User login
In Case You Missed It: COVID
COVID-19 vaccines equally effective in patients on dialysis
Two doses of either the Pfizer-BioNTech COVID-19 vaccine or the Oxford AstraZeneca alternative provide equal and significant protection against severe disease in patients on hemodialysis who have contracted SARS-CoV-2 infection, results of a multicenter observational study indicate.
Following two doses of either vaccine, the risk of hospital admission was 75% lower among vaccinated patients while the risk of death was 88% lower, compared with those who remained unvaccinated.
No difference was seen between the two vaccine types in terms of outcome severity, and there was no loss of protection in patients over the age of 65 or with increasing time since vaccination, the authors add. The need for oxygen and ventilation was also halved among those who had received two shots, compared with those who had not.
“The coronavirus disease 2019 (COVID-19) pandemic has had a devastating effect on the CKD (chronic kidney disease) community, particularly for individuals receiving maintenance dialysis,” Matthew Oliver, MD, University of Toronto, and Peter Blake, MD, Western University, London, Ont., write in an editorial published with the study.
“Overall, [this and other studies] show that COVID-19 vaccination in the maintenance dialysis population provides moderate protection against acquiring SARS-CoV-2 infection but is highly protective against severe outcomes,” they conclude.
The study was published in the June issue of the Clinical Journal of the American Society of Nephrology.
Severe outcomes observed less in patients who tested positive
The cohort included 1,323 patients on hemodialysis who tested positive on PCR testing to SARS-CoV-2 during a surveillance interval between December 2020 and September 2021, report, Damien Ashby, MD, Hammersmith Hospital, London, and colleagues report.
Among those who tested positive, 79% had not been vaccinated, 7% tested positive after their first dose of either vaccine, and 14% tested positive at least 10 days beyond their second dose.
The course of illness was mild in 61% of patients in that they did not require hospital admission, investigators note. Oxygen support was required by 29% of those who tested positive, and 13% died before 28 days, they added. Among those who died within 28 days of testing positive, 90% of the deaths were deemed to be caused by the virus itself.
“Compared with unvaccinated patients, severe COVID-19 outcomes were observed less than half as often in patients testing positive for SARS-Co-V-2 at least 10 days after the second dose,” Dr. Ashby and colleagues emphasize.
“And the protection from severe illness associated with vaccination was most obvious in patients over 65 years, in whom severe COVID-19 outcomes were reduced at least as much after vaccination as in their younger peers,” they add. Following vaccination with the Pfizer-BioNTech vaccine, antibody levels in patients on dialysis were comparable with those of healthy controls.
In contrast, this was not the case for the Oxford AstraZeneca vaccine where neutralizing titers in patients who received the vaccine were less effective against most variants. Despite its ability to produce comparable immunogenicity, the Oxford AstraZeneca vaccine was clearly associated with clinical protection against severe illness, the authors stress.
They also note that their results are relevant to vaccine uptake in the dialysis population where vaccine hesitancy remains a problem. “This study may, therefore, be useful in reducing vaccine hesitancy, which has resulted in low uptake in some countries (for example, Australia, where almost a quarter of patients on dialysis declined),” Dr. Ashby and colleagues point out.
Although significant vulnerability in the dialysis population remains, “this population has much to gain from vaccination, regardless of age or vaccine type,” the authors underscore.
CKD community quick to prioritize vaccine
As the editorialists point out, leaders in the CKD community were quick – and successful – in prioritizing vaccination in the dialysis population right from the beginning of the pandemic. For example, in Ontario, 90% of the maintenance dialysis population had received two doses of a COVID-19 vaccine by September 2021 and 78% had received three doses by January 2022.
Moreover, in Ontario, “our group found that two doses of mRNA vaccine reduced the risk of infection by 69%,” Dr. Oliver and Dr. Blake point out. U.S. researchers also found that the Pfizer mRNA vaccine reduced infection risk from COVID-19 by 79% while the Moderna mRNA vaccine reduced that risk by 73%. Vaccine effectiveness (VE) in the real-world setting indicates that COVID-19 vaccines provide moderate protection against being infected with the SARS-Co-V-2 virus, as the editorialists note.
However, “the VE for preventing severe outcomes is clinically more important for patients on dialysis because their risk of [morbid] events is high,” Dr. Oliver and Dr. Blake write. Indeed, their own study estimated that two doses of an mRNA vaccine reduced severe outcomes by 83%, “a greater benefit than for infection prevention,” they stress.
The editorialists caution that the SARS-CoV-2 virus continues to mutate and serology studies do show that vaccine-induced immunity does wane over time. Thus, while the COVID-19 pandemic is ever-changing, “we should conduct [VE] studies rigorously and expeditiously to bolster the case for prioritizing vaccination in the dialysis population,” Dr. Oliver and Dr. Blake recommend.
