User login
In Case You Missed It: COVID
COVID vaccine response in patients with solid tumors
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long COVID could spell kidney troubles down the line
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Florida doctor won’t treat unvaccinated patients in person
Linda Marraccini, MD, sent a letter to patients that said those who aren’t vaccinated won’t be allowed to book in-person appointments at her practice now that the Food and Drug Administration has fully approved the Pfizer vaccine for general use, according to NBC Miami.
“This is a public health emergency – the health of the public takes priority over the rights of any given individual in this situation,” she wrote in the letter, which was obtained by NBC Miami.
“It appears that there is a lack of selflessness and concern for the burden on the health and well-being of our society from our encounters,” she wrote.
Dr. Marraccini said the policy is in the best interest of her other patients and doesn’t violate the Hippocratic oath. Patients who are having chemotherapy or who have weak immune systems face higher risks of being infected with the coronavirus.
“It’s not fair for people who are unvaccinated to harm other people,” she told Newsweek Sept. 4.
“The Hippocratic oath is very science based,” she said. “I am following the science. I’m applying this to the benefit of the sick.”
Dr. Marraccini said her new policy goes into effect on Sept. 15, and she will continue to see unvaccinated patients virtually during the next month until they find another health care provider.
She said that the response to her decision has been “99.9% favorable” and that she will make exceptions if patients can’t receive a shot because of hardships. Her office also provides the Johnson & Johnson vaccine.
“We’re not going to leave them out there in the cold,” she told Newsweek.
During the past month, COVID-19 cases have surged in Florida, reaching record-level highs of more than 20,000 cases per day. Cases began rising in the summer because of the more contagious Delta variant.
In late August, a group of doctors in southern Florida urged people to get vaccinated, citing their exhaustion and frustration with unvaccinated patients who make up the large majority of COVID-19 hospitalizations, according to Newsweek.
Other doctors have declined to treat unvaccinated patients in recent weeks. Jason Valentine, MD, a doctor in Mobile, Ala., said he would no longer see unvaccinated patients as of Oct. 1, according to AL.com.
Dr. Marraccini urged people to become informed about COVID-19 vaccines, as well as their role in reducing the surge of cases in the state. The pandemic “did not have to go on this long,” she said.
“Responsibility has to do with each individual,” she told Newsweek. “This is a global health issue, and everyone owns part of that responsibility.”
A version of this article first appeared on WebMD.com.
Linda Marraccini, MD, sent a letter to patients that said those who aren’t vaccinated won’t be allowed to book in-person appointments at her practice now that the Food and Drug Administration has fully approved the Pfizer vaccine for general use, according to NBC Miami.
“This is a public health emergency – the health of the public takes priority over the rights of any given individual in this situation,” she wrote in the letter, which was obtained by NBC Miami.
“It appears that there is a lack of selflessness and concern for the burden on the health and well-being of our society from our encounters,” she wrote.
Dr. Marraccini said the policy is in the best interest of her other patients and doesn’t violate the Hippocratic oath. Patients who are having chemotherapy or who have weak immune systems face higher risks of being infected with the coronavirus.
“It’s not fair for people who are unvaccinated to harm other people,” she told Newsweek Sept. 4.
“The Hippocratic oath is very science based,” she said. “I am following the science. I’m applying this to the benefit of the sick.”
Dr. Marraccini said her new policy goes into effect on Sept. 15, and she will continue to see unvaccinated patients virtually during the next month until they find another health care provider.
She said that the response to her decision has been “99.9% favorable” and that she will make exceptions if patients can’t receive a shot because of hardships. Her office also provides the Johnson & Johnson vaccine.
“We’re not going to leave them out there in the cold,” she told Newsweek.
During the past month, COVID-19 cases have surged in Florida, reaching record-level highs of more than 20,000 cases per day. Cases began rising in the summer because of the more contagious Delta variant.
In late August, a group of doctors in southern Florida urged people to get vaccinated, citing their exhaustion and frustration with unvaccinated patients who make up the large majority of COVID-19 hospitalizations, according to Newsweek.
Other doctors have declined to treat unvaccinated patients in recent weeks. Jason Valentine, MD, a doctor in Mobile, Ala., said he would no longer see unvaccinated patients as of Oct. 1, according to AL.com.
Dr. Marraccini urged people to become informed about COVID-19 vaccines, as well as their role in reducing the surge of cases in the state. The pandemic “did not have to go on this long,” she said.
“Responsibility has to do with each individual,” she told Newsweek. “This is a global health issue, and everyone owns part of that responsibility.”
A version of this article first appeared on WebMD.com.
Linda Marraccini, MD, sent a letter to patients that said those who aren’t vaccinated won’t be allowed to book in-person appointments at her practice now that the Food and Drug Administration has fully approved the Pfizer vaccine for general use, according to NBC Miami.
“This is a public health emergency – the health of the public takes priority over the rights of any given individual in this situation,” she wrote in the letter, which was obtained by NBC Miami.
“It appears that there is a lack of selflessness and concern for the burden on the health and well-being of our society from our encounters,” she wrote.
Dr. Marraccini said the policy is in the best interest of her other patients and doesn’t violate the Hippocratic oath. Patients who are having chemotherapy or who have weak immune systems face higher risks of being infected with the coronavirus.
“It’s not fair for people who are unvaccinated to harm other people,” she told Newsweek Sept. 4.
“The Hippocratic oath is very science based,” she said. “I am following the science. I’m applying this to the benefit of the sick.”
Dr. Marraccini said her new policy goes into effect on Sept. 15, and she will continue to see unvaccinated patients virtually during the next month until they find another health care provider.
She said that the response to her decision has been “99.9% favorable” and that she will make exceptions if patients can’t receive a shot because of hardships. Her office also provides the Johnson & Johnson vaccine.
“We’re not going to leave them out there in the cold,” she told Newsweek.
During the past month, COVID-19 cases have surged in Florida, reaching record-level highs of more than 20,000 cases per day. Cases began rising in the summer because of the more contagious Delta variant.
In late August, a group of doctors in southern Florida urged people to get vaccinated, citing their exhaustion and frustration with unvaccinated patients who make up the large majority of COVID-19 hospitalizations, according to Newsweek.
Other doctors have declined to treat unvaccinated patients in recent weeks. Jason Valentine, MD, a doctor in Mobile, Ala., said he would no longer see unvaccinated patients as of Oct. 1, according to AL.com.
Dr. Marraccini urged people to become informed about COVID-19 vaccines, as well as their role in reducing the surge of cases in the state. The pandemic “did not have to go on this long,” she said.
“Responsibility has to do with each individual,” she told Newsweek. “This is a global health issue, and everyone owns part of that responsibility.”
A version of this article first appeared on WebMD.com.
United States reaches 5 million cases of child COVID
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
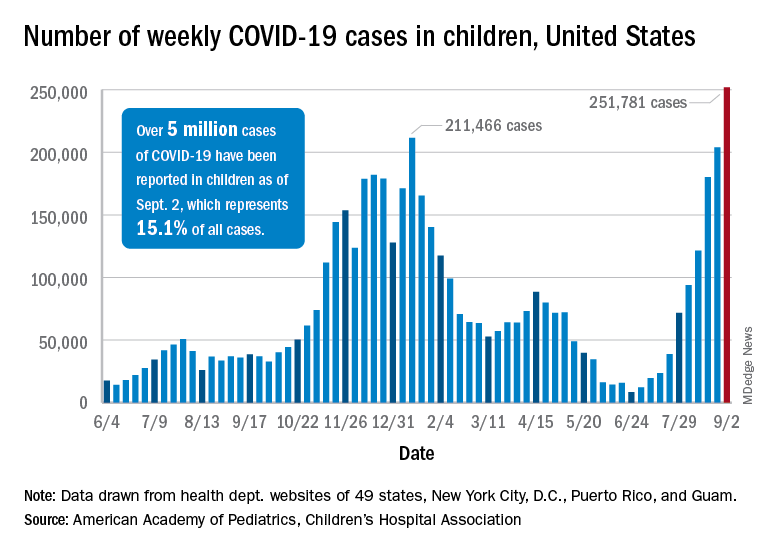
The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
COVID-19 continues to complicate children’s mental health care
The COVID-19 pandemic continues to impact child and adolescent mental health, and clinicians are learning as they go to develop strategies that address the challenges of providing both medical and mental health care to young patients, including those who test positive for COVID-19, according to Hani Talebi, PhD, director of pediatric psychology, and Jorge Ganem, MD, FAAP, director of pediatric hospital medicine, both of the University of Texas at Austin and Dell Children’s Medical Center.
