User login
Oral agent found promising for subset of chronic rhinosinusitis patients
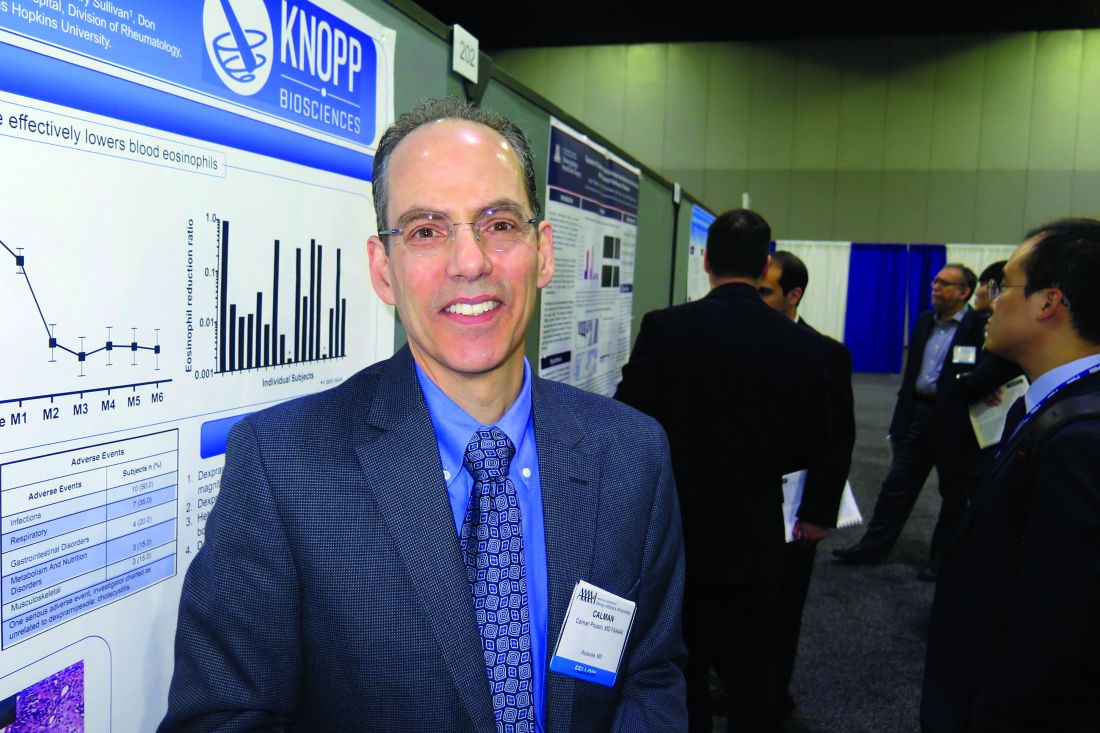
ATLANTA – The use of dexpramipexole by patients with chronic rhinosinusitis was well tolerated and showed robust and tissue eosinophil–lowering activity, according to results from a small study.
Dexpramipexole is an investigational oral agent that has been studied in previous clinical trials for patients with amyotrophic lateral sclerosis, Calman Prussin, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The drug did not meet the clinical endpoint for ALS patients, but its investigators noted that it lowered eosinophil counts by about 50%. “It was a serendipitous finding,” said Dr. Prussin, senior director of clinical and translational medicine for Pittsburgh-based Knopp Biosciences. “We do not have a mechanism of action, but we think it’s working on progenitor cells in the bone marrow.”
In all, 16 of the 20 patients completed the trial. Dr. Prussin and his associates found that the baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than.001). “I don’t think any of us expected to see this,” he said, noting that the drug’s maximal eosinophil-lowering effect was maximal after 2 months. No reduction in total polyp score was observed.
Biopsies conducted in 12 of the patients revealed that polyp tissue eosinophilia was reduced from a mean of 233 to 5 eosinophils/high-powered field, a drop of 97% (P = .001). No serious drug-related adverse effects occurred. The most common adverse event was infection (50%), followed by respiratory symptoms (35%) and gastrointestinal disorders (20%).
Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
ATLANTA – The use of dexpramipexole by patients with chronic rhinosinusitis was well tolerated and showed robust and tissue eosinophil–lowering activity, according to results from a small study.
Dexpramipexole is an investigational oral agent that has been studied in previous clinical trials for patients with amyotrophic lateral sclerosis, Calman Prussin, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The drug did not meet the clinical endpoint for ALS patients, but its investigators noted that it lowered eosinophil counts by about 50%. “It was a serendipitous finding,” said Dr. Prussin, senior director of clinical and translational medicine for Pittsburgh-based Knopp Biosciences. “We do not have a mechanism of action, but we think it’s working on progenitor cells in the bone marrow.”
In all, 16 of the 20 patients completed the trial. Dr. Prussin and his associates found that the baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than.001). “I don’t think any of us expected to see this,” he said, noting that the drug’s maximal eosinophil-lowering effect was maximal after 2 months. No reduction in total polyp score was observed.
Biopsies conducted in 12 of the patients revealed that polyp tissue eosinophilia was reduced from a mean of 233 to 5 eosinophils/high-powered field, a drop of 97% (P = .001). No serious drug-related adverse effects occurred. The most common adverse event was infection (50%), followed by respiratory symptoms (35%) and gastrointestinal disorders (20%).
Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
ATLANTA – The use of dexpramipexole by patients with chronic rhinosinusitis was well tolerated and showed robust and tissue eosinophil–lowering activity, according to results from a small study.
Dexpramipexole is an investigational oral agent that has been studied in previous clinical trials for patients with amyotrophic lateral sclerosis, Calman Prussin, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The drug did not meet the clinical endpoint for ALS patients, but its investigators noted that it lowered eosinophil counts by about 50%. “It was a serendipitous finding,” said Dr. Prussin, senior director of clinical and translational medicine for Pittsburgh-based Knopp Biosciences. “We do not have a mechanism of action, but we think it’s working on progenitor cells in the bone marrow.”
In all, 16 of the 20 patients completed the trial. Dr. Prussin and his associates found that the baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than.001). “I don’t think any of us expected to see this,” he said, noting that the drug’s maximal eosinophil-lowering effect was maximal after 2 months. No reduction in total polyp score was observed.
Biopsies conducted in 12 of the patients revealed that polyp tissue eosinophilia was reduced from a mean of 233 to 5 eosinophils/high-powered field, a drop of 97% (P = .001). No serious drug-related adverse effects occurred. The most common adverse event was infection (50%), followed by respiratory symptoms (35%) and gastrointestinal disorders (20%).
Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: The baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than .001).
Data source: Results from a open-label trial in 16 chronic rhinosinusitis patients who received dexpramipexole 150 mg b.i.d. for 6 months.
Disclosures: Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
Parental perception of asthma may predict future acute visits
ATLANTA – For low-income and minority children with asthma, parents’ perception of a child’s asthma control may be an important predictor of future acute visits, independent of guideline-based criteria for asthma control, judging from the results from a prospective cohort study.
The National Asthma Education and Prevention Program (NAEPP)–based assessment of asthma control incorporates symptoms, nighttime awakenings, and activity interference; short-acting beta 2-agonist use, lung function, and history of exacerbations, “but it does not take into account parental perceptions of asthma control,” lead study author Suzanne Rossi, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “We also know that parental report of symptom frequency and their perception of their child’s asthma control are frequently discordant.”
In an effort to answer these questions, the researchers conducted a prospective cohort study of 150 Baltimore children aged 5-17 years with persistent asthma who had an exacerbation within the past year. After a baseline assessment, clinic visits occurred every 3 months for 1 year. The predictor variable was parental perception of asthma control assessed by the following question: “Do you believe that your child’s asthma was well controlled within the past 4 weeks?” The primary outcome was an acute visit, defined as an unscheduled visit to a physician or an ED visit or a hospitalization. The researchers used generalized estimating equations to relate parental perception of asthma control to future acute visits.
The mean age of patients was 11 years, 57% were male, 91% were African American, and 85% were on public health insurance. In addition, 15% were overweight and 28% were obese. At baseline, patients were using short-acting beta-2 agonists a mean of 4.2 days every 2 weeks, and 96% had an acute visit in the prior 12 months. Only 9% met criteria for well-controlled asthma as defined by NAEPP criteria, 36% were not well controlled, and 55% were poorly controlled.
