User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
How to best use digital technology to help your patients
As psychiatrists, we are increasingly using digital technology, such as e-mail, video conferencing, social media, and text messaging, to communicate with and even treat our patients.1 The benefits of using digital technology for treating patients include, but are not limited to, enhancing access to psychiatric services that are unavailable due to a patient’s geographical location and/or physical disability; providing more cost‐effective delivery of services; and creating more ways for patients to communicate with their physicians.1 While there are benefits to using digital technology, there are also possible repercussions, such as breaches of confidentiality or boundary violations.2 Although there is no evidence-based guidance about how to best use digital technology in patient care,3 the following approaches can help you protect your patients and minimize your liability.
Assess competence. Determine how familiar and comfortable both you and your patient are with the specific software and/or devices you intend to use. Confirm that your patient can access the technology, and inform them of the benefits and risks of using digital technology in their care.1
Create a written policy about your use of digital technology, and review it with all patients to explain how it will be used in their treatment.1 This policy should include a back-up plan in the event of technology failures.1 It should clearly explain that the information gathered with this technology can become part of the patient’s medical record. It should also prohibit patients from using their devices to record other patients in the waiting room or other areas. Such a policy could enhance the protection of private information and help maintain clear boundaries.1 Review and update your policy as often as needed.
Obtain your patients’ written consent to use digital technology. If you want to post information about your patients on social media, obtain their written consent to do so, and mutually agree as to what information would be posted. This should not include their identity or confidential information.1
Do not accept friend requests or contact requests from current or former patients on any social networking platform. Do not follow your patients’ blogs, Twitter accounts, or any other accounts. Be aware that if you and your patients share the same “friend” network on social media, this may create boundary confusion, inappropriate dual relationships, and potential conflicts of interest.1 Keep personal and professional accounts separate to maintain appropriate boundaries and minimize compromising patient confidentiality. Do not post private information on professional practice accounts, and do not link/sync your personal accounts with professional accounts.
Do not store patient information on your personal electronic devices because these devices could be lost or hacked. Avoid contacting your patients via non-secured platforms because doing so could compromise patient confidentiality. Use encrypted software and firewalls for communicating with your patients and storing their information.1 Also, periodically assess your confidentiality policies and procedures to ensure compliance with appropriate statutes and laws.1
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
3. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
As psychiatrists, we are increasingly using digital technology, such as e-mail, video conferencing, social media, and text messaging, to communicate with and even treat our patients.1 The benefits of using digital technology for treating patients include, but are not limited to, enhancing access to psychiatric services that are unavailable due to a patient’s geographical location and/or physical disability; providing more cost‐effective delivery of services; and creating more ways for patients to communicate with their physicians.1 While there are benefits to using digital technology, there are also possible repercussions, such as breaches of confidentiality or boundary violations.2 Although there is no evidence-based guidance about how to best use digital technology in patient care,3 the following approaches can help you protect your patients and minimize your liability.
Assess competence. Determine how familiar and comfortable both you and your patient are with the specific software and/or devices you intend to use. Confirm that your patient can access the technology, and inform them of the benefits and risks of using digital technology in their care.1
Create a written policy about your use of digital technology, and review it with all patients to explain how it will be used in their treatment.1 This policy should include a back-up plan in the event of technology failures.1 It should clearly explain that the information gathered with this technology can become part of the patient’s medical record. It should also prohibit patients from using their devices to record other patients in the waiting room or other areas. Such a policy could enhance the protection of private information and help maintain clear boundaries.1 Review and update your policy as often as needed.
Obtain your patients’ written consent to use digital technology. If you want to post information about your patients on social media, obtain their written consent to do so, and mutually agree as to what information would be posted. This should not include their identity or confidential information.1
Do not accept friend requests or contact requests from current or former patients on any social networking platform. Do not follow your patients’ blogs, Twitter accounts, or any other accounts. Be aware that if you and your patients share the same “friend” network on social media, this may create boundary confusion, inappropriate dual relationships, and potential conflicts of interest.1 Keep personal and professional accounts separate to maintain appropriate boundaries and minimize compromising patient confidentiality. Do not post private information on professional practice accounts, and do not link/sync your personal accounts with professional accounts.
Do not store patient information on your personal electronic devices because these devices could be lost or hacked. Avoid contacting your patients via non-secured platforms because doing so could compromise patient confidentiality. Use encrypted software and firewalls for communicating with your patients and storing their information.1 Also, periodically assess your confidentiality policies and procedures to ensure compliance with appropriate statutes and laws.1
As psychiatrists, we are increasingly using digital technology, such as e-mail, video conferencing, social media, and text messaging, to communicate with and even treat our patients.1 The benefits of using digital technology for treating patients include, but are not limited to, enhancing access to psychiatric services that are unavailable due to a patient’s geographical location and/or physical disability; providing more cost‐effective delivery of services; and creating more ways for patients to communicate with their physicians.1 While there are benefits to using digital technology, there are also possible repercussions, such as breaches of confidentiality or boundary violations.2 Although there is no evidence-based guidance about how to best use digital technology in patient care,3 the following approaches can help you protect your patients and minimize your liability.
Assess competence. Determine how familiar and comfortable both you and your patient are with the specific software and/or devices you intend to use. Confirm that your patient can access the technology, and inform them of the benefits and risks of using digital technology in their care.1
Create a written policy about your use of digital technology, and review it with all patients to explain how it will be used in their treatment.1 This policy should include a back-up plan in the event of technology failures.1 It should clearly explain that the information gathered with this technology can become part of the patient’s medical record. It should also prohibit patients from using their devices to record other patients in the waiting room or other areas. Such a policy could enhance the protection of private information and help maintain clear boundaries.1 Review and update your policy as often as needed.
Obtain your patients’ written consent to use digital technology. If you want to post information about your patients on social media, obtain their written consent to do so, and mutually agree as to what information would be posted. This should not include their identity or confidential information.1
Do not accept friend requests or contact requests from current or former patients on any social networking platform. Do not follow your patients’ blogs, Twitter accounts, or any other accounts. Be aware that if you and your patients share the same “friend” network on social media, this may create boundary confusion, inappropriate dual relationships, and potential conflicts of interest.1 Keep personal and professional accounts separate to maintain appropriate boundaries and minimize compromising patient confidentiality. Do not post private information on professional practice accounts, and do not link/sync your personal accounts with professional accounts.
Do not store patient information on your personal electronic devices because these devices could be lost or hacked. Avoid contacting your patients via non-secured platforms because doing so could compromise patient confidentiality. Use encrypted software and firewalls for communicating with your patients and storing their information.1 Also, periodically assess your confidentiality policies and procedures to ensure compliance with appropriate statutes and laws.1
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
3. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
1. Reamer FG. Evolving standards of care in the age of cybertechnology. Behav Sci Law. 2018;36(2):257-269.
2. Ventola CL. Social media and health care professionals: benefits, risks, and best practices. P T. 2014;39(7):491-499, 520.
3. Logghe HJ, Boeck MA, Gusani NJ, et al. Best practices for surgeons’ social media use: statement of the Resident and Associate Society of the American College of Surgeons. J Am Coll Surg. 2018;226(3):317-327.
Medical ethics in the time of COVID-19
It is clear that the coronavirus 2019 disease (COVID-19) pandemic is one of the most extraordinary epochs of our professional and personal lives. Besides the challenges to the techniques and technologies of care for this illness, we are seeing challenges to the fundamentals of health care, both to the systems whereby it is delivered, and to the ethical principles that guide that delivery. There is unprecedented relevance of certain ethical issues in the practice of medicine, many of which have previously been discussed in classrooms and textbooks, but now are at play in daily practice, particularly at the frontlines of the war against COVID-19.1 In this article, I highlight several ethical dilemmas that are salient to these unique times. Some of the most compelling issues can be sorted into 2 clearly overlapping domains: triage ethics and equity ethics.
Triage ethics
In the areas most greatly affected by the COVID-19 pandemic, scarcity of treatment resources, such as ventilators, is a legitimate concern. French surgeon Dominique Jean Larry was the first to establish medical sorting protocols in the context of the battles of the Napoleonic wars, for which he used the French word triage, meaning “sorting.”2 He articulated 3 prognostic categories: 1) those who would die even with treatment, 2) those who would live without treatment, and 3) those who would die unless treated. Triage decisions arise in the context of insufficient resources, particularly space, staff, and supplies. Although usually identified with disasters, these decisions can arise in other contexts where personnel or technological resources are inadequate. Indeed, one of the first modern incarnations of triage ethics in American civilian life was in the early days of hemodialysis, when so-called “God committees” made complex decisions about which patients would be able to use this new, rare technology.3
Two fundamental moral constructs undergird medical ethics: deontological and utilitarian. The former, in which most clinicians traffic in ordinary practice, is driven by principles or moral rules such as the sanctity of life, the rule of fairness, and the principle of autonomy.4 They apply primarily in the context of treating an individual patient. The utilitarian way of reasoning is not as familiar to clinicians. It is focused on the broader context, the common good, the health of the group. It asks to calculate “the greatest good for the greatest number” as a means of navigating ethical dilemmas.5 The utilitarian perspective is far more familiar to policymakers, health care administrators, and public health professionals. It tends to be anathema to clinicians. However, disasters such as the COVID-19 pandemic ask some clinicians, particularly inpatient physicians, to shift from their usual deontological perspective to a utilitarian one, because triage ethics fundamentally draw on utilitarian reasoning. This can be quite anguishing to clinicians who typically work with individual patients in settings of more adequate, if not abundant, resources. What may feel wrong in a deontological mode can be seen as ethically right in a utilitarian framework.
The Table compares and contrasts these 2 paradigms and how they manifest in the clinical trenches, in a protracted health care crisis with limited resources.
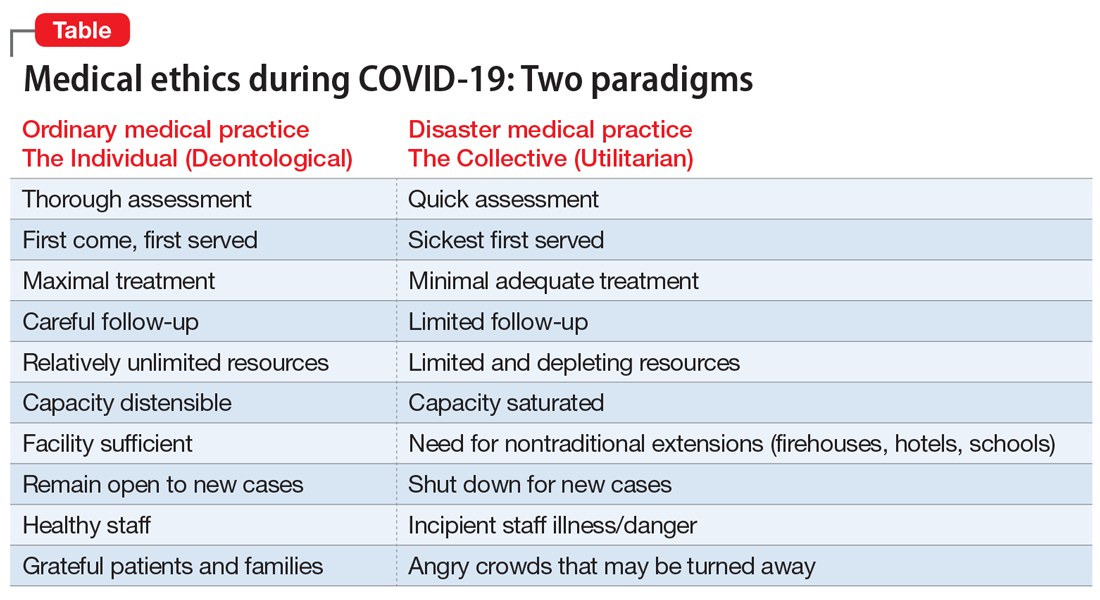
The COVID-19 crisis has produced an unprecedented and extended exposure of clinicians to triage situations in the face of limited resources such as ventilators, personnel, personal protective equipment, etc.6 Numerous possible approaches to deploying limited supplies are being considered. On what basis should such decisions be made? How can fairness be optimally manifest? Some possibilities include:
- first come, first served
- youngest first
- lottery
- short-term survivability
- long-term prognosis for quality of life
- value of a patient to the lives of others (eg, parents, health care workers, vaccine researchers).
One particularly interesting exploration of these questions was done in Maryland and reported in the “Maryland Framework for the Allocation of Scarce Life-sustaining Medical Resources in a Catastrophic Public Health Emergency.”7 This was the product of a multi-year consultation, ending in 2017, with several constituencies, including clinicians, politicians, hospital administrators, and members of the public brainstorming about approaches to allocating a hypothetical scarcity of ventilators. Interestingly, there was one broad consensus among these groups: a ventilator should not be withdrawn from a patient already using it to give to a “better” candidate who comes along later.
Some institutions have developed a method of making triage decisions that takes such decisions out of the hands of individual clinicians and instead assigns them to specialized “triage teams” made up of ethicists and clinicians experienced in critical care, to develop more distance from the emotions at the bedside. To minimize bias, such teams are often insulated from getting personal information about the patient, and receive only acute clinical information.8
Continue to: The pros and cons of these approaches...
The pros and cons of these approaches and the underlying ethical reasoning is beyond the scope of this overview. Policy documents from different states, regions, nations, and institutions have various approaches to making these choices. Presently, there is no coherent national or international agreement on triage ethics.9 It is important, however, that there be transparency in whatever approach an institution adopts for triage decisions.
Equity ethics
Though the equitable distribution of health care delivery has long been a concern, this problem has become magnified by the COVID-19 crisis. Race, sex, age, socioeconomic class, and type of illness have all been perennial sources of division between those who have better or worse access to health care and its outcomes. All of these distinctions have created differentials in rates of cases, hospitalizations, and deaths in the COVID-19 pandemic.10
The shifting of acute health care facilities to mostly COVID-19–related treatment, and postponing less critical and more “elective” care, creates a divide based on illness type. Many facilities have stopped taking admissions for other kinds of cases. This is particularly relevant to psychiatric units, many of which have had to decrease their bed capacities to make all rooms private, and limit their usual treatments offered to inpatients.11 Many long-term units, such as at state hospitals, are closing to new admissions. Many day hospitals and intensive outpatient programs remain closed, not even shifting to telehealth. In areas most affected by COVID-19, some institutions have closed psychiatric wards and reallocated psychiatrists to cover some of the medical units. So the availability of the more intensive, institutionally-based levels of care is significantly reduced, particularly for psychiatric patients.12 These patients already are a disadvantaged population in the distribution of health care resources, and the care of individuals with serious mental illness is more likely to be seen as “nonessential” in this time of suddenly scarcer institutional resources.
One of the cherished ethical values in health care is autonomy, and in a deontological triage environment, honoring patient autonomy is carefully and tenderly administered. However, in a utilitarian-driven triage environment, considerations of the common good can trump autonomy, even in subtle ways that create inequities. Clinicians have been advised to have more frank conversations with patients, particularly those with chronic illnesses, stepping up initiatives to make advanced directives during this crisis, explicitly reminding patients that there may not be enough ventilators for all who need one.13 Some have argued that such physician-initiated conversations can be inherently coercive, making these decisions not as autonomous as it may appear, similar to physicians suggesting medical euthanasia as an option.14 Interestingly, some jurisdictions that offer euthanasia have been suspending such services during the COVID-19 crisis.15 Some hospitals have even wrestled with the possibility that all COVID-19 admissions should be considered “do not resuscitate,” especially because cardiopulmonary resuscitation significantly elevates the risks of viral exposure for the treatment team.16,17 A more explicit example of how current standards protecting patient autonomy may be challenged is patients who are admitted involuntarily to a psychiatric unit. These are patients whose presumptively impaired autonomy is already being overridden by the involuntary nature of the admission. If a psychiatric unit requires admissions to be COVID-19–negative, and if patients refuse COVID-19 testing, should the testing be forced upon them to protect the entire milieu?
Many ethicists are highlighting the embedded equity bias known as “ableism” inherent in triage decisions—implicitly disfavoring resources for patients with COVID-19 who are already physically or intellectually disabled, chronically ill, aged, homeless, psychosocially low functioning, etc.18 Without explicit protections for individuals who are chronically disabled, triage decisions unguided by policy safeguards may reflexively favor the more “abled.” This bias towards the more abled is often inherent in how difficult it is to access health care. It can also be manifested in bedside triage decisions made in the moment by individual clinicians. Many disability rights advocates have been sounding this alarm during the COVID-19 crisis.19
Continue to: A special circumstance of equity...
A special circumstance of equity is arising during this ongoing pandemic—the possibility of treating health care workers as a privileged class. Unlike typical disasters, where health care workers come in afterwards, and therefore are in relatively less danger, pandemics create particularly high risks of danger for such individuals, with repeated exposure to the virus. They are both responders and potential victims. Should they have higher priority for ventilators, vaccines, funding, etc?6 This is a more robust degree of compensatory justice than merely giving appreciation. Giving health care workers such advantages may seem intuitively appealing, but perhaps professionalism and the self-obligation of duty mitigates such claims.20
A unique opportunity
The magnitude and pervasiveness of this pandemic crisis is unique in our lifetimes, as both professionals and as citizens. In the crucible of this extraordinary time, these and other medical ethics dilemmas burn hotter than ever before. Different societies and institutions may come up with different answers, based on their cultures and values. It is important, however, that the venerable ethos of medical ethics, which has evolved through the millennia, codified in oaths, codes, and scholarship, can be a compass at the bedside and in the meetings of legislatures, leaders, and policymakers. Perhaps we can emerge from this time with more clarity about how to balance the preciousness of individual rights with the needs of the common good.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has brought increased attention to triage ethics and equity ethics. There is no coherent national or international agreement on how to best deploy limited supplies such as ventilators and personal protective equipment. Although the equitable distribution of health care delivery has long been a concern, this problem has become magnified by COVID-19. Clinicians may be asked to view health care through the less familiar lens of the common good, as opposed to focusing strictly on an individual patient.
Related Resources
- Johns Hopkins Berman Institute of Bioethics. Coronavirus ethics and policy insights and resources. https://bioethics.jhu.edu/research-and-outreach/covid-19-bioethics-expert-insights/.
- Daugherty-Biddison L, Gwon H, Regenberg A, et al. Maryland framework for the allocation of scarce lifesustaining medical resources in a catastrophic public health emergency. www.law.umaryland.edu/media/SOL/pdfs/Programs/Health-Law/MHECN/ASR%20Framework_Final.pdf.
1. AMA Journal of Ethics. COVID-19 ethics resource center. https://journalofethics.ama-assn.org/COVID-19-ethics-resource-center. Updated May 2020. Accessed May 26, 2020.
2. Skandakalis PN, Lainas P, Zoras O, et al. “To afford the wounded speedy assistance”: Dominique Jean Larrey and Napoleon. World J Surg. 2006;30(8):1392-1399.
3. Ross W. God panels and the history of hemodialysis in America: a cautionary tale. Virtual Mentor. 2012;14(11):890-896.
4. Alexander L, Moore M. Deontological ethics. In: Zalta EN, ed. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/ethics-deontological/. Revised October 17, 2016. Accessed May 26, 2020.
5. Driver J. The history of utilitarianism. In: Zalta EN, ed. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/utilitarianism-history/. Revised September 22, 2014. Accessed May 26, 2020.
6. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med. 2020;382(21):2049-2055.
7. Daugherty-Biddison EL, Faden R, Gwon HW, et al. Too many patients…a framework to guide statewide allocation of scarce mechanical ventilation during disasters. Chest. 2019;155(4):848-854.
8. Dudzinski D, Campelia G, Brazg T. Pandemic resources including COVID-19 materials. Department of Bioethics and Humanities, University of Washington Medicine. http://depts.washington.edu/bhdept/ethics-medicine/bioethics-topics/detail/245. Published April 6, 2020. Accessed May 26, 2020.
9. Antommaria AHM, Gibb TS, McGuire AL, et al; Task Force of the Association of Bioethics Program Directors. Ventilator triage policies during the COVID-19 pandemic at U.S. hospitals associated with members of the Association of Bioethics Program Directors [published online April 24, 2020]. Ann Intern Med. 2020;M20-1738. doi: 10.7326/M20-1738.
10. Cooney E. Who gets hospitalized for COVID-19? Report shows differences by race and sex. STAT. https://www.statnews.com/2020/04/09/hospitalized-COVID-19-patients-differences-by-race-and-sex/. Published April 9, 2020. Accessed May 26, 2020.
11. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. The New Yorker. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed May 26, 2020.
12. American Psychiatric Association Ethics Committee. COVID-19 related opinions of the APA Ethics Committee. American Psychiatric Association. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/Ethics/APA-COVID-19-Ethics-Opinions.pdf. Published May 5, 2020. Accessed May 26, 2020.
13. Wee M. Coronavirus and the misuse of ‘do not resuscitate’ orders. The Spectator. https://www.spectator.co.uk/article/coronavirus-and-the-misuse-of-do-not-resuscitate-orders. Published May 6, 2020. Accessed May 26, 2020.
14. Prokopetz JZ, Lehmann LS. Redefining physicians’ role in assisted dying. N Engl J Med. 2012;367(20):97-99.
15. Yuill K, Boer T. What COVID-19 has revealed about euthanasia. spiked. https://www.spiked-online.com/2020/04/14/COVID-19-has-revealed-the-ugliness-of-euthanasia/. Published April 14, 2020. Accessed May 26, 2020.
16. Plunkett AJ. COVID-19: hospitals should consider CoP carefully before deciding on DNR policy. PSQH. https://www.psqh.com/news/COVID-19-hospitals-should-consider-cop-carefully-before-deciding-on-dnr-policy/. Published March 26, 2020. Accessed May 26, 2020.
17. Kramer DB, Lo B, Dickert NW. CPR in the COVID-19 era: an ethical framework [published online May 6, 2020]. N Engl J Med. doi: 10.1056/NEJMp2010758.
18. Mykitiuk R, Lemmens T. Assessing the value of a life: COVID-19 triage orders mustn’t work against those with disabilities. CBC News. https://www.cbc.ca/news/opinion/opinion-disabled-COVID-19-triage-orders-1.5532137. Published April 19, 2020. Accessed May 26, 2020.
19. Solomon MZ, Wynia MK, Gostin LO. COVID-19 crisis triage—optimizing health outcomes and disability rights [published online May 19, 2020]. N Engl J Med. doi: 10.1056/NEJMp2008300.
20. Appel JM. Ethics consult: who’s first to get COVID-19 Vax? MD/JD bangs gavel. MedPage Today. https://www.medpagetoday.com/infectiousdisease/COVID19/86260. Published May 1, 2020. Accessed May 26, 2020.
It is clear that the coronavirus 2019 disease (COVID-19) pandemic is one of the most extraordinary epochs of our professional and personal lives. Besides the challenges to the techniques and technologies of care for this illness, we are seeing challenges to the fundamentals of health care, both to the systems whereby it is delivered, and to the ethical principles that guide that delivery. There is unprecedented relevance of certain ethical issues in the practice of medicine, many of which have previously been discussed in classrooms and textbooks, but now are at play in daily practice, particularly at the frontlines of the war against COVID-19.1 In this article, I highlight several ethical dilemmas that are salient to these unique times. Some of the most compelling issues can be sorted into 2 clearly overlapping domains: triage ethics and equity ethics.
Triage ethics
In the areas most greatly affected by the COVID-19 pandemic, scarcity of treatment resources, such as ventilators, is a legitimate concern. French surgeon Dominique Jean Larry was the first to establish medical sorting protocols in the context of the battles of the Napoleonic wars, for which he used the French word triage, meaning “sorting.”2 He articulated 3 prognostic categories: 1) those who would die even with treatment, 2) those who would live without treatment, and 3) those who would die unless treated. Triage decisions arise in the context of insufficient resources, particularly space, staff, and supplies. Although usually identified with disasters, these decisions can arise in other contexts where personnel or technological resources are inadequate. Indeed, one of the first modern incarnations of triage ethics in American civilian life was in the early days of hemodialysis, when so-called “God committees” made complex decisions about which patients would be able to use this new, rare technology.3
Two fundamental moral constructs undergird medical ethics: deontological and utilitarian. The former, in which most clinicians traffic in ordinary practice, is driven by principles or moral rules such as the sanctity of life, the rule of fairness, and the principle of autonomy.4 They apply primarily in the context of treating an individual patient. The utilitarian way of reasoning is not as familiar to clinicians. It is focused on the broader context, the common good, the health of the group. It asks to calculate “the greatest good for the greatest number” as a means of navigating ethical dilemmas.5 The utilitarian perspective is far more familiar to policymakers, health care administrators, and public health professionals. It tends to be anathema to clinicians. However, disasters such as the COVID-19 pandemic ask some clinicians, particularly inpatient physicians, to shift from their usual deontological perspective to a utilitarian one, because triage ethics fundamentally draw on utilitarian reasoning. This can be quite anguishing to clinicians who typically work with individual patients in settings of more adequate, if not abundant, resources. What may feel wrong in a deontological mode can be seen as ethically right in a utilitarian framework.
The Table compares and contrasts these 2 paradigms and how they manifest in the clinical trenches, in a protracted health care crisis with limited resources.

The COVID-19 crisis has produced an unprecedented and extended exposure of clinicians to triage situations in the face of limited resources such as ventilators, personnel, personal protective equipment, etc.6 Numerous possible approaches to deploying limited supplies are being considered. On what basis should such decisions be made? How can fairness be optimally manifest? Some possibilities include:
- first come, first served
- youngest first
- lottery
- short-term survivability
- long-term prognosis for quality of life
- value of a patient to the lives of others (eg, parents, health care workers, vaccine researchers).
One particularly interesting exploration of these questions was done in Maryland and reported in the “Maryland Framework for the Allocation of Scarce Life-sustaining Medical Resources in a Catastrophic Public Health Emergency.”7 This was the product of a multi-year consultation, ending in 2017, with several constituencies, including clinicians, politicians, hospital administrators, and members of the public brainstorming about approaches to allocating a hypothetical scarcity of ventilators. Interestingly, there was one broad consensus among these groups: a ventilator should not be withdrawn from a patient already using it to give to a “better” candidate who comes along later.
