User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Drs. Tandon and Constantine reply
We thank Dr. Helmuth and the principal investigators of CATIE for their interest in our article and the opportunity to further clarify a key learning point from CATIE.
Dr. Helmuth acknowledges our balanced review but suggests that cost and metabolic side effects should be considered along with lower EPS liability in selecting antipsychotic therapy. We agree. Avoiding EPS while obtaining a good antipsychotic effect is one key consideration in providing optimal antipsychotic therapy. Other adverse effects, patient preference, cost, and other factors are all important considerations in this complex process of individually optimizing antipsychotic treatment.
The CATIE investigators agree with our interpretation of the study’s principal findings. They take exception, however, to our suggestion that CATIE’s finding of no FGA-SGA difference in EPS and TD may be related to its relatively low assay sensitivity to detect such differences because the sample studied was at low risk for EPS and TD.
While accepting our description of the study sample as accurate, they disagree that it was at low risk for EPS and TD. Patients who have been ill for 16 years and received antipsychotic treatment for an average of 14 years without developing TD or severe EPS (as in CATIE) are by definition at low risk for EPS and TD.
We agree that patients without current TD and who are at risk for developing it comprise the best study population to investigate differential risk for TD; however, patients who have not developed it, despite 14 years of antipsychotic therapy, are at very low risk for developing it at all. First-episode patients without prior antipsychotic exposure would be an optimal study population, but such patients were excluded from CATIE.
Drs. Rosenheck and colleagues do not disagree with any of the other assertions in our article; they are, however, critical of “numerous factual errors in other published critiques of CATIE.” We cannot address such supposed inaccuracies, which are best taken up with authors of those commentaries.
To extract maximum value from this important initiative, we must better understand CATIE’s findings in the context of its study design and the results of other relevant studies. Neither mischaracterization nor overinterpretation of CATIE’s findings helps clinicians, patients, and policy-makers.
The essence of our article was that avoiding motor, cognitive, and affective EPS due to unmodulated dopamine blockade is the key to realizing the “atypical benefits” of a broader spectrum of efficacy and lower risk of TD during antipsychotic therapy. Neither Dr. Helmuth nor Drs. Rosenheck and colleagues appear to disagree with this assertion. Avoiding broadly defined EPS appears to be critical to improving cognition, dysphoria, and negative symptoms with SGAs and FGAs. The lower risk of TD observed with SGAs also appears to be related to the greater ease with which they can provide an equivalent antipsychotic effect without EPS.
Rajiv Tandon, MD
Adjunct professor of psychiatry
University of Florida, Tallahassee
Robert Constantine, PhD
Research associate professor
University of South Florida, Tampa
We thank Dr. Helmuth and the principal investigators of CATIE for their interest in our article and the opportunity to further clarify a key learning point from CATIE.
Dr. Helmuth acknowledges our balanced review but suggests that cost and metabolic side effects should be considered along with lower EPS liability in selecting antipsychotic therapy. We agree. Avoiding EPS while obtaining a good antipsychotic effect is one key consideration in providing optimal antipsychotic therapy. Other adverse effects, patient preference, cost, and other factors are all important considerations in this complex process of individually optimizing antipsychotic treatment.
The CATIE investigators agree with our interpretation of the study’s principal findings. They take exception, however, to our suggestion that CATIE’s finding of no FGA-SGA difference in EPS and TD may be related to its relatively low assay sensitivity to detect such differences because the sample studied was at low risk for EPS and TD.
While accepting our description of the study sample as accurate, they disagree that it was at low risk for EPS and TD. Patients who have been ill for 16 years and received antipsychotic treatment for an average of 14 years without developing TD or severe EPS (as in CATIE) are by definition at low risk for EPS and TD.
We agree that patients without current TD and who are at risk for developing it comprise the best study population to investigate differential risk for TD; however, patients who have not developed it, despite 14 years of antipsychotic therapy, are at very low risk for developing it at all. First-episode patients without prior antipsychotic exposure would be an optimal study population, but such patients were excluded from CATIE.
Drs. Rosenheck and colleagues do not disagree with any of the other assertions in our article; they are, however, critical of “numerous factual errors in other published critiques of CATIE.” We cannot address such supposed inaccuracies, which are best taken up with authors of those commentaries.
To extract maximum value from this important initiative, we must better understand CATIE’s findings in the context of its study design and the results of other relevant studies. Neither mischaracterization nor overinterpretation of CATIE’s findings helps clinicians, patients, and policy-makers.
The essence of our article was that avoiding motor, cognitive, and affective EPS due to unmodulated dopamine blockade is the key to realizing the “atypical benefits” of a broader spectrum of efficacy and lower risk of TD during antipsychotic therapy. Neither Dr. Helmuth nor Drs. Rosenheck and colleagues appear to disagree with this assertion. Avoiding broadly defined EPS appears to be critical to improving cognition, dysphoria, and negative symptoms with SGAs and FGAs. The lower risk of TD observed with SGAs also appears to be related to the greater ease with which they can provide an equivalent antipsychotic effect without EPS.
Rajiv Tandon, MD
Adjunct professor of psychiatry
University of Florida, Tallahassee
Robert Constantine, PhD
Research associate professor
University of South Florida, Tampa
We thank Dr. Helmuth and the principal investigators of CATIE for their interest in our article and the opportunity to further clarify a key learning point from CATIE.
Dr. Helmuth acknowledges our balanced review but suggests that cost and metabolic side effects should be considered along with lower EPS liability in selecting antipsychotic therapy. We agree. Avoiding EPS while obtaining a good antipsychotic effect is one key consideration in providing optimal antipsychotic therapy. Other adverse effects, patient preference, cost, and other factors are all important considerations in this complex process of individually optimizing antipsychotic treatment.
The CATIE investigators agree with our interpretation of the study’s principal findings. They take exception, however, to our suggestion that CATIE’s finding of no FGA-SGA difference in EPS and TD may be related to its relatively low assay sensitivity to detect such differences because the sample studied was at low risk for EPS and TD.
While accepting our description of the study sample as accurate, they disagree that it was at low risk for EPS and TD. Patients who have been ill for 16 years and received antipsychotic treatment for an average of 14 years without developing TD or severe EPS (as in CATIE) are by definition at low risk for EPS and TD.
We agree that patients without current TD and who are at risk for developing it comprise the best study population to investigate differential risk for TD; however, patients who have not developed it, despite 14 years of antipsychotic therapy, are at very low risk for developing it at all. First-episode patients without prior antipsychotic exposure would be an optimal study population, but such patients were excluded from CATIE.
Drs. Rosenheck and colleagues do not disagree with any of the other assertions in our article; they are, however, critical of “numerous factual errors in other published critiques of CATIE.” We cannot address such supposed inaccuracies, which are best taken up with authors of those commentaries.
To extract maximum value from this important initiative, we must better understand CATIE’s findings in the context of its study design and the results of other relevant studies. Neither mischaracterization nor overinterpretation of CATIE’s findings helps clinicians, patients, and policy-makers.
The essence of our article was that avoiding motor, cognitive, and affective EPS due to unmodulated dopamine blockade is the key to realizing the “atypical benefits” of a broader spectrum of efficacy and lower risk of TD during antipsychotic therapy. Neither Dr. Helmuth nor Drs. Rosenheck and colleagues appear to disagree with this assertion. Avoiding broadly defined EPS appears to be critical to improving cognition, dysphoria, and negative symptoms with SGAs and FGAs. The lower risk of TD observed with SGAs also appears to be related to the greater ease with which they can provide an equivalent antipsychotic effect without EPS.
Rajiv Tandon, MD
Adjunct professor of psychiatry
University of Florida, Tallahassee
Robert Constantine, PhD
Research associate professor
University of South Florida, Tampa
Study samples key to assessing risk
We agree with Drs. Tandon’s and Constantine’s explanation of the difference between the results of the CATIE trial1 and previous studies—specifically that CATIE showed no differences between 4 SGAs and an intermediate potency FGA on EPS and tardive dyskinesia (TD). As the authors suggest, these results are best explained by the use of high-dose, high-potency haloperidol as the comparator in pre-CATIE studies, which magnified differences between FGAs and SGAs. A recent study has further suggested that in most of these trials the doses of haloperidol were above FDA-approved levels,2 and few, if any, used prophylactic anticholinergics, further biasing the comparisons.
Drs. Tandon and Constantine further assert that the CATIE sample was at less risk of EPS or TD than previous samples because it excluded first-episode patients and those with TD and addressed a population that had used medications for 14 years without a history of adverse effects. CATIE—like any other ethical human investigation—excluded patients if they had well-documented, drug-related, adverse reactions to any of the proposed treatments.
Many, if not most, FDA registration trials (the source of most data on EPS with SGAs) excluded all patients with previous exposure to the new SGA drugs they tested but did not apply this criterion to patients exposed to older drugs. Thus the trials were more likely to include patients who would have responded poorly to FGAs than those who would have responded poorly to SGAs. Others have recognized that this reduces the validity of such FGA-SGA comparisons.3
Table
Study populations of SGA-FGA comparison trials
| Age | Age at onset | Duration of illness | |
|---|---|---|---|
| CATIE (Lieberman et al, 2005) | 40 | 24 | 16 |
| Olanzapine (Beasley et al, 1998) | 38.6 | 23.9 | 14.7 |
| Risperidone (Csernansky et al, 2002) | 40.3 | 24.4 | 15.9 |
| Amisulpride (Rein and L’Heritier, 1999) | 36 | na | na |
| Ziprasidone (Arato et al, 2002) | 50 | na | na |
| Risperidone (Marder et al, 1994) | 37.4 | 21.7 | 15.7 |
| Risperidone (Marder, 2003) | 43.5 | na | na |
| Aripiprazole (Kane et al, 2002) | 38.6 | 22.3 | 16.3 |
In the Table we present data comparing population characteristics from CATIE, from a meta-analysis that identified all 4 published controlled trials that have examined TD outcomes in FGA and SGAs,4 and from several other well-known comparable trials. The CATIE sample was similar to patients who participated in the other trials in average age, age of onset, and duration of illness.
Beasley et al5 similarly presented an analysis that excluded patients without TD at baseline, which we believe is the optimal population to use when evaluating medication-related risk for TD. CATIE is the only study that conducted a sound randomization comparing FGAs and SGAs in patients without current TD who are at risk for it, and—more than other studies—used an unbiased and thus more informative comparator.
Unfortunately there have been numerous factual errors in published critiques of CATIE. In one, CATIE was deemed disappointing6 because it had “a large percentage of discontinuations for all causes,” but the data presented for comparison were from a 28-week study7—less than half as long as the 72-week CATIE trial. CATIE, in fact, had better overall follow-up rates than the cited study at 28 weeks and also had better long-term follow-up rates than either the paper by Beasley et al5 or by Csernansky et al8—the most often cited “long-term” studies comparing FGAs and SGAs on TD.
Another commentary, like that of Drs. Tandon and Constantine, described CATIE patients as having more chronic illness that those in other trials, with “24 years since first treatment,”9 a misreading of the average age of first onset (which was 24) as if it was the average duration of illness (which was 16 years).
Many commentators have further asserted that because patients with TD at baseline “were not randomly assigned to conventional drugs” the comparison of either outcomes or TD risk was invalid.6,9 As noted above, comparison of side effect risk is properly tested by trials involving patients without that risk at onset.
CATIE represented a major investment of public dollars to learn more about antipsychotic medications. Erroneous critiques needlessly mislead the professional community about what can be learned from this initiative.
The CATIE investigators
Robert Rosenheck, MD, New Haven, CT
T. Scott Stroup, MD, MPH, Chapel Hill, NC
Richard SE Keefe, PhD, Durham, NC
Joseph McEvoy, MD, Durham, NC
Marvin Swartz, MD, Durham, NC
Jeffrey Lieberman, MD, New York, NY
1. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
2. Hugenholtz GWK, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparitor in randomized controlled trials with atypical antipsychotics in schizophrenia: Comparison with officially recommended doses. J Clin Psychiatry 2006;67(6):897-903.
3. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002;159:255-62.
4. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
5. Beasley CM, Dellva MA, Tamura RN, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry 1999;174:23-30.
6. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
7. Breier A, Berg PH, Thakore JH, et al. Olanzapine versus ziprasidone: results of a 28-week double blind study in patients with schizophrenia. Am J Psychiatry 2005;162(10):1879-87.
8. Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002;346:16-22.
9. Kane JM. Commentary on the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). J Clin Psychiatry 2006;67(5):831-2.
We agree with Drs. Tandon’s and Constantine’s explanation of the difference between the results of the CATIE trial1 and previous studies—specifically that CATIE showed no differences between 4 SGAs and an intermediate potency FGA on EPS and tardive dyskinesia (TD). As the authors suggest, these results are best explained by the use of high-dose, high-potency haloperidol as the comparator in pre-CATIE studies, which magnified differences between FGAs and SGAs. A recent study has further suggested that in most of these trials the doses of haloperidol were above FDA-approved levels,2 and few, if any, used prophylactic anticholinergics, further biasing the comparisons.
Drs. Tandon and Constantine further assert that the CATIE sample was at less risk of EPS or TD than previous samples because it excluded first-episode patients and those with TD and addressed a population that had used medications for 14 years without a history of adverse effects. CATIE—like any other ethical human investigation—excluded patients if they had well-documented, drug-related, adverse reactions to any of the proposed treatments.
Many, if not most, FDA registration trials (the source of most data on EPS with SGAs) excluded all patients with previous exposure to the new SGA drugs they tested but did not apply this criterion to patients exposed to older drugs. Thus the trials were more likely to include patients who would have responded poorly to FGAs than those who would have responded poorly to SGAs. Others have recognized that this reduces the validity of such FGA-SGA comparisons.3
Table
Study populations of SGA-FGA comparison trials
| Age | Age at onset | Duration of illness | |
|---|---|---|---|
| CATIE (Lieberman et al, 2005) | 40 | 24 | 16 |
| Olanzapine (Beasley et al, 1998) | 38.6 | 23.9 | 14.7 |
| Risperidone (Csernansky et al, 2002) | 40.3 | 24.4 | 15.9 |
| Amisulpride (Rein and L’Heritier, 1999) | 36 | na | na |
| Ziprasidone (Arato et al, 2002) | 50 | na | na |
| Risperidone (Marder et al, 1994) | 37.4 | 21.7 | 15.7 |
| Risperidone (Marder, 2003) | 43.5 | na | na |
| Aripiprazole (Kane et al, 2002) | 38.6 | 22.3 | 16.3 |
In the Table we present data comparing population characteristics from CATIE, from a meta-analysis that identified all 4 published controlled trials that have examined TD outcomes in FGA and SGAs,4 and from several other well-known comparable trials. The CATIE sample was similar to patients who participated in the other trials in average age, age of onset, and duration of illness.
Beasley et al5 similarly presented an analysis that excluded patients without TD at baseline, which we believe is the optimal population to use when evaluating medication-related risk for TD. CATIE is the only study that conducted a sound randomization comparing FGAs and SGAs in patients without current TD who are at risk for it, and—more than other studies—used an unbiased and thus more informative comparator.
Unfortunately there have been numerous factual errors in published critiques of CATIE. In one, CATIE was deemed disappointing6 because it had “a large percentage of discontinuations for all causes,” but the data presented for comparison were from a 28-week study7—less than half as long as the 72-week CATIE trial. CATIE, in fact, had better overall follow-up rates than the cited study at 28 weeks and also had better long-term follow-up rates than either the paper by Beasley et al5 or by Csernansky et al8—the most often cited “long-term” studies comparing FGAs and SGAs on TD.
Another commentary, like that of Drs. Tandon and Constantine, described CATIE patients as having more chronic illness that those in other trials, with “24 years since first treatment,”9 a misreading of the average age of first onset (which was 24) as if it was the average duration of illness (which was 16 years).
Many commentators have further asserted that because patients with TD at baseline “were not randomly assigned to conventional drugs” the comparison of either outcomes or TD risk was invalid.6,9 As noted above, comparison of side effect risk is properly tested by trials involving patients without that risk at onset.
CATIE represented a major investment of public dollars to learn more about antipsychotic medications. Erroneous critiques needlessly mislead the professional community about what can be learned from this initiative.
The CATIE investigators
Robert Rosenheck, MD, New Haven, CT
T. Scott Stroup, MD, MPH, Chapel Hill, NC
Richard SE Keefe, PhD, Durham, NC
Joseph McEvoy, MD, Durham, NC
Marvin Swartz, MD, Durham, NC
Jeffrey Lieberman, MD, New York, NY
We agree with Drs. Tandon’s and Constantine’s explanation of the difference between the results of the CATIE trial1 and previous studies—specifically that CATIE showed no differences between 4 SGAs and an intermediate potency FGA on EPS and tardive dyskinesia (TD). As the authors suggest, these results are best explained by the use of high-dose, high-potency haloperidol as the comparator in pre-CATIE studies, which magnified differences between FGAs and SGAs. A recent study has further suggested that in most of these trials the doses of haloperidol were above FDA-approved levels,2 and few, if any, used prophylactic anticholinergics, further biasing the comparisons.
Drs. Tandon and Constantine further assert that the CATIE sample was at less risk of EPS or TD than previous samples because it excluded first-episode patients and those with TD and addressed a population that had used medications for 14 years without a history of adverse effects. CATIE—like any other ethical human investigation—excluded patients if they had well-documented, drug-related, adverse reactions to any of the proposed treatments.
Many, if not most, FDA registration trials (the source of most data on EPS with SGAs) excluded all patients with previous exposure to the new SGA drugs they tested but did not apply this criterion to patients exposed to older drugs. Thus the trials were more likely to include patients who would have responded poorly to FGAs than those who would have responded poorly to SGAs. Others have recognized that this reduces the validity of such FGA-SGA comparisons.3
Table
Study populations of SGA-FGA comparison trials
| Age | Age at onset | Duration of illness | |
|---|---|---|---|
| CATIE (Lieberman et al, 2005) | 40 | 24 | 16 |
| Olanzapine (Beasley et al, 1998) | 38.6 | 23.9 | 14.7 |
| Risperidone (Csernansky et al, 2002) | 40.3 | 24.4 | 15.9 |
| Amisulpride (Rein and L’Heritier, 1999) | 36 | na | na |
| Ziprasidone (Arato et al, 2002) | 50 | na | na |
| Risperidone (Marder et al, 1994) | 37.4 | 21.7 | 15.7 |
| Risperidone (Marder, 2003) | 43.5 | na | na |
| Aripiprazole (Kane et al, 2002) | 38.6 | 22.3 | 16.3 |
In the Table we present data comparing population characteristics from CATIE, from a meta-analysis that identified all 4 published controlled trials that have examined TD outcomes in FGA and SGAs,4 and from several other well-known comparable trials. The CATIE sample was similar to patients who participated in the other trials in average age, age of onset, and duration of illness.
Beasley et al5 similarly presented an analysis that excluded patients without TD at baseline, which we believe is the optimal population to use when evaluating medication-related risk for TD. CATIE is the only study that conducted a sound randomization comparing FGAs and SGAs in patients without current TD who are at risk for it, and—more than other studies—used an unbiased and thus more informative comparator.
Unfortunately there have been numerous factual errors in published critiques of CATIE. In one, CATIE was deemed disappointing6 because it had “a large percentage of discontinuations for all causes,” but the data presented for comparison were from a 28-week study7—less than half as long as the 72-week CATIE trial. CATIE, in fact, had better overall follow-up rates than the cited study at 28 weeks and also had better long-term follow-up rates than either the paper by Beasley et al5 or by Csernansky et al8—the most often cited “long-term” studies comparing FGAs and SGAs on TD.
Another commentary, like that of Drs. Tandon and Constantine, described CATIE patients as having more chronic illness that those in other trials, with “24 years since first treatment,”9 a misreading of the average age of first onset (which was 24) as if it was the average duration of illness (which was 16 years).
Many commentators have further asserted that because patients with TD at baseline “were not randomly assigned to conventional drugs” the comparison of either outcomes or TD risk was invalid.6,9 As noted above, comparison of side effect risk is properly tested by trials involving patients without that risk at onset.
CATIE represented a major investment of public dollars to learn more about antipsychotic medications. Erroneous critiques needlessly mislead the professional community about what can be learned from this initiative.
The CATIE investigators
Robert Rosenheck, MD, New Haven, CT
T. Scott Stroup, MD, MPH, Chapel Hill, NC
Richard SE Keefe, PhD, Durham, NC
Joseph McEvoy, MD, Durham, NC
Marvin Swartz, MD, Durham, NC
Jeffrey Lieberman, MD, New York, NY
1. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
2. Hugenholtz GWK, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparitor in randomized controlled trials with atypical antipsychotics in schizophrenia: Comparison with officially recommended doses. J Clin Psychiatry 2006;67(6):897-903.
3. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002;159:255-62.
4. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
5. Beasley CM, Dellva MA, Tamura RN, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry 1999;174:23-30.
6. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
7. Breier A, Berg PH, Thakore JH, et al. Olanzapine versus ziprasidone: results of a 28-week double blind study in patients with schizophrenia. Am J Psychiatry 2005;162(10):1879-87.
8. Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002;346:16-22.
9. Kane JM. Commentary on the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). J Clin Psychiatry 2006;67(5):831-2.
1. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
2. Hugenholtz GWK, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparitor in randomized controlled trials with atypical antipsychotics in schizophrenia: Comparison with officially recommended doses. J Clin Psychiatry 2006;67(6):897-903.
3. Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002;159:255-62.
4. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
5. Beasley CM, Dellva MA, Tamura RN, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry 1999;174:23-30.
6. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
7. Breier A, Berg PH, Thakore JH, et al. Olanzapine versus ziprasidone: results of a 28-week double blind study in patients with schizophrenia. Am J Psychiatry 2005;162(10):1879-87.
8. Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002;346:16-22.
