User login
Aspirin, Vitamin D Levels Protect Against Recurrent CRC
CHICAGO – Both aspirin and higher plasma levels of vitamin D appear to be modestly effective in secondary prevention of colorectal cancer, investigators in a large cohort study and a randomized trial report.
Among more than 25,000 Norwegians with colorectal cancer, aspirin use was associated with a 14% improvement in overall survival, and a 25% improvement in colorectal cancer–specific survival, reported Dr. Simer Bains from the Center for Molecular Medicine Norway at the University of Oslo.
A similar protective effect of plasma 25-hydroxy vitamin D (25[OH]D) was seen in a subanalysis of data from the CALGB/SWOG 80405 trial, which showed that patients in the highest quintile had a significantly longer overall survival, compared with patients in the lowest quintile of 25(OH)D. Whether dietary vitamin D supplementation will have the same effect is unknown, however, said Dr. Kimmie Ng of the Dana-Farber Cancer Institute, Boston, at the annual meeting of the American Society of Clinical Oncology.
“If confirmed, these are very important potential interventions as they are low-cost, over-the-counter options that could have substantial implications for treatment of colon cancer patients, commented Dr. Andrew T. Chan of Massachusetts General Hospital, the invited discussant.
Aspirin study
The benefits of aspirin in primary prevention of colorectal and other cancers have been well documented, but the role of the “wonder drug” in secondary prevention is unclear, Dr. Bains said.
To see whether the use of aspirin after a colorectal cancer diagnosis could make a difference, she and colleagues drew on Norway’s birth-to-death national medical and prescription databases to identify a retrospective cohort of 25,644 patients with colorectal cancer diagnosed from 2004 to 2011. Of this group 6,109 had documented aspirin exposure, defined as a prescription for more than 6 months of aspirin after a diagnosis of colorectal cancer, and 19,535 did not have documented aspirin exposure.
The aspirin user group had a higher mean age than nonusers (74 vs. 70 years), had slightly more men than women (56% vs. 48% men among nonusers), had a higher proportion of well- or moderately differentiated tumors, and less advanced disease stage at diagnosis.
After 9 years of follow-up, there was no difference between the groups in overall survival on univariate analysis. Suspecting that the lack of effect might be attributable to the aspirin users being an older and potentially more fragile group with more comorbidities than nonusers, the authors performed a multivariate regression analysis controlling for age, gender, tumor stage, differentiation, and other drug use and found a hazard ratio for death with aspirin use of 0.86 (P < .001).
In both univariate and multivariate analysis, aspirin use was associated with improved colorectal cancer–specific survival, with HRs of 0.84 and 0.75, respectively (P < .001 for both).
Dr. Bains acknowledged that the study was limited by the lack of randomization and inability to control for over-the-counter aspirin use.
Vitamin D
In a different study, Dr. Ng and colleagues looked at the relationship between plasma 25(OH)D levels in patients with metastatic colorectal cancer enrolled in CALGB/SWOG 80405, which compared the FOLFIRI and FOLFOX regimens plus either cetuximab (Erbitux), bevacizumab (Avastin), or both.
Plasma 25(OH)D levels were measured at baseline by radioimmunoassay before the start of therapy.
The investigators found that among patients in the highest quintile of plasma vitamin D levels, median overall survival was 32.6 months, compared with 24.5 months for patients in the lowest quintile (P = .01).
In a multivariate analysis adjusted for age, sex, race, performance status, chemotherapy backbone regimen, previous adjuvant therapy, RAS mutation status, season of blood draw, region of residency, body mass index and physical activity, the highest levels of 25(OH)D were associated with a 35% improvement in overall survival, compared with the lowest levels (HR 0.65, P for trend = .001).
The investigators found that the association between vitamin D levels and survival persisted across all patient subgroups both before and after adjustment for prognostic factors.
A phase II randomized trial of vitamin D supplementation in patients undergoing adjuvant chemotherapy is currently underway, Dr. Ng noted.
Dr. Chan, the discussant, noted that “vitamin D and aspirin use are among the lifestyle factors most consistently associated with improved outcomes among patients with colorectal cancer in epidemiological studies,” and that “the findings are supported by consistent evidence and biological plausibility.”
The aspirin study was supported by the Research Council of Norway. CALGB/SWOG 80405 was supported by the National Cancer Institute, Southwest Oncology Group, Bristol-Myers Squibb, and Aptuit Inc. Dr. Bains reported no relevant disclosures. Dr Ng reported a consulting or advisory role with several companies, institutional research funding from Genentech/Roche and Pharmaville, and travel expenses from Gilead Sciences. Dr. Chan reported a consulting/advisory role with Bayer Schering Pharma and Pfizer.
CHICAGO – Both aspirin and higher plasma levels of vitamin D appear to be modestly effective in secondary prevention of colorectal cancer, investigators in a large cohort study and a randomized trial report.
Among more than 25,000 Norwegians with colorectal cancer, aspirin use was associated with a 14% improvement in overall survival, and a 25% improvement in colorectal cancer–specific survival, reported Dr. Simer Bains from the Center for Molecular Medicine Norway at the University of Oslo.
A similar protective effect of plasma 25-hydroxy vitamin D (25[OH]D) was seen in a subanalysis of data from the CALGB/SWOG 80405 trial, which showed that patients in the highest quintile had a significantly longer overall survival, compared with patients in the lowest quintile of 25(OH)D. Whether dietary vitamin D supplementation will have the same effect is unknown, however, said Dr. Kimmie Ng of the Dana-Farber Cancer Institute, Boston, at the annual meeting of the American Society of Clinical Oncology.
“If confirmed, these are very important potential interventions as they are low-cost, over-the-counter options that could have substantial implications for treatment of colon cancer patients, commented Dr. Andrew T. Chan of Massachusetts General Hospital, the invited discussant.
Aspirin study
The benefits of aspirin in primary prevention of colorectal and other cancers have been well documented, but the role of the “wonder drug” in secondary prevention is unclear, Dr. Bains said.
To see whether the use of aspirin after a colorectal cancer diagnosis could make a difference, she and colleagues drew on Norway’s birth-to-death national medical and prescription databases to identify a retrospective cohort of 25,644 patients with colorectal cancer diagnosed from 2004 to 2011. Of this group 6,109 had documented aspirin exposure, defined as a prescription for more than 6 months of aspirin after a diagnosis of colorectal cancer, and 19,535 did not have documented aspirin exposure.
The aspirin user group had a higher mean age than nonusers (74 vs. 70 years), had slightly more men than women (56% vs. 48% men among nonusers), had a higher proportion of well- or moderately differentiated tumors, and less advanced disease stage at diagnosis.
After 9 years of follow-up, there was no difference between the groups in overall survival on univariate analysis. Suspecting that the lack of effect might be attributable to the aspirin users being an older and potentially more fragile group with more comorbidities than nonusers, the authors performed a multivariate regression analysis controlling for age, gender, tumor stage, differentiation, and other drug use and found a hazard ratio for death with aspirin use of 0.86 (P < .001).
In both univariate and multivariate analysis, aspirin use was associated with improved colorectal cancer–specific survival, with HRs of 0.84 and 0.75, respectively (P < .001 for both).
Dr. Bains acknowledged that the study was limited by the lack of randomization and inability to control for over-the-counter aspirin use.
Vitamin D
In a different study, Dr. Ng and colleagues looked at the relationship between plasma 25(OH)D levels in patients with metastatic colorectal cancer enrolled in CALGB/SWOG 80405, which compared the FOLFIRI and FOLFOX regimens plus either cetuximab (Erbitux), bevacizumab (Avastin), or both.
Plasma 25(OH)D levels were measured at baseline by radioimmunoassay before the start of therapy.
The investigators found that among patients in the highest quintile of plasma vitamin D levels, median overall survival was 32.6 months, compared with 24.5 months for patients in the lowest quintile (P = .01).
In a multivariate analysis adjusted for age, sex, race, performance status, chemotherapy backbone regimen, previous adjuvant therapy, RAS mutation status, season of blood draw, region of residency, body mass index and physical activity, the highest levels of 25(OH)D were associated with a 35% improvement in overall survival, compared with the lowest levels (HR 0.65, P for trend = .001).
The investigators found that the association between vitamin D levels and survival persisted across all patient subgroups both before and after adjustment for prognostic factors.
A phase II randomized trial of vitamin D supplementation in patients undergoing adjuvant chemotherapy is currently underway, Dr. Ng noted.
Dr. Chan, the discussant, noted that “vitamin D and aspirin use are among the lifestyle factors most consistently associated with improved outcomes among patients with colorectal cancer in epidemiological studies,” and that “the findings are supported by consistent evidence and biological plausibility.”
The aspirin study was supported by the Research Council of Norway. CALGB/SWOG 80405 was supported by the National Cancer Institute, Southwest Oncology Group, Bristol-Myers Squibb, and Aptuit Inc. Dr. Bains reported no relevant disclosures. Dr Ng reported a consulting or advisory role with several companies, institutional research funding from Genentech/Roche and Pharmaville, and travel expenses from Gilead Sciences. Dr. Chan reported a consulting/advisory role with Bayer Schering Pharma and Pfizer.
CHICAGO – Both aspirin and higher plasma levels of vitamin D appear to be modestly effective in secondary prevention of colorectal cancer, investigators in a large cohort study and a randomized trial report.
Among more than 25,000 Norwegians with colorectal cancer, aspirin use was associated with a 14% improvement in overall survival, and a 25% improvement in colorectal cancer–specific survival, reported Dr. Simer Bains from the Center for Molecular Medicine Norway at the University of Oslo.
A similar protective effect of plasma 25-hydroxy vitamin D (25[OH]D) was seen in a subanalysis of data from the CALGB/SWOG 80405 trial, which showed that patients in the highest quintile had a significantly longer overall survival, compared with patients in the lowest quintile of 25(OH)D. Whether dietary vitamin D supplementation will have the same effect is unknown, however, said Dr. Kimmie Ng of the Dana-Farber Cancer Institute, Boston, at the annual meeting of the American Society of Clinical Oncology.
“If confirmed, these are very important potential interventions as they are low-cost, over-the-counter options that could have substantial implications for treatment of colon cancer patients, commented Dr. Andrew T. Chan of Massachusetts General Hospital, the invited discussant.
Aspirin study
The benefits of aspirin in primary prevention of colorectal and other cancers have been well documented, but the role of the “wonder drug” in secondary prevention is unclear, Dr. Bains said.
To see whether the use of aspirin after a colorectal cancer diagnosis could make a difference, she and colleagues drew on Norway’s birth-to-death national medical and prescription databases to identify a retrospective cohort of 25,644 patients with colorectal cancer diagnosed from 2004 to 2011. Of this group 6,109 had documented aspirin exposure, defined as a prescription for more than 6 months of aspirin after a diagnosis of colorectal cancer, and 19,535 did not have documented aspirin exposure.
The aspirin user group had a higher mean age than nonusers (74 vs. 70 years), had slightly more men than women (56% vs. 48% men among nonusers), had a higher proportion of well- or moderately differentiated tumors, and less advanced disease stage at diagnosis.
After 9 years of follow-up, there was no difference between the groups in overall survival on univariate analysis. Suspecting that the lack of effect might be attributable to the aspirin users being an older and potentially more fragile group with more comorbidities than nonusers, the authors performed a multivariate regression analysis controlling for age, gender, tumor stage, differentiation, and other drug use and found a hazard ratio for death with aspirin use of 0.86 (P < .001).
In both univariate and multivariate analysis, aspirin use was associated with improved colorectal cancer–specific survival, with HRs of 0.84 and 0.75, respectively (P < .001 for both).
Dr. Bains acknowledged that the study was limited by the lack of randomization and inability to control for over-the-counter aspirin use.
Vitamin D
In a different study, Dr. Ng and colleagues looked at the relationship between plasma 25(OH)D levels in patients with metastatic colorectal cancer enrolled in CALGB/SWOG 80405, which compared the FOLFIRI and FOLFOX regimens plus either cetuximab (Erbitux), bevacizumab (Avastin), or both.
