User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Getting patients to talk about priapism
CHIEF COMPLAINT: Anxiety and disordered sleep
Mr. Q, a college sophomore, reported symptoms of insomnia, anxiety, and sadness to the university health service. When in bed, he said, he would ruminate about whether he had studied adequately and would ultimately qualify for a graduate program. He exhibited no pervasive sadness, loss of interest or motivation, suicidal ideation, or loss of self-esteem. His medical history revealed no serious illness.
The student health psychiatrist diagnosed Mr. Q as having generalized anxiety disorder. She prescribed trazodone, up to 100 mg/d as needed, for the insomnia. For the next 3 weeks, he took one 25 mg dose each night. After that time, Mr. Q reported that the trazodone alleviated the insomnia and that he felt more rested and could study more effectively. He had stopped taking the medication.
Mr. Q, however, did not tell the health service psychiatrist that he had also experienced an uncomfortable erection that lasted about 4 hours and was not precipitated or accompanied by sexual activity. He finally experienced detumescence after several cold showers. He did not inform her of the episode because he felt embarrassed to discuss “such a thing” with a female physician.
After his anxiety and insomnia resurfaced, Mr. Q was referred to one of the authors.
Why did Mr. Q. develop priapism? How would you counsel him at this point?
Dr. Freed’s and Dr. Muskin’s observations
Priapism refers to a prolonged and painful erection that results from sustained blood flow into the corpora cavernosa. In contrast to a normal erection, both the corpus spongiosum and glans penis remain flaccid. Medical complications and reactions to drugs are well-documented causes.
Table 1
Drugs reported to cause priapism
| Antidepressants |
| Trazodone and, in rare cases, phenelzine and sertraline; bupropion has been associated with clitoral priapism3 |
| Antihypertensives that act via alpha blockade Labetalol, prazosin3-5 |
| Metoclopramide when taken with thioridazine3,4 |
| Sildenafil citrate6 (rare case reports) |
| Substances of abuse |
| Alcohol, marijuana, crack cocaine |
| Typical and atypical antipsychotics |
| Chlorpromazine, clozapine, fluphenazine, haloperidol, mesoridazine, molindone, levomepromazine, perphenazine, promazine, risperidone, thioridazine, thiothixene3-5 |
An erection in priapism may result from sexual stimulation/activity, although this is not typical. Sexually stimulated erections in priapism persist hours after the stimulation ceases.
High-flow priapism is rare, painless, and occurs when well-oxygenated blood stays in the corpora cavernosa. It may result from perineal trauma creating a fistula between an artery and the cavernosa. Because the blood is oxygenated, there is no tissue damage, intervention is not urgent, and the prognosis usually is good.
Low-flow priapism, the more prevalent type, is painful and occurs when venous blood remains in the corpora, resulting in hypoxia and ischemia. Approximately 50% of low-flow priapism cases can result in impotence.1
Because men often are embarrassed by priapism, they may not seek medical attention or mention a prior episode to their physicians. This neglect can be dangerous: Painful erections that persist for more than 4 hours can lead to impotence if left untreated.
The physician must surmount the patient’s reluctance to discuss the symptom. Inquiring about past priapism episodes as part of a complete patient history is essential. We suggest routinely asking patients taking priapism-causing psychotropics (Table 1) if they’ve had a recent erectile problem. Mentioning that a medication can cause uncomfortable and serious sexual side effects may prompt the patient to discuss such problems.
Above all, be direct. A straightforward inquiry about a sensitive medical condition usually draws an honest answer; the patient then realizes the subject is important and should not be embarrassed about it.
After the patient discloses a priapism episode, ask him:
- Was the erection related to sexual activity or desire?
- Were you using any other medications or illicit drugs when the erection occurred?
- Do you have a systemic blood disorder?
- Did you feel any pain during your erection? If so, how long did it persist?
Men who present during a priapism episode should immediately be sent to the ER for urologic treatment. Patients reporting a recent sustained erection should be referred to a urologist if they need to keep taking the priapism-causing drug. Urologic treatment is not necessary if the patient stops the medication and the priapism resolves.
Men who have had at least one past priapism episode and those taking alpha-adrenergic blockers should be instructed to visit the ER immediately if a painful, persistent erection develops. Patients also should be warned not to induce detumescence (such as by taking cold showers, drinking alcohol, or engaging in sexual activity) if the erection persists for more than 2 hours. Any delay in emergency care could lead to impotence.
HISTORY: A probable side effect
Because Mr. Q had no other past erectile problems, we strongly suspected his priapism was medication-induced. He reported he had neither been drinking nor taking illicit drugs or other medications when the erection occurred.
Mr. Q also was convinced that the trazodone had caused the sustained erection. He said, however, he was never informed that priapism was a potential side effect of that medication.
Would you resume trazodone, switch to another sleep-promoting or antianxiety medication, or consider other therapy?
Dr. Freed’s and Dr. Muskin’s observations
The prevalence of priapism is not known, although yearly estimates range from 1/1,000 to 1/10,000 patients who take trazodone.2
Trazodone, an alpha-adrenergic blocker, is most commonly implicated among psychotropics in causing priapism.2 Blockade of alpha-adrenergic receptors in the corpora cavernosa creates a parasympathetic imbalance favoring erection and prevents sympathetic-mediated detumescence. Histaminic, beta-adrenergic, and adrenergic/cholinergic components may also contribute to priapism.
Other medications associated with priapism include antipsychotics, antihypertensives, anticoagulants, some antidepressants, and antiimpotence medications injected into the penis.
Low-flow priapism can also be caused by systemic disorders (Table 2), including malignancies—particularly when a tumor has infiltrated the penis—and carcinoma of the bladder or prostate. Prostatitis has been implicated in some cases.
Table 2
Systemic illnesses and conditions that can cause priapism
|
Because Mr. Q has had at least one priapism episode, we would avoid prescribing any agent with alpha-adrenergic blocking properties.
Could Mr. Q’s response to trazodone have been dose-related? How would you ensure that the patient understands a medication’s risks?
Dr. Freed’s and Dr. Muskin’s observations
No findings indicate that trazodone-related priapism is dose-related. Several cases of men developing sustained priapism—resulting in permanent injury and impotence—have been reported after initial dosages of 25 and 50 mg/d.1,4,7 In a study using the FDA Spontaneous Reporting System, Warner et al found that priapism with trazodone was most likely to occur within the first month of treatment and at dosages 150 mg/d.7 Still other reports indicate that new-onset priapism may occur after years of treatment.3
Priapism refers to a painful, prolonged erection that occurs in the absence of sexual stimulation or does not remit after sexual activity.
Several psychotropic drugs, most often trazodone (Desyrel), can cause priapism. This can occur even if the medication is taken at a low dosage or taken only once.
Individuals who have had prior prolonged erections are more susceptible to priapism. Certain medical conditions, many medications, and substance abuse can also increase the risk of priapism. This effect may be additive.
If the erection lasts more than 2 hours, the patient must obtain emergency care. Impotence has been reported after erections lasting 4 hours or longer.
Mr. Z filed suit in Pennsylvania state court against his pharmacy and emergency room doctor. He alleged that he developed priapism after taking one dose of trazodone for disordered sleep. He subsequently became impotent.
Christopher T. Rhodes, PhD, a professor of pharmaceutics at the University of Rhode Island, was an expert witness in that 2000 trial. According to Dr. Rhodes, court testimony revealed that the ER physician had not informed the patient about the possibility of priapism or about the need to obtain emergency treatment for a sustained erection. Dr. Rhodes adds that the pharmacy handout for trazodone did not list priapism as a possible adverse effect.
The court ruled in favor of the patient, judging that the “quality of advice” was inadequate. The patient was awarded an unspecified sum.
Despite its association with priapism, trazodone is used frequently in men and is a popular medication for disordered sleep. Nierenberg et al demonstrated improved sleep in 67% of depressed patients with insomnia who received trazodone either for depression or disordered sleep.8
When prescribing a priapism-causing agent, make sure the patient understands that erectile effects—though rare—can occur. Consider giving patients an informed consent form explaining the association between psychotropics and priapism and the potential long-term health implications (Box 1). Include the form in the patient’s record for documentation in the event of a malpractice lawsuit (Box 2).
FURTHER TREATMENT: Learning how to cope
Self-hypnosis/relaxation therapy was initiated to address Mr. Q’s anxiety and insomnia. The patient quickly learned the hypnosis techniques and his anxiety/insomnia symptoms began to resolve almost immediately.
Mr. Q’s priapism resolved spontaneously with no apparent erectile dysfunction. He was referred back to the university health service and has been in apparent good health since.
Related resources
- Sleepnet.com. Information on sleep disorders and sleep hygiene. http://www.sleepnet.com/
- National Center on Sleep Disorders Research http://www.nhlbi.nih.gov/about/ncsdr/
Drug brand names
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Labetalol • Trandate
- Levomepromazine • Nozinan
- Mesoridazine • Serentil
- Metoclopramide • Reglan
- Molindone • Lidone
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Prazosin • Minipress
- Promazine • Sparine
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sildenafil citrate • Viagra
- Thioridazine • Mellaril
- Thiothixene • Navane
- Trazodone • Desyrel
Disclosure
Dr. Freed reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Muskin receives research/grant support from Bristol-Myers Squibb Co., is a speaker for and consultant to Bristol-Myers Squibb Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, and Pfizer Inc.; and is a speaker for Cephalon Inc. and Eli Lilly and Co.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. CNS Drugs 1998;9:371-9.
2. Rhodes CT. Trazodone and priapism—implications for responses to adverse events. Clin Res Regulatory Affairs 2001;18:47-52.
3. Compton MT, Miller AH. Priapism associated with conventional and atypical antipsychotic medications: a review. J Clin Psychiatry 2001;62:362-6.
4. Thompson JW, Ware MR, Blashfield RK. Psychotropic medication and priapism: a comprehensive review. J Clin Psychiatry 1990;51:430-3.
5. Reeves RR, Kimble R. Prolonged erections associated with ziprasidone treatment: a case report. J Clin Psychiatry 2003;64:97-8.
6. Sur RL, Kane CJ. Sildenafil citrate-associated priapism. Urology 2000;55:950.-
7. Warner MD, Peabody CA, Whiteford HA, Hollister LE. Trazodone and priapism. J Clin Psychiatry 1987;48:244-5.
8. Nierenberg AA, Adler LA, Peselow E, et al. Trazodone for antidepressant-associated insomnia. Am J Psychiatry 1994;151:1069-72.
CHIEF COMPLAINT: Anxiety and disordered sleep
Mr. Q, a college sophomore, reported symptoms of insomnia, anxiety, and sadness to the university health service. When in bed, he said, he would ruminate about whether he had studied adequately and would ultimately qualify for a graduate program. He exhibited no pervasive sadness, loss of interest or motivation, suicidal ideation, or loss of self-esteem. His medical history revealed no serious illness.
The student health psychiatrist diagnosed Mr. Q as having generalized anxiety disorder. She prescribed trazodone, up to 100 mg/d as needed, for the insomnia. For the next 3 weeks, he took one 25 mg dose each night. After that time, Mr. Q reported that the trazodone alleviated the insomnia and that he felt more rested and could study more effectively. He had stopped taking the medication.
Mr. Q, however, did not tell the health service psychiatrist that he had also experienced an uncomfortable erection that lasted about 4 hours and was not precipitated or accompanied by sexual activity. He finally experienced detumescence after several cold showers. He did not inform her of the episode because he felt embarrassed to discuss “such a thing” with a female physician.
After his anxiety and insomnia resurfaced, Mr. Q was referred to one of the authors.
Why did Mr. Q. develop priapism? How would you counsel him at this point?
Dr. Freed’s and Dr. Muskin’s observations
Priapism refers to a prolonged and painful erection that results from sustained blood flow into the corpora cavernosa. In contrast to a normal erection, both the corpus spongiosum and glans penis remain flaccid. Medical complications and reactions to drugs are well-documented causes.
Table 1
Drugs reported to cause priapism
| Antidepressants |
| Trazodone and, in rare cases, phenelzine and sertraline; bupropion has been associated with clitoral priapism3 |
| Antihypertensives that act via alpha blockade Labetalol, prazosin3-5 |
| Metoclopramide when taken with thioridazine3,4 |
| Sildenafil citrate6 (rare case reports) |
| Substances of abuse |
| Alcohol, marijuana, crack cocaine |
| Typical and atypical antipsychotics |
| Chlorpromazine, clozapine, fluphenazine, haloperidol, mesoridazine, molindone, levomepromazine, perphenazine, promazine, risperidone, thioridazine, thiothixene3-5 |
An erection in priapism may result from sexual stimulation/activity, although this is not typical. Sexually stimulated erections in priapism persist hours after the stimulation ceases.
High-flow priapism is rare, painless, and occurs when well-oxygenated blood stays in the corpora cavernosa. It may result from perineal trauma creating a fistula between an artery and the cavernosa. Because the blood is oxygenated, there is no tissue damage, intervention is not urgent, and the prognosis usually is good.
Low-flow priapism, the more prevalent type, is painful and occurs when venous blood remains in the corpora, resulting in hypoxia and ischemia. Approximately 50% of low-flow priapism cases can result in impotence.1
Because men often are embarrassed by priapism, they may not seek medical attention or mention a prior episode to their physicians. This neglect can be dangerous: Painful erections that persist for more than 4 hours can lead to impotence if left untreated.
The physician must surmount the patient’s reluctance to discuss the symptom. Inquiring about past priapism episodes as part of a complete patient history is essential. We suggest routinely asking patients taking priapism-causing psychotropics (Table 1) if they’ve had a recent erectile problem. Mentioning that a medication can cause uncomfortable and serious sexual side effects may prompt the patient to discuss such problems.
Above all, be direct. A straightforward inquiry about a sensitive medical condition usually draws an honest answer; the patient then realizes the subject is important and should not be embarrassed about it.
After the patient discloses a priapism episode, ask him:
- Was the erection related to sexual activity or desire?
- Were you using any other medications or illicit drugs when the erection occurred?
- Do you have a systemic blood disorder?
- Did you feel any pain during your erection? If so, how long did it persist?
Men who present during a priapism episode should immediately be sent to the ER for urologic treatment. Patients reporting a recent sustained erection should be referred to a urologist if they need to keep taking the priapism-causing drug. Urologic treatment is not necessary if the patient stops the medication and the priapism resolves.
Men who have had at least one past priapism episode and those taking alpha-adrenergic blockers should be instructed to visit the ER immediately if a painful, persistent erection develops. Patients also should be warned not to induce detumescence (such as by taking cold showers, drinking alcohol, or engaging in sexual activity) if the erection persists for more than 2 hours. Any delay in emergency care could lead to impotence.
HISTORY: A probable side effect
Because Mr. Q had no other past erectile problems, we strongly suspected his priapism was medication-induced. He reported he had neither been drinking nor taking illicit drugs or other medications when the erection occurred.
Mr. Q also was convinced that the trazodone had caused the sustained erection. He said, however, he was never informed that priapism was a potential side effect of that medication.
Would you resume trazodone, switch to another sleep-promoting or antianxiety medication, or consider other therapy?
Dr. Freed’s and Dr. Muskin’s observations
The prevalence of priapism is not known, although yearly estimates range from 1/1,000 to 1/10,000 patients who take trazodone.2
Trazodone, an alpha-adrenergic blocker, is most commonly implicated among psychotropics in causing priapism.2 Blockade of alpha-adrenergic receptors in the corpora cavernosa creates a parasympathetic imbalance favoring erection and prevents sympathetic-mediated detumescence. Histaminic, beta-adrenergic, and adrenergic/cholinergic components may also contribute to priapism.
Other medications associated with priapism include antipsychotics, antihypertensives, anticoagulants, some antidepressants, and antiimpotence medications injected into the penis.
Low-flow priapism can also be caused by systemic disorders (Table 2), including malignancies—particularly when a tumor has infiltrated the penis—and carcinoma of the bladder or prostate. Prostatitis has been implicated in some cases.
Table 2
Systemic illnesses and conditions that can cause priapism
|
Because Mr. Q has had at least one priapism episode, we would avoid prescribing any agent with alpha-adrenergic blocking properties.
Could Mr. Q’s response to trazodone have been dose-related? How would you ensure that the patient understands a medication’s risks?
Dr. Freed’s and Dr. Muskin’s observations
No findings indicate that trazodone-related priapism is dose-related. Several cases of men developing sustained priapism—resulting in permanent injury and impotence—have been reported after initial dosages of 25 and 50 mg/d.1,4,7 In a study using the FDA Spontaneous Reporting System, Warner et al found that priapism with trazodone was most likely to occur within the first month of treatment and at dosages 150 mg/d.7 Still other reports indicate that new-onset priapism may occur after years of treatment.3
Priapism refers to a painful, prolonged erection that occurs in the absence of sexual stimulation or does not remit after sexual activity.
Several psychotropic drugs, most often trazodone (Desyrel), can cause priapism. This can occur even if the medication is taken at a low dosage or taken only once.
Individuals who have had prior prolonged erections are more susceptible to priapism. Certain medical conditions, many medications, and substance abuse can also increase the risk of priapism. This effect may be additive.
If the erection lasts more than 2 hours, the patient must obtain emergency care. Impotence has been reported after erections lasting 4 hours or longer.
Mr. Z filed suit in Pennsylvania state court against his pharmacy and emergency room doctor. He alleged that he developed priapism after taking one dose of trazodone for disordered sleep. He subsequently became impotent.
Christopher T. Rhodes, PhD, a professor of pharmaceutics at the University of Rhode Island, was an expert witness in that 2000 trial. According to Dr. Rhodes, court testimony revealed that the ER physician had not informed the patient about the possibility of priapism or about the need to obtain emergency treatment for a sustained erection. Dr. Rhodes adds that the pharmacy handout for trazodone did not list priapism as a possible adverse effect.
The court ruled in favor of the patient, judging that the “quality of advice” was inadequate. The patient was awarded an unspecified sum.
Despite its association with priapism, trazodone is used frequently in men and is a popular medication for disordered sleep. Nierenberg et al demonstrated improved sleep in 67% of depressed patients with insomnia who received trazodone either for depression or disordered sleep.8
When prescribing a priapism-causing agent, make sure the patient understands that erectile effects—though rare—can occur. Consider giving patients an informed consent form explaining the association between psychotropics and priapism and the potential long-term health implications (Box 1). Include the form in the patient’s record for documentation in the event of a malpractice lawsuit (Box 2).
FURTHER TREATMENT: Learning how to cope
Self-hypnosis/relaxation therapy was initiated to address Mr. Q’s anxiety and insomnia. The patient quickly learned the hypnosis techniques and his anxiety/insomnia symptoms began to resolve almost immediately.
Mr. Q’s priapism resolved spontaneously with no apparent erectile dysfunction. He was referred back to the university health service and has been in apparent good health since.
Related resources
- Sleepnet.com. Information on sleep disorders and sleep hygiene. http://www.sleepnet.com/
- National Center on Sleep Disorders Research http://www.nhlbi.nih.gov/about/ncsdr/
Drug brand names
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Labetalol • Trandate
- Levomepromazine • Nozinan
- Mesoridazine • Serentil
- Metoclopramide • Reglan
- Molindone • Lidone
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Prazosin • Minipress
- Promazine • Sparine
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sildenafil citrate • Viagra
- Thioridazine • Mellaril
- Thiothixene • Navane
- Trazodone • Desyrel
Disclosure
Dr. Freed reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Muskin receives research/grant support from Bristol-Myers Squibb Co., is a speaker for and consultant to Bristol-Myers Squibb Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, and Pfizer Inc.; and is a speaker for Cephalon Inc. and Eli Lilly and Co.
CHIEF COMPLAINT: Anxiety and disordered sleep
Mr. Q, a college sophomore, reported symptoms of insomnia, anxiety, and sadness to the university health service. When in bed, he said, he would ruminate about whether he had studied adequately and would ultimately qualify for a graduate program. He exhibited no pervasive sadness, loss of interest or motivation, suicidal ideation, or loss of self-esteem. His medical history revealed no serious illness.
The student health psychiatrist diagnosed Mr. Q as having generalized anxiety disorder. She prescribed trazodone, up to 100 mg/d as needed, for the insomnia. For the next 3 weeks, he took one 25 mg dose each night. After that time, Mr. Q reported that the trazodone alleviated the insomnia and that he felt more rested and could study more effectively. He had stopped taking the medication.
Mr. Q, however, did not tell the health service psychiatrist that he had also experienced an uncomfortable erection that lasted about 4 hours and was not precipitated or accompanied by sexual activity. He finally experienced detumescence after several cold showers. He did not inform her of the episode because he felt embarrassed to discuss “such a thing” with a female physician.
After his anxiety and insomnia resurfaced, Mr. Q was referred to one of the authors.
Why did Mr. Q. develop priapism? How would you counsel him at this point?
Dr. Freed’s and Dr. Muskin’s observations
Priapism refers to a prolonged and painful erection that results from sustained blood flow into the corpora cavernosa. In contrast to a normal erection, both the corpus spongiosum and glans penis remain flaccid. Medical complications and reactions to drugs are well-documented causes.
Table 1
Drugs reported to cause priapism
| Antidepressants |
| Trazodone and, in rare cases, phenelzine and sertraline; bupropion has been associated with clitoral priapism3 |
| Antihypertensives that act via alpha blockade Labetalol, prazosin3-5 |
| Metoclopramide when taken with thioridazine3,4 |
| Sildenafil citrate6 (rare case reports) |
| Substances of abuse |
| Alcohol, marijuana, crack cocaine |
| Typical and atypical antipsychotics |
| Chlorpromazine, clozapine, fluphenazine, haloperidol, mesoridazine, molindone, levomepromazine, perphenazine, promazine, risperidone, thioridazine, thiothixene3-5 |
An erection in priapism may result from sexual stimulation/activity, although this is not typical. Sexually stimulated erections in priapism persist hours after the stimulation ceases.
High-flow priapism is rare, painless, and occurs when well-oxygenated blood stays in the corpora cavernosa. It may result from perineal trauma creating a fistula between an artery and the cavernosa. Because the blood is oxygenated, there is no tissue damage, intervention is not urgent, and the prognosis usually is good.
Low-flow priapism, the more prevalent type, is painful and occurs when venous blood remains in the corpora, resulting in hypoxia and ischemia. Approximately 50% of low-flow priapism cases can result in impotence.1
Because men often are embarrassed by priapism, they may not seek medical attention or mention a prior episode to their physicians. This neglect can be dangerous: Painful erections that persist for more than 4 hours can lead to impotence if left untreated.
The physician must surmount the patient’s reluctance to discuss the symptom. Inquiring about past priapism episodes as part of a complete patient history is essential. We suggest routinely asking patients taking priapism-causing psychotropics (Table 1) if they’ve had a recent erectile problem. Mentioning that a medication can cause uncomfortable and serious sexual side effects may prompt the patient to discuss such problems.
Above all, be direct. A straightforward inquiry about a sensitive medical condition usually draws an honest answer; the patient then realizes the subject is important and should not be embarrassed about it.
After the patient discloses a priapism episode, ask him:
- Was the erection related to sexual activity or desire?
- Were you using any other medications or illicit drugs when the erection occurred?
- Do you have a systemic blood disorder?
- Did you feel any pain during your erection? If so, how long did it persist?
Men who present during a priapism episode should immediately be sent to the ER for urologic treatment. Patients reporting a recent sustained erection should be referred to a urologist if they need to keep taking the priapism-causing drug. Urologic treatment is not necessary if the patient stops the medication and the priapism resolves.
Men who have had at least one past priapism episode and those taking alpha-adrenergic blockers should be instructed to visit the ER immediately if a painful, persistent erection develops. Patients also should be warned not to induce detumescence (such as by taking cold showers, drinking alcohol, or engaging in sexual activity) if the erection persists for more than 2 hours. Any delay in emergency care could lead to impotence.
HISTORY: A probable side effect
Because Mr. Q had no other past erectile problems, we strongly suspected his priapism was medication-induced. He reported he had neither been drinking nor taking illicit drugs or other medications when the erection occurred.
Mr. Q also was convinced that the trazodone had caused the sustained erection. He said, however, he was never informed that priapism was a potential side effect of that medication.