9. Kane JM. Commentary on the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). J Clin Psychiatry 2006;67(5):831-2.
Continued concerns about SGAs
Although the article “Avoiding EPS is key to realizing ‘atypical’ benefits,” by Drs. Rajiv Tandon and Robert J. Constantine (Current Psychiatry, November 2006), is more balanced than some reviews of the CATIE findings, it emphasized avoiding extrapyramidal symptoms (EPS) while ignoring two other features that are important when choosing an antipsychotic.
The first is the propensity for causing weight gain, hyperglycemia, and hyperlipidemia. The CATIE phase 1 investigation showed that second-generation antipsychotics (SGA)—especially olanzapine—are much more likely to cause these health-threatening complications compared with the first-generation antipsychotic (FGA) perphenazine.
The second consideration is cost. I am aware of economic arguments in favor of SGAs, especially if they prevent hospitalizations. However, in light of CATIE and the British CUtLASS 1 studies, it is unconscionable to not consider the huge difference in cost between SGAs and FGAs. Recent reports indicate that SGAs continue to outpace almost all other medications in price increases. This adds to society’s health-cost burden and creates a cruel inequity for those without prescription coverage.
It is an oversimplification of our clinical duty to refer to avoiding EPS as the “key” to antipsychotic treatment. We can only wish it were that simple.
Dennis Helmuth, MD, PhD
Clinical associate professor of psychiatry
Northeastern Ohio Universities College of Medicine,
Wooster, OH
Although the article “Avoiding EPS is key to realizing ‘atypical’ benefits,” by Drs. Rajiv Tandon and Robert J. Constantine (Current Psychiatry, November 2006), is more balanced than some reviews of the CATIE findings, it emphasized avoiding extrapyramidal symptoms (EPS) while ignoring two other features that are important when choosing an antipsychotic.
The first is the propensity for causing weight gain, hyperglycemia, and hyperlipidemia. The CATIE phase 1 investigation showed that second-generation antipsychotics (SGA)—especially olanzapine—are much more likely to cause these health-threatening complications compared with the first-generation antipsychotic (FGA) perphenazine.
The second consideration is cost. I am aware of economic arguments in favor of SGAs, especially if they prevent hospitalizations. However, in light of CATIE and the British CUtLASS 1 studies, it is unconscionable to not consider the huge difference in cost between SGAs and FGAs. Recent reports indicate that SGAs continue to outpace almost all other medications in price increases. This adds to society’s health-cost burden and creates a cruel inequity for those without prescription coverage.
It is an oversimplification of our clinical duty to refer to avoiding EPS as the “key” to antipsychotic treatment. We can only wish it were that simple.
Dennis Helmuth, MD, PhD
Clinical associate professor of psychiatry
Northeastern Ohio Universities College of Medicine,
Wooster, OH
Although the article “Avoiding EPS is key to realizing ‘atypical’ benefits,” by Drs. Rajiv Tandon and Robert J. Constantine (Current Psychiatry, November 2006), is more balanced than some reviews of the CATIE findings, it emphasized avoiding extrapyramidal symptoms (EPS) while ignoring two other features that are important when choosing an antipsychotic.
The first is the propensity for causing weight gain, hyperglycemia, and hyperlipidemia. The CATIE phase 1 investigation showed that second-generation antipsychotics (SGA)—especially olanzapine—are much more likely to cause these health-threatening complications compared with the first-generation antipsychotic (FGA) perphenazine.
The second consideration is cost. I am aware of economic arguments in favor of SGAs, especially if they prevent hospitalizations. However, in light of CATIE and the British CUtLASS 1 studies, it is unconscionable to not consider the huge difference in cost between SGAs and FGAs. Recent reports indicate that SGAs continue to outpace almost all other medications in price increases. This adds to society’s health-cost burden and creates a cruel inequity for those without prescription coverage.
It is an oversimplification of our clinical duty to refer to avoiding EPS as the “key” to antipsychotic treatment. We can only wish it were that simple.
Dennis Helmuth, MD, PhD
Clinical associate professor of psychiatry
Northeastern Ohio Universities College of Medicine,
Wooster, OH
Safety first
Thank you for the interview, “Protect yourself against patient assault,” and the accompanying reprint of Dr. John Battaglia’s article “Is this patient dangerous?” (Current Psychiatry, November 2006). Both give sound clinical guidelines for psychiatrist safety without being insensitive or “blaming the victim” in the case of Dr. Wayne Fenton’s tragic death allegedly at the hands of a patient. Although implicit, however, the need to develop and maintain appropriate boundaries needs to be more explicit and discussed.
From what we know, Dr. Fenton saw the patient in his office on a weekend with no one else present other than the patient’s father, who waited outside. One eulogy said that Dr. Fenton helped install a carpet in a different patient’s residence after the patient was released from the hospital. Both examples surely are instances of going “the extra mile” to help troubled patients, and Dr. Fenton was known as a master clinician who received some of the most difficult cases.
On the other hand, customary boundaries regarding how and where to see patients were not taken. Perhaps Dr. Fenton thought the rewards of breaking these boundaries outweighed the risks. Nevertheless, development and maintenance of boundaries should be undertaken as one way to ensure safety. When making exceptions, extra precaution should be taken.
H. Steven Moffic, MD
Professor of psychiatry and behavioral medicine
Medical College of Wisconsin
Milwaukee
Dr. Battaglia responds
I wholeheartedly agree with Dr. Moffic’s points about the need to take extra precautions when going outside customary boundaries. However, our discipline treads in muddy waters on the issue of what is appropriate when working outside such boundaries.
The extremes of sexual or financial exploitation are clear, but otherwise the entire spectrum of interaction between patient and clinician can be appropriate under certain circumstances. For example, in my work with the Madison (WI) Program of Assertive Community Treatment, I often see patients in their homes, help them with grocery shopping, or assist them with other daily tasks. Although these behaviors do not fit an office model, they are not uncommon in community work and do not necessarily break boundaries.
John Battaglia, MD
Program of Assertive Community Treatment
Associate clinical professor of psychiatry
University of Wisconsin-Madison Medical School
Thank you for the interview, “Protect yourself against patient assault,” and the accompanying reprint of Dr. John Battaglia’s article “Is this patient dangerous?” (Current Psychiatry, November 2006). Both give sound clinical guidelines for psychiatrist safety without being insensitive or “blaming the victim” in the case of Dr. Wayne Fenton’s tragic death allegedly at the hands of a patient. Although implicit, however, the need to develop and maintain appropriate boundaries needs to be more explicit and discussed.
From what we know, Dr. Fenton saw the patient in his office on a weekend with no one else present other than the patient’s father, who waited outside. One eulogy said that Dr. Fenton helped install a carpet in a different patient’s residence after the patient was released from the hospital. Both examples surely are instances of going “the extra mile” to help troubled patients, and Dr. Fenton was known as a master clinician who received some of the most difficult cases.
On the other hand, customary boundaries regarding how and where to see patients were not taken. Perhaps Dr. Fenton thought the rewards of breaking these boundaries outweighed the risks. Nevertheless, development and maintenance of boundaries should be undertaken as one way to ensure safety. When making exceptions, extra precaution should be taken.
H. Steven Moffic, MD
Professor of psychiatry and behavioral medicine
Medical College of Wisconsin
Milwaukee
Dr. Battaglia responds
I wholeheartedly agree with Dr. Moffic’s points about the need to take extra precautions when going outside customary boundaries. However, our discipline treads in muddy waters on the issue of what is appropriate when working outside such boundaries.
The extremes of sexual or financial exploitation are clear, but otherwise the entire spectrum of interaction between patient and clinician can be appropriate under certain circumstances. For example, in my work with the Madison (WI) Program of Assertive Community Treatment, I often see patients in their homes, help them with grocery shopping, or assist them with other daily tasks. Although these behaviors do not fit an office model, they are not uncommon in community work and do not necessarily break boundaries.
John Battaglia, MD
Program of Assertive Community Treatment
Associate clinical professor of psychiatry
University of Wisconsin-Madison Medical School
Thank you for the interview, “Protect yourself against patient assault,” and the accompanying reprint of Dr. John Battaglia’s article “Is this patient dangerous?” (Current Psychiatry, November 2006). Both give sound clinical guidelines for psychiatrist safety without being insensitive or “blaming the victim” in the case of Dr. Wayne Fenton’s tragic death allegedly at the hands of a patient. Although implicit, however, the need to develop and maintain appropriate boundaries needs to be more explicit and discussed.
From what we know, Dr. Fenton saw the patient in his office on a weekend with no one else present other than the patient’s father, who waited outside. One eulogy said that Dr. Fenton helped install a carpet in a different patient’s residence after the patient was released from the hospital. Both examples surely are instances of going “the extra mile” to help troubled patients, and Dr. Fenton was known as a master clinician who received some of the most difficult cases.
On the other hand, customary boundaries regarding how and where to see patients were not taken. Perhaps Dr. Fenton thought the rewards of breaking these boundaries outweighed the risks. Nevertheless, development and maintenance of boundaries should be undertaken as one way to ensure safety. When making exceptions, extra precaution should be taken.
H. Steven Moffic, MD
Professor of psychiatry and behavioral medicine
Medical College of Wisconsin
Milwaukee
Dr. Battaglia responds
I wholeheartedly agree with Dr. Moffic’s points about the need to take extra precautions when going outside customary boundaries. However, our discipline treads in muddy waters on the issue of what is appropriate when working outside such boundaries.
The extremes of sexual or financial exploitation are clear, but otherwise the entire spectrum of interaction between patient and clinician can be appropriate under certain circumstances. For example, in my work with the Madison (WI) Program of Assertive Community Treatment, I often see patients in their homes, help them with grocery shopping, or assist them with other daily tasks. Although these behaviors do not fit an office model, they are not uncommon in community work and do not necessarily break boundaries.
John Battaglia, MD
Program of Assertive Community Treatment
Associate clinical professor of psychiatry
University of Wisconsin-Madison Medical School
Dying too young
The life span of the seriously mentally ill is even shorter than we had thought. Earlier studies showed a loss of 20% of the average life span or 15 to 16 years.1 Recent figures are alarmingly higher, however, and vary from state to state.
Virginia has the "best" mortality rate among the seriously mentally ill with a loss of "only" 13.5 years of potential life, according to the Center for Mental Health Services (CMHS) and the Centers for Disease Control and Prevention (CDC).2 Perhaps persons who suffer from schizophrenia should move to Virgina because the loss of potential life years in other states is much worse:
• Arizona: 31.8 years
• Texas: 29.3 years
• Missouri: 27.9 years
• Utah: 26.9 years
• Oklahoma: 26.3 years.
A recent Ohio study of mortality and medical illness found an average loss of 32 years of life among persons with schizophrenia.3
Why is the life span of mentally ill persons so short? Apart from their high rates of death from unnatural causes (suicide, homicide, and accidents), the most frequent killer is cardiovascular disease. High mortality rates are well-documented in schizophrenia from various ailments but especially heart disease.4 Bipolar disorder and major depression are also associated with high death rates from cardiovascular disease.5
The sad truth is that a “dual neglect” contributes to premature mortality of the seriously mentally ill: the system fails to provide ongoing basic primary healthcare, and patients neglect to seek or adhere to medical care.
Persons with serious mental illness often have risk factors associated with preventable causes of heart disease and stroke, including smoking, obesity, sedentary life styles, and poor nutrition. In addition, the metabolic syndrome—obesity, hypertension, hyperglycemia, and dyslipidemia—is highly associated with schizophrenia,6 bipolar disorder,7 and unipolar depression.8
Cardiovascular risk associated with metabolic syndrome requires ongoing medical follow-up, which many mentally ill patients do not receive. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found shockingly low treatment rates for diabetes, hypertension, and hyperglycemia among outpatients with schizophrenia around the country.9
Let us mobilize to correct this shameful health disparity, one patient at a time. The message to mental health professionals is clear:
• In addition to controlling symptoms of psychosis, mania, depression, or anxiety, routinely screen patients for weight gain, hypertension, high fasting serum glucose, and elevated lipid levels.
• Refer overweight and obese patients to primary care providers, dietitians, and exercise counselors to reduce their cardiovascular risks.
Psychiatrists and nurse practitioners must address both mental and medical health needs when formulating assessments, treatment plans, and patient education. For practical recommendations on managing medical comorbidities, see “7-point checkup for stable schizophrenia outpatients,” by Britton Ashley Arey, MD, and Stephen R. Marder, MD. The public mental health system also could help the seriously mentally ill by integrating primary healthcare with mental healthcare in community settings across the nation. There are no excuses to do anything less.
1. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry 1991;36:239-45.
2. Cotton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost and causes of death among public mental health clients in eight states. Preventing Chronic Disease 2006;3:1-14.
3. Miller B, Paschall CB. Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv 2006;57:1482-7.
4. Meyer JM, Nasrallah HA, eds. Medical illness and schizophrenia. American Psychiatric Publishing, Inc.: Washington DC; 2003.
5. Angst F, Stassen FF, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord 2002;68:167-81.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32.
7. Taylor V, MacQueen G. Associations between bipolar disorder and the metabolic syndrome: a review. J Clin Psychiatry 2006;67:1034-41.
8. Heiskamn TH, Niskanen LK, Hintikka JJ, et al. Metabolic syndrome and depression: a cross-sectional analysis. J Clin Psychiatry 2006;67:1422-7.
9. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006;80:15-22.
The life span of the seriously mentally ill is even shorter than we had thought. Earlier studies showed a loss of 20% of the average life span or 15 to 16 years.1 Recent figures are alarmingly higher, however, and vary from state to state.
Virginia has the "best" mortality rate among the seriously mentally ill with a loss of "only" 13.5 years of potential life, according to the Center for Mental Health Services (CMHS) and the Centers for Disease Control and Prevention (CDC).2 Perhaps persons who suffer from schizophrenia should move to Virgina because the loss of potential life years in other states is much worse:
• Arizona: 31.8 years
• Texas: 29.3 years
• Missouri: 27.9 years
• Utah: 26.9 years
• Oklahoma: 26.3 years.
A recent Ohio study of mortality and medical illness found an average loss of 32 years of life among persons with schizophrenia.3
Why is the life span of mentally ill persons so short? Apart from their high rates of death from unnatural causes (suicide, homicide, and accidents), the most frequent killer is cardiovascular disease. High mortality rates are well-documented in schizophrenia from various ailments but especially heart disease.4 Bipolar disorder and major depression are also associated with high death rates from cardiovascular disease.5
The sad truth is that a “dual neglect” contributes to premature mortality of the seriously mentally ill: the system fails to provide ongoing basic primary healthcare, and patients neglect to seek or adhere to medical care.
Persons with serious mental illness often have risk factors associated with preventable causes of heart disease and stroke, including smoking, obesity, sedentary life styles, and poor nutrition. In addition, the metabolic syndrome—obesity, hypertension, hyperglycemia, and dyslipidemia—is highly associated with schizophrenia,6 bipolar disorder,7 and unipolar depression.8
Cardiovascular risk associated with metabolic syndrome requires ongoing medical follow-up, which many mentally ill patients do not receive. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found shockingly low treatment rates for diabetes, hypertension, and hyperglycemia among outpatients with schizophrenia around the country.9
Let us mobilize to correct this shameful health disparity, one patient at a time. The message to mental health professionals is clear:
• In addition to controlling symptoms of psychosis, mania, depression, or anxiety, routinely screen patients for weight gain, hypertension, high fasting serum glucose, and elevated lipid levels.
• Refer overweight and obese patients to primary care providers, dietitians, and exercise counselors to reduce their cardiovascular risks.
Psychiatrists and nurse practitioners must address both mental and medical health needs when formulating assessments, treatment plans, and patient education. For practical recommendations on managing medical comorbidities, see “7-point checkup for stable schizophrenia outpatients,” by Britton Ashley Arey, MD, and Stephen R. Marder, MD. The public mental health system also could help the seriously mentally ill by integrating primary healthcare with mental healthcare in community settings across the nation. There are no excuses to do anything less.
The life span of the seriously mentally ill is even shorter than we had thought. Earlier studies showed a loss of 20% of the average life span or 15 to 16 years.1 Recent figures are alarmingly higher, however, and vary from state to state.
Virginia has the "best" mortality rate among the seriously mentally ill with a loss of "only" 13.5 years of potential life, according to the Center for Mental Health Services (CMHS) and the Centers for Disease Control and Prevention (CDC).2 Perhaps persons who suffer from schizophrenia should move to Virgina because the loss of potential life years in other states is much worse:
• Arizona: 31.8 years
• Texas: 29.3 years
• Missouri: 27.9 years
• Utah: 26.9 years
• Oklahoma: 26.3 years.
A recent Ohio study of mortality and medical illness found an average loss of 32 years of life among persons with schizophrenia.3
Why is the life span of mentally ill persons so short? Apart from their high rates of death from unnatural causes (suicide, homicide, and accidents), the most frequent killer is cardiovascular disease. High mortality rates are well-documented in schizophrenia from various ailments but especially heart disease.4 Bipolar disorder and major depression are also associated with high death rates from cardiovascular disease.5
The sad truth is that a “dual neglect” contributes to premature mortality of the seriously mentally ill: the system fails to provide ongoing basic primary healthcare, and patients neglect to seek or adhere to medical care.
Persons with serious mental illness often have risk factors associated with preventable causes of heart disease and stroke, including smoking, obesity, sedentary life styles, and poor nutrition. In addition, the metabolic syndrome—obesity, hypertension, hyperglycemia, and dyslipidemia—is highly associated with schizophrenia,6 bipolar disorder,7 and unipolar depression.8
Cardiovascular risk associated with metabolic syndrome requires ongoing medical follow-up, which many mentally ill patients do not receive. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) found shockingly low treatment rates for diabetes, hypertension, and hyperglycemia among outpatients with schizophrenia around the country.9
Let us mobilize to correct this shameful health disparity, one patient at a time. The message to mental health professionals is clear:
• In addition to controlling symptoms of psychosis, mania, depression, or anxiety, routinely screen patients for weight gain, hypertension, high fasting serum glucose, and elevated lipid levels.
• Refer overweight and obese patients to primary care providers, dietitians, and exercise counselors to reduce their cardiovascular risks.
Psychiatrists and nurse practitioners must address both mental and medical health needs when formulating assessments, treatment plans, and patient education. For practical recommendations on managing medical comorbidities, see “7-point checkup for stable schizophrenia outpatients,” by Britton Ashley Arey, MD, and Stephen R. Marder, MD. The public mental health system also could help the seriously mentally ill by integrating primary healthcare with mental healthcare in community settings across the nation. There are no excuses to do anything less.
1. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry 1991;36:239-45.
2. Cotton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost and causes of death among public mental health clients in eight states. Preventing Chronic Disease 2006;3:1-14.
3. Miller B, Paschall CB. Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv 2006;57:1482-7.
4. Meyer JM, Nasrallah HA, eds. Medical illness and schizophrenia. American Psychiatric Publishing, Inc.: Washington DC; 2003.
5. Angst F, Stassen FF, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord 2002;68:167-81.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32.
7. Taylor V, MacQueen G. Associations between bipolar disorder and the metabolic syndrome: a review. J Clin Psychiatry 2006;67:1034-41.
8. Heiskamn TH, Niskanen LK, Hintikka JJ, et al. Metabolic syndrome and depression: a cross-sectional analysis. J Clin Psychiatry 2006;67:1422-7.
9. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006;80:15-22.
1. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry 1991;36:239-45.
2. Cotton CW, Manderscheid RW. Congruencies in increased mortality rates years of potential life lost and causes of death among public mental health clients in eight states. Preventing Chronic Disease 2006;3:1-14.
3. Miller B, Paschall CB. Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv 2006;57:1482-7.
4. Meyer JM, Nasrallah HA, eds. Medical illness and schizophrenia. American Psychiatric Publishing, Inc.: Washington DC; 2003.
5. Angst F, Stassen FF, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34-38 years. J Affect Disord 2002;68:167-81.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32.
7. Taylor V, MacQueen G. Associations between bipolar disorder and the metabolic syndrome: a review. J Clin Psychiatry 2006;67:1034-41.
8. Heiskamn TH, Niskanen LK, Hintikka JJ, et al. Metabolic syndrome and depression: a cross-sectional analysis. J Clin Psychiatry 2006;67:1422-7.
9. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006;80:15-22.
Psychosis: Is it a medical problem?
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
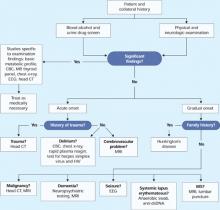
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
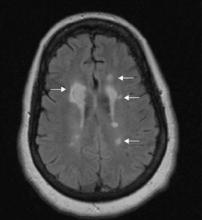
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
Engage resistant patients in collaborative treatment
Whenever you feel you are doing more work than the patient and are more invested than he is, something has gone wrong in collaborative care.
With resistant or hostile patients, fight the urge to move quickly into clinical assessment and to prescribe what you think should be worked on and how. Instead, spend more time—especially when building the treatment alliance in the first 15 minutes (Box1)—exploring their ideas on how, when, and where they feel they can achieve what is most important to them (Table 12).
Resistant patients may have different agendas, but taking a pragmatic approach can merge their goals with yours.
More than 2,000 research publications in the last 30 years prove the clinical importance of the therapeutic alliance.1 When working with resistant patients, keep these points in mind:
Develop a strong alliance early in treatment. “Early” is relative to the length of therapy, but evidence suggests sessions 3 to 5 are a critical window.
The patient’s experience of being understood, supported, and provided with hope depends on the strength of the alliance early in therapy. His or her interpretation of what you do can be different from what you intend. You may be a great clinician but not necessarily for this particular individual at this time, doing the kind of work you do.
Progressively negotiate the quality of the relationship. The patient’s perception of the alliance—not yours—is most influential. Ask specifically if the treatment relationship is working for him or her.
Early in treatment, the alliance itself contributes more to outcomes than do therapeutic techniques and models. First develop a collaborative agreement on the goals and strategies to be used in the therapeutic work.