Plasma 25(OH)D levels were measured at baseline by radioimmunoassay before the start of therapy.
The investigators found that among patients in the highest quintile of plasma vitamin D levels, median overall survival was 32.6 months, compared with 24.5 months for patients in the lowest quintile (P = .01).
In a multivariate analysis adjusted for age, sex, race, performance status, chemotherapy backbone regimen, previous adjuvant therapy, RAS mutation status, season of blood draw, region of residency, body mass index and physical activity, the highest levels of 25(OH)D were associated with a 35% improvement in overall survival, compared with the lowest levels (HR 0.65, P for trend = .001).
The investigators found that the association between vitamin D levels and survival persisted across all patient subgroups both before and after adjustment for prognostic factors.
A phase II randomized trial of vitamin D supplementation in patients undergoing adjuvant chemotherapy is currently underway, Dr. Ng noted.
Dr. Chan, the discussant, noted that “vitamin D and aspirin use are among the lifestyle factors most consistently associated with improved outcomes among patients with colorectal cancer in epidemiological studies,” and that “the findings are supported by consistent evidence and biological plausibility.”
The aspirin study was supported by the Research Council of Norway. CALGB/SWOG 80405 was supported by the National Cancer Institute, Southwest Oncology Group, Bristol-Myers Squibb, and Aptuit Inc. Dr. Bains reported no relevant disclosures. Dr Ng reported a consulting or advisory role with several companies, institutional research funding from Genentech/Roche and Pharmaville, and travel expenses from Gilead Sciences. Dr. Chan reported a consulting/advisory role with Bayer Schering Pharma and Pfizer.
AT THE 2015 ASCO ANNUAL MEETING
Aspirin, vitamin D levels protect against recurrent CRC
CHICAGO – Both aspirin and higher plasma levels of vitamin D appear to be modestly effective in secondary prevention of colorectal cancer, investigators in a large cohort study and a randomized trial report.
Among more than 25,000 Norwegians with colorectal cancer, aspirin use was associated with a 14% improvement in overall survival, and a 25% improvement in colorectal cancer–specific survival, reported Dr. Simer Bains from the Center for Molecular Medicine Norway at the University of Oslo.
A similar protective effect of plasma 25-hydroxy vitamin D (25[OH]D) was seen in a subanalysis of data from the CALGB/SWOG 80405 trial, which showed that patients in the highest quintile had a significantly longer overall survival, compared with patients in the lowest quintile of 25(OH)D. Whether dietary vitamin D supplementation will have the same effect is unknown, however, said Dr. Kimmie Ng of the Dana-Farber Cancer Institute, Boston, at the annual meeting of the American Society of Clinical Oncology.
“If confirmed, these are very important potential interventions as they are low-cost, over-the-counter options that could have substantial implications for treatment of colon cancer patients, commented Dr. Andrew T. Chan of Massachusetts General Hospital, the invited discussant.
Aspirin study
The benefits of aspirin in primary prevention of colorectal and other cancers have been well documented, but the role of the “wonder drug” in secondary prevention is unclear, Dr. Bains said.
To see whether the use of aspirin after a colorectal cancer diagnosis could make a difference, she and colleagues drew on Norway’s birth-to-death national medical and prescription databases to identify a retrospective cohort of 25,644 patients with colorectal cancer diagnosed from 2004 to 2011. Of this group 6,109 had documented aspirin exposure, defined as a prescription for more than 6 months of aspirin after a diagnosis of colorectal cancer, and 19,535 did not have documented aspirin exposure.
The aspirin user group had a higher mean age than nonusers (74 vs. 70 years), had slightly more men than women (56% vs. 48% men among nonusers), had a higher proportion of well- or moderately differentiated tumors, and less advanced disease stage at diagnosis.
After 9 years of follow-up, there was no difference between the groups in overall survival on univariate analysis. Suspecting that the lack of effect might be attributable to the aspirin users being an older and potentially more fragile group with more comorbidities than nonusers, the authors performed a multivariate regression analysis controlling for age, gender, tumor stage, differentiation, and other drug use and found a hazard ratio for death with aspirin use of 0.86 (P < .001).
In both univariate and multivariate analysis, aspirin use was associated with improved colorectal cancer–specific survival, with HRs of 0.84 and 0.75, respectively (P < .001 for both).
Dr. Bains acknowledged that the study was limited by the lack of randomization and inability to control for over-the-counter aspirin use.
Vitamin D
In a different study, Dr. Ng and colleagues looked at the relationship between plasma 25(OH)D levels in patients with metastatic colorectal cancer enrolled in CALGB/SWOG 80405, which compared the FOLFIRI and FOLFOX regimens plus either cetuximab (Erbitux), bevacizumab (Avastin), or both.
Plasma 25(OH)D levels were measured at baseline by radioimmunoassay before the start of therapy.
The investigators found that among patients in the highest quintile of plasma vitamin D levels, median overall survival was 32.6 months, compared with 24.5 months for patients in the lowest quintile (P = .01).
In a multivariate analysis adjusted for age, sex, race, performance status, chemotherapy backbone regimen, previous adjuvant therapy, RAS mutation status, season of blood draw, region of residency, body mass index and physical activity, the highest levels of 25(OH)D were associated with a 35% improvement in overall survival, compared with the lowest levels (HR 0.65, P for trend = .001).
The investigators found that the association between vitamin D levels and survival persisted across all patient subgroups both before and after adjustment for prognostic factors.
A phase II randomized trial of vitamin D supplementation in patients undergoing adjuvant chemotherapy is currently underway, Dr. Ng noted.
Dr. Chan, the discussant, noted that “vitamin D and aspirin use are among the lifestyle factors most consistently associated with improved outcomes among patients with colorectal cancer in epidemiological studies,” and that “the findings are supported by consistent evidence and biological plausibility.”
The aspirin study was supported by the Research Council of Norway. CALGB/SWOG 80405 was supported by the National Cancer Institute, Southwest Oncology Group, Bristol-Myers Squibb, and Aptuit Inc. Dr. Bains reported no relevant disclosures. Dr Ng reported a consulting or advisory role with several companies, institutional research funding from Genentech/Roche and Pharmaville, and travel expenses from Gilead Sciences. Dr. Chan reported a consulting/advisory role with Bayer Schering Pharma and Pfizer.
CHICAGO – Both aspirin and higher plasma levels of vitamin D appear to be modestly effective in secondary prevention of colorectal cancer, investigators in a large cohort study and a randomized trial report.
Among more than 25,000 Norwegians with colorectal cancer, aspirin use was associated with a 14% improvement in overall survival, and a 25% improvement in colorectal cancer–specific survival, reported Dr. Simer Bains from the Center for Molecular Medicine Norway at the University of Oslo.
A similar protective effect of plasma 25-hydroxy vitamin D (25[OH]D) was seen in a subanalysis of data from the CALGB/SWOG 80405 trial, which showed that patients in the highest quintile had a significantly longer overall survival, compared with patients in the lowest quintile of 25(OH)D. Whether dietary vitamin D supplementation will have the same effect is unknown, however, said Dr. Kimmie Ng of the Dana-Farber Cancer Institute, Boston, at the annual meeting of the American Society of Clinical Oncology.
“If confirmed, these are very important potential interventions as they are low-cost, over-the-counter options that could have substantial implications for treatment of colon cancer patients, commented Dr. Andrew T. Chan of Massachusetts General Hospital, the invited discussant.
Aspirin study
The benefits of aspirin in primary prevention of colorectal and other cancers have been well documented, but the role of the “wonder drug” in secondary prevention is unclear, Dr. Bains said.
To see whether the use of aspirin after a colorectal cancer diagnosis could make a difference, she and colleagues drew on Norway’s birth-to-death national medical and prescription databases to identify a retrospective cohort of 25,644 patients with colorectal cancer diagnosed from 2004 to 2011. Of this group 6,109 had documented aspirin exposure, defined as a prescription for more than 6 months of aspirin after a diagnosis of colorectal cancer, and 19,535 did not have documented aspirin exposure.
The aspirin user group had a higher mean age than nonusers (74 vs. 70 years), had slightly more men than women (56% vs. 48% men among nonusers), had a higher proportion of well- or moderately differentiated tumors, and less advanced disease stage at diagnosis.
After 9 years of follow-up, there was no difference between the groups in overall survival on univariate analysis. Suspecting that the lack of effect might be attributable to the aspirin users being an older and potentially more fragile group with more comorbidities than nonusers, the authors performed a multivariate regression analysis controlling for age, gender, tumor stage, differentiation, and other drug use and found a hazard ratio for death with aspirin use of 0.86 (P < .001).
In both univariate and multivariate analysis, aspirin use was associated with improved colorectal cancer–specific survival, with HRs of 0.84 and 0.75, respectively (P < .001 for both).
Dr. Bains acknowledged that the study was limited by the lack of randomization and inability to control for over-the-counter aspirin use.
Vitamin D
In a different study, Dr. Ng and colleagues looked at the relationship between plasma 25(OH)D levels in patients with metastatic colorectal cancer enrolled in CALGB/SWOG 80405, which compared the FOLFIRI and FOLFOX regimens plus either cetuximab (Erbitux), bevacizumab (Avastin), or both.
Plasma 25(OH)D levels were measured at baseline by radioimmunoassay before the start of therapy.
The investigators found that among patients in the highest quintile of plasma vitamin D levels, median overall survival was 32.6 months, compared with 24.5 months for patients in the lowest quintile (P = .01).
In a multivariate analysis adjusted for age, sex, race, performance status, chemotherapy backbone regimen, previous adjuvant therapy, RAS mutation status, season of blood draw, region of residency, body mass index and physical activity, the highest levels of 25(OH)D were associated with a 35% improvement in overall survival, compared with the lowest levels (HR 0.65, P for trend = .001).
The investigators found that the association between vitamin D levels and survival persisted across all patient subgroups both before and after adjustment for prognostic factors.
A phase II randomized trial of vitamin D supplementation in patients undergoing adjuvant chemotherapy is currently underway, Dr. Ng noted.
Dr. Chan, the discussant, noted that “vitamin D and aspirin use are among the lifestyle factors most consistently associated with improved outcomes among patients with colorectal cancer in epidemiological studies,” and that “the findings are supported by consistent evidence and biological plausibility.”
The aspirin study was supported by the Research Council of Norway. CALGB/SWOG 80405 was supported by the National Cancer Institute, Southwest Oncology Group, Bristol-Myers Squibb, and Aptuit Inc. Dr. Bains reported no relevant disclosures. Dr Ng reported a consulting or advisory role with several companies, institutional research funding from Genentech/Roche and Pharmaville, and travel expenses from Gilead Sciences. Dr. Chan reported a consulting/advisory role with Bayer Schering Pharma and Pfizer.
CHICAGO – Both aspirin and higher plasma levels of vitamin D appear to be modestly effective in secondary prevention of colorectal cancer, investigators in a large cohort study and a randomized trial report.
Among more than 25,000 Norwegians with colorectal cancer, aspirin use was associated with a 14% improvement in overall survival, and a 25% improvement in colorectal cancer–specific survival, reported Dr. Simer Bains from the Center for Molecular Medicine Norway at the University of Oslo.
A similar protective effect of plasma 25-hydroxy vitamin D (25[OH]D) was seen in a subanalysis of data from the CALGB/SWOG 80405 trial, which showed that patients in the highest quintile had a significantly longer overall survival, compared with patients in the lowest quintile of 25(OH)D. Whether dietary vitamin D supplementation will have the same effect is unknown, however, said Dr. Kimmie Ng of the Dana-Farber Cancer Institute, Boston, at the annual meeting of the American Society of Clinical Oncology.
“If confirmed, these are very important potential interventions as they are low-cost, over-the-counter options that could have substantial implications for treatment of colon cancer patients, commented Dr. Andrew T. Chan of Massachusetts General Hospital, the invited discussant.
