User login
In Case You Missed It: COVID
COVID-19 Wellbeing
Resources for hospitalists
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
Resources for hospitalists
Resources for hospitalists
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
U.K. variant spreading in the U.S. as COVID mutations raise stakes
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
Brazilian researchers tracking reinfection by new virus variant
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
President Biden to up states’ vaccine supplies, targets more doses
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Weekly COVID-19 cases in children dropped 22%
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
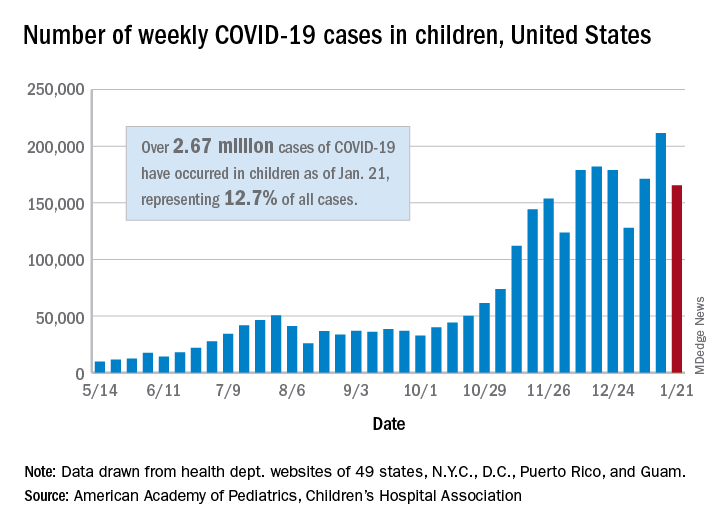
The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
COVID-19 variants may prompt additional Moderna vaccine
As mutated strains of the coronavirus represent new threats in the pandemic, vaccine makers are racing to respond.
Moderna, whose two-dose vaccine has been authorized for use in the United States since Dec. 18, said on Jan. 25 that it is now investigating whether a third dose of the vaccine will better prevent the spread of a variant first seen in South Africa, while it also tests a new vaccine formula for the same purpose.
“Out of an abundance of caution and leveraging the flexibility of our mRNA platform, we are advancing an emerging variant booster candidate against the variant first identified in the Republic of South Africa into the clinic to determine if it will be more effective … against this and potentially future variants,” Moderna CEO Stéphane Bancel said in a statement. Pfizer and BioNTech, whose vaccine was also authorized in December, announced on Jan. 20 that their COVID-19 vaccine creates antibodies that could protect vaccine recipients from the U.K. variant B.1.1.7.
Moderna on Jan. 25 said laboratory tests have shown its COVID-19 vaccine could protect against the U.K. strain but that it is less effective – while still meeting efficacy benchmarks – against the strain identified in South Africa. Data from the study were submitted to a preprint server on Jan. 25 but have not yet been peer reviewed.
“This is not a problem yet,” Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNBC.
“Prepare for it. Sequence these viruses,” he said. “Get ready just in case a variant emerges, which is resistant.”
There were at least 195 confirmed cases of patients infected with the U.K. variant, which is believed to be as much as 70% more transmissible, in the United States as of Jan. 22, according to the Centers for Disease Control and Prevention. No cases from the South African variant have been confirmed in the United States. To try to prevent the variant from entering the country, President Joe Biden plans to ban travel from South Africa, except for American citizens and permanent residents.
The U.S. has reported more than 25 million total COVID-19 cases, according to data from Johns Hopkins University, marking another major milestone during the pandemic.
That means about 1 in 13 people have contracted the virus, or about 7.6% of the U.S. population.
“Twenty-five million cases is an incredible scale of tragedy,” Caitlin Rivers, an epidemiologist at the Johns Hopkins Bloomberg School of Public Health, told The New York Times. She called the pandemic one of the worst public health crises in history.
After the first U.S. case was reported in January 2020, it took more than 9 months to reach 10 million cases in early November. Numbers rose during the holidays, and 10 million more cases were reported by the end of the year.
Following a major surge throughout January 2021, with a peak of more than 300,000 daily cases on some days, the U.S. reached 25 million in about 3 weeks.
Hospitalizations also peaked in early January, with more than 132,000 COVID-19 patients in hospitals across the country, according to the COVID Tracking Project. On Jan. 24, about 111,000 patients were hospitalized, which is the lowest since mid-December.
The U.S. has also reported nearly 420,000 deaths. As recently as the week starting Jan. 17, more than 4,400 deaths were reported in a single day, according to the COVID Tracking Project. Deaths are beginning to drop but still remain above 3,000 daily.
The University of Washington’s Institute for Health Metrics and Evaluation released a new projection Jan. 22 that said new cases would decline steadily in coming weeks. New COVID-19 cases had fallen about 21% in 2 weeks prior to Jan. 25, according to an analysis by The New York Times.
“We’ve been saying since summer that we thought we’d see a peak in January, and I think that, at the national level, we’re around the peak,” Christopher J.L. Murray, MD, director of the institute, told the newspaper.
At the same time, public health officials are concerned that new coronavirus variants could lead to an increase again. Dr. Murray said the variants could “totally change the story.” If the more transmissible strains spread quickly, cases and deaths will surge once more.
“We’re definitely on a downward slope, but I’m worried that the new variants will throw us a curveball in late February or March,” Ms. Rivers told the newspaper.