At the baseline visit, 73% of parents said that their child’s asthma was well controlled, 20% said that it was not well controlled, and 7% were unsure. Of the 136 children who met NAEPP criteria for uncontrolled asthma, 71% had parents who reported that their child’s asthma was well controlled.
The researchers found that on average, children with parents who report uncontrolled asthma were 2.4-fold times more likely to have an acute visit within the next 3 months, compared with children whose parents reported that their child’s asthma was well controlled. The odds ratio remained similar after adjustment for NAEPP-based asthma control, and for age, gender, race, controller medication, insurance, and atopy. Data on hospitalization was excluded because there was insufficient data for analysis.
Dr. Rossi and her associates also found that parental perception of uncontrolled asthma was a predictor of future acute visits among females but not males (odds ratio, 5.3 vs. OR, 1.3, respectively; P = .03), and among those who were overweight or obese but not among those with a normal BMI (OR, 6.2 vs. OR, 1.3; P = .04). Age was not a modifier. She acknowledged certain limitations of the study, including the inability to measure the severity of asthma exacerbation. “Therefore, this primary outcome may reflect parental concern,” Dr. Rossi said. “In addition, these findings may not be generalizable to other pediatric asthma populations. We also had a small sample size for some outcomes such as hospitalization. In terms of future directions, it would be nice to know whether the findings are replicable in other similar populations and in population-based studies. It would be interesting to examine the association with larger study populations to evaluate hospitalizations and to get an assessment of the severity of symptoms associated with the acute visit.”
The National Institute of Allergy and Infectious Diseases, the National Institute of Environmental Health Sciences, and Johns Hopkins University School of Medicine supported the study. Dr. Rossi reported having no financial disclosures.
ATLANTA – For low-income and minority children with asthma, parents’ perception of a child’s asthma control may be an important predictor of future acute visits, independent of guideline-based criteria for asthma control, judging from the results from a prospective cohort study.
The National Asthma Education and Prevention Program (NAEPP)–based assessment of asthma control incorporates symptoms, nighttime awakenings, and activity interference; short-acting beta 2-agonist use, lung function, and history of exacerbations, “but it does not take into account parental perceptions of asthma control,” lead study author Suzanne Rossi, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “We also know that parental report of symptom frequency and their perception of their child’s asthma control are frequently discordant.”
In an effort to answer these questions, the researchers conducted a prospective cohort study of 150 Baltimore children aged 5-17 years with persistent asthma who had an exacerbation within the past year. After a baseline assessment, clinic visits occurred every 3 months for 1 year. The predictor variable was parental perception of asthma control assessed by the following question: “Do you believe that your child’s asthma was well controlled within the past 4 weeks?” The primary outcome was an acute visit, defined as an unscheduled visit to a physician or an ED visit or a hospitalization. The researchers used generalized estimating equations to relate parental perception of asthma control to future acute visits.
The mean age of patients was 11 years, 57% were male, 91% were African American, and 85% were on public health insurance. In addition, 15% were overweight and 28% were obese. At baseline, patients were using short-acting beta-2 agonists a mean of 4.2 days every 2 weeks, and 96% had an acute visit in the prior 12 months. Only 9% met criteria for well-controlled asthma as defined by NAEPP criteria, 36% were not well controlled, and 55% were poorly controlled.
At the baseline visit, 73% of parents said that their child’s asthma was well controlled, 20% said that it was not well controlled, and 7% were unsure. Of the 136 children who met NAEPP criteria for uncontrolled asthma, 71% had parents who reported that their child’s asthma was well controlled.
The researchers found that on average, children with parents who report uncontrolled asthma were 2.4-fold times more likely to have an acute visit within the next 3 months, compared with children whose parents reported that their child’s asthma was well controlled. The odds ratio remained similar after adjustment for NAEPP-based asthma control, and for age, gender, race, controller medication, insurance, and atopy. Data on hospitalization was excluded because there was insufficient data for analysis.
Dr. Rossi and her associates also found that parental perception of uncontrolled asthma was a predictor of future acute visits among females but not males (odds ratio, 5.3 vs. OR, 1.3, respectively; P = .03), and among those who were overweight or obese but not among those with a normal BMI (OR, 6.2 vs. OR, 1.3; P = .04). Age was not a modifier. She acknowledged certain limitations of the study, including the inability to measure the severity of asthma exacerbation. “Therefore, this primary outcome may reflect parental concern,” Dr. Rossi said. “In addition, these findings may not be generalizable to other pediatric asthma populations. We also had a small sample size for some outcomes such as hospitalization. In terms of future directions, it would be nice to know whether the findings are replicable in other similar populations and in population-based studies. It would be interesting to examine the association with larger study populations to evaluate hospitalizations and to get an assessment of the severity of symptoms associated with the acute visit.”
The National Institute of Allergy and Infectious Diseases, the National Institute of Environmental Health Sciences, and Johns Hopkins University School of Medicine supported the study. Dr. Rossi reported having no financial disclosures.
ATLANTA – For low-income and minority children with asthma, parents’ perception of a child’s asthma control may be an important predictor of future acute visits, independent of guideline-based criteria for asthma control, judging from the results from a prospective cohort study.
The National Asthma Education and Prevention Program (NAEPP)–based assessment of asthma control incorporates symptoms, nighttime awakenings, and activity interference; short-acting beta 2-agonist use, lung function, and history of exacerbations, “but it does not take into account parental perceptions of asthma control,” lead study author Suzanne Rossi, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “We also know that parental report of symptom frequency and their perception of their child’s asthma control are frequently discordant.”
In an effort to answer these questions, the researchers conducted a prospective cohort study of 150 Baltimore children aged 5-17 years with persistent asthma who had an exacerbation within the past year. After a baseline assessment, clinic visits occurred every 3 months for 1 year. The predictor variable was parental perception of asthma control assessed by the following question: “Do you believe that your child’s asthma was well controlled within the past 4 weeks?” The primary outcome was an acute visit, defined as an unscheduled visit to a physician or an ED visit or a hospitalization. The researchers used generalized estimating equations to relate parental perception of asthma control to future acute visits.
The mean age of patients was 11 years, 57% were male, 91% were African American, and 85% were on public health insurance. In addition, 15% were overweight and 28% were obese. At baseline, patients were using short-acting beta-2 agonists a mean of 4.2 days every 2 weeks, and 96% had an acute visit in the prior 12 months. Only 9% met criteria for well-controlled asthma as defined by NAEPP criteria, 36% were not well controlled, and 55% were poorly controlled.
At the baseline visit, 73% of parents said that their child’s asthma was well controlled, 20% said that it was not well controlled, and 7% were unsure. Of the 136 children who met NAEPP criteria for uncontrolled asthma, 71% had parents who reported that their child’s asthma was well controlled.
The researchers found that on average, children with parents who report uncontrolled asthma were 2.4-fold times more likely to have an acute visit within the next 3 months, compared with children whose parents reported that their child’s asthma was well controlled. The odds ratio remained similar after adjustment for NAEPP-based asthma control, and for age, gender, race, controller medication, insurance, and atopy. Data on hospitalization was excluded because there was insufficient data for analysis.
Dr. Rossi and her associates also found that parental perception of uncontrolled asthma was a predictor of future acute visits among females but not males (odds ratio, 5.3 vs. OR, 1.3, respectively; P = .03), and among those who were overweight or obese but not among those with a normal BMI (OR, 6.2 vs. OR, 1.3; P = .04). Age was not a modifier. She acknowledged certain limitations of the study, including the inability to measure the severity of asthma exacerbation. “Therefore, this primary outcome may reflect parental concern,” Dr. Rossi said. “In addition, these findings may not be generalizable to other pediatric asthma populations. We also had a small sample size for some outcomes such as hospitalization. In terms of future directions, it would be nice to know whether the findings are replicable in other similar populations and in population-based studies. It would be interesting to examine the association with larger study populations to evaluate hospitalizations and to get an assessment of the severity of symptoms associated with the acute visit.”
The National Institute of Allergy and Infectious Diseases, the National Institute of Environmental Health Sciences, and Johns Hopkins University School of Medicine supported the study. Dr. Rossi reported having no financial disclosures.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Children whose parents reported uncontrolled asthma were 2.4-fold times more likely to have an acute visit within the next 3 months, compared with children whose parents reported that their child’s asthma was well controlled.
Data source: A prospective cohort study of 150 Baltimore children aged 5-17 years with persistent asthma who had an exacerbation within the past year.
Disclosures: The National Institute of Allergy and Infectious Diseases, the National Institute of Environmental Health Sciences, and Johns Hopkins University School of Medicine supported the study. Dr. Rossi reported having no financial disclosures.