Would you resume trazodone, switch to another sleep-promoting or antianxiety medication, or consider other therapy?
Dr. Freed’s and Dr. Muskin’s observations
The prevalence of priapism is not known, although yearly estimates range from 1/1,000 to 1/10,000 patients who take trazodone.2
Trazodone, an alpha-adrenergic blocker, is most commonly implicated among psychotropics in causing priapism.2 Blockade of alpha-adrenergic receptors in the corpora cavernosa creates a parasympathetic imbalance favoring erection and prevents sympathetic-mediated detumescence. Histaminic, beta-adrenergic, and adrenergic/cholinergic components may also contribute to priapism.
Other medications associated with priapism include antipsychotics, antihypertensives, anticoagulants, some antidepressants, and antiimpotence medications injected into the penis.
Low-flow priapism can also be caused by systemic disorders (Table 2), including malignancies—particularly when a tumor has infiltrated the penis—and carcinoma of the bladder or prostate. Prostatitis has been implicated in some cases.
Table 2
Systemic illnesses and conditions that can cause priapism
|
Because Mr. Q has had at least one priapism episode, we would avoid prescribing any agent with alpha-adrenergic blocking properties.
Could Mr. Q’s response to trazodone have been dose-related? How would you ensure that the patient understands a medication’s risks?
Dr. Freed’s and Dr. Muskin’s observations
No findings indicate that trazodone-related priapism is dose-related. Several cases of men developing sustained priapism—resulting in permanent injury and impotence—have been reported after initial dosages of 25 and 50 mg/d.1,4,7 In a study using the FDA Spontaneous Reporting System, Warner et al found that priapism with trazodone was most likely to occur within the first month of treatment and at dosages 150 mg/d.7 Still other reports indicate that new-onset priapism may occur after years of treatment.3
Priapism refers to a painful, prolonged erection that occurs in the absence of sexual stimulation or does not remit after sexual activity.
Several psychotropic drugs, most often trazodone (Desyrel), can cause priapism. This can occur even if the medication is taken at a low dosage or taken only once.
Individuals who have had prior prolonged erections are more susceptible to priapism. Certain medical conditions, many medications, and substance abuse can also increase the risk of priapism. This effect may be additive.
If the erection lasts more than 2 hours, the patient must obtain emergency care. Impotence has been reported after erections lasting 4 hours or longer.
Mr. Z filed suit in Pennsylvania state court against his pharmacy and emergency room doctor. He alleged that he developed priapism after taking one dose of trazodone for disordered sleep. He subsequently became impotent.
Christopher T. Rhodes, PhD, a professor of pharmaceutics at the University of Rhode Island, was an expert witness in that 2000 trial. According to Dr. Rhodes, court testimony revealed that the ER physician had not informed the patient about the possibility of priapism or about the need to obtain emergency treatment for a sustained erection. Dr. Rhodes adds that the pharmacy handout for trazodone did not list priapism as a possible adverse effect.
The court ruled in favor of the patient, judging that the “quality of advice” was inadequate. The patient was awarded an unspecified sum.
Despite its association with priapism, trazodone is used frequently in men and is a popular medication for disordered sleep. Nierenberg et al demonstrated improved sleep in 67% of depressed patients with insomnia who received trazodone either for depression or disordered sleep.8
When prescribing a priapism-causing agent, make sure the patient understands that erectile effects—though rare—can occur. Consider giving patients an informed consent form explaining the association between psychotropics and priapism and the potential long-term health implications (Box 1). Include the form in the patient’s record for documentation in the event of a malpractice lawsuit (Box 2).
FURTHER TREATMENT: Learning how to cope
Self-hypnosis/relaxation therapy was initiated to address Mr. Q’s anxiety and insomnia. The patient quickly learned the hypnosis techniques and his anxiety/insomnia symptoms began to resolve almost immediately.
Mr. Q’s priapism resolved spontaneously with no apparent erectile dysfunction. He was referred back to the university health service and has been in apparent good health since.
Related resources
- Sleepnet.com. Information on sleep disorders and sleep hygiene. http://www.sleepnet.com/
- National Center on Sleep Disorders Research http://www.nhlbi.nih.gov/about/ncsdr/
Drug brand names
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Labetalol • Trandate
- Levomepromazine • Nozinan
- Mesoridazine • Serentil
- Metoclopramide • Reglan
- Molindone • Lidone
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Prazosin • Minipress
- Promazine • Sparine
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sildenafil citrate • Viagra
- Thioridazine • Mellaril
- Thiothixene • Navane
- Trazodone • Desyrel
Disclosure
Dr. Freed reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Muskin receives research/grant support from Bristol-Myers Squibb Co., is a speaker for and consultant to Bristol-Myers Squibb Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, and Pfizer Inc.; and is a speaker for Cephalon Inc. and Eli Lilly and Co.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. CNS Drugs 1998;9:371-9.
2. Rhodes CT. Trazodone and priapism—implications for responses to adverse events. Clin Res Regulatory Affairs 2001;18:47-52.
3. Compton MT, Miller AH. Priapism associated with conventional and atypical antipsychotic medications: a review. J Clin Psychiatry 2001;62:362-6.
4. Thompson JW, Ware MR, Blashfield RK. Psychotropic medication and priapism: a comprehensive review. J Clin Psychiatry 1990;51:430-3.
5. Reeves RR, Kimble R. Prolonged erections associated with ziprasidone treatment: a case report. J Clin Psychiatry 2003;64:97-8.
6. Sur RL, Kane CJ. Sildenafil citrate-associated priapism. Urology 2000;55:950.-
7. Warner MD, Peabody CA, Whiteford HA, Hollister LE. Trazodone and priapism. J Clin Psychiatry 1987;48:244-5.
8. Nierenberg AA, Adler LA, Peselow E, et al. Trazodone for antidepressant-associated insomnia. Am J Psychiatry 1994;151:1069-72.
1. Weiner DM, Lowe FC. Psychotropic drug-induced priapism. CNS Drugs 1998;9:371-9.
2. Rhodes CT. Trazodone and priapism—implications for responses to adverse events. Clin Res Regulatory Affairs 2001;18:47-52.
3. Compton MT, Miller AH. Priapism associated with conventional and atypical antipsychotic medications: a review. J Clin Psychiatry 2001;62:362-6.
4. Thompson JW, Ware MR, Blashfield RK. Psychotropic medication and priapism: a comprehensive review. J Clin Psychiatry 1990;51:430-3.
5. Reeves RR, Kimble R. Prolonged erections associated with ziprasidone treatment: a case report. J Clin Psychiatry 2003;64:97-8.
6. Sur RL, Kane CJ. Sildenafil citrate-associated priapism. Urology 2000;55:950.-
7. Warner MD, Peabody CA, Whiteford HA, Hollister LE. Trazodone and priapism. J Clin Psychiatry 1987;48:244-5.
8. Nierenberg AA, Adler LA, Peselow E, et al. Trazodone for antidepressant-associated insomnia. Am J Psychiatry 1994;151:1069-72.
Cyber self-help
Patients who dread the stigma of in-person psychotherapy are substituting traditional “couch trips” with computer sessions.
Computer-based therapy programs, either online or on CD-ROM or DVD, have become popular adjuncts to traditional therapy for patients with mild depression or anxiety. For example, more than 17,000 users visited the MoodGYM site within 6 months, and more than 20% of these users stayed on the site for 16 minutes or more.1
Computerized psychotherapy has demonstrated numerous benefits in clinical studies and may reduce the time a therapist needs to spend with the patient.
How computer therapy works
Computer-based psychotherapy has its roots in the ELIZA2 program developed in 1966 to study natural language communication between man and machine.3 Users simply write a normal sentence, and ELIZA responds appropriately.
The original ELIZA program, which works via text parsing, is limited in its ability to respond. For example, a user who types in “I feel depressed every day” may repeatedly get a response such as “Are you sure?”
Today’s programs are more sophisticated, utilizing specialized heuristic techniques and semantic databases to produce more natural responses to various expressions. Some programs even have audio and video features.
Most computer-based therapy programs employ a cognitive-behavioral treatment model, similar to that used in print workbooks. Several key concepts are presented, such as the relationship between automatic thoughts and feelings; techniques to control these thoughts are highlighted.
Many programs also use common scales to determine depression or anxiety ratings, thus helping the user choose an appropriate module.
Advantages of computer psychotherapy
Computer-based programs offer patients advantages such as:
- Increased comfort. Without the social cues and dynamics that characterize traditional psychotherapy, some patients may disclose feelings online they would feel uncomfortable sharing in person. Online therapy also is immune to the fatigue, illness, boredom, or exploitation that may occur in a relationship with a therapist.
- Flexibility. Users can work the program at home, at their convenience and pace. Responses to exercises also can be stored for future reference.
- Speed of care. Treatment is accessed with minimal delay.
- A greater sense of empowerment. Whereas patients in traditional therapy often feel dependent upon their therapist for direction, computer-based therapy encourages users to take a more active learning role by choosing where to click and how to respond. Patients feel more in control because they are helping themselves.
- Cost-effectiveness. Although price varies, some programs cost about the same as one in-person session. Most programs are sold directly to medical practices.
Disadvantages
Some patients will not benefit from self-help. Those with moderate to severe depression or anxiety may be unable to focus on the material, and inability to navigate the program can increase the patent’s despondency or anxiety. Personality type also may predict lack of response to self-help treatment.4
Patients with poor eyesight, deficient reading skills, and limited computer proficiency are not good candidates for online or CD-ROM-based therapy. Also, some computers may not be sufficiently powerful to run some programs, and not all Internet connections are fast enough to post multimedia features.
Clinical effectiveness
In 1990, Selmi et al5 compared a six-session, cognitive-behavioral therapy (CBT) course in CD-ROM with six therapist-administered CBT sessions and a control group. An experimenter assisted with computer operation, and both courses followed an identical treatment model and required homework. Patients in both treatment groups demonstrated significant improvement based on Beck Depression Inventory and Automatic Thoughts Questionnaire scores.
Each group comprised only 12 patients, most of whom were young and well-educated. Still, Selmi et al provided initial evidence of computer-based therapy’s effectiveness and these findings have been replicated in subsequent studies. A meta-analysis of 16 studies6 found that CD-ROM-based and therapist-administered CBT work equally well in clinically depressed and anxious outpatients. More studies are needed to determine optimal levels of therapist involvement.
More research also is needed to gauge the effectiveness of Internet-based therapy programs (Table). Clarke et al randomized 144 out of 299 patients in a nonprofit health maintenance organization to online therapy with the Overcoming Depression on the Internet (ODIN) program or to treatment as usual. The study demonstrated no effect for ODIN, perhaps because of severity of depression or infrequent access to the site.7
Table
Samples of computer-based psychotherapy programs
| Beating the Blues http://www.ultrasis.co.uk/products/btb/btb.html |
| BT STEPS http://www.healthtechsys.com/ivr/btsteps/btsteps.html |
| Behavioral Self-Control |
| Program for Windows http://www.behaviortherapy.com/software.htm#software |
| Calipso http://www.calipso.co.uk/mainframe.htm |
| FearFighter http://www.fearfighter.com |
| Good Days Ahead http://www.mindstreet.com |
| MoodGYM http://moodgym.anu.edu.au |
| Overcoming Depression http://www.maiw.com/main.html |
| Overcoming Depression on the Internet (ODIN) https://www.kpchr.org/feelbetter/ |
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
1. Christensen H, Griffiths K, Korten A. Web-based cognitive behavior therapy: analysis of site usage and changes in depression and anxiety scores. J Med Internet Res 2002;4(1):e3. Available at: http://www.jmir.org/2002/1/e3/index.htm. Accessed July 1, 2003.
2. ELIZA. Available at: http://www-ai.ijs.si/eliza/eliza.html. Accessed July 1, 2003.
3. ELIZA- a computer program for the study of natural language communication between man and machine. Communications of the Association for Computing Machinery 1966;9(1):35-6.Available at: http://i5.nyu.edu/~mm64/x52.9265/january1966.html. Accessed July 1, 2003.
4. Beutler LE, Engle D, Mohr D, et al. Predictors of differential response to cognitive, experiential, and self-directed psychotherapeutic procedures. J Consult Clin Psychol 1991;59:333-40.
5. Selmi PM, Klein MH, Greist JH, et al. Computer-administered cognitive-behavioral therapy for depression. Am J Psychiatry 1990;147:51-6.
6. Kaltenthaler E, Shackley P, Stevens K, et al. A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety. Health Technol Assess 2002;6(22):1-89.
7. Clarke G, Reid E, Eubanks D, et al. Overcoming Depression on the Internet (ODIN): a randomized trial of an Internet depression skills intervention program. J Med Internet Res 2002;4(3):e14.-Available at: http://www.jmir.org/2002/3/e14/index.htm. Accessed July 1, 2003.
Patients who dread the stigma of in-person psychotherapy are substituting traditional “couch trips” with computer sessions.
Computer-based therapy programs, either online or on CD-ROM or DVD, have become popular adjuncts to traditional therapy for patients with mild depression or anxiety. For example, more than 17,000 users visited the MoodGYM site within 6 months, and more than 20% of these users stayed on the site for 16 minutes or more.1
Computerized psychotherapy has demonstrated numerous benefits in clinical studies and may reduce the time a therapist needs to spend with the patient.
How computer therapy works
Computer-based psychotherapy has its roots in the ELIZA2 program developed in 1966 to study natural language communication between man and machine.3 Users simply write a normal sentence, and ELIZA responds appropriately.
The original ELIZA program, which works via text parsing, is limited in its ability to respond. For example, a user who types in “I feel depressed every day” may repeatedly get a response such as “Are you sure?”
Today’s programs are more sophisticated, utilizing specialized heuristic techniques and semantic databases to produce more natural responses to various expressions. Some programs even have audio and video features.
Most computer-based therapy programs employ a cognitive-behavioral treatment model, similar to that used in print workbooks. Several key concepts are presented, such as the relationship between automatic thoughts and feelings; techniques to control these thoughts are highlighted.
Many programs also use common scales to determine depression or anxiety ratings, thus helping the user choose an appropriate module.
Advantages of computer psychotherapy
Computer-based programs offer patients advantages such as:
- Increased comfort. Without the social cues and dynamics that characterize traditional psychotherapy, some patients may disclose feelings online they would feel uncomfortable sharing in person. Online therapy also is immune to the fatigue, illness, boredom, or exploitation that may occur in a relationship with a therapist.
- Flexibility. Users can work the program at home, at their convenience and pace. Responses to exercises also can be stored for future reference.
- Speed of care. Treatment is accessed with minimal delay.
- A greater sense of empowerment. Whereas patients in traditional therapy often feel dependent upon their therapist for direction, computer-based therapy encourages users to take a more active learning role by choosing where to click and how to respond. Patients feel more in control because they are helping themselves.
- Cost-effectiveness. Although price varies, some programs cost about the same as one in-person session. Most programs are sold directly to medical practices.
Disadvantages
Some patients will not benefit from self-help. Those with moderate to severe depression or anxiety may be unable to focus on the material, and inability to navigate the program can increase the patent’s despondency or anxiety. Personality type also may predict lack of response to self-help treatment.4
Patients with poor eyesight, deficient reading skills, and limited computer proficiency are not good candidates for online or CD-ROM-based therapy. Also, some computers may not be sufficiently powerful to run some programs, and not all Internet connections are fast enough to post multimedia features.
Clinical effectiveness
In 1990, Selmi et al5 compared a six-session, cognitive-behavioral therapy (CBT) course in CD-ROM with six therapist-administered CBT sessions and a control group. An experimenter assisted with computer operation, and both courses followed an identical treatment model and required homework. Patients in both treatment groups demonstrated significant improvement based on Beck Depression Inventory and Automatic Thoughts Questionnaire scores.
Each group comprised only 12 patients, most of whom were young and well-educated. Still, Selmi et al provided initial evidence of computer-based therapy’s effectiveness and these findings have been replicated in subsequent studies. A meta-analysis of 16 studies6 found that CD-ROM-based and therapist-administered CBT work equally well in clinically depressed and anxious outpatients. More studies are needed to determine optimal levels of therapist involvement.
More research also is needed to gauge the effectiveness of Internet-based therapy programs (Table). Clarke et al randomized 144 out of 299 patients in a nonprofit health maintenance organization to online therapy with the Overcoming Depression on the Internet (ODIN) program or to treatment as usual. The study demonstrated no effect for ODIN, perhaps because of severity of depression or infrequent access to the site.7
Table
Samples of computer-based psychotherapy programs
| Beating the Blues http://www.ultrasis.co.uk/products/btb/btb.html |
| BT STEPS http://www.healthtechsys.com/ivr/btsteps/btsteps.html |
| Behavioral Self-Control |
| Program for Windows http://www.behaviortherapy.com/software.htm#software |
| Calipso http://www.calipso.co.uk/mainframe.htm |
| FearFighter http://www.fearfighter.com |
| Good Days Ahead http://www.mindstreet.com |
| MoodGYM http://moodgym.anu.edu.au |
| Overcoming Depression http://www.maiw.com/main.html |
| Overcoming Depression on the Internet (ODIN) https://www.kpchr.org/feelbetter/ |
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Patients who dread the stigma of in-person psychotherapy are substituting traditional “couch trips” with computer sessions.
Computer-based therapy programs, either online or on CD-ROM or DVD, have become popular adjuncts to traditional therapy for patients with mild depression or anxiety. For example, more than 17,000 users visited the MoodGYM site within 6 months, and more than 20% of these users stayed on the site for 16 minutes or more.1
Computerized psychotherapy has demonstrated numerous benefits in clinical studies and may reduce the time a therapist needs to spend with the patient.
How computer therapy works
Computer-based psychotherapy has its roots in the ELIZA2 program developed in 1966 to study natural language communication between man and machine.3 Users simply write a normal sentence, and ELIZA responds appropriately.
The original ELIZA program, which works via text parsing, is limited in its ability to respond. For example, a user who types in “I feel depressed every day” may repeatedly get a response such as “Are you sure?”
Today’s programs are more sophisticated, utilizing specialized heuristic techniques and semantic databases to produce more natural responses to various expressions. Some programs even have audio and video features.
Most computer-based therapy programs employ a cognitive-behavioral treatment model, similar to that used in print workbooks. Several key concepts are presented, such as the relationship between automatic thoughts and feelings; techniques to control these thoughts are highlighted.
Many programs also use common scales to determine depression or anxiety ratings, thus helping the user choose an appropriate module.
Advantages of computer psychotherapy
Computer-based programs offer patients advantages such as:
- Increased comfort. Without the social cues and dynamics that characterize traditional psychotherapy, some patients may disclose feelings online they would feel uncomfortable sharing in person. Online therapy also is immune to the fatigue, illness, boredom, or exploitation that may occur in a relationship with a therapist.
- Flexibility. Users can work the program at home, at their convenience and pace. Responses to exercises also can be stored for future reference.
- Speed of care. Treatment is accessed with minimal delay.
- A greater sense of empowerment. Whereas patients in traditional therapy often feel dependent upon their therapist for direction, computer-based therapy encourages users to take a more active learning role by choosing where to click and how to respond. Patients feel more in control because they are helping themselves.
- Cost-effectiveness. Although price varies, some programs cost about the same as one in-person session. Most programs are sold directly to medical practices.
Disadvantages
Some patients will not benefit from self-help. Those with moderate to severe depression or anxiety may be unable to focus on the material, and inability to navigate the program can increase the patent’s despondency or anxiety. Personality type also may predict lack of response to self-help treatment.4
Patients with poor eyesight, deficient reading skills, and limited computer proficiency are not good candidates for online or CD-ROM-based therapy. Also, some computers may not be sufficiently powerful to run some programs, and not all Internet connections are fast enough to post multimedia features.
Clinical effectiveness
In 1990, Selmi et al5 compared a six-session, cognitive-behavioral therapy (CBT) course in CD-ROM with six therapist-administered CBT sessions and a control group. An experimenter assisted with computer operation, and both courses followed an identical treatment model and required homework. Patients in both treatment groups demonstrated significant improvement based on Beck Depression Inventory and Automatic Thoughts Questionnaire scores.
Each group comprised only 12 patients, most of whom were young and well-educated. Still, Selmi et al provided initial evidence of computer-based therapy’s effectiveness and these findings have been replicated in subsequent studies. A meta-analysis of 16 studies6 found that CD-ROM-based and therapist-administered CBT work equally well in clinically depressed and anxious outpatients. More studies are needed to determine optimal levels of therapist involvement.
More research also is needed to gauge the effectiveness of Internet-based therapy programs (Table). Clarke et al randomized 144 out of 299 patients in a nonprofit health maintenance organization to online therapy with the Overcoming Depression on the Internet (ODIN) program or to treatment as usual. The study demonstrated no effect for ODIN, perhaps because of severity of depression or infrequent access to the site.7
Table
Samples of computer-based psychotherapy programs
| Beating the Blues http://www.ultrasis.co.uk/products/btb/btb.html |
| BT STEPS http://www.healthtechsys.com/ivr/btsteps/btsteps.html |
| Behavioral Self-Control |
| Program for Windows http://www.behaviortherapy.com/software.htm#software |
| Calipso http://www.calipso.co.uk/mainframe.htm |
| FearFighter http://www.fearfighter.com |
| Good Days Ahead http://www.mindstreet.com |
| MoodGYM http://moodgym.anu.edu.au |
| Overcoming Depression http://www.maiw.com/main.html |
| Overcoming Depression on the Internet (ODIN) https://www.kpchr.org/feelbetter/ |
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
1. Christensen H, Griffiths K, Korten A. Web-based cognitive behavior therapy: analysis of site usage and changes in depression and anxiety scores. J Med Internet Res 2002;4(1):e3. Available at: http://www.jmir.org/2002/1/e3/index.htm. Accessed July 1, 2003.
2. ELIZA. Available at: http://www-ai.ijs.si/eliza/eliza.html. Accessed July 1, 2003.
3. ELIZA- a computer program for the study of natural language communication between man and machine. Communications of the Association for Computing Machinery 1966;9(1):35-6.Available at: http://i5.nyu.edu/~mm64/x52.9265/january1966.html. Accessed July 1, 2003.
4. Beutler LE, Engle D, Mohr D, et al. Predictors of differential response to cognitive, experiential, and self-directed psychotherapeutic procedures. J Consult Clin Psychol 1991;59:333-40.
5. Selmi PM, Klein MH, Greist JH, et al. Computer-administered cognitive-behavioral therapy for depression. Am J Psychiatry 1990;147:51-6.
6. Kaltenthaler E, Shackley P, Stevens K, et al. A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety. Health Technol Assess 2002;6(22):1-89.
7. Clarke G, Reid E, Eubanks D, et al. Overcoming Depression on the Internet (ODIN): a randomized trial of an Internet depression skills intervention program. J Med Internet Res 2002;4(3):e14.-Available at: http://www.jmir.org/2002/3/e14/index.htm. Accessed July 1, 2003.
1. Christensen H, Griffiths K, Korten A. Web-based cognitive behavior therapy: analysis of site usage and changes in depression and anxiety scores. J Med Internet Res 2002;4(1):e3. Available at: http://www.jmir.org/2002/1/e3/index.htm. Accessed July 1, 2003.
2. ELIZA. Available at: http://www-ai.ijs.si/eliza/eliza.html. Accessed July 1, 2003.
3. ELIZA- a computer program for the study of natural language communication between man and machine. Communications of the Association for Computing Machinery 1966;9(1):35-6.Available at: http://i5.nyu.edu/~mm64/x52.9265/january1966.html. Accessed July 1, 2003.
4. Beutler LE, Engle D, Mohr D, et al. Predictors of differential response to cognitive, experiential, and self-directed psychotherapeutic procedures. J Consult Clin Psychol 1991;59:333-40.
5. Selmi PM, Klein MH, Greist JH, et al. Computer-administered cognitive-behavioral therapy for depression. Am J Psychiatry 1990;147:51-6.
6. Kaltenthaler E, Shackley P, Stevens K, et al. A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety. Health Technol Assess 2002;6(22):1-89.
7. Clarke G, Reid E, Eubanks D, et al. Overcoming Depression on the Internet (ODIN): a randomized trial of an Internet depression skills intervention program. J Med Internet Res 2002;4(3):e14.-Available at: http://www.jmir.org/2002/3/e14/index.htm. Accessed July 1, 2003.