Table 1
How to merge the reluctant patient’s goals with clinical needs assessment
| Questions to prioritize patient goals | Questions for clinical needs assessment | Merging patient goals with assessed needs | |
|---|---|---|---|
| What? | What does the patient want the most? What undesired consequences will occur if s/he does not get help? | What does the clinical assessment indicate s/he needs? What obstacles/assets do you need to address to help her/him get what s/he wants? | What treatment contract will drive the treatment plan and organize treatment priorities? |
| Why? | Why did s/he seek help now? Has s/he realized or been told s/he is at risk to lose freedom, health, a relationship, or a job? How committed to change is s/he? | Why are the assessed obstacles and resources important to include in a treatment plan? What diagnostic, function, or severity problems do assessment data reveal? | Is the treatment plan linked to what s/he wants? Does s/he accept that the treatment priorities will help her/him get what s/he wants? |
| How? | How will s/he achieve the most important goal? Must you try her/his preferred treatment before s/he accepts methods you prescribe? | How will you develop patient buy-in and get her/him to accept the plan? | Does s/he believe your strategies will help get what s/he wants? Will s/he be actively invested or passively compliant in treatment? |
| Where? | Where is s/he willing to be treated? Does s/he have strong preferences (such as about group treatment or residential programs)? | Where is the appropriate setting for treatment? What is indicated by the placement criteria? | Refer her/him to the level of care that merges his/her preferences with what is clinically indicated and likely to be effective |
| When? | When does s/he want to begin treatment? Is s/he feeling pressure to start? How badly does s/he want treatment, or is s/he just complying? | When should treatment begin, based on your assessment? What are realistic expectations and milestones in the process? | How urgent is treatment? What is the process? What is expected from referral? |
| Source: Adapted from reference 2, Table 3. | |||
What does the patient want?
When a patient is difficult to engage, begin by listening for the most important concern that brought him to your office.
He may be depressed, anxious, or tired, but exploring why he decided to seek help now (“My wife said she would leave me if I didn’t get help”) reveals what is most important. The “treatment contract,” then, is helping this patient save his marriage.
Initial engagement
Collaborative treatment begins with a genuinely interested dialogue about what prompted the patient’s visit.
Therapist: “Thank you for choosing to work with me. How may I serve you? What is the most important thing you want me to help you with?”
Mr. L: “I didn’t choose you; they made me come.”
T: “I didn’t see anyone drag you in. What would happen if you had not come today?”
Mr. L: “I might lose my job. I came because my boss told me to.”
Focus on what the patient wants, not just what others have said he or she needs (treatment for substance abuse, angry outbursts, conflict at work). The patient may want to stay out of jail, keep his job or relationship, regain custody of his or her children, obtain housing, or get people to “leave me alone and quit locking me up against my will.” Although the patient’s problem may be obvious to us, he needs “discovery” work, not “recovery” work.
Why has the patient come now? What is his highest priority? Can we help him discover the link between his drinking or anger that affects his work performance?
Therapist: “So you want to get the boss off your back. You want people to leave you alone. You feel people treat you unfairly and want them to stop. But why did you come today and not last week or last month?”
Mr. L: “I came now because yesterday my boss said I could lose my job if I didn’t get some help.”
T: “So what you want most importantly is to keep your job, is that it?”
Mr. L: “Well yeah, but I don’t have a drinking problem or any problem with my temper. They’re just overreacting. It wasn’t as bad as they said.”
T: “OK, I am willing to work on helping you keep your job if that’s what is most important to you. Do you know what you are doing that makes them think you have a drinking or anger problem?
Mr. L: “All I did was come in late a couple of times and got into a little argument with a couple of people.”
T: “If we are going to help you keep your job, we could spend our time talking about how unfair your boss is and how she’s misjudging you. Or we could work to show her that she has you all wrong and that you are a productive worker who does not have a substance or anger problem.
“Let’s think together how we could gather the data that would prove you don’t have a substance problem. If all that data is squeaky clean, then I can write a very supportive letter to your boss and tell her all is well. If, however, we discover you do have a problem, I can still write a very supportive letter. But we’ll have to show her how you are taking care of any problems that interfere with your work performance.”
Reframe the presenting complaint
Few patients present fully ready to work on definitive behavioral health recovery. If patients at least attend sessions or talk with you, they must be motivated to do something. Otherwise, they would not show up.
Our task is help patients such as Mr. L get what they want, not what we think they should want. Eventually you will get to explore the patient’s substance use, impulse or parenting problems, mental health symptoms, or communication problems, but this discussion will be in the service of allying with his or her goals.
Rather than viewing patients as unmotivated or resistant, think of resistance as an interactional process. “If we are going to stop them from locking you up,” you might say, “let’s talk about what you are doing that makes you look like you are dangerous and out of control. And when you were not locked away, let’s think of how you kept ‘them’ off your back.”
Instead of interpreting resistance as pathology, view the behavior as an opportunity to understand and respond to the patient’s stage of readiness to change.
Stages of change
By being “difficult,” patients are often declaring that they are not invested in what you think the problem is or in working on that problem. Resistance thrives when we and the patient have not allied around a common goal and are at different stages of change. Think of the therapeutic alliance in the context of the widely-used and well-researched Transtheoretical Model’s stages of change:3,4
Precontemplation. A person at this stage is not considering the possibility of change, although others are aware of a problem. He or she will seldom appear for treatment without coercion. The person could benefit from nonthreatening information to raise awareness of a possible “problem” and possibilities for change.
Contemplation. The person is ambivalent, undecided, vacillating about whether he has a “problem.” He wants to change, but this desire exists simultaneously with resistance to change. Motivational strategies can be useful, but aggressive or premature confrontation could provoke strong resistance and defensive behaviors. Many persons at this stage have indefinite plans to take action in the next 6 months or so.
Preparation takes the person from the contemplation stage to specific steps to solve the problem in the action stage. He or she develops increasing confidence in the decision to change and takes the first steps on the road to action. Most people at this stage plan to take action within 1 month and are making final adjustments before beginning to change.
Action. The person takes specific actions intended to bring about change. This busiest stage of change is characterized by overt modification of behavior and surroundings and requires the greatest time and energy. Support and encouragement are crucial to prevent drop-out and regression in readiness to change.
Maintenance. Goals at this stage are to sustain the changes accomplished by previous action and to prevent relapse. Maintaining new behaviors requires different skills than were needed to initiate change. Gains are consolidated. “Maintenance” is not a static stage; it can last 6 months or up to a lifetime. The patient learns new coping and problem-solving strategies, replaces problem behaviors with a healthier life style, and works through emotional triggers of relapse.
Relapse/recycling can happen but is not inevitable. When setbacks occur, help the patient avoid becoming stuck, discouraged, or demoralized, and help him learn from relapse before committing to a new action cycle. Conduct a comprehensive, multidimensional assessment to explore all reasons for relapse.
Termination is the ultimate goal: to exit the cycle of change without fear of relapse. Certain problems may be terminated or merely kept in remission through maintenance strategies.
Match strategies with stages
Discovery planning. Engaging patients in collaborative care starts with honoring their stages of change and working with them and their families on different tasks for each stage of change.4-6 A patient such as Mr. L, for example—who is at an early stage of change and thinks he has an “unfair boss problem” (not an alcohol problem) or a “nagging wife problem” (not an anger or domestic violence problem)—needs a discovery, drop-out prevention plan.
The cause of the patient’s work or relationship problem may be obvious to you, but a patient in early stages of change resists that information and, if pressed, gets frustrated and leaves treatment. A “discovery” treatment plan embraces the patient’s views and could be focused, for example, on gathering data that would prove to the employer that there is not an alcohol problem.
If random breath alcohol testing, feedback from family, and review of past job losses all prove negative for alcohol problems, the patient would have data to support his or her view that he does not have an alcohol problem. If, however, this exploration reveals an alcohol problem, the patient “discovers” he has more of a problem than he thought. For this plan, the challenge is to keep Mr. L engaged long enough to discover the connection between his alcohol problems and his employment or marital problems.
Recovery planning. On the other hand, a person at the action stage who wants to avoid becoming depressed again or wishes to live a life of sobriety collaborates on a recovery, relapse prevention plan. This patient is committed to developing the knowledge and skills to prevent relapse and open to whatever will promote health and well-being.
The Transtheoretical Model’s 9 processes of change3,4 inform which interventions might be most effective at various stages of change (Table 2). For example, a patient might be proud of the fact that “I can hold my liquor and drink everyone under the table.” Consciousness-raising—such as by explaining that his high alcohol tolerance is a danger signal, not a beneficial ability—can enhance his change process. Even if he is not ready to commit to definitive change, at least exploring whether he has a problem may move him from precontemplation to a contemplation stage of change. Table 3 shows how other processes of change can help motivate patients in later stages of change.4,5
Table 2
Transtheoretical model’s 9 processes of change: What happens at each step
| Process of change | The person… |
|---|---|
| Conscious-raising | becomes aware of a problem from education, advice, self-awareness, or feedback from others |
| Social liberation | begins to think about change because external forces raise awareness (a ban on smoking in restaurants, for example, can heighten awareness that one has a smoking problem) |
| Emotional arousal | becomes more convinced of the need to change when faced with a strong and sudden emotional experience related to the problem (such as death of a loved one) |
| Self-reevaluation | examines his or her values to see whether or not the behavior conflicts with what is important to him or her |
| Commitment | accepts responsibility for changing and affirms to self and others the decision to change |
| Reward | uses self-praise, positive feedback from others, improved well-being or financial security, “natural highs,” and other reinforcing benefits to consolidate change |
| Countering | substitutes other responses to counter unhealthy choices and behavior (such as relaxation techniques to combat angry outbursts or urges to resume smoking) |
| Environmental control | changes surrounding people, places, or things to reduce the risk of continuing or resuming the problem behavior |
| Helping relationships | seeks assistance from trusted friends, professionals, spiritual advisors, or significant others to initiate and sustain the change process |
| Source: Adapted and reprinted with permission from reference 4. | |
Table 3
Strategies and interventions to motivate patients at each stage of change
| Stage of change | Most useful processes of change (see Table 2) | Goal of treatment | Strategies and interventions |
|---|---|---|---|
| Precontemplation | Consciousness-raising Social liberation | To raise doubt |
|
| Contemplation | Consciousness-raising Social liberation Emotional arousal Self-reevaluation | To tip the balance |
|
| Preparation | Social liberation Emotional arousal Self-reevaluation Commitment | To determine best course |
|
| Action | Social liberation Commitment Reward Countering Environment control Helping relationships | To take steps to change |
|
| Maintenance | Commitment Countering Environment control Helping relationships | To prevent relapse |
|
| Relapse/recycling | Depends on stage to which patient returned | To renew processes of change |
|
- Scott D. Miller, PhD. Institute for the Study of Therapeutic Change, Chicago, IL. (773) 404-5130; www.talkingcure.com.
- Miller WR, Rollnick S, Moyers TB. Motivational interviewing: professional training videotape series. Albuquerque, NM: University of New Mexico, 1998. Six videotapes (about $130 total); (505) 768-0279 or 0100.
- Miller WR, consensus panel chair. Treatment improvement protocol. Enhancing motivation for change in substance abuse treatment. Rockville, MD; Center for Substance Abuse Treatment, 1999. DHHS Publication 99-3354. Available from the National Clearinghouse for Alcohol/Drug Information, Rockville, MD; (800) 729-6686.
Dr. Mee-Lee is a board-certified psychiatrist and is certified by examination of the American Society of Addiction Medicine (ASAM). He is based in Davis, CA, and is involved in full-time training and consulting. For information, visit www.DMLMD.com.
1. Horvath AO, Bedi RP. The alliance. In: Norcross JC, ed. Psychotherapy relationships that work: therapist contributions and responsiveness to patients. New York: Oxford University Press; 2002.
2. Mee-Lee D. Treatment planning for dual disorders. Psychiatr Rehabil Skills 2001;5(1):52-79.
3. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: applications to addictive behaviors. Am Psychol 1992;47:1102-14.
4. Prochaska JO, Norcross JC, DiClemente CC. Changing for good. New York: Avon Books; 1994:54.
5. DiClemente CC. Conceptual models and applied research: the ongoing contribution of the transtheoretical model. J Addict Nurs 2005;16:5-12.
6. Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991:18.
Whenever you feel you are doing more work than the patient and are more invested than he is, something has gone wrong in collaborative care.
With resistant or hostile patients, fight the urge to move quickly into clinical assessment and to prescribe what you think should be worked on and how. Instead, spend more time—especially when building the treatment alliance in the first 15 minutes (Box1)—exploring their ideas on how, when, and where they feel they can achieve what is most important to them (Table 12).
Resistant patients may have different agendas, but taking a pragmatic approach can merge their goals with yours.
More than 2,000 research publications in the last 30 years prove the clinical importance of the therapeutic alliance.1 When working with resistant patients, keep these points in mind:
Develop a strong alliance early in treatment. “Early” is relative to the length of therapy, but evidence suggests sessions 3 to 5 are a critical window.
The patient’s experience of being understood, supported, and provided with hope depends on the strength of the alliance early in therapy. His or her interpretation of what you do can be different from what you intend. You may be a great clinician but not necessarily for this particular individual at this time, doing the kind of work you do.
Progressively negotiate the quality of the relationship. The patient’s perception of the alliance—not yours—is most influential. Ask specifically if the treatment relationship is working for him or her.
Early in treatment, the alliance itself contributes more to outcomes than do therapeutic techniques and models. First develop a collaborative agreement on the goals and strategies to be used in the therapeutic work.
Table 1
How to merge the reluctant patient’s goals with clinical needs assessment
| Questions to prioritize patient goals | Questions for clinical needs assessment | Merging patient goals with assessed needs | |
|---|---|---|---|
| What? | What does the patient want the most? What undesired consequences will occur if s/he does not get help? | What does the clinical assessment indicate s/he needs? What obstacles/assets do you need to address to help her/him get what s/he wants? | What treatment contract will drive the treatment plan and organize treatment priorities? |
| Why? | Why did s/he seek help now? Has s/he realized or been told s/he is at risk to lose freedom, health, a relationship, or a job? How committed to change is s/he? | Why are the assessed obstacles and resources important to include in a treatment plan? What diagnostic, function, or severity problems do assessment data reveal? | Is the treatment plan linked to what s/he wants? Does s/he accept that the treatment priorities will help her/him get what s/he wants? |
| How? | How will s/he achieve the most important goal? Must you try her/his preferred treatment before s/he accepts methods you prescribe? | How will you develop patient buy-in and get her/him to accept the plan? | Does s/he believe your strategies will help get what s/he wants? Will s/he be actively invested or passively compliant in treatment? |
| Where? | Where is s/he willing to be treated? Does s/he have strong preferences (such as about group treatment or residential programs)? | Where is the appropriate setting for treatment? What is indicated by the placement criteria? | Refer her/him to the level of care that merges his/her preferences with what is clinically indicated and likely to be effective |
| When? | When does s/he want to begin treatment? Is s/he feeling pressure to start? How badly does s/he want treatment, or is s/he just complying? | When should treatment begin, based on your assessment? What are realistic expectations and milestones in the process? | How urgent is treatment? What is the process? What is expected from referral? |
| Source: Adapted from reference 2, Table 3. | |||
What does the patient want?
When a patient is difficult to engage, begin by listening for the most important concern that brought him to your office.
He may be depressed, anxious, or tired, but exploring why he decided to seek help now (“My wife said she would leave me if I didn’t get help”) reveals what is most important. The “treatment contract,” then, is helping this patient save his marriage.
Initial engagement
Collaborative treatment begins with a genuinely interested dialogue about what prompted the patient’s visit.
Therapist: “Thank you for choosing to work with me. How may I serve you? What is the most important thing you want me to help you with?”
Mr. L: “I didn’t choose you; they made me come.”
T: “I didn’t see anyone drag you in. What would happen if you had not come today?”
Mr. L: “I might lose my job. I came because my boss told me to.”
Focus on what the patient wants, not just what others have said he or she needs (treatment for substance abuse, angry outbursts, conflict at work). The patient may want to stay out of jail, keep his job or relationship, regain custody of his or her children, obtain housing, or get people to “leave me alone and quit locking me up against my will.” Although the patient’s problem may be obvious to us, he needs “discovery” work, not “recovery” work.
Why has the patient come now? What is his highest priority? Can we help him discover the link between his drinking or anger that affects his work performance?
Therapist: “So you want to get the boss off your back. You want people to leave you alone. You feel people treat you unfairly and want them to stop. But why did you come today and not last week or last month?”
Mr. L: “I came now because yesterday my boss said I could lose my job if I didn’t get some help.”
T: “So what you want most importantly is to keep your job, is that it?”
Mr. L: “Well yeah, but I don’t have a drinking problem or any problem with my temper. They’re just overreacting. It wasn’t as bad as they said.”
T: “OK, I am willing to work on helping you keep your job if that’s what is most important to you. Do you know what you are doing that makes them think you have a drinking or anger problem?
Mr. L: “All I did was come in late a couple of times and got into a little argument with a couple of people.”
T: “If we are going to help you keep your job, we could spend our time talking about how unfair your boss is and how she’s misjudging you. Or we could work to show her that she has you all wrong and that you are a productive worker who does not have a substance or anger problem.
“Let’s think together how we could gather the data that would prove you don’t have a substance problem. If all that data is squeaky clean, then I can write a very supportive letter to your boss and tell her all is well. If, however, we discover you do have a problem, I can still write a very supportive letter. But we’ll have to show her how you are taking care of any problems that interfere with your work performance.”
Reframe the presenting complaint
Few patients present fully ready to work on definitive behavioral health recovery. If patients at least attend sessions or talk with you, they must be motivated to do something. Otherwise, they would not show up.
Our task is help patients such as Mr. L get what they want, not what we think they should want. Eventually you will get to explore the patient’s substance use, impulse or parenting problems, mental health symptoms, or communication problems, but this discussion will be in the service of allying with his or her goals.
Rather than viewing patients as unmotivated or resistant, think of resistance as an interactional process. “If we are going to stop them from locking you up,” you might say, “let’s talk about what you are doing that makes you look like you are dangerous and out of control. And when you were not locked away, let’s think of how you kept ‘them’ off your back.”
Instead of interpreting resistance as pathology, view the behavior as an opportunity to understand and respond to the patient’s stage of readiness to change.
Stages of change
By being “difficult,” patients are often declaring that they are not invested in what you think the problem is or in working on that problem. Resistance thrives when we and the patient have not allied around a common goal and are at different stages of change. Think of the therapeutic alliance in the context of the widely-used and well-researched Transtheoretical Model’s stages of change:3,4
Precontemplation. A person at this stage is not considering the possibility of change, although others are aware of a problem. He or she will seldom appear for treatment without coercion. The person could benefit from nonthreatening information to raise awareness of a possible “problem” and possibilities for change.
Contemplation. The person is ambivalent, undecided, vacillating about whether he has a “problem.” He wants to change, but this desire exists simultaneously with resistance to change. Motivational strategies can be useful, but aggressive or premature confrontation could provoke strong resistance and defensive behaviors. Many persons at this stage have indefinite plans to take action in the next 6 months or so.
Preparation takes the person from the contemplation stage to specific steps to solve the problem in the action stage. He or she develops increasing confidence in the decision to change and takes the first steps on the road to action. Most people at this stage plan to take action within 1 month and are making final adjustments before beginning to change.
Action. The person takes specific actions intended to bring about change. This busiest stage of change is characterized by overt modification of behavior and surroundings and requires the greatest time and energy. Support and encouragement are crucial to prevent drop-out and regression in readiness to change.
Maintenance. Goals at this stage are to sustain the changes accomplished by previous action and to prevent relapse. Maintaining new behaviors requires different skills than were needed to initiate change. Gains are consolidated. “Maintenance” is not a static stage; it can last 6 months or up to a lifetime. The patient learns new coping and problem-solving strategies, replaces problem behaviors with a healthier life style, and works through emotional triggers of relapse.
Relapse/recycling can happen but is not inevitable. When setbacks occur, help the patient avoid becoming stuck, discouraged, or demoralized, and help him learn from relapse before committing to a new action cycle. Conduct a comprehensive, multidimensional assessment to explore all reasons for relapse.
Termination is the ultimate goal: to exit the cycle of change without fear of relapse. Certain problems may be terminated or merely kept in remission through maintenance strategies.
Match strategies with stages
Discovery planning. Engaging patients in collaborative care starts with honoring their stages of change and working with them and their families on different tasks for each stage of change.4-6 A patient such as Mr. L, for example—who is at an early stage of change and thinks he has an “unfair boss problem” (not an alcohol problem) or a “nagging wife problem” (not an anger or domestic violence problem)—needs a discovery, drop-out prevention plan.
The cause of the patient’s work or relationship problem may be obvious to you, but a patient in early stages of change resists that information and, if pressed, gets frustrated and leaves treatment. A “discovery” treatment plan embraces the patient’s views and could be focused, for example, on gathering data that would prove to the employer that there is not an alcohol problem.
If random breath alcohol testing, feedback from family, and review of past job losses all prove negative for alcohol problems, the patient would have data to support his or her view that he does not have an alcohol problem. If, however, this exploration reveals an alcohol problem, the patient “discovers” he has more of a problem than he thought. For this plan, the challenge is to keep Mr. L engaged long enough to discover the connection between his alcohol problems and his employment or marital problems.
Recovery planning. On the other hand, a person at the action stage who wants to avoid becoming depressed again or wishes to live a life of sobriety collaborates on a recovery, relapse prevention plan. This patient is committed to developing the knowledge and skills to prevent relapse and open to whatever will promote health and well-being.
