User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Is it bipolar depression? ‘WHIPLASHED’ aids diagnosis
Despite much education and research, bipolar disorder is still under-recognized and inappropriately treated in many clinical settings.1 Bipolar and unipolar depression display similar symptoms, making correct diagnosis difficult. The differential diagnosis is especially problematic in patients suffering a first major depressive episode, when there is no clear history of mania or hypomania.
Nevertheless, bipolar depression does have telltale signs—remembered with the mnemonic WHIPLASHED—to guide diagnosis.2-8
Worse or “wired” when taking antidepressants. The patient complains of feeling “antsy” or being agitated or unable to sleep when taking traditional anti-depressants.
Look for numerous failed antidepressant trials, apparent tolerance to antidepressants that does not resolve with increased dose, and antidepressant-induced mania or mood cycle acceleration.
Hypomania, hyperthymic temperament, or mood swings in a patient’s history. Patients with hyperthymic temperament show persistent traits such as intense optimism, increased energy, reduced need for sleep, extroversion, and overconfidence.
Ask about periods of elevated mood or energy that might not fit formal DSM-IV-TR criteria for hypomania—such as episodes that last only a day or two. Mood lability in younger patients can be especially dramatic and poorly demarcated.
Irritable, hostile, or mixed features. Some patients show one or more hypomanic features, such as racing thoughts when depressed.
Psychomotor retardation appears more common in bipolar I depression than in unipolar major depression. Psychomotor agitation, however, is more likely in bipolar II than in unipolar major depression.
Loaded family history of mood swings, frank bipolar disorder, or affective illness. A family history of comorbid mood disorder and alcoholism may also point to bipolarity.
Abrupt onset and/or termination of depressive bouts or relatively brief episodes (
Seasonal or postpartum depression. “Winter-type” seasonal affective disorder—feeling depressed in the fall and winter, hypomanic in the spring—and postpartum psychosis have clinical and epidemiologic links with bipolar disorder.
Hyperphagia and hypersomnia—sometimes termed atypical features—are common in bipolar depression. Paradoxically, hypersomnia may co-exist with psychomotor agitation in bipolar II patients, resulting in so-called “sleepy speeders.”
Early age of onset. Major depression that appears before age 25—especially with psychotic features—may herald subsequent bipolarity.
Delusions, hallucinations, or other psychotic features are more common in bipolar than in unipolar depression.
Acknowledgment
The author thanks Nassir Ghaemi, MD, and Jim Phelps, MD, for suggesting modifications to the mnemonic.
1. Phelps JR, Ghaemi SN. Improving the diagnosis of bipolar disorder: predictive value of screening tests. J Affect Disord 2006;92(2-3):141-8.
2. Thase ME. Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry 2005;13(5):257-71.
3. Benazzi F, Akiskal H. Irritable-hostile depression: further validation as a bipolar depressive mixed state. J Affect Disord 2005;84(2-3):197-207.
4. Pies R. The “softer” end of the bipolar spectrum. J Psychiatr Pract 2002;8(4):189-95.
5. Albanese MJ, Pies R. The bipolar patient with comorbid substance use disorder: recognition and management. CNS Drugs 2004;18(9):585-96.
6. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-92.
7. Hantouche EG, Akiskal HS. Bipolar II vs. unipolar depression: psychopathologic differentiation by dimensional measures. J Affect Disord 2005;84(2-3):127-32.
8. Mitchell PB, Wilhelm K, Parker G, et al. The clinical features of bipolar depression: a comparison with matched major depressive disorder patients. J Clin Psychiatry 2001;62(3):212-6.
Dr. Pies is clinical professor of psychiatry, Tufts University School of Medicine, Boston, MA.
Despite much education and research, bipolar disorder is still under-recognized and inappropriately treated in many clinical settings.1 Bipolar and unipolar depression display similar symptoms, making correct diagnosis difficult. The differential diagnosis is especially problematic in patients suffering a first major depressive episode, when there is no clear history of mania or hypomania.
Nevertheless, bipolar depression does have telltale signs—remembered with the mnemonic WHIPLASHED—to guide diagnosis.2-8
Worse or “wired” when taking antidepressants. The patient complains of feeling “antsy” or being agitated or unable to sleep when taking traditional anti-depressants.
Look for numerous failed antidepressant trials, apparent tolerance to antidepressants that does not resolve with increased dose, and antidepressant-induced mania or mood cycle acceleration.
Hypomania, hyperthymic temperament, or mood swings in a patient’s history. Patients with hyperthymic temperament show persistent traits such as intense optimism, increased energy, reduced need for sleep, extroversion, and overconfidence.
Ask about periods of elevated mood or energy that might not fit formal DSM-IV-TR criteria for hypomania—such as episodes that last only a day or two. Mood lability in younger patients can be especially dramatic and poorly demarcated.
Irritable, hostile, or mixed features. Some patients show one or more hypomanic features, such as racing thoughts when depressed.
Psychomotor retardation appears more common in bipolar I depression than in unipolar major depression. Psychomotor agitation, however, is more likely in bipolar II than in unipolar major depression.
Loaded family history of mood swings, frank bipolar disorder, or affective illness. A family history of comorbid mood disorder and alcoholism may also point to bipolarity.
Abrupt onset and/or termination of depressive bouts or relatively brief episodes (
Seasonal or postpartum depression. “Winter-type” seasonal affective disorder—feeling depressed in the fall and winter, hypomanic in the spring—and postpartum psychosis have clinical and epidemiologic links with bipolar disorder.
Hyperphagia and hypersomnia—sometimes termed atypical features—are common in bipolar depression. Paradoxically, hypersomnia may co-exist with psychomotor agitation in bipolar II patients, resulting in so-called “sleepy speeders.”
Early age of onset. Major depression that appears before age 25—especially with psychotic features—may herald subsequent bipolarity.
Delusions, hallucinations, or other psychotic features are more common in bipolar than in unipolar depression.
Acknowledgment
The author thanks Nassir Ghaemi, MD, and Jim Phelps, MD, for suggesting modifications to the mnemonic.
Despite much education and research, bipolar disorder is still under-recognized and inappropriately treated in many clinical settings.1 Bipolar and unipolar depression display similar symptoms, making correct diagnosis difficult. The differential diagnosis is especially problematic in patients suffering a first major depressive episode, when there is no clear history of mania or hypomania.
Nevertheless, bipolar depression does have telltale signs—remembered with the mnemonic WHIPLASHED—to guide diagnosis.2-8
Worse or “wired” when taking antidepressants. The patient complains of feeling “antsy” or being agitated or unable to sleep when taking traditional anti-depressants.
Look for numerous failed antidepressant trials, apparent tolerance to antidepressants that does not resolve with increased dose, and antidepressant-induced mania or mood cycle acceleration.
Hypomania, hyperthymic temperament, or mood swings in a patient’s history. Patients with hyperthymic temperament show persistent traits such as intense optimism, increased energy, reduced need for sleep, extroversion, and overconfidence.
Ask about periods of elevated mood or energy that might not fit formal DSM-IV-TR criteria for hypomania—such as episodes that last only a day or two. Mood lability in younger patients can be especially dramatic and poorly demarcated.
Irritable, hostile, or mixed features. Some patients show one or more hypomanic features, such as racing thoughts when depressed.
Psychomotor retardation appears more common in bipolar I depression than in unipolar major depression. Psychomotor agitation, however, is more likely in bipolar II than in unipolar major depression.
Loaded family history of mood swings, frank bipolar disorder, or affective illness. A family history of comorbid mood disorder and alcoholism may also point to bipolarity.
Abrupt onset and/or termination of depressive bouts or relatively brief episodes (
Seasonal or postpartum depression. “Winter-type” seasonal affective disorder—feeling depressed in the fall and winter, hypomanic in the spring—and postpartum psychosis have clinical and epidemiologic links with bipolar disorder.
Hyperphagia and hypersomnia—sometimes termed atypical features—are common in bipolar depression. Paradoxically, hypersomnia may co-exist with psychomotor agitation in bipolar II patients, resulting in so-called “sleepy speeders.”
Early age of onset. Major depression that appears before age 25—especially with psychotic features—may herald subsequent bipolarity.
Delusions, hallucinations, or other psychotic features are more common in bipolar than in unipolar depression.
Acknowledgment
The author thanks Nassir Ghaemi, MD, and Jim Phelps, MD, for suggesting modifications to the mnemonic.
1. Phelps JR, Ghaemi SN. Improving the diagnosis of bipolar disorder: predictive value of screening tests. J Affect Disord 2006;92(2-3):141-8.
2. Thase ME. Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry 2005;13(5):257-71.
3. Benazzi F, Akiskal H. Irritable-hostile depression: further validation as a bipolar depressive mixed state. J Affect Disord 2005;84(2-3):197-207.
4. Pies R. The “softer” end of the bipolar spectrum. J Psychiatr Pract 2002;8(4):189-95.
5. Albanese MJ, Pies R. The bipolar patient with comorbid substance use disorder: recognition and management. CNS Drugs 2004;18(9):585-96.
6. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-92.
7. Hantouche EG, Akiskal HS. Bipolar II vs. unipolar depression: psychopathologic differentiation by dimensional measures. J Affect Disord 2005;84(2-3):127-32.
8. Mitchell PB, Wilhelm K, Parker G, et al. The clinical features of bipolar depression: a comparison with matched major depressive disorder patients. J Clin Psychiatry 2001;62(3):212-6.
Dr. Pies is clinical professor of psychiatry, Tufts University School of Medicine, Boston, MA.
1. Phelps JR, Ghaemi SN. Improving the diagnosis of bipolar disorder: predictive value of screening tests. J Affect Disord 2006;92(2-3):141-8.
2. Thase ME. Bipolar depression: issues in diagnosis and treatment. Harv Rev Psychiatry 2005;13(5):257-71.
3. Benazzi F, Akiskal H. Irritable-hostile depression: further validation as a bipolar depressive mixed state. J Affect Disord 2005;84(2-3):197-207.
4. Pies R. The “softer” end of the bipolar spectrum. J Psychiatr Pract 2002;8(4):189-95.
5. Albanese MJ, Pies R. The bipolar patient with comorbid substance use disorder: recognition and management. CNS Drugs 2004;18(9):585-96.
6. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-92.
7. Hantouche EG, Akiskal HS. Bipolar II vs. unipolar depression: psychopathologic differentiation by dimensional measures. J Affect Disord 2005;84(2-3):127-32.
8. Mitchell PB, Wilhelm K, Parker G, et al. The clinical features of bipolar depression: a comparison with matched major depressive disorder patients. J Clin Psychiatry 2001;62(3):212-6.
Dr. Pies is clinical professor of psychiatry, Tufts University School of Medicine, Boston, MA.
How dopamine drives cocaine craving
Fighting cravings’ intense desire and obsessive thinking may be an addict’s most formidable challenge.1 Patients in recovery—desperate to stop abusing the substance—cannot control themselves after the craving is triggered. Remarkably, even after years of abstinence, cues reminding the addict of the substance—smells, sounds, or familiar surroundings—can ignite cravings and lead to relapse.
Dopamine and dope
A recent imaging study suggests that dopamine may be the culprit behind cravings. Research with cocaine and rodents suggests that dopamine released in the dorsal striatum is associated with drug-seeking behavior. Measuring craving in a rodent is impossible, but a recent imaging study examined how drug cues affect the brains of drug-addicted humans (Figure 1).2
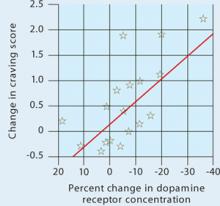
Figure 1 Dopamine increase is associated with drug craving
Changes in craving, as measured by Cocaine Craving Questionnaire scores, correlated with increased dopamine concentration in the putamen and caudate.
Source: Reference 2Volkow et al2 injected 18 cocaine-addicted patients with a dopamine D2 ligand that competes with endogenous dopamine and can be seen on positron emission tomography (PET). PET scans were then taken while each patient viewed a video of nature scenery (control) and then while watching scenes of drug preparation and simulated crack cocaine smoking.
When the control scan was subtracted from the cocaine-cued scan, the dorsal striatum—activated by the cocaine preparation cues—stood out (Figure 2), suggesting the neurobiological mechanisms responsible for craving.
The dorsal striatum is thought to be involved with selecting and initiating actions. In this study, the cocaine video caused a release of dopamine into the dorsal striatum and a desire for the drug. In an earlier study, hungry subjects who were shown food cues also showed increased dopamine activity in the dorsal striatum in association with a desire for food.3
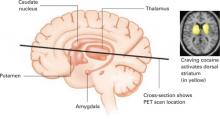
Figure 2 Dopamine release in the dorsal striatum is linked with craving
When cocaine-addicted patients watched a video depicting drug preparation and simulated crack cocaine smoking, PET scans of their brains showed dopamine release in the dorsal striatum.
Source: Reference 2Taken together, these studies suggest that dopamine in the dorsal striatum mediates craving for a desired object. The primary source of this neurotransmitter in the dorsal striatum is dopamine cells in the substantia nigra. The visual stimulus must activate these neurons in the substantia nigra to induce craving.
Caving into cravings
Desire precedes action and motivates behavior necessary for survival. Cocaine addiction apparently usurps the neurobiological mechanisms that motivate individuals to seek sustenance.
Developing an effective treatment for cocaine craving is a high priority at the National Institute on Drug Abuse.4 Medications including modafinil, propranolol, and disulfiram have been found effective for cocaine addiction in randomized, controlled trials, although none are FDA-approved for this use.4
One could speculate that antipsychotics—which are potent dopamine receptor blockers—might calm the cravings associated with cocaine addiction. Unfortunately, it is not that simple. Older antipsychotics might increase substance use in patients with schizophrenia and substance abuse.5 However, compelling evidence suggests that clozapine can reduce drug and alcohol use in dually diagnosed patients with schizophrenia.6 This provides some hope that the newer antipsychotic medications could provide a broad spectrum of pharmacologic activity that has the capacity to cool off cravings that stimulate drug-seeking behavior.
Drug brand names
- Clozapine • Clozaril
- Disulfiram • Antabuse
- Modafinil • Provigil
- Propranolol • Inderal
1. Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 2005;5(1):9-19.
2. Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 2006;26(24):6583-8.
3. Volkow ND, Wang GJ, Fowler JS, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002;44(3):175-80.
4. O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry 2005;162(8):1423-31.
5. Green AI. Treatment of schizophrenia and comorbid substance abuse: pharmacologic approaches. J Clin Psychiatry 2006;67(suppl7):31-5.
6. Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 2000;26(2):441-9.
Fighting cravings’ intense desire and obsessive thinking may be an addict’s most formidable challenge.1 Patients in recovery—desperate to stop abusing the substance—cannot control themselves after the craving is triggered. Remarkably, even after years of abstinence, cues reminding the addict of the substance—smells, sounds, or familiar surroundings—can ignite cravings and lead to relapse.
Dopamine and dope
A recent imaging study suggests that dopamine may be the culprit behind cravings. Research with cocaine and rodents suggests that dopamine released in the dorsal striatum is associated with drug-seeking behavior. Measuring craving in a rodent is impossible, but a recent imaging study examined how drug cues affect the brains of drug-addicted humans (Figure 1).2
Figure 1 Dopamine increase is associated with drug craving
Changes in craving, as measured by Cocaine Craving Questionnaire scores, correlated with increased dopamine concentration in the putamen and caudate.
Source: Reference 2Volkow et al2 injected 18 cocaine-addicted patients with a dopamine D2 ligand that competes with endogenous dopamine and can be seen on positron emission tomography (PET). PET scans were then taken while each patient viewed a video of nature scenery (control) and then while watching scenes of drug preparation and simulated crack cocaine smoking.
When the control scan was subtracted from the cocaine-cued scan, the dorsal striatum—activated by the cocaine preparation cues—stood out (Figure 2), suggesting the neurobiological mechanisms responsible for craving.
The dorsal striatum is thought to be involved with selecting and initiating actions. In this study, the cocaine video caused a release of dopamine into the dorsal striatum and a desire for the drug. In an earlier study, hungry subjects who were shown food cues also showed increased dopamine activity in the dorsal striatum in association with a desire for food.3
Figure 2 Dopamine release in the dorsal striatum is linked with craving
When cocaine-addicted patients watched a video depicting drug preparation and simulated crack cocaine smoking, PET scans of their brains showed dopamine release in the dorsal striatum.
Source: Reference 2Taken together, these studies suggest that dopamine in the dorsal striatum mediates craving for a desired object. The primary source of this neurotransmitter in the dorsal striatum is dopamine cells in the substantia nigra. The visual stimulus must activate these neurons in the substantia nigra to induce craving.
Caving into cravings
Desire precedes action and motivates behavior necessary for survival. Cocaine addiction apparently usurps the neurobiological mechanisms that motivate individuals to seek sustenance.
