User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
The injustice of pre-authorization
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Time series analysis of poison control data
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
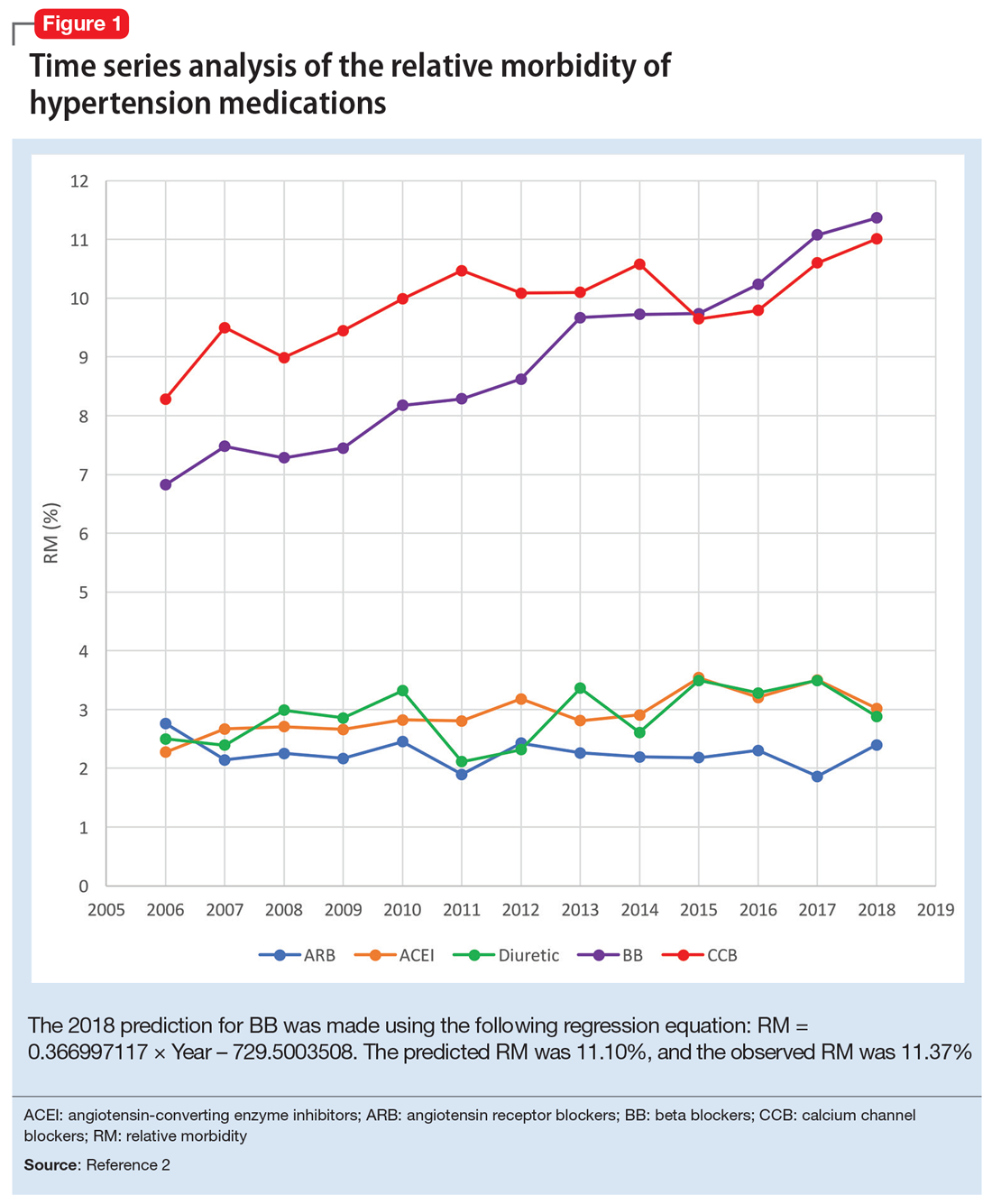
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).
Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
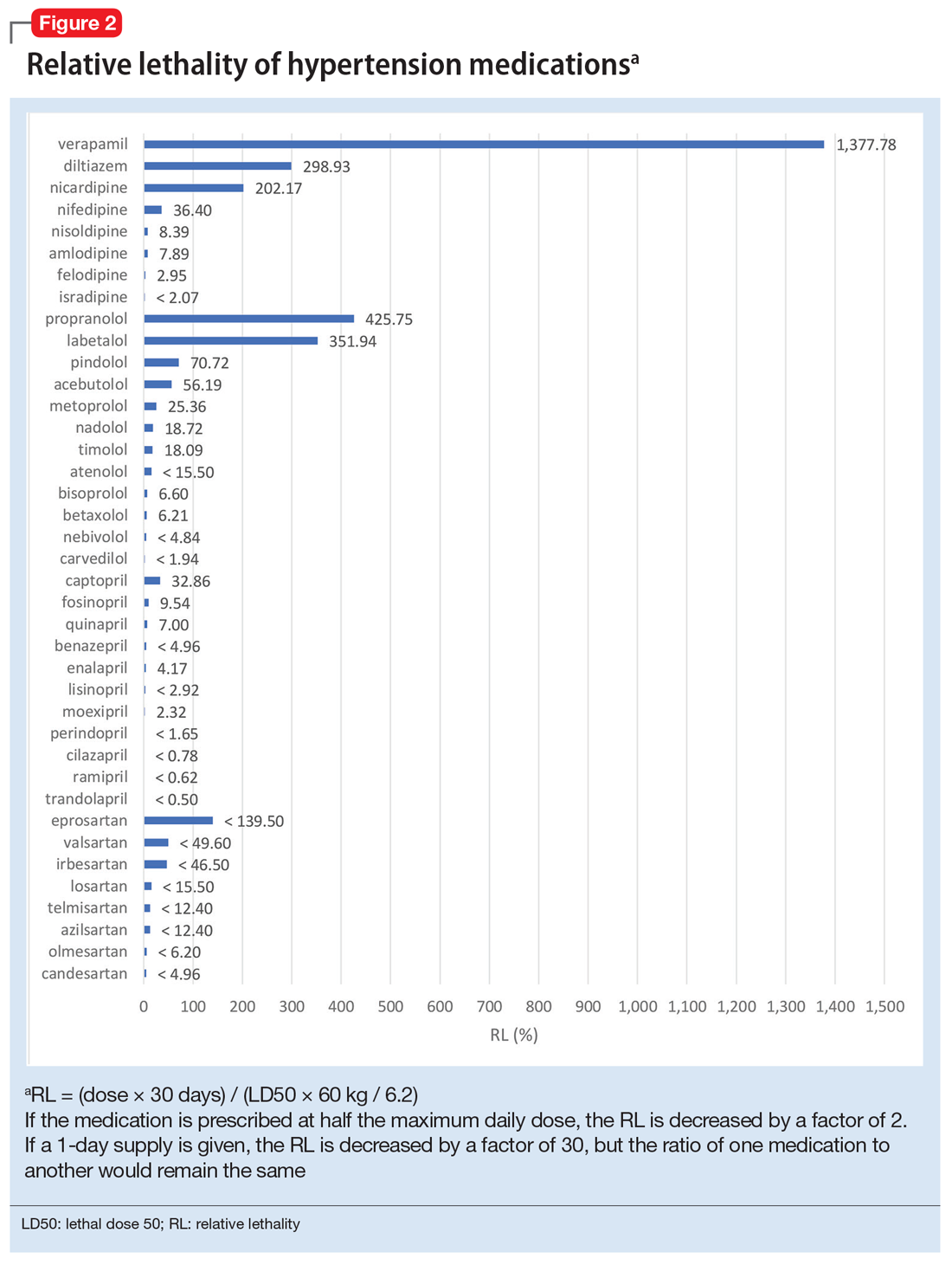
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.
These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
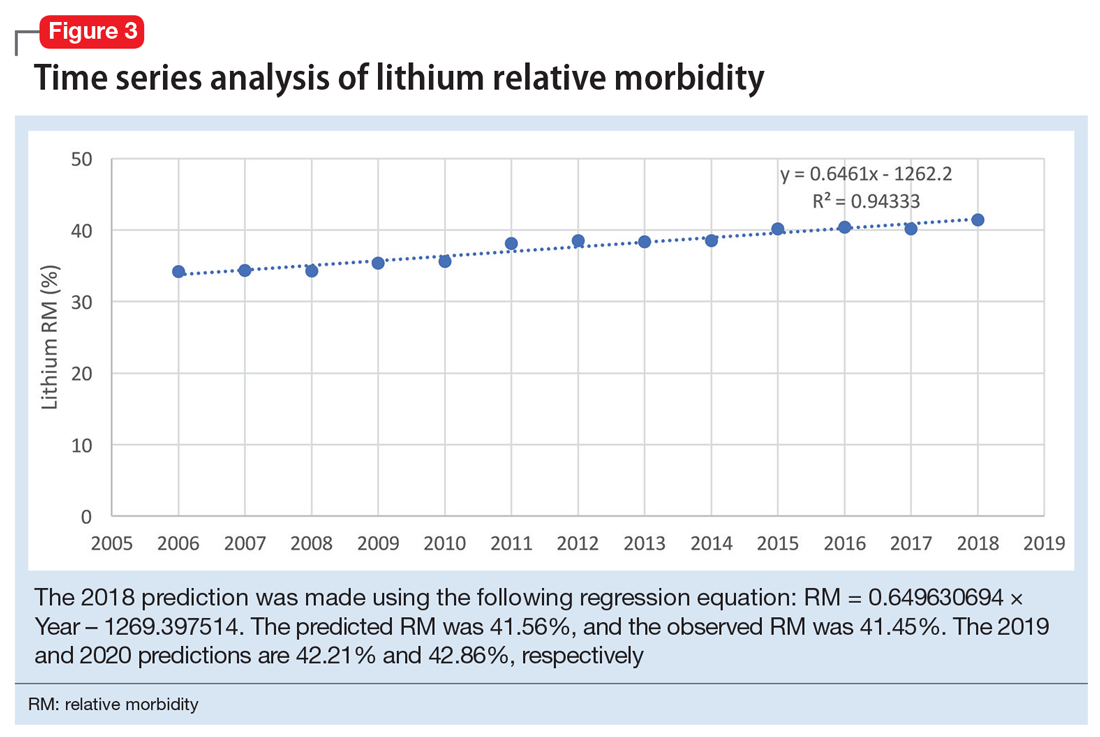
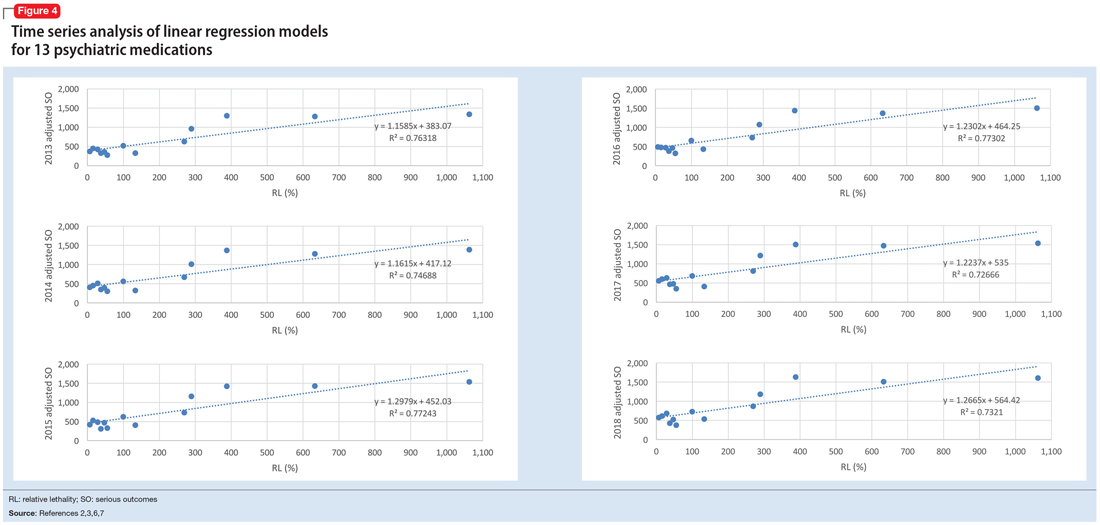
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).
Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).
Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.
These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).
Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).
Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.
These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).
Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
Telepsychiatry during COVID-19: Understanding the rules
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
The resident’s role in combating burnout among medical students
Burnout among health care professionals has been increasingly recognized by the medical community over the past several years. The concern for burnout among medical students is equally serious. In this article, I review the prevalence of burnout among medical students, and the personal and clinical effects they experience. I also discuss how as psychiatry residents we can be more effective in preventing and identifying medical student burnout.
An underappreciated problem
Burnout has been defined as long-term unresolvable job stress that leads to exhaustion and feeling overwhelmed, cynical, and detached from work, and lacking a sense of personal accomplishment. It can lead to depression, anxiety, and suicidal ideation—one survey found that 5.8% of medical students had experienced suicidal ideation at some point in the previous 12 months.1 Burnout affects not only the individual, but also his/her team and patients. One study found that compared to medical students who didn’t report burnout, medical students who did had lower scores on measures of empathy and professionalism.2
While burnout among physicians and residents has received increasing attention, it often may go unrecognized and unreported in medical students. A literature review that included 51 studies found 28% to 45% of medical students report burnout.3 In a survey at one institution, 60% of medical students reported burnout.4 It is evident that medical schools have an important role in helping to minimize burnout rates in their students, and many schools are working toward this goal. However, what happens when students leave the classroom setting for clinical rotations?
A recent study found burnout among medical students peaks during the third year of medical school.5 This is when students are on their clinical rotations, new to the hospital environment, and without the inherent structure and support of being at school.
How residents can help
Like most medical students, while on my clinical rotations, I spent most of my day with residents, and I believe residents can help to both recognize burnout in medical students and prevent it.
The first step in addressing this problem is to understand why it occurs. A survey of medical students showed that inadequate sleep and decreased exercise play a significant role in burnout rates.6 Another study found a correlation between burnout and feeling emotionally exhausted and a decreased perceived quality of life.7 A medical student I recently worked with stated, “How can you not feel burnt out? Juggling work hours, studying, debt, health, and trying to have a life… something always gets dropped.”
So as residents, what can we do to identify and assist medical students who are experiencing burnout, or are at risk of getting there? When needed, we can utilize our psychiatry training to assess our students for depression and substance use disorders, and connect them with appropriate resources. When identifying a medical student with burnout, I believe it can become necessary to notify the attending, the site director responsible for the student, and often the school, so that the student has access to all available resources.
Continue to: It's as important to be proactive...
It’s as important to be proactive as it is to be reactive. Engaging in regular check-ins with our students about self-care and workload, as well as asking about how they are feeling, can offer them opportunities to talk about issues that they might not be getting anywhere else. One medical student I worked with told me, “It’s easy to fade into the background as the student, or to feel like I can’t complain because this is just how medical school is supposed to be.” We have the ability to change this notion with each student we work with.
It is likely that as residents we have worked with a student struggling with burnout without even realizing it. I believe we can play an important role in helping to prevent burnout by identifying at-risk students, offering assistance, and encouraging them to seek professional help. Someone’s life may depend on it.
1. Dyrbye L, Thomas M, Massie F, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334-341.
2. Brazeau C, Schroeder R, Rovi S. Relationships between medical student burnout, empathy, and professionalism climate. Acad Med. 2010;85(suppl 10):S33-S36. doi: 10.1097/ACM.0b013e3181ed4c47.
3. IsHak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;1(2):236-242.
4. Chang E, Eddins-Folensbee F, Coverdale J. Survey of the prevalence of burnout, stress, depression, and the use of supports by medical students at one school. Acad Psychiatry. 2012;36(3):177-182.
5. Hansell MW, Ungerleider RM, Brooks CA, et al. Temporal trends in medical student burnout. Fam Med. 2019;51(5):399-404.
6. Wolf M, Rosenstock J. Inadequate sleep and exercise associated with burnout and depression among medical students. Acad Psychiatry. 2017;41(2):174-179.
7. Colby L, Mareka M, Pillay S, et al. The association between the levels of burnout and quality of life among fourth-year medical students at the University of the Free State. S Afr J Psychiatr. 2018;24:1101.
Burnout among health care professionals has been increasingly recognized by the medical community over the past several years. The concern for burnout among medical students is equally serious. In this article, I review the prevalence of burnout among medical students, and the personal and clinical effects they experience. I also discuss how as psychiatry residents we can be more effective in preventing and identifying medical student burnout.
An underappreciated problem
Burnout has been defined as long-term unresolvable job stress that leads to exhaustion and feeling overwhelmed, cynical, and detached from work, and lacking a sense of personal accomplishment. It can lead to depression, anxiety, and suicidal ideation—one survey found that 5.8% of medical students had experienced suicidal ideation at some point in the previous 12 months.1 Burnout affects not only the individual, but also his/her team and patients. One study found that compared to medical students who didn’t report burnout, medical students who did had lower scores on measures of empathy and professionalism.2
While burnout among physicians and residents has received increasing attention, it often may go unrecognized and unreported in medical students. A literature review that included 51 studies found 28% to 45% of medical students report burnout.3 In a survey at one institution, 60% of medical students reported burnout.4 It is evident that medical schools have an important role in helping to minimize burnout rates in their students, and many schools are working toward this goal. However, what happens when students leave the classroom setting for clinical rotations?
A recent study found burnout among medical students peaks during the third year of medical school.5 This is when students are on their clinical rotations, new to the hospital environment, and without the inherent structure and support of being at school.
How residents can help
Like most medical students, while on my clinical rotations, I spent most of my day with residents, and I believe residents can help to both recognize burnout in medical students and prevent it.