Survey finds chronic rhinosinusitis common, burdensome
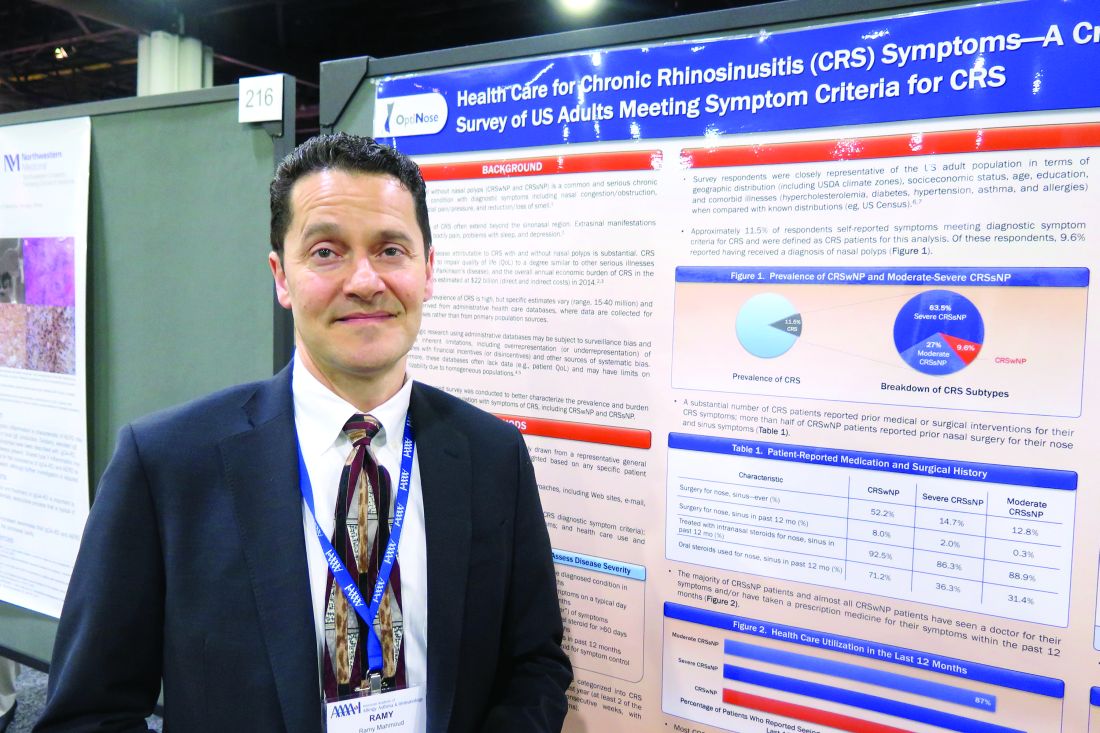
ATLANTA – Results from a large cross-sectional survey of adults who meet diagnostic symptom criteria for chronic rhinosinusitis (CRS) reveal that most report suffering significant symptoms, high rates of physician visits, and dissatisfaction with current intranasal steroid sprays.
“Chronic rhinosinusitis is common, it’s severe, and people are seeking a lot of medical care,” study author Ramy A. Mahmoud, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “It’s causing a lot of disruption in their lives, and they’re not satisfied with currently available intranasal steroids.”
In an effort to better characterize the prevalence and burden of illness in adults with symptoms of CRS, Dr. Mahmoud and his associates conducted a population-based survey of 10,336 U.S. adults drawn from a representative general panel of 4.3 million. They recruited participants via websites, email, phone, online communities, and social networks and collected information about nasal symptoms, frequency, duration, and severity/bother of nasal symptoms and health care use and treatments.
The researchers found that almost 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS. Of these, 63.5% were classified as having severe CRS without known nasal polyps (CRSsNP), 27% had moderate CRSsNP, and 9.6% had CRS with known nasal polyps (CRSwNP). In addition, 87%-99% of patients reported seeing a physician for their symptoms and/or taking a prescription medicine for their CRS symptoms in the past year. In fact, 60% of CRSwNP patients, 58% of severe CRSsNP patients, and 33% of moderate CRSsNP patients said they made more than five physician visits in the past year for nose and sinus symptoms.
The frequency of key CRS symptoms was similar in patients with and without nasal polyps, except that those with severe CRSsNP reported higher rates of facial pain/pressure (in the range of 60%-65%) and loss of smell/taste (46%-56%). The most frequently reported core symptoms were nasal congestion (94%-97%) and drainage (89%-92%). Despite currently available treatment options, up to 46% of CRS patients reported that they remain “extremely” affected by symptoms, and 79% of all CRS patients expressed concern about flare-ups. In addition, a large proportion of respondents expressed frustration with the inadequate symptom relief produced by their current intranasal corticosteroid (87% of CRSwNP patients, 86% of severe CRSsNP patients, and 63% of moderate CRSsNP patients).
Dr. Mahmoud said that the findings indicate a role for more and/or improved treatment options to meet the needs of this patient population. “People who have CRS have it pretty bad,” he said. “Very few people are not seeking a lot of medical care for their symptoms.”
The study was funded by OptiNose.
ATLANTA – Results from a large cross-sectional survey of adults who meet diagnostic symptom criteria for chronic rhinosinusitis (CRS) reveal that most report suffering significant symptoms, high rates of physician visits, and dissatisfaction with current intranasal steroid sprays.
“Chronic rhinosinusitis is common, it’s severe, and people are seeking a lot of medical care,” study author Ramy A. Mahmoud, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “It’s causing a lot of disruption in their lives, and they’re not satisfied with currently available intranasal steroids.”
In an effort to better characterize the prevalence and burden of illness in adults with symptoms of CRS, Dr. Mahmoud and his associates conducted a population-based survey of 10,336 U.S. adults drawn from a representative general panel of 4.3 million. They recruited participants via websites, email, phone, online communities, and social networks and collected information about nasal symptoms, frequency, duration, and severity/bother of nasal symptoms and health care use and treatments.
The researchers found that almost 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS. Of these, 63.5% were classified as having severe CRS without known nasal polyps (CRSsNP), 27% had moderate CRSsNP, and 9.6% had CRS with known nasal polyps (CRSwNP). In addition, 87%-99% of patients reported seeing a physician for their symptoms and/or taking a prescription medicine for their CRS symptoms in the past year. In fact, 60% of CRSwNP patients, 58% of severe CRSsNP patients, and 33% of moderate CRSsNP patients said they made more than five physician visits in the past year for nose and sinus symptoms.
The frequency of key CRS symptoms was similar in patients with and without nasal polyps, except that those with severe CRSsNP reported higher rates of facial pain/pressure (in the range of 60%-65%) and loss of smell/taste (46%-56%). The most frequently reported core symptoms were nasal congestion (94%-97%) and drainage (89%-92%). Despite currently available treatment options, up to 46% of CRS patients reported that they remain “extremely” affected by symptoms, and 79% of all CRS patients expressed concern about flare-ups. In addition, a large proportion of respondents expressed frustration with the inadequate symptom relief produced by their current intranasal corticosteroid (87% of CRSwNP patients, 86% of severe CRSsNP patients, and 63% of moderate CRSsNP patients).
Dr. Mahmoud said that the findings indicate a role for more and/or improved treatment options to meet the needs of this patient population. “People who have CRS have it pretty bad,” he said. “Very few people are not seeking a lot of medical care for their symptoms.”
The study was funded by OptiNose.
ATLANTA – Results from a large cross-sectional survey of adults who meet diagnostic symptom criteria for chronic rhinosinusitis (CRS) reveal that most report suffering significant symptoms, high rates of physician visits, and dissatisfaction with current intranasal steroid sprays.
“Chronic rhinosinusitis is common, it’s severe, and people are seeking a lot of medical care,” study author Ramy A. Mahmoud, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “It’s causing a lot of disruption in their lives, and they’re not satisfied with currently available intranasal steroids.”
In an effort to better characterize the prevalence and burden of illness in adults with symptoms of CRS, Dr. Mahmoud and his associates conducted a population-based survey of 10,336 U.S. adults drawn from a representative general panel of 4.3 million. They recruited participants via websites, email, phone, online communities, and social networks and collected information about nasal symptoms, frequency, duration, and severity/bother of nasal symptoms and health care use and treatments.
