User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Triple reuptake inhibitors: What to expect from ‘mega-antidepressants’
The first triple reuptake inhibitors are at least several years from approval, but this novel antidepressant class represents an intriguing strategy for treating depression. Several pharmaceutical companies are developing these compounds—with at least one in phase-III clinical trials.
Adding a dopamine reuptake component to serotonin and norepinephrine reuptake blockade could result in an antidepressant with a more rapid onset of action, greater efficacy, and fewer side effects. This article updates what is known about triple reuptake inhibitors and suggests their potential role among first-line antidepressants and in treating patients who have not responded adequately to existing agents.
Role of Monoamines in Depression
Remission—the absence of depression signs and symptoms—is the optimum goal in treating depression. Patients who do not meet this goal are more likely to relapse and to relapse more rapidly than those whose symptoms are treated to remission. Incomplete response rates and delayed onset of action limit the efficacy of available antidepressants (Box).1-6
Antidepressant response. An estimated 60% to 70% of depressed patients respond to antidepressants, but only 20% to 40% achieve remission; 15% of depressed patients do not respond to any available antidepressants.1,2
Delayed onset of action with most antidepressants means that depression does not improve noticeably for at least 1 week and typically 3 weeks or more.3,4 Many patients remain greatly impaired during this “therapeutic lag” and can perceive that the medication isn’t helping them with signs and symptoms such as:
- persistent sad mood
- decreased interest in pleasurable activities (anhedonia)
- changes in body weight or appetite
- changes in sleep patterns
- difficulty thinking or concentrating
- feelings of worthlessness or guilt
- low energy, fatigue, or increased agitation
- recurrent thoughts of death or suicide
- poor self-esteem.
Newer antidepressants such as selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, and dual serotonin/norepinephrine reuptake inhibitors generally are better tolerated and easier to use than tricyclics and monoamine oxidase inhibitors. Even the newer antidepressants can cause side effects such as weight gain and sexual dysfunction, however, and might not be more efficacious than older antidepressants.5,6
Three major mechanisms account for the acute actions of antidepressants on monoamines:
- inhibition of monoamine oxidase, the enzyme that degrades serotonin, norepinephrine, and dopamine
- blockade of neurotransmitter reuptake by binding to transporters
- antagonism of presynaptic neurotransmitter receptors, resulting in an increase in neurotransmitter release.4
Monoamine oxidase inhibitors (MAOIs)—the only available antidepressants known to elevate synaptic levels of norepinephrine, serotonin, and dopamine—are recommended for use in appropriately selected, treatment-resistant patients.8 Meta-analysis of clinical trial data suggests that MAOIs such as phenelzine are particularly effective in outpatients with atypical depression features.9 Even so, the clinical usefulness of MAOIs is limited by their potential for serious drug-drug interactions.
Other mechanisms. Because the synaptic actions of available antidepressants on monoamine neurotransmission occur within hours of administration, the several-week delay in onset of therapeutic action suggests that monoamine dysfunction might not be solely responsible for depression’s pathophysiology. Recent investigations suggest that chronic antidepressant use could cause alterations in gene expression, neuronal plasticity, and downstream signaling pathways that underlie the therapeutic effect.10
Even so, medications that target serotonin, norepinephrine, and dopamine remain mainstays of depression pharmacotherapy.
Triple Reuptake Inhibitors
Novel antidepressants that do not involve direct action on monoamines are under investigation (Table 1). Several compound classes—such as neurokinin and glucocorticoid receptor antagonists—are reported to be in phase-III clinical trials, with projected approval within 5 years.11
Table 1
Mechanisms of select antidepressants in development
| Drug class/target | Proposed mechanism of action |
|---|---|
| Triple reuptake inhibitors | Block serotonin, norepinephrine, and dopamine reuptake |
| CRF1 antagonists | Block receptors for corticotropin releasing factor; regulate HPA axis |
| NK receptor antagonists | Block substance P receptor; offer antidepressant/anxiolytic properties |
| Glutamate acting drugs | Block or modulate NMDA receptor |
| Anti-glucocorticoid agents | Block glucocorticoid receptors; modulate HPA axis feedback |
| cAMP signal transduction | Increases cAMP levels; may affect neuroplasticity |
| cAMP: Cyclic adenosine monophosphate | |
| CRF: Corticotropin releasing factor | |
| HPA: Hypothalamic-pituitary-adrenal (axis) | |
| NK: Neurokinin | |
| NMDA: N-methyl-D-aspartate | |
11 that simultaneously block serotonin, norepinephrine, and dopamine transporters.6,12-15 These broad-spectrum, “triple reuptake inhibitors” are thought to have a more-rapid onset of action and greater efficacy than single or dual reuptake inhibitors.15,16
Triple-action rationale. Some trials have shown antidepressants that act on multiple transporters or receptors to be more effective in treating depressed patients and to have a more-rapid onset of action, compared with single-action drugs such as selective serotonin reuptake inhibitors (SSRIs).7 Adding dopaminergic drugs to serotonergic and/or noradrenergic antidepressants also has been shown to boost the response of patients who were treatment-resistant or partial responders.17
Co-administering dopamine receptor agonists such as bromocriptine, pergolide, or pramipexole (D3 receptor-preferring) with traditional antidepressants has improved clinical symptoms in depressed patients.13 In a retrospective chart review, Clinical Global Impressions (CGI)-Improvement Scale scores were shown to improve with adjunctive pramipexole in 6 of 12 patients with bipolar depression and 8 of 20 patients with unipolar depression.18 Other studies have shown that bromocriptine19 and pergolide20 can improve refractory depression in patients receiving concurrent traditional antidepressants.
Similarly, pramipexole monotherapy has been shown to improve depressive symptoms—including anhedonia—in patients with Parkinson’s disease.21,22 In a 14-week, randomized open-label trial of depressed Parkinson’s patients without motor complications, pramipexole was compared with sertraline for improvement of depressive symptoms. Hamilton Depression Rating Scale (HAM-D) scores decreased in both treatment groups, but the proportion of recovered patients (defined as HAM-D ≤8) was significantly greater in the pramipexole-treated group.21
Dopamine agonists such as pergolide have been associated with valvular heart disease in Parkinson’s patients, which may limit these agents’ usefulness in depression treatment.23
Rank order of potency. To provide the most effective depression treatment, would a compound with equal affinity for all 3 transporters be preferable to a drug with greater affinity for 1 or 2 of the transporters compared with the third? The answer is unknown, as the optimum rank order of potency for inhibiting serotonin, norepinephrine, and dopamine transporters is not yet clear.
Having choices of agents with differing transport inhibition profiles (Table 2)6,13-15 might allow clinicians greater treatment flexibility, however. This could be an advantage when individualizing regimens to target specific symptoms of depression or other psychiatric disorders, such as attention-deficit/hyperactivity disorder.14
Table 2
Neurotransmitter uptake inhibition: Differences in transporter binding expected to broaden therapeutic options
| Transporter binding affinity* | |||
|---|---|---|---|
| Compound | Serotonin | Norepinephrine | Dopamine |
| Investigational triple reuptake inhibitors | |||
| PRC025 | 6 | 19 | 100 |
| PRC050 | 6 | 0.4 | 120 |
| DOV 21,947 | 99 | 262 | 213 |
| DOV 102,677 | 740 | 1,030 | 222 |
| DOV 216,303† | 14 | 20 | 78 |
| Reference antidepressants | |||
| Paroxetine | 0.13 | 40 | 490 |
| Imipramine | 1.4 | 37 | 8,500 |
| Setraline | 0.29 | 420 | 25 |
| Bupropion | 9,100 | 52,000 | 520 |
| Venlafaxine | 9 | 1,060 | 9,300 |
| * Affinity of each compound for binding to serotonin, norepinephrine, and dopamine transporters, as expressed by equilibrium dissociation constants (Kd) in nM. | |||
| † Data reflect inhibition of neurotransmitter reuptake. | |||
| Source: References 6,13-15 | |||
Dopamine and Depression
What can we expect of a “mega-antidepressant” that inhibits serotonin, norepinephrine, and dopamine reuptake? The answer lies in understanding dopamine’s role in depression and antidepressant treatment.
Dopamine plays a part in the underlying pathophysiology of depression and in the action of antidepressant treatments—regardless of their acute mechanism—according to the literature.24,25
Depression pathophysiology. Mesocorticolimbic dopaminergic circuits originating in the ventral tegmental area and projecting to cortical and sub-cortical structures (such as the prefrontal cortex and nucleus accumbens) are important in mediating reward and incentive behavior, attention, addiction, and emotions.26 Deficits in this pathway can contribute to depressive symptoms, particularly anhedonia.3,4
Alterations in dopamine pathways also appear to contribute to depression’s pathophysiology. Compared with nondepressed persons, depressed and/or suicidal patients have been shown to have:
- lower levels of dopamine and its metabolite homovanillic acid25,27-29
- increased dopamine D2/D3 receptor binding30,31 and reduced dopamine transporter activity.31,32
- alter dopaminergic neurotransmission24
- potentiate dopamine signaling (in preclinical studies) by increasing postsynaptic mesolimbic dopamine receptor sensitivity.13,24
Potential Clinical Effects
Clinical evidence. To date, one triple reuptake inhibitor has been studied clinically and reported in the literature. This investigational compound—identified as DOV 216,303—has been found to be safe and well-tolerated when tested in small samples of normal volunteers and depressed individuals.
Healthy male volunteers were given DOV 216,303 in single doses from 5 to 150 mg or multiple doses of 50, 75, or 100 mg for 10 days. Some participants reported gastrointestinal side effects, although only at the highest doses tested.
In a multicenter comparison trial, 67 depressed patients received DOV 216,303 (50 mg bid; 36 patients) or citalopram (20 mg bid; 31 patients) for 2 weeks. After 1 week, both treatments produced comparable reductions in the primary outcome measure (HAM-D scores). Improvements also were seen in secondary measures (CGI scale and Beck Depression Inventory).14 The authors noted that the starting citalopram dosage was higher than typically is given.
By acutely blocking dopamine reuptake, triple reuptake inhibitors could have immediate effects on dopamine-related depression symptoms such as anhedonia, rather than requiring a lead-in of chronic dosing common to other antidepressant classes.16 A triple reuptake inhibitor also might:
- address a broader range of depression symptoms, compared with a single or dual reuptake inhibitor
- be useful for treating substance abuse if it can substitute for addictive compounds at the dopamine transporter without possessing reinforcing characteristics.
Side effects observed with SSRIs, such as sexual dysfunction and weight gain, can be related to a continuous, high occupancy of serotonin transporters. This effect might not occur with a triple reuptake inhibitor and the incidence of serotonin-associated side effects might be lower.14
Related resources
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry 2006;67(suppl 6):3-8.
- American College of Neuropsychopharmacology. Neuropsychopharmacology: the fifth generation of progress. www.acnp.org/Default.aspx?Page=5thGenerationChapters.
- Bromocriptine • Parlodel
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Paroxetine • Paxil
- Pergolide • Permax
- Phenelzine • Nardil
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Shaw and Dr. Boules report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Richelson is a consultant to Eli Lilly and Company.
1. Holtzheimer PE, 3rd, Nemeroff CB. Advances in the treatment of depression. NeuroRx 2006;3(1):42-56.
2. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord 2004;80(2-3):135-44.
3. Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry 2001;25(4):781-823.
4. Richelson E. Pharmacology of antidepressants. Mayo Clin Proc 2001;76(5):511-27.
5. Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci 2002;5(suppl):1068-70.
6. Popik P, Krawczyk M, Golembiowska K, et al. Pharmacological profile of the “triple” monoamine neurotransmitter uptake inhibitor, DOV 102,677. Cell Mol Neurobiol 2006;26(4-6):855-71.
7. Richelson E. Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry 2003;64(suppl 13):5-12.
8. Trivedi MH, Kleiber BA. Using treatment algorithms for the effective management of treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 18):25-9.
9. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology 1995;12(3):185-219.
10. Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev. Neurosci 2001;2(5):343-51.
11. Rosack J. Companies desperately seek antidepressant breakthrough. Psychiatry News 2006;41:22-37.
12. Carlier PR, Lo MM, Lo PC, et al. Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett 1998;8(5):487-92.
13. Shaw AM, Boules M, Zhang Y, et al. Antidepressant-like effects of novel triple reuptake inhibitors, PRC025 and PRC050. Eur J Pharmacol 2007;555(1):30-6.
14. Skolnick P, Krieter P, Tizzano J, et al. Preclinical and clinical pharmacology of DOV 216,303, a “triple” reuptake inhibitor. CNS Drug Rev 2006;12(2):123-34.
15. Skolnick P, Popik P, Janowsky A, et al. Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur J Pharmacol 2003;461(2-3):99-104.
16. Skolnick P, Popik P, Janowsky A, et al. “Broad spectrum” antidepressants: is more better for the treatment of depression? Life Sci 2003;73(25):3175-9.
17. Fava M. Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 18):4-11.
18. Sporn J, Ghaemi SN, Sambur MR, et al. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann Clin Psychiatry 2000;12(3):137-40.
19. Inoue T, Tsuchiya K, Miura J, et al. Bromocriptine treatment of tricyclic and heterocyclic antidepressant-resistant depression. Biol Psychiatry 1996;40(2):151-3.
20. Bouckoms A, Mangini L. Pergolide: an antidepressant adjuvant for mood disorders? Psychopharmacol Bull 1993;29(2):207-11.
21. Barone P, Scarzella L, Marconi R, et al. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol 2006;253(5):601-7.
22. Lemke MR, Brecht HM, Koester J, Reichmann H. Effects of the dopamine agonist pramipexole on depression, anhedonia, and motor functioning in Parkinson’s disease. J Neurol Sci 2006;248(1-2):266-70.
23. Zanettini R, Antonini A, Gatto G, et al. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med 2007;356(1):39-46.
24. D’Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 2000;405(1-3):365-73.
25. Papakostas GI. Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol 2006;16(6):391-402.
26. Greene JG. Gene expression profiles of brain dopamine neurons and relevance to neuropsychiatric disease. J Physiol 2006;575(Pt2):411-6.
27. Engstrom G, Alling C, Blennow K, et al. Reduced cerebrospinal HVA concentrations and HVA/5-HIAA ratios in suicide attempters. Monoamine metabolites in 120 suicide attempters and 47 controls. Eur Neuropsychopharmacol 1999;9(5):399-405.
28. Hamner MB, Diamond BI. Plasma dopamine and norepinephrine correlations with psychomotor retardation, anxiety, and depression in non-psychotic depressed patients: a pilot study. Psychiatry Res 1996;64(3):209-11.
29. Mitani H, Shirayama Y, Yamada T, Kawahara R. Plasma levels of homovanillic acid, 5-hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):531-4.
30. Klimek V, Schenck JE, Han H, et al. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry 2002;52(7):740-8.
31. Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med 1997;27(6):1247-56.
32. Meyer JH, Kruger S, Wilson AA, et al. Lower dopamine transporter binding potential in striatum during depression. Neuroreport 2001;12(18):4121-5.
33 Volkow ND, Wang GJ, Fowler JS, et al. The slow and long-lasting blockade of dopamine transporters in human brain induced by the new antidepressant drug radafaxine predict poor reinforcing effects. Biol Psychiatry 2005;57(6):640-6.
The first triple reuptake inhibitors are at least several years from approval, but this novel antidepressant class represents an intriguing strategy for treating depression. Several pharmaceutical companies are developing these compounds—with at least one in phase-III clinical trials.
Adding a dopamine reuptake component to serotonin and norepinephrine reuptake blockade could result in an antidepressant with a more rapid onset of action, greater efficacy, and fewer side effects. This article updates what is known about triple reuptake inhibitors and suggests their potential role among first-line antidepressants and in treating patients who have not responded adequately to existing agents.
Role of Monoamines in Depression
Remission—the absence of depression signs and symptoms—is the optimum goal in treating depression. Patients who do not meet this goal are more likely to relapse and to relapse more rapidly than those whose symptoms are treated to remission. Incomplete response rates and delayed onset of action limit the efficacy of available antidepressants (Box).1-6
Antidepressant response. An estimated 60% to 70% of depressed patients respond to antidepressants, but only 20% to 40% achieve remission; 15% of depressed patients do not respond to any available antidepressants.1,2
Delayed onset of action with most antidepressants means that depression does not improve noticeably for at least 1 week and typically 3 weeks or more.3,4 Many patients remain greatly impaired during this “therapeutic lag” and can perceive that the medication isn’t helping them with signs and symptoms such as:
- persistent sad mood
- decreased interest in pleasurable activities (anhedonia)
- changes in body weight or appetite
- changes in sleep patterns
- difficulty thinking or concentrating
- feelings of worthlessness or guilt
- low energy, fatigue, or increased agitation
- recurrent thoughts of death or suicide
- poor self-esteem.
Newer antidepressants such as selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, and dual serotonin/norepinephrine reuptake inhibitors generally are better tolerated and easier to use than tricyclics and monoamine oxidase inhibitors. Even the newer antidepressants can cause side effects such as weight gain and sexual dysfunction, however, and might not be more efficacious than older antidepressants.5,6
Three major mechanisms account for the acute actions of antidepressants on monoamines:
- inhibition of monoamine oxidase, the enzyme that degrades serotonin, norepinephrine, and dopamine
- blockade of neurotransmitter reuptake by binding to transporters
- antagonism of presynaptic neurotransmitter receptors, resulting in an increase in neurotransmitter release.4
Monoamine oxidase inhibitors (MAOIs)—the only available antidepressants known to elevate synaptic levels of norepinephrine, serotonin, and dopamine—are recommended for use in appropriately selected, treatment-resistant patients.8 Meta-analysis of clinical trial data suggests that MAOIs such as phenelzine are particularly effective in outpatients with atypical depression features.9 Even so, the clinical usefulness of MAOIs is limited by their potential for serious drug-drug interactions.
