User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Laparoscopic sleeve gastrectomy: Comorbidity benefits fade with time
Five years after laparoscopic sleeve gastrectomy, patients will have regained, on average, about half of their preop excess weight, according to an Israeli investigation published online Aug. 5 in JAMA Surgery.
Things went better at first for the 443 patients in the study; at 1 year follow-up, they had lost, on average, 76.8% of their excess weight, but then it started to come back. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%. The failure rate – the number of patients with a percentage of excess weight loss less than 50% – increased from 13.3% at 1 year to 21.1% at 3 years and 38.5% at 5 years (JAMA Surg. 2015 Aug. 5. doi:10.1001/jamasurg.2015.2202).
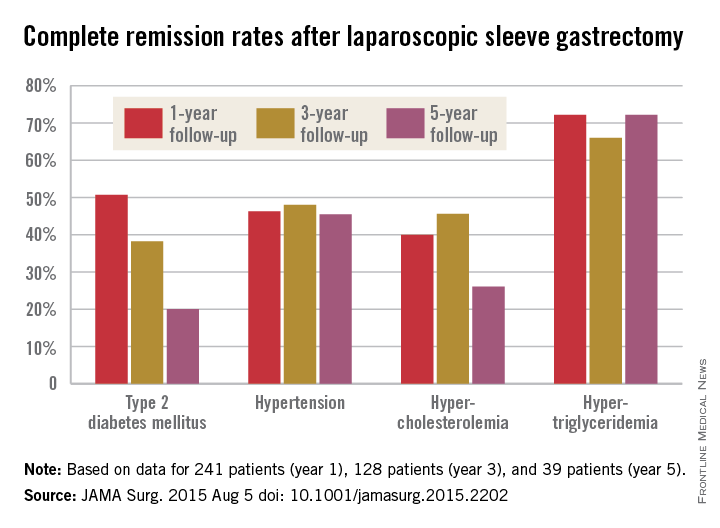
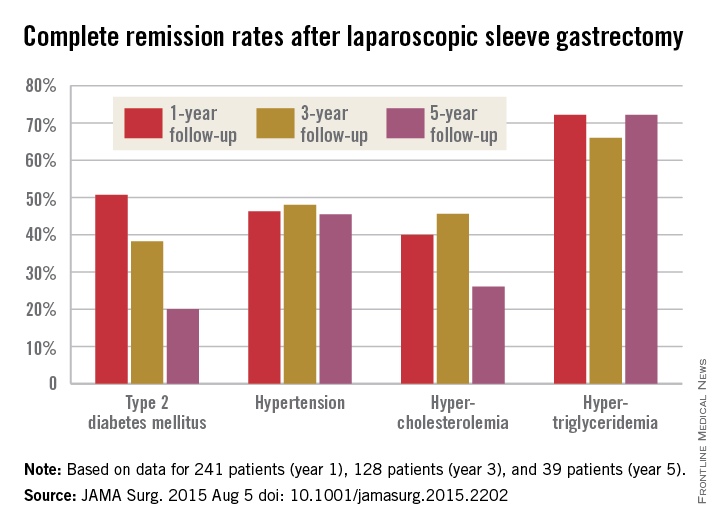
The story was similar for obesity-related comorbidities; early gains eroded with time. Complete remission of diabetes, for instance, was maintained by 50.7% of patients at 1 year, 38.2% at 3 years, and just 20.0% at 5 years. Likewise, a drop in LDL cholesterol from baseline was significant at years 1 and 3, but not 5. Meanwhile, laparoscopic sleeve gastrectomy (LSG) didn’t significantly improve total cholesterol over baseline, and triglyceride improvements began to fade after year 1.
“In our opinion, the presence of obesity-related comorbidities should play a major role when choosing the appropriate procedure for a specific patient. For example, performing an operation that yields a low resolution rate of hyperlipidemia translates into lifelong medical treatment in a young patient with significant hyperlipidemia. In that case, a malabsorptive procedure might be more beneficial than LSG. If the recurrence of obesity is known to be followed by the remittance of an existing comorbidity in a specific procedure, an alternative procedure should be considered. The weight loss durability failure of almost 40% at 5 years of follow-up of LSG should be one of the deciding factors in such cases,” said senior investigator and bariatric surgeon Dr. Andrei Keidar of Beilinson Hospital in Petah Tikva, Israel, and his colleagues.
LSG is becoming more popular in part because it’s easier to learn and less disruptive than gastric bypass, but there are not enough data on long-term outcomes; the investigators sought to fill the gaps.
The average age in the study was 42.2 years; mean body mass index was 43.9 kg/m2, and mean preop excess weight was 51.2 kg. The majority of subjects were women. The operations were performed from 2006 to 2013, and there was considerable loss to follow-up during the project.
Baseline triglycerides followed overall trends with a drop from a mean of 155.2 mg/dL to 106.3 mg/dL at year 1, followed by a tick upward to 107.2 mg/dL at year 3 and 126.4 mg/dL at year 5.
The mean preop HDL cholesterol of 46.7 mg/dL rose to 52.8 mg/dL at year 1 and remained at about that level at 5 years. Improvements in hypertension were fairly durable, as well, with remission in 46.3% of patients at 1 year, 48.0% at 3 years, and 45.5% at 5 years.
“Surprisingly, our results showed that none of the changes in obesity-related comorbidity status correlated with” the amount of “excess weight prior to the surgery,” the investigators noted.
The authors didn’t compare LSG to other bariatric surgeries, but did note that in 2012, the American Society for Metabolic and Bariatric Surgery found that short-term weight loss and improvement in comorbidities was better with LSG than with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric bypass. On the other hand, RYGB has been found to beat LSG on lipid fraction improvements and short term control of type 2 diabetes.
The investigators had no disclosures.
Laparoscopic sleeve gastrectomy [has evolved] very quickly during the last several years into the dominant procedure in use despite a complete void of information about the longer-term effects. [The investigators try] to address this ... but clearly raise more issues than they can answer.

|
Dr. Anita P. Courcoulas |
It is unclear whether current studies will address critical questions about the long-term outcomes of bariatric surgery, including the sustainability of weight loss and comorbidity control and long-term complication rates. The answers will likely be generated over time not only by ... large-scale efforts but also by thoughtful inference that will be made through pooled analyses of data like that from [this study] and from many other disparate randomized and nonrandomized studies of bariatric surgery. It will take time, patience, and a willingness to avoid a rush to judgment. In the meantime, clinicians and prospective patients will need to discuss and weigh the evidence in a dynamic exchange driven not always by final conclusions but by the most current available data.
Dr. Anita Courcoulas is professor of surgery and chief of the section of minimally invasive bariatric and general surgery at the University of Pittsburgh. She reported receiving grants from Nutrisystem, Ethicon, and Covidien and serving as a project consultant for Ethicon and Apollo Endosurgery. She made her comments in an editorial that accompanied the study.
Laparoscopic sleeve gastrectomy [has evolved] very quickly during the last several years into the dominant procedure in use despite a complete void of information about the longer-term effects. [The investigators try] to address this ... but clearly raise more issues than they can answer.

|
Dr. Anita P. Courcoulas |
It is unclear whether current studies will address critical questions about the long-term outcomes of bariatric surgery, including the sustainability of weight loss and comorbidity control and long-term complication rates. The answers will likely be generated over time not only by ... large-scale efforts but also by thoughtful inference that will be made through pooled analyses of data like that from [this study] and from many other disparate randomized and nonrandomized studies of bariatric surgery. It will take time, patience, and a willingness to avoid a rush to judgment. In the meantime, clinicians and prospective patients will need to discuss and weigh the evidence in a dynamic exchange driven not always by final conclusions but by the most current available data.
Dr. Anita Courcoulas is professor of surgery and chief of the section of minimally invasive bariatric and general surgery at the University of Pittsburgh. She reported receiving grants from Nutrisystem, Ethicon, and Covidien and serving as a project consultant for Ethicon and Apollo Endosurgery. She made her comments in an editorial that accompanied the study.
Laparoscopic sleeve gastrectomy [has evolved] very quickly during the last several years into the dominant procedure in use despite a complete void of information about the longer-term effects. [The investigators try] to address this ... but clearly raise more issues than they can answer.

|
Dr. Anita P. Courcoulas |
It is unclear whether current studies will address critical questions about the long-term outcomes of bariatric surgery, including the sustainability of weight loss and comorbidity control and long-term complication rates. The answers will likely be generated over time not only by ... large-scale efforts but also by thoughtful inference that will be made through pooled analyses of data like that from [this study] and from many other disparate randomized and nonrandomized studies of bariatric surgery. It will take time, patience, and a willingness to avoid a rush to judgment. In the meantime, clinicians and prospective patients will need to discuss and weigh the evidence in a dynamic exchange driven not always by final conclusions but by the most current available data.
Dr. Anita Courcoulas is professor of surgery and chief of the section of minimally invasive bariatric and general surgery at the University of Pittsburgh. She reported receiving grants from Nutrisystem, Ethicon, and Covidien and serving as a project consultant for Ethicon and Apollo Endosurgery. She made her comments in an editorial that accompanied the study.
Five years after laparoscopic sleeve gastrectomy, patients will have regained, on average, about half of their preop excess weight, according to an Israeli investigation published online Aug. 5 in JAMA Surgery.
Things went better at first for the 443 patients in the study; at 1 year follow-up, they had lost, on average, 76.8% of their excess weight, but then it started to come back. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%. The failure rate – the number of patients with a percentage of excess weight loss less than 50% – increased from 13.3% at 1 year to 21.1% at 3 years and 38.5% at 5 years (JAMA Surg. 2015 Aug. 5. doi:10.1001/jamasurg.2015.2202).
The story was similar for obesity-related comorbidities; early gains eroded with time. Complete remission of diabetes, for instance, was maintained by 50.7% of patients at 1 year, 38.2% at 3 years, and just 20.0% at 5 years. Likewise, a drop in LDL cholesterol from baseline was significant at years 1 and 3, but not 5. Meanwhile, laparoscopic sleeve gastrectomy (LSG) didn’t significantly improve total cholesterol over baseline, and triglyceride improvements began to fade after year 1.

“In our opinion, the presence of obesity-related comorbidities should play a major role when choosing the appropriate procedure for a specific patient. For example, performing an operation that yields a low resolution rate of hyperlipidemia translates into lifelong medical treatment in a young patient with significant hyperlipidemia. In that case, a malabsorptive procedure might be more beneficial than LSG. If the recurrence of obesity is known to be followed by the remittance of an existing comorbidity in a specific procedure, an alternative procedure should be considered. The weight loss durability failure of almost 40% at 5 years of follow-up of LSG should be one of the deciding factors in such cases,” said senior investigator and bariatric surgeon Dr. Andrei Keidar of Beilinson Hospital in Petah Tikva, Israel, and his colleagues.
LSG is becoming more popular in part because it’s easier to learn and less disruptive than gastric bypass, but there are not enough data on long-term outcomes; the investigators sought to fill the gaps.
The average age in the study was 42.2 years; mean body mass index was 43.9 kg/m2, and mean preop excess weight was 51.2 kg. The majority of subjects were women. The operations were performed from 2006 to 2013, and there was considerable loss to follow-up during the project.
Baseline triglycerides followed overall trends with a drop from a mean of 155.2 mg/dL to 106.3 mg/dL at year 1, followed by a tick upward to 107.2 mg/dL at year 3 and 126.4 mg/dL at year 5.
The mean preop HDL cholesterol of 46.7 mg/dL rose to 52.8 mg/dL at year 1 and remained at about that level at 5 years. Improvements in hypertension were fairly durable, as well, with remission in 46.3% of patients at 1 year, 48.0% at 3 years, and 45.5% at 5 years.
“Surprisingly, our results showed that none of the changes in obesity-related comorbidity status correlated with” the amount of “excess weight prior to the surgery,” the investigators noted.
The authors didn’t compare LSG to other bariatric surgeries, but did note that in 2012, the American Society for Metabolic and Bariatric Surgery found that short-term weight loss and improvement in comorbidities was better with LSG than with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric bypass. On the other hand, RYGB has been found to beat LSG on lipid fraction improvements and short term control of type 2 diabetes.
The investigators had no disclosures.
Five years after laparoscopic sleeve gastrectomy, patients will have regained, on average, about half of their preop excess weight, according to an Israeli investigation published online Aug. 5 in JAMA Surgery.
Things went better at first for the 443 patients in the study; at 1 year follow-up, they had lost, on average, 76.8% of their excess weight, but then it started to come back. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%. The failure rate – the number of patients with a percentage of excess weight loss less than 50% – increased from 13.3% at 1 year to 21.1% at 3 years and 38.5% at 5 years (JAMA Surg. 2015 Aug. 5. doi:10.1001/jamasurg.2015.2202).
The story was similar for obesity-related comorbidities; early gains eroded with time. Complete remission of diabetes, for instance, was maintained by 50.7% of patients at 1 year, 38.2% at 3 years, and just 20.0% at 5 years. Likewise, a drop in LDL cholesterol from baseline was significant at years 1 and 3, but not 5. Meanwhile, laparoscopic sleeve gastrectomy (LSG) didn’t significantly improve total cholesterol over baseline, and triglyceride improvements began to fade after year 1.

“In our opinion, the presence of obesity-related comorbidities should play a major role when choosing the appropriate procedure for a specific patient. For example, performing an operation that yields a low resolution rate of hyperlipidemia translates into lifelong medical treatment in a young patient with significant hyperlipidemia. In that case, a malabsorptive procedure might be more beneficial than LSG. If the recurrence of obesity is known to be followed by the remittance of an existing comorbidity in a specific procedure, an alternative procedure should be considered. The weight loss durability failure of almost 40% at 5 years of follow-up of LSG should be one of the deciding factors in such cases,” said senior investigator and bariatric surgeon Dr. Andrei Keidar of Beilinson Hospital in Petah Tikva, Israel, and his colleagues.