The researchers found that almost 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS. Of these, 63.5% were classified as having severe CRS without known nasal polyps (CRSsNP), 27% had moderate CRSsNP, and 9.6% had CRS with known nasal polyps (CRSwNP). In addition, 87%-99% of patients reported seeing a physician for their symptoms and/or taking a prescription medicine for their CRS symptoms in the past year. In fact, 60% of CRSwNP patients, 58% of severe CRSsNP patients, and 33% of moderate CRSsNP patients said they made more than five physician visits in the past year for nose and sinus symptoms.
The frequency of key CRS symptoms was similar in patients with and without nasal polyps, except that those with severe CRSsNP reported higher rates of facial pain/pressure (in the range of 60%-65%) and loss of smell/taste (46%-56%). The most frequently reported core symptoms were nasal congestion (94%-97%) and drainage (89%-92%). Despite currently available treatment options, up to 46% of CRS patients reported that they remain “extremely” affected by symptoms, and 79% of all CRS patients expressed concern about flare-ups. In addition, a large proportion of respondents expressed frustration with the inadequate symptom relief produced by their current intranasal corticosteroid (87% of CRSwNP patients, 86% of severe CRSsNP patients, and 63% of moderate CRSsNP patients).
Dr. Mahmoud said that the findings indicate a role for more and/or improved treatment options to meet the needs of this patient population. “People who have CRS have it pretty bad,” he said. “Very few people are not seeking a lot of medical care for their symptoms.”
The study was funded by OptiNose.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Nearly 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS.
Data source: A population-based survey of 10,336 US adults drawn from a representative general panel of 4.3 million.
Disclosures: The study was funded by OptiNose.
How to ‘de-label’ penicillin allergies in the hospital
ATLANTA – With the help of a pharmacist and the electronic health records system, Rochester (N.Y.) General Hospital ruled out penicillin allergies in 47 of 50 adult inpatients, and successfully transitioned them from second-line antibiotics to less expensive and more effective beta-lactam options.
The hospital’s EHR system generated a daily list of patients with a history of penicillin allergy who were on second-line options – vancomycin, daptomycin, aztreonam, linezolid, or moxifloxacin – for infections that would be better treated with beta-lactam antibiotics. An infectious disease pharmacist reviewed their history; if reported penicillin reactions were limited to a nonspecific rash more than 5 years earlier or seemed to be IgE mediated, patients were skin-prick tested to see if they really were allergic. Almost all of the 47 patients who had negative tests were switched to either a penicillin-based regimen or a cephalosporin.
Two weeks after discharge on phone follow-up, just one reported a problem: a mild rash that cleared up when he finished his course.
“We are not a major academic center, and have to prioritize what resources we have,” said Dr. Ramsey, who did most of the skin-prick tests. Triaging patients for testing based on antibiotic use is “a good way to start. We are getting this off the ground at our institution. I think it’s a good model for non-academic community hospitals,” like Rochester General, a 528-bed tertiary care center.
It’s no secret that penicillin allergies are extremely overdiagnosed, and that 90% or more of patients who carry the label – both inside and outside of hospitals – really aren’t allergic. Even so, patients are rarely tested to confirm the allergy, and instead end up on expensive second-line options that don’t work as well. They “wind up back in the hospital in a month,” Dr. Ramsey said.
The problem was a frequent topic of discussion at the American Academy of Allergy, Asthma and Immunology annual meeting, where she reported her findings. The Rochester General approach was of great interest to her audience, judging from the number of people who asked for more details after the presentation.
Most of the subjects were on the internal medicine service; the rest were surgery patients. Skin/soft tissue, bloodstream, respiratory, and intra-abdominal infections were the most common.
The investigators excluded patients with a history of severe allergic reactions, such as joint swelling or skin sloughing, as well as patients with a flat, itchy, non-urticarial rash after penicillin less than 5 years earlier. ICU patients were excluded due to consent issues.
Also for safety, the team did a one-time amoxicillin oral challenge before patients were switched to a cephalosporin.
An audience member said he’d heard of pushback on such efforts because patients are being moved to less expensive antibiotics, so hospitals won’t be able to bill as much.
Dr. Ramsey replied that Rochester General has been “extremely supportive of our efforts, because we have a good case for improving clinical outcomes with this program.” Meanwhile, the two infectious disease pharmacists at the hospital “have embraced this fully. It makes their job easier down the road because most of these patients are frequent fliers.”
Dr. Ramsey had no relevant financial disclosures.
ATLANTA – With the help of a pharmacist and the electronic health records system, Rochester (N.Y.) General Hospital ruled out penicillin allergies in 47 of 50 adult inpatients, and successfully transitioned them from second-line antibiotics to less expensive and more effective beta-lactam options.
The hospital’s EHR system generated a daily list of patients with a history of penicillin allergy who were on second-line options – vancomycin, daptomycin, aztreonam, linezolid, or moxifloxacin – for infections that would be better treated with beta-lactam antibiotics. An infectious disease pharmacist reviewed their history; if reported penicillin reactions were limited to a nonspecific rash more than 5 years earlier or seemed to be IgE mediated, patients were skin-prick tested to see if they really were allergic. Almost all of the 47 patients who had negative tests were switched to either a penicillin-based regimen or a cephalosporin.
Two weeks after discharge on phone follow-up, just one reported a problem: a mild rash that cleared up when he finished his course.
“We are not a major academic center, and have to prioritize what resources we have,” said Dr. Ramsey, who did most of the skin-prick tests. Triaging patients for testing based on antibiotic use is “a good way to start. We are getting this off the ground at our institution. I think it’s a good model for non-academic community hospitals,” like Rochester General, a 528-bed tertiary care center.
It’s no secret that penicillin allergies are extremely overdiagnosed, and that 90% or more of patients who carry the label – both inside and outside of hospitals – really aren’t allergic. Even so, patients are rarely tested to confirm the allergy, and instead end up on expensive second-line options that don’t work as well. They “wind up back in the hospital in a month,” Dr. Ramsey said.
The problem was a frequent topic of discussion at the American Academy of Allergy, Asthma and Immunology annual meeting, where she reported her findings. The Rochester General approach was of great interest to her audience, judging from the number of people who asked for more details after the presentation.
Most of the subjects were on the internal medicine service; the rest were surgery patients. Skin/soft tissue, bloodstream, respiratory, and intra-abdominal infections were the most common.
The investigators excluded patients with a history of severe allergic reactions, such as joint swelling or skin sloughing, as well as patients with a flat, itchy, non-urticarial rash after penicillin less than 5 years earlier. ICU patients were excluded due to consent issues.
Also for safety, the team did a one-time amoxicillin oral challenge before patients were switched to a cephalosporin.
An audience member said he’d heard of pushback on such efforts because patients are being moved to less expensive antibiotics, so hospitals won’t be able to bill as much.
Dr. Ramsey replied that Rochester General has been “extremely supportive of our efforts, because we have a good case for improving clinical outcomes with this program.” Meanwhile, the two infectious disease pharmacists at the hospital “have embraced this fully. It makes their job easier down the road because most of these patients are frequent fliers.”
Dr. Ramsey had no relevant financial disclosures.
ATLANTA – With the help of a pharmacist and the electronic health records system, Rochester (N.Y.) General Hospital ruled out penicillin allergies in 47 of 50 adult inpatients, and successfully transitioned them from second-line antibiotics to less expensive and more effective beta-lactam options.
The hospital’s EHR system generated a daily list of patients with a history of penicillin allergy who were on second-line options – vancomycin, daptomycin, aztreonam, linezolid, or moxifloxacin – for infections that would be better treated with beta-lactam antibiotics. An infectious disease pharmacist reviewed their history; if reported penicillin reactions were limited to a nonspecific rash more than 5 years earlier or seemed to be IgE mediated, patients were skin-prick tested to see if they really were allergic. Almost all of the 47 patients who had negative tests were switched to either a penicillin-based regimen or a cephalosporin.
Two weeks after discharge on phone follow-up, just one reported a problem: a mild rash that cleared up when he finished his course.
“We are not a major academic center, and have to prioritize what resources we have,” said Dr. Ramsey, who did most of the skin-prick tests. Triaging patients for testing based on antibiotic use is “a good way to start. We are getting this off the ground at our institution. I think it’s a good model for non-academic community hospitals,” like Rochester General, a 528-bed tertiary care center.
It’s no secret that penicillin allergies are extremely overdiagnosed, and that 90% or more of patients who carry the label – both inside and outside of hospitals – really aren’t allergic. Even so, patients are rarely tested to confirm the allergy, and instead end up on expensive second-line options that don’t work as well. They “wind up back in the hospital in a month,” Dr. Ramsey said.