Other mechanisms. Because the synaptic actions of available antidepressants on monoamine neurotransmission occur within hours of administration, the several-week delay in onset of therapeutic action suggests that monoamine dysfunction might not be solely responsible for depression’s pathophysiology. Recent investigations suggest that chronic antidepressant use could cause alterations in gene expression, neuronal plasticity, and downstream signaling pathways that underlie the therapeutic effect.10
Even so, medications that target serotonin, norepinephrine, and dopamine remain mainstays of depression pharmacotherapy.
Triple Reuptake Inhibitors
Novel antidepressants that do not involve direct action on monoamines are under investigation (Table 1). Several compound classes—such as neurokinin and glucocorticoid receptor antagonists—are reported to be in phase-III clinical trials, with projected approval within 5 years.11
Table 1
Mechanisms of select antidepressants in development
| Drug class/target | Proposed mechanism of action |
|---|---|
| Triple reuptake inhibitors | Block serotonin, norepinephrine, and dopamine reuptake |
| CRF1 antagonists | Block receptors for corticotropin releasing factor; regulate HPA axis |
| NK receptor antagonists | Block substance P receptor; offer antidepressant/anxiolytic properties |
| Glutamate acting drugs | Block or modulate NMDA receptor |
| Anti-glucocorticoid agents | Block glucocorticoid receptors; modulate HPA axis feedback |
| cAMP signal transduction | Increases cAMP levels; may affect neuroplasticity |
| cAMP: Cyclic adenosine monophosphate | |
| CRF: Corticotropin releasing factor | |
| HPA: Hypothalamic-pituitary-adrenal (axis) | |
| NK: Neurokinin | |
| NMDA: N-methyl-D-aspartate | |
11 that simultaneously block serotonin, norepinephrine, and dopamine transporters.6,12-15 These broad-spectrum, “triple reuptake inhibitors” are thought to have a more-rapid onset of action and greater efficacy than single or dual reuptake inhibitors.15,16
Triple-action rationale. Some trials have shown antidepressants that act on multiple transporters or receptors to be more effective in treating depressed patients and to have a more-rapid onset of action, compared with single-action drugs such as selective serotonin reuptake inhibitors (SSRIs).7 Adding dopaminergic drugs to serotonergic and/or noradrenergic antidepressants also has been shown to boost the response of patients who were treatment-resistant or partial responders.17
Co-administering dopamine receptor agonists such as bromocriptine, pergolide, or pramipexole (D3 receptor-preferring) with traditional antidepressants has improved clinical symptoms in depressed patients.13 In a retrospective chart review, Clinical Global Impressions (CGI)-Improvement Scale scores were shown to improve with adjunctive pramipexole in 6 of 12 patients with bipolar depression and 8 of 20 patients with unipolar depression.18 Other studies have shown that bromocriptine19 and pergolide20 can improve refractory depression in patients receiving concurrent traditional antidepressants.
Similarly, pramipexole monotherapy has been shown to improve depressive symptoms—including anhedonia—in patients with Parkinson’s disease.21,22 In a 14-week, randomized open-label trial of depressed Parkinson’s patients without motor complications, pramipexole was compared with sertraline for improvement of depressive symptoms. Hamilton Depression Rating Scale (HAM-D) scores decreased in both treatment groups, but the proportion of recovered patients (defined as HAM-D ≤8) was significantly greater in the pramipexole-treated group.21
Dopamine agonists such as pergolide have been associated with valvular heart disease in Parkinson’s patients, which may limit these agents’ usefulness in depression treatment.23
Rank order of potency. To provide the most effective depression treatment, would a compound with equal affinity for all 3 transporters be preferable to a drug with greater affinity for 1 or 2 of the transporters compared with the third? The answer is unknown, as the optimum rank order of potency for inhibiting serotonin, norepinephrine, and dopamine transporters is not yet clear.
Having choices of agents with differing transport inhibition profiles (Table 2)6,13-15 might allow clinicians greater treatment flexibility, however. This could be an advantage when individualizing regimens to target specific symptoms of depression or other psychiatric disorders, such as attention-deficit/hyperactivity disorder.14
Table 2
Neurotransmitter uptake inhibition: Differences in transporter binding expected to broaden therapeutic options
| Transporter binding affinity* | |||
|---|---|---|---|
| Compound | Serotonin | Norepinephrine | Dopamine |
| Investigational triple reuptake inhibitors | |||
| PRC025 | 6 | 19 | 100 |
| PRC050 | 6 | 0.4 | 120 |
| DOV 21,947 | 99 | 262 | 213 |
| DOV 102,677 | 740 | 1,030 | 222 |
| DOV 216,303† | 14 | 20 | 78 |
| Reference antidepressants | |||
| Paroxetine | 0.13 | 40 | 490 |
| Imipramine | 1.4 | 37 | 8,500 |
| Setraline | 0.29 | 420 | 25 |
| Bupropion | 9,100 | 52,000 | 520 |
| Venlafaxine | 9 | 1,060 | 9,300 |
| * Affinity of each compound for binding to serotonin, norepinephrine, and dopamine transporters, as expressed by equilibrium dissociation constants (Kd) in nM. | |||
| † Data reflect inhibition of neurotransmitter reuptake. | |||
| Source: References 6,13-15 | |||
Dopamine and Depression
What can we expect of a “mega-antidepressant” that inhibits serotonin, norepinephrine, and dopamine reuptake? The answer lies in understanding dopamine’s role in depression and antidepressant treatment.
Dopamine plays a part in the underlying pathophysiology of depression and in the action of antidepressant treatments—regardless of their acute mechanism—according to the literature.24,25
Depression pathophysiology. Mesocorticolimbic dopaminergic circuits originating in the ventral tegmental area and projecting to cortical and sub-cortical structures (such as the prefrontal cortex and nucleus accumbens) are important in mediating reward and incentive behavior, attention, addiction, and emotions.26 Deficits in this pathway can contribute to depressive symptoms, particularly anhedonia.3,4
Alterations in dopamine pathways also appear to contribute to depression’s pathophysiology. Compared with nondepressed persons, depressed and/or suicidal patients have been shown to have:
- lower levels of dopamine and its metabolite homovanillic acid25,27-29
- increased dopamine D2/D3 receptor binding30,31 and reduced dopamine transporter activity.31,32
- alter dopaminergic neurotransmission24
- potentiate dopamine signaling (in preclinical studies) by increasing postsynaptic mesolimbic dopamine receptor sensitivity.13,24
Potential Clinical Effects
Clinical evidence. To date, one triple reuptake inhibitor has been studied clinically and reported in the literature. This investigational compound—identified as DOV 216,303—has been found to be safe and well-tolerated when tested in small samples of normal volunteers and depressed individuals.
Healthy male volunteers were given DOV 216,303 in single doses from 5 to 150 mg or multiple doses of 50, 75, or 100 mg for 10 days. Some participants reported gastrointestinal side effects, although only at the highest doses tested.
In a multicenter comparison trial, 67 depressed patients received DOV 216,303 (50 mg bid; 36 patients) or citalopram (20 mg bid; 31 patients) for 2 weeks. After 1 week, both treatments produced comparable reductions in the primary outcome measure (HAM-D scores). Improvements also were seen in secondary measures (CGI scale and Beck Depression Inventory).14 The authors noted that the starting citalopram dosage was higher than typically is given.
By acutely blocking dopamine reuptake, triple reuptake inhibitors could have immediate effects on dopamine-related depression symptoms such as anhedonia, rather than requiring a lead-in of chronic dosing common to other antidepressant classes.16 A triple reuptake inhibitor also might:
- address a broader range of depression symptoms, compared with a single or dual reuptake inhibitor
- be useful for treating substance abuse if it can substitute for addictive compounds at the dopamine transporter without possessing reinforcing characteristics.
Side effects observed with SSRIs, such as sexual dysfunction and weight gain, can be related to a continuous, high occupancy of serotonin transporters. This effect might not occur with a triple reuptake inhibitor and the incidence of serotonin-associated side effects might be lower.14
Related resources
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry 2006;67(suppl 6):3-8.
- American College of Neuropsychopharmacology. Neuropsychopharmacology: the fifth generation of progress. www.acnp.org/Default.aspx?Page=5thGenerationChapters.
- Bromocriptine • Parlodel
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Paroxetine • Paxil
- Pergolide • Permax
- Phenelzine • Nardil
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Shaw and Dr. Boules report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Richelson is a consultant to Eli Lilly and Company.
The first triple reuptake inhibitors are at least several years from approval, but this novel antidepressant class represents an intriguing strategy for treating depression. Several pharmaceutical companies are developing these compounds—with at least one in phase-III clinical trials.
Adding a dopamine reuptake component to serotonin and norepinephrine reuptake blockade could result in an antidepressant with a more rapid onset of action, greater efficacy, and fewer side effects. This article updates what is known about triple reuptake inhibitors and suggests their potential role among first-line antidepressants and in treating patients who have not responded adequately to existing agents.
Role of Monoamines in Depression
Remission—the absence of depression signs and symptoms—is the optimum goal in treating depression. Patients who do not meet this goal are more likely to relapse and to relapse more rapidly than those whose symptoms are treated to remission. Incomplete response rates and delayed onset of action limit the efficacy of available antidepressants (Box).1-6
Antidepressant response. An estimated 60% to 70% of depressed patients respond to antidepressants, but only 20% to 40% achieve remission; 15% of depressed patients do not respond to any available antidepressants.1,2
Delayed onset of action with most antidepressants means that depression does not improve noticeably for at least 1 week and typically 3 weeks or more.3,4 Many patients remain greatly impaired during this “therapeutic lag” and can perceive that the medication isn’t helping them with signs and symptoms such as:
- persistent sad mood
- decreased interest in pleasurable activities (anhedonia)
- changes in body weight or appetite
- changes in sleep patterns
- difficulty thinking or concentrating
- feelings of worthlessness or guilt
- low energy, fatigue, or increased agitation
- recurrent thoughts of death or suicide
- poor self-esteem.
Newer antidepressants such as selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, and dual serotonin/norepinephrine reuptake inhibitors generally are better tolerated and easier to use than tricyclics and monoamine oxidase inhibitors. Even the newer antidepressants can cause side effects such as weight gain and sexual dysfunction, however, and might not be more efficacious than older antidepressants.5,6
Three major mechanisms account for the acute actions of antidepressants on monoamines:
- inhibition of monoamine oxidase, the enzyme that degrades serotonin, norepinephrine, and dopamine
- blockade of neurotransmitter reuptake by binding to transporters
- antagonism of presynaptic neurotransmitter receptors, resulting in an increase in neurotransmitter release.4
Monoamine oxidase inhibitors (MAOIs)—the only available antidepressants known to elevate synaptic levels of norepinephrine, serotonin, and dopamine—are recommended for use in appropriately selected, treatment-resistant patients.8 Meta-analysis of clinical trial data suggests that MAOIs such as phenelzine are particularly effective in outpatients with atypical depression features.9 Even so, the clinical usefulness of MAOIs is limited by their potential for serious drug-drug interactions.
Other mechanisms. Because the synaptic actions of available antidepressants on monoamine neurotransmission occur within hours of administration, the several-week delay in onset of therapeutic action suggests that monoamine dysfunction might not be solely responsible for depression’s pathophysiology. Recent investigations suggest that chronic antidepressant use could cause alterations in gene expression, neuronal plasticity, and downstream signaling pathways that underlie the therapeutic effect.10
Even so, medications that target serotonin, norepinephrine, and dopamine remain mainstays of depression pharmacotherapy.
Triple Reuptake Inhibitors
Novel antidepressants that do not involve direct action on monoamines are under investigation (Table 1). Several compound classes—such as neurokinin and glucocorticoid receptor antagonists—are reported to be in phase-III clinical trials, with projected approval within 5 years.11
Table 1
Mechanisms of select antidepressants in development
| Drug class/target | Proposed mechanism of action |
|---|---|
| Triple reuptake inhibitors | Block serotonin, norepinephrine, and dopamine reuptake |
| CRF1 antagonists | Block receptors for corticotropin releasing factor; regulate HPA axis |
| NK receptor antagonists | Block substance P receptor; offer antidepressant/anxiolytic properties |
| Glutamate acting drugs | Block or modulate NMDA receptor |
| Anti-glucocorticoid agents | Block glucocorticoid receptors; modulate HPA axis feedback |
| cAMP signal transduction | Increases cAMP levels; may affect neuroplasticity |
| cAMP: Cyclic adenosine monophosphate | |
| CRF: Corticotropin releasing factor | |
| HPA: Hypothalamic-pituitary-adrenal (axis) | |
| NK: Neurokinin | |
| NMDA: N-methyl-D-aspartate | |
11 that simultaneously block serotonin, norepinephrine, and dopamine transporters.6,12-15 These broad-spectrum, “triple reuptake inhibitors” are thought to have a more-rapid onset of action and greater efficacy than single or dual reuptake inhibitors.15,16
Triple-action rationale. Some trials have shown antidepressants that act on multiple transporters or receptors to be more effective in treating depressed patients and to have a more-rapid onset of action, compared with single-action drugs such as selective serotonin reuptake inhibitors (SSRIs).7 Adding dopaminergic drugs to serotonergic and/or noradrenergic antidepressants also has been shown to boost the response of patients who were treatment-resistant or partial responders.17
Co-administering dopamine receptor agonists such as bromocriptine, pergolide, or pramipexole (D3 receptor-preferring) with traditional antidepressants has improved clinical symptoms in depressed patients.13 In a retrospective chart review, Clinical Global Impressions (CGI)-Improvement Scale scores were shown to improve with adjunctive pramipexole in 6 of 12 patients with bipolar depression and 8 of 20 patients with unipolar depression.18 Other studies have shown that bromocriptine19 and pergolide20 can improve refractory depression in patients receiving concurrent traditional antidepressants.
Similarly, pramipexole monotherapy has been shown to improve depressive symptoms—including anhedonia—in patients with Parkinson’s disease.21,22 In a 14-week, randomized open-label trial of depressed Parkinson’s patients without motor complications, pramipexole was compared with sertraline for improvement of depressive symptoms. Hamilton Depression Rating Scale (HAM-D) scores decreased in both treatment groups, but the proportion of recovered patients (defined as HAM-D ≤8) was significantly greater in the pramipexole-treated group.21
Dopamine agonists such as pergolide have been associated with valvular heart disease in Parkinson’s patients, which may limit these agents’ usefulness in depression treatment.23
Rank order of potency. To provide the most effective depression treatment, would a compound with equal affinity for all 3 transporters be preferable to a drug with greater affinity for 1 or 2 of the transporters compared with the third? The answer is unknown, as the optimum rank order of potency for inhibiting serotonin, norepinephrine, and dopamine transporters is not yet clear.
Having choices of agents with differing transport inhibition profiles (Table 2)6,13-15 might allow clinicians greater treatment flexibility, however. This could be an advantage when individualizing regimens to target specific symptoms of depression or other psychiatric disorders, such as attention-deficit/hyperactivity disorder.14
Table 2
Neurotransmitter uptake inhibition: Differences in transporter binding expected to broaden therapeutic options
| Transporter binding affinity* | |||
|---|---|---|---|
| Compound | Serotonin | Norepinephrine | Dopamine |
| Investigational triple reuptake inhibitors | |||
| PRC025 | 6 | 19 | 100 |
| PRC050 | 6 | 0.4 | 120 |
| DOV 21,947 | 99 | 262 | 213 |
| DOV 102,677 | 740 | 1,030 | 222 |
| DOV 216,303† | 14 | 20 | 78 |
| Reference antidepressants | |||
| Paroxetine | 0.13 | 40 | 490 |
| Imipramine | 1.4 | 37 | 8,500 |
| Setraline | 0.29 | 420 | 25 |
| Bupropion | 9,100 | 52,000 | 520 |
| Venlafaxine | 9 | 1,060 | 9,300 |
| * Affinity of each compound for binding to serotonin, norepinephrine, and dopamine transporters, as expressed by equilibrium dissociation constants (Kd) in nM. | |||
| † Data reflect inhibition of neurotransmitter reuptake. | |||
| Source: References 6,13-15 | |||
Dopamine and Depression
What can we expect of a “mega-antidepressant” that inhibits serotonin, norepinephrine, and dopamine reuptake? The answer lies in understanding dopamine’s role in depression and antidepressant treatment.
Dopamine plays a part in the underlying pathophysiology of depression and in the action of antidepressant treatments—regardless of their acute mechanism—according to the literature.24,25
Depression pathophysiology. Mesocorticolimbic dopaminergic circuits originating in the ventral tegmental area and projecting to cortical and sub-cortical structures (such as the prefrontal cortex and nucleus accumbens) are important in mediating reward and incentive behavior, attention, addiction, and emotions.26 Deficits in this pathway can contribute to depressive symptoms, particularly anhedonia.3,4
Alterations in dopamine pathways also appear to contribute to depression’s pathophysiology. Compared with nondepressed persons, depressed and/or suicidal patients have been shown to have:
- lower levels of dopamine and its metabolite homovanillic acid25,27-29
- increased dopamine D2/D3 receptor binding30,31 and reduced dopamine transporter activity.31,32
- alter dopaminergic neurotransmission24
- potentiate dopamine signaling (in preclinical studies) by increasing postsynaptic mesolimbic dopamine receptor sensitivity.13,24
Potential Clinical Effects
Clinical evidence. To date, one triple reuptake inhibitor has been studied clinically and reported in the literature. This investigational compound—identified as DOV 216,303—has been found to be safe and well-tolerated when tested in small samples of normal volunteers and depressed individuals.