The first step in addressing this problem is to understand why it occurs. A survey of medical students showed that inadequate sleep and decreased exercise play a significant role in burnout rates.6 Another study found a correlation between burnout and feeling emotionally exhausted and a decreased perceived quality of life.7 A medical student I recently worked with stated, “How can you not feel burnt out? Juggling work hours, studying, debt, health, and trying to have a life… something always gets dropped.”
So as residents, what can we do to identify and assist medical students who are experiencing burnout, or are at risk of getting there? When needed, we can utilize our psychiatry training to assess our students for depression and substance use disorders, and connect them with appropriate resources. When identifying a medical student with burnout, I believe it can become necessary to notify the attending, the site director responsible for the student, and often the school, so that the student has access to all available resources.
Continue to: It's as important to be proactive...
It’s as important to be proactive as it is to be reactive. Engaging in regular check-ins with our students about self-care and workload, as well as asking about how they are feeling, can offer them opportunities to talk about issues that they might not be getting anywhere else. One medical student I worked with told me, “It’s easy to fade into the background as the student, or to feel like I can’t complain because this is just how medical school is supposed to be.” We have the ability to change this notion with each student we work with.
It is likely that as residents we have worked with a student struggling with burnout without even realizing it. I believe we can play an important role in helping to prevent burnout by identifying at-risk students, offering assistance, and encouraging them to seek professional help. Someone’s life may depend on it.
Burnout among health care professionals has been increasingly recognized by the medical community over the past several years. The concern for burnout among medical students is equally serious. In this article, I review the prevalence of burnout among medical students, and the personal and clinical effects they experience. I also discuss how as psychiatry residents we can be more effective in preventing and identifying medical student burnout.
An underappreciated problem
Burnout has been defined as long-term unresolvable job stress that leads to exhaustion and feeling overwhelmed, cynical, and detached from work, and lacking a sense of personal accomplishment. It can lead to depression, anxiety, and suicidal ideation—one survey found that 5.8% of medical students had experienced suicidal ideation at some point in the previous 12 months.1 Burnout affects not only the individual, but also his/her team and patients. One study found that compared to medical students who didn’t report burnout, medical students who did had lower scores on measures of empathy and professionalism.2
While burnout among physicians and residents has received increasing attention, it often may go unrecognized and unreported in medical students. A literature review that included 51 studies found 28% to 45% of medical students report burnout.3 In a survey at one institution, 60% of medical students reported burnout.4 It is evident that medical schools have an important role in helping to minimize burnout rates in their students, and many schools are working toward this goal. However, what happens when students leave the classroom setting for clinical rotations?
A recent study found burnout among medical students peaks during the third year of medical school.5 This is when students are on their clinical rotations, new to the hospital environment, and without the inherent structure and support of being at school.
How residents can help
Like most medical students, while on my clinical rotations, I spent most of my day with residents, and I believe residents can help to both recognize burnout in medical students and prevent it.
The first step in addressing this problem is to understand why it occurs. A survey of medical students showed that inadequate sleep and decreased exercise play a significant role in burnout rates.6 Another study found a correlation between burnout and feeling emotionally exhausted and a decreased perceived quality of life.7 A medical student I recently worked with stated, “How can you not feel burnt out? Juggling work hours, studying, debt, health, and trying to have a life… something always gets dropped.”
So as residents, what can we do to identify and assist medical students who are experiencing burnout, or are at risk of getting there? When needed, we can utilize our psychiatry training to assess our students for depression and substance use disorders, and connect them with appropriate resources. When identifying a medical student with burnout, I believe it can become necessary to notify the attending, the site director responsible for the student, and often the school, so that the student has access to all available resources.
Continue to: It's as important to be proactive...
It’s as important to be proactive as it is to be reactive. Engaging in regular check-ins with our students about self-care and workload, as well as asking about how they are feeling, can offer them opportunities to talk about issues that they might not be getting anywhere else. One medical student I worked with told me, “It’s easy to fade into the background as the student, or to feel like I can’t complain because this is just how medical school is supposed to be.” We have the ability to change this notion with each student we work with.
It is likely that as residents we have worked with a student struggling with burnout without even realizing it. I believe we can play an important role in helping to prevent burnout by identifying at-risk students, offering assistance, and encouraging them to seek professional help. Someone’s life may depend on it.
1. Dyrbye L, Thomas M, Massie F, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334-341.
2. Brazeau C, Schroeder R, Rovi S. Relationships between medical student burnout, empathy, and professionalism climate. Acad Med. 2010;85(suppl 10):S33-S36. doi: 10.1097/ACM.0b013e3181ed4c47.
3. IsHak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;1(2):236-242.
4. Chang E, Eddins-Folensbee F, Coverdale J. Survey of the prevalence of burnout, stress, depression, and the use of supports by medical students at one school. Acad Psychiatry. 2012;36(3):177-182.
5. Hansell MW, Ungerleider RM, Brooks CA, et al. Temporal trends in medical student burnout. Fam Med. 2019;51(5):399-404.
6. Wolf M, Rosenstock J. Inadequate sleep and exercise associated with burnout and depression among medical students. Acad Psychiatry. 2017;41(2):174-179.
7. Colby L, Mareka M, Pillay S, et al. The association between the levels of burnout and quality of life among fourth-year medical students at the University of the Free State. S Afr J Psychiatr. 2018;24:1101.
1. Dyrbye L, Thomas M, Massie F, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334-341.
2. Brazeau C, Schroeder R, Rovi S. Relationships between medical student burnout, empathy, and professionalism climate. Acad Med. 2010;85(suppl 10):S33-S36. doi: 10.1097/ACM.0b013e3181ed4c47.
3. IsHak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;1(2):236-242.
4. Chang E, Eddins-Folensbee F, Coverdale J. Survey of the prevalence of burnout, stress, depression, and the use of supports by medical students at one school. Acad Psychiatry. 2012;36(3):177-182.
5. Hansell MW, Ungerleider RM, Brooks CA, et al. Temporal trends in medical student burnout. Fam Med. 2019;51(5):399-404.
6. Wolf M, Rosenstock J. Inadequate sleep and exercise associated with burnout and depression among medical students. Acad Psychiatry. 2017;41(2):174-179.
7. Colby L, Mareka M, Pillay S, et al. The association between the levels of burnout and quality of life among fourth-year medical students at the University of the Free State. S Afr J Psychiatr. 2018;24:1101.
Life during COVID-19: A pandemic of silence
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
Neuropsychiatric manifestations of COVID-19
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
Time to retire haloperidol?
For more than half a century, haloperidol has been used as a first-line medication for psychiatric agitation constituting a “behavioral emergency” when a patient cannot or will not take oral medication. Today, haloperidol is most commonly administered as an IM injection along with an anticholinergic medication to minimize extrapyramidal symptoms (EPS) and a benzodiazepine for additional sedation. The multiple-medication “cocktail” is often referred to by double-entendre nicknames, such as “B-52” or “5250” (ie, haloperidol, 5 mg; lorazepam, 2 mg; and
Earlier evidence of haloperidol’s efficacy
The initial “discovery” of antipsychotic medications was made in 1951 based on the inadvertent observation that chlorpromazine had the potential to calm surgical patients with autonomic activation. This calming effect, described as “désintéressment” (meaning a kind of “indifference to the world”),1 resulted in a new class of medications replacing barbiturates and bromides as go-to options to achieve “rapid tranquilization” of psychiatric agitation.2 Although the ability of antipsychotic medications to gradually reduce positive symptoms, such as delusions and hallucinations, has been attributed to dopamine (D2) antagonism, their more immediate sedating and anti-agitation effects are the result of broader effects as histamine (H1) and alpha-1 adrenergic antagonists.
In the 1970s, haloperidol emerged as a first-line option to manage agitation due to its IM and IV availability, as well as its relative lack of sedation and orthostasis compared with low-potency D2 antagonists such as chlorpromazine. However, haloperidol was observed to have a significant risk of acute EPS, including dystonic reactions.2 From the 1970s to the 1990s, numerous prospective clinical trials of haloperidol for the treatment of acute psychotic agitation, including several randomized controlled trials (RCTs) comparing haloperidol to lorazepam, were conducted.3 The design and outcomes of the haloperidol vs lorazepam RCTs were fairly consistent4-7:
- adult participants with acute agitation and a variety of psychiatric diagnoses, for whom informed consent often was waived due to agitation severity
- randomization to either IM haloperidol, 5 mg, or IM lorazepam, 2 mg, administered every 30 minutes until agitation resolved
- behavioral outcomes measured over several hours using various rating scales, without consistent assessment of EPS
- equivalent efficacy of haloperidol and lorazepam, with symptom resolution usually achieved after 1 to 2 doses (in 30 to 60 minutes), but sometimes longer
- anticholinergic “rescue” allowed for EPS, but not administered prophylactically
- EPS, including dystonia and akathisia, were significantly more frequent with haloperidol compared with lorazepam.8
In recognition of the greater risk of EPS with haloperidol compared with lorazepam, and the fact that most study participants were already taking standing doses of antipsychotic medications, some researchers have recommended using benzodiazepines alone as the optimal treatment for agitation.4,9 A 2012 Cochrane review concluded that the involuntary use of haloperidol alone “could be considered unethical.”10,11 However, other studies that examined the combination of haloperidol and lorazepam compared with either medication alone found that the combination of the 2 medications was associated with a more rapid resolution of symptoms, which suggests a superior synergistic effect.6,7,12 By the late 1990s, combined haloperidol and lorazepam, often mixed within a single injection, became the most common strategy to achieve rapid tranquilization in the psychiatric emergency setting.13 However, while the combination has been justified as a way to reduce the antipsychotic medication dose and EPS risk,2 few studies have compared combinations containing <5 mg of haloperidol. As a result, the apparent superiority of combined haloperidol and lorazepam compared with either medication alone may be a simple cumulative dose effect rather than true synergism. It is also important to note that adding lorazepam to haloperidol does not mitigate the risk of EPS such as dystonia in the absence of anticholinergic medication.8 To date, however, there have been no clinical trials investigating the efficacy of IM haloperidol, lorazepam, and
Newer RCTs tell a different story
With the availability of second-generation antipsychotics (SGAs) in IM formulations, clinical trials over the past 2 decades have focused on comparing SGAs with haloperidol alone as the “gold standard” control for acute agitation. Compared with previous trials of
- Study participants who signed informed consent (and were likely less agitated)
- IM haloperidol doses typically >5 mg (eg, 6.5 to 10 mg).
As with studies comparing lorazepam with haloperidol, the results of these RCTs revealed that IM
An updated 2017 Cochrane review of haloperidol for psychosis-induced aggression or agitation concluded that9:
- haloperidol is an effective intervention, although the evidence is “weak”
- significant treatment effects may take as long as 1 to 2 hours following multiple IM injections
- in contrast to SGAs, treatment with haloperidol carries a significant risk of EPS
- adding a benzodiazepine “does not have strong evidence of benefit and carries risk of additional harm.”
Continue to: Haloperidol's well-known toxicity
Haloperidol’s well-known toxicity
Haloperidol has been associated with numerous adverse effects:
Akathisia and other acute EPS. Treatment with even a single dose of IM haloperidol can result in acute EPS, including dystonia and akathisia. At best, such adverse effects are subjectively troubling and unpleasant; at worst, akathisia can exacerbate and be mistaken for agitation, leading to administration of more medication23 and the possible development of suicidal or violent behavior.24-25 In the studies reviewed above, the overall rate of EPS was as high as 21% after treatment with haloperidol,16 with parkinsonism occurring in up to 17% of patients,19 dystonia in up to 11%,7 and akathisia in up to 10%.15 However, because specific EPS were assessed inconsistently, and sometimes not at all, the rate of akathisia—arguably the most relevant and counter-therapeutic adverse effect related to agitation—remains unclear.
In another study that specifically assessed for akathisia in patients treated with haloperidol, up to 40% experienced akathisia 6 hours after a single oral dose of 5 mg.26 Even a single dose of IV
Although anticholinergic medications or benzodiazepinesare often administered as part of a haloperidol “cocktail,” these medications often do not adequately resolve emergent akathisia.26,28 No clinical trials of IM haloperidol combined with benztropine or diphenhydramine have been published, but several studies suggest that combining haloperidol with
Cardiotoxicity. Although low-potency antipsychotic medications such as
Continue to: Although there is no direct evidence...
Although there is no direct evidence that the cardiac risks associated with IV haloperidol apply to IM administration, epidemiologic studies indicate that oral haloperidol carries an elevated risk of ventricular arrhythmia and sudden cardiac death,35,36 with 1 study reporting greater risk compared with other SGAs.37 Haloperidol, whether administered orally or IM, may therefore be an especially poor choice for patients with agitation who are at risk for arrhythmia, including those with relevant medical comorbidities or delirium.34
Neuronal cell death. Several lines of research evidence have demonstrated that haloperidol can cause cellular injury or death in neuronal tissue in a dose-dependent fashion through a variety of mechanisms.38 By contrast, SGAs have been shown to have neuroprotective effects.39 While these findings have mostly come from studies conducted in animals or in vitro human tumor cell lines, some researchers have nonetheless called for haloperidol to be banned, noting that if its neurotoxic effects were more widely known, “we would realize what a travesty it is to use [such] a brain-unfriendly drug.”40
Several reasonable alternatives
Echoing the earlier Cochrane review of haloperidol for psychosis-induced aggression or agitation,10 a 2017 update concluded, “If no other alternative exists, sole use of intramuscular haloperidol could be life-saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical.”9
What then are reasonable alternatives to replace IM haloperidol for agitation? Clinicians should consider the following nonpharmacologic and pharmacologic interventions:
Nonpharmacologic interventions. Several behavioral interventions have been demonstrated to be effective for managing acute agitation, including verbal de-escalation, enhanced “programming” on the inpatient units, and the judicious use of seclusion.41-43 While such interventions may demand additional staff or resources, they have the potential to lower long-term costs, reduce injuries to patients and staff, and improve the quality of care.43 The use of IM haloperidol as a form of “chemical restraint” does not represent standard-of-care treatment,3 and from an ethical perspective, should never be implemented punitively or to compensate for substandard care in the form of inadequate staffing or staff training.
Continue to: Benzodiazepines
Benzodiazepines. Lorazepam offers an attractive alternative to haloperidol without the risk of EPS.2,4,8 However, lorazepam alone may be perceived as less efficacious than a haloperidol “cocktail” because it represents less overall medication. Some evidence has suggested that lorazepam, 4 mg, might be the most appropriate dose, although it has only rarely been studied in clinical trials of acute agitation.3
Respiratory depression is frequently cited as an argument against using lorazepam for agitation, as if the therapeutic window is extremely narrow with ineffectiveness at 2 mg, but potential lethality beyond that dose. In fact, serious respiratory depression with lorazepam is unlikely in the absence of chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, or concomitant alcohol or other sedative use.46 Case reports have documented therapeutic lorazepam dosing of 2 to 4 mg every 2 hours up to 20 to 30 mg/d in patients with manic agitation.47 Even in patients with COPD, significant respiratory depression tends not to occur at doses <8 mg.48 A more evidence-based concern about lorazepam dosing is that 2 mg might be ineffective in patients with established tolerance. For example, 1 report described a patient in acute alcohol withdrawal who required dosing lorazepam to 1,600 mg within 24 hours.49 Collectively, these reports suggest that lorazepam has a much wider therapeutic window than is typically perceived, and that dosing with 3 to 4 mg IM is a reasonable option for agitation when 2 mg is likely to be inadequate.
Paradoxical disinhibition is another concern that might prevent benzodiazepines from being used alone as a first-line intervention for emergency treatment of agitation. However, similar to respiratory depression, this adverse event is relatively rare and tends to occur in children and geriatric patients, individuals intoxicated with alcohol or other sedatives, and patients with brain injury, developmental delay, or dementia.23,46 Although exacerbation of aggression has not been demonstrated in the RCTs examining benzodiazepines for agitation reviewed above, based on other research, some clinicians have expressed concerns about the potential for benzodiazepines to exacerbate aggression in patients with impulse control disorders and a history of violent behavior.50
The 2005 Expert Consensus Panel for Behavioral Emergencies51 recommended the use of lorazepam alone over haloperidol for agitation for patients for whom the diagnosis is unknown or includes the following:
- stimulant intoxication
- personality disorder
- comorbid obesity
- comorbid cardiac arrhythmia
- a history of akathisia and other EPS
- a history of amenorrhea/galactorrhea
- a history of seizures.
In surveys, patients have ranked lorazepam as the preferred medication for emergency agitation, whereas haloperidol was ranked as one of the least-preferred options.51,52
Continue to: Second-generation antipsychotics
Second-generation antipsychotics. The SGAs available in IM formulations, such as aripiprazole, olanzapine, and ziprasidone, have been shown to be at least as effective as haloperidol for the treatment of acute agitation (in 2015, the short-acting injectable formulation of aripiprazole was discontinued in the United States independent of safety or efficacy issues53). A review of RCTs examining IM SGAs for the treatment of agitation concluded that the number needed to treat for response compared with placebo was 5 for aripiprazole, 3 for olanzapine, and 3 for ziprasidone.54 In terms of safety, a meta-analysis of studies examining IM medications for agitation confirmed that the risk of acute EPS, including dystonia, akathisia, and parkinsonism, is significantly lower with SGAs compared with haloperidol.55 An RCT comparing IM ziprasidone with haloperidol found equivalently modest effects on QTc prolongation.56 Therefore, SGAs are an obvious and evidence-based option for replacing haloperidol as a treatment for acute agitation.
