User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Methotrexate is a safe and efficacious alternative to ciclosporin in children with severe AD
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Key clinical point: Both ciclosporin and methotrexate were effective against severe atopic dermatitis (AD) in children, but ciclosporin resulted in a more rapid response whereas methotrexate led to more sustained disease control even after treatment discontinuation.
Major finding: At 12 weeks, a significantly higher proportion of patients achieved 50% improvement in Objective Severity Scoring of Atopic Dermatitis scores (o-SCORAD-50) with ciclosporin vs methotrexate (P = .012). However, at 60 weeks, the proportion of patients who achieved o-SCORAD-50 was higher with methotrexate vs ciclosporin (P = .022). Adverse event rates were comparable in both groups.
Study details: The TREatment of severe Atopic Eczema Trial included 103 children with severe AD (age 2-16 years) who were unresponsive to topical treatments and were randomly assigned to receive ciclosporin or methotrexate.
Disclosures: This study was funded by the UK Medical Research Council/National Institute for Health Research (NIHR). Some authors, including the lead author, declared receiving consulting fees, advisory fees, or research funding from various sources, including UK NIHR.
Source: Flohr C et al and the TREAT Trial Investigators. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre, parallel group, assessor-blinded clinical trial. Br J Dermatol. 2023 (Sep 19). doi: 10.1093/bjd/ljad281
Children with atopic dermatitis more prone to allergic contact dermatitis
Key clinical point: Compared with children without atopic dermatitis (AD), those with AD are significantly more likely to have positive patch tests (PPT) and respond to ≥1 allergen on patch testing.
Major finding: Children with vs without AD were significantly more likely to have a longer duration of dermatitis (P < .0001), >1 PPT result (P = .005), a greater number of PPT overall (P = .012), and a more generalized distribution of dermatitis (P = .001) as well as PPT to bacitracin (P = .030), carba mix (diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethyldithiocarbamate) (P = .025), and cocamidopropyl betaine (P = .0007).
Study details: This retrospective case-control study included 615 children with AD and 297 children without AD.
Disclosures: This study was supported by the Dermatology Foundation, Evanston, IL. The authors declared no conflicts of interest.
Source: Johnson H et al. Prevalence of allergic contact dermatitis in children with and without atopic dermatitis: A multicenter retrospective case-control study. J Am Acad Dermatol. 2023;89(5):1007-1014 (Sep 25). doi: 10.1016/j.jaad.2023.06.048
Key clinical point: Compared with children without atopic dermatitis (AD), those with AD are significantly more likely to have positive patch tests (PPT) and respond to ≥1 allergen on patch testing.
Major finding: Children with vs without AD were significantly more likely to have a longer duration of dermatitis (P < .0001), >1 PPT result (P = .005), a greater number of PPT overall (P = .012), and a more generalized distribution of dermatitis (P = .001) as well as PPT to bacitracin (P = .030), carba mix (diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethyldithiocarbamate) (P = .025), and cocamidopropyl betaine (P = .0007).
Study details: This retrospective case-control study included 615 children with AD and 297 children without AD.
Disclosures: This study was supported by the Dermatology Foundation, Evanston, IL. The authors declared no conflicts of interest.
Source: Johnson H et al. Prevalence of allergic contact dermatitis in children with and without atopic dermatitis: A multicenter retrospective case-control study. J Am Acad Dermatol. 2023;89(5):1007-1014 (Sep 25). doi: 10.1016/j.jaad.2023.06.048
Key clinical point: Compared with children without atopic dermatitis (AD), those with AD are significantly more likely to have positive patch tests (PPT) and respond to ≥1 allergen on patch testing.
Major finding: Children with vs without AD were significantly more likely to have a longer duration of dermatitis (P < .0001), >1 PPT result (P = .005), a greater number of PPT overall (P = .012), and a more generalized distribution of dermatitis (P = .001) as well as PPT to bacitracin (P = .030), carba mix (diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethyldithiocarbamate) (P = .025), and cocamidopropyl betaine (P = .0007).
Study details: This retrospective case-control study included 615 children with AD and 297 children without AD.
Disclosures: This study was supported by the Dermatology Foundation, Evanston, IL. The authors declared no conflicts of interest.
Source: Johnson H et al. Prevalence of allergic contact dermatitis in children with and without atopic dermatitis: A multicenter retrospective case-control study. J Am Acad Dermatol. 2023;89(5):1007-1014 (Sep 25). doi: 10.1016/j.jaad.2023.06.048
What’s Eating You? Phlebotomine Sandflies and Leishmania Parasites
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4 Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
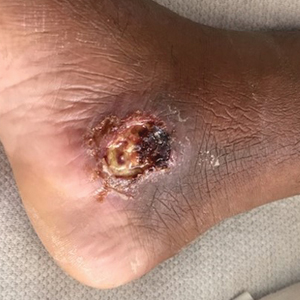
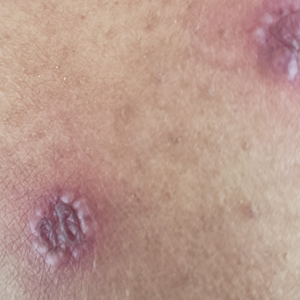
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.