Healthy male volunteers were given DOV 216,303 in single doses from 5 to 150 mg or multiple doses of 50, 75, or 100 mg for 10 days. Some participants reported gastrointestinal side effects, although only at the highest doses tested.
In a multicenter comparison trial, 67 depressed patients received DOV 216,303 (50 mg bid; 36 patients) or citalopram (20 mg bid; 31 patients) for 2 weeks. After 1 week, both treatments produced comparable reductions in the primary outcome measure (HAM-D scores). Improvements also were seen in secondary measures (CGI scale and Beck Depression Inventory).14 The authors noted that the starting citalopram dosage was higher than typically is given.
By acutely blocking dopamine reuptake, triple reuptake inhibitors could have immediate effects on dopamine-related depression symptoms such as anhedonia, rather than requiring a lead-in of chronic dosing common to other antidepressant classes.16 A triple reuptake inhibitor also might:
- address a broader range of depression symptoms, compared with a single or dual reuptake inhibitor
- be useful for treating substance abuse if it can substitute for addictive compounds at the dopamine transporter without possessing reinforcing characteristics.
Side effects observed with SSRIs, such as sexual dysfunction and weight gain, can be related to a continuous, high occupancy of serotonin transporters. This effect might not occur with a triple reuptake inhibitor and the incidence of serotonin-associated side effects might be lower.14
Related resources
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry 2006;67(suppl 6):3-8.
- American College of Neuropsychopharmacology. Neuropsychopharmacology: the fifth generation of progress. www.acnp.org/Default.aspx?Page=5thGenerationChapters.
- Bromocriptine • Parlodel
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Paroxetine • Paxil
- Pergolide • Permax
- Phenelzine • Nardil
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Sertraline • Zoloft
- Venlafaxine • Effexor
Dr. Shaw and Dr. Boules report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Richelson is a consultant to Eli Lilly and Company.
1. Holtzheimer PE, 3rd, Nemeroff CB. Advances in the treatment of depression. NeuroRx 2006;3(1):42-56.
2. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord 2004;80(2-3):135-44.
3. Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry 2001;25(4):781-823.
4. Richelson E. Pharmacology of antidepressants. Mayo Clin Proc 2001;76(5):511-27.
5. Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci 2002;5(suppl):1068-70.
6. Popik P, Krawczyk M, Golembiowska K, et al. Pharmacological profile of the “triple” monoamine neurotransmitter uptake inhibitor, DOV 102,677. Cell Mol Neurobiol 2006;26(4-6):855-71.
7. Richelson E. Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry 2003;64(suppl 13):5-12.
8. Trivedi MH, Kleiber BA. Using treatment algorithms for the effective management of treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 18):25-9.
9. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology 1995;12(3):185-219.
10. Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev. Neurosci 2001;2(5):343-51.
11. Rosack J. Companies desperately seek antidepressant breakthrough. Psychiatry News 2006;41:22-37.
12. Carlier PR, Lo MM, Lo PC, et al. Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett 1998;8(5):487-92.
13. Shaw AM, Boules M, Zhang Y, et al. Antidepressant-like effects of novel triple reuptake inhibitors, PRC025 and PRC050. Eur J Pharmacol 2007;555(1):30-6.
14. Skolnick P, Krieter P, Tizzano J, et al. Preclinical and clinical pharmacology of DOV 216,303, a “triple” reuptake inhibitor. CNS Drug Rev 2006;12(2):123-34.
15. Skolnick P, Popik P, Janowsky A, et al. Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur J Pharmacol 2003;461(2-3):99-104.
16. Skolnick P, Popik P, Janowsky A, et al. “Broad spectrum” antidepressants: is more better for the treatment of depression? Life Sci 2003;73(25):3175-9.
17. Fava M. Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 18):4-11.
18. Sporn J, Ghaemi SN, Sambur MR, et al. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann Clin Psychiatry 2000;12(3):137-40.
19. Inoue T, Tsuchiya K, Miura J, et al. Bromocriptine treatment of tricyclic and heterocyclic antidepressant-resistant depression. Biol Psychiatry 1996;40(2):151-3.
20. Bouckoms A, Mangini L. Pergolide: an antidepressant adjuvant for mood disorders? Psychopharmacol Bull 1993;29(2):207-11.
21. Barone P, Scarzella L, Marconi R, et al. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol 2006;253(5):601-7.
22. Lemke MR, Brecht HM, Koester J, Reichmann H. Effects of the dopamine agonist pramipexole on depression, anhedonia, and motor functioning in Parkinson’s disease. J Neurol Sci 2006;248(1-2):266-70.
23. Zanettini R, Antonini A, Gatto G, et al. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med 2007;356(1):39-46.
24. D’Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 2000;405(1-3):365-73.
25. Papakostas GI. Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol 2006;16(6):391-402.
26. Greene JG. Gene expression profiles of brain dopamine neurons and relevance to neuropsychiatric disease. J Physiol 2006;575(Pt2):411-6.
27. Engstrom G, Alling C, Blennow K, et al. Reduced cerebrospinal HVA concentrations and HVA/5-HIAA ratios in suicide attempters. Monoamine metabolites in 120 suicide attempters and 47 controls. Eur Neuropsychopharmacol 1999;9(5):399-405.
28. Hamner MB, Diamond BI. Plasma dopamine and norepinephrine correlations with psychomotor retardation, anxiety, and depression in non-psychotic depressed patients: a pilot study. Psychiatry Res 1996;64(3):209-11.
29. Mitani H, Shirayama Y, Yamada T, Kawahara R. Plasma levels of homovanillic acid, 5-hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):531-4.
30. Klimek V, Schenck JE, Han H, et al. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry 2002;52(7):740-8.
31. Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med 1997;27(6):1247-56.
32. Meyer JH, Kruger S, Wilson AA, et al. Lower dopamine transporter binding potential in striatum during depression. Neuroreport 2001;12(18):4121-5.
33 Volkow ND, Wang GJ, Fowler JS, et al. The slow and long-lasting blockade of dopamine transporters in human brain induced by the new antidepressant drug radafaxine predict poor reinforcing effects. Biol Psychiatry 2005;57(6):640-6.
1. Holtzheimer PE, 3rd, Nemeroff CB. Advances in the treatment of depression. NeuroRx 2006;3(1):42-56.
2. Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord 2004;80(2-3):135-44.
3. Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry 2001;25(4):781-823.
4. Richelson E. Pharmacology of antidepressants. Mayo Clin Proc 2001;76(5):511-27.
5. Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci 2002;5(suppl):1068-70.
6. Popik P, Krawczyk M, Golembiowska K, et al. Pharmacological profile of the “triple” monoamine neurotransmitter uptake inhibitor, DOV 102,677. Cell Mol Neurobiol 2006;26(4-6):855-71.
7. Richelson E. Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry 2003;64(suppl 13):5-12.
8. Trivedi MH, Kleiber BA. Using treatment algorithms for the effective management of treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 18):25-9.
9. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology 1995;12(3):185-219.
10. Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev. Neurosci 2001;2(5):343-51.
11. Rosack J. Companies desperately seek antidepressant breakthrough. Psychiatry News 2006;41:22-37.
12. Carlier PR, Lo MM, Lo PC, et al. Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett 1998;8(5):487-92.
13. Shaw AM, Boules M, Zhang Y, et al. Antidepressant-like effects of novel triple reuptake inhibitors, PRC025 and PRC050. Eur J Pharmacol 2007;555(1):30-6.
14. Skolnick P, Krieter P, Tizzano J, et al. Preclinical and clinical pharmacology of DOV 216,303, a “triple” reuptake inhibitor. CNS Drug Rev 2006;12(2):123-34.
15. Skolnick P, Popik P, Janowsky A, et al. Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur J Pharmacol 2003;461(2-3):99-104.
16. Skolnick P, Popik P, Janowsky A, et al. “Broad spectrum” antidepressants: is more better for the treatment of depression? Life Sci 2003;73(25):3175-9.
17. Fava M. Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 18):4-11.
18. Sporn J, Ghaemi SN, Sambur MR, et al. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann Clin Psychiatry 2000;12(3):137-40.
19. Inoue T, Tsuchiya K, Miura J, et al. Bromocriptine treatment of tricyclic and heterocyclic antidepressant-resistant depression. Biol Psychiatry 1996;40(2):151-3.
20. Bouckoms A, Mangini L. Pergolide: an antidepressant adjuvant for mood disorders? Psychopharmacol Bull 1993;29(2):207-11.
21. Barone P, Scarzella L, Marconi R, et al. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol 2006;253(5):601-7.
22. Lemke MR, Brecht HM, Koester J, Reichmann H. Effects of the dopamine agonist pramipexole on depression, anhedonia, and motor functioning in Parkinson’s disease. J Neurol Sci 2006;248(1-2):266-70.
23. Zanettini R, Antonini A, Gatto G, et al. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med 2007;356(1):39-46.
24. D’Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol 2000;405(1-3):365-73.
25. Papakostas GI. Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol 2006;16(6):391-402.
26. Greene JG. Gene expression profiles of brain dopamine neurons and relevance to neuropsychiatric disease. J Physiol 2006;575(Pt2):411-6.
27. Engstrom G, Alling C, Blennow K, et al. Reduced cerebrospinal HVA concentrations and HVA/5-HIAA ratios in suicide attempters. Monoamine metabolites in 120 suicide attempters and 47 controls. Eur Neuropsychopharmacol 1999;9(5):399-405.
28. Hamner MB, Diamond BI. Plasma dopamine and norepinephrine correlations with psychomotor retardation, anxiety, and depression in non-psychotic depressed patients: a pilot study. Psychiatry Res 1996;64(3):209-11.
29. Mitani H, Shirayama Y, Yamada T, Kawahara R. Plasma levels of homovanillic acid, 5-hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2006;30(3):531-4.
30. Klimek V, Schenck JE, Han H, et al. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry 2002;52(7):740-8.
31. Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med 1997;27(6):1247-56.
32. Meyer JH, Kruger S, Wilson AA, et al. Lower dopamine transporter binding potential in striatum during depression. Neuroreport 2001;12(18):4121-5.
33 Volkow ND, Wang GJ, Fowler JS, et al. The slow and long-lasting blockade of dopamine transporters in human brain induced by the new antidepressant drug radafaxine predict poor reinforcing effects. Biol Psychiatry 2005;57(6):640-6.
Avoiding managed care’s pitfalls and pratfalls
Physician career satisfaction has declined since the mid-1990s, and managed care gets much of the blameInstant Poll). Doctors associate managed care with:
- loss of autonomy3
- increased paperwork
- less time with patients
- frustrating phone calls with insurance company representatives.
Patients come to us with many types of medical insurance, and we often are unsure if they have adequate coverage. The stakes are high; career satisfaction and practice viability depend on prompt reimbursement without hassle, and successful outcomes depend on patients getting the care they need.
The “pitfalls” of managed care in mental health practice can be minimized, however. This article describes how to decrease the frustration, time, and effort you spend obtaining authorizations and receiving reimbursement.
Case manager: Nurse or social worker employed by a health maintenance organization (HMO) who processes, reviews, and authorizes claims. Can play a clinical role with severely ill patients, helping to coordinate their care, enrolling them in wellness or disease management programs, or advocating for them within the medical system.
Claims review: Method of reviewing an enrollee’s health care service claims before reimbursement. Purpose is to validate the medical necessity of provided services and ensure that cost of service is not excessive.
Medical necessity: Services that: 1) are appropriate and necessary for diagnosis or treatment of a medical condition; 2) are provided for diagnosis and treatment of a medical condition; 3) meet standards of good medical practice within the medical community; and 4) are the appropriate level of intensity to meet the patient’s need.
Summary plan description (SPD): Document developed by an employer or government entity that details an insurance plan’s medical insurance benefits and coverage limitations.
Third-party payer: HMO or other managed care entity that administers a health care plan and arranges for payment of medical services. Clinicians are the “first party,” and patients are the “second party.”
Utilization review (UR): Prospective, concurrent, or retrospective review of medical care for appropriateness of services delivered to a patient. In hospitals, includes review of admissions, services provided, length of stay, and discharge practices. UR usually involves protocols and guidelines to track, review, and render opinions about patient care. Claims for care that fall outside these guidelines risk being denied.
Managed care primer
Understanding how managed care works can help you develop more positive interactions with these systems. Managed care exists to help control medical costs. Restricting services is one method of medical cost control, but employers and government payers also are interested in improving disease prevention, recognition, and management.
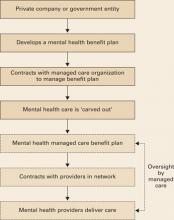
Delivering health benefits. The process begins when a business or government entity develops a summary plan description (SPD) (Box 1), defining what medical and mental health care services a policy covers (Figure).
A managed care company—typically a health maintenance organization (HMO) or similar entity—enters into a contract to implement and manage health care benefits for all persons covered by the plan. Because of their complexity, mental health services are usually “carved out” (Box 2)4 to a mental health specialty managed care company.
Utilization review. Whenever you deliver mental health care, your claims and treatment plans are scrutinized through utilization review (UR). Before authorizing payment, reviewers typically screen claims to:
- verify that the patient’s benefit plan covers the service
- establish the service’s medical necessity
- ensure that the treatment meets accepted standards of care.
UR staff in most managed care companies have administrative authority to authorize care, but care can be denied only by a medical director—typically a psychiatrist who reviews the clinical information submitted by the provider.
Figure Model of a typical managed care plan
Example 1
Claim authorization. A psychiatrist evaluates a patient and diagnoses major depression. The psychiatrist submits a treatment plan to the managed care company requesting authorization for 6 medication management and 10 psychotherapy sessions for the next 12 months. A UR representative reviews the plan, verifies the patient has appropriate coverage, and authorizes the sessions.
Example 2
Claim denial. A psychiatrist evaluates a patient and diagnoses major depression. The psychiatrist submits a treatment plan to the managed care company, requesting authorization for 10 EEG biofeedback sessions to treat the depression. A UR representative examines the plan and asks the medical director to review it. The medical director denies authorization because EEG biofeedback does not meet accepted standards for depression treatment.
Negotiating exceptions. Managed care companies must follow the letter of the benefits they administer. Some employers, however, allow for a “benefit exception” when practitioners identify patient services not covered under the benefit that, if implemented, might improve care and reduce costs.
If you encounter a situation where you believe plan limitations might adversely affect patient outcome and expense, point it out to the managed care company and ask if the patient’s plan allows benefit exceptions. Some plans do not allow for partial hospitalization, for example. If a patient with frequent inpatient admissions could be cared for in the less-intensive environment of a partial hospital program, the managed care case manager might approach the employer and suggest a benefit exception for this patient.
Common reasons claims are denied
Coverage limitations. Insurance plans often exclude or restrict particular services and limit certain types of care such as chemical dependency treatment or psychological testing. Managed care companies do not write the plans they manage and cannot authorize care that the benefit does not cover.
In “carve-out” plans, HMOs and insurance companies that do not have in-house expertise in mental health care or chemical dependency treatment “carve out” these services so that coverage is managed separately from the medical benefit. Carved-out services typically are delivered exclusively by designated providers or groups that contract with the HMO to provide mental health care to members.
Carve-outs have led to concerns about parity, particularly when mental health benefits are reduced or restricted compared with other medical benefits in the plan.
In a “carve-in” plan, mental health care remains within the overall health care coverage, which can facilitate collaboration between mental health and medical care providers. Parity for mental health care may be less of an issue than with “carve-out” plans, but “carve-in” plans might not be equipped to adequately meet the needs of patients with serious, persistent mental illness.4
Recommendation. Make sure you and your patients understand their benefit limitations. All employees with health insurance receive a summary plan description (SPD), which outlines benefit coverage and limitations. Encourage patients to read this document and contact their managed care companies with questions about coverage. Also ensure that your office managers:
- become familiar with SPDs for commonly encountered plans in your practice
- proactively verify patient benefit coverage.
Medical necessity. Practitioners in inpatient or residential settings experience the highest rates of denials of requested care on grounds that care is not medically necessary. Managed care company guidelines specify criteria that must be met—such as active suicidality, disorganized thinking, or significant medical co-morbidity—before care can be authorized.
Recommendation. Document specifically and concisely in daily notes why a patient requires the care you are providing. Managed care company guidelines are only guidelines; when the patient does not meet criteria, the medical director is more likely to authorize care if you clearly state the rationale for that level of care.
Patient is not progressing. Medical and mental health care is expected to provide a therapeutic outcome. When little progress is being made toward treatment goals, managed care companies have an obligation to the payers they represent to ensure that patients receive effective care.
Recommendation. Set realistic goals in the treatment plan. If a patient has a chronic condition and is not expected to improve but requires ongoing care to maintain function, state this in the treatment plan. If a patient is not making progress toward treatment goals, explain the reason and how you are addressing it.
No prior authorization. Some services—such as psychometric testing, inpatient care, or residential treatment—require prior authorization for reimbursement. Particularly for expensive treatments, payers require prior authorization so that utilization review occurs while care is being delivered, as opposed to afterward when costs are more difficult to contain.
Recommendation. Be familiar with prior authorization policies of common plans in your practice. If you discover you have provided care that required prior authorization, make a good-faith effort to submit clinical notes and explain why you did not request prior authorization. Many managed care companies will authorize payment after the fact if care was necessary and reasonable.
Duplication of services. Psychiatric patients often move from practice to practice, sometimes several times a year, without telling clinicians. Thus, laboratory testing, psychometric testing, and other diagnostic services may be repeated. Managed care companies take the stance that duplication of services is expensive and unnecessary, whereas you may argue that you must have adequate information to care for patients appropriately.
Recommendation. When assessing new patients, make it a priority for your office staff to ask patients about care they have received in the last year and obtain records from other providers. If you duplicate services, explain in your treatment plan why it was necessary for your patient’s care.