A version of this article first appeared on WebMD.com.
As mutated strains of the coronavirus represent new threats in the pandemic, vaccine makers are racing to respond.
Moderna, whose two-dose vaccine has been authorized for use in the United States since Dec. 18, said on Jan. 25 that it is now investigating whether a third dose of the vaccine will better prevent the spread of a variant first seen in South Africa, while it also tests a new vaccine formula for the same purpose.
“Out of an abundance of caution and leveraging the flexibility of our mRNA platform, we are advancing an emerging variant booster candidate against the variant first identified in the Republic of South Africa into the clinic to determine if it will be more effective … against this and potentially future variants,” Moderna CEO Stéphane Bancel said in a statement. Pfizer and BioNTech, whose vaccine was also authorized in December, announced on Jan. 20 that their COVID-19 vaccine creates antibodies that could protect vaccine recipients from the U.K. variant B.1.1.7.
Moderna on Jan. 25 said laboratory tests have shown its COVID-19 vaccine could protect against the U.K. strain but that it is less effective – while still meeting efficacy benchmarks – against the strain identified in South Africa. Data from the study were submitted to a preprint server on Jan. 25 but have not yet been peer reviewed.
“This is not a problem yet,” Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNBC.
“Prepare for it. Sequence these viruses,” he said. “Get ready just in case a variant emerges, which is resistant.”
There were at least 195 confirmed cases of patients infected with the U.K. variant, which is believed to be as much as 70% more transmissible, in the United States as of Jan. 22, according to the Centers for Disease Control and Prevention. No cases from the South African variant have been confirmed in the United States. To try to prevent the variant from entering the country, President Joe Biden plans to ban travel from South Africa, except for American citizens and permanent residents.
The U.S. has reported more than 25 million total COVID-19 cases, according to data from Johns Hopkins University, marking another major milestone during the pandemic.
That means about 1 in 13 people have contracted the virus, or about 7.6% of the U.S. population.
“Twenty-five million cases is an incredible scale of tragedy,” Caitlin Rivers, an epidemiologist at the Johns Hopkins Bloomberg School of Public Health, told The New York Times. She called the pandemic one of the worst public health crises in history.
After the first U.S. case was reported in January 2020, it took more than 9 months to reach 10 million cases in early November. Numbers rose during the holidays, and 10 million more cases were reported by the end of the year.
Following a major surge throughout January 2021, with a peak of more than 300,000 daily cases on some days, the U.S. reached 25 million in about 3 weeks.
Hospitalizations also peaked in early January, with more than 132,000 COVID-19 patients in hospitals across the country, according to the COVID Tracking Project. On Jan. 24, about 111,000 patients were hospitalized, which is the lowest since mid-December.
The U.S. has also reported nearly 420,000 deaths. As recently as the week starting Jan. 17, more than 4,400 deaths were reported in a single day, according to the COVID Tracking Project. Deaths are beginning to drop but still remain above 3,000 daily.
The University of Washington’s Institute for Health Metrics and Evaluation released a new projection Jan. 22 that said new cases would decline steadily in coming weeks. New COVID-19 cases had fallen about 21% in 2 weeks prior to Jan. 25, according to an analysis by The New York Times.
“We’ve been saying since summer that we thought we’d see a peak in January, and I think that, at the national level, we’re around the peak,” Christopher J.L. Murray, MD, director of the institute, told the newspaper.
At the same time, public health officials are concerned that new coronavirus variants could lead to an increase again. Dr. Murray said the variants could “totally change the story.” If the more transmissible strains spread quickly, cases and deaths will surge once more.
“We’re definitely on a downward slope, but I’m worried that the new variants will throw us a curveball in late February or March,” Ms. Rivers told the newspaper.
A version of this article first appeared on WebMD.com.
As mutated strains of the coronavirus represent new threats in the pandemic, vaccine makers are racing to respond.
Moderna, whose two-dose vaccine has been authorized for use in the United States since Dec. 18, said on Jan. 25 that it is now investigating whether a third dose of the vaccine will better prevent the spread of a variant first seen in South Africa, while it also tests a new vaccine formula for the same purpose.
“Out of an abundance of caution and leveraging the flexibility of our mRNA platform, we are advancing an emerging variant booster candidate against the variant first identified in the Republic of South Africa into the clinic to determine if it will be more effective … against this and potentially future variants,” Moderna CEO Stéphane Bancel said in a statement. Pfizer and BioNTech, whose vaccine was also authorized in December, announced on Jan. 20 that their COVID-19 vaccine creates antibodies that could protect vaccine recipients from the U.K. variant B.1.1.7.
Moderna on Jan. 25 said laboratory tests have shown its COVID-19 vaccine could protect against the U.K. strain but that it is less effective – while still meeting efficacy benchmarks – against the strain identified in South Africa. Data from the study were submitted to a preprint server on Jan. 25 but have not yet been peer reviewed.
“This is not a problem yet,” Paul Offit, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNBC.
“Prepare for it. Sequence these viruses,” he said. “Get ready just in case a variant emerges, which is resistant.”
There were at least 195 confirmed cases of patients infected with the U.K. variant, which is believed to be as much as 70% more transmissible, in the United States as of Jan. 22, according to the Centers for Disease Control and Prevention. No cases from the South African variant have been confirmed in the United States. To try to prevent the variant from entering the country, President Joe Biden plans to ban travel from South Africa, except for American citizens and permanent residents.