Developing an effective treatment for cocaine craving is a high priority at the National Institute on Drug Abuse.4 Medications including modafinil, propranolol, and disulfiram have been found effective for cocaine addiction in randomized, controlled trials, although none are FDA-approved for this use.4
One could speculate that antipsychotics—which are potent dopamine receptor blockers—might calm the cravings associated with cocaine addiction. Unfortunately, it is not that simple. Older antipsychotics might increase substance use in patients with schizophrenia and substance abuse.5 However, compelling evidence suggests that clozapine can reduce drug and alcohol use in dually diagnosed patients with schizophrenia.6 This provides some hope that the newer antipsychotic medications could provide a broad spectrum of pharmacologic activity that has the capacity to cool off cravings that stimulate drug-seeking behavior.
Drug brand names
- Clozapine • Clozaril
- Disulfiram • Antabuse
- Modafinil • Provigil
- Propranolol • Inderal
Fighting cravings’ intense desire and obsessive thinking may be an addict’s most formidable challenge.1 Patients in recovery—desperate to stop abusing the substance—cannot control themselves after the craving is triggered. Remarkably, even after years of abstinence, cues reminding the addict of the substance—smells, sounds, or familiar surroundings—can ignite cravings and lead to relapse.
Dopamine and dope
A recent imaging study suggests that dopamine may be the culprit behind cravings. Research with cocaine and rodents suggests that dopamine released in the dorsal striatum is associated with drug-seeking behavior. Measuring craving in a rodent is impossible, but a recent imaging study examined how drug cues affect the brains of drug-addicted humans (Figure 1).2
Figure 1 Dopamine increase is associated with drug craving
Changes in craving, as measured by Cocaine Craving Questionnaire scores, correlated with increased dopamine concentration in the putamen and caudate.
Source: Reference 2Volkow et al2 injected 18 cocaine-addicted patients with a dopamine D2 ligand that competes with endogenous dopamine and can be seen on positron emission tomography (PET). PET scans were then taken while each patient viewed a video of nature scenery (control) and then while watching scenes of drug preparation and simulated crack cocaine smoking.
When the control scan was subtracted from the cocaine-cued scan, the dorsal striatum—activated by the cocaine preparation cues—stood out (Figure 2), suggesting the neurobiological mechanisms responsible for craving.
The dorsal striatum is thought to be involved with selecting and initiating actions. In this study, the cocaine video caused a release of dopamine into the dorsal striatum and a desire for the drug. In an earlier study, hungry subjects who were shown food cues also showed increased dopamine activity in the dorsal striatum in association with a desire for food.3
Figure 2 Dopamine release in the dorsal striatum is linked with craving
When cocaine-addicted patients watched a video depicting drug preparation and simulated crack cocaine smoking, PET scans of their brains showed dopamine release in the dorsal striatum.
Source: Reference 2Taken together, these studies suggest that dopamine in the dorsal striatum mediates craving for a desired object. The primary source of this neurotransmitter in the dorsal striatum is dopamine cells in the substantia nigra. The visual stimulus must activate these neurons in the substantia nigra to induce craving.
Caving into cravings
Desire precedes action and motivates behavior necessary for survival. Cocaine addiction apparently usurps the neurobiological mechanisms that motivate individuals to seek sustenance.
Developing an effective treatment for cocaine craving is a high priority at the National Institute on Drug Abuse.4 Medications including modafinil, propranolol, and disulfiram have been found effective for cocaine addiction in randomized, controlled trials, although none are FDA-approved for this use.4
One could speculate that antipsychotics—which are potent dopamine receptor blockers—might calm the cravings associated with cocaine addiction. Unfortunately, it is not that simple. Older antipsychotics might increase substance use in patients with schizophrenia and substance abuse.5 However, compelling evidence suggests that clozapine can reduce drug and alcohol use in dually diagnosed patients with schizophrenia.6 This provides some hope that the newer antipsychotic medications could provide a broad spectrum of pharmacologic activity that has the capacity to cool off cravings that stimulate drug-seeking behavior.
Drug brand names
- Clozapine • Clozaril
- Disulfiram • Antabuse
- Modafinil • Provigil
- Propranolol • Inderal
1. Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 2005;5(1):9-19.
2. Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 2006;26(24):6583-8.
3. Volkow ND, Wang GJ, Fowler JS, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002;44(3):175-80.
4. O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry 2005;162(8):1423-31.
5. Green AI. Treatment of schizophrenia and comorbid substance abuse: pharmacologic approaches. J Clin Psychiatry 2006;67(suppl7):31-5.
6. Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 2000;26(2):441-9.
1. Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 2005;5(1):9-19.
2. Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 2006;26(24):6583-8.
3. Volkow ND, Wang GJ, Fowler JS, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 2002;44(3):175-80.
4. O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry 2005;162(8):1423-31.
5. Green AI. Treatment of schizophrenia and comorbid substance abuse: pharmacologic approaches. J Clin Psychiatry 2006;67(suppl7):31-5.
6. Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 2000;26(2):441-9.
DO MALPRACTICE SAFEGUARDS WORK?
Regarding “To protect and serve: Psychiatrists’ duty to patients” (Malpractice Verdicts, Current Psychiatry, December 2006), Dr. Jon Grant missed the significance of the cited case.
The case is in appeal; many of the “unavailable facts” referred to actually strongly support the defendant’s position that no negligence or malpractice occurred.
Unfortunately, regardless of how well one follows the safeguards listed in the article, the nature of our tort system renders the facts presented in court irrelevant in the face of a jury’s emotional reaction.
The article fosters a potentially harmful idea that if the clinician is careful, he can avoid losing in court. Unless the medical community and the community at large confront how issues like this are resolved in court, we will continue to see larger awards based on spurious arguments.
Paul P. Shultz, ACSW
Farmington Hills, MI
Dr. Grant responds
Considerable research has addressed the tort system and whether juries or bench trials result in different outcomes. This research suggests that juries usually are not emotional and malpractice verdicts often are worse for defendants during a bench trial. I refer the author to: Deborah JM, Barry KA. Is the tort system in crisis? New empirical evidence, 60 Ohio St LJ 315 (1999) and Clermont KM, Eisenberg T. Trial by jury or judge: transcending empiricism, 77 Cornell L Rev 1124 (1992).
Because one cannot predict jury or bench trial outcomes and tort reform is a complex topic, clinicians should continue to document fully and properly their diagnosis and treatment.
Jon E. Grant, JD, MD, MPH
Associate professor of psychiatry
University of Minnesota Medical Center
Minneapolis
Regarding “To protect and serve: Psychiatrists’ duty to patients” (Malpractice Verdicts, Current Psychiatry, December 2006), Dr. Jon Grant missed the significance of the cited case.
The case is in appeal; many of the “unavailable facts” referred to actually strongly support the defendant’s position that no negligence or malpractice occurred.
Unfortunately, regardless of how well one follows the safeguards listed in the article, the nature of our tort system renders the facts presented in court irrelevant in the face of a jury’s emotional reaction.
The article fosters a potentially harmful idea that if the clinician is careful, he can avoid losing in court. Unless the medical community and the community at large confront how issues like this are resolved in court, we will continue to see larger awards based on spurious arguments.
Paul P. Shultz, ACSW
Farmington Hills, MI
Dr. Grant responds
Considerable research has addressed the tort system and whether juries or bench trials result in different outcomes. This research suggests that juries usually are not emotional and malpractice verdicts often are worse for defendants during a bench trial. I refer the author to: Deborah JM, Barry KA. Is the tort system in crisis? New empirical evidence, 60 Ohio St LJ 315 (1999) and Clermont KM, Eisenberg T. Trial by jury or judge: transcending empiricism, 77 Cornell L Rev 1124 (1992).
Because one cannot predict jury or bench trial outcomes and tort reform is a complex topic, clinicians should continue to document fully and properly their diagnosis and treatment.
Jon E. Grant, JD, MD, MPH
Associate professor of psychiatry
University of Minnesota Medical Center
Minneapolis
Regarding “To protect and serve: Psychiatrists’ duty to patients” (Malpractice Verdicts, Current Psychiatry, December 2006), Dr. Jon Grant missed the significance of the cited case.
The case is in appeal; many of the “unavailable facts” referred to actually strongly support the defendant’s position that no negligence or malpractice occurred.
Unfortunately, regardless of how well one follows the safeguards listed in the article, the nature of our tort system renders the facts presented in court irrelevant in the face of a jury’s emotional reaction.
The article fosters a potentially harmful idea that if the clinician is careful, he can avoid losing in court. Unless the medical community and the community at large confront how issues like this are resolved in court, we will continue to see larger awards based on spurious arguments.
Paul P. Shultz, ACSW
Farmington Hills, MI
Dr. Grant responds
Considerable research has addressed the tort system and whether juries or bench trials result in different outcomes. This research suggests that juries usually are not emotional and malpractice verdicts often are worse for defendants during a bench trial. I refer the author to: Deborah JM, Barry KA. Is the tort system in crisis? New empirical evidence, 60 Ohio St LJ 315 (1999) and Clermont KM, Eisenberg T. Trial by jury or judge: transcending empiricism, 77 Cornell L Rev 1124 (1992).
Because one cannot predict jury or bench trial outcomes and tort reform is a complex topic, clinicians should continue to document fully and properly their diagnosis and treatment.
Jon E. Grant, JD, MD, MPH
Associate professor of psychiatry
University of Minnesota Medical Center
Minneapolis
DANGERS OF STIMULANT PATCH MISUSE
In “New warning on stimulants for ADHD: Cause for alarm?” (Current Psychiatry, October 2006), Drs. Lenard Adler and Anthony Rostain discuss new FDA warnings on stimulant use in ADHD. However, the development of a transdermal form of methylphenidate raises issues about potential toxicity.
Transdermal methylphenidate is designed to produce safe, sustained levels of medication when used as indicated. However, chewing, sucking, or swallowing the patch can lead to sudden, potentially toxic increases in methylphenidate levels.1 Even repositioning or reattaching a patch that has come off or scratching a patch that itches can disrupt the control membrane and cause increased and unregulated exposure to the highly concentrated drug.2,3
Although the likelihood of such misuse might be remote, this risk depends on the patient’s and his peer group’s knowledge about the dangers of patch misuse. Just telling the patient about the risks of misuse could inspire him or her to intentionally misuse the patch. Yet ethics and the law require full disclosure of these risks to the patient and parents of minor patients. In the case of children, the school should be informed as well.
Physicians need to know when not to prescribe transdermal stimulant medication. Transdermal patch misuse is expected to be higher in those with substance abuse or suicidal impulses and in highly impulsive or oppositional patients. Also be careful when using transdermal medications in inpatient psychiatric units or on residential substance abuse units where unpredictable behaviors might be magnified. At home, small children might find and ingest used patches discarded by another family member.2
Used transdermal patches may retain a large portion of the drug and are a potential source of abuse.3 For example, the amount of residual drug in a clonidine patch varies from 20% to 70% even after 7 days of use.3
When a person swallows a transdermal patch, exposure to the drug reservoir may be maximized and the situation may become more urgent. An ICU technique used in children called whole bowel irrigation might help reduce toxicity by expediting the patch’s movement through the bowel.3
A. Preston West, MD
Staff psychiatrist
West Los Angeles Veterans Affairs Health Care Center
Los Angeles
1. Harris JM. Clonidine patch toxicity. DICP 1990;24(12):1191-3.
2. Broderick-Cantwell JJ. Case study: accidental clonidine patch overdose in attention deficit/hyperactivity disorder patients. J Am Acad Child Adolesc Psychiatry 1999;38(1):95-8.
3. Horowitz R, Mazor SS, Aks SE, Leikin JB. Accidental clonidine patch ingestion in a child. Am J Ther 2005;12:272-4.
In “New warning on stimulants for ADHD: Cause for alarm?” (Current Psychiatry, October 2006), Drs. Lenard Adler and Anthony Rostain discuss new FDA warnings on stimulant use in ADHD. However, the development of a transdermal form of methylphenidate raises issues about potential toxicity.
Transdermal methylphenidate is designed to produce safe, sustained levels of medication when used as indicated. However, chewing, sucking, or swallowing the patch can lead to sudden, potentially toxic increases in methylphenidate levels.1 Even repositioning or reattaching a patch that has come off or scratching a patch that itches can disrupt the control membrane and cause increased and unregulated exposure to the highly concentrated drug.2,3
Although the likelihood of such misuse might be remote, this risk depends on the patient’s and his peer group’s knowledge about the dangers of patch misuse. Just telling the patient about the risks of misuse could inspire him or her to intentionally misuse the patch. Yet ethics and the law require full disclosure of these risks to the patient and parents of minor patients. In the case of children, the school should be informed as well.
Physicians need to know when not to prescribe transdermal stimulant medication. Transdermal patch misuse is expected to be higher in those with substance abuse or suicidal impulses and in highly impulsive or oppositional patients. Also be careful when using transdermal medications in inpatient psychiatric units or on residential substance abuse units where unpredictable behaviors might be magnified. At home, small children might find and ingest used patches discarded by another family member.2
Used transdermal patches may retain a large portion of the drug and are a potential source of abuse.3 For example, the amount of residual drug in a clonidine patch varies from 20% to 70% even after 7 days of use.3
When a person swallows a transdermal patch, exposure to the drug reservoir may be maximized and the situation may become more urgent. An ICU technique used in children called whole bowel irrigation might help reduce toxicity by expediting the patch’s movement through the bowel.3
A. Preston West, MD
Staff psychiatrist
West Los Angeles Veterans Affairs Health Care Center
Los Angeles
In “New warning on stimulants for ADHD: Cause for alarm?” (Current Psychiatry, October 2006), Drs. Lenard Adler and Anthony Rostain discuss new FDA warnings on stimulant use in ADHD. However, the development of a transdermal form of methylphenidate raises issues about potential toxicity.
Transdermal methylphenidate is designed to produce safe, sustained levels of medication when used as indicated. However, chewing, sucking, or swallowing the patch can lead to sudden, potentially toxic increases in methylphenidate levels.1 Even repositioning or reattaching a patch that has come off or scratching a patch that itches can disrupt the control membrane and cause increased and unregulated exposure to the highly concentrated drug.2,3
Although the likelihood of such misuse might be remote, this risk depends on the patient’s and his peer group’s knowledge about the dangers of patch misuse. Just telling the patient about the risks of misuse could inspire him or her to intentionally misuse the patch. Yet ethics and the law require full disclosure of these risks to the patient and parents of minor patients. In the case of children, the school should be informed as well.
Physicians need to know when not to prescribe transdermal stimulant medication. Transdermal patch misuse is expected to be higher in those with substance abuse or suicidal impulses and in highly impulsive or oppositional patients. Also be careful when using transdermal medications in inpatient psychiatric units or on residential substance abuse units where unpredictable behaviors might be magnified. At home, small children might find and ingest used patches discarded by another family member.2
Used transdermal patches may retain a large portion of the drug and are a potential source of abuse.3 For example, the amount of residual drug in a clonidine patch varies from 20% to 70% even after 7 days of use.3
When a person swallows a transdermal patch, exposure to the drug reservoir may be maximized and the situation may become more urgent. An ICU technique used in children called whole bowel irrigation might help reduce toxicity by expediting the patch’s movement through the bowel.3
A. Preston West, MD
Staff psychiatrist
West Los Angeles Veterans Affairs Health Care Center
Los Angeles
1. Harris JM. Clonidine patch toxicity. DICP 1990;24(12):1191-3.
2. Broderick-Cantwell JJ. Case study: accidental clonidine patch overdose in attention deficit/hyperactivity disorder patients. J Am Acad Child Adolesc Psychiatry 1999;38(1):95-8.
3. Horowitz R, Mazor SS, Aks SE, Leikin JB. Accidental clonidine patch ingestion in a child. Am J Ther 2005;12:272-4.
1. Harris JM. Clonidine patch toxicity. DICP 1990;24(12):1191-3.
2. Broderick-Cantwell JJ. Case study: accidental clonidine patch overdose in attention deficit/hyperactivity disorder patients. J Am Acad Child Adolesc Psychiatry 1999;38(1):95-8.
3. Horowitz R, Mazor SS, Aks SE, Leikin JB. Accidental clonidine patch ingestion in a child. Am J Ther 2005;12:272-4.
ANTIPSYCHOTICS FOR DELIRIUM
In “Antipsychotics for patients without psychosis?” (Current Psychiatry, December 2006) Drs. Fabien Trémeau and Leslie Citrome discuss use of second-generation antipsychotics (SGAs) for nonpsychotic illnesses. We would like to address another area of successful clinical antipsychotic use that is not supported by well-designed studies.
Delirium is a neuropsychiatric disorder of abrupt onset and fluctuating course, with disturbances in consciousness, attention, cognition, and perception. The prevalence of psychotic symptoms ranges from 3% to 67.9% in delirium patients; these symptoms are less likely to be seen in the hypoactive subtype.1
Antipsychotics are widely used to treat delirium presenting with or without psychotic symptoms, despite lack of FDA approval for this indication. Studies on use of SGAs for delirium are limited to case reports, retrospective studies, and open-label trials2—none of which differentiate between delirium subtypes and phenomenology.