Aspirin study
The benefits of aspirin in primary prevention of colorectal and other cancers have been well documented, but the role of the “wonder drug” in secondary prevention is unclear, Dr. Bains said.
To see whether the use of aspirin after a colorectal cancer diagnosis could make a difference, she and colleagues drew on Norway’s birth-to-death national medical and prescription databases to identify a retrospective cohort of 25,644 patients with colorectal cancer diagnosed from 2004 to 2011. Of this group 6,109 had documented aspirin exposure, defined as a prescription for more than 6 months of aspirin after a diagnosis of colorectal cancer, and 19,535 did not have documented aspirin exposure.
The aspirin user group had a higher mean age than nonusers (74 vs. 70 years), had slightly more men than women (56% vs. 48% men among nonusers), had a higher proportion of well- or moderately differentiated tumors, and less advanced disease stage at diagnosis.
After 9 years of follow-up, there was no difference between the groups in overall survival on univariate analysis. Suspecting that the lack of effect might be attributable to the aspirin users being an older and potentially more fragile group with more comorbidities than nonusers, the authors performed a multivariate regression analysis controlling for age, gender, tumor stage, differentiation, and other drug use and found a hazard ratio for death with aspirin use of 0.86 (P < .001).
In both univariate and multivariate analysis, aspirin use was associated with improved colorectal cancer–specific survival, with HRs of 0.84 and 0.75, respectively (P < .001 for both).
Dr. Bains acknowledged that the study was limited by the lack of randomization and inability to control for over-the-counter aspirin use.
Vitamin D
In a different study, Dr. Ng and colleagues looked at the relationship between plasma 25(OH)D levels in patients with metastatic colorectal cancer enrolled in CALGB/SWOG 80405, which compared the FOLFIRI and FOLFOX regimens plus either cetuximab (Erbitux), bevacizumab (Avastin), or both.
Plasma 25(OH)D levels were measured at baseline by radioimmunoassay before the start of therapy.
The investigators found that among patients in the highest quintile of plasma vitamin D levels, median overall survival was 32.6 months, compared with 24.5 months for patients in the lowest quintile (P = .01).
In a multivariate analysis adjusted for age, sex, race, performance status, chemotherapy backbone regimen, previous adjuvant therapy, RAS mutation status, season of blood draw, region of residency, body mass index and physical activity, the highest levels of 25(OH)D were associated with a 35% improvement in overall survival, compared with the lowest levels (HR 0.65, P for trend = .001).
The investigators found that the association between vitamin D levels and survival persisted across all patient subgroups both before and after adjustment for prognostic factors.
A phase II randomized trial of vitamin D supplementation in patients undergoing adjuvant chemotherapy is currently underway, Dr. Ng noted.
Dr. Chan, the discussant, noted that “vitamin D and aspirin use are among the lifestyle factors most consistently associated with improved outcomes among patients with colorectal cancer in epidemiological studies,” and that “the findings are supported by consistent evidence and biological plausibility.”
The aspirin study was supported by the Research Council of Norway. CALGB/SWOG 80405 was supported by the National Cancer Institute, Southwest Oncology Group, Bristol-Myers Squibb, and Aptuit Inc. Dr. Bains reported no relevant disclosures. Dr Ng reported a consulting or advisory role with several companies, institutional research funding from Genentech/Roche and Pharmaville, and travel expenses from Gilead Sciences. Dr. Chan reported a consulting/advisory role with Bayer Schering Pharma and Pfizer.
AT THE 2015 ASCO ANNUAL MEETING
Key clinical point: Aspirin and plasma vitamin D appear to offer survival advantages for patients with colorectal cancer.
Major finding: In a multivariable analysis aspirin use after diagnosis was associated with improved overall survival (HR = 0.86) and colorectal cancer–specific survival (HR = .75). Highest vitamin D levels were associated with a 35% reduction in risk of death vs. lowest levels.
Data source: Retrospective cohort study of 25,644 patients (aspirin). Randomized controlled trial with 2,334 patients (vitamin D).
Disclosures: The aspirin study was supported by the Research Council of Norway. CALGB/SWOG 80405 was supported by the National Cancer Institute, Southwest Oncology Group, Bristol-Myers Squibb, and Aptuit Inc. Dr. Bains reported no relevant disclosures. Dr Ng reported a consulting or advisory role with several companies, institutional research funding from Genentech/Roche and Pharmaville, and travel expenses from Gilead Sciences. Dr. Chan reported a consulting/advisory role with Bayer Schering Pharma and Pfizer.
Vemurafenib After Ipilimumab Linked to Rash
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
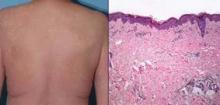
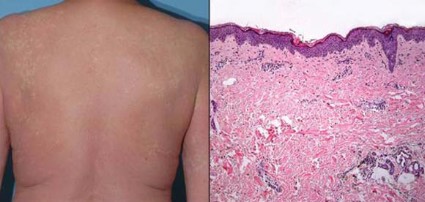
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
CHICAGO – The sequencing and timing of two new targeted therapies for melanoma may have important implications for the development of serious skin toxicity, according to one center’s experience.
Investigators at Memorial Sloan-Kettering Cancer Center in New York retrospectively identified 16 patients treated there for BRAFV600E-mutant metastatic melanoma who received vemurafenib (Zelboraf) after ipilimumab (Yervoy).
Vemurafenib is an inhibitor of the BRAF kinase. Ipilimumab blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA 4), which normally acts as a key checkpoint or brake in the immune system.
Four of the patients (25%) developed a grade 3 maculopapular rash, according to data reported in a poster session at the annual meeting of the American Society of Clinical Oncology.
Biopsy findings suggested these were drug hypersensitivity reactions, and analyses showed that grade 3 rash was much more likely when vemurafenib was given within 1 month of stopping ipilimumab as compared with later (100% vs. 8%, P = .007).
"It’s interesting to speculate that loss of checkpoint inhibition by ipilimumab might predispose patients to drug reactions," lead investigator Dr. James J. Harding commented in an interview, while cautioning that the study was very small and retrospective.
"The take-home message is these agents, both of which improve overall survival, will be used in sequence. It’s not clear if there is a benefit of sequencing one before the other or combining them – that will be studied prospectively," he noted, as in the case of an ongoing phase I-II trial looking at the two drugs together (NCT01400451).
"Until more data are available, it’s possible that there may be a significant maculopapular rash if you give vemurafenib within a month of ipilimumab. In almost all cases, a dose interruption followed by dose reduction is acceptable," he added.
"One thing that people need to remember is that if you give vemurafenib after ipilimumab, you are giving a combination therapy because the ipilimumab half-life is 2 weeks," noted discussant Dr. Mario Sznol, vice-chief of medical oncology with the Yale Medical Group in New Haven, CT.
"I would have hoped that we would have seen really dramatic antitumor effects with this combination, especially in the patients who were treated soon after their last dose of ipilimumab. And in fact that’s not what we saw," he added. "I don’t think this curve [waterfall plot] looks much better than what we would have seen with vemurafenib alone in this population of patients," with no apparent difference for patients receiving vemurafenib within 45 days of ipilimumab and the rest.
"So it’s just a warning that there will be sequence issues and toxicity interactions, and we really need to know the biology when we combine these agents," Dr. Sznol concluded. "We may do better with this combination, but we may not. We may need to use this in combination with other agents."
Of the 16 patients studied, 13 (81%) developed any-grade skin rash on vemurafenib, making this by far the most common adverse event observed. (For comparison, the rate of skin rash with vemurafenib was 37% in the BRIM-3 trial and 52% in the BRIM-2 trial.)
The cases of grade 3 rash developed within 6-8 days of starting vemurafenib and began as a pruritic eruption on the neck or chest that rapidly expanded to involve the back, trunk, and extremities. The incidence seen was triple that in the BRIM-3 trial (25% vs. 8%, P = .02).
Biopsies, performed in two of the four patients, revealed spongiotic and perivascular dermatitis with eosinophils, consistent with drug hypersensitivity reaction.
Although the time elapsed since the prior ipilimumab influenced the development of grade 3 rash, the dose of prior ipilimumab, number of doses, and immune-related adverse events did not.
None of the rashes progressed to anaphylaxis or Stevens-Johnson syndrome. Steroids appeared to be largely ineffective, according to Dr. Harding; one patient developed the rash while already taking steroids, and another was given steroids with little to no improvement.
"We essentially stopped the vemurafenib and then redosed it 11 days later [after the rash resolved]. And, with the exception of one patient, all of the patients tolerated it well and were able to continue," he reported.
The objective overall response rate with vemurafenib was 50%, similar to what was seen in the prior phase II and III trials of the drug.
Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Four patients (25%) developed grade 3 maculopapular skin rash that histologically had features of a drug hypersensitivity rash.
Data Source: A single-center retrospective case series of 16 patients with BRAFV600E-mutant metastatic melanoma treated with vemurafenib after ipilimumab
Disclosures: Dr. Harding disclosed no relevant conflicts of interest. Dr. Sznol disclosed that he is a consultant to Abbott Laboratories, Anaeropharma Science, BioVex, Bristol-Myers Squibb, Genesis Biopharma, Genzyme, and Prometheus; receives honoraria from Prometheus; and receives research funding from Bristol-Myers Squibb.
Acetyl-l-Carnitine Yields Mixed Results for Chemo-Induced Neuropathy
CHICAGO – The impact of acetyl-l-carnitine on chemotherapy-induced peripheral neuropathy may depend largely on the clinical context and patient population, a pair of phase III trials suggests.
Acetyl-l-carnitine (ALC), a natural substance marketed over the counter as a dietary supplement, is popular among cancer patients as a result of preclinical and early-phase data in chemotherapy-related neuropathy and also a study in patients with diabetes-related peripheral neuropathy.
But in a trial among 409 U.S. women receiving adjuvant chemotherapy for breast cancer, those who took ALC not only had no decrease in the development of peripheral neuropathy symptoms relative to peers who were given a placebo, but actually had an increase. And they had a higher rate of serious neuropathy, too.
In contrast, in a trial among more than 200 Chinese patients with various cancers who had peripheral neuropathy from previous chemotherapy, those who took ALC were more likely than those who took a placebo to have an improvement of at least one grade in their neuropathy. They also were more likely to have improvements in fatigue and strength.
Taken together, the two trials, which were reported in a poster discussion session at the annual meeting of the American Society of Clinical Oncology, provide yet another cautionary lesson on the complexity of combining conventional and complementary therapies.
"The use of ALC for prevention is not recommended, and I would say, based on [these results], should be cautioned against. It will be interesting to see the carnitine data and to understand, as much as possible, why the trial was negative," commented Debra L. Barton, Ph.D., of the Mayo Clinic in Rochester, Minn., who was invited to discuss the research. "Further studies are needed to really understand if ALC should be used to treat peripheral neuropathy."
ALC for Prevention of Peripheral Neuropathy
In the first trial, Southwest Oncology Group (SWOG) protocol S0715, investigators led by Dr. Dawn L. Hershman randomized women receiving adjuvant taxane chemotherapy for early breast cancer evenly to either oral ALC 1,000 mg three times daily or matching placebo, for 24 weeks.
Compared with their counterparts in the placebo group, women in the ALC group were more likely to have a greater than 5-point adjusted decrease on the neurotoxicity subscale of the Functional Assessment of Cancer Therapy–Taxane (FACT-NTX) instrument at 12 weeks (odds ratio, 1.48; P = .08) and also at 24 weeks (38% vs. 28%; OR, 1.57; P = .05).
This magnitude of worsening is clinically meaningful, maintained Dr. Hershman of Columbia University in New York, "so this is not like a lot of studies where you find a statistically significant difference that’s not clinically meaningful."
In addition, the incidence of grade 3/4 neurotoxicity was 3.8% with ALC, much higher than the 0.5% seen with placebo.