In a presentation at the 2021 virtual Pediatric Hospital Medicine conference, Dr. Talebi and Dr. Ganem shared their experiences in identifying the impact of the pandemic on mental health services in a freestanding hospital, and synthesizing inpatient mental health care and medical care outside of a dedicated mental health unit.
Mental health is a significant pediatric issue; approximately one in five children have a diagnosable mental or behavioral health problem, but nearly two-thirds get little or no help, Dr. Talebi said. “COVID-19 has only exacerbated these mental health challenges,” he said.
He noted that beginning in April 2020, the proportion of children’s mental health-related emergency department visits increased and remained elevated through the spring, summer, and fall of 2020, as families fearful of COVID-19 avoided regular hospital visits.
Data suggest that up to 50% of all adolescent psychiatric crises that led to inpatient admissions were related in some way to COVID-19, Dr. Talebi said. In addition, “individuals with a recent diagnosis of a mental health disorder are at increased risk for COVID-19 infection,” and the risk is even higher among women and African Americans, he said.
The past year significantly impacted the mental wellbeing of parents and children, Dr. Talebi said. He cited a June 2020 study in Pediatrics in which 27% of parents reported worsening mental health for themselves, and 14% reported worsening behavioral health for their children. Ongoing issues including food insecurity, loss of regular child care, and an overall “very disorienting experience in the day-to-day” compromised the mental health of families, Dr. Talebi emphasized. Children isolated at home were not meeting developmental milestones that organically occur when socializing with peers, parents didn’t know how to handle some of their children’s issues without support from schools, and many people were struggling with other preexisting health conditions, he said.
This confluence of factors helped drive a surge in emergency department visits, meaning longer wait times and concerns about meeting urgent medical and mental health needs while maintaining safety, he added.
Parents and children waited longer to seek care, and community hospitals such as Dell Children’s Medical Center were faced with children in the emergency department with crisis-level mental health issues, along with children already waiting in the ED to address medical emergencies. All these patients had to be tested for COVID-19 and managed accordingly, Dr. Talebi noted.
Dr. Talebi emphasized the need for clinically robust care of the children who were in isolation for 10 days on the medical unit, waiting to test negative. New protocols were created for social workers to conduct daily safety checks, and to develop regular schedules for screening, “so they are having an experience on the medical floors similar to what they would have in a mental health unit,” he said.
Dr. Ganem reflected on the logistical challenges of managing mental health care while observing COVID-19 safety protocols. “COVID-19 added a new wrinkle of isolation,” he said. As institutional guidelines on testing and isolation evolved, negative COVID-19 tests were required for admission to the mental health units both in the hospital and throughout the region. Patients who tested positive had to be quarantined for 10 days, at which time they could be admitted to a mental health unit if necessary, he said.
Dr. Ganem shared details of some strategies adopted by Dell Children’s. He explained that the COVID-19 psychiatry patient workflow started with an ED evaluation, followed by medical clearance and consideration for admission.
“There was significant coordination between the social worker in the emergency department and the psychiatry social worker,” he said.
Key elements of the treatment plan for children with positive COVID-19 tests included an “interprofessional huddle” to coordinate the plan of care, goals for admission, and goals for safety, Dr. Ganem said.
Patients who required admission were expected to have an initial length of stay of 72 hours, and those who tested positive for COVID-19 were admitted to a medical unit with COVID-19 isolation, he said.
Once a patient is admitted, an RN activates a suicide prevention pathway, and an interprofessional team meets to determine what patients need for safe and effective discharge, said Dr. Ganem. He cited the SAFE-T protocol (Suicide Assessment Five-step Evaluation and Triage) as one of the tools used to determine safe discharge criteria. Considerations on the SAFE-T list include family support, an established outpatient therapist and psychiatrist, no suicide attempts prior to the current admission, or a low lethality attempt, and access to partial hospitalization or intensive outpatient programs.
Patients who could not be discharged because of suicidality or inadequate support or concerns about safety at home were considered for inpatient admission. Patients with COVID-19–positive tests who had continued need for inpatient mental health services could be transferred to an inpatient mental health unit after a 10-day quarantine.
Overall, “this has been a continuum of lessons learned, with some things we know now that we didn’t know in April or May of 2020,” Dr. Ganem said. Early in the pandemic, the focus was on minimizing risk, securing personal protective equipment, and determining who provided services in a patient’s room. “We developed new paradigms on the fly,” he said, including the use of virtual visits, which included securing and cleaning devices, as well as learning how to use them in this setting,” he said.
More recently, the emphasis has been on providing services to patients before they need to visit the hospital, rather than automatically admitting any patients with suicidal ideation and a positive COVID-19 test, Dr. Ganem said.
Dr. Talebi and Dr. Ganem had no financial conflicts to disclose. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The COVID-19 pandemic continues to impact child and adolescent mental health, and clinicians are learning as they go to develop strategies that address the challenges of providing both medical and mental health care to young patients, including those who test positive for COVID-19, according to Hani Talebi, PhD, director of pediatric psychology, and Jorge Ganem, MD, FAAP, director of pediatric hospital medicine, both of the University of Texas at Austin and Dell Children’s Medical Center.
In a presentation at the 2021 virtual Pediatric Hospital Medicine conference, Dr. Talebi and Dr. Ganem shared their experiences in identifying the impact of the pandemic on mental health services in a freestanding hospital, and synthesizing inpatient mental health care and medical care outside of a dedicated mental health unit.
Mental health is a significant pediatric issue; approximately one in five children have a diagnosable mental or behavioral health problem, but nearly two-thirds get little or no help, Dr. Talebi said. “COVID-19 has only exacerbated these mental health challenges,” he said.
He noted that beginning in April 2020, the proportion of children’s mental health-related emergency department visits increased and remained elevated through the spring, summer, and fall of 2020, as families fearful of COVID-19 avoided regular hospital visits.
Data suggest that up to 50% of all adolescent psychiatric crises that led to inpatient admissions were related in some way to COVID-19, Dr. Talebi said. In addition, “individuals with a recent diagnosis of a mental health disorder are at increased risk for COVID-19 infection,” and the risk is even higher among women and African Americans, he said.
The past year significantly impacted the mental wellbeing of parents and children, Dr. Talebi said. He cited a June 2020 study in Pediatrics in which 27% of parents reported worsening mental health for themselves, and 14% reported worsening behavioral health for their children. Ongoing issues including food insecurity, loss of regular child care, and an overall “very disorienting experience in the day-to-day” compromised the mental health of families, Dr. Talebi emphasized. Children isolated at home were not meeting developmental milestones that organically occur when socializing with peers, parents didn’t know how to handle some of their children’s issues without support from schools, and many people were struggling with other preexisting health conditions, he said.
This confluence of factors helped drive a surge in emergency department visits, meaning longer wait times and concerns about meeting urgent medical and mental health needs while maintaining safety, he added.
Parents and children waited longer to seek care, and community hospitals such as Dell Children’s Medical Center were faced with children in the emergency department with crisis-level mental health issues, along with children already waiting in the ED to address medical emergencies. All these patients had to be tested for COVID-19 and managed accordingly, Dr. Talebi noted.
Dr. Talebi emphasized the need for clinically robust care of the children who were in isolation for 10 days on the medical unit, waiting to test negative. New protocols were created for social workers to conduct daily safety checks, and to develop regular schedules for screening, “so they are having an experience on the medical floors similar to what they would have in a mental health unit,” he said.
Dr. Ganem reflected on the logistical challenges of managing mental health care while observing COVID-19 safety protocols. “COVID-19 added a new wrinkle of isolation,” he said. As institutional guidelines on testing and isolation evolved, negative COVID-19 tests were required for admission to the mental health units both in the hospital and throughout the region. Patients who tested positive had to be quarantined for 10 days, at which time they could be admitted to a mental health unit if necessary, he said.
Dr. Ganem shared details of some strategies adopted by Dell Children’s. He explained that the COVID-19 psychiatry patient workflow started with an ED evaluation, followed by medical clearance and consideration for admission.
“There was significant coordination between the social worker in the emergency department and the psychiatry social worker,” he said.
Key elements of the treatment plan for children with positive COVID-19 tests included an “interprofessional huddle” to coordinate the plan of care, goals for admission, and goals for safety, Dr. Ganem said.
Patients who required admission were expected to have an initial length of stay of 72 hours, and those who tested positive for COVID-19 were admitted to a medical unit with COVID-19 isolation, he said.
Once a patient is admitted, an RN activates a suicide prevention pathway, and an interprofessional team meets to determine what patients need for safe and effective discharge, said Dr. Ganem. He cited the SAFE-T protocol (Suicide Assessment Five-step Evaluation and Triage) as one of the tools used to determine safe discharge criteria. Considerations on the SAFE-T list include family support, an established outpatient therapist and psychiatrist, no suicide attempts prior to the current admission, or a low lethality attempt, and access to partial hospitalization or intensive outpatient programs.