Some institutions have developed a method of making triage decisions that takes such decisions out of the hands of individual clinicians and instead assigns them to specialized “triage teams” made up of ethicists and clinicians experienced in critical care, to develop more distance from the emotions at the bedside. To minimize bias, such teams are often insulated from getting personal information about the patient, and receive only acute clinical information.8
Continue to: The pros and cons of these approaches...
The pros and cons of these approaches and the underlying ethical reasoning is beyond the scope of this overview. Policy documents from different states, regions, nations, and institutions have various approaches to making these choices. Presently, there is no coherent national or international agreement on triage ethics.9 It is important, however, that there be transparency in whatever approach an institution adopts for triage decisions.
Equity ethics
Though the equitable distribution of health care delivery has long been a concern, this problem has become magnified by the COVID-19 crisis. Race, sex, age, socioeconomic class, and type of illness have all been perennial sources of division between those who have better or worse access to health care and its outcomes. All of these distinctions have created differentials in rates of cases, hospitalizations, and deaths in the COVID-19 pandemic.10
The shifting of acute health care facilities to mostly COVID-19–related treatment, and postponing less critical and more “elective” care, creates a divide based on illness type. Many facilities have stopped taking admissions for other kinds of cases. This is particularly relevant to psychiatric units, many of which have had to decrease their bed capacities to make all rooms private, and limit their usual treatments offered to inpatients.11 Many long-term units, such as at state hospitals, are closing to new admissions. Many day hospitals and intensive outpatient programs remain closed, not even shifting to telehealth. In areas most affected by COVID-19, some institutions have closed psychiatric wards and reallocated psychiatrists to cover some of the medical units. So the availability of the more intensive, institutionally-based levels of care is significantly reduced, particularly for psychiatric patients.12 These patients already are a disadvantaged population in the distribution of health care resources, and the care of individuals with serious mental illness is more likely to be seen as “nonessential” in this time of suddenly scarcer institutional resources.
One of the cherished ethical values in health care is autonomy, and in a deontological triage environment, honoring patient autonomy is carefully and tenderly administered. However, in a utilitarian-driven triage environment, considerations of the common good can trump autonomy, even in subtle ways that create inequities. Clinicians have been advised to have more frank conversations with patients, particularly those with chronic illnesses, stepping up initiatives to make advanced directives during this crisis, explicitly reminding patients that there may not be enough ventilators for all who need one.13 Some have argued that such physician-initiated conversations can be inherently coercive, making these decisions not as autonomous as it may appear, similar to physicians suggesting medical euthanasia as an option.14 Interestingly, some jurisdictions that offer euthanasia have been suspending such services during the COVID-19 crisis.15 Some hospitals have even wrestled with the possibility that all COVID-19 admissions should be considered “do not resuscitate,” especially because cardiopulmonary resuscitation significantly elevates the risks of viral exposure for the treatment team.16,17 A more explicit example of how current standards protecting patient autonomy may be challenged is patients who are admitted involuntarily to a psychiatric unit. These are patients whose presumptively impaired autonomy is already being overridden by the involuntary nature of the admission. If a psychiatric unit requires admissions to be COVID-19–negative, and if patients refuse COVID-19 testing, should the testing be forced upon them to protect the entire milieu?
Many ethicists are highlighting the embedded equity bias known as “ableism” inherent in triage decisions—implicitly disfavoring resources for patients with COVID-19 who are already physically or intellectually disabled, chronically ill, aged, homeless, psychosocially low functioning, etc.18 Without explicit protections for individuals who are chronically disabled, triage decisions unguided by policy safeguards may reflexively favor the more “abled.” This bias towards the more abled is often inherent in how difficult it is to access health care. It can also be manifested in bedside triage decisions made in the moment by individual clinicians. Many disability rights advocates have been sounding this alarm during the COVID-19 crisis.19
Continue to: A special circumstance of equity...
A special circumstance of equity is arising during this ongoing pandemic—the possibility of treating health care workers as a privileged class. Unlike typical disasters, where health care workers come in afterwards, and therefore are in relatively less danger, pandemics create particularly high risks of danger for such individuals, with repeated exposure to the virus. They are both responders and potential victims. Should they have higher priority for ventilators, vaccines, funding, etc?6 This is a more robust degree of compensatory justice than merely giving appreciation. Giving health care workers such advantages may seem intuitively appealing, but perhaps professionalism and the self-obligation of duty mitigates such claims.20
A unique opportunity
The magnitude and pervasiveness of this pandemic crisis is unique in our lifetimes, as both professionals and as citizens. In the crucible of this extraordinary time, these and other medical ethics dilemmas burn hotter than ever before. Different societies and institutions may come up with different answers, based on their cultures and values. It is important, however, that the venerable ethos of medical ethics, which has evolved through the millennia, codified in oaths, codes, and scholarship, can be a compass at the bedside and in the meetings of legislatures, leaders, and policymakers. Perhaps we can emerge from this time with more clarity about how to balance the preciousness of individual rights with the needs of the common good.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has brought increased attention to triage ethics and equity ethics. There is no coherent national or international agreement on how to best deploy limited supplies such as ventilators and personal protective equipment. Although the equitable distribution of health care delivery has long been a concern, this problem has become magnified by COVID-19. Clinicians may be asked to view health care through the less familiar lens of the common good, as opposed to focusing strictly on an individual patient.
Related Resources
- Johns Hopkins Berman Institute of Bioethics. Coronavirus ethics and policy insights and resources. https://bioethics.jhu.edu/research-and-outreach/covid-19-bioethics-expert-insights/.
- Daugherty-Biddison L, Gwon H, Regenberg A, et al. Maryland framework for the allocation of scarce lifesustaining medical resources in a catastrophic public health emergency. www.law.umaryland.edu/media/SOL/pdfs/Programs/Health-Law/MHECN/ASR%20Framework_Final.pdf.
It is clear that the coronavirus 2019 disease (COVID-19) pandemic is one of the most extraordinary epochs of our professional and personal lives. Besides the challenges to the techniques and technologies of care for this illness, we are seeing challenges to the fundamentals of health care, both to the systems whereby it is delivered, and to the ethical principles that guide that delivery. There is unprecedented relevance of certain ethical issues in the practice of medicine, many of which have previously been discussed in classrooms and textbooks, but now are at play in daily practice, particularly at the frontlines of the war against COVID-19.1 In this article, I highlight several ethical dilemmas that are salient to these unique times. Some of the most compelling issues can be sorted into 2 clearly overlapping domains: triage ethics and equity ethics.
Triage ethics
In the areas most greatly affected by the COVID-19 pandemic, scarcity of treatment resources, such as ventilators, is a legitimate concern. French surgeon Dominique Jean Larry was the first to establish medical sorting protocols in the context of the battles of the Napoleonic wars, for which he used the French word triage, meaning “sorting.”2 He articulated 3 prognostic categories: 1) those who would die even with treatment, 2) those who would live without treatment, and 3) those who would die unless treated. Triage decisions arise in the context of insufficient resources, particularly space, staff, and supplies. Although usually identified with disasters, these decisions can arise in other contexts where personnel or technological resources are inadequate. Indeed, one of the first modern incarnations of triage ethics in American civilian life was in the early days of hemodialysis, when so-called “God committees” made complex decisions about which patients would be able to use this new, rare technology.3
Two fundamental moral constructs undergird medical ethics: deontological and utilitarian. The former, in which most clinicians traffic in ordinary practice, is driven by principles or moral rules such as the sanctity of life, the rule of fairness, and the principle of autonomy.4 They apply primarily in the context of treating an individual patient. The utilitarian way of reasoning is not as familiar to clinicians. It is focused on the broader context, the common good, the health of the group. It asks to calculate “the greatest good for the greatest number” as a means of navigating ethical dilemmas.5 The utilitarian perspective is far more familiar to policymakers, health care administrators, and public health professionals. It tends to be anathema to clinicians. However, disasters such as the COVID-19 pandemic ask some clinicians, particularly inpatient physicians, to shift from their usual deontological perspective to a utilitarian one, because triage ethics fundamentally draw on utilitarian reasoning. This can be quite anguishing to clinicians who typically work with individual patients in settings of more adequate, if not abundant, resources. What may feel wrong in a deontological mode can be seen as ethically right in a utilitarian framework.
The Table compares and contrasts these 2 paradigms and how they manifest in the clinical trenches, in a protracted health care crisis with limited resources.

The COVID-19 crisis has produced an unprecedented and extended exposure of clinicians to triage situations in the face of limited resources such as ventilators, personnel, personal protective equipment, etc.6 Numerous possible approaches to deploying limited supplies are being considered. On what basis should such decisions be made? How can fairness be optimally manifest? Some possibilities include:
- first come, first served
- youngest first
- lottery
- short-term survivability
- long-term prognosis for quality of life
- value of a patient to the lives of others (eg, parents, health care workers, vaccine researchers).
One particularly interesting exploration of these questions was done in Maryland and reported in the “Maryland Framework for the Allocation of Scarce Life-sustaining Medical Resources in a Catastrophic Public Health Emergency.”7 This was the product of a multi-year consultation, ending in 2017, with several constituencies, including clinicians, politicians, hospital administrators, and members of the public brainstorming about approaches to allocating a hypothetical scarcity of ventilators. Interestingly, there was one broad consensus among these groups: a ventilator should not be withdrawn from a patient already using it to give to a “better” candidate who comes along later.
Some institutions have developed a method of making triage decisions that takes such decisions out of the hands of individual clinicians and instead assigns them to specialized “triage teams” made up of ethicists and clinicians experienced in critical care, to develop more distance from the emotions at the bedside. To minimize bias, such teams are often insulated from getting personal information about the patient, and receive only acute clinical information.8
Continue to: The pros and cons of these approaches...
The pros and cons of these approaches and the underlying ethical reasoning is beyond the scope of this overview. Policy documents from different states, regions, nations, and institutions have various approaches to making these choices. Presently, there is no coherent national or international agreement on triage ethics.9 It is important, however, that there be transparency in whatever approach an institution adopts for triage decisions.
Equity ethics
Though the equitable distribution of health care delivery has long been a concern, this problem has become magnified by the COVID-19 crisis. Race, sex, age, socioeconomic class, and type of illness have all been perennial sources of division between those who have better or worse access to health care and its outcomes. All of these distinctions have created differentials in rates of cases, hospitalizations, and deaths in the COVID-19 pandemic.10
The shifting of acute health care facilities to mostly COVID-19–related treatment, and postponing less critical and more “elective” care, creates a divide based on illness type. Many facilities have stopped taking admissions for other kinds of cases. This is particularly relevant to psychiatric units, many of which have had to decrease their bed capacities to make all rooms private, and limit their usual treatments offered to inpatients.11 Many long-term units, such as at state hospitals, are closing to new admissions. Many day hospitals and intensive outpatient programs remain closed, not even shifting to telehealth. In areas most affected by COVID-19, some institutions have closed psychiatric wards and reallocated psychiatrists to cover some of the medical units. So the availability of the more intensive, institutionally-based levels of care is significantly reduced, particularly for psychiatric patients.12 These patients already are a disadvantaged population in the distribution of health care resources, and the care of individuals with serious mental illness is more likely to be seen as “nonessential” in this time of suddenly scarcer institutional resources.
One of the cherished ethical values in health care is autonomy, and in a deontological triage environment, honoring patient autonomy is carefully and tenderly administered. However, in a utilitarian-driven triage environment, considerations of the common good can trump autonomy, even in subtle ways that create inequities. Clinicians have been advised to have more frank conversations with patients, particularly those with chronic illnesses, stepping up initiatives to make advanced directives during this crisis, explicitly reminding patients that there may not be enough ventilators for all who need one.13 Some have argued that such physician-initiated conversations can be inherently coercive, making these decisions not as autonomous as it may appear, similar to physicians suggesting medical euthanasia as an option.14 Interestingly, some jurisdictions that offer euthanasia have been suspending such services during the COVID-19 crisis.15 Some hospitals have even wrestled with the possibility that all COVID-19 admissions should be considered “do not resuscitate,” especially because cardiopulmonary resuscitation significantly elevates the risks of viral exposure for the treatment team.16,17 A more explicit example of how current standards protecting patient autonomy may be challenged is patients who are admitted involuntarily to a psychiatric unit. These are patients whose presumptively impaired autonomy is already being overridden by the involuntary nature of the admission. If a psychiatric unit requires admissions to be COVID-19–negative, and if patients refuse COVID-19 testing, should the testing be forced upon them to protect the entire milieu?
Many ethicists are highlighting the embedded equity bias known as “ableism” inherent in triage decisions—implicitly disfavoring resources for patients with COVID-19 who are already physically or intellectually disabled, chronically ill, aged, homeless, psychosocially low functioning, etc.18 Without explicit protections for individuals who are chronically disabled, triage decisions unguided by policy safeguards may reflexively favor the more “abled.” This bias towards the more abled is often inherent in how difficult it is to access health care. It can also be manifested in bedside triage decisions made in the moment by individual clinicians. Many disability rights advocates have been sounding this alarm during the COVID-19 crisis.19
Continue to: A special circumstance of equity...
A special circumstance of equity is arising during this ongoing pandemic—the possibility of treating health care workers as a privileged class. Unlike typical disasters, where health care workers come in afterwards, and therefore are in relatively less danger, pandemics create particularly high risks of danger for such individuals, with repeated exposure to the virus. They are both responders and potential victims. Should they have higher priority for ventilators, vaccines, funding, etc?6 This is a more robust degree of compensatory justice than merely giving appreciation. Giving health care workers such advantages may seem intuitively appealing, but perhaps professionalism and the self-obligation of duty mitigates such claims.20
A unique opportunity
The magnitude and pervasiveness of this pandemic crisis is unique in our lifetimes, as both professionals and as citizens. In the crucible of this extraordinary time, these and other medical ethics dilemmas burn hotter than ever before. Different societies and institutions may come up with different answers, based on their cultures and values. It is important, however, that the venerable ethos of medical ethics, which has evolved through the millennia, codified in oaths, codes, and scholarship, can be a compass at the bedside and in the meetings of legislatures, leaders, and policymakers. Perhaps we can emerge from this time with more clarity about how to balance the preciousness of individual rights with the needs of the common good.
Bottom Line
The coronavirus disease 2019 (COVID-19) pandemic has brought increased attention to triage ethics and equity ethics. There is no coherent national or international agreement on how to best deploy limited supplies such as ventilators and personal protective equipment. Although the equitable distribution of health care delivery has long been a concern, this problem has become magnified by COVID-19. Clinicians may be asked to view health care through the less familiar lens of the common good, as opposed to focusing strictly on an individual patient.
Related Resources
- Johns Hopkins Berman Institute of Bioethics. Coronavirus ethics and policy insights and resources. https://bioethics.jhu.edu/research-and-outreach/covid-19-bioethics-expert-insights/.
- Daugherty-Biddison L, Gwon H, Regenberg A, et al. Maryland framework for the allocation of scarce lifesustaining medical resources in a catastrophic public health emergency. www.law.umaryland.edu/media/SOL/pdfs/Programs/Health-Law/MHECN/ASR%20Framework_Final.pdf.
1. AMA Journal of Ethics. COVID-19 ethics resource center. https://journalofethics.ama-assn.org/COVID-19-ethics-resource-center. Updated May 2020. Accessed May 26, 2020.
2. Skandakalis PN, Lainas P, Zoras O, et al. “To afford the wounded speedy assistance”: Dominique Jean Larrey and Napoleon. World J Surg. 2006;30(8):1392-1399.
3. Ross W. God panels and the history of hemodialysis in America: a cautionary tale. Virtual Mentor. 2012;14(11):890-896.
4. Alexander L, Moore M. Deontological ethics. In: Zalta EN, ed. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/ethics-deontological/. Revised October 17, 2016. Accessed May 26, 2020.
5. Driver J. The history of utilitarianism. In: Zalta EN, ed. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/utilitarianism-history/. Revised September 22, 2014. Accessed May 26, 2020.
6. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med. 2020;382(21):2049-2055.
7. Daugherty-Biddison EL, Faden R, Gwon HW, et al. Too many patients…a framework to guide statewide allocation of scarce mechanical ventilation during disasters. Chest. 2019;155(4):848-854.
8. Dudzinski D, Campelia G, Brazg T. Pandemic resources including COVID-19 materials. Department of Bioethics and Humanities, University of Washington Medicine. http://depts.washington.edu/bhdept/ethics-medicine/bioethics-topics/detail/245. Published April 6, 2020. Accessed May 26, 2020.
9. Antommaria AHM, Gibb TS, McGuire AL, et al; Task Force of the Association of Bioethics Program Directors. Ventilator triage policies during the COVID-19 pandemic at U.S. hospitals associated with members of the Association of Bioethics Program Directors [published online April 24, 2020]. Ann Intern Med. 2020;M20-1738. doi: 10.7326/M20-1738.
10. Cooney E. Who gets hospitalized for COVID-19? Report shows differences by race and sex. STAT. https://www.statnews.com/2020/04/09/hospitalized-COVID-19-patients-differences-by-race-and-sex/. Published April 9, 2020. Accessed May 26, 2020.
11. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. The New Yorker. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed May 26, 2020.
12. American Psychiatric Association Ethics Committee. COVID-19 related opinions of the APA Ethics Committee. American Psychiatric Association. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/Ethics/APA-COVID-19-Ethics-Opinions.pdf. Published May 5, 2020. Accessed May 26, 2020.
13. Wee M. Coronavirus and the misuse of ‘do not resuscitate’ orders. The Spectator. https://www.spectator.co.uk/article/coronavirus-and-the-misuse-of-do-not-resuscitate-orders. Published May 6, 2020. Accessed May 26, 2020.
14. Prokopetz JZ, Lehmann LS. Redefining physicians’ role in assisted dying. N Engl J Med. 2012;367(20):97-99.
15. Yuill K, Boer T. What COVID-19 has revealed about euthanasia. spiked. https://www.spiked-online.com/2020/04/14/COVID-19-has-revealed-the-ugliness-of-euthanasia/. Published April 14, 2020. Accessed May 26, 2020.
16. Plunkett AJ. COVID-19: hospitals should consider CoP carefully before deciding on DNR policy. PSQH. https://www.psqh.com/news/COVID-19-hospitals-should-consider-cop-carefully-before-deciding-on-dnr-policy/. Published March 26, 2020. Accessed May 26, 2020.
17. Kramer DB, Lo B, Dickert NW. CPR in the COVID-19 era: an ethical framework [published online May 6, 2020]. N Engl J Med. doi: 10.1056/NEJMp2010758.
18. Mykitiuk R, Lemmens T. Assessing the value of a life: COVID-19 triage orders mustn’t work against those with disabilities. CBC News. https://www.cbc.ca/news/opinion/opinion-disabled-COVID-19-triage-orders-1.5532137. Published April 19, 2020. Accessed May 26, 2020.
19. Solomon MZ, Wynia MK, Gostin LO. COVID-19 crisis triage—optimizing health outcomes and disability rights [published online May 19, 2020]. N Engl J Med. doi: 10.1056/NEJMp2008300.
20. Appel JM. Ethics consult: who’s first to get COVID-19 Vax? MD/JD bangs gavel. MedPage Today. https://www.medpagetoday.com/infectiousdisease/COVID19/86260. Published May 1, 2020. Accessed May 26, 2020.
1. AMA Journal of Ethics. COVID-19 ethics resource center. https://journalofethics.ama-assn.org/COVID-19-ethics-resource-center. Updated May 2020. Accessed May 26, 2020.
2. Skandakalis PN, Lainas P, Zoras O, et al. “To afford the wounded speedy assistance”: Dominique Jean Larrey and Napoleon. World J Surg. 2006;30(8):1392-1399.
3. Ross W. God panels and the history of hemodialysis in America: a cautionary tale. Virtual Mentor. 2012;14(11):890-896.
4. Alexander L, Moore M. Deontological ethics. In: Zalta EN, ed. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/ethics-deontological/. Revised October 17, 2016. Accessed May 26, 2020.
5. Driver J. The history of utilitarianism. In: Zalta EN, ed. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/utilitarianism-history/. Revised September 22, 2014. Accessed May 26, 2020.
6. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med. 2020;382(21):2049-2055.
7. Daugherty-Biddison EL, Faden R, Gwon HW, et al. Too many patients…a framework to guide statewide allocation of scarce mechanical ventilation during disasters. Chest. 2019;155(4):848-854.
8. Dudzinski D, Campelia G, Brazg T. Pandemic resources including COVID-19 materials. Department of Bioethics and Humanities, University of Washington Medicine. http://depts.washington.edu/bhdept/ethics-medicine/bioethics-topics/detail/245. Published April 6, 2020. Accessed May 26, 2020.
9. Antommaria AHM, Gibb TS, McGuire AL, et al; Task Force of the Association of Bioethics Program Directors. Ventilator triage policies during the COVID-19 pandemic at U.S. hospitals associated with members of the Association of Bioethics Program Directors [published online April 24, 2020]. Ann Intern Med. 2020;M20-1738. doi: 10.7326/M20-1738.
10. Cooney E. Who gets hospitalized for COVID-19? Report shows differences by race and sex. STAT. https://www.statnews.com/2020/04/09/hospitalized-COVID-19-patients-differences-by-race-and-sex/. Published April 9, 2020. Accessed May 26, 2020.
11. Gessen M. Why psychiatric wards are uniquely vulnerable to the coronavirus. The New Yorker. https://www.newyorker.com/news/news-desk/why-psychiatric-wards-are-uniquely-vulnerable-to-the-coronavirus. Published April 21, 2020. Accessed May 26, 2020.
12. American Psychiatric Association Ethics Committee. COVID-19 related opinions of the APA Ethics Committee. American Psychiatric Association. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/Ethics/APA-COVID-19-Ethics-Opinions.pdf. Published May 5, 2020. Accessed May 26, 2020.
13. Wee M. Coronavirus and the misuse of ‘do not resuscitate’ orders. The Spectator. https://www.spectator.co.uk/article/coronavirus-and-the-misuse-of-do-not-resuscitate-orders. Published May 6, 2020. Accessed May 26, 2020.
14. Prokopetz JZ, Lehmann LS. Redefining physicians’ role in assisted dying. N Engl J Med. 2012;367(20):97-99.
15. Yuill K, Boer T. What COVID-19 has revealed about euthanasia. spiked. https://www.spiked-online.com/2020/04/14/COVID-19-has-revealed-the-ugliness-of-euthanasia/. Published April 14, 2020. Accessed May 26, 2020.
16. Plunkett AJ. COVID-19: hospitals should consider CoP carefully before deciding on DNR policy. PSQH. https://www.psqh.com/news/COVID-19-hospitals-should-consider-cop-carefully-before-deciding-on-dnr-policy/. Published March 26, 2020. Accessed May 26, 2020.
17. Kramer DB, Lo B, Dickert NW. CPR in the COVID-19 era: an ethical framework [published online May 6, 2020]. N Engl J Med. doi: 10.1056/NEJMp2010758.
18. Mykitiuk R, Lemmens T. Assessing the value of a life: COVID-19 triage orders mustn’t work against those with disabilities. CBC News. https://www.cbc.ca/news/opinion/opinion-disabled-COVID-19-triage-orders-1.5532137. Published April 19, 2020. Accessed May 26, 2020.
19. Solomon MZ, Wynia MK, Gostin LO. COVID-19 crisis triage—optimizing health outcomes and disability rights [published online May 19, 2020]. N Engl J Med. doi: 10.1056/NEJMp2008300.
20. Appel JM. Ethics consult: who’s first to get COVID-19 Vax? MD/JD bangs gavel. MedPage Today. https://www.medpagetoday.com/infectiousdisease/COVID19/86260. Published May 1, 2020. Accessed May 26, 2020.
Anorexia nervosa and COVID-19
Recent concerns surrounding coronavirus disease 2019 (COVID-19) make it timely to reexamine the complex findings related to eating disorders and the immune system, and the risks for and detection of infection in patients with anorexia nervosa (AN) and similar disorders. To date, there are no published studies evaluating patients with eating disorders and COVID-19. However, it may be helpful to review the data on the infectious process in this patient population to improve patient communication, enhance surveillance and detection, and possibly reduce morbidity and mortality.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) issued warnings that individuals who are older, have underlying medical conditions, and/or are immunocompromised face the greatest risk of serious complications and death as a result of COVID-19, the disease process caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to malnutrition, patients with eating disorders, especially AN, may be perceived to have an increased risk of medical conditions and infection. Despite many studies on specific changes and differences in the immune system of patients with eating disorders, the consequences of these changes remain controversial and inconclusive.
This article reviews research on eating disorders, focusing on published data regarding the effects of AN on the immune system, susceptibility to infections, infectious detection, and morbidity. We also discuss clinical considerations related to COVID-19 and patients with AN.
Infection risks: Conflicting data
In a 1981 study that included 9 participants, Golla et al1 concluded that patients with AN may have “resistance” to infections based on a suggested protective factor within the immune system of these patients. Because this study has been cited repeatedly in multiple articles about AN and cell-mediated immunity,2-7 some clinicians have accepted this evidence of resistance to infection in patients with AN, which may lower their suspicion for and detection of infections in patients with AN.
However, studies published both before and after Golla et al1 have shown statistically significant results that contradict those researchers’ conclusion. A study that compared the medical records of 68 patients with AN with those who did not have AN found no significant difference, and concluded that the rate of infection among patients with AN is the same as among controls.8 These researchers noted that infection rates may be higher among patients with later-stage, more severe AN. In a 1986 study of 12 patients with AN, Cason et al9 concluded that while cellular immunity function is abnormal in patients with AN, their results were not compatible with prior studies that suggested AN patients were more resistant to infection.1,2,8
More recently, researchers compared 1,592 patients with eating disorders with 6,368 matched controls; they reviewed prescriptions of antibacterial, antifungal, and antiviral medications as a measure of infection rates.10 Compared with controls, patients with binge eating disorder (BED), patients with bulimia nervosa (BN), and males with AN more often received prescriptions for antimicrobial medications. There was no statistically significant difference between controls and females with AN, which is consistent with other reports of no increased or decreased risk of infection among females with AN. In terms of antiviral use, this study showed an increased prescription of antivirals only in the BN group.