The U.S. has reported more than 25 million total COVID-19 cases, according to data from Johns Hopkins University, marking another major milestone during the pandemic.
That means about 1 in 13 people have contracted the virus, or about 7.6% of the U.S. population.
“Twenty-five million cases is an incredible scale of tragedy,” Caitlin Rivers, an epidemiologist at the Johns Hopkins Bloomberg School of Public Health, told The New York Times. She called the pandemic one of the worst public health crises in history.
After the first U.S. case was reported in January 2020, it took more than 9 months to reach 10 million cases in early November. Numbers rose during the holidays, and 10 million more cases were reported by the end of the year.
Following a major surge throughout January 2021, with a peak of more than 300,000 daily cases on some days, the U.S. reached 25 million in about 3 weeks.
Hospitalizations also peaked in early January, with more than 132,000 COVID-19 patients in hospitals across the country, according to the COVID Tracking Project. On Jan. 24, about 111,000 patients were hospitalized, which is the lowest since mid-December.
The U.S. has also reported nearly 420,000 deaths. As recently as the week starting Jan. 17, more than 4,400 deaths were reported in a single day, according to the COVID Tracking Project. Deaths are beginning to drop but still remain above 3,000 daily.
The University of Washington’s Institute for Health Metrics and Evaluation released a new projection Jan. 22 that said new cases would decline steadily in coming weeks. New COVID-19 cases had fallen about 21% in 2 weeks prior to Jan. 25, according to an analysis by The New York Times.
“We’ve been saying since summer that we thought we’d see a peak in January, and I think that, at the national level, we’re around the peak,” Christopher J.L. Murray, MD, director of the institute, told the newspaper.
At the same time, public health officials are concerned that new coronavirus variants could lead to an increase again. Dr. Murray said the variants could “totally change the story.” If the more transmissible strains spread quickly, cases and deaths will surge once more.
“We’re definitely on a downward slope, but I’m worried that the new variants will throw us a curveball in late February or March,” Ms. Rivers told the newspaper.
A version of this article first appeared on WebMD.com.
Even patients with cancer in remission at risk for severe COVID-19
It’s been shown that hospitalized cancer patients and those undergoing active treatment are at high risk for severe COVID-19 complications. A new study shows that patients with cancer in remission are at higher risk, too.
For the study, investigators from the University of Pennsylvania, Philadelphia, analyzed 323 patients with SARS-CoV-2 infection in a research database with more than 4,800 patients. About 20% of database patients were Black, but they accounted for almost 65% of the infections, reflecting previous reports of increased risk for COVID among Black people.
A total of 67 of the infected patients had cancer, including 18 patients with active cancer and 49 patients whose cancer was in remission. After adjusting for demographics, smoking status, and comorbidities, a diagnosis of cancer more than doubled the odds of hospitalization and increased the odds of 30-day mortality nearly sixfold.
Worse outcomes were more strongly associated with active cancer, but patients whose cancer was in remission were also at higher risk than patients who did not have cancer.
It’s not only “patients hospitalized or on treatment ... all oncology patients need to take significant precautions during the pandemic to protect themselves,” senior investigator Kara Maxwell, MD, PhD, hematologist/oncologist and assistant professor at the University of Pennsylvania, said in a press release.
The study was published online on Jan. 21 in JNCI Cancer Spectrum.
The good news is that steps to prevent SARS-CoV-2 infection work, suggests a second report from the University of Pennsylvania. Among 124 cancer patients who underwent outpatient infusions from May to October 2020, not a single one experienced seroconversion over a median of 13 clinical visits. That second study was published on Jan. 16 in medRxiv and is pending peer review.
The zero seroconversion rate likely reflects “the success of transmission mitigation measures within health care facilities,” wrote investigators led by Lova Sun, MD, a hematology/oncology fellow at the University of Pennsylvania.
Like many institutions, the University of Pennsylvania Health System (Penn Medicine) is aggressive in protecting outpatients against the virus, the authors wrote. Among other steps, patients are queried about symptoms and contacts before their office visit, and their temperature is taken when they come in. Masks are worn, check-in is contactless, the number of visitors is limited, and patients who test positive are treated in a separate space.
In addition, patients in the study also reported that they wore masks and practiced social distancing in their daily lives.
Approached for comment, hematologist/oncologist Charles Shapiro, MD, a professor at the Icahn School of Medicine at Mount Sinai and director of translational breast cancer research at Mount Sinai Hospital, both in New York, said he wasn’t surprised that the prevention measures followed at Penn Medicine work. They are very similar to the measures followed at Mount Sinai oncology clinics, and “there’ve been very few COVID cases in our shop,” he added.
The bigger take-home message from both studies is that cancer patients, regardless of their age or if they are in remission, should be prioritized for vaccination against COVID-19, which is the best way to mitigate risk. “I strongly urge my patients to get it” if they can, he said.
The problem in New York is that immunizations are largely limited to people aged 65 years and older. Younger cancer patients are left out, and access has been spotty for all patients. “Vaccine is available one day, then not the next. It’s disheartening,” Dr. Shapiro said in an interview. “Hopefully, with the new administration, this will smooth out,” and the age limit will drop.
The study was supported by the National Institutes of Health, among other organizations. Dr. Lova, Dr. Maxwell, and Dr. Shapiro have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s been shown that hospitalized cancer patients and those undergoing active treatment are at high risk for severe COVID-19 complications. A new study shows that patients with cancer in remission are at higher risk, too.