Patients in the ALC group also had scores on the FACT trial outcome index subscale (FACT-TOI), an overall measure of function, that were on average 3.5 points lower (worse) than those among their placebo counterparts (P = .03). There were no significant differences between groups in terms of fatigue and other toxicities.
The investigators have collected biosamples and will be assessing potential biological correlates with peripheral neuropathy outcomes, according to Dr. Hershman.
"We are looking at DNA, oxidative stress, and carnitine levels to better understand the mechanisms of chemotherapy-induced peripheral neuropathy to begin with, because there is not a whole lot known in terms of mechanism," she said. "If we can figure out what makes people worse, then we will maybe be able to figure out how to make people better from a more mechanistic standpoint, because there are very few drugs to treat chemotherapy-induced peripheral neuropathy."
An obvious concern from the trial’s findings is that ALC may somehow potentiate the neurotoxic effects of taxanes. "Based on these data, physicians should be telling patients not to take ALC during adjuvant chemotherapy," Dr. Hershman concluded. "You need to talk to patients. We know from the literature that overwhelmingly large number of patients take supplements during chemotherapy and afterward, many of which have not been tested. It’s important to get that history from patients."
Dr. Barton, the discussant, praised the trial’s rigorous methodology and proposed that there may have been several reasons for the lack of ALC benefit in preventing neuropathy, despite compelling earlier data.
Previous prevention research was done in animals and thus may not translate to humans, she said. And a positive trial for treatment in humans used intravenous administration, which may result in different bioavailability. Finally, "ALC capsules needed to be taken three times a day, and they are rather large, and these patients were, after all, on chemotherapy. They were likely nauseated [and] dyspeptic, and taking what some might call a horse pill three times a day could not have been an easy task. The study did use pill diaries, but we know those aren’t a perfect tool for adherence."
"The great thing is that the study collected blood and they are able to look at carnitine levels," Dr. Barton said. "So if carnitine is up in the group that got acetyl-carnitine and not in the group that got placebo, well, I think that pretty much confirms that this just didn’t work."
ALC for Treatment of Peripheral Neuropathy
In the second trial, protocol ZHAOKE-2007L03540, investigators led by Dr. Yuanjue Sun of the Sixth Affiliated Hospital of Shanghai (China) Jiao Tong University, enrolled 239 patients who had cancer of various types and stages, had completed chemotherapy, and had had at least grade 2 peripheral neuropathy for up to 6 months.
They were randomly assigned to receive either oral ALC at a dose of 3 g/day or matching placebo, for 8 weeks, with outcomes assessed at clinic visits or by telephone.
Analyses showed that compared with their counterparts in the placebo group, patients in the ALC group were more likely to have had an improvement of at least one grade in their neuropathy, both at 8 weeks (51% vs. 24%; P less than .001) and at 12 weeks (58% vs. 40%; P less than .001).
In terms of secondary outcomes, the ALC group was also more likely to have had an improvement in cancer-related fatigue (31% vs. 20%; P = .048), physical strength (29% vs. 13%; P = .02), and electrophysiology in peripheral nerves (75% vs. 58%; P = .02).
The two groups had statistically indistinguishable rates of adverse events (20% vs. 15%) and adverse reactions (6% vs. 5%). The most common events were gastrointestinal ones and skin allergies.
"This is the first time to confirm that ALC has a positive effect to cure chemotherapy-induced peripheral neuropathy in the Chinese population," Dr. Sun commented through a translator.
"I think the very important thing for this trial is, it is a different kind of patient population. Before this, most clinical trials were performed in [whites] or maybe Americans. This is an only-Asian [population]," he noted, and it is possible that there are genetic differences in how ALC is metabolized.
Dr. Barton, the discussant, took a cautionary view, saying that "there are some things to consider before going out and telling patients to consider acetyl-carnitine for their peripheral neuropathy."
It was unclear from the results reported whether the two treatment groups were well balanced and what criteria were used to define improvement for the secondary outcomes, she noted. Additionally, "outcome measures were all provider graded, [and there were] no self-report measures, so it is difficult to understand the impact of treatment on symptoms, particularly from the patient perspective," she noted.
Dr. Hershman, Dr. Sun, and Dr. Barton disclosed no relevant conflicts of interest; the ZHAOKE-2007L03540 trial was sponsored by Lee’s Pharmaceutical Limited.
CHICAGO – The impact of acetyl-l-carnitine on chemotherapy-induced peripheral neuropathy may depend largely on the clinical context and patient population, a pair of phase III trials suggests.
Acetyl-l-carnitine (ALC), a natural substance marketed over the counter as a dietary supplement, is popular among cancer patients as a result of preclinical and early-phase data in chemotherapy-related neuropathy and also a study in patients with diabetes-related peripheral neuropathy.
But in a trial among 409 U.S. women receiving adjuvant chemotherapy for breast cancer, those who took ALC not only had no decrease in the development of peripheral neuropathy symptoms relative to peers who were given a placebo, but actually had an increase. And they had a higher rate of serious neuropathy, too.
In contrast, in a trial among more than 200 Chinese patients with various cancers who had peripheral neuropathy from previous chemotherapy, those who took ALC were more likely than those who took a placebo to have an improvement of at least one grade in their neuropathy. They also were more likely to have improvements in fatigue and strength.
Taken together, the two trials, which were reported in a poster discussion session at the annual meeting of the American Society of Clinical Oncology, provide yet another cautionary lesson on the complexity of combining conventional and complementary therapies.
"The use of ALC for prevention is not recommended, and I would say, based on [these results], should be cautioned against. It will be interesting to see the carnitine data and to understand, as much as possible, why the trial was negative," commented Debra L. Barton, Ph.D., of the Mayo Clinic in Rochester, Minn., who was invited to discuss the research. "Further studies are needed to really understand if ALC should be used to treat peripheral neuropathy."
ALC for Prevention of Peripheral Neuropathy
In the first trial, Southwest Oncology Group (SWOG) protocol S0715, investigators led by Dr. Dawn L. Hershman randomized women receiving adjuvant taxane chemotherapy for early breast cancer evenly to either oral ALC 1,000 mg three times daily or matching placebo, for 24 weeks.
Compared with their counterparts in the placebo group, women in the ALC group were more likely to have a greater than 5-point adjusted decrease on the neurotoxicity subscale of the Functional Assessment of Cancer Therapy–Taxane (FACT-NTX) instrument at 12 weeks (odds ratio, 1.48; P = .08) and also at 24 weeks (38% vs. 28%; OR, 1.57; P = .05).
This magnitude of worsening is clinically meaningful, maintained Dr. Hershman of Columbia University in New York, "so this is not like a lot of studies where you find a statistically significant difference that’s not clinically meaningful."
In addition, the incidence of grade 3/4 neurotoxicity was 3.8% with ALC, much higher than the 0.5% seen with placebo.
Patients in the ALC group also had scores on the FACT trial outcome index subscale (FACT-TOI), an overall measure of function, that were on average 3.5 points lower (worse) than those among their placebo counterparts (P = .03). There were no significant differences between groups in terms of fatigue and other toxicities.
The investigators have collected biosamples and will be assessing potential biological correlates with peripheral neuropathy outcomes, according to Dr. Hershman.
"We are looking at DNA, oxidative stress, and carnitine levels to better understand the mechanisms of chemotherapy-induced peripheral neuropathy to begin with, because there is not a whole lot known in terms of mechanism," she said. "If we can figure out what makes people worse, then we will maybe be able to figure out how to make people better from a more mechanistic standpoint, because there are very few drugs to treat chemotherapy-induced peripheral neuropathy."
An obvious concern from the trial’s findings is that ALC may somehow potentiate the neurotoxic effects of taxanes. "Based on these data, physicians should be telling patients not to take ALC during adjuvant chemotherapy," Dr. Hershman concluded. "You need to talk to patients. We know from the literature that overwhelmingly large number of patients take supplements during chemotherapy and afterward, many of which have not been tested. It’s important to get that history from patients."
Dr. Barton, the discussant, praised the trial’s rigorous methodology and proposed that there may have been several reasons for the lack of ALC benefit in preventing neuropathy, despite compelling earlier data.
Previous prevention research was done in animals and thus may not translate to humans, she said. And a positive trial for treatment in humans used intravenous administration, which may result in different bioavailability. Finally, "ALC capsules needed to be taken three times a day, and they are rather large, and these patients were, after all, on chemotherapy. They were likely nauseated [and] dyspeptic, and taking what some might call a horse pill three times a day could not have been an easy task. The study did use pill diaries, but we know those aren’t a perfect tool for adherence."
"The great thing is that the study collected blood and they are able to look at carnitine levels," Dr. Barton said. "So if carnitine is up in the group that got acetyl-carnitine and not in the group that got placebo, well, I think that pretty much confirms that this just didn’t work."
ALC for Treatment of Peripheral Neuropathy
In the second trial, protocol ZHAOKE-2007L03540, investigators led by Dr. Yuanjue Sun of the Sixth Affiliated Hospital of Shanghai (China) Jiao Tong University, enrolled 239 patients who had cancer of various types and stages, had completed chemotherapy, and had had at least grade 2 peripheral neuropathy for up to 6 months.
They were randomly assigned to receive either oral ALC at a dose of 3 g/day or matching placebo, for 8 weeks, with outcomes assessed at clinic visits or by telephone.
Analyses showed that compared with their counterparts in the placebo group, patients in the ALC group were more likely to have had an improvement of at least one grade in their neuropathy, both at 8 weeks (51% vs. 24%; P less than .001) and at 12 weeks (58% vs. 40%; P less than .001).
In terms of secondary outcomes, the ALC group was also more likely to have had an improvement in cancer-related fatigue (31% vs. 20%; P = .048), physical strength (29% vs. 13%; P = .02), and electrophysiology in peripheral nerves (75% vs. 58%; P = .02).
The two groups had statistically indistinguishable rates of adverse events (20% vs. 15%) and adverse reactions (6% vs. 5%). The most common events were gastrointestinal ones and skin allergies.
"This is the first time to confirm that ALC has a positive effect to cure chemotherapy-induced peripheral neuropathy in the Chinese population," Dr. Sun commented through a translator.
"I think the very important thing for this trial is, it is a different kind of patient population. Before this, most clinical trials were performed in [whites] or maybe Americans. This is an only-Asian [population]," he noted, and it is possible that there are genetic differences in how ALC is metabolized.
Dr. Barton, the discussant, took a cautionary view, saying that "there are some things to consider before going out and telling patients to consider acetyl-carnitine for their peripheral neuropathy."
It was unclear from the results reported whether the two treatment groups were well balanced and what criteria were used to define improvement for the secondary outcomes, she noted. Additionally, "outcome measures were all provider graded, [and there were] no self-report measures, so it is difficult to understand the impact of treatment on symptoms, particularly from the patient perspective," she noted.
Dr. Hershman, Dr. Sun, and Dr. Barton disclosed no relevant conflicts of interest; the ZHAOKE-2007L03540 trial was sponsored by Lee’s Pharmaceutical Limited.
CHICAGO – The impact of acetyl-l-carnitine on chemotherapy-induced peripheral neuropathy may depend largely on the clinical context and patient population, a pair of phase III trials suggests.
Acetyl-l-carnitine (ALC), a natural substance marketed over the counter as a dietary supplement, is popular among cancer patients as a result of preclinical and early-phase data in chemotherapy-related neuropathy and also a study in patients with diabetes-related peripheral neuropathy.
But in a trial among 409 U.S. women receiving adjuvant chemotherapy for breast cancer, those who took ALC not only had no decrease in the development of peripheral neuropathy symptoms relative to peers who were given a placebo, but actually had an increase. And they had a higher rate of serious neuropathy, too.
In contrast, in a trial among more than 200 Chinese patients with various cancers who had peripheral neuropathy from previous chemotherapy, those who took ALC were more likely than those who took a placebo to have an improvement of at least one grade in their neuropathy. They also were more likely to have improvements in fatigue and strength.