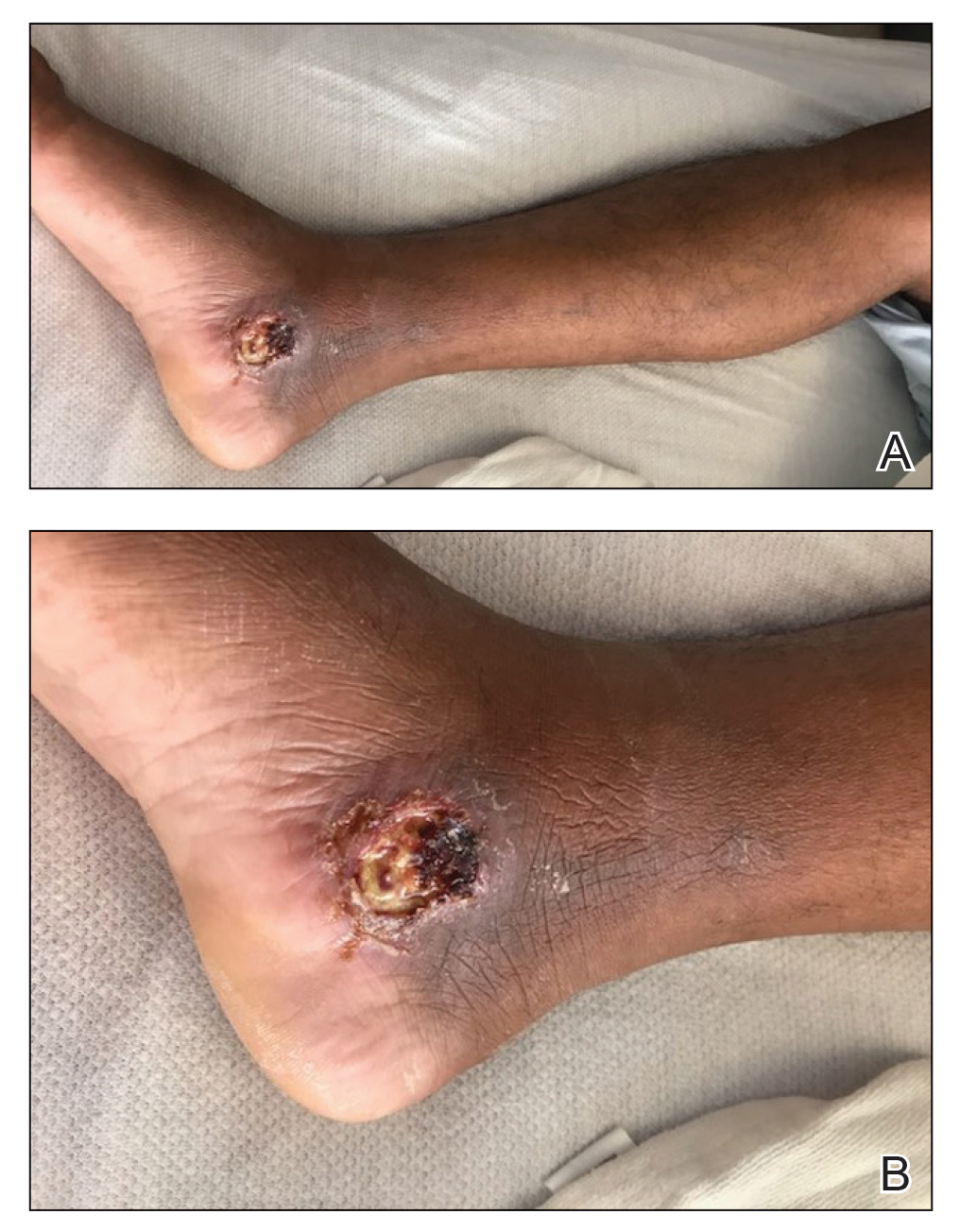
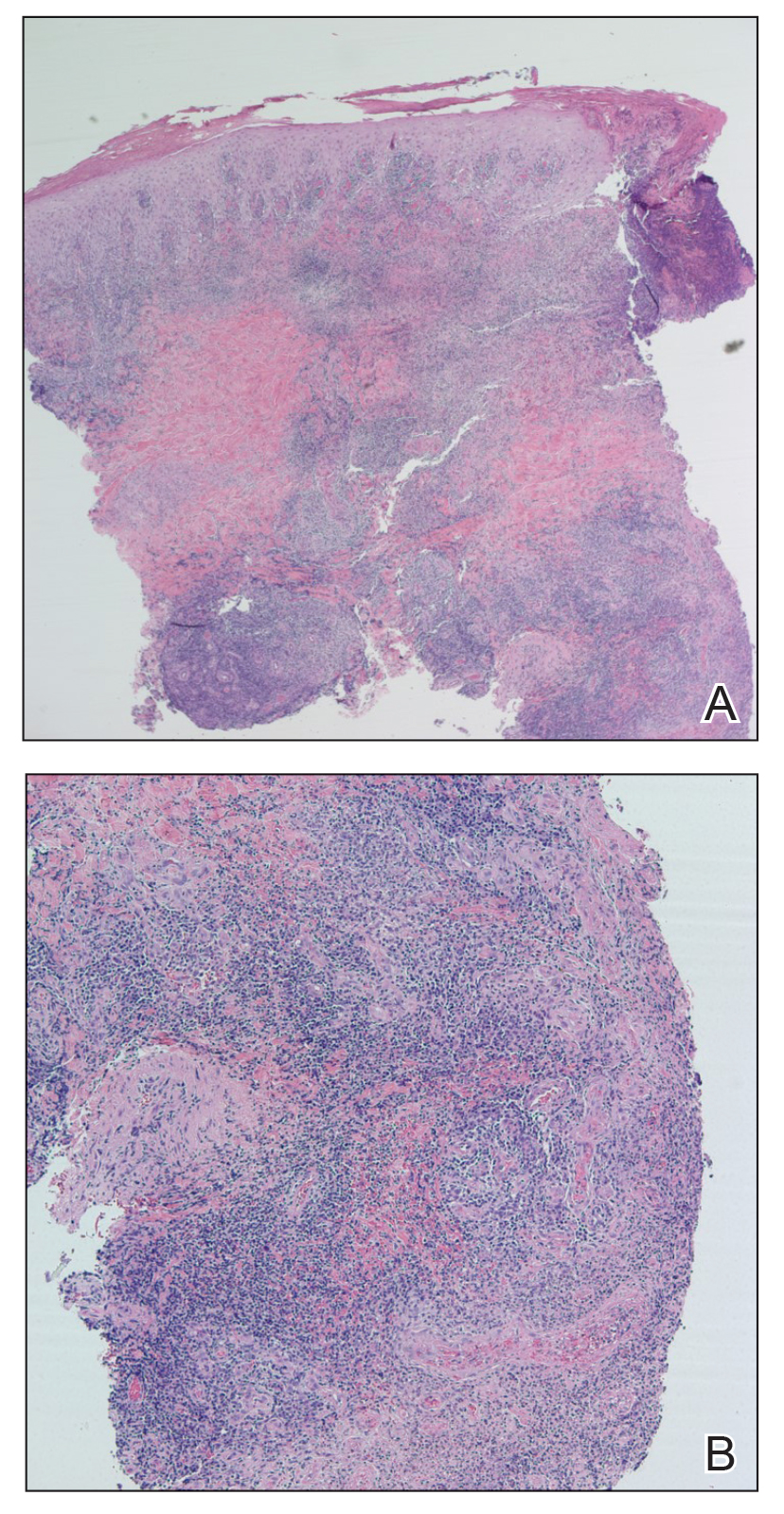
Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.
Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4 Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4 Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
Practice Points
- The Phlebotomus and Lutzomyia genera of sandflies are vectors of Leishmania parasites, which can result in an array of clinical findings associated with leishmaniasis.
- Treatment options for leishmaniasis differ based on whether the infection is considered uncomplicated or complicated, which depends on the species of Leishmania; the number, size, and location of the lesion(s); and host immune status.