Double-blind, randomized studies are needed to assess the efficacy and safety of SGAs in delirium and assist the clinician’s evidence-based decision making.
Yesne Alici Evcimen, MD
William S. Breitbart, MD
Department of psychiatry and behavioral sciences
Memorial Sloan-Kettering Cancer Center
New York
In “Antipsychotics for patients without psychosis?” (Current Psychiatry, December 2006) Drs. Fabien Trémeau and Leslie Citrome discuss use of second-generation antipsychotics (SGAs) for nonpsychotic illnesses. We would like to address another area of successful clinical antipsychotic use that is not supported by well-designed studies.
Delirium is a neuropsychiatric disorder of abrupt onset and fluctuating course, with disturbances in consciousness, attention, cognition, and perception. The prevalence of psychotic symptoms ranges from 3% to 67.9% in delirium patients; these symptoms are less likely to be seen in the hypoactive subtype.1
Antipsychotics are widely used to treat delirium presenting with or without psychotic symptoms, despite lack of FDA approval for this indication. Studies on use of SGAs for delirium are limited to case reports, retrospective studies, and open-label trials2—none of which differentiate between delirium subtypes and phenomenology.
Double-blind, randomized studies are needed to assess the efficacy and safety of SGAs in delirium and assist the clinician’s evidence-based decision making.
Yesne Alici Evcimen, MD
William S. Breitbart, MD
Department of psychiatry and behavioral sciences
Memorial Sloan-Kettering Cancer Center
New York
In “Antipsychotics for patients without psychosis?” (Current Psychiatry, December 2006) Drs. Fabien Trémeau and Leslie Citrome discuss use of second-generation antipsychotics (SGAs) for nonpsychotic illnesses. We would like to address another area of successful clinical antipsychotic use that is not supported by well-designed studies.
Delirium is a neuropsychiatric disorder of abrupt onset and fluctuating course, with disturbances in consciousness, attention, cognition, and perception. The prevalence of psychotic symptoms ranges from 3% to 67.9% in delirium patients; these symptoms are less likely to be seen in the hypoactive subtype.1
Antipsychotics are widely used to treat delirium presenting with or without psychotic symptoms, despite lack of FDA approval for this indication. Studies on use of SGAs for delirium are limited to case reports, retrospective studies, and open-label trials2—none of which differentiate between delirium subtypes and phenomenology.
Double-blind, randomized studies are needed to assess the efficacy and safety of SGAs in delirium and assist the clinician’s evidence-based decision making.
Yesne Alici Evcimen, MD
William S. Breitbart, MD
Department of psychiatry and behavioral sciences
Memorial Sloan-Kettering Cancer Center
New York
TRANSSEXUALISM TX: WHY NOW?
“Gender dysphoria: ‘I’m a man, but…,’” offers an excellent overview of gender identity disorder (Current Psychiatry, December 2006).
However, in my 10 plus years as medical director in a clinic specializing in gender disorders, I’d be sure to ask Mr. C, “Why are you coming to us now?” I would want to know why he brought the problem to the endocrinologist at that time and why he wants to see a psychiatrist after struggling with gender identity for so many years. These answers might help confirm the diagnosis and guide treatment, but the author never addresses this issue.
I question the statement, “Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home.” Given he is 65 and happily married, a limited change might be satisfactory. Moreover, using hormones often enhances transsexuals’ mental well-being.
I’m not sure what to make of his later emerging hypomania. Was it present when he first sought help? Has the aripiprazole prescribed by the primary psychiatrist lessened his gender concerns? If so, then the primary transsexualism diagnosis is questionable and hormone therapy is not indicated.
H. Steven Moffic, MD
Professor of psychiatry and behavioral medicine
Medical College of Wisconsin
Milwaukee
Dr. Martin responds
There are no easy answers when treating these individuals, and much depends on the clinician’s comfort level with the stated problem. I agree that, for many, hormones can improve their well-being. For Mr. C, however, the transvestism seemed to be the overriding factor, and I did not feel comfortable prescribing hormones for a paraphilia after one consultation. I referred the patient to a gender therapist to explore the issue further.
Dr. Moffic’s question regarding why the patient is seeking treatment late in life is a worthy one. When I asked Mr. C he could only say he was finally comfortable bringing the problem out in the open.
Mr. C’s gender issues did not subside with aripiprazole, and the intensity of his gender concerns showed no clear relationship to his underlying cycling disorder. No symptoms of mania were evident when I first met with him.
Kari Ann Martin, MD
Instructor of psychiatry
Department of psychiatry and psychology
Mayo Clinic
Scottsdale, AZ
“Gender dysphoria: ‘I’m a man, but…,’” offers an excellent overview of gender identity disorder (Current Psychiatry, December 2006).
However, in my 10 plus years as medical director in a clinic specializing in gender disorders, I’d be sure to ask Mr. C, “Why are you coming to us now?” I would want to know why he brought the problem to the endocrinologist at that time and why he wants to see a psychiatrist after struggling with gender identity for so many years. These answers might help confirm the diagnosis and guide treatment, but the author never addresses this issue.
I question the statement, “Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home.” Given he is 65 and happily married, a limited change might be satisfactory. Moreover, using hormones often enhances transsexuals’ mental well-being.
I’m not sure what to make of his later emerging hypomania. Was it present when he first sought help? Has the aripiprazole prescribed by the primary psychiatrist lessened his gender concerns? If so, then the primary transsexualism diagnosis is questionable and hormone therapy is not indicated.
H. Steven Moffic, MD
Professor of psychiatry and behavioral medicine
Medical College of Wisconsin
Milwaukee
Dr. Martin responds
There are no easy answers when treating these individuals, and much depends on the clinician’s comfort level with the stated problem. I agree that, for many, hormones can improve their well-being. For Mr. C, however, the transvestism seemed to be the overriding factor, and I did not feel comfortable prescribing hormones for a paraphilia after one consultation. I referred the patient to a gender therapist to explore the issue further.
Dr. Moffic’s question regarding why the patient is seeking treatment late in life is a worthy one. When I asked Mr. C he could only say he was finally comfortable bringing the problem out in the open.
Mr. C’s gender issues did not subside with aripiprazole, and the intensity of his gender concerns showed no clear relationship to his underlying cycling disorder. No symptoms of mania were evident when I first met with him.
Kari Ann Martin, MD
Instructor of psychiatry
Department of psychiatry and psychology
Mayo Clinic
Scottsdale, AZ
“Gender dysphoria: ‘I’m a man, but…,’” offers an excellent overview of gender identity disorder (Current Psychiatry, December 2006).
However, in my 10 plus years as medical director in a clinic specializing in gender disorders, I’d be sure to ask Mr. C, “Why are you coming to us now?” I would want to know why he brought the problem to the endocrinologist at that time and why he wants to see a psychiatrist after struggling with gender identity for so many years. These answers might help confirm the diagnosis and guide treatment, but the author never addresses this issue.
I question the statement, “Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home.” Given he is 65 and happily married, a limited change might be satisfactory. Moreover, using hormones often enhances transsexuals’ mental well-being.
I’m not sure what to make of his later emerging hypomania. Was it present when he first sought help? Has the aripiprazole prescribed by the primary psychiatrist lessened his gender concerns? If so, then the primary transsexualism diagnosis is questionable and hormone therapy is not indicated.
H. Steven Moffic, MD
Professor of psychiatry and behavioral medicine
Medical College of Wisconsin
Milwaukee
Dr. Martin responds
There are no easy answers when treating these individuals, and much depends on the clinician’s comfort level with the stated problem. I agree that, for many, hormones can improve their well-being. For Mr. C, however, the transvestism seemed to be the overriding factor, and I did not feel comfortable prescribing hormones for a paraphilia after one consultation. I referred the patient to a gender therapist to explore the issue further.
Dr. Moffic’s question regarding why the patient is seeking treatment late in life is a worthy one. When I asked Mr. C he could only say he was finally comfortable bringing the problem out in the open.
Mr. C’s gender issues did not subside with aripiprazole, and the intensity of his gender concerns showed no clear relationship to his underlying cycling disorder. No symptoms of mania were evident when I first met with him.
Kari Ann Martin, MD
Instructor of psychiatry
Department of psychiatry and psychology
Mayo Clinic
Scottsdale, AZ
Off-label prescribing
“Off-label” may evoke an uncomfortable sense of therapeutic mischief, yet the term describes a vital evolution of scientific discovery in pharmacotherapy. Because no FDA-approved drugs are available for many psychiatric disorders, patients would suffer needlessly if psychotropics were not used off-label.
The Agency for Healthcare Research and Quality recently reported on the “Efficacy and comparative effectiveness of off-label use of atypical antipsychotics.”1 Its findings confirm other published studies of the wide-spread off-label uses of second-generation antipsychotics (SGAs). In Georgia’s Medicaid system, for example, a large proportion of antipsychotics, antidepressants, and mood stabilizers are prescribed off-label.2
Clinicians, in fact, use psychotropics off-label for many legitimate reasons, including:
No other options. In a recent study,3 we found that only 12% of DSM-IV-TR categories have an approved drug, leaving 88% of psychiatric disorders with no “official” pharmacologic treatment. Obviously, compassionate practitioners use whatever is available to alleviate the suffering of the many psychiatric patients for whom no drug has been approved.
Through trial and error over time, clinicians have found multiple uses for SGAs and other psychotropics in many symptoms or diagnoses. Clinicians have engaged in this necessary innovative process for years—even decades—before some diagnostic categories eventually obtained an FDA-approved drug.
In my opinion, this process is vital to the scientific “discovery” process that precedes controlled clinical trials that ultimately confirm what clinicians have collectively observed. It also is a vital scientific partnership between clinicians who generate hypotheses about additional drug efficacies and researchers who test these hypotheses to produce evidence-based findings.
‘Real-world’ clinical issues. On-label psychotropic use is supported by short-term studies of very “clean” samples of patients, who often are not representative of community-based practice. When the drug is launched in the “real world,” however, it is used in much more complicated patients who may be treatment-resistant and have comorbid medical or psychiatric disorders or substance abuse.
Clinicians often find that a higher (off-label) dose can be more effective for real-world patients than the lower doses that worked in FDA-required pre-approval trials. Thus, off-label use of a high-dose SGA may have better efficacy in some patients than the narrow range of approved dosages.
Maintenance therapy dilemmas. Years may pass before we see maintenance studies for an antipsychotic that has been approved for acute treatment of schizophrenia or mania. But clinicians are highly unlikely to discontinue that drug after a patient successfully responds within a few weeks. Thus, we essentially practice off-label psychopharmacology whenever we maintain a patient on a drug approved only for acute uses.
Combination therapies. No antipsychotic combinations are approved for schizophrenia, yet more than one-third of chronic schizophrenia patients in the United States are concurrently receiving 2 or more concurrent SGAs.4 Combining antipsychotics is often regarded as dubious off-label polypharmacy, yet clinicians stand by their observations that patients who do not improve with 1 drug may respond when another is added.
Although combination pharmacotherapy is not supported by credible evidence—controlled trials of 2 SGAs vs 1 combined with a placebo—clinicians again might be discovering options for treatment-resistant or refractory patients before FDA trials are conducted.
Simpler dosing for better adherence. A drug may be approved for twice-daily (bid) administration, yet clinicians might soon discover that prescribing it once daily (qd) is equally or even more effective because of improved patient adherence. Off-label dosing may be rational and even better than the official dose schedule, yet a drug company might never go through the costly process of repeating its clinical trial to demonstrate that bid and qd dosing are equivalent. Thus, practitioners will continue to use the drug off-label based on clinical experience, not on research data.
Scientific implications. Aside from advancing psychopharmacologic practices and discovering new treatments, off-label data also could shed light on a potential shared neurobiology among psychiatric disorders. Off-label prescribing ultimately might help us reconceptualize the overlapping neural pathways of several axis I and axis II disorders, all of which appear to be improved by the same pharmacologic agent such as an atypical antipsychotic. It might even prompt us to coin a new name for antipsychotics, such as “neurostabilizers.”
Write and tell me what term you would coin for a class of drugs with multiple psychiatric uses.
1. Shekele P, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of off-label use of atypical antipsychotics. Executive summary. No. 6 (AHRQ Pub. No. 07-EHC003-1). Rockville, MD: Agency for Healthcare Research and Quality; January 2007. Available at: http://effectivehealthcare.ahrq.gov/repFiles/Atypical_Executive_Summary.pdf. Accessed February 8, 2007.
2. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry 2006;67:972-82.
3. Devulapalli KK, Nasrallah HA. An analysis of current indications and common off-label uses of psychotropic medications. Submitted for publication.
IMS data, 2005.
“Off-label” may evoke an uncomfortable sense of therapeutic mischief, yet the term describes a vital evolution of scientific discovery in pharmacotherapy. Because no FDA-approved drugs are available for many psychiatric disorders, patients would suffer needlessly if psychotropics were not used off-label.
The Agency for Healthcare Research and Quality recently reported on the “Efficacy and comparative effectiveness of off-label use of atypical antipsychotics.”1 Its findings confirm other published studies of the wide-spread off-label uses of second-generation antipsychotics (SGAs). In Georgia’s Medicaid system, for example, a large proportion of antipsychotics, antidepressants, and mood stabilizers are prescribed off-label.2
Clinicians, in fact, use psychotropics off-label for many legitimate reasons, including:
No other options. In a recent study,3 we found that only 12% of DSM-IV-TR categories have an approved drug, leaving 88% of psychiatric disorders with no “official” pharmacologic treatment. Obviously, compassionate practitioners use whatever is available to alleviate the suffering of the many psychiatric patients for whom no drug has been approved.
Through trial and error over time, clinicians have found multiple uses for SGAs and other psychotropics in many symptoms or diagnoses. Clinicians have engaged in this necessary innovative process for years—even decades—before some diagnostic categories eventually obtained an FDA-approved drug.
In my opinion, this process is vital to the scientific “discovery” process that precedes controlled clinical trials that ultimately confirm what clinicians have collectively observed. It also is a vital scientific partnership between clinicians who generate hypotheses about additional drug efficacies and researchers who test these hypotheses to produce evidence-based findings.
‘Real-world’ clinical issues. On-label psychotropic use is supported by short-term studies of very “clean” samples of patients, who often are not representative of community-based practice. When the drug is launched in the “real world,” however, it is used in much more complicated patients who may be treatment-resistant and have comorbid medical or psychiatric disorders or substance abuse.
Clinicians often find that a higher (off-label) dose can be more effective for real-world patients than the lower doses that worked in FDA-required pre-approval trials. Thus, off-label use of a high-dose SGA may have better efficacy in some patients than the narrow range of approved dosages.
Maintenance therapy dilemmas. Years may pass before we see maintenance studies for an antipsychotic that has been approved for acute treatment of schizophrenia or mania. But clinicians are highly unlikely to discontinue that drug after a patient successfully responds within a few weeks. Thus, we essentially practice off-label psychopharmacology whenever we maintain a patient on a drug approved only for acute uses.
Combination therapies. No antipsychotic combinations are approved for schizophrenia, yet more than one-third of chronic schizophrenia patients in the United States are concurrently receiving 2 or more concurrent SGAs.4 Combining antipsychotics is often regarded as dubious off-label polypharmacy, yet clinicians stand by their observations that patients who do not improve with 1 drug may respond when another is added.
Although combination pharmacotherapy is not supported by credible evidence—controlled trials of 2 SGAs vs 1 combined with a placebo—clinicians again might be discovering options for treatment-resistant or refractory patients before FDA trials are conducted.
Simpler dosing for better adherence. A drug may be approved for twice-daily (bid) administration, yet clinicians might soon discover that prescribing it once daily (qd) is equally or even more effective because of improved patient adherence. Off-label dosing may be rational and even better than the official dose schedule, yet a drug company might never go through the costly process of repeating its clinical trial to demonstrate that bid and qd dosing are equivalent. Thus, practitioners will continue to use the drug off-label based on clinical experience, not on research data.
Scientific implications. Aside from advancing psychopharmacologic practices and discovering new treatments, off-label data also could shed light on a potential shared neurobiology among psychiatric disorders. Off-label prescribing ultimately might help us reconceptualize the overlapping neural pathways of several axis I and axis II disorders, all of which appear to be improved by the same pharmacologic agent such as an atypical antipsychotic. It might even prompt us to coin a new name for antipsychotics, such as “neurostabilizers.”
Write and tell me what term you would coin for a class of drugs with multiple psychiatric uses.
“Off-label” may evoke an uncomfortable sense of therapeutic mischief, yet the term describes a vital evolution of scientific discovery in pharmacotherapy. Because no FDA-approved drugs are available for many psychiatric disorders, patients would suffer needlessly if psychotropics were not used off-label.
