User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Neuropathic pain puts cancer survivors out of work
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
AMSTERDAM – Five years after a cancer diagnosis, patients who report having chronic neuropathic pain are twice as likely to be out of work as patients who report having no neuropathic pain, authors of a large longitudinal study said.
“For middle-term cancer survivors, suffering from chronic neuropathic pain unfortunately predicts labor-market exit,” said Marc-Karim Bendiane, from Aix-Marseille University in Marseille, France.
Pain is still frequently underdiagnosed, poorly managed, and undertreated among cancer survivors, and there is a need for alternatives to analgesics for control of chronic neuropathic pain (CNP), Mr. Bendiane said at an annual congress sponsored by the European Cancer Organisation.
Mr. Bendiane and colleagues used data from VICAN, a longitudinal survey of issues of concern to cancer survivors 2 years and 5 years after a diagnosis. The cohort consists of patients diagnosed with cancers who comprise 88% of all cancer diagnoses in France, including cancers of the breast; colon and rectum; lip, oral cavity, and pharynx; kidney; cervix; endometrium; non-Hodgkin lymphoma; melanoma; thyroid; bladder; and prostate.
To assess CNP, the researchers used data from a seven-item questionnaire designed to identify neuropathic characteristics of pain experienced by patients in the 2 weeks prior to a comprehensive patient interview.
Of the 982 patients who were working at the time of diagnosis, 36% reported pain within the previous 2 weeks, and of this group, 79% had chronic pain of neuropathic origin. CNP was more common in women than in men (P less than .01); in college-educated people, compared with less-educated people (P less than .001); those who had undergone chemotherapy, compared with no chemotherapy (P less than .001); and those who had radiotherapy vs. no radiotherapy (P less than .001).
For each cancer site, the prevalence of CNP among 5-year cancer survivors was substantially higher than the overall prevalence in France of 7%. For example, 34% of patients with cancers of the cervix and endometrium reported CNP, as did 29.9% of patients who survived cancers of the lip, oral cavity, and pharynx, 32.1% of lung cancer survivors, and 32.7% of breast cancer survivors.
Five years after diagnosis, 22.6% of patients who had been employed in 2010 were out of work in 2015.
The presence of CNP was associated with a nearly twofold greater risk of unemployment (adjusted odds ratio, 1.96; P less than .001) in a multivariate logistic regression analysis comparing employed and unemployed patients and controlling for social and demographic characteristics, job characteristics at diagnosis, and medical factors such as tumor site, prognosis, and treatment type.
The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
Key clinical point: Chronic neuropathic pain is a barrier to employment for many cancer survivors.
Major finding: Cancer survivors with chronic neuropathic pain were twice as likely to be unemployed 5 years after diagnosis as patients with no pain.
Data source: Longitudinal study of French cancer survivors.
Disclosures: The French National Cancer Institute and INSERM, the National Institute for Research in Health and Medicine, supported the study. The investigators reported no conflicts of interest.
Long view shows doubling of survival in non-Hodgkin lymphoma
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).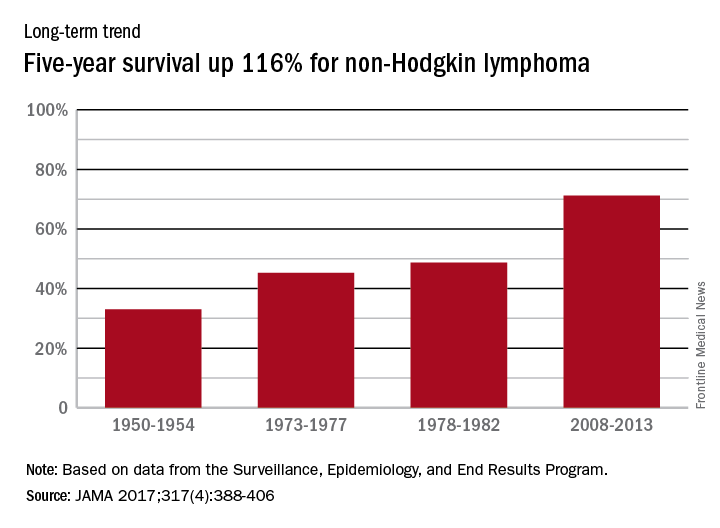
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
FROM JAMA
Hodgkin lymphoma survival has nearly tripled since the 1950s
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.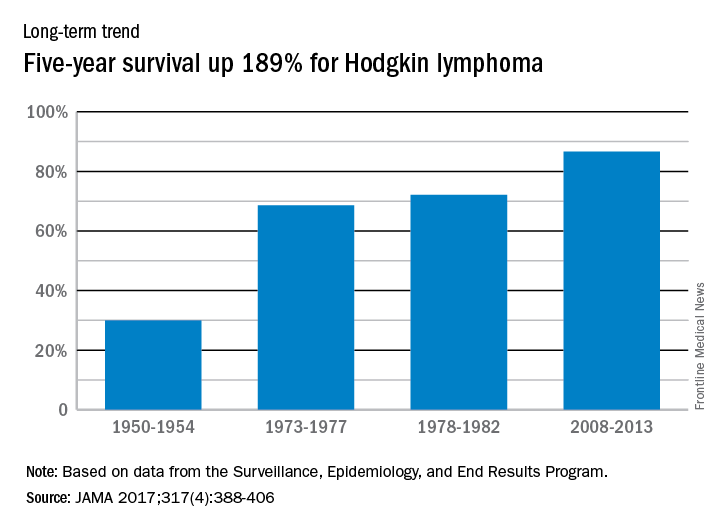
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
Five-year relative survival for Hodgkin lymphoma increased 189% over the approximately 60 years from the early 1950s to 2013, according to investigators looking at data from the Surveillance, Epidemiology, and End Results Program.