Patients who could not be discharged because of suicidality or inadequate support or concerns about safety at home were considered for inpatient admission. Patients with COVID-19–positive tests who had continued need for inpatient mental health services could be transferred to an inpatient mental health unit after a 10-day quarantine.
Overall, “this has been a continuum of lessons learned, with some things we know now that we didn’t know in April or May of 2020,” Dr. Ganem said. Early in the pandemic, the focus was on minimizing risk, securing personal protective equipment, and determining who provided services in a patient’s room. “We developed new paradigms on the fly,” he said, including the use of virtual visits, which included securing and cleaning devices, as well as learning how to use them in this setting,” he said.
More recently, the emphasis has been on providing services to patients before they need to visit the hospital, rather than automatically admitting any patients with suicidal ideation and a positive COVID-19 test, Dr. Ganem said.
Dr. Talebi and Dr. Ganem had no financial conflicts to disclose. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The COVID-19 pandemic continues to impact child and adolescent mental health, and clinicians are learning as they go to develop strategies that address the challenges of providing both medical and mental health care to young patients, including those who test positive for COVID-19, according to Hani Talebi, PhD, director of pediatric psychology, and Jorge Ganem, MD, FAAP, director of pediatric hospital medicine, both of the University of Texas at Austin and Dell Children’s Medical Center.
In a presentation at the 2021 virtual Pediatric Hospital Medicine conference, Dr. Talebi and Dr. Ganem shared their experiences in identifying the impact of the pandemic on mental health services in a freestanding hospital, and synthesizing inpatient mental health care and medical care outside of a dedicated mental health unit.
Mental health is a significant pediatric issue; approximately one in five children have a diagnosable mental or behavioral health problem, but nearly two-thirds get little or no help, Dr. Talebi said. “COVID-19 has only exacerbated these mental health challenges,” he said.
He noted that beginning in April 2020, the proportion of children’s mental health-related emergency department visits increased and remained elevated through the spring, summer, and fall of 2020, as families fearful of COVID-19 avoided regular hospital visits.
Data suggest that up to 50% of all adolescent psychiatric crises that led to inpatient admissions were related in some way to COVID-19, Dr. Talebi said. In addition, “individuals with a recent diagnosis of a mental health disorder are at increased risk for COVID-19 infection,” and the risk is even higher among women and African Americans, he said.
The past year significantly impacted the mental wellbeing of parents and children, Dr. Talebi said. He cited a June 2020 study in Pediatrics in which 27% of parents reported worsening mental health for themselves, and 14% reported worsening behavioral health for their children. Ongoing issues including food insecurity, loss of regular child care, and an overall “very disorienting experience in the day-to-day” compromised the mental health of families, Dr. Talebi emphasized. Children isolated at home were not meeting developmental milestones that organically occur when socializing with peers, parents didn’t know how to handle some of their children’s issues without support from schools, and many people were struggling with other preexisting health conditions, he said.
This confluence of factors helped drive a surge in emergency department visits, meaning longer wait times and concerns about meeting urgent medical and mental health needs while maintaining safety, he added.
Parents and children waited longer to seek care, and community hospitals such as Dell Children’s Medical Center were faced with children in the emergency department with crisis-level mental health issues, along with children already waiting in the ED to address medical emergencies. All these patients had to be tested for COVID-19 and managed accordingly, Dr. Talebi noted.
Dr. Talebi emphasized the need for clinically robust care of the children who were in isolation for 10 days on the medical unit, waiting to test negative. New protocols were created for social workers to conduct daily safety checks, and to develop regular schedules for screening, “so they are having an experience on the medical floors similar to what they would have in a mental health unit,” he said.
Dr. Ganem reflected on the logistical challenges of managing mental health care while observing COVID-19 safety protocols. “COVID-19 added a new wrinkle of isolation,” he said. As institutional guidelines on testing and isolation evolved, negative COVID-19 tests were required for admission to the mental health units both in the hospital and throughout the region. Patients who tested positive had to be quarantined for 10 days, at which time they could be admitted to a mental health unit if necessary, he said.
Dr. Ganem shared details of some strategies adopted by Dell Children’s. He explained that the COVID-19 psychiatry patient workflow started with an ED evaluation, followed by medical clearance and consideration for admission.
“There was significant coordination between the social worker in the emergency department and the psychiatry social worker,” he said.
Key elements of the treatment plan for children with positive COVID-19 tests included an “interprofessional huddle” to coordinate the plan of care, goals for admission, and goals for safety, Dr. Ganem said.
Patients who required admission were expected to have an initial length of stay of 72 hours, and those who tested positive for COVID-19 were admitted to a medical unit with COVID-19 isolation, he said.
Once a patient is admitted, an RN activates a suicide prevention pathway, and an interprofessional team meets to determine what patients need for safe and effective discharge, said Dr. Ganem. He cited the SAFE-T protocol (Suicide Assessment Five-step Evaluation and Triage) as one of the tools used to determine safe discharge criteria. Considerations on the SAFE-T list include family support, an established outpatient therapist and psychiatrist, no suicide attempts prior to the current admission, or a low lethality attempt, and access to partial hospitalization or intensive outpatient programs.
Patients who could not be discharged because of suicidality or inadequate support or concerns about safety at home were considered for inpatient admission. Patients with COVID-19–positive tests who had continued need for inpatient mental health services could be transferred to an inpatient mental health unit after a 10-day quarantine.
Overall, “this has been a continuum of lessons learned, with some things we know now that we didn’t know in April or May of 2020,” Dr. Ganem said. Early in the pandemic, the focus was on minimizing risk, securing personal protective equipment, and determining who provided services in a patient’s room. “We developed new paradigms on the fly,” he said, including the use of virtual visits, which included securing and cleaning devices, as well as learning how to use them in this setting,” he said.
More recently, the emphasis has been on providing services to patients before they need to visit the hospital, rather than automatically admitting any patients with suicidal ideation and a positive COVID-19 test, Dr. Ganem said.
Dr. Talebi and Dr. Ganem had no financial conflicts to disclose. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
FROM PHM 2021
Anakinra improved survival in hospitalized COVID-19 patients
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
FROM NATURE MEDICINE
Addressing vaccine hesitancy with patients
Breakthrough with empathy and compassion
The COVID-19 pandemic is a worldwide tragedy. In the beginning there was a lack of testing, personal protective equipment, COVID tests, and support for health care workers and patients. As 2020 came to a close, the world was given a glimpse of hope with the development of a vaccine against the deadly virus. Many world citizens celebrated the scientific accomplishment and began to breathe a sigh of relief that there was an end in sight. However, the development and distribution of the COVID-19 vaccine revealed a new challenge, vaccine hesitancy.
Community members, young healthy people, and even critically ill hospitalized patients who have the fortune of surviving acute illness are hesitant to the COVID-19 vaccine. I recently cared for a critically ill young patient who was intubated for days with status asthmaticus, one of the worst cases I’d ever seen. She was extubated and made a full recovery. Prior to discharge I asked if she wanted the first dose of the COVID-19 vaccine and she said, “No.” I was shocked. This was an otherwise healthy 30-something-year-old who was lucky enough to survive without any underlying infection in the setting of severe obstructive lung disease. A co-infection with COVID-19 would be disastrous and increase her mortality. I had a long talk at the bedside and asked the reason for her hesitancy. Her answer left me speechless, “I don’t know, I just don’t want to.” I ultimately convinced her that contracting COVID-19 would be a fate worse than she could imagine, and she agreed to the vaccine prior to discharge. This interaction made me ponder – “why are our patients, friends, and family members hesitant about receiving a lifesaving vaccine, especially when they are aware of how sick they or others can become without it?”
According to the World Health Organization, vaccine hesitancy refers to a delay in acceptance or refusal of vaccines despite availability of vaccine services. Vaccine hesitancy is complex and context specific, varying across time, place, and vaccines. It is influenced by factors such as complacency, convenience, and confidence.1 No vaccine is 100% effective. However, throughout history, the work of scientists and doctors to create vaccines saved millions of lives and revolutionized global health. Arguably, the single most life-saving innovation in the history of medicine, vaccines have eradicated smallpox, protected against whooping cough (1914), diphtheria (1926), tetanus (1938), influenza (1945) and mumps (1948), polio (1955), measles (1963), and rubella (1969), and worldwide vaccination rates increased dramatically thanks to successful global health campaigns.2 However, there was a paradox of vaccine success. As terrifying diseases decreased in prevalence, so did the fear of these diseases and their effects – paralysis, brain damage, blindness, and death. This gave birth to a new challenge in modern medicine, vaccine hesitancy – a privilege of first world nations.
