User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
COVID-19 anticoagulation trials ‘paused’ for futility, safety
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
COVID-19–induced drop in first measles vaccinations sparks resurgence concerns
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
FROM PEDIATRICS
Latest rise in child COVID-19 cases is relatively small
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
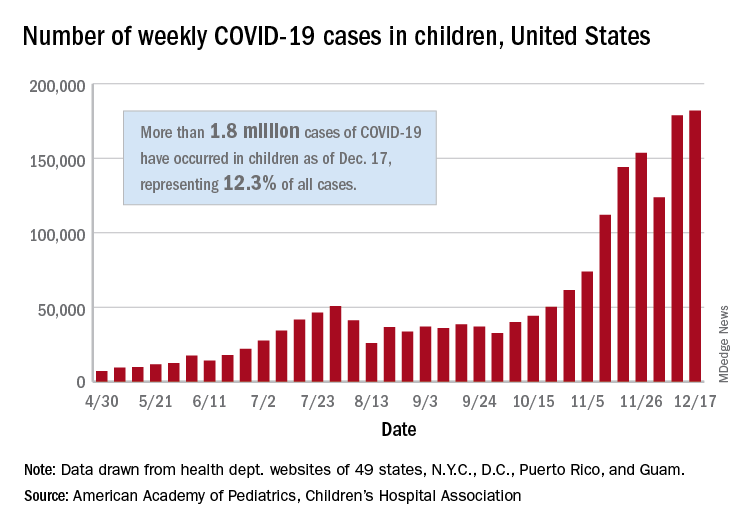
There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
Strategies for tracking SARS-CoV-2 could help detect next pandemic
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Doctors publish paper on COVID-19 protocol; Experts unconvinced
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
COVID-19 variant sparks U.K. travel restrictions
Researchers have detected a highly contagious coronavirus variant in the United Kingdom, leading Prime Minister Boris Johnson to shut down parts of the country and triggering other nations to impose travel and shipping restrictions on England.
Mr. Johnson held a crisis meeting with ministers Monday after Saturday’s shutdown announcement. The prime minister said in a nationally televised address that this coronavirus variant may be “up to 70% more transmissible than the old variant” and was probably responsible for an increase in cases in southeastern England.
“There is still much we don’t know. While we are fairly certain the variant is transmitted more quickly, there is no evidence to suggest that it is more lethal or causes more severe illness. Equally there is no evidence to suggest the vaccine will be any less effective against the new variant,” he said.
Public Health England says it is working to learn as much about the variant as possible. “We know that mortality is a lagging indicator, and we will need to continually monitor this over the coming weeks,” the agency says.
That scientific uncertainty about the variant’s threat shook European nations that were rushing to ship goods to England in advance of a Dec. 31 Brexit deadline. Under Brexit, which is short for “British exit,” the United Kingdom will leave the European Union on Jan. 31, 2020. Until then, the two sides will come up with new trade and security relationships.
European Union members Austria, Belgium, Bulgaria, France, Germany, Ireland, Italy, and the Netherlands announced travel restrictions hours after Johnson’s speech.
Those restrictions created food uncertainty across the U.K., which imports about a quarter of its food from the EU, according to The New York Times. Long lines of trucks heading to ports in the U.K. came to a standstill on major roads such as the M20 near Kent and the Port of Dover.
Outside Europe, Canada, India, Iran, Israel, Hong Kong, Saudi Arabia, and Turkey banned all incoming flights from the U.K. And more bans could come.
The U.S. reaction
The United States has not imposed any new limits on travel with the United Kingdom, although New York Gov. Andrew Cuomo (D) has requested all passengers bound for John F. Kennedy International Airport from the U.K. be tested before boarding and a new travel ban be placed for Europe. He says the federal government must take action now to avoid a crisis situation like the one New York experienced in March and April.
“The United States has a number of flights coming in from the U.K. each day, and we have done absolutely nothing,” Mr. Cuomo said in a statement on the governor’s webpage. “To me, this is reprehensible because this is what happened in the spring. How many times in life do you have to make the same mistake before you learn?”
Leading U.S. health officials have downplayed the dangers of the virus.