During 1950-1954, the 5-year relative survival rate for Hodgkin lymphoma was 30%, compared with 86.6% in 2008-2013, said Ali H. Mokdad, PhD, and his associates at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
In 2014, mortality for Hodgkin lymphoma was 0.4 per 100,000 population, which put it 27th among the 29 included cancers, with about 36,000 years of life lost, which was 26th of the 29 cancers, Dr. Mokdad and his associates said. This part of their study used deidentified death records from the National Center for Health Statistics and population counts from the Census Bureau, the NCHS, and the Human Mortality Database.
FROM JAMA
Circulating DNA catches lymphoma relapse early
A newer technique aimed at detect circulating tumor DNA in the blood – cancer personalized profiling by deep sequencing (CAPP-Seq) – detected recurrence of diffuse large B cell lymphoma more than 6 months earlier than radiographic findings in a study at Stanford (Calif.) University, where the technique was invented.
The findings signal another win for “liquid biopsy,” the measurement of tumor DNA circulating in the blood, which is rapidly emerging as a quick and powerful tool for the diagnosis of a range of cancers and tumor subtypes, and prediction of tumor behavior and treatment response. Investigators at Stanford and elsewhere are studying liquid biopsy not only for lymphoma, but also for colorectal, thyroid, breast, prostate, and most other cancers. The Stanford team recently reported that its circulating DNA-detecting CAPP-Seq technique also helps in lung cancer.
In the new study, Stanford used CAPP-Seq (Cancer Personalized Profiling by deep Sequencing), which it called “an ultrasensitive capture-based targeted sequencing method” to analyze 166 plasma and 118 tissue samples from 92 patients with diffuse large B cell lymphoma (DLBCL) at diagnosis and various point afterward. The team compared the results to radiologic, and other standard diagnostic and monitoring techniques (Sci Transl Med. 2016 Nov 9;8[364]:364ra155).
At diagnosis, the amount of circulating DNA (ctDNA) correlated strongly with clinical indices and was independently predictive of patient outcomes; “whereas 100% of pretreatment samples had detectable ctDNA, only 37% of samples had abnormally high serum” lactate dehydrogenase, currently the most commonly used biomarker for DLBCL, said investigators, led by research fellow Florian Scherer, MD.
The group detected ctDNA in 73% of patients (8/11) who eventually relapsed a mean of 188 days before relapse was detected by standard-of-care radiologic techniques.
CAPP-Seq identified nine patients with a particular type of activated B cell-like tumor, for whom ibrutinib (Imbruvica) is particularly effective; ctDNA also predicted the transformation of indolent follicular lymphoma to DLBCL “with high sensitivity and specificity,” the group reported.
Stanford anticipates “ctDNA will have broad utility for dissecting tumor heterogeneity within and between patients with lymphomas and other cancer types, with applications for the identification of adverse risk groups, the discovery of resistance mechanisms to diverse therapies, and the development of risk-adapted therapeutics.”
The team said its approach “outperformed immunoglobulin sequencing and radiographic imaging for the detection of minimal residual disease and facilitated noninvasive identification of emergent resistance mutations to targeted therapies.” Meanwhile, while biomarkers hold “great promise for risk stratification and therapeutic targeting,” they are “currently difficult to measure in clinical settings,” the investigators said.
Roche bought the rights to CAPP-Seq from Stanford in 2015. Several authors are coinventors on patent applications for CAPP-Seq and also Roche consultants. Two are employees. Dr. Scherer had no disclosures. The work was funded by Stanford, the American Society of Hematology, the National Cancer Institute, and others.
A newer technique aimed at detect circulating tumor DNA in the blood – cancer personalized profiling by deep sequencing (CAPP-Seq) – detected recurrence of diffuse large B cell lymphoma more than 6 months earlier than radiographic findings in a study at Stanford (Calif.) University, where the technique was invented.
The findings signal another win for “liquid biopsy,” the measurement of tumor DNA circulating in the blood, which is rapidly emerging as a quick and powerful tool for the diagnosis of a range of cancers and tumor subtypes, and prediction of tumor behavior and treatment response. Investigators at Stanford and elsewhere are studying liquid biopsy not only for lymphoma, but also for colorectal, thyroid, breast, prostate, and most other cancers. The Stanford team recently reported that its circulating DNA-detecting CAPP-Seq technique also helps in lung cancer.
In the new study, Stanford used CAPP-Seq (Cancer Personalized Profiling by deep Sequencing), which it called “an ultrasensitive capture-based targeted sequencing method” to analyze 166 plasma and 118 tissue samples from 92 patients with diffuse large B cell lymphoma (DLBCL) at diagnosis and various point afterward. The team compared the results to radiologic, and other standard diagnostic and monitoring techniques (Sci Transl Med. 2016 Nov 9;8[364]:364ra155).
At diagnosis, the amount of circulating DNA (ctDNA) correlated strongly with clinical indices and was independently predictive of patient outcomes; “whereas 100% of pretreatment samples had detectable ctDNA, only 37% of samples had abnormally high serum” lactate dehydrogenase, currently the most commonly used biomarker for DLBCL, said investigators, led by research fellow Florian Scherer, MD.
The group detected ctDNA in 73% of patients (8/11) who eventually relapsed a mean of 188 days before relapse was detected by standard-of-care radiologic techniques.
CAPP-Seq identified nine patients with a particular type of activated B cell-like tumor, for whom ibrutinib (Imbruvica) is particularly effective; ctDNA also predicted the transformation of indolent follicular lymphoma to DLBCL “with high sensitivity and specificity,” the group reported.
Stanford anticipates “ctDNA will have broad utility for dissecting tumor heterogeneity within and between patients with lymphomas and other cancer types, with applications for the identification of adverse risk groups, the discovery of resistance mechanisms to diverse therapies, and the development of risk-adapted therapeutics.”
The team said its approach “outperformed immunoglobulin sequencing and radiographic imaging for the detection of minimal residual disease and facilitated noninvasive identification of emergent resistance mutations to targeted therapies.” Meanwhile, while biomarkers hold “great promise for risk stratification and therapeutic targeting,” they are “currently difficult to measure in clinical settings,” the investigators said.
