetal life, however, tiny testicles in the male begin to produce large amounts of testosterone, which changes the brain and body to male. Thus, sex-specific genes and hormones guide aspects of the first phase of brain development.1
Table 1
Female hormonal development: Gestation to puberty
| Stage/age | Hormonal events | Effect on female brain |
|---|---|---|
| Gestation | Components of reproductive axis form in early embryonic development; at 8 weeks, testosterone from fetal testicles begins to change female-type brain areas to male | Unperturbed by testosterone, brain continues to develop along female lines |
| Birth to age 24 months | Hormone-secreting placenta detaches at birth, dramatically increasing GnRH and LH/FSH and driving infant gonads to produce estrogen in girls or testosterone in boys (“infantile puberty”) | Abundant ovarian estrogen secretion enhances development of brain circuits, such as those associated with reproduction, maternal behavior, and social relatedness |
| Age 24 months to prepuberty | “Brakes” put on GnRH and LH/FSH pulsatile brain cells | “Juvenile pause” begins, with constant low estrogen secretion in girls by 24 months (in boys, “brakes” are on by 12 months) |
| Puberty | “Brakes” released on GnRH and LH/FSH neurons, reactivating reproductive axis | Ovary resumes estrogen production (“adolescent puberty”); increase in estrogen, progesterone, and testosterone stimulates brain circuit development; unipolar depression rates increase to 2:1 (female to male) by age 15 |
| GnRH: gonadotropin-releasing hormone; LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Source: References 4,5 | ||
Infantile puberty and the second phase of brain development begin in early childhood, as the ovaries and testicles start to produce large amounts of estrogen and testosterone soon after birth.
Puberty launches the final brain development phase. Up to 2 years before menstruation begins, pulsatile gonadotropin-releasing hormone cells in the hypothalamus wake up and start stimulating the ovaries to produce estrogen, thrusting the girl brain into puberty (Figure). The teen girl brain begins to experience not only estrogen surges from the ovary but progesterone and testosterone surges as well.
Although brain size and basic circuitry are mostly set by age 5, puberty stimulates new brain cells and increases myelin production.2 Faster myelinated connections between emotionally impulsive limbic brain areas such as the amygdala and sensible, cognitive areas such as the prefrontal cortex are not finished until the early 20s.3
Hormonal changes at puberty
The female brain is remodeled during puberty, leading to sexually dimorphic brain activation and development that further differentiates it from the male brain.4
Estrogen surges are associated with increased production of neurohormones and neurochemicals, such as:
- oxytocin, which reinforces social bonding and intimacy
- dopamine, which stimulates motivation and pleasure circuits in the brain.
Hormonal changes and brain development alter gene expression and affect neurodevelopment. These events may trigger a first depression in pubertal girls with a family history of mood disorder (Table 1).4,5 Although menarche has begun at an average age of 12 in the United States for decades, the most recent National Health and Examination Survey (NHANES) shows puberty onset in girls is occurring earlier (Table 2).6-9
Tanner stage—a measure of pubertal status—is a more accurate predictor of depression in teen girls than age.10 Pubertal transition to Tanner stage 3 (development of pubic and axillary hair and breast buds) is associated with a sharp increase in depression rates. Girls at stage 3 and higher are approximately 3 times more likely to be depressed than girls at stages 1 or 2.11
Pubic hair, breast development, and menstruation are markers for underlying hormonal changes (Table 3).4,5 The onset of estrogen, progesterone, and testosterone surges closely correlates with the difference in depression rates between pre- and postpubertal girls.12 After estrogen and progesterone surges begin at puberty, negative emotions exert an increased activating effect on the female brain,13 and social stressors more deeply affect girls than they do boys. This may explain why girls are more susceptible to depression when a friendship fails.14
CASE CONTINUED: Boy troubles
Kate tells you that in 9th grade she and her best friend, Ellen, would talk about boys for hours after school and try on sexually provocative outfits. They both liked Matt, a 10th grader, so when he asked Kate out, Ellen stopped speaking to her. Kate and Matt began some heavy petting, and Kate said she felt selfish and guilty about hurting Ellen. But when girls at school began spreading rumors that Kate was a “slut,” Kate blamed Ellen and told her, “I hate you!”
Soon after, Matt broke up with Kate. Distraught, she dreaded going to school and cried in her room at night for several weeks. She became chronically tired and had difficulty concentrating in class. She ruminated about losing Matt and worried that she was too fat, too ugly, or too flat-chested. She missed Ellen and felt no one liked her.
Table 2
Puberty’s developmental milestones in U.S. girls (averages)
| Correlate | African Americans | Whites | School grade* |
|---|---|---|---|
| Breast bud development | Age 9 | Age 10 | 4th to 5th |
| Girls with puberty onset by age 8 | 32% | 11% | 3rd |
| Girls with puberty onset by age 10 | 76% | 53% | 5th |
| Menarche onset | Age 12.1 | Age 12.6 | 7th |
| Tanner stage 5† onset | Age 13.9 | Age 15.5 | 8th to 9th |
| * Approximate grade level for age groups | |||
| † Pubic hair and breast development reach adult stage | |||
| Source: Data from references 6-9, including the Pediatric Research in Office Settings network and Third National Health and Nutrition Examination Survey, 1988-1994. | |||
Figure Hypothalamic-pituitary-ovarian axis: Turned on at puberty in girls
Puberty onset stimulates depression in genetically vulnerable girls; more likely after Tanner stage 3 (development of pubic and axillary hair and breast buds).
Male vs female teen brains
Depression after a relationship failure in teen girls often begins with ruminative thoughts about her flaws, mistakes, or appearance. These negative thoughts may preoccupy her day and night. Teen girls often feel confused by contradictory social pressures to look and dress provocatively but resist having sex. A sexual encounter can trigger shame and fear.
Although clinical and developmental studies indicate that teen girls respond more dramatically to relationship troubles than boys, the brain and hormone differences responsible for these effects remain unclear. Male hormones hugely increase in boys at puberty—up to 25-fold between ages 9 and 15—but do not cycle. Male brains do not have the same capacity as female brains to respond to cyclical hormonal activity because exposure to androgens during fetal development eliminates this ability. The fetal testosterone surge causes the area associated with sexual pursuit to double in the male brain.
Outside of fertility considerations, Baron-Cohen et al15 suggest that male brain circuits have been formed by fetal testosterone to focus more on systematization—which emphasizes figuring out how things work and performing tasks—rather than empathy and bonding in relationships. This difference has been shown in neuroimaging studies comparing the genders’ attentional systems.16,17 In contrast to the systematizing male brain, female brains are more likely to activate the mirror neuron system—the area required for empathizing.18
Female brains, of course, respond to cyclical hormonal activity. However, the regular monthly waves of estrogen and progesterone do not affect all female brains the same. A subset of women who experience premenstrual dysphoric disorder appear to have brains that trigger depressed moods and irritability during the last 2 weeks of the menstrual cycle.19 A genetic difference in these women is suspected as the culprit; these genes may affect the way their brains metabolize progesterone.
CASE CONTINUED: An overdose of stress
Kate’s poor concentration lingered, and her grades continued to drop. She tells you her parents were having marital problems and she did not want to bother them with her difficulties. Two days before her period was due, she learned she had failed 2 classes. That night, as she got some acetaminophen for a headache, she impulsively took the rest of the bottle.
After swallowing the pills, Kate panicked. She forced herself to vomit and tearfully told her parents what she had done. They took her to the emergency room, where she was medically stabilized, evaluated by a psychiatrist, and referred to you for outpatient treatment.
Treatment recommendations
A combination of factors—genetic, hormonal, and neurodevelopmental—probably contributed to Kate’s acute depressed mood and overdose. Thus, to treat depression in adolescent girls, emerging evidence supports:
- stabilizing hormonal fluctuations such as rapidly falling progesterone just before the start of menstrual periods with an extended-cycle contraceptive (we would try an ethinyl estradiol/levonorgestrel combination such as Seasonale®)
- treating depressive symptoms with a selective serotonin reuptake inhibitor such as citalopram, 10 mg once daily, with careful monitoring for suicidal thoughts or behavior
- providing tools to manage stress and impulsive behavior through weekly psychotherapy (such as cognitive-behavioral therapy, dialectical behavioral therapy, or supportive therapy).
Genetic factors. Kate’s mother’s history of postpartum depression suggests genetic risk for Kate. Studies have found that the expression of particular genes—such as the serotonin transporter (5-HTT) gene—may be associated with depression. Staley et al20 found that depressed women show a significantly greater decrease in 5-HTT availability in the diencephalon (forebrain region containing the thalamus, hypothalamus, and part of the pituitary gland) when compared with healthy women and depressed men.
Table 3
3 stages of girls’ gonadal development
| Stage | Timing | Developmental events |
|---|---|---|
| Adrenarche | Onset around age 6, peaks by age 20 | Rise in weak androgens (DHEA and DHEAS) from adrenal gland results in pubic and axillary hair and increases likelihood of acne |
| Gonadarche | Usually ~2 years before menarche | Pulses of GnRH, LH/FSH lead to increased estrogen, which stimulates breast development, widening of hips, and increased subcutaneous fat deposition |
| Menarche | Relatively late in puberty (usually not before Tanner stage 4) | “Monthly” cycle established; ovarian estrogen pulses in response to GnRH and FSH, the LH surge, and ovulation; progesterone produced after ovulation |
| DHEA: dehydroepiandrosterone; DHEAS: dehydroepiandrosterone sulfate; GnRH: gonadotropin-releasing hormone; | ||
| LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Tanner stage 4: pubic hair and breast development typical of middle to late adolescence (ages 12 to 17) | ||
| Source: References 4,5 | ||
Although men and women have the same 5-HTT gene, women may possess a gender-specific factor—such as estrogen or progesterone—that differentially alters this and other genes’ expression in women with depression. Individuals who carry a short version of the gene may be at particular risk of becoming depressed when exposed to stressful life events.
Caspi et al21 found a polymorphism in the 5-HTT gene on chromosome 17 that can manifest differentially based on environmental factors. In this study, individuals with 2 copies of the long version of this gene were relatively resistant to stressful life events, whereas those with 1 or 2 copies of the short version were highly sensitive to stressful life events. The depression rate in short-gene individuals was:
- 9% in those who had not experienced stressful life events
- nearly 40% in those who had experienced ≥4 stressful life events.
Hormonal and stress factors. Stress responsiveness becomes sexually dimorphic at puberty. Compared with men, women are:
- at greater risk after puberty for heightened stress responsiveness, which is associated with major depressive disorder
- 3 times more likely to develop depression after a stressful life event.22
Women’s and men’s different biological responses to stress might be related to the gender-specific hormones that emerge during puberty. Kate could be at increased risk for depression—especially immediately before her period—if she inherited a stress-sensitive gene and now has increased stress sensitivity triggered by the hormones of puberty.23
Neurodevelopmental factors. Dorsolateral prefrontal cortex circuits associated with making good decisions and weighing the consequences of actions are immature in the adolescent and the last part of the brain to undergo myelination.24-26 Teens are well-known for erratic, emotionally driven behaviors.27,28 Kate’s impulsive overdose exemplifies the consequences of emotional reactivity without the benefit of inhibitory mature brain connections.
Related resources
- Brizendine L. Teen girl brain. In: The female brain. New York: Morgan Road Books; 2006:31-56. www.thefemalebrain.com.
- Strauch B. The primal teen: what discoveries about the teenage brain tell us about our kids. New York: Doubleday; 2003.
- Harter S. Self and identity development. In: Feldman S, Elliott G, eds. At the threshold: the developing adolescent. Cambridge, MA: Harvard University Press; 1990:352-87.
Drug brand names
- Ethinyl estradiol/levonorgestrel • Seasonale
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Kate, age 14, is referred for follow-up treatment of depression after she impulsively swallowed a bottle of acetaminophen. She says she is in academic trouble and has no friends. Kate describes her childhood as mostly happy except for her parents’ arguments. Her medical history indicates she began developing breasts at age 10 and had her first menstrual period at age 12.
Her father is largely absent, traveling and working long hours. Her mother developed postpartum depression and stopped working after Kate’s younger brother was born.
Girls and boys show similar depression risks during childhood, but girls are twice as likely as boys to become clinically depressed after puberty. The key to treating depression in teen girls is to recognize that brain development and fluctuating hormones can influence behavior in ways that confuse them and the people around them. Successfully treating teen girls’ depression may require a gender-specific approach.
3 stages of brain development
Fetal differentiation. All brains start out with female-type brain circuits. At 8 weeks of fetal life, however, tiny testicles in the male begin to produce large amounts of testosterone, which changes the brain and body to male. Thus, sex-specific genes and hormones guide aspects of the first phase of brain development.1
Table 1
Female hormonal development: Gestation to puberty
| Stage/age | Hormonal events | Effect on female brain |
|---|---|---|
| Gestation | Components of reproductive axis form in early embryonic development; at 8 weeks, testosterone from fetal testicles begins to change female-type brain areas to male | Unperturbed by testosterone, brain continues to develop along female lines |
| Birth to age 24 months | Hormone-secreting placenta detaches at birth, dramatically increasing GnRH and LH/FSH and driving infant gonads to produce estrogen in girls or testosterone in boys (“infantile puberty”) | Abundant ovarian estrogen secretion enhances development of brain circuits, such as those associated with reproduction, maternal behavior, and social relatedness |
| Age 24 months to prepuberty | “Brakes” put on GnRH and LH/FSH pulsatile brain cells | “Juvenile pause” begins, with constant low estrogen secretion in girls by 24 months (in boys, “brakes” are on by 12 months) |
| Puberty | “Brakes” released on GnRH and LH/FSH neurons, reactivating reproductive axis | Ovary resumes estrogen production (“adolescent puberty”); increase in estrogen, progesterone, and testosterone stimulates brain circuit development; unipolar depression rates increase to 2:1 (female to male) by age 15 |
| GnRH: gonadotropin-releasing hormone; LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Source: References 4,5 | ||
Infantile puberty and the second phase of brain development begin in early childhood, as the ovaries and testicles start to produce large amounts of estrogen and testosterone soon after birth.
Puberty launches the final brain development phase. Up to 2 years before menstruation begins, pulsatile gonadotropin-releasing hormone cells in the hypothalamus wake up and start stimulating the ovaries to produce estrogen, thrusting the girl brain into puberty (Figure). The teen girl brain begins to experience not only estrogen surges from the ovary but progesterone and testosterone surges as well.
Although brain size and basic circuitry are mostly set by age 5, puberty stimulates new brain cells and increases myelin production.2 Faster myelinated connections between emotionally impulsive limbic brain areas such as the amygdala and sensible, cognitive areas such as the prefrontal cortex are not finished until the early 20s.3
Hormonal changes at puberty
The female brain is remodeled during puberty, leading to sexually dimorphic brain activation and development that further differentiates it from the male brain.4
Estrogen surges are associated with increased production of neurohormones and neurochemicals, such as:
- oxytocin, which reinforces social bonding and intimacy
- dopamine, which stimulates motivation and pleasure circuits in the brain.
Hormonal changes and brain development alter gene expression and affect neurodevelopment. These events may trigger a first depression in pubertal girls with a family history of mood disorder (Table 1).4,5 Although menarche has begun at an average age of 12 in the United States for decades, the most recent National Health and Examination Survey (NHANES) shows puberty onset in girls is occurring earlier (Table 2).6-9
Tanner stage—a measure of pubertal status—is a more accurate predictor of depression in teen girls than age.10 Pubertal transition to Tanner stage 3 (development of pubic and axillary hair and breast buds) is associated with a sharp increase in depression rates. Girls at stage 3 and higher are approximately 3 times more likely to be depressed than girls at stages 1 or 2.11
Pubic hair, breast development, and menstruation are markers for underlying hormonal changes (Table 3).4,5 The onset of estrogen, progesterone, and testosterone surges closely correlates with the difference in depression rates between pre- and postpubertal girls.12 After estrogen and progesterone surges begin at puberty, negative emotions exert an increased activating effect on the female brain,13 and social stressors more deeply affect girls than they do boys. This may explain why girls are more susceptible to depression when a friendship fails.14
CASE CONTINUED: Boy troubles
Kate tells you that in 9th grade she and her best friend, Ellen, would talk about boys for hours after school and try on sexually provocative outfits. They both liked Matt, a 10th grader, so when he asked Kate out, Ellen stopped speaking to her. Kate and Matt began some heavy petting, and Kate said she felt selfish and guilty about hurting Ellen. But when girls at school began spreading rumors that Kate was a “slut,” Kate blamed Ellen and told her, “I hate you!”
Soon after, Matt broke up with Kate. Distraught, she dreaded going to school and cried in her room at night for several weeks. She became chronically tired and had difficulty concentrating in class. She ruminated about losing Matt and worried that she was too fat, too ugly, or too flat-chested. She missed Ellen and felt no one liked her.
Table 2
Puberty’s developmental milestones in U.S. girls (averages)
| Correlate | African Americans | Whites | School grade* |
|---|---|---|---|
| Breast bud development | Age 9 | Age 10 | 4th to 5th |
| Girls with puberty onset by age 8 | 32% | 11% | 3rd |
| Girls with puberty onset by age 10 | 76% | 53% | 5th |
| Menarche onset | Age 12.1 | Age 12.6 | 7th |
| Tanner stage 5† onset | Age 13.9 | Age 15.5 | 8th to 9th |
| * Approximate grade level for age groups | |||
| † Pubic hair and breast development reach adult stage | |||
| Source: Data from references 6-9, including the Pediatric Research in Office Settings network and Third National Health and Nutrition Examination Survey, 1988-1994. | |||
Figure Hypothalamic-pituitary-ovarian axis: Turned on at puberty in girls
Puberty onset stimulates depression in genetically vulnerable girls; more likely after Tanner stage 3 (development of pubic and axillary hair and breast buds).
Male vs female teen brains
Depression after a relationship failure in teen girls often begins with ruminative thoughts about her flaws, mistakes, or appearance. These negative thoughts may preoccupy her day and night. Teen girls often feel confused by contradictory social pressures to look and dress provocatively but resist having sex. A sexual encounter can trigger shame and fear.
Although clinical and developmental studies indicate that teen girls respond more dramatically to relationship troubles than boys, the brain and hormone differences responsible for these effects remain unclear. Male hormones hugely increase in boys at puberty—up to 25-fold between ages 9 and 15—but do not cycle. Male brains do not have the same capacity as female brains to respond to cyclical hormonal activity because exposure to androgens during fetal development eliminates this ability. The fetal testosterone surge causes the area associated with sexual pursuit to double in the male brain.
Outside of fertility considerations, Baron-Cohen et al15 suggest that male brain circuits have been formed by fetal testosterone to focus more on systematization—which emphasizes figuring out how things work and performing tasks—rather than empathy and bonding in relationships. This difference has been shown in neuroimaging studies comparing the genders’ attentional systems.16,17 In contrast to the systematizing male brain, female brains are more likely to activate the mirror neuron system—the area required for empathizing.18
Female brains, of course, respond to cyclical hormonal activity. However, the regular monthly waves of estrogen and progesterone do not affect all female brains the same. A subset of women who experience premenstrual dysphoric disorder appear to have brains that trigger depressed moods and irritability during the last 2 weeks of the menstrual cycle.19 A genetic difference in these women is suspected as the culprit; these genes may affect the way their brains metabolize progesterone.
CASE CONTINUED: An overdose of stress
Kate’s poor concentration lingered, and her grades continued to drop. She tells you her parents were having marital problems and she did not want to bother them with her difficulties. Two days before her period was due, she learned she had failed 2 classes. That night, as she got some acetaminophen for a headache, she impulsively took the rest of the bottle.
After swallowing the pills, Kate panicked. She forced herself to vomit and tearfully told her parents what she had done. They took her to the emergency room, where she was medically stabilized, evaluated by a psychiatrist, and referred to you for outpatient treatment.
Treatment recommendations
A combination of factors—genetic, hormonal, and neurodevelopmental—probably contributed to Kate’s acute depressed mood and overdose. Thus, to treat depression in adolescent girls, emerging evidence supports:
- stabilizing hormonal fluctuations such as rapidly falling progesterone just before the start of menstrual periods with an extended-cycle contraceptive (we would try an ethinyl estradiol/levonorgestrel combination such as Seasonale®)
- treating depressive symptoms with a selective serotonin reuptake inhibitor such as citalopram, 10 mg once daily, with careful monitoring for suicidal thoughts or behavior
- providing tools to manage stress and impulsive behavior through weekly psychotherapy (such as cognitive-behavioral therapy, dialectical behavioral therapy, or supportive therapy).
Genetic factors. Kate’s mother’s history of postpartum depression suggests genetic risk for Kate. Studies have found that the expression of particular genes—such as the serotonin transporter (5-HTT) gene—may be associated with depression. Staley et al20 found that depressed women show a significantly greater decrease in 5-HTT availability in the diencephalon (forebrain region containing the thalamus, hypothalamus, and part of the pituitary gland) when compared with healthy women and depressed men.
Table 3
3 stages of girls’ gonadal development
| Stage | Timing | Developmental events |
|---|---|---|
| Adrenarche | Onset around age 6, peaks by age 20 | Rise in weak androgens (DHEA and DHEAS) from adrenal gland results in pubic and axillary hair and increases likelihood of acne |
| Gonadarche | Usually ~2 years before menarche | Pulses of GnRH, LH/FSH lead to increased estrogen, which stimulates breast development, widening of hips, and increased subcutaneous fat deposition |
| Menarche | Relatively late in puberty (usually not before Tanner stage 4) | “Monthly” cycle established; ovarian estrogen pulses in response to GnRH and FSH, the LH surge, and ovulation; progesterone produced after ovulation |
| DHEA: dehydroepiandrosterone; DHEAS: dehydroepiandrosterone sulfate; GnRH: gonadotropin-releasing hormone; | ||
| LH/FSH: luteinizing hormone/follicle-stimulating hormone | ||
| Tanner stage 4: pubic hair and breast development typical of middle to late adolescence (ages 12 to 17) | ||
| Source: References 4,5 | ||
Although men and women have the same 5-HTT gene, women may possess a gender-specific factor—such as estrogen or progesterone—that differentially alters this and other genes’ expression in women with depression. Individuals who carry a short version of the gene may be at particular risk of becoming depressed when exposed to stressful life events.
Caspi et al21 found a polymorphism in the 5-HTT gene on chromosome 17 that can manifest differentially based on environmental factors. In this study, individuals with 2 copies of the long version of this gene were relatively resistant to stressful life events, whereas those with 1 or 2 copies of the short version were highly sensitive to stressful life events. The depression rate in short-gene individuals was:
- 9% in those who had not experienced stressful life events
- nearly 40% in those who had experienced ≥4 stressful life events.
Hormonal and stress factors. Stress responsiveness becomes sexually dimorphic at puberty. Compared with men, women are:
- at greater risk after puberty for heightened stress responsiveness, which is associated with major depressive disorder
- 3 times more likely to develop depression after a stressful life event.22
Women’s and men’s different biological responses to stress might be related to the gender-specific hormones that emerge during puberty. Kate could be at increased risk for depression—especially immediately before her period—if she inherited a stress-sensitive gene and now has increased stress sensitivity triggered by the hormones of puberty.23
Neurodevelopmental factors. Dorsolateral prefrontal cortex circuits associated with making good decisions and weighing the consequences of actions are immature in the adolescent and the last part of the brain to undergo myelination.24-26 Teens are well-known for erratic, emotionally driven behaviors.27,28 Kate’s impulsive overdose exemplifies the consequences of emotional reactivity without the benefit of inhibitory mature brain connections.
Related resources
- Brizendine L. Teen girl brain. In: The female brain. New York: Morgan Road Books; 2006:31-56. www.thefemalebrain.com.
- Strauch B. The primal teen: what discoveries about the teenage brain tell us about our kids. New York: Doubleday; 2003.
- Harter S. Self and identity development. In: Feldman S, Elliott G, eds. At the threshold: the developing adolescent. Cambridge, MA: Harvard University Press; 1990:352-87.
Drug brand names
- Ethinyl estradiol/levonorgestrel • Seasonale
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci 2004;5(9):701-8.
2. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 1996;6(4):551-60.
3. Yurgelun-Todd DA. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. In press.
4. Cameron J. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Ann NY Acad Sci 2004;1021:110-23.
5. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
6. Biro F, Huang B, Crawford P, et al. Pubertal correlates in black and white girls. J Pediatr 2006;148(2):234-40.
7. Herman-Giddens M, Kaplowitz P, Wasserman R. Navigating the recent articles on girls’ puberty in pediatrics: what do we know and where do we go from here? Pediatrics 2004;113(4):911-7.
8. Herman-Giddens M, Slora E, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997;99(4):505-12.
9. Wu T, Mendola P, Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics 2002;110(4):752-7.
10. Rapkin A, Tsao J, Turk N, et al. Relationships among self-rated Tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol 2006;19:181-7.
11. Angold A, Costello E, Worthman C. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 1998;28:51-61.
12. Angold A, Costello E, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol Med 1999;29:1043-53.
13. Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage 2006;32(2):854-62.
14. McClure EB, Parrish JM, Nelson EE, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol. In press.
15. Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci 2003;358(1430):361-74.
16. Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 2005;28(3):618-26.
17. Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept Mot Skills 2004;99(2):371-91.
18. Cheng YW, Tzeng OJ, Decety J, et al. Gender differences in the human mirror system: a magnetoencephalography study. Neuroreport 2006;17(11):1115-9.
19. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and those without premenstrual syndrome. N Engl J Med 1998;338(4):209-16.
20. Staley J, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59:40-7.
21. Caspi A, Sugden K, Moffitt T, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9.
22. Maciejewski P, Prigerson H, Mazure C. Sex differences in event-related risk for major depression. Psychol Med 2001;31(4):593-604.
23. Seeman M. Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997;154(12):1641-7.
24. Kupfer D, Woodward H. Adolescent development and the regulation of behavior and emotion. Ann NY Acad Sci 2004;1021:320-2.
25. Kelley A, Schochet T, Landry C. Risk taking and novelty seeking in adolescence. Ann NY Acad Sci 2004;1021:27-32.
26. Ellis L, Rothbart M, Posner M. Individual differences in executive attention predict self-regulation and adolescent psychosocial behaviors. Ann NY Acad Sci 2004;1021:337-40.
27. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
28. Pelkonen M, Marttunen M. Child and adolescent suicide: epidemiology, risk factors, and approaches to prevention. Paediatr Drugs 2003;5(4):243-65.
1. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci 2004;5(9):701-8.
2. Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex 1996;6(4):551-60.
3. Yurgelun-Todd DA. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. In press.
4. Cameron J. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Ann NY Acad Sci 2004;1021:110-23.
5. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
6. Biro F, Huang B, Crawford P, et al. Pubertal correlates in black and white girls. J Pediatr 2006;148(2):234-40.
7. Herman-Giddens M, Kaplowitz P, Wasserman R. Navigating the recent articles on girls’ puberty in pediatrics: what do we know and where do we go from here? Pediatrics 2004;113(4):911-7.
8. Herman-Giddens M, Slora E, Wasserman R, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997;99(4):505-12.
9. Wu T, Mendola P, Buck G. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics 2002;110(4):752-7.
10. Rapkin A, Tsao J, Turk N, et al. Relationships among self-rated Tanner staging, hormones, and psychosocial factors in healthy female adolescents. J Pediatr Adolesc Gynecol 2006;19:181-7.
11. Angold A, Costello E, Worthman C. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med 1998;28:51-61.
12. Angold A, Costello E, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol Med 1999;29:1043-53.
13. Hofer A, Siedentopf CM, Ischebeck A, et al. Gender differences in regional cerebral activity during the perception of emotion: a functional MRI study. Neuroimage 2006;32(2):854-62.
14. McClure EB, Parrish JM, Nelson EE, et al. Responses to conflict and cooperation in adolescents with anxiety and mood disorders. J Abnorm Child Psychol. In press.
15. Baron-Cohen S, Richler J, Bisarya D, et al. The systemizing quotient: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos Trans R Soc Lond B Biol Sci 2003;358(1430):361-74.
16. Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 2005;28(3):618-26.
17. Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Percept Mot Skills 2004;99(2):371-91.
18. Cheng YW, Tzeng OJ, Decety J, et al. Gender differences in the human mirror system: a magnetoencephalography study. Neuroreport 2006;17(11):1115-9.
19. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and those without premenstrual syndrome. N Engl J Med 1998;338(4):209-16.
20. Staley J, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry 2006;59:40-7.
21. Caspi A, Sugden K, Moffitt T, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386-9.
22. Maciejewski P, Prigerson H, Mazure C. Sex differences in event-related risk for major depression. Psychol Med 2001;31(4):593-604.
23. Seeman M. Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997;154(12):1641-7.
24. Kupfer D, Woodward H. Adolescent development and the regulation of behavior and emotion. Ann NY Acad Sci 2004;1021:320-2.
25. Kelley A, Schochet T, Landry C. Risk taking and novelty seeking in adolescence. Ann NY Acad Sci 2004;1021:27-32.
26. Ellis L, Rothbart M, Posner M. Individual differences in executive attention predict self-regulation and adolescent psychosocial behaviors. Ann NY Acad Sci 2004;1021:337-40.
27. Dahl R. Adolescent brain development: a period of vulnerabilities and opportunities. Ann NY Acad Sci 2004;1021:1-22.
28. Pelkonen M, Marttunen M. Child and adolescent suicide: epidemiology, risk factors, and approaches to prevention. Paediatr Drugs 2003;5(4):243-65.
How to control weight gain when prescribing antidepressants
Hear Dr. Schwartz's strategies for monitoring patients during antidepressant therapy and for motivating them to lose weight. Click here.
Weight gain occurs with most antidepressants but is frequently overlooked, perhaps because clinicians are focused instead on metabolic effects of antipsychotics and mood stabilizers. Patients taking antidepressants often complain of weight gain, however, and many of the drugs’ FDA-approved package inserts acknowledge this effect.