The problem was a frequent topic of discussion at the American Academy of Allergy, Asthma and Immunology annual meeting, where she reported her findings. The Rochester General approach was of great interest to her audience, judging from the number of people who asked for more details after the presentation.
Most of the subjects were on the internal medicine service; the rest were surgery patients. Skin/soft tissue, bloodstream, respiratory, and intra-abdominal infections were the most common.
The investigators excluded patients with a history of severe allergic reactions, such as joint swelling or skin sloughing, as well as patients with a flat, itchy, non-urticarial rash after penicillin less than 5 years earlier. ICU patients were excluded due to consent issues.
Also for safety, the team did a one-time amoxicillin oral challenge before patients were switched to a cephalosporin.
An audience member said he’d heard of pushback on such efforts because patients are being moved to less expensive antibiotics, so hospitals won’t be able to bill as much.
Dr. Ramsey replied that Rochester General has been “extremely supportive of our efforts, because we have a good case for improving clinical outcomes with this program.” Meanwhile, the two infectious disease pharmacists at the hospital “have embraced this fully. It makes their job easier down the road because most of these patients are frequent fliers.”
Dr. Ramsey had no relevant financial disclosures.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Forty-seven of 50 inpatients turned out not to have a penicillin allergy on skin testing, and were switched to beta-lactam antibiotics. Two weeks after discharge, just one reported a problem, a mild rash. The hospital saved close to $40,000.
Data source: Pilot project at non-academic hospital.
Disclosures: Dr. Ramsey had no relevant financial disclosures.
Chronic rhinosinusitis associated with poor sleep quality
ATLANTA – Answers on a popular self-reported sleep questionnaire correlated positively with sinonasal inflammation, suggesting that patients with chronic rhinosinusitis should be assessed for sleep-related problems, results from a single-center study showed.
“We need to be recognizing the symptoms of chronic rhinosinusitis patients more in order to help them improve their quality of life,” lead study author Jessica Hui, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Asking them about sleep is important.”
Dr. Hui, who is a second-year pediatrics resident at Rush University Medical Center, reported that CRS patients had significant worse sleep quality, compared with controls (a mean PSQI score of 7.44 vs. 3.31, respectively) and that a higher Lund-Mackay Score correlated with greater PSQI (Pearson correlation coefficient of 0.25; P = .03).
The mean age of CRS cases without sleep disruption was 12.08 years, while the mean age of CRS cases with sleep disruption was 34.74 years.
Poor sleep quality was also associated with higher pain index scores (Pearson correlation coefficient of 0.35; P = .002) and higher scores on the SNOT-22 (Pearson correlation coefficient of 0.25; P = .025). The researchers observed that CRS patients without nasal polyps trended towards a higher PSQI, compared with controls (a mean of 8.14 vs. 6.36; P=0.10). Sinus histopathology variables and comorbid diseases did not correlate with PSQI scores.
Dr. Hui reported having no financial disclosures.
ATLANTA – Answers on a popular self-reported sleep questionnaire correlated positively with sinonasal inflammation, suggesting that patients with chronic rhinosinusitis should be assessed for sleep-related problems, results from a single-center study showed.
“We need to be recognizing the symptoms of chronic rhinosinusitis patients more in order to help them improve their quality of life,” lead study author Jessica Hui, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Asking them about sleep is important.”
Dr. Hui, who is a second-year pediatrics resident at Rush University Medical Center, reported that CRS patients had significant worse sleep quality, compared with controls (a mean PSQI score of 7.44 vs. 3.31, respectively) and that a higher Lund-Mackay Score correlated with greater PSQI (Pearson correlation coefficient of 0.25; P = .03).
The mean age of CRS cases without sleep disruption was 12.08 years, while the mean age of CRS cases with sleep disruption was 34.74 years.
Poor sleep quality was also associated with higher pain index scores (Pearson correlation coefficient of 0.35; P = .002) and higher scores on the SNOT-22 (Pearson correlation coefficient of 0.25; P = .025). The researchers observed that CRS patients without nasal polyps trended towards a higher PSQI, compared with controls (a mean of 8.14 vs. 6.36; P=0.10). Sinus histopathology variables and comorbid diseases did not correlate with PSQI scores.
Dr. Hui reported having no financial disclosures.
ATLANTA – Answers on a popular self-reported sleep questionnaire correlated positively with sinonasal inflammation, suggesting that patients with chronic rhinosinusitis should be assessed for sleep-related problems, results from a single-center study showed.
“We need to be recognizing the symptoms of chronic rhinosinusitis patients more in order to help them improve their quality of life,” lead study author Jessica Hui, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Asking them about sleep is important.”
Dr. Hui, who is a second-year pediatrics resident at Rush University Medical Center, reported that CRS patients had significant worse sleep quality, compared with controls (a mean PSQI score of 7.44 vs. 3.31, respectively) and that a higher Lund-Mackay Score correlated with greater PSQI (Pearson correlation coefficient of 0.25; P = .03).
The mean age of CRS cases without sleep disruption was 12.08 years, while the mean age of CRS cases with sleep disruption was 34.74 years.
Poor sleep quality was also associated with higher pain index scores (Pearson correlation coefficient of 0.35; P = .002) and higher scores on the SNOT-22 (Pearson correlation coefficient of 0.25; P = .025). The researchers observed that CRS patients without nasal polyps trended towards a higher PSQI, compared with controls (a mean of 8.14 vs. 6.36; P=0.10). Sinus histopathology variables and comorbid diseases did not correlate with PSQI scores.
Dr. Hui reported having no financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: CRS patients had significant worse sleep quality, compared with controls (a mean Pittsburgh Sleep Quality Index score of 7.44 vs. 3.31, respectively).
Data source: A cohort study of 125 CRS patients with refractory disease and 41 controls.
Disclosures: Dr. Hui reported having no financial disclosures.
Oral immunotherapy induced wheat allergy tolerance
ATLANTA – Oral immunotherapy with wheat gluten flour decreased wheat reactions in some allergic patients, according to a trial reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Wheat allergy is fairly common in young children; most people outgrow it. For some, though, it remains a dangerous problem, especially because wheat is almost impossible to avoid, said senior investigator Hugh Sampson, MD, the Kurt Hirschhorn Professor of Pediatrics at the Icahn School of Medicine at Mount Sinai and director of the Jaffe Food Allergy Institute at Mount Sinai Hospital, New York.
For example, one of the study subjects stopped for a bite on the way to the clinic. “He thought he ate a wheat-free breakfast but ended up arriving with anaphylaxis,” the physician said.
Gluten is the protein-rich allergenic part of wheat; using gluten flour instead of regular flour allowed for smaller, more convenient doses. It was sprinkled over applesauce and other foods. Twelve of the 23 gluten flour subjects (52%), but no one in the placebo group, tolerated a challenge of 4,443 mg at 1 year.
Next, placebo subjects were started on their own gluten flour regimen, but they shot for a higher maximum dose of 3,870 mg/day. The original gluten group stayed on their dose, a maximum of 1,445 mg/day.
The higher dose was more effective at 2-year follow-up; 7 of 23 patients (30%) in the 1,445-mg group tolerated a challenge of 7,443 mg at 2 years, versus 12 of 21 patients (57%) in the 3,870-mg group.
Just over 10% of the doses triggered adverse reactions. Most of the reactions were mild – itching in the throat or mouth, nausea, and the like – but epinephrine was needed after 0.05% of the doses. The adverse reaction rate was similar to that with other forms of oral immunotherapy, and there were no statistically significant differences in the number of reactions between the low- and high-dose gluten groups.
For anyone who reads the study and thinks about running to the grocery store for gluten flour, Dr. Sampson cautioned against it. There’s no Food and Drug Administration–approved product, and, more importantly, “you can run into [serious medical] problems” if, for instance, immunotherapy triggers anaphylaxis with too much exercise afterward.
The study “has nothing to do with” the kind of gluten intolerance that’s led to an explosion in gluten-free products in recent years, he said. “Our study was directed at IgE-mediated reactions. Celiac disease has a very different mechanism.”
Also, only a few people remained tolerant after being backed off wheat immunotherapy for a couple of months. There’s no such thing as a cure for food allergies at this point.
“We are trying to get people into remission. Nobody yet has demonstrated that you can make a permanent change in somebody [who] is hypersensitive, even to a bee sting,” Dr. Sampson said.