The Transtheoretical Model’s 9 processes of change3,4 inform which interventions might be most effective at various stages of change (Table 2). For example, a patient might be proud of the fact that “I can hold my liquor and drink everyone under the table.” Consciousness-raising—such as by explaining that his high alcohol tolerance is a danger signal, not a beneficial ability—can enhance his change process. Even if he is not ready to commit to definitive change, at least exploring whether he has a problem may move him from precontemplation to a contemplation stage of change. Table 3 shows how other processes of change can help motivate patients in later stages of change.4,5
Table 2
Transtheoretical model’s 9 processes of change: What happens at each step
| Process of change | The person… |
|---|---|
| Conscious-raising | becomes aware of a problem from education, advice, self-awareness, or feedback from others |
| Social liberation | begins to think about change because external forces raise awareness (a ban on smoking in restaurants, for example, can heighten awareness that one has a smoking problem) |
| Emotional arousal | becomes more convinced of the need to change when faced with a strong and sudden emotional experience related to the problem (such as death of a loved one) |
| Self-reevaluation | examines his or her values to see whether or not the behavior conflicts with what is important to him or her |
| Commitment | accepts responsibility for changing and affirms to self and others the decision to change |
| Reward | uses self-praise, positive feedback from others, improved well-being or financial security, “natural highs,” and other reinforcing benefits to consolidate change |
| Countering | substitutes other responses to counter unhealthy choices and behavior (such as relaxation techniques to combat angry outbursts or urges to resume smoking) |
| Environmental control | changes surrounding people, places, or things to reduce the risk of continuing or resuming the problem behavior |
| Helping relationships | seeks assistance from trusted friends, professionals, spiritual advisors, or significant others to initiate and sustain the change process |
| Source: Adapted and reprinted with permission from reference 4. | |
Table 3
Strategies and interventions to motivate patients at each stage of change
| Stage of change | Most useful processes of change (see Table 2) | Goal of treatment | Strategies and interventions |
|---|---|---|---|
| Precontemplation | Consciousness-raising Social liberation | To raise doubt |
|
| Contemplation | Consciousness-raising Social liberation Emotional arousal Self-reevaluation | To tip the balance |
|
| Preparation | Social liberation Emotional arousal Self-reevaluation Commitment | To determine best course |
|
| Action | Social liberation Commitment Reward Countering Environment control Helping relationships | To take steps to change |
|
| Maintenance | Commitment Countering Environment control Helping relationships | To prevent relapse |
|
| Relapse/recycling | Depends on stage to which patient returned | To renew processes of change |
|
- Scott D. Miller, PhD. Institute for the Study of Therapeutic Change, Chicago, IL. (773) 404-5130; www.talkingcure.com.
- Miller WR, Rollnick S, Moyers TB. Motivational interviewing: professional training videotape series. Albuquerque, NM: University of New Mexico, 1998. Six videotapes (about $130 total); (505) 768-0279 or 0100.
- Miller WR, consensus panel chair. Treatment improvement protocol. Enhancing motivation for change in substance abuse treatment. Rockville, MD; Center for Substance Abuse Treatment, 1999. DHHS Publication 99-3354. Available from the National Clearinghouse for Alcohol/Drug Information, Rockville, MD; (800) 729-6686.
Dr. Mee-Lee is a board-certified psychiatrist and is certified by examination of the American Society of Addiction Medicine (ASAM). He is based in Davis, CA, and is involved in full-time training and consulting. For information, visit www.DMLMD.com.
Whenever you feel you are doing more work than the patient and are more invested than he is, something has gone wrong in collaborative care.
With resistant or hostile patients, fight the urge to move quickly into clinical assessment and to prescribe what you think should be worked on and how. Instead, spend more time—especially when building the treatment alliance in the first 15 minutes (Box1)—exploring their ideas on how, when, and where they feel they can achieve what is most important to them (Table 12).
Resistant patients may have different agendas, but taking a pragmatic approach can merge their goals with yours.
More than 2,000 research publications in the last 30 years prove the clinical importance of the therapeutic alliance.1 When working with resistant patients, keep these points in mind:
Develop a strong alliance early in treatment. “Early” is relative to the length of therapy, but evidence suggests sessions 3 to 5 are a critical window.
The patient’s experience of being understood, supported, and provided with hope depends on the strength of the alliance early in therapy. His or her interpretation of what you do can be different from what you intend. You may be a great clinician but not necessarily for this particular individual at this time, doing the kind of work you do.
Progressively negotiate the quality of the relationship. The patient’s perception of the alliance—not yours—is most influential. Ask specifically if the treatment relationship is working for him or her.
Early in treatment, the alliance itself contributes more to outcomes than do therapeutic techniques and models. First develop a collaborative agreement on the goals and strategies to be used in the therapeutic work.
Table 1
How to merge the reluctant patient’s goals with clinical needs assessment
| Questions to prioritize patient goals | Questions for clinical needs assessment | Merging patient goals with assessed needs | |
|---|---|---|---|
| What? | What does the patient want the most? What undesired consequences will occur if s/he does not get help? | What does the clinical assessment indicate s/he needs? What obstacles/assets do you need to address to help her/him get what s/he wants? | What treatment contract will drive the treatment plan and organize treatment priorities? |
| Why? | Why did s/he seek help now? Has s/he realized or been told s/he is at risk to lose freedom, health, a relationship, or a job? How committed to change is s/he? | Why are the assessed obstacles and resources important to include in a treatment plan? What diagnostic, function, or severity problems do assessment data reveal? | Is the treatment plan linked to what s/he wants? Does s/he accept that the treatment priorities will help her/him get what s/he wants? |
| How? | How will s/he achieve the most important goal? Must you try her/his preferred treatment before s/he accepts methods you prescribe? | How will you develop patient buy-in and get her/him to accept the plan? | Does s/he believe your strategies will help get what s/he wants? Will s/he be actively invested or passively compliant in treatment? |
| Where? | Where is s/he willing to be treated? Does s/he have strong preferences (such as about group treatment or residential programs)? | Where is the appropriate setting for treatment? What is indicated by the placement criteria? | Refer her/him to the level of care that merges his/her preferences with what is clinically indicated and likely to be effective |
| When? | When does s/he want to begin treatment? Is s/he feeling pressure to start? How badly does s/he want treatment, or is s/he just complying? | When should treatment begin, based on your assessment? What are realistic expectations and milestones in the process? | How urgent is treatment? What is the process? What is expected from referral? |
| Source: Adapted from reference 2, Table 3. | |||
What does the patient want?
When a patient is difficult to engage, begin by listening for the most important concern that brought him to your office.
He may be depressed, anxious, or tired, but exploring why he decided to seek help now (“My wife said she would leave me if I didn’t get help”) reveals what is most important. The “treatment contract,” then, is helping this patient save his marriage.
Initial engagement
Collaborative treatment begins with a genuinely interested dialogue about what prompted the patient’s visit.
Therapist: “Thank you for choosing to work with me. How may I serve you? What is the most important thing you want me to help you with?”
Mr. L: “I didn’t choose you; they made me come.”
T: “I didn’t see anyone drag you in. What would happen if you had not come today?”
Mr. L: “I might lose my job. I came because my boss told me to.”
Focus on what the patient wants, not just what others have said he or she needs (treatment for substance abuse, angry outbursts, conflict at work). The patient may want to stay out of jail, keep his job or relationship, regain custody of his or her children, obtain housing, or get people to “leave me alone and quit locking me up against my will.” Although the patient’s problem may be obvious to us, he needs “discovery” work, not “recovery” work.
Why has the patient come now? What is his highest priority? Can we help him discover the link between his drinking or anger that affects his work performance?
Therapist: “So you want to get the boss off your back. You want people to leave you alone. You feel people treat you unfairly and want them to stop. But why did you come today and not last week or last month?”
Mr. L: “I came now because yesterday my boss said I could lose my job if I didn’t get some help.”
T: “So what you want most importantly is to keep your job, is that it?”
Mr. L: “Well yeah, but I don’t have a drinking problem or any problem with my temper. They’re just overreacting. It wasn’t as bad as they said.”
T: “OK, I am willing to work on helping you keep your job if that’s what is most important to you. Do you know what you are doing that makes them think you have a drinking or anger problem?
Mr. L: “All I did was come in late a couple of times and got into a little argument with a couple of people.”
T: “If we are going to help you keep your job, we could spend our time talking about how unfair your boss is and how she’s misjudging you. Or we could work to show her that she has you all wrong and that you are a productive worker who does not have a substance or anger problem.
“Let’s think together how we could gather the data that would prove you don’t have a substance problem. If all that data is squeaky clean, then I can write a very supportive letter to your boss and tell her all is well. If, however, we discover you do have a problem, I can still write a very supportive letter. But we’ll have to show her how you are taking care of any problems that interfere with your work performance.”
Reframe the presenting complaint
Few patients present fully ready to work on definitive behavioral health recovery. If patients at least attend sessions or talk with you, they must be motivated to do something. Otherwise, they would not show up.
Our task is help patients such as Mr. L get what they want, not what we think they should want. Eventually you will get to explore the patient’s substance use, impulse or parenting problems, mental health symptoms, or communication problems, but this discussion will be in the service of allying with his or her goals.
Rather than viewing patients as unmotivated or resistant, think of resistance as an interactional process. “If we are going to stop them from locking you up,” you might say, “let’s talk about what you are doing that makes you look like you are dangerous and out of control. And when you were not locked away, let’s think of how you kept ‘them’ off your back.”
Instead of interpreting resistance as pathology, view the behavior as an opportunity to understand and respond to the patient’s stage of readiness to change.
Stages of change
By being “difficult,” patients are often declaring that they are not invested in what you think the problem is or in working on that problem. Resistance thrives when we and the patient have not allied around a common goal and are at different stages of change. Think of the therapeutic alliance in the context of the widely-used and well-researched Transtheoretical Model’s stages of change:3,4
Precontemplation. A person at this stage is not considering the possibility of change, although others are aware of a problem. He or she will seldom appear for treatment without coercion. The person could benefit from nonthreatening information to raise awareness of a possible “problem” and possibilities for change.
Contemplation. The person is ambivalent, undecided, vacillating about whether he has a “problem.” He wants to change, but this desire exists simultaneously with resistance to change. Motivational strategies can be useful, but aggressive or premature confrontation could provoke strong resistance and defensive behaviors. Many persons at this stage have indefinite plans to take action in the next 6 months or so.
Preparation takes the person from the contemplation stage to specific steps to solve the problem in the action stage. He or she develops increasing confidence in the decision to change and takes the first steps on the road to action. Most people at this stage plan to take action within 1 month and are making final adjustments before beginning to change.
Action. The person takes specific actions intended to bring about change. This busiest stage of change is characterized by overt modification of behavior and surroundings and requires the greatest time and energy. Support and encouragement are crucial to prevent drop-out and regression in readiness to change.
Maintenance. Goals at this stage are to sustain the changes accomplished by previous action and to prevent relapse. Maintaining new behaviors requires different skills than were needed to initiate change. Gains are consolidated. “Maintenance” is not a static stage; it can last 6 months or up to a lifetime. The patient learns new coping and problem-solving strategies, replaces problem behaviors with a healthier life style, and works through emotional triggers of relapse.
Relapse/recycling can happen but is not inevitable. When setbacks occur, help the patient avoid becoming stuck, discouraged, or demoralized, and help him learn from relapse before committing to a new action cycle. Conduct a comprehensive, multidimensional assessment to explore all reasons for relapse.
Termination is the ultimate goal: to exit the cycle of change without fear of relapse. Certain problems may be terminated or merely kept in remission through maintenance strategies.
Match strategies with stages
Discovery planning. Engaging patients in collaborative care starts with honoring their stages of change and working with them and their families on different tasks for each stage of change.4-6 A patient such as Mr. L, for example—who is at an early stage of change and thinks he has an “unfair boss problem” (not an alcohol problem) or a “nagging wife problem” (not an anger or domestic violence problem)—needs a discovery, drop-out prevention plan.
The cause of the patient’s work or relationship problem may be obvious to you, but a patient in early stages of change resists that information and, if pressed, gets frustrated and leaves treatment. A “discovery” treatment plan embraces the patient’s views and could be focused, for example, on gathering data that would prove to the employer that there is not an alcohol problem.
If random breath alcohol testing, feedback from family, and review of past job losses all prove negative for alcohol problems, the patient would have data to support his or her view that he does not have an alcohol problem. If, however, this exploration reveals an alcohol problem, the patient “discovers” he has more of a problem than he thought. For this plan, the challenge is to keep Mr. L engaged long enough to discover the connection between his alcohol problems and his employment or marital problems.
Recovery planning. On the other hand, a person at the action stage who wants to avoid becoming depressed again or wishes to live a life of sobriety collaborates on a recovery, relapse prevention plan. This patient is committed to developing the knowledge and skills to prevent relapse and open to whatever will promote health and well-being.
The Transtheoretical Model’s 9 processes of change3,4 inform which interventions might be most effective at various stages of change (Table 2). For example, a patient might be proud of the fact that “I can hold my liquor and drink everyone under the table.” Consciousness-raising—such as by explaining that his high alcohol tolerance is a danger signal, not a beneficial ability—can enhance his change process. Even if he is not ready to commit to definitive change, at least exploring whether he has a problem may move him from precontemplation to a contemplation stage of change. Table 3 shows how other processes of change can help motivate patients in later stages of change.4,5
Table 2
Transtheoretical model’s 9 processes of change: What happens at each step
| Process of change | The person… |
|---|---|
| Conscious-raising | becomes aware of a problem from education, advice, self-awareness, or feedback from others |
| Social liberation | begins to think about change because external forces raise awareness (a ban on smoking in restaurants, for example, can heighten awareness that one has a smoking problem) |
| Emotional arousal | becomes more convinced of the need to change when faced with a strong and sudden emotional experience related to the problem (such as death of a loved one) |
| Self-reevaluation | examines his or her values to see whether or not the behavior conflicts with what is important to him or her |
| Commitment | accepts responsibility for changing and affirms to self and others the decision to change |
| Reward | uses self-praise, positive feedback from others, improved well-being or financial security, “natural highs,” and other reinforcing benefits to consolidate change |
| Countering | substitutes other responses to counter unhealthy choices and behavior (such as relaxation techniques to combat angry outbursts or urges to resume smoking) |
| Environmental control | changes surrounding people, places, or things to reduce the risk of continuing or resuming the problem behavior |
| Helping relationships | seeks assistance from trusted friends, professionals, spiritual advisors, or significant others to initiate and sustain the change process |
| Source: Adapted and reprinted with permission from reference 4. | |
Table 3
Strategies and interventions to motivate patients at each stage of change
| Stage of change | Most useful processes of change (see Table 2) | Goal of treatment | Strategies and interventions |
|---|---|---|---|
| Precontemplation | Consciousness-raising Social liberation | To raise doubt |
|
| Contemplation | Consciousness-raising Social liberation Emotional arousal Self-reevaluation | To tip the balance |
|
| Preparation | Social liberation Emotional arousal Self-reevaluation Commitment | To determine best course |
|
| Action | Social liberation Commitment Reward Countering Environment control Helping relationships | To take steps to change |
|
| Maintenance | Commitment Countering Environment control Helping relationships | To prevent relapse |
|
| Relapse/recycling | Depends on stage to which patient returned | To renew processes of change |
|
- Scott D. Miller, PhD. Institute for the Study of Therapeutic Change, Chicago, IL. (773) 404-5130; www.talkingcure.com.
- Miller WR, Rollnick S, Moyers TB. Motivational interviewing: professional training videotape series. Albuquerque, NM: University of New Mexico, 1998. Six videotapes (about $130 total); (505) 768-0279 or 0100.
- Miller WR, consensus panel chair. Treatment improvement protocol. Enhancing motivation for change in substance abuse treatment. Rockville, MD; Center for Substance Abuse Treatment, 1999. DHHS Publication 99-3354. Available from the National Clearinghouse for Alcohol/Drug Information, Rockville, MD; (800) 729-6686.
Dr. Mee-Lee is a board-certified psychiatrist and is certified by examination of the American Society of Addiction Medicine (ASAM). He is based in Davis, CA, and is involved in full-time training and consulting. For information, visit www.DMLMD.com.
1. Horvath AO, Bedi RP. The alliance. In: Norcross JC, ed. Psychotherapy relationships that work: therapist contributions and responsiveness to patients. New York: Oxford University Press; 2002.
2. Mee-Lee D. Treatment planning for dual disorders. Psychiatr Rehabil Skills 2001;5(1):52-79.
3. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: applications to addictive behaviors. Am Psychol 1992;47:1102-14.
4. Prochaska JO, Norcross JC, DiClemente CC. Changing for good. New York: Avon Books; 1994:54.
5. DiClemente CC. Conceptual models and applied research: the ongoing contribution of the transtheoretical model. J Addict Nurs 2005;16:5-12.
6. Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991:18.
1. Horvath AO, Bedi RP. The alliance. In: Norcross JC, ed. Psychotherapy relationships that work: therapist contributions and responsiveness to patients. New York: Oxford University Press; 2002.
2. Mee-Lee D. Treatment planning for dual disorders. Psychiatr Rehabil Skills 2001;5(1):52-79.
3. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: applications to addictive behaviors. Am Psychol 1992;47:1102-14.
4. Prochaska JO, Norcross JC, DiClemente CC. Changing for good. New York: Avon Books; 1994:54.
5. DiClemente CC. Conceptual models and applied research: the ongoing contribution of the transtheoretical model. J Addict Nurs 2005;16:5-12.
6. Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991:18.
Dependent personality disorder: Effective time-limited therapy
Some dependent patients are needy, clingy, and insecure—unable to make the smallest decisions without inordinate advice and reassurance—whereas others are less easy to recognize. Dependency can be expressed in many different ways: obvious or subtle, maladaptive or adaptive.
Dependent psychotherapy patients are compliant and eager to please but can have difficulty terminating treatment. This article offers recommendations for clinical work with dependent adults to help you:
- assess and diagnose dependent personality disorder (DPD)
- distinguish unhealthy from healthy dependency
- provide effective psychotherapy for DPD in inpatient and outpatient settings.
What is a dependent personality?
DPD is diagnosed when an individual exhibits long-standing, inflexible dependency that creates difficulties in social, sexual, and occupational functioning, according to DSM-IV-TR.1 DPD’s essential feature is a pervasive and excessive need to be taken care of that leads to submissive and clinging behavior and fears of separation, beginning by early adulthood and present in a variety of contexts. To receive a DPD diagnosis, a patient must show 5 of 8 possible symptoms (Table 1).
Table 1
Symptoms of dependent personality disorder (DPD)*
| Difficulty making everyday decisions without excessive advice and reassurance |
| Needing others to assume responsibility for most major areas of life |
| Difficulty expressing disagreement because of a fear of disapproval |
| Difficulty initiating projects or doing things on one’s own |
| Going to excessive lengths to obtain nurturance and support from others |
| Feeling uncomfortable or helpless when alone |
| Urgently seeking another relationship as a source of care and support when a close relationship ends |
| Being unrealistically preoccupied with fears of being left to care for oneself |
| * 5 of 8 symptoms required for DPD |
| Source: Adapted from DSM-IV-TR |
Who has DPD? One of the more common Axis II disorders, DPD is not distributed equally across the population. No studies have assessed the impact of age on DPD risk, but variables that affect DPD prevalence include:
- gender (women are far more likely than men to receive a DPD diagnosis)
- practice setting (DPD is more prevalent in rehabilitation and psychiatric inpatient settings than in outpatient practices)
- race and ethnicity (dependency may be less prevalent in African-American than in Caucasian adults).3,4
Interpersonal, intrapsychic dynamics
DPD is viewed as having 4 related components:4,5
- Cognitive: A perception of oneself as powerless and ineffectual plus the belief that other people are comparatively confident and competent.
- Motivational: A strong desire to maintain close ties with protectors and caregivers.
- Emotional: Fear of abandonment or rejection; anxiety about evaluation by authority figures.
- Behavioral: A pattern of relationship-facilitating behavior designed to minimize the possibility of abandonment and rejection.
Interpersonal strategies. Dependent persons use interpersonal strategies to strengthen social ties and minimize the possibility of being rejected or abandoned (Table 2). Some strategies involve behavior that is active and assertive—even quite aggressive.8 Therefore, dependency does not necessarily equate with passivity.
Table 2
Self-presentation strategies
used by dependent persons to facilitate relationships
| Strategy | Goal | Typical behaviors |
|---|---|---|
| Supplication | Appear helpless and vulnerable | Submissiveness, self-deprecation |
| Ingratiation | Create indebtedness | Ego-bolstering, performing favors |
| Exemplification | Exploit others’ Guilt | Providing help, emphasizing effort and sacrifices |
| Self-promotion | Emphasize personal worth | Performance claims, exaggeration of accomplishments |
| Intimidation | Frighten and control others | Anger displays, breakdown threats |
What causes DPD?
Three theoretical frameworks have been used to explain the development and dynamics of DPD. Each suggests intervention techniques for dealing with dependency-related problems.
Psychodynamic. Psychodynamic theorists conceptualize problematic dependency in terms of dependency conflicts (such as conflicts between a desire to be cared for and an urge to dominate and compete). Ego defenses used to manage the affect associated with these conflicts (such as denial or projection) help determine the manner in which underlying dependency needs are expressed.9
Cognitive. Cognitive theorists regard problematic dependency as stemming from self-defeating thought patterns,10 including:
- helplessness-inducing automatic thoughts (reflexive thoughts that reflect the person’s lack of self-confidence)
- negative self-statements (self-deprecating internal monologues in which dependent persons reaffirm their perceived lack of competence and skill).
Diagnosis and assessment
Three principles guide the diagnosis of DPD.
- Dependency, as noted, is not always characterized by passivity. Dependent patients may use active, dramatic self-presentation strategies—such as breakdown threats or parasuicide attempts—to protect themselves from being abandoned.4,8
- Self-reports do not always give a true picture. Because dependency may be viewed as a sign of weakness and immaturity, many adults—especially men—are reluctant to acknowledge dependent thoughts and feelings.12 Interviewing knowledgeable informants can be enlightening.