Interacting with managed care
Unfortunately, clinicians and managed care company representatives often view their relationship as adversarial (Box 3). Yes, some managed care representatives unfairly limit mental health care, and some clinicians maximize income potential by overbilling or providing unnecessary care. In our experience, however, most people on both sides of the equation are doing their jobs fairly and reasonably.
Medical care is expensive. Managed care’s role is not to deny care to patients who need it but to help the fiduciaries they represent—private companies or government entities—ensure that appropriate and economically responsible care is delivered. When you interact with managed care companies, keep 3 principles in mind:
Use common courtesy. Standing up for your patients and practice is reasonable and appropriate. At the same time, treating managed care representatives respectfully and professionally will go a long way as you advocate for your patients.
Document clearly and concisely. Documenting your impressions, goals, and care plans succinctly and well in your notes will save you and the managed care company time, frustration, and dollars. Managed care representatives do not want to review illegible, poorly organized, or overly inclusive documentation.
Some practitioners choose to practice outside of managed care networks—such as in fee for service—thus freeing themselves from guidelines and care limitations associated with managed care. Most health plans permit patients to seek treatment from out-of-network practitioners, although usually with higher out-of-pocket expenses.
Out-of-network practitioners who submit claims to managed care companies must follow many of the in-network rules, such as establishing medical necessity, submitting treatment plans, and undergoing utilization review.
Advantages of being an out-of-network provider—especially in a fee-for-service model—include practice independence, freedom from managed care paperwork, and the possibility of increased revenue by not having to accept reimbursement rates set by managed care contracts.
Disadvantages include potentially seeing fewer patients because of higher out-of-network costs, excluding lower-income patients, and receiving fewer referrals from managed care companies.
Out-of-network practitioners also have not gone through managed care companies’ credentialing, a process that assures patients that network practitioners are licensed and have not had serious quality-of-care or malpractice events that might adversely affect patient care.
Denials take managed care representatives more time than approvals. These busy people often look for reasons to approve reasonable care rather than to deny unreasonable care. If your documentation is clear and practice patterns are sound, your inpatient and outpatient treatment plans are much more likely to avoid the harsh scrutiny of the authorization denial process.
Managed care companies rely on the information you provide. The most common reason for denials being reversed on appeal is that additional information unavailable to the company at the initial review has been provided in the appeals process.
Learn from denials. Whenever you are issued a care denial, find out why. If a pattern emerges, you might need to change your practice or accept that certain types of care will not be covered routinely. For example, you might obtain psychological testing for every patient, whereas many managed care companies authorize testing only in specific circumstances. Thus, you could:
- modify use of testing
- or accept that this practice will not always be reimbursed.
Wellness and prevention programs
Managed care plays an important role in developing and implementing wellness, disease prevention, and disease management programs for employers and government entities. These patient programs reduce health care costs, decrease time away from work (absenteeism), and improve productivity (“presentee-ism”).5,6 Benefit plans often provide free programs and offer financial incentives for patients’ participation.
Health risk assessments, life-style coaching, and condition-specific management programs—such as for diabetes care, smoking cessation, or depression treatment—are becoming common in employee benefit packages. These programs try to improve patients’ health through care coordination with the patients’ health care providers.
As programs are developed, you can expect to regularly receive clinical information about your patients from managed care case managers who are trying to integrate their programs with your patients’ care. Case managers’ goal is to improve clinical outcomes through initiatives such as treatment adherence, patient education, and early detection of treatment resistance or symptom relapse. To take advantage of these resources, be aware of available programs and consider referring patients into them.
Related resources
- Fauman MA. Negotiating managed care: a manual for clinicians. Washington, D.C.: American Psychiatric Publishing; 2002.
- Tuckfelt S, Fink J, Prince Warren M. The psychotherapist’s guide to managed care in the 21st century. Northvale, NJ: Jason Aronson; 1997.
Disclosure
Dr. Sutor is a practicing psychiatrist at the Mayo Clinic, Rochester, MN, and has been assistant medical director for behavioral health at MMSI (the Mayo Clinic’s managed care entity) since 2001.
1. Landon BE, Reschovsky J, Blumenthal D. Changes in career satisfaction among primary care and specialist physicians, 1997-2001. JAMA 2003;289:442-9.
2. Iglehart JK. Health policy report: managed care and mental health. N Engl J Med 1996;334(2):131-5.
3. Wells LA. Psychiatry, managed care, and crooked thinking. Mayo Clin Proc 1998;73(5):483-7.
4. Bartels SJ, Levine KJ, Shea D. Community-based long-term care for older patients with severe and persistent mental illness in the era of managed care. Psychiatr Serv 1999;50:1189-97.
5. Rost K, Smith JL, Dickinson M. The effect of improving primary care depression management on employee absenteeism and productivity. A randomized trial. Medical Care 2004;42(12):1202-10.
6. Keitner GI, Ryan CE, Solomon DA. Realistic expectations and a disease management model for depressed patients with persistent symptoms. J Clin Psychiatry 2006;67(9):1412-21.
Physician career satisfaction has declined since the mid-1990s, and managed care gets much of the blameInstant Poll). Doctors associate managed care with:
- loss of autonomy3
- increased paperwork
- less time with patients
- frustrating phone calls with insurance company representatives.
Patients come to us with many types of medical insurance, and we often are unsure if they have adequate coverage. The stakes are high; career satisfaction and practice viability depend on prompt reimbursement without hassle, and successful outcomes depend on patients getting the care they need.
The “pitfalls” of managed care in mental health practice can be minimized, however. This article describes how to decrease the frustration, time, and effort you spend obtaining authorizations and receiving reimbursement.
Case manager: Nurse or social worker employed by a health maintenance organization (HMO) who processes, reviews, and authorizes claims. Can play a clinical role with severely ill patients, helping to coordinate their care, enrolling them in wellness or disease management programs, or advocating for them within the medical system.
Claims review: Method of reviewing an enrollee’s health care service claims before reimbursement. Purpose is to validate the medical necessity of provided services and ensure that cost of service is not excessive.
Medical necessity: Services that: 1) are appropriate and necessary for diagnosis or treatment of a medical condition; 2) are provided for diagnosis and treatment of a medical condition; 3) meet standards of good medical practice within the medical community; and 4) are the appropriate level of intensity to meet the patient’s need.
Summary plan description (SPD): Document developed by an employer or government entity that details an insurance plan’s medical insurance benefits and coverage limitations.
Third-party payer: HMO or other managed care entity that administers a health care plan and arranges for payment of medical services. Clinicians are the “first party,” and patients are the “second party.”
Utilization review (UR): Prospective, concurrent, or retrospective review of medical care for appropriateness of services delivered to a patient. In hospitals, includes review of admissions, services provided, length of stay, and discharge practices. UR usually involves protocols and guidelines to track, review, and render opinions about patient care. Claims for care that fall outside these guidelines risk being denied.
Managed care primer
Understanding how managed care works can help you develop more positive interactions with these systems. Managed care exists to help control medical costs. Restricting services is one method of medical cost control, but employers and government payers also are interested in improving disease prevention, recognition, and management.
Delivering health benefits. The process begins when a business or government entity develops a summary plan description (SPD) (Box 1), defining what medical and mental health care services a policy covers (Figure).
A managed care company—typically a health maintenance organization (HMO) or similar entity—enters into a contract to implement and manage health care benefits for all persons covered by the plan. Because of their complexity, mental health services are usually “carved out” (Box 2)4 to a mental health specialty managed care company.
Utilization review. Whenever you deliver mental health care, your claims and treatment plans are scrutinized through utilization review (UR). Before authorizing payment, reviewers typically screen claims to:
- verify that the patient’s benefit plan covers the service
- establish the service’s medical necessity
- ensure that the treatment meets accepted standards of care.
UR staff in most managed care companies have administrative authority to authorize care, but care can be denied only by a medical director—typically a psychiatrist who reviews the clinical information submitted by the provider.
Figure Model of a typical managed care plan
Example 1
Claim authorization. A psychiatrist evaluates a patient and diagnoses major depression. The psychiatrist submits a treatment plan to the managed care company requesting authorization for 6 medication management and 10 psychotherapy sessions for the next 12 months. A UR representative reviews the plan, verifies the patient has appropriate coverage, and authorizes the sessions.
Example 2
Claim denial. A psychiatrist evaluates a patient and diagnoses major depression. The psychiatrist submits a treatment plan to the managed care company, requesting authorization for 10 EEG biofeedback sessions to treat the depression. A UR representative examines the plan and asks the medical director to review it. The medical director denies authorization because EEG biofeedback does not meet accepted standards for depression treatment.
Negotiating exceptions. Managed care companies must follow the letter of the benefits they administer. Some employers, however, allow for a “benefit exception” when practitioners identify patient services not covered under the benefit that, if implemented, might improve care and reduce costs.
If you encounter a situation where you believe plan limitations might adversely affect patient outcome and expense, point it out to the managed care company and ask if the patient’s plan allows benefit exceptions. Some plans do not allow for partial hospitalization, for example. If a patient with frequent inpatient admissions could be cared for in the less-intensive environment of a partial hospital program, the managed care case manager might approach the employer and suggest a benefit exception for this patient.
Common reasons claims are denied
Coverage limitations. Insurance plans often exclude or restrict particular services and limit certain types of care such as chemical dependency treatment or psychological testing. Managed care companies do not write the plans they manage and cannot authorize care that the benefit does not cover.
In “carve-out” plans, HMOs and insurance companies that do not have in-house expertise in mental health care or chemical dependency treatment “carve out” these services so that coverage is managed separately from the medical benefit. Carved-out services typically are delivered exclusively by designated providers or groups that contract with the HMO to provide mental health care to members.
Carve-outs have led to concerns about parity, particularly when mental health benefits are reduced or restricted compared with other medical benefits in the plan.
In a “carve-in” plan, mental health care remains within the overall health care coverage, which can facilitate collaboration between mental health and medical care providers. Parity for mental health care may be less of an issue than with “carve-out” plans, but “carve-in” plans might not be equipped to adequately meet the needs of patients with serious, persistent mental illness.4
Recommendation. Make sure you and your patients understand their benefit limitations. All employees with health insurance receive a summary plan description (SPD), which outlines benefit coverage and limitations. Encourage patients to read this document and contact their managed care companies with questions about coverage. Also ensure that your office managers:
- become familiar with SPDs for commonly encountered plans in your practice
- proactively verify patient benefit coverage.
Medical necessity. Practitioners in inpatient or residential settings experience the highest rates of denials of requested care on grounds that care is not medically necessary. Managed care company guidelines specify criteria that must be met—such as active suicidality, disorganized thinking, or significant medical co-morbidity—before care can be authorized.
Recommendation. Document specifically and concisely in daily notes why a patient requires the care you are providing. Managed care company guidelines are only guidelines; when the patient does not meet criteria, the medical director is more likely to authorize care if you clearly state the rationale for that level of care.
Patient is not progressing. Medical and mental health care is expected to provide a therapeutic outcome. When little progress is being made toward treatment goals, managed care companies have an obligation to the payers they represent to ensure that patients receive effective care.
Recommendation. Set realistic goals in the treatment plan. If a patient has a chronic condition and is not expected to improve but requires ongoing care to maintain function, state this in the treatment plan. If a patient is not making progress toward treatment goals, explain the reason and how you are addressing it.
No prior authorization. Some services—such as psychometric testing, inpatient care, or residential treatment—require prior authorization for reimbursement. Particularly for expensive treatments, payers require prior authorization so that utilization review occurs while care is being delivered, as opposed to afterward when costs are more difficult to contain.
Recommendation. Be familiar with prior authorization policies of common plans in your practice. If you discover you have provided care that required prior authorization, make a good-faith effort to submit clinical notes and explain why you did not request prior authorization. Many managed care companies will authorize payment after the fact if care was necessary and reasonable.
Duplication of services. Psychiatric patients often move from practice to practice, sometimes several times a year, without telling clinicians. Thus, laboratory testing, psychometric testing, and other diagnostic services may be repeated. Managed care companies take the stance that duplication of services is expensive and unnecessary, whereas you may argue that you must have adequate information to care for patients appropriately.
Recommendation. When assessing new patients, make it a priority for your office staff to ask patients about care they have received in the last year and obtain records from other providers. If you duplicate services, explain in your treatment plan why it was necessary for your patient’s care.
Interacting with managed care
Unfortunately, clinicians and managed care company representatives often view their relationship as adversarial (Box 3). Yes, some managed care representatives unfairly limit mental health care, and some clinicians maximize income potential by overbilling or providing unnecessary care. In our experience, however, most people on both sides of the equation are doing their jobs fairly and reasonably.
Medical care is expensive. Managed care’s role is not to deny care to patients who need it but to help the fiduciaries they represent—private companies or government entities—ensure that appropriate and economically responsible care is delivered. When you interact with managed care companies, keep 3 principles in mind:
Use common courtesy. Standing up for your patients and practice is reasonable and appropriate. At the same time, treating managed care representatives respectfully and professionally will go a long way as you advocate for your patients.
Document clearly and concisely. Documenting your impressions, goals, and care plans succinctly and well in your notes will save you and the managed care company time, frustration, and dollars. Managed care representatives do not want to review illegible, poorly organized, or overly inclusive documentation.
Some practitioners choose to practice outside of managed care networks—such as in fee for service—thus freeing themselves from guidelines and care limitations associated with managed care. Most health plans permit patients to seek treatment from out-of-network practitioners, although usually with higher out-of-pocket expenses.
Out-of-network practitioners who submit claims to managed care companies must follow many of the in-network rules, such as establishing medical necessity, submitting treatment plans, and undergoing utilization review.
Advantages of being an out-of-network provider—especially in a fee-for-service model—include practice independence, freedom from managed care paperwork, and the possibility of increased revenue by not having to accept reimbursement rates set by managed care contracts.
Disadvantages include potentially seeing fewer patients because of higher out-of-network costs, excluding lower-income patients, and receiving fewer referrals from managed care companies.
Out-of-network practitioners also have not gone through managed care companies’ credentialing, a process that assures patients that network practitioners are licensed and have not had serious quality-of-care or malpractice events that might adversely affect patient care.
Denials take managed care representatives more time than approvals. These busy people often look for reasons to approve reasonable care rather than to deny unreasonable care. If your documentation is clear and practice patterns are sound, your inpatient and outpatient treatment plans are much more likely to avoid the harsh scrutiny of the authorization denial process.
Managed care companies rely on the information you provide. The most common reason for denials being reversed on appeal is that additional information unavailable to the company at the initial review has been provided in the appeals process.
Learn from denials. Whenever you are issued a care denial, find out why. If a pattern emerges, you might need to change your practice or accept that certain types of care will not be covered routinely. For example, you might obtain psychological testing for every patient, whereas many managed care companies authorize testing only in specific circumstances. Thus, you could:
- modify use of testing
- or accept that this practice will not always be reimbursed.
Wellness and prevention programs
Managed care plays an important role in developing and implementing wellness, disease prevention, and disease management programs for employers and government entities. These patient programs reduce health care costs, decrease time away from work (absenteeism), and improve productivity (“presentee-ism”).5,6 Benefit plans often provide free programs and offer financial incentives for patients’ participation.
Health risk assessments, life-style coaching, and condition-specific management programs—such as for diabetes care, smoking cessation, or depression treatment—are becoming common in employee benefit packages. These programs try to improve patients’ health through care coordination with the patients’ health care providers.
As programs are developed, you can expect to regularly receive clinical information about your patients from managed care case managers who are trying to integrate their programs with your patients’ care. Case managers’ goal is to improve clinical outcomes through initiatives such as treatment adherence, patient education, and early detection of treatment resistance or symptom relapse. To take advantage of these resources, be aware of available programs and consider referring patients into them.
Related resources
- Fauman MA. Negotiating managed care: a manual for clinicians. Washington, D.C.: American Psychiatric Publishing; 2002.
- Tuckfelt S, Fink J, Prince Warren M. The psychotherapist’s guide to managed care in the 21st century. Northvale, NJ: Jason Aronson; 1997.
Disclosure
Dr. Sutor is a practicing psychiatrist at the Mayo Clinic, Rochester, MN, and has been assistant medical director for behavioral health at MMSI (the Mayo Clinic’s managed care entity) since 2001.
Physician career satisfaction has declined since the mid-1990s, and managed care gets much of the blameInstant Poll). Doctors associate managed care with:
- loss of autonomy3
- increased paperwork
- less time with patients
- frustrating phone calls with insurance company representatives.
Patients come to us with many types of medical insurance, and we often are unsure if they have adequate coverage. The stakes are high; career satisfaction and practice viability depend on prompt reimbursement without hassle, and successful outcomes depend on patients getting the care they need.
The “pitfalls” of managed care in mental health practice can be minimized, however. This article describes how to decrease the frustration, time, and effort you spend obtaining authorizations and receiving reimbursement.
Case manager: Nurse or social worker employed by a health maintenance organization (HMO) who processes, reviews, and authorizes claims. Can play a clinical role with severely ill patients, helping to coordinate their care, enrolling them in wellness or disease management programs, or advocating for them within the medical system.
Claims review: Method of reviewing an enrollee’s health care service claims before reimbursement. Purpose is to validate the medical necessity of provided services and ensure that cost of service is not excessive.
Medical necessity: Services that: 1) are appropriate and necessary for diagnosis or treatment of a medical condition; 2) are provided for diagnosis and treatment of a medical condition; 3) meet standards of good medical practice within the medical community; and 4) are the appropriate level of intensity to meet the patient’s need.
Summary plan description (SPD): Document developed by an employer or government entity that details an insurance plan’s medical insurance benefits and coverage limitations.
Third-party payer: HMO or other managed care entity that administers a health care plan and arranges for payment of medical services. Clinicians are the “first party,” and patients are the “second party.”