Several other studies examining the rate of infection in patients with AN concluded that there is neither an increased nor decreased rate of infection in patients with AN, and that the rate of infection in this population is similar to that of the general population.8,10-12 Because studies that have included patients with AN have evaluated only symptomatic viral infections, some researchers have proposed that patients with AN may show lower rates of symptomatic viral infection but higher rates of asymptomatic infection, as evidenced by higher viral titers.6 Further research is required. Despite controversy regarding infection rates, studies have found that patients with AN have increased rates of morbidity and mortality from infections.6,12-16
Continue to: Obstacles to detecting infections
Obstacles to detecting infections
Several factors can complicate the surveillance and detection of infections in patients with eating disorders, especially those with AN. These include:
- an accepted predisposition to infection secondary to malnutrition
- a lack of visual or reported infectious symptoms
- misrepresentation and assumptions from published research.
Clinicians who report fewer observed cases of infections among patients with AN may be overlooking comorbid disease processes due to a bias from the literature and/or a lack of awareness of symptom parameters in patients with AN.
Features of AN include a loss of adipose tissue responsible for pro-inflammatory cytokines, and excessive exercise, which stimulates anti-inflammatory myokines. This can modulate the experience of illness that impacts the core features of disease,17 possibly reducing symptomatic presentation of infections.
Fever. The presence and intensity of fever may be altered in patients with eating disorders, especially those with AN. In a study of 311 inpatients with AN, researchers found that patients with AN had a significant delay in fever response in AN.12 Of 23 patients with an active bacterial infection, all but 5 had a fever <37°C, with some as low as 35.5°C. A detectable fever response and unexplained fevers were found in 2 of the 6 patients with a viral infection. A series of case studies found that patients with AN with bacterial infections also had a delayed fever response.18
For patients with infections that commonly present with fever, such as COVID-19, a delayed fever response can delay or evade the detection of infection, thus increasing potential complications as well viral exposure to others. Thus, clinicians should use caution when ruling out COVID-19 or other infections because of a lack of significant fever.
Continue to: Overlapping symptoms
Overlapping symptoms. The symptoms of viral infection can mimic the symptoms of AN, which further complicates screening and diagnosis of infection in these patients. Although up to 80% of individuals infected with COVID-19 may be asymptomatic or have a mild presentation, the most common reported symptoms are fever (92.6%), shortness of breath (50.8%), expectoration (41.4%), fatigue (46.4%), dry cough (33.3%), and myalgia (21.4%).19-21 Gastrointestinal (GI) symptoms have been reported in patients with COVID-19, as well as a loss of taste and smell.
Commonly reported physical symptoms of AN include an intolerance to cold, general fatigue, muscle aches and pains, restlessness, emesis, and a multitude of GI complaints. Patients with AN also have been reported to experience shortness of breath due to conditions such as respiratory muscle weakness,22 nutritional emphysema,23 and anxiety and panic attack.24 These conditions could lead to an increased susceptibility to COVID-19 and increased complications during treatment. Cardiac abnormalities, which are common in patients with AN and BN, may increase the risk of adverse events. While these symptoms may be an important part of screening for diseases such as COVID-19, suspicion of infection also may be lower because of the overlap of AN symptomology, underlying conditions, and a delayed fever response.
Laboratory findings. Laboratory testing results for patients with COVID-19 include lower lymphocyte counts, higher leukocyte counts, elevated levels of infection-related biomarkers and inflammatory cytokines, and significantly decreased T-cell counts.19 Similar values are also found in patients with AN.
The similar clinical presentations and laboratory values of AN and COVID-19 could lead to delayed diagnosis, increased disease transmission, cross-contamination of facilities, and higher incidences of medical complications and mortality.
The immunology of AN and correlations with COVID-19
Many studies examining the immune system of patients with eating disorders, especially those with AN, have discovered changes and differences in both cell-mediated and humoral response to infections.1,3,5,7,9,11,16,21,25-27 Whether these differences represent a dysfunctional immune system, an immunocompromised state, or even a protective factor remains unclear.
Continue to: While some studies have reported...
While some studies have reported that AN represents an immunocompromised state, others describe the immune system of patients with AN as dysfunctional or simply altered.9,11,22,28 Some studies have found that patients with AN had delayed reactions to pathogen skin exposures compared with healthy controls, which provides evidence of an impaired cell-mediated immune system.9,27,29
Some studies have considered the consequences of infection and immunologic findings as markers of or contributing to the onset of AN.2,30,31 Numerous studies have noted abnormalities in AN with regards to cell-mediated immunity, the humoral system, the lymphoreticular system, and the innate immune system, and potential contributions from increased oxidative stress, a chronically activated sympathetic nervous system and hypothalamic-pituitary-adrenal axis, altered intestinal microbiota, and an abnormal bone marrow microenvironment.2
Box 1
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new beta-coronavirus that is still being studied for its effects on the immune system. It may take years to fully understand the nature of the pathogen and the response of the human immune system. To better understand COVID19, researchers have been turning to what they learned from the past outbreaks of severe acute respiratory syndrome (SARS) in 2003- 2004 and Middle East respiratory syndrome (MERS) in 2011, both caused by betacoronaviruses with a zoonotic origin.25,32
The proposed pathogenesis for infection of SARS-CoV-2 is similar to SARS and occurs when aerosolized droplets containing the virus enter the host.32 While currently there is only initial data on the host innate immune status of patients infected with SARS-CoV-2, initial findings of a report on 99 cases in Wuhan, China included increased total neutrophils (38%), reduced total lymphocytes (35%), increased serum interleukin-6 (52%), and increased C-reactive protein (84%).33 Additional findings were decreased percentages of monocytes, eosinophils, and basophils, as well as significantly decreased levels of cytokines and T-cells in more severe cases.19 Past research with SARS reported similar T-cell findings, with a more frequent CD8+ response and a greater magnitude of CD4+.34
Box 119,25,32-34 describes some of the initial immunologic findings reported in patients with COVID-19. In Box 2,5,8,11,13,14,19,26,28,35-40 we discuss reports that describe the immunologic overlay of COVID-19 and AN.
Box 2
Leukopenia (low leukocyte levels) is a common finding in patients with anorexia nervosa (AN),8 and often leads clinicians to lower their suspicion for infection. A 2008 Hungarian study that evaluated lymphocyte activation parameters and clinical status in 11 adolescents (10 girls and 1 boy) with AN, 12 obese adolescents, and 10 healthy controls did not find any association between the variables.35 While many studies have focused on adults, it is important to note that leukopenia is a common finding in adolescents (age 12 to 17) with AN.36
Leukocyte counts are elevated in coronavirus disease 2019 (COVID-19), possibly offsetting AN’s leukopenia. In addition, neutrophil counts are elevated and monocyte, eosinophil, basophil, and especially lymphocyte counts are significantly decreased. A meta-analysis that included 22 studies and 924 participants (512 with AN and 412 controls) examined common inflammatory cytokine findings in patients with AN.11 Compared with healthy controls, patients with AN had significantly elevated levels of tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-1, IL-6, and TNF-receptor II, and significantly decreased levels of C-reactive protein and IL-6 receptor. Elevated levels of TNF-alpha and IL-6 also have been reported in patients with COVID-19.19 These findings may mask suspicion for infection in patients with AN.19
In patients with AN and those with bulimia nervosa, CD4+-to-CD8+ ratios also have been found to be low as a result of normal-tohigher levels of CD4+ cells and lower levels of CD8+ cells.36-39 Researchers have also proposed that the lymphocytosis observed in AN is a result of increased naïve CD4+.36 In AN, total lymphocyte counts have been found to correlate positively with a patient’s body mass index (BMI), while the CD4+ T-lymphocyte correlated negatively with BMI and were critically low in patients with severe malnutrition.26,40 In patients with COVID-19, CD4+ levels have reported to be within normal range, naïve CD4+ cells were elevated, and CD8+ cells were slightly decreased,19 which is similar to the findings in AN.
Fewer studies have evaluated humoral immune response in AN, and results have varied. One study (N = 46) found elevated B-cell counts in adolescents with AN-restricting type,36 while another (N = 40) reported normal levels of B-cells.5 Specific decreases in immunoglobulin (Ig) G and IgM have also been reported in AN, while IgA, IgG, and IgM usually are normal in COVID-19.19
Despite differences in immune system function, cellular immunity appears to remain relatively intact in patients with AN, but can become compromised with severe malnutrition or with advanced weight loss.28,40 This compromised immunity related to severe AN with a very low BMI likely leads to the increased morbidity and mortality.8,13,14
Malnutrition and the immune system
Differences in the type of malnutrition observed in low-weight patients with AN may help explain why patients with AN can maintain a relatively intact cell-mediated immune system.1 Protein-energy malnutrition (PEM), which is found in typical states of starvation, consists of deficiencies in multiple vitamins, protein, and energy (caloric content), whereas the dietary habits of patients with AN usually result in a deficiency of carbohydrates and fats.41 Studies that examined the impact of PEM on immunity to influenza infection have suggested that balanced protein energy replenishment may be a strategy for boosting immunity against influenza viral infections.42 However, carbohydrates are the primary nutrients for human bone marrow fat cells, which play a crucial role in the maturation of white blood cells. This may account for the leukopenia that is common in patients with AN.6,43 The protein-sparing aspect of the typical AN diet may account for the immune system changes observed in patients with AN.44
Although some studies have proposed that immune deficiencies observed in patients with AN are secondary to malnutrition and return to normal with refeeding,5,40,45 others have concluded that immune function is not compromised by factors such as nutritional status or body weight in AN.26,43,46
Continue to: Clinical considerations
Clinical considerations
Neither the CDC nor the WHO have issued a specific protocol for monitoring for and treating COVID-19 in patients with eating disorders; however, the guidelines offered by these organizations for the general population should be followed for patients with eating disorders.
When screening a patient with an eating disorder, keep in mind that the symptoms of eating disorders, such as AN, may mimic an infectious process. Mood symptoms, such as depression or anxiety, could represent physiological responses to infection. Patients with GI symptoms that typically are considered part of the pathology of an eating disorder should be more carefully considered for COVID-19. Monitor a patient’s basal body temperature, and be mindful that a patient with AN may exhibit a delayed fever response. Be vigilant for a recent loss of taste or smell, which should raise suspicion for COVID-19. When monitoring vital signs, pay careful attention for any decompensation in a patient’s pulse oximetry. Whenever possible, order COVID-19 testing for any patient you suspect may be infected.
Outpatient clinicians should work closely in a collaborative manner with a patient’s eating disorder treatment team. Psychiatrists, primary care physicians, psychotherapists, nutritionists, and other clinicians should all follow CDC/WHO guidelines regarding COVID-19, provide surveillance, and communicate any suspicions to the medical team. Eating disorder treatment programs, including residential centers, partial hospital programs (PHP), and intensive outpatient programs (IOP), must enhance monitoring for COVID-19, and exercise caution by practicing social distancing and providing adequate personal protective equipment for patients and staff. To reduce the spread of COVID-19, many IOPs and PHPs have transitioned to virtual treatment. Residential centers must carefully screen patients before admission to weigh the risks and benefits of inpatient vs outpatient care.
Bottom Line
Differences in the immune system of patients with an eating disorder do not necessarily confer a higher or lower risk of infection. Symptoms of some infections can mimic the symptoms of anorexia nervosa. Recognizing infections in patients with eating disorders is critical because compared with the general population, they have higher rates of infection-related morbidity and mortality.
Related Resources
- Congress J, Madaan V. 6 ‘M’s to keep in mind when you next see a patient with anorexia nervosa. Current Psychiatry. 2014;13(5):58-59.
- Westmoreland P. Eating disorders: Masterclass lecture part I. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/eating-disorders-masterclass-lecture-part-i.
1. Golla JA, Larson LA, Anderson CF, et al. An immunological assessment of patients with anorexia nervosa. Am J Clin Nutr. 1981;34(12):2756-2762.
2. Gibson D, Mehler PS. Anorexia nervosa and the immune system—a narrative review. J Clin Med. 2019;8(11):1915. doi: 10.3390/jcm8111915.
3. Słotwin
4. Nova E, Samartín S, Gómez S, et al. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur J Clin Nutr. 2002;56(suppl 3):S34-S37.
5. Allende LM, Corell A, Manzanares J, et al. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94(4):543-551.
6. Brown RF, Bartrop R, Birmingham CL. Immunological disturbance and infectious disease in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20(3):117-128.
7. Polack E, Nahmod VE, Emeric-Sauval E, et al. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J Clin Immunol. 1993;13(6):445-451.
8. Bowers TK, Eckert E. Leukopenia in anorexia nervosa. Lack of increased risk of infection. Arch Intern Med. 1978;138(10):1520-1523.
9. Cason J, Ainley CC, Wolstencroft RA, et al. Cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1986;64(2):370-375.
10. Raevuori A, Lukkariniemi L, Suokas JT, et al. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int J Eat Disord. 2016;49(6):542-552.
11. Solmi M, Veronese N, Favaro A, et al. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237-252.
12. Brown RF, Bartrop R, Beumont P, et al. Bacterial infections in anorexia nervosa: delayed recognition increases complications. Int J Eat Disord. 2005;37(3):261-265.
13. Theander S. Anorexia nervosa. A psychiatric investigation of 94 female patients. Acta Psychiatr Scand Suppl. 1970;214:1-194.
14. Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973;117(3):435-449.
15. Copeland PM, Herzog DB. Hypoglycemia and death in anorexia nervosa. Psychother Psychosom. 1987;48(1-4):146-150.
16. Devuyst O, Lambert M, Rodhain J, et al. Haematological changes and infectious complications in anorexia nervosa: a case-control study. Q J Med. 1993;86(12):791-799.
17. Pisetsky DS, Trace SE, Brownley KA, et al. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: an exploratory study. Cytokine. 2014;69(1):110-115.
18. Birmingham CL, Hodgson DM, Fung J, et al. Reduced febrile response to bacterial infection in anorexia nervosa patients. Int J Eat Disord. 2003;34(2):269-272.
19. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online March 12, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa248.
20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
21. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
22. Birmingham CL, Tan AO. Respiratory muscle weakness and anorexia nervosa. Int J Eat Disord. 2003;33(2):230-233.
23. Cook VJ, Coxson HO, Mason AG, et al. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can Respir J. 2001;8(5):361-365.
24. Khalsa SS, Hassanpour MS, Strober M, et al. Interoceptive anxiety and body representation in anorexia nervosa [published online September 21, 2018]. Front Psychiatry. 2018;9:444. doi: 10.3389/fpsyt.2018.00444.
25. van West D, Maes M. Cytokines in de obsessief compulsieve stoornis en in anorexia nervosa: een overzicht. Acta Neuropsychiatr. 1999;11(4):125-129.
26. Komorowska-Pietrzykowska R, Rajewski A, Wiktorowicz K, et al. Czynnos
27. Marcos A, Varela P, Toro O, et al. Interactions between nutrition and immunity in anorexia nervosa: a 1-y follow-up study. Am J Clin Nutr. 1997;66(2):485S-490S.
28. Pertschuk MJ, Crosby LO, Barot L, et al. Immunocompetency in anorexia nervosa. Am J Clin Nutr. 1982;35(5):968-972.
29. Varela P, Marcos A, Navarro MP. Zinc status in anorexia nervosa. Ann Nutr Metab. 1992;36(4):197-202.
30. Breithaupt L, Köhler-Forsberg O, Larsen JT, et al. Association of exposure to infections in childhood with risk of eating disorders in adolescent girls. JAMA Psychiatry. 2019;76(8):800-809.
31. Brambilla F, Monti D, Franceschi C. Plasma concentrations of interleukin-1-beta, interleukin-6 and tumor necrosis factor-alpha, and of their soluble receptors and receptor antagonist in anorexia nervosa. Psychiatry Res. 2001;103(2-3):107-114.
32. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic [published online February 27, 2020]. Asian Pac J Allergy Immunol. doi: 10.12932/AP-200220-0772.
33. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
34. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490-5500.
35. Páli AA, Pászthy B. Az immunrendszer muködésének megváltozása a táplálkozási magatartás zavarai esetén [Changes of the immune functions in patients with eating disorders]. Ideggyogy Sz. 2008;61(11-12):381‐384.
36. Elegido A, Graell M, Andrés P, et al. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr Res. 2017;39:43-50.
37. Marcos A, Varela P, Santacruz I, et al. Nutritional status and immunocompetence in eating disorders. A comparative study. Eur J Clin Nutr. 1993;47(11):787-793.
38. Mustafa A, Ward A, Treasure J, et al. T lymphocyte subpopulations in anorexia nervosa and refeeding. Clin Immunol Immunopathol. 1997;82(3):282-289.
39. Nagata T, Kiriike N, Tobitani W, et al. Lymphocyte subset, lymphocyte proliferative response, and soluble interleukin-2 receptor in anorexic patients. Biol Psychiatry. 1999;45(4):471-474.
40. Saito H, Nomura K, Hotta M, et al. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. 2007;40(6):575-579.
41. Nova E, Varela P, López-Vidriero I, et al. A one-year follow-up study in anorexia nervosa. Dietary pattern and anthropometrical evolution. Eur J Clin Nutr. 2001;55(7):547-554.
42. Taylor AK, Cao W, Vora KP, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis. 2013;207(3):501-510.
43. Mant MJ, Faragher BS. The hematology of anorexia nervosa. Br J Haematol. 1972;23(6):737-749.
44. Marcos A. The immune system in eating disorders: an overview. Nutrition. 1997;13(10):853-862.
45. Schattner A, Tepper R, Steinbock M, et al. TNF, interferon-gamma and cell-mediated cytotoxicity in anorexia nervosa; effect of refeeding. J Clin Lab Immunol. 1990;32(4):183-184.
46. Nagata T, Tobitani W, Kiriike N, et al. Capacity to produce cytokines during weight restoration in patients with anorexia nervosa. Psychosom Med. 1999;61(3):371-377.
Recent concerns surrounding coronavirus disease 2019 (COVID-19) make it timely to reexamine the complex findings related to eating disorders and the immune system, and the risks for and detection of infection in patients with anorexia nervosa (AN) and similar disorders. To date, there are no published studies evaluating patients with eating disorders and COVID-19. However, it may be helpful to review the data on the infectious process in this patient population to improve patient communication, enhance surveillance and detection, and possibly reduce morbidity and mortality.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) issued warnings that individuals who are older, have underlying medical conditions, and/or are immunocompromised face the greatest risk of serious complications and death as a result of COVID-19, the disease process caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to malnutrition, patients with eating disorders, especially AN, may be perceived to have an increased risk of medical conditions and infection. Despite many studies on specific changes and differences in the immune system of patients with eating disorders, the consequences of these changes remain controversial and inconclusive.
This article reviews research on eating disorders, focusing on published data regarding the effects of AN on the immune system, susceptibility to infections, infectious detection, and morbidity. We also discuss clinical considerations related to COVID-19 and patients with AN.
Infection risks: Conflicting data
In a 1981 study that included 9 participants, Golla et al1 concluded that patients with AN may have “resistance” to infections based on a suggested protective factor within the immune system of these patients. Because this study has been cited repeatedly in multiple articles about AN and cell-mediated immunity,2-7 some clinicians have accepted this evidence of resistance to infection in patients with AN, which may lower their suspicion for and detection of infections in patients with AN.
However, studies published both before and after Golla et al1 have shown statistically significant results that contradict those researchers’ conclusion. A study that compared the medical records of 68 patients with AN with those who did not have AN found no significant difference, and concluded that the rate of infection among patients with AN is the same as among controls.8 These researchers noted that infection rates may be higher among patients with later-stage, more severe AN. In a 1986 study of 12 patients with AN, Cason et al9 concluded that while cellular immunity function is abnormal in patients with AN, their results were not compatible with prior studies that suggested AN patients were more resistant to infection.1,2,8
More recently, researchers compared 1,592 patients with eating disorders with 6,368 matched controls; they reviewed prescriptions of antibacterial, antifungal, and antiviral medications as a measure of infection rates.10 Compared with controls, patients with binge eating disorder (BED), patients with bulimia nervosa (BN), and males with AN more often received prescriptions for antimicrobial medications. There was no statistically significant difference between controls and females with AN, which is consistent with other reports of no increased or decreased risk of infection among females with AN. In terms of antiviral use, this study showed an increased prescription of antivirals only in the BN group.
Several other studies examining the rate of infection in patients with AN concluded that there is neither an increased nor decreased rate of infection in patients with AN, and that the rate of infection in this population is similar to that of the general population.8,10-12 Because studies that have included patients with AN have evaluated only symptomatic viral infections, some researchers have proposed that patients with AN may show lower rates of symptomatic viral infection but higher rates of asymptomatic infection, as evidenced by higher viral titers.6 Further research is required. Despite controversy regarding infection rates, studies have found that patients with AN have increased rates of morbidity and mortality from infections.6,12-16
Continue to: Obstacles to detecting infections
Obstacles to detecting infections
Several factors can complicate the surveillance and detection of infections in patients with eating disorders, especially those with AN. These include:
- an accepted predisposition to infection secondary to malnutrition
- a lack of visual or reported infectious symptoms
- misrepresentation and assumptions from published research.
Clinicians who report fewer observed cases of infections among patients with AN may be overlooking comorbid disease processes due to a bias from the literature and/or a lack of awareness of symptom parameters in patients with AN.
Features of AN include a loss of adipose tissue responsible for pro-inflammatory cytokines, and excessive exercise, which stimulates anti-inflammatory myokines. This can modulate the experience of illness that impacts the core features of disease,17 possibly reducing symptomatic presentation of infections.
Fever. The presence and intensity of fever may be altered in patients with eating disorders, especially those with AN. In a study of 311 inpatients with AN, researchers found that patients with AN had a significant delay in fever response in AN.12 Of 23 patients with an active bacterial infection, all but 5 had a fever <37°C, with some as low as 35.5°C. A detectable fever response and unexplained fevers were found in 2 of the 6 patients with a viral infection. A series of case studies found that patients with AN with bacterial infections also had a delayed fever response.18
For patients with infections that commonly present with fever, such as COVID-19, a delayed fever response can delay or evade the detection of infection, thus increasing potential complications as well viral exposure to others. Thus, clinicians should use caution when ruling out COVID-19 or other infections because of a lack of significant fever.
Continue to: Overlapping symptoms
Overlapping symptoms. The symptoms of viral infection can mimic the symptoms of AN, which further complicates screening and diagnosis of infection in these patients. Although up to 80% of individuals infected with COVID-19 may be asymptomatic or have a mild presentation, the most common reported symptoms are fever (92.6%), shortness of breath (50.8%), expectoration (41.4%), fatigue (46.4%), dry cough (33.3%), and myalgia (21.4%).19-21 Gastrointestinal (GI) symptoms have been reported in patients with COVID-19, as well as a loss of taste and smell.
Commonly reported physical symptoms of AN include an intolerance to cold, general fatigue, muscle aches and pains, restlessness, emesis, and a multitude of GI complaints. Patients with AN also have been reported to experience shortness of breath due to conditions such as respiratory muscle weakness,22 nutritional emphysema,23 and anxiety and panic attack.24 These conditions could lead to an increased susceptibility to COVID-19 and increased complications during treatment. Cardiac abnormalities, which are common in patients with AN and BN, may increase the risk of adverse events. While these symptoms may be an important part of screening for diseases such as COVID-19, suspicion of infection also may be lower because of the overlap of AN symptomology, underlying conditions, and a delayed fever response.
Laboratory findings. Laboratory testing results for patients with COVID-19 include lower lymphocyte counts, higher leukocyte counts, elevated levels of infection-related biomarkers and inflammatory cytokines, and significantly decreased T-cell counts.19 Similar values are also found in patients with AN.
The similar clinical presentations and laboratory values of AN and COVID-19 could lead to delayed diagnosis, increased disease transmission, cross-contamination of facilities, and higher incidences of medical complications and mortality.
The immunology of AN and correlations with COVID-19
Many studies examining the immune system of patients with eating disorders, especially those with AN, have discovered changes and differences in both cell-mediated and humoral response to infections.1,3,5,7,9,11,16,21,25-27 Whether these differences represent a dysfunctional immune system, an immunocompromised state, or even a protective factor remains unclear.
Continue to: While some studies have reported...
While some studies have reported that AN represents an immunocompromised state, others describe the immune system of patients with AN as dysfunctional or simply altered.9,11,22,28 Some studies have found that patients with AN had delayed reactions to pathogen skin exposures compared with healthy controls, which provides evidence of an impaired cell-mediated immune system.9,27,29
Some studies have considered the consequences of infection and immunologic findings as markers of or contributing to the onset of AN.2,30,31 Numerous studies have noted abnormalities in AN with regards to cell-mediated immunity, the humoral system, the lymphoreticular system, and the innate immune system, and potential contributions from increased oxidative stress, a chronically activated sympathetic nervous system and hypothalamic-pituitary-adrenal axis, altered intestinal microbiota, and an abnormal bone marrow microenvironment.2
Box 1
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new beta-coronavirus that is still being studied for its effects on the immune system. It may take years to fully understand the nature of the pathogen and the response of the human immune system. To better understand COVID19, researchers have been turning to what they learned from the past outbreaks of severe acute respiratory syndrome (SARS) in 2003- 2004 and Middle East respiratory syndrome (MERS) in 2011, both caused by betacoronaviruses with a zoonotic origin.25,32
The proposed pathogenesis for infection of SARS-CoV-2 is similar to SARS and occurs when aerosolized droplets containing the virus enter the host.32 While currently there is only initial data on the host innate immune status of patients infected with SARS-CoV-2, initial findings of a report on 99 cases in Wuhan, China included increased total neutrophils (38%), reduced total lymphocytes (35%), increased serum interleukin-6 (52%), and increased C-reactive protein (84%).33 Additional findings were decreased percentages of monocytes, eosinophils, and basophils, as well as significantly decreased levels of cytokines and T-cells in more severe cases.19 Past research with SARS reported similar T-cell findings, with a more frequent CD8+ response and a greater magnitude of CD4+.34
Box 119,25,32-34 describes some of the initial immunologic findings reported in patients with COVID-19. In Box 2,5,8,11,13,14,19,26,28,35-40 we discuss reports that describe the immunologic overlay of COVID-19 and AN.