For the study, investigators from the University of Pennsylvania, Philadelphia, analyzed 323 patients with SARS-CoV-2 infection in a research database with more than 4,800 patients. About 20% of database patients were Black, but they accounted for almost 65% of the infections, reflecting previous reports of increased risk for COVID among Black people.
A total of 67 of the infected patients had cancer, including 18 patients with active cancer and 49 patients whose cancer was in remission. After adjusting for demographics, smoking status, and comorbidities, a diagnosis of cancer more than doubled the odds of hospitalization and increased the odds of 30-day mortality nearly sixfold.
Worse outcomes were more strongly associated with active cancer, but patients whose cancer was in remission were also at higher risk than patients who did not have cancer.
It’s not only “patients hospitalized or on treatment ... all oncology patients need to take significant precautions during the pandemic to protect themselves,” senior investigator Kara Maxwell, MD, PhD, hematologist/oncologist and assistant professor at the University of Pennsylvania, said in a press release.
The study was published online on Jan. 21 in JNCI Cancer Spectrum.
The good news is that steps to prevent SARS-CoV-2 infection work, suggests a second report from the University of Pennsylvania. Among 124 cancer patients who underwent outpatient infusions from May to October 2020, not a single one experienced seroconversion over a median of 13 clinical visits. That second study was published on Jan. 16 in medRxiv and is pending peer review.
The zero seroconversion rate likely reflects “the success of transmission mitigation measures within health care facilities,” wrote investigators led by Lova Sun, MD, a hematology/oncology fellow at the University of Pennsylvania.
Like many institutions, the University of Pennsylvania Health System (Penn Medicine) is aggressive in protecting outpatients against the virus, the authors wrote. Among other steps, patients are queried about symptoms and contacts before their office visit, and their temperature is taken when they come in. Masks are worn, check-in is contactless, the number of visitors is limited, and patients who test positive are treated in a separate space.
In addition, patients in the study also reported that they wore masks and practiced social distancing in their daily lives.
Approached for comment, hematologist/oncologist Charles Shapiro, MD, a professor at the Icahn School of Medicine at Mount Sinai and director of translational breast cancer research at Mount Sinai Hospital, both in New York, said he wasn’t surprised that the prevention measures followed at Penn Medicine work. They are very similar to the measures followed at Mount Sinai oncology clinics, and “there’ve been very few COVID cases in our shop,” he added.
The bigger take-home message from both studies is that cancer patients, regardless of their age or if they are in remission, should be prioritized for vaccination against COVID-19, which is the best way to mitigate risk. “I strongly urge my patients to get it” if they can, he said.
The problem in New York is that immunizations are largely limited to people aged 65 years and older. Younger cancer patients are left out, and access has been spotty for all patients. “Vaccine is available one day, then not the next. It’s disheartening,” Dr. Shapiro said in an interview. “Hopefully, with the new administration, this will smooth out,” and the age limit will drop.
The study was supported by the National Institutes of Health, among other organizations. Dr. Lova, Dr. Maxwell, and Dr. Shapiro have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s been shown that hospitalized cancer patients and those undergoing active treatment are at high risk for severe COVID-19 complications. A new study shows that patients with cancer in remission are at higher risk, too.
For the study, investigators from the University of Pennsylvania, Philadelphia, analyzed 323 patients with SARS-CoV-2 infection in a research database with more than 4,800 patients. About 20% of database patients were Black, but they accounted for almost 65% of the infections, reflecting previous reports of increased risk for COVID among Black people.
A total of 67 of the infected patients had cancer, including 18 patients with active cancer and 49 patients whose cancer was in remission. After adjusting for demographics, smoking status, and comorbidities, a diagnosis of cancer more than doubled the odds of hospitalization and increased the odds of 30-day mortality nearly sixfold.
Worse outcomes were more strongly associated with active cancer, but patients whose cancer was in remission were also at higher risk than patients who did not have cancer.
It’s not only “patients hospitalized or on treatment ... all oncology patients need to take significant precautions during the pandemic to protect themselves,” senior investigator Kara Maxwell, MD, PhD, hematologist/oncologist and assistant professor at the University of Pennsylvania, said in a press release.
The study was published online on Jan. 21 in JNCI Cancer Spectrum.
The good news is that steps to prevent SARS-CoV-2 infection work, suggests a second report from the University of Pennsylvania. Among 124 cancer patients who underwent outpatient infusions from May to October 2020, not a single one experienced seroconversion over a median of 13 clinical visits. That second study was published on Jan. 16 in medRxiv and is pending peer review.
The zero seroconversion rate likely reflects “the success of transmission mitigation measures within health care facilities,” wrote investigators led by Lova Sun, MD, a hematology/oncology fellow at the University of Pennsylvania.
Like many institutions, the University of Pennsylvania Health System (Penn Medicine) is aggressive in protecting outpatients against the virus, the authors wrote. Among other steps, patients are queried about symptoms and contacts before their office visit, and their temperature is taken when they come in. Masks are worn, check-in is contactless, the number of visitors is limited, and patients who test positive are treated in a separate space.
In addition, patients in the study also reported that they wore masks and practiced social distancing in their daily lives.