Need to increase vaccine acceptance
Commenting on the study, Uwe K.H. Korst from Bensheim, Germany, notes that COVID-19 is a daily reminder of how fragile life is for people with CKD. “Daily, the virus continues its horrific and unprecedented course through immunocompromised and immunosuppressed patients with kidney disease,” he writes.
Thus, Mr. Korst continues to call for additional education for health care professionals, patients, and the public to increase vaccine acceptance as well as more research to better understand the virus and its long-term consequences.
“Finally, patients need to express their needs, and physicians need to listen to patients’ voices,” Mr. Korst advises.
Dr. Oliver is a contracted medical lead of Ontario Renal Network and owner of Oliver Medical Management for which he holds patents and has received royalties. He has also reported receiving honoraria for speaking from Baxter Healthcare and participating in advisory boards for Amgen and Janssen. Dr. Blake has reported receiving honoraria from Baxter Global for speaking engagements and serves on the editorial board for the American Journal of Nephrology. Dr. Ashby and Dr. Korst have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two doses of either the Pfizer-BioNTech COVID-19 vaccine or the Oxford AstraZeneca alternative provide equal and significant protection against severe disease in patients on hemodialysis who have contracted SARS-CoV-2 infection, results of a multicenter observational study indicate.
Following two doses of either vaccine, the risk of hospital admission was 75% lower among vaccinated patients while the risk of death was 88% lower, compared with those who remained unvaccinated.
No difference was seen between the two vaccine types in terms of outcome severity, and there was no loss of protection in patients over the age of 65 or with increasing time since vaccination, the authors add. The need for oxygen and ventilation was also halved among those who had received two shots, compared with those who had not.
“The coronavirus disease 2019 (COVID-19) pandemic has had a devastating effect on the CKD (chronic kidney disease) community, particularly for individuals receiving maintenance dialysis,” Matthew Oliver, MD, University of Toronto, and Peter Blake, MD, Western University, London, Ont., write in an editorial published with the study.
“Overall, [this and other studies] show that COVID-19 vaccination in the maintenance dialysis population provides moderate protection against acquiring SARS-CoV-2 infection but is highly protective against severe outcomes,” they conclude.
The study was published in the June issue of the Clinical Journal of the American Society of Nephrology.
Severe outcomes observed less in patients who tested positive
The cohort included 1,323 patients on hemodialysis who tested positive on PCR testing to SARS-CoV-2 during a surveillance interval between December 2020 and September 2021, report, Damien Ashby, MD, Hammersmith Hospital, London, and colleagues report.
Among those who tested positive, 79% had not been vaccinated, 7% tested positive after their first dose of either vaccine, and 14% tested positive at least 10 days beyond their second dose.
The course of illness was mild in 61% of patients in that they did not require hospital admission, investigators note. Oxygen support was required by 29% of those who tested positive, and 13% died before 28 days, they added. Among those who died within 28 days of testing positive, 90% of the deaths were deemed to be caused by the virus itself.
“Compared with unvaccinated patients, severe COVID-19 outcomes were observed less than half as often in patients testing positive for SARS-Co-V-2 at least 10 days after the second dose,” Dr. Ashby and colleagues emphasize.
“And the protection from severe illness associated with vaccination was most obvious in patients over 65 years, in whom severe COVID-19 outcomes were reduced at least as much after vaccination as in their younger peers,” they add. Following vaccination with the Pfizer-BioNTech vaccine, antibody levels in patients on dialysis were comparable with those of healthy controls.
In contrast, this was not the case for the Oxford AstraZeneca vaccine where neutralizing titers in patients who received the vaccine were less effective against most variants. Despite its ability to produce comparable immunogenicity, the Oxford AstraZeneca vaccine was clearly associated with clinical protection against severe illness, the authors stress.
They also note that their results are relevant to vaccine uptake in the dialysis population where vaccine hesitancy remains a problem. “This study may, therefore, be useful in reducing vaccine hesitancy, which has resulted in low uptake in some countries (for example, Australia, where almost a quarter of patients on dialysis declined),” Dr. Ashby and colleagues point out.
Although significant vulnerability in the dialysis population remains, “this population has much to gain from vaccination, regardless of age or vaccine type,” the authors underscore.
CKD community quick to prioritize vaccine
As the editorialists point out, leaders in the CKD community were quick – and successful – in prioritizing vaccination in the dialysis population right from the beginning of the pandemic. For example, in Ontario, 90% of the maintenance dialysis population had received two doses of a COVID-19 vaccine by September 2021 and 78% had received three doses by January 2022.