Roche bought the rights to CAPP-Seq from Stanford in 2015. Several authors are coinventors on patent applications for CAPP-Seq and also Roche consultants. Two are employees. Dr. Scherer had no disclosures. The work was funded by Stanford, the American Society of Hematology, the National Cancer Institute, and others.
A newer technique aimed at detect circulating tumor DNA in the blood – cancer personalized profiling by deep sequencing (CAPP-Seq) – detected recurrence of diffuse large B cell lymphoma more than 6 months earlier than radiographic findings in a study at Stanford (Calif.) University, where the technique was invented.
The findings signal another win for “liquid biopsy,” the measurement of tumor DNA circulating in the blood, which is rapidly emerging as a quick and powerful tool for the diagnosis of a range of cancers and tumor subtypes, and prediction of tumor behavior and treatment response. Investigators at Stanford and elsewhere are studying liquid biopsy not only for lymphoma, but also for colorectal, thyroid, breast, prostate, and most other cancers. The Stanford team recently reported that its circulating DNA-detecting CAPP-Seq technique also helps in lung cancer.
In the new study, Stanford used CAPP-Seq (Cancer Personalized Profiling by deep Sequencing), which it called “an ultrasensitive capture-based targeted sequencing method” to analyze 166 plasma and 118 tissue samples from 92 patients with diffuse large B cell lymphoma (DLBCL) at diagnosis and various point afterward. The team compared the results to radiologic, and other standard diagnostic and monitoring techniques (Sci Transl Med. 2016 Nov 9;8[364]:364ra155).
At diagnosis, the amount of circulating DNA (ctDNA) correlated strongly with clinical indices and was independently predictive of patient outcomes; “whereas 100% of pretreatment samples had detectable ctDNA, only 37% of samples had abnormally high serum” lactate dehydrogenase, currently the most commonly used biomarker for DLBCL, said investigators, led by research fellow Florian Scherer, MD.
The group detected ctDNA in 73% of patients (8/11) who eventually relapsed a mean of 188 days before relapse was detected by standard-of-care radiologic techniques.
CAPP-Seq identified nine patients with a particular type of activated B cell-like tumor, for whom ibrutinib (Imbruvica) is particularly effective; ctDNA also predicted the transformation of indolent follicular lymphoma to DLBCL “with high sensitivity and specificity,” the group reported.
Stanford anticipates “ctDNA will have broad utility for dissecting tumor heterogeneity within and between patients with lymphomas and other cancer types, with applications for the identification of adverse risk groups, the discovery of resistance mechanisms to diverse therapies, and the development of risk-adapted therapeutics.”
The team said its approach “outperformed immunoglobulin sequencing and radiographic imaging for the detection of minimal residual disease and facilitated noninvasive identification of emergent resistance mutations to targeted therapies.” Meanwhile, while biomarkers hold “great promise for risk stratification and therapeutic targeting,” they are “currently difficult to measure in clinical settings,” the investigators said.
Roche bought the rights to CAPP-Seq from Stanford in 2015. Several authors are coinventors on patent applications for CAPP-Seq and also Roche consultants. Two are employees. Dr. Scherer had no disclosures. The work was funded by Stanford, the American Society of Hematology, the National Cancer Institute, and others.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Major finding: Circulating tumor DNA was found in 73% of relapse patients a mean of 188 days before relapse was detected by standard-of-care radiologic techniques. Circulating tumor DNA was found in the plasma of 100% of patients at diagnosis, but only 37% had abnormally high serum lactate dehydrogenase, currently the most commonly used biomarker.
Data source: Analysis of 166 plasma and 118 tissue samples from 92 patients with diffuse large B cell lymphoma.
Disclosures: Roche bought the rights to CAPP-Seq from Stanford (Calif.) University in 2015. Several authors are coinventors on patent applications for CAPP-Seq and also Roche consults. Two are employees. The work was funded by Stanford, the American Society of Hematology, the National Cancer Institute, and others.
FDA approves ibrutinib for refractory MZL
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
[email protected]
On Twitter @HematologyNews1
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
[email protected]
On Twitter @HematologyNews1
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
[email protected]
On Twitter @HematologyNews1
VIDEO: First multicenter trial of CAR T cells shows response in DLBCL
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
VIDEO: Obinutuzumab bests rituximab for PFS in follicular lymphoma
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Antibody face-off in follicular lymphoma gives PFS, but not OS, edge to obinutuzumab
SAN DIEGO – Obinutuzumab, a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, offered a progression-free survival (PFS) edge over rituximab when combined with standard chemotherapy in patients with previously untreated advanced follicular lymphoma.
But other clinicians and investigators who
attended the presentation of the GALLIUM data at a plenary session during the American Society of Hematology annual meeting indicated that despite the data, they weren’t ready to make a switch to the newer, costlier antibody.
“I feel that it is not convincing for practice-changing,” said Kanti R. Rai, MD, professor of medicine and molecular medicine at Hofstra University, Hempstead, N.Y.
“Unless we have evidence of a survival advantage in indolent disease, progression-free survivorship is not an adequate reason to jump to another antibody,” he said in an interview.
In GALLIUM, the primary endpoint of investigator-assessed 3-year PFS at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio of 0.68 favoring obinutuzumab (P = .0012).
Respective 3-year overall survival rates were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
Indolent lymphoma trial
The GALLIUM trial is a phase III study comparing obinutuzumab with rituximab when paired with one of three standard chemotherapy regimens for indolent non-Hodgkin lymphomas, including follicular lymphoma and splenic, nodal, or extranodal marginal zone lymphoma. Dr. Marcus presented data on patients with follicular lymphoma only.
The antibodies were delivered in combination with either CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone; 33.1% of patients), CVP (cyclophosphamide, vincristine, prednisone; 9.8%) or bendamustine alone (B; 57.1%) as the chemotherapy backbone. The choice of regimen was at the discretion of the treating center.