Approached for comment, hematologist/oncologist Charles Shapiro, MD, a professor at the Icahn School of Medicine at Mount Sinai and director of translational breast cancer research at Mount Sinai Hospital, both in New York, said he wasn’t surprised that the prevention measures followed at Penn Medicine work. They are very similar to the measures followed at Mount Sinai oncology clinics, and “there’ve been very few COVID cases in our shop,” he added.
The bigger take-home message from both studies is that cancer patients, regardless of their age or if they are in remission, should be prioritized for vaccination against COVID-19, which is the best way to mitigate risk. “I strongly urge my patients to get it” if they can, he said.
The problem in New York is that immunizations are largely limited to people aged 65 years and older. Younger cancer patients are left out, and access has been spotty for all patients. “Vaccine is available one day, then not the next. It’s disheartening,” Dr. Shapiro said in an interview. “Hopefully, with the new administration, this will smooth out,” and the age limit will drop.
The study was supported by the National Institutes of Health, among other organizations. Dr. Lova, Dr. Maxwell, and Dr. Shapiro have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than one-third of COVID-19 infections are asymptomatic: Review
A systematic review suggests at least one-third of SARS-CoV-2 infections occur in people who never develop symptoms, providing strong evidence for the prevalence of asymptomatic infections.
The finding that nearly one in three infected people remain symptom free suggests testing should be changed, the investigators noted.
“To reduce transmission from people who are presymptomatic or asymptomatic, we need to shift our testing focus to at-home screening,” lead author Daniel Oran, AM, said in an interview. “Inexpensive rapid antigen tests, provided to millions of people for frequent use, could help us significantly reduce the spread of the virus.”
The systematic review was published online Jan. 22 in Annals of Internal Medicine.
The findings come at a dire time when the official number of COVID-19 cases in the United States exceeds 25 million for the first time. Public health officials have raised concerns about more transmissible, and possibly more deadly, variants of SARS-CoV-2, while a new presidential administration tries to meet the challenge of improving vaccine distribution and acceptance rates.
The results also build on earlier findings from the same research team – Mr. Oran and senior author Eric Topol, MD – that published a review article looking at asymptomatic COVID-19 cases. Even though initial data were more limited, they likewise suggested a broader scope of testing is warranted, pointing out that asymptomatic individuals can transmit SARS-CoV-2 for up to 14 days. Dr. Topol is also editor in chief of Medscape.
In the current systematic review, the highest-quality evidence comes from large studies in England and Spain. The nationally representative evidence included serologic surveys from more than 365,000 people in England and more than 61,000 in Spain. When analyzed separately, about the same proportion of asymptomatic cases emerged: 32.4% in England and 33% in Spain.
“It was really remarkable to find that nationwide antibody testing studies in England and Spain – including hundreds of thousands of people – produced nearly identical results: About one-third of the SARS-CoV-2 infections were completely asymptomatic,” said Mr. Oran, a researcher at Scripps Research Translational Institute in La Jolla, Calif.
The systematic review included 43 studies with PCR testing for active SARS-CoV-2 infection and another 18 with antibody results that indicated present or previous infection. The studies were published up until Nov. 17, 2020.
An appreciation for asymptomatic transmission of SARS-CoV-2 infection has come a long way from initial dismissals about its importance, Dr. Topol noted via Twitter. “When Dr. @camilla_rothe reported an asymptomatic transmission a year ago, the @NEJM report was refuted and disparaged. She was later named a TIME 100 Person of the Year.”
Not symptomatic vs. never symptomatic
The term “asymptomatic” could be misleading because some people in this group do progress to develop signs of infection. This “presymptomatic” group of patients is likely a minority, the authors noted. Longitudinal studies indicate that about three-quarters of people who are asymptomatic with SARS-CoV-2 remain so.
Dr. Topol anticipated the one-third asymptomatic finding could draw some feedback about distinguishing asymptomatic from presymptomatic individuals. He tweeted, “Some will argue that there is admixture with presymptomatic cases, but review of all the data supports this estimate as being a conservative one.”
The heterogeneity of the settings, populations and other features of the studies prevented the authors from performing a meta-analysis of the findings.
Home is where the test is
Based on their findings, Mr. Oran and Dr. Topol believe “that COVID-19 control strategies must be altered, taking into account the prevalence and transmission risk of asymptomatic SARS-CoV-2 infection.” They suggested frequent use of inexpensive, rapid home tests to identify people who are asymptomatic or presymptomatic, along with programs and housing provided by the government to offer financial assistance and allow this group of people to isolate themselves.
Further research is warranted to determine if and how well vaccines for SARS-CoV-2 prevent asymptomatic infection.
Dr. Topol and Mr. Oran created a short video to highlight the findings from their systematic review.
The study was supported by a grant from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
A systematic review suggests at least one-third of SARS-CoV-2 infections occur in people who never develop symptoms, providing strong evidence for the prevalence of asymptomatic infections.
The finding that nearly one in three infected people remain symptom free suggests testing should be changed, the investigators noted.
“To reduce transmission from people who are presymptomatic or asymptomatic, we need to shift our testing focus to at-home screening,” lead author Daniel Oran, AM, said in an interview. “Inexpensive rapid antigen tests, provided to millions of people for frequent use, could help us significantly reduce the spread of the virus.”
The systematic review was published online Jan. 22 in Annals of Internal Medicine.