LSG is becoming more popular in part because it’s easier to learn and less disruptive than gastric bypass, but there are not enough data on long-term outcomes; the investigators sought to fill the gaps.
The average age in the study was 42.2 years; mean body mass index was 43.9 kg/m2, and mean preop excess weight was 51.2 kg. The majority of subjects were women. The operations were performed from 2006 to 2013, and there was considerable loss to follow-up during the project.
Baseline triglycerides followed overall trends with a drop from a mean of 155.2 mg/dL to 106.3 mg/dL at year 1, followed by a tick upward to 107.2 mg/dL at year 3 and 126.4 mg/dL at year 5.
The mean preop HDL cholesterol of 46.7 mg/dL rose to 52.8 mg/dL at year 1 and remained at about that level at 5 years. Improvements in hypertension were fairly durable, as well, with remission in 46.3% of patients at 1 year, 48.0% at 3 years, and 45.5% at 5 years.
“Surprisingly, our results showed that none of the changes in obesity-related comorbidity status correlated with” the amount of “excess weight prior to the surgery,” the investigators noted.
The authors didn’t compare LSG to other bariatric surgeries, but did note that in 2012, the American Society for Metabolic and Bariatric Surgery found that short-term weight loss and improvement in comorbidities was better with LSG than with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric bypass. On the other hand, RYGB has been found to beat LSG on lipid fraction improvements and short term control of type 2 diabetes.
The investigators had no disclosures.
FROM JAMA SURGERY
Key clinical point: Laparoscopic sleeve gastrectomy (LSG) might not be the best surgical choice for bariatric patients with significant hyperlipidemia.
Major finding: One year after LSG, patients lost, on average, 76.8% of their excess weight. At 3 years, patients were free of 69.7% of their excess weight, and at 5 years, just 56.1%.
Data source: Retrospective study of 443 Israeli laparoscopic sleeve gastrectomies.
Disclosures: The investigators had no disclosures.
Lifestyle changes, surgical weight loss benefit NAFLD
Losing weight through lifestyle changes can significantly improve several measures of nonalcoholic fatty liver disease, particularly if patients lose at least 10% of their body weight, and bariatric surgery is a valid alternative if diet and exercise fail, according to two studies reported in the August issue of Gastroenterology (2015 Apr. 9 [doi:10.1053/j.gastro.2015.04.005]).
Global rates of nonalcoholic fatty liver disease (NAFLD) are up because of the “parallel epidemics” of obesity and type 2 diabetes mellitus, said Dr. Eduardo Vilar-Gomez of the National Institute of Hepatology in Havana, Cuba, who authored the study of lifestyle changes. There are no approved therapies for the more aggressive form of NAFLD, steatohepatitis, he and his associates said. In past studies, patients who lost about 7%-10% of their body weight substantially improved their NAFLD activity score and had reductions in steatosis, lobular inflammation, and ballooning, but the results did not extend to fibrosis, and prospective studies of the effect of lifestyle changes on histology are lacking, the researchers added .
To fill the gap, they followed 293 adults with histologically confirmed nonalcoholic steatohepatitis who completed a 52-week lifestyle intervention program that included keeping a food diary, restricting saturated fats to less than 10% of total intake, and walking at least 200 minutes a week. Patients had not received hypolipidemic treatment in the preceding 3 months and were not allowed to take insulin sensitizers or vitamin E, both of which are potentially beneficial for nonalcoholic steatohepatitis, the investigators said.
At the end of the yearlong program, steatohepatitis had resolved in 25% of patients, 47% had lower NAFLD activity scores, and 19% had regression of fibrosis, the researchers reported. Although only 30% of participants lost at least 5% of their body weight, weight loss correlated positively with resolution of steatohepatitis and with 2-point reductions in histologic activity scores (P <.001). Among patients who lost at least 10% of their body weight, 90% had resolution of steatohepatitis, 45% had regression of fibrosis, and all had improved histologic activity scores, even if they had negative risk factors such as female sex, a baseline body mass index of at least 35 kg/m2, and a baseline fasting glucose level of at least 5.5 mmol/L, the investigators added. “Our findings support the current recommendation for weight loss using lifestyle modification as the first step in the management of patients with nonalcoholic steatohepatitis,” they wrote.
But what if lifestyle changes fail? The impact of bariatric surgery on nonalcoholic steatohepatitis has not been well studied, said Dr. Guillaume Lassailly at CHRU Lille (France). He and his associates therefore followed 109 morbidly obese patients (BMI ≥40 kg/m2) with biopsy-confirmed nonalcoholic steatohepatitis who underwent bariatric surgery at a single tertiary hospital (Gastroenterology 2015 Apr. 25 [doi:10.1053/j.gastro.2015.04.014]) .
One year after surgery, 85% of patients had achieved disease resolution (95% confidence interval, 75.8%-92.2%), the investigators reported. Stratifying patients by baseline Brunt scores showed that those with milder presurgical disease were more likely to have complete resolution than were patients whose disease was severe (94% vs. 70%; P <.05), the researchers added. Histologic analyses supported the findings, revealing steatosis in 60% of presurgical tissue samples, compared with 10% of samples taken a year after surgery. In addition, average NAFLD disease scores dropped from 5 to 1 (P <.001), hepatocyte ballooning decreased in 84% of samples, lobular inflammation decreased in 67%, and Metavir fibrosis scores dropped in one-third of specimens.
Notably, BMI scores for patients with persistent postsurgical disease dropped by an average of only 9.1, compared with 12.3 for patients whose disease resolved (P = .005), said the researchers. Gastric bypass surgery achieved greater weight loss and improvements in disease status, compared with laparoscopic banding, they added. “The encouraging results of the present study suggest that bariatric surgery should be tested in multicenter, randomized controlled trials in morbidly or severely obese patients with nonalcoholic steatohepatitis who did not respond to lifestyle therapy,” they wrote.
The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
Losing weight through lifestyle changes can significantly improve several measures of nonalcoholic fatty liver disease, particularly if patients lose at least 10% of their body weight, and bariatric surgery is a valid alternative if diet and exercise fail, according to two studies reported in the August issue of Gastroenterology (2015 Apr. 9 [doi:10.1053/j.gastro.2015.04.005]).
Global rates of nonalcoholic fatty liver disease (NAFLD) are up because of the “parallel epidemics” of obesity and type 2 diabetes mellitus, said Dr. Eduardo Vilar-Gomez of the National Institute of Hepatology in Havana, Cuba, who authored the study of lifestyle changes. There are no approved therapies for the more aggressive form of NAFLD, steatohepatitis, he and his associates said. In past studies, patients who lost about 7%-10% of their body weight substantially improved their NAFLD activity score and had reductions in steatosis, lobular inflammation, and ballooning, but the results did not extend to fibrosis, and prospective studies of the effect of lifestyle changes on histology are lacking, the researchers added .
To fill the gap, they followed 293 adults with histologically confirmed nonalcoholic steatohepatitis who completed a 52-week lifestyle intervention program that included keeping a food diary, restricting saturated fats to less than 10% of total intake, and walking at least 200 minutes a week. Patients had not received hypolipidemic treatment in the preceding 3 months and were not allowed to take insulin sensitizers or vitamin E, both of which are potentially beneficial for nonalcoholic steatohepatitis, the investigators said.
At the end of the yearlong program, steatohepatitis had resolved in 25% of patients, 47% had lower NAFLD activity scores, and 19% had regression of fibrosis, the researchers reported. Although only 30% of participants lost at least 5% of their body weight, weight loss correlated positively with resolution of steatohepatitis and with 2-point reductions in histologic activity scores (P <.001). Among patients who lost at least 10% of their body weight, 90% had resolution of steatohepatitis, 45% had regression of fibrosis, and all had improved histologic activity scores, even if they had negative risk factors such as female sex, a baseline body mass index of at least 35 kg/m2, and a baseline fasting glucose level of at least 5.5 mmol/L, the investigators added. “Our findings support the current recommendation for weight loss using lifestyle modification as the first step in the management of patients with nonalcoholic steatohepatitis,” they wrote.
But what if lifestyle changes fail? The impact of bariatric surgery on nonalcoholic steatohepatitis has not been well studied, said Dr. Guillaume Lassailly at CHRU Lille (France). He and his associates therefore followed 109 morbidly obese patients (BMI ≥40 kg/m2) with biopsy-confirmed nonalcoholic steatohepatitis who underwent bariatric surgery at a single tertiary hospital (Gastroenterology 2015 Apr. 25 [doi:10.1053/j.gastro.2015.04.014]) .
One year after surgery, 85% of patients had achieved disease resolution (95% confidence interval, 75.8%-92.2%), the investigators reported. Stratifying patients by baseline Brunt scores showed that those with milder presurgical disease were more likely to have complete resolution than were patients whose disease was severe (94% vs. 70%; P <.05), the researchers added. Histologic analyses supported the findings, revealing steatosis in 60% of presurgical tissue samples, compared with 10% of samples taken a year after surgery. In addition, average NAFLD disease scores dropped from 5 to 1 (P <.001), hepatocyte ballooning decreased in 84% of samples, lobular inflammation decreased in 67%, and Metavir fibrosis scores dropped in one-third of specimens.
Notably, BMI scores for patients with persistent postsurgical disease dropped by an average of only 9.1, compared with 12.3 for patients whose disease resolved (P = .005), said the researchers. Gastric bypass surgery achieved greater weight loss and improvements in disease status, compared with laparoscopic banding, they added. “The encouraging results of the present study suggest that bariatric surgery should be tested in multicenter, randomized controlled trials in morbidly or severely obese patients with nonalcoholic steatohepatitis who did not respond to lifestyle therapy,” they wrote.
The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
Losing weight through lifestyle changes can significantly improve several measures of nonalcoholic fatty liver disease, particularly if patients lose at least 10% of their body weight, and bariatric surgery is a valid alternative if diet and exercise fail, according to two studies reported in the August issue of Gastroenterology (2015 Apr. 9 [doi:10.1053/j.gastro.2015.04.005]).
Global rates of nonalcoholic fatty liver disease (NAFLD) are up because of the “parallel epidemics” of obesity and type 2 diabetes mellitus, said Dr. Eduardo Vilar-Gomez of the National Institute of Hepatology in Havana, Cuba, who authored the study of lifestyle changes. There are no approved therapies for the more aggressive form of NAFLD, steatohepatitis, he and his associates said. In past studies, patients who lost about 7%-10% of their body weight substantially improved their NAFLD activity score and had reductions in steatosis, lobular inflammation, and ballooning, but the results did not extend to fibrosis, and prospective studies of the effect of lifestyle changes on histology are lacking, the researchers added .
To fill the gap, they followed 293 adults with histologically confirmed nonalcoholic steatohepatitis who completed a 52-week lifestyle intervention program that included keeping a food diary, restricting saturated fats to less than 10% of total intake, and walking at least 200 minutes a week. Patients had not received hypolipidemic treatment in the preceding 3 months and were not allowed to take insulin sensitizers or vitamin E, both of which are potentially beneficial for nonalcoholic steatohepatitis, the investigators said.
At the end of the yearlong program, steatohepatitis had resolved in 25% of patients, 47% had lower NAFLD activity scores, and 19% had regression of fibrosis, the researchers reported. Although only 30% of participants lost at least 5% of their body weight, weight loss correlated positively with resolution of steatohepatitis and with 2-point reductions in histologic activity scores (P <.001). Among patients who lost at least 10% of their body weight, 90% had resolution of steatohepatitis, 45% had regression of fibrosis, and all had improved histologic activity scores, even if they had negative risk factors such as female sex, a baseline body mass index of at least 35 kg/m2, and a baseline fasting glucose level of at least 5.5 mmol/L, the investigators added. “Our findings support the current recommendation for weight loss using lifestyle modification as the first step in the management of patients with nonalcoholic steatohepatitis,” they wrote.
But what if lifestyle changes fail? The impact of bariatric surgery on nonalcoholic steatohepatitis has not been well studied, said Dr. Guillaume Lassailly at CHRU Lille (France). He and his associates therefore followed 109 morbidly obese patients (BMI ≥40 kg/m2) with biopsy-confirmed nonalcoholic steatohepatitis who underwent bariatric surgery at a single tertiary hospital (Gastroenterology 2015 Apr. 25 [doi:10.1053/j.gastro.2015.04.014]) .
One year after surgery, 85% of patients had achieved disease resolution (95% confidence interval, 75.8%-92.2%), the investigators reported. Stratifying patients by baseline Brunt scores showed that those with milder presurgical disease were more likely to have complete resolution than were patients whose disease was severe (94% vs. 70%; P <.05), the researchers added. Histologic analyses supported the findings, revealing steatosis in 60% of presurgical tissue samples, compared with 10% of samples taken a year after surgery. In addition, average NAFLD disease scores dropped from 5 to 1 (P <.001), hepatocyte ballooning decreased in 84% of samples, lobular inflammation decreased in 67%, and Metavir fibrosis scores dropped in one-third of specimens.
Notably, BMI scores for patients with persistent postsurgical disease dropped by an average of only 9.1, compared with 12.3 for patients whose disease resolved (P = .005), said the researchers. Gastric bypass surgery achieved greater weight loss and improvements in disease status, compared with laparoscopic banding, they added. “The encouraging results of the present study suggest that bariatric surgery should be tested in multicenter, randomized controlled trials in morbidly or severely obese patients with nonalcoholic steatohepatitis who did not respond to lifestyle therapy,” they wrote.
The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Losing weight through lifestyle changes or bariatric surgery can significantly improve several measures of nonalcoholic steatohepatitis.
Major finding: A yearlong diet and exercise program led to resolution of nonalcoholic steatohepatitis in 25% of patients, while bariatric surgery achieved that outcome for 85% of patients in a separate study.
