User login
HCV Hub
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
direct-acting antivirals
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
VIDEO: Longer HCV therapy worked even after short-term failures
SAN FRANCISCO – Patients with hepatitis C who fail short-course antiviral treatment may still be able to achieve a cure using longer-duration therapies plus ribavirin, a small study has demonstrated.
The C-SWIFT study, an earlier trial of HCV patients undergoing a 4-week, triple-therapy regimen with sofosbuvir, elbasvir, and grazoprevir, resulted in a cure rate of only about 40% – not the result for which the researchers had hoped.
So, “the idea of this current trial was to take those patients and put them on a regimen that would ultimately give them sustained virologic response or virologic cure,” explained Dr. Eric Lawitz of the Texas Liver Institute, San Antonio. “Within these shortened durations, could we re-treat the patients with the same regimen, extending therapy to 12 weeks and adding ribavirin?”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Lawitz discussed the study’s findings and whether retreatment can deliver a cure in patients who’d earlier failed with shorter treatment regimens.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Patients with hepatitis C who fail short-course antiviral treatment may still be able to achieve a cure using longer-duration therapies plus ribavirin, a small study has demonstrated.
The C-SWIFT study, an earlier trial of HCV patients undergoing a 4-week, triple-therapy regimen with sofosbuvir, elbasvir, and grazoprevir, resulted in a cure rate of only about 40% – not the result for which the researchers had hoped.
So, “the idea of this current trial was to take those patients and put them on a regimen that would ultimately give them sustained virologic response or virologic cure,” explained Dr. Eric Lawitz of the Texas Liver Institute, San Antonio. “Within these shortened durations, could we re-treat the patients with the same regimen, extending therapy to 12 weeks and adding ribavirin?”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Lawitz discussed the study’s findings and whether retreatment can deliver a cure in patients who’d earlier failed with shorter treatment regimens.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Patients with hepatitis C who fail short-course antiviral treatment may still be able to achieve a cure using longer-duration therapies plus ribavirin, a small study has demonstrated.
The C-SWIFT study, an earlier trial of HCV patients undergoing a 4-week, triple-therapy regimen with sofosbuvir, elbasvir, and grazoprevir, resulted in a cure rate of only about 40% – not the result for which the researchers had hoped.
So, “the idea of this current trial was to take those patients and put them on a regimen that would ultimately give them sustained virologic response or virologic cure,” explained Dr. Eric Lawitz of the Texas Liver Institute, San Antonio. “Within these shortened durations, could we re-treat the patients with the same regimen, extending therapy to 12 weeks and adding ribavirin?”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Lawitz discussed the study’s findings and whether retreatment can deliver a cure in patients who’d earlier failed with shorter treatment regimens.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2015
Multitargeted drug combination promising when new HCV antivirals fail
SAN FRANCISCO – A five-drug regimen of direct-acting antiviral agents with diverse targets appears to be highly effective as a retreatment in patients infected with the hepatitis C virus patients who had virologic failure on a first-line direct-acting antiviral (DAA) regimen, according to results of a small study presented as a late-breaker at the annual meeting of the American Association for the Study of Liver Diseases (AASLD).
“Retreatment options for [hepatitis C virus (HCV)–infected] patients who fail treatment with DAA regimens are not yet clearly defined,” reported Dr. Fred Poordad, Texas Liver Institute, University of Texas Health Science Center, San Antonio. The five-drug regimen in this study was characterized as “promising.”
The study enrolled patients with genotype (GT) 1 infection who had virologic failure on a previous DAA treatment regimen. All were treated with ombitasvir with ritonavir-boosted paritaprevir (OBV/PTV/r) plus dasabuvir (DSV) and sofosbuvir (SOF). Those with GT1a infection and cirrhosis also received ribavirin and were treated for 24 weeks. All others were treated for 12 weeks.
Twenty-two patients were enrolled. The primary endpoint was sustained virologic response (SVR), defined as HCV RNA level below 25 IU/mL at 4 weeks after completion of the antiviral regimen. The DAA regimens on which patients had previously failed included OBV/PTV/r plus DSV in 14 patients, OBV/PTV/r in 2 patients, telaprevir in 2 patients, SOF in 2 patients, and a simeprevir-based regimen in 2 patients.
Fourteen of 15 (97%) patients treated for 12 weeks achieved SVR12. Of the seven patients treated for 24 weeks, all had HCV suppressed below the limit of detection while on therapy. The SVR4 rate is 100%, with final SVR results pending.
Neither of the two serious side effects – pneumonia and cellulitis – were considered to be drug related, but the patient who developed pneumonia discontinued the study regimen at week 10 without virologic failure.
Virologic failure to DAAs that inhibit NS5A, a protein important to virus assembly, is often associated with resistance-associated variants in genes that code for this protein. According to Dr. Poordad, these variants “have been shown to persist up to 96 weeks posttreatment.” As a result, patients who fail a first-line DAA “are likely to require a multitargeted approach to retreat infection.”
Although this study is small, the very high SVR rates support this premise.
Dr. Poordad reported financial relationships with Abbott/AbbVie, Achillion, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech, Gilead, Inhibitex, Idenix, Janssen, Merck, Novartis, Pfizer, Pharmasset, Salix, Tibotec/Janssen, and Vertex.
SAN FRANCISCO – A five-drug regimen of direct-acting antiviral agents with diverse targets appears to be highly effective as a retreatment in patients infected with the hepatitis C virus patients who had virologic failure on a first-line direct-acting antiviral (DAA) regimen, according to results of a small study presented as a late-breaker at the annual meeting of the American Association for the Study of Liver Diseases (AASLD).
“Retreatment options for [hepatitis C virus (HCV)–infected] patients who fail treatment with DAA regimens are not yet clearly defined,” reported Dr. Fred Poordad, Texas Liver Institute, University of Texas Health Science Center, San Antonio. The five-drug regimen in this study was characterized as “promising.”
The study enrolled patients with genotype (GT) 1 infection who had virologic failure on a previous DAA treatment regimen. All were treated with ombitasvir with ritonavir-boosted paritaprevir (OBV/PTV/r) plus dasabuvir (DSV) and sofosbuvir (SOF). Those with GT1a infection and cirrhosis also received ribavirin and were treated for 24 weeks. All others were treated for 12 weeks.
Twenty-two patients were enrolled. The primary endpoint was sustained virologic response (SVR), defined as HCV RNA level below 25 IU/mL at 4 weeks after completion of the antiviral regimen. The DAA regimens on which patients had previously failed included OBV/PTV/r plus DSV in 14 patients, OBV/PTV/r in 2 patients, telaprevir in 2 patients, SOF in 2 patients, and a simeprevir-based regimen in 2 patients.
Fourteen of 15 (97%) patients treated for 12 weeks achieved SVR12. Of the seven patients treated for 24 weeks, all had HCV suppressed below the limit of detection while on therapy. The SVR4 rate is 100%, with final SVR results pending.
Neither of the two serious side effects – pneumonia and cellulitis – were considered to be drug related, but the patient who developed pneumonia discontinued the study regimen at week 10 without virologic failure.
Virologic failure to DAAs that inhibit NS5A, a protein important to virus assembly, is often associated with resistance-associated variants in genes that code for this protein. According to Dr. Poordad, these variants “have been shown to persist up to 96 weeks posttreatment.” As a result, patients who fail a first-line DAA “are likely to require a multitargeted approach to retreat infection.”
Although this study is small, the very high SVR rates support this premise.
Dr. Poordad reported financial relationships with Abbott/AbbVie, Achillion, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech, Gilead, Inhibitex, Idenix, Janssen, Merck, Novartis, Pfizer, Pharmasset, Salix, Tibotec/Janssen, and Vertex.
SAN FRANCISCO – A five-drug regimen of direct-acting antiviral agents with diverse targets appears to be highly effective as a retreatment in patients infected with the hepatitis C virus patients who had virologic failure on a first-line direct-acting antiviral (DAA) regimen, according to results of a small study presented as a late-breaker at the annual meeting of the American Association for the Study of Liver Diseases (AASLD).
“Retreatment options for [hepatitis C virus (HCV)–infected] patients who fail treatment with DAA regimens are not yet clearly defined,” reported Dr. Fred Poordad, Texas Liver Institute, University of Texas Health Science Center, San Antonio. The five-drug regimen in this study was characterized as “promising.”
The study enrolled patients with genotype (GT) 1 infection who had virologic failure on a previous DAA treatment regimen. All were treated with ombitasvir with ritonavir-boosted paritaprevir (OBV/PTV/r) plus dasabuvir (DSV) and sofosbuvir (SOF). Those with GT1a infection and cirrhosis also received ribavirin and were treated for 24 weeks. All others were treated for 12 weeks.