5 fundamentals of managing adult ADHD
As a psychiatrist specializing in college health, I see 40 to 50 young adults yearly with undiagnosed attention-deficit/hyperactivity disorder (ADHD). I have found that understanding five fundamentals of ADHD is key to recognizing this disorder in adults.
- There is no “adult onset” ADHD. Although ADHD may manifest itself differently in adults than in children, studies indicate that the disorder is a continuation of childhood ADHD rather than a discrete adult disorder. Clinicians thus need to establish that adult patients exhibited symptomatic and functional impairment before age 7 (as per DSM-IV), although some experts suggest preadolescence as a cutoff.1
- Most people do not “outgrow” ADHD. We once assumed that most patients with ADHD became asymptomatic as they matured from adolescence into adulthood. Research reveals that hyperactivity and impulsivity decline over time but inattention and executive dysfunction usually persist into adulthood.2 These residual deficits cause continued vocational, academic, and interpersonal difficulties.
- ADHD can mimic other psychiatric disorders. The hyperkinesis, impulsivity, and inattention that are the essence of ADHD are also commonly observed in adults with anxiety disorders, mood disorders, substance abuse problems, and learning disorders. Patients who present with atypical affective or anxiety symptoms or learning problems, or who do not respond to conventional treatments, should be screened for ADHD.
- The genetic apple does not fall far from the tree in ADHD. Many adults with ADHD are identified in middle age after their children are diagnosed. Adoption data and multiple twin studies have placed the heritability of ADHD at approximately 75%,3 putting first-degree relatives at fairly high predisposition.
- Stimulant medications do not promote substance abuse in ADHD patients. Stimulant medication is more likely to reduce the risk of substance abuse in ADHD than enhance it.4 For patients at high-risk for substance abuse disorders, however, atomoxetine and bupropion offer nonstimulant alternatives. Also, the newer, longer-acting dextroamphetamine/amphetamine and methylphenidate preparations are more difficult to abuse because of their slow-release mechanisms.
1. Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criteria for attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1997;36:1204-10.
2. Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into adulthood as a function of reporting sources and definition of disorder. J Abnorm Psychol 2002;111:279-89.
3. Sprich S, Biederman J, Crawford MH, et al. Adoptive and biological families of children and adolescents with ADHD. J Am Acad. Child Adolesc Psychiatry 2000;143:1432-7.
4. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
Dr. Anders is clinical assistant professor of psychiatry, University Health Services, University of Wisconsin, Madison.
As a psychiatrist specializing in college health, I see 40 to 50 young adults yearly with undiagnosed attention-deficit/hyperactivity disorder (ADHD). I have found that understanding five fundamentals of ADHD is key to recognizing this disorder in adults.
- There is no “adult onset” ADHD. Although ADHD may manifest itself differently in adults than in children, studies indicate that the disorder is a continuation of childhood ADHD rather than a discrete adult disorder. Clinicians thus need to establish that adult patients exhibited symptomatic and functional impairment before age 7 (as per DSM-IV), although some experts suggest preadolescence as a cutoff.1
- Most people do not “outgrow” ADHD. We once assumed that most patients with ADHD became asymptomatic as they matured from adolescence into adulthood. Research reveals that hyperactivity and impulsivity decline over time but inattention and executive dysfunction usually persist into adulthood.2 These residual deficits cause continued vocational, academic, and interpersonal difficulties.
- ADHD can mimic other psychiatric disorders. The hyperkinesis, impulsivity, and inattention that are the essence of ADHD are also commonly observed in adults with anxiety disorders, mood disorders, substance abuse problems, and learning disorders. Patients who present with atypical affective or anxiety symptoms or learning problems, or who do not respond to conventional treatments, should be screened for ADHD.
- The genetic apple does not fall far from the tree in ADHD. Many adults with ADHD are identified in middle age after their children are diagnosed. Adoption data and multiple twin studies have placed the heritability of ADHD at approximately 75%,3 putting first-degree relatives at fairly high predisposition.
- Stimulant medications do not promote substance abuse in ADHD patients. Stimulant medication is more likely to reduce the risk of substance abuse in ADHD than enhance it.4 For patients at high-risk for substance abuse disorders, however, atomoxetine and bupropion offer nonstimulant alternatives. Also, the newer, longer-acting dextroamphetamine/amphetamine and methylphenidate preparations are more difficult to abuse because of their slow-release mechanisms.
As a psychiatrist specializing in college health, I see 40 to 50 young adults yearly with undiagnosed attention-deficit/hyperactivity disorder (ADHD). I have found that understanding five fundamentals of ADHD is key to recognizing this disorder in adults.
- There is no “adult onset” ADHD. Although ADHD may manifest itself differently in adults than in children, studies indicate that the disorder is a continuation of childhood ADHD rather than a discrete adult disorder. Clinicians thus need to establish that adult patients exhibited symptomatic and functional impairment before age 7 (as per DSM-IV), although some experts suggest preadolescence as a cutoff.1
- Most people do not “outgrow” ADHD. We once assumed that most patients with ADHD became asymptomatic as they matured from adolescence into adulthood. Research reveals that hyperactivity and impulsivity decline over time but inattention and executive dysfunction usually persist into adulthood.2 These residual deficits cause continued vocational, academic, and interpersonal difficulties.
- ADHD can mimic other psychiatric disorders. The hyperkinesis, impulsivity, and inattention that are the essence of ADHD are also commonly observed in adults with anxiety disorders, mood disorders, substance abuse problems, and learning disorders. Patients who present with atypical affective or anxiety symptoms or learning problems, or who do not respond to conventional treatments, should be screened for ADHD.
- The genetic apple does not fall far from the tree in ADHD. Many adults with ADHD are identified in middle age after their children are diagnosed. Adoption data and multiple twin studies have placed the heritability of ADHD at approximately 75%,3 putting first-degree relatives at fairly high predisposition.
- Stimulant medications do not promote substance abuse in ADHD patients. Stimulant medication is more likely to reduce the risk of substance abuse in ADHD than enhance it.4 For patients at high-risk for substance abuse disorders, however, atomoxetine and bupropion offer nonstimulant alternatives. Also, the newer, longer-acting dextroamphetamine/amphetamine and methylphenidate preparations are more difficult to abuse because of their slow-release mechanisms.
1. Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criteria for attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1997;36:1204-10.
2. Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into adulthood as a function of reporting sources and definition of disorder. J Abnorm Psychol 2002;111:279-89.
3. Sprich S, Biederman J, Crawford MH, et al. Adoptive and biological families of children and adolescents with ADHD. J Am Acad. Child Adolesc Psychiatry 2000;143:1432-7.
4. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
Dr. Anders is clinical assistant professor of psychiatry, University Health Services, University of Wisconsin, Madison.
1. Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criteria for attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1997;36:1204-10.
2. Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into adulthood as a function of reporting sources and definition of disorder. J Abnorm Psychol 2002;111:279-89.
3. Sprich S, Biederman J, Crawford MH, et al. Adoptive and biological families of children and adolescents with ADHD. J Am Acad. Child Adolesc Psychiatry 2000;143:1432-7.
4. Biederman J, Wilens T, Mick E, et al. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics 1999;104:e20.-
Dr. Anders is clinical assistant professor of psychiatry, University Health Services, University of Wisconsin, Madison.
Treating bipolar disorder during pregnancy: No time for endless debate
To me, the main difference between MDs and PhDs* is that MDs—at some point—must stop gathering data and make decisions.
I once heard Dr. Albert (Mickey) Stunkard say that when he was a physician fellow at Stanford University’s Center for Advanced Studies in the Behavioral Sciences he was at first energized—and a little intimidated—by the scintillating conversations taking place around him. Eventually, though, all the discourse reminded him of those long, philosophical discussions he and his classmates had had in their college dorms (“Well, on one hand you have communism, and on the other hand you have fascism… ”).
Physicians do not have the luxury of endless debate. At some point, we need to do something or else let our patients die of old age while waiting. One issue about which I have had to make decisions over the years—and which has troubled me the most—is whether to treat pregnant patients with psychotropics. Generally, I try to avoid using drugs in these cases, but sometimes I decide that the mother’s need for drug therapy outweighs the potential risks to her offspring.
Dr. Lori Altshuler and colleagues’ article in this issue is the best summary I have seen of what is known about the risks of using psychotropics in pregnant bipolar women. Each time I treat a woman with bipolar disorder, I will remember this discussion and the algorithm these authors suggest for making therapeutic decisions.
This excellent article may not be the final word on the subject. It can, however, help us with an important clinical decision we often have to make—and that is what Current Psychiatry is all about.
To me, the main difference between MDs and PhDs* is that MDs—at some point—must stop gathering data and make decisions.
I once heard Dr. Albert (Mickey) Stunkard say that when he was a physician fellow at Stanford University’s Center for Advanced Studies in the Behavioral Sciences he was at first energized—and a little intimidated—by the scintillating conversations taking place around him. Eventually, though, all the discourse reminded him of those long, philosophical discussions he and his classmates had had in their college dorms (“Well, on one hand you have communism, and on the other hand you have fascism… ”).
Physicians do not have the luxury of endless debate. At some point, we need to do something or else let our patients die of old age while waiting. One issue about which I have had to make decisions over the years—and which has troubled me the most—is whether to treat pregnant patients with psychotropics. Generally, I try to avoid using drugs in these cases, but sometimes I decide that the mother’s need for drug therapy outweighs the potential risks to her offspring.
Dr. Lori Altshuler and colleagues’ article in this issue is the best summary I have seen of what is known about the risks of using psychotropics in pregnant bipolar women. Each time I treat a woman with bipolar disorder, I will remember this discussion and the algorithm these authors suggest for making therapeutic decisions.
This excellent article may not be the final word on the subject. It can, however, help us with an important clinical decision we often have to make—and that is what Current Psychiatry is all about.
To me, the main difference between MDs and PhDs* is that MDs—at some point—must stop gathering data and make decisions.
I once heard Dr. Albert (Mickey) Stunkard say that when he was a physician fellow at Stanford University’s Center for Advanced Studies in the Behavioral Sciences he was at first energized—and a little intimidated—by the scintillating conversations taking place around him. Eventually, though, all the discourse reminded him of those long, philosophical discussions he and his classmates had had in their college dorms (“Well, on one hand you have communism, and on the other hand you have fascism… ”).
Physicians do not have the luxury of endless debate. At some point, we need to do something or else let our patients die of old age while waiting. One issue about which I have had to make decisions over the years—and which has troubled me the most—is whether to treat pregnant patients with psychotropics. Generally, I try to avoid using drugs in these cases, but sometimes I decide that the mother’s need for drug therapy outweighs the potential risks to her offspring.
Dr. Lori Altshuler and colleagues’ article in this issue is the best summary I have seen of what is known about the risks of using psychotropics in pregnant bipolar women. Each time I treat a woman with bipolar disorder, I will remember this discussion and the algorithm these authors suggest for making therapeutic decisions.
This excellent article may not be the final word on the subject. It can, however, help us with an important clinical decision we often have to make—and that is what Current Psychiatry is all about.
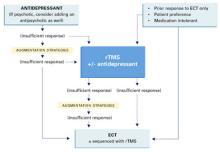
Therapy-resistant major depression The attraction of magnetism: How effective—and safe—is rTMS?
Using magnets to improve health is sometimes hawked in dubious classified ads and “infomercials.” However, a legitimate use of magnetism—repetitive transcranial magnetic stimulation (rTMS)—is showing promise in treating severe depression (Box) 1-4 and other psychiatric disorders.
Patients or their families are likely to ask psychiatrists about rTMS as more becomes known about this investigational technology. Drawing from our experience and the evidence, we offer an update on whether rTMS may be an alternative for treating depression and address issues that must be resolved before it could be used in clinical practice.
WHAT IS RTMS?
rTMS consists of a series of magnetic pulses produced by a stimulator, which can be adjusted for:
- coil type and placement
- stimulation site, intensity, frequency, and number
- amount of time between stimulations
- treatment duration.
In 1985, Barker and colleagues developed single-pulse transcranial magnetic stimulation to examine motor cortex function.1 The single-pulse mechanism they discovered was subsequently adapted to deliver repetitive pulses and is referred to as repetitive transcranial magnetic stimulation (rTMS).
How rTMS works. Transcranial magnetic stimulation uses an electromagnetic coil applied to the head to produce an intense, localized, fluctuating magnetic field that passes unimpeded into a small area of the brain, inducing an electrical current. This results in neuronal depolarization in a localized area under the coil, and possibly distal effects as well.2 During the neurophysiological studies, it was discovered that subjects also experienced a change in mood.
Antidepressant effects. Similar physiologic effects induced by rTMS, electroconvulsive therapy (ECT), and antidepressants on the endocrine system, sleep parameters, and biochemical measures suggest antidepressant properties.3 In 1993, the first published study examining rTMS in psychiatric patients reported reduced depressive symptoms in two subjects.4 Since then, several clinical trials have examined rTMS’ antidepressive effects. In 2001, Canada’s Health Ministry approved rTMS for treating major depression. In the United States, rTMS remains investigational and is FDA-approved only for clinical trials.
Coil type and placement. Initial studies involved stimulation—typically low-frequency—over the vertex, but most subsequent rTMS trials in depression have stimulated the left dorsolateral prefrontal cortex. Neuroimaging studies have shown prefrontal functioning abnormalities in depressed subjects, and it is hypothesized that stimulating this area (plus possible distal effects) may produce an antidepressant effect.5
Various configurations have been used, but circular and figure-eight-shaped coils are most common. These flat coils are made of tightly wound ferromagnetic material such as copper, enclosed in a heavy plastic cover. With the figure-eight coil, the intersection of the two loops produces the strongest magnetic field.
Stimulation site. Stimulation intensity depends on the individual’s motor threshold, and the site can be determined visually or electrophysiologically.
- With the visual method, the motor threshold over the left primary motor cortex site for the first dorsal interosseous muscle (FDI) or the abductor pollius brevis (APB) is determined by iteration. This involves placing the coil at a progression of sites and increasing stimulation intensity until reliable (in 5 of 10 stimulations) contractions are seen in the right FDI or APB.
- Similarly, the electrophysiologic method uses 5 of 10 motorevoked potentials of 50 microvolts to locate the site.
The only small trial that compared visual and electrophysiologic site determination showed similar results with both methods.6 The most common stimulation site is the left dorsolateral prefrontal cortex, 5 cm anterior and parasagittal to the FDI or APB motor cortex. Alternately, frameless stereotactic systems or the international 10-20 proportional system used in EEG labs have been recommended to target sites more accurately.
Stimulus intensity. Each individual’s motor threshold determines stimulus intensity. Using functional MRI studies, researchers from the Medical University of South Carolina concluded that higher stimulation intensity relative to the motor threshold may have a more robust effect, as the magnetic field declines with distance from the coil.7 However, intensities >120% of the motor threshold are generally avoided because of possible increased seizure risk.9
Frequency of stimulation. Most researchers apply frequencies of 1 to 20 Hz over the left dorsolateral prefrontal cortex, but also use lower frequencies (<1 Hz) over the right dorsolateral prefrontal cortex. Using higher frequencies in major depression is attractive in theory because of:
- the reported association of decreased regional cerebral blood flow with hypometabolism in the left dorsolateral prefrontal cortex
- higher-frequency stimulation’s ability to produce temporary excitation and neuronal depolarization.
Number of stimulations. The number of stimulations is determined by frequency (Hz) and stimulation train duration (for example, 10 Hz for 5 seconds equals 50 stimulations). A typical treatment session incorporates 10 to 30 stimulation trains several seconds apart (the inter-train interval). Thus, a typical session delivers 1,000 to 1,200 stimulations. In studies of unmedicated depressed patients, the total number of stimulations has varied from 8,000 to 32,000 per treatment course.
Duration between two stimulation trains. Chen et al have demonstrated that shorter (<1 second) inter-train intervals increase seizure risk with higher frequencies (such as 20 Hz) and intensities (>100% of motor threshold) of stimulation.9 Based on their studies with healthy volunteers, they recommended several “safe” ranges (such as 5 seconds at 110% of motor threshold). Most trials use 30- to 60-second inter-train intervals.
Most treatments continue 2 to 4 weeks, Monday through Friday, although more frequent treatments are being studied.
EFFICACY FOR DEPRESSION
Most studies of rTMS in depression have compared real rTMS to a sham control or electroconvulsive therapy (ECT).
In earlier studies, the sham procedure typically involved tilting the coil away from the skull. This method has been questioned, however, because of evidence of neuronal depolarization.10
More recent sham coils mimic the real coils’ sound and sensation, without magnetic stimulation.
Despite these methodologic problems and some mixed results, depressed patients receiving rTMS show more favorable results than those receiving sham rTMS.11,12 Several meta-analyses have attempted to quantify rTMS’ efficacy for depression:
- Holtzheimer et al concluded that rTMS was statistically superior to sham rTMS, but the clinical significance of these findings was modest in a population of mostly outpatients with less-severe depression.13
- Burt et al found a statistically strong antidepressant effect, but its magnitude varied and few of the studies yielded a substantial clinical response or remission. The team also noted that rTMS’ long-term efficacy or adverse effects are unknown.14
- Kozel et al concluded that left prefrontal rTMS rendered a significant antidepressant effect with measurable clinical improvement.15
- Gershon et al16 supported an antidepressant effect for rTMS when compared with sham rTMS or ECT.
Ongoing rTMS research includes subjects with many types of mild to severe psychiatric illnesses, including major depression, obsessive-compulsive disorder, and psychosis. Typically, patients referred for experimental approaches have not responded to or tolerated available treatments. Exclusion criteria used by most rTMS studies are listed in the Table.
Table
Medical conditions that preclude use of rTMS
| Serious medical conditions History of seizures Increased intracranial pressure Serious head trauma |
| Myocardial infarction within the past 6 months |
| Pregnancy or childbearing potential (unless reliable contraception is being used) |
| Intracranial metallic implants |
| Pacemakers or other implanted devices |
rTMS vs. ECT. Four randomized, controlled trials have compared rTMS with ECT for treating severely ill, often medication-resistant patients.17-20 Although their methodologies differed, all four studies concluded that rTMS and ECT offer similar efficacy, except that rTMS may be less effective for treating psychotic depression.
One study found ECT more effective than rTMS for psychotic depression, although the patients who received ECT were also treated with antipsychotics and/or antidepressants.17 Our study,19 which did not use these agents, has not corroborated this observation. Preliminary data also indicate comparable relapse rates following acute ECT and rTMS when subjects are followed on maintenance medication.21
ADVERSE EFFECTS
The potential adverse effects of new treatments must always be considered. Thus far, rTMS appears to produce minimal, relatively benign complications, including:
- mild discomfort at the stimulation site
- localized muscle twitching during stimulation
- mild post-treatment headaches—believed caused by muscle contractions—which usually respond to aspirin or acetaminophen
- treatment stimulation-related seizures (rarely).8
The rTMS device makes a loud clicking noise, and subjects wear protective ear plugs during treatment.
Patient experience. The first rTMS session—during which the patient’s motor threshold is determined—can last up to 45 minutes. Subsequent sessions are usually 15 to 20 minutes. Patients are typically apprehensive before the first session but become more relaxed with experience and tolerate the treatments easily.
During the procedure, many patients describe a tapping sensation on the forehead, and some experience slight muscle twitching around the eye or corner of the mouth. As the coil warms, the skin it touches sometimes flushes pink, although this does not seem to bother our patients. They can return to their daily routines immediately after a session.
rTMS for major depression. In our experience, rTMS may help patients with major depression. For example, one patient diagnosed with a major depressive episode with psychotic features was referred to our study comparing rTMS with ECT.19 Her depression had lasted several months, with partial response to ECT treatments. She signed informed consent and was randomly assigned to receive rTMS treatment.
At study admission, the patient’s Hamilton Depression Rating Scale (HDRS) score was 48, indicating moderate to severe depression. Following 10 rTMS sessions, her HDRS score had dropped to 2, with remission of symptoms. No follow-up results were documented.
Cognitive effects. Whereas mood disorders are associated with medication-independent neuropsychological deficits, most studies have found no adverse cognitive effects with rTMS.22 Indeed, some of our rTMS patients have improved in certain cognitive tests, although this may be explained by test-retest effects or better attention and concentration associated with mood improvement.
Figure Potential roles for rTMS in treating major depression
Solid lines represent current standards of practice. Dotted lines represent hypothetical roles for rTMS.
Source: Adapted and reprinted with permission from Dowd et al. Is repetitive transcranial magnetic stimulation an alternative to ECTfor the treatment of depression? Contemp Psychiatry 2002;1:1-10.
POTENTIAL ROLE FOR rTMS
Today’s standard treatment of major depressive episodes begins with an antidepressant (plus an antipsychotic, if necessary) and proceeds to augmentation strategies if response is insufficient. rTMS may one day become an augmentation or monotherapy option for patients who do not respond sufficiently to standard treatments (Figure).
ECT treatment may be initiated if a patient has had a prior good response to ECT, is intolerant to medication, or prefers ECT. In that case, rTMS may be used as an alternate initial treatment or with ECT. Thus, rTMS may be used:
- to augment antidepressants
- as an alternative to antidepressants or ECT
- or sequentially with ECT.
Before that can happen, however, optimal treatment parameters need to be clarified by larger, well-designed, controlled studies comparing rTMS to a valid sham treatment, antidepressants, and ECT.
Related resources
- International Society for Transcranial Stimulation. www.ists.unibe.ch/
- Repetitive Transcranial Magnetic Stimulation Research Clinic at Yale-New Haven Psychiatric Hospital.
Disclosure
The authors report that they have no proprietary interest in the technology discussed in this article.
1. Barker A, Jalinous R, Freeston I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106-7.
2. Lisanby SH, Datto CJ, Szuba MP. ECT and rTMS: past, present, and future. Depress Anxiety 2000;12:115-17.
3. Post A, Keck PE, Jr. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001;35:193-215.
4. Holfich G, Kasper S, Hufnagel A, et al. Application of transcranial magnetic stimulation in treatment of drug resistant major depression—a report of two cases. Human Psychopharmacol 1993;8:361-5.
5. George MS, Nahas Z, Speer AM, et al. Transcranial magnetic stimulation—a new method for investigating the neuroanatomy of depression. In: Ebert D, Ebmeier K (eds). New models for depression. New York: Karger, 1998;94-122.
6. Pridmore A, Americo Fernandes Filho J, Nahas Z, et al. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 1998;14(1):25-7.
7. Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;13:376-84.
8. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16.
9. Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105:415-21.
10. Loo CK, Taylor JL, Gandevia SC, et al. Transcranial magnetic stimulation in controlled treatment studies: Are some “sham” forms active? Biol Psychiatry. 2000;47:325-31.
11. George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000;48:962-70.
12. Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000;47:332-7.
13. Holtzheimer PE, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001;35:149-69.
14. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. Int J Neuropsychopharmacol 2002;5:73-103.
15. Kozel FE, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270-5.
16. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am JPsychiatry 2003;160(5):835-45.
17. Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000;47:314-24.
18. Pridmore S, Bruno R, Turnier-Shea Y, et al. Comparison of unlimited numbers of rapid transcranial magnetic stimulation and ECT treatment sessions in major depression episodes. Int J Neuropsychopharmacol 2000;3:129-34.
19. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002;51:659-67
20. Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;53:324-31.
21. Dannon PH, Dolberg OT, Schreiber S, Grunhaus L. Three and six month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biol Psychiatry 2002;15:687-90.
22. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiology (in press).
Using magnets to improve health is sometimes hawked in dubious classified ads and “infomercials.” However, a legitimate use of magnetism—repetitive transcranial magnetic stimulation (rTMS)—is showing promise in treating severe depression (Box) 1-4 and other psychiatric disorders.
Patients or their families are likely to ask psychiatrists about rTMS as more becomes known about this investigational technology. Drawing from our experience and the evidence, we offer an update on whether rTMS may be an alternative for treating depression and address issues that must be resolved before it could be used in clinical practice.
WHAT IS RTMS?
rTMS consists of a series of magnetic pulses produced by a stimulator, which can be adjusted for:
- coil type and placement
- stimulation site, intensity, frequency, and number
- amount of time between stimulations
- treatment duration.