Data source: Two prospective uncontrolled cohort studies of 402 total adults with nonalcoholic steatohepatitis (the more severe form of nonalcoholic fatty liver disease).
Disclosures: The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
FDA approves ReShape intragastric balloon device for weight loss
The first intragastric balloon–based device designed to help obese people lose weight has been approved by the Food and Drug Administration, providing a treatment option that is less invasive than bariatric surgery and gastric banding.
The FDA approved the ReShape Integrated Dual Balloon System on July 28, for “weight reduction when used in conjunction with diet and exercise, in obese patients with a body mass index (BMI) of 30 kg/m2-40 kg/m2 and one or more obesity-related comorbid conditions,” in adults who have not been able to lose weight with diet and exercise alone, according to the agency’s approval letter. Laparoscopic gastric banding is indicated for patients with a BMI of at least 40 kg/m2 (or at least 30 kg/m2 in people with one or more obesity-related comorbidities) and bariatric surgery is usually recommended for patients with a BMI of at least 40 kg/m2 (or at least 35 kg/m2 in people with at least one obesity-related comorbidity).
The ReShape device is made up of two attached balloons that are placed in the stomach through a minimally invasive endoscopic procedure, where they are filled with about 2 cups of saline and methylene blue dye, under mild sedation; the balloons are sealed with mineral oil and left in place for up to 6 months. If a balloon ruptures, the dye appears in the urine. When it is time to remove the balloons, they are deflated then removed using another endoscopic procedure.
The device was evaluated in a pivotal study at eight U.S. sites of over 300 mostly female obese patients whose mean age was about 44 years; their mean weight was about 209-213 pounds, and their mean BMI was about 35 kg/m2; 187 received the device and 139 had the endoscopy only. All participants were on a medically managed diet and exercise program. At 6 months, those in the device group had lost a mean of about 24% of their weight, vs. a mean of about 11% among controls, a statistically significant difference (P = .0041). Those who had lost weight at 6 months “maintained 60% of this weight loss through 48 weeks of follow-up,” according to the FDA.
After placement of the device, common adverse events were vomiting, nausea, and abdominal pain, but most symptoms resolved within 30 days, according to the FDA. The development of gastric ulcerations is described as the “most worrisome” device-related risk, but “there were no unanticipated adverse device effects, no deaths, no intestinal obstructions, and no gastric perforations” in the study.
Among the 265 patients who received the device (those initially enrolled in the pivotal trial plus 78 who were in the control group and opted to receive the device after the first 6 months), 20 (7.5%) experienced severe adverse events; vomiting was the most common, in 4.5%. Serious events included gastric ulcers in two patients (0.8%) at 19 and at 97 days after the device was placed; in both cases, the device was removed. Almost 15% of those who received the device had to have it removed because of an adverse event. The rate of gastric ulcers after a minor change was made to the device was 10%; and the rate of balloon deflations without migration was 6%.
The FDA summary of the approval refers to the “marginal benefit of weight loss” among those in the treatment group, compared with controls, but adds that the decision to approve the device “is based in part on the limited options available to patients with mild to moderate obesity who have failed other means for conservative weight loss.” While the effectiveness of the device is better than what would be expected with diet and exercise or pharmacologic therapy,” it is “substantially less than what would be expected with gastric banding or other surgical interventions.” The list of contraindications includes previous gastrointestinal surgery “with sequelae,” such as an obstruction or adhesions; previous bariatric surgery; any GI inflammatory disease, severe coagulopathy; and women who are pregnant or breastfeeding.
“The company plans to make the ReShape procedure available to patients first in select markets, as physicians and allied health professionals are trained in the procedure and support program to optimize patient outcome,” according to the company’s statement announcing approval.
The ReShape device has been available in Europe since 2007.
Information posted by the FDA, including labeling for professionals, is available at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P140012.
The first intragastric balloon–based device designed to help obese people lose weight has been approved by the Food and Drug Administration, providing a treatment option that is less invasive than bariatric surgery and gastric banding.
The FDA approved the ReShape Integrated Dual Balloon System on July 28, for “weight reduction when used in conjunction with diet and exercise, in obese patients with a body mass index (BMI) of 30 kg/m2-40 kg/m2 and one or more obesity-related comorbid conditions,” in adults who have not been able to lose weight with diet and exercise alone, according to the agency’s approval letter. Laparoscopic gastric banding is indicated for patients with a BMI of at least 40 kg/m2 (or at least 30 kg/m2 in people with one or more obesity-related comorbidities) and bariatric surgery is usually recommended for patients with a BMI of at least 40 kg/m2 (or at least 35 kg/m2 in people with at least one obesity-related comorbidity).
The ReShape device is made up of two attached balloons that are placed in the stomach through a minimally invasive endoscopic procedure, where they are filled with about 2 cups of saline and methylene blue dye, under mild sedation; the balloons are sealed with mineral oil and left in place for up to 6 months. If a balloon ruptures, the dye appears in the urine. When it is time to remove the balloons, they are deflated then removed using another endoscopic procedure.
The device was evaluated in a pivotal study at eight U.S. sites of over 300 mostly female obese patients whose mean age was about 44 years; their mean weight was about 209-213 pounds, and their mean BMI was about 35 kg/m2; 187 received the device and 139 had the endoscopy only. All participants were on a medically managed diet and exercise program. At 6 months, those in the device group had lost a mean of about 24% of their weight, vs. a mean of about 11% among controls, a statistically significant difference (P = .0041). Those who had lost weight at 6 months “maintained 60% of this weight loss through 48 weeks of follow-up,” according to the FDA.
After placement of the device, common adverse events were vomiting, nausea, and abdominal pain, but most symptoms resolved within 30 days, according to the FDA. The development of gastric ulcerations is described as the “most worrisome” device-related risk, but “there were no unanticipated adverse device effects, no deaths, no intestinal obstructions, and no gastric perforations” in the study.
Among the 265 patients who received the device (those initially enrolled in the pivotal trial plus 78 who were in the control group and opted to receive the device after the first 6 months), 20 (7.5%) experienced severe adverse events; vomiting was the most common, in 4.5%. Serious events included gastric ulcers in two patients (0.8%) at 19 and at 97 days after the device was placed; in both cases, the device was removed. Almost 15% of those who received the device had to have it removed because of an adverse event. The rate of gastric ulcers after a minor change was made to the device was 10%; and the rate of balloon deflations without migration was 6%.
The FDA summary of the approval refers to the “marginal benefit of weight loss” among those in the treatment group, compared with controls, but adds that the decision to approve the device “is based in part on the limited options available to patients with mild to moderate obesity who have failed other means for conservative weight loss.” While the effectiveness of the device is better than what would be expected with diet and exercise or pharmacologic therapy,” it is “substantially less than what would be expected with gastric banding or other surgical interventions.” The list of contraindications includes previous gastrointestinal surgery “with sequelae,” such as an obstruction or adhesions; previous bariatric surgery; any GI inflammatory disease, severe coagulopathy; and women who are pregnant or breastfeeding.
“The company plans to make the ReShape procedure available to patients first in select markets, as physicians and allied health professionals are trained in the procedure and support program to optimize patient outcome,” according to the company’s statement announcing approval.
The ReShape device has been available in Europe since 2007.
Information posted by the FDA, including labeling for professionals, is available at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P140012.
The first intragastric balloon–based device designed to help obese people lose weight has been approved by the Food and Drug Administration, providing a treatment option that is less invasive than bariatric surgery and gastric banding.
The FDA approved the ReShape Integrated Dual Balloon System on July 28, for “weight reduction when used in conjunction with diet and exercise, in obese patients with a body mass index (BMI) of 30 kg/m2-40 kg/m2 and one or more obesity-related comorbid conditions,” in adults who have not been able to lose weight with diet and exercise alone, according to the agency’s approval letter. Laparoscopic gastric banding is indicated for patients with a BMI of at least 40 kg/m2 (or at least 30 kg/m2 in people with one or more obesity-related comorbidities) and bariatric surgery is usually recommended for patients with a BMI of at least 40 kg/m2 (or at least 35 kg/m2 in people with at least one obesity-related comorbidity).
The ReShape device is made up of two attached balloons that are placed in the stomach through a minimally invasive endoscopic procedure, where they are filled with about 2 cups of saline and methylene blue dye, under mild sedation; the balloons are sealed with mineral oil and left in place for up to 6 months. If a balloon ruptures, the dye appears in the urine. When it is time to remove the balloons, they are deflated then removed using another endoscopic procedure.
The device was evaluated in a pivotal study at eight U.S. sites of over 300 mostly female obese patients whose mean age was about 44 years; their mean weight was about 209-213 pounds, and their mean BMI was about 35 kg/m2; 187 received the device and 139 had the endoscopy only. All participants were on a medically managed diet and exercise program. At 6 months, those in the device group had lost a mean of about 24% of their weight, vs. a mean of about 11% among controls, a statistically significant difference (P = .0041). Those who had lost weight at 6 months “maintained 60% of this weight loss through 48 weeks of follow-up,” according to the FDA.
After placement of the device, common adverse events were vomiting, nausea, and abdominal pain, but most symptoms resolved within 30 days, according to the FDA. The development of gastric ulcerations is described as the “most worrisome” device-related risk, but “there were no unanticipated adverse device effects, no deaths, no intestinal obstructions, and no gastric perforations” in the study.
Among the 265 patients who received the device (those initially enrolled in the pivotal trial plus 78 who were in the control group and opted to receive the device after the first 6 months), 20 (7.5%) experienced severe adverse events; vomiting was the most common, in 4.5%. Serious events included gastric ulcers in two patients (0.8%) at 19 and at 97 days after the device was placed; in both cases, the device was removed. Almost 15% of those who received the device had to have it removed because of an adverse event. The rate of gastric ulcers after a minor change was made to the device was 10%; and the rate of balloon deflations without migration was 6%.
The FDA summary of the approval refers to the “marginal benefit of weight loss” among those in the treatment group, compared with controls, but adds that the decision to approve the device “is based in part on the limited options available to patients with mild to moderate obesity who have failed other means for conservative weight loss.” While the effectiveness of the device is better than what would be expected with diet and exercise or pharmacologic therapy,” it is “substantially less than what would be expected with gastric banding or other surgical interventions.” The list of contraindications includes previous gastrointestinal surgery “with sequelae,” such as an obstruction or adhesions; previous bariatric surgery; any GI inflammatory disease, severe coagulopathy; and women who are pregnant or breastfeeding.
“The company plans to make the ReShape procedure available to patients first in select markets, as physicians and allied health professionals are trained in the procedure and support program to optimize patient outcome,” according to the company’s statement announcing approval.
The ReShape device has been available in Europe since 2007.
Information posted by the FDA, including labeling for professionals, is available at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P140012.
Extreme weight states may activate the HPA axis
Cortisol measures in women vary across the weight spectrum, with the lowest levels occurring in overweight-class 1 obese women, and the highest levels occurring in those with anorexia nervosa, according to findings from a cross-sectional study.
Cortisol levels rise with more significant obesity – but not to levels as high as those seen in women with anorexia nervosa, suggesting that extreme underweight and overweight states may activate the hypothalamic-pituitary-adrenal (HPA) axis and that hypercortisolemia may contribute to increased adiposity in those with caloric excess, Dr. Melanie Schorr and her colleagues at Massachusetts General Hospital and Harvard Medical School, Boston, reported online in the Journal of Clinical Endocrinology & Metabolism.
Among the 60 women, aged 18-45 years, who were included in the study, 21 were overweight/obese, 18 had anorexia nervosa, and 21 were of normal weight. A U-shaped relationship was seen between cortisol measures and body mass index (most notably between urinary free cortisol/creatinine clearance [UFC/CrCl] and BMI and between mean overnight serum cortisol and BMI, r = 0.55 and 0.66, respectively), and between cortisol measures and visceral adipose tissue and total fat mass (for example, r = 0.50 for UFC/CrCl and adipose tissue, and 0.61 for UFC/CrCl and total fat mass), and either no relationship or a weak negative linear relationship was observed between lean mass and cortisol measures (for example, r = –0.34 for UFC/CrCL and lean mass). The latter “may be because it is the adipose component that is associated with cortisol measures or because hypercortisolemia contributes to muscle wasting,” the investigators wrote (J. Clin. Endocrinol. Metab. 2015 July [doi:10.1210/JC.2015-2078]).
They also noted that cortisol measures were negatively associated with bone mineral density across the weight spectrum between urinary free cortisol/creatinine clearance and lean body mass, suggesting that “relative hypercortisolemia may contribute to bone loss in the setting of both caloric restriction and excess.”
“Given the fact that obesity has reached epidemic proportions and significantly increases the risk of the metabolic syndrome and cardiovascular disease among other comorbidities, insight into the factors that contribute to obesity and/or its complications may have important therapeutic implications,” they concluded.
The authors reported having no disclosures.
Cortisol measures in women vary across the weight spectrum, with the lowest levels occurring in overweight-class 1 obese women, and the highest levels occurring in those with anorexia nervosa, according to findings from a cross-sectional study.
Cortisol levels rise with more significant obesity – but not to levels as high as those seen in women with anorexia nervosa, suggesting that extreme underweight and overweight states may activate the hypothalamic-pituitary-adrenal (HPA) axis and that hypercortisolemia may contribute to increased adiposity in those with caloric excess, Dr. Melanie Schorr and her colleagues at Massachusetts General Hospital and Harvard Medical School, Boston, reported online in the Journal of Clinical Endocrinology & Metabolism.
Among the 60 women, aged 18-45 years, who were included in the study, 21 were overweight/obese, 18 had anorexia nervosa, and 21 were of normal weight. A U-shaped relationship was seen between cortisol measures and body mass index (most notably between urinary free cortisol/creatinine clearance [UFC/CrCl] and BMI and between mean overnight serum cortisol and BMI, r = 0.55 and 0.66, respectively), and between cortisol measures and visceral adipose tissue and total fat mass (for example, r = 0.50 for UFC/CrCl and adipose tissue, and 0.61 for UFC/CrCl and total fat mass), and either no relationship or a weak negative linear relationship was observed between lean mass and cortisol measures (for example, r = –0.34 for UFC/CrCL and lean mass). The latter “may be because it is the adipose component that is associated with cortisol measures or because hypercortisolemia contributes to muscle wasting,” the investigators wrote (J. Clin. Endocrinol. Metab. 2015 July [doi:10.1210/JC.2015-2078]).