Taken together, the two trials, which were reported in a poster discussion session at the annual meeting of the American Society of Clinical Oncology, provide yet another cautionary lesson on the complexity of combining conventional and complementary therapies.
"The use of ALC for prevention is not recommended, and I would say, based on [these results], should be cautioned against. It will be interesting to see the carnitine data and to understand, as much as possible, why the trial was negative," commented Debra L. Barton, Ph.D., of the Mayo Clinic in Rochester, Minn., who was invited to discuss the research. "Further studies are needed to really understand if ALC should be used to treat peripheral neuropathy."
ALC for Prevention of Peripheral Neuropathy
In the first trial, Southwest Oncology Group (SWOG) protocol S0715, investigators led by Dr. Dawn L. Hershman randomized women receiving adjuvant taxane chemotherapy for early breast cancer evenly to either oral ALC 1,000 mg three times daily or matching placebo, for 24 weeks.
Compared with their counterparts in the placebo group, women in the ALC group were more likely to have a greater than 5-point adjusted decrease on the neurotoxicity subscale of the Functional Assessment of Cancer Therapy–Taxane (FACT-NTX) instrument at 12 weeks (odds ratio, 1.48; P = .08) and also at 24 weeks (38% vs. 28%; OR, 1.57; P = .05).
This magnitude of worsening is clinically meaningful, maintained Dr. Hershman of Columbia University in New York, "so this is not like a lot of studies where you find a statistically significant difference that’s not clinically meaningful."
In addition, the incidence of grade 3/4 neurotoxicity was 3.8% with ALC, much higher than the 0.5% seen with placebo.
Patients in the ALC group also had scores on the FACT trial outcome index subscale (FACT-TOI), an overall measure of function, that were on average 3.5 points lower (worse) than those among their placebo counterparts (P = .03). There were no significant differences between groups in terms of fatigue and other toxicities.
The investigators have collected biosamples and will be assessing potential biological correlates with peripheral neuropathy outcomes, according to Dr. Hershman.
"We are looking at DNA, oxidative stress, and carnitine levels to better understand the mechanisms of chemotherapy-induced peripheral neuropathy to begin with, because there is not a whole lot known in terms of mechanism," she said. "If we can figure out what makes people worse, then we will maybe be able to figure out how to make people better from a more mechanistic standpoint, because there are very few drugs to treat chemotherapy-induced peripheral neuropathy."
An obvious concern from the trial’s findings is that ALC may somehow potentiate the neurotoxic effects of taxanes. "Based on these data, physicians should be telling patients not to take ALC during adjuvant chemotherapy," Dr. Hershman concluded. "You need to talk to patients. We know from the literature that overwhelmingly large number of patients take supplements during chemotherapy and afterward, many of which have not been tested. It’s important to get that history from patients."
Dr. Barton, the discussant, praised the trial’s rigorous methodology and proposed that there may have been several reasons for the lack of ALC benefit in preventing neuropathy, despite compelling earlier data.
Previous prevention research was done in animals and thus may not translate to humans, she said. And a positive trial for treatment in humans used intravenous administration, which may result in different bioavailability. Finally, "ALC capsules needed to be taken three times a day, and they are rather large, and these patients were, after all, on chemotherapy. They were likely nauseated [and] dyspeptic, and taking what some might call a horse pill three times a day could not have been an easy task. The study did use pill diaries, but we know those aren’t a perfect tool for adherence."
"The great thing is that the study collected blood and they are able to look at carnitine levels," Dr. Barton said. "So if carnitine is up in the group that got acetyl-carnitine and not in the group that got placebo, well, I think that pretty much confirms that this just didn’t work."
ALC for Treatment of Peripheral Neuropathy
In the second trial, protocol ZHAOKE-2007L03540, investigators led by Dr. Yuanjue Sun of the Sixth Affiliated Hospital of Shanghai (China) Jiao Tong University, enrolled 239 patients who had cancer of various types and stages, had completed chemotherapy, and had had at least grade 2 peripheral neuropathy for up to 6 months.
They were randomly assigned to receive either oral ALC at a dose of 3 g/day or matching placebo, for 8 weeks, with outcomes assessed at clinic visits or by telephone.
Analyses showed that compared with their counterparts in the placebo group, patients in the ALC group were more likely to have had an improvement of at least one grade in their neuropathy, both at 8 weeks (51% vs. 24%; P less than .001) and at 12 weeks (58% vs. 40%; P less than .001).
In terms of secondary outcomes, the ALC group was also more likely to have had an improvement in cancer-related fatigue (31% vs. 20%; P = .048), physical strength (29% vs. 13%; P = .02), and electrophysiology in peripheral nerves (75% vs. 58%; P = .02).
The two groups had statistically indistinguishable rates of adverse events (20% vs. 15%) and adverse reactions (6% vs. 5%). The most common events were gastrointestinal ones and skin allergies.
"This is the first time to confirm that ALC has a positive effect to cure chemotherapy-induced peripheral neuropathy in the Chinese population," Dr. Sun commented through a translator.
"I think the very important thing for this trial is, it is a different kind of patient population. Before this, most clinical trials were performed in [whites] or maybe Americans. This is an only-Asian [population]," he noted, and it is possible that there are genetic differences in how ALC is metabolized.
Dr. Barton, the discussant, took a cautionary view, saying that "there are some things to consider before going out and telling patients to consider acetyl-carnitine for their peripheral neuropathy."
It was unclear from the results reported whether the two treatment groups were well balanced and what criteria were used to define improvement for the secondary outcomes, she noted. Additionally, "outcome measures were all provider graded, [and there were] no self-report measures, so it is difficult to understand the impact of treatment on symptoms, particularly from the patient perspective," she noted.
Dr. Hershman, Dr. Sun, and Dr. Barton disclosed no relevant conflicts of interest; the ZHAOKE-2007L03540 trial was sponsored by Lee’s Pharmaceutical Limited.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Patients taking ALC for prevention were more likely to have a greater than 5-point worsening of FACT-NTX score (38% vs. 28%), whereas patients taking ALC for treatment were more likely to have an improvement of at least one grade in neuropathy (51% vs. 24%).
Data Source: Investigators presented separate, randomized, placebo-controlled phase III trials among 410 women receiving adjuvant taxane chemotherapy for breast cancer and 239 patients with cancer and chemotherapy-induced peripheral neuropathy.
Disclosures: Dr. Hershman, Dr. Sun, and Dr. Barton disclosed no relevant conflicts of interest; the ZHAOKE-2007L03540 trial was sponsored by Lee’s Pharmaceutical Limited.
ASCO 2012: Radium-223 and Prostate Cancer Survival
Dr. Chris Parker of Royal Marsden Hospital in London discusses the ALSYMPCA trial of radium-223 (Alpharadin), the first agent to improve prostate cancer survival by targeting bone metastases.
Dr. Chris Parker of Royal Marsden Hospital in London discusses the ALSYMPCA trial of radium-223 (Alpharadin), the first agent to improve prostate cancer survival by targeting bone metastases.
Dr. Chris Parker of Royal Marsden Hospital in London discusses the ALSYMPCA trial of radium-223 (Alpharadin), the first agent to improve prostate cancer survival by targeting bone metastases.
When the Cancer Patient Isn't a Kid Anymore
CHICAGO – Every parent of a teenager has heard some variation of the demand, "Stop treating me like a kid!" The same can be said for adolescent and young adult survivors of childhood cancers, investigators say.
With childhood cancer survivorship rates hovering around 80% (according to Surveillance, Epidemiology and End Results data from 1996 to 2003), many patients are outgrowing their pediatricians and their pediatric oncologists. The patients still need regular follow-up, but just who will do that follow-up and how thoroughly is still an open question, said Dr. Karim Thomas Sadak, a pediatric oncology fellow at the Center for Cancer and Blood Disorders at Children’s National Medical Center in Washington, D.C.
He and his colleagues surveyed 103 cancer survivors aged 16-24 years and asked them to identify what they found to be the most important factors in their decision to make the transition from a survivorship program at a children’s hospital to a similar program at an adult institution.
"They told us some things we were very surprised to hear about their preferences for care. By far, the most commonly selected component of their clinical care that was rated as very important was the acceptability of their insurance. We might expect to hear that from 30-year-olds, but these people are most likely on their parents’ insurance, as they are under 25," Dr. Sadak said in an interview at the annual meeting of the American Society of Clinical Oncology.
Based on the responses, Dr. Sadak and his associates asked a social worker who helps young patients make the transition to adult care to discuss insurance options with patients and their parents before and during patient visits and in follow-up.
"She gained knowledge about different insurances, reimbursements, copays, and policy issues related to survivorship care, and then she was able to proactively address these concerns with survivors," he said.
Survivors also rated flexible scheduling and comprehensive care as either very important or important. Paradoxically, while 97% of responders said that they wanted to make a transition that promoted independence, 96% wanted their primary childhood cancer provider present during the transition.
Conversely, "availability of vocational training and peer networking as well as considering readiness and a gradual introduction appear to be least important" factors in the decision to make the transition, Dr. Sadak said.
Internists Willing but Uncertain
In a different study, investigators from the United States and Canada surveyed U.S. internists about their knowledge of caring for childhood cancer survivors and found that most general internists said they are willing to follow adolescent and young adult survivors of childhood cancers but many also said that they would feel more comfortable doing so in collaboration with a cancer center.
Internists need to know that there are a wide variety of resources available to help them care for such patients, said coauthor Dr. Eugene Suh, a fellow in pediatric oncology at the University of Chicago Medical Center.
"Internists are really good at gathering information, but I don’t think they necessarily know that this information is out there to help guide them in taking care of these cancer survivors," he said in an interview.
In their survey of a random sample of 2,000 U.S. general internists, 1,025 of whom responded, the investigators found that 72% of those who had seen pediatric cancer survivors in the past 5 years said they never received a treatment summary or survivorship care plan documenting diagnosis, cancer therapy, and plan for follow-up.
Additionally, on a 7-point scale rating familiarity with the Children’s Oncology Group (COG) long-term follow-up guidelines, most respondents reported being "very unfamiliar" (mean score of 5.2 points) with the recommendations.
When presented with a clinical vignette of a female survivor of Hodgkin’s lymphoma treated at age 16 with 25 Gy of mantle radiation and cumulative doses of doxorubicin 150 mg/m2 and cyclophosphamide 15 g/m2, 73% did not recommend yearly breast cancer surveillance, 85% did not recommend cardiac surveillance, and 23% did not recommend thyroid surveillance.
The investigators plan to conduct intervention studies aimed at improving general physicians’ comfort with and knowledge of long-term care for survivors.
Research Resource
One source for survivorship studies could be vertically integrated health care systems, said Dr. Robert M. Cooper and colleagues from Kaiser Permanente Southern California in Los Angeles.
They found that, 5 years after diagnosis, 77% of 4,782 adolescent and young adult cancer patients were still being cared for by Kaiser physicians, as were 62% at 10 years.
"The lengthy insurance retention of adolescent/young adult cancer survivors makes a vertically integrated medical care system an ideal population laboratory for adolescent/young adult cancer survivorship research," they wrote in a poster presented at ASCO 2012.
Patient, Advocate for Thyself
Dr. Sadak said that patients also have to be willing to step up to the plate and act as their own best advocates.
"At some point, we as the provider want to educate the patient ... to have some kind of responsibility for their own health care," he said. "The question is, at what age? It may not be 16, 17, or 18, especially in a population that’s been through a serious illness like childhood cancer. The bonds that these patients and their parents have created are very strong. We have to respect that, while still encouraging the survivor to take responsibility for his health."
Dr. Sadak’s study was funded by a Children’s Health Center Board grant. Dr. Suh’s study was funded by the National Institutes of Health. Dr. Cooper’s study was supported by Kaiser Permanente. All authors reported having no relevant conflicts of interest.
CHICAGO – Every parent of a teenager has heard some variation of the demand, "Stop treating me like a kid!" The same can be said for adolescent and young adult survivors of childhood cancers, investigators say.