The study results are big enough to protect wheat-allergic people from accidental exposure. In the case of the study subject who reacted to wheat in his breakfast, he probably reacted to far less than 7,443 mg of wheat protein – about the amount in a plate of pasta – before immunotherapy. No one with a wheat allergy is intentionally going to order something like that, Dr. Sampson said.
The next step is industry funding for a larger trial. “These studies are expensive, and we hope somebody will take up the torch. We have to get industry involved,” he said.
Private philanthropies funded the work. Dr. Sampson is chief scientific officer for DBV Technologies, a company developing a patch for peanut allergies.
ATLANTA – Oral immunotherapy with wheat gluten flour decreased wheat reactions in some allergic patients, according to a trial reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Wheat allergy is fairly common in young children; most people outgrow it. For some, though, it remains a dangerous problem, especially because wheat is almost impossible to avoid, said senior investigator Hugh Sampson, MD, the Kurt Hirschhorn Professor of Pediatrics at the Icahn School of Medicine at Mount Sinai and director of the Jaffe Food Allergy Institute at Mount Sinai Hospital, New York.
For example, one of the study subjects stopped for a bite on the way to the clinic. “He thought he ate a wheat-free breakfast but ended up arriving with anaphylaxis,” the physician said.
Gluten is the protein-rich allergenic part of wheat; using gluten flour instead of regular flour allowed for smaller, more convenient doses. It was sprinkled over applesauce and other foods. Twelve of the 23 gluten flour subjects (52%), but no one in the placebo group, tolerated a challenge of 4,443 mg at 1 year.
Next, placebo subjects were started on their own gluten flour regimen, but they shot for a higher maximum dose of 3,870 mg/day. The original gluten group stayed on their dose, a maximum of 1,445 mg/day.
The higher dose was more effective at 2-year follow-up; 7 of 23 patients (30%) in the 1,445-mg group tolerated a challenge of 7,443 mg at 2 years, versus 12 of 21 patients (57%) in the 3,870-mg group.
Just over 10% of the doses triggered adverse reactions. Most of the reactions were mild – itching in the throat or mouth, nausea, and the like – but epinephrine was needed after 0.05% of the doses. The adverse reaction rate was similar to that with other forms of oral immunotherapy, and there were no statistically significant differences in the number of reactions between the low- and high-dose gluten groups.
For anyone who reads the study and thinks about running to the grocery store for gluten flour, Dr. Sampson cautioned against it. There’s no Food and Drug Administration–approved product, and, more importantly, “you can run into [serious medical] problems” if, for instance, immunotherapy triggers anaphylaxis with too much exercise afterward.
The study “has nothing to do with” the kind of gluten intolerance that’s led to an explosion in gluten-free products in recent years, he said. “Our study was directed at IgE-mediated reactions. Celiac disease has a very different mechanism.”
Also, only a few people remained tolerant after being backed off wheat immunotherapy for a couple of months. There’s no such thing as a cure for food allergies at this point.
“We are trying to get people into remission. Nobody yet has demonstrated that you can make a permanent change in somebody [who] is hypersensitive, even to a bee sting,” Dr. Sampson said.
The study results are big enough to protect wheat-allergic people from accidental exposure. In the case of the study subject who reacted to wheat in his breakfast, he probably reacted to far less than 7,443 mg of wheat protein – about the amount in a plate of pasta – before immunotherapy. No one with a wheat allergy is intentionally going to order something like that, Dr. Sampson said.
The next step is industry funding for a larger trial. “These studies are expensive, and we hope somebody will take up the torch. We have to get industry involved,” he said.
Private philanthropies funded the work. Dr. Sampson is chief scientific officer for DBV Technologies, a company developing a patch for peanut allergies.
ATLANTA – Oral immunotherapy with wheat gluten flour decreased wheat reactions in some allergic patients, according to a trial reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Wheat allergy is fairly common in young children; most people outgrow it. For some, though, it remains a dangerous problem, especially because wheat is almost impossible to avoid, said senior investigator Hugh Sampson, MD, the Kurt Hirschhorn Professor of Pediatrics at the Icahn School of Medicine at Mount Sinai and director of the Jaffe Food Allergy Institute at Mount Sinai Hospital, New York.
For example, one of the study subjects stopped for a bite on the way to the clinic. “He thought he ate a wheat-free breakfast but ended up arriving with anaphylaxis,” the physician said.
Gluten is the protein-rich allergenic part of wheat; using gluten flour instead of regular flour allowed for smaller, more convenient doses. It was sprinkled over applesauce and other foods. Twelve of the 23 gluten flour subjects (52%), but no one in the placebo group, tolerated a challenge of 4,443 mg at 1 year.
Next, placebo subjects were started on their own gluten flour regimen, but they shot for a higher maximum dose of 3,870 mg/day. The original gluten group stayed on their dose, a maximum of 1,445 mg/day.
The higher dose was more effective at 2-year follow-up; 7 of 23 patients (30%) in the 1,445-mg group tolerated a challenge of 7,443 mg at 2 years, versus 12 of 21 patients (57%) in the 3,870-mg group.
Just over 10% of the doses triggered adverse reactions. Most of the reactions were mild – itching in the throat or mouth, nausea, and the like – but epinephrine was needed after 0.05% of the doses. The adverse reaction rate was similar to that with other forms of oral immunotherapy, and there were no statistically significant differences in the number of reactions between the low- and high-dose gluten groups.
For anyone who reads the study and thinks about running to the grocery store for gluten flour, Dr. Sampson cautioned against it. There’s no Food and Drug Administration–approved product, and, more importantly, “you can run into [serious medical] problems” if, for instance, immunotherapy triggers anaphylaxis with too much exercise afterward.
The study “has nothing to do with” the kind of gluten intolerance that’s led to an explosion in gluten-free products in recent years, he said. “Our study was directed at IgE-mediated reactions. Celiac disease has a very different mechanism.”
Also, only a few people remained tolerant after being backed off wheat immunotherapy for a couple of months. There’s no such thing as a cure for food allergies at this point.
“We are trying to get people into remission. Nobody yet has demonstrated that you can make a permanent change in somebody [who] is hypersensitive, even to a bee sting,” Dr. Sampson said.
The study results are big enough to protect wheat-allergic people from accidental exposure. In the case of the study subject who reacted to wheat in his breakfast, he probably reacted to far less than 7,443 mg of wheat protein – about the amount in a plate of pasta – before immunotherapy. No one with a wheat allergy is intentionally going to order something like that, Dr. Sampson said.
The next step is industry funding for a larger trial. “These studies are expensive, and we hope somebody will take up the torch. We have to get industry involved,” he said.
Private philanthropies funded the work. Dr. Sampson is chief scientific officer for DBV Technologies, a company developing a patch for peanut allergies.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Twelve of the 23 gluten flour subjects (52%), but no one in the placebo group, tolerated a challenge dose of 4,443 mg at 1 year.
Data source: A randomized trial of 46 wheat-allergic patients.
Disclosures: Private philanthropies funded the work. The senior investigator is chief scientific officer for DBV Technologies, a company developing a patch for peanut allergies.
Send children who had anaphylaxis home after 4 hours
ATLANTA – Four hours in the ED are enough to observe most children after anaphylaxis; if they are doing well, they don’t need to be admitted for longer observation, according to Children’s Hospital of Philadelphia investigators.
The right amount of time for observation after anaphylaxis has been debated for years. The current recommendation is 4-8 hours for both children and adults, but it’s a broad range, and some still argue for 24 hours. At least at the Children’s Hospital of Philadelphia (CHOP), much more than 4 hours means that patients need to be admitted to an inpatient floor, or a special extend-care ED unit.
In the fall of 2014, “we decided as a compromise not to go all the way down to 2.5 hours” in the ED, “but to stay within guidelines and go for 4 hours,” so long as kids don’t have asthma or need two epinephrine injections in the ED or have other risks for biphasic reactions, said lead investigator and pediatrician Juhee Lee, MD.
The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED physicians opted for 4-hour observation in 2014, a near 60% reduction.
Shorter observation was safe; 1% of patients (1/76) came back to the ED within 72 hours before the change, versus 3% (5/194) afterward, a statistically insignificant difference. None of the revisits in either cohort was for severe anaphylaxis symptoms.
“It was surprising to see how significantly we could reduce the admission rate. A 4-hour length of observation appears safe and could drastically improve ED patient flow and decrease hospitalization rates,” Dr. Lee said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
About two-thirds of the ED visits in both groups were for food reactions, most commonly peanut and tree nut allergies.