A total of 1,202 patients with follicular lymphoma were enrolled and randomized to treatment and were included in an intention-to-treat analysis.
The treatment arms were well balanced with regard to distribution of patients characteristics, with approximately 21% in each arm having Follicular Lymphoma International Prognostic Index low-risk disease; 37% having intermediate-risk disease; and 34% having high-risk disease.
Roughly half of patients in each arm had bone marrow involvement, and two-thirds had extranodal involvement.
Obinutuzumab was dosed 1,000 mg IV on days 1, 8, and 15 of cycle one, and either on day 1 of cycles two through eight every 3 weeks, or every 4 weeks during cycles two through six.
Overall response rates at the end of induction were 86.9% with rituximab and 88.5% with obinutuzumab, with complete responses of 23.8% and 19.5%, respectively.
As noted before, investigator-assessed PFS favored obinutuzumab, as did PFS assessed by independent reviewer, at 81.9% vs. 77.9% for rituximab (HR, 0.71; P = .0138).
The newer antibody also had a slight edge in time to new treatment, with 87.1% of patients on obinutuzumab not starting on new therapy, compared with 81.2% of patients on rituximab.
More bendamustine deaths
Nearly all patients in each arm had an adverse event, with grade 3 or greater events occurring in 74.6% of patients on obinutuzumab vs. 67.8% on rituximab. Rates of neutropenia, leukopenia, febrile neutropenia, infusion reactions, and thrombocytopenia were all slightly higher with obinutuzumab. Grade 3 or greater infections occurred in 20% with obinutuzumab, compared with 15.6% with rituximab.
“What we did note, however, was a high level of mortality in patients receiving either obinutuzumab-based therapy or rituximab-based therapy, which were no different between the two arms and were somewhat higher than one might expect from patients receiving induction treatment in follicular lymphoma. Hence, we did a more detailed analysis of safety by treatment regimen,” Dr. Marcus said.
There were more deaths among patients treated with bendamustine (5.6% for patients in the B-obinutuzumab cohort, and 4.4% of patients in the B-rituximab cohort) vs. 1.6% and 2.0%, respectively, for patients on CHOP, and 1.6 and 1.8% for patients on CVP.
Dose effect?
John P. Leonard, MD, from Cornell University, New York , who introduced Dr. Marcus, commented that PFS may not be the ideal endpoint for patients with follicular lymphoma.
He pointed out that in trials comparing rituximab with obinutuzumab for other diseases, results have been mixed, with obinutuzumab showing superiority in chronic lymphocytic leukemia, but in data presented elsewhere at ASH 2016, obinutuzumab was not superior to rituximab for treatment of diffuse large B-cell lymphoma.
“One question is whether obinutuzumab, which is generally administered at a higher mg dose to patients, is in fact a better antibody or if it is in fact a dose effect,” he said.
In response to a similar question following his presentation, Dr. Marcus replied that, despite sharing a target, the two antibodies are different, with different mechanisms of action. He also noted that there is no evidence to suggest that rituximab potency would be greater in follicular lymphoma if it were given at higher doses.
The GALLIUM trial is sponsored by Hoffmann-La Roche, Dr, Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
SAN DIEGO – Obinutuzumab, a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, offered a progression-free survival (PFS) edge over rituximab when combined with standard chemotherapy in patients with previously untreated advanced follicular lymphoma.
But other clinicians and investigators who
attended the presentation of the GALLIUM data at a plenary session during the American Society of Hematology annual meeting indicated that despite the data, they weren’t ready to make a switch to the newer, costlier antibody.
“I feel that it is not convincing for practice-changing,” said Kanti R. Rai, MD, professor of medicine and molecular medicine at Hofstra University, Hempstead, N.Y.
“Unless we have evidence of a survival advantage in indolent disease, progression-free survivorship is not an adequate reason to jump to another antibody,” he said in an interview.
In GALLIUM, the primary endpoint of investigator-assessed 3-year PFS at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio of 0.68 favoring obinutuzumab (P = .0012).
Respective 3-year overall survival rates were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
Indolent lymphoma trial
The GALLIUM trial is a phase III study comparing obinutuzumab with rituximab when paired with one of three standard chemotherapy regimens for indolent non-Hodgkin lymphomas, including follicular lymphoma and splenic, nodal, or extranodal marginal zone lymphoma. Dr. Marcus presented data on patients with follicular lymphoma only.
The antibodies were delivered in combination with either CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone; 33.1% of patients), CVP (cyclophosphamide, vincristine, prednisone; 9.8%) or bendamustine alone (B; 57.1%) as the chemotherapy backbone. The choice of regimen was at the discretion of the treating center.
A total of 1,202 patients with follicular lymphoma were enrolled and randomized to treatment and were included in an intention-to-treat analysis.
The treatment arms were well balanced with regard to distribution of patients characteristics, with approximately 21% in each arm having Follicular Lymphoma International Prognostic Index low-risk disease; 37% having intermediate-risk disease; and 34% having high-risk disease.
Roughly half of patients in each arm had bone marrow involvement, and two-thirds had extranodal involvement.
Obinutuzumab was dosed 1,000 mg IV on days 1, 8, and 15 of cycle one, and either on day 1 of cycles two through eight every 3 weeks, or every 4 weeks during cycles two through six.
Overall response rates at the end of induction were 86.9% with rituximab and 88.5% with obinutuzumab, with complete responses of 23.8% and 19.5%, respectively.
As noted before, investigator-assessed PFS favored obinutuzumab, as did PFS assessed by independent reviewer, at 81.9% vs. 77.9% for rituximab (HR, 0.71; P = .0138).
The newer antibody also had a slight edge in time to new treatment, with 87.1% of patients on obinutuzumab not starting on new therapy, compared with 81.2% of patients on rituximab.