“We don’t know that it’s more dangerous, and very importantly, we have not seen a single mutation yet that would make it evade the vaccine,” U.S. Assistant Secretary of Health and Human Services Adm. Brett Giroir, MD, said Sunday on ABC’s This Week with George Stephanopoulos. “I can’t say that won’t happen in the future, but right now it looks like the vaccine will cover everything that we see.”
Dr. Giroir said the HHS and other U.S. government agencies will monitor the variant.
“Viruses mutate,” he said. “We’ve seen almost 4,000 different mutations among this virus. There is no indication that the mutation right now that they’re talking about is overcoming England.”
Where did the variant come from?
Public Health England says the coronavirus variant had existed in the U.K. since September and circulated at very low levels until mid-November.
“The increase in cases linked to the new variant first came to light in late November when PHE was investigating why infection rates in Kent were not falling despite national restrictions. We then discovered a cluster linked to this variant spreading rapidly into London and Essex,” the agency said.
Public Health England says there’s no evidence the new variant is resistant to the Pfizer-BioNTech vaccine, which is now being given across the country to high-priority groups such as health care workers.
An article in The BMJ, a British medical journal, says the variant was first detected by Covid-19 Genomics UK, a consortium that tests the random genetic sequencing of positive COVID-19 samples around the U.K. The variant cases were mostly in the southeast of England.
A University of Birmingham professor said in a Dec. 15 briefing that the variant accounts for 20% of viruses sequenced in Norfolk, 10% in Essex, and 3% in Suffolk. “There are no data to suggest it had been imported from abroad, so it is likely to have evolved in the U.K.,” he said.
The variant is named VUI-202012/01, for the first “variant under investigation” in December 2020, BMJ says. It’s defined by a set of 17 mutations, with the most significant mutation in the spike protein the virus uses to bind to the human ACE2 receptor.
“Changes in this part of spike protein may, in theory, result in the virus becoming more infectious and spreading more easily between people,” the article says.
The European Centre for Disease Prevention and Control says the variant emerged during the time of year when people usually socialize more.
“There is no indication at this point of increased infection severity associated with the new variant,” the agency said. “A few cases with the new variant have to date been reported by Denmark and the Netherlands and, according to media reports, in Belgium.”
Mr. Johnson announced tighter restrictions on England’s hardest-hit areas, such as the southeast and east of England, where new coronavirus cases have continued to rise. And he said people must cut back on their Christmas socializing.
“In England, those living in tier 4 areas should not mix with anyone outside their own household at Christmas, though support bubbles will remain in place for those at particular risk of loneliness or isolation,” he said.
A version of this article first appeared on WebMD.com.
Researchers have detected a highly contagious coronavirus variant in the United Kingdom, leading Prime Minister Boris Johnson to shut down parts of the country and triggering other nations to impose travel and shipping restrictions on England.
Mr. Johnson held a crisis meeting with ministers Monday after Saturday’s shutdown announcement. The prime minister said in a nationally televised address that this coronavirus variant may be “up to 70% more transmissible than the old variant” and was probably responsible for an increase in cases in southeastern England.
“There is still much we don’t know. While we are fairly certain the variant is transmitted more quickly, there is no evidence to suggest that it is more lethal or causes more severe illness. Equally there is no evidence to suggest the vaccine will be any less effective against the new variant,” he said.
Public Health England says it is working to learn as much about the variant as possible. “We know that mortality is a lagging indicator, and we will need to continually monitor this over the coming weeks,” the agency says.
That scientific uncertainty about the variant’s threat shook European nations that were rushing to ship goods to England in advance of a Dec. 31 Brexit deadline. Under Brexit, which is short for “British exit,” the United Kingdom will leave the European Union on Jan. 31, 2020. Until then, the two sides will come up with new trade and security relationships.
European Union members Austria, Belgium, Bulgaria, France, Germany, Ireland, Italy, and the Netherlands announced travel restrictions hours after Johnson’s speech.
Those restrictions created food uncertainty across the U.K., which imports about a quarter of its food from the EU, according to The New York Times. Long lines of trucks heading to ports in the U.K. came to a standstill on major roads such as the M20 near Kent and the Port of Dover.
Outside Europe, Canada, India, Iran, Israel, Hong Kong, Saudi Arabia, and Turkey banned all incoming flights from the U.K. And more bans could come.
The U.S. reaction
The United States has not imposed any new limits on travel with the United Kingdom, although New York Gov. Andrew Cuomo (D) has requested all passengers bound for John F. Kennedy International Airport from the U.K. be tested before boarding and a new travel ban be placed for Europe. He says the federal government must take action now to avoid a crisis situation like the one New York experienced in March and April.
“The United States has a number of flights coming in from the U.K. each day, and we have done absolutely nothing,” Mr. Cuomo said in a statement on the governor’s webpage. “To me, this is reprehensible because this is what happened in the spring. How many times in life do you have to make the same mistake before you learn?”
Leading U.S. health officials have downplayed the dangers of the virus.
“We don’t know that it’s more dangerous, and very importantly, we have not seen a single mutation yet that would make it evade the vaccine,” U.S. Assistant Secretary of Health and Human Services Adm. Brett Giroir, MD, said Sunday on ABC’s This Week with George Stephanopoulos. “I can’t say that won’t happen in the future, but right now it looks like the vaccine will cover everything that we see.”
Dr. Giroir said the HHS and other U.S. government agencies will monitor the variant.
“Viruses mutate,” he said. “We’ve seen almost 4,000 different mutations among this virus. There is no indication that the mutation right now that they’re talking about is overcoming England.”
Where did the variant come from?
Public Health England says the coronavirus variant had existed in the U.K. since September and circulated at very low levels until mid-November.
“The increase in cases linked to the new variant first came to light in late November when PHE was investigating why infection rates in Kent were not falling despite national restrictions. We then discovered a cluster linked to this variant spreading rapidly into London and Essex,” the agency said.
Public Health England says there’s no evidence the new variant is resistant to the Pfizer-BioNTech vaccine, which is now being given across the country to high-priority groups such as health care workers.
An article in The BMJ, a British medical journal, says the variant was first detected by Covid-19 Genomics UK, a consortium that tests the random genetic sequencing of positive COVID-19 samples around the U.K. The variant cases were mostly in the southeast of England.
A University of Birmingham professor said in a Dec. 15 briefing that the variant accounts for 20% of viruses sequenced in Norfolk, 10% in Essex, and 3% in Suffolk. “There are no data to suggest it had been imported from abroad, so it is likely to have evolved in the U.K.,” he said.
The variant is named VUI-202012/01, for the first “variant under investigation” in December 2020, BMJ says. It’s defined by a set of 17 mutations, with the most significant mutation in the spike protein the virus uses to bind to the human ACE2 receptor.
“Changes in this part of spike protein may, in theory, result in the virus becoming more infectious and spreading more easily between people,” the article says.
The European Centre for Disease Prevention and Control says the variant emerged during the time of year when people usually socialize more.
“There is no indication at this point of increased infection severity associated with the new variant,” the agency said. “A few cases with the new variant have to date been reported by Denmark and the Netherlands and, according to media reports, in Belgium.”
Mr. Johnson announced tighter restrictions on England’s hardest-hit areas, such as the southeast and east of England, where new coronavirus cases have continued to rise. And he said people must cut back on their Christmas socializing.
“In England, those living in tier 4 areas should not mix with anyone outside their own household at Christmas, though support bubbles will remain in place for those at particular risk of loneliness or isolation,” he said.
A version of this article first appeared on WebMD.com.
Researchers have detected a highly contagious coronavirus variant in the United Kingdom, leading Prime Minister Boris Johnson to shut down parts of the country and triggering other nations to impose travel and shipping restrictions on England.
Mr. Johnson held a crisis meeting with ministers Monday after Saturday’s shutdown announcement. The prime minister said in a nationally televised address that this coronavirus variant may be “up to 70% more transmissible than the old variant” and was probably responsible for an increase in cases in southeastern England.
“There is still much we don’t know. While we are fairly certain the variant is transmitted more quickly, there is no evidence to suggest that it is more lethal or causes more severe illness. Equally there is no evidence to suggest the vaccine will be any less effective against the new variant,” he said.
Public Health England says it is working to learn as much about the variant as possible. “We know that mortality is a lagging indicator, and we will need to continually monitor this over the coming weeks,” the agency says.
That scientific uncertainty about the variant’s threat shook European nations that were rushing to ship goods to England in advance of a Dec. 31 Brexit deadline. Under Brexit, which is short for “British exit,” the United Kingdom will leave the European Union on Jan. 31, 2020. Until then, the two sides will come up with new trade and security relationships.
European Union members Austria, Belgium, Bulgaria, France, Germany, Ireland, Italy, and the Netherlands announced travel restrictions hours after Johnson’s speech.
Those restrictions created food uncertainty across the U.K., which imports about a quarter of its food from the EU, according to The New York Times. Long lines of trucks heading to ports in the U.K. came to a standstill on major roads such as the M20 near Kent and the Port of Dover.
Outside Europe, Canada, India, Iran, Israel, Hong Kong, Saudi Arabia, and Turkey banned all incoming flights from the U.K. And more bans could come.
The U.S. reaction
The United States has not imposed any new limits on travel with the United Kingdom, although New York Gov. Andrew Cuomo (D) has requested all passengers bound for John F. Kennedy International Airport from the U.K. be tested before boarding and a new travel ban be placed for Europe. He says the federal government must take action now to avoid a crisis situation like the one New York experienced in March and April.
“The United States has a number of flights coming in from the U.K. each day, and we have done absolutely nothing,” Mr. Cuomo said in a statement on the governor’s webpage. “To me, this is reprehensible because this is what happened in the spring. How many times in life do you have to make the same mistake before you learn?”
Leading U.S. health officials have downplayed the dangers of the virus.
“We don’t know that it’s more dangerous, and very importantly, we have not seen a single mutation yet that would make it evade the vaccine,” U.S. Assistant Secretary of Health and Human Services Adm. Brett Giroir, MD, said Sunday on ABC’s This Week with George Stephanopoulos. “I can’t say that won’t happen in the future, but right now it looks like the vaccine will cover everything that we see.”
Dr. Giroir said the HHS and other U.S. government agencies will monitor the variant.
“Viruses mutate,” he said. “We’ve seen almost 4,000 different mutations among this virus. There is no indication that the mutation right now that they’re talking about is overcoming England.”
Where did the variant come from?
Public Health England says the coronavirus variant had existed in the U.K. since September and circulated at very low levels until mid-November.
“The increase in cases linked to the new variant first came to light in late November when PHE was investigating why infection rates in Kent were not falling despite national restrictions. We then discovered a cluster linked to this variant spreading rapidly into London and Essex,” the agency said.
Public Health England says there’s no evidence the new variant is resistant to the Pfizer-BioNTech vaccine, which is now being given across the country to high-priority groups such as health care workers.
An article in The BMJ, a British medical journal, says the variant was first detected by Covid-19 Genomics UK, a consortium that tests the random genetic sequencing of positive COVID-19 samples around the U.K. The variant cases were mostly in the southeast of England.
A University of Birmingham professor said in a Dec. 15 briefing that the variant accounts for 20% of viruses sequenced in Norfolk, 10% in Essex, and 3% in Suffolk. “There are no data to suggest it had been imported from abroad, so it is likely to have evolved in the U.K.,” he said.
The variant is named VUI-202012/01, for the first “variant under investigation” in December 2020, BMJ says. It’s defined by a set of 17 mutations, with the most significant mutation in the spike protein the virus uses to bind to the human ACE2 receptor.
“Changes in this part of spike protein may, in theory, result in the virus becoming more infectious and spreading more easily between people,” the article says.
The European Centre for Disease Prevention and Control says the variant emerged during the time of year when people usually socialize more.
“There is no indication at this point of increased infection severity associated with the new variant,” the agency said. “A few cases with the new variant have to date been reported by Denmark and the Netherlands and, according to media reports, in Belgium.”
Mr. Johnson announced tighter restrictions on England’s hardest-hit areas, such as the southeast and east of England, where new coronavirus cases have continued to rise. And he said people must cut back on their Christmas socializing.
“In England, those living in tier 4 areas should not mix with anyone outside their own household at Christmas, though support bubbles will remain in place for those at particular risk of loneliness or isolation,” he said.
A version of this article first appeared on WebMD.com.
Tier 4 lockdown in England as virus variant spreading fast
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
CDC identifies next priority groups for COVID-19 vaccine
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 ‘far more serious’ than flu, inpatient data confirm
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second COVID-19 vaccine ready for use, CDC panel says
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.