Two-thirds of patients with major depression present with weight loss, and gaining weight can be associated with successful treatment. Weight gain is of concern—and likely to be drug-induced—if it exceeds the disease-induced weight loss and continues after depressive symptoms improve.
Weight may change early or late during antidepressant treatment, and gaining in the first weeks usually predicts future gains.1 Patients who are overweight when treatment begins are especially at risk if given weight-promoting agents. This article:
- compares antidepressant effects on patient weight
- discusses mechanisms by which antidepressants may cause weight gain
- outlines a plan to prevent excess weight gain when patients start antidepressant therapy
- recommends diet, exercise, cognitive-behavioral therapy (CBT), and medications for overweight patients on long-term antidepressant treatment.
Weight-gain potential by class
Unlike antipsychotics, antidepressants have not been associated in clinical trials with causing metabolic syndrome and diabetes. Even so, certain antidepressants can cause clinically significant and perhaps more insidious weight gain when compared with some second-generation antipsychotics (SGAs). For example, SGAs on average may cause 2.3 kg/month weight gain during the first 12 weeks of treatment, and mirtazapine caused 3 kg weight gain in a recent 6-week trial.2,3
Tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) may pose a greater weight-gain risk than newer antidepressants, but selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been clinically noted to cause weight gain over time (Table 1).4-16
SSRIs. Weight gain associated with long-term SSRI use seems clinically apparent, but the evidence is preliminary.
Paroxetine seems to be the SSRI most likely to cause weight gain. A 26- to 32-week comparison trial by Fava et al10 showed that weight gain risk with SSRI therapy varies with the drug used. In this trial, 284 patients with major depressive disorder were randomly assigned to double-blind treatment with paroxetine, sertraline, or fluoxetine:
- More of those taking paroxetine gained >7% in weight from baseline, and their weight gain was statistically significant.
- Sertraline-treated patients had modest, nonsignificant weight gain.
- Fluoxetine-treated patients had modest, nonsignificant weight loss.
Using paroxetine with an antipsychotic can be especially problematic. Fukowi and Murai17 described 2 cases in which adding paroxetine to risperidone caused severe weight gain (13.5 kg to >14 kg) in 4 to 5 months.
Citalopram may cause a 1- to 1.5-kg weight gain over 1 year,8 whereas fluvoxamine has been shown not to affect weight in obese patients.11 Citalopram (like TCAs) can cause carbohydrate craving and early weight gain.18 Escitalopram caused a modest (0.5 kg) weight gain in elderly patients during an 8-week trial.13
Initial weight loss followed by overall weight gain after 1 year of SSRI treatment is a common clinical finding that was not noted in initial acute SSRI drug trials. In a comparison of fluoxetine’s acute and long-term effects,19 839 patients experiencing a major depressive episode were first treated with open-label fluoxetine, 20 mg/d. After 12 weeks, 395 patients who met criteria for remission were randomly assigned to continue with placebo or fluoxetine, 20 mg/d, for 14, 38, or 50 weeks.
In the acute phase, a small but statistically significant weight loss (mean 0.35 kg, P
- 1.1 kg at 26 weeks (P
- 2.2 kg at 38 weeks (P
- 3.1 kg at 50 weeks (P
The authors concluded that the weight gain—similar with fluoxetine or placebo—was probably associated with recovery from depression rather than fluoxetine treatment, although this was not a controlled variable in the study.
Table 1
Long-term effects of antidepressants on body weight, by class*
| Class | Effect (gain, loss, or neutral) |
|---|---|
| MAOIs | Moderate gain overall |
| Phenelzine: greatest gain in MAOI class | |
| Transdermal selegiline: appears neutral | |
| Novel antidepressants | Bupropion: weight loss4 |
| Mirtazapine: greatest potential for gain among antidepressants5 | |
| Nefazodone: neutral6 | |
| Trazodone: modest gain7 | |
| SSRIs | Citalopram: modest gain8 |
| Escitalopram: modest gain9 | |
| Fluoxetine: modest loss acutely10 | |
| Fluvoxamine: neutral11 | |
| Paroxetine: greatest gain in SSRI class10 | |
| Sertraline: modest gain10 | |
| SNRIs | Duloxetine: modest gain12 |
| Venlafaxine: modest gain (controversial)13 | |
| TCAs | Amitriptyline: gain14 |
| Imipramine: gain15 | |
| Nortriptyline: neutral16 | |
| * Information is a general representation of available literature, gathered from many studies with differing designs. Consult original reports for specific data on dosing, patient populations, treatment durations, and weigh changes. | |
| MAOIs: monoamine oxidase inhibitors; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | |
Causes of weight gain
Serotonin. Appetite is controlled by cultural, psychological, neurochemical, and metabolic factors. Among neurochemical factors, serotonin helps regulate appetite and is the neurotransmitter most often manipulated in depression treatment.
Serotonin receptor agonists such as fenfluramine and dexfenfluramine have an acute anorexigenic effect. In rats, 5-HT2c receptor agonism decreases eating behavior, and mice lacking 5-HT2c receptors are obese.20 This may explain why SGAs or antidepressants that block 5-HT2c pose the greatest risk of weight gain.
SSRI or SNRI treatment might increase serotonin in the synaptic cleft, allowing 5-HT2c receptor down-regulation that is slower than—but similar in effect to—the acute 5-HT2c blockade caused by the SGAs.21 Weight gain from SSRI use reflects on these medications’ multiple serotonergic mechanisms. Serotonin appears to regulate carbohydrate intake and can increase food intake.22
Nefazodone and trazodone block 5HT2a receptors potently, and the norepinephrine (nefazodone only) and serotonin reuptake pumps (both agents) less potently. Differences in their mechanism (nefazodone increases norepinephrine) and lack of 5-HT2c blockade might be responsible for their reported weight neutrality.
Tricyclics block differing ratios of norepinephrine and serotonin reuptake pumps, resulting in postsynaptic serotonergic and adrenergic receptor desensitization and, later, down-regulation. TCAs with higher serotonin reuptake blockade may increase weight through this desensitization.
TCAs also affect appetite by blocking histaminergic (H1) pathways. Drugs with high affinity for blocking H1 receptors have been associated with carbohydrate craving18 and low satiety rates that allow increased calorie intake. TCAs have antimuscarinic, antihistaminic, and alpha adrenoceptor-blocking actions, all of which may contribute to weight gain.
In theory, beta-3 adrenergic receptors in adipose tissue may play a role in weight control by converting fat into heat and energy, especially in response to norepinephrine. TCAs or SNRIs that favor a noradrenergic profile may promote weight loss or neutrality. The relatively weight-neutral selegiline patch, which avoids first-pass metabolism and active adverse metabolites, also may use this mechanism.23
Mirtazapine blocks presynaptic alpha-2 and postsynaptic 5HT2a, 5HT2c, and 5HT3 serotonin receptors as well as H1 histamine receptors. Both 5HT2c and H1 blockade result in weight gain, the drug’s most apparent adverse effect. This mechanism is similar to that of the SGA olanzapine.
TNF-α. Obese persons have increased plasma levels of TNF-α and its soluble receptor (sTNF-R p75), which may induce insulin resistance. Activation of the TNF-α system, such as by amitriptyline or mirtazapine, may promote weight gain.24
Preventing weight gain
Early intervention is key to preventing drug-related weight gain and treating obesity. Provide informed consent and psychoeducation when prescribing antidepressants. In patients at metabolic risk, consider using weight-neutral or weight-loss agents (Table 2),4-16 and monitor for weight gain (Table 3). At-risk patients have:
- abdominal obesity (waist circumference >40 inches [102 cm] in men, >35 inches [88 cm] in women, or waist-to-hip ratio >0.9 in women and >1.0 in men)
- hyperlipidemia
- elevated body mass index (BMI [overweight=BMI 25 to 30 kg/m2, obesity=BMI >30 kg/m2])
- hypertension
- diabetes mellitus or impaired glucose tolerance
- history of stroke or cardiovascular disease
- family history of obesity, hypertension, diabetes, or hyperlipidemia.
Use SGA guidelines? Consider following modified American Diabetes Association guidelines for metabolic monitoring of patients treated with SGAs.25 We suggest that you follow SGA guidelines as a default when using mirtazapine—which is pharmacodynamically the most similar to SGAs—and TCAs. For patients taking other antidepressants, we recommend that you:
- measure blood pressure and weight, and calculate BMI often
- instruct patients to weigh themselves at home at least weekly in the morning and to report gains >5 lbs.
An overall 10-lb weight gain is clinically significant in most patients and calls for a management plan. Abdominal girth often increases as part of metabolic syndrome. If you choose to measure this variable and are uncomfortable reaching around patients while measuring, allow patients to apply the tape measure themselves.
Lab tests. Obtain fasting glucose and lipid levels at baseline for most patients and then quarterly in those with initial weight gain, medical comorbidities, or family history of hypertension, hypercholesterolemia, or diabetes. Many clinicians also screen for hypothyroidism and anemia, and these tests may be added. For patients without metabolic risk factors taking SSRIs and SNRIs, start quarterly draws if weight increases rapidly by >5 lbs or if BMI approaches ≥30 kg/m2. Tracking fasting triglycerides can serve as a sentinel for metabolic syndrome, which sometimes occurs before substantial weight gain or hyperglycemia.
Table 2
Antidepressants’ relative long-term effects on body weight
| Effect | Antidepressants |
|---|---|
| Loss | Bupropion,4 fluoxetine10 |
| Gain | Modest: citalopram,8 duloxetine,12 escitalopram,9 sertraline,10 trazodone,7 venlafaxine13 |
| Relatively more: amitriptyline,14 imipramine,15 mirtazapine,5 paroxetine,10 phenelzine | |
| Neutral | Fluvoxamine,11 nefazodone,6 nortriptyline16 |
| Information is a general representation of available literature, gathered from many studies with differing designs. Consult original reports for specific data on dosing, patient populations, treatment durations, and weight changes. | |
Table 3
Using antidepressants in patients at metabolic risk for weight gain
|
Dietary measures
If weight gain has occurred, a safe initial goal for patients is to lose 0.5% to 1% initial body weight per week—or 5% to 10% of weight across several months. Diet and exercise produce maximal benefit but require commitment and motivation, which are often difficult or impossible for depressed patients. Encouraging the patient’s efforts is worthwhile; if intervention is postponed until remission is achieved, weight gain may be substantially higher and more difficult to treat.
Cutting fat and calories. The first step in losing weight is to restrict high-fat and high-calorie foods and eat smaller portions. If this fails, then switch the patient to a low- or very-low-calorie diet, which provides a quick initial weight loss. This can motivate the patient but should be tried only under a physician’s supervision.
Many patients benefit from structured commercial weight-loss programs, but the likelihood of regaining the weight is high if stopped. These programs typically recommend 1,200 kcal/day for women and 1,800 kcal/day for men, with 55% of calories from carbohydrates, about 25% to 35% from protein, and 10% to 25% from fat.
In a study of 100 patients, those on 2 liquid meal replacements per day plus snacks and 1 low-fat meal (approximately 1,200 to 1,500 kcal/day) lost considerable weight in the first 3 months but regained some weight later. Many maintained weight loss on 1 liquid meal replacement per day plus snacks and 2 low-fat meals.26
Low- and very-low-calorie diets are indicated for patients with BMI >35 kg/m2:
- in whom conservative treatment (a portion-controlled, low-fat diet) has failed
- and who are willing to maintain at least 1 year of treatment and major lifestyle changes.
A low-calorie diet provides ≥1,000 kcal/day; very low-calorie diets may provide ≤800 kcal/day and rely mostly on liquid meal replacements. This semi-starvation can produce fatigue, weakness, lightheadedness, and changes in vital signs, including blood pressure, heart rate, and respiratory rate. For this reason, extreme diets require a team approach with the primary care clinician and a dietitian.
Among mentally healthy patients following very-low-calorie diets in clinical trials, 90% lose ≥10 kg and 50% lose ≥20 kg in the first 4 to 6 months.27 Most weight loss occurs in the first 12 to 16 weeks, after which an ad libitum low-fat, high-fiber diet can be used.
Exercise has physiologic and psychological benefits, including inhibiting food intake and promoting a sense of self-control. Physical exercise increases insulin sensitivity and reduces the risk of secondary medical problems, such as heart disease. Walking ≥40 minutes daily produces maximal benefit, but walking even 30 minutes 3 times a week can help maintain weight.
CBT. Eating habits can be changed through identifying lifestyle behaviors to be modified, setting goals, modifying triggers of excessive eating, and reinforcing desired behavior with CBT. Gradual but consistent behavior change leads to healthier eating habits, exercise, and weight loss. Behavior modification alone can generate a weight loss of 0.5 kg to 0.7 kg per week.28
A study of 6 schizophrenia patients (mean age 37) examined CBT effects on weight gain associated with clozapine (n=4) or olanzapine (n=2). Mean BMI decreased from 29.6 kg/m2 to 25.1 kg/m2 after 7 to 9 sessions of individual CBT, followed by 16 biweekly group sessions that focused on weight reduction and weight maintenance. A dietician provided detailed counseling.28
Using medications for weight loss
Switching. To avoid polypharmacy, consider switching the patient to a weight-neutral or weight-losing antidepressant, such as bupropion. Keep in mind when switching medications, however, that the next agent with less weight-gain potential might not deliver comparable antidepressant efficacy.
Antiobesity drugs. Short of switching, an antiobesity drug (Table 4)29-31 or off-label intervention (Table 5)32-36 may be warranted. Antiobesity drugs should not be used as primary therapy for obesity. Their use may be warranted, however, for psychiatric patients who:
- are unable to fully participate in diet and exercise programs because of symptoms (such as cognitive impairment or severe negative symptoms)
- lack social support (such as reflected by financial problems, homelessness, or poor compliance with treatment recommendations).
Generally, we reserve antiobesity drugs for patients with BMI >30 kg/m2 (or >27 kg/m2 in patients with diabetes, hyperlipidemia, or cardiovascular disease). Before adding these agents to a psychotropic regimen, however, review the relative risks and benefits with the patient and his or her primary care physician.
The goal of pharmacotherapy is for the patient to lose 5% to 10% of baseline weight in 3 to 6 months. Failure to achieve this goal is an indication to stop the medication. A plateau in weight loss after 6 to 9 months is expected and is not cause for discontinuation. If successful, drug treatment may be continued indefinitely, and both physician and patient must understand the intention to treat long-term. Most patients regain weight upon discontinuation.
Table 4
Medications indicated for treating obesity
| Drug/mechanism | Indication/dosage | Evidence of efficacy, safety | Comment |
|---|---|---|---|
| Sibutramine (sympathomimetic; serotonergic, noradrenergic reuptake inhibitor) | Obesity (5 to 20 mg/d) | ↓10% to 15% of body weight in 1 year;29 safety, efficacy beyond 1 year undetermined | ↓ triglycerides, total cholesterol, LDL cholesterol ↑ HDL cholesterol Monitor for serotonin syndrome when used with serotonergic psychotropics |
| Orlistat (inhibits gastric and pancreatic lipases by binding to these enzymes in the gut) | Obesity (120 mg tid with meals; take other drugs 1 hour pre- or post-orlistat) | ↓9% to 10% of body weight in 1 year;30 safety, efficacy beyond 2 years undetermined | ↓ triglycerides, total cholesterol, LDL cholesterol ↑ HDL cholesterol Lower risk of drug interactions than with sibutramine; GI side effects; multivitamin required |
| Rimonabant (investigational, pending FDA approval; selective type 1 cannabinoid receptor blocker) | Obesity (20 mg/d) (pending approval) | Reduced weight, improved heart disease risk factors in obese patients with metabolic syndrome or >1 cardiovascular risk factors (1-2 years)31 | Generally well-tolerated; mild nausea most common side effect |
| * Many studies in this table were conducted in patients taking second-generation antipsychotics for schizophrenia or bipolar disorder. Results may not apply to antidepressant-induced weight gain. | |||
| GI: gastrointestinal; HDL: high-density lipoprotein; LDL: low-density lipoprotein | |||
Table 5
Medications used ‘off label’ for treating obesity
| Drug/mechanism | Indication/dosage | Evidence of efficacy, safety | Comment |
|---|---|---|---|
| Amantadine (antiviral agent; may potentiate dopaminergic function) | Influenza A prophylaxis and Parkinson’s disease (300 mg/d, with olanzapine) | ↓3.5 kg over 3 to 6 months (study of 12 patients)32 | Patients had gained a mean 7.3 kg during olanzapine treatment |
| Nizatidine (histamine-2 receptor antagonist) | Duodenal ulcer; GERD (600 mg/d as prophylaxis with olanzapine) | ↓2.5 kg with nizatidine; ↑5.5 kg with placebo33 (16-week RCT) | Unknown effectiveness when used as prophylaxis with antidepressants; can cause delirium, especially in older patients |
| Naltrexone (opioid antagonist; decreases craving for sweet, fatty foods caused by TCAs and lithium) | Alcohol, narcotics addiction (50 mg/d) | TCA-induced weight gain reversed, then resumed after drug was stopped (8-patient trial)34 | Small mean weight loss compared with previous drug-induced weight gain; no adverse effects seen on depressive symptoms |
| Topiramate (anticonvulsant) | Epilepsy, migraine (100 to 400 mg/d as adjunct to antipsychotics) | ↓10 to 15 lbs in 33% to 55% of bipolar disorder patients35 | May serve dual purpose in treating obese patients with affective disorders; fatigue, cognitive dulling, ataxia, glaucoma, oligohydrosis, acidosis are possible |
| Metformin (biguanide antihyperglycemic) | Type 2 diabetes (500 mg tid as adjunct to antipsychotics) | 15 of 19 patients who gained 10% in body weight taking SGAs lost weight with add-on metformin (12-week, open-label trial) | Sporadic diarrhea in some patients; risk of lactic acidosis (tests unremarkable in this small trial)36 |
| * Many studies in this table were conducted in patients taking second-generation antipsychotics for schizophrenia or bipolar disorder. Results may not apply to antidepressant-induced weight gain. | |||
| GERD: gastroesophageal reflux disease; RCT: randomized, double-blind, placebo-controlled trial; SGAs: second-generation antipsychotics; TCAs: tricyclic antidepressants | |||
Related resources
- Centers for Disease Control and Prevention. Overweight and obesity: contributing factors. www.cdc.gov/nccdphp/dnpa/obesity/contributing_factors.htm.
- Mathur R. What causes obesity? www.medicinenet.com/obesity_weight_loss/page2.htm.
Drug brand names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Metformin • Glucophage
- Mirtazapine • Remeron
- Naltrexone • ReVia
- Nefazodone • Serzone
- Nizatidine • Axid
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Paroxetine • Paxil
- Phenelzine • Nardil
- Selegiline (transdermal) • EMSAM
- Sertraline • Zoloft
- Sibutramine • Meridian
- Topiramate • Topamax
- Trazodone • Desyrel
- Venlafaxine • Effexor
Disclosure
Dr. Schwartz has received grants from or served as a consultant to AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Jazz Pharmaceuticals, Pfizer Inc., and Wyeth.
Other authors report no financial relationship with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Himmerich H, Schuld A, Haack M, et al. Early prediction of changes in weight during six weeks of treatment with antidepressants. J Psychiatr Res 2004;38(5):485-9.
2. Wetterling T. Bodyweight gain with atypical antipsychotics. A comparative review. Drug Saf 2001;24(1):59-73.
3. Laimer M, Kramer-Reinstadler K, Rauchenzauner M, et al. Effect of mirtazapine treatment on body composition and metabolism. J Clin Psychiatry 2006;67(3):421-4.
4. Chouinard G. Bupropion and amitriptyline in the treatment of depressed patients. J Clin Psychiatry 1983;44:121-9.
5. Ribeiro L, Busnello JV, Kauer-Sant’Anna M, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Brazil J Med Biol Res 2001;34:1303-7.
6. Davis R, Whittington R, Bryson HM. Nefazodone. A review of its pharmacology and clinical efficacy in the management of major depression. Drugs 1997;54:186-7.
7. Weisler RH, Johnston JA, Lineberry CG, et al. Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychiatry 1994;14:170-9.
8. Leinonen E, Skarstein J, Behnke K, et al. For the Nordic Antidepressant Study Group. Efficacy and tolerability of mirtazapine versus citalopram: a double blind, randomized study in patients with major depressive disorder. Int Clin Psychopharm 1999;14(6):329-37.
9. Kasper S, Lemming OM, de Swart H. Escitalopram in the long-term treatment of major depressive disorder in elderly patients. Neuropsychobiology 2006;54(3):152-9.
10. Fava M, Judge R, Hoog SL, et al. Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long term treatment. J Clin Psychiatry 2000;61(11):863-7.
11. Abell CA, Farquhar DL, Galloway SM, et al. Placebo-controlled, double-blind trial of fluvoxamine maleate in the obese. J Psychosomat Res 1986;30:143-6.
12. Wise TN, Perahia DG, Pangallo BA, et al. Effects of the antidepressant duloxetine on body weight: analyses of 10 clinical studies. Prim Care Companion J Clin Psychiatry 2006;8(5):269-78.
13. Silverstone PH, Ravindran A. Once daily velafaxine extended release compared with fluoxetine in outpatients with depression and anxiety. J Clin Psychiatry 2000;61(suppl 2):20-5.
14. Zetin M, Frost NR, Brumfield D, et al. Amitriptyline stimulates weight gain in hemodialysis patients. Clin Nephrol 1982;18:79-82.
15. Fernstrom MH, Krowinski RL, Kupfer DJ. Chronic imipramine treatment and weight gain. Psychiatr Res 1986;17:269-73.
16. Prince JB, Wilens TE, Biederman J, et al. A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2000;10:193-204.
17. Fukui H, Murai T. Severe weight gain induced by combination treatment with risperidone and paroxetine. Clin Neuropharmacol 2002;25(5):269-71.
18. Bouwer CD, Harvey BH. Phasic craving for carbohydrate observed with citalopram. Int Clin Psychopharmacol 1996;11:273-8.
19. Michelson D, Amsterdam J, Quitkin FM, et al. Changes in weight during a 1-year trial of fluoxetine. Am J Psychiatry 1999;156(8):1170-6.
20. Curzon G, Gibson EL, Oluyomi AO. Appetite suppression by commonly used drugs depends on 5HT availability. Trends Pharmacol Sci 1998;13:12-25.
21. De Vry J, Schreiber R. Effects of selected serotonin 5-HT1 and 5-HT2 receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev 2000;24:341-53.
22. Bickerdike HJ, Vickers SP, Dourish CT. 5HT2c receptor modulation and the treatment of obesity. Diabetes Obes Metab 1999;1:207-14.
23. Strosberg AD, Pietri-Rouxel F. Function and regulation of beta-3 adrenoceptor. Trends Pharmacol Sci 1996;17:373-81.
24. Kraus T, Haack M, Schuld A, et al. Body weight, the tumor necrosis factor system, and leptin production during treatment with mirtazapine or venlafaxine. Pharmacopsychiatry 2002;35(6):220-5.
25. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
26. Ditschuneit HH, Flechtner-Mors M, Johnson TD, et al. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clinical Nutrition 1999;69:198-204.
27. Wadden TA. Evidence for success of calorie restriction in weight control; summary data from clinical research studies. In: Scannell SM, ed. Methods for voluntary weight loss and control. Bethesda, MD: U.S. Department of Health and Human Services 1992;64-74.
28. Umbricht D, Flury H, Bridler R. Cognitive behavior therapy for weight gain. Am J Psychiatry 2001;158:971-2.
29. Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight loss. Obes Res 1999;7(2):189-98.
30. Van Gaal LF, Broom JI, Enzi G, et al. Efficacy and tolerability of orlistat in the treatment of obesity: a 6-month dose-ranging study. Eur J Clin Pharmacol 1998;54:125-32.
31. Kakafika AI, Mikhailidis DP, Karagiannis A, et al. The role of endocannabinoid system blockade in the treatment of metabolic syndrome. J Clin Pharmacol [serial online]. March 28, 2007.
32. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11(6):181-2.
33. Breier A, Tanaka Y, Roychowdhury S, et al. Nizatidine for the prevention of weight gain during olanzapine treatment in schizophrenia and related disorders: a randomised controlled double-blind study. Presented at: Meeting of the Colleges of Psychiatric and Neurologic Pharmacists; March 23-26, 2001; San Antonio, TX.
34. Zimmermann U, Rechlin T, Plaskacewicz GJ. Effect of naltrexone on weight gain and food craving induced by tricyclic antidepressants and lithium: an open study. Biol Psychiatry 1997;41(6):747-9.
35. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychiatry 2002;22(4):431-5.
36. Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry 2002;159:655-7.
Hear Dr. Schwartz's strategies for monitoring patients during antidepressant therapy and for motivating them to lose weight. Click here.
Weight gain occurs with most antidepressants but is frequently overlooked, perhaps because clinicians are focused instead on metabolic effects of antipsychotics and mood stabilizers. Patients taking antidepressants often complain of weight gain, however, and many of the drugs’ FDA-approved package inserts acknowledge this effect.
Two-thirds of patients with major depression present with weight loss, and gaining weight can be associated with successful treatment. Weight gain is of concern—and likely to be drug-induced—if it exceeds the disease-induced weight loss and continues after depressive symptoms improve.
Weight may change early or late during antidepressant treatment, and gaining in the first weeks usually predicts future gains.1 Patients who are overweight when treatment begins are especially at risk if given weight-promoting agents. This article:
- compares antidepressant effects on patient weight
- discusses mechanisms by which antidepressants may cause weight gain
- outlines a plan to prevent excess weight gain when patients start antidepressant therapy
- recommends diet, exercise, cognitive-behavioral therapy (CBT), and medications for overweight patients on long-term antidepressant treatment.
Weight-gain potential by class
Unlike antipsychotics, antidepressants have not been associated in clinical trials with causing metabolic syndrome and diabetes. Even so, certain antidepressants can cause clinically significant and perhaps more insidious weight gain when compared with some second-generation antipsychotics (SGAs). For example, SGAs on average may cause 2.3 kg/month weight gain during the first 12 weeks of treatment, and mirtazapine caused 3 kg weight gain in a recent 6-week trial.2,3
Tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) may pose a greater weight-gain risk than newer antidepressants, but selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been clinically noted to cause weight gain over time (Table 1).4-16
SSRIs. Weight gain associated with long-term SSRI use seems clinically apparent, but the evidence is preliminary.
Paroxetine seems to be the SSRI most likely to cause weight gain. A 26- to 32-week comparison trial by Fava et al10 showed that weight gain risk with SSRI therapy varies with the drug used. In this trial, 284 patients with major depressive disorder were randomly assigned to double-blind treatment with paroxetine, sertraline, or fluoxetine:
- More of those taking paroxetine gained >7% in weight from baseline, and their weight gain was statistically significant.
- Sertraline-treated patients had modest, nonsignificant weight gain.
- Fluoxetine-treated patients had modest, nonsignificant weight loss.
Using paroxetine with an antipsychotic can be especially problematic. Fukowi and Murai17 described 2 cases in which adding paroxetine to risperidone caused severe weight gain (13.5 kg to >14 kg) in 4 to 5 months.
Citalopram may cause a 1- to 1.5-kg weight gain over 1 year,8 whereas fluvoxamine has been shown not to affect weight in obese patients.11 Citalopram (like TCAs) can cause carbohydrate craving and early weight gain.18 Escitalopram caused a modest (0.5 kg) weight gain in elderly patients during an 8-week trial.13
Initial weight loss followed by overall weight gain after 1 year of SSRI treatment is a common clinical finding that was not noted in initial acute SSRI drug trials. In a comparison of fluoxetine’s acute and long-term effects,19 839 patients experiencing a major depressive episode were first treated with open-label fluoxetine, 20 mg/d. After 12 weeks, 395 patients who met criteria for remission were randomly assigned to continue with placebo or fluoxetine, 20 mg/d, for 14, 38, or 50 weeks.
In the acute phase, a small but statistically significant weight loss (mean 0.35 kg, P
- 1.1 kg at 26 weeks (P
- 2.2 kg at 38 weeks (P
- 3.1 kg at 50 weeks (P
The authors concluded that the weight gain—similar with fluoxetine or placebo—was probably associated with recovery from depression rather than fluoxetine treatment, although this was not a controlled variable in the study.
Table 1
Long-term effects of antidepressants on body weight, by class*
| Class | Effect (gain, loss, or neutral) |
|---|---|
| MAOIs | Moderate gain overall |
| Phenelzine: greatest gain in MAOI class | |
| Transdermal selegiline: appears neutral | |
| Novel antidepressants | Bupropion: weight loss4 |
| Mirtazapine: greatest potential for gain among antidepressants5 | |
| Nefazodone: neutral6 | |
| Trazodone: modest gain7 | |
| SSRIs | Citalopram: modest gain8 |
| Escitalopram: modest gain9 | |
| Fluoxetine: modest loss acutely10 | |
| Fluvoxamine: neutral11 | |
| Paroxetine: greatest gain in SSRI class10 | |
| Sertraline: modest gain10 | |
| SNRIs | Duloxetine: modest gain12 |
| Venlafaxine: modest gain (controversial)13 | |
| TCAs | Amitriptyline: gain14 |
| Imipramine: gain15 | |
| Nortriptyline: neutral16 | |
| * Information is a general representation of available literature, gathered from many studies with differing designs. Consult original reports for specific data on dosing, patient populations, treatment durations, and weigh changes. | |
| MAOIs: monoamine oxidase inhibitors; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | |
Causes of weight gain
Serotonin. Appetite is controlled by cultural, psychological, neurochemical, and metabolic factors. Among neurochemical factors, serotonin helps regulate appetite and is the neurotransmitter most often manipulated in depression treatment.