Utilization review (UR): Prospective, concurrent, or retrospective review of medical care for appropriateness of services delivered to a patient. In hospitals, includes review of admissions, services provided, length of stay, and discharge practices. UR usually involves protocols and guidelines to track, review, and render opinions about patient care. Claims for care that fall outside these guidelines risk being denied.
Managed care primer
Understanding how managed care works can help you develop more positive interactions with these systems. Managed care exists to help control medical costs. Restricting services is one method of medical cost control, but employers and government payers also are interested in improving disease prevention, recognition, and management.
Delivering health benefits. The process begins when a business or government entity develops a summary plan description (SPD) (Box 1), defining what medical and mental health care services a policy covers (Figure).
A managed care company—typically a health maintenance organization (HMO) or similar entity—enters into a contract to implement and manage health care benefits for all persons covered by the plan. Because of their complexity, mental health services are usually “carved out” (Box 2)4 to a mental health specialty managed care company.
Utilization review. Whenever you deliver mental health care, your claims and treatment plans are scrutinized through utilization review (UR). Before authorizing payment, reviewers typically screen claims to:
- verify that the patient’s benefit plan covers the service
- establish the service’s medical necessity
- ensure that the treatment meets accepted standards of care.
UR staff in most managed care companies have administrative authority to authorize care, but care can be denied only by a medical director—typically a psychiatrist who reviews the clinical information submitted by the provider.
Figure Model of a typical managed care plan
Example 1
Claim authorization. A psychiatrist evaluates a patient and diagnoses major depression. The psychiatrist submits a treatment plan to the managed care company requesting authorization for 6 medication management and 10 psychotherapy sessions for the next 12 months. A UR representative reviews the plan, verifies the patient has appropriate coverage, and authorizes the sessions.
Example 2
Claim denial. A psychiatrist evaluates a patient and diagnoses major depression. The psychiatrist submits a treatment plan to the managed care company, requesting authorization for 10 EEG biofeedback sessions to treat the depression. A UR representative examines the plan and asks the medical director to review it. The medical director denies authorization because EEG biofeedback does not meet accepted standards for depression treatment.
Negotiating exceptions. Managed care companies must follow the letter of the benefits they administer. Some employers, however, allow for a “benefit exception” when practitioners identify patient services not covered under the benefit that, if implemented, might improve care and reduce costs.
If you encounter a situation where you believe plan limitations might adversely affect patient outcome and expense, point it out to the managed care company and ask if the patient’s plan allows benefit exceptions. Some plans do not allow for partial hospitalization, for example. If a patient with frequent inpatient admissions could be cared for in the less-intensive environment of a partial hospital program, the managed care case manager might approach the employer and suggest a benefit exception for this patient.
Common reasons claims are denied
Coverage limitations. Insurance plans often exclude or restrict particular services and limit certain types of care such as chemical dependency treatment or psychological testing. Managed care companies do not write the plans they manage and cannot authorize care that the benefit does not cover.
In “carve-out” plans, HMOs and insurance companies that do not have in-house expertise in mental health care or chemical dependency treatment “carve out” these services so that coverage is managed separately from the medical benefit. Carved-out services typically are delivered exclusively by designated providers or groups that contract with the HMO to provide mental health care to members.
Carve-outs have led to concerns about parity, particularly when mental health benefits are reduced or restricted compared with other medical benefits in the plan.
In a “carve-in” plan, mental health care remains within the overall health care coverage, which can facilitate collaboration between mental health and medical care providers. Parity for mental health care may be less of an issue than with “carve-out” plans, but “carve-in” plans might not be equipped to adequately meet the needs of patients with serious, persistent mental illness.4
Recommendation. Make sure you and your patients understand their benefit limitations. All employees with health insurance receive a summary plan description (SPD), which outlines benefit coverage and limitations. Encourage patients to read this document and contact their managed care companies with questions about coverage. Also ensure that your office managers:
- become familiar with SPDs for commonly encountered plans in your practice
- proactively verify patient benefit coverage.
Medical necessity. Practitioners in inpatient or residential settings experience the highest rates of denials of requested care on grounds that care is not medically necessary. Managed care company guidelines specify criteria that must be met—such as active suicidality, disorganized thinking, or significant medical co-morbidity—before care can be authorized.
Recommendation. Document specifically and concisely in daily notes why a patient requires the care you are providing. Managed care company guidelines are only guidelines; when the patient does not meet criteria, the medical director is more likely to authorize care if you clearly state the rationale for that level of care.
Patient is not progressing. Medical and mental health care is expected to provide a therapeutic outcome. When little progress is being made toward treatment goals, managed care companies have an obligation to the payers they represent to ensure that patients receive effective care.
Recommendation. Set realistic goals in the treatment plan. If a patient has a chronic condition and is not expected to improve but requires ongoing care to maintain function, state this in the treatment plan. If a patient is not making progress toward treatment goals, explain the reason and how you are addressing it.
No prior authorization. Some services—such as psychometric testing, inpatient care, or residential treatment—require prior authorization for reimbursement. Particularly for expensive treatments, payers require prior authorization so that utilization review occurs while care is being delivered, as opposed to afterward when costs are more difficult to contain.
Recommendation. Be familiar with prior authorization policies of common plans in your practice. If you discover you have provided care that required prior authorization, make a good-faith effort to submit clinical notes and explain why you did not request prior authorization. Many managed care companies will authorize payment after the fact if care was necessary and reasonable.
Duplication of services. Psychiatric patients often move from practice to practice, sometimes several times a year, without telling clinicians. Thus, laboratory testing, psychometric testing, and other diagnostic services may be repeated. Managed care companies take the stance that duplication of services is expensive and unnecessary, whereas you may argue that you must have adequate information to care for patients appropriately.
Recommendation. When assessing new patients, make it a priority for your office staff to ask patients about care they have received in the last year and obtain records from other providers. If you duplicate services, explain in your treatment plan why it was necessary for your patient’s care.
Interacting with managed care
Unfortunately, clinicians and managed care company representatives often view their relationship as adversarial (Box 3). Yes, some managed care representatives unfairly limit mental health care, and some clinicians maximize income potential by overbilling or providing unnecessary care. In our experience, however, most people on both sides of the equation are doing their jobs fairly and reasonably.
Medical care is expensive. Managed care’s role is not to deny care to patients who need it but to help the fiduciaries they represent—private companies or government entities—ensure that appropriate and economically responsible care is delivered. When you interact with managed care companies, keep 3 principles in mind:
Use common courtesy. Standing up for your patients and practice is reasonable and appropriate. At the same time, treating managed care representatives respectfully and professionally will go a long way as you advocate for your patients.
Document clearly and concisely. Documenting your impressions, goals, and care plans succinctly and well in your notes will save you and the managed care company time, frustration, and dollars. Managed care representatives do not want to review illegible, poorly organized, or overly inclusive documentation.
Some practitioners choose to practice outside of managed care networks—such as in fee for service—thus freeing themselves from guidelines and care limitations associated with managed care. Most health plans permit patients to seek treatment from out-of-network practitioners, although usually with higher out-of-pocket expenses.
Out-of-network practitioners who submit claims to managed care companies must follow many of the in-network rules, such as establishing medical necessity, submitting treatment plans, and undergoing utilization review.
Advantages of being an out-of-network provider—especially in a fee-for-service model—include practice independence, freedom from managed care paperwork, and the possibility of increased revenue by not having to accept reimbursement rates set by managed care contracts.
Disadvantages include potentially seeing fewer patients because of higher out-of-network costs, excluding lower-income patients, and receiving fewer referrals from managed care companies.
Out-of-network practitioners also have not gone through managed care companies’ credentialing, a process that assures patients that network practitioners are licensed and have not had serious quality-of-care or malpractice events that might adversely affect patient care.
Denials take managed care representatives more time than approvals. These busy people often look for reasons to approve reasonable care rather than to deny unreasonable care. If your documentation is clear and practice patterns are sound, your inpatient and outpatient treatment plans are much more likely to avoid the harsh scrutiny of the authorization denial process.
Managed care companies rely on the information you provide. The most common reason for denials being reversed on appeal is that additional information unavailable to the company at the initial review has been provided in the appeals process.
Learn from denials. Whenever you are issued a care denial, find out why. If a pattern emerges, you might need to change your practice or accept that certain types of care will not be covered routinely. For example, you might obtain psychological testing for every patient, whereas many managed care companies authorize testing only in specific circumstances. Thus, you could:
- modify use of testing
- or accept that this practice will not always be reimbursed.
Wellness and prevention programs
Managed care plays an important role in developing and implementing wellness, disease prevention, and disease management programs for employers and government entities. These patient programs reduce health care costs, decrease time away from work (absenteeism), and improve productivity (“presentee-ism”).5,6 Benefit plans often provide free programs and offer financial incentives for patients’ participation.
Health risk assessments, life-style coaching, and condition-specific management programs—such as for diabetes care, smoking cessation, or depression treatment—are becoming common in employee benefit packages. These programs try to improve patients’ health through care coordination with the patients’ health care providers.
As programs are developed, you can expect to regularly receive clinical information about your patients from managed care case managers who are trying to integrate their programs with your patients’ care. Case managers’ goal is to improve clinical outcomes through initiatives such as treatment adherence, patient education, and early detection of treatment resistance or symptom relapse. To take advantage of these resources, be aware of available programs and consider referring patients into them.
Related resources
- Fauman MA. Negotiating managed care: a manual for clinicians. Washington, D.C.: American Psychiatric Publishing; 2002.
- Tuckfelt S, Fink J, Prince Warren M. The psychotherapist’s guide to managed care in the 21st century. Northvale, NJ: Jason Aronson; 1997.
Disclosure
Dr. Sutor is a practicing psychiatrist at the Mayo Clinic, Rochester, MN, and has been assistant medical director for behavioral health at MMSI (the Mayo Clinic’s managed care entity) since 2001.
1. Landon BE, Reschovsky J, Blumenthal D. Changes in career satisfaction among primary care and specialist physicians, 1997-2001. JAMA 2003;289:442-9.
2. Iglehart JK. Health policy report: managed care and mental health. N Engl J Med 1996;334(2):131-5.
3. Wells LA. Psychiatry, managed care, and crooked thinking. Mayo Clin Proc 1998;73(5):483-7.
4. Bartels SJ, Levine KJ, Shea D. Community-based long-term care for older patients with severe and persistent mental illness in the era of managed care. Psychiatr Serv 1999;50:1189-97.
5. Rost K, Smith JL, Dickinson M. The effect of improving primary care depression management on employee absenteeism and productivity. A randomized trial. Medical Care 2004;42(12):1202-10.
6. Keitner GI, Ryan CE, Solomon DA. Realistic expectations and a disease management model for depressed patients with persistent symptoms. J Clin Psychiatry 2006;67(9):1412-21.
1. Landon BE, Reschovsky J, Blumenthal D. Changes in career satisfaction among primary care and specialist physicians, 1997-2001. JAMA 2003;289:442-9.
2. Iglehart JK. Health policy report: managed care and mental health. N Engl J Med 1996;334(2):131-5.
3. Wells LA. Psychiatry, managed care, and crooked thinking. Mayo Clin Proc 1998;73(5):483-7.
4. Bartels SJ, Levine KJ, Shea D. Community-based long-term care for older patients with severe and persistent mental illness in the era of managed care. Psychiatr Serv 1999;50:1189-97.
5. Rost K, Smith JL, Dickinson M. The effect of improving primary care depression management on employee absenteeism and productivity. A randomized trial. Medical Care 2004;42(12):1202-10.
6. Keitner GI, Ryan CE, Solomon DA. Realistic expectations and a disease management model for depressed patients with persistent symptoms. J Clin Psychiatry 2006;67(9):1412-21.
Psychiatric assessment: A word to the WISE
Most clinicians can easily identify the biological and psychological aspects of mental illness, but the social components often are overlooked.1 These include a negative life event, familial or interpersonal stressor, environmental difficulty or deficiency, or inadequate social support.2
We have found that these significant psychosocial factors listed in DSM-IV-TR2 can be easily assessed using the mnemonic, “Family and friends with a WISE HALO.”
Family and friends. Stressful events include family disruption by divorce or separation; illness or death of family members; neglect; emotional, physical or sexual abuse; remarriage of a parent; or birth or adoption of a new sibling.
Work. Stressors associated with work include actual or perceived job loss, difficult working conditions, irregular schedules, difficulty getting along with superiors or coworkers, and job dissatisfaction.
Income. Poverty and inadequate finances can influence the patient’s mental health.
Social environment. Problems with living alone, poor support, difficulty with acculturation, and discrimination are some possible difficulties.
Education. Learning problems, conflicts with teachers and classmates, bullying, and illiteracy could harm your patient’s mental health.
Housing. Stressors include homelessness, unsafe neighborhoods, and problems with a landlord.
Access to health care services. Inadequate access to health care, lack of medical insurance, and absence of transportation can influence your patient’s care.
Legal. Arrest, incarceration, ongoing lawsuits, and being the perpetrator or victim of a crime are included here.
Others. This catchall category includes exposure to disasters or wars and unavailability of social services.
After identifying the psychosocial issues affecting your patient, assimilate this information into a biopsychosocial formulation and treatment plan. Interventions could include referrals for individual and family therapy, bereavement and support groups, recreational therapy, or to subsidized housing programs or job training.
1. Campbell WH, Rohrbaugh RM. The biopsychosocial formulation manual. A guide for mental health professionals. New York: Routledge; 2006:63-70.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
Dr. Madaan is a fellow in child and adolescent psychiatry at Creighton University, Omaha, NE.
Dr. Kohli is a practicing family physician in Amherst, VA.
Dr. Khurana is a clinical observer and visiting researcher at Children’s Hospital, Omaha, NE.
Most clinicians can easily identify the biological and psychological aspects of mental illness, but the social components often are overlooked.1 These include a negative life event, familial or interpersonal stressor, environmental difficulty or deficiency, or inadequate social support.2
We have found that these significant psychosocial factors listed in DSM-IV-TR2 can be easily assessed using the mnemonic, “Family and friends with a WISE HALO.”
Family and friends. Stressful events include family disruption by divorce or separation; illness or death of family members; neglect; emotional, physical or sexual abuse; remarriage of a parent; or birth or adoption of a new sibling.
Work. Stressors associated with work include actual or perceived job loss, difficult working conditions, irregular schedules, difficulty getting along with superiors or coworkers, and job dissatisfaction.
Income. Poverty and inadequate finances can influence the patient’s mental health.
Social environment. Problems with living alone, poor support, difficulty with acculturation, and discrimination are some possible difficulties.
Education. Learning problems, conflicts with teachers and classmates, bullying, and illiteracy could harm your patient’s mental health.
Housing. Stressors include homelessness, unsafe neighborhoods, and problems with a landlord.
Access to health care services. Inadequate access to health care, lack of medical insurance, and absence of transportation can influence your patient’s care.
Legal. Arrest, incarceration, ongoing lawsuits, and being the perpetrator or victim of a crime are included here.
Others. This catchall category includes exposure to disasters or wars and unavailability of social services.
After identifying the psychosocial issues affecting your patient, assimilate this information into a biopsychosocial formulation and treatment plan. Interventions could include referrals for individual and family therapy, bereavement and support groups, recreational therapy, or to subsidized housing programs or job training.
Most clinicians can easily identify the biological and psychological aspects of mental illness, but the social components often are overlooked.1 These include a negative life event, familial or interpersonal stressor, environmental difficulty or deficiency, or inadequate social support.2
We have found that these significant psychosocial factors listed in DSM-IV-TR2 can be easily assessed using the mnemonic, “Family and friends with a WISE HALO.”
Family and friends. Stressful events include family disruption by divorce or separation; illness or death of family members; neglect; emotional, physical or sexual abuse; remarriage of a parent; or birth or adoption of a new sibling.
Work. Stressors associated with work include actual or perceived job loss, difficult working conditions, irregular schedules, difficulty getting along with superiors or coworkers, and job dissatisfaction.
Income. Poverty and inadequate finances can influence the patient’s mental health.
Social environment. Problems with living alone, poor support, difficulty with acculturation, and discrimination are some possible difficulties.
Education. Learning problems, conflicts with teachers and classmates, bullying, and illiteracy could harm your patient’s mental health.
Housing. Stressors include homelessness, unsafe neighborhoods, and problems with a landlord.
Access to health care services. Inadequate access to health care, lack of medical insurance, and absence of transportation can influence your patient’s care.
Legal. Arrest, incarceration, ongoing lawsuits, and being the perpetrator or victim of a crime are included here.
Others. This catchall category includes exposure to disasters or wars and unavailability of social services.
After identifying the psychosocial issues affecting your patient, assimilate this information into a biopsychosocial formulation and treatment plan. Interventions could include referrals for individual and family therapy, bereavement and support groups, recreational therapy, or to subsidized housing programs or job training.
1. Campbell WH, Rohrbaugh RM. The biopsychosocial formulation manual. A guide for mental health professionals. New York: Routledge; 2006:63-70.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
Dr. Madaan is a fellow in child and adolescent psychiatry at Creighton University, Omaha, NE.
Dr. Kohli is a practicing family physician in Amherst, VA.
Dr. Khurana is a clinical observer and visiting researcher at Children’s Hospital, Omaha, NE.
1. Campbell WH, Rohrbaugh RM. The biopsychosocial formulation manual. A guide for mental health professionals. New York: Routledge; 2006:63-70.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
Dr. Madaan is a fellow in child and adolescent psychiatry at Creighton University, Omaha, NE.
Dr. Kohli is a practicing family physician in Amherst, VA.
Dr. Khurana is a clinical observer and visiting researcher at Children’s Hospital, Omaha, NE.
Limits of care: What events can you prevent?