Moreover, in Ontario, “our group found that two doses of mRNA vaccine reduced the risk of infection by 69%,” Dr. Oliver and Dr. Blake point out. U.S. researchers also found that the Pfizer mRNA vaccine reduced infection risk from COVID-19 by 79% while the Moderna mRNA vaccine reduced that risk by 73%. Vaccine effectiveness (VE) in the real-world setting indicates that COVID-19 vaccines provide moderate protection against being infected with the SARS-Co-V-2 virus, as the editorialists note.
However, “the VE for preventing severe outcomes is clinically more important for patients on dialysis because their risk of [morbid] events is high,” Dr. Oliver and Dr. Blake write. Indeed, their own study estimated that two doses of an mRNA vaccine reduced severe outcomes by 83%, “a greater benefit than for infection prevention,” they stress.
The editorialists caution that the SARS-CoV-2 virus continues to mutate and serology studies do show that vaccine-induced immunity does wane over time. Thus, while the COVID-19 pandemic is ever-changing, “we should conduct [VE] studies rigorously and expeditiously to bolster the case for prioritizing vaccination in the dialysis population,” Dr. Oliver and Dr. Blake recommend.
Need to increase vaccine acceptance
Commenting on the study, Uwe K.H. Korst from Bensheim, Germany, notes that COVID-19 is a daily reminder of how fragile life is for people with CKD. “Daily, the virus continues its horrific and unprecedented course through immunocompromised and immunosuppressed patients with kidney disease,” he writes.
Thus, Mr. Korst continues to call for additional education for health care professionals, patients, and the public to increase vaccine acceptance as well as more research to better understand the virus and its long-term consequences.
“Finally, patients need to express their needs, and physicians need to listen to patients’ voices,” Mr. Korst advises.
Dr. Oliver is a contracted medical lead of Ontario Renal Network and owner of Oliver Medical Management for which he holds patents and has received royalties. He has also reported receiving honoraria for speaking from Baxter Healthcare and participating in advisory boards for Amgen and Janssen. Dr. Blake has reported receiving honoraria from Baxter Global for speaking engagements and serves on the editorial board for the American Journal of Nephrology. Dr. Ashby and Dr. Korst have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two doses of either the Pfizer-BioNTech COVID-19 vaccine or the Oxford AstraZeneca alternative provide equal and significant protection against severe disease in patients on hemodialysis who have contracted SARS-CoV-2 infection, results of a multicenter observational study indicate.
Following two doses of either vaccine, the risk of hospital admission was 75% lower among vaccinated patients while the risk of death was 88% lower, compared with those who remained unvaccinated.
No difference was seen between the two vaccine types in terms of outcome severity, and there was no loss of protection in patients over the age of 65 or with increasing time since vaccination, the authors add. The need for oxygen and ventilation was also halved among those who had received two shots, compared with those who had not.
“The coronavirus disease 2019 (COVID-19) pandemic has had a devastating effect on the CKD (chronic kidney disease) community, particularly for individuals receiving maintenance dialysis,” Matthew Oliver, MD, University of Toronto, and Peter Blake, MD, Western University, London, Ont., write in an editorial published with the study.
“Overall, [this and other studies] show that COVID-19 vaccination in the maintenance dialysis population provides moderate protection against acquiring SARS-CoV-2 infection but is highly protective against severe outcomes,” they conclude.
The study was published in the June issue of the Clinical Journal of the American Society of Nephrology.
Severe outcomes observed less in patients who tested positive
The cohort included 1,323 patients on hemodialysis who tested positive on PCR testing to SARS-CoV-2 during a surveillance interval between December 2020 and September 2021, report, Damien Ashby, MD, Hammersmith Hospital, London, and colleagues report.
Among those who tested positive, 79% had not been vaccinated, 7% tested positive after their first dose of either vaccine, and 14% tested positive at least 10 days beyond their second dose.
The course of illness was mild in 61% of patients in that they did not require hospital admission, investigators note. Oxygen support was required by 29% of those who tested positive, and 13% died before 28 days, they added. Among those who died within 28 days of testing positive, 90% of the deaths were deemed to be caused by the virus itself.
“Compared with unvaccinated patients, severe COVID-19 outcomes were observed less than half as often in patients testing positive for SARS-Co-V-2 at least 10 days after the second dose,” Dr. Ashby and colleagues emphasize.
“And the protection from severe illness associated with vaccination was most obvious in patients over 65 years, in whom severe COVID-19 outcomes were reduced at least as much after vaccination as in their younger peers,” they add. Following vaccination with the Pfizer-BioNTech vaccine, antibody levels in patients on dialysis were comparable with those of healthy controls.