Twenty-two patients were enrolled. The primary endpoint was sustained virologic response (SVR), defined as HCV RNA level below 25 IU/mL at 4 weeks after completion of the antiviral regimen. The DAA regimens on which patients had previously failed included OBV/PTV/r plus DSV in 14 patients, OBV/PTV/r in 2 patients, telaprevir in 2 patients, SOF in 2 patients, and a simeprevir-based regimen in 2 patients.
Fourteen of 15 (97%) patients treated for 12 weeks achieved SVR12. Of the seven patients treated for 24 weeks, all had HCV suppressed below the limit of detection while on therapy. The SVR4 rate is 100%, with final SVR results pending.
Neither of the two serious side effects – pneumonia and cellulitis – were considered to be drug related, but the patient who developed pneumonia discontinued the study regimen at week 10 without virologic failure.
Virologic failure to DAAs that inhibit NS5A, a protein important to virus assembly, is often associated with resistance-associated variants in genes that code for this protein. According to Dr. Poordad, these variants “have been shown to persist up to 96 weeks posttreatment.” As a result, patients who fail a first-line DAA “are likely to require a multitargeted approach to retreat infection.”
Although this study is small, the very high SVR rates support this premise.
Dr. Poordad reported financial relationships with Abbott/AbbVie, Achillion, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech, Gilead, Inhibitex, Idenix, Janssen, Merck, Novartis, Pfizer, Pharmasset, Salix, Tibotec/Janssen, and Vertex.
AT THE LIVER MEETING 2015
Key clinical point: A five-drug antiviral combination appears to be effective for retreatment of patients with hepatitis C virus infection who have failed direct-acting antivirals.
Major finding: The five-drug regimen achieved a sustained virologic response in 100% of patients treated for 12 weeks.
Data source: Single-arm, open-label study.
Disclosures: Dr. Poordad reported financial relationships with Abbott/AbbVie, Achillion, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech, Gilead, Inhibitex, Idenix, Janssen, Merck, Novartis, Pfizer, Pharmasset, Salix, Tibotec/Janssen, and Vertex.
Regimen effective in genotype 3 HCV despite advanced cirrhosis
SAN FRANCISCO – Sustained virologic response in patients with genotype 3 hepatitis C virus infection and advanced fibrosis or cirrhosis was achieved in 88% of those treated with a daclatasvir-based triple drug regimen for 12 weeks and 96% of those treated 16 weeks, according to findings from a phase IIIb study presented as a late breaker at the annual meeting of the American Association for the Study of Liver Diseases.
In this multinational trial, called ALLY-3+, 50 patients with genotype 3 hepatitis C virus (HCV) infection were randomized to 12 weeks or 16 weeks of a triple drug regimen containing 60 mg once-daily of daclatasvir (DCV), 400 mg once-daily of sofosbuvir (SOF), and weight-based ribavirin (RBV), reported Dr. Vincent Leroy, Centre Hospitalier Universitaire (CHU) de Grenoble, La Tronche, France. Of those enrolled, 74% were treatment experienced, of which 5 patients (10%) had relapsed on SOF plus RBV. Most patients (72%) had cirrhosis and 52% had a baseline viral load greater than 6 million IU/mL.
The primary endpoint was sustained virologic response (SVR), which was evaluated at 4 weeks post treatment.
By intention-to-treat analysis, the SVR rates were 88% for the 24 patients randomized to the 12-week regimen and 92% for the 26 patients randomized to the 16-week regimen. Four of the 5 patients with prior relapse on SOF/RBV treated for 16 weeks achieved SVR.
Of those with cirrhosis, the SVR endpoint was reached in 83% of those treated for 12 weeks and 89% of those treated for 16 weeks. Of those with advanced fibrosis, the SVR rates were 100% whether they were treated for 12 or 16 weeks.
Although there were no virologic breakthroughs, relapse was observed in two of those treated for 16 weeks and two of those treated for 12 weeks.
The treatment was well tolerated, according to Dr. Leroy. He reported that there were no discontinuations due to adverse events. The most common adverse events were insomnia in 30%, fatigue in 26%, and headache in 24%. One patient experienced a grade 3 reduction in hemoglobin.
The ALLY-3+ study follows the previously reported ALLY-3 trial in which the study combination was DCV and SOF alone. This combination produced SVR in 96% of those genotype 3 patients without cirrhosis after 12 weeks of therapy. However, the SVR rate fell to 63% in those with cirrhosis. The aim of this study was to determine whether the addition of RBV could improve SVR rates in patients with genotype 3 HCV.
“HCV genotype-3-infected patients are a challenging population in urgent need of optimally effective therapies,” Dr. Leroy explained. Although ALLY3 demonstrated that high rates of SVR can be achieved in this population in the absence of cirrhosis with DCV + SOF, ALLY-3+ demonstrates that the addition of RBV in those with cirrhosis brings SVR rates to comparable rates of SVR, particularly when treatment is extended to 16 weeks. Dr. Leroy called this triple-drug regimen “generally well tolerated.”
“These results are quite impressive in a difficult group of patients,” said, Dr. Anna S.F. Lok, professor of hepatology, University of Michigan, Ann Arbor, and moderator of the late-breaker oral sessions.
Dr. Leroy reported a financial relationship with AbbVie, Bristol-Myers Squibb (the trial sponsor), Gilead, Janssen, and Merck Sharpe & Dohme.
SAN FRANCISCO – Sustained virologic response in patients with genotype 3 hepatitis C virus infection and advanced fibrosis or cirrhosis was achieved in 88% of those treated with a daclatasvir-based triple drug regimen for 12 weeks and 96% of those treated 16 weeks, according to findings from a phase IIIb study presented as a late breaker at the annual meeting of the American Association for the Study of Liver Diseases.
In this multinational trial, called ALLY-3+, 50 patients with genotype 3 hepatitis C virus (HCV) infection were randomized to 12 weeks or 16 weeks of a triple drug regimen containing 60 mg once-daily of daclatasvir (DCV), 400 mg once-daily of sofosbuvir (SOF), and weight-based ribavirin (RBV), reported Dr. Vincent Leroy, Centre Hospitalier Universitaire (CHU) de Grenoble, La Tronche, France. Of those enrolled, 74% were treatment experienced, of which 5 patients (10%) had relapsed on SOF plus RBV. Most patients (72%) had cirrhosis and 52% had a baseline viral load greater than 6 million IU/mL.
The primary endpoint was sustained virologic response (SVR), which was evaluated at 4 weeks post treatment.
By intention-to-treat analysis, the SVR rates were 88% for the 24 patients randomized to the 12-week regimen and 92% for the 26 patients randomized to the 16-week regimen. Four of the 5 patients with prior relapse on SOF/RBV treated for 16 weeks achieved SVR.
Of those with cirrhosis, the SVR endpoint was reached in 83% of those treated for 12 weeks and 89% of those treated for 16 weeks. Of those with advanced fibrosis, the SVR rates were 100% whether they were treated for 12 or 16 weeks.
Although there were no virologic breakthroughs, relapse was observed in two of those treated for 16 weeks and two of those treated for 12 weeks.
The treatment was well tolerated, according to Dr. Leroy. He reported that there were no discontinuations due to adverse events. The most common adverse events were insomnia in 30%, fatigue in 26%, and headache in 24%. One patient experienced a grade 3 reduction in hemoglobin.
The ALLY-3+ study follows the previously reported ALLY-3 trial in which the study combination was DCV and SOF alone. This combination produced SVR in 96% of those genotype 3 patients without cirrhosis after 12 weeks of therapy. However, the SVR rate fell to 63% in those with cirrhosis. The aim of this study was to determine whether the addition of RBV could improve SVR rates in patients with genotype 3 HCV.
“HCV genotype-3-infected patients are a challenging population in urgent need of optimally effective therapies,” Dr. Leroy explained. Although ALLY3 demonstrated that high rates of SVR can be achieved in this population in the absence of cirrhosis with DCV + SOF, ALLY-3+ demonstrates that the addition of RBV in those with cirrhosis brings SVR rates to comparable rates of SVR, particularly when treatment is extended to 16 weeks. Dr. Leroy called this triple-drug regimen “generally well tolerated.”
“These results are quite impressive in a difficult group of patients,” said, Dr. Anna S.F. Lok, professor of hepatology, University of Michigan, Ann Arbor, and moderator of the late-breaker oral sessions.