Psychotic patient declines hospital admission, drives into an office building
Cook County (IL) Circuit Court
The patient, age 43, had been treated for mental illness for many years. He was voluntarily admitted to a hospital under the care of his psychiatrist, and was discharged at his own request a few days later. He had improved and was not considered a candidate for involuntary admission because he was not a danger to himself or others.
The patient then informed the psychiatrist that he did not want to continue treatment and said he had an appointment with a new psychiatrist within 2 weeks.
Five days later, the patient went to another hospital for voluntary admission. He was seen by an emergency room physician, who determined the patient was a candidate for voluntary admission. The patient, however, decided to leave the hospital while a bed was being arranged.
Two days later, the patient began having auditory and visual hallucinations. He then drove his car through the glass doors of an office building. No one was injured, but the patient was arrested and convicted of felony damage to property.
In his suit, the patient alleged his longtime psychiatrist was negligent and failed to properly treat him to avoid development of hallucinations. The psychiatrist argued that involuntary admission was not indicated and that the care given was appropriate.
- A defense verdict was returned
Patient commits suicide after discharge
Cook County (IL) Circuit Court
A patient, age 45, committed suicide by taking lethal doses of medication prescribed by her psychiatrist. The patient had suffered from severe depression, personality disorder, and substance abuse. The day before her death, she went to a hospital emergency room, where she was assessed for suicide and released without the psychiatrist having been notified.
The patient’s family claimed that the psychiatrist was negligent because he did not adequately assess or monitor the patient’s clinical condition at sufficient intervals over the 3 months preceding her suicide. The family also alleged that the psychiatrist prescribed oxycodone inappropriately.
The psychiatrist argued that proper care was given and that the patient failed to provide a complete, accurate medical history at the emergency room visit and did not to consent to admission.
- A defense verdict was returned
Could admission have prevented patient’s suicide?
Douglas County (NE) District Court
A patient in his mid-60s with a history of depression committed suicide with a gunshot wound to the head. Before his suicide, the patient was seeing a psychiatrist and psychologist for depression and emotional problems.
The patient’s family alleged the psychiatrist failed to diagnose the severity of the patient’s problems and admit him to a hospital for treatment and observation. The psychiatrist and psychologist denied negligence.
- A defense verdict was returned
Medical malpractice law is constantly evolving to determine what constitutes “negligent care.” The legal standard requires a patient who brings a negligence claim against a psychiatrist to prove:
- a relationship between patient and psychiatrist such that a duty of care exists
- the duty was breached—meaning the standard of care was not met
- the breach of duty caused the injury.
Relationship rules
The first case highlights issues surrounding the patient-psychiatrist relationship. In general, once you have agreed to treat a patient, a doctor-patient relationship and duty of care exists.
In the first case, the patient informed his longtime psychiatrist that he no longer wanted to continue care after discharge. A psychiatrist who terminates a doctor-patient relationship should provide written notice, an explanation of termination, and referrals and continue to care for the patient for a reasonable period.1 No such duty exists, however, when the patient ends treatment. Courts have found that the patient has not been abandoned when he or she voluntarily and unilaterally terminates the relationship.2,3
The relationship ends the moment the patient terminates care, unless the patient is not competent to make that unilateral decision. In that situation, your duty of care to the patient continues.2 When a competent patient terminates care, document the date and time of termination and the patient’s competence.
When relationships begin
The patient in the first case had an appointment with a new psychiatrist within 2 weeks. Is the new psychiatrist liable for what happens in the intervening period or does the relationship begin when the patient has been examined or treated? The legal question of when a physician-patient relationship is created remains problematic. Standards vary from state to state, but general principles offer some guidance.
The physician-patient relationship is a contract. The court would examine parties’ actions to ascertain their intent to determine if the patient reasonably believed that the physician—by actions or words—agreed to provide necessary medical care. Additionally, whether a relationship exists depends on the specific facts and circumstances of each situation.
There is some authority, across many jurisdictions, that a physician-patient relationship is established only when a physician conducts the initial history and physical examination. In some cases, however, the relationship has been found to exist at an earlier point, such as when a physician gave a referred patient an appointment for a consultation. When in doubt, assume the relationship exists.4
Duty of care
These cases raise areas where possible duty of care was breached:
- negligent prescription of medication
- failure to assess suicidal thinking.
Assessing suicide risk. Negligence in the second and third cases is based upon failure to assess suicidal thoughts. The legal system recognizes that psychiatrists cannot predict suicide,6 and mistakes in clinical judgment are not the same as negligence. Psychiatrists, however, are required to assess suicide risk and intervene appropriately.
When defending a negligence claim, the profession’s custom—reflected by the standard of care common to others with the practitioner’s training—is the benchmark against which the courts measure negligence. Therefore, take steps determined appropriate by the profession and document this risk assessment.7 For example, ask the patient about:
- suicidal thoughts and intent
- stressors
- history of suicidal behavior/attempts
- substance use
- signs and symptoms of depression
- bipolar disorder
- psychosis.8
Prescriptions. No clear line defines negligence when potentially dangerous medications are prescribed to a suicidal patient. Some psychiatrists dispense limited quantities of medications and see the patient weekly to monitor mood and medication. But even then a psychiatrist cannot prevent suicide—for example, the patient may have multiple prescribers or hoard medications. The concept of “sufficient intervals” to see a patient is determined case-by-case.
Documentation. Make suicide assessments an ongoing process. Document all aspects of the patient’s care, stability, and suicide risk, and reasons for the visit intervals. Indicate in the records your risk-benefit assessment in making treatment decisions.
Cases are selected byfrom Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Drug brand name
- Oxycodone • Percocet
1. American Medical Association Code of Medical Ethics, Opinion 8.115.
2. Knapp v. Eppright, 783 SW2d 293 (Tex 1989).
3. Saunders v. Tisher (Maine Sup. Jud. Ct. 2006).
4. Physicians Risk Management Update. The physician-patient relationship: when does it begin? Available at: http://www.phyins.com/pi/risk/updates/mayjun04.html. Accessed December 28, 2006.
5. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC; 2006. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed December 28, 2006.
6. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
7. Packman WL, Pennuto TO, Bongar B, Orthwein J. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
8. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
9. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
Psychotic patient declines hospital admission, drives into an office building
Cook County (IL) Circuit Court
The patient, age 43, had been treated for mental illness for many years. He was voluntarily admitted to a hospital under the care of his psychiatrist, and was discharged at his own request a few days later. He had improved and was not considered a candidate for involuntary admission because he was not a danger to himself or others.
The patient then informed the psychiatrist that he did not want to continue treatment and said he had an appointment with a new psychiatrist within 2 weeks.
Five days later, the patient went to another hospital for voluntary admission. He was seen by an emergency room physician, who determined the patient was a candidate for voluntary admission. The patient, however, decided to leave the hospital while a bed was being arranged.
Two days later, the patient began having auditory and visual hallucinations. He then drove his car through the glass doors of an office building. No one was injured, but the patient was arrested and convicted of felony damage to property.
In his suit, the patient alleged his longtime psychiatrist was negligent and failed to properly treat him to avoid development of hallucinations. The psychiatrist argued that involuntary admission was not indicated and that the care given was appropriate.
- A defense verdict was returned
Patient commits suicide after discharge
Cook County (IL) Circuit Court
A patient, age 45, committed suicide by taking lethal doses of medication prescribed by her psychiatrist. The patient had suffered from severe depression, personality disorder, and substance abuse. The day before her death, she went to a hospital emergency room, where she was assessed for suicide and released without the psychiatrist having been notified.
The patient’s family claimed that the psychiatrist was negligent because he did not adequately assess or monitor the patient’s clinical condition at sufficient intervals over the 3 months preceding her suicide. The family also alleged that the psychiatrist prescribed oxycodone inappropriately.
The psychiatrist argued that proper care was given and that the patient failed to provide a complete, accurate medical history at the emergency room visit and did not to consent to admission.
- A defense verdict was returned
Could admission have prevented patient’s suicide?
Douglas County (NE) District Court
A patient in his mid-60s with a history of depression committed suicide with a gunshot wound to the head. Before his suicide, the patient was seeing a psychiatrist and psychologist for depression and emotional problems.
The patient’s family alleged the psychiatrist failed to diagnose the severity of the patient’s problems and admit him to a hospital for treatment and observation. The psychiatrist and psychologist denied negligence.
- A defense verdict was returned
Medical malpractice law is constantly evolving to determine what constitutes “negligent care.” The legal standard requires a patient who brings a negligence claim against a psychiatrist to prove:
- a relationship between patient and psychiatrist such that a duty of care exists
- the duty was breached—meaning the standard of care was not met
- the breach of duty caused the injury.
Relationship rules
The first case highlights issues surrounding the patient-psychiatrist relationship. In general, once you have agreed to treat a patient, a doctor-patient relationship and duty of care exists.
In the first case, the patient informed his longtime psychiatrist that he no longer wanted to continue care after discharge. A psychiatrist who terminates a doctor-patient relationship should provide written notice, an explanation of termination, and referrals and continue to care for the patient for a reasonable period.1 No such duty exists, however, when the patient ends treatment. Courts have found that the patient has not been abandoned when he or she voluntarily and unilaterally terminates the relationship.2,3
The relationship ends the moment the patient terminates care, unless the patient is not competent to make that unilateral decision. In that situation, your duty of care to the patient continues.2 When a competent patient terminates care, document the date and time of termination and the patient’s competence.
When relationships begin
The patient in the first case had an appointment with a new psychiatrist within 2 weeks. Is the new psychiatrist liable for what happens in the intervening period or does the relationship begin when the patient has been examined or treated? The legal question of when a physician-patient relationship is created remains problematic. Standards vary from state to state, but general principles offer some guidance.
The physician-patient relationship is a contract. The court would examine parties’ actions to ascertain their intent to determine if the patient reasonably believed that the physician—by actions or words—agreed to provide necessary medical care. Additionally, whether a relationship exists depends on the specific facts and circumstances of each situation.
There is some authority, across many jurisdictions, that a physician-patient relationship is established only when a physician conducts the initial history and physical examination. In some cases, however, the relationship has been found to exist at an earlier point, such as when a physician gave a referred patient an appointment for a consultation. When in doubt, assume the relationship exists.4
Duty of care
These cases raise areas where possible duty of care was breached:
- negligent prescription of medication
- failure to assess suicidal thinking.
Assessing suicide risk. Negligence in the second and third cases is based upon failure to assess suicidal thoughts. The legal system recognizes that psychiatrists cannot predict suicide,6 and mistakes in clinical judgment are not the same as negligence. Psychiatrists, however, are required to assess suicide risk and intervene appropriately.
When defending a negligence claim, the profession’s custom—reflected by the standard of care common to others with the practitioner’s training—is the benchmark against which the courts measure negligence. Therefore, take steps determined appropriate by the profession and document this risk assessment.7 For example, ask the patient about:
- suicidal thoughts and intent
- stressors
- history of suicidal behavior/attempts
- substance use
- signs and symptoms of depression
- bipolar disorder
- psychosis.8
Prescriptions. No clear line defines negligence when potentially dangerous medications are prescribed to a suicidal patient. Some psychiatrists dispense limited quantities of medications and see the patient weekly to monitor mood and medication. But even then a psychiatrist cannot prevent suicide—for example, the patient may have multiple prescribers or hoard medications. The concept of “sufficient intervals” to see a patient is determined case-by-case.
Documentation. Make suicide assessments an ongoing process. Document all aspects of the patient’s care, stability, and suicide risk, and reasons for the visit intervals. Indicate in the records your risk-benefit assessment in making treatment decisions.
Cases are selected byfrom Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Drug brand name
- Oxycodone • Percocet
Psychotic patient declines hospital admission, drives into an office building
Cook County (IL) Circuit Court
The patient, age 43, had been treated for mental illness for many years. He was voluntarily admitted to a hospital under the care of his psychiatrist, and was discharged at his own request a few days later. He had improved and was not considered a candidate for involuntary admission because he was not a danger to himself or others.
The patient then informed the psychiatrist that he did not want to continue treatment and said he had an appointment with a new psychiatrist within 2 weeks.
Five days later, the patient went to another hospital for voluntary admission. He was seen by an emergency room physician, who determined the patient was a candidate for voluntary admission. The patient, however, decided to leave the hospital while a bed was being arranged.
Two days later, the patient began having auditory and visual hallucinations. He then drove his car through the glass doors of an office building. No one was injured, but the patient was arrested and convicted of felony damage to property.
In his suit, the patient alleged his longtime psychiatrist was negligent and failed to properly treat him to avoid development of hallucinations. The psychiatrist argued that involuntary admission was not indicated and that the care given was appropriate.
- A defense verdict was returned
Patient commits suicide after discharge
Cook County (IL) Circuit Court
A patient, age 45, committed suicide by taking lethal doses of medication prescribed by her psychiatrist. The patient had suffered from severe depression, personality disorder, and substance abuse. The day before her death, she went to a hospital emergency room, where she was assessed for suicide and released without the psychiatrist having been notified.
The patient’s family claimed that the psychiatrist was negligent because he did not adequately assess or monitor the patient’s clinical condition at sufficient intervals over the 3 months preceding her suicide. The family also alleged that the psychiatrist prescribed oxycodone inappropriately.
The psychiatrist argued that proper care was given and that the patient failed to provide a complete, accurate medical history at the emergency room visit and did not to consent to admission.
- A defense verdict was returned
Could admission have prevented patient’s suicide?
Douglas County (NE) District Court
A patient in his mid-60s with a history of depression committed suicide with a gunshot wound to the head. Before his suicide, the patient was seeing a psychiatrist and psychologist for depression and emotional problems.
The patient’s family alleged the psychiatrist failed to diagnose the severity of the patient’s problems and admit him to a hospital for treatment and observation. The psychiatrist and psychologist denied negligence.
- A defense verdict was returned
Medical malpractice law is constantly evolving to determine what constitutes “negligent care.” The legal standard requires a patient who brings a negligence claim against a psychiatrist to prove:
- a relationship between patient and psychiatrist such that a duty of care exists
- the duty was breached—meaning the standard of care was not met
- the breach of duty caused the injury.
Relationship rules
The first case highlights issues surrounding the patient-psychiatrist relationship. In general, once you have agreed to treat a patient, a doctor-patient relationship and duty of care exists.
In the first case, the patient informed his longtime psychiatrist that he no longer wanted to continue care after discharge. A psychiatrist who terminates a doctor-patient relationship should provide written notice, an explanation of termination, and referrals and continue to care for the patient for a reasonable period.1 No such duty exists, however, when the patient ends treatment. Courts have found that the patient has not been abandoned when he or she voluntarily and unilaterally terminates the relationship.2,3
The relationship ends the moment the patient terminates care, unless the patient is not competent to make that unilateral decision. In that situation, your duty of care to the patient continues.2 When a competent patient terminates care, document the date and time of termination and the patient’s competence.
When relationships begin
The patient in the first case had an appointment with a new psychiatrist within 2 weeks. Is the new psychiatrist liable for what happens in the intervening period or does the relationship begin when the patient has been examined or treated? The legal question of when a physician-patient relationship is created remains problematic. Standards vary from state to state, but general principles offer some guidance.
The physician-patient relationship is a contract. The court would examine parties’ actions to ascertain their intent to determine if the patient reasonably believed that the physician—by actions or words—agreed to provide necessary medical care. Additionally, whether a relationship exists depends on the specific facts and circumstances of each situation.
There is some authority, across many jurisdictions, that a physician-patient relationship is established only when a physician conducts the initial history and physical examination. In some cases, however, the relationship has been found to exist at an earlier point, such as when a physician gave a referred patient an appointment for a consultation. When in doubt, assume the relationship exists.4
Duty of care
These cases raise areas where possible duty of care was breached:
- negligent prescription of medication
- failure to assess suicidal thinking.
Assessing suicide risk. Negligence in the second and third cases is based upon failure to assess suicidal thoughts. The legal system recognizes that psychiatrists cannot predict suicide,6 and mistakes in clinical judgment are not the same as negligence. Psychiatrists, however, are required to assess suicide risk and intervene appropriately.
When defending a negligence claim, the profession’s custom—reflected by the standard of care common to others with the practitioner’s training—is the benchmark against which the courts measure negligence. Therefore, take steps determined appropriate by the profession and document this risk assessment.7 For example, ask the patient about:
- suicidal thoughts and intent
- stressors
- history of suicidal behavior/attempts
- substance use
- signs and symptoms of depression
- bipolar disorder
- psychosis.8
Prescriptions. No clear line defines negligence when potentially dangerous medications are prescribed to a suicidal patient. Some psychiatrists dispense limited quantities of medications and see the patient weekly to monitor mood and medication. But even then a psychiatrist cannot prevent suicide—for example, the patient may have multiple prescribers or hoard medications. The concept of “sufficient intervals” to see a patient is determined case-by-case.
Documentation. Make suicide assessments an ongoing process. Document all aspects of the patient’s care, stability, and suicide risk, and reasons for the visit intervals. Indicate in the records your risk-benefit assessment in making treatment decisions.
Cases are selected byfrom Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Drug brand name
- Oxycodone • Percocet
1. American Medical Association Code of Medical Ethics, Opinion 8.115.
2. Knapp v. Eppright, 783 SW2d 293 (Tex 1989).
3. Saunders v. Tisher (Maine Sup. Jud. Ct. 2006).
4. Physicians Risk Management Update. The physician-patient relationship: when does it begin? Available at: http://www.phyins.com/pi/risk/updates/mayjun04.html. Accessed December 28, 2006.
5. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC; 2006. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed December 28, 2006.
6. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
7. Packman WL, Pennuto TO, Bongar B, Orthwein J. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
8. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
9. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
1. American Medical Association Code of Medical Ethics, Opinion 8.115.
2. Knapp v. Eppright, 783 SW2d 293 (Tex 1989).
3. Saunders v. Tisher (Maine Sup. Jud. Ct. 2006).
4. Physicians Risk Management Update. The physician-patient relationship: when does it begin? Available at: http://www.phyins.com/pi/risk/updates/mayjun04.html. Accessed December 28, 2006.