In contrast, this was not the case for the Oxford AstraZeneca vaccine where neutralizing titers in patients who received the vaccine were less effective against most variants. Despite its ability to produce comparable immunogenicity, the Oxford AstraZeneca vaccine was clearly associated with clinical protection against severe illness, the authors stress.
They also note that their results are relevant to vaccine uptake in the dialysis population where vaccine hesitancy remains a problem. “This study may, therefore, be useful in reducing vaccine hesitancy, which has resulted in low uptake in some countries (for example, Australia, where almost a quarter of patients on dialysis declined),” Dr. Ashby and colleagues point out.
Although significant vulnerability in the dialysis population remains, “this population has much to gain from vaccination, regardless of age or vaccine type,” the authors underscore.
CKD community quick to prioritize vaccine
As the editorialists point out, leaders in the CKD community were quick – and successful – in prioritizing vaccination in the dialysis population right from the beginning of the pandemic. For example, in Ontario, 90% of the maintenance dialysis population had received two doses of a COVID-19 vaccine by September 2021 and 78% had received three doses by January 2022.
Moreover, in Ontario, “our group found that two doses of mRNA vaccine reduced the risk of infection by 69%,” Dr. Oliver and Dr. Blake point out. U.S. researchers also found that the Pfizer mRNA vaccine reduced infection risk from COVID-19 by 79% while the Moderna mRNA vaccine reduced that risk by 73%. Vaccine effectiveness (VE) in the real-world setting indicates that COVID-19 vaccines provide moderate protection against being infected with the SARS-Co-V-2 virus, as the editorialists note.
However, “the VE for preventing severe outcomes is clinically more important for patients on dialysis because their risk of [morbid] events is high,” Dr. Oliver and Dr. Blake write. Indeed, their own study estimated that two doses of an mRNA vaccine reduced severe outcomes by 83%, “a greater benefit than for infection prevention,” they stress.
The editorialists caution that the SARS-CoV-2 virus continues to mutate and serology studies do show that vaccine-induced immunity does wane over time. Thus, while the COVID-19 pandemic is ever-changing, “we should conduct [VE] studies rigorously and expeditiously to bolster the case for prioritizing vaccination in the dialysis population,” Dr. Oliver and Dr. Blake recommend.
Need to increase vaccine acceptance
Commenting on the study, Uwe K.H. Korst from Bensheim, Germany, notes that COVID-19 is a daily reminder of how fragile life is for people with CKD. “Daily, the virus continues its horrific and unprecedented course through immunocompromised and immunosuppressed patients with kidney disease,” he writes.
Thus, Mr. Korst continues to call for additional education for health care professionals, patients, and the public to increase vaccine acceptance as well as more research to better understand the virus and its long-term consequences.
“Finally, patients need to express their needs, and physicians need to listen to patients’ voices,” Mr. Korst advises.
Dr. Oliver is a contracted medical lead of Ontario Renal Network and owner of Oliver Medical Management for which he holds patents and has received royalties. He has also reported receiving honoraria for speaking from Baxter Healthcare and participating in advisory boards for Amgen and Janssen. Dr. Blake has reported receiving honoraria from Baxter Global for speaking engagements and serves on the editorial board for the American Journal of Nephrology. Dr. Ashby and Dr. Korst have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CDC says about 20% get long COVID. New models try to define it
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
The latest on COVID-19 and the heart in children
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Children & COVID: Rise in new cases slows
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.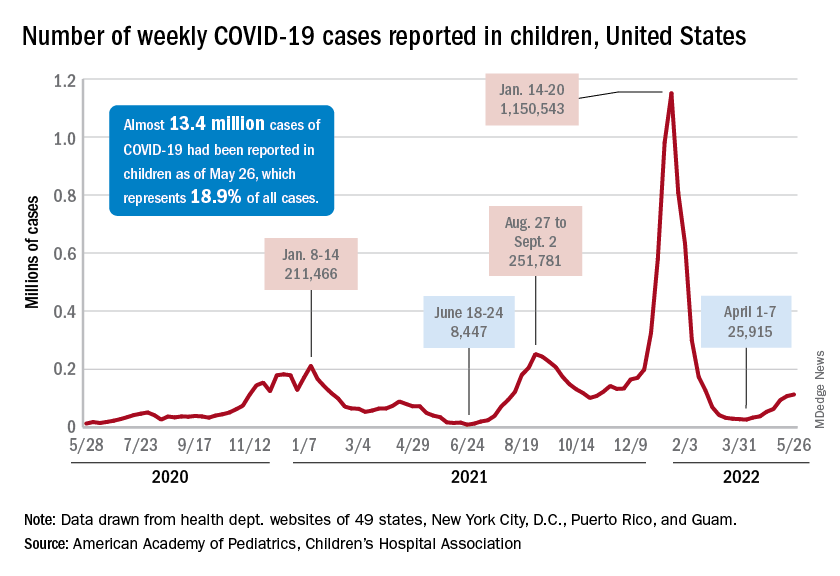
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
Long COVID neuropsychiatric deficits greater than expected
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
AT APA 2022
Most COVID long-haulers suffer long-term debilitating neurologic symptoms
Most COVID-19 long-haulers continue to have brain fog, fatigue, and compromised quality of life more than a year after the initial infection, results from the most extensive follow-up to date of a group of long COVID patients show.