Dr. Leroy reported a financial relationship with AbbVie, Bristol-Myers Squibb (the trial sponsor), Gilead, Janssen, and Merck Sharpe & Dohme.
SAN FRANCISCO – Sustained virologic response in patients with genotype 3 hepatitis C virus infection and advanced fibrosis or cirrhosis was achieved in 88% of those treated with a daclatasvir-based triple drug regimen for 12 weeks and 96% of those treated 16 weeks, according to findings from a phase IIIb study presented as a late breaker at the annual meeting of the American Association for the Study of Liver Diseases.
In this multinational trial, called ALLY-3+, 50 patients with genotype 3 hepatitis C virus (HCV) infection were randomized to 12 weeks or 16 weeks of a triple drug regimen containing 60 mg once-daily of daclatasvir (DCV), 400 mg once-daily of sofosbuvir (SOF), and weight-based ribavirin (RBV), reported Dr. Vincent Leroy, Centre Hospitalier Universitaire (CHU) de Grenoble, La Tronche, France. Of those enrolled, 74% were treatment experienced, of which 5 patients (10%) had relapsed on SOF plus RBV. Most patients (72%) had cirrhosis and 52% had a baseline viral load greater than 6 million IU/mL.
The primary endpoint was sustained virologic response (SVR), which was evaluated at 4 weeks post treatment.
By intention-to-treat analysis, the SVR rates were 88% for the 24 patients randomized to the 12-week regimen and 92% for the 26 patients randomized to the 16-week regimen. Four of the 5 patients with prior relapse on SOF/RBV treated for 16 weeks achieved SVR.
Of those with cirrhosis, the SVR endpoint was reached in 83% of those treated for 12 weeks and 89% of those treated for 16 weeks. Of those with advanced fibrosis, the SVR rates were 100% whether they were treated for 12 or 16 weeks.
Although there were no virologic breakthroughs, relapse was observed in two of those treated for 16 weeks and two of those treated for 12 weeks.
The treatment was well tolerated, according to Dr. Leroy. He reported that there were no discontinuations due to adverse events. The most common adverse events were insomnia in 30%, fatigue in 26%, and headache in 24%. One patient experienced a grade 3 reduction in hemoglobin.
The ALLY-3+ study follows the previously reported ALLY-3 trial in which the study combination was DCV and SOF alone. This combination produced SVR in 96% of those genotype 3 patients without cirrhosis after 12 weeks of therapy. However, the SVR rate fell to 63% in those with cirrhosis. The aim of this study was to determine whether the addition of RBV could improve SVR rates in patients with genotype 3 HCV.
“HCV genotype-3-infected patients are a challenging population in urgent need of optimally effective therapies,” Dr. Leroy explained. Although ALLY3 demonstrated that high rates of SVR can be achieved in this population in the absence of cirrhosis with DCV + SOF, ALLY-3+ demonstrates that the addition of RBV in those with cirrhosis brings SVR rates to comparable rates of SVR, particularly when treatment is extended to 16 weeks. Dr. Leroy called this triple-drug regimen “generally well tolerated.”
“These results are quite impressive in a difficult group of patients,” said, Dr. Anna S.F. Lok, professor of hepatology, University of Michigan, Ann Arbor, and moderator of the late-breaker oral sessions.
Dr. Leroy reported a financial relationship with AbbVie, Bristol-Myers Squibb (the trial sponsor), Gilead, Janssen, and Merck Sharpe & Dohme.
AT THE LIVER MEETING 2015
Key clinical point: Daclatasvir plus sofosbuvir and ribavirin (DCV/SOF/RBV) achieves high sustained virologic response (SVR) rates in patients with hepatitis C virus (HCV) infection and advanced compensated fibrosis or cirrhosis.
Major finding: SVR rates in a phase IIIb trial with DCV/SOF/RBV climbed from 88% for those treated for 12 weeks to 96% for those treated for 16 weeks.
Data source: Multicenter open-label phase IIIb trial.
Disclosures: Dr. Leroy reported a financial relationship with AbbVie, Bristol-Myers Squibb (the trial sponsor), Gilead, Janssen, and Merck Sharpe & Dohme.
VIDEO: Antigen test could simplify HCV screening
SAN FRANCISCO – Hepatitis C testing can be time consuming, expensive, and not widely available, but a new test focused on hepatitis C antigen detection might simplify the process and reduce its costs.
“Our goal was to develop a simple, one-stop test, “said Dr. Ke-Qin Hu of UC Irvine Medical Center, Orange, Calif. “Hepatitis C virus antigens would be good candidates for this.”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Hu discussed the findings from studies of the antigen test, and the test’s potential to improve hepatitis C screening and diagnosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Hepatitis C testing can be time consuming, expensive, and not widely available, but a new test focused on hepatitis C antigen detection might simplify the process and reduce its costs.
“Our goal was to develop a simple, one-stop test, “said Dr. Ke-Qin Hu of UC Irvine Medical Center, Orange, Calif. “Hepatitis C virus antigens would be good candidates for this.”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Hu discussed the findings from studies of the antigen test, and the test’s potential to improve hepatitis C screening and diagnosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – Hepatitis C testing can be time consuming, expensive, and not widely available, but a new test focused on hepatitis C antigen detection might simplify the process and reduce its costs.
“Our goal was to develop a simple, one-stop test, “said Dr. Ke-Qin Hu of UC Irvine Medical Center, Orange, Calif. “Hepatitis C virus antigens would be good candidates for this.”
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Hu discussed the findings from studies of the antigen test, and the test’s potential to improve hepatitis C screening and diagnosis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2015
VIDEO: Inappropriate hepatitis A, B tests may be widespread in hospitals
SAN FRANCISCO – A majority of inpatients with suspected acute hepatitis A and acute hepatitis B underwent inappropriate ordering of lab tests for those conditions, a study in one U.S. medical center showed.
“We looked at all the patients that came into our hospital who had acute hepatitis A or acute hepatitis B panels ordered,” explained Dr. Kamran Hussaini of Saint Louis (Mo.) University.
How frequently were those labs ordered inappropriately? “What we found was that the majority of patients who had acute hepatitis A and acute hepatitis B labs had ALT [alanine aminotransferase] and AST [aspartate aminotransferase] levels that were below 100,” Dr. Hussaini said.
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Hussaini discussed the study’s findings, what may be driving physicians to order inappropriate and costly hepatitis testing, and which strategies could reduce those trends.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – A majority of inpatients with suspected acute hepatitis A and acute hepatitis B underwent inappropriate ordering of lab tests for those conditions, a study in one U.S. medical center showed.
“We looked at all the patients that came into our hospital who had acute hepatitis A or acute hepatitis B panels ordered,” explained Dr. Kamran Hussaini of Saint Louis (Mo.) University.
How frequently were those labs ordered inappropriately? “What we found was that the majority of patients who had acute hepatitis A and acute hepatitis B labs had ALT [alanine aminotransferase] and AST [aspartate aminotransferase] levels that were below 100,” Dr. Hussaini said.
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Hussaini discussed the study’s findings, what may be driving physicians to order inappropriate and costly hepatitis testing, and which strategies could reduce those trends.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – A majority of inpatients with suspected acute hepatitis A and acute hepatitis B underwent inappropriate ordering of lab tests for those conditions, a study in one U.S. medical center showed.
“We looked at all the patients that came into our hospital who had acute hepatitis A or acute hepatitis B panels ordered,” explained Dr. Kamran Hussaini of Saint Louis (Mo.) University.
How frequently were those labs ordered inappropriately? “What we found was that the majority of patients who had acute hepatitis A and acute hepatitis B labs had ALT [alanine aminotransferase] and AST [aspartate aminotransferase] levels that were below 100,” Dr. Hussaini said.
In an interview at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Hussaini discussed the study’s findings, what may be driving physicians to order inappropriate and costly hepatitis testing, and which strategies could reduce those trends.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE LIVER MEETING 2015
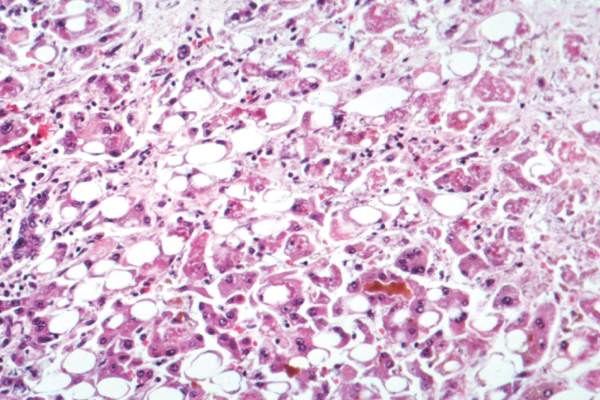
Novel botanical drug reduces hepatic fat content
SAN FRANCISCO – Twelve weeks of treatment with a novel botanical drug extracted from Magnolia officinalis safely and significantly reduced hepatic fat content in a dose-dependent manner among patients in a randomized, placebo-controlled phase II trial.