Unfortunately, for clinicians hoping to replace haloperidol within a multiple-medication IM “cocktail,” there have been no published controlled trials of SGAs combined with benzodiazepines. Although a short report indicated that aripiprazole and lorazepam are chemically compatible to be combined within a single injection,57 the package insert for aripiprazole warns that “If parenteral benzodiazepine therapy is deemed necessary in addition to ABILIFY injection treatment, patients should be monitored for excessive sedation and for orthostatic hypotension.”58 The package insert for olanzapine likewise lists the combination of lorazepam and olanzapine as a drug interaction that can potentiate sedation, and the manufacturer issued specific warnings about parenteral combination.59,60 A single published case of significant hypotension with combined IM olanzapine and lorazepam,60 together with the fact that IM olanzapine can cause hypotension by itself,61 has discouraged the coadministration of these medications. Nonetheless, the combination is used in some emergency settings, with several retrospective studies failing to provide evidence of hypotension or respiratory depression as adverse effects.62-64
Droperidol.
Over the past decade, however, droperidol has returned to the US market68 and its IV and IM usage has been revitalized for managing patients with agitation within or en route to the ED. Studies have demonstrated droperidol efficacy comparable to midazolam, ziprasidone, or olanzapine, as well as effectiveness as an IV adjunct to midazolam.69-71 In contrast to the FDA black-box warning, retrospective studies and RCTs of both IV and IM droperidol suggest that QTc prolongation and torsades de pointes are rare events that do not occur any more frequently than they do with haloperidol, even at doses >10 mg.72,73 However, in studies involving patients with drug intoxication and treatment with multiple medications, oversedation to the point of needing rescue intervention was reported. In an emergency setting where these issues are relatively easily managed, such risks may be better tolerated than in psychiatric settings.
With earlier studies examining the use of droperidol in an acute psychiatric setting that reported a more rapid onset of action than haloperidol,65-67 a 2016 Cochrane review concluded that there was high-quality evidence to support droperidol’s use for psychosis-induced agitation.74 However, a 2015 RCT comparing IM droperidol, 10 mg, to haloperidol, 10 mg, found equivalent efficacy and response times (with maximal response occurring within 2 hours) and concluded that droperidol had no advantage over haloperidol.75 Because none of the clinical trials that evaluated droperidol have included assessments for EPS, its risk of akathisia remains uncertain.
Continue to: Ketamine
Ketamine. In recent years, ketamine has been used to treat acute agitation within or en route to the ED. Preliminary observational studies support ketamine’s efficacy when administered via IV or IM routes,76 with more rapid symptomatic improvement compared with haloperidol, lorazepam, or midazolam alone.77 Reported adverse effects of ketamine include dissociation, psychotic exacerbation, and respiratory depression,76 although 1 small naturalistic study found no evidence of exacerbation of psychotic or other psychiatric symptoms.78 An ongoing RCT is comparing IM ketamine, 5 mg/kg, to combined IM haloperidol, 5 mg, and midazolam, 5 mg.79 Although various ketamine formulations are increasingly being used in psychiatry, active psychosis is generally regarded as a contraindication. It is premature to recommend parenteral ketamine administration for agitation within most psychiatric settings until more research on safety has been completed.
Haloperidol, or something else? Practical considerations
Consider the following factors when deciding whether to use haloperidol or one of its alternatives:
Limitations of the evidence. Modern clinical trials requiring informed consent often do not include the kind of severe agitation that clinicians encounter in acute psychiatric, emergency, or forensic settings. In addition, standard interventions, such as 3-medication haloperidol “cocktails,” have not been evaluated in clinical trials. Clinicians are therefore often in the dark about optimal evidence-based practices.
Treatment goals. Psychiatric agitation has many causes, with a range of severity that warrants a commensurate range of responses. Protocols for managing acute agitation should include graded interventions that begin with nonpharmacologic interventions and voluntary oral medications, and move to involuntary IM medications when necessary.
While treatment guidelines clearly recommend against IM medications as “chemical restraint” with a goal of sedating a patient until he/she is unconscious,3,51 such outcomes are nonetheless often sought by staff who are concerned about the risk of injuries during a behavioral emergency. In such instances, the risks of violence towards patients and staff may outweigh concerns about adverse effects in a risk-benefit analysis. Consequently, clinicians may be prone to “skip over” graded interventions because they assume they “won’t work” in favor of administering involuntary multiple-medication haloperidol “cocktails” despite risks of excess sedation, EPS, and cardiotoxicity. Treatment settings should critically evaluate such biased preferences, with a goal of developing tailored, evidence-based strategies that maximize benefits while minimizing excess sedation and other untoward adverse effects, with an eye towards promoting better overall patient care and reducing length of stay.42,43,80
Continue to: Limitations of available medications
Limitations of available medications. There is no perfect medication for the management of acute agitation. Evidence indicates that pharmacologic options take 15 minutes to several hours to resolve acute agitation, even potentially more rapid-acting medications such as midazolam and droperidol. This is well beyond most clinicians’ desired window for response time in a behavioral emergency. Multiple-medication “cocktails” may be used with the hope of hastening response time, but may not achieve this goal at the expense of increasing the risk of adverse effects and the likelihood that a patient will remain sedated for a prolonged time. In the real world, this often means that by the time a psychiatrist comes to evaluate a patient who has been given emergency medications, the patient cannot be aroused for an interview. Ideally, medications would calm an agitated patient rapidly, without excess or prolonged sedation.80 Less-sedating SGAs, such as ziprasidone, might have this potential, but can sometimes be perceived as ineffective.
Avoiding akathisia. Akathisia’s potential to worsen and be mistaken for agitation makes it an especially concerning, if underappreciated, adverse effect of haloperidol that is often not adequately assessed in clinical trials or practice. In light of evidence that akathisia can occur in nearly half of patients receiving a single 5 mg-dose of haloperidol, it is difficult to justify the use of this medication for agitation when equally effective options exist with a lower risk of EPS.
While haloperidol-induced akathisia could in theory be mitigated by adding anticholinergic medications or benzodiazepines, previous studies have found that such strategies have limited effectiveness compared to “gold standard” treatment with propranolol.28,81,82 Furthermore, the half-lives of anticholinergic medications, such as benztropine or diphenhydramine, are significantly shorter than that of a single dose of haloperidol, which can be as long as 37 hours.83 Therefore, akathisia and other EPS could emerge or worsen several hours or even days after receiving an IM haloperidol “cocktail” as the shorter-acting medications wear off. Akathisia is best minimized by avoiding FGAs, such as haloperidol, when treating acute agitation.
Promoting adherence. Although haloperidol is often recommended for acute agitation in patients with schizophrenia or bipolar disorder on the basis that it would treat the underlying condition, many patients who receive IM medications for acute agitation are already prescribed standing doses of oral medication, which increases the risk of cumulative toxicity. In addition, receiving a medication likely to cause acute EPS that is ranked near the bottom of patient preferences may erode the potential for a therapeutic alliance and hamper longer-term antipsychotic medication adherence.
Time for a change
For nearly half a century, haloperidol has been a “gold standard” intervention for IM control in patients with agitation. However, given its potential to produce adverse effects, including a significant risk of akathisia that can worsen agitation, along with the availability of newer pharmacologic options that are at least as effective (Table 1, and Table 2), haloperidol should be retired as a first-line medication for the treatment of agitation. Clinicians would benefit from RCTs investigating the safety and efficacy of novel interventions including frequently-used, but untested medication combinations, as well as nonpharmacologic interventions.
Continue to: Bottom Line
Bottom Line
Although there is no perfect IM medication to treat acute agitation, haloperidol’s higher risk of adverse effects relative to newer alternatives suggest that it should no longer be considered a first-line intervention.
Related Resources
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 1: onset of efficacy. J Emerg Med. 2018;54(3):364-374.
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 2: safety. J Emerg Med. 2018;54(4): 522-532.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Chlorpromazine • Thorazine
Diphenhydramine • Benadryl
Droperidol • Inapsine
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Midazolam • Versed
Olanzapine • Zyprexa
Prochlorperazine • Compazine
Promethazine • Phenergan
Propranolol • Inderal, Pronol
Ziprasidone • Geodon
1. Shorter E. A history of psychiatry. New York, NY: John Wiley & Sons, Inc.; 1997:249.
2. Salzman C, Green AI, Rodriguez-Villa F, et al. Benzodiazepines combined with neuroleptics for management of severe disruptive behavior. Psychosomatics. 1986;27(suppl 1):17-22.
3. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatr. 2000;61(suppl 14):11-20.
4. Salzman C, Solomon D, Miyawaki E, et al. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. J Clin Psychiatr. 1991:52(4):177-180.
5. Allen MH, Currier GW, Hughes DH, et al. The expert consensus guideline series: treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1-88; quiz 89-90.
6. Foster S, Kessel J, Berman ME, et al. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. Int Clin Psychopharmacol. 1997;12(3):175-179.
7. Garza-Trevino WS, Hollister LE, Overall JE, et al. Efficacy of combinations of intramuscular antipsychotics and sedative-hypnotics for control of psychotic agitation. Am J Psychiatr. 1989:146(12):1598-1601.
8. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective double-blind, emergency study. Am J Emerg Med 1997;15(4):335-340.
9. Ostinelli EG, Brooke-Powney MJ, Li X, et al. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2017; 7:CD009377. doi: 10.1002/14651858.CD009377.pub3.
10. Powney MJ, Adams CE, Jones H. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2012;11:CD009377. doi: 10.1002/14651858.CD009377.pub2.
11. Citrome L. Review: limited evidence on effects of haloperidol alone for rapid tranquillisation in psychosis-induced aggression. Evid Based Ment Health. 2013;16(2):47.
12. Bienek SA, Ownby R, Penalver A, et al. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacother. 1998;18(1):57-62.
13. Binder RL, McNiel DE. Contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatric Serv. 1999;50(2):1553-1554.
14. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl). 2006;188(3):281-292.
15. Tran-Johnson TK, Sack DA, Marcus RN, et al. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatr. 2007;68(1):111-119.
16. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatr. 2000;61(12):933-941.
17. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
18. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatr. 2001;158(7):1149-1151.
19. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psych. 2002;59(5):441-448.
20. Hsu W, Huang S, Lee B, et al. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30(3):230-234.
21. Chan H, Ree S, Su L, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34(3):355-358.
22. Baldaçara L, Sanches M, Cordeiro DC, et al. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Braz J Psychiatry. 2011;33(1):30-39.
23. Hillard JR. Defusing patient violence. Current Psychiatry. 2002;1(4):22-29.
24. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
25. Eikelenboom-Schieveld SJM, Lucire Y, Fogleman JC. The relevance of cytochrome P450 polymorphism in forensic medicine and akathisia-related violence and suicide. J Forens Leg Med. 2016;41:65-71.
26. Van Putten T, May PRA, Marder SR. Akathisia with haloperidol and thiothixene. Arch Gen Psych. 1984;41:1036-1039.
27. Drotts DL, Vinson DR. Prochlorperazine induced akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
28. Salem H, Negpal C, Pigott T. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
29. Huf G, Coutinho ESF, Adams CE. Rapid tranquilization in psychiatric emergency settings in Brazil: pragmatic randomized controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):869.
30. Mantovani C, Labate CM, Sponholz A, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33(3):306-312.
31. Darwish H, Grant R, Haslam R, et al. Promethazine-induced acute dystonic reactions. Am J Dis Child. 1980;134(10):990-991.
32. Jyothi CH, Rudraiah HGM, Vidya HK, et al. Promethazine induced acute dystonia: a case report. Manipal J Med Sci. 2016;1(2):63-64.
33. Ames D, Carr-Lopez SM, Gutierrez MA, et al. Detecting and managing adverse effects of antipsychotic medications: current state of play. Psychiatr Clin North Am. 2016;39(2):275-311.
34. Meyer-Massetti C, Cheng CM, Sharpe MA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5(4):E8-E16. doi: 10.1002/jhm.691.
35. Wu C, Tsai Y, Tsai H. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Dis. 2015;4(2):e001568. doi: 10.1161/JAHA.114.001568.
36. Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointe, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(1):105-122.
37. Leonard CE, Freeman CP, Newcomb CW, et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiol. 2013;suppl 10(6):1-9.
38. Nasrallah H, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatr. 2017;29(3):195-202.
39. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
40. Nasrallah HA. Haloperidol clearly is neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
41. Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Comm Psychiatr. 1993;44(2):125-133.
42. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172.
43. Vieta E, Garriga M, Cardete L, et al. Protocol for the management of psychiatric patients with psychomotor agitation. BMC Psychiatr. 2017;17:328.
44. Nobay F, Simon BC, Levitt A, et al. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744-749.
45. Klein LR, Driver BE, Miner JR, et al. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374-385.
46. Hillard JR. Emergency treatment of acute psychosis. J Clin Psychiatr. 1998;59(suppl 1):57-60.
47. Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. J Clin Psychopharmacol. 1985;5(2):109-110.
48. Denaut M, Yernault JC, De Coster A. Double-blind comparison of the respiratory effects of parenteral lorazepam and diazepam in patients with chronic obstructive lung disease. Curr Med Res Opin. 1975;2(10):611-615.
49. Kahn DR, Barnhorst AV, Bourgeois JA. A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours. CNS Spectr. 2009;14(7):385-389.
50. Jones KA. Benzodiazepines: their role in aggression and why GPs should prescribe with caution. Austral Fam Physician. 2011;40(11):862-865.
51. Allen MH, Currier GW, Carpenter D, et al. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(suppl 1):5-108.
52. Allen MH, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract. 2003;9(1):39-58.
53. Han DH. Some Abilify formulations to discontinue in 2015. MPR. https://www.empr.com/home/news/some-abilify-formulations-to-discontinue-in-2015/. Published January 13, 2015. Accessed April 17, 2020.
54. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
55. Satterthwaite TD, Wolf DH, Rosenheck RA, et al. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment for agitation. J Clin Psychiatr. 2008;69(12):1869-1879.
56. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491.
57. Kovalick LJ, Pikalov AA, Ni N, et al. Short-term physical compatibility of intramuscular aripiprazole with intramuscular lorazepam. Am J Health-Syst Pharm. 2008;65(21):2007-2008.
58. Abilify [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
59. Zyprexa [package insert]. Indianapolis, IN: Lilly Research Laboratories; 2005.
60. Zacher JL, Roche-Desilets J. Hypotension secondary to the combination of intramuscular olanzapine and intramuscular lorazepam. J Clin Psychiatr. 2005;66(12):1614-1615.
61. Marder SR, Sorsaburu S, Dunayevich E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatr 2010;71(4):433-441.
62. Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2012;43(5):790-797.
63. Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olanzapine with benzodiazepines in emergency department patients. J Emerg Med. 2012;43(5):889-896.
64. Williams AM. Coadministration of intramuscular olanzapine and benzodiazepines in agitated patients with mental illness. Ment Health Clin [Internet]. 2018;8(5):208-213.
65. Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45(7):298-299.
66. Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407-413.
67. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16(4):567-573.
68. Boyer EW. Droperidol is back (and here’s what you need to know). ACEP Now. https://www.acepnow.com/article/droperidol-is-back-and-heres-what-you-need-to-know/. Published September 16, 2019. Accessed April 17, 2020.
69. Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167-1172.
70. Chan EW, Taylor DM, Knott JC, et al. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61(1):72-81.
71. Isbister GK, Calver LA, Page CB, et al. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392-401.
72. Macht M, Mull AC, McVaney KE, et al. Comparison of droperidol and haloperidol for use by paramedics assessment of safety and effectiveness. Prehosp Emerg Care. 2014;18(3):375-380.
73. Calver L, Page CB, Downes MA, et al. The safety and effectiveness of droperidol for sedation of acute behavioral disturbance in the emergency department. Ann Emerg Med. 2015;66(3):230-238.
74. Kohokar MA, Rathbone J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2016;12:CD002830.
75. Calver L, Drinkwater V, Gupta R, et al. Droperidol v. haloperidol for sedation of aggressive behavior in acute mental health: randomized controlled trial. Brit J Psychiatr. 2015;206(3):223-228.
76. Hopper AB, Vilke GM, Castillo EM, et al. Ketamine use for acute agitation in the emergency department. J Emerg Med. 2015;48(6):712-719.
77. Riddell J, Tran A, Bengiamin R, et al. Ketamine as a first-line treatment for severely agitated emergency department patients. Am J Emerg Med. 2017;35:1000-1004.
78. Lebin JA, Akhavan AR, Hippe DS, et al. Psychiatric outcomes of patients with severe agitation following administration of prehospital ketamine. Acad Emerg Med. 2019;26(8):889-896.
79. Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department (RACKED): a randomized controlled trial protocol. Trials. 2018;19(1):651.
80. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatr. 2016;17(2):86-128.
81. Adler L, Angrist B, Peselow E, et al. Efficacy of propranolol in neuroleptic-induced akathesia. J Clin Psychopharmacol. 1985;5(3):164-166.
82. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
83. de Leon J, Diaz FJ, Wedlund P, et al. Haloperidol half-life after chronic dosing. J Clin Psychopharmacol. 2004;24(6):656-660.
For more than half a century, haloperidol has been used as a first-line medication for psychiatric agitation constituting a “behavioral emergency” when a patient cannot or will not take oral medication. Today, haloperidol is most commonly administered as an IM injection along with an anticholinergic medication to minimize extrapyramidal symptoms (EPS) and a benzodiazepine for additional sedation. The multiple-medication “cocktail” is often referred to by double-entendre nicknames, such as “B-52” or “5250” (ie, haloperidol, 5 mg; lorazepam, 2 mg; and
Earlier evidence of haloperidol’s efficacy
The initial “discovery” of antipsychotic medications was made in 1951 based on the inadvertent observation that chlorpromazine had the potential to calm surgical patients with autonomic activation. This calming effect, described as “désintéressment” (meaning a kind of “indifference to the world”),1 resulted in a new class of medications replacing barbiturates and bromides as go-to options to achieve “rapid tranquilization” of psychiatric agitation.2 Although the ability of antipsychotic medications to gradually reduce positive symptoms, such as delusions and hallucinations, has been attributed to dopamine (D2) antagonism, their more immediate sedating and anti-agitation effects are the result of broader effects as histamine (H1) and alpha-1 adrenergic antagonists.