Most patients continue to experience debilitating neurologic symptoms an average of 15 months from symptom onset, Igor Koralnik, MD, who oversees the Neuro COVID-19 Clinic at Northwestern Medicine in Chicago, said during a press briefing.
Surprisingly, in some cases, new symptoms appear that didn’t exist before, including variation of heart rate and blood pressure, and gastrointestinal symptoms, indicating there may be a late appearance in dysfunction of the autonomic nervous system in those patients, Dr. Koralnik said.
The study was published online in Annals of Clinical and Translational Neurology.
Evolving symptoms
The investigators evaluated the evolution of neurologic symptoms in 52 adults who had mild COVID-19 symptoms and were not admitted to the hospital.
Their mean age was 43 years, 73% were women and 77% had received a COVID-19 vaccine. These patients have now been followed for between 11 and 18 months since their initial infection.
Overall, between first and follow-up evaluations, there was no significant change in the frequency of most neurologic symptoms, including brain fog (81% vs. 71%), numbness/tingling (69% vs. 65%), headache (67% vs. 54%), dizziness (50% vs. 54%), blurred vision (34% vs. 44%), tinnitus (33% vs. 42%), and fatigue (87% vs. 81%).
The only neurologic symptoms that decreased over time were loss of taste (63% vs. 27%) and smell (58% vs. 21%).
Conversely, heart rate and blood pressure variation (35% vs. 56%) and gastrointestinal symptoms (27% vs. 48%; P = .04) increased at follow-up evaluations.
Patients reported subjective improvements in their recovery, cognitive function and fatigue, but quality of life measures remained lower than the average population of the United States.
There was a neutral effect of COVID vaccination on long COVID symptoms – it didn’t cure long COVID or make long COVID worse, which is a reason given by some long-haulers for not getting vaccinated, Dr. Koralnik told the briefing.
Therefore, “we continue to encourage our patients to get vaccinated and boosted according to the Centers for Disease Control and Prevention recommendation,” he said.
Escape from the ‘pit of despair’
To date, the Northwestern Medicine Neuro COVID-19 Clinic has treated nearly 1,400 COVID long-haulers from across the United States.
Emily Caffee, a physical therapist from Wheaton, Ill., is one of them.
Speaking at the briefing, the 36-year-old described her saga and roller coaster of recovering from long COVID in three acts: her initial infection, followed by a descent into a pit of physical and emotional despair, followed by her eventual escape from that pit more than two years later.
Following a fairly mild case of COVID, Ms. Caffee said worsening neurologic symptoms forced her to take medical leave from her very physical and cognitively demanding job.
Ms. Caffee said she experienced crushing fatigue and brain fog, as well as rapid heart rate and blood pressure changes going from sitting to standing position.
She went from being a competitive athlete to someone who could barely get off the couch or empty the dishwasher.
With the ongoing help of her medical team, she slowly returned to daily activities and eventually to work on a limited basis.
Today, Ms. Caffee says she’s 90%-95% better but still she has some lingering symptoms and does not yet feel like her pre-COVID self.
It’s been a very slow climb out of the pit, Ms. Caffee said.
This study has no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Most COVID-19 long-haulers continue to have brain fog, fatigue, and compromised quality of life more than a year after the initial infection, results from the most extensive follow-up to date of a group of long COVID patients show.
Most patients continue to experience debilitating neurologic symptoms an average of 15 months from symptom onset, Igor Koralnik, MD, who oversees the Neuro COVID-19 Clinic at Northwestern Medicine in Chicago, said during a press briefing.
Surprisingly, in some cases, new symptoms appear that didn’t exist before, including variation of heart rate and blood pressure, and gastrointestinal symptoms, indicating there may be a late appearance in dysfunction of the autonomic nervous system in those patients, Dr. Koralnik said.
The study was published online in Annals of Clinical and Translational Neurology.
Evolving symptoms
The investigators evaluated the evolution of neurologic symptoms in 52 adults who had mild COVID-19 symptoms and were not admitted to the hospital.
Their mean age was 43 years, 73% were women and 77% had received a COVID-19 vaccine. These patients have now been followed for between 11 and 18 months since their initial infection.