Serotonin receptor agonists such as fenfluramine and dexfenfluramine have an acute anorexigenic effect. In rats, 5-HT2c receptor agonism decreases eating behavior, and mice lacking 5-HT2c receptors are obese.20 This may explain why SGAs or antidepressants that block 5-HT2c pose the greatest risk of weight gain.
SSRI or SNRI treatment might increase serotonin in the synaptic cleft, allowing 5-HT2c receptor down-regulation that is slower than—but similar in effect to—the acute 5-HT2c blockade caused by the SGAs.21 Weight gain from SSRI use reflects on these medications’ multiple serotonergic mechanisms. Serotonin appears to regulate carbohydrate intake and can increase food intake.22
Nefazodone and trazodone block 5HT2a receptors potently, and the norepinephrine (nefazodone only) and serotonin reuptake pumps (both agents) less potently. Differences in their mechanism (nefazodone increases norepinephrine) and lack of 5-HT2c blockade might be responsible for their reported weight neutrality.
Tricyclics block differing ratios of norepinephrine and serotonin reuptake pumps, resulting in postsynaptic serotonergic and adrenergic receptor desensitization and, later, down-regulation. TCAs with higher serotonin reuptake blockade may increase weight through this desensitization.
TCAs also affect appetite by blocking histaminergic (H1) pathways. Drugs with high affinity for blocking H1 receptors have been associated with carbohydrate craving18 and low satiety rates that allow increased calorie intake. TCAs have antimuscarinic, antihistaminic, and alpha adrenoceptor-blocking actions, all of which may contribute to weight gain.
In theory, beta-3 adrenergic receptors in adipose tissue may play a role in weight control by converting fat into heat and energy, especially in response to norepinephrine. TCAs or SNRIs that favor a noradrenergic profile may promote weight loss or neutrality. The relatively weight-neutral selegiline patch, which avoids first-pass metabolism and active adverse metabolites, also may use this mechanism.23
Mirtazapine blocks presynaptic alpha-2 and postsynaptic 5HT2a, 5HT2c, and 5HT3 serotonin receptors as well as H1 histamine receptors. Both 5HT2c and H1 blockade result in weight gain, the drug’s most apparent adverse effect. This mechanism is similar to that of the SGA olanzapine.
TNF-α. Obese persons have increased plasma levels of TNF-α and its soluble receptor (sTNF-R p75), which may induce insulin resistance. Activation of the TNF-α system, such as by amitriptyline or mirtazapine, may promote weight gain.24
Preventing weight gain
Early intervention is key to preventing drug-related weight gain and treating obesity. Provide informed consent and psychoeducation when prescribing antidepressants. In patients at metabolic risk, consider using weight-neutral or weight-loss agents (Table 2),4-16 and monitor for weight gain (Table 3). At-risk patients have:
- abdominal obesity (waist circumference >40 inches [102 cm] in men, >35 inches [88 cm] in women, or waist-to-hip ratio >0.9 in women and >1.0 in men)
- hyperlipidemia
- elevated body mass index (BMI [overweight=BMI 25 to 30 kg/m2, obesity=BMI >30 kg/m2])
- hypertension
- diabetes mellitus or impaired glucose tolerance
- history of stroke or cardiovascular disease
- family history of obesity, hypertension, diabetes, or hyperlipidemia.
Use SGA guidelines? Consider following modified American Diabetes Association guidelines for metabolic monitoring of patients treated with SGAs.25 We suggest that you follow SGA guidelines as a default when using mirtazapine—which is pharmacodynamically the most similar to SGAs—and TCAs. For patients taking other antidepressants, we recommend that you:
- measure blood pressure and weight, and calculate BMI often
- instruct patients to weigh themselves at home at least weekly in the morning and to report gains >5 lbs.
An overall 10-lb weight gain is clinically significant in most patients and calls for a management plan. Abdominal girth often increases as part of metabolic syndrome. If you choose to measure this variable and are uncomfortable reaching around patients while measuring, allow patients to apply the tape measure themselves.
Lab tests. Obtain fasting glucose and lipid levels at baseline for most patients and then quarterly in those with initial weight gain, medical comorbidities, or family history of hypertension, hypercholesterolemia, or diabetes. Many clinicians also screen for hypothyroidism and anemia, and these tests may be added. For patients without metabolic risk factors taking SSRIs and SNRIs, start quarterly draws if weight increases rapidly by >5 lbs or if BMI approaches ≥30 kg/m2. Tracking fasting triglycerides can serve as a sentinel for metabolic syndrome, which sometimes occurs before substantial weight gain or hyperglycemia.
Table 2
Antidepressants’ relative long-term effects on body weight
| Effect | Antidepressants |
|---|---|
| Loss | Bupropion,4 fluoxetine10 |
| Gain | Modest: citalopram,8 duloxetine,12 escitalopram,9 sertraline,10 trazodone,7 venlafaxine13 |
| Relatively more: amitriptyline,14 imipramine,15 mirtazapine,5 paroxetine,10 phenelzine | |
| Neutral | Fluvoxamine,11 nefazodone,6 nortriptyline16 |
| Information is a general representation of available literature, gathered from many studies with differing designs. Consult original reports for specific data on dosing, patient populations, treatment durations, and weight changes. | |
Table 3
Using antidepressants in patients at metabolic risk for weight gain
|
Dietary measures
If weight gain has occurred, a safe initial goal for patients is to lose 0.5% to 1% initial body weight per week—or 5% to 10% of weight across several months. Diet and exercise produce maximal benefit but require commitment and motivation, which are often difficult or impossible for depressed patients. Encouraging the patient’s efforts is worthwhile; if intervention is postponed until remission is achieved, weight gain may be substantially higher and more difficult to treat.
Cutting fat and calories. The first step in losing weight is to restrict high-fat and high-calorie foods and eat smaller portions. If this fails, then switch the patient to a low- or very-low-calorie diet, which provides a quick initial weight loss. This can motivate the patient but should be tried only under a physician’s supervision.
Many patients benefit from structured commercial weight-loss programs, but the likelihood of regaining the weight is high if stopped. These programs typically recommend 1,200 kcal/day for women and 1,800 kcal/day for men, with 55% of calories from carbohydrates, about 25% to 35% from protein, and 10% to 25% from fat.
In a study of 100 patients, those on 2 liquid meal replacements per day plus snacks and 1 low-fat meal (approximately 1,200 to 1,500 kcal/day) lost considerable weight in the first 3 months but regained some weight later. Many maintained weight loss on 1 liquid meal replacement per day plus snacks and 2 low-fat meals.26
Low- and very-low-calorie diets are indicated for patients with BMI >35 kg/m2:
- in whom conservative treatment (a portion-controlled, low-fat diet) has failed
- and who are willing to maintain at least 1 year of treatment and major lifestyle changes.
A low-calorie diet provides ≥1,000 kcal/day; very low-calorie diets may provide ≤800 kcal/day and rely mostly on liquid meal replacements. This semi-starvation can produce fatigue, weakness, lightheadedness, and changes in vital signs, including blood pressure, heart rate, and respiratory rate. For this reason, extreme diets require a team approach with the primary care clinician and a dietitian.
Among mentally healthy patients following very-low-calorie diets in clinical trials, 90% lose ≥10 kg and 50% lose ≥20 kg in the first 4 to 6 months.27 Most weight loss occurs in the first 12 to 16 weeks, after which an ad libitum low-fat, high-fiber diet can be used.
Exercise has physiologic and psychological benefits, including inhibiting food intake and promoting a sense of self-control. Physical exercise increases insulin sensitivity and reduces the risk of secondary medical problems, such as heart disease. Walking ≥40 minutes daily produces maximal benefit, but walking even 30 minutes 3 times a week can help maintain weight.
CBT. Eating habits can be changed through identifying lifestyle behaviors to be modified, setting goals, modifying triggers of excessive eating, and reinforcing desired behavior with CBT. Gradual but consistent behavior change leads to healthier eating habits, exercise, and weight loss. Behavior modification alone can generate a weight loss of 0.5 kg to 0.7 kg per week.28
A study of 6 schizophrenia patients (mean age 37) examined CBT effects on weight gain associated with clozapine (n=4) or olanzapine (n=2). Mean BMI decreased from 29.6 kg/m2 to 25.1 kg/m2 after 7 to 9 sessions of individual CBT, followed by 16 biweekly group sessions that focused on weight reduction and weight maintenance. A dietician provided detailed counseling.28
Using medications for weight loss
Switching. To avoid polypharmacy, consider switching the patient to a weight-neutral or weight-losing antidepressant, such as bupropion. Keep in mind when switching medications, however, that the next agent with less weight-gain potential might not deliver comparable antidepressant efficacy.
Antiobesity drugs. Short of switching, an antiobesity drug (Table 4)29-31 or off-label intervention (Table 5)32-36 may be warranted. Antiobesity drugs should not be used as primary therapy for obesity. Their use may be warranted, however, for psychiatric patients who:
- are unable to fully participate in diet and exercise programs because of symptoms (such as cognitive impairment or severe negative symptoms)
- lack social support (such as reflected by financial problems, homelessness, or poor compliance with treatment recommendations).
Generally, we reserve antiobesity drugs for patients with BMI >30 kg/m2 (or >27 kg/m2 in patients with diabetes, hyperlipidemia, or cardiovascular disease). Before adding these agents to a psychotropic regimen, however, review the relative risks and benefits with the patient and his or her primary care physician.
The goal of pharmacotherapy is for the patient to lose 5% to 10% of baseline weight in 3 to 6 months. Failure to achieve this goal is an indication to stop the medication. A plateau in weight loss after 6 to 9 months is expected and is not cause for discontinuation. If successful, drug treatment may be continued indefinitely, and both physician and patient must understand the intention to treat long-term. Most patients regain weight upon discontinuation.
Table 4
Medications indicated for treating obesity
| Drug/mechanism | Indication/dosage | Evidence of efficacy, safety | Comment |
|---|---|---|---|
| Sibutramine (sympathomimetic; serotonergic, noradrenergic reuptake inhibitor) | Obesity (5 to 20 mg/d) | ↓10% to 15% of body weight in 1 year;29 safety, efficacy beyond 1 year undetermined | ↓ triglycerides, total cholesterol, LDL cholesterol ↑ HDL cholesterol Monitor for serotonin syndrome when used with serotonergic psychotropics |
| Orlistat (inhibits gastric and pancreatic lipases by binding to these enzymes in the gut) | Obesity (120 mg tid with meals; take other drugs 1 hour pre- or post-orlistat) | ↓9% to 10% of body weight in 1 year;30 safety, efficacy beyond 2 years undetermined | ↓ triglycerides, total cholesterol, LDL cholesterol ↑ HDL cholesterol Lower risk of drug interactions than with sibutramine; GI side effects; multivitamin required |
| Rimonabant (investigational, pending FDA approval; selective type 1 cannabinoid receptor blocker) | Obesity (20 mg/d) (pending approval) | Reduced weight, improved heart disease risk factors in obese patients with metabolic syndrome or >1 cardiovascular risk factors (1-2 years)31 | Generally well-tolerated; mild nausea most common side effect |
| * Many studies in this table were conducted in patients taking second-generation antipsychotics for schizophrenia or bipolar disorder. Results may not apply to antidepressant-induced weight gain. | |||
| GI: gastrointestinal; HDL: high-density lipoprotein; LDL: low-density lipoprotein | |||
Table 5
Medications used ‘off label’ for treating obesity
| Drug/mechanism | Indication/dosage | Evidence of efficacy, safety | Comment |
|---|---|---|---|
| Amantadine (antiviral agent; may potentiate dopaminergic function) | Influenza A prophylaxis and Parkinson’s disease (300 mg/d, with olanzapine) | ↓3.5 kg over 3 to 6 months (study of 12 patients)32 | Patients had gained a mean 7.3 kg during olanzapine treatment |
| Nizatidine (histamine-2 receptor antagonist) | Duodenal ulcer; GERD (600 mg/d as prophylaxis with olanzapine) | ↓2.5 kg with nizatidine; ↑5.5 kg with placebo33 (16-week RCT) | Unknown effectiveness when used as prophylaxis with antidepressants; can cause delirium, especially in older patients |
| Naltrexone (opioid antagonist; decreases craving for sweet, fatty foods caused by TCAs and lithium) | Alcohol, narcotics addiction (50 mg/d) | TCA-induced weight gain reversed, then resumed after drug was stopped (8-patient trial)34 | Small mean weight loss compared with previous drug-induced weight gain; no adverse effects seen on depressive symptoms |
| Topiramate (anticonvulsant) | Epilepsy, migraine (100 to 400 mg/d as adjunct to antipsychotics) | ↓10 to 15 lbs in 33% to 55% of bipolar disorder patients35 | May serve dual purpose in treating obese patients with affective disorders; fatigue, cognitive dulling, ataxia, glaucoma, oligohydrosis, acidosis are possible |
| Metformin (biguanide antihyperglycemic) | Type 2 diabetes (500 mg tid as adjunct to antipsychotics) | 15 of 19 patients who gained 10% in body weight taking SGAs lost weight with add-on metformin (12-week, open-label trial) | Sporadic diarrhea in some patients; risk of lactic acidosis (tests unremarkable in this small trial)36 |
| * Many studies in this table were conducted in patients taking second-generation antipsychotics for schizophrenia or bipolar disorder. Results may not apply to antidepressant-induced weight gain. | |||
| GERD: gastroesophageal reflux disease; RCT: randomized, double-blind, placebo-controlled trial; SGAs: second-generation antipsychotics; TCAs: tricyclic antidepressants | |||
Related resources
- Centers for Disease Control and Prevention. Overweight and obesity: contributing factors. www.cdc.gov/nccdphp/dnpa/obesity/contributing_factors.htm.
- Mathur R. What causes obesity? www.medicinenet.com/obesity_weight_loss/page2.htm.
Drug brand names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Metformin • Glucophage
- Mirtazapine • Remeron
- Naltrexone • ReVia
- Nefazodone • Serzone
- Nizatidine • Axid
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Paroxetine • Paxil
- Phenelzine • Nardil
- Selegiline (transdermal) • EMSAM
- Sertraline • Zoloft
- Sibutramine • Meridian
- Topiramate • Topamax
- Trazodone • Desyrel
- Venlafaxine • Effexor
Disclosure
Dr. Schwartz has received grants from or served as a consultant to AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Jazz Pharmaceuticals, Pfizer Inc., and Wyeth.
Other authors report no financial relationship with any companies whose products are mentioned in this article or with manufacturers of competing products.
Hear Dr. Schwartz's strategies for monitoring patients during antidepressant therapy and for motivating them to lose weight. Click here.
Weight gain occurs with most antidepressants but is frequently overlooked, perhaps because clinicians are focused instead on metabolic effects of antipsychotics and mood stabilizers. Patients taking antidepressants often complain of weight gain, however, and many of the drugs’ FDA-approved package inserts acknowledge this effect.
Two-thirds of patients with major depression present with weight loss, and gaining weight can be associated with successful treatment. Weight gain is of concern—and likely to be drug-induced—if it exceeds the disease-induced weight loss and continues after depressive symptoms improve.
Weight may change early or late during antidepressant treatment, and gaining in the first weeks usually predicts future gains.1 Patients who are overweight when treatment begins are especially at risk if given weight-promoting agents. This article:
- compares antidepressant effects on patient weight
- discusses mechanisms by which antidepressants may cause weight gain
- outlines a plan to prevent excess weight gain when patients start antidepressant therapy
- recommends diet, exercise, cognitive-behavioral therapy (CBT), and medications for overweight patients on long-term antidepressant treatment.
Weight-gain potential by class
Unlike antipsychotics, antidepressants have not been associated in clinical trials with causing metabolic syndrome and diabetes. Even so, certain antidepressants can cause clinically significant and perhaps more insidious weight gain when compared with some second-generation antipsychotics (SGAs). For example, SGAs on average may cause 2.3 kg/month weight gain during the first 12 weeks of treatment, and mirtazapine caused 3 kg weight gain in a recent 6-week trial.2,3
Tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) may pose a greater weight-gain risk than newer antidepressants, but selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been clinically noted to cause weight gain over time (Table 1).4-16
SSRIs. Weight gain associated with long-term SSRI use seems clinically apparent, but the evidence is preliminary.
Paroxetine seems to be the SSRI most likely to cause weight gain. A 26- to 32-week comparison trial by Fava et al10 showed that weight gain risk with SSRI therapy varies with the drug used. In this trial, 284 patients with major depressive disorder were randomly assigned to double-blind treatment with paroxetine, sertraline, or fluoxetine:
- More of those taking paroxetine gained >7% in weight from baseline, and their weight gain was statistically significant.
- Sertraline-treated patients had modest, nonsignificant weight gain.
- Fluoxetine-treated patients had modest, nonsignificant weight loss.
Using paroxetine with an antipsychotic can be especially problematic. Fukowi and Murai17 described 2 cases in which adding paroxetine to risperidone caused severe weight gain (13.5 kg to >14 kg) in 4 to 5 months.
Citalopram may cause a 1- to 1.5-kg weight gain over 1 year,8 whereas fluvoxamine has been shown not to affect weight in obese patients.11 Citalopram (like TCAs) can cause carbohydrate craving and early weight gain.18 Escitalopram caused a modest (0.5 kg) weight gain in elderly patients during an 8-week trial.13
Initial weight loss followed by overall weight gain after 1 year of SSRI treatment is a common clinical finding that was not noted in initial acute SSRI drug trials. In a comparison of fluoxetine’s acute and long-term effects,19 839 patients experiencing a major depressive episode were first treated with open-label fluoxetine, 20 mg/d. After 12 weeks, 395 patients who met criteria for remission were randomly assigned to continue with placebo or fluoxetine, 20 mg/d, for 14, 38, or 50 weeks.
In the acute phase, a small but statistically significant weight loss (mean 0.35 kg, P
- 1.1 kg at 26 weeks (P
- 2.2 kg at 38 weeks (P
- 3.1 kg at 50 weeks (P
The authors concluded that the weight gain—similar with fluoxetine or placebo—was probably associated with recovery from depression rather than fluoxetine treatment, although this was not a controlled variable in the study.
Table 1
Long-term effects of antidepressants on body weight, by class*
| Class | Effect (gain, loss, or neutral) |
|---|---|
| MAOIs | Moderate gain overall |
| Phenelzine: greatest gain in MAOI class | |
| Transdermal selegiline: appears neutral | |
| Novel antidepressants | Bupropion: weight loss4 |
| Mirtazapine: greatest potential for gain among antidepressants5 | |
| Nefazodone: neutral6 | |
| Trazodone: modest gain7 | |
| SSRIs | Citalopram: modest gain8 |
| Escitalopram: modest gain9 | |
| Fluoxetine: modest loss acutely10 | |
| Fluvoxamine: neutral11 | |
| Paroxetine: greatest gain in SSRI class10 | |
| Sertraline: modest gain10 | |
| SNRIs | Duloxetine: modest gain12 |
| Venlafaxine: modest gain (controversial)13 | |
| TCAs | Amitriptyline: gain14 |
| Imipramine: gain15 | |
| Nortriptyline: neutral16 | |
| * Information is a general representation of available literature, gathered from many studies with differing designs. Consult original reports for specific data on dosing, patient populations, treatment durations, and weigh changes. | |
| MAOIs: monoamine oxidase inhibitors; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | |
Causes of weight gain
Serotonin. Appetite is controlled by cultural, psychological, neurochemical, and metabolic factors. Among neurochemical factors, serotonin helps regulate appetite and is the neurotransmitter most often manipulated in depression treatment.
Serotonin receptor agonists such as fenfluramine and dexfenfluramine have an acute anorexigenic effect. In rats, 5-HT2c receptor agonism decreases eating behavior, and mice lacking 5-HT2c receptors are obese.20 This may explain why SGAs or antidepressants that block 5-HT2c pose the greatest risk of weight gain.
SSRI or SNRI treatment might increase serotonin in the synaptic cleft, allowing 5-HT2c receptor down-regulation that is slower than—but similar in effect to—the acute 5-HT2c blockade caused by the SGAs.21 Weight gain from SSRI use reflects on these medications’ multiple serotonergic mechanisms. Serotonin appears to regulate carbohydrate intake and can increase food intake.22
Nefazodone and trazodone block 5HT2a receptors potently, and the norepinephrine (nefazodone only) and serotonin reuptake pumps (both agents) less potently. Differences in their mechanism (nefazodone increases norepinephrine) and lack of 5-HT2c blockade might be responsible for their reported weight neutrality.
Tricyclics block differing ratios of norepinephrine and serotonin reuptake pumps, resulting in postsynaptic serotonergic and adrenergic receptor desensitization and, later, down-regulation. TCAs with higher serotonin reuptake blockade may increase weight through this desensitization.
TCAs also affect appetite by blocking histaminergic (H1) pathways. Drugs with high affinity for blocking H1 receptors have been associated with carbohydrate craving18 and low satiety rates that allow increased calorie intake. TCAs have antimuscarinic, antihistaminic, and alpha adrenoceptor-blocking actions, all of which may contribute to weight gain.
In theory, beta-3 adrenergic receptors in adipose tissue may play a role in weight control by converting fat into heat and energy, especially in response to norepinephrine. TCAs or SNRIs that favor a noradrenergic profile may promote weight loss or neutrality. The relatively weight-neutral selegiline patch, which avoids first-pass metabolism and active adverse metabolites, also may use this mechanism.23
Mirtazapine blocks presynaptic alpha-2 and postsynaptic 5HT2a, 5HT2c, and 5HT3 serotonin receptors as well as H1 histamine receptors. Both 5HT2c and H1 blockade result in weight gain, the drug’s most apparent adverse effect. This mechanism is similar to that of the SGA olanzapine.
TNF-α. Obese persons have increased plasma levels of TNF-α and its soluble receptor (sTNF-R p75), which may induce insulin resistance. Activation of the TNF-α system, such as by amitriptyline or mirtazapine, may promote weight gain.24
Preventing weight gain
Early intervention is key to preventing drug-related weight gain and treating obesity. Provide informed consent and psychoeducation when prescribing antidepressants. In patients at metabolic risk, consider using weight-neutral or weight-loss agents (Table 2),4-16 and monitor for weight gain (Table 3). At-risk patients have:
- abdominal obesity (waist circumference >40 inches [102 cm] in men, >35 inches [88 cm] in women, or waist-to-hip ratio >0.9 in women and >1.0 in men)
- hyperlipidemia
- elevated body mass index (BMI [overweight=BMI 25 to 30 kg/m2, obesity=BMI >30 kg/m2])
- hypertension
- diabetes mellitus or impaired glucose tolerance
- history of stroke or cardiovascular disease
- family history of obesity, hypertension, diabetes, or hyperlipidemia.
Use SGA guidelines? Consider following modified American Diabetes Association guidelines for metabolic monitoring of patients treated with SGAs.25 We suggest that you follow SGA guidelines as a default when using mirtazapine—which is pharmacodynamically the most similar to SGAs—and TCAs. For patients taking other antidepressants, we recommend that you:
- measure blood pressure and weight, and calculate BMI often
- instruct patients to weigh themselves at home at least weekly in the morning and to report gains >5 lbs.
An overall 10-lb weight gain is clinically significant in most patients and calls for a management plan. Abdominal girth often increases as part of metabolic syndrome. If you choose to measure this variable and are uncomfortable reaching around patients while measuring, allow patients to apply the tape measure themselves.
Lab tests. Obtain fasting glucose and lipid levels at baseline for most patients and then quarterly in those with initial weight gain, medical comorbidities, or family history of hypertension, hypercholesterolemia, or diabetes. Many clinicians also screen for hypothyroidism and anemia, and these tests may be added. For patients without metabolic risk factors taking SSRIs and SNRIs, start quarterly draws if weight increases rapidly by >5 lbs or if BMI approaches ≥30 kg/m2. Tracking fasting triglycerides can serve as a sentinel for metabolic syndrome, which sometimes occurs before substantial weight gain or hyperglycemia.
Table 2
Antidepressants’ relative long-term effects on body weight
| Effect | Antidepressants |
|---|---|
| Loss | Bupropion,4 fluoxetine10 |
| Gain | Modest: citalopram,8 duloxetine,12 escitalopram,9 sertraline,10 trazodone,7 venlafaxine13 |
| Relatively more: amitriptyline,14 imipramine,15 mirtazapine,5 paroxetine,10 phenelzine | |
| Neutral | Fluvoxamine,11 nefazodone,6 nortriptyline16 |
| Information is a general representation of available literature, gathered from many studies with differing designs. Consult original reports for specific data on dosing, patient populations, treatment durations, and weight changes. | |
Table 3
Using antidepressants in patients at metabolic risk for weight gain
|
Dietary measures
If weight gain has occurred, a safe initial goal for patients is to lose 0.5% to 1% initial body weight per week—or 5% to 10% of weight across several months. Diet and exercise produce maximal benefit but require commitment and motivation, which are often difficult or impossible for depressed patients. Encouraging the patient’s efforts is worthwhile; if intervention is postponed until remission is achieved, weight gain may be substantially higher and more difficult to treat.
Cutting fat and calories. The first step in losing weight is to restrict high-fat and high-calorie foods and eat smaller portions. If this fails, then switch the patient to a low- or very-low-calorie diet, which provides a quick initial weight loss. This can motivate the patient but should be tried only under a physician’s supervision.
Many patients benefit from structured commercial weight-loss programs, but the likelihood of regaining the weight is high if stopped. These programs typically recommend 1,200 kcal/day for women and 1,800 kcal/day for men, with 55% of calories from carbohydrates, about 25% to 35% from protein, and 10% to 25% from fat.
In a study of 100 patients, those on 2 liquid meal replacements per day plus snacks and 1 low-fat meal (approximately 1,200 to 1,500 kcal/day) lost considerable weight in the first 3 months but regained some weight later. Many maintained weight loss on 1 liquid meal replacement per day plus snacks and 2 low-fat meals.26
Low- and very-low-calorie diets are indicated for patients with BMI >35 kg/m2:
- in whom conservative treatment (a portion-controlled, low-fat diet) has failed
- and who are willing to maintain at least 1 year of treatment and major lifestyle changes.
A low-calorie diet provides ≥1,000 kcal/day; very low-calorie diets may provide ≤800 kcal/day and rely mostly on liquid meal replacements. This semi-starvation can produce fatigue, weakness, lightheadedness, and changes in vital signs, including blood pressure, heart rate, and respiratory rate. For this reason, extreme diets require a team approach with the primary care clinician and a dietitian.
Among mentally healthy patients following very-low-calorie diets in clinical trials, 90% lose ≥10 kg and 50% lose ≥20 kg in the first 4 to 6 months.27 Most weight loss occurs in the first 12 to 16 weeks, after which an ad libitum low-fat, high-fiber diet can be used.
Exercise has physiologic and psychological benefits, including inhibiting food intake and promoting a sense of self-control. Physical exercise increases insulin sensitivity and reduces the risk of secondary medical problems, such as heart disease. Walking ≥40 minutes daily produces maximal benefit, but walking even 30 minutes 3 times a week can help maintain weight.
CBT. Eating habits can be changed through identifying lifestyle behaviors to be modified, setting goals, modifying triggers of excessive eating, and reinforcing desired behavior with CBT. Gradual but consistent behavior change leads to healthier eating habits, exercise, and weight loss. Behavior modification alone can generate a weight loss of 0.5 kg to 0.7 kg per week.28
A study of 6 schizophrenia patients (mean age 37) examined CBT effects on weight gain associated with clozapine (n=4) or olanzapine (n=2). Mean BMI decreased from 29.6 kg/m2 to 25.1 kg/m2 after 7 to 9 sessions of individual CBT, followed by 16 biweekly group sessions that focused on weight reduction and weight maintenance. A dietician provided detailed counseling.28
Using medications for weight loss
Switching. To avoid polypharmacy, consider switching the patient to a weight-neutral or weight-losing antidepressant, such as bupropion. Keep in mind when switching medications, however, that the next agent with less weight-gain potential might not deliver comparable antidepressant efficacy.
Antiobesity drugs. Short of switching, an antiobesity drug (Table 4)29-31 or off-label intervention (Table 5)32-36 may be warranted. Antiobesity drugs should not be used as primary therapy for obesity. Their use may be warranted, however, for psychiatric patients who:
- are unable to fully participate in diet and exercise programs because of symptoms (such as cognitive impairment or severe negative symptoms)
- lack social support (such as reflected by financial problems, homelessness, or poor compliance with treatment recommendations).
Generally, we reserve antiobesity drugs for patients with BMI >30 kg/m2 (or >27 kg/m2 in patients with diabetes, hyperlipidemia, or cardiovascular disease). Before adding these agents to a psychotropic regimen, however, review the relative risks and benefits with the patient and his or her primary care physician.
The goal of pharmacotherapy is for the patient to lose 5% to 10% of baseline weight in 3 to 6 months. Failure to achieve this goal is an indication to stop the medication. A plateau in weight loss after 6 to 9 months is expected and is not cause for discontinuation. If successful, drug treatment may be continued indefinitely, and both physician and patient must understand the intention to treat long-term. Most patients regain weight upon discontinuation.