5. American Psychiatric Association. Principles of medical ethics with annotations especially applicable to psychiatry. Washington, DC; 2006. Available at: http://www.psych.org/psych_pract/ethics/ppaethics.cfm. Accessed December 28, 2006.
6. Pokorny A. Prediction of suicide in psychiatric patients. Report of a prospective study. Arch Gen Psychiatry 1983;40(3):249-57.
7. Packman WL, Pennuto TO, Bongar B, Orthwein J. Legal issues of professional negligence in suicide cases. Behav Sci Law 2004;22:697-713.
8. Simon RI. The suicidal patient. In: Lifson LE, Simon RI, eds. The mental health practitioner and the law: a comprehensive handbook. Cambridge, MA: Harvard University Press; 1998:166-86.
9. Maunz v. Perales, 276 Kan. 313, 76 P.3d 1027 (Kan 2003).
Effects of infertility
In “How to treat depression, stress associated with infertility treatment” (Current Psychiatry, October 2006), the authors refer to problems associated with infertility—including adverse psychological sequelae.
The authors, however, make only passing reference to potential problems and adverse psychological effects of infertility treatment—including hyperovulatory drugs; destruction, wasting, or freezing human embryos; multifetal pregnancy reduction; and the risk of prematurity for babies conceived through in vitro fertilization nor do they mention the inherent problem with many assisted reproductive technologies that treat the product of conception as a commodity rather than a person. This perception fosters a culture that says children are a “right” in the fertility clinic and a “burden” in the abortion clinic.
The authors contrast doctrinal dictates with the dreams of prospective parents without alluding to the fact that moral rules serve as principles of human conduct to prevent the adverse psychological and spiritual consequences of immoral behavior.
Thomas K. Nelson, MD
Assistant professor of psychiatry
Mayo Clinic College of Medicine
Scottsdale, AZ
In “How to treat depression, stress associated with infertility treatment” (Current Psychiatry, October 2006), the authors refer to problems associated with infertility—including adverse psychological sequelae.
The authors, however, make only passing reference to potential problems and adverse psychological effects of infertility treatment—including hyperovulatory drugs; destruction, wasting, or freezing human embryos; multifetal pregnancy reduction; and the risk of prematurity for babies conceived through in vitro fertilization nor do they mention the inherent problem with many assisted reproductive technologies that treat the product of conception as a commodity rather than a person. This perception fosters a culture that says children are a “right” in the fertility clinic and a “burden” in the abortion clinic.
The authors contrast doctrinal dictates with the dreams of prospective parents without alluding to the fact that moral rules serve as principles of human conduct to prevent the adverse psychological and spiritual consequences of immoral behavior.
Thomas K. Nelson, MD
Assistant professor of psychiatry
Mayo Clinic College of Medicine
Scottsdale, AZ
In “How to treat depression, stress associated with infertility treatment” (Current Psychiatry, October 2006), the authors refer to problems associated with infertility—including adverse psychological sequelae.
The authors, however, make only passing reference to potential problems and adverse psychological effects of infertility treatment—including hyperovulatory drugs; destruction, wasting, or freezing human embryos; multifetal pregnancy reduction; and the risk of prematurity for babies conceived through in vitro fertilization nor do they mention the inherent problem with many assisted reproductive technologies that treat the product of conception as a commodity rather than a person. This perception fosters a culture that says children are a “right” in the fertility clinic and a “burden” in the abortion clinic.
The authors contrast doctrinal dictates with the dreams of prospective parents without alluding to the fact that moral rules serve as principles of human conduct to prevent the adverse psychological and spiritual consequences of immoral behavior.
Thomas K. Nelson, MD
Assistant professor of psychiatry
Mayo Clinic College of Medicine
Scottsdale, AZ
'Meth' and psychosis
In “The ‘meth’ epidemic: Acute intoxication” (Current Psychiatry, November 2006), Drs. J. Michael Bostwick and Timothy W. Lineberry address the striking similarities between schizophrenia symptoms and the residual psychotic features after methamphetamine use. Several risk factors have been associated with methamphetamine psychosis, especially in recently published studies.
One such study associated increased met allele frequency of the catechol-O-methyl transferase with methamphetamine psychosis.1 Another recent study also suggested that Ala/Val polymorphism of the superoxide dismutase 2 (SOD2) gene could be a risk factor for developing methamphetamine psychosis.2 SOD2 normally protects cells from free radical damage.
Premorbid brain dysfunction and schizotypal and schizoid personality disorders also have been considered risk factors for psychosis among methamphetamine users.3,4
Adegboyega Oyemade, MD
Addiction psychiatry fellow
Yale University
New Haven, CT
1. Suzuki A, Nakamura K, Sekine Y, et al. An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatric genetics 2006;16(4):133-8.
2. Nakamura K, Chen CK, Sekine Y, et al. An association analysis of SOD2 variants with methamphetamine psychosis in Japanese and Taiwanese populations. Human genetics 2006;120(2):243-52.
3. Fujii D. Risk factors for treatment-resistive methamphetamine psychosis. J Neuropsychiatry Clin Neurosci 2002;14:239-40.
4. Chen C, Lin S, Sham P, et al. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychological Medicine 2003;33(8):1407-14.
In “The ‘meth’ epidemic: Acute intoxication” (Current Psychiatry, November 2006), Drs. J. Michael Bostwick and Timothy W. Lineberry address the striking similarities between schizophrenia symptoms and the residual psychotic features after methamphetamine use. Several risk factors have been associated with methamphetamine psychosis, especially in recently published studies.
One such study associated increased met allele frequency of the catechol-O-methyl transferase with methamphetamine psychosis.1 Another recent study also suggested that Ala/Val polymorphism of the superoxide dismutase 2 (SOD2) gene could be a risk factor for developing methamphetamine psychosis.2 SOD2 normally protects cells from free radical damage.
Premorbid brain dysfunction and schizotypal and schizoid personality disorders also have been considered risk factors for psychosis among methamphetamine users.3,4
Adegboyega Oyemade, MD
Addiction psychiatry fellow
Yale University
New Haven, CT
In “The ‘meth’ epidemic: Acute intoxication” (Current Psychiatry, November 2006), Drs. J. Michael Bostwick and Timothy W. Lineberry address the striking similarities between schizophrenia symptoms and the residual psychotic features after methamphetamine use. Several risk factors have been associated with methamphetamine psychosis, especially in recently published studies.
One such study associated increased met allele frequency of the catechol-O-methyl transferase with methamphetamine psychosis.1 Another recent study also suggested that Ala/Val polymorphism of the superoxide dismutase 2 (SOD2) gene could be a risk factor for developing methamphetamine psychosis.2 SOD2 normally protects cells from free radical damage.
Premorbid brain dysfunction and schizotypal and schizoid personality disorders also have been considered risk factors for psychosis among methamphetamine users.3,4
Adegboyega Oyemade, MD
Addiction psychiatry fellow
Yale University
New Haven, CT
1. Suzuki A, Nakamura K, Sekine Y, et al. An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatric genetics 2006;16(4):133-8.
2. Nakamura K, Chen CK, Sekine Y, et al. An association analysis of SOD2 variants with methamphetamine psychosis in Japanese and Taiwanese populations. Human genetics 2006;120(2):243-52.
3. Fujii D. Risk factors for treatment-resistive methamphetamine psychosis. J Neuropsychiatry Clin Neurosci 2002;14:239-40.
4. Chen C, Lin S, Sham P, et al. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychological Medicine 2003;33(8):1407-14.
1. Suzuki A, Nakamura K, Sekine Y, et al. An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatric genetics 2006;16(4):133-8.
2. Nakamura K, Chen CK, Sekine Y, et al. An association analysis of SOD2 variants with methamphetamine psychosis in Japanese and Taiwanese populations. Human genetics 2006;120(2):243-52.
3. Fujii D. Risk factors for treatment-resistive methamphetamine psychosis. J Neuropsychiatry Clin Neurosci 2002;14:239-40.
4. Chen C, Lin S, Sham P, et al. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychological Medicine 2003;33(8):1407-14.
Discontinuation symptoms
In “6 safety rules for tapering antidepressants” (Pearls, Current Psychiatry, November 2006), Richard C. Shelton, MD, mentioned that discontinuation symptoms associated with serotonin reuptake inhibitors (SRIs) “appear to be more common and severe with short-acting drugs such as venlafaxine and paroxetine,” but occur with fluoxetine as well.
Although possible, this statement could be misleading because studies comparing discontinuation symptoms among SRIs have not shown clinically significant discontinuation symptoms with fluoxetine. The authors of these studies have hypothesized that the lack of discontinuation symptoms is because of the long half-life of fluoxetine (2 to 4 days) and its active metabolite, norfluoxetine (7 to 9 days).1-4
Dr. Shelton also wrote, “infants born to mothers taking antidepressants can exhibit discontinuation symptoms.” The fact that these neonatal serotonergic symptoms occur equally with fluoxetine and shorter half-life SRIs argues against these being discontinuation symptoms.5,6
Clinicians should recognize that this is a controversial topic and that experts are not comfortable characterizing such effects as discontinuation symptoms rather than symptoms secondary to serotonin exposure.
Scott A. Freeman, MD
Assistant professor of clinical psychiatry
University of Arizona
College of Medicine
Tucson, AZ
References
1. Zajecka J, Fawcett J, Amsterdam J, et al. Safety of abrupt discontinuation of fluoxetine in randomized, placebo-controlled study. J Clin Psychopharm 1998;18(3):193-7.
2. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry 1998;44(2):77-87.
3. Hindmarch I, Kimber S, Cockle SM. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharm 2000;15(6):305-18.
4. Judge R, Parry MG, Quail D, et al. Discontinuation symptoms: comparison of brief interruption in fluoxetine and paroxetine treatment. Int Clin Psychopharm 2002;17(5):217-25.
5. Oberlander TE, Misri S, Fitzgerald CE. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65:230-7.
6. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 2005;293(19):2372-83.
Dr. Shelton responds
Dr. Freeman raises important issues. In fact, discontinuation symptoms with long-acting drugs such as fluoxetine—if they occur—are generally much milder than those observed with drugs with shorter half-lives. However, some studies examining comparative risks simply have been too short to pick up any discontinuation symptoms with longer-acting drugs. For example, in the Judge et al article cited by Dr. Freeman, the discontinuation periods were 3 to 5 days.
The Rosenbaum et al study of 242 patients on paroxetine, sertraline, or fluoxetine discontinued for 5 to 8 days was more informative. The total Discontinuation-Emergent Signs and Symptoms score between baseline and endpoint changed the most in paroxetine, the least in fluoxetine, and sertraline scored in the middle.
Although the total score comparing baseline to endpoint did not differ with fluoxetine, certain discontinuation symptoms were relatively frequent. For example, agitation was found in 25% of patients, emotional lability in 21%, and confusion in 19%. This suggests that discontinuation symptoms may indeed be “clinically significant.”
Similarly, in the Zajecka et al study, patients treated with fluoxetine were randomly assigned to continuation fluoxetine or placebo for 6 weeks. There were few differences between the groups, suggesting that the discontinuation syndrome did not occur with cessation of fluoxetine. However, there was a small but significant increase in dizziness at 4 and 6 weeks and rhinitis at 6 weeks.
These studies yield at least 2 conclusions:
- Discontinuation reactions with fluoxetine tend to be very mild when they occur, an idea consistent with my recommendation to substitute fluoxetine to assist tapering other drugs.
- Reactions, when they occur, happen much later than with short-acting drugs, which is consistent with fluoxetine’s long half-life.
Regarding paroxetine, Sanz et al2 reported that in the World Health Organization’s database of adverse events, 64 of 93 cases of post-natal complications associated with SRI treatment were with paroxetine, compared to 14 with fluoxetine, 9 with sertraline, and 7 with citalopram. The authors described the problem as related to “discontinuation” after delivery. Haddad et al3 described some of the symptoms associated with neonatal complications as “serotonin syndrome.”
Taken as a whole, however, the reported symptoms have been a combination of signs of acute toxicity—such as seizures, hypertonus, jaundice, and hypoglycemia—and more typical discontinuation symptoms, including respiratory distress and hyperventilation. The notion that these reactions are related to serotonin syndrome seems unsupported, given that they occur more often with short half-life paroxetine than with long half-life drugs.
Richard C. Shelton, MD
James G. Blakemore Research Professor
Professor of pharmacology
Department of psychiatry
Vanderbilt University
Nashville, TN
1. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry 2006;67(suppl 4):3-7.
2. Sanz EJ, de las Cuevas C, Kiuru A, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 2005;365:482-7.
3. Haddad PM, Pal BR, Clark P, et al. Neonatal symptoms following maternal paroxetine treatment: serotonin toxicity or paroxetine discontinuation syndrome? J Psychopharmacol 2005;19:554-7.
In “6 safety rules for tapering antidepressants” (Pearls, Current Psychiatry, November 2006), Richard C. Shelton, MD, mentioned that discontinuation symptoms associated with serotonin reuptake inhibitors (SRIs) “appear to be more common and severe with short-acting drugs such as venlafaxine and paroxetine,” but occur with fluoxetine as well.
Although possible, this statement could be misleading because studies comparing discontinuation symptoms among SRIs have not shown clinically significant discontinuation symptoms with fluoxetine. The authors of these studies have hypothesized that the lack of discontinuation symptoms is because of the long half-life of fluoxetine (2 to 4 days) and its active metabolite, norfluoxetine (7 to 9 days).1-4
Dr. Shelton also wrote, “infants born to mothers taking antidepressants can exhibit discontinuation symptoms.” The fact that these neonatal serotonergic symptoms occur equally with fluoxetine and shorter half-life SRIs argues against these being discontinuation symptoms.5,6
Clinicians should recognize that this is a controversial topic and that experts are not comfortable characterizing such effects as discontinuation symptoms rather than symptoms secondary to serotonin exposure.
Scott A. Freeman, MD
Assistant professor of clinical psychiatry
University of Arizona
College of Medicine
Tucson, AZ
References
1. Zajecka J, Fawcett J, Amsterdam J, et al. Safety of abrupt discontinuation of fluoxetine in randomized, placebo-controlled study. J Clin Psychopharm 1998;18(3):193-7.
2. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry 1998;44(2):77-87.
3. Hindmarch I, Kimber S, Cockle SM. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharm 2000;15(6):305-18.
4. Judge R, Parry MG, Quail D, et al. Discontinuation symptoms: comparison of brief interruption in fluoxetine and paroxetine treatment. Int Clin Psychopharm 2002;17(5):217-25.
5. Oberlander TE, Misri S, Fitzgerald CE. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65:230-7.
6. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 2005;293(19):2372-83.
Dr. Shelton responds
Dr. Freeman raises important issues. In fact, discontinuation symptoms with long-acting drugs such as fluoxetine—if they occur—are generally much milder than those observed with drugs with shorter half-lives. However, some studies examining comparative risks simply have been too short to pick up any discontinuation symptoms with longer-acting drugs. For example, in the Judge et al article cited by Dr. Freeman, the discontinuation periods were 3 to 5 days.
The Rosenbaum et al study of 242 patients on paroxetine, sertraline, or fluoxetine discontinued for 5 to 8 days was more informative. The total Discontinuation-Emergent Signs and Symptoms score between baseline and endpoint changed the most in paroxetine, the least in fluoxetine, and sertraline scored in the middle.
Although the total score comparing baseline to endpoint did not differ with fluoxetine, certain discontinuation symptoms were relatively frequent. For example, agitation was found in 25% of patients, emotional lability in 21%, and confusion in 19%. This suggests that discontinuation symptoms may indeed be “clinically significant.”
Similarly, in the Zajecka et al study, patients treated with fluoxetine were randomly assigned to continuation fluoxetine or placebo for 6 weeks. There were few differences between the groups, suggesting that the discontinuation syndrome did not occur with cessation of fluoxetine. However, there was a small but significant increase in dizziness at 4 and 6 weeks and rhinitis at 6 weeks.
These studies yield at least 2 conclusions:
- Discontinuation reactions with fluoxetine tend to be very mild when they occur, an idea consistent with my recommendation to substitute fluoxetine to assist tapering other drugs.
- Reactions, when they occur, happen much later than with short-acting drugs, which is consistent with fluoxetine’s long half-life.
Regarding paroxetine, Sanz et al2 reported that in the World Health Organization’s database of adverse events, 64 of 93 cases of post-natal complications associated with SRI treatment were with paroxetine, compared to 14 with fluoxetine, 9 with sertraline, and 7 with citalopram. The authors described the problem as related to “discontinuation” after delivery. Haddad et al3 described some of the symptoms associated with neonatal complications as “serotonin syndrome.”
Taken as a whole, however, the reported symptoms have been a combination of signs of acute toxicity—such as seizures, hypertonus, jaundice, and hypoglycemia—and more typical discontinuation symptoms, including respiratory distress and hyperventilation. The notion that these reactions are related to serotonin syndrome seems unsupported, given that they occur more often with short half-life paroxetine than with long half-life drugs.
Richard C. Shelton, MD
James G. Blakemore Research Professor
Professor of pharmacology
Department of psychiatry
Vanderbilt University
Nashville, TN
In “6 safety rules for tapering antidepressants” (Pearls, Current Psychiatry, November 2006), Richard C. Shelton, MD, mentioned that discontinuation symptoms associated with serotonin reuptake inhibitors (SRIs) “appear to be more common and severe with short-acting drugs such as venlafaxine and paroxetine,” but occur with fluoxetine as well.