More bendamustine deaths
Nearly all patients in each arm had an adverse event, with grade 3 or greater events occurring in 74.6% of patients on obinutuzumab vs. 67.8% on rituximab. Rates of neutropenia, leukopenia, febrile neutropenia, infusion reactions, and thrombocytopenia were all slightly higher with obinutuzumab. Grade 3 or greater infections occurred in 20% with obinutuzumab, compared with 15.6% with rituximab.
“What we did note, however, was a high level of mortality in patients receiving either obinutuzumab-based therapy or rituximab-based therapy, which were no different between the two arms and were somewhat higher than one might expect from patients receiving induction treatment in follicular lymphoma. Hence, we did a more detailed analysis of safety by treatment regimen,” Dr. Marcus said.
There were more deaths among patients treated with bendamustine (5.6% for patients in the B-obinutuzumab cohort, and 4.4% of patients in the B-rituximab cohort) vs. 1.6% and 2.0%, respectively, for patients on CHOP, and 1.6 and 1.8% for patients on CVP.
Dose effect?
John P. Leonard, MD, from Cornell University, New York , who introduced Dr. Marcus, commented that PFS may not be the ideal endpoint for patients with follicular lymphoma.
He pointed out that in trials comparing rituximab with obinutuzumab for other diseases, results have been mixed, with obinutuzumab showing superiority in chronic lymphocytic leukemia, but in data presented elsewhere at ASH 2016, obinutuzumab was not superior to rituximab for treatment of diffuse large B-cell lymphoma.
“One question is whether obinutuzumab, which is generally administered at a higher mg dose to patients, is in fact a better antibody or if it is in fact a dose effect,” he said.
In response to a similar question following his presentation, Dr. Marcus replied that, despite sharing a target, the two antibodies are different, with different mechanisms of action. He also noted that there is no evidence to suggest that rituximab potency would be greater in follicular lymphoma if it were given at higher doses.
The GALLIUM trial is sponsored by Hoffmann-La Roche, Dr, Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
SAN DIEGO – Obinutuzumab, a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, offered a progression-free survival (PFS) edge over rituximab when combined with standard chemotherapy in patients with previously untreated advanced follicular lymphoma.
But other clinicians and investigators who
attended the presentation of the GALLIUM data at a plenary session during the American Society of Hematology annual meeting indicated that despite the data, they weren’t ready to make a switch to the newer, costlier antibody.
“I feel that it is not convincing for practice-changing,” said Kanti R. Rai, MD, professor of medicine and molecular medicine at Hofstra University, Hempstead, N.Y.
“Unless we have evidence of a survival advantage in indolent disease, progression-free survivorship is not an adequate reason to jump to another antibody,” he said in an interview.
In GALLIUM, the primary endpoint of investigator-assessed 3-year PFS at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio of 0.68 favoring obinutuzumab (P = .0012).
Respective 3-year overall survival rates were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
Indolent lymphoma trial
The GALLIUM trial is a phase III study comparing obinutuzumab with rituximab when paired with one of three standard chemotherapy regimens for indolent non-Hodgkin lymphomas, including follicular lymphoma and splenic, nodal, or extranodal marginal zone lymphoma. Dr. Marcus presented data on patients with follicular lymphoma only.
The antibodies were delivered in combination with either CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone; 33.1% of patients), CVP (cyclophosphamide, vincristine, prednisone; 9.8%) or bendamustine alone (B; 57.1%) as the chemotherapy backbone. The choice of regimen was at the discretion of the treating center.
A total of 1,202 patients with follicular lymphoma were enrolled and randomized to treatment and were included in an intention-to-treat analysis.
The treatment arms were well balanced with regard to distribution of patients characteristics, with approximately 21% in each arm having Follicular Lymphoma International Prognostic Index low-risk disease; 37% having intermediate-risk disease; and 34% having high-risk disease.
Roughly half of patients in each arm had bone marrow involvement, and two-thirds had extranodal involvement.
Obinutuzumab was dosed 1,000 mg IV on days 1, 8, and 15 of cycle one, and either on day 1 of cycles two through eight every 3 weeks, or every 4 weeks during cycles two through six.
Overall response rates at the end of induction were 86.9% with rituximab and 88.5% with obinutuzumab, with complete responses of 23.8% and 19.5%, respectively.
As noted before, investigator-assessed PFS favored obinutuzumab, as did PFS assessed by independent reviewer, at 81.9% vs. 77.9% for rituximab (HR, 0.71; P = .0138).
The newer antibody also had a slight edge in time to new treatment, with 87.1% of patients on obinutuzumab not starting on new therapy, compared with 81.2% of patients on rituximab.
More bendamustine deaths
Nearly all patients in each arm had an adverse event, with grade 3 or greater events occurring in 74.6% of patients on obinutuzumab vs. 67.8% on rituximab. Rates of neutropenia, leukopenia, febrile neutropenia, infusion reactions, and thrombocytopenia were all slightly higher with obinutuzumab. Grade 3 or greater infections occurred in 20% with obinutuzumab, compared with 15.6% with rituximab.
“What we did note, however, was a high level of mortality in patients receiving either obinutuzumab-based therapy or rituximab-based therapy, which were no different between the two arms and were somewhat higher than one might expect from patients receiving induction treatment in follicular lymphoma. Hence, we did a more detailed analysis of safety by treatment regimen,” Dr. Marcus said.
There were more deaths among patients treated with bendamustine (5.6% for patients in the B-obinutuzumab cohort, and 4.4% of patients in the B-rituximab cohort) vs. 1.6% and 2.0%, respectively, for patients on CHOP, and 1.6 and 1.8% for patients on CVP.
Dose effect?
John P. Leonard, MD, from Cornell University, New York , who introduced Dr. Marcus, commented that PFS may not be the ideal endpoint for patients with follicular lymphoma.
He pointed out that in trials comparing rituximab with obinutuzumab for other diseases, results have been mixed, with obinutuzumab showing superiority in chronic lymphocytic leukemia, but in data presented elsewhere at ASH 2016, obinutuzumab was not superior to rituximab for treatment of diffuse large B-cell lymphoma.