The findings come at a dire time when the official number of COVID-19 cases in the United States exceeds 25 million for the first time. Public health officials have raised concerns about more transmissible, and possibly more deadly, variants of SARS-CoV-2, while a new presidential administration tries to meet the challenge of improving vaccine distribution and acceptance rates.
The results also build on earlier findings from the same research team – Mr. Oran and senior author Eric Topol, MD – that published a review article looking at asymptomatic COVID-19 cases. Even though initial data were more limited, they likewise suggested a broader scope of testing is warranted, pointing out that asymptomatic individuals can transmit SARS-CoV-2 for up to 14 days. Dr. Topol is also editor in chief of Medscape.
In the current systematic review, the highest-quality evidence comes from large studies in England and Spain. The nationally representative evidence included serologic surveys from more than 365,000 people in England and more than 61,000 in Spain. When analyzed separately, about the same proportion of asymptomatic cases emerged: 32.4% in England and 33% in Spain.
“It was really remarkable to find that nationwide antibody testing studies in England and Spain – including hundreds of thousands of people – produced nearly identical results: About one-third of the SARS-CoV-2 infections were completely asymptomatic,” said Mr. Oran, a researcher at Scripps Research Translational Institute in La Jolla, Calif.
The systematic review included 43 studies with PCR testing for active SARS-CoV-2 infection and another 18 with antibody results that indicated present or previous infection. The studies were published up until Nov. 17, 2020.
An appreciation for asymptomatic transmission of SARS-CoV-2 infection has come a long way from initial dismissals about its importance, Dr. Topol noted via Twitter. “When Dr. @camilla_rothe reported an asymptomatic transmission a year ago, the @NEJM report was refuted and disparaged. She was later named a TIME 100 Person of the Year.”
Not symptomatic vs. never symptomatic
The term “asymptomatic” could be misleading because some people in this group do progress to develop signs of infection. This “presymptomatic” group of patients is likely a minority, the authors noted. Longitudinal studies indicate that about three-quarters of people who are asymptomatic with SARS-CoV-2 remain so.
Dr. Topol anticipated the one-third asymptomatic finding could draw some feedback about distinguishing asymptomatic from presymptomatic individuals. He tweeted, “Some will argue that there is admixture with presymptomatic cases, but review of all the data supports this estimate as being a conservative one.”
The heterogeneity of the settings, populations and other features of the studies prevented the authors from performing a meta-analysis of the findings.
Home is where the test is
Based on their findings, Mr. Oran and Dr. Topol believe “that COVID-19 control strategies must be altered, taking into account the prevalence and transmission risk of asymptomatic SARS-CoV-2 infection.” They suggested frequent use of inexpensive, rapid home tests to identify people who are asymptomatic or presymptomatic, along with programs and housing provided by the government to offer financial assistance and allow this group of people to isolate themselves.
Further research is warranted to determine if and how well vaccines for SARS-CoV-2 prevent asymptomatic infection.
Dr. Topol and Mr. Oran created a short video to highlight the findings from their systematic review.
The study was supported by a grant from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
A systematic review suggests at least one-third of SARS-CoV-2 infections occur in people who never develop symptoms, providing strong evidence for the prevalence of asymptomatic infections.
The finding that nearly one in three infected people remain symptom free suggests testing should be changed, the investigators noted.
“To reduce transmission from people who are presymptomatic or asymptomatic, we need to shift our testing focus to at-home screening,” lead author Daniel Oran, AM, said in an interview. “Inexpensive rapid antigen tests, provided to millions of people for frequent use, could help us significantly reduce the spread of the virus.”
The systematic review was published online Jan. 22 in Annals of Internal Medicine.
The findings come at a dire time when the official number of COVID-19 cases in the United States exceeds 25 million for the first time. Public health officials have raised concerns about more transmissible, and possibly more deadly, variants of SARS-CoV-2, while a new presidential administration tries to meet the challenge of improving vaccine distribution and acceptance rates.
The results also build on earlier findings from the same research team – Mr. Oran and senior author Eric Topol, MD – that published a review article looking at asymptomatic COVID-19 cases. Even though initial data were more limited, they likewise suggested a broader scope of testing is warranted, pointing out that asymptomatic individuals can transmit SARS-CoV-2 for up to 14 days. Dr. Topol is also editor in chief of Medscape.
In the current systematic review, the highest-quality evidence comes from large studies in England and Spain. The nationally representative evidence included serologic surveys from more than 365,000 people in England and more than 61,000 in Spain. When analyzed separately, about the same proportion of asymptomatic cases emerged: 32.4% in England and 33% in Spain.
“It was really remarkable to find that nationwide antibody testing studies in England and Spain – including hundreds of thousands of people – produced nearly identical results: About one-third of the SARS-CoV-2 infections were completely asymptomatic,” said Mr. Oran, a researcher at Scripps Research Translational Institute in La Jolla, Calif.
The systematic review included 43 studies with PCR testing for active SARS-CoV-2 infection and another 18 with antibody results that indicated present or previous infection. The studies were published up until Nov. 17, 2020.
An appreciation for asymptomatic transmission of SARS-CoV-2 infection has come a long way from initial dismissals about its importance, Dr. Topol noted via Twitter. “When Dr. @camilla_rothe reported an asymptomatic transmission a year ago, the @NEJM report was refuted and disparaged. She was later named a TIME 100 Person of the Year.”