Table 4
Medications indicated for treating obesity
| Drug/mechanism | Indication/dosage | Evidence of efficacy, safety | Comment |
|---|---|---|---|
| Sibutramine (sympathomimetic; serotonergic, noradrenergic reuptake inhibitor) | Obesity (5 to 20 mg/d) | ↓10% to 15% of body weight in 1 year;29 safety, efficacy beyond 1 year undetermined | ↓ triglycerides, total cholesterol, LDL cholesterol ↑ HDL cholesterol Monitor for serotonin syndrome when used with serotonergic psychotropics |
| Orlistat (inhibits gastric and pancreatic lipases by binding to these enzymes in the gut) | Obesity (120 mg tid with meals; take other drugs 1 hour pre- or post-orlistat) | ↓9% to 10% of body weight in 1 year;30 safety, efficacy beyond 2 years undetermined | ↓ triglycerides, total cholesterol, LDL cholesterol ↑ HDL cholesterol Lower risk of drug interactions than with sibutramine; GI side effects; multivitamin required |
| Rimonabant (investigational, pending FDA approval; selective type 1 cannabinoid receptor blocker) | Obesity (20 mg/d) (pending approval) | Reduced weight, improved heart disease risk factors in obese patients with metabolic syndrome or >1 cardiovascular risk factors (1-2 years)31 | Generally well-tolerated; mild nausea most common side effect |
| * Many studies in this table were conducted in patients taking second-generation antipsychotics for schizophrenia or bipolar disorder. Results may not apply to antidepressant-induced weight gain. | |||
| GI: gastrointestinal; HDL: high-density lipoprotein; LDL: low-density lipoprotein | |||
Table 5
Medications used ‘off label’ for treating obesity
| Drug/mechanism | Indication/dosage | Evidence of efficacy, safety | Comment |
|---|---|---|---|
| Amantadine (antiviral agent; may potentiate dopaminergic function) | Influenza A prophylaxis and Parkinson’s disease (300 mg/d, with olanzapine) | ↓3.5 kg over 3 to 6 months (study of 12 patients)32 | Patients had gained a mean 7.3 kg during olanzapine treatment |
| Nizatidine (histamine-2 receptor antagonist) | Duodenal ulcer; GERD (600 mg/d as prophylaxis with olanzapine) | ↓2.5 kg with nizatidine; ↑5.5 kg with placebo33 (16-week RCT) | Unknown effectiveness when used as prophylaxis with antidepressants; can cause delirium, especially in older patients |
| Naltrexone (opioid antagonist; decreases craving for sweet, fatty foods caused by TCAs and lithium) | Alcohol, narcotics addiction (50 mg/d) | TCA-induced weight gain reversed, then resumed after drug was stopped (8-patient trial)34 | Small mean weight loss compared with previous drug-induced weight gain; no adverse effects seen on depressive symptoms |
| Topiramate (anticonvulsant) | Epilepsy, migraine (100 to 400 mg/d as adjunct to antipsychotics) | ↓10 to 15 lbs in 33% to 55% of bipolar disorder patients35 | May serve dual purpose in treating obese patients with affective disorders; fatigue, cognitive dulling, ataxia, glaucoma, oligohydrosis, acidosis are possible |
| Metformin (biguanide antihyperglycemic) | Type 2 diabetes (500 mg tid as adjunct to antipsychotics) | 15 of 19 patients who gained 10% in body weight taking SGAs lost weight with add-on metformin (12-week, open-label trial) | Sporadic diarrhea in some patients; risk of lactic acidosis (tests unremarkable in this small trial)36 |
| * Many studies in this table were conducted in patients taking second-generation antipsychotics for schizophrenia or bipolar disorder. Results may not apply to antidepressant-induced weight gain. | |||
| GERD: gastroesophageal reflux disease; RCT: randomized, double-blind, placebo-controlled trial; SGAs: second-generation antipsychotics; TCAs: tricyclic antidepressants | |||
Related resources
- Centers for Disease Control and Prevention. Overweight and obesity: contributing factors. www.cdc.gov/nccdphp/dnpa/obesity/contributing_factors.htm.
- Mathur R. What causes obesity? www.medicinenet.com/obesity_weight_loss/page2.htm.
Drug brand names
- Amantadine • Symmetrel
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Metformin • Glucophage
- Mirtazapine • Remeron
- Naltrexone • ReVia
- Nefazodone • Serzone
- Nizatidine • Axid
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Paroxetine • Paxil
- Phenelzine • Nardil
- Selegiline (transdermal) • EMSAM
- Sertraline • Zoloft
- Sibutramine • Meridian
- Topiramate • Topamax
- Trazodone • Desyrel
- Venlafaxine • Effexor
Disclosure
Dr. Schwartz has received grants from or served as a consultant to AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Forest Pharmaceuticals, GlaxoSmithKline, Jazz Pharmaceuticals, Pfizer Inc., and Wyeth.
Other authors report no financial relationship with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Himmerich H, Schuld A, Haack M, et al. Early prediction of changes in weight during six weeks of treatment with antidepressants. J Psychiatr Res 2004;38(5):485-9.
2. Wetterling T. Bodyweight gain with atypical antipsychotics. A comparative review. Drug Saf 2001;24(1):59-73.
3. Laimer M, Kramer-Reinstadler K, Rauchenzauner M, et al. Effect of mirtazapine treatment on body composition and metabolism. J Clin Psychiatry 2006;67(3):421-4.
4. Chouinard G. Bupropion and amitriptyline in the treatment of depressed patients. J Clin Psychiatry 1983;44:121-9.
5. Ribeiro L, Busnello JV, Kauer-Sant’Anna M, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Brazil J Med Biol Res 2001;34:1303-7.
6. Davis R, Whittington R, Bryson HM. Nefazodone. A review of its pharmacology and clinical efficacy in the management of major depression. Drugs 1997;54:186-7.
7. Weisler RH, Johnston JA, Lineberry CG, et al. Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychiatry 1994;14:170-9.
8. Leinonen E, Skarstein J, Behnke K, et al. For the Nordic Antidepressant Study Group. Efficacy and tolerability of mirtazapine versus citalopram: a double blind, randomized study in patients with major depressive disorder. Int Clin Psychopharm 1999;14(6):329-37.
9. Kasper S, Lemming OM, de Swart H. Escitalopram in the long-term treatment of major depressive disorder in elderly patients. Neuropsychobiology 2006;54(3):152-9.
10. Fava M, Judge R, Hoog SL, et al. Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long term treatment. J Clin Psychiatry 2000;61(11):863-7.
11. Abell CA, Farquhar DL, Galloway SM, et al. Placebo-controlled, double-blind trial of fluvoxamine maleate in the obese. J Psychosomat Res 1986;30:143-6.
12. Wise TN, Perahia DG, Pangallo BA, et al. Effects of the antidepressant duloxetine on body weight: analyses of 10 clinical studies. Prim Care Companion J Clin Psychiatry 2006;8(5):269-78.
13. Silverstone PH, Ravindran A. Once daily velafaxine extended release compared with fluoxetine in outpatients with depression and anxiety. J Clin Psychiatry 2000;61(suppl 2):20-5.
14. Zetin M, Frost NR, Brumfield D, et al. Amitriptyline stimulates weight gain in hemodialysis patients. Clin Nephrol 1982;18:79-82.
15. Fernstrom MH, Krowinski RL, Kupfer DJ. Chronic imipramine treatment and weight gain. Psychiatr Res 1986;17:269-73.
16. Prince JB, Wilens TE, Biederman J, et al. A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2000;10:193-204.
17. Fukui H, Murai T. Severe weight gain induced by combination treatment with risperidone and paroxetine. Clin Neuropharmacol 2002;25(5):269-71.
18. Bouwer CD, Harvey BH. Phasic craving for carbohydrate observed with citalopram. Int Clin Psychopharmacol 1996;11:273-8.
19. Michelson D, Amsterdam J, Quitkin FM, et al. Changes in weight during a 1-year trial of fluoxetine. Am J Psychiatry 1999;156(8):1170-6.
20. Curzon G, Gibson EL, Oluyomi AO. Appetite suppression by commonly used drugs depends on 5HT availability. Trends Pharmacol Sci 1998;13:12-25.
21. De Vry J, Schreiber R. Effects of selected serotonin 5-HT1 and 5-HT2 receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev 2000;24:341-53.
22. Bickerdike HJ, Vickers SP, Dourish CT. 5HT2c receptor modulation and the treatment of obesity. Diabetes Obes Metab 1999;1:207-14.
23. Strosberg AD, Pietri-Rouxel F. Function and regulation of beta-3 adrenoceptor. Trends Pharmacol Sci 1996;17:373-81.
24. Kraus T, Haack M, Schuld A, et al. Body weight, the tumor necrosis factor system, and leptin production during treatment with mirtazapine or venlafaxine. Pharmacopsychiatry 2002;35(6):220-5.
25. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
26. Ditschuneit HH, Flechtner-Mors M, Johnson TD, et al. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clinical Nutrition 1999;69:198-204.
27. Wadden TA. Evidence for success of calorie restriction in weight control; summary data from clinical research studies. In: Scannell SM, ed. Methods for voluntary weight loss and control. Bethesda, MD: U.S. Department of Health and Human Services 1992;64-74.
28. Umbricht D, Flury H, Bridler R. Cognitive behavior therapy for weight gain. Am J Psychiatry 2001;158:971-2.
29. Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight loss. Obes Res 1999;7(2):189-98.
30. Van Gaal LF, Broom JI, Enzi G, et al. Efficacy and tolerability of orlistat in the treatment of obesity: a 6-month dose-ranging study. Eur J Clin Pharmacol 1998;54:125-32.
31. Kakafika AI, Mikhailidis DP, Karagiannis A, et al. The role of endocannabinoid system blockade in the treatment of metabolic syndrome. J Clin Pharmacol [serial online]. March 28, 2007.
32. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11(6):181-2.
33. Breier A, Tanaka Y, Roychowdhury S, et al. Nizatidine for the prevention of weight gain during olanzapine treatment in schizophrenia and related disorders: a randomised controlled double-blind study. Presented at: Meeting of the Colleges of Psychiatric and Neurologic Pharmacists; March 23-26, 2001; San Antonio, TX.
34. Zimmermann U, Rechlin T, Plaskacewicz GJ. Effect of naltrexone on weight gain and food craving induced by tricyclic antidepressants and lithium: an open study. Biol Psychiatry 1997;41(6):747-9.
35. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychiatry 2002;22(4):431-5.
36. Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry 2002;159:655-7.
1. Himmerich H, Schuld A, Haack M, et al. Early prediction of changes in weight during six weeks of treatment with antidepressants. J Psychiatr Res 2004;38(5):485-9.
2. Wetterling T. Bodyweight gain with atypical antipsychotics. A comparative review. Drug Saf 2001;24(1):59-73.
3. Laimer M, Kramer-Reinstadler K, Rauchenzauner M, et al. Effect of mirtazapine treatment on body composition and metabolism. J Clin Psychiatry 2006;67(3):421-4.
4. Chouinard G. Bupropion and amitriptyline in the treatment of depressed patients. J Clin Psychiatry 1983;44:121-9.
5. Ribeiro L, Busnello JV, Kauer-Sant’Anna M, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Brazil J Med Biol Res 2001;34:1303-7.
6. Davis R, Whittington R, Bryson HM. Nefazodone. A review of its pharmacology and clinical efficacy in the management of major depression. Drugs 1997;54:186-7.
7. Weisler RH, Johnston JA, Lineberry CG, et al. Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychiatry 1994;14:170-9.
8. Leinonen E, Skarstein J, Behnke K, et al. For the Nordic Antidepressant Study Group. Efficacy and tolerability of mirtazapine versus citalopram: a double blind, randomized study in patients with major depressive disorder. Int Clin Psychopharm 1999;14(6):329-37.
9. Kasper S, Lemming OM, de Swart H. Escitalopram in the long-term treatment of major depressive disorder in elderly patients. Neuropsychobiology 2006;54(3):152-9.
10. Fava M, Judge R, Hoog SL, et al. Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long term treatment. J Clin Psychiatry 2000;61(11):863-7.
11. Abell CA, Farquhar DL, Galloway SM, et al. Placebo-controlled, double-blind trial of fluvoxamine maleate in the obese. J Psychosomat Res 1986;30:143-6.
12. Wise TN, Perahia DG, Pangallo BA, et al. Effects of the antidepressant duloxetine on body weight: analyses of 10 clinical studies. Prim Care Companion J Clin Psychiatry 2006;8(5):269-78.
13. Silverstone PH, Ravindran A. Once daily velafaxine extended release compared with fluoxetine in outpatients with depression and anxiety. J Clin Psychiatry 2000;61(suppl 2):20-5.
14. Zetin M, Frost NR, Brumfield D, et al. Amitriptyline stimulates weight gain in hemodialysis patients. Clin Nephrol 1982;18:79-82.
15. Fernstrom MH, Krowinski RL, Kupfer DJ. Chronic imipramine treatment and weight gain. Psychiatr Res 1986;17:269-73.
16. Prince JB, Wilens TE, Biederman J, et al. A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2000;10:193-204.
17. Fukui H, Murai T. Severe weight gain induced by combination treatment with risperidone and paroxetine. Clin Neuropharmacol 2002;25(5):269-71.
18. Bouwer CD, Harvey BH. Phasic craving for carbohydrate observed with citalopram. Int Clin Psychopharmacol 1996;11:273-8.
19. Michelson D, Amsterdam J, Quitkin FM, et al. Changes in weight during a 1-year trial of fluoxetine. Am J Psychiatry 1999;156(8):1170-6.
20. Curzon G, Gibson EL, Oluyomi AO. Appetite suppression by commonly used drugs depends on 5HT availability. Trends Pharmacol Sci 1998;13:12-25.
21. De Vry J, Schreiber R. Effects of selected serotonin 5-HT1 and 5-HT2 receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev 2000;24:341-53.
22. Bickerdike HJ, Vickers SP, Dourish CT. 5HT2c receptor modulation and the treatment of obesity. Diabetes Obes Metab 1999;1:207-14.
23. Strosberg AD, Pietri-Rouxel F. Function and regulation of beta-3 adrenoceptor. Trends Pharmacol Sci 1996;17:373-81.
24. Kraus T, Haack M, Schuld A, et al. Body weight, the tumor necrosis factor system, and leptin production during treatment with mirtazapine or venlafaxine. Pharmacopsychiatry 2002;35(6):220-5.
25. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
26. Ditschuneit HH, Flechtner-Mors M, Johnson TD, et al. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clinical Nutrition 1999;69:198-204.
27. Wadden TA. Evidence for success of calorie restriction in weight control; summary data from clinical research studies. In: Scannell SM, ed. Methods for voluntary weight loss and control. Bethesda, MD: U.S. Department of Health and Human Services 1992;64-74.
28. Umbricht D, Flury H, Bridler R. Cognitive behavior therapy for weight gain. Am J Psychiatry 2001;158:971-2.
29. Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine produces dose-related weight loss. Obes Res 1999;7(2):189-98.
30. Van Gaal LF, Broom JI, Enzi G, et al. Efficacy and tolerability of orlistat in the treatment of obesity: a 6-month dose-ranging study. Eur J Clin Pharmacol 1998;54:125-32.
31. Kakafika AI, Mikhailidis DP, Karagiannis A, et al. The role of endocannabinoid system blockade in the treatment of metabolic syndrome. J Clin Pharmacol [serial online]. March 28, 2007.
32. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11(6):181-2.
33. Breier A, Tanaka Y, Roychowdhury S, et al. Nizatidine for the prevention of weight gain during olanzapine treatment in schizophrenia and related disorders: a randomised controlled double-blind study. Presented at: Meeting of the Colleges of Psychiatric and Neurologic Pharmacists; March 23-26, 2001; San Antonio, TX.
34. Zimmermann U, Rechlin T, Plaskacewicz GJ. Effect of naltrexone on weight gain and food craving induced by tricyclic antidepressants and lithium: an open study. Biol Psychiatry 1997;41(6):747-9.
35. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychiatry 2002;22(4):431-5.
36. Morrison JA, Cottingham EM, Barton BA. Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry 2002;159:655-7.
Stalking intervention: Know the 5 stalker types, safety strategies for victims
A patient, a colleague, or perhaps you have been stalked. The chances of a woman being stalked are an estimated 1 in 14; for men, it is 1 in 50.1 Fearful stalking victims may restrict their lives, change jobs, and curtail social activities to protect themselves from unwanted attention, physical assault, or even murder. They may develop anxiety, depression, or posttraumatic stress disorder (PTSD).2,3
Historical, clinical, and behavioral factors increase a stalker’s risk for committing violence (Table 1).2-7 As a psychiatrist, you may be asked to consult with local law enforcement and stalking victims to assess and manage victims’ risk. To best protect them, be aware of:
- 5 types of stalkers and their typical response to management strategies
- legal and safety issues to consider before taking actions that might endanger stalking victims
- strategies to help victims protect themselves
- interventions for victims and stalkers.
Table 1
Factors that increase the risk of violence
| Factor type | Features |
|---|---|
| Historical | Ex-intimate partner |
| Previous violence | |
| Criminal record (especially violent crimes) | |
| Previous threats (especially specific or face-to-face) | |
| Clinical | “Rejected” or “predatory” stalker type |
| Substance use | |
| Narcissism, entitlement | |
| Personality disorder with anger or behavioral instability | |
| Depression with suicidal ideas | |
| Behavioral | Access to weapons |
| Proximity to victim | |
| Victim in a new relationship | |
| Has already taken actions on plans/threats | |
| Researching the victim | |
| Unconcerned with negative consequences | |
| Risk factors for homicide or serious physical harm: | |
| |
| Source: References 2-7 | |
Stalker types
Mullen et al8 developed a clinically oriented, validated stalker classification system to identify an individual stalker’s type, risks, and probable responses to management interventions (Table 2).
Rejected stalkers—the most common and dangerous type—pursue the victim, often a former intimate partner, after a relationship ends. They often acknowledge a complex and volatile mix of desire for reconciliation and revenge. These stalkers likely have a history of criminal assault.
Rejected stalkers appear to respond best to a combination of coordinated legal sanctions and mental health intervention. Because they are most likely to be violent, rejected stalkers need intensive probation or parole supervision.5
Intimacy-seeking stalkers want an intimate relationship with a victim they believe is their “true love” and tend to imbue their victims with special desirability, excellence, and other qualities consistent with their belief of romanticized love. Most have erotomanic delusions, and the rest have morbid infatuations with the victim. Intimacy-seeking stalkers typically are unperturbed by legal sanctions, viewing them as the price to pay for “true love.” They often require court-mandated psychiatric treatment.
Incompetent stalkers know the victim is disinterested but forge ahead in hopes that their behavior will lead to a relationship. Their stalking can be viewed as crude or “incompetent” attempts to court the victim. Incompetent stalkers often are intellectually limited; they feel entitled to a partner but because of underdeveloped social skills are unable to build upon lesser forms of social interaction. Unlike intimacy-seekers, incompetent stalkers do not endow the victim with unique qualities.
In addition to needing legal sanctions and possible mental health treatment, incompetent stalkers often require social skills training. Otherwise, they are likely to continue their pattern of stalking with other victims.
Resentful stalkers who suffer from mental illness generally require court-ordered psychiatric treatment but are difficult to engage in therapy. Legal sanctions may inflame this type of stalker.
Predatory stalkers prepare for a sexual assault. They stalk to discover the victim’s vulnerabilities and seldom give warnings, so the victim is often unaware of the danger.
Predatory stalkers frequently suffer from paraphilias and have prior convictions for sexual offenses. They must be secured in a correctional or forensic setting to address their paraphilias and propensity for violence.
Table 2
Identifying types of stalkers
| Type | Traits and behaviors |
|---|---|
| Rejected | Pursues former intimate partner |
| Desires reconciliation and/or revenge | |
| Criminal assault history | |
| Personality disorders predominate | |
| Intimacy-seeking | Desires relationship with “true love” |
| Oblivious to victim response | |
| Most have erotomanic delusions | |
| Endows victim with unique qualities | |
| Incompetent | Acknowledges victim’s disinterest |
| Hopes behavior leads to intimacy | |
| Does not endow victim with unique qualities | |
| Low IQ, socially inept, entitled | |
| Resentful | Feels persecuted and desires retribution |
| Intends to frighten or distress | |
| Specific or general grievance | |
| Paranoid diagnoses | |
| Predatory | Preparing for sexual attack |
| Stalks to study and observe | |
| Paraphilias, prior sexual offenses are common | |
| No warnings before attack | |
| Source: Reference 8 | |
Managing victims’ risk
Effectively managing a victim’s stalking risk is a dynamic process. It is critical to use professional judgment in a flexible manner and to work as a team with professionals from other agencies (Box).9-12
Intervention dilemma. Before taking any action, consider that taking direct measures against the stalker to reduce stalking may increase the risk of violence.10 A law enforcement intervention may provoke a stalker by challenging or humiliating him or her. Therefore, there is no single best approach to risk management. Consider the significance of individual-specific nuances, and solicit input from different disciplines. In some cases, no direct action may be preferable.
Protective orders. Obtaining a protective order may or may not be helpful. Most domestic violence research indicates that such orders protect abused women.13 This is important because stalking by a former intimate partner often occurs in relationships characterized by domestic violence.14 In addition to potentially preventing stalking behavior, a protective order may provide legal evidence of the course of stalking, as well as document a “fearful victim,” which is required by law to obtain a criminal conviction.
No conclusive studies have investigated the effectiveness of protective orders specifically related to stalkers, so consider the stalker’s reaction to previous orders.15 Counsel a victim who obtains a protective order against a former intimate partner to avoid developing a false sense of security. Rejected stalkers who have considerable emotional investment in the relationship may not be deterred by the threat of criminal sanctions. Furthermore, stalkers who are psychotic may misperceive and disregard criminal injunctions. In rare cases, a protective order may escalate stalking and violence.15
Dramatic moments. Advise a victim to remain vigilant during “dramatic moments” when violence risk may be especially heightened.15 These include:
- arrests
- issuance of protective orders
- court hearings
- custody hearings
- anniversary dates
- family-oriented holidays.
Encourage a victim who is especially concerned about an impending dramatic moment to prepare by:
- arranging to be out of town on that date
- notifying law enforcement and victim advocates.
A multidisciplinary approach is the most effective way to reduce stalking violence risk. In addition to mental health professionals, an effective team usually includes law enforcement and criminal justice personnel, attorneys, security specialists/private investigators, victim advocates, and the victim and his or her social network.
The victim can increase the chances that officials will view his or her case as a priority by establishing rapport with the senior police official and district attorney assigned to the case.10,11 Such rapport also allows the victim to learn about the laws and resources available for managing stalking risk.
A multidisciplinary team can assess and manage risk, provide education, and support victims. One well-established anti-stalking team—the San Diego Stalking Strike Force—meets monthly to evaluate cases.12 Members also are on-call for emergencies. By exchanging information monthly, the case manager and parole agent enhance stalker supervision.
In court, advocacy is critical. The consultant psychiatrist or victim advocate can educate the court that stalking is not a “lovers’ spat” (in the case of the rejected stalker) or mere nuisance behavior (in the case of other stalker types). The victim and psychiatrist may need to mobilize resources and promote collaboration among professionals in communities that do not have advocates or anti-stalking services.
Treating victims’ symptoms
As a result of the risks they face, stalking victims often suffer significant “social damage.” To cope with being stalked, many victims must make substantial life changes, such as relocating or finding new employment. They may need to restrict outings, adapt security measures, and take time off from work.16 This social damage and anxiety may predispose them to substance abuse.17
Stalking victims also experience emotional distress.3,18 They commonly report symptoms of anxiety disorders, in particular PTSD, and one-quarter experience depression and suicidal ruminations.19 Victims who perceive their stalking as severe report elevated levels of helplessness, anxiety, PTSD, and depression.20
Few studies focus on the duration of victims’ symptoms or their successful treatment.21 Mullen8 has recommended a comprehensive approach that includes education, supportive counseling, psychotherapy, and pharmacotherapy. In particular, cognitive-oriented therapy can target common issues such as anxiety leading to feelings of loss of control and associated avoidance. Pharmacotherapy for anxiety or depressive symptoms follows recommended treatment guidelines.
Because the stalking and associated stress may have an adverse impact on the victim’s personal relationships, partner and family therapy may be necessary. Support organizations for stalking victims, such as Survivors of Stalking, can provide education, safety information, and emotional support.
Improving victims’ safety
Coach a victim to take responsibility for his or her safety by becoming familiar with local stalking laws, resources, and law enforcement policies.13,22 Emphasize that a victim must be assertive to ensure that safety measures are in place (Table 3).3,8,10,15,18
As soon as unwanted pursuit is apparent, the victim should unequivocally tell the stalker that no relationship is wanted.8 This message must be firm, reasonable, and as clear as possible. The victim should not attempt to deliver the message gently or let the stalker “down easy.” Otherwise, the stalker may believe the victim is ambivalent about the decision and will continue or redouble his or her efforts.
After delivering this message, the victim should not engage in any further discussion or initiate contact with the stalker. The victim must avoid all contact to minimize the effects of “intermittent positive reinforcement.”15
The victim should document and preserve evidence by recording the dates and times of each unwanted contact, including vandalism, in an “incident log” or journal. Encourage him or her to photograph and note the date of any property damage. This documentation will help establish a clear course of illegal conduct and can prove invaluable to police and prosecution efforts.
The victim should preserve any evidence—including gifts, mementos, and other materials—by placing it in a plastic bag labeled with the date, time, and place it was received. Encourage the victim to:
- resist the urge to discard evidence that may evoke feelings of fear, shame, or disgust
- avoid handling evidence, and store it in a secure location.
- establish a post office box to prevent someone from stealing mail containing personal information
- shred personal mail instead of placing it in the trash.
It is essential for the victim to form a network of trusted social contacts who will provide a “safety net.” Informing family, friends, co-workers, and neighbors about stalking and its potentially serious consequences may reduce the risk that they might inadvertently disclose a victim’s personal information to the stalker.8 The victim can distribute a photo of the stalker to members of the safety network, as well as co-workers, with instructions to call the victim if the stalker is spotted.
Security experts often advise victims not to adhere to their usual, predictable routines by, for example, taking different daily travel routes or being prepared to go out of town at short notice.2 Victims should also make contingency plans in case their social supports are unavailable in an emergency. Victim advocacy agents can give information about services and locations of local “safe houses” or domestic violence shelters.
Table 3
Victim safety strategies
|
| Source: References 3,8,10,15,18 |
Treating stalkers
Failing to treat a mentally ill stalker may result in continued risk to the victim. For example, an intimacy-seeking stalker with erotomanic delusions who is confined without treatment likely will be released with no significant reduction in risk. No reliable outcome data exist on treatment for stalkers, however, so you must rely on empirically derived clinical data.
If you work with stalkers, you must be familiar with your state’s duty-to-protect statutes and relevant case law related to stalking so you can discuss legal obligations with the stalker before beginning treatment.
Most stalkers will be difficult to engage in treatment because they have been compelled by a court order to seek therapy. Initially you are likely to encounter the stalker’s striking lack of insight into the nature and consequences of this behavior. The stalker may seek validation for his or her actions while demonstrating little interest in ending the obsessional behavior. Expect well-entrenched defenses of denial, rationalization, and minimization.
A comprehensive description of treatment for stalkers is beyond the scope of this article. However, clinicians with experience treating stalkers recommend the following interventions:4
- thorough psychiatric assessment and diagnosis
- treatment of Axis I or II pathology
- cognitive-behavioral therapy to focus on the stalker’s misperceptions
- motivational interviewing techniques to help the stalker appreciate the need for intervention
- victim empathy development
- social skills enhancement
- periodic risk assessments.
- Stalking Resource Center, National Center for Victims of Crime. www.ncvc.org/src.
- Pinals DA, ed. Stalking: a psychiatric perspective. New York: Oxford University Press; 2007.
- The Antistalking Web Site resources. www.antistalking.com/resource.htm.
- U.S. Department of Justice anti-stalking resources. www.usdoj.gov/ovw/stalking.htm.
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Basile KC, Swahn MH, Chen J, Saltzman LE. Stalking in the United States: recent national prevalence estimates. Am J Prev Med 2006;31(2):172-5.
2. McEwan T, Mullen PE, Purcell R. Identifying risk factors in stalking: a review of current research. Int J Law Psychiatry 2007;30:1-9.
3. Spitzberg BH, Cupach WR. The state of the art of stalking: taking stock of the emerging literature. Aggression and Violent Behavior 2007;12:64-86.
4. Mullen P, Mackenzie R, Ogloff JR, et al. Assessing and managing the risks in the stalking situation. J Am Acad Psychiatry Law 2006;34:439-50.
5. Mohandie K, Meloy JR, McGowan MG, Williams J. The RECON typology of stalking: reliability and validity based upon a large sample of North American stalkers. J Forensic Sci 2006;51(1):147-55.
6. James DV, Farnham FR. Stalking and serious violence. J Am Acad Psychiatry Law 2003;31(4):432-9.
7. McFarlane J, Campbell JC, Watson K. Intimate partner stalking and femicide: urgent implications for women’s safety. Behav Sci Law 2002;20(1-2):51-68.
8. Mullen PE, Pathé M, Purcell R. Stalkers and their victims. Cambridge, UK: Cambridge University Press; 2000.
9. Binder RL. Commentary: the importance of professional judgment in evaluation of stalking and threatening situations. J Am Acad Psychiatry Law 2006;34(4):451-4.
10. White S, Cawood J. Threat management of stalking cases. In: Meloy JR, ed. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:295-314.
11. Orion D. I know you really love me: a psychiatrist’s journal of erotomania, stalking, and obsessive love. New York: Macmillan; 1997.
12. Maxey W. The San Diego stalking strike force: a multi-disciplinary approach to assessing and managing stalking and threat cases. Journal of Threat Assessment 2002;2(1):43-53.
13. McFarlane J, Malecha A, Gist J, et al. Protection orders and intimate partner violence: an 18-month study of 150 black, Hispanic, and white women. Am J Public Health 2004;94(4):613-8.
14. Melton HC. Predicting the occurrence of stalking in relationships characterized by domestic violence. J Interpers Violence 2007;22(1):3-25.
15. Meloy JR. The clinical risk management of stalking: “someone is watching over me….” Am J Psychother 1997;51(2):174-84.
16. Purcell R, Pathé M, Mullen PE. When do repeated intrusions become stalking? J Forensic Psychiatry Psychol 2004;15(4):571-3.
17. Pathé M. Surviving stalking. Cambridge, UK: Cambridge University Press; 2002.
18. Kamphuis JH, Emmelkamp PMG. Traumatic distress among support-seeking female victims of stalking. Am J Psychiatry 2001;158:795-8.