Overall, between first and follow-up evaluations, there was no significant change in the frequency of most neurologic symptoms, including brain fog (81% vs. 71%), numbness/tingling (69% vs. 65%), headache (67% vs. 54%), dizziness (50% vs. 54%), blurred vision (34% vs. 44%), tinnitus (33% vs. 42%), and fatigue (87% vs. 81%).
The only neurologic symptoms that decreased over time were loss of taste (63% vs. 27%) and smell (58% vs. 21%).
Conversely, heart rate and blood pressure variation (35% vs. 56%) and gastrointestinal symptoms (27% vs. 48%; P = .04) increased at follow-up evaluations.
Patients reported subjective improvements in their recovery, cognitive function and fatigue, but quality of life measures remained lower than the average population of the United States.
There was a neutral effect of COVID vaccination on long COVID symptoms – it didn’t cure long COVID or make long COVID worse, which is a reason given by some long-haulers for not getting vaccinated, Dr. Koralnik told the briefing.
Therefore, “we continue to encourage our patients to get vaccinated and boosted according to the Centers for Disease Control and Prevention recommendation,” he said.
Escape from the ‘pit of despair’
To date, the Northwestern Medicine Neuro COVID-19 Clinic has treated nearly 1,400 COVID long-haulers from across the United States.
Emily Caffee, a physical therapist from Wheaton, Ill., is one of them.
Speaking at the briefing, the 36-year-old described her saga and roller coaster of recovering from long COVID in three acts: her initial infection, followed by a descent into a pit of physical and emotional despair, followed by her eventual escape from that pit more than two years later.
Following a fairly mild case of COVID, Ms. Caffee said worsening neurologic symptoms forced her to take medical leave from her very physical and cognitively demanding job.
Ms. Caffee said she experienced crushing fatigue and brain fog, as well as rapid heart rate and blood pressure changes going from sitting to standing position.
She went from being a competitive athlete to someone who could barely get off the couch or empty the dishwasher.
With the ongoing help of her medical team, she slowly returned to daily activities and eventually to work on a limited basis.
Today, Ms. Caffee says she’s 90%-95% better but still she has some lingering symptoms and does not yet feel like her pre-COVID self.
It’s been a very slow climb out of the pit, Ms. Caffee said.
This study has no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Most COVID-19 long-haulers continue to have brain fog, fatigue, and compromised quality of life more than a year after the initial infection, results from the most extensive follow-up to date of a group of long COVID patients show.
Most patients continue to experience debilitating neurologic symptoms an average of 15 months from symptom onset, Igor Koralnik, MD, who oversees the Neuro COVID-19 Clinic at Northwestern Medicine in Chicago, said during a press briefing.
Surprisingly, in some cases, new symptoms appear that didn’t exist before, including variation of heart rate and blood pressure, and gastrointestinal symptoms, indicating there may be a late appearance in dysfunction of the autonomic nervous system in those patients, Dr. Koralnik said.
The study was published online in Annals of Clinical and Translational Neurology.
Evolving symptoms
The investigators evaluated the evolution of neurologic symptoms in 52 adults who had mild COVID-19 symptoms and were not admitted to the hospital.
Their mean age was 43 years, 73% were women and 77% had received a COVID-19 vaccine. These patients have now been followed for between 11 and 18 months since their initial infection.
Overall, between first and follow-up evaluations, there was no significant change in the frequency of most neurologic symptoms, including brain fog (81% vs. 71%), numbness/tingling (69% vs. 65%), headache (67% vs. 54%), dizziness (50% vs. 54%), blurred vision (34% vs. 44%), tinnitus (33% vs. 42%), and fatigue (87% vs. 81%).
The only neurologic symptoms that decreased over time were loss of taste (63% vs. 27%) and smell (58% vs. 21%).
Conversely, heart rate and blood pressure variation (35% vs. 56%) and gastrointestinal symptoms (27% vs. 48%; P = .04) increased at follow-up evaluations.
Patients reported subjective improvements in their recovery, cognitive function and fatigue, but quality of life measures remained lower than the average population of the United States.
There was a neutral effect of COVID vaccination on long COVID symptoms – it didn’t cure long COVID or make long COVID worse, which is a reason given by some long-haulers for not getting vaccinated, Dr. Koralnik told the briefing.
Therefore, “we continue to encourage our patients to get vaccinated and boosted according to the Centers for Disease Control and Prevention recommendation,” he said.
Escape from the ‘pit of despair’
To date, the Northwestern Medicine Neuro COVID-19 Clinic has treated nearly 1,400 COVID long-haulers from across the United States.
Emily Caffee, a physical therapist from Wheaton, Ill., is one of them.