In 1985, Barker and colleagues developed single-pulse transcranial magnetic stimulation to examine motor cortex function.1 The single-pulse mechanism they discovered was subsequently adapted to deliver repetitive pulses and is referred to as repetitive transcranial magnetic stimulation (rTMS).
How rTMS works. Transcranial magnetic stimulation uses an electromagnetic coil applied to the head to produce an intense, localized, fluctuating magnetic field that passes unimpeded into a small area of the brain, inducing an electrical current. This results in neuronal depolarization in a localized area under the coil, and possibly distal effects as well.2 During the neurophysiological studies, it was discovered that subjects also experienced a change in mood.
Antidepressant effects. Similar physiologic effects induced by rTMS, electroconvulsive therapy (ECT), and antidepressants on the endocrine system, sleep parameters, and biochemical measures suggest antidepressant properties.3 In 1993, the first published study examining rTMS in psychiatric patients reported reduced depressive symptoms in two subjects.4 Since then, several clinical trials have examined rTMS’ antidepressive effects. In 2001, Canada’s Health Ministry approved rTMS for treating major depression. In the United States, rTMS remains investigational and is FDA-approved only for clinical trials.
Coil type and placement. Initial studies involved stimulation—typically low-frequency—over the vertex, but most subsequent rTMS trials in depression have stimulated the left dorsolateral prefrontal cortex. Neuroimaging studies have shown prefrontal functioning abnormalities in depressed subjects, and it is hypothesized that stimulating this area (plus possible distal effects) may produce an antidepressant effect.5
Various configurations have been used, but circular and figure-eight-shaped coils are most common. These flat coils are made of tightly wound ferromagnetic material such as copper, enclosed in a heavy plastic cover. With the figure-eight coil, the intersection of the two loops produces the strongest magnetic field.
Stimulation site. Stimulation intensity depends on the individual’s motor threshold, and the site can be determined visually or electrophysiologically.
- With the visual method, the motor threshold over the left primary motor cortex site for the first dorsal interosseous muscle (FDI) or the abductor pollius brevis (APB) is determined by iteration. This involves placing the coil at a progression of sites and increasing stimulation intensity until reliable (in 5 of 10 stimulations) contractions are seen in the right FDI or APB.
- Similarly, the electrophysiologic method uses 5 of 10 motorevoked potentials of 50 microvolts to locate the site.
The only small trial that compared visual and electrophysiologic site determination showed similar results with both methods.6 The most common stimulation site is the left dorsolateral prefrontal cortex, 5 cm anterior and parasagittal to the FDI or APB motor cortex. Alternately, frameless stereotactic systems or the international 10-20 proportional system used in EEG labs have been recommended to target sites more accurately.
Stimulus intensity. Each individual’s motor threshold determines stimulus intensity. Using functional MRI studies, researchers from the Medical University of South Carolina concluded that higher stimulation intensity relative to the motor threshold may have a more robust effect, as the magnetic field declines with distance from the coil.7 However, intensities >120% of the motor threshold are generally avoided because of possible increased seizure risk.9
Frequency of stimulation. Most researchers apply frequencies of 1 to 20 Hz over the left dorsolateral prefrontal cortex, but also use lower frequencies (<1 Hz) over the right dorsolateral prefrontal cortex. Using higher frequencies in major depression is attractive in theory because of:
- the reported association of decreased regional cerebral blood flow with hypometabolism in the left dorsolateral prefrontal cortex
- higher-frequency stimulation’s ability to produce temporary excitation and neuronal depolarization.
Number of stimulations. The number of stimulations is determined by frequency (Hz) and stimulation train duration (for example, 10 Hz for 5 seconds equals 50 stimulations). A typical treatment session incorporates 10 to 30 stimulation trains several seconds apart (the inter-train interval). Thus, a typical session delivers 1,000 to 1,200 stimulations. In studies of unmedicated depressed patients, the total number of stimulations has varied from 8,000 to 32,000 per treatment course.
Duration between two stimulation trains. Chen et al have demonstrated that shorter (<1 second) inter-train intervals increase seizure risk with higher frequencies (such as 20 Hz) and intensities (>100% of motor threshold) of stimulation.9 Based on their studies with healthy volunteers, they recommended several “safe” ranges (such as 5 seconds at 110% of motor threshold). Most trials use 30- to 60-second inter-train intervals.
Most treatments continue 2 to 4 weeks, Monday through Friday, although more frequent treatments are being studied.
EFFICACY FOR DEPRESSION
Most studies of rTMS in depression have compared real rTMS to a sham control or electroconvulsive therapy (ECT).
In earlier studies, the sham procedure typically involved tilting the coil away from the skull. This method has been questioned, however, because of evidence of neuronal depolarization.10
More recent sham coils mimic the real coils’ sound and sensation, without magnetic stimulation.
Despite these methodologic problems and some mixed results, depressed patients receiving rTMS show more favorable results than those receiving sham rTMS.11,12 Several meta-analyses have attempted to quantify rTMS’ efficacy for depression:
- Holtzheimer et al concluded that rTMS was statistically superior to sham rTMS, but the clinical significance of these findings was modest in a population of mostly outpatients with less-severe depression.13
- Burt et al found a statistically strong antidepressant effect, but its magnitude varied and few of the studies yielded a substantial clinical response or remission. The team also noted that rTMS’ long-term efficacy or adverse effects are unknown.14
- Kozel et al concluded that left prefrontal rTMS rendered a significant antidepressant effect with measurable clinical improvement.15
- Gershon et al16 supported an antidepressant effect for rTMS when compared with sham rTMS or ECT.
Ongoing rTMS research includes subjects with many types of mild to severe psychiatric illnesses, including major depression, obsessive-compulsive disorder, and psychosis. Typically, patients referred for experimental approaches have not responded to or tolerated available treatments. Exclusion criteria used by most rTMS studies are listed in the Table.
Table
Medical conditions that preclude use of rTMS
| Serious medical conditions History of seizures Increased intracranial pressure Serious head trauma |
| Myocardial infarction within the past 6 months |
| Pregnancy or childbearing potential (unless reliable contraception is being used) |
| Intracranial metallic implants |
| Pacemakers or other implanted devices |
rTMS vs. ECT. Four randomized, controlled trials have compared rTMS with ECT for treating severely ill, often medication-resistant patients.17-20 Although their methodologies differed, all four studies concluded that rTMS and ECT offer similar efficacy, except that rTMS may be less effective for treating psychotic depression.
One study found ECT more effective than rTMS for psychotic depression, although the patients who received ECT were also treated with antipsychotics and/or antidepressants.17 Our study,19 which did not use these agents, has not corroborated this observation. Preliminary data also indicate comparable relapse rates following acute ECT and rTMS when subjects are followed on maintenance medication.21
ADVERSE EFFECTS
The potential adverse effects of new treatments must always be considered. Thus far, rTMS appears to produce minimal, relatively benign complications, including:
- mild discomfort at the stimulation site
- localized muscle twitching during stimulation
- mild post-treatment headaches—believed caused by muscle contractions—which usually respond to aspirin or acetaminophen
- treatment stimulation-related seizures (rarely).8
The rTMS device makes a loud clicking noise, and subjects wear protective ear plugs during treatment.
Patient experience. The first rTMS session—during which the patient’s motor threshold is determined—can last up to 45 minutes. Subsequent sessions are usually 15 to 20 minutes. Patients are typically apprehensive before the first session but become more relaxed with experience and tolerate the treatments easily.
During the procedure, many patients describe a tapping sensation on the forehead, and some experience slight muscle twitching around the eye or corner of the mouth. As the coil warms, the skin it touches sometimes flushes pink, although this does not seem to bother our patients. They can return to their daily routines immediately after a session.
rTMS for major depression. In our experience, rTMS may help patients with major depression. For example, one patient diagnosed with a major depressive episode with psychotic features was referred to our study comparing rTMS with ECT.19 Her depression had lasted several months, with partial response to ECT treatments. She signed informed consent and was randomly assigned to receive rTMS treatment.
At study admission, the patient’s Hamilton Depression Rating Scale (HDRS) score was 48, indicating moderate to severe depression. Following 10 rTMS sessions, her HDRS score had dropped to 2, with remission of symptoms. No follow-up results were documented.
Cognitive effects. Whereas mood disorders are associated with medication-independent neuropsychological deficits, most studies have found no adverse cognitive effects with rTMS.22 Indeed, some of our rTMS patients have improved in certain cognitive tests, although this may be explained by test-retest effects or better attention and concentration associated with mood improvement.
Figure Potential roles for rTMS in treating major depression
Solid lines represent current standards of practice. Dotted lines represent hypothetical roles for rTMS.
Source: Adapted and reprinted with permission from Dowd et al. Is repetitive transcranial magnetic stimulation an alternative to ECTfor the treatment of depression? Contemp Psychiatry 2002;1:1-10.
POTENTIAL ROLE FOR rTMS
Today’s standard treatment of major depressive episodes begins with an antidepressant (plus an antipsychotic, if necessary) and proceeds to augmentation strategies if response is insufficient. rTMS may one day become an augmentation or monotherapy option for patients who do not respond sufficiently to standard treatments (Figure).
ECT treatment may be initiated if a patient has had a prior good response to ECT, is intolerant to medication, or prefers ECT. In that case, rTMS may be used as an alternate initial treatment or with ECT. Thus, rTMS may be used:
- to augment antidepressants
- as an alternative to antidepressants or ECT
- or sequentially with ECT.
Before that can happen, however, optimal treatment parameters need to be clarified by larger, well-designed, controlled studies comparing rTMS to a valid sham treatment, antidepressants, and ECT.
Related resources
- International Society for Transcranial Stimulation. www.ists.unibe.ch/
- Repetitive Transcranial Magnetic Stimulation Research Clinic at Yale-New Haven Psychiatric Hospital.
Disclosure
The authors report that they have no proprietary interest in the technology discussed in this article.
Using magnets to improve health is sometimes hawked in dubious classified ads and “infomercials.” However, a legitimate use of magnetism—repetitive transcranial magnetic stimulation (rTMS)—is showing promise in treating severe depression (Box) 1-4 and other psychiatric disorders.
Patients or their families are likely to ask psychiatrists about rTMS as more becomes known about this investigational technology. Drawing from our experience and the evidence, we offer an update on whether rTMS may be an alternative for treating depression and address issues that must be resolved before it could be used in clinical practice.
WHAT IS RTMS?
rTMS consists of a series of magnetic pulses produced by a stimulator, which can be adjusted for:
- coil type and placement
- stimulation site, intensity, frequency, and number
- amount of time between stimulations
- treatment duration.
In 1985, Barker and colleagues developed single-pulse transcranial magnetic stimulation to examine motor cortex function.1 The single-pulse mechanism they discovered was subsequently adapted to deliver repetitive pulses and is referred to as repetitive transcranial magnetic stimulation (rTMS).
How rTMS works. Transcranial magnetic stimulation uses an electromagnetic coil applied to the head to produce an intense, localized, fluctuating magnetic field that passes unimpeded into a small area of the brain, inducing an electrical current. This results in neuronal depolarization in a localized area under the coil, and possibly distal effects as well.2 During the neurophysiological studies, it was discovered that subjects also experienced a change in mood.
Antidepressant effects. Similar physiologic effects induced by rTMS, electroconvulsive therapy (ECT), and antidepressants on the endocrine system, sleep parameters, and biochemical measures suggest antidepressant properties.3 In 1993, the first published study examining rTMS in psychiatric patients reported reduced depressive symptoms in two subjects.4 Since then, several clinical trials have examined rTMS’ antidepressive effects. In 2001, Canada’s Health Ministry approved rTMS for treating major depression. In the United States, rTMS remains investigational and is FDA-approved only for clinical trials.
Coil type and placement. Initial studies involved stimulation—typically low-frequency—over the vertex, but most subsequent rTMS trials in depression have stimulated the left dorsolateral prefrontal cortex. Neuroimaging studies have shown prefrontal functioning abnormalities in depressed subjects, and it is hypothesized that stimulating this area (plus possible distal effects) may produce an antidepressant effect.5
Various configurations have been used, but circular and figure-eight-shaped coils are most common. These flat coils are made of tightly wound ferromagnetic material such as copper, enclosed in a heavy plastic cover. With the figure-eight coil, the intersection of the two loops produces the strongest magnetic field.
Stimulation site. Stimulation intensity depends on the individual’s motor threshold, and the site can be determined visually or electrophysiologically.
- With the visual method, the motor threshold over the left primary motor cortex site for the first dorsal interosseous muscle (FDI) or the abductor pollius brevis (APB) is determined by iteration. This involves placing the coil at a progression of sites and increasing stimulation intensity until reliable (in 5 of 10 stimulations) contractions are seen in the right FDI or APB.
- Similarly, the electrophysiologic method uses 5 of 10 motorevoked potentials of 50 microvolts to locate the site.
The only small trial that compared visual and electrophysiologic site determination showed similar results with both methods.6 The most common stimulation site is the left dorsolateral prefrontal cortex, 5 cm anterior and parasagittal to the FDI or APB motor cortex. Alternately, frameless stereotactic systems or the international 10-20 proportional system used in EEG labs have been recommended to target sites more accurately.
Stimulus intensity. Each individual’s motor threshold determines stimulus intensity. Using functional MRI studies, researchers from the Medical University of South Carolina concluded that higher stimulation intensity relative to the motor threshold may have a more robust effect, as the magnetic field declines with distance from the coil.7 However, intensities >120% of the motor threshold are generally avoided because of possible increased seizure risk.9
Frequency of stimulation. Most researchers apply frequencies of 1 to 20 Hz over the left dorsolateral prefrontal cortex, but also use lower frequencies (<1 Hz) over the right dorsolateral prefrontal cortex. Using higher frequencies in major depression is attractive in theory because of:
- the reported association of decreased regional cerebral blood flow with hypometabolism in the left dorsolateral prefrontal cortex
- higher-frequency stimulation’s ability to produce temporary excitation and neuronal depolarization.
Number of stimulations. The number of stimulations is determined by frequency (Hz) and stimulation train duration (for example, 10 Hz for 5 seconds equals 50 stimulations). A typical treatment session incorporates 10 to 30 stimulation trains several seconds apart (the inter-train interval). Thus, a typical session delivers 1,000 to 1,200 stimulations. In studies of unmedicated depressed patients, the total number of stimulations has varied from 8,000 to 32,000 per treatment course.
Duration between two stimulation trains. Chen et al have demonstrated that shorter (<1 second) inter-train intervals increase seizure risk with higher frequencies (such as 20 Hz) and intensities (>100% of motor threshold) of stimulation.9 Based on their studies with healthy volunteers, they recommended several “safe” ranges (such as 5 seconds at 110% of motor threshold). Most trials use 30- to 60-second inter-train intervals.
Most treatments continue 2 to 4 weeks, Monday through Friday, although more frequent treatments are being studied.
EFFICACY FOR DEPRESSION
Most studies of rTMS in depression have compared real rTMS to a sham control or electroconvulsive therapy (ECT).
In earlier studies, the sham procedure typically involved tilting the coil away from the skull. This method has been questioned, however, because of evidence of neuronal depolarization.10
More recent sham coils mimic the real coils’ sound and sensation, without magnetic stimulation.
Despite these methodologic problems and some mixed results, depressed patients receiving rTMS show more favorable results than those receiving sham rTMS.11,12 Several meta-analyses have attempted to quantify rTMS’ efficacy for depression:
- Holtzheimer et al concluded that rTMS was statistically superior to sham rTMS, but the clinical significance of these findings was modest in a population of mostly outpatients with less-severe depression.13
- Burt et al found a statistically strong antidepressant effect, but its magnitude varied and few of the studies yielded a substantial clinical response or remission. The team also noted that rTMS’ long-term efficacy or adverse effects are unknown.14
- Kozel et al concluded that left prefrontal rTMS rendered a significant antidepressant effect with measurable clinical improvement.15
- Gershon et al16 supported an antidepressant effect for rTMS when compared with sham rTMS or ECT.
Ongoing rTMS research includes subjects with many types of mild to severe psychiatric illnesses, including major depression, obsessive-compulsive disorder, and psychosis. Typically, patients referred for experimental approaches have not responded to or tolerated available treatments. Exclusion criteria used by most rTMS studies are listed in the Table.
Table
Medical conditions that preclude use of rTMS
| Serious medical conditions History of seizures Increased intracranial pressure Serious head trauma |
| Myocardial infarction within the past 6 months |
| Pregnancy or childbearing potential (unless reliable contraception is being used) |
| Intracranial metallic implants |
| Pacemakers or other implanted devices |
rTMS vs. ECT. Four randomized, controlled trials have compared rTMS with ECT for treating severely ill, often medication-resistant patients.17-20 Although their methodologies differed, all four studies concluded that rTMS and ECT offer similar efficacy, except that rTMS may be less effective for treating psychotic depression.
One study found ECT more effective than rTMS for psychotic depression, although the patients who received ECT were also treated with antipsychotics and/or antidepressants.17 Our study,19 which did not use these agents, has not corroborated this observation. Preliminary data also indicate comparable relapse rates following acute ECT and rTMS when subjects are followed on maintenance medication.21
ADVERSE EFFECTS
The potential adverse effects of new treatments must always be considered. Thus far, rTMS appears to produce minimal, relatively benign complications, including:
- mild discomfort at the stimulation site
- localized muscle twitching during stimulation
- mild post-treatment headaches—believed caused by muscle contractions—which usually respond to aspirin or acetaminophen
- treatment stimulation-related seizures (rarely).8
The rTMS device makes a loud clicking noise, and subjects wear protective ear plugs during treatment.
Patient experience. The first rTMS session—during which the patient’s motor threshold is determined—can last up to 45 minutes. Subsequent sessions are usually 15 to 20 minutes. Patients are typically apprehensive before the first session but become more relaxed with experience and tolerate the treatments easily.
During the procedure, many patients describe a tapping sensation on the forehead, and some experience slight muscle twitching around the eye or corner of the mouth. As the coil warms, the skin it touches sometimes flushes pink, although this does not seem to bother our patients. They can return to their daily routines immediately after a session.
rTMS for major depression. In our experience, rTMS may help patients with major depression. For example, one patient diagnosed with a major depressive episode with psychotic features was referred to our study comparing rTMS with ECT.19 Her depression had lasted several months, with partial response to ECT treatments. She signed informed consent and was randomly assigned to receive rTMS treatment.
At study admission, the patient’s Hamilton Depression Rating Scale (HDRS) score was 48, indicating moderate to severe depression. Following 10 rTMS sessions, her HDRS score had dropped to 2, with remission of symptoms. No follow-up results were documented.
Cognitive effects. Whereas mood disorders are associated with medication-independent neuropsychological deficits, most studies have found no adverse cognitive effects with rTMS.22 Indeed, some of our rTMS patients have improved in certain cognitive tests, although this may be explained by test-retest effects or better attention and concentration associated with mood improvement.
Figure Potential roles for rTMS in treating major depression
Solid lines represent current standards of practice. Dotted lines represent hypothetical roles for rTMS.
Source: Adapted and reprinted with permission from Dowd et al. Is repetitive transcranial magnetic stimulation an alternative to ECTfor the treatment of depression? Contemp Psychiatry 2002;1:1-10.
POTENTIAL ROLE FOR rTMS
Today’s standard treatment of major depressive episodes begins with an antidepressant (plus an antipsychotic, if necessary) and proceeds to augmentation strategies if response is insufficient. rTMS may one day become an augmentation or monotherapy option for patients who do not respond sufficiently to standard treatments (Figure).
ECT treatment may be initiated if a patient has had a prior good response to ECT, is intolerant to medication, or prefers ECT. In that case, rTMS may be used as an alternate initial treatment or with ECT. Thus, rTMS may be used:
- to augment antidepressants
- as an alternative to antidepressants or ECT
- or sequentially with ECT.
Before that can happen, however, optimal treatment parameters need to be clarified by larger, well-designed, controlled studies comparing rTMS to a valid sham treatment, antidepressants, and ECT.
Related resources
- International Society for Transcranial Stimulation. www.ists.unibe.ch/
- Repetitive Transcranial Magnetic Stimulation Research Clinic at Yale-New Haven Psychiatric Hospital.
Disclosure
The authors report that they have no proprietary interest in the technology discussed in this article.
1. Barker A, Jalinous R, Freeston I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106-7.
2. Lisanby SH, Datto CJ, Szuba MP. ECT and rTMS: past, present, and future. Depress Anxiety 2000;12:115-17.
3. Post A, Keck PE, Jr. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001;35:193-215.
4. Holfich G, Kasper S, Hufnagel A, et al. Application of transcranial magnetic stimulation in treatment of drug resistant major depression—a report of two cases. Human Psychopharmacol 1993;8:361-5.
5. George MS, Nahas Z, Speer AM, et al. Transcranial magnetic stimulation—a new method for investigating the neuroanatomy of depression. In: Ebert D, Ebmeier K (eds). New models for depression. New York: Karger, 1998;94-122.
6. Pridmore A, Americo Fernandes Filho J, Nahas Z, et al. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 1998;14(1):25-7.
7. Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;13:376-84.
8. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16.
9. Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105:415-21.
10. Loo CK, Taylor JL, Gandevia SC, et al. Transcranial magnetic stimulation in controlled treatment studies: Are some “sham” forms active? Biol Psychiatry. 2000;47:325-31.
11. George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000;48:962-70.
12. Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000;47:332-7.
13. Holtzheimer PE, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001;35:149-69.
14. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. Int J Neuropsychopharmacol 2002;5:73-103.
15. Kozel FE, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270-5.
16. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am JPsychiatry 2003;160(5):835-45.
17. Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000;47:314-24.
18. Pridmore S, Bruno R, Turnier-Shea Y, et al. Comparison of unlimited numbers of rapid transcranial magnetic stimulation and ECT treatment sessions in major depression episodes. Int J Neuropsychopharmacol 2000;3:129-34.
19. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002;51:659-67
20. Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;53:324-31.
21. Dannon PH, Dolberg OT, Schreiber S, Grunhaus L. Three and six month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biol Psychiatry 2002;15:687-90.
22. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiology (in press).
1. Barker A, Jalinous R, Freeston I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106-7.
2. Lisanby SH, Datto CJ, Szuba MP. ECT and rTMS: past, present, and future. Depress Anxiety 2000;12:115-17.
3. Post A, Keck PE, Jr. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001;35:193-215.
4. Holfich G, Kasper S, Hufnagel A, et al. Application of transcranial magnetic stimulation in treatment of drug resistant major depression—a report of two cases. Human Psychopharmacol 1993;8:361-5.
5. George MS, Nahas Z, Speer AM, et al. Transcranial magnetic stimulation—a new method for investigating the neuroanatomy of depression. In: Ebert D, Ebmeier K (eds). New models for depression. New York: Karger, 1998;94-122.
6. Pridmore A, Americo Fernandes Filho J, Nahas Z, et al. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 1998;14(1):25-7.
7. Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;13:376-84.
8. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16.
9. Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105:415-21.
10. Loo CK, Taylor JL, Gandevia SC, et al. Transcranial magnetic stimulation in controlled treatment studies: Are some “sham” forms active? Biol Psychiatry. 2000;47:325-31.
11. George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000;48:962-70.
12. Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000;47:332-7.
13. Holtzheimer PE, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001;35:149-69.
14. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. Int J Neuropsychopharmacol 2002;5:73-103.
15. Kozel FE, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270-5.
16. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am JPsychiatry 2003;160(5):835-45.
17. Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000;47:314-24.
18. Pridmore S, Bruno R, Turnier-Shea Y, et al. Comparison of unlimited numbers of rapid transcranial magnetic stimulation and ECT treatment sessions in major depression episodes. Int J Neuropsychopharmacol 2000;3:129-34.
19. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002;51:659-67
20. Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;53:324-31.
21. Dannon PH, Dolberg OT, Schreiber S, Grunhaus L. Three and six month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biol Psychiatry 2002;15:687-90.
22. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiology (in press).
Writing in the palm of your hand
Psyber Psychiatry, February. A few voice command programs also are available for Pocket PC devices ARTrecognition, VoiceLookup, PDsay, and VoiceContact allow you to use voice commands to lookup information and launch tasks.