19. Pathé M, Mullen PE. The impact of stalkers on their victims. Br J Psychiatry 1997;170:12-7.
20. Turmanis SA, Brown RI. The stalking and harassment behavior scale: measuring the incidence, nature, and severity of stalking and relational harassment and their psychological effects. Psychol Psychother 2006;79(Pt 2):183-98.
21. Ashmore R, Jones J, Jackson A, Smoyak S. A survey of mental health nurses’ experiences of stalking. J Psychiatr Ment Health Nurs 2006;13:562-9.
22. De Becker G. The gift of fear: survival signals that protect us from violence. New York: Dell Publishing; 1997.
A patient, a colleague, or perhaps you have been stalked. The chances of a woman being stalked are an estimated 1 in 14; for men, it is 1 in 50.1 Fearful stalking victims may restrict their lives, change jobs, and curtail social activities to protect themselves from unwanted attention, physical assault, or even murder. They may develop anxiety, depression, or posttraumatic stress disorder (PTSD).2,3
Historical, clinical, and behavioral factors increase a stalker’s risk for committing violence (Table 1).2-7 As a psychiatrist, you may be asked to consult with local law enforcement and stalking victims to assess and manage victims’ risk. To best protect them, be aware of:
- 5 types of stalkers and their typical response to management strategies
- legal and safety issues to consider before taking actions that might endanger stalking victims
- strategies to help victims protect themselves
- interventions for victims and stalkers.
Table 1
Factors that increase the risk of violence
| Factor type | Features |
|---|---|
| Historical | Ex-intimate partner |
| Previous violence | |
| Criminal record (especially violent crimes) | |
| Previous threats (especially specific or face-to-face) | |
| Clinical | “Rejected” or “predatory” stalker type |
| Substance use | |
| Narcissism, entitlement | |
| Personality disorder with anger or behavioral instability | |
| Depression with suicidal ideas | |
| Behavioral | Access to weapons |
| Proximity to victim | |
| Victim in a new relationship | |
| Has already taken actions on plans/threats | |
| Researching the victim | |
| Unconcerned with negative consequences | |
| Risk factors for homicide or serious physical harm: | |
| |
| Source: References 2-7 | |
Stalker types
Mullen et al8 developed a clinically oriented, validated stalker classification system to identify an individual stalker’s type, risks, and probable responses to management interventions (Table 2).
Rejected stalkers—the most common and dangerous type—pursue the victim, often a former intimate partner, after a relationship ends. They often acknowledge a complex and volatile mix of desire for reconciliation and revenge. These stalkers likely have a history of criminal assault.
Rejected stalkers appear to respond best to a combination of coordinated legal sanctions and mental health intervention. Because they are most likely to be violent, rejected stalkers need intensive probation or parole supervision.5
Intimacy-seeking stalkers want an intimate relationship with a victim they believe is their “true love” and tend to imbue their victims with special desirability, excellence, and other qualities consistent with their belief of romanticized love. Most have erotomanic delusions, and the rest have morbid infatuations with the victim. Intimacy-seeking stalkers typically are unperturbed by legal sanctions, viewing them as the price to pay for “true love.” They often require court-mandated psychiatric treatment.
Incompetent stalkers know the victim is disinterested but forge ahead in hopes that their behavior will lead to a relationship. Their stalking can be viewed as crude or “incompetent” attempts to court the victim. Incompetent stalkers often are intellectually limited; they feel entitled to a partner but because of underdeveloped social skills are unable to build upon lesser forms of social interaction. Unlike intimacy-seekers, incompetent stalkers do not endow the victim with unique qualities.
In addition to needing legal sanctions and possible mental health treatment, incompetent stalkers often require social skills training. Otherwise, they are likely to continue their pattern of stalking with other victims.
Resentful stalkers who suffer from mental illness generally require court-ordered psychiatric treatment but are difficult to engage in therapy. Legal sanctions may inflame this type of stalker.
Predatory stalkers prepare for a sexual assault. They stalk to discover the victim’s vulnerabilities and seldom give warnings, so the victim is often unaware of the danger.
Predatory stalkers frequently suffer from paraphilias and have prior convictions for sexual offenses. They must be secured in a correctional or forensic setting to address their paraphilias and propensity for violence.
Table 2
Identifying types of stalkers
| Type | Traits and behaviors |
|---|---|
| Rejected | Pursues former intimate partner |
| Desires reconciliation and/or revenge | |
| Criminal assault history | |
| Personality disorders predominate | |
| Intimacy-seeking | Desires relationship with “true love” |
| Oblivious to victim response | |
| Most have erotomanic delusions | |
| Endows victim with unique qualities | |
| Incompetent | Acknowledges victim’s disinterest |
| Hopes behavior leads to intimacy | |
| Does not endow victim with unique qualities | |
| Low IQ, socially inept, entitled | |
| Resentful | Feels persecuted and desires retribution |
| Intends to frighten or distress | |
| Specific or general grievance | |
| Paranoid diagnoses | |
| Predatory | Preparing for sexual attack |
| Stalks to study and observe | |
| Paraphilias, prior sexual offenses are common | |
| No warnings before attack | |
| Source: Reference 8 | |
Managing victims’ risk
Effectively managing a victim’s stalking risk is a dynamic process. It is critical to use professional judgment in a flexible manner and to work as a team with professionals from other agencies (Box).9-12
Intervention dilemma. Before taking any action, consider that taking direct measures against the stalker to reduce stalking may increase the risk of violence.10 A law enforcement intervention may provoke a stalker by challenging or humiliating him or her. Therefore, there is no single best approach to risk management. Consider the significance of individual-specific nuances, and solicit input from different disciplines. In some cases, no direct action may be preferable.
Protective orders. Obtaining a protective order may or may not be helpful. Most domestic violence research indicates that such orders protect abused women.13 This is important because stalking by a former intimate partner often occurs in relationships characterized by domestic violence.14 In addition to potentially preventing stalking behavior, a protective order may provide legal evidence of the course of stalking, as well as document a “fearful victim,” which is required by law to obtain a criminal conviction.
No conclusive studies have investigated the effectiveness of protective orders specifically related to stalkers, so consider the stalker’s reaction to previous orders.15 Counsel a victim who obtains a protective order against a former intimate partner to avoid developing a false sense of security. Rejected stalkers who have considerable emotional investment in the relationship may not be deterred by the threat of criminal sanctions. Furthermore, stalkers who are psychotic may misperceive and disregard criminal injunctions. In rare cases, a protective order may escalate stalking and violence.15
Dramatic moments. Advise a victim to remain vigilant during “dramatic moments” when violence risk may be especially heightened.15 These include:
- arrests
- issuance of protective orders
- court hearings
- custody hearings
- anniversary dates
- family-oriented holidays.
Encourage a victim who is especially concerned about an impending dramatic moment to prepare by:
- arranging to be out of town on that date
- notifying law enforcement and victim advocates.
A multidisciplinary approach is the most effective way to reduce stalking violence risk. In addition to mental health professionals, an effective team usually includes law enforcement and criminal justice personnel, attorneys, security specialists/private investigators, victim advocates, and the victim and his or her social network.
The victim can increase the chances that officials will view his or her case as a priority by establishing rapport with the senior police official and district attorney assigned to the case.10,11 Such rapport also allows the victim to learn about the laws and resources available for managing stalking risk.
A multidisciplinary team can assess and manage risk, provide education, and support victims. One well-established anti-stalking team—the San Diego Stalking Strike Force—meets monthly to evaluate cases.12 Members also are on-call for emergencies. By exchanging information monthly, the case manager and parole agent enhance stalker supervision.
In court, advocacy is critical. The consultant psychiatrist or victim advocate can educate the court that stalking is not a “lovers’ spat” (in the case of the rejected stalker) or mere nuisance behavior (in the case of other stalker types). The victim and psychiatrist may need to mobilize resources and promote collaboration among professionals in communities that do not have advocates or anti-stalking services.
Treating victims’ symptoms
As a result of the risks they face, stalking victims often suffer significant “social damage.” To cope with being stalked, many victims must make substantial life changes, such as relocating or finding new employment. They may need to restrict outings, adapt security measures, and take time off from work.16 This social damage and anxiety may predispose them to substance abuse.17
Stalking victims also experience emotional distress.3,18 They commonly report symptoms of anxiety disorders, in particular PTSD, and one-quarter experience depression and suicidal ruminations.19 Victims who perceive their stalking as severe report elevated levels of helplessness, anxiety, PTSD, and depression.20
Few studies focus on the duration of victims’ symptoms or their successful treatment.21 Mullen8 has recommended a comprehensive approach that includes education, supportive counseling, psychotherapy, and pharmacotherapy. In particular, cognitive-oriented therapy can target common issues such as anxiety leading to feelings of loss of control and associated avoidance. Pharmacotherapy for anxiety or depressive symptoms follows recommended treatment guidelines.
Because the stalking and associated stress may have an adverse impact on the victim’s personal relationships, partner and family therapy may be necessary. Support organizations for stalking victims, such as Survivors of Stalking, can provide education, safety information, and emotional support.
Improving victims’ safety
Coach a victim to take responsibility for his or her safety by becoming familiar with local stalking laws, resources, and law enforcement policies.13,22 Emphasize that a victim must be assertive to ensure that safety measures are in place (Table 3).3,8,10,15,18
As soon as unwanted pursuit is apparent, the victim should unequivocally tell the stalker that no relationship is wanted.8 This message must be firm, reasonable, and as clear as possible. The victim should not attempt to deliver the message gently or let the stalker “down easy.” Otherwise, the stalker may believe the victim is ambivalent about the decision and will continue or redouble his or her efforts.
After delivering this message, the victim should not engage in any further discussion or initiate contact with the stalker. The victim must avoid all contact to minimize the effects of “intermittent positive reinforcement.”15
The victim should document and preserve evidence by recording the dates and times of each unwanted contact, including vandalism, in an “incident log” or journal. Encourage him or her to photograph and note the date of any property damage. This documentation will help establish a clear course of illegal conduct and can prove invaluable to police and prosecution efforts.
The victim should preserve any evidence—including gifts, mementos, and other materials—by placing it in a plastic bag labeled with the date, time, and place it was received. Encourage the victim to:
- resist the urge to discard evidence that may evoke feelings of fear, shame, or disgust
- avoid handling evidence, and store it in a secure location.
- establish a post office box to prevent someone from stealing mail containing personal information
- shred personal mail instead of placing it in the trash.
It is essential for the victim to form a network of trusted social contacts who will provide a “safety net.” Informing family, friends, co-workers, and neighbors about stalking and its potentially serious consequences may reduce the risk that they might inadvertently disclose a victim’s personal information to the stalker.8 The victim can distribute a photo of the stalker to members of the safety network, as well as co-workers, with instructions to call the victim if the stalker is spotted.
Security experts often advise victims not to adhere to their usual, predictable routines by, for example, taking different daily travel routes or being prepared to go out of town at short notice.2 Victims should also make contingency plans in case their social supports are unavailable in an emergency. Victim advocacy agents can give information about services and locations of local “safe houses” or domestic violence shelters.
Table 3
Victim safety strategies
|
| Source: References 3,8,10,15,18 |
Treating stalkers
Failing to treat a mentally ill stalker may result in continued risk to the victim. For example, an intimacy-seeking stalker with erotomanic delusions who is confined without treatment likely will be released with no significant reduction in risk. No reliable outcome data exist on treatment for stalkers, however, so you must rely on empirically derived clinical data.
If you work with stalkers, you must be familiar with your state’s duty-to-protect statutes and relevant case law related to stalking so you can discuss legal obligations with the stalker before beginning treatment.
Most stalkers will be difficult to engage in treatment because they have been compelled by a court order to seek therapy. Initially you are likely to encounter the stalker’s striking lack of insight into the nature and consequences of this behavior. The stalker may seek validation for his or her actions while demonstrating little interest in ending the obsessional behavior. Expect well-entrenched defenses of denial, rationalization, and minimization.
A comprehensive description of treatment for stalkers is beyond the scope of this article. However, clinicians with experience treating stalkers recommend the following interventions:4
- thorough psychiatric assessment and diagnosis
- treatment of Axis I or II pathology
- cognitive-behavioral therapy to focus on the stalker’s misperceptions
- motivational interviewing techniques to help the stalker appreciate the need for intervention
- victim empathy development
- social skills enhancement
- periodic risk assessments.
- Stalking Resource Center, National Center for Victims of Crime. www.ncvc.org/src.
- Pinals DA, ed. Stalking: a psychiatric perspective. New York: Oxford University Press; 2007.
- The Antistalking Web Site resources. www.antistalking.com/resource.htm.
- U.S. Department of Justice anti-stalking resources. www.usdoj.gov/ovw/stalking.htm.
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
A patient, a colleague, or perhaps you have been stalked. The chances of a woman being stalked are an estimated 1 in 14; for men, it is 1 in 50.1 Fearful stalking victims may restrict their lives, change jobs, and curtail social activities to protect themselves from unwanted attention, physical assault, or even murder. They may develop anxiety, depression, or posttraumatic stress disorder (PTSD).2,3
Historical, clinical, and behavioral factors increase a stalker’s risk for committing violence (Table 1).2-7 As a psychiatrist, you may be asked to consult with local law enforcement and stalking victims to assess and manage victims’ risk. To best protect them, be aware of:
- 5 types of stalkers and their typical response to management strategies
- legal and safety issues to consider before taking actions that might endanger stalking victims
- strategies to help victims protect themselves
- interventions for victims and stalkers.
Table 1
Factors that increase the risk of violence
| Factor type | Features |
|---|---|
| Historical | Ex-intimate partner |
| Previous violence | |
| Criminal record (especially violent crimes) | |
| Previous threats (especially specific or face-to-face) | |
| Clinical | “Rejected” or “predatory” stalker type |
| Substance use | |
| Narcissism, entitlement | |
| Personality disorder with anger or behavioral instability | |
| Depression with suicidal ideas | |
| Behavioral | Access to weapons |
| Proximity to victim | |
| Victim in a new relationship | |
| Has already taken actions on plans/threats | |
| Researching the victim | |
| Unconcerned with negative consequences | |
| Risk factors for homicide or serious physical harm: | |
| |
| Source: References 2-7 | |
Stalker types
Mullen et al8 developed a clinically oriented, validated stalker classification system to identify an individual stalker’s type, risks, and probable responses to management interventions (Table 2).
Rejected stalkers—the most common and dangerous type—pursue the victim, often a former intimate partner, after a relationship ends. They often acknowledge a complex and volatile mix of desire for reconciliation and revenge. These stalkers likely have a history of criminal assault.
Rejected stalkers appear to respond best to a combination of coordinated legal sanctions and mental health intervention. Because they are most likely to be violent, rejected stalkers need intensive probation or parole supervision.5
Intimacy-seeking stalkers want an intimate relationship with a victim they believe is their “true love” and tend to imbue their victims with special desirability, excellence, and other qualities consistent with their belief of romanticized love. Most have erotomanic delusions, and the rest have morbid infatuations with the victim. Intimacy-seeking stalkers typically are unperturbed by legal sanctions, viewing them as the price to pay for “true love.” They often require court-mandated psychiatric treatment.
Incompetent stalkers know the victim is disinterested but forge ahead in hopes that their behavior will lead to a relationship. Their stalking can be viewed as crude or “incompetent” attempts to court the victim. Incompetent stalkers often are intellectually limited; they feel entitled to a partner but because of underdeveloped social skills are unable to build upon lesser forms of social interaction. Unlike intimacy-seekers, incompetent stalkers do not endow the victim with unique qualities.
In addition to needing legal sanctions and possible mental health treatment, incompetent stalkers often require social skills training. Otherwise, they are likely to continue their pattern of stalking with other victims.
Resentful stalkers who suffer from mental illness generally require court-ordered psychiatric treatment but are difficult to engage in therapy. Legal sanctions may inflame this type of stalker.
Predatory stalkers prepare for a sexual assault. They stalk to discover the victim’s vulnerabilities and seldom give warnings, so the victim is often unaware of the danger.
Predatory stalkers frequently suffer from paraphilias and have prior convictions for sexual offenses. They must be secured in a correctional or forensic setting to address their paraphilias and propensity for violence.
Table 2
Identifying types of stalkers
| Type | Traits and behaviors |
|---|---|
| Rejected | Pursues former intimate partner |
| Desires reconciliation and/or revenge | |
| Criminal assault history | |
| Personality disorders predominate | |
| Intimacy-seeking | Desires relationship with “true love” |
| Oblivious to victim response | |
| Most have erotomanic delusions | |
| Endows victim with unique qualities | |
| Incompetent | Acknowledges victim’s disinterest |
| Hopes behavior leads to intimacy | |
| Does not endow victim with unique qualities | |
| Low IQ, socially inept, entitled | |
| Resentful | Feels persecuted and desires retribution |
| Intends to frighten or distress | |
| Specific or general grievance | |
| Paranoid diagnoses | |
| Predatory | Preparing for sexual attack |
| Stalks to study and observe | |
| Paraphilias, prior sexual offenses are common | |
| No warnings before attack | |
| Source: Reference 8 | |
Managing victims’ risk
Effectively managing a victim’s stalking risk is a dynamic process. It is critical to use professional judgment in a flexible manner and to work as a team with professionals from other agencies (Box).9-12
Intervention dilemma. Before taking any action, consider that taking direct measures against the stalker to reduce stalking may increase the risk of violence.10 A law enforcement intervention may provoke a stalker by challenging or humiliating him or her. Therefore, there is no single best approach to risk management. Consider the significance of individual-specific nuances, and solicit input from different disciplines. In some cases, no direct action may be preferable.
Protective orders. Obtaining a protective order may or may not be helpful. Most domestic violence research indicates that such orders protect abused women.13 This is important because stalking by a former intimate partner often occurs in relationships characterized by domestic violence.14 In addition to potentially preventing stalking behavior, a protective order may provide legal evidence of the course of stalking, as well as document a “fearful victim,” which is required by law to obtain a criminal conviction.
No conclusive studies have investigated the effectiveness of protective orders specifically related to stalkers, so consider the stalker’s reaction to previous orders.15 Counsel a victim who obtains a protective order against a former intimate partner to avoid developing a false sense of security. Rejected stalkers who have considerable emotional investment in the relationship may not be deterred by the threat of criminal sanctions. Furthermore, stalkers who are psychotic may misperceive and disregard criminal injunctions. In rare cases, a protective order may escalate stalking and violence.15
Dramatic moments. Advise a victim to remain vigilant during “dramatic moments” when violence risk may be especially heightened.15 These include:
- arrests
- issuance of protective orders
- court hearings
- custody hearings
- anniversary dates
- family-oriented holidays.
Encourage a victim who is especially concerned about an impending dramatic moment to prepare by:
- arranging to be out of town on that date
- notifying law enforcement and victim advocates.
A multidisciplinary approach is the most effective way to reduce stalking violence risk. In addition to mental health professionals, an effective team usually includes law enforcement and criminal justice personnel, attorneys, security specialists/private investigators, victim advocates, and the victim and his or her social network.
The victim can increase the chances that officials will view his or her case as a priority by establishing rapport with the senior police official and district attorney assigned to the case.10,11 Such rapport also allows the victim to learn about the laws and resources available for managing stalking risk.
A multidisciplinary team can assess and manage risk, provide education, and support victims. One well-established anti-stalking team—the San Diego Stalking Strike Force—meets monthly to evaluate cases.12 Members also are on-call for emergencies. By exchanging information monthly, the case manager and parole agent enhance stalker supervision.
In court, advocacy is critical. The consultant psychiatrist or victim advocate can educate the court that stalking is not a “lovers’ spat” (in the case of the rejected stalker) or mere nuisance behavior (in the case of other stalker types). The victim and psychiatrist may need to mobilize resources and promote collaboration among professionals in communities that do not have advocates or anti-stalking services.
Treating victims’ symptoms
As a result of the risks they face, stalking victims often suffer significant “social damage.” To cope with being stalked, many victims must make substantial life changes, such as relocating or finding new employment. They may need to restrict outings, adapt security measures, and take time off from work.16 This social damage and anxiety may predispose them to substance abuse.17
Stalking victims also experience emotional distress.3,18 They commonly report symptoms of anxiety disorders, in particular PTSD, and one-quarter experience depression and suicidal ruminations.19 Victims who perceive their stalking as severe report elevated levels of helplessness, anxiety, PTSD, and depression.20
Few studies focus on the duration of victims’ symptoms or their successful treatment.21 Mullen8 has recommended a comprehensive approach that includes education, supportive counseling, psychotherapy, and pharmacotherapy. In particular, cognitive-oriented therapy can target common issues such as anxiety leading to feelings of loss of control and associated avoidance. Pharmacotherapy for anxiety or depressive symptoms follows recommended treatment guidelines.
Because the stalking and associated stress may have an adverse impact on the victim’s personal relationships, partner and family therapy may be necessary. Support organizations for stalking victims, such as Survivors of Stalking, can provide education, safety information, and emotional support.
Improving victims’ safety
Coach a victim to take responsibility for his or her safety by becoming familiar with local stalking laws, resources, and law enforcement policies.13,22 Emphasize that a victim must be assertive to ensure that safety measures are in place (Table 3).3,8,10,15,18
As soon as unwanted pursuit is apparent, the victim should unequivocally tell the stalker that no relationship is wanted.8 This message must be firm, reasonable, and as clear as possible. The victim should not attempt to deliver the message gently or let the stalker “down easy.” Otherwise, the stalker may believe the victim is ambivalent about the decision and will continue or redouble his or her efforts.
After delivering this message, the victim should not engage in any further discussion or initiate contact with the stalker. The victim must avoid all contact to minimize the effects of “intermittent positive reinforcement.”15
The victim should document and preserve evidence by recording the dates and times of each unwanted contact, including vandalism, in an “incident log” or journal. Encourage him or her to photograph and note the date of any property damage. This documentation will help establish a clear course of illegal conduct and can prove invaluable to police and prosecution efforts.
The victim should preserve any evidence—including gifts, mementos, and other materials—by placing it in a plastic bag labeled with the date, time, and place it was received. Encourage the victim to:
- resist the urge to discard evidence that may evoke feelings of fear, shame, or disgust
- avoid handling evidence, and store it in a secure location.
- establish a post office box to prevent someone from stealing mail containing personal information
- shred personal mail instead of placing it in the trash.
It is essential for the victim to form a network of trusted social contacts who will provide a “safety net.” Informing family, friends, co-workers, and neighbors about stalking and its potentially serious consequences may reduce the risk that they might inadvertently disclose a victim’s personal information to the stalker.8 The victim can distribute a photo of the stalker to members of the safety network, as well as co-workers, with instructions to call the victim if the stalker is spotted.
Security experts often advise victims not to adhere to their usual, predictable routines by, for example, taking different daily travel routes or being prepared to go out of town at short notice.2 Victims should also make contingency plans in case their social supports are unavailable in an emergency. Victim advocacy agents can give information about services and locations of local “safe houses” or domestic violence shelters.
Table 3
Victim safety strategies
|
| Source: References 3,8,10,15,18 |
Treating stalkers
Failing to treat a mentally ill stalker may result in continued risk to the victim. For example, an intimacy-seeking stalker with erotomanic delusions who is confined without treatment likely will be released with no significant reduction in risk. No reliable outcome data exist on treatment for stalkers, however, so you must rely on empirically derived clinical data.
If you work with stalkers, you must be familiar with your state’s duty-to-protect statutes and relevant case law related to stalking so you can discuss legal obligations with the stalker before beginning treatment.
Most stalkers will be difficult to engage in treatment because they have been compelled by a court order to seek therapy. Initially you are likely to encounter the stalker’s striking lack of insight into the nature and consequences of this behavior. The stalker may seek validation for his or her actions while demonstrating little interest in ending the obsessional behavior. Expect well-entrenched defenses of denial, rationalization, and minimization.
A comprehensive description of treatment for stalkers is beyond the scope of this article. However, clinicians with experience treating stalkers recommend the following interventions:4
- thorough psychiatric assessment and diagnosis
- treatment of Axis I or II pathology
- cognitive-behavioral therapy to focus on the stalker’s misperceptions
- motivational interviewing techniques to help the stalker appreciate the need for intervention
- victim empathy development
- social skills enhancement
- periodic risk assessments.
- Stalking Resource Center, National Center for Victims of Crime. www.ncvc.org/src.
- Pinals DA, ed. Stalking: a psychiatric perspective. New York: Oxford University Press; 2007.
- The Antistalking Web Site resources. www.antistalking.com/resource.htm.
- U.S. Department of Justice anti-stalking resources. www.usdoj.gov/ovw/stalking.htm.
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Basile KC, Swahn MH, Chen J, Saltzman LE. Stalking in the United States: recent national prevalence estimates. Am J Prev Med 2006;31(2):172-5.
2. McEwan T, Mullen PE, Purcell R. Identifying risk factors in stalking: a review of current research. Int J Law Psychiatry 2007;30:1-9.
3. Spitzberg BH, Cupach WR. The state of the art of stalking: taking stock of the emerging literature. Aggression and Violent Behavior 2007;12:64-86.
4. Mullen P, Mackenzie R, Ogloff JR, et al. Assessing and managing the risks in the stalking situation. J Am Acad Psychiatry Law 2006;34:439-50.
5. Mohandie K, Meloy JR, McGowan MG, Williams J. The RECON typology of stalking: reliability and validity based upon a large sample of North American stalkers. J Forensic Sci 2006;51(1):147-55.
6. James DV, Farnham FR. Stalking and serious violence. J Am Acad Psychiatry Law 2003;31(4):432-9.
7. McFarlane J, Campbell JC, Watson K. Intimate partner stalking and femicide: urgent implications for women’s safety. Behav Sci Law 2002;20(1-2):51-68.
8. Mullen PE, Pathé M, Purcell R. Stalkers and their victims. Cambridge, UK: Cambridge University Press; 2000.
9. Binder RL. Commentary: the importance of professional judgment in evaluation of stalking and threatening situations. J Am Acad Psychiatry Law 2006;34(4):451-4.
10. White S, Cawood J. Threat management of stalking cases. In: Meloy JR, ed. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:295-314.
11. Orion D. I know you really love me: a psychiatrist’s journal of erotomania, stalking, and obsessive love. New York: Macmillan; 1997.
12. Maxey W. The San Diego stalking strike force: a multi-disciplinary approach to assessing and managing stalking and threat cases. Journal of Threat Assessment 2002;2(1):43-53.
13. McFarlane J, Malecha A, Gist J, et al. Protection orders and intimate partner violence: an 18-month study of 150 black, Hispanic, and white women. Am J Public Health 2004;94(4):613-8.
14. Melton HC. Predicting the occurrence of stalking in relationships characterized by domestic violence. J Interpers Violence 2007;22(1):3-25.
15. Meloy JR. The clinical risk management of stalking: “someone is watching over me….” Am J Psychother 1997;51(2):174-84.
16. Purcell R, Pathé M, Mullen PE. When do repeated intrusions become stalking? J Forensic Psychiatry Psychol 2004;15(4):571-3.
17. Pathé M. Surviving stalking. Cambridge, UK: Cambridge University Press; 2002.
18. Kamphuis JH, Emmelkamp PMG. Traumatic distress among support-seeking female victims of stalking. Am J Psychiatry 2001;158:795-8.
19. Pathé M, Mullen PE. The impact of stalkers on their victims. Br J Psychiatry 1997;170:12-7.
20. Turmanis SA, Brown RI. The stalking and harassment behavior scale: measuring the incidence, nature, and severity of stalking and relational harassment and their psychological effects. Psychol Psychother 2006;79(Pt 2):183-98.
21. Ashmore R, Jones J, Jackson A, Smoyak S. A survey of mental health nurses’ experiences of stalking. J Psychiatr Ment Health Nurs 2006;13:562-9.
22. De Becker G. The gift of fear: survival signals that protect us from violence. New York: Dell Publishing; 1997.
1. Basile KC, Swahn MH, Chen J, Saltzman LE. Stalking in the United States: recent national prevalence estimates. Am J Prev Med 2006;31(2):172-5.
2. McEwan T, Mullen PE, Purcell R. Identifying risk factors in stalking: a review of current research. Int J Law Psychiatry 2007;30:1-9.
3. Spitzberg BH, Cupach WR. The state of the art of stalking: taking stock of the emerging literature. Aggression and Violent Behavior 2007;12:64-86.
4. Mullen P, Mackenzie R, Ogloff JR, et al. Assessing and managing the risks in the stalking situation. J Am Acad Psychiatry Law 2006;34:439-50.
5. Mohandie K, Meloy JR, McGowan MG, Williams J. The RECON typology of stalking: reliability and validity based upon a large sample of North American stalkers. J Forensic Sci 2006;51(1):147-55.
6. James DV, Farnham FR. Stalking and serious violence. J Am Acad Psychiatry Law 2003;31(4):432-9.
7. McFarlane J, Campbell JC, Watson K. Intimate partner stalking and femicide: urgent implications for women’s safety. Behav Sci Law 2002;20(1-2):51-68.
8. Mullen PE, Pathé M, Purcell R. Stalkers and their victims. Cambridge, UK: Cambridge University Press; 2000.
9. Binder RL. Commentary: the importance of professional judgment in evaluation of stalking and threatening situations. J Am Acad Psychiatry Law 2006;34(4):451-4.
10. White S, Cawood J. Threat management of stalking cases. In: Meloy JR, ed. The psychology of stalking: clinical and forensic perspectives. San Diego, CA: Academic Press; 1998:295-314.
11. Orion D. I know you really love me: a psychiatrist’s journal of erotomania, stalking, and obsessive love. New York: Macmillan; 1997.
12. Maxey W. The San Diego stalking strike force: a multi-disciplinary approach to assessing and managing stalking and threat cases. Journal of Threat Assessment 2002;2(1):43-53.
13. McFarlane J, Malecha A, Gist J, et al. Protection orders and intimate partner violence: an 18-month study of 150 black, Hispanic, and white women. Am J Public Health 2004;94(4):613-8.