In 73 subjects with nonalcoholic fatty liver disease (NAFLD) who were randomized to receive 300 mg daily of the botanical drug (tablet), 100 mg daily, or placebo; hepatic fat content after 12 weeks was reduced by 12.14% with 300 mg daily and by 3.21% with 100 mg daily; fat content increased by 7.56% with placebo, Dr. Yeon Kim reported at the annual meeting of the American Association for the Study of Liver Diseases.
Serum aspartate aminotransferase and alanine aminotransferase were also reduced in the active treatment groups, and other factors, including cholesterol, triglycerides, free fatty acids, homeostatic model assessment (HOMA) insulin resistance, and body mass index, remained unchanged in both the treatment and placebo groups.
No drug-related safety issues occurred during the study, said Dr. Kim of Huons Co. Ltd., Ansan, South Korea.
Magnolia officinalis is a traditional herbal medicine that has been used to treat various liver diseases. Given the demand for new drugs for the treatment of NAFLD, Dr. Kim and his colleagues aimed to evaluate the effect and safety of the botanical tablet for the treatment of NAFLD.
Subjects were diagnosed with NAFLD by ultrasonic examination, and treatment was given twice daily for 12 weeks. Pre- and posttreatment hepatic fat content was measured using magnetic resonance spectroscopy.
The findings show that even with short treatment duration, this novel botanical drug was associated with hepatic fat content reduction without any negative lipid profile, body mass index change, or adverse effects, Dr. Kim said, concluding that larger extended trials to assess its long-term efficacy are warranted.
Dr. Kim reported having no disclosures.
SAN FRANCISCO – Twelve weeks of treatment with a novel botanical drug extracted from Magnolia officinalis safely and significantly reduced hepatic fat content in a dose-dependent manner among patients in a randomized, placebo-controlled phase II trial.
In 73 subjects with nonalcoholic fatty liver disease (NAFLD) who were randomized to receive 300 mg daily of the botanical drug (tablet), 100 mg daily, or placebo; hepatic fat content after 12 weeks was reduced by 12.14% with 300 mg daily and by 3.21% with 100 mg daily; fat content increased by 7.56% with placebo, Dr. Yeon Kim reported at the annual meeting of the American Association for the Study of Liver Diseases.
Serum aspartate aminotransferase and alanine aminotransferase were also reduced in the active treatment groups, and other factors, including cholesterol, triglycerides, free fatty acids, homeostatic model assessment (HOMA) insulin resistance, and body mass index, remained unchanged in both the treatment and placebo groups.
No drug-related safety issues occurred during the study, said Dr. Kim of Huons Co. Ltd., Ansan, South Korea.
Magnolia officinalis is a traditional herbal medicine that has been used to treat various liver diseases. Given the demand for new drugs for the treatment of NAFLD, Dr. Kim and his colleagues aimed to evaluate the effect and safety of the botanical tablet for the treatment of NAFLD.
Subjects were diagnosed with NAFLD by ultrasonic examination, and treatment was given twice daily for 12 weeks. Pre- and posttreatment hepatic fat content was measured using magnetic resonance spectroscopy.
The findings show that even with short treatment duration, this novel botanical drug was associated with hepatic fat content reduction without any negative lipid profile, body mass index change, or adverse effects, Dr. Kim said, concluding that larger extended trials to assess its long-term efficacy are warranted.
Dr. Kim reported having no disclosures.
SAN FRANCISCO – Twelve weeks of treatment with a novel botanical drug extracted from Magnolia officinalis safely and significantly reduced hepatic fat content in a dose-dependent manner among patients in a randomized, placebo-controlled phase II trial.
In 73 subjects with nonalcoholic fatty liver disease (NAFLD) who were randomized to receive 300 mg daily of the botanical drug (tablet), 100 mg daily, or placebo; hepatic fat content after 12 weeks was reduced by 12.14% with 300 mg daily and by 3.21% with 100 mg daily; fat content increased by 7.56% with placebo, Dr. Yeon Kim reported at the annual meeting of the American Association for the Study of Liver Diseases.
Serum aspartate aminotransferase and alanine aminotransferase were also reduced in the active treatment groups, and other factors, including cholesterol, triglycerides, free fatty acids, homeostatic model assessment (HOMA) insulin resistance, and body mass index, remained unchanged in both the treatment and placebo groups.
No drug-related safety issues occurred during the study, said Dr. Kim of Huons Co. Ltd., Ansan, South Korea.
Magnolia officinalis is a traditional herbal medicine that has been used to treat various liver diseases. Given the demand for new drugs for the treatment of NAFLD, Dr. Kim and his colleagues aimed to evaluate the effect and safety of the botanical tablet for the treatment of NAFLD.
Subjects were diagnosed with NAFLD by ultrasonic examination, and treatment was given twice daily for 12 weeks. Pre- and posttreatment hepatic fat content was measured using magnetic resonance spectroscopy.
The findings show that even with short treatment duration, this novel botanical drug was associated with hepatic fat content reduction without any negative lipid profile, body mass index change, or adverse effects, Dr. Kim said, concluding that larger extended trials to assess its long-term efficacy are warranted.
Dr. Kim reported having no disclosures.
AT THE LIVER MEETING 2015
Key clinical point: Twelve weeks of treatment with a novel botanical drug extracted from Magnolia officinalis safely and significantly reduced hepatic fat content in a dose-dependent manner among patients in a randomized, placebo-controlled phase II trial.
Major finding: Hepatic fat content was reduced by 12.14% and 3.21% with 300 and 100 mg daily of the botanical, respectively, compared with an increase of 7.56% with placebo.
Data source: A randomized, placebo-controlled phase II study of 73 patients.
Disclosures: Dr. Kim reported having no disclosures.
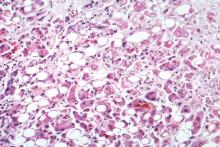
Cysteamine bitartrate provides no histologic improvement in children with NAFLD
SAN FRANCISCO – One year of treatment with delayed-release cysteamine bitartrate was safe and associated with substantial and rapid improvements in liver enzymes in children with nonalcoholic fatty liver disease, but did not improve liver histology.
That finding emerged from the randomized, placebo-controlled phase IIb Cysteamine Bitartrate Delayed-Release for the Treatment of Nonalcoholic Fatty Liver Disease trial (CyNCh).
The rate of histologic improvement in 88 children with nonalcoholic fatty liver disease (NAFLD) who received twice-daily oral delayed-release cysteamine bitartrate (CyB) in the trial was 28%, compared with 22% in 81 children who received placebo (relative improvement ratio, 1.3). An intent-to-treat analysis showed no significant difference between CyB and placebo with respect to initial vs. end-of-treatment improvement in steatosis (30% vs. 41%), ballooning (19% vs. 26%), lobular inflammation (36% vs. 21%), or fibrosis (28% vs. 28%), Dr. Jeffrey B. Schwimmer of the University of California, San Diego, said at the annual meeting of the American Association for the Study of Liver Diseases.
CyB was, however, associated with greater mean change in alanine aminotransferase (–53 vs. –8 U/L) and aspartate aminotransferase (–31 vs. –4 U/L), compared with placebo, he noted, adding that the reduction in aminotransferases with CyB occurred within 4 weeks and was sustained through 52 weeks of treatment.
No changes were seen in serum lipids, cholesterol, or insulin sensitivity, and there were no differences in adverse events with CyB vs. placebo.
NAFLD is the most common cause of chronic liver disease in children, yet there is no approved treatment for the disease in this population, Dr. Schwimmer said.
The current study was initiated based on preliminary evidence showing decreased alanine aminotransferase and aspartate aminotransferase, and increased adiponectin in children treated with cysteamine in a 6-month pilot study. Subjects were children aged 8-17 years from June 2012 to August 2015 with an NAFLD activity score (NAS) of 4 or less. They were randomized to receive twice daily oral CyB at a dose of 300 mg if weight was 65 kg or less, 375 mg if weight was greater than 65 kg to 80 kg, or 450 mg if weight was greater than 80 kg, or to receive matching placebo. All received standardized lifestyle advice.