They also noted that cortisol measures were negatively associated with bone mineral density across the weight spectrum between urinary free cortisol/creatinine clearance and lean body mass, suggesting that “relative hypercortisolemia may contribute to bone loss in the setting of both caloric restriction and excess.”
“Given the fact that obesity has reached epidemic proportions and significantly increases the risk of the metabolic syndrome and cardiovascular disease among other comorbidities, insight into the factors that contribute to obesity and/or its complications may have important therapeutic implications,” they concluded.
The authors reported having no disclosures.
Cortisol measures in women vary across the weight spectrum, with the lowest levels occurring in overweight-class 1 obese women, and the highest levels occurring in those with anorexia nervosa, according to findings from a cross-sectional study.
Cortisol levels rise with more significant obesity – but not to levels as high as those seen in women with anorexia nervosa, suggesting that extreme underweight and overweight states may activate the hypothalamic-pituitary-adrenal (HPA) axis and that hypercortisolemia may contribute to increased adiposity in those with caloric excess, Dr. Melanie Schorr and her colleagues at Massachusetts General Hospital and Harvard Medical School, Boston, reported online in the Journal of Clinical Endocrinology & Metabolism.
Among the 60 women, aged 18-45 years, who were included in the study, 21 were overweight/obese, 18 had anorexia nervosa, and 21 were of normal weight. A U-shaped relationship was seen between cortisol measures and body mass index (most notably between urinary free cortisol/creatinine clearance [UFC/CrCl] and BMI and between mean overnight serum cortisol and BMI, r = 0.55 and 0.66, respectively), and between cortisol measures and visceral adipose tissue and total fat mass (for example, r = 0.50 for UFC/CrCl and adipose tissue, and 0.61 for UFC/CrCl and total fat mass), and either no relationship or a weak negative linear relationship was observed between lean mass and cortisol measures (for example, r = –0.34 for UFC/CrCL and lean mass). The latter “may be because it is the adipose component that is associated with cortisol measures or because hypercortisolemia contributes to muscle wasting,” the investigators wrote (J. Clin. Endocrinol. Metab. 2015 July [doi:10.1210/JC.2015-2078]).
They also noted that cortisol measures were negatively associated with bone mineral density across the weight spectrum between urinary free cortisol/creatinine clearance and lean body mass, suggesting that “relative hypercortisolemia may contribute to bone loss in the setting of both caloric restriction and excess.”
“Given the fact that obesity has reached epidemic proportions and significantly increases the risk of the metabolic syndrome and cardiovascular disease among other comorbidities, insight into the factors that contribute to obesity and/or its complications may have important therapeutic implications,” they concluded.
The authors reported having no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Key clinical point: The variation in cortisol measures suggest that extreme underweight and overweight states may activate the hypothalamic-pituitary-adrenal axis.
Major finding: A U-shaped relationship was seen between cortisol measures and BMI (most notably between urinary free cortisol/creatinine clearance [UFC/CrCl] and BMI, and between mean overnight serum cortisol and BMI, R = 0.55 and 0.66, respectively).
Data source: A cross-sectional study of 60 women.
Disclosures: The authors reported having no disclosures.
Reducing soda consumption could mean lower type 2 diabetes incidence
Drinking sugar-sweetened beverages is associated with a greater incidence of type 2 diabetes, independent of obesity, according to a systematic review and meta-analysis.
The researchers analyzed data from prospective design studies that assessed the consumption of beverages and incident type 2 diabetes, recruited adults free of diabetes, and followed study participants for at least 2 years. The data came from 17 cohorts comprising 38,253 cases of type 2 diabetes over 10,126,756 person-years. Using risk estimates, sampling weights, and population size, the researchers estimated the absolute number of incidents of type 2 diabetes over 10 years, the number of incidents of type 2 diabetes attributable to consumption of sugar-sweetened beverages, and the proportion of the disease incidents attributable to drinking of sugar-sweetened beverages. These estimations assumed causality and no change in individuals’ characteristics over time.
Higher consumption of sugar-sweetened beverages by one serving per day was associated with an 18% greater incidence of type 2 diabetes (95% confidence interval, 8.8%-28%; I2 [for heterogeneity] = 89%), before adjustment for adiposity. When adjusted for potential mediation and confounding by adiposity, the association was weakened to a 13% greater incidence of the disease.
Associations between drinking of artificially sweetened beverages and fruit juice were also found, but findings for artificially sweetened beverages were likely affected by publication bias and residual confounding; for fruit juice, the positive association was not stable and sensitive to study design.
“Under assumption of causality for the association of consumption of sugar-sweetened beverages with incident type 2 diabetes, we provided efficacy estimates that over 10 years two million type 2 diabetes events in the U.S. and 80,000 in the U.K. would be related to consumption of sugar-sweetened beverages,” according to Dr. Fumiaki Imamura of the University of Cambridge School of Clinical Medicine, and his colleagues.
“Future work should seek to improve precision of evidence and characterize efficacy and effectiveness of policy interventions for different populations,” according to the researchers.
Read the full study in the BMJ (doi:10.1136/bmj.h3576).
Drinking sugar-sweetened beverages is associated with a greater incidence of type 2 diabetes, independent of obesity, according to a systematic review and meta-analysis.
The researchers analyzed data from prospective design studies that assessed the consumption of beverages and incident type 2 diabetes, recruited adults free of diabetes, and followed study participants for at least 2 years. The data came from 17 cohorts comprising 38,253 cases of type 2 diabetes over 10,126,756 person-years. Using risk estimates, sampling weights, and population size, the researchers estimated the absolute number of incidents of type 2 diabetes over 10 years, the number of incidents of type 2 diabetes attributable to consumption of sugar-sweetened beverages, and the proportion of the disease incidents attributable to drinking of sugar-sweetened beverages. These estimations assumed causality and no change in individuals’ characteristics over time.
Higher consumption of sugar-sweetened beverages by one serving per day was associated with an 18% greater incidence of type 2 diabetes (95% confidence interval, 8.8%-28%; I2 [for heterogeneity] = 89%), before adjustment for adiposity. When adjusted for potential mediation and confounding by adiposity, the association was weakened to a 13% greater incidence of the disease.
Associations between drinking of artificially sweetened beverages and fruit juice were also found, but findings for artificially sweetened beverages were likely affected by publication bias and residual confounding; for fruit juice, the positive association was not stable and sensitive to study design.
“Under assumption of causality for the association of consumption of sugar-sweetened beverages with incident type 2 diabetes, we provided efficacy estimates that over 10 years two million type 2 diabetes events in the U.S. and 80,000 in the U.K. would be related to consumption of sugar-sweetened beverages,” according to Dr. Fumiaki Imamura of the University of Cambridge School of Clinical Medicine, and his colleagues.
“Future work should seek to improve precision of evidence and characterize efficacy and effectiveness of policy interventions for different populations,” according to the researchers.
Read the full study in the BMJ (doi:10.1136/bmj.h3576).
Drinking sugar-sweetened beverages is associated with a greater incidence of type 2 diabetes, independent of obesity, according to a systematic review and meta-analysis.
The researchers analyzed data from prospective design studies that assessed the consumption of beverages and incident type 2 diabetes, recruited adults free of diabetes, and followed study participants for at least 2 years. The data came from 17 cohorts comprising 38,253 cases of type 2 diabetes over 10,126,756 person-years. Using risk estimates, sampling weights, and population size, the researchers estimated the absolute number of incidents of type 2 diabetes over 10 years, the number of incidents of type 2 diabetes attributable to consumption of sugar-sweetened beverages, and the proportion of the disease incidents attributable to drinking of sugar-sweetened beverages. These estimations assumed causality and no change in individuals’ characteristics over time.
Higher consumption of sugar-sweetened beverages by one serving per day was associated with an 18% greater incidence of type 2 diabetes (95% confidence interval, 8.8%-28%; I2 [for heterogeneity] = 89%), before adjustment for adiposity. When adjusted for potential mediation and confounding by adiposity, the association was weakened to a 13% greater incidence of the disease.
Associations between drinking of artificially sweetened beverages and fruit juice were also found, but findings for artificially sweetened beverages were likely affected by publication bias and residual confounding; for fruit juice, the positive association was not stable and sensitive to study design.
“Under assumption of causality for the association of consumption of sugar-sweetened beverages with incident type 2 diabetes, we provided efficacy estimates that over 10 years two million type 2 diabetes events in the U.S. and 80,000 in the U.K. would be related to consumption of sugar-sweetened beverages,” according to Dr. Fumiaki Imamura of the University of Cambridge School of Clinical Medicine, and his colleagues.
“Future work should seek to improve precision of evidence and characterize efficacy and effectiveness of policy interventions for different populations,” according to the researchers.
Read the full study in the BMJ (doi:10.1136/bmj.h3576).
FROM THE BMJ
LBW, unhealthy lifestyle together increase type 2 diabetes risk
A low birth weight and an unhealthy lifestyle increase risk of type 2 diabetes more than either factor alone, according to Dr. Yanping Li of Harvard T.H. Chan School of Public Health, Boston, and associates.
In a study of 11, 709 cases of type 2 diabetes, the odds ratio for type 2 diabetes as a result of low birth weight alone was 1.45 per kg under normal birth weight, and for unhealthy lifestyle alone, the OR was 2.1. When both factors were present, the OR increased significantly to 2.86.
If both factors were present, unhealthy lifestyle had significantly more impact on diabetes development, with 59% of the attributable proportion of joint effect, compared with just 22% for low birth weight and 18% for the interaction between the two.
“The finding suggests that most cases of type 2 diabetes could be prevented by the adoption of a healthier lifestyle, but simultaneous improvement of both prenatal and postnatal factors could further prevent additional cases,” the investigators concluded.
Find the full study in the BMJ (doi:10.1136/bmj.h3672).
A low birth weight and an unhealthy lifestyle increase risk of type 2 diabetes more than either factor alone, according to Dr. Yanping Li of Harvard T.H. Chan School of Public Health, Boston, and associates.
In a study of 11, 709 cases of type 2 diabetes, the odds ratio for type 2 diabetes as a result of low birth weight alone was 1.45 per kg under normal birth weight, and for unhealthy lifestyle alone, the OR was 2.1. When both factors were present, the OR increased significantly to 2.86.
If both factors were present, unhealthy lifestyle had significantly more impact on diabetes development, with 59% of the attributable proportion of joint effect, compared with just 22% for low birth weight and 18% for the interaction between the two.
“The finding suggests that most cases of type 2 diabetes could be prevented by the adoption of a healthier lifestyle, but simultaneous improvement of both prenatal and postnatal factors could further prevent additional cases,” the investigators concluded.
Find the full study in the BMJ (doi:10.1136/bmj.h3672).
A low birth weight and an unhealthy lifestyle increase risk of type 2 diabetes more than either factor alone, according to Dr. Yanping Li of Harvard T.H. Chan School of Public Health, Boston, and associates.
In a study of 11, 709 cases of type 2 diabetes, the odds ratio for type 2 diabetes as a result of low birth weight alone was 1.45 per kg under normal birth weight, and for unhealthy lifestyle alone, the OR was 2.1. When both factors were present, the OR increased significantly to 2.86.
If both factors were present, unhealthy lifestyle had significantly more impact on diabetes development, with 59% of the attributable proportion of joint effect, compared with just 22% for low birth weight and 18% for the interaction between the two.
“The finding suggests that most cases of type 2 diabetes could be prevented by the adoption of a healthier lifestyle, but simultaneous improvement of both prenatal and postnatal factors could further prevent additional cases,” the investigators concluded.
Find the full study in the BMJ (doi:10.1136/bmj.h3672).
CABG costs more in patients with diabetes
The rate of diabetic coronary artery bypass graft patients has increased more than fivefold in recent decades, and these patients are more likely to have worse outcomes and higher treatment costs, a study showed.
The percentage of patients who had diabetes among all those undergoing coronary artery bypass grafting (CABG) increased from 7% in the 1970s to 37% in the 2000s, according to a database study of 55,501 patients operated on at the Cleveland Clinic.
Patients were identified and preoperative, operative, and postoperative variables were identified, resulting in 45,139 nondiabetic patients assessed and 10,362 diabetic patients (defined as those diabetic patients pharmacologically treated with either insulin or an oral agent) evaluated. The endpoints assessed were in-hospital adverse outcomes as determined by the Society of Thoracic Surgeons National Database, in-hospital direct technical costs, and time-related mortality, according to Dr. Sajjad Raza and his colleagues at the Cleveland Clinic in the August issue of the Journal of Thoracic and Cardiovascular Surgery (150:294-301).
Compared with nondiabetics, diabetic patients undergoing CABG were older and were more likely to be overweight, to be women, and to have a history of heart failure, peripheral arterial disease, carotid disease, hypertension, renal failure, stroke, and advanced coronary artery disease. Over time, the cardiovascular risk profile of the entire population changed, becoming even more pronounced for all patients, but more so for diabetics.
Overall long-term survival at 6 months and at 1, 5 10, 15, and 20 years for diabetic patients was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with 97%, 97%, 90%, 76%, 59%, and 42% for nondiabetic patients, a significant difference at P <.0001.
Propensity matching of similar diabetic and nondiabetic patients showed that deep sternal wound infection and stroke occurred significantly more often in diabetics, although there were no significant differences in cost remaining after matching, even though the length of stay greater than 14 days remained higher for diabetic patients.