Vaccines saved countless lives and improved health and wellbeing around the world for decades. However, to prevent the morbidity and mortality associated with vaccine-preventable diseases and their complications, and optimize control of vaccine-preventable diseases in communities, high vaccination rates must be achieved. Enter the COVID-19 pandemic, the creation of the COVID-19 vaccine, and vaccine hesitancy.
The question we ask ourselves as health care providers is ‘how do we convince the skeptics and those opposed to vaccination to take the vaccine?’ The answer is complicated. If you are like me, you’ve had many conversations with people – friends, patients, family members, who are resistant to the vaccine. Very often the facts are not well received, and those discussions end in argument, high emotions, and broken relationships. With the delta variant of COVID-19 on the rise, spreading aggressively among the unvaccinated, and increased hospitalizations, we foresee the reoccurrence of overwhelmed health systems and a continued death toll.
The new paradox we are faced with is that people choose to believe fiction versus fact, despite the real life evidence of the severe health effects and increased deaths related to COVID-19. Do these skeptics simply have a cavalier attitude towards not only their own life, but the lives of others? Or, is there something deeper? It is not enough to tell people that the vaccines are proven safe3 and are more widely available than ever. It is not enough to tell people that they can die of COVID-19 – they already know that. Emotional pleas to family members are falling on deaf ears. This past month, when asking patients why they don’t want the vaccine, many have no real legitimate health-related reason and respond with a simple, “I don’t want to.” So, how do we get through to the unvaccinated?
A compassionate approach
We navigate these difficult conversations over time with the approach of compassion and empathy, not hostility or bullying. As health care providers, we start by being good empathic listeners. Similar to when we have advance care planning and code status conversations, we cannot enter the dialogue with our intention, beliefs, or formulated goals for that person. We have to listen without judgement to the wide range of reasons why others are reluctant or unwilling to get the vaccine – historical mistrust, political identity, religious reasons, short-term side effects that may cause them to lose a day or two of work – and understand that for each person their reasons are different. The point is to not assume that you know or understand what barriers and beliefs they have towards vaccination, but to meet them at their point of view and listen while keeping your own emotions level and steady.
Identifying the reason for vaccine hesitancy is the first step to getting the unvaccinated closer to vaccination. Ask open ended questions: “Can you help me understand, what is your hesitancy to the vaccine?”; “What about the vaccine worries you?”; “What have you heard about/know about the COVID-19 vaccine?”; or “Can you tell me more about why you feel that way?” As meticulous as it sounds, we have to go back to the basics of patient interviewing.
It is important to remember that this is not a debate and escalation to arguments will certainly backfire. Think about any time you disagreed with someone on a topic. Did criticizing, blaming, and shaming ever convince you to change your beliefs or behaviors? The likely answer is, “No.” Avoid the “backfire effect”– which is when giving people facts disproving their “incorrect” beliefs can actually reinforce those beliefs. The more people are confronted with facts at odds with their opinions, the stronger they cling to those opinions. If you want them to change their mind, you cannot approach the conversation as a debate. You are having this vaccine discussion to try to meet the other person where they are, understand their position, and talk with them, and not at them, about their concerns.
As leaders in health care, we have to be willing to give up control and lead with empathy. We have to show others that we hear them, believe their concerns, and acknowledge that their beliefs are valid to them as individuals. Even if you disagree, this is not the place to let anger, disappointment, or resentment take a front seat. This is about balance, and highlighting the autonomy in decision making that the other person has to make a choice. Be humble in these conversations and avoid condescending tones or statements.
We already know that you are a caring health care provider. As hospitalists, we are frontline providers who have seen unnecessary deaths and illness due to COVID-19. You are passionate and motivated because you are committed to your oath to save lives. However, you have to check your own feelings and remember that you are not speaking with an unvaccinated person to make them get vaccinated, but rather to understand their cognitive process and hopefully walk with them down a path that provides them with a clarity of options they truly have. Extend empathy and they will see your motivation is rooted in good-heartedness and a concern for their wellbeing.
If someone admits to reasons for avoiding vaccination that are not rooted in any fact, then guide them to the best resources. Our health care system recently released a COVID-19 fact versus myth handout called Trust the Facts. This could be the kind of vetted resource you offer. Guide them to accredited websites, such as the World Health Organization, the Center for Disease Control, or their local and state departments of health to help debunk fiction by reviewing it with them. Discuss myths such as, ‘the vaccine will cause infertility,’ ‘the vaccine will give me COVID,’ ‘the vaccine was rushed and is not safe,’ ‘the vaccine is not needed if I am young and healthy,’ ‘the vaccine has a microchip,’ etc. Knowledge is power and disinformation is deadly, but how facts are presented will make the biggest difference in how others receive them, so remember your role is not to argue with these statements, but rather to provide perspective without agreeing or disagreeing.
Respond to their concerns with statements such as, “I hear you…it sounds like you are worried/fearful/mistrusting about the side effects/safety/efficacy of the vaccine…can we talk more about that?” Ask them where these concerns come from – the news, social media, an article, word of mouth, friends, or family. Ask them about the information they have and show genuine interest that you want to see it from their perspective. This is the key to compassionate and empathic dialogue – you relinquish your intentions.
Once you know or unveil their reasons for hesitancy, ask them what they would like to see with regards to COVID-19 and ending the pandemic. Would they like to get back to a new normal, to visit family members, to travel once again, to not have to wear masks and quarantine? What do they want for themselves, their families, communities, the country, or even the world? The goal is to find something in our shared humanity, to connect on a deeper level so they start to open up and let down walls, and find something you both see eye-to-eye on. Know your audience and speak to what serves them. To effectively persuade someone to come around to your point of view starts with recognizing the root of the disagreement and trying to overcome it before trying to change the person’s mind, understanding both the logic and the emotion that’s driving their decision making.4
Building trust
Reminding patients, friends or family members that their health and well-being means a lot to you can also be a strategy to keeping the conversation open and friendly. Sharing stories as hospitalists caring for many critically ill COVID patients or patients who died alone due to COVID-19, and the trauma you experienced as a health care provider feeling paralyzed by the limitations of modern medicine against the deadly virus, will only serve to humanize you in such an interaction.
Building trust will also increase vaccine willingness. This will require a concerted effort by scientists, doctors, and health care systems to engage with community leaders and members. To address hesitancy, the people we serve have to hear those local, personal, and relatable stories about vaccinations, and how it benefits not just themselves, but others around them in their community. As part of the #VaxUp campaign in Virginia, community and physician leaders shared their stories of hesitancy and motivation surrounding the vaccine. These are real people in the community discussing why getting vaccinated is so important and what helped them make an informed decision. I discussed my own hesitancy and concerns and also tackled a few vaccine myths.
As vaccinated health care workers or community leaders, you are living proof of the benefits of getting the COVID vaccine. Focus on the positives but also be honest. If your second shot gave you fevers, chills, or myalgias, then admit it and share how you overcame these expected reactions. Refocus on the safety of the vaccine and the fact that it is freely available to all people. Maybe the person you are speaking with doesn’t know where or how to get an appointment to get vaccinated. Help them find the nearest place to get an appointment and identify barriers they may have in transportation, child, or senior care to leave home safely to get vaccinated, or physical conditions that are preventing them from receiving the vaccine. Share that being vaccinated protects you from contracting the virus and spreading it to loved ones. Focus on how a fully vaccinated community and country can open up opportunities to heal and connect as a society, spend time with family/friends in another county or state, hold a newborn grandchild, or even travel outside the U.S.
There is no guarantee that you will be able to persuade someone to get vaccinated. It’s possible the outcome of your conversation will not result in the other person changing their mind in that moment. That doesn’t mean that you failed, because you started the dialogue and planted the seed. If you are a vaccinated health care provider, your words have influence and power, and we are obliged by our positions to have responsibility for the health of our communities. Don’t be discouraged, as it is through caring, compassionate, respectful, and empathic conversations that your influence will make the most difference in these relationships as you continue to advocate for all human life.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the Medical Executive Committee.
References
1. World Health Organization. Report of the SAGE working group on vaccine hesitancy. Oct 2014. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
2. Hsu JL. A brief history of vaccines: Smallpox to the present. S D Med. 2013;Spec no:33-7. PMID: 23444589.
3. Chiu A, Bever L. Are they experimental? Can they alter DNA? Experts tackle lingering coronavirus vaccine fears. The Washington Post. 2021 May 14. https://www.washingtonpost.com/lifestyle/2021/05/14/safe-fast-vaccine-fear-infertility-dna/
4. Huang L. Edge: Turning Adversity into Advantage. New York: Portfolio/Penguin, 2020.
Breakthrough with empathy and compassion
Breakthrough with empathy and compassion
The COVID-19 pandemic is a worldwide tragedy. In the beginning there was a lack of testing, personal protective equipment, COVID tests, and support for health care workers and patients. As 2020 came to a close, the world was given a glimpse of hope with the development of a vaccine against the deadly virus. Many world citizens celebrated the scientific accomplishment and began to breathe a sigh of relief that there was an end in sight. However, the development and distribution of the COVID-19 vaccine revealed a new challenge, vaccine hesitancy.