Histologic improvement was defined as a decrease in NAS of at least 2 points without worsening of fibrosis.
The lessons learned in CyNCh should guide future clinical trials for pediatric NAFLD, Dr. Schwimmer concluded.
He reported having no disclosures.
SAN FRANCISCO – One year of treatment with delayed-release cysteamine bitartrate was safe and associated with substantial and rapid improvements in liver enzymes in children with nonalcoholic fatty liver disease, but did not improve liver histology.
That finding emerged from the randomized, placebo-controlled phase IIb Cysteamine Bitartrate Delayed-Release for the Treatment of Nonalcoholic Fatty Liver Disease trial (CyNCh).
The rate of histologic improvement in 88 children with nonalcoholic fatty liver disease (NAFLD) who received twice-daily oral delayed-release cysteamine bitartrate (CyB) in the trial was 28%, compared with 22% in 81 children who received placebo (relative improvement ratio, 1.3). An intent-to-treat analysis showed no significant difference between CyB and placebo with respect to initial vs. end-of-treatment improvement in steatosis (30% vs. 41%), ballooning (19% vs. 26%), lobular inflammation (36% vs. 21%), or fibrosis (28% vs. 28%), Dr. Jeffrey B. Schwimmer of the University of California, San Diego, said at the annual meeting of the American Association for the Study of Liver Diseases.
CyB was, however, associated with greater mean change in alanine aminotransferase (–53 vs. –8 U/L) and aspartate aminotransferase (–31 vs. –4 U/L), compared with placebo, he noted, adding that the reduction in aminotransferases with CyB occurred within 4 weeks and was sustained through 52 weeks of treatment.
No changes were seen in serum lipids, cholesterol, or insulin sensitivity, and there were no differences in adverse events with CyB vs. placebo.
NAFLD is the most common cause of chronic liver disease in children, yet there is no approved treatment for the disease in this population, Dr. Schwimmer said.
The current study was initiated based on preliminary evidence showing decreased alanine aminotransferase and aspartate aminotransferase, and increased adiponectin in children treated with cysteamine in a 6-month pilot study. Subjects were children aged 8-17 years from June 2012 to August 2015 with an NAFLD activity score (NAS) of 4 or less. They were randomized to receive twice daily oral CyB at a dose of 300 mg if weight was 65 kg or less, 375 mg if weight was greater than 65 kg to 80 kg, or 450 mg if weight was greater than 80 kg, or to receive matching placebo. All received standardized lifestyle advice.
Histologic improvement was defined as a decrease in NAS of at least 2 points without worsening of fibrosis.
The lessons learned in CyNCh should guide future clinical trials for pediatric NAFLD, Dr. Schwimmer concluded.
He reported having no disclosures.
SAN FRANCISCO – One year of treatment with delayed-release cysteamine bitartrate was safe and associated with substantial and rapid improvements in liver enzymes in children with nonalcoholic fatty liver disease, but did not improve liver histology.
That finding emerged from the randomized, placebo-controlled phase IIb Cysteamine Bitartrate Delayed-Release for the Treatment of Nonalcoholic Fatty Liver Disease trial (CyNCh).
The rate of histologic improvement in 88 children with nonalcoholic fatty liver disease (NAFLD) who received twice-daily oral delayed-release cysteamine bitartrate (CyB) in the trial was 28%, compared with 22% in 81 children who received placebo (relative improvement ratio, 1.3). An intent-to-treat analysis showed no significant difference between CyB and placebo with respect to initial vs. end-of-treatment improvement in steatosis (30% vs. 41%), ballooning (19% vs. 26%), lobular inflammation (36% vs. 21%), or fibrosis (28% vs. 28%), Dr. Jeffrey B. Schwimmer of the University of California, San Diego, said at the annual meeting of the American Association for the Study of Liver Diseases.
CyB was, however, associated with greater mean change in alanine aminotransferase (–53 vs. –8 U/L) and aspartate aminotransferase (–31 vs. –4 U/L), compared with placebo, he noted, adding that the reduction in aminotransferases with CyB occurred within 4 weeks and was sustained through 52 weeks of treatment.
No changes were seen in serum lipids, cholesterol, or insulin sensitivity, and there were no differences in adverse events with CyB vs. placebo.
NAFLD is the most common cause of chronic liver disease in children, yet there is no approved treatment for the disease in this population, Dr. Schwimmer said.
The current study was initiated based on preliminary evidence showing decreased alanine aminotransferase and aspartate aminotransferase, and increased adiponectin in children treated with cysteamine in a 6-month pilot study. Subjects were children aged 8-17 years from June 2012 to August 2015 with an NAFLD activity score (NAS) of 4 or less. They were randomized to receive twice daily oral CyB at a dose of 300 mg if weight was 65 kg or less, 375 mg if weight was greater than 65 kg to 80 kg, or 450 mg if weight was greater than 80 kg, or to receive matching placebo. All received standardized lifestyle advice.
Histologic improvement was defined as a decrease in NAS of at least 2 points without worsening of fibrosis.
The lessons learned in CyNCh should guide future clinical trials for pediatric NAFLD, Dr. Schwimmer concluded.
He reported having no disclosures.
AT THE LIVER MEETING 2015
Key clinical point: One year of treatment with delayed-release cysteamine bitartrate was safe and associated with substantial and rapid improvements in liver enzymes in children with nonalcoholic fatty liver disease, but did not improve liver histology.
Major finding: The rate of histologic improvement was 28% vs. 22% with CyB vs. placebo (relative improvement ratio, 1.3).
Data source: A randomized, placebo-controlled phase IIb study of 169 children.
Disclosures: Dr. Schwimmer reported having no disclosures.
ASTRAL-1: High SVR12 with sofosbuvir/velpatasvir combo for HCV
SAN FRANCISCO – Once-daily treatment with fixed-dose combination sofosbuvir/velpatasvir for 12 weeks was well tolerated and resulted in high sustained virologic response rates in hepatitis C–infected patients in the phase III ASTRAL-1 study.
Of 740 patients with genotype 1, 2, 4, 5, or 6 hepatitis C virus (HCV) infection, with or without cirrhosis, who were enrolled at 81 sites in North America, Europe, or Hong Kong, 624 were randomized to receive active treatment with 400 mg of sofosbuvir (SOF) and 100 mg of velpatasvir (VEL) daily for 12 weeks, and 116 received placebo.
Of the patients in the SOF/VEL group, 53% had genotype 1 disease, 17% had genotype 2, 19% had genotype 4, 6% had genotype 5, and 7% had genotype 6; all genotype 5 patients were in the treatment group, and genotype 3 patients are being evaluated in a separate study, Dr. Jordan J. Feld reported at the annual meeting of the American Association for the Study of Liver Diseases.
The overall sustained virologic response at 12 or more weeks after treatment (SVR12) among treated patients was 99%, thus the study met the prespecified primary efficacy endpoint of 85% SVR12, said Dr. Feld of Toronto Western Hospital Liver Centre, Ontario.
The SVR12 was 98.5% and 97.1% in patients with genotypes 1 and 5, respectively, and 100% in those with genotypes 2, 4, and 6, respectively. The percentage of patients with cirrhosis in those with genotypes 1, 2, 4, 5, and 6 was 22.3%, 9.6%, 23.3%, 14.3%, and 14.6%, respectively (19.4% overall).
Two patients, both with genotype 1 infection (0.6% of genotype 1 patients), experienced virologic relapse. One had genotype 1b infection and was a treatment-experienced patient with cirrhosis, and 1 was a genotype 1a treatment-naive patient without cirrhosis.
None of the other patients, including 48 with cirrhosis, experienced virologic failure, but 4 were considered to have not achieved SVR12 for nonvirologic reasons, including loss to follow-up.
Overall, the type, frequency, and severity of adverse events and laboratory abnormalities were similar in the treatment and placebo group patients, Dr. Feld said, noting that severe adverse events occurred in 15 (2.4%) of the treatment group patients, and in none of the placebo-treated patients, but none of the severe adverse events were considered to be related to the study drug combination.
SOF/VEL combination therapy is an all-oral single tablet regimen shown in phase II studies to result in high SVR12 in patients with genotype 1-6 HCV. Those findings were confirmed in this phase III, placebo-controlled, double-blind study involving both treatment-naive and treatment-experienced genotype 1, 2, 4, 5, and 6 HCV patients with and without cirrhosis.
The current findings demonstrate that the regimen is “simple, safe, and highly effective,” Dr. Feld concluded.