Among diabetics, overall survival at 6 months and at 1, 5, 10, 15, and 20 years after CABG was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with overall survival in nondiabetics at 97%, 97%, 90%, 76%, 59%, and 42%, respectively, a significant difference (P <.0001).
“Although long-term survival after CABG is worse in diabetics and high-risk nondiabetics, it is important to note that, in general, high-risk patients reap the greatest survival benefit from CABG. Moreover, using surgical techniques that are associated with better long-term survival after CABG in diabetics could further enhance this survival benefit,” Dr. Raza and his colleagues wrote.
“Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival,” they added. “Diabetic patients and those with a similar high-risk profile set to undergo CABG should be made aware that their risks of postoperative complications are higher than average, and measures should be taken to reduce their postoperative complications,” Dr. Raza and his colleagues concluded.
The authors reported that they had no relevant conflicts of interest.
Patients with diabetes, with or without metabolic syndrome, represent an increasing challenge for cardiac surgery. CABG has been shown to convey a mortality benefit in such patients who also have multivessel disease. This study confirms what most clinicians already know – that the outcomes of patients with diabetes are worse than those in nondiabetic patients, according to Dr. Mani Arsalan and Dr. Michael Mack. “What is particularly important about this study, however, is that it is a single institutional experience with known surgical excellence and a very meticulous and complete outcomes database,” they wrote (J. Thorac. Cardiovasc. Surg. 2015;150:284-5).
Given their findings and the fact that CABG can be expected to remain the mainstay of treatment of multivessel disease in diabetics because of the results of the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, surgeons should pay increased attention to the details of the procedure for these patients. There should be an increased use of bilateral internal mammary arteries, which has been distressingly low, and yet can provide a 23% mortality benefit. “Two arteries are better than one.” Despite the increased risk of deep sternal infection, “the use of skeletonized bilateral internal mammary arteries in young, nonobese diabetic patients with a greater than 10-year life expectancy seems a reasonable risk to take,” Dr. Arsalan and Dr. Mack wrote. In addition, where possible, reaching satisfactory glycemic control before surgery can help decrease early complications. “The weight may be increasingly on our patients, but the real weight is on us as surgeons to help improve their early and long-term survival,” they concluded.
Dr. Arsalan and Dr. Mack are cardiovascular surgeons at Baylor Scott & White Health, Dallas. Their remarks were part of an invited commentary published with the paper.
Patients with diabetes, with or without metabolic syndrome, represent an increasing challenge for cardiac surgery. CABG has been shown to convey a mortality benefit in such patients who also have multivessel disease. This study confirms what most clinicians already know – that the outcomes of patients with diabetes are worse than those in nondiabetic patients, according to Dr. Mani Arsalan and Dr. Michael Mack. “What is particularly important about this study, however, is that it is a single institutional experience with known surgical excellence and a very meticulous and complete outcomes database,” they wrote (J. Thorac. Cardiovasc. Surg. 2015;150:284-5).
Given their findings and the fact that CABG can be expected to remain the mainstay of treatment of multivessel disease in diabetics because of the results of the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, surgeons should pay increased attention to the details of the procedure for these patients. There should be an increased use of bilateral internal mammary arteries, which has been distressingly low, and yet can provide a 23% mortality benefit. “Two arteries are better than one.” Despite the increased risk of deep sternal infection, “the use of skeletonized bilateral internal mammary arteries in young, nonobese diabetic patients with a greater than 10-year life expectancy seems a reasonable risk to take,” Dr. Arsalan and Dr. Mack wrote. In addition, where possible, reaching satisfactory glycemic control before surgery can help decrease early complications. “The weight may be increasingly on our patients, but the real weight is on us as surgeons to help improve their early and long-term survival,” they concluded.
Dr. Arsalan and Dr. Mack are cardiovascular surgeons at Baylor Scott & White Health, Dallas. Their remarks were part of an invited commentary published with the paper.
Patients with diabetes, with or without metabolic syndrome, represent an increasing challenge for cardiac surgery. CABG has been shown to convey a mortality benefit in such patients who also have multivessel disease. This study confirms what most clinicians already know – that the outcomes of patients with diabetes are worse than those in nondiabetic patients, according to Dr. Mani Arsalan and Dr. Michael Mack. “What is particularly important about this study, however, is that it is a single institutional experience with known surgical excellence and a very meticulous and complete outcomes database,” they wrote (J. Thorac. Cardiovasc. Surg. 2015;150:284-5).
Given their findings and the fact that CABG can be expected to remain the mainstay of treatment of multivessel disease in diabetics because of the results of the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, surgeons should pay increased attention to the details of the procedure for these patients. There should be an increased use of bilateral internal mammary arteries, which has been distressingly low, and yet can provide a 23% mortality benefit. “Two arteries are better than one.” Despite the increased risk of deep sternal infection, “the use of skeletonized bilateral internal mammary arteries in young, nonobese diabetic patients with a greater than 10-year life expectancy seems a reasonable risk to take,” Dr. Arsalan and Dr. Mack wrote. In addition, where possible, reaching satisfactory glycemic control before surgery can help decrease early complications. “The weight may be increasingly on our patients, but the real weight is on us as surgeons to help improve their early and long-term survival,” they concluded.
Dr. Arsalan and Dr. Mack are cardiovascular surgeons at Baylor Scott & White Health, Dallas. Their remarks were part of an invited commentary published with the paper.
The rate of diabetic coronary artery bypass graft patients has increased more than fivefold in recent decades, and these patients are more likely to have worse outcomes and higher treatment costs, a study showed.
The percentage of patients who had diabetes among all those undergoing coronary artery bypass grafting (CABG) increased from 7% in the 1970s to 37% in the 2000s, according to a database study of 55,501 patients operated on at the Cleveland Clinic.
Patients were identified and preoperative, operative, and postoperative variables were identified, resulting in 45,139 nondiabetic patients assessed and 10,362 diabetic patients (defined as those diabetic patients pharmacologically treated with either insulin or an oral agent) evaluated. The endpoints assessed were in-hospital adverse outcomes as determined by the Society of Thoracic Surgeons National Database, in-hospital direct technical costs, and time-related mortality, according to Dr. Sajjad Raza and his colleagues at the Cleveland Clinic in the August issue of the Journal of Thoracic and Cardiovascular Surgery (150:294-301).
Compared with nondiabetics, diabetic patients undergoing CABG were older and were more likely to be overweight, to be women, and to have a history of heart failure, peripheral arterial disease, carotid disease, hypertension, renal failure, stroke, and advanced coronary artery disease. Over time, the cardiovascular risk profile of the entire population changed, becoming even more pronounced for all patients, but more so for diabetics.
Overall long-term survival at 6 months and at 1, 5 10, 15, and 20 years for diabetic patients was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with 97%, 97%, 90%, 76%, 59%, and 42% for nondiabetic patients, a significant difference at P <.0001.
Propensity matching of similar diabetic and nondiabetic patients showed that deep sternal wound infection and stroke occurred significantly more often in diabetics, although there were no significant differences in cost remaining after matching, even though the length of stay greater than 14 days remained higher for diabetic patients.
Among diabetics, overall survival at 6 months and at 1, 5, 10, 15, and 20 years after CABG was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with overall survival in nondiabetics at 97%, 97%, 90%, 76%, 59%, and 42%, respectively, a significant difference (P <.0001).
“Although long-term survival after CABG is worse in diabetics and high-risk nondiabetics, it is important to note that, in general, high-risk patients reap the greatest survival benefit from CABG. Moreover, using surgical techniques that are associated with better long-term survival after CABG in diabetics could further enhance this survival benefit,” Dr. Raza and his colleagues wrote.
“Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival,” they added. “Diabetic patients and those with a similar high-risk profile set to undergo CABG should be made aware that their risks of postoperative complications are higher than average, and measures should be taken to reduce their postoperative complications,” Dr. Raza and his colleagues concluded.
The authors reported that they had no relevant conflicts of interest.
The rate of diabetic coronary artery bypass graft patients has increased more than fivefold in recent decades, and these patients are more likely to have worse outcomes and higher treatment costs, a study showed.
The percentage of patients who had diabetes among all those undergoing coronary artery bypass grafting (CABG) increased from 7% in the 1970s to 37% in the 2000s, according to a database study of 55,501 patients operated on at the Cleveland Clinic.
Patients were identified and preoperative, operative, and postoperative variables were identified, resulting in 45,139 nondiabetic patients assessed and 10,362 diabetic patients (defined as those diabetic patients pharmacologically treated with either insulin or an oral agent) evaluated. The endpoints assessed were in-hospital adverse outcomes as determined by the Society of Thoracic Surgeons National Database, in-hospital direct technical costs, and time-related mortality, according to Dr. Sajjad Raza and his colleagues at the Cleveland Clinic in the August issue of the Journal of Thoracic and Cardiovascular Surgery (150:294-301).
Compared with nondiabetics, diabetic patients undergoing CABG were older and were more likely to be overweight, to be women, and to have a history of heart failure, peripheral arterial disease, carotid disease, hypertension, renal failure, stroke, and advanced coronary artery disease. Over time, the cardiovascular risk profile of the entire population changed, becoming even more pronounced for all patients, but more so for diabetics.
Overall long-term survival at 6 months and at 1, 5 10, 15, and 20 years for diabetic patients was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with 97%, 97%, 90%, 76%, 59%, and 42% for nondiabetic patients, a significant difference at P <.0001.
Propensity matching of similar diabetic and nondiabetic patients showed that deep sternal wound infection and stroke occurred significantly more often in diabetics, although there were no significant differences in cost remaining after matching, even though the length of stay greater than 14 days remained higher for diabetic patients.
Among diabetics, overall survival at 6 months and at 1, 5, 10, 15, and 20 years after CABG was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with overall survival in nondiabetics at 97%, 97%, 90%, 76%, 59%, and 42%, respectively, a significant difference (P <.0001).
“Although long-term survival after CABG is worse in diabetics and high-risk nondiabetics, it is important to note that, in general, high-risk patients reap the greatest survival benefit from CABG. Moreover, using surgical techniques that are associated with better long-term survival after CABG in diabetics could further enhance this survival benefit,” Dr. Raza and his colleagues wrote.
“Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival,” they added. “Diabetic patients and those with a similar high-risk profile set to undergo CABG should be made aware that their risks of postoperative complications are higher than average, and measures should be taken to reduce their postoperative complications,” Dr. Raza and his colleagues concluded.
The authors reported that they had no relevant conflicts of interest.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The percentage of CABG patients with diabetes increased from 7% in the 1970s to 37% in the 2000s. The risk/benefit ratio warrants greater use of bilateral mammary arteries except in obese women with diabetes.
Major finding: Diabetic patients had significantly worse outcomes than nondiabetics with regard to hospital death, deep sternal wound infections, strokes, and renal failure as well as hospital stay and costs.
Data source: A retrospective analysis of a prospective database of patients undergoing first-time CABG at the Cleveland Clinic from 1972 to 2011.
Disclosures: The authors reported that they had no relevant conflicts of interest.
Tailored family counseling reduces children’s BMI gain
Short but regular meetings aimed at helping families develop healthier eating and activity habits can lead to modest but significant drops in overweight children’s body mass index (BMI), a recent study found.
The intervention families also made small gains in healthier habits, such as slightly increasing their fruit and vegetable intake.
“Our data demonstrate that providing families of young overweight children with regular low-intensity support can make small but significant differences to body weight and lifestyle behaviors over 2 years,” wrote Rachael W. Taylor, Ph.D., of the University of Otago in Dunedin, New Zealand, and her associates. “Children in the tailored condition had smaller gains in BMI, were more physically active, had improved diets, and reported that fewer noncore foods were available in the home than children in usual care” (Pediatrics 2015 July 20 [doi:10.1542/peds.2015-0595]).
The researchers randomly assigned 206 children, aged 4-8 years and nearly all falling at or above the 85th percentile for their BMI, to usual care or to a set of regular personalized sessions of healthy lifestyle counseling. The families in the latter tailored intervention met with an multidisciplinary team – usually including both parents, a mentor, a dietitian, an exercise specialist, and a clinical psychologist – one time at baseline. They then met monthly the first year and quarterly the second year with a mentor. Throughout the program they received family-based, personalized guidance, set goals to work toward using behavioral strategies, and received resources as needed.
Those receiving usual care met with a researcher for 30-45 minutes at baseline and again for 15-30 minutes 6 months later. The researcher gave them generalized advice about children’s eating, physical activity, and sleep as well as personalized feedback about the diet and activity habits of their children. Total contact time in the extended intervention was 6-7 hours per family, compared with 45-75 minutes in the usual care group.
At 24 months, 181 children (88%) remained in the program. Children in the tailored program had a BMI 0.34 points lower and a BMI z score 0.12 points lower than did those in the usual care group, after accounting for baseline characteristics. The waist circumferences of children in the tailored program was also 1.5 cm smaller than were the children in the usual care group. The tailored program children also were more physically active than were the other children, but their moderate-vigorous physical activity, sedentary time, and sleep didn’t differ. Families in the tailored program had slightly higher fruit and vegetable intake.
The research was supported by the Health Research Council of New Zealand with additional funding from a Freemasons New Zealand Fellowship and a KPS Research Fellowship. The authors reported no disclosures.
Short but regular meetings aimed at helping families develop healthier eating and activity habits can lead to modest but significant drops in overweight children’s body mass index (BMI), a recent study found.
The intervention families also made small gains in healthier habits, such as slightly increasing their fruit and vegetable intake.
“Our data demonstrate that providing families of young overweight children with regular low-intensity support can make small but significant differences to body weight and lifestyle behaviors over 2 years,” wrote Rachael W. Taylor, Ph.D., of the University of Otago in Dunedin, New Zealand, and her associates. “Children in the tailored condition had smaller gains in BMI, were more physically active, had improved diets, and reported that fewer noncore foods were available in the home than children in usual care” (Pediatrics 2015 July 20 [doi:10.1542/peds.2015-0595]).