Community members, young healthy people, and even critically ill hospitalized patients who have the fortune of surviving acute illness are hesitant to the COVID-19 vaccine. I recently cared for a critically ill young patient who was intubated for days with status asthmaticus, one of the worst cases I’d ever seen. She was extubated and made a full recovery. Prior to discharge I asked if she wanted the first dose of the COVID-19 vaccine and she said, “No.” I was shocked. This was an otherwise healthy 30-something-year-old who was lucky enough to survive without any underlying infection in the setting of severe obstructive lung disease. A co-infection with COVID-19 would be disastrous and increase her mortality. I had a long talk at the bedside and asked the reason for her hesitancy. Her answer left me speechless, “I don’t know, I just don’t want to.” I ultimately convinced her that contracting COVID-19 would be a fate worse than she could imagine, and she agreed to the vaccine prior to discharge. This interaction made me ponder – “why are our patients, friends, and family members hesitant about receiving a lifesaving vaccine, especially when they are aware of how sick they or others can become without it?”
According to the World Health Organization, vaccine hesitancy refers to a delay in acceptance or refusal of vaccines despite availability of vaccine services. Vaccine hesitancy is complex and context specific, varying across time, place, and vaccines. It is influenced by factors such as complacency, convenience, and confidence.1 No vaccine is 100% effective. However, throughout history, the work of scientists and doctors to create vaccines saved millions of lives and revolutionized global health. Arguably, the single most life-saving innovation in the history of medicine, vaccines have eradicated smallpox, protected against whooping cough (1914), diphtheria (1926), tetanus (1938), influenza (1945) and mumps (1948), polio (1955), measles (1963), and rubella (1969), and worldwide vaccination rates increased dramatically thanks to successful global health campaigns.2 However, there was a paradox of vaccine success. As terrifying diseases decreased in prevalence, so did the fear of these diseases and their effects – paralysis, brain damage, blindness, and death. This gave birth to a new challenge in modern medicine, vaccine hesitancy – a privilege of first world nations.
Vaccines saved countless lives and improved health and wellbeing around the world for decades. However, to prevent the morbidity and mortality associated with vaccine-preventable diseases and their complications, and optimize control of vaccine-preventable diseases in communities, high vaccination rates must be achieved. Enter the COVID-19 pandemic, the creation of the COVID-19 vaccine, and vaccine hesitancy.
The question we ask ourselves as health care providers is ‘how do we convince the skeptics and those opposed to vaccination to take the vaccine?’ The answer is complicated. If you are like me, you’ve had many conversations with people – friends, patients, family members, who are resistant to the vaccine. Very often the facts are not well received, and those discussions end in argument, high emotions, and broken relationships. With the delta variant of COVID-19 on the rise, spreading aggressively among the unvaccinated, and increased hospitalizations, we foresee the reoccurrence of overwhelmed health systems and a continued death toll.
The new paradox we are faced with is that people choose to believe fiction versus fact, despite the real life evidence of the severe health effects and increased deaths related to COVID-19. Do these skeptics simply have a cavalier attitude towards not only their own life, but the lives of others? Or, is there something deeper? It is not enough to tell people that the vaccines are proven safe3 and are more widely available than ever. It is not enough to tell people that they can die of COVID-19 – they already know that. Emotional pleas to family members are falling on deaf ears. This past month, when asking patients why they don’t want the vaccine, many have no real legitimate health-related reason and respond with a simple, “I don’t want to.” So, how do we get through to the unvaccinated?
A compassionate approach
We navigate these difficult conversations over time with the approach of compassion and empathy, not hostility or bullying. As health care providers, we start by being good empathic listeners. Similar to when we have advance care planning and code status conversations, we cannot enter the dialogue with our intention, beliefs, or formulated goals for that person. We have to listen without judgement to the wide range of reasons why others are reluctant or unwilling to get the vaccine – historical mistrust, political identity, religious reasons, short-term side effects that may cause them to lose a day or two of work – and understand that for each person their reasons are different. The point is to not assume that you know or understand what barriers and beliefs they have towards vaccination, but to meet them at their point of view and listen while keeping your own emotions level and steady.
Identifying the reason for vaccine hesitancy is the first step to getting the unvaccinated closer to vaccination. Ask open ended questions: “Can you help me understand, what is your hesitancy to the vaccine?”; “What about the vaccine worries you?”; “What have you heard about/know about the COVID-19 vaccine?”; or “Can you tell me more about why you feel that way?” As meticulous as it sounds, we have to go back to the basics of patient interviewing.
It is important to remember that this is not a debate and escalation to arguments will certainly backfire. Think about any time you disagreed with someone on a topic. Did criticizing, blaming, and shaming ever convince you to change your beliefs or behaviors? The likely answer is, “No.” Avoid the “backfire effect”– which is when giving people facts disproving their “incorrect” beliefs can actually reinforce those beliefs. The more people are confronted with facts at odds with their opinions, the stronger they cling to those opinions. If you want them to change their mind, you cannot approach the conversation as a debate. You are having this vaccine discussion to try to meet the other person where they are, understand their position, and talk with them, and not at them, about their concerns.
As leaders in health care, we have to be willing to give up control and lead with empathy. We have to show others that we hear them, believe their concerns, and acknowledge that their beliefs are valid to them as individuals. Even if you disagree, this is not the place to let anger, disappointment, or resentment take a front seat. This is about balance, and highlighting the autonomy in decision making that the other person has to make a choice. Be humble in these conversations and avoid condescending tones or statements.
We already know that you are a caring health care provider. As hospitalists, we are frontline providers who have seen unnecessary deaths and illness due to COVID-19. You are passionate and motivated because you are committed to your oath to save lives. However, you have to check your own feelings and remember that you are not speaking with an unvaccinated person to make them get vaccinated, but rather to understand their cognitive process and hopefully walk with them down a path that provides them with a clarity of options they truly have. Extend empathy and they will see your motivation is rooted in good-heartedness and a concern for their wellbeing.
If someone admits to reasons for avoiding vaccination that are not rooted in any fact, then guide them to the best resources. Our health care system recently released a COVID-19 fact versus myth handout called Trust the Facts. This could be the kind of vetted resource you offer. Guide them to accredited websites, such as the World Health Organization, the Center for Disease Control, or their local and state departments of health to help debunk fiction by reviewing it with them. Discuss myths such as, ‘the vaccine will cause infertility,’ ‘the vaccine will give me COVID,’ ‘the vaccine was rushed and is not safe,’ ‘the vaccine is not needed if I am young and healthy,’ ‘the vaccine has a microchip,’ etc. Knowledge is power and disinformation is deadly, but how facts are presented will make the biggest difference in how others receive them, so remember your role is not to argue with these statements, but rather to provide perspective without agreeing or disagreeing.
Respond to their concerns with statements such as, “I hear you…it sounds like you are worried/fearful/mistrusting about the side effects/safety/efficacy of the vaccine…can we talk more about that?” Ask them where these concerns come from – the news, social media, an article, word of mouth, friends, or family. Ask them about the information they have and show genuine interest that you want to see it from their perspective. This is the key to compassionate and empathic dialogue – you relinquish your intentions.
Once you know or unveil their reasons for hesitancy, ask them what they would like to see with regards to COVID-19 and ending the pandemic. Would they like to get back to a new normal, to visit family members, to travel once again, to not have to wear masks and quarantine? What do they want for themselves, their families, communities, the country, or even the world? The goal is to find something in our shared humanity, to connect on a deeper level so they start to open up and let down walls, and find something you both see eye-to-eye on. Know your audience and speak to what serves them. To effectively persuade someone to come around to your point of view starts with recognizing the root of the disagreement and trying to overcome it before trying to change the person’s mind, understanding both the logic and the emotion that’s driving their decision making.4
Building trust
Reminding patients, friends or family members that their health and well-being means a lot to you can also be a strategy to keeping the conversation open and friendly. Sharing stories as hospitalists caring for many critically ill COVID patients or patients who died alone due to COVID-19, and the trauma you experienced as a health care provider feeling paralyzed by the limitations of modern medicine against the deadly virus, will only serve to humanize you in such an interaction.