In the 1970s, haloperidol emerged as a first-line option to manage agitation due to its IM and IV availability, as well as its relative lack of sedation and orthostasis compared with low-potency D2 antagonists such as chlorpromazine. However, haloperidol was observed to have a significant risk of acute EPS, including dystonic reactions.2 From the 1970s to the 1990s, numerous prospective clinical trials of haloperidol for the treatment of acute psychotic agitation, including several randomized controlled trials (RCTs) comparing haloperidol to lorazepam, were conducted.3 The design and outcomes of the haloperidol vs lorazepam RCTs were fairly consistent4-7:
- adult participants with acute agitation and a variety of psychiatric diagnoses, for whom informed consent often was waived due to agitation severity
- randomization to either IM haloperidol, 5 mg, or IM lorazepam, 2 mg, administered every 30 minutes until agitation resolved
- behavioral outcomes measured over several hours using various rating scales, without consistent assessment of EPS
- equivalent efficacy of haloperidol and lorazepam, with symptom resolution usually achieved after 1 to 2 doses (in 30 to 60 minutes), but sometimes longer
- anticholinergic “rescue” allowed for EPS, but not administered prophylactically
- EPS, including dystonia and akathisia, were significantly more frequent with haloperidol compared with lorazepam.8
In recognition of the greater risk of EPS with haloperidol compared with lorazepam, and the fact that most study participants were already taking standing doses of antipsychotic medications, some researchers have recommended using benzodiazepines alone as the optimal treatment for agitation.4,9 A 2012 Cochrane review concluded that the involuntary use of haloperidol alone “could be considered unethical.”10,11 However, other studies that examined the combination of haloperidol and lorazepam compared with either medication alone found that the combination of the 2 medications was associated with a more rapid resolution of symptoms, which suggests a superior synergistic effect.6,7,12 By the late 1990s, combined haloperidol and lorazepam, often mixed within a single injection, became the most common strategy to achieve rapid tranquilization in the psychiatric emergency setting.13 However, while the combination has been justified as a way to reduce the antipsychotic medication dose and EPS risk,2 few studies have compared combinations containing <5 mg of haloperidol. As a result, the apparent superiority of combined haloperidol and lorazepam compared with either medication alone may be a simple cumulative dose effect rather than true synergism. It is also important to note that adding lorazepam to haloperidol does not mitigate the risk of EPS such as dystonia in the absence of anticholinergic medication.8 To date, however, there have been no clinical trials investigating the efficacy of IM haloperidol, lorazepam, and
Newer RCTs tell a different story
With the availability of second-generation antipsychotics (SGAs) in IM formulations, clinical trials over the past 2 decades have focused on comparing SGAs with haloperidol alone as the “gold standard” control for acute agitation. Compared with previous trials of
- Study participants who signed informed consent (and were likely less agitated)
- IM haloperidol doses typically >5 mg (eg, 6.5 to 10 mg).
As with studies comparing lorazepam with haloperidol, the results of these RCTs revealed that IM
An updated 2017 Cochrane review of haloperidol for psychosis-induced aggression or agitation concluded that9:
- haloperidol is an effective intervention, although the evidence is “weak”
- significant treatment effects may take as long as 1 to 2 hours following multiple IM injections
- in contrast to SGAs, treatment with haloperidol carries a significant risk of EPS
- adding a benzodiazepine “does not have strong evidence of benefit and carries risk of additional harm.”
Continue to: Haloperidol's well-known toxicity
Haloperidol’s well-known toxicity
Haloperidol has been associated with numerous adverse effects:
Akathisia and other acute EPS. Treatment with even a single dose of IM haloperidol can result in acute EPS, including dystonia and akathisia. At best, such adverse effects are subjectively troubling and unpleasant; at worst, akathisia can exacerbate and be mistaken for agitation, leading to administration of more medication23 and the possible development of suicidal or violent behavior.24-25 In the studies reviewed above, the overall rate of EPS was as high as 21% after treatment with haloperidol,16 with parkinsonism occurring in up to 17% of patients,19 dystonia in up to 11%,7 and akathisia in up to 10%.15 However, because specific EPS were assessed inconsistently, and sometimes not at all, the rate of akathisia—arguably the most relevant and counter-therapeutic adverse effect related to agitation—remains unclear.
In another study that specifically assessed for akathisia in patients treated with haloperidol, up to 40% experienced akathisia 6 hours after a single oral dose of 5 mg.26 Even a single dose of IV
Although anticholinergic medications or benzodiazepinesare often administered as part of a haloperidol “cocktail,” these medications often do not adequately resolve emergent akathisia.26,28 No clinical trials of IM haloperidol combined with benztropine or diphenhydramine have been published, but several studies suggest that combining haloperidol with
Cardiotoxicity. Although low-potency antipsychotic medications such as
Continue to: Although there is no direct evidence...
Although there is no direct evidence that the cardiac risks associated with IV haloperidol apply to IM administration, epidemiologic studies indicate that oral haloperidol carries an elevated risk of ventricular arrhythmia and sudden cardiac death,35,36 with 1 study reporting greater risk compared with other SGAs.37 Haloperidol, whether administered orally or IM, may therefore be an especially poor choice for patients with agitation who are at risk for arrhythmia, including those with relevant medical comorbidities or delirium.34
Neuronal cell death. Several lines of research evidence have demonstrated that haloperidol can cause cellular injury or death in neuronal tissue in a dose-dependent fashion through a variety of mechanisms.38 By contrast, SGAs have been shown to have neuroprotective effects.39 While these findings have mostly come from studies conducted in animals or in vitro human tumor cell lines, some researchers have nonetheless called for haloperidol to be banned, noting that if its neurotoxic effects were more widely known, “we would realize what a travesty it is to use [such] a brain-unfriendly drug.”40
Several reasonable alternatives
Echoing the earlier Cochrane review of haloperidol for psychosis-induced aggression or agitation,10 a 2017 update concluded, “If no other alternative exists, sole use of intramuscular haloperidol could be life-saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical.”9
What then are reasonable alternatives to replace IM haloperidol for agitation? Clinicians should consider the following nonpharmacologic and pharmacologic interventions:
Nonpharmacologic interventions. Several behavioral interventions have been demonstrated to be effective for managing acute agitation, including verbal de-escalation, enhanced “programming” on the inpatient units, and the judicious use of seclusion.41-43 While such interventions may demand additional staff or resources, they have the potential to lower long-term costs, reduce injuries to patients and staff, and improve the quality of care.43 The use of IM haloperidol as a form of “chemical restraint” does not represent standard-of-care treatment,3 and from an ethical perspective, should never be implemented punitively or to compensate for substandard care in the form of inadequate staffing or staff training.
Continue to: Benzodiazepines
Benzodiazepines. Lorazepam offers an attractive alternative to haloperidol without the risk of EPS.2,4,8 However, lorazepam alone may be perceived as less efficacious than a haloperidol “cocktail” because it represents less overall medication. Some evidence has suggested that lorazepam, 4 mg, might be the most appropriate dose, although it has only rarely been studied in clinical trials of acute agitation.3
Respiratory depression is frequently cited as an argument against using lorazepam for agitation, as if the therapeutic window is extremely narrow with ineffectiveness at 2 mg, but potential lethality beyond that dose. In fact, serious respiratory depression with lorazepam is unlikely in the absence of chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, or concomitant alcohol or other sedative use.46 Case reports have documented therapeutic lorazepam dosing of 2 to 4 mg every 2 hours up to 20 to 30 mg/d in patients with manic agitation.47 Even in patients with COPD, significant respiratory depression tends not to occur at doses <8 mg.48 A more evidence-based concern about lorazepam dosing is that 2 mg might be ineffective in patients with established tolerance. For example, 1 report described a patient in acute alcohol withdrawal who required dosing lorazepam to 1,600 mg within 24 hours.49 Collectively, these reports suggest that lorazepam has a much wider therapeutic window than is typically perceived, and that dosing with 3 to 4 mg IM is a reasonable option for agitation when 2 mg is likely to be inadequate.
Paradoxical disinhibition is another concern that might prevent benzodiazepines from being used alone as a first-line intervention for emergency treatment of agitation. However, similar to respiratory depression, this adverse event is relatively rare and tends to occur in children and geriatric patients, individuals intoxicated with alcohol or other sedatives, and patients with brain injury, developmental delay, or dementia.23,46 Although exacerbation of aggression has not been demonstrated in the RCTs examining benzodiazepines for agitation reviewed above, based on other research, some clinicians have expressed concerns about the potential for benzodiazepines to exacerbate aggression in patients with impulse control disorders and a history of violent behavior.50
The 2005 Expert Consensus Panel for Behavioral Emergencies51 recommended the use of lorazepam alone over haloperidol for agitation for patients for whom the diagnosis is unknown or includes the following:
- stimulant intoxication
- personality disorder
- comorbid obesity
- comorbid cardiac arrhythmia
- a history of akathisia and other EPS
- a history of amenorrhea/galactorrhea
- a history of seizures.
In surveys, patients have ranked lorazepam as the preferred medication for emergency agitation, whereas haloperidol was ranked as one of the least-preferred options.51,52
Continue to: Second-generation antipsychotics
Second-generation antipsychotics. The SGAs available in IM formulations, such as aripiprazole, olanzapine, and ziprasidone, have been shown to be at least as effective as haloperidol for the treatment of acute agitation (in 2015, the short-acting injectable formulation of aripiprazole was discontinued in the United States independent of safety or efficacy issues53). A review of RCTs examining IM SGAs for the treatment of agitation concluded that the number needed to treat for response compared with placebo was 5 for aripiprazole, 3 for olanzapine, and 3 for ziprasidone.54 In terms of safety, a meta-analysis of studies examining IM medications for agitation confirmed that the risk of acute EPS, including dystonia, akathisia, and parkinsonism, is significantly lower with SGAs compared with haloperidol.55 An RCT comparing IM ziprasidone with haloperidol found equivalently modest effects on QTc prolongation.56 Therefore, SGAs are an obvious and evidence-based option for replacing haloperidol as a treatment for acute agitation.
Unfortunately, for clinicians hoping to replace haloperidol within a multiple-medication IM “cocktail,” there have been no published controlled trials of SGAs combined with benzodiazepines. Although a short report indicated that aripiprazole and lorazepam are chemically compatible to be combined within a single injection,57 the package insert for aripiprazole warns that “If parenteral benzodiazepine therapy is deemed necessary in addition to ABILIFY injection treatment, patients should be monitored for excessive sedation and for orthostatic hypotension.”58 The package insert for olanzapine likewise lists the combination of lorazepam and olanzapine as a drug interaction that can potentiate sedation, and the manufacturer issued specific warnings about parenteral combination.59,60 A single published case of significant hypotension with combined IM olanzapine and lorazepam,60 together with the fact that IM olanzapine can cause hypotension by itself,61 has discouraged the coadministration of these medications. Nonetheless, the combination is used in some emergency settings, with several retrospective studies failing to provide evidence of hypotension or respiratory depression as adverse effects.62-64
Droperidol.
Over the past decade, however, droperidol has returned to the US market68 and its IV and IM usage has been revitalized for managing patients with agitation within or en route to the ED. Studies have demonstrated droperidol efficacy comparable to midazolam, ziprasidone, or olanzapine, as well as effectiveness as an IV adjunct to midazolam.69-71 In contrast to the FDA black-box warning, retrospective studies and RCTs of both IV and IM droperidol suggest that QTc prolongation and torsades de pointes are rare events that do not occur any more frequently than they do with haloperidol, even at doses >10 mg.72,73 However, in studies involving patients with drug intoxication and treatment with multiple medications, oversedation to the point of needing rescue intervention was reported. In an emergency setting where these issues are relatively easily managed, such risks may be better tolerated than in psychiatric settings.
With earlier studies examining the use of droperidol in an acute psychiatric setting that reported a more rapid onset of action than haloperidol,65-67 a 2016 Cochrane review concluded that there was high-quality evidence to support droperidol’s use for psychosis-induced agitation.74 However, a 2015 RCT comparing IM droperidol, 10 mg, to haloperidol, 10 mg, found equivalent efficacy and response times (with maximal response occurring within 2 hours) and concluded that droperidol had no advantage over haloperidol.75 Because none of the clinical trials that evaluated droperidol have included assessments for EPS, its risk of akathisia remains uncertain.
Continue to: Ketamine
Ketamine. In recent years, ketamine has been used to treat acute agitation within or en route to the ED. Preliminary observational studies support ketamine’s efficacy when administered via IV or IM routes,76 with more rapid symptomatic improvement compared with haloperidol, lorazepam, or midazolam alone.77 Reported adverse effects of ketamine include dissociation, psychotic exacerbation, and respiratory depression,76 although 1 small naturalistic study found no evidence of exacerbation of psychotic or other psychiatric symptoms.78 An ongoing RCT is comparing IM ketamine, 5 mg/kg, to combined IM haloperidol, 5 mg, and midazolam, 5 mg.79 Although various ketamine formulations are increasingly being used in psychiatry, active psychosis is generally regarded as a contraindication. It is premature to recommend parenteral ketamine administration for agitation within most psychiatric settings until more research on safety has been completed.
Haloperidol, or something else? Practical considerations
Consider the following factors when deciding whether to use haloperidol or one of its alternatives:
Limitations of the evidence. Modern clinical trials requiring informed consent often do not include the kind of severe agitation that clinicians encounter in acute psychiatric, emergency, or forensic settings. In addition, standard interventions, such as 3-medication haloperidol “cocktails,” have not been evaluated in clinical trials. Clinicians are therefore often in the dark about optimal evidence-based practices.
Treatment goals. Psychiatric agitation has many causes, with a range of severity that warrants a commensurate range of responses. Protocols for managing acute agitation should include graded interventions that begin with nonpharmacologic interventions and voluntary oral medications, and move to involuntary IM medications when necessary.
While treatment guidelines clearly recommend against IM medications as “chemical restraint” with a goal of sedating a patient until he/she is unconscious,3,51 such outcomes are nonetheless often sought by staff who are concerned about the risk of injuries during a behavioral emergency. In such instances, the risks of violence towards patients and staff may outweigh concerns about adverse effects in a risk-benefit analysis. Consequently, clinicians may be prone to “skip over” graded interventions because they assume they “won’t work” in favor of administering involuntary multiple-medication haloperidol “cocktails” despite risks of excess sedation, EPS, and cardiotoxicity. Treatment settings should critically evaluate such biased preferences, with a goal of developing tailored, evidence-based strategies that maximize benefits while minimizing excess sedation and other untoward adverse effects, with an eye towards promoting better overall patient care and reducing length of stay.42,43,80
Continue to: Limitations of available medications
Limitations of available medications. There is no perfect medication for the management of acute agitation. Evidence indicates that pharmacologic options take 15 minutes to several hours to resolve acute agitation, even potentially more rapid-acting medications such as midazolam and droperidol. This is well beyond most clinicians’ desired window for response time in a behavioral emergency. Multiple-medication “cocktails” may be used with the hope of hastening response time, but may not achieve this goal at the expense of increasing the risk of adverse effects and the likelihood that a patient will remain sedated for a prolonged time. In the real world, this often means that by the time a psychiatrist comes to evaluate a patient who has been given emergency medications, the patient cannot be aroused for an interview. Ideally, medications would calm an agitated patient rapidly, without excess or prolonged sedation.80 Less-sedating SGAs, such as ziprasidone, might have this potential, but can sometimes be perceived as ineffective.
Avoiding akathisia. Akathisia’s potential to worsen and be mistaken for agitation makes it an especially concerning, if underappreciated, adverse effect of haloperidol that is often not adequately assessed in clinical trials or practice. In light of evidence that akathisia can occur in nearly half of patients receiving a single 5 mg-dose of haloperidol, it is difficult to justify the use of this medication for agitation when equally effective options exist with a lower risk of EPS.
While haloperidol-induced akathisia could in theory be mitigated by adding anticholinergic medications or benzodiazepines, previous studies have found that such strategies have limited effectiveness compared to “gold standard” treatment with propranolol.28,81,82 Furthermore, the half-lives of anticholinergic medications, such as benztropine or diphenhydramine, are significantly shorter than that of a single dose of haloperidol, which can be as long as 37 hours.83 Therefore, akathisia and other EPS could emerge or worsen several hours or even days after receiving an IM haloperidol “cocktail” as the shorter-acting medications wear off. Akathisia is best minimized by avoiding FGAs, such as haloperidol, when treating acute agitation.
Promoting adherence. Although haloperidol is often recommended for acute agitation in patients with schizophrenia or bipolar disorder on the basis that it would treat the underlying condition, many patients who receive IM medications for acute agitation are already prescribed standing doses of oral medication, which increases the risk of cumulative toxicity. In addition, receiving a medication likely to cause acute EPS that is ranked near the bottom of patient preferences may erode the potential for a therapeutic alliance and hamper longer-term antipsychotic medication adherence.
Time for a change
For nearly half a century, haloperidol has been a “gold standard” intervention for IM control in patients with agitation. However, given its potential to produce adverse effects, including a significant risk of akathisia that can worsen agitation, along with the availability of newer pharmacologic options that are at least as effective (Table 1, and Table 2), haloperidol should be retired as a first-line medication for the treatment of agitation. Clinicians would benefit from RCTs investigating the safety and efficacy of novel interventions including frequently-used, but untested medication combinations, as well as nonpharmacologic interventions.
Continue to: Bottom Line
Bottom Line
Although there is no perfect IM medication to treat acute agitation, haloperidol’s higher risk of adverse effects relative to newer alternatives suggest that it should no longer be considered a first-line intervention.
Related Resources
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 1: onset of efficacy. J Emerg Med. 2018;54(3):364-374.
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 2: safety. J Emerg Med. 2018;54(4): 522-532.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Chlorpromazine • Thorazine
Diphenhydramine • Benadryl
Droperidol • Inapsine
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Midazolam • Versed
Olanzapine • Zyprexa
Prochlorperazine • Compazine
Promethazine • Phenergan
Propranolol • Inderal, Pronol
Ziprasidone • Geodon
For more than half a century, haloperidol has been used as a first-line medication for psychiatric agitation constituting a “behavioral emergency” when a patient cannot or will not take oral medication. Today, haloperidol is most commonly administered as an IM injection along with an anticholinergic medication to minimize extrapyramidal symptoms (EPS) and a benzodiazepine for additional sedation. The multiple-medication “cocktail” is often referred to by double-entendre nicknames, such as “B-52” or “5250” (ie, haloperidol, 5 mg; lorazepam, 2 mg; and
Earlier evidence of haloperidol’s efficacy
The initial “discovery” of antipsychotic medications was made in 1951 based on the inadvertent observation that chlorpromazine had the potential to calm surgical patients with autonomic activation. This calming effect, described as “désintéressment” (meaning a kind of “indifference to the world”),1 resulted in a new class of medications replacing barbiturates and bromides as go-to options to achieve “rapid tranquilization” of psychiatric agitation.2 Although the ability of antipsychotic medications to gradually reduce positive symptoms, such as delusions and hallucinations, has been attributed to dopamine (D2) antagonism, their more immediate sedating and anti-agitation effects are the result of broader effects as histamine (H1) and alpha-1 adrenergic antagonists.
In the 1970s, haloperidol emerged as a first-line option to manage agitation due to its IM and IV availability, as well as its relative lack of sedation and orthostasis compared with low-potency D2 antagonists such as chlorpromazine. However, haloperidol was observed to have a significant risk of acute EPS, including dystonic reactions.2 From the 1970s to the 1990s, numerous prospective clinical trials of haloperidol for the treatment of acute psychotic agitation, including several randomized controlled trials (RCTs) comparing haloperidol to lorazepam, were conducted.3 The design and outcomes of the haloperidol vs lorazepam RCTs were fairly consistent4-7:
- adult participants with acute agitation and a variety of psychiatric diagnoses, for whom informed consent often was waived due to agitation severity
- randomization to either IM haloperidol, 5 mg, or IM lorazepam, 2 mg, administered every 30 minutes until agitation resolved
- behavioral outcomes measured over several hours using various rating scales, without consistent assessment of EPS
- equivalent efficacy of haloperidol and lorazepam, with symptom resolution usually achieved after 1 to 2 doses (in 30 to 60 minutes), but sometimes longer
- anticholinergic “rescue” allowed for EPS, but not administered prophylactically
- EPS, including dystonia and akathisia, were significantly more frequent with haloperidol compared with lorazepam.8
In recognition of the greater risk of EPS with haloperidol compared with lorazepam, and the fact that most study participants were already taking standing doses of antipsychotic medications, some researchers have recommended using benzodiazepines alone as the optimal treatment for agitation.4,9 A 2012 Cochrane review concluded that the involuntary use of haloperidol alone “could be considered unethical.”10,11 However, other studies that examined the combination of haloperidol and lorazepam compared with either medication alone found that the combination of the 2 medications was associated with a more rapid resolution of symptoms, which suggests a superior synergistic effect.6,7,12 By the late 1990s, combined haloperidol and lorazepam, often mixed within a single injection, became the most common strategy to achieve rapid tranquilization in the psychiatric emergency setting.13 However, while the combination has been justified as a way to reduce the antipsychotic medication dose and EPS risk,2 few studies have compared combinations containing <5 mg of haloperidol. As a result, the apparent superiority of combined haloperidol and lorazepam compared with either medication alone may be a simple cumulative dose effect rather than true synergism. It is also important to note that adding lorazepam to haloperidol does not mitigate the risk of EPS such as dystonia in the absence of anticholinergic medication.8 To date, however, there have been no clinical trials investigating the efficacy of IM haloperidol, lorazepam, and
Newer RCTs tell a different story
With the availability of second-generation antipsychotics (SGAs) in IM formulations, clinical trials over the past 2 decades have focused on comparing SGAs with haloperidol alone as the “gold standard” control for acute agitation. Compared with previous trials of
- Study participants who signed informed consent (and were likely less agitated)
- IM haloperidol doses typically >5 mg (eg, 6.5 to 10 mg).
As with studies comparing lorazepam with haloperidol, the results of these RCTs revealed that IM
An updated 2017 Cochrane review of haloperidol for psychosis-induced aggression or agitation concluded that9:
- haloperidol is an effective intervention, although the evidence is “weak”
- significant treatment effects may take as long as 1 to 2 hours following multiple IM injections
- in contrast to SGAs, treatment with haloperidol carries a significant risk of EPS
- adding a benzodiazepine “does not have strong evidence of benefit and carries risk of additional harm.”
Continue to: Haloperidol's well-known toxicity
Haloperidol’s well-known toxicity
Haloperidol has been associated with numerous adverse effects:
Akathisia and other acute EPS. Treatment with even a single dose of IM haloperidol can result in acute EPS, including dystonia and akathisia. At best, such adverse effects are subjectively troubling and unpleasant; at worst, akathisia can exacerbate and be mistaken for agitation, leading to administration of more medication23 and the possible development of suicidal or violent behavior.24-25 In the studies reviewed above, the overall rate of EPS was as high as 21% after treatment with haloperidol,16 with parkinsonism occurring in up to 17% of patients,19 dystonia in up to 11%,7 and akathisia in up to 10%.15 However, because specific EPS were assessed inconsistently, and sometimes not at all, the rate of akathisia—arguably the most relevant and counter-therapeutic adverse effect related to agitation—remains unclear.
In another study that specifically assessed for akathisia in patients treated with haloperidol, up to 40% experienced akathisia 6 hours after a single oral dose of 5 mg.26 Even a single dose of IV
Although anticholinergic medications or benzodiazepinesare often administered as part of a haloperidol “cocktail,” these medications often do not adequately resolve emergent akathisia.26,28 No clinical trials of IM haloperidol combined with benztropine or diphenhydramine have been published, but several studies suggest that combining haloperidol with
Cardiotoxicity. Although low-potency antipsychotic medications such as
Continue to: Although there is no direct evidence...
Although there is no direct evidence that the cardiac risks associated with IV haloperidol apply to IM administration, epidemiologic studies indicate that oral haloperidol carries an elevated risk of ventricular arrhythmia and sudden cardiac death,35,36 with 1 study reporting greater risk compared with other SGAs.37 Haloperidol, whether administered orally or IM, may therefore be an especially poor choice for patients with agitation who are at risk for arrhythmia, including those with relevant medical comorbidities or delirium.34
Neuronal cell death. Several lines of research evidence have demonstrated that haloperidol can cause cellular injury or death in neuronal tissue in a dose-dependent fashion through a variety of mechanisms.38 By contrast, SGAs have been shown to have neuroprotective effects.39 While these findings have mostly come from studies conducted in animals or in vitro human tumor cell lines, some researchers have nonetheless called for haloperidol to be banned, noting that if its neurotoxic effects were more widely known, “we would realize what a travesty it is to use [such] a brain-unfriendly drug.”40
Several reasonable alternatives
Echoing the earlier Cochrane review of haloperidol for psychosis-induced aggression or agitation,10 a 2017 update concluded, “If no other alternative exists, sole use of intramuscular haloperidol could be life-saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical.”9
What then are reasonable alternatives to replace IM haloperidol for agitation? Clinicians should consider the following nonpharmacologic and pharmacologic interventions:
Nonpharmacologic interventions. Several behavioral interventions have been demonstrated to be effective for managing acute agitation, including verbal de-escalation, enhanced “programming” on the inpatient units, and the judicious use of seclusion.41-43 While such interventions may demand additional staff or resources, they have the potential to lower long-term costs, reduce injuries to patients and staff, and improve the quality of care.43 The use of IM haloperidol as a form of “chemical restraint” does not represent standard-of-care treatment,3 and from an ethical perspective, should never be implemented punitively or to compensate for substandard care in the form of inadequate staffing or staff training.
Continue to: Benzodiazepines
Benzodiazepines. Lorazepam offers an attractive alternative to haloperidol without the risk of EPS.2,4,8 However, lorazepam alone may be perceived as less efficacious than a haloperidol “cocktail” because it represents less overall medication. Some evidence has suggested that lorazepam, 4 mg, might be the most appropriate dose, although it has only rarely been studied in clinical trials of acute agitation.3
Respiratory depression is frequently cited as an argument against using lorazepam for agitation, as if the therapeutic window is extremely narrow with ineffectiveness at 2 mg, but potential lethality beyond that dose. In fact, serious respiratory depression with lorazepam is unlikely in the absence of chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, or concomitant alcohol or other sedative use.46 Case reports have documented therapeutic lorazepam dosing of 2 to 4 mg every 2 hours up to 20 to 30 mg/d in patients with manic agitation.47 Even in patients with COPD, significant respiratory depression tends not to occur at doses <8 mg.48 A more evidence-based concern about lorazepam dosing is that 2 mg might be ineffective in patients with established tolerance. For example, 1 report described a patient in acute alcohol withdrawal who required dosing lorazepam to 1,600 mg within 24 hours.49 Collectively, these reports suggest that lorazepam has a much wider therapeutic window than is typically perceived, and that dosing with 3 to 4 mg IM is a reasonable option for agitation when 2 mg is likely to be inadequate.
Paradoxical disinhibition is another concern that might prevent benzodiazepines from being used alone as a first-line intervention for emergency treatment of agitation. However, similar to respiratory depression, this adverse event is relatively rare and tends to occur in children and geriatric patients, individuals intoxicated with alcohol or other sedatives, and patients with brain injury, developmental delay, or dementia.23,46 Although exacerbation of aggression has not been demonstrated in the RCTs examining benzodiazepines for agitation reviewed above, based on other research, some clinicians have expressed concerns about the potential for benzodiazepines to exacerbate aggression in patients with impulse control disorders and a history of violent behavior.50
The 2005 Expert Consensus Panel for Behavioral Emergencies51 recommended the use of lorazepam alone over haloperidol for agitation for patients for whom the diagnosis is unknown or includes the following:
- stimulant intoxication
- personality disorder
- comorbid obesity
- comorbid cardiac arrhythmia
- a history of akathisia and other EPS
- a history of amenorrhea/galactorrhea
- a history of seizures.
In surveys, patients have ranked lorazepam as the preferred medication for emergency agitation, whereas haloperidol was ranked as one of the least-preferred options.51,52
Continue to: Second-generation antipsychotics
Second-generation antipsychotics. The SGAs available in IM formulations, such as aripiprazole, olanzapine, and ziprasidone, have been shown to be at least as effective as haloperidol for the treatment of acute agitation (in 2015, the short-acting injectable formulation of aripiprazole was discontinued in the United States independent of safety or efficacy issues53). A review of RCTs examining IM SGAs for the treatment of agitation concluded that the number needed to treat for response compared with placebo was 5 for aripiprazole, 3 for olanzapine, and 3 for ziprasidone.54 In terms of safety, a meta-analysis of studies examining IM medications for agitation confirmed that the risk of acute EPS, including dystonia, akathisia, and parkinsonism, is significantly lower with SGAs compared with haloperidol.55 An RCT comparing IM ziprasidone with haloperidol found equivalently modest effects on QTc prolongation.56 Therefore, SGAs are an obvious and evidence-based option for replacing haloperidol as a treatment for acute agitation.
Unfortunately, for clinicians hoping to replace haloperidol within a multiple-medication IM “cocktail,” there have been no published controlled trials of SGAs combined with benzodiazepines. Although a short report indicated that aripiprazole and lorazepam are chemically compatible to be combined within a single injection,57 the package insert for aripiprazole warns that “If parenteral benzodiazepine therapy is deemed necessary in addition to ABILIFY injection treatment, patients should be monitored for excessive sedation and for orthostatic hypotension.”58 The package insert for olanzapine likewise lists the combination of lorazepam and olanzapine as a drug interaction that can potentiate sedation, and the manufacturer issued specific warnings about parenteral combination.59,60 A single published case of significant hypotension with combined IM olanzapine and lorazepam,60 together with the fact that IM olanzapine can cause hypotension by itself,61 has discouraged the coadministration of these medications. Nonetheless, the combination is used in some emergency settings, with several retrospective studies failing to provide evidence of hypotension or respiratory depression as adverse effects.62-64
Droperidol.
Over the past decade, however, droperidol has returned to the US market68 and its IV and IM usage has been revitalized for managing patients with agitation within or en route to the ED. Studies have demonstrated droperidol efficacy comparable to midazolam, ziprasidone, or olanzapine, as well as effectiveness as an IV adjunct to midazolam.69-71 In contrast to the FDA black-box warning, retrospective studies and RCTs of both IV and IM droperidol suggest that QTc prolongation and torsades de pointes are rare events that do not occur any more frequently than they do with haloperidol, even at doses >10 mg.72,73 However, in studies involving patients with drug intoxication and treatment with multiple medications, oversedation to the point of needing rescue intervention was reported. In an emergency setting where these issues are relatively easily managed, such risks may be better tolerated than in psychiatric settings.
With earlier studies examining the use of droperidol in an acute psychiatric setting that reported a more rapid onset of action than haloperidol,65-67 a 2016 Cochrane review concluded that there was high-quality evidence to support droperidol’s use for psychosis-induced agitation.74 However, a 2015 RCT comparing IM droperidol, 10 mg, to haloperidol, 10 mg, found equivalent efficacy and response times (with maximal response occurring within 2 hours) and concluded that droperidol had no advantage over haloperidol.75 Because none of the clinical trials that evaluated droperidol have included assessments for EPS, its risk of akathisia remains uncertain.
Continue to: Ketamine
Ketamine. In recent years, ketamine has been used to treat acute agitation within or en route to the ED. Preliminary observational studies support ketamine’s efficacy when administered via IV or IM routes,76 with more rapid symptomatic improvement compared with haloperidol, lorazepam, or midazolam alone.77 Reported adverse effects of ketamine include dissociation, psychotic exacerbation, and respiratory depression,76 although 1 small naturalistic study found no evidence of exacerbation of psychotic or other psychiatric symptoms.78 An ongoing RCT is comparing IM ketamine, 5 mg/kg, to combined IM haloperidol, 5 mg, and midazolam, 5 mg.79 Although various ketamine formulations are increasingly being used in psychiatry, active psychosis is generally regarded as a contraindication. It is premature to recommend parenteral ketamine administration for agitation within most psychiatric settings until more research on safety has been completed.
Haloperidol, or something else? Practical considerations
Consider the following factors when deciding whether to use haloperidol or one of its alternatives:
Limitations of the evidence. Modern clinical trials requiring informed consent often do not include the kind of severe agitation that clinicians encounter in acute psychiatric, emergency, or forensic settings. In addition, standard interventions, such as 3-medication haloperidol “cocktails,” have not been evaluated in clinical trials. Clinicians are therefore often in the dark about optimal evidence-based practices.
Treatment goals. Psychiatric agitation has many causes, with a range of severity that warrants a commensurate range of responses. Protocols for managing acute agitation should include graded interventions that begin with nonpharmacologic interventions and voluntary oral medications, and move to involuntary IM medications when necessary.
While treatment guidelines clearly recommend against IM medications as “chemical restraint” with a goal of sedating a patient until he/she is unconscious,3,51 such outcomes are nonetheless often sought by staff who are concerned about the risk of injuries during a behavioral emergency. In such instances, the risks of violence towards patients and staff may outweigh concerns about adverse effects in a risk-benefit analysis. Consequently, clinicians may be prone to “skip over” graded interventions because they assume they “won’t work” in favor of administering involuntary multiple-medication haloperidol “cocktails” despite risks of excess sedation, EPS, and cardiotoxicity. Treatment settings should critically evaluate such biased preferences, with a goal of developing tailored, evidence-based strategies that maximize benefits while minimizing excess sedation and other untoward adverse effects, with an eye towards promoting better overall patient care and reducing length of stay.42,43,80
Continue to: Limitations of available medications
Limitations of available medications. There is no perfect medication for the management of acute agitation. Evidence indicates that pharmacologic options take 15 minutes to several hours to resolve acute agitation, even potentially more rapid-acting medications such as midazolam and droperidol. This is well beyond most clinicians’ desired window for response time in a behavioral emergency. Multiple-medication “cocktails” may be used with the hope of hastening response time, but may not achieve this goal at the expense of increasing the risk of adverse effects and the likelihood that a patient will remain sedated for a prolonged time. In the real world, this often means that by the time a psychiatrist comes to evaluate a patient who has been given emergency medications, the patient cannot be aroused for an interview. Ideally, medications would calm an agitated patient rapidly, without excess or prolonged sedation.80 Less-sedating SGAs, such as ziprasidone, might have this potential, but can sometimes be perceived as ineffective.
Avoiding akathisia. Akathisia’s potential to worsen and be mistaken for agitation makes it an especially concerning, if underappreciated, adverse effect of haloperidol that is often not adequately assessed in clinical trials or practice. In light of evidence that akathisia can occur in nearly half of patients receiving a single 5 mg-dose of haloperidol, it is difficult to justify the use of this medication for agitation when equally effective options exist with a lower risk of EPS.
While haloperidol-induced akathisia could in theory be mitigated by adding anticholinergic medications or benzodiazepines, previous studies have found that such strategies have limited effectiveness compared to “gold standard” treatment with propranolol.28,81,82 Furthermore, the half-lives of anticholinergic medications, such as benztropine or diphenhydramine, are significantly shorter than that of a single dose of haloperidol, which can be as long as 37 hours.83 Therefore, akathisia and other EPS could emerge or worsen several hours or even days after receiving an IM haloperidol “cocktail” as the shorter-acting medications wear off. Akathisia is best minimized by avoiding FGAs, such as haloperidol, when treating acute agitation.
Promoting adherence. Although haloperidol is often recommended for acute agitation in patients with schizophrenia or bipolar disorder on the basis that it would treat the underlying condition, many patients who receive IM medications for acute agitation are already prescribed standing doses of oral medication, which increases the risk of cumulative toxicity. In addition, receiving a medication likely to cause acute EPS that is ranked near the bottom of patient preferences may erode the potential for a therapeutic alliance and hamper longer-term antipsychotic medication adherence.
Time for a change
For nearly half a century, haloperidol has been a “gold standard” intervention for IM control in patients with agitation. However, given its potential to produce adverse effects, including a significant risk of akathisia that can worsen agitation, along with the availability of newer pharmacologic options that are at least as effective (Table 1, and Table 2), haloperidol should be retired as a first-line medication for the treatment of agitation. Clinicians would benefit from RCTs investigating the safety and efficacy of novel interventions including frequently-used, but untested medication combinations, as well as nonpharmacologic interventions.
Continue to: Bottom Line
Bottom Line
Although there is no perfect IM medication to treat acute agitation, haloperidol’s higher risk of adverse effects relative to newer alternatives suggest that it should no longer be considered a first-line intervention.
Related Resources
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 1: onset of efficacy. J Emerg Med. 2018;54(3):364-374.
- Zun LS. Evidence-based review of pharmacotherapy for acute agitation. Part 2: safety. J Emerg Med. 2018;54(4): 522-532.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Chlorpromazine • Thorazine
Diphenhydramine • Benadryl
Droperidol • Inapsine
Haloperidol • Haldol
Ketamine • Ketalar
Lorazepam • Ativan
Midazolam • Versed
Olanzapine • Zyprexa
Prochlorperazine • Compazine
Promethazine • Phenergan
Propranolol • Inderal, Pronol
Ziprasidone • Geodon
1. Shorter E. A history of psychiatry. New York, NY: John Wiley & Sons, Inc.; 1997:249.
2. Salzman C, Green AI, Rodriguez-Villa F, et al. Benzodiazepines combined with neuroleptics for management of severe disruptive behavior. Psychosomatics. 1986;27(suppl 1):17-22.
3. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatr. 2000;61(suppl 14):11-20.
4. Salzman C, Solomon D, Miyawaki E, et al. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. J Clin Psychiatr. 1991:52(4):177-180.
5. Allen MH, Currier GW, Hughes DH, et al. The expert consensus guideline series: treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1-88; quiz 89-90.
6. Foster S, Kessel J, Berman ME, et al. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. Int Clin Psychopharmacol. 1997;12(3):175-179.
7. Garza-Trevino WS, Hollister LE, Overall JE, et al. Efficacy of combinations of intramuscular antipsychotics and sedative-hypnotics for control of psychotic agitation. Am J Psychiatr. 1989:146(12):1598-1601.
8. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective double-blind, emergency study. Am J Emerg Med 1997;15(4):335-340.
9. Ostinelli EG, Brooke-Powney MJ, Li X, et al. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2017; 7:CD009377. doi: 10.1002/14651858.CD009377.pub3.
10. Powney MJ, Adams CE, Jones H. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2012;11:CD009377. doi: 10.1002/14651858.CD009377.pub2.
11. Citrome L. Review: limited evidence on effects of haloperidol alone for rapid tranquillisation in psychosis-induced aggression. Evid Based Ment Health. 2013;16(2):47.
12. Bienek SA, Ownby R, Penalver A, et al. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacother. 1998;18(1):57-62.
13. Binder RL, McNiel DE. Contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatric Serv. 1999;50(2):1553-1554.
14. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl). 2006;188(3):281-292.
15. Tran-Johnson TK, Sack DA, Marcus RN, et al. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatr. 2007;68(1):111-119.
16. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatr. 2000;61(12):933-941.
17. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
18. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatr. 2001;158(7):1149-1151.
19. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psych. 2002;59(5):441-448.
20. Hsu W, Huang S, Lee B, et al. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30(3):230-234.
21. Chan H, Ree S, Su L, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34(3):355-358.
22. Baldaçara L, Sanches M, Cordeiro DC, et al. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Braz J Psychiatry. 2011;33(1):30-39.
23. Hillard JR. Defusing patient violence. Current Psychiatry. 2002;1(4):22-29.
24. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
25. Eikelenboom-Schieveld SJM, Lucire Y, Fogleman JC. The relevance of cytochrome P450 polymorphism in forensic medicine and akathisia-related violence and suicide. J Forens Leg Med. 2016;41:65-71.
26. Van Putten T, May PRA, Marder SR. Akathisia with haloperidol and thiothixene. Arch Gen Psych. 1984;41:1036-1039.
27. Drotts DL, Vinson DR. Prochlorperazine induced akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
28. Salem H, Negpal C, Pigott T. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
29. Huf G, Coutinho ESF, Adams CE. Rapid tranquilization in psychiatric emergency settings in Brazil: pragmatic randomized controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):869.
30. Mantovani C, Labate CM, Sponholz A, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33(3):306-312.
31. Darwish H, Grant R, Haslam R, et al. Promethazine-induced acute dystonic reactions. Am J Dis Child. 1980;134(10):990-991.
32. Jyothi CH, Rudraiah HGM, Vidya HK, et al. Promethazine induced acute dystonia: a case report. Manipal J Med Sci. 2016;1(2):63-64.
33. Ames D, Carr-Lopez SM, Gutierrez MA, et al. Detecting and managing adverse effects of antipsychotic medications: current state of play. Psychiatr Clin North Am. 2016;39(2):275-311.
34. Meyer-Massetti C, Cheng CM, Sharpe MA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5(4):E8-E16. doi: 10.1002/jhm.691.
35. Wu C, Tsai Y, Tsai H. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Dis. 2015;4(2):e001568. doi: 10.1161/JAHA.114.001568.
36. Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointe, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(1):105-122.
37. Leonard CE, Freeman CP, Newcomb CW, et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiol. 2013;suppl 10(6):1-9.
38. Nasrallah H, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatr. 2017;29(3):195-202.
39. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
40. Nasrallah HA. Haloperidol clearly is neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
41. Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Comm Psychiatr. 1993;44(2):125-133.
42. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172.
43. Vieta E, Garriga M, Cardete L, et al. Protocol for the management of psychiatric patients with psychomotor agitation. BMC Psychiatr. 2017;17:328.
44. Nobay F, Simon BC, Levitt A, et al. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744-749.
45. Klein LR, Driver BE, Miner JR, et al. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374-385.
46. Hillard JR. Emergency treatment of acute psychosis. J Clin Psychiatr. 1998;59(suppl 1):57-60.
47. Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. J Clin Psychopharmacol. 1985;5(2):109-110.
48. Denaut M, Yernault JC, De Coster A. Double-blind comparison of the respiratory effects of parenteral lorazepam and diazepam in patients with chronic obstructive lung disease. Curr Med Res Opin. 1975;2(10):611-615.
49. Kahn DR, Barnhorst AV, Bourgeois JA. A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours. CNS Spectr. 2009;14(7):385-389.
50. Jones KA. Benzodiazepines: their role in aggression and why GPs should prescribe with caution. Austral Fam Physician. 2011;40(11):862-865.
51. Allen MH, Currier GW, Carpenter D, et al. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(suppl 1):5-108.
52. Allen MH, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract. 2003;9(1):39-58.
53. Han DH. Some Abilify formulations to discontinue in 2015. MPR. https://www.empr.com/home/news/some-abilify-formulations-to-discontinue-in-2015/. Published January 13, 2015. Accessed April 17, 2020.
54. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
55. Satterthwaite TD, Wolf DH, Rosenheck RA, et al. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment for agitation. J Clin Psychiatr. 2008;69(12):1869-1879.
56. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491.
57. Kovalick LJ, Pikalov AA, Ni N, et al. Short-term physical compatibility of intramuscular aripiprazole with intramuscular lorazepam. Am J Health-Syst Pharm. 2008;65(21):2007-2008.
58. Abilify [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
59. Zyprexa [package insert]. Indianapolis, IN: Lilly Research Laboratories; 2005.
60. Zacher JL, Roche-Desilets J. Hypotension secondary to the combination of intramuscular olanzapine and intramuscular lorazepam. J Clin Psychiatr. 2005;66(12):1614-1615.
61. Marder SR, Sorsaburu S, Dunayevich E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatr 2010;71(4):433-441.
62. Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2012;43(5):790-797.
63. Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olanzapine with benzodiazepines in emergency department patients. J Emerg Med. 2012;43(5):889-896.
64. Williams AM. Coadministration of intramuscular olanzapine and benzodiazepines in agitated patients with mental illness. Ment Health Clin [Internet]. 2018;8(5):208-213.
65. Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45(7):298-299.
66. Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407-413.
67. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16(4):567-573.
68. Boyer EW. Droperidol is back (and here’s what you need to know). ACEP Now. https://www.acepnow.com/article/droperidol-is-back-and-heres-what-you-need-to-know/. Published September 16, 2019. Accessed April 17, 2020.
69. Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167-1172.
70. Chan EW, Taylor DM, Knott JC, et al. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61(1):72-81.
71. Isbister GK, Calver LA, Page CB, et al. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392-401.
72. Macht M, Mull AC, McVaney KE, et al. Comparison of droperidol and haloperidol for use by paramedics assessment of safety and effectiveness. Prehosp Emerg Care. 2014;18(3):375-380.
73. Calver L, Page CB, Downes MA, et al. The safety and effectiveness of droperidol for sedation of acute behavioral disturbance in the emergency department. Ann Emerg Med. 2015;66(3):230-238.
74. Kohokar MA, Rathbone J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2016;12:CD002830.
75. Calver L, Drinkwater V, Gupta R, et al. Droperidol v. haloperidol for sedation of aggressive behavior in acute mental health: randomized controlled trial. Brit J Psychiatr. 2015;206(3):223-228.
76. Hopper AB, Vilke GM, Castillo EM, et al. Ketamine use for acute agitation in the emergency department. J Emerg Med. 2015;48(6):712-719.
77. Riddell J, Tran A, Bengiamin R, et al. Ketamine as a first-line treatment for severely agitated emergency department patients. Am J Emerg Med. 2017;35:1000-1004.
78. Lebin JA, Akhavan AR, Hippe DS, et al. Psychiatric outcomes of patients with severe agitation following administration of prehospital ketamine. Acad Emerg Med. 2019;26(8):889-896.
79. Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department (RACKED): a randomized controlled trial protocol. Trials. 2018;19(1):651.
80. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatr. 2016;17(2):86-128.
81. Adler L, Angrist B, Peselow E, et al. Efficacy of propranolol in neuroleptic-induced akathesia. J Clin Psychopharmacol. 1985;5(3):164-166.
82. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
83. de Leon J, Diaz FJ, Wedlund P, et al. Haloperidol half-life after chronic dosing. J Clin Psychopharmacol. 2004;24(6):656-660.
1. Shorter E. A history of psychiatry. New York, NY: John Wiley & Sons, Inc.; 1997:249.
2. Salzman C, Green AI, Rodriguez-Villa F, et al. Benzodiazepines combined with neuroleptics for management of severe disruptive behavior. Psychosomatics. 1986;27(suppl 1):17-22.
3. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatr. 2000;61(suppl 14):11-20.
4. Salzman C, Solomon D, Miyawaki E, et al. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. J Clin Psychiatr. 1991:52(4):177-180.
5. Allen MH, Currier GW, Hughes DH, et al. The expert consensus guideline series: treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1-88; quiz 89-90.
6. Foster S, Kessel J, Berman ME, et al. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. Int Clin Psychopharmacol. 1997;12(3):175-179.
7. Garza-Trevino WS, Hollister LE, Overall JE, et al. Efficacy of combinations of intramuscular antipsychotics and sedative-hypnotics for control of psychotic agitation. Am J Psychiatr. 1989:146(12):1598-1601.
8. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective double-blind, emergency study. Am J Emerg Med 1997;15(4):335-340.
9. Ostinelli EG, Brooke-Powney MJ, Li X, et al. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2017; 7:CD009377. doi: 10.1002/14651858.CD009377.pub3.
10. Powney MJ, Adams CE, Jones H. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database Syst Rev. 2012;11:CD009377. doi: 10.1002/14651858.CD009377.pub2.
11. Citrome L. Review: limited evidence on effects of haloperidol alone for rapid tranquillisation in psychosis-induced aggression. Evid Based Ment Health. 2013;16(2):47.
12. Bienek SA, Ownby R, Penalver A, et al. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacother. 1998;18(1):57-62.
13. Binder RL, McNiel DE. Contemporary practices in managing acutely violent patients in 20 psychiatric emergency rooms. Psychiatric Serv. 1999;50(2):1553-1554.
14. Andrezina R, Josiassen RC, Marcus RN, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl). 2006;188(3):281-292.
15. Tran-Johnson TK, Sack DA, Marcus RN, et al. Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatr. 2007;68(1):111-119.
16. Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatr. 2000;61(12):933-941.
17. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl). 2005;178(4):514-523.
18. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatr. 2001;158(7):1149-1151.
19. Breier A, Meehan K, Birkett M, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psych. 2002;59(5):441-448.
20. Hsu W, Huang S, Lee B, et al. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. J Clin Psychopharmacol. 2010;30(3):230-234.
21. Chan H, Ree S, Su L, et al. A double-blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. J Clin Psychopharmacol. 2014;34(3):355-358.
22. Baldaçara L, Sanches M, Cordeiro DC, et al. Rapid tranquilization for agitated patients in emergency psychiatric rooms: a randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Braz J Psychiatry. 2011;33(1):30-39.
23. Hillard JR. Defusing patient violence. Current Psychiatry. 2002;1(4):22-29.
24. Seemüller F, Schennach R, Mayr A, et al. Akathisia and suicidal ideation in first-episode schizophrenia. J Clin Psychopharmacol. 2012;32(5):694-698.
25. Eikelenboom-Schieveld SJM, Lucire Y, Fogleman JC. The relevance of cytochrome P450 polymorphism in forensic medicine and akathisia-related violence and suicide. J Forens Leg Med. 2016;41:65-71.
26. Van Putten T, May PRA, Marder SR. Akathisia with haloperidol and thiothixene. Arch Gen Psych. 1984;41:1036-1039.
27. Drotts DL, Vinson DR. Prochlorperazine induced akathisia in emergency patients. Ann Emerg Med. 1999;34(4):469-475.
28. Salem H, Negpal C, Pigott T. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol. 2017;15(5):789-798.
29. Huf G, Coutinho ESF, Adams CE. Rapid tranquilization in psychiatric emergency settings in Brazil: pragmatic randomized controlled trial of intramuscular haloperidol versus intramuscular haloperidol plus promethazine. BMJ. 2007;335(7625):869.
30. Mantovani C, Labate CM, Sponholz A, et al. Are low doses of antipsychotics effective in the management of psychomotor agitation? A randomized, rated-blind trial of 4 intramuscular interventions. J Clin Psychopharmacol. 2013;33(3):306-312.
31. Darwish H, Grant R, Haslam R, et al. Promethazine-induced acute dystonic reactions. Am J Dis Child. 1980;134(10):990-991.
32. Jyothi CH, Rudraiah HGM, Vidya HK, et al. Promethazine induced acute dystonia: a case report. Manipal J Med Sci. 2016;1(2):63-64.
33. Ames D, Carr-Lopez SM, Gutierrez MA, et al. Detecting and managing adverse effects of antipsychotic medications: current state of play. Psychiatr Clin North Am. 2016;39(2):275-311.
34. Meyer-Massetti C, Cheng CM, Sharpe MA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med. 2010;5(4):E8-E16. doi: 10.1002/jhm.691.
35. Wu C, Tsai Y, Tsai H. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: a nation-wide case-crossover study. J Am Heart Dis. 2015;4(2):e001568. doi: 10.1161/JAHA.114.001568.
36. Beach SR, Celano CM, Sugrue AM, et al. QT prolongation, torsades de pointe, and psychotropic medications: a 5-year update. Psychosomatics. 2018;59(1):105-122.
37. Leonard CE, Freeman CP, Newcomb CW, et al. Antipsychotics and the risks of sudden cardiac death and all-cause death: cohort studies in Medicaid and dually-eligible Medicaid-Medicare beneficiaries of five states. J Clin Exp Cardiol. 2013;suppl 10(6):1-9.
38. Nasrallah H, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatr. 2017;29(3):195-202.
39. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
40. Nasrallah HA. Haloperidol clearly is neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
41. Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Comm Psychiatr. 1993;44(2):125-133.
42. Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165-172.
43. Vieta E, Garriga M, Cardete L, et al. Protocol for the management of psychiatric patients with psychomotor agitation. BMC Psychiatr. 2017;17:328.
44. Nobay F, Simon BC, Levitt A, et al. A prospective, double-blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Acad Emerg Med. 2004;11(7):744-749.
45. Klein LR, Driver BE, Miner JR, et al. Intramuscular midazolam, olanzapine, ziprasidone, or haloperidol for treating acute agitation in the emergency department. Ann Emerg Med. 2018;72(4):374-385.
46. Hillard JR. Emergency treatment of acute psychosis. J Clin Psychiatr. 1998;59(suppl 1):57-60.
47. Modell JG, Lenox RH, Weiner S. Inpatient clinical trial of lorazepam for the management of manic agitation. J Clin Psychopharmacol. 1985;5(2):109-110.
48. Denaut M, Yernault JC, De Coster A. Double-blind comparison of the respiratory effects of parenteral lorazepam and diazepam in patients with chronic obstructive lung disease. Curr Med Res Opin. 1975;2(10):611-615.
49. Kahn DR, Barnhorst AV, Bourgeois JA. A case of alcohol withdrawal requiring 1,600 mg of lorazepam in 24 hours. CNS Spectr. 2009;14(7):385-389.
50. Jones KA. Benzodiazepines: their role in aggression and why GPs should prescribe with caution. Austral Fam Physician. 2011;40(11):862-865.