The CHOP ED also changed how its clinicians treat anaphylaxis over the study period. Children used to get antihistamines and steroids in the ED, and were sent home with 5 days’ worth of both. They also left the ED with a prescription for epinephrine, but not an actual autoinjector.
Now, CHOP lets providers decide if children need antihistamines and steroids in the ED, and sends most home with an EpiPen.
Dr. Lee didn’t have any disclosures. There was no outside funding.
ATLANTA – Four hours in the ED are enough to observe most children after anaphylaxis; if they are doing well, they don’t need to be admitted for longer observation, according to Children’s Hospital of Philadelphia investigators.
The right amount of time for observation after anaphylaxis has been debated for years. The current recommendation is 4-8 hours for both children and adults, but it’s a broad range, and some still argue for 24 hours. At least at the Children’s Hospital of Philadelphia (CHOP), much more than 4 hours means that patients need to be admitted to an inpatient floor, or a special extend-care ED unit.
In the fall of 2014, “we decided as a compromise not to go all the way down to 2.5 hours” in the ED, “but to stay within guidelines and go for 4 hours,” so long as kids don’t have asthma or need two epinephrine injections in the ED or have other risks for biphasic reactions, said lead investigator and pediatrician Juhee Lee, MD.
The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED physicians opted for 4-hour observation in 2014, a near 60% reduction.
Shorter observation was safe; 1% of patients (1/76) came back to the ED within 72 hours before the change, versus 3% (5/194) afterward, a statistically insignificant difference. None of the revisits in either cohort was for severe anaphylaxis symptoms.
“It was surprising to see how significantly we could reduce the admission rate. A 4-hour length of observation appears safe and could drastically improve ED patient flow and decrease hospitalization rates,” Dr. Lee said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
About two-thirds of the ED visits in both groups were for food reactions, most commonly peanut and tree nut allergies.
The CHOP ED also changed how its clinicians treat anaphylaxis over the study period. Children used to get antihistamines and steroids in the ED, and were sent home with 5 days’ worth of both. They also left the ED with a prescription for epinephrine, but not an actual autoinjector.
Now, CHOP lets providers decide if children need antihistamines and steroids in the ED, and sends most home with an EpiPen.
Dr. Lee didn’t have any disclosures. There was no outside funding.
ATLANTA – Four hours in the ED are enough to observe most children after anaphylaxis; if they are doing well, they don’t need to be admitted for longer observation, according to Children’s Hospital of Philadelphia investigators.
The right amount of time for observation after anaphylaxis has been debated for years. The current recommendation is 4-8 hours for both children and adults, but it’s a broad range, and some still argue for 24 hours. At least at the Children’s Hospital of Philadelphia (CHOP), much more than 4 hours means that patients need to be admitted to an inpatient floor, or a special extend-care ED unit.
In the fall of 2014, “we decided as a compromise not to go all the way down to 2.5 hours” in the ED, “but to stay within guidelines and go for 4 hours,” so long as kids don’t have asthma or need two epinephrine injections in the ED or have other risks for biphasic reactions, said lead investigator and pediatrician Juhee Lee, MD.
The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED physicians opted for 4-hour observation in 2014, a near 60% reduction.
Shorter observation was safe; 1% of patients (1/76) came back to the ED within 72 hours before the change, versus 3% (5/194) afterward, a statistically insignificant difference. None of the revisits in either cohort was for severe anaphylaxis symptoms.
“It was surprising to see how significantly we could reduce the admission rate. A 4-hour length of observation appears safe and could drastically improve ED patient flow and decrease hospitalization rates,” Dr. Lee said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
About two-thirds of the ED visits in both groups were for food reactions, most commonly peanut and tree nut allergies.
The CHOP ED also changed how its clinicians treat anaphylaxis over the study period. Children used to get antihistamines and steroids in the ED, and were sent home with 5 days’ worth of both. They also left the ED with a prescription for epinephrine, but not an actual autoinjector.
Now, CHOP lets providers decide if children need antihistamines and steroids in the ED, and sends most home with an EpiPen.
Dr. Lee didn’t have any disclosures. There was no outside funding.
Key clinical point:
Major finding: The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED doctors opted for 4-hour observation in 2014, a near 60% reduction.
Data source: A study of 182 children who underwent 8-hour observation and 257 children who underwent 4-hour observation at the Children’s Hospital of Philadelphia ED.
Disclosures: The lead investigator didn’t have any disclosures. There was no outside funding.
Gender impacts risk of atopic diseases
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001).
Data source: A retrospective review of 113 children who were evaluated for a history of allergic rhinitis, eczema, asthma, food allergies, and IgE, the percentage of eosinophils, and absolute eosinophil counts.
Disclosures: Dr. Nagarajan reported having no financial disclosures.
In children, peanut IgE levels increase over time
ATLANTA – Within a cohort of children diagnosed with peanut allergy who were followed since 2001, peanut IgE levels increased significantly over time in all races, according to a preliminary analysis of data.
In an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology, lead study author Yasmin Hamzavi, MD, said that recent publications have implicated race as a factor in food sensitization, but how this may impact the management of food allergy has not been elucidated. “The novel aspect of this study is that we are not just looking at the baseline peanut IgE levels between the races, but at the rate of change in peanut IgE over time between the different races,” said Dr. Hamzavi, a fellow in the division of allergy and immunology at Northwell Health, Great Neck, N.Y. “My question was, does the level of peanut IgE decrease or increase faster in one race versus another?”
A significant increase in peanut IgE over time was observed among all races (P less than .0002). In addition, white and Asian children showed an increasing trend in peanut IgE, while black children demonstrated a decreasing trend over time (P less than .099), a finding that Dr. Hamzavi described as “surprising and unusual.” She called for larger studies exploring factors for the noted increase among all races, such as changes in testing methods, food avoidance, and increasing sensitization. “Understanding the changes in peanut sensitization over time is a crucial step in determining the likelihood of clinical reactivity,” she said.
Dr. Hamzavi is reviewing data for a similar analysis of children with milk and egg allergy. She reported having no financial disclosures.
ATLANTA – Within a cohort of children diagnosed with peanut allergy who were followed since 2001, peanut IgE levels increased significantly over time in all races, according to a preliminary analysis of data.
In an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology, lead study author Yasmin Hamzavi, MD, said that recent publications have implicated race as a factor in food sensitization, but how this may impact the management of food allergy has not been elucidated. “The novel aspect of this study is that we are not just looking at the baseline peanut IgE levels between the races, but at the rate of change in peanut IgE over time between the different races,” said Dr. Hamzavi, a fellow in the division of allergy and immunology at Northwell Health, Great Neck, N.Y. “My question was, does the level of peanut IgE decrease or increase faster in one race versus another?”
A significant increase in peanut IgE over time was observed among all races (P less than .0002). In addition, white and Asian children showed an increasing trend in peanut IgE, while black children demonstrated a decreasing trend over time (P less than .099), a finding that Dr. Hamzavi described as “surprising and unusual.” She called for larger studies exploring factors for the noted increase among all races, such as changes in testing methods, food avoidance, and increasing sensitization. “Understanding the changes in peanut sensitization over time is a crucial step in determining the likelihood of clinical reactivity,” she said.
Dr. Hamzavi is reviewing data for a similar analysis of children with milk and egg allergy. She reported having no financial disclosures.
ATLANTA – Within a cohort of children diagnosed with peanut allergy who were followed since 2001, peanut IgE levels increased significantly over time in all races, according to a preliminary analysis of data.
In an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology, lead study author Yasmin Hamzavi, MD, said that recent publications have implicated race as a factor in food sensitization, but how this may impact the management of food allergy has not been elucidated. “The novel aspect of this study is that we are not just looking at the baseline peanut IgE levels between the races, but at the rate of change in peanut IgE over time between the different races,” said Dr. Hamzavi, a fellow in the division of allergy and immunology at Northwell Health, Great Neck, N.Y. “My question was, does the level of peanut IgE decrease or increase faster in one race versus another?”
A significant increase in peanut IgE over time was observed among all races (P less than .0002). In addition, white and Asian children showed an increasing trend in peanut IgE, while black children demonstrated a decreasing trend over time (P less than .099), a finding that Dr. Hamzavi described as “surprising and unusual.” She called for larger studies exploring factors for the noted increase among all races, such as changes in testing methods, food avoidance, and increasing sensitization. “Understanding the changes in peanut sensitization over time is a crucial step in determining the likelihood of clinical reactivity,” she said.