Speaking at the briefing, the 36-year-old described her saga and roller coaster of recovering from long COVID in three acts: her initial infection, followed by a descent into a pit of physical and emotional despair, followed by her eventual escape from that pit more than two years later.
Following a fairly mild case of COVID, Ms. Caffee said worsening neurologic symptoms forced her to take medical leave from her very physical and cognitively demanding job.
Ms. Caffee said she experienced crushing fatigue and brain fog, as well as rapid heart rate and blood pressure changes going from sitting to standing position.
She went from being a competitive athlete to someone who could barely get off the couch or empty the dishwasher.
With the ongoing help of her medical team, she slowly returned to daily activities and eventually to work on a limited basis.
Today, Ms. Caffee says she’s 90%-95% better but still she has some lingering symptoms and does not yet feel like her pre-COVID self.
It’s been a very slow climb out of the pit, Ms. Caffee said.
This study has no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF CLINICAL AND TRANSLATIONAL NEUROLOGY
Researchers find a pathway to prevent COVID infection
What’s more, they have succeeded in closing the lock to block the virus and prevent it from interacting with the cell, thereby preventing infection.
UCLouvain emphasized that this discovery, which was published in Nature Communications, is sparking hope that an aerosol antiviral therapy can be developed that would eradicate the virus in the case of an infection or a high-risk contact.
For 2 years, the team under David Alsteens, PhD, a researcher at the UCLouvain Institute of Biomolecular Science and Technology, has been working hard to understand the precise molecular mechanisms the virus uses to infect a cell. They investigated the interaction between sialic acids, a kind of sugar residue present on the surface of cells, and the SARS-CoV-2 spike (S) protein to clarify its role in the infection process.
It was already known that the function of the sugar residues that coat the cells is to promote cell recognition, thus enabling, in particular, viruses to identify their targets more easily, but also to provide them with a point of attachment and to facilitate infection of the cells.
The researchers have now revealed a variant of these sugars that interacts more strongly with the S protein than other sugars do.
In other words, the university explained, they found the set of keys that allows the virus to open the cell door. So, the researchers decided to catch the virus in its own trap, by preventing it from attaching to its host cell. To do this, they blocked the S protein’s points of attachment, thus suppressing any interaction with the cell surface, as if a padlock had been placed on the lock on the cell’s entry door.
Th researchers added that the advantage of this discovery is that it acts on the virus, irrespective of mutations.
The team of researchers will now conduct tests on mice to apply this blocking of virus binding sites and observe whether it works on the body. The results should make it possible to develop an antiviral therapy administered by aerosol in the case of infection or at-risk contact.
This discovery is also of interest for the future to counter other viruses with similar attachment factors.
This article was translated from MediQuality; a version appeared on Medscape.com.
What’s more, they have succeeded in closing the lock to block the virus and prevent it from interacting with the cell, thereby preventing infection.
UCLouvain emphasized that this discovery, which was published in Nature Communications, is sparking hope that an aerosol antiviral therapy can be developed that would eradicate the virus in the case of an infection or a high-risk contact.
For 2 years, the team under David Alsteens, PhD, a researcher at the UCLouvain Institute of Biomolecular Science and Technology, has been working hard to understand the precise molecular mechanisms the virus uses to infect a cell. They investigated the interaction between sialic acids, a kind of sugar residue present on the surface of cells, and the SARS-CoV-2 spike (S) protein to clarify its role in the infection process.
It was already known that the function of the sugar residues that coat the cells is to promote cell recognition, thus enabling, in particular, viruses to identify their targets more easily, but also to provide them with a point of attachment and to facilitate infection of the cells.
The researchers have now revealed a variant of these sugars that interacts more strongly with the S protein than other sugars do.
In other words, the university explained, they found the set of keys that allows the virus to open the cell door. So, the researchers decided to catch the virus in its own trap, by preventing it from attaching to its host cell. To do this, they blocked the S protein’s points of attachment, thus suppressing any interaction with the cell surface, as if a padlock had been placed on the lock on the cell’s entry door.
Th researchers added that the advantage of this discovery is that it acts on the virus, irrespective of mutations.
The team of researchers will now conduct tests on mice to apply this blocking of virus binding sites and observe whether it works on the body. The results should make it possible to develop an antiviral therapy administered by aerosol in the case of infection or at-risk contact.
This discovery is also of interest for the future to counter other viruses with similar attachment factors.
This article was translated from MediQuality; a version appeared on Medscape.com.
What’s more, they have succeeded in closing the lock to block the virus and prevent it from interacting with the cell, thereby preventing infection.
UCLouvain emphasized that this discovery, which was published in Nature Communications, is sparking hope that an aerosol antiviral therapy can be developed that would eradicate the virus in the case of an infection or a high-risk contact.