The Agency for Healthcare Research and Quality recently reported on the “Efficacy and comparative effectiveness of off-label use of atypical antipsychotics.”1 Its findings confirm other published studies of the wide-spread off-label uses of second-generation antipsychotics (SGAs). In Georgia’s Medicaid system, for example, a large proportion of antipsychotics, antidepressants, and mood stabilizers are prescribed off-label.2
Clinicians, in fact, use psychotropics off-label for many legitimate reasons, including:
No other options. In a recent study,3 we found that only 12% of DSM-IV-TR categories have an approved drug, leaving 88% of psychiatric disorders with no “official” pharmacologic treatment. Obviously, compassionate practitioners use whatever is available to alleviate the suffering of the many psychiatric patients for whom no drug has been approved.
Through trial and error over time, clinicians have found multiple uses for SGAs and other psychotropics in many symptoms or diagnoses. Clinicians have engaged in this necessary innovative process for years—even decades—before some diagnostic categories eventually obtained an FDA-approved drug.
In my opinion, this process is vital to the scientific “discovery” process that precedes controlled clinical trials that ultimately confirm what clinicians have collectively observed. It also is a vital scientific partnership between clinicians who generate hypotheses about additional drug efficacies and researchers who test these hypotheses to produce evidence-based findings.
‘Real-world’ clinical issues. On-label psychotropic use is supported by short-term studies of very “clean” samples of patients, who often are not representative of community-based practice. When the drug is launched in the “real world,” however, it is used in much more complicated patients who may be treatment-resistant and have comorbid medical or psychiatric disorders or substance abuse.
Clinicians often find that a higher (off-label) dose can be more effective for real-world patients than the lower doses that worked in FDA-required pre-approval trials. Thus, off-label use of a high-dose SGA may have better efficacy in some patients than the narrow range of approved dosages.
Maintenance therapy dilemmas. Years may pass before we see maintenance studies for an antipsychotic that has been approved for acute treatment of schizophrenia or mania. But clinicians are highly unlikely to discontinue that drug after a patient successfully responds within a few weeks. Thus, we essentially practice off-label psychopharmacology whenever we maintain a patient on a drug approved only for acute uses.
Combination therapies. No antipsychotic combinations are approved for schizophrenia, yet more than one-third of chronic schizophrenia patients in the United States are concurrently receiving 2 or more concurrent SGAs.4 Combining antipsychotics is often regarded as dubious off-label polypharmacy, yet clinicians stand by their observations that patients who do not improve with 1 drug may respond when another is added.
Although combination pharmacotherapy is not supported by credible evidence—controlled trials of 2 SGAs vs 1 combined with a placebo—clinicians again might be discovering options for treatment-resistant or refractory patients before FDA trials are conducted.
Simpler dosing for better adherence. A drug may be approved for twice-daily (bid) administration, yet clinicians might soon discover that prescribing it once daily (qd) is equally or even more effective because of improved patient adherence. Off-label dosing may be rational and even better than the official dose schedule, yet a drug company might never go through the costly process of repeating its clinical trial to demonstrate that bid and qd dosing are equivalent. Thus, practitioners will continue to use the drug off-label based on clinical experience, not on research data.
Scientific implications. Aside from advancing psychopharmacologic practices and discovering new treatments, off-label data also could shed light on a potential shared neurobiology among psychiatric disorders. Off-label prescribing ultimately might help us reconceptualize the overlapping neural pathways of several axis I and axis II disorders, all of which appear to be improved by the same pharmacologic agent such as an atypical antipsychotic. It might even prompt us to coin a new name for antipsychotics, such as “neurostabilizers.”
Write and tell me what term you would coin for a class of drugs with multiple psychiatric uses.
1. Shekele P, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of off-label use of atypical antipsychotics. Executive summary. No. 6 (AHRQ Pub. No. 07-EHC003-1). Rockville, MD: Agency for Healthcare Research and Quality; January 2007. Available at: http://effectivehealthcare.ahrq.gov/repFiles/Atypical_Executive_Summary.pdf. Accessed February 8, 2007.
2. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry 2006;67:972-82.
3. Devulapalli KK, Nasrallah HA. An analysis of current indications and common off-label uses of psychotropic medications. Submitted for publication.
IMS data, 2005.
1. Shekele P, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of off-label use of atypical antipsychotics. Executive summary. No. 6 (AHRQ Pub. No. 07-EHC003-1). Rockville, MD: Agency for Healthcare Research and Quality; January 2007. Available at: http://effectivehealthcare.ahrq.gov/repFiles/Atypical_Executive_Summary.pdf. Accessed February 8, 2007.
2. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry 2006;67:972-82.
3. Devulapalli KK, Nasrallah HA. An analysis of current indications and common off-label uses of psychotropic medications. Submitted for publication.
IMS data, 2005.
‘Killer trolls’: One older man’s battle
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
Dissecting clinical trials with ‘number needed to treat’
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia, you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—two tools of evidence-based medicine (EBM, Box 11,2)—can help answer these questions. This article shows how to calculate NNT and NNH, then applies these tools to published results from CATIE phases 1 and 2.
Evidence-based medicine (EBM) is a process by which a clinician extracts information from the medical literature and applies it in day-to-day patient treatment. Gray and Pinson1 summarize EBM’s 5 steps as:
- formulate the question
- search for answers
- appraise the evidence
- apply the results
- assess the outcome.
This is not a trivial task. To help clinicians, EBM pioneers such as Gordon Guyatt, MD, MSc, and Drummond Rennie, MD, have published useful, readable, short reviews of EBM methods in the “Users’ Guides to the Medical Literature” in the Journal of the American Medical Association.2
Internet resources also are available, including:
- Centre for Evidence-Based Medicine, University of Toronto. www.cebm.utoronto.ca
- Eskind Biomedical Library, Vanderbilt University. Evidence-based knowledge portal. www.mc.vanderbilt.edu/biolib/ebmportal/login.html
- Hayward Medical Communications. Evidence-Based Medicine: What is…? series. www.evidence-based-medicine.co.uk/What_is_series.html.
What is nnt?
NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant.
NNT allows us to place a number on how often we can expect to see a difference between two interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between two treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with one intervention versus another (an NNT of5), the result will likely influence day-to-day practice. Together with calculating a confidence interval (Box 2),3 the NNT can help you judge the clinical significance of a statistically significant result.
Calculating number needed to treat (NNT) or number needed to harm (NNH) does not tell you whether the result is statistically significant. You can find out by examining a range of values called the confidence interval (CI).
An NNT with a 95% CI means that the truth probably lies between the lower and upper bounds of the interval with a probability of 95%. A 95% CI with an NNT of 5 to 15 means we have an NNT that with 95% certainty falls between 5 and 15.
Formulas can be used to calculate CIs.3 One useful online calculator is available at: www.cebm.utoronto.ca/practise/ca/statscal.
Sometimes the lower bound of a CI is a negative number and the upper bound is a positive number (such as –10 to +10). This occurs when the result is not statistically significant. Having a negative number and a positive number in the CI means when comparing intervention A to intervention B, intervention A might be better than B, or B might be better than A. We could not conclude that a difference exists between the two interventions.
Calculating nnt and nnh
NNT and NNH are easy to calculate:
- First determine the difference between the frequencies of the outcome of interest for two interventions.
- Then calculate the reciprocal of this difference.
- Difference in response rates=0.75–0.55=0.20
- NNT=1/0.20=5.
Interpreting the importance of NNT values is easy, too. The smaller the NNT, the larger the clinical difference between interventions; the larger the NNT, the smaller the difference.
- An NNT of 100 or more usually means little difference exists between interventions for the outcome of interest.
- An NNT of 2 would be hugely important and is rarely encountered.
Example. We can calculate the NNT (actually, NNH) for risk of new-onset diabetes mellitus attributable to second-generation antipsychotics (SGAs), using data from a study that compared diabetes rates in patients given SGAs versus conventional antipsychotics.4 Differences in new-onset diabetes rates across ≤25 months were 2.03%, 0.80%, 0.63%, and 0.05% for clozapine, quetiapine, olanzapine, and risperidone, respectively, versus first-generation antipsychotics (FGAs).
The NNH for clozapine compared with FGAs is 1/0.0203=49. This means you would need to treat 49 patients with clozapine instead of an FGA for up to 25 months to encounter 1 extra case of new-onset diabetes mellitus. NNH calculations for quetiapine, olanzapine, and risperidone compared with FGAs would be 125, 159, and 2,000, respectively.
Applying nnt and nnh to catie
An ongoing controversy in schizophrenia treatment is the relative merit of using the more-expensive SGAs versus FGAs. The National Institute of Mental Health-funded CATIE study addressed this issue.5-7
In CATIE phase 1, which was double-blinded, 1,493 patients with schizophrenia were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who discontinued phase 1 before 18 months could participate in phase 2, where 543 patients were randomly assigned to 1 of 5 SGAs that they did not receive in phase 1. Those who prematurely discontinued phase 2 were offered open-label treatment with one or two antipsychotics. When they enrolled, patients were told these switches were possible.
Nearly one-half of all patients who enrolled finished 18 months of follow-up. What resulted, however, was a morass of percentages and p values that were misinterpreted by various parties—including The New York Times, which published an article headlined, “Little difference found in schizophrenia drugs.”8 We can apply NNT and NNH to the CATIE study results, however, and discover that:
- important differences do exist between the drugs tested
- these differences are clinically and statistically significant.3
- lack of efficacy
- poor tolerability
- patient decision.
- NNT=1/(difference in discontinuation rates)=1/(0.82 - 0.64)=1/0.18=5.6. By convention, we round up to the next whole number, in this case 6. This means that for every 6 patients randomized to olanzapine treatment, 1 extra patient completed phase 1 on his or her initially initial medication, compared with patients randomized to quetiapine treatment.
In measuring the number of hospitalizations for exacerbation of schizophrenia symptoms per total person-year of exposure, NNT ranged from 3 to 7 in favor of olanzapine compared with the other antipsychotics. This means that for every 3 to 7 patients treated with olanzapine versus another antipsychotic, 1 hospitalization was avoided.
Tolerability. Calculating NNH can show how often you could expect specific tolerability outcomes when comparing medications. In CATIE, differences in tolerability emerged among the medications, and each antipsychotic had a unique profile of relative strengths and weaknesses that can be expressed in NNT and NNH. For example, in CATIE phase 1:
- For every 5 to 8 patients treated with olanzapine compared to other antipsychotics, 1 additional patient gained >7% in body weight (NNH is 5 to 8; not corrected for duration of exposure to the medication)
- For every 13 to 18 patients treated with olanzapine versus another antipsychotic, 1 additional patient discontinued because of weight gain or metabolic effects.
Potential pitfalls
Different studies can provide different estimates of outcomes such as response, remission, hospitalization, or adverse events. Two studies of the risk of new-onset diabetes with antipsychotics demonstrate that these differences can be difficult to interpret, particularly when populations and study designs differ.
- A Department of Veterans Affairs study of data on 56,849 patients4 produced an NNH of 159 when olanzapine was compared with conventional antipsychotics, meaning 1 extra case of new-onset diabetes was encountered for every 159 patients treated with olanzapine compared to conventional antipsychotics.
- In the CATIE study,5 examining new prescriptions of antidiabetic agents yields an NNH of 61 when olanzapine is compared with perphenazine, meaning that 1 extra case of a new prescription of an antidiabetic agent was encountered for every 61 patients treated with olanzapine versus perphenazine.
NNT and NNH are best calculated from well-controlled clinical trials. However, the underlying study design and potential biases may affect how NNT and NNH apply to clinical practice. A more complete discussion of the CATIE NNT and NNH secondary analysis can be found elsewhere,3 but issues to consider include the impact of differential switching9 and the possible effects of dosages.10
Related resources
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM, 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Citrome receives research support from AstraZeneca Pharmaceuticals, Barr Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer. He is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer, and a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Eli Lilly and Company, and Pfizer.
1. Gray GE, Pinson LA. Evidence-based medicine and psychiatric practice. Psychiatr Q 2003;74(4):387-99.
2. Guyatt GH, Rennie D. Users’ guides to the medical literature [editorial]. JAMA 1993;270(17):2096-7.
3. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract 2006;60(8):933-40.
4. Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004;161(9):1709-11.
5. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
6. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163(4):600-10.
7. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163(4):611-22.
8. Carey B. Little difference found in schizophrenia drugs. The New York Times. September 20, 2005.
9. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163(12):2090-5.
10. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry 2002;10(5):280-91.
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia, you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—two tools of evidence-based medicine (EBM, Box 11,2)—can help answer these questions. This article shows how to calculate NNT and NNH, then applies these tools to published results from CATIE phases 1 and 2.
Evidence-based medicine (EBM) is a process by which a clinician extracts information from the medical literature and applies it in day-to-day patient treatment. Gray and Pinson1 summarize EBM’s 5 steps as:
- formulate the question
- search for answers
- appraise the evidence
- apply the results
- assess the outcome.
This is not a trivial task. To help clinicians, EBM pioneers such as Gordon Guyatt, MD, MSc, and Drummond Rennie, MD, have published useful, readable, short reviews of EBM methods in the “Users’ Guides to the Medical Literature” in the Journal of the American Medical Association.2
Internet resources also are available, including:
- Centre for Evidence-Based Medicine, University of Toronto. www.cebm.utoronto.ca
- Eskind Biomedical Library, Vanderbilt University. Evidence-based knowledge portal. www.mc.vanderbilt.edu/biolib/ebmportal/login.html
- Hayward Medical Communications. Evidence-Based Medicine: What is…? series. www.evidence-based-medicine.co.uk/What_is_series.html.
What is nnt?
NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant.
NNT allows us to place a number on how often we can expect to see a difference between two interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between two treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with one intervention versus another (an NNT of5), the result will likely influence day-to-day practice. Together with calculating a confidence interval (Box 2),3 the NNT can help you judge the clinical significance of a statistically significant result.
Calculating number needed to treat (NNT) or number needed to harm (NNH) does not tell you whether the result is statistically significant. You can find out by examining a range of values called the confidence interval (CI).
An NNT with a 95% CI means that the truth probably lies between the lower and upper bounds of the interval with a probability of 95%. A 95% CI with an NNT of 5 to 15 means we have an NNT that with 95% certainty falls between 5 and 15.
Formulas can be used to calculate CIs.3 One useful online calculator is available at: www.cebm.utoronto.ca/practise/ca/statscal.
Sometimes the lower bound of a CI is a negative number and the upper bound is a positive number (such as –10 to +10). This occurs when the result is not statistically significant. Having a negative number and a positive number in the CI means when comparing intervention A to intervention B, intervention A might be better than B, or B might be better than A. We could not conclude that a difference exists between the two interventions.
Calculating nnt and nnh
NNT and NNH are easy to calculate:
- First determine the difference between the frequencies of the outcome of interest for two interventions.
- Then calculate the reciprocal of this difference.
- Difference in response rates=0.75–0.55=0.20
- NNT=1/0.20=5.
Interpreting the importance of NNT values is easy, too. The smaller the NNT, the larger the clinical difference between interventions; the larger the NNT, the smaller the difference.
- An NNT of 100 or more usually means little difference exists between interventions for the outcome of interest.
- An NNT of 2 would be hugely important and is rarely encountered.
Example. We can calculate the NNT (actually, NNH) for risk of new-onset diabetes mellitus attributable to second-generation antipsychotics (SGAs), using data from a study that compared diabetes rates in patients given SGAs versus conventional antipsychotics.4 Differences in new-onset diabetes rates across ≤25 months were 2.03%, 0.80%, 0.63%, and 0.05% for clozapine, quetiapine, olanzapine, and risperidone, respectively, versus first-generation antipsychotics (FGAs).
The NNH for clozapine compared with FGAs is 1/0.0203=49. This means you would need to treat 49 patients with clozapine instead of an FGA for up to 25 months to encounter 1 extra case of new-onset diabetes mellitus. NNH calculations for quetiapine, olanzapine, and risperidone compared with FGAs would be 125, 159, and 2,000, respectively.
Applying nnt and nnh to catie
An ongoing controversy in schizophrenia treatment is the relative merit of using the more-expensive SGAs versus FGAs. The National Institute of Mental Health-funded CATIE study addressed this issue.5-7
In CATIE phase 1, which was double-blinded, 1,493 patients with schizophrenia were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who discontinued phase 1 before 18 months could participate in phase 2, where 543 patients were randomly assigned to 1 of 5 SGAs that they did not receive in phase 1. Those who prematurely discontinued phase 2 were offered open-label treatment with one or two antipsychotics. When they enrolled, patients were told these switches were possible.
Nearly one-half of all patients who enrolled finished 18 months of follow-up. What resulted, however, was a morass of percentages and p values that were misinterpreted by various parties—including The New York Times, which published an article headlined, “Little difference found in schizophrenia drugs.”8 We can apply NNT and NNH to the CATIE study results, however, and discover that:
- important differences do exist between the drugs tested
- these differences are clinically and statistically significant.3
- lack of efficacy
- poor tolerability
- patient decision.
- NNT=1/(difference in discontinuation rates)=1/(0.82 - 0.64)=1/0.18=5.6. By convention, we round up to the next whole number, in this case 6. This means that for every 6 patients randomized to olanzapine treatment, 1 extra patient completed phase 1 on his or her initially initial medication, compared with patients randomized to quetiapine treatment.