“One question is whether obinutuzumab, which is generally administered at a higher mg dose to patients, is in fact a better antibody or if it is in fact a dose effect,” he said.
In response to a similar question following his presentation, Dr. Marcus replied that, despite sharing a target, the two antibodies are different, with different mechanisms of action. He also noted that there is no evidence to suggest that rituximab potency would be greater in follicular lymphoma if it were given at higher doses.
The GALLIUM trial is sponsored by Hoffmann-La Roche, Dr, Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
AT ASH 2016
Key clinical point: Obinutuzumab plus chemotherapy was associated with better 3-year progression-free survival in patients with untreated follicular lymphoma.
Major finding: Obinutuzumab/chemo was associated with a hazard ratio for investigator-assessed PFS of 0.68 (P = .0012)
Data source: Randomized phase III trial in 1202 patients with previously untreated follicular lymphoma.
Disclosures: The GALLIUM trial was sponsored by Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
Worse outcomes for double-hit lymphomas after ASCT
Patients with double-hit lymphomas (DHLs) and double-expressor lymphomas (DELs) have inferior outcomes after undergoing autologous stem cell transplantation (ASCT), according to a new study published in the Journal of Clinical Oncology.
The worst outcomes were observed in patients with concurrent DELs and DHLs, and this “supports the concept that the double-hit/double-expressor biology appears to render DLBCL resistant to and less likely to be cured by chemotherapy,” write Alex Herrera, MD, an oncologist at the City of Hope, Duarte, Calif., and his colleagues.
But that said, a significant proportion of patients with relapsed/refractory DEL did experience durable remissions following ASCT, particularly those with isolated DEL without DHL.
This suggests that “the presence of DEL alone should not be considered a contraindication to ASCT,” the authors wrote (J Clin Oncol. 2016 Oct. 24. doi: 10.1200/JCO.2016.68.2740).
DHLs and DELs are subtypes of diffuse large B-cell lymphoma (DLBCL), and while they are associated with poor outcomes after standard chemoimmunotherapy, data remain limited as to outcomes of patients with relapsed or refractory disease who undergo ASCT.
The retrospective multicenter study included 117 patients with chemotherapy-sensitive relapsed/refractory DLBCL who underwent ASCT and had archival tumor material available. DEL with MYC/BCL2 coexpression was observed in 52 patients (44%) while 15 patients expressed MYC-R (13%), of whom 12 (10%) had DHL.
The median follow-up time was 45 months for survivors, and the 4-year progression-free survival (PFS) and overall survival (OS) were 54% for the entire cohort.
The 4-year PFS and OS in patients with DHL was worse as compared to those without DHL; 28% vs. 57% (P = .013), and 25% vs. 66% (P less than .001), respectively.
Those with DHL had poorer PFS (28%) and OS (25%), compared with patients with DEL but not DHL (PFS, 53% and OS, 61%) as well as patients with neither DEL nor DHL (PFS, 60% and OS, 70%; three-way P value for PFS, P = .013; OS, P = .002).
Patients with concurrent DEL and DHL had the poorest outcome, with a 4-year PFS of 0%.
After researchers adjusted for clinical characteristics, the only factors that remained significantly associated with PFS were DEL (hazard ratio, 1.8; P = .035) and DHL (HR, 2.9; P = .009). Factors that were significantly associated with OS were DHL (HR, 3.4; P = .004) and remission status at ASCT (HR for partial response, 2.4; P = .007).
Overall, patients with DHL were less likely to achieve a complete response following salvage therapy, and those with DEL and patients with DHL had a shorter time to relapse after induction therapy.
“Although some patients with relapsed/refractory DHL had long-term remission after ASCT (isolated DHL without DEL), the low survival rate in this group argues that alternative transplantation strategies, including allogeneic hematopoietic stem cell transplantation or peri-ASCT relapse prevention strategies should be studied,” they concluded.
Recognizing that the majority of patients with double-hit or double-expressor lymphoma will relapse after R-CHOP, this study evaluates the efficacy of autologous stem cell transplantation as a salvage modality. This is a carefully conducted, albeit retrospective, analysis of patients with relapsed or refractory double-hit or double-expressor lymphoma undergoing autologous hematopoietic stem cell transplantation (auto-HCT) at two high-volume institutions.
Although it is perhaps not surprising that double-hit and double-expressor phenotypes confer inferior outcomes, it is worth examining these issues in some detail. The first issue is that the authors have defined categories that are not recognized by the World Health Organization, but are routinely seen in clinical practice.
For example, it might be assumed that all patients with double-hit lymphoma will have MYC/BCL2 protein expression and, therefore, also have double-expressor lymphoma, but some patients with double-hit lymphoma do not have protein expression of MYC/BCL2 and these patients may have better outcomes than patients whose tumors display both double-hit and double-expressor characteristics.
A second caveat to interpreting the results is that the study population does not reflect the true denominator of all patients with relapsed diffuse large B-cell lymphoma, because only chemotherapy-sensitive patients undergoing auto-HCT were included.
So, should patients with relapsed double-hit and double-expressor lymphoma be offered auto-HCT? What are the alternatives to auto-HCT? Unfortunately, there are no clear answers to these questions, although the surprisingly excellent outcomes for patients without either of these features (70% long-term survival) suggest that there is a group of patients for whom auto-HCT remains an effective and standard tool. For double-hit and double-expressor lymphoma, a clinical trial based on specific biologic changes in individual patients is the ideal but is far from reality at this point.
Overall, despite being a retrospective series with a high attrition rate based on tissue availability, the central review of pathology, uniform assessment of double-hit and double-expressor features, and mature follow-up of 45 months makes this a thought-provoking and timely paper.