- Dependency’s severity varies over time and across situations. Depressive episodes are associated with temporary increases in self-reported dependency. Even modest mood changes can amplify dependency.13,14
Questionnaires do not allow you to probe and follow-up, but paper-and-pencil tools are relatively inexpensive and efficient. They also avoid reliability problems that can occur with structured interviews. The 2 self-report instruments used most often to diagnose DPD are:
Differential diagnosis
DPD must be distinguished from Axis I and II syndromes with often-overlapping symptoms and similar presentations. These include:
- mood disorders, panic disorder, agoraphobia, and dependency arising from one or more general medical conditions
- borderline personality disorder, histrionic personality disorder, and avoidant personality disorder.1
Axis II comorbidity patterns likely reflect the generalized, nonspecific nature of personality pathology and the fact that patients may show personality disorder symptoms in one or more diagnostic categories.
DPD Treatment
Dependency is associated with patient cooperativeness and conscientiousness.3,4,16,17 Compared with nondependent patients, those with dependent personalities:
- delay less time before seeking treatment for psychological or medical symptoms
- adhere more conscientiously to psychotherapeutic and psychotropic regimens
- miss fewer therapy sessions
- show higher rates of treatment completion in outpatient individual and group therapy.
Psychotherapy. Traditional psychotherapies—psychodynamic, cognitive, behavioral—modestly improve DPD symptoms.19 Most effective has been psychotherapy that combines various modalities.2,4,20 Five interventions (Table 3) have been shown to:
- help the patient and therapist identify aspects of the patient’s environment that propagate dependent behavior
- provide the patient with coping skills needed to more effectively control dependency-related impulses.
Table 3
5 useful psychotherapeutic methods for dependent patients
| Explore key relationships from the patient’s past that reinforced dependent behavior; determine if similar patterns occur in present relationships |
| Examine his or her ‘helpless self-concept,’ dependency’s key cognitive component (Tip: Asking the patient to write a selfdescription can be useful) |
| Make explicit any self-denigrating statements that propagate the patient’s feelings of helplessness and vulnerability; challenge these statements when appropriate |
| Help the patient gain insight into the ways he or she expresses dependency needs in different situations (and more-flexible, adaptive ways he or she could express these needs) |
| Use in-session role play and betweensessions homework to help the patient build coping skills that will enable him or her to function more autonomously |
Limitations and caveats
Clinical work with DPD patients traditionally has focused on diminishing problematic dependency. Recent research suggests, however, that expressing dependency strivings in a flexible, situation-appropriate manner can strengthen interpersonal ties and facilitate adaptation and healthy psychological functioning.2,5 Thus, the most effective interventions emphasize replacing unhealthy, maladaptive dependency with flexible, adaptive dependency.
Beyond the strategies summarized in Table 3, several other considerations—such as setting limits—are important in managing DPD and in minimizing therapeutic obstacles and impasses.
Set firm limits on after-hours contact early in treatment. Unless you set firm limits at the outset of therapy, dependent patients tend to have a higher-than-average number of “pseudo-emergencies” and make frequent requests for between-sessions contact.
In inpatient settings, patients with DPD receive more consultations and psychotropic medications than do non-DPD patients with similar demographic and diagnostic profiles, and their treatment costs can become excessive.21
Shift responsibility. When you provide adequate structure early in treatment, the dependent patient will feel secure enough to open up and disclose troubling thoughts and feelings. Then, as therapy progresses, help the patient experience autonomy and competence within the therapeutic milieu by gradually requiring him or her to take on increasing responsibility for structuring treatment.10
Beware of countertransference. Many therapists infantilize dependent patients, and exploitation or abuse—financial or sexual—may follow. You must acknowledge and confront these problematic feelings when they occur, either in formal clinical supervision or in informal consultation with other mental health professionals.4,21
Two countertransference reactions are particularly common (and problematic) in therapeutic work with dependent patients:
- the fantasy of insatiability (believing that no matter how much support and reassurance the patient receives, it will never be enough)
- the fantasy of permanence (believing the patient will become so comfortable in therapy’s protective cocoon that he or she will never leave treatment).
Because some studies suggest that dependent patients may be at increased risk for suicide, monitor them continuously for negative indicators.8,9 Five danger signs (Table 4) suggest an increased risk of self-destructive behavior in dependent patients.
Table 4
5 warning signs of self-destructive behavior in dependent patients
| Recent relationship conflict or interpersonal loss |
| Excessive or unrealistic jealousy |
| Poor impulse control |
| Difficulty modulating negative emotions |
| Previous suicide attempts or suicidal gestures |
Because dependent persons often construct interpersonal milieus that foster and propagate their dependency, concurrent marital and/or family therapy may be warranted to disrupt entrenched dysfunctional patterns.16,17 Examine the rewards dependent patients obtain for behaving helpless and vulnerable and ways in which their dependency may reward friends, family members, and coworkers.
Related resources
- Baltes MM. The many faces of dependency in old age. Cambridge, UK: Cambridge University Press; 1996.
- Bornstein RF. The dependent personality: developmental, social, and clinical perspectives. Psychol Bull 1992;112(1):3-23.
- Millon T. Disorders of personality: DSM-IV and beyond. New York: Wiley; 1996.
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Bornstein RF, Languirand MA. Healthy dependency: leaning on others without losing yourself. New York, NY: Newmarket Press; 2003.
3. Bornstein RF. The dependent personality. New York: Guilford Press; 1993.
4. Bornstein RF. The dependent patient: a practitioner’s guide. Washington, DC: American Psychological Association; 2005.
5. Pincus AL, Wilson KR. Interpersonal variability in dependent personality. J Pers 2001;69(2):223-51.
6. Pincus AL, Gurtman MB. The three faces of interpersonal dependency: structural analysis of self-report dependency measures. J Pers Soc Psychol 1995;69(4):744-58.
7. Overholser JC. The dependent personality and interpersonal problems. J Nerv Ment Dis 1996;184(1)1:8-16.
8. Bornstein RF. The complex relationship between dependency and domestic violence: converging psychological factors and social forces. Am Psychol 2006;61(6):595-606.
9. Coen SJ. The misuse of persons: analyzing pathological dependency. Hillsdale, NJ: Analytic Press; 1992.
10. Overholser JC, Fine MA. Cognitive-behavioral treatment of excessive interpersonal dependency: a four-stage psychotherapy model. Journal of Cognitive Psychotherapy 1994;8(1):55-70.
11. Turkat ID. The personality disorders: a psychological approach to clinical management. New York, NY: Pergamon Press; 1990.
12. Bornstein RF. Criterion validity of objective and projective dependency tests: a meta-analytic assessment of behavioral prediction. Psychological Assessment 1999;11(1):48-57.
13. Birtchnell J. The measurement of dependence by questionnaire. Journal of Personality Disorders 1991;5(3):281-95.
14. Nietzel MT, Harris MJ. Relationship of dependency and achievement/autonomy to depression. Clinical Psychology Review 1990;10:279-97.
15. Bornstein RF. Dependent personality disorder in the DSM-IV and beyond. Clinical Psychology: Science and Practice 1997;4(2):175-87.
16. Ryder RD, Parry-Jones WL. Fears of dependence and its value in working with adolescents. J Adolesc 1982;5(1):71-8.
17. Tait M. Dependence: a means or an impediment to growth? British Journal of Guidance and Counselling. 1997;25(1):17-26.
18. Black DW, Monahan P, Wesner R, et al. The effects of fluvoxamine, cognitive therapy, and placebo on abnormal personality traits in 44 patients with panic disorder. Journal of Personality Disorders. 1996;10(2):185-94.
19. Rathus JH, Sanderson WC, Miller AL, Wetzler S. Impact of personality functioning on cognitive behavioral treatment of panic disorder: a preliminary report. Journal of Personality Disorders 1995;9(2):160-8.
20. Bornstein RF. Integrating cognitive and existential treatment strategies in psychotherapy with dependent patients. Journal of Contemporary Psychotherapy 2004;34(4):293-309.
21. Abramson PR, Cloud MY, Keese N, Keese R. How much is too much? Dependency in a psychotherapeutic relationship. Am J Psychother 1994;48(2):294-301.
Some dependent patients are needy, clingy, and insecure—unable to make the smallest decisions without inordinate advice and reassurance—whereas others are less easy to recognize. Dependency can be expressed in many different ways: obvious or subtle, maladaptive or adaptive.
Dependent psychotherapy patients are compliant and eager to please but can have difficulty terminating treatment. This article offers recommendations for clinical work with dependent adults to help you:
- assess and diagnose dependent personality disorder (DPD)
- distinguish unhealthy from healthy dependency
- provide effective psychotherapy for DPD in inpatient and outpatient settings.
What is a dependent personality?
DPD is diagnosed when an individual exhibits long-standing, inflexible dependency that creates difficulties in social, sexual, and occupational functioning, according to DSM-IV-TR.1 DPD’s essential feature is a pervasive and excessive need to be taken care of that leads to submissive and clinging behavior and fears of separation, beginning by early adulthood and present in a variety of contexts. To receive a DPD diagnosis, a patient must show 5 of 8 possible symptoms (Table 1).
Table 1
Symptoms of dependent personality disorder (DPD)*
| Difficulty making everyday decisions without excessive advice and reassurance |
| Needing others to assume responsibility for most major areas of life |
| Difficulty expressing disagreement because of a fear of disapproval |
| Difficulty initiating projects or doing things on one’s own |
| Going to excessive lengths to obtain nurturance and support from others |
| Feeling uncomfortable or helpless when alone |
| Urgently seeking another relationship as a source of care and support when a close relationship ends |
| Being unrealistically preoccupied with fears of being left to care for oneself |
| * 5 of 8 symptoms required for DPD |
| Source: Adapted from DSM-IV-TR |
Who has DPD? One of the more common Axis II disorders, DPD is not distributed equally across the population. No studies have assessed the impact of age on DPD risk, but variables that affect DPD prevalence include:
- gender (women are far more likely than men to receive a DPD diagnosis)
- practice setting (DPD is more prevalent in rehabilitation and psychiatric inpatient settings than in outpatient practices)
- race and ethnicity (dependency may be less prevalent in African-American than in Caucasian adults).3,4
Interpersonal, intrapsychic dynamics
DPD is viewed as having 4 related components:4,5
- Cognitive: A perception of oneself as powerless and ineffectual plus the belief that other people are comparatively confident and competent.
- Motivational: A strong desire to maintain close ties with protectors and caregivers.
- Emotional: Fear of abandonment or rejection; anxiety about evaluation by authority figures.
- Behavioral: A pattern of relationship-facilitating behavior designed to minimize the possibility of abandonment and rejection.
Interpersonal strategies. Dependent persons use interpersonal strategies to strengthen social ties and minimize the possibility of being rejected or abandoned (Table 2). Some strategies involve behavior that is active and assertive—even quite aggressive.8 Therefore, dependency does not necessarily equate with passivity.
Table 2
Self-presentation strategies
used by dependent persons to facilitate relationships
| Strategy | Goal | Typical behaviors |
|---|---|---|
| Supplication | Appear helpless and vulnerable | Submissiveness, self-deprecation |
| Ingratiation | Create indebtedness | Ego-bolstering, performing favors |
| Exemplification | Exploit others’ Guilt | Providing help, emphasizing effort and sacrifices |
| Self-promotion | Emphasize personal worth | Performance claims, exaggeration of accomplishments |
| Intimidation | Frighten and control others | Anger displays, breakdown threats |
What causes DPD?
Three theoretical frameworks have been used to explain the development and dynamics of DPD. Each suggests intervention techniques for dealing with dependency-related problems.
Psychodynamic. Psychodynamic theorists conceptualize problematic dependency in terms of dependency conflicts (such as conflicts between a desire to be cared for and an urge to dominate and compete). Ego defenses used to manage the affect associated with these conflicts (such as denial or projection) help determine the manner in which underlying dependency needs are expressed.9
Cognitive. Cognitive theorists regard problematic dependency as stemming from self-defeating thought patterns,10 including:
- helplessness-inducing automatic thoughts (reflexive thoughts that reflect the person’s lack of self-confidence)
- negative self-statements (self-deprecating internal monologues in which dependent persons reaffirm their perceived lack of competence and skill).
Diagnosis and assessment
Three principles guide the diagnosis of DPD.
- Dependency, as noted, is not always characterized by passivity. Dependent patients may use active, dramatic self-presentation strategies—such as breakdown threats or parasuicide attempts—to protect themselves from being abandoned.4,8
- Self-reports do not always give a true picture. Because dependency may be viewed as a sign of weakness and immaturity, many adults—especially men—are reluctant to acknowledge dependent thoughts and feelings.12 Interviewing knowledgeable informants can be enlightening.
- Dependency’s severity varies over time and across situations. Depressive episodes are associated with temporary increases in self-reported dependency. Even modest mood changes can amplify dependency.13,14
Questionnaires do not allow you to probe and follow-up, but paper-and-pencil tools are relatively inexpensive and efficient. They also avoid reliability problems that can occur with structured interviews. The 2 self-report instruments used most often to diagnose DPD are:
Differential diagnosis
DPD must be distinguished from Axis I and II syndromes with often-overlapping symptoms and similar presentations. These include:
- mood disorders, panic disorder, agoraphobia, and dependency arising from one or more general medical conditions
- borderline personality disorder, histrionic personality disorder, and avoidant personality disorder.1
Axis II comorbidity patterns likely reflect the generalized, nonspecific nature of personality pathology and the fact that patients may show personality disorder symptoms in one or more diagnostic categories.
DPD Treatment
Dependency is associated with patient cooperativeness and conscientiousness.3,4,16,17 Compared with nondependent patients, those with dependent personalities:
- delay less time before seeking treatment for psychological or medical symptoms
- adhere more conscientiously to psychotherapeutic and psychotropic regimens
- miss fewer therapy sessions
- show higher rates of treatment completion in outpatient individual and group therapy.
Psychotherapy. Traditional psychotherapies—psychodynamic, cognitive, behavioral—modestly improve DPD symptoms.19 Most effective has been psychotherapy that combines various modalities.2,4,20 Five interventions (Table 3) have been shown to:
- help the patient and therapist identify aspects of the patient’s environment that propagate dependent behavior
- provide the patient with coping skills needed to more effectively control dependency-related impulses.
Table 3
5 useful psychotherapeutic methods for dependent patients
| Explore key relationships from the patient’s past that reinforced dependent behavior; determine if similar patterns occur in present relationships |
| Examine his or her ‘helpless self-concept,’ dependency’s key cognitive component (Tip: Asking the patient to write a selfdescription can be useful) |
| Make explicit any self-denigrating statements that propagate the patient’s feelings of helplessness and vulnerability; challenge these statements when appropriate |
| Help the patient gain insight into the ways he or she expresses dependency needs in different situations (and more-flexible, adaptive ways he or she could express these needs) |
| Use in-session role play and betweensessions homework to help the patient build coping skills that will enable him or her to function more autonomously |
Limitations and caveats
Clinical work with DPD patients traditionally has focused on diminishing problematic dependency. Recent research suggests, however, that expressing dependency strivings in a flexible, situation-appropriate manner can strengthen interpersonal ties and facilitate adaptation and healthy psychological functioning.2,5 Thus, the most effective interventions emphasize replacing unhealthy, maladaptive dependency with flexible, adaptive dependency.
Beyond the strategies summarized in Table 3, several other considerations—such as setting limits—are important in managing DPD and in minimizing therapeutic obstacles and impasses.
Set firm limits on after-hours contact early in treatment. Unless you set firm limits at the outset of therapy, dependent patients tend to have a higher-than-average number of “pseudo-emergencies” and make frequent requests for between-sessions contact.
In inpatient settings, patients with DPD receive more consultations and psychotropic medications than do non-DPD patients with similar demographic and diagnostic profiles, and their treatment costs can become excessive.21
Shift responsibility. When you provide adequate structure early in treatment, the dependent patient will feel secure enough to open up and disclose troubling thoughts and feelings. Then, as therapy progresses, help the patient experience autonomy and competence within the therapeutic milieu by gradually requiring him or her to take on increasing responsibility for structuring treatment.10
Beware of countertransference. Many therapists infantilize dependent patients, and exploitation or abuse—financial or sexual—may follow. You must acknowledge and confront these problematic feelings when they occur, either in formal clinical supervision or in informal consultation with other mental health professionals.4,21
Two countertransference reactions are particularly common (and problematic) in therapeutic work with dependent patients:
- the fantasy of insatiability (believing that no matter how much support and reassurance the patient receives, it will never be enough)
- the fantasy of permanence (believing the patient will become so comfortable in therapy’s protective cocoon that he or she will never leave treatment).
Because some studies suggest that dependent patients may be at increased risk for suicide, monitor them continuously for negative indicators.8,9 Five danger signs (Table 4) suggest an increased risk of self-destructive behavior in dependent patients.
Table 4
5 warning signs of self-destructive behavior in dependent patients
| Recent relationship conflict or interpersonal loss |
| Excessive or unrealistic jealousy |
| Poor impulse control |
| Difficulty modulating negative emotions |
| Previous suicide attempts or suicidal gestures |
Because dependent persons often construct interpersonal milieus that foster and propagate their dependency, concurrent marital and/or family therapy may be warranted to disrupt entrenched dysfunctional patterns.16,17 Examine the rewards dependent patients obtain for behaving helpless and vulnerable and ways in which their dependency may reward friends, family members, and coworkers.
Related resources
- Baltes MM. The many faces of dependency in old age. Cambridge, UK: Cambridge University Press; 1996.
- Bornstein RF. The dependent personality: developmental, social, and clinical perspectives. Psychol Bull 1992;112(1):3-23.
- Millon T. Disorders of personality: DSM-IV and beyond. New York: Wiley; 1996.
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Some dependent patients are needy, clingy, and insecure—unable to make the smallest decisions without inordinate advice and reassurance—whereas others are less easy to recognize. Dependency can be expressed in many different ways: obvious or subtle, maladaptive or adaptive.
Dependent psychotherapy patients are compliant and eager to please but can have difficulty terminating treatment. This article offers recommendations for clinical work with dependent adults to help you:
- assess and diagnose dependent personality disorder (DPD)
- distinguish unhealthy from healthy dependency
- provide effective psychotherapy for DPD in inpatient and outpatient settings.
What is a dependent personality?
DPD is diagnosed when an individual exhibits long-standing, inflexible dependency that creates difficulties in social, sexual, and occupational functioning, according to DSM-IV-TR.1 DPD’s essential feature is a pervasive and excessive need to be taken care of that leads to submissive and clinging behavior and fears of separation, beginning by early adulthood and present in a variety of contexts. To receive a DPD diagnosis, a patient must show 5 of 8 possible symptoms (Table 1).
Table 1
Symptoms of dependent personality disorder (DPD)*
| Difficulty making everyday decisions without excessive advice and reassurance |
| Needing others to assume responsibility for most major areas of life |
| Difficulty expressing disagreement because of a fear of disapproval |
| Difficulty initiating projects or doing things on one’s own |
| Going to excessive lengths to obtain nurturance and support from others |
| Feeling uncomfortable or helpless when alone |
| Urgently seeking another relationship as a source of care and support when a close relationship ends |
| Being unrealistically preoccupied with fears of being left to care for oneself |
| * 5 of 8 symptoms required for DPD |
| Source: Adapted from DSM-IV-TR |
Who has DPD? One of the more common Axis II disorders, DPD is not distributed equally across the population. No studies have assessed the impact of age on DPD risk, but variables that affect DPD prevalence include:
- gender (women are far more likely than men to receive a DPD diagnosis)
- practice setting (DPD is more prevalent in rehabilitation and psychiatric inpatient settings than in outpatient practices)
- race and ethnicity (dependency may be less prevalent in African-American than in Caucasian adults).3,4
Interpersonal, intrapsychic dynamics
DPD is viewed as having 4 related components:4,5
- Cognitive: A perception of oneself as powerless and ineffectual plus the belief that other people are comparatively confident and competent.
- Motivational: A strong desire to maintain close ties with protectors and caregivers.
- Emotional: Fear of abandonment or rejection; anxiety about evaluation by authority figures.
- Behavioral: A pattern of relationship-facilitating behavior designed to minimize the possibility of abandonment and rejection.
Interpersonal strategies. Dependent persons use interpersonal strategies to strengthen social ties and minimize the possibility of being rejected or abandoned (Table 2). Some strategies involve behavior that is active and assertive—even quite aggressive.8 Therefore, dependency does not necessarily equate with passivity.
Table 2
Self-presentation strategies
used by dependent persons to facilitate relationships
| Strategy | Goal | Typical behaviors |
|---|---|---|
| Supplication | Appear helpless and vulnerable | Submissiveness, self-deprecation |
| Ingratiation | Create indebtedness | Ego-bolstering, performing favors |
| Exemplification | Exploit others’ Guilt | Providing help, emphasizing effort and sacrifices |
| Self-promotion | Emphasize personal worth | Performance claims, exaggeration of accomplishments |
| Intimidation | Frighten and control others | Anger displays, breakdown threats |
What causes DPD?
Three theoretical frameworks have been used to explain the development and dynamics of DPD. Each suggests intervention techniques for dealing with dependency-related problems.
Psychodynamic. Psychodynamic theorists conceptualize problematic dependency in terms of dependency conflicts (such as conflicts between a desire to be cared for and an urge to dominate and compete). Ego defenses used to manage the affect associated with these conflicts (such as denial or projection) help determine the manner in which underlying dependency needs are expressed.9
Cognitive. Cognitive theorists regard problematic dependency as stemming from self-defeating thought patterns,10 including:
- helplessness-inducing automatic thoughts (reflexive thoughts that reflect the person’s lack of self-confidence)
- negative self-statements (self-deprecating internal monologues in which dependent persons reaffirm their perceived lack of competence and skill).
Diagnosis and assessment
Three principles guide the diagnosis of DPD.
- Dependency, as noted, is not always characterized by passivity. Dependent patients may use active, dramatic self-presentation strategies—such as breakdown threats or parasuicide attempts—to protect themselves from being abandoned.4,8
- Self-reports do not always give a true picture. Because dependency may be viewed as a sign of weakness and immaturity, many adults—especially men—are reluctant to acknowledge dependent thoughts and feelings.12 Interviewing knowledgeable informants can be enlightening.