- All US practitioners should be aware of this pathogen, especially with regard to patients who have a history of travel to other countries. Health care professionals in states such as Texas and Oklahoma should be especially cognizant because these constitute one of the few areas in the United States where locally acquired cases of leishmaniasis have been reported.
Atopic Dermatitis Triggered by Omalizumab and Treated With Dupilumab
To the Editor:
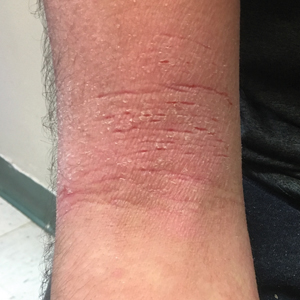
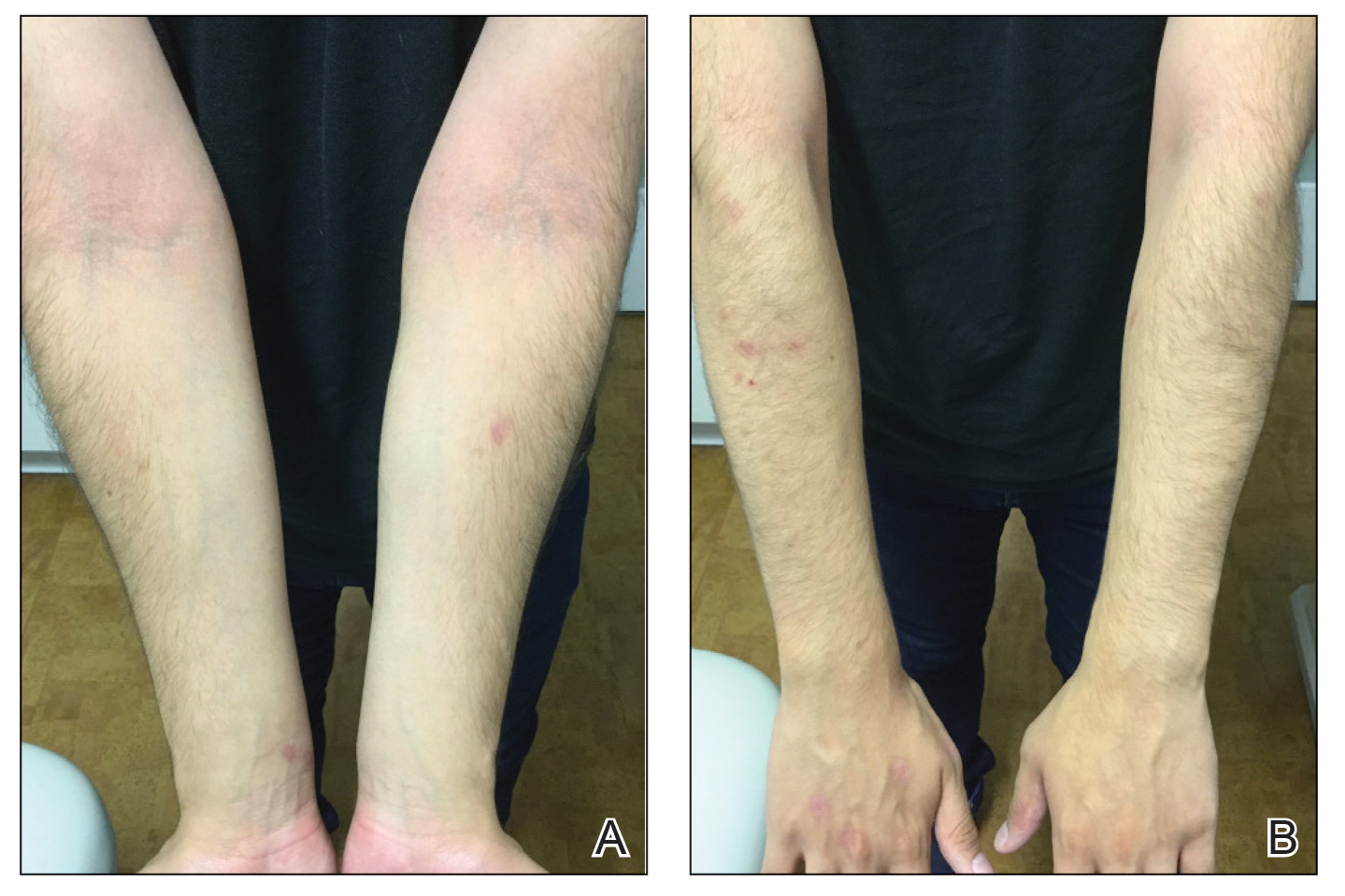
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

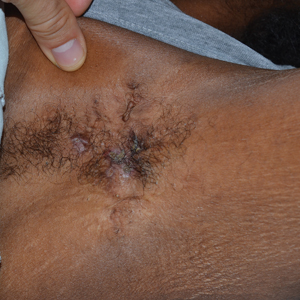
Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.
Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
Practice Points
- Monoclonal antibodies are promising therapies for atopic conditions, although its efficacy for atopic dermatitis (AD) is debated and the side-effect profile is not entirely known.
- Omalizumab may cause a paradoxical exacerbation of AD in select patients analogous to tumor necrosis factor α inhibitor–induced psoriasis.
Suits or joggers? A doctor’s dress code
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Tender Nodular Lesions in the Axilla and Vulva
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
A 28-year-old woman presented with tender burning lesions of the left axillary and vaginal skin that had worsened over the last year. Her medical history was notable for hidradenitis suppurativa, which had been present since adolescence, as well as pulmonary Langerhans cell histiocytosis diagnosed 7 years prior to the current presentation after a spontaneous pneumothorax that eventually led to a pulmonary transplantation 3 years prior. The patient’s Langerhans cell histiocytosis was believed to have resolved without treatment after smoking cessation. Physical examination revealed nodular inflammation and scarring with deep undermining along the left axilla as well as swelling of the mons pubis with erosive skin lesions in the surrounding vaginal area. Bilateral cervical, axillary, inguinal, supraclavicular, and femoral lymph node chains were negative for adenopathy. A shave biopsy was performed on the axillary nodule.
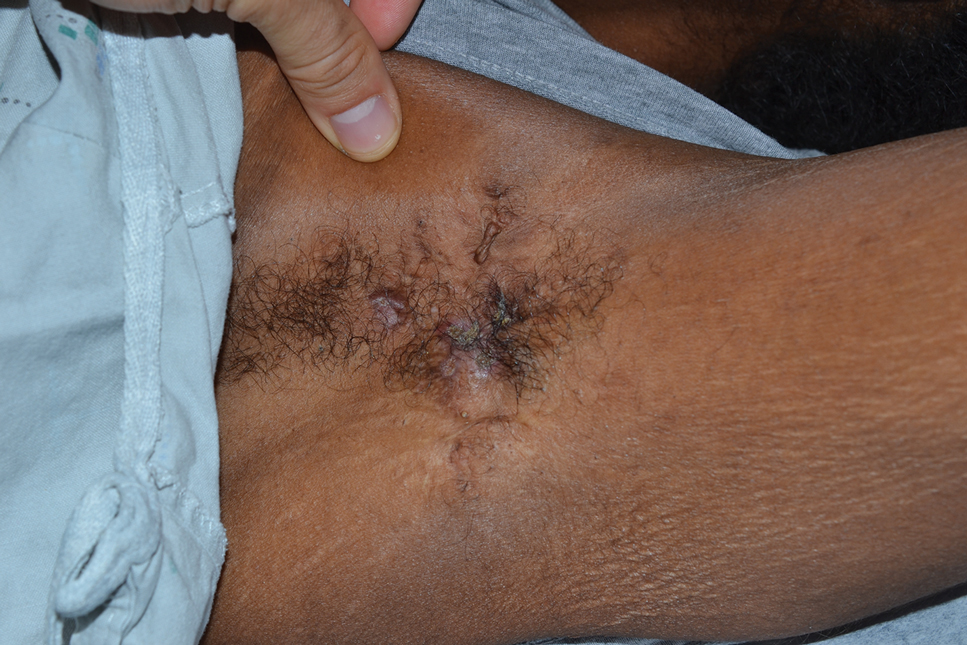
Cutaneous Presentation of Metastatic Salivary Duct Carcinoma
To the Editor:
Metastatic spread of salivary duct carcinoma (SDC) to the skin is rare. Diagnosing SDC can be challenging because the cutaneous manifestations of this disease are variable and include nodules, papules, and erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles. We describe a case of cutaneous metastatic SDC that originated from the parotid gland and presented with 2 distinct cutaneous findings: sharply demarcated erythematous plaques and focally hemorrhagic angiomatous papules.