Building trust will also increase vaccine willingness. This will require a concerted effort by scientists, doctors, and health care systems to engage with community leaders and members. To address hesitancy, the people we serve have to hear those local, personal, and relatable stories about vaccinations, and how it benefits not just themselves, but others around them in their community. As part of the #VaxUp campaign in Virginia, community and physician leaders shared their stories of hesitancy and motivation surrounding the vaccine. These are real people in the community discussing why getting vaccinated is so important and what helped them make an informed decision. I discussed my own hesitancy and concerns and also tackled a few vaccine myths.
As vaccinated health care workers or community leaders, you are living proof of the benefits of getting the COVID vaccine. Focus on the positives but also be honest. If your second shot gave you fevers, chills, or myalgias, then admit it and share how you overcame these expected reactions. Refocus on the safety of the vaccine and the fact that it is freely available to all people. Maybe the person you are speaking with doesn’t know where or how to get an appointment to get vaccinated. Help them find the nearest place to get an appointment and identify barriers they may have in transportation, child, or senior care to leave home safely to get vaccinated, or physical conditions that are preventing them from receiving the vaccine. Share that being vaccinated protects you from contracting the virus and spreading it to loved ones. Focus on how a fully vaccinated community and country can open up opportunities to heal and connect as a society, spend time with family/friends in another county or state, hold a newborn grandchild, or even travel outside the U.S.
There is no guarantee that you will be able to persuade someone to get vaccinated. It’s possible the outcome of your conversation will not result in the other person changing their mind in that moment. That doesn’t mean that you failed, because you started the dialogue and planted the seed. If you are a vaccinated health care provider, your words have influence and power, and we are obliged by our positions to have responsibility for the health of our communities. Don’t be discouraged, as it is through caring, compassionate, respectful, and empathic conversations that your influence will make the most difference in these relationships as you continue to advocate for all human life.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the Medical Executive Committee.
References
1. World Health Organization. Report of the SAGE working group on vaccine hesitancy. Oct 2014. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
2. Hsu JL. A brief history of vaccines: Smallpox to the present. S D Med. 2013;Spec no:33-7. PMID: 23444589.
3. Chiu A, Bever L. Are they experimental? Can they alter DNA? Experts tackle lingering coronavirus vaccine fears. The Washington Post. 2021 May 14. https://www.washingtonpost.com/lifestyle/2021/05/14/safe-fast-vaccine-fear-infertility-dna/
4. Huang L. Edge: Turning Adversity into Advantage. New York: Portfolio/Penguin, 2020.
The COVID-19 pandemic is a worldwide tragedy. In the beginning there was a lack of testing, personal protective equipment, COVID tests, and support for health care workers and patients. As 2020 came to a close, the world was given a glimpse of hope with the development of a vaccine against the deadly virus. Many world citizens celebrated the scientific accomplishment and began to breathe a sigh of relief that there was an end in sight. However, the development and distribution of the COVID-19 vaccine revealed a new challenge, vaccine hesitancy.
Community members, young healthy people, and even critically ill hospitalized patients who have the fortune of surviving acute illness are hesitant to the COVID-19 vaccine. I recently cared for a critically ill young patient who was intubated for days with status asthmaticus, one of the worst cases I’d ever seen. She was extubated and made a full recovery. Prior to discharge I asked if she wanted the first dose of the COVID-19 vaccine and she said, “No.” I was shocked. This was an otherwise healthy 30-something-year-old who was lucky enough to survive without any underlying infection in the setting of severe obstructive lung disease. A co-infection with COVID-19 would be disastrous and increase her mortality. I had a long talk at the bedside and asked the reason for her hesitancy. Her answer left me speechless, “I don’t know, I just don’t want to.” I ultimately convinced her that contracting COVID-19 would be a fate worse than she could imagine, and she agreed to the vaccine prior to discharge. This interaction made me ponder – “why are our patients, friends, and family members hesitant about receiving a lifesaving vaccine, especially when they are aware of how sick they or others can become without it?”
According to the World Health Organization, vaccine hesitancy refers to a delay in acceptance or refusal of vaccines despite availability of vaccine services. Vaccine hesitancy is complex and context specific, varying across time, place, and vaccines. It is influenced by factors such as complacency, convenience, and confidence.1 No vaccine is 100% effective. However, throughout history, the work of scientists and doctors to create vaccines saved millions of lives and revolutionized global health. Arguably, the single most life-saving innovation in the history of medicine, vaccines have eradicated smallpox, protected against whooping cough (1914), diphtheria (1926), tetanus (1938), influenza (1945) and mumps (1948), polio (1955), measles (1963), and rubella (1969), and worldwide vaccination rates increased dramatically thanks to successful global health campaigns.2 However, there was a paradox of vaccine success. As terrifying diseases decreased in prevalence, so did the fear of these diseases and their effects – paralysis, brain damage, blindness, and death. This gave birth to a new challenge in modern medicine, vaccine hesitancy – a privilege of first world nations.
Vaccines saved countless lives and improved health and wellbeing around the world for decades. However, to prevent the morbidity and mortality associated with vaccine-preventable diseases and their complications, and optimize control of vaccine-preventable diseases in communities, high vaccination rates must be achieved. Enter the COVID-19 pandemic, the creation of the COVID-19 vaccine, and vaccine hesitancy.
The question we ask ourselves as health care providers is ‘how do we convince the skeptics and those opposed to vaccination to take the vaccine?’ The answer is complicated. If you are like me, you’ve had many conversations with people – friends, patients, family members, who are resistant to the vaccine. Very often the facts are not well received, and those discussions end in argument, high emotions, and broken relationships. With the delta variant of COVID-19 on the rise, spreading aggressively among the unvaccinated, and increased hospitalizations, we foresee the reoccurrence of overwhelmed health systems and a continued death toll.
The new paradox we are faced with is that people choose to believe fiction versus fact, despite the real life evidence of the severe health effects and increased deaths related to COVID-19. Do these skeptics simply have a cavalier attitude towards not only their own life, but the lives of others? Or, is there something deeper? It is not enough to tell people that the vaccines are proven safe3 and are more widely available than ever. It is not enough to tell people that they can die of COVID-19 – they already know that. Emotional pleas to family members are falling on deaf ears. This past month, when asking patients why they don’t want the vaccine, many have no real legitimate health-related reason and respond with a simple, “I don’t want to.” So, how do we get through to the unvaccinated?
A compassionate approach
We navigate these difficult conversations over time with the approach of compassion and empathy, not hostility or bullying. As health care providers, we start by being good empathic listeners. Similar to when we have advance care planning and code status conversations, we cannot enter the dialogue with our intention, beliefs, or formulated goals for that person. We have to listen without judgement to the wide range of reasons why others are reluctant or unwilling to get the vaccine – historical mistrust, political identity, religious reasons, short-term side effects that may cause them to lose a day or two of work – and understand that for each person their reasons are different. The point is to not assume that you know or understand what barriers and beliefs they have towards vaccination, but to meet them at their point of view and listen while keeping your own emotions level and steady.
Identifying the reason for vaccine hesitancy is the first step to getting the unvaccinated closer to vaccination. Ask open ended questions: “Can you help me understand, what is your hesitancy to the vaccine?”; “What about the vaccine worries you?”; “What have you heard about/know about the COVID-19 vaccine?”; or “Can you tell me more about why you feel that way?” As meticulous as it sounds, we have to go back to the basics of patient interviewing.
It is important to remember that this is not a debate and escalation to arguments will certainly backfire. Think about any time you disagreed with someone on a topic. Did criticizing, blaming, and shaming ever convince you to change your beliefs or behaviors? The likely answer is, “No.” Avoid the “backfire effect”– which is when giving people facts disproving their “incorrect” beliefs can actually reinforce those beliefs. The more people are confronted with facts at odds with their opinions, the stronger they cling to those opinions. If you want them to change their mind, you cannot approach the conversation as a debate. You are having this vaccine discussion to try to meet the other person where they are, understand their position, and talk with them, and not at them, about their concerns.
As leaders in health care, we have to be willing to give up control and lead with empathy. We have to show others that we hear them, believe their concerns, and acknowledge that their beliefs are valid to them as individuals. Even if you disagree, this is not the place to let anger, disappointment, or resentment take a front seat. This is about balance, and highlighting the autonomy in decision making that the other person has to make a choice. Be humble in these conversations and avoid condescending tones or statements.
We already know that you are a caring health care provider. As hospitalists, we are frontline providers who have seen unnecessary deaths and illness due to COVID-19. You are passionate and motivated because you are committed to your oath to save lives. However, you have to check your own feelings and remember that you are not speaking with an unvaccinated person to make them get vaccinated, but rather to understand their cognitive process and hopefully walk with them down a path that provides them with a clarity of options they truly have. Extend empathy and they will see your motivation is rooted in good-heartedness and a concern for their wellbeing.