14. Melton HC. Predicting the occurrence of stalking in relationships characterized by domestic violence. J Interpers Violence 2007;22(1):3-25.
15. Meloy JR. The clinical risk management of stalking: “someone is watching over me….” Am J Psychother 1997;51(2):174-84.
16. Purcell R, Pathé M, Mullen PE. When do repeated intrusions become stalking? J Forensic Psychiatry Psychol 2004;15(4):571-3.
17. Pathé M. Surviving stalking. Cambridge, UK: Cambridge University Press; 2002.
18. Kamphuis JH, Emmelkamp PMG. Traumatic distress among support-seeking female victims of stalking. Am J Psychiatry 2001;158:795-8.
19. Pathé M, Mullen PE. The impact of stalkers on their victims. Br J Psychiatry 1997;170:12-7.
20. Turmanis SA, Brown RI. The stalking and harassment behavior scale: measuring the incidence, nature, and severity of stalking and relational harassment and their psychological effects. Psychol Psychother 2006;79(Pt 2):183-98.
21. Ashmore R, Jones J, Jackson A, Smoyak S. A survey of mental health nurses’ experiences of stalking. J Psychiatr Ment Health Nurs 2006;13:562-9.
22. De Becker G. The gift of fear: survival signals that protect us from violence. New York: Dell Publishing; 1997.
Treating posttraumatic stress in motor vehicle accident survivors
Stopped at a red light, Mr. O glances in the rearview mirror and sees headlights coming up fast. The sport utility vehicle behind him is not slowing down. He braces himself as the SUV plows into the back of his car, snapping his head back and forth violently.
As white smoke fills his eyes and lungs. Mr. O realizes he has been pushed into the intersection, and for a moment thinks about never seeing his wife and children again. As he hears tires screeching, his car is struck by a truck.
Mr. O does not die, as he feared, but 6 months later he is “just not ready” to return to work. The doctor who is treating his whiplash injury refers him for evaluation of lingering anxiety.
Posttraumatic stress disorder (PTSD) resulting from a motor vehicle accident (MVA) can have a persistent disabling effect. To help you effectively treat patients such as Mr. O, this article examines:
- common PTSD symptoms in accident survivors
- recommended diagnostic interviews and assessment tools
- techniques for using psychotherapy to overcome residual PTSD symptoms.
CASE CONTINUED: Lingering impairment
In the 6 months since the accident, Mr. O’s sleep is disrupted by pain and worry; when he can sleep, he frequently has nightmares about the accident. Mr. O feels anxious and irritable, and thoughts of that evening play over and over in his mind.
Mr. O doesn’t like to talk about the accident and has not resumed driving. He avoids all but required trips, such as to doctors’ appointments, which he endures with extreme anxiety. Whenever his wife drives without him, he insists that she immediately call him when she reaches her destination. At the same time, he feels emotionally distant from her and the children. He shows little interest in hobbies he’d previously enjoyed.
3 symptom clusters of PTSD
To meet DSM-IV-TR criteria for PTSD, a person must have experienced, witnessed, or been confronted by an event that involved actual or threatened death or serious injury, to which he responded with intense fear, helplessness, or horror.1 PTSD’s 3 symptom clusters—reexperiencing, avoidance/numbing, and hyperarousal—encompass 17 core symptoms, and a patient must exhibit at least the minimum number of symptoms from each cluster (Table 1).
MVA survivors with PTSD often have intrusive memories and nightmares. They might avoid talking about the accident and resist or abstain from driving or traveling by car. They often fear and avoid people, places, activities, and reminders of the MVA that can trigger upsetting reactions, such as anxiety, tachycardia, and panic. They may be irritable, detached, or estranged from loved ones, or have difficulty sleeping or concentrating. These symptoms must persist for ≥30 days and cause clinically significant distress and impaired functioning for a person to meet the criteria for chronic PTSD.
Table 1
Patients experience 3 ‘clusters’ of PTSD symptoms
| Symptom cluster | Symptoms |
|---|---|
| Reexperiencing (≥1 required) |
|
| Avoidance/numbing (≥3 required) |
|
| Hyperarousal (≥2 required) |
|
| Note: In addition to having the minimum number of symptoms from each cluster as indicated above, for a patient to meet PTSD criteria, symptoms must cause clinically significant distress and impairment in functioning. | |
| PTSD: posttraumatic stress disorder | |
| Source: DSM-IV-TR | |
CASE CONTINUED: Reaching a diagnosis
Using a combination of interviews and self-report measures, the psychiatrist diagnoses Mr. O with chronic PTSD. Since the MVA, Mr. O has developed the required number of reexperiencing, avoidance/numbing, and hyperarousal symptoms. These symptoms have persisted for >30 days and significantly impair his functioning.
Use multiple assessment tools
To assess an MVA survivor for PTSD and related problems, we advocate using a combination of:
- unstructured clinical interviews
- structured clinical interviews
- self-report measures.
Also collect information from collateral sources, such as patients’ spouses or significant others, when appropriate and available.
In an unstructured interview, obtain:
- a thorough, detailed description of the MVA, including what occurred and the patient’s thoughts and feelings during and since the accident
- a description of physical injuries, medical treatments, and medication use.
This information can rule out physical causes of PTSD-like symptoms, such as a traumatic brain injury that results in concentration difficulties and irritability. Also assess the MVA’s effect on travel behavior because this information will help inform treatment.
Structured diagnostic interviews are straightforward and easy to administer with minimal training. We prefer the 30-question Clinician Administered PTSD Scale (CAPS) because evidence supports its reliability and validity.2,3 Use the CAPS to rate intensity and frequency of the 17 core PTSD symptoms over the past week, month, or lifetime. The CAPS can be scored for a PTSD diagnosis and for symptom severity. This tool’s drawback is that it takes 30 to 60 minutes to administer and a few more minutes to score.
Self-report measures are quick to administer and score and provide valuable information about symptom presence and severity.4 We recommend the PTSD Checklist (PCL), a widely used measure that has been shown to reliably and validly assess MVA-related PTSD.5,6 Consisting of 17 items corresponding to the DSM-IV-TR PTSD symptoms, the PCL takes about 5 minutes to complete and 1 or 2 minutes to score. A score ≥44 is a highly accurate indication of PTSD.6
Patients with MVA-related PTSD often have psychiatric comorbidities.7 The most frequently diagnosed are:
- major depressive disorder (in about one-half of persons with MVA-related PTSD)
- anxiety disorders, such as generalized anxiety disorder (in about one-third)
- chronic pain
- alcohol or other substance abuse.
We use the Structured Clinical Interview for DSM-IV (SCID) to diagnose comorbid conditions.8 If you do not have time to administer a structured clinical interview, we recommend using psychometrically sound self-report measures, such as the Beck Depression Inventory9 and the State Trait Anxiety Inventory.10
Length of time since the MVA gives a good indication of how likely PTSD is to remit without intervention. Longitudinal studies have found that within 1 year, PTSD will remit without intervention in nearly two-thirds of those diagnosed within 1 to 4 months of the MVA. PTSD that persists after 1 year is much less likely to resolve without treatment.11 Other predictors of PTSD persistence include:
- lack of physical recovery
- major depression within the first 2 months of the MVA
- current major depression
- alcohol abuse before the MVA
- perceived vulnerability during the MVA
- poor family relationships after the MVA.11
PTSD symptoms that initially do not meet diagnostic criteria (subsyndromal PTSD) can worsen in the first year postMVA and lead to a diagnosis of delayed-onset PTSD.12 Having less social support and experiencing additional life stressors—such as another accident, worsening physical health, or change in job—can contribute to delayed-onset PTSD.
CASE CONTINUED: Overcoming fears with psychotherapy
As part of cognitive-behavioral therapy (CBT), the therapist teaches Mr. O a simple breathing exercise to reduce anxiety. He also leads Mr. O through a progression of imaginal and in vivo exposure exercises. The former involves having the patient think about provocative situations in a graded fashion, from easiest to most difficult, while in the psychiatrist’s office. The latter involves having Mr. O seek out red lights—first as a passenger in a vehicle, then as a driver with a passenger, and then while driving alone—until they no longer cause distress.
The American Psychiatric Association,13 Veterans Affairs/Department of Defense,14 International Society of Traumatic Stress Studies,15 and other organizations recommend CBT to treat PTSD.16 Randomized, controlled trials and other evidence support CBT’s efficacy for MVA-related PTSD.11,17
Before implementing CBT, cultivate a strong therapeutic relationship with MVA survivors. The exercises may be acutely distressing, and you will be asking them to complete between-session practice tasks.
CBT for MVA-related PTSD can be delivered to individuals or groups,18 typically in 8 to 16 weekly or semi-weekly, 60- to 90-minute sessions. (Table 2) explains which elements of CBT address specific PTSD symptoms.11
Therapy usually begins with psychoeducation about PTSD symptoms and expected reactions to trauma (the “flight, fight, or freeze” response) to normalize these reactions and place them within the cognitive-behavioral conceptualization. Teach your patients that avoiding memories and reminders of the trauma maintains PTSD and that they must overcome avoidance for treatment to be successful. Note that avoidance can be subtle, such as a patient going to a feared place but distracting himself while there.
CBT for PTSD often includes teaching an anxiety management skill (Box). Imaginal and in vivo exercises also are usually part of treatment.
In imaginal exposure, patients repeatedly and fully confront their frightening memories within session by recounting as much detail about the MVA as possible, including what they were sensing, feeling, and thinking. This description of the MVA can be recorded during the session or written outside of therapy and read aloud by the patient during sessions.
Either way, assign your patients to review the written or recorded account 2 to 3 times per day between sessions. Repeating this exercise results in habituation to these memories, and the thoughts will evoke progressively less distress.
In vivo exposure is designed to extinguish the conditioned associations patients formed during the MVA. Travel-related anxiety is the primary focus of in vivo exposure because almost all patients experience it.11
This type of exposure therapy uses a fear hierarchy—a list of feared MVA reminders. Patients rate each reminder using a distress scale, such as the Subjective Units of Discomfort Scale (SUDS). Together the therapist and patient agree on a situation in the fear hierarchy that the patient feels able to confront in person without escaping. Patients confront the situation until their distress scale score declines by at least half, repeatedly addressing each item on the hierarchy until they have overcome the most frightening reminders. Consider recruiting patients’ family or friends to help complete these homework exercises.
Typically taught early in the course of cognitive-behavioral therapy, an anxiety management skill gives the patient an easy-to-use, effective way to reduce hyperarousal symptoms.
Anxiety management skills range from simple paced diaphragmatic breathing—where the patient learns to breathe from the abdomen, inhaling and exhaling to a count of 3—to more involved techniques, such as progressive muscle relaxation, when patients systemically tense and relax designated muscle groups in a sequential, articulated fashion.
The patient can use an anxiety management skill to lower basal physical arousal and acute arousal brought on by a stressful experience, such as confronting a reminder of the motor vehicle accident.
Cognitive therapy typically is conducted simultaneously with the other therapeutic components. Early in therapy, the clinician assesses patients’ beliefs related to the accident (such as “The world is very dangerous” or “I have no control over what happens on the road”) and their psychological experiences (“I will lose control of my emotions if I think about it”) and challenges the veracity of these assumptions by bringing up these distortions and statements as they occur within the treatment session. By using forms designed to identify thoughts and beliefs that produce anxiety, patients learn to monitor and challenge their maladaptive thoughts, in essence becoming their own cognitive therapists.
Scheduling pleasant events—assigning patients to participate in activities they previously enjoyed but have discontinued—has been used effectively to treat depression.19 For MVA survivors, this therapy is designed to target PTSD’s numbing symptoms by increasing patients’ social support and resilience.
Patients initially may need some cajoling, but once they begin pleasant activities they often find the experience reinforcing and mood-enhancing, which increases their future participation.
Although pharmacologic therapy for PTSD is beyond the scope of this article, antidepressants—including selective serotonin reuptake inhibitors (such as paroxetine and sertraline), tricyclics, and monoamine oxidase inhibitors—have been shown to effectively treat PTSD.20 For some patients, a combination of medication and psychotherapy may be best.
Patients with MVA-related PTSD often present other problems, including chronic pain, sleep problems, and generalized anxiety. How—and even if—to address these problems in therapy for PTSD is a matter of clinical judgment. Some evidence suggests that CBT can help improve comorbid conditions.7,21
Table 2
Cognitive-behavioral therapy: What’s effective for MVA-related PTSD
| Symptom cluster | CBT component that targets it |
|---|---|
| Reexperiencing | In vivo and imaginal exposure |
| Avoidance | In vivo exposure (for MVA reminders) Imaginal exposure (for MVA memories and related affect) |
| Numbing | Pleasant events scheduling |
| Hyperarousal | Anxiety management skills training |
| All symptom clusters | Psychoeducation about PTSD |
| All symptom clusters | Cognitive therapy |
| Note: Although listed as targeting specific symptom clusters, CBT components have an effect across all clusters. | |
| CBT: cognitive-behavior therapy; MVA: motor vehicle accident; PTSD: posttraumatic stress disorder | |
| Source: Reference 11 | |
CASE CONTINUED: Getting back on the road
After 4 months of CBT, Mr. O’s symptoms have resolved to the point where he is able to drive and return to work. When confronted with situations that had been problematic, Mr. O uses the CBT tools he learned to monitor thoughts and reactions that previously led to distress. With each change and improvement he feels a growing sense of confidence.
Related resources
- National Center for Posttraumatic Stress Disorder. U.S. Department of Veterans Affairs. www.ncptsd.va.gov.
- Hickling EJ, Blanchard EB. Overcoming the trauma of your motor vehicle accident: a cognitive behavioral treatment program, therapist guide. New York: Oxford University Press; 2006.
- Follette VM, Ruzek JI, Abueg FR. Cognitive-behavioral therapies for trauma, 2nd ed. New York: Guilford Press; 1998.
Drug brand names
- Paroxetine • Paxil
- Sertraline • Zoloft
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Blake AT, Weathers F, Nagy L, et al. Clinician administered PTSD scale for DSM-IV (CAPS). Boston, MA: National Center for Post-traumatic Stress Disorder, Behavioral Science Division; 1998.
3. Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 2001;13(3):132-56.
4. Shear MK, Feske U, Brown C, et al. Anxiety disorders measures. In: Rush AJ Jr, Pincus HA, First MB, et al, eds. Handbook of psychiatric measures. Washington, DC: American Psychiatric Press; 2000:549-89.
5. Weathers FW, Litz BT, Herman DS, et al. The PTSD checklist: reliability, validity&diagnostic utility. Paper presented at: annual meeting of the International Society for Traumatic Stress Studies; October 1993; San Antonio, TX.
6. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996;34:669-73.
7. Blanchard EB, Hickling EJ, Freidenberg BM, et al. Two studies of the psychiatric morbidity among motor vehicle accident survivors 1 year after the crash. Behav Res Ther 2004;42:569-83.
8. Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-IV—non-patient version. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996.
9. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;5:561-71.
10. Spielberger CD, Gorsuch RL, Lushune RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970.
11. Blanchard EB, Hickling EJ. After the crash: assessment and treatment of motor vehicle accident survivors. Washington, D.C.: American Psychological Association; 2004.
12. Buckley T, Blanchard EB, Hickling EJ. A prospective examination of delayed onset PTSD secondary to motor vehicle accidents. J Abnorm Psychol 1998;107:508-19.
13. Ursano RJ, Bell C, Eth S, et al. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry 2004;161:3-31.
14. Veterans Health Administration. Management of posttraumatic stress (Office of Quality and Performance Publication #10Q-CPG/PTSD-04). Washington, DC: Veterans Administration, Department of Defense Clinical Practice Guideline Working Group; 2003. Available at: http://www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm. Accessed March 21, 2007.
15. Foa EB, Keane TJ, Friedman MJ. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. New York: Guilford Press; 2000.
16. Bradley R, Greene J, Russ E, et al. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 2005;162:214-27.
17. Ehlers A, Clark DM. Early psychological interventions for adult survivors of trauma: a review. Biol Psychiatry 2003;53:817-26.
18. Beck GJ, Coffey SF. Group cognitive behavioral treatment for PTSD: treatment of motor vehicle accident survivors. Cogn Behav Pract 2004;12:267-77.
19. Jacobson NS, Dobson KS, Truax PA, et al. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol 1996;64:295-304.
20. Davidson J, Bernik M, Connor K, et al. A new treatment algorithm for posttraumatic stress disorder. Psychiatr Ann 2005;35:887-900.
21. Shipherd JC, Beck JG, Hamblen JL, et al. A preliminary examination of treatment for posttraumatic stress disorder in chronic pain patients: a case study. J Trauma Stress 2003;16(5):451-7.
Stopped at a red light, Mr. O glances in the rearview mirror and sees headlights coming up fast. The sport utility vehicle behind him is not slowing down. He braces himself as the SUV plows into the back of his car, snapping his head back and forth violently.
As white smoke fills his eyes and lungs. Mr. O realizes he has been pushed into the intersection, and for a moment thinks about never seeing his wife and children again. As he hears tires screeching, his car is struck by a truck.
Mr. O does not die, as he feared, but 6 months later he is “just not ready” to return to work. The doctor who is treating his whiplash injury refers him for evaluation of lingering anxiety.
Posttraumatic stress disorder (PTSD) resulting from a motor vehicle accident (MVA) can have a persistent disabling effect. To help you effectively treat patients such as Mr. O, this article examines:
- common PTSD symptoms in accident survivors
- recommended diagnostic interviews and assessment tools
- techniques for using psychotherapy to overcome residual PTSD symptoms.
CASE CONTINUED: Lingering impairment
In the 6 months since the accident, Mr. O’s sleep is disrupted by pain and worry; when he can sleep, he frequently has nightmares about the accident. Mr. O feels anxious and irritable, and thoughts of that evening play over and over in his mind.
Mr. O doesn’t like to talk about the accident and has not resumed driving. He avoids all but required trips, such as to doctors’ appointments, which he endures with extreme anxiety. Whenever his wife drives without him, he insists that she immediately call him when she reaches her destination. At the same time, he feels emotionally distant from her and the children. He shows little interest in hobbies he’d previously enjoyed.
3 symptom clusters of PTSD
To meet DSM-IV-TR criteria for PTSD, a person must have experienced, witnessed, or been confronted by an event that involved actual or threatened death or serious injury, to which he responded with intense fear, helplessness, or horror.1 PTSD’s 3 symptom clusters—reexperiencing, avoidance/numbing, and hyperarousal—encompass 17 core symptoms, and a patient must exhibit at least the minimum number of symptoms from each cluster (Table 1).
MVA survivors with PTSD often have intrusive memories and nightmares. They might avoid talking about the accident and resist or abstain from driving or traveling by car. They often fear and avoid people, places, activities, and reminders of the MVA that can trigger upsetting reactions, such as anxiety, tachycardia, and panic. They may be irritable, detached, or estranged from loved ones, or have difficulty sleeping or concentrating. These symptoms must persist for ≥30 days and cause clinically significant distress and impaired functioning for a person to meet the criteria for chronic PTSD.
Table 1
Patients experience 3 ‘clusters’ of PTSD symptoms
| Symptom cluster | Symptoms |
|---|---|
| Reexperiencing (≥1 required) |
|
| Avoidance/numbing (≥3 required) |
|
| Hyperarousal (≥2 required) |
|
| Note: In addition to having the minimum number of symptoms from each cluster as indicated above, for a patient to meet PTSD criteria, symptoms must cause clinically significant distress and impairment in functioning. | |
| PTSD: posttraumatic stress disorder | |
| Source: DSM-IV-TR | |
CASE CONTINUED: Reaching a diagnosis
Using a combination of interviews and self-report measures, the psychiatrist diagnoses Mr. O with chronic PTSD. Since the MVA, Mr. O has developed the required number of reexperiencing, avoidance/numbing, and hyperarousal symptoms. These symptoms have persisted for >30 days and significantly impair his functioning.
Use multiple assessment tools
To assess an MVA survivor for PTSD and related problems, we advocate using a combination of:
- unstructured clinical interviews
- structured clinical interviews
- self-report measures.
Also collect information from collateral sources, such as patients’ spouses or significant others, when appropriate and available.
In an unstructured interview, obtain:
- a thorough, detailed description of the MVA, including what occurred and the patient’s thoughts and feelings during and since the accident
- a description of physical injuries, medical treatments, and medication use.
This information can rule out physical causes of PTSD-like symptoms, such as a traumatic brain injury that results in concentration difficulties and irritability. Also assess the MVA’s effect on travel behavior because this information will help inform treatment.
Structured diagnostic interviews are straightforward and easy to administer with minimal training. We prefer the 30-question Clinician Administered PTSD Scale (CAPS) because evidence supports its reliability and validity.2,3 Use the CAPS to rate intensity and frequency of the 17 core PTSD symptoms over the past week, month, or lifetime. The CAPS can be scored for a PTSD diagnosis and for symptom severity. This tool’s drawback is that it takes 30 to 60 minutes to administer and a few more minutes to score.
Self-report measures are quick to administer and score and provide valuable information about symptom presence and severity.4 We recommend the PTSD Checklist (PCL), a widely used measure that has been shown to reliably and validly assess MVA-related PTSD.5,6 Consisting of 17 items corresponding to the DSM-IV-TR PTSD symptoms, the PCL takes about 5 minutes to complete and 1 or 2 minutes to score. A score ≥44 is a highly accurate indication of PTSD.6
Patients with MVA-related PTSD often have psychiatric comorbidities.7 The most frequently diagnosed are:
- major depressive disorder (in about one-half of persons with MVA-related PTSD)
- anxiety disorders, such as generalized anxiety disorder (in about one-third)
- chronic pain
- alcohol or other substance abuse.
We use the Structured Clinical Interview for DSM-IV (SCID) to diagnose comorbid conditions.8 If you do not have time to administer a structured clinical interview, we recommend using psychometrically sound self-report measures, such as the Beck Depression Inventory9 and the State Trait Anxiety Inventory.10
Length of time since the MVA gives a good indication of how likely PTSD is to remit without intervention. Longitudinal studies have found that within 1 year, PTSD will remit without intervention in nearly two-thirds of those diagnosed within 1 to 4 months of the MVA. PTSD that persists after 1 year is much less likely to resolve without treatment.11 Other predictors of PTSD persistence include:
- lack of physical recovery
- major depression within the first 2 months of the MVA
- current major depression
- alcohol abuse before the MVA
- perceived vulnerability during the MVA
- poor family relationships after the MVA.11
PTSD symptoms that initially do not meet diagnostic criteria (subsyndromal PTSD) can worsen in the first year postMVA and lead to a diagnosis of delayed-onset PTSD.12 Having less social support and experiencing additional life stressors—such as another accident, worsening physical health, or change in job—can contribute to delayed-onset PTSD.
CASE CONTINUED: Overcoming fears with psychotherapy
As part of cognitive-behavioral therapy (CBT), the therapist teaches Mr. O a simple breathing exercise to reduce anxiety. He also leads Mr. O through a progression of imaginal and in vivo exposure exercises. The former involves having the patient think about provocative situations in a graded fashion, from easiest to most difficult, while in the psychiatrist’s office. The latter involves having Mr. O seek out red lights—first as a passenger in a vehicle, then as a driver with a passenger, and then while driving alone—until they no longer cause distress.
The American Psychiatric Association,13 Veterans Affairs/Department of Defense,14 International Society of Traumatic Stress Studies,15 and other organizations recommend CBT to treat PTSD.16 Randomized, controlled trials and other evidence support CBT’s efficacy for MVA-related PTSD.11,17
Before implementing CBT, cultivate a strong therapeutic relationship with MVA survivors. The exercises may be acutely distressing, and you will be asking them to complete between-session practice tasks.
CBT for MVA-related PTSD can be delivered to individuals or groups,18 typically in 8 to 16 weekly or semi-weekly, 60- to 90-minute sessions. (Table 2) explains which elements of CBT address specific PTSD symptoms.11
Therapy usually begins with psychoeducation about PTSD symptoms and expected reactions to trauma (the “flight, fight, or freeze” response) to normalize these reactions and place them within the cognitive-behavioral conceptualization. Teach your patients that avoiding memories and reminders of the trauma maintains PTSD and that they must overcome avoidance for treatment to be successful. Note that avoidance can be subtle, such as a patient going to a feared place but distracting himself while there.
CBT for PTSD often includes teaching an anxiety management skill (Box). Imaginal and in vivo exercises also are usually part of treatment.
In imaginal exposure, patients repeatedly and fully confront their frightening memories within session by recounting as much detail about the MVA as possible, including what they were sensing, feeling, and thinking. This description of the MVA can be recorded during the session or written outside of therapy and read aloud by the patient during sessions.
Either way, assign your patients to review the written or recorded account 2 to 3 times per day between sessions. Repeating this exercise results in habituation to these memories, and the thoughts will evoke progressively less distress.
In vivo exposure is designed to extinguish the conditioned associations patients formed during the MVA. Travel-related anxiety is the primary focus of in vivo exposure because almost all patients experience it.11
This type of exposure therapy uses a fear hierarchy—a list of feared MVA reminders. Patients rate each reminder using a distress scale, such as the Subjective Units of Discomfort Scale (SUDS). Together the therapist and patient agree on a situation in the fear hierarchy that the patient feels able to confront in person without escaping. Patients confront the situation until their distress scale score declines by at least half, repeatedly addressing each item on the hierarchy until they have overcome the most frightening reminders. Consider recruiting patients’ family or friends to help complete these homework exercises.
Typically taught early in the course of cognitive-behavioral therapy, an anxiety management skill gives the patient an easy-to-use, effective way to reduce hyperarousal symptoms.
Anxiety management skills range from simple paced diaphragmatic breathing—where the patient learns to breathe from the abdomen, inhaling and exhaling to a count of 3—to more involved techniques, such as progressive muscle relaxation, when patients systemically tense and relax designated muscle groups in a sequential, articulated fashion.
The patient can use an anxiety management skill to lower basal physical arousal and acute arousal brought on by a stressful experience, such as confronting a reminder of the motor vehicle accident.
Cognitive therapy typically is conducted simultaneously with the other therapeutic components. Early in therapy, the clinician assesses patients’ beliefs related to the accident (such as “The world is very dangerous” or “I have no control over what happens on the road”) and their psychological experiences (“I will lose control of my emotions if I think about it”) and challenges the veracity of these assumptions by bringing up these distortions and statements as they occur within the treatment session. By using forms designed to identify thoughts and beliefs that produce anxiety, patients learn to monitor and challenge their maladaptive thoughts, in essence becoming their own cognitive therapists.
Scheduling pleasant events—assigning patients to participate in activities they previously enjoyed but have discontinued—has been used effectively to treat depression.19 For MVA survivors, this therapy is designed to target PTSD’s numbing symptoms by increasing patients’ social support and resilience.
Patients initially may need some cajoling, but once they begin pleasant activities they often find the experience reinforcing and mood-enhancing, which increases their future participation.
Although pharmacologic therapy for PTSD is beyond the scope of this article, antidepressants—including selective serotonin reuptake inhibitors (such as paroxetine and sertraline), tricyclics, and monoamine oxidase inhibitors—have been shown to effectively treat PTSD.20 For some patients, a combination of medication and psychotherapy may be best.
Patients with MVA-related PTSD often present other problems, including chronic pain, sleep problems, and generalized anxiety. How—and even if—to address these problems in therapy for PTSD is a matter of clinical judgment. Some evidence suggests that CBT can help improve comorbid conditions.7,21
Table 2
Cognitive-behavioral therapy: What’s effective for MVA-related PTSD
| Symptom cluster | CBT component that targets it |
|---|---|
| Reexperiencing | In vivo and imaginal exposure |
| Avoidance | In vivo exposure (for MVA reminders) Imaginal exposure (for MVA memories and related affect) |
| Numbing | Pleasant events scheduling |
| Hyperarousal | Anxiety management skills training |
| All symptom clusters | Psychoeducation about PTSD |
| All symptom clusters | Cognitive therapy |
| Note: Although listed as targeting specific symptom clusters, CBT components have an effect across all clusters. | |
| CBT: cognitive-behavior therapy; MVA: motor vehicle accident; PTSD: posttraumatic stress disorder | |
| Source: Reference 11 | |
CASE CONTINUED: Getting back on the road
After 4 months of CBT, Mr. O’s symptoms have resolved to the point where he is able to drive and return to work. When confronted with situations that had been problematic, Mr. O uses the CBT tools he learned to monitor thoughts and reactions that previously led to distress. With each change and improvement he feels a growing sense of confidence.
Related resources
- National Center for Posttraumatic Stress Disorder. U.S. Department of Veterans Affairs. www.ncptsd.va.gov.
- Hickling EJ, Blanchard EB. Overcoming the trauma of your motor vehicle accident: a cognitive behavioral treatment program, therapist guide. New York: Oxford University Press; 2006.
- Follette VM, Ruzek JI, Abueg FR. Cognitive-behavioral therapies for trauma, 2nd ed. New York: Guilford Press; 1998.
Drug brand names
- Paroxetine • Paxil
- Sertraline • Zoloft
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Stopped at a red light, Mr. O glances in the rearview mirror and sees headlights coming up fast. The sport utility vehicle behind him is not slowing down. He braces himself as the SUV plows into the back of his car, snapping his head back and forth violently.
As white smoke fills his eyes and lungs. Mr. O realizes he has been pushed into the intersection, and for a moment thinks about never seeing his wife and children again. As he hears tires screeching, his car is struck by a truck.
Mr. O does not die, as he feared, but 6 months later he is “just not ready” to return to work. The doctor who is treating his whiplash injury refers him for evaluation of lingering anxiety.
Posttraumatic stress disorder (PTSD) resulting from a motor vehicle accident (MVA) can have a persistent disabling effect. To help you effectively treat patients such as Mr. O, this article examines:
- common PTSD symptoms in accident survivors
- recommended diagnostic interviews and assessment tools
- techniques for using psychotherapy to overcome residual PTSD symptoms.