A 60-year-old man presented with a persistent polymorphous pruritic eruption of several months’ duration involving the entire face, ears, neck, and upper chest. He had a history of unspecified adenocarcinoma of the parotid gland diagnosed 2 years prior and underwent multiple treatment cycles with several chemotherapeutic agents over the course of 18 months. Physical examination showed erythematous papules and nodules on the face and neck with slight overlying scale. Sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules were noted on the neck and chest (Figure 1). Two 4-mm punch biopsies were sampled from representative nodular areas. Histopathology showed multiple round solid-tumor nodules with central necrosis in the superficial and deep dermis that were not associated with the overlying epidermis (Figures 2A and 2B). The tumor cells appeared polygonal and contained ample eosinophilic cytoplasm. Tumor nuclei showed marked pleomorphism, and numerous atypical mitotic figures were readily identifiable (Figure 2C). There was diffuse cytoplasmic staining with cytokeratin 7 and nuclear staining with androgen receptor (Figure 2D). These findings were consistent with a diagnosis of SDC metastatic to the skin.
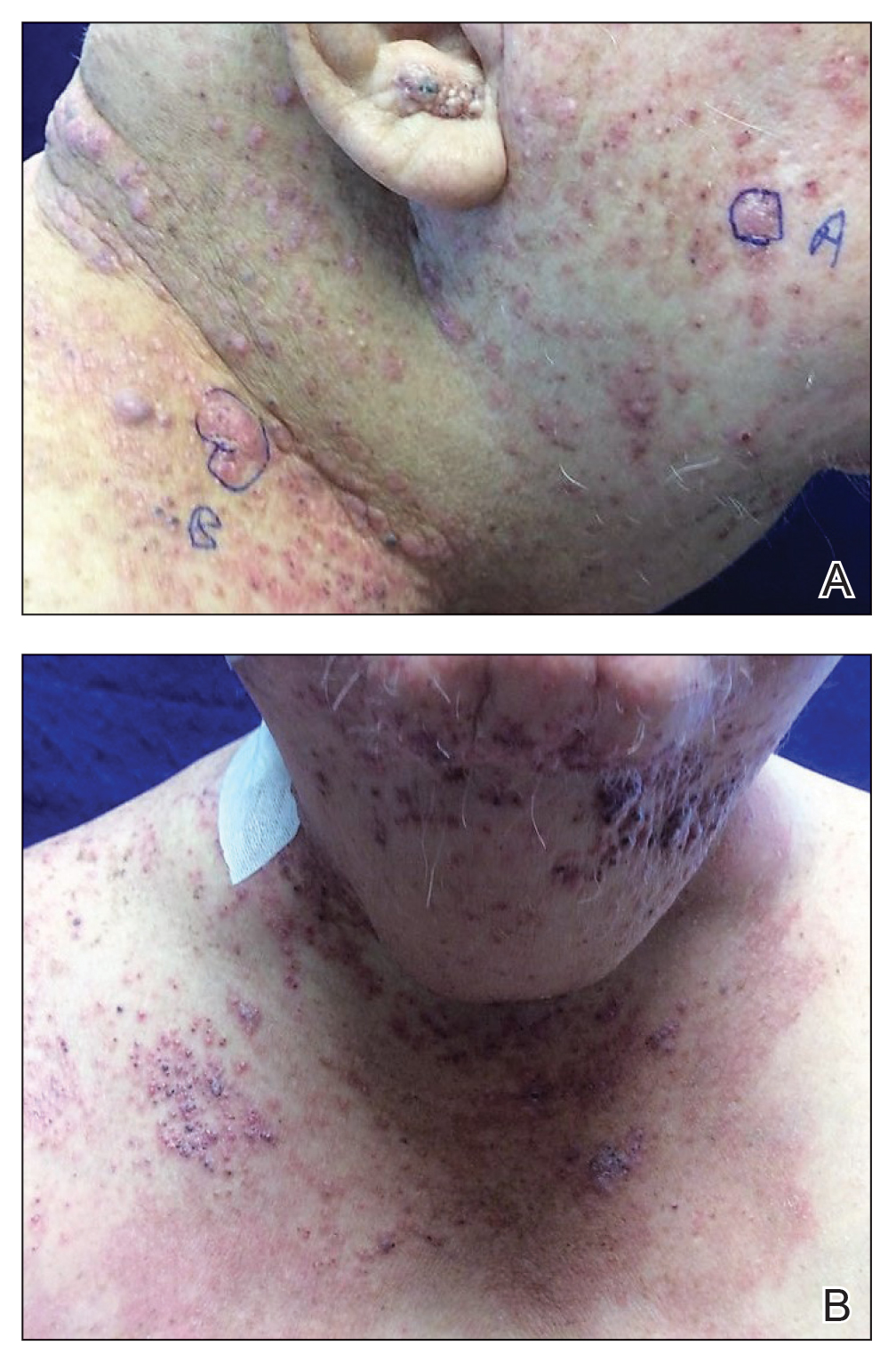
The patient underwent 8 cycles of docetaxel chemotherapy. With disease progression, the chemotherapy regimen was changed to gemcitabine and methotrexate. The patient continued to experience disease progression and died 9 months after diagnosis of skin metastases.
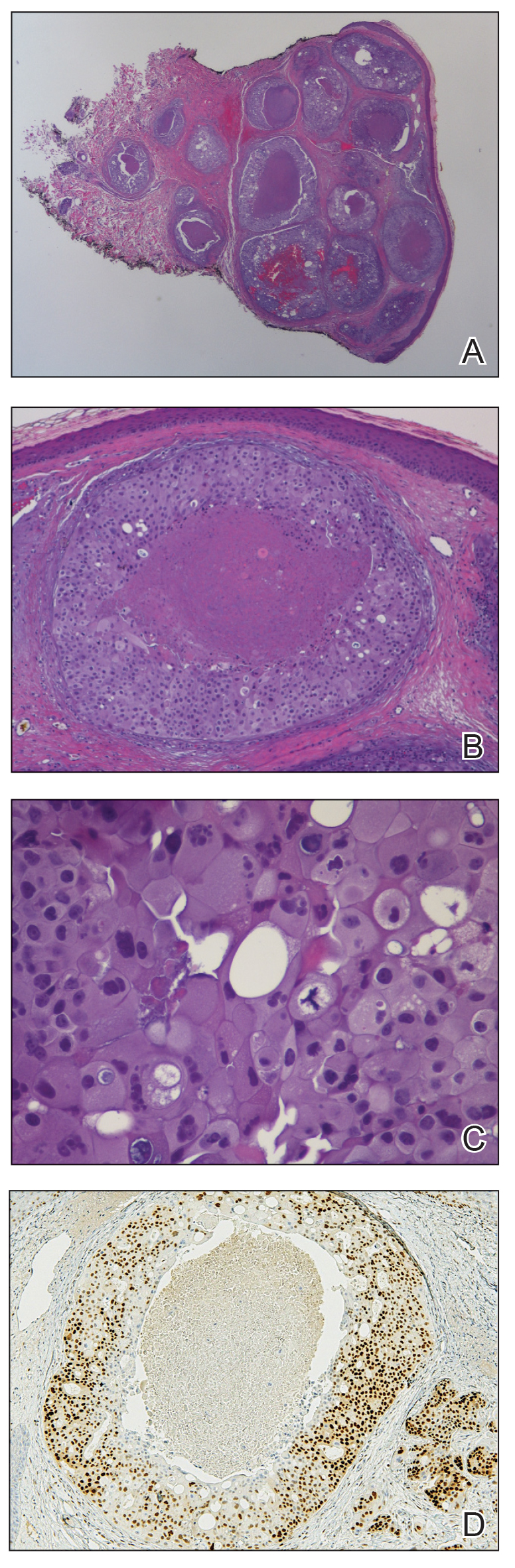
Salivary duct carcinoma is rare and is estimated to represent 1% to 3% of all salivary malignancies.1 It is a highly aggressive form of salivary gland carcinoma and is associated with a poor clinical outcome. The 3-year overall survival rate for stage I disease is 42% and only 23% for stage IV disease.2 Salivary duct carcinoma has a high rate of distant metastasis,3 but cases of cutaneous metastases are rare.3-8 Previously reported cases of SDC that metastasized to the skin originated from the parotid gland (n=6) and submandibular gland (n=1).3
The diagnosis of cutaneous metastases is challenging due to the variability of the skin manifestations. Three cases described small firm nodules in patients,3-5 while others presented with purpuric papules and pseudovesicles.6-8 Our patient presented with sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules, which further emphasizes the capricious nature of skin findings.
The morphology of SDC is strikingly similar to ductal adenocarcinoma of the breast, which can lead to diagnostic confusion. Both carcinomas may show oncocytic cells, ductal formations, and cribriform structures with central comedo necrosis. Moreover, immunohistochemical features overlap, including positive staining for cytokeratin 7 and gross cystic disease fluid protein 15. Positive immunohistochemistry with androgen receptor is consistent with SDC but also can be expressed in some cases of breast carcinoma.9,10 Therefore, the diagnosis of cutaneous involvement from metastatic SDC requires not just an evaluation of the pathologic features but careful attention to the clinical history and a thorough staging evaluation.
- D’heygere E, Meulemans J, Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26:142-151.
- Gilbert MR, Sharma A, Schmitt NC, et al. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489-495.
- Chakari W, Andersen L, Andersen JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Tok J, Kao GF, Berberian BJ, et al. Cutaneous metastasis from a parotid adenocarcinoma. Report of a case with immunohistochemical findings and review of the literature. Am J Dermatopathol. 1995;17:303-306.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Hafiji J, Rytina E, Jani P, et al. A rare cutaneous presentation of metastatic parotid adenocarcinoma. Australas J Dermatol. 2013;54:E40-E42.
- Zanca A, Ferracini U, Bertazzoni MG. Telangiectatic metastasis from ductal carcinoma of the parotid gland. J Am Acad Dermatol. 1993;28:113-114.
- Brys´ M, Wójcik M, Romanowicz-Makowska H, et al. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2002;128:85-90.
- Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288-294.
To the Editor:
Metastatic spread of salivary duct carcinoma (SDC) to the skin is rare. Diagnosing SDC can be challenging because the cutaneous manifestations of this disease are variable and include nodules, papules, and erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles. We describe a case of cutaneous metastatic SDC that originated from the parotid gland and presented with 2 distinct cutaneous findings: sharply demarcated erythematous plaques and focally hemorrhagic angiomatous papules.
A 60-year-old man presented with a persistent polymorphous pruritic eruption of several months’ duration involving the entire face, ears, neck, and upper chest. He had a history of unspecified adenocarcinoma of the parotid gland diagnosed 2 years prior and underwent multiple treatment cycles with several chemotherapeutic agents over the course of 18 months. Physical examination showed erythematous papules and nodules on the face and neck with slight overlying scale. Sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules were noted on the neck and chest (Figure 1). Two 4-mm punch biopsies were sampled from representative nodular areas. Histopathology showed multiple round solid-tumor nodules with central necrosis in the superficial and deep dermis that were not associated with the overlying epidermis (Figures 2A and 2B). The tumor cells appeared polygonal and contained ample eosinophilic cytoplasm. Tumor nuclei showed marked pleomorphism, and numerous atypical mitotic figures were readily identifiable (Figure 2C). There was diffuse cytoplasmic staining with cytokeratin 7 and nuclear staining with androgen receptor (Figure 2D). These findings were consistent with a diagnosis of SDC metastatic to the skin.