Gilead Sciences sponsored ASTRAL-1. Dr. Feld disclosed ties with Merck, Janssen, Gilead, AbbVie, Theravance, Bristol-Myers Squibb, and Boehringer Ingelheim.
SAN FRANCISCO – Once-daily treatment with fixed-dose combination sofosbuvir/velpatasvir for 12 weeks was well tolerated and resulted in high sustained virologic response rates in hepatitis C–infected patients in the phase III ASTRAL-1 study.
Of 740 patients with genotype 1, 2, 4, 5, or 6 hepatitis C virus (HCV) infection, with or without cirrhosis, who were enrolled at 81 sites in North America, Europe, or Hong Kong, 624 were randomized to receive active treatment with 400 mg of sofosbuvir (SOF) and 100 mg of velpatasvir (VEL) daily for 12 weeks, and 116 received placebo.
Of the patients in the SOF/VEL group, 53% had genotype 1 disease, 17% had genotype 2, 19% had genotype 4, 6% had genotype 5, and 7% had genotype 6; all genotype 5 patients were in the treatment group, and genotype 3 patients are being evaluated in a separate study, Dr. Jordan J. Feld reported at the annual meeting of the American Association for the Study of Liver Diseases.
The overall sustained virologic response at 12 or more weeks after treatment (SVR12) among treated patients was 99%, thus the study met the prespecified primary efficacy endpoint of 85% SVR12, said Dr. Feld of Toronto Western Hospital Liver Centre, Ontario.
The SVR12 was 98.5% and 97.1% in patients with genotypes 1 and 5, respectively, and 100% in those with genotypes 2, 4, and 6, respectively. The percentage of patients with cirrhosis in those with genotypes 1, 2, 4, 5, and 6 was 22.3%, 9.6%, 23.3%, 14.3%, and 14.6%, respectively (19.4% overall).
Two patients, both with genotype 1 infection (0.6% of genotype 1 patients), experienced virologic relapse. One had genotype 1b infection and was a treatment-experienced patient with cirrhosis, and 1 was a genotype 1a treatment-naive patient without cirrhosis.
None of the other patients, including 48 with cirrhosis, experienced virologic failure, but 4 were considered to have not achieved SVR12 for nonvirologic reasons, including loss to follow-up.
Overall, the type, frequency, and severity of adverse events and laboratory abnormalities were similar in the treatment and placebo group patients, Dr. Feld said, noting that severe adverse events occurred in 15 (2.4%) of the treatment group patients, and in none of the placebo-treated patients, but none of the severe adverse events were considered to be related to the study drug combination.
SOF/VEL combination therapy is an all-oral single tablet regimen shown in phase II studies to result in high SVR12 in patients with genotype 1-6 HCV. Those findings were confirmed in this phase III, placebo-controlled, double-blind study involving both treatment-naive and treatment-experienced genotype 1, 2, 4, 5, and 6 HCV patients with and without cirrhosis.
The current findings demonstrate that the regimen is “simple, safe, and highly effective,” Dr. Feld concluded.
Gilead Sciences sponsored ASTRAL-1. Dr. Feld disclosed ties with Merck, Janssen, Gilead, AbbVie, Theravance, Bristol-Myers Squibb, and Boehringer Ingelheim.
SAN FRANCISCO – Once-daily treatment with fixed-dose combination sofosbuvir/velpatasvir for 12 weeks was well tolerated and resulted in high sustained virologic response rates in hepatitis C–infected patients in the phase III ASTRAL-1 study.
Of 740 patients with genotype 1, 2, 4, 5, or 6 hepatitis C virus (HCV) infection, with or without cirrhosis, who were enrolled at 81 sites in North America, Europe, or Hong Kong, 624 were randomized to receive active treatment with 400 mg of sofosbuvir (SOF) and 100 mg of velpatasvir (VEL) daily for 12 weeks, and 116 received placebo.
Of the patients in the SOF/VEL group, 53% had genotype 1 disease, 17% had genotype 2, 19% had genotype 4, 6% had genotype 5, and 7% had genotype 6; all genotype 5 patients were in the treatment group, and genotype 3 patients are being evaluated in a separate study, Dr. Jordan J. Feld reported at the annual meeting of the American Association for the Study of Liver Diseases.
The overall sustained virologic response at 12 or more weeks after treatment (SVR12) among treated patients was 99%, thus the study met the prespecified primary efficacy endpoint of 85% SVR12, said Dr. Feld of Toronto Western Hospital Liver Centre, Ontario.
The SVR12 was 98.5% and 97.1% in patients with genotypes 1 and 5, respectively, and 100% in those with genotypes 2, 4, and 6, respectively. The percentage of patients with cirrhosis in those with genotypes 1, 2, 4, 5, and 6 was 22.3%, 9.6%, 23.3%, 14.3%, and 14.6%, respectively (19.4% overall).
Two patients, both with genotype 1 infection (0.6% of genotype 1 patients), experienced virologic relapse. One had genotype 1b infection and was a treatment-experienced patient with cirrhosis, and 1 was a genotype 1a treatment-naive patient without cirrhosis.
None of the other patients, including 48 with cirrhosis, experienced virologic failure, but 4 were considered to have not achieved SVR12 for nonvirologic reasons, including loss to follow-up.
Overall, the type, frequency, and severity of adverse events and laboratory abnormalities were similar in the treatment and placebo group patients, Dr. Feld said, noting that severe adverse events occurred in 15 (2.4%) of the treatment group patients, and in none of the placebo-treated patients, but none of the severe adverse events were considered to be related to the study drug combination.
SOF/VEL combination therapy is an all-oral single tablet regimen shown in phase II studies to result in high SVR12 in patients with genotype 1-6 HCV. Those findings were confirmed in this phase III, placebo-controlled, double-blind study involving both treatment-naive and treatment-experienced genotype 1, 2, 4, 5, and 6 HCV patients with and without cirrhosis.
The current findings demonstrate that the regimen is “simple, safe, and highly effective,” Dr. Feld concluded.
Gilead Sciences sponsored ASTRAL-1. Dr. Feld disclosed ties with Merck, Janssen, Gilead, AbbVie, Theravance, Bristol-Myers Squibb, and Boehringer Ingelheim.
AT THE LIVER MEETING 2015
Key clinical point: Once-daily treatment with fixed-dose combination sofosbuvir/velpatasvir for 12 weeks was well tolerated and resulted in high sustained virologic response rates in hepatitis C–infected patients in the phase III ASTRAL-1 study.
Major finding: The overall SVR12 among treated patients was 99%.
Data source: The phase III ASTRAL-1 trial of 740 patients.
Disclosures: Gilead Sciences sponsored ASTRAL-1. Dr. Feld disclosed ties with Merck, Janssen, Gilead, AbbVie, Theravance, Bristol-Myers Squibb, and Boehringer Ingelheim.
Cellular therapy benefit limited to young alcoholic hepatitis patients
SAN FRANCISCO – The only patients with alcoholic hepatitis to derive a survival benefit from extracorporeal hepatocellular therapy with C3A hepatoma cells were those who were young and had low Model for End-Stage Liver Disease (MELD) scores, according to data from a randomized trial presented as a late breaker at the annual meeting of the American Association for the Study of Liver Diseases.
The evidence of benefit in younger patients was drawn from a post-hoc analysis of a negative late-breaker trial presented by Dr. David Reich, vice chair, department of surgery, Drexel University, Philadelphia. A new trial is now being planned to target this subgroup of patients.
In this multinational randomized trial, called VTI-208, 203 patients with alcoholic hepatitis were randomized to the experimental therapy or to a control arm. Those in the experimental arm received C3A hepatoma cells delivered continuously for 3-5 days by an extracorporeal liver assist device (ELAD) along with standard of care. Patients in the control arm received standard of care alone. The primary endpoint of this study, with 40 participating centers worldwide, was overall survival (OS).
In an intent-to-treat analysis, OS did not differ significantly for ELAD relative to standard of care (52.1% vs. 52.3%). However, when patients were stratified by baseline MELD score and age, there were trends for an OS advantage in those receiving ELAD over standard of care for MELD scores less than 28 (71% vs. 57%; P = .077) and for subjects with an age below the median (67% vs. 55%; P = .167).
When both factors were considered together (MELD score less than 28 and an age below the median), the advantage of ELAD over standard of care reached significance (100% vs. 76%; P = .006). This subgroup analysis was not prespecified in the trial design, making this difference hypothesis generating,
The findings “suggest that ELAD may be a favorable alcoholic hepatitis treatment modality in younger patients with sufficient renal function and less severe coagulopathy,” Dr. Reich reported. “A study to confirm the survival benefit in this population is in preparation and is scheduled to start in 2016.”