Dr. Hamzavi is reviewing data for a similar analysis of children with milk and egg allergy. She reported having no financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: A significant increase in peanut IgE over time was observed among white, black, and Asian children (P less than .0002).
Data source: A retrospective review of 193 children diagnosed with peanut allergy .
Disclosures: Dr. Hamzavi reported having no financial disclosures.
Tree nut allergy responds to immunotherapy
AT 2017 AAAAI ANNUAL MEETING
ATLANTA – Long-term walnut oral immunotherapy induces clinically relevant treatment response in children with tree nut allergy, results from a small ongoing study showed.
“Tree nut allergy is a generally life-long and potentially life-threatening disorder without an active therapy,” study author Amy M. Scurlock, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Significant clinical, immunologic, and serologic cross reactivity has been described among tree nut families. Multi–tree nut allergen sensitization is common with 46% reporting allergy to more than one tree nut.”
Dr. Scurlock reported on results from 9 of 14 children with allergy to walnuts and another test tree nut (pecans, cashews, hazelnuts, or pistachios) who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment. Walnut and test tree nut desensitization oral food challenges were performed by 142 weeks. If they passed, the subjects stopped their treatment for four weeks. Next, they underwent another oral food challenge to determine if they continued to be sensitized or if they had developed sustained unresponsiveness.
The median age of the 14 randomized subjects was 9 years, 75% were male, and 9 (64%) underwent an oral food challenge by week 142. (Two subjects dropped out after randomization, and three have yet to reach the week 142 time point.) Desensitization to both walnut and a test tree nut was observed in seven out of the nine subjects (78%). After 4 weeks off of walnut oral immunotherapy, four out of those seven patients who were desensitized (57%) also demonstrated sustained unresponsiveness to both walnuts and test tree nuts, and six out of seven subjects (86%) had sustained unresponsiveness to just walnuts.
“I am always amazed by the commitment of our food allergic subjects and their families in immunotherapy trials, and this study is no exception,” Dr. Scurlock commented. “Subjects had to undergo an increased number of oral food challenges (walnut, test tree nut, placebo) at protocol-specified time points in addition to daily home dosing. While walnut oral immunotherapy was generally well tolerated, we frequently observed oral allergy/itching associated with dosing in our cohort, which was an atopic group (75% allergic rhinitis). These symptoms can complicate assessment during dosing and oral food challenges. We observed that, with long-term therapy, there were some subjects who developed ‘dosing fatigue’ that could adversely affect adherence. Future studies will need to focus on strategies that optimize long-term sustainability/tolerability of dosing.”
She acknowledged certain limitations of the study, including its single-center design and small sample size. “While the findings are encouraging and similar to outcomes observed in other oral immunotherapy trials, further study in larger cohorts is critical before advancing toward broad clinical implementation. Specific issues regarding complexity of cross-reactivity and the efficacy of specific tree nuts to induce immunomodulation across tree nut families require future study. In addition, improving the long-term sustainability/tolerability of dosing and examining novel approaches is important.”
Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.
AT 2017 AAAAI ANNUAL MEETING
ATLANTA – Long-term walnut oral immunotherapy induces clinically relevant treatment response in children with tree nut allergy, results from a small ongoing study showed.
“Tree nut allergy is a generally life-long and potentially life-threatening disorder without an active therapy,” study author Amy M. Scurlock, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Significant clinical, immunologic, and serologic cross reactivity has been described among tree nut families. Multi–tree nut allergen sensitization is common with 46% reporting allergy to more than one tree nut.”
Dr. Scurlock reported on results from 9 of 14 children with allergy to walnuts and another test tree nut (pecans, cashews, hazelnuts, or pistachios) who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment. Walnut and test tree nut desensitization oral food challenges were performed by 142 weeks. If they passed, the subjects stopped their treatment for four weeks. Next, they underwent another oral food challenge to determine if they continued to be sensitized or if they had developed sustained unresponsiveness.
The median age of the 14 randomized subjects was 9 years, 75% were male, and 9 (64%) underwent an oral food challenge by week 142. (Two subjects dropped out after randomization, and three have yet to reach the week 142 time point.) Desensitization to both walnut and a test tree nut was observed in seven out of the nine subjects (78%). After 4 weeks off of walnut oral immunotherapy, four out of those seven patients who were desensitized (57%) also demonstrated sustained unresponsiveness to both walnuts and test tree nuts, and six out of seven subjects (86%) had sustained unresponsiveness to just walnuts.
“I am always amazed by the commitment of our food allergic subjects and their families in immunotherapy trials, and this study is no exception,” Dr. Scurlock commented. “Subjects had to undergo an increased number of oral food challenges (walnut, test tree nut, placebo) at protocol-specified time points in addition to daily home dosing. While walnut oral immunotherapy was generally well tolerated, we frequently observed oral allergy/itching associated with dosing in our cohort, which was an atopic group (75% allergic rhinitis). These symptoms can complicate assessment during dosing and oral food challenges. We observed that, with long-term therapy, there were some subjects who developed ‘dosing fatigue’ that could adversely affect adherence. Future studies will need to focus on strategies that optimize long-term sustainability/tolerability of dosing.”
She acknowledged certain limitations of the study, including its single-center design and small sample size. “While the findings are encouraging and similar to outcomes observed in other oral immunotherapy trials, further study in larger cohorts is critical before advancing toward broad clinical implementation. Specific issues regarding complexity of cross-reactivity and the efficacy of specific tree nuts to induce immunomodulation across tree nut families require future study. In addition, improving the long-term sustainability/tolerability of dosing and examining novel approaches is important.”
Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.
AT 2017 AAAAI ANNUAL MEETING
ATLANTA – Long-term walnut oral immunotherapy induces clinically relevant treatment response in children with tree nut allergy, results from a small ongoing study showed.
“Tree nut allergy is a generally life-long and potentially life-threatening disorder without an active therapy,” study author Amy M. Scurlock, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Significant clinical, immunologic, and serologic cross reactivity has been described among tree nut families. Multi–tree nut allergen sensitization is common with 46% reporting allergy to more than one tree nut.”
Dr. Scurlock reported on results from 9 of 14 children with allergy to walnuts and another test tree nut (pecans, cashews, hazelnuts, or pistachios) who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment. Walnut and test tree nut desensitization oral food challenges were performed by 142 weeks. If they passed, the subjects stopped their treatment for four weeks. Next, they underwent another oral food challenge to determine if they continued to be sensitized or if they had developed sustained unresponsiveness.
The median age of the 14 randomized subjects was 9 years, 75% were male, and 9 (64%) underwent an oral food challenge by week 142. (Two subjects dropped out after randomization, and three have yet to reach the week 142 time point.) Desensitization to both walnut and a test tree nut was observed in seven out of the nine subjects (78%). After 4 weeks off of walnut oral immunotherapy, four out of those seven patients who were desensitized (57%) also demonstrated sustained unresponsiveness to both walnuts and test tree nuts, and six out of seven subjects (86%) had sustained unresponsiveness to just walnuts.
“I am always amazed by the commitment of our food allergic subjects and their families in immunotherapy trials, and this study is no exception,” Dr. Scurlock commented. “Subjects had to undergo an increased number of oral food challenges (walnut, test tree nut, placebo) at protocol-specified time points in addition to daily home dosing. While walnut oral immunotherapy was generally well tolerated, we frequently observed oral allergy/itching associated with dosing in our cohort, which was an atopic group (75% allergic rhinitis). These symptoms can complicate assessment during dosing and oral food challenges. We observed that, with long-term therapy, there were some subjects who developed ‘dosing fatigue’ that could adversely affect adherence. Future studies will need to focus on strategies that optimize long-term sustainability/tolerability of dosing.”
She acknowledged certain limitations of the study, including its single-center design and small sample size. “While the findings are encouraging and similar to outcomes observed in other oral immunotherapy trials, further study in larger cohorts is critical before advancing toward broad clinical implementation. Specific issues regarding complexity of cross-reactivity and the efficacy of specific tree nuts to induce immunomodulation across tree nut families require future study. In addition, improving the long-term sustainability/tolerability of dosing and examining novel approaches is important.”
Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.
Key clinical point:
Major finding: Of nine subjects who underwent walnut and test tree nut desensitization oral food challenges by week 142, desensitization to both was observed in seven (78%).
Data source: A review of 14 children with allergy to walnuts and another test tree nut who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment.
Disclosures: Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.