With childhood cancer survivorship rates hovering around 80% (according to Surveillance, Epidemiology and End Results data from 1996 to 2003), many patients are outgrowing their pediatricians and their pediatric oncologists. The patients still need regular follow-up, but just who will do that follow-up and how thoroughly is still an open question, said Dr. Karim Thomas Sadak, a pediatric oncology fellow at the Center for Cancer and Blood Disorders at Children’s National Medical Center in Washington, D.C.
He and his colleagues surveyed 103 cancer survivors aged 16-24 years and asked them to identify what they found to be the most important factors in their decision to make the transition from a survivorship program at a children’s hospital to a similar program at an adult institution.
"They told us some things we were very surprised to hear about their preferences for care. By far, the most commonly selected component of their clinical care that was rated as very important was the acceptability of their insurance. We might expect to hear that from 30-year-olds, but these people are most likely on their parents’ insurance, as they are under 25," Dr. Sadak said in an interview at the annual meeting of the American Society of Clinical Oncology.
Based on the responses, Dr. Sadak and his associates asked a social worker who helps young patients make the transition to adult care to discuss insurance options with patients and their parents before and during patient visits and in follow-up.
"She gained knowledge about different insurances, reimbursements, copays, and policy issues related to survivorship care, and then she was able to proactively address these concerns with survivors," he said.
Survivors also rated flexible scheduling and comprehensive care as either very important or important. Paradoxically, while 97% of responders said that they wanted to make a transition that promoted independence, 96% wanted their primary childhood cancer provider present during the transition.
Conversely, "availability of vocational training and peer networking as well as considering readiness and a gradual introduction appear to be least important" factors in the decision to make the transition, Dr. Sadak said.
Internists Willing but Uncertain
In a different study, investigators from the United States and Canada surveyed U.S. internists about their knowledge of caring for childhood cancer survivors and found that most general internists said they are willing to follow adolescent and young adult survivors of childhood cancers but many also said that they would feel more comfortable doing so in collaboration with a cancer center.
Internists need to know that there are a wide variety of resources available to help them care for such patients, said coauthor Dr. Eugene Suh, a fellow in pediatric oncology at the University of Chicago Medical Center.
"Internists are really good at gathering information, but I don’t think they necessarily know that this information is out there to help guide them in taking care of these cancer survivors," he said in an interview.
In their survey of a random sample of 2,000 U.S. general internists, 1,025 of whom responded, the investigators found that 72% of those who had seen pediatric cancer survivors in the past 5 years said they never received a treatment summary or survivorship care plan documenting diagnosis, cancer therapy, and plan for follow-up.
Additionally, on a 7-point scale rating familiarity with the Children’s Oncology Group (COG) long-term follow-up guidelines, most respondents reported being "very unfamiliar" (mean score of 5.2 points) with the recommendations.
When presented with a clinical vignette of a female survivor of Hodgkin’s lymphoma treated at age 16 with 25 Gy of mantle radiation and cumulative doses of doxorubicin 150 mg/m2 and cyclophosphamide 15 g/m2, 73% did not recommend yearly breast cancer surveillance, 85% did not recommend cardiac surveillance, and 23% did not recommend thyroid surveillance.
The investigators plan to conduct intervention studies aimed at improving general physicians’ comfort with and knowledge of long-term care for survivors.
Research Resource
One source for survivorship studies could be vertically integrated health care systems, said Dr. Robert M. Cooper and colleagues from Kaiser Permanente Southern California in Los Angeles.
They found that, 5 years after diagnosis, 77% of 4,782 adolescent and young adult cancer patients were still being cared for by Kaiser physicians, as were 62% at 10 years.
"The lengthy insurance retention of adolescent/young adult cancer survivors makes a vertically integrated medical care system an ideal population laboratory for adolescent/young adult cancer survivorship research," they wrote in a poster presented at ASCO 2012.
Patient, Advocate for Thyself
Dr. Sadak said that patients also have to be willing to step up to the plate and act as their own best advocates.
"At some point, we as the provider want to educate the patient ... to have some kind of responsibility for their own health care," he said. "The question is, at what age? It may not be 16, 17, or 18, especially in a population that’s been through a serious illness like childhood cancer. The bonds that these patients and their parents have created are very strong. We have to respect that, while still encouraging the survivor to take responsibility for his health."
Dr. Sadak’s study was funded by a Children’s Health Center Board grant. Dr. Suh’s study was funded by the National Institutes of Health. Dr. Cooper’s study was supported by Kaiser Permanente. All authors reported having no relevant conflicts of interest.
CHICAGO – Every parent of a teenager has heard some variation of the demand, "Stop treating me like a kid!" The same can be said for adolescent and young adult survivors of childhood cancers, investigators say.
With childhood cancer survivorship rates hovering around 80% (according to Surveillance, Epidemiology and End Results data from 1996 to 2003), many patients are outgrowing their pediatricians and their pediatric oncologists. The patients still need regular follow-up, but just who will do that follow-up and how thoroughly is still an open question, said Dr. Karim Thomas Sadak, a pediatric oncology fellow at the Center for Cancer and Blood Disorders at Children’s National Medical Center in Washington, D.C.
He and his colleagues surveyed 103 cancer survivors aged 16-24 years and asked them to identify what they found to be the most important factors in their decision to make the transition from a survivorship program at a children’s hospital to a similar program at an adult institution.
"They told us some things we were very surprised to hear about their preferences for care. By far, the most commonly selected component of their clinical care that was rated as very important was the acceptability of their insurance. We might expect to hear that from 30-year-olds, but these people are most likely on their parents’ insurance, as they are under 25," Dr. Sadak said in an interview at the annual meeting of the American Society of Clinical Oncology.
Based on the responses, Dr. Sadak and his associates asked a social worker who helps young patients make the transition to adult care to discuss insurance options with patients and their parents before and during patient visits and in follow-up.
"She gained knowledge about different insurances, reimbursements, copays, and policy issues related to survivorship care, and then she was able to proactively address these concerns with survivors," he said.
Survivors also rated flexible scheduling and comprehensive care as either very important or important. Paradoxically, while 97% of responders said that they wanted to make a transition that promoted independence, 96% wanted their primary childhood cancer provider present during the transition.
Conversely, "availability of vocational training and peer networking as well as considering readiness and a gradual introduction appear to be least important" factors in the decision to make the transition, Dr. Sadak said.
Internists Willing but Uncertain
In a different study, investigators from the United States and Canada surveyed U.S. internists about their knowledge of caring for childhood cancer survivors and found that most general internists said they are willing to follow adolescent and young adult survivors of childhood cancers but many also said that they would feel more comfortable doing so in collaboration with a cancer center.
Internists need to know that there are a wide variety of resources available to help them care for such patients, said coauthor Dr. Eugene Suh, a fellow in pediatric oncology at the University of Chicago Medical Center.
"Internists are really good at gathering information, but I don’t think they necessarily know that this information is out there to help guide them in taking care of these cancer survivors," he said in an interview.
In their survey of a random sample of 2,000 U.S. general internists, 1,025 of whom responded, the investigators found that 72% of those who had seen pediatric cancer survivors in the past 5 years said they never received a treatment summary or survivorship care plan documenting diagnosis, cancer therapy, and plan for follow-up.
Additionally, on a 7-point scale rating familiarity with the Children’s Oncology Group (COG) long-term follow-up guidelines, most respondents reported being "very unfamiliar" (mean score of 5.2 points) with the recommendations.
When presented with a clinical vignette of a female survivor of Hodgkin’s lymphoma treated at age 16 with 25 Gy of mantle radiation and cumulative doses of doxorubicin 150 mg/m2 and cyclophosphamide 15 g/m2, 73% did not recommend yearly breast cancer surveillance, 85% did not recommend cardiac surveillance, and 23% did not recommend thyroid surveillance.
The investigators plan to conduct intervention studies aimed at improving general physicians’ comfort with and knowledge of long-term care for survivors.
Research Resource
One source for survivorship studies could be vertically integrated health care systems, said Dr. Robert M. Cooper and colleagues from Kaiser Permanente Southern California in Los Angeles.
They found that, 5 years after diagnosis, 77% of 4,782 adolescent and young adult cancer patients were still being cared for by Kaiser physicians, as were 62% at 10 years.
"The lengthy insurance retention of adolescent/young adult cancer survivors makes a vertically integrated medical care system an ideal population laboratory for adolescent/young adult cancer survivorship research," they wrote in a poster presented at ASCO 2012.
Patient, Advocate for Thyself
Dr. Sadak said that patients also have to be willing to step up to the plate and act as their own best advocates.
"At some point, we as the provider want to educate the patient ... to have some kind of responsibility for their own health care," he said. "The question is, at what age? It may not be 16, 17, or 18, especially in a population that’s been through a serious illness like childhood cancer. The bonds that these patients and their parents have created are very strong. We have to respect that, while still encouraging the survivor to take responsibility for his health."
Dr. Sadak’s study was funded by a Children’s Health Center Board grant. Dr. Suh’s study was funded by the National Institutes of Health. Dr. Cooper’s study was supported by Kaiser Permanente. All authors reported having no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Among adolescent and young adult cancer survivors, 97% said acceptance of their insurance was important or very important for making the transition to an adult physician’s care.
Data Source: Investigators surveyed 103 survivors, aged 16-24 years, of pediatric cancers.
Disclosures: Dr. Sadak’s study was funded by a Children’s Health Center Board grant. Dr. Suh’s study was funded by the NIH. The study by Dr. Cooper and colleagues was supported by Kaiser Permanente. All authors reported having no relevant conflicts of interest.
ASCO 2012: Three Causes for Optimism in Lung Cancer
Dr. David R. Spigel, program director for lung cancer research at the Sarah Cannon Research Institute in Nashville, discusses three early studies that point to better outcomes with molecularly target agents. The first suggests that an approved drug, crizotinib (Xalkori), might be effective in patients with the ROS1 mutation. The others showcase novel agents, introducing the possibility of immunotherapy by targeting PD1 and of benefiting patients with KRAS mutations by adding selumetinib to chemotherapy.
Dr. David R. Spigel, program director for lung cancer research at the Sarah Cannon Research Institute in Nashville, discusses three early studies that point to better outcomes with molecularly target agents. The first suggests that an approved drug, crizotinib (Xalkori), might be effective in patients with the ROS1 mutation. The others showcase novel agents, introducing the possibility of immunotherapy by targeting PD1 and of benefiting patients with KRAS mutations by adding selumetinib to chemotherapy.
Dr. David R. Spigel, program director for lung cancer research at the Sarah Cannon Research Institute in Nashville, discusses three early studies that point to better outcomes with molecularly target agents. The first suggests that an approved drug, crizotinib (Xalkori), might be effective in patients with the ROS1 mutation. The others showcase novel agents, introducing the possibility of immunotherapy by targeting PD1 and of benefiting patients with KRAS mutations by adding selumetinib to chemotherapy.
ASCO 2012: Radiotherapy for Locally Advanced Prostate Cancer
Dr. Malcolm Mason of Cardiff University in Wales discusses his report that adding radiotherapy to androgen deprivation therapy for locally advanced prostate cancer produced a durable survival benefit compared with hormone therapy alone. Findings come from the final 10-year analysis of a phase III intergroup trial carried out by the U.K. Medical Research Council, the National Cancer Institute of Canada, and the Southwest Oncology Group.
Dr. Malcolm Mason of Cardiff University in Wales discusses his report that adding radiotherapy to androgen deprivation therapy for locally advanced prostate cancer produced a durable survival benefit compared with hormone therapy alone. Findings come from the final 10-year analysis of a phase III intergroup trial carried out by the U.K. Medical Research Council, the National Cancer Institute of Canada, and the Southwest Oncology Group.
Dr. Malcolm Mason of Cardiff University in Wales discusses his report that adding radiotherapy to androgen deprivation therapy for locally advanced prostate cancer produced a durable survival benefit compared with hormone therapy alone. Findings come from the final 10-year analysis of a phase III intergroup trial carried out by the U.K. Medical Research Council, the National Cancer Institute of Canada, and the Southwest Oncology Group.