Box 2
Leukopenia (low leukocyte levels) is a common finding in patients with anorexia nervosa (AN),8 and often leads clinicians to lower their suspicion for infection. A 2008 Hungarian study that evaluated lymphocyte activation parameters and clinical status in 11 adolescents (10 girls and 1 boy) with AN, 12 obese adolescents, and 10 healthy controls did not find any association between the variables.35 While many studies have focused on adults, it is important to note that leukopenia is a common finding in adolescents (age 12 to 17) with AN.36
Leukocyte counts are elevated in coronavirus disease 2019 (COVID-19), possibly offsetting AN’s leukopenia. In addition, neutrophil counts are elevated and monocyte, eosinophil, basophil, and especially lymphocyte counts are significantly decreased. A meta-analysis that included 22 studies and 924 participants (512 with AN and 412 controls) examined common inflammatory cytokine findings in patients with AN.11 Compared with healthy controls, patients with AN had significantly elevated levels of tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-1, IL-6, and TNF-receptor II, and significantly decreased levels of C-reactive protein and IL-6 receptor. Elevated levels of TNF-alpha and IL-6 also have been reported in patients with COVID-19.19 These findings may mask suspicion for infection in patients with AN.19
In patients with AN and those with bulimia nervosa, CD4+-to-CD8+ ratios also have been found to be low as a result of normal-tohigher levels of CD4+ cells and lower levels of CD8+ cells.36-39 Researchers have also proposed that the lymphocytosis observed in AN is a result of increased naïve CD4+.36 In AN, total lymphocyte counts have been found to correlate positively with a patient’s body mass index (BMI), while the CD4+ T-lymphocyte correlated negatively with BMI and were critically low in patients with severe malnutrition.26,40 In patients with COVID-19, CD4+ levels have reported to be within normal range, naïve CD4+ cells were elevated, and CD8+ cells were slightly decreased,19 which is similar to the findings in AN.
Fewer studies have evaluated humoral immune response in AN, and results have varied. One study (N = 46) found elevated B-cell counts in adolescents with AN-restricting type,36 while another (N = 40) reported normal levels of B-cells.5 Specific decreases in immunoglobulin (Ig) G and IgM have also been reported in AN, while IgA, IgG, and IgM usually are normal in COVID-19.19
Despite differences in immune system function, cellular immunity appears to remain relatively intact in patients with AN, but can become compromised with severe malnutrition or with advanced weight loss.28,40 This compromised immunity related to severe AN with a very low BMI likely leads to the increased morbidity and mortality.8,13,14
Malnutrition and the immune system
Differences in the type of malnutrition observed in low-weight patients with AN may help explain why patients with AN can maintain a relatively intact cell-mediated immune system.1 Protein-energy malnutrition (PEM), which is found in typical states of starvation, consists of deficiencies in multiple vitamins, protein, and energy (caloric content), whereas the dietary habits of patients with AN usually result in a deficiency of carbohydrates and fats.41 Studies that examined the impact of PEM on immunity to influenza infection have suggested that balanced protein energy replenishment may be a strategy for boosting immunity against influenza viral infections.42 However, carbohydrates are the primary nutrients for human bone marrow fat cells, which play a crucial role in the maturation of white blood cells. This may account for the leukopenia that is common in patients with AN.6,43 The protein-sparing aspect of the typical AN diet may account for the immune system changes observed in patients with AN.44
Although some studies have proposed that immune deficiencies observed in patients with AN are secondary to malnutrition and return to normal with refeeding,5,40,45 others have concluded that immune function is not compromised by factors such as nutritional status or body weight in AN.26,43,46
Continue to: Clinical considerations
Clinical considerations
Neither the CDC nor the WHO have issued a specific protocol for monitoring for and treating COVID-19 in patients with eating disorders; however, the guidelines offered by these organizations for the general population should be followed for patients with eating disorders.
When screening a patient with an eating disorder, keep in mind that the symptoms of eating disorders, such as AN, may mimic an infectious process. Mood symptoms, such as depression or anxiety, could represent physiological responses to infection. Patients with GI symptoms that typically are considered part of the pathology of an eating disorder should be more carefully considered for COVID-19. Monitor a patient’s basal body temperature, and be mindful that a patient with AN may exhibit a delayed fever response. Be vigilant for a recent loss of taste or smell, which should raise suspicion for COVID-19. When monitoring vital signs, pay careful attention for any decompensation in a patient’s pulse oximetry. Whenever possible, order COVID-19 testing for any patient you suspect may be infected.
Outpatient clinicians should work closely in a collaborative manner with a patient’s eating disorder treatment team. Psychiatrists, primary care physicians, psychotherapists, nutritionists, and other clinicians should all follow CDC/WHO guidelines regarding COVID-19, provide surveillance, and communicate any suspicions to the medical team. Eating disorder treatment programs, including residential centers, partial hospital programs (PHP), and intensive outpatient programs (IOP), must enhance monitoring for COVID-19, and exercise caution by practicing social distancing and providing adequate personal protective equipment for patients and staff. To reduce the spread of COVID-19, many IOPs and PHPs have transitioned to virtual treatment. Residential centers must carefully screen patients before admission to weigh the risks and benefits of inpatient vs outpatient care.
Bottom Line
Differences in the immune system of patients with an eating disorder do not necessarily confer a higher or lower risk of infection. Symptoms of some infections can mimic the symptoms of anorexia nervosa. Recognizing infections in patients with eating disorders is critical because compared with the general population, they have higher rates of infection-related morbidity and mortality.
Related Resources
- Congress J, Madaan V. 6 ‘M’s to keep in mind when you next see a patient with anorexia nervosa. Current Psychiatry. 2014;13(5):58-59.
- Westmoreland P. Eating disorders: Masterclass lecture part I. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/eating-disorders-masterclass-lecture-part-i.
Recent concerns surrounding coronavirus disease 2019 (COVID-19) make it timely to reexamine the complex findings related to eating disorders and the immune system, and the risks for and detection of infection in patients with anorexia nervosa (AN) and similar disorders. To date, there are no published studies evaluating patients with eating disorders and COVID-19. However, it may be helpful to review the data on the infectious process in this patient population to improve patient communication, enhance surveillance and detection, and possibly reduce morbidity and mortality.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) issued warnings that individuals who are older, have underlying medical conditions, and/or are immunocompromised face the greatest risk of serious complications and death as a result of COVID-19, the disease process caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Due to malnutrition, patients with eating disorders, especially AN, may be perceived to have an increased risk of medical conditions and infection. Despite many studies on specific changes and differences in the immune system of patients with eating disorders, the consequences of these changes remain controversial and inconclusive.
This article reviews research on eating disorders, focusing on published data regarding the effects of AN on the immune system, susceptibility to infections, infectious detection, and morbidity. We also discuss clinical considerations related to COVID-19 and patients with AN.
Infection risks: Conflicting data
In a 1981 study that included 9 participants, Golla et al1 concluded that patients with AN may have “resistance” to infections based on a suggested protective factor within the immune system of these patients. Because this study has been cited repeatedly in multiple articles about AN and cell-mediated immunity,2-7 some clinicians have accepted this evidence of resistance to infection in patients with AN, which may lower their suspicion for and detection of infections in patients with AN.
However, studies published both before and after Golla et al1 have shown statistically significant results that contradict those researchers’ conclusion. A study that compared the medical records of 68 patients with AN with those who did not have AN found no significant difference, and concluded that the rate of infection among patients with AN is the same as among controls.8 These researchers noted that infection rates may be higher among patients with later-stage, more severe AN. In a 1986 study of 12 patients with AN, Cason et al9 concluded that while cellular immunity function is abnormal in patients with AN, their results were not compatible with prior studies that suggested AN patients were more resistant to infection.1,2,8
More recently, researchers compared 1,592 patients with eating disorders with 6,368 matched controls; they reviewed prescriptions of antibacterial, antifungal, and antiviral medications as a measure of infection rates.10 Compared with controls, patients with binge eating disorder (BED), patients with bulimia nervosa (BN), and males with AN more often received prescriptions for antimicrobial medications. There was no statistically significant difference between controls and females with AN, which is consistent with other reports of no increased or decreased risk of infection among females with AN. In terms of antiviral use, this study showed an increased prescription of antivirals only in the BN group.
Several other studies examining the rate of infection in patients with AN concluded that there is neither an increased nor decreased rate of infection in patients with AN, and that the rate of infection in this population is similar to that of the general population.8,10-12 Because studies that have included patients with AN have evaluated only symptomatic viral infections, some researchers have proposed that patients with AN may show lower rates of symptomatic viral infection but higher rates of asymptomatic infection, as evidenced by higher viral titers.6 Further research is required. Despite controversy regarding infection rates, studies have found that patients with AN have increased rates of morbidity and mortality from infections.6,12-16
Continue to: Obstacles to detecting infections
Obstacles to detecting infections
Several factors can complicate the surveillance and detection of infections in patients with eating disorders, especially those with AN. These include:
- an accepted predisposition to infection secondary to malnutrition
- a lack of visual or reported infectious symptoms
- misrepresentation and assumptions from published research.
Clinicians who report fewer observed cases of infections among patients with AN may be overlooking comorbid disease processes due to a bias from the literature and/or a lack of awareness of symptom parameters in patients with AN.
Features of AN include a loss of adipose tissue responsible for pro-inflammatory cytokines, and excessive exercise, which stimulates anti-inflammatory myokines. This can modulate the experience of illness that impacts the core features of disease,17 possibly reducing symptomatic presentation of infections.
Fever. The presence and intensity of fever may be altered in patients with eating disorders, especially those with AN. In a study of 311 inpatients with AN, researchers found that patients with AN had a significant delay in fever response in AN.12 Of 23 patients with an active bacterial infection, all but 5 had a fever <37°C, with some as low as 35.5°C. A detectable fever response and unexplained fevers were found in 2 of the 6 patients with a viral infection. A series of case studies found that patients with AN with bacterial infections also had a delayed fever response.18
For patients with infections that commonly present with fever, such as COVID-19, a delayed fever response can delay or evade the detection of infection, thus increasing potential complications as well viral exposure to others. Thus, clinicians should use caution when ruling out COVID-19 or other infections because of a lack of significant fever.
Continue to: Overlapping symptoms
Overlapping symptoms. The symptoms of viral infection can mimic the symptoms of AN, which further complicates screening and diagnosis of infection in these patients. Although up to 80% of individuals infected with COVID-19 may be asymptomatic or have a mild presentation, the most common reported symptoms are fever (92.6%), shortness of breath (50.8%), expectoration (41.4%), fatigue (46.4%), dry cough (33.3%), and myalgia (21.4%).19-21 Gastrointestinal (GI) symptoms have been reported in patients with COVID-19, as well as a loss of taste and smell.
Commonly reported physical symptoms of AN include an intolerance to cold, general fatigue, muscle aches and pains, restlessness, emesis, and a multitude of GI complaints. Patients with AN also have been reported to experience shortness of breath due to conditions such as respiratory muscle weakness,22 nutritional emphysema,23 and anxiety and panic attack.24 These conditions could lead to an increased susceptibility to COVID-19 and increased complications during treatment. Cardiac abnormalities, which are common in patients with AN and BN, may increase the risk of adverse events. While these symptoms may be an important part of screening for diseases such as COVID-19, suspicion of infection also may be lower because of the overlap of AN symptomology, underlying conditions, and a delayed fever response.
Laboratory findings. Laboratory testing results for patients with COVID-19 include lower lymphocyte counts, higher leukocyte counts, elevated levels of infection-related biomarkers and inflammatory cytokines, and significantly decreased T-cell counts.19 Similar values are also found in patients with AN.
The similar clinical presentations and laboratory values of AN and COVID-19 could lead to delayed diagnosis, increased disease transmission, cross-contamination of facilities, and higher incidences of medical complications and mortality.
The immunology of AN and correlations with COVID-19
Many studies examining the immune system of patients with eating disorders, especially those with AN, have discovered changes and differences in both cell-mediated and humoral response to infections.1,3,5,7,9,11,16,21,25-27 Whether these differences represent a dysfunctional immune system, an immunocompromised state, or even a protective factor remains unclear.
Continue to: While some studies have reported...
While some studies have reported that AN represents an immunocompromised state, others describe the immune system of patients with AN as dysfunctional or simply altered.9,11,22,28 Some studies have found that patients with AN had delayed reactions to pathogen skin exposures compared with healthy controls, which provides evidence of an impaired cell-mediated immune system.9,27,29
Some studies have considered the consequences of infection and immunologic findings as markers of or contributing to the onset of AN.2,30,31 Numerous studies have noted abnormalities in AN with regards to cell-mediated immunity, the humoral system, the lymphoreticular system, and the innate immune system, and potential contributions from increased oxidative stress, a chronically activated sympathetic nervous system and hypothalamic-pituitary-adrenal axis, altered intestinal microbiota, and an abnormal bone marrow microenvironment.2
Box 1
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new beta-coronavirus that is still being studied for its effects on the immune system. It may take years to fully understand the nature of the pathogen and the response of the human immune system. To better understand COVID19, researchers have been turning to what they learned from the past outbreaks of severe acute respiratory syndrome (SARS) in 2003- 2004 and Middle East respiratory syndrome (MERS) in 2011, both caused by betacoronaviruses with a zoonotic origin.25,32
The proposed pathogenesis for infection of SARS-CoV-2 is similar to SARS and occurs when aerosolized droplets containing the virus enter the host.32 While currently there is only initial data on the host innate immune status of patients infected with SARS-CoV-2, initial findings of a report on 99 cases in Wuhan, China included increased total neutrophils (38%), reduced total lymphocytes (35%), increased serum interleukin-6 (52%), and increased C-reactive protein (84%).33 Additional findings were decreased percentages of monocytes, eosinophils, and basophils, as well as significantly decreased levels of cytokines and T-cells in more severe cases.19 Past research with SARS reported similar T-cell findings, with a more frequent CD8+ response and a greater magnitude of CD4+.34
Box 119,25,32-34 describes some of the initial immunologic findings reported in patients with COVID-19. In Box 2,5,8,11,13,14,19,26,28,35-40 we discuss reports that describe the immunologic overlay of COVID-19 and AN.
Box 2
Leukopenia (low leukocyte levels) is a common finding in patients with anorexia nervosa (AN),8 and often leads clinicians to lower their suspicion for infection. A 2008 Hungarian study that evaluated lymphocyte activation parameters and clinical status in 11 adolescents (10 girls and 1 boy) with AN, 12 obese adolescents, and 10 healthy controls did not find any association between the variables.35 While many studies have focused on adults, it is important to note that leukopenia is a common finding in adolescents (age 12 to 17) with AN.36
Leukocyte counts are elevated in coronavirus disease 2019 (COVID-19), possibly offsetting AN’s leukopenia. In addition, neutrophil counts are elevated and monocyte, eosinophil, basophil, and especially lymphocyte counts are significantly decreased. A meta-analysis that included 22 studies and 924 participants (512 with AN and 412 controls) examined common inflammatory cytokine findings in patients with AN.11 Compared with healthy controls, patients with AN had significantly elevated levels of tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-1, IL-6, and TNF-receptor II, and significantly decreased levels of C-reactive protein and IL-6 receptor. Elevated levels of TNF-alpha and IL-6 also have been reported in patients with COVID-19.19 These findings may mask suspicion for infection in patients with AN.19
In patients with AN and those with bulimia nervosa, CD4+-to-CD8+ ratios also have been found to be low as a result of normal-tohigher levels of CD4+ cells and lower levels of CD8+ cells.36-39 Researchers have also proposed that the lymphocytosis observed in AN is a result of increased naïve CD4+.36 In AN, total lymphocyte counts have been found to correlate positively with a patient’s body mass index (BMI), while the CD4+ T-lymphocyte correlated negatively with BMI and were critically low in patients with severe malnutrition.26,40 In patients with COVID-19, CD4+ levels have reported to be within normal range, naïve CD4+ cells were elevated, and CD8+ cells were slightly decreased,19 which is similar to the findings in AN.
Fewer studies have evaluated humoral immune response in AN, and results have varied. One study (N = 46) found elevated B-cell counts in adolescents with AN-restricting type,36 while another (N = 40) reported normal levels of B-cells.5 Specific decreases in immunoglobulin (Ig) G and IgM have also been reported in AN, while IgA, IgG, and IgM usually are normal in COVID-19.19
Despite differences in immune system function, cellular immunity appears to remain relatively intact in patients with AN, but can become compromised with severe malnutrition or with advanced weight loss.28,40 This compromised immunity related to severe AN with a very low BMI likely leads to the increased morbidity and mortality.8,13,14
Malnutrition and the immune system
Differences in the type of malnutrition observed in low-weight patients with AN may help explain why patients with AN can maintain a relatively intact cell-mediated immune system.1 Protein-energy malnutrition (PEM), which is found in typical states of starvation, consists of deficiencies in multiple vitamins, protein, and energy (caloric content), whereas the dietary habits of patients with AN usually result in a deficiency of carbohydrates and fats.41 Studies that examined the impact of PEM on immunity to influenza infection have suggested that balanced protein energy replenishment may be a strategy for boosting immunity against influenza viral infections.42 However, carbohydrates are the primary nutrients for human bone marrow fat cells, which play a crucial role in the maturation of white blood cells. This may account for the leukopenia that is common in patients with AN.6,43 The protein-sparing aspect of the typical AN diet may account for the immune system changes observed in patients with AN.44
Although some studies have proposed that immune deficiencies observed in patients with AN are secondary to malnutrition and return to normal with refeeding,5,40,45 others have concluded that immune function is not compromised by factors such as nutritional status or body weight in AN.26,43,46
Continue to: Clinical considerations
Clinical considerations
Neither the CDC nor the WHO have issued a specific protocol for monitoring for and treating COVID-19 in patients with eating disorders; however, the guidelines offered by these organizations for the general population should be followed for patients with eating disorders.
When screening a patient with an eating disorder, keep in mind that the symptoms of eating disorders, such as AN, may mimic an infectious process. Mood symptoms, such as depression or anxiety, could represent physiological responses to infection. Patients with GI symptoms that typically are considered part of the pathology of an eating disorder should be more carefully considered for COVID-19. Monitor a patient’s basal body temperature, and be mindful that a patient with AN may exhibit a delayed fever response. Be vigilant for a recent loss of taste or smell, which should raise suspicion for COVID-19. When monitoring vital signs, pay careful attention for any decompensation in a patient’s pulse oximetry. Whenever possible, order COVID-19 testing for any patient you suspect may be infected.
Outpatient clinicians should work closely in a collaborative manner with a patient’s eating disorder treatment team. Psychiatrists, primary care physicians, psychotherapists, nutritionists, and other clinicians should all follow CDC/WHO guidelines regarding COVID-19, provide surveillance, and communicate any suspicions to the medical team. Eating disorder treatment programs, including residential centers, partial hospital programs (PHP), and intensive outpatient programs (IOP), must enhance monitoring for COVID-19, and exercise caution by practicing social distancing and providing adequate personal protective equipment for patients and staff. To reduce the spread of COVID-19, many IOPs and PHPs have transitioned to virtual treatment. Residential centers must carefully screen patients before admission to weigh the risks and benefits of inpatient vs outpatient care.
Bottom Line
Differences in the immune system of patients with an eating disorder do not necessarily confer a higher or lower risk of infection. Symptoms of some infections can mimic the symptoms of anorexia nervosa. Recognizing infections in patients with eating disorders is critical because compared with the general population, they have higher rates of infection-related morbidity and mortality.
Related Resources
- Congress J, Madaan V. 6 ‘M’s to keep in mind when you next see a patient with anorexia nervosa. Current Psychiatry. 2014;13(5):58-59.
- Westmoreland P. Eating disorders: Masterclass lecture part I. Psychcast (podcast). https://www.mdedge.com/podcasts/psychcast/eating-disorders-masterclass-lecture-part-i.
1. Golla JA, Larson LA, Anderson CF, et al. An immunological assessment of patients with anorexia nervosa. Am J Clin Nutr. 1981;34(12):2756-2762.
2. Gibson D, Mehler PS. Anorexia nervosa and the immune system—a narrative review. J Clin Med. 2019;8(11):1915. doi: 10.3390/jcm8111915.
3. Słotwin
4. Nova E, Samartín S, Gómez S, et al. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur J Clin Nutr. 2002;56(suppl 3):S34-S37.
5. Allende LM, Corell A, Manzanares J, et al. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94(4):543-551.
6. Brown RF, Bartrop R, Birmingham CL. Immunological disturbance and infectious disease in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20(3):117-128.
7. Polack E, Nahmod VE, Emeric-Sauval E, et al. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J Clin Immunol. 1993;13(6):445-451.
8. Bowers TK, Eckert E. Leukopenia in anorexia nervosa. Lack of increased risk of infection. Arch Intern Med. 1978;138(10):1520-1523.
9. Cason J, Ainley CC, Wolstencroft RA, et al. Cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1986;64(2):370-375.
10. Raevuori A, Lukkariniemi L, Suokas JT, et al. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int J Eat Disord. 2016;49(6):542-552.
11. Solmi M, Veronese N, Favaro A, et al. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237-252.
12. Brown RF, Bartrop R, Beumont P, et al. Bacterial infections in anorexia nervosa: delayed recognition increases complications. Int J Eat Disord. 2005;37(3):261-265.
13. Theander S. Anorexia nervosa. A psychiatric investigation of 94 female patients. Acta Psychiatr Scand Suppl. 1970;214:1-194.
14. Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973;117(3):435-449.
15. Copeland PM, Herzog DB. Hypoglycemia and death in anorexia nervosa. Psychother Psychosom. 1987;48(1-4):146-150.
16. Devuyst O, Lambert M, Rodhain J, et al. Haematological changes and infectious complications in anorexia nervosa: a case-control study. Q J Med. 1993;86(12):791-799.
17. Pisetsky DS, Trace SE, Brownley KA, et al. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: an exploratory study. Cytokine. 2014;69(1):110-115.
18. Birmingham CL, Hodgson DM, Fung J, et al. Reduced febrile response to bacterial infection in anorexia nervosa patients. Int J Eat Disord. 2003;34(2):269-272.
19. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online March 12, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa248.
20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
21. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
22. Birmingham CL, Tan AO. Respiratory muscle weakness and anorexia nervosa. Int J Eat Disord. 2003;33(2):230-233.
23. Cook VJ, Coxson HO, Mason AG, et al. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can Respir J. 2001;8(5):361-365.
24. Khalsa SS, Hassanpour MS, Strober M, et al. Interoceptive anxiety and body representation in anorexia nervosa [published online September 21, 2018]. Front Psychiatry. 2018;9:444. doi: 10.3389/fpsyt.2018.00444.
25. van West D, Maes M. Cytokines in de obsessief compulsieve stoornis en in anorexia nervosa: een overzicht. Acta Neuropsychiatr. 1999;11(4):125-129.
26. Komorowska-Pietrzykowska R, Rajewski A, Wiktorowicz K, et al. Czynnos
27. Marcos A, Varela P, Toro O, et al. Interactions between nutrition and immunity in anorexia nervosa: a 1-y follow-up study. Am J Clin Nutr. 1997;66(2):485S-490S.
28. Pertschuk MJ, Crosby LO, Barot L, et al. Immunocompetency in anorexia nervosa. Am J Clin Nutr. 1982;35(5):968-972.
29. Varela P, Marcos A, Navarro MP. Zinc status in anorexia nervosa. Ann Nutr Metab. 1992;36(4):197-202.
30. Breithaupt L, Köhler-Forsberg O, Larsen JT, et al. Association of exposure to infections in childhood with risk of eating disorders in adolescent girls. JAMA Psychiatry. 2019;76(8):800-809.
31. Brambilla F, Monti D, Franceschi C. Plasma concentrations of interleukin-1-beta, interleukin-6 and tumor necrosis factor-alpha, and of their soluble receptors and receptor antagonist in anorexia nervosa. Psychiatry Res. 2001;103(2-3):107-114.
32. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic [published online February 27, 2020]. Asian Pac J Allergy Immunol. doi: 10.12932/AP-200220-0772.
33. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
34. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490-5500.
35. Páli AA, Pászthy B. Az immunrendszer muködésének megváltozása a táplálkozási magatartás zavarai esetén [Changes of the immune functions in patients with eating disorders]. Ideggyogy Sz. 2008;61(11-12):381‐384.
36. Elegido A, Graell M, Andrés P, et al. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr Res. 2017;39:43-50.
37. Marcos A, Varela P, Santacruz I, et al. Nutritional status and immunocompetence in eating disorders. A comparative study. Eur J Clin Nutr. 1993;47(11):787-793.
38. Mustafa A, Ward A, Treasure J, et al. T lymphocyte subpopulations in anorexia nervosa and refeeding. Clin Immunol Immunopathol. 1997;82(3):282-289.
39. Nagata T, Kiriike N, Tobitani W, et al. Lymphocyte subset, lymphocyte proliferative response, and soluble interleukin-2 receptor in anorexic patients. Biol Psychiatry. 1999;45(4):471-474.
40. Saito H, Nomura K, Hotta M, et al. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. 2007;40(6):575-579.
41. Nova E, Varela P, López-Vidriero I, et al. A one-year follow-up study in anorexia nervosa. Dietary pattern and anthropometrical evolution. Eur J Clin Nutr. 2001;55(7):547-554.
42. Taylor AK, Cao W, Vora KP, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis. 2013;207(3):501-510.
43. Mant MJ, Faragher BS. The hematology of anorexia nervosa. Br J Haematol. 1972;23(6):737-749.
44. Marcos A. The immune system in eating disorders: an overview. Nutrition. 1997;13(10):853-862.
45. Schattner A, Tepper R, Steinbock M, et al. TNF, interferon-gamma and cell-mediated cytotoxicity in anorexia nervosa; effect of refeeding. J Clin Lab Immunol. 1990;32(4):183-184.
46. Nagata T, Tobitani W, Kiriike N, et al. Capacity to produce cytokines during weight restoration in patients with anorexia nervosa. Psychosom Med. 1999;61(3):371-377.
1. Golla JA, Larson LA, Anderson CF, et al. An immunological assessment of patients with anorexia nervosa. Am J Clin Nutr. 1981;34(12):2756-2762.
2. Gibson D, Mehler PS. Anorexia nervosa and the immune system—a narrative review. J Clin Med. 2019;8(11):1915. doi: 10.3390/jcm8111915.
3. Słotwin
4. Nova E, Samartín S, Gómez S, et al. The adaptive response of the immune system to the particular malnutrition of eating disorders. Eur J Clin Nutr. 2002;56(suppl 3):S34-S37.