If someone admits to reasons for avoiding vaccination that are not rooted in any fact, then guide them to the best resources. Our health care system recently released a COVID-19 fact versus myth handout called Trust the Facts. This could be the kind of vetted resource you offer. Guide them to accredited websites, such as the World Health Organization, the Center for Disease Control, or their local and state departments of health to help debunk fiction by reviewing it with them. Discuss myths such as, ‘the vaccine will cause infertility,’ ‘the vaccine will give me COVID,’ ‘the vaccine was rushed and is not safe,’ ‘the vaccine is not needed if I am young and healthy,’ ‘the vaccine has a microchip,’ etc. Knowledge is power and disinformation is deadly, but how facts are presented will make the biggest difference in how others receive them, so remember your role is not to argue with these statements, but rather to provide perspective without agreeing or disagreeing.
Respond to their concerns with statements such as, “I hear you…it sounds like you are worried/fearful/mistrusting about the side effects/safety/efficacy of the vaccine…can we talk more about that?” Ask them where these concerns come from – the news, social media, an article, word of mouth, friends, or family. Ask them about the information they have and show genuine interest that you want to see it from their perspective. This is the key to compassionate and empathic dialogue – you relinquish your intentions.
Once you know or unveil their reasons for hesitancy, ask them what they would like to see with regards to COVID-19 and ending the pandemic. Would they like to get back to a new normal, to visit family members, to travel once again, to not have to wear masks and quarantine? What do they want for themselves, their families, communities, the country, or even the world? The goal is to find something in our shared humanity, to connect on a deeper level so they start to open up and let down walls, and find something you both see eye-to-eye on. Know your audience and speak to what serves them. To effectively persuade someone to come around to your point of view starts with recognizing the root of the disagreement and trying to overcome it before trying to change the person’s mind, understanding both the logic and the emotion that’s driving their decision making.4
Building trust
Reminding patients, friends or family members that their health and well-being means a lot to you can also be a strategy to keeping the conversation open and friendly. Sharing stories as hospitalists caring for many critically ill COVID patients or patients who died alone due to COVID-19, and the trauma you experienced as a health care provider feeling paralyzed by the limitations of modern medicine against the deadly virus, will only serve to humanize you in such an interaction.
Building trust will also increase vaccine willingness. This will require a concerted effort by scientists, doctors, and health care systems to engage with community leaders and members. To address hesitancy, the people we serve have to hear those local, personal, and relatable stories about vaccinations, and how it benefits not just themselves, but others around them in their community. As part of the #VaxUp campaign in Virginia, community and physician leaders shared their stories of hesitancy and motivation surrounding the vaccine. These are real people in the community discussing why getting vaccinated is so important and what helped them make an informed decision. I discussed my own hesitancy and concerns and also tackled a few vaccine myths.
As vaccinated health care workers or community leaders, you are living proof of the benefits of getting the COVID vaccine. Focus on the positives but also be honest. If your second shot gave you fevers, chills, or myalgias, then admit it and share how you overcame these expected reactions. Refocus on the safety of the vaccine and the fact that it is freely available to all people. Maybe the person you are speaking with doesn’t know where or how to get an appointment to get vaccinated. Help them find the nearest place to get an appointment and identify barriers they may have in transportation, child, or senior care to leave home safely to get vaccinated, or physical conditions that are preventing them from receiving the vaccine. Share that being vaccinated protects you from contracting the virus and spreading it to loved ones. Focus on how a fully vaccinated community and country can open up opportunities to heal and connect as a society, spend time with family/friends in another county or state, hold a newborn grandchild, or even travel outside the U.S.
There is no guarantee that you will be able to persuade someone to get vaccinated. It’s possible the outcome of your conversation will not result in the other person changing their mind in that moment. That doesn’t mean that you failed, because you started the dialogue and planted the seed. If you are a vaccinated health care provider, your words have influence and power, and we are obliged by our positions to have responsibility for the health of our communities. Don’t be discouraged, as it is through caring, compassionate, respectful, and empathic conversations that your influence will make the most difference in these relationships as you continue to advocate for all human life.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the Medical Executive Committee.
References
1. World Health Organization. Report of the SAGE working group on vaccine hesitancy. Oct 2014. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
2. Hsu JL. A brief history of vaccines: Smallpox to the present. S D Med. 2013;Spec no:33-7. PMID: 23444589.
3. Chiu A, Bever L. Are they experimental? Can they alter DNA? Experts tackle lingering coronavirus vaccine fears. The Washington Post. 2021 May 14. https://www.washingtonpost.com/lifestyle/2021/05/14/safe-fast-vaccine-fear-infertility-dna/
4. Huang L. Edge: Turning Adversity into Advantage. New York: Portfolio/Penguin, 2020.
Large study affirms what we already know: Masks work to prevent COVID-19
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
Health care–associated infections spiked in 2020 in U.S. hospitals
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Beta-blocker reduces lung inflammation in critical COVID-19
In a small study, intravenous administration of the beta-blocker metoprolol to critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) safely blunted lung inflammation associated with the disease.
Metoprolol administration also resulted in better oxygenation and fewer days on intensive mechanical ventilation and in the ICU, compared with no treatment.
These data suggest that metoprolol repurposing for the treatment of ARDS in COVID-19 patients is a safe and inexpensive strategy with the potential to improve outcomes, the researchers said.
“Metoprolol repurposing for the treatment of ARDS associated with COVID-19 is a safe and cheap intervention that can help to alleviate the massive personal and health care burden associated with the pandemic,” they concluded.
The results, from the MADRID-COVID pilot trial from Agustin Clemente-Moragon, BSc, Centro National de Investigaciones Cardiovasculares, Madrid, and colleagues, were published online Aug. 30, 2021, in the Journal of the American College of Cardiology.
In previous work, the researchers showed that metoprolol, but not other clinically available intravenous beta-blockers, abrogates neutrophil-driven exacerbated inflammation, neutrophil-platelet interaction, and formation of neutrophil extracellular traps in a mouse model of acute lung injury.
These results prompted the current pilot trial in 20 patients, ages 18-80 years, with COVID-19–associated ARDS.
Randomization was stratified by age (59 and younger vs. 60 and older), history of hypertension (yes or no), and circulating neutrophil counts (<6,000 vs. ≥6,000). Bronchoalveolar lavage (BAL) fluid and blood samples were obtained from patients at randomization and 24 hours after the third metoprolol dose in the treatment group, and on day 4 in controls.
Because of the cardiovascular effects of metoprolol, patients were monitored invasively and by echocardiography, the authors noted.
As expected, metoprolol significantly reduced heart rate (P < .01) and systolic blood pressure (P < .05), although both remained within the physiological range. Echocardiography showed no deterioration of cardiac function after metoprolol treatment.
To assess the ability of metoprolol to address neutrophil-mediated exacerbated lung inflammation, the researchers analyzed leukocyte populations in BAL samples by flow cytometry at baseline and on day 4.
At baseline, the metoprolol and control groups showed no differences in BAL neutrophil content. But on day 4, after 3 days of treatment with metoprolol, neutrophil content was significantly lower in the metoprolol group (median, 14.3 neutrophils/mcL) than in the control group (median, 397 neutrophils/mcL).
Metoprolol-treated patients also had lower total inflammatory-cell content and lower monocyte/macrophage content. Lymphocytes did not differ between the groups.
The investigators also explored the impact of metoprolol on the chemokine, monocyte chemoattractant protein–1 (MCP-1), as it has been shown to promote pulmonary fibrosis in late-stage ARDS.
They found that MCP-1 was significantly attenuated after 3 days of metoprolol treatment. At baseline, the median MCP-1 level was 298 pg/mL; on day 4 after metoprolol, it was 203 pg/mL (P = .009).
MCP-1 levels remained unchanged in control patients.
An elegant study
In an accompanying editorial, Mourad H. Senussi, MD, assistant professor at Baylor College of Medicine, Houston, wrote: “Although the study has a small sample size, we commend the authors, who attempt to shed light on the important pathophysiological underpinnings that help establish biological plausibility for this inexpensive, safe, and widely available medication.”
In an interview with this news organization, Dr. Senussi added that metoprolol is not itself something primarily used to treat COVID-19 per se. “Rather, the drug blunts the sympathetic-host response. There is a fine balance between that sympathetic surge that is helpful to the body, and then a sympathetic surge that if left unchecked, can lead to significant damage. And so, I think this study really shows that medications like metoprolol can help blunt that initial sympathetic effect.”
A larger study is “absolutely” warranted, he added, “this is a drug that is readily available, safe, and inexpensive. The study design here was simple and most importantly, showed biological plausibility.”