Superiority of Dose-Dense Chemo Upheld for Ovarian Cancer
CHICAGO – The superiority of dose-dense paclitaxel-plus-carboplatin chemotherapy stood up in the long term for women with ovarian or related cancers in a randomized trial.
Investigators from the Japanese Gynecologic Oncology Group 3016 study reported statistically significant reductions in risks of progression and of death with dose-dense chemotherapy, compared with a conventional regimen, at a median follow-up of 6.4 years.
Women who were given dose-dense chemotherapy had the risk of progression or death cut by 24% and the risk of death cut by 21%, according to results reported at the annual meeting of the American Society of Clinical Oncology. This followed respective reductions of 29% and 25% at a median follow-up of 2.4 years (Lancet 2009;374:1331-8).
In subgroup analyses, the survival benefit of dose-dense chemotherapy was significant only among women who had residual disease after debulking surgery measuring greater than 1 cm, or serous histology.
"Dose-dense [paclitaxel plus carboplatin] improved long-term progression-free survival and overall survival in patients with advanced epithelial ovarian cancer," said first author Dr. Noriyuki Katsumata of the Nippon Medical School–Musashi-Kosugi Hospital in Kawasaki, Japan.
"Neither dose-dense nor conventional treatment seemed effective against clear-cell or mucinous ovarian carcinoma, which suggests that other treatment strategies are needed," he added.
The study "is a major contribution to our understanding of dose-dense therapy and whether or not it should be used," said the invited discussant, Dr. Jonathan S. Berek, director of the Stanford (Calif.) Women’s Cancer Center. In particular, the overall survival benefit "is a very important observation for our patients and our specialty."
However, the trial was done in a Japanese population, he cautioned. "Maybe there are racial differences. We’ll have to wait for confirmatory trials to see whether there is any difference. There may be none, but we’ll see."
"One of the questions I’d like to raise is, how does this compare to the advantage that we saw with intraperitoneal therapy, and what should we recommend to our patients while we await more confirmatory and comparative data?" Dr. Berek said.
He noted that a variety of trials – GOG (Gynecologic Oncology Group)–0252, GOG-0262, ICON8, MITO-7, and iPocc – will better clarify and compare the merits of dose-dense and intraperitoneal approaches, sometimes in combination with the antiangiogenic agent bevacizumab (Avastin).
"I suspect in the next 2-3 years, the head-to-head comparison of the dose-dense regimens and the intraperitoneal regimens will emerge, and we will probably have at least some clear answers about whether it’s route of administration or frequency of administration, or in some cases, both" that optimizes efficacy, Dr. Berek concluded.
The 637 women accrued to the Japanese trial, also known as the NOVEL study, had advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer and had undergone debulking surgery. The large majority (84%) had serous tumor histology.
They were randomly assigned to paclitaxel (Taxol) plus carboplatin given according to a conventional schedule (paclitaxel, 180 mg/m2; carboplatin, AUC [area under the curve] = 6; each given once every 3 weeks) or according to a dose-dense schedule (paclitaxel, 80 mg/m2 weekly; carboplatin, AUC = 6 once every 3 weeks), in both arms for six to nine cycles.
The updated results showed that relative to conventional chemotherapy, dose-dense chemotherapy conferred better median progression-free survival (28.2 vs. 17.5 months, respectively; hazard ratio, 0.76; P = .004) and overall survival (not reached vs. 62.2 months; HR, 0.79; P = .04). At this point, 58.7% of the dose-dense group and 51.1% of the conventional therapy group were alive.
In analyses stratified by the extent of residual disease after debulking surgery, the overall survival benefit of dose-dense chemotherapy was significant only for women who had residual disease measuring greater than 1 cm (median, 51.2 months vs. 33.5 months; HR, 0.75; P = .03) or serous histology (median, not reached vs. 61.2 months; HR, 0.76; P = .03).
In a multivariate analysis, dose-dense chemotherapy was a significant independent predictor of overall survival, along with ovarian disease site, earlier stage, less residual disease, better performance status, and a paclitaxel relative dose intensity of at least 80%.
Dr. Katsumata disclosed no relevant conflicts of interest. Dr. Berek disclosed no relevant conflicts of interest.
CHICAGO – The superiority of dose-dense paclitaxel-plus-carboplatin chemotherapy stood up in the long term for women with ovarian or related cancers in a randomized trial.
Investigators from the Japanese Gynecologic Oncology Group 3016 study reported statistically significant reductions in risks of progression and of death with dose-dense chemotherapy, compared with a conventional regimen, at a median follow-up of 6.4 years.
Women who were given dose-dense chemotherapy had the risk of progression or death cut by 24% and the risk of death cut by 21%, according to results reported at the annual meeting of the American Society of Clinical Oncology. This followed respective reductions of 29% and 25% at a median follow-up of 2.4 years (Lancet 2009;374:1331-8).
In subgroup analyses, the survival benefit of dose-dense chemotherapy was significant only among women who had residual disease after debulking surgery measuring greater than 1 cm, or serous histology.
"Dose-dense [paclitaxel plus carboplatin] improved long-term progression-free survival and overall survival in patients with advanced epithelial ovarian cancer," said first author Dr. Noriyuki Katsumata of the Nippon Medical School–Musashi-Kosugi Hospital in Kawasaki, Japan.
"Neither dose-dense nor conventional treatment seemed effective against clear-cell or mucinous ovarian carcinoma, which suggests that other treatment strategies are needed," he added.
The study "is a major contribution to our understanding of dose-dense therapy and whether or not it should be used," said the invited discussant, Dr. Jonathan S. Berek, director of the Stanford (Calif.) Women’s Cancer Center. In particular, the overall survival benefit "is a very important observation for our patients and our specialty."
However, the trial was done in a Japanese population, he cautioned. "Maybe there are racial differences. We’ll have to wait for confirmatory trials to see whether there is any difference. There may be none, but we’ll see."
"One of the questions I’d like to raise is, how does this compare to the advantage that we saw with intraperitoneal therapy, and what should we recommend to our patients while we await more confirmatory and comparative data?" Dr. Berek said.
He noted that a variety of trials – GOG (Gynecologic Oncology Group)–0252, GOG-0262, ICON8, MITO-7, and iPocc – will better clarify and compare the merits of dose-dense and intraperitoneal approaches, sometimes in combination with the antiangiogenic agent bevacizumab (Avastin).
"I suspect in the next 2-3 years, the head-to-head comparison of the dose-dense regimens and the intraperitoneal regimens will emerge, and we will probably have at least some clear answers about whether it’s route of administration or frequency of administration, or in some cases, both" that optimizes efficacy, Dr. Berek concluded.
The 637 women accrued to the Japanese trial, also known as the NOVEL study, had advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer and had undergone debulking surgery. The large majority (84%) had serous tumor histology.
They were randomly assigned to paclitaxel (Taxol) plus carboplatin given according to a conventional schedule (paclitaxel, 180 mg/m2; carboplatin, AUC [area under the curve] = 6; each given once every 3 weeks) or according to a dose-dense schedule (paclitaxel, 80 mg/m2 weekly; carboplatin, AUC = 6 once every 3 weeks), in both arms for six to nine cycles.
The updated results showed that relative to conventional chemotherapy, dose-dense chemotherapy conferred better median progression-free survival (28.2 vs. 17.5 months, respectively; hazard ratio, 0.76; P = .004) and overall survival (not reached vs. 62.2 months; HR, 0.79; P = .04). At this point, 58.7% of the dose-dense group and 51.1% of the conventional therapy group were alive.
In analyses stratified by the extent of residual disease after debulking surgery, the overall survival benefit of dose-dense chemotherapy was significant only for women who had residual disease measuring greater than 1 cm (median, 51.2 months vs. 33.5 months; HR, 0.75; P = .03) or serous histology (median, not reached vs. 61.2 months; HR, 0.76; P = .03).
In a multivariate analysis, dose-dense chemotherapy was a significant independent predictor of overall survival, along with ovarian disease site, earlier stage, less residual disease, better performance status, and a paclitaxel relative dose intensity of at least 80%.
Dr. Katsumata disclosed no relevant conflicts of interest. Dr. Berek disclosed no relevant conflicts of interest.
CHICAGO – The superiority of dose-dense paclitaxel-plus-carboplatin chemotherapy stood up in the long term for women with ovarian or related cancers in a randomized trial.
Investigators from the Japanese Gynecologic Oncology Group 3016 study reported statistically significant reductions in risks of progression and of death with dose-dense chemotherapy, compared with a conventional regimen, at a median follow-up of 6.4 years.
Women who were given dose-dense chemotherapy had the risk of progression or death cut by 24% and the risk of death cut by 21%, according to results reported at the annual meeting of the American Society of Clinical Oncology. This followed respective reductions of 29% and 25% at a median follow-up of 2.4 years (Lancet 2009;374:1331-8).
In subgroup analyses, the survival benefit of dose-dense chemotherapy was significant only among women who had residual disease after debulking surgery measuring greater than 1 cm, or serous histology.
"Dose-dense [paclitaxel plus carboplatin] improved long-term progression-free survival and overall survival in patients with advanced epithelial ovarian cancer," said first author Dr. Noriyuki Katsumata of the Nippon Medical School–Musashi-Kosugi Hospital in Kawasaki, Japan.
"Neither dose-dense nor conventional treatment seemed effective against clear-cell or mucinous ovarian carcinoma, which suggests that other treatment strategies are needed," he added.
The study "is a major contribution to our understanding of dose-dense therapy and whether or not it should be used," said the invited discussant, Dr. Jonathan S. Berek, director of the Stanford (Calif.) Women’s Cancer Center. In particular, the overall survival benefit "is a very important observation for our patients and our specialty."
However, the trial was done in a Japanese population, he cautioned. "Maybe there are racial differences. We’ll have to wait for confirmatory trials to see whether there is any difference. There may be none, but we’ll see."
"One of the questions I’d like to raise is, how does this compare to the advantage that we saw with intraperitoneal therapy, and what should we recommend to our patients while we await more confirmatory and comparative data?" Dr. Berek said.
He noted that a variety of trials – GOG (Gynecologic Oncology Group)–0252, GOG-0262, ICON8, MITO-7, and iPocc – will better clarify and compare the merits of dose-dense and intraperitoneal approaches, sometimes in combination with the antiangiogenic agent bevacizumab (Avastin).
"I suspect in the next 2-3 years, the head-to-head comparison of the dose-dense regimens and the intraperitoneal regimens will emerge, and we will probably have at least some clear answers about whether it’s route of administration or frequency of administration, or in some cases, both" that optimizes efficacy, Dr. Berek concluded.
The 637 women accrued to the Japanese trial, also known as the NOVEL study, had advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer and had undergone debulking surgery. The large majority (84%) had serous tumor histology.
They were randomly assigned to paclitaxel (Taxol) plus carboplatin given according to a conventional schedule (paclitaxel, 180 mg/m2; carboplatin, AUC [area under the curve] = 6; each given once every 3 weeks) or according to a dose-dense schedule (paclitaxel, 80 mg/m2 weekly; carboplatin, AUC = 6 once every 3 weeks), in both arms for six to nine cycles.
The updated results showed that relative to conventional chemotherapy, dose-dense chemotherapy conferred better median progression-free survival (28.2 vs. 17.5 months, respectively; hazard ratio, 0.76; P = .004) and overall survival (not reached vs. 62.2 months; HR, 0.79; P = .04). At this point, 58.7% of the dose-dense group and 51.1% of the conventional therapy group were alive.
In analyses stratified by the extent of residual disease after debulking surgery, the overall survival benefit of dose-dense chemotherapy was significant only for women who had residual disease measuring greater than 1 cm (median, 51.2 months vs. 33.5 months; HR, 0.75; P = .03) or serous histology (median, not reached vs. 61.2 months; HR, 0.76; P = .03).