5. Allende LM, Corell A, Manzanares J, et al. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94(4):543-551.
6. Brown RF, Bartrop R, Birmingham CL. Immunological disturbance and infectious disease in anorexia nervosa: a review. Acta Neuropsychiatr. 2008;20(3):117-128.
7. Polack E, Nahmod VE, Emeric-Sauval E, et al. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J Clin Immunol. 1993;13(6):445-451.
8. Bowers TK, Eckert E. Leukopenia in anorexia nervosa. Lack of increased risk of infection. Arch Intern Med. 1978;138(10):1520-1523.
9. Cason J, Ainley CC, Wolstencroft RA, et al. Cell-mediated immunity in anorexia nervosa. Clin Exp Immunol. 1986;64(2):370-375.
10. Raevuori A, Lukkariniemi L, Suokas JT, et al. Increased use of antimicrobial medication in bulimia nervosa and binge-eating disorder prior to the eating disorder treatment. Int J Eat Disord. 2016;49(6):542-552.
11. Solmi M, Veronese N, Favaro A, et al. Inflammatory cytokines and anorexia nervosa: a meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology. 2015;51:237-252.
12. Brown RF, Bartrop R, Beumont P, et al. Bacterial infections in anorexia nervosa: delayed recognition increases complications. Int J Eat Disord. 2005;37(3):261-265.
13. Theander S. Anorexia nervosa. A psychiatric investigation of 94 female patients. Acta Psychiatr Scand Suppl. 1970;214:1-194.
14. Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973;117(3):435-449.
15. Copeland PM, Herzog DB. Hypoglycemia and death in anorexia nervosa. Psychother Psychosom. 1987;48(1-4):146-150.
16. Devuyst O, Lambert M, Rodhain J, et al. Haematological changes and infectious complications in anorexia nervosa: a case-control study. Q J Med. 1993;86(12):791-799.
17. Pisetsky DS, Trace SE, Brownley KA, et al. The expression of cytokines and chemokines in the blood of patients with severe weight loss from anorexia nervosa: an exploratory study. Cytokine. 2014;69(1):110-115.
18. Birmingham CL, Hodgson DM, Fung J, et al. Reduced febrile response to bacterial infection in anorexia nervosa patients. Int J Eat Disord. 2003;34(2):269-272.
19. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online March 12, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa248.
20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
21. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
22. Birmingham CL, Tan AO. Respiratory muscle weakness and anorexia nervosa. Int J Eat Disord. 2003;33(2):230-233.
23. Cook VJ, Coxson HO, Mason AG, et al. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can Respir J. 2001;8(5):361-365.
24. Khalsa SS, Hassanpour MS, Strober M, et al. Interoceptive anxiety and body representation in anorexia nervosa [published online September 21, 2018]. Front Psychiatry. 2018;9:444. doi: 10.3389/fpsyt.2018.00444.
25. van West D, Maes M. Cytokines in de obsessief compulsieve stoornis en in anorexia nervosa: een overzicht. Acta Neuropsychiatr. 1999;11(4):125-129.
26. Komorowska-Pietrzykowska R, Rajewski A, Wiktorowicz K, et al. Czynnos
27. Marcos A, Varela P, Toro O, et al. Interactions between nutrition and immunity in anorexia nervosa: a 1-y follow-up study. Am J Clin Nutr. 1997;66(2):485S-490S.
28. Pertschuk MJ, Crosby LO, Barot L, et al. Immunocompetency in anorexia nervosa. Am J Clin Nutr. 1982;35(5):968-972.
29. Varela P, Marcos A, Navarro MP. Zinc status in anorexia nervosa. Ann Nutr Metab. 1992;36(4):197-202.
30. Breithaupt L, Köhler-Forsberg O, Larsen JT, et al. Association of exposure to infections in childhood with risk of eating disorders in adolescent girls. JAMA Psychiatry. 2019;76(8):800-809.
31. Brambilla F, Monti D, Franceschi C. Plasma concentrations of interleukin-1-beta, interleukin-6 and tumor necrosis factor-alpha, and of their soluble receptors and receptor antagonist in anorexia nervosa. Psychiatry Res. 2001;103(2-3):107-114.
32. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic [published online February 27, 2020]. Asian Pac J Allergy Immunol. doi: 10.12932/AP-200220-0772.
33. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
34. Li CK, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181(8):5490-5500.
35. Páli AA, Pászthy B. Az immunrendszer muködésének megváltozása a táplálkozási magatartás zavarai esetén [Changes of the immune functions in patients with eating disorders]. Ideggyogy Sz. 2008;61(11-12):381‐384.
36. Elegido A, Graell M, Andrés P, et al. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr Res. 2017;39:43-50.
37. Marcos A, Varela P, Santacruz I, et al. Nutritional status and immunocompetence in eating disorders. A comparative study. Eur J Clin Nutr. 1993;47(11):787-793.
38. Mustafa A, Ward A, Treasure J, et al. T lymphocyte subpopulations in anorexia nervosa and refeeding. Clin Immunol Immunopathol. 1997;82(3):282-289.
39. Nagata T, Kiriike N, Tobitani W, et al. Lymphocyte subset, lymphocyte proliferative response, and soluble interleukin-2 receptor in anorexic patients. Biol Psychiatry. 1999;45(4):471-474.
40. Saito H, Nomura K, Hotta M, et al. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. 2007;40(6):575-579.
41. Nova E, Varela P, López-Vidriero I, et al. A one-year follow-up study in anorexia nervosa. Dietary pattern and anthropometrical evolution. Eur J Clin Nutr. 2001;55(7):547-554.
42. Taylor AK, Cao W, Vora KP, et al. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J Infect Dis. 2013;207(3):501-510.
43. Mant MJ, Faragher BS. The hematology of anorexia nervosa. Br J Haematol. 1972;23(6):737-749.
44. Marcos A. The immune system in eating disorders: an overview. Nutrition. 1997;13(10):853-862.
45. Schattner A, Tepper R, Steinbock M, et al. TNF, interferon-gamma and cell-mediated cytotoxicity in anorexia nervosa; effect of refeeding. J Clin Lab Immunol. 1990;32(4):183-184.
46. Nagata T, Tobitani W, Kiriike N, et al. Capacity to produce cytokines during weight restoration in patients with anorexia nervosa. Psychosom Med. 1999;61(3):371-377.
Telepsychiatry: What you need to know
The need for mental health services has never been greater. Unfortunately, many patients have limited access to psychiatric treatment, especially those who live in rural areas. Telepsychiatry—the delivery of psychiatric services through telecommunications technology, usually video conferencing—may help address this problem. Even before the onset of the coronavirus disease 2019 (COVID-19) pandemic, telepsychiatry was becoming increasingly common. A survey of US mental health facilities found that the proportion of facilities offering telepsychiatry nearly doubled from 2010 to 2017, from 15.2% to 29.2%.1
In this article, we describe examples of where and how telepsychiatry is being used successfully, and its potential advantages. We discuss concerns about its use, its impact on the therapeutic alliance, and patients’ and clinicians’ perceptions of it. We also discuss the legal, technological, and financial aspects of using telepsychiatry. With an increased understanding of these issues, psychiatric clinicians will be better able to integrate telepsychiatry into their practices.
How and where is telepsychiatry being used
In addition to being used to provide psychotherapy, telepsychiatry is being employed for diagnosis and evaluation; clinical consultations; research; supervision, mentoring, and education of trainees; development of treatment programs; and public health. Telepsychiatry is an excellent mechanism to provide high-level second opinions to primary care physicians and psychiatrists on complex cases for both diagnostic purposes and treatment.
Evidence suggests that telepsychiatry can play a beneficial role in a variety of settings, and for a range of patient populations.
Emergency departments (EDs). Using telepsychiatry for psychiatric consultations in EDs could result in a quicker disposition of patients and reduced crowding and wait times. A survey of on-call clinicians in a pediatric ED found that using telepsychiatry for on-site psychiatric consultations decreased patients’ length of stay, improved resident on-call burden, and reduced factors related to physician burnout.2 In this study, telepsychiatry use reduced travel for face-to-face evaluations by 75% and saved more than 2 hours per call day.2
Medical clinics. Using telepsychiatry to deliver cognitive-behavioral therapy significantly reduced symptoms of depression or anxiety among 203 primary care patients.3 Incorporating telepsychiatry into existing integrated primary care settings is becoming more common. For example, an integrated-care model that includes telepsychiatry is serving the needs of complex patients in a high-volume, urban primary care clinic in Colorado.4
Assertive Community Treatment (ACT) teams. Telepsychiatry is being used by ACT teams for crisis intervention and to reduce inpatient hospitalizations.5
Continue to: Correctional facilities
Correctional facilities. With the downsizing and closure of many state psychiatric hospitals across the United States over the last several decades, jails and prisons have become de facto mental health hospitals. This situation presents many challenges, including access to mental health care and the need to avoid medications with the potential for abuse. Using telepsychiatry for psychiatric consultations in correctional facilities can improve access to mental health care.
Geriatric patients.
Children and adolescents. The Michigan Child Collaborative Care (MC3) program is a telepsychiatry consultation service that has been able to provide cost-effective, timely, remote consultation to primary care clinicians who care for youth and perinatal women.8 New York has a pediatric collaborative care program, the Child and Adolescent Psychiatry for Primary Care (CAP PC), that incorporates telepsychiatry consultations for families who live >1 hour away from one of the program’s treatment sites.9
Patients with cancer. A literature review that included 9 studies found no statistically significant differences between standard face-to-face interventions and telepsychiatry for improving quality-of-life scores among patients receiving treatment for cancer.10
Patients with insomnia. Cognitive-behavioral therapy for insomnia (CBT-I) is often recommended as a first-line treatment, but is not available for many patients. A recent study showed that CBT-I provided via telepsychiatry for patients with shift work sleep disorder was as effective as face-to-face therapy.11 Increasing the availability of this treatment could decrease reliance on pharmacotherapy for sleep.
Patients with opioid use disorder (OUD). Treatment for patients with OUD is limited by access to, and availability of, psychiatric clinicians. Telepsychiatry can help bridge this gap. One example of such use is in Ontario, Canada, where more than 10,000 patients with concurrent opiate abuse and other mental health disorders have received care via telepsychiatry since 2008.12
Continue to: Increasing access to cost-effective care where it is needed most
Increasing access to cost-effective care where it is needed most
There is a crisis in mental health care in rural areas of the United States. A study assessing delivery of care to US residents who live in rural areas found these patients’ mental health–related quality of life was 2.5 standard deviations below the national mean.13 Additionally, the need for treatment is expected to rise as the number of psychiatrists falls. According to a 2017 National Council for Behavioral Health report,14 by 2025, demand may outstrip supply by 6,090 to 15,600 psychiatrists. While telepsychiatry cannot improve this shortage per se, it can help increase access to psychiatric services. The potential benefits of telepsychiatry for patients are summarized in Table 1.15
Telepsychiatry may be more cost-effective than traditional face-to-face treatment. A cost analysis of an expanding, multistate behavioral telehealth intervention program for rural American Indian/Alaska Native populations found substantial cost savings associated with telepsychiatry.16 In this analysis, the estimated cost efficiencies of telepsychiatry were more evident in rural communities, and having a multistate center was less expensive than each state operating independently.16
Most importantly, evidence suggests that treatment delivered via telepsychiatry is at least as effective as traditional face-to-face care. In a review that included >150 studies, Bashshur et al17 concluded, “Effective approaches to the long-term management of mental illness include monitoring, surveillance, mental health promotion, mental illness prevention, and biopsychosocial treatment programs. The empirical evidence … demonstrates the capability of [telepsychiatry] to perform these functions more efficiently and as well as or more effectively than in-person care
Clinician and patient attitudes toward telepsychiatry
Clinicians have legitimate concerns about the quality of care being delivered when using telepsychiatry. Are patients satisfied with treatment delivered via telepsychiatry? Can a therapeutic alliance be established and maintained? It appears that clinicians may have more concerns than patients do.18
A study of telepsychiatry consultations for patients in rural primary care clinics performed by clinicians at an urban health center found that patients and clinicians were highly satisfied with telepsychiatry.19 Both patients and clinicians believed that telepsychiatry provided patients with better access to care. There was a high degree of agreement between patients and clinician responses.19
Continue to: In a review of...
In a review of 452 telepsychiatry studies, Hubley et al20 focused on satisfaction, reliability, treatment outcomes, implementation outcomes, cost effectiveness, and legal issues. They concluded that patients and clinicians are generally satisfied with telepsychiatry services. Interestingly, clinicians expressed more concerns about the potential adverse effects of telepsychiatry on therapeutic rapport. Hubley et al20 found no published reports of adverse events associated with telepsychiatry use.
In a study of school-based telepsychiatry in an urban setting, Mayworm et al21 found that patients were highly satisfied with both in-person and telepsychiatry services, and there were no significant differences in preference. This study also found that telepsychiatry services were more time-efficient than in-person services.
A study of using telepsychiatry to treat unipolar depression found that patient satisfaction scores improved with increasing number of video-based sessions, and were similar among all age groups.22 An analysis of this study found that total satisfaction scores were higher for patients than for clinicians.23
In a study of satisfaction with telepsychiatry among community-dwelling older veterans, 90% of participants reported liking or even preferring telepsychiatry, even though the experience was novel for most of them.24
As always, patients’ preferences need to be kept in mind when considering what services can and should be provided via telepsychiatry, because not all patients will find it acceptable. For example, in a study of veterans’ attitudes toward treatment via telepsychiatry, Goetter et al25 found that interest was mixed. Twenty-six percent of patients were “not at all comfortable,” while 13% were “extremely comfortable” using telepsychiatry from home. Notably, 33% indicated a clear preference for telepsychiatry compared to in-person mental health visits.
Continue to: Legal aspects of telepsychiatry
Legal aspects of telepsychiatry
Box 1
As part of the efforts to contain the spread of coronavirus disease 2019 (COVID-19), the use of telemedicine, including telepsychiatry, has increased substantially. Here are a few key facts to keep in mind while practicing telepsychiatry during this pandemic:
- The Centers for Medicare and Medicaid Services relaxed requirements for telehealth starting March 6, 2020 and for the duration of the COVID-19 Public Health Emergency. Under this new waiver, Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patient’s places of residence. For details, see www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. This fact sheet reviews relevant information, including billing codes.
- Health Insurance Portability and Accountability Act requirements, specifically those for secure communications, will not be enforced when telehealth is used under the new waiver. Because of this, popular but unsecure software applications, such as Apple’s FaceTime, Microsoft’s Teams, or Facebook’s Messenger, WhatsApp, and Messenger Rooms, can be used.
- Informed consent for the use of telepsychiatry in this situation should be obtained from the patient or his/her guardian, and documented in the patient’s medical record. For example: “Informed consent received for providing services via video teleconferencing to the home in order to protect the patient from COVID-19 exposure. Confidentiality issues were discussed.”
Licensure. State licensing and medical regulatory organizations consider the care provided via telepsychiatry to be rendered where the patient is physically located when services are rendered. Because of this, psychiatrists who use telepsychiatry generally need to hold a license in the state where their patients are located, regardless of where the psychiatrist is located.
Some states offer special telemedicine licenses. Typically, these licenses allow clinicians to practice across state lines without having to obtain a full professional license from the state. Be sure to check with the relevant state medical board where you intend to practice.
Because state laws related to telepsychiatry are continuously evolving, we suggest that clinicians continually check these laws and obtain a regulatory response in writing so there is ongoing documentation. For more information on this topic, see “Telepsychiatry during COVID-19: Understanding the rules” at MDedge.com/psychiatry.
Malpractice insurance. Some insurance companies offer coverage that includes the practice of telepsychiatry, whereas other carriers require the purchase of additional coverage for telepsychiatry. There may be additional requirements for practicing across state lines. Be sure to check with your insurer.
Continue to: Technical requirements and costs
Technical requirements and costs
In order to perform telepsychiatry, one needs Internet access, appropriate hardware such as a desktop or laptop computer or tablet, and a video conferencing application. Software must be HIPAA-compliant, although this requirement is not being enforced during the COVID-19 pandemic. Several popular video conferencing platforms were designed for or have versions suitable for telemedicine, including Zoom, Doxy.me, Vidyo, and Skype.
The use of different electronic health record (EHR) systems by various health care systems is a barrier to using telepsychiatry.
Box 2
The North Carolina Statewide Telepsychiatry Program (NC-STeP) began in 2013 by providing telepsychiatry services in hospital emergency departments (EDs) to individuals experiencing an acute behavioral health crisis. In 2018, the program expanded to include community-based primary care sites using a “hybrid” collaborative-care model. This model benefits patients by improving access to mental health specialty care; reducing the need for trips to the ED and inpatient admissions, thus decompressing EDs; improving compliance with treatment; reducing delays in care; reducing stigma; and improving continuity of care and follow-up. East Carolina University’s Center for Telepsychiatry and E-Behavioral Health is the home for this program, which is connecting hospital EDs and community-based primary care sites across North Carolina.
NC-STeP provides patients with a faceto-face interaction with a clinician through real-time video conferencing that is facilitated using mobile carts and desktop units. A web portal combines scheduling, electronic medical records, health information exchange functions, and data management systems.
NC-STeP has significantly reduced patient length of stay in EDs, provided cost savings to the health care delivery system through overturned involuntary commitments, improved ED throughout, and reduced patient boarding time; and has achieved high rates of patient, staff, and clinician satisfaction. Highlights of the program include:
- 57 hospitals and 8 communitybased sites in the network (as of January 1, 2020)
- 8 clinical hubs are operational, with 53 consultant clinicians
- 40,573 telepsychiatry assessments (as of January 1, 2020)
- 5,631 involuntary commitments overturned, thus preventing unnecessary hospitalizations representing a saving of $30,407,400 to the state
- Since program inception, >40% of ED patients who received telepsychiatry services were discharged to home
- 32% of the patients served had no insurance coverage
- Currently, the average consult elapsed time (in queue to consult complete) is 3 hours 9 minutes.
For more information about this program, see www.ecu.edu/cs-dhs/ncstep.
Our practice has extensive experience with telepsychiatry (Box 3), and for us, the specific costs associated with providing telepsychiatry services include maintenance of infrastructure and the purchase of hardware (eg, computers, smartphones, tablets), a video conferencing application (some free versions are available), EHR systems, and Internet access.
Box 3
Our practice (Rural Psychiatry Associates, Grand Forks, North Dakota) and our close associates have provided telepsychiatry services to >200 mental health clinics, hospitals, Native American villages, prisons, and nursing homes, mostly in rural and underserved areas. To provide these services, in addition to physicians, we also utilize nurse practitioners and physician assistants, for whom we provide extensive education, training, and supervision. We also provide education to the staff at the facilities where we provide services.
For nursing homes, we often use what is referred to as a “blended mode,” where we combine telepsychiatry visits with in-person, on-site visits, alternating monthly. In this model, we also typically alternate one physician with one nonphysician clinician at each facility. For continuity of care, the same clinicians service the same facilities. For very distant facilities with only a few patients, only telepsychiatry is utilized. However, initial services are always provided by a physician to establish a relationship, discuss policies and procedures, and evaluate patients face-to-face.
Telepsychiatry is increasingly used for education and mentoring. We have found telepsychiatry to be especially useful when working with psychiatric residents on a realtime basis as they evaluate and treat patients at a different location.
Reimbursement for telepsychiatry
Private insurance reimbursement for treatment delivered via telepsychiatry obviously depends on the specific insurance company. Some facilities, such as nursing homes, hospitals, medical clinics, and correctional facilities, offer lump-sum fees to clinicians for providing contracted services. Some clinicians are providing telepsychiatry as direct-bill or concierge services, which require direct payment from the patient without any reimbursement from insurance.
Medicare Part B covers some telepsychiatry services, but only under certain conditions.28 Previously, reimbursement was limited to services provided to patients who live in rural areas. However, on November 1, 2019, eligibility for telehealth services for Medicare Advantage (MA) recipients was expanded to include patients in both urban and rural locations. Patients covered by MA also can receive telehealth services from their home, instead of having to drive to a Centers for Medicare and Medicaid Services–qualified telehealth service center.
Continue to: Medicaid is the single...
Medicaid is the single largest payer for mental health services in the United States,29 and all Medicaid programs reimburse for some telepsychiatry services. As with all Medicaid health care, fees paid for telepsychiatry are state-specific. Since 2013, several state Medicaid programs, including New York,30 have expanded the list of eligible telehealth sites to include schools, thereby giving children virtual access to mental health clinicians.
Getting started
Clinicians who are interested in starting to provide treatment via telepsychiatry can begin by reviewing the American Psychiatric Association’s Telepsychiatry Toolkit at www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit. This toolkit, which is being continually updated, features numerous training videos for clinicians new to telepsychiatry, such as Learning To Do Telemental Health (www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/learning-telemental-health) and The Credentialing Process (www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/credentialing-process). Before starting, also consider reviewing the steps listed in Table 2.
Bottom Line
Evidence suggests telepsychiatry can be beneficial for a wide range of patient populations and settings. Most patients accept its use, and some actually prefer it to face-to-face care. Telepsychiatry may be especially useful for patients who have limited access to psychiatric treatment, such as those who live in rural areas. Factors to consider before incorporating telepsychiatry into your practice include addressing various legal, technological, and financial requirements.
Related Resources
- Von Hafften A. Telepsychiatry practice guidelines. American Psychiatric Association. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/practice-guidelines.
- Centers for Disease Control and Prevention. Telehealth and telemedicine: a research anthology of law and policy resources. https://www.cdc.gov/phlp/publications/topic/anthologies/anthologies-telehealth.html. Reviewed July 31, 2019.
- American Telemedicine Association. https://www.americantelemed.org/.
1. Spivak S, Spivak A, Cullen B, et al. Telepsychiatry use in U.S. mental health facilities, 2010-2017. Psychiatr Serv. 2019;71(2):appips201900261. doi: 10.1176/appi.ps.201900261.
2. Reliford A, Adebanjo B. Use of telepsychiatry in pediatric emergency room to decrease length of stay for psychiatric patients, improve resident on-call burden, and reduce factors related to physician burnout. Telemed J E Health. 2019;25(9):828-832.
3. Mathiasen K, Riper H, Andersen TE, et al. Guided internet-based cognitive behavioral therapy for adult depression and anxiety in routine secondary care: observational study. J Med Internet Res. 2018;20(11):e10927. doi: 10.2196/10927.
4. Waugh M, Calderone J, Brown Levey S, et al. Using telepsychiatry to enrich existing integrated primary care. Telemed J E Health. 2019;25(8):762-768.
5. Swanson CL, Trestman RL. Rural assertive community treatment and telepsychiatry. J Psychiatr Pract. 2018;24(4):269-273.
6. Gentry MT, Lapid MI, Rummans TA. Geriatric telepsychiatry: systematic review and policy considerations. Am J Geriatr Psychiatry. 2019;27(2):109-127.
7. Christensen LF, Moller AM, Hansen JP, et al. Patients’ and providers’ experiences with video consultations used in the treatment of older patients with unipolar depression: a systematic review. J Psychiatr Ment Health Nurs. 2020;27(3):258-271.
8. Marcus S, Malas N, Dopp R, et al. The Michigan Child Collaborative Care program: building a telepsychiatry consultation service. Psychiatr Serv. 2019;70(9):849-852.
9. Kaye DL, Fornari V, Scharf M, et al. Description of a multi-university education and collaborative care child psychiatry access program: New York State’s CAP PC. Gen Hosp Psychiatry. 2017;48:32-36.
10. Larson JL, Rosen AB, Wilson FA. The effect of telehealth interventions on quality of life of cancer patients: a systematic review and meta-analysis. Telemed J E Health. 2018;24(6):397-405.
11. Peter L, Reindl R, Zauter S, et al. Effectiveness of an online CBT-I intervention and a face-to-face treatment for shift work sleep disorder: a comparison of sleep diary data. Int J Environ Res Public Health. 2019;16(17):E3081. doi: 10.3390/ijerph16173081.
12. LaBelle B, Franklyn AM, Pkh Nguyen V, et al. Characterizing the use of telepsychiatry for patients with opioid use disorder and cooccurring mental health disorders in Ontario, Canada. Int J Telemed Appl. 2018;2018(3):1-7.
13. Fortney JC, Heagerty PJ, Bauer AM, et al. Study to promote innovation in rural integrated telepsychiatry (SPIRIT): rationale and design of a randomized comparative effectiveness trial of managing complex psychiatric disorders in rural primary care clinics. Contemp Clin Trials. 2020;90:105873. doi: 10.1016/j.cct.2019.105873.
14. Weiner S. Addressing the escalating psychiatrist shortage. AAMC. https://www.aamc.org/news-insights/addressing-escalating-psychiatrist-shortage. Published February 12, 2018. Accessed May 14, 2020.
15. American Psychiatric Association. What is telepsychiatry? https://www.psychiatry.org/patients-families/what-is-telepsychiatry. Published 2017. Accessed May 14, 2020.
16. Yilmaz SK, Horn BP, Fore C, et al. An economic cost analysis of an expanding, multi-state behavioural telehealth intervention. J Telemed Telecare. 2019;25(6):353-364.
17. Bashshur RL, Shannon GW, Bashshur N, et al. The empirical evidence for telemedicine interventions in mental disorders. Telemed J E Health. 2016;22(2):87-113.
18. Lopez A, Schwenk S, Schneck CD, et al. Technology-based mental health treatment and the impact on the therapeutic alliance. Curr Psychiatry Rep. 2019;21(8):76.
19. Schubert NJ, Backman PJ, Bhatla R, et al. Telepsychiatry and patient-provider concordance. Can J Rural Med. 2019;24(3):75-82.
20. Hubley S, Lynch SB, Schneck C, et al. Review of key telepsychiatry outcomes. World J Psychiatry. 2016;6(2):269-282.
21. Mayworm AM, Lever N, Gloff N, et al. School-based telepsychiatry in an urban setting: efficiency and satisfaction with care. Telemed J E Health. 2020;26(4):446-454.
22. Christensen LF, Gildberg FA, Sibbersen C, et al. Videoconferences and treatment of depression: satisfaction score correlated with number of sessions attended but not with age [published online October 31, 2019]. Telemed J E Health. 2019. doi: 10.1089/tmj.2019.0129.