Dr. Senussi also noted that, although the benefit was noted in COVID-19 patients, the study sets the groundwork for further research in the use of beta-blockade in the critically ill. “Further studies are needed to elucidate and identify where along the inflammatory spectrum these critically ill patients lie, which patients would benefit from beta-blockers, and at what time point during their hospital stay.”
The MADRID-COVID authors and Dr. Senussi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small study, intravenous administration of the beta-blocker metoprolol to critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) safely blunted lung inflammation associated with the disease.
Metoprolol administration also resulted in better oxygenation and fewer days on intensive mechanical ventilation and in the ICU, compared with no treatment.
These data suggest that metoprolol repurposing for the treatment of ARDS in COVID-19 patients is a safe and inexpensive strategy with the potential to improve outcomes, the researchers said.
“Metoprolol repurposing for the treatment of ARDS associated with COVID-19 is a safe and cheap intervention that can help to alleviate the massive personal and health care burden associated with the pandemic,” they concluded.
The results, from the MADRID-COVID pilot trial from Agustin Clemente-Moragon, BSc, Centro National de Investigaciones Cardiovasculares, Madrid, and colleagues, were published online Aug. 30, 2021, in the Journal of the American College of Cardiology.
In previous work, the researchers showed that metoprolol, but not other clinically available intravenous beta-blockers, abrogates neutrophil-driven exacerbated inflammation, neutrophil-platelet interaction, and formation of neutrophil extracellular traps in a mouse model of acute lung injury.
These results prompted the current pilot trial in 20 patients, ages 18-80 years, with COVID-19–associated ARDS.
Randomization was stratified by age (59 and younger vs. 60 and older), history of hypertension (yes or no), and circulating neutrophil counts (<6,000 vs. ≥6,000). Bronchoalveolar lavage (BAL) fluid and blood samples were obtained from patients at randomization and 24 hours after the third metoprolol dose in the treatment group, and on day 4 in controls.
Because of the cardiovascular effects of metoprolol, patients were monitored invasively and by echocardiography, the authors noted.
As expected, metoprolol significantly reduced heart rate (P < .01) and systolic blood pressure (P < .05), although both remained within the physiological range. Echocardiography showed no deterioration of cardiac function after metoprolol treatment.
To assess the ability of metoprolol to address neutrophil-mediated exacerbated lung inflammation, the researchers analyzed leukocyte populations in BAL samples by flow cytometry at baseline and on day 4.
At baseline, the metoprolol and control groups showed no differences in BAL neutrophil content. But on day 4, after 3 days of treatment with metoprolol, neutrophil content was significantly lower in the metoprolol group (median, 14.3 neutrophils/mcL) than in the control group (median, 397 neutrophils/mcL).
Metoprolol-treated patients also had lower total inflammatory-cell content and lower monocyte/macrophage content. Lymphocytes did not differ between the groups.
The investigators also explored the impact of metoprolol on the chemokine, monocyte chemoattractant protein–1 (MCP-1), as it has been shown to promote pulmonary fibrosis in late-stage ARDS.
They found that MCP-1 was significantly attenuated after 3 days of metoprolol treatment. At baseline, the median MCP-1 level was 298 pg/mL; on day 4 after metoprolol, it was 203 pg/mL (P = .009).
MCP-1 levels remained unchanged in control patients.
An elegant study
In an accompanying editorial, Mourad H. Senussi, MD, assistant professor at Baylor College of Medicine, Houston, wrote: “Although the study has a small sample size, we commend the authors, who attempt to shed light on the important pathophysiological underpinnings that help establish biological plausibility for this inexpensive, safe, and widely available medication.”
In an interview with this news organization, Dr. Senussi added that metoprolol is not itself something primarily used to treat COVID-19 per se. “Rather, the drug blunts the sympathetic-host response. There is a fine balance between that sympathetic surge that is helpful to the body, and then a sympathetic surge that if left unchecked, can lead to significant damage. And so, I think this study really shows that medications like metoprolol can help blunt that initial sympathetic effect.”
A larger study is “absolutely” warranted, he added, “this is a drug that is readily available, safe, and inexpensive. The study design here was simple and most importantly, showed biological plausibility.”
Dr. Senussi also noted that, although the benefit was noted in COVID-19 patients, the study sets the groundwork for further research in the use of beta-blockade in the critically ill. “Further studies are needed to elucidate and identify where along the inflammatory spectrum these critically ill patients lie, which patients would benefit from beta-blockers, and at what time point during their hospital stay.”
The MADRID-COVID authors and Dr. Senussi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small study, intravenous administration of the beta-blocker metoprolol to critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) safely blunted lung inflammation associated with the disease.
Metoprolol administration also resulted in better oxygenation and fewer days on intensive mechanical ventilation and in the ICU, compared with no treatment.
These data suggest that metoprolol repurposing for the treatment of ARDS in COVID-19 patients is a safe and inexpensive strategy with the potential to improve outcomes, the researchers said.
“Metoprolol repurposing for the treatment of ARDS associated with COVID-19 is a safe and cheap intervention that can help to alleviate the massive personal and health care burden associated with the pandemic,” they concluded.
The results, from the MADRID-COVID pilot trial from Agustin Clemente-Moragon, BSc, Centro National de Investigaciones Cardiovasculares, Madrid, and colleagues, were published online Aug. 30, 2021, in the Journal of the American College of Cardiology.
In previous work, the researchers showed that metoprolol, but not other clinically available intravenous beta-blockers, abrogates neutrophil-driven exacerbated inflammation, neutrophil-platelet interaction, and formation of neutrophil extracellular traps in a mouse model of acute lung injury.
These results prompted the current pilot trial in 20 patients, ages 18-80 years, with COVID-19–associated ARDS.
Randomization was stratified by age (59 and younger vs. 60 and older), history of hypertension (yes or no), and circulating neutrophil counts (<6,000 vs. ≥6,000). Bronchoalveolar lavage (BAL) fluid and blood samples were obtained from patients at randomization and 24 hours after the third metoprolol dose in the treatment group, and on day 4 in controls.
Because of the cardiovascular effects of metoprolol, patients were monitored invasively and by echocardiography, the authors noted.
As expected, metoprolol significantly reduced heart rate (P < .01) and systolic blood pressure (P < .05), although both remained within the physiological range. Echocardiography showed no deterioration of cardiac function after metoprolol treatment.
To assess the ability of metoprolol to address neutrophil-mediated exacerbated lung inflammation, the researchers analyzed leukocyte populations in BAL samples by flow cytometry at baseline and on day 4.
At baseline, the metoprolol and control groups showed no differences in BAL neutrophil content. But on day 4, after 3 days of treatment with metoprolol, neutrophil content was significantly lower in the metoprolol group (median, 14.3 neutrophils/mcL) than in the control group (median, 397 neutrophils/mcL).
Metoprolol-treated patients also had lower total inflammatory-cell content and lower monocyte/macrophage content. Lymphocytes did not differ between the groups.
The investigators also explored the impact of metoprolol on the chemokine, monocyte chemoattractant protein–1 (MCP-1), as it has been shown to promote pulmonary fibrosis in late-stage ARDS.
They found that MCP-1 was significantly attenuated after 3 days of metoprolol treatment. At baseline, the median MCP-1 level was 298 pg/mL; on day 4 after metoprolol, it was 203 pg/mL (P = .009).
MCP-1 levels remained unchanged in control patients.
An elegant study
In an accompanying editorial, Mourad H. Senussi, MD, assistant professor at Baylor College of Medicine, Houston, wrote: “Although the study has a small sample size, we commend the authors, who attempt to shed light on the important pathophysiological underpinnings that help establish biological plausibility for this inexpensive, safe, and widely available medication.”
In an interview with this news organization, Dr. Senussi added that metoprolol is not itself something primarily used to treat COVID-19 per se. “Rather, the drug blunts the sympathetic-host response. There is a fine balance between that sympathetic surge that is helpful to the body, and then a sympathetic surge that if left unchecked, can lead to significant damage. And so, I think this study really shows that medications like metoprolol can help blunt that initial sympathetic effect.”
A larger study is “absolutely” warranted, he added, “this is a drug that is readily available, safe, and inexpensive. The study design here was simple and most importantly, showed biological plausibility.”
Dr. Senussi also noted that, although the benefit was noted in COVID-19 patients, the study sets the groundwork for further research in the use of beta-blockade in the critically ill. “Further studies are needed to elucidate and identify where along the inflammatory spectrum these critically ill patients lie, which patients would benefit from beta-blockers, and at what time point during their hospital stay.”
The MADRID-COVID authors and Dr. Senussi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.