The patient underwent 8 cycles of docetaxel chemotherapy. With disease progression, the chemotherapy regimen was changed to gemcitabine and methotrexate. The patient continued to experience disease progression and died 9 months after diagnosis of skin metastases.

Salivary duct carcinoma is rare and is estimated to represent 1% to 3% of all salivary malignancies.1 It is a highly aggressive form of salivary gland carcinoma and is associated with a poor clinical outcome. The 3-year overall survival rate for stage I disease is 42% and only 23% for stage IV disease.2 Salivary duct carcinoma has a high rate of distant metastasis,3 but cases of cutaneous metastases are rare.3-8 Previously reported cases of SDC that metastasized to the skin originated from the parotid gland (n=6) and submandibular gland (n=1).3
The diagnosis of cutaneous metastases is challenging due to the variability of the skin manifestations. Three cases described small firm nodules in patients,3-5 while others presented with purpuric papules and pseudovesicles.6-8 Our patient presented with sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules, which further emphasizes the capricious nature of skin findings.
The morphology of SDC is strikingly similar to ductal adenocarcinoma of the breast, which can lead to diagnostic confusion. Both carcinomas may show oncocytic cells, ductal formations, and cribriform structures with central comedo necrosis. Moreover, immunohistochemical features overlap, including positive staining for cytokeratin 7 and gross cystic disease fluid protein 15. Positive immunohistochemistry with androgen receptor is consistent with SDC but also can be expressed in some cases of breast carcinoma.9,10 Therefore, the diagnosis of cutaneous involvement from metastatic SDC requires not just an evaluation of the pathologic features but careful attention to the clinical history and a thorough staging evaluation.
To the Editor:
Metastatic spread of salivary duct carcinoma (SDC) to the skin is rare. Diagnosing SDC can be challenging because the cutaneous manifestations of this disease are variable and include nodules, papules, and erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles. We describe a case of cutaneous metastatic SDC that originated from the parotid gland and presented with 2 distinct cutaneous findings: sharply demarcated erythematous plaques and focally hemorrhagic angiomatous papules.
A 60-year-old man presented with a persistent polymorphous pruritic eruption of several months’ duration involving the entire face, ears, neck, and upper chest. He had a history of unspecified adenocarcinoma of the parotid gland diagnosed 2 years prior and underwent multiple treatment cycles with several chemotherapeutic agents over the course of 18 months. Physical examination showed erythematous papules and nodules on the face and neck with slight overlying scale. Sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules were noted on the neck and chest (Figure 1). Two 4-mm punch biopsies were sampled from representative nodular areas. Histopathology showed multiple round solid-tumor nodules with central necrosis in the superficial and deep dermis that were not associated with the overlying epidermis (Figures 2A and 2B). The tumor cells appeared polygonal and contained ample eosinophilic cytoplasm. Tumor nuclei showed marked pleomorphism, and numerous atypical mitotic figures were readily identifiable (Figure 2C). There was diffuse cytoplasmic staining with cytokeratin 7 and nuclear staining with androgen receptor (Figure 2D). These findings were consistent with a diagnosis of SDC metastatic to the skin.

The patient underwent 8 cycles of docetaxel chemotherapy. With disease progression, the chemotherapy regimen was changed to gemcitabine and methotrexate. The patient continued to experience disease progression and died 9 months after diagnosis of skin metastases.

Salivary duct carcinoma is rare and is estimated to represent 1% to 3% of all salivary malignancies.1 It is a highly aggressive form of salivary gland carcinoma and is associated with a poor clinical outcome. The 3-year overall survival rate for stage I disease is 42% and only 23% for stage IV disease.2 Salivary duct carcinoma has a high rate of distant metastasis,3 but cases of cutaneous metastases are rare.3-8 Previously reported cases of SDC that metastasized to the skin originated from the parotid gland (n=6) and submandibular gland (n=1).3
The diagnosis of cutaneous metastases is challenging due to the variability of the skin manifestations. Three cases described small firm nodules in patients,3-5 while others presented with purpuric papules and pseudovesicles.6-8 Our patient presented with sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules, which further emphasizes the capricious nature of skin findings.
The morphology of SDC is strikingly similar to ductal adenocarcinoma of the breast, which can lead to diagnostic confusion. Both carcinomas may show oncocytic cells, ductal formations, and cribriform structures with central comedo necrosis. Moreover, immunohistochemical features overlap, including positive staining for cytokeratin 7 and gross cystic disease fluid protein 15. Positive immunohistochemistry with androgen receptor is consistent with SDC but also can be expressed in some cases of breast carcinoma.9,10 Therefore, the diagnosis of cutaneous involvement from metastatic SDC requires not just an evaluation of the pathologic features but careful attention to the clinical history and a thorough staging evaluation.
- D’heygere E, Meulemans J, Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26:142-151.
- Gilbert MR, Sharma A, Schmitt NC, et al. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489-495.
- Chakari W, Andersen L, Andersen JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Tok J, Kao GF, Berberian BJ, et al. Cutaneous metastasis from a parotid adenocarcinoma. Report of a case with immunohistochemical findings and review of the literature. Am J Dermatopathol. 1995;17:303-306.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Hafiji J, Rytina E, Jani P, et al. A rare cutaneous presentation of metastatic parotid adenocarcinoma. Australas J Dermatol. 2013;54:E40-E42.
- Zanca A, Ferracini U, Bertazzoni MG. Telangiectatic metastasis from ductal carcinoma of the parotid gland. J Am Acad Dermatol. 1993;28:113-114.