Entry criteria for this study included a MELD score less than 35, a bilirubin greater than 8 mg/dL, and no other serious concomitant disease. Of patients randomized, 120 had a MELD score less than 28 and 101 were below the median age.
The expectation of benefit from C3A hepatoma cells was derived from evidence that these express immunomodulatory proteins and growth factors support hepatocellular function, according to Dr. Reich. The difference in survival in the younger patients was observed at 91 days. Dr. Reich reported that survival was “fairly stable” after 100 days in all patient groups.
“Alcoholic hepatitis results from hepatic inflammation, oxidative damage, cholestasis, and apoptosis, all of which create a vicious cycle that leads to liver dysfunction and secondary organ failure associated with poor prognosis,” Dr. Reich explained. It was hoped that ELAD delivery of C3A cells could modify this process.
Further study in subgroups that may benefit are encouraged by the safety profile observed in this study. There were no significant differences in serious adverse events observed in the two study arms, Dr. Reich reported.
Dr. Reich reported a financial relationship with Neuwave and VTI Corporation, the sponsor of the randomized study.
SAN FRANCISCO – The only patients with alcoholic hepatitis to derive a survival benefit from extracorporeal hepatocellular therapy with C3A hepatoma cells were those who were young and had low Model for End-Stage Liver Disease (MELD) scores, according to data from a randomized trial presented as a late breaker at the annual meeting of the American Association for the Study of Liver Diseases.
The evidence of benefit in younger patients was drawn from a post-hoc analysis of a negative late-breaker trial presented by Dr. David Reich, vice chair, department of surgery, Drexel University, Philadelphia. A new trial is now being planned to target this subgroup of patients.
In this multinational randomized trial, called VTI-208, 203 patients with alcoholic hepatitis were randomized to the experimental therapy or to a control arm. Those in the experimental arm received C3A hepatoma cells delivered continuously for 3-5 days by an extracorporeal liver assist device (ELAD) along with standard of care. Patients in the control arm received standard of care alone. The primary endpoint of this study, with 40 participating centers worldwide, was overall survival (OS).
In an intent-to-treat analysis, OS did not differ significantly for ELAD relative to standard of care (52.1% vs. 52.3%). However, when patients were stratified by baseline MELD score and age, there were trends for an OS advantage in those receiving ELAD over standard of care for MELD scores less than 28 (71% vs. 57%; P = .077) and for subjects with an age below the median (67% vs. 55%; P = .167).
When both factors were considered together (MELD score less than 28 and an age below the median), the advantage of ELAD over standard of care reached significance (100% vs. 76%; P = .006). This subgroup analysis was not prespecified in the trial design, making this difference hypothesis generating,
The findings “suggest that ELAD may be a favorable alcoholic hepatitis treatment modality in younger patients with sufficient renal function and less severe coagulopathy,” Dr. Reich reported. “A study to confirm the survival benefit in this population is in preparation and is scheduled to start in 2016.”
Entry criteria for this study included a MELD score less than 35, a bilirubin greater than 8 mg/dL, and no other serious concomitant disease. Of patients randomized, 120 had a MELD score less than 28 and 101 were below the median age.
The expectation of benefit from C3A hepatoma cells was derived from evidence that these express immunomodulatory proteins and growth factors support hepatocellular function, according to Dr. Reich. The difference in survival in the younger patients was observed at 91 days. Dr. Reich reported that survival was “fairly stable” after 100 days in all patient groups.
“Alcoholic hepatitis results from hepatic inflammation, oxidative damage, cholestasis, and apoptosis, all of which create a vicious cycle that leads to liver dysfunction and secondary organ failure associated with poor prognosis,” Dr. Reich explained. It was hoped that ELAD delivery of C3A cells could modify this process.
Further study in subgroups that may benefit are encouraged by the safety profile observed in this study. There were no significant differences in serious adverse events observed in the two study arms, Dr. Reich reported.
Dr. Reich reported a financial relationship with Neuwave and VTI Corporation, the sponsor of the randomized study.
SAN FRANCISCO – The only patients with alcoholic hepatitis to derive a survival benefit from extracorporeal hepatocellular therapy with C3A hepatoma cells were those who were young and had low Model for End-Stage Liver Disease (MELD) scores, according to data from a randomized trial presented as a late breaker at the annual meeting of the American Association for the Study of Liver Diseases.
The evidence of benefit in younger patients was drawn from a post-hoc analysis of a negative late-breaker trial presented by Dr. David Reich, vice chair, department of surgery, Drexel University, Philadelphia. A new trial is now being planned to target this subgroup of patients.
In this multinational randomized trial, called VTI-208, 203 patients with alcoholic hepatitis were randomized to the experimental therapy or to a control arm. Those in the experimental arm received C3A hepatoma cells delivered continuously for 3-5 days by an extracorporeal liver assist device (ELAD) along with standard of care. Patients in the control arm received standard of care alone. The primary endpoint of this study, with 40 participating centers worldwide, was overall survival (OS).
In an intent-to-treat analysis, OS did not differ significantly for ELAD relative to standard of care (52.1% vs. 52.3%). However, when patients were stratified by baseline MELD score and age, there were trends for an OS advantage in those receiving ELAD over standard of care for MELD scores less than 28 (71% vs. 57%; P = .077) and for subjects with an age below the median (67% vs. 55%; P = .167).
When both factors were considered together (MELD score less than 28 and an age below the median), the advantage of ELAD over standard of care reached significance (100% vs. 76%; P = .006). This subgroup analysis was not prespecified in the trial design, making this difference hypothesis generating,
The findings “suggest that ELAD may be a favorable alcoholic hepatitis treatment modality in younger patients with sufficient renal function and less severe coagulopathy,” Dr. Reich reported. “A study to confirm the survival benefit in this population is in preparation and is scheduled to start in 2016.”
Entry criteria for this study included a MELD score less than 35, a bilirubin greater than 8 mg/dL, and no other serious concomitant disease. Of patients randomized, 120 had a MELD score less than 28 and 101 were below the median age.
The expectation of benefit from C3A hepatoma cells was derived from evidence that these express immunomodulatory proteins and growth factors support hepatocellular function, according to Dr. Reich. The difference in survival in the younger patients was observed at 91 days. Dr. Reich reported that survival was “fairly stable” after 100 days in all patient groups.
“Alcoholic hepatitis results from hepatic inflammation, oxidative damage, cholestasis, and apoptosis, all of which create a vicious cycle that leads to liver dysfunction and secondary organ failure associated with poor prognosis,” Dr. Reich explained. It was hoped that ELAD delivery of C3A cells could modify this process.
Further study in subgroups that may benefit are encouraged by the safety profile observed in this study. There were no significant differences in serious adverse events observed in the two study arms, Dr. Reich reported.
Dr. Reich reported a financial relationship with Neuwave and VTI Corporation, the sponsor of the randomized study.
FROM THE LIVER MEETING 2015
Key clinical point:Extracorporeal hepatocellular therapy with human C3A hepatoma cells did not provide an overall survival benefit in patients with alcoholic hepatitis.
Major finding: In a post hoc analysis of an otherwise negative trial, C3A cell infusion was only associated with a survival advantage in younger, healthier patients (100% vs. 73%; P = .006).
Data source: Randomized, multicenter trial.
Disclosures: Dr. Reich reported a financial relationship with Neuwave and VTI Corporation, the sponsor of the randomized study.
High SVR achieved in decompensated HCV patients with 12-week therapy
SAN FRANCISCO – In patients infected with hepatitis C virus (HCV) who have decompensated liver disease, a 12-week combination drug regimen with velpatasvir and sofosbuvir (VEL/SOF) with or without ribavirin (VEL/SOF/R) achieved an overall sustained virologic response (SVR) rate of 94%, according to a late-breaker trial presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase III trial, called ASTRAL-4, randomized patients with HCV genotypes 1, 2, 3, 4, and 6 to one of three regimens. The simplest regimen, which required a single daily pill, achieved 100% SVR rates in genotypes 2 and 4, according to Dr. Michael R. Charlton of Intermountain Medical Center, Salt Lake City, Utah.
In this trial, 267 patients with HCV and decompensated liver disease were randomized to 12 weeks of 400 mg sofosbuvir and 100 mg velpatasvir in a fixed-dose single-pill combination, 12 weeks of SOF/VEL plus weight-based ribavirin (VEL/SOF/R), or 24 weeks of VEL/SOF. The majority (55%) were treatment experienced. Patients with hepatocellular carcinoma or prior liver transplant were excluded. Most (78%) had genotype 1 HCV. Only one patient had genotype 6.
On VEL/SOF alone for 12 weeks, the SVR rates ranged from 100% in patients with genotypes 2 or 4 HCV to 88% in genotype 1 and 50% in genotype 3. With the addition of ribavirin, the 12-week SVR rates climbed to 96% in the genotype1 group and 85% in genotype 3 group. After 24 weeks of VEL/SOF, the single genotype 6 patient in this trial achieved SVR.
SVR rates in those receiving VEL/SOF for 24 weeks were not much better than those achieved by the 12-week regimen. While SVR on the extended regimen was 100% in those with genotype 4 (as it was after 12 weeks), it remained 50% in genotype 3, which was achieved after 12 weeks. The SVR rate after 24 weeks VEL/SOF was 75% in genotype 2 patients and 92% in genotype 1 patients.
All regimens were well tolerated. Although 18% of patients experienced serious adverse events, all but one was considered to be unrelated to therapy. Most adverse events overall were consistent with the underlying liver disease or with the known side effects of ribavirin.
“Among patients who achieved SVR, 47% had improvements in Child-Pugh score and 57% had improvements in MELD scores 12 weeks after treatment, largely driven by increases in albumin and decreases in bilirubin,” Dr. Charlton said.
Emphasizing that the current treatment options for HCV with decompensated liver disease are “limited,” Dr. Charlton indicated that the SVR rates achieved with velpatasvir, a pangenotypic HCV NS5A inhibitor, in a fixed-dose pill with sofosbuvir are encouraging. Although the addition of ribavirin may be required for optimal SVR in some genotypes, several HCV subgroups appear to be adequately treated with a single once-daily pill taken over 12 weeks.
Gilead Sciences petitioned the Food and Drug Administration in October for approval of a fixed-dose combination of sofosbuvir/velpatasvir, according to a statement from the company.
Dr. Charlton reported financial relationships with Abbvie, Gilead, Janssen, Merck, and Novartis.
SAN FRANCISCO – In patients infected with hepatitis C virus (HCV) who have decompensated liver disease, a 12-week combination drug regimen with velpatasvir and sofosbuvir (VEL/SOF) with or without ribavirin (VEL/SOF/R) achieved an overall sustained virologic response (SVR) rate of 94%, according to a late-breaker trial presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase III trial, called ASTRAL-4, randomized patients with HCV genotypes 1, 2, 3, 4, and 6 to one of three regimens. The simplest regimen, which required a single daily pill, achieved 100% SVR rates in genotypes 2 and 4, according to Dr. Michael R. Charlton of Intermountain Medical Center, Salt Lake City, Utah.
In this trial, 267 patients with HCV and decompensated liver disease were randomized to 12 weeks of 400 mg sofosbuvir and 100 mg velpatasvir in a fixed-dose single-pill combination, 12 weeks of SOF/VEL plus weight-based ribavirin (VEL/SOF/R), or 24 weeks of VEL/SOF. The majority (55%) were treatment experienced. Patients with hepatocellular carcinoma or prior liver transplant were excluded. Most (78%) had genotype 1 HCV. Only one patient had genotype 6.
On VEL/SOF alone for 12 weeks, the SVR rates ranged from 100% in patients with genotypes 2 or 4 HCV to 88% in genotype 1 and 50% in genotype 3. With the addition of ribavirin, the 12-week SVR rates climbed to 96% in the genotype1 group and 85% in genotype 3 group. After 24 weeks of VEL/SOF, the single genotype 6 patient in this trial achieved SVR.
SVR rates in those receiving VEL/SOF for 24 weeks were not much better than those achieved by the 12-week regimen. While SVR on the extended regimen was 100% in those with genotype 4 (as it was after 12 weeks), it remained 50% in genotype 3, which was achieved after 12 weeks. The SVR rate after 24 weeks VEL/SOF was 75% in genotype 2 patients and 92% in genotype 1 patients.
All regimens were well tolerated. Although 18% of patients experienced serious adverse events, all but one was considered to be unrelated to therapy. Most adverse events overall were consistent with the underlying liver disease or with the known side effects of ribavirin.
“Among patients who achieved SVR, 47% had improvements in Child-Pugh score and 57% had improvements in MELD scores 12 weeks after treatment, largely driven by increases in albumin and decreases in bilirubin,” Dr. Charlton said.
Emphasizing that the current treatment options for HCV with decompensated liver disease are “limited,” Dr. Charlton indicated that the SVR rates achieved with velpatasvir, a pangenotypic HCV NS5A inhibitor, in a fixed-dose pill with sofosbuvir are encouraging. Although the addition of ribavirin may be required for optimal SVR in some genotypes, several HCV subgroups appear to be adequately treated with a single once-daily pill taken over 12 weeks.
Gilead Sciences petitioned the Food and Drug Administration in October for approval of a fixed-dose combination of sofosbuvir/velpatasvir, according to a statement from the company.
Dr. Charlton reported financial relationships with Abbvie, Gilead, Janssen, Merck, and Novartis.
SAN FRANCISCO – In patients infected with hepatitis C virus (HCV) who have decompensated liver disease, a 12-week combination drug regimen with velpatasvir and sofosbuvir (VEL/SOF) with or without ribavirin (VEL/SOF/R) achieved an overall sustained virologic response (SVR) rate of 94%, according to a late-breaker trial presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase III trial, called ASTRAL-4, randomized patients with HCV genotypes 1, 2, 3, 4, and 6 to one of three regimens. The simplest regimen, which required a single daily pill, achieved 100% SVR rates in genotypes 2 and 4, according to Dr. Michael R. Charlton of Intermountain Medical Center, Salt Lake City, Utah.
In this trial, 267 patients with HCV and decompensated liver disease were randomized to 12 weeks of 400 mg sofosbuvir and 100 mg velpatasvir in a fixed-dose single-pill combination, 12 weeks of SOF/VEL plus weight-based ribavirin (VEL/SOF/R), or 24 weeks of VEL/SOF. The majority (55%) were treatment experienced. Patients with hepatocellular carcinoma or prior liver transplant were excluded. Most (78%) had genotype 1 HCV. Only one patient had genotype 6.
On VEL/SOF alone for 12 weeks, the SVR rates ranged from 100% in patients with genotypes 2 or 4 HCV to 88% in genotype 1 and 50% in genotype 3. With the addition of ribavirin, the 12-week SVR rates climbed to 96% in the genotype1 group and 85% in genotype 3 group. After 24 weeks of VEL/SOF, the single genotype 6 patient in this trial achieved SVR.
SVR rates in those receiving VEL/SOF for 24 weeks were not much better than those achieved by the 12-week regimen. While SVR on the extended regimen was 100% in those with genotype 4 (as it was after 12 weeks), it remained 50% in genotype 3, which was achieved after 12 weeks. The SVR rate after 24 weeks VEL/SOF was 75% in genotype 2 patients and 92% in genotype 1 patients.
All regimens were well tolerated. Although 18% of patients experienced serious adverse events, all but one was considered to be unrelated to therapy. Most adverse events overall were consistent with the underlying liver disease or with the known side effects of ribavirin.
“Among patients who achieved SVR, 47% had improvements in Child-Pugh score and 57% had improvements in MELD scores 12 weeks after treatment, largely driven by increases in albumin and decreases in bilirubin,” Dr. Charlton said.
Emphasizing that the current treatment options for HCV with decompensated liver disease are “limited,” Dr. Charlton indicated that the SVR rates achieved with velpatasvir, a pangenotypic HCV NS5A inhibitor, in a fixed-dose pill with sofosbuvir are encouraging. Although the addition of ribavirin may be required for optimal SVR in some genotypes, several HCV subgroups appear to be adequately treated with a single once-daily pill taken over 12 weeks.
Gilead Sciences petitioned the Food and Drug Administration in October for approval of a fixed-dose combination of sofosbuvir/velpatasvir, according to a statement from the company.
Dr. Charlton reported financial relationships with Abbvie, Gilead, Janssen, Merck, and Novartis.
AT THE LIVER MEETING 2015
Key clinical point: High sustained viral response rates were achieved across hepatitis C virus genotypes with a two-drug fixed-dose combination that included a NS5A inhibitor.
Major finding: The overall SVR rate was 94% after 12 weeks of a fixed-dose combination of velpatasvir and sofosbuvir with ribavirin.
Data source: Multicenter, randomized, phase III study.
Disclosures: Dr. Charlton reported financial relationships with Abbvie, Gilead, Janssen, Merck, and Novartis.