- Dependency’s severity varies over time and across situations. Depressive episodes are associated with temporary increases in self-reported dependency. Even modest mood changes can amplify dependency.13,14
Questionnaires do not allow you to probe and follow-up, but paper-and-pencil tools are relatively inexpensive and efficient. They also avoid reliability problems that can occur with structured interviews. The 2 self-report instruments used most often to diagnose DPD are:
Differential diagnosis
DPD must be distinguished from Axis I and II syndromes with often-overlapping symptoms and similar presentations. These include:
- mood disorders, panic disorder, agoraphobia, and dependency arising from one or more general medical conditions
- borderline personality disorder, histrionic personality disorder, and avoidant personality disorder.1
Axis II comorbidity patterns likely reflect the generalized, nonspecific nature of personality pathology and the fact that patients may show personality disorder symptoms in one or more diagnostic categories.
DPD Treatment
Dependency is associated with patient cooperativeness and conscientiousness.3,4,16,17 Compared with nondependent patients, those with dependent personalities:
- delay less time before seeking treatment for psychological or medical symptoms
- adhere more conscientiously to psychotherapeutic and psychotropic regimens
- miss fewer therapy sessions
- show higher rates of treatment completion in outpatient individual and group therapy.
Psychotherapy. Traditional psychotherapies—psychodynamic, cognitive, behavioral—modestly improve DPD symptoms.19 Most effective has been psychotherapy that combines various modalities.2,4,20 Five interventions (Table 3) have been shown to:
- help the patient and therapist identify aspects of the patient’s environment that propagate dependent behavior
- provide the patient with coping skills needed to more effectively control dependency-related impulses.
Table 3
5 useful psychotherapeutic methods for dependent patients
| Explore key relationships from the patient’s past that reinforced dependent behavior; determine if similar patterns occur in present relationships |
| Examine his or her ‘helpless self-concept,’ dependency’s key cognitive component (Tip: Asking the patient to write a selfdescription can be useful) |
| Make explicit any self-denigrating statements that propagate the patient’s feelings of helplessness and vulnerability; challenge these statements when appropriate |
| Help the patient gain insight into the ways he or she expresses dependency needs in different situations (and more-flexible, adaptive ways he or she could express these needs) |
| Use in-session role play and betweensessions homework to help the patient build coping skills that will enable him or her to function more autonomously |
Limitations and caveats
Clinical work with DPD patients traditionally has focused on diminishing problematic dependency. Recent research suggests, however, that expressing dependency strivings in a flexible, situation-appropriate manner can strengthen interpersonal ties and facilitate adaptation and healthy psychological functioning.2,5 Thus, the most effective interventions emphasize replacing unhealthy, maladaptive dependency with flexible, adaptive dependency.
Beyond the strategies summarized in Table 3, several other considerations—such as setting limits—are important in managing DPD and in minimizing therapeutic obstacles and impasses.
Set firm limits on after-hours contact early in treatment. Unless you set firm limits at the outset of therapy, dependent patients tend to have a higher-than-average number of “pseudo-emergencies” and make frequent requests for between-sessions contact.
In inpatient settings, patients with DPD receive more consultations and psychotropic medications than do non-DPD patients with similar demographic and diagnostic profiles, and their treatment costs can become excessive.21
Shift responsibility. When you provide adequate structure early in treatment, the dependent patient will feel secure enough to open up and disclose troubling thoughts and feelings. Then, as therapy progresses, help the patient experience autonomy and competence within the therapeutic milieu by gradually requiring him or her to take on increasing responsibility for structuring treatment.10
Beware of countertransference. Many therapists infantilize dependent patients, and exploitation or abuse—financial or sexual—may follow. You must acknowledge and confront these problematic feelings when they occur, either in formal clinical supervision or in informal consultation with other mental health professionals.4,21
Two countertransference reactions are particularly common (and problematic) in therapeutic work with dependent patients:
- the fantasy of insatiability (believing that no matter how much support and reassurance the patient receives, it will never be enough)
- the fantasy of permanence (believing the patient will become so comfortable in therapy’s protective cocoon that he or she will never leave treatment).
Because some studies suggest that dependent patients may be at increased risk for suicide, monitor them continuously for negative indicators.8,9 Five danger signs (Table 4) suggest an increased risk of self-destructive behavior in dependent patients.
Table 4
5 warning signs of self-destructive behavior in dependent patients
| Recent relationship conflict or interpersonal loss |
| Excessive or unrealistic jealousy |
| Poor impulse control |
| Difficulty modulating negative emotions |
| Previous suicide attempts or suicidal gestures |
Because dependent persons often construct interpersonal milieus that foster and propagate their dependency, concurrent marital and/or family therapy may be warranted to disrupt entrenched dysfunctional patterns.16,17 Examine the rewards dependent patients obtain for behaving helpless and vulnerable and ways in which their dependency may reward friends, family members, and coworkers.
Related resources
- Baltes MM. The many faces of dependency in old age. Cambridge, UK: Cambridge University Press; 1996.
- Bornstein RF. The dependent personality: developmental, social, and clinical perspectives. Psychol Bull 1992;112(1):3-23.
- Millon T. Disorders of personality: DSM-IV and beyond. New York: Wiley; 1996.
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Bornstein RF, Languirand MA. Healthy dependency: leaning on others without losing yourself. New York, NY: Newmarket Press; 2003.
3. Bornstein RF. The dependent personality. New York: Guilford Press; 1993.
4. Bornstein RF. The dependent patient: a practitioner’s guide. Washington, DC: American Psychological Association; 2005.
5. Pincus AL, Wilson KR. Interpersonal variability in dependent personality. J Pers 2001;69(2):223-51.
6. Pincus AL, Gurtman MB. The three faces of interpersonal dependency: structural analysis of self-report dependency measures. J Pers Soc Psychol 1995;69(4):744-58.
7. Overholser JC. The dependent personality and interpersonal problems. J Nerv Ment Dis 1996;184(1)1:8-16.
8. Bornstein RF. The complex relationship between dependency and domestic violence: converging psychological factors and social forces. Am Psychol 2006;61(6):595-606.
9. Coen SJ. The misuse of persons: analyzing pathological dependency. Hillsdale, NJ: Analytic Press; 1992.
10. Overholser JC, Fine MA. Cognitive-behavioral treatment of excessive interpersonal dependency: a four-stage psychotherapy model. Journal of Cognitive Psychotherapy 1994;8(1):55-70.
11. Turkat ID. The personality disorders: a psychological approach to clinical management. New York, NY: Pergamon Press; 1990.
12. Bornstein RF. Criterion validity of objective and projective dependency tests: a meta-analytic assessment of behavioral prediction. Psychological Assessment 1999;11(1):48-57.
13. Birtchnell J. The measurement of dependence by questionnaire. Journal of Personality Disorders 1991;5(3):281-95.
14. Nietzel MT, Harris MJ. Relationship of dependency and achievement/autonomy to depression. Clinical Psychology Review 1990;10:279-97.
15. Bornstein RF. Dependent personality disorder in the DSM-IV and beyond. Clinical Psychology: Science and Practice 1997;4(2):175-87.
16. Ryder RD, Parry-Jones WL. Fears of dependence and its value in working with adolescents. J Adolesc 1982;5(1):71-8.
17. Tait M. Dependence: a means or an impediment to growth? British Journal of Guidance and Counselling. 1997;25(1):17-26.
18. Black DW, Monahan P, Wesner R, et al. The effects of fluvoxamine, cognitive therapy, and placebo on abnormal personality traits in 44 patients with panic disorder. Journal of Personality Disorders. 1996;10(2):185-94.
19. Rathus JH, Sanderson WC, Miller AL, Wetzler S. Impact of personality functioning on cognitive behavioral treatment of panic disorder: a preliminary report. Journal of Personality Disorders 1995;9(2):160-8.
20. Bornstein RF. Integrating cognitive and existential treatment strategies in psychotherapy with dependent patients. Journal of Contemporary Psychotherapy 2004;34(4):293-309.
21. Abramson PR, Cloud MY, Keese N, Keese R. How much is too much? Dependency in a psychotherapeutic relationship. Am J Psychother 1994;48(2):294-301.
1. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Bornstein RF, Languirand MA. Healthy dependency: leaning on others without losing yourself. New York, NY: Newmarket Press; 2003.
3. Bornstein RF. The dependent personality. New York: Guilford Press; 1993.
4. Bornstein RF. The dependent patient: a practitioner’s guide. Washington, DC: American Psychological Association; 2005.
5. Pincus AL, Wilson KR. Interpersonal variability in dependent personality. J Pers 2001;69(2):223-51.
6. Pincus AL, Gurtman MB. The three faces of interpersonal dependency: structural analysis of self-report dependency measures. J Pers Soc Psychol 1995;69(4):744-58.
7. Overholser JC. The dependent personality and interpersonal problems. J Nerv Ment Dis 1996;184(1)1:8-16.
8. Bornstein RF. The complex relationship between dependency and domestic violence: converging psychological factors and social forces. Am Psychol 2006;61(6):595-606.
9. Coen SJ. The misuse of persons: analyzing pathological dependency. Hillsdale, NJ: Analytic Press; 1992.
10. Overholser JC, Fine MA. Cognitive-behavioral treatment of excessive interpersonal dependency: a four-stage psychotherapy model. Journal of Cognitive Psychotherapy 1994;8(1):55-70.
11. Turkat ID. The personality disorders: a psychological approach to clinical management. New York, NY: Pergamon Press; 1990.
12. Bornstein RF. Criterion validity of objective and projective dependency tests: a meta-analytic assessment of behavioral prediction. Psychological Assessment 1999;11(1):48-57.
13. Birtchnell J. The measurement of dependence by questionnaire. Journal of Personality Disorders 1991;5(3):281-95.
14. Nietzel MT, Harris MJ. Relationship of dependency and achievement/autonomy to depression. Clinical Psychology Review 1990;10:279-97.
15. Bornstein RF. Dependent personality disorder in the DSM-IV and beyond. Clinical Psychology: Science and Practice 1997;4(2):175-87.
16. Ryder RD, Parry-Jones WL. Fears of dependence and its value in working with adolescents. J Adolesc 1982;5(1):71-8.
17. Tait M. Dependence: a means or an impediment to growth? British Journal of Guidance and Counselling. 1997;25(1):17-26.
18. Black DW, Monahan P, Wesner R, et al. The effects of fluvoxamine, cognitive therapy, and placebo on abnormal personality traits in 44 patients with panic disorder. Journal of Personality Disorders. 1996;10(2):185-94.
19. Rathus JH, Sanderson WC, Miller AL, Wetzler S. Impact of personality functioning on cognitive behavioral treatment of panic disorder: a preliminary report. Journal of Personality Disorders 1995;9(2):160-8.
20. Bornstein RF. Integrating cognitive and existential treatment strategies in psychotherapy with dependent patients. Journal of Contemporary Psychotherapy 2004;34(4):293-309.
21. Abramson PR, Cloud MY, Keese N, Keese R. How much is too much? Dependency in a psychotherapeutic relationship. Am J Psychother 1994;48(2):294-301.
7-point checkup: Defuse cardiovascular and psychiatric risks in schizophrenia outpatients
Mr. K, age 34, has been hospitalized 4 times in 5 years for acute exacerbations of schizophrenia caused by medication nonadherence. This time he reports he discontinued antipsychotic therapy because he was “tired of taking medications every day.”
He spent 2 weeks in the acute inpatient psychiatric unit and restarted olanzapine—titrated to 15 mg/d—to which he responded well. When he presented to our outpatient clinic for follow-up, Mr. K reported adhering to his medications and denied positive symptoms. He complained of mild daytime sedation but no other side effects.
Schizophrenia patients spend most of their lives stable, rather than hospitalized for acute psychotic episodes. While stable, they continue to require close attention, and medical issues are particularly important during this time. Outpatient maintenance—such as optimizing antipsychotic therapy, offering psychosocial interventions, and monitoring physical health and well-being—provides opportunities to improve the course of illness for patients such as Mr. K.
This article describes a 7-point checkup to keep schizophrenia outpatients stable. It can help you maintain or improve patients’ function, prevent relapse, and monitor for adverse effects (Table 1).
Table 1
7-point checkup for assessing the stable schizophrenia patient
| 1. | Evaluate positive, negative, and cognitive symptoms |
| 2. | Monitor level of adherence |
| 3. | Evaluate weight, cardiovascular risk factors, and other medical parameters |
| 4. | Examine for extrapyramidal symptoms or tardive dyskinesia |
| 5. | Evaluate for comorbid mood symptoms and substance use |
| 6. | Look for prodromal symptoms that may signal relapse |
| 7. | Evaluate psychosocial interventions |
1. Symptom clusters
For several years, Mr. K worked as a research technician in a university lab, maintained an apartment, and attended to activities of daily living while taking olanzapine, 15 mg nightly. After discontinuing his medication, he reported auditory hallucinations, paranoid delusions, ideas of reference, and grossly disorganized thinking and behavior. He also was using marijuana daily, which exacerbated his psychotic symptoms and paranoia.
Addressing schizophrenia’s symptom clusters (Table 2) is key to improving patients’ social and occupational function and quality of life. Mr. K no longer has hallucinations, delusions, or disorganized thinking or behavior, but our evaluation shows his improvements are limited to schizophrenia’s positive symptoms.
Table 2
Schizophrenia’s 4 symptom clusters*
| Positive symptoms | Delusions, hallucinations, disorganization |
| Negative symptoms | Blunted affect, alogia, avolition, anhedonia |
| Cognitive symptoms | Attention, memory, executive functions (such as abstraction) |
| Affective symptoms | Dysphoria, suicidality, hopelessness |
| * Interaction of symptoms contributes to social and occupational dysfunction and adversely affects work and interpersonal relationships and self-care. | |
Cognitive symptoms. Mr. K’s concentration, attention, and memory are impaired, which interferes with his work. His ability to abstract is not impaired.
Affective symptoms. Mr. K denies signs or symptoms of depression, mania, hopelessness, or thoughts of wanting to hurt himself or anyone else.
Because antipsychotics do not adequately treat negative and cognitive symptoms, we will address these symptom clusters with psychosocial interventions.
2. Adherence
Nonadherence to medication is the most common cause of relapse and rehospitalization for patients with schizophrenia.1 We find the following strategies helpful when encouraging, maintaining, and monitoring adherence to medications.
Normalize adherence and enlist patient participation.
We inform Mr. K that most patients have trouble taking medications every day and ask how he remembers. Brainstorming with patients on ways to remember to take medications increases their likelihood of participating.
Try reminder strategies. Recommend pillboxes, alarms, and other aids. Consider enlisting family members to help patients remember to take their medications every day.
Monitor prescriptions. We limit Mr. K’s prescription to 1 month so that we can assess the timeliness and consistency of refills.
Educate. Emphasize the link between adherence and wellness, and nonadherence and relapse-—which Mr. K has clearly demonstrated. We repeat this lesson on multiple visits and counsel any involved family members as well, so that everyone understands the importance of adherence to the patient’s continued well-being.
Look for signs of nonadherence. Because Mr. K discontinued his medications without telling his physician, we remain vigilant for signs of nonadherence—such as reemergence of hallucinations, delusions, paranoia, or ideas of reference—or sudden disappearance of a side effect—such as daytime sedation, which he has consistently reported with olanzapine.
Discontinuation rates in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) suggest that many stable schizophrenia outpatients are dissatisfied with their medications. Most patients in all treatment groups changed their medications during the 18-month National Institute of Mental Health-sponsored trial.2
SGAs versus FGAs. Schizophrenia patients may be more likely to tolerate second-generation antipsychotics (SGAs) than first-generation antipsychotics (FGAs) because of FGAs’ higher risk of movement side effects such as akathisia. Some data suggest that patients find SGAs more tolerable overall, leading to lower discontinuation rates.3
Recent evidence, however, has cast doubt on the idea that SGAs are clinically superior to FGAs. The CUtLASS trial,4 for example, found no quality-of-life differences in patients using either class. Both FGAs and SGAs reduce the risk of relapse in stable patients, although SGAs may have an advantage over FGAs in preventing relapse. An analysis by Leucht et al5 found lower relapse/treatment failure rates in 6 placebo-controlled SGA trials (total 983 patients), compared with 11 FGA trials (total 2,032 patients).
Although the data comparing SGAs with FGAs are controversial, SGAs seem to have lower EPS potential. Regardless of the type of antipsychotic, however, studies have shown that patients who relapse while taking antipsychotics have less-severe episodes than those who relapse after discontinuing their medications.
For patients who frequently forget or incorrectly take oral medications, long-acting depot antipsychotics may increase adherence and decrease relapse rates during the stable phase.6
3. Weight gain, cardiovascular risk
Cardiovascular disease is the leading cause of death among persons with schizophrenia,7,8 whose life span is 10 to 20 years shorter than the population at large.9 Schizophrenia patients may be genetically predisposed to cardiovascular disease and metabolic syndrome (Table 3),10 exacerbated by typically sedentary lifestyles, high smoking rates, and poor diets. Certain SGAs add to the risk of weight gain and metabolic abnormalities.11
Table 3
Does your patient have metabolic syndrome?
| Metabolic syndrome is defined as having any 3 of these findings: | |
| Abdominal obesity | Waist circumference |
| >102 cm (40 in) in men | |
| >88 cm (35 in) in women | |
| Elevated triglycerides | ≥150 mg/dL |
| Low HDL | |
| Hypertension | ≥130/85 mm/Hg |
| Hyperglycemia | Fasting blood glucose ≥110 mg/dL |
| Source: National Cholesterol Education Program Adult Treatment Panel III guidelines, reference 10 | |
Table 4
Monitoring protocol for patients taking second-generation antipsychotics*
| Short-term | Long-term | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 4 wk | 8 wk | 12 wk | Quarterly | Annually | Every 5 yrs | |
| Personal/family history | X | X | |||||
| Weight (BMI) | X | X | X | X | X | ||
| Waist circumference | X | X | |||||
| Blood pressure | X | X | X | ||||
| Fasting plasma glucose | X | X | X | ||||
| Fasting lipid profile | X | X | X | ||||
| * More-frequent assessments may be warranted, based on clinical status. | |||||||
| BMI: body mass index | |||||||
| Source: Reference 12 | |||||||
- using treatments that do not increase the risk for cardiovascular disease
- switching to agents with less weight gain liability if a patient’s weight increases >7% or his or her body mass index (BMI) increases 1 unit during antipsychotic therapy.
Mr. K remained stable on olanzapine for several years, but his blood glucose, cholesterol, and triglycerides have risen dramatically and he has gained 40 lbs. Although we were concerned that he might not respond as well to another SGA, we reviewed the risks and benefits with specific concern about his cardiovascular health. As a result, we switched him to aripiprazole, 15 mg/d, with close monitoring and supervision for signs of relapse.
SGAs’ heterogeneity. The ADA/APA statement and multiple clinical trials support differential risks of weight gain and metabolic abnormalities with SGAs (Table 5). Patients in CATIE phase 1 who were randomly assigned to olanzapine experienced greater total weight gain and monthly weight gain (mean +2 lb/month) than patients taking any other antipsychotic.13 In an analysis of change from baseline to last observation, 30% of patients in the olanzapine group gained >7% of their baseline weight, compared with 7% to 16% of patients taking other antipsychotics.
Table 5
Differential risks of weigh gain and metabolic abnormalities with SGAs
| Risk | Antipsychotics |
|---|---|
| Highest | Clozapine, olanzapine |
| Intermediate/low | Risperidone, quetiapine |
| Lowest | Aripiprazole, ziprasidone |
| SGAs: Second-generation antipsychotics | |
Mr. K remains free of positive symptoms after taking aripiprazole for several months. He complains less of daytime sedation and has lost >10 lbs. We are awaiting repeat glucose, cholesterol, and triglyceride serum levels. We will continue to monitor his weight and metabolic values and ensure that he receives primary care follow-up.
4. Eps and tardive dyskinesia
Compared with FGAs, SGAs may be associated with a lower incidence of tardive dyskinesia (TD) and extrapyramidal symptoms (EPS). Even so, routine screening for EPS and TD remains necessary, according to the Mount Sinai consensus conference on physical health monitoring of patients with schizophrenia.16 At every visit, we observe Mr. K for:
- facial movements (excessive blinking, puckering, lip smacking, sucking, or grimacing)
- decreased arm swing while walking or choreoathetoid-like or writhing limb or trunk movements.
In addition to screening for metabolic syndrome, EPS, and movement disorders, the Mount Sinai consensus conference16 offers guidelines for monitoring prolactin, cardiac, and ocular changes in patients taking antipsychotics.
5. Mood disorders, substance abuse
Screen schizophrenia outpatients for signs of anxiety, depression, mania, and substance abuse at every visit. An antidepressant trial is recommended for depressive episodes even if antipsychotic therapy has adequately reduced positive psychotic symptoms.17 This strategy can prevent depressive relapses and might help prevent psychotic relapse as well.18 Also consider adjunctive therapy such as:
- mood stabilizers for affective instability
- benzodiazepines for short-term anxiety or agitation
- tricyclics or selective serotonin reuptake inhibitors for comorbid anxiety disorders such as obsessive-compulsive or panic disorder.19
Because of Mr. K’s history of marijuana abuse, we tell him we will use random urine toxicology screens. We also order urine toxicology if patients behave abnormally or appear impaired. We counsel patients and families about the link between substance use and psychotic decompensation, which Mr. K has demonstrated several times.
We emphasize that the goal of repeated questioning and screening is to ensure Mr. K’s well-being. If he is using substances, we will refer him to the help he needs.
6. Prodromal signs of relapse
Mr. K has reported decreased sleep, increased irritability, increased social isolation, and some agitation before his acute psychotic decompensations. These symptoms form his prodrome for relapse, which we routinely assess at follow-up visits.