Not symptomatic vs. never symptomatic
The term “asymptomatic” could be misleading because some people in this group do progress to develop signs of infection. This “presymptomatic” group of patients is likely a minority, the authors noted. Longitudinal studies indicate that about three-quarters of people who are asymptomatic with SARS-CoV-2 remain so.
Dr. Topol anticipated the one-third asymptomatic finding could draw some feedback about distinguishing asymptomatic from presymptomatic individuals. He tweeted, “Some will argue that there is admixture with presymptomatic cases, but review of all the data supports this estimate as being a conservative one.”
The heterogeneity of the settings, populations and other features of the studies prevented the authors from performing a meta-analysis of the findings.
Home is where the test is
Based on their findings, Mr. Oran and Dr. Topol believe “that COVID-19 control strategies must be altered, taking into account the prevalence and transmission risk of asymptomatic SARS-CoV-2 infection.” They suggested frequent use of inexpensive, rapid home tests to identify people who are asymptomatic or presymptomatic, along with programs and housing provided by the government to offer financial assistance and allow this group of people to isolate themselves.
Further research is warranted to determine if and how well vaccines for SARS-CoV-2 prevent asymptomatic infection.
Dr. Topol and Mr. Oran created a short video to highlight the findings from their systematic review.
The study was supported by a grant from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Vaccines may not be as effective against variants
The current COVID-19 vaccines may not be as effective against new coronavirus variants, but they should be powerful enough to still be beneficial, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during a news briefing on Jan. 21.
Both vaccines from Pfizer-BioNTech and Moderna have such high efficacy rates that it creates a “cushion effect,” he said, meaning that new variants will likely only diminish vaccine efficacy slightly.
“Bottom line: We’re paying very close attention to it,” he said. “There are alternative plans if we ever have to modify the vaccine.”
The U.S. has reported 144 cases of the B.1.1.7 variant, which was first identified in the United Kingdom, according to the latest update from the CDC. So far, no cases of the variant strain identified in South Africa have been reported in the U.S., but Dr. Fauci said public health officials are looking for it.
“We’re following very carefully the one in South Africa, which is a little bit more concerning, but nonetheless not something that we don’t think we can handle,” he said.
Despite challenges with vaccine distribution and administration, the U.S. “can and should” vaccinate 70% to 85% of adults by the end of the summer, Dr. Fauci told CNN. If that happens, people could begin to return to some sense of normalcy by the fall, he added.
“When you put ... the pedal to the floor, you can get it done,” he said.
If the U.S. administered one million shots per day, it would take until the end of 2021 to fully vaccine 75% of adults, according to a CNN analysis. Dr. Fauci said he believes the U.S. can give more than one million shots per day. An updated tally from the CDC showed that 1.6 million shots were given in the past 24 hours, which was the largest single-day increase yet reported.
“I’d like it to be a lot more,” Dr. Fauci told CNN. “If we can do better than that, which I personally think we likely will, then great.”
A version of this article first appeared on WebMD.com.
The current COVID-19 vaccines may not be as effective against new coronavirus variants, but they should be powerful enough to still be beneficial, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during a news briefing on Jan. 21.
Both vaccines from Pfizer-BioNTech and Moderna have such high efficacy rates that it creates a “cushion effect,” he said, meaning that new variants will likely only diminish vaccine efficacy slightly.
“Bottom line: We’re paying very close attention to it,” he said. “There are alternative plans if we ever have to modify the vaccine.”
The U.S. has reported 144 cases of the B.1.1.7 variant, which was first identified in the United Kingdom, according to the latest update from the CDC. So far, no cases of the variant strain identified in South Africa have been reported in the U.S., but Dr. Fauci said public health officials are looking for it.
“We’re following very carefully the one in South Africa, which is a little bit more concerning, but nonetheless not something that we don’t think we can handle,” he said.
Despite challenges with vaccine distribution and administration, the U.S. “can and should” vaccinate 70% to 85% of adults by the end of the summer, Dr. Fauci told CNN. If that happens, people could begin to return to some sense of normalcy by the fall, he added.
“When you put ... the pedal to the floor, you can get it done,” he said.
If the U.S. administered one million shots per day, it would take until the end of 2021 to fully vaccine 75% of adults, according to a CNN analysis. Dr. Fauci said he believes the U.S. can give more than one million shots per day. An updated tally from the CDC showed that 1.6 million shots were given in the past 24 hours, which was the largest single-day increase yet reported.
“I’d like it to be a lot more,” Dr. Fauci told CNN. “If we can do better than that, which I personally think we likely will, then great.”
A version of this article first appeared on WebMD.com.
The current COVID-19 vaccines may not be as effective against new coronavirus variants, but they should be powerful enough to still be beneficial, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during a news briefing on Jan. 21.
Both vaccines from Pfizer-BioNTech and Moderna have such high efficacy rates that it creates a “cushion effect,” he said, meaning that new variants will likely only diminish vaccine efficacy slightly.
“Bottom line: We’re paying very close attention to it,” he said. “There are alternative plans if we ever have to modify the vaccine.”
The U.S. has reported 144 cases of the B.1.1.7 variant, which was first identified in the United Kingdom, according to the latest update from the CDC. So far, no cases of the variant strain identified in South Africa have been reported in the U.S., but Dr. Fauci said public health officials are looking for it.
“We’re following very carefully the one in South Africa, which is a little bit more concerning, but nonetheless not something that we don’t think we can handle,” he said.