Sonali M. Smith, MD, is from the University of Chicago, and has disclosed a consulting or advisory role with Genentech, Seattle Genetics, TG Therapeutics, Gilead Sciences, Immunogenix, Pharmacyclics, NanoString Technologies, Genmab, Juno Therapeutics, Abbvie, and Portola Pharmaceuticals. These remarks were taken from the editorial accompanying Dr. Herrara’s report (J Clin Oncol. 2016 Oct 17. doi: 10.1200/JCO.2016.70.0625).
Recognizing that the majority of patients with double-hit or double-expressor lymphoma will relapse after R-CHOP, this study evaluates the efficacy of autologous stem cell transplantation as a salvage modality. This is a carefully conducted, albeit retrospective, analysis of patients with relapsed or refractory double-hit or double-expressor lymphoma undergoing autologous hematopoietic stem cell transplantation (auto-HCT) at two high-volume institutions.
Although it is perhaps not surprising that double-hit and double-expressor phenotypes confer inferior outcomes, it is worth examining these issues in some detail. The first issue is that the authors have defined categories that are not recognized by the World Health Organization, but are routinely seen in clinical practice.
For example, it might be assumed that all patients with double-hit lymphoma will have MYC/BCL2 protein expression and, therefore, also have double-expressor lymphoma, but some patients with double-hit lymphoma do not have protein expression of MYC/BCL2 and these patients may have better outcomes than patients whose tumors display both double-hit and double-expressor characteristics.
A second caveat to interpreting the results is that the study population does not reflect the true denominator of all patients with relapsed diffuse large B-cell lymphoma, because only chemotherapy-sensitive patients undergoing auto-HCT were included.
So, should patients with relapsed double-hit and double-expressor lymphoma be offered auto-HCT? What are the alternatives to auto-HCT? Unfortunately, there are no clear answers to these questions, although the surprisingly excellent outcomes for patients without either of these features (70% long-term survival) suggest that there is a group of patients for whom auto-HCT remains an effective and standard tool. For double-hit and double-expressor lymphoma, a clinical trial based on specific biologic changes in individual patients is the ideal but is far from reality at this point.
Overall, despite being a retrospective series with a high attrition rate based on tissue availability, the central review of pathology, uniform assessment of double-hit and double-expressor features, and mature follow-up of 45 months makes this a thought-provoking and timely paper.
Sonali M. Smith, MD, is from the University of Chicago, and has disclosed a consulting or advisory role with Genentech, Seattle Genetics, TG Therapeutics, Gilead Sciences, Immunogenix, Pharmacyclics, NanoString Technologies, Genmab, Juno Therapeutics, Abbvie, and Portola Pharmaceuticals. These remarks were taken from the editorial accompanying Dr. Herrara’s report (J Clin Oncol. 2016 Oct 17. doi: 10.1200/JCO.2016.70.0625).
Recognizing that the majority of patients with double-hit or double-expressor lymphoma will relapse after R-CHOP, this study evaluates the efficacy of autologous stem cell transplantation as a salvage modality. This is a carefully conducted, albeit retrospective, analysis of patients with relapsed or refractory double-hit or double-expressor lymphoma undergoing autologous hematopoietic stem cell transplantation (auto-HCT) at two high-volume institutions.
Although it is perhaps not surprising that double-hit and double-expressor phenotypes confer inferior outcomes, it is worth examining these issues in some detail. The first issue is that the authors have defined categories that are not recognized by the World Health Organization, but are routinely seen in clinical practice.
For example, it might be assumed that all patients with double-hit lymphoma will have MYC/BCL2 protein expression and, therefore, also have double-expressor lymphoma, but some patients with double-hit lymphoma do not have protein expression of MYC/BCL2 and these patients may have better outcomes than patients whose tumors display both double-hit and double-expressor characteristics.
A second caveat to interpreting the results is that the study population does not reflect the true denominator of all patients with relapsed diffuse large B-cell lymphoma, because only chemotherapy-sensitive patients undergoing auto-HCT were included.
So, should patients with relapsed double-hit and double-expressor lymphoma be offered auto-HCT? What are the alternatives to auto-HCT? Unfortunately, there are no clear answers to these questions, although the surprisingly excellent outcomes for patients without either of these features (70% long-term survival) suggest that there is a group of patients for whom auto-HCT remains an effective and standard tool. For double-hit and double-expressor lymphoma, a clinical trial based on specific biologic changes in individual patients is the ideal but is far from reality at this point.
Overall, despite being a retrospective series with a high attrition rate based on tissue availability, the central review of pathology, uniform assessment of double-hit and double-expressor features, and mature follow-up of 45 months makes this a thought-provoking and timely paper.
Sonali M. Smith, MD, is from the University of Chicago, and has disclosed a consulting or advisory role with Genentech, Seattle Genetics, TG Therapeutics, Gilead Sciences, Immunogenix, Pharmacyclics, NanoString Technologies, Genmab, Juno Therapeutics, Abbvie, and Portola Pharmaceuticals. These remarks were taken from the editorial accompanying Dr. Herrara’s report (J Clin Oncol. 2016 Oct 17. doi: 10.1200/JCO.2016.70.0625).
Patients with double-hit lymphomas (DHLs) and double-expressor lymphomas (DELs) have inferior outcomes after undergoing autologous stem cell transplantation (ASCT), according to a new study published in the Journal of Clinical Oncology.
The worst outcomes were observed in patients with concurrent DELs and DHLs, and this “supports the concept that the double-hit/double-expressor biology appears to render DLBCL resistant to and less likely to be cured by chemotherapy,” write Alex Herrera, MD, an oncologist at the City of Hope, Duarte, Calif., and his colleagues.
But that said, a significant proportion of patients with relapsed/refractory DEL did experience durable remissions following ASCT, particularly those with isolated DEL without DHL.
This suggests that “the presence of DEL alone should not be considered a contraindication to ASCT,” the authors wrote (J Clin Oncol. 2016 Oct. 24. doi: 10.1200/JCO.2016.68.2740).
DHLs and DELs are subtypes of diffuse large B-cell lymphoma (DLBCL), and while they are associated with poor outcomes after standard chemoimmunotherapy, data remain limited as to outcomes of patients with relapsed or refractory disease who undergo ASCT.