In measuring the number of hospitalizations for exacerbation of schizophrenia symptoms per total person-year of exposure, NNT ranged from 3 to 7 in favor of olanzapine compared with the other antipsychotics. This means that for every 3 to 7 patients treated with olanzapine versus another antipsychotic, 1 hospitalization was avoided.
Tolerability. Calculating NNH can show how often you could expect specific tolerability outcomes when comparing medications. In CATIE, differences in tolerability emerged among the medications, and each antipsychotic had a unique profile of relative strengths and weaknesses that can be expressed in NNT and NNH. For example, in CATIE phase 1:
- For every 5 to 8 patients treated with olanzapine compared to other antipsychotics, 1 additional patient gained >7% in body weight (NNH is 5 to 8; not corrected for duration of exposure to the medication)
- For every 13 to 18 patients treated with olanzapine versus another antipsychotic, 1 additional patient discontinued because of weight gain or metabolic effects.
Potential pitfalls
Different studies can provide different estimates of outcomes such as response, remission, hospitalization, or adverse events. Two studies of the risk of new-onset diabetes with antipsychotics demonstrate that these differences can be difficult to interpret, particularly when populations and study designs differ.
- A Department of Veterans Affairs study of data on 56,849 patients4 produced an NNH of 159 when olanzapine was compared with conventional antipsychotics, meaning 1 extra case of new-onset diabetes was encountered for every 159 patients treated with olanzapine compared to conventional antipsychotics.
- In the CATIE study,5 examining new prescriptions of antidiabetic agents yields an NNH of 61 when olanzapine is compared with perphenazine, meaning that 1 extra case of a new prescription of an antidiabetic agent was encountered for every 61 patients treated with olanzapine versus perphenazine.
NNT and NNH are best calculated from well-controlled clinical trials. However, the underlying study design and potential biases may affect how NNT and NNH apply to clinical practice. A more complete discussion of the CATIE NNT and NNH secondary analysis can be found elsewhere,3 but issues to consider include the impact of differential switching9 and the possible effects of dosages.10
Related resources
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM, 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Citrome receives research support from AstraZeneca Pharmaceuticals, Barr Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer. He is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer, and a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Eli Lilly and Company, and Pfizer.
Clinical trials produce a mountain of data that can be difficult to interpret and apply to clinical practice. When reading about studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia, you may wonder:
- How large is the effect being measured?
- Is it clinically important?
- Are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?
Number needed to treat (NNT) and number needed to harm (NNH)—two tools of evidence-based medicine (EBM, Box 11,2)—can help answer these questions. This article shows how to calculate NNT and NNH, then applies these tools to published results from CATIE phases 1 and 2.
Evidence-based medicine (EBM) is a process by which a clinician extracts information from the medical literature and applies it in day-to-day patient treatment. Gray and Pinson1 summarize EBM’s 5 steps as:
- formulate the question
- search for answers
- appraise the evidence
- apply the results
- assess the outcome.
This is not a trivial task. To help clinicians, EBM pioneers such as Gordon Guyatt, MD, MSc, and Drummond Rennie, MD, have published useful, readable, short reviews of EBM methods in the “Users’ Guides to the Medical Literature” in the Journal of the American Medical Association.2
Internet resources also are available, including:
- Centre for Evidence-Based Medicine, University of Toronto. www.cebm.utoronto.ca
- Eskind Biomedical Library, Vanderbilt University. Evidence-based knowledge portal. www.mc.vanderbilt.edu/biolib/ebmportal/login.html
- Hayward Medical Communications. Evidence-Based Medicine: What is…? series. www.evidence-based-medicine.co.uk/What_is_series.html.
What is nnt?
NNT helps us gauge effect size—or clinical significance. It is different from knowing if a clinical trial result is statistically significant.
NNT allows us to place a number on how often we can expect to see a difference between two interventions. If we see a therapeutic difference once every 100 patients (an NNT of 100), the difference between two treatments is not of great concern under most circumstances. But if a difference in outcome is seen once in every 5 patients being treated with one intervention versus another (an NNT of5), the result will likely influence day-to-day practice. Together with calculating a confidence interval (Box 2),3 the NNT can help you judge the clinical significance of a statistically significant result.
Calculating number needed to treat (NNT) or number needed to harm (NNH) does not tell you whether the result is statistically significant. You can find out by examining a range of values called the confidence interval (CI).
An NNT with a 95% CI means that the truth probably lies between the lower and upper bounds of the interval with a probability of 95%. A 95% CI with an NNT of 5 to 15 means we have an NNT that with 95% certainty falls between 5 and 15.
Formulas can be used to calculate CIs.3 One useful online calculator is available at: www.cebm.utoronto.ca/practise/ca/statscal.
Sometimes the lower bound of a CI is a negative number and the upper bound is a positive number (such as –10 to +10). This occurs when the result is not statistically significant. Having a negative number and a positive number in the CI means when comparing intervention A to intervention B, intervention A might be better than B, or B might be better than A. We could not conclude that a difference exists between the two interventions.
Calculating nnt and nnh
NNT and NNH are easy to calculate:
- First determine the difference between the frequencies of the outcome of interest for two interventions.
- Then calculate the reciprocal of this difference.
- Difference in response rates=0.75–0.55=0.20
- NNT=1/0.20=5.
Interpreting the importance of NNT values is easy, too. The smaller the NNT, the larger the clinical difference between interventions; the larger the NNT, the smaller the difference.
- An NNT of 100 or more usually means little difference exists between interventions for the outcome of interest.
- An NNT of 2 would be hugely important and is rarely encountered.
Example. We can calculate the NNT (actually, NNH) for risk of new-onset diabetes mellitus attributable to second-generation antipsychotics (SGAs), using data from a study that compared diabetes rates in patients given SGAs versus conventional antipsychotics.4 Differences in new-onset diabetes rates across ≤25 months were 2.03%, 0.80%, 0.63%, and 0.05% for clozapine, quetiapine, olanzapine, and risperidone, respectively, versus first-generation antipsychotics (FGAs).
The NNH for clozapine compared with FGAs is 1/0.0203=49. This means you would need to treat 49 patients with clozapine instead of an FGA for up to 25 months to encounter 1 extra case of new-onset diabetes mellitus. NNH calculations for quetiapine, olanzapine, and risperidone compared with FGAs would be 125, 159, and 2,000, respectively.
Applying nnt and nnh to catie
An ongoing controversy in schizophrenia treatment is the relative merit of using the more-expensive SGAs versus FGAs. The National Institute of Mental Health-funded CATIE study addressed this issue.5-7
In CATIE phase 1, which was double-blinded, 1,493 patients with schizophrenia were randomly assigned to 1 of 5 antipsychotics—perphenazine, olanzapine, quetiapine, risperidone, or ziprasidone—for up to 18 months. Patients who discontinued phase 1 before 18 months could participate in phase 2, where 543 patients were randomly assigned to 1 of 5 SGAs that they did not receive in phase 1. Those who prematurely discontinued phase 2 were offered open-label treatment with one or two antipsychotics. When they enrolled, patients were told these switches were possible.
Nearly one-half of all patients who enrolled finished 18 months of follow-up. What resulted, however, was a morass of percentages and p values that were misinterpreted by various parties—including The New York Times, which published an article headlined, “Little difference found in schizophrenia drugs.”8 We can apply NNT and NNH to the CATIE study results, however, and discover that:
- important differences do exist between the drugs tested
- these differences are clinically and statistically significant.3
- lack of efficacy
- poor tolerability
- patient decision.
- NNT=1/(difference in discontinuation rates)=1/(0.82 - 0.64)=1/0.18=5.6. By convention, we round up to the next whole number, in this case 6. This means that for every 6 patients randomized to olanzapine treatment, 1 extra patient completed phase 1 on his or her initially initial medication, compared with patients randomized to quetiapine treatment.
In measuring the number of hospitalizations for exacerbation of schizophrenia symptoms per total person-year of exposure, NNT ranged from 3 to 7 in favor of olanzapine compared with the other antipsychotics. This means that for every 3 to 7 patients treated with olanzapine versus another antipsychotic, 1 hospitalization was avoided.
Tolerability. Calculating NNH can show how often you could expect specific tolerability outcomes when comparing medications. In CATIE, differences in tolerability emerged among the medications, and each antipsychotic had a unique profile of relative strengths and weaknesses that can be expressed in NNT and NNH. For example, in CATIE phase 1:
- For every 5 to 8 patients treated with olanzapine compared to other antipsychotics, 1 additional patient gained >7% in body weight (NNH is 5 to 8; not corrected for duration of exposure to the medication)
- For every 13 to 18 patients treated with olanzapine versus another antipsychotic, 1 additional patient discontinued because of weight gain or metabolic effects.
Potential pitfalls
Different studies can provide different estimates of outcomes such as response, remission, hospitalization, or adverse events. Two studies of the risk of new-onset diabetes with antipsychotics demonstrate that these differences can be difficult to interpret, particularly when populations and study designs differ.
- A Department of Veterans Affairs study of data on 56,849 patients4 produced an NNH of 159 when olanzapine was compared with conventional antipsychotics, meaning 1 extra case of new-onset diabetes was encountered for every 159 patients treated with olanzapine compared to conventional antipsychotics.
- In the CATIE study,5 examining new prescriptions of antidiabetic agents yields an NNH of 61 when olanzapine is compared with perphenazine, meaning that 1 extra case of a new prescription of an antidiabetic agent was encountered for every 61 patients treated with olanzapine versus perphenazine.
NNT and NNH are best calculated from well-controlled clinical trials. However, the underlying study design and potential biases may affect how NNT and NNH apply to clinical practice. A more complete discussion of the CATIE NNT and NNH secondary analysis can be found elsewhere,3 but issues to consider include the impact of differential switching9 and the possible effects of dosages.10
Related resources
- Guyatt G, Rennie D. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago: AMA Press; 2001.
- Straus SE, Richardson WS, Glasziou P, et al. Evidence-based medicine: how to practice and teach EBM, 3rd ed. Edinburgh, UK: Elsevier/Churchill Livingstone; 2005.
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Citrome receives research support from AstraZeneca Pharmaceuticals, Barr Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Forest Pharmaceuticals, Janssen Pharmaceutica, and Pfizer. He is a consultant to Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Jazz Pharmaceuticals, and Pfizer, and a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Eli Lilly and Company, and Pfizer.
1. Gray GE, Pinson LA. Evidence-based medicine and psychiatric practice. Psychiatr Q 2003;74(4):387-99.
2. Guyatt GH, Rennie D. Users’ guides to the medical literature [editorial]. JAMA 1993;270(17):2096-7.
3. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract 2006;60(8):933-40.
4. Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004;161(9):1709-11.
5. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
6. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163(4):600-10.
7. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163(4):611-22.
8. Carey B. Little difference found in schizophrenia drugs. The New York Times. September 20, 2005.
9. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163(12):2090-5.
10. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry 2002;10(5):280-91.
1. Gray GE, Pinson LA. Evidence-based medicine and psychiatric practice. Psychiatr Q 2003;74(4):387-99.
2. Guyatt GH, Rennie D. Users’ guides to the medical literature [editorial]. JAMA 1993;270(17):2096-7.
3. Citrome L, Stroup TS. Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract 2006;60(8):933-40.
4. Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004;161(9):1709-11.
5. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353(12):1209-23.
6. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163(4):600-10.
7. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163(4):611-22.
8. Carey B. Little difference found in schizophrenia drugs. The New York Times. September 20, 2005.
9. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163(12):2090-5.
10. Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry 2002;10(5):280-91.
How to help patients with olfactory reference syndrome
Ms. A, a 21-year-old teacher, recalled always having been “sensitive,” but when she started her first job at age 19 she began to believe that she emitted an offensive odor. She experienced thoughts that she passed offensive flatus, her breath had a fecal odor, and people noticed and were offended.
Gradually Ms. A became more convinced of these distressing beliefs and began to think that she permeated fecal odor through her skin. She also became sure that colleagues were talking about her and that they complained about her “disgusting” smell.
Patients with olfactory reference syndrome (ORS) falsely believe they emit an offensive body odor. Prominent referential thinking—believing that other people perceive the odor—also is common. To introduce you to ORS, we discuss its clinical diagnosis and treatment based on our review of several hundred cases, including the largest reported series of patients with ORS.1-4
Olfactory reference syndrome (ORS) has been described around the world for more than a century. In 1891, Potts described a delusional 50-year-old man who “had been troubled for the past three months with smelling a very bad odor, which he likened to that of a ‘back-house,’ and which came from his own person. [He believed] this smell was so very strong that other men objected to working with him….”
Despite its long history, the syndrome’s prevalence is not well-established. ORS probably is underdiagnosed and more common than generally recognized:
- In a tertiary psychiatry unit in London, 0.5% of 2,000 patients who were not systematically screened for ORS spontaneously reported ORS symptoms.
- In a self-report survey of 2,481 students in Japan, 2.1% had been concerned with emitting a strange bodily odor during the past year.
- In a study in a dental clinic in Japan, the majority of patients with a primary complaint of halitosis actually had “imaginary halitosis” (another term for ORS).
Clinical features of ors
Ms. A quit her job and felt confident enough to work again only when she performed a 2-hour daily cleansing routine, doused herself in per-fume, and placed an incontinence pad in her underwear. Despite these precautions, she still thought her colleagues avoided her.
She always averted her mouth when speaking, held her hand in front of her mouth, and sat far from others and close to the door in meetings. She tried to keep meeting room doors open and believed that colleagues held their hands to their noses to “protect” themselves from her odor.
ORS symptoms are most often reported as beginning when patients are in their mid 20s, although some reports suggest onset during puberty or adolescence.4 In clinical series, the ratio of men to women is approximately 2:1.
Preoccupation. Individuals with ORS are preoccupied with the belief that they emit an unpleasant or offensive body odor (Box 1),1,2,5-9 most commonly:
Although most reports suggest that patients focus on one odor, some describe being concerned about several smells simultaneously or different odors over time.10,11
Referential thinking. As the syndrome’s name implies, many ORS patients have delusions of reference, falsely believing that other people perceive the odor.3 They misinterpret the behavior of others, assuming it is a reaction to how the patient smells (Box 2).2,3
They may misperceive comments (such as, “It’s stuffy in here”), receiving perfume or soap as a gift, or behaviors such as people sniffing, touching or rubbing their nose, clearing their throat, opening a window to get fresh air, putting a newspaper in front of their face, or looking or moving toward or away from the patient.1,3,5,7
Because they are ashamed, embarrassed, and concerned about offending others with their odor,13 many patients engage in repetitive and “safety” behaviors intended to check, eliminate, or camouflage the supposed odor (Box 3).1,3,7,12,14
DSM-IV-TR classifies olfactory reference syndrome (ORS) as a delusional disorder, somatic type (the modern equivalent of monosymptomatic hypochondriacal psychosis). ORS also is mentioned in the text on social anxiety disorder. ORS may not be diagnosed if:
- criterion A for schizophrenia has ever been met
- or if symptoms are due to the direct physiologic effects of a substance or a general medical condition, such as a brain tumor or temporal lobe epilepsy.
Many patients report being able to smell the imagined odor, suggesting that they experience an olfactory hallucination. Pryse-Phillips described the olfactory hallucinations of her 36 ORS case patients as “a real and immediate perception… often perceived in the absence of other odors.”
ORS generally is regarded as delusional, with possible secondary illusional misinterpretations and referential thinking. ORS beliefs usually appear to be of delusional intensity, although some patients may have some—although limited—insight (that is, overvalued ideation).
Functional impairment. Individuals with ORS often avoid other people or believe that others avoid them.12 They are typically embarrassed and worried that others will be offended by the smell. They may:
- avoid activities such as dating
- break off engagements
- refuse to travel
- move to another town
- become housebound.3,7,10,12
The distress and impaired functioning may lead to psychiatric hospitalization, depression, suicidal ideation, suicide attempts, and completed suicide.7,10,12,15 Pryse-Phillips studied 36 patients with ORS and reported:
- nearly one-half (43%) experienced “suicidal ideas or action”
- 2 (5.6%) committed suicide.3
Some authors have questioned whether ORS can transform into schizophrenia, but others have found little evidence for this.3,6
Psychiatric comorbidity. Depression is mentioned most often in the literature.12,13 In Pryse-Phillips’ 36 ORS patients (who did not have a “primary” depressive disorder), depression symptoms tended to be severe.3 The depression generally is considered secondary to ORS, although Pryse-Phillips evaluated 50 additional patients with ORS symptoms whom she considered to have a “primary” depressive disorder.3 Other psychiatric comorbidities include bipolar disorder, personality disorder, schizophrenia, hypochondriasis, alcohol and/or drug abuse, obsessive-compulsive disorder (OCD), and body dysmorphic disorder.3,7,15 In a study of 200 individuals with body dysmorphic disorder, 8 had comorbid ORS.16
Diagnosing ORS
Clinical clues to ORS (Table 1) probably are not present in all patients and some are not specific to ORS. They appear to be common features of the illness, however, and may alert you to its presence. Our clinical impression is that many patients with ORS are secretive about their symptoms because they are ashamed of them. Thus, you need to be alert to clues and specifically inquire about ORS symptoms to detect its presence.