Despite challenges with vaccine distribution and administration, the U.S. “can and should” vaccinate 70% to 85% of adults by the end of the summer, Dr. Fauci told CNN. If that happens, people could begin to return to some sense of normalcy by the fall, he added.
“When you put ... the pedal to the floor, you can get it done,” he said.
If the U.S. administered one million shots per day, it would take until the end of 2021 to fully vaccine 75% of adults, according to a CNN analysis. Dr. Fauci said he believes the U.S. can give more than one million shots per day. An updated tally from the CDC showed that 1.6 million shots were given in the past 24 hours, which was the largest single-day increase yet reported.
“I’d like it to be a lot more,” Dr. Fauci told CNN. “If we can do better than that, which I personally think we likely will, then great.”
A version of this article first appeared on WebMD.com.
ColCORONA: Colchicine reduces complications in outpatient COVID-19
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.
The oral, anti-inflammatory drug colchicine can prevent complications and hospitalizations in nonhospitalized patients newly diagnosed with COVID-19, according to a press release from the ColCORONA trial investigators.
After 1 month of therapy, there was a 21% risk reduction in the primary composite endpoint of death or hospitalizations that missed statistical significance, compared with placebo among 4,488 outpatients enrolled in the global, phase 3 trial.
After excluding 329 patients without a confirmatory polymerase chain reaction test, however, the use of colchicine was reported to significantly reduce hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%.
“We believe that this is a medical breakthrough. There’s no approved therapy to prevent complications of COVID-19 in outpatients, to prevent them from reaching the hospital,” lead investigator Jean-Claude Tardif, MD, from the Montreal Heart Institute, said in an interview.
“I know that several countries will be reviewing the data very rapidly and that Greece approved it today,” he said. “So this is providing hope for patients.”
Having been burned by hydroxychloroquine and other treatments brought forth without peer review, the response to the announcement was tempered by a desire for more details.
Asked for comment, Steven E. Nissen, MD, of the Cleveland Clinic Foundation, was cautious. “The press release about the trial is vague and lacks details such as hazard ratios, confidence intervals, and P values,” he said in an interview.
“It is impossible to evaluate the results of this trial without these details. It is also uncertain how rigorously data were collected,” he added. “We’ll need to see the manuscript to adequately interpret the results.”
The evidence in the press release is hard to interpret, but early intervention with anti-inflammatory therapy has considerable biologic appeal in COVID, said Paul Ridker, MD, MPH, who led the pivotal CANTOS trial of the anti-inflammatory drug canakinumab in the post-MI setting, and is also chair of the ACTIV-4B trial currently investigating anticoagulants and antithrombotics in outpatient COVID-19.
“Colchicine is both inexpensive and generally well tolerated, and the apparent benefits so far reported are substantial,” Dr. Ridker, from Brigham and Women’s Hospital in Boston, said in an interview. “We are eager to see the full data as rapidly as possible.”
The commonly used gout and rheumatic disease agent costs about 26 cents in Canada and between $4 and $6 in the United States. As previously reported, it reduced the time to clinical deterioration and hospital stay but not mortality in the 105-patient Greek Study in the Effects of Colchicine in COVID-19 Complications Prevention (GRECCO-19) study.
Dr. Tardif said he’s looking forward to having the data in the public domain and that they acted swiftly because the evidence was “clinically persuasive” and “the health system is congested now.”
“We received the results Friday, Jan. 22 at 5 p.m., an hour later we were in meetings with our data safety monitoring board [DSMB], 2 hours later we issued a press release, and a day later we’re submitting a full manuscript to a major scientific journal, so I don’t know if anyone has done this at this speed,” he said. “So we are actually very proud of what we did.”
ColCORONA was designed to enroll 6,000 outpatients, at least 40 years of age, who were diagnosed with COVID-19 infection within the previous 24 hours, and had a least one high-risk criterion, including age at least 70 years, body mass index of at least 30 kg/m2, diabetes mellitus, uncontrolled hypertension, known respiratory disease, heart failure or coronary disease, fever of at least 38.4° C within the last 48 hours, dyspnea at presentation, bicytopenia, pancytopenia, or the combination of high neutrophil count and low lymphocyte count.
Participants were randomly assigned to receive either placebo or colchicine 0.5 mg twice daily for 3 days and then once daily for another 27 days.
The number needed to prevent one COVID-19 complication is about 60 patients, Dr. Tardif said.
Colchicine was well tolerated and resulted in fewer serious adverse events than with placebo, he said. Diarrhea occurred more often with colchicine, but there was no increase in pneumonia. Caution should be used, however, in treating patients with severe renal disease.
Dr. Tardif said he would not prescribe colchicine to an 18-year-old COVID outpatient who doesn’t have any concomitant diseases, but would for those meeting the study protocol.
“As long as a patient appears to me to be at risk of a complication, I would prescribe it, without a doubt,” he said. “I can tell you that when we held the meeting with the DSMB Friday evening, I actually put each member on the spot and asked them: ‘If it were you – not even treating a patient, but if you had COVID today, would you take it based on the data you’ve seen?’ and all of the DSMB members said they would.
“So we’ll have that debate in the public domain when the paper is out, but I believe most physicians will use it to treat their patients.”
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the U.S. National Heart, Lung, and Blood Institute; Montreal philanthropist Sophie Desmarais; and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators.
A version of this article first appeared on Medscape.com.