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure:
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Psyber Psychiatry, February. A few voice command programs also are available for Pocket PC devices ARTrecognition, VoiceLookup, PDsay, and VoiceContact allow you to use voice commands to lookup information and launch tasks.
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure:
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Psyber Psychiatry, February. A few voice command programs also are available for Pocket PC devices ARTrecognition, VoiceLookup, PDsay, and VoiceContact allow you to use voice commands to lookup information and launch tasks.
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Disclosure:
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Taking the ‘ouch’ out of IM antipsychotics
Long-acting intramuscular (IM) antipsychotics are necessary for patients who do not respond to—or comply with—oral medication regimens. We can make these injections less painful, provided that agitation does not complicate treatment.
In 25 years of practice, I’ve discovered the following ways to diminish the pain of injection:
- Inject into the deltoid’s posterior aspect. Nociceptive pain fibers may be less dense in the posterior versus the anterior deltoid. I try to inject latitudinally about 1 cm behind the deltoid midline and longitudinally about 5 cm below the acromioclavicular joint.
- Inject into the lateral gluteusto avoid stimulating the sciatic nerve that runs down the medial gluteus.
- Have the patient fold his or her arm across the lap.Muscles that are relaxed before injection are less likely to hurt afterward. The arm’s flexed position will help relax the deltoid.
- Massage the muscle area overlying the injection sitefor about 10 seconds before injecting. This further relaxes the muscle.
- Inject slowly—about 30 seconds per cc. A faster injection can increase pain.
- Inject air into the vial before withdrawing. Commonly used injectable psychiatric drugs are based in sesame oil. Withdrawing these viscous medications through the perforation site can be difficult if the vial is partially evacuated and the remaining fluid is under negative atmospheric pressure.
Some clinicians use the “Z technique” to prevent backflow when injecting IM antipsychotics. With this method, skin and subcutaneous tissue are retracted to avoid creating a straight-line needle tract that would allow the ready backflow of injected material.
I feel this method is unnecessary for decanoate preparations; they are viscous enough to prevent significant backflow provided the injection is slowly administered.
To IM or not to IM
Where possible, administering medications subcutaneously instead of intramuscularly can also reduce pain.
Contrary to popular belief, fluphenazine decanoate can be administered subcutaneously, using a 5/8-inch, 22-gauge needle for patients who fear long needles or are exquisitely sensitive to pain. IM administration is required for haloperidol decanoate, however.
50 vs. 100 mg/cc
Choice of preparation can also promote post-injection comfort. I have heard patients occasionally complain of lingering muscular discomfort after receiving the 100 mg/cc haloperidol decanoate preparation, but I have never heard such complaints after administering haloperidol, 50 mg/cc, or fluphenazine, 25 mg/cc.
Drug brand names
- Fluphenazine • Prolixin
- Haloperidol • Haldol
Dr. Fleishman is a staff psychiatrist at St. Francis Memorial Hospital, San Francisco, CA.
Long-acting intramuscular (IM) antipsychotics are necessary for patients who do not respond to—or comply with—oral medication regimens. We can make these injections less painful, provided that agitation does not complicate treatment.
In 25 years of practice, I’ve discovered the following ways to diminish the pain of injection:
- Inject into the deltoid’s posterior aspect. Nociceptive pain fibers may be less dense in the posterior versus the anterior deltoid. I try to inject latitudinally about 1 cm behind the deltoid midline and longitudinally about 5 cm below the acromioclavicular joint.
- Inject into the lateral gluteusto avoid stimulating the sciatic nerve that runs down the medial gluteus.
- Have the patient fold his or her arm across the lap.Muscles that are relaxed before injection are less likely to hurt afterward. The arm’s flexed position will help relax the deltoid.
- Massage the muscle area overlying the injection sitefor about 10 seconds before injecting. This further relaxes the muscle.
- Inject slowly—about 30 seconds per cc. A faster injection can increase pain.
- Inject air into the vial before withdrawing. Commonly used injectable psychiatric drugs are based in sesame oil. Withdrawing these viscous medications through the perforation site can be difficult if the vial is partially evacuated and the remaining fluid is under negative atmospheric pressure.
Some clinicians use the “Z technique” to prevent backflow when injecting IM antipsychotics. With this method, skin and subcutaneous tissue are retracted to avoid creating a straight-line needle tract that would allow the ready backflow of injected material.
I feel this method is unnecessary for decanoate preparations; they are viscous enough to prevent significant backflow provided the injection is slowly administered.
To IM or not to IM
Where possible, administering medications subcutaneously instead of intramuscularly can also reduce pain.
Contrary to popular belief, fluphenazine decanoate can be administered subcutaneously, using a 5/8-inch, 22-gauge needle for patients who fear long needles or are exquisitely sensitive to pain. IM administration is required for haloperidol decanoate, however.
50 vs. 100 mg/cc
Choice of preparation can also promote post-injection comfort. I have heard patients occasionally complain of lingering muscular discomfort after receiving the 100 mg/cc haloperidol decanoate preparation, but I have never heard such complaints after administering haloperidol, 50 mg/cc, or fluphenazine, 25 mg/cc.
Drug brand names
- Fluphenazine • Prolixin
- Haloperidol • Haldol
Long-acting intramuscular (IM) antipsychotics are necessary for patients who do not respond to—or comply with—oral medication regimens. We can make these injections less painful, provided that agitation does not complicate treatment.
In 25 years of practice, I’ve discovered the following ways to diminish the pain of injection:
- Inject into the deltoid’s posterior aspect. Nociceptive pain fibers may be less dense in the posterior versus the anterior deltoid. I try to inject latitudinally about 1 cm behind the deltoid midline and longitudinally about 5 cm below the acromioclavicular joint.
- Inject into the lateral gluteusto avoid stimulating the sciatic nerve that runs down the medial gluteus.
- Have the patient fold his or her arm across the lap.Muscles that are relaxed before injection are less likely to hurt afterward. The arm’s flexed position will help relax the deltoid.
- Massage the muscle area overlying the injection sitefor about 10 seconds before injecting. This further relaxes the muscle.
- Inject slowly—about 30 seconds per cc. A faster injection can increase pain.
- Inject air into the vial before withdrawing. Commonly used injectable psychiatric drugs are based in sesame oil. Withdrawing these viscous medications through the perforation site can be difficult if the vial is partially evacuated and the remaining fluid is under negative atmospheric pressure.
Some clinicians use the “Z technique” to prevent backflow when injecting IM antipsychotics. With this method, skin and subcutaneous tissue are retracted to avoid creating a straight-line needle tract that would allow the ready backflow of injected material.
I feel this method is unnecessary for decanoate preparations; they are viscous enough to prevent significant backflow provided the injection is slowly administered.
To IM or not to IM
Where possible, administering medications subcutaneously instead of intramuscularly can also reduce pain.
Contrary to popular belief, fluphenazine decanoate can be administered subcutaneously, using a 5/8-inch, 22-gauge needle for patients who fear long needles or are exquisitely sensitive to pain. IM administration is required for haloperidol decanoate, however.
50 vs. 100 mg/cc
Choice of preparation can also promote post-injection comfort. I have heard patients occasionally complain of lingering muscular discomfort after receiving the 100 mg/cc haloperidol decanoate preparation, but I have never heard such complaints after administering haloperidol, 50 mg/cc, or fluphenazine, 25 mg/cc.
Drug brand names
- Fluphenazine • Prolixin
- Haloperidol • Haldol
Dr. Fleishman is a staff psychiatrist at St. Francis Memorial Hospital, San Francisco, CA.
Dr. Fleishman is a staff psychiatrist at St. Francis Memorial Hospital, San Francisco, CA.
Visual hallucinations and drug therapy
In your “Cases That Test Your Skills” article on Charles Bonnet syndrome (CBS) (Current Psychiatry, May 2003), the authors briefly mention substance-induced psychosis in the differential diagnosis of visual hallucinations.
Ms. K was taking two antimuscarinic drugs, tolterodine and oxybutynin; I would not have accepted CBS as a diagnosis until the role of these drugs was clarified.
One patient developed visual hallucinations when her urologist started her on imipramine for urinary incontinence. Interestingly, once she was reassured that imipramine was causing this effect, she chose to keep taking it. She preferred occasional hallucinations to incontinence.
William Braden MD
Providence, RI
The authors did not adequately consider anticholinergic toxicity from a longstanding medication regimen as a possible cause of Ms. K’s visual hallucinations.
It is true that Ms. K had been taking tolterodine and oxybutynin, both anticholinergic agents, for more than 2 years without apparent adverse effects. However, that does not rule out the possibility that her advancing age and other factors increased her vulnerability to such toxicity. While her normal Mini-Mental State Examination score and clear sensorium do rule out frank delirium, anticholinergic toxicity can occur without gross cognitive impairment.
Robert L. Marcus, MD
Dix Hills, NY
The author responds
Drs. Braden and Marcus raise valid concerns about the effects of tolterodine and oxybutynin on Ms. K’s visual hallucinations. Benzodiazepines, tricyclic antidepressants, analgesics, beta-blockers, and antimuscarinics have all been linked to substance-induced psychosis, particularly in older patients.
We felt comfortable excluding these medications as playing a role in Ms. K’s visual hallucinations. She had tolerated these agents well with no changes for more than 2 years, so visual hallucinations as a sudden adverse effect seemed unlikely. Looking back, maybe anticholinergic toxicity could have been considered as a possible cause.
Drs. Braden and Marcus remind us that when evaluating apparent psychosis in older psychiatric patients, we should rule out causes such as medications and illegal substances. When alternative explanations are exhausted, however, a diagnosis of CBS may be warranted in the visually impaired patient who retains cognitive function.
Lee I. Kubersky
Third-year medical student
University of Medicine and Dentistry of New Jersey
Cooper Hospital/University Medical Center
Camden, NJ
In your “Cases That Test Your Skills” article on Charles Bonnet syndrome (CBS) (Current Psychiatry, May 2003), the authors briefly mention substance-induced psychosis in the differential diagnosis of visual hallucinations.
Ms. K was taking two antimuscarinic drugs, tolterodine and oxybutynin; I would not have accepted CBS as a diagnosis until the role of these drugs was clarified.
One patient developed visual hallucinations when her urologist started her on imipramine for urinary incontinence. Interestingly, once she was reassured that imipramine was causing this effect, she chose to keep taking it. She preferred occasional hallucinations to incontinence.
William Braden MD
Providence, RI
The authors did not adequately consider anticholinergic toxicity from a longstanding medication regimen as a possible cause of Ms. K’s visual hallucinations.
It is true that Ms. K had been taking tolterodine and oxybutynin, both anticholinergic agents, for more than 2 years without apparent adverse effects. However, that does not rule out the possibility that her advancing age and other factors increased her vulnerability to such toxicity. While her normal Mini-Mental State Examination score and clear sensorium do rule out frank delirium, anticholinergic toxicity can occur without gross cognitive impairment.
Robert L. Marcus, MD
Dix Hills, NY
The author responds
Drs. Braden and Marcus raise valid concerns about the effects of tolterodine and oxybutynin on Ms. K’s visual hallucinations. Benzodiazepines, tricyclic antidepressants, analgesics, beta-blockers, and antimuscarinics have all been linked to substance-induced psychosis, particularly in older patients.
We felt comfortable excluding these medications as playing a role in Ms. K’s visual hallucinations. She had tolerated these agents well with no changes for more than 2 years, so visual hallucinations as a sudden adverse effect seemed unlikely. Looking back, maybe anticholinergic toxicity could have been considered as a possible cause.
Drs. Braden and Marcus remind us that when evaluating apparent psychosis in older psychiatric patients, we should rule out causes such as medications and illegal substances. When alternative explanations are exhausted, however, a diagnosis of CBS may be warranted in the visually impaired patient who retains cognitive function.
Lee I. Kubersky
Third-year medical student
University of Medicine and Dentistry of New Jersey
Cooper Hospital/University Medical Center
Camden, NJ
In your “Cases That Test Your Skills” article on Charles Bonnet syndrome (CBS) (Current Psychiatry, May 2003), the authors briefly mention substance-induced psychosis in the differential diagnosis of visual hallucinations.
Ms. K was taking two antimuscarinic drugs, tolterodine and oxybutynin; I would not have accepted CBS as a diagnosis until the role of these drugs was clarified.
One patient developed visual hallucinations when her urologist started her on imipramine for urinary incontinence. Interestingly, once she was reassured that imipramine was causing this effect, she chose to keep taking it. She preferred occasional hallucinations to incontinence.
William Braden MD
Providence, RI
The authors did not adequately consider anticholinergic toxicity from a longstanding medication regimen as a possible cause of Ms. K’s visual hallucinations.
It is true that Ms. K had been taking tolterodine and oxybutynin, both anticholinergic agents, for more than 2 years without apparent adverse effects. However, that does not rule out the possibility that her advancing age and other factors increased her vulnerability to such toxicity. While her normal Mini-Mental State Examination score and clear sensorium do rule out frank delirium, anticholinergic toxicity can occur without gross cognitive impairment.
Robert L. Marcus, MD
Dix Hills, NY
The author responds
Drs. Braden and Marcus raise valid concerns about the effects of tolterodine and oxybutynin on Ms. K’s visual hallucinations. Benzodiazepines, tricyclic antidepressants, analgesics, beta-blockers, and antimuscarinics have all been linked to substance-induced psychosis, particularly in older patients.
We felt comfortable excluding these medications as playing a role in Ms. K’s visual hallucinations. She had tolerated these agents well with no changes for more than 2 years, so visual hallucinations as a sudden adverse effect seemed unlikely. Looking back, maybe anticholinergic toxicity could have been considered as a possible cause.
Drs. Braden and Marcus remind us that when evaluating apparent psychosis in older psychiatric patients, we should rule out causes such as medications and illegal substances. When alternative explanations are exhausted, however, a diagnosis of CBS may be warranted in the visually impaired patient who retains cognitive function.
Lee I. Kubersky
Third-year medical student
University of Medicine and Dentistry of New Jersey
Cooper Hospital/University Medical Center
Camden, NJ
Irritable bowel syndrome and psychiatric illness: Three clinical challenges
Psychiatrists often treat patients with irritable bowel syndrome (IBS) and an accompanying mental illness. Knowledge of available treatments and communication with the referring doctor are crucial to treating both the IBS symptoms and the comorbidity.
This article presents three cases that illustrate the challenges of identifying target symptoms, avoiding drug-drug interactions, ruling out serious underlying medical problems, and formulating treatment.
WHO GETS IBS?
Approximately 12% of the United States population reports IBS symptoms (abdominal pain, bloating, altered bowel habits).1 These symptoms begin before age 35 in most patients and during childhood in some. Onset after age 65 is rare.
IBS is common among patients with alcohol abuse disorder (32%),2 chronic fatigue syndrome (92%), fibromyalgia (77%), or temporomandibular joint syndrome (64%).3 Seventy percent of patients with IBS are women.4 Chronic pelvic pain, dyspareunia, dysmenorrhea, or a history of abdominal surgeries are risk factors for IBS in women.
LINK BETWEEN IBS AND MENTAL ILLNESS
Although mental illness often coexists with IBS, no cause-effect relationship has been shown.5
IBS is often preceded by stressful life events, such as family death or divorce,3 and some believe IBS is a precursor to numerous psychiatric disorders. Generalized anxiety disorder, major depression, panic disorder, social phobia, somatization disorder, or dysthymia have been diagnosed in most IBS patients.2
CASE 1: IBS AND DEPRESSION
Ms. R, age 55, has had IBS for 10 years. She has occasional diarrhea and abdominal cramps relieved by bowel movements. She is taking a bulking agent but still sometimes suffers abdominal pain.
She is referred to a psychiatrist after complaining of fatigue, loss of interest in hobbies, and crying spells for 2 months. She denies suicidal ideations. Her referring physician reports that she is taking conjugated estrogens to manage menopause symptoms. She denies any recent stressful life events. Thyroid function, glucose, and CBC are normal.
The challenge: Deciding which to treat first—the IBS symptoms or the depression—and how.
Discussion: The predominant symptom (in Ms. R’s case, abdominal pain) can help determine choice of medication. Bulk-forming agents, antispasmodics, barbiturates, benzodiazepines, and serotonin reuptake inhibitors have historically been used to treat IBS,6 but scant evidence supports their use.
Obtaining a thorough prescription history from the primary care physician, OB/GYN, and other treatment team members is critical before formulating a treatment plan. Ms. R’s estrogen use will not affect the choice of psychotropic or IBS medication because there are no significant interactions between estrogen and these classes of drugs.
Ms. R’s abdominal pain and depression can be treated simultaneously. Randomized, controlled trials have demonstrated that tricyclic antidepressants reduce abdominal pain and that behavioral therapy (relaxation therapy, hypnotherapy, and cognitive-behavioral therapy) may relieve individual IBS symptoms.7
Case 1 concluded: After reviewing Ms. R’s medications, the psychiatrist starts:
- desipramine, 50 mg at bedtime, to minimize anticholinergic side effects
- and short-term psychotherapy, which helped her identify support mechanisms and ways to better balance her life stresses.
After 6 weeks, her Beck Depression Inventory score improved from 30 at baseline to 8. She reports her abdominal pain is “the best it has been in 10 years.” Six months after diagnosis, she continues to take desipramine and is doing well.
CASE 2: IBS, DEPRESSION, AND PSYCHOSIS
Ms. H, age 32, is referred to a psychiatrist for treatment of depression with paranoid features.
Four years ago, a gastroenterologist diagnosed her as having IBS. She experiences frequent diarrhea and lower abdominal cramping. For 2 years she has been taking the antimuscarinic dicyclomine, 10 mg tid, which has provided some relief from her cramps. An estimated 20 diarrhea attacks per day leaves her housebound much of the time, however.
She reports fatigue, loss of interest in hobbies across 2 months, and paranoid thinking. She denies hallucinations or delusions but believes that her teenage children are discussing her “sickness” and plotting to “drive her crazy.” She is not suicidal.
The challenge: Treating Ms. H’s depression and paranoia while avoiding drug-drug interactions.
Discussion: Adverse drug-drug interactions can occur when prescribing psychotropics to patients with IBS (Table 1). Additive constipation, diarrhea, abdominal pain, and sedation are common interactions between psychotropics and the 5-HT3 antagonists and 5HT4 agonists commonly prescribed for IBS.
Table 1
Interactions between psychotropics and agents prescribed for IBS
| Antispasmodics | Benzodiazepines | SSRIs | Tricyclics | |
|---|---|---|---|---|
| MAOIs | Additive sedation | Additive dizziness, sedation, dry mouth, | Contraindicated–hyperpyrexia and severe neurologic effects | Contraindicated–hyperpyrexia, seizures, and death |
| SSRIs | Additive sedation | Additive sedation | —- | Increased tricyclic levels with concurrent use |
| Tricyclics | Additive sedation, dry mouth | Additive sedation | Additive sedation, dry mouth, increased tricyclic levels | —- |
| Anticonvulsants | Additive sedation | Additive sedation | Increased levels of anticonvulsants | Additive sedation, dry mouth, constipation |
| Benzodiazepines | Additive sedation | —- | Additive sedation and dry mouth | Additive sedation |
| Buspirone | Additive sedation, dizziness | Additive sedation | Additive sedation, dizziness, nausea | Additive sedation, dry mouth, constipation, increased tricyclic level |
| Traditional antipsychotics | Additive sedation, CNS effects | Additive sedation, CNS effects | Additive sedation, dizziness | Additive sedation and anticholinergic effects; increased tricyclic level |
| Atypical antipsychotics | Additive sedation, CNS effects | Contraindicated–respiratory and cardiovascular collapse | Elevated antipsychotic levels | Levels of both drugs increased |
| Aripiprazole | Somnolence,constipation | Additive sedation | Increased blood levels of aripiprazole | Increased sedation and anticholinergic effects |
| Psychotropics and 5-HT3 antagonists taken concomitantly typically lead to additive constipation and abdominal pain. | ||||
| Psychotropics and 5-HT4 agonists taken concomitantly typically lead to additive diarrhea and/or abdominal pain. | ||||
| Source: Physician’s Desk Reference. Mobile PDR release version 32. Database version 437. Montvale, NJ: Thomson Healthcare 2003. | ||||
- Hematochezia
- Weight loss < 10 pounds
- Family history of colon cancer
- Recurrent fever
- Anemia
- Chronic severe diarrhea
Source: American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Am J Gastroenterol. 2002;97:S1-S5.
Other than fiber supplements, most traditional IBS medications are sedating and are associated with anticholinergic side effects. In Ms. H’s case, extreme caution is necessary before prescribing an antidepressant or antipsychotic because of dicyclomine’s additive sedating effects.
Case 2 concluded: After a thorough initial patient interview, the psychiatrist elects to treat Ms. H’s major depression with an antidepressant but delays the use of an antipsychotic to avoid additive sedation.
After talking with Ms. H’s family physician, the psychiatrist stops her dicyclomine and starts sertraline, 100 mg/d. She tolerates the sertraline well and the dosage is titrated across 1 month to 200 mg/d.
Four weeks later, Ms. H’s Beck Depression Inventory score has improved from 26 at baseline to 5, but her paranoid thoughts and frequent diarrhea persist. The psychiatrist adds low-dose olanzapine (5 mg at bedtime) to minimize extrapyramidal side effects. One month later, her depression and paranoia have resolved.
Ms. H’s gastroenterologist instructs her to begin taking alosetron, 1 mg bid, for her continued frequent diarrhea. Adding this agent to her sertraline/olanzapine regimen can lead to additive constipation and abdominal pain, so the psychiatrist monitors her psychiatric medications. One month later, she reports that her affect is much improved and her diarrhea is “gone.”
CASE 3: DEPRESSION AND ABDOMINAL PAIN
Mr. J, age 52, has had depression for 1 year. His depressive symptoms have improved significantly on fluoxetine, 20 mg/d; he once again enjoys life and has a more positive outlook.
The patient was in reasonably good health until about 1 month ago, when he began to experience abdominal pain. He has lost 14 lbs over the past month. He is not taking other medications.
The challenge: Find the cause of Mr. J’s persistent abdominal pain without undermining depression therapy.
Discussion: Although Mr. J’s symptoms might be side effects of fluoxetine, his abdominal pain and weight loss >10 lbs within 1 month are cause for concern. The American College of Gastroenterology has identified six alarm symptoms that could point to a serious medical problem in patients with severe abdominal pain (Box).7
Patients who exhibit any of these symptoms should be referred for endoscopic and stool studies. Colon cancer screening should be considered for all patients age 50 and older.
Patients with IBS usually present first to their primary care physicians with abdominal pain and altered bowel habits. These symptoms can occur in many gastrointestinal and systemic illnesses (Table 2).8
Table 2
Diagnosing irritable bowel syndrome: What to rule out
| Differential diagnosis | Examples |
|---|---|
| Inflammatory bowel disease | Crohn’s disease, ulcerative colitis |
| Medication effects | Laxatives, constipating agents |
| Infections | Parasitic, bacterial, viral, opportunistic |
| Malabsorption syndromes | Celiac disease, pancreatic insufficiency |
| Endocrine disorders | Hypothyroidism, hyperthyroidism, diabetes, Addison’s disease |
| Endocrine tumors (extremely uncommon) | Gastrinoma, carcinoid |
| Colorectal carcinoma | Adenocarcinoma, villous adenoma |
| Intestinal pseudo-obstruction | Diabetes, scleroderma |
| Lactose intolerance | —- |
| Psychiatric disorders | Depression, anxiety, somatization disorders |
| Source: Dalton CB, Drossman D. Am Fam Physician. 1997;55(3):875-80. | |
Case 3 concluded: The psychiatrist and primary care physician consult a gastroenterologist, who performs a colonoscopy and identifies a resectable Duke’s Class B adenocarcinoma in the transverse colon. A partial colectomy is performed.
Three years later, Mr. J is cancer-free and his depression is stable. The psychiatrist advises him to keep taking fluoxetine, 20 mg/d, because the stress of his cancer therapy increases the risk of depression recurrence.
Related resources
- National Institute of Diabetes and Digestive and Kidney Diseases —Irritable Bowel Syndrome www.niddk.nih.gov/health/digest/pubs/irrbowel/irrbowel.htm
Drug brand names
- Alosetron • Lotronex
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Desipramine • Norpramin
- Dicyclomine • Bentyl
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Sertraline • Zoloft
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Locke GR, 3rd. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1-19.
2. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry. 1997;42:835-40.
3. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221-7.
4. Smith RP. Lower gastrointestinal disease in women. Obstet Gynecol Clin North Am. 2001;28:351-62.
5. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313-276.
6. Mobile PDR Release Version 32. Database Version 437. An abbreviated, up-to-date version of the PDR onto computing devices. Thomson Healthcare, Ortho-Biotech Oncology, 2003.
7. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1-S5.
8. Dalton CB, Drossman D. Diagnosis and treatment of irritable bowel syndrome. Am Fam Physician. 1997;55(3):875-80.
Psychiatrists often treat patients with irritable bowel syndrome (IBS) and an accompanying mental illness. Knowledge of available treatments and communication with the referring doctor are crucial to treating both the IBS symptoms and the comorbidity.
This article presents three cases that illustrate the challenges of identifying target symptoms, avoiding drug-drug interactions, ruling out serious underlying medical problems, and formulating treatment.
WHO GETS IBS?
Approximately 12% of the United States population reports IBS symptoms (abdominal pain, bloating, altered bowel habits).1 These symptoms begin before age 35 in most patients and during childhood in some. Onset after age 65 is rare.
IBS is common among patients with alcohol abuse disorder (32%),2 chronic fatigue syndrome (92%), fibromyalgia (77%), or temporomandibular joint syndrome (64%).3 Seventy percent of patients with IBS are women.4 Chronic pelvic pain, dyspareunia, dysmenorrhea, or a history of abdominal surgeries are risk factors for IBS in women.
LINK BETWEEN IBS AND MENTAL ILLNESS
Although mental illness often coexists with IBS, no cause-effect relationship has been shown.5
IBS is often preceded by stressful life events, such as family death or divorce,3 and some believe IBS is a precursor to numerous psychiatric disorders. Generalized anxiety disorder, major depression, panic disorder, social phobia, somatization disorder, or dysthymia have been diagnosed in most IBS patients.2
CASE 1: IBS AND DEPRESSION
Ms. R, age 55, has had IBS for 10 years. She has occasional diarrhea and abdominal cramps relieved by bowel movements. She is taking a bulking agent but still sometimes suffers abdominal pain.
She is referred to a psychiatrist after complaining of fatigue, loss of interest in hobbies, and crying spells for 2 months. She denies suicidal ideations. Her referring physician reports that she is taking conjugated estrogens to manage menopause symptoms. She denies any recent stressful life events. Thyroid function, glucose, and CBC are normal.
The challenge: Deciding which to treat first—the IBS symptoms or the depression—and how.
Discussion: The predominant symptom (in Ms. R’s case, abdominal pain) can help determine choice of medication. Bulk-forming agents, antispasmodics, barbiturates, benzodiazepines, and serotonin reuptake inhibitors have historically been used to treat IBS,6 but scant evidence supports their use.
Obtaining a thorough prescription history from the primary care physician, OB/GYN, and other treatment team members is critical before formulating a treatment plan. Ms. R’s estrogen use will not affect the choice of psychotropic or IBS medication because there are no significant interactions between estrogen and these classes of drugs.
Ms. R’s abdominal pain and depression can be treated simultaneously. Randomized, controlled trials have demonstrated that tricyclic antidepressants reduce abdominal pain and that behavioral therapy (relaxation therapy, hypnotherapy, and cognitive-behavioral therapy) may relieve individual IBS symptoms.7
Case 1 concluded: After reviewing Ms. R’s medications, the psychiatrist starts:
- desipramine, 50 mg at bedtime, to minimize anticholinergic side effects
- and short-term psychotherapy, which helped her identify support mechanisms and ways to better balance her life stresses.
After 6 weeks, her Beck Depression Inventory score improved from 30 at baseline to 8. She reports her abdominal pain is “the best it has been in 10 years.” Six months after diagnosis, she continues to take desipramine and is doing well.
CASE 2: IBS, DEPRESSION, AND PSYCHOSIS
Ms. H, age 32, is referred to a psychiatrist for treatment of depression with paranoid features.
Four years ago, a gastroenterologist diagnosed her as having IBS. She experiences frequent diarrhea and lower abdominal cramping. For 2 years she has been taking the antimuscarinic dicyclomine, 10 mg tid, which has provided some relief from her cramps. An estimated 20 diarrhea attacks per day leaves her housebound much of the time, however.
She reports fatigue, loss of interest in hobbies across 2 months, and paranoid thinking. She denies hallucinations or delusions but believes that her teenage children are discussing her “sickness” and plotting to “drive her crazy.” She is not suicidal.
The challenge: Treating Ms. H’s depression and paranoia while avoiding drug-drug interactions.
Discussion: Adverse drug-drug interactions can occur when prescribing psychotropics to patients with IBS (Table 1). Additive constipation, diarrhea, abdominal pain, and sedation are common interactions between psychotropics and the 5-HT3 antagonists and 5HT4 agonists commonly prescribed for IBS.
Table 1
Interactions between psychotropics and agents prescribed for IBS
| Antispasmodics | Benzodiazepines | SSRIs | Tricyclics | |
|---|---|---|---|---|
| MAOIs | Additive sedation | Additive dizziness, sedation, dry mouth, | Contraindicated–hyperpyrexia and severe neurologic effects | Contraindicated–hyperpyrexia, seizures, and death |
| SSRIs | Additive sedation | Additive sedation | —- | Increased tricyclic levels with concurrent use |
| Tricyclics | Additive sedation, dry mouth | Additive sedation | Additive sedation, dry mouth, increased tricyclic levels | —- |
| Anticonvulsants | Additive sedation | Additive sedation | Increased levels of anticonvulsants | Additive sedation, dry mouth, constipation |
| Benzodiazepines | Additive sedation | —- | Additive sedation and dry mouth | Additive sedation |
| Buspirone | Additive sedation, dizziness | Additive sedation | Additive sedation, dizziness, nausea | Additive sedation, dry mouth, constipation, increased tricyclic level |
| Traditional antipsychotics | Additive sedation, CNS effects | Additive sedation, CNS effects | Additive sedation, dizziness | Additive sedation and anticholinergic effects; increased tricyclic level |
| Atypical antipsychotics | Additive sedation, CNS effects | Contraindicated–respiratory and cardiovascular collapse | Elevated antipsychotic levels | Levels of both drugs increased |
| Aripiprazole | Somnolence,constipation | Additive sedation | Increased blood levels of aripiprazole | Increased sedation and anticholinergic effects |
| Psychotropics and 5-HT3 antagonists taken concomitantly typically lead to additive constipation and abdominal pain. | ||||
| Psychotropics and 5-HT4 agonists taken concomitantly typically lead to additive diarrhea and/or abdominal pain. | ||||
| Source: Physician’s Desk Reference. Mobile PDR release version 32. Database version 437. Montvale, NJ: Thomson Healthcare 2003. | ||||
- Hematochezia
- Weight loss < 10 pounds
- Family history of colon cancer
- Recurrent fever
- Anemia
- Chronic severe diarrhea
Source: American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Am J Gastroenterol. 2002;97:S1-S5.
Other than fiber supplements, most traditional IBS medications are sedating and are associated with anticholinergic side effects. In Ms. H’s case, extreme caution is necessary before prescribing an antidepressant or antipsychotic because of dicyclomine’s additive sedating effects.
Case 2 concluded: After a thorough initial patient interview, the psychiatrist elects to treat Ms. H’s major depression with an antidepressant but delays the use of an antipsychotic to avoid additive sedation.
After talking with Ms. H’s family physician, the psychiatrist stops her dicyclomine and starts sertraline, 100 mg/d. She tolerates the sertraline well and the dosage is titrated across 1 month to 200 mg/d.
Four weeks later, Ms. H’s Beck Depression Inventory score has improved from 26 at baseline to 5, but her paranoid thoughts and frequent diarrhea persist. The psychiatrist adds low-dose olanzapine (5 mg at bedtime) to minimize extrapyramidal side effects. One month later, her depression and paranoia have resolved.
Ms. H’s gastroenterologist instructs her to begin taking alosetron, 1 mg bid, for her continued frequent diarrhea. Adding this agent to her sertraline/olanzapine regimen can lead to additive constipation and abdominal pain, so the psychiatrist monitors her psychiatric medications. One month later, she reports that her affect is much improved and her diarrhea is “gone.”
CASE 3: DEPRESSION AND ABDOMINAL PAIN
Mr. J, age 52, has had depression for 1 year. His depressive symptoms have improved significantly on fluoxetine, 20 mg/d; he once again enjoys life and has a more positive outlook.
The patient was in reasonably good health until about 1 month ago, when he began to experience abdominal pain. He has lost 14 lbs over the past month. He is not taking other medications.
The challenge: Find the cause of Mr. J’s persistent abdominal pain without undermining depression therapy.
Discussion: Although Mr. J’s symptoms might be side effects of fluoxetine, his abdominal pain and weight loss >10 lbs within 1 month are cause for concern. The American College of Gastroenterology has identified six alarm symptoms that could point to a serious medical problem in patients with severe abdominal pain (Box).7
Patients who exhibit any of these symptoms should be referred for endoscopic and stool studies. Colon cancer screening should be considered for all patients age 50 and older.
Patients with IBS usually present first to their primary care physicians with abdominal pain and altered bowel habits. These symptoms can occur in many gastrointestinal and systemic illnesses (Table 2).8
Table 2
Diagnosing irritable bowel syndrome: What to rule out
| Differential diagnosis | Examples |
|---|---|
| Inflammatory bowel disease | Crohn’s disease, ulcerative colitis |
| Medication effects | Laxatives, constipating agents |
| Infections | Parasitic, bacterial, viral, opportunistic |
| Malabsorption syndromes | Celiac disease, pancreatic insufficiency |
| Endocrine disorders | Hypothyroidism, hyperthyroidism, diabetes, Addison’s disease |
| Endocrine tumors (extremely uncommon) | Gastrinoma, carcinoid |
| Colorectal carcinoma | Adenocarcinoma, villous adenoma |
| Intestinal pseudo-obstruction | Diabetes, scleroderma |
| Lactose intolerance | —- |
| Psychiatric disorders | Depression, anxiety, somatization disorders |
| Source: Dalton CB, Drossman D. Am Fam Physician. 1997;55(3):875-80. | |
Case 3 concluded: The psychiatrist and primary care physician consult a gastroenterologist, who performs a colonoscopy and identifies a resectable Duke’s Class B adenocarcinoma in the transverse colon. A partial colectomy is performed.
Three years later, Mr. J is cancer-free and his depression is stable. The psychiatrist advises him to keep taking fluoxetine, 20 mg/d, because the stress of his cancer therapy increases the risk of depression recurrence.
Related resources
- National Institute of Diabetes and Digestive and Kidney Diseases —Irritable Bowel Syndrome www.niddk.nih.gov/health/digest/pubs/irrbowel/irrbowel.htm
Drug brand names
- Alosetron • Lotronex
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Desipramine • Norpramin
- Dicyclomine • Bentyl
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Sertraline • Zoloft
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatrists often treat patients with irritable bowel syndrome (IBS) and an accompanying mental illness. Knowledge of available treatments and communication with the referring doctor are crucial to treating both the IBS symptoms and the comorbidity.
This article presents three cases that illustrate the challenges of identifying target symptoms, avoiding drug-drug interactions, ruling out serious underlying medical problems, and formulating treatment.
WHO GETS IBS?
Approximately 12% of the United States population reports IBS symptoms (abdominal pain, bloating, altered bowel habits).1 These symptoms begin before age 35 in most patients and during childhood in some. Onset after age 65 is rare.
IBS is common among patients with alcohol abuse disorder (32%),2 chronic fatigue syndrome (92%), fibromyalgia (77%), or temporomandibular joint syndrome (64%).3 Seventy percent of patients with IBS are women.4 Chronic pelvic pain, dyspareunia, dysmenorrhea, or a history of abdominal surgeries are risk factors for IBS in women.
LINK BETWEEN IBS AND MENTAL ILLNESS
Although mental illness often coexists with IBS, no cause-effect relationship has been shown.5
IBS is often preceded by stressful life events, such as family death or divorce,3 and some believe IBS is a precursor to numerous psychiatric disorders. Generalized anxiety disorder, major depression, panic disorder, social phobia, somatization disorder, or dysthymia have been diagnosed in most IBS patients.2
CASE 1: IBS AND DEPRESSION
Ms. R, age 55, has had IBS for 10 years. She has occasional diarrhea and abdominal cramps relieved by bowel movements. She is taking a bulking agent but still sometimes suffers abdominal pain.
She is referred to a psychiatrist after complaining of fatigue, loss of interest in hobbies, and crying spells for 2 months. She denies suicidal ideations. Her referring physician reports that she is taking conjugated estrogens to manage menopause symptoms. She denies any recent stressful life events. Thyroid function, glucose, and CBC are normal.
The challenge: Deciding which to treat first—the IBS symptoms or the depression—and how.
Discussion: The predominant symptom (in Ms. R’s case, abdominal pain) can help determine choice of medication. Bulk-forming agents, antispasmodics, barbiturates, benzodiazepines, and serotonin reuptake inhibitors have historically been used to treat IBS,6 but scant evidence supports their use.
Obtaining a thorough prescription history from the primary care physician, OB/GYN, and other treatment team members is critical before formulating a treatment plan. Ms. R’s estrogen use will not affect the choice of psychotropic or IBS medication because there are no significant interactions between estrogen and these classes of drugs.
Ms. R’s abdominal pain and depression can be treated simultaneously. Randomized, controlled trials have demonstrated that tricyclic antidepressants reduce abdominal pain and that behavioral therapy (relaxation therapy, hypnotherapy, and cognitive-behavioral therapy) may relieve individual IBS symptoms.7
Case 1 concluded: After reviewing Ms. R’s medications, the psychiatrist starts:
- desipramine, 50 mg at bedtime, to minimize anticholinergic side effects
- and short-term psychotherapy, which helped her identify support mechanisms and ways to better balance her life stresses.
After 6 weeks, her Beck Depression Inventory score improved from 30 at baseline to 8. She reports her abdominal pain is “the best it has been in 10 years.” Six months after diagnosis, she continues to take desipramine and is doing well.
CASE 2: IBS, DEPRESSION, AND PSYCHOSIS
Ms. H, age 32, is referred to a psychiatrist for treatment of depression with paranoid features.
Four years ago, a gastroenterologist diagnosed her as having IBS. She experiences frequent diarrhea and lower abdominal cramping. For 2 years she has been taking the antimuscarinic dicyclomine, 10 mg tid, which has provided some relief from her cramps. An estimated 20 diarrhea attacks per day leaves her housebound much of the time, however.
She reports fatigue, loss of interest in hobbies across 2 months, and paranoid thinking. She denies hallucinations or delusions but believes that her teenage children are discussing her “sickness” and plotting to “drive her crazy.” She is not suicidal.
The challenge: Treating Ms. H’s depression and paranoia while avoiding drug-drug interactions.
Discussion: Adverse drug-drug interactions can occur when prescribing psychotropics to patients with IBS (Table 1). Additive constipation, diarrhea, abdominal pain, and sedation are common interactions between psychotropics and the 5-HT3 antagonists and 5HT4 agonists commonly prescribed for IBS.
Table 1
Interactions between psychotropics and agents prescribed for IBS
| Antispasmodics | Benzodiazepines | SSRIs | Tricyclics | |
|---|---|---|---|---|
| MAOIs | Additive sedation | Additive dizziness, sedation, dry mouth, | Contraindicated–hyperpyrexia and severe neurologic effects | Contraindicated–hyperpyrexia, seizures, and death |
| SSRIs | Additive sedation | Additive sedation | —- | Increased tricyclic levels with concurrent use |
| Tricyclics | Additive sedation, dry mouth | Additive sedation | Additive sedation, dry mouth, increased tricyclic levels | —- |
| Anticonvulsants | Additive sedation | Additive sedation | Increased levels of anticonvulsants | Additive sedation, dry mouth, constipation |
| Benzodiazepines | Additive sedation | —- | Additive sedation and dry mouth | Additive sedation |
| Buspirone | Additive sedation, dizziness | Additive sedation | Additive sedation, dizziness, nausea | Additive sedation, dry mouth, constipation, increased tricyclic level |
| Traditional antipsychotics | Additive sedation, CNS effects | Additive sedation, CNS effects | Additive sedation, dizziness | Additive sedation and anticholinergic effects; increased tricyclic level |
| Atypical antipsychotics | Additive sedation, CNS effects | Contraindicated–respiratory and cardiovascular collapse | Elevated antipsychotic levels | Levels of both drugs increased |
| Aripiprazole | Somnolence,constipation | Additive sedation | Increased blood levels of aripiprazole | Increased sedation and anticholinergic effects |
| Psychotropics and 5-HT3 antagonists taken concomitantly typically lead to additive constipation and abdominal pain. | ||||
| Psychotropics and 5-HT4 agonists taken concomitantly typically lead to additive diarrhea and/or abdominal pain. | ||||
| Source: Physician’s Desk Reference. Mobile PDR release version 32. Database version 437. Montvale, NJ: Thomson Healthcare 2003. | ||||
- Hematochezia
- Weight loss < 10 pounds
- Family history of colon cancer
- Recurrent fever
- Anemia
- Chronic severe diarrhea
Source: American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Am J Gastroenterol. 2002;97:S1-S5.
Other than fiber supplements, most traditional IBS medications are sedating and are associated with anticholinergic side effects. In Ms. H’s case, extreme caution is necessary before prescribing an antidepressant or antipsychotic because of dicyclomine’s additive sedating effects.
Case 2 concluded: After a thorough initial patient interview, the psychiatrist elects to treat Ms. H’s major depression with an antidepressant but delays the use of an antipsychotic to avoid additive sedation.
After talking with Ms. H’s family physician, the psychiatrist stops her dicyclomine and starts sertraline, 100 mg/d. She tolerates the sertraline well and the dosage is titrated across 1 month to 200 mg/d.
Four weeks later, Ms. H’s Beck Depression Inventory score has improved from 26 at baseline to 5, but her paranoid thoughts and frequent diarrhea persist. The psychiatrist adds low-dose olanzapine (5 mg at bedtime) to minimize extrapyramidal side effects. One month later, her depression and paranoia have resolved.
Ms. H’s gastroenterologist instructs her to begin taking alosetron, 1 mg bid, for her continued frequent diarrhea. Adding this agent to her sertraline/olanzapine regimen can lead to additive constipation and abdominal pain, so the psychiatrist monitors her psychiatric medications. One month later, she reports that her affect is much improved and her diarrhea is “gone.”
CASE 3: DEPRESSION AND ABDOMINAL PAIN
Mr. J, age 52, has had depression for 1 year. His depressive symptoms have improved significantly on fluoxetine, 20 mg/d; he once again enjoys life and has a more positive outlook.
The patient was in reasonably good health until about 1 month ago, when he began to experience abdominal pain. He has lost 14 lbs over the past month. He is not taking other medications.
The challenge: Find the cause of Mr. J’s persistent abdominal pain without undermining depression therapy.
Discussion: Although Mr. J’s symptoms might be side effects of fluoxetine, his abdominal pain and weight loss >10 lbs within 1 month are cause for concern. The American College of Gastroenterology has identified six alarm symptoms that could point to a serious medical problem in patients with severe abdominal pain (Box).7
Patients who exhibit any of these symptoms should be referred for endoscopic and stool studies. Colon cancer screening should be considered for all patients age 50 and older.
Patients with IBS usually present first to their primary care physicians with abdominal pain and altered bowel habits. These symptoms can occur in many gastrointestinal and systemic illnesses (Table 2).8
Table 2
Diagnosing irritable bowel syndrome: What to rule out
| Differential diagnosis | Examples |
|---|---|
| Inflammatory bowel disease | Crohn’s disease, ulcerative colitis |
| Medication effects | Laxatives, constipating agents |
| Infections | Parasitic, bacterial, viral, opportunistic |
| Malabsorption syndromes | Celiac disease, pancreatic insufficiency |
| Endocrine disorders | Hypothyroidism, hyperthyroidism, diabetes, Addison’s disease |
| Endocrine tumors (extremely uncommon) | Gastrinoma, carcinoid |
| Colorectal carcinoma | Adenocarcinoma, villous adenoma |
| Intestinal pseudo-obstruction | Diabetes, scleroderma |
| Lactose intolerance | —- |
| Psychiatric disorders | Depression, anxiety, somatization disorders |
| Source: Dalton CB, Drossman D. Am Fam Physician. 1997;55(3):875-80. | |
Case 3 concluded: The psychiatrist and primary care physician consult a gastroenterologist, who performs a colonoscopy and identifies a resectable Duke’s Class B adenocarcinoma in the transverse colon. A partial colectomy is performed.
Three years later, Mr. J is cancer-free and his depression is stable. The psychiatrist advises him to keep taking fluoxetine, 20 mg/d, because the stress of his cancer therapy increases the risk of depression recurrence.
Related resources
- National Institute of Diabetes and Digestive and Kidney Diseases —Irritable Bowel Syndrome www.niddk.nih.gov/health/digest/pubs/irrbowel/irrbowel.htm
Drug brand names
- Alosetron • Lotronex
- Aripiprazole • Abilify
- Buspirone • BuSpar
- Desipramine • Norpramin
- Dicyclomine • Bentyl
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Sertraline • Zoloft
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Locke GR, 3rd. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1-19.
2. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry. 1997;42:835-40.
3. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221-7.
4. Smith RP. Lower gastrointestinal disease in women. Obstet Gynecol Clin North Am. 2001;28:351-62.
5. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313-276.
6. Mobile PDR Release Version 32. Database Version 437. An abbreviated, up-to-date version of the PDR onto computing devices. Thomson Healthcare, Ortho-Biotech Oncology, 2003.
7. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1-S5.
8. Dalton CB, Drossman D. Diagnosis and treatment of irritable bowel syndrome. Am Fam Physician. 1997;55(3):875-80.
1. Locke GR, 3rd. The epidemiology of functional gastrointestinal disorders in North America. Gastroenterol Clin North Am. 1996;25:1-19.
2. Goldberg J, Davidson P. A biopsychosocial understanding of the irritable bowel syndrome: a review. Can J Psychiatry. 1997;42:835-40.
3. Aaron LA, Burke MM, Buchwald D. Overlapping conditions among chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221-7.
4. Smith RP. Lower gastrointestinal disease in women. Obstet Gynecol Clin North Am. 2001;28:351-62.
5. Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313-276.
6. Mobile PDR Release Version 32. Database Version 437. An abbreviated, up-to-date version of the PDR onto computing devices. Thomson Healthcare, Ortho-Biotech Oncology, 2003.
7. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1-S5.
8. Dalton CB, Drossman D. Diagnosis and treatment of irritable bowel syndrome. Am Fam Physician. 1997;55(3):875-80.
How to avoid ethnic bias when diagnosing schizophrenia
In patients with psychotic symptoms, why are African-Americans more likely than whites to be diagnosed with schizophrenia? After more than 30 years of debate, some answers—and remedies for the problem—are becoming clear.
In psychiatry, where interpersonal interactions are key to eliciting diagnostic symptoms and signs, there is an intrinsic risk of misinterpretation when clinician and patient are of different cultural, ethnic, or socioeconomic backgrounds. This article analyzes four factors that contribute to misinterpretation and to ethnic misdiagnosis of schizophrenia. Culturally sensitive strategies are offered to avoid diagnostic bias in clinical practice.
SCHIZOPHRENIA MISDIAGNOSIS
Large epidemiologic studies report similar rates of schizophrenia and bipolar disorder in African-American and white populations.1 Although patients of both races have been wrongly diagnosed with schizophrenia, the pattern is stronger and more persistent in African-Americans.
Two (or more) of the following, each present for a significant portion of time during a 1-month period (or less if successfully treated):
- Delusions
- Hallucinations
- Disorganized speech (eg, frequent derailment or incoherence)
- Grossly disorganized or catatonic behavior
- Negative symptoms (ie, affective flattening, alogia, or avolition)
Note: Only one Criterion A symptom is required if delusions are bizarre or hallucinations consist of a voice keeping up a running commentary on the person’s behavior or thoughts, or two or more voices conversing with each other.