Criteria. DSM-IV-TR and ICD-10 lack specific diagnostic criteria for ORS, instead applying criteria for delusional disorder. One problem with this approach is that delusional disorder criteria specify that any co-occurring mood symptoms must be brief relative to the duration of the delusional periods. This requirement may not be valid when applied to ORS.
In our experience, some patients have protracted depressive symptoms that appear secondary to “primary” ORS symptoms, and another diagnosis—such as psychotic depression—does not appear to account for their symptoms.
We propose working diagnostic criteria for ORS (Table 2), which are similar to those proposed by Lochner and Stein17 and require empiric validation. Suggested questions for the patient interview (Table 3) can help you identify and diagnose ORS.
Differential diagnosis. Keep in mind that a false belief that one emits a bad smell may be a symptom of schizophrenia, and this would trump an ORS diagnosis if other schizophrenia symptoms are present. Some patients with severe depression may believe they smell bad as part of a nihilistic delusional belief system (such as in Cotard’s syndrome—nihilistic delusions in severe depression).
Whether to conceptualize a false belief about body odor as a symptom of depression or as ORS with comorbid or secondary depression may be unclear from case to case.
Table 1
Clinical clues to the presence of olfactory reference syndrome
| Referential thinking. Interpreting actions of others—such as opening a window, moving away, putting a hand to their nose, or making comments related to odors—as evidence that the person smells offensive |
| Excessive attempts to ‘disguise’ the smell, such as washing routines, clothes changing, clothes laundering, or using abundant perfume, deodorant, mouthwash, mints, or other forms of camouflage |
| Other excessive and repetitive behaviors, such as checking for or asking other people for reassurance about the odor |
| Social anxiety or avoidance of social activities, relationships, work, school, or other daily activities |
| Requests for treatment for the perceived odor from dentists, gastroenterologists, proctologists, or other nonpsychiatric physicians despite a negative medical workup |
Working diagnostic criteria for olfactory reference syndrome
| A. | Persistent false belief that one emits a malodorous smell; this belief may encompass a range of insight and does not have to be delusional |
| B. | The belief is time-consuming and preoccupies the individual for at least 1 hour per day |
| C. | The belief causes clinically significant distress or results in significant impairment in social, occupational, or other important areas of functioning |
| D. | The belief is not better accounted for by another mental disorder or a general medical condition |
Table 3
Diagnosing ORS: Suggested questions for patient interview
|
Treatment-seeking behavior
Ms. A consulted several proctologists and a dentist but was not convinced by their reassurance and continued to believe she “stank.” Her relationship with her boyfriend suffered because she continually asked for reassurance about how she smelled and avoided sexual intercourse because of her odor concerns.
Eventually she confronted her boss about her belief that her coworkers were complaining about her smell. Despite reassurance that she didn’t smell bad, she left her job.
Excessive showering or washing are among the repetitive, ritualistic or “safety” behaviors many patients with ORS engage in to check, eliminate, or camouflage supposed odor. Frequent clothes changing or laundering also is common.
Camouflaging attempts may include excessive use of deodorant, soap, cologne, powder, mints, mouthwash, or toothpaste; wearing layers of clothing; or smoking.
Many patients frequently check for the odor or its source (such as trying to smell their own breath or checking the anal area for seepage). Some patients use the toilet excessively or eat a special diet to try to minimize the smell. Others repeatedly seek reassurance about how they smell.
Avoidance behaviors are common and include sitting far from other people, moving as little as possible to avoid spreading the supposed odor, or averting the head or covering the mouth.
Convincing patients such as Ms. A of the falsity of their beliefs can be difficult,1 and some succeed in having medical procedures or surgery, such as excision of tonsils or axillary glands.3,7,12 To our knowledge, controlled prospective studies of nonpsychiatric treatments have not been done, but it appears that such treatments usually are ineffective.1,3,6,9
Psychiatric interventions. Convincing patients with ORS to obtain mental health treatment can be difficult.2,6 Patients with delusional halitosis “would rather go in search of a ‘better dentist’ than go to a psychiatrist.”1
To get patients to accept psychiatric treatment, we suggest an approach similar to that recommended for body dysmorphic disorder. It may be helpful, for example, to focus on the distress and disability caused by the odor preoccupation, rather than on whether the patient actually smells bad.
Medication and psychotherapy
Limited evidence. The ORS treatment literature is very limited, consisting largely of case reports and small case series. To our knowledge, no controlled treatment trials have been done, no treatments have been compared head to head, and most studies did not use standardized measurements of psychopathology.
Published data therefore must be interpreted cautiously. Some medication reports used relatively low doses and short treatment durations (although what constitutes an adequate therapeutic trial for ORS is not known). Psychotherapy reports often did not specify details of the intervention or the number and duration of sessions. It is not known whether adding a cognitive component to behavioral therapy enhances efficacy, and the combination of psychotherapy and medication has not been studied systematically. More methodologically rigorous treatment studies are needed.
Because of space limitations, we cite representative case reports in the following section of this treatment review, rather than all of the cases found in our literature search.
Antidepressants. Although most ORS patients are delusional, serotonin reuptake inhibitor (SRI) monotherapy has been reported to be efficacious in 10 of 15 cases (67%). Most of these patients received clomipramine.18 In reports of non-SRI antidepressants, 6 of 15 cases (43%) responded. Some patients’ symptoms responded to an antidepressant after failing to respond to antipsychotic treatment.19
Antipsychotics. Pimozide is the most studied medication for ORS, with 15 of 31 cases (48%) responding.2,20 In a series of 12 patients, pimozide responders received 2 to 4 mg/d, except for one patient who needed 6 mg/d.21 Patients usually responded within 1 to 4 weeks (an average time to response was not reported). In 2 of these cases, ORS symptoms recurred after pimozide was discontinued and then remitted again after it was restarted.21 In another report,2 7 of 14 patients (50%) responded to pimozide.
Clinicians using other first-generation antipsychotics (trifluoperazine, thioridazine, and chlorpromazine) reported a positive response in only 2 of 19 cases (11%).12,22
Combination therapy. Ten of 17 cases (59%) of ORS responded to combined treatment with an antidepressant and an antipsychotic.2,12
Other somatic treatments. Several reports found benzodiazepine monotherapy lacked efficacy, as was the case for electroconvulsive therapy.12,15 One report noted an unsuccessful outcome with leucotomy and a partial response with bilateral partial division of the thalamo-frontal tract.15
Psychosocial treatment. All reports of psychosocial therapies are single cases or small series, and none used a control intervention.2,7,14
Behavioral treatment has been efficacious, although patients require months to years to habituate. Several reports totalling 14 patients describe behavioral treatment over weeks to months.7,23 These treatments involved exposure to avoided social situations and response prevention, which consisted of refraining from repetitive or camouflaging behaviors such as showering, visits to the toilet, or deodorant use. Gomez-Perez and colleagues23 noted that exposure therapy was less effective for ORS than for social phobia or OCD.
One report described a patient with flatulence concerns who responded to a paradoxical intention consisting of instructions to emit gas as soon as it was experienced; at 1-year follow-up, her symptoms had not recurred.24
Psychodynamic interventions show no benefit for ORS symptoms.
Treatment summary
Ms. A became increasingly despondent and depressed. She eventually sought the help of her family doctor, who referred her to a psychiatrist. With a combination of a serotonergic antidepressant (escitalopram, 40 mg/d), a low-dose atypical antipsychotic (quetiapine, 50 mg at night), and cognitive-behavioral therapy, she started to re-engage in daily activities. During 6 months of treatment, the intensity of her belief about having body odor abated.
Limited data support the use of SRI monotherapy or an SRI plus an antipsychotic. Using SRI monotherapy for delusional patients may sound counterintuitive, but this approach appears efficacious for patients with delusional body dysmorphic disorder, which has similarities to ORS.17,25,26
Clinically, we have found the use of atypical antipsychotics as an adjunct to SRIs to be helpful, although this strategy has not been subjected to clinical trials. Pimozide alone or in combination with an antidepressant also appears promising, as does exposure and response prevention. Do not combine pimozide with clomipramine because of the risk of cardiac toxicity.
Related resources
- Phillips KA, Gunderson C, Gruber U, Castle DJ. Delusions of body malodour: the olfactory reference syndrome. In: Brewer W, Castle D, Pantelis C. Olfaction and the brain. Cambridge, UK: Cambridge University Press; 2006:334-53.
- Pryse-Phillips W. An olfactory reference syndrome. Acta Psychiatr Scand 1971;47:484-509.
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Escitalopram • Lexapro
- Pimozide • Orap
- Quetiapine • Seroquel
- Thioridazine • Mellaril
- Trifluoperazine • Stelazine
Dr. Phillips receives research support from the National Institute of Mental Health, the Food and Drug Administration, UCB Pharma, and Forest Pharmaceuticals.
Dr. Castle receives research support from Janssen-Cilag; is a consultant to Eli Lilly and Company., Bristol-Myers Squibb, and Lundbeck; and is a speaker for Eli Lilly and Co., sanofi-aventis, Bristol-Myers Squibb, Janssen-Cilag, Lundbeck, and Organon.
Acknowledgment
The authors would like to thank Craig Gunderson, MD, and Uschi Gruber, MB, for their assistance with a literature search on olfactory reference syndrome.
1. Iwu CO, Akpata O. Delusional halitosis. Review of the literature and analysis of 32 cases. Br Dent J 1989;167:294-6.
2. Osman AA. Monosymptomatic hypochondriacal psychosis in developing countries. Br J Psychiatry 1991;159:428-31.
3. Pryse-Phillips W. An olfactory reference syndrome. Acta Psychiatr Scand 1971;47:484-509.
4. Yamada M, Shigemoto T, Kashiwamura K, et al. Fear of emitting bad odors. Bull Yamaguchi Med Sch 1977;24:141-61.
5. Potts CS. Two cases of hallucination of smell. University of Pennsylvania Medical Magazine 1891;226.-
6. Forte FS. Olfactory hallucinations as a proctologic manifestation of early schizophrenia. Am J Surg 1952;84:620-2.
7. Marks I, Mishan J. Dysmorphophobic avoidance with disturbed bodily perception: a pilot study of exposure therapy. Br J Psychiatry 1988;152:674-8.
8. Kasahara Y, Kenji S. Ereuthophobia and allied conditions: a contribution toward the psychopathological and cross-cultural study of a borderline state. In: Arieti S, ed. The world biennial of psychiatry in psychotherapy. New York, NY: Basic Books, 1971.
9. Iwakura M, Yasuno Y, Shimura M, Sakamoto S. Clinical characteristics of halitosis: differences in two patient groups with primary and secondary complaints of halitosis. J Dent Res 1994;73:1568-74.
10. Johanson E. Mild paranoia. Acta Psychiatr Scand 1964;40:13-4.
11. Sutton RL. Bromidrosiphobia. JAMA 1919;72:1267-8.
12. Malasi TH, El-Hilu SR, Mirza IA, Fakhr El-Islam M. Olfactory delusional syndrome with various aetiologies. Br J Psychiatry 1990;156:256-60.
13. Alvarez WC. Practical leads to puzzling diagnoses. Philadelphia, PA: JB Lippincott; 1958.
14. Brotman AW, Jenike MA. Monosymptomatic hypochondriasis treated with tricyclic antidepressants. Am J Psychiatry 1984;141:1608-9.
15. Videbech T. Chronic olfactory paranoid syndromes. Acta Psychiatr Scand 1966;42:183-213.
16. Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosom 2005;46:317-32.
17. Lochner C, Stein DJ. Olfactory reference syndrome: diagnostic criteria and differential diagnosis. J Postgrad Med 2003;49:328-31.
18. Dominguez RA, Puig A. Olfactory reference syndrome responds to clomipramine but not fluoxetine: a case report. J Clin Psychiatry 1997;58:497-8.
19. Ross CA, Siddiqui AR, Matas M. DSM-III. Problems in diagnosis of paranoia and obsessive-compulsive disorder. Can J Psychiatry 1987;32:146-8.
20. Ulzen TPM. Pimozide-responsive monosymptomatic hypochondriacal psychosis in an adolescent. Can J Psychiatry 1993;38:153-4.
21. Riding J, Munro A. Pimozide in the treatment of monosymptomatic hypochondriacal psychosis. Acta Psychiatr Scand 1975;52:23-30.
22. Kong SG, Tan KH. Monosymptomatic hypochondriacal psychosis: a report of 3 cases. Singapore Med J 1984;25:432-5.
23. Gomen-Perez JD, Marks IM, Gutierrez-Fisac JL. Dysmorphophobia: clinical features and outcome with behavior therapy. Eur Psychiatry 1994;9:229-35.
24. Milan MA, Kolko DJ. Paradoxical intention in the treatment of obsessional flatulence ruminations. J Behav Ther Exp Psychiatry 1982;13:167-72.
25. Phillips KA. The broken mirror: understanding and treating body dysmorphic disorder. New York, NY: Oxford University Press; 1996 (revised and expanded edition, 2005; Japanese edition, 1999).
26. Marks IM. Fears, phobias, and rituals. Oxford, UK: Oxford University Press; 1987.
Ms. A, a 21-year-old teacher, recalled always having been “sensitive,” but when she started her first job at age 19 she began to believe that she emitted an offensive odor. She experienced thoughts that she passed offensive flatus, her breath had a fecal odor, and people noticed and were offended.
Gradually Ms. A became more convinced of these distressing beliefs and began to think that she permeated fecal odor through her skin. She also became sure that colleagues were talking about her and that they complained about her “disgusting” smell.
Patients with olfactory reference syndrome (ORS) falsely believe they emit an offensive body odor. Prominent referential thinking—believing that other people perceive the odor—also is common. To introduce you to ORS, we discuss its clinical diagnosis and treatment based on our review of several hundred cases, including the largest reported series of patients with ORS.1-4
Olfactory reference syndrome (ORS) has been described around the world for more than a century. In 1891, Potts described a delusional 50-year-old man who “had been troubled for the past three months with smelling a very bad odor, which he likened to that of a ‘back-house,’ and which came from his own person. [He believed] this smell was so very strong that other men objected to working with him….”
Despite its long history, the syndrome’s prevalence is not well-established. ORS probably is underdiagnosed and more common than generally recognized:
- In a tertiary psychiatry unit in London, 0.5% of 2,000 patients who were not systematically screened for ORS spontaneously reported ORS symptoms.
- In a self-report survey of 2,481 students in Japan, 2.1% had been concerned with emitting a strange bodily odor during the past year.
- In a study in a dental clinic in Japan, the majority of patients with a primary complaint of halitosis actually had “imaginary halitosis” (another term for ORS).
Clinical features of ors
Ms. A quit her job and felt confident enough to work again only when she performed a 2-hour daily cleansing routine, doused herself in per-fume, and placed an incontinence pad in her underwear. Despite these precautions, she still thought her colleagues avoided her.
She always averted her mouth when speaking, held her hand in front of her mouth, and sat far from others and close to the door in meetings. She tried to keep meeting room doors open and believed that colleagues held their hands to their noses to “protect” themselves from her odor.
ORS symptoms are most often reported as beginning when patients are in their mid 20s, although some reports suggest onset during puberty or adolescence.4 In clinical series, the ratio of men to women is approximately 2:1.
Preoccupation. Individuals with ORS are preoccupied with the belief that they emit an unpleasant or offensive body odor (Box 1),1,2,5-9 most commonly:
Although most reports suggest that patients focus on one odor, some describe being concerned about several smells simultaneously or different odors over time.10,11
Referential thinking. As the syndrome’s name implies, many ORS patients have delusions of reference, falsely believing that other people perceive the odor.3 They misinterpret the behavior of others, assuming it is a reaction to how the patient smells (Box 2).2,3
They may misperceive comments (such as, “It’s stuffy in here”), receiving perfume or soap as a gift, or behaviors such as people sniffing, touching or rubbing their nose, clearing their throat, opening a window to get fresh air, putting a newspaper in front of their face, or looking or moving toward or away from the patient.1,3,5,7
Because they are ashamed, embarrassed, and concerned about offending others with their odor,13 many patients engage in repetitive and “safety” behaviors intended to check, eliminate, or camouflage the supposed odor (Box 3).1,3,7,12,14
DSM-IV-TR classifies olfactory reference syndrome (ORS) as a delusional disorder, somatic type (the modern equivalent of monosymptomatic hypochondriacal psychosis). ORS also is mentioned in the text on social anxiety disorder. ORS may not be diagnosed if:
- criterion A for schizophrenia has ever been met
- or if symptoms are due to the direct physiologic effects of a substance or a general medical condition, such as a brain tumor or temporal lobe epilepsy.
Many patients report being able to smell the imagined odor, suggesting that they experience an olfactory hallucination. Pryse-Phillips described the olfactory hallucinations of her 36 ORS case patients as “a real and immediate perception… often perceived in the absence of other odors.”
ORS generally is regarded as delusional, with possible secondary illusional misinterpretations and referential thinking. ORS beliefs usually appear to be of delusional intensity, although some patients may have some—although limited—insight (that is, overvalued ideation).
Functional impairment. Individuals with ORS often avoid other people or believe that others avoid them.12 They are typically embarrassed and worried that others will be offended by the smell. They may:
- avoid activities such as dating
- break off engagements
- refuse to travel
- move to another town
- become housebound.3,7,10,12
The distress and impaired functioning may lead to psychiatric hospitalization, depression, suicidal ideation, suicide attempts, and completed suicide.7,10,12,15 Pryse-Phillips studied 36 patients with ORS and reported:
- nearly one-half (43%) experienced “suicidal ideas or action”
- 2 (5.6%) committed suicide.3
Some authors have questioned whether ORS can transform into schizophrenia, but others have found little evidence for this.3,6
Psychiatric comorbidity. Depression is mentioned most often in the literature.12,13 In Pryse-Phillips’ 36 ORS patients (who did not have a “primary” depressive disorder), depression symptoms tended to be severe.3 The depression generally is considered secondary to ORS, although Pryse-Phillips evaluated 50 additional patients with ORS symptoms whom she considered to have a “primary” depressive disorder.3 Other psychiatric comorbidities include bipolar disorder, personality disorder, schizophrenia, hypochondriasis, alcohol and/or drug abuse, obsessive-compulsive disorder (OCD), and body dysmorphic disorder.3,7,15 In a study of 200 individuals with body dysmorphic disorder, 8 had comorbid ORS.16
Diagnosing ORS
Clinical clues to ORS (Table 1) probably are not present in all patients and some are not specific to ORS. They appear to be common features of the illness, however, and may alert you to its presence. Our clinical impression is that many patients with ORS are secretive about their symptoms because they are ashamed of them. Thus, you need to be alert to clues and specifically inquire about ORS symptoms to detect its presence.
Criteria. DSM-IV-TR and ICD-10 lack specific diagnostic criteria for ORS, instead applying criteria for delusional disorder. One problem with this approach is that delusional disorder criteria specify that any co-occurring mood symptoms must be brief relative to the duration of the delusional periods. This requirement may not be valid when applied to ORS.
In our experience, some patients have protracted depressive symptoms that appear secondary to “primary” ORS symptoms, and another diagnosis—such as psychotic depression—does not appear to account for their symptoms.
We propose working diagnostic criteria for ORS (Table 2), which are similar to those proposed by Lochner and Stein17 and require empiric validation. Suggested questions for the patient interview (Table 3) can help you identify and diagnose ORS.
Differential diagnosis. Keep in mind that a false belief that one emits a bad smell may be a symptom of schizophrenia, and this would trump an ORS diagnosis if other schizophrenia symptoms are present. Some patients with severe depression may believe they smell bad as part of a nihilistic delusional belief system (such as in Cotard’s syndrome—nihilistic delusions in severe depression).
Whether to conceptualize a false belief about body odor as a symptom of depression or as ORS with comorbid or secondary depression may be unclear from case to case.
Table 1
Clinical clues to the presence of olfactory reference syndrome
| Referential thinking. Interpreting actions of others—such as opening a window, moving away, putting a hand to their nose, or making comments related to odors—as evidence that the person smells offensive |
| Excessive attempts to ‘disguise’ the smell, such as washing routines, clothes changing, clothes laundering, or using abundant perfume, deodorant, mouthwash, mints, or other forms of camouflage |
| Other excessive and repetitive behaviors, such as checking for or asking other people for reassurance about the odor |
| Social anxiety or avoidance of social activities, relationships, work, school, or other daily activities |
| Requests for treatment for the perceived odor from dentists, gastroenterologists, proctologists, or other nonpsychiatric physicians despite a negative medical workup |
Working diagnostic criteria for olfactory reference syndrome
| A. | Persistent false belief that one emits a malodorous smell; this belief may encompass a range of insight and does not have to be delusional |
| B. | The belief is time-consuming and preoccupies the individual for at least 1 hour per day |
| C. | The belief causes clinically significant distress or results in significant impairment in social, occupational, or other important areas of functioning |
| D. | The belief is not better accounted for by another mental disorder or a general medical condition |
Table 3
Diagnosing ORS: Suggested questions for patient interview
|
Treatment-seeking behavior
Ms. A consulted several proctologists and a dentist but was not convinced by their reassurance and continued to believe she “stank.” Her relationship with her boyfriend suffered because she continually asked for reassurance about how she smelled and avoided sexual intercourse because of her odor concerns.
Eventually she confronted her boss about her belief that her coworkers were complaining about her smell. Despite reassurance that she didn’t smell bad, she left her job.
Excessive showering or washing are among the repetitive, ritualistic or “safety” behaviors many patients with ORS engage in to check, eliminate, or camouflage supposed odor. Frequent clothes changing or laundering also is common.
Camouflaging attempts may include excessive use of deodorant, soap, cologne, powder, mints, mouthwash, or toothpaste; wearing layers of clothing; or smoking.
Many patients frequently check for the odor or its source (such as trying to smell their own breath or checking the anal area for seepage). Some patients use the toilet excessively or eat a special diet to try to minimize the smell. Others repeatedly seek reassurance about how they smell.
Avoidance behaviors are common and include sitting far from other people, moving as little as possible to avoid spreading the supposed odor, or averting the head or covering the mouth.
Convincing patients such as Ms. A of the falsity of their beliefs can be difficult,1 and some succeed in having medical procedures or surgery, such as excision of tonsils or axillary glands.3,7,12 To our knowledge, controlled prospective studies of nonpsychiatric treatments have not been done, but it appears that such treatments usually are ineffective.1,3,6,9
Psychiatric interventions. Convincing patients with ORS to obtain mental health treatment can be difficult.2,6 Patients with delusional halitosis “would rather go in search of a ‘better dentist’ than go to a psychiatrist.”1
To get patients to accept psychiatric treatment, we suggest an approach similar to that recommended for body dysmorphic disorder. It may be helpful, for example, to focus on the distress and disability caused by the odor preoccupation, rather than on whether the patient actually smells bad.
Medication and psychotherapy
Limited evidence. The ORS treatment literature is very limited, consisting largely of case reports and small case series. To our knowledge, no controlled treatment trials have been done, no treatments have been compared head to head, and most studies did not use standardized measurements of psychopathology.
Published data therefore must be interpreted cautiously. Some medication reports used relatively low doses and short treatment durations (although what constitutes an adequate therapeutic trial for ORS is not known). Psychotherapy reports often did not specify details of the intervention or the number and duration of sessions. It is not known whether adding a cognitive component to behavioral therapy enhances efficacy, and the combination of psychotherapy and medication has not been studied systematically. More methodologically rigorous treatment studies are needed.
Because of space limitations, we cite representative case reports in the following section of this treatment review, rather than all of the cases found in our literature search.
Antidepressants. Although most ORS patients are delusional, serotonin reuptake inhibitor (SRI) monotherapy has been reported to be efficacious in 10 of 15 cases (67%). Most of these patients received clomipramine.18 In reports of non-SRI antidepressants, 6 of 15 cases (43%) responded. Some patients’ symptoms responded to an antidepressant after failing to respond to antipsychotic treatment.19
Antipsychotics. Pimozide is the most studied medication for ORS, with 15 of 31 cases (48%) responding.2,20 In a series of 12 patients, pimozide responders received 2 to 4 mg/d, except for one patient who needed 6 mg/d.21 Patients usually responded within 1 to 4 weeks (an average time to response was not reported). In 2 of these cases, ORS symptoms recurred after pimozide was discontinued and then remitted again after it was restarted.21 In another report,2 7 of 14 patients (50%) responded to pimozide.
Clinicians using other first-generation antipsychotics (trifluoperazine, thioridazine, and chlorpromazine) reported a positive response in only 2 of 19 cases (11%).12,22
Combination therapy. Ten of 17 cases (59%) of ORS responded to combined treatment with an antidepressant and an antipsychotic.2,12
Other somatic treatments. Several reports found benzodiazepine monotherapy lacked efficacy, as was the case for electroconvulsive therapy.12,15 One report noted an unsuccessful outcome with leucotomy and a partial response with bilateral partial division of the thalamo-frontal tract.15
Psychosocial treatment. All reports of psychosocial therapies are single cases or small series, and none used a control intervention.2,7,14
Behavioral treatment has been efficacious, although patients require months to years to habituate. Several reports totalling 14 patients describe behavioral treatment over weeks to months.7,23 These treatments involved exposure to avoided social situations and response prevention, which consisted of refraining from repetitive or camouflaging behaviors such as showering, visits to the toilet, or deodorant use. Gomez-Perez and colleagues23 noted that exposure therapy was less effective for ORS than for social phobia or OCD.
One report described a patient with flatulence concerns who responded to a paradoxical intention consisting of instructions to emit gas as soon as it was experienced; at 1-year follow-up, her symptoms had not recurred.24
Psychodynamic interventions show no benefit for ORS symptoms.
Treatment summary
Ms. A became increasingly despondent and depressed. She eventually sought the help of her family doctor, who referred her to a psychiatrist. With a combination of a serotonergic antidepressant (escitalopram, 40 mg/d), a low-dose atypical antipsychotic (quetiapine, 50 mg at night), and cognitive-behavioral therapy, she started to re-engage in daily activities. During 6 months of treatment, the intensity of her belief about having body odor abated.
Limited data support the use of SRI monotherapy or an SRI plus an antipsychotic. Using SRI monotherapy for delusional patients may sound counterintuitive, but this approach appears efficacious for patients with delusional body dysmorphic disorder, which has similarities to ORS.17,25,26
Clinically, we have found the use of atypical antipsychotics as an adjunct to SRIs to be helpful, although this strategy has not been subjected to clinical trials. Pimozide alone or in combination with an antidepressant also appears promising, as does exposure and response prevention. Do not combine pimozide with clomipramine because of the risk of cardiac toxicity.
Related resources
- Phillips KA, Gunderson C, Gruber U, Castle DJ. Delusions of body malodour: the olfactory reference syndrome. In: Brewer W, Castle D, Pantelis C. Olfaction and the brain. Cambridge, UK: Cambridge University Press; 2006:334-53.
- Pryse-Phillips W. An olfactory reference syndrome. Acta Psychiatr Scand 1971;47:484-509.
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Escitalopram • Lexapro
- Pimozide • Orap
- Quetiapine • Seroquel
- Thioridazine • Mellaril
- Trifluoperazine • Stelazine
Dr. Phillips receives research support from the National Institute of Mental Health, the Food and Drug Administration, UCB Pharma, and Forest Pharmaceuticals.
Dr. Castle receives research support from Janssen-Cilag; is a consultant to Eli Lilly and Company., Bristol-Myers Squibb, and Lundbeck; and is a speaker for Eli Lilly and Co., sanofi-aventis, Bristol-Myers Squibb, Janssen-Cilag, Lundbeck, and Organon.
Acknowledgment
The authors would like to thank Craig Gunderson, MD, and Uschi Gruber, MB, for their assistance with a literature search on olfactory reference syndrome.
Ms. A, a 21-year-old teacher, recalled always having been “sensitive,” but when she started her first job at age 19 she began to believe that she emitted an offensive odor. She experienced thoughts that she passed offensive flatus, her breath had a fecal odor, and people noticed and were offended.
Gradually Ms. A became more convinced of these distressing beliefs and began to think that she permeated fecal odor through her skin. She also became sure that colleagues were talking about her and that they complained about her “disgusting” smell.
Patients with olfactory reference syndrome (ORS) falsely believe they emit an offensive body odor. Prominent referential thinking—believing that other people perceive the odor—also is common. To introduce you to ORS, we discuss its clinical diagnosis and treatment based on our review of several hundred cases, including the largest reported series of patients with ORS.1-4
Olfactory reference syndrome (ORS) has been described around the world for more than a century. In 1891, Potts described a delusional 50-year-old man who “had been troubled for the past three months with smelling a very bad odor, which he likened to that of a ‘back-house,’ and which came from his own person. [He believed] this smell was so very strong that other men objected to working with him….”
Despite its long history, the syndrome’s prevalence is not well-established. ORS probably is underdiagnosed and more common than generally recognized:
- In a tertiary psychiatry unit in London, 0.5% of 2,000 patients who were not systematically screened for ORS spontaneously reported ORS symptoms.
- In a self-report survey of 2,481 students in Japan, 2.1% had been concerned with emitting a strange bodily odor during the past year.
- In a study in a dental clinic in Japan, the majority of patients with a primary complaint of halitosis actually had “imaginary halitosis” (another term for ORS).
Clinical features of ors
Ms. A quit her job and felt confident enough to work again only when she performed a 2-hour daily cleansing routine, doused herself in per-fume, and placed an incontinence pad in her underwear. Despite these precautions, she still thought her colleagues avoided her.
She always averted her mouth when speaking, held her hand in front of her mouth, and sat far from others and close to the door in meetings. She tried to keep meeting room doors open and believed that colleagues held their hands to their noses to “protect” themselves from her odor.
ORS symptoms are most often reported as beginning when patients are in their mid 20s, although some reports suggest onset during puberty or adolescence.4 In clinical series, the ratio of men to women is approximately 2:1.
Preoccupation. Individuals with ORS are preoccupied with the belief that they emit an unpleasant or offensive body odor (Box 1),1,2,5-9 most commonly:
Although most reports suggest that patients focus on one odor, some describe being concerned about several smells simultaneously or different odors over time.10,11
Referential thinking. As the syndrome’s name implies, many ORS patients have delusions of reference, falsely believing that other people perceive the odor.3 They misinterpret the behavior of others, assuming it is a reaction to how the patient smells (Box 2).2,3
They may misperceive comments (such as, “It’s stuffy in here”), receiving perfume or soap as a gift, or behaviors such as people sniffing, touching or rubbing their nose, clearing their throat, opening a window to get fresh air, putting a newspaper in front of their face, or looking or moving toward or away from the patient.1,3,5,7
Because they are ashamed, embarrassed, and concerned about offending others with their odor,13 many patients engage in repetitive and “safety” behaviors intended to check, eliminate, or camouflage the supposed odor (Box 3).1,3,7,12,14
DSM-IV-TR classifies olfactory reference syndrome (ORS) as a delusional disorder, somatic type (the modern equivalent of monosymptomatic hypochondriacal psychosis). ORS also is mentioned in the text on social anxiety disorder. ORS may not be diagnosed if:
- criterion A for schizophrenia has ever been met
- or if symptoms are due to the direct physiologic effects of a substance or a general medical condition, such as a brain tumor or temporal lobe epilepsy.
Many patients report being able to smell the imagined odor, suggesting that they experience an olfactory hallucination. Pryse-Phillips described the olfactory hallucinations of her 36 ORS case patients as “a real and immediate perception… often perceived in the absence of other odors.”
ORS generally is regarded as delusional, with possible secondary illusional misinterpretations and referential thinking. ORS beliefs usually appear to be of delusional intensity, although some patients may have some—although limited—insight (that is, overvalued ideation).
Functional impairment. Individuals with ORS often avoid other people or believe that others avoid them.12 They are typically embarrassed and worried that others will be offended by the smell. They may:
- avoid activities such as dating
- break off engagements
- refuse to travel
- move to another town
- become housebound.3,7,10,12
The distress and impaired functioning may lead to psychiatric hospitalization, depression, suicidal ideation, suicide attempts, and completed suicide.7,10,12,15 Pryse-Phillips studied 36 patients with ORS and reported:
- nearly one-half (43%) experienced “suicidal ideas or action”
- 2 (5.6%) committed suicide.3
Some authors have questioned whether ORS can transform into schizophrenia, but others have found little evidence for this.3,6
Psychiatric comorbidity. Depression is mentioned most often in the literature.12,13 In Pryse-Phillips’ 36 ORS patients (who did not have a “primary” depressive disorder), depression symptoms tended to be severe.3 The depression generally is considered secondary to ORS, although Pryse-Phillips evaluated 50 additional patients with ORS symptoms whom she considered to have a “primary” depressive disorder.3 Other psychiatric comorbidities include bipolar disorder, personality disorder, schizophrenia, hypochondriasis, alcohol and/or drug abuse, obsessive-compulsive disorder (OCD), and body dysmorphic disorder.3,7,15 In a study of 200 individuals with body dysmorphic disorder, 8 had comorbid ORS.16
Diagnosing ORS
Clinical clues to ORS (Table 1) probably are not present in all patients and some are not specific to ORS. They appear to be common features of the illness, however, and may alert you to its presence. Our clinical impression is that many patients with ORS are secretive about their symptoms because they are ashamed of them. Thus, you need to be alert to clues and specifically inquire about ORS symptoms to detect its presence.