We question him about sleep, social contacts, irritability, and agitation; assess psychotic symptoms; and observe thought processes and behaviors. If a patient endorses or displays prodromal signs of relapse, we consider:
- Is he taking the medication?
- Is he abusing substances?
- Does a change in dosage (actual or a cytochrome P450-mediated drug-drug interaction) explain a decrease in efficacy?
- Is he under stress and need an increased antipsychotic dosage?
- Might psychosocial interventions (support groups, cognitive-behavioral therapy, family involvement, etc.) help him deal with symptoms, decrease stress, or avoid an exacerbation?
- Has medication been optimized (correct dosing, long enough duration), or does the patient need an increased dosage or a different antipsychotic?
7. Psychosocial interventions
Combining medications with psychosocial programs is most effective for maintaining remission and improving patients’ social and occupational functioning.17 For Mr. K we recommend these interventions:
Social skills training. Mr. K now meets with other schizophrenia patients and a moderator to set long-term goals and smaller, attainable goals as homework assignments each week. Patients get feedback, positive reinforcement, and the opportunity to practice new skills. Mr. K is working on improving family relationships, furthering his career, and improving his interpersonal skills.
Family therapy and education. We have met with Mr. K’s mother several times, and she now visits him regularly and speaks with him on the phone at least once a week. She attends Support and Family Education and National Alliance on Mental Illness meetings to increase her understanding of her son’s illness.
Alcoholics Anonymous/Marijuana Anonymous. We refer Mr. K to AA/MA groups; he attends 2 to 3 times per week and has a sponsor. He is not sober yet but has dramatically cut back his substance use and continues to express motivation to quit.
Related resources
- Torrey EF. Surviving schizophrenia: a manual for families, patients, and providers, 5th ed. New York: Harper Collins; 2006.
- Mueser KT, Gingerich S. The complete family guide to schizophrenia: helping your loved one get the most out of life. New York: Guilford Press; 2006.
- National Alliance on Mental Illness. Resources for schizophrenia patients and their families. www.nami.org.
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Arey reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Marder receives research/grant support from Janssen Pharmaceutica and Eli Lilly and Co. and is a consultant or speaker for Bristol-Myers Squibb Co., Otsuka America Pharmaceutical, Pfizer, Solvay Pharmaceuticals, Merck and Co., and Roche.
1. Weiden P, Glazer M. Assessment and treatment selection for “revolving door” inpatients with schizophrenia. Psychiatr Q. 1997;68(4):377-92.
2. Lieberman JA, Stroup TS, McEvoy JP, et al. for the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;22;353(12):1209-23.
3. Marder SR, Glynn SM, Wirshing WC, et al. Maintenance treatment of schizophrenia with risperidone or haloperidol: 2-year outcomes. Am J Psychiatry 2003;160:1405-12.
4. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry 2006;63:1079-87.
5. Leucht S, Barnes TR, Kissling W, et al. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry 2003;160:1209-22.
6. Davis JM, Kane JM, Marder SR, et al. Dose response of prophylactic antipsychotics. J Clin Psychiatry 1993;54(3, suppl):24-30.
7. Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry 1997;171:502-8.
8. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry 1998;173:11-53.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis [serial online] 2006 Apr [date cited]. Available from: http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htm.
10. NCEP Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143-421.
11. Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry 2006;51(8):480-91.
12. et al. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
13. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
14. Stroup TS, Liberman JA, McEvoy JP, et al. for the CATIE Investigators. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:4:611-22.
15. McQuade RD, Stock E, Marcus R, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry 2004;65(suppl 18):47-56.
16. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-49.
17. Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004;30:193-217.
18. Siris SG, Morgan V, Fagerstrom R, et al. Adjunctive imipramine in the treatment of postpsychotic depression. Arch Gen Psychiatry 1987;44:533-9.
19. Braga RJ, Petrides G, Figueira I. Anxiety disorders in schizophrenia. Compr Psychiatry 2004;45(6):460-8.
Mr. K, age 34, has been hospitalized 4 times in 5 years for acute exacerbations of schizophrenia caused by medication nonadherence. This time he reports he discontinued antipsychotic therapy because he was “tired of taking medications every day.”
He spent 2 weeks in the acute inpatient psychiatric unit and restarted olanzapine—titrated to 15 mg/d—to which he responded well. When he presented to our outpatient clinic for follow-up, Mr. K reported adhering to his medications and denied positive symptoms. He complained of mild daytime sedation but no other side effects.
Schizophrenia patients spend most of their lives stable, rather than hospitalized for acute psychotic episodes. While stable, they continue to require close attention, and medical issues are particularly important during this time. Outpatient maintenance—such as optimizing antipsychotic therapy, offering psychosocial interventions, and monitoring physical health and well-being—provides opportunities to improve the course of illness for patients such as Mr. K.
This article describes a 7-point checkup to keep schizophrenia outpatients stable. It can help you maintain or improve patients’ function, prevent relapse, and monitor for adverse effects (Table 1).
Table 1
7-point checkup for assessing the stable schizophrenia patient
| 1. | Evaluate positive, negative, and cognitive symptoms |
| 2. | Monitor level of adherence |
| 3. | Evaluate weight, cardiovascular risk factors, and other medical parameters |
| 4. | Examine for extrapyramidal symptoms or tardive dyskinesia |
| 5. | Evaluate for comorbid mood symptoms and substance use |
| 6. | Look for prodromal symptoms that may signal relapse |
| 7. | Evaluate psychosocial interventions |
1. Symptom clusters
For several years, Mr. K worked as a research technician in a university lab, maintained an apartment, and attended to activities of daily living while taking olanzapine, 15 mg nightly. After discontinuing his medication, he reported auditory hallucinations, paranoid delusions, ideas of reference, and grossly disorganized thinking and behavior. He also was using marijuana daily, which exacerbated his psychotic symptoms and paranoia.
Addressing schizophrenia’s symptom clusters (Table 2) is key to improving patients’ social and occupational function and quality of life. Mr. K no longer has hallucinations, delusions, or disorganized thinking or behavior, but our evaluation shows his improvements are limited to schizophrenia’s positive symptoms.
Table 2
Schizophrenia’s 4 symptom clusters*
| Positive symptoms | Delusions, hallucinations, disorganization |
| Negative symptoms | Blunted affect, alogia, avolition, anhedonia |
| Cognitive symptoms | Attention, memory, executive functions (such as abstraction) |
| Affective symptoms | Dysphoria, suicidality, hopelessness |
| * Interaction of symptoms contributes to social and occupational dysfunction and adversely affects work and interpersonal relationships and self-care. | |
Cognitive symptoms. Mr. K’s concentration, attention, and memory are impaired, which interferes with his work. His ability to abstract is not impaired.
Affective symptoms. Mr. K denies signs or symptoms of depression, mania, hopelessness, or thoughts of wanting to hurt himself or anyone else.
Because antipsychotics do not adequately treat negative and cognitive symptoms, we will address these symptom clusters with psychosocial interventions.
2. Adherence
Nonadherence to medication is the most common cause of relapse and rehospitalization for patients with schizophrenia.1 We find the following strategies helpful when encouraging, maintaining, and monitoring adherence to medications.
Normalize adherence and enlist patient participation.
We inform Mr. K that most patients have trouble taking medications every day and ask how he remembers. Brainstorming with patients on ways to remember to take medications increases their likelihood of participating.
Try reminder strategies. Recommend pillboxes, alarms, and other aids. Consider enlisting family members to help patients remember to take their medications every day.
Monitor prescriptions. We limit Mr. K’s prescription to 1 month so that we can assess the timeliness and consistency of refills.
Educate. Emphasize the link between adherence and wellness, and nonadherence and relapse-—which Mr. K has clearly demonstrated. We repeat this lesson on multiple visits and counsel any involved family members as well, so that everyone understands the importance of adherence to the patient’s continued well-being.
Look for signs of nonadherence. Because Mr. K discontinued his medications without telling his physician, we remain vigilant for signs of nonadherence—such as reemergence of hallucinations, delusions, paranoia, or ideas of reference—or sudden disappearance of a side effect—such as daytime sedation, which he has consistently reported with olanzapine.
Discontinuation rates in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) suggest that many stable schizophrenia outpatients are dissatisfied with their medications. Most patients in all treatment groups changed their medications during the 18-month National Institute of Mental Health-sponsored trial.2
SGAs versus FGAs. Schizophrenia patients may be more likely to tolerate second-generation antipsychotics (SGAs) than first-generation antipsychotics (FGAs) because of FGAs’ higher risk of movement side effects such as akathisia. Some data suggest that patients find SGAs more tolerable overall, leading to lower discontinuation rates.3
Recent evidence, however, has cast doubt on the idea that SGAs are clinically superior to FGAs. The CUtLASS trial,4 for example, found no quality-of-life differences in patients using either class. Both FGAs and SGAs reduce the risk of relapse in stable patients, although SGAs may have an advantage over FGAs in preventing relapse. An analysis by Leucht et al5 found lower relapse/treatment failure rates in 6 placebo-controlled SGA trials (total 983 patients), compared with 11 FGA trials (total 2,032 patients).
Although the data comparing SGAs with FGAs are controversial, SGAs seem to have lower EPS potential. Regardless of the type of antipsychotic, however, studies have shown that patients who relapse while taking antipsychotics have less-severe episodes than those who relapse after discontinuing their medications.
For patients who frequently forget or incorrectly take oral medications, long-acting depot antipsychotics may increase adherence and decrease relapse rates during the stable phase.6
3. Weight gain, cardiovascular risk
Cardiovascular disease is the leading cause of death among persons with schizophrenia,7,8 whose life span is 10 to 20 years shorter than the population at large.9 Schizophrenia patients may be genetically predisposed to cardiovascular disease and metabolic syndrome (Table 3),10 exacerbated by typically sedentary lifestyles, high smoking rates, and poor diets. Certain SGAs add to the risk of weight gain and metabolic abnormalities.11
Table 3
Does your patient have metabolic syndrome?
| Metabolic syndrome is defined as having any 3 of these findings: | |
| Abdominal obesity | Waist circumference |
| >102 cm (40 in) in men | |
| >88 cm (35 in) in women | |
| Elevated triglycerides | ≥150 mg/dL |
| Low HDL | |
| Hypertension | ≥130/85 mm/Hg |
| Hyperglycemia | Fasting blood glucose ≥110 mg/dL |
| Source: National Cholesterol Education Program Adult Treatment Panel III guidelines, reference 10 | |
Table 4
Monitoring protocol for patients taking second-generation antipsychotics*
| Short-term | Long-term | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 4 wk | 8 wk | 12 wk | Quarterly | Annually | Every 5 yrs | |
| Personal/family history | X | X | |||||
| Weight (BMI) | X | X | X | X | X | ||
| Waist circumference | X | X | |||||
| Blood pressure | X | X | X | ||||
| Fasting plasma glucose | X | X | X | ||||
| Fasting lipid profile | X | X | X | ||||
| * More-frequent assessments may be warranted, based on clinical status. | |||||||
| BMI: body mass index | |||||||
| Source: Reference 12 | |||||||
- using treatments that do not increase the risk for cardiovascular disease
- switching to agents with less weight gain liability if a patient’s weight increases >7% or his or her body mass index (BMI) increases 1 unit during antipsychotic therapy.
Mr. K remained stable on olanzapine for several years, but his blood glucose, cholesterol, and triglycerides have risen dramatically and he has gained 40 lbs. Although we were concerned that he might not respond as well to another SGA, we reviewed the risks and benefits with specific concern about his cardiovascular health. As a result, we switched him to aripiprazole, 15 mg/d, with close monitoring and supervision for signs of relapse.
SGAs’ heterogeneity. The ADA/APA statement and multiple clinical trials support differential risks of weight gain and metabolic abnormalities with SGAs (Table 5). Patients in CATIE phase 1 who were randomly assigned to olanzapine experienced greater total weight gain and monthly weight gain (mean +2 lb/month) than patients taking any other antipsychotic.13 In an analysis of change from baseline to last observation, 30% of patients in the olanzapine group gained >7% of their baseline weight, compared with 7% to 16% of patients taking other antipsychotics.
Table 5
Differential risks of weigh gain and metabolic abnormalities with SGAs
| Risk | Antipsychotics |
|---|---|
| Highest | Clozapine, olanzapine |
| Intermediate/low | Risperidone, quetiapine |
| Lowest | Aripiprazole, ziprasidone |
| SGAs: Second-generation antipsychotics | |
Mr. K remains free of positive symptoms after taking aripiprazole for several months. He complains less of daytime sedation and has lost >10 lbs. We are awaiting repeat glucose, cholesterol, and triglyceride serum levels. We will continue to monitor his weight and metabolic values and ensure that he receives primary care follow-up.
4. Eps and tardive dyskinesia
Compared with FGAs, SGAs may be associated with a lower incidence of tardive dyskinesia (TD) and extrapyramidal symptoms (EPS). Even so, routine screening for EPS and TD remains necessary, according to the Mount Sinai consensus conference on physical health monitoring of patients with schizophrenia.16 At every visit, we observe Mr. K for:
- facial movements (excessive blinking, puckering, lip smacking, sucking, or grimacing)
- decreased arm swing while walking or choreoathetoid-like or writhing limb or trunk movements.
In addition to screening for metabolic syndrome, EPS, and movement disorders, the Mount Sinai consensus conference16 offers guidelines for monitoring prolactin, cardiac, and ocular changes in patients taking antipsychotics.
5. Mood disorders, substance abuse
Screen schizophrenia outpatients for signs of anxiety, depression, mania, and substance abuse at every visit. An antidepressant trial is recommended for depressive episodes even if antipsychotic therapy has adequately reduced positive psychotic symptoms.17 This strategy can prevent depressive relapses and might help prevent psychotic relapse as well.18 Also consider adjunctive therapy such as:
- mood stabilizers for affective instability
- benzodiazepines for short-term anxiety or agitation
- tricyclics or selective serotonin reuptake inhibitors for comorbid anxiety disorders such as obsessive-compulsive or panic disorder.19
Because of Mr. K’s history of marijuana abuse, we tell him we will use random urine toxicology screens. We also order urine toxicology if patients behave abnormally or appear impaired. We counsel patients and families about the link between substance use and psychotic decompensation, which Mr. K has demonstrated several times.
We emphasize that the goal of repeated questioning and screening is to ensure Mr. K’s well-being. If he is using substances, we will refer him to the help he needs.
6. Prodromal signs of relapse
Mr. K has reported decreased sleep, increased irritability, increased social isolation, and some agitation before his acute psychotic decompensations. These symptoms form his prodrome for relapse, which we routinely assess at follow-up visits.
We question him about sleep, social contacts, irritability, and agitation; assess psychotic symptoms; and observe thought processes and behaviors. If a patient endorses or displays prodromal signs of relapse, we consider:
- Is he taking the medication?
- Is he abusing substances?
- Does a change in dosage (actual or a cytochrome P450-mediated drug-drug interaction) explain a decrease in efficacy?
- Is he under stress and need an increased antipsychotic dosage?
- Might psychosocial interventions (support groups, cognitive-behavioral therapy, family involvement, etc.) help him deal with symptoms, decrease stress, or avoid an exacerbation?
- Has medication been optimized (correct dosing, long enough duration), or does the patient need an increased dosage or a different antipsychotic?
7. Psychosocial interventions
Combining medications with psychosocial programs is most effective for maintaining remission and improving patients’ social and occupational functioning.17 For Mr. K we recommend these interventions:
Social skills training. Mr. K now meets with other schizophrenia patients and a moderator to set long-term goals and smaller, attainable goals as homework assignments each week. Patients get feedback, positive reinforcement, and the opportunity to practice new skills. Mr. K is working on improving family relationships, furthering his career, and improving his interpersonal skills.
Family therapy and education. We have met with Mr. K’s mother several times, and she now visits him regularly and speaks with him on the phone at least once a week. She attends Support and Family Education and National Alliance on Mental Illness meetings to increase her understanding of her son’s illness.
Alcoholics Anonymous/Marijuana Anonymous. We refer Mr. K to AA/MA groups; he attends 2 to 3 times per week and has a sponsor. He is not sober yet but has dramatically cut back his substance use and continues to express motivation to quit.
Related resources
- Torrey EF. Surviving schizophrenia: a manual for families, patients, and providers, 5th ed. New York: Harper Collins; 2006.
- Mueser KT, Gingerich S. The complete family guide to schizophrenia: helping your loved one get the most out of life. New York: Guilford Press; 2006.
- National Alliance on Mental Illness. Resources for schizophrenia patients and their families. www.nami.org.
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Arey reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Marder receives research/grant support from Janssen Pharmaceutica and Eli Lilly and Co. and is a consultant or speaker for Bristol-Myers Squibb Co., Otsuka America Pharmaceutical, Pfizer, Solvay Pharmaceuticals, Merck and Co., and Roche.
Mr. K, age 34, has been hospitalized 4 times in 5 years for acute exacerbations of schizophrenia caused by medication nonadherence. This time he reports he discontinued antipsychotic therapy because he was “tired of taking medications every day.”
He spent 2 weeks in the acute inpatient psychiatric unit and restarted olanzapine—titrated to 15 mg/d—to which he responded well. When he presented to our outpatient clinic for follow-up, Mr. K reported adhering to his medications and denied positive symptoms. He complained of mild daytime sedation but no other side effects.
Schizophrenia patients spend most of their lives stable, rather than hospitalized for acute psychotic episodes. While stable, they continue to require close attention, and medical issues are particularly important during this time. Outpatient maintenance—such as optimizing antipsychotic therapy, offering psychosocial interventions, and monitoring physical health and well-being—provides opportunities to improve the course of illness for patients such as Mr. K.
This article describes a 7-point checkup to keep schizophrenia outpatients stable. It can help you maintain or improve patients’ function, prevent relapse, and monitor for adverse effects (Table 1).
Table 1
7-point checkup for assessing the stable schizophrenia patient
| 1. | Evaluate positive, negative, and cognitive symptoms |
| 2. | Monitor level of adherence |
| 3. | Evaluate weight, cardiovascular risk factors, and other medical parameters |
| 4. | Examine for extrapyramidal symptoms or tardive dyskinesia |
| 5. | Evaluate for comorbid mood symptoms and substance use |
| 6. | Look for prodromal symptoms that may signal relapse |
| 7. | Evaluate psychosocial interventions |
1. Symptom clusters
For several years, Mr. K worked as a research technician in a university lab, maintained an apartment, and attended to activities of daily living while taking olanzapine, 15 mg nightly. After discontinuing his medication, he reported auditory hallucinations, paranoid delusions, ideas of reference, and grossly disorganized thinking and behavior. He also was using marijuana daily, which exacerbated his psychotic symptoms and paranoia.
Addressing schizophrenia’s symptom clusters (Table 2) is key to improving patients’ social and occupational function and quality of life. Mr. K no longer has hallucinations, delusions, or disorganized thinking or behavior, but our evaluation shows his improvements are limited to schizophrenia’s positive symptoms.
Table 2
Schizophrenia’s 4 symptom clusters*
| Positive symptoms | Delusions, hallucinations, disorganization |
| Negative symptoms | Blunted affect, alogia, avolition, anhedonia |
| Cognitive symptoms | Attention, memory, executive functions (such as abstraction) |
| Affective symptoms | Dysphoria, suicidality, hopelessness |
| * Interaction of symptoms contributes to social and occupational dysfunction and adversely affects work and interpersonal relationships and self-care. | |
Cognitive symptoms. Mr. K’s concentration, attention, and memory are impaired, which interferes with his work. His ability to abstract is not impaired.
Affective symptoms. Mr. K denies signs or symptoms of depression, mania, hopelessness, or thoughts of wanting to hurt himself or anyone else.
Because antipsychotics do not adequately treat negative and cognitive symptoms, we will address these symptom clusters with psychosocial interventions.
2. Adherence
Nonadherence to medication is the most common cause of relapse and rehospitalization for patients with schizophrenia.1 We find the following strategies helpful when encouraging, maintaining, and monitoring adherence to medications.
Normalize adherence and enlist patient participation.
We inform Mr. K that most patients have trouble taking medications every day and ask how he remembers. Brainstorming with patients on ways to remember to take medications increases their likelihood of participating.
Try reminder strategies. Recommend pillboxes, alarms, and other aids. Consider enlisting family members to help patients remember to take their medications every day.
Monitor prescriptions. We limit Mr. K’s prescription to 1 month so that we can assess the timeliness and consistency of refills.
Educate. Emphasize the link between adherence and wellness, and nonadherence and relapse-—which Mr. K has clearly demonstrated. We repeat this lesson on multiple visits and counsel any involved family members as well, so that everyone understands the importance of adherence to the patient’s continued well-being.
Look for signs of nonadherence. Because Mr. K discontinued his medications without telling his physician, we remain vigilant for signs of nonadherence—such as reemergence of hallucinations, delusions, paranoia, or ideas of reference—or sudden disappearance of a side effect—such as daytime sedation, which he has consistently reported with olanzapine.
Discontinuation rates in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) suggest that many stable schizophrenia outpatients are dissatisfied with their medications. Most patients in all treatment groups changed their medications during the 18-month National Institute of Mental Health-sponsored trial.2
SGAs versus FGAs. Schizophrenia patients may be more likely to tolerate second-generation antipsychotics (SGAs) than first-generation antipsychotics (FGAs) because of FGAs’ higher risk of movement side effects such as akathisia. Some data suggest that patients find SGAs more tolerable overall, leading to lower discontinuation rates.3
Recent evidence, however, has cast doubt on the idea that SGAs are clinically superior to FGAs. The CUtLASS trial,4 for example, found no quality-of-life differences in patients using either class. Both FGAs and SGAs reduce the risk of relapse in stable patients, although SGAs may have an advantage over FGAs in preventing relapse. An analysis by Leucht et al5 found lower relapse/treatment failure rates in 6 placebo-controlled SGA trials (total 983 patients), compared with 11 FGA trials (total 2,032 patients).