295.30 Paranoid type
A type of schizophrenia in which the following criteria are met:
- Preoccupation with one or more delusions or frequent auditory hallucinations
- None of the following is prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect.
Source: DSM-IV-TR
In the 1970s, Simon et al2 studied 192 hospitalized patients, of whom all African-Americans and 85% of whites had been identified clinically as having schizophrenia. Using a structured interview, the researchers found that only 40% of the African Americans and 50% of the whites met diagnostic criteria for schizophrenia. African-Americans with mood disorders were found to be at particular risk of schizophrenia misdiagnosis.
In the 1980s, among 76 patients with a clinical diagnosis of schizophrenia, Mukherjee et al3 diagnosed one-half (52%) with bipolar disorder using a structured clinical interview. Schizophrenia misdiagnoses were more common in African-Americans (86%) and Hispanics (83%) than in whites (51%). In particular, African-Americans were most likely to be misdiagnosed with paranoid schizophrenia. African-Americans complained more commonly than whites of auditory hallucinations, which may represent an ethnic difference in symptomatic presentation of psychotic mood disorders.
In the 1990s, colleagues and I conducted two studies—one of 173 patients in a Tennessee psychiatric hospital4 and the other of 490 patients in an Ohio psychiatric emergency service5—and found yet again that African-Americans were more likely than whites to be diagnosed with schizophrenia. In the hospital study, higher rates of schizophrenia diagnosis were associated with lower rates of mood disorder diagnosis. This inverse relationship implied that African-Americans with mood disorders were being misdiagnosed with schizophrenia.
Men were more likely than women to be diagnosed with schizophrenia, suggesting that African-American men were most likely to be misdiagnosed. When adjustments were made for gender, black women were found to be at higher risk for misdiagnosis than white women.
Lawson et al6 extended this research in a population-based study of African-Americans living in Tennessee. They found that African-Americans constituted 16% of the state’s population but 48% of psychiatric inpatients diagnosed with schizophrenia and 37% of psychiatric outpatients.
CONSEQUENCES OF INACCURATE DIAGNOSIS
Differentiating between schizophrenia (Box 1) and a psychotic mood disorder (Box 2) is more than a semantic exercise. Schizophrenia implies a chronic, unremitting, debilitating illness that worsens over time. Though this perception of schizophrenia is not entirely accurate, in clinical practice its diagnosis imparts a bleak prognosis that may lower the clinician’s expectations for the patient.7
Schizophrenia misdiagnosis also may lead the psychiatrist to rely excessively on antipsychotics, rather than attempting thymoleptic and psychotherapy trials. Studies suggest that African-American patients are more likely than similar white patients to receive antipsychotics4,8,9 and less likely to receive psychotherapy.5,10
Reasons why African-Americans are often misdiagnosed with schizophrenia remain unclear but probably include four contributing factors:
- differences in symptom presentation compared with whites
- failure by clinicians to identify affective symptoms in African-Americans
- minority patients’ wariness when dealing with health services
- and racial stereotyping.
DIFFERENCES IN SYMPTOM EXPRESSION
African-American patients with mood disorders or schizophrenia are more likely than are similar white patients to complain of auditory hallucinations.11-13 For example, Strakowski et al14 examined 330 patients with nonaffective and psychotic diagnoses in a study that was used to develop DSM-IV criteria for schizophrenia. Auditory hallucinations were rated as more severe in African-American than in similar white patients.
MAJOR DEPRESSIVE EPISODE
Five or more of the following symptoms present during the same 2-week period and representing a change from previous functioning; must include either depressed mood or loss of interest or pleasure.
- Depressed mood
- Markedly diminished interest or pleasure
- Significant weight loss
- Insomnia or hypersomnia
- Psychomotor agitation or retardation
- Fatigue
- Feelings of worthlessness or excessive guilt
- Diminished ability to concentrate
SEVERE MAJOR DEPRESSION WITH PSYCHOTIC FEATURES
Mood-congruent
Delusions or hallucinations whose content is entirely consistent with the typical depressive themes of personal inadequacy, guilt, disease, death, nihilism, or deserved punishment
Mood-incongruent
Delusions or hallucinations whose content does not involve typical depressive themes. Includes symptoms such as persecutory delusions, thought insertion, thought broadcasting, and delusions of control
Source: DSM-IV-TR
African-American patients also are more likely than whites to exhibit so-called Schneiderian first-rank symptoms of schizophrenia,15 including:
- delusions of thought broadcasting or insertion
- auditory hallucinations of voices conversing about the patient in the third person.
These symptoms were once used to diagnose schizophrenia, but their lack of specificity has been well documented.2,16 First-rank symptoms of schizophrenia depend on the specific form of the hallucination or delusion, are likely to be influenced by a patient’s culture, and may be misleading in multicultural populations. Though first-rank symptoms now occupy a minor role in U.S. diagnostic systems, they might continue to sway clinicians—even when using structured diagnostic interviews—to inappropriately diagnose schizophrenia in lieu of affective disorders in minority patients.15
To extend this finding, our group16 studied rates and severity of affective and psychotic symptoms—particularly first-rank symptoms—in 100 patients with psychotic mania who met DSM-III-R criteria for bipolar disorder (80%) or schizoaffective disorder, bipolar type (20%) as determined by structured diagnostic interview. No differences in affective symptoms between African-American and white patients were seen. African-Americans were more likely to endorse auditory hallucinations and to report severe auditory hallucinations of voices commenting on their behavior—the only first-rank symptom on which they differed from whites.
Though their affective symptoms were similar, African-Americans were significantly more likely than whites to have been diagnosed with a schizophrenia-spectrum disorder. Because misdiagnosis of African-Americans could not be explained by psychotic symptoms—which were as severe as those of white patients—these findings suggest other mechanisms were at work.
UNIDENTIFIED AFFECTIVE SYMPTOMS
Underidentification of mood disorders in African-American patients may also lead to over-diagnosis of schizophrenia. In a sample of 99 patients, colleagues and I17 compared clinical diagnoses made in a psychiatric emergency service with those by research investigators using a structured clinical interview. Reasons for diagnostic differences were identified and divided into two categories:
- the same symptoms were recorded but applied differently to diagnostic criteria (criterion variance)
- different information was recorded, which led to diagnostic discrepancies (information variance).
Differences occurred significantly more often in African-American than in white patients, but only information variance was associated with ethnicity. This suggests that clinicians are less likely to elicit appropriate information from African-American than from white psychiatric patients. The fact that researchers obtained this information during diagnostic interviews suggests that the patients could provide it when given appropriate prompts. Specifically, affective symptoms were less likely to be elicited by clinicians than by researchers.
PATIENT WARINESS
Minority patients, when interacting with clinicians of the majority population, may project “protective wariness.”18 Specific behaviors include hesitancy or reluctance to fully engage with the care provider as a precaution against being exploited or harmed. Cultural misunderstandings19 and patient concerns about past reports of minorities receiving substandard or unethical health care20 may contribute to this behavior.
Whaley21 compared nonpathologic distrust and paranoia in 404 community-living African-Americans and whites. Some were healthy, and some had diagnoses of schizophrenia or depression. African-Americans—particularly those with psychiatric disorders—showed higher levels of distrust than whites. Distrust was also associated with depression in African-Americans but not in whites. Whaley concluded that:
- depressed African-Americans may exhibit more distrust toward clinicians than do whites
- this distrust puts African-Americans at risk of being perceived as paranoid and being misdiagnosed with paranoid schizophrenia.
Table
Remedial actions to avoid ethnic bias in diagnosing schizophrenia
| Problem | Remedies |
|---|---|
| Failure to recognize differences in symptom expression | Become familiar with ethnic differences in how patients describe symptoms Incorporate structured interviews or rating scales into the clinical assessment |
| Failure to elicit affective symptoms | Incorporate structured interviews or rating scales into the clinical assessment Maintain a high index of suspicion for affective symptoms (see Box 2) |
| Misinterpreted protective wariness | Clarify the patient’s degree of suspicion; consider this in the historical context of abuses toward minorities by majority populations Become familiar with ethnic differences in how symptoms are described |
| Covert and overt stereotyping and cultural insensitivity | Review practice patterns Consult with culturally sensitive clinicians as necessary |
Though Whaley did not report differences in distrust between African-American men and women, others have noted that distrust of health providers may be more common in minority men.18
RACIAL STEREOTYPING
Compared with similar white men, African-American men with mental disorders are more likely to be:
- referred for mental health care through social and legal—rather than medical—systems and to be involuntarily committed
- perceived as violent—even though controlled research suggests they are not. This misperception can lead to excessive medication and restraints.22
Differential treatment of African-American men may create a cycle of distrust, hostility, and additional inappropriate treatment. Together, these factors may increase the risk that African-American men will be misdiagnosed with schizophrenia.
Past racism in biomedical and psychiatric practice and research has been documented23,24 and more recently reviewed by Lawson.19 Historically, African-Americans were perceived to have a “primitive psychic” nature that was thought to be more susceptible to schizophrenia than depression.19 Whether these or similar racist stereotypes continue to inject ethnic bias into clinical assessment requires further study.
WHERE DO WE GO FROM HERE?
Although research into methods to eliminate ethnicity bias is sparse, the work reviewed in this article suggests ways that psychiatrists can minimize this problem (Table).
Obtain comprehensive information. Use structured interviews, such as the Structured Clinical Interview for DSM-IV (SCID), and rating scales, such as the Hamilton Depression Scale, which require clinicians to ask about all types of symptoms, particularly affective symptoms.
Review treatment records. Review your practice patterns for evidence of schizophrenia over-diagnosis in African-Americans or other ethnic groups. Examine ethnic differences in legal referrals or use of restraints or seclusion, which may indicate an ethnic bias in how threats are perceived. Only by being aware of bias can one correct it.
Become familiar with cultural and ethnic differences in idioms of distress. Specifically, review research in cultural psychiatry to identify potential differences among cultural groups in how they describe psychiatric symptoms. Talk with colleagues or friends from other cultural groups, and read literature from different ethnic perspectives to increase your cultural sensitivity.
Consult with psychiatrists with expertise in cultural variability of clinical presentation when the diagnosis or threat assessment seems unclear. Consultation is recommended if a patient’s diagnosis is uncertain or if you detect bias in your practice.
These interventions require clinicians to become familiar with psychosocial differences in how patients of various cultural and ethnic groups express psychiatric symptoms. With this understanding, we can better engage wary patients, obtain valid information, and improve clinical practice and patient outcomes.
Finally, psychiatry’s diagnostic systems need to continually address how patient assessment is influenced by ethnicity, culture, gender, and other socio-demographic factors. Studies are needed to examine the contributions of multiple factors—such as symptom differences and stereotyping—that contribute to ethnic-related diagnostic disparities.
Related resources
- Paul AM. Painting insanity black: Why are there more black schizophrenics? Salon.com Dec. 1, 1999. http://www.salon.com/books/it/1999/12/01/schizo/index.html
- Alarcon RD, Westermeyer J, Foulks EF, Ruiz P. Clinical relevance of contemporary cultural psychiatry. J Nerv Ment Dis 1999;187: 465-71.
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health 2003;93:200-8.
- Lin KM, Smith MW, Ortiz V. Culture and psychopharmacology. Psychiatr Clin North Am 2001; 24:523-38.
Acknowledgement
Preparation of this manuscript was supported in part by National Institutes of Health grant MH56352.
1. Robins LN, Regier DA (eds). Psychiatric disorders in America: the Epidemiologic Catchment Area study. New York: The Free Press. 1991.
2. Simon RJ, Fleiss JL, Gurland BJ, et al. Depression and schizophrenia in hospitalized black and white mental patients. Arch Gen Psychiatry 1973;28:509-12.
3. Mukherjee S, Shukla S, Woodle J, et al. Misdiagnosis of schizophrenia in bipolar patients: a multiethnic comparison. Am J Psychiatry 1983;140:1571-4.
4. Strakowski SM, Shelton RC, Kolbrener ML. The effects of race and comorbidity on clinical diagnosis in patients with psychosis. J Clin Psychiatry 1993;54:96-102.
5. Strakowski SM, Lonczak HS, Sax KW, et al. The effects of race on diagnosis and disposition from a psychiatric emergency service. J Clin Psychiatry 1995;56:101-7.
6. Lawson WB, Hepler N, Holladay J, Cuffel B. Race as a factor in inpatient and outpatient admissions and diagnosis. Hosp Comm Psychiatry 1994;45:72-4.
7. Hoffman H, Kupper Z, Kunz B. Hopelessness and its impact on rehabilitation outcome in schizophrenia—an exploratory study. Schizophr Res 2000;43:147-58.
8. Walkup JT, McAlpine DD, Olfson M, et al. Patients with schizophrenia at risk for excessive antipsychotic dosing. J Clin Psychiatry 2000;61:344-8.
9. Segal SP, Bola JR, Watson MA. Race, quality of care, and antipsychotic prescribing practices in psychiatric emergency services. Psychiatr Serv 1996;47:282-6.
10. Flaskerud JH, Hu L. Racial/ethnic identity and amount and type of psychiatric treatment. Am J Psychiatry 1992;149:379-84.
11. Adebimpe VR, Klein HE, Fried J. Hallucinations and delusions in black psychiatric patients. J Natl Med Assoc 1981;73:517-20.
12. Adebimpe VR, Chu CC, Klein HE, Lange MH. Racial and geographic differences in the psychopathology of schizophrenia. Am J Psychiatry 1982;139:888-91.
13. Fabrega H, Jr, Mezzich J, Ulrich RF. Black-white differences in psychopathology in an urban psychiatric population. Compr Psychiatry 1988;29:285-97.
14. Strakowski SM, Flaum M, Amador X, et al. Racial differences in the diagnosis of psychosis. Schizophr Res 1996;21:117-24.
15. Schneider K. Clinical psychopathology (translated by Hamilton MW). New York: Grune and Stratton, 1959.
16. Strakowski SM, McElroy SL, Keck PE, Jr, West SA. Racial influence on diagnosis in psychotic mania. J Aff Disord. 1996;39:157-62.
17. Strakowski SM, Hawkins JM, Keck PE, Jr, et al. The effects of race and information variance on disagreement between psychiatric emergency service and research diagnoses in first-episode psychosis. J Clin Psychiatry 1997;58:457-63.
18. Jones BE, Gray BA. Problems in diagnosing schizophrenia and affective disorders among blacks. Hosp Comm Psychiatry 1986;37:61-5.
19. Neighbors HW, Jackson JS, Campbell L, Williams D. The influence of racial factors on psychiatric diagnosis: A review and suggestions for research. Comm Ment Health J 1989;25:301-11.
20. Lawson WB. Racial and ethnic factors in psychiatric research. Hosp Comm Psychiatry 1986;37:50-4.
21. Whaley AL. Ethnicity/race, paranoia, and psychiatric diagnoses: Clinician bias versus sociocultural differences. J Psychopathol Behav Assess 1997;19:1-20.
22. Lawson WB, Yesavage JA, Werner RD. Race, violence, and psychopathology. J Clin Psychiatry 1984;45:294-7.
23. Spurlock J. Psychiatric states. In: Williams RA (ed). Textbook of black-related diseases. New York: McGraw-Hill, 1975.
24. Thomas A, Sillen S. Racism and psychiatry. New York: Brunner/Mazel, 1972.
In patients with psychotic symptoms, why are African-Americans more likely than whites to be diagnosed with schizophrenia? After more than 30 years of debate, some answers—and remedies for the problem—are becoming clear.
In psychiatry, where interpersonal interactions are key to eliciting diagnostic symptoms and signs, there is an intrinsic risk of misinterpretation when clinician and patient are of different cultural, ethnic, or socioeconomic backgrounds. This article analyzes four factors that contribute to misinterpretation and to ethnic misdiagnosis of schizophrenia. Culturally sensitive strategies are offered to avoid diagnostic bias in clinical practice.
SCHIZOPHRENIA MISDIAGNOSIS
Large epidemiologic studies report similar rates of schizophrenia and bipolar disorder in African-American and white populations.1 Although patients of both races have been wrongly diagnosed with schizophrenia, the pattern is stronger and more persistent in African-Americans.
Two (or more) of the following, each present for a significant portion of time during a 1-month period (or less if successfully treated):
- Delusions
- Hallucinations
- Disorganized speech (eg, frequent derailment or incoherence)
- Grossly disorganized or catatonic behavior
- Negative symptoms (ie, affective flattening, alogia, or avolition)
Note: Only one Criterion A symptom is required if delusions are bizarre or hallucinations consist of a voice keeping up a running commentary on the person’s behavior or thoughts, or two or more voices conversing with each other.
295.30 Paranoid type
A type of schizophrenia in which the following criteria are met:
- Preoccupation with one or more delusions or frequent auditory hallucinations
- None of the following is prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect.
Source: DSM-IV-TR
In the 1970s, Simon et al2 studied 192 hospitalized patients, of whom all African-Americans and 85% of whites had been identified clinically as having schizophrenia. Using a structured interview, the researchers found that only 40% of the African Americans and 50% of the whites met diagnostic criteria for schizophrenia. African-Americans with mood disorders were found to be at particular risk of schizophrenia misdiagnosis.
In the 1980s, among 76 patients with a clinical diagnosis of schizophrenia, Mukherjee et al3 diagnosed one-half (52%) with bipolar disorder using a structured clinical interview. Schizophrenia misdiagnoses were more common in African-Americans (86%) and Hispanics (83%) than in whites (51%). In particular, African-Americans were most likely to be misdiagnosed with paranoid schizophrenia. African-Americans complained more commonly than whites of auditory hallucinations, which may represent an ethnic difference in symptomatic presentation of psychotic mood disorders.
In the 1990s, colleagues and I conducted two studies—one of 173 patients in a Tennessee psychiatric hospital4 and the other of 490 patients in an Ohio psychiatric emergency service5—and found yet again that African-Americans were more likely than whites to be diagnosed with schizophrenia. In the hospital study, higher rates of schizophrenia diagnosis were associated with lower rates of mood disorder diagnosis. This inverse relationship implied that African-Americans with mood disorders were being misdiagnosed with schizophrenia.
Men were more likely than women to be diagnosed with schizophrenia, suggesting that African-American men were most likely to be misdiagnosed. When adjustments were made for gender, black women were found to be at higher risk for misdiagnosis than white women.
Lawson et al6 extended this research in a population-based study of African-Americans living in Tennessee. They found that African-Americans constituted 16% of the state’s population but 48% of psychiatric inpatients diagnosed with schizophrenia and 37% of psychiatric outpatients.
CONSEQUENCES OF INACCURATE DIAGNOSIS
Differentiating between schizophrenia (Box 1) and a psychotic mood disorder (Box 2) is more than a semantic exercise. Schizophrenia implies a chronic, unremitting, debilitating illness that worsens over time. Though this perception of schizophrenia is not entirely accurate, in clinical practice its diagnosis imparts a bleak prognosis that may lower the clinician’s expectations for the patient.7
Schizophrenia misdiagnosis also may lead the psychiatrist to rely excessively on antipsychotics, rather than attempting thymoleptic and psychotherapy trials. Studies suggest that African-American patients are more likely than similar white patients to receive antipsychotics4,8,9 and less likely to receive psychotherapy.5,10
Reasons why African-Americans are often misdiagnosed with schizophrenia remain unclear but probably include four contributing factors:
- differences in symptom presentation compared with whites
- failure by clinicians to identify affective symptoms in African-Americans
- minority patients’ wariness when dealing with health services
- and racial stereotyping.
DIFFERENCES IN SYMPTOM EXPRESSION
African-American patients with mood disorders or schizophrenia are more likely than are similar white patients to complain of auditory hallucinations.11-13 For example, Strakowski et al14 examined 330 patients with nonaffective and psychotic diagnoses in a study that was used to develop DSM-IV criteria for schizophrenia. Auditory hallucinations were rated as more severe in African-American than in similar white patients.
MAJOR DEPRESSIVE EPISODE
Five or more of the following symptoms present during the same 2-week period and representing a change from previous functioning; must include either depressed mood or loss of interest or pleasure.
- Depressed mood
- Markedly diminished interest or pleasure
- Significant weight loss
- Insomnia or hypersomnia
- Psychomotor agitation or retardation
- Fatigue
- Feelings of worthlessness or excessive guilt
- Diminished ability to concentrate
SEVERE MAJOR DEPRESSION WITH PSYCHOTIC FEATURES
Mood-congruent
Delusions or hallucinations whose content is entirely consistent with the typical depressive themes of personal inadequacy, guilt, disease, death, nihilism, or deserved punishment
Mood-incongruent
Delusions or hallucinations whose content does not involve typical depressive themes. Includes symptoms such as persecutory delusions, thought insertion, thought broadcasting, and delusions of control
Source: DSM-IV-TR
African-American patients also are more likely than whites to exhibit so-called Schneiderian first-rank symptoms of schizophrenia,15 including:
- delusions of thought broadcasting or insertion
- auditory hallucinations of voices conversing about the patient in the third person.
These symptoms were once used to diagnose schizophrenia, but their lack of specificity has been well documented.2,16 First-rank symptoms of schizophrenia depend on the specific form of the hallucination or delusion, are likely to be influenced by a patient’s culture, and may be misleading in multicultural populations. Though first-rank symptoms now occupy a minor role in U.S. diagnostic systems, they might continue to sway clinicians—even when using structured diagnostic interviews—to inappropriately diagnose schizophrenia in lieu of affective disorders in minority patients.15
To extend this finding, our group16 studied rates and severity of affective and psychotic symptoms—particularly first-rank symptoms—in 100 patients with psychotic mania who met DSM-III-R criteria for bipolar disorder (80%) or schizoaffective disorder, bipolar type (20%) as determined by structured diagnostic interview. No differences in affective symptoms between African-American and white patients were seen. African-Americans were more likely to endorse auditory hallucinations and to report severe auditory hallucinations of voices commenting on their behavior—the only first-rank symptom on which they differed from whites.
Though their affective symptoms were similar, African-Americans were significantly more likely than whites to have been diagnosed with a schizophrenia-spectrum disorder. Because misdiagnosis of African-Americans could not be explained by psychotic symptoms—which were as severe as those of white patients—these findings suggest other mechanisms were at work.
UNIDENTIFIED AFFECTIVE SYMPTOMS
Underidentification of mood disorders in African-American patients may also lead to over-diagnosis of schizophrenia. In a sample of 99 patients, colleagues and I17 compared clinical diagnoses made in a psychiatric emergency service with those by research investigators using a structured clinical interview. Reasons for diagnostic differences were identified and divided into two categories:
- the same symptoms were recorded but applied differently to diagnostic criteria (criterion variance)
- different information was recorded, which led to diagnostic discrepancies (information variance).
Differences occurred significantly more often in African-American than in white patients, but only information variance was associated with ethnicity. This suggests that clinicians are less likely to elicit appropriate information from African-American than from white psychiatric patients. The fact that researchers obtained this information during diagnostic interviews suggests that the patients could provide it when given appropriate prompts. Specifically, affective symptoms were less likely to be elicited by clinicians than by researchers.
PATIENT WARINESS
Minority patients, when interacting with clinicians of the majority population, may project “protective wariness.”18 Specific behaviors include hesitancy or reluctance to fully engage with the care provider as a precaution against being exploited or harmed. Cultural misunderstandings19 and patient concerns about past reports of minorities receiving substandard or unethical health care20 may contribute to this behavior.
Whaley21 compared nonpathologic distrust and paranoia in 404 community-living African-Americans and whites. Some were healthy, and some had diagnoses of schizophrenia or depression. African-Americans—particularly those with psychiatric disorders—showed higher levels of distrust than whites. Distrust was also associated with depression in African-Americans but not in whites. Whaley concluded that:
- depressed African-Americans may exhibit more distrust toward clinicians than do whites
- this distrust puts African-Americans at risk of being perceived as paranoid and being misdiagnosed with paranoid schizophrenia.