Criteria. DSM-IV-TR and ICD-10 lack specific diagnostic criteria for ORS, instead applying criteria for delusional disorder. One problem with this approach is that delusional disorder criteria specify that any co-occurring mood symptoms must be brief relative to the duration of the delusional periods. This requirement may not be valid when applied to ORS.
In our experience, some patients have protracted depressive symptoms that appear secondary to “primary” ORS symptoms, and another diagnosis—such as psychotic depression—does not appear to account for their symptoms.
We propose working diagnostic criteria for ORS (Table 2), which are similar to those proposed by Lochner and Stein17 and require empiric validation. Suggested questions for the patient interview (Table 3) can help you identify and diagnose ORS.
Differential diagnosis. Keep in mind that a false belief that one emits a bad smell may be a symptom of schizophrenia, and this would trump an ORS diagnosis if other schizophrenia symptoms are present. Some patients with severe depression may believe they smell bad as part of a nihilistic delusional belief system (such as in Cotard’s syndrome—nihilistic delusions in severe depression).
Whether to conceptualize a false belief about body odor as a symptom of depression or as ORS with comorbid or secondary depression may be unclear from case to case.
Table 1
Clinical clues to the presence of olfactory reference syndrome
| Referential thinking. Interpreting actions of others—such as opening a window, moving away, putting a hand to their nose, or making comments related to odors—as evidence that the person smells offensive |
| Excessive attempts to ‘disguise’ the smell, such as washing routines, clothes changing, clothes laundering, or using abundant perfume, deodorant, mouthwash, mints, or other forms of camouflage |
| Other excessive and repetitive behaviors, such as checking for or asking other people for reassurance about the odor |
| Social anxiety or avoidance of social activities, relationships, work, school, or other daily activities |
| Requests for treatment for the perceived odor from dentists, gastroenterologists, proctologists, or other nonpsychiatric physicians despite a negative medical workup |
Working diagnostic criteria for olfactory reference syndrome
| A. | Persistent false belief that one emits a malodorous smell; this belief may encompass a range of insight and does not have to be delusional |
| B. | The belief is time-consuming and preoccupies the individual for at least 1 hour per day |
| C. | The belief causes clinically significant distress or results in significant impairment in social, occupational, or other important areas of functioning |
| D. | The belief is not better accounted for by another mental disorder or a general medical condition |
Table 3
Diagnosing ORS: Suggested questions for patient interview
|
Treatment-seeking behavior
Ms. A consulted several proctologists and a dentist but was not convinced by their reassurance and continued to believe she “stank.” Her relationship with her boyfriend suffered because she continually asked for reassurance about how she smelled and avoided sexual intercourse because of her odor concerns.
Eventually she confronted her boss about her belief that her coworkers were complaining about her smell. Despite reassurance that she didn’t smell bad, she left her job.
Excessive showering or washing are among the repetitive, ritualistic or “safety” behaviors many patients with ORS engage in to check, eliminate, or camouflage supposed odor. Frequent clothes changing or laundering also is common.
Camouflaging attempts may include excessive use of deodorant, soap, cologne, powder, mints, mouthwash, or toothpaste; wearing layers of clothing; or smoking.
Many patients frequently check for the odor or its source (such as trying to smell their own breath or checking the anal area for seepage). Some patients use the toilet excessively or eat a special diet to try to minimize the smell. Others repeatedly seek reassurance about how they smell.
Avoidance behaviors are common and include sitting far from other people, moving as little as possible to avoid spreading the supposed odor, or averting the head or covering the mouth.
Convincing patients such as Ms. A of the falsity of their beliefs can be difficult,1 and some succeed in having medical procedures or surgery, such as excision of tonsils or axillary glands.3,7,12 To our knowledge, controlled prospective studies of nonpsychiatric treatments have not been done, but it appears that such treatments usually are ineffective.1,3,6,9
Psychiatric interventions. Convincing patients with ORS to obtain mental health treatment can be difficult.2,6 Patients with delusional halitosis “would rather go in search of a ‘better dentist’ than go to a psychiatrist.”1
To get patients to accept psychiatric treatment, we suggest an approach similar to that recommended for body dysmorphic disorder. It may be helpful, for example, to focus on the distress and disability caused by the odor preoccupation, rather than on whether the patient actually smells bad.
Medication and psychotherapy
Limited evidence. The ORS treatment literature is very limited, consisting largely of case reports and small case series. To our knowledge, no controlled treatment trials have been done, no treatments have been compared head to head, and most studies did not use standardized measurements of psychopathology.
Published data therefore must be interpreted cautiously. Some medication reports used relatively low doses and short treatment durations (although what constitutes an adequate therapeutic trial for ORS is not known). Psychotherapy reports often did not specify details of the intervention or the number and duration of sessions. It is not known whether adding a cognitive component to behavioral therapy enhances efficacy, and the combination of psychotherapy and medication has not been studied systematically. More methodologically rigorous treatment studies are needed.
Because of space limitations, we cite representative case reports in the following section of this treatment review, rather than all of the cases found in our literature search.
Antidepressants. Although most ORS patients are delusional, serotonin reuptake inhibitor (SRI) monotherapy has been reported to be efficacious in 10 of 15 cases (67%). Most of these patients received clomipramine.18 In reports of non-SRI antidepressants, 6 of 15 cases (43%) responded. Some patients’ symptoms responded to an antidepressant after failing to respond to antipsychotic treatment.19
Antipsychotics. Pimozide is the most studied medication for ORS, with 15 of 31 cases (48%) responding.2,20 In a series of 12 patients, pimozide responders received 2 to 4 mg/d, except for one patient who needed 6 mg/d.21 Patients usually responded within 1 to 4 weeks (an average time to response was not reported). In 2 of these cases, ORS symptoms recurred after pimozide was discontinued and then remitted again after it was restarted.21 In another report,2 7 of 14 patients (50%) responded to pimozide.
Clinicians using other first-generation antipsychotics (trifluoperazine, thioridazine, and chlorpromazine) reported a positive response in only 2 of 19 cases (11%).12,22
Combination therapy. Ten of 17 cases (59%) of ORS responded to combined treatment with an antidepressant and an antipsychotic.2,12
Other somatic treatments. Several reports found benzodiazepine monotherapy lacked efficacy, as was the case for electroconvulsive therapy.12,15 One report noted an unsuccessful outcome with leucotomy and a partial response with bilateral partial division of the thalamo-frontal tract.15
Psychosocial treatment. All reports of psychosocial therapies are single cases or small series, and none used a control intervention.2,7,14
Behavioral treatment has been efficacious, although patients require months to years to habituate. Several reports totalling 14 patients describe behavioral treatment over weeks to months.7,23 These treatments involved exposure to avoided social situations and response prevention, which consisted of refraining from repetitive or camouflaging behaviors such as showering, visits to the toilet, or deodorant use. Gomez-Perez and colleagues23 noted that exposure therapy was less effective for ORS than for social phobia or OCD.
One report described a patient with flatulence concerns who responded to a paradoxical intention consisting of instructions to emit gas as soon as it was experienced; at 1-year follow-up, her symptoms had not recurred.24
Psychodynamic interventions show no benefit for ORS symptoms.
Treatment summary
Ms. A became increasingly despondent and depressed. She eventually sought the help of her family doctor, who referred her to a psychiatrist. With a combination of a serotonergic antidepressant (escitalopram, 40 mg/d), a low-dose atypical antipsychotic (quetiapine, 50 mg at night), and cognitive-behavioral therapy, she started to re-engage in daily activities. During 6 months of treatment, the intensity of her belief about having body odor abated.
Limited data support the use of SRI monotherapy or an SRI plus an antipsychotic. Using SRI monotherapy for delusional patients may sound counterintuitive, but this approach appears efficacious for patients with delusional body dysmorphic disorder, which has similarities to ORS.17,25,26
Clinically, we have found the use of atypical antipsychotics as an adjunct to SRIs to be helpful, although this strategy has not been subjected to clinical trials. Pimozide alone or in combination with an antidepressant also appears promising, as does exposure and response prevention. Do not combine pimozide with clomipramine because of the risk of cardiac toxicity.
Related resources
- Phillips KA, Gunderson C, Gruber U, Castle DJ. Delusions of body malodour: the olfactory reference syndrome. In: Brewer W, Castle D, Pantelis C. Olfaction and the brain. Cambridge, UK: Cambridge University Press; 2006:334-53.
- Pryse-Phillips W. An olfactory reference syndrome. Acta Psychiatr Scand 1971;47:484-509.
- Chlorpromazine • Thorazine
- Clomipramine • Anafranil
- Escitalopram • Lexapro
- Pimozide • Orap
- Quetiapine • Seroquel
- Thioridazine • Mellaril
- Trifluoperazine • Stelazine
Dr. Phillips receives research support from the National Institute of Mental Health, the Food and Drug Administration, UCB Pharma, and Forest Pharmaceuticals.
Dr. Castle receives research support from Janssen-Cilag; is a consultant to Eli Lilly and Company., Bristol-Myers Squibb, and Lundbeck; and is a speaker for Eli Lilly and Co., sanofi-aventis, Bristol-Myers Squibb, Janssen-Cilag, Lundbeck, and Organon.
Acknowledgment
The authors would like to thank Craig Gunderson, MD, and Uschi Gruber, MB, for their assistance with a literature search on olfactory reference syndrome.
1. Iwu CO, Akpata O. Delusional halitosis. Review of the literature and analysis of 32 cases. Br Dent J 1989;167:294-6.
2. Osman AA. Monosymptomatic hypochondriacal psychosis in developing countries. Br J Psychiatry 1991;159:428-31.
3. Pryse-Phillips W. An olfactory reference syndrome. Acta Psychiatr Scand 1971;47:484-509.
4. Yamada M, Shigemoto T, Kashiwamura K, et al. Fear of emitting bad odors. Bull Yamaguchi Med Sch 1977;24:141-61.
5. Potts CS. Two cases of hallucination of smell. University of Pennsylvania Medical Magazine 1891;226.-
6. Forte FS. Olfactory hallucinations as a proctologic manifestation of early schizophrenia. Am J Surg 1952;84:620-2.
7. Marks I, Mishan J. Dysmorphophobic avoidance with disturbed bodily perception: a pilot study of exposure therapy. Br J Psychiatry 1988;152:674-8.
8. Kasahara Y, Kenji S. Ereuthophobia and allied conditions: a contribution toward the psychopathological and cross-cultural study of a borderline state. In: Arieti S, ed. The world biennial of psychiatry in psychotherapy. New York, NY: Basic Books, 1971.
9. Iwakura M, Yasuno Y, Shimura M, Sakamoto S. Clinical characteristics of halitosis: differences in two patient groups with primary and secondary complaints of halitosis. J Dent Res 1994;73:1568-74.
10. Johanson E. Mild paranoia. Acta Psychiatr Scand 1964;40:13-4.
11. Sutton RL. Bromidrosiphobia. JAMA 1919;72:1267-8.
12. Malasi TH, El-Hilu SR, Mirza IA, Fakhr El-Islam M. Olfactory delusional syndrome with various aetiologies. Br J Psychiatry 1990;156:256-60.
13. Alvarez WC. Practical leads to puzzling diagnoses. Philadelphia, PA: JB Lippincott; 1958.
14. Brotman AW, Jenike MA. Monosymptomatic hypochondriasis treated with tricyclic antidepressants. Am J Psychiatry 1984;141:1608-9.
15. Videbech T. Chronic olfactory paranoid syndromes. Acta Psychiatr Scand 1966;42:183-213.
16. Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosom 2005;46:317-32.
17. Lochner C, Stein DJ. Olfactory reference syndrome: diagnostic criteria and differential diagnosis. J Postgrad Med 2003;49:328-31.
18. Dominguez RA, Puig A. Olfactory reference syndrome responds to clomipramine but not fluoxetine: a case report. J Clin Psychiatry 1997;58:497-8.
19. Ross CA, Siddiqui AR, Matas M. DSM-III. Problems in diagnosis of paranoia and obsessive-compulsive disorder. Can J Psychiatry 1987;32:146-8.
20. Ulzen TPM. Pimozide-responsive monosymptomatic hypochondriacal psychosis in an adolescent. Can J Psychiatry 1993;38:153-4.
21. Riding J, Munro A. Pimozide in the treatment of monosymptomatic hypochondriacal psychosis. Acta Psychiatr Scand 1975;52:23-30.
22. Kong SG, Tan KH. Monosymptomatic hypochondriacal psychosis: a report of 3 cases. Singapore Med J 1984;25:432-5.
23. Gomen-Perez JD, Marks IM, Gutierrez-Fisac JL. Dysmorphophobia: clinical features and outcome with behavior therapy. Eur Psychiatry 1994;9:229-35.
24. Milan MA, Kolko DJ. Paradoxical intention in the treatment of obsessional flatulence ruminations. J Behav Ther Exp Psychiatry 1982;13:167-72.
25. Phillips KA. The broken mirror: understanding and treating body dysmorphic disorder. New York, NY: Oxford University Press; 1996 (revised and expanded edition, 2005; Japanese edition, 1999).
26. Marks IM. Fears, phobias, and rituals. Oxford, UK: Oxford University Press; 1987.
1. Iwu CO, Akpata O. Delusional halitosis. Review of the literature and analysis of 32 cases. Br Dent J 1989;167:294-6.
2. Osman AA. Monosymptomatic hypochondriacal psychosis in developing countries. Br J Psychiatry 1991;159:428-31.
3. Pryse-Phillips W. An olfactory reference syndrome. Acta Psychiatr Scand 1971;47:484-509.
4. Yamada M, Shigemoto T, Kashiwamura K, et al. Fear of emitting bad odors. Bull Yamaguchi Med Sch 1977;24:141-61.
5. Potts CS. Two cases of hallucination of smell. University of Pennsylvania Medical Magazine 1891;226.-
6. Forte FS. Olfactory hallucinations as a proctologic manifestation of early schizophrenia. Am J Surg 1952;84:620-2.
7. Marks I, Mishan J. Dysmorphophobic avoidance with disturbed bodily perception: a pilot study of exposure therapy. Br J Psychiatry 1988;152:674-8.
8. Kasahara Y, Kenji S. Ereuthophobia and allied conditions: a contribution toward the psychopathological and cross-cultural study of a borderline state. In: Arieti S, ed. The world biennial of psychiatry in psychotherapy. New York, NY: Basic Books, 1971.
9. Iwakura M, Yasuno Y, Shimura M, Sakamoto S. Clinical characteristics of halitosis: differences in two patient groups with primary and secondary complaints of halitosis. J Dent Res 1994;73:1568-74.
10. Johanson E. Mild paranoia. Acta Psychiatr Scand 1964;40:13-4.
11. Sutton RL. Bromidrosiphobia. JAMA 1919;72:1267-8.
12. Malasi TH, El-Hilu SR, Mirza IA, Fakhr El-Islam M. Olfactory delusional syndrome with various aetiologies. Br J Psychiatry 1990;156:256-60.
13. Alvarez WC. Practical leads to puzzling diagnoses. Philadelphia, PA: JB Lippincott; 1958.
14. Brotman AW, Jenike MA. Monosymptomatic hypochondriasis treated with tricyclic antidepressants. Am J Psychiatry 1984;141:1608-9.
15. Videbech T. Chronic olfactory paranoid syndromes. Acta Psychiatr Scand 1966;42:183-213.
16. Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosom 2005;46:317-32.
17. Lochner C, Stein DJ. Olfactory reference syndrome: diagnostic criteria and differential diagnosis. J Postgrad Med 2003;49:328-31.
18. Dominguez RA, Puig A. Olfactory reference syndrome responds to clomipramine but not fluoxetine: a case report. J Clin Psychiatry 1997;58:497-8.
19. Ross CA, Siddiqui AR, Matas M. DSM-III. Problems in diagnosis of paranoia and obsessive-compulsive disorder. Can J Psychiatry 1987;32:146-8.
20. Ulzen TPM. Pimozide-responsive monosymptomatic hypochondriacal psychosis in an adolescent. Can J Psychiatry 1993;38:153-4.
21. Riding J, Munro A. Pimozide in the treatment of monosymptomatic hypochondriacal psychosis. Acta Psychiatr Scand 1975;52:23-30.
22. Kong SG, Tan KH. Monosymptomatic hypochondriacal psychosis: a report of 3 cases. Singapore Med J 1984;25:432-5.
23. Gomen-Perez JD, Marks IM, Gutierrez-Fisac JL. Dysmorphophobia: clinical features and outcome with behavior therapy. Eur Psychiatry 1994;9:229-35.
24. Milan MA, Kolko DJ. Paradoxical intention in the treatment of obsessional flatulence ruminations. J Behav Ther Exp Psychiatry 1982;13:167-72.
25. Phillips KA. The broken mirror: understanding and treating body dysmorphic disorder. New York, NY: Oxford University Press; 1996 (revised and expanded edition, 2005; Japanese edition, 1999).
26. Marks IM. Fears, phobias, and rituals. Oxford, UK: Oxford University Press; 1987.