In a multivariate analysis, dose-dense chemotherapy was a significant independent predictor of overall survival, along with ovarian disease site, earlier stage, less residual disease, better performance status, and a paclitaxel relative dose intensity of at least 80%.
Dr. Katsumata disclosed no relevant conflicts of interest. Dr. Berek disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: With a median follow-up of 6.4 years, median overall survival was not reached with dose-dense paclitaxel-plus-carboplatin chemotherapy vs. 62.2 months with a conventional schedule (HR, 0.79; P = .04).
Data Source: The randomized JGOG 3016/NOVEL randomized trial evaluated 637 women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Disclosures: Dr. Katsumata disclosed no relevant conflicts of interest. Dr. Berek disclosed no relevant conflicts of interest.
Blinatumomab Gets Complete Response in Acute Lymphoblastic Leukemia
CHICAGO – The novel antibody blinatumomab continues to induce high complete remission rates in adults with relapsed or refractory B-precursor acute lymphoblastic leukemia.
In a phase II study with a dose-finding phase, 26 of 36 patients treated at various dose levels had a complete response (CR) or a complete response with partial hematologic recovery (CRh) within two treatment cycles, Dr. Max S. Topp reported at the annual meeting of the American Society of Clinical Oncology. Of 23 patients treated at the optimal dose, 17 had a CR or CRh, he said.
All but two of the patients with responses to the drug also had a molecular remission, he noted, and 13 patients went on to receive an allogeneic stem cell transplant after achieving a CR or CRh.
"We reached a very high rate of hematological and molecular remission in patients with blinatumomab," said Dr. Topp of the University of Würzburg (Germany).
The median duration of complete hematologic remission among all patients treated in the dose-finding phase was 8.9 months (median follow-up, 4.5 months). Median overall survival among patients treated at all dose levels was 9 months (median follow-up, 10.7 months).
Blinatumomab is a bispecific T-cell engager (BiTE) antibody designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. It showed good activity in a phase I clinical trial in patients with relapsed non-Hodgkin’s lymphoma, as well as in a study of patients with B-lineage acute lymphoblastic leukemia (ALL) who were positive for minimal residual disease (J. Clin. Oncol. 2011;29:2493-8). Dr. Topp reported earlier results of the current MT103-206 study at the 2011 annual meeting of the American Society of Hematology.
The current trial was an open-label, multicenter phase II study of blinatumomab in patients with relapsed/refractory B-precursor ALL, or Philadelphia chromosome–positive ALL, who were ineligible for tyrosine kinase inhibitors or who were in relapse following an allogeneic stem cell transplant.
The trial had a dose-finding run-in phase with four patient cohorts. Dr. Topp provided updated outcomes data on cohorts 2a and 3 (the extension phase), in which patients received the selected dose schedule: an initial dose of 5 mcg/m2 IV daily for the first week of cycle 1, followed by 15 mcg/m2 per day for weeks 2-4 of every 4-week cycle, and every subsequent cycle. Patients had 2 weeks off between each cycle.
Patients who had a CR or CRh in the first two treatment cycles underwent consolidation with three additional cycles of blinatumomab and allogeneic stem cell transplant.
Medically important safety events to date include the cytokine release syndrome in three patients, two of whom had a high tumor burden with no cytoreductive prephase; and these patients required temporary treatment interruption. The third patient had a milder form of the syndrome and was managed with supportive medication, with no discontinuation of blinatumomab.
Six patients had central nervous system adverse events – three seizures and three cases of encephalopathy – that were reversible with treatment interruption. All six were eventually continued on the 5-mg/m2 dose, but two had recurrence and permanently stopped treatment. One patient stopped because of a fungal infection that proved to be fatal.
Pyrexia, headache, tremor, and fatigue were the most common treatment-emergent adverse events, but there were only four grade 3 or higher reactions, including one increase in cytokine release syndrome. Most of the events occurred at the start of the first cycle.
The invited discussant, Dr. Bruno Medeiros of Stanford (Calif.) University, commented that blinatumomab appears to have good single-agent activity in relapsed or refractory ALL and is safe before allogeneic transplant. This agent, and another novel antibody against acute lymphocytic leukemia, inotuzumab, may also be effective when combined with chemotherapy or as single agents in first-line therapy, he said.
A global phase II study of blinatumomab in patients with relapsed or refractory ALL is underway.
The study was funded by Micromet, which has been acquired by Amgen. Dr. Topp disclosed having a consultant or advisory role and receiving other remuneration from Micromet. Dr. Medeiros disclosed ties with Millennium, Celgene, and Novartis.
CHICAGO – The novel antibody blinatumomab continues to induce high complete remission rates in adults with relapsed or refractory B-precursor acute lymphoblastic leukemia.
In a phase II study with a dose-finding phase, 26 of 36 patients treated at various dose levels had a complete response (CR) or a complete response with partial hematologic recovery (CRh) within two treatment cycles, Dr. Max S. Topp reported at the annual meeting of the American Society of Clinical Oncology. Of 23 patients treated at the optimal dose, 17 had a CR or CRh, he said.
All but two of the patients with responses to the drug also had a molecular remission, he noted, and 13 patients went on to receive an allogeneic stem cell transplant after achieving a CR or CRh.
"We reached a very high rate of hematological and molecular remission in patients with blinatumomab," said Dr. Topp of the University of Würzburg (Germany).
The median duration of complete hematologic remission among all patients treated in the dose-finding phase was 8.9 months (median follow-up, 4.5 months). Median overall survival among patients treated at all dose levels was 9 months (median follow-up, 10.7 months).
Blinatumomab is a bispecific T-cell engager (BiTE) antibody designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. It showed good activity in a phase I clinical trial in patients with relapsed non-Hodgkin’s lymphoma, as well as in a study of patients with B-lineage acute lymphoblastic leukemia (ALL) who were positive for minimal residual disease (J. Clin. Oncol. 2011;29:2493-8). Dr. Topp reported earlier results of the current MT103-206 study at the 2011 annual meeting of the American Society of Hematology.
The current trial was an open-label, multicenter phase II study of blinatumomab in patients with relapsed/refractory B-precursor ALL, or Philadelphia chromosome–positive ALL, who were ineligible for tyrosine kinase inhibitors or who were in relapse following an allogeneic stem cell transplant.
The trial had a dose-finding run-in phase with four patient cohorts. Dr. Topp provided updated outcomes data on cohorts 2a and 3 (the extension phase), in which patients received the selected dose schedule: an initial dose of 5 mcg/m2 IV daily for the first week of cycle 1, followed by 15 mcg/m2 per day for weeks 2-4 of every 4-week cycle, and every subsequent cycle. Patients had 2 weeks off between each cycle.
Patients who had a CR or CRh in the first two treatment cycles underwent consolidation with three additional cycles of blinatumomab and allogeneic stem cell transplant.
Medically important safety events to date include the cytokine release syndrome in three patients, two of whom had a high tumor burden with no cytoreductive prephase; and these patients required temporary treatment interruption. The third patient had a milder form of the syndrome and was managed with supportive medication, with no discontinuation of blinatumomab.
Six patients had central nervous system adverse events – three seizures and three cases of encephalopathy – that were reversible with treatment interruption. All six were eventually continued on the 5-mg/m2 dose, but two had recurrence and permanently stopped treatment. One patient stopped because of a fungal infection that proved to be fatal.
Pyrexia, headache, tremor, and fatigue were the most common treatment-emergent adverse events, but there were only four grade 3 or higher reactions, including one increase in cytokine release syndrome. Most of the events occurred at the start of the first cycle.
The invited discussant, Dr. Bruno Medeiros of Stanford (Calif.) University, commented that blinatumomab appears to have good single-agent activity in relapsed or refractory ALL and is safe before allogeneic transplant. This agent, and another novel antibody against acute lymphocytic leukemia, inotuzumab, may also be effective when combined with chemotherapy or as single agents in first-line therapy, he said.
A global phase II study of blinatumomab in patients with relapsed or refractory ALL is underway.
The study was funded by Micromet, which has been acquired by Amgen. Dr. Topp disclosed having a consultant or advisory role and receiving other remuneration from Micromet. Dr. Medeiros disclosed ties with Millennium, Celgene, and Novartis.
CHICAGO – The novel antibody blinatumomab continues to induce high complete remission rates in adults with relapsed or refractory B-precursor acute lymphoblastic leukemia.
In a phase II study with a dose-finding phase, 26 of 36 patients treated at various dose levels had a complete response (CR) or a complete response with partial hematologic recovery (CRh) within two treatment cycles, Dr. Max S. Topp reported at the annual meeting of the American Society of Clinical Oncology. Of 23 patients treated at the optimal dose, 17 had a CR or CRh, he said.
All but two of the patients with responses to the drug also had a molecular remission, he noted, and 13 patients went on to receive an allogeneic stem cell transplant after achieving a CR or CRh.
"We reached a very high rate of hematological and molecular remission in patients with blinatumomab," said Dr. Topp of the University of Würzburg (Germany).
The median duration of complete hematologic remission among all patients treated in the dose-finding phase was 8.9 months (median follow-up, 4.5 months). Median overall survival among patients treated at all dose levels was 9 months (median follow-up, 10.7 months).
Blinatumomab is a bispecific T-cell engager (BiTE) antibody designed to direct cytotoxic T cells to cancer cells expressing the CD19 receptor. It showed good activity in a phase I clinical trial in patients with relapsed non-Hodgkin’s lymphoma, as well as in a study of patients with B-lineage acute lymphoblastic leukemia (ALL) who were positive for minimal residual disease (J. Clin. Oncol. 2011;29:2493-8). Dr. Topp reported earlier results of the current MT103-206 study at the 2011 annual meeting of the American Society of Hematology.
The current trial was an open-label, multicenter phase II study of blinatumomab in patients with relapsed/refractory B-precursor ALL, or Philadelphia chromosome–positive ALL, who were ineligible for tyrosine kinase inhibitors or who were in relapse following an allogeneic stem cell transplant.
The trial had a dose-finding run-in phase with four patient cohorts. Dr. Topp provided updated outcomes data on cohorts 2a and 3 (the extension phase), in which patients received the selected dose schedule: an initial dose of 5 mcg/m2 IV daily for the first week of cycle 1, followed by 15 mcg/m2 per day for weeks 2-4 of every 4-week cycle, and every subsequent cycle. Patients had 2 weeks off between each cycle.
Patients who had a CR or CRh in the first two treatment cycles underwent consolidation with three additional cycles of blinatumomab and allogeneic stem cell transplant.
Medically important safety events to date include the cytokine release syndrome in three patients, two of whom had a high tumor burden with no cytoreductive prephase; and these patients required temporary treatment interruption. The third patient had a milder form of the syndrome and was managed with supportive medication, with no discontinuation of blinatumomab.
Six patients had central nervous system adverse events – three seizures and three cases of encephalopathy – that were reversible with treatment interruption. All six were eventually continued on the 5-mg/m2 dose, but two had recurrence and permanently stopped treatment. One patient stopped because of a fungal infection that proved to be fatal.
Pyrexia, headache, tremor, and fatigue were the most common treatment-emergent adverse events, but there were only four grade 3 or higher reactions, including one increase in cytokine release syndrome. Most of the events occurred at the start of the first cycle.
The invited discussant, Dr. Bruno Medeiros of Stanford (Calif.) University, commented that blinatumomab appears to have good single-agent activity in relapsed or refractory ALL and is safe before allogeneic transplant. This agent, and another novel antibody against acute lymphocytic leukemia, inotuzumab, may also be effective when combined with chemotherapy or as single agents in first-line therapy, he said.
A global phase II study of blinatumomab in patients with relapsed or refractory ALL is underway.
The study was funded by Micromet, which has been acquired by Amgen. Dr. Topp disclosed having a consultant or advisory role and receiving other remuneration from Micromet. Dr. Medeiros disclosed ties with Millennium, Celgene, and Novartis.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY