User login
Research and Reviews for the Practicing Oncologist
EMR Tracks Quality in Palliative Care
The purpose of both the electronic medical record and research in quality of care is to guide clinicians toward better practices. At the eighth annual Chicago Supportive Oncology Conference, Dr. Susan Block and Dr. Sydney Dy discussed why measuring quality in palliative care is so important, yet so difficult to do.
The purpose of both the electronic medical record and research in quality of care is to guide clinicians toward better practices. At the eighth annual Chicago Supportive Oncology Conference, Dr. Susan Block and Dr. Sydney Dy discussed why measuring quality in palliative care is so important, yet so difficult to do.
The purpose of both the electronic medical record and research in quality of care is to guide clinicians toward better practices. At the eighth annual Chicago Supportive Oncology Conference, Dr. Susan Block and Dr. Sydney Dy discussed why measuring quality in palliative care is so important, yet so difficult to do.
Missed ASCO? Here Are The Highlights
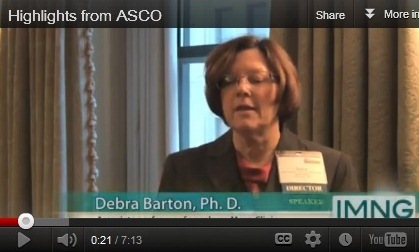
We caught up with Debra L Barton, PhD, RN at the 8th Annual Chicago Supportive Oncology Conference. We asked what she believed were the highlights from the 2012 American Society of Clinical Oncology annual meeting in the area of patient and survivor care research. Dr. Barton is involved in research at the Mayo Clinic centering on symptom management in cancer survivors. See the November/December 2012 of The Journal of Supportive Oncology for additional ASCO highlights.
We caught up with Debra L Barton, PhD, RN at the 8th Annual Chicago Supportive Oncology Conference. We asked what she believed were the highlights from the 2012 American Society of Clinical Oncology annual meeting in the area of patient and survivor care research. Dr. Barton is involved in research at the Mayo Clinic centering on symptom management in cancer survivors. See the November/December 2012 of The Journal of Supportive Oncology for additional ASCO highlights.
We caught up with Debra L Barton, PhD, RN at the 8th Annual Chicago Supportive Oncology Conference. We asked what she believed were the highlights from the 2012 American Society of Clinical Oncology annual meeting in the area of patient and survivor care research. Dr. Barton is involved in research at the Mayo Clinic centering on symptom management in cancer survivors. See the November/December 2012 of The Journal of Supportive Oncology for additional ASCO highlights.
Research Revealing Cognitive Dysfunction Causes, Treatments
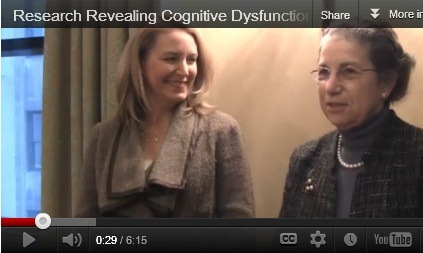
Cognitive dysfunction is common among cancer patients. However, oncologists don't yet know enough about how to treat the condition or what causes it. Dr. Patricia Ganz and Dr. Lynne Wagner discussed the most recent studies in cognitive dysfunction at the eighth annual Chicago Supportive Oncology Conference.
Cognitive dysfunction is common among cancer patients. However, oncologists don't yet know enough about how to treat the condition or what causes it. Dr. Patricia Ganz and Dr. Lynne Wagner discussed the most recent studies in cognitive dysfunction at the eighth annual Chicago Supportive Oncology Conference.
Cognitive dysfunction is common among cancer patients. However, oncologists don't yet know enough about how to treat the condition or what causes it. Dr. Patricia Ganz and Dr. Lynne Wagner discussed the most recent studies in cognitive dysfunction at the eighth annual Chicago Supportive Oncology Conference.
Lower Treatment Cost Doesn't Mean Lower Quality Care
As health care facilities across the country are finding ways to cut costs without sacrificing quality care, the Memorial Sloan-Kettering Cancer Center decided not to offer the drug ziv-aflibercept (Zaltrap) for the treatment of colorectal cancer.
At the eighth annual Chicago Supportive Oncology Conference, Dr. Thomas Smith said the center's decision could be a game-changer. Meanwhile, Dr. Anthony Back stressed the importance of talking to patients about cost of treatment.
As health care facilities across the country are finding ways to cut costs without sacrificing quality care, the Memorial Sloan-Kettering Cancer Center decided not to offer the drug ziv-aflibercept (Zaltrap) for the treatment of colorectal cancer.
At the eighth annual Chicago Supportive Oncology Conference, Dr. Thomas Smith said the center's decision could be a game-changer. Meanwhile, Dr. Anthony Back stressed the importance of talking to patients about cost of treatment.
As health care facilities across the country are finding ways to cut costs without sacrificing quality care, the Memorial Sloan-Kettering Cancer Center decided not to offer the drug ziv-aflibercept (Zaltrap) for the treatment of colorectal cancer.
At the eighth annual Chicago Supportive Oncology Conference, Dr. Thomas Smith said the center's decision could be a game-changer. Meanwhile, Dr. Anthony Back stressed the importance of talking to patients about cost of treatment.
Why do cancer patients smoke and what can providers do about it?
Despite the widespread dissemination of information about the health risks associated with smoking, many cancer patients continue to smoke, which results in a decreased quality of life, an increased probability of cancer recurrence, and a decreased survival time. Efficacious interventions are available to assist cancer patients to quit smoking, yet smoking cessation interventions are often not implemented. This review describes how clinicians, administrators, insurers, and purchasers can encourage a culture of health care in which tobacco cessation interventions are implemented consistent with evidence-based standards of care. Implementing efficacious tobacco cessation interventions can reduce morbidity and mortality among cancer patients...
*Click on the link to the left of this introduction for a PDF of the full article.
Despite the widespread dissemination of information about the health risks associated with smoking, many cancer patients continue to smoke, which results in a decreased quality of life, an increased probability of cancer recurrence, and a decreased survival time. Efficacious interventions are available to assist cancer patients to quit smoking, yet smoking cessation interventions are often not implemented. This review describes how clinicians, administrators, insurers, and purchasers can encourage a culture of health care in which tobacco cessation interventions are implemented consistent with evidence-based standards of care. Implementing efficacious tobacco cessation interventions can reduce morbidity and mortality among cancer patients...
*Click on the link to the left of this introduction for a PDF of the full article.
Despite the widespread dissemination of information about the health risks associated with smoking, many cancer patients continue to smoke, which results in a decreased quality of life, an increased probability of cancer recurrence, and a decreased survival time. Efficacious interventions are available to assist cancer patients to quit smoking, yet smoking cessation interventions are often not implemented. This review describes how clinicians, administrators, insurers, and purchasers can encourage a culture of health care in which tobacco cessation interventions are implemented consistent with evidence-based standards of care. Implementing efficacious tobacco cessation interventions can reduce morbidity and mortality among cancer patients...
*Click on the link to the left of this introduction for a PDF of the full article.
Dasatinib in the first-line treatment of chronic myeloid leukemia
Dasatinib has been approved for first-line treatment of chronic-phase chronic myeloid leukemia by the Food and Drug Administration and is recommended as a first-line treatment option by the National Comprehensive Cancer Network. Based on in vitro data, dasatinib seems to be less susceptible to the resistance mechanisms that affect imatinib. Dasatinib is an effective second-line treatment in patients who are resistant to imatinib. First-line clinical data show that dasatinib provides more rapid and deeper degrees of response than does imatinib, which may correlate with improvements in long-term patient outcome. Grade 1 or 2 cytopenias are the most common adverse events of first-line dasatinib treatment. In a phase 3 comparison with imatinib, several types of nonhematologic adverse events were less frequent in the dasatinib arm; frequencies of grade 3 and 4 events were 2%. Among patients with a minimum follow-up of 24 months, grade 1 or 2 pleural effusion was reported in 14% of dasatinib-treated patients and was manageable in almost all cases; no grade 3 or 4 pleural effusion occurred. Prompt and effective monitoring and management of dasatinib toxicities is essential to minimize intolerance and nonadherence to therapy. Patient education is important to increase the likelihood of prompt management and provide reassurance. Recommendations for patient monitoring, management, and education are provided.
*For a PDF of the full article, click on the link to the left of this introduction.
Dasatinib has been approved for first-line treatment of chronic-phase chronic myeloid leukemia by the Food and Drug Administration and is recommended as a first-line treatment option by the National Comprehensive Cancer Network. Based on in vitro data, dasatinib seems to be less susceptible to the resistance mechanisms that affect imatinib. Dasatinib is an effective second-line treatment in patients who are resistant to imatinib. First-line clinical data show that dasatinib provides more rapid and deeper degrees of response than does imatinib, which may correlate with improvements in long-term patient outcome. Grade 1 or 2 cytopenias are the most common adverse events of first-line dasatinib treatment. In a phase 3 comparison with imatinib, several types of nonhematologic adverse events were less frequent in the dasatinib arm; frequencies of grade 3 and 4 events were 2%. Among patients with a minimum follow-up of 24 months, grade 1 or 2 pleural effusion was reported in 14% of dasatinib-treated patients and was manageable in almost all cases; no grade 3 or 4 pleural effusion occurred. Prompt and effective monitoring and management of dasatinib toxicities is essential to minimize intolerance and nonadherence to therapy. Patient education is important to increase the likelihood of prompt management and provide reassurance. Recommendations for patient monitoring, management, and education are provided.
*For a PDF of the full article, click on the link to the left of this introduction.
Dasatinib has been approved for first-line treatment of chronic-phase chronic myeloid leukemia by the Food and Drug Administration and is recommended as a first-line treatment option by the National Comprehensive Cancer Network. Based on in vitro data, dasatinib seems to be less susceptible to the resistance mechanisms that affect imatinib. Dasatinib is an effective second-line treatment in patients who are resistant to imatinib. First-line clinical data show that dasatinib provides more rapid and deeper degrees of response than does imatinib, which may correlate with improvements in long-term patient outcome. Grade 1 or 2 cytopenias are the most common adverse events of first-line dasatinib treatment. In a phase 3 comparison with imatinib, several types of nonhematologic adverse events were less frequent in the dasatinib arm; frequencies of grade 3 and 4 events were 2%. Among patients with a minimum follow-up of 24 months, grade 1 or 2 pleural effusion was reported in 14% of dasatinib-treated patients and was manageable in almost all cases; no grade 3 or 4 pleural effusion occurred. Prompt and effective monitoring and management of dasatinib toxicities is essential to minimize intolerance and nonadherence to therapy. Patient education is important to increase the likelihood of prompt management and provide reassurance. Recommendations for patient monitoring, management, and education are provided.
*For a PDF of the full article, click on the link to the left of this introduction.
Community Oncology Podcast - Cetuximab and FOLFIRI in colon cancer
Cetuximab plus FOLFIRI as a first-line therapy in metastatic colon cancer, skin toxicities associated with EGFR inhibitors, and treating hypertension in cancer patients are among the highlights of this month’s podcast, featuring Dr. David Henry, editor-in-chief of Community Oncology.
Cetuximab plus FOLFIRI as a first-line therapy in metastatic colon cancer, skin toxicities associated with EGFR inhibitors, and treating hypertension in cancer patients are among the highlights of this month’s podcast, featuring Dr. David Henry, editor-in-chief of Community Oncology.
Cetuximab plus FOLFIRI as a first-line therapy in metastatic colon cancer, skin toxicities associated with EGFR inhibitors, and treating hypertension in cancer patients are among the highlights of this month’s podcast, featuring Dr. David Henry, editor-in-chief of Community Oncology.
50 Practical Medication Tips at End of Life
Patients with a life-limiting illness frequently experience pain and other symptoms. It is important to pay close attention when medication therapy is used to manage these symptoms. Occasionally, practitioners need to be creative in selecting, dosing, administering, and discontinuing medications at the end of life because of the patient’s changing health care needs.
In the video below, Dr. Kathryn Walker and Dr. Lynn McPherson of the University of Maryland discuss the role of the pharmacist in the hospital and hospice settings, as well as a few of their favorite medication tips and tricks in end-of-life care.
This article offers practical end-of-life medication tips including, but not limited to, medication administration; guidance on how to increase and decrease doses; medication selection for difficult to-treat patients; alternative dosage formulations; routes of medication administration; debridement medication regimens; and appropriate drug therapy selection. Dr. McPherson and Dr. Walker discuss how to deal with changing the goals of care for your dying patients and their families. They offer suggestions on how to integrate some helpful end-of-life medication tips into your practice.
*For a PDF of the full article, click on the link to the left of this introduction.
Patients with a life-limiting illness frequently experience pain and other symptoms. It is important to pay close attention when medication therapy is used to manage these symptoms. Occasionally, practitioners need to be creative in selecting, dosing, administering, and discontinuing medications at the end of life because of the patient’s changing health care needs.
In the video below, Dr. Kathryn Walker and Dr. Lynn McPherson of the University of Maryland discuss the role of the pharmacist in the hospital and hospice settings, as well as a few of their favorite medication tips and tricks in end-of-life care.
This article offers practical end-of-life medication tips including, but not limited to, medication administration; guidance on how to increase and decrease doses; medication selection for difficult to-treat patients; alternative dosage formulations; routes of medication administration; debridement medication regimens; and appropriate drug therapy selection. Dr. McPherson and Dr. Walker discuss how to deal with changing the goals of care for your dying patients and their families. They offer suggestions on how to integrate some helpful end-of-life medication tips into your practice.
*For a PDF of the full article, click on the link to the left of this introduction.
Patients with a life-limiting illness frequently experience pain and other symptoms. It is important to pay close attention when medication therapy is used to manage these symptoms. Occasionally, practitioners need to be creative in selecting, dosing, administering, and discontinuing medications at the end of life because of the patient’s changing health care needs.
In the video below, Dr. Kathryn Walker and Dr. Lynn McPherson of the University of Maryland discuss the role of the pharmacist in the hospital and hospice settings, as well as a few of their favorite medication tips and tricks in end-of-life care.
This article offers practical end-of-life medication tips including, but not limited to, medication administration; guidance on how to increase and decrease doses; medication selection for difficult to-treat patients; alternative dosage formulations; routes of medication administration; debridement medication regimens; and appropriate drug therapy selection. Dr. McPherson and Dr. Walker discuss how to deal with changing the goals of care for your dying patients and their families. They offer suggestions on how to integrate some helpful end-of-life medication tips into your practice.
*For a PDF of the full article, click on the link to the left of this introduction.
Cetuximab plus FOLFIRI in first-line treatment of KRAS mutation-negative, EGFR-positive metastatic colorectal cancer
In July 2012, cetuximab was approved for use in combination with FOLFIRI (irinotecan, 5-fluorouracil, leucovorin) for first-line treatment of patients with KRAS mutation-negative (wild-type), EGFR-expressing metastatic colorectal cancer (mCRC) as determined by Food and Drug Administration-approved tests. A companion diagnostic, Therascreen KRAS RGQ PCR Kit for determining KRAS mutation status was approved concurrently with the cetuximab approval. The test is a real-time polymerase chain reaction assay that detects 7 mutations of the KRAS gene; tumors with none of these mutations are considered wild-type KRAS tumors.
*For PDFs of the full article and accompanying Commentary, click on the links to the left of this introduction.
In July 2012, cetuximab was approved for use in combination with FOLFIRI (irinotecan, 5-fluorouracil, leucovorin) for first-line treatment of patients with KRAS mutation-negative (wild-type), EGFR-expressing metastatic colorectal cancer (mCRC) as determined by Food and Drug Administration-approved tests. A companion diagnostic, Therascreen KRAS RGQ PCR Kit for determining KRAS mutation status was approved concurrently with the cetuximab approval. The test is a real-time polymerase chain reaction assay that detects 7 mutations of the KRAS gene; tumors with none of these mutations are considered wild-type KRAS tumors.
*For PDFs of the full article and accompanying Commentary, click on the links to the left of this introduction.
In July 2012, cetuximab was approved for use in combination with FOLFIRI (irinotecan, 5-fluorouracil, leucovorin) for first-line treatment of patients with KRAS mutation-negative (wild-type), EGFR-expressing metastatic colorectal cancer (mCRC) as determined by Food and Drug Administration-approved tests. A companion diagnostic, Therascreen KRAS RGQ PCR Kit for determining KRAS mutation status was approved concurrently with the cetuximab approval. The test is a real-time polymerase chain reaction assay that detects 7 mutations of the KRAS gene; tumors with none of these mutations are considered wild-type KRAS tumors.
*For PDFs of the full article and accompanying Commentary, click on the links to the left of this introduction.
Management of dermatological toxicities in patients receiving EGFR inhibitors
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.
Patients receiving treatment with epidermal growth factor receptor inhibitors often experience dermatological toxicities. The majority of patients develop skin rash, and may also experience adverse nail and periungual alterations. EGFR inhibitors have become part of the standard of care for several solid tumors, including metastatic colorectal cancer, cancers of the head and neck, and non small-cell lung cancer, thus adequate management of these side effects is necessary to ensure patient compliance to therapy, as well as to maximize patient comfort and quality of life. This review presents a protocol our center optimized to successfully manage cetuximab-associated acneiform rash and nail toxicities.
Click on the PDF icon at the top of this introduction to read the full article.