The researchers randomly assigned 206 children, aged 4-8 years and nearly all falling at or above the 85th percentile for their BMI, to usual care or to a set of regular personalized sessions of healthy lifestyle counseling. The families in the latter tailored intervention met with an multidisciplinary team – usually including both parents, a mentor, a dietitian, an exercise specialist, and a clinical psychologist – one time at baseline. They then met monthly the first year and quarterly the second year with a mentor. Throughout the program they received family-based, personalized guidance, set goals to work toward using behavioral strategies, and received resources as needed.
Those receiving usual care met with a researcher for 30-45 minutes at baseline and again for 15-30 minutes 6 months later. The researcher gave them generalized advice about children’s eating, physical activity, and sleep as well as personalized feedback about the diet and activity habits of their children. Total contact time in the extended intervention was 6-7 hours per family, compared with 45-75 minutes in the usual care group.
At 24 months, 181 children (88%) remained in the program. Children in the tailored program had a BMI 0.34 points lower and a BMI z score 0.12 points lower than did those in the usual care group, after accounting for baseline characteristics. The waist circumferences of children in the tailored program was also 1.5 cm smaller than were the children in the usual care group. The tailored program children also were more physically active than were the other children, but their moderate-vigorous physical activity, sedentary time, and sleep didn’t differ. Families in the tailored program had slightly higher fruit and vegetable intake.
The research was supported by the Health Research Council of New Zealand with additional funding from a Freemasons New Zealand Fellowship and a KPS Research Fellowship. The authors reported no disclosures.
Short but regular meetings aimed at helping families develop healthier eating and activity habits can lead to modest but significant drops in overweight children’s body mass index (BMI), a recent study found.
The intervention families also made small gains in healthier habits, such as slightly increasing their fruit and vegetable intake.
“Our data demonstrate that providing families of young overweight children with regular low-intensity support can make small but significant differences to body weight and lifestyle behaviors over 2 years,” wrote Rachael W. Taylor, Ph.D., of the University of Otago in Dunedin, New Zealand, and her associates. “Children in the tailored condition had smaller gains in BMI, were more physically active, had improved diets, and reported that fewer noncore foods were available in the home than children in usual care” (Pediatrics 2015 July 20 [doi:10.1542/peds.2015-0595]).
The researchers randomly assigned 206 children, aged 4-8 years and nearly all falling at or above the 85th percentile for their BMI, to usual care or to a set of regular personalized sessions of healthy lifestyle counseling. The families in the latter tailored intervention met with an multidisciplinary team – usually including both parents, a mentor, a dietitian, an exercise specialist, and a clinical psychologist – one time at baseline. They then met monthly the first year and quarterly the second year with a mentor. Throughout the program they received family-based, personalized guidance, set goals to work toward using behavioral strategies, and received resources as needed.
Those receiving usual care met with a researcher for 30-45 minutes at baseline and again for 15-30 minutes 6 months later. The researcher gave them generalized advice about children’s eating, physical activity, and sleep as well as personalized feedback about the diet and activity habits of their children. Total contact time in the extended intervention was 6-7 hours per family, compared with 45-75 minutes in the usual care group.
At 24 months, 181 children (88%) remained in the program. Children in the tailored program had a BMI 0.34 points lower and a BMI z score 0.12 points lower than did those in the usual care group, after accounting for baseline characteristics. The waist circumferences of children in the tailored program was also 1.5 cm smaller than were the children in the usual care group. The tailored program children also were more physically active than were the other children, but their moderate-vigorous physical activity, sedentary time, and sleep didn’t differ. Families in the tailored program had slightly higher fruit and vegetable intake.
The research was supported by the Health Research Council of New Zealand with additional funding from a Freemasons New Zealand Fellowship and a KPS Research Fellowship. The authors reported no disclosures.
FROM PEDIATRICS
Key clinical point: Regular support to families can reduce weight in overweight children.
Major finding: After 2 years, children in the intervention group had 0.34 lower BMIs and 0.12 lower BMI z scores than did those in usual care.
Data source: The findings are based on a randomized, nonblinded 2-year trial of 206 mild to moderately overweight children, aged 4-8 years, in New Zealand.
Disclosures: The research was supported by the Health Research Council of New Zealand with additional funding from a Freemasons New Zealand Fellowship and a KPS Research Fellowship. The authors reported no disclosures.
A call to action on metabolic syndrome and pediatric psoriasis
VANCOUVER – Dermatologists and primary care physicians working collaboratively have a golden opportunity to improve the long-term health of pediatric psoriasis patients by addressing their predisposition to components of the metabolic syndrome, Dr. Amy S. Paller declared at the World Congress of Dermatology.
“I think we as dermatologists should be in touch with the primary care doctors of every one of our children with psoriasis. Together, we should be thinking about whether the child has metabolic issues and working jointly to most effectively counsel and evaluate these children for their potential risk for these metabolic disorders,” said Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University, Chicago.
Pediatric psoriasis is commonly associated with other comorbid conditions in addition to metabolic disorders. But the metabolic syndrome has recently become the focus of increasing attention given that cardiovascular disease is the No. 1 cause of death in the United States, and it appears that children with psoriasis may be getting a jump start on the atherosclerotic process.
By now, it’s well established that plaque psoriasis in adults is strongly associated with increased risks of diabetes, obesity, dyslipidemia, the metabolic syndrome, and cardiovascular disease. Mounting evidence indicates children and adolescents with psoriasis face the same risks.
Everyone knows how difficult it can be to make the long-term lifestyle changes that reverse obesity and its related metabolic disorders. But dermatologists, pediatricians, and family physicians have some leverage when it comes to pediatric psoriasis.
“Think about the fact that 30% of children with psoriasis have a first-degree relative with psoriasis, usually a parent. I think we need to think about counseling young adults with psoriasis early on, especially if that adult is overweight or obese, about the need for adopting a healthy lifestyle. If they do that, it’s not just for themselves but for their children, and we just might prevent pediatric psoriasis in that family or temper its severity through that healthy lifestyle intervention,” Dr. Paller continued.
The hope is that effectively addressing the metabolic comorbidities of pediatric psoriasis will modulate and improve the skin disease; in other words, that weight loss could improve psoriasis. As yet, however, that’s just a hope, as there is no persuasive supporting evidence.
“We’re looking towards ongoing adult trials to give us some clues about whether that’s the case,” she said.
Evidence for comorbidities
Some of the key evidence regarding the metabolic comorbidities of pediatric psoriasis comes from a landmark German epidemiologic study involving 33,981 pediatric psoriasis patients. The prevalence of psoriasis in German youth rose linearly from 0.12% at age 1 year to 1.2% at age 18. Pediatric psoriasis patients had significantly higher rates of diabetes, hyperlipidemia, obesity, and hypertension than did nonpsoriatic controls (Br. J. Dermatol. 2010;162:633-6).
A Kaiser Permanente study of nearly 711,000 youths aged 2-19 years showed that those who were overweight were 2.8-fold more likely than normal-weight youth to have severe or widespread psoriasis, while those who were moderately obese were at 2.9-fold increased risk and extremely obese youth were at 4.2-fold increased risk. Among adolescents, having psoriasis was associated with significantly higher mean total and LDL cholesterol, triglycerides, and alanine aminotransferase levels (J. Pediatr. 2011;159:577-83).
Recent evidence suggests that even before increased levels of LDL cholesterol and triglycerides are apparent in children with psoriasis, abnormalities in lipid function are present and may potentially serve as a novel marker for early cardiovascular risk. Dr. Paller cited a study presented by Dr. Wynnis L. Tom of Rady Children’s Hospital, San Diego, at the 2015 annual meeting of the Society for Investigative Dermatology. The case-control study included 50 children with psoriasis and 50 matched controls with a mean age of 13 years.
Like other investigators, Dr. Tom found that the psoriatic children had higher waist/hip ratios and more insulin resistance. While fasting lipid levels didn’t differ between the two groups, the psoriasis patients had significantly higher levels of atherogenic apolipoprotein B, fewer of the particularly cardioprotective large-size HDL particles, and reduced HDL efflux capacity. Stay tuned regarding these potential early markers, Dr. Paller advised.
She was lead author of a 409-patient international study that showed the risks of obesity and a high waist circumference rise with greater severity of pediatric psoriasis. Children with severe psoriasis were at 4.92-fold increased risk of obesity, compared with controls, while even those with mild psoriasis were at 3.6-fold increased risk (JAMA Dermatol. 2013;149:166-76).
Which comes first?
The question arises: Which comes first in children, the excess adiposity or the psoriasis? Dr. Paller said that although the final word isn’t in, she and her coworkers found in a pilot study of 27 overweight or obese children with psoriasis that excess adiposity typically came first. Moreover, among the roughly one-half of children with a family history of obesity, onset of psoriasis occurred a full 3 years earlier than in those without a positive family history (JAMA Dermatol. 2014;150:573-4).
In another small study, this by investigators at Tufts University, Boston, 6 of 20 children with psoriasis (30%) met criteria for the metabolic syndrome, compared with just 1 of 20 matched nonpsoriatic controls (Pediatr. Dermatol. 2013;30:700-5).
Dr. Paller said that if dermatologists and primary care physicians are to successfully collaborate in tackling the comorbid metabolic disorders associated with pediatric psoriasis, a prerequisite is that dermatologists are going to have to do a better job of educating their primary care colleagues about the skin disease as manifest in children.
“I think it’s very important that pediatricians are aware that psoriasis is a risk factor for metabolic syndrome. But pediatric psoriasis is often misdiagnosed by primary care physicians who mistake it for eczema or tinea infection or contact dermatitis,” according to the pediatric dermatologist.
In one eye-catching Australian study, she noted, a mere 9% of patients with pediatric psoriasis were correctly diagnosed before referral to a dermatologist (Australas. J. Dermatol. 2012;53:98-105).
Pediatric psoriasis: not just skin deep
In addition to the increased risk of metabolic disorders faced by pediatric psoriasis patients, other common comorbidities include depression, anxiety disorders, impaired self-esteem and quality of life, arthritis, and Crohn’s disease, Dr. Paller observed.
• Quality of life. “The quality of life impact of psoriasis is profound. It’s a highly visible disorder, which affects the development of self-esteem and social relationships,” Dr. Paller said.
Investigators at Texas A&M University applied the Pediatric Quality of Life Inventory Version 4.0 to 208 patients aged 2-17 years with moderate to severe psoriasis and compared the results to published data on children with arthritis, asthma, diabetes, and psychiatric disorders. Health-related quality of life turned out to be more impaired in the psoriasis patients than in those with diabetes. The quality-of-life impairment associated with pediatric psoriasis was comparable to that of having asthma or arthritis, albeit not as severe as for pediatric psychiatric disorders (Eur. J. Pediatr. 2012;171:485-92).
• Psychiatric disorders. A study of more than 7,400 pediatric psoriasis patients concluded they had an adjusted 25% increased risk of developing depression, compared with psoriasis-free controls, as well as a 32% increased risk of anxiety disorders and a 55% greater risk of bipolar disorder (J. Am. Acad. Dermatol. 2012;67:651-7.e2).
• Psoriatic arthritis. An estimated 1 in 10 U.S. children with psoriasis report having arthritis, often classified as juvenile idiopathic arthritis (JAMA Dermatol. 2013;149:1180-5).
• Crohn’s disease. A large German epidemiologic study concluded that psoriasis was associated with a 3.69-fold increased risk of Crohn’s disease. There was no increased risk of ulcerative colitis (Br. J. Dermatol. 2010;162:633-6).
Dr. Paller reported receiving research grants from Amgen and Leo and serving as a consultant to AbbVie.
VANCOUVER – Dermatologists and primary care physicians working collaboratively have a golden opportunity to improve the long-term health of pediatric psoriasis patients by addressing their predisposition to components of the metabolic syndrome, Dr. Amy S. Paller declared at the World Congress of Dermatology.
“I think we as dermatologists should be in touch with the primary care doctors of every one of our children with psoriasis. Together, we should be thinking about whether the child has metabolic issues and working jointly to most effectively counsel and evaluate these children for their potential risk for these metabolic disorders,” said Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University, Chicago.
Pediatric psoriasis is commonly associated with other comorbid conditions in addition to metabolic disorders. But the metabolic syndrome has recently become the focus of increasing attention given that cardiovascular disease is the No. 1 cause of death in the United States, and it appears that children with psoriasis may be getting a jump start on the atherosclerotic process.
By now, it’s well established that plaque psoriasis in adults is strongly associated with increased risks of diabetes, obesity, dyslipidemia, the metabolic syndrome, and cardiovascular disease. Mounting evidence indicates children and adolescents with psoriasis face the same risks.
Everyone knows how difficult it can be to make the long-term lifestyle changes that reverse obesity and its related metabolic disorders. But dermatologists, pediatricians, and family physicians have some leverage when it comes to pediatric psoriasis.
“Think about the fact that 30% of children with psoriasis have a first-degree relative with psoriasis, usually a parent. I think we need to think about counseling young adults with psoriasis early on, especially if that adult is overweight or obese, about the need for adopting a healthy lifestyle. If they do that, it’s not just for themselves but for their children, and we just might prevent pediatric psoriasis in that family or temper its severity through that healthy lifestyle intervention,” Dr. Paller continued.
The hope is that effectively addressing the metabolic comorbidities of pediatric psoriasis will modulate and improve the skin disease; in other words, that weight loss could improve psoriasis. As yet, however, that’s just a hope, as there is no persuasive supporting evidence.
“We’re looking towards ongoing adult trials to give us some clues about whether that’s the case,” she said.
Evidence for comorbidities
Some of the key evidence regarding the metabolic comorbidities of pediatric psoriasis comes from a landmark German epidemiologic study involving 33,981 pediatric psoriasis patients. The prevalence of psoriasis in German youth rose linearly from 0.12% at age 1 year to 1.2% at age 18. Pediatric psoriasis patients had significantly higher rates of diabetes, hyperlipidemia, obesity, and hypertension than did nonpsoriatic controls (Br. J. Dermatol. 2010;162:633-6).
A Kaiser Permanente study of nearly 711,000 youths aged 2-19 years showed that those who were overweight were 2.8-fold more likely than normal-weight youth to have severe or widespread psoriasis, while those who were moderately obese were at 2.9-fold increased risk and extremely obese youth were at 4.2-fold increased risk. Among adolescents, having psoriasis was associated with significantly higher mean total and LDL cholesterol, triglycerides, and alanine aminotransferase levels (J. Pediatr. 2011;159:577-83).
Recent evidence suggests that even before increased levels of LDL cholesterol and triglycerides are apparent in children with psoriasis, abnormalities in lipid function are present and may potentially serve as a novel marker for early cardiovascular risk. Dr. Paller cited a study presented by Dr. Wynnis L. Tom of Rady Children’s Hospital, San Diego, at the 2015 annual meeting of the Society for Investigative Dermatology. The case-control study included 50 children with psoriasis and 50 matched controls with a mean age of 13 years.
Like other investigators, Dr. Tom found that the psoriatic children had higher waist/hip ratios and more insulin resistance. While fasting lipid levels didn’t differ between the two groups, the psoriasis patients had significantly higher levels of atherogenic apolipoprotein B, fewer of the particularly cardioprotective large-size HDL particles, and reduced HDL efflux capacity. Stay tuned regarding these potential early markers, Dr. Paller advised.
She was lead author of a 409-patient international study that showed the risks of obesity and a high waist circumference rise with greater severity of pediatric psoriasis. Children with severe psoriasis were at 4.92-fold increased risk of obesity, compared with controls, while even those with mild psoriasis were at 3.6-fold increased risk (JAMA Dermatol. 2013;149:166-76).
Which comes first?
The question arises: Which comes first in children, the excess adiposity or the psoriasis? Dr. Paller said that although the final word isn’t in, she and her coworkers found in a pilot study of 27 overweight or obese children with psoriasis that excess adiposity typically came first. Moreover, among the roughly one-half of children with a family history of obesity, onset of psoriasis occurred a full 3 years earlier than in those without a positive family history (JAMA Dermatol. 2014;150:573-4).
In another small study, this by investigators at Tufts University, Boston, 6 of 20 children with psoriasis (30%) met criteria for the metabolic syndrome, compared with just 1 of 20 matched nonpsoriatic controls (Pediatr. Dermatol. 2013;30:700-5).
Dr. Paller said that if dermatologists and primary care physicians are to successfully collaborate in tackling the comorbid metabolic disorders associated with pediatric psoriasis, a prerequisite is that dermatologists are going to have to do a better job of educating their primary care colleagues about the skin disease as manifest in children.
“I think it’s very important that pediatricians are aware that psoriasis is a risk factor for metabolic syndrome. But pediatric psoriasis is often misdiagnosed by primary care physicians who mistake it for eczema or tinea infection or contact dermatitis,” according to the pediatric dermatologist.
In one eye-catching Australian study, she noted, a mere 9% of patients with pediatric psoriasis were correctly diagnosed before referral to a dermatologist (Australas. J. Dermatol. 2012;53:98-105).
Pediatric psoriasis: not just skin deep
In addition to the increased risk of metabolic disorders faced by pediatric psoriasis patients, other common comorbidities include depression, anxiety disorders, impaired self-esteem and quality of life, arthritis, and Crohn’s disease, Dr. Paller observed.
• Quality of life. “The quality of life impact of psoriasis is profound. It’s a highly visible disorder, which affects the development of self-esteem and social relationships,” Dr. Paller said.
Investigators at Texas A&M University applied the Pediatric Quality of Life Inventory Version 4.0 to 208 patients aged 2-17 years with moderate to severe psoriasis and compared the results to published data on children with arthritis, asthma, diabetes, and psychiatric disorders. Health-related quality of life turned out to be more impaired in the psoriasis patients than in those with diabetes. The quality-of-life impairment associated with pediatric psoriasis was comparable to that of having asthma or arthritis, albeit not as severe as for pediatric psychiatric disorders (Eur. J. Pediatr. 2012;171:485-92).
• Psychiatric disorders. A study of more than 7,400 pediatric psoriasis patients concluded they had an adjusted 25% increased risk of developing depression, compared with psoriasis-free controls, as well as a 32% increased risk of anxiety disorders and a 55% greater risk of bipolar disorder (J. Am. Acad. Dermatol. 2012;67:651-7.e2).
• Psoriatic arthritis. An estimated 1 in 10 U.S. children with psoriasis report having arthritis, often classified as juvenile idiopathic arthritis (JAMA Dermatol. 2013;149:1180-5).
• Crohn’s disease. A large German epidemiologic study concluded that psoriasis was associated with a 3.69-fold increased risk of Crohn’s disease. There was no increased risk of ulcerative colitis (Br. J. Dermatol. 2010;162:633-6).
Dr. Paller reported receiving research grants from Amgen and Leo and serving as a consultant to AbbVie.
VANCOUVER – Dermatologists and primary care physicians working collaboratively have a golden opportunity to improve the long-term health of pediatric psoriasis patients by addressing their predisposition to components of the metabolic syndrome, Dr. Amy S. Paller declared at the World Congress of Dermatology.
“I think we as dermatologists should be in touch with the primary care doctors of every one of our children with psoriasis. Together, we should be thinking about whether the child has metabolic issues and working jointly to most effectively counsel and evaluate these children for their potential risk for these metabolic disorders,” said Dr. Paller, professor and chair of the department of dermatology and professor of pediatrics at Northwestern University, Chicago.
Pediatric psoriasis is commonly associated with other comorbid conditions in addition to metabolic disorders. But the metabolic syndrome has recently become the focus of increasing attention given that cardiovascular disease is the No. 1 cause of death in the United States, and it appears that children with psoriasis may be getting a jump start on the atherosclerotic process.
By now, it’s well established that plaque psoriasis in adults is strongly associated with increased risks of diabetes, obesity, dyslipidemia, the metabolic syndrome, and cardiovascular disease. Mounting evidence indicates children and adolescents with psoriasis face the same risks.
Everyone knows how difficult it can be to make the long-term lifestyle changes that reverse obesity and its related metabolic disorders. But dermatologists, pediatricians, and family physicians have some leverage when it comes to pediatric psoriasis.
“Think about the fact that 30% of children with psoriasis have a first-degree relative with psoriasis, usually a parent. I think we need to think about counseling young adults with psoriasis early on, especially if that adult is overweight or obese, about the need for adopting a healthy lifestyle. If they do that, it’s not just for themselves but for their children, and we just might prevent pediatric psoriasis in that family or temper its severity through that healthy lifestyle intervention,” Dr. Paller continued.
The hope is that effectively addressing the metabolic comorbidities of pediatric psoriasis will modulate and improve the skin disease; in other words, that weight loss could improve psoriasis. As yet, however, that’s just a hope, as there is no persuasive supporting evidence.
“We’re looking towards ongoing adult trials to give us some clues about whether that’s the case,” she said.
Evidence for comorbidities
Some of the key evidence regarding the metabolic comorbidities of pediatric psoriasis comes from a landmark German epidemiologic study involving 33,981 pediatric psoriasis patients. The prevalence of psoriasis in German youth rose linearly from 0.12% at age 1 year to 1.2% at age 18. Pediatric psoriasis patients had significantly higher rates of diabetes, hyperlipidemia, obesity, and hypertension than did nonpsoriatic controls (Br. J. Dermatol. 2010;162:633-6).
A Kaiser Permanente study of nearly 711,000 youths aged 2-19 years showed that those who were overweight were 2.8-fold more likely than normal-weight youth to have severe or widespread psoriasis, while those who were moderately obese were at 2.9-fold increased risk and extremely obese youth were at 4.2-fold increased risk. Among adolescents, having psoriasis was associated with significantly higher mean total and LDL cholesterol, triglycerides, and alanine aminotransferase levels (J. Pediatr. 2011;159:577-83).
Recent evidence suggests that even before increased levels of LDL cholesterol and triglycerides are apparent in children with psoriasis, abnormalities in lipid function are present and may potentially serve as a novel marker for early cardiovascular risk. Dr. Paller cited a study presented by Dr. Wynnis L. Tom of Rady Children’s Hospital, San Diego, at the 2015 annual meeting of the Society for Investigative Dermatology. The case-control study included 50 children with psoriasis and 50 matched controls with a mean age of 13 years.
Like other investigators, Dr. Tom found that the psoriatic children had higher waist/hip ratios and more insulin resistance. While fasting lipid levels didn’t differ between the two groups, the psoriasis patients had significantly higher levels of atherogenic apolipoprotein B, fewer of the particularly cardioprotective large-size HDL particles, and reduced HDL efflux capacity. Stay tuned regarding these potential early markers, Dr. Paller advised.
She was lead author of a 409-patient international study that showed the risks of obesity and a high waist circumference rise with greater severity of pediatric psoriasis. Children with severe psoriasis were at 4.92-fold increased risk of obesity, compared with controls, while even those with mild psoriasis were at 3.6-fold increased risk (JAMA Dermatol. 2013;149:166-76).
Which comes first?
The question arises: Which comes first in children, the excess adiposity or the psoriasis? Dr. Paller said that although the final word isn’t in, she and her coworkers found in a pilot study of 27 overweight or obese children with psoriasis that excess adiposity typically came first. Moreover, among the roughly one-half of children with a family history of obesity, onset of psoriasis occurred a full 3 years earlier than in those without a positive family history (JAMA Dermatol. 2014;150:573-4).
In another small study, this by investigators at Tufts University, Boston, 6 of 20 children with psoriasis (30%) met criteria for the metabolic syndrome, compared with just 1 of 20 matched nonpsoriatic controls (Pediatr. Dermatol. 2013;30:700-5).
Dr. Paller said that if dermatologists and primary care physicians are to successfully collaborate in tackling the comorbid metabolic disorders associated with pediatric psoriasis, a prerequisite is that dermatologists are going to have to do a better job of educating their primary care colleagues about the skin disease as manifest in children.
“I think it’s very important that pediatricians are aware that psoriasis is a risk factor for metabolic syndrome. But pediatric psoriasis is often misdiagnosed by primary care physicians who mistake it for eczema or tinea infection or contact dermatitis,” according to the pediatric dermatologist.
In one eye-catching Australian study, she noted, a mere 9% of patients with pediatric psoriasis were correctly diagnosed before referral to a dermatologist (Australas. J. Dermatol. 2012;53:98-105).
Pediatric psoriasis: not just skin deep
In addition to the increased risk of metabolic disorders faced by pediatric psoriasis patients, other common comorbidities include depression, anxiety disorders, impaired self-esteem and quality of life, arthritis, and Crohn’s disease, Dr. Paller observed.
• Quality of life. “The quality of life impact of psoriasis is profound. It’s a highly visible disorder, which affects the development of self-esteem and social relationships,” Dr. Paller said.
Investigators at Texas A&M University applied the Pediatric Quality of Life Inventory Version 4.0 to 208 patients aged 2-17 years with moderate to severe psoriasis and compared the results to published data on children with arthritis, asthma, diabetes, and psychiatric disorders. Health-related quality of life turned out to be more impaired in the psoriasis patients than in those with diabetes. The quality-of-life impairment associated with pediatric psoriasis was comparable to that of having asthma or arthritis, albeit not as severe as for pediatric psychiatric disorders (Eur. J. Pediatr. 2012;171:485-92).
• Psychiatric disorders. A study of more than 7,400 pediatric psoriasis patients concluded they had an adjusted 25% increased risk of developing depression, compared with psoriasis-free controls, as well as a 32% increased risk of anxiety disorders and a 55% greater risk of bipolar disorder (J. Am. Acad. Dermatol. 2012;67:651-7.e2).
• Psoriatic arthritis. An estimated 1 in 10 U.S. children with psoriasis report having arthritis, often classified as juvenile idiopathic arthritis (JAMA Dermatol. 2013;149:1180-5).
• Crohn’s disease. A large German epidemiologic study concluded that psoriasis was associated with a 3.69-fold increased risk of Crohn’s disease. There was no increased risk of ulcerative colitis (Br. J. Dermatol. 2010;162:633-6).
Dr. Paller reported receiving research grants from Amgen and Leo and serving as a consultant to AbbVie.
EXPERT ANALYSIS FROM WCD 2015
Targeting gut microbiome boosted metformin tolerance
Boston – A purified food supplement designed to alter gut bacterial composition improved both metformin tolerance and fasting glucose levels among people with type 2 diabetes, according to results from a small randomized study presented at the annual scientific sessions of the American Diabetes Association.
Research into how diabetes affects and is affected by the gut microbiome is still in its infancy. However, compared with nondiabetics, people with type 2 diabetes have been shown to have microbiomes richer in certain types of bacteria than others, and these altered bacterial profiles may be linked to changes in insulin sensitivity, glucose regulation, and methane production.
Mark L Heiman, Ph.D., of MicroBiome Therapeutics in New Orleans, designed the intervention, a powder comprising three purified food ingredients: inulin from agave, beta-glucan from oatmeal, and polyphenols derived from blueberry skins. These were hypothesized to alter gut microbial composition by stimulating blooms of microbes that can generate short-chain fatty acids, displacing some bacteria-producing lactic acid; increase viscosity in the colon (allowing for sequestering of bile acids); and combat oxidative stress and methane production.
Though first investigated as a monotherapy for use in people with prediabetes, the so-called gut microbiome modulator NM504 was found incidentally to improve metformin tolerance in people with newly diagnosed type 2 diabetes (Benef. Microbes 2014;5:29-32), and researchers hypothesized that metformin likely interacts with microbiota in the colon, resulting in an abundance of lactate-producing bacteria. Lactic acid production in the colon, exacerbated by starch or sugar consumption, is a known contributor to adverse GI side effects in people taking metformin.