- Brys´ M, Wójcik M, Romanowicz-Makowska H, et al. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2002;128:85-90.
- Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288-294.
- D’heygere E, Meulemans J, Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26:142-151.
- Gilbert MR, Sharma A, Schmitt NC, et al. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489-495.
- Chakari W, Andersen L, Andersen JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Tok J, Kao GF, Berberian BJ, et al. Cutaneous metastasis from a parotid adenocarcinoma. Report of a case with immunohistochemical findings and review of the literature. Am J Dermatopathol. 1995;17:303-306.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Hafiji J, Rytina E, Jani P, et al. A rare cutaneous presentation of metastatic parotid adenocarcinoma. Australas J Dermatol. 2013;54:E40-E42.
- Zanca A, Ferracini U, Bertazzoni MG. Telangiectatic metastasis from ductal carcinoma of the parotid gland. J Am Acad Dermatol. 1993;28:113-114.
- Brys´ M, Wójcik M, Romanowicz-Makowska H, et al. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2002;128:85-90.
- Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288-294.
Practice Points
- Skin manifestations of metastatic salivary duct carcinoma can be variable, ranging from nodules to erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles.
- The specific clinical findings as well as histologic and immunohistochemical characteristics can aid in the diagnosis of this rare disease.
Iododerma Simulating Cryptococcal Infection
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-
The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.
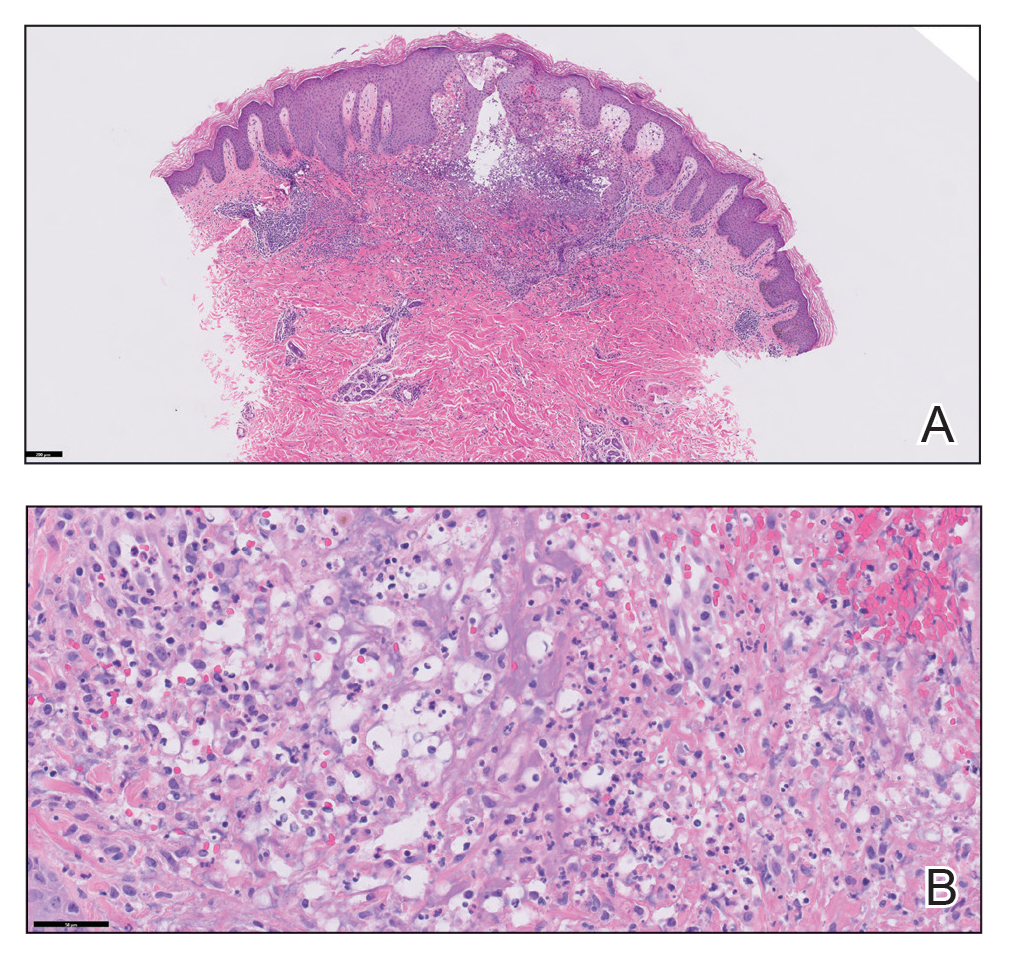
Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
Practice Points
- Halogenodermas are rare cutaneous reactions to excess exposure to or ingestion of halogen-containing drugs or substances such as bromine, iodine (iododerma), fluorine, and rarely lithium.
- The clinical presentation of a halogenoderma varies; the most characteristic manifestation is a vegetative or exudative plaque with a peripheral rim of pustules.
- Histologically, lesions of a halogenoderma are characterized by pseudoepitheliomatous hyperplasia associated with numerous intraepidermal microabscesses overlying a dense mixed inflammatory infiltrate of neutrophils, plasma cells, eosinophils, histiocytes, and scattered multinucleated giant cells.
- Rarely, the dermal infiltrate of a halogenoderma contains abundant acellular bodies surrounded by capsulelike vacuolated spaces mimicking Cryptococcus neoformans.
Roflumilast side effect benefits patients with psoriasis and overweight/obesity
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
Novel triple-threat approach to acne beats placebo
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.