51. Allen MH, Currier GW, Carpenter D, et al. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(suppl 1):5-108.
52. Allen MH, Carpenter D, Sheets JL, et al. What do consumers say they want and need during a psychiatric emergency? J Psychiatr Pract. 2003;9(1):39-58.
53. Han DH. Some Abilify formulations to discontinue in 2015. MPR. https://www.empr.com/home/news/some-abilify-formulations-to-discontinue-in-2015/. Published January 13, 2015. Accessed April 17, 2020.
54. Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876-1885.
55. Satterthwaite TD, Wolf DH, Rosenheck RA, et al. A meta-analysis of the risk of acute extrapyramidal symptoms with intramuscular antipsychotics for the treatment for agitation. J Clin Psychiatr. 2008;69(12):1869-1879.
56. Miceli JJ, Tensfeldt TG, Shiovitz T, et al. Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther. 2010;32(3):472-491.
57. Kovalick LJ, Pikalov AA, Ni N, et al. Short-term physical compatibility of intramuscular aripiprazole with intramuscular lorazepam. Am J Health-Syst Pharm. 2008;65(21):2007-2008.
58. Abilify [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
59. Zyprexa [package insert]. Indianapolis, IN: Lilly Research Laboratories; 2005.
60. Zacher JL, Roche-Desilets J. Hypotension secondary to the combination of intramuscular olanzapine and intramuscular lorazepam. J Clin Psychiatr. 2005;66(12):1614-1615.
61. Marder SR, Sorsaburu S, Dunayevich E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatr 2010;71(4):433-441.
62. Wilson MP, MacDonald K, Vilke GM, et al. A comparison of the safety of olanzapine and haloperidol in combination with benzodiazepines in emergency department patients with acute agitation. J Emerg Med. 2012;43(5):790-797.
63. Wilson MP, MacDonald K, Vilke GM, et al. Potential complications of combining intramuscular olanzapine with benzodiazepines in emergency department patients. J Emerg Med. 2012;43(5):889-896.
64. Williams AM. Coadministration of intramuscular olanzapine and benzodiazepines in agitated patients with mental illness. Ment Health Clin [Internet]. 2018;8(5):208-213.
65. Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45(7):298-299.
66. Thomas H, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21(4):407-413.
67. Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16(4):567-573.
68. Boyer EW. Droperidol is back (and here’s what you need to know). ACEP Now. https://www.acepnow.com/article/droperidol-is-back-and-heres-what-you-need-to-know/. Published September 16, 2019. Accessed April 17, 2020.
69. Martel M, Sterzinger A, Miner J, et al. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12(12):1167-1172.
70. Chan EW, Taylor DM, Knott JC, et al. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61(1):72-81.
71. Isbister GK, Calver LA, Page CB, et al. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56(4):392-401.
72. Macht M, Mull AC, McVaney KE, et al. Comparison of droperidol and haloperidol for use by paramedics assessment of safety and effectiveness. Prehosp Emerg Care. 2014;18(3):375-380.
73. Calver L, Page CB, Downes MA, et al. The safety and effectiveness of droperidol for sedation of acute behavioral disturbance in the emergency department. Ann Emerg Med. 2015;66(3):230-238.
74. Kohokar MA, Rathbone J. Droperidol for psychosis-induced aggression or agitation. Cochrane Database Syst Rev. 2016;12:CD002830.
75. Calver L, Drinkwater V, Gupta R, et al. Droperidol v. haloperidol for sedation of aggressive behavior in acute mental health: randomized controlled trial. Brit J Psychiatr. 2015;206(3):223-228.
76. Hopper AB, Vilke GM, Castillo EM, et al. Ketamine use for acute agitation in the emergency department. J Emerg Med. 2015;48(6):712-719.
77. Riddell J, Tran A, Bengiamin R, et al. Ketamine as a first-line treatment for severely agitated emergency department patients. Am J Emerg Med. 2017;35:1000-1004.
78. Lebin JA, Akhavan AR, Hippe DS, et al. Psychiatric outcomes of patients with severe agitation following administration of prehospital ketamine. Acad Emerg Med. 2019;26(8):889-896.
79. Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department (RACKED): a randomized controlled trial protocol. Trials. 2018;19(1):651.
80. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatr. 2016;17(2):86-128.
81. Adler L, Angrist B, Peselow E, et al. Efficacy of propranolol in neuroleptic-induced akathesia. J Clin Psychopharmacol. 1985;5(3):164-166.
82. Adler LA, Reiter S, Corwin J, et al. Neuroleptic-induced akathisia: propranolol versus benztropine. Biol Psychiatry. 1988;23(2):211-213.
83. de Leon J, Diaz FJ, Wedlund P, et al. Haloperidol half-life after chronic dosing. J Clin Psychopharmacol. 2004;24(6):656-660.
COVID-19 in the era of loneliness
The natural state of human beings is to live together and function as organized groups. The beginnings of communities have primeval origins; evolutionarily, societies that worked together were more productive, efficient and—probably most important—safer. Thousands of years of evolution have ingrained these behaviors as part of our genetic constitution and developmental process. Social integration and acceptance thus are an integral part of basic human behavior and provide a sense of protection, pleasure, and purpose in life.
Unfortunately, the social isolation necessary to address the coronavirus disease 2019 (COVID-19) pandemic is preventing this integration, and is likely to worsen what some have called an epidemic of loneliness. As mental health clinicians, we need to use technology to strengthen our patients’ social support systems.
Loneliness: A growing problem
Changes in society over the last few decades have led to increased isolation. In the last 50 years, there has been a rise in single-person households in the United States. This is most common in large cities, where the prevalence is approximately 40%.1 The average number of confidants or the size of an American’s social network reduced by more than one-third from 1985 to 2009.2 In a study published in 2018, the health service company Cigna used the UCLA Loneliness Scale to survey >20,000 American adults.3 Nearly half of respondents reported always feeling alone (46%) or left out (47%), and individuals age 18 to 22 were the loneliest age group and claimed to be in worse health than older age groups. Furthermore, the results suggested that people who felt lonelier were more likely to have poor sleep and be less physically active. Americans who lived with others were less likely to report feeling lonely, except for single parents living only with their children. The results also showed that people who engage in meaningful interactions with others had lower loneliness scores and perceived that they were in better overall health.3
Studies have consistently demonstrated a link between loneliness and health problems such as cardiovascular disease, substance use disorders (SUDs), and mood disorders. A 2010 meta-analysis of 148 prospective studies with 308,849 participants found that the influence of social relationships on the risk of mortality is comparable to well-established risk factors for mortality such as smoking and alcohol consumption.4 These findings were confirmed in a 2015 meta-analysis that included 70 studies with 3.4 million participants followed for an average of 7 years. 5
Loneliness has been identified as a social determinant of health and is considered by many to be epidemic in proportion in developed countries. According to a 2019 Business Insider survey, almost 20% of US health care leaders planned to address social isolation in the next 12 months.6
Increased vulnerability during COVID-19 isolation
The forced quarantines and social distancing imposed by the COVID-19 crisis are likely to further exacerbate the loneliness epidemic. Hopefully, this increased isolation will not last more than several months, and its effect on chronic medical illnesses will be minor. However, for patients with mental illness, this further isolation, in conjunction with rising societal anxiety and fear of the potentially devastating financial consequences, could worsen their illness, and might even lead to suicidal ideation or behavior.
Individuals with SUDs are particularly vulnerable to the social limitations required by COVID-19. While social isolation is essential to limit the spread of COVID-19, this restriction poses unique challenges for these patients because connection and social support are important aspects of achieving and maintaining sobriety.7
Continue to: A call to action
A call to action
As mental health clinicians, we need to proactively engage with our patients to develop a plan to strengthen their social support systems. This may mean suggesting that they stay in contact with their network of people via video conferencing or by using the phone. We need to identify high-risk patients and continue to provide treatment via telepsychiatry. This is especially necessary to prevent relapse among patients with SUDs or mood disorders, and to minimize the risk of suicide.
We are ethically required to provide an atmosphere of trust, safety, and social inclusion by using resources, such as telehealth, video conferencing, and other online tools, to ameliorate the short- and long-term impact of COVID-19 isolation. Providing avenues that are easily accessible, are supportive, and maintain standards of care are essential. These resources should be implemented as early as possible to avoid negative outcomes regarding both COVID-19 and mental health.
There is also a significant risk that once circumstances improve, there will be a surge in the number of patients seeking a higher level of mental health care. Our actions and preparedness today will define the trajectory of our patients’ mental health in the future, potentially for years to come. While presently we are forced to be reactive, hopefully what is borne out of this crisis will translate into proactive measures for future crises.
Let this brief commentary serve as a call to action. As society finds ways to work from home, mental health clinicians need to lead the charge to use these same technologies to increase our patients’ social interactions. If we do not find ways to address the mental health burden of the COVID-19 pandemic, who will? We are all part of the mental health community, and we need to continue to function as an organized group, as has been the natural state of human beings for thousands of years.
Bottom Line
The social isolation required to limit the spread of the coronavirus disease 2019 pandemic is likely to increase loneliness, particularly among vulnerable patients with mood disorders and/or substance use disorders. As mental health clinicians, we need to work to strengthen our patients’ social support systems using resources such as video conferencing and other technologies.
Related Resources
- Cacioppo S, Grippo AJ, London S, et al. Loneliness: clinical import and interventions. Perspect Psychol Sci. 2015;10(2):238-249.
- Geriatric loneliness with Dr. Steven Wengel. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/geriatricloneliness-dr-steven-wengel. Published April 1, 2020.
1. Howe N. Millennials and the loneliness epidemic. Forbes. https://www.forbes.com/sites/neilhowe/2019/05/03/millennials-and-the-loneliness-epidemic/. Published May 3, 2019. Accessed April 10, 2020.
2. The Economist. All the lonely people: loneliness is a serious public-health problem. https://www.economist.com/international/2018/09/01/loneliness-is-a-serious-public-health-problem. Published September 1, 2018. Accessed April 10, 2020.
3. Cigna. New Cigna study reveals loneliness at epidemic levels in America. https://www.cigna.com/newsroom/news-releases/2018/new-cigna-study-reveals-loneliness-at-epidemic-levels-in-america. Published May 1, 2018. Accessed April 10, 2020.
4. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316.
5. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
6. Finley D. How increased social distancing for the coronavirus could spur a loneliness epidemic. Business Insider. https://www.businessinsider.com/coronavirus-could-spur-loneliness-epidemic-2020-3. Published March 16, 2020. Accessed April 10, 2020.
7. Roy L. Addiction treatment facilities: are they prepared for the COVID-19 coronavirus outbreak? Forbes. https://www.forbes.com/sites/lipiroy/2020/03/16/addiction-treatment-facilities-are-they-prepared-for-covid-19/#555149b544ea. Published March 16, 2020. Accessed April 10, 2020.
The natural state of human beings is to live together and function as organized groups. The beginnings of communities have primeval origins; evolutionarily, societies that worked together were more productive, efficient and—probably most important—safer. Thousands of years of evolution have ingrained these behaviors as part of our genetic constitution and developmental process. Social integration and acceptance thus are an integral part of basic human behavior and provide a sense of protection, pleasure, and purpose in life.
Unfortunately, the social isolation necessary to address the coronavirus disease 2019 (COVID-19) pandemic is preventing this integration, and is likely to worsen what some have called an epidemic of loneliness. As mental health clinicians, we need to use technology to strengthen our patients’ social support systems.
Loneliness: A growing problem
Changes in society over the last few decades have led to increased isolation. In the last 50 years, there has been a rise in single-person households in the United States. This is most common in large cities, where the prevalence is approximately 40%.1 The average number of confidants or the size of an American’s social network reduced by more than one-third from 1985 to 2009.2 In a study published in 2018, the health service company Cigna used the UCLA Loneliness Scale to survey >20,000 American adults.3 Nearly half of respondents reported always feeling alone (46%) or left out (47%), and individuals age 18 to 22 were the loneliest age group and claimed to be in worse health than older age groups. Furthermore, the results suggested that people who felt lonelier were more likely to have poor sleep and be less physically active. Americans who lived with others were less likely to report feeling lonely, except for single parents living only with their children. The results also showed that people who engage in meaningful interactions with others had lower loneliness scores and perceived that they were in better overall health.3
Studies have consistently demonstrated a link between loneliness and health problems such as cardiovascular disease, substance use disorders (SUDs), and mood disorders. A 2010 meta-analysis of 148 prospective studies with 308,849 participants found that the influence of social relationships on the risk of mortality is comparable to well-established risk factors for mortality such as smoking and alcohol consumption.4 These findings were confirmed in a 2015 meta-analysis that included 70 studies with 3.4 million participants followed for an average of 7 years. 5
Loneliness has been identified as a social determinant of health and is considered by many to be epidemic in proportion in developed countries. According to a 2019 Business Insider survey, almost 20% of US health care leaders planned to address social isolation in the next 12 months.6
Increased vulnerability during COVID-19 isolation
The forced quarantines and social distancing imposed by the COVID-19 crisis are likely to further exacerbate the loneliness epidemic. Hopefully, this increased isolation will not last more than several months, and its effect on chronic medical illnesses will be minor. However, for patients with mental illness, this further isolation, in conjunction with rising societal anxiety and fear of the potentially devastating financial consequences, could worsen their illness, and might even lead to suicidal ideation or behavior.
Individuals with SUDs are particularly vulnerable to the social limitations required by COVID-19. While social isolation is essential to limit the spread of COVID-19, this restriction poses unique challenges for these patients because connection and social support are important aspects of achieving and maintaining sobriety.7
Continue to: A call to action
A call to action
As mental health clinicians, we need to proactively engage with our patients to develop a plan to strengthen their social support systems. This may mean suggesting that they stay in contact with their network of people via video conferencing or by using the phone. We need to identify high-risk patients and continue to provide treatment via telepsychiatry. This is especially necessary to prevent relapse among patients with SUDs or mood disorders, and to minimize the risk of suicide.
We are ethically required to provide an atmosphere of trust, safety, and social inclusion by using resources, such as telehealth, video conferencing, and other online tools, to ameliorate the short- and long-term impact of COVID-19 isolation. Providing avenues that are easily accessible, are supportive, and maintain standards of care are essential. These resources should be implemented as early as possible to avoid negative outcomes regarding both COVID-19 and mental health.
There is also a significant risk that once circumstances improve, there will be a surge in the number of patients seeking a higher level of mental health care. Our actions and preparedness today will define the trajectory of our patients’ mental health in the future, potentially for years to come. While presently we are forced to be reactive, hopefully what is borne out of this crisis will translate into proactive measures for future crises.
Let this brief commentary serve as a call to action. As society finds ways to work from home, mental health clinicians need to lead the charge to use these same technologies to increase our patients’ social interactions. If we do not find ways to address the mental health burden of the COVID-19 pandemic, who will? We are all part of the mental health community, and we need to continue to function as an organized group, as has been the natural state of human beings for thousands of years.
Bottom Line
The social isolation required to limit the spread of the coronavirus disease 2019 pandemic is likely to increase loneliness, particularly among vulnerable patients with mood disorders and/or substance use disorders. As mental health clinicians, we need to work to strengthen our patients’ social support systems using resources such as video conferencing and other technologies.
Related Resources
- Cacioppo S, Grippo AJ, London S, et al. Loneliness: clinical import and interventions. Perspect Psychol Sci. 2015;10(2):238-249.
- Geriatric loneliness with Dr. Steven Wengel. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/geriatricloneliness-dr-steven-wengel. Published April 1, 2020.
The natural state of human beings is to live together and function as organized groups. The beginnings of communities have primeval origins; evolutionarily, societies that worked together were more productive, efficient and—probably most important—safer. Thousands of years of evolution have ingrained these behaviors as part of our genetic constitution and developmental process. Social integration and acceptance thus are an integral part of basic human behavior and provide a sense of protection, pleasure, and purpose in life.
Unfortunately, the social isolation necessary to address the coronavirus disease 2019 (COVID-19) pandemic is preventing this integration, and is likely to worsen what some have called an epidemic of loneliness. As mental health clinicians, we need to use technology to strengthen our patients’ social support systems.
Loneliness: A growing problem
Changes in society over the last few decades have led to increased isolation. In the last 50 years, there has been a rise in single-person households in the United States. This is most common in large cities, where the prevalence is approximately 40%.1 The average number of confidants or the size of an American’s social network reduced by more than one-third from 1985 to 2009.2 In a study published in 2018, the health service company Cigna used the UCLA Loneliness Scale to survey >20,000 American adults.3 Nearly half of respondents reported always feeling alone (46%) or left out (47%), and individuals age 18 to 22 were the loneliest age group and claimed to be in worse health than older age groups. Furthermore, the results suggested that people who felt lonelier were more likely to have poor sleep and be less physically active. Americans who lived with others were less likely to report feeling lonely, except for single parents living only with their children. The results also showed that people who engage in meaningful interactions with others had lower loneliness scores and perceived that they were in better overall health.3
Studies have consistently demonstrated a link between loneliness and health problems such as cardiovascular disease, substance use disorders (SUDs), and mood disorders. A 2010 meta-analysis of 148 prospective studies with 308,849 participants found that the influence of social relationships on the risk of mortality is comparable to well-established risk factors for mortality such as smoking and alcohol consumption.4 These findings were confirmed in a 2015 meta-analysis that included 70 studies with 3.4 million participants followed for an average of 7 years. 5
Loneliness has been identified as a social determinant of health and is considered by many to be epidemic in proportion in developed countries. According to a 2019 Business Insider survey, almost 20% of US health care leaders planned to address social isolation in the next 12 months.6
Increased vulnerability during COVID-19 isolation
The forced quarantines and social distancing imposed by the COVID-19 crisis are likely to further exacerbate the loneliness epidemic. Hopefully, this increased isolation will not last more than several months, and its effect on chronic medical illnesses will be minor. However, for patients with mental illness, this further isolation, in conjunction with rising societal anxiety and fear of the potentially devastating financial consequences, could worsen their illness, and might even lead to suicidal ideation or behavior.