The retrospective multicenter study included 117 patients with chemotherapy-sensitive relapsed/refractory DLBCL who underwent ASCT and had archival tumor material available. DEL with MYC/BCL2 coexpression was observed in 52 patients (44%) while 15 patients expressed MYC-R (13%), of whom 12 (10%) had DHL.
The median follow-up time was 45 months for survivors, and the 4-year progression-free survival (PFS) and overall survival (OS) were 54% for the entire cohort.
The 4-year PFS and OS in patients with DHL was worse as compared to those without DHL; 28% vs. 57% (P = .013), and 25% vs. 66% (P less than .001), respectively.
Those with DHL had poorer PFS (28%) and OS (25%), compared with patients with DEL but not DHL (PFS, 53% and OS, 61%) as well as patients with neither DEL nor DHL (PFS, 60% and OS, 70%; three-way P value for PFS, P = .013; OS, P = .002).
Patients with concurrent DEL and DHL had the poorest outcome, with a 4-year PFS of 0%.
After researchers adjusted for clinical characteristics, the only factors that remained significantly associated with PFS were DEL (hazard ratio, 1.8; P = .035) and DHL (HR, 2.9; P = .009). Factors that were significantly associated with OS were DHL (HR, 3.4; P = .004) and remission status at ASCT (HR for partial response, 2.4; P = .007).
Overall, patients with DHL were less likely to achieve a complete response following salvage therapy, and those with DEL and patients with DHL had a shorter time to relapse after induction therapy.
“Although some patients with relapsed/refractory DHL had long-term remission after ASCT (isolated DHL without DEL), the low survival rate in this group argues that alternative transplantation strategies, including allogeneic hematopoietic stem cell transplantation or peri-ASCT relapse prevention strategies should be studied,” they concluded.
Patients with double-hit lymphomas (DHLs) and double-expressor lymphomas (DELs) have inferior outcomes after undergoing autologous stem cell transplantation (ASCT), according to a new study published in the Journal of Clinical Oncology.
The worst outcomes were observed in patients with concurrent DELs and DHLs, and this “supports the concept that the double-hit/double-expressor biology appears to render DLBCL resistant to and less likely to be cured by chemotherapy,” write Alex Herrera, MD, an oncologist at the City of Hope, Duarte, Calif., and his colleagues.
But that said, a significant proportion of patients with relapsed/refractory DEL did experience durable remissions following ASCT, particularly those with isolated DEL without DHL.
This suggests that “the presence of DEL alone should not be considered a contraindication to ASCT,” the authors wrote (J Clin Oncol. 2016 Oct. 24. doi: 10.1200/JCO.2016.68.2740).
DHLs and DELs are subtypes of diffuse large B-cell lymphoma (DLBCL), and while they are associated with poor outcomes after standard chemoimmunotherapy, data remain limited as to outcomes of patients with relapsed or refractory disease who undergo ASCT.
The retrospective multicenter study included 117 patients with chemotherapy-sensitive relapsed/refractory DLBCL who underwent ASCT and had archival tumor material available. DEL with MYC/BCL2 coexpression was observed in 52 patients (44%) while 15 patients expressed MYC-R (13%), of whom 12 (10%) had DHL.
The median follow-up time was 45 months for survivors, and the 4-year progression-free survival (PFS) and overall survival (OS) were 54% for the entire cohort.
The 4-year PFS and OS in patients with DHL was worse as compared to those without DHL; 28% vs. 57% (P = .013), and 25% vs. 66% (P less than .001), respectively.
Those with DHL had poorer PFS (28%) and OS (25%), compared with patients with DEL but not DHL (PFS, 53% and OS, 61%) as well as patients with neither DEL nor DHL (PFS, 60% and OS, 70%; three-way P value for PFS, P = .013; OS, P = .002).
Patients with concurrent DEL and DHL had the poorest outcome, with a 4-year PFS of 0%.
After researchers adjusted for clinical characteristics, the only factors that remained significantly associated with PFS were DEL (hazard ratio, 1.8; P = .035) and DHL (HR, 2.9; P = .009). Factors that were significantly associated with OS were DHL (HR, 3.4; P = .004) and remission status at ASCT (HR for partial response, 2.4; P = .007).
Overall, patients with DHL were less likely to achieve a complete response following salvage therapy, and those with DEL and patients with DHL had a shorter time to relapse after induction therapy.
“Although some patients with relapsed/refractory DHL had long-term remission after ASCT (isolated DHL without DEL), the low survival rate in this group argues that alternative transplantation strategies, including allogeneic hematopoietic stem cell transplantation or peri-ASCT relapse prevention strategies should be studied,” they concluded.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: The 4-year progression-free survival in patients with DEL vs. non-DEL was 48% versus 59% (P = .049), and the 4-year OS was 56% vs. 67% (P = .10).
Data source: Retrospective, multicenter study that included 117 patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma who underwent ASCT.
Disclosures: The study was funded by a Conquer Cancer Foundation/ASCO Young Investigator Award and National Cancer Institute Grants; the Dana-Farber Cancer Institute Award Fund for Collaborative Research Initiatives in Hematologic Oncology; the Harold and Virginia Lash/David Lash Fund for Lymphoma Research; and NCI Grant No. P30CA033572 for work performed in the COH Pathology Core. Dr. Herrera reports receiving research funding from Seattle Genetics, Pharmacyclics, Genentech, Immune Design, and Sequenta, and received travel, accommodations, and expenses from Bristol-Myers Squibb. Several coauthors also report relationships with industry.
Hepatitis infection raises non-Hodgkin lymphoma risk in HIV patients
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma.
Major finding: Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk and those with hepatitis C showed a 73% greater risk of non-Hodgkin lymphoma, compared to treated individuals with neither coinfection.
Data source: A cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe.
Disclosures: The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.