Although the data comparing SGAs with FGAs are controversial, SGAs seem to have lower EPS potential. Regardless of the type of antipsychotic, however, studies have shown that patients who relapse while taking antipsychotics have less-severe episodes than those who relapse after discontinuing their medications.
For patients who frequently forget or incorrectly take oral medications, long-acting depot antipsychotics may increase adherence and decrease relapse rates during the stable phase.6
3. Weight gain, cardiovascular risk
Cardiovascular disease is the leading cause of death among persons with schizophrenia,7,8 whose life span is 10 to 20 years shorter than the population at large.9 Schizophrenia patients may be genetically predisposed to cardiovascular disease and metabolic syndrome (Table 3),10 exacerbated by typically sedentary lifestyles, high smoking rates, and poor diets. Certain SGAs add to the risk of weight gain and metabolic abnormalities.11
Table 3
Does your patient have metabolic syndrome?
| Metabolic syndrome is defined as having any 3 of these findings: | |
| Abdominal obesity | Waist circumference |
| >102 cm (40 in) in men | |
| >88 cm (35 in) in women | |
| Elevated triglycerides | ≥150 mg/dL |
| Low HDL | |
| Hypertension | ≥130/85 mm/Hg |
| Hyperglycemia | Fasting blood glucose ≥110 mg/dL |
| Source: National Cholesterol Education Program Adult Treatment Panel III guidelines, reference 10 | |
Table 4
Monitoring protocol for patients taking second-generation antipsychotics*
| Short-term | Long-term | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 4 wk | 8 wk | 12 wk | Quarterly | Annually | Every 5 yrs | |
| Personal/family history | X | X | |||||
| Weight (BMI) | X | X | X | X | X | ||
| Waist circumference | X | X | |||||
| Blood pressure | X | X | X | ||||
| Fasting plasma glucose | X | X | X | ||||
| Fasting lipid profile | X | X | X | ||||
| * More-frequent assessments may be warranted, based on clinical status. | |||||||
| BMI: body mass index | |||||||
| Source: Reference 12 | |||||||
- using treatments that do not increase the risk for cardiovascular disease
- switching to agents with less weight gain liability if a patient’s weight increases >7% or his or her body mass index (BMI) increases 1 unit during antipsychotic therapy.
Mr. K remained stable on olanzapine for several years, but his blood glucose, cholesterol, and triglycerides have risen dramatically and he has gained 40 lbs. Although we were concerned that he might not respond as well to another SGA, we reviewed the risks and benefits with specific concern about his cardiovascular health. As a result, we switched him to aripiprazole, 15 mg/d, with close monitoring and supervision for signs of relapse.
SGAs’ heterogeneity. The ADA/APA statement and multiple clinical trials support differential risks of weight gain and metabolic abnormalities with SGAs (Table 5). Patients in CATIE phase 1 who were randomly assigned to olanzapine experienced greater total weight gain and monthly weight gain (mean +2 lb/month) than patients taking any other antipsychotic.13 In an analysis of change from baseline to last observation, 30% of patients in the olanzapine group gained >7% of their baseline weight, compared with 7% to 16% of patients taking other antipsychotics.
Table 5
Differential risks of weigh gain and metabolic abnormalities with SGAs
| Risk | Antipsychotics |
|---|---|
| Highest | Clozapine, olanzapine |
| Intermediate/low | Risperidone, quetiapine |
| Lowest | Aripiprazole, ziprasidone |
| SGAs: Second-generation antipsychotics | |
Mr. K remains free of positive symptoms after taking aripiprazole for several months. He complains less of daytime sedation and has lost >10 lbs. We are awaiting repeat glucose, cholesterol, and triglyceride serum levels. We will continue to monitor his weight and metabolic values and ensure that he receives primary care follow-up.
4. Eps and tardive dyskinesia
Compared with FGAs, SGAs may be associated with a lower incidence of tardive dyskinesia (TD) and extrapyramidal symptoms (EPS). Even so, routine screening for EPS and TD remains necessary, according to the Mount Sinai consensus conference on physical health monitoring of patients with schizophrenia.16 At every visit, we observe Mr. K for:
- facial movements (excessive blinking, puckering, lip smacking, sucking, or grimacing)
- decreased arm swing while walking or choreoathetoid-like or writhing limb or trunk movements.
In addition to screening for metabolic syndrome, EPS, and movement disorders, the Mount Sinai consensus conference16 offers guidelines for monitoring prolactin, cardiac, and ocular changes in patients taking antipsychotics.
5. Mood disorders, substance abuse
Screen schizophrenia outpatients for signs of anxiety, depression, mania, and substance abuse at every visit. An antidepressant trial is recommended for depressive episodes even if antipsychotic therapy has adequately reduced positive psychotic symptoms.17 This strategy can prevent depressive relapses and might help prevent psychotic relapse as well.18 Also consider adjunctive therapy such as:
- mood stabilizers for affective instability
- benzodiazepines for short-term anxiety or agitation
- tricyclics or selective serotonin reuptake inhibitors for comorbid anxiety disorders such as obsessive-compulsive or panic disorder.19
Because of Mr. K’s history of marijuana abuse, we tell him we will use random urine toxicology screens. We also order urine toxicology if patients behave abnormally or appear impaired. We counsel patients and families about the link between substance use and psychotic decompensation, which Mr. K has demonstrated several times.
We emphasize that the goal of repeated questioning and screening is to ensure Mr. K’s well-being. If he is using substances, we will refer him to the help he needs.
6. Prodromal signs of relapse
Mr. K has reported decreased sleep, increased irritability, increased social isolation, and some agitation before his acute psychotic decompensations. These symptoms form his prodrome for relapse, which we routinely assess at follow-up visits.
We question him about sleep, social contacts, irritability, and agitation; assess psychotic symptoms; and observe thought processes and behaviors. If a patient endorses or displays prodromal signs of relapse, we consider:
- Is he taking the medication?
- Is he abusing substances?
- Does a change in dosage (actual or a cytochrome P450-mediated drug-drug interaction) explain a decrease in efficacy?
- Is he under stress and need an increased antipsychotic dosage?
- Might psychosocial interventions (support groups, cognitive-behavioral therapy, family involvement, etc.) help him deal with symptoms, decrease stress, or avoid an exacerbation?
- Has medication been optimized (correct dosing, long enough duration), or does the patient need an increased dosage or a different antipsychotic?
7. Psychosocial interventions
Combining medications with psychosocial programs is most effective for maintaining remission and improving patients’ social and occupational functioning.17 For Mr. K we recommend these interventions:
Social skills training. Mr. K now meets with other schizophrenia patients and a moderator to set long-term goals and smaller, attainable goals as homework assignments each week. Patients get feedback, positive reinforcement, and the opportunity to practice new skills. Mr. K is working on improving family relationships, furthering his career, and improving his interpersonal skills.
Family therapy and education. We have met with Mr. K’s mother several times, and she now visits him regularly and speaks with him on the phone at least once a week. She attends Support and Family Education and National Alliance on Mental Illness meetings to increase her understanding of her son’s illness.
Alcoholics Anonymous/Marijuana Anonymous. We refer Mr. K to AA/MA groups; he attends 2 to 3 times per week and has a sponsor. He is not sober yet but has dramatically cut back his substance use and continues to express motivation to quit.
Related resources
- Torrey EF. Surviving schizophrenia: a manual for families, patients, and providers, 5th ed. New York: Harper Collins; 2006.
- Mueser KT, Gingerich S. The complete family guide to schizophrenia: helping your loved one get the most out of life. New York: Guilford Press; 2006.
- National Alliance on Mental Illness. Resources for schizophrenia patients and their families. www.nami.org.
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Dr. Arey reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Marder receives research/grant support from Janssen Pharmaceutica and Eli Lilly and Co. and is a consultant or speaker for Bristol-Myers Squibb Co., Otsuka America Pharmaceutical, Pfizer, Solvay Pharmaceuticals, Merck and Co., and Roche.
1. Weiden P, Glazer M. Assessment and treatment selection for “revolving door” inpatients with schizophrenia. Psychiatr Q. 1997;68(4):377-92.
2. Lieberman JA, Stroup TS, McEvoy JP, et al. for the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;22;353(12):1209-23.
3. Marder SR, Glynn SM, Wirshing WC, et al. Maintenance treatment of schizophrenia with risperidone or haloperidol: 2-year outcomes. Am J Psychiatry 2003;160:1405-12.
4. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry 2006;63:1079-87.
5. Leucht S, Barnes TR, Kissling W, et al. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry 2003;160:1209-22.
6. Davis JM, Kane JM, Marder SR, et al. Dose response of prophylactic antipsychotics. J Clin Psychiatry 1993;54(3, suppl):24-30.
7. Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry 1997;171:502-8.
8. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry 1998;173:11-53.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis [serial online] 2006 Apr [date cited]. Available from: http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htm.
10. NCEP Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143-421.
11. Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry 2006;51(8):480-91.
12. et al. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
13. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
14. Stroup TS, Liberman JA, McEvoy JP, et al. for the CATIE Investigators. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:4:611-22.
15. McQuade RD, Stock E, Marcus R, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry 2004;65(suppl 18):47-56.
16. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-49.
17. Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004;30:193-217.
18. Siris SG, Morgan V, Fagerstrom R, et al. Adjunctive imipramine in the treatment of postpsychotic depression. Arch Gen Psychiatry 1987;44:533-9.
19. Braga RJ, Petrides G, Figueira I. Anxiety disorders in schizophrenia. Compr Psychiatry 2004;45(6):460-8.
1. Weiden P, Glazer M. Assessment and treatment selection for “revolving door” inpatients with schizophrenia. Psychiatr Q. 1997;68(4):377-92.
2. Lieberman JA, Stroup TS, McEvoy JP, et al. for the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;22;353(12):1209-23.
3. Marder SR, Glynn SM, Wirshing WC, et al. Maintenance treatment of schizophrenia with risperidone or haloperidol: 2-year outcomes. Am J Psychiatry 2003;160:1405-12.
4. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry 2006;63:1079-87.
5. Leucht S, Barnes TR, Kissling W, et al. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry 2003;160:1209-22.
6. Davis JM, Kane JM, Marder SR, et al. Dose response of prophylactic antipsychotics. J Clin Psychiatry 1993;54(3, suppl):24-30.
7. Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry 1997;171:502-8.
8. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry 1998;173:11-53.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis [serial online] 2006 Apr [date cited]. Available from: http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htm.
10. NCEP Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143-421.
11. Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry 2006;51(8):480-91.
12. et al. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
13. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
14. Stroup TS, Liberman JA, McEvoy JP, et al. for the CATIE Investigators. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:4:611-22.
15. McQuade RD, Stock E, Marcus R, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry 2004;65(suppl 18):47-56.
16. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-49.
17. Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004;30:193-217.
18. Siris SG, Morgan V, Fagerstrom R, et al. Adjunctive imipramine in the treatment of postpsychotic depression. Arch Gen Psychiatry 1987;44:533-9.
19. Braga RJ, Petrides G, Figueira I. Anxiety disorders in schizophrenia. Compr Psychiatry 2004;45(6):460-8.
Are irreputable health sites hurting your patients?
Web sites that offer questionable information about psychiatric illnesses and treatments can sway patients toward unproven, often worthless “remedies.” These sites may present themselves as patient resources but instead are promoting political or antipsychiatry agendas or selling unregulated, untested therapies.
Don’t let unscrupulous sites fool your patients. This article offers tools to help patients find evidence-based mental health information from objective, reputable sites.
Why counsel patients on web use?
Bad information can be harmful. I have lost many patients to follow-up because they discovered an unsubstantiated treatment complication or off-the-wall “remedy” on an antipsychiatry or antimedication site.
Years ago, I treated another mental health clinician. After she viewed an antimedication site, she was convinced that her bipolar disorder had “run its course” and stopped treatment, even though she had suffered a severe manic episode 1 year earlier. Another doctor treated her as if her bipolar disorder had been “cured.”
I resolved never to let patients troll the Internet for medical information without rudimentary guidance.
Most patients do not know how to analyze medical information. In medical school we learned—by implementing dictums of evidence-based medicine—where to find clinical information and how to assess its quality and objectivity. Most patients have not received such training.
Patients need our support. Most patients seeing a psychiatrist for the first time are anxious and fearful of what they might find out about themselves or their lives. Exploring their inner worlds is routine to us, but unsettling and disorienting to them. Unfiltered, uncensored Web sites prey upon new patients by offering a ready source of comfort.
Guiding new patients during this vulnerable time can cement the doctor-patient relationship and prevent faulty information from jeopardizing recovery. Patients who do not receive emotional support could turn to a Church of Scientology site—such as http://psychiatrysucks.com—or one of many other antipsychiatry sites to fill the void.
Encourage patients to describe their anxieties and trepidations toward their illnesses and medications. Help them explore questions about trust and hope, and anticipate and solicit questions resulting from their Internet exploration.
Setting web search guidelines
When new patients ask where to find information on their disorder or treatment, suggest the National Institutes of Health’s Web site, which offers a wealth of current information written in plain English, and links to databases, such as Medline and ongoing clinical trials.
Then give patients basic guidelines for broader Internet exploration. Warn them against sites that post personal attacks, exude a zealous tone, or present extreme positions or statements. Sites infused with fervor—positive or negative—should always warrant suspicion.
For more subtle concerns about quality of information, encourage patients to ask the following six questions—easily recalled with the acronym NO BASH (Table)—when visiting a mental health site:
Table
NO BASH: 6 questions to ask when perusing a health site
| 1. Is the site Networked? |
| 2. Is the information Objective? |
| 3. Is the content Balanced? |
| 4. Does the site’s author make Accusations? |
| 5. Is the site Selling something? |
| 6. Is the site ‘Hyperholy’? |
- IS THE SITE NETWORKED?
- IS THE INFORMATION OBJECTIVE?
- IS THE CONTENT BALANCED?
- DOES THE SITE’S AUTHOR MAKE ACCUSATIONS?
- IS THE SITE SELLING SOMETHING?
- IS THE SITE ‘HYPERHOLY’?
Other considerations
Also consider the site’s domain designation:
- sites with the .edu domain—operated by educational institutions—are most reliable
- .com designates a commercial site that is generally geared to selling goods or services and might or might not support psychiatric treatment
- .net and .org sites tend to be noncommercial, although some might be antipsychiatry.
Also steer patients to health care sites that display the HON Code seal of the Health On the Net Foundation (HON). HON, a nonprofit international organization that promotes development of useful, reliable online medical and health information, certifies health sites that meet its rigorous ethical standards (see Related resources).
- Health Care on the Net Foundation code of conduct (HON code) for medical and health Web sites. www.hon.ch/HONcode/Conduct.html.
Disclosure
Dr. Montgomery reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Web sites that offer questionable information about psychiatric illnesses and treatments can sway patients toward unproven, often worthless “remedies.” These sites may present themselves as patient resources but instead are promoting political or antipsychiatry agendas or selling unregulated, untested therapies.
Don’t let unscrupulous sites fool your patients. This article offers tools to help patients find evidence-based mental health information from objective, reputable sites.
Why counsel patients on web use?
Bad information can be harmful. I have lost many patients to follow-up because they discovered an unsubstantiated treatment complication or off-the-wall “remedy” on an antipsychiatry or antimedication site.
Years ago, I treated another mental health clinician. After she viewed an antimedication site, she was convinced that her bipolar disorder had “run its course” and stopped treatment, even though she had suffered a severe manic episode 1 year earlier. Another doctor treated her as if her bipolar disorder had been “cured.”
I resolved never to let patients troll the Internet for medical information without rudimentary guidance.
Most patients do not know how to analyze medical information. In medical school we learned—by implementing dictums of evidence-based medicine—where to find clinical information and how to assess its quality and objectivity. Most patients have not received such training.
Patients need our support. Most patients seeing a psychiatrist for the first time are anxious and fearful of what they might find out about themselves or their lives. Exploring their inner worlds is routine to us, but unsettling and disorienting to them. Unfiltered, uncensored Web sites prey upon new patients by offering a ready source of comfort.
Guiding new patients during this vulnerable time can cement the doctor-patient relationship and prevent faulty information from jeopardizing recovery. Patients who do not receive emotional support could turn to a Church of Scientology site—such as http://psychiatrysucks.com—or one of many other antipsychiatry sites to fill the void.
Encourage patients to describe their anxieties and trepidations toward their illnesses and medications. Help them explore questions about trust and hope, and anticipate and solicit questions resulting from their Internet exploration.
Setting web search guidelines
When new patients ask where to find information on their disorder or treatment, suggest the National Institutes of Health’s Web site, which offers a wealth of current information written in plain English, and links to databases, such as Medline and ongoing clinical trials.
Then give patients basic guidelines for broader Internet exploration. Warn them against sites that post personal attacks, exude a zealous tone, or present extreme positions or statements. Sites infused with fervor—positive or negative—should always warrant suspicion.
For more subtle concerns about quality of information, encourage patients to ask the following six questions—easily recalled with the acronym NO BASH (Table)—when visiting a mental health site:
Table
NO BASH: 6 questions to ask when perusing a health site
| 1. Is the site Networked? |
| 2. Is the information Objective? |
| 3. Is the content Balanced? |
| 4. Does the site’s author make Accusations? |
| 5. Is the site Selling something? |
| 6. Is the site ‘Hyperholy’? |
- IS THE SITE NETWORKED?
- IS THE INFORMATION OBJECTIVE?
- IS THE CONTENT BALANCED?
- DOES THE SITE’S AUTHOR MAKE ACCUSATIONS?
- IS THE SITE SELLING SOMETHING?
- IS THE SITE ‘HYPERHOLY’?
Other considerations
Also consider the site’s domain designation:
- sites with the .edu domain—operated by educational institutions—are most reliable
- .com designates a commercial site that is generally geared to selling goods or services and might or might not support psychiatric treatment
- .net and .org sites tend to be noncommercial, although some might be antipsychiatry.
Also steer patients to health care sites that display the HON Code seal of the Health On the Net Foundation (HON). HON, a nonprofit international organization that promotes development of useful, reliable online medical and health information, certifies health sites that meet its rigorous ethical standards (see Related resources).
- Health Care on the Net Foundation code of conduct (HON code) for medical and health Web sites. www.hon.ch/HONcode/Conduct.html.
Disclosure
Dr. Montgomery reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Web sites that offer questionable information about psychiatric illnesses and treatments can sway patients toward unproven, often worthless “remedies.” These sites may present themselves as patient resources but instead are promoting political or antipsychiatry agendas or selling unregulated, untested therapies.
Don’t let unscrupulous sites fool your patients. This article offers tools to help patients find evidence-based mental health information from objective, reputable sites.
Why counsel patients on web use?
Bad information can be harmful. I have lost many patients to follow-up because they discovered an unsubstantiated treatment complication or off-the-wall “remedy” on an antipsychiatry or antimedication site.
Years ago, I treated another mental health clinician. After she viewed an antimedication site, she was convinced that her bipolar disorder had “run its course” and stopped treatment, even though she had suffered a severe manic episode 1 year earlier. Another doctor treated her as if her bipolar disorder had been “cured.”
I resolved never to let patients troll the Internet for medical information without rudimentary guidance.
Most patients do not know how to analyze medical information. In medical school we learned—by implementing dictums of evidence-based medicine—where to find clinical information and how to assess its quality and objectivity. Most patients have not received such training.
Patients need our support. Most patients seeing a psychiatrist for the first time are anxious and fearful of what they might find out about themselves or their lives. Exploring their inner worlds is routine to us, but unsettling and disorienting to them. Unfiltered, uncensored Web sites prey upon new patients by offering a ready source of comfort.
Guiding new patients during this vulnerable time can cement the doctor-patient relationship and prevent faulty information from jeopardizing recovery. Patients who do not receive emotional support could turn to a Church of Scientology site—such as http://psychiatrysucks.com—or one of many other antipsychiatry sites to fill the void.
Encourage patients to describe their anxieties and trepidations toward their illnesses and medications. Help them explore questions about trust and hope, and anticipate and solicit questions resulting from their Internet exploration.
Setting web search guidelines
When new patients ask where to find information on their disorder or treatment, suggest the National Institutes of Health’s Web site, which offers a wealth of current information written in plain English, and links to databases, such as Medline and ongoing clinical trials.
Then give patients basic guidelines for broader Internet exploration. Warn them against sites that post personal attacks, exude a zealous tone, or present extreme positions or statements. Sites infused with fervor—positive or negative—should always warrant suspicion.
For more subtle concerns about quality of information, encourage patients to ask the following six questions—easily recalled with the acronym NO BASH (Table)—when visiting a mental health site:
Table
NO BASH: 6 questions to ask when perusing a health site
| 1. Is the site Networked? |
| 2. Is the information Objective? |
| 3. Is the content Balanced? |
| 4. Does the site’s author make Accusations? |
| 5. Is the site Selling something? |
| 6. Is the site ‘Hyperholy’? |
- IS THE SITE NETWORKED?
- IS THE INFORMATION OBJECTIVE?
- IS THE CONTENT BALANCED?
- DOES THE SITE’S AUTHOR MAKE ACCUSATIONS?
- IS THE SITE SELLING SOMETHING?
- IS THE SITE ‘HYPERHOLY’?
Other considerations
Also consider the site’s domain designation:
- sites with the .edu domain—operated by educational institutions—are most reliable
- .com designates a commercial site that is generally geared to selling goods or services and might or might not support psychiatric treatment
- .net and .org sites tend to be noncommercial, although some might be antipsychiatry.
Also steer patients to health care sites that display the HON Code seal of the Health On the Net Foundation (HON). HON, a nonprofit international organization that promotes development of useful, reliable online medical and health information, certifies health sites that meet its rigorous ethical standards (see Related resources).
- Health Care on the Net Foundation code of conduct (HON code) for medical and health Web sites. www.hon.ch/HONcode/Conduct.html.
Disclosure
Dr. Montgomery reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.