Individuals with SUDs are particularly vulnerable to the social limitations required by COVID-19. While social isolation is essential to limit the spread of COVID-19, this restriction poses unique challenges for these patients because connection and social support are important aspects of achieving and maintaining sobriety.7
Continue to: A call to action
A call to action
As mental health clinicians, we need to proactively engage with our patients to develop a plan to strengthen their social support systems. This may mean suggesting that they stay in contact with their network of people via video conferencing or by using the phone. We need to identify high-risk patients and continue to provide treatment via telepsychiatry. This is especially necessary to prevent relapse among patients with SUDs or mood disorders, and to minimize the risk of suicide.
We are ethically required to provide an atmosphere of trust, safety, and social inclusion by using resources, such as telehealth, video conferencing, and other online tools, to ameliorate the short- and long-term impact of COVID-19 isolation. Providing avenues that are easily accessible, are supportive, and maintain standards of care are essential. These resources should be implemented as early as possible to avoid negative outcomes regarding both COVID-19 and mental health.
There is also a significant risk that once circumstances improve, there will be a surge in the number of patients seeking a higher level of mental health care. Our actions and preparedness today will define the trajectory of our patients’ mental health in the future, potentially for years to come. While presently we are forced to be reactive, hopefully what is borne out of this crisis will translate into proactive measures for future crises.
Let this brief commentary serve as a call to action. As society finds ways to work from home, mental health clinicians need to lead the charge to use these same technologies to increase our patients’ social interactions. If we do not find ways to address the mental health burden of the COVID-19 pandemic, who will? We are all part of the mental health community, and we need to continue to function as an organized group, as has been the natural state of human beings for thousands of years.
Bottom Line
The social isolation required to limit the spread of the coronavirus disease 2019 pandemic is likely to increase loneliness, particularly among vulnerable patients with mood disorders and/or substance use disorders. As mental health clinicians, we need to work to strengthen our patients’ social support systems using resources such as video conferencing and other technologies.
Related Resources
- Cacioppo S, Grippo AJ, London S, et al. Loneliness: clinical import and interventions. Perspect Psychol Sci. 2015;10(2):238-249.
- Geriatric loneliness with Dr. Steven Wengel. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/geriatricloneliness-dr-steven-wengel. Published April 1, 2020.
1. Howe N. Millennials and the loneliness epidemic. Forbes. https://www.forbes.com/sites/neilhowe/2019/05/03/millennials-and-the-loneliness-epidemic/. Published May 3, 2019. Accessed April 10, 2020.
2. The Economist. All the lonely people: loneliness is a serious public-health problem. https://www.economist.com/international/2018/09/01/loneliness-is-a-serious-public-health-problem. Published September 1, 2018. Accessed April 10, 2020.
3. Cigna. New Cigna study reveals loneliness at epidemic levels in America. https://www.cigna.com/newsroom/news-releases/2018/new-cigna-study-reveals-loneliness-at-epidemic-levels-in-america. Published May 1, 2018. Accessed April 10, 2020.
4. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316.
5. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
6. Finley D. How increased social distancing for the coronavirus could spur a loneliness epidemic. Business Insider. https://www.businessinsider.com/coronavirus-could-spur-loneliness-epidemic-2020-3. Published March 16, 2020. Accessed April 10, 2020.
7. Roy L. Addiction treatment facilities: are they prepared for the COVID-19 coronavirus outbreak? Forbes. https://www.forbes.com/sites/lipiroy/2020/03/16/addiction-treatment-facilities-are-they-prepared-for-covid-19/#555149b544ea. Published March 16, 2020. Accessed April 10, 2020.
1. Howe N. Millennials and the loneliness epidemic. Forbes. https://www.forbes.com/sites/neilhowe/2019/05/03/millennials-and-the-loneliness-epidemic/. Published May 3, 2019. Accessed April 10, 2020.
2. The Economist. All the lonely people: loneliness is a serious public-health problem. https://www.economist.com/international/2018/09/01/loneliness-is-a-serious-public-health-problem. Published September 1, 2018. Accessed April 10, 2020.
3. Cigna. New Cigna study reveals loneliness at epidemic levels in America. https://www.cigna.com/newsroom/news-releases/2018/new-cigna-study-reveals-loneliness-at-epidemic-levels-in-america. Published May 1, 2018. Accessed April 10, 2020.
4. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316.
5. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227-237.
6. Finley D. How increased social distancing for the coronavirus could spur a loneliness epidemic. Business Insider. https://www.businessinsider.com/coronavirus-could-spur-loneliness-epidemic-2020-3. Published March 16, 2020. Accessed April 10, 2020.
7. Roy L. Addiction treatment facilities: are they prepared for the COVID-19 coronavirus outbreak? Forbes. https://www.forbes.com/sites/lipiroy/2020/03/16/addiction-treatment-facilities-are-they-prepared-for-covid-19/#555149b544ea. Published March 16, 2020. Accessed April 10, 2020.
COVID-19: A psychiatry resident’s perspective
During these unprecedented times, venturing into the unknown of the coronavirus disease 2019 (COVID-19) pandemic, a feeling of impending doom prevails. Almost all of us have been restricted to our homes. Although the physical dimensions of what we call home may vary, the meaning of this restriction is fairly universal. No matter how our sociodemographics differ, with no guidance for this situation from anything even remotely comparable in the past, our lives have been transformed into a work in progress.
During this pandemic, I have observed a wide range of human emotions and behavior—many of them familiar and predictable, some abysmal, and some inspiring.
’Why should I care?’
On December 31, 2019, health officials in China informed the World Health Organization about a pneumonia-like presentation in a group of people in Wuhan. On January 7, 2020, a novel coronavirus was identified as the cause, and the first death was reported a few days later. In the following days and weeks the disease rapidly spread, as did the growing sense that this was not a typical virus.
While these events occurred, the rest of the world was in what I call a ”Why should I care?” mode. Most humans tend to suffer from this indifference. This has been observed repeatedly through the years, such as when the Ebola outbreak occurred in Africa in 2014-2016. It was only when cases started to develop in Europe and the United States that other countries started to pay attention. A similar phenomenon has been observed every time we’ve faced a global outbreak (avian influenza, Middle East respiratory syndrome, etc.).
When are we going to learn? It is time to realize that global borders are more porous than we think, and human interactions cannot be blocked by any wall. When a catastrophic event, outbreak, or disaster starts in any part of the world, it is naive to assume that we will not be affected. We will eventually be affected—the only question is how, when, and to what extent? We are always all in this together.
An abundance of ignorance and stupidity
Within a few weeks of the first reports from China, cases of COVID-19 were reported in South Korea, Italy, Spain, Germany, and many other countries. Slowly, COVID-19 reached the United States, which as of mid-April had the highest number of cases worldwide. When COVID-19 hit the United States, the response was that of shock and anger. How could this happen to us? Why is the government not doing anything?
Amidst this pandemonium, ignorance and stupidity of the highest degree were commonplace. This was not restricted to any particular country or region. Almost 2 months into the pandemic, the Ministry of Tourism in my home country of Nepal declared Nepal a ”coronavirus-free zone” and took measures to bring in tourists, focusing specifically on China, where COVID-19 had already killed hundreds. In India, some people were drinking cow urine in hopes of warding off the virus. In the United Sates, thousands of young people flocked to beaches for Spring Break, disregarding measures for social distancing. ”If I get corona, I get corona,” one young man said in an interview that went viral. Personally, I have encountered people who responded to this pandemic by saying the disease was ”cooties” or ”just a flu,” and dismissing it with ”If I die from this, I die.”
Continue to: Rising panic and fear
Rising panic and fear
For most people, seeing COVID-19 at their doorstep triggered a panic, and sent many into a frenzy of buying and hoarding. Once again, we proved that people everywhere are equally stupid, as toilet paper began to vanish from stores across the globe. And yet, this again was a moment when some people began to experience a false sense of immunity: ”I have enough food, money, and toilet paper to last me for 2 years. Why should I be worried?”
When the numbers of COVID-19 deaths in Europe were first reported, the fear became palpable. In Italy and Spain, towns were locked down, and tens of thousands of people (mostly older adults) have died. It was truly heartbreaking to see people alone and at their weakest with no family members allowed to be by their side.
A glimmer of hope
Despite all of this, there were superheroes—the nurses, physicians, allied health professionals, first responders, store workers, restaurant workers, delivery personnel, and others who didn’t have the option of staying home, or who volunteered to help people in need. In moments like this, the actions of these individuals give us hope, reminding us that the human spirit is resilient, and that we will get through this.
A rotation in the emergency department during COVID-19
As a psychiatry resident, it is unlikely that my peers and I face the same risks as our colleagues in other medical specialities. But those of us who happened to be in medical rotations during this time have had the chance to experience this very closely. My personal experience, albeit a brief one, of working in an emergency department with suspected COVID-19 patients has been sobering. Watching nurses and physicians walk into a room wearing personal protective equipment, fearful inside but with a reassuring smile for a scared patient, definitely was one of the most compelling moments of my life. Living in a distant land, with my daughter, wife, parents, and extended family back home in Nepal, has made this even more challenging.
We will overcome this as we have overcome previous challenges in the past. There will be death and chaos, but we will prevail. The only thing is to ask ourselves: How do we want to continue living when this is over?
During these unprecedented times, venturing into the unknown of the coronavirus disease 2019 (COVID-19) pandemic, a feeling of impending doom prevails. Almost all of us have been restricted to our homes. Although the physical dimensions of what we call home may vary, the meaning of this restriction is fairly universal. No matter how our sociodemographics differ, with no guidance for this situation from anything even remotely comparable in the past, our lives have been transformed into a work in progress.
During this pandemic, I have observed a wide range of human emotions and behavior—many of them familiar and predictable, some abysmal, and some inspiring.
’Why should I care?’
On December 31, 2019, health officials in China informed the World Health Organization about a pneumonia-like presentation in a group of people in Wuhan. On January 7, 2020, a novel coronavirus was identified as the cause, and the first death was reported a few days later. In the following days and weeks the disease rapidly spread, as did the growing sense that this was not a typical virus.
While these events occurred, the rest of the world was in what I call a ”Why should I care?” mode. Most humans tend to suffer from this indifference. This has been observed repeatedly through the years, such as when the Ebola outbreak occurred in Africa in 2014-2016. It was only when cases started to develop in Europe and the United States that other countries started to pay attention. A similar phenomenon has been observed every time we’ve faced a global outbreak (avian influenza, Middle East respiratory syndrome, etc.).
When are we going to learn? It is time to realize that global borders are more porous than we think, and human interactions cannot be blocked by any wall. When a catastrophic event, outbreak, or disaster starts in any part of the world, it is naive to assume that we will not be affected. We will eventually be affected—the only question is how, when, and to what extent? We are always all in this together.
An abundance of ignorance and stupidity
Within a few weeks of the first reports from China, cases of COVID-19 were reported in South Korea, Italy, Spain, Germany, and many other countries. Slowly, COVID-19 reached the United States, which as of mid-April had the highest number of cases worldwide. When COVID-19 hit the United States, the response was that of shock and anger. How could this happen to us? Why is the government not doing anything?
Amidst this pandemonium, ignorance and stupidity of the highest degree were commonplace. This was not restricted to any particular country or region. Almost 2 months into the pandemic, the Ministry of Tourism in my home country of Nepal declared Nepal a ”coronavirus-free zone” and took measures to bring in tourists, focusing specifically on China, where COVID-19 had already killed hundreds. In India, some people were drinking cow urine in hopes of warding off the virus. In the United Sates, thousands of young people flocked to beaches for Spring Break, disregarding measures for social distancing. ”If I get corona, I get corona,” one young man said in an interview that went viral. Personally, I have encountered people who responded to this pandemic by saying the disease was ”cooties” or ”just a flu,” and dismissing it with ”If I die from this, I die.”
Continue to: Rising panic and fear
Rising panic and fear
For most people, seeing COVID-19 at their doorstep triggered a panic, and sent many into a frenzy of buying and hoarding. Once again, we proved that people everywhere are equally stupid, as toilet paper began to vanish from stores across the globe. And yet, this again was a moment when some people began to experience a false sense of immunity: ”I have enough food, money, and toilet paper to last me for 2 years. Why should I be worried?”
When the numbers of COVID-19 deaths in Europe were first reported, the fear became palpable. In Italy and Spain, towns were locked down, and tens of thousands of people (mostly older adults) have died. It was truly heartbreaking to see people alone and at their weakest with no family members allowed to be by their side.
A glimmer of hope
Despite all of this, there were superheroes—the nurses, physicians, allied health professionals, first responders, store workers, restaurant workers, delivery personnel, and others who didn’t have the option of staying home, or who volunteered to help people in need. In moments like this, the actions of these individuals give us hope, reminding us that the human spirit is resilient, and that we will get through this.
A rotation in the emergency department during COVID-19
As a psychiatry resident, it is unlikely that my peers and I face the same risks as our colleagues in other medical specialities. But those of us who happened to be in medical rotations during this time have had the chance to experience this very closely. My personal experience, albeit a brief one, of working in an emergency department with suspected COVID-19 patients has been sobering. Watching nurses and physicians walk into a room wearing personal protective equipment, fearful inside but with a reassuring smile for a scared patient, definitely was one of the most compelling moments of my life. Living in a distant land, with my daughter, wife, parents, and extended family back home in Nepal, has made this even more challenging.
We will overcome this as we have overcome previous challenges in the past. There will be death and chaos, but we will prevail. The only thing is to ask ourselves: How do we want to continue living when this is over?
During these unprecedented times, venturing into the unknown of the coronavirus disease 2019 (COVID-19) pandemic, a feeling of impending doom prevails. Almost all of us have been restricted to our homes. Although the physical dimensions of what we call home may vary, the meaning of this restriction is fairly universal. No matter how our sociodemographics differ, with no guidance for this situation from anything even remotely comparable in the past, our lives have been transformed into a work in progress.
During this pandemic, I have observed a wide range of human emotions and behavior—many of them familiar and predictable, some abysmal, and some inspiring.
’Why should I care?’
On December 31, 2019, health officials in China informed the World Health Organization about a pneumonia-like presentation in a group of people in Wuhan. On January 7, 2020, a novel coronavirus was identified as the cause, and the first death was reported a few days later. In the following days and weeks the disease rapidly spread, as did the growing sense that this was not a typical virus.
While these events occurred, the rest of the world was in what I call a ”Why should I care?” mode. Most humans tend to suffer from this indifference. This has been observed repeatedly through the years, such as when the Ebola outbreak occurred in Africa in 2014-2016. It was only when cases started to develop in Europe and the United States that other countries started to pay attention. A similar phenomenon has been observed every time we’ve faced a global outbreak (avian influenza, Middle East respiratory syndrome, etc.).
When are we going to learn? It is time to realize that global borders are more porous than we think, and human interactions cannot be blocked by any wall. When a catastrophic event, outbreak, or disaster starts in any part of the world, it is naive to assume that we will not be affected. We will eventually be affected—the only question is how, when, and to what extent? We are always all in this together.
An abundance of ignorance and stupidity
Within a few weeks of the first reports from China, cases of COVID-19 were reported in South Korea, Italy, Spain, Germany, and many other countries. Slowly, COVID-19 reached the United States, which as of mid-April had the highest number of cases worldwide. When COVID-19 hit the United States, the response was that of shock and anger. How could this happen to us? Why is the government not doing anything?
Amidst this pandemonium, ignorance and stupidity of the highest degree were commonplace. This was not restricted to any particular country or region. Almost 2 months into the pandemic, the Ministry of Tourism in my home country of Nepal declared Nepal a ”coronavirus-free zone” and took measures to bring in tourists, focusing specifically on China, where COVID-19 had already killed hundreds. In India, some people were drinking cow urine in hopes of warding off the virus. In the United Sates, thousands of young people flocked to beaches for Spring Break, disregarding measures for social distancing. ”If I get corona, I get corona,” one young man said in an interview that went viral. Personally, I have encountered people who responded to this pandemic by saying the disease was ”cooties” or ”just a flu,” and dismissing it with ”If I die from this, I die.”
Continue to: Rising panic and fear
Rising panic and fear
For most people, seeing COVID-19 at their doorstep triggered a panic, and sent many into a frenzy of buying and hoarding. Once again, we proved that people everywhere are equally stupid, as toilet paper began to vanish from stores across the globe. And yet, this again was a moment when some people began to experience a false sense of immunity: ”I have enough food, money, and toilet paper to last me for 2 years. Why should I be worried?”
When the numbers of COVID-19 deaths in Europe were first reported, the fear became palpable. In Italy and Spain, towns were locked down, and tens of thousands of people (mostly older adults) have died. It was truly heartbreaking to see people alone and at their weakest with no family members allowed to be by their side.
A glimmer of hope
Despite all of this, there were superheroes—the nurses, physicians, allied health professionals, first responders, store workers, restaurant workers, delivery personnel, and others who didn’t have the option of staying home, or who volunteered to help people in need. In moments like this, the actions of these individuals give us hope, reminding us that the human spirit is resilient, and that we will get through this.
A rotation in the emergency department during COVID-19
As a psychiatry resident, it is unlikely that my peers and I face the same risks as our colleagues in other medical specialities. But those of us who happened to be in medical rotations during this time have had the chance to experience this very closely. My personal experience, albeit a brief one, of working in an emergency department with suspected COVID-19 patients has been sobering. Watching nurses and physicians walk into a room wearing personal protective equipment, fearful inside but with a reassuring smile for a scared patient, definitely was one of the most compelling moments of my life. Living in a distant land, with my daughter, wife, parents, and extended family back home in Nepal, has made this even more challenging.
We will overcome this as we have overcome previous challenges in the past. There will be death and chaos, but we will prevail. The only thing is to ask ourselves: How do we want to continue living when this is over?