Table
Remedial actions to avoid ethnic bias in diagnosing schizophrenia
| Problem | Remedies |
|---|---|
| Failure to recognize differences in symptom expression | Become familiar with ethnic differences in how patients describe symptoms Incorporate structured interviews or rating scales into the clinical assessment |
| Failure to elicit affective symptoms | Incorporate structured interviews or rating scales into the clinical assessment Maintain a high index of suspicion for affective symptoms (see Box 2) |
| Misinterpreted protective wariness | Clarify the patient’s degree of suspicion; consider this in the historical context of abuses toward minorities by majority populations Become familiar with ethnic differences in how symptoms are described |
| Covert and overt stereotyping and cultural insensitivity | Review practice patterns Consult with culturally sensitive clinicians as necessary |
Though Whaley did not report differences in distrust between African-American men and women, others have noted that distrust of health providers may be more common in minority men.18
RACIAL STEREOTYPING
Compared with similar white men, African-American men with mental disorders are more likely to be:
- referred for mental health care through social and legal—rather than medical—systems and to be involuntarily committed
- perceived as violent—even though controlled research suggests they are not. This misperception can lead to excessive medication and restraints.22
Differential treatment of African-American men may create a cycle of distrust, hostility, and additional inappropriate treatment. Together, these factors may increase the risk that African-American men will be misdiagnosed with schizophrenia.
Past racism in biomedical and psychiatric practice and research has been documented23,24 and more recently reviewed by Lawson.19 Historically, African-Americans were perceived to have a “primitive psychic” nature that was thought to be more susceptible to schizophrenia than depression.19 Whether these or similar racist stereotypes continue to inject ethnic bias into clinical assessment requires further study.
WHERE DO WE GO FROM HERE?
Although research into methods to eliminate ethnicity bias is sparse, the work reviewed in this article suggests ways that psychiatrists can minimize this problem (Table).
Obtain comprehensive information. Use structured interviews, such as the Structured Clinical Interview for DSM-IV (SCID), and rating scales, such as the Hamilton Depression Scale, which require clinicians to ask about all types of symptoms, particularly affective symptoms.
Review treatment records. Review your practice patterns for evidence of schizophrenia over-diagnosis in African-Americans or other ethnic groups. Examine ethnic differences in legal referrals or use of restraints or seclusion, which may indicate an ethnic bias in how threats are perceived. Only by being aware of bias can one correct it.
Become familiar with cultural and ethnic differences in idioms of distress. Specifically, review research in cultural psychiatry to identify potential differences among cultural groups in how they describe psychiatric symptoms. Talk with colleagues or friends from other cultural groups, and read literature from different ethnic perspectives to increase your cultural sensitivity.
Consult with psychiatrists with expertise in cultural variability of clinical presentation when the diagnosis or threat assessment seems unclear. Consultation is recommended if a patient’s diagnosis is uncertain or if you detect bias in your practice.
These interventions require clinicians to become familiar with psychosocial differences in how patients of various cultural and ethnic groups express psychiatric symptoms. With this understanding, we can better engage wary patients, obtain valid information, and improve clinical practice and patient outcomes.
Finally, psychiatry’s diagnostic systems need to continually address how patient assessment is influenced by ethnicity, culture, gender, and other socio-demographic factors. Studies are needed to examine the contributions of multiple factors—such as symptom differences and stereotyping—that contribute to ethnic-related diagnostic disparities.
Related resources
- Paul AM. Painting insanity black: Why are there more black schizophrenics? Salon.com Dec. 1, 1999. http://www.salon.com/books/it/1999/12/01/schizo/index.html
- Alarcon RD, Westermeyer J, Foulks EF, Ruiz P. Clinical relevance of contemporary cultural psychiatry. J Nerv Ment Dis 1999;187: 465-71.
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health 2003;93:200-8.
- Lin KM, Smith MW, Ortiz V. Culture and psychopharmacology. Psychiatr Clin North Am 2001; 24:523-38.
Acknowledgement
Preparation of this manuscript was supported in part by National Institutes of Health grant MH56352.
In patients with psychotic symptoms, why are African-Americans more likely than whites to be diagnosed with schizophrenia? After more than 30 years of debate, some answers—and remedies for the problem—are becoming clear.
In psychiatry, where interpersonal interactions are key to eliciting diagnostic symptoms and signs, there is an intrinsic risk of misinterpretation when clinician and patient are of different cultural, ethnic, or socioeconomic backgrounds. This article analyzes four factors that contribute to misinterpretation and to ethnic misdiagnosis of schizophrenia. Culturally sensitive strategies are offered to avoid diagnostic bias in clinical practice.
SCHIZOPHRENIA MISDIAGNOSIS
Large epidemiologic studies report similar rates of schizophrenia and bipolar disorder in African-American and white populations.1 Although patients of both races have been wrongly diagnosed with schizophrenia, the pattern is stronger and more persistent in African-Americans.
Two (or more) of the following, each present for a significant portion of time during a 1-month period (or less if successfully treated):
- Delusions
- Hallucinations
- Disorganized speech (eg, frequent derailment or incoherence)
- Grossly disorganized or catatonic behavior
- Negative symptoms (ie, affective flattening, alogia, or avolition)
Note: Only one Criterion A symptom is required if delusions are bizarre or hallucinations consist of a voice keeping up a running commentary on the person’s behavior or thoughts, or two or more voices conversing with each other.
295.30 Paranoid type
A type of schizophrenia in which the following criteria are met:
- Preoccupation with one or more delusions or frequent auditory hallucinations
- None of the following is prominent: disorganized speech, disorganized or catatonic behavior, or flat or inappropriate affect.
Source: DSM-IV-TR
In the 1970s, Simon et al2 studied 192 hospitalized patients, of whom all African-Americans and 85% of whites had been identified clinically as having schizophrenia. Using a structured interview, the researchers found that only 40% of the African Americans and 50% of the whites met diagnostic criteria for schizophrenia. African-Americans with mood disorders were found to be at particular risk of schizophrenia misdiagnosis.
In the 1980s, among 76 patients with a clinical diagnosis of schizophrenia, Mukherjee et al3 diagnosed one-half (52%) with bipolar disorder using a structured clinical interview. Schizophrenia misdiagnoses were more common in African-Americans (86%) and Hispanics (83%) than in whites (51%). In particular, African-Americans were most likely to be misdiagnosed with paranoid schizophrenia. African-Americans complained more commonly than whites of auditory hallucinations, which may represent an ethnic difference in symptomatic presentation of psychotic mood disorders.
In the 1990s, colleagues and I conducted two studies—one of 173 patients in a Tennessee psychiatric hospital4 and the other of 490 patients in an Ohio psychiatric emergency service5—and found yet again that African-Americans were more likely than whites to be diagnosed with schizophrenia. In the hospital study, higher rates of schizophrenia diagnosis were associated with lower rates of mood disorder diagnosis. This inverse relationship implied that African-Americans with mood disorders were being misdiagnosed with schizophrenia.
Men were more likely than women to be diagnosed with schizophrenia, suggesting that African-American men were most likely to be misdiagnosed. When adjustments were made for gender, black women were found to be at higher risk for misdiagnosis than white women.
Lawson et al6 extended this research in a population-based study of African-Americans living in Tennessee. They found that African-Americans constituted 16% of the state’s population but 48% of psychiatric inpatients diagnosed with schizophrenia and 37% of psychiatric outpatients.
CONSEQUENCES OF INACCURATE DIAGNOSIS
Differentiating between schizophrenia (Box 1) and a psychotic mood disorder (Box 2) is more than a semantic exercise. Schizophrenia implies a chronic, unremitting, debilitating illness that worsens over time. Though this perception of schizophrenia is not entirely accurate, in clinical practice its diagnosis imparts a bleak prognosis that may lower the clinician’s expectations for the patient.7
Schizophrenia misdiagnosis also may lead the psychiatrist to rely excessively on antipsychotics, rather than attempting thymoleptic and psychotherapy trials. Studies suggest that African-American patients are more likely than similar white patients to receive antipsychotics4,8,9 and less likely to receive psychotherapy.5,10
Reasons why African-Americans are often misdiagnosed with schizophrenia remain unclear but probably include four contributing factors:
- differences in symptom presentation compared with whites
- failure by clinicians to identify affective symptoms in African-Americans
- minority patients’ wariness when dealing with health services
- and racial stereotyping.
DIFFERENCES IN SYMPTOM EXPRESSION
African-American patients with mood disorders or schizophrenia are more likely than are similar white patients to complain of auditory hallucinations.11-13 For example, Strakowski et al14 examined 330 patients with nonaffective and psychotic diagnoses in a study that was used to develop DSM-IV criteria for schizophrenia. Auditory hallucinations were rated as more severe in African-American than in similar white patients.
MAJOR DEPRESSIVE EPISODE
Five or more of the following symptoms present during the same 2-week period and representing a change from previous functioning; must include either depressed mood or loss of interest or pleasure.
- Depressed mood
- Markedly diminished interest or pleasure
- Significant weight loss
- Insomnia or hypersomnia
- Psychomotor agitation or retardation
- Fatigue
- Feelings of worthlessness or excessive guilt
- Diminished ability to concentrate
SEVERE MAJOR DEPRESSION WITH PSYCHOTIC FEATURES
Mood-congruent
Delusions or hallucinations whose content is entirely consistent with the typical depressive themes of personal inadequacy, guilt, disease, death, nihilism, or deserved punishment
Mood-incongruent
Delusions or hallucinations whose content does not involve typical depressive themes. Includes symptoms such as persecutory delusions, thought insertion, thought broadcasting, and delusions of control
Source: DSM-IV-TR
African-American patients also are more likely than whites to exhibit so-called Schneiderian first-rank symptoms of schizophrenia,15 including:
- delusions of thought broadcasting or insertion
- auditory hallucinations of voices conversing about the patient in the third person.
These symptoms were once used to diagnose schizophrenia, but their lack of specificity has been well documented.2,16 First-rank symptoms of schizophrenia depend on the specific form of the hallucination or delusion, are likely to be influenced by a patient’s culture, and may be misleading in multicultural populations. Though first-rank symptoms now occupy a minor role in U.S. diagnostic systems, they might continue to sway clinicians—even when using structured diagnostic interviews—to inappropriately diagnose schizophrenia in lieu of affective disorders in minority patients.15
To extend this finding, our group16 studied rates and severity of affective and psychotic symptoms—particularly first-rank symptoms—in 100 patients with psychotic mania who met DSM-III-R criteria for bipolar disorder (80%) or schizoaffective disorder, bipolar type (20%) as determined by structured diagnostic interview. No differences in affective symptoms between African-American and white patients were seen. African-Americans were more likely to endorse auditory hallucinations and to report severe auditory hallucinations of voices commenting on their behavior—the only first-rank symptom on which they differed from whites.
Though their affective symptoms were similar, African-Americans were significantly more likely than whites to have been diagnosed with a schizophrenia-spectrum disorder. Because misdiagnosis of African-Americans could not be explained by psychotic symptoms—which were as severe as those of white patients—these findings suggest other mechanisms were at work.
UNIDENTIFIED AFFECTIVE SYMPTOMS
Underidentification of mood disorders in African-American patients may also lead to over-diagnosis of schizophrenia. In a sample of 99 patients, colleagues and I17 compared clinical diagnoses made in a psychiatric emergency service with those by research investigators using a structured clinical interview. Reasons for diagnostic differences were identified and divided into two categories:
- the same symptoms were recorded but applied differently to diagnostic criteria (criterion variance)
- different information was recorded, which led to diagnostic discrepancies (information variance).
Differences occurred significantly more often in African-American than in white patients, but only information variance was associated with ethnicity. This suggests that clinicians are less likely to elicit appropriate information from African-American than from white psychiatric patients. The fact that researchers obtained this information during diagnostic interviews suggests that the patients could provide it when given appropriate prompts. Specifically, affective symptoms were less likely to be elicited by clinicians than by researchers.
PATIENT WARINESS
Minority patients, when interacting with clinicians of the majority population, may project “protective wariness.”18 Specific behaviors include hesitancy or reluctance to fully engage with the care provider as a precaution against being exploited or harmed. Cultural misunderstandings19 and patient concerns about past reports of minorities receiving substandard or unethical health care20 may contribute to this behavior.
Whaley21 compared nonpathologic distrust and paranoia in 404 community-living African-Americans and whites. Some were healthy, and some had diagnoses of schizophrenia or depression. African-Americans—particularly those with psychiatric disorders—showed higher levels of distrust than whites. Distrust was also associated with depression in African-Americans but not in whites. Whaley concluded that:
- depressed African-Americans may exhibit more distrust toward clinicians than do whites
- this distrust puts African-Americans at risk of being perceived as paranoid and being misdiagnosed with paranoid schizophrenia.
Table
Remedial actions to avoid ethnic bias in diagnosing schizophrenia
| Problem | Remedies |
|---|---|
| Failure to recognize differences in symptom expression | Become familiar with ethnic differences in how patients describe symptoms Incorporate structured interviews or rating scales into the clinical assessment |
| Failure to elicit affective symptoms | Incorporate structured interviews or rating scales into the clinical assessment Maintain a high index of suspicion for affective symptoms (see Box 2) |
| Misinterpreted protective wariness | Clarify the patient’s degree of suspicion; consider this in the historical context of abuses toward minorities by majority populations Become familiar with ethnic differences in how symptoms are described |
| Covert and overt stereotyping and cultural insensitivity | Review practice patterns Consult with culturally sensitive clinicians as necessary |
Though Whaley did not report differences in distrust between African-American men and women, others have noted that distrust of health providers may be more common in minority men.18
RACIAL STEREOTYPING
Compared with similar white men, African-American men with mental disorders are more likely to be:
- referred for mental health care through social and legal—rather than medical—systems and to be involuntarily committed
- perceived as violent—even though controlled research suggests they are not. This misperception can lead to excessive medication and restraints.22
Differential treatment of African-American men may create a cycle of distrust, hostility, and additional inappropriate treatment. Together, these factors may increase the risk that African-American men will be misdiagnosed with schizophrenia.
Past racism in biomedical and psychiatric practice and research has been documented23,24 and more recently reviewed by Lawson.19 Historically, African-Americans were perceived to have a “primitive psychic” nature that was thought to be more susceptible to schizophrenia than depression.19 Whether these or similar racist stereotypes continue to inject ethnic bias into clinical assessment requires further study.
WHERE DO WE GO FROM HERE?
Although research into methods to eliminate ethnicity bias is sparse, the work reviewed in this article suggests ways that psychiatrists can minimize this problem (Table).
Obtain comprehensive information. Use structured interviews, such as the Structured Clinical Interview for DSM-IV (SCID), and rating scales, such as the Hamilton Depression Scale, which require clinicians to ask about all types of symptoms, particularly affective symptoms.
Review treatment records. Review your practice patterns for evidence of schizophrenia over-diagnosis in African-Americans or other ethnic groups. Examine ethnic differences in legal referrals or use of restraints or seclusion, which may indicate an ethnic bias in how threats are perceived. Only by being aware of bias can one correct it.
Become familiar with cultural and ethnic differences in idioms of distress. Specifically, review research in cultural psychiatry to identify potential differences among cultural groups in how they describe psychiatric symptoms. Talk with colleagues or friends from other cultural groups, and read literature from different ethnic perspectives to increase your cultural sensitivity.
Consult with psychiatrists with expertise in cultural variability of clinical presentation when the diagnosis or threat assessment seems unclear. Consultation is recommended if a patient’s diagnosis is uncertain or if you detect bias in your practice.
These interventions require clinicians to become familiar with psychosocial differences in how patients of various cultural and ethnic groups express psychiatric symptoms. With this understanding, we can better engage wary patients, obtain valid information, and improve clinical practice and patient outcomes.
Finally, psychiatry’s diagnostic systems need to continually address how patient assessment is influenced by ethnicity, culture, gender, and other socio-demographic factors. Studies are needed to examine the contributions of multiple factors—such as symptom differences and stereotyping—that contribute to ethnic-related diagnostic disparities.
Related resources
- Paul AM. Painting insanity black: Why are there more black schizophrenics? Salon.com Dec. 1, 1999. http://www.salon.com/books/it/1999/12/01/schizo/index.html
- Alarcon RD, Westermeyer J, Foulks EF, Ruiz P. Clinical relevance of contemporary cultural psychiatry. J Nerv Ment Dis 1999;187: 465-71.
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health 2003;93:200-8.
- Lin KM, Smith MW, Ortiz V. Culture and psychopharmacology. Psychiatr Clin North Am 2001; 24:523-38.
Acknowledgement
Preparation of this manuscript was supported in part by National Institutes of Health grant MH56352.
1. Robins LN, Regier DA (eds). Psychiatric disorders in America: the Epidemiologic Catchment Area study. New York: The Free Press. 1991.
2. Simon RJ, Fleiss JL, Gurland BJ, et al. Depression and schizophrenia in hospitalized black and white mental patients. Arch Gen Psychiatry 1973;28:509-12.
3. Mukherjee S, Shukla S, Woodle J, et al. Misdiagnosis of schizophrenia in bipolar patients: a multiethnic comparison. Am J Psychiatry 1983;140:1571-4.
4. Strakowski SM, Shelton RC, Kolbrener ML. The effects of race and comorbidity on clinical diagnosis in patients with psychosis. J Clin Psychiatry 1993;54:96-102.
5. Strakowski SM, Lonczak HS, Sax KW, et al. The effects of race on diagnosis and disposition from a psychiatric emergency service. J Clin Psychiatry 1995;56:101-7.
6. Lawson WB, Hepler N, Holladay J, Cuffel B. Race as a factor in inpatient and outpatient admissions and diagnosis. Hosp Comm Psychiatry 1994;45:72-4.
7. Hoffman H, Kupper Z, Kunz B. Hopelessness and its impact on rehabilitation outcome in schizophrenia—an exploratory study. Schizophr Res 2000;43:147-58.
8. Walkup JT, McAlpine DD, Olfson M, et al. Patients with schizophrenia at risk for excessive antipsychotic dosing. J Clin Psychiatry 2000;61:344-8.
9. Segal SP, Bola JR, Watson MA. Race, quality of care, and antipsychotic prescribing practices in psychiatric emergency services. Psychiatr Serv 1996;47:282-6.
10. Flaskerud JH, Hu L. Racial/ethnic identity and amount and type of psychiatric treatment. Am J Psychiatry 1992;149:379-84.
11. Adebimpe VR, Klein HE, Fried J. Hallucinations and delusions in black psychiatric patients. J Natl Med Assoc 1981;73:517-20.
12. Adebimpe VR, Chu CC, Klein HE, Lange MH. Racial and geographic differences in the psychopathology of schizophrenia. Am J Psychiatry 1982;139:888-91.
13. Fabrega H, Jr, Mezzich J, Ulrich RF. Black-white differences in psychopathology in an urban psychiatric population. Compr Psychiatry 1988;29:285-97.
14. Strakowski SM, Flaum M, Amador X, et al. Racial differences in the diagnosis of psychosis. Schizophr Res 1996;21:117-24.
15. Schneider K. Clinical psychopathology (translated by Hamilton MW). New York: Grune and Stratton, 1959.
16. Strakowski SM, McElroy SL, Keck PE, Jr, West SA. Racial influence on diagnosis in psychotic mania. J Aff Disord. 1996;39:157-62.
17. Strakowski SM, Hawkins JM, Keck PE, Jr, et al. The effects of race and information variance on disagreement between psychiatric emergency service and research diagnoses in first-episode psychosis. J Clin Psychiatry 1997;58:457-63.
18. Jones BE, Gray BA. Problems in diagnosing schizophrenia and affective disorders among blacks. Hosp Comm Psychiatry 1986;37:61-5.
19. Neighbors HW, Jackson JS, Campbell L, Williams D. The influence of racial factors on psychiatric diagnosis: A review and suggestions for research. Comm Ment Health J 1989;25:301-11.
20. Lawson WB. Racial and ethnic factors in psychiatric research. Hosp Comm Psychiatry 1986;37:50-4.
21. Whaley AL. Ethnicity/race, paranoia, and psychiatric diagnoses: Clinician bias versus sociocultural differences. J Psychopathol Behav Assess 1997;19:1-20.
22. Lawson WB, Yesavage JA, Werner RD. Race, violence, and psychopathology. J Clin Psychiatry 1984;45:294-7.
23. Spurlock J. Psychiatric states. In: Williams RA (ed). Textbook of black-related diseases. New York: McGraw-Hill, 1975.
24. Thomas A, Sillen S. Racism and psychiatry. New York: Brunner/Mazel, 1972.
1. Robins LN, Regier DA (eds). Psychiatric disorders in America: the Epidemiologic Catchment Area study. New York: The Free Press. 1991.
2. Simon RJ, Fleiss JL, Gurland BJ, et al. Depression and schizophrenia in hospitalized black and white mental patients. Arch Gen Psychiatry 1973;28:509-12.
3. Mukherjee S, Shukla S, Woodle J, et al. Misdiagnosis of schizophrenia in bipolar patients: a multiethnic comparison. Am J Psychiatry 1983;140:1571-4.
4. Strakowski SM, Shelton RC, Kolbrener ML. The effects of race and comorbidity on clinical diagnosis in patients with psychosis. J Clin Psychiatry 1993;54:96-102.
5. Strakowski SM, Lonczak HS, Sax KW, et al. The effects of race on diagnosis and disposition from a psychiatric emergency service. J Clin Psychiatry 1995;56:101-7.
6. Lawson WB, Hepler N, Holladay J, Cuffel B. Race as a factor in inpatient and outpatient admissions and diagnosis. Hosp Comm Psychiatry 1994;45:72-4.
7. Hoffman H, Kupper Z, Kunz B. Hopelessness and its impact on rehabilitation outcome in schizophrenia—an exploratory study. Schizophr Res 2000;43:147-58.
8. Walkup JT, McAlpine DD, Olfson M, et al. Patients with schizophrenia at risk for excessive antipsychotic dosing. J Clin Psychiatry 2000;61:344-8.
9. Segal SP, Bola JR, Watson MA. Race, quality of care, and antipsychotic prescribing practices in psychiatric emergency services. Psychiatr Serv 1996;47:282-6.
10. Flaskerud JH, Hu L. Racial/ethnic identity and amount and type of psychiatric treatment. Am J Psychiatry 1992;149:379-84.
11. Adebimpe VR, Klein HE, Fried J. Hallucinations and delusions in black psychiatric patients. J Natl Med Assoc 1981;73:517-20.
12. Adebimpe VR, Chu CC, Klein HE, Lange MH. Racial and geographic differences in the psychopathology of schizophrenia. Am J Psychiatry 1982;139:888-91.
13. Fabrega H, Jr, Mezzich J, Ulrich RF. Black-white differences in psychopathology in an urban psychiatric population. Compr Psychiatry 1988;29:285-97.
14. Strakowski SM, Flaum M, Amador X, et al. Racial differences in the diagnosis of psychosis. Schizophr Res 1996;21:117-24.
15. Schneider K. Clinical psychopathology (translated by Hamilton MW). New York: Grune and Stratton, 1959.
16. Strakowski SM, McElroy SL, Keck PE, Jr, West SA. Racial influence on diagnosis in psychotic mania. J Aff Disord. 1996;39:157-62.
17. Strakowski SM, Hawkins JM, Keck PE, Jr, et al. The effects of race and information variance on disagreement between psychiatric emergency service and research diagnoses in first-episode psychosis. J Clin Psychiatry 1997;58:457-63.
18. Jones BE, Gray BA. Problems in diagnosing schizophrenia and affective disorders among blacks. Hosp Comm Psychiatry 1986;37:61-5.
19. Neighbors HW, Jackson JS, Campbell L, Williams D. The influence of racial factors on psychiatric diagnosis: A review and suggestions for research. Comm Ment Health J 1989;25:301-11.
20. Lawson WB. Racial and ethnic factors in psychiatric research. Hosp Comm Psychiatry 1986;37:50-4.
21. Whaley AL. Ethnicity/race, paranoia, and psychiatric diagnoses: Clinician bias versus sociocultural differences. J Psychopathol Behav Assess 1997;19:1-20.
22. Lawson WB, Yesavage JA, Werner RD. Race, violence, and psychopathology. J Clin Psychiatry 1984;45:294-7.
23. Spurlock J. Psychiatric states. In: Williams RA (ed). Textbook of black-related diseases. New York: McGraw-Hill, 1975.
24. Thomas A, Sillen S. Racism and psychiatry. New York: Brunner/Mazel, 1972.