Although possible, this statement could be misleading because studies comparing discontinuation symptoms among SRIs have not shown clinically significant discontinuation symptoms with fluoxetine. The authors of these studies have hypothesized that the lack of discontinuation symptoms is because of the long half-life of fluoxetine (2 to 4 days) and its active metabolite, norfluoxetine (7 to 9 days).1-4
Dr. Shelton also wrote, “infants born to mothers taking antidepressants can exhibit discontinuation symptoms.” The fact that these neonatal serotonergic symptoms occur equally with fluoxetine and shorter half-life SRIs argues against these being discontinuation symptoms.5,6
Clinicians should recognize that this is a controversial topic and that experts are not comfortable characterizing such effects as discontinuation symptoms rather than symptoms secondary to serotonin exposure.
Scott A. Freeman, MD
Assistant professor of clinical psychiatry
University of Arizona
College of Medicine
Tucson, AZ
References
1. Zajecka J, Fawcett J, Amsterdam J, et al. Safety of abrupt discontinuation of fluoxetine in randomized, placebo-controlled study. J Clin Psychopharm 1998;18(3):193-7.
2. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry 1998;44(2):77-87.
3. Hindmarch I, Kimber S, Cockle SM. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharm 2000;15(6):305-18.
4. Judge R, Parry MG, Quail D, et al. Discontinuation symptoms: comparison of brief interruption in fluoxetine and paroxetine treatment. Int Clin Psychopharm 2002;17(5):217-25.
5. Oberlander TE, Misri S, Fitzgerald CE. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65:230-7.
6. Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 2005;293(19):2372-83.
Dr. Shelton responds
Dr. Freeman raises important issues. In fact, discontinuation symptoms with long-acting drugs such as fluoxetine—if they occur—are generally much milder than those observed with drugs with shorter half-lives. However, some studies examining comparative risks simply have been too short to pick up any discontinuation symptoms with longer-acting drugs. For example, in the Judge et al article cited by Dr. Freeman, the discontinuation periods were 3 to 5 days.
The Rosenbaum et al study of 242 patients on paroxetine, sertraline, or fluoxetine discontinued for 5 to 8 days was more informative. The total Discontinuation-Emergent Signs and Symptoms score between baseline and endpoint changed the most in paroxetine, the least in fluoxetine, and sertraline scored in the middle.
Although the total score comparing baseline to endpoint did not differ with fluoxetine, certain discontinuation symptoms were relatively frequent. For example, agitation was found in 25% of patients, emotional lability in 21%, and confusion in 19%. This suggests that discontinuation symptoms may indeed be “clinically significant.”
Similarly, in the Zajecka et al study, patients treated with fluoxetine were randomly assigned to continuation fluoxetine or placebo for 6 weeks. There were few differences between the groups, suggesting that the discontinuation syndrome did not occur with cessation of fluoxetine. However, there was a small but significant increase in dizziness at 4 and 6 weeks and rhinitis at 6 weeks.
These studies yield at least 2 conclusions:
- Discontinuation reactions with fluoxetine tend to be very mild when they occur, an idea consistent with my recommendation to substitute fluoxetine to assist tapering other drugs.
- Reactions, when they occur, happen much later than with short-acting drugs, which is consistent with fluoxetine’s long half-life.
Regarding paroxetine, Sanz et al2 reported that in the World Health Organization’s database of adverse events, 64 of 93 cases of post-natal complications associated with SRI treatment were with paroxetine, compared to 14 with fluoxetine, 9 with sertraline, and 7 with citalopram. The authors described the problem as related to “discontinuation” after delivery. Haddad et al3 described some of the symptoms associated with neonatal complications as “serotonin syndrome.”
Taken as a whole, however, the reported symptoms have been a combination of signs of acute toxicity—such as seizures, hypertonus, jaundice, and hypoglycemia—and more typical discontinuation symptoms, including respiratory distress and hyperventilation. The notion that these reactions are related to serotonin syndrome seems unsupported, given that they occur more often with short half-life paroxetine than with long half-life drugs.
Richard C. Shelton, MD
James G. Blakemore Research Professor
Professor of pharmacology
Department of psychiatry
Vanderbilt University
Nashville, TN
1. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry 2006;67(suppl 4):3-7.
2. Sanz EJ, de las Cuevas C, Kiuru A, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 2005;365:482-7.
3. Haddad PM, Pal BR, Clark P, et al. Neonatal symptoms following maternal paroxetine treatment: serotonin toxicity or paroxetine discontinuation syndrome? J Psychopharmacol 2005;19:554-7.
1. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry 2006;67(suppl 4):3-7.
2. Sanz EJ, de las Cuevas C, Kiuru A, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 2005;365:482-7.
3. Haddad PM, Pal BR, Clark P, et al. Neonatal symptoms following maternal paroxetine treatment: serotonin toxicity or paroxetine discontinuation syndrome? J Psychopharmacol 2005;19:554-7.
REM while ‘awake’
I read with great interest Dr. Henry Nasrallah’s editorial, “Pathways of pleasure and pain,” and his postscript describing dopamine’s role in regulating the sleep-wake cycle (Current Psychiatry, November 2006). I am a psychiatrist and regularly observe my father who suffers from Parkinson’s disease caused by stroke, not progressive idiopathic Parkinson’s.
My father has episodes of what I call “REM awake.” He is awake and appears alert but responds to dream content as if it is real. When this happens he has rhythmic eye blinking and his gait is improved but not normal. He no longer gets “stuck” when trying to stand and walk, possibly because he does not remember that he cannot do this easily. He does not recall the episode when he is awake.
My father can rapidly transition out of “REM awake” when I have him close his eyes, relax, then stretch. His vital signs do not change, and EEG does not show a seizure.
When he “wakes” he cannot get up from his chair without great difficulty but is aware of his surroundings. This is his baseline. I therefore believe the process Dr. Nasrallah described in mice is present and observable in humans.
Kathleen Stack, MD
Hampton, VA
I read with great interest Dr. Henry Nasrallah’s editorial, “Pathways of pleasure and pain,” and his postscript describing dopamine’s role in regulating the sleep-wake cycle (Current Psychiatry, November 2006). I am a psychiatrist and regularly observe my father who suffers from Parkinson’s disease caused by stroke, not progressive idiopathic Parkinson’s.
My father has episodes of what I call “REM awake.” He is awake and appears alert but responds to dream content as if it is real. When this happens he has rhythmic eye blinking and his gait is improved but not normal. He no longer gets “stuck” when trying to stand and walk, possibly because he does not remember that he cannot do this easily. He does not recall the episode when he is awake.
My father can rapidly transition out of “REM awake” when I have him close his eyes, relax, then stretch. His vital signs do not change, and EEG does not show a seizure.
When he “wakes” he cannot get up from his chair without great difficulty but is aware of his surroundings. This is his baseline. I therefore believe the process Dr. Nasrallah described in mice is present and observable in humans.
Kathleen Stack, MD
Hampton, VA
I read with great interest Dr. Henry Nasrallah’s editorial, “Pathways of pleasure and pain,” and his postscript describing dopamine’s role in regulating the sleep-wake cycle (Current Psychiatry, November 2006). I am a psychiatrist and regularly observe my father who suffers from Parkinson’s disease caused by stroke, not progressive idiopathic Parkinson’s.
My father has episodes of what I call “REM awake.” He is awake and appears alert but responds to dream content as if it is real. When this happens he has rhythmic eye blinking and his gait is improved but not normal. He no longer gets “stuck” when trying to stand and walk, possibly because he does not remember that he cannot do this easily. He does not recall the episode when he is awake.
My father can rapidly transition out of “REM awake” when I have him close his eyes, relax, then stretch. His vital signs do not change, and EEG does not show a seizure.
When he “wakes” he cannot get up from his chair without great difficulty but is aware of his surroundings. This is his baseline. I therefore believe the process Dr. Nasrallah described in mice is present and observable in humans.
Kathleen Stack, MD
Hampton, VA
Irrational beliefs: A ubiquitous human trait
The human brain’s ability to think is a dramatic evolutionary leap above all other living creatures. But this cognitive power has a down side: our brains appear to be wired to generate and harbor false beliefs. Severe cases are pejoratively labeled “delusions” and usually lead to psychiatric care. But what about less-severe false and irrational beliefs that are common in the “normal” population?
Consider superstitions. How many of your friends expect the worst after seeing a black cat cross their path, adamantly avoid a hotel room on the 13th floor, knock on wood to ward off evil, or are convinced that breaking a mirror brings bad luck?
Let’s keep going. How many of your coworkers believe astrology determines their destiny or that clairvoyance and reincarnation are real? I’ll bet you know dozens of ordinary, “healthy” people who tenaciously believe in ghosts, the devil, and angels.
You certainly have met people who are convinced objects can be levitated through mental forces. What about the many who believe dreams provide information about the future or that fortune-tellers can predict the future by palm-reading, tarot cards, or gazing into a crystal ball?
Take athletes. Many have odd beliefs that shape their behavior before, during, and after games to help them win. They may wear a lucky item of clothing at every game, wear their socks inside out, bounce the ball a specific number of times before a free throw, or slap the goalie’s pad for good luck. Some believe they will play better if they force themselves to eat a certain number of candy bars or vomit before each game or tuck their shirts in on the right side but not on the left.
How about college students who insist on using a specific “lucky pen” during an exam or believe that singing a certain song before a test will help get them a good grade? Ask gamblers what they believe can make them win at poker, craps, roulette, or slot machines, and you will hear a flurry of bizarre beliefs. And denying the potential for harm or death is a common false belief among persons who engage in high-risk and dangerous sports or behaviors.
Suspicious theories or religious beliefs proliferate when humans feel helpless. We accept “conspiracy theories” whenever a major event (such as landing on the moon) or a calamity (assassination of a President, terrorist attack, or massive flooding after a hurricane) occurs. Many people believe in UFOs or that aliens abduct innocent people, molest them aboard a space ship, then return them to Earth to tell others about their traumatic memories.
Finally, various nonpsychotic disorders are associated with illogical, irrational, or false beliefs:
- Individuals with anxiety disorders may firmly believe that a plane will crash if they board it or that a bridge will collapse if they drive over it.
- Successful people who become clinically depressed often express the (false) belief that they are stupid, failures, worthless, or bankrupt.
- Pretty women with body dysmorphic disorder are convinced they are ugly.
- Anorexic adults who have shriveled to 75 pounds firmly believe they are fat.
- A person with obsessive-compulsive disorder is convinced that something terrible will happen if he does not flush the toilet 9 times every time he uses it.
What does it all mean? Overwhelming evidence shows that odd, irrational, false, or bizarre beliefs are ubiquitous among people regarded as ordinary and sane.
Does generating false beliefs have a useful or adaptive evolutionary value? Could the magical belief in miracles have evolved in the human brain to instill hope during dark hours or to help us confront the inevitability of death? Could this “mental software”—designed to cope with inevitable existential tribulations—have mutated into unwarranted superstitious and supernatural beliefs in everyday life?
Until neuroscience research untangles the enigmas of human thoughts and beliefs, let us remember that patients with schizophrenia’s implausible delusions, mania’s grandiose delusions, or psychotic depression’s somatic delusions are merely extreme variants on a continuum of irrational beliefs on which all of us “sane” individuals also belong.
The human brain’s ability to think is a dramatic evolutionary leap above all other living creatures. But this cognitive power has a down side: our brains appear to be wired to generate and harbor false beliefs. Severe cases are pejoratively labeled “delusions” and usually lead to psychiatric care. But what about less-severe false and irrational beliefs that are common in the “normal” population?
Consider superstitions. How many of your friends expect the worst after seeing a black cat cross their path, adamantly avoid a hotel room on the 13th floor, knock on wood to ward off evil, or are convinced that breaking a mirror brings bad luck?
Let’s keep going. How many of your coworkers believe astrology determines their destiny or that clairvoyance and reincarnation are real? I’ll bet you know dozens of ordinary, “healthy” people who tenaciously believe in ghosts, the devil, and angels.
You certainly have met people who are convinced objects can be levitated through mental forces. What about the many who believe dreams provide information about the future or that fortune-tellers can predict the future by palm-reading, tarot cards, or gazing into a crystal ball?
Take athletes. Many have odd beliefs that shape their behavior before, during, and after games to help them win. They may wear a lucky item of clothing at every game, wear their socks inside out, bounce the ball a specific number of times before a free throw, or slap the goalie’s pad for good luck. Some believe they will play better if they force themselves to eat a certain number of candy bars or vomit before each game or tuck their shirts in on the right side but not on the left.
How about college students who insist on using a specific “lucky pen” during an exam or believe that singing a certain song before a test will help get them a good grade? Ask gamblers what they believe can make them win at poker, craps, roulette, or slot machines, and you will hear a flurry of bizarre beliefs. And denying the potential for harm or death is a common false belief among persons who engage in high-risk and dangerous sports or behaviors.
Suspicious theories or religious beliefs proliferate when humans feel helpless. We accept “conspiracy theories” whenever a major event (such as landing on the moon) or a calamity (assassination of a President, terrorist attack, or massive flooding after a hurricane) occurs. Many people believe in UFOs or that aliens abduct innocent people, molest them aboard a space ship, then return them to Earth to tell others about their traumatic memories.
Finally, various nonpsychotic disorders are associated with illogical, irrational, or false beliefs:
- Individuals with anxiety disorders may firmly believe that a plane will crash if they board it or that a bridge will collapse if they drive over it.
- Successful people who become clinically depressed often express the (false) belief that they are stupid, failures, worthless, or bankrupt.
- Pretty women with body dysmorphic disorder are convinced they are ugly.
- Anorexic adults who have shriveled to 75 pounds firmly believe they are fat.
- A person with obsessive-compulsive disorder is convinced that something terrible will happen if he does not flush the toilet 9 times every time he uses it.
What does it all mean? Overwhelming evidence shows that odd, irrational, false, or bizarre beliefs are ubiquitous among people regarded as ordinary and sane.
Does generating false beliefs have a useful or adaptive evolutionary value? Could the magical belief in miracles have evolved in the human brain to instill hope during dark hours or to help us confront the inevitability of death? Could this “mental software”—designed to cope with inevitable existential tribulations—have mutated into unwarranted superstitious and supernatural beliefs in everyday life?
Until neuroscience research untangles the enigmas of human thoughts and beliefs, let us remember that patients with schizophrenia’s implausible delusions, mania’s grandiose delusions, or psychotic depression’s somatic delusions are merely extreme variants on a continuum of irrational beliefs on which all of us “sane” individuals also belong.
The human brain’s ability to think is a dramatic evolutionary leap above all other living creatures. But this cognitive power has a down side: our brains appear to be wired to generate and harbor false beliefs. Severe cases are pejoratively labeled “delusions” and usually lead to psychiatric care. But what about less-severe false and irrational beliefs that are common in the “normal” population?
Consider superstitions. How many of your friends expect the worst after seeing a black cat cross their path, adamantly avoid a hotel room on the 13th floor, knock on wood to ward off evil, or are convinced that breaking a mirror brings bad luck?
Let’s keep going. How many of your coworkers believe astrology determines their destiny or that clairvoyance and reincarnation are real? I’ll bet you know dozens of ordinary, “healthy” people who tenaciously believe in ghosts, the devil, and angels.
You certainly have met people who are convinced objects can be levitated through mental forces. What about the many who believe dreams provide information about the future or that fortune-tellers can predict the future by palm-reading, tarot cards, or gazing into a crystal ball?
Take athletes. Many have odd beliefs that shape their behavior before, during, and after games to help them win. They may wear a lucky item of clothing at every game, wear their socks inside out, bounce the ball a specific number of times before a free throw, or slap the goalie’s pad for good luck. Some believe they will play better if they force themselves to eat a certain number of candy bars or vomit before each game or tuck their shirts in on the right side but not on the left.
How about college students who insist on using a specific “lucky pen” during an exam or believe that singing a certain song before a test will help get them a good grade? Ask gamblers what they believe can make them win at poker, craps, roulette, or slot machines, and you will hear a flurry of bizarre beliefs. And denying the potential for harm or death is a common false belief among persons who engage in high-risk and dangerous sports or behaviors.
Suspicious theories or religious beliefs proliferate when humans feel helpless. We accept “conspiracy theories” whenever a major event (such as landing on the moon) or a calamity (assassination of a President, terrorist attack, or massive flooding after a hurricane) occurs. Many people believe in UFOs or that aliens abduct innocent people, molest them aboard a space ship, then return them to Earth to tell others about their traumatic memories.
Finally, various nonpsychotic disorders are associated with illogical, irrational, or false beliefs:
- Individuals with anxiety disorders may firmly believe that a plane will crash if they board it or that a bridge will collapse if they drive over it.
- Successful people who become clinically depressed often express the (false) belief that they are stupid, failures, worthless, or bankrupt.
- Pretty women with body dysmorphic disorder are convinced they are ugly.
- Anorexic adults who have shriveled to 75 pounds firmly believe they are fat.
- A person with obsessive-compulsive disorder is convinced that something terrible will happen if he does not flush the toilet 9 times every time he uses it.
What does it all mean? Overwhelming evidence shows that odd, irrational, false, or bizarre beliefs are ubiquitous among people regarded as ordinary and sane.
Does generating false beliefs have a useful or adaptive evolutionary value? Could the magical belief in miracles have evolved in the human brain to instill hope during dark hours or to help us confront the inevitability of death? Could this “mental software”—designed to cope with inevitable existential tribulations—have mutated into unwarranted superstitious and supernatural beliefs in everyday life?
Until neuroscience research untangles the enigmas of human thoughts and beliefs, let us remember that patients with schizophrenia’s implausible delusions, mania’s grandiose delusions, or psychotic depression’s somatic delusions are merely extreme variants on a continuum of irrational beliefs on which all of us “sane” individuals also belong.
Did antismoking therapy make him sick?
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.