Dr. Heiman presented results from a randomized, placebo-controlled, single-center crossover study in 10 people with type 2 diabetes (8 female) with difficulty tolerating metformin due to GI symptoms or who had discontinued previous therapy because of these symptoms (J. Diabetes Sci. Technol. 2015;9:808-14).
Subjects were randomly assigned metformin (500 mg twice daily in the first week titrated to three times daily in the second week) alongside the experimental product or placebo. They were asked to record their glucose and adverse GI symptoms (including stool consistency, urgency to evacuate, bloating sensation, and flatulence) every day; metformin tolerance was measured by these symptoms using a scoring system previously validated in patients with irritable bowel syndrome.
After 2 weeks of treatment and a washout period of 2 weeks with no treatment, subjects’ initial treatment assignments were reversed for an additional 2 weeks, and outcomes were recorded.
The combination of metformin and NM504 resulted in improved tolerance score of metformin, compared with placebo (6.78 ± 0.65 [mean ± standard error of the mean] vs. 4.45 ± 0.69, P = .0006). Mean fasting glucose levels were significantly lower with the metformin/NM504 combination (121.3 ± 7.8 mg/dL) than with metformin-placebo (151.9 ± 7.8 mg/dL), (P < .02).
It is unclear whether the improved glucose was a result of improved availability of metformin or an independent effect of the intervention. Last year Dr. Heiman presented results from a previous study of 28 adults with prediabetes randomized to NM504 monotherapy or placebo who saw significantly lower blood sugar levels at 120 and 180 minutes after a glucose challenge, compared with patients taking placebo. This was presented at the International Congress of Endocrinology’s annual meeting (ICE-Endo) 2014 meeting in Chicago; Dr. Heiman noted that the results are awaiting review and perhaps publication.
Dr. Heiman commented that the glucose-regulating effect seen in the metformin study “appeared somewhat durable, even after the 2-week washout period” among subjects switching from intervention to placebo; however, further studies would be needed to determine its effect on insulin sensitivity. Fecal microbial composition was not investigated before and after treatment.
Dr. Daniel Hsia of Pennington Biomedical Research Center, in Baton Rouge, La., a study coauthor, said in an interview that although larger trials would be needed to validate the results and better understand the activity of the intervention in the gut, the findings were “promising” for a proof-of-concept study.
“At this point, we’re not going to start using this in every single diabetes patient on metformin, but it’s very encouraging that you could potentially make it more accessible. Metformin is a very good agent with one of the longest histories. It’s cheap, it can be used in combination with a lot of other medications, but a certain percentage of people will have to stop metformin altogether due to side effects.” Metformin is also approved for children with type 2 diabetes as young as 10 years, he pointed out. “The fact that [NM504] is food and you could add this to the regimen makes it very appealing” for use in adolescent patients, he said.
Dr. Heiman, a former obesity researcher at Eli Lilly, said that interventions to manipulate gut microbiota were worthy of deeper investigation. “In the past we were content to say ‘you’ve got bacteria to help you digest food’ – but little was known about what they do, whether they convert sugars to short-chain fatty acids, whether they might send out signals that may, for example, make you crave sugar. The exciting things is learning what all the signals are, and how they communicate with the body,” he said.
MicroBiome Therapeutics, owner of a patent on the investigational product, sponsored the study. Dr Heiman is an employee of MicroBiome Therapeutics, and another coauthor disclosed funding from the company. Dr. Hsia and three other coauthors reported no conflicts of interest.
Boston – A purified food supplement designed to alter gut bacterial composition improved both metformin tolerance and fasting glucose levels among people with type 2 diabetes, according to results from a small randomized study presented at the annual scientific sessions of the American Diabetes Association.
Research into how diabetes affects and is affected by the gut microbiome is still in its infancy. However, compared with nondiabetics, people with type 2 diabetes have been shown to have microbiomes richer in certain types of bacteria than others, and these altered bacterial profiles may be linked to changes in insulin sensitivity, glucose regulation, and methane production.
Mark L Heiman, Ph.D., of MicroBiome Therapeutics in New Orleans, designed the intervention, a powder comprising three purified food ingredients: inulin from agave, beta-glucan from oatmeal, and polyphenols derived from blueberry skins. These were hypothesized to alter gut microbial composition by stimulating blooms of microbes that can generate short-chain fatty acids, displacing some bacteria-producing lactic acid; increase viscosity in the colon (allowing for sequestering of bile acids); and combat oxidative stress and methane production.
Though first investigated as a monotherapy for use in people with prediabetes, the so-called gut microbiome modulator NM504 was found incidentally to improve metformin tolerance in people with newly diagnosed type 2 diabetes (Benef. Microbes 2014;5:29-32), and researchers hypothesized that metformin likely interacts with microbiota in the colon, resulting in an abundance of lactate-producing bacteria. Lactic acid production in the colon, exacerbated by starch or sugar consumption, is a known contributor to adverse GI side effects in people taking metformin.
Dr. Heiman presented results from a randomized, placebo-controlled, single-center crossover study in 10 people with type 2 diabetes (8 female) with difficulty tolerating metformin due to GI symptoms or who had discontinued previous therapy because of these symptoms (J. Diabetes Sci. Technol. 2015;9:808-14).
Subjects were randomly assigned metformin (500 mg twice daily in the first week titrated to three times daily in the second week) alongside the experimental product or placebo. They were asked to record their glucose and adverse GI symptoms (including stool consistency, urgency to evacuate, bloating sensation, and flatulence) every day; metformin tolerance was measured by these symptoms using a scoring system previously validated in patients with irritable bowel syndrome.
After 2 weeks of treatment and a washout period of 2 weeks with no treatment, subjects’ initial treatment assignments were reversed for an additional 2 weeks, and outcomes were recorded.
The combination of metformin and NM504 resulted in improved tolerance score of metformin, compared with placebo (6.78 ± 0.65 [mean ± standard error of the mean] vs. 4.45 ± 0.69, P = .0006). Mean fasting glucose levels were significantly lower with the metformin/NM504 combination (121.3 ± 7.8 mg/dL) than with metformin-placebo (151.9 ± 7.8 mg/dL), (P < .02).
It is unclear whether the improved glucose was a result of improved availability of metformin or an independent effect of the intervention. Last year Dr. Heiman presented results from a previous study of 28 adults with prediabetes randomized to NM504 monotherapy or placebo who saw significantly lower blood sugar levels at 120 and 180 minutes after a glucose challenge, compared with patients taking placebo. This was presented at the International Congress of Endocrinology’s annual meeting (ICE-Endo) 2014 meeting in Chicago; Dr. Heiman noted that the results are awaiting review and perhaps publication.
Dr. Heiman commented that the glucose-regulating effect seen in the metformin study “appeared somewhat durable, even after the 2-week washout period” among subjects switching from intervention to placebo; however, further studies would be needed to determine its effect on insulin sensitivity. Fecal microbial composition was not investigated before and after treatment.
Dr. Daniel Hsia of Pennington Biomedical Research Center, in Baton Rouge, La., a study coauthor, said in an interview that although larger trials would be needed to validate the results and better understand the activity of the intervention in the gut, the findings were “promising” for a proof-of-concept study.
“At this point, we’re not going to start using this in every single diabetes patient on metformin, but it’s very encouraging that you could potentially make it more accessible. Metformin is a very good agent with one of the longest histories. It’s cheap, it can be used in combination with a lot of other medications, but a certain percentage of people will have to stop metformin altogether due to side effects.” Metformin is also approved for children with type 2 diabetes as young as 10 years, he pointed out. “The fact that [NM504] is food and you could add this to the regimen makes it very appealing” for use in adolescent patients, he said.
Dr. Heiman, a former obesity researcher at Eli Lilly, said that interventions to manipulate gut microbiota were worthy of deeper investigation. “In the past we were content to say ‘you’ve got bacteria to help you digest food’ – but little was known about what they do, whether they convert sugars to short-chain fatty acids, whether they might send out signals that may, for example, make you crave sugar. The exciting things is learning what all the signals are, and how they communicate with the body,” he said.
MicroBiome Therapeutics, owner of a patent on the investigational product, sponsored the study. Dr Heiman is an employee of MicroBiome Therapeutics, and another coauthor disclosed funding from the company. Dr. Hsia and three other coauthors reported no conflicts of interest.
Boston – A purified food supplement designed to alter gut bacterial composition improved both metformin tolerance and fasting glucose levels among people with type 2 diabetes, according to results from a small randomized study presented at the annual scientific sessions of the American Diabetes Association.
Research into how diabetes affects and is affected by the gut microbiome is still in its infancy. However, compared with nondiabetics, people with type 2 diabetes have been shown to have microbiomes richer in certain types of bacteria than others, and these altered bacterial profiles may be linked to changes in insulin sensitivity, glucose regulation, and methane production.
Mark L Heiman, Ph.D., of MicroBiome Therapeutics in New Orleans, designed the intervention, a powder comprising three purified food ingredients: inulin from agave, beta-glucan from oatmeal, and polyphenols derived from blueberry skins. These were hypothesized to alter gut microbial composition by stimulating blooms of microbes that can generate short-chain fatty acids, displacing some bacteria-producing lactic acid; increase viscosity in the colon (allowing for sequestering of bile acids); and combat oxidative stress and methane production.
Though first investigated as a monotherapy for use in people with prediabetes, the so-called gut microbiome modulator NM504 was found incidentally to improve metformin tolerance in people with newly diagnosed type 2 diabetes (Benef. Microbes 2014;5:29-32), and researchers hypothesized that metformin likely interacts with microbiota in the colon, resulting in an abundance of lactate-producing bacteria. Lactic acid production in the colon, exacerbated by starch or sugar consumption, is a known contributor to adverse GI side effects in people taking metformin.
Dr. Heiman presented results from a randomized, placebo-controlled, single-center crossover study in 10 people with type 2 diabetes (8 female) with difficulty tolerating metformin due to GI symptoms or who had discontinued previous therapy because of these symptoms (J. Diabetes Sci. Technol. 2015;9:808-14).
Subjects were randomly assigned metformin (500 mg twice daily in the first week titrated to three times daily in the second week) alongside the experimental product or placebo. They were asked to record their glucose and adverse GI symptoms (including stool consistency, urgency to evacuate, bloating sensation, and flatulence) every day; metformin tolerance was measured by these symptoms using a scoring system previously validated in patients with irritable bowel syndrome.
After 2 weeks of treatment and a washout period of 2 weeks with no treatment, subjects’ initial treatment assignments were reversed for an additional 2 weeks, and outcomes were recorded.
The combination of metformin and NM504 resulted in improved tolerance score of metformin, compared with placebo (6.78 ± 0.65 [mean ± standard error of the mean] vs. 4.45 ± 0.69, P = .0006). Mean fasting glucose levels were significantly lower with the metformin/NM504 combination (121.3 ± 7.8 mg/dL) than with metformin-placebo (151.9 ± 7.8 mg/dL), (P < .02).
It is unclear whether the improved glucose was a result of improved availability of metformin or an independent effect of the intervention. Last year Dr. Heiman presented results from a previous study of 28 adults with prediabetes randomized to NM504 monotherapy or placebo who saw significantly lower blood sugar levels at 120 and 180 minutes after a glucose challenge, compared with patients taking placebo. This was presented at the International Congress of Endocrinology’s annual meeting (ICE-Endo) 2014 meeting in Chicago; Dr. Heiman noted that the results are awaiting review and perhaps publication.
Dr. Heiman commented that the glucose-regulating effect seen in the metformin study “appeared somewhat durable, even after the 2-week washout period” among subjects switching from intervention to placebo; however, further studies would be needed to determine its effect on insulin sensitivity. Fecal microbial composition was not investigated before and after treatment.
Dr. Daniel Hsia of Pennington Biomedical Research Center, in Baton Rouge, La., a study coauthor, said in an interview that although larger trials would be needed to validate the results and better understand the activity of the intervention in the gut, the findings were “promising” for a proof-of-concept study.
“At this point, we’re not going to start using this in every single diabetes patient on metformin, but it’s very encouraging that you could potentially make it more accessible. Metformin is a very good agent with one of the longest histories. It’s cheap, it can be used in combination with a lot of other medications, but a certain percentage of people will have to stop metformin altogether due to side effects.” Metformin is also approved for children with type 2 diabetes as young as 10 years, he pointed out. “The fact that [NM504] is food and you could add this to the regimen makes it very appealing” for use in adolescent patients, he said.
Dr. Heiman, a former obesity researcher at Eli Lilly, said that interventions to manipulate gut microbiota were worthy of deeper investigation. “In the past we were content to say ‘you’ve got bacteria to help you digest food’ – but little was known about what they do, whether they convert sugars to short-chain fatty acids, whether they might send out signals that may, for example, make you crave sugar. The exciting things is learning what all the signals are, and how they communicate with the body,” he said.
MicroBiome Therapeutics, owner of a patent on the investigational product, sponsored the study. Dr Heiman is an employee of MicroBiome Therapeutics, and another coauthor disclosed funding from the company. Dr. Hsia and three other coauthors reported no conflicts of interest.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: NM504, a food-derived product intended to stimulate gut production of beneficial bacteria, improved tolerance to metformin in people with type 2 diabetes.
Major finding: Patients previously reporting discontinuation of metformin therapy were significantly more tolerant to metformin (P = .0006) when randomized to an experimental agent hypothesized to alter gut microbial composition.
Data source: A small (n = 10) randomized trial in an academic clinical setting; patients were randomized 1:1 to receive an escalating dose of metformin plus placebo or NM504 for 2 weeks. After 2-week interruption, treatment assignments were switched for all patients.
Disclosures: The manufacturer sponsored the study of the proprietary investigational product. The lead investigator is a company employee and one of the coauthors disclosed a financial relationship.