23. Christensen LF, Gildberg FA, Sibbersen C, et al. Disagreement in satisfaction between patients and providers in the use of videoconferences by depressed adults. Telemed J E Health. 2020;26(5):614-620.
24. Hantke N, Lajoy M, Gould CE, et al. Patient satisfaction with geriatric psychiatry services via video teleconference. Am J Geriatr Psychiatry. 2020;28(4):491-494.
25. Goetter EM, Blackburn AM, Bui E, et al. Veterans’ prospective attitudes about mental health treatment using telehealth. J Psychosoc Nurs Ment Health Serv. 2019;57(9):38-43.
26. Vanderpool D. Top 10 myths about telepsychiatry. Innov Clin Neurosci. 2017;14(9-10):13-15.
27. Butterfield A. Telepsychiatric evaluation and consultation in emergency care settings. Child Adolesc Psychiatr Clin N Am. 2018;27(3):467-478.
28. Medicare.gov. Telehealth. https://www.medicare.gov/coverage/telehealth. Accessed May 14, 2020.
29. Centers for Medicare & Medicaid Services. Behavioral Health Services. https://www.medicaid.gov/medicaid/benefits/bhs/index.html. Accessed May 14, 2020.
30. New York Pub Health Law §2999-cc (2017).
The need for mental health services has never been greater. Unfortunately, many patients have limited access to psychiatric treatment, especially those who live in rural areas. Telepsychiatry—the delivery of psychiatric services through telecommunications technology, usually video conferencing—may help address this problem. Even before the onset of the coronavirus disease 2019 (COVID-19) pandemic, telepsychiatry was becoming increasingly common. A survey of US mental health facilities found that the proportion of facilities offering telepsychiatry nearly doubled from 2010 to 2017, from 15.2% to 29.2%.1
In this article, we describe examples of where and how telepsychiatry is being used successfully, and its potential advantages. We discuss concerns about its use, its impact on the therapeutic alliance, and patients’ and clinicians’ perceptions of it. We also discuss the legal, technological, and financial aspects of using telepsychiatry. With an increased understanding of these issues, psychiatric clinicians will be better able to integrate telepsychiatry into their practices.
How and where is telepsychiatry being used
In addition to being used to provide psychotherapy, telepsychiatry is being employed for diagnosis and evaluation; clinical consultations; research; supervision, mentoring, and education of trainees; development of treatment programs; and public health. Telepsychiatry is an excellent mechanism to provide high-level second opinions to primary care physicians and psychiatrists on complex cases for both diagnostic purposes and treatment.
Evidence suggests that telepsychiatry can play a beneficial role in a variety of settings, and for a range of patient populations.
Emergency departments (EDs). Using telepsychiatry for psychiatric consultations in EDs could result in a quicker disposition of patients and reduced crowding and wait times. A survey of on-call clinicians in a pediatric ED found that using telepsychiatry for on-site psychiatric consultations decreased patients’ length of stay, improved resident on-call burden, and reduced factors related to physician burnout.2 In this study, telepsychiatry use reduced travel for face-to-face evaluations by 75% and saved more than 2 hours per call day.2
Medical clinics. Using telepsychiatry to deliver cognitive-behavioral therapy significantly reduced symptoms of depression or anxiety among 203 primary care patients.3 Incorporating telepsychiatry into existing integrated primary care settings is becoming more common. For example, an integrated-care model that includes telepsychiatry is serving the needs of complex patients in a high-volume, urban primary care clinic in Colorado.4
Assertive Community Treatment (ACT) teams. Telepsychiatry is being used by ACT teams for crisis intervention and to reduce inpatient hospitalizations.5
Continue to: Correctional facilities
Correctional facilities. With the downsizing and closure of many state psychiatric hospitals across the United States over the last several decades, jails and prisons have become de facto mental health hospitals. This situation presents many challenges, including access to mental health care and the need to avoid medications with the potential for abuse. Using telepsychiatry for psychiatric consultations in correctional facilities can improve access to mental health care.
Geriatric patients.
Children and adolescents. The Michigan Child Collaborative Care (MC3) program is a telepsychiatry consultation service that has been able to provide cost-effective, timely, remote consultation to primary care clinicians who care for youth and perinatal women.8 New York has a pediatric collaborative care program, the Child and Adolescent Psychiatry for Primary Care (CAP PC), that incorporates telepsychiatry consultations for families who live >1 hour away from one of the program’s treatment sites.9
Patients with cancer. A literature review that included 9 studies found no statistically significant differences between standard face-to-face interventions and telepsychiatry for improving quality-of-life scores among patients receiving treatment for cancer.10
Patients with insomnia. Cognitive-behavioral therapy for insomnia (CBT-I) is often recommended as a first-line treatment, but is not available for many patients. A recent study showed that CBT-I provided via telepsychiatry for patients with shift work sleep disorder was as effective as face-to-face therapy.11 Increasing the availability of this treatment could decrease reliance on pharmacotherapy for sleep.
Patients with opioid use disorder (OUD). Treatment for patients with OUD is limited by access to, and availability of, psychiatric clinicians. Telepsychiatry can help bridge this gap. One example of such use is in Ontario, Canada, where more than 10,000 patients with concurrent opiate abuse and other mental health disorders have received care via telepsychiatry since 2008.12
Continue to: Increasing access to cost-effective care where it is needed most
Increasing access to cost-effective care where it is needed most
There is a crisis in mental health care in rural areas of the United States. A study assessing delivery of care to US residents who live in rural areas found these patients’ mental health–related quality of life was 2.5 standard deviations below the national mean.13 Additionally, the need for treatment is expected to rise as the number of psychiatrists falls. According to a 2017 National Council for Behavioral Health report,14 by 2025, demand may outstrip supply by 6,090 to 15,600 psychiatrists. While telepsychiatry cannot improve this shortage per se, it can help increase access to psychiatric services. The potential benefits of telepsychiatry for patients are summarized in Table 1.15
Telepsychiatry may be more cost-effective than traditional face-to-face treatment. A cost analysis of an expanding, multistate behavioral telehealth intervention program for rural American Indian/Alaska Native populations found substantial cost savings associated with telepsychiatry.16 In this analysis, the estimated cost efficiencies of telepsychiatry were more evident in rural communities, and having a multistate center was less expensive than each state operating independently.16
Most importantly, evidence suggests that treatment delivered via telepsychiatry is at least as effective as traditional face-to-face care. In a review that included >150 studies, Bashshur et al17 concluded, “Effective approaches to the long-term management of mental illness include monitoring, surveillance, mental health promotion, mental illness prevention, and biopsychosocial treatment programs. The empirical evidence … demonstrates the capability of [telepsychiatry] to perform these functions more efficiently and as well as or more effectively than in-person care
Clinician and patient attitudes toward telepsychiatry
Clinicians have legitimate concerns about the quality of care being delivered when using telepsychiatry. Are patients satisfied with treatment delivered via telepsychiatry? Can a therapeutic alliance be established and maintained? It appears that clinicians may have more concerns than patients do.18
A study of telepsychiatry consultations for patients in rural primary care clinics performed by clinicians at an urban health center found that patients and clinicians were highly satisfied with telepsychiatry.19 Both patients and clinicians believed that telepsychiatry provided patients with better access to care. There was a high degree of agreement between patients and clinician responses.19
Continue to: In a review of...
In a review of 452 telepsychiatry studies, Hubley et al20 focused on satisfaction, reliability, treatment outcomes, implementation outcomes, cost effectiveness, and legal issues. They concluded that patients and clinicians are generally satisfied with telepsychiatry services. Interestingly, clinicians expressed more concerns about the potential adverse effects of telepsychiatry on therapeutic rapport. Hubley et al20 found no published reports of adverse events associated with telepsychiatry use.
In a study of school-based telepsychiatry in an urban setting, Mayworm et al21 found that patients were highly satisfied with both in-person and telepsychiatry services, and there were no significant differences in preference. This study also found that telepsychiatry services were more time-efficient than in-person services.
A study of using telepsychiatry to treat unipolar depression found that patient satisfaction scores improved with increasing number of video-based sessions, and were similar among all age groups.22 An analysis of this study found that total satisfaction scores were higher for patients than for clinicians.23
In a study of satisfaction with telepsychiatry among community-dwelling older veterans, 90% of participants reported liking or even preferring telepsychiatry, even though the experience was novel for most of them.24
As always, patients’ preferences need to be kept in mind when considering what services can and should be provided via telepsychiatry, because not all patients will find it acceptable. For example, in a study of veterans’ attitudes toward treatment via telepsychiatry, Goetter et al25 found that interest was mixed. Twenty-six percent of patients were “not at all comfortable,” while 13% were “extremely comfortable” using telepsychiatry from home. Notably, 33% indicated a clear preference for telepsychiatry compared to in-person mental health visits.
Continue to: Legal aspects of telepsychiatry
Legal aspects of telepsychiatry
Box 1
As part of the efforts to contain the spread of coronavirus disease 2019 (COVID-19), the use of telemedicine, including telepsychiatry, has increased substantially. Here are a few key facts to keep in mind while practicing telepsychiatry during this pandemic:
- The Centers for Medicare and Medicaid Services relaxed requirements for telehealth starting March 6, 2020 and for the duration of the COVID-19 Public Health Emergency. Under this new waiver, Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patient’s places of residence. For details, see www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. This fact sheet reviews relevant information, including billing codes.
- Health Insurance Portability and Accountability Act requirements, specifically those for secure communications, will not be enforced when telehealth is used under the new waiver. Because of this, popular but unsecure software applications, such as Apple’s FaceTime, Microsoft’s Teams, or Facebook’s Messenger, WhatsApp, and Messenger Rooms, can be used.
- Informed consent for the use of telepsychiatry in this situation should be obtained from the patient or his/her guardian, and documented in the patient’s medical record. For example: “Informed consent received for providing services via video teleconferencing to the home in order to protect the patient from COVID-19 exposure. Confidentiality issues were discussed.”
Licensure. State licensing and medical regulatory organizations consider the care provided via telepsychiatry to be rendered where the patient is physically located when services are rendered. Because of this, psychiatrists who use telepsychiatry generally need to hold a license in the state where their patients are located, regardless of where the psychiatrist is located.
Some states offer special telemedicine licenses. Typically, these licenses allow clinicians to practice across state lines without having to obtain a full professional license from the state. Be sure to check with the relevant state medical board where you intend to practice.
Because state laws related to telepsychiatry are continuously evolving, we suggest that clinicians continually check these laws and obtain a regulatory response in writing so there is ongoing documentation. For more information on this topic, see “Telepsychiatry during COVID-19: Understanding the rules” at MDedge.com/psychiatry.
Malpractice insurance. Some insurance companies offer coverage that includes the practice of telepsychiatry, whereas other carriers require the purchase of additional coverage for telepsychiatry. There may be additional requirements for practicing across state lines. Be sure to check with your insurer.
Continue to: Technical requirements and costs
Technical requirements and costs
In order to perform telepsychiatry, one needs Internet access, appropriate hardware such as a desktop or laptop computer or tablet, and a video conferencing application. Software must be HIPAA-compliant, although this requirement is not being enforced during the COVID-19 pandemic. Several popular video conferencing platforms were designed for or have versions suitable for telemedicine, including Zoom, Doxy.me, Vidyo, and Skype.
The use of different electronic health record (EHR) systems by various health care systems is a barrier to using telepsychiatry.
Box 2
The North Carolina Statewide Telepsychiatry Program (NC-STeP) began in 2013 by providing telepsychiatry services in hospital emergency departments (EDs) to individuals experiencing an acute behavioral health crisis. In 2018, the program expanded to include community-based primary care sites using a “hybrid” collaborative-care model. This model benefits patients by improving access to mental health specialty care; reducing the need for trips to the ED and inpatient admissions, thus decompressing EDs; improving compliance with treatment; reducing delays in care; reducing stigma; and improving continuity of care and follow-up. East Carolina University’s Center for Telepsychiatry and E-Behavioral Health is the home for this program, which is connecting hospital EDs and community-based primary care sites across North Carolina.
NC-STeP provides patients with a faceto-face interaction with a clinician through real-time video conferencing that is facilitated using mobile carts and desktop units. A web portal combines scheduling, electronic medical records, health information exchange functions, and data management systems.
NC-STeP has significantly reduced patient length of stay in EDs, provided cost savings to the health care delivery system through overturned involuntary commitments, improved ED throughout, and reduced patient boarding time; and has achieved high rates of patient, staff, and clinician satisfaction. Highlights of the program include:
- 57 hospitals and 8 communitybased sites in the network (as of January 1, 2020)
- 8 clinical hubs are operational, with 53 consultant clinicians
- 40,573 telepsychiatry assessments (as of January 1, 2020)
- 5,631 involuntary commitments overturned, thus preventing unnecessary hospitalizations representing a saving of $30,407,400 to the state
- Since program inception, >40% of ED patients who received telepsychiatry services were discharged to home
- 32% of the patients served had no insurance coverage
- Currently, the average consult elapsed time (in queue to consult complete) is 3 hours 9 minutes.
For more information about this program, see www.ecu.edu/cs-dhs/ncstep.
Our practice has extensive experience with telepsychiatry (Box 3), and for us, the specific costs associated with providing telepsychiatry services include maintenance of infrastructure and the purchase of hardware (eg, computers, smartphones, tablets), a video conferencing application (some free versions are available), EHR systems, and Internet access.
Box 3
Our practice (Rural Psychiatry Associates, Grand Forks, North Dakota) and our close associates have provided telepsychiatry services to >200 mental health clinics, hospitals, Native American villages, prisons, and nursing homes, mostly in rural and underserved areas. To provide these services, in addition to physicians, we also utilize nurse practitioners and physician assistants, for whom we provide extensive education, training, and supervision. We also provide education to the staff at the facilities where we provide services.
For nursing homes, we often use what is referred to as a “blended mode,” where we combine telepsychiatry visits with in-person, on-site visits, alternating monthly. In this model, we also typically alternate one physician with one nonphysician clinician at each facility. For continuity of care, the same clinicians service the same facilities. For very distant facilities with only a few patients, only telepsychiatry is utilized. However, initial services are always provided by a physician to establish a relationship, discuss policies and procedures, and evaluate patients face-to-face.
Telepsychiatry is increasingly used for education and mentoring. We have found telepsychiatry to be especially useful when working with psychiatric residents on a realtime basis as they evaluate and treat patients at a different location.
Reimbursement for telepsychiatry
Private insurance reimbursement for treatment delivered via telepsychiatry obviously depends on the specific insurance company. Some facilities, such as nursing homes, hospitals, medical clinics, and correctional facilities, offer lump-sum fees to clinicians for providing contracted services. Some clinicians are providing telepsychiatry as direct-bill or concierge services, which require direct payment from the patient without any reimbursement from insurance.
Medicare Part B covers some telepsychiatry services, but only under certain conditions.28 Previously, reimbursement was limited to services provided to patients who live in rural areas. However, on November 1, 2019, eligibility for telehealth services for Medicare Advantage (MA) recipients was expanded to include patients in both urban and rural locations. Patients covered by MA also can receive telehealth services from their home, instead of having to drive to a Centers for Medicare and Medicaid Services–qualified telehealth service center.
Continue to: Medicaid is the single...
Medicaid is the single largest payer for mental health services in the United States,29 and all Medicaid programs reimburse for some telepsychiatry services. As with all Medicaid health care, fees paid for telepsychiatry are state-specific. Since 2013, several state Medicaid programs, including New York,30 have expanded the list of eligible telehealth sites to include schools, thereby giving children virtual access to mental health clinicians.
Getting started
Clinicians who are interested in starting to provide treatment via telepsychiatry can begin by reviewing the American Psychiatric Association’s Telepsychiatry Toolkit at www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit. This toolkit, which is being continually updated, features numerous training videos for clinicians new to telepsychiatry, such as Learning To Do Telemental Health (www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/learning-telemental-health) and The Credentialing Process (www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/credentialing-process). Before starting, also consider reviewing the steps listed in Table 2.
Bottom Line
Evidence suggests telepsychiatry can be beneficial for a wide range of patient populations and settings. Most patients accept its use, and some actually prefer it to face-to-face care. Telepsychiatry may be especially useful for patients who have limited access to psychiatric treatment, such as those who live in rural areas. Factors to consider before incorporating telepsychiatry into your practice include addressing various legal, technological, and financial requirements.
Related Resources
- Von Hafften A. Telepsychiatry practice guidelines. American Psychiatric Association. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/practice-guidelines.
- Centers for Disease Control and Prevention. Telehealth and telemedicine: a research anthology of law and policy resources. https://www.cdc.gov/phlp/publications/topic/anthologies/anthologies-telehealth.html. Reviewed July 31, 2019.
- American Telemedicine Association. https://www.americantelemed.org/.
The need for mental health services has never been greater. Unfortunately, many patients have limited access to psychiatric treatment, especially those who live in rural areas. Telepsychiatry—the delivery of psychiatric services through telecommunications technology, usually video conferencing—may help address this problem. Even before the onset of the coronavirus disease 2019 (COVID-19) pandemic, telepsychiatry was becoming increasingly common. A survey of US mental health facilities found that the proportion of facilities offering telepsychiatry nearly doubled from 2010 to 2017, from 15.2% to 29.2%.1
In this article, we describe examples of where and how telepsychiatry is being used successfully, and its potential advantages. We discuss concerns about its use, its impact on the therapeutic alliance, and patients’ and clinicians’ perceptions of it. We also discuss the legal, technological, and financial aspects of using telepsychiatry. With an increased understanding of these issues, psychiatric clinicians will be better able to integrate telepsychiatry into their practices.
How and where is telepsychiatry being used
In addition to being used to provide psychotherapy, telepsychiatry is being employed for diagnosis and evaluation; clinical consultations; research; supervision, mentoring, and education of trainees; development of treatment programs; and public health. Telepsychiatry is an excellent mechanism to provide high-level second opinions to primary care physicians and psychiatrists on complex cases for both diagnostic purposes and treatment.
Evidence suggests that telepsychiatry can play a beneficial role in a variety of settings, and for a range of patient populations.
Emergency departments (EDs). Using telepsychiatry for psychiatric consultations in EDs could result in a quicker disposition of patients and reduced crowding and wait times. A survey of on-call clinicians in a pediatric ED found that using telepsychiatry for on-site psychiatric consultations decreased patients’ length of stay, improved resident on-call burden, and reduced factors related to physician burnout.2 In this study, telepsychiatry use reduced travel for face-to-face evaluations by 75% and saved more than 2 hours per call day.2
Medical clinics. Using telepsychiatry to deliver cognitive-behavioral therapy significantly reduced symptoms of depression or anxiety among 203 primary care patients.3 Incorporating telepsychiatry into existing integrated primary care settings is becoming more common. For example, an integrated-care model that includes telepsychiatry is serving the needs of complex patients in a high-volume, urban primary care clinic in Colorado.4
Assertive Community Treatment (ACT) teams. Telepsychiatry is being used by ACT teams for crisis intervention and to reduce inpatient hospitalizations.5
Continue to: Correctional facilities
Correctional facilities. With the downsizing and closure of many state psychiatric hospitals across the United States over the last several decades, jails and prisons have become de facto mental health hospitals. This situation presents many challenges, including access to mental health care and the need to avoid medications with the potential for abuse. Using telepsychiatry for psychiatric consultations in correctional facilities can improve access to mental health care.
Geriatric patients.
Children and adolescents. The Michigan Child Collaborative Care (MC3) program is a telepsychiatry consultation service that has been able to provide cost-effective, timely, remote consultation to primary care clinicians who care for youth and perinatal women.8 New York has a pediatric collaborative care program, the Child and Adolescent Psychiatry for Primary Care (CAP PC), that incorporates telepsychiatry consultations for families who live >1 hour away from one of the program’s treatment sites.9
Patients with cancer. A literature review that included 9 studies found no statistically significant differences between standard face-to-face interventions and telepsychiatry for improving quality-of-life scores among patients receiving treatment for cancer.10
Patients with insomnia. Cognitive-behavioral therapy for insomnia (CBT-I) is often recommended as a first-line treatment, but is not available for many patients. A recent study showed that CBT-I provided via telepsychiatry for patients with shift work sleep disorder was as effective as face-to-face therapy.11 Increasing the availability of this treatment could decrease reliance on pharmacotherapy for sleep.
Patients with opioid use disorder (OUD). Treatment for patients with OUD is limited by access to, and availability of, psychiatric clinicians. Telepsychiatry can help bridge this gap. One example of such use is in Ontario, Canada, where more than 10,000 patients with concurrent opiate abuse and other mental health disorders have received care via telepsychiatry since 2008.12
Continue to: Increasing access to cost-effective care where it is needed most
Increasing access to cost-effective care where it is needed most
There is a crisis in mental health care in rural areas of the United States. A study assessing delivery of care to US residents who live in rural areas found these patients’ mental health–related quality of life was 2.5 standard deviations below the national mean.13 Additionally, the need for treatment is expected to rise as the number of psychiatrists falls. According to a 2017 National Council for Behavioral Health report,14 by 2025, demand may outstrip supply by 6,090 to 15,600 psychiatrists. While telepsychiatry cannot improve this shortage per se, it can help increase access to psychiatric services. The potential benefits of telepsychiatry for patients are summarized in Table 1.15
Telepsychiatry may be more cost-effective than traditional face-to-face treatment. A cost analysis of an expanding, multistate behavioral telehealth intervention program for rural American Indian/Alaska Native populations found substantial cost savings associated with telepsychiatry.16 In this analysis, the estimated cost efficiencies of telepsychiatry were more evident in rural communities, and having a multistate center was less expensive than each state operating independently.16
Most importantly, evidence suggests that treatment delivered via telepsychiatry is at least as effective as traditional face-to-face care. In a review that included >150 studies, Bashshur et al17 concluded, “Effective approaches to the long-term management of mental illness include monitoring, surveillance, mental health promotion, mental illness prevention, and biopsychosocial treatment programs. The empirical evidence … demonstrates the capability of [telepsychiatry] to perform these functions more efficiently and as well as or more effectively than in-person care
Clinician and patient attitudes toward telepsychiatry
Clinicians have legitimate concerns about the quality of care being delivered when using telepsychiatry. Are patients satisfied with treatment delivered via telepsychiatry? Can a therapeutic alliance be established and maintained? It appears that clinicians may have more concerns than patients do.18
A study of telepsychiatry consultations for patients in rural primary care clinics performed by clinicians at an urban health center found that patients and clinicians were highly satisfied with telepsychiatry.19 Both patients and clinicians believed that telepsychiatry provided patients with better access to care. There was a high degree of agreement between patients and clinician responses.19
Continue to: In a review of...
In a review of 452 telepsychiatry studies, Hubley et al20 focused on satisfaction, reliability, treatment outcomes, implementation outcomes, cost effectiveness, and legal issues. They concluded that patients and clinicians are generally satisfied with telepsychiatry services. Interestingly, clinicians expressed more concerns about the potential adverse effects of telepsychiatry on therapeutic rapport. Hubley et al20 found no published reports of adverse events associated with telepsychiatry use.
In a study of school-based telepsychiatry in an urban setting, Mayworm et al21 found that patients were highly satisfied with both in-person and telepsychiatry services, and there were no significant differences in preference. This study also found that telepsychiatry services were more time-efficient than in-person services.
A study of using telepsychiatry to treat unipolar depression found that patient satisfaction scores improved with increasing number of video-based sessions, and were similar among all age groups.22 An analysis of this study found that total satisfaction scores were higher for patients than for clinicians.23
In a study of satisfaction with telepsychiatry among community-dwelling older veterans, 90% of participants reported liking or even preferring telepsychiatry, even though the experience was novel for most of them.24
As always, patients’ preferences need to be kept in mind when considering what services can and should be provided via telepsychiatry, because not all patients will find it acceptable. For example, in a study of veterans’ attitudes toward treatment via telepsychiatry, Goetter et al25 found that interest was mixed. Twenty-six percent of patients were “not at all comfortable,” while 13% were “extremely comfortable” using telepsychiatry from home. Notably, 33% indicated a clear preference for telepsychiatry compared to in-person mental health visits.
Continue to: Legal aspects of telepsychiatry
Legal aspects of telepsychiatry
Box 1
As part of the efforts to contain the spread of coronavirus disease 2019 (COVID-19), the use of telemedicine, including telepsychiatry, has increased substantially. Here are a few key facts to keep in mind while practicing telepsychiatry during this pandemic:
- The Centers for Medicare and Medicaid Services relaxed requirements for telehealth starting March 6, 2020 and for the duration of the COVID-19 Public Health Emergency. Under this new waiver, Medicare can pay for office, hospital, and other visits furnished via telehealth across the country and including in patient’s places of residence. For details, see www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. This fact sheet reviews relevant information, including billing codes.
- Health Insurance Portability and Accountability Act requirements, specifically those for secure communications, will not be enforced when telehealth is used under the new waiver. Because of this, popular but unsecure software applications, such as Apple’s FaceTime, Microsoft’s Teams, or Facebook’s Messenger, WhatsApp, and Messenger Rooms, can be used.
- Informed consent for the use of telepsychiatry in this situation should be obtained from the patient or his/her guardian, and documented in the patient’s medical record. For example: “Informed consent received for providing services via video teleconferencing to the home in order to protect the patient from COVID-19 exposure. Confidentiality issues were discussed.”
Licensure. State licensing and medical regulatory organizations consider the care provided via telepsychiatry to be rendered where the patient is physically located when services are rendered. Because of this, psychiatrists who use telepsychiatry generally need to hold a license in the state where their patients are located, regardless of where the psychiatrist is located.
Some states offer special telemedicine licenses. Typically, these licenses allow clinicians to practice across state lines without having to obtain a full professional license from the state. Be sure to check with the relevant state medical board where you intend to practice.
Because state laws related to telepsychiatry are continuously evolving, we suggest that clinicians continually check these laws and obtain a regulatory response in writing so there is ongoing documentation. For more information on this topic, see “Telepsychiatry during COVID-19: Understanding the rules” at MDedge.com/psychiatry.
Malpractice insurance. Some insurance companies offer coverage that includes the practice of telepsychiatry, whereas other carriers require the purchase of additional coverage for telepsychiatry. There may be additional requirements for practicing across state lines. Be sure to check with your insurer.