CASE CONTINUED: Lingering impairment
In the 6 months since the accident, Mr. O’s sleep is disrupted by pain and worry; when he can sleep, he frequently has nightmares about the accident. Mr. O feels anxious and irritable, and thoughts of that evening play over and over in his mind.
Mr. O doesn’t like to talk about the accident and has not resumed driving. He avoids all but required trips, such as to doctors’ appointments, which he endures with extreme anxiety. Whenever his wife drives without him, he insists that she immediately call him when she reaches her destination. At the same time, he feels emotionally distant from her and the children. He shows little interest in hobbies he’d previously enjoyed.
3 symptom clusters of PTSD
To meet DSM-IV-TR criteria for PTSD, a person must have experienced, witnessed, or been confronted by an event that involved actual or threatened death or serious injury, to which he responded with intense fear, helplessness, or horror.1 PTSD’s 3 symptom clusters—reexperiencing, avoidance/numbing, and hyperarousal—encompass 17 core symptoms, and a patient must exhibit at least the minimum number of symptoms from each cluster (Table 1).
MVA survivors with PTSD often have intrusive memories and nightmares. They might avoid talking about the accident and resist or abstain from driving or traveling by car. They often fear and avoid people, places, activities, and reminders of the MVA that can trigger upsetting reactions, such as anxiety, tachycardia, and panic. They may be irritable, detached, or estranged from loved ones, or have difficulty sleeping or concentrating. These symptoms must persist for ≥30 days and cause clinically significant distress and impaired functioning for a person to meet the criteria for chronic PTSD.
Table 1
Patients experience 3 ‘clusters’ of PTSD symptoms
| Symptom cluster | Symptoms |
|---|---|
| Reexperiencing (≥1 required) |
|
| Avoidance/numbing (≥3 required) |
|
| Hyperarousal (≥2 required) |
|
| Note: In addition to having the minimum number of symptoms from each cluster as indicated above, for a patient to meet PTSD criteria, symptoms must cause clinically significant distress and impairment in functioning. | |
| PTSD: posttraumatic stress disorder | |
| Source: DSM-IV-TR | |
CASE CONTINUED: Reaching a diagnosis
Using a combination of interviews and self-report measures, the psychiatrist diagnoses Mr. O with chronic PTSD. Since the MVA, Mr. O has developed the required number of reexperiencing, avoidance/numbing, and hyperarousal symptoms. These symptoms have persisted for >30 days and significantly impair his functioning.
Use multiple assessment tools
To assess an MVA survivor for PTSD and related problems, we advocate using a combination of:
- unstructured clinical interviews
- structured clinical interviews
- self-report measures.
Also collect information from collateral sources, such as patients’ spouses or significant others, when appropriate and available.
In an unstructured interview, obtain:
- a thorough, detailed description of the MVA, including what occurred and the patient’s thoughts and feelings during and since the accident
- a description of physical injuries, medical treatments, and medication use.
This information can rule out physical causes of PTSD-like symptoms, such as a traumatic brain injury that results in concentration difficulties and irritability. Also assess the MVA’s effect on travel behavior because this information will help inform treatment.
Structured diagnostic interviews are straightforward and easy to administer with minimal training. We prefer the 30-question Clinician Administered PTSD Scale (CAPS) because evidence supports its reliability and validity.2,3 Use the CAPS to rate intensity and frequency of the 17 core PTSD symptoms over the past week, month, or lifetime. The CAPS can be scored for a PTSD diagnosis and for symptom severity. This tool’s drawback is that it takes 30 to 60 minutes to administer and a few more minutes to score.
Self-report measures are quick to administer and score and provide valuable information about symptom presence and severity.4 We recommend the PTSD Checklist (PCL), a widely used measure that has been shown to reliably and validly assess MVA-related PTSD.5,6 Consisting of 17 items corresponding to the DSM-IV-TR PTSD symptoms, the PCL takes about 5 minutes to complete and 1 or 2 minutes to score. A score ≥44 is a highly accurate indication of PTSD.6
Patients with MVA-related PTSD often have psychiatric comorbidities.7 The most frequently diagnosed are:
- major depressive disorder (in about one-half of persons with MVA-related PTSD)
- anxiety disorders, such as generalized anxiety disorder (in about one-third)
- chronic pain
- alcohol or other substance abuse.
We use the Structured Clinical Interview for DSM-IV (SCID) to diagnose comorbid conditions.8 If you do not have time to administer a structured clinical interview, we recommend using psychometrically sound self-report measures, such as the Beck Depression Inventory9 and the State Trait Anxiety Inventory.10
Length of time since the MVA gives a good indication of how likely PTSD is to remit without intervention. Longitudinal studies have found that within 1 year, PTSD will remit without intervention in nearly two-thirds of those diagnosed within 1 to 4 months of the MVA. PTSD that persists after 1 year is much less likely to resolve without treatment.11 Other predictors of PTSD persistence include:
- lack of physical recovery
- major depression within the first 2 months of the MVA
- current major depression
- alcohol abuse before the MVA
- perceived vulnerability during the MVA
- poor family relationships after the MVA.11
PTSD symptoms that initially do not meet diagnostic criteria (subsyndromal PTSD) can worsen in the first year postMVA and lead to a diagnosis of delayed-onset PTSD.12 Having less social support and experiencing additional life stressors—such as another accident, worsening physical health, or change in job—can contribute to delayed-onset PTSD.
CASE CONTINUED: Overcoming fears with psychotherapy
As part of cognitive-behavioral therapy (CBT), the therapist teaches Mr. O a simple breathing exercise to reduce anxiety. He also leads Mr. O through a progression of imaginal and in vivo exposure exercises. The former involves having the patient think about provocative situations in a graded fashion, from easiest to most difficult, while in the psychiatrist’s office. The latter involves having Mr. O seek out red lights—first as a passenger in a vehicle, then as a driver with a passenger, and then while driving alone—until they no longer cause distress.
The American Psychiatric Association,13 Veterans Affairs/Department of Defense,14 International Society of Traumatic Stress Studies,15 and other organizations recommend CBT to treat PTSD.16 Randomized, controlled trials and other evidence support CBT’s efficacy for MVA-related PTSD.11,17
Before implementing CBT, cultivate a strong therapeutic relationship with MVA survivors. The exercises may be acutely distressing, and you will be asking them to complete between-session practice tasks.
CBT for MVA-related PTSD can be delivered to individuals or groups,18 typically in 8 to 16 weekly or semi-weekly, 60- to 90-minute sessions. (Table 2) explains which elements of CBT address specific PTSD symptoms.11
Therapy usually begins with psychoeducation about PTSD symptoms and expected reactions to trauma (the “flight, fight, or freeze” response) to normalize these reactions and place them within the cognitive-behavioral conceptualization. Teach your patients that avoiding memories and reminders of the trauma maintains PTSD and that they must overcome avoidance for treatment to be successful. Note that avoidance can be subtle, such as a patient going to a feared place but distracting himself while there.
CBT for PTSD often includes teaching an anxiety management skill (Box). Imaginal and in vivo exercises also are usually part of treatment.
In imaginal exposure, patients repeatedly and fully confront their frightening memories within session by recounting as much detail about the MVA as possible, including what they were sensing, feeling, and thinking. This description of the MVA can be recorded during the session or written outside of therapy and read aloud by the patient during sessions.
Either way, assign your patients to review the written or recorded account 2 to 3 times per day between sessions. Repeating this exercise results in habituation to these memories, and the thoughts will evoke progressively less distress.
In vivo exposure is designed to extinguish the conditioned associations patients formed during the MVA. Travel-related anxiety is the primary focus of in vivo exposure because almost all patients experience it.11
This type of exposure therapy uses a fear hierarchy—a list of feared MVA reminders. Patients rate each reminder using a distress scale, such as the Subjective Units of Discomfort Scale (SUDS). Together the therapist and patient agree on a situation in the fear hierarchy that the patient feels able to confront in person without escaping. Patients confront the situation until their distress scale score declines by at least half, repeatedly addressing each item on the hierarchy until they have overcome the most frightening reminders. Consider recruiting patients’ family or friends to help complete these homework exercises.
Typically taught early in the course of cognitive-behavioral therapy, an anxiety management skill gives the patient an easy-to-use, effective way to reduce hyperarousal symptoms.
Anxiety management skills range from simple paced diaphragmatic breathing—where the patient learns to breathe from the abdomen, inhaling and exhaling to a count of 3—to more involved techniques, such as progressive muscle relaxation, when patients systemically tense and relax designated muscle groups in a sequential, articulated fashion.
The patient can use an anxiety management skill to lower basal physical arousal and acute arousal brought on by a stressful experience, such as confronting a reminder of the motor vehicle accident.
Cognitive therapy typically is conducted simultaneously with the other therapeutic components. Early in therapy, the clinician assesses patients’ beliefs related to the accident (such as “The world is very dangerous” or “I have no control over what happens on the road”) and their psychological experiences (“I will lose control of my emotions if I think about it”) and challenges the veracity of these assumptions by bringing up these distortions and statements as they occur within the treatment session. By using forms designed to identify thoughts and beliefs that produce anxiety, patients learn to monitor and challenge their maladaptive thoughts, in essence becoming their own cognitive therapists.
Scheduling pleasant events—assigning patients to participate in activities they previously enjoyed but have discontinued—has been used effectively to treat depression.19 For MVA survivors, this therapy is designed to target PTSD’s numbing symptoms by increasing patients’ social support and resilience.
Patients initially may need some cajoling, but once they begin pleasant activities they often find the experience reinforcing and mood-enhancing, which increases their future participation.
Although pharmacologic therapy for PTSD is beyond the scope of this article, antidepressants—including selective serotonin reuptake inhibitors (such as paroxetine and sertraline), tricyclics, and monoamine oxidase inhibitors—have been shown to effectively treat PTSD.20 For some patients, a combination of medication and psychotherapy may be best.
Patients with MVA-related PTSD often present other problems, including chronic pain, sleep problems, and generalized anxiety. How—and even if—to address these problems in therapy for PTSD is a matter of clinical judgment. Some evidence suggests that CBT can help improve comorbid conditions.7,21
Table 2
Cognitive-behavioral therapy: What’s effective for MVA-related PTSD
| Symptom cluster | CBT component that targets it |
|---|---|
| Reexperiencing | In vivo and imaginal exposure |
| Avoidance | In vivo exposure (for MVA reminders) Imaginal exposure (for MVA memories and related affect) |
| Numbing | Pleasant events scheduling |
| Hyperarousal | Anxiety management skills training |
| All symptom clusters | Psychoeducation about PTSD |
| All symptom clusters | Cognitive therapy |
| Note: Although listed as targeting specific symptom clusters, CBT components have an effect across all clusters. | |
| CBT: cognitive-behavior therapy; MVA: motor vehicle accident; PTSD: posttraumatic stress disorder | |
| Source: Reference 11 | |
CASE CONTINUED: Getting back on the road
After 4 months of CBT, Mr. O’s symptoms have resolved to the point where he is able to drive and return to work. When confronted with situations that had been problematic, Mr. O uses the CBT tools he learned to monitor thoughts and reactions that previously led to distress. With each change and improvement he feels a growing sense of confidence.
Related resources
- National Center for Posttraumatic Stress Disorder. U.S. Department of Veterans Affairs. www.ncptsd.va.gov.
- Hickling EJ, Blanchard EB. Overcoming the trauma of your motor vehicle accident: a cognitive behavioral treatment program, therapist guide. New York: Oxford University Press; 2006.
- Follette VM, Ruzek JI, Abueg FR. Cognitive-behavioral therapies for trauma, 2nd ed. New York: Guilford Press; 1998.
Drug brand names
- Paroxetine • Paxil
- Sertraline • Zoloft
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Blake AT, Weathers F, Nagy L, et al. Clinician administered PTSD scale for DSM-IV (CAPS). Boston, MA: National Center for Post-traumatic Stress Disorder, Behavioral Science Division; 1998.
3. Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 2001;13(3):132-56.
4. Shear MK, Feske U, Brown C, et al. Anxiety disorders measures. In: Rush AJ Jr, Pincus HA, First MB, et al, eds. Handbook of psychiatric measures. Washington, DC: American Psychiatric Press; 2000:549-89.
5. Weathers FW, Litz BT, Herman DS, et al. The PTSD checklist: reliability, validity&diagnostic utility. Paper presented at: annual meeting of the International Society for Traumatic Stress Studies; October 1993; San Antonio, TX.
6. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996;34:669-73.
7. Blanchard EB, Hickling EJ, Freidenberg BM, et al. Two studies of the psychiatric morbidity among motor vehicle accident survivors 1 year after the crash. Behav Res Ther 2004;42:569-83.
8. Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-IV—non-patient version. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996.
9. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;5:561-71.
10. Spielberger CD, Gorsuch RL, Lushune RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970.
11. Blanchard EB, Hickling EJ. After the crash: assessment and treatment of motor vehicle accident survivors. Washington, D.C.: American Psychological Association; 2004.
12. Buckley T, Blanchard EB, Hickling EJ. A prospective examination of delayed onset PTSD secondary to motor vehicle accidents. J Abnorm Psychol 1998;107:508-19.
13. Ursano RJ, Bell C, Eth S, et al. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry 2004;161:3-31.
14. Veterans Health Administration. Management of posttraumatic stress (Office of Quality and Performance Publication #10Q-CPG/PTSD-04). Washington, DC: Veterans Administration, Department of Defense Clinical Practice Guideline Working Group; 2003. Available at: http://www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm. Accessed March 21, 2007.
15. Foa EB, Keane TJ, Friedman MJ. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. New York: Guilford Press; 2000.
16. Bradley R, Greene J, Russ E, et al. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 2005;162:214-27.
17. Ehlers A, Clark DM. Early psychological interventions for adult survivors of trauma: a review. Biol Psychiatry 2003;53:817-26.
18. Beck GJ, Coffey SF. Group cognitive behavioral treatment for PTSD: treatment of motor vehicle accident survivors. Cogn Behav Pract 2004;12:267-77.
19. Jacobson NS, Dobson KS, Truax PA, et al. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol 1996;64:295-304.
20. Davidson J, Bernik M, Connor K, et al. A new treatment algorithm for posttraumatic stress disorder. Psychiatr Ann 2005;35:887-900.
21. Shipherd JC, Beck JG, Hamblen JL, et al. A preliminary examination of treatment for posttraumatic stress disorder in chronic pain patients: a case study. J Trauma Stress 2003;16(5):451-7.
1. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Blake AT, Weathers F, Nagy L, et al. Clinician administered PTSD scale for DSM-IV (CAPS). Boston, MA: National Center for Post-traumatic Stress Disorder, Behavioral Science Division; 1998.
3. Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 2001;13(3):132-56.
4. Shear MK, Feske U, Brown C, et al. Anxiety disorders measures. In: Rush AJ Jr, Pincus HA, First MB, et al, eds. Handbook of psychiatric measures. Washington, DC: American Psychiatric Press; 2000:549-89.
5. Weathers FW, Litz BT, Herman DS, et al. The PTSD checklist: reliability, validity&diagnostic utility. Paper presented at: annual meeting of the International Society for Traumatic Stress Studies; October 1993; San Antonio, TX.
6. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996;34:669-73.
7. Blanchard EB, Hickling EJ, Freidenberg BM, et al. Two studies of the psychiatric morbidity among motor vehicle accident survivors 1 year after the crash. Behav Res Ther 2004;42:569-83.
8. Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-IV—non-patient version. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996.
9. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;5:561-71.
10. Spielberger CD, Gorsuch RL, Lushune RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970.
11. Blanchard EB, Hickling EJ. After the crash: assessment and treatment of motor vehicle accident survivors. Washington, D.C.: American Psychological Association; 2004.
12. Buckley T, Blanchard EB, Hickling EJ. A prospective examination of delayed onset PTSD secondary to motor vehicle accidents. J Abnorm Psychol 1998;107:508-19.
13. Ursano RJ, Bell C, Eth S, et al. Practice guidelines for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry 2004;161:3-31.
14. Veterans Health Administration. Management of posttraumatic stress (Office of Quality and Performance Publication #10Q-CPG/PTSD-04). Washington, DC: Veterans Administration, Department of Defense Clinical Practice Guideline Working Group; 2003. Available at: http://www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm. Accessed March 21, 2007.
15. Foa EB, Keane TJ, Friedman MJ. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. New York: Guilford Press; 2000.
16. Bradley R, Greene J, Russ E, et al. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 2005;162:214-27.
17. Ehlers A, Clark DM. Early psychological interventions for adult survivors of trauma: a review. Biol Psychiatry 2003;53:817-26.
18. Beck GJ, Coffey SF. Group cognitive behavioral treatment for PTSD: treatment of motor vehicle accident survivors. Cogn Behav Pract 2004;12:267-77.
19. Jacobson NS, Dobson KS, Truax PA, et al. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol 1996;64:295-304.
20. Davidson J, Bernik M, Connor K, et al. A new treatment algorithm for posttraumatic stress disorder. Psychiatr Ann 2005;35:887-900.
21. Shipherd JC, Beck JG, Hamblen JL, et al. A preliminary examination of treatment for posttraumatic stress disorder in chronic pain patients: a case study. J Trauma Stress 2003;16(5):451-7.
Telemedicine: Right for you and your patients?
Most mental health patients are appropriate candidates for telemedicine services—using telecommunication technologies to provide healthcare from a distance—if they agree to this treatment modality.
Psychiatry has an advantage over other specialties because we need only an image—not complex monitoring equipment—to evaluate and treat our patients. We can manage medications and perform consultation, psychological testing, and individual, family, or group psychotherapy using telehealth technologies.
Consider 3 factors before implementing a telemental health program:
What does your patient need? The nature of needed mental health services and point of service suggest which technology to choose. Determine if the barrier to an office visit is geographic or if the patient’s mobility is limited by a comorbidity.
Use videoconferencing or videophones? Videoconferencing equipment used for telemental health applications—a video screen, camera, speakers, and software—provides a clear image, but usually requires dedicated space, integrated services digital network (ISDN) lines, and technical support. These factors can limit clinical applications to “hub and spoke”1 programs that require patients to travel to a central location where videoconferencing technology is available.
Videophones are portable, affordable, durable, and work over conventional telephone lines. These devices resemble a desk telephone with a small screen and built-in camera. Videophones are ideal for:
- community case management2
- settings where space and budgets are limited.
Small image size and narrow bandwidth limit some clinical assessments such as evaluating negative symptoms of schizophrenia or medication-induced movement disorders. Videophones require a power source and a conventional telephone line. Cellular phones do not support videophone technology, which can be a problem for many patients who lack access to conventional telephone services.
Patient selection. Acutely agitated patients and those who pose a danger to themselves or others require face-to-face evaluation. Patients with hearing or vision deficits, delusions, ideas of reference, or hallucinations are not candidates for telehealth treatment.
Telehealth equipment does not allow you to evaluate subtle psychiatric signs such as affect, speech cadence, and certain movement disorders.
Nonverbal information can be crucial, such as in a multicultural environment. You might need periodic face-to-face evaluations to ensure that you do not miss nuances or “back channel” communication3 such as pauses, speech cadence, or gestures.
1. Rothchild E. Telepsychiatry: why do it? Psychiatr Ann 1999;29(7):394-401.
2. Nieves JE. Videophones and psychiatry. Clinical Psychiatric News 2006;34(3):22.-
3. Cukor P, Baer L, Willis BS, et al. Use of videophones and low-cost standard telephone lines to provide social presence in telepsychiatry. Telemed J 1998;4(4):313-21.
Dr. Nieves is associate clinical professor of psychiatry, Eastern Virginia Medical School, and staff psychiatrist, Veterans Administration Medical Center, Hampton, VA.
Most mental health patients are appropriate candidates for telemedicine services—using telecommunication technologies to provide healthcare from a distance—if they agree to this treatment modality.
Psychiatry has an advantage over other specialties because we need only an image—not complex monitoring equipment—to evaluate and treat our patients. We can manage medications and perform consultation, psychological testing, and individual, family, or group psychotherapy using telehealth technologies.
Consider 3 factors before implementing a telemental health program:
What does your patient need? The nature of needed mental health services and point of service suggest which technology to choose. Determine if the barrier to an office visit is geographic or if the patient’s mobility is limited by a comorbidity.
Use videoconferencing or videophones? Videoconferencing equipment used for telemental health applications—a video screen, camera, speakers, and software—provides a clear image, but usually requires dedicated space, integrated services digital network (ISDN) lines, and technical support. These factors can limit clinical applications to “hub and spoke”1 programs that require patients to travel to a central location where videoconferencing technology is available.
Videophones are portable, affordable, durable, and work over conventional telephone lines. These devices resemble a desk telephone with a small screen and built-in camera. Videophones are ideal for:
- community case management2
- settings where space and budgets are limited.
Small image size and narrow bandwidth limit some clinical assessments such as evaluating negative symptoms of schizophrenia or medication-induced movement disorders. Videophones require a power source and a conventional telephone line. Cellular phones do not support videophone technology, which can be a problem for many patients who lack access to conventional telephone services.
Patient selection. Acutely agitated patients and those who pose a danger to themselves or others require face-to-face evaluation. Patients with hearing or vision deficits, delusions, ideas of reference, or hallucinations are not candidates for telehealth treatment.
Telehealth equipment does not allow you to evaluate subtle psychiatric signs such as affect, speech cadence, and certain movement disorders.
Nonverbal information can be crucial, such as in a multicultural environment. You might need periodic face-to-face evaluations to ensure that you do not miss nuances or “back channel” communication3 such as pauses, speech cadence, or gestures.
Most mental health patients are appropriate candidates for telemedicine services—using telecommunication technologies to provide healthcare from a distance—if they agree to this treatment modality.
Psychiatry has an advantage over other specialties because we need only an image—not complex monitoring equipment—to evaluate and treat our patients. We can manage medications and perform consultation, psychological testing, and individual, family, or group psychotherapy using telehealth technologies.
Consider 3 factors before implementing a telemental health program:
What does your patient need? The nature of needed mental health services and point of service suggest which technology to choose. Determine if the barrier to an office visit is geographic or if the patient’s mobility is limited by a comorbidity.
Use videoconferencing or videophones? Videoconferencing equipment used for telemental health applications—a video screen, camera, speakers, and software—provides a clear image, but usually requires dedicated space, integrated services digital network (ISDN) lines, and technical support. These factors can limit clinical applications to “hub and spoke”1 programs that require patients to travel to a central location where videoconferencing technology is available.
Videophones are portable, affordable, durable, and work over conventional telephone lines. These devices resemble a desk telephone with a small screen and built-in camera. Videophones are ideal for:
- community case management2
- settings where space and budgets are limited.
Small image size and narrow bandwidth limit some clinical assessments such as evaluating negative symptoms of schizophrenia or medication-induced movement disorders. Videophones require a power source and a conventional telephone line. Cellular phones do not support videophone technology, which can be a problem for many patients who lack access to conventional telephone services.
Patient selection. Acutely agitated patients and those who pose a danger to themselves or others require face-to-face evaluation. Patients with hearing or vision deficits, delusions, ideas of reference, or hallucinations are not candidates for telehealth treatment.
Telehealth equipment does not allow you to evaluate subtle psychiatric signs such as affect, speech cadence, and certain movement disorders.
Nonverbal information can be crucial, such as in a multicultural environment. You might need periodic face-to-face evaluations to ensure that you do not miss nuances or “back channel” communication3 such as pauses, speech cadence, or gestures.
1. Rothchild E. Telepsychiatry: why do it? Psychiatr Ann 1999;29(7):394-401.
2. Nieves JE. Videophones and psychiatry. Clinical Psychiatric News 2006;34(3):22.-
3. Cukor P, Baer L, Willis BS, et al. Use of videophones and low-cost standard telephone lines to provide social presence in telepsychiatry. Telemed J 1998;4(4):313-21.
Dr. Nieves is associate clinical professor of psychiatry, Eastern Virginia Medical School, and staff psychiatrist, Veterans Administration Medical Center, Hampton, VA.
1. Rothchild E. Telepsychiatry: why do it? Psychiatr Ann 1999;29(7):394-401.
2. Nieves JE. Videophones and psychiatry. Clinical Psychiatric News 2006;34(3):22.-
3. Cukor P, Baer L, Willis BS, et al. Use of videophones and low-cost standard telephone lines to provide social presence in telepsychiatry. Telemed J 1998;4(4):313-21.
Dr. Nieves is associate clinical professor of psychiatry, Eastern Virginia Medical School, and staff psychiatrist, Veterans Administration Medical Center, Hampton, VA.
Make ADHD treatment as effective as possible
Clinical practice guidelines (CPGs) for the diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) in children and adults represent a consensus on the minimal standards and most reasonable, evidence-based practices.1-3 ADHD is too complex for any set of guidelines to address every situation, but CPGs are an excellent starting point for the conscientious practitioner who wants to make ADHD treatment as effective as possible.
Obtain a copy of the CPG that best fits your patients. Several are available for free at www.pediatrics.org/cgi/content/full/105/5/1158 (children) and www.aacap.org/galleries/PracticeParameters/New_ADHD_Parameter.pdf (children, adolescents, and adults).
Use a validated rating scale to confirm your clinical judgment and monitor treatment progress. Several rating scales for childhood psychiatric conditions are available at www.massgeneral.org/schoolpsychiatry/screeningtools_table.asp.
For adults with suspected ADHD, consider asking those who knew the patient as a child to fill out the Adult ADHD Self-Report Scale—available at www.med.nyu.edu/psych/assets/adhdscreen18.pdf—and corroborate the patient’s memory of childhood symptoms. This step is not always necessary, however, because adults with ADHD have been shown to adequately report childhood impairment.4
Start treatment with stimulant medications unless there are clinical reasons to avoid them, such as active substance abuse, glaucoma, or unstablized bipolar disorder. CPGs note that many FDA contraindications for stimulants have little basis in practice or research. These drugs therefore can be used as first-line treatment of ADHD in patients with comorbid tics, anxiety disorders, seizures, stabilized bipolar disorder, carefully monitored substance abuse, and during pregnancy.
Nineteen medications are FDA-approved for ADHD, and 18 are delivery systems of amphetamine or methylphenidate. In large groups, both chemicals have:
- similar effect size (about 0.95)
- the same side effects
- a response rate of 70% to 75%, which increases to 80% to 90% when both are tried.5
Although studies do not show either molecule to be more effective, individuals usually have a clear preference based on how well the medication manages their target symptoms.
Adjust medication according to the patient’s target symptoms. This process educates the patient about why he or she should take the medication. Remember that the patient with ADHD rarely seeks treatment; the primary motivation usually comes from parents or significant others.
Asking “What bothers you the most about your ADHD, and what do you want to get fixed today?” speaks to how the patient can benefit from therapy and indicates what symptoms he or she should look for. Remember, these patients always have had ADHD; they do not know what is possible with treatment.
This answer also tells you what the patient—as opposed to the family—defines as success and reveals his or her motivation to adhere to the medication. Particularly when treating adolescents, get a list of target symptoms from them and their parents because the lists may be different. Unless both the parents and adolescent are satisfied, one might sabotage therapy.
Fine-tune the medication for optimal relief of target symptoms. Although this seems obvious, the prevailing practice pattern is to increase the dosage until the first sign of improvement and then stop. This practice forfeits many potential benefits of medication. Instead, increase the dosage by the lowest increment available as long as the patient:
- reports clear improvement of his or her target symptoms with each dosage increase
- experiences no side effects other than a mild loss of appetite.
When the patient no longer sees improvement, the lowest dose that resolved the target symptoms will be that individual’s optimal dose.
1. Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158-70.
2. Dulcan M, Dunne JE, Ayres W, et al. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997;(suppl 10):S85-S121.
3. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;(suppl 2):S26-S49.
4. Murphy P, Schachar R. Uses of self-ratings in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry 2000;157:1156-9.
5. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-13.
Dr. Dodson is in private practice in Denver, CO.
Clinical practice guidelines (CPGs) for the diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) in children and adults represent a consensus on the minimal standards and most reasonable, evidence-based practices.1-3 ADHD is too complex for any set of guidelines to address every situation, but CPGs are an excellent starting point for the conscientious practitioner who wants to make ADHD treatment as effective as possible.
Obtain a copy of the CPG that best fits your patients. Several are available for free at www.pediatrics.org/cgi/content/full/105/5/1158 (children) and www.aacap.org/galleries/PracticeParameters/New_ADHD_Parameter.pdf (children, adolescents, and adults).
Use a validated rating scale to confirm your clinical judgment and monitor treatment progress. Several rating scales for childhood psychiatric conditions are available at www.massgeneral.org/schoolpsychiatry/screeningtools_table.asp.
For adults with suspected ADHD, consider asking those who knew the patient as a child to fill out the Adult ADHD Self-Report Scale—available at www.med.nyu.edu/psych/assets/adhdscreen18.pdf—and corroborate the patient’s memory of childhood symptoms. This step is not always necessary, however, because adults with ADHD have been shown to adequately report childhood impairment.4
Start treatment with stimulant medications unless there are clinical reasons to avoid them, such as active substance abuse, glaucoma, or unstablized bipolar disorder. CPGs note that many FDA contraindications for stimulants have little basis in practice or research. These drugs therefore can be used as first-line treatment of ADHD in patients with comorbid tics, anxiety disorders, seizures, stabilized bipolar disorder, carefully monitored substance abuse, and during pregnancy.
Nineteen medications are FDA-approved for ADHD, and 18 are delivery systems of amphetamine or methylphenidate. In large groups, both chemicals have:
- similar effect size (about 0.95)
- the same side effects
- a response rate of 70% to 75%, which increases to 80% to 90% when both are tried.5
Although studies do not show either molecule to be more effective, individuals usually have a clear preference based on how well the medication manages their target symptoms.
Adjust medication according to the patient’s target symptoms. This process educates the patient about why he or she should take the medication. Remember that the patient with ADHD rarely seeks treatment; the primary motivation usually comes from parents or significant others.
Asking “What bothers you the most about your ADHD, and what do you want to get fixed today?” speaks to how the patient can benefit from therapy and indicates what symptoms he or she should look for. Remember, these patients always have had ADHD; they do not know what is possible with treatment.
This answer also tells you what the patient—as opposed to the family—defines as success and reveals his or her motivation to adhere to the medication. Particularly when treating adolescents, get a list of target symptoms from them and their parents because the lists may be different. Unless both the parents and adolescent are satisfied, one might sabotage therapy.
Fine-tune the medication for optimal relief of target symptoms. Although this seems obvious, the prevailing practice pattern is to increase the dosage until the first sign of improvement and then stop. This practice forfeits many potential benefits of medication. Instead, increase the dosage by the lowest increment available as long as the patient:
- reports clear improvement of his or her target symptoms with each dosage increase
- experiences no side effects other than a mild loss of appetite.
When the patient no longer sees improvement, the lowest dose that resolved the target symptoms will be that individual’s optimal dose.
Clinical practice guidelines (CPGs) for the diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD) in children and adults represent a consensus on the minimal standards and most reasonable, evidence-based practices.1-3 ADHD is too complex for any set of guidelines to address every situation, but CPGs are an excellent starting point for the conscientious practitioner who wants to make ADHD treatment as effective as possible.
Obtain a copy of the CPG that best fits your patients. Several are available for free at www.pediatrics.org/cgi/content/full/105/5/1158 (children) and www.aacap.org/galleries/PracticeParameters/New_ADHD_Parameter.pdf (children, adolescents, and adults).
Use a validated rating scale to confirm your clinical judgment and monitor treatment progress. Several rating scales for childhood psychiatric conditions are available at www.massgeneral.org/schoolpsychiatry/screeningtools_table.asp.
For adults with suspected ADHD, consider asking those who knew the patient as a child to fill out the Adult ADHD Self-Report Scale—available at www.med.nyu.edu/psych/assets/adhdscreen18.pdf—and corroborate the patient’s memory of childhood symptoms. This step is not always necessary, however, because adults with ADHD have been shown to adequately report childhood impairment.4
Start treatment with stimulant medications unless there are clinical reasons to avoid them, such as active substance abuse, glaucoma, or unstablized bipolar disorder. CPGs note that many FDA contraindications for stimulants have little basis in practice or research. These drugs therefore can be used as first-line treatment of ADHD in patients with comorbid tics, anxiety disorders, seizures, stabilized bipolar disorder, carefully monitored substance abuse, and during pregnancy.
Nineteen medications are FDA-approved for ADHD, and 18 are delivery systems of amphetamine or methylphenidate. In large groups, both chemicals have:
- similar effect size (about 0.95)
- the same side effects
- a response rate of 70% to 75%, which increases to 80% to 90% when both are tried.5
Although studies do not show either molecule to be more effective, individuals usually have a clear preference based on how well the medication manages their target symptoms.
Adjust medication according to the patient’s target symptoms. This process educates the patient about why he or she should take the medication. Remember that the patient with ADHD rarely seeks treatment; the primary motivation usually comes from parents or significant others.
Asking “What bothers you the most about your ADHD, and what do you want to get fixed today?” speaks to how the patient can benefit from therapy and indicates what symptoms he or she should look for. Remember, these patients always have had ADHD; they do not know what is possible with treatment.
This answer also tells you what the patient—as opposed to the family—defines as success and reveals his or her motivation to adhere to the medication. Particularly when treating adolescents, get a list of target symptoms from them and their parents because the lists may be different. Unless both the parents and adolescent are satisfied, one might sabotage therapy.
Fine-tune the medication for optimal relief of target symptoms. Although this seems obvious, the prevailing practice pattern is to increase the dosage until the first sign of improvement and then stop. This practice forfeits many potential benefits of medication. Instead, increase the dosage by the lowest increment available as long as the patient:
- reports clear improvement of his or her target symptoms with each dosage increase
- experiences no side effects other than a mild loss of appetite.
When the patient no longer sees improvement, the lowest dose that resolved the target symptoms will be that individual’s optimal dose.
1. Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158-70.
2. Dulcan M, Dunne JE, Ayres W, et al. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997;(suppl 10):S85-S121.
3. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;(suppl 2):S26-S49.
4. Murphy P, Schachar R. Uses of self-ratings in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry 2000;157:1156-9.
5. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-13.
Dr. Dodson is in private practice in Denver, CO.
1. Committee on Quality Improvement, Subcommittee on Attention-Deficit/Hyperactivity Disorder. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000;105:1158-70.
2. Dulcan M, Dunne JE, Ayres W, et al. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997;(suppl 10):S85-S121.
3. Greenhill LL, Pliszka S, Dulcan MK, et al. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;(suppl 2):S26-S49.
4. Murphy P, Schachar R. Uses of self-ratings in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry 2000;157:1156-9.
5. Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996;35:1304-13.
Dr. Dodson is in private practice in Denver, CO.
Caring for your patient after discharge
Improper treatment of depression,
psychosis blamed for suicide
Kings County (NY) Supreme Court
A 52-year-old patient with a history of mental illness was hospitalized for treatment of major depression with recurrent psychotic features. After release she underwent counseling with a psychiatrist at a mental health center.
One month after discharge the patient was rehospitalized for 2 weeks. After this release she resumed counseling at the mental health center.
Six months later the patient’s husband telephoned the center and reported that the patient needed further treatment. The husband was instructed to bring the patient to a hospital, but he did not do so. The next day the patient committed suicide by jumping from a fourth-floor window.
The case went to trial against the psychiatrist, a social worker, and the mental health center. The patient’s family claimed that she should have been prescribed antidepressant medication, enrolled in family therapy, and received immediate care when her husband telephoned the mental health center with concerns. The psychiatrist, a social worker, and the mental health center argued that the patient was properly treated and medication was prescribed. They counterclaimed that the husband was negligent toward his wife by failing to take her to the hospital as instructed.
A $75,000 settlement was reached with the social worker prior to the verdict. Remaining parties reached a $650,000/$250,000 high/low agreement.
- A defense verdict was returned
A woman with prescription drug abuse
commits suicide 19 days after discharge
Floyd County (GA) Superior Court
A patient, in her early 40s, was under a psychiatrist’s care and admitted to an acute care psychiatric facility for prescription drug abuse. The patient was discharged from the psychiatric facility with instructions to continue outpatient therapy with the psychiatrist. The patient committed suicide 19 days later.
The patient’s family alleged that the psychiatrist failed to properly diagnose and treat the patient’s mental condition, arguing that the clinician should not have discharged the patient from the acute care psychiatric facility while she experienced drug withdrawal symptoms and depression. The psychiatrist claimed that the patient was treated properly for substance abuse, and depression was secondary and related to drug abuse. The psychiatrist also said that the patient received a comprehensive discharge plan, which included follow-up treatment with him and counselors.
- A defense verdict was returned
Suicide rates are highest immediately after hospital discharge.1,2 Inadequate follow-up care or discharge planning may increase the risk for suicide.3 A recent study of 121,933 psychiatric patients at VA hospitals found that 481 (0.4%) died of suicide within 1 year of discharge; 46% of those deaths occurred within the first 3 months. Patients who stayed less than 14 days or had poor continuity of care had a higher risk of suicide.4
Discharge may form the basis for a negligence claim if the release is not a valid exercise in professional judgment. In Bell vs New York City Health and Hospitals Corporation, a patient attempted suicide after hospital discharge. He was released despite suicidal ideation and psychosis. Citing the lack of a well documented psychiatric examination, the court found the hospital negligent because the psychiatrist failed to investigate the patient’s psychiatric history and delusions or an incident when the patient was restrained the night before.5,6
The courts have not found psychiatrists negligent when they perform a risk assessment and reasonably conclude that the benefits of release outweigh the risks.7
Reasonable protection
Two factors determine liability in suicide cases: forseeability and reasonable care.
Forseeability refers to the reasonable evaluation of suicide potential based on a risk assessment. Failure to perform and document this assessment may be evidence of negligence.
Document in your risk assessment the patient’s:
- short-term suicide risk factors (Box 1)
- suicidal thoughts, plans, intents, and actions
- feelings of hopelessness
- substance abuse
- evidence of poor impulse control8,9
- protective factors such as coping and survival skills, family responsibilities, child-related concerns, and moral/ religious beliefs.10,11
- Panic attacks
- Anxiety
- Loss of pleasure
- Diminished concentration
- Depressive turmoil
- Insomnia
Source: Reference 12
In the second case, reasonable care encompasses a discharge plan and continuity of care. Discharge plans should include safety precautions and treatment. Follow-up after discharge ensures that the treatment plan has been carried out. Educate family members about monitoring the patient, communicating observations about changes or concerns, and safeguarding the home, such as removing firearms (Box 2).13
- Emphasize the need for follow-up therapy and/or medication adherence
- Inform the patient and family of crisis management procedures and steps. Patient needs to know how to the contact treatment provider and what to do when the clinician is not immediately accessible in an emergency
- Obtain the patient’s permission for you to talk with family members as is clinically necessary
- Instruct the family to monitor the patient and communicate changes or concerns to the outpatient providers
- Enlist the family to help safeguard the home, for example, removing firearms
- Evaluate the patient’s understanding and acceptance of the aftercare plan.
Family members should be aware of any problems in the patient’s understanding or acceptance of the plan.
Source: Reference 9
- information sources (such as patient report, family report) the psychiatrist used when deciding to discharge the patient
- factors that went into the decision to discharge (such as response to medications)
- how these factors were balanced against the option of keeping the patient in the hospital.
Comparative negligence. In some suicide cases, courts have allowed a comparative negligence defense, either against the family or the patient. In Maunz vs Perales, the psychiatrist instructed the patient’s family to remove all guns from the home, referred the patient to an outpatient clinic, advised the family to make an appointment 1 week later, and then discharged the patient. The next day, the patient bought a gun and shot himself.
The court held that “people generally have a duty to exercise ordinary care for their own safety. To rule otherwise would make the doctor the absolute insurer of any patient exhibiting suicidal tendencies. The consequence of such a ruling would be that no health care provider would want to risk the liability exposure in treating such a patient, and, thus, suicidal persons would be denied necessary treatment.”5,15
1. Geddes JR, Juszczak E, O’Brien F, et al. Suicide in the 12 months after discharge from psychiatric hospital in Scotland, 1968-1992. BMJ 1995;311:357-60.
2. Roy A. Risk factors for suicide in psychiatric patients. Arch Gen Psychiatry 1982;39:1089-95.
3. Oquendo MA, Kamali M, Ellis SP, et al. Adequacy of antidepressant treatment after discharge and the occurrence of suicidal acts in major depression: a prospective study. Am J Psychiatry 2002;159:1746-51.
4. Desai RA, Dausey DJ, Rosenheck RA. Mental health service delivery and suicide risk: the role of individual patient and facility factors. Am J Psychiatry 2005;162:311-18.
5. Packman WL, Pennuto TO, Bongar B, et al. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
6. Bell v. New York City Health and Hospitals Corporation 456 NYS2d 787 (1982).
7. Johnson v. United States, 409 F. Supp. 1283 (D Fla 1981).
8. Simon RI. Commentary: medical errors, sentinel events, and malpractice. J Am Acad Psychiatry Law 2006;34:99-100.
9. Berman AL. Risk management with suicidal patients. J Clin Psychol 2006;62:171-84.
10. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol 1983;51:276-86.
11. Simon RI. Suicide risk assessment: is clinical experience enough? J Am Acad Psychiatry Law 2006;34:276-8.
12. Fawcett J, Scheftner WA, Fogg I, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry 1990;147:1189-45.
13. Abille v. United States, 482 F. Supp. 703 (ND Cal 1980).
14. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
15. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Dr. Grant is associate professor of psychiatry, University of Minnesota Medical Center, Minneapolis
Improper treatment of depression,
psychosis blamed for suicide
Kings County (NY) Supreme Court
A 52-year-old patient with a history of mental illness was hospitalized for treatment of major depression with recurrent psychotic features. After release she underwent counseling with a psychiatrist at a mental health center.
One month after discharge the patient was rehospitalized for 2 weeks. After this release she resumed counseling at the mental health center.
Six months later the patient’s husband telephoned the center and reported that the patient needed further treatment. The husband was instructed to bring the patient to a hospital, but he did not do so. The next day the patient committed suicide by jumping from a fourth-floor window.
The case went to trial against the psychiatrist, a social worker, and the mental health center. The patient’s family claimed that she should have been prescribed antidepressant medication, enrolled in family therapy, and received immediate care when her husband telephoned the mental health center with concerns. The psychiatrist, a social worker, and the mental health center argued that the patient was properly treated and medication was prescribed. They counterclaimed that the husband was negligent toward his wife by failing to take her to the hospital as instructed.
A $75,000 settlement was reached with the social worker prior to the verdict. Remaining parties reached a $650,000/$250,000 high/low agreement.
- A defense verdict was returned
A woman with prescription drug abuse
commits suicide 19 days after discharge
Floyd County (GA) Superior Court
A patient, in her early 40s, was under a psychiatrist’s care and admitted to an acute care psychiatric facility for prescription drug abuse. The patient was discharged from the psychiatric facility with instructions to continue outpatient therapy with the psychiatrist. The patient committed suicide 19 days later.
The patient’s family alleged that the psychiatrist failed to properly diagnose and treat the patient’s mental condition, arguing that the clinician should not have discharged the patient from the acute care psychiatric facility while she experienced drug withdrawal symptoms and depression. The psychiatrist claimed that the patient was treated properly for substance abuse, and depression was secondary and related to drug abuse. The psychiatrist also said that the patient received a comprehensive discharge plan, which included follow-up treatment with him and counselors.
- A defense verdict was returned
Suicide rates are highest immediately after hospital discharge.1,2 Inadequate follow-up care or discharge planning may increase the risk for suicide.3 A recent study of 121,933 psychiatric patients at VA hospitals found that 481 (0.4%) died of suicide within 1 year of discharge; 46% of those deaths occurred within the first 3 months. Patients who stayed less than 14 days or had poor continuity of care had a higher risk of suicide.4
Discharge may form the basis for a negligence claim if the release is not a valid exercise in professional judgment. In Bell vs New York City Health and Hospitals Corporation, a patient attempted suicide after hospital discharge. He was released despite suicidal ideation and psychosis. Citing the lack of a well documented psychiatric examination, the court found the hospital negligent because the psychiatrist failed to investigate the patient’s psychiatric history and delusions or an incident when the patient was restrained the night before.5,6
The courts have not found psychiatrists negligent when they perform a risk assessment and reasonably conclude that the benefits of release outweigh the risks.7
Reasonable protection
Two factors determine liability in suicide cases: forseeability and reasonable care.
Forseeability refers to the reasonable evaluation of suicide potential based on a risk assessment. Failure to perform and document this assessment may be evidence of negligence.
Document in your risk assessment the patient’s:
- short-term suicide risk factors (Box 1)
- suicidal thoughts, plans, intents, and actions
- feelings of hopelessness
- substance abuse
- evidence of poor impulse control8,9
- protective factors such as coping and survival skills, family responsibilities, child-related concerns, and moral/ religious beliefs.10,11
- Panic attacks
- Anxiety
- Loss of pleasure
- Diminished concentration
- Depressive turmoil
- Insomnia
Source: Reference 12
In the second case, reasonable care encompasses a discharge plan and continuity of care. Discharge plans should include safety precautions and treatment. Follow-up after discharge ensures that the treatment plan has been carried out. Educate family members about monitoring the patient, communicating observations about changes or concerns, and safeguarding the home, such as removing firearms (Box 2).13
- Emphasize the need for follow-up therapy and/or medication adherence
- Inform the patient and family of crisis management procedures and steps. Patient needs to know how to the contact treatment provider and what to do when the clinician is not immediately accessible in an emergency
- Obtain the patient’s permission for you to talk with family members as is clinically necessary
- Instruct the family to monitor the patient and communicate changes or concerns to the outpatient providers
- Enlist the family to help safeguard the home, for example, removing firearms
- Evaluate the patient’s understanding and acceptance of the aftercare plan.
Family members should be aware of any problems in the patient’s understanding or acceptance of the plan.
Source: Reference 9
- information sources (such as patient report, family report) the psychiatrist used when deciding to discharge the patient
- factors that went into the decision to discharge (such as response to medications)
- how these factors were balanced against the option of keeping the patient in the hospital.
Comparative negligence. In some suicide cases, courts have allowed a comparative negligence defense, either against the family or the patient. In Maunz vs Perales, the psychiatrist instructed the patient’s family to remove all guns from the home, referred the patient to an outpatient clinic, advised the family to make an appointment 1 week later, and then discharged the patient. The next day, the patient bought a gun and shot himself.
The court held that “people generally have a duty to exercise ordinary care for their own safety. To rule otherwise would make the doctor the absolute insurer of any patient exhibiting suicidal tendencies. The consequence of such a ruling would be that no health care provider would want to risk the liability exposure in treating such a patient, and, thus, suicidal persons would be denied necessary treatment.”5,15
Improper treatment of depression,
psychosis blamed for suicide
Kings County (NY) Supreme Court
A 52-year-old patient with a history of mental illness was hospitalized for treatment of major depression with recurrent psychotic features. After release she underwent counseling with a psychiatrist at a mental health center.
One month after discharge the patient was rehospitalized for 2 weeks. After this release she resumed counseling at the mental health center.
Six months later the patient’s husband telephoned the center and reported that the patient needed further treatment. The husband was instructed to bring the patient to a hospital, but he did not do so. The next day the patient committed suicide by jumping from a fourth-floor window.
The case went to trial against the psychiatrist, a social worker, and the mental health center. The patient’s family claimed that she should have been prescribed antidepressant medication, enrolled in family therapy, and received immediate care when her husband telephoned the mental health center with concerns. The psychiatrist, a social worker, and the mental health center argued that the patient was properly treated and medication was prescribed. They counterclaimed that the husband was negligent toward his wife by failing to take her to the hospital as instructed.
A $75,000 settlement was reached with the social worker prior to the verdict. Remaining parties reached a $650,000/$250,000 high/low agreement.
- A defense verdict was returned
A woman with prescription drug abuse
commits suicide 19 days after discharge
Floyd County (GA) Superior Court
A patient, in her early 40s, was under a psychiatrist’s care and admitted to an acute care psychiatric facility for prescription drug abuse. The patient was discharged from the psychiatric facility with instructions to continue outpatient therapy with the psychiatrist. The patient committed suicide 19 days later.
The patient’s family alleged that the psychiatrist failed to properly diagnose and treat the patient’s mental condition, arguing that the clinician should not have discharged the patient from the acute care psychiatric facility while she experienced drug withdrawal symptoms and depression. The psychiatrist claimed that the patient was treated properly for substance abuse, and depression was secondary and related to drug abuse. The psychiatrist also said that the patient received a comprehensive discharge plan, which included follow-up treatment with him and counselors.
- A defense verdict was returned
Suicide rates are highest immediately after hospital discharge.1,2 Inadequate follow-up care or discharge planning may increase the risk for suicide.3 A recent study of 121,933 psychiatric patients at VA hospitals found that 481 (0.4%) died of suicide within 1 year of discharge; 46% of those deaths occurred within the first 3 months. Patients who stayed less than 14 days or had poor continuity of care had a higher risk of suicide.4
Discharge may form the basis for a negligence claim if the release is not a valid exercise in professional judgment. In Bell vs New York City Health and Hospitals Corporation, a patient attempted suicide after hospital discharge. He was released despite suicidal ideation and psychosis. Citing the lack of a well documented psychiatric examination, the court found the hospital negligent because the psychiatrist failed to investigate the patient’s psychiatric history and delusions or an incident when the patient was restrained the night before.5,6
The courts have not found psychiatrists negligent when they perform a risk assessment and reasonably conclude that the benefits of release outweigh the risks.7
Reasonable protection
Two factors determine liability in suicide cases: forseeability and reasonable care.
Forseeability refers to the reasonable evaluation of suicide potential based on a risk assessment. Failure to perform and document this assessment may be evidence of negligence.
Document in your risk assessment the patient’s:
- short-term suicide risk factors (Box 1)
- suicidal thoughts, plans, intents, and actions
- feelings of hopelessness
- substance abuse
- evidence of poor impulse control8,9
- protective factors such as coping and survival skills, family responsibilities, child-related concerns, and moral/ religious beliefs.10,11
- Panic attacks
- Anxiety
- Loss of pleasure
- Diminished concentration
- Depressive turmoil
- Insomnia
Source: Reference 12
In the second case, reasonable care encompasses a discharge plan and continuity of care. Discharge plans should include safety precautions and treatment. Follow-up after discharge ensures that the treatment plan has been carried out. Educate family members about monitoring the patient, communicating observations about changes or concerns, and safeguarding the home, such as removing firearms (Box 2).13
- Emphasize the need for follow-up therapy and/or medication adherence
- Inform the patient and family of crisis management procedures and steps. Patient needs to know how to the contact treatment provider and what to do when the clinician is not immediately accessible in an emergency
- Obtain the patient’s permission for you to talk with family members as is clinically necessary
- Instruct the family to monitor the patient and communicate changes or concerns to the outpatient providers
- Enlist the family to help safeguard the home, for example, removing firearms
- Evaluate the patient’s understanding and acceptance of the aftercare plan.
Family members should be aware of any problems in the patient’s understanding or acceptance of the plan.
Source: Reference 9
- information sources (such as patient report, family report) the psychiatrist used when deciding to discharge the patient
- factors that went into the decision to discharge (such as response to medications)
- how these factors were balanced against the option of keeping the patient in the hospital.
Comparative negligence. In some suicide cases, courts have allowed a comparative negligence defense, either against the family or the patient. In Maunz vs Perales, the psychiatrist instructed the patient’s family to remove all guns from the home, referred the patient to an outpatient clinic, advised the family to make an appointment 1 week later, and then discharged the patient. The next day, the patient bought a gun and shot himself.
The court held that “people generally have a duty to exercise ordinary care for their own safety. To rule otherwise would make the doctor the absolute insurer of any patient exhibiting suicidal tendencies. The consequence of such a ruling would be that no health care provider would want to risk the liability exposure in treating such a patient, and, thus, suicidal persons would be denied necessary treatment.”5,15
1. Geddes JR, Juszczak E, O’Brien F, et al. Suicide in the 12 months after discharge from psychiatric hospital in Scotland, 1968-1992. BMJ 1995;311:357-60.
2. Roy A. Risk factors for suicide in psychiatric patients. Arch Gen Psychiatry 1982;39:1089-95.
3. Oquendo MA, Kamali M, Ellis SP, et al. Adequacy of antidepressant treatment after discharge and the occurrence of suicidal acts in major depression: a prospective study. Am J Psychiatry 2002;159:1746-51.
4. Desai RA, Dausey DJ, Rosenheck RA. Mental health service delivery and suicide risk: the role of individual patient and facility factors. Am J Psychiatry 2005;162:311-18.
5. Packman WL, Pennuto TO, Bongar B, et al. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
6. Bell v. New York City Health and Hospitals Corporation 456 NYS2d 787 (1982).
7. Johnson v. United States, 409 F. Supp. 1283 (D Fla 1981).
8. Simon RI. Commentary: medical errors, sentinel events, and malpractice. J Am Acad Psychiatry Law 2006;34:99-100.
9. Berman AL. Risk management with suicidal patients. J Clin Psychol 2006;62:171-84.
10. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol 1983;51:276-86.
11. Simon RI. Suicide risk assessment: is clinical experience enough? J Am Acad Psychiatry Law 2006;34:276-8.
12. Fawcett J, Scheftner WA, Fogg I, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry 1990;147:1189-45.
13. Abille v. United States, 482 F. Supp. 703 (ND Cal 1980).
14. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
15. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Dr. Grant is associate professor of psychiatry, University of Minnesota Medical Center, Minneapolis
1. Geddes JR, Juszczak E, O’Brien F, et al. Suicide in the 12 months after discharge from psychiatric hospital in Scotland, 1968-1992. BMJ 1995;311:357-60.
2. Roy A. Risk factors for suicide in psychiatric patients. Arch Gen Psychiatry 1982;39:1089-95.
3. Oquendo MA, Kamali M, Ellis SP, et al. Adequacy of antidepressant treatment after discharge and the occurrence of suicidal acts in major depression: a prospective study. Am J Psychiatry 2002;159:1746-51.
4. Desai RA, Dausey DJ, Rosenheck RA. Mental health service delivery and suicide risk: the role of individual patient and facility factors. Am J Psychiatry 2005;162:311-18.
5. Packman WL, Pennuto TO, Bongar B, et al. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
6. Bell v. New York City Health and Hospitals Corporation 456 NYS2d 787 (1982).
7. Johnson v. United States, 409 F. Supp. 1283 (D Fla 1981).
8. Simon RI. Commentary: medical errors, sentinel events, and malpractice. J Am Acad Psychiatry Law 2006;34:99-100.
9. Berman AL. Risk management with suicidal patients. J Clin Psychol 2006;62:171-84.
10. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol 1983;51:276-86.
11. Simon RI. Suicide risk assessment: is clinical experience enough? J Am Acad Psychiatry Law 2006;34:276-8.
12. Fawcett J, Scheftner WA, Fogg I, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry 1990;147:1189-45.
13. Abille v. United States, 482 F. Supp. 703 (ND Cal 1980).
14. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
15. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Dr. Grant is associate professor of psychiatry, University of Minnesota Medical Center, Minneapolis
To split or not to split?
In “Pros and cons of pill splitting” (Pearls, Current Psychiatry, December 2006), Drs. Rakesh Jain and Shailesh Jain listed Eskalith CR in the “Do not split” category.
Although it is a controlled-release preparation, the tablet is scored, which implies that it can be split. In fact, over the years my patients have split it without problems. Am I missing something?
James W. Jefferson, MD
Distinguished senior scientist
Madison Institute of Medicine
Clinical professor of psychiatry
University of Wisconsin Medical School
Madison, WI
Drs. Jain and Jain Respond
Dr. Jefferson is correct that Eskalith CR is scored and we too have asked patients to split the pill for many years with no apparent difficulties.
However, the latest Eskalith CR package insert states, “When patients require closer titration than that available with doses of Eskalith CR in increments of 450 mg, immediate-release capsules should be used.” This statement implies that splitting this pill is not recommended.
Additionally, GlaxoSmithKline—the manufacturer of Eskalith CR—informed us that the pill is scored to facilitate dissolution studies on split pills, but these studies were never conducted. Therefore, the company cannot recommend pill splitting for Eskalith CR.
Although splitting Eskalith CR pills is common, based on the information above we were conservative in our recommendation and put this medication in the “Do not split” column.
Rakesh Jain, MD, MPH
Director of psychopharmacology research
R/D Clinical Research, Inc.
Lake Jackson, TX
Shailesh Jain, MD, MPH
Assistant professor of psychiatry
University of Texas Medical School
at San Antonio
In “Pros and cons of pill splitting” (Pearls, Current Psychiatry, December 2006), Drs. Rakesh Jain and Shailesh Jain listed Eskalith CR in the “Do not split” category.
Although it is a controlled-release preparation, the tablet is scored, which implies that it can be split. In fact, over the years my patients have split it without problems. Am I missing something?
James W. Jefferson, MD
Distinguished senior scientist
Madison Institute of Medicine
Clinical professor of psychiatry
University of Wisconsin Medical School
Madison, WI
Drs. Jain and Jain Respond
Dr. Jefferson is correct that Eskalith CR is scored and we too have asked patients to split the pill for many years with no apparent difficulties.
However, the latest Eskalith CR package insert states, “When patients require closer titration than that available with doses of Eskalith CR in increments of 450 mg, immediate-release capsules should be used.” This statement implies that splitting this pill is not recommended.
Additionally, GlaxoSmithKline—the manufacturer of Eskalith CR—informed us that the pill is scored to facilitate dissolution studies on split pills, but these studies were never conducted. Therefore, the company cannot recommend pill splitting for Eskalith CR.
Although splitting Eskalith CR pills is common, based on the information above we were conservative in our recommendation and put this medication in the “Do not split” column.
Rakesh Jain, MD, MPH
Director of psychopharmacology research
R/D Clinical Research, Inc.
Lake Jackson, TX
Shailesh Jain, MD, MPH
Assistant professor of psychiatry
University of Texas Medical School
at San Antonio
In “Pros and cons of pill splitting” (Pearls, Current Psychiatry, December 2006), Drs. Rakesh Jain and Shailesh Jain listed Eskalith CR in the “Do not split” category.
Although it is a controlled-release preparation, the tablet is scored, which implies that it can be split. In fact, over the years my patients have split it without problems. Am I missing something?
James W. Jefferson, MD
Distinguished senior scientist
Madison Institute of Medicine
Clinical professor of psychiatry
University of Wisconsin Medical School
Madison, WI
Drs. Jain and Jain Respond
Dr. Jefferson is correct that Eskalith CR is scored and we too have asked patients to split the pill for many years with no apparent difficulties.
However, the latest Eskalith CR package insert states, “When patients require closer titration than that available with doses of Eskalith CR in increments of 450 mg, immediate-release capsules should be used.” This statement implies that splitting this pill is not recommended.
Additionally, GlaxoSmithKline—the manufacturer of Eskalith CR—informed us that the pill is scored to facilitate dissolution studies on split pills, but these studies were never conducted. Therefore, the company cannot recommend pill splitting for Eskalith CR.
Although splitting Eskalith CR pills is common, based on the information above we were conservative in our recommendation and put this medication in the “Do not split” column.
Rakesh Jain, MD, MPH
Director of psychopharmacology research
R/D Clinical Research, Inc.
Lake Jackson, TX
Shailesh Jain, MD, MPH
Assistant professor of psychiatry
University of Texas Medical School
at San Antonio