For 2 years, the team under David Alsteens, PhD, a researcher at the UCLouvain Institute of Biomolecular Science and Technology, has been working hard to understand the precise molecular mechanisms the virus uses to infect a cell. They investigated the interaction between sialic acids, a kind of sugar residue present on the surface of cells, and the SARS-CoV-2 spike (S) protein to clarify its role in the infection process.
It was already known that the function of the sugar residues that coat the cells is to promote cell recognition, thus enabling, in particular, viruses to identify their targets more easily, but also to provide them with a point of attachment and to facilitate infection of the cells.
The researchers have now revealed a variant of these sugars that interacts more strongly with the S protein than other sugars do.
In other words, the university explained, they found the set of keys that allows the virus to open the cell door. So, the researchers decided to catch the virus in its own trap, by preventing it from attaching to its host cell. To do this, they blocked the S protein’s points of attachment, thus suppressing any interaction with the cell surface, as if a padlock had been placed on the lock on the cell’s entry door.
Th researchers added that the advantage of this discovery is that it acts on the virus, irrespective of mutations.
The team of researchers will now conduct tests on mice to apply this blocking of virus binding sites and observe whether it works on the body. The results should make it possible to develop an antiviral therapy administered by aerosol in the case of infection or at-risk contact.
This discovery is also of interest for the future to counter other viruses with similar attachment factors.
This article was translated from MediQuality; a version appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Births jump for first time since 2014
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 million live births occurred in the United States in 2021, the largest increase in the nation’s birth rate since 2014, according to the U.S. Centers for Disease Control and Prevention.
Provisional data showed a 1% uptick in births, to 3.66 million, after 6 years of dropping by approximately 2% per year. The gains were concentrated among birthing people ages 25 and older. Teenage births, on the other hand, are at their lowest level since the 1990s, according to the CDC. The agency reported a record 6% decrease in births for teenagers aged 15 to 19 years between 2020 and 2021. Women ages 20 to 25 years also had a record decrease in births of 4% during that period.
Brady E. Hamilton, PhD, of the CDC’s National Center for Health Statistics, and the lead author of the new report, said the rise in births points to childbearing that was postponed during the pandemic. Data from 2021 showed a 4% drop in the nation’s birth rate between 2019 and 2020.
“The option to forgo birth is not always viable for older women, but you saw a lot of that during the pandemic,” Dr. Hamilton said. “Events happened related to job security and the economy that caused people to wait to have a child.”
Dr. Hamilton said more data are needed to determine the full impact of increased overall birth rates on individuals. The final report, which will be released in July, will delve deeper into the influence increased birth rates had on demographics and preterm births, which Dr. Hamilton and his team found have increased by 4%.
“For those beginning to have children, we see these trends, but it will be interesting to see what happens to younger women in the future,” Dr. Hamilton said. “Once we have the final data for 2021, we will be able to see a more detailed pattern emerge and draw conclusions from that.”
Dr. Hamilton has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases keep rising past 100,000
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.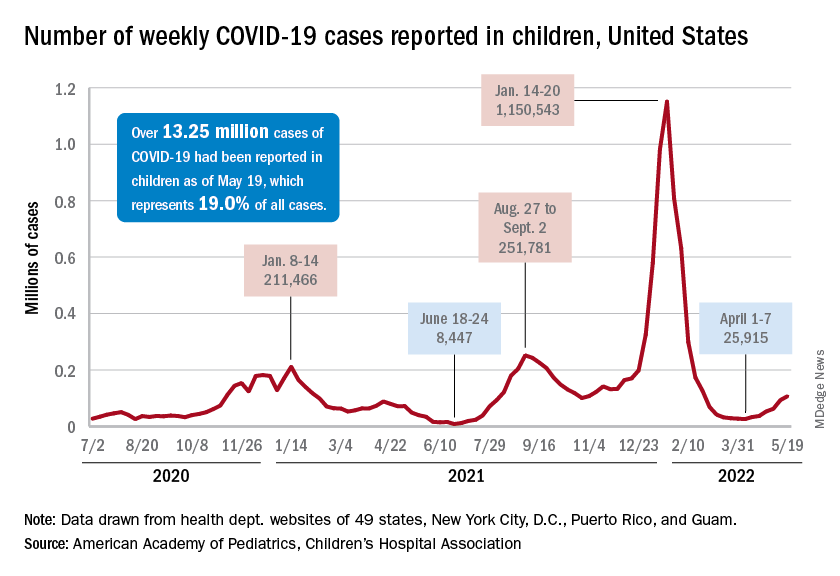
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
FDA, AMA prepare for potential COVID-19 shots for children younger than 6
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.