Continue to: Technical requirements and costs
Technical requirements and costs
In order to perform telepsychiatry, one needs Internet access, appropriate hardware such as a desktop or laptop computer or tablet, and a video conferencing application. Software must be HIPAA-compliant, although this requirement is not being enforced during the COVID-19 pandemic. Several popular video conferencing platforms were designed for or have versions suitable for telemedicine, including Zoom, Doxy.me, Vidyo, and Skype.
The use of different electronic health record (EHR) systems by various health care systems is a barrier to using telepsychiatry.
Box 2
The North Carolina Statewide Telepsychiatry Program (NC-STeP) began in 2013 by providing telepsychiatry services in hospital emergency departments (EDs) to individuals experiencing an acute behavioral health crisis. In 2018, the program expanded to include community-based primary care sites using a “hybrid” collaborative-care model. This model benefits patients by improving access to mental health specialty care; reducing the need for trips to the ED and inpatient admissions, thus decompressing EDs; improving compliance with treatment; reducing delays in care; reducing stigma; and improving continuity of care and follow-up. East Carolina University’s Center for Telepsychiatry and E-Behavioral Health is the home for this program, which is connecting hospital EDs and community-based primary care sites across North Carolina.
NC-STeP provides patients with a faceto-face interaction with a clinician through real-time video conferencing that is facilitated using mobile carts and desktop units. A web portal combines scheduling, electronic medical records, health information exchange functions, and data management systems.
NC-STeP has significantly reduced patient length of stay in EDs, provided cost savings to the health care delivery system through overturned involuntary commitments, improved ED throughout, and reduced patient boarding time; and has achieved high rates of patient, staff, and clinician satisfaction. Highlights of the program include:
- 57 hospitals and 8 communitybased sites in the network (as of January 1, 2020)
- 8 clinical hubs are operational, with 53 consultant clinicians
- 40,573 telepsychiatry assessments (as of January 1, 2020)
- 5,631 involuntary commitments overturned, thus preventing unnecessary hospitalizations representing a saving of $30,407,400 to the state
- Since program inception, >40% of ED patients who received telepsychiatry services were discharged to home
- 32% of the patients served had no insurance coverage
- Currently, the average consult elapsed time (in queue to consult complete) is 3 hours 9 minutes.
For more information about this program, see www.ecu.edu/cs-dhs/ncstep.
Our practice has extensive experience with telepsychiatry (Box 3), and for us, the specific costs associated with providing telepsychiatry services include maintenance of infrastructure and the purchase of hardware (eg, computers, smartphones, tablets), a video conferencing application (some free versions are available), EHR systems, and Internet access.
Box 3
Our practice (Rural Psychiatry Associates, Grand Forks, North Dakota) and our close associates have provided telepsychiatry services to >200 mental health clinics, hospitals, Native American villages, prisons, and nursing homes, mostly in rural and underserved areas. To provide these services, in addition to physicians, we also utilize nurse practitioners and physician assistants, for whom we provide extensive education, training, and supervision. We also provide education to the staff at the facilities where we provide services.
For nursing homes, we often use what is referred to as a “blended mode,” where we combine telepsychiatry visits with in-person, on-site visits, alternating monthly. In this model, we also typically alternate one physician with one nonphysician clinician at each facility. For continuity of care, the same clinicians service the same facilities. For very distant facilities with only a few patients, only telepsychiatry is utilized. However, initial services are always provided by a physician to establish a relationship, discuss policies and procedures, and evaluate patients face-to-face.
Telepsychiatry is increasingly used for education and mentoring. We have found telepsychiatry to be especially useful when working with psychiatric residents on a realtime basis as they evaluate and treat patients at a different location.
Reimbursement for telepsychiatry
Private insurance reimbursement for treatment delivered via telepsychiatry obviously depends on the specific insurance company. Some facilities, such as nursing homes, hospitals, medical clinics, and correctional facilities, offer lump-sum fees to clinicians for providing contracted services. Some clinicians are providing telepsychiatry as direct-bill or concierge services, which require direct payment from the patient without any reimbursement from insurance.
Medicare Part B covers some telepsychiatry services, but only under certain conditions.28 Previously, reimbursement was limited to services provided to patients who live in rural areas. However, on November 1, 2019, eligibility for telehealth services for Medicare Advantage (MA) recipients was expanded to include patients in both urban and rural locations. Patients covered by MA also can receive telehealth services from their home, instead of having to drive to a Centers for Medicare and Medicaid Services–qualified telehealth service center.
Continue to: Medicaid is the single...
Medicaid is the single largest payer for mental health services in the United States,29 and all Medicaid programs reimburse for some telepsychiatry services. As with all Medicaid health care, fees paid for telepsychiatry are state-specific. Since 2013, several state Medicaid programs, including New York,30 have expanded the list of eligible telehealth sites to include schools, thereby giving children virtual access to mental health clinicians.
Getting started
Clinicians who are interested in starting to provide treatment via telepsychiatry can begin by reviewing the American Psychiatric Association’s Telepsychiatry Toolkit at www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit. This toolkit, which is being continually updated, features numerous training videos for clinicians new to telepsychiatry, such as Learning To Do Telemental Health (www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/learning-telemental-health) and The Credentialing Process (www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/credentialing-process). Before starting, also consider reviewing the steps listed in Table 2.
Bottom Line
Evidence suggests telepsychiatry can be beneficial for a wide range of patient populations and settings. Most patients accept its use, and some actually prefer it to face-to-face care. Telepsychiatry may be especially useful for patients who have limited access to psychiatric treatment, such as those who live in rural areas. Factors to consider before incorporating telepsychiatry into your practice include addressing various legal, technological, and financial requirements.
Related Resources
- Von Hafften A. Telepsychiatry practice guidelines. American Psychiatric Association. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/practice-guidelines.
- Centers for Disease Control and Prevention. Telehealth and telemedicine: a research anthology of law and policy resources. https://www.cdc.gov/phlp/publications/topic/anthologies/anthologies-telehealth.html. Reviewed July 31, 2019.
- American Telemedicine Association. https://www.americantelemed.org/.
1. Spivak S, Spivak A, Cullen B, et al. Telepsychiatry use in U.S. mental health facilities, 2010-2017. Psychiatr Serv. 2019;71(2):appips201900261. doi: 10.1176/appi.ps.201900261.
2. Reliford A, Adebanjo B. Use of telepsychiatry in pediatric emergency room to decrease length of stay for psychiatric patients, improve resident on-call burden, and reduce factors related to physician burnout. Telemed J E Health. 2019;25(9):828-832.
3. Mathiasen K, Riper H, Andersen TE, et al. Guided internet-based cognitive behavioral therapy for adult depression and anxiety in routine secondary care: observational study. J Med Internet Res. 2018;20(11):e10927. doi: 10.2196/10927.
4. Waugh M, Calderone J, Brown Levey S, et al. Using telepsychiatry to enrich existing integrated primary care. Telemed J E Health. 2019;25(8):762-768.
5. Swanson CL, Trestman RL. Rural assertive community treatment and telepsychiatry. J Psychiatr Pract. 2018;24(4):269-273.
6. Gentry MT, Lapid MI, Rummans TA. Geriatric telepsychiatry: systematic review and policy considerations. Am J Geriatr Psychiatry. 2019;27(2):109-127.
7. Christensen LF, Moller AM, Hansen JP, et al. Patients’ and providers’ experiences with video consultations used in the treatment of older patients with unipolar depression: a systematic review. J Psychiatr Ment Health Nurs. 2020;27(3):258-271.
8. Marcus S, Malas N, Dopp R, et al. The Michigan Child Collaborative Care program: building a telepsychiatry consultation service. Psychiatr Serv. 2019;70(9):849-852.
9. Kaye DL, Fornari V, Scharf M, et al. Description of a multi-university education and collaborative care child psychiatry access program: New York State’s CAP PC. Gen Hosp Psychiatry. 2017;48:32-36.
10. Larson JL, Rosen AB, Wilson FA. The effect of telehealth interventions on quality of life of cancer patients: a systematic review and meta-analysis. Telemed J E Health. 2018;24(6):397-405.
11. Peter L, Reindl R, Zauter S, et al. Effectiveness of an online CBT-I intervention and a face-to-face treatment for shift work sleep disorder: a comparison of sleep diary data. Int J Environ Res Public Health. 2019;16(17):E3081. doi: 10.3390/ijerph16173081.
12. LaBelle B, Franklyn AM, Pkh Nguyen V, et al. Characterizing the use of telepsychiatry for patients with opioid use disorder and cooccurring mental health disorders in Ontario, Canada. Int J Telemed Appl. 2018;2018(3):1-7.
13. Fortney JC, Heagerty PJ, Bauer AM, et al. Study to promote innovation in rural integrated telepsychiatry (SPIRIT): rationale and design of a randomized comparative effectiveness trial of managing complex psychiatric disorders in rural primary care clinics. Contemp Clin Trials. 2020;90:105873. doi: 10.1016/j.cct.2019.105873.
14. Weiner S. Addressing the escalating psychiatrist shortage. AAMC. https://www.aamc.org/news-insights/addressing-escalating-psychiatrist-shortage. Published February 12, 2018. Accessed May 14, 2020.
15. American Psychiatric Association. What is telepsychiatry? https://www.psychiatry.org/patients-families/what-is-telepsychiatry. Published 2017. Accessed May 14, 2020.
16. Yilmaz SK, Horn BP, Fore C, et al. An economic cost analysis of an expanding, multi-state behavioural telehealth intervention. J Telemed Telecare. 2019;25(6):353-364.
17. Bashshur RL, Shannon GW, Bashshur N, et al. The empirical evidence for telemedicine interventions in mental disorders. Telemed J E Health. 2016;22(2):87-113.
18. Lopez A, Schwenk S, Schneck CD, et al. Technology-based mental health treatment and the impact on the therapeutic alliance. Curr Psychiatry Rep. 2019;21(8):76.
19. Schubert NJ, Backman PJ, Bhatla R, et al. Telepsychiatry and patient-provider concordance. Can J Rural Med. 2019;24(3):75-82.
20. Hubley S, Lynch SB, Schneck C, et al. Review of key telepsychiatry outcomes. World J Psychiatry. 2016;6(2):269-282.
21. Mayworm AM, Lever N, Gloff N, et al. School-based telepsychiatry in an urban setting: efficiency and satisfaction with care. Telemed J E Health. 2020;26(4):446-454.
22. Christensen LF, Gildberg FA, Sibbersen C, et al. Videoconferences and treatment of depression: satisfaction score correlated with number of sessions attended but not with age [published online October 31, 2019]. Telemed J E Health. 2019. doi: 10.1089/tmj.2019.0129.
23. Christensen LF, Gildberg FA, Sibbersen C, et al. Disagreement in satisfaction between patients and providers in the use of videoconferences by depressed adults. Telemed J E Health. 2020;26(5):614-620.
24. Hantke N, Lajoy M, Gould CE, et al. Patient satisfaction with geriatric psychiatry services via video teleconference. Am J Geriatr Psychiatry. 2020;28(4):491-494.
25. Goetter EM, Blackburn AM, Bui E, et al. Veterans’ prospective attitudes about mental health treatment using telehealth. J Psychosoc Nurs Ment Health Serv. 2019;57(9):38-43.
26. Vanderpool D. Top 10 myths about telepsychiatry. Innov Clin Neurosci. 2017;14(9-10):13-15.
27. Butterfield A. Telepsychiatric evaluation and consultation in emergency care settings. Child Adolesc Psychiatr Clin N Am. 2018;27(3):467-478.
28. Medicare.gov. Telehealth. https://www.medicare.gov/coverage/telehealth. Accessed May 14, 2020.
29. Centers for Medicare & Medicaid Services. Behavioral Health Services. https://www.medicaid.gov/medicaid/benefits/bhs/index.html. Accessed May 14, 2020.
30. New York Pub Health Law §2999-cc (2017).
1. Spivak S, Spivak A, Cullen B, et al. Telepsychiatry use in U.S. mental health facilities, 2010-2017. Psychiatr Serv. 2019;71(2):appips201900261. doi: 10.1176/appi.ps.201900261.
2. Reliford A, Adebanjo B. Use of telepsychiatry in pediatric emergency room to decrease length of stay for psychiatric patients, improve resident on-call burden, and reduce factors related to physician burnout. Telemed J E Health. 2019;25(9):828-832.
3. Mathiasen K, Riper H, Andersen TE, et al. Guided internet-based cognitive behavioral therapy for adult depression and anxiety in routine secondary care: observational study. J Med Internet Res. 2018;20(11):e10927. doi: 10.2196/10927.
4. Waugh M, Calderone J, Brown Levey S, et al. Using telepsychiatry to enrich existing integrated primary care. Telemed J E Health. 2019;25(8):762-768.
5. Swanson CL, Trestman RL. Rural assertive community treatment and telepsychiatry. J Psychiatr Pract. 2018;24(4):269-273.
6. Gentry MT, Lapid MI, Rummans TA. Geriatric telepsychiatry: systematic review and policy considerations. Am J Geriatr Psychiatry. 2019;27(2):109-127.
7. Christensen LF, Moller AM, Hansen JP, et al. Patients’ and providers’ experiences with video consultations used in the treatment of older patients with unipolar depression: a systematic review. J Psychiatr Ment Health Nurs. 2020;27(3):258-271.
8. Marcus S, Malas N, Dopp R, et al. The Michigan Child Collaborative Care program: building a telepsychiatry consultation service. Psychiatr Serv. 2019;70(9):849-852.
9. Kaye DL, Fornari V, Scharf M, et al. Description of a multi-university education and collaborative care child psychiatry access program: New York State’s CAP PC. Gen Hosp Psychiatry. 2017;48:32-36.
10. Larson JL, Rosen AB, Wilson FA. The effect of telehealth interventions on quality of life of cancer patients: a systematic review and meta-analysis. Telemed J E Health. 2018;24(6):397-405.
11. Peter L, Reindl R, Zauter S, et al. Effectiveness of an online CBT-I intervention and a face-to-face treatment for shift work sleep disorder: a comparison of sleep diary data. Int J Environ Res Public Health. 2019;16(17):E3081. doi: 10.3390/ijerph16173081.
12. LaBelle B, Franklyn AM, Pkh Nguyen V, et al. Characterizing the use of telepsychiatry for patients with opioid use disorder and cooccurring mental health disorders in Ontario, Canada. Int J Telemed Appl. 2018;2018(3):1-7.
13. Fortney JC, Heagerty PJ, Bauer AM, et al. Study to promote innovation in rural integrated telepsychiatry (SPIRIT): rationale and design of a randomized comparative effectiveness trial of managing complex psychiatric disorders in rural primary care clinics. Contemp Clin Trials. 2020;90:105873. doi: 10.1016/j.cct.2019.105873.
14. Weiner S. Addressing the escalating psychiatrist shortage. AAMC. https://www.aamc.org/news-insights/addressing-escalating-psychiatrist-shortage. Published February 12, 2018. Accessed May 14, 2020.
15. American Psychiatric Association. What is telepsychiatry? https://www.psychiatry.org/patients-families/what-is-telepsychiatry. Published 2017. Accessed May 14, 2020.
16. Yilmaz SK, Horn BP, Fore C, et al. An economic cost analysis of an expanding, multi-state behavioural telehealth intervention. J Telemed Telecare. 2019;25(6):353-364.
17. Bashshur RL, Shannon GW, Bashshur N, et al. The empirical evidence for telemedicine interventions in mental disorders. Telemed J E Health. 2016;22(2):87-113.
18. Lopez A, Schwenk S, Schneck CD, et al. Technology-based mental health treatment and the impact on the therapeutic alliance. Curr Psychiatry Rep. 2019;21(8):76.
19. Schubert NJ, Backman PJ, Bhatla R, et al. Telepsychiatry and patient-provider concordance. Can J Rural Med. 2019;24(3):75-82.
20. Hubley S, Lynch SB, Schneck C, et al. Review of key telepsychiatry outcomes. World J Psychiatry. 2016;6(2):269-282.
21. Mayworm AM, Lever N, Gloff N, et al. School-based telepsychiatry in an urban setting: efficiency and satisfaction with care. Telemed J E Health. 2020;26(4):446-454.
22. Christensen LF, Gildberg FA, Sibbersen C, et al. Videoconferences and treatment of depression: satisfaction score correlated with number of sessions attended but not with age [published online October 31, 2019]. Telemed J E Health. 2019. doi: 10.1089/tmj.2019.0129.
23. Christensen LF, Gildberg FA, Sibbersen C, et al. Disagreement in satisfaction between patients and providers in the use of videoconferences by depressed adults. Telemed J E Health. 2020;26(5):614-620.
24. Hantke N, Lajoy M, Gould CE, et al. Patient satisfaction with geriatric psychiatry services via video teleconference. Am J Geriatr Psychiatry. 2020;28(4):491-494.
25. Goetter EM, Blackburn AM, Bui E, et al. Veterans’ prospective attitudes about mental health treatment using telehealth. J Psychosoc Nurs Ment Health Serv. 2019;57(9):38-43.
26. Vanderpool D. Top 10 myths about telepsychiatry. Innov Clin Neurosci. 2017;14(9-10):13-15.
27. Butterfield A. Telepsychiatric evaluation and consultation in emergency care settings. Child Adolesc Psychiatr Clin N Am. 2018;27(3):467-478.
28. Medicare.gov. Telehealth. https://www.medicare.gov/coverage/telehealth. Accessed May 14, 2020.
29. Centers for Medicare & Medicaid Services. Behavioral Health Services. https://www.medicaid.gov/medicaid/benefits/bhs/index.html. Accessed May 14, 2020.
30. New York Pub Health Law §2999-cc (2017).
Changes in patient behavior during COVID-19: What I’ve observed
Unprecedented circumstances, extraordinary times, continental shift, life-altering experience—the descriptions of the coronavirus disease 2019 (COVID-19) pandemic have been endless, and accurate. Every clinician who has cared for patients during these trying times has noticed new patterns in patient behavior. Psychiatrists are acutely aware of the emotional, behavioral, and cognitive methods that patients are using to protect themselves from the chaos around them, and the ways in which they process a societal catastrophe such as COVID-19 (Figure). Here are some new patterns I have noticed among my own patients.
Physical and emotional separation
I first noticed the changes in my patients’ behavior at the front desk, where they now spend less time talking with the staff. They bring their own pens for filling out the paperwork, avoid touching items around them, and try to keep social interactions brief and to the point. Patients have been more cooperative about scheduling and rescheduling their appointments. They have generally been nicer to the staff, frequently thanking us for the work we do, and verbalizing their support for health care professionals in general.
Patients have been more supportive of their family members and other patients in the clinic, with some noticeable exceptions, such as maintaining social distancing for their own comfort and safety. Some patients wear face masks not just for safety but also to separate themselves and hide their emotions from the world. This allows them to feel more emotionally secure when interacting with other people.
The use of telehealth has given many patients the security of not having to leave their home, and the decreased need for travel adds to their comfort.
Changes I didn’t expect
The COVID-19 pandemic has resulted in some unexpected changes in my patients. Only a minority of my patients have expressed increased anxiety, while most have become less anxious overall on issues other than the pandemic. Many of my patients who have stressful jobs, especially teachers, say they feel more comfortable working from home and have less anxiety and depression because they are removed from their daily stressors. There also has been an increase in patients’ use of humor, including inappropriate humor, to defend against their fear of COVID-19.
Our clinic is a multidisciplinary facility that specializes in integrating mental and physical health treatments for pain, and for some patients, increased anxiety is clearly associated with an increase in pain. However, during the COVID-19 pandemic, patients have recognized this connection and verbalized their concerns. Some somatic patients have had a decrease in their physical symptoms, including chronic pain, because they see that the whole world is not well, which somehow helps to validate their concerns.
The changes in our patients’ psychological well-being will likely continue to morph as we enter a more stable period. The eventual resolution of the pandemic will bring further changes to our patients’ emotional lives. As we go through these times together, we will continue to uncover new ways that our patients will use to defend themselves against stress and adversities.
Unprecedented circumstances, extraordinary times, continental shift, life-altering experience—the descriptions of the coronavirus disease 2019 (COVID-19) pandemic have been endless, and accurate. Every clinician who has cared for patients during these trying times has noticed new patterns in patient behavior. Psychiatrists are acutely aware of the emotional, behavioral, and cognitive methods that patients are using to protect themselves from the chaos around them, and the ways in which they process a societal catastrophe such as COVID-19 (Figure). Here are some new patterns I have noticed among my own patients.

Physical and emotional separation
I first noticed the changes in my patients’ behavior at the front desk, where they now spend less time talking with the staff. They bring their own pens for filling out the paperwork, avoid touching items around them, and try to keep social interactions brief and to the point. Patients have been more cooperative about scheduling and rescheduling their appointments. They have generally been nicer to the staff, frequently thanking us for the work we do, and verbalizing their support for health care professionals in general.
Patients have been more supportive of their family members and other patients in the clinic, with some noticeable exceptions, such as maintaining social distancing for their own comfort and safety. Some patients wear face masks not just for safety but also to separate themselves and hide their emotions from the world. This allows them to feel more emotionally secure when interacting with other people.
The use of telehealth has given many patients the security of not having to leave their home, and the decreased need for travel adds to their comfort.
Changes I didn’t expect
The COVID-19 pandemic has resulted in some unexpected changes in my patients. Only a minority of my patients have expressed increased anxiety, while most have become less anxious overall on issues other than the pandemic. Many of my patients who have stressful jobs, especially teachers, say they feel more comfortable working from home and have less anxiety and depression because they are removed from their daily stressors. There also has been an increase in patients’ use of humor, including inappropriate humor, to defend against their fear of COVID-19.
Our clinic is a multidisciplinary facility that specializes in integrating mental and physical health treatments for pain, and for some patients, increased anxiety is clearly associated with an increase in pain. However, during the COVID-19 pandemic, patients have recognized this connection and verbalized their concerns. Some somatic patients have had a decrease in their physical symptoms, including chronic pain, because they see that the whole world is not well, which somehow helps to validate their concerns.
The changes in our patients’ psychological well-being will likely continue to morph as we enter a more stable period. The eventual resolution of the pandemic will bring further changes to our patients’ emotional lives. As we go through these times together, we will continue to uncover new ways that our patients will use to defend themselves against stress and adversities.
Unprecedented circumstances, extraordinary times, continental shift, life-altering experience—the descriptions of the coronavirus disease 2019 (COVID-19) pandemic have been endless, and accurate. Every clinician who has cared for patients during these trying times has noticed new patterns in patient behavior. Psychiatrists are acutely aware of the emotional, behavioral, and cognitive methods that patients are using to protect themselves from the chaos around them, and the ways in which they process a societal catastrophe such as COVID-19 (Figure). Here are some new patterns I have noticed among my own patients.

Physical and emotional separation
I first noticed the changes in my patients’ behavior at the front desk, where they now spend less time talking with the staff. They bring their own pens for filling out the paperwork, avoid touching items around them, and try to keep social interactions brief and to the point. Patients have been more cooperative about scheduling and rescheduling their appointments. They have generally been nicer to the staff, frequently thanking us for the work we do, and verbalizing their support for health care professionals in general.
Patients have been more supportive of their family members and other patients in the clinic, with some noticeable exceptions, such as maintaining social distancing for their own comfort and safety. Some patients wear face masks not just for safety but also to separate themselves and hide their emotions from the world. This allows them to feel more emotionally secure when interacting with other people.
The use of telehealth has given many patients the security of not having to leave their home, and the decreased need for travel adds to their comfort.
Changes I didn’t expect
The COVID-19 pandemic has resulted in some unexpected changes in my patients. Only a minority of my patients have expressed increased anxiety, while most have become less anxious overall on issues other than the pandemic. Many of my patients who have stressful jobs, especially teachers, say they feel more comfortable working from home and have less anxiety and depression because they are removed from their daily stressors. There also has been an increase in patients’ use of humor, including inappropriate humor, to defend against their fear of COVID-19.
Our clinic is a multidisciplinary facility that specializes in integrating mental and physical health treatments for pain, and for some patients, increased anxiety is clearly associated with an increase in pain. However, during the COVID-19 pandemic, patients have recognized this connection and verbalized their concerns. Some somatic patients have had a decrease in their physical symptoms, including chronic pain, because they see that the whole world is not well, which somehow helps to validate their concerns.
The changes in our patients’ psychological well-being will likely continue to morph as we enter a more stable period. The eventual resolution of the pandemic will bring further changes to our patients’ emotional lives. As we go through these times together, we will continue to uncover new ways that our patients will use to defend themselves against stress and adversities.
An unexplained exacerbation of depression, anxiety, and panic
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.
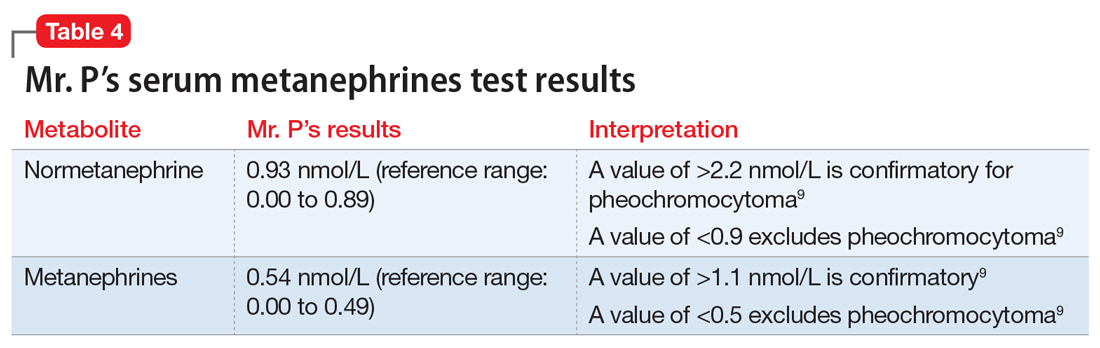
After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.

After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
CASE Depression, anxiety, and panic attacks
At the urging of his parents Mr. P, age 33, presents to the partial hospitalization program (PHP) for worsening depression and anxiety, daily panic attacks with accompanying diaphoresis and headache, and the possibility that he may have taken an overdose of zolpidem. Mr. P denies taking an intentional overdose of zolpidem, claiming instead that he was having a sleep-walking episode and did not realize how many pills he took.
In addition to daily panic attacks, Mr. P reports having trouble falling asleep, overwhelming sadness, and daily passive suicidal ideation without a plan or active intent.
Mr. P cannot identify a specific trigger to this most recent exacerbation of depressed/anxious mood, but instead describes it as slowly building over the past 6 to 8 months. Mr. P says the panic attacks occur without warning and states, “I feel like my heart is going to jump out of my chest; I get a terrible headache, and I sweat like crazy. Sometimes I just feel like I’m about to pass out or die.” Although these episodes had been present for approximately 2 years, they now occur almost daily.
HISTORY Inconsistent adherence
For the last year, Mr. P had been taking alprazolam, 0.5 mg twice daily, and paroxetine, 20 mg/d, and these medications provided moderate relief of his depressive/anxious symptoms. However, he stopped taking both medications approximately 3 or 4 weeks ago when he ran out. He also takes propranolol, 20 mg/d, sporadically, for hypertension. In the past, he had been prescribed carvedilol, clonidine, and lisinopril—all with varying degrees of relief of his hypertension. He denies a family history of hypertension or any other chronic or acute health problems. He reports that he has been sober from alcohol for 19 months but smokes 1 to 2 marijuana cigarettes a day.
EVALUATION Elevated blood pressure and pulse
Mr. P’s physical examination and medical review of systems are unremarkable, except for an elevated blood pressure (190/110 mm Hg) and pulse (92 beats per minute); he also has a headache. A repeat blood pressure test later in the day is 172/94 mm Hg, with a pulse of 100 beats per minute. His urine drug screen is positive only for delta-9-tetrahydrocannabinol (THC).
[polldaddy:10558304]
The author’s observations
A CBC with differential is helpful for ruling out infection and anemia as causes of anxiety and depression.1 In Mr. P’s case, there were no concerning symptoms that pointed to anemia or infection as likely causes of his anxiety, depression, or panic attacks. A TSH level also would be reasonable, because hyperthyroidism can present as anxiety, while hyperthyroidism or hypothyroidism each can present as depression.1 However, both Mr. P’s medical history and physical examination were not concerning for thyroid disease, making it unlikely that he had either of those conditions. A review of Mr. P’s medical records indicated that within the past 6 months, his primary care physician (PCP) had ordered a CBC and TSH test; the results of both were within normal limits.
Serum porphyrin tests can exclude porphyria as a contributor to Mr. P’s anxiety and depression. Porphyrias are a group of 8 inherited disorders that involve accumulation of heme precursors (porphyrins) in the CNS and subcutaneous tissue.2 Collectively, porphyrias affect approximately 1 in 200,000 people.2 Anxiety and depression are strongly associated with porphyria, but do not occur secondary to the illness; depression and anxiety appear to be intrinsic personality features in people with porphyria.3 Skin lesions and abdominal pain are the most common symptoms,3 and there is a higher incidence of hypertension in people with porphyria than in the general population.4 Mr. P does not report any heritable disorders, nor does he appear to have any CNS disturbance or unusual cutaneous lesions, which makes it unlikely that this disorder is related to his psychiatric symptoms.
Continue to: A serum metanephrines test measures...
A serum metanephrines test measures the metabolites of epinephrine and norepinephrine. These catecholamines are produced in excess by an adrenal or extra-adrenal tumor seen in pheochromocytoma. The classic triad of symptoms of pheochromocytoma are hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic (Table 15-7). This type of tumor is rare, with an annual incidence of only 2 to 8 cases per 1 million individuals. Among people with hypertension, the annual incidence is 0.1% to 1.0%, and for those with an adrenal mass, the annual incidence is 5% (Table 26,8). Autopsy studies suggest that up to 50% of pheochromocytomas are undiagnosed.8 Left untreated, pheochromocytoma can result in hypertensive crisis, arrhythmia, myocardial infarction, multisystem organ failure, and premature death.7 Table 36,7 highlights some causes of false-positive serum on metanephrines testing.
EVALUATION Metanephrines testing
Mr. P has what appears to be treatment-resistant hypertension, accompanied by the classic symptoms observed in most patients with pheochromocytoma. Because Mr. P is participating in the PHP 6 days per week for 6 hours each day, visiting his PCP would be inconvenient, so the treatment team orders the serum metanephrines test. If a positive result is found, Mr. P will be referred to his PCP for further assessment and follow-up care with endocrinology.
TREATMENT Pharmacotherapy to target anxiety and panic
Next, the treatment team establishes a safety plan for Mr. P, and restarts paroxetine, 20 mg/d, to target his depressed and anxious mood. Alprazolam, 0.5 mg twice daily, is started to target anxious mood and panic symptoms, and to allow time for the anxiolytic properties of the paroxetine to become fully effective. The alprazolam will be tapered and stopped after 2 weeks. Mr. P is started on hydroxyzine, 1 to 2 25-mg tablets 2 to 3 times daily as needed for anxious mood and panic symptoms.
The serum metanephrines test results are equivocal, with a slight elevation of both epinephrine and norepinephrine that is too low to confirm a diagnosis of pheochromocytoma but too elevated to exclude it (Table 49). With Mr. P’s consent, the treatment team contacts his PCP and convey the results of this test. Mr. P schedules an appointment with his PCP for the following week for further assessment and confirmatory pheochromocytoma testing.

After 1 week, Mr. P remains anxious, with a slight reduction in panic attacks from multiple attacks each day to 3 or 4 attacks per week. The team considers adding an additional anxiolytic agent.
[polldaddy:10558305]
Continue to: The author's observations
The author’s observations
The triad of symptoms in pheochromocytoma results directly from the intermittent release of catecholamines into systemic circulation. Surges of epinephrine and norepinephrine lead to headaches, palpitations, diaphoresis, and (less commonly) gastrointestinal symptoms such as nausea, vomiting, and constipation. Persistent or episodic hypertension may be present, with 13% of patients maintaining a normal blood pressure.5-7 Patients with pheochromocytoma-related anxiety typically have substantial or complete resolution of anxiety and panic attacks after tumor resection.6,8,10
Because of their ability to raise catecholamine levels, several medications, including some psychotropics, can lead to false-positive results on serum and urine metanephrines testing. Tricyclic antidepressants and beta-blockers can cause false-positive results on plasma assays, while buspirone can cause false-positives on urinalysis assays.5 Trazodone, on the other hand, exhibits no catecholaminergic activity and its alpha-1 adrenergic antagonism may actually have some benefit in pheochromocytoma.11 Alpha-1 adrenergic antagonism with doxazosin, prazosin, or terazosin is the first-line of treatment in reducing pheochromocytoma-related hypertension.7 Treatment with a beta-blocker is safe only after alpha-adrenergic blockade occurs. While beta-blockers are useful for reducing the palpitations and anxiety observed in patients with pheochromocytoma, they must not be used alone due to the risk of hypertensive crisis resulting from unopposed alpha-adrenergic agonist activated vasoconstriction.5,7
TREATMENT CBT provides benefit
Mr. P decides against receiving an additional agent for anxiety and instead decides to wait for the outcome of the confirmatory pheochromocytoma testing. He continues to take alprazolam, and both his depressed mood and anxiety improve. His panic attacks continue to lessen, and he appears to benefit from cognitive-behavioral therapy provided during group therapy. Mr. P is advised by his PCP to taper and stop the alprazolam 3 to 5 days before his 24-hour urine metanephrines test because benzodiazepines can lead to false-positive results on a urinalysis assay.7
OUTCOME Remission of anxiety and depression
Mr. P has a repeat serum metanephrines test and a 24-hour urinalysis assay. Both are negative for pheochromocytoma. His PCP refers him to cardiology for management of treatment-resistant hypertension. He is discharged from the PHP and continues psychotherapy for depression and anxiety in an intensive outpatient program (IOP). Throughout his PHP and IOP treatments, he continues to take paroxetine and hydroxyzine. He achieves a successful remission of his anxiety and depression, with partial but significant remission of his panic attacks.
The author’s observations
Although Mr. P did not have pheochromocytoma, it is important to rule out this rare condition in patients who present with treatment-resistant hypertension and/or treatment-resistant anxiety.
Continue to: Bottom Line
Bottom Line
Pheochromocytoma is a tumor of the adrenal gland. The classic triad of symptoms of this rare condition is hypertension, sweating, and headache; approximately 30% of patients report significant anxiety and panic. Several medications, including tricyclic antidepressants, beta-blockers, and buspirone, can lead to false-positive results on the serum and urine metanephrines testing used to diagnose pheochromocytoma.
Related Resources
- National Organization for Rare Disorders. Rare Disease Database: pheochromocytoma. www.rarediseases.org/rare-diseases/pheochromocytoma/.
- Young WF Jr. Clinical presentation and diagnosis of pheochromocytoma. UpToDate. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-pheochromocytoma. Published January 2020.
Drug Brand Names
Alprazolam • Xanax
Amitriptyline • Elavil
Buspirone • Buspar
Carvedilol • Coreg
Clonidine • Catapres
Doxazosin • Cardura
Hydroxyzine • Vistaril
Lisinopril • Prinivil, Zestril
Paroxetine • Paxil
Prazosin • Minipress
Propranolol • Inderal
Terazosin • Hytrin
Trazodone • Desyrel
Zolpidem • Ambien
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
1. Morrison J. When psychological problems mask medical disorders: a guide for psychotherapists. 2nd ed. New York, NY: The Guilford Press; 2015.
2. American Porphyria Foundation. About porphyria. https://porphyriafoundation.org/patients/about-porphyria. Accessed May 13, 2020.
3. Millward L, Kelly P, King A, et al. Anxiety and depression in the acute porphyrias. J Inherit Metab Dis. 2005;28(6):1099-1107.
4. Bonkovsky H, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med. 2014;127(12):1233-1241.
5. Tsirlin A, Oo Y, Sharma R, et al. Pheochromocytoma: a review. Maturitas. 2014;77(3):229-238.
6. Leung A, Zun L, Nordstrom K, et al. Psychiatric emergencies for physicians: clinical management and approach to distinguishing pheochromocytoma from psychiatric and thyrotoxic diseases in the emergency room. J Emerg Med. 2017;53(5):712-716.
7. Garg M, Kharb S, Brar KS, et al. Medical management of pheochromocytoma: role of the endocrinologist. Indian J Endocrinol and Metab. 2011;15(suppl 4):S329-S336. doi: 10.4103/2230-8210.86976.
8. Zardawi I. Phaeochromocytoma masquerading as anxiety and depression. Am J Case Rep. 2013;14:161-163.
9. ARUP Laboratories. Test directory. https://www.aruplab.com. Accessed February 11, 2020.
10. Sriram P, Raghavan V. Pheochromocytoma presenting as anxiety disorder: a case report. Asian J Psychiatr. 2017;29:83-84.
11. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press; 2013.
Cannabidiol for psychosis: A review of 4 studies
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
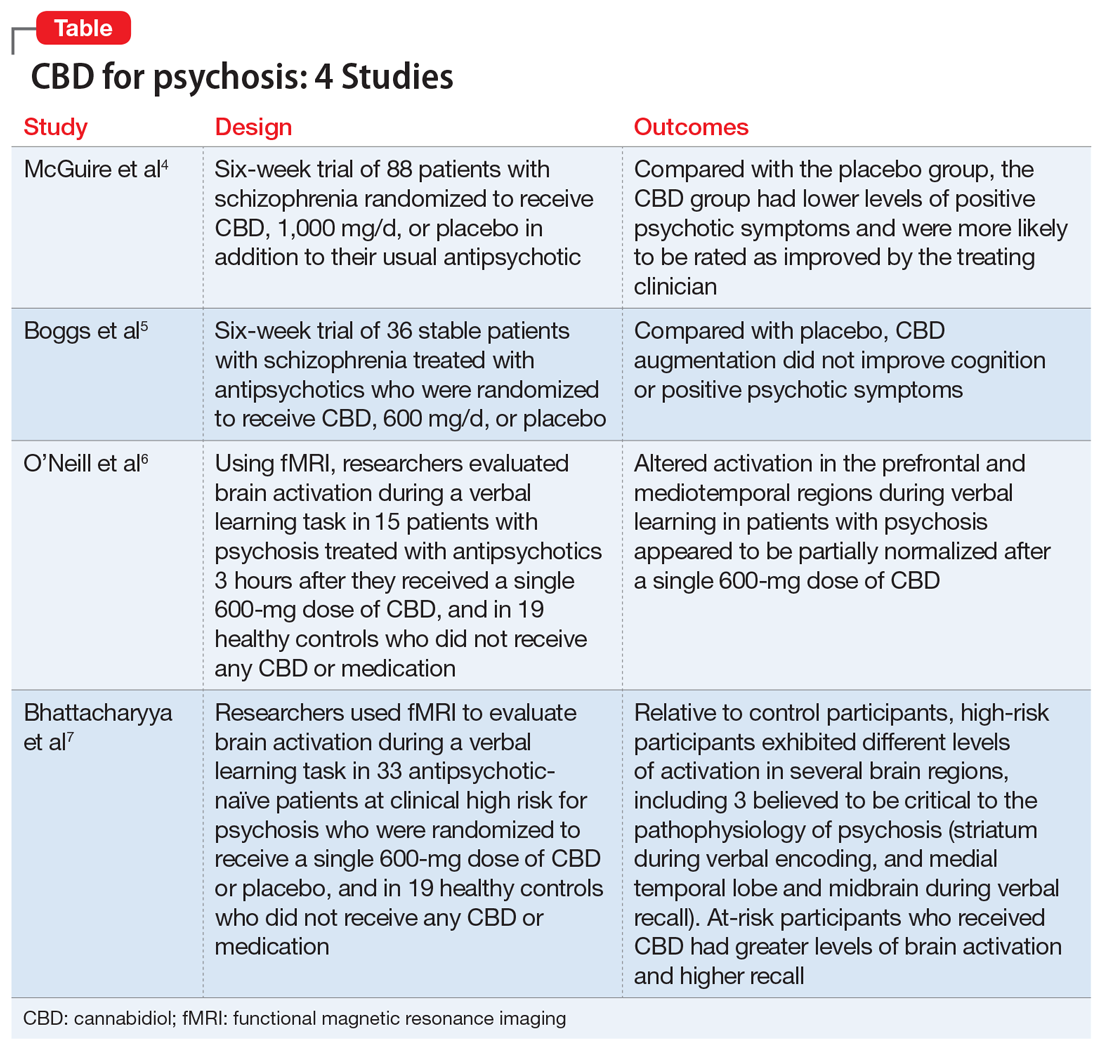
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7

Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7

Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
Stop calling it ‘behavioral health’: Psychiatry is much more
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
Psychiatry has been historically plagued by absurd misnomers. It started with the laughable “mental hygiene,” coined by William Sweetser, MD, in 1843, 1 year before the original 13 members of the Association of Medical Superintendents of American Institutions for the Insane established what in 1921 was renamed the American Psychiatric Association. Mental hygiene evokes an image of psychiatrists scrubbing the brains of mentally ill patients with soap and water! That term was neither medically nor scientifically appropriate, but it stuck for decades.
Enter “mental health.” In 1949, the National Institute of Mental Health was established. It is the 5th oldest of the 27 Institutes and Centers of the National Institutes of Health. Then, in 1963, Congress passed the Community Mental Health Act, which established Community Mental Health Centers around the country. It is perplexing that the term “health” was used instead of “illness,” when psychiatry is a medical specialty that treats mental disorders. Health is certainly the goal of all medical specialties, but cardiology was never called “heart health,” neurology was never called “brain health,” and pediatrics was never called “children’s health.” Like all its sister medical specialties, psychiatry treats disease and syndromes, but somehow, it has been transmogrified into “mental health.” Perhaps it was meant to be a euphemism to disguise and avert the unfortunate stigma associated with mental illness back during the institutionalization era.
The advent of ‘behavioral health’
Then suddenly, the term “behavioral health” was coined and began to be used as a substitute for psychiatry, further distorting psychiatry’s medical identity. Behavioral health is completely different from psychiatry. It refers to healthy behaviors that people should uphold throughout their lives to maintain their overall health and well-being, including eating a balanced diet, exercising regularly, avoiding tobacco and drugs of abuse, practicing safe sex, and establishing meaningful social relationships. So behavioral health promotes a healthy lifestyle, and that could very aptly apply to cardiology, pulmonology, nephrology, or hepatology, where good nutrition and avoiding weight gain, smoking, and sedentary living can reduce the risk for various medical diseases and early mortality. For dermatologists, behavioral health is avoiding sunburn, and for dentists, it is regular brushing and flossing.
Thus, behavioral health is a term that broadly promotes physical health and well-being, and should not be conflated with mental disorders. It is by no means synonymous with psychiatry, a medical discipline that addresses serious disorders of thought, emotions, affect, delusions, hallucinations, suicide, homicide, impulsivity, obsessions and compulsions, motivation, memory, attention, and judgment. Psychiatry is far more than behaviors that promote healthy living. Psychiatry contends with acute and chronic mental disorders, similar to other chronic medical conditions such as chronic heart, lung, gastrointestinal, or kidney diseases. Psychiatric disorders can emerge in individuals despite—and irrespective of—a healthy lifestyle promoted by behavioral health. Most psychiatric disorders have been shown to be highly genetic, and can be triggered by gene-environment interactions, even in the context of a healthful life that behavioral health advocates and fecundates.
I dislike conspiracy theories, but it is legitimate to inquire: Was there a “malicious intent” by insurance companies and managed-care entities when they abruptly replaced the medically accurate term “psychiatry” with the counterfactual “behavioral health”? Did they intend to portray psychiatry as somehow “different” from other medical specialties? Did this phraseological acrobatics facilitate and justify the carving out of psychiatric and addiction care, cursed with an anemic budget and absence of parity for persons with psychiatric brain disorders? Somehow, using behavioral health instead of psychiatry has the unfortunate connotation that patients with mental illness are “misbehaving” by not practicing healthy living, rather than being genuinely medically ill through no fault of their own. That’s a surreptitious de-medicalization of psychiatric brain disorders. It is very likely that the same companies that propagated behavioral health are the ones who came up with the demeaning term “providers,” which lumps physicians with nonphysicians, diluting the medical identify of psychiatrists, and implying a non-equivalence of psychiatric disorders with other medical conditions, which perpetuates stigma.
An erroneous epithet
We are psychiatric physicians, not “behavioral health advisors.” We are graduates of medical schools where we had clinical psychiatric experiences rotating with internal medicine, surgery, obstetrics and gynecology, and pediatrics. We did not have behavioral health rotations. And after graduating with an MD, we spent 4 additional years in psychiatric residency training, not behavioral health training, and we treated very sick patients in emergency departments and on inpatient units, not on behavioral health wards. We receive our board certification from the American Board of Psychiatry and Neurology, not from a behavioral health board. As psychiatrists, we are regularly consulted on the cases of medical and surgical patients who develop psychiatric disorders, which has absolutely nothing to do with behavioral health. Our psychiatric outpatient clinics require extensive medical knowledge and psychopharmacological skills, not behavioral health.
As part of our work as physicians and psychiatrists, we do counsel patients on adopting a healthy lifestyle because many of them have comorbid medical conditions such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, asthma, and kidney and gastrointestinal disorders. We practice collaborative care with primary care physicians so we can jointly manage patients’ physical and mental disorders, and help them optimize their lifestyles. Thus, behavioral health is a tiny component of what psychiatrists do, and it does not come close to defining our comprehensive medical care. Similarly, neurologists and cardiologists should not be labeled as behavior health specialties simply because they counsel their patients on how to lower the risk of strokes or heart attacks due to unhealthy lifestyles.
So, let’s call a spade a spade. Psychiatry is psychiatric medical care, not behavioral health. Let’s abandon this erroneous epithet and change the signs outside hospitals and clinics to “psychiatric medicine” facilities. I guarantee that orthopedists would not like it all if you call their specialty “bone health,” and may break your leg if you label their discipline “bone hygiene”… after washing it with soap and water, of course!
The injustice of pre-authorization
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
I agree with Dr. Nasrallah’s clear description of the malign nature of the pre-authorization system, as described in his editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
As an example of the latter, I was recently told by a pharmacist that I needed to call the insurer to justify why a patient was going from a prescription for #30 citalopram to #45 citalopram. The request had triggered a quantity limit. The pharmacist had explained to the insurer that more pills were required because the dosage was being lowered from 40 to 30 mg/d. Because there are no 30-mg tablets available, it made most sense for the patient to take one and a half 20-mg tablets, which totals 45 pills per month.
The insurer—probably a screener, not a pharmacist—would not accept that explanation and insisted that I call them myself. I bitterly resented how casually the insurer expected busy doctors to interrupt their clinical work to comply with arbitrary micromanagement of pill quantities! I’ve seldom seen such nonsense in more than 40 years of practice.
When doctors call these insurers, they are connected to a screener, but never a pharmacist. The screener asks a series of questions prompted by a computer. We give them verbal answers, but they don’t comprehend what they input into their system. The reasons we give to the screener may not even make it into the report that the screener passes on to the staff member who makes the decision. The doctor is not told what is in the report, or who is reviewing it. So much for transparency in this era that supposedly values it!
In any case, answering all the computer-prompted questions can take a long time. And time, as we know (but they do not), is not elastic.
Serious consequences may ensue if an insurer denies coverage for the doctor’s first choice. Many patients cannot afford to pay hundreds of extra dollars out of pocket. The insurer may ask the doctor to choose a different medication. Aside from the disrespect for the doctor’s decision implied by such a request, another problem is that the patient knows the new medication is his/her doctor’s second (or third) choice. Any positive placebo effect that may have existed before has now been lost. Most doctors would be glad to have a positive placebo effect augmenting the physiologic effects of the medication, especially when the patient is already feeling helpless or hopeless. These negative feelings would likely increase when the patient feels pressured into starting a medication that they know was their doctor’s second choice.
These are just a few reasons pre-authorization is a horrid system; Dr. Nasrallah covered many others in his editorial. The system, as currently structured, needs to be eliminated.
Arthur Mode, MD
Private psychiatric practice
Falls Church, Virginia
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Hooray for...
Hooray for Dr. Nasrallah’s editorial about pre-authorization! I worry, however, that he missed some important considerations.
He writes, “The welfare of the patient is not on the insurance company’s radar screen, perhaps because it is crowded out by dollar signs.” But the welfare of the patient is exactly what is on their radar screens! If the patient dies, the insurance company profits, because it will not have to pay for treatment. This is like having a Red Sox employee manage the Yankees, except we are talking about human lives, not baseball games. Dr. Nasrallah asks (but does not answer), “How did for-profit insurance companies empower themselves to tyrannize clinical practice so that the treatment administered isn’t customized to the patient’s need but instead to fatten the profits of the insurance company?” The answer: Physicians let them. Many physicians are paid by insurers directly or through work for clinics or hospitals. He who pays the piper calls the tune. And because employers often select the insurer, patients have no say.
Honesty is most important. Pre-authorization is a dishonest term, because pre-authorization actually is pre-denial. The term pre-authorization should be replaced by “pre-denial.” It is also fraudulent when insurance companies call themselves health care companies, because they only provide insurance, not health care. Similarly, the term “evidence-based medicine” is typically only an excuse that insurers use to refuse to cover the cost of treatment. In another scenario of Dr. Nasrallah’s patient with treatment-resistant depression who responded to modafinil, what if the evidence for using this medication was based on the patient’s psychiatric history alone, without any evidence from a meta-analysis of randomized controlled trials? That would not be “evidence-based” in the dishonest world of insurance. Evidence to insurers does not include what is evident in the patient’s response to a given treatment.
What about amnesty, especially for physicians who work in the so-called pre-authorization denial business? Some even claim to be peers (ie, the “peer to peer reviews” they conduct) and insist they cannot be on speakerphone, so that their identity is kept secret from the patient. Not all of these “physicians” are incompetent. Not all of them have criminal minds or lack empathy. Some may have had exceptional circumstances leading them to such a profession, which Dr. Nasrallah correctly notes as felonious behavior. For these physicians, I think some kind of amnesty program would be appropriate, rather than prosecution.
John Jacobs, MD
Private psychiatric practice
Manchester, New Hampshire
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: I have just finished reading...
I have just finished reading Dr. Nasrallah’s editorial about pre-authorization. I agree with everything he said, but I do have a couple of comments:
1. Many of our colleagues do not accept insurance because their practices operate on a cash basis. This seems to obviate the problem of pre-authorization, and suggests that if we truly want to get rid of pre-authorization, we should get rid of insurance.
2. In practices that do not accept insurance, some patients may be filing their own insurance claims. Do you have any information on this approach? Are patients able to apply pressure to their insurance companies? Do patients get frustrated with their insurance companies and pay cash, rather than trying to negotiate with their insurance companies?
Katherine Hankins, MD
Private psychiatric practice
Omaha, Nebraska
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Why not address...
Why not address the underlying (and actual) cause of the “pre-authorization” scam/scandal: the private health insurance industry.
Other countries in the western world have figured out how to provide guaranteed health care to their citizens without resorting to a costly insurance industry. This parasitic business suborns 10% to 20% of the health care bill while wasting our money on withholding health care deemed “not eligible” for patients who need it. Meanwhile, the executives who manage this insurance racket are paid enormous salaries not to deliver services.
Moreover, we reap a double loss to the health care system because hospitals must employ a building full of clerks to submit (and then, when rejected, re-submit) bills for reimbursement of hospital charges.
Franz Kafka would immediately grasp the despicable workings of this self-serving scheme.
David Link, MD
Associate Professor of Pediatrics
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to all my colleagues who commented on (and unanimously agreed with) my editorial. It is clearly one of the most outrageous hurdles that all psychiatric practitioners face every day.
For the sake of our patients who deserve optimal medical care (laboratory tests, procedures, and medications), insurance companies must be tightly regulated to avoid second-guessing the treating clinicians, and readily cover what is prescribed. Some patients who can afford it resort to paying out of pocket for privacy reasons or for rapid access to psychiatric care, and may or may not file for insurance coverage, but they will certainly receive what their psychiatrist deems appropriate after a direct evaluation.
I hope the American Psychiatric Association and American Medical Association will continue to forcefully pursue legislation to eliminate pre-authorization and restore some sanity to the critical process of good clinical care.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri